Abstract
Hydrophobic interactions govern specificity for natural antimicrobial peptides. No such relationship has been established for synthetic peptoids that mimic antimicrobial peptides. Peptoid macrocycles synthesized with five different aromatic groups are investigated by minimum inhibitory and hemolytic concentration assays, epifluorescence microscopy, atomic force microscopy, and X-ray reflectivity. Peptoid hydrophobicity is determined using high performance liquid chromatography. Disruption of bacterial but not eukaryotic lipid membranes is demonstrated on the solid supported lipid bilayers and Langmuir monolayers. X-ray reflectivity studies demonstrate that intercalation of peptoids with zwitterionic or negatively charged lipid membranes is found to be regulated by hydrophobicity. Critical levels of peptoid selectivity are demonstrated and found to be modulated by their hydrophobic groups. It is suggested that peptoids may follow different optimization schemes as compared to their natural analogues.
Keywords: Antimicrobial peptoids, lipid membranes, CYTOTOXICITY, AFM, HYDROPHOBICITY, X-ray scattering
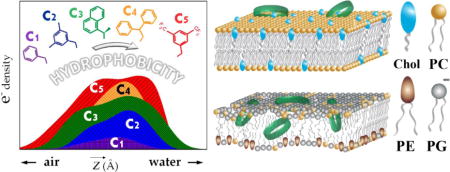
Graphical abstract
1. Introduction
Antimicrobial peptides (AMPs) are a component of innate immunity that exhibit broad-spectrum antimicrobial activity [1, 2]. AMPs target bacterial cells by electrostatic interactions between the positively charged peptide moieties and the negatively charged lipid headgroups [3, 4]. The surface of nearly all bacteria has a net negative charge, while eukaryotic membranes are typically zwitterionic [5, 6]. Selectivity is largely thought to be due to elementary electrostatics [7]. However, antimicrobial peptides are still capable of interacting with mammalian cells by permeating into the hydrophobic core of lipid bilayer [8]. Links between cytotoxicity and peptide structural differences are not well understood yet [9–11]. Larger hydrophobic content of an AMP enhances its antimicrobial activity up to a critical level [12, 13]. Further increases in net hydrophobicity are associated with the reduced selectivity [14, 15]. For example, magainin-II displays bactericidal activity against Gram-negative species, whereas its analogs with higher numbers of non-polar moieties also inhibit growth of both Gram-positive bacteria and eukaryotic cells [16, 17].
Comparative analysis of a novel family of synthetic AMPs composed of oligo-lysine sequences adjacent to an Ala/Trp/Phe linker shows two steps in membrane insertion [18]. The first step corresponds to the minimal hydrophobicity required for peptides to be transferred from the aqueous environment to the negatively charged lipid bilayer, whereas reaching the second critical point allows AMP to interact with neutral lipids [19]. Stark et al. demonstrated that by increasing the number of cationic residues the peptide sequence can be converted into a highly active amphipathic molecule [20]. If average hydrophobicity is above the second critical point, the peptides become capable of disrupting eukaryotic membranes [21].
Clinical application of AMPs has been unsuccessful [22, 23]. Developing novel synthetic molecules that demonstrate high selectivity and activity is therefore crucial [24]. In poly-N-substituted glycines, or “peptoids”, the amino acid side-chains are linked to the peptide backbone trough amide nitrogen rather than the α-carbon [25, 26]. Peptoids display high antimicrobial efficacy in vitro and in vivo [27–29] accompanied by the resistivity towards proteolytic degradation [30]. Creating peptoid-based antimicrobials suitable as therapeutics requires that cytotoxic effects be minimal. The mechanisms of interaction between peptoids and membrane lipids remain poorly defined. Better understanding of their structure-function relationships is needed [31].
Peptoid macrocycles have previously demonstrated superior antimicrobial potency as compared to the linear AMP mimics [32–35]. Conformational constraint achieved by macrocyclization facilitates peptoids penetration through the hydrophobic core of membrane bilayer [36, 37]. Five macrocyclic peptoid hexamers with different aromatic groups, hereafter referred to C1-C5 are used for this study (Fig. 1A). To compare peptide vs. peptoid backbone an amphiphilic cyclopeptide gramicidin S composed of a ten amino acids sequence – cyclo-(Val-Orn-Leu-D-Phe-Pro)2 [38] is tested along with decameric peptoid macrocycle C1dec. Peptoid monomer subunits with designators are depicted in Fig. 1B.
Fig. 1.
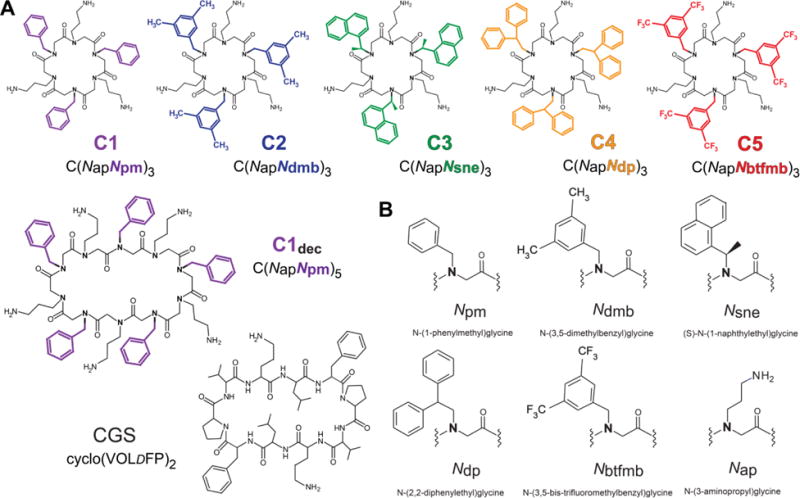
(A) Molecular structures of peptoids and gramicidin S (CGS). Aromatic groups are highlighted in: C1 – magenta; C2 – blue; C3 – green; C4 – orange and C5 – red. The color designation is consistent throughout the text. O – ornithine, DF – D-phenylalanine residues. (B) Nomenclature and abbreviations for peptoid monomer subunits.
Bacteriostatic effect of antimicrobials is assessed by minimum inhibitory concentration (MIC) against methicillin-resistant Staphylococcus aureus, or MRSA, which is a typical risk-associated pathogenic BSL-2 microorganism. MRSA infections pose a serious threat for public health and are difficult to be cured due to the outstanding resistivity towards antibiotics currently in use [39]. Antimicrobial agents recommended by Infectious Diseases Society of America (IDSA) for their treatment include vancomycin, daptomycin and tetracycline antibiotics [40]. MIC values for vancomycin against MRSA USA300 Los Angeles County clone (LAC) range from 0.71 to 0.89 μgmL−1 [41, 42]. However, the reported clinical efficacy is only 35-57% [43, 44] with a high risk of drug-induced hematologic disorders [45, 46]. In vitro cytotoxicity is determined as the rate of hemolysis on human erythrocytes. The sensitivity of red blood cells is a major clinical parameter by antimicrobial therapies.
We investigate the molecular basis for peptoid interactions with bacterial and eukaryotic lipid membranes on the sub-nanometer scale using Langmuir monolayers and solid-supported bilayers as a model system. Our results unravel the role of peptoid hydrophobic content for their selectivity between pathogen and host cells.
2. Materials and methods
2.1. Peptide/peptoid synthesis
Sequence-specific peptoids macrocycles have been synthesized on 2-chlorotrityl resin, using previously described “sub-monomer chemistry” approach followed by macrocyclization of linear precursors [47]. Sequential steps of bromoacylation and nucleophilic displacement by a primary amine forms the side chain of interest. To achieve ‘head-to-tail’ cyclization, N-acetylated linear precursors were synthesized on 2-chlorotrityl chloride resin to generate free N-terminal amino and C-terminal carboxylic acid groups, which are then cleaved with 20% hexafluoroisopropanol and dichloromethane. Reaction of macrocyclization was conducted by benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP) as an acylating agent [32]. Gramicidin S was synthesized according to the standard Fmoc solid-phase peptide synthesis protocols [48].
2.2. High-performance liquid chromatography
The retention times (tR) characterized by reversed-phase high-performance liquid chromatography (RP-HPLC) and calculated mass ratios of hydrophobic active groups are used to evaluate peptoid hydrophobicity. RP-HPLC analysis was performed by using a C18 column operated under a 5 to 95% acetonitrile in water (0.1% TFA) gradient at a 0.7 mL min−1 flow rate monitored by UV absorbance at 214 nm [49].
2.3. Antibacterial assays
Methicillin-resistant Staphylococcus aureus (MRSA) pulse-field gel electrophoresis types USA300 (strain LAC) [50] were grown in tryptic soy broth (BD Biosciences) as previously described [51]. Antimicrobial susceptibility has been evaluated using the broth-macrodilution procedure in 96-well plates outlined in document M07-A7 of the CLSI [52]. After incubation at 37°C for 18 to 24 hours in either LB (Luria-Bertani) or RPMI media, the turbidity of each sample was analyzed, and the minimum inhibitory concentration (MIC) was defined as the lowest concentration of peptoid resulting in an optically clear bacterial culture. Experiments were conducted in three independent replicates of three parallel trials to ensure statistical significance.
2.4. Rate of hemolysis
Hemolytic concentrations have been determined against fresh human erythrocytes in PBS (phosphate-buffered saline) solution. Treatment of erythrocytes with a detergent (1% Triton X-100) solution and incubation in the vehicle buffer (PBS) were used as a positive and negative control, respectively. Percentages of hemolysis caused by each of the compounds have been defined as [(A – Atestblank)/(Acontrol – Ablank)] × 100, where A is the absorbance of the test well; Acontrol – the average absorbance of wells with erythrocytes in pure PBS; Atestblank – the average absorbance of erythrocytes exposed to PBS and peptoids, and Ablank – the average absorbance of wells with PBS alone [49]. Experiments were conducted in three independent replicates of three parallel trials.
2.5. Langmuir insertion assays
Monolayers composed of dipalmitoyl-sn-glycero-3 phosphatidylcholine (DPPC) and cholesterol (1:1 molar ratio) were employed to construct model system mimicking the eukaryotic (mammalian) plasma membrane [53]. The interactions of peptoids with Staphylococcus aureus were modeled using monolayers composed of anionic 1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol (DPPG) [54]. The reason behind this choice is as follows: phosphatidylglycerol is the most abundant phospholipid in nearly all Gram-negative bacteria, while PC and cholesterol in a specific proportion constitute the major lipid fraction of mammalian cell membranes [55, 56].
The instrumental setup consisted of a custom-made Teflon Langmuir trough equipped with two Teflon barriers controlled by motors for symmetric compression or expansion of monolayers at the air-liquid interface. The monolayer surface pressure was measured by a stationary Wilhelmy plate accompanied with the sensor system (Riegler & Kirstein, Potsdam, Germany) and kept at a constant pressure by a pressure-area feedback loop throughout the duration of experiment. Dulbecco’s phosphate buffered saline (DPBS) (Invitrogen, Carlsbad, CA) without calcium and magnesium ions was used as the subphase, with the temperature being maintained at 23° +/− 0.5°C. To reduce fluctuations and maintain stability, the entire equipment was mounted on a vibration isolation stage (Newport Corporation, Irvine, CA).
All lipids were purchased from Avanti Polar Lipids (Alabaster, AL) and used without further purification. Lipids were deposited onto the aqueous surface from chloroform and chloroform/methanol 2:1 (v/v %) solutions (Sigma-Aldrich, St. Louis, MO) and equilibrated, followed by compression to the surface pressure of 30 mN m–1, which is mimetic of natural biomembranes [57]. Peptoid solutions dissolved in pure water were then uniformly injected into the subphase underneath the monolayer using a micro-syringe with an “L-shaped” needle (VDRL needle; Hamilton Robotics, Reno, NV) up to the final concentration corresponding to 20% of their MICs against S. aureus. As injected molecules start interacting with lipid monolayer the surface pressure increases, and is accompanied by barrier expansion. The effective relative change in area per individual lipid molecule (ΔA/A) was monitored continually until the equilibrium has been reached to characterize the insertion of peptoids into lipid monolayer.
2.6. Epifluorescence microscopy
EFM provides insight into the lateral morphology of the lipid layer by imaging its fluorescence distribution. Fluorescence image contrast arises due to different phase densities and partitioning characteristics of the dye in coexisting phases. Because of steric hindrance, lipid-linked Texas Red molecules cannot diffuse into the highly ordered domains of liquid-condensed phase, which remain uncolored (black), and stay in the surrounding fluid regions of liquid-expended phase rendering it bright (orange). Upon introducing surface active compounds the phase separation of lipids is perturbed by the inserted molecules.
Experiments were performed as previously described [58]. The trough used for Langmuir isotherm experiments was equipped with Nikon 80i epifluorescence microscope to monitor the phase morphology evolution of the lipid film. Lipid-linked dye – Texas Red-DHPE (Invitrogen, Carlsbad, CA; 1 mol %) was pre-mixed with the stock phospholipid solutions prior to spreading. Data for excitation between 530 and 590 nm and emission between 610 and 690 nm were gathered via an HYQ Texas red filter cube. Images captured sequentially at the intervals of 30 sec during initial 15 minutes after introducing antimicrobials by the Cascade: 1K digital imaging system (Photometrics, Tucson, AZ) and analyzed using MetaMorph Microscopy Automation & Image Acquisition and Analysis Software 7.0 (Molecular Devices, LLC, Sunnyvale, CA). A resistively heated indium tin oxide-coated glass plate (Delta Technologies, Ltd., Loveland, CO) was placed over the trough to minimize dust contamination, air convection, and evaporative losses as well as to prevent condensation of water on the microscope objective. Epifluorescence microscopy permits the monolayer structure to be observed over a large lateral area (0.16 mm2), while isotherm data are collected concurrently, and detects evolution of the lipid ordered domains over time.
2.7 Specular X-ray reflectivity
X-ray scattering on Langmuir monolayers was carried out using liquid surface diffractometer at the 9-ID-B* and 15-ID-C (ChemMatCARS) beamlines at the Advanced Photon Source, Argonne National Laboratory. The trough was mounted in a hermetic helium-filled canister, where the oxygen level in was constantly monitored to be <1% in order to minimize scattering background. A cryogenically cooled double-crystal Si (111) monochromator was used to select a wavelength of λ = 0.9202 Å (1.24 Å at 15-ID). According to the standard notation, the incident X-ray beam strikes horizontal surface of liquid sample at low angle α, close to the critical angle for this wavelength. The scattered beam makes an angle β to the surface and an azimuthal angle ψ to the plane of incidence. In specular X-ray reflectivity (XR) design, α = β; ψ = 0 and the intensity of the reflected beam is measured as a function of the incident angle over the wave vector transfer qz = (4π/λ) sin(α) along the surface normal. The background subtraction was done by collecting off-specular signal at fixed distances from the incidence plane. The range of measured angles corresponded to qz values from 0.01 to 0.7 Å−1 [59]. The data were analyzed by both model-dependent (MD) (employing RFIT2000 by Oleg Konovalov, ESRF) and model-independent (MI) [60] fitting yielding very similar models [61].
2.8 XR model analysis
The interface of lipid monolayers is modeled as a stack of slabs (boxes), each of electron density (ρ), and thickness (l). A slab corresponds to a specific region of the lipid molecule, e.g. alkyl chains or outer polar moieties. The final fit was achieved by minimizing the χ2 value while ensuring that parameters obtained were physically consistent. The extra electrons per lipid molecule contributed by inserted peptoids to each slab are calculated using the following formula:
where lslab and ρslab are thickness and electron density; Alipid + ΔAlipid is the area per lipid molecule upon insertion and N initial e−slab - the number of electrons in the slab in the original untreated monolayer. The lipid-to-drug ratio in the film was estimated as:
where Ndrug e− is the number of electrons per drug molecule (for polar slabs the 50% hydration of polar moieties is assumed). The lower lipid-to-drug ratio denotes higher concentration of inserted compound molecules in the monolayer signifying higher surface activity. All XR measurements have been done in triplicate to ensure reproducibility. The error for contributed extra electrons was defined as the square root of the total number of electrons divided by the square root of the number of experimental attempts.
2.9 Atomic force microscopy
AFM measurements were performed on solid-supported lipid bilayers (SLBs) using a MFP-3D-BIO atomic force microscope from Asylum Research (Oxford Instruments) (Santa Barbra, CA). Small unilamellar vesicles (SUVs) composed of DPPG/DOPG and DPPC/DOPC/cholesterol in equimolar concentrations have been used for preparing SLBs mimicking bacterial and mammalian cell membranes, respectively. SUVs were prepared by extrusion. Lipids pre-diluted in organic solvent (chloroform or 65:35:8 %vol chloroform/methanol/water solution) were mixed in the desired proportion and set on a rotary evaporator overnight for desiccation. A Tris-buffer containing Ca2+ has been added to the dried lipids, vigorously vortexed and heated above the gel-liquid crystal transition temperature (Tm). Samples are run through membranes with pore size of 400 and 100 nm until transparency of solution was achieved. Formation of SLBs was performed by liposome fusion and rupture on muscovite (mica) support. SUVs were incubated for 5 hours at 45°C on a freshly cleaved mica sheet attached to a glass disk inside the AFM fluid cell followed by washing out of unattached vesicles. After topographical scanning of region with sufficient bilayer coverage, the peptoid solution was injected and the same region was scanned to monitor changes in the bilayer morphology.
3. Results
3.1. Peptoid hydrophobicity
Hydrophobicity of a chemical compound is related to transfer free energy from a polar medium to non-polar one. Hydrophobicity of a peptide/peptoid is usually evaluated as a sum of transfer free energies for particular monomer subunits. Ranked collections of the water-to-bilayer transfer energies of the twenty natural amino acid side chains are called a “hydrophobicity scales”. The first of them was proposed by Nozaki and Tanford in 1971 and to date more than 100 experimental and knowledge-based (calculated) scales are available [62]. These are highly dependent on the type of immiscible phases; it can be either two isotropic solvents (i.e., water/octanol, water/cyclohexane) or water with included anisotropic structures such as micelles, liposomes and planar membranes. The methods for estimating transfer free energy (HPLC, accessible surface area, site-directed mutagenesis etc.) and the physicochemical conditions including pH or ionic strength may also vary between different experimental systems [63, 64].
Longer times of retention in HPLC column suggest a higher hydrophobic moment of the molecule. Furthermore, a simple and straightforward approach to evaluate mean hydrophobicity along with the Eisenberg’s consensus scale calculations [65] is defining the mass ratio of non-polar moieties to the total molecular weight (Mnon-polar) by counting individual atomic masses. Replacement of aromatic side-chains in peptoid macrocycles results in the gradual increase of both characteristics. Retention times (tR) range between 6 min for C1 and 7.5 min for C5. The order of tR correlates to the mass ratio of the aromatic groups (from 35%to 60%) and follows the order C1<C2<C3<C4<C5. The hydrophobic moment and mean hydrophobicity of gramicidin S are similar to those by peptoid macrocycle of comparable ring size. C1dec displays 1.5 times longer tR as compared to its hexameric counterpart – C1 while having nearly identical Mnon-polar. Retention times along with the mass ratio of aromatic groups per molecule are listed in Table 1.
Table 1.
Hydrophobic properties of peptide and peptoid macrocycles.
| Compound | sequence | Molecular Mass (calc.)[a] | hydrophobicity
|
|
|---|---|---|---|---|
| tR[min][b] | Mnon-polar[c] | |||
| C1 | C(NapNpm)3 | 784.4 | ≤ 6.00 | 0.348 |
| C2 | C(NapNdmb)3 | 868.5 | 6.592 | 0.411 |
| C3 | C(NapNsne)3 | 976.5 | 6.956 | 0.482 |
| C4 | C(NapNdp)3 | 1054.6 | 7.301 | 0.512 |
| C5 | C(NapNbtfmb)3 | 1192.4 | 7.375 | 0.571 |
| C1dec | C(NapNpm)5 | 1306.7 | ~9.00 | 0.348 |
| CGS | cyclo(VOLDFP)2 | 1141.7 | 8.512 | 0.335 |
Calculated molecular masses (m/z) for charged ions [M + H+].
RP-HPLC-retention time.
Mass ratio of non-polar (aromatic and/or aliphatic) side-chains in the molecule.
3.2. In vitro activity
All studied peptoids display a broad-spectrum activity against Gram-positive and Gram-negative bacteria, as previously shown [34, 37]. Table 2 represents a comparative set of data on inhibitory and toxicity levels of common antimicrobial agents.
Table 2.
Bacteriostatic and hemolytic activities of antimicrobials.
| Peptoid | MIC[μgmL−1][a] | HC10[μgmL−1][b] | SI[c] | Reference |
|---|---|---|---|---|
| C1 | 500 | 250 | 0.5 | This study |
| C2 | 3.9 | 125 | 32 | This study |
| C3 | 3.9 | N/D | N/D | This study |
| C4 | 3.9 | 31.3 | 8 | This study |
| C5 | 3.9 | 31.3 | 8 | This study |
| C1dec | 7.8 | 250 | 32 | This study |
| AMPs | ||||
| Gramicidin S | 15.6 | >15.6 | >1 | This study |
| LL-37 | 32 | 167.5 | 5.2 | [74, 75] |
| Melittin | 0.78 | 1.78 | 2.3 | [68, 76] |
| lipopeptides | ||||
| Daptomycin | 16 | >400 | >25 | [71, 73] |
| Polymyxin B | 8-64 | >40 | 0.65-5 | [70, 72] |
| β-lactam antibiotics | ||||
| Penicillin[d] | 128 | >4 | >0.03 | [69] |
Minimum inhibitory concentrations against methicillin-resistant Staphylococcus aureus (MRSA) USA300.
Concentrations at which 10% of hemolysis is observed.
Selectivity index: HC10/MIC against MRSA USA300.
Penicillin V (phenoxymethylpenicillin).
C1, which has the lowest hydrophobic moment, appears to be inactive against MRSA. C2-C5 all have equivalent bacteriostatic effect (MIC eq. 3.9 μg mL−1). Inhibition of bacterial growth by C1dec is considerably higher than by peptoid analogue of a shorter ring size. Concentrations characterized by 10% of hemolysis (HC10) range between 250 μg mL−1 and 31 μg mL−1 from C1/C1dec to C4/C5. This is comparable level of efficacy to the most first- and second-line treatment antibiotics currently in use [66, 67] and a variety of known AMPs. We define the selectivity index (SI) of antimicrobials as hemolytic concentration divided by the MIC against S. aureus, sometimes referred to as the selectivity ratio. SI indicates to the ability of the drug to inhibit bacterial growth while minimizing cytotoxic effects to the host cells. SI values range between 0.5 and 32, with the highest ones corresponding to the compounds C2 and C1dec. CGS, which has very similar hydrophobic characteristics to C1dec, displays significantly less selectivity in vitro. Interestingly, non-peptoid antimicrobials with the mass ratio of hydrophobic groups to be more than 50%, such as penicillin antibiotics or melittin, are highly cytotoxic towards human erythrocytes (SI ≤ 2.0) [68, 69], while peptoids with similar levels of mean hydrophobicity (C4/C5) have a four-fold higher selectivity index (SI = 8). The cell-specificity of lipopeptides with low Mnon-polar (<20%), in turn, varies widely indicating that the mean hydrophobicity alone cannot provide an accurate prediction of antibacterial properties [70–73]. The curve of selectivity index is plotted in Fig. 2. The shaded area represents the optimal hydrophobicity zone characterized by highest MIC to HC10 ratio, i. e. “therapeutic window”.
Fig. 2.

Correlation between antimicrobial and hemolytic properties of peptoid macrocycles. The peak of SI curve indicates selectivity maximum resided within the “therapeutic window” (gray-shaded) of optimal hydrophobicity
3.3. Constant pressure insertion assays and epifluorescence microscopy
Langmuir monolayers of DPPG and DPPC/Cholesterol (1:1 molar ratio) are constructed to model bacterial and eukaryotic cell membranes, respectively. Insertion isotherms for C4 displaying bulky biphenyl groups show that it readily incorporates into both DPPG and DPPC/cholesterol monolayers making up to 95% and 40% increase in area per lipid molecule, respectively (Fig. 3, top right). In turn, C1 which has its side-chains containing a single phenyl ring permeates the anionic lipid film to a much lesser extent resulting in only 14% area increase, and does not interact with DPPC/cholesterol at all.
Fig. 3.

Changes in area per lipid molecule (ΔA/A) upon peptoid incorporation into DPPG monolayer (top left). Insertion isotherms for compounds with high (C4, orange) and low (C1, magenta) hydrophobicity (top right). Time-resolved changes in lateral morphology of monolayer (bottom). Texas Red-DHPE fluorescence probe (1 mol %) is added to the lipid solutions.
Epifluorescence microscopy indicates that C4 acts at least four times faster than C1 (Fig. 3, bottom). The rapid deterioration of monolayer structure starts immediately after peptoid was introduced and is completed in less than 5 minutes. The laterally ordered domains of DPPG in this case, disappear by fading rather than by decreasing in diameter, which is likely due to the penetration of biphenyl rings through the liquid-condensed phase. A summary of relative increase in area per lipid molecule for all peptoids is presented in Fig. 3 (top left).
3.4. Surface X-ray scattering
The membrane localization of peptoids can be directly extrapolated from changes in electron density profiles by X-ray reflectivity (Fig. 4A-B). Insertion of peptoid macrocycles into DPPG monolayers is characterized by a larger bump of electron density in the left region of the curve (see Fig. 4C). Data modeling shows that an increase in electron density of the slab closest to the interface directly correlates with peptoid hydrophobicity (Table 3). Starting from C3, it goes above the standard electron density of lipid alkyl chains (ρT = 0.312 e−/Å3) and reaches a maximum value for the most hydrophobic C5 indicating substantial insertion into the hydrophobic portion of DPPG. If the inserted molecules stayed in the outer region of lipid monolayer, the expanded area, instead, would result in a drop of the electron density within the upper slab as observed for CGS (ρT = 0.295 e−/Å3), likely associated with the molecular tilt of lipid alkyl “tails”.
Fig. 4.

(A) Electron density profile of pure DPPG monolayer overlapped with the molecular cartoon for clarity. Red lines represent Parratt-based fit modeling the lipid film as two boxes of fixed electron density. (B) Fresnel-divided reflectivity curves before and after peptoid insertion. The scatter plots show experimental values, while solid lines are the best fits of the models to the experimental data. XR curves are vertically shifted for clarity. (C) Corresponding electron density profiles of Langmuir monolayers normalized over the relative area increase per lipid molecule. (D) Distribution of peptoid electron density normalized over the ΔA across the surface. The color palette for peptoids designation is as follows: C1 – magenta; C2 – blue; C3 – green; C4 – orange and C5 – red.
Table 3.
Box-modeled XR data.
| Sample | «Tails»
|
«Headgroups»
|
Alipid [Å2]/ΔAlipid | Lipid-to-drug ratio | ||||
|---|---|---|---|---|---|---|---|---|
| lT (Å) | ρT (e−/Å3) | extra e− | lH (Å) | ρH (e−/Å3) | extra e− | |||
| DPPG | 16.5 | 0.312 | – | 8.3 | 0.477 | – | 47/− | N/A |
| + C1 | 13 | 0.313 | 0 | 9.1 | 0.451 | 61±5 | 54/0.14 | 7.13 |
| + C2 | 11.6 | 0.308 | 0 | 8.7 | 0.448 | 108±6 | 69/0.46 | 4.47 |
| + C3 | 15.3 | 0.316 | 142±7 | 8.7 | 0.455 | 208±8 | 82/0.75 | 1.55 |
| + C4 | 12.1 | 0.381 | 167±8 | 6 | 0.438 | 82±5 | 92/0.95 | 2.33 |
| + C5 | 15.5 | 0.372 | 283±10 | 8.8 | 0.422 | 188±8 | 94/1.00 | 1.33 |
| + C1dec | 11.0 | 0.340 | 96±6 | 7.9 | 0.394 | 134±7 | 94/1.00 | 2.7 |
| + CGS | 12.6 | 0.295 | 34±3 | 8.2 | 0.460 | 135±7 | 78/0.66 | 4.3 |
Subscripts: T – alkyl chains/“tails”; H- headgroups
The number of inserted molecules characterized inversely by lipid-to-drug ratio rises up along with the growth of peptoid hydrophobicity. The higher value for C4 in this case can be explained by the steric hindrance impeding incorporation of bulky biphenyl groups into tightly packed gel phase of alkyl chains, yet not really affecting the bacteriostatic or membrane disruptive activity. The lipid-to-drug ratio for C1dec is considerably lower than those displayed by both CGS and C1. These observations allow us to conclude that peptoid hydrophobic moment, rather than the mean hydrophobicity is responsible for their interaction with lipid monolayers. Furthermore, the superior insertion of peptoid over the peptide macrocycle indicates the importance of other structural characteristics, such as chirality and backbone monomer sequence. Addition of fluorinated residues does not change the peptoid mode of action as compared to homologues with similar hydrophobicity. This was a surprising outcome, as fluorination is a typical optimization strategy in small molecule drugs.
C1 and C2 are solely partitioned within the bottom region corresponding to the hydrated lipid headgroups. C3 molecules are split nearly equally between the slabs, while about 60% of electrons by C4 and C5 are attributed to the upper hydrophobic core of monolayer. For C5, the total number of contributed electrons is higher, likely, due to the presence of “heavy” 3,5-bis-trifluoremethylbenzene groups. Interestingly, while the numbers of extra electrons by C1dec and CGS within the polar glycerophosphate layer are roughly equal, the limited capability of peptide to permeate into saturated fatty acids is characterized by a three times lower contribution of electrons to the upper slab. Fig. 4D represents a distribution map of peptoid electron density normalized over the relative area increase per lipid molecule. Excess density of C3-C5 spans the entirety of lipid monolayer.
3.5. Interaction with lipid bilayers
The specificity of peptoid macrocycles towards the anionic vs. neutral solid-supported lipid bilayers (SLBs) is demonstrated by atomic force microscopy. Incorporation of unsaturated lipids in the solid-supported bilayer system better mimics the fluid-gel phase coexistence in biological membranes than the Langmuir monolayers alone. Saturated lipids are preferred for monolayer work, since C=C bonds of lipid alkyl chains oxidize when exposed to the air. The AFM data support the hypothesis of peptoid aggregation at anionic, lipid interfaces. Patches of DPPG/DOPG bilayer become smaller after compound with hydrophobic groups of average size (C3) is introduced into the fluid system (Fig. 5A, left). An overall increase in the height of these regions is detected (Fig. 5A, right). Peptoid does not induce any changes in morphology or height profiles of DPPC/DOPC/cholesterol SLB (see Fig. 5B). Although the complete detachment of DPPG/DOPG bilayer is not observed, it appears structurally roughened with a large number of local defects. An illustrative sketch of peptoid selective interaction with bacterial over eukaryotic model membranes is shown in Fig. 6. In tandem to the previously published work these results allow us to propose pore-forming mechanism of membrane destabilization by macrocyclic peptoids [37].
Fig. 5.
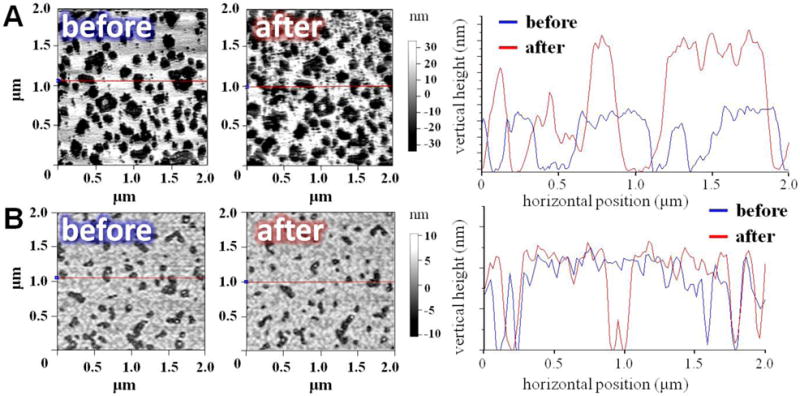
(A) DPPG/DOPG and (B) DPPC/DOPC/cholesterol bilayers before and after injection of peptoid macrocycle (C3) measured using AFM. The map distribution of bilayer patches within the scanned area (left) corresponds to the height profile (right).
Fig. 6.

Cartoon scheme of molecular selectivity between eukaryotic (top) and bacterial (bottom) lipid membranes by macrocyclic peptoids of optimal hydrophobicity. Approx. 1/3 of inserted molecules reside within the aliphatic chains of negatively charged phospholipids. PC – phosphatidylcholine; PG – phosphatidylglycerol; PE – phosphatidylethanolamine; CH – cholesterol.
4. Discussion
Hydrophobic interactions drive antimicrobial molecules to move from the aqueous environment into membranes. Excessive hydrophobicity increases cytotoxicity for natural peptides [77]. The balance between hydrophobic and polar content is important for modulating selectivity of antimicrobial peptides [78, 79], and non-peptidic therapeutic agents [80, 81]. Circular dichroism and ATR-FTIR studies of linear cationic AMPs in model membrane bilayers demonstrated that peptides with large hydrophobic surface undergo conformational changes from random coil to the α-helical and from α-helix to β-sheet secondary structure when placed into the non-polar environment and are more likely to form aggregates than their less hydrophobic analogues [82]. This is attributable to a charge compensatory effect. After peptides bind to the negatively charged lipids on a bacterial surface, a dehydrated environment is created and favors formation of β-strand aggregates [21, 83]. Similar behavior is also observed by viral fusion peptides from HIV-1 gp41 modulated by the level of cholesterol in targeted membrane [84, 85].
For peptides with cyclic structure, conformational transition may not be feasible. The optimal balance between their functional activity and undesired cytotoxic effect is not determined by one single factor, but rather depends on a combination of (i) backbone ring size, (ii) hydrophobic content; (iii) distribution of positive charges [9]. The substitution of ornithine with lysine in CGS reduces the antimicrobial activity [86]. In contrast, for the diastereoisomers of larger-size peptide GS14 the distortion of amphipathic topology contributes to their bacteriostatic properties with a decreased rate of hemolysis [87]. Dathe with coworkers show that addition of fluorophore to the arginine- and tryptophan-rich macrocyclic hexapeptides characterized by longer retention times makes them inactive against both Gram-positive and Gram-negative bacteria, while hemolytic concentrations are not necessarily affected by the increase in hydrophobicity [88]. Calorimetric measurements on liposome models ascertain lipid clustering modulated by hydrophobic amino acid residues to precede membrane permeation [89]. The mechanisms contributing to this function remain poorly understood.
Our results suggest that peptoid macrocycles obey unique design principles. Previous studies on α-helical peptoids mimicking cationic AMPs reveal a linear relationship between the number of hydrophobic residues and activity against S. aureus. No such correlation with peptoid antimicrobial potency against Gram-negative bacteria is observed. Surprisingly, the peptide analogues demonstrate greater selectivity as compared to synthetic mimics [90]. A recent work by Bolt et al. links peptoid hydrophobicity to the cytotoxicity against human fibroblasts and hepatocytes with the optimal retention times to be between 15 and 20 min [91]. For linear peptide-peptoid hybrids the highest selectivity ratio corresponds to dodecameric compounds displaying tR ~ 18 min with the significant loss of cell-specificity when tR is above 20 min [92].
Macrocyclization allows peptoids to have less hydrophobic character, while retaining their antimicrobial properties. Membrane selectivity by small peptoid macrocycles correlates to the level of their intercalation within the lipid hydrophobic core, modulated by aromatic residues. One needs to note that artificial lipid membranes represent a simplistic model of targeted cell surface and do not address the complexity of cellular envelope organization. Two critical points of hydrophobicity are observed for macrocyclic hexamers. The first corresponds to tR eq. 6.5 min, where the capability of inhibiting bacteria growth is accompanied by moderate levels of hemolysis. Beyond this point, the hydrophobic forces dominate the interaction, corroborated by the localization of inserted peptoids primarily within the lipid alkyl chains rather than only the polar lipid headgroups. The second critical point is at tR ~ 7.3 min characterized by a reduction in hemolytic concentration. Peptoids with the hydrophobic moieties occupying more than a half of their molecular mass are found to readily interact with both negatively charged and zwitterionic model membranes beyond this point. The optimum ratio between antimicrobial and hemolytic effects of macrocyclic hexamers used in this study lies within a relatively narrow therapeutic window with the lower and upper limits observed for C1 and C4, respectively. Increasing aromatic group sizes results in the increase of tR by ~ 0.15 min per side chain, which is four-fold less than the effect of a single-residue substitution with diphenylethyl in linear peptoids [90]. We note these trends may vary between target microorganisms and for other synthetic molecules of different structural schemes.
5. Conclusions
Peptoid hydrophobicity is demonstrated to modulate their specificity towards anionic over neutral lipid membranes via a two-step mechanism. Mechanistic data obtained on SLBs and Langmuir monolayers agree well with the levels of bacteriostatic and cytotoxic activities observed in vitro. Penetration of peptoid macrocycles through the entire membrane is not essential to kill bacterial cells. Peptoids binding to the negatively charged membrane surface leads to cellular depolarization and inhibits biological function. The hydrophobic moieties of molecules with the maximized selectivity index occupy between 35% and 50% of their mass. The design of macrocyclic compounds with small incremental modifications of aromatic groups will provide an effective treatment for any given pathogen. We anticipate our findings to have an impact on optimization of peptoids as future antimicrobial therapeutics.
Highlights.
Membrane specificity of peptoids is modulated by their hydrophobic properties via a two-step mechanism.
The optimal ratio between antimicrobial and hemolytic effects is characterized by peptoid intercalation into zwitterionic and negatively charged lipid matrix.
The maximized selectivity index for small cyclic peptoids implies the hydrophobic moieties to occupy between 35% and 50% of their molecular masses.
Acknowledgments
This research was supported by the NIH (R01 AI073892, D.G.), NSF (CHE-1507946, K.K.) and DARPA (W911NF-09-1-378 D.G.). ChemMatCARS Sector 15 is supported by the National Science Foundation under grant number NSF/CHE-1346572. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. M. W. Martynowycz was partially supported by the NSF via a fellowship through the Adler Planetary & Astronomy Museum, the laboratory-graduate fellowship at Argonne National Laboratory, and the Dean’s fellowship at the Illinois Institute of Technology.
Footnotes
FORMERLY 9-ID-C
Author Contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.
Conflict of interest
The authors declare no competing financial or otherwise interest.
References
- 1.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 2.Diamond G, Beckloff N, Weinberg A, Kisich KO. The roles of antimicrobial peptides in innate host defense. Current pharmaceutical design. 2009;15:2377–2392. doi: 10.2174/138161209788682325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hancock RE, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends in microbiology. 2000;8:402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 4.Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clinical microbiology reviews. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epand RM, Rotem S, Mor A, Berno B, Epand RF. Bacterial membranes as predictors of antimicrobial potency. Journal of the American Chemical Society. 2008;130:14346–14352. doi: 10.1021/ja8062327. [DOI] [PubMed] [Google Scholar]
- 6.Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harbor perspectives in biology. 2010;2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nature reviews Molecular cell biology. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuzaki K. Control of cell selectivity of antimicrobial peptides. Biochimica et biophysica acta. 2009;1788:1687–1692. doi: 10.1016/j.bbamem.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Yin LM, Edwards MA, Li J, Yip CM, Deber CM. Roles of hydrophobicity and charge distribution of cationic antimicrobial peptides in peptide-membrane interactions. The Journal of biological chemistry. 2012;287:7738–7745. doi: 10.1074/jbc.M111.303602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henriksen JR, Etzerodt T, Gjetting T, Andresen TL. Side chain hydrophobicity modulates therapeutic activity and membrane selectivity of antimicrobial peptide mastoparan-X. PloS one. 2014;9:e91007. doi: 10.1371/journal.pone.0091007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyu Y, Yang Y, Lyu X, Dong N, Shan A. Antimicrobial activity, improved cell selectivity and mode of action of short PMAP-36-derived peptides against bacteria and Candida. Scientific reports. 2016;6:27258. doi: 10.1038/srep27258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dathe M, Wieprecht T, Nikolenko H, Handel L, Maloy WL, MacDonald DL, Beyermann M, Bienert M. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS letters. 1997;403:208–212. doi: 10.1016/s0014-5793(97)00055-0. [DOI] [PubMed] [Google Scholar]
- 13.Asthana N, Yadav SP, Ghosh JK. Dissection of antibacterial and toxic activity of melittin: a leucine zipper motif plays a crucial role in determining its hemolytic activity but not antibacterial activity. The Journal of biological chemistry. 2004;279:55042–55050. doi: 10.1074/jbc.M408881200. [DOI] [PubMed] [Google Scholar]
- 14.Kustanovich I, Shalev DE, Mikhlin M, Gaidukov L, Mor A. Structural requirements for potent versus selective cytotoxicity for antimicrobial dermaseptin S4 derivatives. The Journal of biological chemistry. 2002;277:16941–16951. doi: 10.1074/jbc.M111071200. [DOI] [PubMed] [Google Scholar]
- 15.Clark KS, Svetlovics J, McKeown AN, Huskins L, Almeida PF. What determines the activity of antimicrobial and cytolytic peptides in model membranes. Biochemistry. 2011;50:7919–7932. doi: 10.1021/bi200873u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wieprecht T, Dathe M, Beyermann M, Krause E, Maloy WL, MacDonald DL, Bienert M. Peptide hydrophobicity controls the activity and selectivity of magainin 2 amide in interaction with membranes. Biochemistry. 1997;36:6124–6132. doi: 10.1021/bi9619987. [DOI] [PubMed] [Google Scholar]
- 17.Tachi T, Epand RF, Epand RM, Matsuzaki K. Position-dependent hydrophobicity of the antimicrobial magainin peptide affects the mode of peptide-lipid interactions and selective toxicity. Biochemistry. 2002;41:10723–10731. doi: 10.1021/bi0256983. [DOI] [PubMed] [Google Scholar]
- 18.Glukhov E, Stark M, Burrows LL, Deber CM. Basis for selectivity of cationic antimicrobial peptides for bacterial versus mammalian membranes. The Journal of biological chemistry. 2005;280:33960–33967. doi: 10.1074/jbc.M507042200. [DOI] [PubMed] [Google Scholar]
- 19.Glukhov E, Burrows LL, Deber CM. Membrane interactions of designed cationic antimicrobial peptides: the two thresholds. Biopolymers. 2008;89:360–371. doi: 10.1002/bip.20917. [DOI] [PubMed] [Google Scholar]
- 20.Stark M, Liu LP, Deber CM. Cationic hydrophobic peptides with antimicrobial activity. Antimicrobial agents and chemotherapy. 2002;46:3585–3590. doi: 10.1128/AAC.46.11.3585-3590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollmann A, Martinez M, Noguera ME, Augusto MT, Disalvo A, Santos NC, Semorile L, Maffia PC. Role of amphipathicity and hydrophobicity in the balance between hemolysis and peptide-membrane interactions of three related antimicrobial peptides. Colloids and surfaces B Biointerfaces. 2016;141:528–536. doi: 10.1016/j.colsurfb.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Andres E, Dimarcq JL. Cationic antimicrobial peptides: update of clinical development. Journal of internal medicine. 2004;255:519–520. doi: 10.1046/j.1365-2796.2003.01278.x. [DOI] [PubMed] [Google Scholar]
- 23.Gordon YJ, Romanowski EG, McDermott AM. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Current eye research. 2005;30:505–515. doi: 10.1080/02713680590968637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters BM, Shirtliff ME, Jabra-Rizk MA. Antimicrobial peptides: primeval molecules or future drugs? PLoS pathogens. 2010;6:e1001067. doi: 10.1371/journal.ppat.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon RJ, Kania RS, Zuckermann RN, Huebner VD, Jewell DA, Banville S, Ng S, Wang L, Rosenberg S, Marlowe CK, et al. Peptoids: a modular approach to drug discovery. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:9367–9371. doi: 10.1073/pnas.89.20.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dohm MT, Kapoor R, Barron AE. Peptoids: bio-inspired polymers as potential pharmaceuticals. Current pharmaceutical design. 2011;17:2732–2747. doi: 10.2174/138161211797416066. [DOI] [PubMed] [Google Scholar]
- 27.Goodson B, Ehrhardt A, Ng S, Nuss J, Johnson K, Giedlin M, Yamamoto R, Moos WH, Krebber A, Ladner M, Giacona MB, Vitt C, Winter J. Characterization of novel antimicrobial peptoids. Antimicrobial agents and chemotherapy. 1999;43:1429–1434. doi: 10.1128/aac.43.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chongsiriwatana NP, Patch JA, Czyzewski AM, Dohm MT, Ivankin A, Gidalevitz D, Zuckermann RN, Barron AE. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2794–2799. doi: 10.1073/pnas.0708254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Czyzewski AM, Jenssen H, Fjell CD, Waldbrook M, Chongsiriwatana NP, Yuen E, Hancock RE, Barron AE. In Vivo, in vitro, and in silico characterization of peptoids as antimicrobial agents. PloS one. 2016;11:e0135961. doi: 10.1371/journal.pone.0135961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller SM, Simon RJ, Ng S, Zuckermann RN, Kerr JM, Moos WH. Proteolytic studies of homologous peptide and N-substituted glycine peptoid oligomers. Bioorg Med Chem Lett. 1994;4:2657–2662. [Google Scholar]
- 31.Fowler SA, Blackwell HE. Structure-function relationships in peptoids: recent advances toward deciphering the structural requirements for biological function. Organic & biomolecular chemistry. 2009;7:1508–1524. doi: 10.1039/b817980h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin SB, Yoo B, Todaro LJ, Kirshenbaum K. Cyclic peptoids. Journal of the American Chemical Society. 2007;129:3218–3225. doi: 10.1021/ja066960o. [DOI] [PubMed] [Google Scholar]
- 33.Yoo B, Shin SB, Huang ML, Kirshenbaum K. Peptoid macrocycles: making the rounds with peptidomimetic oligomers. Chemistry. 2010;16:5528–5537. doi: 10.1002/chem.200903549. [DOI] [PubMed] [Google Scholar]
- 34.Huang ML, Shin SB, Benson MA, Torres VJ, Kirshenbaum K. A comparison of linear and cyclic peptoid oligomers as potent antimicrobial agents. ChemMedChem. 2012;7:114–122. doi: 10.1002/cmdc.201100358. [DOI] [PubMed] [Google Scholar]
- 35.Andreev K, Kosutic M, Ivankin A, Huang M, Kirschenbaum K, Gidalevitz D. Role of the cyclization in de novo design of antimicrobial peptide mimics. Biophysical journal. 2012;102:493a–493a. [Google Scholar]
- 36.Andreev K, Lingaraju M, Ivankin A, Huang M, Kirshenbaum K, Gidalevitz D. Membrane interactions of antimicrobial peptoids - restriction of conformational flexibility as a strategy to enhance activity. Biophysical journal. 2013;104:598a–598a. [Google Scholar]
- 37.Andreev K, Martynowycz MW, Ivankin A, Huang ML, Kuzmenko I, Meron M, Lin B, Kirshenbaum K, Gidalevitz D. Cyclization improves membrane permeation by antimicrobial peptoids. Langmuir: the ACS journal of surfaces and colloids. 2016;32:12905–12913. doi: 10.1021/acs.langmuir.6b03477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gause GF. Gramicidin S. Lancet. 1946;2:46. [PubMed] [Google Scholar]
- 39.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. Jama. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, M JR, Talan DA, Chambers HF. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011;52:e18–55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 41.Placencia FX, Kong L, Weisman LE. Treatment of methicillin-resistant Staphylococcus aureus in neonatal mice: lysostaphin versus vancomycin. Pediatric research. 2009;65:420–424. doi: 10.1203/PDR.0b013e3181994a53. [DOI] [PubMed] [Google Scholar]
- 42.Mohamed MF, Abdelkhalek A, Seleem MN. Evaluation of short synthetic antimicrobial peptides for treatment of drug-resistant and intracellular Staphylococcus aureus. Scientific reports. 2016;6:29707. doi: 10.1038/srep29707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rello J, Torres A, Ricart M, Valles J, Gonzalez J, Artigas A, Rodriguez-Roisin R. Ventilator-associated pneumonia by Staphylococcus aureus. Comparison of methicillin-resistant and methicillin-sensitive episodes. American journal of respiratory and critical care medicine. 1994;150:1545–1549. doi: 10.1164/ajrccm.150.6.7952612. [DOI] [PubMed] [Google Scholar]
- 44.Sader HS, Fey PD, Limaye AP, Madinger N, Pankey G, Rahal J, Rybak MJ, Snydman DR, Steed LL, Waites K, Jones RN. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrobial agents and chemotherapy. 2009;53:4127–4132. doi: 10.1128/AAC.00616-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Von Drygalski A, Curtis BR, Bougie DW, McFarland JG, Ahl S, Limbu I, Baker KR, Aster RH. Vancomycin-induced immune thrombocytopenia. The New England journal of medicine. 2007;356:904–910. doi: 10.1056/NEJMoa065066. [DOI] [PubMed] [Google Scholar]
- 46.Gniadek TJ, Arndt PA, Leger RM, Zydowicz D, Cheng EY, Zantek ND. Drug-induced immune hemolytic anemia associated with anti-vancomycin complicated by a paraben antibody. Transfusion. 2018;58:181–188. doi: 10.1111/trf.14362. [DOI] [PubMed] [Google Scholar]
- 47.Zuckermann RN, Kerr JM, Kent SBH, Moos WH. Efficient method for the preparation of peptoids [oligo(N-substituted glycines)] by submonomer solid-phase synthesis. Journal of the American Chemical Society. 1992;114:10646–10647. [Google Scholar]
- 48.Wadhwani P, Afonin S, Ieronimo M, Buerck J, Ulrich AS. Optimized protocol for synthesis of cyclic gramicidin S: starting amino acid is key to high yield. The Journal of organic chemistry. 2006;71:55–61. doi: 10.1021/jo051519m. [DOI] [PubMed] [Google Scholar]
- 49.Huang ML, Benson MA, Shin SBY, Torres VJ, Kirshenbaum K. Amphiphilic cyclic peptoids that exhibit antimicrobial activity by disrupting Staphylococcus aureus membranes. European Journal of Organic Chemistry. 2013;2013:3560–3566. [Google Scholar]
- 50.Kennedy AD, Otto M, Braughton KR, Whitney AR, Chen L, Mathema B, Mediavilla JR, Byrne KA, Parkins LD, Tenover FC, Kreiswirth BN, Musser JM, DeLeo FR. Epidemic community-associated methicillin-resistant Staphylococcus aureus: recent clonal expansion and diversification. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1327–1332. doi: 10.1073/pnas.0710217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alonzo F, 3rd, Benson MA, Chen J, Novick RP, Shopsin B, Torres VJ. Staphylococcus aureus leucocidin ED contributes to systemic infection by targeting neutrophils and promoting bacterial growth in vivo. Molecular microbiology. 2012;83:423–435. doi: 10.1111/j.1365-2958.2011.07942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clinical and Laboratory Standards Institute (formerly NCCLS) Approved Standard. Seventh. M7-A7, Clinical and Laboratory Standards Institute; Wayne, PA: 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. [Google Scholar]
- 53.Marangoni MN, Martynowycz MW, Kuzmenko I, Braun D, Polak PE, Weinberg G, Rubinstein I, Gidalevitz D, Feinstein DL. Membrane cholesterol modulates superwarfarin toxicity. Biophysical journal. 2016;110:1777–1788. doi: 10.1016/j.bpj.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andreev K, Bianchi C, Laursen JS, Citterio L, Hein-Kristensen L, Gram L, Kuzmenko I, Olsen CA, Gidalevitz D. Guanidino groups greatly enhance the action of antimicrobial peptidomimetics against bacterial cytoplasmic membranes. Biochimica et biophysica acta. 2014;1838:2492–2502. doi: 10.1016/j.bbamem.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spector AA, Yorek MA. Membrane lipid composition and cellular function. Journal of lipid research. 1985;26:1015–1035. [PubMed] [Google Scholar]
- 56.Peetla C, Stine A, Labhasetwar V. Biophysical interactions with model lipid membranes: applications in drug discovery and drug delivery. Molecular pharmaceutics. 2009;6:1264–1276. doi: 10.1021/mp9000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marsh D. Lateral pressure in membranes. Biochimica et biophysica acta. 1996;1286:183–223. doi: 10.1016/s0304-4157(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 58.Neville F, Cahuzac M, Konovalov O, Ishitsuka Y, Lee KY, Kuzmenko I, Kale GM, Gidalevitz D. Lipid headgroup discrimination by antimicrobial peptide LL-37: insight into mechanism of action. Biophysical journal. 2006;90:1275–1287. doi: 10.1529/biophysj.105.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alsnielsen J, Jacquemain D, Kjaer K, Leveiller F, Lahav M, Leiserowitz L. Principles and applications of grazing-incidence X-ray and neutron-scattering from ordered molecular monolayers at the air-water-interface. Physics Reports-Review Section of Physics Letters. 1994;246:252–313. [Google Scholar]
- 60.Danauskas SM, Li DX, Meron M, Lin BH, Lee KYC. Stochastic fitting of specular X-ray reflectivity data using StochFit. J Appl Crystallogr. 2008;41:1187–1193. [Google Scholar]
- 61.Nobre TM, Martynowycz MW, Andreev K, Kuzmenko I, Nikaido H, Gidalevitz D. Modification of Salmonella lipopolysaccharides prevents the outer membrane penetration of novobiocin. Biophysical journal. 2015;109:2537–2545. doi: 10.1016/j.bpj.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nozaki Y, Tanford C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. Establishment of a hydrophobicity scale. The Journal of biological chemistry. 1971;246:2211–2217. [PubMed] [Google Scholar]
- 63.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. Journal of molecular biology. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 64.Wimley WC, White SH. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nature structural biology. 1996;3:842–848. doi: 10.1038/nsb1096-842. [DOI] [PubMed] [Google Scholar]
- 65.Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annual review of biochemistry. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- 66.Diederen BM, van Duijn I, Willemse P, Kluytmans JA. In vitro activity of daptomycin against methicillin-resistant Staphylococcus aureus, including heterogeneously glycopeptide-resistant strains. Antimicrobial agents and chemotherapy. 2006;50:3189–3191. doi: 10.1128/AAC.00526-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Humphries RM, Pollett S, Sakoulas G. A current perspective on daptomycin for the clinical microbiologist. Clinical microbiology reviews. 2013;26:759–780. doi: 10.1128/CMR.00030-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan H, Li S, Sun X, Mi H, He B. Individual substitution analogs of Mel(12-26), melittin’s C-terminal 15-residue peptide: their antimicrobial and hemolytic actions. FEBS letters. 2003;554:100–104. doi: 10.1016/s0014-5793(03)01113-x. [DOI] [PubMed] [Google Scholar]
- 69.Le CF, Yusof MY, Hassan H, Sekaran SD. In vitro properties of designed antimicrobial peptides that exhibit potent antipneumococcal activity and produces synergism in combination with penicillin. Scientific reports. 2015;5:9761. doi: 10.1038/srep09761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boyen F, Verstappen KM, De Bock M, Duim B, Weese JS, Schwarz S, Haesebrouck F, Wagenaar JA. In vitro antimicrobial activity of miconazole and polymyxin B against canine meticillin-resistant Staphylococcus aureus and meticillin-resistant Staphylococcus pseudintermedius isolates. Veterinary dermatology. 2012;23:381–385. e370. doi: 10.1111/j.1365-3164.2012.01040.x. [DOI] [PubMed] [Google Scholar]
- 71.Liu R, Chen X, Chakraborty S, Lemke JJ, Hayouka Z, Chow C, Welch RA, Weisblum B, Masters KS, Gellman SH. Tuning the biological activity profile of antibacterial polymers via subunit substitution pattern. Journal of the American Chemical Society. 2014;136:4410–4418. doi: 10.1021/ja500367u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nasompag S, Dechsiri P, Hongsing N, Phonimdaeng P, Daduang S, Klaynongsruang S, Camesano TA, Patramanon R. Effect of acyl chain length on therapeutic activity and mode of action of the CX-KYR-NH2 antimicrobial lipopeptide. Biochimica et biophysica acta. 2015;1848:2351–2364. doi: 10.1016/j.bbamem.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 73.Niu H, Yee R, Cui P, Tian L, Zhang S, Shi W, Sullivan D, Zhu B, Zhang W, Zhang Y. Identification of agents active against methicillin-resistant Staphylococcus aureus USA300 from a clinical compound library. Pathogens. 2017;6 doi: 10.3390/pathogens6030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deslouches B, Steckbeck JD, Craigo JK, Doi Y, Mietzner TA, Montelaro RC. Rational design of engineered cationic antimicrobial peptides consisting exclusively of arginine and tryptophan, and their activity against multidrug-resistant pathogens. Antimicrobial agents and chemotherapy. 2013;57:2511–2521. doi: 10.1128/AAC.02218-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murugan RN, Jacob B, Ahn M, Hwang E, Sohn H, Park HN, Lee E, Seo JH, Cheong C, Nam KY, Hyun JK, Jeong KW, Kim Y, Shin SY, Bang JK. De novo design and synthesis of ultra-short peptidomimetic antibiotics having dual antimicrobial and anti-inflammatory activities. PloS one. 2013;8:e80025. doi: 10.1371/journal.pone.0080025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi JH, Jang AY, Lin S, Lim S, Kim D, Park K, Han SM, Yeo JH, Seo HS. Melittin, a honeybee venomderived antimicrobial peptide, may target methicillinresistant Staphylococcus aureus. Molecular medicine reports. 2015;12:6483–6490. doi: 10.3892/mmr.2015.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kondejewski LH, Lee DL, Jelokhani-Niaraki M, Farmer SW, Hancock RE, Hodges RS. Optimization of microbial specificity in cyclic peptides by modulation of hydrophobicity within a defined structural framework. The Journal of biological chemistry. 2002;277:67–74. doi: 10.1074/jbc.M107825200. [DOI] [PubMed] [Google Scholar]
- 78.Nielsen SL, Frimodt-Moller N, Kragelund BB, Hansen PR. Structure-activity study of the antibacterial peptide fallaxin. Protein science: a publication of the Protein Society. 2007;16:1969–1976. doi: 10.1110/ps.072966007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Munk JK, Ritz C, Fliedner FP, Frimodt-Moller N, Hansen PR. Novel method to identify the optimal antimicrobial peptide in a combination matrix, using anoplin as an example. Antimicrobial agents and chemotherapy. 2014;58:1063–1070. doi: 10.1128/AAC.02369-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuroda K, Caputo GA, DeGrado WF. The role of hydrophobicity in the antimicrobial and hemolytic activities of polymethacrylate derivatives. Chemistry. 2009;15:1123–1133. doi: 10.1002/chem.200801523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koh JJ, Zou H, Lin S, Lin H, Soh RT, Lim FH, Koh WL, Li J, Lakshminarayanan R, Verma C, Tan DT, Cao D, Beuerman RW, Liu S. Nonpeptidic amphiphilic xanthone derivatives: Structure-activity relationship and membrane-targeting properties. Journal of medicinal chemistry. 2016;59:171–193. doi: 10.1021/acs.jmedchem.5b01500. [DOI] [PubMed] [Google Scholar]
- 82.Chen Y, Guarnieri MT, Vasil AI, Vasil ML, Mant CT, Hodges RS. Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides. Antimicrobial agents and chemotherapy. 2007;51:1398–1406. doi: 10.1128/AAC.00925-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maturana P, Martinez M, Noguera ME, Santos NC, Disalvo EA, Semorile L, Maffia PC, Hollmann A. Lipid selectivity in novel antimicrobial peptides: Implication on antimicrobial and hemolytic activity. Colloids and surfaces B, Biointerfaces. 2017;153:152–159. doi: 10.1016/j.colsurfb.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 84.Ivankin A, Apellaniz B, Gidalevitz D, Nieva JL. Mechanism of membrane perturbation by the HIV-1 gp41 membrane-proximal external region and its modulation by cholesterol. Biochimica et biophysica acta. 2012;1818:2521–2528. doi: 10.1016/j.bbamem.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ivankin A, Kuzmenko I, Gidalevitz D. Cholesterol mediates membrane curvature during fusion events. Physical review letters. 2012;108:238103. doi: 10.1103/PhysRevLett.108.238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jelokhani-Niaraki M, Kondejewski LH, Farmer SW, Hancock RE, Kay CM, Hodges RS. Diastereoisomeric analogues of gramicidin S: structure, biologicalactivity and interaction with lipid bilayers. The Biochemical journal. 2000;349 Pt 3:747–755. doi: 10.1042/bj3490747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kondejewski LH, Jelokhani-Niaraki M, Farmer SW, Lix B, Kay CM, Sykes BD, Hancock RE, Hodges RS. Dissociation of antimicrobial and hemolytic activities in cyclic peptide diastereomers by systematic alterations in amphipathicity. The Journal of biological chemistry. 1999;274:13181–13192. doi: 10.1074/jbc.274.19.13181. [DOI] [PubMed] [Google Scholar]
- 88.Scheinpflug K, Nikolenko H, Komarov IV, Rautenbach M, Dathe M. What goes around comes around-a comparative study of the influence of chemical modifications on the antimicrobial properties of small cyclic peptides. Pharmaceuticals (Basel) 2013;6:1130–1144. doi: 10.3390/ph6091130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Finger S, Kerth A, Dathe M, Blume A. The efficacy of trivalent cyclic hexapeptides to induce lipid clustering in PG/PE membranes correlates with their antimicrobial activity. Biochimica et biophysica acta. 2015;1848:2998–3006. doi: 10.1016/j.bbamem.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 90.Mojsoska B, Zuckermann RN, Jenssen H. Structure-activity relationship study of novel peptoids that mimic the structure of antimicrobial peptides. Antimicrobial agents and chemotherapy. 2015;59:4112–4120. doi: 10.1128/AAC.00237-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bolt HL, Eggimann GA, Jahoda CAB, Zuckermann RN, Sharples GJ, Cobb SL. Exploring the links between peptoid antibacterial activity and toxicity. Medchemcomm. 2017;8:886–896. doi: 10.1039/c6md00648e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jahnsen RD, Sandberg-Schaal A, Vissing KJ, Nielsen HM, Frimodt-Moller N, Franzyk H. Tailoring cytotoxicity of antimicrobial peptidomimetics with high activity against multidrug-resistant Escherichia coli. Journal of medicinal chemistry. 2014;57:2864–2873. doi: 10.1021/jm401335p. [DOI] [PubMed] [Google Scholar]


