Abstract
It is now generally accepted that diabetes increases the risk for cognitive impairment, but the precise mechanisms are poorly understood. A critical problem in linking diabetes to cognitive impairment is that patients often have multiple comorbidities (e.g., obesity, hypertension) that have been independently linked to cognitive deficits. In the study reported here we focused on young adults with and without type 1 diabetes who were virtually free of such comorbidities. The two groups were matched on major health and demographic factors, and all participants completed a verbal working memory task during magnetoencephalographic brain imaging. We hypothesized that patients would have altered neural dynamics in verbal working memory processing and that these differences would directly relate to clinical disease measures. Accordingly, we found that patients had significantly stronger neural responses in the superior parietal cortices during memory encoding and significantly weaker activity in parietal-occipital regions during maintenance compared with control subjects. Moreover, disease duration and glycemic control were both significantly correlated with neural responses in various brain regions. In conclusion, young healthy adults with type 1 diabetes already have aberrant neural processing relative to their peers without diabetes, using compensatory responses to perform the task, and glucose management and duration may play a central role.
Introduction
The human brain is one of the most metabolically active organs in the body, so it follows that glucose metabolism dysregulation, a hallmark of diabetes, would cause a variety of deleterious effects on neural and cognitive processes. Accordingly, several metrics of brain structure, including white matter tract integrity, white matter hyperintensities, and gray matter volume, are known to be abnormal in patients with type 1 diabetes (1–3). Early studies of brain function also found impairments in this patient group (4–6). Such brain abnormalities likely underlie the various neuropsychological impairments that have been described in patients with type 1 diabetes, including deficits in measures of intelligence, attention, psychomotor speed, visual perception, cognitive flexibility, and executive functioning (7,8), but to date, links between brain aberrations and cognitive decline in these patients have not been firmly established. The existence of neuropsychological deficits in this population is well appreciated, however, and several studies have connected these deficits to glycemic control, disease duration, major comorbidities, and other disease factors (9,10) and have begun to distinguish the cognitive functions most severely affected (11).
Executive functioning, including processes such as working memory, appears to be uniquely impaired in patients with type 1 diabetes as shown by recent meta-analyses (10,11). Working memory is generally defined as the mechanism by which information is temporarily stored and/or transformed for utilization toward current goals and/or processes (12,13). Thus, working memory plays a critical role in daily mental function and supports higher-order cognitive processes such as decision-making and language comprehension. The brain regions and responses serving working memory processing have been widely studied in healthy (13–17) and diseased populations (18–21), but to date, far fewer studies have been conducted in patients with diabetes.
Electrophysiological studies of verbal working memory in healthy participants have shown strong neural responses across left hemispheric brain regions known to be central to language processing, including left superior temporal cortices, the left supramarginal gyrus, and the left prefrontal cortex (16,22). These studies have also shown robust activity in visual occipital areas, parietal cortices, and left frontotemporal cortices, and, importantly, response strength across these regions was highly dynamic and varied by phase of working memory processing. Interestingly, recent work has also shown increased activity in homolog right prefrontal and temporal cortices of older control subjects and patients with psychiatric or neurological conditions, which is thought to reflect compensatory activity that enables adequate task performance (19–22). Although research has been limited, these compensatory activation patterns have also been found using functional MRI (fMRI) and verbal working memory tasks in patients with type 1 diabetes (5).
In the current study, we used magnetoencephalography (MEG) to identify dynamic alterations in cortical neurophysiology between patients with type 1 diabetes and demographically matched control subjects without diabetes. MEG is an emerging technique that measures neural activity directly and offers a finer temporal resolution than comparable methods such as fMRI. The millisecond-scale time resolution of MEG provides the ability to examine differences in the phases of working memory processing (e.g., encoding vs. maintenance) and thus allows more subtle differences to be identified between groups. This enhanced sensitivity was especially important in the current study, because our patient group was relatively young, free of common comorbid conditions, and was examined in the normoglycemia range. We focused on this group of patients because it would allow for stronger conclusions about the unique impact of the disease on brain physiology.
Participants in each group completed a Sternberg-type working memory task during MEG, and we followed a data-driven approach to identify and image the oscillatory neural responses serving working memory processes. Subsequently, we used time-series analysis methods to examine the neural dynamics underlying encoding and maintenance operations. We hypothesized that patients with diabetes would exhibit increased recruitment of regions involved in working memory processing during the encoding and maintenance phases and that the specific regions being recruited would vary across these phases. Furthermore, we hypothesized that measures of disease duration and glycemic control would relate to brain activity, such that greater disease duration and higher HbA1c levels would result in the activation of compensatory mechanisms, such as hyperactivity in core working memory brain regions and/or increased recruitment of right hemispheric homolog brain regions, to maintain behavioral performance levels.
Research Design and Methods
Participants
The study recruited 38 patients (14 females) with type 1 diabetes and no known comorbidities for participation in the study (age range: 19–35 years). A demographically matched control group (n = 38) was also enrolled for comparison. The two groups were matched on age, sex, education, BMI, ethnicity, and handedness. Participants with type 1 diabetes were recruited from the University of Nebraska Medical Center (UNMC) Diabetes Clinic. All patients were receiving insulin therapy in the form of basal-bolus therapy delivered via insulin pump or insulin injections. Control subjects were recruited from the greater Omaha area.
Exclusionary criteria for both groups included any medical diagnosis primarily affecting central nervous system function (e.g., psychiatric and/or neurological disease); known brain neoplasm or lesion; history of significant head trauma; current substance dependence; pregnancy or lactation; any hospitalization within the previous 3 months; any type of cancer; treatment with antipsychotics, antidepressants, and related medications known to affect brain function, with the exception of as needed antidepressants after a 24-h washout period; current or prior treatment with statins; and ferromagnetic implants. Additional exclusionary criteria for patients included the presence of micro- or macrovascular disease, defined as a urinary albumin-to-creatine ratio of >30 μg albumin/mg creatinine in the previous 12 months; hypertension (blood pressure >130/85 mmHg); kidney disease, defined by glomerular filtration rate <60 mL/min/1.73 m2; aspartate transaminase–to–alanine transaminase ratio >2 units/L; a severe hypoglycemic episode within the past 3 months, defined as an event requiring third-party assistance; and untreated thyroid and/or vitamin B12 deficiency. Written informed consent was obtained from each participant following the guidelines of the UNMC Institutional Review Board, which approved the study protocol.
Laboratory Blood Tests
Before MEG, participants underwent a panel of blood tests according to the standards of care described by the American Diabetes Association. These tests included measurements of glycated hemoglobin (HbA1c), creatinine, glucose, aspartate transaminase, alanine transaminase, albumin-to-creatinine ratio, thyroid-stimulating hormone, and vitamin B12 (see Table 1). Demographic and medical history data were collected, and patients were given a brief questionnaire about the number of hypoglycemic episodes they experience per week. On the day of the MEG, patients checked their blood glucose level using a point-of-care device. Those whose measurements were within the 70–200 mg/dL range started their MEG session shortly thereafter, whereas those whose measurements were 55–70 mg/dL were asked to raise their blood glucose to the normal range, and after 1 h in the normal range, these participants started their MEG session. Participants whose blood glucose levels were <55 mg/dL—because values lower than this threshold equate to clinically significant hypoglycemia—or >200 mg/dL were rescheduled for a different day at least 1 week later.
Table 1.
Demographics and laboratory test results
| Patients (n = 33) | Control subjects (n = 34) | |
|---|---|---|
| Age (years) | 26.1 (5.0) | 26.3 (3.8) |
| Sex | ||
| Male | 20 | 24 |
| Female | 13 | 10 |
| Handedness | ||
| Right | 28 | 30 |
| Left | 5 | 4 |
| Disease duration (years) | 12.39 (7.76) | — |
| HbA1c (%) | 7.78 (1.37) | — |
| HbA1c (mmol/mol) | 62.0 (15.0) | — |
| Hypoglycemic episodes per week (n) | 3.05 (3.04) | — |
| Creatinine (mg/dL) | 0.84 (0.013) | — |
| Glucose (mg/dL) | 142.69 (38.29) | — |
| Albumin-to-creatinine ratio (μg albumin/mg creatinine) | 8.30 (6.86) | — |
| Thyroid-stimulating hormone (mcIU/mL) | 2.72 (1.93) | — |
| Vitamin B12 (pg/mL) | 485.06 (218.86) | — |
Values are depicted as n or as mean (SD).
Working Memory Task Experiment
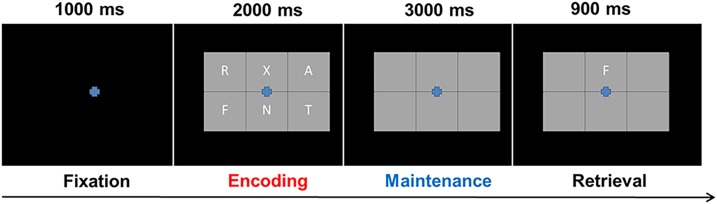
During the MEG session, participants were seated in a nonmagnetic chair and instructed to fixate on a crosshair presented centrally for 1.0 s. A grid containing six letters was then presented for 2.0 s (encoding). These letters then disappeared from the grid, and 3.0 s later (maintenance phase), a single “probe” letter appeared for 0.9 s (retrieval phase) (Fig. 1). Participants were instructed to respond with a button press indicating whether the probe letter was one of the six letters previously presented. Each trial lasted 6.9 s, including a 1.0-s prestimulus fixation. Each participant completed 128 trials. Our group has validated this same task in several previous studies (16,21,22).
Figure 1.
Task paradigm. Presentation of stimuli during the verbal working memory task started with a 1,000 ms fixation cross, followed by the appearance of a six-letter grid for 2,000 ms (encoding period), an empty grid for 3,000 ms (maintenance period), and finally, the probe letter for 900 ms (retrieval). During the retrieval phase, participants were to respond with a finger tap indicating whether the probe letter was present in the previously shown six-letter grid.
MEG Methods and Analyses
The MEG methods and statistical analyses used in this study closely correspond to those used in previous normative studies by our group (23,24). Briefly, MEG recordings were conducted within a magnetically shielded room using an Elekta MEG system with 306 magnetic sensors (Elekta, Helsinki, Finland). MEG data were sampled at 1 kHz using an acquisition bandwidth of 0.1–330 Hz, individually corrected for head motion (offline), and subjected to noise reduction using the signal space separation method with a temporal extension (25). Each participant’s MEG data were then coregistered with structural T1-weighted MRI data.
After removal of cardio artifacts, the continuous magnetic time series was divided into epochs of 6.9-s duration, with the baseline defined as −0.4 to 0.0 s before initial stimulus onset. Artifact-free epochs were transformed into the time-frequency domain using complex demodulation, and the resulting spectral power estimations per sensor were averaged over trials to generate time-frequency plots of mean spectral density. These sensor-level data were normalized using the respective bin’s baseline power, which was calculated as the mean power during the −0.4- to 0.0-s period.
The precise time-frequency windows used for imaging were determined by statistical analysis of the sensor-level spectrograms across the array of gradiometers during the 5-s “encoding” and “maintenance” time window. Each data point in the spectrogram was initially evaluated using a mass univariate approach based on the general linear model (GLM) and then corrected for multiple comparisons in stage two. First, one-sample t tests were conducted on each data point, and a threshold of the output spectrograms of t values was set at P < 0.05 to define time-frequency bins containing potentially significant oscillatory deviations across all participants. In stage two, time-frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also significant, and a cluster value was derived by summing all of the t values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster values, and the significance level of the observed clusters was tested directly using this distribution (16,26,27). Based on these analyses, the time-frequency windows that contained significant oscillatory events across all participants during the encoding and maintenance phases (see results) were subjected to beamforming.
Cortical networks were imaged at 4.0 × 4.0 × 4.0-mm resolution using the dynamic imaging of coherent sources beamformer (28,29), which uses spatial filters in the frequency domain to calculate source power for the entire brain volume. Following convention, we computed the noise-normalized source power per voxel in each participant using active (i.e., task) and passive (i.e., baseline) periods of equal duration and bandwidth. Such images are typically referred to as pseudo-t maps, with units (i.e., pseudo-t) that reflect noise-normalized power differences per voxel. All source imaging used Brain Electrical Source Analysis (BESA) version 6.1 software (BESA GmbH, Gräfelfing, Germany). Before statistical analysis, each participant’s functional MEG images were transformed into standardized Montreal Institute space using the transform that was previously applied to the structural images and then spatially resampled (23,24). The resulting three-dimensional maps of brain activity were averaged across participants in each group to assess the neuroanatomical basis of significant oscillatory responses identified through the sensor-level analysis. These images were also statistically evaluated using a mixed effects, mass univariate approach based on the GLM and a whole-brain correlational approach. The effect of group (type 1 diabetes/control subjects) was determined using two-tailed independent-samples t tests per time-frequency bin. Whole-brain correlation maps were computed using the functional images and clinical metrics, including disease duration and HbA1c, partialling out age because it is a confounding factor with duration and HbA1c. Statistical comparisons for the time-series analyses used an average value over the maintenance period for each participant for group-level t test comparisons for tests of significance. All output statistical maps were displayed as a function of the alpha level, thresholded at P < 0.01, and adjusted for multiple comparisons using a spatial extent threshold (i.e., cluster restriction; k = 300) based on the theory of Gaussian random fields (30).
Results
Demographic, Behavioral, and Laboratory Results
Five participants from the patient group and four participants from the matched control subjects were excluded at the data analysis phase due to artifactual MEG data. The final study group included 33 participants with type 1 diabetes (13 females; mean age, 26.1 years; SD 5.0) and 34 demographically matched control subjects (10 females; mean age, 26.3 years; SD 3.8). Average education level was 16.2 years (SD 1.7) for patients and 17.4 years (SD 3.0) for control subjects; this difference was not significant. The mean disease duration in patients was 12.4 years (SD 7.8), and the mean HbA1c was 7.78% (SD 1.37). Other blood laboratory test values are reported in Table 1. As was expected, both groups performed the working memory task reasonably well, with a mean accuracy rate of 85.53% (SD 12.34) and no between-group differences (t 65 = 1.08, P = 0.283). Note that equivalent performance across groups was by design, because accuracy differences would have confounded our main MEG results (in that case, significant neural differences could reflect performance disparities and not the effect of disease).
Sensor-Level Results
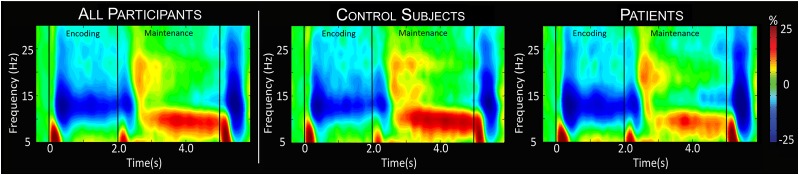
To identify the precise time-frequency bins for follow-up beamforming analyses, the sensor-level time-frequency spectrograms were statistically evaluated using t tests comparing the active period to the baseline period followed up with nonparametric permutation testing for multiple comparisons correction. These analyses indicated that across patients and control subjects, there was a significant decrease in 9–16 Hz (extended alpha) activity throughout the encoding phase of the task in sensors over posterior and left hemispheric brain regions (P < 0.001, corrected) (Fig. 2). This activity began ∼200 ms after the onset of the encoding grid and extended until ∼2,200 ms. This was followed by a significant increase in 8–11 Hz (low alpha; P < 0.001, corrected) (Fig. 2) activity that began at ∼2,400 ms and stretched throughout most of the maintenance phase, peaking in more posterior sensors bilaterally. These significant time-frequency windows were imaged using beamforming to enable group-level statistical modeling to occur in anatomical space. To this end, we imaged data from 200 to 2,200 ms in the 9–16 Hz range and from 2,200 to 5,000 ms in the 8–11 Hz range using discrete 400-ms windows to enable the neural dynamics to be statistically probed.
Figure 2.
Time-frequency spectrograms. Grand- (far left) and group-averaged (middle and right) time-frequency spectrograms from a representative parieto-occipital MEG sensor. Time is shown on the x-axis in seconds, and frequency is shown on the y-axis in Hz. The colors reflect power increases (red) and decreases (blue) relative to the baseline, with the scale bar shown to the far right. Time-frequency windows for source imaging (beamforming) were derived from statistical analysis of the sensor-level spectrogram data across all participants. Thus, the separate group-level spectrograms are provided for visualization purposes only. Weaker low alpha responses in patients can be discerned throughout most of the maintenance phase.
Dynamic Functional Imaging Analysis
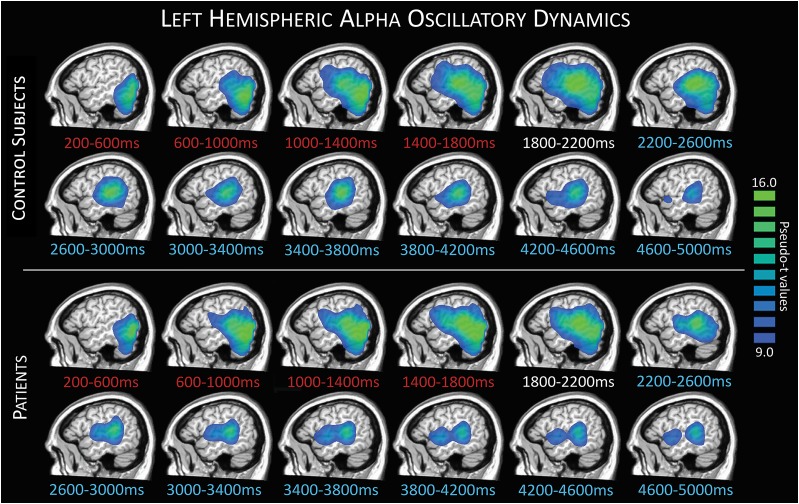
To evaluate the brain dynamics serving working memory performance, we initially examined the time course of activity in each group (Fig. 3). Neural responses during the encoding period were generally similar across groups, with some divergence emerging during maintenance. Specifically, we found a strong decrease in extended alpha activity (9–16 Hz) during encoding, beginning in occipital regions and quickly spreading to more anterior regions, including superior temporal and inferior prefrontal regions. In patients, two distinct peaks could be observed in the 8–11 Hz range during most of the maintenance phase, strongest in the left superior temporal region with a weaker more anterior peak in left inferior frontal regions. In contrast, a left superior temporal peak dominated the maintenance period in control subjects, although a smaller inferior frontal peak could also be discerned. The overall pattern and progression of the left hemispheric activity were generally similar between groups, with specific regional differences expanded upon below.
Figure 3.
Left hemisphere response dynamics. Group-averaged images per time window during encoding (red text), transition (white text), and maintenance (blue text) periods depict the evolution of responses during task performance. Control group averages are shown in the top panel, with patient group averages below. A strong decrease in alpha activity relative to the baseline can be seen during encoding time bins, starting in occipital and stretching forward to more parietal, superior temporal, and inferior frontal regions through later encoding, transition, and into maintenance.
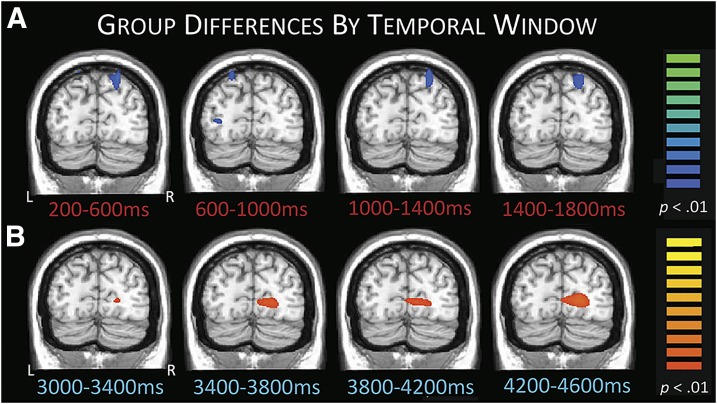
Next, we compared the two groups to identify regions with statistical differences in each time window. These analyses revealed that patients exhibited significantly stronger decreases in extended alpha (9–16 Hz) relative to control subjects in bilateral parietal and left lateral occipital cortices during encoding (P < 0.01, corrected) (Fig. 4A). This pattern of response differences stretched into the transition period (data not shown). During maintenance, patients had a significantly weaker increase in low alpha (8–11 Hz) in the right occipital region compared with control subjects (Fig. 4B), and this continued throughout most of maintenance (P < 0.01, corrected).
Figure 4.
Group differences in occipital activity during encoding and maintenance. A: Patients exhibited stronger decreases in extended alpha activity relative to control subjects in superior parietal regions throughout the encoding period, likely reflecting compensatory activity. B: Patients also had weaker low alpha responses (i.e., alpha synchronization) compared with control subjects during the maintenance phase in occipital areas, likely reflecting weakened suppression of incoming visual information. This pattern held throughout most of maintenance, with some representative time windows shown. All images have been thresholded at P < 0.01, corrected; scale bars to the right. L, left; R, right.
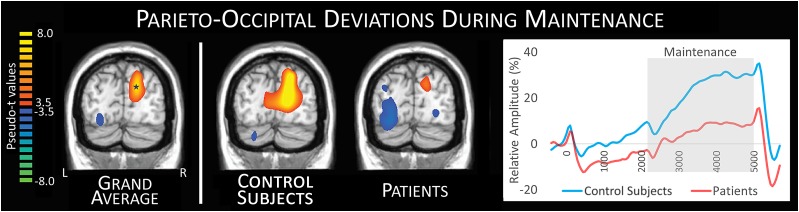
The alpha increase observed during the maintenance phase has been described in several recent articles (16,22) and was of particular interest in this study because it has been linked to the ability to maintain representations in the face of incoming, distracting visual information (14,15,31,32). Given this, we compiled the grand average image of patients and control subjects and extracted the time series of the peak voxel, which revealed significantly stronger 8–11 Hz alpha activity in the control subjects relative to the patients within the maintenance period (t65 = 3.39, P = 0.001) (Fig. 5).
Figure 5.
Time series of parieto-occipital differences during maintenance. The grand average image compiled from patients and control subjects (far left) illustrates the characteristic parieto-occipital low alpha increase (synchronization) during maintenance, which is thought to reflect the inhibition of incoming visual information. This grand-averaged image was used to identify the peak voxel (14, −80, 25) of this response, and the time series of this voxel was then extracted for group comparisons. This analysis revealed that control subjects had a sustained, stronger low alpha response throughout the maintenance period relative to patients with type 1 diabetes (far right). In this plot, time is shown on the x-axis in ms, amplitude is shown on the y-axis in percent change from baseline, and the gray box reflects the maintenance period where the response amplitude significantly (P = 0.001) differed between groups. L, left; R, right.
Whole-Brain Correlations
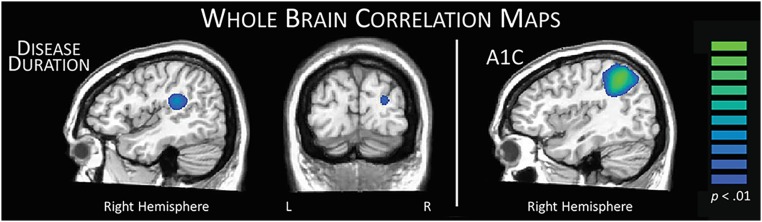
Lastly, we computed maps of whole-brain correlations using the disease duration, HbA1c, and MEG images averaged across encoding and maintenance periods in patients. Because disease duration and HbA1c both correlated with age (r = 0.41, P = 0.020 and r = −0.46, P = 0.007, respectively), all correlation maps were generated with age as a covariate to partial out the influence of age. Supporting a meaningful relationship between right hemispheric activity and disease markers, these analyses indicated greater right hemispheric involvement in task performance with increases in disease duration and HbA1c (Fig. 6). For example, with increasing disease duration, stronger decreases in extended alpha activity were found in the right temporal parietal junction (r = −0.52, P = 0.003) and parieto-occipital region (r = −0.48, P = 0.005). Moreover, increased HbA1c values were associated with stronger decreases in right parietal regions (r = −0.52, P = 0.002). Neither disease duration nor HbA1c correlated with task performance, measured by accuracy (r = −0.08, P = 0.683 and r = −0.10, P = 0.572, respectively).
Figure 6.
Neuroclinical correlation maps. Left: The duration of disease in patients with type 1 diabetes was significantly correlated with response amplitude across the encoding and maintenance periods in two distinct regions. The right temporoparietal negative correlation (peak voxel: r = −0.52, P = 0.003) with disease duration likely reflects compensatory activity in right hemispheric homolog areas, which gets stronger with longer disease duration. However, the negative correlation between parieto-occipital activity and time since diagnosis (peak voxel: r = −0.48, P = 0.005) likely reflects aberrantly decreased occipital inhibition with disease progression. Right: The HbA1c level in patients was negatively correlated with right parietal activity during encoding and maintenance (peak voxel: r = −0.52, P = 0.002), such that as HbA1c levels increased, there were greater decreases in alpha relative to the baseline, again suggesting compensatory activity. All correlations were performed using age as a covariate of no interest, because it was found to correlate with both disease duration and HbA1c levels. L, left; R, right.
Discussion
We used high-density MEG to investigate the impact of type 1 diabetes on the neurophysiology serving working memory processing in young adults. In both groups, our data indicated a sharp decrease in extended alpha activity that began in occipital regions during encoding. This decrease in alpha activity spread to include left temporal and prefrontal cortices during the latter half of encoding, narrowed to low alpha during early maintenance, and dissipated during the latter half of maintenance. This pattern of left hemispheric oscillatory activity corroborates and extends previous imaging work from our laboratory and others, which has shown that verbal working memory activates a network of left hemispheric language regions. As for group differences, patients with type 1 diabetes exhibited stronger oscillatory alpha responses relative to control subjects in bilateral superior parietal cortices during encoding and weaker low alpha responses in occipital cortices during maintenance. Finally, our results indicated that duration of diabetes and glycemic regulation (HbA1c) were both correlated with oscillatory MEG responses across the time series. Below, we discuss the implications of these findings for understanding the impact of type 1 diabetes on the brain networks serving working memory processing and the roles of glycemic regulation and the duration of disease.
The alpha responses observed throughout the task period in both groups have been linked to a wide range of cognitive processes involved in working memory and other executive functions. These primarily left-lateralized decreases in superior temporal and inferior prefrontal regions have been previously connected to the Baddeley model of working memory, specifically to the phonological loop and central executive subcomponents (12,13,16,17,22,33). The phonological loop represents vocal and subvocal rehearsal mechanisms to increase the likelihood of maintaining the information (33,34). Interestingly, we found that patients with type 1 diabetes exhibited stronger alpha oscillations relative to control subjects in the superior parietal cortices throughout most of the encoding period. This brain region, along with the supramarginal gyrus, has been repeatedly linked to phonological loop processes (16,21,22), and in our study, these differences emerged in the left superior parietal as well as in its right hemisphere homolog. We propose that these differences reflect compensatory activity in the patients with diabetes, because control subjects also activated these regions during the encoding period and they are known to be central to working memory processing.
In regard to maintenance, increased posterior occipital alpha activity is thought to be a mechanism for inhibiting incoming (distracting) visual information, which would facilitate the maintenance of relevant memory representations in the more anterior, left hemisphere regions discussed above (14,15,31,32). Both groups exhibited increased occipital alpha during most of the maintenance period, but this increase was significantly weaker in patients. Basically, patients were able to perform at nearly the same level as control subjects despite this aberrant occipital processing, so it is plausible that they compensated through increased processing in other nodes involved in working memory processing. Similar findings of aberrant processing in one brain area and compensatory processing in other brain regions serving working memory processing has been reported in the aging literature (22). Given the age of our participants, future studies should probe the impact of aging on these brain networks in patients with type 1 diabetes, because it is probable that the common compensatory mechanisms of healthy aging may be exhausted by the time these patients reach older adulthood.
Consistent with our MEG/HbA1c correlation findings, previous behavioral studies have linked deficits in working memory processing with glycemic control (35). For example, intensive regulation of glycemic control in adolescents with type 1 diabetes (36) and adults with type 2 diabetes (37) has been shown to improve working memory task performance, although there is also a risk of increased hypoglycemic episodes with intensive treatment regimens, which may again lead to worse cognitive outcomes long-term (6,37). Unawareness of hypoglycemia, another long-term factor, has also been shown to induce further cognitive impairment. This hypothesis posits that as cognitive deficits arise, they affect the patient’s ability to control glycemic levels, altering the awareness of the glycemic state and leaving the dysglycemia untreated, which ultimately leads to further cognitive decline (38,39). This cyclical decline increases the likelihood of devastating cognitive outcomes, such as mild cognitive impairment and Alzheimer disease (38). Apathy toward the disease and nonadherence to treatment regimens have also been shown to produce poor cognitive outcomes in children and adults with diabetes (40–43). These previous findings clearly illuminate the intricate interrelationship between disease duration and glucose management, and thus, it was unsurprising that both correlated with neural activity related to working memory in the current study. The areas affected have been previously linked to working memory processing, as the occipital region has been tightly connected to blocking incoming visual information that could disturb representations, whereas the right temporoparietal area has been linked with compensatory rehearsal processes in older adults (22). However, given that our findings are correlational, future studies should consider directly comparing good and poor glycemic management groups to ensure these neurological differences are definitive.
In conclusion, we found aberrant processing in the networks serving working memory in young adults with type 1 diabetes who were otherwise healthy. These patients exhibited stronger responses in the superior parietal area during encoding and a diminished alpha response in the parieto-occipital cortices during maintenance. Overall, this pattern of neural dynamics was closer to that of an aging population, with increased right hemispheric involvement during encoding. We suggest that these young patients may already be compensating for decreased neural resources or efficiency by using other network resources and that this may leave less capacity for compensation later in life. Compensatory capacity may be especially affected in those with poor glycemic control, because this has been shown to greatly influence patient outcomes (e.g., cognition) in later adulthood.
Before closing, it is important to recognize some limitations of this study, including the specific focus on working memory, the young age of the participants, and that the participants were clear of virtually all common comorbidities. Although these limitations were by design, they still limit the generalizability of the results.
To close, we found that aberrant neurophysiological activity during working memory processing is already present in young adults with type 1 diabetes and that duration of disease and glycemic control play a key role. These findings add to the body of literature implicating these specific disease factors in cognitive dysfunction, with clear neurophysiological differences emerging even before behavioral differences and likely worsening with age. Future studies should more directly examine the role of glycemic control and the impact of aging.
Article Information
Funding. This work was supported by National Institutes of Health grants F31-AG-055332 (to A.I.W.) and R01-MH103220 (to T.W.W.), National Science Foundation grant 1539067 (to T.W.W.), and by a Research Support Fund grant from the Nebraska Health System and the University of Nebraska Medical Center. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Duality of Interest. C.V.D. is a consultant with Novo Nordisk and receives research funding from Theracos and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. C.M.E., A.I.W., A.L.P., E.H.-G., T.J.M., C.V.D., and T.W.W. contributed to data analysis and interpretation. C.M.E., E.H.-G., A.T.D., C.V.D., and T.W.W. contributed to article revisions. C.M.E., G.H.L., K.L.B., A.T.D., and C.V.D. collected data and assisted in participant recruitment. C.M.E. and T.W.W. drafted the article. A.L.P., E.H.-G., K.L.B., A.T.D., C.V.D., and T.W.W. contributed to experimental design. All authors take responsibility for the contents of this article. T.W.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 2017 Annual Meeting of the Organization for Human Brain Mapping, Vancouver, BC, Canada, 25–29 June 2017.
References
- 1.Biessels GJ, Reijmer YD. Brain changes underlying cognitive dysfunction in diabetes: what can we learn from MRI? Diabetes 2014;63:2244–2252 [DOI] [PubMed] [Google Scholar]
- 2.Moran C, Beare R, Phan T, et al. . Neuroimaging and its relevance to understanding pathways linking diabetes and cognitive dysfunction. J Alzheimers Dis 2017;59:405–419 [DOI] [PubMed] [Google Scholar]
- 3.Nunley KA, Ryan CM, Orchard TJ, et al. . White matter hyperintensities in middle-aged adults with childhood-onset type 1 diabetes. Neurology 2015;84:2062–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolo NR, Musen G, Jacobson AM, et al. . Brain activation during working memory is altered in patients with type 1 diabetes during hypoglycemia. Diabetes 2011;60:3256–3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guàrdia-Olmos J, Gallardo-Moreno GB, Gudayol-Ferré E, Peró-Cebollero M, González-Garrido AA. Effect of verbal task complexity in a working memory paradigm in patients with type 1 diabetes. A fMRI study. PLoS One 2017;12:e0178172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rooijackers HM, Wiegers EC, Tack CJ, van der Graaf M, de Galan BE. Brain glucose metabolism during hypoglycemia in type 1 diabetes: insights from functional and metabolic neuroimaging studies. Cell Mol Life Sci 2016;73:705–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet 2012;379:2291–2299 [DOI] [PubMed] [Google Scholar]
- 8.Ryan CM, van Duinkerken E, Rosano C. Neurocognitive consequences of diabetes. Am Psychol 2016;71:563–576 [DOI] [PubMed] [Google Scholar]
- 9.Moheet A, Mangia S, Seaquist ER. Impact of diabetes on cognitive function and brain structure. Ann N Y Acad Sci 2015;1353:60–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tonoli C, Heyman E, Roelands B, et al. . Type 1 diabetes-associated cognitive decline: a meta-analysis and update of the current literature. J Diabetes 2014;6:499–513 [DOI] [PubMed] [Google Scholar]
- 11.Broadley MM, White MJ, Andrew B. A systematic review and meta-analysis of executive function performance in type 1 diabetes mellitus. Psychosom Med 2017;79:684–696 [DOI] [PubMed] [Google Scholar]
- 12.Baddeley A. Working memory. Science 1992;255:556–559 [DOI] [PubMed] [Google Scholar]
- 13.D’Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci 2007;362:761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnefond M, Jensen O. Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Curr Biol 2012;22:1969–1974 [DOI] [PubMed] [Google Scholar]
- 15.Händel BF, Haarmeier T, Jensen O. Alpha oscillations correlate with the successful inhibition of unattended stimuli. J Cogn Neurosci 2011;23:2494–2502 [DOI] [PubMed] [Google Scholar]
- 16.Heinrichs-Graham E, Wilson TW. Spatiotemporal oscillatory dynamics during the encoding and maintenance phases of a visual working memory task. Cortex 2015;69:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rottschy C, Langner R, Dogan I, et al. . Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage 2012;60:830–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDermott TJ, Badura-Brack AS, Becker KM, et al. . Attention training improves aberrant neural dynamics during working memory processing in veterans with PTSD. Cogn Affect Behav Neurosci 2016;16:1140–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDermott TJ, Badura-Brack AS, Becker KM, et al. . Male veterans with PTSD exhibit aberrant neural dynamics during working memory processing: an MEG study. J Psychiatry Neurosci 2016;41:251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiesman AI, Heinrichs-Graham E, McDermott TJ, Santamaria PM, Gendelman HE, Wilson TW. Quiet connections: reduced fronto-temporal connectivity in nondemented Parkinson’s disease during working memory encoding. Hum Brain Mapp 2016;37:3224–3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson TW, Proskovec AL, Heinrichs-Graham E, et al. . Aberrant neuronal dynamics during working memory operations in the aging HIV-infected brain. Sci Rep 2017;7:41568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proskovec AL, Heinrichs-Graham E, Wilson TW. Aging modulates the oscillatory dynamics underlying successful working memory encoding and maintenance. Hum Brain Mapp 2016;37:2348–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDermott TJ, Wiesman AI, Proskovec AL, Heinrichs-Graham E, Wilson TW. Spatiotemporal oscillatory dynamics of visual selective attention during a flanker task. Neuroimage 2017;156:277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiesman AI, Heinrichs-Graham E, Proskovec AL, McDermott TJ, Wilson TW. Oscillations during observations: dynamic oscillatory networks serving visuospatial attention. Hum Brain Mapp 2017;38:5128–5140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 2006;51:1759–1768 [DOI] [PubMed] [Google Scholar]
- 26.Ernst MD. Permutation methods: a basis for exact inference. Stat Sci 2004;19:676–685 [Google Scholar]
- 27.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 2007;164:177–190 [DOI] [PubMed] [Google Scholar]
- 28.Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci U S A 2001;98:694–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp 2005;25:199–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 1996;4:58–73 [DOI] [PubMed] [Google Scholar]
- 31.Bonnefond M, Jensen O. The role of gamma and alpha oscillations for blocking out distraction. Commun Integr Biol 2013;6:e22702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Payne L, Guillory S, Sekuler R. Attention-modulated alpha-band oscillations protect against intrusion of irrelevant information. J Cogn Neurosci 2013;25:1463–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci 2000;4:417–423 [DOI] [PubMed] [Google Scholar]
- 34.Baddeley A. Working memory: the interface between memory and cognition. J Cogn Neurosci 1992;4:281–288 [DOI] [PubMed] [Google Scholar]
- 35.Pappas C, Andel R, Infurna FJ, Seetharaman S. Glycated haemoglobin (HbA1c), diabetes and trajectories of change in episodic memory performance. J Epidemiol Community Health 2017;71:115–120 [DOI] [PubMed] [Google Scholar]
- 36.Knight S, Northam E, Donath S, et al. . Improvements in cognition, mood and behaviour following commencement of continuous subcutaneous insulin infusion therapy in children with type 1 diabetes mellitus: a pilot study. Diabetologia 2009;52:193–198 [DOI] [PubMed] [Google Scholar]
- 37.Ryan CM, Freed MI, Rood JA, Cobitz AR, Waterhouse BR, Strachan MW. Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes Care 2006;29:345–351 [DOI] [PubMed] [Google Scholar]
- 38.Grober E, Hall CB, Hahn SR, Lipton RB. Memory impairment and executive dysfunction are associated with inadequately controlled diabetes in older adults. J Prim Care Community Health 2011;2:229–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansen TI, Olsen SE, Haferstrom ECD, et al. . Cognitive deficits associated with impaired awareness of hypoglycaemia in type 1 diabetes. Diabetologia 2017;60:971–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brock LL, Brock CD, Thiedke CC. Executive function and medical non-adherence: a different perspective. Int J Psychiatry Med 2011;42:105–115 [DOI] [PubMed] [Google Scholar]
- 41.McNally K, Rohan J, Pendley JS, Delamater A, Drotar D. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care 2010;33:1159–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padala PR, Desouza CV, Almeida S, et al. . The impact of apathy on glycemic control in diabetes: a cross-sectional study. Diabetes Res Clin Pract 2008;79:37–41 [DOI] [PubMed] [Google Scholar]
- 43.Soutor SA, Chen R, Streisand R, Kaplowitz P, Holmes CS. Memory matters: developmental differences in predictors of diabetes care behaviors. J Pediatr Psychol 2004;29:493–505 [DOI] [PubMed] [Google Scholar]