Abstract
Objective
This systematic examination and meta-analysis examined the scope and variation of the worldwide double burden of diseases and identified related socio-demographic factors.
Design
We searched PubMed for studies published in English from January 1, 2000, through September 28, 2016, that reported on double disease burden. Twenty-nine studies from 18 high-, middle- and low-income countries met inclusion criteria and provided 71 obesity-undernutrition ratios, which were included in meta-regression analysis.
Results
All high-income countries had a much higher prevalence of obesity than undernutrition (i.e., all the obesity/undernutrition ratios > 1); 55% of the ratios in lower-middle-and low-income countries were < 1, but only 28% in upper-middle-income countries. Meta-analysis showed a pooled obesity-undernutrition ratio of 4.3 (95% CI = 3.1–5.5), which varied by country income level, subjects’ age, and over time. The average ratio was higher in high- rather than in lower-middle and low-income countries (β [SE] = 10.8 [2.6]); in adults vs. children (7.1 [2.2]), and in data collected since 2000 vs. before 2000 (5.2 [1.5]; all p values < 0.05).
Conclusions
There are considerable differences in the obesity vs. undernutrition ratios and in their prevalence by country income level, age groups, and over time, which may be a consequence of the cumulative exposure to an obesogenic environment.
Keywords: double burden, undernutrition, obesity, overweight, worldwide
INTRODUCTION
The double burden of malnutrition is defined by the World Health Organization (WHO) as the coexistence of undernutrition along with overweight/obesity or diet-related non-communicable diseases (NCDs).1 Several studies have examined this double burden, especially in low- and middle-income countries. Most are based on data from cross-sectional surveys or country-level aggregating statistics dating from the early 2000s.2–5 In general, the studies indicate that a double burden is not exclusive to urban areas and those with high income status, but also happens in rural areas and those with low income status.6, 7 Economic development, poverty, urbanization, and lifestyle changes8 are most commonly suggested as reasons for this. This body of research is also pertinent to the developmental origins of adult disease (e.g., the Baker hypothesis, metabolic programming, thrifty phenotype, etc.),9 which helps explain the coexistence of high-prevalence undernutrition and obesity as well as lifestyle-related chronic diseases such as cardiovascular diseases and diabetes.
The reported prevalence of undernutrition and obesity has varied considerably across available studies, which may be due to the diverse study populations, study designs, outcome measures, and analytic methods. There are very few systematic reviews examining the diversity and range of the double burden of diseases and its variation by population characteristics.7, 10 The present study is such an examination and meta-analysis, designed to elucidate the degree of the worldwide double burden and its variations and to identify attributable socio-demographic factors (e.g., subjects’ sex, age, country income level, and secular trends).
METHODS AND MATERIALS
Literature Search Strategy
We searched PubMed for relevant articles published in English from January 1, 2000, through September 28, 2016. We developed search strategies based on various combinations of related key words for double burden, obesity, malnutrition, undernutrition, and nutritional transition. The initial search yielded 700 publications, which led to 274 studies after removing duplicates from multiple search results. A title and abstract screening was performed to exclude irrelevant publications, which resulted in 219 studies. After two coauthors performed a second round of full-text screening for eligibility, 29 final papers met our inclusion criteria (see Appendix 1). These studies were from 18 high-, middle- and low-income countries: Bangladesh (n = 2), Brazil (2), Chile (2), China (3), India (3), Indonesia (2), Iran (3), Mexico (2), and 10 other countries (one study each from Albania, Colombia, Ecuador, Gambia, Guatemala, Libya, Nigeria, Peru, South Africa, and the USA).
Inclusion and Exclusion Criteria
Studies included in the review met the following inclusion criteria: a) published in English and since 2000, b) reported both prevalence of malnutrition/undernutrition and overweight/obesity or diet-related NCDs, c) original study, d) in the general population, e) focused on one country or population, f) having a total number of study subjects > 5000, and g) available in full text.
Studies were excluded if: a) no report on prevalence of malnutrition/undernutrition, overweight/obesity, or diet-related NCDs, b) not an original study (review papers, using aggregated data [from the United Nations (UN), WHO, etc.]), c) not using a general population (refugees, island tribes, infants under 2 years old only, etc.), d) having mixed countries or study samples, e) having a total study subject of < 5000, or f) full text was not available.
Data Extraction
Data were extracted and arranged following PRISMA guidelines into standardized data extraction form by two of our investigators. Information extracted included publication year; study setting; country; study subjects’ demographics; source for classification of underweight, overweight, and obesity; patterns of undernutrition, obesity, and diet-related NCDs; and suggested driving force behind the double burden. Sex- and age-specific undernutrition and obesity values were specifically abstracted unless the articles reported an overall value for the whole study subject group. To maximize comparability, we chose the values using overweight/obesity and underweight as the main outcomes in data extraction rather than overweight, obesity, stunting, wasting, anemia, or other body compositions if a study used multiple measures, since 82.8% of articles fit this description. If an article reported outcomes in multiple years, we selected time points of up to four years with regular intervals to ensure studies received balanced weights in meta-analysis.
Key Study Variables
The key outcome variables were (a) undernutrition and obesity/overweight prevalence as reported in the articles and (b) our calculated obesity-undernutrition ratio (see below). Key exposure variables included the study population’s characteristics, time span, country’s level of economic development (income level), and world region.
1) Disease ratio of obesity and over-nutrition-related NCDs to undernutrition (obesity-undernutrition ratio)
The majority of the 71 data points from 29 studies reported underweight (76.1%), stunted (15.5%), and others (8.4%) as the measures of undernutrition, and overweight/obesity (73.2%), obesity (18.3%), and overweight (8.5%) as the indices of obesity. No study reported the prevalence of diet-related NCDs. To show the coexisting disease burden of undernutrition and obesity, we calculated their ratio. Among 71 obesity-undernutrition ratios for various groups (by country, sex, age, and data collection year), the majority of the obesity-undernutrition ratio information (53.5%) was presented in terms of the prevalence of overweight/obesity and underweight.
2) Country’s level of economic development and world region
The 18 countries covered by these 29 studies were classified into three categories based on gross national income (GNI) per capita, using the World Bank Atlas method for the 2017 fiscal year. High-income countries were those with a GNI per capita of $12,476 or higher (Chile and the US); upper-middle-income countries were those with a GNI per capita from $4,036 to $12,475 (Albania, Brazil, China, Colombia, Ecuador, Iran, Libya, Mexico, Peru, and South Africa); lower-middle- and low-income countries had a GNI per capita of $4,035 or less (Bangladesh, Guatemala, India, Indonesia, and Nigeria).11
In addition, these 18 countries were grouped regionally as Asia, Eastern Europe, Middle East, Africa, North America, South America, Central America, or Oceania according to the UN’s country grouping.12 We considered diversity of populations by world region of study rather than by racial groups represented. This was because some countries have multiple ethnic groups, but the information on racial composition reported in most studies was limited.
3) Sex, age, and secular trends
To better attribute double burden estimates, papers were categorized by sex as “male only,” “female only,” or “both/non-specific.” We examined the relationships between age and data collection year with the obesity-undernutrition ratio by nonparametric smoothing curve using the LOWESS method13 and categorized age and data collection year at the points of slope change into three age groups (below 9, between 9–29, and above 30 years) and two data collection year groups (before 2000 and since 2000). These age and data collection year categories were subsequently used in regression models given their non-linear relationships with the obesity-undernutrition ratio.
Statistical analysis
First we examined the range of the prevalence and the obesity-undernutrition ratios by study subject characteristics (i.e., sex, age) and by type of countries (economic development level and geographic location).
In conducting a test for heterogeneity in our meta-regression analysis, we found strong evidence of heterogeneity in the obesity-undernutrition ratio between studies (using the “meta” command in STATA, Q = 39551.5, df = 70, p < 0.001; I2 = 99.8%). So meta-regression models with random effects were conducted for analyzing the associations between the obesity-undernutrition ratio and the study characteristics of interest. This approach provided restricted maximum likelihood estimates of regression parameters, and random effects allowed for the potential variations in the associations between the obesity-undernutrition ratio and the study characteristics across the multiple studies. To give more weight to study estimates with better precision (e.g., smaller standard errors, etc.), sample size was considered relative to the weighting factor and used for meta-regression models with random effects using the “metareg” command in STATA.
The meta-regression model with random effects was used to determine which factors explained the heterogeneity. We included variables that had been suggested in the literature to be contributing factors to the heterogeneity of the double burden of diseases, such as age,14 country income level,3 and secular trends.15, 16 Sex, study setting, and different types of undernutrition and obesity measures did not have significant associations with the obesity-undernutrition ratio.
The differences in pooled mean estimates of obesity-undernutrition ratios by country income level were presented in separate forest plots. All analysis was performed using Stata Release 14 (College Station, TX). Statistical significance was set at P < 0.05.
RESULTS
Characteristics of studies
The 29 studies included were from 18 countries worldwide, which differed greatly. Ten studies were conducted in Asia, seven in South America, four in Africa, three studies each in the Middle East and Central America, and one each from North America and Eastern Europe. Most studies were conducted in middle-income countries (n = 25, 86%), one in a low-income country (Gambia17), and three in high-income countries (Chile,15, 16 and the USA18).
Table 1 summarizes the main characteristics and findings of the 29 studies, ordered by target age group, world region (Asia, Middle East, Africa, Europe, and South/ Central/ North America), country, and publication year. Study subjects’ age and sex composition varied. Results were reported in 14 studies for children, in nine studies for adults, and six studies for both children and adults. Seven studies included female adults aged 15 and above, accounting for the child-bearing period.
Table 1.
Main characteristics of the 29 studies included in this study regarding double burden of disease*
| Reference | Country/ world region |
Study setting | Overall study subjects |
Prevalence of undernutrition (%) |
Prevalence of obesity (%) |
Classification of weight status† |
Obesity/ undernutrition ratio‡ |
|---|---|---|---|---|---|---|---|
| 1) Among children (14 studies) | |||||||
| Zhang et al., 201631 | China | China Health and Nutrition Survey 1991– 2009 | N= 11,630 M, F; < 18 y Rural area | UW: 7.8 Stunted: 32.2 | OWB: 14.6 | Chinese growth reference; Stunt (HAZ < −2); UW (WAZ < −2); OW/OB (BMIZ > 1) | 1.9 |
| Zhang et al., 201534 | China | National Surveys on Chinese Students' Constitution and Health, Shandong 2010 | N= 42,348 M, F; 7–18 y | UW (M, F) 9.8, 15.1 | OW (M, F) 16.3, 10.7 OB (M, F) 6.4, 2.1 | IOTF | M: 2.3, F: 0.8 |
| Sandjaja et al., 201335 | Indonesia | South East Asian Nutrition Survey 2011 | N= 7,211 M, F; 1–12 y | Stunting, UW rural: 39.2, 28.9 urban: 25.1, 19.2 | OW, OB rural: 3.2, 1.8 urban: 5.6, 5.1 | WHO 2006 & 2007, stunting (HAZ < −2) | Rural: 0.2 Urban: 0.2 |
| Kelishadi et al., 201530 | Iran | Childhood and Adolescence Surveillance & Prevention of Adult NCD study (CASPIAN-IV) 2011–2012 | N= 23,043 M, F; 6–18 y | UW: 9.8 | OW: 13.0 OB: 6.6 Abdominal OB: 17.6 | UW (< 5th); OW (85–95th); OB (> 95th %tile) in WHO standard curves | 2.0 |
| Rahmanian et al., 201453 | Iran | CASPIAN-III 2009–2010 | N= 5,088 M,F; 10–18 y | UW M: 20.0, F: 15.0 | OW, OB M: 6.5, 10.0 F: 8.5, 8.1 | WHO | M: 0.8 F: 1.1 |
| Motlagh et al., 201133 | Iran | Mandatory National Screening before Elementary School 2008 | N= 862,433 M, F; 6 y | UW: 19.1 Short stature: 6.5 | OW: 12.8, OB: 3.4 | CDC, short stature (HAZ < −2) | 0.8 |
| Adel el et al., 200854 | Libya | National Representative Survey 1995 | N= 5,348 M, F; < 5 y | UW: 4.3 Wasted: 3.7 Stunted: 20.7 | OWB: 16.2 | WHO 2006, UW (WAZ < −2), stunting (HAZ < −2), wasting (WHZ < −2) | 3.8 |
| Armstrong et al., 201655 | South Africa | Discovery Vitality Health of the Nation Study 2001–2004 | N=10,285M, F; 6–13 y | Stunted: 8.1 Wasted: 5.4 UW: 5.8 | OWB: 15.4 | WHO 2007 & IOTF | 2.7 |
| Hyska et al., 201437 | Albania | Nationwide Cross-Sectional Study 2013 | N= 5,810 M,F; 7–9.9 y | UW M: 1.5, F: 1.9 | OW, OB M: 14.5, 9.8 F: 13.3, 5.5 | IOTF | M: 16.2 F: 9.9 |
| Stanojevic et al., 200716 | Chile | Junta Nacional de Jardines Infantiles Preschool Program 1996– 2004 | N= 19,082 – 29,217 M, F; 3–4 y | Stunting 1996: 2.2 2000: 1.8 2004: 1.7 | OW, OB 1996: 22.7, 8.4 2000: 23.6, 9.3 2004: 22.7, 9.0 | WHO 1977 reference, stunting (HAZ < −2), overweight (1 < WHZ ≤ 2), obesity (WHZ > 2) | 1996: 14.1 2000: 18.3 2004: 18.6 |
| Kain et al., 200515 | Chile | National Annual School Survey 1987–2002 | N= 154,000 – 180,000 M, F; 6 y | UW (M, F) 1987: 4.2, 3.9 1990: 3.5, 3.1 1996: 3.1, 2.9 2002: 3.3, 3.0 | OB (M, F) 1987: 5.5, 4.8 1990: 7.8, 6.7 1996: 11.4, 10.6 2002: 14.5, 13.2 | CDC | OB: UW 1987: 1.3 1990: 2.2 1996: 3.7 2002: 4.4 |
| Kroker-Lobos et al., 201425 | Mexico | National Health and Nutrition Surveys 2006, 2012 | N= 78,581 M, F; All age | Stunting in 2006, 2012: < 5 y: 15.5, 13.6 5–11 y: 10.1, 6.9 | OW in 2006, 2012 < 5 y: 8.3, 9.0 OWB in 2006, 2012 5–11 y: 34.8, 34.4 | For child: WHO 2006 & 2007 For adult: WHO | < 5 y 2006: 0.5 2012: 0.7 5–11 y 2006: 3.4 2012: 5.0 |
| Flores et al., 200938 | Mexico | Mexican National Health & Nutrition Survey 2006 | N= 8,716 M, F; 5–11 y | Stunting: 9.9 | OW: 16.2, OB: 9.0 | IOTF, stunting (HAZ < −2) | OWB: stunt = 2.5 |
| Iriart et al., 201318 | USA | National Health and Nutrition Examination Survey 2003–2010 | N= 14,710 M, F; 2–19 y | Stunting M: 3.5, F: 3.5 | OWB M: 32.8, F: 32.0 | CDC | M: 9.4 F: 9.1 |
| 2) Among adults (9 studies) | |||||||
| Kamal et al., 201532 | Bangla-desh | Bangladesh Demographic and Health Survey 2011 | N=16,273 F; 15–49 y | UW: 24.2 | OW: 26.3 OB: 2.9 | UW (BMI < 18.5), OW (23–29.9), OB(≥ 30 kg/m2) | 1.2 |
| Shafique et al., 200722 | Bangla-desh | Nutritional Surveillance Project 2000–2004 | N= 282,182 F; 15–45 y | UW rural:38.8, urban: 29.7 | OW, OB Rural: 3.7, 0.4 Urban: 8.0, 1.1 | WHO | Rural:0.1 Urban: 0.3 |
| Xu et al., 201556 | China | China’s National Health Service Survey, Shaanxi 2013 | N= 37,902 M, F; ≥ 18 y | UW: M- 7.2, F- 10.9 | OW: M- 15.0, F- 13.3 OB: M- 1.0, F- 1.1 | WHO | M: 2.2 F: 1.3 |
| Sengupta et al., 201526 | India | National Family Health Survey 1998–99 & 2005–06 | N=89,199 (NFHS2), 93,724 (NFHS3) F; 15–49 y | UW: NFHS2- 35.8 NFHS3- 32.3 | OW; OB NFHS2- 13.7; 4.8 NFHS3- 17.0; 7.1 | WHO’s Asian specific BMI cut-off | NFHS2: 0.5 NFHS3: 0.7 |
| Gaur et al., 201321 | India | National Family Health Survey of India 2005–06 | N= 19,488 F; 15–49 y | UW (Slum, Non-slum) 15–24 y: 39.1, 32.5 25–34 y: 18.1, 15.6 35–44 y: 11.7, 7.0 45–49 y: 9.4, 4.5 | OWB (Slum, Non-slum) 15–24 y: 7.5, 11.0 25–34 y: 27.0, 31.9 35–44 y: 38.6, 47.5 45–49 y: 49.4, 52.9 | WHO | Non-slum 15–24y: 0.3 25–34y: 2.0 35–44y: 6.8 45–49y: 11.8 |
| Subramanian et al., 200736 | India | Indian National Family Health Survey 1998–99 | N= 77,220 F; 15–49 y | 32.1 | OW: 9.6 OB: 2.7 | WHO | 0.4 |
| Van der Sande et al., 200117 | Gambia | Community-based Survey 1996-1997 | N= 5373 M, F; ≥15 y | UW: 17.9 | OB: 4.0 | WHO | OB: UW 0.2 |
| Kandala et al., 201657 | Nigeria | Nigerian Demographic and Health Survey 2008 | N= 27,967 F; 15–49 y | UW: 12.0 | OW: 15.7 OB: 5.2 OWB: 20.9 | WHO | 1.7 |
| Monteiro et al., 200427 | Brazil | Population-based Household Surveys 1975, 1989, 1997 | N= 52,565 F; > 20 y | UW 1975: 12.7 1989: 6.6 1997: 6.0 | OB 1975: 7.4 1989: 12.3 1997: 12.7 | WHO | OB: UW 1975: 0.6 1989: 1.9 1997: 2.1 |
| 3) Mixed (children and adults, 6 studies) | |||||||
| Vaezghasemi et al., 201429 | Indonesia | Indonesian Family Life Survey 2007 | N= 42,755 M, F; All age | UW Rural: 20.0, Urban: 18.0 | OWB Rural: 17.0, Urban: 25.0 | WHO | Rural: 0.9 Urban: 1.4 |
| Conde et al., 201423 | Brazil | Brazilian National Surveys 1974–2009 | N=188,488 M, F; All age | UW (M, F) in adult (≥ 20y) 1974: 7.7, 11.6 2009: 1.8, 3.6 | OWB (M, F) in adult (≥ 20y) 1974: 17.9, 26.9 2009: 50.1, 48.0 | WHO | M, F 1974: 2.3, 2.3 2009: 27.8, 13.3 |
| Sarmiento et al., 201424 | Colombia | Colombian Demographic/ Health Survey & National Nutritional Survey | N= 160,696 M, F; All age | < 5 y stunting: 13.2 5–11 y: NR 18–64 y UW: 2.8 | < 5 y OB: 5.2 5–11 y OB: 4.6 18-64 y OWB: 51.2 | For child: WHO 2006 & 2007, stunting (HAZ < −2) For adult: WHO | 18–64 y: 18.3 |
| Freire et al., 201419 | Ecuador | Ecuadorian National Health and Nutrition Survey 2012 | N= 39,337 women (12–49 y) and child (0–11 y) | UW, Anemia < 5y: 25.3, NR 5–11 y: NR, 3.5 12–49 y: NR, 15.4 | OW, OB < 5 y (OWB): 8.6 5–11 y: 19.5, 10.9 12–49 y: 34.2, 20.5 | For child: WHO 2006 & 2007 For adult: WHO | < 5 y: 0.3 |
| Loret de Mola et al., 201428 | Peru | Peruvian National Demographic & Family Health Surveys 1996–2011 | N= 165,118 women (20–49 y) and child (< 5 y) | UW (2000, 2007, 2011) < 5 y: 5.3, 5.0, 4.1 20–49 y: NA | OW (2000, 2007, 2011) < 5 y: 14.8, 11.2, 9.7 20–49 y: 37.4, 37.4, 38.9 | WHO | OW: UW in < 5 y 2000: 2.8 2007: 2.2 2011: 2.4 |
| Ramirez-Zea et al., 201420 | Guatemala | National Maternal and Child Health Surveys 2008 | N= 25,297 women (15–49 y) and child (0–5 y) | UW, stunting or short stature < 5 y: 1.0, 48.0 15–49 y: 2.6, 28.3 | OW, OB < 5 y: 3.9, 1.1 15–49 y: 32.2, 17.2 | For child: WHO 2006 & Stunting/ short stature (HAZ < −2 or height < 145 cm); For adult: UW (BMI < 18.5), OW (25–29.9), OB(≥ 30 kg/m2) | < 5 y: 5.0 15–49 y: 19.0 |
BMIZ: body-mass-index Z-scores, CDC: Center for Disease Control, F: female, HAZ: height-for-age Z-scores, IOTF: International Obesity Task Force, M: male, m: month, NR: not reported, OB: obese, OW: overweight, OWB: overweight or obese, R: rural, U: urban, UW: underweight, WAZ: weight-for-age Z-scores, WHO: World Health Organization, WHZ: weight-for-height Z-scores, y: year
Contents were ordered by study subject’s age (child, adult, and mixed) and world region (Asia, Middle East, Africa, Europe, and South, Central, and North America).
Classification of overweight and obesity: CDC: underweight (BMI < 5th percentile), overweight (≥ 85th and < 95th percentile), obesity (≥ 95th percentile) in CDC Growth Charts; IOTF: corresponding to BMI classification of underweight (BMI < 18 kg/m2), overweight (25–29.9 kg/m2) and obesity (≥ 30 kg/m2) at age 18 in the IOTF sex-age-specific BMI cut-offs; WHO: underweight (BMI < 18 kg/m2), overweight (25–29.9 kg/m2), obesity (≥ 30 kg/m2); WHO’s Asian specific BMI cut-off: underweight (BMI < 18.5 kg/m2), overweight (23–27.4 kg/m2), obesity (≥ 27.5kg/m2); WHO 2006 for child: underweight (BMIZ < −2SD), overweight (> 2SD and ≤ 3SD), obesity (> 3SD) in children aged < 5 y by WHO Child Growth Standards; WHO 2007 for child: underweight (BMIZ < −2SD), overweight (1SD < BMI Z ≤ 2SD), obesity (> 2SD) in children aged 5–19 y by WHO Reference 2007.
Obesity/ undernutrition ratio was calculated as the prevalence of overweight/obesity vs. underweight; otherwise we indicated used measures in the table.
All studies were based on a cross-sectional design (27 studies used national-level survey data with health, nutrition, or education program perspectives). Data in studies were collected from 1974 to 2012. No study examined the double burden within individuals across the life-course. Six studies provided series of data by multiple years.
Findings of the double burden
The prevalence of obesity varied from 4.0% (for obesity17) to 54.7% (overweight and obesity19), while undernutrition (underweight) varied from 1.0%20 to 39.1%.21 The obesity-undernutrition ratio ranged from 0.122 to 27.8.23 A few papers compared the undernutrition and obesity status between age groups, male vs. female, urban vs. rural, slum vs. non-slum, and over time as well. Young children were more likely to be at risk of undernutrition, while adults had higher obesity prevalence (e.g., 13.2% of children aged < 5 were stunted in their growth, while 51.2% of adults aged 18–64 were overweight or obese24). Steep increases of the obesity-undernutrition ratio were shown across age groups20, 21, 25 (e.g., 5.0 in children aged < 5 vs. 19.0 in adults aged 15–4920) and years of data collection15, 16, 23, 25–27 (e.g., 0.6 in 1975 vs. 2.1 in 199727). However, one study had similar obesity-undernutrition ratios by years among children younger than 5 years old.28 Obesity was more prevalent in urban and high socio-economic status (SES) populations, while undernutrition was more prevalent in rural and low-SES populations. The obesity-undernutrition ratio was higher in urban22, 29 (e.g., urban 1.4 vs. rural 0.9) and non-slum areas than the ratio in rural and slum areas.21
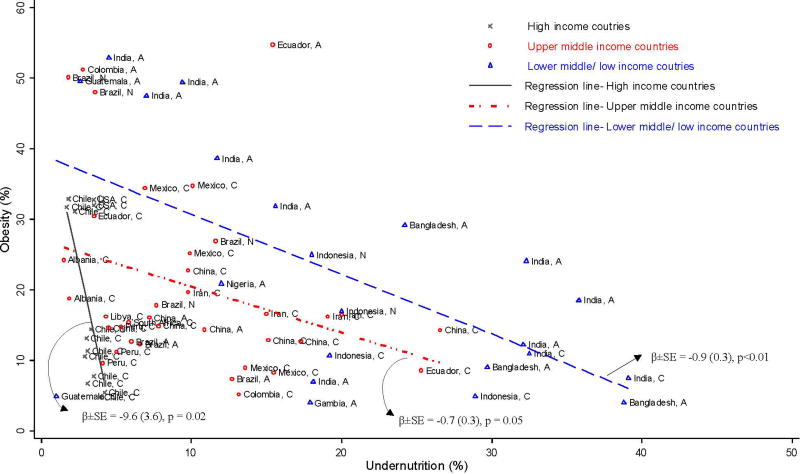
Figure 1 shows three major groups in the association between the prevalence of undernutrition and obesity by country income level. First, studies from lower-middle- and low-income countries had higher undernutrition, and data points were grouped in the right side of the graph compared to those from upper-middle- and high-income countries. Although data from lower-middle and low-income countries were scattered widely, the majority of adult data tended to have higher obesity but lower undernutrition compared to children.
Figure 1.
Double burden of undernutrition and obesity in 18 countries by country economic development level: coexistence of undernutrition and obesity
Second, data from high-income countries had lower undernutrition than others, located on the left side of the graph, but data points varied by obesity level, which was distinguished by year of data collection (1987 to 2006). Studies with recent data had a higher prevalence of obesity compared to others.
Finally, most data from upper-middle-income countries were between high- and lower-middle- and low-income countries. The majority of adult data had lower undernutrition and higher obesity and was thus positioned to the left side of the graph compared to children.
Pooled estimates of obesity-undernutrition ratio and its associated factors in meta-analysis
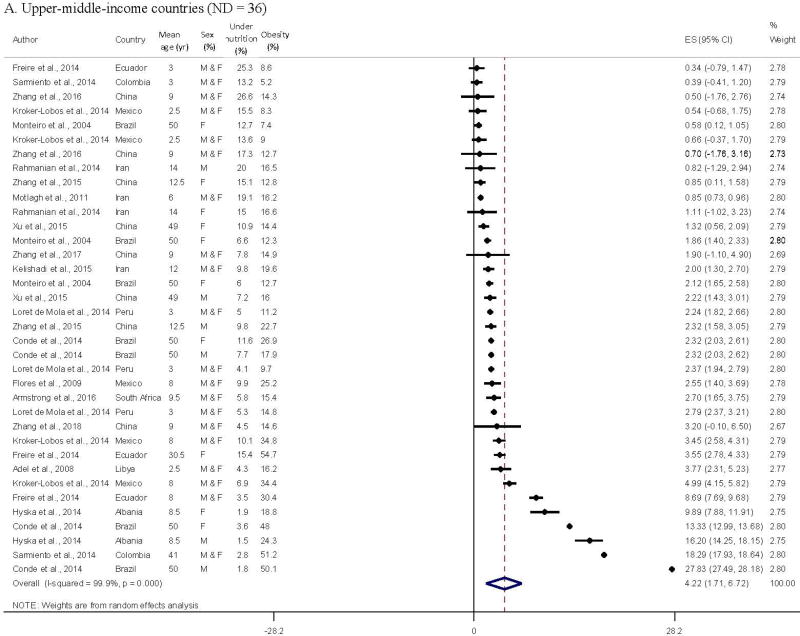
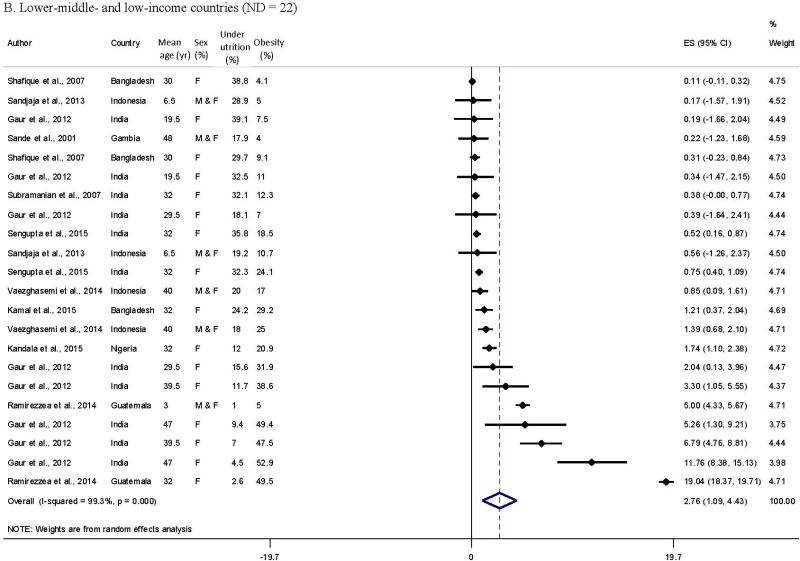
The pooled obesity-undernutrition ratio was 4.3 (95% CI = 2.9–5.7), with significant heterogeneity between studies from the meta-analysis of 71 data points from 29 studies. The forest plots by country income level in Figure 2 indicated general patterns in the obesity-undernutrition ratio by age group (adult/child), country, and over time. All high-income countries had a much higher prevalence of obesity than of undernutrition (the ratio > 1 [Appendix 2]; pooled ratio = 7.1 (95% CI = 4.8–9.4); obesity-undernutrition correlation coefficient r = −0.61, p = 0.02); 55.0% of the data from lower-middle- and low-income countries had a lower prevalence of obesity than undernutrition (the ratio < 1; r = −0.61, p = 0.002); while the ratios of upper-middle-income countries were between the two groups (27.8% of data had the ratio < 1; r = −0.32, p = 0.05).
Figure 2.
Comparing meta-analysis results of reported obesity-undernutrition ratio in middle- and low-income countries
A. Upper-middle-income countries (ND = 36)
B. Lower-middle- and low-income countries (ND = 22)
The obesity-undernutrition ratios among children, in Central/South America, and using recent data were higher than those among with adults, in Asia and the Middle East, and using older data in upper-middle-income countries (number of data points = 36 out of 16 studies). The obesity-undernutrition ratio varied from 0.319 to 27.8,23 and their pooled ratio was 4.2 (95% CI = 1.7–6.7) in upper-middle-income countries.
Most of the obesity-undernutrition ratio in lower-middle- and low-income countries (22 data points taken from 10 studies) were from Asian countries. Their ratio was generally ordered by subject age. Children had a lower ratio than adults. The obesity-undernutrition ratio varied from 0.122 to 19.0,20 and their pooled ratio was 2.8 (95% CI = 1.1–4.4) in lower-middle- and low-income countries.
In the meta-regression model, the obesity-undernutrition ratio was significantly associated with country income level, subject age, and over time (Table 2). The average obesity-undernutrition ratio was significantly higher in the group aged 30 years and above compared to those aged 8 years and below (β = 7.1, SE = 2.2, p < 0.01). Also, the ratio increased by country income level: low- and lower-middle-income countries had a 10.8 (SE = 2.6, p < 0.001) lower ratio and upper-middle-income countries had a 7.7 (SE = 2.2, p < 0.001) lower ratio than high-income countries. The ratio using data collected since 2000 was 5.2 (SE = 1.5, p < 0.001) higher than that in previous years.
Table 2.
Results of meta-regression analysis: Economic and socio-demographic factors associated with the obesity-undernutrition ratios based on reported prevalence in 29 studies*
| Predictors | Beta | Standard Error | p value |
|---|---|---|---|
| Sex | |||
| Male (ref, ND = 11) | |||
| Female (ND = 31) | −1.77 | 1.96 | 0.37 |
| Male and Female (ND = 29) | 1.24 | 2.15 | 0.56 |
| Age group | |||
| ≤ 8 years old (ref, ND = 27) | |||
| 9–29 years old (ND = 18) | 1.97 | 1.89 | 0.30 |
| ≥ 30 years old (ND = 26) | 7.09 | 2.15 | 0.002 |
| Country income level | |||
| High (ref, ND = 13) | |||
| Upper-middle (ND = 36) | −7.67 | 2.18 | < 0.001 |
| Lower-middle and low (ND = 22) | −10.78 | 2.59 | < 0.001 |
| Time trend | |||
| Data collection before 2000 (ref, ND = 18) | |||
| Data collection since 2000 (ND = 53) | 5.24 | 1.53 | < 0.001 |
ND: number of data points.
71 data points from 29 studies were analyzed using a Stata “Metareg” command with dummy variables of age group, sex, country income level, and secular trend.
Age, country income, and secular trend were categorized at the points of slope change into two/three groups. These categories were subsequently used in regression models given their non-linear relationship of age with the obesity-undernutrition ratio.
Factors associated with double burden of diseases worldwide
We gained insight into the driving factors behind the double burden of diseases from 29 studies. These studies discussed possible causes from three perspectives: lifestyle changes in diets and physical activity, urbanization and economic development, and policy in individual countries. In regard to lifestyle, researchers attributed the double burden to more sedentary jobs and activities with computers and TV,29, 30 a decreased amount of physical activity due to more automobile use and less walking,19, 22, 28, 31–33 as well as diet shift including less fruits/vegetables intake19 but increased carbohydrate/high-caloric-beverage consumption25, 28, 32 than before. As the effects of urbanization and economic development, researchers noted the roots of the double burden lying in increased purchasing power along with more capital-intensive agriculture28, 29, 31, 34 and cheap, energy-dense food production,22, 26 alongside increased food prices and food insecurity among the low-SES population due to income inequality through urbanization.17, 35, 36 Studies suggested that educating people for better health awareness,21 putting more focus on preventing obesity and related chronic disease than on undernutrition,20, 25, 33, 37 and developing appropriate healthcare structures in particular for low-SES populations through fortification, policy, and government programs19, 33, 38 would be beneficial to reduce the double burden of diseases.
DISCUSSION
Along with the spread of the obesity epidemic worldwide, in 2014 the WHO declared that the double burden of diseases was an important public health issue. The double burden is characterized by the coexistence of both undernutrition and obesity or diet-related non-communicable chronic diseases. NCDs are responsible of two-thirds of the 57 million global deaths annually, and about 80% of these are in low- and middle-income countries.2 Several studies have examined the double burden of diseases in low-and middle-income countries by using data from cross-sectional surveys or using country-level aggregating statistics since the early 2000s.39–41 However, the reported prevalence of undernutrition and obesity varied considerably in these studies.
Our systematic review summarized the global double-burden of malnutrition in typical patterns using an obesity-undernutrition ratio. All high-income countries had a higher obesity prevalence than undernutrition; however, 55.0% of the data from lower-middle- and low-income countries had a higher undernutrition prevalence than obesity; and upper-middle-income countries were between the two groups. The pooled obesity-undernutrition ratio was 4.3 (95% CI = 2.9–5.7) in the 29 studies from 18 countries, and the ratio varied by country income level, age, and over time. The obesity-undernutrition ratio was significantly lower in middle-income countries vs. high-income countries. In contrast, a higher obesity-undernutrition ratio was found among 30-year-olds and older than among 8-year-olds and younger, as well as in studies using data collected since 2000 as compared to that before 2000 (p < 0.01).
According to a recent systematic analysis, the global overweight/obesity rate in 2013 was estimated as 37.5% in adults and 13.2% in children.42 Overweight/obesity rose by 27.5% for adults and 47.1% for children between 1980 and 2013, and obesity is still more prevalent in developed countries than in developing countries (e.g., obesity rate among adults in 2012 or the nearest year: the United States—35.3%, Australia—28.3%, Canada—25.4%, the United Kingdom—24.7%43), although the age-standardized obesity prevalence in both regions has been increasing.
On the other hand, low- and middle-income countries are experiencing the industrialization and urbanization that drive the epidemic of NCDs, as well as facing the unfinished agenda of combatting infectious diseases. Malnutrition and infection in early life may increase the risk of NCDs in later life.3 For example, one review paper reported that 11 mega-countries (> 100 million inhabitants), which were mostly low- or middle-income countries, were facing rapid epidemiologic, nutritional, and physical activity transitions because of changes in food systems and unhealthy lifestyles along with urbanization. As an example of low-income countries, Ethiopia and Nepal had an increased prevalence of overweight and a reduced prevalence of underweight between the 1990s–2000s. In particular, the annualized change in prevalence of overweight among women aged 19–49 was higher in urban than rural areas (Ethiopia: 0.36 vs. 0.18, Nepal: 1.33 vs. 0.87)44 based on data collected from nationally representative samples, although the change in underweight was the same or smaller in urban vs. rural areas. Regional differences within a low-income country are also important to consider in the global double burden of diseases.
There are several possible interpretations of the significant heterogeneity in the magnitude of the obesity-undernutrition ratio reported in this study based on subject age, country income level, and over time. First, the higher obesity-undernutrition ratio among adults (≥ 30 years old) vs. in younger groups, in more recent data (collected ≥ 2000) vs. previous studies, and in higher-income vs. lower-income countries may be a consequence not just of economic developmental factors but also of the cumulative impact of longer exposure to an obesogenic environment, which encourages people to eat unhealthily and not to exercise enough. Such prolonged obesogenic exposure may create a more pronounced risk for obesity in adults,45, 46 thus increasing the ratio.
In addition, we found that North, South, and Central American countries reported higher obesity-undernutrition ratios than other regions. The prevalence of overweight/obesity was highest in the Americas (62% for overweight in both sexes, and 26% for obesity) and was the lowest in South East Asia (14% overweight in both sexes, and 3% for obesity).47 Increased urbanization may lead to the integration of a region into international markets and reduce the consumption of traditional dishes based on cereals, legumes, fresh fruits and vegetables, while increasing the consumption of ultra-processed products with high amounts of sugars, salt, and fats—a “westernized diet.”48, 49 Over the past decade, fast-growing economies buoyed by a commodities boom, including countries such as Mexico, Colombia, and Brazil, have seen a rising middle class that has consumed more processed food and has had an increasingly sedentary lifestyle.50, 51
The well-documented lifestyle changes regarding food consumption and physical activity, as well as urbanization, economic development, and health policy in many countries have contributed to the double burden. This is relevant to the recent conclusions of a WHO International Agency for Research on Cancer (IARC) Expert Panel. They examined and reported on the evidence regarding energy balance and obesity, with a focus on low- and middle-income countries.52 Poor-quality diet and unbalanced energy intake could cause the coexistence of undernutrition and over-nutrition. Educational interventions and policies to improve health awareness and limit consumption of sugar-sweetened beverages and high-calorie-dense foods are desired to prevent a higher double burden in the future.
Beside its strengths, this study has some limitations. First, most identified studies were conducted in middle-income countries, and very few studies were from high-income countries, so the latter study samples might not be representative. This is due to the fact that undernutrition is not a major concern in high-income countries. Second, we could not investigate the effects of population SES on the obesity-undernutrition ratio since the studies either did not include SES measures or they used different SES measures (e.g., education level, residence area, etc.). Third, many studies are cross-sectional studies. Future research should use longitudinal data to examine changes over time in diverse groups regarding the double burden of diseases and its associated factors. Defining the relationship between health policies and disease prevention programs in promoting healthy lifestyles would help fight the double burden of diseases, especially among the countries undergoing rapid social transitions.
CONCLUSION
Many of the studies we identified have reported the coexistence of undernutrition and obesity in middle-income countries, but the obesity-undernutrition ratios have differed considerably by country economic development levels, age groups, and over time. Higher obesity-undernutrition ratios were found in adults, in high-income countries, and in studies using more recent data. Further research is needed to better understand the contributions of various factors to the double disease burden, especially since limited research has been conducted using longitudinal data and in low-income countries. National policies and intervention programs are needed to address the challenges, especially in those countries facing rapid social economic transitions.
Supplementary Material
Table 3.
Suggested factors contributing to double burden of disease worldwide*
| Categories | Examples and related studies | Countries | Number of studies |
|---|---|---|---|
| Lifestyle changes in diets and physical activity |
|
China, Bangladesh, Ecuador, Indonesia, Iran, Mexico, Peru | 9 |
| Urbanization/Economic development |
|
Bangladesh, Brazil, China Gambia, India, Indonesia, Peru, Vietnam | 11 |
| Policy |
|
Albania, Ecuador Guatemala, Iran, Mexico | 6 |
| Other |
|
India | 1 |
Results were based on the 29 studies we reviewed.
Acknowledgments
The study was supported in part by research grants from the Chinese Medical Board (CMB 16-262), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH, U54HD070725). We would like to thank Dr. Brenda Denzler for her assistance in improving the manuscript and thank Dr. Hong Xue for his comments to help improve the study.
Footnotes
Conflicts of interest:
No conflicts of interest was declared.
Authors’ contributions
YW conceived the research idea, directed the study, secured research funding, provided administrative support, interpreted the analysis results, and wrote the manuscript. JM was responsible for the study design, literature search, data collection and analysis, and drafting the manuscript. LS contributed to the literature search and data collection as well as in drafting the manuscript. YZ critically revised the manuscript. All authors contributed to study design, data analysis, and writing of the manuscript. YW and JM had full access to the data used in the study and had final responsibility for the decision to submit for publication.
References
- 1.World Health Organization (WHO) Double burden of malnutrition. [Accessed Jan 29, 2017]; Available: http://www.who.int/nutrition/double-burden-malnutrition/en/
- 2.Barquera S, Pedroza-Tobias A, Medina C. Cardiovascular diseases in mega-countries: the challenges of the nutrition, physical activity and epidemiologic transitions, and the double burden of disease. Curr Opin Lipidol. 2016;27(4):329–344. doi: 10.1097/MOL.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bygbjerg IC. Double burden of noncommunicable and infectious diseases in developing countries. Science. 2012;337(6101):1499–1501. doi: 10.1126/science.1223466. [DOI] [PubMed] [Google Scholar]
- 4.Piernas C, Wang D, Du S, Zhang B, Wang Z, Su C, et al. The double burden of under- and overnutrition and nutrient adequacy among Chinese preschool and school-aged children in 2009–2011. Eur J Clin Nutr. 2015;69(12):1323–1329. doi: 10.1038/ejcn.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doak CM, Adair LS, Monteiro C, Popkin BM. Overweight and underweight coexist within households in Brazil, China and Russia. J Nutr. 2000;130(12):2965–2971. doi: 10.1093/jn/130.12.2965. [DOI] [PubMed] [Google Scholar]
- 6.Oddo VM, Rah JH, Semba RD, Sun K, Akhter N, Sari M, et al. Predictors of maternal and child double burden of malnutrition in rural Indonesia and Bangladesh. Am J Clin Nutr. 2012;95(4):951–958. doi: 10.3945/ajcn.111.026070. [DOI] [PubMed] [Google Scholar]
- 7.Kimani-Murage EW. Exploring the paradox: double burden of malnutrition in rural South Africa. Glob Health Action. 2013;6:19249. doi: 10.3402/gha.v6i0.19249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolcic I. Double burden of malnutrition: A silent driver of double burden of disease in low- and middle-income countries. J Glob Health. 2012;2(2):020303. doi: 10.7189/jogh.02.020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uauy R, Kain J, Corvalan C. How can the Developmental Origins of Health and Disease (DOHaD) hypothesis contribute to improving health in developing countries? Am J Clin Nutr. 2011;94(6 Suppl):1759S–1764S. doi: 10.3945/ajcn.110.000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dans A, Ng N, Varghese C, Tai ES, Firestone R, Bonita R. The rise of chronic non-communicable diseases in southeast Asia: time for action. Lancet. 2011;377(9766):680–689. doi: 10.1016/S0140-6736(10)61506-1. [DOI] [PubMed] [Google Scholar]
- 11.World Bank Country and lending Groups. Country classification. [Accessed Jan 29 2017]; Available: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 12.United nations Country Grouping. Wolrd Region. [Accessed Jan 29 2017]; Available: http://www.internetworldstats.com/list1.htm#EE.
- 13.Hamilton L. Statistics with Stata. Version 10. Cengage Learning Inc: Belmont. 2009:230. [Google Scholar]
- 14.Aitsi-Selmi A. Households with a stunted child and obese mother: trends and child feeding practices in a middle-income country, 1992–2008. Matern Child Health J. 2015;19(6):1284–1291. doi: 10.1007/s10995-014-1634-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kain J, Uauy R, Lera L, Taibo M, Albala C. Trends in height and BMI of 6-year-old children during the nutrition transition in Chile. Obes Res. 2005;13(12):2178–2186. doi: 10.1038/oby.2005.270. [DOI] [PubMed] [Google Scholar]
- 16.Stanojevic S, Kain J, Uauy R. The association between changes in height and obesity in Chilean preschool children: 1996–2004. Obesity (Silver Spring) 2007;15(4):1012–1022. doi: 10.1038/oby.2007.611. [DOI] [PubMed] [Google Scholar]
- 17.van der Sande MA, Ceesay SM, Milligan PJ, Nyan OA, Banya WA, Prentice A, et al. Obesity and undernutrition and cardiovascular risk factors in rural and urban Gambian communities. Am J Public Health. 2001;91(10):1641–1644. doi: 10.2105/ajph.91.10.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iriart C, Boursaw B, Rodrigues GP, Handal AJ. Obesity and malnutrition among Hispanic children in the United States: double burden on health inequities. Rev Panam Salud Publica. 2013;34(4):235–243. [PubMed] [Google Scholar]
- 19.Freire WB, Silva-Jaramillo KM, Ramirez-Luzuriaga MJ, Belmont P, Waters WF. The double burden of undernutrition and excess body weight in Ecuador. Am J Clin Nutr. 2014;100(6):1636s–1643s. doi: 10.3945/ajcn.114.083766. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez-Zea M, Kroker-Lobos MF, Close-Fernandez R, Kanter R. The double burden of malnutrition in indigenous and nonindigenous Guatemalan populations. Am J Clin Nutr. 2014;100(6):1644s–1651s. doi: 10.3945/ajcn.114.083857. [DOI] [PubMed] [Google Scholar]
- 21.Gaur K, Keshri K, Joe W. Does living in slums or non-slums influence women's nutritional status? Evidence from Indian mega-cities. Soc Sci Med. 2013;77:137–146. doi: 10.1016/j.socscimed.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Shafique S, Akhter N, Stallkamp G, de Pee S, Panagides D, Bloem MW. Trends of under- and overweight among rural and urban poor women indicate the double burden of malnutrition in Bangladesh. Int J Epidemiol. 2007;36(2):449–457. doi: 10.1093/ije/dyl306. [DOI] [PubMed] [Google Scholar]
- 23.Conde WL, Monteiro CA. Nutrition transition and double burden of undernutrition and excess of weight in Brazil. Am J Clin Nutr. 2014;100(6):1617s–1622s. doi: 10.3945/ajcn.114.084764. [DOI] [PubMed] [Google Scholar]
- 24.Sarmiento OL, Parra DC, Gonzalez SA, Gonzalez-Casanova I, Forero AY, Garcia J. The dual burden of malnutrition in Colombia. Am J Clin Nutr. 2014;100(6):1628s–1635s. doi: 10.3945/ajcn.114.083816. [DOI] [PubMed] [Google Scholar]
- 25.Kroker-Lobos MF, Pedroza-Tobias A, Pedraza LS, Rivera JA. The double burden of undernutrition and excess body weight in Mexico. Am J Clin Nutr. 2014;100(6):1652s–1658s. doi: 10.3945/ajcn.114.083832. [DOI] [PubMed] [Google Scholar]
- 26.Sengupta A, Angeli F, Syamala TS, Dagnelie PC, van Schayck CP. Overweight and obesity prevalence among Indian women by place of residence and socio-economic status: Contrasting patterns from 'underweight states' and 'overweight states' of India. Soc Sci Med. 2015;138:161–169. doi: 10.1016/j.socscimed.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Monteiro CA, Conde WL, Popkin BM. The burden of disease from undernutrition and overnutrition in countries undergoing rapid nutrition transition: a view from Brazil. Am J Public Health. 2004;94(3):433–434. doi: 10.2105/ajph.94.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loret de Mola C, Quispe R, Valle GA, Poterico JA. Nutritional transition in children under five years and women of reproductive age: a 15-years trend analysis in Peru. PLoS One. 2014;9(3):e92550. doi: 10.1371/journal.pone.0092550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaezghasemi M, Ohman A, Eriksson M, Hakimi M, Weinehall L, Kusnanto H, et al. The effect of gender and social capital on the dual burden of malnutrition: a multilevel study in Indonesia. PLoS One. 2014;9(8):e103849. doi: 10.1371/journal.pone.0103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelishadi R, Motlagh ME, Bahreynian M, Gharavi MJ, Kabir K, Ardalan G, et al. Methodology and Early Findings of the Assessment of Determinants of Weight Disorders among Iranian Children and Adolescents: The Childhood and Adolescence Surveillance and PreventIon of Adult Noncommunicable Disease-IV Study. Int J Prev Med. 2015;6:77. doi: 10.4103/2008-7802.162953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang N, Becares L, Chandola T. Patterns and Determinants of Double-Burden of Malnutrition among Rural Children: Evidence from China. PLoS One. 2016;11(7):e0158119. doi: 10.1371/journal.pone.0158119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamal SM, Hassan CH, Alam GM. Dual burden of underweight and overweight among women in Bangladesh: patterns, prevalence, and sociodemographic correlates. J Health Popul Nutr. 2015;33(1):92–105. [PMC free article] [PubMed] [Google Scholar]
- 33.Motlagh ME, Kelishadi R, Amirkhani MA, Ziaoddini H, Dashti M, Aminaee T, et al. Double burden of nutritional disorders in young Iranian children: findings of a nationwide screening survey. Public Health Nutr. 2011;14(4):605–610. doi: 10.1017/S1368980010002399. [DOI] [PubMed] [Google Scholar]
- 34.Zhang YX, Lin M, Sun GZ. The double burden of overweight and thinness among children and adolescents in Shandong China. Int J Cardiol. 2015;184:380–381. doi: 10.1016/j.ijcard.2015.02.076. [DOI] [PubMed] [Google Scholar]
- 35.Sandjaja S, Budiman B, Harahap H, Ernawati F, Soekatri M, Widodo Y, et al. Food consumption and nutritional and biochemical status of 0.5-12-year-old Indonesian children: the SEANUTS study. Br J Nutr. 2013;110(Suppl 3):S11–20. doi: 10.1017/S0007114513002109. [DOI] [PubMed] [Google Scholar]
- 36.Subramanian SV, Kawachi I, Smith GD. Income inequality and the double burden of under- and overnutrition in India. J Epidemiol Community Health. 2007;61(9):802–809. doi: 10.1136/jech.2006.053801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hyska J, Mersini E, Mone I, Burazeri G. Prevalence and demographic correlates of overweight and obesity among children in a transitional southeastern European population. J Community Health. 2014;39(5):828–834. doi: 10.1007/s10900-014-9888-9. [DOI] [PubMed] [Google Scholar]
- 38.Flores M, Macias N, Rivera M, Barquera S, Hernandez L, Garcia-Guerra A, et al. Energy and nutrient intake among Mexican school-aged children, Mexican National Health and Nutrition Survey 2006. Salud Publica Mex. 2009;51(Suppl 4):S540–550. doi: 10.1590/s0036-36342009001000009. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Chen HJ, Shaikh S, Mathur P. Is obesity becoming a public health problem in India? Examine the shift from under- to overnutrition problems over time. Obes Rev. 2009;10(4):456–474. doi: 10.1111/j.1467-789X.2009.00568.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Mi J, Shan XY, Wang QJ, Ge KY. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes (Lond) 2007;31(1):177–188. doi: 10.1038/sj.ijo.0803354. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Wang L, Qu W. New national data show alarming increase in obesity and noncommunicable chronic diseases in China. Eur J Clin Nutr. 2017;71(1):149–150. doi: 10.1038/ejcn.2016.171. [DOI] [PubMed] [Google Scholar]
- 42.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.OECD. Obesity Update- Obesity rate (%) among adults, 2012 or nearest year, June 2014. [cited 2017 Jul.3] Available from: http://www.oecd.org/health/Obesity-Update-2014.pdf.
- 44.Popkin BM, Slining MM. New dynamics in global obesity facing low- and middle-income countries. Obes Rev. 2013;14(Suppl 2):11–20. doi: 10.1111/obr.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G. Cumulative life course and adult socioeconomic status and markers of inflammation in adulthood. J Epidemiol Community Health. 2008;62(6):484–491. doi: 10.1136/jech.2006.054106. [DOI] [PubMed] [Google Scholar]
- 46.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8(4):253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Global Health Observatory (GHO) Data. Obesity: situation and trends. [Accessed Mar 13, 2017]; Available: http://www.who.int/gho/ncd/risk_factors/obesity_text/en/
- 48.Reuters HEALTH NEWS. Fri Feb 13, 2015. Obesity weighs on Latin America after success in fight against hunger. [Accessed Mar 13, 2017]; Available: http://www.reuters.com/article/us-latam-obesity-idUSKBN0LH13520150213.
- 49.Doak CM, Adair LS, Bentley M, Monteiro C, Popkin BM. The dual burden household and the nutrition transition paradox. Int J Obes (Lond) 2005;29(1):129–136. doi: 10.1038/sj.ijo.0802824. [DOI] [PubMed] [Google Scholar]
- 50.Kain J, Vio F, Albala C. Obesity trends and determinant factors in Latin America. Cad Saude Publica. 2003;19(Suppl 1):S77–86. doi: 10.1590/s0102-311x2003000700009. [DOI] [PubMed] [Google Scholar]
- 51.Jaacks LM, Slining MM, Popkin BM. Recent trends in the prevalence of under- and overweight among adolescent girls in low- and middle-income countries. Pediatr Obes. 2015;10(6):428–435. doi: 10.1111/ijpo.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romieu I, Dossus L, Barquera S, Blottiere HM, Franks PW, Gunter M, et al. Energy balance and obesity: what are the main drivers? Cancer Causes Control. 2017;28(3):247–258. doi: 10.1007/s10552-017-0869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rahmanian M, Kelishadi R, Qorbani M, Motlagh ME, Shafiee G, Aminaee T, et al. Dual burden of body weight among Iranian children and adolescents in 2003 and 2010: the CASPIAN-III study. Arch Med Sci. 2014;10(1):96–103. doi: 10.5114/aoms.2014.40735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adel el T, Marie-Francoise RC, Mahmud Salaheddin M, Najeeb E, Ahmed AM, Ibrahim B, et al. Nutritional status of under-five children in libya; a national population-based survey. Libyan J Med. 2008;3(1):13–19. doi: 10.4176/071006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Armstrong ME, Lambert MI, Lambert EV. Relationships between different nutritional anthropometric statuses and health-related fitness of South African primary school children. Ann Hum Biol. 2016:1–6. doi: 10.1080/03014460.2016.1224386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu Y, Zhou Z, Li Y, Yang J, Guo X, Gao J, et al. Exploring the nonlinear relationship between body mass index and health-related quality of life among adults: a cross-sectional study in Shaanxi Province, China. Health Qual Life Outcomes. 2015;13:153. doi: 10.1186/s12955-015-0347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kandala NB, Emina JB. The dual burden of nutrition transition among women in sub-Saharan Africa: A case study of underweight in Nigeria. J Biosoc Sci. 2016;48(4):486–501. doi: 10.1017/S0021932015000334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.