Abstract
This review summarizes current advances in dental pulp stem cells (DPSCs) and their potential applications in the nervous diseases. Injured adult mammalian nervous system has a limited regenerative capacity due to an insufficient pool of precursor cells in both central and peripheral nervous systems. Nerve growth is also constrained by inhibitory factors (associated with central myelin) and barrier tissues (glial scarring). Stem cells, possessing the capacity of self-renewal and multicellular differentiation, promise new therapeutic strategies for overcoming these impediments to neural regeneration. Dental pulp stem cells (DPSCs) derive from a cranial neural crest lineage, retain a remarkable potential for neuronal differentiation, and additionally express multiple factors that are suitable for neuronal and axonal regeneration. DPSCs can also express immunomodulatory factors that stimulate formation of blood vessels and enhance regeneration and repair of injured nerve. These unique properties together with their ready accessibility make DPSCs an attractive cell source for tissue engineering in injured and diseased nervous systems. In this review, we interrogate the neuronal differentiation potential as well as the neuroprotective, neurotrophic, angiogenic, and immunomodulatory properties of DPSCs and its application in the injured nervous system. Taken together, DPSCs are an ideal stem cell resource for therapeutic approaches to neural repair and regeneration in nerve diseases.
1. Introduction
Traumatic events, iatrogenic injuries, and neurodegenerative diseases can lead to axonal degeneration, inflammation, neuron death, and cytoarchitectural malformation in both the peripheral nervous system (PNS) and central nervous system (CNS) [1–6]. Conventional medical therapies have limited efficacy in supporting functional recovery from nervous damage since the mature nervous system lacks the necessary precursor cells to generate new neurons and glial cells [7]. Recently, stem cell-based strategies in combination with novel technologies (e.g., precisely controlled hydrogels) have heralded potential new therapeutic approaches for addressing nerve regeneration and repair [8–11].
Mesenchymal stem cells (MSCs) harvested from adult tissues are potentially an important therapeutic cell source for treatment of CNS and PNS perturbations since they possess the capacity for both neuronal and glial differentiation. MSCs also express numerous anti-inflammatory and neurotrophic factors supporting nerve repair [8–14]. These multipotent stem cells are present in bone marrow [15, 16], adipose tissue [17, 18], umbilical cord [19, 20], and dental tissue [21–25]. Dental pulp stem cells (DPSCs) can readily be obtained from the third molars, usually discarded as medical waste. DPSCs have MSC-like characteristics such as the ability for self-renewal and multilineage differentiation. These dental pulp-derived MSCs avoid ethical concerns when sourced from other tissue, and they can be obtained without unnecessary invasive procedures, for example, MSCs collected from bone marrow or adipose tissue [9, 26–28]. DPSCs can differentiate into neuron-like cells and secrete neurotrophic factors such as neurotrophin (NT) [29, 30]. In addition, DPSCs express neuron-related markers even before being induced to neuronal differentiation [29, 31, 32]. Taken together, these unique properties make DPSCs an excellent candidate for stem cell-related therapies in nerve diseases.
2. Dental Pulp Stem Cells (DPSCs)
2.1. The Characteristics of DPSCs
The basic tooth structure consists of an outer enamel layer, a middle dentin layer, and an inner dental pulp layer. It develops from both cranial neural crest-derived mesenchymal stem cells (MSCs) and oral-derived epithelial stem cells in the early stages of embryogenesis [33–35]. Dental pulp, a soft connective tissue containing blood vessels, nerves, and mesenchymal tissue, has a central role in primary and secondary tooth development and ongoing maintenance for instance in reaction to caries [36, 37]. Stem cells can be isolated from the dental pulp tissue and possess MSC-like characteristics including self-renewal and multipotency [21, 38–40]. The first dental pulp-related stem cells were isolated from the third molar dental pulp by Gronthos et al. in 2000 [21]. Subsequently, it was reported that DPSCs could also be isolated from other dental pulps including human exfoliated deciduous teeth [22], human permanent and primary teeth [41], and supernumerary teeth [42]. Meanwhile, they are featured by high-proliferative capacity [43–47]. Most importantly, compared with collection procedures of other tissue-derived stem cells, the collection of DPSCs involves none harm to the donor or invasive surgical procedures [27, 40].
There are currently no specific biomarkers that uniquely define DPSCs. They express MSC-like phenotypic markers such as CD27, CD29, CD44, CD73, CD90, CD105, CD146, CD166, CD271, and STRO-1. Yet they do not express CD34, CD45, CD14, or CD19 and HLA-DR surface molecules [38, 39, 48]. Similar to embryonic stem cells, DPSCs express stemness-related markers such as Oct-4, Nanog, and Sox-2, as well as the cytoskeleton-related markers (Nestin and Vimentin) [29, 49, 50]. In addition, DPSCs express other cranial neural crest cell-related neural markers such as glial fibrillary acidic protein (GFAP), β-III tubulin, and microtubule-associated protein-2 (MAP-2) [29, 50, 51].
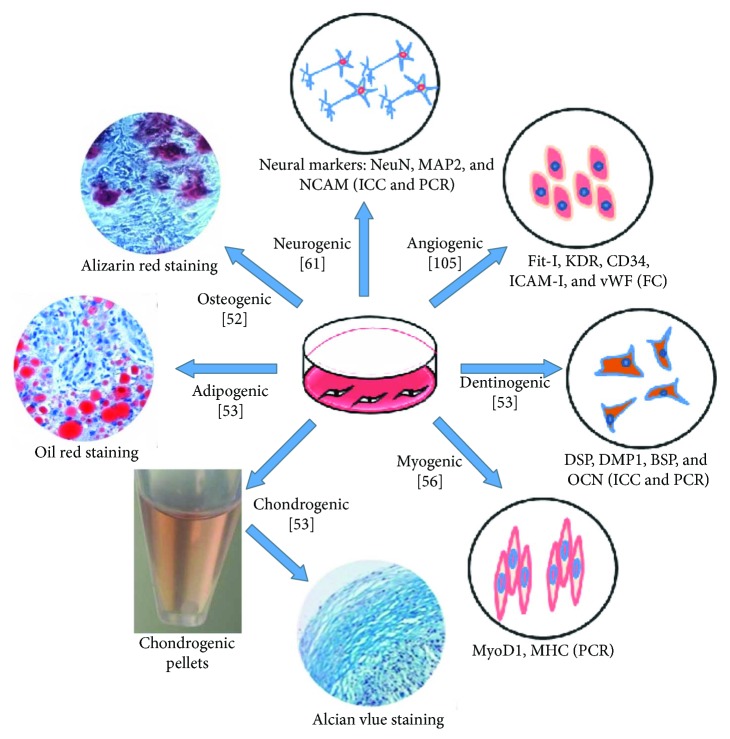
DPSCs are multipotent and can be induced to differentiate into cells for osteogenesis [52], chondrogenesis [53], adipogenesis [53], neurogenesis [54], dentinogenesis [53], odontogenesis [55], and myogenic lineages [56] (Figure 1). Using classic reprogramming factors (e.g., Oct3/4, Sox2, Klf4, and c-MYC), human DPSCs can be converted into induced pluripotent stem cells (iPSCs) [57, 58]. iPSCs exhibit the characteristics of embryonic stem cells and can differentiate into all three germ layers [59, 60]. Human DPSCs have a higher reprogramming efficiency than human dermal fibroblasts because they have a rapid proliferation rate and endogenously express high levels of the reprogramming factors c-MYC and Klf4 [57]. Therefore, DPSCs are potentially an important patient-specific cell source of iPSCs for clinical applications, regenerative medicine, and tissue engineering.
Figure 1.
Multidifferentiation potential of DPSCs. DPSCs possess MSC-like properties and are multipotent. NCAM: neural cell adhesion molecule; MAP2: microtubule-associated protein 2; NeuN: neuron-specific nuclear protein; Fit-I: VEGF receptor 1; KDR: VEGF receptor 2; CD34: cluster of differentiation 34; ICAM-I: intercellular cell adhesion molecule-1; vWF: von Willebrand factor, DSP: dentin sialoprotein, DMP1: dentin matrix acidic phosphoprotein 1, BSP: bone sialoprotein, OCN: osteocalcin, MyoD1: myoblast determination protein 1; MHC: major histocompatibility complex; PCR: polymerase chain reaction; FC: flow cytometry; ICC: immunocytochemical.
2.2. Neuronal Differentiation of DPSCs
DPSCs arise from the cranial neural crest and possess neuron-like characteristics that facilitate their in vitro induction into functional neurons. Numerous protocols have been developed to differentiate DPSCs into neurons. Typically, such methods rely on growth factors and various small molecules including basic fibroblast growth factor (bFGF) [61, 62], epidermal growth factor (EGF) [63], nerve growth factor (NGF) [62, 64], brain-derived neurotrophic factor (BDNF) [65], glial cell line-derived neurotrophic factor (GDNF) [66], sonic hedgehog [66], neurotrophin 3 (NT-3) [61], retinoic acid (RA) [63], forskolin [50, 67], and heparin [66] as well as culture supplements such as B27 [61], insulin-transferrin-sodium selenite (ITS) [54], nonessential amino acids [66], and N2 [61, 66]. Under controlled in vitro conditions (e.g., spheroid suspension culture in serum-free media), it is possible to differentiate DPSCs into neural lineages that expressed numerous neural markers [61, 63, 64, 68]. Chun et al. have demonstrated that DPSCs could be differentiated into dopaminergic neural cells by the formation of neurosphere [69]. However, huge variations exist in the neural differentiation of DPSCs due to alterations made to the culture of neurosphere, which indicates a delicate regulatory approach is necessary to achieve target differentiation. It is controversial on the timing of neurosphere formation. The study of Gervois et al. showed that it formed in the initial phase during a neural induction [61], whereas studies of Karbanova et al. observed that the neurosphere formed in a rather late phase during the differentiation [70].
Nevertheless, it is possible to bypass neurosphere formation by using endogenous environmental cues and directly differentiate DPSCs into motor and dopaminergic neuronal sublineages [65, 71]. Studies of Chang et al. reported that DPSCs could be directly differentiated into motor neurons by growth factors and small molecules, for example, BDNF and all-trans retinoic acid [71]. Gnanasegaran et al. demonstrated that DPSCs could be induced to differentiate into dopaminergic-like cells by multistage inductive protocols [72]. The study of Singh et al. showed that DPSCs are induced by a two-step method to generate functional dopaminergic neurons: FGF2 first with an addition of BDNF on 9th day. Furthermore, when induced, DPSCs showed much more distinct neuronal characteristics comparing to the other tissue-derived MSCs, for example, bone marrow and adipose tissue [73]. In addition, DPSCs could be differentiated into spiral ganglion neuron-like cells by treating with BDNF, NT-3, and GDNF [74].
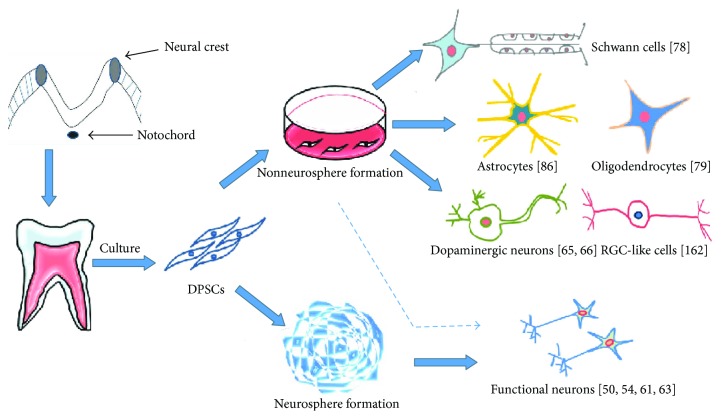
Typically, a successful neuronal differentiation of DPSCs is confirmed by the increased expression of neuronal markers such as NeuN [61], neurofilament-200 [54], MAP-2 [61, 75], synaptophysin [61], and neural cell adhesion molecules [76]. Few studies have used ultrastructural and/or electrophysiological analyses to confirm the state of differentiation [61]. Previous studies focus on differentiation directions: DPSCs could be differentiated into either neuronal precursor cells (rather than mature neurons capable of generating action potentials) or immature Schwann cells and oligodendrocytes that can support nerve regeneration [77–80] (Figure 2). Recently, research has evolved into in-depth studies on functional and mechanism of DPSC-differentiated neurons. A series of studies have explored the functional activities of DPSC-differentiated neurons in voltage-gated sodium and potassium channels as well the neuronal marker expressions, indicating a successful differentiation is active and functional new neurons have emerged [50, 54, 67]. Further, these predifferentiated DPSCs have been traced and proved well integrated into the central nervous tissue when transplanted in animal models [54, 67]. In summary, versatile differentiations of DPSCs depend on inductive protocols. They can be differentiated into neurons, dopaminergic-like cells, Schwann cells, and oligodendrocytes. Thus, DPSCs are an attractive cell source for stem cell therapy to treat the nervous diseases.
Figure 2.
Neural differentiation potential of DPSCs. DPSCs can be induced to differentiate into neural cell lineages including Schwann cells, astrocytes, and dopaminergic neurons.
2.3. Neuroprotective and Neurotrophic Properties of DPSCs
The efficacy of stem cell therapies in nervous diseases is strongly influenced by trophic factors, for example, BDNF, GDNF, NGF, NT-3, vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF) [29, 30]. The expression of these trophic factors by DPSCs is remarkably higher than those of MSCs derived from bone marrow (BMSCs) and adipose tissue [9, 30]. Further in vivo study also demonstrates a more efficient secretion of BDNF and GDNF than BMSCs [81]. These findings confirm that in comparison to other MSCs, DPSCs exhibit superior neuroprotective and neural supportive properties in response to injuries and pathologies of the nervous system. DPSCs have the ability to reduce neurodegeneration in the early stages of neuronal apoptosis and promote motor and sensory neuron survival in spinal cord injury (SCI) by the secretion of BDNF and NGF [82, 83]. Furthermore, trophic factors secreted by DPSCs promoted axon regeneration despite the presence of axon growth inhibitors in the completely transected spinal cord model of SCI [84, 85]. DPSCs also provided both direct and indirect protections against cell death by secreting cytoprotective factors in an ischemic astrocyte model of injury [86, 87]. Compared with other stem cells (DFSCs, SCAP, and BMSCs), DPSCs have shown a higher cytokine expression facilitating neuronal differentiations [88].
2.4. Angiogenic Properties of DPSCs
In general, the human body needs abundant nutrition and blood supply in order to maintain its tissues and organs in a healthy condition. The sprouting of new capillaries from existing blood vessels during inflammation and hypoxic conditions depends on the expression and secretion of specific angiogenic trophic factors [89, 90]. Some MSCs are able to promote therapeutic angiogenesis by the secretion of angiogenic growth factors and by differentiating into endothelial cells [91–93]. In particular, DPSCs have been found to secrete and produce abundant angiogenic factors, for example, colony-stimulating factor, interleukin-8, angiogenin, endothelin-1, angiopoietin-1, and insulin-like growth factor binding protein-3 [94–96]. DPSCs also secrete and express other stimulatory growth factors such as VEGF, PDGF, bFGF, and NGF [19, 30, 97]. Synergistically, these factors can promote proliferation and survival of vascular endothelial cells [98, 99] as well as endothelial tubulogenesis [100]. Both the formation and function of new blood vessels are improved by either injection of DPSCs into neuronal disease models or transplantation of DPSCs into ischemia and myocardial infarction animal models [101, 102]. Moreover, Nam et al. observed that by coinjection of DPSCs and HUVECs into immunodeficient mice, microvessel-like structures would be formed, which illustrated that DPSCs could perform as perivascular cells for in vivo angiogenesis [103]. DPSCs also have the ability to differentiate into endothelial-like cells. When incubated with VEGF, the expression of VEGFR1, VEGFR2, von Willebrand factor, and CD54 is increased [104, 105]. These VEGF-induced DPSCs exhibited endothelial features and formed capillary-like structures when cultured on a fibrin clot [105]. More recently, a structured dentin-/pulp-like tissue with vasculatures has been created using DPSCs via 3D print technique, suggesting a new direction for customized application for individual design of defect repair [106].
2.5. Immunomodulatory Properties of DPSCs
MSCs exhibit some immunomodulatory and anti-inflammatory factors, for example, interleukin-10 (IL-10) [107], hepatocyte growth factor (HGF), [108], transforming growth factor-β (TGF-β) [109], and prostaglandin E2 [110]. MSCs can act as an immunosuppressive agent by modulating the immune response in inflammatory or autoimmune diseases [111, 112]. DPSCs also have immunomodulatory properties associated with expression of soluble factors that inhibit T cell function. For instance, it has been reported that DPSCs express interleukin-8 (IL-8), interleukin-6 (IL-6), and TGF-β via Toll-like receptor (TLR) 4 during neuroinflammation in neurodegenerative diseases [8, 113]. An upregulated expression of TLR4 appeared to increase the expression of IL-8 in DPSCs [114], particularly in SCI crush injury where IL-8 preserves axon integrity and decreases cavitation [115, 116]. DPSCs also express TGF-β, HGF, and indoleamine 2,3-dioxygenase (IDO) without prior activation [117, 118] and suppress the proliferation of peripheral blood mononuclear cells and the activation of T cells [119, 120]. Coculture of DPSCs and T cells promoted T cell secretion of human leukocyte antigen-G, vascular adhesion molecule-1, intracellular adhesion molecule-1, IL-6, TGF-β, HGF, and IL-10, while it downregulated proinflammatory cytokines such as IL-2, IL-6 receptor, IL-12, IL-17A, and tumor necrosis factor-α (TNF-α) [121]. It was reported that the proliferation of T cells was inhibited by over 90% when cocultured with DPSCs in vitro [8, 122]. In addition, recent studies demonstrated that human and rat DPSCs were able to induce FasL-mediated apoptosis of IL-17 T-helper cells, and rat DPSCs exhibited a very strong ameliorating effect on DSS-induced colitis in mice [123, 124]. The study of Hong et al. reported that DPSCs could modulate immune tolerance by increasing CD4+CD25+FoxP3+ regulatory T cells. The results of the intraperitoneal injection of DPSCs into Balb/c(H-2d) mice demonstrated that DPSCs had a meaningful effect on mixed lymphocyte reaction [125]. Studies of Kwack et al. demonstrated that DPSCs could inhibit acute allogeneic immune responses by the release of TGF-β as a result of allogeneic stimulation of T lymphocytes and provide a novel insight for the allogeneic transplantation of DPSCs in future clinical use [120]. Recent animal studies conclude that DPSCs could modulate immune tolerance and influence apoptosis via T cells and lymphocytes.
3. Dental Pulp Stem Cells (DPSCs) and Central Nervous System Diseases
Traumatic damage to the brain and spinal cord leading to a CNS dysfunction, stroke, Parkinson's disease, Alzheimer's disease, and retinal injury is a common central nervous system disease. The CNS typically has a poor ability to repair and regenerate new neurons because of its limited pool of precursor cells [126, 127], expression of myelin-associated growth inhibitory factors [128], and the inherent propensity of resident glial cells to form scar tissue [129]. At present, it is very difficult to treat CNS diseases with conventional clinical therapies. Some studies have suggested that stem cell treatment may offer a novel therapeutic strategy for CNS disease [127, 130]. The hope is that the application of exogenous stem cells (particularly DPSCs) will lead to both regeneration of new neural precursor cells and their enhanced neuronal and glial differentiation as well as to survival and maintenance of existing neural cells through secretion of trophic factors [29, 30, 40].
3.1. DPSCs and SCI
SCI in humans can cause partial or complete loss of motor and sensory function that reduces the quality of an individual's life and leads to an economic burden on society [124, 131]. SCI involves an initial primary tissue disruption (e.g., mechanical damage to nerve cells and blood vessels) and then a secondary injury caused by neuroinflammatory responses (e.g., excitotoxicity, blood-brain barrier disruption, oxidative stress, and apoptosis) [132, 133]. Because of their neural crest lineage, DPSCs have championed as preferred stem cells for SCI therapies supported by growing evidence of DPSCs differentiating into neuron-like and oligodendrocyte-like cells that may promote axonal regeneration and tissue repair after SCI [28, 127, 134, 135]. DPSCs also reduce secondary inflammatory injury, which facilitates axonal regeneration and reduces progressive hemorrhagic necrosis associated with interleukin-1β (IL-1β), ras homolog gene family member A (RhoA), and sulfonylurea receptor1 (SUR1) expression [136]. When transplanted together with artificial scaffolds such as chitosan, DPSCs promoted motor functional recovery and inhibited cell apoptosis after SCI by secreting BDNF, GDNF, and NT-3 and reducing the expression of active-caspase 3 [8, 137].
3.2. DPSCs and Stroke
Stroke is an ischemic cerebrovascular condition that leads to brain damage, long-term disability, and even death [138]. Due to prolonged period of insufficient blood supply and poor oxygen perfusion, damages on affected brain are irreversible. There are unfortunately few effective strategies that can reverse the damage effect on the brain or restore one's function to prestrike level [139]. Recent studies indicate that stem cell therapy may present a novel strategy for stroke treatment due to the multipotency, immunomodulatory, and neuroprotective and angiogenic properties of these cells [140, 141]. Some in vivo studies have shown that transplantation of DPSCs into the ischemic areas of middle cerebral artery occlusion (MCAO) in Sprague-Dawley (SD) rats promoted locomotor functional recovery and decreased infarct areas by their differentiation into dopaminergic neurons and secretion of neurotrophic factors [102, 142]. DPSC transplantation into ischemic areas of focal cerebral ischemia in rats led to expression of proangiogenic factors that supported dense capillary formation and renormalization of blood flow [143]. Intracerebral transplantation of DPSCs into regions of focal cerebral ischemia in rodent models promoted forelimb sensory and motor functional recovery at 4 weeks posttreatment [140]. DPSCs also provided cytoprotection for astrocytes by reducing reactive gliosis and preventing free radical and IL-1β secretion within in vitro ischemic models [86]. Thus, DPSCs may play an immunomodulatory role to promote functional recovery after ischemic stroke.
3.3. DPSCs and Parkinson's Disease
Parkinson's disease (PD) is a progressive neurodegenerative condition associated with loss of nigrostriatal dopaminergic (DA) neurons that leads to muscle rigidity, bradykinesia, resting tremor, and postural instability [144]. Stem cell-based therapies hold some promise as a novel strategy for PD treatment [145]. DPSCs can be induced to differentiate into dopamine expressing DA neuron-like cells in vitro by using experimental cell induction media [65]. Intrathecal transplantation of DPSCs into the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine- (MPTP-) induced old-aged mouse model of PD promoted recovery of behavioral deficits, restored DA functions, and attenuated MPTP-induced damage by reducing the secretion of proinflammatory factors such as IL-1α, IL-1β, IL6, IL8, and TNF-α and by upregulating the expression levels of anti-inflammatory factors such as IL2, IL4, and TNF-β [146]. DPSCs also showed neuroimmunomodulatory activity in an in vitro model of PD by reducing MPTP-induced deficits associated with reactive oxygen species, DNA damage, and nitric oxide release [146, 147]. DPSCs also promoted survival of DA neurons and enhanced nigrostriatal tract functional recovery in a 6-hydroxydopamine- (6-OHDA-) induced PD rat model by 6 weeks posttransplantation [148]. Some studies have also shown that DPSCs reduced 6-OHDA-induced damage in the in vitro model of PD [69, 145]. The clinical use of DPSCs may be a promising approach for treating PD in the future.
3.4. DPSCs and Alzheimer's Disease
Alzheimer's disease (AD) is a progressive neurodegenerative condition caused by the loss of neurons, intracellular neurofibrillary tangles, and deposition of insoluble β-amyloid peptides in the brain [149, 150]. Clinical symptoms of AD include memory loss, cognitive deficits, and linguistic disorders [150]. Recently, several studies reported that stem cell-based therapies in both in vitro and in vivo models of AD improved AD-induced pathologies and behavioral deficits [151–153]. DPSCs promoted neuronal repair and regeneration by restoring cytoskeletal structure, protecting microtubule stability, and reducing tau phosphorylation in the okadaic acid- (OA-) induced cellular model of AD [154]. DPSCs can also reduce amyloid beta (Aβ) peptide-induced cytotoxicity and apoptosis in the AD cellular model by secreting higher levels of VEGF, fractalkine, RANTES, fms-related tyrosine kinase 3, and monocyte chemotactic protein 1 [155, 156]. These results suggest that DPSCs are a promising cell source for secretome-based treatment of AD.
3.5. DPSCs and Retinal Injury
The retina is a part of the CNS and is composed of photoreceptors, bipolar cells, and retinal ganglion cells (RGCs) [43, 157]. Head injuries can cause traumatic optic neuropathy (TON) while ocular chronic degenerative diseases such as glaucoma lead to the slow loss of RGCs [158]. Retinal and optic nerve injuries have a limited capacity to repair and regenerate because of axon growth inhibitory molecules and reduced production of neurotrophic growth factors [7, 159]. One study reported that DPSC transplantation into the vitreous of optic nerve injury rat model could promote axonal regeneration and RGC survival by a neurotrophin-mediated mechanism [83]. This same study revealed that DPSCs were more beneficial for axonal regeneration than BMSCs because of their higher secretion of neurotrophin factors. A subsequent report showed that intravitreal transplantation of DPSCs in an animal model of glaucoma maintained visual function up to 35 days after treatment by preventing RGC death [160]. Although not assessed in vivo, some in vitro studies have reported that DPSCs can be induced to differentiate into both RGC-like and photoreceptor cells [161, 162]. Taken together, these results suggest that DPSCs may become an important cell source for stem cell-based therapies in ocular diseases.
4. DPSCs and Peripheral Nerve Injury
Peripheral nerve injury caused by traumatic accidents and iatrogenic damage often accompanies physical disability and neuropathic pain. There are many current clinical treatments including direct end-to-end nerve suturing, nerve grafts, and nerve conduits containing growth-stimulatory biomaterials to repair and regenerate injured peripheral nerves [163–165]. Among them, autologous nerve grafting is the gold standard therapy for the long gap of peripheral nerve deficits [166, 167]. However, there are some disadvantages which restrict the clinical use of autografting, such as donor nerve availability and morphometric mismatching [168–171]. With the development of nerve tissue engineering and stem cell-related therapy, various novel nerve conduits in combination with stem cells are providing alternate strategies and approaches for the treatment of peripheral nerve injury [165, 172]. Some studies suggest that DPSC-embedded biomaterial nerve conduits such as polylactic glycolic acid tubes have the ability to promote regeneration of injured facial nerve and to improve functional recovery comparable to that of autografts [173]. Collagen conduits loaded with Schwann-like cells induced from DPSCs in vitro have facilitated repair and regeneration of 15 mm sciatic nerve defects [174]. In another report, differentiated DPSCs combined with collagen scaffolds exhibited Schwann cell-related properties and promoted axonal outgrowth and myelination in 2D or 3D culture conditions of an in vitro model [78]. Moreover, DPSCs transfected with oligodendrocyte lineage transcription factor 2 differentiated into functional oligodendrocytes in vitro and promoted injured peripheral nerve repair and regeneration in a mouse model [175]. DPSCs transplanted into diabetic rats secreted various cytokines that modulated the proportions of M1/M2 macrophages and provided beneficial anti-inflammatory effects in diabetic polyneuropathy [176].
In summary, DPSCs have the capacity to differentiate into Schwann-like and oligodendrocyte-like cells and they secrete neurotrophic factors that provide neuroprotection and modulate the immune response. These cells are poised to become a promising cell source for peripheral nerve injury treatment in the future.
5. Conclusions and Future Insights
This review summarizes the neuronal differentiation potential, neuroprotective features, and neurotrophic, angiogenic, and immunomodulatory properties of DPSCs in the pathological and injured nervous system. DPSCs have the biological properties of MSCs and possess a considerable capacity to differentiate into neuron-like cells and secrete neuron-related trophic factors due to their cranial neural crest origin. DPSCs are able to express neuronal markers without preinduced differentiation. Thus, both nondifferentiated and differentiated DPSCs are emerging as new cell sources for the treatment of nervous system deficits associated with SCI, stroke, AD, PD, and long gaps of peripheral nerve injury. DPSCs have several advantages over other exogenous stem cells for nervous system therapies because they are easily harvested without highly invasive surgery, have low immunogenicity, and arise from a neural crest origin that facilitates their neural differentiation. Moreover, after lentiviral transfection with Lin28, Nanog, Oct4, and Sox2 or retroviral transfection with Oct3/4, Sox2, and Klf4, DPSCs can be reprogrammed to generate an embryoid body of iPSCs. The DPSC-derived iPSCs have ability to differentiate into β-III tubulin neuron-like cells and tyrosine hydroxylase-positive dopaminergic neuron-like cells and may become another DPSC-related cell sources for the treatment of nervous system diseases in the future.
Because of the vascularization and immunomodulatory properties of DPSCs, these cells can both directly and indirectly stimulate formation of new blood vessels and enhance blood flow to injury sites. In addition to their roles in regeneration and repair of injured neural tissue (Table 1), therapies using DPSCs are emerging as a promising novel strategy for treating other brain conditions and syndromes such as traumatic brain injury, multiple sclerosis, and autism spectrum disorders.
Table 1.
Examples for the beneficial of DPSCs on the central nervous system (CNS) diseases and the peripheral nervous system (PNS) diseases.
| Type of diseases | Author | Differentiated status of DPSCs | Delivery method | Function of DPSCs | References |
|---|---|---|---|---|---|
| The central nervous system (CNS) diseases | |||||
|
| |||||
| Spinal cord injury (SCI) | Yamamoto et al. | Undifferentiated | DPSC transplantation | DPSCs inhibited massive SCI-induced apoptosis, preserved neural fibers and myelin, regenerated transected axons, and replaced damaged cells by differentiating into oligodendrocytes | [134] |
| Yang et al. | Undifferentiated | DPSCs transplanted with cell pellets | DPSCs reduced inflammatory injury, promoted axonal regeneration, and reduced progressive hemorrhagic necrosis after SCI by inhibiting IL-1β, RhoA, and SUR1 expression | [136] | |
| Zhang et al. | Undifferentiated | DPSCs transplanted with chitosan-scaffold | DPSCs promoted motor functional recovery and inhibited cell apoptosis after SCI through secreting BDNF, GDNF, NT-3 and reducing the expression of active-caspase 3 | [137] | |
|
| |||||
| Stroke | Song et al. | Undifferentiated | DPSCs cocultured with the conditioned medium in vitro | DPSCs conferred superior cytoprotection against cell death by reducing reactive gliosis and suppressing free radical and proinflammatory cytokine expression | [86] |
| Song et al. | Undifferentiated | Intravenous DPSC injection | DPSCs reduced the infarct volume of SD rats after middle cerebral artery occlusion (MCAO) due to high angiogenesis and neurogenic differentiation and reduction of reactive gliosis | [87] | |
| Sugiyama et al. | Dental pulp-derived CD31(−)/CD146(−) side population (SP) stem cells | CD31(−)/CD146(−) SP cells transplantation | DPSCs promoted migration and differentiation of the endogenous neuronal progenitor cells and induced vasculogenesis and ameliorated ischemic brain injury of SD rats after transient middle cerebral artery occlusion (TMCAO) | [102] | |
| Yang et al. | Dental pulp-derived neuronal stem cells (tNSCs) | tNSC transplantation | Transplanted tNSC promoted function recovery after MCAO because of possessing hypoimmunogenic properties and immune modulation abilities | [142] | |
| Leong et al. | Undifferentiated | Intracerebral DPSC transplantation | DPSCs enhanced the recovery of poststroke sensorimotor deficits owing to differentiation into astrocytes and mediation through DPSC-dependent paracrine effects | [143] | |
|
| |||||
| Parkinson's disease (PD) | Kanafi et al. | Dopaminergic cell-type differentiated | DPSCs were induced in vitro | DPSCs showed efficient propensity towards functional dopaminergic cell type | [65] |
| Chun et al. | Dopaminergic neurons differentiated | DPSCs were treated with the dopaminergic neuron differentiation kit in vitro | DPSCs could differentiate into dopaminergic neural cells under experimental cell differentiation conditions | [69] | |
| Gnanasegaran et al. | Undifferentiated | Intrathecal DPSC transplantation into a mouse model of PD in vitro | DPSCs could treat the PD by regulating inflammatory mediators such as reducing the secretions of proinflammatory factors (IL-1α, IL-1β, IL6, IL8, and TNF-α) and upregulating the expression levels of anti-inflammatory factors (IL2, IL4, and TNF-β) | [146] | |
| Gnanasegaran et al. | DAergic-like cells differentiated | DPSCs were cultured in a system which consists of neuron and microglia in vitro | DPSCs were shown to have immunomodulatory capacities to reduce 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine- (MPTP-) induced deficits such as reactive oxygen species, DNA damages, and nitric oxide release | [147] | |
|
| |||||
| Alzheimer's disease (AD) | Wang et al. | Undifferentiated | DPSCs cocultured with okadaic acid- (OA-) induced cellular model of AD in vitro | DPSC-treated cells had the morphology of restored neurons, elongated dendrites, densely arranged microfilaments, and thickened microtubular fibrils | [154] |
| Ahmed et al. | Undifferentiated | DPSCs cocultured with amyloid beta (Aβ) peptide-induced cellular model of AD in vitro | DPSCs secreted and produced numerous vascular endothelial growth factor (VEGF), fractalkine, RANTES, fms-related tyrosine kinase 3 (FLT-3), and monocyte chemotactic protein 1 (MCP-1) | [155] | |
|
| |||||
| Retinal injury | Mead et al. | Undifferentiated | Intravitreal DPSC transplantation | DPSCs produced and secreted lots of neurotrophins in order to promote neuritogenesis/axogenesis of retinal cells | [83] |
| Mead et al. | Undifferentiated | Intravitreal DPSC transplantation | DPSC provided protection from retinal ganglion cell (RGC) loss and retinal nerve fiber layer thickness (RNFL) thinning and preserved RGC function | [160] | |
| Bray et al. | Undifferentiated | DPSCs cocultured with the conditioned media which were obtained from organotypic explants from damaged rat retinas in vitro | DPSCs had ability to promote neurodifferentiation and expression of retinal neuronal markers in order to cure the rat retinas | [161] | |
|
| |||||
| The peripheral nervous system (PNS) diseases | |||||
|
| |||||
| Facial nerve defect | Sasaki et al. | Undifferentiated | DPSCs transplanted with poly-dl-lactide-coglycolide (PLGA) and collagen gel | DPSCs promoted the axon regeneration and myelinated nerve formation | [173] |
| Sciatic nerve defect | Sanen et al. | Schwann cell-type differentiated | DPSCs transplanted with NeuraWrap™ conduits | DPSCs promoted in growing neurites, myelinated nerve, and newly blood vessel formation and survival | [174] |
| Sciatic nerve defect | Askari et al. | Oligodendrocyte progenitor cell- (OPC-) type differentiated | DPSC-induced OPC transplantation | DPSCs could be differentiated into functional oligodendrocytes | [175] |
| Sciatic nerve defect | Omi et al. | Undifferentiated | DPSC transplantation | DPSCs increased the gene expression of interleukin-10 and promoted macrophages polarization towards anti-inflammatory M2 phenotypes | [176] |
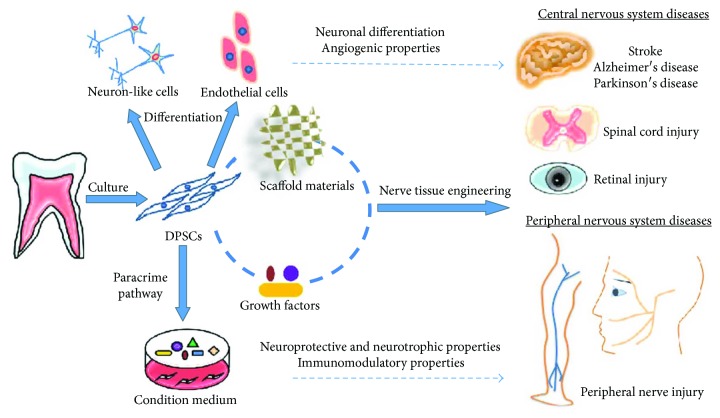
However, despite the functional advantages of using DPSCs for the treatment of nervous system injuries and diseases, there remain significant roadblocks with respect to overcoming the nervous system's seemingly inherent and immutable resistance to regeneration and repair. Nerve tissue engineering approaches are now beginning to adopt combinatorial strategies that involve simultaneous manipulations to cells, growth factors, and scaffolds in order to circumvent the recalcitrant nature of the nervous system (Figure 3). In particular, novel scaffolds such as hydrogels have a 3D porous structure and good cytocompatibility that can be used to provide an in vivo-like microenvironment and structural support for cell adhesion, proliferation, and growth. Scaffolds can be designed to embed biological important macromolecules such as bFGF and NGF and to precisely tune their diffusion rate and enzymatic degradation. Seed cells such as DPSCs have beneficial effects on neural regeneration and repair associated with their neural differentiation potential and their neurotrophic, angiogenic, and immunomodulatory properties. Therefore, the spatiotemporal combination of DPSCs, scaffolds, and growth factors provides a promising strategy for treating nervous system-related diseases and injuries in future clinical approaches.
Figure 3.
Tissue-engineered constructs of DPSCs, scaffolds, and growth factors and their applications in nervous system diseases. In the constructs, scaffolds can provide biomimetic environments and structural support for cell survival and proliferation. Growth factors can promote neuronal cell proliferation and survival in vivo and in vitro. DPSCs can enhance neuronal regeneration and repair due to their neuronal differentiation potential and their neurotrophic, neuroprotective, angiogenic, and immunomodulatory properties.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81601626 to Huaqiong Li), the UQDVCR (610709 to Qingsong Ye), the Wenzhou Medical University (QTJ16026 to Lihua Luo), and Research and Development Program Project of Xiangyang (20270268020 to Lihua Luo).
Contributor Information
Huaqiong Li, Email: lihq@wibe.ac.cn.
Qingsong Ye, Email: qingsongye@hotmail.com.
Conflicts of Interest
The authors declare that they have no competing interests regarding the publication of this paper.
Authors' Contributions
Lihua Luo and Yan He contributed equally to this work.
References
- 1.Kahveci K., Dincer M., Doger C., Yarici A. K. Traumatic brain injury and palliative care: a retrospective analysis of 49 patients receiving palliative care during 2013–2016 in Turkey. 2017;12(1):77–83. doi: 10.4103/1673-5374.198987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciaramitaro P., Mondelli M., Logullo F., et al. Traumatic peripheral nerve injuries: epidemiological findings, neuropathic pain and quality of life in 158 patients. 2010;15(2):120–127. doi: 10.1111/j.1529-8027.2010.00260.x. [DOI] [PubMed] [Google Scholar]
- 3.Esquenazi Y., Park S. H., Kline D. G., Kim D. H. Surgical management and outcome of iatrogenic radial nerve injection injuries. 2016;142:98–103. doi: 10.1016/j.clineuro.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Baumard J., Lesourd M., Remigereau C., et al. Tool use in neurodegenerative diseases: planning or technical reasoning? 2017 doi: 10.1111/jnp.12121. [DOI] [PubMed] [Google Scholar]
- 5.Rolfe A., Sun D. Stem cell therapy in brain trauma: implications for repair and regeneration of injured brain in experimental TBI models. In: Kobeissy F. H., editor. Boca Raton, FL, USA: CRC Press/Taylor & Francis; 2015. pp. 587–596. [PubMed] [Google Scholar]
- 6.Hong H., Kim B. S., Im H. I. Pathophysiological role of neuroinflammation in neurodegenerative diseases and psychiatric disorders. 2016;20(Supplement 1):S2–S7. doi: 10.5213/inj.1632604.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry M., Ahmed Z., Lorber B., Douglas M., Logan A. Regeneration of axons in the visual system. 2008;26(2-3):147–174. [PubMed] [Google Scholar]
- 8.Bianco J., De Berdt P., Deumens R., des Rieux A. Taking a bite out of spinal cord injury: do dental stem cells have the teeth for it? 2016;73(7):1413–1437. doi: 10.1007/s00018-015-2126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caseiro A. R., Pereira T., Ivanova G., Luís A. L., Maurício A. C. Neuromuscular regeneration: perspective on the application of mesenchymal stem cells and their secretion products. 2016;2016:16. doi: 10.1155/2016/9756973.9756973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mead B., Berry M., Logan A., Scott R. A. H., Leadbeater W., Scheven B. A. Stem cell treatment of degenerative eye disease. 2015;14(3):243–257. doi: 10.1016/j.scr.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghasemi Hamidabadi H., Rezvani Z., Nazm Bojnordi M., et al. Chitosan-intercalated montmorillonite/poly(vinyl alcohol) nanofibers as a platform to guide neuronlike differentiation of human dental pulp stem cells. 2017;9(13):11392–11404. doi: 10.1021/acsami.6b14283. [DOI] [PubMed] [Google Scholar]
- 12.Ferroni L., Gardin C., Tocco I., et al. Potential for neural differentiation of mesenchymal stem cells. 2012;129:89–115. doi: 10.1007/10_2012_152. [DOI] [PubMed] [Google Scholar]
- 13.Neirinckx V., Coste C., Rogister B., Wislet-Gendebien S. Concise review: adult mesenchymal stem cells, adult neural crest stem cells, and therapy of neurological pathologies: a state of play. 2013;2(4):284–296. doi: 10.5966/sctm.2012-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teixeira F. G., Carvalho M. M., Sousa N., Salgado A. J. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? 2013;70(20):3871–3882. doi: 10.1007/s00018-013-1290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdallah B. M., Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. 2008;15(2):109–116. doi: 10.1038/sj.gt.3303067. [DOI] [PubMed] [Google Scholar]
- 16.Ohishi M., Schipani E. Bone marrow mesenchymal stem cells. 2010;109(2):277–282. doi: 10.1002/jcb.22399. [DOI] [PubMed] [Google Scholar]
- 17.Jin H., Bae Y., Kim M., et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. 2013;14(9):17986–18001. doi: 10.3390/ijms140917986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim M. H., Ong W. K., Sugii S. The current landscape of adipose-derived stem cells in clinical applications. 2014;16, article e8 doi: 10.1017/erm.2014.8. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro J., Gartner A., Pereira T., et al. Chapter four - perspectives of employing mesenchymal stem cells from the Wharton’s jelly of the umbilical cord for peripheral nerve repair. 2013;108:79–120. doi: 10.1016/B978-0-12-410499-0.00004-6. [DOI] [PubMed] [Google Scholar]
- 20.Frausin S., Viventi S., Verga Falzacappa L., et al. Wharton’s jelly derived mesenchymal stromal cells: biological properties, induction of neuronal phenotype and current applications in neurodegeneration research. 2015;117(4-5):329–338. doi: 10.1016/j.acthis.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Gronthos S., Mankani M., Brahim J., Robey P. G., Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miura M., Gronthos S., Zhao M., et al. SHED: stem cells from human exfoliated deciduous teeth. 2003;100(10):5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonoyama W., Liu Y., Yamaza T., et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. 2008;34(2):166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seo B. M., Miura M., Gronthos S., et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. 2004;364(9429):149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 25.Morsczeck C., Gotz W., Schierholz J., et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. 2005;24(2):155–165. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Collart-Dutilleul P. Y., Chaubron F., De Vos J., Cuisinier F. J. Allogenic banking of dental pulp stem cells for innovative therapeutics. 2015;7(7):1010–1021. doi: 10.4252/wjsc.v7.i7.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geng Y. W., Zhang Z., Liu M. Y., Hu W. P. Differentiation of human dental pulp stem cells into neuronal by resveratrol. 2017;41(12):1391–1398. doi: 10.1002/cbin.10835. [DOI] [PubMed] [Google Scholar]
- 28.Hidalgo San Jose L., Stephens P., Song B., Barrow D. Microfluidic encapsulation supports stem cell viability, proliferation, and neuronal differentiation. 2018;24(3):158–170. doi: 10.1089/ten.tec.2017.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakai K., Yamamoto A., Matsubara K., et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. 2012;122(1):80–90. doi: 10.1172/JCI59251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mead B., Logan A., Berry M., Leadbeater W., Scheven B. A. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. 2014;9(10, article e109305) doi: 10.1371/journal.pone.0109305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foudah D., Monfrini M., Donzelli E., et al. Expression of neural markers by undifferentiated mesenchymal-like stem cells from different sources. 2014;2014:16. doi: 10.1155/2014/987678.987678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ullah I., Park J. M., Kang Y. H., et al. Transplantation of human dental pulp-derived stem cells or differentiated neuronal cells from human dental pulp-derived stem cells identically enhances regeneration of the injured peripheral nerve. 2017;26(17):1247–1257. doi: 10.1089/scd.2017.0068. [DOI] [PubMed] [Google Scholar]
- 33.Chai Y., Jiang X., Ito Y., et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. 2000;127(8):1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- 34.Shi S., Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. 2003;18(4):696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 35.Karaoz E., Demircan P. C., Saglam O., Aksoy A., Kaymaz F., Duruksu G. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. 2011;136(4):455–473. doi: 10.1007/s00418-011-0858-3. [DOI] [PubMed] [Google Scholar]
- 36.Martens W., Bronckaers A., Politis C., Jacobs R., Lambrichts I. Dental stem cells and their promising role in neural regeneration: an update. 2013;17(9):1969–1983. doi: 10.1007/s00784-013-1030-3. [DOI] [PubMed] [Google Scholar]
- 37.Kuang R., Zhang Z., Jin X., et al. Nanofibrous spongy microspheres for the delivery of hypoxia-primed human dental pulp stem cells to regenerate vascularized dental pulp. 2016;33:225–234. doi: 10.1016/j.actbio.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gronthos S., Brahim J., Li W., et al. Stem cell properties of human dental pulp stem cells. 2002;81(8):531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 39.Kawashima N. Characterisation of dental pulp stem cells: a new horizon for tissue regeneration? 2012;57(11):1439–1458. doi: 10.1016/j.archoralbio.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Xiao L., Ide R., Saiki C., Kumazawa Y., Okamura H. Human dental pulp cells differentiate toward neuronal cells and promote neuroregeneration in adult organotypic hippocampal slices in vitro. 2017;18(8):p. 1745. doi: 10.3390/ijms18081745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karaoz E., Dogan B. N., Aksoy A., et al. Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. 2010;133(1):95–112. doi: 10.1007/s00418-009-0646-5. [DOI] [PubMed] [Google Scholar]
- 42.Huang A. H.-C., Chen Y.-K., Lin L.-M., Shieh T.-Y., Chan A. W.-S. Isolation and characterization of dental pulp stem cells from a supernumerary tooth. 2008;37(9):571–574. doi: 10.1111/j.1600-0714.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- 43.Rai S., Kaur S., Kaur M., Arora S. P. Redefining the potential applications of dental stem cells: an asset for future. 2012;18(3):276–284. doi: 10.4103/0971-6866.107976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kabir R., Gupta M., Aggarwal A., Sharma D., Sarin A., Kola M. Z. Imperative role of dental pulp stem cells in regenerative therapies: a systematic review. 2014;20(1):1–8. doi: 10.4103/1117-6806.127092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torkzaban P., Saffarpour A., Bidgoli M., Sohilifar S. In vitro evaluation of isolation possibility of stem cells from intra oral soft tissue and comparison of them with bone marrow stem cells. 2012;9(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- 46.Stanko P., Kaiserova K., Altanerova V., Altaner C. Comparison of human mesenchymal stem cells derived from dental pulp, bone marrow, adipose tissue, and umbilical cord tissue by gene expression. 2014;158(3):373–377. doi: 10.5507/bp.2013.078. [DOI] [PubMed] [Google Scholar]
- 47.Chalisserry E. P., Nam S. Y., Park S. H., Anil S. Therapeutic potential of dental stem cells. 2017;8 doi: 10.1177/2041731417702531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmieri A., Pezzetti F., Graziano A., et al. Comparison between osteoblasts derived from human dental pulp stem cells and osteosarcoma cell lines. 2008;32(7):733–738. doi: 10.1016/j.cellbi.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Cheng P.-H., Snyder B., Fillos D., Ibegbu C. C., Huang A., Chan A. W. S. Postnatal stem/progenitor cells derived from the dental pulp of adult chimpanzee. 2008;9(1):p. 20. doi: 10.1186/1471-2121-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiraly M., Porcsalmy B., Pataki A., et al. Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. 2009;55(5):323–332. doi: 10.1016/j.neuint.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 51.Feng X., Xing J., Feng G., et al. Age-dependent impaired neurogenic differentiation capacity of dental stem cell is associated with Wnt/β-catenin signaling. 2013;33(8):1023–1031. doi: 10.1007/s10571-013-9965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu L., Ling J., Wei X., Wu L., Xiao Y. Stem cell regulatory gene expression in human adult dental pulp and periodontal ligament cells undergoing odontogenic/osteogenic differentiation. 2009;35(10):1368–1376. doi: 10.1016/j.joen.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 53.Iohara K., Zheng L., Ito M., Tomokiyo A., Matsushita K., Nakashima M. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. 2006;24(11):2493–2503. doi: 10.1634/stemcells.2006-0161. [DOI] [PubMed] [Google Scholar]
- 54.Arthur A., Rychkov G., Shi S., Koblar S. A., Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. 2008;26(7):1787–1795. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y. X., Ma Z. F., Huo N., et al. Porcine tooth germ cell conditioned medium can induce odontogenic differentiation of human dental pulp stem cells. 2011;5(5):354–362. doi: 10.1002/term.321. [DOI] [PubMed] [Google Scholar]
- 56.Zhang W., Walboomers X. F., Shi S., Fan M., Jansen J. A. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. 2006;12(10):2813–2823. doi: 10.1089/ten.2006.12.2813. [DOI] [PubMed] [Google Scholar]
- 57.Yan X., Qin H., Qu C., Tuan R. S., Shi S., Huang G. T. J. iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. 2010;19(4):469–480. doi: 10.1089/scd.2009.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamaoki N., Takahashi K., Tanaka T., et al. Dental pulp cells for induced pluripotent stem cell banking. 2010;89(8):773–778. doi: 10.1177/0022034510366846. [DOI] [PubMed] [Google Scholar]
- 59.Nordin N., Lai M. I., Veerakumarasivam A., et al. Induced pluripotent stem cells: history, properties and potential applications. 2011;66(1):4–9. [PubMed] [Google Scholar]
- 60.Malhotra N. Induced pluripotent stem (iPS) cells in dentistry: a review. 2016;9(2):176–185. doi: 10.15283/ijsc16029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gervois P., Struys T., Hilkens P., et al. Neurogenic maturation of human dental pulp stem cells following neurosphere generation induces morphological and electrophysiological characteristics of functional neurons. 2015;24(3):296–311. doi: 10.1089/scd.2014.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang J., Lian M., Cao P., et al. Effects of nerve growth factor and basic fibroblast growth factor promote human dental pulp stem cells to neural differentiation. 2017;42(4):1015–1025. doi: 10.1007/s11064-016-2134-3. [DOI] [PubMed] [Google Scholar]
- 63.Osathanon T., Sawangmake C., Nowwarote N., Pavasant P. Neurogenic differentiation of human dental pulp stem cells using different induction protocols. 2014;20(4):352–358. doi: 10.1111/odi.12119. [DOI] [PubMed] [Google Scholar]
- 64.Xiao L., Tsutsui T. Characterization of human dental pulp cells-derived spheroids in serum-free medium: stem cells in the core. 2013;114(11):2624–2636. doi: 10.1002/jcb.24610. [DOI] [PubMed] [Google Scholar]
- 65.Kanafi M., Majumdar D., Bhonde R., Gupta P., Datta I. Midbrain cues dictate differentiation of human dental pulp stem cells towards functional dopaminergic neurons. 2014;229(10):1369–1377. doi: 10.1002/jcp.24570. [DOI] [PubMed] [Google Scholar]
- 66.Chang C. C., Chang K. C., Tsai S. J., Chang H. H., Lin C. P. Neurogenic differentiation of dental pulp stem cells to neuron-like cells in dopaminergic and motor neuronal inductive media. 2014;113(12):956–965. doi: 10.1016/j.jfma.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Kiraly M., Kadar K., Horvathy D. B., et al. Integration of neuronally predifferentiated human dental pulp stem cells into rat brain in vivo. 2011;59(3):371–381. doi: 10.1016/j.neuint.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 68.Fatima N., Khan A. A., Vishwakarma S. K. Immunophenotypic and molecular analysis of human dental pulp stem cells potential for neurogenic differentiation. 2017;8(1):81–89. doi: 10.4103/ccd.ccd_998_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chun S. Y., Soker S., Jang Y. J., Kwon T. G., Yoo E. S. Differentiation of human dental pulp stem cells into dopaminergic neuron-like cells in vitro. 2016;31(2):171–177. doi: 10.3346/jkms.2016.31.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karbanova J., Soukup T., Suchanek J., Pytlik R., Corbeil D., Mokry J. Characterization of dental pulp stem cells from impacted third molars cultured in low serum-containing medium. 2011;193(6):344–365. doi: 10.1159/000321160. [DOI] [PubMed] [Google Scholar]
- 71.Lu Y., Yuan X., Ou Y., et al. Autophagy and apoptosis during adult adipose-derived stromal cells differentiation into neuron-like cells in vitro. 2012;7(16):1205–1212. doi: 10.3969/j.issn.1673-5374.2012.16.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gnanasegaran N., Govindasamy V., Kathirvaloo P., Musa S., Abu Kasim N. H. Effects of cell cycle phases on the induction of dental pulp stem cells toward dopaminergic-like cells. 2018;12(2):e881–e893. doi: 10.1002/term.2401. [DOI] [PubMed] [Google Scholar]
- 73.Singh M., Kakkar A., Sharma R., et al. Synergistic effect of BDNF and FGF2 in efficient generation of functional dopaminergic neurons from human mesenchymal stem cells. 2017;7(1, article 10378) doi: 10.1038/s41598-017-11028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gonmanee T., Thonabulsombat C., Vongsavan K., Sritanaudomchai H. Differentiation of stem cells from human deciduous and permanent teeth into spiral ganglion neuron-like cells. 2018;88:34–41. doi: 10.1016/j.archoralbio.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 75.Feng X., Lu X., Huang D., et al. 3D porous chitosan scaffolds suit survival and neural differentiation of dental pulp stem cells. 2014;34(6):859–870. doi: 10.1007/s10571-014-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ustiashvili M., Kordzaia D., Mamaladze M., Jangavadze M., Sanodze L. Investigation of functional activity human dental pulp stem cells at acute and chronic pulpitis. 2014;9(234):19–24. [PubMed] [Google Scholar]
- 77.Heng B. C., Lim L. W., Wu W., Zhang C. An overview of protocols for the neural induction of dental and oral stem cells in vitro. 2016;22(3):220–250. doi: 10.1089/ten.teb.2015.0488. [DOI] [PubMed] [Google Scholar]
- 78.Martens W., Sanen K., Georgiou M., et al. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. 2014;28(4):1634–1643. doi: 10.1096/fj.13-243980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Askari N., Yaghoobi M. M., Shamsara M., Esmaeili-Mahani S. Human dental pulp stem cells differentiate into oligodendrocyte progenitors using the expression of olig2 transcription factor. 2014;200(2):93–103. doi: 10.1159/000381668. [DOI] [PubMed] [Google Scholar]
- 80.Goorha S., Reiter L. T. Culturing and neuronal differentiation of human dental pulp stem cells. 2017;92:21.6.1–21.6.10. doi: 10.1002/cphg.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Y. H., Li J. E., Liu W. W. Comparing the effect of neurotrophic factor induced MSCs (BMSC and DPSC) on the expression of myelin proteins Nogo-A and OMgp in a glaucoma rat model. 2017;10(3):4705–4713. [Google Scholar]
- 82.Nosrat I. V., Widenfalk J., Olson L., Nosrat C. A. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. 2001;238(1):120–132. doi: 10.1006/dbio.2001.0400. [DOI] [PubMed] [Google Scholar]
- 83.Mead B., Logan A., Berry M., Leadbeater W., Scheven B. A. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. 2013;54(12):7544–7556. doi: 10.1167/iovs.13-13045. [DOI] [PubMed] [Google Scholar]
- 84.Arthur A., Shi S., Zannettino A. C. W., Fujii N., Gronthos S., Koblar S. A. Implanted adult human dental pulp stem cells induce endogenous axon guidance. 2009;27(9):2229–2237. doi: 10.1002/stem.138. [DOI] [PubMed] [Google Scholar]
- 85.Kolar M. K., Itte V. N., Kingham P. J., Novikov L. N., Wiberg M., Kelk P. The neurotrophic effects of different human dental mesenchymal stem cells. 2017;7(1, article 12605) doi: 10.1038/s41598-017-12969-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song M., Jue S. S., Cho Y. A., Kim E. C. Comparison of the effects of human dental pulp stem cells and human bone marrow-derived mesenchymal stem cells on ischemic human astrocytes in vitro. 2015;93(6):973–983. doi: 10.1002/jnr.23569. [DOI] [PubMed] [Google Scholar]
- 87.Song M., Lee J. H., Bae J., Bu Y., Kim E. C. Human dental pulp stem cells are more effective than human bone marrow-derived mesenchymal stem cells in cerebral ischemic injury. 2017;26(6):1001–1016. doi: 10.3727/096368916X694391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumar A., Kumar V., Rattan V., Jha V., Bhattacharyya S. Secretome cues modulate the neurogenic potential of bone marrow and dental stem cells. 2017;54(6):4672–4682. doi: 10.1007/s12035-016-0011-3. [DOI] [PubMed] [Google Scholar]
- 89.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. 2000;6(4):389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 90.Folkman J. Tumor angiogenesis: therapeutic implications. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 91.Giordano A., Galderisi U., Marino I. R. From the laboratory bench to the patient’s bedside: an update on clinical trials with mesenchymal stem cells. 2007;211(1):27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 92.Psaltis P. J., Zannettino A. C. W., Worthley S. G., Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. 2008;26(9):2201–2210. doi: 10.1634/stemcells.2008-0428. [DOI] [PubMed] [Google Scholar]
- 93.Sieveking D. P., Ng M. K. Cell therapies for therapeutic angiogenesis: back to the bench. 2009;14(2):153–166. doi: 10.1177/1358863X08098698. [DOI] [PubMed] [Google Scholar]
- 94.Hilkens P., Fanton Y., Martens W., et al. Pro-angiogenic impact of dental stem cells in vitro and in vivo. 2014;12(3):778–790. doi: 10.1016/j.scr.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 95.Bronckaers A., Hilkens P., Fanton Y., et al. Angiogenic properties of human dental pulp stem cells. 2013;8(8, article e71104) doi: 10.1371/journal.pone.0071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ratajczak J., Bronckaers A., Dillen Y., et al. The neurovascular properties of dental stem cells and their importance in dental tissue engineering. 2016;2016:17. doi: 10.1155/2016/9762871.9762871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tran-Hung L., Laurent P., Camps J., About I. Quantification of angiogenic growth factors released by human dental cells after injury. 2008;53(1):9–13. doi: 10.1016/j.archoralbio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 98.Aranha A. M. F., Zhang Z., Neiva K. G., Costa C. A. S., Hebling J., Nör J. E. Hypoxia enhances the angiogenic potential of human dental pulp cells. 2010;36(10):1633–1637. doi: 10.1016/j.joen.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 99.Iohara K., Zheng L., Wake H., et al. A novel stem cell source for vasculogenesis in ischemia: subfraction of side population cells from dental pulp. 2008;26(9):2408–2418. doi: 10.1634/stemcells.2008-0393. [DOI] [PubMed] [Google Scholar]
- 100.Tran-Hung L., Mathieu S., About I. Role of human pulp fibroblasts in angiogenesis. 2006;85(9):819–823. doi: 10.1177/154405910608500908. [DOI] [PubMed] [Google Scholar]
- 101.Gandia C., Armiñan A., García-Verdugo J. M., et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. 2008;26(3):638–645. doi: 10.1634/stemcells.2007-0484. [DOI] [PubMed] [Google Scholar]
- 102.Sugiyama M., Iohara K., Wakita H., et al. Dental pulp-derived CD31−/CD146− side population stem/progenitor cells enhance recovery of focal cerebral ischemia in rats. 2011;17(9-10):1303–1311. doi: 10.1089/ten.tea.2010.0306. [DOI] [PubMed] [Google Scholar]
- 103.Nam H., Kim G. H., Bae Y. K., et al. Angiogenic capacity of dental pulp stem cell regulated by SDF-1α-CXCR4 axis. 2017;2017:10. doi: 10.1155/2017/8085462.8085462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.d'Aquino R., Graziano A., Sampaolesi M., et al. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. 2007;14(6):1162–1171. doi: 10.1038/sj.cdd.4402121. [DOI] [PubMed] [Google Scholar]
- 105.Marchionni C., Bonsi L., Alviano F., et al. Angiogenic potential of human dental pulp stromal (stem) cells. 2009;22(3):699–706. doi: 10.1177/039463200902200315. [DOI] [PubMed] [Google Scholar]
- 106.Hilkens P., Bronckaers A., Ratajczak J., Gervois P., Wolfs E., Lambrichts I. The angiogenic potential of DPSCs and SCAPs in an in vivo model of dental pulp regeneration. 2017;2017:14. doi: 10.1155/2017/2582080.2582080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deuse T., Stubbendorff M., Tang-Quan K., et al. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. 2011;20(5):655–667. doi: 10.3727/096368910X536473. [DOI] [PubMed] [Google Scholar]
- 108.Kang J. W., Koo H. C., Hwang S. Y., et al. Immunomodulatory effects of human amniotic membrane-derived mesenchymal stem cells. 2012;13(1):23–31. doi: 10.4142/jvs.2012.13.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Comite P., Cobianchi L., Avanzini M. A., et al. Immunomodulatory properties of porcine, bone marrow-derived multipotent mesenchymal stromal cells and comparison with their human counterpart. 2011;57(2) Supplement [PubMed] [Google Scholar]
- 110.Soleymaninejadian E., Pramanik K., Samadian E. Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. 2012;67(1):1–8. doi: 10.1111/j.1600-0897.2011.01069.x. [DOI] [PubMed] [Google Scholar]
- 111.Pourgholaminejad A., Aghdami N., Baharvand H., Moazzeni S. M. The effect of pro-inflammatory cytokines on immunophenotype, differentiation capacity and immunomodulatory functions of human mesenchymal stem cells. 2016;85:51–60. doi: 10.1016/j.cyto.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 112.De Miguel M. P., Fuentes-Julián S., Blázquez-Martínez A., et al. Immunosuppressive properties of mesenchymal stem cells: advances and applications. 2012;12(5):574–591. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- 113.Rajput I. R., Hussain A., Li Y. L., et al. Saccharomyces boulardii and bacillus subtilis B10 modulate TLRs mediated signaling to induce immunity by chicken BMDCs. 2014;115(1):189–198. doi: 10.1002/jcb.24650. [DOI] [PubMed] [Google Scholar]
- 114.He W., Qu T., Yu Q., et al. LPS induces IL-8 expression through TLR4, MyD88, NF-kappaB and MAPK pathways in human dental pulp stem cells. 2013;46(2):128–136. doi: 10.1111/j.1365-2591.2012.02096.x. [DOI] [PubMed] [Google Scholar]
- 115.Heiman A., Pallottie A., Heary R. F., Elkabes S. Toll-like receptors in central nervous system injury and disease: a focus on the spinal cord. 2014;42:232–245. doi: 10.1016/j.bbi.2014.06.203. [DOI] [PubMed] [Google Scholar]
- 116.Guth L., Zhang Z., DiProspero N. A., Joubin K., Fitch M. T. Spinal cord injury in the rat: treatment with bacterial lipopolysaccharide and indomethacin enhances cellular repair and locomotor function. 1994;126(1):76–87. doi: 10.1006/exnr.1994.1043. [DOI] [PubMed] [Google Scholar]
- 117.Tomic S., Djokic J., Vasilijic S., et al. Immunomodulatory properties of mesenchymal stem cells derived from dental pulp and dental follicle are susceptible to activation by toll-like receptor agonists. 2011;20(4):695–708. doi: 10.1089/scd.2010.0145. [DOI] [PubMed] [Google Scholar]
- 118.Ozdemir A. T., Ozgul Ozdemir R. B., Kirmaz C., et al. The paracrine immunomodulatory interactions between the human dental pulp derived mesenchymal stem cells and CD4 T cell subsets. 2016;310:108–115. doi: 10.1016/j.cellimm.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 119.Sugita S., Kamao H., Iwasaki Y., et al. Inhibition of T-cell activation by retinal pigment epithelial cells derived from induced pluripotent stem cells. 2015;56(2):1051–1062. doi: 10.1167/iovs.14-15619. [DOI] [PubMed] [Google Scholar]
- 120.Kwack K. H., Lee J. M., Park S. H., Lee H. W. Human dental pulp stem cells suppress alloantigen-induced immunity by stimulating T cells to release transforming growth factor beta. 2017;43(1):100–108. doi: 10.1016/j.joen.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 121.Demircan P. C., Sariboyaci A. E., Unal Z. S., Gacar G., Subasi C., Karaoz E. Immunoregulatory effects of human dental pulp-derived stem cells on T cells: comparison of transwell co-culture and mixed lymphocyte reaction systems. 2011;13(10):1205–1220. doi: 10.3109/14653249.2011.605351. [DOI] [PubMed] [Google Scholar]
- 122.Pierdomenico L., Bonsi L., Calvitti M., et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. 2005;80(6):836–842. doi: 10.1097/01.tp.0000173794.72151.88. [DOI] [PubMed] [Google Scholar]
- 123.Zhao Y., Wang L., Jin Y., Shi S. Fas ligand regulates the immunomodulatory properties of dental pulp stem cells. 2012;91(10):948–954. doi: 10.1177/0022034512458690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Földes A., Kádár K., Kerémi B., et al. Mesenchymal stem cells of dental origin-their potential for antiinflammatory and regenerative actions in brain and gut damage. 2016;14(8):914–934. doi: 10.2174/1570159X14666160121115210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hong J. W., Lim J. H., Chung C. J., et al. Immune tolerance of human dental pulp-derived mesenchymal stem cells mediated by CD4+CD25+FoxP3+ regulatory T-Cells and induced by TGF-β1 and IL-10. 2017;58(5):1031–1039. doi: 10.3349/ymj.2017.58.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mead B., Logan A., Berry M., Leadbeater W., Scheven B. A. Concise review: dental pulp stem cells: a novel cell therapy for retinal and central nervous system repair. 2017;35(1):61–67. doi: 10.1002/stem.2398. [DOI] [PubMed] [Google Scholar]
- 127.Varga G., Gerber G. Mesenchymal stem cells of dental origin as promising tools for neuroregeneration. 2014;5(2):p. 61. doi: 10.1186/scrt450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schwab M. E. Myelin-associated inhibitors of neurite growth and regeneration in the CNS. 1990;13(11):452–456. doi: 10.1016/0166-2236(90)90098-U. [DOI] [PubMed] [Google Scholar]
- 129.Stichel C. C., Muller H. W. The CNS lesion scar: new vistas on an old regeneration barrier. 1998;294(1):1–9. doi: 10.1007/s004410051151. [DOI] [PubMed] [Google Scholar]
- 130.Gervois P., Wolfs E., Dillen Y., et al. Paracrine maturation and migration of SH-SY5Y cells by dental pulp stem cells. 2017;96(6):654–662. doi: 10.1177/0022034517690491. [DOI] [PubMed] [Google Scholar]
- 131.Khazaeipour Z., Norouzi-Javidan A., Kaveh M., Khanzadeh Mehrabani F., Kazazi E., Emami-Razavi S. H. Psychosocial outcomes following spinal cord injury in Iran. 2014;37(3):338–345. doi: 10.1179/2045772313Y.0000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huang H., Chen H.-Q., Gul J., Yul R.-H. Comparative study of hyperbaric oxygen therapy and conventional drug treatment on spinal cord injury at different therapeutic windows. 2011;6(5):1117–1122. [Google Scholar]
- 133.Jiang Y., Gong F. L., Zhao G. B., Li J. Chrysin suppressed inflammatory responses and the inducible nitric oxide synthase pathway after spinal cord injury in rats. 2014;15(7):12270–12279. doi: 10.3390/ijms150712270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yamamoto A., Matsubara K., Kano F., Sakai K. Analysis of the neuroregenerative activities of mesenchymal stem cells in functional recovery after rat spinal cord injury. 2014;1213:321–328. doi: 10.1007/978-1-4939-1453-1_26. [DOI] [PubMed] [Google Scholar]
- 135.Yamamoto A., Sakai K., Matsubara K., Kano F., Ueda M. Multifaceted neuro-regenerative activities of human dental pulp stem cells for functional recovery after spinal cord injury. 2014;78:16–20. doi: 10.1016/j.neures.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 136.Yang C., Li X., Sun L., Guo W., Tian W. Potential of human dental stem cells in repairing the complete transection of rat spinal cord. 2017;14(2, article 026005) doi: 10.1088/1741-2552/aa596b. [DOI] [PubMed] [Google Scholar]
- 137.Zhang J., Lu X., Feng G., et al. Chitosan scaffolds induce human dental pulp stem cells to neural differentiation: potential roles for spinal cord injury therapy. 2016;366(1):129–142. doi: 10.1007/s00441-016-2402-1. [DOI] [PubMed] [Google Scholar]
- 138.Sughrue M. E., Mehra A., Connolly E. S., Jr, D’Ambrosio A. L. Anti-adhesion molecule strategies as potential neuroprotective agents in cerebral ischemia: a critical review of the literature. 2004;53(10):497–508. doi: 10.1007/s00011-004-1282-0. [DOI] [PubMed] [Google Scholar]
- 139.Hossmann K. A. Pathophysiology and therapy of experimental stroke. 2006;26(7-8):1055–1081. doi: 10.1007/s10571-006-9008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Leong W. K., Lewis M. D., Koblar S. A. Concise review: preclinical studies on human cell-based therapy in rodent ischemic stroke models: where are we now after a decade? 2013;31(6):1040–1043. doi: 10.1002/stem.1348. [DOI] [PubMed] [Google Scholar]
- 141.Lemmens R., Steinberg G. K. Stem cell therapy for acute cerebral injury: what do we know and what will the future bring? 2013;26(6):617–625. doi: 10.1097/WCO.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang K. L., Chen M. F., Liao C. H., Pang C. Y., Lin P. Y. A simple and efficient method for generating Nurr1-positive neuronal stem cells from human wisdom teeth (tNSC) and the potential of tNSC for stroke therapy. 2009;11(5):606–617. doi: 10.1080/14653240902806994. [DOI] [PubMed] [Google Scholar]
- 143.Leong W. K., Henshall T. L., Arthur A., et al. Human adult dental pulp stem cells enhance poststroke functional recovery through non-neural replacement mechanisms. 2012;1(3):177–187. doi: 10.5966/sctm.2011-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dauer W., Przedborski S. Parkinson’s disease: mechanisms and models. 2003;39(6):889–909. doi: 10.1016/S0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 145.Wang Y., Chen S., Yang D., Le W. D. Stem cell transplantation: a promising therapy for Parkinson’s disease. 2007;2(3):243–250. doi: 10.1007/s11481-007-9074-2. [DOI] [PubMed] [Google Scholar]
- 146.Gnanasegaran N., Govindasamy V., Simon C., et al. Effect of dental pulp stem cells in MPTP-induced old-aged mice model. 2017;47(6):403–414. doi: 10.1111/eci.12753. [DOI] [PubMed] [Google Scholar]
- 147.Gnanasegaran N., Govindasamy V., Mani V., Abu Kasim N. H. Neuroimmunomodulatory properties of DPSCs in an in vitro model of Parkinson’s disease. 2017;69(9):689–699. doi: 10.1002/iub.1655. [DOI] [PubMed] [Google Scholar]
- 148.Fujii H., Matsubara K., Sakai K., et al. Dopaminergic differentiation of stem cells from human deciduous teeth and their therapeutic benefits for Parkinsonian rats. 2015;1613:59–72. doi: 10.1016/j.brainres.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 149.Citron M. Alzheimer’s disease: strategies for disease modification. 2010;9(5):387–398. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]
- 150.Huang Y., Mucke L. Alzheimer mechanisms and therapeutic strategies. 2012;148(6):1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li M., Guo K., Ikehara S. Stem cell treatment for Alzheimer’s disease. 2014;15(10):19226–19238. doi: 10.3390/ijms151019226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shin J. Y., Park H. J., Kim H. N., et al. Mesenchymal stem cells enhance autophagy and increase β-amyloid clearance in Alzheimer disease models. 2014;10(1):32–44. doi: 10.4161/auto.26508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Apel C., Forlenza O. V., de Paula V. J. R., et al. The neuroprotective effect of dental pulp cells in models of Alzheimer’s and Parkinson’s disease. 2009;116(1):71–78. doi: 10.1007/s00702-008-0135-3. [DOI] [PubMed] [Google Scholar]
- 154.Wang F., Jia Y., Liu J., et al. Dental pulp stem cells promote regeneration of damaged neuron cells on the cellular model of Alzheimer’s disease. 2017;41(6):639–650. doi: 10.1002/cbin.10767. [DOI] [PubMed] [Google Scholar]
- 155.Ahmed N. E.-M. B., Murakami M., Hirose Y., Nakashima M. Therapeutic potential of dental pulp stem cell secretome for Alzheimer’s disease treatment: an in vitro study. 2016;2016:11. doi: 10.1155/2016/8102478.8102478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mita T., Furukawa-Hibi Y., Takeuchi H., et al. Conditioned medium from the stem cells of human dental pulp improves cognitive function in a mouse model of Alzheimer’s disease. 2015;293:189–197. doi: 10.1016/j.bbr.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 157.Reh T. A., Fischer A. J. Retinal stem cells. 2006;419:52–73. doi: 10.1016/S0076-6879(06)19003-5. [DOI] [PubMed] [Google Scholar]
- 158.Munemasa Y., Kitaoka Y. Autophagy in axonal degeneration in glaucomatous optic neuropathy. 2015;47:1–18. doi: 10.1016/j.preteyeres.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 159.Richardson P. M., McGuinness U. M., Aguayo A. J. Axons from CNS neurones regenerate into PNS grafts. 1980;284(5753):264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- 160.Mead B., Hill L. J., Blanch R. J., et al. Mesenchymal stromal cell–mediated neuroprotection and functional preservation of retinal ganglion cells in a rodent model of glaucoma. 2016;18(4):487–496. doi: 10.1016/j.jcyt.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 161.Bray A. F., Cevallos R. R., Gazarian K., Lamas M. Human dental pulp stem cells respond to cues from the rat retina and differentiate to express the retinal neuronal marker rhodopsin. 2014;280:142–155. doi: 10.1016/j.neuroscience.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 162.Roozafzoon R., Lashay A., Vasei M., et al. Dental pulp stem cells differentiation into retinal ganglion-like cells in a three dimensional network. 2015;457(2):154–160. doi: 10.1016/j.bbrc.2014.12.069. [DOI] [PubMed] [Google Scholar]
- 163.Battiston B., Geuna S., Ferrero M., Tos P. Nerve repair by means of tubulization: literature review and personal clinical experience comparing biological and synthetic conduits for sensory nerve repair. 2005;25(4):258–267. doi: 10.1002/micr.20127. [DOI] [PubMed] [Google Scholar]
- 164.Matsuyama T., Mackay M., Midha R. Peripheral nerve repair and grafting techniques: a review. 2000;40(4):187–199. doi: 10.2176/nmc.40.187. [DOI] [PubMed] [Google Scholar]
- 165.Pfister B. J., Gordon T., Loverde J. R., Kochar A. S., Mackinnon S. E., Cullen D. K. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. 2011;39(2):81–124. doi: 10.1615/CritRevBiomedEng.v39.i2.20. [DOI] [PubMed] [Google Scholar]
- 166.Tamaki T., Hirata M., Nakajima N., et al. A long-gap peripheral nerve injury therapy using human skeletal muscle-derived stem cells (Sk-SCs): an achievement of significant morphological, numerical and functional recovery. 2016;11(11, article e0166639) doi: 10.1371/journal.pone.0166639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pertici V., Laurin J., Feron F., Marqueste T., Decherchi P. Functional recovery after repair of peroneal nerve gap using different collagen conduits. 2014;156(5):1029–1040. doi: 10.1007/s00701-014-2009-9. [DOI] [PubMed] [Google Scholar]
- 168.Lee S. K., Wolfe S. W. Peripheral nerve injury and repair. 2000;8(4):243–252. doi: 10.5435/00124635-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 169.Alluin O., Wittmann C., Marqueste T., et al. Functional recovery after peripheral nerve injury and implantation of a collagen guide. 2009;30(3):363–373. doi: 10.1016/j.biomaterials.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 170.Dai L. G., Huang G. S., Hsu S. H. Sciatic nerve regeneration by cocultured Schwann cells and stem cells on microporous nerve conduits. 2013;22(11):2029–2039. doi: 10.3727/096368912X658953. [DOI] [PubMed] [Google Scholar]
- 171.Faroni A., Mobasseri S. A., Kingham P. J., Reid A. J. Peripheral nerve regeneration: experimental strategies and future perspectives. 2015;82-83:160–167. doi: 10.1016/j.addr.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 172.Gärtner A., Amorim I., Almeida A., et al. Promoting nerve regeneration in a neurotmesis rat model using poly(DL-lactide-ɛ-caprolactone) membranes and mesenchymal stem cells from the Wharton’s jelly: in vitro and in vivo analysis. 2014;2014:17. doi: 10.1155/2014/302659.302659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sasaki R., Aoki S., Yamato M., et al. PLGA artificial nerve conduits with dental pulp cells promote facial nerve regeneration. 2011;5(10):823–830. doi: 10.1002/term.387. [DOI] [PubMed] [Google Scholar]
- 174.Sanen K., Martens W., Georgiou M., Ameloot M., Lambrichts I., Phillips J. Engineered neural tissue with Schwann cell differentiated human dental pulp stem cells: potential for peripheral nerve repair? 2017;11(12):3362–3372. doi: 10.1002/term.2249. [DOI] [PubMed] [Google Scholar]
- 175.Askari N., Yaghoobi M. M., Shamsara M., Esmaeili-Mahani S. Tetracycline-regulated expression of OLIG2 gene in human dental pulp stem cells lead to mouse sciatic nerve regeneration upon transplantation. 2015;305:197–208. doi: 10.1016/j.neuroscience.2015.07.088. [DOI] [PubMed] [Google Scholar]
- 176.Omi M., Hata M., Nakamura N., et al. Transplantation of dental pulp stem cells suppressed inflammation in sciatic nerves by promoting macrophage polarization towards anti-inflammation phenotypes and ameliorated diabetic polyneuropathy. 2016;7(4):485–496. doi: 10.1111/jdi.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]