Abstract
Studies in the fruit fly Drosophila melanogaster have provided many fundamental insights into the genetic regulation of neural development, including the identification and characterization of evolutionarily conserved axon guidance pathways and their roles in important guidance decisions. Due to its highly organized and fast-developing embryonic nervous system, relatively small number of neurons, and molecular and genetic tools for identifying, labeling, and manipulating individual neurons or small neuronal subsets, studies of axon guidance in the Drosophila embryonic CNS have allowed researchers to dissect these genetic mechanisms with a high degree of precision. In this review, we discuss the major axon guidance pathways that regulate midline crossing of axons and the formation and guidance of longitudinal axon tracts, two processes that contribute to the development of the precise three-dimensional structure of the insect nerve cord. We focus particularly on recent insights into the roles and regulation of canonical midline axon guidance pathways, and on additional factors and pathways that have recently been shown to contribute to axon guidance decisions at and near the midline.
Keywords: Axon guidance, Drosophila, midline, Slit, Robo, Netrin, Frazzled
1. Introduction
During embryonic development, the nervous system must construct itself by orchestrating not only the specification of numerous types of neurons, but also the ways in which membrane processes from these neurons (axons and dendrites) connect to each other and to non-neural cells. Though relatively simple by comparison with more sophisticated nervous systems such as those of vertebrates, the larval nervous system in the fruit fly Drosophila melanogaster must nonetheless allow the newly-hatched animal to crawl freely while seeking food and avoiding predators and nociceptive stimuli. This requires the same underlying neural and muscular processes as more complex behaviors in vertebrate animals, including detecting, processing, and integrating sensory information from a number of sensory modalities (touch, temperature, light, smell) and responding with coordinated muscle contraction [1,2].
Like its eventual adult form, the Drosophila larva has a segmented body plan. This extends to the embryonic central nervous system (CNS), which exhibits a segmentally-repeated pattern of bilaterally symmetric neuromeres (or segmental ganglia) in the ventral nerve cord (VNC, analogous to the dorsal spinal cord in vertebrates) (Figure 1A). Each abdominal hemisegment contains the same set of approximately 300 neurons (36 motor neurons plus around 270 uniquely identifiable interneurons) [3,4], each of which make stereotyped and reproducible axon guidance decisions from segment to segment and animal to animal (Figure 1B). This makes it possible to examine the same identifiable neurons facing the same guidance decisions in multiple segments and multiple animals across different genetic backgrounds, which greatly facilitates the quantitative examination of individual developmental outcomes.
Figure 1. Structure of the Drosophila embryonic CNS, and identifiable subsets of neurons.
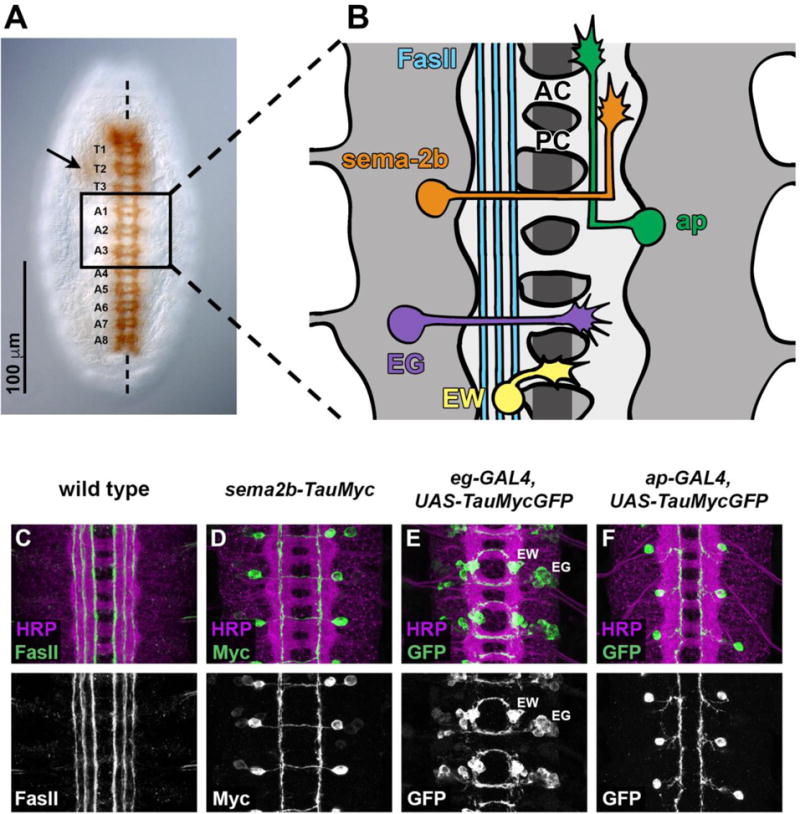
(A) Ventral view of a mature Drosophila embryo, stained with the monoclonal antibody BP102, which labels all of the CNS axons. The segmentally-repeated pattern of ganglia in the ventral nerve cord is apparent. T1–T3 indicate thoracic segments; A1–A8 indicate abdominal segments. Arrow indicates position of the brain, which is located more dorsally and out of the focal plane. Dashed vertical line indicates the midline.
(B) Schematic of three adjacent segments from the abdominal ventral nerve cord, illustrating identifiable neuronal subsets and their axon projection patterns. Anterior (AC) and posterior (PC) commissures are labeled in the top segment. FasII-positive axon pathways (blue) consist of ipsilateral and post-crossing commissural axons, and form in discrete medial, intermediate, and lateral zones within the neuropile. A subset of commissural neurons is labeled by the sema2b-TauMyc reporter transgene (orange); their axons cross the midline in the anterior commissure and form a longitudinal pathway in the intermediate region. Two subsets of commissural neurons and their axons are labeled by the eg-GAL4 reporter transgene: the EG axons (purple) cross the midline in the anterior commissure and the EW axons (yellow) cross the midline in the posterior commissure. A subset of ipsilateral neurons is labeled by the ap-GAL4 reporter transgene (green); their axons project towards the midline, then turn without crossing and project anteriorly in the medial region of the neuropile.
(C–F) Confocal micrographs of wild type (A) or transgenic (C–F) embryos stained with the indicated antibodies. Anti-HRP (magenta) labels all CNS axons; anti-FasII (green in C) labels FasII-positive longitudinal pathways; anti-Myc (green in D) or anti-GFP (green in E,F) labels the cell bodies and axons of transgenically-labeled neurons carrying each of the transgenes described above.
The fruit fly has long been an attractive model system for studies in genetics and molecular biology, and the adoption of genetic screening methods for studying developmental biology in the 1970s and 1980s [5] allowed developmental neuroscientists to apply this powerful technique to the study of axon guidance [6,7]. Because of the relatively simple and stereotyped structure of the embryonic nervous system, the fast developmental time, ease of culture, large number of offspring, and powerful genetic tools, many fundamental insights into basic axon guidance mechanisms and evolutionarily conserved molecules and pathways have been achieved using the fly embryonic CNS as a model [8,9]. One of the most fundamental of axon guidance decisions, and one that many axons in the CNS of bilaterian animals must face, is whether or not to cross the midline. This decision has important consequences for integration of sensory cues, information processing, and neuromuscular coordination between the two sides of the body, and misregulation of midline crossing during development can lead to a number of neurological disorders in humans [10–12].
Compared to vertebrate systems, a smaller number of signaling pathways regulate guidance at and around the midline in insects, but these core signaling pathways are evolutionarily conserved in other animals including humans [13]. This has contributed to the relative ease in which genetic studies can be carried out in flies, where loss-of-function phenotypes are often stronger and more straightforward to interpret thanks to a relative lack of genetic redundancy compared to vertebrate genomes. In addition, a number of subset-specific antibodies and genetic tools for labeling subsets of commissural (midline-crossing) or ipsilateral (non-midline-crossing) neurons and their axons (such as TauMyc and GAL4/UAS transgenic reporter lines) allow precise, reproducible, and quantitative examination of midline crossing and axon pathway formation in the Drosophila embryonic CNS (Figure 1).
In this review, we discuss our current understanding of the signaling pathways and molecular mechanisms that regulate axon guidance in the Drosophila embryonic CNS, focusing on two key guidance decisions that are essential for assembling the precise three-dimensional structure of the insect ventral nerve cord: midline crossing and longitudinal pathway formation. We discuss recent insights into how axon guidance receptors signal in response to their respective ligands, ligand-dependent versus -independent guidance decisions and signaling mechanisms, and the regulation of and crosstalk between major attractive and repulsive signaling pathways. A list of guidance pathway components and their proposed roles is compiled in Table 1.
Table 1.
Components of axon guidance pathways involved in midline crossing, midline repulsion, commissure choice, and guidance of longitudinal axons in the Drosophila embryonic CNS.
| Protein | Role(s) | Pathway or cognate ligand/receptor | References |
|---|---|---|---|
| Ligands | Cognate receptor(s) | ||
| Amalgam (Ama) | midline crossing | Nrt | Liebl et al. [164] |
| Hedgehog (Hh) | midline crossing | Ptc | Ricolo et al. [66] |
| Netrin A (NetA) | midline crossing, longitudinal axon guidance | Fra, other (unknown) | Mitchell et al. [26], Harris et al. [25], Hiramoto et al. [160] |
| Netrin B (NetB) | midline crossing, longitudinal axon guidance | Fra, Dscam | Mitchell et al. [26], Harris et al. [25], Hiramoto et al. [160], Andrews et al. [45] |
| Sema-1a | lateral pathway formation, D/V patterning | PlexA | Yu et al. [76], Zlatic et al. [81] |
| Sema-2a | intermediate pathway formation, midline crossing, D/V patterning | PlexB, Sema-1a | Wu et al. [80], Hernandez-Fleming et al. [84], Zlatic et al. [81] |
| Sema-2b | intermediate pathway formation, midline crossing | PlexB, Sema-1a | Wu et al. [80], Hernandez-Fleming et al. [84], Zlatic et al. [81] |
| Slit | midline repulsion, longitudinal axon growth, longitudinal pathway formation | Robo1, Robo2, Robo3, Dscam | Kidd et al. [98], Brose et al. [87], Hiramoto et al. [161], Alavi et al. [56] |
| Turtle (Tutl) | midline crossing | unknown | Bodily et al [48], Al-Anzi et al [47] |
| Wnt5 | commissure choice | Drl | Yoshikawa et al [144] |
| Receptors | Cognate ligand(s) | ||
| Derailed (Drl) | commissure choice | Wnt5 | Callahan et al [145] |
| Down syndrome cell adhesion molecule (Dscam) | midline crossing, longitudinal axon growth | SlitN, NetB | Andrews et al. [45], Alavi et al. [56] |
| Flamingo (Fmi) | midline crossing | unknown | Organisti et al. [42] |
| Frazzled (Fra) | midline crossing, comm transcription, longitudinal axon guidance (Net redistribution) | NetA, NetB, other (unknown) | Kolodziej et al. [23], Yang et al. [36], Hiramoto et al. [160] |
| Neurotactin (Nrt) | midline crossing | Ama | Liebl et al. [164] |
| Patched (Ptc) | midline crossing | Hh | Ricolo et al. [66] |
| Plexin A (PlexA) | lateral pathway formation, D/V patterning | Sema-1a | Winberg et al. [77], Zlatic et al. [81] |
| Plexin B (PlexB) | intermediate pathway formation, D/V patterning | Sema-2a, Sema-2b | Ayoob et al. [79], Zlatic et al. [81] |
| Robo1 | midline repulsion, longitudinal axon growth | Slit | Kidd et al. [86], Hiramoto et al. [161], Alavi et al. [56] |
| Robo2 | midline repulsion, lateral pathway formation | Slit | Simpson et al. [99], Simpson et al. [102], Rajagopalan et al. [100], Rajagopalan et al. [103] |
| Robo3 | intermediate pathway formation | Slit | Simpson et al. [102], Rajagopalan et al. [103] |
| Sema-1a | midline crossing | Sema-2a, Sema-2b | Hernandez-Fleming et al. [84] |
| Downstream effectors and pathway regulators | Associated pathway(s) | ||
| Abelson tyrosine kinase (Abl) | midline crossing | Net-Fra, Slit-Robo, Ama-Nrt | Bashaw et al. [101], Forsthoefel et al. [33], O’Donnell et al. [35] |
| Beta-spectrin | midline repulsion | Slit-Robo | Garbe et al. [153] |
| Calmodulin (CaM) | midline crossing | unknown | Hsouna et al. [165] |
| Canoe (Cno) | midline repulsion | Slit-Robo | Slovakova et al. [132] |
| Commissureless (Comm) | midline crossing | Slit-Robo, Fra (Net-independent) | Kidd et al. [119], Keleman et al. [120,121], Gilestro et al. [122], Yang et al. [36] |
| Dally-like protein (Dlp) | midline repulsion | Slit-Robo | Johnson et al. [118] |
| Dreadlocks (Dock) | midline repulsion | Slit-Robo | Fan et al. [137] |
| Enabled (Ena) | midline crossing, midline repulsion | Net-Fra, Slit-Robo | Bashaw et al. [101], Forsthoefel et al. [33] |
| GEF64C | midline crossing | unknown | Bashaw et al. [166] |
| Kuzbanian (Kuz) | midline repulsion | Slit-Robo | Coleman et al. [135] |
| Mummy (Mmy) | midline repulsion | Slit-Robo | Manavalan et al. [114] |
| Mushroom body defect (Mud) | midline crossing | Frizzled | Cate et al. [43] |
| Myosin II | midline crossing | Net-Fra | Dorsten et al. [167] |
| Neurexin IV (Nrx IV) | midline repulsion | Slit-Robo | Banerjee et al. [168] |
| Pak | midline repulsion | Slit-Robo | Fan et al. [137] |
| Presenilin (Psn) | midline attraction | Fra (Net-independent) | Neuhaus-Follini et al. [37] |
| RhoGAPp190 | Sema2-Sema1a | Hernandez-Fleming et al. [84] | |
| Robo2 | midline crossing | Slit-Robo | Spitzweck et al. [44], Evans et al. [104,125] |
| RPTP10D | midline repulsion | Slit-Robo | Sun et al. [134] |
| RPTP69D | midline repulsion | Slit-Robo | Sun et al. [134] |
| Son of sevenless (Sos) | midline repulsion | Slit-Robo | Yang et al. [138] |
| Src42A | midline crossing, inhibition of midline crossing, commissure choice | Hh, Wnt5-Drl, other (unknown) | Ricolo et al. [66], Wouda et al [149], O’Donnell et al. [169] |
| Src64B | inhibition of midline crossing, commissure choice | Wnt5-Drl, unknown | Wouda et al [149], O’Donnell et al. [169] |
| Syndecan (Sdc) | midline crossing | Slit-Robo | Johnson et al. [118], Steigemann et al. [117] |
| Trio | midline crossing | Net-Fra, other (unknown) | Forsthoefel et al. [33] |
| Vilse/CrGAP | midline repulsion | Slit-Robo | Hu et al. [170], Lundstrom et al. [171] |
2. Midline attraction
The majority of neuronal axons in the Drosophila embryonic CNS are commissural; that is, they cross the midline to innervate synaptic targets on the opposite (contralateral) side of the body [3]. A key early step in axon guidance for commissural neurons is for their axons to orient toward, then grow toward and across the midline. The midline thus represents a key organizing center for embryonic axon guidance, and specialized glial cells located at the midline (known as midline glia) produce both attractive and repulsive signaling cues which promote or inhibit midline crossing of pathfinding axons, respectively [13,14]. Axons approaching, crossing, then leaving the midline must control their responses to various attractive and repulsive cues with a high degree of temporal precision, to ensure that once a commissural axon enters the midline, it will continue across and exit before proceeding on the next leg of its journey, never to re-cross. Non-crossing, or ipsilateral, axons must either ignore attractive cues or respond preferentially to repulsive cues, keeping them on the same side of the body as they originated.
2.1. Netrin-Frazzled/DCC-mediated midline attraction
Midline attraction and midline crossing of axons in Drosophila is influenced by a number of molecules and signaling pathways; chief among these is the Netrin-Frazzled/DCC pathway, which is evolutionarily conserved across bilaterian animals and promotes midline crossing of axons in a wide variety of animal groups [15–26]. In Drosophila, two functionally redundant Netrin ligands (NetA and NetB) signal through their receptor Frazzled (Fra, a member of the DCC/Deleted in Colorectal Cancer family) to promote midline crossing [23,25,26]. Initial models considered Netrins as long-range chemoattractants, secreted by and diffusing away from midline cells to attract commissural axons toward the midline at a distance. However, this model has been contradicted by evidence from both Drosophila and vertebrates, suggesting that Netrins may instead act as short-range or contact-dependent cues. In Drosophila, tethering Netrin to the cell membrane does not block its function in commissure formation, suggesting that it does not need to diffuse from the cells producing it at the midline [27]. Even in embryos lacking both Netrin genes, commissural axons are able to orient toward the midline. This indicates that Netrin acts as a short-range permissive cue to promote growth across the midline in Drosophila, rather than a long-range chemoattractant to draw commissural axons toward the midline [27]. Also in Drosophila, Net and Fra regulate axon-target adhesion in R8 photoreceptor axons, supporting a role for local Net-Fra signaling in contexts outside of the embryonic CNS [28]. Recent data from studies in mouse embryos suggest that mammalian Netrin-1 likewise may not act as a long-range chemoattractant, but rather may promote midline crossing through local contact-dependent adhesion [29–31]. A study of cell death in Netrin mutants in Drosophila suggests that NetB also acts as a neurotrophic factor, promoting neuronal survival in addition to midline attraction, and that blocking apoptosis in neurons can rescue midline crossing defects seen in NetAB mutants [32].
Abelson tyrosine kinase (Abl) and the guanine nucleotide-exchange factor Trio both influence axon pathfinding at the Drosophila embryonic CNS midline, and appear to act at least in part downstream of Netrin and Frazzled [33–35]. Abl and Trio physically interact with the cytoplasmic domain of Fra. An increase in Abl expression in cells increases tyrosine phosphorylation of Fra and Trio. This indicates that Abl, Trio and Fra function together in commissure formation. It can also be resolved that Abl and Trio function downstream of at least one other receptor that has a pro-crossing role in regulating commissure formation [33].
2.2. Netrin-independent transcriptional regulation by Frazzled
In addition to acting as a Netrin receptor to mediate midline attractive signaling, Drosophila Fra also functions as a transcriptional activator to regulate expression of commisureless (comm), a key modulator of axonal responsiveness at the midline [36]. Proteolysis of Fra in response to an uncharacterized but Netrin-independent signal releases the Fra intracellular domain (ICD), which moves to the nucleus and is sufficient to activate comm transcription and promote midline crossing. Fra regulation of comm expression relies on the conserved P3 motif within the Fra ICD, which functions as a transcriptional activator [37] and has also been implicated in attractive signaling by Fra [38]. The Fra ortholog in mosquito (Aedes aegypti) is also required for midline crossing, and promotes transcription of the comm2 gene, suggesting that Frazzled’s dual role in attractive signaling and transcriptional activation is evolutionarily conserved, at least within a subset of insects [22,39]. Vertebrate orthologs of Fra (DCC and Neogenin) can also regulate transcription via nuclear localization of their ICDs [40,41], although the in vivo transcriptional targets of these receptors have not yet been identified, and comm orthologs do not appear to be present in vertebrates.
2.3. Signaling pathways acting in parallel to Net-Fra to promote midline crossing
Although the Net-Fra pathway appears to be the major midline attractive pathway in Drosophila, many commissural axons cross the midline in the absence of Net-Fra signaling [23,25,26], suggesting the presence of additional pathways which can promote midline crossing independently of Net or Fra. Mutations in Abl or trio enhance midline crossing defects in a fra null mutant, indicating that they function in a parallel pathway in addition to any role they might have downstream of fra [33]. Similarly, mutations in a number of other genes result in a commissureless or near-commissureless phenotype when introduced into NetAB or fra mutants; notably, most of these mutations cause little or no detectable defects in midline crossing on their own, suggesting that they may act redundantly to Net-Fra signaling to promote midline crossing (or that their individual mutant phenotypes are too mild to detect) [42–45].
The transmembrane cadherin flamingo (Fmi) can mediate both a homophilic and heterophilic cell–cell interaction, as well as transmit signals on the interior of the cell. The Fmi extracellular domain has been shown to be essential for promoting cell adhesion in vitro. Although fmi single mutants do not display any midline crossing defects, mutations in fmi strongly enhance both the fra and NetAB mutant phenotypes [42]. Restoring expression of Fmi broadly in neurons could rescue this enhancement, but not restoring Fmi in a restricted set of commissural neurons. The intracellular domain of Fmi was also required for rescue, suggesting that Fmi must interact with cytoplasmic components for proper attractive function, perhaps by mediating cell-cell adhesion in commissural neurons [42].
The microtubule-binding protein Mushroom body defect (Mud) is expressed in post-mitotic neurons of the ventral nerve cord throughout embryogenesis. Mud is able to regulate the orientation of microtubule-based spindle fiber formation, and its function in neurons may be to connect information about polarity with actin motor complexes in order to orient microtubule structures within neurons to encourage directed growth [43]. Deletion of mud alone has little or no effect on midline crossing, but strongly enhances midline crossing defects of NetAB mutants. Mud has been associated with signaling downstream of Frizzled (Fz) [46], and genetic interaction experiments suggest that Fz and Mud may function in the same pathway to promote midline crossing, parallel to Net and Fra, and could cooperate with Fmi [42,43].
The immunoglobulin (Ig) superfamily gene turtle (tutl, also known as Dasm1) encodes multiple transmembrane and secreted Tutl isoforms expressed throughout the developing embryonic CNS, in both neurons and glial cells [47,48]. tutl mutants display a number of axon guidance phenotypes, including gaps in the longitudinal connectives, thin or absent commissures, and ectopic midline crossing of axons [47]. Loss of tutl dominantly enhances NetAB midline crossing phenotypes, indicating that it promotes midline crossing independently of Netrins. No genetic interactions were detected between tutl and either slit or robo1, suggesting that Tutl is unlikely to act as a direct inhibitor of Slit-Robo repulsion to promote midline crossing [47]. Secreted and transmembrane isoforms of Tutl were able to rescue midline crossing defects in tutl mutants when expressed in neurons or midline glia, consistent with a non-autonomous role for Tutl in axon guidance, perhaps as a ligand for an unknown cognate receptor [47]. Outside of the CNS, Tutl has been proposed to regulate photoreceptor axon targeting via homophilic cell-cell interactions [49], and to interact with the transmembrane Ig protein Borderless (Bdl) to promote glial ensheathment of photoreceptor axons [50]. Whether these mechanisms might also account for Tutl’s influence on midline crossing in the embryonic CNS remains to be determined.
Down syndrome cell adhesion molecule (Dscam) in Drosophila is a contact-dependent homophilic cell repulsive molecule with thousands of alternatively spliced isoforms [51–55]. Dscam is involved in axon guidance and targeting, segregation of axon branches, and dendritic patterning [51,56–60]. Dscam has been shown to bind Netrin proteins in an evolutionarily conserved manner, with similar affinity to Net-Fra binding [45,61,62]. It is suggested that Dscam acts in vivo as a required receptor facilitating attraction to Net. However, there is a dramatic reduction in number of midline crossing axons in Dscam/fra double mutants significantly greater than seen in net mutants. This suggests that Dscam most likely participates in Net-dependent and independent pathways, both participating in pro-crossing roles [45]. Dscam also plays a role in longitudinal axon guidance in the fly embryonic CNS, where it appears to cooperate with Robo1 in response to Slit (see section 5.3 below).
Drosophila Hedgehog (Hh) is a classical morphogen that is required for the specification of the midline glia, the cells that commissural axons grow towards [63]. In vertebrates, the Hh ortholog Sonic Hedgehog (Shh) has been identified as a midline-derived chemoattractant for commissural axons that acts in parallel to Netrin to promote midline crossing [64]. Drosophila hh mutants show strong CNS patterning defects, likely due to its role in cell fate specification [65]. Ricolo et al [66]used a temperature-sensitive allele of hh to show that removing hh function specifically in later embryos (after its patterning role has been fulfilled) resulted in defective commissural axon guidance, suggesting that hh also plays a role in midline crossing in the fly embryo. Hh is produced at the Drosophila CNS midline at low levels, and the Hh receptors Patched (Ptc) and Smoothened (Smo) are also found localized to commissural axons. Gain of function experiments suggested that Hh can attract commissural axons in Drosophila, acting in parallel to the Net-Fra pathway and likely through a non-canonical signaling pathway involving Src42A [66].
Semaphorin (Sema) family proteins are one of the four “classical” axon guidance ligands (along with Netrins, Slits, and Ephrins) [67,68]. Semaphorins are multifunctional axon guidance proteins, in some contexts act as ligands to signal through their canonical Plexin and Neuropilin family receptors, and in other contexts can act as receptors in response to Plexins or other Semaphorins as ligands (reverse signaling) [68]. Mammalian Sema-3A, signaling through Plexin-A1 and Neuropilin-2, has been shown regulate midline crossing in the vertebrate spinal cord, primarily in the context of repulsion of post-commissural axons [69,70]. In Drosophila, Semas have mostly been known for their roles in axon guidance outside of the embryonic CNS (including axon and dendrite patterning in the olfactory lobe [71–73], guidance of sensory axons [74], photoreceptor targeting [75], motor axon fasciculation and targeting [76–79]) and in axon fasciculation and longitudinal pathway formation in the CNS [77,79–81]. There are five Sema family members in Drosophila (Sema-1a, Sema-1b, Sema-2a, Sema-2b, and Sema-5c), and the transmembrane Semaphorin Sema-1a acts as a repulsive/de-adhesive signal during motor axon guidance, where it acts as a receptor to activate reverse signaling in response to PlexA [75,76,82,83]. Recent evidence reveals that Sema-1a also acts in parallel to Net-Fra to promote midline crossing, acting cell autonomously in response to secreted Sema2 ligands instead of Sema-1a’s canonical binding partner PlexA [84]. Sema-1a promotes midline crossing through an attractive mechanism mediated by RhoGAPp190, in contrast to its repulsive signaling in other contexts which involves the downstream effectors Pebble (Pbl), Varicose (Var), and Cheerio (Cher) [82,83].
3. Midline repulsion
While many individual molecules and a variety of signaling pathways have been identified which promote midline crossing in the fly embryonic CNS, midline repulsion of axons in the Drosophila embryonic CNS appears to be exclusively under control of the midline repellant ligand Slit and its Roundabout (Robo) family receptors. Like the Net-Fra pathway, the Slit-Robo pathway is evolutionarily conserved across bilaterian animals and appears to have a universal role in regulating midline crossing of axons [85–96]. Drosophila slit mutants suffer a complete absence of midline repulsion, causing all CNS axons to collapse at the midline [97,98]; this severe effect is phenocopied in embryos lacking the two midline repulsive Slit receptors robo1 and robo2 [99,100]. Members of the Robo receptor family have an evolutionarily conserved “5+3” protein structure, shared by all three Drosophila Robos: five immunoglobulin-like (Ig) domains, three fibronectin type III (Fn) repeats, a transmembrane domain and two to four conserved cytoplasmic motifs (CC0, CC1, CC2, and CC3) [85,86,101]. Drosophila Robo1 has all four cytoplasmic motifs, while Robo2 and Robo3 only have CC0 and CC1 [99,100,102,103]. Despite the conserved protein structure, these receptors differ from each other in localization and function. All three are localized to longitudinal axon segments and absent from commissures, but they differ in their localization to discrete medial-lateral regions within the neuropile. The innermost medial region contains only Robo1, the intermediate region combines Robo1 and Robo3, and all three receptors are present in the outermost lateral region [102,103]. Robo1 and Robo2 both function in midline repulsion [99,100]. However, while this appears to be Robo1’s sole function in the embryonic CNS, Robo2 also promotes midline crossing and contributes to lateral positioning of axon pathways in the embryonic nerve cord [44,102–104]. Robo3 appears to have no role in midline repulsion, and instead is dedicated to positioning of longitudinal axon pathways and sensory axon terminals in the embryonic nerve cord [44,102,103,105].
3.1 Slit is secreted at the midline and binds to the Ig1 domain of all Robo family members
Since Slit and Robo were identified as a ligand-receptor pair in 1999, a series of genetic interaction and in vitro biochemical studies have pinpointed the binding site to the Ig1 domain of Robo1 and the second leucine-rich repeat (LRR D2) of Slit [87,88,98,106–111]. However, it was only recently formally demonstrated that Slit binding to Robo1’s Ig1 domain is required in vivo for Robo1’s midline repulsive function [112].
Slit is produced and secreted by midline glia, and is detectable on the surface of both midline cells and longitudinal axons which express the Slit-binding Robo receptors [81,98,113]. The secretion of Slit is dependent upon a glycosylation event mediated by Mummy (mmy), a gene that encodes the only known uridine diphosphate-N-acetylglucosamine (UDP-GlcNac) diphosphorylase in Drosophila. In mmy mutants, where glycosylation of Slit cannot be completed, Slit is only found at the VNC midline and is completely absent on longitudinal and commissural axon tracts. However, this only interferes with the ligand’s ability to be secreted, and not its ability to bind Robo1 [114]. Slit and Robo are known to interact with heparin, which can form a ternary complex with Slit and Robo and influence their affinity for each other [111,115,116]. Disruption of genes encoding heparin sulfate proteoglycans (HSPGs) in Drosophila can also lead to ectopic midline crossing of axons, and the HSPG Syndecan (Sdc) can influence the extracellular distribution of Slit in the embryonic nerve cord [113,117,118].
3.2 Early Robo1 inhibition allows axons to initially cross midline
Many axons need to cross the midline in order to innervate the contralateral side of the body and carry out proper motor functions. This necessitates precise temporal regulation of Slit-Robo repulsion. In Drosophila pre-crossing commissural axons, Commissureless (Comm) protein limits the amount of Robo1 on the growth cone surface by endosomal sorting [119–121]. In comm mutants, Robo1 is constitutively trafficked to the surface of growth cones, preventing normally commissural axons from crossing the midline and producing the commissureless phenotype for which comm is named [6]. Transcription of comm is tightly regulated and highly dynamic throughout embryogenesis, turning on during a brief window as each commissural neuron approaches and crosses the midline, then extinguishing shortly after [121]. It is not yet known what external signal activates comm expression in individual commissural neurons, although the mechanism involves Netrin-independent proteolysis of Fra and the nuclear localization of the Fra ICD [36,37]. When Comm protein is present, it is co-localized with Robo1 in vesicles targeted for lysosomal degradation by Comm’s cytoplasmic targeting sequence [120–122]. Within commissural neurons, Comm and Robo1 are trafficked through multiple compartments before reaching the late endosome. Throughout this process Comm predominantly interacts with Rab7 and Shrub-containing vesicles [123]. As Shrub is associated with the formation of multivesicular bodies (MVBs) within late endosomes, this could indicate that Comm may be retained within MVBs before transport to the lysosome [124]. Interestingly, Rab7 activity is necessary to allow Robo1 to reach the growth cone surface, however, when co-expressed with Comm this function is overridden. After crossing, Comm expression is terminated and Robo1 protein is able to return to growth cones and prevent axons from recrossing the midline.
Even in the presence of Comm, a small amount of Robo1 escapes degradation and reaches the growth cone surface as axons are crossing the midline [86]. This surface-localized pool of Robo1 appears to be kept inactive by trans interactions with the Ig1 and Ig2 domains of Robo2 [125], which can antagonize Robo1 and promote midline crossing non-autonomously in addition to its canonical role in midline repulsion [44,99,125]. The binding location on Robo1 for this inhibitory interaction is still unknown.
Two additional genes related to comm (comm2 and comm3) are present in Drosophila, although their function has not been characterized [121]. Some or all of the three Drosophila comm genes are conserved in other insects, and the comm2 gene in mosquitoes may function similarly to Drosophila comm [39,126]. Comm orthologs have not been identified outside of insects, although a number of vertebrate proteins have been identified that may act analogously to Comm to regulate intracellular trafficking of Robo receptors in commissural neurons [127–129]. In vertebrates, RabGDI acts as a temporal regulator to control Robo1 expression at the growth cone surface and prevent premature response to Slit [129]. Pre-crossing commissural axons do not express RabGDI and are attracted to Netrin cues present at the midline. Once the growth cone interacts with the floor plate, expression of RabGDI is activated. RabGDI cooperates with calsyntenin 1 within Rab11-positive vesicles allowing for rapid insertion of Robo1 into the membrane by vesicle fusion [128]. The accumulation of Robo1 at the growth cone surface sensitizes the axon to negative cues present at the midline and enables expulsion from the floorplate. Once on the contralateral side of the body, axons continue to express RabGDI which enhances Robo1’s midline repulsive function and prevents ectopic re-crossing.
While Comm and RabGDI regulate Robo1 by different mechanisms, both allow commissural axons to initially cross the midline by preventing premature repulsion in response to Slit. Of note, a single RabGDI orthologue, Gdi, exists in Drosophila and shares a high degree of amino acid sequence similarity with vertebrate RabGDI [130]. However, Gdi has only been shown to be critical for pupal case and pole cell formation [131]. Whether Gdi can regulate Drosophila Robo1 like its vertebrate counterpart has yet to be determined, if the need for such regulation even exists due to the presence of Comm.
The vertebrate proline rich and Gla domain gene PRRG4 shares some sequence similarity with Drosophila comm (in particular the functionally critical GLPSYDEAL motif [121]), and misexpression of PRRG4 in Drosophila neurons caused ectopic midline crossing [127]. In cultured cells, PRRG4 was able to prevent surface localization of mammalian Robo1, similar to Comm’s effect on Drosophila Robo1 [121,127]. Whether PRRG4 might be a true (if cryptic) ortholog of Comm, or instead an independently evolved protein with functional analogy to Comm is unclear.
3.3 Factors that stabilize Robo1 on axons and enhance midline repulsion
In contrast to the factors described above which negatively regulate Robo1’s surface expression or activity, others have been identified which enhance Robo1’s midline repulsive activity by promoting or stabilizing Robo1 levels at the cell surface. While Mummy (Mmy) has not been shown to directly glycosylate Robo1, mmy mutant stage 12–14 embryos show significantly reduced amounts of Robo1 protein present on axons. Observations of Robo2 and Robo3 protein levels in mmy mutants yielded similar results. This data indicates that Mmy acts in an indirect manner to regulate and maintain the abundance of all three Robo receptors via an unknown, slit-independent mechanism. Ectopic midline crossing and longitudinal pathway defects are observed in mmy mutant embryos, suggesting that Mmy may influence multiple Robo-dependent axon guidance decisions [114].
Another effector that influences Robo1 signaling is Canoe (Cno). During early stages of embryogenesis, Cno is expressed in the ipsilateral axons, while in later stages Cno is expressed in commissural axons that have already crossed the midline once. The expression pattern alone indicates a role in midline repulsion – preventing early ipsilateral axons from crossing and late commissural axons from re-crossing the midline ectopically – and is mechanistically reminiscent of vertebrate RabGDI. cno mutant embryos display a variety of axon guidance defects, including axon stalling, ectopic midline crossing, and defasciculation, consistent with multiple roles for Cno in early axon guidance events. Genetic interaction and in vitro experiments suggest that Cno forms a complex with Robo1 in vivo which is required for the receptor’s localization and midline repulsive function [132].
Outside of the embryonic VNC, the receptor tyrosine phosphatase RPTP69D directly binds to Robo3 to increase surface protein levels and enhance axonal response to Slit and thus its function in axon growth in the adult brain [133]. RPTP10D and RPTP69D have previously been shown to be important for embryonic neural development by interacting with the Slit-Robo pathway [134]. It will be interesting to see if RPTP69D or other RPTPs can similarly regulate surface levels of Robo proteins in embryonic neurons.
3.4 Robo1 signaling downstream of Slit binding
According to the current model, after the receptor binds Slit at the midline, Robo1 must undergo two processes to activate its midline repulsive function: cleavage and clathrin-dependent endocytosis. First, the metalloprotease Kuzbanian (kuz) cleaves Robo1 near the transmembrane domain, effectively shedding the receptor’s ectodomain [135]. The exact site of kuz cleavage remains unknown as the enzyme’s substrate specificity is not well characterized, but the cleavage site must be located at some point between the first Fn repeat and the transmembrane domain as Coleman et al. were able to create an uncleavable form of Robo1 by switching Robo1’s three Fn repeats and juxtamembrane region with the corresponding regions of Frazzled (fra) [135]. This shedding event causes a conformational change in the receptor allowing downstream cytoplasmic domains to interact with Son of Sevenless (Sos) via the Dreadlocks (Dock) adaptor protein. Following cleavage, Robo1 is endocytosed and trafficked from early to late endosomes. Genetic interaction studies suggest that this endocytic event contributes to receptor activation by positively regulating midline repulsion [136]. This is accomplished through a network of downstream effectors recruited to the receptor’s CC2 or CC3 motifs after slit stimulation. Abelson (Abl), Enabled (Ena), Dock and Cno directly bind to the cytoplasmic domain of Robo1 [101,132,137–139]. Slit-dependent Robo1 endocytosis to the early endosome is essential for recruitment of Sos to this complex, which interacts with Dock and regulates local Rac activation via its DH RhoGEF domain [136,138].
Robo2 appears to act similarly to Robo1 to signal midline repulsion in response to Slit; it is co-expressed with Robo1 in ipsilateral pioneer neurons during early stages of CNS development, and axons inappropriately cross the midline in robo2 mutants (although to a far lesser extent than in robo1 mutants) [99,100]. Despite this, very little is known about the signaling mechanisms or cytoplasmic effectors of Robo2 in the context of midline repulsion. Robo2 lacks the CC2 and CC3 motifs that mediate interactions with known downstream components of Robo1 signaling, suggesting that it may signal through a distinct set of downstream factors to mediate midline repulsion. Similarly, it is not yet known if endocytosis and/or proteolytic processing are required for midline repulsive signaling by Robo2.
3.5 Functional relevance of Robo ectodomain elements
While all three Drosophila Robo receptors share the conserved 5 Ig + 3 Fn ectodomain structure that is characteristic of Robo family proteins, our current understanding of the individual roles each of these domains play in midline repulsion in vivo is limited. In vitro studies of both insect and vertebrate Robo receptors have established Ig1’s role in binding Slit ligands [109–111], and recent in vivo studies have confirmed the functional importance of Drosophila Robo1 Ig1 in Slit-dependent midline repulsion in the fly embryonic CNS [112,140]. Despite their evolutionary conservation, the four remaining Ig domains in Drosophila Robo1 (Ig2–Ig5) are individually dispensable for Slit binding and midline repulsive signaling by Robo1 in embryonic neurons. Ig2–Ig5 are also not required for proper expression, axonal localization, or commissural clearance of Robo1 protein in vivo, and endosomal sorting by Comm is unaffected in Robo1 variants lacking any of the five individual Ig domains [140]. Current studies in our lab indicate that each of the three Fn domains (Fn1–Fn3) are also individually dispensable for Slit binding and midline repulsive activity, but have revealed a requirement for Fn3 for commissural clearance and downregulation by Comm (H.E.B., unpublished).
Although nearly all Robo receptors share the same ectodomain structure, there are examples of Robo family proteins that lack various domains: in the silkworm Bombyx mori, two orthologs of Robo1 (BmRobo1a and BmRobo1b) lack the Ig5 and Fn1 domains [93], and the vertebrate Robo4/Magic Roundabout receptor is missing Ig3–Ig5 and Fn1 [141]. Although it retains all eight ectodomain elements, the mammalian Robo3/Rig-1 protein is not a Slit receptor; it has acquired sequence changes in Ig1 that prevent Slit binding, and instead binds the novel ligand NELL2 in an Fn-dependent manner [142,143]. Notably, Robo3/Rig-1 orthologs from non-mammalian vertebrate species (zebrafish, Xenopus, and chick) retain the ability to bind Slit, suggesting that a “signaling switch” in Robo3 occurred at some point in the mammalian lineage [143].
Drosophila Robo2 is a multi-functional receptor, able to promote midline crossing in some neurons while inhibiting crossing in others [44,125]. Robo2 also regulates the formation and lateral position of longitudinal axon pathways (see section 5.2 below) [44,102–104]. Gain-of-function studies suggest that the Ig1 domain of Robo2 (which, like in Robo1, is necessary for Slit binding) is required for its midline repulsive activity, but at least partially dispensable for its pro-crossing role. Instead, Ig2 is critical for promotion of midline crossing by Robo2 [125]. The Ig1 and Ig3 domains of Robo2 have both been implicated in Robo2’s role in lateral positioning, and Ig3 in particular appears to influence the multimerization of Robo2 [104].
4. Choice of commissure
In each segment of the Drosophila embryonic ventral nerve cord, commissural axons cross the midline in the anterior commissure (AC) or posterior commissure (PC) [3]. Choice of commissure is a stereotypical decision, such that the same identifiable neuron will project its axon into the same commissure in each segment (Figure 1). Whether an axon crosses the midline in the AC or PC is regulated by the secreted ligand Wnt5 and its receptor Derailed (Drl). Wnt5 is expressed in neurons located adjacent to the PC [144], and repels Drl-expressing axons which cross the midline in the AC [145]. Drl expression is tightly regulated, and is only detectable on axons as they are crossing the midline [146]. In wnt5 or drl mutants, axons that normally project through the AC mis-project into the PC or wander between the AC and PC as they cross the midline [144,146], while misexpression of Wnt5 or Drl can induce PC axons to switch to the AC [144,146]. It is unclear whether the PC represents a “default” pathway for commissural axons, or whether additional signaling pathways specifically instruct axons to cross via the AC in the absence of Wnt5-Drl signaling.
Drl is a member of the “related to tyrosine kinases” (RYK) family of receptors, and signals axon repulsion independently of its atypical tyrosine kinase domain [147]. Instead, the Wnt5-Drl signaling pathway involves the non-receptor tyrosine kinases Src42A and Src64B through a mechanism that includes Wnt5-induced homodimerization of Drl [148,149]. In a recent study, Long et al. [150] used a series of chimeric receptors to demonstrate that the cytoplasmic domains of three known repulsive axon guidance receptors in Drosophila (Robo1, Drl, and Unc5) can act equivalently to repel axons in three distinct axon guidance contexts (midline crossing, commissure choice, and CNS exit). This intriguing result suggests that these three receptors could signal through a common cytoplasmic signaling pathway which may involve the downstream effector Trio [150].
5. Longitudinal axon guidance
5.1. Post-crossing guidance of longitudinal axons
After crossing the midline, commissural axons gain sensitivity to Slit by expressing Robo1 on their growth cones, preventing them from re-crossing. Once on the contralateral side of the midline, many commissural axons make an anterior or posterior turn and extend along longitudinal axon pathways at specific dorsal-ventral and medial-lateral positions within the neuropile [3,81,151]. These pathways initially form as pioneer axons from adjacent segments grow towards each other, then contact and grow along each other to form continuous longitudinal axon bundles (or fascicles) during the early stages of axon guidance [152]. As neural development progresses, later-developing axons select from and fasciculate with these pathways as they extend towards their ultimate synaptic targets. It appears that repulsive signaling via the Slit-Robo pathway is required for the maintenance of ipsilateral (and presumably post-crossing commissural) axon pathways, via a mechanism that involves the cytoskeletal protein beta-spectrin and may be distinct from the repulsive signaling that occurs during axon pathfinding [153].
5.2. Robo receptors regulate medial-lateral position of longitudinal axon pathways
The dorsal-ventral and medial-lateral positions of longitudinal axon pathways in the Drosophila embryonic CNS are patterned by orthogonal gradients of Slit (medial-lateral) and Sema (dorsal-ventral) ligands [81]; the same cues also regulate the positions of neuronal dendrites and sensory axon terminals within the same three-dimensional neuropile [105,154]. In addition to their canonical role in Slit-dependent midline repulsion, Robo receptors in Drosophila also play an important role in specifying the formation and medial-lateral positioning of longitudinal axon pathways [44,102–104]. While Robo1 appears dedicated to midline repulsion, Robo2 is required for the formation of longitudinal pathways in the lateral region of the neuropile, while Robo3 promotes pathway formation in the intermediate region [102,103]. While both Robo1 and Robo2 can substitute for Robo3 to specify intermediate pathways, neither Robo1 nor Robo3 can rescue Robo2’s role in lateral pathway formation, suggesting that Robo2 acts through a distinct mechanism [44]. Notably, Robo2 and Robo3 orthologs are not conserved outside of insects, and a single ancestral Robo2/3 receptor appears to combine the activities of both Robo2 and Robo3 in the flour beetle Tribolium castaneum [90]. Tribolium Robo2/3 can substitute for Drosophila Robo3 to promote intermediate pathway formation, suggesting that the underlying mechanism is conserved and pre-dates the gene duplication event that produced robo2 and robo3 in dipteran insects [155]. Chimeric receptor experiments indicate that the ectodomain of Robo2 (in particular, the Ig1 and Ig3 domains) is important for specifying its lateral positioning activity [44,104], yet the mechanism(s) underlying Robo2’s and Robo3’s control of lateral position in Drosophila remain poorly characterized; while initial models posited that this activity was in response to a midline-derived Slit gradient [102,103], no direct evidence has shown that Slit binding by either Robo2 or Robo3 is required for their respective roles. Similarly, while vertebrate Robo1 and Robo2 receptors influence the positions of longitudinal axon tracts in the hindbrain and spinal cord, it is not clear whether they do so through the same mechanism(s) as Drosophila Robo2 and Robo3 [88,156–158].
5.3. Semaphorins and their Plexin receptors pattern the dorsal-ventral axis and promote longitudinal pathway formation
Drosophila Plexin receptors (PlexA and PlexB) are required for proper formation and fasciculation of lateral (PlexA) and intermediate (PlexB) longitudinal pathways in the embryonic nerve cord [77,79], and the PlexA ligand Sema-1a is also required for the proper formation of lateral axon pathways [76] in addition to its role in promoting midline crossing via reverse signaling in response to Sema-2s [84]. Both Sema-1a and Sema-2a exhibit restricted expression patterns along the dorsal-ventral axis in the developing neuropile, with Sema-1a highest in dorsolateral regions and Sema-2a forming a gradient with the highest in the central region and lower levels in dorsal and ventral regions [81]. This Sema expression restricts Plexin-expressing sensory axon terminals to specific regions within the neuropile, presumably the same regions that their post-synaptic dendritic targets are located [81]. Although the two secreted Semaphorins in Drosophila (Sema-2a and Sema-2b) are both ligands of PlexB, they serve distinct guidance functions in the embryonic CNS [80]. PlexB appears to integrate Sema-2a repulsion and Sema-2b attraction to synergistically manage construction of specific CNS longitudinal projections and select sensory afferent innervation within that same CNS region. Manipulations of the PlexB receptor signaling results in larval sensory-dependent behavioral deficits. This suggests that in conjunction with longer range Slit, semaphorins ensure the accuracy of CNS interneuron projection organization and sensory afferent targeting in the CNS [80].
5.4. Crossing the segment boundary
Longitudinal axon pathways ensure connectivity between adjacent segments, which is critical for neuromuscular coordination along the anterior-posterior axis and the patterns of peristaltic muscle contraction that characterize the movement of the Drosophila larva [159]. A number of signaling pathways that regulate midline crossing also contribute to growth across the segment boundary. Hiramoto and colleagues [160,161] suggested a model whereby Fra acts to re-localize Netrin proteins secreted by midline cells to longitudinal axons, where it can act to guide longitudinal axons anteriorly towards the next segment [160]. To prevent these axons from then turning towards the midline source of Netrin, Slit and Robo act to suppress responsiveness to Netrin via a G protein-dependent signaling pathway [161]. Kuzina and colleagues proposed that this Netrin redistribution mechanism depends on a meshwork of neuronal projections at the segment boundary, whose production is stimulated by Notch signaling [162]. Recent evidence suggests that Dscam is also involved in promoting axon growth across segment boundaries, where it acts in a complex with Robo1 to bind a proteolytically processed form of Slit (SlitN) and modulate the activity of the Robo-Slit complex to change the canonical activity of the complex from repulsion to outgrowth promotion [56]. Dscam had previously been shown to act as a Slit receptor to promote axon collateral formation in mechanosensory axons in the adult fly, in concert with RPTP69D [163].
6. Summary and conclusions
Thanks to its relatively simple and highly organized embryonic nervous system and the highly stereotyped nature of individual axon guidance decisions in identifiable and easily labeled and manipulated subsets of neurons, the fruit fly Drosophila melanogaster is a powerful system for studying basic questions in developmental neuroscience. Genetic studies of axon guidance in Drosophila over the last two and a half decades have revealed that a seemingly simple binary decision, whether or not to cross the midline, is regulated by a complex and intricate underlying network involving multiple signaling pathways and regulatory and crosstalk mechanisms. Thanks to the broad evolutionary conservation of these pathways and many of their individual components, studies of midline axon guidance in the fruit fly have provided powerful insights into general developmental principles that have a high degree of relevance for understanding development in disease in other animal groups, including humans.
Acknowledgments
This work was supported by the National Institutes of Health [grant number R15 NS098406], and by funding provided by the University of Arkansas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ohyama T, Jovanic T, Denisov G, Dang TC, Hoffmann D, Kerr RA, et al. High-throughput analysis of stimulus-evoked behaviors in Drosophila larva reveals multiple modality-specific escape strategies. PLoS ONE. 2013;8:e71706. doi: 10.1371/journal.pone.0071706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida-Carvalho MJ, Berh D, Braun A, Chen YC, Eichler K, Eschbach C, et al. The Ol1mpiad: concordance of behavioural faculties of stage 1 and stage 3 Drosophila larvae. Journal of Experimental Biology. 2017;220:2452–2475. doi: 10.1242/jeb.156646. [DOI] [PubMed] [Google Scholar]
- 3.Rickert C, Kunz T, Harris KL, Whitington PM, Technau GM. Morphological characterization of the entire interneuron population reveals principles of neuromere organization in the ventral nerve cord of Drosophila. J Neurosci. 2011;31:15870–15883. doi: 10.1523/JNEUROSCI.4009-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogulja-Ortmann A, Lüer K, Seibert J, Rickert C, Technau GM. Programmed cell death in the embryonic central nervous system of Drosophila melanogaster. 2007;134:105–116. doi: 10.1242/dev.02707. [DOI] [PubMed] [Google Scholar]
- 5.Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 6.Seeger M, Tear G, Ferres-Marco D, Goodman CS. Mutations affecting growth cone guidance in Drosophila: genes necessary for guidance toward or away from the midline. Neuron. 1993;10:409–426. doi: 10.1016/0896-6273(93)90330-t. [DOI] [PubMed] [Google Scholar]
- 7.Van Vactor D, Sink H, Fambrough D, Tsoo R, Goodman CS. Genes that control neuromuscular specificity in Drosophila. Cell. 1993;73:1137–1153. doi: 10.1016/0092-8674(93)90643-5. [DOI] [PubMed] [Google Scholar]
- 8.Sánchez-Soriano N, Tear G, Whitington PM, Prokop A. Drosophila as a genetic and cellular model for studies on axonal growth. Neural Development. 2007;2:9. doi: 10.1186/1749-8104-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sánchez-Soriano N, Gonçalves-Pimentel C, Beaven R, Haessler U, Ofner-Ziegenfuss L, Ballestrem C, et al. Drosophila growth cones: a genetically tractable platform for the analysis of axonal growth dynamics. Dev Neurobiol. 2010;70:58–71. doi: 10.1002/dneu.20762. [DOI] [PubMed] [Google Scholar]
- 10.Engle EC. Human genetic disorders of axon guidance. Cold Spring Harbor Perspectives in Biology. 2010;2:a001784. doi: 10.1101/cshperspect.a001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nugent AA, Kolpak AL, Engle EC. Human disorders of axon guidance. Curr Opin Neurobiol. 2012;22:837–843. doi: 10.1016/j.conb.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izzi L, Charron F. Midline axon guidance and human genetic disorders. Clinical Genetics. 2011;80:226–234. doi: 10.1111/j.1399-0004.2011.01735.x. [DOI] [PubMed] [Google Scholar]
- 13.Evans TA, Bashaw GJ. Axon guidance at the midline: of mice and flies. Curr Opin Neurobiol. 2010;20:79–85. doi: 10.1016/j.conb.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickson BJ, Zou Y. Navigating Intermediate Targets: The Nervous System Midline. Cold Spring Harbor Perspectives in Biology. 2010;2:a002055–a002055. doi: 10.1101/cshperspect.a002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai Wing Sun K, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. 2011;138:2153–2169. doi: 10.1242/dev.044529. [DOI] [PubMed] [Google Scholar]
- 16.Linne V, Stollewerk A. Conserved and novel functions for Netrin in the formation of the axonal scaffold and glial sheath cells in spiders. Dev Biol. 2011;353:134–146. doi: 10.1016/j.ydbio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Ishii N, Wadsworth WG, Stern BD, Culotti JG, Hedgecock EM. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron. 1992;9:873–881. doi: 10.1016/0896-6273(92)90240-e. [DOI] [PubMed] [Google Scholar]
- 18.Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, et al. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 19.Chan SS, Zheng H, Su MW, Wilk R, Killeen MT, Hedgecock EM, et al. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. doi: 10.1016/s0092-8674(00)81337-9. [DOI] [PubMed] [Google Scholar]
- 20.Cebrià F, Newmark PA. Planarian homologs of netrin and netrin receptor are required for proper regeneration of the central nervous system and the maintenance of nervous system architecture. 2005;132:3691–3703. doi: 10.1242/dev.01941. [DOI] [PubMed] [Google Scholar]
- 21.Simanton WL, Clark SM, Clemons A, Jacowski C, Farrell-VanZomeren A, Beach P, et al. Conservation of arthropod midline netrin accumulation revealed with a cross-reactive antibody provides evidence for midline cell homology. Evol Dev. 2009;11:260–268. doi: 10.1111/j.1525-142X.2009.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clemons A, Haugen M, Le C, Mori A, Tomchaney M, Severson DW, et al. siRNA-mediated gene targeting in Aedes aegypti embryos reveals that frazzled regulates vector mosquito CNS development. PLoS ONE. 2011;6:e16730. doi: 10.1371/journal.pone.0016730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolodziej PA, Timpe LC, Mitchell KJ, Fried SR, Goodman CS, Jan LY, et al. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- 24.Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 25.Harris R, Sabatelli LM, Seeger MA. Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila Netrin/UNC-6 homologs. Neuron. 1996;17:217–228. doi: 10.1016/s0896-6273(00)80154-3. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell KJ, Doyle JL, Serafini T, Kennedy TE, Tessier-Lavigne M, Goodman CS, et al. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron. 1996;17:203–215. doi: 10.1016/s0896-6273(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 27.Brankatschk M, Dickson BJ. Netrins guide Drosophila commissural axons at short range. Nat Neurosci. 2006;9:188–194. doi: 10.1038/nn1625. [DOI] [PubMed] [Google Scholar]
- 28.Akin O, Zipursky SL. Frazzled promotes growth cone attachment at the source of a Netrin gradient in the Drosophila visual system. Elife. 2016;5 doi: 10.7554/eLife.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varadarajan SG, Kong JH, Phan KD, Kao TJ, Panaitof SC, Cardin J, et al. Netrin1 Produced by Neural Progenitors, Not Floor Plate Cells, Is Required for Axon Guidance in the Spinal Cord. Neuron. 2017;94:790–799.e3. doi: 10.1016/j.neuron.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dominici C, Moreno-Bravo JA, Puiggros SR, Rappeneau Q, Rama N, Vieugue P, et al. Floor-plate-derived netrin-1 is dispensable for commissural axon guidance. Nature. 2017;545:350–354. doi: 10.1038/nature22331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varadarajan SG, Butler SJ. Netrin1 establishes multiple boundaries for axon growth in the developing spinal cord. Dev Biol. 2017;430:177–187. doi: 10.1016/j.ydbio.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newquist G, Drennan JM, Lamanuzzi M, Walker K, Clemens JC, Kidd T. Blocking apoptotic signaling rescues axon guidance in Netrin mutants. Cell Rep. 2013;3:595–606. doi: 10.1016/j.celrep.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forsthoefel DJ, Liebl EC, Kolodziej PA, Seeger MA. The Abelson tyrosine kinase, the Trio GEF and Enabled interact with the Netrin receptor Frazzled in Drosophila. 2005;132:1983–1994. doi: 10.1242/dev.01736. [DOI] [PubMed] [Google Scholar]
- 34.Dorsten JN, Varughese BE, Karmo S, Seeger MA, VanBerkum MFA. In the absence of frazzled over-expression of Abelson tyrosine kinase disrupts commissure formation and causes axons to leave the embryonic CNS. PLoS ONE. 2010;5:e9822. doi: 10.1371/journal.pone.0009822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Donnell MP, Bashaw GJ. Distinct functional domains of the Abelson tyrosine kinase control axon guidance responses to Netrin and Slit to regulate the assembly of neural circuits. 2013;140:2724–2733. doi: 10.1242/dev.093831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L, Garbe DS, Bashaw GJ. A Frazzled/DCC-Dependent Transcriptional Switch Regulates Midline Axon Guidance. Science. 2009 doi: 10.1126/science.1171320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neuhaus-Follini A, Bashaw GJ. The Intracellular Domain of the Frazzled/DCC Receptor Is a Transcription Factor Required for Commissural Axon Guidance. Neuron. 2015;87:751–763. doi: 10.1016/j.neuron.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garbe DS, O’Donnell M, Bashaw GJ. Cytoplasmic domain requirements for Frazzled-mediated attractive axon turning at the Drosophila midline. 2007;134:4325–4334. doi: 10.1242/dev.012872. [DOI] [PubMed] [Google Scholar]
- 39.Sarro J, Andrews E, Sun L, Behura SK, Tan JC, Zeng E, et al. Requirement for commissureless2 function during dipteran insect nerve cord development. Dev Dyn. 2013:n/a–n/a. doi: 10.1002/dvdy.24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taniguchi Y, Kim SH, Sisodia SS. Presenilin-dependent “gamma-secretase” processing of deleted in colorectal cancer (DCC) J Biol Chem. 2003;278:30425–30428. doi: 10.1074/jbc.C300239200. [DOI] [PubMed] [Google Scholar]
- 41.Goldschneider D, Rama N, Guix C, Mehlen P. The neogenin intracellular domain regulates gene transcription via nuclear translocation. Mol Cell Biol. 2008;28:4068–4079. doi: 10.1128/MCB.02114-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Organisti C, Hein I, Grunwald Kadow IC, Suzuki T. Flamingo, a seven-pass transmembrane cadherin, cooperates with Netrin/Frazzled in Drosophilamidline guidance, Genes. Cells. 2014:n/a–n/a. doi: 10.1111/gtc.12202. [DOI] [PubMed] [Google Scholar]
- 43.Cate S, Gajendra S, Alsbury S, Raabe T, Tear G, Mitchell KJ. Mushroom body defect is required in parallel to Netrin for midline axon guidance in Drosophila. 2016;143:972–977. doi: 10.1242/dev.129684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spitzweck B, Brankatschk M, Dickson BJ. Distinct Protein Domains and Expression Patterns Confer Divergent Axon Guidance Functions for Drosophila Robo Receptors. Cell. 2010;140:409–420. doi: 10.1016/j.cell.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Andrews GL, Tanglao S, Farmer WT, Morin S, Brotman S, Berberoglu MA, et al. Dscam guides embryonic axons by Netrin-dependent and -independent functions. 2008;135:3839–3848. doi: 10.1242/dev.023739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ségalen M, Johnston CA, Martin CA, Dumortier JG, Prehoda KE, David NB, et al. The Fz-Dsh planar cell polarity pathway induces oriented cell division via Mud/NuMA in Drosophila and zebrafish. Dev Cell. 2010;19:740–752. doi: 10.1016/j.devcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Anzi B, Wyman RJ. The Drosophila immunoglobulin gene turtle encodes guidance molecules involved in axon pathfinding. Neural Development. 2009;4:31. doi: 10.1186/1749-8104-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bodily KD, Morrison CM, Renden RB, Broadie K. A novel member of the Ig superfamily, turtle, is a CNS-specific protein required for coordinated motor control. J Neurosci. 2001;21:3113–3125. doi: 10.1523/JNEUROSCI.21-09-03113.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferguson K, Long H, Cameron S, Chang WT, Rao Y. The conserved Ig superfamily member Turtle mediates axonal tiling in Drosophila. J Neurosci. 2009;29:14151–14159. doi: 10.1523/JNEUROSCI.2497-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y, Cameron S, Chang WT, Rao Y. Turtle interacts with borderless in regulating glial extension and axon ensheathment. Molecular Brain. 2017;10:17. doi: 10.1186/s13041-017-0299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmucker D, Clemens JC, Shu H, Worby CA, Xiao J, Muda M, et al. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell. 2000;101:671–684. doi: 10.1016/s0092-8674(00)80878-8. [DOI] [PubMed] [Google Scholar]
- 52.Wojtowicz WM, Flanagan JJ, Millard SS, Zipursky SL, Clemens JC. Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell. 2004;118:619–633. doi: 10.1016/j.cell.2004.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wojtowicz WM, Wu W, Andre I, Qian B, Baker D, Zipursky SL. A vast repertoire of Dscam binding specificities arises from modular interactions of variable Ig domains. Cell. 2007;130:1134–1145. doi: 10.1016/j.cell.2007.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sawaya MR, Wojtowicz WM, Andre I, Qian B, Wu W, Baker D, et al. A double S shape provides the structural basis for the extraordinary binding specificity of Dscam isoforms. Cell. 2008;134:1007–1018. doi: 10.1016/j.cell.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meijers R, Puettmann-Holgado R, Skiniotis G, Liu JH, Walz T, Wang JH, et al. Structural basis of Dscam isoform specificity. Nature. 2007;449:487–491. doi: 10.1038/nature06147. [DOI] [PubMed] [Google Scholar]
- 56.Alavi M, Song M, King G, Gillis T, Propst R. Dscam1 Forms a Complex with Robo1 and the N-Terminal Fragment of Slit to Promote the Growth of Longitudinal Axons. PLoS Biol. 2016 doi: 10.1371/journal.pbio.1002560.s013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soba P, Zhu S, Emoto K, Younger S, Yang SJ, Yu HH, et al. Drosophila sensory neurons require Dscam for dendritic self-avoidance and proper dendritic field organization. Neuron. 2007;54:403–416. doi: 10.1016/j.neuron.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hughes ME, Bortnick R, Tsubouchi A, Bäumer P, Kondo M, Uemura T, et al. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron. 2007;54:417–427. doi: 10.1016/j.neuron.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hummel T, Vasconcelos ML, Clemens JC, Fishilevich Y, Vosshall LB, Zipursky SL. Axonal targeting of olfactory receptor neurons in Drosophila is controlled by Dscam. Neuron. 2003;37:221–231. doi: 10.1016/s0896-6273(02)01183-2. [DOI] [PubMed] [Google Scholar]
- 60.Zhu H, Hummel T, Clemens JC, Berdnik D, Zipursky SL, Luo L. Dendritic patterning by Dscam and synaptic partner matching in the Drosophila antennal lobe. Nat Neurosci. 2006;9:349–355. doi: 10.1038/nn1652. [DOI] [PubMed] [Google Scholar]
- 61.Liu G, Li W, Wang L, Kar A, Guan KL, Rao Y, et al. DSCAM functions as a netrin receptor in commissural axon pathfinding. Proc Natl Acad Sci USA. 2009;106:2951–2956. doi: 10.1073/pnas.0811083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li W, Guan KL. The Down syndrome cell adhesion molecule (DSCAM) interacts with and activates Pak. J Biol Chem. 2004;279:32824–32831. doi: 10.1074/jbc.M401878200. [DOI] [PubMed] [Google Scholar]
- 63.Hummel T, Schimmelpfeng K, Klämbt C. Commissure formation in the embryonic CNS of Drosophila. Dev Biol. 1999;209:381–398. doi: 10.1006/dbio.1999.9235. [DOI] [PubMed] [Google Scholar]
- 64.Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- 65.Merianda TT, Botta V, Bhat KM. Patched regulation of axon guidance is by specifying neural identity in the Drosophila nerve cord. Dev Genes Evol. 2005;215:285–296. doi: 10.1007/s00427-005-0475-z. [DOI] [PubMed] [Google Scholar]
- 66.Ricolo D, Butí E, Araújo SJ. Drosophila melanogaster Hedgehog cooperates with Frazzled to guide axons through a non-canonical signalling pathway. Mech Dev. 2015;137:11–22. doi: 10.1016/j.mod.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 67.Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alto LT, Terman JR. Semaphorins and their Signaling Mechanisms. Methods Mol Biol. 2017;1493:1–25. doi: 10.1007/978-1-4939-6448-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nawabi H, Briançon-Marjollet A, Clark C, Sanyas I, Takamatsu H, Okuno T, et al. A midline switch of receptor processing regulates commissural axon guidance in vertebrates. Genes & Development. 2010;24:396–410. doi: 10.1101/gad.542510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zou Y, Stoeckli ET, Chen H, Tessier-Lavigne M. Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell. 2000;102:363–375. doi: 10.1016/s0092-8674(00)00041-6. [DOI] [PubMed] [Google Scholar]
- 71.Komiyama T, Sweeney LB, Schuldiner O, Garcia KC, Luo L. Graded expression of semaphorin-1a cell-autonomously directs dendritic targeting of olfactory projection neurons. Cell. 2007;128:399–410. doi: 10.1016/j.cell.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 72.Sweeney LB, Couto A, Chou YH, Berdnik D, Dickson BJ, Luo L, et al. Temporal target restriction of olfactory receptor neurons by Semaphorin-1a/PlexinA-mediated axon-axon interactions. Neuron. 2007;53:185–200. doi: 10.1016/j.neuron.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 73.Sweeney LB, Chou YH, Wu Z, Joo W, Komiyama T, Potter CJ, et al. Secreted semaphorins from degenerating larval ORN axons direct adult projection neuron dendrite targeting. Neuron. 2011;72:734–747. doi: 10.1016/j.neuron.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bates KE, Whitington PM. Semaphorin 2a secreted by oenocytes signals through plexin B and plexin A to guide sensory axons in the Drosophila embryo. Dev Biol. 2007;302:522–535. doi: 10.1016/j.ydbio.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 75.Yu L, Zhou Y, Cheng S, Rao Y. Plexin a-semaphorin-1a reverse signaling regulates photoreceptor axon guidance in Drosophila. J Neurosci. 2010;30:12151–12156. doi: 10.1523/JNEUROSCI.1494-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu HH, Araj HH, Ralls SA, Kolodkin AL. The transmembrane Semaphorin Sema I is required in Drosophila for embryonic motor and CNS axon guidance. Neuron. 1998;20:207–220. doi: 10.1016/s0896-6273(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 77.Winberg ML, Noordermeer JN, Tamagnone L, Comoglio PM, Spriggs MK, Tessier-Lavigne M, et al. Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell. 1998;95:903–916. doi: 10.1016/s0092-8674(00)81715-8. [DOI] [PubMed] [Google Scholar]
- 78.Yu HH, Huang AS, Kolodkin AL. Semaphorin-1a acts in concert with the cell adhesion molecules fasciclin II and connectin to regulate axon fasciculation in Drosophila. Genetics. 2000;156:723–731. doi: 10.1093/genetics/156.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ayoob JC, Terman JR, Kolodkin AL. Drosophila Plexin B is a Sema-2a receptor required for axon guidance, 133. 2006:2125–2135. doi: 10.1242/dev.02380. [DOI] [PubMed] [Google Scholar]
- 80.Wu Z, Sweeney LB, Ayoob JC, Chak K, Andreone BJ, Ohyama T, et al. A combinatorial semaphorin code instructs the initial steps of sensory circuit assembly in the Drosophila CNS. Neuron. 2011;70:281–298. doi: 10.1016/j.neuron.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zlatic M, Li F, Strigini M, Grueber W, Bate M. Positional cues in the Drosophila nerve cord: semaphorins pattern the dorso-ventral axis. PLoS Biol. 2009;7:e1000135. doi: 10.1371/journal.pbio.1000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jeong S, Juhaszova K, Kolodkin AL. The Control of semaphorin-1a-mediated reverse signaling by opposing pebble and RhoGAPp190 functions in drosophila. Neuron. 2012;76:721–734. doi: 10.1016/j.neuron.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jeong S, Yang DS, Hong YG, Mitchell SP, Brown MP, Kolodkin AL. Varicose and cheerio collaborate with pebble to mediate semaphorin-1a reverse signaling in Drosophila. Proceedings of the National Academy of Sciences. 2017;11:201713010. doi: 10.1073/pnas.1713010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hernandez-Fleming M, Rohrbach EW, Bashaw GJ. Sema-1a Reverse Signaling Promotes Midline Crossing in Response to Secreted Semaphorins. Cell Rep. 2017;18:174–184. doi: 10.1016/j.celrep.2016.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dickson BJ, Gilestro GF. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol. 2006;22:651–675. doi: 10.1146/annurev.cellbio.21.090704.151234. [DOI] [PubMed] [Google Scholar]
- 86.Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, et al. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- 87.Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, et al. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 88.Long H, Sabatier C, Ma L, Plump AS, Yuan W, Ornitz DM, et al. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- 89.Fricke C, Lee JS, Geiger-Rudolph S, Bonhoeffer F, Chien CB. astray, a zebrafish roundabout homolog required for retinal axon guidance. Science. 2001;292:507–510. doi: 10.1126/science.1059496. [DOI] [PubMed] [Google Scholar]
- 90.Evans TA, Bashaw GJ. Slit/Robo-mediated axon guidance in Tribolium and Drosophila: Divergent genetic programs build insect nervous systems. Dev Biol. 2012;363:266–278. doi: 10.1016/j.ydbio.2011.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cebrià F, Guo T, Jopek J, Newmark PA. Regeneration and maintenance of the planarian midline is regulated by a slit orthologue. Dev Biol. 2007;307:394–406. doi: 10.1016/j.ydbio.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cebrià F, Newmark PA. Morphogenesis defects are associated with abnormal nervous system regeneration following roboA RNAi in planarians. 2007;134:833–837. doi: 10.1242/dev.02794. [DOI] [PubMed] [Google Scholar]
- 93.Li XT, Yu Q, Zhou QS, Zhao X, Liu ZY, Cui WZ, et al. BmRobo1a and BmRobo1b control axon repulsion in the silkworm Bombyx mori. Gene. 2016;577:215–220. doi: 10.1016/j.gene.2015.11.044. [DOI] [PubMed] [Google Scholar]
- 94.Li XT, Yu Q, Zhou QS, Zhao X, Liu ZY, Cui WZ, et al. BmRobo2/3 is required for axon guidance in the silkworm Bombyx mori. Gene. 2015 doi: 10.1016/j.gene.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 95.Yu Q, Li XT, Liu C, Cui WZ, Mu ZM, Zhao X, et al. Evolutionarily Conserved Repulsive Guidance Role of Slit in the Silkworm Bombyx mori. PLoS ONE. 2014;9:e109377. doi: 10.1371/journal.pone.0109377.s002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zallen JA, Yi BA, Bargmann CI. The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell. 1998;92:217–227. doi: 10.1016/s0092-8674(00)80916-2. [DOI] [PubMed] [Google Scholar]
- 97.Rothberg JM, Jacobs JR, Goodman CS, Artavanis-Tsakonas S. slit: an extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes & Development. 1990;4:2169–2187. doi: 10.1101/gad.4.12a.2169. [DOI] [PubMed] [Google Scholar]
- 98.Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 99.Simpson JH, Kidd T, Bland KS, Goodman CS. Short-range and long-range guidance by slit and its Robo receptors. Robo and Robo2 play distinct roles in midline guidance. Neuron. 2000;28:753–766. doi: 10.1016/s0896-6273(00)00151-3. [DOI] [PubMed] [Google Scholar]
- 100.Rajagopalan S, Nicolas E, Vivancos V, Berger J, Dickson BJ. Crossing the midline: roles and regulation of Robo receptors. Neuron. 2000;28:767–777. doi: 10.1016/s0896-6273(00)00152-5. [DOI] [PubMed] [Google Scholar]
- 101.Bashaw GJ, Kidd T, Murray D, Pawson T, Goodman CS. Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell. 2000;101:703–715. doi: 10.1016/s0092-8674(00)80883-1. [DOI] [PubMed] [Google Scholar]
- 102.Simpson JH, Bland KS, Fetter RD, Goodman CS. Short-range and long-range guidance by Slit and its Robo receptors: a combinatorial code of Robo receptors controls lateral position. Cell. 2000;103:1019–1032. doi: 10.1016/s0092-8674(00)00206-3. [DOI] [PubMed] [Google Scholar]
- 103.Rajagopalan S, Vivancos V, Nicolas E, Dickson BJ. Selecting a longitudinal pathway: Robo receptors specify the lateral position of axons in the Drosophila CNS. Cell. 2000;103:1033–1045. doi: 10.1016/s0092-8674(00)00207-5. [DOI] [PubMed] [Google Scholar]
- 104.Evans TA, Bashaw GJ. Functional diversity of Robo receptor immunoglobulin domains promotes distinct axon guidance decisions. Curr Biol. 2010;20:567–572. doi: 10.1016/j.cub.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zlatic M, Landgraf M, Bate M. Genetic specification of axonal arbors: atonal regulates robo3 to position terminal branches in the Drosophila nervous system. Neuron. 2003;37:41–51. doi: 10.1016/s0896-6273(02)01131-5. [DOI] [PubMed] [Google Scholar]
- 106.Battye R, Stevens A, Perry RL, Jacobs JR. Repellent signaling by Slit requires the leucine-rich repeats. J Neurosci. 2001;21:4290–4298. doi: 10.1523/JNEUROSCI.21-12-04290.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen JH, Wen L, Dupuis S, Wu JY, Rao Y. The N-terminal leucine-rich regions in Slit are sufficient to repel olfactory bulb axons and subventricular zone neurons. J Neurosci. 2001;21:1548–1556. doi: 10.1523/JNEUROSCI.21-05-01548.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Howitt JA, Clout NJ, Hohenester E. Binding site for Robo receptors revealed by dissection of the leucine-rich repeat region of Slit. Embo J. 2004;23:4406–4412. doi: 10.1038/sj.emboj.7600446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu Z, Patel K, Schmidt H, Andrews W, Pini A, Sundaresan V. Extracellular Ig domains 1 and 2 of Robo are important for ligand (Slit) binding. Mol Cell Neurosci. 2004;26:232–240. doi: 10.1016/j.mcn.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 110.Morlot C, Thielens NM, Ravelli RBG, Hemrika W, Romijn RA, Gros P, et al. Structural insights into the Slit-Robo complex. Proc Natl Acad Sci USA. 2007;104:14923–14928. doi: 10.1073/pnas.0705310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fukuhara N, Howitt JA, Hussain SA, Hohenester E. Structural and functional analysis of slit and heparin binding to immunoglobulin-like domains 1 and 2 of Drosophila Robo. J Biol Chem. 2008;283:16226–16234. doi: 10.1074/jbc.M800688200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brown HE, Reichert MC, Evans TA. Slit Binding via the Ig1 Domain Is Essential for Midline Repulsion by Drosophila Robo1 but Dispensable for Receptor Expression, Localization, and Regulation in Vivo, G3:. Genes|Genomes|Genetics. 2015;5:2429–2439. doi: 10.1534/g3.115.022327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smart AD, Course MM, Rawson JM, Selleck SB, Van Vactor D, Johnson KG. Heparan sulfate proteoglycan specificity during axon pathway formation in the Drosophila embryo. Dev Neurobiol. 2011;71:608–618. doi: 10.1002/dneu.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Manavalan MA, Jayasinghe VR, Grewal R, Bhat KM. The glycosylation pathway is required for the secretion of Slit and for the maintenance of the Slit receptor Robo on axons. Sci Signal. 2017;10 doi: 10.1126/scisignal.aam5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hussain SA, Piper M, Fukuhara N, Strochlic L, Cho G, Howitt JA, et al. A molecular mechanism for the heparan sulfate dependence of slit-robo signaling. J Biol Chem. 2006;281:39693–39698. doi: 10.1074/jbc.M609384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ahmed YA, Yates EA, Moss DJ, Loeven MA, Hussain SA, Hohenester E, et al. Panels of chemically-modified heparin polysaccharides and natural heparan sulfate saccharides both exhibit differences in binding to Slit and Robo, as well as variation between protein binding and cellular activity. Mol Biosyst. 2016 doi: 10.1039/c6mb00432f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Steigemann P, Molitor A, Fellert S, Jäckle H, Vorbrüggen G. Heparan sulfate proteoglycan syndecan promotes axonal and myotube guidance by slit/robo signaling. Curr Biol. 2004;14:225–230. doi: 10.1016/j.cub.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 118.Johnson KG, Ghose A, Epstein E, Lincecum J, O’Connor MB, Van Vactor D. Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr Biol. 2004;14:499–504. doi: 10.1016/j.cub.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 119.Kidd T, Russell C, Goodman CS, Tear G. Dosage-sensitive and complementary functions of roundabout and commissureless control axon crossing of the CNS midline. Neuron. 1998;20:25–33. doi: 10.1016/s0896-6273(00)80431-6. [DOI] [PubMed] [Google Scholar]
- 120.Keleman K, Ribeiro C, Dickson BJ. Comm function in commissural axon guidance: cell-autonomous sorting of Robo in vivo. Nat Neurosci. 2005;8:156–163. doi: 10.1038/nn1388. [DOI] [PubMed] [Google Scholar]
- 121.Keleman K, Rajagopalan S, Cleppien D, Teis D, Paiha K, Huber LA, et al. Comm sorts robo to control axon guidance at the Drosophila midline. Cell. 2002;110:415–427. doi: 10.1016/s0092-8674(02)00901-7. [DOI] [PubMed] [Google Scholar]
- 122.Gilestro GF. Redundant mechanisms for regulation of midline crossing in Drosophila. PLoS ONE. 2008;3:e3798. doi: 10.1371/journal.pone.0003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.van den Brink DM, Banerji O, Tear G. Commissureless regulation of axon outgrowth across the midline is independent of Rab function. PLoS ONE. 2013;8:e64427. doi: 10.1371/journal.pone.0064427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sweeney NT, Brenman JE, Jan YN, Gao FB. The coiled-coil protein shrub controls neuronal morphogenesis in Drosophila. Current Biology. 2006;16:1006–1011. doi: 10.1016/j.cub.2006.03.067. [DOI] [PubMed] [Google Scholar]
- 125.Evans TA, Santiago C, Arbeille E, Bashaw GJ. Robo2 acts in trans to inhibit Slit-Robo1 repulsion in pre-crossing commissural axons. Elife. 2015;4:e08407. doi: 10.7554/eLife.08407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Behura SK, Haugen M, Flannery E, Sarro J, Tessier CR, Severson DW, et al. Comparative Genomic Analysis of Drosophila melanogaster and Vector Mosquito Developmental Genes. PLoS ONE. 2011;6:e21504. doi: 10.1371/journal.pone.0021504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Justice ED, Barnum SJ, Kidd T. The WAGR syndrome gene PRRG4 is a functional homologue of the commissureless axon guidance gene. PLoS Genet. 2017;13:e1006865. doi: 10.1371/journal.pgen.1006865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Alther TA, Domanitskaya E, Stoeckli ET. Calsyntenin 1-mediated trafficking of axon guidance receptors regulates the switch in axonal responsiveness at a choice point. 2016;143:994–1004. doi: 10.1242/dev.127449. [DOI] [PubMed] [Google Scholar]
- 129.Philipp M, Niederkofler V, Debrunner M, Alther T, Kunz B, Stoeckli ET. RabGDI controls axonal midline crossing by regulating Robo1 surface expression. Neural Development. 2012;7:36. doi: 10.1186/1749-8104-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zahner JE, Cheney CM. A Drosophila homolog of bovine smg p25a GDP dissociation inhibitor undergoes a shift in isoelectric point in the developmental mutant quartet. Mol Cell Biol. 1993;13:217–227. doi: 10.1128/mcb.13.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ricard CS, Jakubowski JM, Verbsky JW, Barbieri MA, Lewis WM, Fernandez GE, et al. Drosophila rab GDI mutants disrupt development but have normal Rab membrane extraction. Genesis. 2001;31:17–29. doi: 10.1002/gene.10000. [DOI] [PubMed] [Google Scholar]
- 132.Slováková J, Speicher S, Sánchez-Soriano N, Prokop A, Carmena A. The actin-binding protein Canoe/AF-6 forms a complex with Robo and is required for Slit-Robo signaling during axon pathfinding at the CNS midline. J Neurosci. 2012;32:10035–10044. doi: 10.1523/JNEUROSCI.6342-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oliva C, Soldano A, Mora N, De Geest N, Claeys A, Erfurth ML, et al. Regulation of Drosophila Brain Wiring by Neuropil Interactions via a Slit-Robo-RPTP Signaling Complex. Dev Cell. 2016;39:267–278. doi: 10.1016/j.devcel.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sun Q, Bahri S, Schmid A, Chia W, Zinn K. Receptor tyrosine phosphatases regulate axon guidance across the midline of the Drosophila embryo. 2000;127:801–812. doi: 10.1242/dev.127.4.801. [DOI] [PubMed] [Google Scholar]
- 135.Coleman HA, Labrador JP, Chance RK, Bashaw GJ. The Adam family metalloprotease Kuzbanian regulates the cleavage of the roundabout receptor to control axon repulsion at the midline. 2010;137:2417–2426. doi: 10.1242/dev.047993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chance RK, Bashaw GJ. Slit-Dependent Endocytic Trafficking of the Robo Receptor Is Required for Son of Sevenless Recruitment and Midline Axon Repulsion. PLoS Genet. 2015;11:e1005402. doi: 10.1371/journal.pgen.1005402.s006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fan X, Labrador JP, Hing H, Bashaw GJ. Slit stimulation recruits Dock and Pak to the roundabout receptor and increases Rac activity to regulate axon repulsion at the CNS midline. Neuron. 2003;40:113–127. doi: 10.1016/s0896-6273(03)00591-9. [DOI] [PubMed] [Google Scholar]
- 138.Yang L, Bashaw GJ. Son of sevenless directly links the Robo receptor to rac activation to control axon repulsion at the midline. Neuron. 2006;52:595–607. doi: 10.1016/j.neuron.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 139.Hsouna A, Kim YS, VanBerkum MFA. Abelson tyrosine kinase is required to transduce midline repulsive cues. J Neurobiol. 2003;57:15–30. doi: 10.1002/neu.10232. [DOI] [PubMed] [Google Scholar]
- 140.Reichert MC, Brown HE, Evans TA. In vivo functional analysis of Drosophila Robo1 immunoglobulin-like domains. Neural Development. 2016;11:15. doi: 10.1186/s13064-016-0071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huminiecki L, Gorn M, Suchting S, Poulsom R, Bicknell R. Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics. 2002;79:547–552. doi: 10.1006/geno.2002.6745. [DOI] [PubMed] [Google Scholar]
- 142.Jaworski A, Tom I, Tong RK, Gildea HK, Koch AW, Gonzalez LC, et al. Operational redundancy in axon guidance through the multifunctional receptor Robo3 and its ligand NELL2. Science. 2015;350:961–965. doi: 10.1126/science.aad2615. [DOI] [PubMed] [Google Scholar]
- 143.Zelina P, Blockus H, Zagar Y, Péres A, Friocourt F, Wu Z, et al. Signaling switch of the axon guidance receptor Robo3 during vertebrate evolution. Neuron. 2014;84:1258–1272. doi: 10.1016/j.neuron.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 144.Yoshikawa S, McKinnon RD, Kokel M, Thomas JB. Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature. 2003;422:583–588. doi: 10.1038/nature01522. [DOI] [PubMed] [Google Scholar]
- 145.Callahan CA, Muralidhar MG, Lundgren SE, Scully AL, Thomas JB. Control of neuronal pathway selection by a Drosophila receptor protein-tyrosine kinase family member. Nature. 1995;376:171–174. doi: 10.1038/376171a0. [DOI] [PubMed] [Google Scholar]
- 146.Bonkowsky JL, Yoshikawa S, O’Keefe D, Scully AL, Thomas JB. Axon routing across the midline controlled by the Drosophila Derailed receptor. Nature. 1999;402:540–544. doi: 10.1038/990122. [DOI] [PubMed] [Google Scholar]
- 147.Yoshikawa S, Bonkowsky JL, Kokel M, Shyn S, Thomas JB. The derailed guidance receptor does not require kinase activity in vivo. J Neurosci. 2001;21:RC119. doi: 10.1523/JNEUROSCI.21-01-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Petrova IM, Lahaye LL, Martiáñez T, de Jong AWM, Malessy MJ, Verhaagen J, et al. Homodimerization of the Wnt receptor DERAILED recruits the Src family kinase SRC64B. Mol Cell Biol. 2013;33:4116–4127. doi: 10.1128/MCB.00169-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wouda RR, Bansraj MRKS, de Jong AWM, Noordermeer JN, Fradkin LG. Src family kinases are required for WNT5 signaling through the Derailed/RYK receptor in the Drosophila embryonic central nervous system. 2008;135:2277–2287. doi: 10.1242/dev.017319. [DOI] [PubMed] [Google Scholar]
- 150.Long H, Yoshikawa S, Thomas JB. Equivalent Activities of Repulsive Axon Guidance Receptors. J Neurosci. 2016;36:1140–1150. doi: 10.1523/JNEUROSCI.3406-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Landgraf M, Sánchez-Soriano N, Technau GM, Urban J, Prokop A. Charting the Drosophila neuropile: a strategy for the standardised characterisation of genetically amenable neurites. Dev Biol. 2003;260:207–225. doi: 10.1016/s0012-1606(03)00215-x. [DOI] [PubMed] [Google Scholar]
- 152.Jacobs JR, Goodman CS. Embryonic development of axon pathways in the Drosophila CNS. II. Behavior of pioneer growth cones. J Neurosci. 1989;9:2412–2422. doi: 10.1523/JNEUROSCI.09-07-02412.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Garbe DS, Das A, Dubreuil RR, Bashaw GJ. beta-Spectrin functions independently of Ankyrin to regulate the establishment and maintenance of axon connections in the Drosophila embryonic CNS. 2007;134:273–284. doi: 10.1242/dev.02653. [DOI] [PubMed] [Google Scholar]
- 154.Mauss A, Tripodi M, Evers JF, Landgraf M. Midline signalling systems direct the formation of a neural map by dendritic targeting in the Drosophila motor system. PLoS Biol. 2009;7:e1000200. doi: 10.1371/journal.pbio.1000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Evans TA. CRISPR-based gene replacement reveals evolutionarily conserved axon guidance functions of Drosophila Robo3 and Tribolium Robo2/3. EvoDevo. 2017;8:1–10. doi: 10.1186/s13227-017-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jaworski A, Long H, Tessier-Lavigne M. Collaborative and specialized functions of robo1 and robo2 in spinal commissural axon guidance. J Neurosci. 2010;30:9445–9453. doi: 10.1523/JNEUROSCI.6290-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Farmer WT, Altick AL, Nural HF, Dugan JP, Kidd T, Charron F, et al. Pioneer longitudinal axons navigate using floor plate and Slit/Robo signals. 2008;135:3643–3653. doi: 10.1242/dev.023325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim M, Roesener AP, Mendonca PRF, Mastick GS. Robo1 and Robo2 have distinct roles in pioneer longitudinal axon guidance. Dev Biol. 2011 doi: 10.1016/j.ydbio.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Heckscher ES, Lockery SR, Doe CQ. Characterization of Drosophila larval crawling at the level of organism, segment, and somatic body wall musculature. J Neurosci. 2012;32:12460–12471. doi: 10.1523/JNEUROSCI.0222-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hiramoto M, Hiromi Y, Giniger E, Hotta Y. The Drosophila Netrin receptor Frazzled guides axons by controlling Netrin distribution. Nature. 2000;406:886–889. doi: 10.1038/35022571. [DOI] [PubMed] [Google Scholar]
- 161.Hiramoto M, Hiromi Y. ROBO directs axon crossing of segmental boundaries by suppressing responsiveness to relocalized Netrin. Nat Neurosci. 2006;9:58–66. doi: 10.1038/nn1612. [DOI] [PubMed] [Google Scholar]
- 162.Kuzina I, Song JK, Giniger E. How Notch establishes longitudinal axon connections between successive segments of the Drosophila CNS. 2011;138:1839–1849. doi: 10.1242/dev.062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dascenco D, Erfurth ML, Izadifar A, Song M, Sachse S. Slit and Receptor Tyrosine Phosphatase 69D Confer Spatial Specificity to Axon Branching via Dscam1. Cell. 2015 doi: 10.1016/j.cell.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liebl EC, Rowe RG, Forsthoefel DJ, Stammler AL, Schmidt ER, Turski ML, et al. Interactions between the secreted protein Amalgam, its transmembrane receptor Neurotactin and the Abelson tyrosine kinase affect axon pathfinding. 2003;130:3217–3226. doi: 10.1242/dev.00545. [DOI] [PubMed] [Google Scholar]
- 165.Hsouna A, VanBerkum MFA. Abelson tyrosine kinase and Calmodulin interact synergistically to transduce midline guidance cues in the Drosophila embryonic CNS. Int J Dev Neurosci. 2008;26:345–354. doi: 10.1016/j.ijdevneu.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 166.Bashaw GJ, Hu H, Nobes CD, Goodman CS. A novel Dbl family RhoGEF promotes Rho-dependent axon attraction to the central nervous system midline in Drosophila and overcomes Robo repulsion. The Journal of Cell Biology. 2001;155:1117–1122. doi: 10.1083/jcb.200110077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dorsten JN, Kolodziej PA, VanBerkum MFA. Frazzled regulation of myosin II activity in the Drosophila embryonic CNS. Dev Biol. 2007;308:120–132. doi: 10.1016/j.ydbio.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 168.Banerjee S, Blauth K, Peters K, Rogers SL, Fanning AS, Bhat MA. Drosophila Neurexin IV interacts with roundabout and is required for repulsive midline axon guidance. J Neurosci. 2010;30:5653–5667. doi: 10.1523/JNEUROSCI.6187-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.O’Donnell MP, Bashaw GJ. Src Inhibits Midline Axon Crossing Independent of Frazzled/Deleted in Colorectal Carcinoma (DCC) Receptor Tyrosine Phosphorylation. J Neurosci. 2013;33:305–314. doi: 10.1523/JNEUROSCI.2756-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hu H, Li M, Labrador JP, McEwen J, Lai EC, Goodman CS, et al. Cross GTPase-activating protein (CrossGAP)/Vilse links the Roundabout receptor to Rac to regulate midline repulsion. Proc Natl Acad Sci USA. 2005;102:4613–4618. doi: 10.1073/pnas.0409325102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lundström A, Gallio M, Englund C, Steneberg P, Hemphälä J, Aspenström P, et al. Vilse, a conserved Rac/Cdc42 GAP mediating Robo repulsion in tracheal cells and axons. Genes & Development. 2004;18:2161–2171. doi: 10.1101/gad.310204. [DOI] [PMC free article] [PubMed] [Google Scholar]


