Abstract
After synthesis, proteins are folded into their native conformations aided by molecular chaperones. Dysfunction in folding caused by genetic mutations in numerous genes causes protein conformational diseases. Membrane proteins are more prone to misfolding due to their more intricate folding than soluble proteins. Misfolded proteins are detected by the cellular quality control systems, especially in the endoplasmic reticulum, and proteins may be retained there for eventual degradation by the ubiquitin-proteasome system or through autophagy. Some misfolded proteins aggregate, leading to pathologies in numerous neurological diseases. In vitro, modulating mutant protein folding by altering molecular chaperone expression can ameliorate some misfolding. Some small molecules known as chemical chaperones also correct mutant protein misfolding in vitro and in vivo. However, due to their lack of specificity, their potential as therapeutics is limited. Another class of compounds, known as pharmacological chaperones (pharmacoperones), binds with high specificity to misfolded proteins, either as enzyme substrates or receptor ligands, leading to decreased folding energy barriers and correction of the misfolding. Because many of the misfolded proteins are misrouted but do not have defects in function per se, pharmacoperones have promising potential in advancing to the clinic as therapeutics, since correcting routing may ameliorate the underlying mechanism of disease. This review will comprehensively summarize this exciting area of research, surveying the literature from in vitro studies in cell lines to transgenic animal models and clinical trials in several protein misfolding diseases.
I. INTRODUCTION TO PROTEOSTASIS
Homeostasis is the essence of all physiological processes in the animal. In healthy animals, perturbation of any physiological parameter will result in a series of adaptations seeking the return to pre-perturbation level of the particular physiological parameter. If homeostasis cannot be achieved (dyshomeostasis), pathology will ensue, especially after a significant time has passed.
Not only is homeostasis important at the organismal level, but it is also important at the level of individual cells. Proteostasis or protein homeostasis refers to the fact that at the cellular and subcellular (organelle) levels, it is essential to maintain homeostasis of proteins, with protein production, folding, and disposal reaching a balance (20, 216, 319). When stressed, either due to synthesis of misfolded/misassembled protein or other environmental stress such as increased temperature or oxidative stress, heat shock response (230, 299) and unfolded protein response (UPR) (151, 306, 384) are activated and the expression of molecular chaperones is increased, aiding in the folding of misfolded proteins, preventing the accumulation of misfolded proteins, and accelerating the degradation of misfolded proteins. Protein synthesis is also decreased by decreased gene transcription and translation. However, when misfolded proteins do accumulate in the endoplasmic reticulum (ER), therefore leading to persistent ER stress (317, 385), prolonged UPR activation will cause intracellular accumulation of reactive oxygen species (oxidative stress) and consequent cell death (146).
Aging is associated with loss of proteostasis network capacity, reduced activation of the normal protective mechanisms, resulting in increasing difficulty in maintaining proteostasis (25, 140); hence, aging is accompanied by increased incidence of chronic diseases, such as metabolic and neurodegenerative diseases, and some forms of cancer (140, 154) occur. In humans, there are about two missense mutations per gene (318), and ~25–30% of these missense mutations likely affect protein stability or folding (261). With additional mistakes incorporated in gene transcription and splicing, translation, and posttranslational modification and targeting (92), these represent a constant challenge for the cellular proteostasis machinery. In young animals, these challenges are handled ably, but in aging animals, with decreased capacity to respond to these stresses, age-related diseases gradually manifest, especially if there is a genetic component, such as in familial amyotrophic lateral sclerosis (ALS), Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, and other neurodegenerative disease (25, 92, 140, 216). Neurons, due to their structure and inability to regenerate, are the most sensitive to the accumulation of the misfolded proteins (92).
Considering that an average cell contains 10,000–20,000 different proteins (“the proteome”), maintaining the proper balance (concentration), localization, and integrity of these proteins is a daunting challenge for the cell. This is true since many proteins are only marginally stable and are prone to misfolding and aggregation, especially when the cells are faced with exogenous (such as heat shock) or endogenous (such as metabolic) stress conditions (140). To facilitate the maintenance of proteostasis, human cells employ ~1,400 proteins, including 332 molecular chaperones (comprising the chaperome) and their regulators, proteins involved in defending oxidative stress, and protein degradation machinery [ubiquitin-proteasome degradation system (UPS) and autophagy] (40, 140, 205). To maintain proteostasis, cells maintain a delicate balance between protein synthesis, folding, and degradation (132), using a three-pronged strategy of prevention, repair, and damage control (308).
The UPS is known to be critically important in the clearance of soluble monomeric misfolded proteins through ER-associated degradation (ERAD) (249). Misfolded proteins are retro-translocated into the cytosol through the translocon (188, 288), and degraded by the UPS (67, 124, 150, 310). However, UPS is not effective against insoluble protein aggregates (171). Indeed, UPS activity is frequently severely impaired in the presence of aggregated proteins, even in compartments where there are no protein aggregates, suggesting that it is not simply that the aggregates occupy all the proteasomes in the cell (28). The proteasome is also unable to digest proteins with polyglutamine (polyQ) expansion. Autophagy is used to degrade the aggregated proteins (274, 296; for review, see Refs. 86, 215, 265).
When the misfolded proteins overwhelm the cellular machinery for protein folding, proteotoxicity is observed (152, 153). Molecular chaperones antagonize proteotoxicity (18, 21, 39, 40, 71). At the organismal level, proteotoxic stress in one tissue can modulate the proteostasis activity in other tissues through transcellular chaperone signaling (360, 376, 377).
II. QUALITY CONTROL
Proteins of the secretory pathway that are folded in the ER are constantly monitored by the highly redundant ER quality control system (57, 149). Any features indicative of non-native conformation such as hydrophobic patches exposed on the surface of soluble proteins and mobile loops as well as reduced compactness (globular proteins have a smaller surface area in the native state than in the nonnative state) or increased β-sheet content will result in the protein being retained in the ER and prevented from further forward trafficking to its normal destination. Even in wild-type (WT) proteins, a significant fraction (up to 30%) is misfolded and/or aggregated, despite help of molecular chaperones, and these misfolded proteins are rapidly (within minutes of their synthesis) degraded by the UPS (325). Several G protein-coupled receptors (GPCRs), such as the δ-opioid receptor (282), GnRHR (368), prostaglandin D2 receptor 1 (275), melanocortin-3 receptor (348), melanocortin-4 receptor (356), CB1 cannabinoid receptor (5), V2R (399), olfactory receptor (239), and D2 dopamine receptor (104), are inefficiently expressed at the plasma membrane.
For proteins with a linear sequence, the sequon, Asn-X-Ser/Thr (where X is any amino acid except proline), potential N-linked glycosylation can occur on the amide group of asparagine. It is estimated that more than half of all proteins are glycoproteins and more than 90% of the glycoproteins have N-linked glycosylation (11). N-linked glycosylation is a cotranslational modification that is initiated at the luminal face of the ER when the nascent protein is emerging from the ER membrane. Carbohydrates have different compositions in the ER and post-ER locations, and some chaperones such as calnexin and calreticulin recognize the carbohydrates to exert quality control (53, 346, 363, 389).
For proteins in the secretory pathway, nascent proteins are cotranslationally inserted into the ER lumen where the initial folding occurs. There, resident molecular chaperones facilitate the folding of the proteins into their native conformation (120, 141, 143) based on the primary structure of the proteins as elucidated by the elegant studies of Anfinsen (7). Molecular chaperones also prevent the aggregation of folding intermediates with hydrophobic patches exposed by masking these patches and preventing inappropriate liaisons similar to the human chaperones at high school proms. Some molecular chaperones can serve as disaggregases [such as heat shock protein (HSP) 104] (276, 330), disassociating the aggregated proteins into soluble intermediates, and by cooperating with conventional chaperones such as HSP70 and HSP40, promoting the folding of intermediates (123; reviewed in Ref. 36). It has been suggested that designer protein disaggregase might be developed as a therapeutic for numerous currently incurable neurodegenerative diseases (329).
Proteins that cannot achieve the native conformations have two potential fates: targeting for degradation or undergoing aggregation leading to accumulation of the misfolded protein in the intracellular or extracellular spaces. In the first instance, a loss-of-function disease ensues, whereas the latter causes a gain of function. It is critical to differentiate between these two fates because different strategies are devised for treating loss-of-function versus gain-of-function diseases. Most diseases associated with inactivating GPCRs cause loss of function; few of them cause aggregation, with rhodopsin representing one prominent example. Mutations in α1-antitrypsin (AAT) cause the mutant proteins to polymerize in the ER of hepatocytes. These intracellular polymers accumulated in the ER are toxic, causing chronic liver diseases, whereas systemic deficiency of AAT also causes pulmonary emphysema.
A. Quality Control at the ER
The ER is the site of synthesis and folding of membrane and secretory proteins (273). On the basis of results obtained in yeast (121), it is estimated that about one-third of all eukaryotic proteins, including all cell-surface and secretory proteins and proteins located in compartments along the exocytic or endocytic pathways, are folded and matured in the ER before exiting the ER and reaching their final destinations (373). Quality control checkpoints within the ER have been known for more than three decades (233, 311; for detailed and excellent reviews, see Refs. 93–95, 110, 148, 149, 211, 346, 409). The ER has a mechanism that prevents the transport of misfolded proteins from exiting the ER and reaching their final destination. Calnexin and calreticulin are involved in the ER retention of glycoproteins of the secretory pathway. They bind to glycoproteins with monoglucosylated glycans (137, 147) so that these glycoproteins can be reglucosylated by the UDP-glucose:glycoprotein glucosyltransferase, an ER sensor for misfolded proteins (339). UDP-glucose:glycoprotein glucosyltransferase only glucosylates misfolded (but not native) glycoproteins by recognizing the innermost N-acetylglucosamine unit of the oligosaccharide and exposed hydrophobic amino acids in the misfolded proteins (339).
Hydrophobic amino acids exposed at the surface are detected as misfolded by the ER quality control. Molecular chaperones transiently shield these exposed amino acids that should have been buried inside in the native conformation (143). The formation of intra- and interchain disulfide bonds in the ER is important for many proteins of the secretory pathway to mature and achieve native conformation (252). Aberrant disulfide bonds are recognized as misfolded and retained for further folding, aided by molecular chaperones and folding catalysts such as protein disulfide isomerase and ERp57 (22, 125, 391).
These and other multiple mechanisms in the ER differentiate properly folded and misfolded proteins. The misfolded proteins are retained in the ER (for example by rebinding of general molecular chaperones or interaction with specific folding factors for some proteins), prevented from trafficking onward, retro-translocated into the cytoplasm through the translocon, and degraded by the UPS system (67, 94, 95, 122, 149, 150, 211, 292, 334). Both integral membrane proteins and other proteins of the secretory pathway undergo this ER quality control. The protein fate is determined by folding, chaperone binding, and tagging for degradation by poly-ubiquitination (132, 211).
Although stringent, quality control at the ER is not foolproof. Some misfolded proteins can evade the ER quality control monitoring and escape the ER. Some misfolded proteins are transiently diverted to the ER/Golgi intermediate compartment (ERGIC) and cis-Golgi apparatus (48, 85, 238, 255, 269) before being retro-translocated to the ER for ERAD (402) or sorting to lysosome for degradation, or forming toxic aggregates. Indeed, it has been shown that traffic between the ER and the Golgi is required for ERAD (reviewed in Ref. 364). It is also possible that proteins that already exited the ER become misfolded during the trafficking. Therefore, there is additional quality control machinery to monitor and correct misfolding that occurs beyond the ER in the secretory pathway (16, 364).
B. Quality Control at the Golgi Apparatus
First described by Camillo Golgi of the University of Padua as an “internal reticular apparatus” (126), the Golgi apparatus has two functions in the secretory pathway: 1) sorting proteins into their various final destinations, accomplished by the trans-Golgi-network, and 2) modifying the proteins and lipids passing through it, performed in the rest of the Golgi. As the central hub of the secretory pathway, the Golgi cisternae play a critical role in the health of cellular homeostasis. This structure receives the products from the ER and processes them to the mature form for further trafficking to the final destinations in the secretory pathway (411). The majority of the sorting decisions are made in the Golgi apparatus, especially in the trans-Golgi network. One well-studied example is the extensive trimming of high-mannose N-glycans derived from the ER in the cis-Golgi. These glycoproteins are sensitive to endoglycosidase H (Endo H) in the ER but become insensitive to Endo H at the Golgi and beyond. Rather, because of new sugars added, glycoproteins become sensitive to neuraminidase. Several conformational features, such as not partitioning into the appropriate lipid microdomains or protein aggregation, are recognized as misfolded and identified by the Golgi quality control, tagged with ubiquitin, and targeted to the endosomal degradation system for disposal (16).
Quality control at the Golgi is demonstrated by early studies showing that some laboratory-generated mutant proteins of the secretory pathway, especially proteins with abnormal transmembrane domains, can escape the ER but are retained in the Golgi (113, 131, 144, 258), suggesting that these proteins can reach conformations that are recognized as native by the ER machinery but are recognized as nonnative by the Golgi quality control machinery. This was subsequently demonstrated in naturally occurring mutations; for example, retention in the Golgi accounts for a portion of the intracellular retention of mutant GPCRs (88, 353, 393).
Mutations in genes for the machinery involved in the trimming, including enzymes, nucleotide sugar transporters, or recycling/reorganization components [such as conserved oligomeric Golgi (COG) complex, SEC, golgins], can all affect glycosylation and cause human illness, currently numbering in several dozens of inherited diseases (108, 109, 369). Common diseases such as diabetes and cancer are also associated with protein glycosylation defect (108, 109, 369). Mutations in COG impair the retrograde vesicular traffic of resident Golgi proteins needed to maintain normal Golgi structure and function (370).
C. Quality Control at the Plasma Membrane
For plasma membrane proteins that have already passed the ER and Golgi quality controls and reached the plasma membrane, they are subject to another round of quality control at the plasma membrane and potentially cleared from the plasma membrane if found to be misfolded. Chaperones, cochaperones, and E2 ubiquitin-conjugating and E3 ubiquitin-ligating enzymes have been identified to promote the lysosomal degradation of defective ΔF508 CFTR proteins at the plasma membrane (266). Similar quality control has been found for other proteins such as CD4, mutant dopamine D4.4 and vasopressin V2 receptors (V2R) (10), and human ether-a-go-go-related gene (hERG) K+ channel (8). Nonnative membrane proteins that escape the ER quality control monitoring or are generated in post-ER compartments are eliminated from the plasma membrane by this mechanism (9, 266). However, membrane proteins with a misfolded cytoplasmic domain might not be efficiently recognized and degraded by the quality control mechanisms at the plasma membrane and cytoplasm, although the misfolded domain is exposed to the cytoplasm (227).
Quality control at the plasma membrane, like quality control at the other locations, is a double-edged sword. Whereas it protects cells from damaged proteins, it also eliminates proteins that are functional but are mildly misfolded. This presents another hurdle in designing potential therapeutics such as pharmacological chaperones to effectively treat protein conformational diseases.
D. Quality Control at the Mitochondrion
The mitochondrion, representing the cellular machinery for generating the energy currency, ATP, consists of four compartments, including the outer mitochondrial membrane, the intermembrane space, inner mitochondrial membrane, and mitochondrial matrix. Quality control components in the mitochondria consist of molecular chaperones such as HSP60 and HSP70 that aid in the folding, especially in the matrix, and proteases and ubiquitin ligases that target the permanently misfolded proteins for degradation (19, 136). Misfolded proteins in the matrix are retro-translocated into the cytosol for degradation, akin to the retro-translocation of misfolded proteins in the ER for degradation in the ERAD process (231). In addition to sharing some core components, the cytosolic UPS and the mitochondria-associated degradation pathway are also tightly interconnected functionally, and their activities are reciprocally modulated (38). Mitochondrial quality surveillance systems contribute to quality control in extra-mitochondrial compartments, acting on cytosolic or ER substrates; therefore, it is crucial for the maintenance of health at the whole cell level (38, 79). Similarly, several cytosolic pathways, including mitochondrial precursor overaccumulation stress (204), the UPR activated by mistargeting of proteins (396), and the mitochondria to cytosol stress response (386), seek to restore proteostasis and reduce proteotoxicity of mitochondrial insults by affecting cytosolic protein translation, folding, and degradation (79).
The UPR also operates in the mitochondria. It is a protective transcriptional response changing the expression of mitochondrial proteostasis genes (including chaperones and proteases) via mitochondrial-to-nuclear signaling (295, 327), that is, protein misfolding or misassembly in the mitochondrial matrix will result in upregulation of the expression of nuclear genes encoding mitochondrial chaperones and proteases (412). The promoters of these genes also harbor stress elements similar to the genes upregulated with UPR in other organelles such as the ER. In addition, the accumulation of unfolded or misfolded proteins in mitochondria triggers mitophagy, the selective degradation of mitochondria by autophagy (185). The mitochondrial kinase PINK1 and the E3 ubiquitin ligase PARK2/Parkin remove depolarized mitochondria, reducing the unfolded or misfolded protein load. Mutations in PARK6 (encoding PINK1) or PARK2 (encoding Parkin) cause familial Parkinson’s disease (208, 372), highlighting the importance of mitochondrial quality control in health and disease (reviewed in Ref. 287).
The UPR likely evolved just to respond to transient disruption of homeostasis (220). Prolonged activation of the mitochondrial UPR can shift a protective mechanism to a potentially harmful one (229). For example, in the presence of deleterious mitochondrial DNA that caused reduced oxidative phosphorylation, the cells seek to recover oxidative phosphorylation activity by constitutive activation of mitochondrial UPR. However, the persistent mitochondrial UPR propagates or maintains the deleterious mitochondrial DNA by promoting mitochondrial biogenesis and dynamics, potentially underlying mitochondrial diseases and aging-associated accumulation of mutated mitochondrial DNA (229).
Reduced activity in degradation (such as due to impaired function of mitochondrial proteases) with consequent proteotoxicity is associated with aging and pathogenesis of many diseases such as neurodegenerative disorders, metabolic syndrome, and cancer (294, 328). Restoring proteostasis by either reducing protein translation (such as by inhibiting the mammalian target of rapamycin pathway) or targeting the UPS represents promising therapeutic options for mitochondrial disease treatment. This has been demonstrated with rapamycin (to inhibit protein translation) in rodent models of Parkinson’s disease (184), Leigh syndrome (191, 192), and focal-segmental-glomerulosclerosis (278) or by restoring proteasome function (222, 323). It has been suggested that the benefits of a high dose of rapamycin outweigh the side effects in mitochondrial disease (190).
E. Quality Control at Other Organelles
Another quality control process, called regulation of aberrant protein production (RAPP), is described for mutations in the sequence coding for the signal peptide that results in defective interaction of the nascent peptide with signal recognition particle, leading to increased degradation of the mRNA encoding the mutant protein by Argonaute2, hence decreased translation of the mutant protein (198).
F. Quality Control at the Extracellular Fluid
In addition to the critical importance of proteostasis inside the cells, proteostasis in the extracellular fluid is also critically important. Some of the most debilitating and intractable diseases are caused by misfolding and aggregation of proteins in the extracellular fluid. Alzheimer’s disease, type 2 diabetes mellitus, and prion disease are some examples. Recent studies reveal a growing list of abundant chaperones that are constitutively secreted to the extracellular space where they act as sensors and the machinery for disposal of misfolded proteins (400). They bind to the hydrophobic patches exposed on the misfolded proteins, preventing their aggregation to form insoluble deposits and diminishing their toxicity towards cells. They can also transport the soluble misfolded proteins into the lysosome via receptor-mediated endocytosis for degradation. The extracellular chaperones currently identified are clusterin, haptoglobin, α2-macroglobulin, apolipoprotein E, caseins, and fibronogen. Clusterin, haptoglobin, α2-macroglobulin, and caseins serve as holdases (similar to the intracellular small heat shock proteins) (162), protecting proteins from aggregation and precipitation but directly refolding the misfolded proteins, whereas apolipoprotein E and fibrinogen stabilize proteins in solution (80). Some of the extracellular chaperones are frequently found in extracellular deposits, upregulated in stress or disease conditions, or are genetically associated with some of the diseases mentioned earlier in this paragraph, especially with Alzeheimer’s disease (139, 217, 342).
III. PROTEIN CONFORMATIONAL DISEASES
Protein conformational diseases denote the diseases caused by proteins encoded by mutant genes that cannot achieve native conformation resulting in either loss of function or gain of function, leading to human illness. It is estimated that there are hundreds of protein conformational diseases, including many of the diseases not caused by an infectious agent (371). Here we highlight some prominent examples.
A. Familial Hypercholesterolemia
A well-established protein conformation disease is familial hypercholesterolemia caused by mutations in low-density lipoprotein (LDL) receptor (LDLR) gene (44, 156, 225, 362). The LDLR transports the blood cholesterol to the inside of the cells through endocytosis. Plasma LDL-cholesterol levels in patients with heterozygous mutation in LDLR (300–500 mg/dl) are twice that of the healthy people. Those in patients who have homozygous or compound heterozygous mutations in LDLR (600−1,200 mg/dl) are dramatically higher than those in healthy individuals. The increased levels of LDL-cholesterol lead to tendon xanthoma and atherosclerosis in heterozygous carriers and premature death from heart attack in homozygous or compound heterozygous patients (156). About 53% of the LDLR mutations belong to class 2, where the receptor protein is synthesized in the ER as a partially glycosylated form of 120 kDa, but unlike the WT LDLR that quickly exits the ER and becomes the fully glycosylated 160-kDa form, is retained in the ER and degraded. These mutations can be missense or small inframe deletion mutations. These pioneering studies represent some of the earliest examples of protein misfolding as a root cause of human disease.
Other earlier examples include insulin-resistant diabetes due to mutation in the insulin receptor gene (1), sucrase-isomaltase deficiency due to mutation in the SI gene (232), leukocyte adhesion deficiency due to mutations in the common β-subunit (CD18) (207), osteogenesis imperfecta due to mutations in genes encoding pro-α-l collagen and pro-α-2 collagen (COLIA1 and COLIA2) (290), as well as pulmonary emphysema and chronic liver disease due to mutations in α1-antitrypsin gene (AAT) (37, 78).
Studies on α1-antitrypsin mutants, especially the major form identified from patients, the so-called Z allele that changes E342 to lysine, showed that due to defective folding (234, 406), E342K does not achieve mature glycosylation (for example containing sialic acid) (97, 183). It is sequestered in the ER and not secreted in similar fashion to the WT α1-antitrypsin (280, 380). This results in low plasma concentrations of functionally active α1-antitrypsin. Subsequently, the ER-retained form is quickly degraded by the proteasome (293). If this degradation is defective (for example, in some patients with severe liver disease associated with α1-antitrypsin deficiency), the mutant α1-antitrypsin that accumulated in the ER is toxic to hepatocytes causing chronic liver injury and hepatocellular carcinoma (398).
B. Cystic Fibrosis
Cystic fibrosis (CF) is another prominent example of a protein conformational disease. Confocal microscopy on primary cultures of airway epithelia in nonpermeabilized cells using antibody directed against an extracellular epitope of CF transmembrane conductance regulator (CFTR) showed that CFTR is located in the apical membrane in non-CF subjects. In contrast, it is either absent or expressed at much lower levels in CF patients, suggesting that at least some CFTR mutants are defective in trafficking to the plasma membrane (84). The misfolded ΔF508 CFTR is retained in the ER by Hsp70 and its co-chaperone CHIP, retro-translocated into the cytosol, tagged with ubiquitin, and degraded by the proteasome (182, 246, 388). When the degradation is blocked by proteasome inhibitor, misfolded CFTR aggregates form aggresomes (114, 195, 390).
The aggresome, a juxtanuclear inclusion body structure, is formed in some cases of expression of misfolded proteins, especially when the proteasome activity is inhibited (114, 195, 390; reviewed in Refs. 209, 213). In transgenic mice expressing mutant superoxide dismutase (SOD) linked to autosomal dominant familial ALS, mutant SOD aggregate into high-molecular-weight insoluble protein complexes (IPCs) resembling aggresomes that are detectable in spinal cord extracts several months before the appearance of motor neuron pathology (194). It has been suggested that the aggresome is cytoprotective, facilitating the degradation of toxic aggregates by recruiting the cytoplasmic misfolded proteins there (359, 367).
C. Prion Disease and Other Neurodegenerative Diseases
Prion proteins exist in normal healthy cells. However, as first suggested by the Nobel Laureate Stanley Prusiner (291), when misfolded, the disease-causing version aggregates and exerts dominant negative effect on the normal prion, leading to the misfolding of the normal prion. Several neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, ALS or Lou Gehrig’s disease, are also caused by misfolded proteins (amyloid-β peptide and phosphorylated tau, α-synuclein, huntingtin, SOD, respectively), similar to prion diseases. These protein aggregates form toxic inclusions in specific regions of the brain. Aging is an independent risk factor for many of the neurodegenerative diseases. Aging is associated with a decreased capacity for maintaining proteostasis (91) and altered expression of chaperome, which is exacerbated in neurodegenerative diseases (40).
The polyglutamine expansion encoded by CAG trinucleotide repeats in unrelated proteins causes nine neurodegenerative disorders, including spinal and bulbar muscular atrophy (SBMA, also known as Kennedy's disease) caused by mutations in the androgen receptor; Huntington's disease caused by mutations in Huntingtin; six spinocerebellar ataxias caused by mutations in ataxin1, ataxin2, ataxin3, CACNA1A, and TATA-binding protein, respectively; and dentatorubrol-pallidoluysian atrophy (DRPLA) caused by mutations in the DRPLA gene (268). The dynamic nature of these unstable mutations is responsible for the variable phenotype even within a single family.
Many of these misfolded proteins form toxic aggregates; therefore, the mutant protein is usually dominant negative over the WT protein. The accumulation of the toxic aggregates initiates a vicious cycle that feeds on itself causing increasing aggregation and disruption of the proteome, finally manifesting as a pathology. One mechanism of the toxicity is that protein aggregation directly causes an impairment of the UPS function (26). In vitro, transient expression of two unrelated aggregation-prone proteins almost completely inhibits the UPS (26). Dominant negative activity is also the cause of several endocrine diseases, such as neurogenic diabetes insipidus caused by mutations in the AVP gene (encoding arginine vasopressin), growth hormone deficiency caused by the GH1 gene (encoding growth hormone 1), thyroid hormone resistance caused by mutations in the THRB gene (encoding thyroid hormone receptor β), and familial hypocalciuric hypercalcemia caused by mutations in the CASR gene (encoding the calcium-sensing receptor) (reviewed in Ref. 285). In some genes, both haploinsufficiency and dominant negative activity may cause the disease. For example, although heterodimerization causing dominant negative activity has been observed in many GPCRs (reviewed in Refs. 42, 250, 349, 353), in the MC4R, the vast majority of the heterozygous mutations do not cause dominant negative activity, even though they may be retained intracellularly (reviewed in Refs. 351, 352).
D. Diseases Caused by Mutations in GPCR Genes
GPCRs comprise the largest family of membrane proteins, regulating almost all physiological processes. In humans, there are 799 GPCRs, with about half encoding olfactory receptors. They mediate the actions of diverse extracellular signals, including photons, ions, odorants, amino acids, fatty acids, nucleotides, peptides, and large glycoproteins (223). They are the leading therapeutic targets in the pharmaceutical market, accounting for >30% of sales, including some of the most commonly prescribed medications (270). Numerous genetic diseases are caused by inactivating or activating mutations in GPCRs (347, 349).
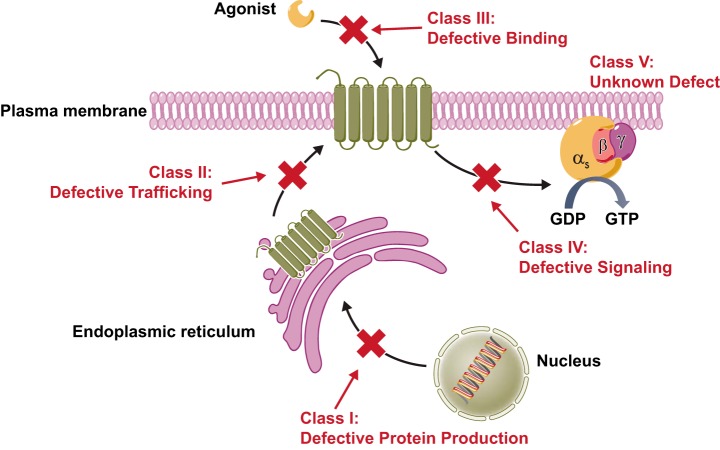
These mutations cause diseases by four major pathways, and we categorize these mutations into four classes (FIGURE 1): class I, decreased mRNA levels with consequent decreased proteins levels, mostly caused by nonsense and frameshift mutations; class II, receptors that are retained intracellularly, primarily in the ER with some mutants retained in the Golgi apparatus; class III, mutant receptors that reach the cell surface but are defective in binding to the ligand; and class IV, mutant receptors that reach the cell surface, bind to the ligand but are unable to transform the ligand binding into intracellular signaling (351, 356), although defective constitutive signaling has also been found. Functional studies, especially in receptors with numerous mutations, show that the most common defect is intracellular retention (class II) (349, 353). In some cases, although the mutations were identified from patients with a particular disease, the mutant receptors have no obvious defect; we classify these mutants as class V.
FIGURE 1.
Classification of naturally occurring inactivating mutations of GPCRs. [From Tao and Conn (353). Copyright 2014 The Endocrine Society.]
E. Diseases Caused by Mutations in Polypeptide Hormone Genes
Polypeptide hormones that are secreted and circulating in the blood are also prototypic examples of proteins of the secretory pathway. They are usually produced as preprohormones in the ER of the endocrine cells and processed by protease into mature hormones. There are also excellent examples of mutations in these preprohormone genes that cause the encoded protein to be misfolded, and the mature hormone molecules fail to be secreted. Whereas mutations in V2 vasopressin receptor or aquaporin cause nephrogenic diabetes insipidus, neurogenic (familial neurohypophysial) diabetes insipidus is caused by mutations in the AVP gene that encodes the arginine vasopressin (AVP) (169, 251). This disease is inherited in an autosomal dominant fashion, suggesting potential neuronal toxicity of mutant proteins. Expression studies in Neuro2A neuroblastoma cells show that mutant AVPs are retained in the ER, not trafficked through the secretory pathway and leads to decreased secretion of AVP (168, 262). ER retention of mutant AVP is confirmed in a transgenic rat model expressing a mutant AVP (331). Dominant AVP mutants form disulfide-linked aggregates in the ER that has a fibrillar substructure, resembling other neurodegenerative diseases that have fibrillar protein aggregates (33). Differentiated Neuro2A cells expressing mutant AVPs have decreased viability, suggesting that the intracellular accumulation of mutant AVPs is toxic, leading to progressive neuronal cell loss (168) that is confirmed in a transgenic mouse model (316). Indeed, the mutant AVPs dimerize with WT AVP and exerts dominant negative effect on WT AVP secretion (170).
These are just some of the prominent examples of protein conformational diseases. Many more are described in the literature that are not elaborated herein. Interested readers are directed to the following classical as well as current review articles on this topic for further information, with some of these articles focused on loss-of-function mutations and others focused on gain-of-function mutations (14, 15, 41, 56, 64, 65, 70, 129, 142, 212, 279, 312, 338, 344, 371, 392).
IV. MOLECULAR CHAPERONES AS TOOLS AND POTENTIAL THERAPEUTICS FOR CORRECTING MISFOLDED PROTEINS CAUSING PROTEIN CONFORMATIONAL DISEASES
Molecular chaperones are defined as proteins that assist the folding or assembly of other proteins but are not present in the final functional protein (96). They have been traditionally credited with performing two functions: preventing aggregation of folding intermediates or unfolded proteins and facilitating the folding of misfolded proteins. Subsequently, some chaperones are shown to serve as disaggregases, breaking up protein aggregates (276). In mammalian cells, HSP110, HSP70, or Hsc70 and HSP40 work together to slowly dissolve large disordered aggregates, suggesting that HSP110 fulfils a subset of HSP104 activities in mammals (330).
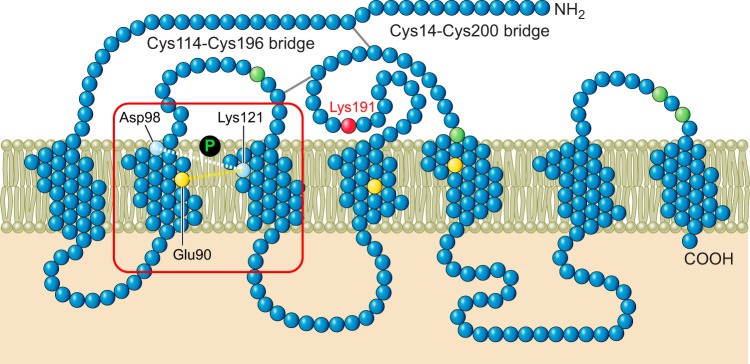
Disulfide bond, covalent interaction between two cysteine residues, is an important co- and posttranslational modification for proteins of the secretory pathway, enhancing structural stability and promoting the assembly of multiprotein complexes. Correct disulfide bond formation is critical for proteins to assume native conformation (103), and aberrant disulfide bonds contribute to protein aggregation, especially in neurological disorders such as Alzheimer’s disease, Parkinson’s disease, and ALS (6). FIGURE 2 depicts two disulfide bonds that are essential for proteins to assume native conformation of the GnRHR. Members of the protein disulfide isomerase (PDI) family and ERp57 catalyze the formation of disulfides in the oxidizing environment of the ER (391). PDI family members are important players in both the formation and reduction of disulfides for correct folding of proteins entering the ER, exhibiting both oxidase activity (introducing disulfides into proteins) and isomerase activity (promoting the rearrangement of incorrect disulfides to correct disulfides) (391). During the normal folding process, cysteine residues that are close to each other can form disulfides in the folding intermediates, although some of these disulfides will not be found in the final native conformation. Some PDI enzymes will reduce non-native disulfides so that native disulfides can form. In misfolded proteins, non-native disulfides are not broken down; rather, they are retained. A tightly controlled redox homeostasis is necessary for the formation of correct disulfide bonding in the ER (206).
FIGURE 2.
Serpentine cartoon of the GnRHR. Highlighted are two disulfide bridges that are required for passage to the plasma membrane and the site of binding of many pharmacoperones of the GnRHR. The position of the 114–196 bridge is common in GPCRs while the 14–200 bridge is uncommon, but its formation is required for the human (but not rat or mouse) GnRHR to traffic correctly.
Variants in PDI are associated with ALS, causing motor dysfunction (394). These PDI variants can impair synaptic protein expression and compromise neuromuscular junction integrity (394). Aberrant disulfide bond formation has been found in mutant rhodopsin (87), MC4R (357), α1-antitrypsin (309), as well as many other proteins.
Some rhodopsin mutants are misfolded, retained in the ER, and form cytoplasmic ubiquitinated inclusions, aggresome-like structure. The immunoglobulin heavy chain binding protein (BiP), the 78-kDa glucose-regulated protein (259), prevents rhodopsin aggregation, with overexpression of BiP decreasing mutant rhodopsin aggregation and inhibition of BiP resulting in enhanced aggregation (17).
HSP90 has also been shown to be important for the folding of GPCRs. For example, with the MC4R, overexpression of HSC70 increases cell surface expression of WT and mutant receptors and mutant receptor signaling, whereas expression of a cochaperone that accelerates the degradation of HSC70 clients, HSJ1b, decreases MC4R expression (248). Inhibition of endogenous HSP90 by geldanamycin decreases MC4R levels, and expression of the HSP90 cochaperone Aha1 (activator of HSP90 ATPase) increases MC4R levels (248).
The molecular chaperones produced endogenously can facilitate the folding of all nascent proteins; therefore, there is no specificity (TABLE 1). Although it is possible to modulate the expression of molecular chaperones to partially correct misfolded proteins in vitro in cell models and in vivo in animal models, the therapeutic potential in humans is limited due to the lack of specificity.
Table 1.
Summary of molecular, chemical, and pharmacological chaperones
| Chaperone | Source | Specificity | Examples |
|---|---|---|---|
| Molecular chaperone | Endogenous | Nonspecific | Calnexin, calreticulin, PDI, ERp57, HSP |
| Chemical chaperone | Exogenous | Nonspecific | Glycerol, DMSO, TMAO, PBA |
| Pharmacological chaperone | Exogenous | Specific | Enzyme substrate, receptor agonist, antagonist, and allosteric modulator |
DMSO, dimethyl sulfoxide; HSP, heat shock protein; PBA, 4-phenylbutyric acid; PDI, protein disulfide isomerase; TMAO, trimethylamine-N-oxide.
V. CHEMICAL CHAPERONES AS TOOLS AND POTENTIAL THERAPEUTICS FOR CORRECTING MISFOLDED PROTEINS CAUSING PROTEIN CONFORMATIONAL DISEASES
High osmotic pressure conditions cause some organisms, including bacteria, plants, and invertebrate and vertebrate animals, to accumulate low-molecular-weight components, neutral substances such as polyhydric alcohols, sugars, amino acids, and related compounds at moderate to high concentrations (up to several molar) to raise the osmotic pressure in the cytoplasm (405). These organic osmolytes, together with the inorganic ions, account for the majority of osmotic force, contributing to the internal environment where biochemical reactions occur. The major organic osmolytes include polyhydric alcohols (polyols), such as glycerol and sucrose; some free amino acids and derivatives such as taurine and β-alanine; as well as urea and methylamines such as trimethylamine-N-oxide (TMAO), betaine, and sarcosine (405). The organic osmolytes have been shown to be able to stabilize proteins by minimizing the water-protein interface area and shifting the balance towards the native state rather than the denatured state (13). Among the organic methylamines, the fully methylated TMAO is the most potent in stabilizing macromolecules (405).
Glycerol changes the solvent properties of water. It was shown that glycerol is largely excluded from the ordered vicinal water around proteins (115, 116). Therefore, addition of glycerol to a protein solution (or intracellularly) changes the thermodynamics, favoring the native conformation (405). With ΔF508 CFTR, incubation with 10% glycerol induces the EndoH-resistant fraction that bears complex N-linked oligosaccharides, with increased cAMP-stimulated channel activity, suggesting that the glycerol-rescued fraction not only exits the ER but also reaches the plasma membrane where it forms functional channels (321). The effects of glycerol are evident only in temperature-sensitive mutants (83, 321). In addition to glycerol, the other cellular osmolyte TMAO, as well as deuterated water, has similar chaperone effects (43).
Mutations in aquaporin-2 (AQP2) cause non-X-linked nephrogenic diabetes insipidus. Some mutant AQP2 are primarily retained in the ER, with reticular and perinuclear vesicular localization (345). Glycerol, TMAO, and DMSO induce an almost complete redistribution of some AQP2 mutants from the ER to the membrane/endosome fractions, where they function as water channels (345).
Sodium 4-phenylbutyrate (PBA) is a new drug recently approved by the United States Food and Drug Administration (FDA) for treating urea cycle disorders. With ΔF508 CFTR, in vitro studies showed that PBA, by downregulating HSC70, induces the trafficking of the mutant CFTR to the plasma membrane with functional restoration of chloride channel activity (313, 315). Further genomic and proteomic analyses identified the proteins modulated by PBA, especially chaperones associated with ERAD, highlighting the importance of members of HSP70 family (332, 333, 395). Randomized, double-blind, placebo-controlled clinical trials show that Buphenyl, the FDA-approved PBA for treating urea cycle disorders by scavenging ammonia, causes statistically significant partial induction of chloride transport in patients homozygous for ΔF508 CFTR, hence a viable therapeutic approach for CF (314, 407).
Heterozygous mutations in bone morphogenetic protein (BMP) type II receptor (BMPR-II) cause familial pulmonary arterial hypertension. Some mutations in BMPR-II cause the mutant receptors to be unable to traffic to the plasma membrane. In vitro studies demonstrated that several chemical chaperones, including thapsigargin, glycerol, or PBA, cause a marked increase in cell surface expression of a mutant BMPR-II, C118W, that is usually retained in the ER (337). The rescued mutant receptor is competent in responding to BMP stimulation with increased phosphorylation of Smad1/5. For kinase-deficient BMPR-II mutants, although BPA can rescue it to the cell surface, there is no response to BMP stimulation with increased Smad1/5 phosphorylation. In addition, chemical chaperones also increase the cell surface expression of WT BMPR-II (337), suggesting that WT BMPR-II folding and trafficking are not optimal, a theme prevalent in many proteins of the secretory pathway.
In cell and mouse models, PBA was shown to result in partial correction of Z α1-antitrypsin (47). However, in a preliminary open label study on 10 patients, oral administration of 4-PBA for 14 days does not result in significant increase in blood α1-antitrypsin levels, despite significant symptomatic and metabolic side effects (361).
In studies with mutant V2Rs, only one of the nine mutants studied has increased cell surface expression after treatment with chemical chaperones including glycerol and DMSO (302). Growing the cells at 27°C or treatment with other osmolytes has no effect on mutant receptor maturation and cell surface density (302). Treatment of four mutant MC4Rs with PBA showed that only one is rescued to the cell surface, whereas it has no effect on the other three mutant receptors (128). Compared with data generated with pharmacoperones (see below), these results suggest that chemical chaperones are effective in only limited mutants and the efficacy is less dramatic compared with pharmacoperones.
Mutation in preproparathyroid hormone (PPTH) causes autosomal dominant familial isolated hypoparathyroidism (AD-FIH). The mutant hormone is trapped intracellularly, primarily in the ER, causing ER stress and inducing apoptosis (82). PBA decreases intracellular accumulation and restores normal secretion of the mutant hormone, together with attenuated ER stress and apoptosis, suggesting that chemical chaperones might serve as a therapeutic option for treating this rare disease (82). PBA also increases mutant Factor H secretion, the deficiency of which is associated with higher risk for infections and kidney diseases (4).
In addition to serving as a chemical chaperone, PBA also attenuates the UPR activation (23). It ameliorates ER stress that is critically involved in the pathogenesis of obesity and type 2 diabetes mellitus (271); hence, PBA restores glucose homeostasis in mouse diabetes models and inhibits adipogenesis (23, 272).
In summary, chemical chaperones such as PBA are safe and effective in human therapeutics. When used at the proper concentration, they can potentially ameliorate a number of diseases. For example, it was recently shown that in adipocytes, very low concentrations of PBA inhibit only pathologic ER stress and UPR induced by glucotoxic insults, leaving the physiologically activated response during differentiation unaltered (235). An important question is whether systemic administration of chemical chaperones will shift the set point of proteostasis in the whole organism resulting in undesired side effects. Similar to the molecular chaperones, the lack of specificity limits the therapeutic potential of chemical chaperones (TABLE 1).
VI. PHARMACOLOGICAL CHAPERONES AS POTENTIAL THERAPEUTICS FOR CORRECTING MISFOLDED PROTEINS CAUSING PROTEIN CONFORMATIONAL DISEASES
Pharmacological chaperones, or pharmacoperones (pharmacochaperones), are small molecules that specifically bind to the target protein, stabilizing the native conformation or facilitating the folding of non-native intermediates into native conformation (12, 70, 77, 99, 193, 237, 353, 368). Although detected by the ER quality control system as misfolded, the mutant proteins sometimes retain function. ER quality control does not detect newly folded proteins for function; some mutant proteins can fold normally, hence not defective in trafficking but defective in function. For example, class III and IV GPCR mutants are localized at the cell surface but either cannot bind the ligand (class III) or can bind the ligand but cannot activate downstream signaling (349, 351, 353). P556L TSHR and Δ88–92 MC4R are examples of class III mutations (89, 130), whereas I183N MC3R is a class IV mutation (355). Pharmacoperones cannot correct these class III and class IV mutants.
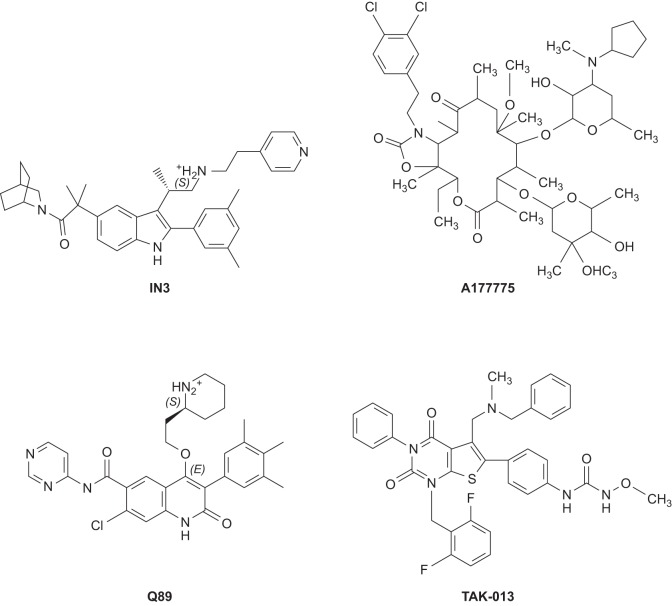
Pharmacoperones need to be able to cross the plasma and ER membranes, specifically bind to the misfolded proteins in the ER, and escort them out of the ER by promoting the folding of the mutant protein. Usually, there is a positive correlation between the binding affinity and chaperoning potency, with the compounds exhibiting higher affinity showing higher chaperoning activity (175). FIGURE 3 shows several pharmacoperones for the GnRHR. Although they come from diverse chemical groups, the GnRHR recognizes them as specific agonists.
FIGURE 3.
Target-specific peptidomimetic antagonists that rescue GnRHR mutants. Target specific pharmacoperones of the GnRHR. These are small hydrophobic drugs that diffuse into the cell and serve as templates to fold or refold misfolded mutants.
However, there is a trade-off between affinity and in vivo utility, especially with those inhibitors (for enzymes) or antagonists (for receptors) serving as pharmacoperones, because in vivo, these pharmacoperones will need to be displaced from the clients once reaching the final destination. When the affinity is too high, the displacement will be difficult, diminishing potential therapeutic utility. Pharmacoperones do not have to be antagonists; agonists and allosteric modulators can also serve as pharmacoperones (158, 180, 353).
Pharmacoperones can be identified in at least two ways. One is through optimization of natural or synthetic substrates, inhibitors, or ligands, and the other approach is through screening of libraries.
A. Cystic Fibrosis
CF is an autosomal recessive fatal genetic disease, affecting multiple organs, including the lungs, intestine, liver, pancreas, and other exocrine glands as well as the male reproductive tract (resulting in infertility in the homozygous males). It is the most common genetic disease in Caucasians, affecting 1 in every 2,000 live births. Recurrent bacterial infection of the lungs due to disruption of the bronchial mucociliary escalator, leading to progressive bronchiectasis and respiratory failure, is usually the cause of death in CF patients. The genetic cause of CF was first revealed in 1989 with the cloning of the CFTR gene and identification of mutations in CFTR (199, 300). CFTR is a large gene, spanning almost 250 kb, with 24 exons, encoding a protein of 1,480 amino acids. The CFTR is a transmembrane protein that serves as a chloride channel, transporting chloride ion across the cell membrane (reviewed in Ref. 210). So far more than 1,900 CFTR mutations have been identified (updated at www.genet.sickkids.on.ca/cftr).
The most common mutation in CFTR, ΔF508, resulting from an inframe deletion of three nucleotides, is found in ~70% of the CF chromosomes (199). Subsequent studies quickly identified the defect of this common CFTR mutant: defective intracellular transport and processing of CFTR is the molecular basis for most cystic fibrosis (63). Unlike the WT CFTR, ΔF508 is expressed as an incompletely glycosylated protein that is sensitive to EndoH digestion, suggesting that it is retained in the ER, whereas the mature form of the WT CFTR is resistant to EndoH treatment but sensitive to neuraminidase digestion, suggesting that it has already exited the ER. The ΔF508 CFTR is temperature sensitive: incubation of the cells expressing ΔF508 CFTR at reduced temperatures enhances the maturation of the mutant protein (83), highlighting that this mutant CFTR can be corrected. Of special relevance for clinical care is that restoration of partial (6–25%) membrane expression can lead to dramatic improvement in patients’ health (189, 410).
After successful correction by decreased temperature and chemical chaperones was demonstrated, extensive studies were performed to identify small molecule compounds that can serve as pharmacoperones, correcting ΔF508 CFTR trafficking defect (54, 55, 90, 236, 277, 304, 305, 320, 375, 387, 408; reviewed in Refs. 138, 240). These studies led to the identification of chemically diverse compounds, including a promising drug candidate, VX-809/Lumacaftor, that was tested in clinical trials. Unfortunately, when administered alone, VX-809 leads to no significant clinical improvement in ΔF508-CFTR patients (68), although combination with a CFTR potentiator VX-770 results in a small improvement in lung function in these patients. One drawback is that VX-770 has been shown to decrease the rescue effect of VX-809 on the functional expression of ΔF508-CFTR at the plasma membrane (66, 379). More potent pharmacoperones and other combination protocols targeting different mechanisms are needed (286, 366, 378).
B. Lysosomal Storage Disorders
Lysosomal storage disorders (LSDs) are a group of more than 50 disorders, occurring in from 1:1,500 to 7,000 live births (340). LSDs are generally characterized by the deficiency in a lysosomal enzyme, resulting in the accumulation of the macromolecular substrates and progressive deterioration in the patients, leading to organ dysfunctions and premature death (247). The deficiency of different lysosomal enzymes results in the accumulation of various glycosphingolipids, causing distinct diseases (such as Fabry disease or Gaucher disease). These rare inborn errors of metabolism disorders exhibit a broad spectrum of phenotypes. In severe cases, the lysosomal build-up of undigested substrate starts prenatally. In many cases, onset happens in early infancy in patients with mutations resulting in very low residual enzyme activity. On the other end of the spectrum, some patients have late-onset disease due to less severe mutation with higher residual enzyme activity. Analysis of the correlation between residual enzyme activity and disease severity suggested that a modest restoration of 10–15% of enzyme activity may be sufficient to prevent the pathogenesis of LSD (100, 226, 326); it is not necessary to restore enzyme activity to the normal level for a significant clinical benefit. This also explains that the penetrance is not complete in many cases of mutations with residual enzyme activity.
Current treatment strategies include enzyme replacement therapy, substrate reduction therapy, bone marrow transplantation, with gene and stem cell therapies actively investigated. Another strategy is using small molecule inhibitors at subinhibitory concentration, a paradoxical approach (99). This approach was first studied in Fabry disease, a disorder of glycosphingolipid metabolism caused by deficiency of lysosomal α-galactosidase A (α-Gal A) encoded by the GLA gene, resulting in renal failure, myocardial infarction and stroke, and premature death (118). More than 400 mutations in GLA, primarily missense mutations with some nonsense mutations and indels of single amino acids, have been identified (247). Fabry disease is one of the few X-linked LSDs, although female heterozygous patients are also affected, likely due to random X chromosome inactivation. Currently, the main lines of treatment are enzyme replacement or substrate reduction therapy (247). Some of the mutant enzymes in Fabry disease have kinetic properties similar to those for WT α-Gal A, but they are less stable and form aggregates in the ER and have accelerated degradation, resulting in enzyme deficiency.
Fan et al. (101) showed that in Fabry lymphoblasts, a potent competitive inhibitor of α-Gal A, 1-deoxy-galactonojirimycin (DGJ), enhances α-Gal A activity when used at subinhibitory intracellular concentrations by enhancing transport and maturation of the WT and mutant enzymes [later it was shown that the pharmacoperone releases the mutants α-Gal A from BiP (401)]. In transgenic mice overexpressing a mutant α-Gal A, DGJ is able to substantially increase the enzyme activity in some organs when administered orally (101). This pioneering study demonstrated that paradoxically, competitive inhibitors can treat genetic metabolic diseases caused by enzyme deficiency (reviewed in Ref. 99). Subsequent studies with a large panel of mutants showed that DGJ can substantially increase the enzyme activity of up to 60% of mutations from both classic and cardiac forms of Fabry disease patients, suggesting that it may be used to treat many Fabry disease patients with missense mutations (165, 397). In vivo experiments in transgenic mice expressing mutant human α-Gal A showed that DGJ is safe and effective in restoring enzyme activity in multiple tissues and decreasing substrate storage (166, 202).
Clinical proof of concept for the pharmacoperone approach has been reported in a patient with the cardiac variant of Fabry disease, harboring G328R mutation that retains residual α-Gal A activity (112). Infusion of the weak inhibitor of α-Gal A, galactose, is safe and effective therapeutically, with marked improvement in cardiac contractility (112). The cardiac variant of Fabry disease, in contrast to the classic form of the disease, has later onset and milder phenotype, without the severe vascular phenotype that usually results in death in early adulthood. Previous in vitro studies showed that galactose increases the activities of these variants (167, 267). However, in a clinical trial, pharmacoperone treatment in patients with mutant α-Gal A does not result in significant decrease in the number of globotriaosylceremide inclusions per kidney interstitial capillary, although there may be improvements in gastrointestinal symptoms (119).
Since the original studies of Fan et al. (101), similar findings have been reported for Gaucher disease (200, 228, 322), Tay-Sachs and Sandhoff diseases (365), GM1-gangliosidosis (244), Pompe disease (201), and Schindler/Kanzaki disease (69). For example, Gaucher disease, the most prevalent lysosomal storage disease, is caused by mutations in the gene encoding β-glucocerebrosidase, GBA1, leading to accumulation of glucosylceramide in the macrophages. Mutant enzymes are retained in the ER and degraded by the proteasome (307, 324). Both inhibitors and noninhibitors serving as pharmacoperones have been identified, rescuing some of the mutant enzymes to the lysosome (3, 61, 200, 228, 322, 341; reviewed in Ref. 196), although some other effects such as shifting the pH optimum have also been observed (341). Because of the inverse relationship between β-glucocerebrosidase activity and α-synuclein aggregation (linked to Parkinson’s disease) (245), enhancement of β-glucocerebrosidase activity, especially by noninhibitory pharmacoperones, might have implications for the treatment of Parkinson’s disease, as recently demonstrated in induced pluripotent dopaminergic stem cells from Gaucher disease patients (2). Treatment of these cells with noninhibitory pharmacoperone NCGC607 leads to decreased α-synuclein levels in these cells, with potential utility for treating Parkinson’s disease and other synucleinopathies (2). In vivo, an earlier study showed that an inhibitory pharmacoperone, isofagomine, when administered orally, can improve motor function, abolish microglial inflammation in the substantia nigra, and decrease the number of small α-synuclein aggregates (298). Since the transgenic mice overexpress WT human β-glucocerebrosidase, this represents another example of the imperfect maturation of WT protein.
Schindler/Kanzaki disease is an inherited neurological disease caused by mutation in a gene encoding a lysosomal enzyme, α-N-acetylgalactosaminidase (α-NAGAL), NAGA. Both DGJ and DGJNAc (2-acetamido-1,2-dideoxy-d-galactonojirimycin, a specific inhibitor for α-NAGAL) can bind to the mutant human α-NAGAL, stabilizing and chaperoning it to the lysosome (69).
A distinct advantage of pharmacoperones over enzyme replacement is the ability to cross the blood-brain barrier compared with the inability of enzymes to do so, therefore better suited for LSDs that have neurological dysfunctions. However, pharmacoperones cannot correct all mutant enzymes (27, 69), correcting ~50% of mutant enzymes causing Fabry disease. Another interesting point raised is the specificity of the pharmacoperones. DGJ is a pharmacoperone in clinical trial for Fabry disease; its actions on α-NAGAL therefore represent an off-target effect, a potential source for side effects (69).
Interestingly, the WT enzymes are not folded optimally which can be improved with pharmacoperones (69, 101, 289). For example, pharmacoperones increase the maturation of recombinant human α-glucosidase and α-galactosidase A when co-administered (289). Therapeutically, this is important because although pharmacoperones can be used in patients with misfolded enzymes, in patients that have almost no residual enzyme activities, pharmacoperones alone are likely not effective in treating the patients. They will need enzyme replacement. However, even in these patients, pharmacoperones co-administration will likely increase the efficacy of the enzymes administered, significantly reducing the cost of treatment.
C. p53 and Cancer
The tumor suppressor p53, a DNA-binding transcription factor, is a key regulator of the cell’s defense against cancer pathogenesis. p53 and associated cell-cycle pathways arrest the cell cycle and induce senescence and apoptosis in an emerging cancerous cell and upon cellular stress by controlling gene transcription as well as a variety of other mechanisms (381, 382). For cancer pathogenesis, p53 or its pathways must be inactivated by mutation. Indeed, loss of p53 function is found in almost all human tumors, with mutation in TP53 (that encodes p53) observed in ~50% of human cancers, almost exclusively in the core DNA-binding domain (35, 186, 187), whereas in the remaining tumors, p53-associated pathways are deficient. Many oncogenic p53 missense mutants have decreased stability, with increased elimination by denaturation and aggregation (46). Methods to stabilize p53 mutants can potentially reactivate apoptotic signaling pathways in tumor cells, hence being used to treat cancer with these destabilizing p53 mutations.
Binding of a specific double-stranded DNA (45), heparin, or a peptide derived from p53-binding protein (111) can stabilize mutant p53. The peptide, acting as a chaperone, even restores sequence-specific DNA binding activity of a highly destabilized mutant I195T to almost that of the WT p53 level (111). Importantly, small molecule compounds have been identified, either by chemical library screening or virtual screening and rational drug design, that can act as pharmacoperones, specifically stabilizing p53 mutants, including some frequently found in human cancers (35, 49, 50, 106, 218, 219, 301; reviewed in Ref. 51). These compounds inhibit tumor cell growth both in vitro and in vivo; they also work synergistically with traditional chemotherapeutic drugs (52). One pharmacoperone, APR-246, shows promising results in a phase I/II clinical trial, being safe and effective in inducing cell cycle arrest, apoptosis, and target gene expression (224). Precision medicine targeting the specific mutation in TP53 is feasible.
D. Diseases Caused by Mutations in GPCR Genes
Morello et al. (256) reported the utility of pharmacoperones for AVPR2 mutations associated with nephrogenic diabetes insipidus. In this study, selective nonpeptidic V2R antagonists SR121463A and VPA-985 were shown to be able to correct eight naturally occurring AVPR2 mutations, and this effect is not blocked by peptide antagonists, suggesting that the site of action is intracellular. It was suggested that because of their hydrophobic nature, SR121463A and VPA-985 can pass through the plasma and ER membranes and bind to the misfolded mutants in the ER and rescue them onto the cell surface. Once they reach the cell surface, at least some of them are functional in responding to agonist stimulation. Since this study, pharmacoperones have been identified for both naturally occurring and laboratory-generated mutant GPCRs (TABLE 2) (reviewed in Refs. 75, 76, 353). FIGURE 4 demonstrates the cellular mechanism of pharmacoperone action.
Table 2.
Pharmacoperones for misfolded GPCRs
| Receptor | Diseases or Function | Reference Nos. |
|---|---|---|
| Rhodopsin | Retinitis pigmentosa | 263, 264 |
| V2R | Nephrogenic diabetes insipidus | 256 |
| GnRHR | Hypogonadotropic hypogonadism | 177, 221 |
| LHCGR | Hypergonadotropic hypogonadism | 260 |
| CaSR | Familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism | 160 |
| FSHR | Hypergonadotropic hypogonadism | 178 |
| MC4R | Obesity | 102, 297 |
| δ-Opioid receptor | Pain perception | 281 |
| µ-Opioid receptor | Pain perception | 60 |
| κ-Opioid receptor | Pain perception | 62 |
| D4 dopamine receptor | Idiopathic Parkinson's disease | 374 |
| MCHR | Energy homeostasis | 98 |
| Kinin B1 receptor | Allergic airway disease | 105 |
| V1a receptor | Arterial hypertension, congestive heart failure, and peripheral vascular disease | 145 |
| V1b/V3 receptor | Stress response | 303 |
| A1 adenosine receptor | Type 2 diabetes and cardio- and neuroprotection | 243 |
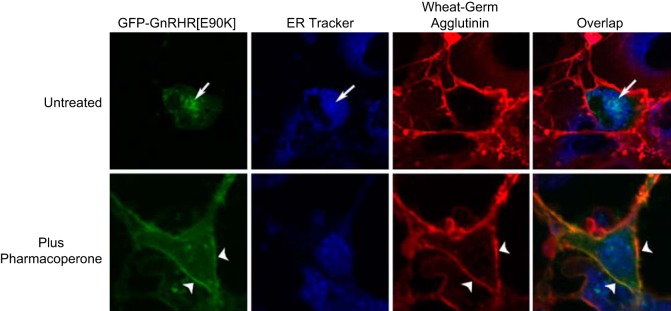
FIGURE 4.
Cellular demonstration of the underlying mechanism of pharmacoperone rescue. Confocal images of Cos-7 cells transiently cotransfected with the GFP-tagged human GnRHR in green and the dominant-negative human GnRHR (E90K) and labeled with ER tracker (blue) and wheat-germ agglutinin Alexa-633 (red). The yellow color shows the overlap between the plasma membrane marker and the mutant receptor. Treatment with 1 μg/ml IN3 for 4 h allows the mutant receptor to leave the ER and move to the plasma membrane (arrowheads). [Modified from Janovick et al. (172). Copyright 2007 Elsevier.]
In vitro studies on multiple mutations revealed that some mutants do not signal even after being rescued to the cell surface, suggesting additional defects in ligand binding and/or signaling. For example, in the MC4R, we show that G98R can be efficiently rescued to the cell surface with two pharmacoperones, ML00253764 and Ipsen 5i, but no signaling can be obtained (350, 354). In the GnRHR, one mutant cannot be rescued. Therefore in vitro experiments can be used to predict which patients will benefit from the pharmacoperone treatment. Even in previously undruggable GPCR, the Frizzled4 (Fz4) receptor, pharmacoperones were identified for a misfolded mutant (117).
The first clinical trial of this strategy was again reported in nephrogenic diabetes insipidus patients (31). Short-term treatment with a nonpeptidic V1a receptor antagonist (SR49059) in X-linked nephrogenic diabetes insipidus patients results in decreased urine volume and water intake and increased urine osmolality (31). Although the clinical trial was ended due to hepatic toxicity (31), it showed that indeed this approach is clinically feasible. Similarly, in vivo studies in mice with a knockin mutation of GnRHR, pulsatile administration of an antagonist that serves as a pharmacoperone rescues the mutant GnRHR, E90K, from ER to the plasma membrane, which responds to the stimulation to the endogenous agonist, resulting in restored steroidogenesis and spermatogenesis, at least partially, and serum androgen completely (181). Similar in vivo experiments in other GPCRs where pharmacoperones have been identified are eagerly awaited.
E. Miscellaneous Diseases
Tyrosinase is the key enzyme in melanin synthesis; mutations in tyrosinase (TYR) gene are associated with oculocutaneous albinism type 1. The mutant tyrosinases are retained in the ER and have prolonged association with calnexin and calreticulin (32, 135). Interestingly, in advanced and metastatic melanoma, tyrosinase activity is also diminished due to retention of tyrosinase in the ER (134). Tyrosinase catalyzes the oxidation of tyrosine and l-3,4-dihyroxyphenylalanine (DOPA) to dopaquinone (335). Both the substrate tyrosine and the cofactor DOPA are required for the promotion of folding, ER exit, and transport to Golgi apparatus of tyrosinase, serving as pharmacoperones (133). The effect of DOPA is evident both co- and posttranslationally, promoting the folding of both nascent and preexistent tyrosinase (133). It is suggested that these compounds stabilize the tyrosinase so that it cannot serve as the substrate for UDP-glucose:glycoprotein glucosyltransferase to be reglucosylated, hence released from the ER (133).
The sulfonylurea receptor 1 (SUR1), a member of the multispanning transmembrane proteins of the ATP-binding cassette (ABC) family (like CFTR), oligomerizes with inwardly rectifying K+ (Kir) channel Kir6.2 to form ATP-sensitive potassium channel (KATP) to regulate insulin secretion in pancreatic β cells in response to blood glucose concentration. Loss-of-function mutations in genes encoding either the SUR1 (ABCC8) or Kir6.2 (KCNJ11) channel subunit of the KATP cause congenital hyperinsulinemia or persistent hyperinsulinemic hypoglycemia of infancy (164). Some of the causative mutations in SUR1 result in the mutant channel proteins failing to reach the cell surface, although they retain at least partial channel activity if coaxed to the cell surface (for example, by masking the ER retention motif through site-directed mutagenesis) (57, 358). This is because the structural features required for trafficking are different from those required for proper channel function (58). Interestingly, sulfonylureas, KATP channel antagonists that have long been used to treat type 2 diabetes, can correct the trafficking defects in some of these mutations (403, 404).
Mutations in phenylalanine hydroxylase (PAH) cause the inborn error of metabolism, phenylketonuria (OMIM no. 261600), with l-Phe accumulating in the blood that is toxic to the brain. Of the more than 500 disease-causing mutations (see PAH Mutation Analysis Consortium database at http://www.pahdb.mcgill.edu/; Ref. 155), the most common defect is misfolding and consequent accelerated turnover, leading to loss of enzymatic activity (283). High-throughput screening identified compounds that could serve as pharmacoperones for the mutant PAH, hence promising alternative treatments for phenylketonuria (284).
Menkes disease is a neurodegenerative disorder with severe neurological symptoms such as seizures, lethargy, and hypotonia, resulting in death in early childhood. It is caused by copper deficiency due to mutations in ATP7A that codes for the copper ATPase, copper-transporting ATPase 1 (383). Copper-transporting ATPase 1 that is localized in the trans-Golgi network functions as an intracellular pump to transport copper into the trans-Golgi network for incorporation into copper-requiring enzymes; those localized on the plasma membrane are involved in copper transport out of the cells (241). Some of the mutant copper-transporting ATPase 1s are misfolded and retained in the ER (203). The mutants are temperature sensitive and can be corrected by chemical chaperones, further supporting the suggestion that the ER-retained mutant is misfolded (203). In vitro, copper supplementation in the culture media results in the correction of the mislocalization (203). Therefore, the substrate for the transporter serves as a pharmacoperone, facilitating the folding of the mutant protein. In Menkes patients with residual copper-transport activity, early treatment with copper injections can normalize clinical outcomes and increase survival (197).
In summary, compared with the nonspecific molecular chaperones and chemical chaperones, pharmacoperones have significant therapeutic potential due to their specificity (TABLE 1). Pharmacoperones only affect the folding of the target protein, be it enzyme or receptor, without affecting the folding of other proteins. Therefore, they are expected to have significantly less rates of deleterious side effects.
It should be mentioned that pharmacoperones can be a double-edged sword. They might account for some of the unexplained side effects of current drugs by increasing the expression levels of drug targets (364). In these cases, selectively interfering with the biosynthesis of drug targets in the ER and inducing the quality control system to block their transport or accelerate their degradation might alleviate these side effects, complementing the current pharmacotherapy (364).
VII. HIGH-THROUGHPUT SCREENS TO IDENTIFY DRUGS FOR PROTEIN CONFORMATIONAL DISEASES
Many of the current studies on pharmacoperones are the result of investigations on known compounds, be they substrates or ligands. Many of these compounds are also antagonists that block the rescued protein function, although a few agonists have also been found to act as pharmacoperones. High-throughput screening to identify additional small molecules that correct the folding defect without blocking protein function has been employed in a few cases.
The word pharmacoperone refers to small molecules that rescue misfolded mutants and restore them to function (77). These are usually hydrophobic structures that enter cells by diffusion and then function as “molecular templates” to promote correct folding of proteins (73, 176, 177). Such putative drugs can correct the trafficking of mistrafficked proteins that do not ordinarily reach their normal site of function (29, 30, 368), and in principle, these drugs can treat disease (59). Science writers have welcomed this approach (24), noting that it is a practical “alternative (to gene therapy),” one that serves as a means of “skirting gene therapy to correct genetic defects.” It is also worth noting that using pharmacoperones to correct defective protein folding is less of a challenge than it is to totally replace a defective gene by genetic engineering or to use enzyme replacement therapy. Moreover, remediation of the defective proteins (from the ER or otherwise) removes retained material that can be toxic or that can activate the UPR and result in other undesirable actions. An in vivo proof of principle was recently published (163).
One could envision “lifestyle drugs” given in vitamin supplements that might preclude misfolding that leads to diseases such as Parkinson’s disease (caused by misfolded α-synuclein), cataracts (misfolded lens crystallins) (107), or Alzheimer’s disease (misfolded amyloid) (343)). For these reasons, identification of such agents by high-throughput screening can be a potentially fruitful effort.
A. Design of Screens for Pharmacoperones for GPCRs
In the case of screens for two GPCRs, GnRHR (74) and V2R (180, 336), the screens employ stable HeLa cell lines expressing the misfolded mutants of these receptors, human GnRHR E90K or human V2R Y128S, respectively. These mutants are expressed under the control of the tet (tetracycline) (OFF) transactivator. Accordingly, when tetracycline or doxycycline is present in the medium, the receptor gene is not expressed and the mutant protein does not accumulate in the cell (214). There is literally no detectable receptor gene or protein expression, as measured by either real-time polymerase chain reaction or by measuring protein expression (72, 179) when these antibiotics are included in the medium. Removal of antibiotic supplements rapidly restores gene expression of these genes. Not surprisingly, antibiotic coculture results in a cell that is identical to those used in the primary screen, but one that lacks mutant expression. Accordingly, cells cultured with tetracycline or doxycycline may be used as negative control lines. For both of these GPCRs, the activation of second messengers can be assessed and used as an indicator of cellular rescue.
It was clear from the earlier studies that misfolded GnRHR and the V2R mutants are retained in the ER and do not traffic to the plasma membrane. As such, second messengers are not generated upon agonist treatment. Both assays show gain of activity in the presence of pharmacoperone drugs which restore proper folding of the receptors and enable correct trafficking and activation by their natural agonists and second messenger coupling. For V2R, which is Gαs coupled (34), we measure the amount of cAMP produced when the cells are in the presence of vasopressin. Compounds that correct the misfolding can potentially result in vasopressin activation of the Gs-coupled system, thereby increasing the adenylate cyclase activity and production of cAMP. For GnRHR, which couples through Gαq (161), we measure the amount of calcium released into the cell from the ER. Compounds that correct the misfolded GnRHR mutants can potentially result in GnRH activation of phospholipase C and accumulation of inositol trisphosphate 3 within the cells which then triggers the release of Ca2+ from the ER stores.
B. Design of Screens for Pharmacoperones for Soluble Enzymes
Hyperoxalosis is a deadly disease with limited therapeutic options, such as lifetime dialysis or dual-organ transplantation. Treatments for hyperoxalosis also include vitamin B6 therapy, but only a small percentage of patients respond to this approach (253, 254). Primary hyperoxaluria type I (PH1) is an autosomal recessive disorder of glyoxylate metabolism caused by misrouting of liver peroxisomal alanine:glyoxylate aminotransferase (AGT). When active AGT is absent in the peroxisome, glyoxylate is oxidized to oxalate in the cytosol and is excreted by the kidneys, resulting in increased oxalate concentration in the urine and deposition of insoluble calcium oxalate crystals. Systemic oxalosis is potentially lethal (81). Common treatments are directed at decreasing oxalate in the body, often by dialysis or by kidney transplantation.
Looking for drugs that promote the distribution of soluble enzymes to a cellular compartment required a fundamentally different approach than redistribution of a GPCR from ER retention to the plasma membrane. A cell-based high content assay (242) was miniaturized into a 1,536-well plate format and used to screen ~4,000 pharmacologically relevant compounds including FDA-, European Union-, and Japanese-approved drugs (157). This assay employs CHO cells stably expressing AGT-170, a mutant that predominantly resides in the mitochondria instead of peroxisome as the WT AGT. The high content assay optically monitors the relocation of the mutant AGT to the peroxisomes through automated image acquisition and analysis. Two analogs were identified by this approach, demonstrating the applicability of this assay for large-scale high-throughput screens. One of these drugs, monensin, was further characterized in Western blot assays to confirm that partial proper relocation of AGT in cells occurred (157).
In summary, traditionally pharmacoperones were discovered based on known properties of chemicals such as enzyme substrates (for enzymes) or receptor ligands (for receptors). However, with the advances in high-throughput and ultra-high-throughput screening, screening libraries for novel pharmacoperones with more ideal profiles (e.g., not acting as enzyme inhibitors for enzymes or antagonists for receptors) will be increasingly important to move this field forward towards translational research from the bench into the bedside. A multidisciplinary team including medicinal chemists will be needed to exploit this opportunity.
C. Potential Therapeutic Problems With Hits
Almost all existing pharmacoperones for GPCRs were selected from antagonist screens for receptors, with only a few examples of agonists or allosteric modulators serving as pharmacoperones (158, 159, 180; reviewed in Ref. 353). This occurred because investigators selected agents that interacted with the protein of interest, but did not activate it. Dual antagonist-pharmacoperone activity is problematic since these drugs present a complex pharmacology, with the antagonists competing with endogenous agonists following receptor rescue. This competition presents the requirement for episodic administration and washout to enable the receptor to be activated by endogenous agonists (181). The availability of non-antagonists will facilitate oral dosing. Agonists can also serve as pharmacoperones. However, agonists stimulate the signaling, and consequent internalization and downregulation of the receptor therefore can potentially decrease the expression of the normal WT receptor (158). For misfolded receptors, the net expression on the cell surface is also the balance of the chaperoning activity promoting the forward trafficking and the post-signaling downregulation. For these reasons, pharmacoperone drugs that stabilize the receptor or mutant but are not agonists or antagonists are a desirable goal in high-throughput screening work. We have recently shown that in a large screen for pharmacoperone activity, there is no correlation between pharmacoperone and antagonist activities (180). Accordingly, identification of non-antagonist pharmacoperones should be viewed as an achievable goal.
There are two remaining observations that are significant to mention. First, it is clear that pharmacoperone drugs need not be present at the precise time of protein synthesis (172, 173). This means that ER-accumulated misfolded and mistrafficked mutants need not be (first) degraded and subsequently replaced by recently synthesized proteins in the presence of pharmacoperone. Second, while pharmacoperones are specific for individual proteins, those that rescue one mutant of an individual protein frequently rescue multiple mutants of the same protein, likely by stabilizing a core region that makes the protein acceptable to the quality control system of the cell. This observation improves the therapeutic reach of these drugs (172–175, 256, 257), since each mutant of an individual protein will not require a separate drug. However, there are also cases where some mutants cannot be rescued to the correct location, likely due to the severe defect in folding, or are not functional despite being corrected to the proper location. For example, G98R MC4R can be efficiently escorted to the cell surface using pharmacoperones but cannot initiate signaling upon agonist stimulation (159, 350, 354).
For lysosomal enzymes, mutations of which cause lysosomal storage diseases, pharmacoperones that are not enzyme inhibitors also have therapeutic advantage over pharmacoperones that are also enzyme inhibitors (196). For pharmacoperones that are also enzyme inhibitors, after the mutant enzyme reaches the lysosome, the bound pharmacoperone has to be outcompeted by the substrate because the pharmacoperone is bound to the active site of the mutant enzyme. For pharmacoperones that are not enzyme inhibitors and are not bound to the active site of the mutant enzyme, the mutant enzyme can be functional without the need for the pharmacoperone to be displaced by the substrate because the active site is free for the substrate to bind. This has been demonstrated in a cellular model of Gaucher disease with compounds derived from high-throughput screening (3, 127).
The arrival of validated screens for non-antagonist pharmacoperones will likely herald new advances in this novel approach to the rescue of mistrafficked proteins.
VIII. CONCLUSIONS
The average human cell has 10,000–20,000 different proteins at their typical concentrations and locales. To maintain proteostasis, cells have stringent quality control mechanisms in different locations, ensuring balance in protein synthesis, folding, trafficking, and degradation. Although essential for homeostasis, this stringent monitoring results in many mutant proteins being detected as misfolded, therefore retained in the ER and some other locales, although the mutant proteins might be perfectly functional, be it as enzyme, or receptor, or as an ion channel, if they could reach their normal destination. Although molecular or chemical chaperones can help the mutant proteins to fold and are useful tools for studying mutant protein folding, they are of limited value as a therapeutic in patients due to the nonspecific action. Pharmacoperones have great potential because different from molecular and chemical chaperones, they can act as specific folding templates for the mutant proteins, helping them reach their native conformation, passing the quality control monitoring, and reaching their normal destination. Some of the rescued mutants might be functional. Because the pharmacoperones are small molecules, they can be administered orally, reaching all parts of the body including the central nervous system at much lower costs than alternative treatments such as enzyme replacement therapy and are much safer than gene therapy. Pharmacoperones have great potential for all protein conformational diseases, especially for some of the orphan diseases where there are no current therapeutics.
GRANTS
Work cited in this article was supported by National Institutes of Health Grants OD012220, DK085040, DK099090, and DK103591 (to P. M. Conn) as well as American Diabetes Association Grant 1-12-BS212 and Auburn University (to Y.-X. Tao).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We apologize to all colleagues whose relevant work could not be cited due to space limitations. This manuscript is to honor the fond memory of Dr. P. Michael Conn, who died while the manuscript was under review. He was a great mentor, colleague, and friend to Y.-X. Tao. Y.-X. Tao gratefully acknowledges Dr. Timothy D. Braden (Auburn University, Auburn, AL) and Dr. Carl A. Pinkert (Univ. of Alabama, Tuscaloosa, AL) for expert editing of the manuscript.
Address for reprint requests and other correspondence: Y.-X. Tao, Dept. of Anatomy, Physiology and Pharmacology, College of Veterinary Medicine, Auburn University, Auburn, AL 36849-5519 (e-mail: taoyaxi@auburn.edu).
REFERENCES
- 1.Accili D, Frapier C, Mosthaf L, McKeon C, Elbein SC, Permutt MA, Ramos E, Lander E, Ullrich A, Taylor SI. A mutation in the insulin receptor gene that impairs transport of the receptor to the plasma membrane and causes insulin-resistant diabetes. EMBO J 8: 2509–2517, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aflaki E, Borger DK, Moaven N, Stubblefield BK, Rogers SA, Patnaik S, Schoenen FJ, Westbroek W, Zheng W, Sullivan P, Fujiwara H, Sidhu R, Khaliq ZM, Lopez GJ, Goldstein DS, Ory DS, Marugan J, Sidransky E. A new glucocerebrosidase chaperone reduces α-synuclein and glycolipid levels in iPSC-derived dopaminergic neurons from patients with Gaucher disease and parkinsonism. J Neurosci 36: 7441–7452, 2016. doi: 10.1523/JNEUROSCI.0636-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aflaki E, Stubblefield BK, Maniwang E, Lopez G, Moaven N, Goldin E, Marugan J, Patnaik S, Dutra A, Southall N, Zheng W, Tayebi N, Sidransky E. Macrophage models of Gaucher disease for evaluating disease pathogenesis and candidate drugs. Sci Transl Med 6: 240ra73, 2014. doi: 10.1126/scitranslmed.3008659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albuquerque JA, Lamers ML, Castiblanco-Valencia MM, Dos Santos M, Isaac L. Chemical chaperones curcumin and 4-phenylbutyric acid improve secretion of mutant factor H R127H by fibroblasts from a factor H-deficient patient. J Immunol 189: 3242–3248, 2012. doi: 10.4049/jimmunol.1201418. [DOI] [PubMed] [Google Scholar]
- 5.Andersson H, D’Antona AM, Kendall DA, Von Heijne G, Chin CN. Membrane assembly of the cannabinoid receptor 1: impact of a long N-terminal tail. Mol Pharmacol 64: 570–577, 2003. doi: 10.1124/mol.64.3.570. [DOI] [PubMed] [Google Scholar]
- 6.Andreu CI, Woehlbier U, Torres M, Hetz C. Protein disulfide isomerases in neurodegeneration: from disease mechanisms to biomedical applications. FEBS Lett 586: 2826–2834, 2012. doi: 10.1016/j.febslet.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Anfinsen CB. Principles that govern the folding of protein chains. Science 181: 223–230, 1973. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- 8.Apaja PM, Foo B, Okiyoneda T, Valinsky WC, Barriere H, Atanasiu R, Ficker E, Lukacs GL, Shrier A. Ubiquitination-dependent quality control of hERG K+ channel with acquired and inherited conformational defect at the plasma membrane. Mol Biol Cell 24: 3787–3804, 2013. doi: 10.1091/mbc.E13-07-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apaja PM, Lukacs GL. Protein homeostasis at the plasma membrane. Physiology (Bethesda) 29: 265–277, 2014. doi: 10.1152/physiol.00058.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apaja PM, Xu H, Lukacs GL. Quality control for unfolded proteins at the plasma membrane. J Cell Biol 191: 553–570, 2010. doi: 10.1083/jcb.201006012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta 1473: 4–8, 1999. doi: 10.1016/S0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 12.Arakawa T, Ejima D, Kita Y, Tsumoto K. Small molecule pharmacological chaperones: from thermodynamic stabilization to pharmaceutical drugs. Biochim Biophys Acta 1764: 1677–1687, 2006. doi: 10.1016/j.bbapap.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Arakawa T, Timasheff SN. The stabilization of proteins by osmolytes. Biophys J 47: 411–414, 1985. doi: 10.1016/S0006-3495(85)83932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aridor M, Hannan LA. Traffic jam: a compendium of human diseases that affect intracellular transport processes. Traffic 1: 836–851, 2000. doi: 10.1034/j.1600-0854.2000.011104.x. [DOI] [PubMed] [Google Scholar]
- 15.Aridor M, Hannan LA. Traffic jams II: an update of diseases of intracellular transport. Traffic 3: 781–790, 2002. doi: 10.1034/j.1600-0854.2002.31103.x. [DOI] [PubMed] [Google Scholar]
- 16.Arvan P, Zhao X, Ramos-Castaneda J, Chang A. Secretory pathway quality control operating in Golgi, plasmalemmal, and endosomal systems. Traffic 3: 771–780, 2002. doi: 10.1034/j.1600-0854.2002.31102.x. [DOI] [PubMed] [Google Scholar]
- 17.Athanasiou D, Kosmaoglou M, Kanuga N, Novoselov SS, Paton AW, Paton JC, Chapple JP, Cheetham ME. BiP prevents rod opsin aggregation. Mol Biol Cell 23: 3522–3531, 2012. doi: 10.1091/mbc.E12-02-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of α-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science 295: 865–868, 2002. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 19.Baker MJ, Palmer CS, Stojanovski D. Mitochondrial protein quality control in health and disease. Br J Pharmacol 171: 1870–1889, 2014. doi: 10.1111/bph.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science 319: 916–919, 2008. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 21.Balchin D, Hayer-Hartl M, Hartl FU. In vivo aspects of protein folding and quality control. Science 353: aac4354, 2016. doi: 10.1126/science.aac4354. [DOI] [PubMed] [Google Scholar]
- 22.Bardwell JC, McGovern K, Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell 67: 581–589, 1991. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- 23.Basseri S, Lhoták S, Sharma AM, Austin RC. The chemical chaperone 4-phenylbutyrate inhibits adipogenesis by modulating the unfolded protein response. J Lipid Res 50: 2486–2501, 2009. doi: 10.1194/jlr.M900216-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellotti V, Nuvolone M, Giorgetti S, Obici L, Palladini G, Russo P, Lavatelli F, Perfetti V, Merlini G. The workings of the amyloid diseases. Ann Med 39: 200–207, 2007. doi: 10.1080/07853890701206887. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Zvi A, Miller EA, Morimoto RI. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci USA 106: 14914–14919, 2009. doi: 10.1073/pnas.0902882106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292: 1552–1555, 2001. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- 27.Benjamin ER, Flanagan JJ, Schilling A, Chang HH, Agarwal L, Katz E, Wu X, Pine C, Wustman B, Desnick RJ, Lockhart DJ, Valenzano KJ. The pharmacological chaperone 1-deoxygalactonojirimycin increases α-galactosidase A levels in Fabry patient cell lines. J Inherit Metab Dis 32: 424–440, 2009. doi: 10.1007/s10545-009-1077-0. [DOI] [PubMed] [Google Scholar]
- 28.Bennett EJ, Bence NF, Jayakumar R, Kopito RR. Global impairment of the ubiquitin-proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol Cell 17: 351–365, 2005. doi: 10.1016/j.molcel.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 29.Bernier V, Lagacé M, Bichet DG, Bouvier M. Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol Metab 15: 222–228, 2004. doi: 10.1016/j.tem.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Bernier V, Lagacé M, Bichet DG, Bouvier M. Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol Metab 15: 222–228, 2004. doi: 10.1016/j.tem.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Bernier V, Morello JP, Zarruk A, Debrand N, Salahpour A, Lonergan M, Arthus MF, Laperrière A, Brouard R, Bouvier M, Bichet DG. Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. J Am Soc Nephrol 17: 232–243, 2006. doi: 10.1681/ASN.2005080854. [DOI] [PubMed] [Google Scholar]
- 32.Berson JF, Frank DW, Calvo PA, Bieler BM, Marks MS. A common temperature-sensitive allelic form of human tyrosinase is retained in the endoplasmic reticulum at the nonpermissive temperature. J Biol Chem 275: 12281–12289, 2000. doi: 10.1074/jbc.275.16.12281. [DOI] [PubMed] [Google Scholar]
- 33.Birk J, Friberg MA, Prescianotto-Baschong C, Spiess M, Rutishauser J. Dominant pro-vasopressin mutants that cause diabetes insipidus form disulfide-linked fibrillar aggregates in the endoplasmic reticulum. J Cell Sci 122: 3994–4002, 2009. doi: 10.1242/jcs.051136. [DOI] [PubMed] [Google Scholar]
- 34.Birnbaumer M. Vasopressin receptors. Trends Endocrinol Metab 11: 406–410, 2000. doi: 10.1016/S1043-2760(00)00304-0. [DOI] [PubMed] [Google Scholar]
- 35.Boeckler FM, Joerger AC, Jaggi G, Rutherford TJ, Veprintsev DB, Fersht AR. Targeted rescue of a destabilized mutant of p53 by an in silico screened drug. Proc Natl Acad Sci USA 105: 10360–10365, 2008. doi: 10.1073/pnas.0805326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bösl B, Grimminger V, Walter S. The molecular chaperone Hsp104–a molecular machine for protein disaggregation. J Struct Biol 156: 139–148, 2006. doi: 10.1016/j.jsb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Brantly M, Courtney M, Crystal RG. Repair of the secretion defect in the Z form of α 1-antitrypsin by addition of a second mutation. Science 242: 1700–1702, 1988. doi: 10.1126/science.2904702. [DOI] [PubMed] [Google Scholar]
- 38.Braun RJ, Westermann B. With the help of MOM: mitochondrial contributions to cellular quality control. Trends Cell Biol 27: 441–452, 2017. doi: 10.1016/j.tcb.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Brehme M, Voisine C. Model systems of protein-misfolding diseases reveal chaperone modifiers of proteotoxicity. Dis Model Mech 9: 823–838, 2016. doi: 10.1242/dmm.024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brehme M, Voisine C, Rolland T, Wachi S, Soper JH, Zhu Y, Orton K, Villella A, Garza D, Vidal M, Ge H, Morimoto RI. A chaperome subnetwork safeguards proteostasis in aging and neurodegenerative disease. Cell Reports 9: 1135–1150, 2014. doi: 10.1016/j.celrep.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bross P, Corydon TJ, Andresen BS, Jørgensen MM, Bolund L, Gregersen N. Protein misfolding and degradation in genetic diseases. Hum Mutat 14: 186–198, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 42.Brothers SP, Cornea A, Janovick JA, Conn PM. Human ‘loss-of-function’ GnRH receptor mutants retain wild type receptors in the endoplasmic reticulum: molecular basis of the dominant-negative effect. Mol Endocrinol 18: 1787–1797, 2004. doi: 10.1210/me.2004-0091. [DOI] [PubMed] [Google Scholar]
- 43.Brown CR, Hong-Brown LQ, Biwersi J, Verkman AS, Welch WJ. Chemical chaperones correct the mutant phenotype of the delta F508 cystic fibrosis transmembrane conductance regulator protein. Cell Stress Chaperones 1: 117–125, 1996. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science 232: 34–47, 1986. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 45.Bullock AN, Henckel J, DeDecker BS, Johnson CM, Nikolova PV, Proctor MR, Lane DP, Fersht AR. Thermodynamic stability of wild-type and mutant p53 core domain. Proc Natl Acad Sci USA 94: 14338–14342, 1997. doi: 10.1073/pnas.94.26.14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bullock AN, Henckel J, Fersht AR. Quantitative analysis of residual folding and DNA binding in mutant p53 core domain: definition of mutant states for rescue in cancer therapy. Oncogene 19: 1245–1256, 2000. doi: 10.1038/sj.onc.1203434. [DOI] [PubMed] [Google Scholar]
- 47.Burrows JA, Willis LK, Perlmutter DH. Chemical chaperones mediate increased secretion of mutant α 1-antitrypsin (α 1-AT) Z: A potential pharmacological strategy for prevention of liver injury and emphysema in α 1-AT deficiency. Proc Natl Acad Sci USA 97: 1796–1801, 2000. doi: 10.1073/pnas.97.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buscà R, Martínez M, Vilella E, Pognonec P, Deeb S, Auwerx J, Reina M, Vilaró S. The mutation Gly142-->Glu in human lipoprotein lipase produces a missorted protein that is diverted to lysosomes. J Biol Chem 271: 2139–2146, 1996. doi: 10.1074/jbc.271.4.2139. [DOI] [PubMed] [Google Scholar]
- 49.Bykov VJ, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, Bergman J, Wiman KG, Selivanova G. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med 8: 282–288, 2002. doi: 10.1038/nm0302-282. [DOI] [PubMed] [Google Scholar]
- 50.Bykov VJ, Issaeva N, Zache N, Shilov A, Hultcrantz M, Bergman J, Selivanova G, Wiman KG. Reactivation of mutant p53 and induction of apoptosis in human tumor cells by maleimide analogs. J Biol Chem 280: 30384–30391, 2005. doi: 10.1074/jbc.M501664200. [DOI] [PubMed] [Google Scholar]
- 51.Bykov VJ, Wiman KG. Mutant p53 reactivation by small molecules makes its way to the clinic. FEBS Lett 588: 2622–2627, 2014. doi: 10.1016/j.febslet.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 52.Bykov VJ, Zache N, Stridh H, Westman J, Bergman J, Selivanova G, Wiman KG. PRIMA-1(MET) synergizes with cisplatin to induce tumor cell apoptosis. Oncogene 24: 3484–3491, 2005. doi: 10.1038/sj.onc.1208419. [DOI] [PubMed] [Google Scholar]
- 53.Caramelo JJ, Parodi AJ. Getting in and out from calnexin/calreticulin cycles. J Biol Chem 283: 10221–10225, 2008. doi: 10.1074/jbc.R700048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carlile GW, Robert R, Goepp J, Matthes E, Liao J, Kus B, Macknight SD, Rotin D, Hanrahan JW, Thomas DY. Ibuprofen rescues mutant cystic fibrosis transmembrane conductance regulator trafficking. J Cyst Fibros 14: 16–25, 2015. doi: 10.1016/j.jcf.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Carlile GW, Robert R, Zhang D, Teske KA, Luo Y, Hanrahan JW, Thomas DY. Correctors of protein trafficking defects identified by a novel high-throughput screening assay. ChemBioChem 8: 1012–1020, 2007. doi: 10.1002/cbic.200700027. [DOI] [PubMed] [Google Scholar]
- 56.Carrell RW, Lomas DA. Conformational disease. Lancet 350: 134–138, 1997. doi: 10.1016/S0140-6736(97)02073-4. [DOI] [PubMed] [Google Scholar]
- 57.Cartier EA, Conti LR, Vandenberg CA, Shyng SL. Defective trafficking and function of KATP channels caused by a sulfonylurea receptor 1 mutation associated with persistent hyperinsulinemic hypoglycemia of infancy. Proc Natl Acad Sci USA 98: 2882–2887, 2001. doi: 10.1073/pnas.051499698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cartier EA, Shen S, Shyng SL. Modulation of the trafficking efficiency and functional properties of ATP-sensitive potassium channels through a single amino acid in the sulfonylurea receptor. J Biol Chem 278: 7081–7090, 2003. doi: 10.1074/jbc.M211395200. [DOI] [PubMed] [Google Scholar]
- 59.Castro-Fernández C, Maya-Núñez G, Conn PM. Beyond the signal sequence: protein routing in health and disease. Endocr Rev 26: 479–503, 2005. doi: 10.1210/er.2004-0010. [DOI] [PubMed] [Google Scholar]
- 60.Chaipatikul V, Erickson-Herbrandson LJ, Loh HH, Law PY. Rescuing the traffic-deficient mutants of rat μ-opioid receptors with hydrophobic ligands. Mol Pharmacol 64: 32–41, 2003. doi: 10.1124/mol.64.1.32. [DOI] [PubMed] [Google Scholar]
- 61.Chang HH, Asano N, Ishii S, Ichikawa Y, Fan JQ. Hydrophilic iminosugar active-site-specific chaperones increase residual glucocerebrosidase activity in fibroblasts from Gaucher patients. FEBS J 273: 4082–4092, 2006. doi: 10.1111/j.1742-4658.2006.05410.x. [DOI] [PubMed] [Google Scholar]
- 62.Chen Y, Chen C, Wang Y, Liu-Chen LY. Ligands regulate cell surface level of the human κ opioid receptor by activation-induced down-regulation and pharmacological chaperone-mediated enhancement: differential effects of nonpeptide and peptide agonists. J Pharmacol Exp Ther 319: 765–775, 2006. doi: 10.1124/jpet.106.107987. [DOI] [PubMed] [Google Scholar]
- 63.Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O’Riordan CR, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63: 827–834, 1990. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- 64.Chiti F, Dobson CM. Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade. Annu Rev Biochem 86: 27–68, 2017. doi: 10.1146/annurev-biochem-061516-045115. [DOI] [PubMed] [Google Scholar]
- 65.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 75: 333–366, 2006. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 66.Cholon DM, Quinney NL, Fulcher ML, Esther CR Jr, Das J, Dokholyan NV, Randell SH, Boucher RC, Gentzsch M. Potentiator ivacaftor abrogates pharmacological correction of ΔF508 CFTR in cystic fibrosis. Sci Transl Med 6: 246ra96, 2014. doi: 10.1126/scitranslmed.3008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ciechanover A. Intracellular protein degradation: from a vague idea, through the lysosome and the ubiquitin-proteasome system, and onto human diseases and drug targeting (Nobel lecture). Angew Chem Int Ed Engl 44: 5944–5967, 2005. doi: 10.1002/anie.200501428. [DOI] [PubMed] [Google Scholar]
- 68.Clancy JP, Rowe SM, Accurso FJ, Aitken ML, Amin RS, Ashlock MA, Ballmann M, Boyle MP, Bronsveld I, Campbell PW, De Boeck K, Donaldson SH, Dorkin HL, Dunitz JM, Durie PR, Jain M, Leonard A, McCoy KS, Moss RB, Pilewski JM, Rosenbluth DB, Rubenstein RC, Schechter MS, Botfield M, Ordoñez CL, Spencer-Green GT, Vernillet L, Wisseh S, Yen K, Konstan MW. Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax 67: 12–18, 2012. doi: 10.1136/thoraxjnl-2011-200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clark NE, Metcalf MC, Best D, Fleet GWJ, Garman SC. Pharmacological chaperones for human α-N-acetylgalactosaminidase. Proc Natl Acad Sci USA 109: 17400–17405, 2012. doi: 10.1073/pnas.1203924109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cohen FE, Kelly JW. Therapeutic approaches to protein-misfolding diseases. Nature 426: 905–909, 2003. doi: 10.1038/nature02265. [DOI] [PubMed] [Google Scholar]
- 71.Cohen SIA, Arosio P, Presto J, Kurudenkandy FR, Biverstal H, Dolfe L, Dunning C, Yang X, Frohm B, Vendruscolo M, Johansson J, Dobson CM, Fisahn A, Knowles TPJ, Linse S. A molecular chaperone breaks the catalytic cycle that generates toxic Aβ oligomers. Nat Struct Mol Biol 22: 207–213, 2015. doi: 10.1038/nsmb.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conn PM, Janovick JA. Pharmacoperone identification for therapeutic rescue of misfolded mutant proteins. Front Endocrinol (Lausanne) 2: 6, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Conn PM, Leaños-Miranda A, Janovick JA. Protein origami: therapeutic rescue of misfolded gene products. Mol Interv 2: 308–316, 2002. doi: 10.1124/mi.2.5.308. [DOI] [PubMed] [Google Scholar]
- 74.Conn PM, Smith E, Spicer T, Chase P, Scampavia L, Janovick JA. A phenotypic high throughput screening assay for the identification of pharmacoperones for the gonadotropin releasing hormone receptor. Assay Drug Dev Technol 12: 238–246, 2014. doi: 10.1089/adt.2014.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Conn PM, Smithson DC, Hodder PS, Stewart MD, Behringer RR, Smith E, Ulloa-Aguirre A, Janovick JA. Transitioning pharmacoperones to therapeutic use: in vivo proof-of-principle and design of high throughput screens. Pharmacol Res 83: 38–51, 2014. doi: 10.1016/j.phrs.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Conn PM, Ulloa-Aguirre A, Ito J, Janovick JA. G protein-coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacol Rev 59: 225–250, 2007. doi: 10.1124/pr.59.3.2. [DOI] [PubMed] [Google Scholar]
- 77.Conn PM, Ulloa-Aguirre A, Janovick JA. “Pharmacoperone”: what’s in a word? Pharmacol Res 83: 1–2, 2014. doi: 10.1016/j.phrs.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 78.Crystal RG. α 1-Antitrypsin deficiency, emphysema, and liver disease. Genetic basis and strategies for therapy. J Clin Invest 85: 1343–1352, 1990. doi: 10.1172/JCI114578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.D’Amico D, Sorrentino V, Auwerx J. Cytosolic proteostasis networks of the mitochondrial stress response. Trends Biochem Sci 42: 712–725, 2017. doi: 10.1016/j.tibs.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 80.Dabbs RA, Wyatt AR, Yerbury JJ, Ecroyd H, Wilson MR. Extracellular chaperones. Top Curr Chem 328: 241–268, 2013. doi: 10.1007/128_2011_262. [DOI] [PubMed] [Google Scholar]
- 81.Danpure CJ. Molecular etiology of primary hyperoxaluria type 1: new directions for treatment. Am J Nephrol 25: 303–310, 2005. doi: 10.1159/000086362. [DOI] [PubMed] [Google Scholar]
- 82.Datta R, Waheed A, Shah GN, Sly WS. Signal sequence mutation in autosomal dominant form of hypoparathyroidism induces apoptosis that is corrected by a chemical chaperone. Proc Natl Acad Sci USA 104: 19989–19994, 2007. doi: 10.1073/pnas.0708725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature 358: 761–764, 1992. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- 84.Denning GM, Ostedgaard LS, Welsh MJ. Abnormal localization of cystic fibrosis transmembrane conductance regulator in primary cultures of cystic fibrosis airway epithelia. J Cell Biol 118: 551–559, 1992. doi: 10.1083/jcb.118.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deschênes SM, Walcott JL, Wexler TL, Scherer SS, Fischbeck KH. Altered trafficking of mutant connexin32. J Neurosci 17: 9077–9084, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ding WX, Yin XM. Sorting, recognition and activation of the misfolded protein degradation pathways through macroautophagy and the proteasome. Autophagy 4: 141–150, 2008. doi: 10.4161/auto.5190. [DOI] [PubMed] [Google Scholar]
- 87.Doi T, Molday RS, Khorana HG. Role of the intradiscal domain in rhodopsin assembly and function. Proc Natl Acad Sci USA 87: 4991–4995, 1990. doi: 10.1073/pnas.87.13.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dong C, Wu G. Regulation of anterograde transport of α2-adrenergic receptors by the N termini at multiple intracellular compartments. J Biol Chem 281: 38543–38554, 2006. doi: 10.1074/jbc.M605734200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Donohoue PA, Tao YX, Collins M, Yeo GSH, O’Rahilly S, Segaloff DL. Deletion of codons 88-92 of the melanocortin-4 receptor gene: a novel deleterious mutation in an obese female. J Clin Endocrinol Metab 88: 5841–5845, 2003. doi: 10.1210/jc.2003-030903. [DOI] [PubMed] [Google Scholar]
- 90.Dormer RL, Dérand R, McNeilly CM, Mettey Y, Bulteau-Pignoux L, Métayé T, Vierfond JM, Gray MA, Galietta LJ, Morris MR, Pereira MM, Doull IJ, Becq F, McPherson MA. Correction of delF508-CFTR activity with benzo(c)quinolizinium compounds through facilitation of its processing in cystic fibrosis airway cells. J Cell Sci 114: 4073–4081, 2001. [DOI] [PubMed] [Google Scholar]
- 91.Douglas PM, Dillin A. Protein homeostasis and aging in neurodegeneration. J Cell Biol 190: 719–729, 2010. doi: 10.1083/jcb.201005144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Drummond DA, Wilke CO. Mistranslation-induced protein misfolding as a dominant constraint on coding-sequence evolution. Cell 134: 341–352, 2008. doi: 10.1016/j.cell.2008.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ellgaard L, Helenius A. ER quality control: towards an understanding at the molecular level. Curr Opin Cell Biol 13: 431–437, 2001. doi: 10.1016/S0955-0674(00)00233-7. [DOI] [PubMed] [Google Scholar]
- 94.Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 4: 181–191, 2003. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 95.Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science 286: 1882–1888, 1999. doi: 10.1126/science.286.5446.1882. [DOI] [PubMed] [Google Scholar]
- 96.Ellis J. Proteins as molecular chaperones. Nature 328: 378–379, 1987. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- 97.Eriksson S, Larsson C. Purification and partial characterization of pas-positive inclusion bodies from the liver in alpha 1-antitrypsin deficiency. N Engl J Med 292: 176–180, 1975. doi: 10.1056/NEJM197501232920403. [DOI] [PubMed] [Google Scholar]
- 98.Fan J, Perry SJ, Gao Y, Schwarz DA, Maki RA. A point mutation in the human melanin concentrating hormone receptor 1 reveals an important domain for cellular trafficking. Mol Endocrinol 19: 2579–2590, 2005. doi: 10.1210/me.2004-0301. [DOI] [PubMed] [Google Scholar]
- 99.Fan JQ. A contradictory treatment for lysosomal storage disorders: inhibitors enhance mutant enzyme activity. Trends Pharmacol Sci 24: 355–360, 2003. doi: 10.1016/S0165-6147(03)00158-5. [DOI] [PubMed] [Google Scholar]
- 100.Fan JQ. A counterintuitive approach to treat enzyme deficiencies: use of enzyme inhibitors for restoring mutant enzyme activity. Biol Chem 389: 1–11, 2008. doi: 10.1515/BC.2008.009. [DOI] [PubMed] [Google Scholar]
- 101.Fan JQ, Ishii S, Asano N, Suzuki Y. Accelerated transport and maturation of lysosomal α-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat Med 5: 112–115, 1999. doi: 10.1038/4801. [DOI] [PubMed] [Google Scholar]
- 102.Fan ZC, Tao YX. Functional characterization and pharmacological rescue of melanocortin-4 receptor mutations identified from obese patients. J Cell Mol Med 13, 9B: 3268–3282, 2009. doi: 10.1111/j.1582-4934.2009.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feige MJ, Hendershot LM. Disulfide bonds in ER protein folding and homeostasis. Curr Opin Cell Biol 23: 167–175, 2011. doi: 10.1016/j.ceb.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fishburn CS, Elazar Z, Fuchs S. Differential glycosylation and intracellular trafficking for the long and short isoforms of the D2 dopamine receptor. J Biol Chem 270: 29819–29824, 1995. doi: 10.1074/jbc.270.50.29819. [DOI] [PubMed] [Google Scholar]
- 105.Fortin JP, Dziadulewicz EK, Gera L, Marceau F. A nonpeptide antagonist reveals a highly glycosylated state of the rabbit kinin B1 receptor. Mol Pharmacol 69: 1146–1157, 2006. doi: 10.1124/mol.105.019976. [DOI] [PubMed] [Google Scholar]
- 106.Foster BA, Coffey HA, Morin MJ, Rastinejad F. Pharmacological rescue of mutant p53 conformation and function. Science 286: 2507–2510, 1999. doi: 10.1126/science.286.5449.2507. [DOI] [PubMed] [Google Scholar]
- 107.Frederikse PH. Amyloid-like protein structure in mammalian ocular lenses. Curr Eye Res 20: 462–468, 2000. doi: 10.1076/0271-3683(200006)2061-YFT462. [DOI] [PubMed] [Google Scholar]
- 108.Freeze HH. Genetic defects in the human glycome. Nat Rev Genet 7: 537–551, 2006. doi: 10.1038/nrg1894. [DOI] [PubMed] [Google Scholar]
- 109.Freeze HH, Ng BG. Golgi glycosylation and human inherited diseases. Cold Spring Harb Perspect Biol 3: a005371, 2011. doi: 10.1101/cshperspect.a005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Frenkel Z, Shenkman M, Kondratyev M, Lederkremer GZ. Separate roles and different routing of calnexin and ERp57 in ER quality control revealed by interactions with asialoglycoprotein receptor chains. Mol Cell Biol 15: 2133–2142, 2004. doi: 10.1091/mbc.E03-12-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Friedler A, Hansson LO, Veprintsev DB, Freund SM, Rippin TM, Nikolova PV, Proctor MR, Rüdiger S, Fersht AR. A peptide that binds and stabilizes p53 core domain: chaperone strategy for rescue of oncogenic mutants. Proc Natl Acad Sci USA 99: 937–942, 2002. doi: 10.1073/pnas.241629998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Frustaci A, Chimenti C, Ricci R, Natale L, Russo MA, Pieroni M, Eng CM, Desnick RJ. Improvement in cardiac function in the cardiac variant of Fabry’s disease with galactose-infusion therapy. N Engl J Med 345: 25–32, 2001. doi: 10.1056/NEJM200107053450104. [DOI] [PubMed] [Google Scholar]
- 113.Gabel CA, Bergmann JE. Processing of the asparagine-linked oligosaccharides of secreted and intracellular forms of the vesicular stomatitis virus G protein: in vivo evidence of Golgi apparatus compartmentalization. J Cell Biol 101: 460–469, 1985. doi: 10.1083/jcb.101.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.García-Mata R, Bebök Z, Sorscher EJ, Sztul ES. Characterization and dynamics of aggresome formation by a cytosolic GFP-chimera. J Cell Biol 146: 1239–1254, 1999. doi: 10.1083/jcb.146.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gekko K, Timasheff SN. Mechanism of protein stabilization by glycerol: preferential hydration in glycerol-water mixtures. Biochemistry 20: 4667–4676, 1981. doi: 10.1021/bi00519a023. [DOI] [PubMed] [Google Scholar]
- 116.Gekko K, Timasheff SN. Thermodynamic and kinetic examination of protein stabilization by glycerol. Biochemistry 20: 4677–4686, 1981. doi: 10.1021/bi00519a024. [DOI] [PubMed] [Google Scholar]
- 117.Generoso SF, Giustiniano M, La Regina G, Bottone S, Passacantilli S, Di Maro S, Cassese H, Bruno A, Mallardo M, Dentice M, Silvestri R, Marinelli L, Sarnataro D, Bonatti S, Novellino E, Stornaiuolo M. Pharmacological folding chaperones act as allosteric ligands of Frizzled4. Nat Chem Biol 11: 280–286, 2015. doi: 10.1038/nchembio.1770. [DOI] [PubMed] [Google Scholar]
- 118.Germain DP. Fabry disease. Orphanet J Rare Dis 5: 30, 2010. doi: 10.1186/1750-1172-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Germain DP, Hughes DA, Nicholls K, Bichet DG, Giugliani R, Wilcox WR, Feliciani C, Shankar SP, Ezgu F, Amartino H, Bratkovic D, Feldt-Rasmussen U, Nedd K, Sharaf El Din U, Lourenco CM, Banikazemi M, Charrow J, Dasouki M, Finegold D, Giraldo P, Goker-Alpan O, Longo N, Scott CR, Torra R, Tuffaha A, Jovanovic A, Waldek S, Packman S, Ludington E, Viereck C, Kirk J, Yu J, Benjamin ER, Johnson F, Lockhart DJ, Skuban N, Castelli J, Barth J, Barlow C, Schiffmann R. Treatment of Fabry’s disease with the pharmacologic chaperone migalastat. N Engl J Med 375: 545–555, 2016. doi: 10.1056/NEJMoa1510198. [DOI] [PubMed] [Google Scholar]
- 120.Gething M-J, Sambrook J. Protein folding in the cell. Nature 355: 33–45, 1992. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 121.Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, Weissman JS. Global analysis of protein expression in yeast. Nature 425: 737–741, 2003. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 122.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82: 373–428, 2002. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 123.Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell 94: 73–82, 1998. doi: 10.1016/S0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- 124.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature 426: 895–899, 2003. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 125.Goldberger RF, Epstein CJ, Anfinsen CB. Acceleration of reactivation of reduced bovine pancreatic ribonuclease by a microsomal system from rat liver. J Biol Chem 238: 628–635, 1963. [PubMed] [Google Scholar]
- 126.Goldfischer S. The internal reticular apparatus of Camillo Golgi: a complex, heterogeneous organelle, enriched in acid, neutral, and alkaline phosphatases, and involved in glycosylation, secretion, membrane flow, lysosome formation, and intracellular digestion. J Histochem Cytochem 30: 717–733, 1982. doi: 10.1177/30.7.6286754. [DOI] [PubMed] [Google Scholar]
- 127.Goldin E, Zheng W, Motabar O, Southall N, Choi JH, Marugan J, Austin CP, Sidransky E. High throughput screening for small molecule therapy for Gaucher disease using patient tissue as the source of mutant glucocerebrosidase. PLoS One 7: e29861, 2012. doi: 10.1371/journal.pone.0029861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Granell S, Mohammad S, Ramanagoudr-Bhojappa R, Baldini G. Obesity-linked variants of melanocortin-4 receptor are misfolded in the endoplasmic reticulum and can be rescued to the cell surface by a chemical chaperone. Mol Endocrinol 24: 1805–1821, 2010. doi: 10.1210/me.2010-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gregersen N, Bross P, Vang S, Christensen JH. Protein misfolding and human disease. Annu Rev Genomics Hum Genet 7: 103–124, 2006. doi: 10.1146/annurev.genom.7.080505.115737. [DOI] [PubMed] [Google Scholar]
- 130.Gu WX, Du GG, Kopp P, Rentoumis A, Albanese C, Kohn LD, Madison LD, Jameson JL. The thyrotropin (TSH) receptor transmembrane domain mutation (Pro556-Leu) in the hypothyroid hyt/hyt mouse results in plasma membrane targeting but defective TSH binding. Endocrinology 136: 3146–3153, 1995. doi: 10.1210/endo.136.7.7789342. [DOI] [PubMed] [Google Scholar]
- 131.Guan JL, Rose JK. Conversion of a secretory protein into a transmembrane protein results in its transport to the Golgi complex but not to the cell surface. Cell 37: 779–787, 1984. doi: 10.1016/0092-8674(84)90413-6. [DOI] [PubMed] [Google Scholar]
- 132.Guerriero CJ, Brodsky JL. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiol Rev 92: 537–576, 2012. doi: 10.1152/physrev.00027.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Halaban R, Cheng E, Svedine S, Aron R, Hebert DN. Proper folding and endoplasmic reticulum to Golgi transport of tyrosinase are induced by its substrates, DOPA and tyrosine. J Biol Chem 276: 11933–11938, 2001. doi: 10.1074/jbc.M008703200. [DOI] [PubMed] [Google Scholar]
- 134.Halaban R, Cheng E, Zhang Y, Moellmann G, Hanlon D, Michalak M, Setaluri V, Hebert DN. Aberrant retention of tyrosinase in the endoplasmic reticulum mediates accelerated degradation of the enzyme and contributes to the dedifferentiated phenotype of amelanotic melanoma cells. Proc Natl Acad Sci USA 94: 6210–6215, 1997. doi: 10.1073/pnas.94.12.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Halaban R, Svedine S, Cheng E, Smicun Y, Aron R, Hebert DN. Endoplasmic reticulum retention is a common defect associated with tyrosinase-negative albinism. Proc Natl Acad Sci USA 97: 5889–5894, 2000. doi: 10.1073/pnas.97.11.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hammerling BC, Gustafsson AB. Mitochondrial quality control in the myocardium: cooperation between protein degradation and mitophagy. J Mol Cell Cardiol 75: 122–130, 2014. doi: 10.1016/j.yjmcc.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hammond C, Braakman I, Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci USA 91: 913–917, 1994. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hanrahan JW, Sampson HM, Thomas DY. Novel pharmacological strategies to treat cystic fibrosis. Trends Pharmacol Sci 34: 119–125, 2013. doi: 10.1016/j.tips.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 139.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schürmann B, Heun R, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Hüll M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O’Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet 41: 1088–1093, 2009. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hartl FU. Cellular homeostasis and aging. Annu Rev Biochem 85: 1–4, 2016. doi: 10.1146/annurev-biochem-011116-110806. [DOI] [PubMed] [Google Scholar]
- 141.Hartl FU. Molecular chaperones in cellular protein folding. Nature 381: 571–579, 1996. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 142.Hartl FU. Protein misfolding diseases. Annu Rev Biochem 86: 21–26, 2017. doi: 10.1146/annurev-biochem-061516-044518. [DOI] [PubMed] [Google Scholar]
- 143.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature 475: 324–332, 2011. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 144.Hauri HP, Roth J, Sterchi EE, Lentze MJ. Transport to cell surface of intestinal sucrase-isomaltase is blocked in the Golgi apparatus in a patient with congenital sucrase-isomaltase deficiency. Proc Natl Acad Sci USA 82: 4423–4427, 1985. doi: 10.1073/pnas.82.13.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hawtin SR. Pharmacological chaperone activity of SR49059 to functionally recover misfolded mutations of the vasopressin V1a receptor. J Biol Chem 281: 14604–14614, 2006. doi: 10.1074/jbc.M511610200. [DOI] [PubMed] [Google Scholar]
- 146.Haynes CM, Titus EA, Cooper AA. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell 15: 767–776, 2004. doi: 10.1016/j.molcel.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 147.Hebert DN, Foellmer B, Helenius A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell 81: 425–433, 1995. doi: 10.1016/0092-8674(95)90395-X. [DOI] [PubMed] [Google Scholar]
- 148.Hebert DN, Molinari M. Flagging and docking: dual roles for N-glycans in protein quality control and cellular proteostasis. Trends Biochem Sci 37: 404–410, 2012. doi: 10.1016/j.tibs.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hebert DN, Molinari M. In and out of the ER: protein folding, quality control, degradation, and related human diseases. Physiol Rev 87: 1377–1408, 2007. doi: 10.1152/physrev.00050.2006. [DOI] [PubMed] [Google Scholar]
- 150.Hershko A. The ubiquitin system for protein degradation and some of its roles in the control of the cell-division cycle (Nobel lecture). Angew Chem Int Ed Engl 44: 5932–5943, 2005. doi: 10.1002/anie.200501724. [DOI] [PubMed] [Google Scholar]
- 151.Hetz C, Martinon F, Rodriguez D, Glimcher LH. The unfolded protein response: integrating stress signals through the stress sensor IRE1α. Physiol Rev 91: 1219–1243, 2011. doi: 10.1152/physrev.00001.2011. [DOI] [PubMed] [Google Scholar]
- 152.Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell 66: 191–197, 1991. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- 153.Hill SM, Hanzén S, Nyström T. Restricted access: spatial sequestration of damaged proteins during stress and aging. EMBO Rep 18: 377–391, 2017. doi: 10.15252/embr.201643458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hipp MS, Park SH, Hartl FU. Proteostasis impairment in protein-misfolding and -aggregation diseases. Trends Cell Biol 24: 506–514, 2014. doi: 10.1016/j.tcb.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 155.Hoang L, Byck S, Prevost L, Scriver CR. PAH Mutation Analysis Consortium Database: a database for disease-producing and other allelic variation at the human PAH locus. Nucleic Acids Res 24: 127–131, 1996. doi: 10.1093/nar/24.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hobbs HH, Russell DW, Brown MS, Goldstein JL. The LDL receptor locus in familial hypercholesterolemia: mutational analysis of a membrane protein. Annu Rev Genet 24: 133–170, 1990. doi: 10.1146/annurev.ge.24.120190.001025. [DOI] [PubMed] [Google Scholar]
- 157.Hou S, Madoux F, Scampavia L, Janovick JA, Conn PM, Spicer TP. Drug library screening for the identification of ionophores that correct the mistrafficking disorder associated with oxalosis kidney disease. SLAS Discov 22: 887–896, 2017. doi: 10.1177/2472555217689992. [DOI] [PubMed] [Google Scholar]
- 158.Huang H, Tao YX. A small molecule agonist THIQ as a novel pharmacoperone for intracellularly retained melanocortin-4 receptor mutants. Int J Biol Sci 10: 817–824, 2014. doi: 10.7150/ijbs.9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Huang H, Wang W, Tao YX. Pharmacological chaperones for the misfolded melanocortin-4 receptor associated with human obesity. Biochim Biophys Acta 1863, 10 Pt A: 2496–2507, 2017. doi: 10.1016/j.bbadis.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 160.Huang Y, Breitwieser GE. Rescue of calcium-sensing receptor mutants by allosteric modulators reveals a conformational checkpoint in receptor biogenesis. J Biol Chem 282: 9517–9525, 2007. doi: 10.1074/jbc.M609045200. [DOI] [PubMed] [Google Scholar]
- 161.Huckle WR, Conn PM. Molecular mechanism of gonadotropin releasing hormone action. II. The effector system. Endocr Rev 9: 387–395, 1988. doi: 10.1210/edrv-9-4-387. [DOI] [PubMed] [Google Scholar]
- 162.Humphreys DT, Carver JA, Easterbrook-Smith SB, Wilson MR. Clusterin has chaperone-like activity similar to that of small heat shock proteins. J Biol Chem 274: 6875–6881, 1999. doi: 10.1074/jbc.274.11.6875. [DOI] [PubMed] [Google Scholar]
- 163.Hunter PJ. Receptor redemption: skirting gene therapy to correct genetic defects. Scientist 18: 30, 2004. [Google Scholar]
- 164.Huopio H, Shyng SL, Otonkoski T, Nichols CG. K(ATP) channels and insulin secretion disorders. Am J Physiol Endocrinol Metab 283: E207–E216, 2002. doi: 10.1152/ajpendo.00047.2002. [DOI] [PubMed] [Google Scholar]
- 165.Ishii S, Chang HH, Kawasaki K, Yasuda K, Wu HL, Garman SC, Fan JQ. Mutant α-galactosidase A enzymes identified in Fabry disease patients with residual enzyme activity: biochemical characterization and restoration of normal intracellular processing by 1-deoxygalactonojirimycin. Biochem J 406: 285–295, 2007. doi: 10.1042/BJ20070479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ishii S, Chang HH, Yoshioka H, Shimada T, Mannen K, Higuchi Y, Taguchi A, Fan JQ. Preclinical efficacy and safety of 1-deoxygalactonojirimycin in mice for Fabry disease. J Pharmacol Exp Ther 328: 723–731, 2009. doi: 10.1124/jpet.108.149054. [DOI] [PubMed] [Google Scholar]
- 167.Ishii S, Kase R, Sakuraba H, Suzuki Y. Characterization of a mutant α-galactosidase gene product for the late-onset cardiac form of Fabry disease. Biochem Biophys Res Commun 197: 1585–1589, 1993. doi: 10.1006/bbrc.1993.2659. [DOI] [PubMed] [Google Scholar]
- 168.Ito M, Jameson JL, Ito M. Molecular basis of autosomal dominant neurohypophyseal diabetes insipidus. Cellular toxicity caused by the accumulation of mutant vasopressin precursors within the endoplasmic reticulum. J Clin Invest 99: 1897–1905, 1997. doi: 10.1172/JCI119357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ito M, Mori Y, Oiso Y, Saito H. A single base substitution in the coding region for neurophysin II associated with familial central diabetes insipidus. J Clin Invest 87: 725–728, 1991. doi: 10.1172/JCI115052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ito M, Yu RN, Jameson JL, Ito M. Mutant vasopressin precursors that cause autosomal dominant neurohypophyseal diabetes insipidus retain dimerization and impair the secretion of wild-type proteins. J Biol Chem 274: 9029–9037, 1999. doi: 10.1074/jbc.274.13.9029. [DOI] [PubMed] [Google Scholar]
- 171.Iwata A, Riley BE, Johnston JA, Kopito RR. HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin. J Biol Chem 280: 40282–40292, 2005. doi: 10.1074/jbc.M508786200. [DOI] [PubMed] [Google Scholar]
- 172.Janovick JA, Brothers SP, Cornea A, Bush E, Goulet MT, Ashton WT, Sauer DR, Haviv F, Greer J, Conn PM. Refolding of misfolded mutant GPCR: post-translational pharmacoperone action in vitro. Mol Cell Endocrinol 272: 77–85, 2007. doi: 10.1016/j.mce.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Janovick JA, Brothers SP, Cornea A, Bush E, Goulet MT, Ashton WT, Sauer DR, Haviv F, Greer J, Conn PM. Refolding of misfolded mutant GPCR: post-translational pharmacoperone action in vitro. Mol Cell Endocrinol 272: 77–85, 2007. doi: 10.1016/j.mce.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Janovick JA, Goulet M, Bush E, Greer J, Wettlaufer DG, Conn PM. Structure-activity relations of successful pharmacologic chaperones for rescue of naturally occurring and manufactured mutants of the gonadotropin-releasing hormone receptor. J Pharmacol Exp Ther 305: 608–614, 2003. doi: 10.1124/jpet.102.048454. [DOI] [PubMed] [Google Scholar]
- 175.Janovick JA, Goulet M, Bush E, Greer J, Wettlaufer DG, Conn PM. Structure-activity relations of successful pharmacologic chaperones for rescue of naturally occurring and manufactured mutants of the gonadotropin-releasing hormone receptor. J Pharmacol Exp Ther 305: 608–614, 2003. doi: 10.1124/jpet.102.048454. [DOI] [PubMed] [Google Scholar]
- 176.Janovick JA, Maya-Nunez G, Conn PM. Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab 87: 3255–3262, 2002. doi: 10.1210/jcem.87.7.8582. [DOI] [PubMed] [Google Scholar]
- 177.Janovick JA, Maya-Nunez G, Conn PM. Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab 87: 3255–3262, 2002. doi: 10.1210/jcem.87.7.8582. [DOI] [PubMed] [Google Scholar]
- 178.Janovick JA, Maya-Núñez G, Ulloa-Aguirre A, Huhtaniemi IT, Dias JA, Verbost P, Conn PM. Increased plasma membrane expression of human follicle-stimulating hormone receptor by a small molecule thienopyr(im)idine. Mol Cell Endocrinol 298: 84–88, 2009. doi: 10.1016/j.mce.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Janovick JA, Park BS, Conn PM. Therapeutic rescue of misfolded mutants: validation of primary high throughput screens for identification of pharmacoperone drugs. PLoS One 6: e22784, 2011. doi: 10.1371/journal.pone.0022784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Janovick JA, Spicer TP, Smith E, Bannister TD, Kenakin T, Scampavia L, Conn PM. Receptor antagonism/agonism can be uncoupled from pharmacoperone activity. Mol Cell Endocrinol 434: 176–185, 2016. doi: 10.1016/j.mce.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Janovick JA, Stewart MD, Jacob D, Martin LD, Deng JM, Stewart CA, Wang Y, Cornea A, Chavali L, Lopez S, Mitalipov S, Kang E, Lee HS, Manna PR, Stocco DM, Behringer RR, Conn PM. Restoration of testis function in hypogonadotropic hypogonadal mice harboring a misfolded GnRHR mutant by pharmacoperone drug therapy. Proc Natl Acad Sci USA 110: 21030–21035, 2013. doi: 10.1073/pnas.1315194110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jensen TJ, Loo MA, Pind S, Williams DB, Goldberg AL, Riordan JR. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell 83: 129–135, 1995. doi: 10.1016/0092-8674(95)90241-4. [DOI] [PubMed] [Google Scholar]
- 183.Jeppsson JO, Larsson C, Eriksson S. Characterization of alpha1-antitrypsin in the inclusion bodies from the liver in alpha1-antitrypsin deficiency. N Engl J Med 293: 576–579, 1975. doi: 10.1056/NEJM197509182931203. [DOI] [PubMed] [Google Scholar]
- 184.Jiang J, Jiang J, Zuo Y, Gu Z. Rapamycin protects the mitochondria against oxidative stress and apoptosis in a rat model of Parkinson’s disease. Int J Mol Med 31: 825–832, 2013. doi: 10.3892/ijmm.2013.1280. [DOI] [PubMed] [Google Scholar]
- 185.Jin SM, Youle RJ. The accumulation of misfolded proteins in the mitochondrial matrix is sensed by PINK1 to induce PARK2/Parkin-mediated mitophagy of polarized mitochondria. Autophagy 9: 1750–1757, 2013. doi: 10.4161/auto.26122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Joerger AC, Fersht AR. The p53 pathway: origins, inactivation in cancer, and emerging therapeutic approaches. Annu Rev Biochem 85: 375–404, 2016. doi: 10.1146/annurev-biochem-060815-014710. [DOI] [PubMed] [Google Scholar]
- 187.Joerger AC, Fersht AR. Structure-function-rescue: the diverse nature of common p53 cancer mutants. Oncogene 26: 2226–2242, 2007. doi: 10.1038/sj.onc.1210291. [DOI] [PubMed] [Google Scholar]
- 188.Johnson AE, van Waes MA. The translocon: a dynamic gateway at the ER membrane. Annu Rev Cell Dev Biol 15: 799–842, 1999. doi: 10.1146/annurev.cellbio.15.1.799. [DOI] [PubMed] [Google Scholar]
- 189.Johnson LG, Olsen JC, Sarkadi B, Moore KL, Swanstrom R, Boucher RC. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat Genet 2: 21–25, 1992. doi: 10.1038/ng0992-21. [DOI] [PubMed] [Google Scholar]
- 190.Johnson SC, Kaeberlein M. Rapamycin in aging and disease: maximizing efficacy while minimizing side effects. Oncotarget 7: 44876–44878, 2016. doi: 10.18632/oncotarget.10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Johnson SC, Yanos ME, Bitto A, Castanza A, Gagnidze A, Gonzalez B, Gupta K, Hui J, Jarvie C, Johnson BM, Letexier N, McCanta L, Sangesland M, Tamis O, Uhde L, Van Den Ende A, Rabinovitch PS, Suh Y, Kaeberlein M. Dose-dependent effects of mTOR inhibition on weight and mitochondrial disease in mice. Front Genet 6: 247, 2015. doi: 10.3389/fgene.2015.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Johnson SC, Yanos ME, Kayser EB, Quintana A, Sangesland M, Castanza A, Uhde L, Hui J, Wall VZ, Gagnidze A, Oh K, Wasko BM, Ramos FJ, Palmiter RD, Rabinovitch PS, Morgan PG, Sedensky MM, Kaeberlein M. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science 342: 1524–1528, 2013. doi: 10.1126/science.1244360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Johnson SM, Wiseman RL, Sekijima Y, Green NS, Adamski-Werner SL, Kelly JW. Native state kinetic stabilization as a strategy to ameliorate protein misfolding diseases: a focus on the transthyretin amyloidoses. Acc Chem Res 38: 911–921, 2005. doi: 10.1021/ar020073i. [DOI] [PubMed] [Google Scholar]
- 194.Johnston JA, Dalton MJ, Gurney ME, Kopito RR. Formation of high molecular weight complexes of mutant Cu, Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 97: 12571–12576, 2000. doi: 10.1073/pnas.220417997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol 143: 1883–1898, 1998. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jung O, Patnaik S, Marugan J, Sidransky E, Westbroek W. Progress and potential of non-inhibitory small molecule chaperones for the treatment of Gaucher disease and its implications for Parkinson disease. Expert Rev Proteomics 13: 471–479, 2016. doi: 10.1080/14789450.2016.1174583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kaler SG. ATP7A-related copper transport diseases-emerging concepts and future trends. Nat Rev Neurol 7: 15–29, 2011. doi: 10.1038/nrneurol.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Karamyshev AL, Patrick AE, Karamysheva ZN, Griesemer DS, Hudson H, Tjon-Kon-Sang S, Nilsson I, Otto H, Liu Q, Rospert S, von Heijne G, Johnson AE, Thomas PJ. Inefficient SRP interaction with a nascent chain triggers a mRNA quality control pathway. Cell 156: 146–157, 2014. doi: 10.1016/j.cell.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science 245: 1073–1080, 1989. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 200.Khanna R, Benjamin ER, Pellegrino L, Schilling A, Rigat BA, Soska R, Nafar H, Ranes BE, Feng J, Lun Y, Powe AC, Palling DJ, Wustman BA, Schiffmann R, Mahuran DJ, Lockhart DJ, Valenzano KJ. The pharmacological chaperone isofagomine increases the activity of the Gaucher disease L444P mutant form of β-glucosidase. FEBS J 277: 1618–1638, 2010. doi: 10.1111/j.1742-4658.2010.07588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Khanna R, Flanagan JJ, Feng J, Soska R, Frascella M, Pellegrino LJ, Lun Y, Guillen D, Lockhart DJ, Valenzano KJ. The pharmacological chaperone AT2220 increases recombinant human acid α-glucosidase uptake and glycogen reduction in a mouse model of Pompe disease. PLoS One 7: e40776, 2012. doi: 10.1371/journal.pone.0040776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Khanna R, Soska R, Lun Y, Feng J, Frascella M, Young B, Brignol N, Pellegrino L, Sitaraman SA, Desnick RJ, Benjamin ER, Lockhart DJ, Valenzano KJ. The pharmacological chaperone 1-deoxygalactonojirimycin reduces tissue globotriaosylceramide levels in a mouse model of Fabry disease. Mol Ther 18: 23–33, 2010. doi: 10.1038/mt.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kim BE, Smith K, Meagher CK, Petris MJ. A conditional mutation affecting localization of the Menkes disease copper ATPase. Suppression by copper supplementation. J Biol Chem 277: 44079–44084, 2002. doi: 10.1074/jbc.M208737200. [DOI] [PubMed] [Google Scholar]
- 204.Kim HE, Grant AR, Simic MS, Kohnz RA, Nomura DK, Durieux J, Riera CE, Sanchez M, Kapernick E, Wolff S, Dillin A. Lipid biosynthesis coordinates a mitochondrial-to-cytosolic stress response. Cell 166: 1539–1552.e16, 2016. doi: 10.1016/j.cell.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem 82: 323–355, 2013. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- 206.Kirstein J, Morito D, Kakihana T, Sugihara M, Minnen A, Hipp MS, Nussbaum-Krammer C, Kasturi P, Hartl FU, Nagata K, Morimoto RI. Proteotoxic stress and ageing triggers the loss of redox homeostasis across cellular compartments. EMBO J 34: 2334–2349, 2015. doi: 10.15252/embj.201591711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kishimoto TK, Hollander N, Roberts TM, Anderson DC, Springer TA. Heterogeneous mutations in the beta subunit common to the LFA-1, Mac-1, and p150,95 glycoproteins cause leukocyte adhesion deficiency. Cell 50: 193–202, 1987. doi: 10.1016/0092-8674(87)90215-7. [DOI] [PubMed] [Google Scholar]
- 208.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392: 605–608, 1998. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 209.Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol 10: 524–530, 2000. doi: 10.1016/S0962-8924(00)01852-3. [DOI] [PubMed] [Google Scholar]
- 210.Kopito RR. Biosynthesis and degradation of CFTR. Physiol Rev 79, Suppl: S167–S173, 1999. doi: 10.1152/physrev.1999.79.1.S167. [DOI] [PubMed] [Google Scholar]
- 211.Kopito RR. ER quality control: the cytoplasmic connection. Cell 88: 427–430, 1997. doi: 10.1016/S0092-8674(00)81881-4. [DOI] [PubMed] [Google Scholar]
- 212.Kopito RR, Ron D. Conformational disease. Nat Cell Biol 2: E207–E209, 2000. doi: 10.1038/35041139. [DOI] [PubMed] [Google Scholar]
- 213.Kopito RR, Sitia R. Aggresomes and Russell bodies. Symptoms of cellular indigestion? EMBO Rep 1: 225–231, 2000. doi: 10.1093/embo-reports/kvd052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Krestel HE, Mayford M, Seeburg PH, Sprengel R. A GFP-equipped bidirectional expression module well suited for monitoring tetracycline-regulated gene expression in mouse. Nucleic Acids Res 29: E39, 2001. doi: 10.1093/nar/29.7.e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell 40: 280–293, 2010. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem 84: 435–464, 2015. doi: 10.1146/annurev-biochem-060614-033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fiévet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, de Pancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossù P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanché H, Dartigues JF, Tzourio C, Gut I, Van Broeckhoven C, Alpérovitch A, Lathrop M, Amouyel P; European Alzheimer’s Disease Initiative Investigators . Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet 41: 1094–1099, 2009. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 218.Lambert JM, Gorzov P, Veprintsev DB, Söderqvist M, Segerbäck D, Bergman J, Fersht AR, Hainaut P, Wiman KG, Bykov VJ. PRIMA-1 reactivates mutant p53 by covalent binding to the core domain. Cancer Cell 15: 376–388, 2009. doi: 10.1016/j.ccr.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 219.Lambert JM, Moshfegh A, Hainaut P, Wiman KG, Bykov VJ. Mutant p53 reactivation by PRIMA-1MET induces multiple signaling pathways converging on apoptosis. Oncogene 29: 1329–1338, 2010. doi: 10.1038/onc.2009.425. [DOI] [PubMed] [Google Scholar]
- 220.Lamech LT, Haynes CM. The unpredictability of prolonged activation of stress response pathways. J Cell Biol 209: 781–787, 2015. doi: 10.1083/jcb.201503107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Leaños-Miranda A, Janovick JA, Conn PM. Receptor-misrouting: an unexpectedly prevalent and rescuable etiology in gonadotropin-releasing hormone receptor-mediated hypogonadotropic hypogonadism. J Clin Endocrinol Metab 87: 4825–4828, 2002. doi: 10.1210/jc.2002-020961. [DOI] [PubMed] [Google Scholar]
- 222.Lee BH, Lee MJ, Park S, Oh DC, Elsasser S, Chen PC, Gartner C, Dimova N, Hanna J, Gygi SP, Wilson SM, King RW, Finley D. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature 467: 179–184, 2010. doi: 10.1038/nature09299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lefkowitz RJ. A brief history of G-protein coupled receptors (Nobel Lecture). Angew Chem Int Ed Engl 52: 6366–6378, 2013. doi: 10.1002/anie.201301924. [DOI] [PubMed] [Google Scholar]
- 224.Lehmann S, Bykov VJ, Ali D, Andrén O, Cherif H, Tidefelt U, Uggla B, Yachnin J, Juliusson G, Moshfegh A, Paul C, Wiman KG, Andersson PO. Targeting p53 in vivo: a first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. J Clin Oncol 30: 3633–3639, 2012. doi: 10.1200/JCO.2011.40.7783. [DOI] [PubMed] [Google Scholar]
- 225.Lehrman MA, Schneider WJ, Brown MS, Davis CG, Elhammer A, Russell DW, Goldstein JL. The Lebanese allele at the low density lipoprotein receptor locus. Nonsense mutation produces truncated receptor that is retained in endoplasmic reticulum. J Biol Chem 262: 401–410, 1987. [PubMed] [Google Scholar]
- 226.Leinekugel P, Michel S, Conzelmann E, Sandhoff K. Quantitative correlation between the residual activity of beta-hexosaminidase A and arylsulfatase A and the severity of the resulting lysosomal storage disease. Hum Genet 88: 513–523, 1992. doi: 10.1007/BF00219337. [DOI] [PubMed] [Google Scholar]
- 227.Lewis MJ, Pelham HR. Inefficient quality control of thermosensitive proteins on the plasma membrane. PLoS One 4: e5038, 2009. doi: 10.1371/journal.pone.0005038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lieberman RL, Wustman BA, Huertas P, Powe AC Jr, Pine CW, Khanna R, Schlossmacher MG, Ringe D, Petsko GA. Structure of acid β-glucosidase with pharmacological chaperone provides insight into Gaucher disease. Nat Chem Biol 3: 101–107, 2007. doi: 10.1038/nchembio850. [DOI] [PubMed] [Google Scholar]
- 229.Lin YF, Schulz AM, Pellegrino MW, Lu Y, Shaham S, Haynes CM. Maintenance and propagation of a deleterious mitochondrial genome by the mitochondrial unfolded protein response. Nature 533: 416–419, 2016. doi: 10.1038/nature17989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lindquist S. The heat-shock response. Annu Rev Biochem 55: 1151–1191, 1986. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 231.Livnat-Levanon N, Glickman MH. Ubiquitin-proteasome system and mitochondria–reciprocity. Biochim Biophys Acta 1809: 80–87, 2011. doi: 10.1016/j.bbagrm.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 232.Lloyd ML, Olsen WA. A study of the molecular pathology of sucrase-isomaltase deficiency. A defect in the intracellular processing of the enzyme. N Engl J Med 316: 438–442, 1987. doi: 10.1056/NEJM198702193160804. [DOI] [PubMed] [Google Scholar]
- 233.Lodish HF. Transport of secretory and membrane glycoproteins from the rough endoplasmic reticulum to the Golgi. A rate-limiting step in protein maturation and secretion. J Biol Chem 263: 2107–2110, 1988. [PubMed] [Google Scholar]
- 234.Loebermann H, Tokuoka R, Deisenhofer J, Huber R. Human α1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol 177: 531–557, 1984. doi: 10.1016/0022-2836(84)90298-5. [DOI] [PubMed] [Google Scholar]
- 235.Longo M, Spinelli R, D’Esposito V, Zatterale F, Fiory F, Nigro C, Raciti GA, Miele C, Formisano P, Beguinot F, Di Jeso B. Pathologic endoplasmic reticulum stress induced by glucotoxic insults inhibits adipocyte differentiation and induces an inflammatory phenotype. Biochim Biophys Acta 1863, 6 Pt A: 1146–1156, 2016. doi: 10.1016/j.bbamcr.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 236.Loo TW, Bartlett MC, Clarke DM. Rescue of DeltaF508 and other misprocessed CFTR mutants by a novel quinazoline compound. Mol Pharm 2: 407–413, 2005. doi: 10.1021/mp0500521. [DOI] [PubMed] [Google Scholar]
- 237.Loo TW, Clarke DM. Chemical and pharmacological chaperones as new therapeutic agents. Expert Rev Mol Med 9: 1–18, 2007. doi: 10.1017/S1462399407000361. [DOI] [PubMed] [Google Scholar]
- 238.Lotti LV, Torrisi MR, Erra MC, Bonatti S. Morphological analysis of the transfer of VSV ts-045 G glycoprotein from the endoplasmic reticulum to the intermediate compartment in vero cells. Exp Cell Res 227: 323–331, 1996. doi: 10.1006/excr.1996.0281. [DOI] [PubMed] [Google Scholar]
- 239.Lu M, Staszewski L, Echeverri F, Xu H, Moyer BD. Endoplasmic reticulum degradation impedes olfactory G-protein coupled receptor functional expression. BMC Cell Biol 5: 34, 2004. doi: 10.1186/1471-2121-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lukacs GL, Verkman AS. CFTR: folding, misfolding and correcting the ΔF508 conformational defect. Trends Mol Med 18: 81–91, 2012. doi: 10.1016/j.molmed.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol Rev 87: 1011–1046, 2007. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- 242.Madoux F, Janovick JA, Smithson D, Fargue S, Danpure CJ, Scampavia L, Chen YT, Spicer TP, Conn PM. Development of a phenotypic high-content assay to identify pharmacoperone drugs for the treatment of primary hyperoxaluria type 1 by high-throughput screening. Assay Drug Dev Technol 13: 16–24, 2015. doi: 10.1089/adt.2014.627. [DOI] [PubMed] [Google Scholar]
- 243.Málaga-Diéguez L, Yang Q, Bauer J, Pankevych H, Freissmuth M, Nanoff C. Pharmacochaperoning of the A1 adenosine receptor is contingent on the endoplasmic reticulum. Mol Pharmacol 77: 940–952, 2010. doi: 10.1124/mol.110.063511. [DOI] [PubMed] [Google Scholar]
- 244.Matsuda J, Suzuki O, Oshima A, Yamamoto Y, Noguchi A, Takimoto K, Itoh M, Matsuzaki Y, Yasuda Y, Ogawa S, Sakata Y, Nanba E, Higaki K, Ogawa Y, Tominaga L, Ohno K, Iwasaki H, Watanabe H, Brady RO, Suzuki Y. Chemical chaperone therapy for brain pathology in G(M1)-gangliosidosis. Proc Natl Acad Sci USA 100: 15912–15917, 2003. doi: 10.1073/pnas.2536657100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, Sidransky E, Grabowski GA, Krainc D. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146: 37–52, 2011. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Meacham GC, Patterson C, Zhang W, Younger JM, Cyr DM. The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat Cell Biol 3: 100–105, 2001. doi: 10.1038/35050509. [DOI] [PubMed] [Google Scholar]
- 247.Mehta A, Beck M, Eyskens F, Feliciani C, Kantola I, Ramaswami U, Rolfs A, Rivera A, Waldek S, Germain DP. Fabry disease: a review of current management strategies. QJM 103: 641–659, 2010. doi: 10.1093/qjmed/hcq117. [DOI] [PubMed] [Google Scholar]
- 248.Meimaridou E, Gooljar SB, Ramnarace N, Anthonypillai L, Clark AJ, Chapple JP. The cytosolic chaperone Hsc70 promotes traffic to the cell surface of intracellular retained melanocortin-4 receptor mutants. Mol Endocrinol 25: 1650–1660, 2011. doi: 10.1210/me.2011-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: the long road to destruction. Nat Cell Biol 7: 766–772, 2005. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- 250.Milligan G. A day in the life of a G protein-coupled receptor: the contribution to function of G protein-coupled receptor dimerization. Br J Pharmacol 153, Suppl 1: S216–S229, 2008. doi: 10.1038/sj.bjp.0707490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Moeller HB, Rittig S, Fenton RA. Nephrogenic diabetes insipidus: essential insights into the molecular background and potential therapies for treatment. Endocr Rev 34: 278–301, 2013. doi: 10.1210/er.2012-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Molinari M, Helenius A. Glycoproteins form mixed disulphides with oxidoreductases during folding in living cells. Nature 402: 90–93, 1999. doi: 10.1038/47062. [DOI] [PubMed] [Google Scholar]
- 253.Monico CG, Olson JB, Milliner DS. Implications of genotype and enzyme phenotype in pyridoxine response of patients with type I primary hyperoxaluria. Am J Nephrol 25: 183–188, 2005. doi: 10.1159/000085411. [DOI] [PubMed] [Google Scholar]
- 254.Monico CG, Rossetti S, Olson JB, Milliner DS. Pyridoxine effect in type I primary hyperoxaluria is associated with the most common mutant allele. Kidney Int 67: 1704–1709, 2005. doi: 10.1111/j.1523-1755.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 255.Moolenaar CE, Ouwendijk J, Wittpoth M, Wisselaar HA, Hauri HP, Ginsel LA, Naim HY, Fransen JA. A mutation in a highly conserved region in brush-border sucrase-isomaltase and lysosomal alpha-glucosidase results in Golgi retention. J Cell Sci 110: 557–567, 1997. [DOI] [PubMed] [Google Scholar]
- 256.Morello JP, Salahpour A, Laperrière A, Bernier V, Arthus MF, Lonergan M, Petäjä-Repo U, Angers S, Morin D, Bichet DG, Bouvier M. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J Clin Invest 105: 887–895, 2000. doi: 10.1172/JCI8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Morello JP, Salahpour A, Laperrière A, Bernier V, Arthus MF, Lonergan M, Petäjä-Repo U, Angers S, Morin D, Bichet DG, Bouvier M. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J Clin Invest 105: 887–895, 2000. doi: 10.1172/JCI8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mulders SM, Bichet DG, Rijss JP, Kamsteeg EJ, Arthus MF, Lonergan M, Fujiwara M, Morgan K, Leijendekker R, van der Sluijs P, van Os CH, Deen PM. An aquaporin-2 water channel mutant which causes autosomal dominant nephrogenic diabetes insipidus is retained in the Golgi complex. J Clin Invest 102: 57–66, 1998. doi: 10.1172/JCI2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Munro S, Pelham HR. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell 46: 291–300, 1986. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- 260.Newton CL, Whay AM, McArdle CA, Zhang M, van Koppen CJ, van de Lagemaat R, Segaloff DL, Millar RP. Rescue of expression and signaling of human luteinizing hormone G protein-coupled receptor mutants with an allosterically binding small-molecule agonist. Proc Natl Acad Sci USA 108: 7172–7176, 2011. doi: 10.1073/pnas.1015723108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ng PC, Henikoff S. Predicting the effects of amino acid substitutions on protein function. Annu Rev Genomics Hum Genet 7: 61–80, 2006. doi: 10.1146/annurev.genom.7.080505.115630. [DOI] [PubMed] [Google Scholar]
- 262.Nijenhuis M, Zalm R, Burbach JP. Mutations in the vasopressin prohormone involved in diabetes insipidus impair endoplasmic reticulum export but not sorting. J Biol Chem 274: 21200–21208, 1999. doi: 10.1074/jbc.274.30.21200. [DOI] [PubMed] [Google Scholar]
- 263.Noorwez SM, Kuksa V, Imanishi Y, Zhu L, Filipek S, Palczewski K, Kaushal S. Pharmacological chaperone-mediated in vivo folding and stabilization of the P23H-opsin mutant associated with autosomal dominant retinitis pigmentosa. J Biol Chem 278: 14442–14450, 2003. doi: 10.1074/jbc.M300087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Noorwez SM, Malhotra R, McDowell JH, Smith KA, Krebs MP, Kaushal S. Retinoids assist the cellular folding of the autosomal dominant retinitis pigmentosa opsin mutant P23H. J Biol Chem 279: 16278–16284, 2004. doi: 10.1074/jbc.M312101200. [DOI] [PubMed] [Google Scholar]
- 265.Ohsumi Y. Historical landmarks of autophagy research. Cell Res 24: 9–23, 2014. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Okiyoneda T, Barrière H, Bagdány M, Rabeh WM, Du K, Höhfeld J, Young JC, Lukacs GL. Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science 329: 805–810, 2010. doi: 10.1126/science.1191542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Okumiya T, Ishii S, Takenaka T, Kase R, Kamei S, Sakuraba H, Suzuki Y. Galactose stabilizes various missense mutants of α-galactosidase in Fabry disease. Biochem Biophys Res Commun 214: 1219–1224, 1995. doi: 10.1006/bbrc.1995.2416. [DOI] [PubMed] [Google Scholar]
- 268.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci 30: 575–621, 2007. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 269.Ouwendijk J, Moolenaar CE, Peters WJ, Hollenberg CP, Ginsel LA, Fransen JA, Naim HY. Congenital sucrase-isomaltase deficiency. Identification of a glutamine to proline substitution that leads to a transport block of sucrase-isomaltase in a pre-Golgi compartment. J Clin Invest 97: 633–641, 1996. doi: 10.1172/JCI118459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov 5: 993–996, 2006. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 271.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306: 457–461, 2004. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 272.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Palade G. Intracellular aspects of the process of protein synthesis. Science 189: 347–358, 1975. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- 274.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Øvervatn A, Bjørkøy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282: 24131–24145, 2007. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 275.Parent A, Roy SJ, Iorio-Morin C, Lépine MC, Labrecque P, Gallant MA, Slipetz D, Parent JL. ANKRD13C acts as a molecular chaperone for G protein-coupled receptors. J Biol Chem 285: 40838–40851, 2010. doi: 10.1074/jbc.M110.142257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Parsell DA, Kowal AS, Singer MA, Lindquist S. Protein disaggregation mediated by heat-shock protein Hsp104. Nature 372: 475–478, 1994. doi: 10.1038/372475a0. [DOI] [PubMed] [Google Scholar]
- 277.Pedemonte N, Lukacs GL, Du K, Caci E, Zegarra-Moran O, Galietta LJ, Verkman AS. Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest 115: 2564–2571, 2005. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Peng M, Ostrovsky J, Kwon YJ, Polyak E, Licata J, Tsukikawa M, Marty E, Thomas J, Felix CA, Xiao R, Zhang Z, Gasser DL, Argon Y, Falk MJ. Inhibiting cytosolic translation and autophagy improves health in mitochondrial disease. Hum Mol Genet 24: 4829–4847, 2015. doi: 10.1093/hmg/ddv207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Perlmutter DH. Misfolded proteins in the endoplasmic reticulum. Lab Invest 79: 623–638, 1999. [PubMed] [Google Scholar]
- 280.Perlmutter DH, Kay RM, Cole FS, Rossing TH, Van Thiel D, Colten HR. The cellular defect in α 1-proteinase inhibitor (α 1-PI) deficiency is expressed in human monocytes and in Xenopus oocytes injected with human liver mRNA. Proc Natl Acad Sci USA 82: 6918–6921, 1985. doi: 10.1073/pnas.82.20.6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Petäjä-Repo UE, Hogue M, Bhalla S, Laperrière A, Morello JP, Bouvier M. Ligands act as pharmacological chaperones and increase the efficiency of δ opioid receptor maturation. EMBO J 21: 1628–1637, 2002. doi: 10.1093/emboj/21.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Petaja-Repo UE, Hogue M, Laperriere A, Walker P, Bouvier M. Export from the endoplasmic reticulum represents the limiting step in the maturation and cell surface expression of the human δ opioid receptor. J Biol Chem 275: 13727–13736, 2000. doi: 10.1074/jbc.275.18.13727. [DOI] [PubMed] [Google Scholar]
- 283.Pey AL, Stricher F, Serrano L, Martinez A. Predicted effects of missense mutations on native-state stability account for phenotypic outcome in phenylketonuria, a paradigm of misfolding diseases. Am J Hum Genet 81: 1006–1024, 2007. doi: 10.1086/521879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Pey AL, Ying M, Cremades N, Velazquez-Campoy A, Scherer T, Thöny B, Sancho J, Martinez A. Identification of pharmacological chaperones as potential therapeutic agents to treat phenylketonuria. J Clin Invest 118: 2858–2867, 2008. doi: 10.1172/JCI34355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Phillips JA., III Dominant-negative diabetes insipidus and other endocrinopathies. J Clin Invest 112: 1641–1643, 2003. doi: 10.1172/JCI20441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Phuan PW, Veit G, Tan JA, Finkbeiner WE, Lukacs GL, Verkman AS. Potentiators of defective ΔF508-CFTR gating that do not interfere with corrector action. Mol Pharmacol 88: 791–799, 2015. doi: 10.1124/mol.115.099689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85: 257–273, 2015. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Plemper RK, Böhmler S, Bordallo J, Sommer T, Wolf DH. Mutant analysis links the translocon and BiP to retrograde protein transport for ER degradation. Nature 388: 891–895, 1997. doi: 10.1038/42276. [DOI] [PubMed] [Google Scholar]
- 289.Porto C, Cardone M, Fontana F, Rossi B, Tuzzi MR, Tarallo A, Barone MV, Andria G, Parenti G. The pharmacological chaperone N-butyldeoxynojirimycin enhances enzyme replacement therapy in Pompe disease fibroblasts. Mol Ther 17: 964–971, 2009. doi: 10.1038/mt.2009.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Prockop DJ, Constantinou CD, Dombrowski KE, Hojima Y, Kadler KE, Kuivaniemi H, Tromp G, Vogel BE. Type I procollagen: the gene-protein system that harbors most of the mutations causing osteogenesis imperfecta and probably more common heritable disorders of connective tissue. Am J Med Genet 34: 60–67, 1989. doi: 10.1002/ajmg.1320340112. [DOI] [PubMed] [Google Scholar]
- 291.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science 216: 136–144, 1982. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 292.Qi L, Tsai B, Arvan P. New insights into the physiological role of endoplasmic reticulum-associated degradation. Trends Cell Biol 27: 430–440, 2017. doi: 10.1016/j.tcb.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Qu D, Teckman JH, Omura S, Perlmutter DH. Degradation of a mutant secretory protein, α1-antitrypsin Z, in the endoplasmic reticulum requires proteasome activity. J Biol Chem 271: 22791–22795, 1996. doi: 10.1074/jbc.271.37.22791. [DOI] [PubMed] [Google Scholar]
- 294.Quirós PM, Langer T, López-Otín C. New roles for mitochondrial proteases in health, ageing and disease. Nat Rev Mol Cell Biol 16: 345–359, 2015. doi: 10.1038/nrm3984. [DOI] [PubMed] [Google Scholar]
- 295.Quirós PM, Mottis A, Auwerx J. Mitonuclear communication in homeostasis and stress. Nat Rev Mol Cell Biol 17: 213–226, 2016. doi: 10.1038/nrm.2016.23. [DOI] [PubMed] [Google Scholar]
- 296.Ravikumar B, Duden R, Rubinsztein DC. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet 11: 1107–1117, 2002. doi: 10.1093/hmg/11.9.1107. [DOI] [PubMed] [Google Scholar]
- 297.René P, Le Gouill C, Pogozheva ID, Lee G, Mosberg HI, Farooqi IS, Valenzano KJ, Bouvier M. Pharmacological chaperones restore function to MC4R mutants responsible for severe early-onset obesity. J Pharmacol Exp Ther 335: 520–532, 2010. doi: 10.1124/jpet.110.172098. [DOI] [PubMed] [Google Scholar]
- 298.Richter F, Fleming SM, Watson M, Lemesre V, Pellegrino L, Ranes B, Zhu C, Mortazavi F, Mulligan CK, Sioshansi PC, Hean S, De La Rosa K, Khanna R, Flanagan J, Lockhart DJ, Wustman BA, Clark SW, Chesselet MF. A GCase chaperone improves motor function in a mouse model of synucleinopathy. Neurotherapeutics 11: 840–856, 2014. doi: 10.1007/s13311-014-0294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell 40: 253–266, 2010. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 300.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, Drumm ML, Iannuzzi MC, Collins FS, Tsui LC. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245: 1066–1073, 1989. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 301.Rippin TM, Bykov VJ, Freund SM, Selivanova G, Wiman KG, Fersht AR. Characterization of the p53-rescue drug CP-31398 in vitro and in living cells. Oncogene 21: 2119–2129, 2002. doi: 10.1038/sj.onc.1205362. [DOI] [PubMed] [Google Scholar]
- 302.Robben JH, Sze M, Knoers NV, Deen PM. Rescue of vasopressin V2 receptor mutants by chemical chaperones: specificity and mechanism. Mol Biol Cell 17: 379–386, 2006. doi: 10.1091/mbc.E05-06-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Robert J, Auzan C, Ventura MA, Clauser E. Mechanisms of cell-surface rerouting of an endoplasmic reticulum-retained mutant of the vasopressin V1b/V3 receptor by a pharmacological chaperone. J Biol Chem 280: 42198–42206, 2005. doi: 10.1074/jbc.M510180200. [DOI] [PubMed] [Google Scholar]
- 304.Robert R, Carlile GW, Liao J, Balghi H, Lesimple P, Liu N, Kus B, Rotin D, Wilke M, de Jonge HR, Scholte BJ, Thomas DY, Hanrahan JW. Correction of the Δ phe508 cystic fibrosis transmembrane conductance regulator trafficking defect by the bioavailable compound glafenine. Mol Pharmacol 77: 922–930, 2010. doi: 10.1124/mol.109.062679. [DOI] [PubMed] [Google Scholar]
- 305.Robert R, Carlile GW, Pavel C, Liu N, Anjos SM, Liao J, Luo Y, Zhang D, Thomas DY, Hanrahan JW. Structural analog of sildenafil identified as a novel corrector of the F508del-CFTR trafficking defect. Mol Pharmacol 73: 478–489, 2008. doi: 10.1124/mol.107.040725. [DOI] [PubMed] [Google Scholar]
- 306.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529, 2007. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 307.Ron I, Horowitz M. ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum Mol Genet 14: 2387–2398, 2005. doi: 10.1093/hmg/ddi240. [DOI] [PubMed] [Google Scholar]
- 308.Rongo C. Better to burn out than it is to rust: coordinating cellular redox states during aging and stress. EMBO J 34: 2310–2311, 2015. doi: 10.15252/embj.201592504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Ronzoni R, Berardelli R, Medicina D, Sitia R, Gooptu B, Fra AM. Aberrant disulphide bonding contributes to the ER retention of alpha1-antitrypsin deficiency variants. Hum Mol Genet 25: 642–650, 2016. doi: 10.1093/hmg/ddv501. [DOI] [PubMed] [Google Scholar]
- 310.Rose I. Ubiquitin at Fox Chase (Nobel lecture). Angew Chem Int Ed Engl 44: 5926–5931, 2005. doi: 10.1002/anie.200500995. [DOI] [PubMed] [Google Scholar]
- 311.Rose JK, Doms RW. Regulation of protein export from the endoplasmic reticulum. Annu Rev Cell Biol 4: 257–288, 1988. doi: 10.1146/annurev.cb.04.110188.001353. [DOI] [PubMed] [Google Scholar]
- 312.Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med 10, Suppl: S10–S17, 2004. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 313.Rubenstein RC, Egan ME, Zeitlin PL. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing delta F508-CFTR. J Clin Invest 100: 2457–2465, 1997. doi: 10.1172/JCI119788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Rubenstein RC, Zeitlin PL. A pilot clinical trial of oral sodium 4-phenylbutyrate (Buphenyl) in deltaF508-homozygous cystic fibrosis patients: partial restoration of nasal epithelial CFTR function. Am J Respir Crit Care Med 157: 484–490, 1998. doi: 10.1164/ajrccm.157.2.9706088. [DOI] [PubMed] [Google Scholar]
- 315.Rubenstein RC, Zeitlin PL. Sodium 4-phenylbutyrate downregulates Hsc70: implications for intracellular trafficking of DeltaF508-CFTR. Am J Physiol Cell Physiol 278: C259–C267, 2000. doi: 10.1152/ajpcell.2000.278.2.C259. [DOI] [PubMed] [Google Scholar]
- 316.Russell TA, Ito M, Ito M, Yu RN, Martinson FA, Weiss J, Jameson JL. A murine model of autosomal dominant neurohypophyseal diabetes insipidus reveals progressive loss of vasopressin-producing neurons. J Clin Invest 112: 1697–1706, 2003. doi: 10.1172/JCI200318616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol 14: 20–28, 2004. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 318.Sachidanandam R, Weissman D, Schmidt SC, Kakol JM, Stein LD, Marth G, Sherry S, Mullikin JC, Mortimore BJ, Willey DL, Hunt SE, Cole CG, Coggill PC, Rice CM, Ning Z, Rogers J, Bentley DR, Kwok PY, Mardis ER, Yeh RT, Schultz B, Cook L, Davenport R, Dante M, Fulton L, Hillier L, Waterston RH, McPherson JD, Gilman B, Schaffner S, Van Etten WJ, Reich D, Higgins J, Daly MJ, Blumenstiel B, Baldwin J, Stange-Thomann N, Zody MC, Linton L, Lander ES, Altshuler D; International SNP Map Working Group . A map of human genome sequence variation containing 1.42 million single nucleotide polymorphisms. Nature 409: 928–933, 2001. doi: 10.1038/35057149. [DOI] [PubMed] [Google Scholar]
- 319.Sala AJ, Bott LC, Morimoto RI. Shaping proteostasis at the cellular, tissue, and organismal level. J Cell Biol 216: 1231–1241, 2017. doi: 10.1083/jcb.201612111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Sampson HM, Robert R, Liao J, Matthes E, Carlile GW, Hanrahan JW, Thomas DY. Identification of a NBD1-binding pharmacological chaperone that corrects the trafficking defect of F508del-CFTR. Chem Biol 18: 231–242, 2011. doi: 10.1016/j.chembiol.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 321.Sato S, Ward CL, Krouse ME, Wine JJ, Kopito RR. Glycerol reverses the misfolding phenotype of the most common cystic fibrosis mutation. J Biol Chem 271: 635–638, 1996. doi: 10.1074/jbc.271.2.635. [DOI] [PubMed] [Google Scholar]
- 322.Sawkar AR, Cheng WC, Beutler E, Wong CH, Balch WE, Kelly JW. Chemical chaperones increase the cellular activity of N370S β -glucosidase: a therapeutic strategy for Gaucher disease. Proc Natl Acad Sci USA 99: 15428–15433, 2002. doi: 10.1073/pnas.192582899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Schmidt M, Finley D. Regulation of proteasome activity in health and disease. Biochim Biophys Acta 1843: 13–25, 2014. doi: 10.1016/j.bbamcr.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Schmitz M, Alfalah M, Aerts JM, Naim HY, Zimmer KP. Impaired trafficking of mutants of lysosomal glucocerebrosidase in Gaucher’s disease. Int J Biochem Cell Biol 37: 2310–2320, 2005. doi: 10.1016/j.biocel.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 325.Schubert U, Antón LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature 404: 770–774, 2000. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 326.Schueler UH, Kolter T, Kaneski CR, Zirzow GC, Sandhoff K, Brady RO. Correlation between enzyme activity and substrate storage in a cell culture model system for Gaucher disease. J Inherit Metab Dis 27: 649–658, 2004. doi: 10.1023/B:BOLI.0000042959.44318.7c. [DOI] [PubMed] [Google Scholar]
- 327.Schulz AM, Haynes CM. UPR(mt)-mediated cytoprotection and organismal aging. Biochim Biophys Acta 1847: 1448–1456, 2015. doi: 10.1016/j.bbabio.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Segref A, Kevei É, Pokrzywa W, Schmeisser K, Mansfeld J, Livnat-Levanon N, Ensenauer R, Glickman MH, Ristow M, Hoppe T. Pathogenesis of human mitochondrial diseases is modulated by reduced activity of the ubiquitin/proteasome system. Cell Metab 19: 642–652, 2014. doi: 10.1016/j.cmet.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 329.Shorter J. Designer protein disaggregases to counter neurodegenerative disease. Curr Opin Genet Dev 44: 1–8, 2017. doi: 10.1016/j.gde.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Shorter J. The mammalian disaggregase machinery: Hsp110 synergizes with Hsp70 and Hsp40 to catalyze protein disaggregation and reactivation in a cell-free system. PLoS One 6: e26319, 2011. doi: 10.1371/journal.pone.0026319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Si-Hoe SL, De Bree FM, Nijenhuis M, Davies JE, Howell LM, Tinley H, Waller SJ, Zeng Q, Zalm R, Sonnemans M, Van Leeuwen FW, Burbach JP, Murphy D. Endoplasmic reticulum derangement in hypothalamic neurons of rats expressing a familial neurohypophyseal diabetes insipidus mutant vasopressin transgene. FASEB J 14: 1680–1684, 2000. [DOI] [PubMed] [Google Scholar]
- 332.Singh OV, Pollard HB, Zeitlin PL. Chemical rescue of deltaF508-CFTR mimics genetic repair in cystic fibrosis bronchial epithelial cells. Mol Cell Proteomics 7: 1099–1110, 2008. doi: 10.1074/mcp.M700303-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Singh OV, Vij N, Mogayzel PJ Jr, Jozwik C, Pollard HB, Zeitlin PL. Pharmacoproteomics of 4-phenylbutyrate-treated IB3-1 cystic fibrosis bronchial epithelial cells. J Proteome Res 5: 562–571, 2006. doi: 10.1021/pr050319o. [DOI] [PubMed] [Google Scholar]
- 334.Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature 426: 891–894, 2003. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- 335.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 84: 1155–1228, 2004. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 336.Smith E, Janovick JA, Bannister TD, Shumate J, Scampavia L, Conn PM, Spicer TP. Identification of potential pharmacoperones capable of rescuing the functionality of misfolded vasopressin 2 receptor involved in nephrogenic diabetes insipidus. J Biomol Screen 21: 824–831, 2016. doi: 10.1177/1087057116653925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Sobolewski A, Rudarakanchana N, Upton PD, Yang J, Crilley TK, Trembath RC, Morrell NW. Failure of bone morphogenetic protein receptor trafficking in pulmonary arterial hypertension: potential for rescue. Hum Mol Genet 17: 3180–3190, 2008. doi: 10.1093/hmg/ddn214. [DOI] [PubMed] [Google Scholar]
- 338.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci 4: 49–60, 2003. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 339.Sousa M, Parodi AJ. The molecular basis for the recognition of misfolded glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. EMBO J 14: 4196–4203, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Staretz-Chacham O, Lang TC, LaMarca ME, Krasnewich D, Sidransky E. Lysosomal storage disorders in the newborn. Pediatrics 123: 1191–1207, 2009. doi: 10.1542/peds.2008-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Steet RA, Chung S, Wustman B, Powe A, Do H, Kornfeld SA. The iminosugar isofagomine increases the activity of N370S mutant acid β-glucosidase in Gaucher fibroblasts by several mechanisms. Proc Natl Acad Sci USA 103: 13813–13818, 2006. doi: 10.1073/pnas.0605928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA 90: 1977–1981, 1993. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Sun Y, Breydo L, Makarava N, Yang Q, Bocharova OV, Baskakov IV. Site-specific conformational studies of prion protein (PrP) amyloid fibrils revealed two cooperative folding domains within amyloid structure. J Biol Chem 282: 9090–9097, 2007. doi: 10.1074/jbc.M608623200. [DOI] [PubMed] [Google Scholar]
- 344.Sweeney P, Park H, Baumann M, Dunlop J, Frydman J, Kopito R, McCampbell A, Leblanc G, Venkateswaran A, Nurmi A, Hodgson R. Protein misfolding in neurodegenerative diseases: implications and strategies. Transl Neurodegener 6: 6, 2017. doi: 10.1186/s40035-017-0077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Tamarappoo BK, Verkman AS. Defective aquaporin-2 trafficking in nephrogenic diabetes insipidus and correction by chemical chaperones. J Clin Invest 101: 2257–2267, 1998. doi: 10.1172/JCI2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Tannous A, Pisoni GB, Hebert DN, Molinari M. N-linked sugar-regulated protein folding and quality control in the ER. Semin Cell Dev Biol 41: 79–89, 2015. doi: 10.1016/j.semcdb.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Tao YX. Constitutive activation of G protein-coupled receptors and diseases: insights into mechanisms of activation and therapeutics. Pharmacol Ther 120: 129–148, 2008. doi: 10.1016/j.pharmthera.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Tao YX. Functional characterization of novel melanocortin-3 receptor mutations identified from obese subjects. Biochim Biophys Acta 1772: 1167–1174, 2007. doi: 10.1016/j.bbadis.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 349.Tao YX. Inactivating mutations of G protein-coupled receptors and diseases: structure-function insights and therapeutic implications. Pharmacol Ther 111: 949–973, 2006. doi: 10.1016/j.pharmthera.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 350.Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev 31: 506–543, 2010. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Tao YX. Molecular mechanisms of the neural melanocortin receptor dysfunction in severe early onset obesity. Mol Cell Endocrinol 239: 1–14, 2005. doi: 10.1016/j.mce.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 352.Tao YX. Mutations in melanocortin-4 receptor and human obesity. Prog Mol Biol Transl Sci 88: 173–204, 2009. doi: 10.1016/S1877-1173(09)88006-X. [DOI] [PubMed] [Google Scholar]
- 353.Tao YX, Conn PM. Chaperoning G protein-coupled receptors: from cell biology to therapeutics. Endocr Rev 35: 602–647, 2014. doi: 10.1210/er.2013-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Tao YX, Huang H. Ipsen 5i is a novel potent pharmacoperone for intracellularly retained melanocortin-4 receptor mutants. Front Endocrinol (Lausanne) 5: 131, 2014. doi: 10.3389/fendo.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Tao YX, Segaloff DL. Functional characterization of melanocortin-3 receptor variants identify a loss-of-function mutation involving an amino acid critical for G protein-coupled receptor activation. J Clin Endocrinol Metab 89: 3936–3942, 2004. doi: 10.1210/jc.2004-0367. [DOI] [PubMed] [Google Scholar]
- 356.Tao YX, Segaloff DL. Functional characterization of melanocortin-4 receptor mutations associated with childhood obesity. Endocrinology 144: 4544–4551, 2003. doi: 10.1210/en.2003-0524. [DOI] [PubMed] [Google Scholar]
- 357.Tarnow P, Schoneberg T, Krude H, Gruters A, Biebermann H. Mutationally induced disulfide bond formation within the third extracellular loop causes melanocortin 4 receptor inactivation in patients with obesity. J Biol Chem 278: 48666–48673, 2003. doi: 10.1074/jbc.M309941200. [DOI] [PubMed] [Google Scholar]
- 358.Taschenberger G, Mougey A, Shen S, Lester LB, LaFranchi S, Shyng SL. Identification of a familial hyperinsulinism-causing mutation in the sulfonylurea receptor 1 that prevents normal trafficking and function of KATP channels. J Biol Chem 277: 17139–17146, 2002. doi: 10.1074/jbc.M200363200. [DOI] [PubMed] [Google Scholar]
- 359.Taylor JP, Tanaka F, Robitschek J, Sandoval CM, Taye A, Markovic-Plese S, Fischbeck KH. Aggresomes protect cells by enhancing the degradation of toxic polyglutamine-containing protein. Hum Mol Genet 12: 749–757, 2003. doi: 10.1093/hmg/ddg074. [DOI] [PubMed] [Google Scholar]
- 360.Taylor RC, Dillin A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell 153: 1435–1447, 2013. doi: 10.1016/j.cell.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Teckman JH. Lack of effect of oral 4-phenylbutyrate on serum alpha-1-antitrypsin in patients with alpha-1-antitrypsin deficiency: a preliminary study. J Pediatr Gastroenterol Nutr 39: 34–37, 2004. doi: 10.1097/00005176-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 362.Tolleshaug H, Hobgood KK, Brown MS, Goldstein JL. The LDL receptor locus in familial hypercholesterolemia: multiple mutations disrupt transport and processing of a membrane receptor. Cell 32: 941–951, 1983. doi: 10.1016/0092-8674(83)90079-X. [DOI] [PubMed] [Google Scholar]
- 363.Trombetta ES, Helenius A. Lectins as chaperones in glycoprotein folding. Curr Opin Struct Biol 8: 587–592, 1998. doi: 10.1016/S0959-440X(98)80148-6. [DOI] [PubMed] [Google Scholar]
- 364.Trombetta ES, Parodi AJ. Quality control and protein folding in the secretory pathway. Annu Rev Cell Dev Biol 19: 649–676, 2003. doi: 10.1146/annurev.cellbio.19.110701.153949. [DOI] [PubMed] [Google Scholar]
- 365.Tropak MB, Reid SP, Guiral M, Withers SG, Mahuran D. Pharmacological enhancement of β-hexosaminidase activity in fibroblasts from adult Tay-Sachs and Sandhoff Patients. J Biol Chem 279: 13478–13487, 2004. doi: 10.1074/jbc.M308523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Trzcińska-Daneluti AM, Chen A, Nguyen L, Murchie R, Jiang C, Moffat J, Pelletier L, Rotin D. RNA interference screen to identify kinases that suppress rescue of ΔF508-CFTR. Mol Cell Proteomics 14: 1569–1583, 2015. doi: 10.1074/mcp.M114.046375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Tyedmers J, Mogk A, Bukau B. Cellular strategies for controlling protein aggregation. Nat Rev Mol Cell Biol 11: 777–788, 2010. doi: 10.1038/nrm2993. [DOI] [PubMed] [Google Scholar]
- 368.Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM. Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic 5: 821–837, 2004. doi: 10.1111/j.1600-0854.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 369.Ungar D. Golgi linked protein glycosylation and associated diseases. Semin Cell Dev Biol 20: 762–769, 2009. doi: 10.1016/j.semcdb.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 370.Ungar D, Oka T, Krieger M, Hughson FM. Retrograde transport on the COG railway. Trends Cell Biol 16: 113–120, 2006. doi: 10.1016/j.tcb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 371.Valastyan JS, Lindquist S. Mechanisms of protein-folding diseases at a glance. Dis Model Mech 7: 9–14, 2014. doi: 10.1242/dmm.013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Valente EM, Bentivoglio AR, Dixon PH, Ferraris A, Ialongo T, Frontali M, Albanese A, Wood NW. Localization of a novel locus for autosomal recessive early-onset parkinsonism, PARK6, on human chromosome 1p35-p36. Am J Hum Genet 68: 895–900, 2001. doi: 10.1086/319522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Van Anken E, Braakman I. Versatility of the endoplasmic reticulum protein folding factory. Crit Rev Biochem Mol Biol 40: 191–228, 2005. doi: 10.1080/10409230591008161. [DOI] [PubMed] [Google Scholar]
- 374.Van Craenenbroeck K, Clark SD, Cox MJ, Oak JN, Liu F, Van Tol HH. Folding efficiency is rate-limiting in dopamine D4 receptor biogenesis. J Biol Chem 280: 19350–19357, 2005. doi: 10.1074/jbc.M414043200. [DOI] [PubMed] [Google Scholar]
- 375.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, Decker CJ, Miller M, McCartney J, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu PA. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci USA 108: 18843–18848, 2011. doi: 10.1073/pnas.1105787108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Van Oosten-Hawle P, Morimoto RI. Organismal proteostasis: role of cell-nonautonomous regulation and transcellular chaperone signaling. Genes Dev 28: 1533–1543, 2014. doi: 10.1101/gad.241125.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Van Oosten-Hawle P, Porter RS, Morimoto RI. Regulation of organismal proteostasis by transcellular chaperone signaling. Cell 153: 1366–1378, 2013. doi: 10.1016/j.cell.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Veit G, Avramescu RG, Chiang AN, Houck SA, Cai Z, Peters KW, Hong JS, Pollard HB, Guggino WB, Balch WE, Skach WR, Cutting GR, Frizzell RA, Sheppard DN, Cyr DM, Sorscher EJ, Brodsky JL, Lukacs GL. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol Biol Cell 27: 424–433, 2016. doi: 10.1091/mbc.E14-04-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Veit G, Avramescu RG, Perdomo D, Phuan PW, Bagdany M, Apaja PM, Borot F, Szollosi D, Wu YS, Finkbeiner WE, Hegedus T, Verkman AS, Lukacs GL. Some gating potentiators, including VX-770, diminish ΔF508-CFTR functional expression. Sci Transl Med 6: 246ra97, 2014. doi: 10.1126/scitranslmed.3008889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Verbanac KM, Heath EC. Biosynthesis, processing, and secretion of M and Z variant human α 1-antitrypsin. J Biol Chem 261: 9979–9989, 1986. [PubMed] [Google Scholar]
- 381.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature 408: 307–310, 2000. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 382.Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell 137: 413–431, 2009. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 383.Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet 3: 7–13, 1993. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- 384.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334: 1081–1086, 2011. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 385.Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol 197: 857–867, 2012. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Wang X, Chen XJ. A cytosolic network suppressing mitochondria-mediated proteostatic stress and cell death. Nature 524: 481–484, 2015. doi: 10.1038/nature14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Wang Y, Bartlett MC, Loo TW, Clarke DM. Specific rescue of cystic fibrosis transmembrane conductance regulator processing mutants using pharmacological chaperones. Mol Pharmacol 70: 297–302, 2006. doi: 10.1124/mol.106.023994. [DOI] [PubMed] [Google Scholar]
- 388.Ward CL, Omura S, Kopito RR. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell 83: 121–127, 1995. doi: 10.1016/0092-8674(95)90240-6. [DOI] [PubMed] [Google Scholar]
- 389.Ware FE, Vassilakos A, Peterson PA, Jackson MR, Lehrman MA, Williams DB. The molecular chaperone calnexin binds Glc1Man9GlcNAc2 oligosaccharide as an initial step in recognizing unfolded glycoproteins. J Biol Chem 270: 4697–4704, 1995. doi: 10.1074/jbc.270.9.4697. [DOI] [PubMed] [Google Scholar]
- 390.Wigley WC, Fabunmi RP, Lee MG, Marino CR, Muallem S, DeMartino GN, Thomas PJ. Dynamic association of proteasomal machinery with the centrosome. J Cell Biol 145: 481–490, 1999. doi: 10.1083/jcb.145.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Wilkinson B, Gilbert HF. Protein disulfide isomerase. Biochim Biophys Acta 1699: 35–44, 2004. doi: 10.1016/S1570-9639(04)00063-9. [DOI] [PubMed] [Google Scholar]
- 392.Winklhofer KF, Tatzelt J, Haass C. The two faces of protein misfolding: gain- and loss-of-function in neurodegenerative diseases. EMBO J 27: 336–349, 2008. doi: 10.1038/sj.emboj.7601930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Wise EL, Bonner KT, Williams TJ, Pease JE. A single nucleotide polymorphism in the CCR3 gene ablates receptor export to the plasma membrane. J Allergy Clin Immunol 126: 150–7.e2, 2010. doi: 10.1016/j.jaci.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 394.Woehlbier U, Colombo A, Saaranen MJ, Pérez V, Ojeda J, Bustos FJ, Andreu CI, Torres M, Valenzuela V, Medinas DB, Rozas P, Vidal RL, Lopez-Gonzalez R, Salameh J, Fernandez-Collemann S, Muñoz N, Matus S, Armisen R, Sagredo A, Palma K, Irrazabal T, Almeida S, Gonzalez-Perez P, Campero M, Gao FB, Henny P, van Zundert B, Ruddock LW, Concha ML, Henriquez JP, Brown RH, Hetz C. ALS-linked protein disulfide isomerase variants cause motor dysfunction. EMBO J 35: 845–865, 2016. doi: 10.15252/embj.201592224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Wright JM, Zeitlin PL, Cebotaru L, Guggino SE, Guggino WB. Gene expression profile analysis of 4-phenylbutyrate treatment of IB3-1 bronchial epithelial cell line demonstrates a major influence on heat-shock proteins. Physiol Genomics 16: 204–211, 2004. doi: 10.1152/physiolgenomics.00160.2003. [DOI] [PubMed] [Google Scholar]
- 396.Wrobel L, Topf U, Bragoszewski P, Wiese S, Sztolsztener ME, Oeljeklaus S, Varabyova A, Lirski M, Chroscicki P, Mroczek S, Januszewicz E, Dziembowski A, Koblowska M, Warscheid B, Chacinska A. Mistargeted mitochondrial proteins activate a proteostatic response in the cytosol. Nature 524: 485–488, 2015. doi: 10.1038/nature14951. [DOI] [PubMed] [Google Scholar]
- 397.Wu X, Katz E, Della Valle MC, Mascioli K, Flanagan JJ, Castelli JP, Schiffmann R, Boudes P, Lockhart DJ, Valenzano KJ, Benjamin ER. A pharmacogenetic approach to identify mutant forms of α-galactosidase A that respond to a pharmacological chaperone for Fabry disease. Hum Mutat 32: 965–977, 2011. doi: 10.1002/humu.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Wu Y, Whitman I, Molmenti E, Moore K, Hippenmeyer P, Perlmutter DH. A lag in intracellular degradation of mutant α1-antitrypsin correlates with the liver disease phenotype in homozygous PiZZ α 1-antitrypsin deficiency. Proc Natl Acad Sci USA 91: 9014–9018, 1994. doi: 10.1073/pnas.91.19.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Wüller S, Wiesner B, Löffler A, Furkert J, Krause G, Hermosilla R, Schaefer M, Schülein R, Rosenthal W, Oksche A. Pharmacochaperones post-translationally enhance cell surface expression by increasing conformational stability of wild-type and mutant vasopressin V2 receptors. J Biol Chem 279: 47254–47263, 2004. doi: 10.1074/jbc.M408154200. [DOI] [PubMed] [Google Scholar]
- 400.Wyatt AR, Yerbury JJ, Ecroyd H, Wilson MR. Extracellular chaperones and proteostasis. Annu Rev Biochem 82: 295–322, 2013. doi: 10.1146/annurev-biochem-072711-163904. [DOI] [PubMed] [Google Scholar]
- 401.Yam GH, Zuber C, Roth J. A synthetic chaperone corrects the trafficking defect and disease phenotype in a protein misfolding disorder. FASEB J 19: 12–18, 2005. doi: 10.1096/fj.04-2375com. [DOI] [PubMed] [Google Scholar]
- 402.Yamamoto K, Fujii R, Toyofuku Y, Saito T, Koseki H, Hsu VW, Aoe T. The KDEL receptor mediates a retrieval mechanism that contributes to quality control at the endoplasmic reticulum. EMBO J 20: 3082–3091, 2001. doi: 10.1093/emboj/20.12.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Yan F, Lin CW, Weisiger E, Cartier EA, Taschenberger G, Shyng SL. Sulfonylureas correct trafficking defects of ATP-sensitive potassium channels caused by mutations in the sulfonylurea receptor. J Biol Chem 279: 11096–11105, 2004. doi: 10.1074/jbc.M312810200. [DOI] [PubMed] [Google Scholar]
- 404.Yan FF, Lin YW, MacMullen C, Ganguly A, Stanley CA, Shyng SL. Congenital hyperinsulinism associated ABCC8 mutations that cause defective trafficking of ATP-sensitive K+ channels: identification and rescue. Diabetes 56: 2339–2348, 2007. doi: 10.2337/db07-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN. Living with water stress: evolution of osmolyte systems. Science 217: 1214–1222, 1982. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 406.Yu MH, Lee KN, Kim J. The Z type variation of human alpha 1-antitrypsin causes a protein folding defect. Nat Struct Biol 2: 363–367, 1995. doi: 10.1038/nsb0595-363. [DOI] [PubMed] [Google Scholar]
- 407.Zeitlin PL, Diener-West M, Rubenstein RC, Boyle MP, Lee CK, Brass-Ernst L. Evidence of CFTR function in cystic fibrosis after systemic administration of 4-phenylbutyrate. Mol Ther 6: 119–126, 2002. doi: 10.1006/mthe.2002.0639. [DOI] [PubMed] [Google Scholar]
- 408.Zhang D, Ciciriello F, Anjos SM, Carissimo A, Liao J, Carlile GW, Balghi H, Robert R, Luini A, Hanrahan JW, Thomas DY. Ouabain mimics low temperature rescue of F508del-CFTR in cystic fibrosis epithelial cells. Front Pharmacol 3: 176, 2012. doi: 10.3389/fphar.2012.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Zhang JX, Braakman I, Matlack KE, Helenius A. Quality control in the secretory pathway: the role of calreticulin, calnexin and BiP in the retention of glycoproteins with C-terminal truncations. Mol Biol Cell 8: 1943–1954, 1997. doi: 10.1091/mbc.8.10.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Zhang L, Button B, Gabriel SE, Burkett S, Yan Y, Skiadopoulos MH, Dang YL, Vogel LN, McKay T, Mengos A, Boucher RC, Collins PL, Pickles RJ. CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium. PLoS Biol 7: e1000155, 2009. doi: 10.1371/journal.pbio.1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Zhang X, Wang Y. Glycosylation quality control by the Golgi structure. J Mol Biol 428: 3183–3193, 2016. doi: 10.1016/j.jmb.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. EMBO J 21: 4411–4419, 2002. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]