Abstract
Mercury‐dependent artisanal and small‐scale gold mining (ASGM) is the largest source of mercury pollution on Earth. In this practice, elemental mercury is used to extract gold from ore as an amalgam. The amalgam is typically isolated by hand and then heated—often with a torch or over a stove—to distill the mercury and isolate the gold. Mercury release from tailings and vaporized mercury exceed 1000 tonnes each year from ASGM. The health effects on the miners are dire, with inhaled mercury leading to neurological damage and other health issues. The communities near these mines are also affected due to mercury contamination of water and soil and subsequent accumulation in food staples, such as fish—a major source of dietary protein in many ASGM regions. The risks to children are also substantial, with mercury emissions from ASGM resulting in both physical and mental disabilities and compromised development. Between 10 and 19 million people use mercury to mine for gold in more than 70 countries, making mercury pollution from ASGM a global issue. With the Minamata Convention on Mercury entering force this year, there is political motivation to help overcome the problem of mercury in ASGM. In this effort, chemists can play a central role. Here, the problem of mercury in ASGM is reviewed with a discussion on how the chemistry community can contribute solutions. Introducing portable and low‐cost mercury sensors, inexpensive and scalable remediation technologies, novel methods to prevent mercury uptake in fish and food crops, and efficient and easy‐to‐use mercury‐free mining techniques are all ways in which the chemistry community can help. To meet these challenges, it is critical that new technologies or techniques are low‐cost and adaptable to the remote and under‐resourced areas in which ASGM is most common. The problem of mercury pollution in ASGM is inherently a chemistry problem. We therefore encourage the chemistry community to consider and address this issue that affects the health of millions of people.
Keywords: artisanal and small-scale gold mining (ASGM), gold, mercury, Minamata convention, mining
The Mercury Problem in Artisanal Gold Mining
Mercury‐based artisanal and small‐scale gold mining (ASGM) causes more mercury pollution than any other human activity.1 In this practice, mercury metal is used to extract gold from ore as a stable amalgam. The amalgam is then heated to evaporate the mercury and isolate the gold. While mercury amalgamation has been used for thousands of years to mine gold and silver,2 it is unfortunately still a widespread technique in present‐day artisanal gold mining. Mercury is abundant and inexpensive, sourced through a variety of industrial supply chains or mined directly from cinnabar, making it a readily available tool for mining gold.3 It is estimated that between 410 and 1400 tonnes of mercury are emitted through ASGM each year, accounting for 37 % of global mercury emissions.1, 4 Driven by rising prices in gold (approximately $40,000 USD kg−1 for most of 2017),5 ASGM is widespread, with an estimated 10 to 19 million miners working primarily in Asia, Africa and South America (Figure 1).1, 3c, 4, 6 As many as 5 million women7 and children1a, 8 are involved directly in these mining operations. These miners typically live in impoverished areas and use gold mining as a source of income. In many cases, few other opportunities for employment exist in these regions and ASGM is therefore a critical way to sustain their livelihood.9
Figure 1.
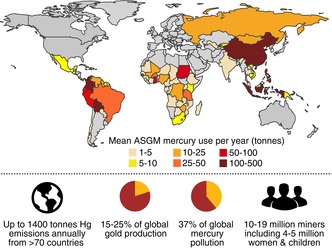
Estimated annual mercury use in artisanal and small‐scale gold mining (ASGM).1b, 4, 6c, 10
These mining activities largely take place in the so‐called “informal” economy in which participants operate unlicensed or without legal authorization—a reason why effective regulation of mercury emissions is extraordinarily difficult.11 Nevertheless, these artisanal miners contribute substantially to the local and global economy, generating approximately 15 to 25 % of the world's gold.1a, 4, 10b So, while each individual mining operation may be relatively small, the practice is widespread. Because of the extraordinary amounts of mercury handled directly by the miners and released into the environment, the burden to human health is staggering.12 Mercury vapor inhaled by miners results in impaired cognitive function, neurological damage, kidney damage and several other health problems.13 In some cases, amalgams are processed near the home or in gold shops in villages or cities, so the mercury vapor generated in the process affects non‐miners living in these areas.10b For children and fetuses, exposure to mercury pollution is especially dangerous as it increases the likelihood of physical deformities, neurological damage and lower IQ.14 These risks of mercury exposure are also compounded by the high levels of mercury that accumulate in fish and other food supplies in ASGM communities.12, 15
The mercury problem in ASGM is a growing crisis in environmental and human well‐being. Rightfully, the issue has garnered attention in both the scientific16 and general news media8b, 17 as ASGM becomes more prevalent and its effects more visible.8b, 17b The damage to cognitive and neurological function of the miners and the physical and mental disabilities prevalent in children near ASGM communities are startlingly clear in these reports.8b, 17b Furthermore, the wider damage to the environment and the transport of high levels of mercury to sites beyond the mine have, in some cases, led to national conflict and military intervention.17a The time to address this problem is now. On August 16th 2017, the Minamata Convention on Mercury was ratified by more than 50 parties to the treaty.18 This milestone brought into force the most comprehensive effort to control the trade, use and emissions of mercury. As ASGM is the largest source of mercury pollution worldwide, reforming this sector is a priority of the Minamata Convention.18 Accordingly, Article 7 of the convention requires signatories to “take steps to reduce, and where feasible eliminate, the use of mercury…and the emissions and releases to the environment of mercury from, such mining and processing.”18 There are also specific provisions for member nations to help educate miners and promote research into sustainable, mercury‐free mining.18 In the accompanying Annex C of the Minamata Convention, further actions are prescribed that include the elimination of four especially problematic activities: whole‐ore amalgamation, open heating of amalgams, heating amalgams in residential areas, and the use of cyanide to extract gold from mercury‐rich tailings (vide infra). Because these goals will likely require advances in environmental chemistry and innovative extractive technologies, it is worthwhile to consider how the chemistry community might contribute to these global initiatives. Finally, the urgency to address these challenges cannot be overstated as the parties to the convention have three years develop and implement a national plan of action.18
The purpose of this Minireview is to outline the process and consequences of mining gold by amalgamation with mercury. Then, we discuss some opportunities for the chemistry community to help solve the mercury problem in ASGM. For instance, there is a need for inexpensive and portable mercury sensors; low‐cost and scalable remediation technology for air, water and soil; mining techniques that minimize mercury exposure and emissions; and reliable and easy‐to‐adopt methods for mercury‐free mining. The majority of ASGM is carried out in developing nations by miners that rely on gold sales for their livelihood. Therefore, any technology designed to solve the mercury problem must be inexpensive and, ideally, support the miners in their trade. Support of these miners through new and sustainable mining practices is critical because they typically live in areas with few other economic opportunities. This is a significant challenge to which chemists can contribute.
The Process: Mining Gold with Mercury
A representative mercury‐based ASGM technique is outlined in Figure 2.19 The reader is referred to leading publications for further details of this process, as well as other techniques for extracting gold through amalgamation with mercury, such as panning and sluicing in alluvial operations, or direct addition of mercury into mining pits.6a,6b, 9, 20 For the so‐called “whole‐ore amalgamation” in Figure 2, rocks and ore are first crushed into small pieces by hand. This material, the consistency of coarse sand, is then added with water into motorized mills or trommels (Figure 2 A). Because water is used, this equipment is often built near waterways such as rivers, which can later be subject to severe mercury pollution. Heavy steel or tungsten bars or balls are also added to the trommels to pulverize the ore upon rotation (Figure 2 A). To extract the gold from the crushed ore, liquid mercury is added directly to the trommel and mixed for several hours. Mercury is frequently used to capture the gold (rather than mercury‐free gravity separation techniques) in cases in which the gold grain is very fine.20d, 21 In each trommel, it is common to add between 0.3 and 1 kg of mercury to 20 kg of crushed ore (Figure 2 B).20d The mercury–gold amalgam is dense and separates from the finer crushed rocks and sand. The amalgam is then isolated by hand and excess liquid mercury is recovered for reuse (Figure 2 C). The amalgamation process may be repeated 3 or 4 times to maximize gold extraction.19a The combined mercury–gold amalgams are then strained through cloth to remove excess liquid mercury and provide a mercury–gold amalgam ball (Figure 2 D). These amalgams are typically between 40 % and 80 % mercury by mass.19a
Figure 2.

Liquid mercury is used in artisanal and small‐scale gold mining. a) Trommels are used to crush rock and mix mercury with ore. b) As much as 1 kg of liquid mercury is added to each trommel along with water and 20 kg of ore. c) Excess mercury is recovered, but mercury‐rich mine tailings are often released directly into the environment. d) Additional mercury is used on the mercury–gold concentrate to form a solid amalgam, which may be isolated by hand. e) The mercury–gold amalgam is heated with a torch to distill the mercury and allow isolation of gold. f) A sample of gold isolated in an artisanal mine. All images are used with permission from Yayasan Tambuhak Sinta (YTS) and Pure Earth.
To recover the gold from the amalgam, the solid is heated to vaporize the mercury (Figure 2 E) and separate it from the gold (Figure 2 F). The heating can be accomplished with a hand torch or a stove.19a In some parts of the world, centralized processing centers in villages carry out this process for a fee.20d This mercury distillation step in the mining process is especially dangerous, because of the exposure to mercury gas that can cause neurological damage. Typically no measures are taken to recover the mercury or prevent mercury inhalation (Figure 2 E). In other cases, a condenser (or retort) is used to recover the liquid mercury for reuse. The use of a retorts is important for lowering the exposure to mercury vapor in mining communities, but many retorts still lead to substantial loss of mercury.19a
The tailings in ASGM are also a major source of mercury pollution. After the amalgam is isolated from the trommel, the leftover crushed ore, water, and unrecovered mercury are either released directly into the environment or processed further in large tanks containing aqueous sodium cyanide that, in the presence of oxygen, extracts unrecovered gold over several days or weeks as the water‐soluble dicyanoaurate(I) [Au(CN)2]−.20d In some new trials to recover mercury, the tailings are passed through sluices.21 Where cyanide tanks are used, the dissolved gold can be recovered by binding to activated charcoal or through precipitation with zinc.20d When cyanide leaching is used to process tailings, the final waste stream is typically dumped in rudimentary tailing ponds or released into waterways—often the same water sources used for irrigation, drinking and fishing. Because significant amounts of mercury are also made water soluble by complexing with cyanide, this practice results in substantial release of highly toxic and mobile mercury species into aquatic environments, which leads to mercury methylation by bacteria and bioaccumulation of methylmercury.19a, 20d
The gold isolated after heating the amalgam will typically contain up to 5 % residual mercury by mass.10b Gold shops will typically purchase the gold and refine it further to remove mercury, again through distillation.3b In some parts of the world, these gold shops also process the full mercury–gold amalgam.10b, 20d These gold shops are additional sources of mercury emissions that are problematic because they are typically located in high population centers.10b The final gold product is melted and molded for further sale on the gold market.
Mercury Emissions from Artisanal and Small‐Scale Gold Mining
In ASGM, the amalgamation process, tailings processing, and gold recovery from the amalgam result in substantial release of mercury into the environment. By some estimates, release of mercury from ASGM exceeds 1 million kg each year.1 This level of mercury pollution may exceed the combined emissions of coal combustion, cement production, chlor‐alkali plant operation, and large‐scale industrial mining and metallurgy.1b It is therefore important to look at each stage of common ASGM practices to identify how mercury is released into the environment so that measures can be taken to prevent such emissions and mitigate harm. The primary sources of these emissions are from tailings discharge to land and water and mercury gas emissions during amalgam roasting.
In the amalgamation process such as the one shown in Figure 2, substantial amounts of mercury can be lost in the tailings. In particular, milling ore and mercury in trommels can result in the formation of tiny mercury droplets that become finely dispersed in the tailings. This “mercury flour” is especially problematic because it can be easily washed away with water and transported far from the mining site (Figure 3 A).22 In some cases, mercury‐rich tailings can travel in rivers hundreds of kilometers from the mine.22c The floured mercury is also difficult to recover because it does not coalesce efficiently.22a,22b In cases in which the tailings are released directly into the environment, the mercury contaminates water and soil, where it is difficult to recover or remediate.21 These tailings may contain between 50 and 5000 mg mercury per kg ore, resulting in a substantial loss of mercury.6a, 23 This lost mercury may also contain up to 14 % gold by mass, so significant amounts of gold are lost in tailings too.22b These tailings are sometimes sold for further processing in large tanks using cyanide leaching.23 The cyanide complexes and dissolves the residual gold. The gold is then recovered by the addition of activated carbon, which is, in turn, isolated and burned (often in open drums).23 As much as 20 g of gold can be isolated for every tonne of tailings.23 Because mercury is also solubilized through cyanide leaching, release of this water into the environment—either through direct release or by way of drainage from unsecured tailing ponds—is a significant source of mercury pollution.20d, 21, 23 The release of mercury from tailings into soil and water is a serious hazard to human health because it can compromise food safety24 and contaminate drinking water.25 Mercury contaminated water used for irrigation also leads to contaminated food crops, such as rice.26
Figure 3.
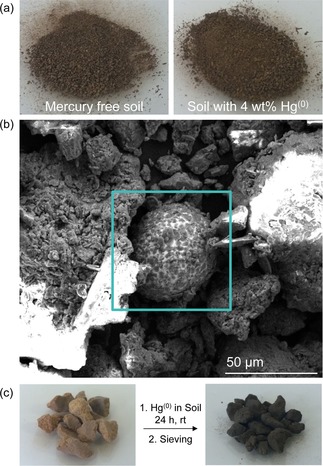
A) When mercury is pulverized in mills, it can form tiny beads that bind to soil. A simulated sample of mercury flour (top right) is shown in which the mercury does not coalesce and it is not visible to the naked eye. The soil containing the floured mercury appears very similar in appearance to mercury‐free soil (top left). B) The floured mercury is observable in the SEM image as microbeads bound to soil. C) A polymer made from sulfur and recycled cooking oil is effective at removing floured mercury from the soil sample. The procedure requires mixing the polymer and soil directly. The polymer changes colour to black as metacinnabar is formed. The polymer‐bound mercury can be isolated by sieving. Neither the polymer nor the polymer bound to mercury are toxic to cells. Images were adapted under a Creative Commons (CC) license.45
Heating mercury–gold amalgams to vaporize mercury is another major source of mercury emissions in ASGM. Mercury gas is harmful to the lungs, kidneys, liver and nervous system,13 so these emissions are especially dangerous. Where miners evaporate mercury from mercury–gold amalgams directly, this is frequently done without proper ventilation or a retort (e.g., Figure 2 E).6b, 9, 10b, 19a The amalgam may even be roasted over stoves in residential kitchens.16c With kilograms of amalgam processed by miners daily, and each amalgam containing 40–80 % mercury by mass, the exposure to mercury is extremely high.10b, 19a, 20d Gold shops and other amalgam smelting centers are also sources of substantial mercury pollution.10b, 19a Poor ventilation and inadequate extractor or fume‐hood equipment puts the shop owners and operators at risk for mercury exposure.10b These facilities are also commonly deficient in appropriate scrubber technology, emitting mercury vapor into the public. Because gold shops are typically located in villages, cities and areas of high population density, public exposure to mercury gas is a serious concern.10b Even with shops equipped with retorts and filters, atmospheric levels of mercury within 10 m of the entrance can be as high as 10–20 ppb and levels exceeding 100 ppb are common in the processing rooms, with both levels far beyond the 1 ppb level considered hazardous to human health by the World Health Organization (WHO).10b When no retorts or air filtration are used, extreme mercury concentrations are inevitable in amalgam roasting because virtually all of the mercury in a kilogram of amalgam is evaporated in less than one hour. Alarmingly, there are reports of mercury gas emissions from gold shops and amalgam processing stations operating in immediate proximity to residential areas,8b, 10b, 19a markets,10b health centers,8b schools,10b public gathering areas,10b playing children19a and breastfeeding mothers.10b
Health and Ecological Impacts
Human health
Mercury poisoning is a tremendous burden to human health, especially in ASGM communities.6c Mercury gas, such as that encountered in ASGM amalgam processing, is readily absorbed in the lungs and then further transported to other organs.13 Elemental mercury is able to cross membranes including the blood–brain barrier and the blood‐placenta barrier, posing a threat to neurological function and fetal development, respectively.13, 15 Acute mercury exposure (for instance to mercury vapor produced from heating mercury–gold amalgam) can lead to tremors, memory loss, respiratory distress and even death.13, 26, 27 Chronic exposure to mercury gas may lead to renal failure, tremors, movement disorders, and various psychosis and memory impairment.13 Inorganic mercury, formed through oxidation of mercury metal lost during ASGM may contaminate water and also lead to kidney damage if consumed.13, 15 Conversion of mercury pollution from ASGM into methyl mercury also poses a tremendous risk as this highly toxic form of mercury accumulates in food supplies, such as fish, crustaceans and mollusks.24a Consumption of methyl mercury is particularly harmful to the central nervous system, causing nerve and brain damage.15 Kidneys are also affected and methylmercury presents an extreme risk to fetal development.15
There are many documented cases of miners in ASGM suffering the effects of prolonged exposure to mercury.12 In addition to the symptoms of mercury intoxication noted above, these individuals suffered from frequent and severe headaches, dizziness, vision and motor disorders, among other health issues.12 Video recorded and published clinical interviews with miners poisoned by mercury show these devastating effects, especially with respect to tremors and movement disorders.26
Another troubling consequence of mercury pollution from ASGM is the effects on embryos, fetuses, and children. Mercury levels in women of child‐bearing age near ASGM activities are often high due to consumption of mercury‐contaminated water, seafood or rice; direct handling of mercury in mining or other gold‐related processing; or through exposure to mercury gas during amalgam processing.7, 8b, 17b Because maternal transfer of mercury to the fetus is efficient for elemental and methylmercury,13, 14, 15 it is perhaps not surprising that children in ASGM communities have high incidence of physical and mental disabilities.3c, 14b,14c News reports on the debilitating effects of mercury poisoning in children of artisanal gold mines (including limb deformities, brain damage, and hydrocephalus) bring this problem into confronting focus.8b, 16c, 17b
With recent estimates indicating there are as many as 19 million artisanal gold miners,4, 6c mercury use and emissions in ASGM is clearly a global health problem.6c
Wildlife and plant health
While the human cost of mercury poisoning in ASGM is the most important and immediate concern, mercury pollution also damages the wider ecosystem—compromising food chains and biodiversity. For example, mercury emissions can adversely affect algal growth; crustacean health; fish growth, brain function, and reproduction; and amphibian larval health and survival.28 It is also known that mercury bio‐accumulates in fish, which then poses a threat to any bird or mammal that consumes it (including humans).28, 29 Harm to these avian and mammalian predators is relevant to ASGM as many of the mining sites are located in highly diverse regions such as the Amazon rainforest (Figure 1). It is also common for the people living near these ASGM areas to eat fish as a major source of dietary protein, which leads to high mercury levels even in non‐miners.16a, 24 In this way, mercury pollution threatens food security.
Aquatic plants are bioaccumulators of mercury and uptake of the heavy metal may, in some cases, compromise plant health. Inorganic mercury in water, for instance, can lead to decreased chlorophyll content and protease activity for floating water cabbage Pistia stratiotes.29c Likewise, the pond weed Elodea densa presented with abnormal mitotic activity upon exposure to methylmercury.29c For terrestrial plants, the uptake and effects of mercury seems to be plant specific and highly dependent on the mercury concentration.30 In fact, mercury‐derived fungicides have long been used to protect wheat seed and sugarcane setts,30 so the effects of mercury on plant health are not universally detrimental. This may be due to low bioavailability of mercury in soil and a tendency for the mercury to accumulate in roots.30 However, mercury vapor uptake through the leaves of both C3 and C4 plants is possible and therefore relevant to ASGM regions.30
For regions of ASGM, an understanding of mercury uptake in plants is important for protecting food crops from contamination, and also for using plants intentionally to remove mercury from soil.30 Regarding crop contamination, mercury uptake in rice in ASGM communities has been documented.26 In these cases rice paddies were irrigated with mercury contaminated water, resulting in mercury levels as high as 1.2 ppm in the edible grain—more than 10 times the limits recommended by the WHO.26 While mercury uptake into crops is clearly undesirable, mercury uptake into non‐edible plants may be a useful way to remediate mercury pollution in water and soil due to ASGM. In one example, it was shown that Siam weed (Chromolaena odorata) could grow in nutrient‐supplemented ASGM tailings and accumulate mercury in its leaves without phytotoxicity.31 In another recent study at a Columbian gold mine, native plant species growing at mercury contaminated sites were also promising leads for removing and accumulating mercury from soil.32 In these and other strategies in phytoremediation of ASGM areas,33 careful planning for the fate of the biomass is required so that the heavy metal is ultimately removed from the environment and not simply re‐emitted by means of combustion, for example.34
Another adverse impact on the environment due to ASGM is deforestation.35 In Peru,17a for example, it was shown that from 2006 to 2009 alone approximately 20 km2 of Amazon forest was cleared each year for ASGM.35 Because this deforestation and mining is unregulated and strongly correlated with rising gold prices, it is unfortunately likely to continue to damage one of the most biodiverse ecosystems in the world.35
Challenges and Opportunities for Chemists
The problem of mercury use and emissions in ASGM is profound. It is also a problem that has existed for many decades and therefore one which has resisted many well‐intentioned interventions by governments, environmental advocates and humanitarian service organizations.9, 36 Stemming mercury pollution in ASGM is not as simple as legally restricting or banning the use of mercury, as such measures have already been implemented in areas with the highest levels of mercury use, with little success.3c, 36, 37 Banning mercury use may also hinder engagement and education of miners working outside the law, so in these cases mercury use continues unabated.36 Poor and ineffective enforcement of legislated mercury controls, lack of direct engagement and support of artisanal miners, the high market value of gold, and a strong black market for mercury trade all but ensure that mercury use in ASGM is likely to continue for years.36, 37, 38 In taking measures to address the mercury problem in ASGM, one must keep in mind that these miners live in some of the poorest areas of the world and have few, if any, options for other employment and income.4 Mining for gold is their livelihood and a means to support their family. This consideration is important because while there are an estimated 10–19 million ASGM workers at any time, 80–100 million people are thought to be directly dependent on the associated income.9 Policies, technologies and service work aimed at lowering the burden of mercury emissions on health and the environment should therefore consider how such measures support the miners.9, 36, 37, 38
The chemistry community can provide important technological advances that may help overcome some aspects of the mercury problem in ASGM. Some specific opportunities and challenges are discussed next to help encourage contributions from the chemistry research community, with an aim to spur eventual collaboration with environmental scientists, public health advocates and even field work with ASGM miners and their supporters. For each of these needs, it must be clear that any potential solution will be easier to implement if it is extremely low in cost, scalable, easy to transport to remote locations, operate with intermittent or no central power supply, require little or no training for operation, and provide immediate and obvious benefit to miners. Only then, will uptake of any technological solution be realistic.9
Low‐cost and portable mercury monitoring
The mercury emissions from ASGM pollute air, water and soil. In many cases, this pollution is a threat to food and water supplies.12, 22c, 24, 25, 26 It is therefore important to have real‐time, portable, and cost‐effective monitoring of mercury levels‐especially in air and water. While portable atomic absorption spectrometers and hand‐held X‐ray fluorescence instruments have been used to monitor mercury pollution at mining sites39 (including ASGM locales),10b, 40 these instruments typically cost thousands of dollars (USD). It would be useful for ASGM communities to have access to low‐cost, low‐maintenance sensors for rapid measurement of mercury levels in air and water.41 Such technologies42 could potentially help the miners, other users of contaminated waterways, and local authorities limit exposure to mercury, provided the sensors require minimal training and are portable or even disposable. As mercury can be interconverted between various oxidation states with various ligands that impact mobility and toxicity,28a the ability to assess speciation is also important. Additionally, such technologies could be coordinated with information campaigns on the dangers of mercury exposure.
Low‐cost point of care diagnostics for mercury exposure
Like mercury analysis in the environment, clinical analysis of mercury exposure typically relies on atomic absorption spectrometers.26 To administer care and medical advice to miners in remote locations, portable and disposable mercury diagnostics might be valuable. In developing such technologies, chemists might consider ways in which breath, saliva, hair or urine might be analyzed in a rapid and cost‐effective fashion.12 These technologies could also be used by the miners to help self‐monitor exposure to mercury.
New strategies for tailings processing
The mercury‐laden tailings in ASGM are one of the more challenging problems for remediation.21 After milling mercury with ore, microbeads of mercury are dispersed in the fine sand and water and can be carried far from the mine when discharged in water courses.22 Many thousands of tonnes of tailings are generated each year in ASGM and removing mercury and recovering residual gold are critical problems in tailing processing. In some cases, the tailings are processed in large vats of aqueous sodium cyanide, which solubilizes gold21 for eventual recovery on a sorbent or through precipitation with zinc.23 Unfortunately, cyanide also complexes with mercury in the tailings, facilitating its transport into the environment through wastewater.23 Mechanical separation of the mercury from the sand and soil in the tailings, for instance on a shaker table, may be useful in recovering mercury and preventing its release in the environment, but such strategies have limited uptake at present.21 Simple and rapid chemical remediation of mercury in tailings (and the recovery of gold) is an outstanding problem in ASGM. Any potential solution must be able to process large volumes of tailings (tonnes), operate on a shorter timescale than cyanide leaching techniques, and facilitate gold recovery at a level that incentivizes uptake. Ideally, the technique should also be environmentally innocuous and only generate discharges that are recyclable or biodegradable.
Soil remediation
Like the tailings problem, remediating mercury‐contaminated soil is a large‐scale problem in ASGM areas. Keeping in mind the limited financial resources of ASGM communities and the governments in these jurisdictions, any strategy for soil remediation must require low‐capital outlay and simple protocols for remediation. Extensive excavation and capping, off‐site disposal, and washing or thermal treatment of soil (all measures taken in wealthy nations)40, 43 are likely impractical solutions for soil remediation in ASGM. Instead, there is a need for the development of soil amendments that can trap and immobilize diverse forms of mercury. These soil additives must be very inexpensive and scalable to have any chance of impact on contaminated areas spanning several square kilometers. These so‐called “in situ remediation” techniques43, 44 must also sequester the mercury in a way that prevents not only leaching into ground water, but also methylation by bacteria and subsequent bioaccumulation. In an effort to address this need, a recent study by our laboratory introduced a mercury sorbent made from recycled cooking oils and sulfur that immobilized floured mercury metal in soil (Figure 3).45 The material, prepared by inverse vulcanization45, 46 of extremely low‐cost feedstocks,45, 46c can be milled with mercury‐contaminated soil to convert the metal to the non‐toxic and highly insoluble metacinnabar (Figure 3).45 Notably, the polymer changes from brown to black as it reacts with the mercury. This is useful because the mercury flour is typically not distinguishable from most soil (Figure 3). While additional field tests are required to ascertain whether the scale and efficiency of this mercury immobilization method can have on ASGM, it is notable that this study explicitly considers cost, scale and ease of operation required for use in ASGM.
Another potential strategy in remediation and reclamation of mercury‐contaminated soil in ASGM areas is through phytoremediation.31, 32, 33, 34 In this technique, plants are used to extract mercury from soil. Improving the efficiency of mercury uptake in the plant and a plan for the fate of mercury‐contaminated biomass are still major (biochemistry and chemistry) issues to be resolved for impact in ASGM. Nevertheless, this strategy is promising in that it is relatively low‐tech and low‐cost in its deployment and may therefore be an important measure to take in remediating mercury‐contaminated soil at ASGM sites.
Water remediation
Several aspects of water remediation are important to areas of ASGM. Tailings are often released into rivers and streams that are sources of water for drinking and irrigation.21 Removal of mercury from this water may therefore help prevent direct consumption of mercury and prevent uptake in crops and aquatic life. For cases in which tailing ponds are employed, mercury removal or immobilization could be important for preventing release into the wider environment.47 For example, the simple addition of calcium hydroxide to ASGM tailing ponds has been shown in field trials to facilitate removal of suspended particles in the water and prevent the methylation of mercury.48
For water used for drinking and irrigation, it may be necessary to develop sorbents that can trap mercury of various forms rapidly and irreversibly. In preparing such sorbents or filtration media, it is critical that the sorbent be effective at trapping inorganic mercury, inorganic mercury bound to organic matter, mercury bound to cyanide, mercury metal and alkylmercury compounds. While inexpensive sorbents for mercury removal from water and soil have been reported,49 these reports typically assess the removal of mercury(II) chloride from water. These model systems rarely reflect the complex matrices encountered in the field.50 It is also known that sorbents effective at removing mercury(II) chloride from water, may fail at sequestering mercury complexed to humic matter.45 It is therefore important that any new sorbents proposed for use in ASGM clearly demonstrate the capability of binding mercury complexed to organic matter. Furthermore, the sorbent should be inexpensive, capture diverse forms of mercury (inorganic, organic, liquid and gas) and be easily monitored for maintenance and replacement.
In recent developments on mercury sorbent technology, low‐cost polymers prepared from elemental sulfur are promising.45, 46c, 51 Because sulfur is produced as a byproduct of the petroleum industry in many millions of tonnes each year, the potential for economical and large‐scale sorbent synthesis is encouraging.46c Importantly, sulfur‐based sorbents have been reported that are rapid,51b–51d,51f and effective against inorganic, organic, and metallic mercury compounds.45 In some cases, trapping mercury produces a color change that may be useful in monitoring the lifetime of water filters and other water purification devices.45, 51a These promising developments aside, there is still an urgent need to adapt these and other technologies to the ASGM mercury problem. Any mercury sorbent for this purpose must be very inexpensive, scalable to kilogram or tonne quantities, portable, easy to use and maintain, and require little or no training for use. Additionally, the rate of mercury sorption should be high and insensitive to other metals and debris found in the polluted water. The high rate of mercury uptake by a sorbent is important for continuous filtration. In the case of remediation of tailing ponds, a method to recover gold will also be useful as it could incentivize deployment of the sorbent and perhaps finance the remediation.
Air‐quality control
In ASGM, there are typically few, if any, measures taken to prevent mercury exposure. The mercury metal and amalgams are handled directly by hand, and heating the amalgam is often done without retorts and proper ventilation. Low‐cost personal protective equipment might be useful for miners to prevent mercury exposure. For instance, inexpensive mercury sorbents could be used to prepare disposable masks that limit the inhalation of mercury vapor during amalgam processing. Likewise, affordable and self‐indicating air filters might improve the air quality in and around gold shops that process amalgam.10b In these cases, rapid capture of mercury vapor is essential. The effectiveness of activated carbon52 and inexpensive sulfur‐based sorbents45, 51c,51f may be useful in meeting this challenge. As noted previously, any new technology for ASGM must provide an immediate and obvious benefit to the miners for uptake to be realistic.9 In the case of disposable masks, this benefit might be the prevention of severe headaches and respiratory distress from acute exposure to mercury vapor: a common issue in ASGM during amalgam processing.13, 19a
Preventing mercury uptake in livestock, fowl and food crops
In addition to accumulation in seafood, mercury pollution can accumulate in food crops, livestock and poultry.24a, 26, 53 Mercury pollution from ASGM is suspected to threaten these food supplies through contamination of groundwater, rivers and lakes used for irrigation and drinking.25a, 26 Thus, there is an opportunity for chemists to introduce soil amendments, sorbents and filters that purify water or otherwise prevent mercury uptake into plants and animals. Such technology would likely require assessment of its effects on plant and animal health. In the case of soil additives or other amendments, the long‐term environmental fate is also an important consideration. Ideally, any measure taken to address this challenge would be deployed in a way that also improves crop yields and health of livestock.
Mercury recycling in gold mining
A retort can be used to recover mercury during amalgam processing.9, 19a The retort is a condenser apparatus that cools mercury vapor produced when the amalgam heated in a sealed crucible or container, returning the metal to its liquid form for reuse.9, 19a When they are used properly, retorts reduce mercury gas emissions, with some designs enabling more than 90 % of mercury recovery.9, 20b,20c, 54 In addition to the health benefits of inhaling less mercury vapor, the miners benefit financially because the mercury can be reused and does not need to be purchased again. Using a retort or other mercury recovery method should be encouraged over the alternative of open‐air amalgam heating. In cases where the amalgam is heated with a torch or over an open flame without a retort, virtually all of the mercury is lost to the atmosphere.9 This open‐air processing accounts for hundreds of tons of mercury emissions each year,10a so even seemingly simple measures like the use of a retort may have a positive impact on miner health and the environment.
Unfortunately, even the introduction and uptake of a retort in ASGM can be fraught with challenges. Training in the use and necessity of retorts (including education on the hazards of mercury gas) are essential.9, 36 Even then, miners often hesitate to use a system in which the amalgam (and therefore gold) are not visible through the entire roasting process, because they fear gold is lost in the opaque metal of common retorts.36 Attempting to overcome this distrust, glass retorts have been introduced, but they tend to break.36 Cost is also an issue with unsuccessful campaigns to promote retort uptake failing because the equipment costs hundreds of dollars (USD).36 Scalability is also important and retorts should accommodate the several kilograms of amalgam that may be processed per day by miners.19a The speed of gold recovery is also important in some locations, as theft of gold during the amalgam decomposition has been reported.9 In a very practical approach, Veiga and associates have worked with artisanal gold miners to train them in the fabrication and use of retorts that can be prepared from simple kitchen supplies or plumbing equipment, costing only a few US dollars per retort.20b,20c, 54 These efforts have led to a measurable reduction in mercury emissions.
Mercury recovery at any stage in the gold mining process is an important capability to protect the environment and health of the miners. This may be a new and simple retort design, extractive technologies or chemistry to separate gold and mercury in the amalgam in a relatively safe way that does not generate mercury vapor, or cost‐effective and simple ways to re‐activate recovered mercury that may contain oxides that hinder its effectiveness in amalgamation.9, 20b Recovery of mercury from tailings is also a critical issue in stemming mercury emissions from ASGM.21 Gravity separation, sluicing, and shaking tables are promising options,21 but there remains an urgent need to recover the vast amounts of mercury lost in the fine sand and slurries of the tailings.
Mercury‐free gold mining
The most important way to address the mercury problem in ASGM is to introduce mercury‐free mining techniques. This may be an obvious goal, but there are a number of criteria that must be met for a mercury‐free technique to be adopted in ASGM. The mercury‐free method must be superior in the rate and yield of gold recovery in comparison to mercury amalgamation; the technique should be affordable and not require a large capital outlay or complex equipment; the mercury‐free method should be easy to learn and not require extensive technical support; and any chemistry used in the extraction of gold should be inexpensive, relatively safe, and generate innocuous and biodegradable waste where possible. It is also extremely important that the miners see an obvious opportunity for financial gain, if any clean mining technique is to be adopted.9
Mercury‐free gold mining is possible. After all, mercury is not used in formalized and regulated gold mining, which instead employs sophisticated ore processing equipment, flotation techniques, and cyanide leaching to recover gold.9 The capital requirements, maintenance and technical skills required for such operations makes it difficult to adopt or adapt these techniques for ASGM.9 Where techniques such as cyanide leaching are used in ASGM, training is critical for the safe and secure use of hundreds of kilograms of sodium or potassium cyanide and the management and discharge of tailings can be a risk to the miners and the environment.9, 55 Nevertheless, while cyanide is highly toxic, it does not persist and accumulate in the environment to the same degree as mercury. Cyanide is also faster and more effective at extracting gold than mercury amalgamation, as demonstrated on ore samples from several ASGM regions.23 Alternative, non‐toxic lixiviants that are as high‐yielding and selective as cyanide in gold extraction would be beneficial to the ASGM sector.55 While thiourea, thiocyanate, thiosulfate, chlorine, bromine and iodine have been explored as reagents to extract and solubilize gold in water, there has been relatively limited use of these lixiviants in current mining operations‐formal or artisanal.11f, 55
Gravity techniques can be used to concentrate gold particles from crushed ore and sediment, but these techniques typically require relatively large gold grain size. Where possible, panning, sluicing and concentration using mechanized shaking tables and spiral concentrators can be used to concentrate gold without mercury.56 These options, however, may not be suitable for all ASGM areas. For instance, the gold grain size in Indonesia is very fine, which makes classic gravity concentration equipment (such as shaking tables, jigs and sluice boxes) relatively inefficient.9, 19a, 23 In some areas of Southeast Africa, gravity concentrators have been demonstrated as a viable alternative to amalgamation.57 In these cases an additional purification of gold concentrate is achieved by removing magnetite and hematite with a magnet.57 While such methods may be unique to the regional mineralogy, this is a promising example of mercury‐free artisanal gold mining that can support the economy of developing communities.
Another recent study in mercury‐free gold extraction involved borax‐facilitated smelting.22b, 58 In this technique, gold concentrates are first prepared by a gravitational separation such as sluicing and panning. Iron shavings can also be removed with a magnet. The gold concentrate is then mixed with an equal mass of borax, which is thought to lower the melting point of the mineral concentrate. Heating the mixture in a crucible provides molten gold, which settles to the bottom of the vessel and solidifies in relatively pure form upon cooling, whereas the borax complexes to silicate and oxide impurities. Trials of this method in ASGM regions of the Philippines, Indonesia, Tanzania, Bolivia and Zimbabwe are encouraging, but faster processing and improved yields are likely required to overcome the continued reliance on mercury.22b, 59 The borax method is also inefficient with concentrates containing high levels of sulfides.11f
There is a pressing need for chemists to develop mercury‐free technologies that simply and reliably extract gold from ore in a time‐ and cost‐effective manner. An additional (chemical) challenge is to design such methods so that any required equipment or reagents are widely available, easy to handle, and safe to use. In‐built mechanisms to recycle any key materials or strategies to ensure minimal impact on the environment are added challenges to which chemists can provide a solution. More generally, chemistry support for the ASGM community (Figure 4) has the potential to improve the well‐being of millions of people.
Figure 4.

A summary of ways in which the chemistry community can support artisanal gold miners’ health and livelihood. In proposing a solution to any one of these problems and challenges, it is essential that the technique, chemistry, and any equipment be low cost, require minimal capital outlay, operate in remote locations, and be easy to use. It is also important that the benefit to the miners be immediately obvious, so expediency in financial returns and health benefits are critical.
Interdisciplinary action is necessary
This Minireview is directed to the attention of the chemistry community to promote awareness and action in helping solve the mercury problem in ASGM. We encourage researchers, funding agencies and journal editors in chemistry and allied fields to consider how they might marshal their resources and expertise to address this global problem. With that said, we emphasize that interdisciplinary collaboration is required for any solution to be effectively implemented. This includes taking into account the extensive efforts of environmental scientists, geologists, and public health and environmental advocates in assessing the scope and severity of the mercury problem in ASGM.1, 6c, 14b,14c, 18 It also means working with the formalized mining sector to help adapt mercury‐free technology and resource management strategies for use in ASGM.9 Chemists may also be in a position to inform or shape policy decisions regarding the regulation of ASGM practices, remediation strategies, and gold and mercury trade.3b, 36, 37, 38, 60 Additionally, the challenges in implementing and enforcing the mandates of the Minamata Convention are manifold and require careful consideration of labor dynamics,3a land and resource rights,61 the relationship between formal and informal mining sectors,11e, 61, 62 and engagement strategies that support rather than alienate miners that rely on gold mining as their only source of income.3a,3b, 20e Ethical dilemmas also arise when restricting or banning mercury without supporting alternative mining methods or establishing other employment opportunities for miners.3b Additionally, cumbersome licensing measures for ASGM and severe restriction of mercury has largely resulted in non‐compliance with the regulations and growing black markets for mercury trade.3a, 20e, 61a, 62b With regards to technical solutions, the most important consideration is that the resources available in ASGM are severely limited. Therefore, any novel technical solution to the problems outlined in this Minireview must therefore be low‐cost, easy to use, and provide immediate and obvious benefit to the miners.9 Likewise, direct fieldwork with miners and their advocates is important so that training and long‐term uptake of new and clean technologies is driven from the ground up.9, 36 We encourage the chemistry community to contribute to this endeavour.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Louisa J. Esdaile earned a Bachelor of Applied Science in Chemistry at Queensland University of Technology (QUT) in 2002. Under the supervision of Associate Professor Dennis Arnold at QUT, Louisa completed her Ph.D. in Organic Chemistry in 2007. During her Ph.D. Louisa developed innovative methods in porphyrin synthesis. From 2007–2009, Louisa worked with Prof. Harry Anderson at the University of Oxford as a post‐doctoral researcher where she studied porphyrin‐based molecular wires. Louisa then moved to Northwestern University to take up a second post‐doctoral research position in the lab of Prof. Sir J. Fraser Stoddart. In the Stoddart lab, Louisa contributed to the design and synthesis of molecular knots, dendrimers and other molecules with interesting topology. After two years at Dow Electronic Materials, and one year of teaching at The University of Tulsa, Louisa moved to Flinders University in Adelaide, Australia where she is an Associate Lecturer and Research Fellow. Her current research interests center on sustainable materials that benefit the environment. Louisa also has interests in the intersection of science and public policy, earning a Masters of Public Administration (Policy) from Flinders University in 2017.

Biographical Information
Justin M. Chalker earned a B.S. in Chemistry and a B.A. in the History and Philosophy of Science at The University of Pittsburgh in 2006. At Pittsburgh, he contributed to the total synthesis of several natural products under the direction of Theodore Cohen. Supported by a Rhodes Scholarship and a National Science Foundation Graduate Research Fellowship, Justin then completed his D.Phil. at the University of Oxford under the supervision of Benjamin Davis where he developed several tools for the site‐selective modification of proteins. In 2012, Justin started his independent career as an assistant professor at The University of Tulsa where he established a diverse research program in organic chemistry, biochemistry and material science. In 2015, Justin moved to Flinders University as a Lecturer in Synthetic Chemistry and recipient of an ARC Discovery Early Career Researcher Award. In 2016, Justin was named Tall Poppy of the Year for South Australia in recognition of his achievements in research, teaching and science communication and in 2017 Justin was promoted to Senior Lecturer. Among his current research priorities are the discovery and development of sustainable materials for pollution control.

Acknowledgements
The authors are grateful for the generous financial support provided by Flinders University, The Australian Research Council (DE150101863), and the Australian Government National Environmental Science Programme Emerging Priorities Funding. We thank Richard Fuller, Andrew McCartor, and Budi Susilorini of Pure Earth and Dr Jossep Frederick William of the Medicuss Foundation for valuable discussions on mercury use, emissions, and human health in artisanal and small‐scale gold mining.
L. J. Esdaile, J. M. Chalker, Chem. Eur. J. 2018, 24, 6905.
Contributor Information
Dr. Louisa J. Esdaile, Email: louisa.esdaile@flinders.edu.au.
Dr. Justin M. Chalker, Email: justin.chalker@flinders.edu.au.
References
- 1.
- 1a. Reducing Mercury in Artisanal and Small-Scale Gold Mining (ASGM), United Nations Environment Programme. Accessed 10 October 2017 from http://web.unep.org/chemicalsandwaste/global-mercury-partnership/reducing-mercury-artisanal-and-small-scale-gold-mining-asgm;
- 1b. Global Mercury Assessment 2013. Sources, Emissions, Releases and Environmental Transport, United Nations Environment Programme, Chemicals Branch, Geneva, Switzerland, 2013.
- 2. de Lacerda L. D., Salomons W., Mercury from Gold and Silver Mining A Chemical Time Bomb?, 1998, Springer, Berlin. [Google Scholar]
- 3.
- 3a. Spiegel S. J., Agrawal S., Mikha D., Vitamerry K., Le Billon P., Veiga M., Konolius K., Paul B., Ecol. Econ. 2018, 144, 1–11; [Google Scholar]
- 3b. Fritz M. M. C., Maxson P. A., Baumgartner R. J., Resour. Policy 2016, 50, 177–192; [Google Scholar]
- 3c. Mercury Trade and Supply in Indonesia Y. Ismawati, K. Zaki, S. Buftheim, M. A. Septiono, A. S. Arif, T. Avianto, Y. Y. Satriyo, W. T. Brahmancha, 2017 Accessed 1 October 2017 from https://www.balifokus.asia/reports.
- 4. Seccatore J., Veiga M., Origliasso C., Marin T., De Tomi G., Sci. Total Environ. 2014, 496, 662–667. [DOI] [PubMed] [Google Scholar]
- 5. World Gold Council Accessed 9 October 2017 from https://www.gold.org/data/gold-price.
- 6.
- 6a. Cordy P., Veiga M. M., Salih I., Al-Saadi S., Console S., Garcia O., Mesa L. A., Velásquez-López P. C., Roeser M., Sci. Total Environ. 2011, 410–411, 154–160; [DOI] [PubMed] [Google Scholar]
- 6b. Veiga M. M., Hinton J. J., Nat. Resour. Forum 2002, 26, 15–26; [Google Scholar]
- 6c. Steckling N., Tobollik M., Plass D., Hornberg C., Ericson B., Fuller R., Bose-O'Reilly S., Ann. Glob. Health 2017, 83, 234–247. [DOI] [PubMed] [Google Scholar]
- 7. Mercury in Women of Child-Bearing Age in 25 Countries, L. Bell, D. Evers, S. Johnson, K. Regan, J. DiGangi, J. Federico, J. Samanek, 2017 Accessed 1 October 2017 from https://www.balifokus.asia/reports.
- 8.
- 8a. Hazardous Child Labor in Small-Scale Gold Mining in the Philippines, J. Kippenberg, M. Wurth, C. Conde, Human Rights Watch 2015 Accessed 10 October 2017 from https://www.hrw.org/report/2015/09/29/what-if-something-went-wrong/hazardous-child-labor-small-scale-gold-mining;
- 8b. Unearthing Toxic Conditions for Impoverished Gold Miners, PBS Newshour, broadcast 17 February 2015. Accessed 10 October 2017 from http://www.pbs.org/newshour/bb/unearthing-toxic-conditions-impoverished-gold-miners/;
- 8c. Indonesia: Child Labor in Small-Scale Gold Mining L. C. Price for the Pulitzer Center. Accessed 10 October 2017 from http://pulitzercenter.org/reporting/indonesia-child-labor-small-scale-gold-mining.
- 9. Hinton J. J., Veiga M. M., Veiga A. T. C., J. Cleaner Prod. 2003, 11, 99–115. [Google Scholar]
- 10.
- 10a. Technical Background Report for the Global Mercury Assessment 2013, United Nations Environment Programme. Accessed 10 October 2017 from http://www.amap.no/documents/doc/Technical-Background-Report-for-the-Global-Mercury-Assessment-2013/848;
- 10b. Cordy P., Veiga M., Crawford B., Garcia O., Gonzalez V., Moraga D., Roeser M., Wip D., Environ. Res. 2013, 125, 82–91. [DOI] [PubMed] [Google Scholar]
- 11.
- 11a. Van Bockstael S., Futures 2014, 62, 10–20; [Google Scholar]
- 11b. Hilson G., Hilson A., Adu-Darko E., J. Rural Stud. 2014, 34, 292–303; [Google Scholar]
- 11c. Hilson G., Hilson A., Maconachie R., McQuilken J., Goumandakoye H., Geoforum 2017, 83, 80–90; [Google Scholar]
- 11d. Hilson G., Maconachie R., Area 2017, 49, 443–451; [Google Scholar]
- 11e. Marshall B. G., Veiga M. M., Extractive Industries Soc. 2017, 4, 300–303; [Google Scholar]
- 11f. Veiga M. M., Angeloci-Santos G., Meech J. A., Extractive Industries Soc. 2014, 1, 351–361. [Google Scholar]
- 12. Gibb H., O'Leary K. G., Environ. Health Perspect. 2014, 122, 667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park J.-D., Zheng W., J. Prev. Med. Public Health 2012, 45, 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.
- 14a. Brown I. A., Austin D. W., Toxicol. Environ. Chem. 2012, 94, 1610–1627; [Google Scholar]
- 14b. Bose-O′Reilly S., Lettmeier B., Gothe R. M., Beinhoff C., Siebert U., Drasch G., Environ. Res. 2008, 107, 89–97; [DOI] [PubMed] [Google Scholar]
- 14c. Bellinger D. C., O'Leary K., Rainis H., Gibb H. J., Environ. Res. 2016, 147, 159–163. [DOI] [PubMed] [Google Scholar]
- 15. Tchounwou P. B., Ayensu W. K., Ninashvili N., Sutton D., Environ. Toxicol. 2003, 18, 149–175. [DOI] [PubMed] [Google Scholar]
- 16.
- 16a. Fraser B., Nature 2016, 534, 162; [DOI] [PubMed] [Google Scholar]
- 16b. Notman N., Chem. World 2016, 13, 54–57; [Google Scholar]
- 16c. The Toxic Toll of Indonesia's Gold Mines, R. C. Paddock, National Geographic (24 May 2016). Accessed 10 October 2017 from http://news.nationalgeographic.com/2016/05/160524-indonesia-toxic-toll/.
- 17.
- 17a.Peru Scrambles to Drive Out Illegal Gold Mining and Save Precious Land, S. Daley, The New York Times (26 July 2016). Accessed 10 October 2017 from http://www.nytimes.com/2016/07/26/world/americas/peru-illegal-gold-mining-latin-america.html;
- 17b. Extracting Gold with Mercury Exacts a Lethal Toll, PBS Newshour, Broadcast 9 Oct 2016. Accessed 10 October 2017 from http://www.pbs.org/newshour/bb/extracting-gold-mercury-exacts-lethal-toll/.
- 18. Minamata Convention on Mercury, United Nations Environment Programme. Accessed 10 October 2017 from http://www.mercuryconvention.org.
- 19.
- 19a. Mercury: The Burning Issue, Yayasan Tambuhak Sinta (YTS). Accessed 2 October 2017 from https://www.youtube.com/watch?v=x9u9ngtoP6Q;
- 19b. Schmidt C. W., Environ. Health Perspect. 2012, 120, 425–429.22169225 [Google Scholar]
- 20.
- 20a. Balzino M., Seccatore J., Marin T., De Tomi G., Veiga M. M., J. Cleaner Prod. 2015, 102, 370–377; [Google Scholar]
- 20b. García O., Veiga M. M., Cordy P., Suescún O. E., Martin Molina J., Roeser M., J. Cleaner Prod. 2015, 90, 244–252; [Google Scholar]
- 20c. Shandro J. A., Veiga M. M., Chouinard R., J. Cleaner Prod. 2009, 17, 525–532; [Google Scholar]
- 20d. Veiga M. M., Angeloci G., Hitch M., Velasquez-Lopez P. C., J. Cleaner Prod. 2014, 64, 535–544; [Google Scholar]
- 20e. Malehase T., Daso A. P., Okonkwo J. O., Environ. Rev. 2017, 25, 218–224. [Google Scholar]
- 21. Mercury: The Tailings Issue, Yayasan Tambuhak Sinta (YTS). Accessed 2 October 2017 from https://www.youtube.com/watch?v=xsXiAAuFLNc&feature=youtu.be.
- 22.
- 22a. Vieira R., J. Cleaner Prod. 2006, 14, 448–454; [Google Scholar]
- 22b. Appel P. W. U., Na-Oy L. D., Indian J. Environ. Prot. 2014, 5, 493–499; [Google Scholar]
- 22c. Diringer S. E., Feingold B. J., Ortiz E. J., Gallis J. A., Araújo-Flores J. M., Berky A., Pan W. K. Y., Hsu-Kim H., Environ. Sci.: Processes Impacts 2015, 17, 478–487. [DOI] [PubMed] [Google Scholar]
- 23. Veiga M. M., Nunes D., Klein B., Shandro J. A., Velasquez P. C., Sousa R. N., J. Cleaner Prod. 2009, 17, 1373–1381. [Google Scholar]
- 24.
- 24a. Reichelt-Brushett A. J., Stone J., Howe P., Thomas B., Clark M., Male Y., Nanlohy A., Butcher P., Environ. Res. 2017, 152, 407–418; [DOI] [PubMed] [Google Scholar]
- 24b.A. Riaz, S. Khan, S. Muhammad, C. Liu, M. T. Shah, M. Tariq, Environ. Geochem. Health 2017, https://doi.org/10.1007/s10653-017-0007-6; [DOI] [PubMed]
- 24c. Salazar-Camacho C., Salas-Moreno M., Marrugo-Madrid S., Marrugo-Negrete J., Díez S., Environ. Int. 2017, 107, 47–54. [DOI] [PubMed] [Google Scholar]
- 25.
- 25a. Kofi Bempah C., Ewusi A., Environ. Monit. Assess. 2016, 188, 261; [DOI] [PubMed] [Google Scholar]
- 25b. Cobbina S. J., Duwiejuah A. B., Quansah R., Obiri S., Bakobie N., Int. J. Environ. Res. Public Health 2015, 12, 10620–10634; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25c. Clifford M. J., Extractive Industries Soc. 2017, 4, 497–505; [Google Scholar]
- 25d. Appleton J. D., Williams T. M., Breward N., Apostol A., Miguel J., Miranda C., Sci. Total Environ. 1999, 228, 95–109. [DOI] [PubMed] [Google Scholar]
- 26. Bose-O′Reilly S., Schierl R., Nowak D., Siebert U., Frederick William J., Owi F. T., Ismawati Ir Y., Environ. Res. 2016, 149, 274–281. [DOI] [PubMed] [Google Scholar]
- 27. Solis M. T., Yuen E., Cortez P. S., Goebel P. J., Am. J. Emerg. Med. 2000, 18, 599–602. [DOI] [PubMed] [Google Scholar]
- 28.
- 28a. Zillioux E. J., Porcella D. B., Benoit J. M., Environ. Toxicol. Chem. 1993, 12, 2245–2264; [Google Scholar]
- 28b. Wolfe M. F., Schwarzbach S., Sulaiman R. A., Environ. Toxicol. Chem. 1998, 17, 146–160. [Google Scholar]
- 29.
- 29a. Scheuhammer A. M., Meyer M. W., Sandheinrich M. B., Murray M. W., Ambio 2007, 36, 12–18; [DOI] [PubMed] [Google Scholar]
- 29b. Scheuhammer A., Braune B., Chan H. M., Frouin H., Krey A., Letcher R., Loseto L., Noël M., Ostertag S., Ross P., Wayland M., Sci. Total Environ. 2015, 509–510, 91–103; [DOI] [PubMed] [Google Scholar]
- 29c. Boening D. W., Chemosphere 2000, 40, 1335–1351. [DOI] [PubMed] [Google Scholar]
- 30. Patra M., Sharma A., Bot. Rev. 2000, 66, 379–422. [Google Scholar]
- 31. Hamzah A., Kusuma Z., Utomo W. H., Guritno B., J. Trop. Agric. 2012, 50, 88–91. [Google Scholar]
- 32. Marrugo-Negrete J., Marrugo-Madrid S., Pinedo-Hernández J., Durango-Hernández J., Díez S., Sci. Total Environ. 2016, 542, 809–816. [DOI] [PubMed] [Google Scholar]
- 33.
- 33a. Muddarisna N., Krisnayanti B. D., Utami S. R., Handayanto E., Appl. Ecol. Env. Sci. 2013, 1, 27–32; [Google Scholar]
- 33b. Muddarisna N., Krisnayanti B. D., Utami S. R., Handayanto E., J. Environ. Sci. Toxicol. Food Technol. 2013, 4, 15–19; [Google Scholar]
- 33c. Muddarisna N., Siahaan B. C., J. Degraded Min. Land Manage. 2014, 2, 251–258; [Google Scholar]
- 33d. Handayanto E., Muddarisna N., Krisnayanti B. D., Adv. Environ. Biol. 2014, 8, 1277–1284. [Google Scholar]
- 34. Anderson C. W. N., J. Degraded Min. Land Manage. 2013, 1, 51–56. [Google Scholar]
- 35. Swenson J. J., Carter C. E., Domec J.-C., Delgado C. I., PLoS One 2011, 6, e18875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hilson G., Sci. Total Environ. 2006, 362, 1–14. [DOI] [PubMed] [Google Scholar]
- 37. Sousa R., Veiga M., Van Zyl D., Telmer K., Spiegel S., Selder J., J. Cleaner Prod. 2011, 19, 742–750. [Google Scholar]
- 38. Clifford M. J., Futures 2014, 62, 106–112. [Google Scholar]
- 39. Radu T., Diamond D., J. Hazard. Mater. 2009, 171, 1168–1171. [DOI] [PubMed] [Google Scholar]
- 40. Guidance on the Identification, Management and Remediation of Mercury Contaminated Sites, IPEN, 2016 Accessed 10 October 2017 from http://ipen.org/documents/ipen-guidance-identification-management-and-remediation-mercury-contaminated-sites.
- 41. The Scientific Legacy of a City Poisoned by Mercury, J. Sokol, The Atlantic (27 September 2017). Accessed 2 October 2017 from https://www.theatlantic.com/science/archive/2017/09/mercury-poisoning-minamata/541191/?utm_source=atlfb.
- 42. Botasini S., Heijo G., Méndez E., Anal. Chim. Acta 2013, 800, 1–11. [DOI] [PubMed] [Google Scholar]
- 43.
- 43a. Xu J., Bravo A. G., Lagerkvist A., Bertilsson S., Sjöblom R., Kumpiene J., Environ. Int. 2015, 74, 42–53; [DOI] [PubMed] [Google Scholar]
- 43b. Wang J., Feng X., Anderson C. W. N., Xing Y., Shang L., J. Hazard. Mater. 2012, 221–222, 1–18. [DOI] [PubMed] [Google Scholar]
- 44. Gong Y., Liu Y., Xiong Z., Kaback D., Zhao D., Nanotechnology 2012, 23, 294007. [DOI] [PubMed] [Google Scholar]
- 45. Worthington M. J. H., Kucera R. L., Albuquerque I. S., Gibson C. T., Sibley A., Slattery A. D., Campbell J. A., Alboaiji S. F. K., Muller K. A., Young J., Adamson N., Gascooke J. R., Jampaiah D., Sabri Y. M., Bhargava S. K., Ippolito S. J., Lewis D. A., Quinton J. S., Ellis A. V., Johs A., Bernardes G. J. L., Chalker J. M., Chem. Eur. J. 2017, 23, 16219–16230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.
- 46a. Chung W. J., Griebel J. J., Kim E. T., Yoon H., Simmonds A. G., Ji H. J., Dirlam P. T., Glass R. S., Wie J. J., Nguyen N. A., Guralnick B. W., Park J., Somogyi Á., Theato P., Mackay M. E., Sung Y.-E., Char K., Pyun J., Nat. Chem. 2013, 5, 518–524; [DOI] [PubMed] [Google Scholar]
- 46b. Griebel J. J., Glass R. S., Char K., Pyun J., Prog. Polym. Sci. 2016, 58, 90–125; [Google Scholar]
- 46c. Worthington M. J. H., Kucera R. L., Chalker J. M., Green Chem. 2017, 19, 2748–2761; [Google Scholar]
- 46d. Hoefling A., Lee Y. J., Theato P., Macromol. Chem. Phys. 2017, 218, 1600303. [Google Scholar]
- 47. Wang Q., Kim D., Dionysiou D. D., Sorial G. A., Timberlake D., Environ. Pollut. 2004, 131, 323–336. [DOI] [PubMed] [Google Scholar]
- 48. Guedron S., Cossa D., Grimaldi M., Charlet L., Appl. Geochem. 2011, 26, 222–229. [Google Scholar]
- 49.
- 49a. Bailey S. E., Olin T. J., Bricka R. M., Adrian D. D., Wat. Res. 1999, 33, 2469–2479; [Google Scholar]
- 49b. Meng X., Hua Z., Dermatas D., Wang W., Kuo H. Y., J. Hazard. Mater. 1998, 57, 231–241. [Google Scholar]
- 50.
- 50a. Aiken G. R., Hsu-Kim H., Ryan J. N., Environ. Sci. Technol. 2011, 45, 3196–3201; [DOI] [PubMed] [Google Scholar]
- 50b. Haitzer M., Aiken G. R., Ryan J. N., Environ. Sci. Technol. 2002, 36, 3564–3570; [DOI] [PubMed] [Google Scholar]
- 50c. Hsu-Kim H., Kucharzyk K. H., Zhang T., Deshusses M. A., Environ. Sci. Technol. 2013, 47, 2441–2456. [DOI] [PubMed] [Google Scholar]
- 51.
- 51a. Crockett M. P., Evans A. M., Worthington M. J. H., Albuquerque I. S., Slattery A. D., Gibson C. T., Campbell J. A., Lewis D. A., Bernardes G. J. L., Chalker J. M., Angew. Chem. Int. Ed. 2016, 55, 1714–1718; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 1746–1750; [Google Scholar]
- 51b. Hasell T., Parker D. J., Jones H. A., McAllister T., Howdle S. M., Chem. Commun. 2016, 52, 5383–5386; [DOI] [PubMed] [Google Scholar]
- 51c. Parker D. J., Jones H. A., Petcher S., Cervini L., Griffin J. M., Akhtar R., Hasell T., J. Mater. Chem. A 2017, 5, 11682–11692; [Google Scholar]
- 51d. Thielke M. W., Bultema L. A., Brauer D. D., Richter B., Fischer M., Theato P., Polymer 2016, 8, 266; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51e. Akay S., Kayan B., Kalderis D., Arslan M., Yagci Y., Kiskan B., J. Appl. Polym. Sci. 2017, 134, 45306; [Google Scholar]
- 51f. Abraham A. M., Kumar S. V., Alhassan S. M., Chem. Eng. J. 2018, 332, 1–7. [Google Scholar]
- 52. Shewchuk S. R., Azargohar R., Dalai A. K., J. Environ. Anal. Toxicol. 2016, 6, 1000379. [Google Scholar]
- 53. Chibunda R. T., Janssen C. R., Food Addit. Contam. Part A 2009, 26, 1482–1487. [DOI] [PubMed] [Google Scholar]
- 54. Spiegel S. J., Savornin O., Shoko D., Veiga M. M., Int. J. Occup. Environ. Health 2006, 12, 215–221. [DOI] [PubMed] [Google Scholar]
- 55. Hilson G., Monhemius A. J., J. Cleaner Prod. 2006, 14, 1158–1167. [Google Scholar]
- 56. Artisanal and Small-Scale Gold Mining Without Mercury, United States Environmental Protection Agency. Accessed 10 October 2017 from http://www.epa.gov/international-cooperation/artisanal-and-small-scale-gold-mining-without-mercury.
- 57. Drace K., Kiefer A. M., Veiga M. M., Williams M. K., Ascari B., Knapper K. A., Logan K. M., Breslin V. M., Skidmore A., Bolt D. A., Geist G., Reidy L., Cizdziel J. V., J. Cleaner Prod. 2012, 32, 88–95. [Google Scholar]
- 58. Gold Extraction with BORAX for Small-Scale Miners–Rather Rich & Healthy than Poor & Poisoned. Accessed 10 October 2017 from https://www.youtube.com/watch?v=X6Sawj0HyF0&feature=youtu.be.
- 59. Køster-Rasmussen R., Westergaard M. L., Brasholt M., Gutierrez R., Jørs E., Thomsen J. F., New Solutions 2016, 25, 567–587. [DOI] [PubMed] [Google Scholar]
- 60. Zolnikov T. R., Sci. Total Environ. 2012, 419, 1–6. [DOI] [PubMed] [Google Scholar]
- 61.
- 61a. Spiegel S., Land Use Policy 2016, 54, 559–573; [Google Scholar]
- 61b. Sippl K., Extractive Industries Soc. 2015, 2, 198–208. [Google Scholar]
- 62.
- 62a. Verbrugge B., Extractive Industries Soc. 2017, 4, 352–360; [Google Scholar]
- 62b. Spiegel S., Keane S., Metcalf S., Veiga M., Environ. Epidemiol. Toxicol. 2015, 17, 765–785. [Google Scholar]


