Abstract
Sleep homeostasis and circadian function are important maintaining factors for optimal health and well-being. Conversely, sleep and circadian disruptions are implicated in a variety of adverse health outcomes including substance use disorders (SUDs). These risks are particularly salient during adolescence. Adolescents require 8–10 hours of sleep per night, although few consistently achieve these durations. A mismatch between developmental changes and social/environmental demands contributes to inadequate sleep. Homeostatic sleep drive takes longer to build, circadian rhythms naturally delay, and sensitivity to the phase-shifting effects of light increases, all of which lead to an evening preference (i.e., chronotype) during adolescence. On the other hand, school start times are often earlier and use of electronic devices at night increases, leading to disrupted sleep and circadian misalignment (i.e., social jet-lag). Social factors (e.g., peer influence) and school demands further impact sleep and circadian rhythms. To cope with sleepiness, many teens regularly consume highly caffeinated energy drinks and other stimulants, creating further disruptions in sleep. Chronic sleep loss and circadian misalignment enhance developmental tendencies towards increase reward sensitivity and impulsivity, increasing the likelihood of engaging in risky behaviors, and exacerbating the vulnerability to substance use and SUDs. We review the neurobiology of brain reward systems and the impact of sleep and circadian rhythms changes on addiction vulnerability in adolescence, and suggest areas that warrant further research.
Keywords: adolescence, circadian, addiction, sleep, reward, circuitry
Introduction
Adolescence is a particularly vulnerable time for initiating drug use and developing SUDs. Early onset of drug abuse is strongly related to the development of lifelong addiction (1,2), and earlier use (11–17 years of age) predicts later dependence (i.e., within 2 years) for almost every drug of abuse (1). Identifying risk factors for substance use and abuse in adolescence is therefore a high public health priority.
Several factors contribute to adolescence being a particularly vulnerable time for the development of substance abuse, including earlier maturation of neural reward systems relative to the development of cognitive control systems, increased sensation-seeking behavior, the combination of increased peer influence and reduced parental monitoring, and greater sensitivity to the rewarding effects of drugs. Our review focuses on factors typically receiving far less attention—sleep and circadian rhythms (Figure 1). We focus on developmental changes to sleep and circadian rhythms during adolescence and how these may impact reward function and substance use. We highlight key findings related to potential genetic and molecular mechanisms underlying sleep and circadian function in relation to reward. Finally, we present an integrative model with parallels between human and animal studies, and discuss implications for intervention and treatments.
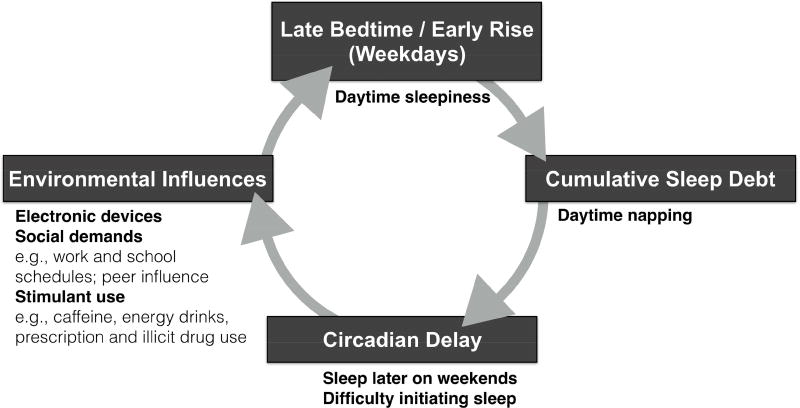
Figure 1.
Factors contributing to sleep deprivation in adolescents are compounded by the consequences of sleep and circadian disruptions.
Reward and Substance Use in Adolescence
Vulnerability for substance use and affective disorders
Initiation of substance use (i.e., first use of alcohol, marijuana, or other drugs) typically occurs during mid-to-late adolescence (~14–18 years), with frequency of use increasing during adolescence into early adulthood, and peaking during their 20’s, then an eventual decline (3). Alcohol and tobacco use remains relatively steady from late adolescence to the mid-30’s, while frequency of use of other substances, such as marijuana or other illicit drugs, declines into adulthood (3). Despite variability in the age of onset and pattern of use of substances, there is a striking epidemiological pattern pointing to adolescence as a vulnerable period for beginning substance use and other risky behaviors. Early initiation of use and factors, such as peer influence and socioeconomic status, increase risk for later substance use problems. From a developmental perspective, substance use during adolescence occurs in the context of normative changes in reward circuitry and behavior, affect, and sleep and circadian function (Figure 2).
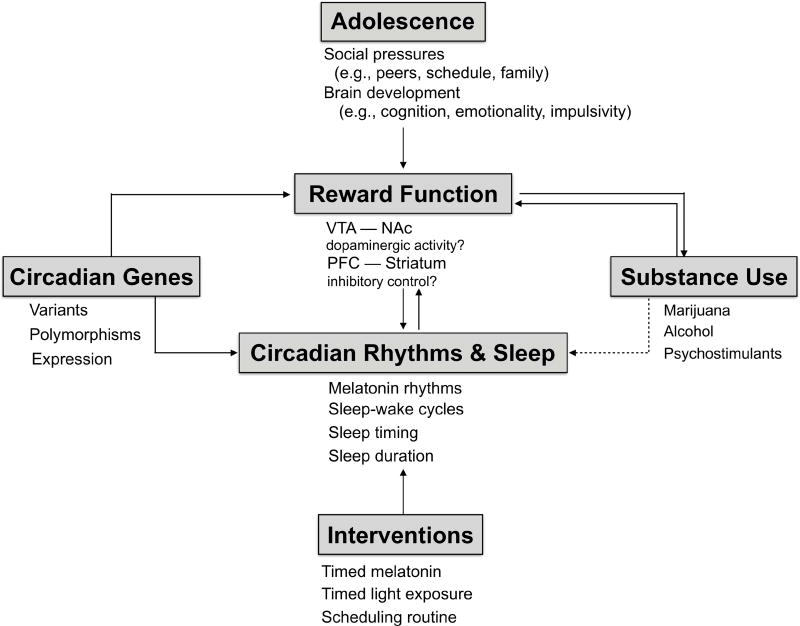
Figure 2.
Working model of the interplay between circadian rhythms, sleep, ‘reward’, and vulnerability to substance use during adolescence. Developmental changes in neural circuitry controlling reward function occurs during adolescence and seems to be altered by sleep deprivation and circadian misalignment, potentially leading to risk-taking, impulsivity, and poor decision making. These changes likely contribute to vulnerability to substance use particularly in adolescents. Rodent studies have demonstrated associations between certain circadian variants, striatal dopamine, and brain response to reward stimuli. Interventions to phase shift, resynchronize, and/or improve sleep timing may be effective for treating mood and substance issues often experienced during adolescence.
Affective problems, such as depression and anxiety, also often emerge during adolescence, both of which are associated with greater rates of substance use (4,5). Rates of comorbidity between affective and SUDs during adolescence range from 9.0–47.9%, and although depression and anxiety typically precede substance problems (5), alcohol and substance use (rather than disorders) may also precede depression (6). As the combination of affective and SUDs involve more severe depressive episodes and higher risk for suicidality (7,8), the development of psychiatric comorbidity has substantial public health significance.
Changes in reward circuitry and social context
Adolescence is marked by dramatic development in frontostriatal reward circuitry, including the ventral striatum (VS), dorsal striatum (DS), and medial prefrontal cortex (mPFC) (9,10). Dopamine availability increases during adolescence (11,12), potentially driving incentive motivation and sensation seeking, and likely underlying vulnerability for risky decisions and substance use. Conceptual models of brain development (13,14) postulate that the combination of altered reactivity of reward- and threat-related circuits, in conjunction with slowly maturing cognitive control, creates developmental risk for heightened emotionality and reward-seeking behaviors. Although debate continues over specific aspects of these models (15,16), there is consensus that the plasticity associated with changes in structure and function of reward circuitry during adolescent development make this a vulnerable period for reward-driven behaviors and problems such as substance use.
Evidence from Animal Models
Animal studies support clinical observations that biological changes during adolescence increase addiction vulnerability. Thus, certain aspects of brain development are conserved across species and may contribute to propensity for adolescents to use drugs. Cortical dendritic spine and synapse numbers increase during early development, then are gradually pruned during adolescence in humans, non-human primates, and rodents (17–22). Dopamine systems involved in salience and reward also undergo changes during adolescence. In adolescence, firing rates of dopamine neurons of the ventral tegmental area (VTA) increase, and dopamine receptor expression peaks (23). Thus, the development of brain reward system is, at least partially, evolutionarily conserved, suggesting mechanisms mediating adolescent vulnerability to substance abuse can be investigated using animal models.
Adolescents may experience the effects of drugs as more positive and the negative effects as less negative relative to adults (24), a theory also supported by animal studies. Adolescent rats voluntarily ingest larger quantities of alcohol than adults using a variety of access paradigms (25–28). Adolescents also require higher doses of alcohol than adults to form a conditioned taste aversion, or to exhibit sedation and motor incoordination (29,30). Thus, adolescent rats appear more vulnerable to high levels of alcohol consumption than adults. Adolescent rats are also more likely to self-administer the cannabinoid receptor agonist WIN-55212-2 (WIN) in higher quantities than adults, with a greater propensity to display relapse-like behavior to WIN-associated cues after adolescent relative to adult WIN self-administration (31). Adolescents also self-administer more cocaine than adults (23), but only when self-administration begins after (not before) the onset of puberty (23,32). While pre-pubertal cocaine use may not differ from adults, cocaine exposure occurring this period does promote the development of habitual response strategies, potentially increasing an individual’s vulnerability to substance abuse as an adult (33,34). In summary, adolescent rodents are willing to self-administer greater quantities of rewarding substances than adults, which may be mediated by an increased drive for rewards, largely supporting the clinical evidence for enhanced vulnerability to substance use during adolescence.
Sleep and Circadian Function in Adolescence: A Novel Risk Factor for Substance Use?
Normative changes in sleep and circadian rhythms
Delays in sleep, circadian preference, and chronotype begin around puberty and reach their maximum around age 20 before beginning a long, slow shift towards earlier timing over the lifespan (35,36). Sleep timing shifts later, i.e., delays, throughout adolescence, with greater delays on weekends, when sleep timing is unconstrained by school schedules (37). Parallel shifts occur in circadian preference, indicated by an increase in self-reported eveningness, and in chronotype (i.e., behavioral manifestations of underlying circadian timing), indicated by later sleep-wake times. Changes in sleep/circadian timing appear to result from both phase delays in the timing of the circadian clock (38), and a slower accumulation in homeostatic sleep drive (37). Some studies suggest that adolescents are more sensitive to light exposure during times of day when light causes delays (evening) and less sensitive when light causes advances (morning) (37) (39). Increasingly pervasive use of electronic devices influence adolescent sleep timing, duration, continuity, and quality by inducing circadian phase delays (40). Behavioral and environmental factors also contribute to changes in adolescent sleep timing, such as social pressure from peers and/or decreasing parental involvement in bedtime routines may lead to later sleep timing and reduced sleep duration (41,42).
Coincident with these changes in sleep timing, adolescents tend to experience reduced sleep duration, increased daytime sleepiness, and increased sleep disturbances (37). Although sleep duration decreases, sleep need—i.e., the amount of sleep required to ensure maximal alertness and performance—appears to remain unchanged (37). Adolescents need 8–10 hours of sleep per night (43), but the vast majority (71%) of high school students sleep less than 8 hours per night, and 44% sleep fewer than 6 hours per night (44). Decreased sleep duration and increased sleep disturbance may be a consequence of delayed sleep timing, conflicting with school start times on weekdays, (typically earlier in high school than in elementary or middle school), resulting in circadian misalignment and sleep loss (38,45). On weekends, adolescents tend to stay up later, and sleep later the next day to make up for lost sleep. Weekend oversleep compounds the phase delay in the clock (46,47), making it even more difficult to get up on Monday morning. Weekday-weekend shifts in sleep are often referred to as “social jet lag” (45,48), a term that aptly captures the vicious cycle of circadian misalignment, sleep disturbance, and sleep loss.
Other endogenous and exogenous sleep changes during adolescence could plausibly contribute to sleep duration and continuity. For example, slow-wave sleep (SWS) decreases ~60% across adolescence (49), possibly reflecting synaptic pruning and resulting efficiency of network connectivity (50,51). This “lightening” of sleep depth also contributes to impaired sleep continuity. Caffeine, alcohol, and other substances, also impact sleep and circadian rhythms—caffeine later in the day disrupts sleep and delays circadian timing (52,53). The combination of endogenous developmental changes, together with social, educational, environmental, and other influences, make adolescence a uniquely vulnerable period for circadian misalignment, sleep disturbance, and sleep loss.
Sleep/circadian vulnerability for substance use
Circadian misalignment, sleep disturbance, and sleep loss during adolescence are associated with increased substance use and related problems. Adolescents with short sleep duration are more likely to use substances—including caffeine, nicotine, alcohol, and illicit drugs (54–56)—and to engage in other risky behaviors (54,57,58). Prospective studies in adolescents implicate short sleep (59,60), and weekend oversleep as a risk for future alcohol and illicit drug use. Certainly, not all adolescents are similarly vulnerable to these changes. Some adolescents have trait-like characteristics that predispose them to engage in (or enjoy) more late-night activities, feel sleepy later in the night, or put themselves in more reward-focused environments, all of which could expose them to experiences that contribute to substance use problems.
Sleep and circadian characteristics typical of circadian misalignment are associated with affective and SUDs. The largest evidence base comes from studies of circadian preference, variously referred to as morningness-eveningness, or chronotype. Circadian preference is partly a consequence of endogenous circadian phase (61) and individual differences in homeostatic sleep drive (62). Cross-sectional studies in adolescents and adults report associations between greater eveningness and more frequent substance use and increased substance problems (63). Recent prospective evidence suggests eveningness predicts increases in substance involvement, and not merely a consequence of staying up late because they are engaging in substance use (64,65).
Some, but not all, studies support a link between social jet lag or circadian misalignment, and adolescent substance involvement. Weekday-weekend sleep differences have been associated with increased risk-taking behaviors, substance use, and depressed mood (66,67). Several longitudinal studies have linked markers of social jet-lag, including larger weekend delays and more variable sleep timing, with greater likelihood of alcohol use 2-years later (59); greater alcohol use disorder symptoms 3- and 5-years later (68); and an earlier onset of alcohol use disorder (69). However, two other longitudinal studies reported that later sleep timing (i.e., eveningness), rather than weekend changes in sleep timing, predicted increases in substance use (64,65).
Effects of sleep and circadian changes on reward function
Multiple lines of evidence support sleep and circadian modulation of reward function, suggesting a mechanistic pathway to substance abuse (Figure 2). Diurnal rhythms have been observed in self-reported positive affect and behavior, and an objective measure of reward activation (70–72). Moreover, eveningness is associated with depression, reduced reward responsiveness, blunted positive affect rhythms, sensation seeking, poor self-regulation, and impulsivity (70,71,73–79). Later bedtimes, more variable bedtimes, and shorter sleep may also contribute to these behaviors (67,78). These changes in sleep timing and chronotype are associated with altered reward-related brain function in adolescents (80–82).
Reward-related brain circuitry may thus underlie the link between adolescent sleep/circadian function and substance and affective disorders. Striatal response to monetary reward and relative glucose metabolism in the striatum and mPFC show diurnal variation—increasing in the afternoon/evening relative to the morning (70,83,84). Furthermore, reactivity in key nodes of reward circuitry is sensitive to circadian disturbance and sleep loss. In early adolescence, larger weekday-weekend differences in sleep timing (i.e., social jet lag) are associated with lower mPFC reactivity to anticipation and receipt of monetary reward, even after adjusting for puberty, gender, and total sleep time (82). Later sleep timing is also associated with reduced mPFC (81) and VS responses to monetary reward (85). Later adolescents (20 year-old males) who were evening types exhibited lower mPFC responses to reward anticipation and higher VS response to reward outcome relative to morning-types, controlling for time of scan (65). In turn, these eveningness-associated patterns of neural response to reward predicted greater concurrent alcohol dependence and alcohol consumption.
Sleep deprivation also disrupts reward neural circuitry, usually enhancing brain reactivity to positive experiences. In response to reward, VS response increases and mPFC decreases in young adults (ages 18–25) after one night of total sleep deprivation (86). In decision-making and reward-processing contexts, sleep deprivation shifts adults from protecting against loss and towards increasing pursuit of gains; increases VS activity during risky decision-making; and decreases insula and orbitofrontal cortical activity to loss (87). Pleasant pictures elicit a similar response (88), with greater mesolimbic network response (putamen and VTA) in sleep-deprived adults. Thus, sleep deprivation enhances mesolimbic reward brain activity and reduces prefrontal cortical response to perform executive functions (88–90). Sleep deprivation may affect brain reactivity differently in adolescents than adults, perhaps related to the shift toward greater neural and behavioral sensitivity to reward during adolescence (9). For instance, sleep restriction is associated with blunted striatal reactivity to reward outcome in adolescents (91), but heightened response in young adults (92).
Naturalistic differences in sleep quality and duration are also associated with function in adolescents’ reward circuitry, which may help to explain the consistent associations of sleep with affective psychopathology and substance use (63). Typically-developing adolescents with shorter sleep duration and lower sleep quality exhibit lower caudate response to reward anticipation (85), greater insula response to risk-taking, and lower functional connectivity between DLPFC and both insula and VS during risk taking (89) (Figure 3). Less effective frontostriatal regulation during rewarding contexts and behavioral compensation for blunted motivation could thus serve as a mechanism for the higher level of risky behaviors in adolescents with short sleep duration (93). Alterations in reward circuitry may also be a mechanism underlying the increased risk of depression associated with insomnia (94). This association appears to be mediated by higher DLPFC response to reward, a pattern consistently associated with adolescent depression (95).
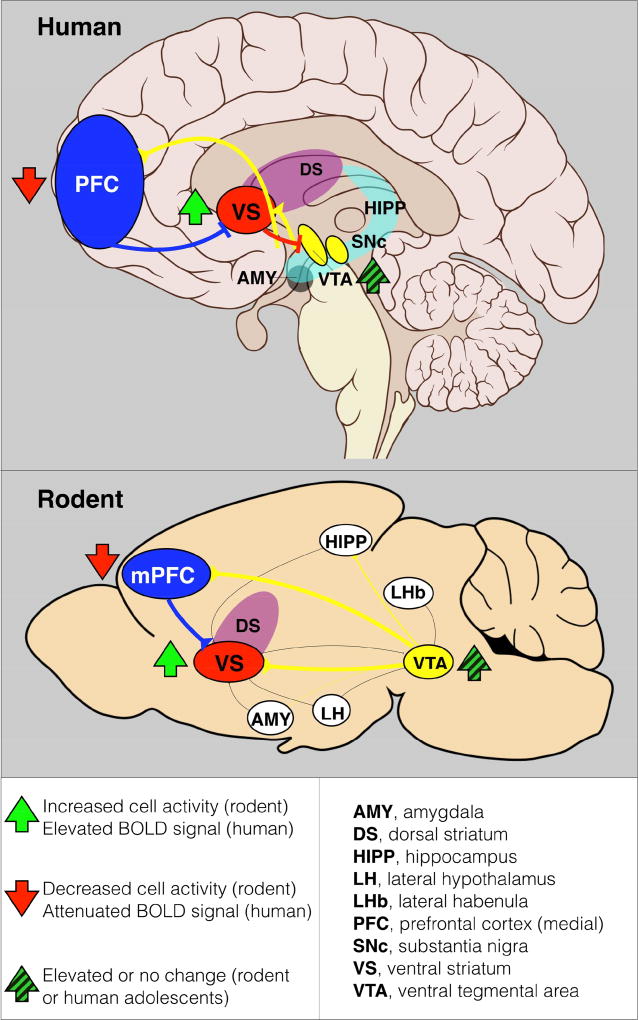
Figure 3.
Sagittal sections of a representative human (A) and rodent (B) brain illustrating major pathways of ‘reward’ neural circuitry. Sleep deprivation and/or circadian misalignment leads to reduced activity of the PFC (in humans, both DLPFC and mPFC) and increased activity of the ventral striatum (VS) or nucleus accumbens (NAc) in response to reward anticipation in adolescents. The imbalance of excitatory and inhibitory signaling is hypothesized to lead to poor executive function, academic performance, and decision making, while increasing impulsivity, risk-taking behavior, all of which likely contribute to a vulnerability to substance use and abuse. In rodents, sleep deprivation and/or circadian disruptions, whether environmental or genetic, usually lead to poor cognitive performance, working memory, and increased impulsivity, risk-taking behavior, and drug self-administration. Yellow pathways represent ascending dopaminergic pathways from the ventral tegmental area (VTA), and blue pathways represent descending glutamatergic pathways from the prefrontal cortex (PFC). AMY, amygdala; DS, dorsal striatum; HIPP, hippocampus; LHb, lateral habenula; LH, lateral hypothalamus; mPFC, medial prefrontal cortex; SNc, substantia nigra.
Changes in sleep and circadian rhythms, in combination with normative changes in reward circuitry, may constitute vulnerability factors for developing affective or substance use problems during adolescence. However, while the effects of circadian misalignment and sleep loss on neural circuits have been described, their cellular and molecular underpinnings remain poorly understood. Initial findings suggest that sleep deprivation reduces dopamine D2/D3 receptors in the VS without affecting basal dopamine release (96); D2 receptors in the VS are closely associated with risk taking behaviors and compulsive drug consumption (97,98). While decreased striatal expression of D2/D3 receptors is a potential mechanism by which sleep deprivation enhances risk and reward seeking, additional experimental studies in humans and animals are needed to better define the mechanisms underlying relationships between sleep, circadian rhythms, and reward. Furthermore, such experimental studies will be necessary to clarify the directionality of processes linking sleep/circadian characteristics, reward function, and substance use. Although we hypothesize that sleep/circadian disturbances influence reward function and, in turn, substance, other models are plausible. For instance, a more sensation-seeking personality may drive some adolescents to stay up later, to engage in rewarding, risky activities, and to thereby sacrifice sleep.
Evidence from animal models
Animal studies have begun to determine the impact of sleep restriction and circadian misalignment on the behavioral responses to drugs of abuse. Acute sleep deprivation or chronic partial sleep restrictions in animals often leads to enhanced risk taking and reward seeking, including cocaine and alcohol intake, sucrose self-administration, as well as intra-cranial self-stimulations (99–103). In various aspects, animal studies have recapitulated the phenomenon of sleep loss-enhanced reward seeking observed in humans. The similarities between the two suggest that there may be hard-wired molecular, cellular, and circuit-based mechanisms across species.
Sleep deprivation may alter synaptic transmission onto the principal neurons of the VS. As in humans, function in frontostriatal reward circuitry—in particular, balance between mPFC and VS—plays a central role in sleep deprivation effects. Glutamate release probability is reduced selectively at mPFC to nucleus accumbens (NAc) synapses following acute sleep deprivation in mice. Reversing this projection-specific synaptic alteration by optogenetically boosting the release probability at mPFC-to-NAc synapses reverses the sleep deprivation-induced enhanced sucrose self-administration (100). These results suggest that a weakening of the mPFC-to-NAc connection may facilitate reward seeking by reducing ‘top-down’ inhibitory control (104–106). It remains to be determined whether such weakening at mPFC-to-NAc pathway alone is sufficient for enhanced reward seeking after acute sleep deprivation (Figure 3). Finally, given the extensive evidence on the importance of this pathway in regulating various drug seeking and relapse (107–111), it is uncertain whether similar mechanisms may underlie enhanced drug and alcohol intake after sleep loss.
Much of the evidence from human imaging studies supports dysfunction in the VTA-PFC-NAc circuit following sleep disturbances and/or circadian misalignment. Each of these regions express circadian genes and maintain rhythms of activity and responses. In humans, rats, and mice, striatal and midbrain activity show diurnal variation (83,84,112–115). In the NAc, rhythms of dopamine, glutamate, and GABA are independent of light input (116,117), and may be controlled by diurnal variations of dopamine transporter activity (118). Sleep loss and/or circadian disruptions also affect these circuits, impacting reward seeking and relapse behaviors (119). For example, acute sleep deprivation leads to relapse of cocaine self-administration in rats via reduced excitatory transmission from mPFC to NAc (100), suggesting excitatory and inhibitory imbalances to the NAc may promote drug self-administration (120,121). Reduced inhibition of the NAc following sleep deprivation is usually associated with reduced PFC activity, and offers a plausible mechanism for elevated VS and reduced frontocortical responses in adolescents with circadian misalignment (80,112,122).
As described above, circadian misalignment can heighten the risk for substance use and abuse (123). Changes in photoperiod and day length are often used to model environmental sleep/circadian disruptions in animals. Repeated shifts of the light-dark schedule decreases and increases alcohol drinking in male and female rats, respectively (124). However, these effects vary by strain and species, as similar paradigms produce little to no effect on alcohol intake in mice. Shorter days (6 hrs light/18 hrs dark) attenuate reinstatement of cocaine seeking behavior in rats. The effect of short days persists even after returning to a standard 12:12 light-dark schedule, and correlates with photoperiodic modulation of tyrosine hydroxylase and dopamine transporter expression in the mPFC and DS (125).
The impact of these environmentally-induced circadian changes likely involves direct and indirect projections from the circadian pacemaker of the suprachiasmatic nucleus (SCN) to reward-related circuitry, including the VTA (126), medial preoptic area and dorsomedial hypothalamus (127). However, other brain regions receiving direct light input from the eye may also contribute to regulation of reward circuitry through non-canonical circadian pathways. For example, the lateral habenula receives direct inputs from both the SCN and the eyes (128), and modulates the activity of dopamine neurons in the VTA (Figure 3), suggesting another potential pathway for light to affect mood and reward-related behaviors (129).
Timing of light exposure (e.g., light at night) also impacts mood and reward circuits and behavior. Even brief exposure to light suppresses melatonin secretion at night. Light exposure at night, whether acute or chronic, alters melatonin release, which affects both sleep and circadian systems. In animals, exposure to aberrant light during early life or adolescence suppresses melatonin rhythms and leads to anxiety and depressive-like behaviors in adulthood (130). Whether aberrant light impacts reward or motivation behaviors is unknown, but changes in melatonin signaling can affect dopamine neurotransmission (131), and behavioral responses to drugs of abuse (132).
Animal studies also support findings in humans associating circadian gene variants with addiction. Variants of PER2 are associated with D2 receptor availability and cocaine addiction vulnerability (133,134); risky alcohol drinking under high psychosocial stress (135); and disrupted sleep (136). Interestingly, in healthy adolescents, specific PER2 alleles are associated with VS and mPFC response to reward outcomes (81). In alcohol-dependent adults, a PER1 variant predicts heavy drinking (137). CLOCK variants, particularly the CLOCK 3111T/C polymorphism, are associated with alcohol dependence (138), and increased risk for comorbid depression and alcohol use disorders (139). Located within the 3’ untranslated region, this polymorphism affects the expression, function, and stability of CLOCK (140). Mice with mutations of any of the core circadian genes, such as Per1, Per2, or Clock, among others, display altered behavioral responses to many drugs of abuse. For example, Clock mutant mice have reduced sleep and disrupted rhythms with increased risk-taking behavior and preference for alcohol and cocaine. Per1 and Per2 mutant mice voluntarily consume more alcohol than wild-type mice (141,142), which is further elevated following stress in Per1 mutant mice (137). Per1 and Per2 within the NAc also regulate anxiety-like behaviors (143), further suggesting Per genes may be important for mood and addiction comorbidity. Other circadian genes, such as Npas2, regulate cocaine reward behaviors, likely via a cell-type specific mechanism within the NAc (144). Circadian genes impact these behaviors through their regulation of neuronal function in reward-related circuitry, including circadian regulation of VTA-mediated dopamine release and responsivity of downstream targets (114,145,146). Additional studies are necessary to fully understand the impact of specific variants identified in human studies on endogenous gene and protein expression (140), and their potential effects during adolescence, since most, if not all, of these studies were completed in adulthood.
Treatment Implications
Chronotherapies, such as bright light therapy and social rhythm therapy, are efficacious treatments for certain mood disorders in adults, and have favorable side effect profiles (147,148). Few studies have evaluated these interventions on mood, sleep problems, and circadian alignment in adolescents. Studies have only focused on teens with depression or delayed phase sleep disorder (149). One randomized cross-over trial of 28 adolescents with depression found BLT (2,500 lux vs. placebo, 50 lux), induced an antidepressant response and a phase advance of cortisol and melatonin rhythms, with no adverse treatment effects (150). Given the promise of these initial findings, further studies are needed to examine the effects of sleep and circadian interventions on a range of outcomes, including reward function, and the vulnerability for substance use and SUDs.
Public policy offers another avenue for intervention. In 2014, the American Academy of Pediatrics stated, “Although a number of factors, including biological changes in sleep associated with puberty, lifestyle choices, and academic demands, negatively affect middle and high school students’ ability to obtain sufficient sleep, the evidence strongly implicates earlier school start times (i.e., before 8:30 am) as a key modifiable contributor to insufficient sleep, as well as circadian rhythm disruption, in this population. Furthermore, a substantial body of research has now demonstrated that delaying school start times is an effective countermeasure to chronic sleep loss and has a wide range of potential benefits to students regarding physical and mental health, safety, and academic achievement.” A combination of later school start times at the population level, and sleep and circadian interventions at the individual level, could help to align adolescent’s biological rhythms with the environment and perhaps mitigate future psychiatric and substance abuse problems.
Summary
Sleep and circadian rhythms undergo developmental changes from childhood through adolescence and into adulthood. During adolescence, an evening preference combined with social demands and other environmental factors, contributes to circadian misalignment and further exacerbates sleep disturbances, impacting neural circuits underlying mood and reward. Adolescent development is associated with enhanced reward sensitivity relative to cognitive control, phase delay in endogenous circadian rhythms, and reduced homeostatic sleep drive. Genetic, environmental and social factors interact with these developmental processes, often resulting in late sleep timing, short sleep duration, and circadian misalignment. These observations lead to a testable hypothesis: That late sleep timing, short sleep duration, and circadian misalignment adversely impact cortico-limbic function in adolescents, further enhancing reward function, impairing cognitive control, and increasing substance use risk. Future studies should examine naturally-occurring sleep and circadian phenotypes, both cross-sectionally and longitudinally, to investigate the neural, molecular, and genetic mechanisms by which sleep/circadian rhythms affect reward function and substance use risk. Furthermore, experimental manipulations of sleep and circadian rhythms can be investigated to examine their effects on reward function and substance use behavior. Such studies could lead to novel strategies for identifying vulnerability and mitigating the risk of developing SUDs in adolescence.
Acknowledgments
Dr. Buysse received consultation fees from Bayer HealthCare, BeHealth Solutions, Cereve, Inc., CME Outfitters, Emmi Solutions, Medscape, and Merck (DJB).
Grant Support:
DA038654, DA041872 (RWL); DA032557 (BPH); MH104418 (EEF); MH077106, DA033064 (PLF); DA041563, DA042029, AA025547 (MMT); AG047139, HL125103 (DJB); DA0358085 (YHH); DA039841, DA039865, DA042886, MH077159, MH106460 (CAM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
All other report no biomedical financial interests or potential conflicts of interest.
References
- 1.Chen CY, Storr CL, Anthony JC. Early-onset drug use and risk for drug dependence problems. Addict Behav. 2009;34:319–322. doi: 10.1016/j.addbeh.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma A, Morrow JD. Neurobiology of Adolescent Substance Use Disorders. Child Adolesc Psychiatr Clin N Am. 2016;25:367–375. doi: 10.1016/j.chc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Public Health. 1995;85:41–47. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kandel DB, Johnson JG, Bird HR, Canino G, Goodman SH, Lahey BB, et al. Psychiatric disorders associated with substance use among children and adolescents: findings from the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) Study. J Abnorm Child Psychol. 1997;25:121–132. doi: 10.1023/a:1025779412167. [DOI] [PubMed] [Google Scholar]
- 5.O'Neil KA, Conner BT, Kendall PC. Internalizing disorders and substance use disorders in youth: comorbidity, risk, temporal order, and implications for intervention. Clin Psychol Rev. 2011;31:104–112. doi: 10.1016/j.cpr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Womack SR, Shaw DS, Weaver CM, Forbes EE. Bidirectional Associations Between Cannabis Use and Depressive Symptoms From Adolescence Through Early Adulthood Among At-Risk Young Men. J Stud Alcohol Drugs. 2016;77:287–297. doi: 10.15288/jsad.2016.77.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alegria AA, Hasin DS, Nunes EV, Liu SM, Davies C, Grant BF, et al. Comorbidity of generalized anxiety disorder and substance use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2010;71:1187–1195. doi: 10.4088/JCP.09m05328gry. quiz 1252-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanco C, Alegria AA, Liu SM, Secades-Villa R, Sugaya L, Davies C, et al. Differences among major depressive disorder with and without co-occurring substance use disorders and substance-induced depressive disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2012;73:865–873. doi: 10.4088/JCP.10m06673. [DOI] [PubMed] [Google Scholar]
- 9.Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, et al. Healthy adolescents' neural response to reward: associations with puberty, positive affect, and depressive symptoms. J Am Acad Child Adolesc Psychiatry. 2010;49:162–172. e161–165. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padmanabhan A, Geier CF, Ordaz SJ, Teslovich T, Luna B. Developmental changes in brain function underlying the influence of reward processing on inhibitory control. Dev Cogn Neurosci. 2011;1:517–529. doi: 10.1016/j.dcn.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luciana M, Collins PF. Incentive Motivation, Cognitive Control, and the Adolescent Brain: Is It Time for a Paradigm Shift? Child Dev Perspect. 2012;6:392–399. doi: 10.1111/j.1750-8606.2012.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padmanabhan A, Luna B. Developmental imaging genetics: linking dopamine function to adolescent behavior. Brain Cogn. 2014;89:27–38. doi: 10.1016/j.bandc.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Rev. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends Cogn Sci. 2012;16:322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, et al. The dual systems model: Review, reappraisal, and reaffirmation. Dev Cogn Neurosci. 2016;17:103–117. doi: 10.1016/j.dcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DePoy LM, Noble B, Allen AG, Gourley SL. Developmentally divergent effects of Rho-kinase inhibition on cocaine- and BDNF-induced behavioral plasticity. Behav Brain Res. 2013;243:171–175. doi: 10.1016/j.bbr.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- 19.Koss WA, Belden CE, Hristov AD, Juraska JM. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse. 2014;68:61–72. doi: 10.1002/syn.21716. [DOI] [PubMed] [Google Scholar]
- 20.Milstein JA, Elnabawi A, Vinish M, Swanson T, Enos JK, Bailey AM, et al. Olanzapine treatment of adolescent rats causes enduring specific memory impairments and alters cortical development and function. PLoS One. 2013;8:e57308. doi: 10.1371/journal.pone.0057308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- 22.Woo TU, Pucak ML, Kye CH, Matus CV, Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80:1149–1158. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]
- 23.Wong WC, Ford KA, Pagels NE, McCutcheon JE, Marinelli M. Adolescents are more vulnerable to cocaine addiction: behavioral and electrophysiological evidence. J Neurosci. 2013;33:4913–4922. doi: 10.1523/JNEUROSCI.1371-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- 26.Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- 27.Ristuccia RC, Spear LP. Adolescent and adult heart rate responses to self-administered ethanol. Alcohol Clin Exp Res. 2008;32:1807–1815. doi: 10.1111/j.1530-0277.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serlin H, Torregrossa MM. Adolescent rats are resistant to forming ethanol seeking habits. Dev Cogn Neurosci. 2015;16:183–190. doi: 10.1016/j.dcn.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcohol Clin Exp Res. 2010;34:2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, et al. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- 31.Kirschmann EK, Pollock MW, Nagarajan V, Torregrossa MM. Effects of Adolescent Cannabinoid Self-Administration in Rats on Addiction-Related Behaviors and Working Memory. Neuropsychopharmacology. 2017;42:989–1000. doi: 10.1038/npp.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madsen HB, Zbukvic IC, Luikinga SJ, Lawrence AJ, Kim JH. Extinction of conditioned cues attenuates incubation of cocaine craving in adolescent and adult rats. Neurobiol Learn Mem. 2016 doi: 10.1016/j.nlm.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 33.DePoy LM, Allen AG, Gourley SL. Adolescent cocaine self-administration induces habit behavior in adulthood: sex differences and structural consequences. Transl Psychiatry. 2016;6:e875. doi: 10.1038/tp.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinton EA, Wheeler MG, Gourley SL. Early-life cocaine interferes with BDNF-mediated behavioral plasticity. Learn Mem. 2014;21:253–257. doi: 10.1101/lm.033290.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Randler C. Morningness-eveningness comparison in adolescents from different countries around the world. Chronobiol Int. 2008;25:1017–1028. doi: 10.1080/07420520802551519. [DOI] [PubMed] [Google Scholar]
- 36.Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, et al. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 37.Carskadon MA. Sleep in adolescents: the perfect storm. Pediatr Clin North Am. 2011;58:637–647. doi: 10.1016/j.pcl.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crowley SJ, Van Reen E, LeBourgeois MK, Acebo C, Tarokh L, Seifer R, et al. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS One. 2014;9:e112199. doi: 10.1371/journal.pone.0112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crowley SJ, Cain SW, Burns AC, Acebo C, Carskadon MA. Increased Sensitivity of the Circadian System to Light in Early/Mid-Puberty. J Clin Endocrinol Metab. 2015;100:4067–4073. doi: 10.1210/jc.2015-2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cain N, Gradisar M. Electronic media use and sleep in school-aged children and adolescents: A review. Sleep Med. 2010;11:735–742. doi: 10.1016/j.sleep.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Bartel KA, Gradisar M, Williamson P. Protective and risk factors for adolescent sleep: a meta-analytic review. Sleep Med Rev. 2015;21:72–85. doi: 10.1016/j.smrv.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Mednick SC, Christakis NA, Fowler JH. The spread of sleep loss influences drug use in adolescent social networks. PLoS One. 2010;5:e9775. doi: 10.1371/journal.pone.0009775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paruthi S, Brooks LJ, D'Ambrosio C, Hall WA, Kotagal S, Lloyd RM, et al. Recommended Amount of Sleep for Pediatric Populations: A Consensus Statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785–786. doi: 10.5664/jcsm.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basch CE, Basch CH, Ruggles KV, Rajan S. Prevalence of sleep duration on an average school night among 4 nationally representative successive samples of American high school students, 2007–2013. Prev Chronic Dis. 2014;11:E216. doi: 10.5888/pcd11.140383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Touitou Y. Adolescent sleep misalignment: a chronic jet lag and a matter of public health. J Physiol Paris. 2013;107:323–326. doi: 10.1016/j.jphysparis.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Crowley SJ, Carskadon MA. Modifications to weekend recovery sleep delay circadian phase in older adolescents. Chronobiol Int. 2010;27:1469–1492. doi: 10.3109/07420528.2010.503293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor SR, Doyle FJ, 3rd, Petzold LR. Oscillator model reduction preserving the phase response: application to the circadian clock. Biophys J. 2008;95:1658–1673. doi: 10.1529/biophysj.107.128678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 49.Baker FC, Willoughby AR, de Zambotti M, Franzen PL, Prouty D, Javitz H, et al. Age-Related Differences in Sleep Architecture and Electroencephalogram in Adolescents in the National Consortium on Alcohol and Neurodevelopment in Adolescence Sample. Sleep. 2016;39:1429–1439. doi: 10.5665/sleep.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feinberg I, Campbell IG. Sleep EEG changes during adolescence: an index of a fundamental brain reorganization. Brain Cogn. 2010;72:56–65. doi: 10.1016/j.bandc.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 51.Saletin JM, van der Helm E, Walker MP. Structural brain correlates of human sleep oscillations. Neuroimage. 2013;83:658–668. doi: 10.1016/j.neuroimage.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burke TM, Markwald RR, McHill AW, Chinoy ED, Snider JA, Bessman SC, et al. Effects of caffeine on the human circadian clock in vivo and in vitro. Sci Transl Med. 2015;7:305ra146. doi: 10.1126/scitranslmed.aac5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drake C, Roehrs T, Shambroom J, Roth T. Caffeine effects on sleep taken 0, 3, or 6 hours before going to bed. J Clin Sleep Med. 2013;9:1195–1200. doi: 10.5664/jcsm.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKnight-Eily LR, Eaton DK, Lowry R, Croft JB, Presley-Cantrell L, Perry GS. Relationships between hours of sleep and health-risk behaviors in US adolescent students. Prev Med. 2011;53:271–273. doi: 10.1016/j.ypmed.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 55.Paiva T, Gaspar T, Matos MG. Mutual relations between sleep deprivation, sleep stealers and risk behaviours in adolescents. Sleep Sci. 2016;9:7–13. doi: 10.1016/j.slsci.2016.02.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sivertsen B, Skogen JC, Jakobsen R, Hysing M. Sleep and use of alcohol and drug in adolescence. A large population-based study of Norwegian adolescents aged 16 to 19 years. Drug Alcohol Depend. 2015;149:180–186. doi: 10.1016/j.drugalcdep.2015.01.045. [DOI] [PubMed] [Google Scholar]
- 57.Thomas AG, Monahan KC, Lukowski AF, Cauffman E. Sleep problems across development: a pathway to adolescent risk taking through working memory. J Youth Adolesc. 2015;44:447–464. doi: 10.1007/s10964-014-0179-7. [DOI] [PubMed] [Google Scholar]
- 58.Wheaton AG, Olsen EO, Miller GF, Croft JB. Sleep Duration and Injury-Related Risk Behaviors Among High School Students--United States, 2007–2013. MMWR Morb Mortal Wkly Rep. 2016;65:337–341. doi: 10.15585/mmwr.mm6513a1. [DOI] [PubMed] [Google Scholar]
- 59.Pasch KE, Latimer LA, Cance JD, Moe SG, Lytle LA. Longitudinal bi-directional relationships between sleep and youth substance use. J Youth Adolesc. 2012;41:1184–1196. doi: 10.1007/s10964-012-9784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberts RE, Roberts CR, Duong HT. Sleepless in adolescence: prospective data on sleep deprivation, health and functioning. J Adolesc. 2009;32:1045–1057. doi: 10.1016/j.adolescence.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duffy JF, Dijk DJ, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med. 1999;47:141–150. [PMC free article] [PubMed] [Google Scholar]
- 62.Mongrain V, Carrier J, Dumont M. Circadian and homeostatic sleep regulation in morningness-eveningness. J Sleep Res. 2006;15:162–166. doi: 10.1111/j.1365-2869.2006.00532.x. [DOI] [PubMed] [Google Scholar]
- 63.Hasler BP, Soehner AM, Clark DB. Sleep and circadian contributions to adolescent alcohol use disorder. Alcohol. 2015;49:377–387. doi: 10.1016/j.alcohol.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tavernier R, Munroe M, Willoughby T. Perceived morningness-eveningness predicts academic adjustment and substance use across university, but social jetlag is not to blame. Chronobiol Int. 2015;32:1233–1245. doi: 10.3109/07420528.2015.1085062. [DOI] [PubMed] [Google Scholar]
- 65.Hasler BP, Casement MD, Sitnick SL, Shaw DS, Forbes EE. Eveningness among late adolescent males predicts neural reactivity to reward and alcohol dependence two years later. Behav Brain Res. 2017;327:112–120. doi: 10.1016/j.bbr.2017.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O'Brien EM, Mindell JA. Sleep and risk-taking behavior in adolescents. Behav Sleep Med. 2005;3:113–133. doi: 10.1207/s15402010bsm0303_1. [DOI] [PubMed] [Google Scholar]
- 67.Pasch KE, Laska MN, Lytle LA, Moe SG. Adolescent sleep, risk behaviors, and depressive symptoms: are they linked? Am J Health Behav. 2010;34:237–248. doi: 10.5993/ajhb.34.2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hasler BP, Martin CS, Wood DS, Rosario B, Clark DB. A longitudinal study of insomnia and other sleep complaints in adolescents with and without alcohol use disorders. Alcohol Clin Exp Res. 2014;38:2225–2233. doi: 10.1111/acer.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hasler BP, Kirisci L, Clark DB. Restless Sleep and Variable Sleep Timing During Late Childhood Accelerate the Onset of Alcohol and Other Drug Involvement. J Stud Alcohol Drugs. 2016;77:649–655. doi: 10.15288/jsad.2016.77.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hasler BP, Germain A, Nofzinger EA, Kupfer DJ, Krafty RT, Rothenberger SD, et al. Chronotype and diurnal patterns of positive affect and affective neural circuitry in primary insomnia. J Sleep Res. 2012;21:515–526. doi: 10.1111/j.1365-2869.2012.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller MA, Rothenberger SD, Hasler BP, Donofry SD, Wong PM, Manuck SB, et al. Chronotype predicts positive affect rhythms measured by ecological momentary assessment. Chronobiol Int. 2015;32:376–384. doi: 10.3109/07420528.2014.983602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB, et al. Nature's clocks and human mood: the circadian system modulates reward motivation. Emotion. 2009;9:705–716. doi: 10.1037/a0017080. [DOI] [PubMed] [Google Scholar]
- 73.Adan A, Natale V, Caci H, Prat G. Relationship between circadian typology and functional and dysfunctional impulsivity. Chronobiol Int. 2010;27:606–619. doi: 10.3109/07420521003663827. [DOI] [PubMed] [Google Scholar]
- 74.Caci H, Robert P, Boyer P. Novelty seekers and impulsive subjects are low in morningness. Eur Psychiatry. 2004;19:79–84. doi: 10.1016/j.eurpsy.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 75.Drennan MD, Klauber MR, Kripke DF, Goyette LM. The effects of depression and age on the Horne-Ostberg morningness-eveningness score. J Affect Disord. 1991;23:93–98. doi: 10.1016/0165-0327(91)90096-b. [DOI] [PubMed] [Google Scholar]
- 76.Hasler BP, Allen JJ, Sbarra DA, Bootzin RR, Bernert RA. Morningness-eveningness and depression: preliminary evidence for the role of the behavioral activation system and positive affect. Psychiatry Res. 2010;176:166–173. doi: 10.1016/j.psychres.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Merikanto I, Kronholm E, Peltonen M, Laatikainen T, Vartiainen E, Partonen T. Circadian preference links to depression in general adult population. J Affect Disord. 2015;188:143–148. doi: 10.1016/j.jad.2015.08.061. [DOI] [PubMed] [Google Scholar]
- 78.Owens JA, Dearth-Wesley T, Lewin D, Gioia G, Whitaker RC. Self-Regulation and Sleep Duration, Sleepiness, and Chronotype in Adolescents. Pediatrics. 2016;138 doi: 10.1542/peds.2016-1406. [DOI] [PubMed] [Google Scholar]
- 79.Tonetti L, Adan A, Caci H, De Pascalis V, Fabbri M, Natale V. Morningness-eveningness preference and sensation seeking. Eur Psychiatry. 2010;25:111–115. doi: 10.1016/j.eurpsy.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 80.Hasler BP, Sitnick SL, Shaw DS, Forbes EE. An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Res. 2013;214:357–364. doi: 10.1016/j.pscychresns.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Forbes EE, Dahl RE, Almeida JR, Ferrell RE, Nimgaonkar VL, Mansour H, et al. PER2 rs2304672 polymorphism moderates circadian-relevant reward circuitry activity in adolescents. Biol Psychiatry. 2012;71:451–457. doi: 10.1016/j.biopsych.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hasler BP, Dahl RE, Holm SM, Jakubcak JL, Ryan ND, Silk JS, et al. Weekend-weekday advances in sleep timing are associated with altered reward-related brain function in healthy adolescents. Biol Psychol. 2012;91:334–341. doi: 10.1016/j.biopsycho.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buysse DJ, Nofzinger EA, Germain A, Meltzer CC, Wood A, Ombao H, et al. Regional brain glucose metabolism during morning and evening wakefulness in humans: preliminary findings. Sleep. 2004;27:1245–1254. doi: 10.1093/sleep/27.7.1245. [DOI] [PubMed] [Google Scholar]
- 84.Germain A, Nofzinger EA, Meltzer CC, Wood A, Kupfer DJ, Moore RY, et al. Diurnal variation in regional brain glucose metabolism in depression. Biol Psychiatry. 2007;62:438–445. doi: 10.1016/j.biopsych.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holm SM, Forbes EE, Ryan ND, Phillips ML, Tarr JA, Dahl RE. Reward-related brain function and sleep in pre/early pubertal and mid/late pubertal adolescents. J Adolesc Health. 2009;45:326–334. doi: 10.1016/j.jadohealth.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mullin BC, Phillips ML, Siegle GJ, Buysse DJ, Forbes EE, Franzen PL. Sleep deprivation amplifies striatal activation to monetary reward. Psychol Med. 2013;43:2215–2225. doi: 10.1017/S0033291712002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Venkatraman V, Chuah YM, Huettel SA, Chee MW. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30:603–609. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- 88.Gujar N, Yoo SS, Hu P, Walker MP. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J Neurosci. 2011;31:4466–4474. doi: 10.1523/JNEUROSCI.3220-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Telzer EH, Fuligni AJ, Lieberman MD, Galvan A. The effects of poor quality sleep on brain function and risk taking in adolescence. Neuroimage. 2013;71:275–283. doi: 10.1016/j.neuroimage.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 91.Franzen PL, Buysse DJ, Forbes EE, Jones NP, Ohlsen TJ, Germain A. Sleep restriction lowers striatal responses to the receipt of monetary reward in adolescents. Sleep. 2016;39S:A93. [Google Scholar]
- 92.Germain A, McNamee R, Khan H, McMakin DL, Franzen P, Forbes EE. Sleep restriction amplifies neural response to reward cues compared to normal sleep and sleep deprivation. Sleep. 2016;39S:A94. [Google Scholar]
- 93.Owens J, Wang G, Lewin D, Skora E, Baylor A. Association between short sleep duration and risk behavior factors in middle school students. Sleep. 2016 doi: 10.1093/sleep/zsw004. [DOI] [PubMed] [Google Scholar]
- 94.Staner L. Comorbidity of insomnia and depression. Sleep Med Rev. 2010;14:35–46. doi: 10.1016/j.smrv.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 95.Forbes EE, Dahl RE. Research Review: altered reward function in adolescent depression: what, when and how? J Child Psychol Psychiatry. 2012;53:3–15. doi: 10.1111/j.1469-7610.2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Volkow ND, Tomasi D, Wang GJ, Telang F, Fowler JS, Logan J, et al. Evidence that sleep deprivation downregulates dopamine D2R in ventral striatum in the human brain. J Neurosci. 2012;32:6711–6717. doi: 10.1523/JNEUROSCI.0045-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Linnet J, Peterson E, Doudet DJ, Gjedde A, Moller A. Dopamine release in ventral striatum of pathological gamblers losing money. Acta Psychiatr Scand. 2010;122:326–333. doi: 10.1111/j.1600-0447.2010.01591.x. [DOI] [PubMed] [Google Scholar]
- 98.Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aalto J, Kiianmaa K. REM-sleep deprivation-induced increase in ethanol intake: role of brain monoaminergic neurons. Alcohol. 1986;3:377–381. doi: 10.1016/0741-8329(86)90057-1. [DOI] [PubMed] [Google Scholar]
- 100.Liu Z, Wang Y, Cai L, Li Y, Chen B, Dong Y, et al. Prefrontal Cortex to Accumbens Projections in Sleep Regulation of Reward. J Neurosci. 2016;36:7897–7910. doi: 10.1523/JNEUROSCI.0347-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McKenna BS, Dickinson DL, Orff HJ, Drummond SP. The effects of one night of sleep deprivation on known-risk and ambiguous-risk decisions. J Sleep Res. 2007;16:245–252. doi: 10.1111/j.1365-2869.2007.00591.x. [DOI] [PubMed] [Google Scholar]
- 102.Puhl MD, Boisvert M, Guan Z, Fang J, Grigson PS. A novel model of chronic sleep restriction reveals an increase in the perceived incentive reward value of cocaine in high drug-taking rats. Pharmacol Biochem Behav. 2013;109:8–15. doi: 10.1016/j.pbb.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Steiner SS, Ellman SJ. Relation between REM sleep and intracranial self-stimulation. Science. 1972;177:1122–1124. doi: 10.1126/science.177.4054.1122. [DOI] [PubMed] [Google Scholar]
- 104.Feil J, Sheppard D, Fitzgerald PB, Yucel M, Lubman DI, Bradshaw JL. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci Biobehav Rev. 2010;35:248–275. doi: 10.1016/j.neubiorev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 105.Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, et al. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science. 2016;351:aac9698. doi: 10.1126/science.aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 107.Chen GJ, Xiong Z, Yan Z. Abeta impairs nicotinic regulation of inhibitory synaptic transmission and interneuron excitability in prefrontal cortex. Mol Neurodegener. 2013;8:3. doi: 10.1186/1750-1326-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.LaLumiere RT, Smith KC, Kalivas PW. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. Eur J Neurosci. 2012;35:614–622. doi: 10.1111/j.1460-9568.2012.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shen Z, Huang P, Qian W, Wang C, Yu H, Yang Y, et al. Severity of dependence modulates smokers' functional connectivity in the reward circuit: a preliminary study. Psychopharmacology (Berl) 2016;233:2129–2137. doi: 10.1007/s00213-016-4262-5. [DOI] [PubMed] [Google Scholar]
- 111.Terraneo A, Leggio L, Saladini M, Ermani M, Bonci A, Gallimberti L. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: A pilot study. Eur Neuropsychopharmacol. 2016;26:37–44. doi: 10.1016/j.euroneuro.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hasler BP, Forbes EE, Franzen PL. Time-of-day differences and short-term stability of the neural response to monetary reward: a pilot study. Psychiatry Res. 2014;224:22–27. doi: 10.1016/j.pscychresns.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dominguez-Lopez S, Howell RD, Lopez-Canul MG, Leyton M, Gobbi G. Electrophysiological characterization of dopamine neuronal activity in the ventral tegmental area across the light-dark cycle. Synapse. 2014;68:454–467. doi: 10.1002/syn.21757. [DOI] [PubMed] [Google Scholar]
- 114.Sidor MM, Spencer SM, Dzirasa K, Parekh PK, Tye KM, Warden MR, et al. Daytime spikes in dopaminergic activity drive rapid mood-cycling in mice. Mol Psychiatry. 2015;20:1406–1419. doi: 10.1038/mp.2014.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao H, Rusak B. Circadian firing-rate rhythms and light responses of rat habenular nucleus neurons in vivo and in vitro. Neuroscience. 2005;132:519–528. doi: 10.1016/j.neuroscience.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 116.Castaneda TR, de Prado BM, Prieto D, Mora F. Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J Pineal Res. 2004;36:177–185. doi: 10.1046/j.1600-079x.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 117.Webb IC, Lehman MN, Coolen LM. Diurnal and circadian regulation of reward-related neurophysiology and behavior. Physiol Behav. 2015;143:58–69. doi: 10.1016/j.physbeh.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 118.Ferris MJ, Espana RA, Locke JL, Konstantopoulos JK, Rose JH, Chen R, et al. Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proc Natl Acad Sci U S A. 2014;111:E2751–2759. doi: 10.1073/pnas.1407935111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Muto V, Jaspar M, Meyer C, Kusse C, Chellappa SL, Degueldre C, et al. Local modulation of human brain responses by circadian rhythmicity and sleep debt. Science. 2016;353:687–690. doi: 10.1126/science.aad2993. [DOI] [PubMed] [Google Scholar]
- 120.Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Salgado S, Kaplitt MG. The Nucleus Accumbens: A Comprehensive Review. Stereotact Funct Neurosurg. 2015;93:75–93. doi: 10.1159/000368279. [DOI] [PubMed] [Google Scholar]
- 122.Hasler BP, Clark DB. Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcohol Clin Exp Res. 2013;37:558–565. doi: 10.1111/acer.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bildt C, Michelsen H. Gender differences in the effects from working conditions on mental health: a 4-year follow-up. Int Arch Occup Environ Health. 2002;75:252–258. doi: 10.1007/s00420-001-0299-8. [DOI] [PubMed] [Google Scholar]
- 124.Logan RW, Williams WP, 3rd, McClung CA. Circadian rhythms and addiction: mechanistic insights and future directions. Behav Neurosci. 2014;128:387–412. doi: 10.1037/a0036268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sorg BA, Stark G, Sergeeva A, Jansen HT. Photoperiodic suppression of drug reinstatement. Neuroscience. 2011;176:284–295. doi: 10.1016/j.neuroscience.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Luo AH, Aston-Jones G. Circuit projection from suprachiasmatic nucleus to ventral tegmental area: a novel circadian output pathway. Eur J Neurosci. 2009;29:748–760. doi: 10.1111/j.1460-9568.2008.06606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mendoza J, Challet E. Circadian insights into dopamine mechanisms. Neuroscience. 2014;282:230–242. doi: 10.1016/j.neuroscience.2014.07.081. [DOI] [PubMed] [Google Scholar]
- 128.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15:443–454. doi: 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bedrosian TA, Nelson RJ. Timing of light exposure affects mood and brain circuits. Transl Psychiatry. 2017;7:e1017. doi: 10.1038/tp.2016.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zisapel N. Melatonin-dopamine interactions: from basic neurochemistry to a clinical setting. Cell Mol Neurobiol. 2001;21:605–616. doi: 10.1023/A:1015187601628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tyhon A, Lakaye B, Adamantidis A, Tirelli E. Amphetamine- and cocaine-induced conditioned place preference and concomitant psychomotor sensitization in mice with genetically inactivated melanin-concentrating hormone MCH(1) receptor. Eur J Pharmacol. 2008;599:72–80. doi: 10.1016/j.ejphar.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 133.Shumay E, Fowler JS, Wang GJ, Logan J, Alia-Klein N, Goldstein RZ, et al. Repeat variation in the human PER2 gene as a new genetic marker associated with cocaine addiction and brain dopamine D2 receptor availability. Transl Psychiatry. 2012;2:e86. doi: 10.1038/tp.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Blomeyer D, Buchmann AF, Lascorz J, Zimmermann US, Esser G, Desrivieres S, et al. Association of PER2 genotype and stressful life events with alcohol drinking in young adults. PLoS One. 2013;8:e59136. doi: 10.1371/journal.pone.0059136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Comasco E, Nordquist N, Gokturk C, Aslund C, Hallman J, Oreland L, et al. The clock gene PER2 and sleep problems: association with alcohol consumption among Swedish adolescents. Ups J Med Sci. 2010;115:41–48. doi: 10.3109/03009731003597127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dong L, Bilbao A, Laucht M, Henriksson R, Yakovleva T, Ridinger M, et al. Effects of the circadian rhythm gene period 1 (per1) on psychosocial stress-induced alcohol drinking. Am J Psychiatry. 2011;168:1090–1098. doi: 10.1176/appi.ajp.2011.10111579. [DOI] [PubMed] [Google Scholar]
- 138.Kovanen L, Saarikoski ST, Haukka J, Pirkola S, Aromaa A, Lonnqvist J, et al. Circadian clock gene polymorphisms in alcohol use disorders and alcohol consumption. Alcohol Alcohol. 2010;45:303–311. doi: 10.1093/alcalc/agq035. [DOI] [PubMed] [Google Scholar]
- 139.Sjoholm LK, Kovanen L, Saarikoski ST, Schalling M, Lavebratt C, Partonen T. CLOCK is suggested to associate with comorbid alcohol use and depressive disorders. J Circadian Rhythms. 2010;8:1. doi: 10.1186/1740-3391-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ozburn AR, Purohit K, Parekh PK, Kaplan GN, Falcon E, Mukherjee S, et al. Functional Implications of the CLOCK 3111T/C Single-Nucleotide Polymorphism. Front Psychiatry. 2016;7:67. doi: 10.3389/fpsyt.2016.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gamsby JJ, Templeton EL, Bonvini LA, Wang W, Loros JJ, Dunlap JC, et al. The circadian Per1 and Per2 genes influence alcohol intake, reinforcement, and blood alcohol levels. Behav Brain Res. 2013;249:15–21. doi: 10.1016/j.bbr.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- 143.Spencer S, Falcon E, Kumar J, Krishnan V, Mukherjee S, Birnbaum SG, et al. Circadian genes Period 1 and Period 2 in the nucleus accumbens regulate anxiety-related behavior. Eur J Neurosci. 2013;37:242–250. doi: 10.1111/ejn.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ozburn AR, Falcon E, Twaddle A, Nugent AL, Gillman AG, Spencer SM, et al. Direct regulation of diurnal Drd3 expression and cocaine reward by NPAS2. Biol Psychiatry. 2015;77:425–433. doi: 10.1016/j.biopsych.2014.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U S A. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Parekh PK, Becker-Krail D, Sundaravelu P, Ishigaki S, Okado H, Sobue G, et al. Altered GluA1 (Gria1) Function and Accumbal Synaptic Plasticity in the ClockDelta19 Model of Bipolar Mania. Biol Psychiatry. 2017 doi: 10.1016/j.biopsych.2017.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wirz-Justice A. Chronobiology and mood disorders. Dialogues Clin Neurosci. 2003;5:315–325. doi: 10.31887/DCNS.2003.5.4/awirzjustice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Frank E. Interpersonal and social rhythm therapy: a means of improving depression and preventing relapse in bipolar disorder. J Clin Psychol. 2007;63:463–473. doi: 10.1002/jclp.20371. [DOI] [PubMed] [Google Scholar]
- 149.Bartlett DJ, Biggs SN, Armstrong SM. Circadian rhythm disorders among adolescents: assessment and treatment options. Med J Aust. 2013;199:S16–20. doi: 10.5694/mja13.10912. [DOI] [PubMed] [Google Scholar]
- 150.Niederhofer H, von Klitzing K. Bright light treatment as mono-therapy of non-seasonal depression for 28 adolescents. Int J Psychiatry Clin Pract. 2012;16:233–237. doi: 10.3109/13651501.2011.625123. [DOI] [PubMed] [Google Scholar]