Abstract
STUDY QUESTION
Why are many sperm required for successful fertilization of oocytes in vitro, even though fertilization occurs in vivo when only a few sperm reach the oocyte?
SUMMARY ANSWER
Creatine produced in the ovary promotes efficient fertilization in vivo; however, in vitro, creatine is not contained in the in vitro fertilization (IVF) medium.
WHAT IS KNOWN ALREADY
The IVF medium enables capacitation of sperm. However, the IVF medium does not fully mimic the in vivo environment during fertilization. Consequently, fertilization in vitro is more inefficient than in the oviduct.
STUDY DESIGN, SIZE, DURATION
Follicular and oviductal fluids were collected and then analyzed for creatine and glucose levels. To determine the physiological functions of creatine, the creatine antagonist 3-guanidinopropionic acid (GPA) was injected into hormonally primed mice. Using conventional IVF protocols, sperm were pre-incubated in IVF medium with creatine and then co-cultured with 10 ovulated cumulus-oocyte complexes (1–1000 per oocyte) in 50 μl medium droplets.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Glucose and creatine levels were measured using commercial enzymatic assay kits. The effect of creatine in vivo was assessed by mating experiments using mice treated with or without GPA just before ovulation. To assess the functions of sperm incubated in IVF medium containing creatine, we analyzed (1) the motility of sperm using computer-assisted sperm assay, (2) the capacitation level of sperm by western blot analyses, and (3) the condition of sperm acrosomes by peanut agglutinin lectin-FITC staining.
MAIN RESULTS AND THE ROLE OF CHANCE
Oviductal creatine levels were significantly increased following ovulation. Injecting mice with GPA just before ovulation significantly reduced the number of fertilized oocytes. The addition of creatine to IVF medium enhanced sperm capacitation by increasing ATP levels. Successful fertilization was achieved with as few as five sperm/oocyte in the creatine group, and the number of fertilized oocytes was significantly higher than in the control without creatine (P < 0.01).
LIMITATIONS, REASONS FOR CAUTION
In the present study, a pharmacological approach, creatine antagonist (GPA) treatment, but not a knockout mouse model, was used to understand the role of creatine in vivo. The role of creatine in fertilization processes can only be shown in a mouse model.
WIDER IMPLICATIONS OF THE FINDINGS
A modified IVF technique using creatine-containing medium was developed and shown to markedly improve fertilization with small numbers of sperm. This approach has the potential to be highly beneficial for human assisted reproductive technologies, especially for patients with a limited number of good quality sperm.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported in part by JSPS KAKENHI Grant numbers JP24688028, JP16H05017 (to M.S.), and JP15J05331 (to T.U.), the Japan Agency for Medical Research and Development (AMED) (16gk0110015h0001 to M.S.), and National Institutes of Health (NIH-HD-076980 to J.S.R). The authors have nothing to disclose.
Keywords: sperm capacitation, creatine, fertilization, ATP production, in vitro fertilization
Introduction
In mammals, a large number of ejaculated sperm enter the uterus. However, only a limited number of sperm with normal morphology, such as an intact acrosome and linear sperm tail, reach the isthmus of oviducts (Overstreet and Cooper, 1975). Most sperm that reach the isthmus bind directly to the surface of oviductal epithelial cells until activation of the fertilization process. Small numbers of sperm reach the oviductal ampulla when ovulated cumulus-oocyte complexes (COCs) enter the oviduct and fertilization occurs quickly (Hunter, 1981). Most sperm that reach the ampulla have undergone physiological changes to prepare them for fertilization, a process known as capacitation. This process includes hyperactivation of sperm motility and preparation of sperm to undergo acrosomal exocytosis. Recently, it was reported that mouse sperm reach each ovulated oocyte one at a time in vivo, indicating that one capacitated sperm is sufficient to complete the fertilization process of each oocyte in vivo (Muro et al., 2016). However, in vitro fertilization (IVF) is much less efficient than that in vivo, because a large number of sperm are required for successful fertilization (Yanagimachi and Chang, 1963). Specifically, 500 motile sperm per oocyte are needed for successful fertilization in conventional IVF protocols for human and mouse oocytes (Fraser and Drury, 1975; Wolf et al., 1984). This finding suggests that the IVF environment is suboptimal and that factors promoting highly efficient fertilization in vivo need to be identified and applied to IVF protocols to improve the success and efficiency of assisted reproductive technology (ART).
To mimic in vivo oviductal conditions during fertilization, Quinn et al. (1985) devised a culture medium based on the composition of human tubal fluid (HTF). The HTF medium induced sperm capacitation in vitro and supported IVF of rodent and human oocytes (Murray and Smith, 1997; Hsu et al., 1999). The medium is a basic physiological pH-buffered balanced salt solution containing glycolytic substrates for ATP production (Quinn et al., 1995). The factors in HTF medium, which initiate sperm capacitation, are Ca2+, HCO3– and albumin (Visconti, 2009). Albumin is important to enhance membrane permeability by facilitating removal of cholesterol from the sperm cell membrane, thereby enhancing membrane permeability (Go and Wolf, 1985). In other species, such as cattle and pigs, modified Bracket and Oliphant’s medium, Tyrode’s basal medium, and medium 199 have often been used for IVF, which contain at least two factors such as glycolytic substrates, HCO3– and albumin (Kuwayama et al., 1992; Shimada et al., 2003; Nedambale et al., 2006). Therefore, IVF media containing glycolytic substrates, Ca2+, HCO3– and albumin (or another absorber of cholesterol, such as cyclodextrin) permit capacitation of sperm for IVF without oviduct fluid (Choi and Toyoda, 1998). Nevertheless, it is thought that IVF media do not fully mimic the in vivo environment during the fertilization process, because a large number of sperm are required to support a high fertilization rate.
Glycolysis produces reactive oxygen species and lactate as by-products of ATP production (Brand and Hermfisse, 1997). The accumulation of reactive oxygen species induces oxidative stress leading to DNA damage responses in sperm as well as reduced mitochondrial activity (Lopes et al., 1998), low ATP production, and decreased sperm motility (Baumber et al., 2000). The accumulation of lactate lowers the cytoplasmic pH level, thereby altering the mechanisms that support sperm motility (Carr et al., 1985). These negative effects on sperm functions contribute to the limited fertilization potential of sperm under current IVF culture conditions, indicating that other factors are needed to improve IVF success. In muscle cells, the creatine pathway regulates the ATP concentration by movement of PO32– between ADP and ATP in a creatine kinase-dependent manner (Harris et al., 1992). When muscle cells are cultured, addition of creatine increases repeated contraction and relaxation without induction of oxidative stress and low intracellular pH (Derave et al., 2003). Interestingly, creatine kinase activity has been reported in human and mouse sperm (Huszar et al., 1990; Wallimann and Hemmer, 1994). However, the functions of the creatine pathway in mammalian sperm during fertilization remain unclear. Therefore, the current study was designed to determine whether creatine is generated in the female reproductive tract and whether creatine kinase is activated in sperm and/or oocytes during fertilization. Based on our data indicating that creatine is present in vivo, we developed a novel efficient IVF culture medium that improved capacitation and fertilization and required only a limited number of sperm in conventional 50 μl HTF droplets used for IVF.
Materials and Methods
Materials
Equine chorionic gonadotropin (eCG) and human chorionic gonadotropin (hCG) were purchased from Asuka Seiyaku (Tokyo, Japan). AMV reverse transcriptase was purchased from Promega (Madison, WI, USA). Routine chemicals and reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA) or Nakarai Chemical Co (Osaka, Japan). Creatine monohydrate was purchased from Wako (Osaka, Japan). 3-Guanidinopropionic acid (GPA) was purchased from Sigma-Aldrich.
Animals
Immature female (3 weeks old), adult female (8–12 weeks old), and 3-month-old male C57BL/6 mice were obtained from Charles River Laboratories Japan. At 23 days of age, immature female mice were injected intraperitoneally (i.p.) with four IU eCG to stimulate follicular growth, followed by 5 IU hCG after 48 h to stimulate ovulation. Animals were housed in the Experiment Animal Center at Hiroshima University under a 14 h light/10 h dark schedule and provided with food and water ad libitum.
Ethical approval
Animals were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals, as approved by the Animal Care and Use Committee at Hiroshima University.
GPA treatments
GPA (0.4 g/kg) was injected into hormonally primed mice at selected time points (Kazak et al., 2015). For in vivo mating experiments, female mice were mated with male mice immediately following the hCG injection, and oocytes were collected at 24 h after hCG stimulation. For in vitro fertilization assays, ovulated oocytes were collected from oviduct ampulla at 16 h after hCG stimulation and then used for IVF.
Sperm preparation
HTF medium was used for sperm incubation and IVF (Quinn et al., 1995). Sperm were collected from the cauda epididymis into 500 μl HTF medium using a 27 G syringe. The sperm were incubated in HTF medium containing 0, 200, 500 or 1000 μM creatine for various time intervals and then analyzed. Some sperm were incubated with 500 μM creatine and either 50 or 500 μM GPA.
Immunofluorescence and peanut agglutinin lectin (PNA) staining of sperm
After incubation, sperm were mounted on glass slides. The sperm were permeabilized by 0.1% (v/v) TritonX-100/phosphate buffered saline (PBS) and fixed with 100% methanol for 10 min at room temperature. For immunofluorescence, sperm were probed with a 1:100-diluted anti-Slc6a8 antibody (ab-62196, Abcam, Cambridge, UK), and the antigens were visualized with Cy3-conjugated goat anti-rabbit IgG (1:200, Sigma-Aldrich). For PNA staining, sperm were incubated with PNA-FITC (Sigma-Aldrich) in PBS for 60 min at 37°C. After washing with PBS, sperm were mounted with VECTASHIELD Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA, USA). Digital images were captured using a BZ-9000 microscope (Keyence Co., Osaka, Japan).
Measurement of the ATP concentration and creatine kinase activity in sperm
After incubation, ATP concentrations in sperm were measured with the Enzylight™ ATP Assay Kit (cat#: EATP-100; Bioassay System, Hayward, CA, USA). Briefly, sperm samples were mixed with assay buffer and substrate, and then luminescence was measured on a luminometer (2030 Multilabel Reader ARVO X4; PerkinElmer Inc., Waltham, MA, USA).
Creatine kinase activity in sperm was measured with the Enzychrom™ Creatine Kinase Assay Kit (cat#: ECPK-100; Bioassay System). Sperm samples were mixed with assay buffer, substrate, and enzyme. The fluorescence was then measured at 20 and 40 min of incubation in a microplate reader at 340 nm.
Analysis of sperm motility using a computer-assisted sperm assay (CASA) system
Sperm were incubated in HTF medium containing 0, 500 or 500 μM creatine and 500 μM GPA for 4 h. Sperm samples (10 μl) were loaded into 10 μm-deep makler counting chambers. Sperm tracks (0.5 s, 45 frames) were captured at 60 Hz using a CASA system (HT CASA-Ceros II; Hamiltan Thorne, MA, USA). More than 200 individual trajectories were recorded.
IVF and embryo transfer
IVF was analyzed as described previously (Shimada et al., 2008). Ten ovulated COCs collected from super-ovulated mice were placed in 50 μl HTF medium for each IVF treatment. Spermatozoa were collected according to sperm preparation. After 60 min of incubation, spermatozoa present in the upper layer of HTF medium were transferred to the fertilization medium at final numbers of 1, 2.5, 5, 50, 500 or 1000 spermatozoa per COC. Oocytes were examined for numbers of pronuclei after 6 h and cultured further in developing medium (KSOM+AA; Millipore, Billerica, MA, USA). Some blastocysts were used for embryo transfer according to De Matos et al. (2008).
RNA extraction and RT-PCR
Total RNA was obtained using the RNAeasy Mini Kit (Qiagen Sciences, Germantown, MD, USA), according to the manufacturer’s instructions, and reverse transcribed using 500 ng poly-dT and 0.25U AMV reverse transcriptase at 42°C for 75 min and then 95°C for 5 min. cDNA and primers shown in Supplementary Table S1 were added to a 15 μl total reaction volume of Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). PCRs were then performed using the StepOne real time PCR system (Applied Biosystems). L19 was used as a control for reaction efficiency and variations in concentrations of mRNA in the original RT reaction.
Western blot analyses
Sperm were lysed by homogenization in sodium dodecyl sulfate (SDS) sample buffer. Extracts resolved by SDS polyacrylamide gel (12.5%) electrophoresis were transferred to polyvinylidene fluoride membranes (GE Bioscience, Newark, NJ, USA). Membranes were blocked in Tris-buffered saline with Tween 20 [TBST; 10 mM Tris (pH 7.5), 150 mM NaCl, and 0.05% (v/v) Tween 20] containing 5% (w/v) bovine serum albumin (Nakarai). Blots were incubated with a 1:1000-diluted anti-phosphotyrosine antibody (8959; Cell Signaling Technology, Inc., MA, USA), anti-Slc6a8 antibody, or anti-tubulin antibody (2148; Cell Signaling Technology, Inc.) overnight at 4°C. After washing in TBST, enhanced chemiluminescence (ECL) detection was performed using an ECL system, according the manufacturer’s specifications (GE Bioscience) and appropriate exposure of the blots to Fuji X-ray film (Fujifilm, Tokyo, Japan). Band intensities were analyzed using a Gel-Pro Analyzer (Media Cybernetics, Rockville, MD, USA).
Measurement of creatine and glucose levels
The fluid contents of follicles and oviducts were collected in 10 μl cold PBS (pH 7.4) using a 27 G syringe. The tissues were homogenized in 200 μl cold PBS. After centrifuging at 14,000g for 5 min, the supernatants were sonicated, and the protein concentration was measured using the DC Protein Assay Kit (Bio-Lad laboratories, Hercules, CA, USA). Samples of 20 μg protein were prepared to detect creatine levels using a Creatine Assay Kit (Abcam) and glucose levels using a glucose estimation kit (BioVision Inc., Mountain View, CA, USA), according to each manufacturer’s protocol.
Statistics
Statistical analyses of data from three or four replicates for comparison were carried out by either Student’s t-test or one-way analysis of variance followed by Tukey’s post hoc test (Statview; Abacus Concepts, Inc., Berkeley, CA).
Results
The creatine pathway is activated in sperm and oocytes in association with increased levels of glucose in the oviduct at the time of fertilization
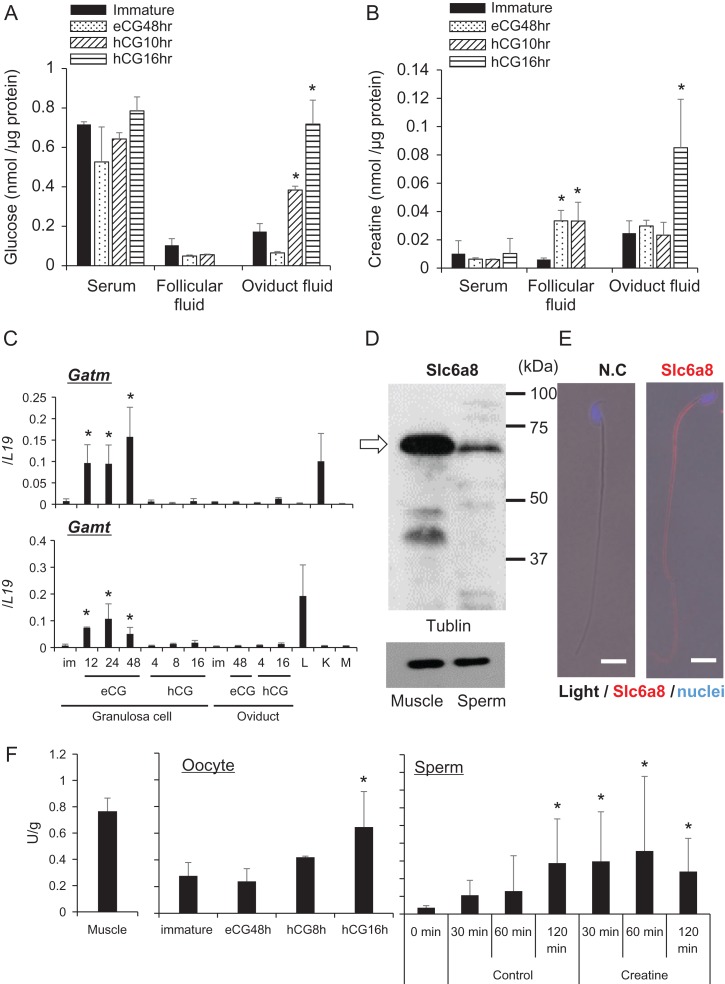
The concentrations of glucose in oviductal and follicular fluids were low compared with their concentrations in serum of mice before hormonal treatments (control) but increased in oviductal fluid at 10 and 16 h after hCG treatment (Fig. 1A). The concentration of creatine in follicular fluid was significantly increased after eCG stimulation, which was maintained but not increased further by hCG. In contrast, the levels of creatine were significantly increased in oviductal fluid after hCG, but not eCG, stimulation (Fig. 1B). Serum levels of creatine did not change in response to any hormonal treatment, indicating that the hormone-induced changes in the ovary and oviduct were tissue-specific. To determine whether the ovary or oviduct produced creatine in response to gonadotropins, the expression of genes encoding creatine-synthesizing enzymes, Gamt coding glycine amidinotransferase and Gatm coding guanidinoacetate methyltransferase, was examined by RT-PCR. Increased expression of these genes was observed in granulosa cells following stimulation by eCG but not hCG. The expression of these genes was not altered in the oviduct by hormonal treatments (Fig. 1C).
Figure 1.
The creatine pathway is activated in sperm and oocytes during fertilization. (A) Glucose levels in serum, follicular fluid, and oviduct fluid. Samples were collected from four female mice at each time point after hormonal treatments. Values are represented as the mean ± standard deviation (SD) of four replicates. *P < 0.05 compared with levels in immature mice. (B) Creatine concentrations in serum, follicular fluid, and oviduct fluid. Samples were collected from four female mice at each time point after hormonal treatments. Values are represented as the mean ± SD of four replicates. *P < 0.05 compared with levels in immature mice. (C) Gatm and Gamt expression levels in granulosa cells and oviducts after hormonal treatments. Expression levels of genes were normalized to those of L19. Values represent the mean ± SD of three replicates. *Denotes a significant difference compared with the level in immature granulosa cells. L: Liver; K: Kidney; M: Muscle. (D) Expression of creatine transporter in mouse sperm. Sperm were collected from the cauda epididymis and cell lysates were prepared for western blot analyses using an anti-Slc6a8 antibody. The white arrow indicates the position of Slc6a8. Tubulin was used as a loading control. Lysates prepared from muscle tissue were used as positive controls. Results are representative of three independent experiments. (E) Localization of creatine transporter in mouse sperm. Sperm were collected from the epididymis into HTF medium and then air dried. Smears were incubated with the anti-Slc6a8 antibody and then a secondary antibody. N.C. refers to the negative control that was incubated without the anti-Slc6a8 antibody. Scale bar indicates 10 μm. (F) Creatine kinase activities in oocytes and sperm. Oocytes were collected from the ovary or oviduct after hormonal treatments. Sperm were collected from the epididymis and then incubated in HTF medium with or without creatine for a maximum of 120 min. Muscle was used as a positive control. Creatine kinase activities were normalized to the protein concentrations. Values represent the mean ± SD of three replicates. *Denotes a significant difference compared with the level in immature females or 0 min.
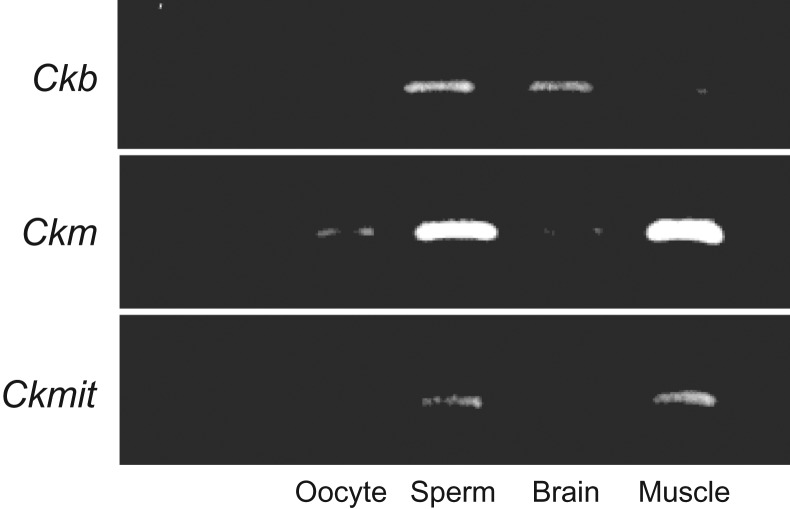
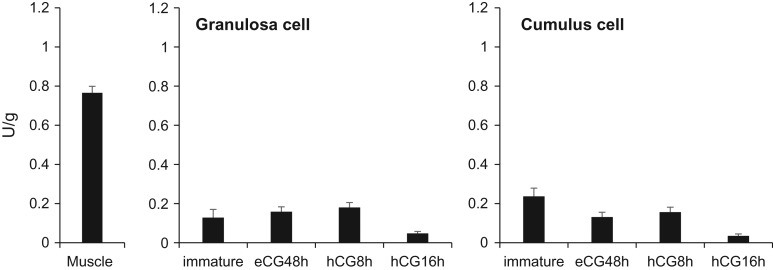
A positive signal for the creatine transporter Slc6a8 was detected at 70 kDa by western blot analyses of lysates prepared from sperm and muscle tissue as a control (Fig. 1D). Slc6a8-positive signals were localized to the sperm tail and midpiece (Fig. 1E). The expression of the muscle-type creatine kinase (Ckm) was detected in matured oocytes. All types of creatine kinase were expressed in sperm (Supplementary Fig. S1). The activity of creatine kinase in mature oocytes collected at 16 h after hCG stimulation was significantly higher than that in immature oocytes. However, the activities in granulosa cells and cumulus cells were not changed after hormonal treatment, and were low as well as that in immature oocytes (Supplementary Fig. S2). Additionally, the activity of creatine kinase in sperm was increased significantly after 120 min of incubation in HTF fertilization medium and occurred earlier in the presence of creatine (Fig. 1F).
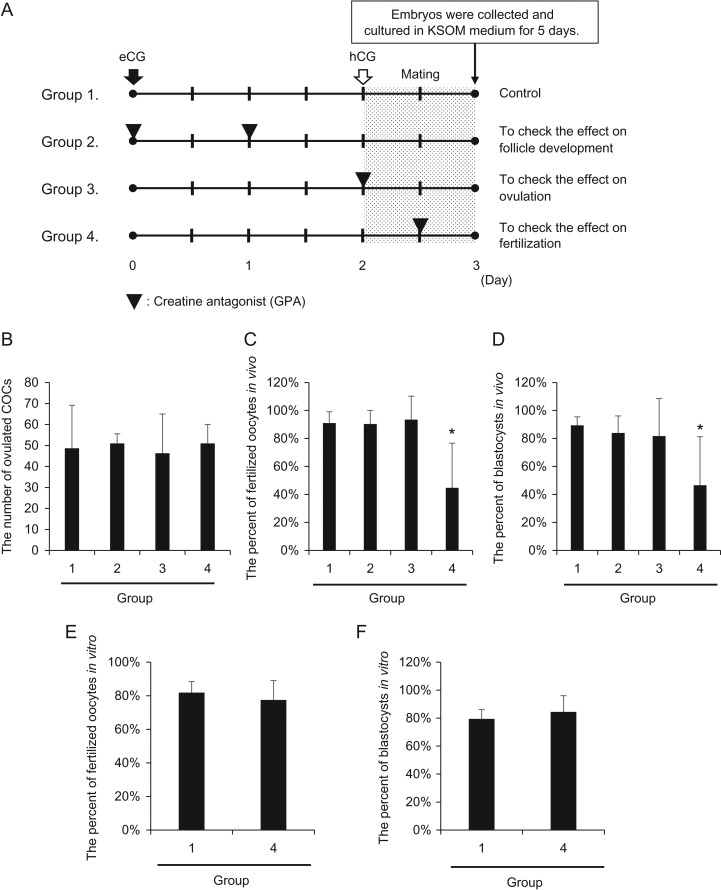
Injection of a creatine antagonist (GPA) into female mice suppresses in vivo fertilization
To determine the physiological functions of the creatine pathway, a creatine antagonist (GPA) was injected at specific time points during the ovulatory period (Fig. 2A). The number of ovulated COCs was not affected by injection of GPA at any time point (Fig. 2B). The numbers of fertilized oocytes and blastocyst stage embryos recovered from mice co-injected with GPA and either eCG or hCG were not significantly decreased compared with those from control mice (Fig. 2C, D). However, when GPA was injected at 12 h after hCG, the number of fertilized oocytes was decreased significantly, and only a limited number of these fertilized oocytes developed to the blastocyst stage (Fig. 2C, D). To determine whether GPA negatively affected the functions of either oocytes or sperm, COCs were collected from mice injected with GPA at 12 h after hCG injection and used for IVF. The numbers of fertilized oocytes and blastocysts were not significantly different between control and GPA treatment groups (Fig. 2E, F).
Figure 2.
Injection of a creatine antagonist (GPA) into female mice suppresses in vivo fertilization. (A) Schematic diagram of GPA treatment of 3-week-old female mice. In the control (group 1), mice were injected ip with four IU eCG followed by five IU hCG after 48 h. To evaluate the effect of creatine on follicle developmental processes, GPA was co-injected with eCG and injected at 24 h after the first eCG injection (group 2). To evaluate the effect of creatine on ovulation processes, GPA was co-injected with hCG (group 3). To evaluate the effect of creatine on fertilization processes, GPA was injected at 10 h after hCG injection (group 4). The female mice were mated with male mice just after hCG stimulation, and fertilized oocytes were collected at 24 h after hCG stimulation. KSOM; potassium-supplemented simplex optimized medium. (B–D) Number of ovulated oocytes (B) and percentages of fertilized oocytes in vivo (C) and blastocysts in vivo per fertilized oocyte (D) when GPA was injected at each selected time point. Black bars indicate the mean value of each percentage. Values represent the mean ± SD of three replicates. *P < 0.05 compared with the control. (E, F) Percentages of fertilized oocytes in vitro (E) and blastocysts per in vitro-fertilized oocyte (F) when cumulus-oocyte complexes (COCs) were collected from GPA-treated mice and then used for IVF. Ovulated oocytes were collected from oviduct ampulla at 16 h after hCG stimulation and used for IVF. Values represent the mean ± SD of three replicates. Black bars indicate the mean value of each percentage.
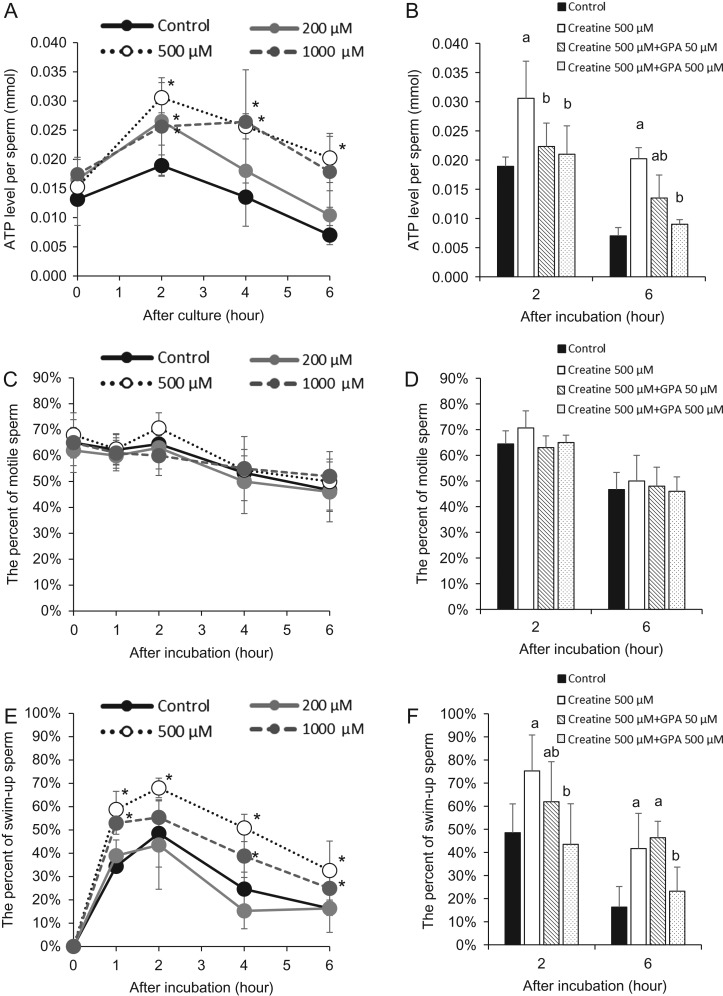
Addition of creatine to IVF medium increases sperm ATP levels and sperm motility
When sperm were cultured in IVF medium containing 200–1000 μM creatine, sperm ATP levels were significantly increased compared with control sperm at 2 h of incubation. Sperm incubated in medium containing the highest levels (500–1000 μM) of creatine continued to exhibit elevated ATP levels for 6 h (Fig. 3A). The addition of GPA reduced the increase in ATP levels stimulated by creatine at 2 and 6 h (Fig. 3B). Although the percentage of motile sperm was not affected by the addition of creatine and/or GPA (Fig. 3C, D), sperm motility measured by swim-up assays was significantly increased within 1 h compared with controls. Specifically, more than half of sperm incubated in medium containing 500–1000 μM creatine were observed in the upper layer within 1 h of incubation, and the percentage was significantly higher than in the controls. Increased sperm motility was maintained in 500–1000 μM creatine groups until 6 h (Fig. 3E). The addition of 500 μM GPA to media containing creatine significantly decreased the percentage of swim-up sperm in upper layers at 2 and 6 h (Fig. 3F).
Figure 3.
Addition of creatine increases ATP levels in sperm and enhances sperm motility in vitro. (A) Changes in ATP levels of sperm after culture in HTF medium with/without creatine. Sperm were collected from the murine epididymis and then treated with 0 (control), 200, 500 or 1000 μM creatine. The experiment was repeated four times using a total of four male mice. Values represent the mean ± SD of four replicates. *P < 0.05 compared with the level of the control. (B) Effects of creatine antagonist GPA (0, 50, or 500 μM) on ATP levels in sperm after culture in HTF medium containing 500 μM creatine. Sperm were collected from the mouse epididymis and cultured in each treatment group. The experiment was repeated four times using sperm from a total of four male mice. Values represent the mean ± SD of four replicates. Different superscripts denote significant differences among the GPA concentrations at each time point (P < 0.05). Control: Sperm were cultured without creatine and GPA. (C) Percentage of motile sperm in HTF medium with/without creatine. Sperm were collected from the murine epididymis and incubated in HTF medium with/without creatine for a maximum of 6 h. The numbers of motile and total sperm were counted at each time point. The percentage of motile sperm was then calculated. The experiment was repeated four times using a total of four male mice. Values represent the mean ± SD of four replicates. (D) Percentages of motile sperm in HTF medium containing creatine with/without creatine antagonist GPA. Sperm were collected from the mouse epididymis and cultured in HTF medium containing 500 μM creatine with various doses of creatine antagonist GPA (0, 50 or 500 μM) for a maximum of 6 h. The numbers of motile and total sperm were counted at each time point. The percentage of motile sperm was then calculated. The experiment was repeated four times using a total of four male mice. Values represent the mean ± SD of four replicates. (E) Percentages of swim-up sperm in HTF medium with/without creatine. Sperm were collected from the murine epididymis and cultured in HTF medium with/without creatine for a maximum of 6 h. The numbers of sperm in the upper layer and total sperm were counted at each time point. The percent of swim-up sperm was then calculated. The experiment was repeated four times using a total of four male mice. Values represent the mean ± SD of four replicates. (F) Percentages of swim-up sperm in HTF medium containing creatine with/without creatine antagonist GPA. Sperm were collected from the mouse epididymis and cultured in HTF medium containing 500 μM creatine with various doses of creatine antagonist GPA (0, 50 or 500 μM) for a maximum of 6 h. The numbers of sperm in the upper layer and total sperm were counted at each time point. The percentage of swim-up sperm was then calculated. The experiment was repeated four times using a total of four male mice. Values represent the mean ± SD of four replicates. Different superscripts denote significant differences among the GPA concentrations at each time point (P < 0.05).
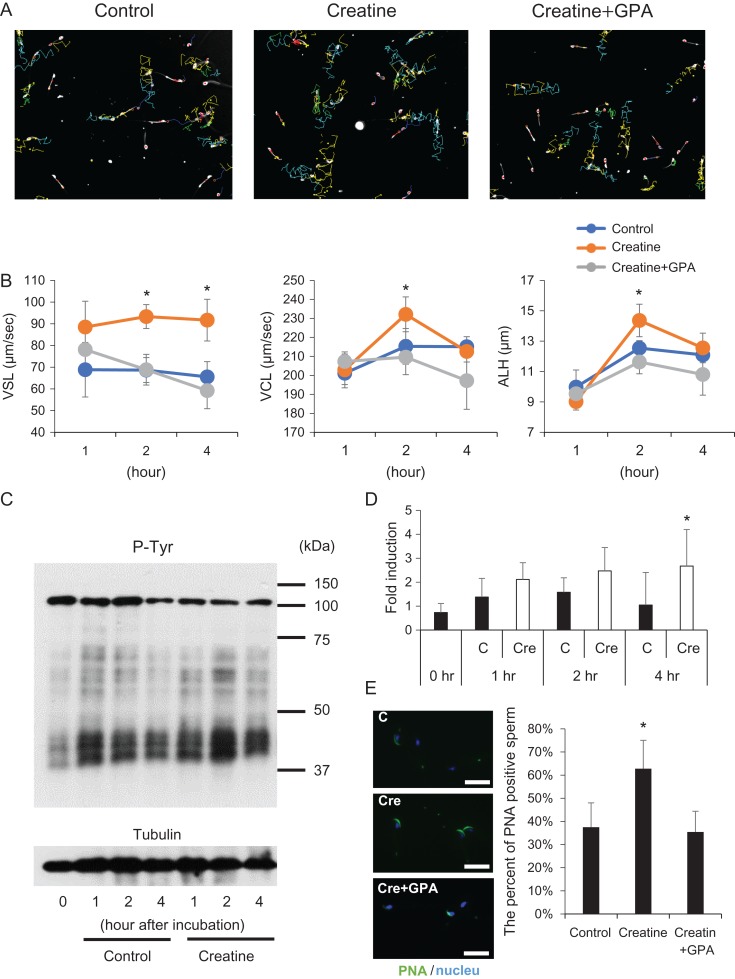
Creatine enhances sperm capacitation
CASA-generated sperm tracks showed different mean velocities and lateral head amplitude values for the different treatments. The track amplitude of sperm incubated with creatine was greater than that of sperm incubated without creatine or with creatine+GPA (Fig. 4A). Straight line velocity (VSL) of sperm was not changed from 1 to 4 h in control medium or medium with creatine+GPA, whereas the VSL of sperm incubated in creatine-containing media reached a maximum level at 1 h, which was maintained up to 4 h. Because the characteristics of hyperactivation are defined as high curvilinear velocity (VCL) and high lateral head movement (ALH) (Suarez, 2008), these were also measured. VCL of sperm incubated with 500 μM creatine was significantly higher than that of sperm incubated without creatine or with creatine+GPA at 2 h. Similar to the change in VCL, ALH of sperm incubated with 500 μM creatine was significantly higher than that of sperm incubated without creatine or with creatine+GPA at 2 h, indicating that creatine enhanced hyperactivation and increased the speed of hyperactivated sperm (Fig. 4B). To determine the effect of creatine on tyrosine phosphorylation, a marker of sperm capacitation, sperm lysates were assayed by western blot analyses using anti-phosphotyrosine antibodies. Positive signals were significantly stronger in creatine-containing IVF medium than in the control at 4 h (Fig. 4C, D). The higher level of tyrosine phosphorylation in the creatine treatment group was associated with the presence of more acrosome-intact (PNA-positive) sperm (Fig. 4E).
Figure 4.
Creatine enhances sperm motility and capacitation. (A) Tracks of sperm incubated with/without 500 μM creatine for 2 h determined using the CASA system. (B) Effect of creatine on straight line velocity (VSL), curvilinear velocity (VCL), and lateral amplitude (ALH). Sperm were cultured in 500 μl HTF medium with/without 500 μM creatine. Some sperm were incubated with 500 μM creatine and 500 μM GPA, and then analyzed by CASA technology. The experiment was repeated four times using a total of four male mice (more than 200 individual trajectories in each treatment group were analyzed per mouse). Values represent the mean ± SD. *Denotes significant differences compared with the control. (C) Induction of protein tyrosine phosphorylation in sperm by creatine. Sperm collected from the epididymis were incubated with 500 μM creatine for 1, 2 or 4 h. Tyrosine phosphorylation (P-Tyr) was detected by an anti-phosphotyrosine antibody. Tubulin was used as a loading control. Results are representative of three independent experiments. (D) Intensity of tyrosine phosphorylation induced by creatine. The intensity of all bands was analyzed using a Gel-Pro Analyzer (Media Cybernetics, MD, USA). Values are the mean ± SD of three replicates. *P < 0.05 compared with the control at same time point. C: Sperm were cultured without creatine. Cre: Sperm treated with 500 μM creatine. (E) Percentage of acrosome-intact sperm after incubation with creatine. Sperm collected from the epididymis were incubated with 500 μM creatine for 4 h. Sperm were air dried and then incubated with peanut agglutinin lectin (PNA)-FITC in PBS, a known marker of intact sperm acrosomes. Values are the mean ± SD of three replicates. *P < 0.05 compared with the control. Scale bar indicates 20 μm.
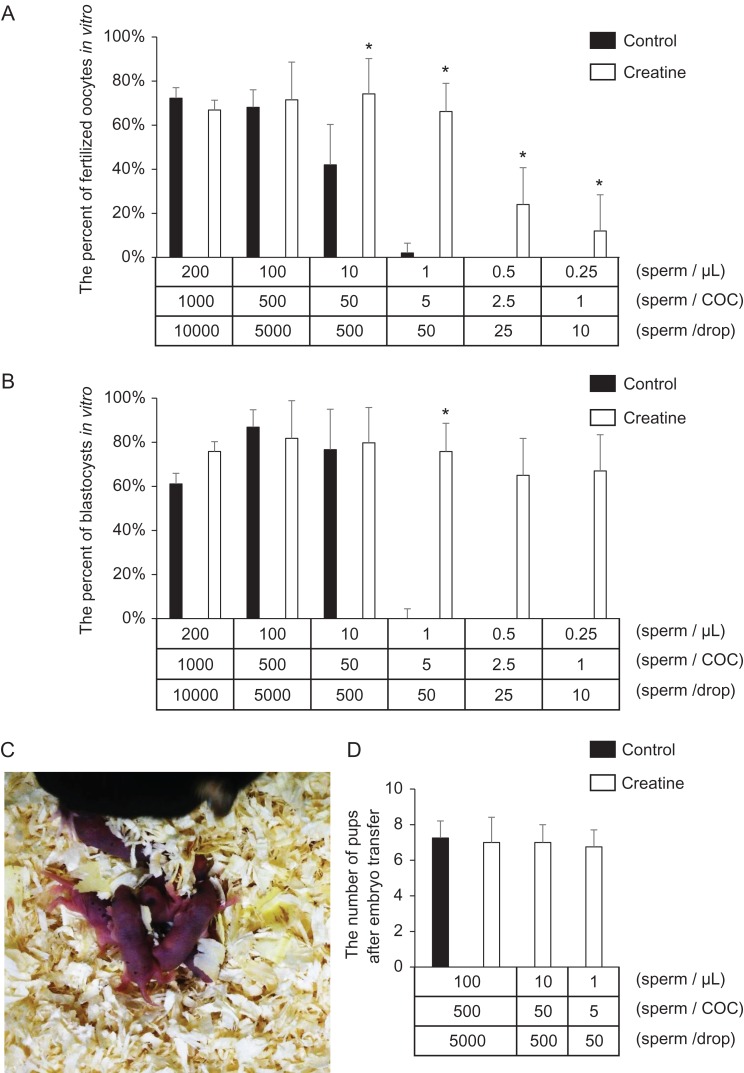
Creatine reduces the number of sperm required for successful fertilization in vitro
Sperm were incubated in IVF control medium or medium containing creatine. From 10 to 10,000 sperm were then added to a 50 μl drop containing 10 ovulated COCs. When more than 500 sperm/oocyte were used for IVF, no significant difference was observed between control and creatine treatment groups. The addition of GPA to creatine-containing medium did not show any effects on fertilization when 500 sperm/oocyte were used for insemination. However, the number of fertilized oocytes in the presence of less than 50 sperm/oocyte was significantly lower in the control group without creatine. Additionally, in the absence of creatine, no fertilized oocytes were observed when there were fewer than 2.5 sperm/oocyte (Fig. 5A; Table I). However, addition of creatine to pre-incubation and IVF drop medium increased fertilization. Both 5 and 50 sperm/oocyte induced successful fertilization, and the number of oocytes fertilized was similar to that when more than 500 sperm/oocyte were added. Although the number of fertilized oocytes was lower when there were fewer than 2.5 sperm/oocyte, fertilized oocytes were still observed when just one sperm/oocyte was used for IVF in the creatine treatment group (Fig. 5A). However, the highly efficient fertilization supported by creatine was significantly suppressed by addition of 500 μM GPA (Table II). Oocytes fertilized in the presence of five sperm in creatine-containing IVF medium developed normally to the blastocyst stage and were born as viable pups after transplantation into recipient pseudopregnant mice (Fig. 5B, C; Table III).
Figure 5.
Creatine permits reduction of the number of sperm required for IVF. (A, B) Fertilization rate (A) and development rate to the blastocyst stage (B) using creatine-containing IVF medium. Ten ovulated COCs were placed in 50 μl HTF medium. Sperm were collected from the epididymis into 500 μl medium with/without creatine. After 60 min of incubation, the sperm were transferred to IVF medium at final numbers of 1, 2.5, 5, 50, 500 or 1000 sperm/COC. At 6 h after insemination, some oocytes were examined for the number of pronuclei. Other oocytes were cultured further in the developing medium to assess development to the blastocyst stage. Values are the mean ± SD of five replicates. *P < 0.05 compared with the control. (C, D) Delivered pups from embryo transfer of blastocyst embryos fertilized by the novel creatine-containing IVF method using only five sperm per oocyte (C), and the average number of pups obtained by embryo transfer (D). Thirty blastocysts at 3.5 days after insemination were surgically transferred into the uterine horns of 2.5 day pseudopregnant females. The number of pups was then recorded. Values are the mean ± SD of three replicates.
Table I.
The fertilization and development rates after IVF using different number of sperm capacitated in mHTF medium contained creatine or not.
| Sperm no. | ||||||||
|---|---|---|---|---|---|---|---|---|
| /μl | /COC | /drop | Total oocyte | 2-cell | (%) | Blastocysts | (%) | |
| 200 | 1000 | 10,000 | Control | 100 | 72 | (72%) | 44 | (61%) |
| Creatine | 94 | 63 | (67%) | 48 | (76%) | |||
| 100 | 500 | 5000 | Control | 83 | 57 | (69%) | 49 | (86%) |
| Creatine | 80 | 58 | (73%) | 48 | (83%) | |||
| 10 | 50 | 500 | Control | 100 | 42 | (42%) | 32 | (76%) |
| Creatine | 115 | 92 | (80%) | 74 | (80%) | |||
| 1 | 5 | 50 | Control | 92 | 4 | (4%) | 1 | (25%) |
| Creatine | 112 | 60 | (54%) | 46 | (77%) | |||
| 0.5 | 2.5 | 25 | Control | 30 | 0 | (0%) | 0 | – |
| Creatine | 70 | 22 | (31%) | 10 | (45%) | |||
| 0.25 | 1 | 10 | Control | 30 | 0 | (0%) | 0 | – |
| Creatine | 70 | 8 | (11%) | 4 | (50%) | |||
Table II.
The fertilization and development rates after IVF using different number of sperm capacitated in mHTF medium contained creatine and creatine antagonist (GPA).
| Sperm no. | (μM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| /μl | /COC | /drop | Creatine | GPA | Total oocyte | 2-cell | (%) | Blastocysts | (%) | |
| 100 | 500 | 5000 | 500 | 0 | 32 | 22 | (69%) | 19 | (86%) | |
| 50 | 35 | 23 | (66%) | 14 | (61%) | |||||
| 500 | 36 | 24 | (67%) | 18 | (75%) | |||||
| 1 | 5 | 50 | 500 | 0 | 32 | 25 | (78%) | 19 | (76%) | |
| 50 | 33 | 13 | (39%) | 9 | (69%) | |||||
| 500 | 33 | 4 | (12%) | 3 | (75%) | |||||
Table III.
The number of embryo recovery after embryo transfer of blastocysts fertilized by conventional IVF using a few number of sperm.
| Sperm no. | ||||||
|---|---|---|---|---|---|---|
| /μl | /COC | /drop | Implanted embryos | Delivered pups | (%) | |
| 100 | 500 | 5000 | Control | 113 | 29 | (26%) |
| Creatine | 120 | 28 | (23%) | |||
| 10 | 50 | 500 | Creatine | 132 | 32 | (24%) |
| 1 | 5 | 50 | Creatine | 110 | 31 | (28%) |
Discussion
Factors within reproductive tracts affect specific steps in fertilization. For example, cystic fibrosis transmembrane conductance regulator (CFTR) and natriuretic peptide up-regulate sperm capacitation and motility (Wang et al., 2003; Kong et al., 2017). Notably, disruption of the Cftr gene in the uterus inhibits sperm migration to the oviduct in vivo (Hodges et al., 2008), indicating that not only male but also female factors are essential for sperm functions and successful fertilization in vivo. In this study, we show that creatine produced by eCG-stimulated granulosa cells is released into oviducts, and uptake of creatine by sperm provides unique mechanisms to induce successful in vivo fertilization.
It is well known that the pattern of sperm flagellar motion is altered from symmetrical to asymmetrical during fertilization (Chang and Suarez, 2011). With the change in motion, the amplitude of the flagellar bend increases (Suarez, 1987). The change in the sperm flagellar beating pattern associated with fertilization is called hyperactivation and is essential for penetration of the zona pellucida (Stauss et al., 1995). Using computer-assisted sperm analysis systems, the characteristics of hyperactivation are defined as high VCL, high ALH and low VSL (Suarez, 2008). In this study, addition of creatine enhanced VCL, VSL and ALH. However, the ratio of VSL to VCL remained the same, indicating that there was no overall change in flagellar bend amplitude but rather higher activity in sperm treated with creatine. Other studies have also reported a positive correlation between the VSL of hyperactivated sperm and the fertilization rate of human sperm under IVF conditions (Hirano et al., 2001). Although it is very difficult to observe specific patterns of sperm motility in the oviduct during fertilization in detail, creatine is present during mouse in vivo fertilization and induces efficient IVF, probably because it enhances and maintains hyperactivated motility of mouse sperm. Thus, creatine is a novel female factor that plays an important role in increasing fertilization by enhancing the ability of sperm to maintain a hyperactivated status.
With the sustained increase of activity in hyperactivated mouse sperm, creatine increased and sustained high ATP levels in sperm. Increased ATP levels in sperm are associated with hyperactivation (Ho et al., 2002). When genes involved in ATP production, such as Gapds coding spermatogenic cell-specific glyceraldehyde 3-phosphate dehydrogenase and Ldhc coding lactate dehydrogenase C, are disrupted in testicular germ cells, the sperm are unable to acquire a hyperactivation status and the male mice are infertile (Miki et al., 2004; Odet et al., 2008). Our results showed that addition of creatine maintained ATP levels in sperm incubated under capacitating conditions, indicating that sperm capacitation might also be sustained and improved by up-regulation of intracellular ATP levels via the creatine pathway. Therefore, creatine appears to be a potent regulator of ATP metabolism in sperm during fertilization, and the positive effects of creatine may improve the success and efficiency of IVF techniques.
IVF has become a standard procedure to facilitate pregnancy for infertile couples (Mouzon et al., 2009) and analyze fertility defects in mutant mice (Hodges et al., 2008). In conventional clinical IVF procedures, human oocytes are inseminated using 50,000 or more motile sperm/oocyte (Bongso et al., 1988; Vitek et al., 2013). In the case of patients in whom the number of motile sperm is less than 8 × 105, intracytoplasmic sperm injection (ICSI) is usually selected as the preferred IVF approach (Hamberger et al., 1998). According to Irahara et al. (2017), about 40% of the total number of cycles in ART in Japan were performed by ICSI in 2014. Furthermore, in the World Collaborative Report on ART 2005, the number of cycles in which ICSI is used was more than the number of cycles performed in which conventional IVF was used in Europe and the United States (Zegers-Hochschild et al., 2014). However, the developmental rate of fertilized oocytes and the clinical pregnancy rate of ICSI are lower than those of IVF (Vanlanduyt et al., 2005; Abdalmageed et al., 2015), and the costs are higher because of the need for manual oocyte injections.
Many researchers have tried to reduce the number of sperm needed for conventional IVF to adapt this technique to infertile male patients with low numbers of motile sperm. However, under the in vitro condition, it is known that more than 600 sperm/oocyte are necessary to achieve a fertilization rate of more than 50% (Siddiquey and Cohen, 1982; Magargee et al., 2000), indicating that the conditions of IVF are quite different from those in vivo because some factors present in vivo are missing in IVF medium. In this study, we showed that addition of creatine to conventional IVF medium prolonged lifespan significantly and maintained more vigorous motility of sperm, thereby permitting a low number of sperm per oocyte (5 sperm/oocyte) to complete successful fertilization in more than 60% of oocytes. Of particular relevance, this approach is remarkably simple compared with other approaches. For example, an IVF method using 5–50 sperm/oocyte has been reported by Hasegawa et al. (2014) using microdroplets. In their method, 10 oocytes are placed into 1 μl HTF medium containing reduced glutathione, and insemination of sperm is performed using a capillary (100 μm diameter) in the immediate vicinity of the oocytes. In contrast, our novel IVF protocol using creatine is easily adaptable to conventional IVF procedures and does not require specific techniques to achieve a high rate of fertilization using a low number of sperm. Because creatine kinase is also expressed in human sperm, this technique should be easily adapted for use in conventional human IVF without requiring ICSI.
Supplementary Figure S1.
The expression of creatine kinase in sperm and oocyte. Because there are three types of brain-type creatine kinase (Ckb), muscle-type (Ckm) and mitochondria-type (Ckmit), the expression of creatine kinase was checked in sperm and oocytes. Oocytes were collected from oviduct at 16 hr after hCG stimulation. Sperm were collected from epididymis and incubated in HTF medium for 1 hr. Total RNA was recovered from oocyte, sperm, brain and muscle, and then used in the RT-PCR study. Brain and muscle were used as positive control.
Supplementary Figure S2.
The activities of creatine kinase in granulosa cells and cumulus cells. The samples were collected after hormonal treatment. Muscle was used as positive control.
In conclusion, creatine enhances fertilization in vivo. Likewise, in vitro, addition of creatine dramatically improves the duration of sperm viability and motility, reduces the number of sperm required for IVF procedures, and promotes blastocyst and normal embryo development leading to the birth of viable offspring. Thus, creatine is a novel factor that improves and sustains sperm capacitation. It is possible that addition of creatine will improve human IVF as a simple and inexpensive approach.
Supplementary Material
Acknowledgements
The authors are grateful to Dr S. Suarez, Emeritus Professor, Cornell University, for insightful comments and suggestions concerning the manuscript.
Authors’ roles
T.U. was responsible for experimental design, data analysis, and writing the manuscript. T.K. and M.G. collected samples and interpreted data. J.S.R. was responsible for planning and discussions, and reviewed the manuscript. M.S. supervised all aspects of this study and wrote the manuscript.
Funding
This work was supported in part by Japan Society for the Promotion of Science KAKENHI Grant numbers JP24688028, JP16H05017 (to M.S.), and JP15J05331 (to T.U.), the Japan Agency for Medical Research and Development (AMED) (16gk0110015h0001 to M.S.) and National Institutes of Health (NIH-HD-076980 to J.S.R.).
Conflict of interest
The authors have nothing to disclose.
References
- Abdalmageed OS, Keyhan S, Acharya KS, Acharya CR, Hurd WW, Muasher SJ. Conventional in vitro fertilization (IVF) versus intracytoplasmic sperm injection (ICSI) for couples with polycystic ovary syndrome (PCOS) and normozoospermic semen: an analysis of 4679 cycles from the SART registry. Fertil Steril 2015;104:e300–e301. [Google Scholar]
- Baumber J, Ball BA, Gravance CG, Medina V, Davies-Morel MCG. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J Androl 2000;21:895–902. [PubMed] [Google Scholar]
- Bongso A, Chye NS, Ratnam S, Sathananthan H, Wong PC. Chromosome anomalies in human oocytes failing to fertilize after insemination in vitro. Hum Reprod 1988;3:645–649. [DOI] [PubMed] [Google Scholar]
- Brand KA, Hermfisse U. Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB J 1997;11:388–395. [DOI] [PubMed] [Google Scholar]
- Carr DW, Usselman MC, Acott TS. Effects of pH, lactate, and viscoelastic drag on sperm motility: a species comparison1. Biol Reprod 1985;33:588–595. [DOI] [PubMed] [Google Scholar]
- Chang H, Suarez SS. Two distinct Ca2+ signaling pathways modulate sperm flagellar beating patterns in mice. Biol Reprod 2011;85:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Toyoda Y. Cyclodextrin removes cholesterol from mouse sperm and induces capacitation in a protein-free medium. Biol Reprod 1998;59:1328–1333. [DOI] [PubMed] [Google Scholar]
- De Matos DG, Miller K, Scott R, Tran CA, Kagan D, Nataraja SG, Clark A, Palmer S. Leukemia inhibitory factor induces cumulus expansion in immature human and mouse oocytes and improves mouse two-cell rate and delivery rates when it is present during mouse in vitro oocyte maturation. Fertil Steril 2008;90:2367–2375. [DOI] [PubMed] [Google Scholar]
- Derave W, Bosch L Van Den, Lemmens G, Eijnde BO, Robberecht W, Hespel P. Skeletal muscle properties in a transgenic mouse model for amyotrophic lateral sclerosis: effects of creatine treatment. Neurobiol Dis 2003;13:264–272. [DOI] [PubMed] [Google Scholar]
- Fraser LR, Drury LM. The relationship between sperm concentration and fertilization in vitro of mouse eggs. Biol Reprod 1975;13:513–518. [DOI] [PubMed] [Google Scholar]
- Go KJ, Wolf DP. Albumin-mediated changes in sperm sterol content during capacitation 1. Biol Reprod 1985;32:145–153. [DOI] [PubMed] [Google Scholar]
- Hamberger L, Lundin K, Sjögren A, Söderlund B. Indications for intracytoplasmic sperm injection. Hum Reprod 1998;13:128–133. [DOI] [PubMed] [Google Scholar]
- Harris RC, Söderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci 1992;83:367–374. [DOI] [PubMed] [Google Scholar]
- Hasegawa A, Mochida K, Tomishima T, Inoue K, Ogura A. Microdroplet in vitro fertilization can reduce the number of spermatozoa necessary for fertilizing oocytes. J Reprod Dev 2014;60:187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y, Shibahara H, Obara H, Suzuki T, Takamizawa S, Yamaguchi C, Tsunoda H, Sato I. ANDROLOGY: relationships between sperm motility characteristics assessed by the computer-aided sperm analysis (CASA) and fertilization rates in vitro. J Assist Reprod Genet 2001;18:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H-C, Granish KA, Suarez SS. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev Biol 2002;250:208–217. [DOI] [PubMed] [Google Scholar]
- Hodges CA, Palmert MR, Drumm ML. Infertility in females with cystic fibrosis is multifactorial: evidence from mouse models. Endocrinology 2008;149:2790–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P-C, Hsu C-C, Guo YL. Hydrogen peroxide induces premature acrosome reaction in rat sperm and reduces their penetration of the zona pellucida. Toxicology 1999;139:93–101. [DOI] [PubMed] [Google Scholar]
- Hunter RH. Sperm transport and reservoirs in the pig oviduct in relation to the time of ovulation. J Reprod Fertil 1981;63:109–117. [DOI] [PubMed] [Google Scholar]
- Huszar G, Vigue L, Corrales M. Sperm creatine kinase activity in fertile and infertile oligospermic men. J Androl 1990;11:40–46. [PubMed] [Google Scholar]
- Irahara M, Kuwahara A, Iwasa T, Ishikawa T, Ishihara O, Kugu K, Sawa R, Banno K, Saito H. Assisted reproductive technology in Japan: a summary report of 1992–2014 by the Ethics Committee, Japan Society of Obstetrics and Gynecology. Reprod Med Biol 2017;16:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, Vetrivelan R, Lu GZ, Laznik-Bogoslavski D, Hasenfuss SC et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 2015;163:643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong N, Xu X, Zhang Y, Wang Y, Hao X, Zhao Y, Qiao J, Xia G, Zhang M. Natriuretic peptide type C induces sperm attraction for fertilization in mouse. Sci Rep 2017;7:39711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwayama M, Hamano S, Nagai T. Vitrification of bovine blastocysts obtained by in vitro culture of oocytes matured and fertilized in vitro. J Reprod Fertil 1992;96:187–193. [DOI] [PubMed] [Google Scholar]
- Lopes S, Jurisicova A, Sun JG, Casper RF. Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa. Hum Reprod 1998;13:896–900. [DOI] [PubMed] [Google Scholar]
- Magargee SF, Cramer PG, Hammerstedt RH. Increased in vitro binding and fertilizing ability of mouse sperm exposed to a synthetic peptide. Mol Reprod Dev 2000;57:406–411. [DOI] [PubMed] [Google Scholar]
- Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O’Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci USA 2004;101:16501–16506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouzon J, de, Lancaster P, Nygren KG, Sullivan E, Zegers-Hochschild F, Mansour R, Ishihara O, Adamson D. World Collaborative Report on assisted reproductive technology, 2002. Hum Reprod 2009;24:2310–2320. [DOI] [PubMed] [Google Scholar]
- Muro Y, Hasuwa H, Isotani A, Miyata H, Yamagata K, Ikawa M, Yanagimachi R, Okabe M. Behavior of mouse spermatozoa in the female reproductive tract from soon after mating to the beginning of fertilization1. Biol Reprod 2016;94:157–163. [DOI] [PubMed] [Google Scholar]
- Murray SC, Smith TT. Sperm interaction with fallopian tube apical membrane enhances sperm motility and delays capacitation. Fertil Steril 1997;68:351–357. [DOI] [PubMed] [Google Scholar]
- Nedambale TL, Du F, Xu J, Chaubal SA, Dinnyes A, Groen W, Faber D, Dobrinsky JR, Yang X, Tian XC. Prolonging bovine sperm–oocyte incubation in modified medium 199 improves embryo development rate and the viability of vitrified blastocysts. Theriogenology 2006;66:1951–1960. [DOI] [PubMed] [Google Scholar]
- Odet F, Duan C, Willis WD, Goulding EH, Kung A, Eddy EM, Goldberg E. Expression of the gene for mouse lactate dehydrogenase C (Ldhc) is required for male fertility. Biol Reprod 2008;79:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet JW, Cooper GW. Reduced sperm motility in the isthmus of the rabbit oviduct. Nature 1975;258:718–719. [DOI] [PubMed] [Google Scholar]
- Quinn P, Kerin JF, Warnes GM. Improved pregnancy rate in human in vitro fertilization with the use of a medium based on the composition of human tubal fluid. Fertil Steril 1985;44:493–498. [DOI] [PubMed] [Google Scholar]
- Quinn P, Moinipanah R, Steinberg JM, Weathersbee PS. Successful human in vitro fertilization using a modified human tubal fluid medium lacking glucose and phosphate ions. Fertil Steril 1995;63:922–924. [DOI] [PubMed] [Google Scholar]
- Shimada M, Nishibori M, Isobe N, Kawano N, Terada T. Luteinizing hormone receptor formation in cumulus cells surrounding porcine oocytes during meiotic maturation of porcine oocytes. Biol Reprod 2003;68:1142–1149. [DOI] [PubMed] [Google Scholar]
- Shimada M, Yanai Y, Okazaki T, Noma N, Kawashima I, Mori T, Richards JS. Hyaluronan fragments generated by sperm-secreted hyaluronidase stimulate cytokine/chemokine production via the TLR2 and TLR4 pathway in cumulus cells of ovulated COCs, which may enhance fertilization. Development 2008;135:2001–2011. [DOI] [PubMed] [Google Scholar]
- Siddiquey AK, Cohen J. In-vitro fertilization in the mouse and the relevance of different sperm/egg concentrations and volumes. J Reprod Fertil 1982;66:237–242. [DOI] [PubMed] [Google Scholar]
- Stauss CR, Votta TJ, Suarez SS. Sperm motility hyperactivation facilitates penetration of the hamster zona pellucida. Biol Reprod 1995;53:1280–1285. [DOI] [PubMed] [Google Scholar]
- Suarez SS. Sperm transport and motility in the mouse oviduct: observations in situ. Biol Reprod 1987;36:203–210. [DOI] [PubMed] [Google Scholar]
- Suarez SS. Control of hyperactivation in sperm. Hum Reprod 2008;14:647–657. [DOI] [PubMed] [Google Scholar]
- Vanlanduyt L, Devos A, Joris H, Verheyen G, Devroey P, Vansteirteghem A. Blastocyst formation in in vitro fertilization versus intracytoplasmic sperm injection cycles: Influence of the fertilization procedure. Fertil Steril 2005;83:1397–1403. [DOI] [PubMed] [Google Scholar]
- Visconti PE. Understanding the molecular basis of sperm capacitation through kinase design. Proc Natl Acad Sci 2009;106:667–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek WS, Galárraga O, Klatsky PC, Robins JC, Carson SA, Blazar AS. Management of the first in vitro fertilization cycle for unexplained infertility: a cost-effectiveness analysis of split in vitro fertilization-intracytoplasmic sperm injection. Fertil Steril 2013;100:1381–1388.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T, Hemmer W. Creatine kinase in non-muscle tissues and cells. Mol Cell Biochem 1994;133–134:193–220. [DOI] [PubMed] [Google Scholar]
- Wang XF, Zhou CX, Shi QX, Yuan YY, Yu MK, Ajonuma LC, Ho LS, Lo PS, Tsang LL, Liu Y et al. Involvement of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Nat Cell Biol 2003;5:902–906. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Byrd W, Dandekar P, Quigley MM. Sperm concentration and the fertilization of human eggs in vitro. Biol Reprod 1984;31:837–848. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R, Chang MC. Fertilization of Hamster eggs in vitro. Nature 1963;200:281–282. [DOI] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Mansour R, Ishihara O, Adamson GD, Mouzon J, de, Nygren KG, Sullivan EA. International Committee for Monitoring Assisted Reproductive Technology: world report on assisted reproductive technology, 2005. Fertil Steril 2014;101:366–378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.