Supplemental Digital Content is available in the text.
Keywords: apixaban, atrial fibrillation, cost-effectiveness analysis, dabigatran, left atrial appendage closure, warfarin
Abstract
Background and Purpose—
Once a patient with atrial fibrillation experiences an embolic event, the risk of a recurrent event increases 2.6-fold. New treatments have emerged as viable treatment alternatives to warfarin for stroke risk reduction in secondary prevention populations. This analysis sought to assess the cost-effectiveness of left atrial appendage closure (LAAC) compared with warfarin and the non–vitamin K antagonist oral anticoagulants dabigatran 150 mg, apixaban and rivaroxaban in the prevention of stroke in nonvalvular atrial fibrillation patients with a prior stroke or transient ischemic attack.
Methods—
A Markov model was constructed using data from the secondary prevention subgroup analyses of the non–vitamin K antagonist oral anticoagulant and LAAC pivotal trials. Costs were from 2016 US Medicare reimbursement rates and the literature. The cost-effectiveness analysis was conducted from a US Medicare perspective over a lifetime (20 years) horizon. The model was populated with a cohort of 10 000 patients aged 70 years with a CHA2DS2-VASc score of 7 (annual stroke risk=9.60%) and HAS-BLED score of 3 (annual bleeding risk=3.74%).
Results—
LAAC achieved cost-effectiveness relative to dabigatran at year 5 and warfarin and apixaban at year 6. At 10 years, LAAC had more quality-adjusted life years (4.986 versus 4.769, 4.869, 4.888, and 4.810) and lower costs ($42 616 versus $53 770, $58 774, $55 656, and $58 655) than warfarin, dabigatran, apixaban, and rivaroxaban, respectively, making LAAC the dominant (more effective and less costly) stroke risk reduction strategy. LAAC remained the dominant strategy over the lifetime analysis.
Conclusions—
Upfront procedure costs initially make LAAC higher cost than warfarin and the non–vitamin K antagonist oral anticoagulants, but within 10 years, LAAC delivers more quality-adjusted life years and has lower total costs, making LAAC the most cost-effective treatment strategy for secondary prevention of stroke in atrial fibrillation.
See related article, p 1315
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and an independent risk factor for stroke.1 Once an AF patient experiences an embolic event, the probability of a second event increases 2.6-fold.2 Traditionally, these patients have been managed with warfarin therapy which may reduce the risk of stroke in AF by two thirds, provided patients are within therapeutic range and adherent to therapy.3 While effective at reducing the risk of ischemic stroke and systemic embolism, warfarin therapy is associated with an increased risk of bleeding, lower quality of life (QoL), and high patient nonadherence.4
Non–vitamin K antagonist oral anticoagulant (NOAC) therapies and device-based left atrial appendage closure (LAAC) are alternatives for stroke prophylaxis in nonvalvular AF. Although long-term data are limited, the NOACs seem to be a safe and an effective alternative to warfarin therapy but are similar in that they are systemic solutions dependent on patient compliance.5–7 Autopsy/echocardiography studies indicate that over 90% of all thrombi in nonvalvular AF patients originate from the left atrial appendage.8,9 LAAC was developed to provide a localized solution independent of patient compliance. LAAC with the WATCHMAN device (Boston Scientific, Marlborough, MA) seals the ostium of the left atrial appendage, preventing the embolization of thrombi formed therein.10 Results from a subgroup analysis of the PROTECT AF trial (WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation) indicated that LAAC also reduces the risk of stroke in the subset of AF patients who had sustained a prior embolic event, with an efficacy similar to that of warfarin.11
Although recent publications explore the cost-effectiveness of LAAC and NOACs, no analysis has yet been undertaken to evaluate the cost-effectiveness of LAAC for the prevention of recurrent stroke in AF.12–15 The objective of this study is to assess the cost-effectiveness of LAAC with WATCHMAN relative to warfarin and the NOACs dabigatran 150 mg (hereafter referred to as dabigatran), rivaroxaban, and apixaban for the secondary prevention of stroke in nonvalvular AF.
Methods
Study Design
A Markov model was developed to assess the cost-effectiveness of LAAC compared with NOACs and warfarin in AF patients with a previous stroke or transient ischemic attack (TIA); NOAC versus warfarin analyses are presented in the online-only Data Supplement. The model was constructed using 3-month cycles and investigated cost-effectiveness at 10 and 20 years, with 20 years representing the lifetime analysis. Cost-effectiveness was reported as an incremental cost-effectiveness ratio (ICER) and evaluated using the conventional US willingness-to-pay threshold of $50 000 per quality-adjusted life year (QALY) gained.16 The analysis took a US Medicare perspective and incorporated costs for each treatment strategy and associated clinical sequelae, including ischemic stroke, TIA, systemic embolism, hemorrhagic stroke, major bleeding, and myocardial infarction.
Markov Model Structure
The model structure and patient pathways are depicted in Figure 1. The structure and assumptions used were adapted from a previously published cost-effectiveness analysis of OACs and LAAC.13 Model parameters are available for other researchers upon request to the corresponding author.
Figure 1.
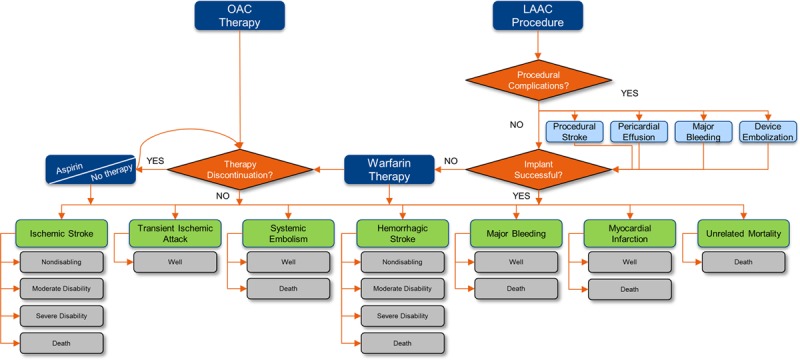
Model schematic. LAAC indicates left atrial appendage closure; and OAC, oral anticoagulant.
Our model included 5 treatment strategies: LAAC with WATCHMAN, adjusted-dose warfarin, dabigatran, apixaban, and rivaroxaban. Patients in the LAAC arm could experience a successful procedure, a successful procedure with complications, or a failed procedure. Procedural outcomes were taken from PROTECT AF. It was assumed that outcomes for the secondary prevention subgroup would not differ from the overall patient population, thereby allowing the inclusion of more events and greater precision in estimates from the total population treated. Procedural events included ischemic stroke (2.4%), major bleeding (2.0%), pericardial effusion (4.8%), and device embolization (1.2%).10 Successfully implanted patients remained in the LAAC treatment arm for life; 93% were assumed to discontinue warfarin after 12 months.11 Patients who were not successfully implanted initiated warfarin therapy.
In the drug arms, patients could discontinue primary therapy because of a bleeding event or nonclinical reasons. Patients who discontinued primary drug therapy were assumed to switch to aspirin. Discontinuation of second-line therapy was assumed to result in no treatment.
Five primary health states existed within the model: Well, Nondisabling, Moderate Disability, Severe Disability, and Death. Patients were assumed to be Well, or in normal good health, upon entering the model. Transitions between health states were driven by clinical events and associated with cost and QoL adjustments. Within each model cycle, patients could experience a clinical event that led to a worse health state or death. Only stroke could alter disability outcomes. All events except for TIA could lead to death. Patients also faced an ongoing risk of death from unrelated causes.
Clinical Events
The base case analysis assumed a 70-year-old patient with a history of stroke and a CHA2DS2-VASc score (congestive heart failure, hypertension, age ≥75 years [double weight], diabetes mellitus, stroke [double weight],vascular disease [coronary artery disease, peripheral artery disease, aortic atherosclerosis], age 65-74 years, and female sex) of 7 (annual stroke risk 9.60%) and a HAS-BLED score (hypertension, abnormal renal and liver function, stroke, bleeding, labile INR, elderly, drugs or alcohol) of 3 (annual bleeding risk 3.74%). The baseline CHA2DS2-VASc score was derived from the CHADS2 (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke [double weight]) patient profile in the secondary prevention subgroup analyses of the pivotal clinical trials.11,17–19 Clinical inputs appear in the online-only Data Supplement.
LAAC events were drawn primarily from a secondary prevention subgroup analysis of PROTECT AF at 5 years of follow-up (Boston Scientific, unpublished data, 2017). Event probabilities for dabigatran, rivaroxaban, and apixaban were taken from the secondary prevention subgroup analyses of their respective pivotal trials.17–19 Event probabilities for warfarin were based on results from the European Atrial Fibrillation Trial on secondary prevention.3,20,21 Event rates from this trial were indirectly compared against event rates from the warfarin arms of the secondary prevention subgroup analyses of PROTECT AF, RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy), ROCKET AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation), and ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation).17–19
The rates of myocardial infarction and bleeding events for LAAC were assumed to correlate to concurrent drug therapy, as LAAC itself has no impact on risk of bleeding or myocardial infarction. Risk of bleeding events for LAAC patients was equal to warfarin for the first 45 days, aspirin and clopidogrel for 45 days to 6 months, and aspirin for the remainder of the model, per the PROTECT AF protocol.10
Warfarin nonclinical discontinuation rates were based on an analysis by Helgason et al22 on the discontinuation of warfarin therapy after a stroke. NOAC discontinuation rates were taken from the overall trial populations, as discontinuation rates specific to the secondary prevention population have not been published.5–7
The probability of ischemic stroke, TIA, hemorrhagic stroke, and death were increased per decade of life to account for the impact of advancing age.23,24 Probability of nonevent death was calculated from US life tables.25
Health State Utilities and Stroke Outcomes
QoL was captured in the model as health utility, which reflects decrements to life quality based on health states. Health utility values are assessed on a scale of 0 to 1, with 1 representing perfect health and 0 representing death. An underlying baseline utility of 0.77 was used to reflect QoL of patients at age 70 with AF and a prior nondisabling stroke.26 This baseline utility was applied as a multiplying factor to utility values associated with each therapy and decremented by 2% per decade to account for a general decline in QoL with advancing age.27 Utility values were assumed to be equal for dabigatran, rivaroxaban, and apixaban. Utility values for Well with warfarin (0.987), Well with NOAC (0.994), and Well with aspirin (0.998) were consistent with values used in other analyses.12–15,26 The utility value for Well with LAAC (0.999) was derived by applying the Nichol ordinary least squares algorithm to Short-Form 12 Health Survey data collected in PROTECT AF.28,29 A one-time disutility of −0.0315 was applied in the first cycle to reflect a temporary reduction in QoL because of the LAAC procedure.30
QoL was also assessed by stroke outcomes, as stroke is the most debilitating and feared consequence of AF.31 The modified Rankin Scale (mRS) was used to characterize stroke outcomes as nondisabling (mRS score of 0–2), moderately disabling (mRS score of 3), severely disabling (mRS score of 4–5), and fatal (mRS score of 6). Stroke outcomes for LAAC were derived from the full PROTECT AF analysis, as the small number of events in the secondary prevention subgroup analysis alone was not sufficient to draw statistically meaningful conclusions (Boston Scientific, unpublished data, 2017). Warfarin and NOAC stroke outcomes were derived from secondary prevention and overall stroke prevention patient population analyses. Stroke outcomes and utility values are reported in the online-only Data Supplement.
Disutilities, a one-time decrement to QoL experienced for 1 model cycle, were applied to account for acute events. Utility decrements were applied for ischemic stroke (−0.139), hemorrhagic stroke (−0.181), major bleeding (−0.181), TIA (−0.103), systemic embolism (−0.120), and myocardial infarction (−0.125).31 QALYs were calculated by multiplying the length of time in a given state by the utility for that state. Future QALYs were discounted at an annual rate of 3.0.
Costs
The economic analysis considered all direct medical costs for the therapies and treatment of associated acute events, as well as costs for long-term disability care (cost inputs are reported in the online-only Data Supplement). Societal costs such as lost productivity were not considered. Acute event costs were taken from US 2016 diagnosis-related group national average values.32 Costs for poststroke inpatient rehabilitation were from 2016 case-mix group reimbursement rates.33 Long-term stroke disability costs were from published literature.34–36
LAAC procedure costs were calculated as a weighted average of the 2 diagnosis-related groups for percutaneous intracardiac procedures (273 and 274) plus the cost of 2 follow-up transesophageal echocardiograms.32,37 Failed procedures were assumed to incur the full procedure cost. LAAC patients also incurred costs for 6 months of drug therapy after the procedure. The annual cost of warfarin therapy was applied to LAAC patients unable to discontinue warfarin at 12 months. Additional costs related to procedural adverse events, including pericardial effusion and bleeding, were added to the baseline cost of the procedure. NOAC costs were calculated as an average of US pharmaceutical wholesale acquisition costs.38 Warfarin therapy costs were from US wholesale acquisition costs plus reimbursement rates for Current Procedural Terminology codes related to international normalized ratio monitoring.37,38 All costs are given in 2016 US dollars and were rounded to the nearest whole dollar. Future costs were discounted at an annual rate of 3%.
Sensitivity Analyses
Uncertainty in model parameters was assessed using one-way and probabilistic sensitivity analyses (PSA). One-way sensitivity analysis is used to determine which model inputs have the greatest impact on model results, and PSA is used to estimate the effect of variation of individual parameters on uncertainty of model results.39 The PSA was based on a Monte Carlo approach with 5000 iterations of the model over the lifetime horizon. The analyses included all event probabilities, costs, and health state utilities for LAAC, NOACs, and warfarin. Inputs were varied within 95% confidence intervals, where available, and by ±20% where confidence intervals were not published. Ranges and distributions for the clinical inputs appear in the online-only Data Supplement. Stroke outcomes assumed a Dirichlet distribution, and health state utilities assumed a beta distribution. All cost parameters were varied by ±20% and assumed to follow a gamma distribution.
Results
Cumulative cost curves for each therapy are in Figure 2; total costs, QALYs, and ICERs at 10 and 20 years are presented in the Table. Results for the NOAC versus warfarin analyses are presented in the online-only Data Supplement. As would be expected, LAAC was the more costly therapy in the early years immediately after implantation (Figure 2). Thereafter, costs for LAAC patients grew at a slower rate than those for warfarin and NOAC patients, with LAAC becoming less costly than OACs between years 5 and 7. LAAC also generated more QALYs than the other therapies. At 10 years, LAAC provided 4.986 QALYs, on average, compared with 4.769, 4.869, 4.888, and 4.810 for warfarin, dabigatran, apixaban, and rivaroxaban, respectively. This trend continued over the 20-year time horizon. At 10 and 20 years, rivaroxaban was the most costly therapy ($58 655 and $88 644) and had the fewest QALYs (4.810 and 5.695), making it a dominated strategy. As a result, it was excluded from the incremental cost-effectiveness analysis and sensitivity analysis.
Figure 2.
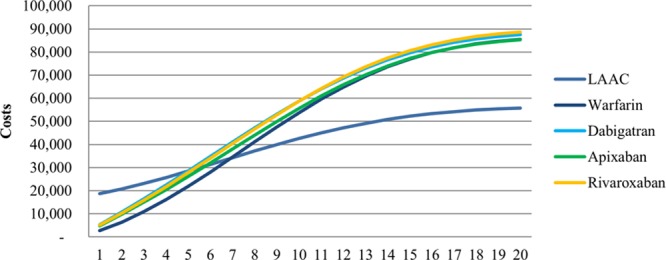
Cumulative costs by therapy over 20 years. LAAC indicates left atrial appendage closure.
Table.
Total Costs and QALYs Over 10 and 20 Years for LAAC, Warfarin, Dabigatran, and Apixaban
LAAC achieved cost-effectiveness relative to dabigatran at year 5 and warfarin and apixaban at year 6. At year 10, LAAC was dominant (more effective and less costly) relative to warfarin, dabigatran, and apixaban. LAAC remained the dominant treatment strategy for the remainder of the 20-year time horizon. The sustained cost-effectiveness performance of LAAC over time was driven by the higher initial costs of the procedure being offset year on year by the QoL decrements and long-term care costs resulting from the moderately and severely disabling strokes. LAAC achieved cost-effectiveness relative to NOACs earlier than warfarin because of the higher annual therapy costs of NOACs.
One-Way Sensitivity Analysis
The cost-effectiveness of LAAC relative to OACs was predictably responsive to variations in individual model parameters. Tornado diagrams depicting the 10 most impactful variables to model results in descending order of influence at 20 years are depicted in the online-only Data Supplement. When comparing LAAC versus warfarin, results were most sensitive to variations in the rate of nondisabling LAAC ischemic stroke, LAAC relative risk of ischemic stroke compared with warfarin, warfarin relative risk of ischemic stroke compared with no therapy, and the health state utility for nondisabling stroke. In all instances, LAAC remained cost saving relative to warfarin. LAAC results relative to dabigatran and apixaban were sensitive to variations in the relative risk of ischemic stroke compared with warfarin, LAAC implant success rate, and the percent of LAAC ischemic strokes that were nondisabling. But in all instances of varying the different inputs, LAAC remained cost saving relative to the NOACs.
Probabilistic Sensitivity Analyses
PSA simulations at 20 years (Figure 3) demonstrated that LAAC had lower average total costs than warfarin, dabigatran, and apixaban. Relative to warfarin, there was a 99.7% probability that LAAC provided more QALYs, 100.0% probability that LAAC was cost saving, and 99.7% overall probability of cost-effectiveness using a willingness-to-pay threshold of $50 000/QALY. At 20 years, there was a 90.4% probability that LAAC was cost-effective relative to dabigatran and 95.0% probability that LAAC was cost-effective relative to apixaban.
Figure 3.
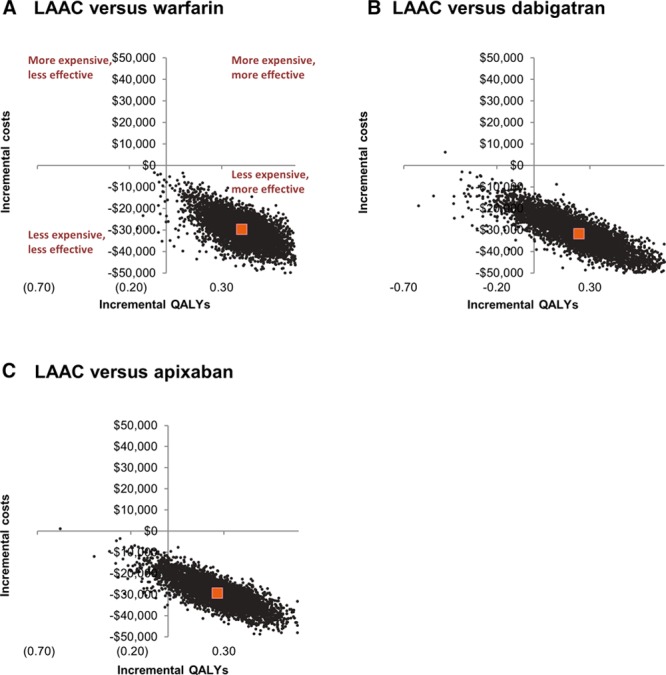
Cost-effectiveness plane at 20 years of left atrial appendage closure (LAAC) vs warfarin, LAAC vs dabigatran, and LAAC vs apixaban. QALY indicates quality-adjusted life year.
Discussion
These analyses indicate that LAAC is a cost-effective strategy for the secondary prevention of stroke in nonvalvular AF patients. LAAC was cost-effective relative to NOACs and warfarin by year 6. LAAC was dominant (more effective and less costly) than NOACs and warfarin by year 10. PSA indicated that these outcomes were robust: at 20 years, LAAC was cost-effective in 99.7% of simulations and cost saving in 100.0% relative to warfarin. Similarly, LAAC was cost-effective compared with dabigatran and apixaban in 90.4% and 95.0% of simulations, respectively. Rivaroxaban was the most costly therapy with the fewest QALYs and was therefore excluded from detailed ICER and sensitivity analyses.
To the best of our knowledge, this is the first analysis to explore the cost-effectiveness of LAAC relative to OACs in AF patients with a previous stroke or TIA. However, Kamel et al14,15 explored the cost-effectiveness of dabigatran and apixaban relative to warfarin in a secondary prevention population over a 20-year lifetime analysis. The dabigatran study found dabigatran to be more expensive than warfarin but also more effective, yielding a lifetime ICER of $25 000/QALY. Similarly, apixaban was found to be more expensive than warfarin but provided an incremental gain of 0.28 QALYs with a lifetime ICER of $11 400/QALY. The lifetime (20 years) findings from our current analysis corroborate these findings, with apixaban dominant to warfarin and dabigatran cost-effective relative to warfarin with an ICER of $11 555/QALY (detailed results appear in the online-only Data Supplement). Given the nature of economic modeling, variation in results is to be expected and, in this instance, is likely because of differences in stroke outcomes by treatment strategy. To explore this further, we substituted our stroke outcomes data with those used by Kamel et al14,15 across all OAC arms in our model. Compared with warfarin, results were consistent (LAAC provided more QALYs [0.535] with lower costs [−$47 720], apixaban provided more QALYs [0.178] with slightly lower costs [−$3253], and dabigatran provided more QALYs [0.139] with somewhat higher costs [$9501]; detailed results appear in the online-only Data Supplement). No published cost-effectiveness analyses of rivaroxaban relative to warfarin in AF patients with a previous stroke or TIA were identified. Taken together, although the acquisition costs for warfarin are low, a growing body of evidence suggests that the newer stroke risk reduction therapies are more cost-effective treatment strategies over the long run.
Drug adherence over a lifetime warrants further consideration. As a device-based solution, LAAC does not experience issues of adherence over the long term. However, adherence of LAAC patients to lifelong aspirin therapy has not been studied. Likewise, the importance of post-LAAC lifelong aspirin therapy is also unknown; an argument could be made the long-term aspirin is either (1) important to reduce noncardioembolic strokes or (2) detrimental by increasing major bleeding. This question can only be assessed by conducting prospective, comparative trials.
Conversely, a great deal is known about warfarin adherence over time. Patients are often permanently transitioned off warfarin therapy after a hemorrhagic stroke and may be either permanently or temporarily taken off OAC after other major bleeding. Helgason et al22 found that over 6 years, 132 of 229 patients (58%) initially prescribed warfarin therapy after a stroke had stopped taking their medication. The subgroup analyses of the NOACs do not provide details about therapy adherence in this population. However, data from the larger trials suggest that discontinuation rates are high. In the RE-LY trial, 21.2% of patients taking dabigatran discontinued therapy at 2 years.5 In the ROCKET AF trial, 23.7% of rivaroxaban patients discontinued therapy by the trial’s conclusion after an average of 590 days of treatment.6 Only the trial of apixaban found that fewer patients discontinued the novel drug than warfarin—and yet, the discontinuation rate was 25.3% over a median of 1.8 years.7 Given the obvious advantage of LAAC in terms of maintaining therapeutic benefit, sensitivity analyses explored the possibility that drug adherence was better than what was modeled in the base case. With the difficulties seen in the real world with maintaining therapy adherence, it is likely that adherence to a pharmaceutical regimen would be lower than what was modeled here, which may even further improve the cost-effectiveness of LAAC compared with pharmaceutical strategies.
In addition, a recent publication highlighted the importance, and difficulty, of assessing patient risk for intracranial hemorrhage before prescribing a lifelong OAC therapeutic regimen, and further suggested that patient subgroups at high risk for hemorrhage may have even more pronounced clinical and cost benefits when treated with LAAC.40 Further research is needed to test this hypothesis and better understand how patient characteristics and risk profiles impact the cost-effectiveness of LAAC. Assessing individual patient characteristics coupled with shared decision-making between treating clinicians and patients is of paramount importance in determining the most appropriate candidates for LAAC.
Limitations
No single randomized controlled trial has evaluated all comparators against one another. Therefore, we used an indirect comparison to estimate the relative effects of each treatment, using warfarin as the common comparator. Furthermore, the body of literature on the secondary prevention of stroke in patients with AF is rather limited. Only 2, relatively small (n=2253 and n=454,41 respectively), trials of warfarin have specifically looked at the secondary prevention population. The focus of these trials was on ischemic events, and therefore, results did not provide a great deal of data on the risk of bleeding in the secondary prevention population, although 1 study found the risk of bleeding increased in line with stroke risk.42
Similarly, the efficacy of LAAC in secondary prevention was taken from a subgroup analysis of PROTECT AF, which included 131 patients with a previous stroke or TIA. Event rates seen in this patient sample, which was followed for 4 to 5 years, have been extrapolated beyond the follow-up period out to 20 years.
In the past 3 years, secondary prevention subgroup analyses of the AF trials for all 3 NOACs have been published. These trials involved larger patient samples (dabigatran: 3623, apixaban: 3436, and rivaroxaban: 7468), providing a more conclusive picture of the clinical efficacy of these new drugs compared with warfarin and LAAC; however, the mean follow-up of these studies is only ≈2 years, and for the purposes of our cost analysis, the data have also been extrapolated out to 20 years. Event rates for OACs are also extrapolated beyond the follow-up period of the clinical trials.
Stroke severity data were taken from the larger PROTECT AF cohort, as the secondary prevention sample size was not large enough to draw any meaningful conclusions about outcomes. These results were extremely favorable to LAAC, with 75% of strokes resulting in functional independence—largely because post-LAAC strokes tend not to be hemorrhagic strokes, which are known to result in more severe disability. Of course, one cannot rule out the possibility that stroke outcomes would be different if using a cohort of only secondary prevention LAAC patients.
Cognitive impairment was not measured in the LAAC or NOAC clinical trials. Accordingly, the impact of cognitive impairment and dementia, which can have a profound impact on patient therapy adherence, clinical outcomes, and QoL, on cost-effectiveness of stroke risk reduction strategies could not be assessed.43 However, we incorporated stroke severity, as based on the poststroke mRS scores, into our analysis.
Finally, clinical probabilities for all treatment arms were taken from multiple clinical studies with different time horizons extrapolated out to 20 years. Model results may not be representative of real-world clinical practice and are specific to that drug or device. It should not be assumed that these data can be extrapolated to other LAAC devices or OACs. In addition, the model reflects the US healthcare system and costs, and the ICERs may not be easily generalizable to other healthcare systems.
Conclusions
While initially more costly than warfarin and the NOACs, LAAC with the WATCHMAN proved to be both a cost-effective and cost-saving treatment strategy. Percutaneous LAAC represents a major change in the management of patients with prior stroke or TIA. It has the inherent benefit of maintaining stroke protection over time without the issue of patient compliance, an important clinical consideration in patients at the highest risk of stroke. These findings should be taken into consideration when formulating policy and practice guidelines for secondary stroke prevention in nonvalvular AF.
Acknowledgments
This study was conceptualized and designed by Dr Reddy, R.L. Akehurst, S.L. Amorosi, M.B. Gavaghan, D.S. Hertz, and D.R. Holmes Jr. Drafting/revising of the article content was done by Dr Reddy, R.L. Akehurst, S.L. Amorosi, M.B. Gavaghan, D.S. Hertz, and D.R. Holmes Jr. The data were analyzed/interpreted by Dr Reddy, R.L. Akehurst, S.L. Amorosi, and M.B. Gavaghan. Principal investigator of this article was Dr Reddy.
Sources of Funding
This work was supported by funding from Boston Scientific which manufactures the WATCHMAN Device.
Disclosures
Dr Reddy, R.L. Akehurst, M.B. Gavaghan, and D.S. Hertz are paid consultants to Boston Scientific. S.L. Amorosi is a full-time employee of Boston Scientific. The other author reports no conflicts.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.117.018825/-/DC1.
References
- 1.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 2.Stroke Prevention in Atrial Fibrillation Investigators. Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet. 1996;348:633–638. doi: 10.1016/S0140-6736(96)03487-3. [PubMed] [Google Scholar]
- 3.European Atrial Fibrillation Trial (EAFT) Study Group. Secondary Prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet. 1993;342:1255–1262. doi: 10.1016/0140-6736(93)92358-Z. [PubMed] [Google Scholar]
- 4.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857e67. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 5.Connelly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 6.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 7.Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 8.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 9.Stoddard MF, Dawkins PR, Price CR, Ammash NM. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: a transesophageal echocardiographic study. J Am Coll Cardiol. 1995;25:452–459. doi: 10.1016/0735-1097(94)00396-8. [DOI] [PubMed] [Google Scholar]
- 10.Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomized non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 11.Reddy VY, Doshi SK, Siever H, Buchbinder M, Neuzil P, Huber K, et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3 year follow-up of the PROTECT AF trial. Circulation. 2013;127:720–729. doi: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 12.Harrington AR, Armstrong EP, Nolan PE, Jr, Malone DC. Cost-effectiveness of apixaban, dabigatran, rivaroxaban and warfarin for stroke prevention in atrial fibrillation. Stroke. 2013;44:1676–1681. doi: 10.1161/STROKEAHA.111.000402. [DOI] [PubMed] [Google Scholar]
- 13.Reddy VY, Akehurst RL, Armstrong SO, Amorosi SL, Beard SM, Holmes DR. Time to cost-effectiveness following stroke reduction strategies in AF: warfarin versus NOACs versus LAA closure. J Am Coll Cardiol. 2015;66:2728–2739. doi: 10.1016/j.jacc.2015.09.084. [DOI] [PubMed] [Google Scholar]
- 14.Kamel H, Johnston C, Easton JD, Kim AS. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in patients with atrial fibrillation and prior stroke or transient ischemic attack. Stroke. 2012;43:881–883. doi: 10.1161/STROKEAHA.111.641027. [DOI] [PubMed] [Google Scholar]
- 15.Kamel H, Easton JD, Johnston SC, Kim AS. Cost-effectiveness of apixaban vs warfarin for secondary stroke prevention in atrial fibrillation. Neurology. 2012;79:1428–1434. doi: 10.1212/WNL.0b013e31826d5fe8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8:165–178. doi: 10.1586/14737167.8.2.165. [DOI] [PubMed] [Google Scholar]
- 17.Diener HC, Connolly SJ, Ezekowitz MD, Wallentin L, Reilly PA, Yang S, et al. RE-LY Study Group. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol. 2010;9:1157–1163. doi: 10.1016/S1474-4422(10)70274-X. doi: 10.1016/S1474-4422(10)70274-X. [DOI] [PubMed] [Google Scholar]
- 18.Hankey GJ, Patel MR, Stevens SR, Becker RC, Breithardt G, Carolei A, et al. ROCKET AF Steering Committee Investigators. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol. 2012;11:315–322. doi: 10.1016/S1474-4422(12)70042-X. doi: 10.1016/S1474-4422(12)70042-X. [DOI] [PubMed] [Google Scholar]
- 19.Easton JD, Lopes RD, Bahit MC, Wojdyla DM, Granger CB, Wallentin L, et al. ARISTOTLE Committees and Investigators. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol. 2012;11:503–511. doi: 10.1016/S1474-4422(12)70092-3. doi: 10.1016/S1474-4422(12)70092-3. [DOI] [PubMed] [Google Scholar]
- 20.Mohr JP, Thompson JL, Lazar RM, Levin B, Sacco RL, Furie KL, et al. Warfarin-Aspirin Recurrent Stroke Study Group. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345:1444–1451. doi: 10.1056/NEJMoa011258. doi: 10.1056/NEJMoa011258. [DOI] [PubMed] [Google Scholar]
- 21.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 22.Helgason CM, Do MA, Nutescu E. Warfarin in patients with stroke and reasons for discontinuation. J Stroke Cerebrovasc Dis. 2004;13:70–73. doi: 10.1016/j.jstrokecerebrovasdis.2004.01.006. doi: 10.1016/j.jstrokecerebrovasdis.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Atrial Fibrillation Investigators. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457. doi: 10.1001/archinte.1994.00420130036007. [PubMed] [Google Scholar]
- 24.Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34:2060–2065. doi: 10.1161/01.STR.0000080678.09344.8D. doi: 10.1161/01.STR.0000080678.09344.8D. [DOI] [PubMed] [Google Scholar]
- 25.Office of the Chief Actuary, Social Security Administration. Actuarial Life Table, Period Life Table. https://www.ssa.gov/oact/STATS/table4c6.html. Accessed January 4, 2018.
- 26.Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med. 1996;156:1829–1836. [PubMed] [Google Scholar]
- 27.Fryback DG, Dunham NC, Palta M, Hanmer J, Buechner J, Cherepanov D, et al. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Med Care. 2007;45:1162–1170. doi: 10.1097/MLR.0b013e31814848f1. doi: 10.1097/MLR.0b013e31814848f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichol MB, Sengupta N, Globe DR. Evaluating quality-adjusted life years: estimation of the health utility index (HUI2) from the SF-36. Med Decis Making. 2001;21:105–112. doi: 10.1177/0272989X0102100203. doi: 10.1177/0272989X0102100203. [DOI] [PubMed] [Google Scholar]
- 29.Alli O, Doshi S, Kar S, Reddy V, Sievert H, Mullin C, et al. Quality of life assessment in the randomized PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) trial of patients at risk for stroke with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2013;61:1790–1798. doi: 10.1016/j.jacc.2013.01.061. doi: 10.1016/j.jacc.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 30.Cohen DJ, Taira DA, Berezin R, Cox DA, Morice MC, Stone GW, et al. Cost-effectiveness of coronary stenting in acute myocardial infarction: results from the stent primary angioplasty in myocardial infarction (stent-PAMI) trial. Circulation. 2001;104:3039–3045. doi: 10.1161/hc5001.100794. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan PW, Arant TW, Ellis SL, Ulrich H. The cost effectiveness of anticoagulation management services for patients with atrial fibrillation and at high risk of stroke in the US. Pharmacoeconomics. 2006;24:1021–1033. doi: 10.2165/00019053-200624100-00009. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Medicare & Medicaid Services. FY 2016 IPPS Final Rule Home Page. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2016-IPPS-Final-Rule-Home-Page.html. Accessed January 4, 2018.
- 33.Centers for Medicare & Medicaid Services. Inpatient Rehabilitation Facility PPS. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientRehabFacPPS/index.html. Accessed January 4, 2018.
- 34.Cipriano LE, Steinberg ML, Gazelle GS, González RG. Comparing and predicting the costs and outcomes of patients with major and minor stroke using the Boston Acute Stroke Imaging Scale neuroimaging classification system. AJNR Am J Neuroradiol. 2009;30:703–709. doi: 10.3174/ajnr.A1441. doi: 10.3174/ajnr.A1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mercaldi CJ, Siu K, Sander SD, Walker DR, Wu Y, Li Q, et al. Long-term costs of ischemic stroke and major bleeding events among Medicare patients with nonvalvular atrial fibrillation. Cardiol Res Pract. 2012;2012:645469. doi: 10.1155/2012/645469. doi: 10.1155/2012/645469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caro JJ, Huybrechts KF. Stroke treatment economic model (STEM): predicting long-term costs from functional status. Stroke. 1999;30:2574–2579. doi: 10.1161/01.str.30.12.2574. [DOI] [PubMed] [Google Scholar]
- 37.TCI SuperCoder, The Coding Institute. CPT® Code Lookup. https://www.supercoder.com/cpt-codes-range. Accessed January 4, 2018.
- 38.ANALYSOURCE – ACTIVE NDCS. DMD America. https://www.analysource.com/qry/as_products.taf?_purgefilter=Y. Accessed January 4, 2018.
- 39.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD ISPOR-SMDM Modeling Good Research Practices Task Force. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–6. Value Health. 2012;15:835–842. doi: 10.1016/j.jval.2012.04.014. doi: 10.1016/j.jval.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 40.Gurol ME. Nonpharmacological management of atrial fibrillation in patients at high intracranial hemorrhage risk. Stroke. 2018;49:247–254. doi: 10.1161/STROKEAHA.117.017081. doi: 10.1161/STROKEAHA.117.017081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morocutti C, Amabile G, Fattapposta F, Nicolosi A, Matteoli S, Trappolini M, et al. Indobufen versus warfarin in the secondary prevention of major vascular events in nonrheumatic atrial fibrillation. SIFA (Studio Italiano Fibrillazione Atriale) Investigators. Stroke. 1997;28:1015–1021. doi: 10.1161/01.str.28.5.1015. [DOI] [PubMed] [Google Scholar]
- 42.Gomes T, Mamdani MM, Holbrook AM, Paterson JM, Hellings C, Juurlink DN. Rates of hemorrhage during warfarin therapy for atrial fibrillation. CMAJ. 2013;185:E121–E127. doi: 10.1503/cmaj.121218. doi: 10.1503/cmaj.121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cacciatore F, Testa G, Langellotto A, Galizia G, Della-Morte D, Gargiulo G, et al. Role of ventricular rate response on dementia in cognitively impaired elderly subjects with atrial fibrillation: a 10-year study. Dement Geriatr Cogn Disord. 2012;34:143–148. doi: 10.1159/000342195. doi: 10.1159/000342195. [DOI] [PubMed] [Google Scholar]


