Abstract
Background
A method of quantifying clinical bleeding in dogs with immune thrombocytopenia (ITP) is needed because ITP patients have variable bleeding tendencies that inconsistently correlate with platelet count. A scoring system will facilitate patient comparisons and allow stratification based on bleeding severity in clinical trials.
Hypothesis/Objectives
To develop and evaluate a bleeding assessment tool for dogs, and a training course for improving its consistent implementation.
Animals
Client‐owned dogs (n = 61) with platelet counts <50,000/μL; 34 classified as primary ITP, 17 as secondary ITP, and 10 as non‐ITP.
Methods
A novel bleeding assessment tool, DOGiBAT, comprising bleeding grades from 0 (none) to 2 (severe) at 9 anatomic sites, was developed. Clinicians and technicians completed a training course and quiz before scoring thrombocytopenic patients. The training course was assessed by randomizing student volunteers to take the quiz with or without prior training. A logistic regression model assessed the association between training and quiz performance. The correlation of DOGiBAT score with platelet count and outcome measures was assessed in the thrombocytopenic dogs.
Results
Clinicians and technicians consistently applied the DOGiBAT, correctly scoring all quiz cases. The odds of trained students answering correctly were higher than those of untrained students (P < .0001). In clinical cases, DOGiBAT score and platelet count were inversely correlated (r s = −0.527, P < .0001), and DOGiBAT directly correlated with transfusion requirements (r s = 0.512, P < .0001) and hospitalization duration (r s = 0.35, P = .006).
Conclusions and Clinical Importance
The DOGiBAT and assessment quiz are simple tools to standardize evaluation of bleeding severity. With further validation, the DOGiBAT may provide a clinically relevant metric to characterize ITP severity and monitor response in treatment trials.
Keywords: bleeding scale, daily canine bleeding assessment tool, immune‐mediated thrombocytopenic purpura, platelets
Abbreviations
- DOGiBAT
daily canine bleeding assessment tool
- IBLS
ITP bleeding scale
- ITP
immune thrombocytopenia
- NI
nonimmune
- PI
primary immune
- SI
secondary immune
1. INTRODUCTION
Immune thrombocytopenia (ITP) is a common cause of severe thrombocytopenia in dogs.1 It occurs when antiplatelet antibodies bound to the platelet surface lead to clearance and premature platelet destruction by the reticuloendothelial system.2, 3 Although ITP causes thrombocytopenia, not all dogs with ITP develop signs of hemorrhage and platelet count does not consistently predict bleeding risk or mortality.4 Human ITP patients also have been shown to have heterogeneous bleeding phenotypes. One study in the human literature reported no association between platelet count and bleeding severity in patients with severe thrombocytopenia (platelet count < 30,000/μL).5
Immunosuppressive doses of glucocorticoids are the current first‐line treatment for dogs with ITP, but few evidence‐based guidelines exist to dictate the timing or choice of adjunctive, second‐line therapies.4, 6, 7 Several studies have used platelet recovery as the main indicator of response to treatment.6, 8, 9 However, treatment to attain a target platelet count has the potential to lead to over‐treatment (ie, higher drug dosages and addition of adjunctive immunosuppressants that may not provide additional benefit in preventing or controlling bleeding). Corticosteroids and combined immunosuppressive drug treatment carry risks including gastrointestinal ulceration,10 hepatotoxicity,11 secondary infections,12, 13, 14 and potential hypercoagulability.15 Because platelet count alone does not reliably predict clinically relevant bleeding in canine or human ITP patients,4, 5 additional clinical and laboratory metrics for objectively gauging bleeding severity are needed to minimize the risk of over‐treatment for an individual patient and to stratify patients for prospective clinical studies. A bleeding score metric would help to objectively assess efficacy of adjunctive therapies in multicenter clinical trials, stratify patients according to their bleeding severity, and identify laboratory variables that predict or correlate with bleeding severity.
Our primary objective was to develop a novel ITP bleeding assessment tool for dogs or “DOGiBAT” to allow more consistent and standardized quantification of bleeding severity in dogs with ITP. Other study objectives were to assess a training course designed to instruct users of the DOGiBAT on its correct implementation, and to apply the DOGiBAT in clinical cases of canine thrombocytopenia to assess the relationship between DOGiBAT and platelet count and as a preliminary evaluation of the relationship between DOGiBAT and patient outcome. Dogs with platelet counts < 50,000/μL of any underlying etiology were included in this pilot project. We hypothesized that the use of the DOGiBAT would allow for consistent bleeding severity scoring among clinicians, and that a training course would improve less clinically experienced users' (veterinary students') ability to correctly assign bleeding severity scores. We also hypothesized that in clinical cases of thrombocytopenia in dogs, DOGiBAT score and platelet count would be inversely correlated, but that this relationship would be lost in severely thrombocytopenic dogs (platelet counts < 30,000/μL). For these cases, no consistent association between platelet count and DOGiBAT score would exist, as described for people with ITP evaluated using a comparable scoring system,5 and based on the observed bleeding heterogeneity of severely thrombocytopenic ITP dogs.1, 3, 4
2. MATERIALS AND METHODS
2.1. Development of DOGiBAT, training course, and case‐based quiz
A novel daily bleeding assessment tool for dogs, the DOGiBAT, was developed for dogs with ITP (Table 1). This tool was modeled after a scoring system designed for human ITP patients, the ITP Bleeding Scale (IBLS).5 The DOGiBAT comprised 9 different anatomic sites (cutaneous, catheter or venipuncture bleeding, oral mucosa, intraocular, epistaxis, gastrointestinal, urinary, pulmonary, and intracranial). Each anatomic site was given a site‐specific bleeding grade of 0 (none), 1 (mild), or 2 (severe), with the exception of pulmonary and intracranial, which were given only grades of 0 (absent) or 2 (suspected/present).
Table 1.
Novel canine daily bleeding score assessment tool, DOGiBAT, developed for canine ITP
| Bleeding grade | |||
|---|---|---|---|
| Site | 0 | 1 | 2 |
| Skin | No | Petechiae/ecchymoses single site | Petechiae/ecchymoses >1 anatomic site |
| Catheter/venipuncture/other cutaneous bleed | No | Self‐limiting and <5 minutes | >5 minutes and/or intervention to control |
| Oral mucosa | No | Petechiae | Frank hemorrhage |
| Intraocular | No | Funduscopic | Hyphema |
| Epistaxis | No | Unilateral and <5 minutes | Bilateral or >5 minutes |
| Gastrointestinal | Occult blood (–); (Hema‐chek™, Siemans Healthcare Diagnostics Inc., Tarrytown, New York) | Occult blood (+); (Hema‐chek™, Siemans Healthcare Diagnostics Inc.) | Hematemesis, hematochezia, melena |
| Urinary | No | Microscopic (dipstick) | Macroscopic |
| Pulmonary hemorrhage (suspected/observed) | No | N/A | Yes |
| Intracranial hemorrhage (suspected/observed) | No | N/A | Yes |
Each anatomic site receives a grade of 0 (none), 1 (mild), or 2 (severe), as detailed above. The grades at each site are totaled to give a maximal DOGiBAT of 18.
A computer‐based training course was developed to train users in the application of the DOGiBAT to clinical cases. The training course initially introduced the participant to general principles of scoring within the DOGiBAT bleeding tool, and then provided pictures from clinical patients demonstrating to the trainee the appearance of each bleeding grade at each anatomic site. Sample slides demonstrating correct scoring of cutaneous hemorrhage are shown in Figure 1, and the complete training course is available in Supporting Information (Figure S1).
Figure 1.
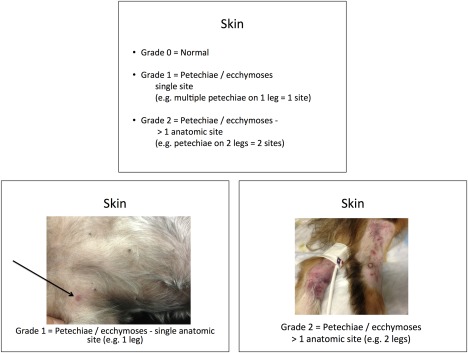
Sample slide from training course instructing the DOGiBAT user on how to correctly score cutaneous bleeding
A case‐based quiz set of still images also was developed, using images from clinical cases. This case‐based quiz was used to assess users' ability to correctly apply the DOGiBAT tool after they had completed the training course. We designed a standard case‐based quiz to allow for consistency over time and across multiple institutions. The quiz provided the user with clinical case descriptions and images to completely score 3 canine thrombocytopenic cases using the DOGiBAT. The complete quiz is available in the supplementary materials, along with the quiz score sheet to record answers, and the answer key to the quiz (Supporting Information Figures S2‐S4).
2.2. Study design
This study consisted of 3 phases, as outlined in Figure 2. In phase 1, clinicians and technicians took the training course, after which they completed a case‐based quiz to assess their ability to correctly implement the DOGiBAT.
Figure 2.
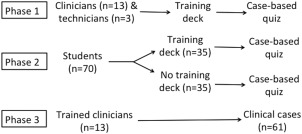
Experimental design showing the 3 phases of this study
In phase 2, equal numbers of third and fourth year veterinary student volunteers (n = 70) were randomized (https://www.randomizer.org) to take the quiz with (n = 35) or without (n = 35) completing the training course to assess the effectiveness of the training course. Veterinary students were selected as representative of a pool of users with a similar level of professional veterinary education. The study was considered exempt by the Iowa State University Institutional Review Board.
In phase 3, the clinicians who had received training on correct implementation of the DOGiBAT in phase 1 applied the DOGiBAT bleeding tool to clinical cases as part of a larger multicenter study of thrombocytopenia in dogs. Canine patients presenting to Iowa State University (ISU), Cornell University Hospital for Animals (CUHA), Cornell University Veterinary Specialists (CUVS), and Veterinary Specialists and Emergency Services (VSES), with body weight > 3 kg and platelet count < 50,000/μL of any underlying etiology were enrolled with client consent. Because the cause of thrombocytopenia was determined in the course of diagnostic evaluation, dogs with immune and non‐ITP were enrolled for compilation of DOGiBAT scores, clinicopathologic variables, and outcome measures. Cases were excluded if they had received glucocorticoids for a period of > 48 hours at the time of enrollment or if they had received any other immunosuppressive therapies. These cases were excluded to enroll a relatively homogeneous population of dogs with newly diagnosed thrombocytopenia.
Enrolled thrombocytopenic dogs were classified into 3 subgroups according to the underlying etiology of the thrombocytopenia, using a classification scheme similar to 1 previously described.16 In brief, dogs were classified into 1 of 3 subgroups as follows: Subgroup 1 (PI)—dogs with primary ITP, in the absence of an underlying cause based on diagnostic imaging and laboratory testing; Subgroup 2 (SI)—dogs with secondary ITP due to identified neoplasia, drug treatment, or infectious disease; and, Subgroup 3 (NI)—dogs with non‐ITP, secondary to bone marrow aplasia based on CBC or bone marrow examination or consumptive coagulopathy associated with an abnormal coagulation profile. Diagnostic testing and treatment decisions were at the discretion of the attending clinician. After study completion and before undertaking data analyses, case records were reviewed and classification was confirmed by consensus among 3 of the authors (K.M. Makielski, D.N. LeVine, and M.B. Brooks) based on all available diagnostic results.
All dogs were scored on admission with the DOGiBAT. The PI cases enrolled at all institutions were scored daily using the DOGiBAT, from time of enrollment until discharge from the hospital, death, or euthanasia, or until a maximum of 7 days. The SI and NI cases were all scored on day 1, with daily scoring until discharge only for cases enrolled at ISU. The score sheet to record daily bleeding scores for clinical cases of ITP using the DOGiBAT is available in the Supporting Information (Figure S5). Platelet counts were obtained either as an automated count on a hematology analyzer (ADVIA 2120, Siemens Healthcare Diagnostics Inc, Tarrytown, New York) as part of a CBC or as a manual platelet count. All automated counts were confirmed by technician review of a blood smear. The study was approved by the Institutional Animal Care and Use Committees of ISU and CUHA.
2.3. Statistical analysis
Sample size calculations were performed using online resources (OpenEpi, Atlanta, Georgia; http://www.stat.ubc.ca/~rollin/stats/ssize/b2.html) to determine the number of students needed per group (students with access to the training course and students who were not given access to the training course) to detect a significant difference in DOGiBAT quiz score with training. Pilot data was utilized from 10 untrained veterinary students who scored 3 cases (cluster size 30) with a baseline correct response rate of 75%. Using independent samples, an alpha = 0.05 and 80% power, the required sample size to detect a significant difference (defined as a 20% increase in number of correct scores) between the 2 groups was calculated. When sample sizes were adjusted for clustering by students (ie, each student answered multiple questions), and assuming an intraclass correlation of 0.75, the required sample size was determined to be 35 students in the trained group and 35 students in the untrained group.
For data analysis of quiz‐taker performance, bleeding grades were converted to a 2‐level outcome variable, where the response was considered either correct (1) or incorrect (0). Quiz site scores were considered correct if they agreed with the investigators' pre‐specified classification. A logistic regression model was used to model factors associated with the outcome (probability of being correct), while adjusting for correlated responses from sites within cases [Statistical Analysis System (SAS) 9.3, SAS Institute Inc, Cary, North Carolina]. The explanatory variables of interest were training or not and grade level of the student (third versus fourth year). Additionally, the interaction between training and grade level was included. Case was used as a random effect whenever appropriate to account for the dependency among observations.
To assess the association between DOGiBAT score and platelet count in the clinical phase of the study, a Spearman correlation coefficient was calculated for each anatomic site and for total DOGiBAT score [Statistical Analysis System (SAS) 9.3, SAS Institute Inc]. Only days that had both a platelet count and a DOGiBAT score performed were included in this analysis. Automated platelet counts that were reported as <10,000/μL or <20,000/μL were converted to 9,000/μL and 19,000/μL, respectively, for data analysis. To compare admission DOGiBAT scores and platelet counts among the 3 groups of dogs (PI, SI, NI), Kruskal‐Wallis tests were performed followed by pairwise comparisons with nonparametric Wilcoxon rank sum tests when differences among all 3 groups were identified. Assumptions of normality were not made. P‐values < .05 were considered significant.
Spearman correlation coefficients were calculated to assess the associations between admission DOGiBAT score (at each anatomic site and total DOGiBAT score) and outcome measures, and between admission platelet count and outcome measures. Outcome measures assessed were transfusion requirements, duration of hospitalization (days), and survival to discharge. For data analysis of outcome measures, transfusion requirements and survival to discharge each were converted to a 2‐level outcome variable. In the case of transfusion requirements, the outcome was whether any volume of any blood product(s) was administered during hospitalization (1) or no blood products were administered (0). For survival, the outcome was death or euthanasia while hospitalized (0) or survival to discharge from the hospital (1). Wilcoxon rank sum tests were performed to compare outcome measures between PI dogs and non‐PI dogs (SI and NI; Prism 6.0, GraphPad Software Inc., La Jolla, CA). P‐values < .05 were considered significant.
3. RESULTS
In the first phase of this project, clinicians (n = 13) and technicians (n = 3) completed a case‐based quiz after training. Clinicians consisted of boarded‐certified small animal specialists (n = 6), residents (n = 5), a rotating intern (n = 1), and an internship‐trained emergency veterinarian (n = 1). Technicians were all licensed veterinary technicians. Clinicians and technicians were able to correctly apply the DOGiBAT after training, scoring 100% of responses correctly.
In the second phase of this project, 70 veterinary students were administered the case‐based quiz, after having been randomized to either receive the training course (n = 35) or no training (n = 35). Of the students who received training, 17/35 were third year veterinary students and 18/35 were fourth year veterinary students. Of the students who received no training, 18/35 were third year veterinary students and 17/35 were fourth year veterinary students. Student enrollment was kept open for a period of 6 months. Therefore, students taking the quiz were at various points of their third and fourth years of veterinary school. Students who received training correctly scored 86.1% (814/945) of responses, compared with only 78.2% (739/945) of responses from students who did not receive training. The odds of trained students giving correct answers were higher than those of untrained students (P < .0001). Numbers and percentages of correct scores from students with and without training at each anatomic site are shown in Table 2. The anatomic site for which training was most important for correct scoring was oral bleeding (82.9% [87/105] with training; 47.6% [50/105] without training; P < .0001). Similarly, correct scoring of ocular (75.2% [79/105] with training; 58.1% [61/105] without training; P = .01) and cutaneous bleeding (84.8% [89/105] with training; 66.7% [70/105] without training; P = .003) were significantly improved by prior training. Regardless of training status, fourth year students were better able to apply the DOGiBAT correctly (84.2% [796/945]) compared with third year students (80.1% [757/945]; P = .015). When analyzed separately, training benefited students regardless of their class year, because training had a significant effect on correct scoring in both the third year (P = .02) and fourth year (P < .0001) veterinary students.
Table 2.
Numbers and percentages of correct scores at each anatomic site on a case‐based quiz from students with and without training
| Trained | Untrained | ||||
|---|---|---|---|---|---|
| DOGiBAT site | #/105 | % | #/105 | % | P‐value |
| Skin | 89 | 84.8 | 70 | 66.7 | .003 |
| Catheter, etc. | 68 | 64.8 | 79 | 75.2 | .115 |
| Oral cavity | 87 | 82.9 | 50 | 47.6 | <.0001 |
| Ocular | 79 | 75.2 | 61 | 58.1 | .01 |
| Epistaxis | 97 | 92.4 | 98 | 93.3 | .665 |
| Gastrointestinal | 99 | 94.3 | 93 | 88.6 | .148 |
| Urinary | 99 | 94.3 | 96 | 91.4 | .39 |
| Pulmonary | 91 | 86.7 | 88 | 83.8 | .456 |
| Intracranial | 105 | 100 | 104 | 99 | .989 |
| Total DOGiBAT | 31 | 29.5 | 11 | 10.5 | <.0001 |
Values for sites significantly affected by training (P < .05) are in bolded text. Total DOGiBAT score (bottom row) was considered correct if each anatomic site was graded correctly, resulting in the correct total for the individual case.
In the third phase of this project, the clinicians (n = 13) trained in phase 1 utilized the DOGiBAT for daily assessment of bleeding in a multicenter cohort study of dogs with thrombocytopenia. Dogs (n = 61) with platelet counts <50,000/μL, regardless of etiology, were enrolled. Enrolled dogs consisted of 31 female dogs (50.8%; 30 spayed, 1 intact) and 30 male dogs (49.2%; 26 castrated, 4 intact). These dogs were classified based on results of diagnostic testing as primary immune (PI)‐mediated (PI; 34/61; 55.7%), secondary immune (SI)‐mediated (SI; 17/61; 27.9%), or nonimmune‐mediated (NI; 10/61; 16.4%). Of the 34 dogs considered PI, this was the first presentation for 32 of the dogs (94.1%), whereas 2/34 (5.9%) had prior treatment for ITP and were presenting for disease relapse. On admission to the hospital, platelet counts were significantly lower in the PI dogs (median, 9,500/μL; range, 0–40,000/μL) than the SI dogs (median, 21,000/μL; range, 0–44,000/μL; P = .034) or NI dogs (median, 26,000/μL; range, 11,000–44,000/μL; P = .003; Figure 3A). Total DOGiBAT scores also were significantly higher in the PI dogs on admission (median, 5; range, 0–12) than in the SI dogs (median, 3; range, 0–12; P = .021) or NI dogs (median, 2; range, 0–10; P = .01; Figure 3B). Admission platelet counts (P = .305) and admission DOGiBAT scores (P = .239) were not significantly different between the SI dogs and the NI dogs. The most common anatomic sites of bleeding on enrollment in the study were cutaneous bleeding (38/61 [62.3%] of enrolled dogs), followed by oral mucosa (31/61 [50.8%]), gastrointestinal (25/61 [41%]) and urinary (25/61 [41%]). A significant negative correlation was found between platelet count and DOGiBAT score in all dogs (r s = −0.527, P < .0001; Figure 4) and when only evaluating PI dogs (r s = −0.476, P < .0001). This significant negative correlation also persisted when only DOGiBAT scores from days when platelet counts <30,000/μL were evaluated, both in all enrolled dogs (r s = −0.435, P < .0001) and in only the PI dogs (r s = −0.319, P = .001).
Figure 3.
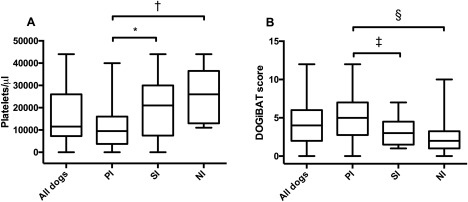
Box plots of platelet counts (A) and DOGiBAT scores (B) on admission in all dogs, dogs with PI, SI, and NI thrombocytopenia. In each case, the box represents the interquartile range, the horizontal line represents the median, and the whiskers represent the data range. *P = .034; †P = .003; ‡P = .021; §P = .01
Figure 4.
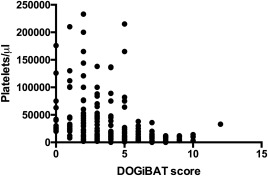
Relationship between total DOGiBAT score and platelet count in all dogs on days of hospitalization where both a platelet count and DOGiBAT score were performed. There was a strong negative correlation between platelet count and DOGiBAT score in all dogs (r s = −0.527, P < .0001)
Outcome measures (administration of blood products, duration of hospitalization in days, and survival to discharge) were assessed in PI dogs and compared with non‐PI (SI and NI) dogs (Table 3). A total of 36 units of blood products was administered (32/36 [88.9%] to PI dogs), with packed red blood cells (31/36 [86.1%]) being the most common type of blood product administered. Thirteen of the 34 PI dogs (38.2%) received blood products during hospitalization, compared with 2/27 non‐PI dogs (7.4%). A significant difference was found in transfusion requirements between PI dogs and non‐PI dogs (P = .007). Duration of hospitalization was similar between PI dogs (median, 4.5; range, 1–12 days) and non‐PI dogs (median, 4; range, 0–11 days; P = .974). Similarly, survival to discharge was similar between PI dogs (31/34 [91.2%] survival) and non‐PI dogs (25/27 [92.6%] survival; P = .999). Of the 5 dogs that did not survive to discharge, 1 (a PI dog) died during hospitalization, and 4 (2 PI dogs and 2 NI dogs) were euthanized, with the reason for euthanasia attributed to poor prognosis.
Table 3.
Dogs with PI thrombocytopenia (PI) and non‐PI dogs (dogs with either SI thrombocytopenia or nonimmune thrombocytopenia) were assessed for outcome measures
| PI dogs (n = 34) | Non‐PI dogs (n = 27) | ||||
|---|---|---|---|---|---|
| Outcome measure | #/34 | % | #/27 | % | P‐value |
| Blood product administration | 13 | 38.2 | 2 | 7.4 | .007 |
| Survival to discharge | 31 | 91.2 | 25 | 92.6 | .999 |
| Outcome measure | Median | Range | Median | Range | P‐value |
| Hospitalization duration (days) | 4.5 | 1–12 | 4 | 0–11 | .974 |
Numbers and percentages of dogs that received blood products during hospitalization and that survived to discharge are presented, along with hospital duration (median and range; days). Values for outcomes that are significantly different between PI and non‐PI dogs (P < .05) are in bolded text.
The correlation between outcome measures and DOGiBAT and platelet count for all dogs and for the PI subgroup analyzed separately is presented in Table 4. In all enrolled dogs, total DOGiBAT score at admission was significantly correlated with blood product administration (r s = 0.512, P < .0001) and duration of hospitalization (r s = 0.35, P = .006). These associations persisted when only PI dogs were evaluated (blood product administration [r s = 0.541, P = .001], duration of hospitalization [r s = 0.452, P = .007]). Total DOGiBAT score at admission was not significantly correlated with survival to discharge in all dogs (r s = −0.168, P = .197) or in only PI dogs (r s = −0.123, P = .49).
Table 4.
Correlation data between clinical outcomes and admission DOGiBAT scores and between clinical outcomes and admission platelet counts in all enrolled dogs (n = 61) and in dogs with PI thrombocytopenia (PI; n = 34)
| All enrolled dogs (n = 61) | PI dogs only (n = 34) | |||||||
|---|---|---|---|---|---|---|---|---|
| Platelet count | Total DOGiBAT | Platelet count | Total DOGiBAT | |||||
| Outcome measure | r s | P‐value | r s | P‐value | r s | P‐value | r s | P‐value |
| Blood product administration | −0.355 | .005 | 0.512 | <.0001 | −0.248 | .164 | 0.541 | .001 |
| Hospitalization duration (days) | −0.224 | .085 | 0.35 | .006 | −0.231 | .196 | 0.452 | .007 |
| Survival to discharge | −0.192 | .142 | −0.168 | .197 | −0.283 | .111 | −0.123 | .49 |
Spearman correlation coefficients (r s) and P‐values considered significant (P < .05) are in bolded text.
A significant negative correlation was detected in all dogs between admission platelet count and blood product administration (r s = −0.355, P = .005), but not the duration of hospitalization (r s = −0.224, P = .085) or survival to discharge (r s = −0.192, P = .142). However, when only PI dogs were evaluated, admission platelet count was not significantly correlated with blood product administration (r s = −0.248, P = .164), duration of hospitalization (r s = −0.231, P = .196), or survival to discharge (r s = −0.283, P = .111). Correlations between outcomes and individual DOGiBAT anatomic sites are provided in the Supporting Information (Tables S1 and S2).
4. DISCUSSION
We developed and evaluated the DOGiBAT, an assessment tool to quantify clinical bleeding in dogs with ITP. The DOGiBAT was first evaluated by use of a case‐based quiz administered to clinicians, technicians, and veterinary students. Clinicians and technicians correctly applied the DOGiBAT to the case‐based quiz with excellent interuser agreement, scoring all answers correctly after receiving training. The training course was shown to improve correct implementation of the DOGiBAT, because veterinary students randomized to receive training before the quiz had significantly higher odds of answering correctly compared to veterinary students randomized to take the quiz without prior training. The final phase of this project was a preliminary evaluation of application of the DOGiBAT by trained clinicians to thrombocytopenic dogs. The potential biological relevance of the DOGiBAT is suggested by its inverse correlation with platelet count. In dogs with primary ITP, admission platelet count was not correlated with any of the evaluated outcome measures (blood product administration, duration of hospitalization, and survival to discharge), supporting the idea that platelet count alone does not consistently predict clinical bleeding in dogs with ITP. However, total DOGiBAT score at admission was significantly correlated with blood product administration and duration of hospitalization in dogs with primary ITP, demonstrating its potential prognostic value.
Immune thrombocytopenia is a common cause of severe thrombocytopenia in dogs, yet individuals with similar platelet counts display variable bleeding tendencies. The exact mechanisms for this bleeding heterogeneity have yet to be determined. Mechanisms proposed in the human literature include differences in platelet activation,17 interference of antibodies with platelet function,18, 19 effects of anemia on platelet function, vascular endothelial abnormalities,20 and presence of procoagulant microparticles.21, 22 Clinical trials in ITP patients, therefore, require not only assessment of platelet count but also a metric of bleeding severity in order to consistently gauge disease severity and response to treatment.
We therefore developed the DOGiBAT as an objective system for scoring clinical bleeding in dogs with ITP. To our knowledge, only 1 prior bleeding score has been reported in the veterinary literature. It was used to score thrombocytopenic dogs in a clinical trial evaluating efficacy of various platelet transfusions, but the scoring system itself did not undergo thorough evaluation in that study.23 Several bleeding scores are used to quantify bleeding in human ITP patients. The DOGiBAT was modeled after the ITP Bleeding Scale (IBLS), a simple yet complete scoring system utilized in the human literature.5 In 1 study of humans with ITP, the IBLS was reported to be associated with platelet count, but this association did not persist for patients with platelet counts < 30,000/μL,5 likely because of the heterogeneity in clinical bleeding seen with severe thrombocytopenia. Since the initial description of the IBLS, several other publications in the human literature have implemented this bleeding score in ITP patients to make correlations between clinical bleeding severity and clinicopathologic variables24, 25, 26 and to assess clinical bleeding in treatment trials.27, 28
Although similar to the IBLS, several modifications tailor the DOGiBAT to ITP in dogs. First, the DOGiBAT does not include grading of historical bleeding, as many canine ITP patients present with acute disease without a prior diagnosis of ITP. Similarly, grading of gynecological bleeding was not included, because the majority of female dogs diagnosed with ITP are spayed.4, 6, 8, 29 Modifications were made to the oral cavity category, because dogs do not typically develop the blood blisters commonly seen in humans with ITP. Finally, a category was added to the DOGiBAT to account for bleeding after a minimally invasive procedure (eg, venipuncture).
The DOGiBAT was first evaluated with clinicians and technicians in referral practices by use of a case‐based quiz utilizing details and images from clinical cases. These clinicians and technicians scored all cases correctly at all sites, demonstrating that the DOGiBAT was easy to employ correctly and consistently. Because the majority of clinicians given the case‐based quiz after training were either board‐certified specialists or residents receiving training in a small animal specialty, this population is likely representative of a referral institution, but may not represent the population of small animal veterinary practitioners as a whole. Further evaluation in a larger population of clinicians in all types of veterinary practices should be considered in future studies using the DOGiBAT.
The training module instructed users on implementation of the DOGiBAT scoring tool. To evaluate the effects of training, veterinary students were utilized as a large population of individuals who likely had less clinical experience than the clinicians in phase 1. Although students were not questioned or selected based on their clinical expertise, it is likely that the students varied in their prior exposure to small animal practice. However, students were randomized to receive the training module before taking the quiz to minimize any confounding effect of prior experience.
We found that students who received training scored more correct responses than those who had received no training. Scoring of 3 anatomic sites (oral cavity, ocular, and cutaneous) was most improved by having access to training. Because some bleeding grades are inherently easier to understand without training (eg, presence or absence of epistaxis) or require more diagnostic acumen (eg, funduscopic bleeding or hyphema), differences among categories were not unexpected. By providing the user a written definition and pictorial example of each bleeding grade at each anatomic site, the training course was able to clarify grading to improve correct scoring at challenging anatomic sites. We also determined that fourth year veterinary students scored more responses correctly compared with third year veterinary students, independent of their access to the training course. This observation is suspected to be a result of the clinical experience that fourth year veterinary students gain from their clinical rotations during their senior year. Although evaluated in a group of students, the training course will likely improve correct implementation of the DOGiBAT among more experienced clinicians as well.
In the third phase of this study, clinicians implemented the DOGiBAT in clinical practice in a pilot study of dogs with thrombocytopenia. Although the DOGiBAT was developed for use in canine ITP patients, dogs with thrombocytopenia of any underlying etiology were enrolled and we performed comparisons of bleed scores and platelet counts among all dogs and specifically for the target ITP population. Because this study represents the initial evaluation of the DOGiBAT scoring system, dogs were excluded if they had received >48 hours of treatment with glucocorticoids or if they had received treatment with additional immunosuppressants, in an attempt to enroll a relatively homogeneous population of treatment naïve dogs.
Most reports of ITP in human patients30, 31 describe an inverse correlation between bleed score and platelet count (ie, a lower platelet count is associated with a higher bleed score). Similarly, we found that dogs with primary ITP demonstrate this inverse relationship, with lower platelet counts and higher DOGiBAT scores than those with secondary ITP and thrombocytopenia of other causes (Figure 3). Unlike 1 study of ITP implementing the IBLS score5 on which the DOGiBAT was modeled, we found that the inverse correlation between DOGiBAT and platelet count persisted for dogs with counts <30,000/μL. Possible reasons for this observation include differences between the 2 studies in their design, patient populations, and the statistical methods used to determine association. The population reported previously consisted of human patients primarily with chronic ITP, enrolled at any visit regardless of platelet count and treatment status.5 To be enrolled in our study, dogs had to have platelet counts <50,000/μL and could not have been receiving glucocorticoids for > 48 hours before enrollment. Therefore, our population was more likely to have acute clinical disease. Additional studies with well‐defined canine and human populations are needed to further assess the relationship between severity of bleeding and platelet count in both species.
When assessing outcome measures in dogs with primary ITP, we found no association between platelet count at admission and blood product administration, survival to discharge, or duration of hospitalization, similar to results of a previous retrospective study of prognostic factors in ITP.4 However, there was a significant correlation between admission DOGiBAT score and both blood product administration and duration of hospitalization (ie, PI dogs with higher DOGiBAT scores at admission were more likely to receive blood products and have a longer duration of hospitalization). Therefore, the DOGiBAT may have potential as a metric of disease severity in dogs with ITP. Reasons that platelet count did not correlate with outcome measures could include other concurrent patient factors such as differences in vascular integrity, differences in autoantibody effect on platelet function, and difference in activation state of the patient's remaining platelets. The fact that platelet count did not correlate with any of the outcome measures examined in PI dogs highlights the importance of a bleeding severity scoring system such as DOGiBAT. Further evaluation in a larger population of dogs with primary ITP is needed to confirm this preliminary finding.
Our study had some limitations. First, the training and evaluation of clinicians' DOGiBAT scoring used digital case‐based materials, rather than physical examination of clinical patients. However, this design allowed for a consistent method of assessing correct implementation of the DOGiBAT across multiple institutions. Future studies could include on‐site clinician assessment of clinical patients to further compare interuser agreement at a single institution.
An inherent feature of the DOGiBAT scoring system is that the total score may not be predictive of morbidity or mortality because hemorrhage at each anatomic site is not equivalent. For example, intracranial hemorrhage is 1 of the most severe clinical sequela of ITP. Melena has been shown to be a negative survival predictor in dogs with ITP,4 and thus a gastrointestinal bleed score of 2 may have more clinical relevance than a cutaneous bleed score of 2. A truly summative score would be ideal, but would require assigning a “severity factor” to each site. In the absence of large follow‐up studies with extensive outcome data, it would be premature to assign such severity factors to the DOGiBAT. Additionally, although the correlation of DOGiBAT scores with outcome measures in ITP dogs may demonstrate its potential prognostic value, this could be the result of a type 1 error (ie, the reporting of a significant relationship when 1 does not actually exist). The relationship between DOGiBAT score and outcome requires further evaluation and validation in additional studies of thrombocytopenic dogs. Additionally, our results do not provide cut‐off values predictive of transfusion requirements or duration of hospitalization.
In conclusion, we have developed the DOGiBAT, a bleed score system that can be used to score canine patients with thrombocytopenia. Adoption of the DOGiBAT scoring system and training course as a component of disease severity assessment may help facilitate future multi‐institution clinical studies of dogs with ITP. Monitoring DOGiBAT scores could be included with other clinicopathologic variables in future studies with the goal of identifying predictors of disease severity and response to treatment and investigating the reasons why ITP patients have variable bleeding tendencies.
WORK PERFORMANCE SITES
Clinical cases were enrolled at Iowa State University (ISU), Cornell University Hospital for Animals (CUHA), Cornell University Veterinary Specialists (CUVS), and Veterinary Specialists and Emergency Services, Rochester, NY (VSES). Clinicians and technicians at these institutions participated in the quiz. Student volunteers from ISU participated in the quiz and evaluation of the training course.
This study was presented as an oral abstract at the 2016 ACVIM Forum, Denver, CO.
CONFLICT OF INTEREST DECLARATION
The authors declare that they have no conflict of interest with the contents of this article.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Figure S1. Complete training course for correct implementation of the canine daily bleeding score assessment tool, DOGiBAT.
Figure S2. Complete case‐based quiz utilized to assess correct implementation of the canine daily bleeding score assessment tool, DOGiBAT.
Figure S3. Score sheet to record answers of the DOGiBAT quiz.
Figure S4. Answer key for the DOGiBAT quiz.
Figure S5. Score sheet to record daily bleeding scores for clinical cases of ITP using the DOGiBAT.
Table S1. Correlation data between clinical outcomes and admission DOGiBAT scores at individual anatomic sites in all enrolled dogs (n = 61). Spearman correlation coefficients (rs) and p‐values considered significant (p < 0.05) are in bolded text.
Table S2. Correlation data between clinical outcomes and admission DOGiBAT scores at individual anatomic sites in only dogs categorized as having primary immune thrombocytopenia (n = 34). Spearman correlation coefficients (rs) and p‐values considered significant (p< 0.05) in bolded text.
ACKNOWLEDGMENTS
The authors thank the clinicians at ISU, CUHA, CUVS, and VSES for enrolling and scoring clinical cases. The authors also thank the clinicians, technicians, and students who participated in the training and the quiz, and Amy Molitaris for assistance in compiling clinical data. Clinical cases were enrolled as part of a larger study of canine immune thrombocytopenia that was supported by a grant from the American Kennel Club Canine Health Foundation (2052). The contents of this publication are solely the responsibility of the authors' and do not necessarily represent the views of the Foundation.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
In phase 2, veterinary student volunteers were administered a case‐based quiz. This study was considered exempt by the ISU Institutional Review Board.
In phase 3, clinical cases of thrombocytopenia were enrolled with client consent. This study was approved by the Institutional Animal Care and Use Committees of ISU and CUHA.
Makielski KM, Brooks MB, Wang C, Cullen JN, O'Connor AM, LeVine DN. Development and implementation of a novel immune thrombocytopenia bleeding score for dogs. J Vet Intern Med. 2018;32:1041–1050. https://doi.org/10.1111/jvim.15089
Funding information American Kennel Club Canine Health Foundation (2052)
REFERENCES
- 1. Botsch V, Kuchenhoff H, Hartmann K, Hirschberger J. Retrospective study of 871 dogs with thrombocytopenia. Vet Rec. 2009;164:647–651. [DOI] [PubMed] [Google Scholar]
- 2. McMillan R. Antiplatelet antibodies in chronic immune thrombocytopenia and their role in platelet destruction and defective platelet production. Hematol Oncol Clin North Am. 2009;23:1163–1175. [DOI] [PubMed] [Google Scholar]
- 3. Lewis DC, Meyers KM. Canine idiopathic thrombocytopenic purpura. J Vet Intern Med. 1996;10:207–218. [DOI] [PubMed] [Google Scholar]
- 4. O'Marra SK, Delaforcade AM, Shaw SP. Treatment and predictors of outcome in dogs with immune‐mediated thrombocytopenia. J Am Vet Med Assoc. 2011;238:346–352. [DOI] [PubMed] [Google Scholar]
- 5. Page LK, Psaila B, Provan D, et al. The immune thrombocytopenic purpura (ITP) bleeding score: assessment of bleeding in patients with ITP. Br J Haematol. 2007;138:245–248. [DOI] [PubMed] [Google Scholar]
- 6. Balog K, Huang AA, Sum SO, Moore GE, Thompson C, Scott‐Moncrieff JC. A prospective randomized clinical trial of vincristine versus human intravenous immunoglobulin for acute adjunctive management of presumptive primary immune‐mediated thrombocytopenia in dogs. J Vet Intern Med. 2013;27:536–541. [DOI] [PubMed] [Google Scholar]
- 7. Putsche JC, Kohn B. Primary immune‐mediated thrombocytopenia in 30 dogs (1997–2003). J Am Anim Hosp Assoc. 2008;44:250–257. [DOI] [PubMed] [Google Scholar]
- 8. Bianco D, Armstrong PJ, Washabau RJ. A prospective, randomized, double‐blinded, placebo‐controlled study of human intravenous immunoglobulin for the acute management of presumptive primary immune‐mediated thrombocytopenia in dogs. J Vet Intern Med. 2009;23:1071–1078. [DOI] [PubMed] [Google Scholar]
- 9. Rozanski EA, Callan MB, Hughes D, Sanders N, Giger U. Comparison of platelet count recovery with use of vincristine and prednisone or prednisone alone for treatment for severe immune‐mediated thrombocytopenia in dogs. J Am Vet Med Assoc. 2002;220:477–481. [DOI] [PubMed] [Google Scholar]
- 10. Boston SE, Moens NM, Kruth SA, Southorn EP. Endoscopic evaluation of the gastroduodenal mucosa to determine the safety of short‐term concurrent administration of meloxicam and dexamethasone in healthy dogs. Am J Vet Res. 2003;64:1369–1375. [DOI] [PubMed] [Google Scholar]
- 11. Wallisch K, Trepanier LA. Incidence, timing, and risk factors of azathioprine hepatotoxicosis in dogs. J Vet Intern Med. 2015;29:513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pritchard JC, Jacob ME, Ward TJ, Parsons CT, Kathariou S, Wood MW. Listeria monocytogenes septicemia in an immunocompromised dog. Vet Clin Pathol. 2016;45:254–259. [DOI] [PubMed] [Google Scholar]
- 13. Smith PM, Haughland SP, Jeffery ND. Brain abscess in a dog immunosuppressed using cyclosporin. Vet J. 2007;173:675–678. [DOI] [PubMed] [Google Scholar]
- 14. Siak MK, Burrows AK. Cutaneous nocardiosis in two dogs receiving ciclosporin therapy for the management of canine atopic dermatitis. Vet Dermatol. 2013;24:453–456. e102–453. [DOI] [PubMed] [Google Scholar]
- 15. O'Marra SK, Shaw SP, deLaforcade AM. Investigating hypercoagulability during treatment for immune‐mediated thrombocytopenia: a pilot study. J Vet Emerg Crit Care (San Antonio) 2012;22:126–130. [DOI] [PubMed] [Google Scholar]
- 16. Bachman DE, Forman MA, Hostutler RA, Corn S, Lin J, Kociba GJ. Prospective diagnostic accuracy evaluation and clinical utilization of a modified assay for platelet‐associated immunoglobulin in thrombocytopenic and nonthrombocytopenic dogs. Vet Clin Pathol. 2015;44:355–368. [DOI] [PubMed] [Google Scholar]
- 17. Panzer S, Rieger M, Vormittag R, Eichelberger B, Dunkler D, Pabinger I. Platelet function to estimate the bleeding risk in autoimmune thrombocytopenia. Eur J Clin Invest. 2007;37:814–819. [DOI] [PubMed] [Google Scholar]
- 18. Chu XX, Hou M, Peng J, et al. Effects of IgG and its F(ab')2 fragments of some patients with idiopathic thrombocytopenic purpura on platelet aggregation. Eur J Haematol. 2006;76:153–159. [DOI] [PubMed] [Google Scholar]
- 19. Olsson A, Andersson PO, Tengborn L, Wadenvik H. Serum from patients with chronic idiopathic thrombocytopenic purpura frequently affect the platelet function. Thromb Res. 2002;107:135–139. [DOI] [PubMed] [Google Scholar]
- 20. Goerge T, Ho‐Tin‐Noe B, Carbo C, et al. Inflammation induces hemorrhage in thrombocytopenia. Blood. 2008;111:4958–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tantawy AA, Matter RM, Hamed AA, Shams El Din El Telbany MA Platelet microparticles in immune thrombocytopenic purpura in pediatrics. Pediatr Hematol Oncol. 2010;27:283–296. [DOI] [PubMed] [Google Scholar]
- 22. Jy W, Horstman LL, Arce M, Ahn YS. Clinical significance of platelet microparticles in autoimmune thrombocytopenias. J Lab Clin Med. 1992;119:334–345. [PubMed] [Google Scholar]
- 23. Davidow EB, Brainard B, Martin LG, et al. Use of fresh platelet concentrate or lyophilized platelets in thrombocytopenic dogs with clinical signs of hemorrhage: a preliminary trial in 37 dogs. J Vet Emerg Crit Care (San Antonio). 2012;22:116–125. [DOI] [PubMed] [Google Scholar]
- 24. Greene LA, Chen S, Seery C, Imahiyerobo AM, Bussel JB. Beyond the platelet count: immature platelet fraction and thromboelastometry correlate with bleeding in patients with immune thrombocytopenia. Br J Haematol. 2014;166:592–600. [DOI] [PubMed] [Google Scholar]
- 25. Goette NP, Glembotsky AC, Lev PR, et al. Platelet apoptosis in adult immune thrombocytopenia: insights into the mechanism of damage triggered by auto‐antibodies. PLoS One. 2016;11:e0160563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Psaila B, Bussel JB, Frelinger AL, Michelson AD. Differences in platelet function in patients with acute myeloid leukemia and myelodysplasia compared to equally thrombocytopenic patients with immune thrombocytopenia. J Thromb Haemost. 2011;9:2302–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liebman HA, Saleh MN, Bussel JB, et al. Comparison of two dosing schedules for subcutaneous injections of low‐dose anti‐CD20 veltuzumab in relapsed immune thrombocytopenia. Haematologica. 2016;101:1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zeller MP, Heddle NM, Kelton JG, et al. Effect of a thrombopoietin receptor agonist on use of intravenous immune globulin in patients with immune thrombocytopenia. Transfusion. 2016;56:73–79. [DOI] [PubMed] [Google Scholar]
- 29. Huang AA, Moore GE, Scott‐Moncrieff JC. Idiopathic immune‐mediated thrombocytopenia and recent vaccination in dogs. J Vet Intern Med. 2012;26:142–148. [DOI] [PubMed] [Google Scholar]
- 30. Neunert C, Noroozi N, Norman G, et al. Severe bleeding events in adults and children with primary immune thrombocytopenia: a systematic review. J Thromb Haemost. 2015;13:457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frelinger AL III, Grace RF, Gerrits AJ, et al. Platelet function tests, independent of platelet count, are associated with bleeding severity in ITP. Blood. 2015;126:873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Figure S1. Complete training course for correct implementation of the canine daily bleeding score assessment tool, DOGiBAT.
Figure S2. Complete case‐based quiz utilized to assess correct implementation of the canine daily bleeding score assessment tool, DOGiBAT.
Figure S3. Score sheet to record answers of the DOGiBAT quiz.
Figure S4. Answer key for the DOGiBAT quiz.
Figure S5. Score sheet to record daily bleeding scores for clinical cases of ITP using the DOGiBAT.
Table S1. Correlation data between clinical outcomes and admission DOGiBAT scores at individual anatomic sites in all enrolled dogs (n = 61). Spearman correlation coefficients (rs) and p‐values considered significant (p < 0.05) are in bolded text.
Table S2. Correlation data between clinical outcomes and admission DOGiBAT scores at individual anatomic sites in only dogs categorized as having primary immune thrombocytopenia (n = 34). Spearman correlation coefficients (rs) and p‐values considered significant (p< 0.05) in bolded text.


