Main Text
We present a theoretical study of the interaction between a protein (diffusing particle) and chromatin (polymer chain). Each monomer is a trap where a particle can transiently bind. We derive, to our knowledge, novel formulas for the transition rate between monomer sites, given a specific polymer configuration, and find that a particle is likely to rapidly rebind many times to its release site before moving to another site. The reattachment probability is larger when the local density around the release site is smaller. Interestingly, for an equilibrated polymer, the transition probability decays as a power law for close monomer-to-monomer distances and reaches an asymptotic value for long distances. By computing the transition rate between monomers, we show that the problem of facilitated search by a protein can be mapped to a continuous-time Markov chain, which we solve. Our findings suggest that proteins may be locally trapped for a time much longer than their dissociation time, whereas their overall motion is ergodic. Our results are corroborated by Brownian simulations.
The interaction of proteins with chromatin regulates many cellular functions. Most DNA-binding proteins interact both non-specifically and transiently (1) with many chromatin sites, as well as specifically and more stably with cognate binding sites. These interactions and chromatin structure are important in governing protein dynamics (2, 3). However, the effect of these transient interactions on protein motion and distribution has not yet been shown from a first principle.
Some aspects of protein interactions with DNA have been studied in the context of the search of a gene promoter site by a transcription factor (TF) (4). It was first noted (5) that the search for a promoter site by a TF would be faster if it involves three-dimensional excursions, as well as sliding of the protein along DNA (6), as was shown in prokaryotes (7). These different types of motion were observed experimentally, leading to massive interest in models of facilitated diffusion (8, 9, 10, 11, 12, 13, 14, 15), and in the impact of a regulating site’s position on transcription (16, 17). In current microscopy experiments, it is impossible to examine the search process to its fullest (18). Thus, we concentrate here on modeling the experimentally observed dynamics of the protein as a diffusing and interacting particle.
We will show that proteins are likely to stay in the proximity of a site for a time much longer than their dissociation time from DNA due to reattachment. Moreover, we find that reattachment depends on the local density around the release site, and that the precise configuration of the polymer impacts the interacting particle’s dynamics. We further find the rates associated with the transition between different monomer sites. Finally, we show that the process as a whole is ergodic; it has no long-time power-law distribution of the residence time at a site as has been previously suggested (19).
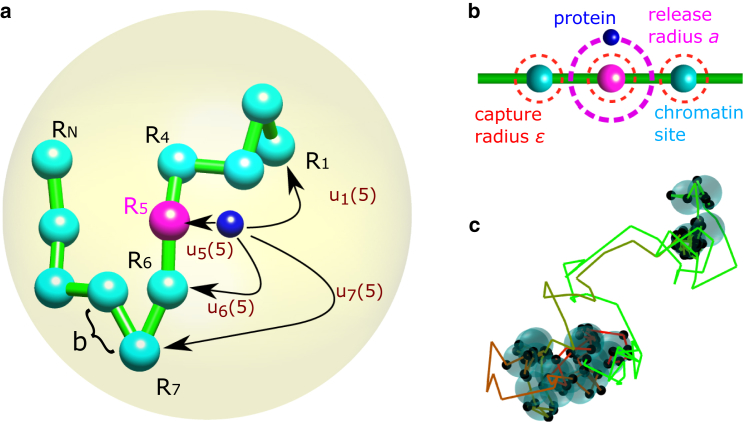
We consider a point particle (protein) placed at a distance a from monomer n (locus), part of a long flexible polymer (20, 21) (chromatin) (Fig. 1 a). The particle diffuses until it encounters monomer l. Absorption occurs at capture radius from l (Fig. 1 b), where the particle remains bound for characteristic time T. Upon dissociation, the particle is placed at a distance a from the monomer l position (Fig. 1 b), with a uniform angular distribution, and starts diffusing again. Although we postulate here two radii, release (a) and capture (ϵ), the effective behavior is equivalent to a model with one release radius and a partially reflecting boundary condition on it. In a partially reflecting model, the particle has a probability of re-absorbing immediately after release. Small re-absorption probability corresponds to . Thus, when the release and capture radii are comparable, the particle spends much time around one monomer cluster, then jumps to another, only to come back to the first (Fig. 1 c).
Figure 1.
Transition between monomer sites. (a) A particle (dark blue) representing a protein interacts with a monomer site (cyan) that serves as a chromatin locus. It detaches from an initial monomer n (magenta) and reattaches to site l with probability without touching other sites along the way. The polymer is inside a confining domain (yellow) of radius A. b is the mean-square displacement of a bond. (b) The particle is released at distance a from the initial site, with uniform angular distribution. It attaches to a site once it is at distance ϵ from it. We depict chromatin as a coarse-grained chain of beads. Each bead is of characteristic size b, representing 3.2 kb and of size 30 nm (41). For the rest of the paper, the characteristic length in our system is b. Hence, for and nm, the release and capture radii are of the order of the size of a protein and the interaction distance. (c) Trajectory of the particle interacting with the monomer sites (cyan). The trajectory color changes with time from red to green. The black dots are attachment points at the monomer site (,, , and ). To see this figure in color, go online.
The probability of the particle arriving at a certain monomer site before another via three-dimensional diffusion depends on their respective initial distance from each other (22). The probability that a particle starting from arrives at monomer l before encountering any other monomer (see Supporting Material) is
| (1) |
where N is the polymer length, is the position of monomer i, is the Neumann Green’s function of the Laplacian in a sphere of radius A, is a constant that depends on the monomers’ positions but not on the initial position, . In writing Eq. 1, we assume that the trapping monomers are well separated. When the ϵ neighborhoods of two monomers merge, the equation can be modified (23).
Proteins move much faster in the nucleus than chromatin. The diffusion coefficient of a chromatin locus can be estimated by inserting a fluorescent tag and following its trajectory (24). Assuming a coarse-grained model of chromatin, it was found that a locus of size has a diffusion coefficient of about D = 10−2 μm2/s (21), whereas proteins move three orders of magnitude faster. for example, 13.5 μm2/s for the c-Myc protein (25). We thus assume that the polymer is fixed at an equilibrium configuration inside the domain while the particle is diffusing.
Assuming that the polymer is equilibrated in the domain, we found the transition probability , that a particle starting in the proximity of site n finds site l first before encountering other monomers, by averaging Eq. 1 with the equilibrium distribution of the polymer in bulk (see the Supporting Material):
| (2) |
where , b is the standard deviation of a bond length (Fig. 1 a), and we assume , with C a numerical factor of order 1. The expression for the end monomers is different (see Supporting Material, Section S4). Although we did not explicitly include a sliding state of the protein along DNA (6), it could be included by modifying the nearest-neighbors transition probability, , and will not qualitatively change our results.
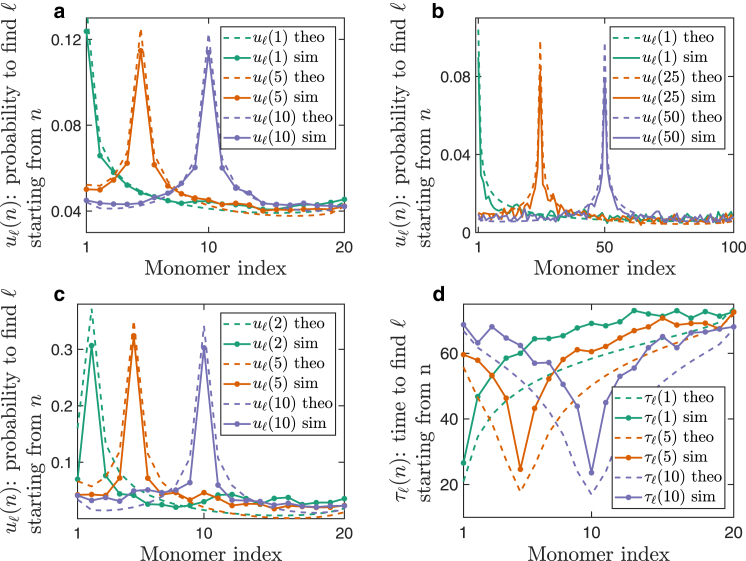
The reattachment probability at the release site is larger than the probability of attaching to faraway sites (Fig. 2 a; Eq. 2), suggesting that once released, the particle is likely to rebind at the same site. For a longer polymer strand, the ratio between the reattachment probability and probability to attachment to a faraway site is larger than for a short polymer (Fig. 2 b). This result is contrary to previous studies that assumed that the transition probability and the for rebinding were equal among all monomers (13, 26, 27, 28, 29, 30, 31, 32), or were related to a Lévy type diffusion of the particle (30).
Figure 2.
Transition between monomer sites. (a) The transition probability starting from sites 1 (turquoise), 5 (orange), or 10 (purple). The polymer has 20 monomer sites (N = 20), and , , and . The solid lines are the result of Brownian simulations, whereas the dashed lines are computed using the analytical formula (Eq. 2). (b) The site transition probability when the polymer is longer (N = 100 sites). (c) The polymer is of length . It is crowded into a small domain, , and the capture radius is . (d) The mean first-transition time, , from site n to site l without interacting with any other site along the way. The full lines are the result of Brownian simulation and the dashed lines are computed using Eq. 5 with the same parameters as in (a). A single time unit is equal to, where D is the diffusion coefficient of the particle. To see this figure in color, go online.
Interestingly, we find that the reattachment probability is minimal for the middle monomer (Eq. 2). To clarify this effect, we estimated the expected number of monomers in a ball around the release monomer, for different monomers along the chain (see Fig. S1). This quantity is akin to the local density around the release site. The monomer density is highest around the middle monomer, which is closest to the polymer center of mass. Thus, the reattachment probability is sensitive to the local density around the release site and not just to the average monomer density in the domain.
Segregation of chromosomes in the nucleus may be the result of self-avoiding interaction (SAI) between them (33). To study how SAI between monomers would modify the behavior of , we performed Brownian simulation (BS) where the monomers interacted through the Lennard-Jones potential (34) in addition to engaging in nearest-neighbors spring interactions (see Supporting Material). The separation of monomers due to SAI decreases the local monomer density around the release point, leading to a larger reattachment probability, , compared to that of a phantom chain (see Fig. S2 a).
The root mean-square distance between monomers scales with their distance along the chain (). This results in the power-law scaling for proximal sites of a phantom polymer (Eq. 2), for which . To study the behavior for an SA polymer, we computed between proximal sites from the BS, averaged over all release sites n, and fitted it: . We found the fitted exponent to be (see Fig. S2 b), as would be expected from the rmsd of an SA polymer, for which is the Flory exponent (35). When a and ϵ are larger, α increases (see Fig. S2 c). This behavior may be explained by studying high-order expansions of in the parameter ϵ.
Computing , we assumed that the polymer configuration is not much affected by confinement (the ratio of gyration radius to domain radius: and for Fig. 2, a and b, respectively). Thus, its equilibrium distribution was well approximated by the equilibrium distribution of a flexible chain in bulk. Since one end of the polymer is anchored at the origin of the domain, when the end-to-end distance () is of the order of the domain radius, the polymer feels the effect of confinement.
In the nucleus, chromosomes are tightly packed and are not in the dilute regime. We thus simulated a polymer in a smaller domain for which and and computed (Fig. 2 c). Interestingly, Eq. 2 still matches the transition probability, even for this moderately crowded polymer. Indeed, when the target monomer l is close by, its distance distribution from the release monomer is not affected much by the presence of confinement. At the same time, the transition probability to faraway monomers weakly depends on the distance between the release site and the target. Thus, it is not much affected by confinement. The validity of Eq. 2 is expected to break for extreme packing ().
To understand the rates involved in the encounters between proteins and chromatin, we computed the conditional mean first-passage time (MFPT), , of a particle from position to site l without encountering other monomers along the way. The conditional dynamics of the particle obeys the Langevin equation (36),
| (3) |
where is a white Gaussian noise, the drift is , and is given by Eq. 2. Since approaches zero when approaches any monomer other than l (see Eq. S5 in the Supporting Material), the drift will diverge for (). Thus, moving according to Eq. 3, the particle experiences a drift pushing it away from all monomers except for its final destination, l.
can be found by solving a boundary value problem (see Eq. S73). It has the approximate solution
| (4) |
where is the eigenvalue of an associated eigenvalue problem (see Supporting Material, Section S5).
Averaging Eq. 4 with the equilibrium polymer configuration, we find an asymptotic formula for the mean conditional transition time starting from monomer n,
| (5) |
where is the Green’s function between monomer n and j positions, averaged over the equilibrium distribution.
Compared to BS, this formula (Eq. 5) matches the simulation, where the difference is up to ∼ of the MFPT (see Fig. 2 d). Interestingly, to the release site () is much faster than to other sites, and the MFPT converges asymptotically when the target monomer is far from the release site (). When , the recapture time is faster with respect to transition time to another site. Indeed, when an interacting protein starts from the boundary layer of the release site, the characteristic recapture rate is significantly higher than the travel time to other sites. Hence, we suggest that when a protein is released from a chromatin site, it would quickly rebind to it with high probability.
We find that the addition of SAI reduces compared to a phantom polymer (Fig. S2 d). The addition of SAI increases the average distance between monomers. Consequently, the eigenvalue is larger (see Eq. S87), resulting in a smaller MFPT (Eq. 5). Therefore, the monomers of an SA polymer fill the domain more optimally than those of a phantom polymer, resulting in a rapid capture.
Since the reattachment rate at a site is larger, we expect that when SAIs are dominant, proteins will spend a larger fraction of their time bound at monomer sites. Heterochromatin is considered to be denser than euchromatin (37). However, the nature of interaction of proteins with heterochromatin is still unclear. We would expect to be smaller in heterochromatin domains and that a protein would “forget” faster its release position. Alternatively, if reattachment occurs with higher probability () in heterochromatin, will be larger in these domains.
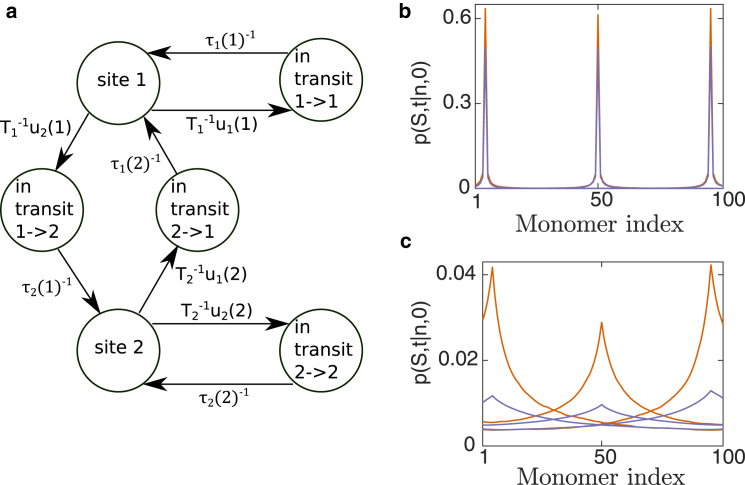
Proteins in the nucleus can be either bound to chromatin or other nuclear compartments, or can stochastically move between association events. We denote the unbound state as “in transit” from one monomer site to another. We assume that at site n, the particle remains bound for a characteristic time, , and leaves with a Poissonian rate. We thus formulated the transition between the different states using a continuous-time Markov chain (CTMC) (Fig. 3 a). We constructed the rate matrix Q between bound states and transit states that depend on , , and (see the Supporting Material). We used Q to find the time evolution of the probability distribution function of the particle,
| (6) |
where is the state vector of the CTMC and is the initial particle distribution.
Figure 3.
Site-site transition time and particle distribution in the domain. (a) Formulating the behavior of a particle (protein) that interacts with the polymer (chromatin), inside a domain (nucleus) as a continuous-time Markov chain. We illustrate it for the case of two binding monomer sites. The particle can be bound at site 1 or 2 and is released with Poissonian dissociation rates and. While diffusing to bind to a site, it is “in transit” and arrives at its destination with probability 1 and rate . (b and c) The monomer is bound at a site for a characteristic time The probability, p, of the particle being in a bound state at the monomer site after 1T (b) or 10T (c) is computed by solving numerically Eq. 6. The monomer starts at a distance a from either monomer 2, 50, or 99. and were estimated from Brownian simulation: , , , and (orange) or (purple). For , there are 100 bound states and in-transit states. To see this figure in color, go online.
We performed BS and estimated numerically the and values, taking (); we estimated starting from site n using Eq. 6. When the reattachment probability is high, the particle remains in proximity to its initial site for a long time (Fig. 3, b and c) compared with T. Thus, the equilibration time of the protein in the domain is much longer than its disassociation rate from a site (). When the reattachment probability is smaller, the particle diffuses farther from its initial site (Fig. 3 c). Interestingly, the residence probability at the original site is not uniform along the chain. Since the middle monomer resides where monomer density is highest, its reattachment probability, , is minimal (Eq. 2).
Using the long-time behavior of , we estimated fraction f of bound particles. For high reattachment probability, , whereas the rest of the probability is in the unbound (in-transit) states. For smaller reattachment probability, . In the Supporting Material, we plotted f for different values of ϵ (see Fig. S3). f estimated using Eq. 6 corresponds to the bound fraction estimated directly with BS.
A naive estimate for the bound fraction, which does not take into account reattachment, can be found through the ratio of the off rate, , and on rate starting from the bulk ( (38)): . We found that the bulk estimate greatly underestimates the bound fraction found from the BS or using the CTMC formalism (see Fig. S3). Thus, starting at the boundary layer of the initial monomer site can impact significantly the fraction of bound proteins. This may be the origin of the observed non-uniform distribution (39) and protein clusters (40) in the nucleus.
To conclude, there is a finite probability, after each release, that the protein diffuses away to another remote site, given that there is a trajectory between them. Thus, the process is ergodic. When the ϵ-neighborhoods of many traps overlap around the released site, such that the particle cannot find a path out, it will be quenched in this area.
Our findings can explain the long residence-time distributions that are observed experimentally (19) without the need for a power-law waiting-time distribution as assumed in a continuous-time random-walk model. As we have shown, escaping a binding site involves several dissociation and association events, with different characteristic rates. Hence, the localization time distribution may not appear to have exponential distribution in experiments.
Since the three-dimensional organization of chromatin guides search of TFs through transient interactions, the number of proteins and their interaction strength is not sufficient to understand their collective behavior. To fully model protein behavior at chromatin loci, one has to study the nature of the local interactions around the site of interest. Based on our model, we can extract directly from microscopy data the interaction parameters of proteins at specific chromatin domains. Thus, we can understand how different proteins “see” chromatin differently.
Author Contributions
A.A. performed the research and wrote the article.
Acknowledgments
The author thanks M. Kardar, D. Holcman, J. Reingruber, and A. K. Chakraborty for their helpful discussions and comments.
I acknowledge financial support from Massachusetts General Hospital (internal fund 214931).
Editor: Andrew Spakowitz.
Footnotes
Supporting Materials and Methods and three figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(18)30057-2.
Supporting Material
References
- 1.Halford S.E., Marko J.F. How do site-specific DNA-binding proteins find their targets? Nucleic Acids Res. 2004;32:3040–3052. doi: 10.1093/nar/gkh624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Misteli T. Protein dynamics: implications for nuclear architecture and gene expression. Science. 2001;291:843–847. doi: 10.1126/science.291.5505.843. [DOI] [PubMed] [Google Scholar]
- 3.Hansen A.S., Pustova I., Darzacq X. CTCF and cohesin regulate chromatin loop stability with distinct dynamics. eLife. 2017;6:e25776. doi: 10.7554/eLife.25776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ptashne M. Gene regulation by proteins acting nearby and at a distance. Nature. 1986;322:697–701. doi: 10.1038/322697a0. [DOI] [PubMed] [Google Scholar]
- 5.Berg O.G., Winter R.B., von Hippel P.H. Diffusion-driven mechanisms of protein translocation on nucleic acids. 1. Models and theory. Biochemistry. 1981;20:6929–6948. doi: 10.1021/bi00527a028. [DOI] [PubMed] [Google Scholar]
- 6.Hammar P., Leroy P., Elf J. The lac repressor displays facilitated diffusion in living cells. Science. 2012;336:1595–1598. doi: 10.1126/science.1221648. [DOI] [PubMed] [Google Scholar]
- 7.Elf J., Li G.-W., Xie X.S. Probing transcription factor dynamics at the single-molecule level in a living cell. Science. 2007;316:1191–1194. doi: 10.1126/science.1141967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slutsky M., Kardar M., Mirny L.A. Diffusion in correlated random potentials, with applications to DNA. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2004;69:061903. doi: 10.1103/PhysRevE.69.061903. [DOI] [PubMed] [Google Scholar]
- 9.Hu T., Grosberg A.Y., Shklovskii B.I. How proteins search for their specific sites on DNA: the role of DNA conformation. Biophys. J. 2006;90:2731–2744. doi: 10.1529/biophysj.105.078162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li G.-W., Berg O.G., Elf J. Effects of macromolecular crowding and DNA looping on gene regulation kinetics. Nat. Phys. 2009;5:294–297. [Google Scholar]
- 11.Bauer M., Metzler R. Generalized facilitated diffusion model for DNA-binding proteins with search and recognition states. Biophys. J. 2012;102:2321–2330. doi: 10.1016/j.bpj.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cartailler J., Reingruber J. Facilitated diffusion framework for transcription factor search with conformational changes. Phys. Biol. 2015;12:046012. doi: 10.1088/1478-3975/12/4/046012. [DOI] [PubMed] [Google Scholar]
- 13.Bénichou O., Kafri Y., Voituriez R. Searching fast for a target on DNA without falling to traps. Phys. Rev. Lett. 2009;103:138102. doi: 10.1103/PhysRevLett.103.138102. [DOI] [PubMed] [Google Scholar]
- 14.Lomholt M.A., van den Broek B., Metzler R. Facilitated diffusion with DNA coiling. Proc. Natl. Acad. Sci. USA. 2009;106:8204–8208. doi: 10.1073/pnas.0903293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koslover E.F., Díaz de la Rosa M.A., Spakowitz A.J. Theoretical and computational modeling of target-site search kinetics in vitro and in vivo. Biophys. J. 2011;101:856–865. doi: 10.1016/j.bpj.2011.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolesov G., Wunderlich Z., Mirny L.A. How gene order is influenced by the biophysics of transcription regulation. Proc. Natl. Acad. Sci. USA. 2007;104:13948–13953. doi: 10.1073/pnas.0700672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godec A., Metzler R. Universal proximity effect in target search kinetics in the few-encounter limit. Phys. Rev. X. 2016;6:041037. [Google Scholar]
- 18.Normanno D., Boudarène L., Dahan M. Probing the target search of DNA-binding proteins in mammalian cells using TetR as model searcher. Nat. Commun. 2015;6:7357. doi: 10.1038/ncomms8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caccianini L., Normanno D., Dahan M. Single molecule study of non-specific binding kinetics of LacI in mammalian cells. Faraday Discuss. 2015;184:393–400. doi: 10.1039/c5fd00112a. [DOI] [PubMed] [Google Scholar]
- 20.Doi M., Edwards S.F. Oxford Clarendon Press; Oxford, United Kingdom: 1986. The Theory of Polymer Dynamics. [Google Scholar]
- 21.Amitai A., Seeber A., Holcman D. Visualization of chromatin decompaction and break site extrusion as predicted by statistical polymer modeling of single-locus trajectories. Cell Rep. 2017;18:1200–1214. doi: 10.1016/j.celrep.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Pulkkinen O., Metzler R. Distance matters: the impact of gene proximity in bacterial gene regulation. Phys. Rev. Lett. 2013;110:198101. doi: 10.1103/PhysRevLett.110.198101. [DOI] [PubMed] [Google Scholar]
- 23.Cheviakov A.F., Ward M.J. Optimizing the principal eigenvalue of the Laplacian in a sphere with interior traps. Math. Comput. Model. 2011;53:1394–1409. [Google Scholar]
- 24.Robinett C.C., Straight A., Belmont A.S. In vivo localization of DNA sequences and visualization of large-scale chromatin organization using lac operator/repressor recognition. J. Cell Biol. 1996;135:1685–1700. doi: 10.1083/jcb.135.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izeddin I., Récamier V., Darzacq X. Single-molecule tracking in live cells reveals distinct target-search strategies of transcription factors in the nucleus. eLife. 2014;3:e02230. doi: 10.7554/eLife.02230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slutsky M., Mirny L.A. Kinetics of protein-DNA interaction: facilitated target location in sequence-dependent potential. Biophys. J. 2004;87:4021–4035. doi: 10.1529/biophysj.104.050765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reingruber J., Holcman D. Transcription factor search for a DNA promoter in a three-state model. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2011;84:020901. doi: 10.1103/PhysRevE.84.020901. [DOI] [PubMed] [Google Scholar]
- 28.Sheinman M., Kafri Y. The effects of intersegmental transfers on target location by proteins. Phys. Biol. 2009;6:016003. doi: 10.1088/1478-3975/6/1/016003. [DOI] [PubMed] [Google Scholar]
- 29.Bénichou O., Chevalier C., Voituriez R. Facilitated diffusion of proteins on chromatin. Phys. Rev. Lett. 2011;106:038102. doi: 10.1103/PhysRevLett.106.038102. [DOI] [PubMed] [Google Scholar]
- 30.Lomholt M.A., Ambjörnsson T., Metzler R. Optimal target search on a fast-folding polymer chain with volume exchange. Phys. Rev. Lett. 2005;95:260603. doi: 10.1103/PhysRevLett.95.260603. [DOI] [PubMed] [Google Scholar]
- 31.Shvets A.A., Kolomeisky A.B. The role of DNA looping in the search for specific targets on DNA by multisite proteins. J. Phys. Chem. Lett. 2016;7:5022–5027. doi: 10.1021/acs.jpclett.6b02371. [DOI] [PubMed] [Google Scholar]
- 32.Mirny L., Slutsky M., Kosmrlj A. How a protein searches for its site on DNA: the mechanism of facilitated diffusion. J. Phys. A Math. Theor. 2009;42:434013. [Google Scholar]
- 33.Amitai A., Holcman D. Polymer physics of nuclear organization and function. Phys. Rep. 2017;678:1–83. [Google Scholar]
- 34.Lennard-Jones J.E. On the determination of molecular fields: II: from the equation of state of a gas. Proc. R. Soc. Lond. A. 1924;106:463–477. [Google Scholar]
- 35.de Gennes P.G. Cornell University Press; Ithaca, NY: 1979. Scaling Concepts in Polymer Physics. [Google Scholar]
- 36.Karlin S., Taylor H. Academic Press; Cambridge, MA: 1981. A Second Course in Stochastic Processes. [Google Scholar]
- 37.Woodcock C.L., Ghosh R.P. Chromatin higher-order structure and dynamics. Cold Spring Harb. Perspect. Biol. 2010;2:a000596. doi: 10.1101/cshperspect.a000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuss Z. Springer-Verlag; New York, NY: 2009. Diffusion and Stochastic Processes. An Analytical Approach. [Google Scholar]
- 39.Knight S.C., Xie L., Tjian R. Dynamics of CRISPR-Cas9 genome interrogation in living cells. Science. 2015;350:823–826. doi: 10.1126/science.aac6572. [DOI] [PubMed] [Google Scholar]
- 40.Cisse I.I., Izeddin I., Darzacq X. Real-time dynamics of RNA polymerase II clustering in live human cells. Science. 2013;341:664–667. doi: 10.1126/science.1239053. [DOI] [PubMed] [Google Scholar]
- 41.Amitai A., Holcman D. Diffusing polymers in confined microdomains and estimation of chromosomal territory sizes from chromosome capture data. Phys. Rev. Lett. 2013;110:248105. doi: 10.1103/PhysRevLett.110.248105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.