MacDougall et al. review the structure and function of the calcium sensor synaptotagmin-7 in exocytosis.
Abstract
Synaptotagmin (Syt) proteins comprise a 17-member family, many of which trigger exocytosis in response to calcium. Historically, most studies have focused on the isoform Syt-1, which serves as the primary calcium sensor in synchronous neurotransmitter release. Recently, Syt-7 has become a topic of broad interest because of its extreme calcium sensitivity and diversity of roles in a wide range of cell types. Here, we review the known and emerging roles of Syt-7 in various contexts and stress the importance of its actions. Unique functions of Syt-7 are discussed in light of recent imaging, electrophysiological, and computational studies. Particular emphasis is placed on Syt-7–dependent regulation of synaptic transmission and neuroendocrine cell secretion. Finally, based on biochemical and structural data, we propose a mechanism to link Syt-7’s role in membrane fusion with its role in subsequent fusion pore expansion via strong calcium-dependent phospholipid binding.
Introduction
Controlled release of neurotransmitters and hormones from secretory cells occurs through the process of exocytosis. Exocytosis is triggered by a rise in intracellular Ca2+ (Douglas and Rubin, 1961; Katz and Miledi, 1965; Augustine and Neher, 1992), with members of the synaptotagmin (Syt) protein family acting as the Ca2+ sensors that trigger vesicle-to-plasma-membrane fusion (Brose et al., 1992). Synaptotagmins interact with soluble N-ethyl maleimide–sensitive factor attachment protein receptor (SNARE) proteins, which constitute the core membrane fusion machinery, as well as anionic phospholipid membranes, as part of the mechanism used to couple Ca2+ binding to opening of the fusion pore (Söllner et al., 1993b; Chapman, 2008; Südhof and Rothman, 2009; Südhof, 2013). There are 17 different isoforms of synaptotagmin present in mammals, 8 of which trigger membrane fusion in response to Ca2+ over a range of affinities and with different activation kinetics (Bhalla et al., 2008; Gustavsson and Han, 2009; Craxton, 2010; Moghadam and Jackson, 2013). A growing number of studies are revealing how different synaptotagmin isoforms exert characteristic effects on the rate and extent of exocytosis, and how synaptotagmin diversity may permit multilayered modulation of content release through isoform-specific effects on fusion dynamics (Schonn et al., 2008; Zhang et al., 2011; Rao et al., 2014, 2017). This review focuses on Syt-7, a high-affinity Ca2+ sensor that has been implicated in regulating exocytosis across a wide range of cellular and physiological contexts.
The synaptotagmins share a common molecular architecture comprising an N-terminal transmembrane domain that anchors the protein to lipid bilayers, a linker region, and tandem cytoplasmic Ca2+ binding domains, termed C2A and C2B (Südhof and Rizo, 1996). Synaptotagmin isoforms possess a range of Ca2+ affinities, with some exhibiting no detectable Ca2+ binding (von Poser et al., 1997; Bhalla et al., 2005; Hui et al., 2005; Wang et al., 2005). The C2A and C2B domains interact with multiple effectors in Ca2+-independent and -dependent ways. Notable interaction partners include the t-SNAREs, syntaxin and SNAP-23/25, on the plasma membrane (Rao et al., 2004; Bhalla et al., 2006; Zhang et al., 2010; Weber et al., 2014; Zhou et al., 2017). Additionally, the C2 domains of synaptotagmins bind membranes containing anionic phospholipids such as phosphatidylserine (PS) and phosphatidylinositol-(4,5)-bisphosphate (Davletov and Südhof, 1993; Chapman and Jahn, 1994; Schiavo et al., 1996; Lynch et al., 2007, 2008; Stein et al., 2007; Wan et al., 2011; Park et al., 2012; van den Bogaart et al., 2012). Binding of Ca2+ to the C2 domains prompts their insertion into lipid bilayers. The interaction of synaptotagmin, Ca2+, and anionic phospholipids is thought to play a key role in stimulus-secretion coupling (Sutton et al., 1995; Bai et al., 2004a; Hui et al., 2006). Among the known synaptotagmin isoforms, Syt-7 has the highest Ca2+ sensitivity, capable of binding anionic phospholipids and stimulating membrane fusion in vitro at Ca2+ concentrations <1 µM (Sugita et al., 2002; Bhalla et al., 2005).
Studies of exocytosis using optical imaging techniques and electrophysiological measurements have provided many examples of how different synaptotagmin isoforms confer distinct effects on exocytosis dynamics by affecting the probability, extent, and rate of content release, as well as determining the choice among alternate fusion modes (Zhang et al., 2011; Rao et al., 2014, 2017). The large variety of synaptotagmin isoforms present in mammalian cells, combined with coexpression of multiple isoforms within the same cell, suggests the potential for fine tuning of secretory outputs in response to different stimuli or physiological circumstances.
Historically, the most widely studied synaptotagmin isoform has been Syt-1, which triggers fast, synchronous neurotransmitter release in neurons and neuroendocrine cells (Geppert et al., 1994). Although the intent of this review is to focus on the properties and activities of Syt-7, reference will occasionally be made to mechanistic studies performed using Syt-1, to the extent that they highlight features that can reasonably be assumed to be shared among synaptotagmin family members. Additionally, it will often prove instructive to compare and contrast Syt-1 and Syt-7 activities to draw attention to what makes Syt-7 stand apart. In many cases, the functions attributed to Syt-7 occur in cells coexpressing Syt-1 (or the closely related Syt-2 or Syt-9 isoforms), and indeed, it is often the juxtaposition of their competing activities that gives rise to the observed effects (Wen et al., 2010; Bacaj et al., 2013; Rao et al., 2014; Luo and Sudhof, 2017). Therefore, an appreciation of the differences between Syt-7 and other isoforms—especially with respect to biochemistry and structure—is necessary for understanding how regulatory functions attributed to Syt-7 become manifest. Syt-7, by dint of the high Ca2+-dependent phospholipid affinity of its C2 domains, is well suited for processes operating at low intracellular Ca2+. As is discussed in this review, such properties provide a mechanistic framework for understanding Syt-7’s actions at synapses, in neuroendocrine tissue, and within a variety of other cellular contexts.
Diverse cellular functions of Syt-7
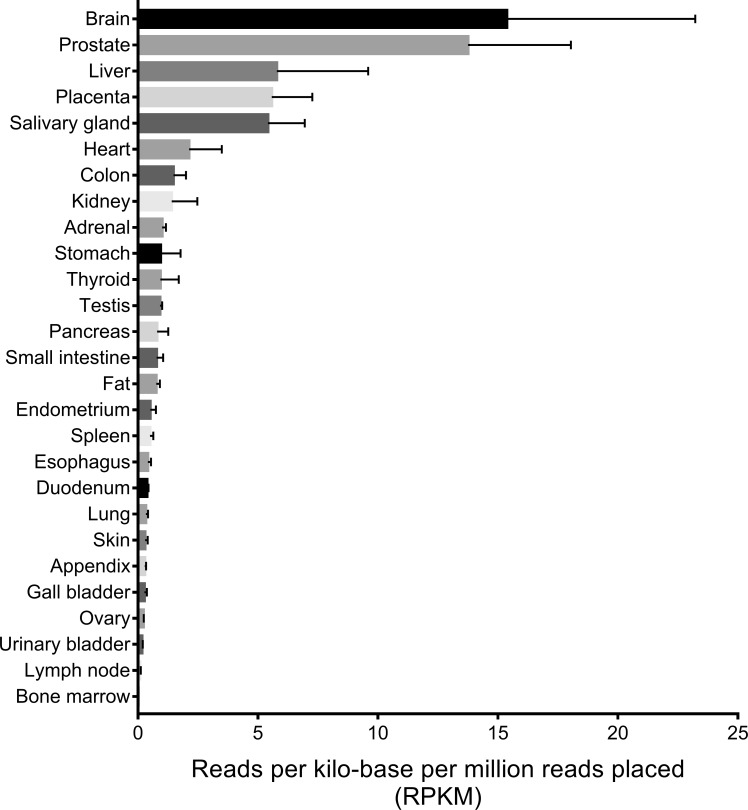
Syt-7 has been shown to act as the Ca2+ sensor for regulated exocytosis in a variety of cellular contexts (Table 1). Expression of Syt-7 is widespread in mammals (Fig. 1). Despite its ubiquitous expression, Syt-7 knockout (KO) mice are viable into adulthood (Martinez et al., 2000). Thus, it is very likely that another isoform compensates for Syt-7’s essential functions.
Table 1. Syt-7 expression and function in various mammalian cells.
| Cell type | Function | Reference |
|---|---|---|
| Acinar cells, pancreas | Not characterized | Falkowski et al., 2011 |
| Acinar cells, parotid | Not characterized | Jo et al., 2006 |
| Adipocytes | Glucose uptake | Li et al., 2007 |
| Alveolar type II cells, lung | Lamellar body exocytosis | Neuland et al., 2014 |
| Cardiac sympathetic nerve terminals | Neuroepinephrine secretion | Shih et al., 2016 |
| Chromaffin/PC12 cells | Stress hormone secretion | Fukuda et al., 2004; Schonn et al., 2008; Zhang et al., 2011; Rao et al., 2014 |
| Fibroblasts | Lysosomal exocytosis and membrane repair | Martinez et al., 2000; Reddy et al., 2001; Chakrabarti et al., 2003 |
| Melanotrophs, intermediate pituitary | Not characterized | Kreft et al., 2003 |
| Macrophages | Phagocytosis | Becker et al., 2009 |
| Migration | Peng et al., 2016 | |
| Neurons | Asynchronous release (although also see Yao et al., 2011; Xue et al., 2015 for role of Doc2) | Bacaj et al., 2013; Weber et al., 2014; Luo et al., 2015; Turecek and Regehr, 2018 |
| Synaptic facilitation | Chen et al., 2017; Jackman and Regehr, 2017; Turecek and Regehr, 2018 | |
| Osteoclasts and osteoblasts | Bone remodeling | Zhao et al., 2008 |
| α Cells, pancreas | Glucagon secretion | Gustavsson et al., 2009 |
| β Cells, pancreas | Insulin secretion | Li et al., 2007; Gustavsson et al., 2008; Dolai et al., 2016 |
| T-cell lymphocyte | Cytolytic T cell responses | Fowler et al., 2007 |
Figure 1.
Syt-7 RNA-seq results from 27 human tissues. Quantitative transcriptomic analysis (RNA-seq) of Syt-7 was performed using NCBI (https://www.ncbi.nlm.nih.gov/gene/9066/?report=expression) with data obtained from Fagerberg et al. (2014), BioProject PRJEB4337, and BioProject PRJEB4337. The relative expression levels are shown in reads per kilobase per million reads placed (RPKM). RPKM is a normalized unit of specific gene transcription. Genes in different tissues were screened and scored based on total RNA level. The scores are normalized to 1 million reads (per million reads placed) to correct for the transcription activity differences in various tissues. The “per million reads placed” scores are divided by the length of the gene (per kilobase). Error bars represent SD.
One of the first characterized cellular functions for Syt-7 was in Ca2+-dependent lysosomal exocytosis (Martinez et al., 2000). During this process, lysosomes fuse with the plasma membrane, providing a means to repair tears or breaks in the membrane after damage (Martinez et al., 2000; Reddy et al., 2001). Lysosomal exocytosis, mediated by Syt-7, is also responsible for supplying excess membrane and releasing bone-degrading molecules at the ruffled border of osteoclasts, which resorb old bone during healing and repair (Zhao et al., 2008). Bone maintenance, remodeling, and repair further depend on secretion of bone matrix proteins from osteoblasts, a process that is mediated by Syt-7 present on secretory vesicles. These functions are compromised in Syt-7 KO mice, which have reduced bone volume and are susceptible to osteoporosis (Zhao et al., 2008). Syt-7–dependent delivery of excess membrane to the cell surface through lysosome exocytosis also figures prominently in neurite outgrowth (Arantes and Andrews, 2006) and the ability of macrophages to engulf and take up foreign particles for phagocytic degradation (Czibener et al., 2006; Becker et al., 2009).
A key role has also been identified for Syt-7 in regulating Ca2+-dependent exocytotic events that contribute to maintenance of glucose homeostasis. Syt-7 positively regulates Ca2+-stimulated secretion of insulin from large dense-core vesicles in pancreatic β cells (Gustavsson et al., 2008) and Ca2+-stimulated release of glucagon from vesicles in pancreatic α-cells (Gustavsson et al., 2009). In adipocytes and skeletal muscle cells, Syt-7 promotes incorporation of the GLUT4 glucose transporter into the plasma membrane (Li et al., 2007). Disruption of these processes in Syt-7 KO mice results in glucose intolerance (Li et al., 2007; Gustavsson et al., 2008). A decrease in glucose-stimulated insulin secretion was also observed after knockdown of Syt-7 in human pancreatic β cells (Dolai et al., 2016).
Ca2+-triggered secretion of vesicle contents depends on Syt-7 in other specialized settings, including release of norepinephrine from cardiac sympathetic nerve terminals (Shih et al., 2016) and release of lytic enzymes from T-cell lymphocytes that destroy target cells (Fowler et al., 2007). The fact that Syt-7 regulates exocytosis in all of these different contexts may indicate a requirement for exocytosis to be activated efficiently by small increases in intracellular Ca2+, for which Syt-7 is particularly well suited given its high Ca2+ sensitivity (Sugita et al., 2002). Although Syt-7 was identified as a key player in controlling the Ca2+-dependent membrane fusion events described above, it may function jointly with other isoforms in mediating exocytosis in these instances. For example, insulin secretion is decreased by only ∼40% in Syt-7 KO mice, suggesting that other Ca2+ sensors—such as Syt-9, which is expressed at comparable levels in pancreatic islets—are likely involved (Gustavsson et al., 2008; Gustavsson and Han, 2009). Syt-7 was identified as the principal Ca2+ sensor behind nearly all glucagon secretion from α-cells, but this appears to be the exception rather than the rule (Gustavsson et al., 2009).
In several cell types, the interplay between the activities of Syt-7 and other synaptotagmin isoforms has been studied to some extent. Syt-7 is highly and ubiquitously expressed in the brain (Fig. 1), where it is commonly found with one or more of the lower-affinity isoforms Syt-1, 2, and/or 9 (Li et al., 1995, 2017; Ullrich and Südhof, 1995; Wen et al., 2010; Bacaj et al., 2013, 2015; Moghadam and Jackson, 2013; Luo et al., 2015). The juxtaposition of these isoforms with Syt-7 may allow for modulation of the secretory response as a function of spatial and temporal variations in the Ca2+ signal and can have interesting physiological consequences (Wen et al., 2010; Bacaj et al., 2013, 2015; Luo et al., 2015; Li et al., 2017; Luo and Sudhof, 2017). It has been hypothesized that combination of synaptotagmins with different Ca2+ sensitivities could afford a range of possibilities for fusion output, based on differential activation of synaptotagmin isoforms according to the strengths or patterns of stimulation in excitable cells (Moghadam and Jackson, 2013).
Syt-7, via interactions with effector proteins, could also regulate exocytosis upstream of the fusion event. Loss of Syt-7 activity in KO cells causes a reduction of sustained release during stimulus trains, which was suggested to result from a role of Syt-7 in replenishment of the readily releasable vesicle pool (Liu et al., 2014). The effect of Syt-7 removal was recapitulated by loss of calmodulin, and Syt-7 was found to associate tightly with calmodulin in a manner that depended on Ca2+ binding to its C2 domains. These data suggest that the Syt-7–calmodulin complex plays a fundamental role in regulating vesicle replenishment at synapses (Liu et al., 2014).
Syt-1 and Syt-7 are the major synaptotagmin isoforms present in adrenal chromaffin cells (Maximov et al., 2008; Schonn et al., 2008; Matsuoka et al., 2011). Chromaffin cells secrete a cocktail of bioactive agents into the bloodstream in response to sympathetic activation (Carmichael and Winkler, 1985). Strong stimulation of secretion from chromaffin cells has revealed rapid and delayed exocytotic components (Heinemann et al., 1993, 1994; Chow et al., 1994), with the initial fast phase corresponding to release from the readily releasable pool (RRP) and a delayed release component from the slowly releasable pool (SRP) of vesicles (Sørensen, 2004; Schonn et al., 2008). Membrane capacitance measurements combined with Ca2+ uncaging in Syt-1 KO mice showed that the rapid phase of release is eliminated in the absence of Syt-1 (Voets et al., 2001; Schonn et al., 2008). The remaining delayed component exhibits a Ca2+ threshold that closely mirrors the low, micromolar-range sensitivity of Syt-7 for binding to phospholipids in the presence of Ca2+ (Sugita et al., 2002). Furthermore, deletion of Syt-7 in a Syt-1 KO background almost completely eliminates the slow phase of release (Schonn et al., 2008). Syt-7 has been hypothesized to affect exocytosis kinetics either by interacting with and modulating the release rate of Syt-1–containing vesicles (Schonn et al., 2008; Weber et al., 2014) or by triggering fusion of a subpopulation of predominantly Syt-7–containing vesicles (Rao et al., 2014). In either case, the combination of Syt-1 and Syt-7 could permit fine-tuning of secretory outputs. For example, the ability of Syt-7 to promote fusion at much lower Ca2+ concentrations may provide the basis for constitutive release of neurotransmitters in response to low-level, basal stimulation (Fulop et al., 2005).
Intracellular localization of Syt-7
The fact that Syt-7 regulates exocytosis in such a wide array of cellular contexts raises the question of whether it imparts control over fusion through a common mechanism in all cases, or whether different functional modes are exploited in different circumstances. One piece of information that seems necessary for answering this question is the subcellular localization of Syt-7. In neurons, based on immunostaining of endogenous Syt-7 combined with light and electron microscopy, Syt-7 was found close to presynaptic active zones but did not appear to overlap with clusters of vesicles (Sugita et al., 2001). Thus, it was concluded that Syt-7 is bound to the plasma membrane, an interpretation further supported by subcellular fractionation measurements (Sugita et al., 2001). Other studies have subsequently reported a plasma membrane localization of Syt-7 in neurons (Virmani et al., 2003; Weber et al., 2014; Jackman et al., 2016).
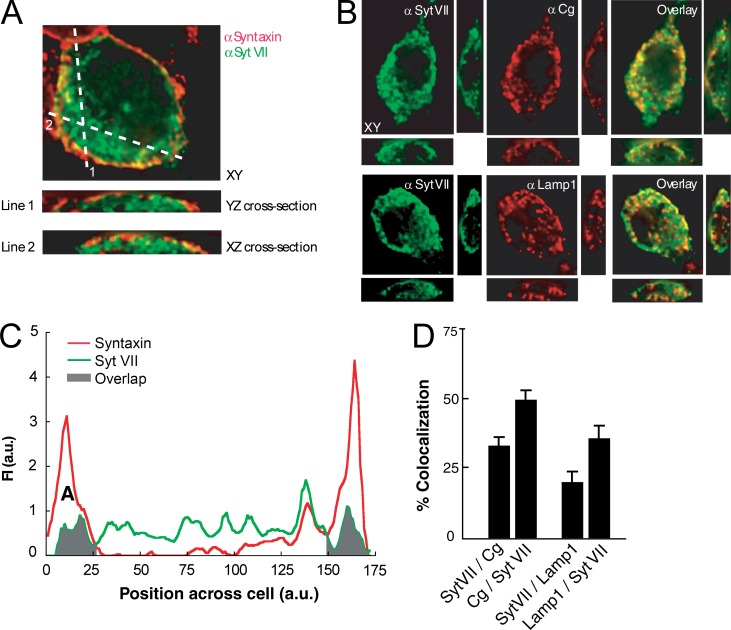
These results stand in sharp contrast with studies indicating that Syt-7 is targeted primarily to internal organelles, including large dense-core vesicles in both primary and immortalized neuroendocrine cells (Fukuda et al., 2002b, 2004; Wang et al., 2005; Tsuboi and Fukuda, 2007; Zhang et al., 2011; Rao et al., 2014, 2017; Dolai et al., 2016) and lysosomes in nonneuronal cells (Martinez et al., 2000; Reddy et al., 2001). One such study performed quantitative immunostaining of tag-free, up-regulated Syt-7 in PC12 cells and found significant colocalization of Syt-7 with vesicle and lysosome markers but minimal overlap with the plasma membrane marker syntaxin-1a (Wang et al., 2005; Fig. 2). Similarly, numerous studies have localized GFP-tagged Syt-7 to the surface of large dense-core vesicles in PC12 cells and chromaffin cells (Fukuda et al., 2004; Zhang et al., 2011; Rao et al., 2014, 2017; Bendahmane et al., 2018). Thus, there is considerable evidence that in neuroendocrine cells Syt-7 does not act as a plasma membrane Ca2+ sensor but instead regulates exocytosis directly from the vesicle surface, perhaps acting similarly to Syt-1 but with a different Ca2+ sensitivity.
Figure 2.
Syt-7 is predominantly localized on large dense-core vesicles and lysosomes in PC12 cells. (A) Syt-7–expressing (green) PC12 cells show low colocalization with a plasma membrane marker, syntaxin 1A (red). (B) Representative immunofluorescence images of Syt-7 and the vesicular protein, chromogranin B (Cg, first row), or the lysosomal protein, Lamp1 (second row). (C) Graphical representation of A. (D) Graphical representation of B, demonstrating significant colocalization of Syt-7 with organelle markers. Adapted with permission from Wang et al. (2005). Error bars are ±SEM.
Wang et al. (2005) found a significant degree of overlap between Syt-7 and Syt-1 in PC12 cells, suggesting that multiple synaptotagmin isoforms can reside on the same secretory vesicle. The divalent metal dependence of catecholamine release in these cells was sensitive to Syt-7 expression level; dose–response curves shifted to lower concentrations when Syt-7 was overexpressed and to higher concentrations when Syt-7 expression was knocked down with RNAi. This correlation suggests that the ratio of Syt-7 to Syt-1 on each vesicle dictates the metal sensitivity of release (Wang et al., 2005). Other studies using fluorophore-tagged synaptotagmin constructs found that Syt-1 and Syt-7 were largely sorted to separate vesicle populations, indicating that different vesicle pools may be endowed with distinct fusion properties via selective isoform sorting (Rao et al., 2014). Colocalization studies of Syt-1, 4, 7, and 9 in PC12 and primary rat chromaffin cells suggested that vesicles can harbor multiple synaptotagmin isoforms (Matsuoka et al., 2011). In PC12s, Syt-1 and Syt-7 have lower colocalization (∼45%) than other isoform pairs. Partial spatial segregation of Syt-1 and Syt-7 could be achieved through differential sorting based on vesicle size, with Syt-1 and Syt-7 showing preference for smaller and larger vesicles, respectively (Zhang et al., 2011). This size preference was also noted in primary rat chromaffin cells (Matsuoka et al., 2011).
The presence of Syt-7 on the plasma membrane versus secretory vesicles, and the degree of cosorting with other synaptotagmin isoforms, could be influenced by a variety of factors. Intracellular trafficking could be affected by experimental variables such as fluorescent protein tags and their placement and/or higher-than-normal protein expression levels causing spillover to nonphysiological sites. Although we have consistently observed overexpressed Syt-7 to have a predominantly vesicular localization (Rao et al., 2014), the relative abundance of protein on the plasma membrane of bovine chromaffin cells is variable from cell preparation to preparation. The balance of its localization may be affected by overexpression, which in turn, has the potential to alter the balance of anterograde and retrograde trafficking reactions within a cell. It is also possible that physiologically relevant differences in Syt-7 trafficking occur according to cell type or context that allow targeting of Syt-7 activity to a specific subcellular location or regulation of its mode of action.
Posttranslational protein modifications may affect the intracellular trafficking of synaptotagmins (Chapman et al., 1996; Veit et al., 1996; Fukuda, 2002; Heindel et al., 2003; Han et al., 2004; Kang et al., 2004; Kanno and Fukuda, 2008; Flannery et al., 2010; Kwon and Chapman, 2012). In one study, N-glycosylation was shown to play a critical role in proper targeting of Syt-1 to vesicles in both neurons and PC12 cells (Han et al., 2004). A point mutation (N24Q) that abolished N-glycosylation altered Syt-1 localization from PC12 secretory vesicles to the plasma membrane. Surprisingly, in Syt-1 KO neurons expressing the glycosylation mutant, synchronous release was restored despite the protein being missorted (Han et al., 2004). A different study showed that vesicular sorting of Syt-1 N- and O-glycosylation mutants was disrupted only when proteins were expressed in a wild-type rather than Syt-1 KO background (Kwon and Chapman, 2012). Thus, glycosylation’s role in proper Syt-1 sorting, and thereby synaptic function, may be context dependent.
Syt-7 also undergoes several posttranslational modifications in the cell. Three cysteine residues located within and adjacent to the Syt-7 transmembrane domain are palmitoylated, and palmitoylation was shown to be necessary for targeting of Syt-7 to lysosomes (Flannery et al., 2010). Potentiation of insulin secretion requires phosphorylation of Syt-7 at Ser103 by protein kinase A, and Syt-7 is likely to be phosphorylated at other sites as well (Wu et al., 2015). Whether posttranslational modifications could account for some of the apparent discrepancies in the literature with respect to Syt-7 localization remains to be determined.
Context-dependent effects of Syt-7 could also be related to alternative splicing. The standard form of Syt-7 is 403 amino acids long, with a molecular weight of ∼45 kD (Gustavsson and Han, 2009). However, multiple Syt-7 protein variants resulting from alternative splicing of the Syt-7 gene primary transcript have been identified. These variants exhibit temporally and spatially regulated patterns of expression during development (Sugita et al., 2001). In rat brain, at least 12 variants have been identified that range in size from 122 to 687 amino acids; some are truncated versions missing the C2 domains, whereas others harbor extended linker regions (Sugita et al., 2001). Truncated and extended isoforms were found to have competing effects on synaptic vesicle recycling in rat hippocampal neurons, in that recycling was enhanced by a truncated isoform and decelerated by an extended-linker isoform (Virmani et al., 2003). In mouse brain, three isoforms have been identified: the dominant canonical isoform, Syt-7α, and two alternate isoforms, Syt-7β and Syt-7γ, whose spacer regions contain insertion sequences of 44 and 116 amino acids, respectively (Fukuda et al., 2002b). Similarly, pancreatic β-cells have been found to express the α, β, and δ isoforms of Syt-7; the δ isoform is a truncated version that localized to a different vesicle population and inhibited insulin secretion upon overexpression in INS-1E cells (Gauthier et al., 2008). The functional differences among isoforms with different linker lengths are not yet well understood.
The role of Syt-7 in synaptic transmission
Although Syt-7 is expressed in nonneural tissues, it is particularly enriched in the brain (Li et al., 1995). Syt-7’s prominent neuronal expression and high Ca2+ affinity immediately led to suggestions that in neurons Syt-7 could serve as a specialized high-affinity sensor to support two forms of presynaptic plasticity that are driven by submicromolar Ca2+ signals: asynchronous release and facilitation (Zucker, 1996; Zucker and Regehr, 2002; Hui et al., 2005).
Asynchronous release refers to the delayed, slow release of neurotransmitter that persists after the end of a single action potential or a train of action potentials. The term originates from early descriptions of evoked release at the frog neuromuscular junction (NMJ; Barrett and Stevens, 1972; Goda and Stevens, 1994). These classic electrophysiological studies demonstrated the possibility of separable Ca2+-dependent release mechanisms based on the fact that exocytosis followed multiple, kinetically distinguishable rates (Goda and Stevens, 1994). Facilitation involves the transient elevation of release probability after an action potential, which can drastically increase the amount of neurotransmitter released if a second action potential arrives shortly after the first (Jackman and Regehr, 2017). Because facilitation and asynchronous release both rely on the same submicromolar Ca2+ signal, reflect a transient increase in release probability, and exhibit similar decay kinetics, it was suggested early on that they might use the same mechanism (Rahamimoff and Yaari, 1973).
Action potentials cause brief, large Ca2+ signals in the immediate vicinity of open voltage-gated Ca2+ channels (Llinás et al., 1992; Atluri and Regehr, 1996; Zenisek et al., 2003). These “nanodomains” of Ca2+ are thought to reach tens or hundreds of micromolar (Simon and Llinás, 1985), which would activate low-affinity Ca2+ sensors such as Syt-1 and Syt-2 to drive synchronous vesicle fusion and neurotransmitter release. Nanodomains quickly collapse at the end of an action potential when voltage-gated Ca2+ channels close, after which a modest amount of residual free Ca2+ persists in the presynaptic terminal before being buffered by endogenous Ca2+-binding proteins, sequestered into intracellular organelles, or extruded (Cooper et al., 1996; Zucker and Regehr, 2002). It is in the context of this “residual Ca2+ hypothesis” that the role of Syt-7 might be best understood, where it acts as a high-affinity sensor to detect modest Ca2+ signals and increase vesicle release probability (Katz and Miledi, 1968; Jackman and Regehr, 2017).
Early studies
Early studies of neuronal synapses detected no discernible effect of Syt-7 on synaptic transmission. At the Drosophila melanogaster larval NMJ, knockdown of Syt-7 caused no change in either the initial release probability or short-term plasticity (Saraswati et al., 2007). Similarly, at inhibitory synapses between cultured mouse cortical neurons, Syt-7 KO had no effect on baseline release, short-term plasticity, or delayed release after train stimulation (asynchronous release; Maximov et al., 2008). These results are somewhat surprising and difficult to explain, given that subsequent studies found that Syt-7 plays a role in both facilitation and asynchronous release. However, in the case of the D. melanogaster NMJ, an earlier study failed to detect presynaptic Syt-7 in wild-type animals, which may explain why Syt-7 knockdown did not change the properties of synaptic transmission (Adolfsen et al., 2004). Similarly, it is not known whether Syt-7 is normally present at inhibitory synapses in cortical cultures. Moreover, cultured neurons often fail to exhibit normal short-term plasticity, possibly because cultured neurons fail to develop the normal complement of synaptic proteins and/or because cultured neurons are often studied using nonphysiological extracellular Ca2+ concentrations.
Asynchronous release
The first indication that Syt-7 plays a role in synaptic transmission came from the zebrafish NMJ, where knockdown of Syt-7 led to a marked decrease in asynchronous release (Wen et al., 2010). At the zebrafish NMJ, prolonged train stimulation results in two separate forms of release. Early in the train, release is synchronized to each action potential. Later in the train, release becomes less synchronized, and more release events occur in between action potentials. Knockdown of Syt-7 left synchronous release intact but abolished asynchronous release. Knockdown of Syt-2 (the dominant low-affinity synaptotagmin isoform at this synapse) had the opposite effect, preserving asynchronous release while abolishing the early synchronous component. Thus at the zebrafish NMJ, these two synaptotagmin isoforms mediate kinetically distinguishable forms of neurotransmitter release.
Syt-7’s role in asynchronous release has been subsequently proposed at many other synapses. These include cultured hippocampal neurons (Bacaj et al., 2013; Li et al., 2017), excitatory synapses between hippocampal CA1 pyramidal neurons and the subiculum (Bacaj et al., 2013), retinal bipolar cell synapses (Luo et al., 2015), the cerebellar basket cell synapse (Chen et al., 2017), the calyx of Held (Luo and Sudhof, 2017), and cerebellar parallel fiber synapse (Turecek and Regehr, 2018; but see Liu et al., 2014; Weber et al., 2014 for conflicting studies). Syt-7–mediated asynchronous release is substantial after action potential trains, which could allow presynaptic cells to exert sustained impact on postsynaptic activity for hundreds of milliseconds (Maximov and Südhof, 2005; Luo et al., 2015).
Facilitation
Syt-7 has recently been shown to be required for synaptic facilitation at multiple synapses in the hippocampus and thalamus (Jackman et al., 2016). At these synapses, facilitation driven by a single action potential causes a ∼2-fold enhancement of synaptic vesicle release, which decays with a time constant of ∼100 ms. In global Syt-7 KO animals, facilitation was completely abolished. Notably, facilitation could be restored in adult animals in a cell-autonomous fashion by virally driven rescue expression of Syt-7, suggesting that the loss of facilitation was not a result of abnormal development in global Syt-7 KO animals (Jackman et al., 2016). Rescue expression of Syt-7 with a mutated Ca2+-binding C2A domain did not restore facilitation, showing that Syt-7’s high-affinity Ca2+ binding is essential for facilitation.
Subsequent studies have revealed a novel function for Syt-7–mediated facilitation. Several synapses in the cerebellum and medial vestibular nucleus display net synaptic depression despite exhibiting prominent Syt-7 expression (Chen et al., 2017; Turecek et al., 2017). Interestingly, these synapses share an uncommon trait: in response to repeated stimulation, they depress to the same steady-state response amplitude regardless of the stimulus frequency. It is possible these synapses have a hidden component of facilitation that perfectly counters depression to create a “frequency-invariant” synapse, because use-dependent vesicle depletion (the primary cause of depression) and use-dependent facilitation (driven by Syt-7) both increase with stimulus frequency. Indeed, at the cerebellar Purkinje cell synapse, prominent facilitation was revealed when the initial release probability was decreased by lowering extracellular Ca2+, and frequency invariance was lost in Syt-7 KO animals (Turecek et al., 2017). Thus, Syt-7 may be responsible for an underappreciated class of synapses that are perfectly able to transmit the rate of presynaptic activity by maintaining constant responses over a wide range of firing frequencies.
Understanding Syt-7 actions
The mechanism by which Syt-7 produces either asynchronous release or facilitation remains to be determined. The conventional view is that it does so from the plasma membrane by increasing the probability of fusion of Syt-1–bearing vesicles (Sugita et al., 2001; Jackman et al., 2016). The structural similarities between Syt-1 and Syt-7 suggest that the two isoforms might use similar mechanisms (discussed in detail in later sections) to drive the fusion of synaptic vesicles with the plasma membrane. Recent studies support the idea that Syt-1 and Syt-7 are equally and independently capable of driving vesicle fusion (Bacaj et al., 2015).
Synaptic vesicle fusion depends critically on Syt-1’s ability to bind anionic lipids in a Ca2+-dependent manner (Chapman, 2008; Jahn and Fasshauer, 2012). Upon binding Ca2+, Syt-1 pulls synaptic vesicles closer to the plasma membrane, which may lower the energy barrier for fusion (Chang et al., 2018). Ca2+-bound C2 domains can also insert deeply into membranes, inducing curvature that further lowers the energy barrier (Martens et al., 2007; Hui et al., 2009). Insertion into the membrane appears to be especially important, as a point mutation to a membrane-inserting hydrophobic residue completely abolishes Syt-1–mediated synaptic vesicle fusion (Bai et al., 2002; Herrick et al., 2006; Paddock et al., 2011).
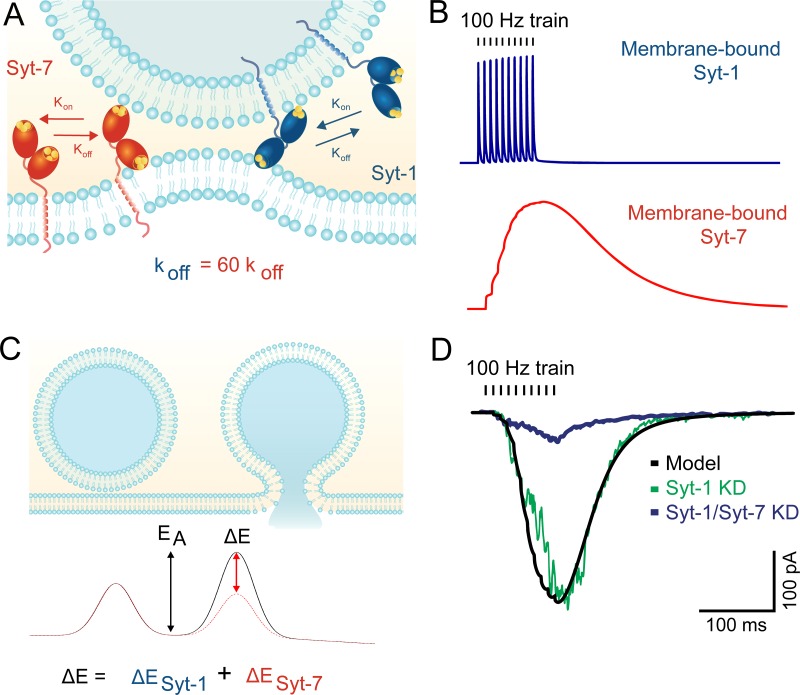
Recently, an author of this review (Jackman et al., 2016) proposed a model of synaptic release based solely on the observed membrane-binding properties of Syt-1 and Syt-7 (Brandt et al., 2012; Fig. 3). According to this model, the 40-kBT energy barrier for fusion (Kuzmin et al., 2001) decreases when presynaptic Ca2+ binds to either Syt-1 or Syt-7. The rate constant for fusion is then predicted by the Arrhenius equation. Syt-1’s low Ca2+ affinity and fast membrane-binding kinetics allow it to quickly bind and unbind from membranes during an action potential, leading to synchronous vesicle fusion. In contrast, Syt-7’s high Ca2+ affinity and slow binding kinetics keep it bound to membranes in between action potentials, which may lower the energy barrier for fusion and facilitate the action of Syt-1 during subsequent action potentials. A necessary constraint of this model is that Syt-7 is less effective than Syt-1 at lowering the energy barrier, as the model otherwise predicts high rates of sustained fusion in between action potentials. However, even in the absence of Ca2+-bound Syt-1, the model predicts that Syt-7 will drive very low rates of fusion for ∼100 ms after an action potential. This could explain Syt-7’s role in asynchronous release. Indeed, when Syt-1 is removed from the model, the predicted fusion rates display striking similarities to Syt-7–driven release recorded from Syt-1 KO neurons (Bacaj et al., 2013).
Figure 3.
Membrane-binding kinetics of Syt-7 may explain its role in asynchronous release. (A) The C2 domains of Syt-1 and Syt-7 both bind membranes in a Ca2+-dependent manner. However, Syt-7 binds Ca2+ with much higher affinity and exhibits far slower unbinding kinetics after Ca2+ levels subside. (B) Modeled membrane binding of the two isoforms in response to a 100-Hz train of action potentials. Syt-1 rapidly releases from membranes in between action potentials, whereas Syt-7 binding continues to increase during the train. See Jackman and Regehr (2017) for modeling parameters. (C) For vesicles to fuse with the plasma membrane, they must overcome the energy barrier associated with merging the two lipid bilayers. Syt-1 (and likely Syt-7) lowers the barrier by bringing the two membranes in close apposition and inducing membrane curvature. (D) Modeling fusion rates without a contribution from Syt-1 (black line) predicts release rates that display similarities to synaptic responses recorded from Syt-1 KO neurons (adapted with permission from Bacaj et al., 2013). Syt-7 knockdown profoundly reduced release, indicating that the asynchronous release is driven mostly by Syt-7.
Until recently, it was unclear whether Syt-7 could simultaneously support both asynchronous release and facilitation. Studies of the role of Syt-7 in asynchronous release were performed using synapses that do not show facilitation (Wen et al., 2010; Bacaj et al., 2013), whereas studies of facilitation were performed at synapses without prominent asynchronous release (Jackman et al., 2016; Turecek et al., 2017). Two recent studies examined synapses that possess asynchronous release and facilitation and found that deletion of Syt-7 profoundly impaired both forms of plasticity (Chen et al., 2017; Turecek and Regehr, 2018). This supports the hypothesis that a single mechanism underlies the two phenomena (Rahamimoff and Yaari, 1973; Van der Kloot and Molgó, 1994). Syt-7 likely produces a prolonged increase in the probability of release after an action potential, which can manifest as either a low rate of sustained fusion (asynchronous release) or dramatic increase in subsequent Syt-1–driven fusion (facilitation). It is interesting to speculate whether asynchronous release might be an inevitable consequence of facilitation, or vice versa.
Other mediators of asynchronous release
Asynchronous release is not completely abolished by the removal of Syt-7 (Bacaj et al., 2013; Chen et al., 2017; Turecek and Regehr, 2018). Thus, it is likely that other presynaptic Ca2+ sensors perform functions that partly or completely overlap with Syt-7. Doc2 proteins are likely candidates for such functions (Yao et al., 2011; Xue et al., 2015). Similar to synaptotagmins, Doc2α and Doc2β possess a pair of Ca2+-binding C2 domains, but they lack a transmembrane domain (Yao et al., 2011). Doc2 interacts with SNARE proteins and membranes in a Ca2+-dependent manner, but with higher Ca2+ affinity and slower binding kinetics than Syt-1 (Yao et al., 2011). Although Doc2 is cytoplasmic, upon binding Ca2+, it translocates to the plasma membrane and contributes to asynchronous release in cultured hippocampal neurons as well as dense-core vesicle exocytosis from chromaffin cells (Xue et al., 2015; Houy et al., 2017).
A small component of facilitation also persists at Syt-7 KO synapses at the cerebellar parallel fiber synapse (Turecek and Regehr, 2018) and calyx of Held (Luo and Sudhof, 2017). The remaining facilitation exhibits a decay that is faster than the facilitation driven by Syt-7. Early studies of facilitation at the frog NMJ detected two distinct components of facilitation, termed F1 and F2 (Mallart and Martin, 1967). These two components of facilitation decayed with time constants roughly equal to facilitation observed in the absence of Syt-7 (F1) and facilitation driven by Syt-7 at wild-type synapses (F2). Revealing the mechanism behind F1 is an important step toward understanding the functional role of this fast form of facilitation in synaptic transmission.
The role of Syt-7 in neuroendocrine cell secretion
Cell-based, biophysical studies on dynamics of the fusion pore have revealed characteristic effects of Syt-7 on the steps of fusion pore opening and subsequent dilation/expansion (Segovia et al., 2010; Zhang et al., 2011; Rao et al., 2014). In considering these effects, it is useful to contrast Syt-7’s effects on the rate and extent of fusion with the corresponding activities of Syt-1, to highlight unique aspects of Syt-7 function. Both isoforms are present in neurons (Geppert et al., 1994; Li et al., 1995; Ullrich and Südhof, 1995; Sugita et al., 2001) and chromaffin cells (Fukuda et al., 2004; Schonn et al., 2008), and differences in fusion pore dynamics suggest that the interplay of their activities can produce multilayered regulatory control over fusion outcomes. In the discussion below, particular emphasis is placed on the role of Syt-7 in the control of exocytosis in adrenomedullary chromaffin cells.
The fusion pore
Vesicle fusion progresses from a prefusion contact point to the eventual generation of a fusion pore, an aqueous connection between the vesicle interior and cell exterior through which cargos are discharged. One of the important intermediates in this pathway is thought to be “hemifusion,” which is operationally defined as lipid mixing without aqueous content mixing (Chernomordik et al., 2006). Studies in reconstituted systems suggest that the prevalence of hemifusion, and the stability of such an intermediate in general, depends on the characteristics of the lipids (intrinsic curvature, liposome size, etc.) and identity of proteins involved in the fusion reaction (Chernomordik et al., 1998; Yoon et al., 2006; Kyoung et al., 2011; Diao et al., 2012; Hernandez et al., 2012). In chromaffin cells, the hemifused state may actually constitute a regulated step in the transition from vesicle–plasma membrane contact to pore opening, or from pore opening to dynamin-dependent fission (Zhao et al., 2016). Whether Syt-1 has a role in these processes besides accelerating the transition from the hemifused state to pore opening is unclear (Kyoung et al., 2011). However, Syt-7 has features that distinguish it from Syt-1 and suggest it may more effectively stabilize highly curved intermediates along the fusion pathway (see Fig. 8). The stabilization of such intermediates may delay the transition from hemifusion to pore formation (Zhao et al., 2016) and/or pore formation to expansion (Rao et al., 2014). Hemifused intermediates may represent energetically favorable sites for dynamin-dependent fission mechanisms (Liu et al., 2011) which work to counter the tendency for pores to expand (Anantharam et al., 2011; Zhao et al., 2016). This idea is supported by evidence showing an increased likelihood of a “kiss-and-run” form of endocytosis occurring at sites of Syt-7 vesicle fusion (Rao et al., 2014).
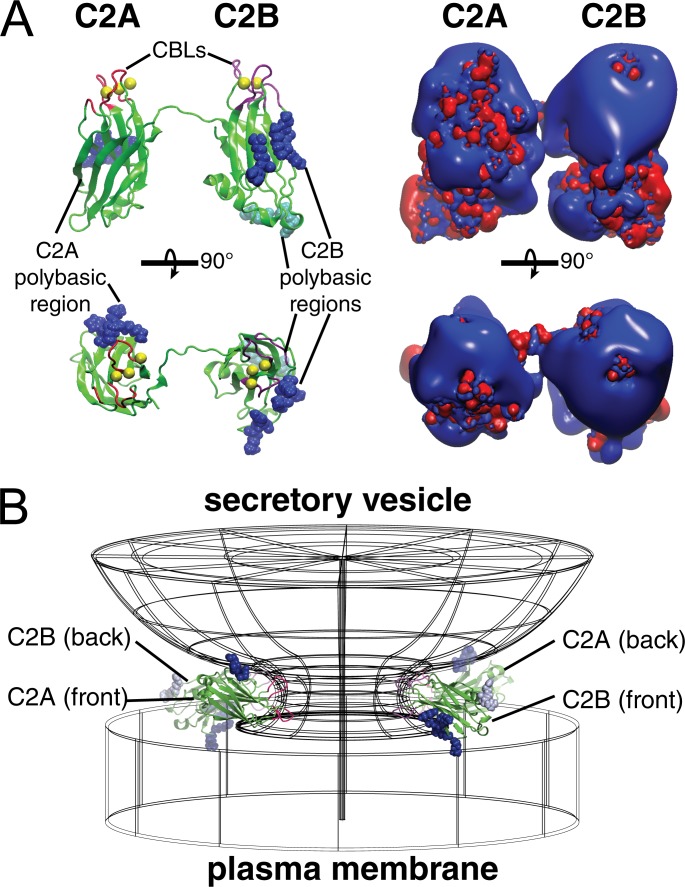
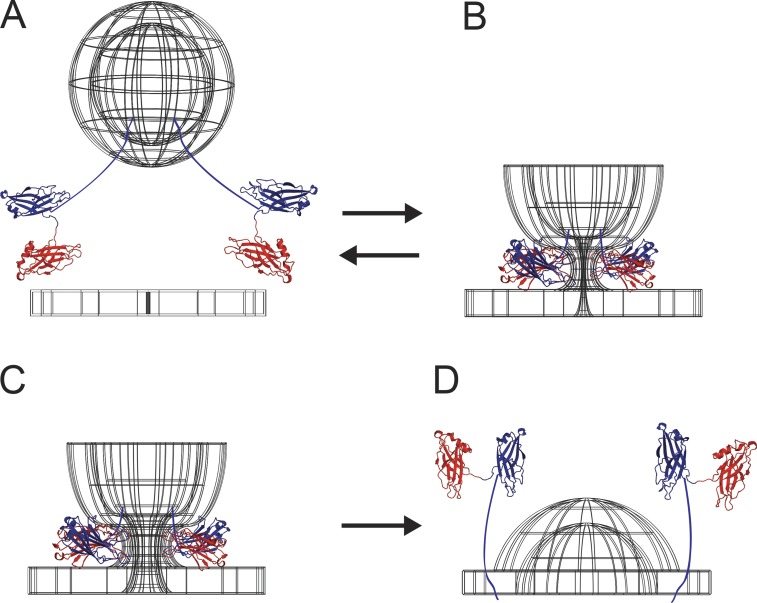
Figure 8.
Structural model for Syt stabilization of fusion pores. (A) Hypothesized relative orientation of C2A and C2B, illustrated from a composite of Syt-7 C2A (PDB accession no. 2D8K) and C2B (Xue et al., 2010) structures. Synaptotagmin C2A and C2B domains orient randomly relative to each other in solution (Choi et al., 2010), but catalysis of fusion is most efficient when the two domains point their CBLs (pink and purple; Ca2+ ions yellow) in the same direction (Bai et al., 2016), suggesting that the transition state involves the membrane-bound protein in an orientation similar to that shown here. Left: Because the C-terminal residues of the C2A domain and N-terminal residues of the C2B domain are connected by the short C2AB flexible linker, the polybasic β-4 strands orient in opposite directions, in the same way that two people standing face-to-face point their left arms in opposite directions (dark blue spheres: K183, K184, H185, K186 on C2A and K315, R316, K319, K320, K321 on C2B). A second basic cluster on C2B may also contribute to bridging as previously shown for Syt-1 (Xue et al., 2008; light blue spheres: R390 and R392). Right: Same views shown as potential maps, calculated using APBS-PDB2PQR (blue: +50 mV; red: −50 mV equipotential contours, assuming 0.15 M KCl, pH 7.4; Baker et al., 2001; Dolinsky et al., 2004). (B) Proposed locations of C2 domains during stabilization of a narrow, high-curvature fusion pore. Here the CBLs insert into the fusion pore neck and stabilize positive curvature in the plane of the pore ring, whereas the polybasic regions interact with anionic lipids on the opposing vesicle and plasma membrane surfaces. The prefusion spacing between vesicle and plasma membranes is roughly ∼2–3 Å, approximately the width of a C2 domain (Diao et al., 2012).
Once formed, the fusion pore can be viewed as a narrow channel that enables the outward flux of neurotransmitter molecules. With amperometry, which allows the most sensitive measure of content flux through an open pore, the nascent pore is characterized by a distinctive “prespike foot” (Wightman et al., 1991; Chow et al., 1992). The prespike foot consists of small-amplitude current flickers that likely correspond to the trickle of neurotransmitter escaping the pore while it is still narrow (Alvarez de Toledo et al., 1993; Zhou et al., 1996). The fusion pore may then expand to permit rapid release of the remaining neurotransmitter contents in a burst represented by a large spike in the amperometric trace (Wightman et al., 1991; Chow et al., 1992; Schroeder et al., 1996); alternatively, it may reseal to produce kiss-and-run events in which only a fraction of the vesicle’s contents is released (Graham et al., 2002; Wang et al., 2003a, 2006; Fulop et al., 2005). These kiss-and-run events are indicated by stand-alone feet in the amperometric traces (Zhou et al., 1996). The narrow angstrom-scale diameter of the fusion pore permits passage of small neurotransmitter molecules and retention of larger peptide cargo molecules (MacDonald et al., 2006). Therefore, kiss-and-run events provide a mechanism for controlling the quantity and identity of content being released. Many of these concepts, including the structure and possible regulators of fusion pores, are reviewed elegantly by Chang et al. (2017).
Amperometric measurements of norepinephrine release from PC12 cells were the first to reveal Syt-dependent effects on control of fusion pore opening and stability (Wang et al., 2001, 2003a,b; Bai et al., 2004b; Zhang et al., 2010). The main effects of overexpressed Syt-7 were an overall increased frequency of fusion events as well as a stabilization of the fusion pore compared with Syt-1 and Syt-9 (Zhang et al., 2010, 2011). The degree of fusion pore stabilization was correlated with in vitro measurements of synaptotagmin binding to PS-containing lipids. In contrast, there was no evidence for a correlation between the strength of Ca2+-independent and -dependent interactions with SNARE proteins syntaxin and SNAP-25 or for the ability of synaptotagmin to facilitate SNARE complex assembly with stabilization of the fusion pore (Zhang et al., 2010). It was concluded that isoform-specific differences in interactions with anionic phospholipid effectors may underlie differences in rates of fusion pore transitions (Zhang et al., 2010).
Regulation of fusion pore expansion
In primary chromaffin cells, endogenous synaptotagmin isoforms number two (Syt-1 and -7; Schonn et al., 2008) or four (Syt-1, -4, -7, and -9; Matsuoka et al., 2011). However, only Syt-1 and Syt-7 have been identified on dense-core vesicles. Isoform-specific effects on the fusion pore, its expansion, and the concomitant release of cargo proteins are evident in chromaffin cells imaged with conventional or polarized TIRF (pTIRF) microscopy (Anantharam et al., 2010; Rao et al., 2014). pTIRF microscopy relies on the use of a lipophilic dye (DiI or DiD) that embeds in the membrane with a fixed orientation. Upon fusion, the dye diffuses from the plasma membrane into the vesicle membrane. Alternate excitation by light polarized parallel or perpendicular to the coverslip and detection of the resultant emissions can be used to identify regions of high curvature and deduce membrane geometries associated with pore expansion (Anantharam et al., 2010).
The stability of localized regions of curvature representing the fused vesicle/plasma membrane domain is substantially enhanced after fusion of Syt-7–bearing vesicles (Fig. 4; Rao et al., 2014). In contrast, indentations associated with Syt-1 fusion events have a much shorter lifetime, suggesting that they are more likely to collapse into the plasma membrane after fusion (Rao et al., 2014). Accordingly, fluorescent cargos are discharged slowly from vesicles bearing fluorescent Syt-7 (Rao et al., 2017; Bendahmane et al., 2018). These results indicate that Syt-7 stabilizes areas of high membrane curvature during exocytosis in cells, which seems to be in agreement with amperometric measurements of fusion pore stabilization (Zhang et al., 2011).
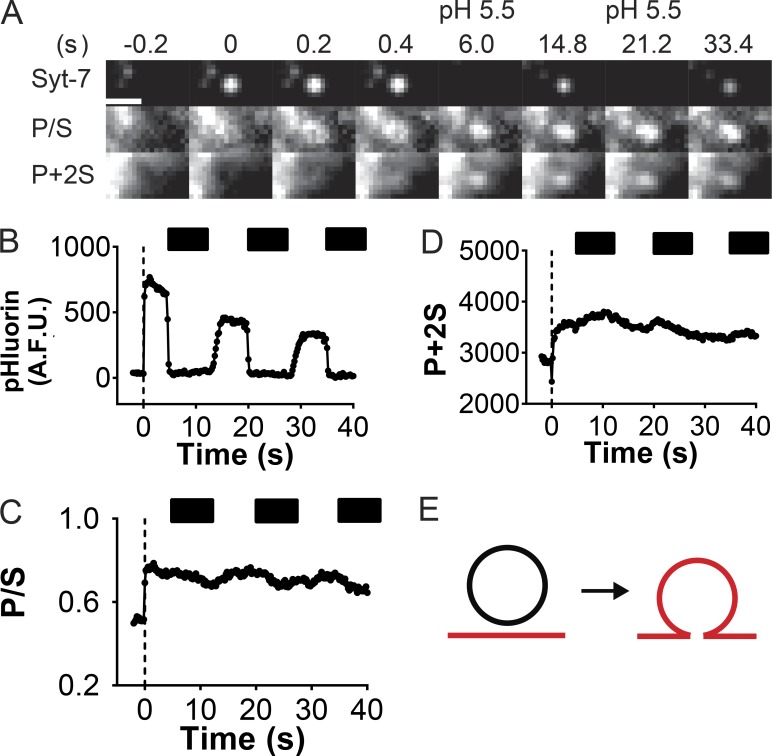
Figure 4.
Fusion pores of Syt-7–bearing dense-core vesicles are characterized by slow expansion. (A) Time series of a pHluorin-tagged Syt-7–bearing vesicle undergoing exocytosis with associated changes in DiD-labeled membrane fluorescence (P/S and P+2S). Bar, 960 nm. The cell was periodically perfused with a low-pH (5.5) solution to verify that the fusion pore was still open (note quenching of pHluorin fluorescence at times 6.0 and 21.2 s). (B–D) Intensity-versus-time curves for images in A. The dotted black line indicates the fusion frame (time 0). Black bars indicate pH 5.5 wash. A.F.U., arbitrary fluorescence units. (E) Simulations based on P/S and P+2S fluorescence emission suggest that the fusion pore is not expanding or is expanding slowly. Modified from Rao et al. (2014).
It should be noted here that the long persistence of fusion-site membrane curvature observed by optical methods represents a much slower process than the transition from prespike foot to burst of neurotransmitter release captured by amperometry recordings, which occurs in a matter of milliseconds (Alvarez de Toledo et al., 1993; Albillos et al., 1997; Alés et al., 1999). Though both phenomena have been interpreted as expansion of the fusion pore, they are likely to represent sequential kinetic steps in the process of exocytosis, and we propose drawing a distinction between them. A fast initial widening of the fusion pore (<3 nm; Albillos et al., 1997) could allow complete release of small neurotransmitters but may not increase the pore diameter to the extent required for passage of larger peptide cargo. This step would occur too quickly to be resolved by the frame rate of the fluorescence measurements, which is on the order of 10 Hz. A subsequent, more extensive opening, which is the step observed by pTIRF, would permit exit of peptides and larger molecules from the vesicle (Taraska et al., 2003; Perrais et al., 2004; MacDonald et al., 2006). This two-step model would be consistent with proposals that the fusion pore acts as a size filter for selective cargo release (Perrais et al., 2004; Moghadam and Jackson, 2013).
Biochemical and optical measurements cited above raise the intriguing possibility that C2 domain lipid interactions regulate structural changes at multiple points during progressive widening of the pore (Wang et al., 2003a,b, 2006; Segovia et al., 2010). In support of this idea, a chimera of Syt-1, whose C2B domain Ca2+-binding loops were exchanged for those of Syt-7, exhibits a higher affinity for anionic phospholipids in the absence of Ca2+ and slower dissociation from phospholipids in the presence of Ca2+ and, importantly, exerts slowing effects on fusion pore expansion and cargo release kinetics compared with the wild-type protein (Bendahmane et al., 2018). The tight binding of the Syt-7 C2 domains to lipids constituting the fusion pore (Voleti et al., 2017) could be a determining factor of pore stabilization, exerting regulatory control over the probabilities for the fusion pore to open, expand, or reseal (Fig. 5). According to this view, it is possible that the remarkably slow rates of pore expansion of Syt-7 vesicles relative to Syt-1 are linked to the slow dissociation of Syt-7 from target membranes (Hui et al., 2005; Rhee et al., 2005; Bendahmane et al., 2018).
Figure 5.
The exocytotic fate of a Syt-bearing dense-core vesicle. (A) A vesicle is shown that has yet to fuse or has already fused and undergone endocytosis (i.e., from B to A). Syt-7’s N terminus is exposed to the vesicle lumen; the C2 domains are exposed to the cytosol. (B) Membrane depolarization and Ca2+ influx lead to fusion and fusion pore formation. To trigger entry into this state, the C2A (blue) and C2B (red) might both penetrate the plasma membrane (Bai and Chapman, 2004) or the C2A and C2B might interact with opposing vesicle and plasma membranes (Herrick et al., 2009). (C) The early fusion pore widens. Continued lipid association of the C2 domains may slow the expansion of the fusion pore (Bendahmane et al., 2018). (D) The C2 domains release the plasma membrane as the fusion pore expands.
TIRF-based measurements of lysosomal exocytosis in mouse embryonic fibroblasts reveal similar inhibitory effects of Syt-7 on fusion pore expansion (Jaiswal et al., 2004). The majority of fusion events from wild-type cells resulted in partial release of lysosome contents. By measuring release of different sized fluorescent dextran molecules, the diameter of the fusion pore during partial release was estimated to be 30 nm, which prevented egress of larger cargoes. However, in Syt-7 KO cells, full release was favored over partial release, and fusion pores were wide enough to permit release of all sizes of dextrans tested. Thus, it was concluded that Syt-7 restricts expansion of the fusion pore during lysosomal exocytosis (Jaiswal et al., 2004, 2009). Interestingly, lysosomal exocytosis was triggered by Ca2+ even in the absence of Syt-7, suggesting either that Syt-7 regulates lysosomal exocytosis in a fundamentally different way from synaptic vesicle and dense-core vesicle exocytosis, where it couples Ca2+ binding to fusion, or that alternative Ca2+ sensors are present that take over in the absence of Syt-7 (Jaiswal et al., 2004).
Dynamics of dense-core vesicles
Recent optical imaging studies in bovine chromaffin cells show how the different Ca2+ sensitivities of Syt-1 and Syt-7 impact fusion kinetics of their respective vesicle populations (Rao et al., 2017). Small elevations from baseline cytosolic Ca2+ levels are generally ineffective at driving fusion of vesicles bearing Syt-1 but much more effective at driving fusion of vesicles bearing Syt-7. This is again consistent with prior in vitro studies, which demonstrated that Syt-7 binds membranes and triggers fusion at far lower Ca2+ concentrations compared with Syt-1 (Hui et al., 2005; Wang et al., 2005; Bhalla et al., 2008). As the Ca2+ concentration rises (in cells, ostensibly as a result of membrane depolarization), both populations of vesicles fuse with higher efficiency. These data show that the response times of Syt-1 versus Syt-7 vesicles are finely tuned to levels of intracellular Ca2+ in a way that reflects the intrinsic biochemical properties of the synaptotagmin isoforms themselves. From these data emerges a model whereby vesicles that fuse in response to mild depolarization typically release cargos through slowly expanding pores, and vesicles that fuse in response to strong depolarization exhibit a range of pore behaviors, including rapid and more complete release of cargos (Fulop et al., 2005, 2008; Fulop and Smith, 2007; Rao et al., 2014).
Vesicles bearing fluorescence-tagged Syt-1 or Syt-7 exhibit differences in their preferred fusion locations within cells and are also characterized by different degrees of mobility in their travel to fusion sites (Rao et al., 2017). A fusion location bias might suggest that Syt isoforms are differentially dependent on Ca2+ channel subtypes for fusion. Bovine chromaffin cells, in which these studies were performed, express P/Q, N-, and L-type channels, with P/Q channels carrying the majority of the Ca2+ current (García et al., 2006). Evidence also exists for direct physical coupling between Syt-1 and channel subtypes (Charvin et al., 1997; Sheng et al., 1997; Wiser et al., 1999; Mahapatra et al., 2012). Based on its lower intrinsic affinity for Ca2+, one might predict that vesicles bearing Syt-1 are closely positioned to channels to maximize probability of release (Young and Neher, 2009). However, multiple in vitro studies demonstrate that Syt-7’s Ca2+ sensitivity for binding membranes exceeds that of Syt-1 by more than an order of magnitude (Bhalla et al., 2005; Hui et al., 2005). Thus, vesicles harboring Syt-7 may not be as dependent on close coupling to channels. The clustered (Syt-7 vesicles) versus dispersed (Syt-1 vesicles) fusion locations of chromaffin vesicles may also reflect the requirement for different target SNAREs or preferential binding to different ordered versus disordered lipid phases (Wan et al., 2011) and not channel locations per se. There is not yet convincing evidence that synaptotagmin isoforms require different SNAREs or accessory proteins for fusion. However, given the number of interfaces over which Syt–SNARE interactions are possible (Zhou et al., 2015, 2017), such differences may play an important role in early or later stages of fusion.
The question of why Syt-bearing vesicles exhibit differences in mobility is an intriguing one (Rao et al., 2017). The most complete biophysical descriptions of vesicle motion, in general, have been provided by Holz, Axelrod, and colleagues (Allersma et al., 2004, 2006; Degtyar et al., 2007; Holz and Axelrod, 2008). The fundamental observation that such behavior is common (Ng et al., 2002, 2003; Griesinger et al., 2005; Shakiryanova et al., 2005; Levitan et al., 2007) challenges the assumption that the final moments (within 100 ms) before fusion are characterized by stable protein–protein/lipid interactions. Travel can occur over large distances (tens to even hundreds of nanometers), and travel even increases in the moments before fusion, suggesting that essential steps necessary to prepare a vesicle for fusion, such as priming, do not require a stable interaction between the vesicle and plasma membrane lipids (Eberhard et al., 1990; Hay et al., 1995; Holz and Axelrod, 2002). On a related note, the observation that vesicles have the capacity to fuse almost immediately after entering the evanescent field—termed “newcomers”—raises questions about what constitutes docking from a molecular or even morphological perspective (Rao et al., 2017).
What, then, are the molecular origins of vesicle mobility, and why do they differentially impinge on vesicles bearing different synaptotagmin isoforms? The motions are clearly greater than electrostatic, van der Waals, or hydrogen bond distances (Allersma et al., 2006; Degtyar et al., 2007), and even the length scale over which SNAREs zipper (Sutton et al., 1998). One presumes that known modulators of motion, including actin and myosin, are somehow involved (Lang et al., 2000; Li et al., 2004; Neco et al., 2004; Desnos et al., 2007; Berberian et al., 2009; Peng et al., 2012; Villanueva et al., 2012); myosin’s activities are modified by Ca2+ and ATP (Murphy et al., 2001; Rosé et al., 2002; Krementsov et al., 2004). Whether these or additional proteins act to affect vesicle mobility to different degrees, or whether different proteins act to promote or hinder travel independently, are questions that have yet to be answered. The fundamental reason mobility may be greater for some vesicles than others may be a practical one. Motion may allow a vesicle to sample new, unexplored regions of the membrane where fruitful interactions leading to fusion are more likely to exist (Degtyar et al., 2007). Based on this logic, larger-scale motions are less evident in Syt-7 bearing vesicles because they are somehow shepherded to regions more “favorable” for fusion. A goal for future studies might be to identify what privileges those regions—including their protein and lipid constituents—as exocytotic sites.
Vesicle pools
The studies referenced above demonstrate the influence of Syt-7 activity on the fusion properties of individual vesicles and suggest the type of behavior that would be expected for vesicles regulated solely or predominantly by one synaptotagmin isoform. However, it may not always be true that each vesicle is paired with a particular synaptotagmin isoform that determines its fusion properties. Electrophysiological studies in chromaffin cells have suggested that Syt-1 and Syt-7 activities jointly contribute to regulation of the same vesicle pools (Schonn et al., 2008). Upon stimulation, exocytosis measured by capacitance and amperometry occurred in stages, with the initial fast phase corresponding to release from the RRP and a delayed release component from the SRP of vesicles (Sørensen, 2004; Schonn et al., 2008). In Syt-1 and Syt-7 double KO cells, both the fast and slow components disappeared, and overall exocytosis was reduced by more than 70%, leaving behind only a very slow secretory component (Schonn et al., 2008). Although it would be tempting to attribute release from the RRP to activation by Syt-1 and release from the SRP to activation by Syt-7, the data suggest a more complicated picture involving overlapping regulatory effects. The fast burst of release is eliminated in Syt-1 KO cells, showing that Syt-1 is indeed required for RRP release. However, Syt-7 concurrently modulates release from this subpool of vesicles as shown by a smaller fast burst of release with a quicker time constant in Syt-7 KO cells compared with wild type. Similarly, although Syt-7 is responsible for triggering the slow burst of exocytosis in a Syt-1 KO background, a minor component of the slow burst phase is still observed in Syt-7 KO cells. This suggests that Syt-1 may also contribute to triggering exocytosis from the SRP (Schonn et al., 2008). Thus, a complex interplay of competing Syt-1 and Syt-7 activities appears to determine the fate of vesicles in both the RRP and SRP.
Structural and biochemical properties of Syt-7
To define the functional properties of Syt-7 and what differentiates those properties from Syt-1 or other isoforms, one should begin by considering the C2 domains that form most of the cytoplasmic region of the protein. Current data suggest that the relative importance of the tandem C2 domains in regulation of exocytosis may differ between Syt-1 and Syt-7. One study addressed this point by comparing exocytosis in wild-type mice, Syt-7 KO mice, and knock-in (KI) mice expressing a mutant in which Ca2+ binding to Syt-7 C2B was disrupted (Segovia et al., 2010). Dual-capacitance and amperometry measurements indicated that the number of exocytotic events was decreased in the Syt-7 KO but not the Syt-7 KI. However, when Ca2+ binding to C2B was disrupted, the fusion pores were prone to closing, yielding an increase in kiss-and-run fusion events indicated by transient increases in membrane capacitance coincident with reduced amplitude amperometric spikes. Thus, C2A activity was deemed necessary and sufficient for fusion pore opening, whereas C2B was additionally required for stabilizing the fusion pore and promoting its expansion (Segovia et al., 2010). Although disruption of Ca2+ binding to Syt-7 C2A but not C2B prevented rescue of asynchronous release in Syt-1 and Syt-7 double-deficient hippocampal neurons, the opposite was true for rescue of Syt-1–mediated fast, synchronous release (Bacaj et al., 2013). Thus, the relative importance of the C2 domains are reversed, with Ca2+ binding to C2A required for Syt-7–triggered release and Ca2+ binding to C2B required for Syt-1–triggered release. In the same study, Syt-7 overexpression reduced the frequency of spontaneous, mini-release in Syt-1 KO neurons (Bacaj et al., 2013). The ability of Syt-7 to clamp miniature inhibitory postsynaptic currents was disrupted by mutation of Ca2+ binding sites in C2A but not C2B, again demonstrating a special importance for the Syt-7 C2A domain.
Comparison of Syt-7 and Syt-1 ion binding
Presumably, Syt-7–specific effects on exocytosis arise from unique structural and biochemical characteristics. This is a puzzling problem given the high degree of sequence and structural similarity between conserved domains in Syt-1 and Syt-7, which suggests that subtle structural variations (Fig. 6) coalesce to produce substantial functional differences (Evans et al., 2015; Voleti et al., 2017; Bendahmane et al., 2018). Perhaps the most important biochemical signature of Syt-7 that sets it apart from the other synaptotagmin isoforms is its extreme Ca2+ sensitivity. Syt-7 stimulation of SNARE-mediated liposome fusion occurs with a 400-fold higher sensitivity to Ca2+ than that of Syt-1 (Bhalla et al., 2005).
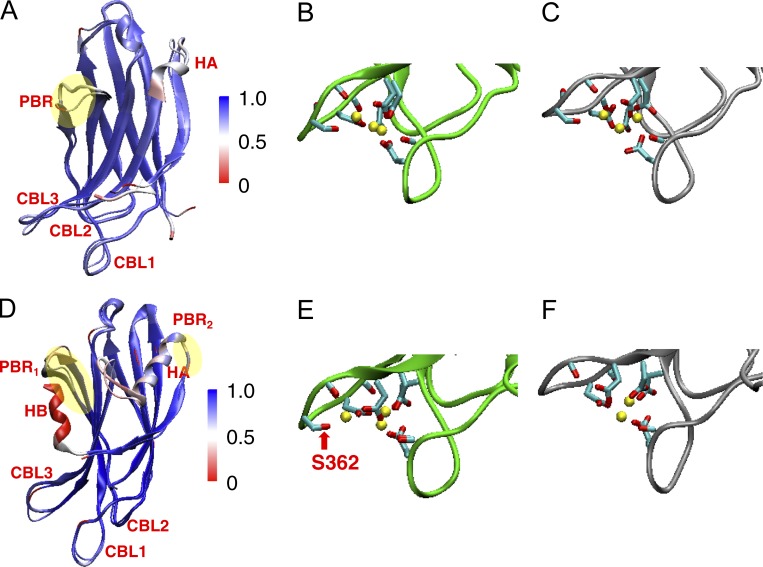
Figure 6.
Conservation of domain structures within the C2A and C2B domains of Syt-1 and Syt-7. (A) Superimposed experimental structures of C2A domains of Syt-7 (PDB accession no. 6ANJ) and Syt-1 (PDB accession no. 1BYN, first frame) aligned using MultiSeq (Shao et al., 1998; Roberts et al., 2006; Voleti et al., 2017). The proteins are shown in cartoon format (wider sheets for Syt-1 and narrower sheets for Syt-7) and are colored according to the Qres values, which measure the backbone structural similarity of each residue between the two aligned structures (blue for high similarity and red for low similarity). The backbone RMSD value is 1.4 Å. The polybasic region (PBR) is highlighted in yellow. The Ca2+-binding loops (CBL1–3) and helix A (HA) are labeled. (B and C) Ca2+-binding loops in Syt-7 (B) and Syt-1 (C) C2A domain experimental structures. The proteins are illustrated in cartoon format as green for Syt-7 and gray for Syt-1. The residues that coordinate the Ca2+ ions (yellow spheres) are shown as sticks (O, red; C, cyan). (D–F) are similar to A–C, but for the C2B domains of Syt-7 (PDB accession no. 3N5A) and Syt-1 (PDB accession no. 1TJX; Cheng et al., 2004; Xue et al., 2010). The backbone RMSD value is 0.9 Å. Highlighted in yellow are the two polybasic regions PBR1 and PBR2; PBR1 maps to β-strand 4 and aligns to the PBR on the C2A domains. Helix B (HB) is presented in Syt-1 and not in Syt-7. The red arrow indicates Ser362, which is present in Syt-7 and helps to coordinate the outermost Ca2+ but is missing in Syt-1.
The Ca2+-sensing ability of the synaptotagmins arises from their C2A and C2B domains, which are members of the protein kinase C family of C2 domains known for binding intracellular membranes in a Ca2+-dependent manner (Nalefski and Falke, 1996). Each C2 domain consists of an eight-stranded β sandwich in an oblong shape ∼4 nm long and 2 nm wide, with flexible loops projecting from each end (Fig. 6, A and D; Corbalan-Garcia and Gómez-Fernández, 2014). Three Ca2+-binding loops located on one end of each C2 domain (CBL1-3 in Fig. 7) contain five conserved aspartate residues that coordinate Ca2+ ions (Fig. 6, B, C, E, and F; and Fig. 7; Sutton et al., 1995). The much higher Ca2+ sensitivity of Syt-7 relative to Syt-1 is surprising considering their high degree of structural similarity. The Syt-7 C2A and C2B domains are essentially superimposable on the corresponding domains of Syt-1, with overlap observed for even many of the individual amino acid side-chain orientations (Xue et al., 2010; Voleti et al., 2017). Furthermore, the Ca2+ affinities of the isolated Syt-1 and Syt-7 C2A domains in solution are nearly identical, even though the Syt-7 C2A domain binds PS-containing membranes at ∼50-fold lower Ca2+ concentrations (Sugita et al., 2002; Maximov et al., 2008). This suggests that the enhanced Ca2+ sensitivity arises from stronger membrane binding by the Ca2+-loaded state of Syt-7 relative to Syt-1 (Brandt et al., 2012; Voleti et al., 2017). In alignments of the respective C2A and C2B domain structures, the most noticeable difference is the presence of a C-terminal α-helix on Syt-1 C2B (the HB helix) that is absent from Syt-7 C2B (Fernandez et al., 2001; Cheng et al., 2004; Xue et al., 2010). Subtle differences also exist in the positioning of residues in the HA helices and loops in both domains, although the latter may be attributable to crystal contacts (Fig. 6, A and D).
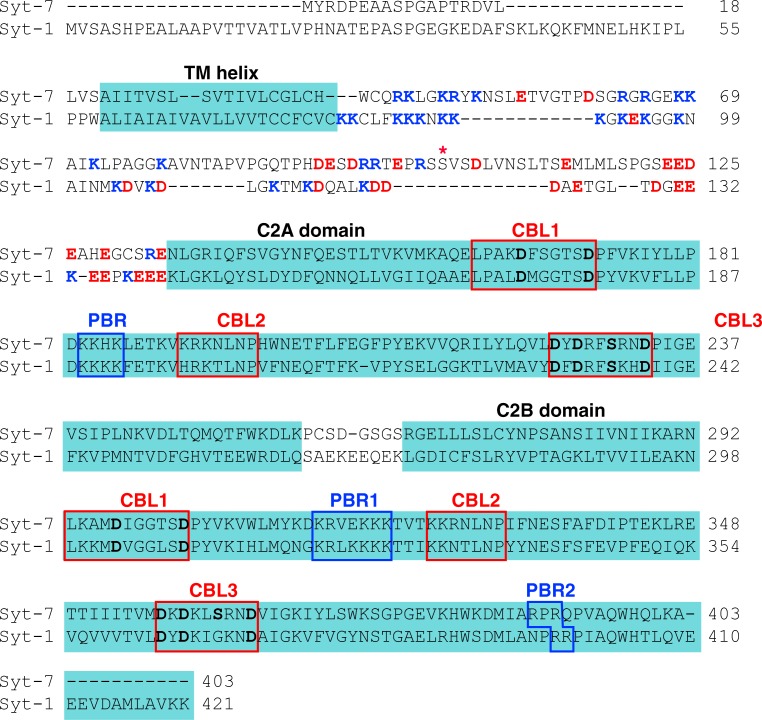
Figure 7.
Amino acid sequence comparison of Syt-7 and Syt-1. The canonical rat sequences (UniProt accession nos. Q62747-1 and P21707-1) were aligned along with the other rat synaptotagmin isoforms plus human Syt-1 and Syt-7 using Clustal Omega (Sievers et al., 2011). The human sequences (not depicted) are identical within the cytoplasmic region except for positions 66 (Ser instead of Gly), 156 (Ile instead of Val), and 325 (Met instead of Lys) of Syt-7. Transmembrane (TM) and C2 domain regions are highlighted (Lu et al., 2014). Lys/Arg (blue) and Asp/Glu (red) residues in the TM-C2A linker region are highlighted (Lai et al., 2013). The Ca2+- and membrane-binding loops (CBL) are boxed in red, with residues in bold whose side chains coordinate Ca2+. Polybasic regions boxed in blue correspond to those indicated in Fig. 6 (A and D). S103 (starred) represents a Syt-7 phosphorylation site (Wu et al., 2015).
Still, even small differences in positioning, dynamics, or flexibility of the loops may endow the isoforms with different divalent ion binding characteristics. Although the solution structure of the Syt-1 C2B domain contains two Ca2+ ions in the Ca2+-binding pocket, the C2B domain from Syt-7 as well as C2A domains from both proteins bind three Ca2+ each (Shao et al., 1998; Fernandez et al., 2001; Xue et al., 2010; Voleti et al., 2017). The Syt-1 C2B domain has been crystallized with three Ca2+ at extremely high Ca2+ concentrations (>50 mM) or when bound to a soluble form of PS, suggesting that the membrane-bound state may involve three Ca2+ (Cheng et al., 2004; Honigmann et al., 2013). The greater Ca2+ binding ability of the Syt-7 C2B domain may arise from the presence of a Ser side chain (Ser362) that is not present in most Syt C2B domains but is conserved among Ca2+-sensitive Syt C2A domains (Zhang et al., 1998; Fernandez et al., 2001; Fig. 6, D and E). The synaptotagmin isoforms also exhibit different divalent ion selectivities, with Syt-7 but not Syt-1 capable of binding liposomes containing 15% PS and stimulating SNARE-mediated membrane fusion with the larger Sr2+ and Ba2+ ions as well as Ca2+ (Bhalla et al., 2005). The ability to bind these larger cations appears to be a property of the Syt-7 metal-binding loops, as an engineered chimera of Syt-1 containing loops from Syt-7 efficiently coupled Sr2+ binding to membrane fusion in vitro and to exocytosis in mouse hippocampal neurons (Evans et al., 2015).
Ca2+ binding to the C2 domains’ loop regions neutralizes the negative charge of the aspartic acid residues and promotes penetration of the Ca2+ binding loops into anionic lipid bilayers (Zhang et al., 1998; Ubach et al., 2001; Herrick et al., 2006; Hui et al., 2006). The synaptotagmin isoforms have been grouped according to the speed with which they dissociate from PS-containing liposomes after chelation of Ca2+ by EDTA, with Syt-7 showing by far the slowest dissociation kinetics (Hui et al., 2005). Electron paramagnetic resonance measurements indicate that the Syt-7 C2A domain may insert more deeply into lipid bilayers than Syt-1 C2A (Osterberg et al., 2015). Biochemical experiments and atomistic molecular dynamics simulations demonstrate a large hydrophobic component to C2A lipid interactions that plays a role in increasing binding stability. A significant contribution to the energetic stabilization arises from the phenylalanine residues at the tips of Ca2+ binding loops 1 and 3 including Phe167, which corresponds to a methionine in Syt-1 (Brandt et al., 2012; Chon et al., 2015; Vermaas and Tajkhorshid, 2017). Recently, a second important residue was also identified in the Syt-7 C2A binding loop: Arg231, whose forked guanidino group binds more efficiently to PS than the lysine found at the corresponding position in Syt-1. Combined mutation of both Arg231 and Phe167 in the C2A domain of Syt-7 significantly weakens its lipid binding, although the mutant Syt-7 C2A domain still binds membranes much more strongly than Syt-1 (Voleti et al., 2017). The same study also reported that the Syt-7 C2B domain binds more tightly to membranes than its counterpart in Syt-1, consistent with molecular modeling results that showed more contacts with PS lipids for both C2 domains of Syt-7 compared with their counterparts in Syt-1 (Vermaas and Tajkhorshid, 2017; Voleti et al., 2017).
Further defining the molecular determinants of Syt-7’s stronger binding and slower dissociation from membranes may be relevant for understanding the mechanisms through which Syt-7 regulates various stages of exocytosis. Indeed, limited mutations that slightly enhance Syt-1’s membrane affinity have outsized effects on fusion pore formation, pore expansion rates, and even the likelihood of fusion (Bendahmane et al., 2018). Isoform-specific effects of synaptotagmins on exocytosis may even be attributable to differentiable, biophysical properties of their respective C2A and C2B domains (Sugita et al., 1996, 2002; Sugita and Südhof, 2000; Hui et al., 2005, 2009; Segovia et al., 2010; Bacaj et al., 2013; Voleti et al., 2017). For example, the Ca2+-saturated Syt-7 C2A domain binds membranes with or without PIP2 much more strongly than does Syt-1 C2A, and its affinity is comparable to that of Syt-7 C2B in the presence of PIP2 (Voleti et al., 2017). In contrast, the C2B domain of Syt-1, in the presence of PIP2 (and Ca2+), binds membranes more strongly than the Syt-1 C2A domain (Voleti et al., 2017). These differences in strength of membrane binding of the Syt-1 versus Syt-7 C2A/B appear to correlate with their relative functional importance during exocytosis (Segovia et al., 2010; Bacaj et al., 2013). On the other hand, the functional importance of the Syt-1 C2A domain may be greater than originally believed; a charge-conserving mutation that blocks Ca2+ binding in this domain decreased the efficiency of synchronous neurotransmitter release by ∼80% (Striegel et al., 2012).
Aside from the C2 domains, synaptotagmins exhibit substantial variation in amino acid sequence in the region between the transmembrane domain and the C2A domain. In Syt-1, the N- and C-terminal portions of this linker region are rich in basic and acidic residues, respectively, and have been suggested to comprise an electrostatic zipper that contributes to regulation of fusion pore opening and protein–lipid binding (Lai et al., 2013; Lu et al., 2014; Fealey et al., 2016). The sequence of the canonical Syt-7 linker region displays a somewhat similar electrostatic pattern, although the overall sequence conservation is low (Fig. 7). The role of this region of Syt-7 is relatively unexplored, other than its role in alternative splicing as discussed above.
Syt-7 protein effectors
Multiple models explain how synaptotagmin regulates exocytosis, but most agree that regulation involves interactions with anionic phospholipids (Stein et al., 2007; Park et al., 2012, 2015), probably in conjunction with SNAREs (Chapman, 2008; Chicka et al., 2008; Paddock et al., 2008; Hui et al., 2009; Südhof and Rothman, 2009; Zhou et al., 2013, 2015, 2017; Brewer et al., 2015). The t-SNAREs syntaxin and SNAP-23/25, together with the vesicle v-SNARE synaptobrevin/VAMP, constitute the minimal complement of proteins required for vesicle fusion in vitro (Söllner et al., 1993a,b; Weber et al., 1998; Tucker et al., 2004). Inclusion of the soluble C2AB fragment of synaptotagmin in an in vitro liposome fusion assay dramatically increases the rate and extent of fusion in the presence of Ca2+ (Tucker et al., 2004; Gaffaney et al., 2008). Syt-7 stimulates SNARE-driven membrane fusion with half-maximum activity at 0.30 µM Ca2+, a Ca2+ sensitivity more than 400 times higher than that of Syt-1, and the highest in the Syt family (Bhalla et al., 2005). Synaptotagmin may exist in dynamic equilibrium between SNARE-bound and unbound states; complexin binding to the SNARE complex might enhance and stabilize the SNARE-bound state of synaptotagmin (Cai et al., 2008; Lin et al., 2013). It should be noted, however, that complexin has also been characterized as a fusion clamp, preventing SNARE zippering without the energy imparted from Syt-Ca2+/membrane binding (Giraudo et al., 2008; Krishnakumar et al., 2011).
Synaptotagmin–SNARE interactions—and in particular, Syt-1–SNARE interactions—have been implicated in both clamping spontaneous release and triggering fusion in response to Ca2+ (Gaffaney et al., 2008; Lynch et al., 2008; Hui et al., 2011; Kim et al., 2012; Bai et al., 2016). Several different interaction surfaces have been identified (Zhou et al., 2015, 2017) with more than one study pinpointing the lysine-rich polybasic patch on the β-4 strand of C2B in binding the syntaxin/SNAP-25 heterodimer in a Ca2+-independent manner (Bai and Chapman, 2004; Bai et al., 2004b; Rickman et al., 2004a). We note that these interactions are highly sensitive to ionic strength, and the physiological significance of these interactions has been the subject of some debate (Park et al., 2015). One possibility is that multiple modes of weak Syt–SNARE interaction may be tuned for rapid reversibility in the crowded and dynamic environment of a fusion pore.
A comparative analysis of Syt-1 and Syt-7 SNARE binding detected no substantial differences in overall SNARE-binding affinity (Rickman et al., 2004b); however, sequence differences could have subtle regulatory effects on Syt–SNARE interactions. For example, the Syt-7 C2A domain binds syntaxin in vitro at low micromolar Ca2+ concentrations, whereas by comparison Syt-1 C2A requires 200 µM Ca2+ (Li et al., 1995). Interestingly, three Ca2+-independent interfaces recently identified in a crystal structure of Syt-1 and SNARE proteins are not conserved in Syt-7 (Zhou et al., 2015). Thus, the relevance of these Syt-1/SNARE complexes to Syt-7 function is not yet clear. However, given the multitude of interfaces identified between Syt-1 C2AB and SNAREs (Zhou et al., 2015, 2017), it is possible that other contacts between Syt-7 and SNARE proteins regulate its isoform-specific functions in the successive steps of docking, priming, fusion, and pore expansion.
Syt-7 functions in processes such as insulin secretion that use the common SNARE isoforms syntaxin-1a, VAMP2, and SNAP-25 (Gaisano, 2017); however, Syt-7 also interacts with SNAP-23. A specific interaction between SNAP-23 and Syt-7 was identified that contributes to docking of Syt-7–containing vesicles (Chieregatti et al., 2004). When SNAP-23 was substituted in place of SNAP-25 in glutamatergic hippocampal neurons, activities associated with Syt-7–mediated fusion were enhanced, including spontaneous release events. The number of spontaneous release events was increased further upon Syt-7 deletion, signifying that Syt-7 clamps release in the absence of stimulation but overall is a leakier Ca2+ sensor than Syt-1. In contrast, Syt-7 KO in the presence of SNAP-25 had little effect on exocytosis. It was concluded that Syt-7 pairs with SNAP-23 and Syt-1 pairs with SNAP-25, and that the specific Syt/SNAP pairing determines fusion kinetics and fidelity (Weber et al., 2014). Optical measurements using pHluorin-tagged synaptotagmins indicated the presence of Syt-1 on vesicles undergoing Syt-7/SNAP-23–mediated fusion, which suggests that, at least in some cases, Syt-7 may not act as the exclusive Ca2+ sensor for fusion events but instead may alter the fusion properties of Syt-1–containing vesicles.
It has also been reported that synaptotagmins, especially Syt-7, undergo Ca2+ dependent homo- and hetero-oligomerization mediated by their C2 domains (Fukuda and Mikoshiba, 2000a,b; Fukuda et al., 2002a). Oligomerization of Syt-1 has long been a subject of debate, with some studies indicating self-association and others suggesting the interactions are mediated by other factors (Desai et al., 2000; Ubach et al., 2001). An author of this review has provided evidence against direct oligomerization of Syt-7 C2 domains (Brandt et al., 2012; Vasquez et al., 2014). Recently, Syt-1 has been observed to form ring-shaped oligomers via cryo-EM, mediated by anionic lipids or polyanionic solutes, the latter of which produces rings that are insensitive to Ca2+ (Wang et al., 2014, 2017). Ring formation is conserved among synaptotagmin isoforms including Syt-7, which forms them somewhat more potently than Syt-1 in the presence of anionic membranes (Zanetti et al., 2016). If synaptotagmins oligomerize in vivo, either directly or indirectly, these structures could serve to regulate steps in the fusion process, and hetero-oligomerization of different synaptotagmin isoforms could provide a means for suppressing or enhancing each other’s activities (Bhalla et al., 2008). However, such rings have not yet been observed in vivo, and the role of synaptotagmin oligomerization in the context of exocytosis is currently unclear.
Syt-7–lipid interactions
The interactions between synaptotagmin C2 domains and anionic phospholipids play crucial roles in Ca2+-triggered fusion. This is a property that Syt-1 and Syt-7 share, suggesting that the two proteins’ membrane interactions may have similar functions in the mechanism of Syt/SNARE/Ca2+-triggered membrane fusion. Syt-1 C2A and C2B domains both bind anionic lipids such as PS in a Ca2+-dependent manner, mainly via interactions with the Ca2+-binding pocket (Zhang et al., 1998; Nalefski et al., 2001; Ubach et al., 2001; Pérez-Lara et al., 2016), and Syt-7 does the same, albeit with greater Ca2+ sensitivity owing to stronger hydrophobic lipid interactions (Sugita et al., 2002; Brandt et al., 2012; Vasquez et al., 2014; Voleti et al., 2017). The C2B domains bind PIP2 at a polybasic region centered on the β-4 strand (PBR1 in Figs. 6 and 7) in a manner that is Ca2+ independent but enhanced by Ca2+ (Tucker et al., 2003; Hui et al., 2006; Radhakrishnan et al., 2009; Voleti et al., 2017). The Syt-7 C2B domain has a robust sensitivity toward PIP2 in vitro (Voleti et al., 2017), even though it contains a Lys-to-Glu substitution at position E318 in the β-4 polybasic region (Fig. 7). Liposome fusion assays are commonly conducted with lipid membranes containing a mixture of PS and phosphatidylcholine, with or without PIP2. Omission of PS and PIP2 from the liposomes results in inability of synaptotagmin to stimulate membrane fusion, indicating anionic lipids to be a critical cofactor for synaptotagmin activity (Bhalla et al., 2005; Kreutzberger et al., 2017). Upon Ca2+ binding to the C2A and C2B domains, they penetrate into the lipid bilayer (Bai et al., 2002; Frazier et al., 2003; Rufener et al., 2005; Osterberg et al., 2015). This allows synaptotagmin to bend and deform membranes, as indicated by tubulation of liposomes initiated by the Syt-1 C2B or C2AB domain (Martens et al., 2007). The ability of Syt-1 to bend membranes and generate positive curvature is suggested to be an essential part of the mechanism used to stimulate membrane fusion, as tubulation-defective mutants preferentially induced fusion of high-curvature vesicles in vitro (Hui et al., 2009). Membrane penetration by the synaptotagmin C2 domains and generation of positive curvature may help to overcome an energy barrier along the pathway to hemifusion and/or fusion, e.g., by stabilizing a highly curved intermediate state (Fig. 8).
Another shared property of Syt-1 and Syt-7 C2 domains is their ability to bridge between membrane bilayers and induce aggregation of liposomes in vitro. The C2AB tandem and isolated C2B domain of Syt-1 induce liposome clustering (Araç et al., 2006; Hui et al., 2011; Seven et al., 2013), and the C2A domain of Syt-7 was recently reported to do the same (Hamilton et al., 2017; Voleti et al., 2017). The liposome aggregation effect has complicated the study of Syt-mediated curvature generation in vitro and led to questions about the importance of membrane bridging in synaptotagmin function. Syt-1 C2AB tandem domains can exhibit a wide range of orientations with respect to each other, both in solution and when bound to liposome membranes, including a bridging conformation in which the Ca2+-binding loops of C2A and C2B point in opposite directions (Herrick et al., 2009; Choi et al., 2010; Seven et al., 2013). Binding to PIP2/syntaxin-1 clusters via the β-4 polybasic region in Syt-1 C2B enhances bridging, as the Ca2+-binding loops insert in trans into PS-containing membranes (Honigmann et al., 2013). A second basic cluster comprising Arg398 and Arg399 (PBR2 in Figs. 6 and 7) also contributes to liposome bridging by Syt-1 C2B (Xue et al., 2008; Honigmann et al., 2013); however, these residues have also been implicated in protein–protein interactions, and how the interactions balance in vivo is not yet clear (Gaffaney et al., 2008; Hui et al., 2011; Zhou et al., 2015).
Central to the bridging question is how the two C2 domains work together, including their relative orientation when bound to membranes. For Syt-1, cis and trans membrane binding exist in a delicate balance, in which relative accessibility of target membranes plays a major role (Vennekate et al., 2012). Both fluorescence of insertion-dependent dyes and electron paramagnetic resonance depth measurements have been used to show that Syt-1 C2A and C2B insert more deeply into liposome membranes when present as a tandem, relative to the individual domains in isolation (Bai et al., 2002; Herrick et al., 2006). These results suggest that the two domains of Syt-1 cooperate to bind and insert into their target membranes, a finding also reported based on force measurements (Takahashi et al., 2010). However, the effect may be different for Syt-7; lateral diffusion measurements of Syt-7 C2A, C2B, and C2AB domains on supported lipid bilayers were consistent with independent membrane interactions of the two domains, indicating the absence of substantial intermolecular contacts of C2A with C2B (Vasquez et al., 2014). Liposome binding measurements from the same study further revealed lack of cooperativity in binding and dissociation of Syt-7 from phospholipid membranes. In principle, cooperativity between Syt-7 C2 domains may be unnecessary because of their strong membrane affinity; in Syt-1, interdomain cooperativity could promote rapid and efficient coinsertion despite the relatively modest membrane affinity of its C2A domain. One recent study probed the relative orientation of the two C2 domains in Syt-1 during membrane binding and exocytosis by systematically replacing the C2A-C2B linker with polyproline helices of varying lengths, each constraining the C2A and C2B domains in a particular relative orientation. Results both in vitro and in neurons showed a clear periodicity, indicating that fusion is most efficient when the two domains point their Ca2+-binding loops in the same direction (Bai et al., 2016). Although the Ca2+-binding loops represent the primary site of membrane interaction, a full picture of synaptotagmin membrane binding requires consideration of the polybasic regions as well as the Ca2+-binding loops on both domains.
Model for fusion pore stabilization
Collectively, the bridging, curvature, and coinsertion data support a structural model in which Syt–lipid interactions stabilize fusion pores by simultaneously inducing positive curvature in the ring of the pore via insertion of the Ca2+-binding loops and bridging between the secretory vesicle and plasma membrane in the orthogonal dimension via polybasic regions on both C2 domains (Fig. 8). This model is similar to the transition state in a previous structural hypothesis of synaptotagmin function (Martens et al., 2007), with the added observation that the polybasic regions of the two domains should be accessible to bind opposing membrane surfaces when their Ca2+-binding loops point in the same direction (Fig. 8 A). Presumably, the β-4 region of C2B (PBR1 in Fig. 6) orients toward the plasma membrane and binds PIP2, whereas the polybasic region of C2A orients toward the vesicle. Although the polybasic region of synaptotagmin C2A domains is not particularly selective for PIP2 binding because of the presence of a glutamate residue at a key position (Guillén et al., 2013), it is nevertheless present in Syt-1 as a region of positive surface charge density and has a greater surface potential in Syt-7 (Chon et al., 2015). The model in Fig. 8 allows for both the Ca2+-binding loops and polybasic regions of C2A and C2B to interact with membranes simultaneously and further predicts that fusion pore stabilization should depend on lipid binding via combined effects of all of these interactions.
It was recently shown that fusion of large dense-core vesicles in chromaffin cells and β-cells, two populations that use Syt-7, proceeds via a hemifused intermediate state (Zhao et al., 2016). The orientation shown in Fig. 8 could conceivably stabilize higher-energy states involved in formation of the hemifused intermediate and/or conversion of the hemifused intermediate to the fully fused state along with other SNARE fusion machinery proteins. Although Fig. 8 omits SNARE complexes and other accessory proteins for clarity, SNARE proteins are well known to form the core of the fusion machinery (Südhof and Rothman, 2009). Proper SNARE assembly is required for fusion, and synaptotagmin was unable to rescue fusion in vitro from a docked state formed by mutant SNARE complexes incapable of complete zippering (Hernandez et al., 2012). Our model is also not intended to reflect stoichiometry of synaptotagmin or SNARE complexes in the fusion pore, which has been a subject of active investigation (Mohrmann and Sørensen, 2012). It is possible that Syt C2AB domains may interact with SNAREs or other proteins during fusion pore stabilization (Dai et al., 2007; Gaffaney et al., 2008). Syt–SNARE interactions are also reported to be associated with clamping fusion before Ca2+ entry (Gaffaney et al., 2008; Zhou et al., 2017) and/or accelerating postfusion pore expansion (Lynch et al., 2008).
The orientation shown in Fig. 8 may represent a high-energy intermediate or transition state and does not specify the trajectory by which this orientation is reached upon Ca2+ entry. One possibility is that upon Ca2+ entry, the two C2 domains initially interact with whatever membrane is closest, and then are funneled together to the nascent fusion pore neck. (We note that in the crystal structure of the “primed” Syt/SNARE/complexin complex, the Ca2+-binding loops of the C2B domain at the tripartite interface orient outward toward the SNARE N termini, an orientation that would place them readily accessible to Ca2+ signals [Zhou et al., 2017].) After initial rapid fusion is a phase of fusion pore expansion, which is also accelerated by synaptotagmin in a Ca2+-dependent manner; the rate of expansion is much greater for Syt-1 vesicles than for Syt-7 vesicles (Rao et al., 2014). For Syt-1, rapid fusion pore expansion appears to involve interaction of the C2 domains with both SNARE proteins and membranes (Lynch et al., 2008). Chimeras between Syt-1 and Syt-7 exhibit intermediate rates of fusion pore expansion (Bendahmane et al., 2018); the intermolecular interactions responsible for this effect are not yet clear.
Conclusion and future directions
Syt-7 was originally identified as a Ca2+ sensor for lysosomal exocytosis, but the repertoire of known Syt-7 activities has since expanded to include roles in secretion from many different cell types (Table 1). Sometimes Syt-7 acts as the principal Ca2+ sensor, such as in glucagon secretion from pancreatic α-cells, but for the most part Syt-7 acts in concert with other, faster synaptotagmin isoforms such as Syt-1 to give rise to nuanced secretory outputs in response to different strengths and rates of stimulation, most notably in chromaffin cells and presynaptic axon terminals of the central nervous system. Biophysical studies have shown that Syt-7 exerts differential control over fusion pore opening and expansion, with stabilization of curved membrane structures such as the fusion pore being a hallmark of Syt-7 activity. The high Ca2+ sensitivity of Syt-7 and the slow dissociation of its C2 domains after penetration into phospholipid bilayers are just two of its well-established biochemical features. Such features are likely to play a role in the mechanisms Syt-7 uses to exert specialized control over exocytosis dynamics, ultimately enabling modulation of the type, rate, and extent of cargo release in a stimulus- and context-dependent manner. Syt-7 also interacts with many different effector molecules, often through the same structural motifs on the C2A and C2B domains. The overlapping nature of these binding interactions suggests that some may be mutually exclusive. Thus, Syt-7 may participate in a stepwise series of interactions with different partners along the trajectory from vesicle docking, to priming, to fusion pore opening, to fusion pore expansion. The timing of the interactions with different effectors, as well as the mechanistic roles they play in regulating exocytosis dynamics, requires further investigation.
Despite the significant progress made, questions remain about Syt-7’s mechanism of action. For example, when do C2 domains insert into the membrane and when are they released relative to fusion pore opening and expansion? Do C2A and C2B domains both penetrate the same membrane, or do they bridge vesicle and plasma membranes? How do C2A and C2B engage their respective effectors, and as mentioned above, do interaction modes change to regulate docking, priming, fusion pore opening, or fusion pore expansion? The ability to start addressing these questions will likely require structural studies of Syt-7 in complex with components of the SNARE apparatus and/or lipid bilayers.
It is also not yet completely understood how the modest sequence and structural differences in the C2 domains of Syt-7 compared with other synaptotagmin isoforms give rise to such substantial differences in fusion pore dynamics. Structural data show highly overlapping folds and positioning of Ca2+ ligands comparing Syt-7 with Syt-1, which offers few clues as to the molecular origin of their biochemical and functional differences. It could be that Syt-1 and Syt-7, though highly similar in structure, exhibit important dynamical differences such as specific intra- and/or interdomain conformational changes that regulate biochemical and functional activities, and we suggest that future studies explore this possibility. Analysis of chimeras containing Syt-7 structural elements grafted onto another synaptotagmin isoform or vice versa may also continue to be useful in teasing apart structure–function relationships.
Several apparent discrepancies regarding Syt-7 function remain incompletely resolved, namely: does endogenous Syt-7 exert its regulatory effects from the vesicle or the plasma membrane, and does it regulate individual fusion events independently or in concert with other isoforms, for example by clamping or otherwise modulating fusion kinetics of Syt-1–containing vesicles? These questions point to the larger issue of whether Syt-7 operates via a common mechanism in all cases. The possibilities described above are not necessarily mutually exclusive. Given the large number of cell types in which Syt-7 is expressed and the multitude of fusion processes in which it participates, it would not be entirely surprising to encounter different Syt-7 trafficking patterns, molecular interaction partners, and splice variants in different contexts that alter, in some way, its mode of action. It is possible that Syt-7’s versatility in regulating exocytosis dynamics in such a wide array of settings may be paralleled by changes in localization and mode of action that are context dependent.
Acknowledgments
The authors thank Drs. Volker Kiessling, Reinhard Jahn, and Josep Rizo for critical feedback and Jasmine Vazquez for illustrations.
Grant support was provided by the National Institutes of Health (GM102866 to J.D. Knight; GM111997 to A. Anantharam) and the American Heart Association (SDG14420049 to A. Anantharam).
The authors declare no competing financial interests.
Author contributions: D.D. MacDougall, S.L. Jackson, J.D. Knight, and A. Anantharam wrote the paper; Z. Lin, N.L. Chon, S.L. Jackman, H. Lin, J.D. Knight, and A. Anantharam prepared tables and figures; J.D. Knight and A. Anantharam assembled and edited the paper.
Lesley C. Anson served as editor.
References
- Adolfsen B., Saraswati S., Yoshihara M., and Littleton J.T.. 2004. Synaptotagmins are trafficked to distinct subcellular domains including the postsynaptic compartment. J. Cell Biol. 166:249–260. 10.1083/jcb.200312054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albillos A., Dernick G., Horstmann H., Almers W., Alvarez de Toledo G., and Lindau M.. 1997. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 389:509–512. 10.1038/39081 [DOI] [PubMed] [Google Scholar]
- Alés E., Tabares L., Poyato J.M., Valero V., Lindau M., and Alvarez de Toledo G.. 1999. High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat. Cell Biol. 1:40–44. 10.1038/9012 [DOI] [PubMed] [Google Scholar]
- Allersma M.W., Wang L., Axelrod D., and Holz R.W.. 2004. Visualization of regulated exocytosis with a granule-membrane probe using total internal reflection microscopy. Mol. Biol. Cell. 15:4658–4668. 10.1091/mbc.e04-02-0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allersma M.W., Bittner M.A., Axelrod D., and Holz R.W.. 2006. Motion matters: Secretory granule motion adjacent to the plasma membrane and exocytosis. Mol. Biol. Cell. 17:2424–2438. 10.1091/mbc.e05-10-0938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez de Toledo G., Fernández-Chacón R., and Fernández J.M.. 1993. Release of secretory products during transient vesicle fusion. Nature. 363:554–558. 10.1038/363554a0 [DOI] [PubMed] [Google Scholar]
- Anantharam A., Onoa B., Edwards R.H., Holz R.W., and Axelrod D.. 2010. Localized topological changes of the plasma membrane upon exocytosis visualized by polarized TIRFM. J. Cell Biol. 188:415–428. 10.1083/jcb.200908010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharam A., Bittner M.A., Aikman R.L., Stuenkel E.L., Schmid S.L., Axelrod D., and Holz R.W.. 2011. A new role for the dynamin GTPase in the regulation of fusion pore expansion. Mol. Biol. Cell. 22:1907–1918. 10.1091/mbc.e11-02-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araç D., Chen X., Khant H.A., Ubach J., Ludtke S.J., Kikkawa M., Johnson A.E., Chiu W., Südhof T.C., and Rizo J.. 2006. Close membrane-membrane proximity induced by Ca(2+)-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat. Struct. Mol. Biol. 13:209–217. 10.1038/nsmb1056 [DOI] [PubMed] [Google Scholar]
- Arantes R.M., and Andrews N.W.. 2006. A role for synaptotagmin VII-regulated exocytosis of lysosomes in neurite outgrowth from primary sympathetic neurons. J. Neurosci. 26:4630–4637. 10.1523/JNEUROSCI.0009-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atluri P.P., and Regehr W.G.. 1996. Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse. J. Neurosci. 16:5661–5671. 10.1523/JNEUROSCI.16-18-05661.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G.J., and Neher E.. 1992. Calcium requirements for secretion in bovine chromaffin cells. J. Physiol. 450:247–271. 10.1113/jphysiol.1992.sp019126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacaj T., Wu D., Yang X., Morishita W., Zhou P., Xu W., Malenka R.C., and Südhof T.C.. 2013. Synaptotagmin-1 and synaptotagmin-7 trigger synchronous and asynchronous phases of neurotransmitter release. Neuron. 80:947–959. 10.1016/j.neuron.2013.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacaj T., Wu D., Burré J., Malenka R.C., Liu X., and Südhof T.C.. 2015. Synaptotagmin-1 and -7 are redundantly essential for maintaining the capacity of the readily-releasable pool of synaptic vesicles. PLoS Biol. 13:e1002267 10.1371/journal.pbio.1002267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J., and Chapman E.R.. 2004. The C2 domains of synaptotagmin—Partners in exocytosis. Trends Biochem. Sci. 29:143–151. 10.1016/j.tibs.2004.01.008 [DOI] [PubMed] [Google Scholar]
- Bai H., Xue R., Bao H., Zhang L., Yethiraj A., Cui Q., and Chapman E.R.. 2016. Different states of synaptotagmin regulate evoked versus spontaneous release. Nat. Commun. 7:10971 10.1038/ncomms10971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J., Wang P., and Chapman E.R.. 2002. C2A activates a cryptic Ca(2+)-triggered membrane penetration activity within the C2B domain of synaptotagmin I. Proc. Natl. Acad. Sci. USA. 99:1665–1670. 10.1073/pnas.032541099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J., Tucker W.C., and Chapman E.R.. 2004a PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 11:36–44. 10.1038/nsmb709 [DOI] [PubMed] [Google Scholar]
- Bai J., Wang C.T., Richards D.A., Jackson M.B., and Chapman E.R.. 2004b Fusion pore dynamics are regulated by synaptotagmin*t-SNARE interactions. Neuron. 41:929–942. 10.1016/S0896-6273(04)00117-5 [DOI] [PubMed] [Google Scholar]
- Baker N.A., Sept D., Joseph S., Holst M.J., and McCammon J.A.. 2001. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA. 98:10037–10041. 10.1073/pnas.181342398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E.F., and Stevens C.F.. 1972. The kinetics of transmitter release at the frog neuromuscular junction. J. Physiol. 227:691–708. 10.1113/jphysiol.1972.sp010054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S.M., Delamarre L., Mellman I., and Andrews N.W.. 2009. Differential role of the Ca(2+) sensor synaptotagmin VII in macrophages and dendritic cells. Immunobiology. 214:495–505. 10.1016/j.imbio.2008.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendahmane M., Bohannon K.P., Bradberry M.M., Rao T.C., Schmidtke M.W., Abbineni P.S., Chon N.L., Tran S., Lin H., Chapman E.R., et al. 2018. The synaptotagmin C2B domain calcium-binding loops modulate the rate of fusion pore expansion. Mol. Biol. Cell. 10.1091/mbc.E17-11-0623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberian K., Torres A.J., Fang Q., Kisler K., and Lindau M.. 2009. F-actin and myosin II accelerate catecholamine release from chromaffin granules. J. Neurosci. 29:863–870. 10.1523/JNEUROSCI.2818-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla A., Tucker W.C., and Chapman E.R.. 2005. Synaptotagmin isoforms couple distinct ranges of Ca2+, Ba2+, and Sr2+ concentration to SNARE-mediated membrane fusion. Mol. Biol. Cell. 16:4755–4764. 10.1091/mbc.e05-04-0277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla A., Chicka M.C., Tucker W.C., and Chapman E.R.. 2006. Ca(2+)-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat. Struct. Mol. Biol. 13:323–330. 10.1038/nsmb1076 [DOI] [PubMed] [Google Scholar]
- Bhalla A., Chicka M.C., and Chapman E.R.. 2008. Analysis of the synaptotagmin family during reconstituted membrane fusion. Uncovering a class of inhibitory isoforms. J. Biol. Chem. 283:21799–21807. 10.1074/jbc.M709628200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt D.S., Coffman M.D., Falke J.J., and Knight J.D.. 2012. Hydrophobic contributions to the membrane docking of synaptotagmin 7 C2A domain: Mechanistic contrast between isoforms 1 and 7. Biochemistry. 51:7654–7664. 10.1021/bi3007115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer K.D., Bacaj T., Cavalli A., Camilloni C., Swarbrick J.D., Liu J., Zhou A., Zhou P., Barlow N., Xu J., et al. 2015. Dynamic binding mode of a Synaptotagmin-1-SNARE complex in solution. Nat. Struct. Mol. Biol. 22:555–564. 10.1038/nsmb.3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N., Petrenko A.G., Südhof T.C., and Jahn R.. 1992. Synaptotagmin: A calcium sensor on the synaptic vesicle surface. Science. 256:1021–1025. 10.1126/science.1589771 [DOI] [PubMed] [Google Scholar]
- Cai H., Reim K., Varoqueaux F., Tapechum S., Hill K., Sørensen J.B., Brose N., and Chow R.H.. 2008. Complexin II plays a positive role in Ca2+-triggered exocytosis by facilitating vesicle priming. Proc. Natl. Acad. Sci. USA. 105:19538–19543. 10.1073/pnas.0810232105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael S.W., and Winkler H.. 1985. The adrenal chromaffin cell. Sci. Am. 253:40–49. 10.1038/scientificamerican0885-40 [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Kobayashi K.S., Flavell R.A., Marks C.B., Miyake K., Liston D.R., Fowler K.T., Gorelick F.S., and Andrews N.W.. 2003. Impaired membrane resealing and autoimmune myositis in synaptotagmin VII–deficient mice. J. Cell Biol. 162:543–549. 10.1083/jcb.200305131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.W., Chiang C.W., and Jackson M.B.. 2017. Fusion pores and their control of neurotransmitter and hormone release. J. Gen. Physiol. 149:301–322. 10.1085/jgp.201611724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Trimbuch T., and Rosenmund C.. 2018. Synaptotagmin-1 drives synchronous Ca2+-triggered fusion by C2B-domain-mediated synaptic-vesicle-membrane attachment. Nat. Neurosci. 21:33–40. 10.1038/s41593-017-0037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E.R. 2008. How does synaptotagmin trigger neurotransmitter release? Annu. Rev. Biochem. 77:615–641. 10.1146/annurev.biochem.77.062005.101135 [DOI] [PubMed] [Google Scholar]
- Chapman E.R., and Jahn R.. 1994. Calcium-dependent interaction of the cytoplasmic region of synaptotagmin with membranes. Autonomous function of a single C2-homologous domain. J. Biol. Chem. 269:5735–5741. [PubMed] [Google Scholar]
- Chapman E.R., Blasi J., An S., Brose N., Johnston P.A., Südhof T.C., and Jahn R.. 1996. Fatty acylation of synaptotagmin in PC12 cells and synaptosomes. Biochem. Biophys. Res. Commun. 225:326–332. 10.1006/bbrc.1996.1174 [DOI] [PubMed] [Google Scholar]
- Charvin N., L’evêque C., Walker D., Berton F., Raymond C., Kataoka M., Shoji-Kasai Y., Takahashi M., De Waard M., and Seagar M.J.. 1997. Direct interaction of the calcium sensor protein synaptotagmin I with a cytoplasmic domain of the alpha1A subunit of the P/Q-type calcium channel. EMBO J. 16:4591–4596. 10.1093/emboj/16.15.4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Satterfield R., Young S.M. Jr., and Jonas P.. 2017. Triple function of synaptotagmin 7 ensures efficiency of high-frequency transmission at central GABAergic synapses. Cell Reports. 21:2082–2089. 10.1016/j.celrep.2017.10.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Sequeira S.M., Malinina L., Tereshko V., Söllner T.H., and Patel D.J.. 2004. Crystallographic identification of Ca2+ and Sr2+ coordination sites in synaptotagmin I C2B domain. Protein Sci. 13:2665–2672. 10.1110/ps.04832604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L.V., Frolov V.A., Leikina E., Bronk P., and Zimmerberg J.. 1998. The pathway of membrane fusion catalyzed by influenza hemagglutinin: Restriction of lipids, hemifusion, and lipidic fusion pore formation. J. Cell Biol. 140:1369–1382. 10.1083/jcb.140.6.1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik L.V., Zimmerberg J., and Kozlov M.M.. 2006. Membranes of the world unite! J. Cell Biol. 175:201–207. 10.1083/jcb.200607083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicka M.C., Hui E., Liu H., and Chapman E.R.. 2008. Synaptotagmin arrests the SNARE complex before triggering fast, efficient membrane fusion in response to Ca2+. Nat. Struct. Mol. Biol. 15:827–835. 10.1038/nsmb.1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieregatti E., Chicka M.C., Chapman E.R., and Baldini G.. 2004. SNAP-23 functions in docking/fusion of granules at low Ca2+. Mol. Biol. Cell. 15:1918–1930. 10.1091/mbc.e03-09-0684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi U.B., Strop P., Vrljic M., Chu S., Brunger A.T., and Weninger K.R.. 2010. Single-molecule FRET-derived model of the synaptotagmin 1-SNARE fusion complex. Nat. Struct. Mol. Biol. 17:318–324. 10.1038/nsmb.1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chon N.L., Osterberg J.R., Henderson J., Khan H.M., Reuter N., Knight J.D., and Lin H.. 2015. Membrane docking of the synaptotagmin 7 C2A domain: Computation reveals interplay between electrostatic and hydrophobic contributions. Biochemistry. 54:5696–5711. 10.1021/acs.biochem.5b00422 [DOI] [PubMed] [Google Scholar]
- Chow R.H., von Rüden L., and Neher E.. 1992. Delay in vesicle fusion revealed by electrochemical monitoring of single secretory events in adrenal chromaffin cells. Nature. 356:60–63. 10.1038/356060a0 [DOI] [PubMed] [Google Scholar]
- Chow R.H., Klingauf J., and Neher E.. 1994. Time course of Ca2+ concentration triggering exocytosis in neuroendocrine cells. Proc. Natl. Acad. Sci. USA. 91:12765–12769. 10.1073/pnas.91.26.12765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R.L., Winslow J.L., Govind C.K., and Atwood H.L.. 1996. Synaptic structural complexity as a factor enhancing probability of calcium-mediated transmitter release. J. Neurophysiol. 75:2451–2466. 10.1152/jn.1996.75.6.2451 [DOI] [PubMed] [Google Scholar]
- Corbalan-Garcia S., and Gómez-Fernández J.C.. 2014. Signaling through C2 domains: More than one lipid target. Biochim. Biophys. Acta. 1838:1536–1547. 10.1016/j.bbamem.2014.01.008 [DOI] [PubMed] [Google Scholar]
- Craxton M. 2010. A manual collection of Syt, Esyt, Rph3a, Rph3al, Doc2, and Dblc2 genes from 46 metazoan genomes—An open access resource for neuroscience and evolutionary biology. BMC Genomics. 11:37 10.1186/1471-2164-11-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czibener C., Sherer N.M., Becker S.M., Pypaert M., Hui E., Chapman E.R., Mothes W., and Andrews N.W.. 2006. Ca2+ and synaptotagmin VII–dependent delivery of lysosomal membrane to nascent phagosomes. J. Cell Biol. 174:997–1007. 10.1083/jcb.200605004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H., Shen N., Araç D., and Rizo J.. 2007. A quaternary SNARE-synaptotagmin-Ca2+-phospholipid complex in neurotransmitter release. J. Mol. Biol. 367:848–863. 10.1016/j.jmb.2007.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletov B.A., and Südhof T.C.. 1993. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J. Biol. Chem. 268:26386–26390. [PubMed] [Google Scholar]
- Degtyar V.E., Allersma M.W., Axelrod D., and Holz R.W.. 2007. Increased motion and travel, rather than stable docking, characterize the last moments before secretory granule fusion. Proc. Natl. Acad. Sci. USA. 104:15929–15934. 10.1073/pnas.0705406104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai R.C., Vyas B., Earles C.A., Littleton J.T., Kowalchyck J.A., Martin T.F., and Chapman E.R.. 2000. The C2B domain of synaptotagmin is a Ca2+-sensing module essential for exocytosis. J. Cell Biol. 150:1125–1136. 10.1083/jcb.150.5.1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnos C., Huet S., Fanget I., Chapuis C., Böttiger C., Racine V., Sibarita J.B., Henry J.P., and Darchen F.. 2007. Myosin va mediates docking of secretory granules at the plasma membrane. J. Neurosci. 27:10636–10645. 10.1523/JNEUROSCI.1228-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao J., Grob P., Cipriano D.J., Kyoung M., Zhang Y., Shah S., Nguyen A., Padolina M., Srivastava A., Vrljic M., et al. 2012. Synaptic proteins promote calcium-triggered fast transition from point contact to full fusion. eLife. 1:e00109 10.7554/eLife.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolai S., Xie L., Zhu D., Liang T., Qin T., Xie H., Kang Y., Chapman E.R., and Gaisano H.Y.. 2016. Synaptotagmin-7 functions to replenish insulin granules for exocytosis in human islet β-cells. Diabetes. 65:1962–1976. 10.2337/db15-1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinsky T.J., Nielsen J.E., McCammon J.A., and Baker N.A.. 2004. PDB2PQR: An automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 32(Web Server):W665–W667. 10.1093/nar/gkh381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W.W., and Rubin R.P.. 1961. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J. Physiol. 159:40–57. 10.1113/jphysiol.1961.sp006791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard D.A., Cooper C.L., Low M.G., and Holz R.W.. 1990. Evidence that the inositol phospholipids are necessary for exocytosis. Loss of inositol phospholipids and inhibition of secretion in permeabilized cells caused by a bacterial phospholipase C and removal of ATP. Biochem. J. 268:15–25. 10.1042/bj2680015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.S., Ruhl D.A., and Chapman E.R.. 2015. An engineered metal sensor tunes the kinetics of synaptic transmission. J. Neurosci. 35:11769–11779. 10.1523/JNEUROSCI.1694-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerberg L., Hallström B.M., Oksvold P., Kampf C., Djureinovic D., Odeberg J., Habuka M., Tahmasebpoor S., Danielsson A., Edlund K., et al. 2014. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteomics. 13:397–406. 10.1074/mcp.M113.035600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski M.A., Thomas D.D., Messenger S.W., Martin T.F., and Groblewski G.E.. 2011. Expression, localization, and functional role for synaptotagmins in pancreatic acinar cells. Am. J. Physiol. Gastrointest. Liver Physiol. 301:G306–G316. 10.1152/ajpgi.00108.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fealey M.E., Mahling R., Rice A.M., Dunleavy K., Kobany S.E., Lohese K.J., Horn B., and Hinderliter A.. 2016. Synaptotagmin I’s intrinsically disordered region interacts with synaptic vesicle lipids and exerts allosteric control over C2A. Biochemistry. 55:2914–2926. 10.1021/acs.biochem.6b00085 [DOI] [PubMed] [Google Scholar]
- Fernandez I., Araç D., Ubach J., Gerber S.H., Shin O., Gao Y., Anderson R.G., Südhof T.C., and Rizo J.. 2001. Three-dimensional structure of the synaptotagmin 1 C2B-domain: synaptotagmin 1 as a phospholipid binding machine. Neuron. 32:1057–1069. 10.1016/S0896-6273(01)00548-7 [DOI] [PubMed] [Google Scholar]
- Flannery A.R., Czibener C., and Andrews N.W.. 2010. Palmitoylation-dependent association with CD63 targets the Ca2+ sensor synaptotagmin VII to lysosomes. J. Cell Biol. 191:599–613. 10.1083/jcb.201003021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler K.T., Andrews N.W., and Huleatt J.W.. 2007. Expression and function of synaptotagmin VII in CTLs. J. Immunol. 178:1498–1504. 10.4049/jimmunol.178.3.1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier A.A., Roller C.R., Havelka J.J., Hinderliter A., and Cafiso D.S.. 2003. Membrane-bound orientation and position of the synaptotagmin I C2A domain by site-directed spin labeling. Biochemistry. 42:96–105. 10.1021/bi0268145 [DOI] [PubMed] [Google Scholar]
- Fukuda M. 2002. Vesicle-associated membrane protein-2/synaptobrevin binding to synaptotagmin I promotes O-glycosylation of synaptotagmin I. J. Biol. Chem. 277:30351–30358. 10.1074/jbc.M204056200 [DOI] [PubMed] [Google Scholar]
- Fukuda M., and Mikoshiba K.. 2000a Calcium-dependent and -independent hetero-oligomerization in the synaptotagmin family. J. Biochem. 128:637–645. 10.1093/oxfordjournals.jbchem.a022796 [DOI] [PubMed] [Google Scholar]
- Fukuda M., and Mikoshiba K.. 2000b Distinct self-oligomerization activities of synaptotagmin family. Unique calcium-dependent oligomerization properties of synaptotagmin VII. J. Biol. Chem. 275:28180–28185. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Katayama E., and Mikoshiba K.. 2002a The calcium-binding loops of the tandem C2 domains of synaptotagmin VII cooperatively mediate calcium-dependent oligomerization. J. Biol. Chem. 277:29315–29320. 10.1074/jbc.M201697200 [DOI] [PubMed] [Google Scholar]
- Fukuda M., Ogata Y., Saegusa C., Kanno E., and Mikoshiba K.. 2002b Alternative splicing isoforms of synaptotagmin VII in the mouse, rat and human. Biochem. J. 365:173–180. 10.1042/bj20011877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M., Kanno E., Satoh M., Saegusa C., and Yamamoto A.. 2004. Synaptotagmin VII is targeted to dense-core vesicles and regulates their Ca2+-dependent exocytosis in PC12 cells. J. Biol. Chem. 279:52677–52684. 10.1074/jbc.M409241200 [DOI] [PubMed] [Google Scholar]
- Fulop T., and Smith C.. 2007. Matching native electrical stimulation by graded chemical stimulation in isolated mouse adrenal chromaffin cells. J. Neurosci. Methods. 166:195–202. 10.1016/j.jneumeth.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T., Radabaugh S., and Smith C.. 2005. Activity-dependent differential transmitter release in mouse adrenal chromaffin cells. J. Neurosci. 25:7324–7332. 10.1523/JNEUROSCI.2042-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T., Doreian B., and Smith C.. 2008. Dynamin I plays dual roles in the activity-dependent shift in exocytic mode in mouse adrenal chromaffin cells. Arch. Biochem. Biophys. 477:146–154. 10.1016/j.abb.2008.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffaney J.D., Dunning F.M., Wang Z., Hui E., and Chapman E.R.. 2008. Synaptotagmin C2B domain regulates Ca2+-triggered fusion in vitro: Critical residues revealed by scanning alanine mutagenesis. J. Biol. Chem. 283:31763–31775. 10.1074/jbc.M803355200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisano H.Y. 2017. Recent new insights into the role of SNARE and associated proteins in insulin granule exocytosis. Diabetes Obes. Metab. 19(Suppl 1):115–123. 10.1111/dom.13001 [DOI] [PubMed] [Google Scholar]
- García A.G., García-De-Diego A.M., Gandía L., Borges R., and García-Sancho J.. 2006. Calcium signaling and exocytosis in adrenal chromaffin cells. Physiol. Rev. 86:1093–1131. 10.1152/physrev.00039.2005 [DOI] [PubMed] [Google Scholar]
- Gauthier B.R., Duhamel D.L., Iezzi M., Theander S., Saltel F., Fukuda M., Wehrle-Haller B., and Wollheim C.B.. 2008. Synaptotagmin VII splice variants alpha, beta, and delta are expressed in pancreatic beta-cells and regulate insulin exocytosis. FASEB J. 22:194–206. 10.1096/fj.07-8333com [DOI] [PubMed] [Google Scholar]
- Geppert M., Goda Y., Hammer R.E., Li C., Rosahl T.W., Stevens C.F., and Südhof T.C.. 1994. Synaptotagmin I: A major Ca2+ sensor for transmitter release at a central synapse. Cell. 79:717–727. 10.1016/0092-8674(94)90556-8 [DOI] [PubMed] [Google Scholar]
- Giraudo C.G., Garcia-Diaz A., Eng W.S., Yamamoto A., Melia T.J., and Rothman J.E.. 2008. Distinct domains of complexins bind SNARE complexes and clamp fusion in vitro. J. Biol. Chem. 283:21211–21219. 10.1074/jbc.M803478200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda Y., and Stevens C.F.. 1994. Two components of transmitter release at a central synapse. Proc. Natl. Acad. Sci. USA. 91:12942–12946. 10.1073/pnas.91.26.12942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M.E., O’Callaghan D.W., McMahon H.T., and Burgoyne R.D.. 2002. Dynamin-dependent and dynamin-independent processes contribute to the regulation of single vesicle release kinetics and quantal size. Proc. Natl. Acad. Sci. USA. 99:7124–7129. 10.1073/pnas.102645099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesinger C.B., Richards C.D., and Ashmore J.F.. 2005. Fast vesicle replenishment allows indefatigable signalling at the first auditory synapse. Nature. 435:212–215. 10.1038/nature03567 [DOI] [PubMed] [Google Scholar]
- Guillén J., Ferrer-Orta C., Buxaderas M., Pérez-Sánchez D., Guerrero-Valero M., Luengo-Gil G., Pous J., Guerra P., Gómez-Fernández J.C., Verdaguer N., and Corbalán-García S.. 2013. Structural insights into the Ca2+ and PI(4,5)P2 binding modes of the C2 domains of rabphilin 3A and synaptotagmin 1. Proc. Natl. Acad. Sci. USA. 110:20503–20508. 10.1073/pnas.1316179110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson N., and Han W.. 2009. Calcium-sensing beyond neurotransmitters: Functions of synaptotagmins in neuroendocrine and endocrine secretion. Biosci. Rep. 29:245–259. 10.1042/BSR20090031 [DOI] [PubMed] [Google Scholar]
- Gustavsson N., Lao Y., Maximov A., Chuang J.C., Kostromina E., Repa J.J., Li C., Radda G.K., Südhof T.C., and Han W.. 2008. Impaired insulin secretion and glucose intolerance in synaptotagmin-7 null mutant mice. Proc. Natl. Acad. Sci. USA. 105:3992–3997. 10.1073/pnas.0711700105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson N., Wei S.H., Hoang D.N., Lao Y., Zhang Q., Radda G.K., Rorsman P., Südhof T.C., and Han W.. 2009. Synaptotagmin-7 is a principal Ca2+ sensor for Ca2+-induced glucagon exocytosis in pancreas. J. Physiol. 587:1169–1178. 10.1113/jphysiol.2008.168005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton D.J., Coffman M.D., Knight J.D., and Reed S.M.. 2017. Lipid-coated gold nanoparticles and FRET allow sensitive monitoring of liposome clustering mediated by the synaptotagmin-7 C2A domain. Langmuir. 33:9222–9230. 10.1021/acs.langmuir.7b01397 [DOI] [PubMed] [Google Scholar]
- Han W., Rhee J.-S., Maximov A., Lao Y., Mashimo T., Rosenmund C., and Südhof T.C.. 2004. N-glycosylation is essential for vesicular targeting of synaptotagmin 1. Neuron. 41:85–99. 10.1016/S0896-6273(03)00820-1 [DOI] [PubMed] [Google Scholar]
- Hay J.C., Fisette P.L., Jenkins G.H., Fukami K., Takenawa T., Anderson R.A., and Martin T.F.J.. 1995. ATP-dependent inositide phosphorylation required for Ca(2+)-activated secretion. Nature. 374:173–177. 10.1038/374173a0 [DOI] [PubMed] [Google Scholar]
- Heindel U., Schmidt M.F., and Veit M.. 2003. Palmitoylation sites and processing of synaptotagmin I, the putative calcium sensor for neurosecretion. FEBS Lett. 544:57–62. 10.1016/S0014-5793(03)00449-6 [DOI] [PubMed] [Google Scholar]
- Heinemann C., von Rüden L., Chow R.H., and Neher E.. 1993. A two-step model of secretion control in neuroendocrine cells. Pflugers Arch. 424:105–112. 10.1007/BF00374600 [DOI] [PubMed] [Google Scholar]
- Heinemann C., Chow R.H., Neher E., and Zucker R.S.. 1994. Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+. Biophys. J. 67:2546–2557. 10.1016/S0006-3495(94)80744-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez J.M., Stein A., Behrmann E., Riedel D., Cypionka A., Farsi Z., Walla P.J., Raunser S., and Jahn R.. 2012. Membrane fusion intermediates via directional and full assembly of the SNARE complex. Science. 336:1581–1584. 10.1126/science.1221976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick D.Z., Sterbling S., Rasch K.A., Hinderliter A., and Cafiso D.S.. 2006. Position of synaptotagmin I at the membrane interface: Cooperative interactions of tandem C2 domains. Biochemistry. 45:9668–9674. 10.1021/bi060874j [DOI] [PubMed] [Google Scholar]
- Herrick D.Z., Kuo W., Huang H., Schwieters C.D., Ellena J.F., and Cafiso D.S.. 2009. Solution and membrane-bound conformations of the tandem C2A and C2B domains of synaptotagmin 1: Evidence for bilayer bridging. J. Mol. Biol. 390:913–923. 10.1016/j.jmb.2009.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz R.W., and Axelrod D.. 2002. Localization of phosphatidylinositol 4,5-P(2) important in exocytosis and a quantitative analysis of chromaffin granule motion adjacent to the plasma membrane. Ann. N. Y. Acad. Sci. 971:232–243. 10.1111/j.1749-6632.2002.tb04467.x [DOI] [PubMed] [Google Scholar]
- Holz R.W., and Axelrod D.. 2008. Secretory granule behaviour adjacent to the plasma membrane before and during exocytosis: Total internal reflection fluorescence microscopy studies. Acta Physiol. (Oxf.). 192:303–307. 10.1111/j.1748-1716.2007.01818.x [DOI] [PubMed] [Google Scholar]
- Honigmann A., van den Bogaart G., Iraheta E., Risselada H.J., Milovanovic D., Mueller V., Müllar S., Diederichsen U., Fasshauer D., Grubmüller H., et al. 2013. Phosphatidylinositol 4,5-bisphosphate clusters act as molecular beacons for vesicle recruitment. Nat. Struct. Mol. Biol. 20:679–686. 10.1038/nsmb.2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houy S., Groffen A.J., Ziomkiewicz I., Verhage M., Pinheiro P.S., and Sørensen J.B.. 2017. Doc2B acts as a calcium sensor for vesicle priming requiring synaptotagmin-1, Munc13-2 and SNAREs. eLife. 6:e27000 10.7554/eLife.27000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E., Bai J., Wang P., Sugimori M., Llinas R.R., and Chapman E.R.. 2005. Three distinct kinetic groupings of the synaptotagmin family: Candidate sensors for rapid and delayed exocytosis. Proc. Natl. Acad. Sci. USA. 102:5210–5214. 10.1073/pnas.0500941102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E., Bai J., and Chapman E.R.. 2006. Ca2+-triggered simultaneous membrane penetration of the tandem C2-domains of synaptotagmin I. Biophys. J. 91:1767–1777. 10.1529/biophysj.105.080325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E., Johnson C.P., Yao J., Dunning F.M., and Chapman E.R.. 2009. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca(2+)-regulated fusion. Cell. 138:709–721. 10.1016/j.cell.2009.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E., Gaffaney J.D., Wang Z., Johnson C.P., Evans C.S., and Chapman E.R.. 2011. Mechanism and function of synaptotagmin-mediated membrane apposition. Nat. Struct. Mol. Biol. 18:813–821. 10.1038/nsmb.2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman S.L., and Regehr W.G.. 2017. The mechanisms and functions of synaptic facilitation. Neuron. 94:447–464. 10.1016/j.neuron.2017.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman S.L., Turecek J., Belinsky J.E., and Regehr W.G.. 2016. The calcium sensor synaptotagmin 7 is required for synaptic facilitation. Nature. 529:88–91. 10.1038/nature16507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., and Fasshauer D.. 2012. Molecular machines governing exocytosis of synaptic vesicles. Nature. 490:201–207. 10.1038/nature11320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal J.K., Chakrabarti S., Andrews N.W., and Simon S.M.. 2004. Synaptotagmin VII restricts fusion pore expansion during lysosomal exocytosis. PLoS Biol. 2:E233 10.1371/journal.pbio.0020233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal J.K., Rivera V.M., and Simon S.M.. 2009. Exocytosis of post-Golgi vesicles is regulated by components of the endocytic machinery. Cell. 137:1308–1319. 10.1016/j.cell.2009.04.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo H., Byun H.M., Kim J.H., Kim M.S., Kim S.H., Hong J.H., Seo J.T., Lee S.I., Shin D.M., and Son H.K.. 2006. Expression of Ca2+-dependent synaptotagmin isoforms in mouse and rat parotid acinar cells. Yonsei Med. J. 47:70–77. 10.3349/ymj.2006.47.1.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R., Swayze R., Lise M.F., Gerrow K., Mullard A., Honer W.G., and El-Husseini A.. 2004. Presynaptic trafficking of synaptotagmin I is regulated by protein palmitoylation. J. Biol. Chem. 279:50524–50536. 10.1074/jbc.M404981200 [DOI] [PubMed] [Google Scholar]
- Kanno E., and Fukuda M.. 2008. Increased plasma membrane localization of O-glycosylation-deficient mutant of synaptotagmin I in PC12 cells. J. Neurosci. Res. 86:1036–1043. 10.1002/jnr.21568 [DOI] [PubMed] [Google Scholar]
- Katz B., and Miledi R.. 1965. The effect of calcium on acetylcholine release from motor nerve terminals. Proc. R. Soc. Lond. B Biol. Sci. 161:496–503. 10.1098/rspb.1965.0017 [DOI] [PubMed] [Google Scholar]
- Katz B., and Miledi R.. 1968. The role of calcium in neuromuscular facilitation. J. Physiol. 195:481–492. 10.1113/jphysiol.1968.sp008469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.Y., Choi B.K., Choi M.G., Kim S.A., Lai Y., Shin Y.K., and Lee N.K.. 2012. Solution single-vesicle assay reveals PIP2-mediated sequential actions of synaptotagmin-1 on SNAREs. EMBO J. 31:2144–2155. 10.1038/emboj.2012.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft M., Kuster V., Grilc S., Rupnik M., Milisav I., and Zorec R.. 2003. Synaptotagmin I increases the probability of vesicle fusion at low [Ca2+] in pituitary cells. Am. J. Physiol. Cell Physiol. 284:C547–C554. 10.1152/ajpcell.00333.2002 [DOI] [PubMed] [Google Scholar]
- Krementsov D.N., Krementsova E.B., and Trybus K.M.. 2004. Myosin V. J. Cell Biol. 164:877–886. 10.1083/jcb.200310065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberger A.J.B., Kiessling V., Liang B., Seelheim P., Jakhanwal S., Jahn R., Castle J.D., and Tamm L.K.. 2017. Reconstitution of calcium-mediated exocytosis of dense-core vesicles. Sci. Adv. 3:e1603208 10.1126/sciadv.1603208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnakumar S.S., Radoff D.T., Kümmel D., Giraudo C.G., Li F., Khandan L., Baguley S.W., Coleman J., Reinisch K.M., Pincet F., and Rothman J.E.. 2011. A conformational switch in complexin is required for synaptotagmin to trigger synaptic fusion. Nat. Struct. Mol. Biol. 18:934–940. 10.1038/nsmb.2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin P.I., Zimmerberg J., Chizmadzhev Y.A., and Cohen F.S.. 2001. A quantitative model for membrane fusion based on low-energy intermediates. Proc. Natl. Acad. Sci. USA. 98:7235–7240. 10.1073/pnas.121191898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S.E., and Chapman E.R.. 2012. Glycosylation is dispensable for sorting of synaptotagmin 1 but is critical for targeting of SV2 and synaptophysin to recycling synaptic vesicles. J. Biol. Chem. 287:35658–35668. 10.1074/jbc.M112.398883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyoung M., Srivastava A., Zhang Y., Diao J., Vrljic M., Grob P., Nogales E., Chu S., and Brunger A.T.. 2011. In vitro system capable of differentiating fast Ca2+-triggered content mixing from lipid exchange for mechanistic studies of neurotransmitter release. Proc. Natl. Acad. Sci. USA. 108:E304–E313. 10.1073/pnas.1107900108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y., Lou X., Jho Y., Yoon T.Y., and Shin Y.K.. 2013. The synaptotagmin 1 linker may function as an electrostatic zipper that opens for docking but closes for fusion pore opening. Biochem. J. 456:25–33. 10.1042/BJ20130949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T., Wacker I., Wunderlich I., Rohrbach A., Giese G., Soldati T., and Almers W.. 2000. Role of actin cortex in the subplasmalemmal transport of secretory granules in PC-12 cells. Biophys. J. 78:2863–2877. 10.1016/S0006-3495(00)76828-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan E.S., Lanni F., and Shakiryanova D.. 2007. In vivo imaging of vesicle motion and release at the Drosophila neuromuscular junction. Nat. Protoc. 2:1117–1125. 10.1038/nprot.2007.142 [DOI] [PubMed] [Google Scholar]
- Li C., Ullrich B., Zhang J.Z., Anderson R.G., Brose N., and Südhof T.C.. 1995. Ca(2+)-dependent and -independent activities of neural and non-neural synaptotagmins. Nature. 375:594–599. 10.1038/375594a0 [DOI] [PubMed] [Google Scholar]
- Li D., Xiong J., Qu A., and Xu T.. 2004. Three-dimensional tracking of single secretory granules in live PC12 cells. Biophys. J. 87:1991–2001. 10.1529/biophysj.104.043281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang P., Xu J., Gorelick F., Yamazaki H., Andrews N., and Desir G.V.. 2007. Regulation of insulin secretion and GLUT4 trafficking by the calcium sensor synaptotagmin VII. Biochem. Biophys. Res. Commun. 362:658–664. 10.1016/j.bbrc.2007.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.C., Chanaday N.L., Xu W., and Kavalali E.T.. 2017. Synaptotagmin-1- and synaptotagmin-7-dependent fusion mechanisms target synaptic vesicles to kinetically distinct endocytic pathways. Neuron. 93:616–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M.Y., Rohan J.G., Cai H., Reim K., Ko C.P., and Chow R.H.. 2013. Complexin facilitates exocytosis and synchronizes vesicle release in two secretory model systems. J. Physiol. 591:2463–2473. 10.1113/jphysiol.2012.244517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Bai H., Hui E., Yang L., Evans C.S., Wang Z., Kwon S.E., and Chapman E.R.. 2014. Synaptotagmin 7 functions as a Ca2+-sensor for synaptic vesicle replenishment. eLife. 3:e01524 10.7554/eLife.01524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.W., Neumann S., Ramachandran R., Ferguson S.M., Pucadyil T.J., and Schmid S.L.. 2011. Differential curvature sensing and generating activities of dynamin isoforms provide opportunities for tissue-specific regulation. Proc. Natl. Acad. Sci. USA. 108:E234–E242. 10.1073/pnas.1102710108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., and Silver R.B.. 1992. Microdomains of high calcium concentration in a presynaptic terminal. Science. 256:677–679. 10.1126/science.1350109 [DOI] [PubMed] [Google Scholar]
- Lu B., Kiessling V., Tamm L.K., and Cafiso D.S.. 2014. The juxtamembrane linker of full-length synaptotagmin 1 controls oligomerization and calcium-dependent membrane binding. J. Biol. Chem. 289:22161–22171. 10.1074/jbc.M114.569327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F., and Sudhof T.C.. 2017. Synaptotagmin-7-mediated asynchronous release boosts high-fidelity synchronous transmission at a central synapse. Neuron. 94:826–839. [DOI] [PubMed] [Google Scholar]
- Luo F., Bacaj T., and Südhof T.C.. 2015. Synaptotagmin-7 is essential for Ca2+-triggered delayed asynchronous release but not for Ca2+-dependent vesicle priming in retinal ribbon synapses. J. Neurosci. 35:11024–11033. 10.1523/JNEUROSCI.0759-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch K.L., Gerona R.R., Larsen E.C., Marcia R.F., Mitchell J.C., and Martin T.F.. 2007. Synaptotagmin C2A loop 2 mediates Ca2+-dependent SNARE interactions essential for Ca2+-triggered vesicle exocytosis. Mol. Biol. Cell. 18:4957–4968. 10.1091/mbc.e07-04-0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch K.L., Gerona R.R., Kielar D.M., Martens S., McMahon H.T., and Martin T.F.. 2008. Synaptotagmin-1 utilizes membrane bending and SNARE binding to drive fusion pore expansion. Mol. Biol. Cell. 19:5093–5103. 10.1091/mbc.e08-03-0235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald P.E., Braun M., Galvanovskis J., and Rorsman P.. 2006. Release of small transmitters through kiss-and-run fusion pores in rat pancreatic beta cells. Cell Metab. 4:283–290. 10.1016/j.cmet.2006.08.011 [DOI] [PubMed] [Google Scholar]
- Mahapatra S., Calorio C., Vandael D.H., Marcantoni A., Carabelli V., and Carbone E.. 2012. Calcium channel types contributing to chromaffin cell excitability, exocytosis and endocytosis. Cell Calcium. 51:321–330. 10.1016/j.ceca.2012.01.005 [DOI] [PubMed] [Google Scholar]
- Mallart A., and Martin A.R.. 1967. Two components of facilitation at the neuromuscular junction of the frog. J. Physiol. 191:19P–20P. [PMC free article] [PubMed] [Google Scholar]
- Martens S., Kozlov M.M., and McMahon H.T.. 2007. How synaptotagmin promotes membrane fusion. Science. 316:1205–1208. 10.1126/science.1142614 [DOI] [PubMed] [Google Scholar]
- Martinez I., Chakrabarti S., Hellevik T., Morehead J., Fowler K., and Andrews N.W.. 2000. Synaptotagmin VII regulates Ca2+-dependent exocytosis of lysosomes in fibroblasts. J. Cell Biol. 148:1141–1149. 10.1083/jcb.148.6.1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka H., Harada K., Nakamura J., Fukuda M., and Inoue M.. 2011. Differential distribution of synaptotagmin-1, -4, -7, and -9 in rat adrenal chromaffin cells. Cell Tissue Res. 344:41–50. 10.1007/s00441-011-1131-8 [DOI] [PubMed] [Google Scholar]
- Maximov A., and Südhof T.C.. 2005. Autonomous function of synaptotagmin 1 in triggering synchronous release independent of asynchronous release. Neuron. 48:547–554. 10.1016/j.neuron.2005.09.006 [DOI] [PubMed] [Google Scholar]
- Maximov A., Lao Y., Li H., Chen X., Rizo J., Sørensen J.B., and Südhof T.C.. 2008. Genetic analysis of synaptotagmin-7 function in synaptic vesicle exocytosis. Proc. Natl. Acad. Sci. USA. 105:3986–3991. 10.1073/pnas.0712372105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadam P.K., and Jackson M.B.. 2013. The functional significance of synaptotagmin diversity in neuroendocrine secretion. Front. Endocrinol. (Lausanne). 4:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrmann R., and Sørensen J.B.. 2012. SNARE requirements en route to exocytosis: From many to few. J. Mol. Neurosci. 48:387–394. 10.1007/s12031-012-9744-2 [DOI] [PubMed] [Google Scholar]
- Murphy C.T., Rock R.S., and Spudich J.A.. 2001. A myosin II mutation uncouples ATPase activity from motility and shortens step size. Nat. Cell Biol. 3:311–315. 10.1038/35060110 [DOI] [PubMed] [Google Scholar]
- Nalefski E.A., and Falke J.J.. 1996. The C2 domain calcium-binding motif: Structural and functional diversity. Protein Sci. 5:2375–2390. 10.1002/pro.5560051201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalefski E.A., Wisner M.A., Chen J.Z., Sprang S.R., Fukuda M., Mikoshiba K., and Falke J.J.. 2001. C2 domains from different Ca2+ signaling pathways display functional and mechanistic diversity. Biochemistry. 40:3089–3100. 10.1021/bi001968a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neco P., Giner D., Viniegra S., Borges R., Villarroel A., and Gutiérrez L.M.. 2004. New roles of myosin II during vesicle transport and fusion in chromaffin cells. J. Biol. Chem. 279:27450–27457. 10.1074/jbc.M311462200 [DOI] [PubMed] [Google Scholar]
- Neuland K., Sharma N., and Frick M.. 2014. Synaptotagmin-7 links fusion-activated Ca2+ entry and fusion pore dilation. J. Cell Sci. 127:5218–5227. 10.1242/jcs.153742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng Y.K., Lu X., and Levitan E.S.. 2002. Physical mobilization of secretory vesicles facilitates neuropeptide release by nerve growth factor-differentiated PC12 cells. J. Physiol. 542:395–402. 10.1113/jphysiol.2002.021733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng Y.K., Lu X., Gulacsi A., Han W., Saxton M.J., and Levitan E.S.. 2003. Unexpected mobility variation among individual secretory vesicles produces an apparent refractory neuropeptide pool. Biophys. J. 84:4127–4134. 10.1016/S0006-3495(03)75137-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterberg J.R., Chon N.L., Boo A., Maynard F.A., Lin H., and Knight J.D.. 2015. Membrane docking of the synaptotagmin 7 C2A domain: Electron paramagnetic resonance measurements show contributions from two membrane binding loops. Biochemistry. 54:5684–5695. 10.1021/acs.biochem.5b00421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock B.E., Striegel A.R., Hui E., Chapman E.R., and Reist N.E.. 2008. Ca2+-dependent, phospholipid-binding residues of synaptotagmin are critical for excitation-secretion coupling in vivo. J. Neurosci. 28:7458–7466. 10.1523/JNEUROSCI.0197-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock B.E., Wang Z., Biela L.M., Chen K., Getzy M.D., Striegel A., Richmond J.E., Chapman E.R., Featherstone D.E., and Reist N.E.. 2011. Membrane penetration by synaptotagmin is required for coupling calcium binding to vesicle fusion in vivo. J. Neurosci. 31:2248–2257. 10.1523/JNEUROSCI.3153-09.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y., Hernandez J.M., van den Bogaart G., Ahmed S., Holt M., Riedel D., and Jahn R.. 2012. Controlling synaptotagmin activity by electrostatic screening. Nat. Struct. Mol. Biol. 19:991–997. 10.1038/nsmb.2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y., Seo J.B., Fraind A., Pérez-Lara A., Yavuz H., Han K., Jung S.R., Kattan I., Walla P.J., Choi M., et al. 2015. Synaptotagmin-1 binds to PIP(2)-containing membrane but not to SNAREs at physiological ionic strength. Nat. Struct. Mol. Biol. 22:815–823. 10.1038/nsmb.3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng A., Rotman Z., Deng P.Y., and Klyachko V.A.. 2012. Differential motion dynamics of synaptic vesicles undergoing spontaneous and activity-evoked endocytosis. Neuron. 73:1108–1115. 10.1016/j.neuron.2012.01.023 [DOI] [PubMed] [Google Scholar]
- Peng X., Moore M., Mathur A., Zhou Y., Sun H., Gan Y., Herazo-Maya J.D., Kaminski N., Hu X., Pan H., et al. 2016. Plexin C1 deficiency permits synaptotagmin 7-mediated macrophage migration and enhances mammalian lung fibrosis. FASEB J. 30:4056–4070. 10.1096/fj.201600373R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Lara Á., Thapa A., Nyenhuis S.B., Nyenhuis D.A., Halder P., Tietzel M., Tittmann K., Cafiso D.S., and Jahn R.. 2016. PtdInsP2 and PtdSer cooperate to trap synaptotagmin-1 to the plasma membrane in the presence of calcium. eLife. 5:e15886 10.7554/eLife.15886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais D., Kleppe I.C., Taraska J.W., and Almers W.. 2004. Recapture after exocytosis causes differential retention of protein in granules of bovine chromaffin cells. J. Physiol. 560:413–428. 10.1113/jphysiol.2004.064410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan A., Stein A., Jahn R., and Fasshauer D.. 2009. The Ca2+ affinity of synaptotagmin 1 is markedly increased by a specific interaction of its C2B domain with phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 284:25749–25760. 10.1074/jbc.M109.042499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R., and Yaari Y.. 1973. Delayed release of transmitter at the frog neuromuscular junction. J. Physiol. 228:241–257. 10.1113/jphysiol.1973.sp010084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.K., Huynh C., Proux-Gillardeaux V., Galli T., and Andrews N.W.. 2004. Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J. Biol. Chem. 279:20471–20479. 10.1074/jbc.M400798200 [DOI] [PubMed] [Google Scholar]
- Rao T.C., Passmore D.R., Peleman A.R., Das M., Chapman E.R., and Anantharam A.. 2014. Distinct fusion properties of synaptotagmin-1 and synaptotagmin-7 bearing dense core granules. Mol. Biol. Cell. 25:2416–2427. 10.1091/mbc.e14-02-0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao T.C., Santana Rodriguez Z., Bradberry M.M., Ranski A.H., Dahl P.J., Schmidtke M.W., Jenkins P.M., Axelrod D., Chapman E.R., Giovannucci D.R., and Anantharam A.. 2017. Synaptotagmin isoforms confer distinct activation kinetics and dynamics to chromaffin cell granules. J. Gen. Physiol. 149:763–780. 10.1085/jgp.201711757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A., Caler E.V., and Andrews N.W.. 2001. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 106:157–169. 10.1016/S0092-8674(01)00421-4 [DOI] [PubMed] [Google Scholar]
- Rhee J.S., Li L.Y., Shin O.H., Rah J.C., Rizo J., Südhof T.C., and Rosenmund C.. 2005. Augmenting neurotransmitter release by enhancing the apparent Ca2+ affinity of synaptotagmin 1. Proc. Natl. Acad. Sci. USA. 102:18664–18669. 10.1073/pnas.0509153102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman C., Archer D.A., Meunier F.A., Craxton M., Fukuda M., Burgoyne R.D., and Davletov B.. 2004a Synaptotagmin interaction with the syntaxin/SNAP-25 dimer is mediated by an evolutionarily conserved motif and is sensitive to inositol hexakisphosphate. J. Biol. Chem. 279:12574–12579. 10.1074/jbc.M310710200 [DOI] [PubMed] [Google Scholar]
- Rickman C., Craxton M., Osborne S., and Davletov B.. 2004b Comparative analysis of tandem C2 domains from the mammalian synaptotagmin family. Biochem. J. 378:681–686. 10.1042/bj20031407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E., Eargle J., Wright D., and Luthey-Schulten Z.. 2006. MultiSeq: Unifying sequence and structure data for evolutionary analysis. BMC Bioinformatics. 7:382 10.1186/1471-2105-7-382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosé S.D., Lejen T., Casaletti L., Larson R.E., Pene T.D., and Trifaró J.M.. 2002. Molecular motors involved in chromaffin cell secretion. Ann. N. Y. Acad. Sci. 971:222–231. 10.1111/j.1749-6632.2002.tb04466.x [DOI] [PubMed] [Google Scholar]
- Rufener E., Frazier A.A., Wieser C.M., Hinderliter A., and Cafiso D.S.. 2005. Membrane-bound orientation and position of the synaptotagmin C2B domain determined by site-directed spin labeling. Biochemistry. 44:18–28. 10.1021/bi048370d [DOI] [PubMed] [Google Scholar]
- Saraswati S., Adolfsen B., and Littleton J.T.. 2007. Characterization of the role of the Synaptotagmin family as calcium sensors in facilitation and asynchronous neurotransmitter release. Proc. Natl. Acad. Sci. USA. 104:14122–14127. 10.1073/pnas.0706711104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G., Gu Q.M., Prestwich G.D., Söllner T.H., and Rothman J.E.. 1996. Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin. Proc. Natl. Acad. Sci. USA. 93:13327–13332. 10.1073/pnas.93.23.13327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonn J.S., Maximov A., Lao Y., Südhof T.C., and Sørensen J.B.. 2008. Synaptotagmin-1 and -7 are functionally overlapping Ca2+ sensors for exocytosis in adrenal chromaffin cells. Proc. Natl. Acad. Sci. USA. 105:3998–4003. 10.1073/pnas.0712373105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder T.J., Borges R., Finnegan J.M., Pihel K., Amatore C., and Wightman R.M.. 1996. Temporally resolved, independent stages of individual exocytotic secretion events. Biophys. J. 70:1061–1068. 10.1016/S0006-3495(96)79652-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia M., Alés E., Montes M.A., Bonifas I., Jemal I., Lindau M., Maximov A., Südhof T.C., and Alvarez de Toledo G.. 2010. Push-and-pull regulation of the fusion pore by synaptotagmin-7. Proc. Natl. Acad. Sci. USA. 107:19032–19037. 10.1073/pnas.1014070107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seven A.B., Brewer K.D., Shi L., Jiang Q.X., and Rizo J.. 2013. Prevalent mechanism of membrane bridging by synaptotagmin-1. Proc. Natl. Acad. Sci. USA. 110:E3243–E3252. 10.1073/pnas.1310327110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakiryanova D., Tully A., Hewes R.S., Deitcher D.L., and Levitan E.S.. 2005. Activity-dependent liberation of synaptic neuropeptide vesicles. Nat. Neurosci. 8:173–178. 10.1038/nn1377 [DOI] [PubMed] [Google Scholar]
- Shao X., Fernandez I., Südhof T.C., and Rizo J.. 1998. Solution structures of the Ca2+-free and Ca2+-bound C2A domain of synaptotagmin I: Does Ca2+ induce a conformational change?. Biochemistry. 37:16106–16115. 10.1021/bi981789h [DOI] [PubMed] [Google Scholar]
- Sheng Z.H., Yokoyama C.T., and Catterall W.A.. 1997. Interaction of the synprint site of N-type Ca2+ channels with the C2B domain of synaptotagmin I. Proc. Natl. Acad. Sci. USA. 94:5405–5410. 10.1073/pnas.94.10.5405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih A.M., Varghese L., Bittar A., Park S.H., Chung J.M., and Shin O.H.. 2016. Dysregulation of norepinephrine release in the absence of functional synaptotagmin 7. J. Cell. Biochem. 117:1446–1453. 10.1002/jcb.25436 [DOI] [PubMed] [Google Scholar]
- Sievers F., Wilm A., Dineen D., Gibson T.J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J., et al. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7:539 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S.M., and Llinás R.R.. 1985. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys. J. 48:485–498. 10.1016/S0006-3495(85)83804-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T., Bennett M.K., Whiteheart S.W., Scheller R.H., and Rothman J.E.. 1993a A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 75:409–418. 10.1016/0092-8674(93)90376-2 [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S.W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., and Rothman J.E.. 1993b SNAP receptors implicated in vesicle targeting and fusion. Nature. 362:318–324. 10.1038/362318a0 [DOI] [PubMed] [Google Scholar]
- Sørensen J.B. 2004. Formation, stabilisation and fusion of the readily releasable pool of secretory vesicles. Pflugers Arch. 448:347–362. 10.1007/s00424-004-1247-8 [DOI] [PubMed] [Google Scholar]
- Stein A., Radhakrishnan A., Riedel D., Fasshauer D., and Jahn R.. 2007. Synaptotagmin activates membrane fusion through a Ca2+-dependent trans interaction with phospholipids. Nat. Struct. Mol. Biol. 14:904–911. 10.1038/nsmb1305 [DOI] [PubMed] [Google Scholar]
- Striegel A.R., Biela L.M., Evans C.S., Wang Z., Delehoy J.B., Sutton R.B., Chapman E.R., and Reist N.E.. 2012. Calcium binding by synaptotagmin’s C2A domain is an essential element of the electrostatic switch that triggers synchronous synaptic transmission. J. Neurosci. 32:1253–1260. 10.1523/JNEUROSCI.4652-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T.C. 2013. A molecular machine for neurotransmitter release: Synaptotagmin and beyond. Nat. Med. 19:1227–1231. 10.1038/nm.3338 [DOI] [PubMed] [Google Scholar]
- Südhof T.C., and Rizo J.. 1996. Synaptotagmins: C2-domain proteins that regulate membrane traffic. Neuron. 17:379–388. 10.1016/S0896-6273(00)80171-3 [DOI] [PubMed] [Google Scholar]
- Südhof T.C., and Rothman J.E.. 2009. Membrane fusion: Grappling with SNARE and SM proteins. Science. 323:474–477. 10.1126/science.1161748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S., and Südhof T.C.. 2000. Specificity of Ca2+-dependent protein interactions mediated by the C2A domains of synaptotagmins. Biochemistry. 39:2940–2949. 10.1021/bi9920984 [DOI] [PubMed] [Google Scholar]
- Sugita S., Hata Y., and Südhof T.C.. 1996. Distinct Ca(2+)-dependent properties of the first and second C2-domains of synaptotagmin I. J. Biol. Chem. 271:1262–1265. 10.1074/jbc.271.3.1262 [DOI] [PubMed] [Google Scholar]
- Sugita S., Han W., Butz S., Liu X., Fernández-Chacón R., Lao Y., and Südhof T.C.. 2001. Synaptotagmin VII as a plasma membrane Ca(2+) sensor in exocytosis. Neuron. 30:459–473. 10.1016/S0896-6273(01)00290-2 [DOI] [PubMed] [Google Scholar]
- Sugita S., Shin O.H., Han W., Lao Y., and Südhof T.C.. 2002. Synaptotagmins form a hierarchy of exocytotic Ca(2+) sensors with distinct Ca(2+) affinities. EMBO J. 21:270–280. 10.1093/emboj/21.3.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R.B., Davletov B.A., Berghuis A.M., Südhof T.C., and Sprang S.R.. 1995. Structure of the first C2 domain of synaptotagmin I: A novel Ca2+/phospholipid-binding fold. Cell. 80:929–938. 10.1016/0092-8674(95)90296-1 [DOI] [PubMed] [Google Scholar]
- Sutton R.B., Fasshauer D., Jahn R., and Brunger A.T.. 1998. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 395:347–353. 10.1038/26412 [DOI] [PubMed] [Google Scholar]
- Takahashi H., Shahin V., Henderson R.M., Takeyasu K., and Edwardson J.M.. 2010. Interaction of synaptotagmin with lipid bilayers, analyzed by single-molecule force spectroscopy. Biophys. J. 99:2550–2558. 10.1016/j.bpj.2010.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraska J.W., Perrais D., Ohara-Imaizumi M., Nagamatsu S., and Almers W.. 2003. Secretory granules are recaptured largely intact after stimulated exocytosis in cultured endocrine cells. Proc. Natl. Acad. Sci. USA. 100:2070–2075. 10.1073/pnas.0337526100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T., and Fukuda M.. 2007. Synaptotagmin VII modulates the kinetics of dense-core vesicle exocytosis in PC12 cells. Genes Cells. 12:511–519. 10.1111/j.1365-2443.2007.01070.x [DOI] [PubMed] [Google Scholar]
- Tucker W.C., Edwardson J.M., Bai J., Kim H.J., Martin T.F., and Chapman E.R.. 2003. Identification of synaptotagmin effectors via acute inhibition of secretion from cracked PC12 cells. J. Cell Biol. 162:199–209. 10.1083/jcb.200302060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker W.C., Weber T., and Chapman E.R.. 2004. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science. 304:435–438. 10.1126/science.1097196 [DOI] [PubMed] [Google Scholar]
- Turecek J., and Regehr W.G.. 2018. Synaptotagmin 7 mediates both facilitation and asynchronous release at granule cell synapses. J. Neurosci. 38:3240–3251. 10.1523/JNEUROSCI.3207-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecek J., Jackman S.L., and Regehr W.G.. 2017. Synaptotagmin 7 confers frequency invariance onto specialized depressing synapses. Nature. 551:503–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubach J., Lao Y., Fernandez I., Arac D., Südhof T.C., and Rizo J.. 2001. The C2B domain of synaptotagmin I is a Ca2+-binding module. Biochemistry. 40:5854–5860. 10.1021/bi010340c [DOI] [PubMed] [Google Scholar]
- Ullrich B., and Südhof T.C.. 1995. Differential distributions of novel synaptotagmins: Comparison to synapsins. Neuropharmacology. 34:1371–1377. 10.1016/0028-3908(95)00132-P [DOI] [PubMed] [Google Scholar]
- van den Bogaart G., Meyenberg K., Diederichsen U., and Jahn R.. 2012. Phosphatidylinositol 4,5-bisphosphate increases Ca2+ affinity of synaptotagmin-1 by 40-fold. J. Biol. Chem. 287:16447–16453. 10.1074/jbc.M112.343418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W., and Molgó J.. 1994. Quantal acetylcholine release at the vertebrate neuromuscular junction. Physiol. Rev. 74:899–991. 10.1152/physrev.1994.74.4.899 [DOI] [PubMed] [Google Scholar]
- Vasquez J.K., Chantranuvatana K., Giardina D.T., Coffman M.D., and Knight J.D.. 2014. Lateral diffusion of proteins on supported lipid bilayers: Additive friction of synaptotagmin 7 C2A-C2B tandem domains. Biochemistry. 53:7904–7913. 10.1021/bi5012223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M., Söllner T.H., and Rothman J.E.. 1996. Multiple palmitoylation of synaptotagmin and the t-SNARE SNAP-25. FEBS Lett. 385:119–123. 10.1016/0014-5793(96)00362-6 [DOI] [PubMed] [Google Scholar]
- Vennekate W., Schröder S., Lin C.C., van den Bogaart G., Grunwald M., Jahn R., and Walla P.J.. 2012. Cis- and trans-membrane interactions of synaptotagmin-1. Proc. Natl. Acad. Sci. USA. 109:11037–11042. 10.1073/pnas.1116326109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermaas J.V., and Tajkhorshid E.. 2017. Differential membrane binding mechanics of synaptotagmin isoforms observed in atomic detail. Biochemistry. 56:281–293. 10.1021/acs.biochem.6b00468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva J., Torregrosa-Hetland C.J., Garcia-Martinez V., del Mar Frances M., Viniegra S., and Gutierrez L.M.. 2012. The F-actin cortex in chromaffin granule dynamics and fusion: a minireview. J. Mol. Neurosci. 48:323–327. [DOI] [PubMed] [Google Scholar]
- Virmani T., Han W., Liu X., Südhof T.C., and Kavalali E.T.. 2003. Synaptotagmin 7 splice variants differentially regulate synaptic vesicle recycling. EMBO J. 22:5347–5357. 10.1093/emboj/cdg514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T., Moser T., Lund P.E., Chow R.H., Geppert M., Südhof T.C., and Neher E.. 2001. Intracellular calcium dependence of large dense-core vesicle exocytosis in the absence of synaptotagmin I. Proc. Natl. Acad. Sci. USA. 98:11680–11685. 10.1073/pnas.201398798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voleti R., Tomchick D.R., Südhof T.C., and Rizo J.. 2017. Exceptionally tight membrane-binding may explain the key role of the synaptotagmin-7 C2A domain in asynchronous neurotransmitter release. Proc. Natl. Acad. Sci. USA. 114:E8518–E8527. 10.1073/pnas.1710708114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Poser C., Ichtchenko K., Shao X., Rizo J., and Südhof T.C.. 1997. The evolutionary pressure to inactivate. A subclass of synaptotagmins with an amino acid substitution that abolishes Ca2+ binding. J. Biol. Chem. 272:14314–14319. 10.1074/jbc.272.22.14314 [DOI] [PubMed] [Google Scholar]
- Wan C., Kiessling V., Cafiso D.S., and Tamm L.K.. 2011. Partitioning of synaptotagmin I C2 domains between liquid-ordered and liquid-disordered inner leaflet lipid phases. Biochemistry. 50:2478–2485. 10.1021/bi101864k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.T., Grishanin R., Earles C.A., Chang P.Y., Martin T.F., Chapman E.R., and Jackson M.B.. 2001. Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science. 294:1111–1115. 10.1126/science.1064002 [DOI] [PubMed] [Google Scholar]
- Wang C.T., Lu J.C., Bai J., Chang P.Y., Martin T.F., Chapman E.R., and Jackson M.B.. 2003a Different domains of synaptotagmin control the choice between kiss-and-run and full fusion. Nature. 424:943–947. 10.1038/nature01857 [DOI] [PubMed] [Google Scholar]
- Wang C.T., Bai J., Chang P.Y., Chapman E.R., and Jackson M.B.. 2006. Synaptotagmin-Ca2+ triggers two sequential steps in regulated exocytosis in rat PC12 cells: Fusion pore opening and fusion pore dilation. J. Physiol. 570:295–307. 10.1113/jphysiol.2005.097378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Bello O., Auclair S.M., Wang J., Coleman J., Pincet F., Krishnakumar S.S., Sindelar C.V., and Rothman J.E.. 2014. Calcium sensitive ring-like oligomers formed by synaptotagmin. Proc. Natl. Acad. Sci. USA. 111:13966–13971. 10.1073/pnas.1415849111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Li F., Bello O.D., Sindelar C.V., Pincet F., Krishnakumar S.S., and Rothman J.E.. 2017. Circular oligomerization is an intrinsic property of synaptotagmin. eLife. 6:e27441 10.7554/eLife.27441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Wang C.T., Bai J., Jackson M.B., and Chapman E.R.. 2003b Mutations in the effector binding loops in the C2A and C2B domains of synaptotagmin I disrupt exocytosis in a nonadditive manner. J. Biol. Chem. 278:47030–47037. 10.1074/jbc.M306728200 [DOI] [PubMed] [Google Scholar]
- Wang P., Chicka M.C., Bhalla A., Richards D.A., and Chapman E.R.. 2005. Synaptotagmin VII is targeted to secretory organelles in PC12 cells, where it functions as a high-affinity calcium sensor. Mol. Cell. Biol. 25:8693–8702. 10.1128/MCB.25.19.8693-8702.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J.P., Toft-Bertelsen T.L., Mohrmann R., Delgado-Martinez I., and Sørensen J.B.. 2014. Synaptotagmin-7 is an asynchronous calcium sensor for synaptic transmission in neurons expressing SNAP-23. PLoS One. 9:e114033 10.1371/journal.pone.0114033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Zemelman B.V., McNew J.A., Westermann B., Gmachl M., Parlati F., Söllner T.H., and Rothman J.E.. 1998. SNAREpins: Minimal machinery for membrane fusion. Cell. 92:759–772. 10.1016/S0092-8674(00)81404-X [DOI] [PubMed] [Google Scholar]
- Wen H., Linhoff M.W., McGinley M.J., Li G.L., Corson G.M., Mandel G., and Brehm P.. 2010. Distinct roles for two synaptotagmin isoforms in synchronous and asynchronous transmitter release at zebrafish neuromuscular junction. Proc. Natl. Acad. Sci. USA. 107:13906–13911. 10.1073/pnas.1008598107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman R.M., Jankowski J.A., Kennedy R.T., Kawagoe K.T., Schroeder T.J., Leszczyszyn D.J., Near J.A., Diliberto E.J. Jr., and Viveros O.H.. 1991. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc. Natl. Acad. Sci. USA. 88:10754–10758. 10.1073/pnas.88.23.10754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiser O., Trus M., Hernández A., Renström E., Barg S., Rorsman P., and Atlas D.. 1999. The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc. Natl. Acad. Sci. USA. 96:248–253. 10.1073/pnas.96.1.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Wei S., Petersen N., Ali Y., Wang X., Bacaj T., Rorsman P., Hong W., Südhof T.C., and Han W.. 2015. Synaptotagmin-7 phosphorylation mediates GLP-1-dependent potentiation of insulin secretion from β-cells. Proc. Natl. Acad. Sci. USA. 112:9996–10001. 10.1073/pnas.1513004112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M., Ma C., Craig T.K., Rosenmund C., and Rizo J.. 2008. The Janus-faced nature of the C(2)B domain is fundamental for synaptotagmin-1 function. Nat. Struct. Mol. Biol. 15:1160–1168. 10.1038/nsmb.1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M., Craig T.K., Shin O.H., Li L., Brautigam C.A., Tomchick D.R., Südhof T.C., Rosenmund C., and Rizo J.. 2010. Structural and mutational analysis of functional differentiation between synaptotagmins-1 and -7. PLoS One. 5:e12544 10.1371/journal.pone.0012544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue R., Gaffaney J.D., and Chapman E.R.. 2015. Structural elements that underlie Doc2β function during asynchronous synaptic transmission. Proc. Natl. Acad. Sci. USA. 112:E4316–E4325. 10.1073/pnas.1502288112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J., Gaffaney J.D., Kwon S.E., and Chapman E.R.. 2011. Doc2 is a Ca2+ sensor required for asynchronous neurotransmitter release. Cell. 147:666–677. 10.1016/j.cell.2011.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T.Y., Okumus B., Zhang F., Shin Y.K., and Ha T.. 2006. Multiple intermediates in SNARE-induced membrane fusion. Proc. Natl. Acad. Sci. USA. 103:19731–19736. 10.1073/pnas.0606032103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S.M. Jr., and Neher E.. 2009. Synaptotagmin has an essential function in synaptic vesicle positioning for synchronous release in addition to its role as a calcium sensor. Neuron. 63:482–496. 10.1016/j.neuron.2009.07.028 [DOI] [PubMed] [Google Scholar]
- Zanetti M.N., Bello O.D., Wang J., Coleman J., Cai Y., Sindelar C.V., Rothman J.E., and Krishnakumar S.S.. 2016. Ring-like oligomers of Synaptotagmins and related C2 domain proteins. eLife. 5:e17262 10.7554/eLife.17262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D., Davila V., Wan L., and Almers W.. 2003. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J. Neurosci. 23:2538–2548. 10.1523/JNEUROSCI.23-07-02538.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Rizo J., and Südhof T.C.. 1998. Mechanism of phospholipid binding by the C2A-domain of synaptotagmin I. Biochemistry. 37:12395–12403. 10.1021/bi9807512 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Hui E., Chapman E.R., and Jackson M.B.. 2010. Regulation of exocytosis and fusion pores by synaptotagmin-effector interactions. Mol. Biol. Cell. 21:2821–2831. 10.1091/mbc.e10-04-0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Wu Y., Wang Z., Dunning F.M., Rehfuss J., Ramanan D., Chapman E.R., and Jackson M.B.. 2011. Release mode of large and small dense-core vesicles specified by different synaptotagmin isoforms in PC12 cells. Mol. Biol. Cell. 22:2324–2336. 10.1091/mbc.e11-02-0159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Ito Y., Chappel J., Andrews N.W., Teitelbaum S.L., and Ross F.P.. 2008. Synaptotagmin VII regulates bone remodeling by modulating osteoclast and osteoblast secretion. Dev. Cell. 14:914–925. 10.1016/j.devcel.2008.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W.D., Hamid E., Shin W., Wen P.J., Krystofiak E.S., Villarreal S.A., Chiang H.C., Kachar B., and Wu L.G.. 2016. Hemi-fused structure mediates and controls fusion and fission in live cells. Nature. 534:548–552. 10.1038/nature18598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A., Brewer K.D., and Rizo J.. 2013. Analysis of SNARE complex/synaptotagmin-1 interactions by one-dimensional NMR spectroscopy. Biochemistry. 52:3446–3456. 10.1021/bi400230u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Lai Y., Bacaj T., Zhao M., Lyubimov A.Y., Uervirojnangkoorn M., Zeldin O.B., Brewster A.S., Sauter N.K., Cohen A.E., et al. 2015. Architecture of the synaptotagmin-SNARE machinery for neuronal exocytosis. Nature. 525:62–67. 10.1038/nature14975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Zhou P., Wang A.L., Wu D., Zhao M., Südhof T.C., and Brunger A.T.. 2017. The primed SNARE-complexin-synaptotagmin complex for neuronal exocytosis. Nature. 548:420–425. 10.1038/nature23484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Misler S., and Chow R.H.. 1996. Rapid fluctuations in transmitter release from single vesicles in bovine adrenal chromaffin cells. Biophys. J. 70:1543–1552. 10.1016/S0006-3495(96)79718-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R.S. 1996. Exocytosis: a molecular and physiological perspective. Neuron. 17:1049–1055. 10.1016/S0896-6273(00)80238-X [DOI] [PubMed] [Google Scholar]
- Zucker R.S., and Regehr W.G.. 2002. Short-term synaptic plasticity. Annu. Rev. Physiol. 64:355–405. 10.1146/annurev.physiol.64.092501.114547 [DOI] [PubMed] [Google Scholar]