Abstract
ATP synthases catalyse the formation of ATP, the most common chemical energy storage unit found in living cells. These enzymes are driven by an electrochemical ion gradient, which allows the catalytic evolution of ATP by a binding change mechanism. Most ATP synthases are capable of catalysing ATP hydrolysis to varying degrees, and to prevent wasteful ATP hydrolysis, bacteria and mitochondria have regulatory mechanisms such as ADP inhibition. Additionally, ɛ subunit inhibition has also been described in three bacterial systems, Escherichia coli, Bacillus PS3 and Caldalkalibacillus thermarum TA2.A1. Previous studies suggest that the ɛ subunit is capable of undergoing an ATP-dependent conformational change from the ATP hydrolytic inhibitory ‘extended’ conformation to the ATP-induced non-inhibitory ‘hairpin’ conformation. A recently published crystal structure of the F1 domain of the C. thermarum TA2.A1 F1Fo ATP synthase revealed a mutant ɛ subunit lacking the ability to bind ATP in a hairpin conformation. This is a surprising observation considering it is an organism that performs no ATP hydrolysis in vivo, and appears to challenge the current dogma on the regulatory role of the ɛ subunit. This has prompted a re-examination of present knowledge of the ɛ subunits role in different organisms. Here, we compare published biochemical, biophysical and structural data involving ɛ subunit-mediated ATP hydrolysis regulation in a variety of organisms, concluding that the ɛ subunit from the bacterial F-type ATP synthases is indeed capable of regulating ATP hydrolysis activity in a wide variety of bacteria, making it a potentially valuable drug target, but its exact role is still under debate.
Keywords: F-type ATP synthases, ɛ subunit, regulation, hydrolysis of ATP, bacterial
1. Introduction
All organisms require ATP, a universal chemical energy storage unit, to carry out and maintain cellular functions. ATP synthases are found in almost all kingdoms of life and are the main ATP synthesizing enzymatic machineries in aerobically growing bacterial, archaeal and eukaryotic cells. Recently, the F-type ATP synthase has been shown to be a novel attractive drug-target against Mycobacterium tuberculosis [1,2], a causative agent of tuberculosis. Owing to different mechanistic modes of regulation between bacterial and eukaryotic respiratory systems [3], the F-type ATP synthase may be an attractive target for novel antimicrobial compounds.
Bacterial ATP synthases comprise the soluble F1 domain [4], harbouring a α3β3 hexameric assembly, the γ subunit central stalk, the ɛ subunit and the δ subunit. The γ and ɛ subunits form a central drive-shaft, connecting the soluble F1 domain to the membrane-embedded Fo domain [5]. Notably, the ɛ subunit of bacteria is the δ subunit in mitochondria, but with a divergent function [5]. The membrane-bound Fo domain harbours a c-ring, where the number of c subunits varies from 8 to 15 among different organisms, yet remains invariant within individual organisms [6–10], the a subunit, which is horizontally aligned to the c-ring [11,12], and the dimeric b2 subunit [13]. The b2 dimer forms a peripheral stalk, connecting the Fo domain with the α3β3 catalytic hexamer via the δ subunit in bacteria [14–16], or the oligomycin-sensitive conferral protein (OSCP) subunit in mitochondrial ATP synthases [11,12,17,18]. A proton- or sodium-motive force drives the rotation of the membrane-embedded c-ring [19] versus the membrane-embedded stator subunits a and b2 [11,12]. The transported ion is dependent on the selectivity of the binding site structure in the c-ring [20]. In the ATP synthesis direction of c-ring rotation (clockwise), a single revolution of the c-ring causes a single revolution of the γ subunit, which in turn induces conformational changes in all three αβ subunits. The net result of this rotation is the catalysis of three ADP and three inorganic phosphate molecules (Pi) into three ATP molecules [21] coupled to the translocation of either H+ or Na+ across the cytoplasmic membrane [22]. In addition, this enzymatic reaction can be driven in the reverse direction (anti-clockwise), hydrolysing ATP [23] and pumping ions into either the periplasm or the P-side of the membrane.
The regulation and prevention of ATP hydrolysis is of critical importance, and bacteria and mitochondria have adapted diverse mechanisms of regulation. To date they have been shown to share a common, yet poorly described Mg-ADP hydrolysis inhibition form of regulation [24,25]; however, they have also developed other more distinct, organism-specific mechanisms. In mitochondria, ATP hydrolysis is controlled by the intrinsic regulatory protein IF1 [26], while most bacteria have been proposed to be regulated via a conformational transition of the ɛ subunit [27]. The exceptions to this are α-proteobacteria, which are regulated by the ζ subunit [28–30]. A recent review comparing the different regulatory mechanisms can be found elsewhere [3]. Owing to the unique function of the ɛ subunit in bacteria that is not present in higher eukaryotes and its potential as a drug target, the core focus of this article is the role of the ɛ subunit in bacterial F1Fo ATP synthase ATP hydrolysis regulation.
The ɛ subunit harbours two domains. The N-terminal domain (NTD) is a rigid β-sheet domain, while the C-terminal domain (CTD) comprises two α-helices connected by a flexible linker [31]. The CTD has been frequently described as having a dynamic nature, with the ability to change conformation depending on the presence or the absence of various concentrations of ATP [32–35]. When these two helices are parallel (i.e. in a ‘down-state’), both spatially localized to the rigid β-sheet domain, the ɛ subunit is described to be in the contracted ‘hairpin conformation’, a state which is able to be induced by ATP binding [31,36–39]. Conversely, when these two helices are in series (i.e. in a ‘rod’), spatially distant from the rigid β-sheet domain, yet parallel to the γ subunit, and reaching into the α3β3 catalytic hexamer (i.e. in an ‘up-state’), the ɛ subunit is described to be in the ‘extended conformation’ [40–42]. In the light of the potential interactions the ɛ subunit may have in each conformation, and the kinetic effects of various mutants, the extended and hairpin conformations are frequently referred to as the ‘inhibitory’ and ‘non-inhibitory’ states, respectively, regarding the ATP hydrolysis ability of the enzyme. In the authors' view, until we fully understand the mechanism, it is prudent to simply refer to these conformations by their shape (‘hairpin’ and ‘extended’) until a solid consensus can be reached on the functional mechanism, or whether the strength of the influence of the regulatory role is simply more species-dependent than previously assumed.
Structures of isolated ɛ subunits from E. coli [36,38], Bacillus PS3 [39] and Thermosynechococcus elongates BP-1 [31] show that the ɛ subunit adopts a hairpin conformation. Conversely, an NMR structure of the c-terminal helix (CTH) truncated ɛ subunit (ɛΔ103–120) from Mycobacterium tuberculosis was found in the extended conformation in the absence of ATP [43], implicating the CTH in a critical role for maintaining the hairpin conformation. This suggests that the ɛ subunit is capable of a dynamic conformational movement. Yet more clearly indicative of conformational dynamics was an E. coli F1 (EF1) ɛγ subunit structure, where the ɛ subunit was found in a ‘half-extended’ conformation [44]. In structures of the whole F1 domain, the ɛ subunit has been found in either the extended or hairpin conformation, as shown in the F1 domain from Bacillus PS3 [41], Paracoccus denitrificans [15], the F1 [40] and F1Fo [16] from E. coli, and the F1 from Caldalkalibacillus thermarum TA2.A1 [45]. However, contrary to the body of evidence supporting an ATP-mediated hairpin conformation, a C. thermarum TA2.A1 ɛ subunit lacking the ability to bind ATP was also found in the hairpin conformation [45]. Taken together, structural studies have revealed a number of ‘snapshots’ from various species, but no complete picture currently exists.
ATP hydrolysis is prevented selectively in several bacterial F1Fo ATP synthases studied to date. In three Bacillus species [46–48], P. denitrificans [49] and two mycobacterial species (M. smegmatis and M. bovis), ATP hydrolysis is selectively prevented [50–52]. It has long been proposed that the extent of ATP hydrolytic inhibition is due to the binding affinity of the ɛ subunit for ATP, a proposal supported by the observation that the ɛ subunit from different organisms binds ATP with different affinities. Isolated ɛ subunits have widely ranging binding affinities, from Bacillus PS3 with an apparent binding constant of 4 µM [53,54] to 2 mM for Bacillus subtilis [55] and 22 mM for E. coli [39]. ATP binding assays have also been carried out for whole F1 complex (α3β3γɛ) from T. elongatus BP-1 [56] and various mycobacterial species [43], but ATP binding was not observed in the measured concentration range.
In the light of these data, the regulatory role of the ɛ subunit in bacterial systems remains controversial. In this study, we compare available biochemical, biophysical and structural data from different bacterial organisms to help clarify the state of the field.
2. Current evidence on the regulatory role of the ɛ subunit
2.1. Biophysical and biochemical experiments indicate a regulatory mechanism dependent on the ɛ subunit
2.1.1. Escherichia coli
To the best of our knowledge, the first suggestion that the ɛ subunit had an inhibition role of ATP hydrolysis in an in vivo setting was described for the EF1 domain [42]. Biochemical cross-linking experiments showed that the ɛ subunit from E. coli could be covalently attached to the α3β3 assembly [57,58], indicating an extended conformation, and was later resolved in an F1 crystal structure [40]. Interestingly, in a different study, the same authors observed that the ɛ-β crosslink (ɛS108C-βD380C) occurs less frequently in the presence of ATP compared with the presence of ADP or the competitive inhibitor AMP-PNP [59]. This led to the hypothesis that the decreased crosslinking might be caused by decreased binding affinities of ADP and AMP-PNP to the CTD of the ɛ subunit, thus allowing the ɛ subunit to stay in the extended conformation.
The conformational transition of the ɛ subunit has also been observed using Förster resonance energy transfer (FRET) experiments [60]. Although this was not immediately evident in initial experiments of the same group [61], this movement has because been independently confirmed using single-molecule FRET studies, supporting the idea of a conformational transition [62]. In single-molecule rotation experiments, the ɛ subunit increases the duration of the pause during the rotational motion of the ATP synthase in hydrolysis direction [63]. ɛS108 (S108A/D) and ɛY114 (Y114A) mutations interact with βE381:Oɛx and γG85:O, respectively (shown in the crystal structure [40]), reducing the inhibitory effect on ATP hydrolysis by the ɛ subunit [64]. Cross-linking was also observed between βE381C/βS383C and ɛS108C, also suppressing ATP hydrolysis activity [65]. This study is supported by further studies describing cross-linking between ɛA117C and cQ42C (revealing the ɛ subunit in a hairpin conformation) and between ɛA118C and γL99C (revealing the ɛ subunit in an extended conformation) [58]. To the best of our knowledge, this is the first report confirming that the ɛ subunit could exist in two distinct structural states. Furthermore, it has been claimed that inhibition caused by the ɛ subunit is separate from Mg-ADP inhibition [66], and a dynamic transition between the released and tightly bound auto-inhibited state by the ɛ subunit has been proposed [66,67]. It has also been proposed that the ɛ subunit fine-tunes fundamental steps in the ATP synthesis direction [34,68].
Lastly, it should be mentioned that the ATP binding affinity of the isolated ɛ subunit from EF1 (22 mM) [39] is below the average physiological bulk ATP concentration in E. coli cells (1.54 mM) [69], making it seem less likely that ATP has a regulatory function by binding to the ɛ subunit under physiological conditions. Nonetheless, we cannot discount the potential influence of localized ATP pools before diffusion away from the ATP synthase, a difficult phenomenon to study with any degree of accuracy.
2.1.2. Bacillus PS3
Both the isolated ɛ subunit from Bacillus PS3 [39,70] and the ɛγ-subunit complex [71] have been shown to bind ATP. Mutations in the CTH of the ɛ subunit from Bacillus PS3 [39] result in decreased ATP binding affinity, supporting the notion that the ɛ subunit from Bacillus PS3 binds ATP [53]. Additionally, using the F1 complex (TF1), Iino et al. [32] monitored extended/hairpin conformation transitions using FRET between labelled residues in the ɛ and/or β subunits. Importantly, this study also revealed that the ɛ subunit has sub-millimolar affinity to ATP at close to the optimal growth temperature of Bacillus PS3, suggesting that the ɛ subunit is an ATP concentration sensor in vivo [32]. However, the time taken for these conformational shifts suggests that the ɛ subunit exerts a slow switch-like regulation of ATP hydrolysis, rather than a rapid movement. Furthermore, Iino et al. [32] observed that the dependence of the ATP synthetic activity of E. coli F1Fo wild-type (WT) versus Δɛ C-terminus on ΔpH and ΔΨ was similar, suggesting that the loss of the ɛ subunit CTH is not rate-limiting for ATP synthesis [32]. Supporting this, single-molecule rotation measurements of TF1 revealed that in the presence of low ATP concentration (200 nM), comparatively more numerous and extended pauses in rotation were observed in the presence of the WT ɛ subunit, than in the absence. However, at a higher ATP concentration (2 µM) the ɛ subunit presence/absence had negligible effect on enzyme kinetics. This study provides support for the slow conformational shift regulation model, and provides insight that the extended conformation may inhibit ATP hydrolysis in TF1. However, in a TF1 harbouring an ɛ subunit truncation mutant, ɛΔCTD, (a stop codon after ɛD87), no obvious differences were observed in rotation from the WT TF1 complex [72].
Interestingly, the crystal structures of the isolated ɛ subunit [39] and in the TF1 complex [41] have both been shown to be capable of taking both extended and hairpin conformations. In the case of the isolated ɛ subunit, in the presence of ATP, the overall structure is very similar to the isolated E. coli ɛ subunit (a hairpin conformation). In the absence of ATP, the NMR structural data were relatively poorly resolved; however, on the basis of the dihedral ϕ/ψ angles, the authors were able to discern that a proportion of the molecules formed a long single helix, supporting the notion that the hairpin structure can transition into an extended helical structure, and that that extension of the structure is what drives the inhibition of ATPase hydrolysis at low ATP concentrations [39]. What this study also revealed is that the conformation shift is likely to be dynamic, supporting a previous finding by Iino et al. [32] and the later observations of Tsumuraya et al. [72]. Recently, the TF1 crystal structure (α3β3γɛ) was solved, where the ɛ subunit was found in the extended conformation, structurally confirming the extended helical structure in a physiological structural context [41]. The crystal contacts, like the ɛ subunit extended conformation E. coli structure, strongly support the notion that the extended conformation inhibits rotation in the hydrolysis direction. Furthermore, mutagenesis experiments revealed that mutations in either the DELSDED motif of the β subunit (DELSEED in mammalian F1), or the terminal helix of the CTD from the ɛ subunit, resulted in an increase in the ability of TF1 to catalyse ATP hydrolysis, supporting the proposed role of the ɛ subunit as an inhibitor of ATP hydrolytic activity. This study also suggests that ATP hydrolysis inhibition caused by the ɛ subunit may be due to an electrostatic interaction between the CTH of the ɛ subunit and the DELSDED motif of the TF1 β subunit [73]. In addition, there is a growing body of evidence supporting the notion that these conformational changes may also be dependent on proton motif force (pmf) [33,74].
It has been proposed that the directionality of the γ subunit directs the conformational state of the ɛ subunit. For this to be feasible, the c-ring must transmit a torque larger than the thermodynamic equilibrium to the γ subunit by an increased pmf (approx. 400 mV) to revoke the ɛ subunit extended conformation and initialize ATP synthesis by the F1 complex [75]. In agreement with this theory, the mutation of critical ATP binding residues, E83 and R92 [53], did not influence the transition from the extended to hairpin conformation. This finding suggests that the nucleotide occupation in the catalytic binding site in the β subunit induces the conformational change of the ɛ subunit [32] to a half-extended conformation, while in a last step ATP may bind to the ɛ subunit, trapping the ɛ subunit in a contracted ATP-bound state. This contracted ATP-bound structure stabilizes the hairpin conformation [35,76]. The justification that has been proposed is that during ATP hydrolysis, the contracted ATP bound state is necessary to prevent uncoupling between ATPase activity and H+ pumping [74]. Furthermore, the ɛ subunit has been proposed to decrease ADP binding affinity to the catalytic site (α3β3) [77], taking a different mode of action from Mg-ADP inhibition of ATP hydrolysis [78], relieving the inhibition state [79]. However, this is difficult to unravel as the extended pauses observed during rotation experiments were at the same angular positions as Mg-ADP inhibition [72].
2.1.3. Caldalkalibacillus thermarum TA2.A1
The thermoalkaliphilic bacterium C. thermarum TA2.A1 grows at 65°C and at pH levels between 7.5 and 10.2 on fermentative substrates [80,81], with highest growth rates aerobically at alkaline pH (9.5) [82]. The pH-dependent growth is caused in part by inefficient ATP synthesis at pH values below 8.5 caused by a lysine residue (K180) in the a subunit of the ATP synthase, allowing proton translocation and thus ATP synthesis optimally under alkaline pH conditions [83].
Given the energetically hostile conditions required for C. thermarum TA2.A1 growth, it is essential that such an organism conserve ATP. Therefore, it is unsurprising that the ATP hydrolysis activity of the F1Fo or F1 ATP synthase from C. thermarum TA2.A1 is suppressed under physiological conditions, identified by biochemical [48,82,83] and single-molecule experiments [84]. Keis et al. [85] demonstrated that in the presence of a low ATP concentration (50 µM), the WT F1Fo and F1 have negligible ATPase activity, while at high ATP concentrations (2 mM) ATP hydrolysis was observed. This hints at a conformational change of the ɛ subunit from the extended to the hairpin conformation upon ATP binding to the catalytic β subunit (as observed in Bacillus PS3 [32]) or ATP binding to the ɛ subunit itself. Conversely, in a recent study, regardless of ATP concentration, negligible ATP hydrolysis was observed in the absence of chemical or mechanical activation [84].
ATP hydrolysis activity is not inhibited if ATP binding residues in the CTH (residues R123 and R127), or if proposed non-ligand binding residues R116, H117, K118 and R119 (TA2F1 Δɛ6A), are mutated to alanine [85], while mutating R116, H117, K118 and R119 simultaneously to alanine still prevented ATP hydrolysis activity at low ATP concentration (50 µM) [81]. Collectively, these data suggest that residues ɛR123 and/or ɛR127 bind to the α3β3γ complex, and could potentially serve to stabilize an inhibitory extended conformation, akin to the interaction between ɛK123 and βD372, or the salt bridge between ɛR122 and βD382 in the ɛ subunit from E. coli and Bacillus PS3, respectively. In contrast to these clear observations, a mixed effect was observed upon trypsin treatment, where the WT TA2F1Fo and TA2F1 complexes are protected by trypsin digestion compared with the TA2F1 Δɛ6A mutant, in which the ɛ subunit was completely degraded. We would like to reinforce the point that in spite of the very same mutant being more active in ATP hydrolysis than the WT TA2F1, no further activation of ATPase activity was observed [85]. The reason for this remains unclear.
The recent structures of the WT TA2F1 and a mutant, in which the ATP binding site is lost (D89A/R92A), revealed the ɛ subunit in a similar hairpin conformation [45], akin to that of the E. coli [36,38] and P. denitrificans [15] ɛ subunits. Notably, the E. coli and Bacillus PS3 ɛ subunits have also been solved in the extended conformation [40,41], so while it is curious that the TA2F1 D89A/R92A mutant resulted in a hairpin conformation, we know from the more vigorously studied EF1 that this is not entirely unexpected as the conformations are clearly dynamic in nature [44]. Having noted this, theoretically, the TA2F1 D89A/R92A mutant should lack the ability to bind ATP, and if this is the regulator inducing the formation of a hairpin conformation, one would expect to find an extended conformation ɛ subunit structure. However, this study clearly demonstrates that the lack of ATP presence, or, more directly, the lack of an apparent ATP-binding motif, appears not to necessarily mean the ɛ subunit will definitively adopt the extended conformation at all times, if indeed it does in TA2F1.
In the context of the previously discussed organisms, the structural role of ɛR126 (Bacillus PS3) and ɛQ127 (E. coli, numbering as in the deposited crystal structure) are not clear as these residues are not resolved in the extended conformation in Bacillus PS3 ɛ subunit [41], nor are there any obvious interactions with the α3β3γ interface in the E. coli ɛ subunit [40]. However, the role of these residues in the ɛ subunit from C. thermarum TA2.A1 in the extended conformation cannot be assumed, as the ɛ subunit was neither resolved [86] nor found to reside in the extended conformation [45] in the conditions used in the presently available crystal structures.
Lastly, while the focus of this review is on ɛ subunit regulation, it should be mentioned that the γ subunit has a potential role in ATP hydrolysis regulation in TA2F1. A mutation changing 8KRRIR12 residues in the γ subunit of TA2F1 to 8QQQIQ12 residues (TA2F1γQ4) resulted in a similarly partially active hydrolytic enzyme with similar ATP hydrolysis kinetics to the TA2F1 Δɛ6A mutant previously mentioned [86].
2.2. Structural properties of the ɛ subunit from bacteria
2.2.1. Comparison of structural features of isolated ɛ subunits in the hairpin conformation
Although certain crystallographic features of the ɛ subunit have been previously discussed in the context of the biochemical and biophysical section of this manuscript, such is the complexity of the topic that a dedicated section must be presented to give the full picture on this topic.
First, we compare the isolated ɛ subunits from different organisms in the context of their highly varied ATP binding affinities. The crystal structure of the ɛ subunit from Bacillus PS3 shows a well-defined ATP binding motif comprising interactions of E83, D89 (backbone), R92, R122 and R126 with ATP [39]. However, the crystal structure was solved as a dimer, and thus does not reflect the monomeric presence of the ɛ subunit in bacterial ATP synthases. Molecular dynamics simulations have since served to refine models of the ATP binding site, and predict where Mg2+ ions bind between ATP:Oα/Oβ [83], which has also been observed in the structure from C. thermarum TA2.A1 [45].
The well-defined binding motif in the ɛ subunit from Bacillus PS3 enables the protein to bind ATP with an affinity of 4 µM at 25°C [53]. Interestingly, under the same conditions, a R103A/R115A double mutant was capable of binding ATP with two orders of magnitude increased affinity (52 nM) [54]. This was proposed to be caused by an increased number of hydrogen-bonds between the protein and the ligand due to a structural rearrangement of the ligand binding site [87]. ATP binding affinities of the ɛ subunit from B. subtilis (2 mM at 25°C) [55], M. tuberculosis (ATP binding was not observed in the measured range) [43] and E. coli (22 mM at 25°C) [39] have also been reported.
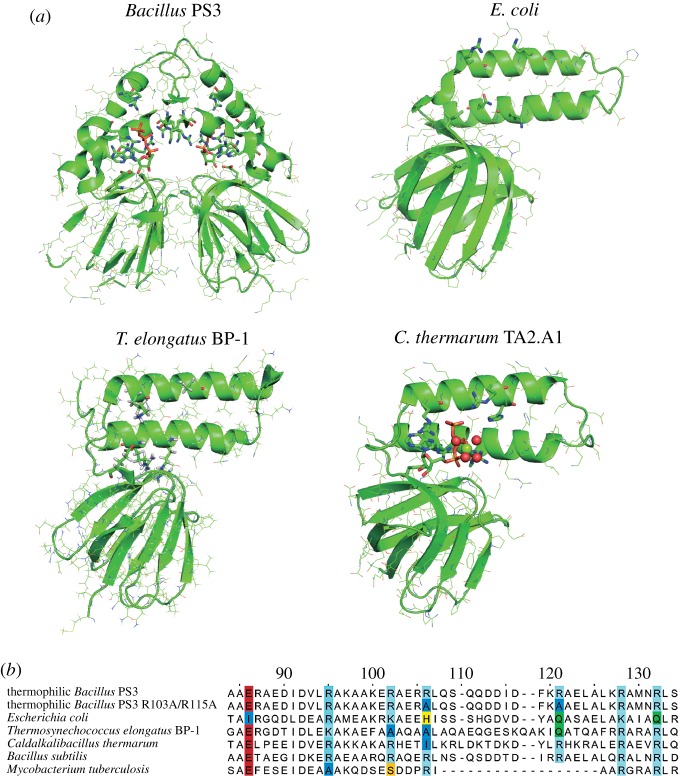
Current structural data (figure 1a) and a sequence alignment (figure 1b) indicate that the proposed ATP binding site composition controls the ligand binding affinity. In the ɛ subunit from Bacillus PS3, positively charged residues can be found [39], some of them exchanged by polar and/or hydrophobic amino acids in the α-helical CTD of the ɛ subunit from E. coli [36,38] or T. elongates BP-1 [31] (see also the sequence alignment in figure 1b). To derive the reasons for the different binding affinities of ATP to the ɛ subunit from different organisms, we have aligned and compared the sequences of ɛ subunits from different organisms with known Kd to bind ATP (figure 1b), and compared them with the most well-described enzyme for these studies, Bacillus PS3. When comparing the ɛ subunit sequences of Bacillus PS3 and B. subtilis, there are no differences in the proposed ATP binding motif, yet they differ 500-fold in their ATP binding affinity (4 µM versus 2 mM for Bacillus PS3 [53] and B. subtilis [55], respectively). This suggests that there are other factors to consider.
Figure 1.
(a) Crystal/NMR structures of the ɛ subunits from Bacillus PS3 (PDB-ID: 2E5Y), Escherichia coli (PDB-ID:1AQT), Thermosynechococcus elongates BP-1 (PDB-ID: 2RQ6) and Caldalkalibacillus thermarum TA2.A1 (PDB-ID: 5HKK—only the ɛ subunit is shown; all other F1 subunits are omitted) in the hairpin conformation. Known and potential binding residues are highlighted. (b) Sequence alignment of the binding site of several ɛ subunits from different organisms. The binding site residues are coloured/highlighted. The sequence alignment was created using Jalview [88].
It has been previously proposed that an allosteric Mg2+ binding site causes a reduction of the ATP binding affinity [89], which is in agreement with the experimentally measured decreasing Kd of the ɛ subunit R84A mutant from Bacillus PS3 [53]. Furthermore, the alignment of the different proposed ATP binding motif residues show that the ɛ subunit from E. coli harbours four divergences from the Bacillus PS3 primary sequence: E83I, R99 K, R122 K and R126Q (in alignment positions 86, 102, 128 and 132, respectively). Mutations of three of these residues (E83, R122 and R126) to alanine have shown a remarkably reduced ability of the ɛ subunit to bind ATP from Bacillus PS3 [53], while the R99A mutation showed a moderate effect in gel-filtration experiments.
Subtle divergences in proposed ATP binding motifs in protein sequence alignments appear to have significant effects. The ɛ subunit from C. thermarum TA2.A1 harbours a divergence in the proposed ATP binding motif from the Bacillus PS3 sequence in position 103 (R103I in respect to Bacillus PS3, position 106 in alignment). Owing to its structural location, this mutation may indeed increase ATP binding affinity, as shown for the R103A/R115A double mutant from the ɛ subunit from Bacillus PS3 [54]. This proposition remains to be experimentally verified. The ɛ subunit sequence from T. elongates BP-1 differs from Bacillus PS3 at positions 95 and 102, which have been shown to reduce ATP binding affinity [53]; however, as these mutations may not be crucial for ATP binding, the ATP binding affinity might theoretically be expected to be in the higher millimolar range. The ɛ subunit from M. tuberculosis also harbours mutations in these positions (R92A and R99S; alignment positions 95 and 102, respectively), which were shown to decrease the ATP binding affinity. When arginine residues (R92 and R99) were mutated to alanine residues in the ɛ subunit of Bacillus PS3, the R92A mutation caused a decreased binding affinity of 40-fold (4 µM of WT versus 160 µM of R92A mutant) [53]. Interestingly, the ɛ subunit from M. tuberculosis also contains a gap of 16 residues in the CTD, thus missing potentially stabilizing hydrophobic interactions. This gap may cause the lack of any observed hairpin conformation, as indicated by small-angle X-ray scattering (SAXS) observations [43], but definitively highlights this ɛ subunit as a unique and promising drug target.
2.2.2. Structural features of the ɛ subunit in a state between the hairpin and extended conformations
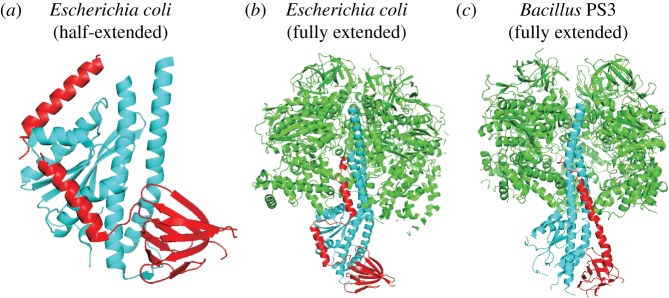
The first structural evidence showing that the ɛ subunit from E. coli has dynamic conformational changes was an X-ray structure capturing a half-extended conformation (or half-hairpin) derived from a γɛ complex (PDB-ID: 1FS0; figure 2a) [44]. This conformation is dramatically different from the other presented structures (isolated ɛ subunits), which have been reported to be in the hairpin conformation by NMR (PDB-ID: 1BSN) [37] and X-ray crystallography (PDB-ID: 1AQT; figure 1a) [38]. These different conformations of the hairpin conformation (PDB-IDs: 1BSN and 1AQT) and the half-extended state (PDB-ID: 1FS0) indicate that protein–protein interactions are likely to stabilize the extended conformation.
Figure 2.
The ɛ subunit from Escherichia coli in (a) the half-extended conformation in the presence of the γ subunit (PDB-ID: 1FS0) and (b) in the extended conformation in presence of α3β3γ (PDB-ID: 3OAA), and (c) the ɛ subunit from Bacillus PS3 in the extended conformation in the presence of α3β3γ (PDB-ID: 4XD7). One β and one α subunit are omitted for clarity in the extended conformation of the ɛ subunit from E. coli and Bacillus PS3, respectively. Subunits α3β3, γ and ɛ are shown in green, cyan and red, respectively, in all panels.
Current ATP fluorescence sensors based on the ɛ subunit show that, in the absence of ATP, an extended conformation will be adopted [69,90,91], an observation supported by the finding that the ɛγ complex from Bacillus PS3 is capable of binding ATP [71]. If the whole F1 domain is present during crystallization, a fully extended state of the ɛ subunit [40] can be observed in F1 from some bacterial species, binding to α3β3γ and thus preventing rotation in the hydrolysis direction. Interestingly, a recent cryo-EM study revealed that the ɛ subunits of all F1Fo particles from E. coli adopt the extended conformation [16]. However, binding conformations of these cryo-EM structures are different from the previously resolved X-ray structures. The structures of the α3β3γɛ complex from E. coli [40] and Bacillus PS3 [41] in the extended conformation are shown in figure 2b,c. Lastly, as the bulk phase ATP concentration in living cells is in the millimolar range (approx. 1.54 mM for the E. coli cell cytoplasm [69]), and the binding constant of ATP to the ɛ subunit from E. coli is 22 mM [39], it would seem logical that the ɛ subunit is predominantly in the extended conformation under physiological conditions. However, two factors cannot be excluded: first, that the ɛ subunit is in a similar conformation to the half-extended conformation, as the ɛ subunit will be released after ATP binding to the β subunit [32]; second, the role or possibility of localized ATP pools prior to diffusion into the bulk phase.
2.2.3. Revisiting the crystal structure from Caldalkalibacillus thermarum TA2.A1
Recent biophysical [32–35,68], crystallographic [40,41] and cryo-EM [16] data support the conclusion that the two C-terminal helicies of the ɛ subunit from different organisms are capable of undergoing a conformational change from a hairpin to an extended conformation. In the crystal structure of the WT TA2F1, the ɛ subunit is found in the hairpin conformation bound to ATP (PDB-ID: 5HKK), as shown previously for the isolated ɛ subunit from Bacillus PS3 [39]. However, unexpectedly, the TA2F1 D89A/R92A mutant is also found in the hairpin conformation, despite ATP not binding to the ɛ subunit in the crystal structure (PDB-ID: 5IK2) [45]. This contradicts our present understanding of the dynamics and regulatory role of the ɛ subunit that have been observed from concerted E. coli and Bacillus PS3 studies. We have made the comparison with Bacillus PS3, as this is more closely related, phylogenetically, to C. thermarum TA2.A1 than E. coli. First, NMR data of TF1 showed the structure was difficult to resolve due to dynamic movements, in the absence of ATP [39], perhaps also due to the lack of true resting conformation, but an extended form was indeed revealed. The C. thermarum TA2.A1 ɛ subunit may simply fall into the hairpin conformation more consistently, providing a tighter trend. This is an interesting, and perhaps unique feature of C. thermarum TA2.A1, because we cannot discount the increase in ATP hydrolysis observed with the γ subunit mutant TA2F1γQ4 compared with the native latent WT activity [86]. Second, the structure is consistent with the rotation studies of Bacillus PS3, where the WT TF1 and TF1 ɛΔCTH also have an identical kinetic profile to the WT enzyme [72].
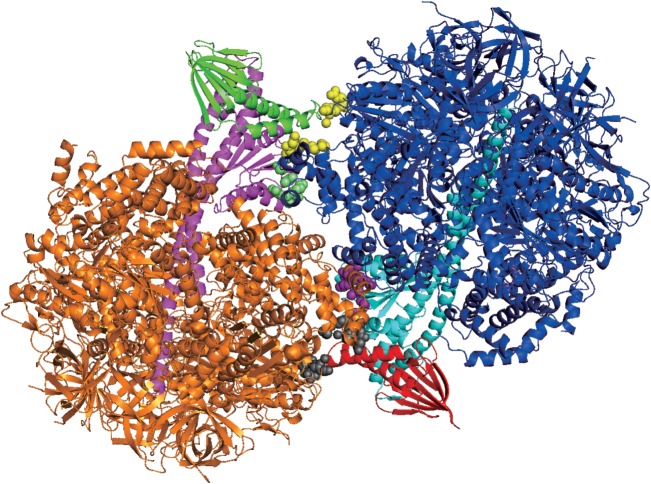
Another possible reason might lie with the technical details in the mutant structure [45]. First, we observe two independently crystallized F1 subunits of the ATP synthases in the unit cell, A and B. Second, we observe crucial interactions between the ɛ and γ subunits of monomer A (spheres; coloured in purple and yellow) with the hexagonal α3β3 assembly of monomer B and vice versa (spheres; coloured in grey and lime), as shown in figure 3. Despite the absence of ATP, these interactions may subtly favour the ɛ subunit in the hairpin conformation, because the hairpin conformation could also be observed for the ATP-free, monomeric, isolated apo structure of the ɛ subunit by NMR spectroscopy [31,36] or X-ray crystallography [38]. Protein–protein interactions, which are prevented in this structure [45] by interactions of the ɛ and γ of monomer A with α3β3 of monomer B, and vice versa, may also be required for the conformational change, as indicated by the crystal structure of the ɛγ-complex from the F-type ATP synthase from E. coli [44]. This is a pertinent point when considering the consistent presence of the hairpin conformation in the absence of the γ subunit when resolving full-length ɛ subunit structures [36,38]. However, to confound the matter, the conformational change from the extended to the hairpin conformation of the ɛ subunit in the γɛ sub-complex is also induced by binding to ATP [71], and is a dynamic movement, and therefore highly conditional. Clearly, there is still much research to conduct before this can be resolved.
Figure 3.
The structural model of TA2F1 D92A/R92A mutant, whose ɛ subunit apparently does not bind ATP, as deposited in the Protein Database (PDB ID: 5IK2). It can be observed that the α3β3 interacts with subunits γ and ɛ of the other ATP synthase. Subunits ɛ, γ and α3β3 are shown in green (red), magenta (cyan) and orange (blue) in cartoon representation, respectively. Contacts of the α3β3 assembly with subunits ɛ and γ in the distance of 5 Å are shown in yellow (grey) and lime (purple), respectively. This figure was created with PyMOL v. 1.7.0 (www.pymol.org) [92]. A zoom-in for the contacts of monomer A and B is shown in electronic supplementary material, figure S1.
3. Conclusion and the 6th antibiotic target space
3.1. Regulatory function of the ɛ subunit
Considering biochemical and biophysical measurements, and the structural data in the hairpin [36,38,39], extended [16,40,41] and half-extended conformations [44], there are various lines of evidence that support the notion that the ɛ subunit of ATP synthases from at least some bacteria regulate ATP hydrolysis activity. However, taking into account the different ATP binding affinities of the ɛ subunit from different organisms, it can be expected that not all bacterial organisms are regulated by ATP binding to the ɛ subunit under physiological conditions (in E. coli, the ɛ subunit has a binding affinity of 22 mM [39]).
ATP binding appears to be a strong influence required to stabilize the hairpin conformation, yet there is still a lack of conclusive evidence on whether ATP synthesis is influenced by the ɛ subunit in E. coli [69,90,91]. Furthermore, it has been proposed that the CTD of the ɛ subunit from E. coli [93] and the ATP-bound down state from the Bacillus PS3 ɛ subunit allow efficient proton coupling to ATP hydrolysis [94]. Together, these data indicate a slightly different working principle in different organisms.
In the authors' view, what is now of intense interest for this micro-field may be the role of the ɛ subunit of P. denitrificans and other α-proteobacteria—in which it has been shown that the ɛ subunit does not inhibit ATP hydrolytic activity, but a novel regulatory subunit, the ζ subunit, that harbours an ATP binding site, is present [29]. It will be of interest to examine whether the ζ subunit inhibition is influenced by the presence of Mg-ATP.
Considering that biochemical experiments demonstrate that the CTD of the ɛ subunit from C. thermarum TA2.A1 has a role in the regulation of ATP hydrolysis activity, but not the TA2F1 Δɛ6A mutant [85], it is curious that the WT ɛ subunit is found predominantly in the hairpin conformation in the absence of ATP, and that the TA2F1 D89A/R92A mutant is in the hairpin conformation despite presumably lacking the ability to bind ATP. It is indeed feasible that the crystal contacts between two neighbouring ATP synthases in the crystal may influence both the TA2F1WT and the D89A/R92A mutant structures obtained [45], and is expected to regulate ATP hydrolysis similarly to other bacteria, such as E. coli or Bacillus PS3. We cannot discount that mechanisms of regulation may be subtle in their diversity. In addition, TA2F1 has seemingly little native ATP hydrolysis activity [84], whereas both the E. coli and Bacillus PS3 enzymes have native ATP hydrolysis activity [95,96], making the C. thermarum TA2.A1 a very interesting enzyme to study to aid in unravelling ɛ subunit regulatory function. At this point, both the structure and function studies suggest a strong role of ADP inhibition [84] and the ɛ subunit as a releasable ‘emergency break’, similar to the suggested role of mammalian IF1 or the alpha-proteobacterial ζ subunit. Yet functional studies clearly demonstrate a role in ATP hydrolysis suppression [85], suggesting a dynamic role that may be precluded by crystallization conditions. The lack of resting conformation shown in the NMR studies of the Bacillus PS3 ɛ subunit support this notion [39]. Taking structural and functional evidence together, a recent claim [45] to have identified the mechanism of the ATP hydrolysis regulation of the C. thermarum TA2.A1 F1 ATPase would seem premature. While the data presented by the authors were crystal structures of excellent quality, the authors did not reveal the role of the ɛ subunit, for which there clearly is a role [85], nor the dynamics of ADP regulation, which is a common mechanism of ATP hydrolysis regulation. Generally, we do not consider observations of TA2F1 structure/function to be dismissive of other studies denoting ɛ subunit regulatory function in other organisms, but they do add valuable insight into the diversity and tuning involved in ATP synthase regulation.
3.2. The 6th antibiotic target space: pathogen ATP synthases as potential drug-targets
Recently, the F1Fo ATP synthases of certain mycobacterial species have been demonstrated to be promising new drug targets. The drug bedaquiline (BDQ; previously known as compound TMC207) has been shown to be a novel antibiotic compound against tuberculosis [1], and to bind to a purified preparation of M. smegmatis and M. tuberculosis c-rings, but relatively poorly to the c subunit ring mutant atpEA63P [2]. To support this, a recent structural study revealed a model of BDQ bound to the c-ring of M. phelei [97]. Comparisons with the possible binding mode of the drug to other c-rings [97] suggest possible reasons for selective binding [98] to various mycobacterial species. Previously, computational models had proposed the drug interacting at the c-ring/a subunit interface [99], while biochemical and structural data from the Grüber group indicate interactions with both the c-ring and the ɛ subunit [43,100]. Oddly, it would seem that the BDQ binding site and the subunits involved are not clear, as available data are inconsistent. This underlines an inherent risk in the use of crystallography, and the requirement for supporting functional studies. It is duly acknowledged that inhibitors carrying charges may also participate in favourable charge–charge interactions with proteins under favourable pH conditions, which may confound results. Full F1Fo time-resolved crystallographic affinity constants in direct comparison with affinity constants from pre-steady-state kinetic measurements could be useful tools to clarify the mechanism and binding mode of BDQ. Despite these confounding results, knowledge about the interactions of BDQ with the c-ring [97] have allowed an intelligent design approach to construct novel potential antibiotic compounds with a reduced backbone while still binding to the ATP synthase of mycobacteria [101].
Mycobacterial species also appear to have developed several mechanisms to prevent wasteful ATP hydrolysis, an activity that could be fatal for non-growing, infective-phase M. tuberculosis. The first regulatory mechanism is Mg2+-ADP, and the second may be using the ɛ subunit [50]; however, a unique loop in the γ subunit may also slow down ATP hydrolysis due to interactions with the c-ring [51], and an extended ɛ subunit CTD may prevent ATP hydrolysis activity due to interactions with the γ subunit [52].
The possible regulatory role of the γ subunit in C. thermarum TA2.A1 [85] must take into account the different elemental mechanistic steps of the bacterial F1 compared with mammalian F1, as there may be some possibilities to selectively inhibit the function of the bacterial enzyme, as mitochondrial (bovine) and bacterial (Bacillus PS3) ATP synthases have different affinities to various compounds [102]. With this in mind, it would seem a feasible suggestion that novel compounds could be designed which may uncouple respiratory-driven ATP synthesis [103] by interacting with the c-ring and the a or γ subunits. In line with this review, a more bacterial-specific possibility would be to develop drug compounds to modulate the state of the ɛ subunit by forcing an unfavourable conformation for the state of bacterial growth (e.g. forcing M. tuberculosis to hydrolyse its ATP while in a slow-growth infective state). Further developments of antimicrobial drugs targeting mycobacterial species may also involve compounds that take advantage of interactions of the unique loop in the γ subunit with the c-ring [51], preventing a rotation in synthesis direction or small organic molecules targeting the interface between subunit γ and the extension of the C-terminal domain of subunit α [52].
Lastly, while developments have certainly been made towards targeting M. tuberculosis, there are several other promising drug targets, such as the F1Fo ATP synthases of Trypanosoma brucei (sleeping sickness) and Fusobacterium nucleatum, that all have novel features. The T. brucei F1Fo ATPase has several unique subunits (e.g. P18) of unknown function [104,105]. Fusobacterium nucleatum has an F1Fo ATP synthase that uses sodium as a coupling ion, a common feature in human pathogen ATP synthases. Structural insights into the c-ring of this pathogenic organism have recently been obtained and may denote a possible drug target [106]. In all cases, the role of the ɛ subunit is undefined, but given its clear regulatory role in catalysis, there is a strong case to support exploring the ɛ subunit function more widely.
Supplementary Material
Data accessibility
This article has no additional data.
Authors' contributions
A.K., M.Z.-Z. and D.G.G.M. all wrote parts of the manuscript.
Competing interests
We have no competing interests.
Funding
This work was supported by a TU Delft StartUP fund (D.G.G.M.) and a Conacyt Postdoctoral Grant (M.Z.-Z.).
References
- 1.Andries K, et al. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307, 223–227. (doi:10.1126/science.1106753) [DOI] [PubMed] [Google Scholar]
- 2.Koul A, et al. 2007. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat. Chem. Biol. 3, 323–324. (doi:10.1038/nchembio884) [DOI] [PubMed] [Google Scholar]
- 3.Krah A. 2015. Linking structural features from mitochondrial and bacterial F-type ATP synthases to their distinct mechanisms of ATPase inhibition. Prog. Biophys. Mol. Biol. 119, 94–102. (doi:10.1016/j.pbiomolbio.2015.06.005) [DOI] [PubMed] [Google Scholar]
- 4.Abrahams JP, Leslie AG, Lutter R, Walker JE. 1994. Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature 370, 621–628. (doi:10.1038/370621a0) [DOI] [PubMed] [Google Scholar]
- 5.Dautant A, Velours J, Giraud M-F. 2010. Crystal structure of the Mg·ADP-inhibited state of the yeast F1c10-ATP synthase. J. Biol. Chem. 285, 29 502–29 510. (doi:10.1074/jbc.M110.124529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stock D, Leslie AG, Walker JE. 1999. Molecular architecture of the rotary motor in ATP synthase. Science 286, 1700–1705. (doi:10.1126/science.286.5445.1700) [DOI] [PubMed] [Google Scholar]
- 7.Meier T, Yu J, Raschle T, Henzen F, Dimroth P, Muller DJ. 2005. Structural evidence for a constant c11 ring stoichiometry in the sodium F-ATP synthase. FEBS J. 272, 5474–5483. (doi:10.1111/j.1742-4658.2005.04940.x) [DOI] [PubMed] [Google Scholar]
- 8.Pogoryelov D, Yu J, Meier T, Vonck J, Dimroth P, Muller DJ. 2005. The c15 ring of the Spirulina platensis F-ATP synthase: F1/F0 symmetry mismatch is not obligatory. EMBO Rep. 6, 1040–1044. (doi:10.1038/sj.embor.7400517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthies D, Preiss L, Klyszejko AL, Muller DJ, Cook GM, Vonck J, Meier T. 2009. The c13 ring from a thermoalkaliphilic ATP synthase reveals an extended diameter due to a special structural region. J. Mol. Biol. 388, 611–618. (doi:10.1016/j.jmb.2009.03.052) [DOI] [PubMed] [Google Scholar]
- 10.Ballhausen B, Altendorf K, Deckers-Hebestreit G. 2009. Constant c10 ring stoichiometry in the Escherichia coli ATP synthase analyzed by cross-linking. J. Bacteriol. 191, 2400–2404. (doi:10.1128/JB.01390-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allegretti M, Klusch N, Mills DJ, Vonck J, Kühlbrandt W, Davies KM. 2015. Horizontal membrane-intrinsic α-helices in the stator a-subunit of an F-type ATP synthase. Nature 521, 237–240. (doi:10.1038/nature14185) [DOI] [PubMed] [Google Scholar]
- 12.Hahn A, Parey K, Bublitz M, Mills DJ, Zickermann V, Vonck J, Kühlbrandt W, Meier T. 2016. Structure of a complete ATP synthase dimer reveals the molecular basis of inner mitochondrial membrane morphology. Mol. Cell 63, 445–456. (doi:10.1016/j.molcel.2016.05.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dmitriev O, Jones PC, Jiang W, Fillingame RH. 1999. Structure of the membrane domain of subunit b of the Escherichia coli F0F1 ATP synthase. J. Biol. Chem. 274, 15 598–15 604. (doi:10.1074/JBC.274.22.15598) [DOI] [PubMed] [Google Scholar]
- 14.Stalz W-D, Greie J-C, Deckers-Hebestreit G, Altendorf K. 2003. Direct interaction of subunits a and b of the F0 complex of Escherichia coli ATP synthase by forming an ab2 subcomplex. J. Biol. Chem. 278, 27 068–27 071. (doi:10.1074/jbc.M302027200) [DOI] [PubMed] [Google Scholar]
- 15.Morales-Rios E, Montgomery MG, Leslie AGW, Walker JE. 2015. Structure of ATP synthase from Paracoccus denitrificans determined by X-ray crystallography at 4.0 Å resolution. Proc. Natl Acad. Sci. USA 112, 13 231–13 236. (doi:10.1073/pnas.1517542112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sobti M, Smits C, Wong AS, Ishmukhametov R, Stock D, Sandin S, Stewart AG. 2016. Cryo-EM structures of the autoinhibited E. coli ATP synthase in three rotational states. Elife 5, e21598 (doi:10.7554/eLife.21598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rees DM, Leslie AGW, Walker JE. 2009. The structure of the membrane extrinsic region of bovine ATP synthase. Proc. Natl Acad. Sci. USA 106, 21 597–21 601. (doi:10.1073/pnas.0910365106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiko C, et al. 2015. Bovine F1Fo ATP synthase monomers bend the lipid bilayer in 2D membrane crystals. Elife 4, e06119 (doi:10.7554/eLife.06119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambongi Y, Iko Y, Tanabe M, Omote H, Iwamoto-Kihara A, Ueda I, Yanagida T, Wada Y, Futai M. 1999. Mechanical rotation of the c subunit oligomer in ATP synthase (F0F1): direct observation. Science 286, 1722–1724. (doi:10.1126/science.286.5445.1722) [DOI] [PubMed] [Google Scholar]
- 20.Krah A, Pogoryelov D, Langer JD, Bond PJ, Meier T, Faraldo-Gómez JD. 2010. Structural and energetic basis for H+ versus Na+ binding selectivity in ATP synthase Fo rotors. Biochim. Biophys. Acta Bioenerg. 1797, 763–772. (doi:10.1016/j.bbabio.2010.04.014) [DOI] [PubMed] [Google Scholar]
- 21.Diez M, et al. 2004. Proton-powered subunit rotation in single membrane-bound F0F1-ATP synthase. Nat. Struct. Mol. Biol. 11, 135–141. (doi:10.1038/nsmb718) [DOI] [PubMed] [Google Scholar]
- 22.Pogoryelov D, Krah A, Langer JD, Yildiz Ö, Faraldo-Gómez JD, Meier T. 2010. Microscopic rotary mechanism of ion translocation in the Fo complex of ATP synthases. Nat. Chem. Biol. 6, 891–899. (doi:10.1038/nchembio.457) [DOI] [PubMed] [Google Scholar]
- 23.Noji H, Yasuda R, Yoshida M, Kinosita K. 1997. Direct observation of the rotation of F1-ATPase. Nature 386, 299–302. (doi:10.1038/386299a0) [DOI] [PubMed] [Google Scholar]
- 24.Hirono-Hara Y, Noji H, Nishiura M, Muneyuki E, Hara KY, Yasuda R, Kinosita K, Yoshida M. 2001. Pause and rotation of F1-ATPase during catalysis. Proc. Natl Acad. Sci. USA 98, 13 649–13 654. (doi:10.1073/pnas.241365698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menz RI, Walker JE, Leslie AGW. 2001. Structure of bovine mitochondrial F1-ATPase with nucleotide bound to all three catalytic sites. Cell 106, 331–341. (doi:10.1016/S0092-8674(01)00452-4) [DOI] [PubMed] [Google Scholar]
- 26.Pullman ME, Monroy GC. 1963. A naturally occurring inhibitor of mitochondrial adenosine triphosphatase. J. Biol. Chem. 238, 3762–3769. [PubMed] [Google Scholar]
- 27.Kato-Yamada Y, Bald D, Koike M, Motohashi K, Hisabori T, Yoshida M. 1999. ɛ Subunit, an endogenous inhibitor of bacterial F1-ATPase, also inhibits F0F1-ATPase. J. Biol. Chem. 274, 33 991–33 994. (doi:10.1074/jbc.274.48.33991) [DOI] [PubMed] [Google Scholar]
- 28.Morales-Ríos E, de la Rosa-Morales F, Mendoza-Hernández G, Rodríguez-Zavala JS, Celis H, Zarco-Zavala M, García-Trejo JJ. 2010. A novel 11-kDa inhibitory subunit in the F1FO ATP synthase of Paracoccus denitrificans and related alpha-proteobacteria. FASEB J. 24, 599–608. (doi:10.1096/fj.09-137356) [DOI] [PubMed] [Google Scholar]
- 29.Zarco-Zavala M, Morales-Ríos E, Mendoza-Hernández G, Ramírez-Silva L, Pérez-Hernández G, García-Trejo JJ. 2014. The ζ subunit of the F1FO-ATP synthase of α-proteobacteria controls rotation of the nanomotor with a different structure. FASEB J. 28, 2146–2157. (doi:10.1096/fj.13-241430) [DOI] [PubMed] [Google Scholar]
- 30.Serrano P, Geralt M, Mohanty B, Wüthrich K. 2014. NMR structures of α-proteobacterial ATPase-pegulating ζ-subunits. J. Mol. Biol. 426, 2547–2553. (doi:10.1016/j.jmb.2014.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yagi H, Konno H, Murakami-Fuse T, Isu A, Oroguchi T, Akutsu H, Ikeguchi M, Hisabori T. 2010. Structural and functional analysis of the intrinsic inhibitor subunit ɛ of F1-ATPase from photosynthetic organisms. Biochem. J. 425, 85–98. (doi:10.1042/BJ20091247) [DOI] [PubMed] [Google Scholar]
- 32.Iino R, Murakami T, Iizuka S, Kato-Yamada Y, Suzuki T, Yoshida M. 2005. Real-time monitoring of conformational dynamics of the epsilon subunit in F1-ATPase. J. Biol. Chem. 280, 40 130–40 134. (doi:10.1074/jbc.M506160200) [DOI] [PubMed] [Google Scholar]
- 33.Feniouk BA, Suzuki T, Yoshida M. 2007. Regulatory interplay between proton motive force, ADP, phosphate, and subunit epsilon in bacterial ATP synthase. J. Biol. Chem. 282, 764–772. (doi:10.1074/jbc.M606321200) [DOI] [PubMed] [Google Scholar]
- 34.Iino R, Hasegawa R, Tabata KV, Noji H. 2009. Mechanism of inhibition by C-terminal alpha-helices of the epsilon subunit of Escherichia coli FoF1-ATP synthase. J. Biol. Chem. 284, 17 457–17 464. (doi:10.1074/jbc.M109.003798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feniouk BA, Kato-Yamada Y, Yoshida M, Suzuki T. 2010. Conformational transitions of subunit epsilon in ATP synthase from thermophilic Bacillus PS3. Biophys. J. 98, 434–442. (doi:10.1016/j.bpj.2009.10.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilkens S, Dahlquist FW, McIntosh LP, Donaldson LW, Capaldi RA. 1995. Structural features of the ɛ subunit of the Escherichia coli ATP synthase determined by NMR spectroscopy. Nat. Struct. Biol. 2, 961–967. (doi:10.1038/nsb1195-961) [DOI] [PubMed] [Google Scholar]
- 37.Wilkens S, Capaldi RA. 1998. Solution structure of the subunit of the F1-ATPase from Escherichia coli and interactions of this subunit with subunits in the complex. J. Biol. Chem. 273, 26 645–26 651. (doi:10.1074/jbc.273.41.26645) [DOI] [PubMed] [Google Scholar]
- 38.Uhlin U, Cox GB, Guss JM. 1997. Crystal structure of the epsilon subunit of the proton-translocating ATP synthase from Escherichia coli. Structure 5, 1219–1230. (doi:10.1016/s0969-2126(97)00272-4) [DOI] [PubMed] [Google Scholar]
- 39.Yagi H, Kajiwara N, Tanaka H, Tsukihara T, Kato-Yamada Y, Yoshida M, Akutsu H. 2007. Structures of the thermophilic F1-ATPase subunit suggesting ATP-regulated arm motion of its C-terminal domain in F1. Proc. Natl Acad. Sci. USA 104, 11 233–11 238. (doi:10.1073/pnas.0701045104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cingolani G, Duncan TM. 2011. Structure of the ATP synthase catalytic complex F1 from Escherichia coli in an autoinhibited conformation. Nat. Struct. Mol. Biol. 18, 701–707. (doi:10.1038/nsmb.2058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shirakihara Y, Shiratori A, Tanikawa H, Nakasako M, Yoshida M, Suzuki T. 2015. Structure of a thermophilic F1-ATPase inhibited by an ɛ-subunit: deeper insight into the ɛ-inhibition mechanism. FEBS J. 282, 2895–2913. (doi:10.1111/febs.13329) [DOI] [PubMed] [Google Scholar]
- 42.Klionsky DJ, Brusilow WS, Simoni RD. 1984. In vivo evidence for the role of the epsilon subunit as an inhibitor of the proton-translocating ATPase of Escherichia coli. J. Bacteriol. 160, 1055–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biukovic G, Basak S, Manimekalai MSS, Rishikesan S, Roessle M, Dick T, Rao SPS, Hunke C, Grüber G. 2013. Variations of subunit ɛ of the Mycobacterium tuberculosis F1Fo ATP synthase and a novel model for mechanism of action of the tuberculosis drug TMC207. Antimicrob. Agents Chemother. 57, 168–176. (doi:10.1128/AAC.01039-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodgers AJ, Wilce MC. 2000. Structure of the gamma-epsilon complex of ATP synthase. Nat. Struct. Biol. 7, 1051–1054. (doi:10.1038/80975) [DOI] [PubMed] [Google Scholar]
- 45.Ferguson SA, Cook GM, Montgomery MG, Leslie AGW, Walker JE. 2016. Regulation of the thermoalkaliphilic F1-ATPase from Caldalkalibacillus thermarum. Proc. Natl Acad. Sci. USA 113, 10 860–10 865. (doi:10.1073/pnas.1612035113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hicks DB, Krulwich TA. 1990. Purification and reconstitution of the F1F0-ATP synthase from alkaliphilic Bacillus firmus OF4. Evidence that the enzyme translocates H+ but not Na +. J. Biol. Chem. 265, 20 547–20 554. [PubMed] [Google Scholar]
- 47.Hoffmann A, Dimroth P. 1990. The ATPase of Bacillus alcalophilus. Purification and properties of the enzyme. Eur. J. Biochem. 194, 423–430. (doi:10.1111/j.1432-1033.1990.tb15635.x) [DOI] [PubMed] [Google Scholar]
- 48.Cook GM, Keis S, Morgan HW, von Ballmoos C, Matthey U, Kaim G, Dimroth P. 2003. Purification and biochemical characterization of the F1Fo-ATP synthase from thermoalkaliphilic Bacillus sp. strain TA2.A1. J. Bacteriol. 185, 4442–4449. (doi:10.1128/JB.185.15.4442-4449.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.García-Trejo JJ, Zarco-Zavala M, Mendoza-Hoffmann F, Hernández-Luna E, Ortega R, Mendoza-Hernández G. 2016. The inhibitory mechanism of the ζ subunit of the F1FO-ATPase nanomotor of Paracoccus denitrificans and related α-Proteobacteria. J. Biol. Chem. 291, 538–546. (doi:10.1074/jbc.M115.688143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haagsma AC, Driessen NN, Hahn M-M, Lill H, Bald D. 2010. ATP synthase in slow- and fast-growing mycobacteria is active in ATP synthesis and blocked in ATP hydrolysis direction. FEMS Microbiol. Lett. 313, 68–74. (doi:10.1111/j.1574-6968.2010.02123.x) [DOI] [PubMed] [Google Scholar]
- 51.Hotra A, Suter M, Biuković G, Ragunathan P, Kundu S, Dick T, Grüber G. 2016. Deletion of a unique loop in the mycobacterial F-ATP synthase γ subunit sheds light on its inhibitory role in ATP hydrolysis-driven H+ pumping. FEBS J. 283, 1947–1961. (doi:10.1111/febs.13715) [DOI] [PubMed] [Google Scholar]
- 52.Ragunathan P, et al. 2017. The uniqueness of subunit α of mycobacterial F-ATP synthases: an evolutionary variant for niche adaptation. J. Biol. Chem. 292, 11 266–11 279. (doi:10.1074/jbc.M117.784959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kato S, Yoshida M, Kato-Yamada Y. 2007. Role of the ɛ subunit of thermophilic F1-ATPase as a sensor for ATP. J. Biol. Chem. 282, 37 618–37 623. (doi:10.1074/jbc.M707509200) [DOI] [PubMed] [Google Scholar]
- 54.Kato-Yamada Y. 2016. High affinity nucleotide-binding mutant of the ɛ subunit of thermophilic F1-ATPase. Biochem. Biophys. Res. Commun. 469, 1129–1132. (doi:10.1016/j.bbrc.2015.12.121) [DOI] [PubMed] [Google Scholar]
- 55.Kato-Yamada Y. 2005. Isolated ɛ subunit of Bacillus subtilis F1-ATPase binds ATP. FEBS Lett. 579, 6875–6878. (doi:10.1016/j.febslet.2005.11.036) [DOI] [PubMed] [Google Scholar]
- 56.Konno H, Murakami-Fuse T, Fujii F, Koyama F, Ueoka-Nakanishi H, Pack C-G, Kinjo M, Hisabori T. 2006. The regulator of the F1 motor: inhibition of rotation of cyanobacterial F1-ATPase by the ɛ subunit. EMBO J. 25, 4596–4604. (doi:10.1038/sj.emboj.7601348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bulygin VV, Duncan TM, Cross RL. 1998. Rotation of the epsilon subunit during catalysis by Escherichia coli FoF1-ATP synthase. J. Biol. Chem. 273, 31 765–31 769. (doi:10.1074/JBC.273.48.31765) [DOI] [PubMed] [Google Scholar]
- 58.Tsunoda SP, Rodgers AJ, Aggeler R, Wilce MC, Yoshida M, Capaldi RA. 2001. Large conformational changes of the epsilon subunit in the bacterial F1F0 ATP synthase provide a ratchet action to regulate this rotary motor enzyme. Proc. Natl Acad. Sci. USA 98, 6560–6564. (doi:10.1073/pnas.111128098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bulygin VV, Duncan TM, Cross RL. 2004. Rotor/Stator interactions of the subunit in Escherichia coli ATP synthase and implications for enzyme regulation. J. Biol. Chem. 279, 35 616–35 621. (doi:10.1074/jbc.M405012200) [DOI] [PubMed] [Google Scholar]
- 60.Bockenhauer SD, Duncan TM, Moerner WE, Börsch M.. 2014. The regulatory switch of F1-ATPase studied by single-molecule FRET in the ABEL trap Proc. SPIE 8950, 89500H (doi:10.1117/12.2042688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zimmermann B, Diez M, Zarrabi N, Gräber P, Börsch M. 2005. Movements of the epsilon-subunit during catalysis and activation in single membrane-bound H(+)-ATP synthase. EMBO J. 24, 2053–2063. (doi:10.1038/sj.emboj.7600682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duncan TM, Düser MG, Heitkamp T, McMillan DGG, Börsch M.2014. Regulatory conformational changes of the ɛ subunit in single FRET-labeled FoF1-ATP synthase. Quantitative Biology—Biomolecules arXiv:1402.3845. [DOI] [PMC free article] [PubMed]
- 63.Nakanishi-Matsui M, Kashiwagi S, Hosokawa H, Cipriano DJ, Dunn SD, Wada Y, Futai M. 2006. Stochastic high-speed rotation of Escherichia coli ATP synthase F1 sector: the epsilon subunit-sensitive rotation. J. Biol. Chem. 281, 4126–4131. (doi:10.1074/jbc.M510090200) [DOI] [PubMed] [Google Scholar]
- 64.Nakanishi-Matsui M, Sekiya M, Yano S, Futai M. 2014. Inhibition of F1 -ATPase rotational catalysis by the carboxyl-terminal domain of the ɛ subunit. J. Biol. Chem. 289, 30 822–30 831. (doi:10.1074/jbc.M114.578872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aggeler R, Haughton MA, Capaldi RA. 1995. Disulfide bond formation between the COOH-terminal domain of the beta subunits and the gamma and epsilon subunits of the Escherichia coli F1-ATPase. J. Biol. Chem. 270, 9185–9191. (doi:10.1074/jbc.270.16.9185) [DOI] [PubMed] [Google Scholar]
- 66.Shah NB, Hutcheon ML, Haarer BK, Duncan TM. 2013. F1-ATPase of Escherichia coli: the ɛ-inhibited state forms after ATP hydrolysis, is distinct from the ADP-inhibited state, and responds dynamically to catalytic site ligands. J. Biol. Chem. 288, 9383–9395. (doi:10.1074/jbc.M113.451583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sekiya M, Hosokawa H, Nakanishi-Matsui M, Al-Shawi MK, Nakamoto RK, Futai M. 2010. Single molecule behavior of inhibited and active states of Escherichia coli ATP synthase F1 rotation. J. Biol. Chem. 285, 42 058–42 067. (doi:10.1074/jbc.M110.176701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shah NB, Duncan TM. 2015. Aerobic growth of Escherichia coli is reduced, and ATP synthesis is selectively inhibited when five C-terminal residues are deleted from the ɛ subunit of ATP synthase. J. Biol. Chem. 290, 21 032–21 041. (doi:10.1074/jbc.M115.665059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yaginuma H, Kawai S, Tabata KV, Tomiyama K, Kakizuka A, Komatsuzaki T, Noji H, Imamura H. 2014. Diversity in ATP concentrations in a single bacterial cell population revealed by quantitative single-cell imaging. Sci. Rep. 4, 6522 (doi:10.1038/srep06522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kato-Yamada Y, Yoshida M. 2003. Isolated epsilon subunit of thermophilic F1-ATPase binds ATP. J. Biol. Chem. 278, 36 013–36 016. (doi:10.1074/jbc.M306140200) [DOI] [PubMed] [Google Scholar]
- 71.Iizuka S, Kato S, Yoshida M, Kato-Yamada Y. 2006. γɛ sub-complex of thermophilic ATP synthase has the ability to bind ATP. Biochem. Biophys. Res. Commun. 349, 1368–1371. (doi:10.1016/j.bbrc.2006.09.001) [DOI] [PubMed] [Google Scholar]
- 72.Tsumuraya M, Furuike S, Adachi K, Kinosita K, Yoshida M. 2009. Effect of ɛ subunit on the rotation of thermophilic Bacillus F1-ATPase. FEBS Lett. 583, 1121–1126. (doi:10.1016/j.febslet.2009.02.038) [DOI] [PubMed] [Google Scholar]
- 73.Hara KY, Kato-Yamada Y, Kikuchi Y, Hisabori T, Yoshida M. 2001. The role of the DELSEED motif of F1-ATPase: propagation of the inhibitory effect of the epsilon subunit. J. Biol. Chem. 276, 23 969–23 973. (doi:10.1074/jbc.M009303200) [DOI] [PubMed] [Google Scholar]
- 74.Suzuki T, Murakami T, Iino R, Suzuki J, Ono S, Shirakihara Y, Yoshida M. 2003. F0F1-ATPase/synthase is geared to the synthesis mode by conformational rearrangement of epsilon subunit in response to proton motive force and ADP/ATP balance. J. Biol. Chem. 278, 46 840–46 846. (doi:10.1074/jbc.M307165200) [DOI] [PubMed] [Google Scholar]
- 75.Feniouk BA, Junge W. 2005. Regulation of the F0F1-ATP synthase: the conformation of subunit ɛ might be determined by directionality of subunit γ rotation. FEBS Lett. 579, 5114–5118. (doi:10.1016/j.febslet.2005.08.030) [DOI] [PubMed] [Google Scholar]
- 76.Saita E, Iino R, Suzuki T, Feniouk BA, Kinosita K, Yoshida M. 2010. Activation and stiffness of the inhibited states of F1-ATPase probed by single-molecule manipulation. J. Biol. Chem. 285, 11 411–11 417. (doi:10.1074/jbc.M109.099143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yasuno T, Muneyuki E, Yoshida M, Kato-Yamada Y. 2009. Modulation of nucleotide binding to the catalytic sites of thermophilic F1-ATPase by the ɛ subunit: implication for the role of the ɛ subunit in ATP synthesis. Biochem. Biophys. Res. Commun. 390, 230–234. (doi:10.1016/j.bbrc.2009.09.092) [DOI] [PubMed] [Google Scholar]
- 78.Haruyama T, Hirono-Hara Y, Kato-Yamada Y. 2010. Inhibition of thermophilic F1-ATPase by the ɛ subunit takes different path from the ADP-Mg inhibition. Biophysics 6, 59–65. (doi:10.2142/biophysics.6.59) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mizumoto J, Kikuchi Y, Nakanishi YH, Mouri N, Cai A, Ohta T, Haruyama T, Kato-Yamada Y. 2013. ɛ subunit of Bacillus subtilis F1-ATPase relieves MgADP inhibition. PLoS ONE 8, 1–11. (doi:10.1371/journal.pone.0073888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olsson K, Keis S, Morgan HW, Dimroth P, Cook GM. 2003. Bioenergetic properties of the thermoalkaliphilic Bacillus sp. strain TA2.A1. J. Bacteriol. 185, 461–465. (doi:10.1128/JB.185.2.461-465.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kalamorz F, et al. 2011. Draft genome sequence of thermoalkaliphilic Calalkalibacillus thermarum strain TA2.A1. J. Bacteriol. 193, 4290–4291. (doi:10.1128/JB.05035-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McMillan DGG, Keis S, Berney M, Cook GM. 2009. Nonfermentative thermoalkaliphilic growth is restricted to alkaline environments. Appl. Environ. Microbiol. 75, 7649–7654. (doi:10.1128/AEM.01639-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McMillan DGG, Keis S, Dimroth P, Cook GM. 2007. A specific adaptation in the a subunit of thermoalkaliphilic F1FO-ATP synthase enables ATP synthesis at high pH but not at neutral pH values. J. Biol. Chem. 282, 17 395–17 404. (doi:10.1074/jbc.M611709200) [DOI] [PubMed] [Google Scholar]
- 84.McMillan DGG, Watanabe R, Ueno H, Cook GM, Noji H. 2016. Biophysical characterization of a thermoalkaliphilic molecular motor with a high stepping torque gives insight into evolutionary ATP synthase adaptation. J. Biol. Chem. 291, 23 965–23 977. (doi:10.1074/jbc.M116.743633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keis S, Stocker A, Dimroth P, Cook GM. 2006. Inhibition of ATP hydrolysis by thermoalkaliphilic F1Fo-ATP synthase is controlled by the C terminus of the epsilon subunit. J. Bacteriol. 188, 3796–3804. (doi:10.1128/JB.00040-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stocker A, Keis S, Vonck J, Cook GM, Dimroth P. 2007. The structural basis for unidirectional rotation of thermoalkaliphilic F1-ATPase. Structure 15, 904–914. (doi:10.1016/j.str.2007.06.009) [DOI] [PubMed] [Google Scholar]
- 87.Krah A, Kato-Yamada Y, Takada S. 2017. The structural basis of a high affinity ATP binding ɛ subunit from a bacterial ATP synthase. PLoS ONE 12, e0177907 (doi:10.1371/journal.pone.0177907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. 2009. Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191. (doi:10.1093/bioinformatics/btp033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krah A, Takada S. 2015. On the Mg2+ binding site of the ɛ subunit from bacterial F-type ATP synthases. Biochim. Biophys. Acta Bioenerg. 1857, 1101–1112. (doi:10.1016/j.bbabio.2015.05.018) [DOI] [PubMed] [Google Scholar]
- 90.Imamura H, Nhat KPH, Togawa H, Saito K, Iino R, Kato-Yamada Y, Nagai T, Noji H. 2009. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc. Natl Acad. Sci. USA 106, 15 651–15 656. (doi:10.1073/pnas.0904764106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoshida T, Kakizuka A, Imamura H. 2016. BTeam, a novel BRET-based biosensor for the accurate quantification of ATP concentration within living cells. Sci. Rep. 6, 39618 (doi:10.1038/srep39618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schrodinger LLC.2015. The PyMOL Molecular Graphics System, Version 1.7. See https://pymol.org .
- 93.Cipriano DJ, Dunn SD. 2006. The role of the epsilon subunit in the Escherichia coli ATP synthase: the C-terminal domain is required for efficient energy coupling. J. Biol. Chem. 281, 501–507. (doi:10.1074/jbc.M509986200) [DOI] [PubMed] [Google Scholar]
- 94.Kadoya F, Kato S, Watanabe K, Kato-Yamada Y. 2011. ATP binding to the ɛ subunit of thermophilic ATP synthase is crucial for efficient coupling of ATPase and H+ pump activities. Biochem. J. 437, 135–140. (doi:10.1042/BJ20110443) [DOI] [PubMed] [Google Scholar]
- 95.Futai M, Sternweis PC, Heppel LA. 1974. Purification and properties of reconstitutively active and inactive adenosinetriphosphatase from Escherichia coli. Proc. Natl Acad. Sci. USA 71, 2725–2729. (doi:10.1073/pnas.71.7.2725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoshida M, Allison WS. 1983. Modulation by ADP and Mg2+ of the inactivation of the F1-ATPase from the thermophilic bacterium, PS3, with dicyclohexylcarbodiimide. J. Biol. Chem. 258, 14 407–14 412. [PubMed] [Google Scholar]
- 97.Preiss L, Langer JD, Yildiz O, Eckhardt-Strelau L, Guillemont JEG, Koul A, Meier T. 2015. Structure of the mycobacterial ATP synthase Fo rotor ring in complex with the anti-TB drug bedaquiline. Sci. Adv. 1, e1500106 (doi:10.1126/sciadv.1500106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haagsma AC, et al. 2009. Selectivity of TMC207 towards mycobacterial ATP synthase compared with that towards the eukaryotic homologue. Antimicrob. Agents Chemother. 53, 1290–1292. (doi:10.1128/AAC.01393-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Jonge MR, Koymans LHM, Guillemont JEG, Koul A, Andries K. 2007. A computational model of the inhibition of Mycobacterium tuberculosis ATPase by a new drug candidate R207910. Proteins Struct. Funct. Bioinform. 67, 971–980. (doi:10.1002/prot.21376) [DOI] [PubMed] [Google Scholar]
- 100.Kundu S, Biukovic G, Grüber G, Dick T. 2016. Bedaquiline targets the ɛ subunit of mycobacterial F-ATP synthase. Antimicrob. Agents Chemother. 60, 6977–6979. (doi:10.1128/AAC.01291-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He C, Preiss L, Wang B, Fu L, Wen H, Zhang X, Cui H, Meier T, Yin D. 2017. Structural simplification of bedaquiline: the discovery of 3-(4-(N, N -Dimethylaminomethyl)phenyl)quinoline-Derived antitubercular lead compounds. ChemMedChem 12, 106–119. (doi:10.1002/cmdc.201600441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gledhill JR, Walker JE. 2005. Inhibition sites in F1-ATPase from bovine heart mitochondria. Biochem. J. 386, 591–598. (doi:10.1042/BJ20041513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hards K, Robson JR, Berney M, Shaw L, Bald D, Koul A, Andries K, Cook GM. 2015. Bactericidal mode of action of bedaquiline. J. Antimicrob. Chemother. 111, 10580–10585. (doi:10.1093/jac/dkv054) [DOI] [PubMed] [Google Scholar]
- 104.Zíková A, Schnaufer A, Dalley RA, Panigrahi AK, Stuart KD, Lukes J. 2009. The F0F1-ATP synthase complex contains novel subunits and is essential for procyclic Trypanosoma brucei. PLoS Pathog. 5, e1000436 (doi:10.1371/journal.ppat.1000436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Šubrtová K, Panicucci B, Zíková A, Anupama A, Stuart K. 2015. ATPaseTb2, a unique membrane-bound FoF1-ATPase component, is essential in bloodstream and dyskinetoplastic trypanosomes. PLOS Pathog. 11, e1004660 (doi:10.1371/journal.ppat.1004660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schulz S, Iglesias-Cans M, Krah A, Yildiz Ö, Leone V, Matthies D, Cook GM, Faraldo-Gómez JD, Meier T. 2013. A new type of Na+-Driven ATP synthase membrane rotor with a two-carboxylate ion-coupling motif. PLoS Biol. 11, e1001596 (doi:10.1371/journal.pbio.1001596) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.