Abstract
Objective
We aimed to evaluate the association between cancer and cerebrovascular disease in a prospective cohort study with adjudicated cerebrovascular diagnoses.
Methods
We analyzed participants from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study who were 45 years and older and had Medicare coverage for 365 days before their baseline study visit. Participants with a history of cancer or cerebrovascular events were excluded. The time-dependent exposure was a new diagnosis of malignant cancer identified through Medicare claims algorithms. Participants were prospectively followed from their baseline study visit (2003–2007) through 2014 for the outcome of a neurologist-adjudicated cerebrovascular event defined as a composite of stroke (ischemic or hemorrhagic) or TIA. Cox regression was used to evaluate the association between a new cancer diagnosis and subsequent cerebrovascular events. Follow-up time was modeled in discrete time periods to fulfill the proportional hazard assumption.
Results
Among 6,602 REGARDS participants who met eligibility criteria, 1,149 were diagnosed with cancer during follow-up. Compared to no cancer, a new cancer diagnosis was associated with subsequent cerebrovascular events in the first 30 days after diagnosis (hazard ratio 6.1, 95% confidence interval 2.7–13.7). This association persisted after adjustment for demographics, region of residence, and vascular risk factors (hazard ratio 6.6, 95% confidence interval 2.7–16.0). There was no association between cancer diagnosis and incident cerebrovascular events beyond 30 days. Cancers considered high risk for venous thromboembolism demonstrated the strongest associations with cerebrovascular event risk.
Conclusion
A new diagnosis of cancer is associated with a substantially increased short-term risk of cerebrovascular events.
Cancer is diagnosed in 1.6 million Americans each year.1 Hypercoagulability is a well-known complication of cancer, and up to 20% of patients with cancer develop venous thromboembolism.2,3 Single-center cohort studies have suggested that incident stroke is common in patients with cancer, and in a large autopsy study, 15% of patients with cancer had evidence of cerebrovascular disease.4–6 Despite these data, cancer is not typically viewed as an independent risk factor for stroke, and patients with cancer are not routinely targeted for stroke prevention strategies.7
Recent data justify a rigorous assessment of the association between cancer and stroke. A Swedish study reported that several cancer types were associated with stroke, but this study was limited to inpatient data, a homogenous population, and retrospective identification of strokes using administrative codes.8 A US study using cancer registry data linked to Medicare claims demonstrated that patients with common solid tumor cancers had an increased risk of stroke, especially in the first 3 months after cancer diagnosis.9 However, this study also relied on claims data to identify strokes, which is less reliable than the gold standard of prospectively adjudicated stroke diagnoses.10,11 Furthermore, because of their dependence on claims-based data, prior studies were unable to comprehensively adjust for stroke risk factors. For example, smoking and obesity, known risk factors for both cancer and stroke, are not reliably recorded in administrative claims.12–15
We used prospectively collected data, including neurologist-adjudicated stroke and TIA diagnoses, from the population-based Reasons for Geographic and Racial Differences in Stroke (REGARDS) study to evaluate the association between cancer and cerebrovascular disease.16 We hypothesized that cancer would be associated with an increased risk of stroke/TIA independent of vascular risk factors.
Methods
Design
This analysis used data from the REGARDS study in conjunction with linked Medicare claims from the Centers for Medicare and Medicaid Services. REGARDS is a nationwide, population-based, prospective cohort study with adjudicated ascertainment of cerebrovascular events.16 Between 2003 and 2007, 30,239 participants aged 45 years and older were enrolled. REGARDS oversampled black participants and those living in the Stroke Belt (Southeastern United States).
Standard protocol approvals, registrations, and patient consents
Participating institutions' review boards approved the REGARDS study protocol. All participants provided written informed consent, including for data linkage with Medicare claims.
Participants
We included participants who had continuous fee-for-service Medicare Parts A and B but not C (Medicare Advantage) coverage for the 365 days before their baseline in-home study visit. Sixty-seven percent of REGARDS participants had linked Medicare data; baseline characteristics were similar between participants linked and not linked to Medicare except those not linked were more often men and black.17 Participants with history of cerebrovascular events or cancer at baseline were excluded. History of stroke/TIA was identified through self-report during the baseline study interview or the presence of a Medicare ICD-9-CM diagnosis code for cerebrovascular disease, 430.xx–438.xx, in the year before baseline study visit. History of cancer was identified through self-report during the baseline study interview or through a Medicare ICD-9-CM code for cancer in the year before the baseline visit (see appendix e-1 for codes, links.lww.com/WNL/A504). In prior research, Medicare claims data identified incident cancer with specificity of ≥98%.18
Measurements
The primary exposure was any new diagnosis of cancer during follow-up except cutaneous basal cell or squamous cell carcinoma. New diagnoses of cancer were defined by ≥1 of the following Medicare claims algorithms: any inpatient or outpatient emergency department claim with ICD-9-CM diagnoses of 140.xx–172.xx, 174.xx–208.xx, or 209.0–209.3 in any diagnosis position; any inpatient or outpatient claim with ICD-9-CM, Healthcare Common Procedure Coding System, or Current Procedural Terminology codes for chemotherapy, radiation, or hormone therapy; or ≥2 outpatient claims with an ICD-9-CM diagnosis of 140.xx–172.xx, 174.xx–208.xx, or 209.0–209.3 in any diagnosis position associated with physician evaluation and management codes 30–365 days apart. In the algorithm requiring ≥2 outpatient claims, the cancer diagnosis was assigned the date of the second cancer claim. No participants included in the current analysis were diagnosed with cancer solely by a claim for hormone therapy.
At baseline, enrolled participants completed a 45-minute telephone interview to provide information on demographics, socioeconomic status, cardiovascular risk factors, and medical history, including history of cancer. Participants then completed an in-home study visit, which included blood pressure measurements, ECG, and collection of urine and blood samples.
The primary outcome was a REGARDS-adjudicated stroke or TIA. After the baseline visit, participants were contacted every 6 months to identify possible cerebrovascular events. These phone interviews included the validated questionnaire for verifying stroke-free status and questioning about whether participants had been hospitalized for cerebrovascular disease since their last follow-up encounter.16,19 When participants reported stroke/TIA symptoms or diagnosis or the interviewer suspected a cerebrovascular event, relevant medical records were reviewed by an expert stroke panel for central adjudication. Stroke events were defined per the World Health Organization (WHO) definition but also included cases with neurologic symptoms lasting <24 hours with brain imaging demonstrating acute ischemia/hemorrhage and cases in which the expert adjudicators believed the event was a likely stroke but clinical information was insufficient for the WHO or imaging-based definitions.16,20 TIA events were defined as neurologic symptoms attributed to ischemia lasting <24 hours and with negative brain imaging for stroke.
Analysis
Descriptive statistics were used to calculate baseline characteristics. Kaplan-Meier statistics were used to calculate the cumulative incidence of cerebrovascular events among participants with and without cancer. Time of study entry for all participants was the date of their baseline REGARDS study visit with new diagnoses of cancer modeled as a time-dependent exposure. Participants who developed cancer during follow-up contributed follow-up time to both the cancer and noncancer groups. Specifically, they contributed follow-up time to the noncancer group from the date of their baseline study visit to the date of their cancer diagnosis, and to the cancer group from their date of cancer diagnosis through the end of study. Among participants with multiple cancers diagnosed during the study period, only the first cancer diagnosis was analyzed. Follow-up was censored when participants developed stroke/TIA, withdrew from the REGARDS study, lost Medicare fee-for-service coverage, or on December 31, 2014.
To account for possible confounding by baseline differences in stroke risk factors between those with and without cancer, we performed multivariable Cox proportional hazard analyses adjusting for age, race, sex, region of residence, annual income, highest education level achieved, systolic and diastolic blood pressure, antihypertensive medication use, left ventricular hypertrophy, diabetes, atrial fibrillation, total and high-density lipoprotein cholesterol, coronary heart disease, estimated glomerular filtration rate, albuminuria, physical activity, body mass index, smoking history (current smoking and smoking pack-years), and alcohol use. Cumulative incidence curves were not parallel in the first year of follow-up, and maximum likelihood estimates analyses showed that the hazard for cerebrovascular events associated with cancer varied as a function of follow-up time, indicating that the proportional hazard assumption was violated for the entirety of participant follow-up. Therefore, we calculated hazard ratios (HRs) during discrete time periods during which the assumption was met. These time periods were 0–30 days, 31–90 days, 91–180 days, 181–365 days, and >365 days; previous analyses reported stable stroke risk among patients with cancer during these time periods, and maximum likelihood estimation techniques confirmed time proportionality.9
Using the multivariable model, we performed 2 sensitivity analyses. First, we restricted the outcome of interest to stroke. Second, we assigned the date of the first outpatient cancer claim as the date of cancer diagnosis.
In addition, we conducted subgroup analyses evaluating cerebrovascular event risk among participants with (1) traditionally high-risk cancers and (2) common cancers (see appendix e-2 for methods, links.lww.com/WNL/A504).
In post hoc sensitivity analyses, we used a matched cohort design to evaluate cerebrovascular event risk in patients with cancer (see appendix e-3 for methods, links.lww.com/WNL/A504).
In exploratory analysis, we evaluated the incidence rate of venous thromboembolism among participants with and without cancer (see appendix e-4 for methods/results, links.lww.com/WNL/A504).
Statistical analyses were performed by H.Z. using SAS version 9.4 (SAS Institute Inc., Cary, NC). The threshold for statistical significance was p < 0.05.
Data availability
The data used in this analysis include potentially identifying participant information as well as restricted Medicare claims data and therefore cannot be made publicly available because of ethical/legal restrictions. However, qualified investigators who have been granted access by the University of Alabama at Birmingham and the Centers for Medicare and Medicaid Services can obtain deidentified data on request at regardsadmin@uab.edu.
Results
Characteristics
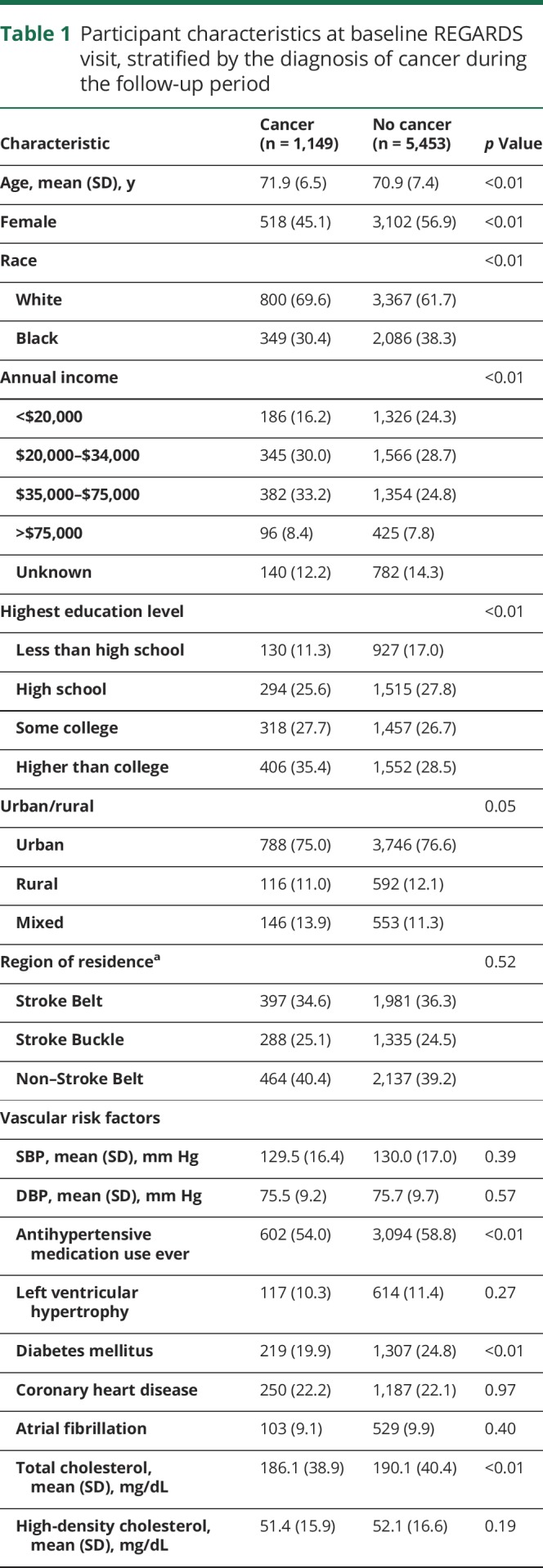
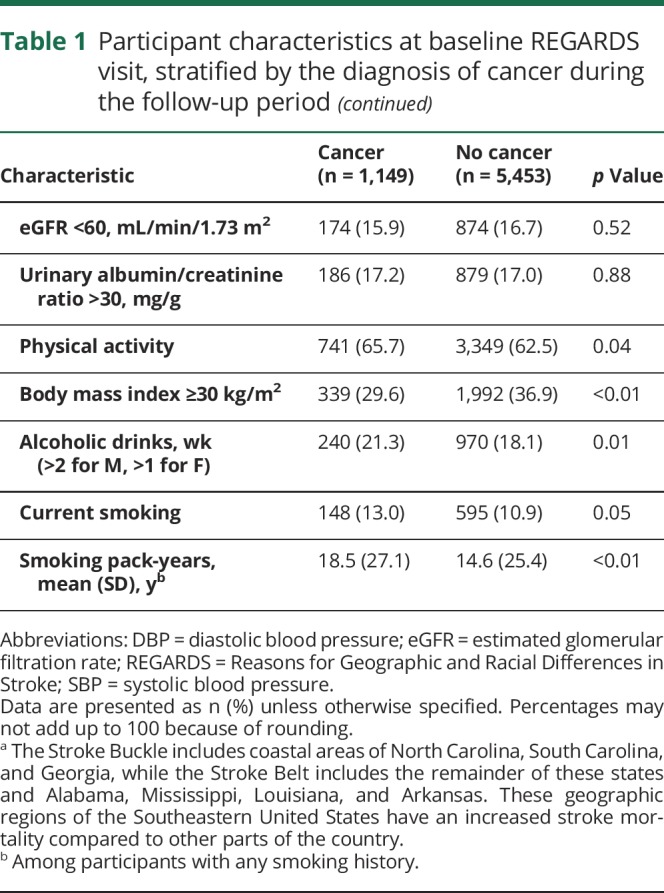
Among 20,403 REGARDS participants with Medicare-linked data, 6,602 met eligibility criteria and were included in this analysis (figure 1). During a median follow-up period of 6.0 years (interquartile range [IQR] 2.3–9.3), 1,149 study participants (17% of cohort) were diagnosed with cancer, including 850 solid tumor cancers, 100 hematologic cancers, 21 primary brain cancers, and 178 cancers of unknown primary site. The breakdown of cancer types was generally representative of national rates; prostate, lung, and breast cancers were the most common primary sites.1 Mean age of patients with cancer at the time of cancer diagnosis was 75.5 years (±7.0). Older age, male sex, white race, higher income, higher education level, mixed urban-rural residence, more physical activity, and higher alcohol and smoking consumption were more common among participants diagnosed with cancer as compared to participants not diagnosed with cancer (table 1). In addition, participants with incident cancer had lower total cholesterol levels and less often had diabetes, a body mass index ≥30 kg/m2, and previously used antihypertensive medication.
Figure 1. Study eligibility criteria.
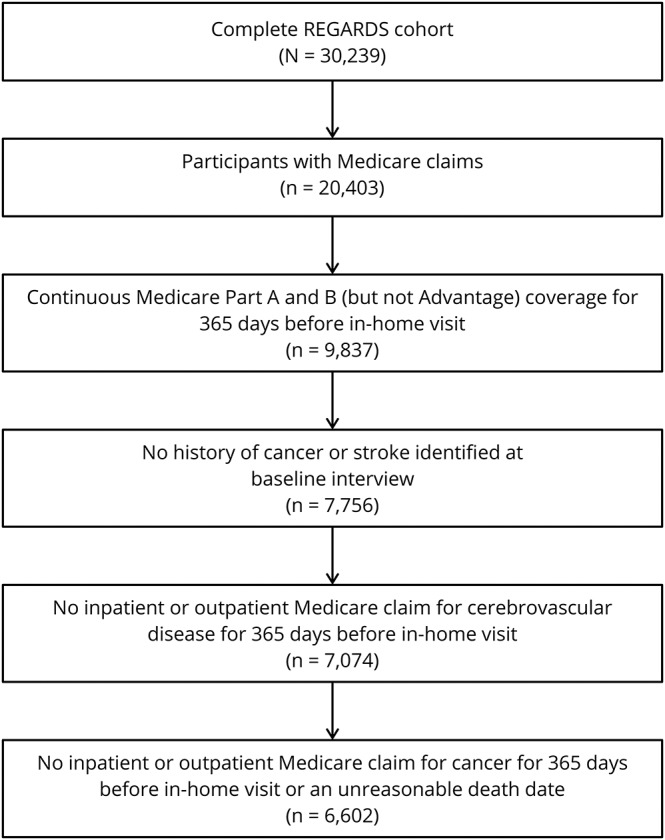
Flow diagram detailing selection criteria for study participants. REGARDS = Reasons for Geographic and Racial Differences in Stroke.
Table 1.
Participant characteristics at baseline REGARDS visit, stratified by the diagnosis of cancer during the follow-up period
Primary analysis
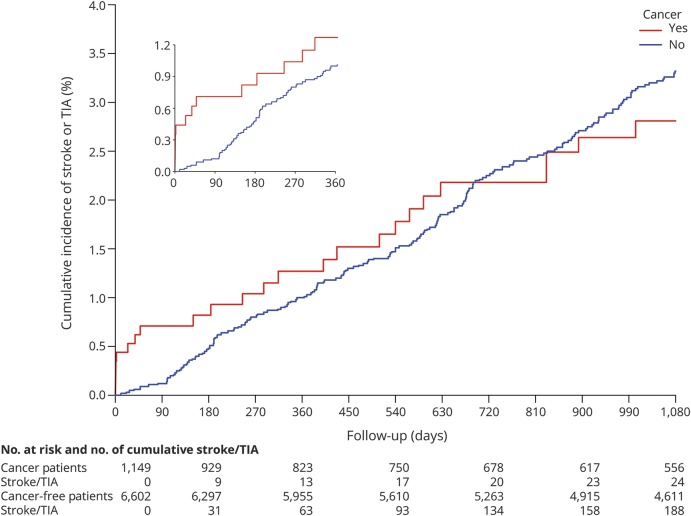
Among participants diagnosed with cancer, median follow-up from time of cancer diagnosis to cerebrovascular event or end of follow-up was 2.9 years (IQR 0.8–5.9), and during this period, 44 participants (3.8%) had a stroke (ischemic n = 34, hemorrhagic n = 1) or TIA (n = 9). Most cerebrovascular events occurred in participants with solid tumor cancers, particularly prostate (n = 10), lung (n = 9), or breast (n = 5) cancer, although 5 participants with hematologic cancers also developed events (table e-1, links.lww.com/WNL/A503). No cerebrovascular events were diagnosed in participants with primary brain cancers. Conversely, among participants not diagnosed with cancer, median follow-up time was 7.3 years (IQR 2.7–9.6), and 412 (6.2%) were diagnosed with cerebrovascular events (92% ischemic). All strokes in both groups were diagnosed using the WHO or tissue-based imaging definitions. Cerebrovascular event mechanisms as defined by the TOAST (Trial of Org 10172 in Acute Stroke Treatment) classification are provided in table e-2. Data on censoring and the timing of stroke events are presented in table 2. The 30-day cumulative incidence of cerebrovascular events was higher in participants who developed cancer vs those who did not (figure 2). Beyond 30 days, the cumulative incidence of cerebrovascular events among the cancer and noncancer groups converged.
Table 2.
Crude number and percentage of cerebrovascular events stratified by diagnosis of cancer and time period
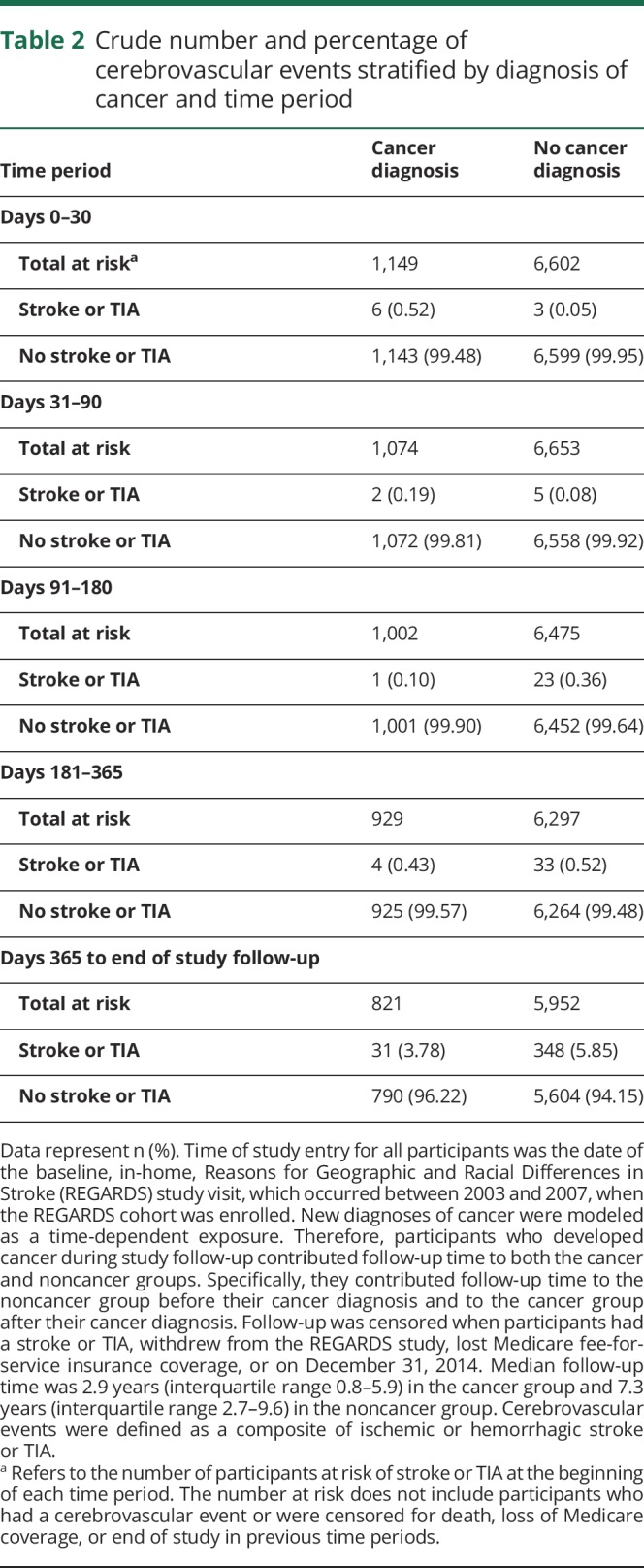
Figure 2. Cumulative incidence of cerebrovascular events among participants with and without a new diagnosis of cancer.
New diagnoses of cancer were modeled as a time-dependent exposure. Participants who developed cancer during study follow-up contributed follow-up time to the noncancer group before their cancer diagnosis and to the cancer group after their diagnosis. Follow-up day 0 refers to the date of the baseline REGARDS study visit for participants without cancer, and to the date of cancer diagnosis for participants who developed cancer. Follow-up was censored when participants experienced stroke or TIA, withdrew from the REGARDS study, left Medicare insurance coverage, or on December 31, 2014. The inset shows the same data on a magnified x-axis up to 360 days of follow-up. REGARDS = Reasons for Geographic and Racial Differences in Stroke.
Compared to no cancer, a new diagnosis of cancer was associated with an increased risk of cerebrovascular events in the first 30 days after diagnosis (HR 6.1, 95% confidence interval [CI] 2.7–13.7) (table 3). This association persisted after adjustment for demographics, region of residence, and vascular risk factors (HR 6.6, 95% CI 2.7–16.0). A similar association was found when the outcome of interest was stroke only (adjusted HR 4.7, 95% CI 1.5–14.6). At later time periods, incident cancer was no longer significantly associated with the development of cerebrovascular events. The results were materially unchanged in a sensitivity analysis that used the date of the first outpatient cancer claim as the date of cancer diagnosis (table e-3, links.lww.com/WNL/A503).
Table 3.
Cox regression analyses evaluating the association between a new cancer diagnosis and cerebrovascular events
Subgroup analyses
Among participants who developed cancers other than prostate (n = 920), the adjusted HR for cerebrovascular events in the first 30 days after cancer diagnosis was 8.3 (95% CI 3.4–20.2). When restricted to lung, colorectal, or pancreatic cancer (n = 257), the adjusted association between cancer and stroke/TIA during the first 30 days after diagnosis was similarly strong (HR 11.1, 95% CI 2.7–44.9). When restricted to cancers with claims for metastases (n = 322), the adjusted HR for cerebrovascular events in the first 30 days after cancer diagnosis was 20.8 (95% CI 7.7–96.5) (table e-4, links.lww.com/WNL/A503). When restricted to cancer types considered high risk for venous thromboembolism (n = 314), the adjusted HR for cerebrovascular events in the first 30 days after cancer diagnosis was 15.1 (95% CI 4.8–47.4) (table 2). In addition, this latter cancer subgroup also demonstrated an increased risk of cerebrovascular events in the 31- to 90-day period following cancer diagnosis (HR 6.0, 95% CI 1.5–24.4). Beyond 90 days from cancer diagnosis, these more aggressive cancer subgroups did not have increased stroke/TIA risk.
Among common specific cancer types, the adjusted HR for cerebrovascular events in the first 30 days after diagnosis was increased for lung (n = 146) (20.5, 95% CI 5.0–83.6) and breast (n = 120) (12.7, 95% CI 1.8–91.5) cancers but not for prostate, colorectal, or pancreatic cancer.
Matched cohort sensitivity analysis
Participants with incident cancer had an increased risk of cerebrovascular events in the first 30 days after their cancer diagnosis when compared to age-, sex-, race-, and education-matched controls without cancer (HR 5.4, 95% CI 2.4–12.5) (table e-5, links.lww.com/WNL/A503). This association persisted after multivariable adjustment (HR 5.9, 95% CI 2.3–15.0).
Discussion
In this nationwide, prospective cohort study with adjudicated stroke and TIA events, a new diagnosis of cancer was associated with a substantially increased short-term risk of cerebrovascular events independent of vascular risk factors. Typically more aggressive solid tumor cancers, particularly lung cancer, and cancer types previously shown to increase venous thromboembolism risk had the strongest association with subsequent stroke/TIA. Known metastatic disease strengthened the short-term association between cancer and cerebrovascular events, implicating cancer stage as a possible mediator of stroke risk. Beyond 30 days after cancer diagnosis, the risk of cerebrovascular events was no longer increased for all patients with cancer, although those with cancers considered high risk for venous thromboembolism did demonstrate increased cerebrovascular risk from 31 to 90 days after diagnosis.
These data corroborate prior studies that reported associations between cancer and stroke using claims data.8,9,21 In a Swedish study that used nationwide claims data to identify strokes, patients diagnosed with cancer from 1987 to 2008 had a significantly increased risk of stroke, particularly in the first 6 months after diagnosis.8 Furthermore, most individual cancer types were associated with increased stroke risk, including cancers unrelated to smoking or sex. Similarly, using US cancer registry data linked to Medicare claims, we reported that patients diagnosed with the 4 most common cancers had an increased risk of stroke compared to control patients without cancer matched by demographics and comorbidities.9 In that study, the HR for stroke in the first month after cancer diagnosis ranged from 1.3 for prostate cancer to 7.4 for lung cancer. One limitation of these prior studies was reliance on administrative diagnosis codes to identify strokes, which could have introduced misclassification leading to false associations. The current study addresses this concern, as cerebrovascular events were prospectively identified and adjudicated by an expert panel, and even with this more robust design, new diagnoses of cancer were independently associated with an increased short-term risk of stroke/TIA.
Various mechanisms might explain the association between cancer and cerebrovascular disease. Cancer often produces a hypercoagulable state through circulating microvesicles and alterations in coagulation function, platelet activity, and endothelial integrity.3,22–24 This hypercoagulability can lead to thrombosis in the heart or cerebral vasculature, thereby causing stroke. In addition, stroke may occur through marantic endocarditis, a manifestation of cancer-mediated hypercoagulability, which, according to autopsy data, may be the most common cause of symptomatic stroke in the cancer population.6 Furthermore, venous thromboembolism, which is common in patients with cancer, could cause cerebrovascular events by paradoxical embolism through patent foramen ovale.2,3,25 Cancer treatments, especially platinum-based compounds and angiogenesis inhibitors, are associated with thrombotic risk, including stroke.26,27 These treatments are frequently used soon after cancer diagnosis when stroke risk appears highest. Patients with primary brain tumors can develop stroke from radiation vasculopathy, complications of surgery, and use of angiogenesis inhibitors, although in the current study, which included 21 such patients, none developed stroke.28 Of note, we found fewer cryptogenic strokes in participants with cancer vs participants without cancer, which contradicts some publications on this topic, and may reflect differences in age and vascular risk factors between groups or the stroke subtype classification methods used by the REGARDS adjudication committee.29 Cancer has also been associated with an increased risk of hemorrhagic stroke, which might be explained by intratumoral hemorrhage and/or the frequent thrombocytopenia attributed to chemotherapy.5,9 Future translational studies are needed to clarify the pathophysiologic basis for the association between cancer and stroke.
This study has limitations. First, incident cancer was identified through a Medicare claims algorithm, and although previous studies have demonstrated high sensitivity and specificity for identifying cancer diagnoses through Medicare claims, it is possible that some cancer diagnoses were missed or incorrect.18 This could have biased the analysis toward not finding an association between cancer and stroke.18 Second, because of our reliance on claims-based data, we do not have clinical data on exact cancer stage, histopathology, and treatments. We also do not know the exact onset of participants' cancers, just when they were diagnosed. It is possible that some cancers were biologically active before diagnosis thereby affecting stroke risk. Third, REGARDS included only black and white US adults, so our results may not apply to other populations. REGARDS also oversampled black participants and those living in the US Stroke Belt; however, the association between cancer and stroke risk should not have been affected by the oversampling because race and region of residence were adjusted for in the multivariable models. Fourth, besides cancer diagnoses, some other covariates likely changed with time, including age, cerebrovascular risk factors and treatments, and cancer screening tools, and changes in these time-dependent factors could have confounded the relationship between cancer and cerebrovascular disease. Detection bias is also a consideration because patients with cancer might be monitored more closely than noncancer patients. Finally, despite a sample size of 6,602 participants, including 1,149 with new cancer diagnoses, there were few strokes/TIAs, which limited the statistical power to detect associations between cancer and cerebrovascular events as evidenced by wide CIs for estimated HRs. While statistical power was not an issue during the first month after cancer diagnosis since the association between cancer and stroke/TIA was highly significant then, it may have hindered the ability to detect a true association between cancer and cerebrovascular events at later time periods, as suggested by the associations seen in the high-risk cancer subgroups.
In a large population-based study, a new diagnosis of cancer was associated with an increased short-term risk of cerebrovascular events independent of vascular risk factors. Stroke/TIA risk was highest among cancer types known to be high risk for venous thromboembolism, and attenuated soon after cancer diagnosis. Randomized trials are needed to determine the optimal stroke prevention strategies in these high-risk patients. In the meantime, physicians should target cardiovascular risk factors in patients newly diagnosed with cancer.
Acknowledgment
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at regardsstudy.org.
Glossary
- CI
confidence interval
- HR
hazard ratio
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IQR
interquartile range
- REGARDS
Reasons for Geographic and Racial Differences in Stroke
- WHO
World Health Organization
Footnotes
CME Course: NPub.org/cmelist
Author contributions
Babak Navi conceived and designed the research, analyzed and interpreted the data, handled funding and supervision, drafted the manuscript, and made critical revision of the manuscript for important intellectual content. George Howard conceived and designed the research, acquired the data, analyzed and interpreted the data, and made critical revision of the manuscript for important intellectual content. Virginia Howard acquired the data, handled funding and supervision, and made critical revision of the manuscript for important intellectual content. Hong Zhao analyzed and interpreted the data, performed statistical analysis, and made critical revision of the manuscript for important intellectual content. Suzanne Judd acquired the data and made critical revision of the manuscript for important intellectual content. Mitchell Elkind analyzed and interpreted the data, handled funding and supervision, and made critical revision of the manuscript for important intellectual content. Costantino Iadecola handled funding and supervision and made critical revision of the manuscript for important intellectual content. Lisa DeAngelis conceived and designed the research, handled funding and supervision, and made critical revision of the manuscript for important intellectual content. Hooman Kamel conceived and designed the research, analyzed and interpreted the data, and made critical revision of the manuscript for important intellectual content. Peter Okin made critical revision of the manuscript for important intellectual content. Susan Gilchrist analyzed and interpreted the data, drafted the manuscript, and made critical revision of the manuscript for important intellectual content. Elsayed Soliman acquired the data and made critical revision of the manuscript for important intellectual content. Mary Cushman acquired the data, handled funding and supervision, and made critical revision of the manuscript for important intellectual content. Paul Muntner analyzed and interpreted the data, handled funding and supervision, and made critical revision of the manuscript for important intellectual content.
Study funding
Supported by NIH NINDS grants U01NS041588 and K23NS091395 and the Florence Gould Endowment for Discovery in Stroke.
Disclosure
B. Navi received research funding from NIH grant K23NS091395 and the Florence Gould Endowment for Discovery in Stroke. G. Howard, V. Howard, H. Zhao, and S. Judd report no disclosures relevant to the manuscript. M. Elkind receives compensation for providing consultative services for Abbott, BioTelemetry/CardioNet, BMS-Pfizer partnership, Boehringer Ingelheim, and Sanofi-Regeneron partnership; receives compensation for serving as an expert witness in litigation for BMS-Sanofi, Merck/Organon, and Hi-Tech Pharmaceuticals; receives royalties from UpToDate for chapters related to stroke; and serves on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association. C. Iadecola is supported by multiple NIH grants and served on the scientific advisory board for Broadview Ventures. L. DeAngelis served on scientific advisory boards for Juno Therapeutics, Sapience, and Roche. H. Kamel is funded by NIH grants K23NS082367, R01NS097443, and the Michael Goldberg Stroke Research Fund; was a consultant for Genentech, Medtronic, or iRhythm; and served on the speakers bureau for Genentech. P. Okin's spouse served on scientific advisory boards for Juno Therapeutics, Sapience, and Roche. S. Gilchrist and E. Soliman report no disclosures relevant to the manuscript. M. Cushman received research funding from the NIH, provided consultative services for Merck, and served on the speakers bureau for Diazyme and diaDexus. P. Muntner received research funding and honoraria from Amgen Inc. Go to Neurology.org/N for full disclosures.
References
- 1.Henley SJ, Singh SD, King J, Wilson RJ, O'Neil ME, Ryerson AB. Invasive cancer incidence and survival—United States, 2012. MMWR Morb Mortal Wkly Rep 2015;64:1353–1358. [DOI] [PubMed] [Google Scholar]
- 2.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005;293:715–722. [DOI] [PubMed] [Google Scholar]
- 3.Gomes M, Khorana AA. Risk assessment for thrombosis in cancer. Semin Thromb Hemost 2014;40:319–324. [DOI] [PubMed] [Google Scholar]
- 4.Navi BB, Singer S, Merkler AE, et al. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology 2014;83:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navi BB, Reichman JS, Berlin D, et al. Intracerebral and subarachnoid hemorrhage in patients with cancer. Neurology 2010;74:494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine 1985;64:16–35. [DOI] [PubMed] [Google Scholar]
- 7.Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:3754–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zoller B, Ji J, Sundquist J, Sundquist K. Risk of haemorrhagic and ischaemic stroke in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer 2012;48:1875–1883. [DOI] [PubMed] [Google Scholar]
- 9.Navi BB, Reiner AS, Kamel H, et al. Association between incident cancer and subsequent stroke. Ann Neurol 2015;77:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumamaru H, Judd SE, Curtis JR, et al. Validity of claims-based stroke algorithms in contemporary Medicare data: Reasons for Geographic and Racial Differences in Stroke (REGARDS) study linked with Medicare claims. Circ Cardiovasc Qual Outcomes 2014;7:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lakshminarayan K, Larson JC, Virnig B, et al. Comparison of Medicare claims versus physician adjudication for identifying stroke outcomes in the Women's Health Initiative. Stroke 2014;45:815–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lloyd JT, Blackwell SA, Wei II, Howell BL, Shrank WH. Validity of a claims-based diagnosis of obesity among Medicare beneficiaries. Eval Health Prof 2015;38:508–517. [DOI] [PubMed] [Google Scholar]
- 13.Desai RJ, Solomon DH, Shadick N, Iannaccone C, Kim SC. Identification of smoking using Medicare data: a validation study of claims-based algorithms. Pharmacoepidemiol Drug Saf 2016;25:472–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkin DM, Boyd L, Walker LC. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer 2011;105(suppl 2):S77–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard VJ, Kleindorfer DO, Judd SE, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol 2011;69:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie F, Colantonio LD, Curtis JR, et al. Linkage of a population-based cohort with primary data collection to Medicare claims: the reasons for geographic and racial differences in stroke study. Am J Epidemiol 2016;184:532–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Setoguchi S, Solomon DH, Glynn RJ, Cook EF, Levin R, Schneeweiss S. Agreement of diagnosis and its date for hematologic malignancies and solid tumors between Medicare claims and cancer registry data. Cancer Causes Control 2007;18:561–569. [DOI] [PubMed] [Google Scholar]
- 19.Jones WJ, Williams LS, Meschia JF. Validating the questionnaire for verifying stroke-free status (QVSFS) by neurological history and examination. Stroke 2001;32:2232–2236. [DOI] [PubMed] [Google Scholar]
- 20.WHO Task Force on Stroke and Other Cerebrovascular Disorders. Stroke–1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and Other Cerebrovascular Disorders. Stroke 1989;20:1407–1431. [DOI] [PubMed] [Google Scholar]
- 21.Chen PC, Muo CH, Lee YT, Yu YH, Sung FC. Lung cancer and incidence of stroke: a population-based cohort study. Stroke 2011;42:3034–3039. [DOI] [PubMed] [Google Scholar]
- 22.Eichinger S. Cancer associated thrombosis: risk factors and outcomes. Thromb Res 2016;140(suppl 1):S12–S17. [DOI] [PubMed] [Google Scholar]
- 23.Manly DA, Wang J, Glover SL, et al. Increased microparticle tissue factor activity in cancer patients with venous thromboembolism. Thromb Res 2010;125:511–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia 2002;4:465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc 1984;59:17–20. [DOI] [PubMed] [Google Scholar]
- 26.Li SH, Chen WH, Tang Y, et al. Incidence of ischemic stroke post-chemotherapy: a retrospective review of 10,963 patients. Clin Neurol Neurosurg 2006;108:150–156. [DOI] [PubMed] [Google Scholar]
- 27.Zuo PY, Chen XL, Liu YW, Xiao CL, Liu CY. Increased risk of cerebrovascular events in patients with cancer treated with bevacizumab: a meta-analysis. PLoS One 2014;9:e102484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parikh NS, Burch JE, Kamel H, DeAngelis LM, Navi BB. Recurrent thromboembolic events after ischemic stroke in patients with primary brain tumors. J Stroke Cerebrovasc Dis 2017;26:2396–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarzbach CJ, Schaefer A, Ebert A, et al. Stroke and cancer: the importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke 2012;43:3029–3034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this analysis include potentially identifying participant information as well as restricted Medicare claims data and therefore cannot be made publicly available because of ethical/legal restrictions. However, qualified investigators who have been granted access by the University of Alabama at Birmingham and the Centers for Medicare and Medicaid Services can obtain deidentified data on request at regardsadmin@uab.edu.