Abstract
OBJECTIVE
To determine if beta-(β)-blockers improve outcomes after acute traumatic brain injury (TBI).
BACKGROUND
There have been no new inpatient pharmacologic therapies to improve TBI outcomes in a half-century. Treatment of TBI patients with β-blockers offers a potentially beneficial approach.
METHODS
Using MEDLINE, EMBASE, and CENTRAL databases, eligible articles for our systematic review and meta-analysis (PROSPERO CRD42016048547) included adult (age≥16 years) blunt trauma patients admitted with TBI. The exposure of interest was β-blocker administration initiated during the hospitalization. Outcomes were mortality, functional measures, quality of life, cardiopulmonary morbidity (e.g. hypotension, bradycardia, bronchospasm, and/or congestive heart failure). Data were analyzed using a random-effects model, and represented by pooled odds ratio (OR) with 95% confidence intervals (CI) and statistical heterogeneity (I2).
RESULTS
Data were extracted from 9 included studies encompassing 2005 unique TBI patients with β-blocker treatment and 6240 unique controls. Exposure to β-blockers after TBI was associated with a reduction of in-hospital mortality (pooled OR 0.39, 95%CI: 0.27–0.56; I2=65%, p<0.00001). None of the included studies examined functional outcome or quality of life measures, and cardiopulmonary adverse events were rarely reported. No clear evidence of reporting bias was identified.
CONCLUSIONS
In adults with acute TBI, observational studies reveal a significant mortality advantage with β-blockers; however, quality of evidence is very low. We conditionally recommend the use of in-hospital β-blockers. However, we recommend further high-quality trials to answer questions about the mechanisms of action, effectiveness on subgroups, dose-response, length of therapy, functional outcome, and quality of life after β-blocker use for TBI.
INTRODUCTION
Traumatic brain injury (TBI) is a public health problem with profound consequences (1). Acute TBI is associated with a hyperadrenergic state that, in the context of a disrupted blood brain barrier, leads to high local norepinephrine levels and increased cerebral metabolic rate (CMR) for both oxygen and glucose. The increased CMR in the injured brain, with defective autoregulation, can exacerbate the pre-existing ischemia and metabolic crisis following TBI (2). This hyperadrenergic state may contribute to increased mortality after TBI (3) and, conversely, patients with low levels of adrenergic stress as evidenced by a normal heart rate may have reduced mortality after TBI (4).
Treatment with beta-adrenergic receptor antagonists offers a potentially beneficial approach to blunting this cascade of sympathetic activation after TBI (3,5). However, β-blockers are negative inotropes and can induce bradycardia. Either adverse effect can lead to hypotension, which is associated with poor outcomes in the TBI population (6–8). β-blockers have been evaluated mostly in retrospective cohort studies and a meta-analysis of the literature through mid-2013 demonstrated a potential mortality benefit with exposure to β-blockers (9). However, additional studies have been published since then and an updated systematic review is required to summarize the current evidence and offer guidance to clinicians. Using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework (10–12), we performed a systematic review, meta-analysis, and guideline that could aid decision-making for in-hospital β-blockers after traumatic brain injury.
METHODS
Objective
Our PICO (Population, Intervention, Comparator, and Outcomes) question was structured as follows:
Population:In adults with acute TBI,
Intervention: in-hospital β-blockers should be used
Comparator: in-hospital β-blockers should not be used
Outcome: To improve mortality, functional outcomes, quality of life outcomes, without worsening cardiopulmonary morbidity (e.g. hypotension, bradycardia, bronchospasm, and/or congestive heart failure).
Study Eligibility
Our protocol was registered with the PROSPERO international prospective register of systematic reviews (Registration Number: CRD42016048547). This study is transparently built upon a previously published systematic review, using similar methods and eligibility criteria(9). We searched for randomized controlled trials (RCTs), quasi-randomized and non-randomized controlled trials, and cohort studies (prospective and retrospective) comparing TBI patients who received in-hospital β-blockers after injury to those who did not. We excluded case reports, letters to the editor, articles in the lay press, abstracts, and review articles. RCTs and observational studies were analyzed separately, as a direct comparison between the estimates of observational studies and RCTs could be misleading.
Population
We included studies that involved adult patients aged ≥ 16 years with acute TBI of any severity requiring hospital admission.
Interventions and Comparators
All forms of in-hospital β-blockers were included, provided they were given during the hospital stay and continued for any duration of time. The comparison group could have received either placebo or no treatment. We included any dose of beta-blockers and planned sensitivity analyses if different dose and regimens were utilized.
Outcome measures
Per GRADE methodology, outcomes were chosen by the team and rated in importance from 1 to 9, with scores of 7–9 representing critical outcomes. The critical outcomes were in-hospital mortality, functional recovery, and quality of life with scores of 9, 8, and 7 respectively. The important (i.e., secondary) outcomes all related to cardiopulmonary morbidity. We broadly accepted functional outcome, as assessed using the Glasgow Outcome Score (GOS) scale, Extended Glasgow Outcome Score (GOSE) scale, Functional Independence Measure (FIM), or Disability Rating Scale (DRS). Similarly, we allowed quality of life metrics that used any standardized scale. Our secondary outcomes consisted of common cardiopulmonary adverse effects of β-blockers, such as cardiac biomarker elevation, arrhythmia, clinically significant hypotension (i.e., systolic blood pressure < 90 mm Hg, which required fluid resuscitation, discontinuation of the study drug, and/or an inotropic agent), clinically significant bradycardia (i.e., bradycardia requiring a temporary pacemaker, a sympathomimetic agent, atropine, or discontinuation of the study drug), bronchospasm, and/or congestive heart failure.
Information Sources
Similar to the original systematic review and meta-analysis on this topic, we searched MEDLINE (from January 1, 1950), EMBASE (from January 1, 1980), and Cochrane Central Register of Controlled Trials (CENTRAL, all years). The search was not restricted by date, language or publication status. The search was last updated on May 9, 2016. The search strategy was based on the MEDLINE search strategy (Supplementary Material, Table: Search Strategy), and was modified as necessary for the other databases. In addition, we searched the reference lists of relevant articles.
Data collection and analysis
Two authors independently examined all of the abstracts of the studies identified by our search and determined the eligibility of each study. Any disagreements were resolved by consensus and including a third author. We scanned the titles and abstracts of every record retrieved to determine which of the studies should be assessed further. If it was clear from the title and abstract that the article was irrelevant, the article was rejected. The full manuscripts of the remaining articles were then retrieved.
Data abstraction forms were created and used to collect the relevant data from the included studies. Two authors independently extracted data on patients, methods, interventions (or exposures in the cohort studies), outcomes and results.
Risk of Bias Assessment
Two authors independently assessed the risk of bias for each included study. Any disagreement was resolved through discussion and consensus. Each included study was classified as an RCT or a cohort study, and the risk of bias was assessed differently for each type of study. For RCTs, we used the Cochrane Collaboration’s tool of assessing risk of bias according to the following domains: sequence generation, allocation concealment, blinding of outcomes, incomplete outcome data, selective outcome reporting, and baseline imbalances (13). For cohort studies, selection of the exposed and unexposed cohorts, the comparability of the cohorts, the assessment of the outcomes, and the adequacy of follow-up were addressed using the Newcastle-Ottawa Scale (NOS, Supplementary Material, Figure 1) (14). The scale was modified to include important TBI prognostic variables (age, pupillary reactivity and Glasgow Coma Scale (GCS) Score) under the comparability category, and therefore allowed the reviewers to optimize the applicability of the scale to the TBI cohort studies. Our selection for these prognostic variables was based on the International Mission for Prognosis and Analysis of Clinical Trials (IMPACT) Core prognostic model (15). When considering comparability in the modified NOS, we assessed whether these important variables were adjusted for in a multivariate analysis (e.g., age, GCS score, pupillary reaction).
Figure 1.
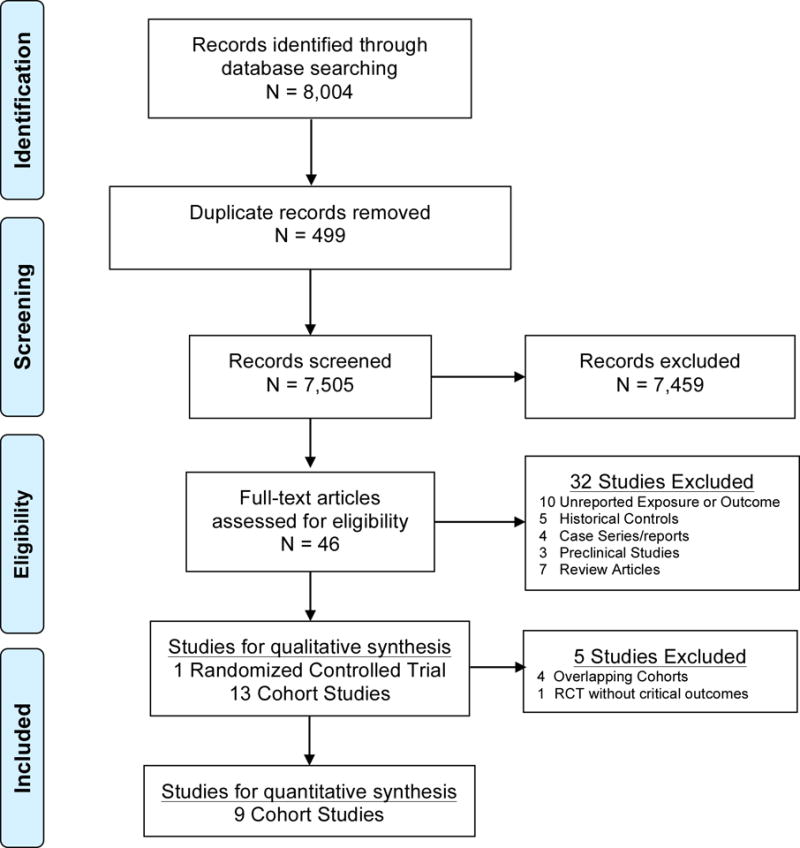
PRISMA flow diagram for systematic review phases of Beta-Blockers after traumatic brain injury
Quantitative Assessment
We calculated the odds ratio (OR) to measure the treatment effect for the dichotomous outcomes with corresponding 95% confidence intervals (CI). The generic inverse variance method was used when the included study reported only the odds ratio (OR) and its standard error. Clinical heterogeneity across the studies was assessed by examining the details of the subjects, the baseline data, and the interventions and the outcomes to determine whether the studies were sufficiently similar. Statistical heterogeneity was determined using the I2 statistic and the Chi-square test. We used a funnel plot to assess for reporting bias (Supplementary Material, Figure 2).
Figure 2.
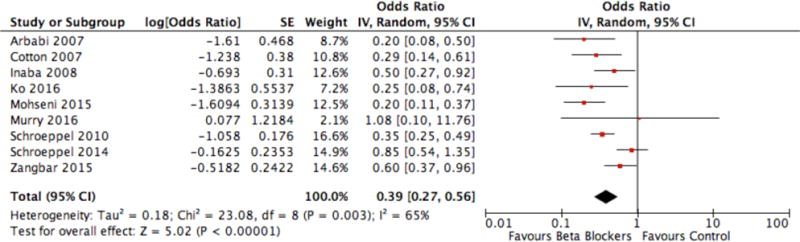
Forest plot of Beta-blocker exposure after acute traumatic brain injury versus no exposure with in-hospital mortality outcome
We used the Review Manager software (RevMan 5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) to conduct a quantitative analysis. We performed a meta-analysis using a random-effect model because there was a suggestion statistical heterogeneity between the studies, although there was no evidence of clinical heterogeneity.
RESULTS
Qualitative Synthesis, Excluded Studies
A total of 8,004 potentially relevant citations were screened for retrieval. 499 duplicates were excluded. 7,459 were excluded after scanning the titles and/or abstracts because they did not meet our inclusion criteria (Figure 1). A total of 46 citations were retrieved for detailed evaluation of the full text articles. We excluded 32 of those citations. Ten studies were excluded as the key exposure or outcome was not reported (16–25). Three were excluded due to the study population (26–28). Five were excluded due to the use of historical controls.(29–33) Four were excluded as they were case series or case reports (34–37). Seven were excluded as they were review articles (2,38–43). Three were excluded as they were studies in animals (44–46). These exclusions left a total of 14 manuscripts, including 1 randomized controlled clinical trial (47) and 13 cohort studies (48–60). This represents 5 new cohorts since our original systematic review.
In this section, we describe the overlapping non-unique cohorts that we excluded from the meta-analysis. There were four overlapping non-unique cohorts derived from the same cohort and were conducted mostly by the same group of investigators (50,57–59). One study cohort (57) was a subgroup of a larger cohort (50) but with a different analysis plan and objectives, specifically to investigate the relationship between troponin elevation and the outcome of severe TBI. Two other cohorts, designed to study the association between atrial arrhythmias and trauma patient outcomes (58) and to evaluate the association between β-blockers and TBI outcomes across different racial groups (59) were both subsets of the same larger cohort (50) study. Therefore, we included only the larger cohort (50) which was more representative of the general TBI population and the primary objective addressed the same question as our review. A similar overlapping example was found between a cohort study including a sample that was more representative of the general TBI population (49) and a cohort designed to examine the relationship between the β-blockers exposure and the outcome of a subgroup of the TBI population who had early cardiac uncoupling (60). Hence, we meta-analyzed 9 unique cohorts (i.e., quantitative synthesis) among the 13 studies identified by qualitative synthesis (Figure 1).
Qualitative Synthesis, Included studies
Descriptive statistics were extracted from the RCT by Cruickshank et al.(47) and each of the 13 cohort studies (Tables 1A and 1B). Again, only data from the 9 unique cohort studies were used for the meta-analysis (Figure 2).
Table 1A.
Detailed Results from Randomized Controlled Trials of Beta-Blockers after TBI
| Author Year (Reference) | N | Intervention | Outcome Measure | Result | P value | Overall Quality Assessment |
|---|---|---|---|---|---|---|
| Cruickshank 1987 (47) | 114 (intervention=56, control= 58) | After initial stabilization: Atenolol 10 mg IV every 6 hours for 3 days then 100 mg PO OD for 4 days Vs. matching placebo | Cardiac morbidity defined as: CK-MB level, noradrenaline level, arrhythmia, ST/T wave changes. Secondary outcomes include: hypotension bronchospasm and heart failure | Lower risk of high CK-MB (i.e. >3% of total CK) level (2/27 vs. 9/30); similar noradrenaline levels; lower risk of supraventricular tachycardia (6/56 vs. 28/58); lower risk of ST/T wave changes (15/56 vs. 26/58), No significant difference in other outcomes: hypotension (5/56 vs. 2/58), bradycardia (6/56 vs. 6/58) heart failure (0/56 vs. 0/58), and bronchospasm (1/56 vs. 0/58) | <0.05 for CK-MB, <0.0001 for supraventricular tachycardia, <0.05 for ST/T wave changes, 0.27 for hypotension, 0.95 for bradycardia, 1.0 for heart failure,0.49 for bronchospasm | Poor |
Table 1B.
Detailed Results from Cohort Studies of Beta-Blockers after TBI
| Author Year (Reference) | N | Intervention | Outcome Measure | Result¶ | P value | Comments |
|---|---|---|---|---|---|---|
| Arbabi 2007 (48) | 605 (exposure=94, control=511) | Any β-blockers given for >24 Hours during hospital stay | In-hospital Mortality | Lower adjusted odds of mortality (9 vs. 27 deaths, OR 0.2)* | <0.0001 | Adjusted for age, ISS, total GCS, Head AIS, SBP |
| Cotton 2007 (49) | 420 (exposure=173, control=247) | Metoprolol, propranolol, labetalol, esmolol, atenolol or sotalol given for >48 Hours during hospital stay for >2 consecutive days | In-hospital Mortality | Lower adjusted odds of mortality (9 vs. 27 deaths, OR 0.29) | <0.0001 | Adjusted for age, race, gender, mechanism of injury, ISS, Revised Trauma Score (RTS), calculated probability of survival using the Trauma Related Injury Severity Score (TRISS) methodology. |
| Inaba 2008 (50) | 1,156 (exposure=203, control=953) | Any β-blockers exposure during ICU stay | In-hospital mortality | Lower adjusted odds of mortality (34 vs. 199 deaths, OR 0.54) | 0.01 | Adjusted for age, total GCS, ISS, Head AIS, hypotension, subarachnoid hemorrhage, basal skull fracture |
| Ko 2016 (51) | 440 (exposure=109, control=331) | Propranolol at 1-mg intravenous every 6 h starting within 12 hours of admission for a minimum of 48 hours. | In-hospital mortality | Lower adjusted odds of mortality (7 vs. 43, OR 0.25) | 0.012 | Adjusted for age, total GCS, ISS, SBP, type of intracranial injury and neurosurgical intervention |
| Mohseni 2015 (52) | 874 (exposure=287, control=587) | Any β-blockers exposure during hospital stay | In-hospital mortality | Lower adjusted odds of mortality (30 vs. 102 deaths, OR 0.20) | 0.001 | Adjusted for age, total GCS, ISS, Head AIS, neurosurgical intervention and type of intracranial injury |
| Murry 2016 (53) | 38 (exposure=28, control=10) | Propranolol at 1 -mg intravenous every 6 h starting within 12 hours of admission for a minimum of 48 hours. | Primary outcomes: hypotension & bradycardia. Secondary outcomes: In-hospital mortality | No difference in hypotension but more bradycardic episodes in the control group. Similar odds of mortality (3 vs. 1 deaths, OR1.08) | 0.60 for hypotension, 0.05 for bradycardia | No adjusted analysis was done |
| Schroeppel 2010 (54) | 2,601 (exposure=506, control=2095) | Atenolol, carvedilol, esmolol, labetolol, metoprolol, nadolol, propranolol or sotalol. Exposure was defined by receiving > one dose of a P- blockers during hospital stay | In-hospital mortality | Lower adjusted odds of mortality (76 vs. 335 deaths, OR 0.35) | <0.0001 | Adjusted for age, total GCS, ISS, blood transfusion |
| Schroeppel 2014 (55) | 1,755 (exposure=427, control=1,328) | Atenolol, carvedilol, esmolol, labetolol, metoprolol, nadolol, propranolol or sotalol. Exposure was defined by receiving > one dose of a P- blockers during hospital stay | In-hospital mortality | Similar adjusted odds of mortality (56 vs. 80 deaths, OR 0.85 95% CI: 0.54–1.35). Lower odds in subgroup of patients who received propranolol (OR 0.2, 95% CI: 0.04–0.92) | Not reported | Adjusted for age, total GCS, Head AIS, admission SBP, blood transfusion |
| Zangbar 2015 (56) | 356 (exposure=178, control=178) | Metoprolol. Exposure was defined as receiving at least one dose during hospital stay. | In-hospital mortality | Lower adjusted odds of mortality (100 vs. 110 deaths, OR 0.79) | 0.04 | Using propensity score matching, patients were matched controlling for age, gender, race, admission vital signs, total GCS, ISS, average heart rate monitored during ICU admission, and standard deviation of heart rate during the ICU admission |
| Salim 2008 (57) | 420 (exposure=91, control=329) | Any fi-blockers exposure during hospital stay | In-hospital mortality | Lower adjusted odds of mortality (22 vs. 118 deaths, OR 0.59), even lower odds in subgroup of patients with elevated troponin during admission (OR 0.38) | 0.09 (0.03) | Adjusted for age, total GCS, ISS, Head AIS, days ventilated, ventilated, peak troponin, admission troponin, subarachnoid hemorrhage, basal skull fracture |
| Hadjizacharia 2011 (58) | 695 (exposure=320, control=375) | Any fi-blockers exposure during ICU stay | In-hospital mortality | Lower odds of mortality (20 vs. 81 deaths, OR 0.30) | Not reported | Not clear if adjusted analysis was done |
| Bukur 2012 (59) | 2,446 (exposure=886, control=1,580) | Any fi-blockers exposure during ICU stay | In-hospital mortality | Lower adjusted odds of mortality (120 vs. 297 deaths, OR 0.63) | 0.001 | Adjusted for age, total GCS, ISS, Head AIS, hypotension, subarachnoid hemorrhage, basal skull fracture |
| Riordan 2007 (60) | 446 (exposure=138, control=308) | Esmolol, propranolol, labetalol, metoprolol, atenolol or carvedilol regardless of dose, duration or route of administration | In-hospital mortality | Lower adjusted odds of mortality (29 vs. 135 deaths, OR 0.83) | Non-significant | Adjusted for age, ISS and length of stay using propensity score methods |
The effect estimate (i.e. odds ratio) compares the exposure group (β-blockers) to the control group (reference).
Number of events (i.e. deaths) was derived from population rates but not directly reported for traumatic brain injury subgroup in this study.
OR: odds ratio; GCS: Glasgow Coma Scale; ISS: Injury Severity Score; AIS: Abbreviated Injury Score; OR: odds ratio; ICU: intensive care unit; CI: confidence interval; SBP: systolic blood pressure
Italics of lower 4 rows of cohorts represent overlapping cohorts from higher rows of original cohorts
The Cruickshank et al. (47) study was a double-blinded placebo-controlled trial, published in 1987, that examined the safety and impact of atenolol on cardiac morbidity of patients with acute TBI(47). This trial included patients with ages of 11–70 years old with acute TBI, admitted to the intensive care or neurosurgical unit of one of four study centers in three European countries. The study drug was initiated immediately after hemodynamic stabilization (mean time was 20.2 hours following injury) (47).
The cohort studies included only hospitalized adult patients with TBI as defined by the Head Abbreviated Injury Scale (AIS) score or by using the International Statistical Classification of Diseases, Ninth Revision (ICD-9CM) code for blunt TBI (48–50,54,57–60). The exposures in the included studies were defined as any β-blockers agent, regardless of dose, route of administration, or pre-hospital exposure. All β-blockers were initiated during the acute in-hospital stay following TBI and continued for a variable length of time. The 9 unique cohort studies included a total of 8,245 patients. All of the cohort studies were conducted in the United States except one (Mohseni et al. (52) was conducted in Sweden). The studies were published between 2007 and 2016.
The RCT by Cruickshank et al. had a high risk of bias because of unclear randomization and allocation concealment method, and incomplete outcome data (Table 2). The risk of bias assessment of the included cohort studies was carried out using a modified NOS. Each one of the 9 cohort studies had a moderate risk of bias and reached scores of 5–7 out of 9 points (Table 3).
Table 2.
Risk of Bias Assessment for a Randomized Controlled Trial (based on Cochrane Collaboration’s tool of assessing risk of bias19) of Beta-Blockers after TBI
| Study, Year | Random Sequence Generation | Allocation concealment | Blinding of Outcome Assessment | Incomplete Outcome data | Selective Reporting | Baseline Imbalance |
|---|---|---|---|---|---|---|
| Cruickshank 1987 (47) | Unclear risk | Unclear risk | Low risk |
High risk (CK-MB was measured in only 60 patients, noradrenaline was measured in only 69 patients, daily ECG was obtained in 104 patients, continuous blood pressure monitoring was available in 3 out of 4 study centers, 3 patients had incomplete outcome data because they were discharged from ICU to floor) |
Low risk |
High risk (more patients with severe traumatic brain injury in the intervention group than in the control group; 14 vs. 6, respectively) |
ICU: intensive care unit; ECG: electrocardiogram.
Table 3.
Risk of Bias Assessment for Cohort Studies (based on modified Newcastle-Ottawa scale) of Beta-Blockers after TBI
| Study | Selection | Comparability | Outcome | Total Score |
|---|---|---|---|---|
| Arbabi et al., 2007(48) | *** | ** | ** | 7/9 |
| Cotton et al., 2007(49) | *** | * | ** | 6/9 |
| Inaba et al., 2008(50) | *** | ** | ** | 7/9 |
| Ko et al. 2016(51) | *** | ** | ** | 7/9 |
| Mohseni et al. 2015(52) | *** | ** | ** | 7/9 |
| Murry et al. 2016(53) | *** | – | ** | 5/9 |
| Schroeppel et al., 2010(54) | *** | ** | ** | 7/9 |
| Schroeppel et al., 2014(55) | *** | ** | ** | 7/9 |
| Zangbar et al. 2015(56) | *** | ** | ** | 7/9 |
| Salim et al., 2008(57) | ** | ** | ** | 6/9 |
| Hadjizacharia et al., 2011(58) | *** | – | ** | 5/9 |
| Bukur et al., 2012(59) | *** | ** | ** | 7/9 |
| Riordan et al., 2007(60) | ** | * | ** | 5/9 |
Lower Total Score means Higher Risk of Bias.
Outcome assessment, Critical Outcomes
Hospital mortality was assessed by all cohort studies but not by the RCT (48–50,54). None of the included studies examined functional outcome or quality of life measures. The findings of the cohort studies are summarized in Table 1B. Of the 9 cohort studies, 8 demonstrated that β-blockers exposure after TBI was associated with older age, higher comorbidity burden and more severe injuries. The investigators of 8 of the 9 cohort studies attempted to adjust for potential confounding variables (Table 1B). Seven of the 8 cohort studies that adjusted for potential confounders showed that β-blockers exposure following TBI was associated with statistically significant lower in-hospital mortality. In a subgroup analysis of the Schroeppel et al. (54) study, propranolol use was associated with lower mortality while use of other β-blockers did not show a significant association with mortality. The other study that showed no difference in mortality did not present an adjusted analysis. In general, propranolol was the most frequently studied, although there are also a limited number of studies employing metoprolol or labetalol.
Outcome assessment, Important Outcomes
Two studies assessed for potential cardiopulmonary adverse events associated with β-blockers therapy in TBI patients (47, 53). Compared to the placebo group in the RCT by Cruickshank et al. (47), the atenolol group had a lower proportion of patients with abnormally high CK-MB cardiac biomarker level (2/27 vs. 9/30, p=0.05) and a lower incidence of supraventricular tachycardia (6/46 vs. 28/49, p<0.0001). There was no significant difference between both groups in terms of the incidence of the other outcomes including hypotension, bradycardia, congestive heart failure and bronchospasm (Table 1A). In the Murry et al. (53) cohort, there was no significant difference in the rate of hypotensive events but more bradycardia episodes (defined as heart rate < 60 beats/min) were recorded in the control group relative to the patients who received propranolol. It was not reported whether these bradycardia events were clinically significant and symptomatic (i.e. events requiring a temporary pacemaker, a sympathomimetic agent, atropine, or discontinuation of the propranolol).
Quantitative assessment (Meta-analysis)
Meta-analysis of the cohort studies (Figure 2) showed that exposure to β-blockers after TBI was associated with a significant reduction in the adjusted odds of in-hospital mortality (9 studies, 8,245 patients, pooled OR 0.39, 95% CI: 0.27–0.56; I2=65%, p<0.00001). None of the included cohort studies adequately described the different severity subgroups of TBI to allow for a subgroup analysis of the relationship between β-blockers therapy and hospital mortality of the different TBI subgroups. No clear evidence of reporting (i.e., publication) bias was noted from the funnel plot (Supplementary Material, Figure 2).
Grading the Evidence
In reference to our critical outcome, hospital mortality, the study designs were observational and retrospective. The risk of bias is serious with flawed measurements of exposure (i.e., no study reported dose or timing) and confounders (i.e. no study reported pre-injury exposure, daily ICU covariates). Furthermore, there is a potential publication bias as the included RCT states: “total deaths and in-hospital deaths will be fully reported elsewhere”, but this critical outcome is not found elsewhere in the literature despite lack of measurable publication bias by funnel plot. Inconsistency is very serious due to wide and unassessed baseline risk factors such as pre-injury cardiopulmonary comorbidities and pre-injury β-blocker use. There is substantial heterogeneity (I2 = 65%) indicating serious statistical inconsistency. Indirectness is very serious due to differences in population (e.g. TBI severity, polytrauma severity, cardiovascular risk factors, age), differences across intervention (e.g., type dose, length, target of β-blocker), and differences across comparator (i.e., reasons for control or non-exposure). Imprecision is also very seriously compromised with inability of the pooled sample to achieve optimal information size. For example, to witness the raw unadjusted mortality effect (16.9% with β-blocker, versus 17.7% with control), using a Type I error of 5%, power of 80%, over 35,000 subjects per arm would be required to enroll in a clinical trial. So, the quality rating for the in-hospital mortality outcome is very low. But, we see a strong association of β-blocker use with our critical outcome of in-hospital mortality (i.e., 61% lower odds of mortality or 2.6 lower odds of mortality), thus upgrading its quality from very low to low. Despite this quality upgrade, the overall quality of evidence across all outcomes ultimately remains very low due to the total lack of evidence for our critical outcomes of functional outcome and quality of life. Our hierarchy of outcomes and summary of findings are detailed in Table 4.
Table 4.
Summary of Findings for Beta-Blockers after TBI
| QUALITY ASSESSMENT | SUMMARY OF FINDINGS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Participants (Studies) |
Study Design |
Inconsistency | Indirectness | Imprecision | Publication Bias |
Overall Quality of Evidence |
Study Event Rates (%) | Relative Effect | Anticipated Absolute Effects |
|
| With β-Blocker after TBI |
No β-Blocker after TBI |
Risk Difference using β-Blocker after TBI |
||||||||
| CRITICAL OUTCOME: In-hospital Mortality | ||||||||||
| 8,245 (9 studies) | Cohorts | Very Serious1 | Very Serious2 | Very Serious3 | Serious4 | ++, low5 | 338/2005 (16.9%)6 |
1103/6240 (17.7%)6 |
0.39 (0.27 to 0.56) | 99 fewer per 1000 (from 69 to 122 fewer) |
|
| ||||||||||
| CRITICAL OUTCOME: Function | ||||||||||
| 0 (0 studies) | N/A | Unable to Assess7 | Unable to Assess7 | Unable to Assess7 | Unable to Assess7 | +, very low | N/A | N/A | N/A | N/A |
|
| ||||||||||
| CRITICAL OUTCOME: Quality of Life | ||||||||||
| 0 (0 studies) | N/A | Unable to Assess7 | Unable to Assess7 | Unable to Assess7 | Unable to Assess7 | +, very low | N/A | N/A | N/A | N/A |
|
| ||||||||||
| IMPORTANT OUTCOME: Cardiac Morbidity by Biomarker/Arrhythmia | ||||||||||
| 114 (1 study) | Randomized Clinical Trial | Unable to Assess7 | Unable to Assess7 | Unable to Assess7 | Unable to Assess7 | +, very low | 2/27 (CK-MB) 6/46 (SVT) | 9/30(CK-MB) 28/49 (SVT) | N/A | N/A |
|
| ||||||||||
| IMPORTANT OUTCOME: Hypotension | ||||||||||
| 152 (2 studies)8 | Randomized Clinical Trial | Unable to Assess3 | Unable to Assess3 | Unable to Assess3 | Unable to Assess3 | +, very low | 5/568 | 2/588 | N/A | N/A |
|
| ||||||||||
| IMPORTANT OUTCOME: Bradycardia | ||||||||||
| 152 (2 studies)8 | Randomized Clinical Trial | Unable to Assess3 | Unable to Assess3 | Unable to Assess3 | Unable to Assess3 | +, very low | 6/568 | 6/568 | N/A | N/A |
|
| ||||||||||
| IMPORTANT OUTCOME: Congestive Heart Failure | ||||||||||
| 114 (1 study) | Randomized Clinical Trial | Unable to Assess3 | Unable to Assess3 | Unable to Assess3 | Unable to Assess3 | +, very low | 0/56 | 0/58 | N/A | N/A |
|
| ||||||||||
| IMPORTANT OUTCOME: Bronchospasm | ||||||||||
| 114 (1 study) | Randomized Clinical Trial | Unable to Assess3 | Unable to Assess3 | Unable to Assess3 | Unable to Assess3 | +, very low | 1/56 | 0/58 | N/A | N/A |
Inconsistency is very serious due to wide and unassessed baseline risk factors such as pre-injury cardiopulmonary comorbidities and pre-injury β-blocker use; substantial heterogeneity (I2 = 65%) indicating serious statistical inconsistency
Indirectness is very serious due to differences in population (e.g. TBI severity, non-TBI severity, age), differences across intervention (e.g., type dose, length, target of β-blocker), and differences across comparator (i.e., reasons for control or non-exposure)
Imprecision is also very seriously compromised with inability of the pooled sample to achieve optimal information size, as using a Type I error of 5%, power of 80%, over 35,000 subjects per arm would be required to enroll in a clinical trial
Known publication bias, as reviewed not but included RCT for this outcome states, “in-hospital deaths will be fully reported elsewhere”, but this critical outcome is not found elsewhere in the literature, despite being unable to quantify publication bias by funnel plot
Upgraded quality of evidence from very low to low quality given the consistent large magnitude of effect, 61% lower odds of mortality or 2.6 lower odds of mortality)
Number of deaths were derived from population rates but not directly reported for 1 of the pooled cohort studies
No published studies or No studies available for comparison
We only report the Randomized Clinical Trial; the other study is a prospective cohort that reports group statistical characteristics but not patient level data for these outcomes
Recommendation
In adults with acute TBI with no contraindications for β-blockers, we conditionally recommend the use of in-hospital β-blockers (Figure 3) provided that hypotension (defined as systolic blood pressure<90mmHg) and symptomatic bradycardia (defined as heart rate<50 with symptoms) are avoided. The evidence is limited about whether these thresholds are too restrictive or irrelevant, but it would be cavalier to employ permissive hypotensive strategies in the face of known TBI outside of clinical trials (6,15). The majority of cohort studies included patients with Head AIS of 4–5. Therefore, we limit our recommendation to patients with severe TBI who are admitted to ICU where monitoring for and prevention of adverse cardiovascular events is feasible. Although this recommendation is based on a synthesis of very low-quality studies, most of these studies demonstrate a consistent effect and do not report significant cardiopulmonary harm from administration of β-blockers. However, we cannot provide a recommendation on when to initiate β-blockers, which β-blockers to use, or how to titrate β-blockers to a specific heart rate, blood pressure, and/or length of time.
Figure 3.

Practice management guideline for Beta-Blockers after traumatic brain injury
*Provided that common ICU complications of hypotension (i.e., usually defined as systolic blood pressure<90mmHg) and symptomatic bradycardia (is, usually defined as heart rate<50 with symptoms) are avoided
DISCUSSION
This multispecialty-authored systematic review, meta-analysis, and guideline identified that quality of evidence is very low for in-hospital β-blockers to reduce mortality after TBI, and supports a weak recommendation for the use of in-hospital β-blockers after acute TBI in adults. Despite the paucity of randomized studies, this systematic review and meta-analysis of the observational data suggests that β-blockers reduce mortality after TBI with no major adverse effects. There are no data on the impact of β-blockers use on functional outcome or quality of life measures in TBI patients (61).
Although the results of this meta-analysis (Figure 2) appear to be quite compelling, with an odds ratio for in-hospital mortality of 0.39 [95%CI 0.27–0.56], one must be careful due to the likelihood of selection bias in most of the included studies due to the lack of randomization. We draw the parallel of this evolving story to the practice-changing Corticosteroid Randomization After Significant Head injury (CRASH) trial published in the Lancet (62), which debunked the decades old practice of corticosteroid treatment after TBI. The pre-trial foundational meta-analysis (63) suggested corticosteroid should be moved forward into the large-scale RCT phase, however it could have been heavily influenced by one study (64,65). As we now know, the CRASH trial found corticosteroids increased mortality, as opposed to the prior notion of survival benefit. Therefore, we can only offer a conditional (i.e., weak) recommendation in favor of in-hospital β-blocker use after TBI. This recommendation is conditional on the avoidance of symptomatic bradycardia and hypotension, which may be associated with poor outcome following TBI (6,15). Furthermore, we limit our recommendation to patients with severe TBI who are admitted to ICU where monitoring for adverse cardiovascular events is feasible.
It is evident that mortality is not a perfect endpoint for patients with TBI, who might survive only to be inflicted with life-long functional impairment of a vegetative or severely disabled state (e.g., RESCUE-ICP (66) and DECRA (67) RCTs). Unfortunately, the value to society and to individuals for small changes in functional status is not well-defined, thus limiting the interpretation of the extended Glasgow Outcome Scale (GOS-E) often used in TBI RCTs (68–71). “The 100 percent failure rate for TBI clinical trials strongly suggests that” future TBI RCTs should use A) “quantitative outcome measures”, B) "require more optimization of dose", and C) “adopt adaptive designs” (72).
A number of ongoing studies will likely provide more insight on the mechanism of action, safety, and efficacy of these agents in the TBI population (Decreasing Adrenergic or Sympathetic Hyperactivity after severe traumatic brain injury using propranolol and clonidine (73), NCT01322048 and NCT02957331 (accessible at https://clinicaltrials.gov), and the AAST multi-center prospective, observational study on immune dysfunction in subjects who present with TBI and receive β-blockers (accessible at http://www.aast.org)). All the studies to date only report the dichotomous use (i.e., yes/no) of β-blockers. There are no real-world data on standardized β-blocker dose-equivalents, or time-varying adjustment accounting for daily confounders of complex ICU care. Although propranolol is a cheap, centrally acting agent with intravenous and oral formulations, perhaps making it easier to initially study, consideration should also be given to determine the comparative effectiveness of other mixed-receptor agents (e.g., labetalol) or rapidly metabolized intravenous agents (e.g., esmolol). We do not know if the survival benefit observed in our analysis may be related to the degree of heart rate control (56) and whether competing pressor use influenced outcomes, as studied in septic ICU populations without TBI (74). Given that the only reported effect is on mortality, future studies should focus on patients with a significant risk of death. These patients could include those requiring ICU care, moderate or higher TBI, specific pathoanatomic classes of intracranial hemorrhage, and/or a combination of prognostic covariates for mortality. These additional studies should help answer questions about β-blocker mechanism of action, while adjusting for covariates (e.g. brain injury severity, polytrauma, associated co-morbidities, daily ICU events) to reveal effects on long-term patient-centered outcomes on cognition, neurologic function, and quality of life. Given the complexity of TBI management as well as subtle possible differences in clinical signs and symptoms, imaging, and genetic variances in the population, a large federally supported trauma consortium should provide the funding and research infrastructure necessary to advance the field in this regard (75).
Supplementary Material
Supplementary Figure 1. Newcastle-Ottawa Scale.
Supplementary Figure 2. Funnel plot of beta-blocker exposure after traumatic brain injury versus no exposure with in-hospital mortality outcome.
Acknowledgments
We would like to thank Elizabeth Uleryk from the Hospital for Sick Children Library for her assistance in designing the literature search strategy. We thank the EAST membership for feedback during this process, and the EAST Guidelines Committee for the pre-submission peer-review.
FUNDING:
Vanderbilt Faculty Research Scholars Program (mbp); National Institutes of Health NHLBI R01HL111111 (mbp), NIGMS R01GM120484 (mbp), and NCATS UL1TR000445 for REDCap (all authors)
Footnotes
PRESENTATION AT: Guidelines Plenary Session, 29th Annual Scientific Assembly of the Eastern Association for the Surgery of Trauma meeting in San Antonio, TX on January 16, 2016.
CONFLICT OF INTEREST WITH OTHER SOURCES OF SUPPORT:
Dr. Patel has been or is supported by the Vanderbilt Institute for Clinical and Translational Research awards (VR1584, VR5351, VR9276, VR12073) via CTSA grant UL1TR000011 (NCRR/NCATS/NIH), a 2013 EAST Trauma Foundation Research Scholarship, and speaker fees from Pfizer. Drs. Haut and Patel have served on the EAST Guidelines Section and Board of Directors. Dr. Haut is the primary investigator of a AHRQ grant (R01HS024547) of a PCORI contract (CE-12–11–4489). Dr. Haut receives book royalties from Lippincott, Williams, Wilkins (“Avoiding Common ICU Errors”), consultant and speaker fees from VHA/Vizient IMPERATIV® Advantage Performance Improvement Collaborative, and consultant and speaker fees for the Illinois Surgical Quality Improvement Collaborative. Dr. Haut was the paid author of a paper commissioned by the National Academies of Medicine.
Contributor Information
Aziz S. Alali, Email: aziz.alali@mail.utoronto.ca.
Kaushik Mukherjee, Email: KMukherjee@llu.edu.
Victoria A. McCredie, Email: Victoria.McCredie@mail.utoronto.ca.
Eyal Golan, Email: golan8@gmail.com.
Prakesh S. Shah, Email: pshah@mtsinai.on.ca.
James M. Bardes, Email: jim.bardes@gmail.com.
Susan E. Hamblin, Email: susan.hamblin@Vanderbilt.Edu.
Elliott R. Haut, Email: ehaut1@jhmi.edu.
James C. Jackson, Email: james.c.jackson@Vanderbilt.Edu.
Kosar Khwaja, Email: dr.k.khwaja@mcgill.ca.
Nimitt J. Patel, Email: nimitt.patel.md@gmail.com.
Satish R. Raj, Email: satish.raj@ucalgary.ca.
Laura D. Wilson, Email: laura-wilson@utulsa.edu.
Avery B. Nathens, Email: avery.nathens@sunnybrook.ca.
Mayur B. Patel, Email: mayur.b.patel@vanderbilt.edu.
References
- 1.Menon DK, Zahed C. Prediction of outcome in severe traumatic brain injury. Current Opinion in Critical Care. 2009 Oct;15(5):437–41. doi: 10.1097/MCC.0b013e3283307a26. [DOI] [PubMed] [Google Scholar]
- 2.Heffernan DS, Inaba K, Arbabi S, Cotton BA. Sympathetic Hyperactivity After Traumatic Brain Injury and the Role of Beta-Blocker Therapy. The Journal of Trauma: Injury, Infection, and Critical Care. 2010 Dec;69(6):1602–9. doi: 10.1097/TA.0b013e3181f2d3e8. [DOI] [PubMed] [Google Scholar]
- 3.Tran TY, Dunne IE, German JW. Beta blockers exposure and traumatic brain injury: a literature review. Neurosurgical Focus. 2008 Oct;25(4):E8. doi: 10.3171/FOC.2008.25.10.E8. [DOI] [PubMed] [Google Scholar]
- 4.Ley EJ, Berry C, Mirocha J, Salim A. Mortality is Reduced for Heart Rate 80 to 89 After Traumatic Brain Injury. Journal of Surgical Research. 2010 Sep;163(1):142–5. doi: 10.1016/j.jss.2010.04.046. [DOI] [PubMed] [Google Scholar]
- 5.Radosevich JJ, Patanwala AE, Erstad BL. Emerging pharmacological agents to improve survival from traumatic brain injury. Brain Inj. 2013 Nov 11;27(13–14):1492–9. doi: 10.3109/02699052.2013.823658. [DOI] [PubMed] [Google Scholar]
- 6.Berry C, Ley EJ, Bukur M, Malinoski D, Margulies DR, Mirocha J, et al. Injury. 11. Vol. 43. Elsevier Ltd; 2012. Nov 1, Redefining hypotension in traumatic brain injury; pp. 1833–7. [DOI] [PubMed] [Google Scholar]
- 7.POISE Study Group. Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008 May 31;371(9627):1839–47. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 8.Fleischmann KE, Beckman JA, Buller CE, Calkins H, Fleisher LA, Freeman WK, et al. 2009 ACCF/AHA Focused Update on Perioperative Beta Blockade. Journal of the American College of Cardiology. 2009 Nov;54(22):2102–28. doi: 10.1016/j.jacc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Alali AS, McCredie VA, Golan E, Shah PS, Nathens AB. Beta Blockers for Acute Traumatic Brain Injury: A Systematic Review and Meta-analysis. Neurocrit Care. 2013 Sep 6;20(3):514–23. doi: 10.1007/s12028-013-9903-5. [DOI] [PubMed] [Google Scholar]
- 10.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. BMJ. 7650. Vol. 336. British Medical Journal Publishing Group; 2008. Apr 26, GRADE: an emerging consensus on rating quality of evidence and strength of recommendations; pp. 924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, et al. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. Journal of Trauma and Acute Care Surgery. 73(5 Suppl 4):S283–7. doi: 10.1097/TA.0b013e31827013e9. [DOI] [PubMed] [Google Scholar]
- 12.Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, et al. BMJ. 7652. Vol. 336. British Medical Journal Publishing Group; 2008. May 10, Going from evidence to recommendations; pp. 1049–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. BMJ. oct 18 2. Vol. 343. British Medical Journal Publishing Group; 2011. The Cochrane Collaboration’r assessing risk of bias in randomised trials; pp. d5928–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. Springer Netherlands. 2010 Jul 22;25(9):603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 15.Panczykowski DM, Puccio AM, Scruggs BJ, Bauer JS, Hricik AJ, Beers SR, et al. Prospective Independent Validation of IMPACT Modeling as a Prognostic Tool in Severe Traumatic Brain Injury. J Neurotrauma. 2012 Jan;29(1):47–52. doi: 10.1089/neu.2010.1482. [DOI] [PubMed] [Google Scholar]
- 16.Ley EJ, Berry C, Mirocha J, Salim A. Mortality is Reduced for Heart Rate 80 to 89 After Traumatic Brain Injury. Journal of Surgical Research. 2010 Sep;163(1):142–5. doi: 10.1016/j.jss.2010.04.046. [DOI] [PubMed] [Google Scholar]
- 17.Neideen T, Lam M, Brasel KJ. Preinjury Beta Blockers are Associated With Increased Mortality in Geriatric Trauma Patients. The Journal of Trauma: Injury, Infection, and Critical Care. 2008 Nov;65(5):1016–20. doi: 10.1097/TA.0b013e3181897eac. [DOI] [PubMed] [Google Scholar]
- 18.Schneider EB, Efron DT, MacKenzie EJ, Rivara FP, Nathens AB, Jurkovich GJ. Premorbid Statin Use Is Associated With Improved Survival and Functional Outcomes in Older Head-Injured Individuals. The Journal of Trauma: Injury, Infection, and Critical Care. 2011 Oct;71(4):815–9. doi: 10.1097/TA.0b013e3182319de5. [DOI] [PubMed] [Google Scholar]
- 19.Robertson CS, Valadka AB, Hannay HJ, Contant CF, Gopinath SP, Cormio M, et al. Prevention of secondary ischemic insults after severe head injury. Critical Care Medicine. 1999 Oct;27(10):2086–95. doi: 10.1097/00003246-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Bukur M, Lustenberger T, Cotton B, Arbabi S, Talving P, Salim A, et al. Beta-blocker exposure in the absence of significant head injuries is associated with reduced mortality in critically ill patients. The American Journal of Surgery. 2012 Nov;204(5):697–703. doi: 10.1016/j.amjsurg.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Evans D, Khoo K, Radulescu A, Cook C, Gerlach A, Papadimos T, et al. Journal of Emergencies, Trauma, and Shock. 4. Vol. 7. Medknow Publications; 2014. Pre-injury beta blocker use does not affect the hyperdynamic response in older trauma patients; pp. 305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman A, Bar-Klein G, Serlin Y, Parmet Y, Heinemann U, Kaufer D. Should losartan be administered following brain injury? Expert Review of Neurotherapeutics. 2014 Nov 26;14(12):1365–75. doi: 10.1586/14737175.2014.972945. [DOI] [PubMed] [Google Scholar]
- 23.Hendén PL, Söndergaard S, Rydenhag B, Reinsfelt B, Ricksten S-E, Åneman A. Can Baroreflex Sensitivity and Heart Rate Variability Predict Late Neurological Outcome in Patients With Traumatic Brain Injury? Journal of Neurosurgical Anesthesiology. 2014 Jan;26(1):50–9. doi: 10.1097/ANA.0b013e3182a47b62. [DOI] [PubMed] [Google Scholar]
- 24.Lewis PR, Dunne CE, Thompson KA, McDonald VS, Calvo RY, Badiee J, et al. Attenuation of cardiovascular stress with sympatholytics does not improve survival in patients with severe isolated traumatic brain injury. Journal of Trauma and Acute Care Surgery. 2016 Apr;80(4):643–7. doi: 10.1097/TA.0000000000000957. [DOI] [PubMed] [Google Scholar]
- 25.Mohseni S, Talving P, Wallin G, Ljungqvist O, Riddez L. Preinjury β-blockade is protective in isolated severe traumatic brain injury. Journal of Trauma and Acute Care Surgery. 2014 Mar;76(3):804–8. doi: 10.1097/TA.0000000000000139. [DOI] [PubMed] [Google Scholar]
- 26.Shoup JP, Winkler J, Czap A, Staff I, Fortunato G, McCullough LD, et al. β-Blockers associated with no class-specific survival benefit in acute intracerebral hemorrhage. Journal of the Neurological Sciences. 2014 Jan;336(1–2):127–31. doi: 10.1016/j.jns.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velayutham PK, Adhikary SD, Babu SK, Vedantam R, Korula G, Ramachandran A. Oxidative stress–associated hypertension in surgically induced brain injury patients: Effects of β-blocker and angiotensin-converting enzyme inhibitor. Journal of Surgical Research. 2013 Jan;179(1):125–31. doi: 10.1016/j.jss.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Webb A, Semple J, Rothwell PM. Greater transmission of aortic pulsatility to the brain on propranolol versus amlodipine: Novel MRI method to measure concurrent beat-to-beat brachial and cerebral arterial pulsatility. Glasgow, UK: pp. 20150338–9. [Google Scholar]
- 29.Naredi S, Olivecrona M, Lindgren C, Ostlund AL, Grände PO, Koskinen LOD. Acta Anaesthesiol Scand. 4. Vol. 45. Munksgaard International Publishers; 2001. Apr, An outcome study of severe traumatic head injury using the “Lund therapy” with low-dose prostacyclin; pp. 402–6. [DOI] [PubMed] [Google Scholar]
- 30.Naredi S, Edén E, Zäll S, Stephensen H, Rydenhag B. Intensive Care Medicine. 5. Vol. 24. Springer-Verlag; 2014. Feb 26, A standardized neurosurgical/neurointensive therapy directed toward vasogenic edema after severe traumatic brain injury: clinical results; pp. 446–51. [DOI] [PubMed] [Google Scholar]
- 31.Grände PO, Nordstrom CH, Asgeirsson B. Current alternative therapy of post-traumatic brain edema. Nord Med. 1994;109(5):157–9. [PubMed] [Google Scholar]
- 32.Eker C, Asgeirsson B, Grande P-O, Schalen W, Nordstrom C-H. Improved outcome after severe head injury with a new therapy based on principles for brain volume regulation and preserved microcirculation. Critical Care Medicine. 1998 Nov;26(11):1881–6. doi: 10.1097/00003246-199811000-00033. [DOI] [PubMed] [Google Scholar]
- 33.Asgeirsson B, Grände PO, Nordstrom CH. Intensive Care Medicine. 4. Vol. 20. Springer-Verlag; 1994. Apr, A new therapy of post-trauma brain oedema based on haemodynamic principles for brain volume regulation; pp. 260–7. [DOI] [PubMed] [Google Scholar]
- 34.Robertson CS, Clifton GL, Taylor AA, Grossman RG. Treatment of hypertension associated with head injury. J Neurosurg. 1983 Sep;59(3):455–60. doi: 10.3171/jns.1983.59.3.0455. [DOI] [PubMed] [Google Scholar]
- 35.Fuchs HJ, Herden HN, Welter J. Effect of metoprolol on the circulation after head trauma. Studies during continuous intravenous administration Deutsche Medizinische Wochenschrift. 105(44):1531–6. doi: 10.1055/s-2008-1070906. [DOI] [PubMed] [Google Scholar]
- 36.Do D, Sheen VL, Bromfield E. Journal of Neurology, Neurosurgery & Psychiatry. 6. Vol. 69. BMJ Publishing Group Ltd; 2000. Dec 1, Treatment of paroxysmal sympathetic storm with labetalol; pp. 832–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asgeirsson B, Grände PO, Nordstrom CH, Berntman L, Messeter K, Ryding E. Acta Anaesthesiol Scand. 3. Vol. 39. Blackwell Publishing Ltd; 1995. Apr, Effects of hypotensive treatment with α 2-agonist and β 1-antagonist on cerebral haemodynamics in severely head injured patients; pp. 347–51. [DOI] [PubMed] [Google Scholar]
- 38.Radosevich JJ, Patanwala AE, Erstad BL. Emerging pharmacological agents to improve survival from traumatic brain injury. Brain Inj. 2013 Nov 11;27(13–14):1492–9. doi: 10.3109/02699052.2013.823658. [DOI] [PubMed] [Google Scholar]
- 39.Xiong Y, Zhang Y, Mahmood A, Chopp M. Investigational agents for treatment of traumatic brain injury. Expert Opinion on Investigational Drugs. 2015 Mar 25;24(6):743–60. doi: 10.1517/13543784.2015.1021919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van den Bergh WM. Pharmacotherapy of traumatic brain injury. Netherlands Journal of Critical Care. 24(1):6–11. [Google Scholar]
- 41.Koskinen LOD, Olivecrona M, Grände PO. Severe traumatic brain injury management and clinical outcome using the Lund concept. Neuroscience. 2014 Dec;283:245–55. doi: 10.1016/j.neuroscience.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 42.Coppola S, Froio S, Chiumello D. Annual Update in Intensive Care and Emergency Medicine 2015. Cham: Springer International Publishing; 2015. β-Blockers in Critically Ill Patients: From Physiology to Clinical Evidence; pp. 139–52. (Annual Update in Intensive Care and Emergency Medicine; vol. 2015) [Google Scholar]
- 43.Griffin GD. Stroke, mTBI, infection, antibiotics and beta blockade: Connecting the dots. Medical Hypotheses. 2015 Aug;85(2):224–9. doi: 10.1016/j.mehy.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Ley EJ, Park R, Dagliyan G, Palestrant D, Miller CM, Conti PS, et al. In Vivo Effect of Propranolol Dose and Timing on Cerebral Perfusion After Traumatic Brain Injury. The Journal of Trauma: Injury, Infection, and Critical Care. 2010 Feb;68(2):353–6. doi: 10.1097/TA.0b013e3181c8269a. [DOI] [PubMed] [Google Scholar]
- 45.Ley EJ, Scehnet J, Park R, Schroff S, Dagliyan G, Conti PS, et al. The In Vivo Effect of Propranolol on Cerebral Perfusion and Hypoxia After Traumatic Brain Injury. The Journal of Trauma: Injury, Infection, and Critical Care. 2009 Jan;66(1):154–61. doi: 10.1097/TA.0b013e31819388be. [DOI] [PubMed] [Google Scholar]
- 46.Song D, Xu J, Du T, Yan E, Hertz L, Walz W, et al. Inhibition of Brain Swelling after Ischemia-Reperfusion by β-Adrenergic Antagonists: Correlation with Increased K +and Decreased Ca 2+Concentrations in Extracellular Fluid. BioMed Research International. 2014;2014(3):1–10. doi: 10.1155/2014/873590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cruickshank J, Degaute J, Kuurne T, Vincent J, Neil-Dwyer G, Hayes Y, et al. Reduction of stress/catecholamine-induced cardiac necrosis by beta 1-selective blockade. The Lancet. 1987 Sep;330(8559):585–9. doi: 10.1016/s0140-6736(87)92984-9. [DOI] [PubMed] [Google Scholar]
- 48.Arbabi S, Campion EM, Hemmila MR, Barker M, Dimo M, Ahrns KS, et al. Beta-Blocker Use is Associated With Improved Outcomes in Adult Trauma Patients. The Journal of Trauma: Injury, Infection, and Critical Care. 2007 Jan;62(1):56–62. doi: 10.1097/TA.0b013e31802d972b. [DOI] [PubMed] [Google Scholar]
- 49.Cotton BA, Snodgrass KB, Fleming SB, Carpenter RO, Kemp CD, Arbogast PG, et al. Beta-Blocker Exposure is Associated With Improved Survival After Severe Traumatic Brain Injury. The Journal of Trauma: Injury, Infection, and Critical Care. 2007 Jan;62(1):26–35. doi: 10.1097/TA.0b013e31802d02d0. [DOI] [PubMed] [Google Scholar]
- 50.Inaba K, Teixeira PGR, David J-S, Chan LS, Salim A, Brown C, et al. Beta-Blockers in Isolated Blunt Head Injury. Journal of the American College of Surgeons. 2008 Mar;206(3):432–8. doi: 10.1016/j.jamcollsurg.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Ko A, Harada MY, Barmparas G, Thomsen GM, Alban RF, Bloom M, et al. Early Propranolol after Traumatic Brain Injury is Associated with Lower Mortality. Journal of Trauma and Acute Care Surgery. 2016 Jan;:1–21. doi: 10.1097/TA.0000000000000959. [DOI] [PubMed] [Google Scholar]
- 52.Mohseni S, Talving P, Thelin EP, Wallin G, Ljungqvist O, Riddez L. World Journal of Surgery. Springer US; 2015. Apr 14, The Effect of β-blockade on Survival After Isolated Severe Traumatic Brain Injury; pp. 1–8. [DOI] [PubMed] [Google Scholar]
- 53.Murry JS, Hoang DM, Barmparas G, Harada MY, Bukur M, Bloom MB, et al. Journal of Surgical Research. 1. Vol. 200. Elsevier Inc; 2016. Jan 1, Prospective evaluation of early propranolol after traumatic brain injury; pp. 221–6. [DOI] [PubMed] [Google Scholar]
- 54.Schroeppel TJ, Fischer PE, Zarzaur BL, Magnotti LJ, Clement LP, Fabian TC, et al. Beta-Adrenergic Blockade and Traumatic Brain Injury: Protective? The Journal of Trauma: Injury, Infection, and Critical Care. 2010 Oct;69(4):776–82. doi: 10.1097/TA.0b013e3181e981b8. [DOI] [PubMed] [Google Scholar]
- 55.Schroeppel TJ, Sharpe JP, Magnotti LJ, Weinberg JA, Clement LP, Croce MA, et al. Traumatic brain injury and β-blockers. Journal of Trauma and Acute Care Surgery. 2014 Feb;76(2):504–9. doi: 10.1097/TA.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 56.Zangbar B, Khalil M, Rhee P, Joseph B, Kulvatunyou N, Tang A, et al. Journal of Surgical Research. 2. Vol. 200. Elsevier Inc; 2016. Feb 1, Metoprolol improves survival in severe traumatic brain injury independent of heart rate control; pp. 586–92. [DOI] [PubMed] [Google Scholar]
- 57.Salim A, Hadjizacharia P, Brown C, Inaba K, Teixeira PGR, Chan L, et al. Significance of Troponin Elevation After Severe Traumatic Brain Injury. The Journal of Trauma: Injury, Infection, and Critical Care. 2008 Jan;64(1):46–52. doi: 10.1097/TA.0b013e31815eb15a. [DOI] [PubMed] [Google Scholar]
- 58.Hadjizacharia P, O’Keeffe T, Brown CVR, Inaba K, Salim A, Chan LS, et al. Incidence, risk factors, and outcomes for atrial arrhythmias in trauma patients. The American Surgeon. 2011 May;77(5):634–9. doi: 10.1177/000313481107700526. [DOI] [PubMed] [Google Scholar]
- 59.Bukur M, Mohseni S, Mosheni S, Ley E, Salim A, Margulies D, et al. Efficacy of beta-blockade after isolated blunt head injury: does race matter? J Trauma Acute Care Surg. 2012 Apr;72(4):1013–8. doi: 10.1097/TA.0b013e318241bc5b. [DOI] [PubMed] [Google Scholar]
- 60.Riordan WP, Cotton BA, Norris PR, Waitman LR, Jenkins JM, Morris JA. Beta-blocker exposure in patients with severe traumatic brain injury (TBI) and cardiac uncoupling. J Trauma. 2007 Sep;63(3) doi: 10.1097/TA.0b013e3181271c34. 503–10–discussion510–1. [DOI] [PubMed] [Google Scholar]
- 61.Hendrick LE, Schroeppel TJ, Sharpe JP, Alsbrook D, Magnotti LJ, Weinberg JA, et al. Impact of Beta-Blockers on Nonhead Injured Trauma Patients. The American Surgeon. 2016 Jul;82(7):575–9. [PubMed] [Google Scholar]
- 62.Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, et al. Effect of intravenous corticosteroids on death within 14 days in 10 008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. The Lancet. 2004 Oct;364(9442):1321–8. doi: 10.1016/S0140-6736(04)17188-2. [DOI] [PubMed] [Google Scholar]
- 63.Alderson P, Roberts I. BMJ. 7098. Vol. 314. BMJ Group; 1997. Jun 28, Corticosteroids in acute traumatic brain injury: systematic review of randomised controlled trials; pp. 1855–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gregson B, Todd NV, Crawford D, Gerber CJ, Fulton B, Tacconi L, et al. CRASH trial is based on problematic meta-analysis. BMJ. 1999 Aug 28;319(7209):578–8. doi: 10.1136/bmj.319.7209.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alderson P. Design of CRASH trial. Evidence shows that quality of trial by Faupel et al is good and therefore should not be excluded. BMJ. 1999 Oct 16;319(7216):1068. doi: 10.1136/bmj.319.7216.1068a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, et al. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. N Engl J Med. 2016 Sep 22;375(12):1119–30. doi: 10.1056/NEJMoa1605215. [DOI] [PubMed] [Google Scholar]
- 67.Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, et al. Decompressive Craniectomy in Diffuse Traumatic Brain Injury. New England Journal of Medicine. 2011 Apr 21;364(16):1493–502. doi: 10.1056/NEJMoa1102077. [DOI] [PubMed] [Google Scholar]
- 68.Nichol A, French C, Little L, Haddad S, Presneill J, Arabi Y, et al. Erythropoeitin in traumatic brain injury (EPO-TBI): A double-blind randomised controlled trial. Lancet. 2015 Dec 19;386(10012):2499–506. doi: 10.1016/S0140-6736(15)00386-4. [DOI] [PubMed] [Google Scholar]
- 69.Wright DW, Yeatts SD, Silbergleit R, Palesch YY, Hertzberg VS, Frankel M, et al. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med. 2014 Dec 25;371(26):2457–66. doi: 10.1056/NEJMoa1404304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skolnick BE, Maas AI, Narayan RK, van der Hoop RG, MacAllister T, Ward JD, et al. A clinical trial of progesterone for severe traumatic brain injury. N Engl J Med. 2014 Dec 25;371(26):2467–76. doi: 10.1056/NEJMoa1411090. [DOI] [PubMed] [Google Scholar]
- 71.Robertson CS, Hannay HJ, Yamal J-M, Gopinath S, Goodman JC, Tilley BC, et al. Effect of Erythropoietin and Transfusion Threshold on Neurological Recovery After Traumatic Brain Injury. JAMA. 2014 Jul 2;312(1):36. doi: 10.1001/jama.2014.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stein D. Opinion: Why Most TBI Studies Fail [Internet] 2016 [cited 2016 Oct 9]. Available from: http://www.the-scientist.com/?articles.view/articleNo/45434/title/Opinion–why-most-tbi-studies-fail/
- 73.Patel MB, McKenna JW, Alvarez JM, Sugiura A, Jenkins JM, Guillamondegui OD, et al. Decreasing adrenergic or sympathetic hyperactivity after severe traumatic brain injury using propranolol and clonidine (DASH After TBI Study): study protocol for a randomized controlled trial. Trials. 2140 ed. BioMed Central. 2012 Sep 26;13(1):232. doi: 10.1186/1745-6215-13-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, et al. JAMA: The Journal of the American Medical Association. 16. Vol. 310. American Medical Association; 2013. Oct 23, Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial; pp. 1683–91. [DOI] [PubMed] [Google Scholar]
- 75.Kochanek PM, Bramlett HM, Dixon CE, Shear DA, Dietrich WD, Schmid KE, et al. Approach to modeling, therapy evaluation, drug selection, and biomarker assessments for a multicenter pre-clinical drug screening consortium. J Neurotrauma. 2016 Mar 15;33(6):513–22. doi: 10.1089/neu.2015.4113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Newcastle-Ottawa Scale.
Supplementary Figure 2. Funnel plot of beta-blocker exposure after traumatic brain injury versus no exposure with in-hospital mortality outcome.


