Key Points
Activation of the Vdr pathway stimulates proliferation of early, but not late, mouse erythroid progenitors in a cell autonomous manner.
Vdr and Gr signaling cooperate to increase the growth of mouse erythroid progenitors.
Abstract
The pathways that regulate the growth of erythroid progenitors are incompletely understood. In a computational analysis of gene expression changes during erythroid ontogeny, the vitamin D receptor (Vdr) nuclear hormone receptor transcription factor gene was identified in fetal and adult stages, but not at the embryonic stage of development. Vdr was expressed in definitive erythroid (EryD) progenitors and was downregulated during their maturation. Activation of Vdr signaling by the vitamin D3 agonist calcitriol increased the outgrowth of EryD colonies from fetal liver and adult bone marrow, maintained progenitor potential, and delayed erythroid maturation, as revealed by clonogenic assays, suspension culture, cell surface phenotype, and gene expression analyses. The early (cKit+CD71lo/neg), but not the late (cKit+CD71hi), EryD progenitor subset of LinnegcKit+ cells was responsive to calcitriol. Culture of cKit+CD71lo/neg progenitors in the presence of both vitamin D3 and glucocorticoid receptor ligands resulted in an increase in proliferation that was at least additive compared with either ligand alone. Lentivirus shRNA-mediated knockdown of Vdr expression abrogated the stimulation of early erythroid progenitor growth by calcitriol. These findings suggest that Vdr has a cell-intrinsic function in early erythroid progenitors. Targeting of downstream components of the Vdr signaling pathway may lead to new approaches for the expansion of erythroid progenitors ex vivo.
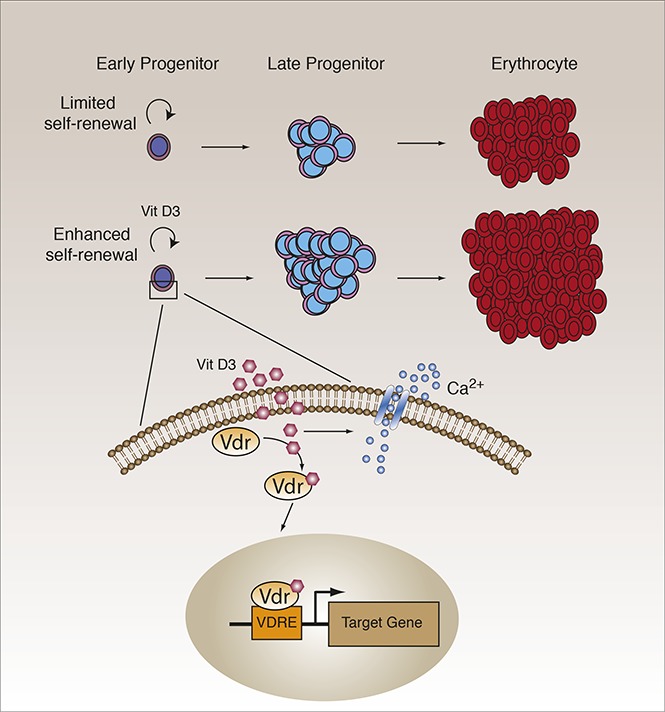
Visual Abstract
Introduction
Erythropoiesis is the process by which red blood cell (RBC) progenitors are produced and undergo terminal differentiation to erythrocytes. During ontogeny, the earliest erythroid progenitors emerge transiently in the yolk sac and differentiate into “primitive” erythroid cells (EryP).1,2 Two sequential waves of definitive erythroid (EryD) progenitors have been identified: the first wave is transient and yolk sac-derived,3 and the second is stable and generated from hematopoietic stem cells in the fetal liver (FL).4,5 Within 2 to 3 days after the onset of FL erythropoiesis, EryD progenitors far outnumber EryP in the circulation.6 Toward the end of gestation, hematopoietic stem cells from the FL migrate to the bone marrow (BM), which becomes and remains the primary site of hematopoiesis, including the production and differentiation of erythroid progenitors, throughout postnatal life (for recent reviews, see Dzierzak and Philipsen7 and Barminko et al8).
Definitive erythropoiesis produces more than 1011 RBCs per day from erythroid progenitors.9 Two distinct EryD progenitors have been defined functionally, using colony assays: the earlier burst-forming unit–erythroid (BFU-E) progenitor undergoes a limited number of self-renewal divisions before giving rise to more mature colony-forming unit–erythroid (CFU-E) progenitors (reviewed in Dzierzak and Philipsen7 and Hattangadi et al10). CFU-E maturation is accompanied by 5 to 6 cell divisions that result in the sequential formation of 5 morphologically distinct populations of erythroblasts.11 With each successive division, the cells accumulate hemoglobin and decrease in size; within their nuclei, loss of nucleoli and chromosomal condensation are observed.2,7,10 Erythroblasts eventually enucleate to form reticulocytes that undergo additional steps in maturation, including clearance of mitochondria and other organelles.12,13 Flow cytometric strategies have been developed to phenotypically identify these distinct stages of erythroid maturation in the mouse.11,14-16
The basal rate of erythropoiesis is determined by the growth and survival of CFU-E progenitors, which are regulated largely by erythropoietin (EPO) and its receptor.10,17,18 Under conditions of stress such as anemia, acute blood loss, or hypoxia, RBC production must increase above the basal rate, with increased self-renewal of BFU-E in response to EPO and a number of other hormones and cytokines (reviewed in Paulson et al19 and Socolovsky20). The pathways that regulate the growth of early erythroid progenitors remain poorly understood.
In a computational search for transcription factor genes that are differentially regulated in the primitive (embryonic)1 and definitive (fetal, adult)21,22 erythroid lineages, we observed that the vitamin D receptor (Vdr) nuclear transcription factor gene is expressed at the fetal and adult, but not the embryonic, stage of erythroid ontogeny. Vdr is related to the glucocorticoid, estrogen, androgen, and other steroid hormone receptors (reviewed in Levin23). Binding of its hormone ligand, vitamin D3, results in formation of heterodimers of Vdr and retinoic acid X receptor (RXR) that translocate into the nucleus.24 Heterodimerization permits high-affinity binding to vitamin D response elements within Vdr target genes.24 The Vdr signaling pathway has been best studied in bone, the immune system, and skin (reviewed in Bouillon et al25). Studies on the response to vitamin D signaling in erythropoiesis have employed mostly erythroleukemia cell lines (eg, Moore et al,26 Waki et al,27 and Alon et al28) and have reached conflicting conclusions. In one study, calcitriol was reported to inhibit erythroid differentiation in human K562 and mouse erythroleukemia cells, but no effect on proliferation was detected.26 In a second study of human cord blood and TF1 cells, proliferation was enhanced by treatment with calcitriol, but erythroid differentiation was not evaluated.28 In yet a third study, both the proliferation and differentiation of mouse erythroleukemia cells were inhibited by calcitriol.27 Improvement in the anemia associated with chronic renal failure has been reported and may involve an increase in the numbers of BFU-Es.29 The role of this pathway in erythropoiesis remains poorly understood.
Here, we report that activation of Vdr by calcitriol, the most biologically active metabolite of vitamin D3,30 stimulates the growth of mouse FL and BM erythroid progenitors, resulting in a large increase in the numbers of mature red blood cells. Vdr is expressed in EryD progenitors and is downregulated during erythroid maturation. The increase in proliferation of progenitors results, at least in part, from maintenance of erythroid progenitor potential and from delayed maturation. The early progenitor CD71lo/neg, but not the late progenitor-containing CD71hi population of Linneg cKit+ cells, is responsive to calcitriol, independent of its calcemic effects. Vdr and the glucocorticoid receptor can cooperate to regulate the proliferation of early progenitors. Lentiviral shRNA-mediated knockdown of Vdr abrogates the stimulation of early progenitor growth by calcitriol. These findings demonstrate that Vdr has an intrinsic function in early erythroid progenitors and may allow the identification of novel targets for therapeutic intervention. Activation of the Vdr pathway offers a new approach for the expansion of EryD progenitors ex vivo.
Materials and methods
Detailed experimental procedures are described in the supplemental Materials.
Preparation of hematopoietic cells
FL (embryonic day 12.5 [E12.5] unless otherwise indicated) or BM (6-8 weeks old) cells were isolated from CD-1 mice (Charles River Laboratories) as described.31-33 Lineage depletion of single-cell suspensions was performed using a Lineage Cell Depletion Kit (Miltenyi Biotec Inc.) according to the manufacturer’s instructions. The Mount Sinai School of Medicine Institutional Animal Care and Use Committee approved this study.
Culture of erythroid progenitors
To expand erythroid progenitors, cells were cultured (37°C, 5% CO2) in serum-free progenitor medium (PM): StemSpan SFEM (Stem Cell Technologies) supplemented with human recombinant EPO (0.5 units/mL; Amgen), mouse stem cell factor (100 ng/mL; Thermo Fisher Scientific), mouse insulin-like growth factor (40 ng/mL; Thermo Fisher Scientific), lipid concentrate (1×; Thermo Fisher Scientific), penicillin/streptomycin (1%; Pen/Strep; Thermo Fisher Scientific), and dexamethasone (10−6 M; Sigma; D2915).
Erythroid maturation culture
Erythroid maturation was initiated by culturing cells in suspension (2.5 × 105/mL, 37°C, 5% CO2) in maturation medium (MM): IMDM (Corning) supplemented with recombinant human EPO (2 U/mL), mouse stem cell factor (100 ng/mL), knockout serum replacement (10%; Thermo Fisher Scientific), fetal bovine plasma-derived serum (5%; Animal Technologies), protein-free hybridoma medium II (10%; Thermo Fisher Scientific), glutamine (1×; Thermo Fisher Scientific), and penicillin/streptomycin (1%).34 Cell density was measured daily. At cell concentrations 4.0 × 106/mL or higher, the cultures were split into 1.0 × 106 cells/mL. Culture medium was supplemented with calcitriol (100 nM, or as indicated; Sigma), calcipotriol (100 nM or as indicated; Cayman Chemical), or dexamethasone (100 nM).
Results
Activation of Vdr signaling by calcitriol stimulates the growth of EryD progenitors
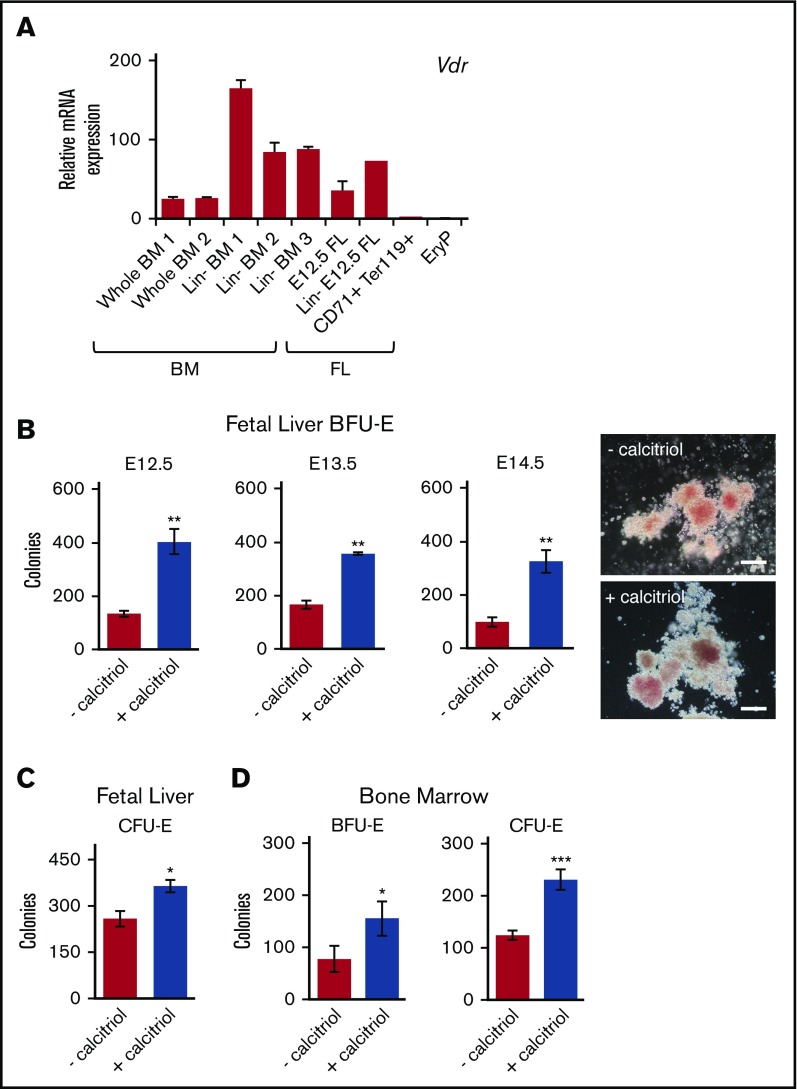
To examine Vdr expression during ontogeny and at different stages of erythroid maturation, RNA was isolated from whole or lineage-depleted (Linneg) BM or FL, maturing Ter119+ FL erythroblasts, and progenitor stage (E8.5) yolk sac EryP1,32,35 and analyzed using quantitative reverse transcription polymerase chain reaction. Vdr mRNA was detected in both whole and Linneg BM and FL, but not in maturing Ter119+ FL erythroblasts or in EryP (Figure 1A). In our earlier microarray analysis of developing primitive erythroid cells, Vdr was also not expressed in EryP progenitors (E7.5-8.5) or erythroblasts (E9.5-12.5).1
Figure 1.
Activation of Vdr signaling stimulates the growth of EryD progenitors in fetal liver and bone marrow. (A) Real-time reverse transcription polymerase chain reaction (RT-PCR) analysis of RNA (10 ng) isolated from cells from BM (whole or Linneg), FL (whole, Linneg, CD71+Ter119+), or E8.5 yolk sac (GFP+ EryP1). Expression was normalized to Ubb. (B) E12.5, E13.5, and E14.5 Linneg FL cells were cultured in methylcellulose (3.3 × 104 cells/mL) in 1 mL total volume (35-mm dish), with or without calcitriol. The number of colonies was increased by treatment with calcitriol (photos of E12.5 Linneg FL BFU-E colonies on the right; scale bars, 100 μm). (For data in histogram, n = 3.) (C) E12.5 Linneg FL cells cultured in methylcellulose (1.6 × 103 cells/mL, 1 mL in 35-mm dish), with or without calcitriol. CFU-E colonies were scored after 2 or 3 days (n = 3). (D) Linneg BM (female) cells cultured in methylcellulose under conditions (see panels B and C) that support the growth of BFU-E or CFU-E, BFU-E (n = 2), and CFU-E (n = 5). Data were analyzed using an unpaired Student t test (**P < .01, B; *P < .05, C-D; ***P < .001, D). Biological replicates are represented. Error bars, ± standard error of the mean (SEM) for panels A-C and D (CFU-E) or standard deviation (SD) for panel D (BFU-E).
Expression of Vdr was higher in the progenitor-enriched Linneg population than in unfractionated tissue from BM or FL (Figure 1A). To determine whether activation of Vdr signaling influences progenitor potential, Linneg E12.5 FL cells were plated in clonogenic assays in the presence or absence of calcitriol and scored for the formation of BFU-E and CFU-E colonies. The numbers of BFU-E were increased by nearly 4-fold when cells were cultured with calcitriol (Figure 1B), suggesting that calcitriol enhances the growth and/or survival of progenitors. Comparable increases in BFU-E numbers were observed for Linneg cells isolated from E13.5 or E14.5 FL (Figure 1B). Not only the numbers of BFU-E, but also of CFU-E, were increased (by ∼40%; Figure 1 C). The response to Vdr signaling was not limited to fetal erythropoiesis, as BFU-E and CFU-E colony numbers from calcitriol-treated Linneg BM cells were also increased (Figure 1D). We have not detected differences between male and female BM cells in these assays (Figure 1B,D). The response to calcitriol stimulation was dose dependent, as shown for CFU-E from BM (supplemental Figure 1C-D).
Signaling through Vdr maintains EryD progenitors and delays their maturation
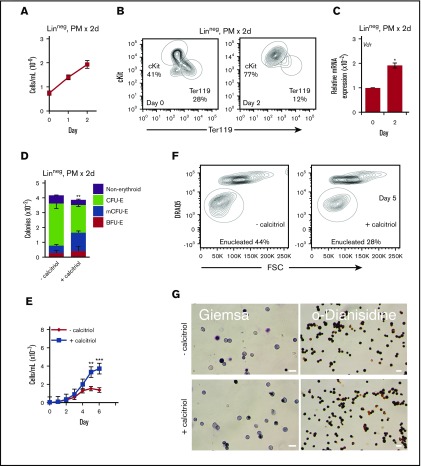
For these studies, we focused on the Linneg fraction of FL, which is the major site of definitive erythropoiesis before birth, and in which the majority of hematopoietic progenitors is committed to the erythroid lineage. To dissect the mechanisms by which calcitriol increases progenitor numbers, we next expanded progenitors in liquid medium before plating in methylcellulose. When E12.5 Linneg FL cells were cultured for 2 days under progenitor conditions (in PM), cell numbers were increased by ∼3-fold (Figure 2A). Before lineage depletion, the majority of FL cells were Ter119+ (∼70%), and a minority were ckit+ (∼10%) (data not shown). After lineage depletion, the Ter119+ cell population was decreased to ∼28% and the fraction of cKit+ cells increased to ∼40%. After 2 days, the frequency of cKit+ cells nearly doubled (to ∼77%), with a corresponding decrease in the frequency of Ter119+ cells (to ∼12%; Figure 2B). Consistent with the increase in the numbers of cKit+ cells, a ∼2-fold increase in Vdr transcription was observed (Figure 2C). To examine the developmental potential of cells cultured in PM, equal numbers of Linneg cells were transferred (after 48 hours in PM) to methylcellulose in the presence or absence of calcitriol. Colony numbers were scored after 8 days. Three types of erythroid colonies were identified: BFU-E, CFU-E, and multi-CFU-E (mCFU-E or late BFU-E, cluster of 3-20 CFU-E; supplemental Figure 2A). The distribution of these colony types, but not their total numbers, was altered when Vdr signaling was activated (Figure 2D), with increased numbers of earlier progenitors (BFU-E and mCFU-E) and decreased numbers of the later progenitors (CFU-E). These findings indicate that culture in PM maintains cKit+ colony-forming erythroid progenitors that can respond to activation of Vdr by calcitriol.
Figure 2.
Activation of Vdr delays maturation of EryD progenitors. (A) Proliferation of E12.5 Linneg FL cells cultured for 2 days under progenitor conditions (in PM; n = 3). (B) Representative flow cytometry analysis of cKit and Ter119 expression for E12.5 FL Linneg cells after 2 days of culture in PM (n = 3). (In contrast with the starting population of FL cells, the Ter119+ cells that remained after lineage depletion showed only very weak Ter119 fluorescence.) (C) Real-time RT-PCR analysis of Vdr RNA (10 ng) from E12.5 FL Linneg cells after 2 days of culture in PM. Expression was normalized to Ubb. (D) Distribution of colonies formed from Linneg progenitors cultured for 2 days in PM and then transferred to methylcellulose (2.0 × 103 cells in 1 mL, 35-mm dish) with or without calcitriol. The distribution of these colony types, but not their total numbers, was altered when Vdr signaling was activated, with increased numbers of earlier progenitors (BFU-E + mCFU-E, 40% vs 18%) and decreased numbers of the later progenitors (CFU-E, 45% vs 69%) (n = 3). (E) Proliferation of E12.5 Linneg cells cultured for 2 days in PM and then transferred to MM with or without calcitriol (n = 5). (F) Representative flow cytometry plots of DRAQ5 staining vs FSC of Ter119+ cells after 5 days of culture in MM, with or without calcitriol. (G) Wright-Giemsa or o-dianisidine staining of cytospun cells from day 6 of culture in MM. Scale bars, 20 μm. Data were analyzed using a 2-way analysis of variance (analysis of variance [ANOVA], E) or an unpaired Student t test (B-D,F; *P < .05, C; **P < .01, D-E; ***P < .001, E). Error bars, ± SEM (n = 3).
When progenitors were cultured in suspension under maturation conditions (in MM) and allowed to mature, cell proliferation continued for 6 days, with a ∼60-fold increase in cell number (supplemental Figure 2B), corresponding to ∼5 cell divisions. In the presence of calcitriol, cell numbers increased by 160- to 180-fold (Figure 2E), suggesting that 1 to 2 additional cell divisions took place during the 6-day period. The increase in cell numbers was dose-dependent (supplemental Figure 3). As expected for maturing cells, the numbers of cKit+ and Ter119+ cells decreased and increased, respectively (supplemental Figure 2C). Cell and nuclear size as well as surface expression of CD44 all decreased as the cells progressed to enucleate (supplemental Figure 2D-G). A maturation delay was observed during the first 4 days of culture in the presence of calcitriol, with lower numbers of Ter119+ cells, higher forward scatter (FSC) and CD44 (supplemental Figure 4A-B), and delayed enucleation (Figure 2F; supplemental Figure 4C). By days 6 to 7, staining with Wright-Giemsa and accumulation of hemoglobin protein were similar in treated and untreated cultures (Figure 2G; supplemental Figure 4D).
The CD71lo/neg population of LinnegcKit+ FL erythroid progenitors is the target of Vdr stimulation
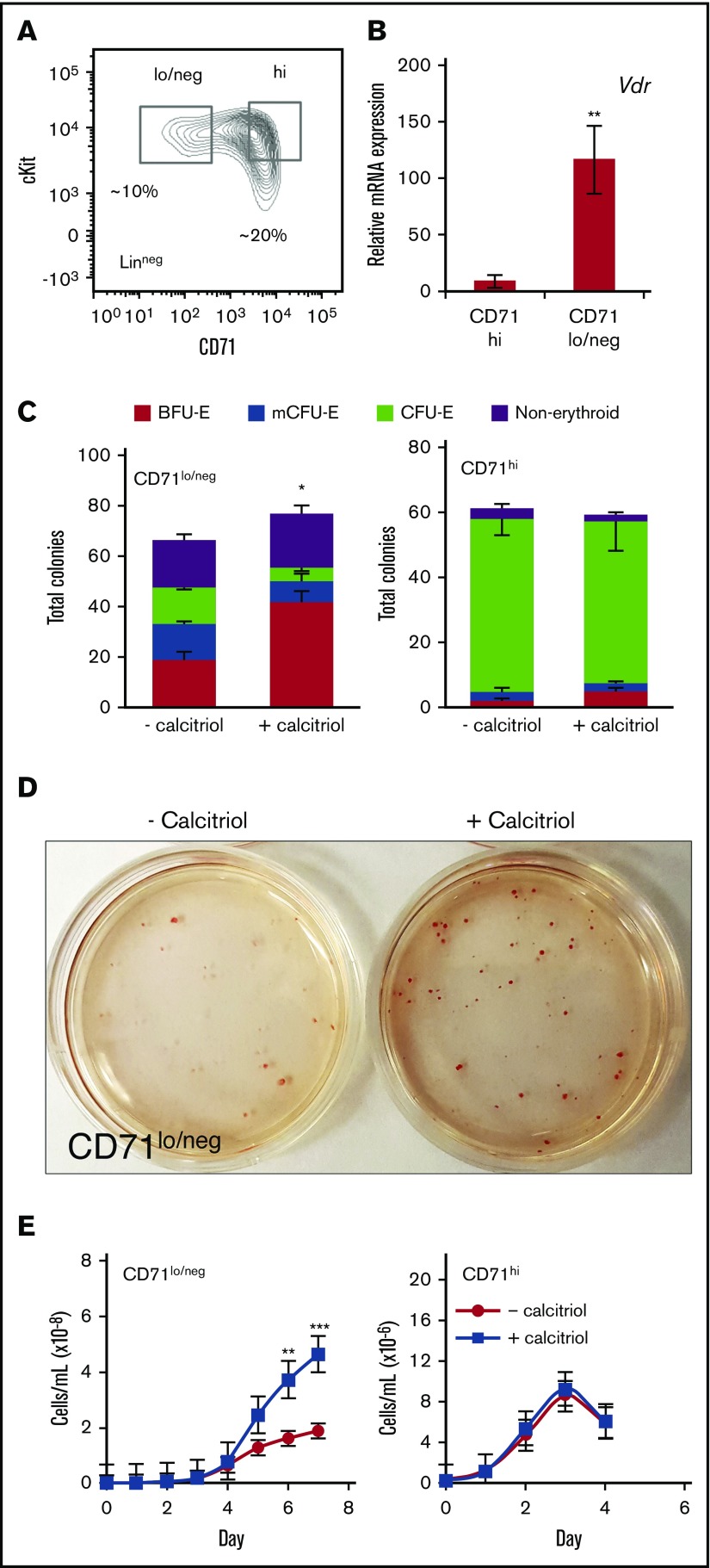
The Linneg population of FL cells contains cKit+ progenitors at different stages of maturation that can be identified prospectively on the basis of their expression of CD71 (Figure 3A); multiple types of hematopoietic progenitors, including early erythroid progenitors, are CD71lo/neg, whereas late erythroid progenitors are CD71hi.14,21,36 To determine which populations of cKit+ cells respond to activation by calcitriol, sorted cells were first analyzed for expression of Vdr, which was significantly higher (∼13-fold) in the CD71lo/neg than in the CD71hi population (Figure 3B). For these studies, cells were plated at low density (500 cells/mL) to facilitate analysis of single-cell contribution to each colony. The CD71lo/neg cells produced BFU-E, mCFU-E, and CFU-E, whereas the CD71hi cells produced mostly CFU-E (Figure 3C). Analysis of colony size (surface area) using ImageJ did not reveal significant differences for the 2 culture conditions (supplemental Figure 5). CD71lo/neg cells, but not CD71hi cells, responded to activation of Vdr, with formation of ∼2.5-fold higher numbers of BFU-E (Figure 3C-D). The colonies were strongly pigmented, indicating production of hemoglobin (Figure 3D). When the 2 sorted cell populations were plated directly into MM, treatment with calcitriol again stimulated proliferation of CD71lo/neg, but not CD71hi, cells (Figure 3E). Similar to the CD71hi population, the ckit+CD71med population formed primarily CFU-E and did not increase proliferation in response to calcitriol (supplemental Figure 6B-C).
Figure 3.
The LinnegcKit+CD71lo/negpopulation of FL erythroid progenitors is the target of signaling through Vdr. (A) Representative flow cytometry plot showing the gating of E12.5 Linneg cKit+ FL cells based on CD71 expression. (B) Real-time RT-PCR analysis of Vdr RNA (10 ng) expression in ckit+CD71lo/neg and ckit+ CD71hi populations. Expression was normalized to Ubb (n = 3). (C) Distribution of colonies formed from ckit+CD71lo/neg and ckit+ CD71hi cells. Cells were sorted and cultured in methylcellulose (125 cells in 250 μL, 24-well dish) with or without calcitriol (n = 3). CD71lo/neg, but not CD71hi, cells responded to activation of Vdr (BFU-E + mCFU-E + calcitriol, 66% vs 49% − calcitriol). CD71hi cells produced comparable numbers of CFU-E in the presence or absence of calcitriol (89% vs 85%, respectively). (D) Photograph of plates of colonies formed from ckit+CD71lo/neg cells cultured in methylcellulose for 8 days. (E) Proliferation of ckit+CD71lo/neg or CD71hi cells cultured in MM with or without calcitriol. Stimulation of Vdr signaling resulted in a ∼2- to 3-fold increase in CD71lo/neg cell proliferation (n = 5). Data were analyzed using an unpaired Student t test (B-C) or 2-way ANOVA (E; *P < .05, C; **P < .01, B,E; ***P < .001, E). Error bars, ± SEM.
As observed for unfractionated Linneg cells, cKit expression in the CD71lo/neg population was prolonged, and fewer Ter119+ erythroblasts formed in response to calcitriol (supplemental Figure 7A). By day 5, cKit expression was lost and the majority of cells were Ter119+ (not shown). However, these Ter119+ cells displayed higher FSC and CD44 expression and less enucleation than the untreated cells, suggesting that they were less mature (supplemental Figure 7B-C).
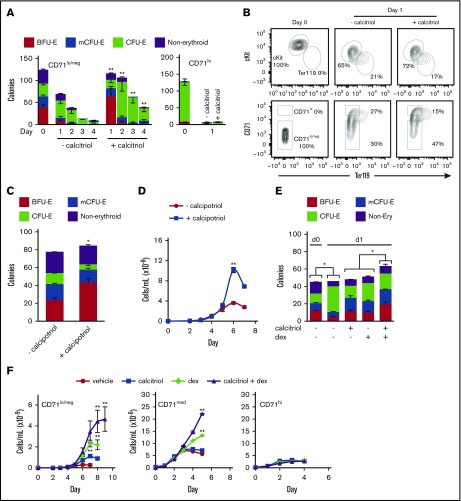
To determine the period over which Vdr signaling can maintain progenitor potential, we cultured cKit+CD71lo/neg and ckit+CD71hi populations in MM in the presence or absence of calcitriol and transferred the cells to methylcellulose cultures after 1, 2, 3, or 4 days (Figure 4A). Both cKit+CD71lo/neg-derived early and late progenitors (Figure 4A) and their surface phenotypes (Figure 4B) were maintained for a longer time and in greater numbers in response to activation of Vdr. CD71hi progenitor potential was lost after 1 day in the presence or absence of calcitriol (Figure 4A; supplemental Figure 7E).
Figure 4.
Vdr signaling maintains erythroid progenitor potential. (A) Colony-forming potential of ckit+CD71lo/neg or ckit+ CD71hi cells cultured under maturation conditions (in MM) with or without calcitriol and then transferred to methylcellulose (250 cells in 250 μL, 24-well dish) on the indicated days (n = 3). (B) Representative flow cytometry analysis of the expression of cKit or CD71 vs Ter119 for ckit+CD71lo/neg cells after 1 day in culture, with or without calcitriol (n = 3). (C) Distribution of colonies formed from ckit+CD71lo/neg cells (125 cells in 250 μL, 24-well dish) cultured in methylcellulose with or without calcipotriol (100 nM), a low calcemic synthetic derivative of calcitriol that is <0.5% to 1% as active as calcitriol in regulating calcium metabolism, because of pharmacokinetic differences38 (n = 3). (D) Proliferation of cKit+CD71lo/neg cells in MM with or without calcipotriol (n = 3). (E) Colony-forming potential of ckit+CD71lo/neg cells cultured in MM with or without dexamethasone (dex), calcitriol, or both ligands (100 nM) for 1 day and then transferred to methylcellulose (125 cells in 250 μL, 24-well dish) (n = 3). BFU-E and mCFU-E numbers were maintained in the presence of either ligand alone but the numbers increased if the 2 ligands were present together. (F) Proliferation of ckit+CD71lo/neg, CD71med, or CD71hi cells cultured in MM with or without calcitriol, dex, or both ligands (100 nM) (n = 3). Data were analyzed using an unpaired Student t test (C,E) or 2-way ANOVA (A,D,F; (*P < .05, C,E; **P < .01, A,D,F). Error bars, ± SEM.
Regulation of erythroid progenitor growth by vitamin D signaling is dependent primarily on the transcriptional activity of Vdr
Calcitriol stimulates Vdr-regulated transcription as well as alterations in Ca2+ flux.37 To distinguish between these effects, the CD71lo/neg population was treated with calcipotriol, a vitamin D3 analog reported to be 100- to 200-fold less potent in its calcemic effects than calcitriol.38 Calcipotriol increased BFU-E numbers by ∼2.5-fold, with a corresponding decrease in the numbers of CFU-E, and increased cell proliferation by ∼2.8-fold (Figure 4C-D), comparable to the effects seen with calcitriol (Figure 3C,E). Therefore, calcium flux is not the primary driver of these effects. As demonstrated by the titration shown in supplemental Figure 7E, the effect of calcipotriol was dose-dependent.
Maintenance of progenitor potential is enhanced by simultaneous activation of Vdr and glucocorticoid receptor signaling
The glucocorticoid receptor (Gr), Similar to Vdr, is a member of the nuclear hormone receptor transcription factor family and has been shown to stimulate the proliferation of cKit+CD71lo/neg cells.21 To determine whether the Vdr and Gr signaling pathways can cooperate to modulate erythroid progenitor growth, cKit+CD71lo/neg cells were cultured with or without calcitriol, dexamethasone, or the 2 ligands in combination. After 24 hours, the cells were plated in methylcellulose to evaluate progenitor potential. In the absence of Vdr or Gr ligand, the numbers of BFU-E and mCFU-E decreased as the numbers of CFU-E increased (Figure 4E). Colony numbers were maintained in the presence of either ligand alone (Figure 4E). When the 2 ligands were present together, the numbers of BFU-E and mCFU-E increased (Figure 4E), suggesting that they function cooperatively.
To evaluate the effect of these steroid hormones on proliferation, cKit+CD71lo/neg cells were cultured for up to 9 days in the presence or absence of calcitriol, dexamethasone, or the 2 ligands in combination. Either ligand alone stimulated cell proliferation (calcitriol ∼3-fold; dexamethasone, ∼8-fold; Figure 4F). In combination, calcitriol and dexamethasone increased cell numbers by ∼17-fold (Figure 4F). Although CD71med cells did not respond to calcitriol alone (supplemental Figure 6B-C), their proliferation was stimulated by dexamethasone and, to an even greater extent, dexamethasone and calcitriol together (Figure 4F). Neither dexamethasone nor calcitriol stimulated proliferation of CD71hi cells (Figure 4F).
Vdr signaling maintains cycling of Linneg cKit+CD71lo/neg progenitors
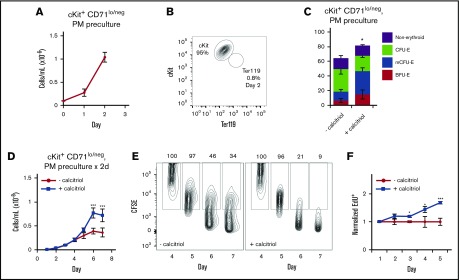
The cKit+CD71lo/neg population of progenitors represents ∼10% of FL Linneg cells (Figure 3A). To obtain sufficient numbers of cells for more detailed analyses, we precultured the cells in PM for 2 days in the absence of calcitriol, resulting in a ∼10-fold increase in cell numbers (Figure 5A). The cells remained almost entirely cKit+ and Ter119neg (Figure 5B); the majority of colony-forming cells were erythroid progenitors (Figure 5C). When precultured cells were then plated in colony assays in the presence or absence of calcitriol, the numbers of early progenitors (BFU-E and mCFU-E) increased (57% vs 28%), and numbers of late progenitors (CFU-E) decreased (48% vs 27%; Figure 5C). These results strongly suggest that calcitriol maintains early progenitors at the expense of late progenitors.
Figure 5.
Vdr signaling maintains cycling of cKit+CD71lo/negcells. (A) Proliferation of cKit+CD71lo/neg cells cultured under progenitor conditions (in PM) for 2 days (n = 3). (B) Representative flow cytometry analysis of cKit and Ter119 expression on cKit+CD71lo/neg cells after 2 days of culture in PM. (C) Distribution of colonies formed from cKit+CD71lo/neg cells after 2 days of culture in methylcellulose (250 cells in 250 μL, 24-well dish), with or without calcitriol (n = 3). The cells were plated at low density to ensure that the colonies would be well separated and that scoring would be accurate. (D) Proliferation of cKit+CD71lo/neg cells cultured for 2 days in PM then transferred to MM with or without calcitriol (n = 5). (E) CD71lo/neg cells on day 4 of culture in MM were labeled with carboxyfluorescein diacetate succinimidyl ester and fluorescence measured on the indicated days. Percentages are shown above each gate. (F) Flow cytometry analysis of EdU incorporation in CD71lo/neg cells cultured in MM on the indicated days (n = 3). Data were analyzed using an unpaired Student t test (C) or a 2-way ANOVA (D-F; *P < .05, C,F; ***P < .001 D,F). Error bars, ± SEM.
When CD71lo/neg cells were cultured in suspension in MM, calcitriol treatment resulted in increased cell numbers (Figure 5D), cell divisions (dilution of carboxyfluorescein diacetate succinimidyl ester; Figure 5E), and DNA synthesis (incorporation of EdU; Figure 5F). In addition, a transient increase in the numbers of cells in S phase and a decrease in the numbers of cells in G0/G1 were observed (days 2-5; supplemental Figure 8). Consistent with these changes, erythroid maturation was delayed, as evidenced by levels of expression of cKit, Ter119, and CD44; cell size (FSC); and frequency of enucleation (supplemental Figure 9A-C). Activation of Vdr signaling did not appear to influence cell survival, as we did not observe significant changes in expression of annexin V or activated caspase 3/7 (not shown). As expected, calcipotriol and dexamethasone (supplemental Figure 9D-E) each increased the proliferation of CD71lo/neg cells. Cells precultured in PM before transfer to MM proliferated more when exposed to both calcitriol and dexamethasone (∼7-fold) than when cultured with either ligand alone (∼2.3, calcitriol; ∼3.2, dexamethasone), as observed for cells that were not precultured (Figure 4F).
Changes in gene expression after activation of Vdr
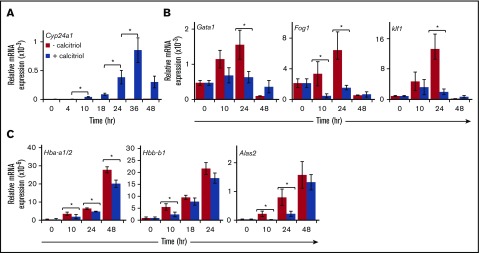
The Vdr transcription factor regulates gene expression in cells in response to binding of vitamin D3 agonists. Cyp24a1 (1,25-dihydroxyvitamin D3 24-hydroxylase), a well-characterized direct target of Vdr,39 was strongly activated in erythroid progenitors cultured in the presence of calcitriol, but was undetectable in its absence (Figure 6A). In contrast, activation of Vdr by calcitriol blocked the upregulation of erythroid transcription factor genes Gata1, Fog1, and Klf1 (Figure 6B) and the erythroid genes Hbb-a1/2, Hbb-b1, and Alas2 (Figure 6C). To our surprise, the expression of genes associated with erythroid progenitors and known to be downregulated during maturation (Gata2, Hopx, and Gcr)21,22 was not affected after activation of Vdr. Expression of these genes decreased to nearly undetectable levels within 24 hours, irrespective of the presence of calcitriol (data not shown).
Figure 6.
Gene expression changes in response to activation of Vdr. Linneg cKit+CD71lo/neg cells were cultured for 2 days under progenitor conditions (PM) and then transferred to maturation conditions (MM) with or without calcitriol. Total RNA was isolated at the indicated times and analyzed (3.3 ng), using real-time RT-PCR. Expression was normalized to Ubb. (A) Expression of the Vdr target gene Cyp24a1. (B) Expression of the erythroid transcription factor genes Gata1, Fog1, and Klf1. (C) Expression of α- and β-like globins and Alas2. n > 4 experiments. Data were analyzed using a 2-way ANOVA (*P < .05). Error bars, ± SEM.
Vdr is required for stimulation of erythroid cell proliferation by calcitriol
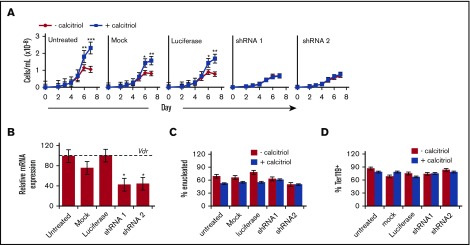
To determine whether the stimulation of erythroid cell proliferation is dependent on Vdr, we used a lentivirus shRNA-mediated knockdown approach. LinnegcKit+CD71lo/neg cells were plated in PM and transduced with shRNA lentiviruses targeting Vdr (shRNA1 or shRNA2) or with a luciferase shRNA virus and were then allowed to mature by culture in MM, in the presence or absence of calcitriol. Mock transduced and untreated cells served as additional controls. Knockdown of Vdr using shRNA1 or shRNA2 abrogated the calcitriol-mediated increase in cell numbers seen in the controls (Figure 7A). Vdr expression in shRNA1 or shRNA2 lentivirus-transduced cells was reduced to ∼40% of its level in the controls (Figure 7B). Consistent with these findings, Vdr knockdown reversed the delay in maturation in response to calcitriol, as evidenced by control levels of enucleation (Figure 7C) and surface expression of Ter119 (Figure 7D). No differences were observed in cell numbers or in the frequencies of cKit+ cells among the various conditions (supplemental Figure 10A-B).
Figure 7.
Stimulation of erythroid progenitor growth by calcitriol is dependent on expression of Vdr. Linneg cKit+CD71lo/neg cells were cultured for 2 days under progenitor conditions (PM) with shRNA lentiviruses targeting Vdr (shRNA1 and shRNA2) or luciferase. Mock treated and untreated cells were included as additional controls. (A) Proliferation of cKit+CD71lo/neg cells cultured in MM with or without calcitriol (n = 3). (B) Analysis of Vdr expression in Linneg cKit+CD71lo/neg cells after 2 days of culture in PM. Vdr expression levels were normalized to Ubb and are presented as percentage expression compared with the luciferase shRNA control (dotted line, 100%). (C-D) Frequency of enucleated (C) and Ter119+ (D) cells after culture for 7 days in MM. Data were analyzed using an unpaired Student t test (B-D) or using a 2-way ANOVA (A; *P < .05, A-B; **P < .01, A; ***P < .001, A). Error bars, ± SEM (n = 3).
Discussion
The mechanisms underlying the development and growth of early erythroid progenitors are poorly understood. Studies in vitro and in vivo have demonstrated that several steroid hormone ligand-activated receptors influence the decision between expansion (glucocorticoid and androgen receptors)22,40-43 and terminal differentiation (thyroid hormone receptor)44,45 of erythroid progenitors. VDR is a transcription factor that is activated by binding to its steroid hormone ligand, vitamin D3. The VDR signaling pathway has been largely unexplored in the erythroid lineage, and the targets of signaling by VDR in erythroid progenitors are unknown. Here, we define a function for vitamin D signaling through VDR in EryD (but not EryP) progenitors. Vdr is not expressed in primitive erythroid progenitors (E7.5-8.5) or erythroblasts (E9.5-12.5; Isern et al1 and this report), and therefore, these cells would not be expected to respond to vitamin D agonists.
Vdr signaling stimulates the growth of erythroid progenitors
Erythroid progenitors are known to respond to a variety of stimuli in vitro and in vivo (reviewed in Hattangadi et al10 and Paulson et al19). We have found that activation of the murine Vdr pathway by vitamin D3 agonists increases the numbers of BFU-E and CFU-E progenitors from fetal liver and adult bone marrow and delays their maturation. Vdr signaling is active in early erythroid progenitors in FL (E12.5-E14.5) and adult BM, but not in maturing erythroid cells. The expansion of erythroid progenitors results, ultimately, in increased numbers of mature erythroid cells.
We note that definitive erythro-myeloid progenitors are present in the FL at E11.5-12.5; however, their numbers decline thereafter, as hematopoietic stem cell-derived erythroid progenitors expand and differentiate.3 The increase in the numbers of BFU-E colonies in response to calcitriol is essentially the same for Linneg cells from E12.5, E13.5, or E14.5 FL. Although some of the responding cells at E12.5 might be erythro-myeloid progenitors, our data clearly indicate that the effect of Vdr activation by calcitriol is not limited to a stage of development when erythro-myeloid progenitors are present. Indeed, erythroid progenitors from adult bone marrow also respond to calcitriol.
Early erythroid progenitors are targets of Vdr signaling
The Linneg population of hematopoietic cells in FL and BM contains early and late cKit+ erythroid progenitors that can be further fractionated on the basis of their expression of CD71.14,15,21,36 In the discussion that follows, we refer to populations based on their immunophenotype and avoid the terms BFU-E and CFU-E.
The CD71lo/neg progenitors (here termed early progenitors) are a heterogeneous population, forming nonerythroid colonies, BFU-E, multi-CFU-E, and CFU-E colonies, whereas CD71hi (late progenitors) form primarily CFU-E colonies (Figure 3C). The primary target of Vdr signaling in fetal liver is the cKit+CD71lo/neg population (early progenitors), but not the cKit+CD71med or cKit+CD71hi populations (late progenitors). In contrast, not only the cKit+CD71lo/neg but also the cKit+CD71med population responds to glucocorticoid signaling (this report and Flygare et al21 and Zhang et al22). Therefore, the vitamin D pathway functions in a more restricted set of progenitors than does the glucocorticoid pathway. It is important to note that we have evaluated not only changes in immunophenotype and cell numbers in response to calcitriol or dexamethasone but also functional changes in progenitor potential (using colony assays). Although the morphologies of the colonies we scored as CFU-E from any of the 3 CD71 populations were indistinguishable, the differences among these populations in their response to calcitriol vs glucocorticoids strongly suggest there must be functional differences among the target cells.
Agonists of the glucocorticoid and vitamin D receptor pathways function together to increase erythroid cell numbers
Treatment of erythroid progenitors from FL with either calcitriol/calcipotriol or dexamethasone increases the numbers of early erythroid progenitors and stimulates cell proliferation during maturation. Therefore, vitamin D agonists may partially substitute for the function of glucocorticoids on early erythroid progenitors. When these steroid hormones are combined, the observed increases are at least additive, and perhaps synergistic. Moreover, although calcitriol alone does not enhance the proliferation of cKit+CD71med cells, its combination with dexamethasone does result in increased cell proliferation, perhaps in part through upregulation of Vdr by Gr.46,47 The cooperative effects of Vdr and Gr observed in our experiments are reminiscent of the reported synergy between dexamethasone and Ppar-α agonists for CD71lo/neg progenitors.47 We have shown that VDR agonists enhance the proliferation of cKit+CD71lo/neg cells. It is possible that Vdr and Gr have unique functions within certain cell subsets and synergistic functions in other subsets within the cKit+CD71lo/neg population.
Transcriptional regulation by Vdr in erythroid progenitors
Vdr and vitamin D3 have both interdependent and independent functions.48-50 Our shRNA knockdown studies revealed that Vdr is necessary for the proliferative response to vitamin D3. Unliganded Vdr is apparently not necessary for the growth of erythroid progenitors, as indicated by the absence of an effect of partial (∼60%) Vdr knockdown in the absence of vitamin D3. However, it is possible that complete Vdr knockdown would reveal a requirement for unliganded Vdr. In any case, these findings point to an erythroid cell-autonomous function for Vdr in response to activation by calcitriol.
Calcipotriol, a vitamin D3 analog with far less potent calcemic effects than calcitriol,37 induces the same changes in the growth of early erythroid progenitors as calcitriol. Therefore, these changes are likely a result of Vdr-regulated transcription, and not to alterations in Ca2+ flux. Whether these genes are direct or indirect targets of Vdr remains to be determined. Although vitamin D response elements can be identified within or at various distances from genes encoding erythroid regulators such as Gata1 or Klf1 (not shown), it is not yet known which of these elements function in erythroid progenitors.
Both the glucocorticoid receptor (Gr) and Vdr are steroid hormone receptors. The regulation of erythroid progenitors by glucocorticoids such as dexamethasone has been well documented in the literature (eg, see Flygare et al,21 Zhang et al,22 and von Lindern et al51). Although DNA-binding mutant Gr mice are not anemic, an erythroid defect was uncovered in response to hypoxic stress.41 As observed for Gr, Vdr is not essential for steady-state erythropoiesis.52-54 Given the similarity in response of erythroid progenitors to glucocorticoids and to vitamin D agonists, and the additive effects of these steroid hormones, it seems likely that Vdr, similar to Gr, plays a role in stress erythropoiesis. It is worth noting that, as stress erythroid progenitors have unique properties, many of the genes involved in the erythroid recovery from stress have little, if any, effect on steady-state erythropoiesis.55,56
Although glucocorticoids are well known to stimulate erythropoiesis, their use in the treatment of anemia is associated with severe adverse effects.57-59 Several studies have reported an association between vitamin D deficiency and anemia (reviewed in Smith and Tangpricha60) and have established that vitamin D3 improves anemia in chronic renal failure and other types of anemia.29,60-62 Vitamin D deficiency is also associated with and is a predictor of anemia in end-stage heart failure.63 Although the results from the present study suggest that vitamin D agonists stimulate erythropoiesis by targeting progenitors, expression of genes known to regulate erythroid progenitors (Gata2, Zfp36l2, Bmi1, and Hopx) was not affected by Vdr signaling. Therefore, other genes must be involved in the Vdr signaling pathway in erythroid progenitors.
In conclusion, these findings support a model in which vitamin D signaling through the Vdr transcription factor stimulates the growth of early erythroid progenitors (see cartoon, supplemental Figure 10). A better understanding of how the growth of erythroid progenitors and the switch from self-renewal to differentiation are controlled might facilitate the development of novel erythropoiesis-stimulating agents)64,65 with fewer adverse effects. Moreover, combined activation of distinct pathways within progenitors might be achieved at lower concentrations of their agonists to achieve maximum therapeutic benefit, and might also be of value for the development of more efficient systems for the generation ex vivo of RBCs (or their progenitors) for transfusion.66,67
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the Mount Sinai Flow Cytometry Shared Resource Facility for assistance with cell sorting. The authors also thank James Bieker, Nithya Gnanapragasam, Diane Krause, and Merav Socolovsky for helpful discussions.
This work was supported by grants from the National Institutes of Health (R01 HL62248 from the National Heart, Lung, and Blood Institute and DK52191 from the National Institute of Diabetes and Digestive and Kidney Diseases) (M.H.B.) and in part by a training grant from the National Institutes of Health, National Heart, Lung, and Blood Institute (T32 HL094283) (J.B.).
Authorship
Contribution: J.B. and M.H.B. designed the experiments, wrote the manuscript, and prepared the figures; J.B. performed most of the experiments; and B.M.R., A.E., and A.N.L. assisted with some of the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Margaret H. Baron, Icahn School of Medicine at Mount Sinai, Box 1079, 1468 Madison Ave, Annenberg 24-04E, New York, NY 10029-6574; e-mail: margaret.baron@mssm.edu.
References
- 1.Isern J, He Z, Fraser ST, et al. Single-lineage transcriptome analysis reveals key regulatory pathways in primitive erythroid progenitors in the mouse embryo. Blood. 2011;117(18):4924-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron MH, Isern J, Fraser ST. The embryonic origins of erythropoiesis in mammals. Blood. 2012;119(21):4828-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGrath KE, Frame JM, Fegan KH, et al. Distinct Sources of Hematopoietic Progenitors Emerge before HSCs and Provide Functional Blood Cells in the Mammalian Embryo. Cell Reports. 2015;11(12):1892-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1(4):291-301. [DOI] [PubMed] [Google Scholar]
- 5.Godin I, Garcia-Porrero JA, Dieterlen-Lièvre F, Cumano A. Stem cell emergence and hemopoietic activity are incompatible in mouse intraembryonic sites. J Exp Med. 1999;190(1):43-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser ST, Isern J, Baron MH. Maturation and enucleation of primitive erythroblasts during mouse embryogenesis is accompanied by changes in cell-surface antigen expression. Blood. 2007;109(1):343-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dzierzak E, Philipsen S. Erythropoiesis: development and differentiation. Cold Spring Harb Perspect Med. 2013;3(4):a011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barminko J, Reinholt B, Baron MH. Development and differentiation of the erythroid lineage in mammals. Dev Comp Immunol. 2016;58:18-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palis J. Primitive and definitive erythropoiesis in mammals. Front Physiol. 2014;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hattangadi SM, Wong P, Zhang L, Flygare J, Lodish HF. From stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood. 2011;118(24):6258-6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen K, Liu J, Heck S, Chasis JA, An X, Mohandas N. Resolving the distinct stages in erythroid differentiation based on dynamic changes in membrane protein expression during erythropoiesis. Proc Natl Acad Sci USA. 2009;106(41):17413-17418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ney PA. Normal and disordered reticulocyte maturation. Curr Opin Hematol. 2011;18(3):152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang YA, Sanalkumar R, O’Geen H, et al. Autophagy driven by a master regulator of hematopoiesis. Mol Cell Biol. 2012;32(1):226-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Socolovsky M, Gross AW, Lodish HF. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood. 2003;102(12):3938-3946. [DOI] [PubMed] [Google Scholar]
- 15.Socolovsky M, Nam H, Fleming MD, Haase VH, Brugnara C, Lodish HF. Ineffective erythropoiesis in Stat5a(-/-)5b(-/-) mice due to decreased survival of early erythroblasts. Blood. 2001;98(12):3261-3273. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Zhang J, Ginzburg Y, et al. Quantitative analysis of murine terminal erythroid differentiation in vivo: novel method to study normal and disordered erythropoiesis. Blood. 2013;121(8):e43-e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jelkmann W. Regulation of erythropoietin production. J Physiol. 2011;589(Pt 6):1251-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bunn HF. Erythropoietin. Cold Spring Harb Perspect Med. 2013;3(3):a011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulson RF, Shi L, Wu DC. Stress erythropoiesis: new signals and new stress progenitor cells. Curr Opin Hematol. 2011;18(3):139-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Socolovsky M. Molecular insights into stress erythropoiesis. Curr Opin Hematol. 2007;14(3):215-224. [DOI] [PubMed] [Google Scholar]
- 21.Flygare J, Rayon Estrada V, Shin C, Gupta S, Lodish HF. HIF1alpha synergizes with glucocorticoids to promote BFU-E progenitor self-renewal. Blood. 2011;117(12):3435-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Prak L, Rayon-Estrada V, et al. ZFP36L2 is required for self-renewal of early burst-forming unit erythroid progenitors. Nature. 2013;499(7456):92-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin ER. Extranuclear steroid receptors are essential for steroid hormone actions. Annu Rev Med. 2015;66(1):271-280. [DOI] [PubMed] [Google Scholar]
- 24.Pike JW, Meyer MB. Fundamentals of vitamin D hormone-regulated gene expression. J Steroid Biochem Mol Biol. 2014;144(Pt A):5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29(6):726-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore DC, Carter DL, Bhandal AK, Studzinski GP. Inhibition by 1,25 dihydroxyvitamin D3 of chemically induced erythroid differentiation of K562 leukemia cells. Blood. 1991;77(7):1452-1461. [PubMed] [Google Scholar]
- 27.Waki M, Inaba M, Hiura Y, et al. Modulation by cAMP of 1alpha,25-dihydroxyvitamin D3 sensitivity of murine erythroleukemia cells. Arch Biochem Biophys. 2001;391(2):265-270. [DOI] [PubMed] [Google Scholar]
- 28.Alon DB, Chaimovitz C, Dvilansky A, et al. Novel role of 1,25(OH)(2)D(3) in induction of erythroid progenitor cell proliferation. Exp Hematol. 2002;30(5):403-409. [DOI] [PubMed] [Google Scholar]
- 29.Aucella F, Scalzulli RP, Gatta G, Vigilante M, Carella AM, Stallone C. Calcitriol increases burst-forming unit-erythroid proliferation in chronic renal failure. A synergistic effect with r-HuEpo. Nephron Clin Pract. 2003;95(4):c121-c127. [DOI] [PubMed] [Google Scholar]
- 30.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10(4):482-496. [DOI] [PubMed] [Google Scholar]
- 31.Baron MH, ed. Developmental Hematopoiesis: Methods and Protocols, vol. 105 Totowa, NJ: Humana Press; 2005 [Google Scholar]
- 32.Isern J, Fraser ST, He Z, Baron MH. The fetal liver is a niche for maturation of primitive erythroid cells. Proc Natl Acad Sci USA. 2008;105(18):6662-6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vacaru AM, Vitale J, Nieves J, Baron MH. Generation of transgenic mouse fluorescent reporter lines for studying hematopoietic development. Methods Mol Biol. 2014;1194:289-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.England SJ, McGrath KE, Frame JM, Palis J. Immature erythroblasts with extensive ex vivo self-renewal capacity emerge from the early mammalian fetus. Blood. 2011;117(9):2708-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fraser ST, Isern J, Baron MH. The use of transgenic fluorescent reporter mouse lines to monitor hematopoietic and erythroid development during embryogenesis. Methods Enzymol. 2010;476:403-427. [DOI] [PubMed] [Google Scholar]
- 36.Terszowski G, Waskow C, Conradt P, et al. Prospective isolation and global gene expression analysis of the erythrocyte colony-forming unit (CFU-E). Blood. 2005;105(5):1937-1945. [DOI] [PubMed] [Google Scholar]
- 37.Dimitrov V, Salehi-Tabar R, An BS, White JH. Non-classical mechanisms of transcriptional regulation by the vitamin D receptor: insights into calcium homeostasis, immune system regulation and cancer chemoprevention. J Steroid Biochem Mol Biol. 2014;144(Pt A):74-80. [DOI] [PubMed] [Google Scholar]
- 38.Kragballe K. Calcipotriol: a new drug for topical psoriasis treatment. Pharmacol Toxicol. 1995;77(4):241-246. [DOI] [PubMed] [Google Scholar]
- 39.Gocek E, Baurska H, Marchwicka A, Marcinkowska E. Regulation of Leukemic Cell Differentiation through the Vitamin D Receptor at the Levels of Intracellular Signal Transduction, Gene Transcription, and Protein Trafficking and Stability. Leuk Res Treatment. 2012;2012:713243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolbus A, Blázquez-Domingo M, Carotta S, et al. Cooperative signaling between cytokine receptors and the glucocorticoid receptor in the expansion of erythroid progenitors: molecular analysis by expression profiling. Blood. 2003;102(9):3136-3146. [DOI] [PubMed] [Google Scholar]
- 41.Bauer A, Tronche F, Wessely O, et al. The glucocorticoid receptor is required for stress erythropoiesis. Genes Dev. 1999;13(22):2996-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leberbauer C, Boulmé F, Unfried G, Huber J, Beug H, Müllner EW. Different steroids co-regulate long-term expansion versus terminal differentiation in primary human erythroid progenitors. Blood. 2005;105(1):85-94. [DOI] [PubMed] [Google Scholar]
- 43.Hwang Y, Futran M, Hidalgo D, et al. Global increase in replication fork speed during a p57KIP2-regulated erythroid cell fate switch. Sci Adv. 2017;3(5):e1700298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kendrick TS, Payne CJ, Epis MR, et al. Erythroid defects in TRalpha-/- mice. Blood. 2008;111(6):3245-3248. [DOI] [PubMed] [Google Scholar]
- 45.Bauer A, Mikulits W, Lagger G, Stengl G, Brosch G, Beug H. The thyroid hormone receptor functions as a ligand-operated developmental switch between proliferation and differentiation of erythroid progenitors. EMBO J. 1998;17(15):4291-4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hidalgo AA, Deeb KK, Pike JW, Johnson CS, Trump DL. Dexamethasone enhances 1alpha,25-dihydroxyvitamin D3 effects by increasing vitamin D receptor transcription. J Biol Chem. 2011;286(42):36228-36237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee HY, Gao X, Barrasa MI, et al. PPAR-α and glucocorticoid receptor synergize to promote erythroid progenitor self-renewal. Nature. 2015;522(7557):474-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willems HM, van den Heuvel EG, Carmeliet G, Schaafsma A, Klein-Nulend J, Bakker AD. VDR dependent and independent effects of 1,25-dihydroxyvitamin D3 on nitric oxide production by osteoblasts. Steroids. 2012;77(1-2):126-131. [DOI] [PubMed] [Google Scholar]
- 49.Dowd DR, MacDonald PN. The 1,25-dihydroxyvitamin D3-independent actions of the vitamin D receptor in skin. J Steroid Biochem Mol Biol. 2010;121(1-2):317-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Engelhard A, Bauer RC, Casta A, Djabali K, Christiano AM. Ligand-independent regulation of the hairless promoter by vitamin D receptor. Photochem Photobiol. 2008;84(2):515-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Lindern M, Zauner W, Mellitzer G, et al. The glucocorticoid receptor cooperates with the erythropoietin receptor and c-Kit to enhance and sustain proliferation of erythroid progenitors in vitro. Blood. 1999;94(2):550-559. [PubMed] [Google Scholar]
- 52.Li YC, Pirro AE, Amling M, et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94(18):9831-9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakai Y, Kishimoto J, Demay MB. Metabolic and cellular analysis of alopecia in vitamin D receptor knockout mice. J Clin Invest. 2001;107(8):961-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Kelly J, Hisatake J, Hisatake Y, Bishop J, Norman A, Koeffler HP. Normal myelopoiesis but abnormal T lymphocyte responses in vitamin D receptor knockout mice. J Clin Invest. 2002;109(8):1091-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lenox LE, Perry JM, Paulson RF. BMP4 and Madh5 regulate the erythroid response to acute anemia. Blood. 2005;105(7):2741-2748. [DOI] [PubMed] [Google Scholar]
- 56.Bennett LF, Liao C, Paulson RF. Stress erythropoiesis model systems. Methods Mol Biol. 2018;1698:91-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96(1):23-43. [DOI] [PubMed] [Google Scholar]
- 58.Zatelli MC, Ambrosio MR, Bondanelli M, Degli Uberti E. Pituitary side effects of old and new drugs. J Endocrinol Invest. 2014;37(10):917-923. [DOI] [PubMed] [Google Scholar]
- 59.Ericson-Neilsen W, Kaye AD. Steroids: pharmacology, complications, and practice delivery issues. Ochsner J. 2014;14(2):203-207. [PMC free article] [PubMed] [Google Scholar]
- 60.Smith EM, Tangpricha V. Vitamin D and anemia: insights into an emerging association. Curr Opin Endocrinol Diabetes Obes. 2015;22(6):432-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kiss Z, Ambrus C, Almasi C, et al. Serum 25(OH)-cholecalciferol concentration is associated with hemoglobin level and erythropoietin resistance in patients on maintenance hemodialysis. Nephron Clin Pract. 2011;117(4):c373-c378. [DOI] [PubMed] [Google Scholar]
- 62.Kumar VA, Kujubu DA, Sim JJ, Rasgon SA, Yang PS. Vitamin D supplementation and recombinant human erythropoietin utilization in vitamin D-deficient hemodialysis patients. J Nephrol. 2011;24(1):98-105. [DOI] [PubMed] [Google Scholar]
- 63.Zittermann A, Jungvogel A, Prokop S, et al. Vitamin D deficiency is an independent predictor of anemia in end-stage heart failure. Clin Res Cardiol. 2011;100(9):781-788. [DOI] [PubMed] [Google Scholar]
- 64.Bunn HF. New agents that stimulate erythropoiesis. Blood. 2007;109(3):868-873. [DOI] [PubMed] [Google Scholar]
- 65.Aapro MS. Editorial: anemia management with erythropoiesis-stimulating agents: a risk-benefit update. Oncologist. 2008;13(Suppl 3):1-3. [DOI] [PubMed] [Google Scholar]
- 66.Arnaud L, Saison C, Helias V, et al. A dominant mutation in the gene encoding the erythroid transcription factor KLF1 causes a congenital dyserythropoietic anemia. Am J Hum Genet. 2010;87(5):721-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Migliaccio AR, Whitsett C, Papayannopoulou T, Sadelain M. The potential of stem cells as an in vitro source of red blood cells for transfusion. Cell Stem Cell. 2012;10(2):115-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.