Abstract
It has been known for a long time that thyroid hormones have prominent effects on hepatic fatty acid and cholesterol synthesis and metabolism. Indeed, hypothyroidism has been associated with increased serum levels of triglycerides and cholesterol as well as non-alcoholic fatty liver disease (NAFLD). Advances in areas such as cell imaging, autophagy and metabolomics have generated a more detailed and comprehensive picture of thyroid-hormone-mediated regulation of hepatic lipid metabolism at the molecular level. In this Review, we describe and summarize the key features of direct thyroid hormone regulation of lipogenesis, fatty acid β-oxidation, cholesterol synthesis and the reverse cholesterol transport pathway in normal and altered thyroid hormone states. Thyroid hormone mediates these effects at the transcriptional and post-translational levels and via autophagy. Given these potentially beneficial effects on lipid metabolism, it is possible that thyroid hormone analogues and/or mimetics might be useful for the treatment of metabolic diseases involving the liver, such as hypercholesterolaemia and NAFLD.
In mammals, thyroid hormones are critical regulators of metabolism, development and growth. Many of the metabolic activities regulated by thyroid hormones are related to the anabolism and/or catabolism of macromolecules that affect energy homeostasis during different nutritional conditions, such as proteins, lipids and carbohydrates. Indeed, it has long been appreciated that the thyroid hormones T3 and T4 have direct effects on both cholesterol and fatty acid synthesis and metabolism. Increased levels of LDL cholesterol and HDL cholesterol in the serum can be associated with hypothyroidism, whereas their levels are decreased by thyroid hormone administration and in hyperthyroidism1. Similarly, serum levels of triglycerides can be increased in hypothyroidism, and the reverse is observed in hyperthyroidism1. High-dose T3 has previously been used to promote weight loss and treat hypercholesterolaemia in patients with obesity2. Although beneficial effects were reported, serious cardiac problems and loss of lean body mass limited any further development of T3 as a therapy2.
Most of the effects of thyroid hormones on hepatic lipid homeostasis are controlled by transcriptional regulation of target genes that are involved in these homeostasis pathways. However, many of the enzymes, transporters, carrier proteins and cell-signalling proteins involved in hepatic lipid homeostasis can also be regulated by metabolite concentration, cellular energy status and post-translational modifications that occur downstream of the transcriptional effects of thyroid hormone3,4. Although much is known about lipid synthesis and metabolism at the biochemical and physiological levels, examination of cell-signalling and metabolomic changes in conjunction with transcriptional effects by thyroid hormones has provided a more detailed understanding of the actions of thyroid hormones on lipids in the liver.
In this Review, we examine the direct thyroid-hormone-mediated effects on hepatic lipogenesis and lipid metabolism, regulation of cholesterol biosynthesis and clearance and thyroid hormone receptor (THR)-independent effects of thyroid hormones on hepatic lipid metabolism as well as the potential application of thyroid hormones and/or thyroid hormone analogues for the treatment of hypercholesterolaemia and non-alcoholic fatty liver disease (NAFLD). Although we primarily focus on the direct effects of thyroid hormone on hepatic lipid metabolism, there is evidence for indirect regulation of hepatic lipid metabolism by thyroid hormone via the central nervous system that might have a notable physiological role5,6.
Hepatic lipid metabolism
Thyroid hormone receptors in hepatic lipid metabolism
The THRs are members of the nuclear hormone receptor family and function as ligand-dependent transcription factors7. There are two THR genes (THRA and THRB) that encode two isoforms, α (THRα) and β (THRβ), respectively. Both isoforms are expressed in most tissues; however, THRβ is the major form expressed in the liver8,9, whereas THRα is highly expressed in heart and bone. THRs are predominantly nuclear owing to nucleo–cytoplasmic shuttling, although a small residual pool of THRs exists in the cytoplasm10. THRs can bind to the thyroid hormone response elements (TREs) of their target genes in the absence of ligand and recruit a co-repressor complex with histone deacetylase activity to repress the transcription of positively regulated genes. Upon ligand binding, co-repressors are released owing to conformational changes in the THR, and a co-activator complex with histone acetyltransferase activity is recruited to the target gene promoter to activate transcription. Additionally, thyroid hormones can regulate transcription by altering the function of other transcription factors11–13 (such as activation of forkhead box protein O1 (FOXO1) by thyroid hormones3,4), modulating cell-signalling cascades through protein–protein interactions (such as the regulation of phosphoinositide 3-kinase (PI3K) by THRβ14) or binding to proteins other than THRs (such as binding to αvβ3 integrin)15.
The role of THRβ in hepatic lipid metabolism was first established in studies of mice with a dominant negative mutation in Thrb (ThrbPV/PV). These mice develop enlarged livers with hepatic steatosis by 4–5 months of age16. The ThrbPV/PV mouse phenotype is attributed to increased peroxisome proliferator-activated receptor-γ (PPARγ) signalling and decreased THR-mediated fatty acid β-oxidation, which leads to lipid accumulation in the liver16. Consistent with these findings, thyroid hormone and THRβ-specific ligands also reduce hepatic triglyceride content17,18. By contrast, mice expressing the dominant negative mutation in the THRα gene locus, ThraPV/PV, and THRα-null mice, all had reduced liver weights and decreased lipid accumulation16,19 owing to decreased lipogenesis, suggesting that THRα is involved in that process. Surprisingly, unlike the ThraPV/PV-mutant mice, male mice with a dominant negative Pro398His mutation introduced into the Thra gene locus exhibit hepatic steatosis20. This increase in steatosis is due to interference in PPARα binding to its promoter response element by the Pro398His mutant receptor, leading to decreased PPARα-mediated transcription of genes encoding proteins involved in fatty acid oxidation20.
The precise mechanism (or mechanisms) that underlies the differences in hepatic lipid metabolism between ThraPV/PV and ThrbPV/PV and the various THRα mutant and knockout mice is not known; however, differential recruitment of co-repressors could have a contributing role. Notably, mice with double and single knockouts of the nuclear co-repressors silencing mediator of retinoid acid and THR (SMRT; also known as NCOR2) and nuclear receptor co-repressor (NCOR1) show notable changes in hepatic lipid synthesis and storage, especially when NCOR1 is knocked out21. In addition to THR, other important regulators of intracellular thyroid hormone levels (such as deiodinases22) and thyroid hormone transporters23 can also regulate hepatic lipid metabolism.
Thyroid hormones and hepatic fatty acid uptake
Thyroid hormones stimulate lipolysis from fat stores in white adipose tissue and from dietary fat sources to generate circulating free fatty acids (FFAs), which are the major source of lipids for the liver (Fig. 1). FFAs enter hepatocytes via protein transporters such as fatty acid transporter proteins (FATPs), liver fatty acid binding proteins (L-FABPs) and fatty acid translocase (FAT; also known as CD36)24. A study from 2009 suggests that fatty acid transporters are regulated by THRs25. Radiolabelled fatty acid infusion studies have shown that fatty acid uptake from triglyceride-rich lipoproteins is increased in the presence of thyroid hormones in a tissue-specific manner as hyperthyroidism increased triglyceride-derived fatty acid uptake in oxidative tissues such as liver and muscle, whereas hypothyroidism increased triglyceride-derived fatty acid uptake in white adipose tissue and decreased its uptake in liver25. In addition, hepatic FAT and FABP expression levels are suppressed in animal models of postnatal hypothyroidism26,27. Although these studies indicate that thyroid hormones might be important in regulating FFA uptake in the liver, the precise mechanisms by which thyroid hormones alter FFA uptake are currently unclear.
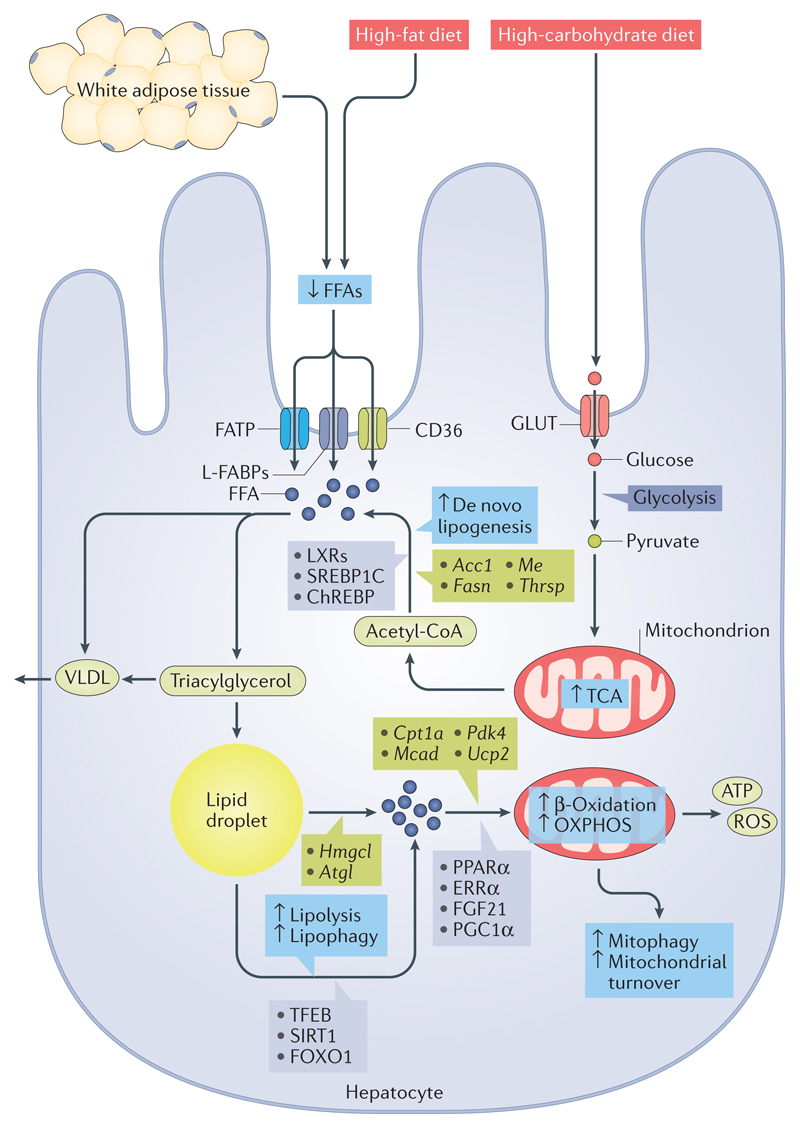
Figure 1. Thyroid hormone effects on hepatic lipid metabolism.
Thyroid hormone stimulates lipolysis from fat stores in white adipose tissue and from dietary fat sources (high-fat diets) to generate free fatty acids (FFAs) that enter the hepatic cells via protein transporters such as fatty acid transporter protein (FATP), liver fatty acid binding protein (L-FABP) and CD36. Thyroid hormone induces de novo lipogenesis (DNL) via the transcription of several key lipogenic genes such as Acc1, Fasn, Me and Thrsp. In addition, thyroid hormone indirectly controls the transcriptional regulation of hepatic DNL by regulating the expression and activities of other transcription factors such as sterol regulatory element-binding protein 1C (SREBP1C), liver X receptors (LXRs) and carbohydrate-responsive element-binding protein (ChREBP). DNL is also driven by the influx of high levels of carbohydrate or glucose derived from high-carbohydrate diets via glucose transporters (GLUTs), which are shuttled to FFA generation. FFAs are typically esterified to triacylglycerol and subsequently packaged into VLDL for export or stored as intracellular lipid droplets. Triacylglycerol stored as lipid droplets can also be hydrolysed back to FFAs via classic lipases and lipophagy (by regulating transcription factor EB (TFEB), NAD-dependent protein deacetylase sirtuin 1 (SIRT1) and forkhead box protein O1 (FOXO1)), undergo mitochondrial β-oxidation by the activity of various co-activators or nuclear receptors (such as peroxisome proliferator-activated receptor-α (PPARα), oestrogen-related receptor-α (ERRα), fibroblast growth factor 21 (FGF21) and PPARγ co-activator 1α (PGC1α)) and target the transcription of genes such as Cpt1a, Mcad (also known as Acadm), Pdk4 and Ucp2. ↑/↓ depicts the positive or negative effect that thyroid hormone action has on the cellular process shown, respectively. OXPHOS, oxidative phosphorylation; ROS, reactive oxygen species; TCA, tricarboxylic acid.
Hepatic lipogenesis and triacylglycerol assembly
Triglyceride production can come from exogenous FFAs in the circulation or intracellular FFAs generated by glycolysis and lipogenesis from glucose supplied by excess dietary intake. The process of converting glucose to fatty acids, termed ‘de novo lipogenesis’, is tightly regulated by hormones and nutritional status. De novo lipogenesis and subsequent triacylglycerol synthesis involve a number of key enzymatic processes, including elongation and desaturation of fatty acid precursors, production of fatty acids, triacylglycerol synthesis and VLDL assembly28. Thyroid hormones are a well-known inducer of hepatic de novo lipogenesis, as they stimulate the transcription of several key genes involved in lipogenesis in rodents such as fatty acid synthase (Fasn), acetyl-CoA carboxylase alpha (Acc1; also known as Acaca), malic enzyme (Me) and thyroid hormone-responsive Spot14 homologue (Thrsp; also known as Spot14) (Fig. 1). After their synthesis, FFAs are typically esterified to triacylglycerol, after which they can be packaged into VLDL, stored as fat droplets or used to make and/or repair cellular constituents.
Thyroid hormones regulate the expression of many of the genes involved in lipogenesis by binding to their specific THR29–32. However, in addition to regulating lipogenic gene expression directly, thyroid hormones also indirectly control the transcriptional regulation of hepatic lipogenesis as a result of their effects on the expression and activities of other transcription factors, such as sterol regulatory element-binding protein 1C (SREBP1C), liver X receptors (LXRs) and carbohydrate-responsive element-binding protein (ChREBP), which all have crucial roles in hepatic lipogenesis33. Thyroid hormones directly induce the gene expression of the LXRs34 and ChREBP35 in hepatic cells via the recruitment of THRs to the promoters of these genes.
The mechanism for the regulation of SREBP1C by thyroid hormone in humans is not known. In mice, one study reported that Srebp1c transcription was negatively regulated by thyroid hormone via a putative negative thyroid hormone response element (nTRE)36, but another group has shown that Srebp1c transcription is also upregulated by non-genomic thyroid hormone signalling37. Although thyroid hormone increases the expression of genes involved in de novo lipogenesis, it does not cause a net increase in mouse hepatic levels of triacylglycerol38. The major reason for this lack of increase is due to upregulated metabolism of FFAs by thyroid hormones; however, downregulation of the key desaturase enzyme stearoyl-CoA desaturase 1 (SCD1) by thyroid hormones as observed in humans might also contribute39. The mechanism by which thyroid hormones downregulate SCD1 in humans is not yet understood, but it seems to occur in a TRE-independent manner39. Similarly, thyroid hormones decrease the activity of glycerol-3-phosphate acyltransferase 3 (GPAT3)40, which is needed for triacylglycerol synthesis in rat hepatocytes.
Thyroid hormones also reduce apolipoprotein B100 (Apo B100) levels in the livers of rats, which decreases the production of VLDL and LDL41. Indeed, in humans, serum levels of triglycerides are normal or slightly decreased in hyperthyroidism, whereas they are normal or increased in hypothyroidism1,42. Thyroid hormones also modulate the relative amounts of circulating lipoproteins as highlighted by the fact that levels of HDL are increased in hypothyroidism owing to the decreased activity of cholesteryl ester transfer protein (CETP) and hepatic lipase1.
In addition to their effects on the neutral lipids and triacylglycerol, thyroid hormones seem to decrease the biosynthesis of hepatic sphingolipid and phospholipid species. In 2005, a report showed that thyroid hormones increase de novo sphingolipid synthesis in the livers of rats43. However, using metabolomics analyses, we found that the administration of thyroid hormones prevents the production of hepatic sphingolipids in rats that are fed a high-fat diet44. Furthermore, thyroid hormones can alter the intracellular concentration of several phospholipid species, such as phosphatidylcholine, phosphatidylserine and cardiolipin45.
Lipolysis and hepatic fat oxidation
Although thyroid hormones stimulate lipogenesis, there is a net reduction in total hepatic triglycerides during hyperthyroidism46 due to fatty acid metabolism occurring at a higher rate than fatty acid synthesis. Mobilization, degradation and β-oxidation of fatty acids by thyroid hormones all contribute to the increased overall rate of fatty acid metabolism (Fig. 1). In particular, thyroid hormones increase the activity of hepatic lipases, lipophagy and mitochondrial oxidation of fatty acids, which are the primary processes used by the liver to reduce steatosis.
The catabolic actions of thyroid hormone on hepatic lipids are primarily mediated by the mobilization of FFAs from stored triacylglycerol and their subsequent β-oxidation. The release of FFAs from triacylglycerol stores in hepatocytes is mediated by the enzymatic activities of cytosolic lipases47. The two major cytosolic lipases in the liver are hepatic lipase and adipose triglyceride lipase (ATGL; also known as PNPLA2). The expression and activity of hepatic lipase are sensitive to thyroid hormone status48. In both animals and humans, hypothyroidism is associated with a decline in hepatic lipase activity, which can be recovered with thyroid hormone replacement therapy49 (Fig. 1). The regulation of ATGL expression and activity by thyroid hormones in hepatic cells is less clear. However, a 2015 study did suggest that thyroid hormones increase the recruitment of ATGL to lipid droplets to facilitate lipolysis50. Zinc-α2-glycoprotein, which is encoded by AZGP1, stimulates lipolysis in humans and induces a reduction in body fat in mice51. Interestingly, thyroid hormones increase the expression of zinc-α2-glycoprotein in hepatic cells, which might also contribute to the lipolytic action of thyroid hormones52.
Regulation of lipophagy by thyroid hormones
Lysosomal acid lipase/cholesteryl ester hydrolase (LAL) is another critical regulator of hepatic triacylglycerol lipolysis in addition to the cytosolic lipases53. The delivery of triacylglycerols to lysosomes is mediated by an autophagic process known as lipophagy54,55. This specific type of autophagy involves the engulfment of triacylglycerol stored in the fat droplets by autophagosomes, followed by autophagosomal–lysosomal fusion that delivers the triacylglycerols to lysosomes for degradation and hydrolysis into FFAs54,55. Thyroid hormones increase the number of lipid-laden autophagosomes and lysosomes in both human hepatic cells and mouse liver in a THR-dependent manner56 (Fig. 1). Moreover, inhibition of autophagy and/or lipophagy in vivo markedly reduces thyroid-hormone-induced acylcarnitine flux and ketogenesis, which is the final step in β-oxidation56. Although the precise mechanism for induction of lipophagy by thyroid hormones is not clear, the induction of β-trophin (C19orf80; also known as ANGPTL8) by thyroid hormones might be a necessary priming step for the recruitment of autophagic machinery to triacylglycerols stored in fat droplets57.
Observations from our group suggest that thyroid hormones also activate lysosomal biogenesis by inhibiting mammalian target of rapamycin complex 1 (MTORC1) activity and activating the transcriptional activity of transcription factor EB (TFEB; R.A.S., B.K.S. and P.M.Y., unpublished observations), which controls the expression of many genes that encode proteins involved in autophagy and lysosomal genes and is known to regulate lipophagy58. Additionally, thyroid hormones activate NAD-dependent protein deacetylase sirtuin 1 (SIRT1) to decrease FOXO1 acetylation and phosphorylation4. These post-translational modifications increase the transcriptional activity and nuclear localization of FOXO1, which, in turn, induces the expression of several genes associated with autophagy59.
Effects of thyroid hormones on peroxisomal fat oxidation
A primary function of peroxisomal β-oxidation is to shorten very long-chain fatty acids (>16 carbon atoms) so that they can be further metabolized within mitochondria. Researchers have known for decades that thyroid hormones regulate both the number and expression levels of the peroxisomal enzymes60–66. However, the mechanisms by which thyroid hormones regulate peroxisome synthesis and function are currently unknown.
Regulation of mitochondrial fatty acid oxidation by thyroid hormones
Mitochondria are the major sites for fatty acid metabolism and are classic targets for thyroid hormone action in the liver67. Thyroid hormones regulate mitochondrial biogenesis and function in hepatocytes via coordinated signals emanating from both the nuclear and mitochondrial genome68. The nuclear regulation of mitochondrial content by thyroid hormones is primarily due to regulation of the PPARγ co-activator 1α (PGC1α)–nuclear respiratory factor 1 (NRF1)–transcription factor A, mitochondrial (mtTFA) axis68. Thyroid hormones are known to increase protein levels of PGC1α, which acts as a co-transcriptional regulation factor that induces mitochondrial biogenesis by activating NRF1 to promote the expression of mtTFA68. In addition to the PGC1α–NRF1–mtTFA axis, THRs have been reported to be localized within mitochondria and to regulate transcription from the mitochondrial genome69. The rate-limiting enzyme for mitochondrial β-oxidation is carnitine O-palmitoyltransferase 1, liver isoform (CPT1-Lα), which is transcriptionally stimulated by thyroid hormones in hepatocytes70 (Fig. 1) and inhibited by malonyl-CoA that is generated by acetyl-CoA carboxylase during fatty acid synthesis. In 2013, thyroid-hormone-mediated activation of SIRT1 activity was shown to induce PGC1α activity and regulate CPT1A mRNA expression71. Thyroid hormones also regulate CPT1A gene expression by increasing PPARα signalling in the liver12. Notably, PPARα is required for thyroid-hormone-mediated induction of fibroblast growth factor 21 (FGF21), a protein that regulates hepatic fat catabolism72. Thyroid hormones increase the expression of other mitochondrial enzymes needed for fatty acid β-oxidation, including medium-chain acyl-CoA dehydrogenase (MCAD)73, pyruvate dehydrogenase kinase isoform 4 (PDK4)74 and mitochondrial uncoupling protein 2 (UCP2)75. Moreover, data from our group suggest that oestrogen-related receptor-α (ERRα; also known as ESRRA) also regulates thyroid-hormone-induced expression of CPT1-L and mitochondrial β-oxidation via PGC1α (R.A.S., B.K.S. and P.M.Y., unpublished observations).
In addition to stimulating mitochondrial activity and fatty acid β-oxidation, thyroid hormones couple lipophagy with the removal of mitochondria that have been damaged by reactive oxygen species (ROS) generated from an increase in oxidative phosphorylation. In 2015, we showed that thyroid hormones maintain the quality of hepatic mitochondria by autophagic removal of mitochondria, known as mitophagy76. ROS generated by thyroid-hormone-mediated oxidative phosphorylation initiate a Ca2+–calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2)–5′-AMP-activated protein kinase (AMPK) signalling cascade. The activation of this signalling cascade results in the activation of serine/threonine-protein kinase ULK1 (a key mitophagic protein), which translocates into the mitochondria. ULK1 recruits autophagy-related proteins (that is, ATG proteins), which are required for nascent autophagosome formation, and initiates mitochondrial clearance76. Additionally, hepatic mitophagy seems to be coupled with mitochondrial biogenesis as both processes are induced by thyroid hormones76,77. This tight association between mitochondrial activity and mitochondrial turnover ensures the maintenance of a healthy mitochondrial pool that can sustain increased lipid handling induced by thyroid hormones.
Cholesterol biosynthesis and clearance
Thyroid hormones help maintain the basal serum levels of cholesterol that are needed to meet the body’s normal requirements for cellular synthesis and turnover (Fig. 2). Thyroid hormones regulate serum levels of cholesterol by stimulating cholesterol biosynthesis, export (primarily as VLDL and LDL), reverse transport from peripheral tissues, hepatic reuptake via LDL receptors (LDLRs) and conversion into bile acids in the liver78. In rats, thyroid hormones induce the expression of hydroxymethylglutaryl-CoA reductase (Hmgcr) and farnesyl pyrophosphate synthetase (Fdps) to promote cholesterol synthesis in the liver79. Thyroid hormones also strongly induce the gene and protein expression of Apo A1 and scavenger receptor class B member 1 (SRB1), which increases cholesterol efflux from peripheral tissues to HDL in the reverse cholesterol transport pathway80,81. Furthermore, thyroid hormones can increase HDL metabolism by stimulating CETP activity82.
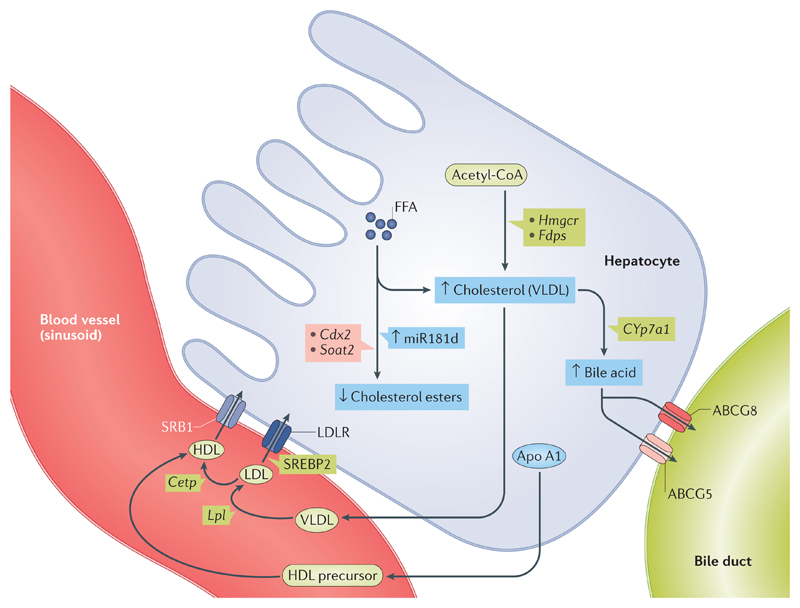
Figure 2. Thyroid hormone regulation of cholesterol biosynthesis and clearance.
Thyroid hormone stimulates cholesterol formation (mostly as VLDL) from its precursors and acetyl-CoA. Thyroid hormone increases the expression of Hmgcr and Fdps to promote hepatic cholesterol synthesis. Thyroid hormone also strongly induces the gene and protein expression of apolipoprotein A1 (Apo A1), scavenger receptor class B member 1 (SRB1) and sterol regulatory element-binding protein 2 (SREBP2), which then increase LDL receptor (LDLR) levels to increase cholesterol efflux from peripheral tissues to HDL through the reverse cholesterol transport pathway. Thyroid hormone increases HDL metabolism by stimulating cholesteryl ester transfer protein (CETP) activity. Thyroid hormone also increases expression of cholesterol 7α-hydroxylase (CYP7A1), which converts cholesterol into bile acids in the reverse cholesterol transport pathway. Thyroid hormone promotes the excretion of bile acids by directly increasing ATP-binding cassette subfamily G member 5/8 (Abcg5/Abcg8) transporter gene transcription. Additionally, thyroid hormone induces miR181d expression, which then decreases the expression of caudal-type homeobox protein 2 (CDX2) transcription factor and the Soat2 gene to inhibit cholesterol ester formation. ↑/↓ shows increase or decrease in thyroid hormone action, respectively. FFA, free fatty acid.
In rats, the major mechanism by which thyroid hormones decrease serum levels of cholesterol is through the induction of hepatic LDLRs to increase cholesterol clearance81. LDLR is also regulated by SREBP2, which itself is transcriptionally regulated by thyroid hormones83 in rodents and humans. Furthermore, thyroid hormones can increase the transcription of both mouse and human LDLR-related protein 1 (LRP1), a lipoprotein involved in the removal of chylomicron remnants and VLDL84. Within the liver, thyroid hormones also increase the expression of rat cholesterol 7α-hydroxylase (CYP7A1), the rate-limiting enzyme that converts cholesterol into bile acids in the reverse cholesterol transport pathway, and decrease expression of Apo B protein, the major apolipoprotein in LDL, to reduce serum levels of LDL cholesterol even further85,86. In addition, thyroid hormones can promote the excretion of bile acids in the liver and intestines, which are the last steps of the reverse cholesterol transport pathway, by stimulating mouse ATP-binding cassette subfamily G member (Abcg5/Abcg8) complex gene transcription, directly and independently from its effects on LXRs87.
Finally, in addition to the transcriptional regulation of genes involved in cholesterol synthesis, reverse cholesterol transport and bile secretion88, thyroid hormones might use microRNAs (miRNAs) to regulate serum levels of cholesterol. Thus, thyroid hormones induce expression of human miR181d, which decreases the expression of caudal-type homeobox protein 2 (CDX2), a transcription factor that activates sterol O-acyltransferase 2 (SOAT2). SOAT2 is critical for the conversion of cholesterol to cholesterol esters89, the preferred form of cholesterol within LDL. This example of thyroid hormones using miRNAs demonstrates that thyroid hormones might use a non-TRE-mediated mechanism to lower serum levels of cholesterol.
Non-transcriptional effects
Previously, research suggested that the only mode of action of thyroid hormones was the transcriptional regulation of target genes via THRs binding to TREs and the recruitment of co-activators to increase RNA polymerase binding to the basal transcriptional protein complex. Surprisingly, T3 and T4 exert biological actions that do not require THRs binding to DNA or the absence of THRs90,91. Thus, T3 activates PI3K–RACα serine/threonine-protein kinase (AKT) signalling via a non-genomic mechanism92. This signalling mechanism has been implicated in the regulation of FASN expression by T3. The inhibition of T3-mediated induction of FASN expression by PI3K and extracellular-signal-regulated kinase 1 (ERK1) inhibitors further suggests that there are other non-transcriptional mechanisms that control hepatic lipogenesis by thyroid hormones32. Thyroid hormones can also regulate hepatic lipid metabolism by activating the cAMP–protein kinase A (PKA) and Ca2+–AMPK pathways93–95.
In addition to T3 and T4, the thyroid hormone derivative 3,5-diiodothyronine has been extensively studied for its ability to regulate hepatic lipid metabolism via non-THR-mediated signalling96. In vitro and in vivo studies show that 3,5-diiodothyronine increases fatty acid oxidation in hepatocytes and supresses the lipogenic pathways97–103. Notably, 3,5-diiodothyronine directly activates SIRT1, which leads to the deacetylation of PGC1α and activation of its transcriptional activity in order to induce expression of the genes required for fatty acid oxidation104. 3,5-Diiodothyronine also modulates the activities and localization of hepatic lipases to increase lipid mobilization from fat droplets50. In addition, in a mouse model of familial hypercholesterolaemia, 3,5-diiodothyronine exerts beneficial effects on lipid metabolism by reducing serum levels of LDL cholesterol by an LDLR-independent mechanism86. Thus far, there is no evidence to suggest that reverse T3 regulates transcription by nuclear THRs or has any non-transcriptional effects on metabolism and/or cell signalling.
TSH and hepatic lipid metabolism
Although hypothyroidism-associated increased hepatosteatosis is thought to result from a decrease in serum levels of thyroid hormone, studies have suggested that when the levels of TSH in the serum are high, TSH binds to TSH receptors in the liver to modulate lipid metabolism. In vivo rodent studies show that TSH receptors are expressed in hepatocytes and can be stimulated by TSH to induce hepatosteatosis via SREBP1C105. TSH also supresses the synthesis of hepatic bile acid via an SREBP2–hepatocyte nuclear factor 4α (HNF4α)–CYP7A1 signalling pathway106. Moreover, TSH inhibits cholesterol synthesis by increasing AMPK-mediated phosphorylation of HMGCR to inhibit HMGCR activity107. Collectively, these findings support the notion that TSH itself can regulate both hepatic lipid and cholesterol homeostasis; however, in vivo studies confirming the direct action of TSH, independent from thyroid hormone, are very difficult to interpret because of the concomitant reduction in the serum levels of thyroid hormone.
Metabolic diseases of the liver
Thyroid hormone effects on hypercholesterolaemia
Since the early 1950s, we have known that thyroid hormone status in humans is inversely related to levels of LDL cholesterol108. Moreover, thyroid hormone supplementation leads to improvements in lipid and lipoprotein profiles in patients with hypothyroidism109. Early studies of levothyroxine and the thyroxine enantiomer dextrothyroxine showed promising effects in reducing serum levels of LDL cholesterol but were discontinued owing to serious adverse effects from cardiac, bone and muscle toxicity110–112. Nonetheless, promising results from these studies led to the development of liver-selective and THR isoform-specific thyroid hormone mimetics as potential lipid-lowering agents113,114. The first liver-selective thyromimetic, 3,3-dibromo-3′-pyridazinone-1-thyronine (L-94901), was described in 1986 (REF. 115) (Table 1). This compound has cholesterol-lowering effects in hypothyroid rats without any deleterious effects on the heart115. Similarly, three other compounds (CGH-509A, CGS-23425 and T-0681) have shown efficacy in lowering serum levels of LDL cholesterol116,117; however, the development of these compounds for clinical use has not been actively pursued.
Table 1. Thyroid hormone analogues and/or mimetics and their biological effects.
| Thyroid hormone analogues and/or mimetics | Biological effects | Species | Refs |
|---|---|---|---|
| L-94901 | Lowers cholesterol | Mouse | 115 |
| CGH-509A | Lowers cholesterol | Rat | 117 |
| CGS-23425 | Lowers cholesterol | Rat | 117 |
| T-0681 | Lowers cholesterol | Mouse | 116 |
| DITPA | Lowers cholesterol | Human | 118 |
| GC-1 (sobetirome) | Lowers cholesterol, triglyceride, blood glucose, adipose tissue and atherosclerosis | Mouse | 119–121 |
| KB-141 | Lowers cholesterol, triglyceride, adipose tissue and blood glucose | Monkey, rat and mouse | 122 |
| KB2115 (eprotirome) | Lowers cholesterol and triglyceride | Human | 123 |
| MGL-3196 | Lowers cholesterol and triglyceride | Human | 124 |
| MB07811 | Lowers cholesterol, triglyceride and blood glucose | Human | 126 |
| 3,5-Diiodothyronine | Lowers blood glucose and triglyceride and improves hepatic insulin resistance | Rat | 44,103 |
2,5-Diiodothyropropionic acid (DITPA) is the first THR-selective thyromimetic to display slightly higher affinity for THRβ than THRα. In a clinical trial that lasted for 6 months, DITPA therapy moderately decreased serum levels of total cholesterol and LDL cholesterol in patients with congestive heart failure118. GC-1 (also known as sobetirome) belongs to the first generation of more specific THRβ agonists and reduces serum levels of cholesterol and triglyceride in animal models of obesity119. In a phase I study, a 2-week treatment regimen of GC-1 reduced serum levels of LDL cholesterol by up to 41% in healthy participants120. Furthermore, GC-1 reduces the cholesterol content in plaques along the aortic arterial walls of apolipoprotein E (APOE)-deficient mice121. Another THRβ-specific thyromimetic, KB-141, decreases plasma levels of cholesterol in both rodents and primates, primarily through stimulation of the reverse cholesterol pathway122. The THRβ-specific analogue KB2115 (also known as eprotirome) has similar effects on plasma levels of cholesterol and is the first thyroid hormone mimetic designed for the treatment of dyslipidaemia that has reached phase III trials. When administered with statin therapy, eprotirome further decreases levels of LDL cholesterol, triglycerides and lipoproteins in patients with hypercholesterolaemia123. Another THRβ-specific thyromimetic, MGL-3196, has been developed for the treatment of hypercholesterolaemia and is currently in a phase I trial124.
Another class of thyromimetics are liver-selective prodrugs and/or their metabolites that bind to THR with a high affinity. Hepatic CYP450 enzymes activate some of these compounds, a process that generates the active metabolites, which have a short half-life. Therefore, most of the thyromimetic actions of this class of thyromimetics are confined to the liver, and thus, adverse effects in non-hepatic tissues are minimized. One such drug, MB07811, is effective in reducing serum levels of LDL cholesterol and total cholesterol in rabbits, dogs and monkeys125. This drug is safe on the basis of a phase Ib clinical study126 and was carried forward for a phase II trial127.
Although the majority of patients with mild hypothyroidism have little or no derangements in serum levels of triglyceride and VLDL1, some patients with severe hypothyroidism exhibit hypertriglyceridaemia1. Similar to levothyroxine replacement in hypothyroidism, thyroid hormone mimetics can also decrease hypertriglyceridaemia, a known risk factor for atherosclerosis that is independent of levels of LDL cholesterol. Thus, GC-1 reduces serum levels of triglyceride by >50–60% in hypothyroid and normal mice128. Similarly, both KB-141 and MB07811 markedly reduce serum levels of triglycerides in normal and obese mice17,129.
Effects of thyroid hormones in NAFLD
NAFLD is a global epidemic with an incidence of 30% or more among adults in both developed and developing countries130. NAFLD is considered to be a hepatic manifestation of the metabolic syndrome and is closely associated with the development of other metabolic risk factors such as type 2 diabetes mellitus, hyperlipidaemia and coronary artery disease131. NAFLD represents a spectrum of liver diseases that includes excessive accumulation of lipids in the hepatocytes that is initially benign (hepatosteatosis) but progresses to a more advanced stage with inflammation (non-alcoholic steatohepatitis (NASH)) and culminates in fibrosis accompanied by increased inflammation, apoptosis and scarring of liver tissue (cirrhosis)132. Patients with NAFLD also have an increased risk of hepatocellular carcinoma133. The long-term complications of NAFLD have made it the most common cause for liver transplantation in the United States133.
Several epidemiological studies conducted in countries from around the world show an inverse relationship between serum levels of thyroid hormone and the incidence of NAFLD134,135. Similarly, Asian patients with NAFLD had significantly lower levels of serum-free T4 than control patients in a cross-sectional study of 878 elderly Chinese euthyroid participants (11.12 ± 1.43 pmol/l versus 11.58 ± 1.47 pmol/l; P < 0.001)136. In another report, subclinical hypothyroidism, even within the range of upper-normal TSH levels, was significantly associated with NAFLD in a concentration-dependent manner137. Overt hypothyroidism is even more closely associated with NAFLD and is a risk factor that is independent from other known metabolic risk factors, thus confirming the strong clinical relationship between these two conditions137,138.
In paediatric populations, children with obesity who have increased TSH levels have more severe hepatosteatosis than children with obesity but normal TSH139. Of note, two studies from 2014 and 2016 demonstrate that serum levels of free T3, free T4 and the free T3:free T4 ratio are inversely associated and that TSH levels are associated with NAFLD in the general population, even among those within the reference range for euthyroid participants140,141. In addition to the deleterious effects of decreased serum levels of thyroid hormone on hepatic lipid homeostasis, it is possible that increased TSH per se might promote the development of NAFLD by stimulating lipogenesis in the liver105. In addition, the intrahepatic thyroid hormone concentration and/or thyroid hormone signalling could be decreased in the livers of patients with NAFLD142–144. Although the cause (or causes) of such resistance to thyroid hormone action in the liver is not clear, studies from the past three decades suggest that intracellular fatty acids impair THR activity145. Additionally, the mRNA and protein expression levels of type 1 iodothyronine deiodinase (DIO1), the enzyme that converts T4 to T3 in the liver, are very sensitive to serum levels of thyroid hormone. Therefore, decreased expression and activity of DIO1 could lead to intrahepatic hypothyroidism by reducing the conversion of T4 to T3. Of note, decreased serum T3 and increased reverse T3 have been reported in patients with advanced NASH146.
Data from our groups suggest that reduced intrahepatic concentrations of thyroid hormone transporters, THR and nuclear co-activators of THR are other mechanisms that could potentially regulate thyroid hormone signalling in NAFLD (R.A.S., B.K.S. and P.M.Y., unpublished observations). Notably, exogenous thyroid hormones, thyroid hormone analogues and a novel glucagon–thyroid hormone hybrid molecule can all reduce hepatosteatosis in NAFLD147,148. Many factors exist that contribute to the progression of NAFLD, such as diet, endocrine status and gene polymorphisms; thus, decreased intrahepatic concentrations of thyroid hormones might be found in only a subset of patients with NAFLD. Further studies of serum markers for thyroid hormone action on hepatic function, such as sex hormone-binding globulin, ferritin, cholesterol or acylcarnitines, could provide potential tools to evaluate intrahepatic thyroid hormone status149,150.
Several preclinical studies of thyroid hormone analogues have demonstrated their efficacy in reducing lipid accumulation in animal models of NAFLD96 (Table 1). GC-1 is a synthetic thyroid hormone analogue that preferentially binds THRβ1 in an isoform-specific manner and has the same affinity for THRβ1 as T3. Similar to T3, GC-1 prevents and reverses hepatosteatosis in rats fed a diet that induces NASH148. Furthermore, GC-1 lowers liver weight, the liver weight:body weight ratio and serum levels of triglycerides in these same animals. In addition to decreasing hepatic lipid accumulation, GC-1 also decreases lipoperoxidation and reduces liver injury, as the increases in serum levels of aspartate transaminase (AST) and alanine transaminase (ALT) fall after GC-1 treatment148. These findings suggest that GC-1 is an excellent thyromimetic for the treatment of NAFLD provided it has the requisite safety profile.
MB07811 is an oral THRβ-specific agonist that targets the liver to reduce hepatic steatosis in rats and mice17. MB07811 reduces hepatic triglycerides by increasing hepatic β-oxidation, mitochondrial respiration rates and expression of genes involved in β-oxidation. The aforementioned thyromimetic KB2115 can also improve NAFLD in rats151. In addition, 12 weeks of therapy with KB2115 lowered serum levels of cholesterol in patients who were taking statins, suggesting that KB2115 is a safe chronic therapy, as the authors of the study did not report cardiac or bone toxicity123. Unfortunately, despite these beneficial effects, the clinical trials of KB2115 were terminated as a parallel 12-month dosing study in dogs showed adverse effects on cartilage152. These findings suggest that thyroid hormone analogues have other adverse effects in addition to those known to occur in bone and heart. Researchers will need to perform careful preclinical testing for adverse effects before clinical studies for other compounds are undertaken.
In 2016, one group reported the generation of a hybrid molecule, which contains both thyroid hormone and glucagon147, that reduces hepatosteatosis in NAFLD without bone or heart adverse effects. This compound has opened an exciting new area for synthetic bi-hormonal therapy of metabolic diseases, as one hormone targets a specific tissue whereas the other has intracellular activity. The thyroid hormone metabolite 3,5-diiodothyronine is also able to reduce hepatic insulin resistance and decrease hepatosteatosis, suggesting that it is an attractive candidate for the treatment of NAFLD, particularly as it does not seem to have the systemic adverse effects of T3 and T4 (REF. 44).
A noteworthy point is that 15% of patients with NASH have hypothyroidism compared with 7.2% of patients with normal liver function153. A 2012 study found that patients with NASH had a higher risk of hypothyroidism than patients with NAFLD without NASH and that hypothyroidism increased the risk of NASH154. A further study showed that NASH and advanced fibrosis occurred more frequently in both patients who were hypothyroid and patients who were subclinically hypothyroid155. These studies suggest that hypothyroidism and subclinical hypothyroidism also increase the risk of NASH in addition to hepatosteatosis142. Although these observations suggest a potential beneficial effect of thyroid hormones on NASH, thus far, there have not been any animal or human interventional studies demonstrating that thyroid hormones or thyroid hormone analogues can prevent or block the progression to NASH.
Decreased levels of thyroid hormone have been associated with an increased incidence of hepatocellular cancer in humans156. In addition, thyroid hormones have been shown to be anti-neoplastic in liver cancers157. Hepatocellular cancer occurs in patients with NASH, and many THR mutations have been reported in patients with hepatocellular cancer158. The presiding hypothesis is that THRs serve as tumour suppressors, primarily by inhibiting WNT signalling, the expression of cyclin-dependent kinase 2 (CDK2) and cyclin E and by stimulating TGFβ signalling. The suppression and stimulation of these signalling pathways are thought to lead to cell cycle arrest at the G1 phase159. Therefore, the suppressive activity of thyroid hormones is hypothesized to be blocked in the presence of mutant THRs. However, there have been no studies that definitively demonstrate that thyroid hormones can prevent hepatocellular cancer in animals or patients with NASH and/or fibrosis.
Conclusions and future perspectives
Advances in our understanding of the cellular and molecular mechanisms of fatty acid and cholesterol synthesis and metabolism have led to a better appreciation for the role of thyroid hormones and THRs in maintaining normal hepatic lipid homeostasis. We now understand the lipid derangements that can occur in hypothyroidism and hyperthyroidism at a deeper, more mechanistic, level. It is possible that some of the control points in the signalling pathways that regulate triglyceride and cholesterol levels within the liver and serum are modulated by thyroid hormones and thus could be potential drug targets for thyromimetics or other drugs. Additionally, serum levels of free T3, free T4 and levels of TSH characterize the sufficiency of the hypothalamic–pituitary–thyroid axis; however, they might not accurately reflect intrahepatic levels of thyroid hormones, which can be reduced in the livers of patients with NAFLD.
Studies from the past several years suggest that thyroid hormone analogues that are specific for THRβ or THRβ in the liver, or analogues that are bi-hormonal, are potential therapies for metabolic conditions such as hypercholesterolaemia and NAFLD. Although the actions of thyroid hormones on hepatic fatty acid and cholesterol metabolism have been topics of interest to basic scientists and clinicians for many years, the new advances in our knowledge in these areas that are presented in this Review provide stronger rationales and tools for using thyroid hormone or thyromimetic drugs to treat hepatic metabolic disorders.
Key points.
Thyroid hormones regulate hepatic lipid metabolism in a cell autonomous manner
Thyroid hormone receptors (THRα and THRβ) differentially regulate hepatic lipid metabolism
Thyroid hormone induces the expression of genes that encode proteins involved in hepatic lipogenesis
Thyroid hormone couples autophagy to mitochondrial fat oxidation to induce ketogenesis
Thyroid hormone induces reverse cholesterol transport
Thyroid hormone analogues and/or mimetics offer therapeutic alternatives for treatment of lipid-associated hepatic pathologies
Acknowledgements
The authors thank their funding agencies, the Singapore Ministry of Health, Ministry of Education and Ministry of Trade, the National Medical Research Council, Singapore, and the Singapore Agency for Science, Technology and Research, for grants NMRC/CSA/0054/2013 (P.M.Y.), NMRC/BNIG/2025/2014 (R.A.S.), IA/I/16/2/502691-Wellcome Trust/DBT India Alliance Intermediate Fellowship (R.A.S.) and NMRC/OFYIRG/0002/2016 (B.K.S.).
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author contributions
R.A.S. and P.M.Y. contributed equally to researching data for the article, discussing the content and writing the manuscript. R.A.S., B.K.S. and P.M.Y. contributed equally to reviewing and/or editing the manuscript before submission.
References
- 1.Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12:287–293. doi: 10.1089/10507250252949405. [DOI] [PubMed] [Google Scholar]
- 2.Krotkiewski M. Thyroid hormones and treatment of obesity. Int J Obes Relat Metab Disord. 2000;24:S116–S119. doi: 10.1038/sj.ijo.0801294. [DOI] [PubMed] [Google Scholar]
- 3.Singh BK, et al. Hepatic FOXO1 target genes are co-regulated by thyroid hormone via RICTOR protein deacetylation and MTORC2-AKT protein inhibition. J Biol Chem. 2016;291:198–214. doi: 10.1074/jbc.M115.668673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh BK, et al. FoxO1 deacetylation regulates thyroid hormone-induced transcription of key hepatic gluconeogenic genes. J Biol Chem. 2013;288:30365–30372. doi: 10.1074/jbc.M113.504845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Sanchez N, et al. Hypothalamic effects of thyroid hormones on metabolism. Best practice and research. Clin Endocrinol Metabolism. 2014;28:703–712. doi: 10.1016/j.beem.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Sanchez N, et al. Hypothalamic AMPK-ER stress-JNK1 axis mediates the central actions of thyroid hormones on energy balance. Cell Metab. 2017;26:212–229. doi: 10.1016/j.cmet.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yen PM, Sinha R. Cellular action of thyroid hormone. Endotext. updated 12 Feb 2000. [Google Scholar]
- 8.Lazar MA. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev. 1993;14:184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- 9.Chamba A, et al. Expression and function of thyroid hormone receptor variants in normal and chronically diseased human liver. J Clin Endocrinol Metab. 1996;81:360–367. doi: 10.1210/jcem.81.1.8550778. [DOI] [PubMed] [Google Scholar]
- 10.Baumann CT, Maruvada P, Hager GL, Yen PM. Nuclear cytoplasmic shuttling by thyroid hormone receptors. multiple protein interactions are required for nuclear retention. J Biol Chem. 2001;276:11237–11245. doi: 10.1074/jbc.M011112200. [DOI] [PubMed] [Google Scholar]
- 11.Davis PJ, Goglia F, Leonard JL. Nongenomic actions of thyroid hormone. Nat Rev Endocrinol. 2016;12:111–121. doi: 10.1038/nrendo.2015.205. [DOI] [PubMed] [Google Scholar]
- 12.Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94:355–382. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flamant F, et al. Thyroid hormone signaling pathways: time for a more precise nomenclature. Endocrinology. 2017;158:2052–2057. doi: 10.1210/en.2017-00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuya F, Hanover JA, Cheng SY. Activation of phosphatidylinositol 3-kinase signaling by a mutant thyroid hormone β receptor. Proc Natl Acad Sci USA. 2006;103:1780–1785. doi: 10.1073/pnas.0510849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin HY, et al. Identification and functions of the plasma membrane receptor for thyroid hormone analogues. Discov Med. 2011;11:337–347. [PubMed] [Google Scholar]
- 16.Araki O, Ying H, Zhu XG, Willingham MC, Cheng SY. Distinct dysregulation of lipid metabolism by unliganded thyroid hormone receptor isoforms. Mol Endocrinol. 2009;23:308–315. doi: 10.1210/me.2008-0311. [This is an important work highlighting the distinct effects of unliganded THRs on lipid metabolism.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cable EE, et al. Reduction of hepatic steatosis in rats and mice after treatment with a liver-targeted thyroid hormone receptor agonist. Hepatology. 2009;49:407–417. doi: 10.1002/hep.22572. [This article presents an interesting study showing the efficacy of liver-targeted thyroid hormone agonist in reducing NAFLD in rodent models.] [DOI] [PubMed] [Google Scholar]
- 18.Erion MD, et al. Targeting thyroid hormone receptor-β agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc Natl Acad Sci USA. 2007;104:15490–15495. doi: 10.1073/pnas.0702759104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jornayvaz FR, et al. Thyroid hormone receptor-α gene knockout mice are protected from diet-induced hepatic insulin resistance. Endocrinology. 2012;153:583–591. doi: 10.1210/en.2011-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu YY, et al. A mutant thyroid hormone receptor α antagonizes peroxisome proliferator-activated receptor α signaling in vivo and impairs fatty acid oxidation. Endocrinology. 2007;148:1206–1217. doi: 10.1210/en.2006-0836. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu H, et al. NCoR1 and SMRT play unique roles in thyroid hormone action in vivo. Mol Cell Biol. 2015;35:555–565. doi: 10.1128/MCB.01208-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fonseca TL, et al. Perinatal deiodinase 2 expression in hepatocytes defines epigenetic susceptibility to liver steatosis and obesity. Proc Natl Acad Sci USA. 2015;112:14018–14023. doi: 10.1073/pnas.1508943112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer zu Schwabedissen HE, et al. Hepatic organic anion transporting polypeptide transporter and thyroid hormone receptor interplay determines cholesterol and glucose homeostasis. Hepatology. 2011;54:644–654. doi: 10.1002/hep.24391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mashek DG. Hepatic fatty acid trafficking: multiple forks in the road. Adv Nutr. 2013;4:697–710. doi: 10.3945/an.113.004648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klieverik LP, et al. Thyroid hormone effects on whole-body energy homeostasis and tissue-specific fatty acid uptake in vivo. Endocrinology. 2009;150:5639–5648. doi: 10.1210/en.2009-0297. [DOI] [PubMed] [Google Scholar]
- 26.Santana-Farre R, et al. Influence of neonatal hypothyroidism on hepatic gene expression and lipid metabolism in adulthood. PLOS ONE. 2012;7:e37386. doi: 10.1371/journal.pone.0037386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakagawa S, Kawashima Y, Hirose A, Kozuka H. Regulation of hepatic level of fatty-acid-binding protein by hormones and clofibric acid in the rat. Biochem J. 1994;297:581–584. doi: 10.1042/bj2970581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czech MP, Tencerova M, Pedersen DJ, Aouadi M. Insulin signalling mechanisms for triacylglycerol storage. Diabetologia. 2013;56:949–964. doi: 10.1007/s00125-013-2869-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell MC, Anderson GW, Mariash CN. Human spot 14 glucose and thyroid hormone response: characterization and thyroid hormone response element identification. Endocrinology. 2003;144:5242–5248. doi: 10.1210/en.2002-0008. [DOI] [PubMed] [Google Scholar]
- 30.Desvergne B, Petty KJ, Nikodem VM. Functional characterization and receptor binding studies of the malic enzyme thyroid hormone response element. J Biol Chem. 1991;266:1008–1013. [PubMed] [Google Scholar]
- 31.Zhang Y, Yin L, Hillgartner FB. Thyroid hormone stimulates acetyl-coA carboxylase-α transcription in hepatocytes by modulating the composition of nuclear receptor complexes bound to a thyroid hormone response element. J Biol Chem. 2001;276:974–983. doi: 10.1074/jbc.M005894200. [DOI] [PubMed] [Google Scholar]
- 32.Radenne A, et al. Hepatic regulation of fatty acid synthase by insulin and T3: evidence for T3 genomic and nongenomic actions. Am J Physiol Endocrinol Metab. 2008;295:E884–E894. doi: 10.1152/ajpendo.90438.2008. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Viscarra J, Kim SJ, Sul HS. Transcriptional regulation of hepatic lipogenesis. Nat Rev Mol Cell Biol. 2015;16:678–689. doi: 10.1038/nrm4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashimoto K, Matsumoto S, Yamada M, Satoh T, Mori M. Liver X receptor-α gene expression is positively regulated by thyroid hormone. Endocrinology. 2007;148:4667–4675. doi: 10.1210/en.2007-0150. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto K, et al. Carbohydrate response element binding protein gene expression is positively regulated by thyroid hormone. Endocrinology. 2009;150:3417–3424. doi: 10.1210/en.2009-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hashimoto K, et al. Mouse sterol response element binding protein-1c gene expression is negatively regulated by thyroid hormone. Endocrinology. 2006;147:4292–4302. doi: 10.1210/en.2006-0116. [DOI] [PubMed] [Google Scholar]
- 37.Gnoni GV, et al. 3,5,3’triiodo-L-thyronine induces SREBP-1 expression by non-genomic actions in human HEP G2 cells. J Cell Physiol. 2012;227:2388–2397. doi: 10.1002/jcp.22974. [DOI] [PubMed] [Google Scholar]
- 38.Yao X, et al. Regulation of fatty acid composition and lipid storage by thyroid hormone in mouse liver. Cell Biosci. 2014;4:38. doi: 10.1186/2045-3701-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hashimoto K, et al. Human stearoyl-CoA desaturase 1 (SCD-1) gene expression is negatively regulated by thyroid hormone without direct binding of thyroid hormone receptor to the gene promoter. Endocrinology. 2013;154:537–549. doi: 10.1210/en.2012-1559. [DOI] [PubMed] [Google Scholar]
- 40.Dang AQ, Faas FH, Carter WJ. Influence of hypo- and hyperthyroidism on rat liver glycerophospholipid metabolism. Lipids. 1985;20:897–902. doi: 10.1007/BF02534774. [DOI] [PubMed] [Google Scholar]
- 41.Davidson NO, Powell LM, Wallis SC, Scott J. Thyroid hormone modulates the introduction of a stop codon in rat liver apolipoprotein B messenger RNA. J Biol Chem. 1988;263:13482–13485. [PubMed] [Google Scholar]
- 42.Abrams JJ, Grundy SM, Ginsberg H. Metabolism of plasma triglycerides in hypothyroidism and hyperthyroidism in man. J Lipid Res. 1981;22:307–322. [PubMed] [Google Scholar]
- 43.Babenko NA. Long- and short-term effects of thyroxine on sphingolipid metabolism in rat liver. Med Sci Monit. 2005;11:BR131–BR138. [PubMed] [Google Scholar]
- 44.Iannucci LF, et al. Metabolomic analysis shows differential hepatic effects of T2 and T3 in rats after short-term feeding with high fat diet. Sci Rep. 2017;7:2023. doi: 10.1038/s41598-017-02205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bucki R, Gorska M, Zendzian-Piotrowska M, Gorski J. Effect of triiodothyronine on the content of phospholipids in the rat liver nuclei. J Physiol Pharmacol. 2000;51:535–540. [PubMed] [Google Scholar]
- 46.Oppenheimer JH, Schwartz HL, Lane JT, Thompson MP. Functional relationship of thyroid hormone-induced lipogenesis, lipolysis, and thermogenesis in the rat. J Clin Invest. 1991;87:125–132. doi: 10.1172/JCI114961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quiroga AD, Lehner R. Liver triacylglycerol lipases. Biochim Biophys Acta. 2012;1821:762–769. doi: 10.1016/j.bbalip.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Kihara S, Wolle J, Ehnholm C, Chan L, Oka K. Regulation of hepatic triglyceride lipase by thyroid hormone in HepG2 cells. J Lipid Res. 1993;34:961–970. [PubMed] [Google Scholar]
- 49.Brenta G, et al. Atherogenic lipoproteins in subclinical hypothyroidism and their relationship with hepatic lipase activity: response to replacement treatment with levothyroxine. Thyroid. 2016;26:365–372. doi: 10.1089/thy.2015.0140. [DOI] [PubMed] [Google Scholar]
- 50.Grasselli E, et al. Triglyceride mobilization from lipid droplets sustains the anti-steatotic action of iodothyronines in cultured rat hepatocytes. Front Physiol. 2015;6:418. doi: 10.3389/fphys.2015.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanchez LM, Chirino AJ, Bjorkman P. Crystal structure of human ZAG, a fat-depleting factor related to MHC molecules. Science. 1999;283:1914–1919. doi: 10.1126/science.283.5409.1914. [DOI] [PubMed] [Google Scholar]
- 52.Simo R, et al. Thyroid hormone upregulates zinc-α2-glycoprotein production in the liver but not in adipose tissue. PLOS ONE. 2014;9:e85753. doi: 10.1371/journal.pone.0085753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reiner Z, et al. Lysosomal acid lipase deficiency — an under-recognized cause of dyslipidaemia and liver dysfunction. Atherosclerosis. 2014;235:21–30. doi: 10.1016/j.atherosclerosis.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Singh R, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cingolani F, Czaja MJ. Regulation and functions of autophagic lipolysis. Trends Endocrinol Metab. 2016;27:696–705. doi: 10.1016/j.tem.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sinha RA, et al. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J Clin Invest. 2012;122:2428–2438. doi: 10.1172/JCI60580. [This study describes the role of autophagy in thyroid-hormone-induced ketogenesis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tseng YH, et al. Chromosome 19 open reading frame 80 is upregulated by thyroid hormone and modulates autophagy and lipid metabolism. Autophagy. 2014;10:20–31. doi: 10.4161/auto.26126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Settembre C, Ballabio A. Lysosome: regulator of lipid degradation pathways. Trends Cell Biol. 2014;24:743–750. doi: 10.1016/j.tcb.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu HY, et al. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J Biol Chem. 2009;284:31484–31492. doi: 10.1074/jbc.M109.033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takeda T, et al. Regulation of rat hepatic peroxisomal enoyl-CoA hydratase-3-hydroxyacyl-CoA dehydrogenase bifunctional enzyme by thyroid hormone. Biochem Biophys Res Commun. 1992;185:211–216. doi: 10.1016/s0006-291x(05)80977-5. [DOI] [PubMed] [Google Scholar]
- 61.Just WW, Hartl FU, Schimassek H. Rat liver peroxisomes. I. New peroxisome population induced by thyroid hormones in the liver of male rats. Eur J Cell Biol. 1982;26:249–254. [PubMed] [Google Scholar]
- 62.Just WW, Hartl FU. Rat liver peroxisomes, II. Stimulation of peroxisomal fatty-acid β-oxidation by thyroid hormones. Hoppe Seylers Z Physiol Chem. 1983;364:1541–1547. doi: 10.1515/bchm2.1983.364.2.1541. [DOI] [PubMed] [Google Scholar]
- 63.Iossa S, et al. Effect of long-term high-fat feeding on energy balance and liver oxidative activity in rats. Br J Nutr. 2000;84:377–385. [PubMed] [Google Scholar]
- 64.Goudonnet H, et al. Differential action of thyroid hormones and chemically related compounds on the activity of UDP-glucuronosyltransferases and cytochrome P-450 isozymes in rat liver. Biochim Biophys Acta. 1990;1035:12–19. doi: 10.1016/0304-4165(90)90167-u. [DOI] [PubMed] [Google Scholar]
- 65.Goglia F, Liverini G, Lanni A, Iossa S, Barletta A. Effects of 3,5,3′-triiodothyronine (T3) on rat liver peroxisomal compartment during cold exposure. Exp Biol. 1989;48:135–140. [PubMed] [Google Scholar]
- 66.Fringes B, Reith A. Time course of peroxisome biogenesis during adaptation to mild hyperthyroidism in rat liver: a morphometric/stereologic study by electron microscopy. Lab Invest. 1982;47:19–26. [PubMed] [Google Scholar]
- 67.Cioffi F, Lanni A, Goglia F. Thyroid hormones, mitochondrial bioenergetics and lipid handling. Curr Opin Endocrinol Diabetes Obes. 2010;17:402–407. doi: 10.1097/MED.0b013e32833cf354. [DOI] [PubMed] [Google Scholar]
- 68.Weitzel JM, Iwen KA. Coordination of mitochondrial biogenesis by thyroid hormone. Mol Cell Endocrinol. 2011;342:1–7. doi: 10.1016/j.mce.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 69.Wrutniak-Cabello C, Casas F, Cabello G. The direct tri-lodothyronine mitochondrial pathway: science or mythology? Thyroid. 2000;10:965–969. doi: 10.1089/thy.2000.10.965. [DOI] [PubMed] [Google Scholar]
- 70.Jackson-Hayes L, et al. A thyroid hormone response unit formed between the promoter and first intron of the carnitine palmitoyltransferase-Iα gene mediates the liver-specific induction by thyroid hormone. J Biol Chem. 2003;278:7964–7972. doi: 10.1074/jbc.M211062200. [DOI] [PubMed] [Google Scholar]
- 71.Thakran S, et al. Role of sirtuin 1 in the regulation of hepatic gene expression by thyroid hormone. J Biol Chem. 2013;288:807–818. doi: 10.1074/jbc.M112.437970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adams AC, et al. Thyroid hormone regulates hepatic expression of fibroblast growth factor 21 in a PPARα-dependent manner. J Biol Chem. 2010;285:14078–14082. doi: 10.1074/jbc.C110.107375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Djouadi F, Riveau B, Merlet-Benichou C, Bastin J. Tissue-specific regulation of medium-chain acyl-CoA dehydrogenase gene by thyroid hormones in the developing rat. Biochem J. 1997;324:289–294. doi: 10.1042/bj3240289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holness MJ, Bulmer K, Smith ND, Sugden MC. Investigation of potential mechanisms regulating protein expression of hepatic pyruvate dehydrogenase kinase isoforms 2 and 4 by fatty acids and thyroid hormone. Biochem J. 2003;369:687–695. doi: 10.1042/BJ20021509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jekabsons MB, Gregoire FM, Schonfeld-Warden NA, Warden CH, Horwitz BA. T(3) stimulates resting metabolism and UCP-2 and UCP-3 mRNA but not nonphosphorylating mitochondrial respiration in mice. Am J Physiol. 1999;277:E380–E389. doi: 10.1152/ajpendo.1999.277.2.E380. [DOI] [PubMed] [Google Scholar]
- 76.Sinha RA, et al. Thyroid hormone induction of mitochondrial activity is coupled to mitophagy via ROS-AMPK-ULK1 signaling. Autophagy. 2015;11:1341–1357. doi: 10.1080/15548627.2015.1061849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lesmana R, et al. Thyroid hormone stimulation of autophagy is essential for mitochondrial biogenesis and activity in skeletal muscle. Endocrinology. 2016;157:23–38. doi: 10.1210/en.2015-1632. [DOI] [PubMed] [Google Scholar]
- 78.Ness GC. Thyroid hormone. Basis for its hypocholesterolemic effect. J Fla Med Assoc. 1991;78:383–385. [PubMed] [Google Scholar]
- 79.Ness GC, Pendleton LC, Li YC, Chiang JY. Effect of thyroid hormone on hepatic cholesterol 7α hydroxylase, LDL receptor, HMG-CoA reductase, farnesyl pyrophosphate synthetase and apolipoprotein A-I mRNA levels in hypophysectomized rats. Biochem Biophys Res Commun. 1990;172:1150–1156. doi: 10.1016/0006-291x(90)91568-d. [DOI] [PubMed] [Google Scholar]
- 80.Mooradian AD, Wong NC, Shah GN. Age-related changes in the responsiveness of apolipoprotein A1 to thyroid hormone. Am J Physiol. 1996;271:R1602–R1607. doi: 10.1152/ajpregu.1996.271.6.R1602. [DOI] [PubMed] [Google Scholar]
- 81.Lopez D, Abisambra Socarras JF, Bedi M, Ness GC. Activation of the hepatic LDL receptor promoter by thyroid hormone. Biochim Biophys Acta. 2007;1771:1216–1225. doi: 10.1016/j.bbalip.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 82.Lagrost L. Regulation of cholesteryl ester transfer protein (CETP) activity: review of in vitro and in vivo studies. Biochim Biophys Acta. 1994;1215:209–236. doi: 10.1016/0005-2760(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 83.Shin DJ, Osborne TF. Thyroid hormone regulation and cholesterol metabolism are connected through sterol regulatory element-binding protein-2 (SREBP-2) J Biol Chem. 2003;278:34114–34118. doi: 10.1074/jbc.M305417200. [This study describes the role of SREBP2 in thyroid-hormone-regulated cholesterol metabolism.] [DOI] [PubMed] [Google Scholar]
- 84.Moon JH, et al. Decreased expression of hepatic low-density lipoprotein receptor-related protein 1 in hypothyroidism: a novel mechanism of atherogenic dyslipidemia in hypothyroidism. Thyroid. 2013;23:1057–1065. doi: 10.1089/thy.2012.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ness GC, Lopez D. Transcriptional regulation of rat hepatic low-density lipoprotein receptor and cholesterol 7α hydroxylase by thyroid hormone. Arch Biochem Biophys. 1995;323:404–408. doi: 10.1006/abbi.1995.0061. [DOI] [PubMed] [Google Scholar]
- 86.Goldberg IJ, et al. Thyroid hormone reduces cholesterol via a non-LDL receptor-mediated pathway. Endocrinology. 2012;153:5143–5149. doi: 10.1210/en.2012-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bonde Y, Plosch T, Kuipers F, Angelin B, Rudling M. Stimulation of murine biliary cholesterol secretion by thyroid hormone is dependent on a functional ABCG5/G8 complex. Hepatology. 2012;56:1828–1837. doi: 10.1002/hep.25861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonde Y, et al. Thyroid hormone reduces PCSK9 and stimulates bile acid synthesis in humans. J Lipid Res. 2014;55:2408–2415. doi: 10.1194/jlr.M051664. [This study describes the effect of thyroid hormone on human proprotein convertase subtilisin/kexin type 9 (PCSK9).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yap CS, Sinha RA, Ota S, Katsuki M, Yen PM. Thyroid hormone negatively regulates CDX2 and SOAT2 mRNA expression via induction of miRNA-181d in hepatic cells. Biochem Biophys Res Commun. 2013;440:635–639. doi: 10.1016/j.bbrc.2013.09.116. [This study highlights the potential role of miRNA in thyroid-hormone-regulated cholesterol metabolism.] [DOI] [PubMed] [Google Scholar]
- 90.Grasselli E, et al. Non-receptor-mediated actions are responsible for the lipid-lowering effects of iodothyronines in FaO rat hepatoma cells. J Endocrinol. 2011;210:59–69. doi: 10.1530/JOE-11-0074. [DOI] [PubMed] [Google Scholar]
- 91.Cordeiro A, Souza LL, Einicker-Lamas M, Pazos-Moura CC. Non-classic thyroid hormone signalling involved in hepatic lipid metabolism. J Endocrinol. 2013;216:R47–R57. doi: 10.1530/JOE-12-0542. [DOI] [PubMed] [Google Scholar]
- 92.Cao X, Kambe F, Moeller LC, Refetoff S, Seo H. Thyroid hormone induces rapid activation of Akt/protein kinase B-mammalian target of rapamycin-p70S6K cascade through phosphatidylinositol 3-kinase in human fibroblasts. Mol Endocrinol. 2005;19:102–112. doi: 10.1210/me.2004-0093. [DOI] [PubMed] [Google Scholar]
- 93.Swierczynski J, et al. Triiodothyronine-induced accumulations of malic enzyme, fatty acid synthase, acetyl-coenzyme A carboxylase, and their mRNAs are blocked by protein kinase inhibitors. Transcription is the affected step. J Biol Chem. 1991;266:17459–17466. [PubMed] [Google Scholar]
- 94.Yamauchi M, et al. Thyroid hormone activates adenosine 5′-monophosphate-activated protein kinase via intracellular calcium mobilization and activation of calcium/calmodulin-dependent protein kinase kinase-β. Mol Endocrinol. 2008;22:893–903. doi: 10.1210/me.2007-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nakamura H, Rue PA, DeGroot LJ. Thyroid hormone increases type I adenosine 3′, 5′-monophosphate-dependent protein kinase and casein kinase activities in rat liver cytosol: analysis of protein kinases by polyacrylamide disc gel electrophoresis. Endocrinology. 1983;112:1427–1433. doi: 10.1210/endo-112-4-1427. [DOI] [PubMed] [Google Scholar]
- 96.Coppola M, et al. Thyroid hormone analogues and derivatives: actions in fatty liver. World J Hepatol. 2014;6:114–129. doi: 10.4254/wjh.v6.i3.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lanni A, et al. 3,5-Diiodo-L-thyronine powerfully reduces adiposity in rats by increasing the burning of fats. FASEB J. 2005;19:1552–1554. doi: 10.1096/fj.05-3977fje. [DOI] [PubMed] [Google Scholar]
- 98.Grasselli E, et al. Direct effects of iodothyronines on excess fat storage in rat hepatocytes. J Hepatol. 2011;54:1230–1236. doi: 10.1016/j.jhep.2010.09.027. [This article describes the role of 3,5-diiodothyronine in reducing hepatic fat.] [DOI] [PubMed] [Google Scholar]
- 99.Cavallo A, et al. 3,5-Diiodo-L-thyronine administration to hypothyroid rats rapidly enhances fatty acid oxidation rate and bioenergetic parameters in liver cells. PLOS ONE. 2013;8:e52328. doi: 10.1371/journal.pone.0052328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grasselli E, et al. 3,5-Diiodo-L-thyronine modifies the lipid droplet composition in a model of hepatosteatosis. Cell Physiol Biochem. 2014;33:344–356. doi: 10.1159/000356674. [DOI] [PubMed] [Google Scholar]
- 101.Vergani L. Lipid lowering effects of iodothyronines: in vivo and in vitro studies on rat liver. World J Hepatol. 2014;6:169–177. doi: 10.4254/wjh.v6.i4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gnocchi D, Massimi M, Alisi A, Incerpi S, Bruscalupi G. Effect of fructose and 3,5-diiodothyronine (3,5-T(2)) on lipid accumulation and insulin signalling in non-alcoholic fatty liver disease (NAFLD)-like rat primary hepatocytes. Horm Metab Res. 2014;46:333–340. doi: 10.1055/s-0034-1371858. [DOI] [PubMed] [Google Scholar]
- 103.Coppola M, Cioffi F, Moreno M, Goglia F, Silvestri E. 3,5-Diiodo-L-thyronine: a possible pharmacological agent? Curr Drug Deliv. 2016;13:330–338. doi: 10.2174/1567201813666151123124340. [DOI] [PubMed] [Google Scholar]
- 104.de Lange P, et al. Nonthyrotoxic prevention of diet-induced insulin resistance by 3,5-diiodo-L-thyronine in rats. Diabetes. 2011;60:2730–2739. doi: 10.2337/db11-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yan F, et al. Thyrotropin increases hepatic triglyceride content through upregulation of SREBP-1c activity. J Hepatol. 2014;61:1358–1364. doi: 10.1016/j.jhep.2014.06.037. [This study describes a direct action of TSH in regulating hepatic lipid metabolism.] [DOI] [PubMed] [Google Scholar]
- 106.Song Y, et al. Thyroid-stimulating hormone regulates hepatic bile acid homeostasis via SREBP-2/HNF-4α/CYP7A1 axis. J Hepatol. 2015;62:1171–1179. doi: 10.1016/j.jhep.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 107.Zhang X, et al. Thyroid-stimulating hormone decreases HMG-CoA reductase phosphorylation via AMP-activated protein kinase in the liver. J Lipid Res. 2015;56:963–971. doi: 10.1194/jlr.M047654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab. 2003;88:2438–2444. doi: 10.1210/jc.2003-030398. [DOI] [PubMed] [Google Scholar]
- 109.Tzotzas T, Krassas GE, Konstantinidis T, Bougoulia M. Changes in lipoprotein(a) levels in overt and subclinical hypothyroidism before and during treatment. Thyroid. 2000;10:803–808. doi: 10.1089/thy.2000.10.803. [DOI] [PubMed] [Google Scholar]
- 110.Sherman SI, et al. Augmented hepatic and skeletal thyromimetic effects of tiratricol in comparison with levothyroxine. J Clin Endocrinol Metab. 1997;82:2153–2158. doi: 10.1210/jcem.82.7.4054. [DOI] [PubMed] [Google Scholar]
- 111.The coronary drug project. Findings leading to further modifications of its protocol with respect to dextrothyroxine. The coronary drug project research group. JAMA. 1972;220:996–1008. [No authors listed.] [PubMed] [Google Scholar]
- 112.Galioni EF, et al. Long-term effect of dried thyroid on serum-lipoprotein and serum-cholesterol levels. Lancet. 1957;272:120–123. doi: 10.1016/s0140-6736(57)90199-x. [DOI] [PubMed] [Google Scholar]
- 113.Baxter JD, Webb P. Thyroid hormone mimetics: potential applications in atherosclerosis, obesity and type 2 diabetes. Nat Rev Drug Discov. 2009;8:308–320. doi: 10.1038/nrd2830. [DOI] [PubMed] [Google Scholar]
- 114.Elbers LP, Kastelein JJ, Sjouke B. Thyroid hormone mimetics: the past, current status and future challenges. Curr Atheroscler Rep. 2016;18:14. doi: 10.1007/s11883-016-0564-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Underwood AH, et al. A thyromimetic that decreases plasma cholesterol levels without increasing cardiac activity. Nature. 1986;324:425–429. doi: 10.1038/324425a0. [DOI] [PubMed] [Google Scholar]
- 116.Tancevski I, et al. The liver-selective thyromimetic T-0681 influences reverse cholesterol transport and atherosclerosis development in mice. PLOS ONE. 2010;5:e8722. doi: 10.1371/journal.pone.0008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Taylor AH, Stephan ZF, Steele RE, Wong NC. Beneficial effects of a novel thyromimetic on lipoprotein metabolism. Mol Pharmacol. 1997;52:542–547. doi: 10.1124/mol.52.3.542. [DOI] [PubMed] [Google Scholar]
- 118.Goldman S, et al. DITPA (3,5-Diiodothyropropionic Acid), a thyroid hormone analog to treat heart failure: phase II trial veterans affairs cooperative study. Circulation. 2009;119:3093–3100. doi: 10.1161/CIRCULATIONAHA.108.834424. [DOI] [PubMed] [Google Scholar]
- 119.Johansson L, et al. Selective thyroid receptor modulation by GC-1 reduces serum lipids and stimulates steps of reverse cholesterol transport in euthyroid mice. Proc Natl Acad Sci USA. 2005;102:10297–10302. doi: 10.1073/pnas.0504379102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tancevski I, Demetz E, Eller P. Sobetirome: a selective thyromimetic for the treatment of dyslipidemia. Recent Pat Cardiovasc Drug Discov. 2011;6:16–19. doi: 10.2174/157489011794578473. [DOI] [PubMed] [Google Scholar]
- 121.Kannisto K, et al. The thyroid receptor β modulator GC-1 reduces atherosclerosis in ApoE deficient mice. Atherosclerosis. 2014;237:544–554. doi: 10.1016/j.atherosclerosis.2014.09.035. [DOI] [PubMed] [Google Scholar]
- 122.Grover GJ, Mellstrom K, Malm J. Development of the thyroid hormone receptor β-subtype agonist KB-141: a strategy for body weight reduction and lipid lowering with minimal cardiac side effects. Cardiovasc Drug Rev. 2005;23:133–148. doi: 10.1111/j.1527-3466.2005.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 123.Ladenson PW, et al. Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N Engl J Med. 2010;362:906–916. doi: 10.1056/NEJMoa0905633. [This study describes the use of a thyroid hormone analogue in treating dyslipidaemia in humans.] [DOI] [PubMed] [Google Scholar]
- 124.Kelly MJ, et al. Discovery of 2-[3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy) phenyl]-3,5-dioxo-2,3,4,5-tetrahydro[1,2,4] triazine-6-carbonitrile (MGL-3196), a highly selective thyroid hormone receptor β agonist in clinical trials for the treatment of dyslipidemia. J Med Chem. 2014;57:3912–3923. doi: 10.1021/jm4019299. [DOI] [PubMed] [Google Scholar]
- 125.Ito BR, et al. Thyroid hormone β receptor activation has additive cholesterol lowering activity in combination with atorvastatin in rabbits, dogs and monkeys. Br J Pharmacol. 2009;156:454–465. doi: 10.1111/j.1750-3639.2009.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Myers C. Metabasis therapeutics announces the publication of pre-clinical findings on MB07811, its product candidate. FierceBiotech. 2009 https://www.fiercebiotech.com/biotech/metabasis-therapeutics-announces-publication-of-pre-clinical-findings-on-mb07811-its. [Google Scholar]
- 127.US National Library of Medicine. ClinicalTrials.gov. 2018 http://clinicaltrials.gov/ct2/show/NCT00879112.
- 128.Trost SU, et al. The thyroid hormone receptor-β-selective agonist GC-1 differentially affects plasma lipids and cardiac activity. Endocrinology. 2000;141:3057–3064. doi: 10.1210/endo.141.9.7681. [DOI] [PubMed] [Google Scholar]
- 129.Bryzgalova G, et al. Anti-obesity, anti-diabetic, and lipid lowering effects of the thyroid receptor β subtype selective agonist KB-141. J Steroid Biochem Mol Biol. 2008;111:262–267. doi: 10.1016/j.jsbmb.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 130.Ahmed M. Non-alcoholic fatty liver disease in 2015. World J Hepatol. 2015;7:1450–1459. doi: 10.4254/wjh.v7.i11.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 132.Caligiuri A, Gentilini A, Marra F. Molecular pathogenesis of NASH. Int J Mol Sci. 2016;17:1575. doi: 10.3390/ijms17091575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pais R, et al. NAFLD and liver transplantation: current burden and expected challenges. J Hepatol. 2016;65:1245–1257. doi: 10.1016/j.jhep.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eshraghian A, Hamidian Jahromi A. Non-alcoholic fatty liver disease and thyroid dysfunction: a systematic review. World J Gastroenterol. 2014;20:8102–8109. doi: 10.3748/wjg.v20.i25.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ludwig U, et al. Subclinical and clinical hypothyroidism and non-alcoholic fatty liver disease: a cross-sectional study of a random population sample aged 18 to 65 years. BMC Endocr Disord. 2015;15:41. doi: 10.1186/s12902-015-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu C, Xu L, Yu C, Miao M, Li Y. Association between thyroid function and nonalcoholic fatty liver disease in euthyroid elderly Chinese. Clin Endocrinol. 2011;75:240–246. doi: 10.1111/j.1365-2265.2011.04016.x. [DOI] [PubMed] [Google Scholar]
- 137.Chung GE, et al. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J Hepatol. 2012;57:150–156. doi: 10.1016/j.jhep.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 138.Bano A, et al. Thyroid function and the risk of nonalcoholic fatty liver disease: The Rotterdam Study. J Clin Endocrinol Metab. 2016;101:3204–3211. doi: 10.1210/jc.2016-1300. [DOI] [PubMed] [Google Scholar]
- 139.Torun E, Ozgen IT, Gokce S, Aydin S, Cesur Y. Thyroid hormone levels in obese children and adolescents with non-alcoholic fatty liver disease. J Clin Res Pediatr Endocrinol. 2014;6:34–39. doi: 10.4274/Jcrpe.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gokmen FY, et al. FT3/FT4 ratio predicts non-alcoholic fatty liver disease independent of metabolic parameters in patients with euthyroidism and hypothyroidism. Clin (Sao Paulo) 2016;71:221–225. doi: 10.6061/clinics/2016(04)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tao Y, Gu H, Wu J, Sui J. Thyroid function is associated with non-alcoholic fatty liver disease in euthyroid subjects. Endocr Res. 2015;40:74–78. doi: 10.3109/07435800.2014.952014. [DOI] [PubMed] [Google Scholar]
- 142.Sinha RA, Yen PM. Thyroid hormone-mediated autophagy and mitochondrial turnover in NAFLD. Cell Biosci. 2016;6:46. doi: 10.1186/s13578-016-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sinha RA, Singh BK, Yen PM. Thyroid hormone regulation of hepatic lipid and carbohydrate metabolism. Trends Endocrinol Metab. 2014;25:538–545. doi: 10.1016/j.tem.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 144.Pihlajamaki J, et al. Thyroid hormone-related regulation of gene expression in human fatty liver. J Clin Endocrinol Metab. 2009;94:3521–3529. doi: 10.1210/jc.2009-0212. [This paper presents an interesting study describing defective hepatic thyroid hormone signalling in human NAFLD.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li QL, Yamamoto N, Inoue A, Morisawa S. Fatty acyl-CoAs are potent inhibitors of the nuclear thyroid hormone receptor in vitro. J Biochem. 1990;107:699–702. doi: 10.1093/oxfordjournals.jbchem.a123111. [DOI] [PubMed] [Google Scholar]
- 146.Bohinc BN, et al. Repair-related activation of hedgehog signaling in stromal cells promotes intrahepatic hypothyroidism. Endocrinology. 2014;155:4591–4601. doi: 10.1210/en.2014-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Finan B, et al. Chemical hybridization of glucagon and thyroid hormone optimizes therapeutic impact for metabolic disease. Cell. 2016;167:843–857. doi: 10.1016/j.cell.2016.09.014. [This study describes a novel approach of using chemical hybridization of thyroid hormone and glucagon for the treatment of metabolic diseases.] [DOI] [PubMed] [Google Scholar]
- 148.Perra A, et al. Thyroid hormone (T3) and TRβ agonist GC-1 inhibit/reverse nonalcoholic fatty liver in rats. FASEB J. 2008;22:2981–2989. doi: 10.1096/fj.08-108464. [DOI] [PubMed] [Google Scholar]
- 149.Refetoff S, Weiss RE, Usala SJ. The syndromes of resistance to thyroid hormone. Endocr Rev. 1993;14:348–399. doi: 10.1210/edrv-14-3-348. [DOI] [PubMed] [Google Scholar]
- 150.Chng CL, et al. Physiological and metabolic changes during the transition from hyperthyroidism to euthyroidism in Graves’ disease. Thyroid. 2016;26:1422–1430. doi: 10.1089/thy.2015.0602. [DOI] [PubMed] [Google Scholar]
- 151.Vatner DF, et al. Thyroid hormone receptor-β agonists prevent hepatic steatosis in fat-fed rats but impair insulin sensitivity via discrete pathways. Am J Physiol Endocrinol Metab. 2013;305:E89–E100. doi: 10.1152/ajpendo.00573.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lammel Lindemann J, Webb P. Sobetirome: the past, present and questions about the future. Expert Opin Ther Targets. 2016;20:145–149. doi: 10.1517/14728222.2016.1090429. [DOI] [PubMed] [Google Scholar]
- 153.Liangpunsakul S, Chalasani N. Is hypothyroidism a risk factor for non-alcoholic steatohepatitis? J Clin Gastroenterol. 2003;37:340–343. doi: 10.1097/00004836-200310000-00014. [DOI] [PubMed] [Google Scholar]
- 154.Pagadala MR, et al. Prevalence of hypothyroidism in nonalcoholic fatty liver disease. Dig Dis Sci. 2012;57:528–534. doi: 10.1007/s10620-011-2006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim D, et al. Subclinical hypothyroidism and low-normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin Gastroenterol Hepatol. 2018;16:123–131. doi: 10.1016/j.cgh.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 156.Hassan MM, et al. Association between hypothyroidism and hepatocellular carcinoma: a case-control study in the United States. Hepatology. 2009;49:1563–1570. doi: 10.1002/hep.22793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Frau C, et al. Local hypothyroidism favors the progression of preneoplastic lesions to hepatocellular carcinoma in rats. Hepatology. 2015;61:249–259. doi: 10.1002/hep.27399. [DOI] [PubMed] [Google Scholar]
- 158.Chan IH, Privalsky ML. Thyroid hormone receptors mutated in liver cancer function as distorted antimorphs. Oncogene. 2006;25:3576–3588. doi: 10.1038/sj.onc.1209389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yen CC, et al. Mediation of the inhibitory effect of thyroid hormone on proliferation of hepatoma cells by transforming growth factor-β. J Mol Endocrinol. 2006;36:9–21. doi: 10.1677/jme.1.01911. [DOI] [PubMed] [Google Scholar]