Abstract
Heterotopic ossification (HO) involves the formation and accumulation of extraskeletal bone tissue at the expense of local tissues including muscles and connective tissues. There are common forms of HO that are triggered by extensive trauma, burns and other bodily insults, and there are also rare congenital severe forms of HO that occur in children with Fibrodysplasia Ossificans Progressiva or Progressive Osseous Heteroplasia. Given that HO is often preceded by inflammation, current treatments usually involve anti-inflammatory drugs alone or in combination with local irradiation, but are not very effective. Recent studies have provided novel insights into the pathogenesis of acquired and genetic forms of HO and have used the information to conceive and test new and more specific therapies in animal models. In this review, I provide salient examples of these exciting and promising advances that are undoubtedly paving the way toward resolution of this debilitating and at times fatal disease.
Introduction
Heterotopic ossification (HO) consists in the formation of bone tissue at extraskeletal anatomical sites at the expense of local tissues, including muscles and connective tissues [1, 2]. Because of their size and locations, the HO masses can hamper routine body functions and physical mobility and lead to chronic pain, joint ankylosis, pressure ulcers, venous thrombosis and other health complications [3]. One form of HO is acquired and common and is triggered by extensive trauma, invasive surgeries, spinal cord and brain injuries, protracted immobilization or deep burns [3–5]. The pathogenesis of acquired HO is not clear, but it is thought that the severe local inflammation triggered by the above and encompassing physical insults would lead to recruitment of progenitor cells, release of as yet unclear pro-skeletogenic factors, derangement of normal tissue repair processes, and formation of heterotopic bone [6–9]. There are also congenital, very severe but rare forms of HO that occur in children with Fibrodysplasia Ossificans Progressiva (FOP) (OMIM 135100) or Progressive Osseous Heteroplasia (POH) (OMIM 166350) [10]. Patients with FOP carry mildly-activating mutations in ACVR1 that encodes the cell surface type I bone morphogenetic protein (BMP) receptor ALK2 (activin receptor-like kinase 2) [11]. Several mutations have been identified so far and they all cluster in the intracellular glycine-serine-rich (GS) domain of ALK2, with the most common mutation being ACVR1R206H that characterizes patients with classic FOP [12]. With regard to POH, patients carry inactivating mutations in GNAS that encodes Gαs, an intracellular protein that mediates and elicits signals from G protein-coupled receptors (GPCRs) [13, 14]. The congenital mutations in FOP and POH are thought to subvert normal homeostatic and cell differentiation mechanisms, leading progenitors to activate skeletogenic processes and causing formation of ectopic endochondral or intramembranous bone, respectively [10]. Both acquired and congenital forms of HO remain clinical challenges since no truly effective treatments have been created so far [15, 16]. In this review, I will describe basic aspects of HO development and exciting novel pathogenic findings from recent studies that have been instrumental in the conception and testing of new treatments in animal studies and in an ongoing phase 2 clinical trial for FOP.
HO development
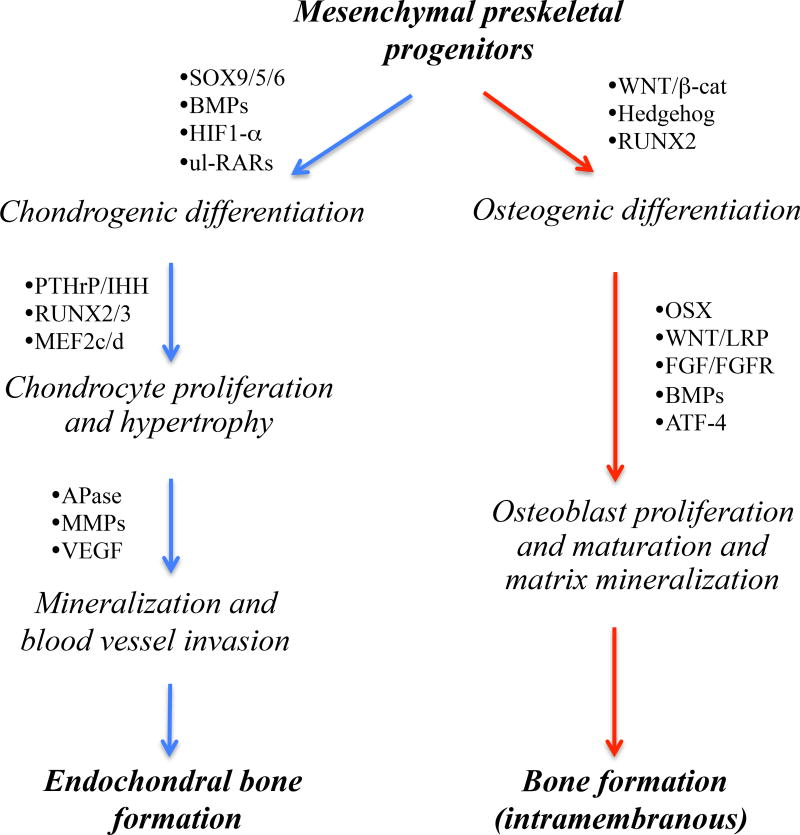
The initiation and growth of injury- or surgery-induced HO tissue masses most often occur via, and replicate, the endochondral ossification process by which the majority of skeletal elements normally form and grow during prenatal and postnatal life [17, 18]. The same endochondral process invariably mediates HO formation in FOP patients [19]. During that multistep developmental process, preskeletal mesenchymal cells undergo condensation and differentiate into chondrocytes that lay down and assemble the cartilaginous rudiments of future skeletal elements. The neoformed chondrocytes become organized into growth plates with typical zones of proliferation, maturation and hypertrophy that sustain growth, expansion and elongation of the skeletal elements and eventually permit the replacement of mineralized hypertrophic cartilage with endochondral bone and marrow. All these steps are recapitulated in the above forms of HO following local inflammation, though tissue and zone structure and configuration are not as distinct and well defined as those in a standard growth plate. In contrast, HO formation in POH as well as in the related disorder Albright Hereditary Osteodystrophy (OMIM 103580) occurs via intramembranous ossification [10]. This process normally subtends the formation of a few skeletal elements including calvaria and mandible, and entails the direct differentiation of condensed mesenchymal cells into osteoblasts without an intermediate cartilaginous step [18]. Much is known about the cellular and molecular mechanisms that regulate the development of chondrocytes and osteoblasts and the processes of endochondral and intramembranous ossification [18, 20–23]. Major regulators involved in these developmental pathways are schematically depicted in Fig. 1. For example, it is well established that the commitment and differentiation of preskeletal mesenchymal cells toward chondrogenesis and endochondral ossification are promoted by: hypoxia; BMP signaling (acting via canonical phosphorylated SMAD1/5/8 signaling); and transcription factors such as SOX trio and unliganded retinoic acid receptors (RARs) [18, 20, 24]. On the other hand, progenitor cell differentiation toward osteogenesis and bone formation are promoted by: Wnt/β-catenin signaling; nuclear factors such as Runx2 and Osterix; and vascularization [18, 25]. Such extensive knowledge has been, and continues to be, of fundamental value and relevance for the conception and design of possible treatments for HO, as elaborated below.
Fig. 1.
Schematic describes the major developmental steps that underlie the differentiation of preskeletal mesenchymal cells toward endochondral (right) and intramembranous (left) ossification. Multiple distinct mechanisms control fate determination and commitment of the progenitor cells to either developmental pathway, including hypoxia and retinoid and Wnt/β-catenin signaling that are particularly relevant to studies discussed in this review article. In many if not all respects, these developmental pathways are recapitulated in the formation of endochondral and intramembranous masses of HO tissue. Thus, specific steps and factors involved in each developmental pathway are being tested and exploited as possible and reasonable therapeutic targets for acquired and/or congenital forms of HO (as described in the text). Note that factors and pathways mediating the transition from mineralized hypertrophic cartilage to endochondral bone are very similar to those regulating osteogenic differentiation (listed on the left). BMP, bone morphogenetic protein; HIF, hypoxia-inducible factor; RAR, retinoic acid receptor; ul-RAR, unliganded RAR; PTHrP, parathyroid hormone-related protein; IHH, indian hedgehog; APase, alkaline phosphatase; MMP, matrix metalloprotease; VEGF, vascular endothelial growth factor; FGF, fibroblast growth factor; FGFR, fibroblast growth factor receptor; and OSX, osterix.
Treatments for acquired HO
Current treatments for acquired HO include systemic administration of non-steroidal anti-inflammatory drugs (NSAIDs), prophylactic low-dose irradiation limited to the involved site, or a combination of both [16, 26]. NSAIDs inhibit the production and release of both physiologic and inflammatory prostaglandins [27], and animals treated with the NSAID drug indomethacin exhibited more resistance to experimental HO (triggered by injury or intramuscular injection of recombinant BMPs) and displayed lower local skeletogenic cell differentiation [28, 29]. Irradiation of the affected site is believed to act broadly and influence mesenchymal cells (MSCs) present in local injured tissues, altering their functioning and/or survival and eventually reducing their ability to undergo skeletogenic differentiation [26, 30, 31]. Such standard common treatments are associated with decreases in HO incidence, but are not always effective and cannot be given to certain patients such as severely-wounded soldiers because of their often extensive wounds and other combat-related injuries [32]. In addition, NSAIDs often elicit side effects that include gastrointestinal pain and ulceration, renal toxicity and lower platelet function [26] that affect patient compliance [33]. Surgery is also used to remove the HO tissue masses, but it requires further hospitalization and can cause complications including infections, blood loss and even another round of HO [5, 34]. It is important to note that these current treatments are generic and do not target specific steps in the skeletogenic processes, possibly limiting effectiveness and applicability and eliciting side effects. Thus, recent new studies such as those described below have shifted away from those general approaches and have aimed to target specific steps in HO development.
To identify new culprits and pathways involved in HO, Peterson et al. [35] collected surgical discard specimens of adipose tissue and adipose-derived MSCs from over 200 burn patients within 3 days from injury; adipose tissue and cells from 35 unaffected individuals served as controls. Global gene array expression analyses revealed that canonical pSMAD1/5/8 signaling and RUNX2 expression were significantly increased in samples from burn patients compared to controls, and there was also a direct link between pSMAD1/5/8 signaling and adenosine receptors. Because purinergic receptors have been implicated in bone development [36, 37], the data indicated that both BMP signaling and ATP activity may play roles in burn-induced HO. To test this thesis, the authors created a mouse model of HO that combined dorsal scalp burn with Achilles tenotomy and elicited tendon-associated HO (forming via endochondral ossification) after 4 to 8 weeks. As in burn patients, the authors found that adipose tissue-derived MSCs harvested soon after burn injury displayed higher levels of both canonical BMP signaling and ATP and greater osteogenic capacity compared to control cells. Extracellular ATP levels could have affected intracellular production of cyclic AMP levels that when high, can inhibit SMAD1/5/8 phosphorylation and osteogenic differentiation [38]. To test relevance to HO, mice receiving burn and tenotomy were treated with apyrase at the burn site to cleave ATP (and thus increase adenosine levels and cyclic AMP production) and were then monitored for HO development over time. Companion mice receiving no apyrase treatment developed extensive HO in their injured Achilles tendon, whereas those treated with apyrase displayed reduced levels of BMP signaling and RUNX2 expression, lower amounts of HO, and improved range-of-motion in the operated limb [35]. The study reveals systemic consequences following HO-eliciting burn injuries and suggests that remote ATP hydrolysis via local apyrase treatment at the burn site may represent a therapy for HO.
Neurological HO is a frequent pathology observed in 10 to 20% of patients with traumatic spinal cord and brain injuries [5, 39]. Genet et al. [40] recently created the first mouse model of spinal cord injury-induced HO that involves spinal cord transection between T7 and T8 combined with cardiotoxin-induced hamstring muscle injury, using 5–6 week-old mice. Intramuscular HO readily developed in the operated mice, but only in those subjected to both spinal cord and muscle injury. Serum from the affected mice stimulated osteogenic cell differentiation in MSC cultures without addition of other stimulating factors. Substance P was present in high amounts in the serum of affected mice, but treatment with a substance P receptor antagonist did not elicit a major decrease in HO. On the other hand, pharmacologic ablation of phagocytic macrophages reduced the amount of HO by nearly 90%. The data indicate that a combination of neurological damage and tissue inflammation drives HO development and that treatments targeting both inflammation and macrophage function could represent more effective methods against HO.
Hypoxia stimulates chondrogenic cell differentiation [41, 42], a reflection of the fact that embryonic preskeletal cell condensations are devoid of blood vessels and that forced induction of blood vessels blocks chondrogenesis and skeletogenesis [43]. Hypoxia inducible factor-1α (Hif1α) is a key signaling mediator of cellular hypoxic responses and is important for chondrogenesis [41, 42]. Thus, Agarwal et al. [44] tested the possibility that targeting Hif1α may represent a treatment for HO. Using the scalp burn-Achilles tendon HO mouse model [35], they found that Hif1α gene expression was indeed elevated at prospective tendon HO sites as indicated by immunostaining and gene expression analyses. Interestingly, a similar HIF1α upregulation was seen in fat tissue from burn patients compared to healthy controls. Systemic treatment of mice with pharmacologic Hif1α inhibitors-PX-478 or rapamycin- led to a significant decrease in tendon-associated HO as indicated by decreased expression of chondrogenic marker genes (Sox9 and aggrecan) and histological and μCT analyses. Notably, there were no major adverse effects of drug treatment on wound healing at the burn site or at the leg tenotomy site. Equally interesting was the finding that HO was substantially reduced in Hif1α-deficient mice in which floxed Hif1α had been conditionally ablated by mating with Prx-Cre mice (targeting the entire limb mesenchymal population) [44]. The study points to the novel possibility that targeting Hif1α may represent a promising new therapy for HO.
Injury to muscles and other soft tissues can cause dystrophic calcification [45] that may predispose the tissues to HO [46, 47], but the mechanisms normally protecting injured tissues from calcification are unclear. Interestingly, Schoenecker and co-workers recently showed for the first time that plasminogen deficiency inhibited bone fracture healing while concurrently promoting HO in neighboring muscles [48]. The negative effects on bone repair were in line with the roles of plasminogen in tissue repair in general [49]. Considering the fact that plasminogen deficiency had been linked to sporadic liver calcification after injury [50], the group set out to directly test plasminogen role in calcification and HO [51]. In global plasminogen-deficient mutant mice, cardiotoxin- or crush-induced muscle injury was associated with dramatic and rapid local dystrophic calcification that was followed by formation of endochondral HO masses over time. These responses were not observed in operated wild type littermates. Experimental down-regulation of α2-antiplasmin expression or treatment with pyrophosphate (a mineralization inhibitor) led to a marked reduction of both calcification and HO in operated plasminogen-deficient mice. This interesting study thus points to the possibility that increasing plasmin activity may represent a new pharmacologic therapy to prevent soft tissue calcification and subsequent HO while eliciting beneficial effects on tissue repair.
Treatments for congenital HO
HO in FOP patients is extremely aggressive and involves the extraskeletal accumulation of large amounts of endochondral bone masses throughout the body, resulting in severe interference with body function and even premature death [19]. HO in these patients is inoperable given that the disease is highly reactive and surgery causes recurrent and even more severe HO. Because the onset of HO formation is often preceded by local inflammation [52], the current standard of care for FOP patients is a brief 4-day course of high-dose corticosteroids normally started within 24 h of a flare-up [15]. The steroid treatment can lower inflammation, swelling and pain, but is not able to consistently reduce the frequency of progression of the flare-up to HO [15]. As indicated above, FOP patients carry mildly-activating mutations in ACVR1 encoding ALK2 [11, 12], a BMP receptor that under normal circumstances exerts pro-chondrogenic and pro-skeletogenic effects when activated by appropriate BMPs [22, 53]. Thus, therapeutic strategies conceived and tested over the last few years have aimed to block the over-active BMP signaling, using FOP mouse models.
In the first ever study of this type, Yu et al. [54] reported that systemic treatment with LDN-193189 –a selective inhibitor of BMP type I receptor kinases- did significantly reduce HO in the limbs of transgenic mice expressing an inducible and constitutive-active Acvr1Q207D transgene [55]. LDN-193189 is a modified version of Dorsomorphin and had been optimized for pharmacokinetic characteristics and stability [56]. The authors found that LDN-193189 treatment reduced pSMAD1/5/8 levels and BMP signaling in the affected limb tissues expressing Acvr1Q207D and, importantly, elicited beneficial effects on limb mobility and function compared to littermates receiving no treatment. In follow-up studies [57, 58], the same group and collaborators developed a more potent drug inhibitor –LDN-212454- that displayed a nearly 4-fold higher selectivity for BMP type I receptor kinases compared to related TGF-β and Activin type I receptors and also exhibited some selectivity for ALK2 versus ALK1 and ALK3. They found that LDN-212454 was able to markedly reduce HO in the Acvr1Q207D-expressing mice, providing an important step ahead toward the ultimate creation of a highly selective and specific drug of this type to treat FOP.
The above study was particularly important also for its first proof-of-principle evidence that drug treatment can largely block genetically-driven HO. However, because the ligands involved in HO induction were not known at the time and because drugs targeting exclusively mutant ALK2 were, and still are, not available, we resorted to a different pharmacologic strategy. It had been known for several years that (i) chondrogenesis requires transcriptional repressor action by unliganded nuclear retinoic acid receptors (RARs) [24] and (ii) treatment with natural retinoid agonists such as all-trans-retinoic acid (and ensuing reversal of transcriptional repression) blocks chondrogenesis in vitro and in vivo [59]. Additionally, genes involved in BMP signaling were found to be the most highly reduced by retinoid agonist treatment of chondrogenic cells, as revealed by transcriptome analyses [60]. Because chondrogenesis normally involves expression of unliganded RARα followed by RARγ [61], we tested the ability of synthetic retinoid agonists selective for either of these receptors to inhibit HO in Acvr1Q207D-expressing mice [62]. We did find that systemic oral or subdermal administration of retinoid agonists powerfully inhibited HO, with the RARγ agonists being far more effective. In vivo and in vitro experiments revealed that the agonists had blocked the chondrogenic phase of HO and that there was no significant rebound effect after drug treatment stoppage. Mechanistically, the agonists acted by blocking expression of master chondrogenic genes such as Sox9 while reducing canonical pSMAD1/5/8-mediated BMP signaling and promoting SMAD degradation via the proteasome. One of the most effective RARγ agonists we identified was Palovarotene [63] and in a recent study, we demonstrated that this same drug effectively prevented HO in a conditional knock-in mouse model expressing ACVR1R206H, the most common mutation in FOP patients associated with classic disease presentation [64]. We found also that Palovarotene was more tolerated by mutant mice than wild type littermates, likely reflecting its ability to normalize BMP signaling in the mutants but lowering it in controls. It is notable and important to point out that our mouse studies have led to the testing of Palovarotene in a FDA-approved double blind and placebo phase 2 clinical trial with FOP patients that was initiated by Clementia Pharmaceuticals in July 2014 (ClinicalTrials.gov identifier NCT02190747) [65]. Clementia announced top-line results in October 2016 indicating that, promisingly, Palovarotene treatment led to reductions in: the fraction of patients developing HO; time of resolution of a flare-up; and patient-reported pain linked to the flare-up area. The trial is ongoing and is in an open-label extension at the moment.
Hatsell et al. recently uncovered an intriguing new aspect of HO pathogenesis in FOP and described a new potential treatment based on it [66]. Using cell lines over-expressing ACVR1R206H, they found that the cells signaled via canonical pSMAD1/5/8 not only in response to various BMPs (including BMP6), but also activin A. This response was not observed in cells over-expressing wild type ACVR1. In these control cells, exogenous activin A interfered with responsiveness to BMP6 and pSMAD1/5/8 signaling (monitored via a BRE-Luc reporter) in agreement with a concurrent study showing that activin A normally has antagonist action on certain BMPs (BMP6 and BMP9) and canonical signaling [67]. Thus, the authors tested whether systemic administration of activin A neutralizing antibodies would be able to inhibit HO in conditional knock-in ACVR1R206H-expressing transgenic mice they originally created, and found that the antibodies were indeed quite effective. In a follow-up study just published, the group asked whether the neo-function of activin A was only needed to initiate HO in FOP model mice or may be needed continuously [68]. They found that expansion of initial HO lesions involved growth and fusion of independent neighboring HO masses. Expansion appeared to dependent on activin A since expansion of pre-existing masses as well as emergence of new ones were both inhibited by activin A antibody treatment. The authors concluded that HO in FOP is initially induced by inflammation and activin A neo-function, and relies on activin A for further growth and expansion. The data provide further strength to the interesting possibility that antibody-based interference with activin A action may represent a novel treatment for HO in FOP patients.
At variance with FOP, HO in Progressive Osseous Heteroplasia (POH) develops via intramembranous ossification that involves the direct differentiation of skeletal progenitor cells into osteoblasts. Clinically, POH is diagnosed in infancy and initially presents with subcutaneous ossification sites that can spread into neighboring muscles and other soft tissues over time. The inactivating mutations in GNAS causing POH have been known for a while, but the molecular mechanisms leading to HO had remained unclear. In a significant step ahead, Regard et al. [69] showed that conditional ablation of Gnasf/f in mouse limb mesenchyme using Prrx1Cre mice caused extraskeletal mineralization and progressive HO of soft tissues. The same was seen when adenoCre particles were injected subcutaneously in 4 week-old Gnasf/f mice. Mutant progenitors isolated from subcutaneous or adipose tissue exhibited accelerated osteogenesis in vitro compared to controls [70]. In mutant cells as well as mutant tissues in vivo, there was up-regulation of hedgehog signaling –as indicated by increased Patched and Gli expression- that could have been pathogenic. Indeed, concurrent conditional ablation of Gnas and Gli2 with Prrx1Cre mice largely rescued the HO phenotype [69]. In sum, the study points to the strong possibility that pharmacologic inhibitors of hedgehog signaling–including those in current clinical use against certain forms of cancer [71]-could represent a powerful treatment for HO in POH.
Conclusions and perspectives
Current clinical treatments for HO are based on the consistent observation that both acquired and genetic forms of HO (at least in FOP) are preceded by local inflammation and thus, the use of anti-inflammatory drugs is justified and can be beneficial. However, there has long be a need to create more effective and specific therapies, and the examples of recent studies described above do provide evidence of progress in this important biomedical arena. This progress was made possible by, and derives directly from, advances in cellular and molecular pathogenesis of HO that have allowed selection of drugs directed toward specific steps in pathogenic cascades, be it anti-Hif-1a drugs and plasmin in acquired HO or activin A antibodies and Palovarotene in FOP. Given the ability of hedgehog signaling to induce osteogenesis [72], it is also possible that hedgehog antagonists could prove effective against HO in POH. We do not know whether any of these putative novel treatments will prove to be effective and safe in patients and which among alternative treatments for the same HO type will turn out to be most effective and safest, though early data on Palovatotene efficacy and safety in the ongoing FOP clinical trial are very encouraging. It should be noted that while I described therapeutic approaches for acquired HO separately from those for congenital forms of HO, this was done for illustration only. Indeed, some of the above novel treatments have actually been tested in both acquired and genetic mouse models, including Hif-1α antagonists and Palovarotene [44, 62]. In addition, it is important to point out other emerging treatments for HO, including interference with macrophage and mast cell function [73] and inhibition of excessive levels of active transforming growth factor-β found in neoforming HO lesions [74]. In sum, the current combination of basic biological analyses with translational medicine approaches is paving the way toward effective therapeutic resolutions of HO in all its forms, providing renewed hope to patients and their families alike that these debilitating diseases will one day be treatable.
Highlights.
-
-
Heterotopic ossification involves formation of extraskeletal bone tissue
-
-
Current drug- and radiation-based treatments are not effective and have side effects
-
-
This review describes recent advances on the pathogenesis of acquired and congenital HO
-
-
The new data and insights are paving the way toward more effective treatments
Acknowledgments
Studies in my laboratory included in this review were supported by grants W81XWH-07-1-0212 and W81XWH-13-2-0076 from the Department of Defense’s Peer Reviewed Orthopaedic Research Program (PRORP) and grant 1RO1 AR071946-01 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. I would like to express my gratitude to colleagues, postdocs and research technicians who participated in our HO studies over the years. Due to the concise nature of this review, not all relevant and deserving literature and authors could be cited.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garland DE. A clinical prospective on common forms of acquired heterotopic ossification. Clin. Orthop. 1991;263:13–29. [PubMed] [Google Scholar]
- 2.Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J. Nucl. Med. 2002;43:346–353. [PubMed] [Google Scholar]
- 3.Vanden Bosshe L, Vanderstraeten G. Heterotopic ossification: a review. J. Rehabil. Med. 2005;37:129–136. doi: 10.1080/16501970510027628. [DOI] [PubMed] [Google Scholar]
- 4.Forsberg JA, Potter BK. Heterotopic ossification in wartime wounds. J. Surg. Orthop. Adv. 2010;19:54–61. [PubMed] [Google Scholar]
- 5.Van Kuijk AA, Geurts AC, van Kuppevelt HJ. Neurogenic heterotopic ossification in spinal cord injury. Spinal Cord. 2002;40:313–326. doi: 10.1038/sj.sc.3101309. [DOI] [PubMed] [Google Scholar]
- 6.Chalmers J, Gray DH, Rush J. Observation on the induction of bone in soft tissues. J. Bone Joint Surg. Br. 1975;57:36–45. [PubMed] [Google Scholar]
- 7.Pape HC, Marsh S, Morley JR, Krettek C, Giannoudis PV. Current concepts in the development of heterotopic ossification. J. Bone Joint Surg. Br. 2004;86:783–787. doi: 10.1302/0301-620x.86b6.15356. [DOI] [PubMed] [Google Scholar]
- 8.Reichel LM, Salisbury E, Moustoukas MJ, Davis AR, Olmsted-David E. Molecular mechanisms of heterotopic ossification. J. Hand Surg. Am. 2014;39:563–566. doi: 10.1016/j.jhsa.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salisbury E, Rodenberg E, Sonnet C, Hipp J, Gannon FH, Vadakkan TJ, Dickinson ME, Olmsted-David EA, Davis AR. Sensory nerve induced inflammation contributes to heterotopic ossification. J. Cell. Biochem. 2011;112:2748–2758. doi: 10.1002/jcb.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan FS, Hahn GV, Zasloff M. Heterotopic ossification: two rare forms and what they can teach us. J. Am. Acad. Orthop. Surg. 1994;2:288–296. doi: 10.5435/00124635-199409000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Shore E, Xu M, Feldman GJ, Fenstermacher DA, Consortium TFIR, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nature Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 12.Pacifici M, Shore EM. Common mutations in ALK2/ACVR1, a multi-facet receptor, have roles in distinct musculoskeletal and neural orphan disorders. Cytokine & Growth Factor Rev. 2016;27:93–104. doi: 10.1016/j.cytogfr.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eddy MC, Jan De Beur SM, Yandow SM, McAllister WH, Shore EM, Kaplan FS, Whyte MP, Levine MA. Deficiency in the alpha-subunit of the stimulatory G protein and severe extraskeletal ossification. J. Bone Min. Res. 2000;15:2074–2083. doi: 10.1359/jbmr.2000.15.11.2074. [DOI] [PubMed] [Google Scholar]
- 14.Shore EM, Ahn J, Jan De Beur SM, Li M, Xu M, McKinlay Gardner MB, Zasloff M, Whyte MP, Levine MA, Kaplan FS. Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. New Engl. J. Med. 2002;346:99–106. doi: 10.1056/NEJMoa011262. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan FS, Shore EM, Pignolo RJ. The medical management of Fibrodysplasia Ossificans Progressiva: current treatment considerations. Clin. Proc. Intl. Clin. Consort. FOP. 2011;4:1–100. [Google Scholar]
- 16.Teasell RW, Mehta S, Aubut JL, Ashe MC, Sequeira K, Macaluso S, Tu L, Team SR. A systematic review of the therapeutic interventions for heterotopic ossification after spinal cord injury. Spinal Cord. 2010;48:512–521. doi: 10.1038/sc.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 18.Lefebvre V, Bhattaram P. Vertebrate skeletogenesis. Curr. Topics Dev. Biol. 2010;90:291–317. doi: 10.1016/S0070-2153(10)90008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan FS, Glaser DL, Hebela N, Shore EM. Heterotopic ossification. J. Am. Acad. Orthop. Surg. 2004;12:116–125. doi: 10.5435/00124635-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Kozhemyakina E, Lassar AB, Zelzer E. A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Development. 2015;142:817–831. doi: 10.1242/dev.105536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 2012;13:27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 22.Salazar VS, Gamer LW, Rosen V. BMP signaling in skeletal development, disease and repair. Nat. Rev. Endocrinology. 2016;12:203–221. doi: 10.1038/nrendo.2016.12. [DOI] [PubMed] [Google Scholar]
- 23.Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell…and more. Endocrine Rev. 2013;34:658–690. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weston AD, Chandraratna RAS, Torchia J, Underhill TM. Requirement for RAR-mediated gene repression in skeletal progenitor differentiation. J. Cell Biol. 2002;158:39–51. doi: 10.1083/jcb.200112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev. Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Sell S, Willms R, Jany R, Esenwein S, Gaissmaier C, Martini F, Bruhn G, Burkhardsmaier F, Bamberg M, Kusswetter W. The suppression of heterotopic ossifications: radiation versus NSAID therapy - A prospective study. J. Arthroplasty. 1998;13:854–859. doi: 10.1016/s0883-5403(98)90189-9. [DOI] [PubMed] [Google Scholar]
- 27.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 28.DiCesare PE, Nimni ME, Pen L, Yazdi M, Cheung DT. Effects of indomethacin on demineralized bone-induced heterotopic ossification in the rat. J. Orthop. Res. 1991;9:855–861. doi: 10.1002/jor.1100090611. [DOI] [PubMed] [Google Scholar]
- 29.Moed BR, Resnick RB, Fakhouri AJ, Nallamothu B, Wagner RA. Effect of two nonsteroidal antinflammatory drugs on heterotopic bone formation in a rabbit model. J. Arthoplasty. 1994;9:81–87. doi: 10.1016/0883-5403(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 30.Craven PL, Urist MR. Osteogenesis by radioisotope labeled cell populations in implants of bone matrix under the influence of ionizing radiation. Clin. Orth. Relat. Res. 1971;76:231–233. doi: 10.1097/00003086-197105000-00030. [DOI] [PubMed] [Google Scholar]
- 31.Sautter-Bihl ML, Liebermeister E, Nanassy A. Radiotherapy as a local treatment option for heterotopic ossifications in patients with spinal cord injury. Spinal Cord. 2000;38:33–36. doi: 10.1038/sj.sc.3100847. [DOI] [PubMed] [Google Scholar]
- 32.Alfieri KA, Forsberg JA, Potter BK. Blast injuries and heterotopic ossification. Bone Joint Res. 2012;1:174–179. doi: 10.1302/2046-3758.18.2000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karunakar MA, Sen A, Bosse MJ, Sims SH, Goulet JA, Kellam JF. Indometacin as prophylaxis for heterotopic ossification after the operative treatment of fractures of the acetabulum. J. Bone Joint Surg. Br. 2006;88:1613–1617. doi: 10.1302/0301-620X.88B12.18151. [DOI] [PubMed] [Google Scholar]
- 34.Meiners T, Abel R, Bohm V, Gerner HJ. Resection of heterotopic ossification of the hip in spinal cord injured patients. Spinal Cord. 1997;35:443–445. doi: 10.1038/sj.sc.3100415. [DOI] [PubMed] [Google Scholar]
- 35.Peterson JR, De La Rosa S, O E, Cilwa KE, Agarwal S, Buchman SR, Cederna PS, Xi C, Morris MD, Herndon DN, et al. Treatment of heterotopic ossification through remote ATP hydrolysis. Science Trans. Med. 2014;6:255ra132. doi: 10.1126/scitranslmed.3008810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowler WB, Buckley KA, Gartland PA, Hipskind RA, Bilbe G, Gallagher JA. Extracellular nucleotide signaling: A mechanism for integrating local and systemic responses in the activation of bone remodeling. Bone. 2001;28:507–512. doi: 10.1016/s8756-3282(01)00430-6. [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki T, Iwasawa M, Nakashima T, Mori S, Shigemoto K, Nakamura H, Katagiri H, Takayanagi H, Tanaka S. Intracellular and extracellular ATP coordinately regulate the inverse correlation between osteoclast survival and bone resorption. J. Biol. Chem. 2012;287:37808–37823. doi: 10.1074/jbc.M112.385369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang S, Kaplan FS, Shore EM. Different roles of GNAS and cAMP signaling during early and late stages of osteogenic differentiation. Horm. Metab. Res. 2012;44:724–731. doi: 10.1055/s-0032-1321845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dizdar D, Tiftik T, Kara M, Tunc H, Ersoz M, Akkus S. Risk factors for developing heterotopic ossification in patients with traumatic brain injury. Brain Inj. 2013;27:807–811. doi: 10.3109/02699052.2013.775490. [DOI] [PubMed] [Google Scholar]
- 40.Genet F, Kulina I, Vaquette C, Torossian F, Millard S, Pettit AR, Sims NA, Anginot A, Guerton B, Winkler IG, et al. Neurological heterotopic ossification following spinal cord injury is triggered by macrophage-mediated inflammation in muscle. J. Pathol. 2015;236:229–240. doi: 10.1002/path.4519. This study describes the first neurological model of HO in mice and the roles of muscle macrophages in pathogenesis. [DOI] [PubMed] [Google Scholar]
- 41.Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight MC, Johnson RS. Hypoxia in is essential for chondrocyte growth cartilage: HIF-1α is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865–2876. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schipani E. Hypoxia and HIF-1alpha in chondrogenesis. Annu N.Y. Acad. Sci. 2006;1068:66–73. doi: 10.1196/annals.1346.009. [DOI] [PubMed] [Google Scholar]
- 43.Yin M, Pacifici M. Vascular regression is required for mesenchymal condensation and chondrogenesis in the developing limb. Dev. Dyn. 2001;222:522–533. doi: 10.1002/dvdy.1212. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal S, Loder S, Brownley C, Cholok D, Mangiavini L, Li J-S, Breuler C, Sung HH, Li S, Ranganathan K, et al. Inhibition of Hif1 prevents both trauma-induced and genetic heterotopic ossification. Proc. Natl. Acad. Sci. USA. 2015;113:E338–E347. doi: 10.1073/pnas.1515397113. This study provides new evidence that Hif1α could serve as a therapeutic target for HO prevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fleish H, Neuman WF. Mechanisms of calcification: roles of collagen, polyphosphates, and phosphatase. Am. J. Physiol. 1961;200:1296–1300. doi: 10.1152/ajplegacy.1961.200.6.1296. [DOI] [PubMed] [Google Scholar]
- 46.Le Nihouannen D, Daculsi G, Saffarzadeh A, Gauthier O, Delplace S, Pilet P, Layrolle P. Ectopic bone formation by microporous calcium phosphate ceramic particles in sheep muscles. Bone. 2005;36:1086–1093. doi: 10.1016/j.bone.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 47.O'Brien EJ, Frank CB, Shrive NG, Hallgrimsson B, D A H. Heterotopic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma. Int. J. Exp. Pathol. 2012;93:319–331. doi: 10.1111/j.1365-2613.2012.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuasa M, Mignemi NA, Nyman JS, Duvall CL, Schwartz HS, Okawa A, Yoshii T, Bhattacharjee G, Zhao C, Bible JE, et al. Fibrinolysis is essential for fracture repair and prevention of heterotopic ossification. J. Clin. Inv. 2015;125:3117–3131. doi: 10.1172/JCI80313. This study shows that fibrinolysis is important for prevention of HO and could be targeted for therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ploplis VA, Carmeliet P, Vazirzadeh S, Van Vlaenderen I, Moons L, Plow EF, Collen D. Effects of disruption of the plasminogen gene on thrombosis, growth, and health in mice. Circulation. 1995;92:2585–2593. doi: 10.1161/01.cir.92.9.2585. [DOI] [PubMed] [Google Scholar]
- 50.Bezerra JA, Bugge TH, Melin-Aldana H, Sabla G, Kombrinck KW, Witte DP, Degen JL. Plasminogen deficiency leads to impaired remodeling after a toxic injury to the liver. Proc. Natl. Acad. Sci. USA. 1999;96:15143–15148. doi: 10.1073/pnas.96.26.15143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mignemi NA, Yuasa M, Baker CE, Moore SN, Ihejirika RC, Oelsner WK, Wallace CS, Yoshii T, Okawa A, Revenko AS, et al. Plasmin prevents dystrophic calcification after muscle injury. J. Bone Min. Res. 2017;32:294–308. doi: 10.1002/jbmr.2973. This study uncovers new evidence that plasmin is important to prevent ectopic calcification and its progression to HO. [DOI] [PubMed] [Google Scholar]
- 52.Shore E, Kaplan FS. Insights from a rare genetic disorder of extra-skeletal bone formation fibrodysplasia ossificans progressiva (FOP) Bone. 2008;43:427–433. doi: 10.1016/j.bone.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang W, Rigueur D, Lyons KM. TFGβ signaling in cartilage development and maintenance. Birth Defects Res. (Part C) 2014;102:37–51. doi: 10.1002/bdrc.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu PB, Deng DY, et al. BMP type I receptor inhibition reduces heterotopic ossification. Nat. Med. 2008;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukuda T, Scott G, Komatsu Y, Araya R, Kawano M, Ray MK, Yamada M, Mishina Y. Generation of a mouse with conditionally activated signaling through the BMP receptor, ALK2. Genesis. 2006;44:159–167. doi: 10.1002/dvg.20201. [DOI] [PubMed] [Google Scholar]
- 56.Cuny GD, Yu PB, Laha JK, Xing X, Liu J-F, Lai CS, Deng DY, Sachidanandan C, Bloch KD, Peterson RT. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg. Med. Chem. Lett. 2008;18:4388–4392. doi: 10.1016/j.bmcl.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohedas AH, Xing X, Armstrong KA, Bullock AN, Cuny GD, Yu PB. Development of an ALK2-biased BMP type I receptor kinase inhibitor. ACS Chem. Biol. 2013;8:1291–1302. doi: 10.1021/cb300655w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanvitale CE, Kerr G, Chaikuad A, Ramel MC, Mohedas AH, Reichert S, Wang Y, Triffitt JT, Cuny GD, Yu PB, et al. A new class of small molecule inhibitor of BMP signaling. PLoS ONE. 2013;8:e62721. doi: 10.1371/journal.pone.0062721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pacifici M, Cossu G, Molinaro M, Tato' F. Vitamin A inhibits chondrogenesis but not myogenesis. Exp. Cell Res. 1980;129:469–474. doi: 10.1016/0014-4827(80)90517-0. [DOI] [PubMed] [Google Scholar]
- 60.Weston AD, Blumberg B, Underhill TM. Active repression by unligated retinoid receptors in development: less is sometimes more. J. Cell Biol. 2003;161:223–228. doi: 10.1083/jcb.200211117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weston AD, Hoffman LM, Underhill TM. Revisiting the role of retinoid signaling in skeletal development. Birth Defects Research Pt. C. 2003;69:156–173. doi: 10.1002/bdrc.10010. [DOI] [PubMed] [Google Scholar]
- 62.Shimono K, Tung W-E, Macolino C, Chi A, Didizian JH, Mundy C, Chandraratna RAS, Mishina Y, Enomoto-Iwamoto M, Pacifici M, et al. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-γ agonists. Nature Med. 2011;17:454–460. doi: 10.1038/nm.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hind M, Stinchcombe S. Palovarotene, a novel retinoic acid receptor gamma agonist for the treatment of emphysema. Curr. Opin. Invest. Drugs. 2009;10:1243–1250. [PubMed] [Google Scholar]
- 64.Chakkalakal SA, Uchibe K, Convente MR, Zhang D, Economides AN, Kaplan FS, Pacifici M, Iwamoto M, Shore EM. Palovarotene inhibits heterotopic ossification and maintains limb mobility and growth in mice with the human ACVR1R206H Fibrodysplasia Ossificans Progressiva (FOP) mutation. J. Bone Min. Res. 2016;31:1–10. doi: 10.1002/jbmr.2820. This study provides the first evidnece that Palovarotene effectively prevents HO in mice bearing the human FOP mutation in ACVR1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pacifici M. Retinoid roles and action in skeletal development and growth provide the rationale for an ongoing heterotopic ossification prevention trial. Bone. 2018 doi: 10.1016/j.bone.2017.08.010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hatsell SJ, Idone V, Alessi Wolken DM, Huang L, Kim HJ, Wang LC, Wen X, Nannuru KC, Jimenez J, Xie L, et al. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Science Trans. Med. 2015;7:303ra137. doi: 10.1126/scitranslmed.aac4358. This seminal study shows for the first time that HO pathogenesis in FOP involves a neo-function of activin A, pointing also to a new therapeutic target. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Olsen OE, Wader KF, Hella H, Mylin AK, Turesson I, Nesthus I, Waage A, Sundan A, Holien T. Activin A inhibits BMP-signaling by binding ACVR2A and ACVR2B. Cell Commun. Signaling. 2015;13:27. doi: 10.1186/s12964-015-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Upadhyay J, Xie L, Huang L, Das N, Stewart RC, Lyon MC, Palmer K, Rajamani S, Graul C, Lobo M, et al. he expansion of heterotopic bone in Fibrodysplasia Ossificans Porgressiva is activin A-dependent. J. Bone Min. Res. 2017 doi: 10.1002/jbmr.3235. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 69.Regard JB, Mahlotra D, Gvozdenovic-Jeremic J, Josey M, Chen M, Weinstein LS, Lu J, Shore E, Kaplan FS, Yang Y. Activation of hedgehog signaling by loss of GNAS causes heterotopic ossification. Nat. Med. 2013;19:1505–1512. doi: 10.1038/nm.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pignolo RJ, Xu M, Russell E, Richardon A, Billings PC, Kaplan FS, Shore EM. Heterozygous activation of Gnas in adipose-derived mesencymal progenitor cells enhances osteoblast differentiation and promotes heterotopic ossification. J. Bone Min. Res. 2011;26:2647–2655. doi: 10.1002/jbmr.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang JY, Mackay-Wiggau JM, Aszterbaum M, Yauch RL, Lindgren J, Chang K, Coppola C, Chanana AM, Marji J, Bickers DR, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N. Engl. J. Med. 2012;366:2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakamura T, Aikawa T, Enomoto-Iwamoto M, Iwamoto M, Higuchi Y, Pacifici M, Kinto N, Yamaguchi A, Noji S, Kurisu K, et al. Induction of osteogenic differentiation by hedgehog proteins. Biochem. Biophys. Res. Commun. 1997;237:465–469. doi: 10.1006/bbrc.1997.7156. [DOI] [PubMed] [Google Scholar]
- 73.Convente MR, Chakkalakal SA, Yang E, Caron R, Zhang D, Kambayashi T, Kaplan FS, Shore EM. Depletion of mast cells and macrophages impairs heterotopic ossification in an Acvr1R206H, mouse model of Fibrodysplasia Ossificans Progressiva. J. Bone Min. Res. 2017 doi: 10.1002/jbmr.3304. (in press) 10.1002/jbmr.3304. This study provides new insights into the roles of inflammatory cells in HO pathogenesis in FOP mice and points to the relevance of these cells as therapeutic targets also. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang X, Li F, Xie L, Crane J, Zhen G, Mishina Y, Deng R, Gao B, Chen H, Liu S, et al. Inhibition of overactive TFG-β attenuates progression of heterotopic ossification in mice. Nat. Communications. 2018;9:551. doi: 10.1038/s41467-018-02988-5. This study shows for the first time that excessive levels of active TGF-β play roles in acquired and genetic forms of HO and represent a new therapeutic target. [DOI] [PMC free article] [PubMed] [Google Scholar]