Abstract
Background: Although a growing body of literature recommends the early initiation of palliative care (PC), the use of PC remains variable.
Objective: The current study sought to describe the use of PC and to identify factors associated with the use of inpatient PC.
Design: Retrospective, cross-sectional analysis of data from the National Inpatient Sample.
Setting and Subjects: Patients admitted with a primary diagnosis of gastrointestinal and/or thoracic cancer from 2012 to 2013.
Measurements: In-hospital length of stay (LOS), morbidity, mortality, and total charges.
Results: A total of 282,899 patients were identified who met inclusion criteria of whom, 24,100 (8.5%) patients received a PC consultation during their inpatient admission. Patients who received PC were more likely to have a longer LOS (LOS >14 days: 5.4% vs. 9.4%) and were more likely to develop a postoperative complication (28.3% vs. 45.9%, both p < 0.001). Inpatient mortality was significantly higher among patients who had received PC than those who did not (5.4% vs. 44.1%, p < 0.001). On multivariable analysis, patient age (age ≥75 years: Odds Ratio [OR] = 2.54, 95% CI: 2.33–2.78), comorbidity (CCI >6: OR = 2.60, 95% CI: 2.48–2.74), and admission to larger hospitals (reference small: OR = 1.20, 95% CI: 1.14–1.25) were associated with greater odds of receiving PC (all p < 0.05). Patients who underwent a major operation during their inpatient admission demonstrated 79% lower odds of receiving PC (OR = 0.21, 95% CI: 0.20–0.22, p < 0.001).
Conclusions: Among patients admitted for cancer, PC services were used in 8.5% of patients during their inpatient admission with surgical patients being 79% less likely to receive a PC consultation. Further research is required to delineate the barriers to the use of PC so as to promote the use of PC among high-risk patients.
Keywords: : cancer, end-of-life, inpatient palliative care, palliative care, surgical palliative care
Introduction
Palliative care (PC) encompasses a spectrum of approaches to delivering care that aims to improve the quality of life of patients with serious life-threatening disease.1 Specifically, the goal of PC is to prevent or treat, as early as possible, the symptoms and side effects of disease and its related treatments.1–3 PC has been recommended as standard care and is increasingly offered to patients with serious illness with observable benefits.4,5 Several previous reports have demonstrated that use of concurrent PC from the point of diagnosis results in a beneficial impact on quality and survival.6–9 Furthermore, PC programs have also been shown to reduce costs of care, be associated with lower rates of emergency room visits, higher satisfaction scores with better quality of life (QOL), and fewer in-hospital deaths.10–13 For example, in a recent systematic review and meta-analysis of existing randomized clinical trials of PC, Kavalieratos et al. demonstrated that PC interventions were associated with improvements in patient QOL and symptom burden, whereas a recent report by May et al. demonstrated that receipt of PC was associated with a 22%–32% lower cost among patients with the largest cost savings observed among patients with greater comorbidity.14,15
Despite the reported benefits associated with the use of PC, previous reports have demonstrated that PC continues to be underused among patients with serious, life-threatening illnesses.5 Furthermore, there is a paucity of nationally representative data evaluating the use of PC consultation among patients admitted for gastrointestinal and thoracic cancers, particularly among patients undergoing surgery.16–18 Most existing studies report on data from patients admitted for the medical management of cancer and are collected from either single-center studies or case series/reports.19 Additionally, existing literature fails to adequately describe/identify patients that are most likely to be referred to PC. Given this, using a nationally representative database, the objectives of this study were to investigate the utilization of PC consultation among patients admitted for the inpatient management of gastrointestinal or thoracic cancer and to identify patient and/or hospital characteristics associated with receipt of a PC consultation. Additionally, we sought to investigate the relationship between PC consultation and clinical and financial outcomes within this patient population.
Methods
Data sources and patient population
This retrospective review was performed using data from the Nationwide Inpatient Sample (NIS) from 2012 to 2013. The NIS is a national representative, all-payer database that collects data from all inpatient discharges across the United States. Collected and maintained by the Agency for Healthcare Research and Quality (AHRQ), the NIS collects data on patient age, sex, race, insurance status, and for each patient record, includes up to 25 diagnostic and procedure codes, coded using the International Classification of Disease, Ninth Revision, Clinical Modification lexicon.20 Additionally, hospital-level characteristics, including hospital region, hospital location (urban vs. rural), hospital bed size, and hospital teaching status are also recorded for each patient.20 Using a stratified sampling methodology based on hospital characteristics, the NIS represents a 20% stratified sample of all hospital discharges in the United States.20
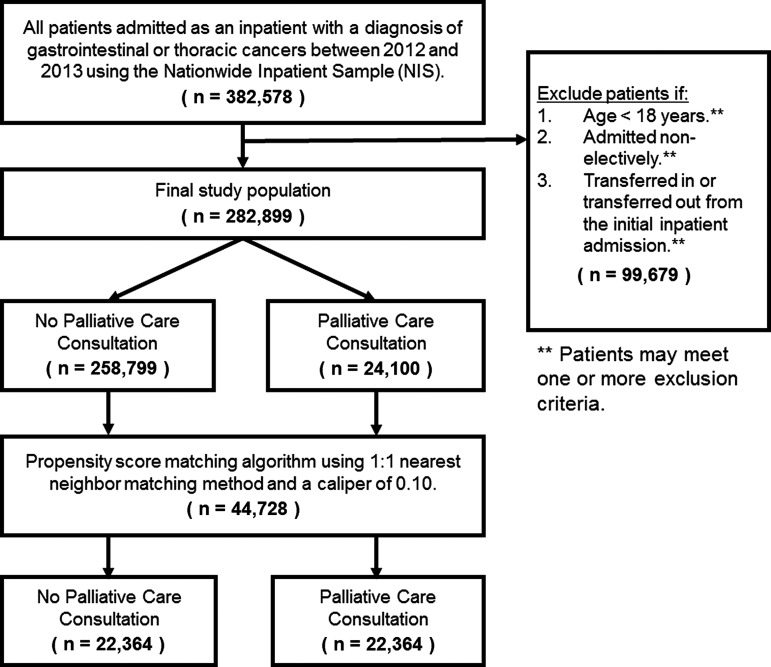
Patients admitted for an inpatient admission with a primary diagnosis of gastrointestinal and/or thoracic cancer were identified using relevant ICD-9-CM diagnosis codes (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/jpm). Patients younger than 18 years of age, patients admitted on an emergent basis, and patients who were either transferred in or transferred out were excluded from our final study population (Fig. 1). Patient comorbidity was calculated using the Charlson Comorbidity Index (CCI); patients were categorized according to their CCI score as either CCI = 2, CCI = 3–6, and CCI >6.21 Patients undergoing a major operative procedure were identified using a previously defined criteria as per the AHRQ.22
FIG. 1.
Derivation of final patient population. The type of primary cancer was determined using relevant International Classification of Disease, Ninth Edition, Clinical Modification (ICD-9-CM) diagnosis codes presented in Supplementary Table S1.
Primary study outcomes
The primary outcomes of the study included the receipt of a PC consultation, development of one or more postoperative/inpatient complications, inpatient length of stay (LOS), inpatient mortality, discharge to additional care, and total charges for the inpatient admission. Use of PC services was identified using the ICD-9-CM diagnosis code “v66.7” and compared between patient groups and the receipt of a major operation during the inpatient admission.23,24 Additional sensitivity analyses were also performed to assess the use of PC services by the type of primary cancer. Inpatient complications were identified using a previously validated set of conditions identified for use in administrative data.25 Specifically, these included surgical site infections, postoperative bleeding, venous thromboembolism (deep venous thrombosis and pulmonary embolism), pneumonia, use of ventilator, myocardial infarction, and cerebrovascular accidents (stroke and transient ischemic attacks).25 Total hospital charges were recorded for the entire inpatient admission and were inflation adjusted using the inflation calculator as provided by the Department of Labor and reported to the nearest 2017 dollar.26
Statistical analyses
Categorical data were expressed as whole numbers and proportions while continuous variables were reported as medians with interquartile range (IQR). Categorical data were compared using Pearson's chi-squared test, whereas continuous data were compared using the Kruskal–Wallis test. Multivariable logistic regression analysis was performed to assess the factors associated with use of PC services. Specifically, our model adjusted for patient characteristics, including patient age, sex, race, insurance status, CCI score, receipt of a major operation during the inpatient admission, and hospital-level characteristics, including hospital region, hospital bed size, and hospital teaching status. Colinearity of variables was examined using variance inflation factor analysis and model fit was evaluated using the Akaike Information Criterion. Results of the multivariable analysis were presented as odds ratios (ORs) with corresponding 95% confidence intervals (95% CIs). To further examine the association between receipt of PC and postoperative outcomes, a propensity score matched analysis was performed. Specifically, patients who received a PC consultation during their inpatient admission were matched to patients who did not receive a PC consultation. Receipt of PC consultation was specified as the dependent variable with patient age, sex, race, insurance status, income quartile, CCI score, and receipt of a major operation during the inpatient admission, hospital region, hospital teaching status, hospital bed size, and LOS were included as independent variables. Patients were matched using a 1:1 nearest neighbor matching algorithm without replacement, with a caliper width of 0.1. Balance of covariates was evaluated using absolute differences. All analyses were performed using STATA version 14.0 (StataCorp, College Station, TX) and a p-value of <0.05 was used to define statistical significance. The study was approved by the Johns Hopkins University Institutional Review Board.
Results
Baseline patient and hospital characteristics
A total of 282,899 patients were identified who met inclusion criteria; 56.6% (n = 160,164) patients were admitted for the management of a gastrointestinal cancer, whereas 43.4% (n = 122,735) patients were admitted for the management of a thoracic malignancy. The median age of all patients was 66 years (IQR: 57–75) with a majority of patients being male (n = 156,105, 55.2%) and Caucasian (n = 194,405, 72.2%, Table 1). Medicare was the most common payor (55.3%, n = 155,977), followed by private insurers (n = 78,094, 27.7%) and Medicaid (30,145, 10.7%). Comorbidities were common with 48.9% of patients presenting with CCI score of 6 or more (CCI = 6: n = 63,267, 22.4%; CCI >6: n = 75,093, 26.5%). Most patients were admitted to a large (n = 180,627, 63.9%) or medium-sized (n = 68,520, 24.2%) hospital based on region-specific hospital bed size categories; 55.4% (n = 156,641) of patients were admitted to an urban teaching facility, whereas 45.7% (urban nonteaching: n = 97,771, 34.6%; rural: n = 28,487, 10.1%) of patients were admitted to either a rural or urban, nonteaching facility.
Table 1.
A Comparison of Patient and Disease Characteristics between Patients Who Received a Palliative Care Consultation During Their Inpatient Admission and Patients Who Did Not
| Characteristic | No palliative care N (%) | Palliative care N (%) | p value | Total N (%) |
|---|---|---|---|---|
| Age group | <0.001 | |||
| 18–44 years | 11,497 (4.4) | 816 (3.4) | 12,313 (4.4) | |
| 45–64 years | 104,249 (40.3) | 8708 (36.1) | 112,957 (39.9) | |
| 65–74 years | 78,590 (30.4) | 6757 (28.0) | 85,347 (30.2) | |
| >74 years | 64,463 (24.9) | 7819 (32.4) | 72,282 (25.6) | |
| Sex | 0.806 | |||
| Male | 142,788 (55.2) | 13,317 (55.3) | 156,105 (55.2) | |
| Female | 116,004 (44.8) | 10,783 (44.7) | 126,787 (44.8) | |
| Race (n = 269,210) | <0.001 | |||
| Caucasian | 178,364 (72.4) | 16,041 (69.9) | 194,405 (72.2) | |
| African American | 32,494 (13.2) | 3226 (14.1) | 35,720 (13.3) | |
| Hispanic | 19,028 (7.7) | 1973 (8.6) | 21,001 (7.8) | |
| Other | 16,358 (6.6) | 1726 (7.5) | 18,084 (6.7) | |
| Insurance | <0.001 | |||
| Private | 71,962 (27.9) | 6132 (25.6) | 78,094 (27.7) | |
| Medicare | 143,064 (55.4) | 12,913 (53.8) | 155,977 (55.3) | |
| Medicaid | 27,462 (10.6) | 2684 (11.2) | 30,145 (10.7) | |
| Other | 15,827 (6.1) | 2261 (9.4) | 18,088 (6.4) | |
| Income zip quartile | <0.001 | |||
| Q1 | 72,901 (28.8) | 6595 (28.0) | 79,496 (28.7) | |
| Q2 | 64,271 (25.4) | 5741 (24.4) | 70,012 (25.3) | |
| Q3 | 60,868 (24.1) | 5757 (24.4) | 66,625 (24.1) | |
| Q4 | 55,028 (21.7) | 5486 (23.3) | 60,514 (21.9) | |
| CCI | <0.001 | |||
| CCI = 2 | 54,977 (21.2) | 2244 (9.3) | 57,221 (20.2) | |
| CCI = 3–5 | 82,299 (31.8) | 5019 (20.8) | 87,318 (30.9) | |
| CCI = 6 | 56,278 (21.8) | 6989 (29.0) | 63,267 (22.4) | |
| CCI >6 | 65,245 (25.2) | 9848 (40.9) | 75,093 (26.5) | |
| Inpatient surgical procedurea | <0.001 | |||
| No | 168,752 (65.2) | 21,596 (89.6) | 190,348 (67.3) | |
| Yes | 90,047 (34.8) | 2504 (10.4) | 92,551 (32.7) | |
| Hospital location | <0.001 | |||
| South | 103,424 (40.0) | 9108 (37.8) | 112,532 (39.8) | |
| Midwest | 57,564 (22.2) | 4915 (20.4) | 62,479 (22.1) | |
| West | 44,794 (17.3) | 5361 (22.2) | 50,155 (17.7) | |
| Northeast | 53,017 (20.5) | 4716 (19.6) | 57,733 (20.4) | |
| Hospital bed size | <0.001 | |||
| Small | 31,137 (12.0) | 2615 (10.9) | 33,752 (11.9) | |
| Medium | 62,505 (24.2) | 6015 (25.0) | 68,520 (24.2) | |
| Large | 165,157 (63.8) | 15,470 (64.2) | 180,627 (63.9) | |
| Hospital teaching status | <0.001 | |||
| Rural | 26,228 (10.1) | 2259 (9.4) | 28,487 (10.1) | |
| Urban nonteaching | 89,632 (34.6) | 8139 (33.8) | 97,771 (34.6) | |
| Urban teaching | 142,939 (55.2) | 13,702 (56.9) | 156,641 (55.4) |
Analysis presented here is for all patients included in the final study cohort (n = 282,899).
Defined using criteria outlined by the Agency for Healthcare Research and Quality (AHRQ).
CCI, Charlson Comorbidity Index.
Among all patients in the study population, 8.5% (n = 24,100) of patients received a PC consultation during their inpatient admission, with the number of patients receiving a PC service observed to increase from 8.2% in 2012 (n = 11,730) to 8.8% in 2013 (n = 12,370, (+7.3%, p < 0.001). Patients receiving a PC service were more likely to be older (age >74: no PC vs. PC: 24.9% vs. 32.4%, p < 0.001), be a member of a racial minority (27.6% vs. 30.2%, p < 0.001), and were more likely to present with preexisting comorbidity compared with those who did not receive PC (CCI = 2: 21.2% vs. 9.3%, CCI >6: 25.2% vs. 40.9%, p < 0.001); minor differences in insurance status, median household income, and hospital characteristics were also observed between the two patient groups. Similar differences in patient and hospital characteristics were also observed when stratified by the receipt of a major operation during the inpatient admission (Supplementary Tables S2 and S3).
PC type of cancer and receipt of major operation during inpatient admission
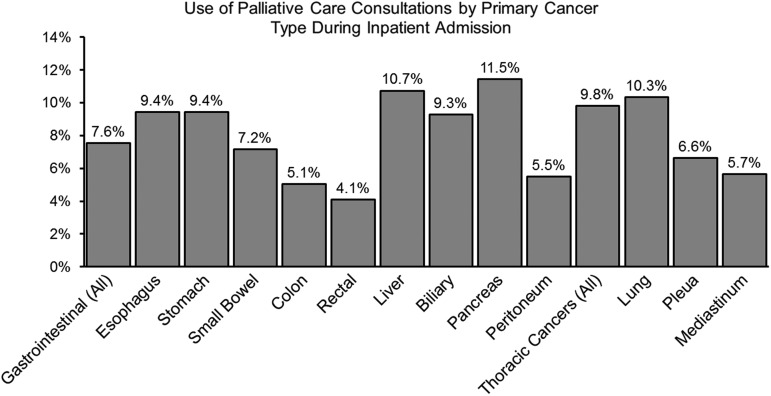
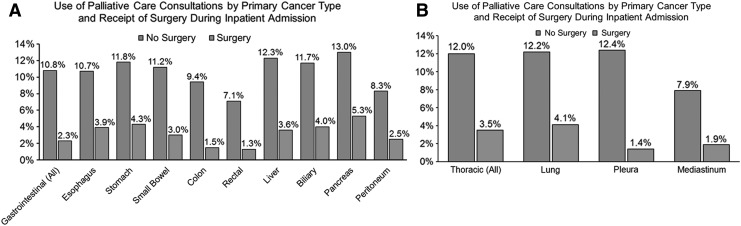
Use of PC services was observed to vary by the type of primary cancer ranging from a low of 4.1% among patients admitted for the management of rectal cancers to a high of 11.5% among patients admitted for the management of pancreatic cancer (Fig. 2). Additionally, use of PC consultations was also observed to be higher among patients who presented with a thoracic malignancy compared with patients admitted for the management of a gastrointestinal cancer (9.8% vs. 7.6%). Use of PC services was over four times higher among patients who did not undergo a major operation during their inpatient admission compared with patients who underwent a major operation (11.4% vs. 2.7%, p < 0.001). This pattern was also observed when stratified by the type of primary cancer (Fig. 3). For example, among patients who were admitted for the management of a gastrointestinal cancer, PC services were used in 10.3% of patients who did not undergo a major operation during the inpatient admission. In contrast, among patients with a gastrointestinal cancer undergoing a major operation during the inpatient admission, only 2.3% received a PC consultation. A similar trend was also observed among patients with a thoracic malignancy.
FIG. 2.
Comparison of the use of palliative care services during the inpatient admission by the type of primary cancer. Analysis presented here is for all patients included in the final study cohort (n = 282,899). The type of primary cancer was determined using relevant International Classification of Disease, Ninth Edition, Clinical Modification (ICD-9-CM) diagnosis codes presented in Supplementary Table S1.
FIG. 3.
Comparison of the use of palliative care services during the inpatient admission by the type of primary cancer and receipt of a major operation during the inpatient admission among (A) patients who presented with a primary gastrointestinal cancer, and (B) patients who presented with a primary thoracic cancer. Analysis presented here is for all patients included in the final study cohort (n = 282,899). The type of primary cancer was determined using relevant International Classification of Disease, Ninth Edition, Clinical Modification (ICD-9-CM) diagnosis codes presented in Supplementary Table S1.
Factors associated with the use of PC
To identify patient and hospital-level characteristics independently associated with use of PC during the inpatient admission, multivariable logistic regression analysis was performed (Table 2). Increasing patient age (age ≥75: OR = 2.54, 95%CI: 2.33–2.78, p < 0.001) and increasing preoperative comorbidity were associated with an increased odds of receiving PC (CCI >6: OR = 2.60, 95%CI: 2.48–2.74, p < 0.001). Similarly, compared with patients admitted to smaller hospitals, patients admitted to larger hospitals (OR = 1.20, 95%CI: 1.41–1.25, p < 0.001) demonstrated greater odds of receiving a PC consultation. Development of an in-hospital complication was associated with 92% (OR = 1.92, CI: 1.86–1.98, p < 0.001) greater odds of receiving a PC service, whereas patients who had a LOS between 10–14 days and patients who had a LOS ≥15 days demonstrated 45% (OR = 1.45, 95%CI: 1.38–2.52, p < 0.001) and 116% (OR = 2.16, 95%CI: 2.04–2.28, p < 0.001) greater odds of receiving PC, respectively. Interestingly, after adjusting for patient and hospital characteristics, patients undergoing a surgical operation during the inpatient admission demonstrated 79% lower odds for receiving a PC consultation compared with patients who did not undergo a surgical procedure during the inpatient admission (OR = 0.21, 95%CI: 0.20–0.22, p < 0.001).
Table 2.
Univariable and Multivariable Analyses of Risk Factors Associated with Receipt of Palliative Care Consultation during Inpatient Admission
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Characteristic | Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value |
| Age group | ||||||
| 18–44 years | Reference | Reference | — | — | ||
| 45–64 years | 1.18 | 1.093–1.268 | <0.001 | 1.18 | 1.089–1.278 | <0.001 |
| 65–74 years | 1.21 | 1.124–1.306 | <0.001 | 1.73 | 1.589–1.889 | <0.001 |
| >74 years | 1.71 | 1.586–1.842 | <0.001 | 2.54 | 2.328–2.775 | <0.001 |
| Sex | ||||||
| Male | Reference | — | — | Reference | — | — |
| Female | 1.00 | 0.971–1.023 | 0.806 | 1.05 | 1.022–1.082 | 0.001 |
| Race | ||||||
| Caucasian | Reference | — | — | Reference | — | — |
| African American | 1.10 | 1.061–1.149 | <0.001 | 1.00 | 0.959–1.046 | 0.945 |
| Hispanic | 1.15 | 1.098–1.211 | <0.001 | 1.07 | 1.017–1.130 | 0.009 |
| Other | 1.17 | 1.114–1.236 | <0.001 | 1.12 | 1.062–1.186 | <0.001 |
| Insurance | ||||||
| Private | Reference | — | — | Reference | — | — |
| Medicare | 1.06 | 1.026–1.093 | <0.001 | 0.65 | 0.619–0.675 | <0.001 |
| Medicaid | 1.15 | 1.093–1.202 | <0.001 | 1.04 | 0.988–1.098 | 0.128 |
| Other | 1.68 | 1.593–1.765 | <0.001 | 1.62 | 1.531–1.711 | <0.001 |
| Income zip quartile | ||||||
| Q1 | Reference | — | — | Reference | — | — |
| Q2 | 0.99 | 0.952–1.025 | 0.501 | 1.01 | 0.974–1.055 | 0.500 |
| Q3 | 1.05 | 1.008–1.085 | 0.018 | 1.07 | 1.025–1.110 | 0.001 |
| Q4 | 1.10 | 1.061–1.144 | <0.001 | 1.13 | 1.087–1.180 | <0.001 |
| CCI | ||||||
| CCI = 2 | Reference | — | — | Reference | — | — |
| CCI = 3–5 | 1.49 | 1.420–1.572 | <0.001 | 1.19 | 1.123–1.251 | <0.001 |
| CCI = 6 | 3.04 | 2.897–3.195 | <0.001 | 2.48 | 2.351–2.609 | <0.001 |
| CCI >6 | 3.70 | 3.527–3.877 | <0.001 | 2.60 | 2.475–2.739 | <0.001 |
| Inpatient surgical procedurea | ||||||
| No | Reference | — | — | Reference | — | — |
| Yes | 0.22 | 0.208–0.227 | <0.001 | 0.21 | 0.199–0.218 | <0.001 |
| Hospital region | ||||||
| South | Reference | — | — | — | — | — |
| Midwest | 0.97 | 0.935–1.005 | 0.094 | — | — | — |
| West | 1.36 | 1.312–1.408 | <0.001 | — | — | — |
| Northeast | 1.01 | 0.974–1.048 | 0.592 | — | — | — |
| Hospital bed size | ||||||
| Small | Reference | — | — | Reference | — | — |
| Medium | 1.15 | 1.092–1.202 | <0.001 | 1.17 | 1.115–1.236 | <0.001 |
| Large | 1.12 | 1.068–1.165 | <0.001 | 1.20 | 1.143–1.254 | <0.001 |
| Hospital teaching status | ||||||
| Rural | Reference | — | — | — | — | — |
| Urban, Nonteaching | 1.05 | 1.004–1.107 | 0.033 | — | — | — |
| Urban, teaching | 1.11 | 1.062–1.166 | <0.001 | — | — | — |
| Length of stay | ||||||
| 0–5 days | Reference | — | — | Reference | — | — |
| 5–9 days | 0.97 | 0.939–0.998 | 0.034 | 1.10 | 1.064–1.136 | <0.001 |
| 10–14 days | 1.31 | 1.258–1.374 | <0.001 | 1.45 | 1.379–1.522 | <0.001 |
| >14 days | 1.84 | 1.754–1.931 | <0.001 | 2.16 | 2.040–2.278 | <0.001 |
| Postoperative complicationb | ||||||
| No | Reference | — | — | Reference | — | — |
| Yes | 2.15 | 2.090–2.205 | <0.001 | 1.92 | 1.864–1.978 | <0.001 |
Analysis presented here is for all patients included in the final study cohort (n = 282,899).
Defined using criteria outlined by the Agency for Healthcare Research and Quality (AHRQ).
Includes Sepsis, Pneumonia, Surgical Site Infection, Gastrointestinal Hemorrhage, Venous Thromboembolism, Respiratory Failure, Renal Failure, Myocardial Infarction, Stroke, and Postoperative Fistula.
Clinical and financial outcomes by the use of PC
Among all patients discharged, the median LOS was 4 days (IQR: 3–7) with 5.8% (n = 16,334) of patients demonstrating a LOS of 15 days or more (Table 3). LOS was observed to be longer among patients who received a PC consultation with the proportion of patients demonstrating a LOS of 15 days or more being higher among patients who received PC (LOS ≥15: 5.4% vs. 9.4%, p < 0.001). During the inpatient admission, 29.8% (n = 84,394) of patients developed an in-hospital complication. Inpatient morbidity was observed to be higher among patients who received PC compared with patients who did not (28.3% vs. 45.9%, p < 0.001). Of note, this effect was more pronounced among patients who underwent a major operation during their inpatient admission (29.9% vs. 65.1%, p < 0.001) compared with those who did not (27.5% vs. 43.7%, p < 0.001, Supplementary Tables S4 and S5).
Table 3.
A Comparison of Clinical and Financial Outcomes between Patients Who Received a Palliative Care Consultation during Their Inpatient Admission and Patients Who Did Not
| Characteristic | No palliative care N (%) | Palliative care N (%) | p value | Total N (%) |
|---|---|---|---|---|
| Length of stay, days, median (IQR) | 4 (3–7) | 5 (2–8) | <0.001 | 4 (3–7) |
| Length of stay | ||||
| 0–5 days | 134,574 (52.0) | 11,791 (48.9) | 146,365 (51.7) | |
| 5–9 days | 87,127 (33.7) | 7388 (30.7) | 94,515 (33.4) | |
| 10–14 days | 23,032 (8.9) | 2653 (11.0) | 25,685 (9.1) | |
| >14 days | 14,066 (5.4) | 2268 (9.4) | 16,334 (5.8) | |
| In-hospital complicationa | 73,330 (28.3) | 11,064 (45.9) | <0.001 | 84,394 (29.8) |
| Death | 13,889 (5.4) | 10,630 (44.1) | <0.001 | 24,519 (8.7) |
| Discharge dispositionb (n = 258,305) | <0.001 | |||
| Routine discharge | 172,484 (70.5) | 3852 (28.6) | 176,336 (68.3) | |
| Discharge with additional care | 70,411 (28.8) | 9173 (68.2) | 79,584 (30.8) | |
| Otherc | 1950 (0.8) | 435 (3.2) | 2385 (0.9) | |
| Total hospital charges, $, median (IQR) | $36,367 ($19,406–$66,298) | $30,259 ($14,169–$62,760) | <0.001 | $35,888 ($18,968–$66,042) |
Analysis presented here is for all patients included in the final study cohort (n = 282,899).
Includes Sepsis, Pneumonia, Surgical Site Infection, Gastrointestinal Hemorrhage, Venous Thromboembolism, Respiratory Failure, Renal Failure, Myocardial Infarction, Stroke, and Postoperative Fistula.
Only among patients who survived to discharge.
Includes leave against medical advice, unknown destination, and transferred to court as defined by the AHRQ.
A total of 24,519 patients (8.7%) died during the index admission. In-hospital mortality was significantly higher among patients who had received a PC consultation compared with those who did not (5.4% vs. 44.1%, p < 0.001); this effect was observed regardless of the receipt of a major operation during the inpatient admission (no major operation: 6.7% vs. 43.9%; major operation: 2.9% vs. 46.0%, both p < 0.001). Among the 258,305 patients who were discharged alive, 68.3% (n = 176,336) underwent a routine discharge to home, whereas 30.8% (n = 79,584) were discharged to additional care facilities/with additional care. Patients who received a PC consultation during the inpatient admission were proportionally more likely to be discharged to additional care compared with patients who did not receive a PC consultation (28.8% vs. 68.2%, p < 0.001), with a comparable effect being observed despite stratification by the receipt of a major operation during the inpatient admission (no major operation: 28.7% vs. 68.4%; major operation: 28.9% vs. 66.0%, both p < 0.001).
Among all patients, the median in-hospital charge for the inpatient admission was $35,888 (IQR: $18,968–$66,042) with patients receiving PC demonstrating a lower median charge compared with patients who did not ($36,367 [IQR: $19,406–$66,298] vs. $30,259 [IQR: $14,169–$62,760], p < 0.001). Interestingly, when stratified by the receipt of a major operation during the inpatient admission, use of PC was associated with a 58.3% higher median charge among patients who underwent a major operation during their inpatient admission ($62,913 [IQR: $40,134–$101,499] vs. $99,604 [IQR: $57,239–$181,070], (+58.3%, p < 0.001).
Propensity score-matched analysis: clinical and financial outcomes by use of PC
To further examine the effects of PC consultations during the inpatient admission, 22,364 patients who received a PC consultation were matched to 22,364 patients who did not receive a PC consultation during their inpatient admission (Supplementary Table S6 and Supplementary Fig. S1). Among the matched cohort, PC consultation was associated with a longer LOS for the index admission (median LOS: 4 days [IQR: 2–7], vs. 5 days [IQR: 2–8], p < 0.001) as well as higher in-hospital morbidity (29.2% vs. 46.0%, p < 0.001, Supplementary Table S7). Furthermore, in-hospital mortality was also higher among patients who received a PC consultation (6.9% vs. 43.9%, p < 0.001); these patients were also more likely to be discharged to additional care from their index admission (32.3% vs. 68.2%, p < 0.001). Of note, minimal differences in total hospital charges were observed between the two patient groups (median hospital charges: $30,220 [IQR:$16,945–$54,868] vs. $30,898 [IQR:$14,518–$63,874], ( = +2.2%, p = 0.051).
Discussion
Results of the current study are consistent with previous reports demonstrating the relatively low use of PC services among patients undergoing surgery compared with patients admitted for the medical management of disease.16,18,27,28 For example, in their recent report of patients receiving treatment at Veterans Health Administration hospitals, Olmsted et al. demonstrated that patients undergoing surgery were 16% less likely to receive PC and/or be admitted to a hospice, whereas Kross et al. demonstrated that among patients admitted to the ICU, patients admitted to the ICU under a surgical service were less likely to receive a PC consultation compared with those admitted to a medicine service.27,28 Similarly, in the current study, odds of receiving a PC consultation were 79% lower among patients who underwent surgery during their admission. Furthermore, use of PC among this subgroup of patients was also observed to be variable by the type of primary cancer ranging from a low of 1.3% among patients undergoing a major operation for rectal cancer to a high of 5.3% among patients undergoing a major operation for pancreatic cancer. Despite a recent statement by the American College of Surgeons recognizing the life-affirming role of PC in the management of surgical patients with severe terminal illness, historically, the use of PC services among surgical patients has been low.29,30 The relatively low use of PC in surgery is likely due to the lack of integration of PC principles into routine surgical practice, a trend that can be attributed to the surgical “rescue culture” as well as surgeons' perceptions regarding error, decisional regret, and responsibility.31–37 Taken together with the previous report, results from the current study highlight that although PC consultations were initiated within subsets of patients undergoing surgery, use of PC remains low among all patients, particularly among patients undergoing surgery. Moving forward, greater emphasis should be placed on changing hospital/surgical culture to ensure the integration of principles of PC into routine surgical practices as well as the timely initiation of PC services for surgical patients.
Although numerous previous reports have demonstrated that the timely initiation of PC services is associated with lower healthcare resources, specifically, shorter LOS, lower healthcare costs, and fewer emergency department visits, in the current study, we observed that patients who received PC were more likely to have a longer LOS, and were proportionally more likely to be discharged to additional care facilities compared with patients who did not receive PC.38–41 Additionally, patients who received a PC consultation were almost twice as likely to have developed a postoperative complication during their admission with the observed in-hospital mortality being approximately fivefold higher among this group of patients. Although we were unable to determine the exact timing of PC consultation relative to the operation performed or postoperative complications, our findings suggest that the inpatient palliative consultations occurred late in the course of illness and close to death. To this point, a recent study by Earle et al. demonstrated that although the proportion of patients using hospice care services has increased in recent years, on average, hospice care is initiated only within the last 3 days of life.42 Similarly, a separate study from Christakis demonstrated that among a study cohort of patient enrolled in hospice care, the median survival after enrollment was only 36 days with 15.6% of patients dying within 7 days of enrollment.43
Interestingly, PC consultations were also associated with lower hospital charges among all patients. Consistent with our findings, a recent analysis from May et al. comparing hospital costs by the receipt of PC demonstrated that use of PC was associated with significant cost savings ranging from 22% to 32%, varying by patient characteristics.14 The effect of PC observed in the current study is likely heterogeneous and may vary according to the multifaceted interaction between patient, disease, and provider level characteristic. To further investigate this, a stratified analysis was performed by the receipt of surgery during the inpatient admission and also performed an additional sensitivity analysis using propensity score matching. Of note, when stratified by the receipt of surgery, PC consultation was associated with higher charges among patients who underwent a surgical resection during their inpatient admission, whereas hospital charges were observed to be comparable among those admitted for the medical management of their disease. While one might intuitively expect PC consultations to result in lower hospital charges due to a lower use of hospital resources, our findings likely suggest that PC consultations may have been initiated very late in disease management, and perhaps after significant hospital resources had already been used to treat the underlying disease and/or the resulting complications. Future research is required to better understand how the effect of PC on hospital costs varies for different combinations of patients, disease, and specialties. Additionally, policies are required that ensure the timely initiation of PC services among patients who benefit most from such interventions. Increased access to PC services among these patients may result in improved quality of care at lower cost to patients, hospitals, and payors.
Results of the current study should be interpreted with several limitations. First, given that the database used in the current analysis is constructed from administrative claims, our analysis is subject to all the potential limitation inherent to administrative data.44 Specifically, these include the potential for miscoding as well as a lack of more granular clinical/disease-specific data.44 For example, in the current study, we were unable to assess differences in PC use by disease severity as measured by tumor stage/grade as well as the extent of disease. As a consequence, there may some residual confounding in our results as more granular details relating to disease severity could not be adjusted for. To account for this residual bias, we performed an additional sensitivity analysis using propensity score matching. Importantly, results from the sensitivity analysis were consistent to those performed for the entire, unmatched cohort. Second, although the use of PC consultation was measured using a previously validated ICD-9-CM diagnosis code, it is possible that this code did not capture all PC consultations, and as a consequence may underestimate the actual use of PC consultations.23 For example, pain specialists who provide PC services may use alternate billing codes for their services rendered and as such may not have been captured in the current analysis.23 It is important to note, however, that estimates for the use of PC in the current study are comparable to previous studies reporting the use of PC to vary from 5.0% to 38.3% among different patient populations.24,28 Third, as the NIS collects data only on whether or not a patient develops an in-hospital complication, we were unable to account for the timing of the in-hospital complication relative to the use of PC. As a result of this, we were unable to further assess the relationship between in-hospital complications and use of PC. Specifically, we were unable to determine whether complications were more common among patients who received PC due to a greater burden of disease, or whether PC consultations were initiated after a serious/terminal in-hospital complication. Lastly, as the NIS reports data only for the inpatient admission, we were unable to track patients longitudinally over time and were therefore unable to report on long-term clinical and financial outcomes such as overall survival and QALYs, as well as compare patient-reported outcomes, including metrics pertaining to post-discharge quality of life and functional status.
In conclusion, using a nationally representative database of patients undergoing gastrointestinal and/or thoracic cancers, the current study demonstrated that PC consultations were used in 8.5% of patients, with patients undergoing surgery being 79% less likely to receive a PC consultation compared with patients who did not undergo surgery. Although patients who received PC were more likely to have developed an in-hospital complication and die during the index hospitalization, results from the current study highlight the potential improvements in care quality and resource use that can be achieved through the integration of PC into routine cancer care.
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
Contents of this article were presented as a poster presentation at the Annual Cancer Symposium of the Society for Surgical Oncology held between March 15 and 18, 2017 in Seattle, WA.
References
- 1.Hui D, Bruera E: Integrating palliative care into the trajectory of cancer care. Nat Rev Clin Oncol 2015;13:159–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocque GB, Cleary JF: Palliative care reduces morbidity and mortality in cancer. Nat Rev Clin Oncol 2012;10:80–89 [DOI] [PubMed] [Google Scholar]
- 3.Hui D, Elsayem A, De la Cruz M, Berger A, et al. : Availability and integration of palliative care at US cancer centers. JAMA 2010;303:1054–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith TJ, Temin S, Alesi ER, et al. : American Society of Clinical Oncology provisional clinical opinion: The integration of palliative care into standard oncology care. J Clin Oncol 2012;30:880–887 [DOI] [PubMed] [Google Scholar]
- 5.Ferris FD, Bruera E, Cherny N, et al. : Palliative cancer care a decade later: Accomplishments, the need, next steps—from the American Society of Clinical Oncology. J Clin Oncol 2009;27:3052–3058 [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann C, Riechelmann R, Krzyzanowska M, et al. : Effectiveness of specialized palliative care: A systematic review. JAMA 2008;299:1698–1709 [DOI] [PubMed] [Google Scholar]
- 7.Glare P, Plakovic K, Schloms A, et al. : Study using the NCCN guidelines for palliative care to screen patients for palliative care needs and referral to palliative care specialists. J Natl Compr Canc Netw 2013;11:1087–1096 [DOI] [PubMed] [Google Scholar]
- 8.Glare PA: Early implementation of palliative care can improve patient outcomes. J Natl Compr Canc Netw 2013;11 Suppl 1:S3–S9 [DOI] [PubMed] [Google Scholar]
- 9.Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med 2010;363:733–742 [DOI] [PubMed] [Google Scholar]
- 10.Higginson IJ, Bausewein C, Reilly CC, et al. : An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: A randomised controlled trial. Lancet Respir Med 2014;2:979–987 [DOI] [PubMed] [Google Scholar]
- 11.Bakitas M, Lyons KD, Hegel MT, et al. : Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: The Project ENABLE II randomized controlled trial. JAMA 2009;302:741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmermann C, Swami N, Krzyzanowska M, et al. : Early palliative care for patients with advanced cancer: A cluster-randomised controlled trial. Lancet (London, England) 2014;383:1721–1730 [DOI] [PubMed] [Google Scholar]
- 13.Temel JS, Greer JA, Admane S, et al. : Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: Results of a randomized study of early palliative care. J Clin Oncol 2011;29:2319–2326 [DOI] [PubMed] [Google Scholar]
- 14.May P, Garrido MM, Cassel JB, et al. : Palliative care teams cost-saving effect is larger for cancer patients with higher numbers of comorbidities. Health Aff 2016;35:44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kavalieratos D, Corbelli J, Zhang D, et al. : Association between palliative care and patient and caregiver outcomes: A systematic review and meta-analysis. JAMA 2016;316:2104–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lilley EJ, Cooper Z, Schwarze ML, Mosenthal AC: Palliative care in surgery. Ann Surg 2017. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lilley EJ, Cooper Z, Schwarze ML, Mosenthal AC: Palliative care in surgery: Defining the research priorities. J Palliat Med 2017;20:702–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lilley EJ, Khan KT, Johnston FM, et al. : Palliative care interventions for surgical patients. JAMA Surg 2016;151:172. [DOI] [PubMed] [Google Scholar]
- 19.Morris RS, Gani F, Hammad AY, et al. : Factors associated with palliative care use in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Res 2017;211:79–86 [DOI] [PubMed] [Google Scholar]
- 20.HCUP-US NIS Overview n.d. www.hcup-us.ahrq.gov/nisoverview.jsp#data (Last accessed January11, 2016)
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 22.Healthcare Cost and Utilization Project (HCUP) NIS Notes n.d. www.hcup-us.ahrq.gov/db/vars/pclassn/nisnote.jsp (Last accessed January8, 2015)
- 23.Hua M, Li G, Clancy C, et al. : Validation of the V66.7 code for palliative care consultation in a single academic medical center. J Palliat Med 2017;20:372–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulvey CL, Smith TJ, Gourin CG: Use of inpatient palliative care services in patients with metastatic incurable head and neck cancer. Head Neck 2016;38:355–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iezzoni LI, Daley J, Heeren T, et al. : Identifying complications of care using administrative data. Med Care 1994;32:700–715 [DOI] [PubMed] [Google Scholar]
- 26.Bureau of Labor Statistics USD of L. CPI Inflation Calculator. n.d. www.bls.gov/data/inflation_calculator.htm (Last accessed February13, 2017)
- 27.Kross EK, Engelberg RA, Downey L, et al. : Differences in end-of-life care in the ICU across patients cared for by medicine, surgery, neurology, and neurosurgery physicians. Chest 2014;145:313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olmsted CL, Johnson AM, Kaboli P, et al. : Use of palliative care and hospice among surgical and medical specialties in the veterans health administration. JAMA Surg 2014;149:1169. [DOI] [PubMed] [Google Scholar]
- 29.Dunn GP: Surgery, palliative care, and the American College of Surgeons. Ann Palliat Med 2015;4:5–9 [DOI] [PubMed] [Google Scholar]
- 30.Task Force on Surgical Palliative care, Committee on Ethics: Statement of principles of palliative care. Bull Am Coll Surg 2005;90:34–35 [PubMed] [Google Scholar]
- 31.Winner M, Wilson A, Ronnekleiv-Kelly S, et al. : A singular hope: How the discussion around cancer surgery sometimes fails. Ann Surg Oncol 2017;24:31–37 [DOI] [PubMed] [Google Scholar]
- 32.Winner M, Wilson A, Yahanda A, et al. : Cancer surgeons' attitudes and practices about discussing the chance of operative “cure.” Surgery 2016;160:1619–1627 [DOI] [PubMed] [Google Scholar]
- 33.Bagante F, Spolverato G, Cucchetti A, et al. : Defining when to offer operative treatment for intrahepatic cholangiocarcinoma: A regret-based decision curves analysis. Surgery 2016;160:106–117 [DOI] [PubMed] [Google Scholar]
- 34.Cassell J, Buchman TG, Streat S, et al. : Surgeons, intensivists, and the covenant of care: Administrative models and values affecting care at the end of life. Crit Care Med 2003;31:1263–1270 [DOI] [PubMed] [Google Scholar]
- 35.Cooper Z, Courtwright A, Karlage A, et al. : Pitfalls in communication that lead to nonbeneficial emergency surgery in elderly patients with serious illness: Description of the problem and elements of a solution. Ann Surg 2014;260:949–957 [DOI] [PubMed] [Google Scholar]
- 36.Schwarze ML, Redmann AJ, Brasel KJ, Alexander GC: The role of surgeon error in withdrawal of postoperative life support. Ann Surg 2012;256:10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwarze ML, Bradley CT, Brasel KJ: Surgical “buy-in”: The contractual relationship between surgeons and patients that influences decisions regarding life-supporting therapy. Crit Care Med 2010;38:843–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.May P, Garrido MM, Cassel JB, et al. : Prospective cohort study of hospital palliative care teams for inpatients with advanced cancer: Earlier consultation is associated with larger cost-saving effect. J Clin Oncol 2015;33:2745–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelley AS, Deb P, Du Q, et al. : Hospice enrollment saves money for medicare and improves care quality across a number of different lengths-of-stay. Health Aff 2013;32:552–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrison RS, Dietrich J, Ladwig S, et al. : Palliative care consultation teams cut hospital costs for medicaid beneficiaries. Health Aff 2011;30:454–463 [DOI] [PubMed] [Google Scholar]
- 41.Adelson K, Paris J, Horton JR, et al. : Standardized criteria for palliative care consultation on a solid tumor oncology service reduces downstream health care use. J Oncol Pract 2017;13:e431–e440 [DOI] [PubMed] [Google Scholar]
- 42.Earle C, Neville B, Landrum M, et al. : Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol 2004;22:315–321 [DOI] [PubMed] [Google Scholar]
- 43.Christakis NA, Escarce JJ: Survival of medicare patients after enrollment in hospice programs. N Engl J Med 1996;335:172–178 [DOI] [PubMed] [Google Scholar]
- 44.Haut ER, Pronovost PJ, Schneider EB: Limitations of administrative databases. JAMA 2012;307:2589; author reply 2589–2590 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.