Abstract
Background
Early-life maltreatment has severe consequences for the affected individual, and it has an impact on the next generation. To improve understanding of the intergenerational effects of abuse, we investigated the consequences of early-life maltreatment on maternal sensitivity and associated brain mechanisms during mother–child interactions.
Methods
In total, 47 mothers (22 with a history of physical and/or sexual childhood abuse and 25 without, all without current mental disorders) took part in a standardized real-life interaction with their 7- to 11-year-old child (not abused) and a subsequent functional imaging script-driven imagery task.
Results
Mothers with early-life maltreatment were less sensitive in real-life mother–child interactions, but while imagining conflictual interactions with their child, they showed increased activation in regions of the salience and emotion-processing network, such as the amygdala, insula and hippocampus. This activation pattern was in contrast to that of mothers without early-life maltreatment, who showed higher activations in those regions in response to pleasant mother–child interactions. Mothers with early-life maltreatment also showed reduced functional connectivity between regions of the salience and the mentalizing networks.
Limitations
Region-of-interest analyses, which were performed in addition to whole-brain analyses, were exploratory in nature, because they were not further controlled for multiple comparisons.
Conclusion
Results suggest that for mothers with early-life maltreatment, conflictual interactions with their child may be more salient and behaviourally relevant than pleasant interactions, and that their salience network is poorly modulated by the brain regions involved in mentalizing processes. This activation pattern offers new insights into the mechanisms behind the intergenerational effects of maltreatment and into options for reducing these effects.
Introduction
Early-life maltreatment (ELM) is estimated to occur in 25% of children1 and has severe consequences for the affected individual, such as reduced theory of mind2 and emotion regulation3 capacities, lower relationship quality4 and increased risk of developing mental disorders.5,6 There is accumulating evidence that ELM has intergenerational impacts, such as effects on the child’s temperament7 and increased risk of child abuse.8 Alterations in maternal caregiving behaviour constitute one possible pathway of this cycle of abuse.
Maternal sensitivity is the capacity to adequately respond to a child’s cues. According to emotional availability scales, a mother with high sensitivity shows positive, creative, authentic and congruent communication with her child.9 Reduced maternal sensitivity, on the other hand, is associated with behaviour problems and deficits in social competence in children,10,11 and poor maternal caregiving is related to risk of psychopathology and response to treatment in offspring.12,13 A growing number of studies shows that ELM enhances the risk of poor maternal sensitivity. Physical childhood abuse has been associated with reduced maternal sensitivity and higher levels of maternal intrusiveness, hostility and more negative emotions toward the child14,15; sexual abuse has been associated with less positive mother–child interactions and higher levels of permissive and harsh parenting.16,17
Caregiving behaviour, such as maternal sensitivity, is regulated by brain circuits, including the salience, reward, empathy and emotion-regulation networks, known to be affected by early-life experiences and the mental health of mothers.18,19 Previous studies have focused primarily on brain responses in healthy, non-maltreated mothers to the cues of either their own or unfamiliar infants. Data suggest that the maternal amygdala response is specific for a mother’s own child, indicating an increased salience and personal relevance of the own infant’s positive cues affecting the mother’s responsiveness to the child.20 Maternal neural response to infant stimuli has also been associated with attachment quality and caregiving behaviour. Mothers of insecurely attached children showed increased amygdala activation to their infant’s crying,21 and mothers’ amygdala responses to negative infant faces has been linked to more intrusive interactions with their own infants.22 Additionally, stronger anterior insula activation in response to negative infant stimuli has been reported in intrusive and depressed mothers.23,24 Hence, increased salience of infants’ negative cues and heightened affective response to them may promote emotionally overwhelmed, intrusive responses in mothers, instead of sensitive exchange and bonding with the child. Furthermore, sensitive mothers show stronger activations in the lateral frontal pole region and inferior frontal gyrus as a result of negative cues from their own infant,23,25 and non-depressed mothers (but not depressed mothers) showed greater response to their infant’s cry in the dorsal anterior cingulate cortex.26 These findings suggest that activations in maternal frontolimbic regions as a result of negative infant cues seem to be crucially involved in regulating and inhibiting automatic negative emotional responses toward their infant (inferior frontal gyrus) and in evaluating the emotional input and selecting a response to the child (dorsal anterior cingulate cortex). Furthermore, the hippocampus may also play a significant role in adaptive maternal behaviour. For instance, Musser and colleagues23 have hypothesized that stronger hippocampal activation in more sensitive mothers in response to their own infant’s cry might point to the ability to recall previous interactions with their child that helped them cope with stressful emotions.
Several studies have linked ELM to functional alterations in brain regions involved in salience and emotion processing, such as increased amygdala activation to (adult) sad faces,27 increased salience of emotionally negative stimuli28 and decreased connectivity within the salience network,29 but very little is known about the neuronal correlates of maternal responsiveness in people with ELM. The only previous studies investigating neural responses to infant cues in traumatized mothers (with posttraumatic stress disorder) showed higher amygdala and insula activations and reduced prefrontal activations to videos of their distressed children compared with healthy controls.30,31 Additionally, to our knowledge, no study up to now has used an ecologically valid paradigm to investigate a complex (namely, conflictual) mother–child interaction to challenge maternal sensitivity and the mother’s ability to regulate her emotions.
In this study, we investigated the neural correlates of maternal responses in mothers with ELM compared to mothers without ELM in response to interactions with their own child and an unfamiliar child. Importantly, to specifically address the consequences of ELM for the neural correlates of mother–child interactions, we included only mothers without current mental disorders and controlled for a lifetime history of mental disorders. First, we assessed maternal sensitivity in a standardized real-life interaction with their own primary-school-aged (7 to 11 years) child. Then, we included mothers in a standardized, script-driven functional imaging paradigm, in which they were instructed to imagine brief episodes of conflictual and pleasant mother–child interactions. We expected mothers with ELM to be less sensitive than mothers without ELM; to show stronger neuronal activations in regions of the salience and emotion-processing networks (amygdala, insula), as well as in the hippocampus; and to exhibit decreased functional connectivity between cortical and subcortical areas in response to conflictual versus pleasant mother–child interactions with their own child. In contrast, we expected conflictual scripts to trigger enhanced activations in regions of the emotion regulation network (particularly inferior frontal gyrus and anterior cingulate cortex) in mothers without ELM. Furthermore, we investigated the association between neuronal activations and maternal sensitivity.
Methods
Participants
Originally, 29 mothers with a history of physical and/or sexual abuse (ELM) and 28 mothers without such a history (controls) participated in the study, along with their 7- to 11-year-old children (German primary-school age). However, because of head movement 10 participants were excluded from further analysis (Appendix 1, available at jpn.ca-170026-a1), so that the final sample contained 22 mothers with ELM and 25 control mothers. Six mothers with ELM reported experiences of neglect or psychological abuse in addition to physical and/or sexual abuse; none of the control mothers reported any type of abuse. None of the mothers had a current mental disorder, but 9 mothers with ELM had had a lifetime diagnosis (Appendix 1). The groups were matched for the mother’s and child’s age, child’s sex and mother’s years of education (Table 1). Exclusion criteria consisted of current mental disorders; neurologic disorders; lifetime diagnosis of schizophrenia, schizoaffective disorder or bipolar disorder; substance use in the previous 6 months; psychotropic medication use; severe medical illness in the mother or child; child endangerment; mother and child not living together. They were assessed by diagnostic interview (see Measures, below) and by directly asking the mothers and children. The controls had no lifetime mental disorders.
Table 1.
Demographic, psychometric and rating characteristics
| Characteristic | ELM (n = 22)* | Controls (n = 25)* | t / χ2 | p value |
|---|---|---|---|---|
| Mothers | ||||
| Age, yr | 39.9 ± 6.6 | 39.6 ± 4.9 | 0.179† | 0.858 |
| Education, yr | 17.9 ± 4.0 | 16.4 ± 3.1 | 1.461† | 0.151 |
| HAM-D score | 2.8 ± 3.7 | 0.9 ± 1.3 | 2.355‡ | 0.027 |
| EAS sensitivity | 4.0 ± 1.0 (2.0–6.0) | 4.5 ± 1.0 (2.5–6.5) | −1.750† | 0.044 (1-tailed) |
| fMRI vividness ratings, all interactions | 4.14 ± 0.58 (2.73–4.88) | 4.30 ± 0.54 (2.67–5.0) | −0.942† | 0.351 |
| fMRI anger ratings, own child conflictual interactions | 4.12 ± 0.70 (2.5–5.0) | 4.41 ± 0.64 (2.5–5.0) | −1.476† | 0.147 |
| fMRI arousal ratings, own child conflictual interactions | 3.92 ± 0.75 (2.5–5.0) | 3.97 ± 0.63 (2.5–5.0) | −0.236† | 0.815 |
| Children | ||||
| Age, yr | 8.1 ± 1.0 | 7.8 ± 1.0 | 0.693† | 0.492 |
| Male/female, % | 45.5/54.5 | 52/48 | 0.201§ | 0.654 |
EAS = Emotional Availability Scales; ELM = early-life maltreatment; fMRI = functional magnetic resonance imaging; HAM-D = Hamilton Rating Scale for Depression; SD = standard deviation.
Data are mean ± SD (range) unless indicated otherwise.
Degrees of freedom = 45.
Degrees of freedom = 25.4.
Degrees of freedom = 1.
The study was performed in accordance with the Declaration of Helsinki and approved by the local ethics committee at the Faculty of Medicine, University of Heidelberg. Participants gave written informed consent before participation and received monetary compensation.
Measures
We assessed maltreatment experiences using the Childhood Experience of Care and Abuse Interview,32 and defined ELM as physical and/or sexual abuse before the age of 18 years according to the interview, which can be regarded as the gold standard in retrospective evaluation of childhood trauma.33 We assessed maternal sensitivity in a standardized real-life mother–child interaction using the Emotional Availability Scales (EAS; Appendix 1).34 We used the Structured Clinical Interview for DSM-IV Axis I35 and the International Personality Disorder Examination36 to assess lifetime axis I and II disorders, and we measured depression using the Hamilton Rating Scale for Depression (HAM-D).37 Interviews were conducted by qualified and trained diagnosticians (Masters degree in psychology).
Functional MRI paradigm
We assessed neural correlates of maternal responses in a script-driven imagery paradigm that consisted of 16 acoustically presented, standardized brief scripts of mother–child interactions. The scripts included 4 experimental conditions — conflictual versus pleasant interactions with one’s own or an unfamiliar child (4 different scripts per condition) — and were presented in a pseudorandomized order in 2 sessions separated by an approximately 8 min break for structural magnetic resonance scanning. Each script started with a 15 s baseline (no stimulation), followed by a 23 s description of a mother–child interaction read by a professional actor, a 15 s postscript imagination phase and then vividness, arousal and anger ratings (1 to 5, none to very much). Participants were instructed to close their eyes, listen to the scripts and vividly imagine the presented interactions. In line with previous studies38–41 and to avoid the effects of different acoustic input, we used only the 15 s imagination phases in the fMRI analyses (Appendix 1).
Functional MRI data acquisition
We performed functional imaging on a 3 T whole-body magnetic resonance scanner (Tim Trio; Siemens) equipped with a 32-channel head coil. In each volume, we acquired 33 transverse slices (slice thickness = 3 mm). We used a T2*-sensitive gradient echo-planar imaging sequence (repetition time = 2000 ms, echo time = 30 ms; flip angle = 78°; field of view = 92 × 192 mm; in-plane resolution 3 × 3 mm). Isotropic high-resolution (1 × 1 × 1 mm3) structural images were recorded using a T1-weighted sagitally oriented MPRAGE sequence.
Statistical analysis
We analyzed group differences (ELM versus controls) in demographic and psychometric data, and in vividness, anger and arousal ratings during the fMRI task using 2-sample t tests for continuous data and χ2 tests for categorical data (IBM SPSS, version 22.0). We also performed analyses of covariance on EAS sensitivity, with mother’s lifetime diagnosis of a mental disorder and child’s age as covariates. With regard to our a priori hypothesis, we chose 1-tailed tests for EAS sensitivity. All other results report 2-tailed tests.
We used statistical parametric mapping (SPM8, Wellcome Department of Imaging Neuroscience) for fMRI data preprocessing (Appendix 1). We constructed a design matrix for each participant by defining the listening and imagination phases of the scripts (convolved with a canonical hemodynamic response function) as separate regressors for the 4 combinations of interaction (conflictual, pleasant) and child identity (own, unfamiliar), as well as 1 regressor each for the baseline and the rating phases. We included 6 movement parameters; fMRI time series were high-pass filtered (cutoff 120 s), and temporal autocorrelation was modelled as a first-order autoregressive process.
We assessed consistent effects across participants and between groups in a random effects multiple regression analysis with 8 contrast images (conflictual–own versus baseline, conflictual–unfamiliar versus baseline, pleasant–own versus baseline, and pleasant–unfamiliar versus baseline for each group), which represented estimated effects of the 3 experimental factors group (ELM, controls), mother–child interaction (conflictual, pleasant) and child identity (own, unfamiliar). We included EAS sensitivity in the multiple regression analysis in condition-specific (group × interaction × child identity) regressors, generating another 8 regressors. We included lifetime diagnosis of a mental disorder and child’s age as covariates of no interest.
To test our hypotheses regarding differences between the ELM and control groups when imagining interactions with their own child, we calculated the 2-way group (ELM, control) × mother–child interaction (conflictual, pleasant) separately for the mother’s own and the unfamiliar child. For whole-brain analyses, we applied a significance level of 5% (family-wise-error correction for multiple comparisons). To this end, we combined a voxel-level threshold of p < 0.005 (uncorrected) with a nonarbitrary cluster-extent threshold. The reasoning behind this was that voxel values in fMRI data are not independent of each other, which should result in spatially extended clusters of significantly activated voxels.42 We determined the cluster-extent threshold (k = 75) resulting in a corrected probability of 5% for false positives using the latest version of the AFNI routine 3dFWHMx to estimate an ACF model on the basis of our data and then entering the resulting parameters (a, b, c) 0.330546, 5.11517, 11.465 into 3dClustSim to estimate the minimum cluster size for our current data. We also performed region-of-interest (ROI) analyses for bilateral amygdala, insula, hippocampus, anterior cingulate cortex and inferior frontal gyrus (pFWE < 0.05; Appendix 1). Regions for ROI analyses were chosen because of their significance for maternal caregiving behaviour, and images were anatomically defined according to Automated Anatomic Labelling using the WFU Pick Atlas Tool and applying a small-volume correction.
Next, we performed generalized psychophysiological interaction (gPPI) analyses43 to test whether the coupling of the amygdala and insula with other brain regions during the imagination of conflictual versus pleasant interactions with one’s own child differed between groups (seed regions: right amygdala, peak voxel x, y, z = 30, −7, −14; right insula, peak voxel x, y, z = 39, 8, 4; left insula, peak voxel x, y, z = −33, −7, 22; Appendix 1) using a multiple regression design as described above (including lifetime diagnosis of a mental disorder and child’s age as covariates of no interest), but without including EAS sensitivity. Results were thresholded using the same procedure as for the functional analyses: that is, resulting in a corrected p value < 0.05 (cluster-extent threshold: k > 75).
Finally, we tested whether neural activations in response to conflictual versus pleasant interactions were associated with EAS sensitivity. To do this, we performed hypothesis-driven ROI analyses for bilateral amygdala, insula, hippocampus, anterior cingulate cortex and inferior frontal gyrus on contrasts comparing conflictual versus pleasant interactions with one’s own child on the regressors parameterizing interindividual differences in EAS sensitivity on task-relevant conditions separately for each group. We then tested whether these effects were specific to one group and significantly weaker in the other. We accomplished this by masking the statistical map describing the (conflictual versus pleasant interaction with own child × EAS sensitivity) effect in the first group with the statistical map describing the ([group × conflictual versus pleasant interaction with own child] × EAS sensitivity) contrast, with the activated voxels having to survive at an uncorrected p value of 0.05 (masking procedure).44
Results
Demographic, psychometric and rating data
As expected, ELM mothers interacted less sensitively with their child than control mothers, according to the EAS (t45 = −1.750, p < 0.05, 1-tailed), even when controlling for the child’s age (F1,44 = 2.864, p < 0.05, 1-tailed). However, when controlling for mothers’ lifetime diagnosis of mental disorder, the group effect (ELM versus control) no longer reached significance (F1,44 = 1.307, p = 0.259), nor did the effect of the covariate lifetime diagnosis (F1,44 = 0.437, p = 0.512). Scores on the HAM-D differed between the ELM and control groups (t25.4 = 2.355, p < 0.05). Nevertheless, mean HAM-D values in both groups were in a very low normal range, without clinical relevance (HAM-D < 10), and none of the mothers fulfilled the criteria for a current depressive episode.
Mothers were able to vividly imagine the mother–child interaction scripts as indicated by mean ± standard deviation vividness ratings of 4.25 ± 0.57 on a scale of 1 to 5 (no significant group difference [t45 = −0.958, p = 0.343]). Across groups, participants indicated higher feelings of anger after conflictual rather than pleasant interactions (t65.254 = 27.388, p < 0.01).
Functional MRI data
Group × mother–child interaction
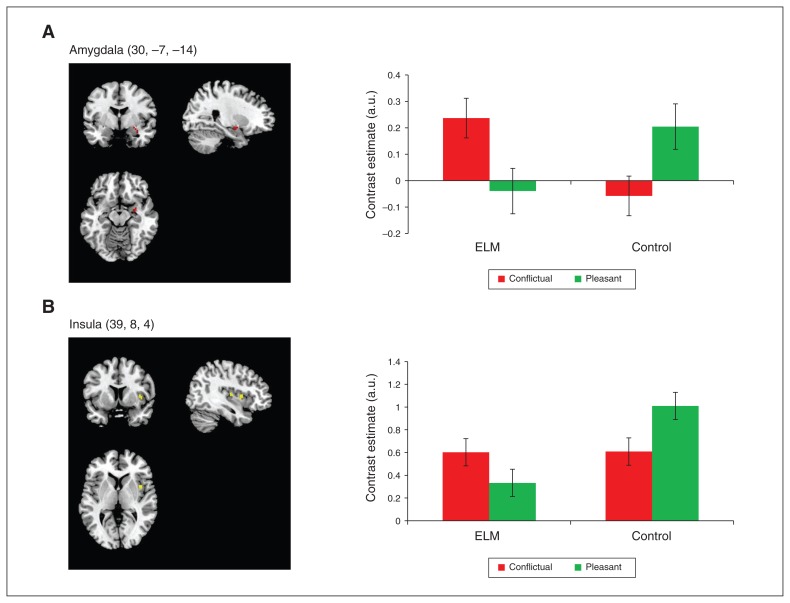
We were particularly interested in the group × mother–child interaction effect, and whether it was related to the child’s identity. As expected, compared with control mothers, whole-brain analyses revealed increased activations in mothers with ELM in the right amygdala (peak voxel: x, y, z = 30, −7, −11) and left insula (peak voxel: x, y, z = −33, −7, 22), and also in the right supplementary motor area (peak voxel: x, y, z = 12, −22, 67) and right middle frontal gyrus (peak voxel: x, y, z = 21, 47, 31) to conflictual versus pleasant interactions with their own child, irrespective of lifetime diagnosis of mental disorder and child’s age ( Table 2). We found the opposite pattern (i.e., stronger activations to pleasant versus conflictual interactions) in controls in the same regions. These results were confirmed and extended in ROI analyses, which showed significantly stronger activations in ELM mothers to conflictual versus pleasant interactions in the right amygdala (Fig. 1A), bilateral hippocampi (pFWE < 0.05), and by trend in the right insula (pFWE = 0.097; Fig. 1B; Table 2). We did not find significant effects in the inferior frontal gyrus or anterior cingulate cortex in emotion-regulation areas. Notably, the described effects were specific to interactions with one’s own child; the group × mother–child interaction contrast for scripts with an unfamiliar child did not result in significant effects. Furthermore, ELM mothers did not show stronger brain activations to pleasant versus conflictual interactions with their own child compared with controls, and controls did not show stronger activations to conflictual versus pleasant interactions compared with ELM mothers.
Table 2.
Peak effects for group (ELM v. control) × mother–child interaction (conflictual v. pleasant)*
| Contrast | Cluster size k | t | p value | Peak voxel MNI, x, y, z (mm) | Anatomic location of peak voxel |
|---|---|---|---|---|---|
| Whole-brain analyses | |||||
| ELM > control: conflictual > pleasant (control > ELM: pleasant > conflictual) with own child | 260 | 4.27 | < 0.001† | −39, −34, −11 | Parahippocampal gyrus |
| 403 | 4.15 | < 0.001† | 12, −22, 67 | Supplementary motor area | |
| 376 | 3.92 | < 0.001† | 30, −7, −11 | Amygdala | |
| 445 | 3.85 | < 0.001† | −33, −7, 22 | Insula | |
| 227 | 3.78 | < 0.001† | 21, 47, 31 | Middle frontal gyrus | |
| 150 | 3.56 | < 0.001† | 12, 8, 40 | Middle cingulate cortex | |
| ELM > control: pleasant > conflictual (control > ELM: conflictual > pleasant) with ownchild | No significant activations | ||||
| ELM > control: conflictual > pleasant (control > ELM: pleasant > conflictual) with unfamiliarchild | No significant activations | ||||
| ELM > control: pleasant > conflictual (control > ELM: conflictual > pleasant) with unfamiliarchild | No significant activations | ||||
| Region-of-interest analyses | |||||
| ELM > control: conflictual > pleasant (control > ELM: pleasant > conflictual) with ownchild | 8 | 3.36 | 0.025‡ | 30, −7, −14 | Amygdala |
| 161 | 3.49 | 0.097‡ | 39, 8, 4 | Insula | |
| 44 | 3.8 | 0.023‡ | −15, −34, 7 | Hippocampus | |
| 32 | 3.68 | 0.033‡ | −24, −22, −11 | Hippocampus | |
| 95 | 3.68 | 0.033‡ | 36, −22, −11 | Hippocampus | |
| ELM: correlation sensitivity × BOLD signal (conflictual > pleasant) masked with correlation sensitivity × (group × interaction) | 10 | 4.01 | 0.021‡ | −27, 14, −17 | Insula |
| 14 | 3.91 | 0.028‡ | 36, 20, −14 | Insula | |
| 6 | 2.98 | 0.069‡ | 27, 2, −20 | Amygdala | |
| Control: correlation sensitivity × BOLD signal (pleasant > conflictual) masked with correlation sensitivity × (group by interaction) | No significant activations | ||||
BOLD = blood oxygen–level dependent; ELM = early-life maltreatment; MNI = Montreal Neurological Institute.
Covariates of interest: lifetime mental disorder and child’s age.
Uncorrected.
Family-wise error corrected.
Fig. 1.
Blood oxygen–level dependent activations in conflictual versus pleasant interactions with the mother’s own child. The figure depicts the group × mother–child interaction contrast. Mothers with early-life maltreatment (ELM) showed increased activations in the (A) amygdala and (B) insula for conflictual versus pleasant interactions with their own child, while control mothers showed the opposite pattern. Contrast estimates (adjusted units) of amygdala and insula are presented, with error bars representing the standard error of the mean. Data are extracted from a 2 mm sphere around the peak voxel. Amygdala (30, −7, −14)
Functional connectivity: group × mother–child interaction
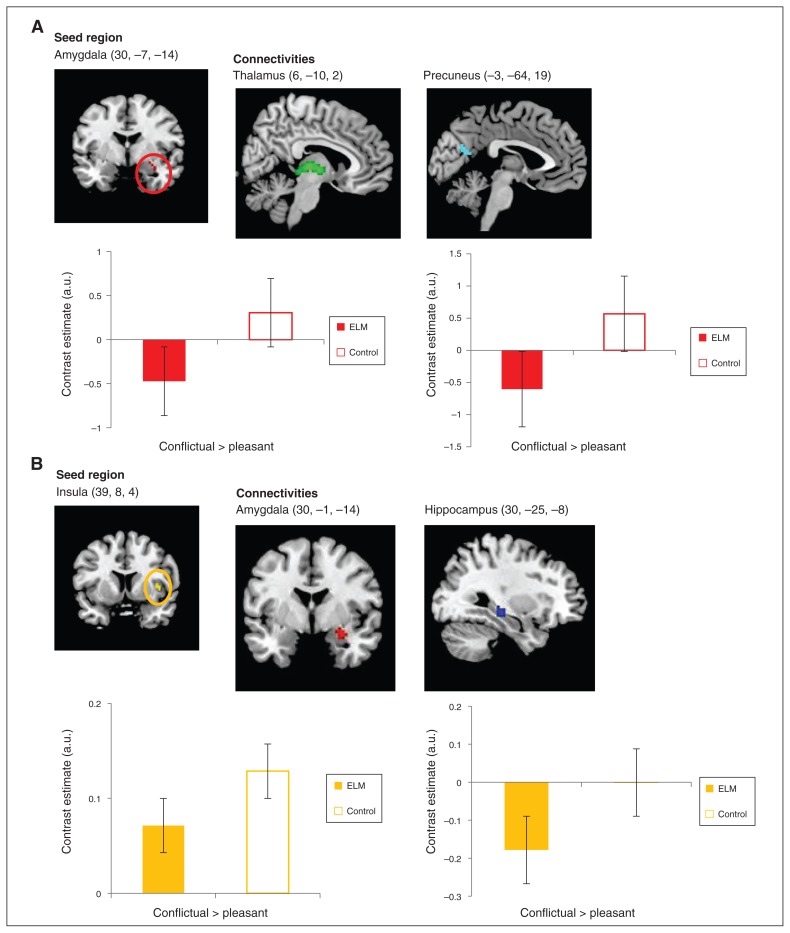
Using the right amygdala as seed region, the gPPI analysis revealed reduced functional connectivity with the thalamus (peak voxel = 6, −10, 2) and precuneus (peak voxel: x, y, z = −3, −64, 19) to conflictual versus pleasant interactions in ELM mothers compared with controls when controlling for the influence of a previous mental disorder and the age of the child. A second gPPI analysis using the right insula as seed region revealed reduced functional connectivity with the right hippocampus (pFWE < 0.05; peak voxel: x, y, z = 30, −25, −8) and by trend with the right amygdala (pFWE = 0.057; peak voxel: x, y, z = 30, −1, −14) in ELM compared with controls in ROI analyses (Table 3 and Fig. 2A and 2B). We found no significant functional connectivity using the left insula as seed region. Again, the functional connectivity analyses were specific to interactions with one’s own child and revealed no significant effect for group × mother–child interaction with an unfamiliar child.
Table 3.
Peak functional connectivity effects for group (ELM v. control) × mother–child interaction (conflictual v. pleasant)*
| Contrast | Cluster size k | t | p value | Peak voxel MNI, x, y, z (mm) | Anatomic location of peak voxel |
|---|---|---|---|---|---|
| Seed: amygdala (30, −7, −14; 2 mm radius sphere) | |||||
| ELM < control: conflictual < pleasant (control > ELM: conflictual > pleasant) with ownchild | 201 | 4.22 | < 0.001† | 6, −10, 2 | Thalamus |
| 109 | 3.44 | < 0.001† | −3, −64, | 19 Precuneus | |
| ELM < control: conflictual < pleasant (control > ELM: conflictual > pleasant) with unfamiliarchild | No significant activations | ||||
| Seed: insula (39, 8, 4; 2 mm radius sphere) ELM < control: conflictual < pleasant (control > ELM: conflictual > pleasant) with ownchild | 18 | 4.05 | 0.012‡ | 30, −25, −8 | Hippocampus |
| 10 | 3.09 | 0.057‡ | 30, −1, −14 | Amygdala | |
| ROI analyses | |||||
| ELM < control: conflictual < pleasant (control > ELM: conflictual > pleasant) with unfamiliarchild | No significant activations | ||||
ELM = early-life maltreatment; MNI = Montreal Neurological Institute; ROI = region of interest.
Covariates of interest: lifetime mental disorder and child’s age.
Uncorrected.
Family-wise error corrected.
Fig. 2.
Reduced functional connectivities in conflictual interactions versus pleasant interactions with their own child in mothers with early-life maltreatment (ELM). In mothers with ELM, interplay (A) between the amygdala (seed region) and the thalamus and precuneus, as well as (B) between the insula (seed region) and the amygdala and hippocampus was diminished. Contrast estimates (adjusted units) depict functional connectivity for conflictual > pleasant interactions with the mother’s own child. Data are extracted from a 2 mm sphere around the peak voxel. Thalamus (6, −10, 2) Precuneus (−3, −64, 19)
Associations with maternal sensitivity
In ELM mothers, EAS sensitivity was positively associated with activations in the bilateral insula (pFWE < 0.05; peak voxel: x, y, z = −27, 14, −17; 36, 20, −14) and by trend in the right amygdala (pFWE = 0.069; peak voxel: x, y, z = 27, 2, −20) to conflictual versus pleasant interactions with their own child. These associations were specific to the ELM group and not found in the control group (Table 2). In the control group, we found no significant associations between maternal sensitivity and activations to pleasant versus conflictual interactions in the predefined ROIs.
Discussion
This study in mothers with a history of ELM sheds light on the effects of ELM on the next generation, because it identified altered activations in the maternal brain in response to imagination of conflictual interactions with the mother’s own child. Consistent with previous reports,15,45 mothers with ELM were less sensitive in a standardized interaction with their child than mothers without ELM. Notably, our data suggest that maternal lifetime diagnosis of a mental disorder in addition to ELM exerted a negative influence on maternal sensitivity. At the neural level, ELM mothers showed stronger activation in the amygdala, insula, hippocampus and supplementary motor area in response to conflictual interactions with their own child, irrespective of lifetime history of a mental disorder. In contrast, mothers without ELM showed higher activation in the amygdala, insula, hippocampus and supplementary motor area when imagining pleasant interactions with their child. Notably, we found no significant activations for mother–child interactions with an unfamiliar child.
The finding of stronger amygdalar and insular activation in response to negative cues is common in people with posttraumatic stress disorder or early-life adversities46–48 and might indicate a sensitization to conflictual or threatening stimuli because of adverse experiences. Additionally, our findings in ELM mothers also parallel previous reports of elevated activations in the insula of intrusive mothers23 and in the amygdala of mothers with insecurely attached children in response to negative cues from their own versus an unfamiliar infant, along with elevated activations in the parahippocampal gyrus.21 The latter has been discussed in line with attachment-related processes, which might bias maternal responses to one’s own child. The present finding of stronger hippocampal activations in mothers with ELM while imagining conflictual versus pleasant interactions with their own child might indicate that negative interactions with their child provide a stronger activation of a social cognitive map, representing behaviourally relevant context factors of acquired social knowledge that guides the mother’s interaction with her child.49–51 In addition to higher hippocampal activation, we found a disturbed interplay between hippocampus and insula. Most interestingly, pleasant interactions with their own child appeared not to work as salient cues in ELM mothers and may therefore not facilitate sensitive caregiving behaviour. Instead, increased activations in the amygdala and insula as well as in the supplementary motor area in ELM mothers indicate a sensitization to and a high salience and behavioural relevance of conflictual rather than pleasant interactions. This is also supported by the results of the connectivity analyses, which suggest a reduced interplay among regions of the salience network, along with a reduced coupling between regions of the salience (amygdala, insula, thalamus) and mentalizing (precuneus) networks during conflictual versus pleasant interactions in ELM mothers. In line with previous reports of ELM-related alterations in functional connectivity between brain regions of emotion, salience and mentalizing networks29,52 the present results may reflect a disturbed interplay within the salience network and a disturbed modulation of the salience network through mentalizing processes that support sensitive maternal responses in conflictual interactions with one’s own child. Notably, control mothers showed an opposite neural response pattern, with increased activations in the insula, amygdala and supplementary motor area in response to pleasant versus conflictual mother–child interactions. This result was consistent with previous findings of enhanced amygdala responses to happy versus sad faces of one’s own infant, but not of an unfamiliar infant, in healthy mothers.20 Supplementary motor area activation may underline the relevance of pleasant interactions for initiating actions and approaching the child.
Finally, in ELM mothers, maternal sensitivity was positively related to bilateral insula and amygdala activations to conflictual versus pleasant interactions. This finding might reflect that a sensitization to conflictual or threatening stimuli after adverse childhood experiences results in being particularly vigilant and responsive to negative interactions with one’s own child but not in responding to pleasant interactions, as is characteristic for control mothers.
We want to stress that in this sample, none of the participating children was abused. When interpreting the results, one should bear in mind that the cycle of abuse was not fulfilled in this sample, but that the findings showed neural and behavioural alterations after ELM nonetheless. These alterations may add to a better understanding of the mechanisms behind the intergenerational effects of adverse childhood experiences, such as physical and sexual abuse.
Our study had several strengths. First, we examined a standardized real-life mother–child interaction as well as fMRI data from a script-driven imagery paradigm depicting mother–child interactions in mothers with ELM and a non-ELM control group. Since lifetime history of a mental disorder influenced maternal sensitivity, we controlled for this in the fMRI analyses, underlining the specificity of the observed neural changes for ELM. Contrary to previous studies,19–26 we focused on mothers of primary-school-aged children, an age group that, to our knowledge, has not been addressed before.
Limitations
Findings should be considered in the light of the study’s limitations, which suggest further studies are needed. First, because of the relatively small sample size, results should be replicated in a larger group. Notably, ROI analyses, which were performed in addition to whole-brain analyses, were exploratory in nature since — except for family-wise error corrections — they were not further controlled for multiple comparisons. Second, ELM was defined as sexual and/or physical abuse before age 18 years, based on an extensive retrospective interview. Despite the advantages of this approach, the interview data and the sample size did not allow for a finer differentiation of age at onset of ELM or inclusion of other trauma types, such as emotional abuse or neglect, which have also been shown to have neurobiological impacts. Third, previous research shows that the function of the parental brain is modified by current mental illness,53 and is programmed early on by psychopathologies, such as depression. 54 This is why we excluded current mental disorders and controlled for a lifetime history of mental disorders. However, although subthreshold depressive symptoms might have exerted an influence on maternal behaviour and brain functioning, they were not controlled for, because they are highly common in people with a history of serious ELM,55–57 and thus represent typical sequelae of ELM rather than a distinct condition. Future studies could compare ELM mothers with and without a history of a mental disorder, making it possible to draw conclusions about the effects of early programming of the maternal brain by depression and other mental disorders. Finally, data based on the imagination of behaviour cannot be equated with real-life behaviour; conclusions about real-life maternal behaviour should be drawn with caution. However, there is accumulating evidence that the imagination of behaviour evokes neural responses that are similar to actual behaviour.58–60 Thus, the imagination-based paradigm does appear to be a valid approach for studying neural correlates of maternal behaviour.
Conclusion
Taken together, the reported findings may suggest that mothers with ELM, irrespective of current or lifetime mental disorders, are sensitized to and experience conflictual interactions with their child as more salient or evaluate them as more relevant for their maternal behaviour, as reflected in higher amygdala, insula and supplementary motor area activity; at the same time, they show impaired modulatory mentalizing processes. In contrast, consistent with previous studies, non-ELM mothers’ amygdala response was more exaggerated in relation to pleasant interactions with their child, favouring positive mother–infant exchange and bonding. Our findings provide new and unique evidence for neuronal mechanisms implicated in the intergenerational effects of experiences of ELM, and we hope that they will stimulate future research into interventions to improve caregiving behaviours in maltreated mothers.
Acknowledgments
The authors thank K. Ueltzhoeffer for his valuable input regarding the analysis, E. Mielke for her help with data collection, and I. Rek and K. Hillmann for participant recruitment and organization. The study was supported by a grant from the German Federal Ministry of Education and Research to SC.H. (BMBF; 01KR1207A; coordinator: RB). The BMBF had no influence on study design or manuscript.
Footnotes
Competing interests: None declared.
Contributors: K. Bertsch, C. Reck, E. Moehler, R. Brunner, F. Bermpohl and S. Herpertz designed the study. C. Neukel, A. Fuchs, A.-L. Zietlow and E. Moehler acquired the data, which C. Neukel, K. Bertsch and S. Herpertz analyzed. C. Neukel wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Butchart A, Mikton C. Global status report on violence prevention 2014. Luxembourg: World Health Organisation; 2014. [Google Scholar]
- 2.Germine L, Dunn EC, McLaughlin KA, et al. Childhood adversity is associated with adult theory of mind and social affiliation, but not face processing. PLoS One. 2015;10:e0129612. doi: 10.1371/journal.pone.0129612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley B, Westen D, Mercer KB, et al. Association between childhood maltreatment and adult emotional dysregulation in a low-income, urban, African American sample: moderation by oxytocin receptor gene. Dev Psychopathol. 2011;23:439–52. doi: 10.1017/S0954579411000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flynn M, Cicchetti D, Rogosch F. The prospective contribution of childhood maltreatment to low self-worth, low relationship quality, and symptomatology across adolescence: a developmental-organizational perspective. Dev Psychol. 2014;50:2165–75. doi: 10.1037/a0037162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teicher MH, Samson JA. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry. 2013;170:1114–33. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry. 2007;64:49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- 7.Lang AJ, Gartstein MA, Rodgers CS, et al. The impact of maternal childhood abuse on parenting and infant temperament. J Child Adolesc Psychiatr Nurs. 2010;23:100–10. doi: 10.1111/j.1744-6171.2010.00229.x. [DOI] [PubMed] [Google Scholar]
- 8.Berlin LJ, Appleyard K, Dodge KA. Intergenerational continuity in child maltreatment: mediating mechanisms and implications for prevention. Child Dev. 2011;82:162–76. doi: 10.1111/j.1467-8624.2010.01547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biringen Z, Easterbrooks MA. Emotional availability. Concept, research, and window on developmental psychopathology. Dev Psychopathol. 2012;24:1–8. doi: 10.1017/S0954579411000617. [DOI] [PubMed] [Google Scholar]
- 10.Leerkes EM, Nayena Blankson A, O’Brien M. Differential effects of maternal sensitivity to infant distress and nondistress on social-emotional functioning. Child Dev. 2009;80:762–75. doi: 10.1111/j.1467-8624.2009.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raby KL, Roisman GI, Fraley RC, et al. The enduring predictive significance of early maternal sensitivity: social and academic competence through age 32 years. Child Dev. 2015;86:695–708. doi: 10.1111/cdev.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halevi G, Djalovski A, Vengrober A, et al. Risk and resilience trajectories in war-exposed children across the first decade of life. J Child Psychol Psychiatry. 2016;57:1183–93. doi: 10.1111/jcpp.12622. [DOI] [PubMed] [Google Scholar]
- 13.Kane JC, Murray LK, Cohen J, et al. Moderators of treatment response to trauma-focused cognitive behavioral therapy among youth in Zambia. J Child Psychol Psychiatry. 2016;57:1194–202. doi: 10.1111/jcpp.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driscoll JR, Easterbrooks M. Young mothers’ play with their toddlers: individual variability as a function of psychosocial factors. Infant Child Dev. 2007;16:649–70. [Google Scholar]
- 15.Moehler E, Biringen Z, Poustka L. Emotional availability in a sample of mothers with a history of abuse. Am J Orthopsychiatry. 2007;77:624–8. doi: 10.1037/0002-9432.77.4.624. [DOI] [PubMed] [Google Scholar]
- 16.DiLillo D, Damashek A. Parenting characteristics of women reporting a history of childhood sexual abuse. Child Maltreat. 2003;8:319–33. doi: 10.1177/1077559503257104. [DOI] [PubMed] [Google Scholar]
- 17.Roberts R, O’Connor T, Dunn J, et al. The effects of child sexual abuse in later family life: mental health, parenting and adjustment of offspring. Child Abuse Negl. 2004;28:525–45. doi: 10.1016/j.chiabu.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Feldman R. The adaptive human parental brain: implications for children’s social development. Trends Neurosci. 2015;38:387–99. doi: 10.1016/j.tins.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Kim P, Strathearn L, Swain JE. The maternal brain and its plasticity in humans. Horm Behav. 2007;77:113–23. doi: 10.1016/j.yhbeh.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strathearn L, Kim S. Mothers’ amygdala response to positive or negative infant affect is modulated by personal relevance. Front Neurosci. 2013;7:176. doi: 10.3389/fnins.2013.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laurent HK, Ablow JC. The missing link: mothers’ neural response to infant cry related to infant attachment behaviors. Infant Behav Dev. 2012;35:761–72. doi: 10.1016/j.infbeh.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim P, Capistrano CG, Erhart A, et al. Socioeconomic disadvantage, neural responses to infant emotions, and emotional availability among first-time new mothers. Behav Brain Res. 2017;325:188–96. doi: 10.1016/j.bbr.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musser ED, Kaiser-Laurent H, Ablow JC. The neural correlates of maternal sensitivity: an fMRI study. Dev Cogn Neurosci. 2012;2:428–36. doi: 10.1016/j.dcn.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan JK, Ambrosia M, Forbes EE, et al. Maternal response to child affect: role of maternal depression and relationship quality. J Affect Disord. 2015;187:106–13. doi: 10.1016/j.jad.2015.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wan MW, Downey D, Strachan H, et al. The neural basis of maternal bonding. PLoS One. 2014;9:e88436. doi: 10.1371/journal.pone.0088436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laurent HK, Ablow JC. A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Soc Cogn Affect Neurosci. 2012;7:125–34. doi: 10.1093/scan/nsq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dannlowski U, Kugel H, Huber F, et al. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum Brain Mapp. 2013;34:2899–909. doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaughlin KA, Peverill M, Gold AL, et al. Child maltreatment and neural systems underlying emotion regulation. J Am Acad Child Adolesc Psychiatry. 2015;54:753–62. doi: 10.1016/j.jaac.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Werff SJ, Pannekoek JN, Veer IM, et al. Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychol Med. 2013;43:1825–36. doi: 10.1017/S0033291712002942. [DOI] [PubMed] [Google Scholar]
- 30.Moser DA, Aue T, Wang Z, et al. Limbic brain responses in mothers with post-traumatic stress disorder and comorbid dissociation to video clips of their children. Stress. 2013;16:493–502. doi: 10.3109/10253890.2013.816280. [DOI] [PubMed] [Google Scholar]
- 31.Schechter DS, Moser DA, Wang Z, et al. An fMRI study of the brain responses of traumatized mothers to viewing their toddlers during separation and play. Soc Cogn Affect Neurosci. 2012;7:969–79. doi: 10.1093/scan/nsr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bifulco A, Brown GW, Harris TO. Childhood Experience of Care and Abuse (CECA): a retrospective interview measure. J Child Psychol Psychiatry. 1994;35:1419–35. doi: 10.1111/j.1469-7610.1994.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 33.Thabrew H, de Sylva S, Romans SE. Evaluating childhood adversity. Adv Psychosom Med. 2012;32:35–57. doi: 10.1159/000330002. [DOI] [PubMed] [Google Scholar]
- 34.Biringen Z. The Emotional Availability (EA) Scales. 4th ed. Boulder (CO): International Center for Excellence in Emotional Availability; 2008. [Google Scholar]
- 35.Sheehan DV, Janavs R, Baker R, et al. International neuropsychiatric interview. Tampa (FL): University of South Florida Press; 1999. [Google Scholar]
- 36.Bronisch T, Mombour W. Comparison of a diagnostic checklist with a structured interview for the assessment of DSM-III-R and ICD-10 personality disorders. Psychopathology. 1994;27:312–20. doi: 10.1159/000284889. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanius RA, Williamson PC, Densmore M, et al. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry. 2001;158:1920–2. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- 39.Lanius RA, Frewen PA, Girotti M, et al. Neural correlates of trauma script-imagery in posttraumatic stress disorder with and without comorbid major depression: a functional MRI investigation. Psychiatry Res. 2007;155:45–56. doi: 10.1016/j.pscychresns.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Daniels JK, Hegadoren KM, Coupland NJ, et al. Neural correlates and predictive power of trait resilience in an acutely traumatized sample: a pilot investigation. J Clin Psychiatry. 2012;73:327–32. doi: 10.4088/JCP.10m06293. [DOI] [PubMed] [Google Scholar]
- 41.Daniels JK, Coupland NJ, Hegadoren KM, et al. Neural and behavioral correlates of peritraumatic dissociation in an acutely traumatized sample. J Clin Psychiatry. 2012;3:420–6. doi: 10.4088/JCP.10m06642. [DOI] [PubMed] [Google Scholar]
- 42.Forman SD, Cohen JD, Fitzgerald M, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 43.McLaren DG, Ries ML, Xu G, et al. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–86. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volman I, Toni I, Verhagen L, et al. Endogenous testosterone modulates prefrontal–amygdala connectivity during social emotional behavior. Cereb Cortex. 2011;21:2282–90. doi: 10.1093/cercor/bhr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pereira J, Vickers K, Atkinson L, et al. Parenting stress mediates between maternal maltreatment history and maternal sensitivity in a community sample. Child Abuse Negl. 2012;36:433–7. doi: 10.1016/j.chiabu.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Fonzo GA, Simmons AN, Thorp SR, et al. Exaggerated and disconnected insular-amygdalar BOLD response to threat-related emotional faces in women with intimate-partner violence PTSD. Biol Psychiatry. 2010;68:433. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hughes KC, Shin LM. Functional neuroimaging studies of posttraumatic stress disorder. Expert Rev Neurother. 2011;11:275–85. doi: 10.1586/ern.10.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Javanbakht A, King AP, Evans GW, et al. Childhood poverty predicts adult amygdala and frontal activity and connectivity in response to emotional faces. Front Behav Neurosci. 2015;9:154. doi: 10.3389/fnbeh.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ekstrom AD, Ranganath C. Space, time and episodic memory: the hippocampus is all over the cognitive map. Hippocampus. 2017;00:1–8. doi: 10.1002/hipo.22750. [DOI] [PubMed] [Google Scholar]
- 50.Montagrin A, Saiote C, Schiller D. The social hippocampus. Hippocampus. 2017 doi: 10.1002/hipo.22797. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 51.Mack ML, Love BC, Preston AR. Building concepts one episode at a time: the hippocampus and concept formation. Neurosci Lett. 2017 Aug 8; doi: 10.1016/j.neulet.2017.07.061. pii: S0304-3940(17)30647-X. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L, Dai Z, Peng H, et al. Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Hum Brain Mapp. 2014;35:1154–66. doi: 10.1002/hbm.22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swain JE, Kim P, Ho SS. Neuroendocrinology of parental response to baby-cry. J Neuroendocrinol. 2011;23:1036–41. doi: 10.1111/j.1365-2826.2011.02212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sundstroem Poromaa I, Comasco E, Georgakis MK, et al. Sex differences in depression during pregnancy and the postpartum period. J Neurosci Res. 2017;95:719–30. doi: 10.1002/jnr.23859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carpenter LL, Shattuck TT, Tyrka AR, et al. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology (Berl) 2011;214:367–75. doi: 10.1007/s00213-010-2007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spertus IL, Yehuda R, Wong CM, et al. Childhood emotional abuse and neglect as predictors of psychological and physical symptoms in women presenting to a primary care practice. Child Abuse Negl. 2003;27:1247–58. doi: 10.1016/j.chiabu.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Silverman AB, Reinherz HZ, Giaconia RM. The long-term sequelae of child and adolescent abuse: a longitudinal community study. Child Abuse Negl. 1996;20:709–23. doi: 10.1016/0145-2134(96)00059-2. [DOI] [PubMed] [Google Scholar]
- 58.Case LK, Pineda J, Ramachandran VS. Common coding and dynamic interactions between observed, imagined, and experienced motor and somatosensory activity. Neuropsychologia. 2015;79:233–45. doi: 10.1016/j.neuropsychologia.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oullier O, Jantzen KJ, Steinberg FL, et al. Neural substrates of real and imagined sensorimotor coordination. Cereb Cortex. 2004;15:975–85. doi: 10.1093/cercor/bhh198. [DOI] [PubMed] [Google Scholar]
- 60.Cichy RM, Heinzle J, Haynes JD. Imagery and perception share cortical representations of content and location. Cereb Cortex. 2011;22:372–80. doi: 10.1093/cercor/bhr106. [DOI] [PubMed] [Google Scholar]