Abstract
Nanobioconjugates using carbon nanotubes (CNTs) are attractive and promising hybrid materials. Various biological applications using the CNT nanobioconjugates, for example, drug delivery systems and nanobiosensors, have been proposed by many authors. Scanning techniques such as scanning electron microscopy (SEM) and scanning probe microscopy (SPM) have advantages to characterize the CNT nanobioconjugates under various conditions, for example, isolated conjugates, conjugates in thin films, and conjugates in living cells. In this review article, almost 300 papers are categorized based on types of CNT applications, and various scanning data are introduced to illuminate merits of scanning techniques.
1. Introduction
1.1. Nanobioconjugate Applications of Carbon Nanotubes
Carbon nanotubes (CNTs) are promising nanomaterials that have extraordinary structures and properties [1–7]. The robust and flexible structures of CNTs may allow the fabrication of various nanoarchitectures. Furthermore, the unique electrical and optical properties of CNTs may permit their use in various applications, such as nanosensors.
One of the important technical milestones for CNT nanotechnology is the separation of CNTs. CNTs synthesized from amorphous carbon have various lengths, diameters, and number of layers. The latter include single-walled CNTs (SWNTs), double-walled CNTs (DWNTs), and multiwalled CNTs (MWNTs). Furthermore, CNTs with various chiralities can be produced. Since the physicochemical properties of CNTs vary according to these factors, methods of isolating single-chirality CNTs have been proposed [8–13].
Among the diverse possible applications of CNTs, biological applications are important [14–35]. Biological applications require soluble CNTs; they often involve aqueous solutions. “Wrapping” techniques are popular approaches to solubilize CNTs [23, 26, 36–52]. When CNT powder is added to a surfactant solution followed by sonication, CNT bundles form and each bundle will become wrapped with surfactant molecules. In addition to surfactants, which were used to first demonstrate the feasibility of wrapping, various other organic molecules including DNA and protein molecules have also been successfully used to wrap CNTs [53–67]. Advantages of the use of DNA molecules have been described by several authors. For example, DNA and SWNTs are specifically related to DNA sequence and CNT chirality [66]. The authors suggested that (TCC)10, (TGA)10, and (CCA)10 have an avid affinity for (9, 1) SWNTs.
Scanning techniques are a powerful means to characterize the structures and physicochemical properties of CNTs and nanobioconjugates of CNTs, such as hybrids of DNA and CNTs (DNA-CNT hybrids). In this paper, various scanning studies of DNA-CNT and other nanobioconjugates of CNTs are categorized based on the types of biological applications. We previously published a review article summarizing scanning probe microscopy (SPM) studies of DNA-CNT hybrids [68]. In that paper, we categorized references based on the types of SPMs. In the present review, the references are categorized based on research targets. This review also includes scanning electron microscopy (SEM) to provide a comprehensive overview of research scanning techniques. The advantages of the scanning techniques in each CNT application are highlighted.
2. Advantages of Scanning Techniques in Studying Nanobioconjugates of CNTs
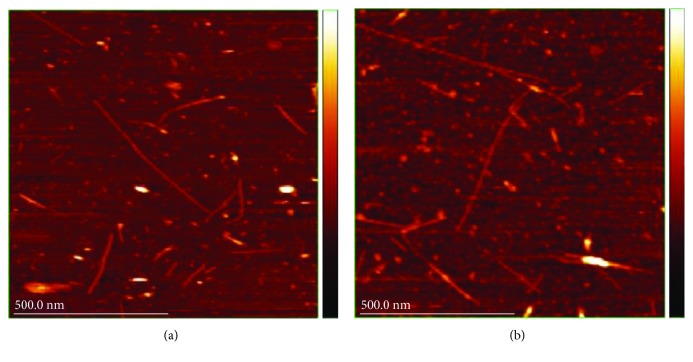
SPM and SEM are reasonable approaches to characterize nanobioconjugates with CNTs, as is transmission electron microscopy (TEM). Among the SPM techniques, atomic force microscopy (AFM) is frequently employed to obtain topographical information of CNT nanobioconjugates. Figure 1 provides example AFM topographs of DNA-SWNTs hybrids in aqueous solution [69]. AFM imaging can clearly reveal rod-like structures of CNTs without complicated sample preparations. The authors found that the heights of the observed hybrids fluctuated according to environmental conditions.
Figure 1.
AFM images reported by Hayashida and Umemura. (a) Hybrids of ssDNA and SWNTs. (b) Hybrids of dsDNA and SWNTs. Observation was carried out in a buffer solution (reprinted from Hayashida and Umemura [69] with permission).
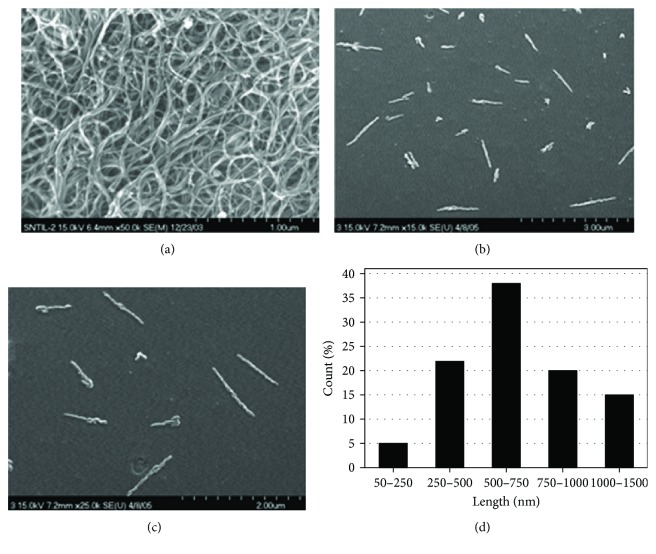
Figure 2 shows SEM images of SWNTs and DNA-SWNT conjugates reported by Nepal et al. [70]. Bare SWNTs were observed as bundled structures (Figure 2(a)), but individual bundles were clearly resolved. In the case of DNA-SWNT conjugates, monodispersed SWNTs were also clearly evident. Furthermore, MWNTs were also observed by SEM and compared with SWNTs. A length distribution analysis determined that sample preparation procedures affected CNT length. TEM has also been used to visualize CNT nanobioconjugates. One study incorporated TEM along with AFM and SEM for similar samples [71].
Figure 2.
SEM images reported by Nepal et al. (a) An SEM image of SWNTs. (b and c) An SEM image of DNA-SWNTs. (d) Length distribution of DNA-SWNTs (reprinted from Nepal et al. [70] with permission).
Thin or thick films containing CNT nanobioconjugates are popular samples as well as isolated conjugates. Their surface morphologies have been mainly studied by SEM [25, 70, 72–82]). Scanning techniques have also been used to examine other various structures of CNT nanobioconjugates [83–91]. CNT nanobioconjugates have also been verified using energy dispersive X-ray spectrometry (EDS) [25, 91–95].
General characterization, which includes the use of scanning techniques, is a fundamental and crucial aspect of CNT-related studies. When bioconjugates of CNTs are isolated on a flat surface, the excellent high resolution afforded by SPM is a big advantage. When bioconjugates of CNTs are prepared as films, SEM and SPM can be selectively employed according to the roughness of the films.
3. CNT Nanobioconjugates for DNA Sensors
While one of the purposes of the aforementioned wrapping techniques is to solubilize CNTs, the wrapped structures also have potential value as nanobiodevices. This section described some notable scanning studies involving DNA wrapping.
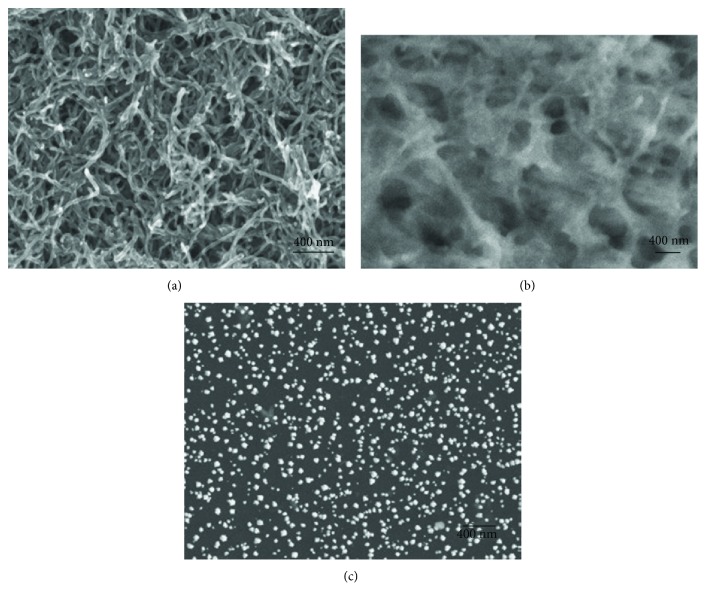
An important application of CNTs for DNA studies is mismatch detection in the hybridization of DNA molecules [96–99]. One study reported on the fabrication of conjugates of MWNTs, gold nanoparticles (Au NPs), and poly(p-aminobenzoic acid) (PABA). After depositing the conjugates on a glassy carbon electrode (GCE) surface, single-stranded DNA (ssDNA) was attached to the conjugates by gold monosulfide bonding [98]. Hybridization between the attached ssDNA and a complementary ssDNA was detected by differential pulse voltammetry. The authors succeeded in detecting three-based mismatched ssDNA. Another study from the same research group expanded the method combining zinc oxide nanowires (ZnONWs) with gold nanoparticles and MWNTs and succeeded in detecting single-mismatched ssDNA [97]. SEM was employed to characterize the functionalized GCE surfaces. Figures 3(a)–3(c) show SEM images of MWNTs/GCE, PABA/MWNTs/GCE, and Au NPs/PABA/MWNTs/GCE, respectively [98]. Although the surface conditions were different among the three samples, surface structures were well characterized by SEM. In particular, distribution of Au NPs on the GCE was clearly visualized. SEM proved advantageous for these samples because of the large depth of field.
Figure 3.
SEM images reported by Wang et al. (a) MWNTs/GCE. (b) PAbA/MWNTs/GCE. (c) Au nanoparticles/PABA/MWNTs/GCE (reprinted from Wang et al. [97] with permission).
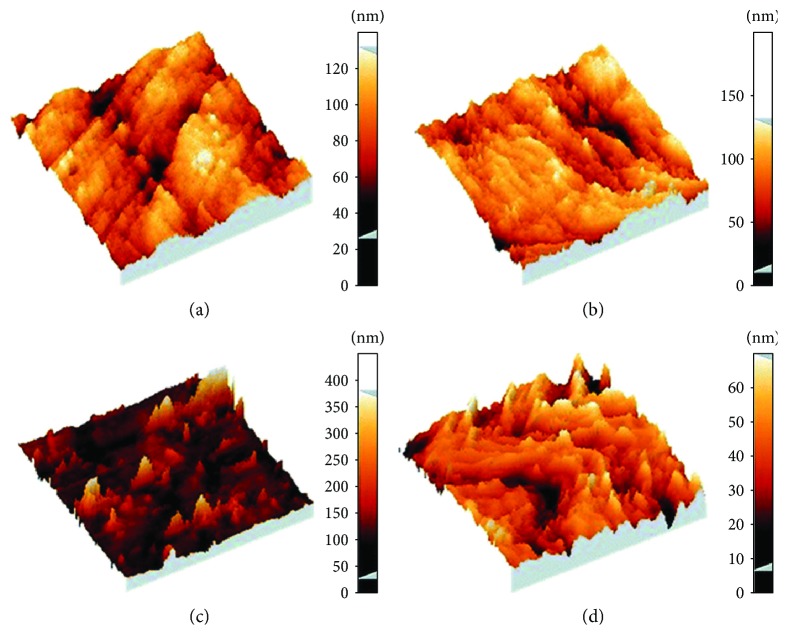
Mismatch detection on GCE has also been accomplished by combining DNA, MWNTs, and [Fe(CN)6]3−/4− [96]. The authors used AFM to characterize the functionalized GCE surfaces (Figure 4).
Figure 4.
AFM images of functionalized GCE surfaces for DNA mismatch detection. (a) Oxidized bare GCE. (b and d) DNA/oxidized GCE. (c) MWNTs/DNA/oxidized GCE. Scan sizes: 5 × 5 μm in (a), (b), and (c). 1 × 1 μm in (d) (reprinted from Shahrokhian et al. [96] with permission).
It is simple to attach DNA molecules to CNT surfaces. Thus, CNTs could potentially be used as DNA sensors. When DNA hybridization is detected electrically, CNT nanobioconjugates for DNA detection are deposited on electrode surfaces. Electrode surfaces are not transparent in many cases. Thus, scanning techniques have obvious advantages over TEM and usual optical microscopes. Compared with SEM images, AFM has the advantage of enabling quantitative information concerning height. In contrast, when electrode surfaces are very rough, SEM is preferred.
Other studies have optically detected DNA hybridization using CNT nanobioconjugates. In this case, isolated DNA-CNT in suspension was used, rather than films on solid electrode surfaces [100–107]. Single-mismatch detection can also be done optically. Optical responses of CNTs, such as absorption and generation of photoluminescence spectra, can be used to detect DNA hybridization. Suspended DNA-CNT conjugates can be deposited on flat surfaces such as a cleaved mica surface for AFM observation.
In the future, direct detection of DNA associated with CNTs could be possible using scanning tunneling microscopy (STM). STM is a SPM-related technique that detects minute electrical signals, such as tunneling currents, between a conductive probe and a sample surface, as does AFM detects forces between a probe and a sample [108, 109]. In these electrical and optical detection techniques, plentiful CNTs are necessary for DNA detection. However, if STM could be adapted for this purpose, a single CNT might be sufficient to detect a single DNA molecule. Detection of DNA reaction with single DNA pairs with a single CNT is the dream of a single-molecule DNA sensor.
4. CNT Nanobioconjugates as Molecular Sensors
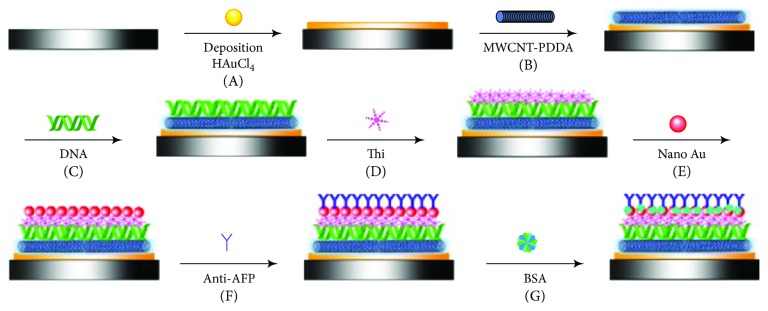
In addition to the detection of DNA hybridization, CNT can detect various biological reactions [94, 96–99, 110–133]). One study described the fabrication of an immunosensor using CNTs (Figure 5) [127]. The authors immobilized MWNTs dispersed with poly(diallydimethlammonium chloride) (PDDA) on an Au nanofilm that had been electrochemically deposited on GCE. DNA and thionine were attached to the functionalized GCE. After depositing Au NPs on the thionine surface, alpha-fetoprotein (AFP) antibody was immobilized on the NPs. The fabricated GCE was available to electrochemically detect AFP using an immunoreaction between the AFP and AFP antibody. SEM observation of the GCE surfaces at each functionalization step was effective to verify their samples.
Figure 5.
Schematic view of the fabrication process of an immunosensor reported by Ran et al. (A) Deposition of Au nanoparticles. (B) Coating of MWNTs-PDDA layer. (C) Immobilization of DNA film. (D) Formation of thionine layer. (E) Assembly of gold nanoparticles. (F) Anti-AFP loading. (G) BSA blocking (reprinted from Ran et al. [127] with permission).
In another study, a lactate biosensor was created by combining a conductive polymer (poly-5,2′-5′,2″-ter-thiophene-3′-carboxylic acid; pTTCA) and MWNTs Rahman et al. [126]. After depositing pTTCA/MWNT films on a gold electrode, lactate dehydrogenase and the oxidized form of nicotinamide adenine dinucleotide (NAD+) were immobilized on the film. SEM was used to characterize the functionalized surfaces. Another recent study from the same laboratory demonstrated uric acid detection by this functionalized electrode [94].
A unique new method for sensor applications has been proposed [80, 124]. The authors deposited DNA-CNT conjugates between two Au electrodes. Since DNA bases have specific affinity with ions, such as Hg(II), Cd(II), and Pb(II), the nanodevice could be utilized as an ionic sensor. SEM was a reasonable method to characterize structures of their nanodevices.
The mechanisms of the electrical and optical responses of CNTs are not fully understood. Experimental data from various biomolecules are important to establish CNT biosensor applications. In this context, sample verification by scanning techniques is obviously important and systematic accumulation of data under similar experimental conditions is expected. The current reality is that studies are conducted using various CNT powders. Furthermore, CNTs with the same product number purchased from the same company can display differences in composition between lots. Hybridization of CNTs and biomolecules can also vary among researchers. Thus, the direct comparison of data obtained by different research groups is difficult.
5. Nanobioconjugates for Cell Researches
The use of CNT nanobioconjugates to study living cells is growing in popularity ([134–139] [28, 85, 140–167]). Scanning techniques are important and powerful tools in verifying samples.
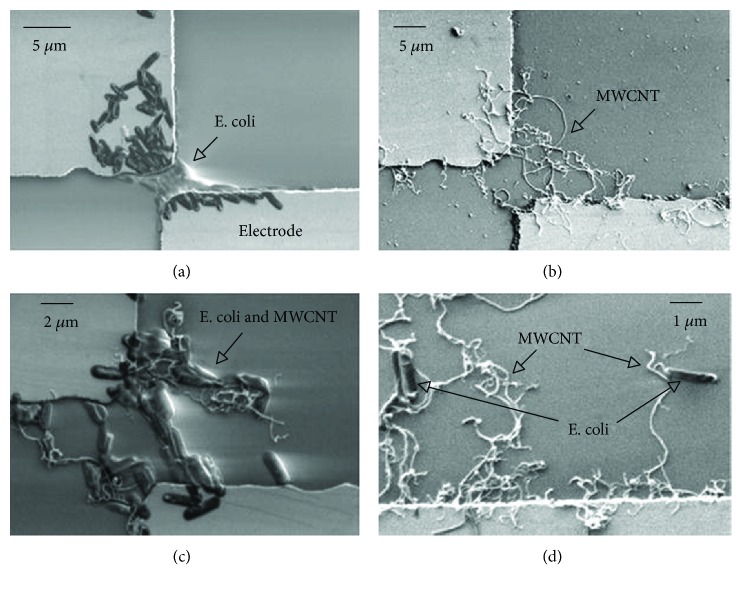
One of the important applications is CNT-mediated drug delivery [155, 161, 168–189]. The fabrication of bio/nanointerfaces of Escherichia coli and MWNTs has been described Suehiro et al. [157]. In the unique approach, a mixture of E. coli and solubilized MWNTs was deposited between two microelectrodes. E. coli was trapped by the MWNTs by dielectrophoresis force. SEM images clearly visualized the bacteria trapped between the electrodes (Figure 6).
Figure 6.
SEM images of trapped E. coli cells and MWNTs between two microelectrodes by DEP force. (a) Trapped cells. (b) Trapped MWNTs. (c) Trapped cells and MWNTs. (d) Cells trapped at the tip of MWNTs (reprinted from Suehiro et al. [157] with permission).
For use in drug delivery, it is crucial to demonstrate that CNTs are not toxic. Many studies that did not utilize scanning techniques have intensively studied the toxicity of CNTs ([135, 169, 190–215]. The use of scanning techniques has proven advantageous for toxicity studies [85, 214, 216–219]. As one example, the effects of MWNTs on human lung epithelial cells evaluated using SEM and other assessments revealed MWNT-mediated cytotoxicity and genotoxicity [135].
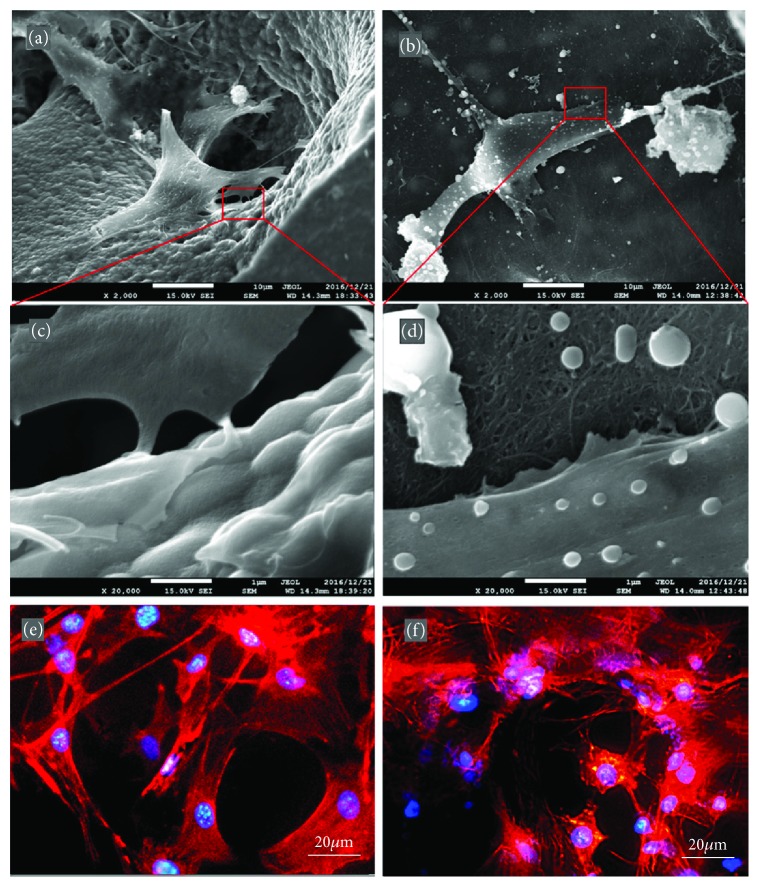
In another study, a three-dimensional (3D) scaffold for bone recognition was fabricated using MWNTs [159]. MWNT networks were prepared with polyacrylonitrile (PAN) followed by the addition and mixing of polymethyl-methacrylate (PMMA) microspheres to fabricate microporous structures of PMAA, PAN, and MWNTs. Figure 7 shows SEM and fluorescent microscope images of MC3T3-E1 cells that had spread on the fabricated structures. Figures 7(a), 7(c), and 7(e) are images of IP-CHA, an established commercial product used as the control. Figures 7(b), 7(d), and 7(f) depict the results from similar experiments with the aforementioned CNTp nanoporous scaffolds. SEM clearly revealed the adherence of cells to both scaffold surfaces. The authors described the advantages of CNTp scaffolds based on various characterization experiments.
Figure 7.
SEM and fluorescence microscope images of MC3T3-E1 cells on IP-CHA and CNTp scaffolds. (a, c, and e) On IP-CHA. (b, d, and f) On CNTp. (a, b, c, and d) IP-CHA and (b and d) CNTp. Magnification: 2000x for (a and c), 20,000x for (c and d). (e and f) Cells were labeled for actin filaments (red) and nucleus (blue) (reprinted from Tanaka et al. [159] with permission).
In cell studies, microscale elevation changes are expected. In particular, when cells are mixed with CNT nanobioconjugates, the heights of the hybridized objects can exceed 20 microns. SEM has proved useful to discern this topography. However, SEM observation of the behavior of living cells with CNTs is difficult since the examination is typically carried out in vacuum. Although environmental and atmospheric pressure SEMs are available, there are various constraints to their use. It is anticipated that SPM will soon be amenable for the time-lapse observation of living cells with CNT nanobioconjugates. Then, optical microscopes will truly be competitive observation tools.
6. CNT Nanobioconjugates as Sharp Probes
The small diameters of CNTs could be well suited to their use as sharp probes. The use of CNTs as SPM probes is one of the important scanning applications [136, 220–230].
Improvement of AFM resolution using a CNT tip is the most typical approach. In many applications, a single CNT is attached to the top of the usual AFM tip. In one study, an MWNT tip was used for AFM as well as scanning tunneling microscopy (STM) [220]. Hafner et al. demonstrated the direct growth of MWNT on an SPM tip [221]. Stevens et al. applied an MWNT AFM tip to observe 2 nm diameter iridium particles on mica surfaces in aqueous solutions [225].
Another important application is the use of CNTs as an “injector” for living cells. The attachment of cargo for drug delivery via disulfide bonding has been proposed [136]. The authors fabricated an AFM tip with an MWNT using SEM with a manipulator. Then, hybrids of quantum dot (QD) and streptavidin were attached on the MWNT surfaces using a crosslinker containing disulfide bonding. The functionalized AFM tip was injected into HeLa cells under AFM guidance, and QD was spontaneously released.
Various uses of CNTs as a sharp SPM probe can be envisioned. Single-cell surgery using drug delivery CNT probes is of one use. If induced pluripotent stem cells could be managed using this approach, it would be a fundamentally important advancement in medicine. Such applications demand the establishment of means of mass production of SPM probes with a CNT tip. CNT tips are currently handmade. Thus, for now, the accumulation of huge experimental data is difficult.
7. Characterization of CNT Nanobioconjugates by Scanning Techniques
Electrical properties of CNTs are defined due to their chirality. STM is a powerful tool to investigate the electrical properties of individual CNTs [231–234]. In one study, hybrids of DNA and MWNTs were observed by STM and scanning tunneling spectroscopy (STS) profiles were affected by the attachment of DNA molecules to MWNTs [231]. STS of SWNTs wrapped with ssDNA can be affected by DNA sequences [234]. A theoretical model has been proposed to explain the experimental STM and STS results [233].
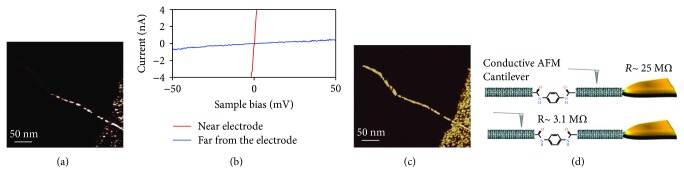
Another scanning technique to investigate electrical properties of CNT nanobioconjugates is conductive AFM [235–237]. In one study, molecular transport junctions (MTJs) were fabricated. Two metallic SWNTs wrapped with DNA molecules were connected with p-phenylenediamine (PPD), and the resistance of the MTJs was measured by conductive AFM (Figure 8) [237].
Figure 8.
Representative conductive AFM image of a MTJ formed using PPD as the molecular linker and interfaced to a macroscopic metal electrode. (b) Representative I–V curves recorded at selected points across the MTJ: red line for measurements in close proximity to the macroscopic electrode and blue line for measurements at the far end from the macroscopic electrode. (c) Phase AFM image of the MTJ shown in (a). (d) Schematic representation of the conductive AFM measurements on the MTJs (reprinted from Zhu et al. [237] with permission).
The electrical properties of CNTs can be studied using two approaches. The precise assessment of the electrical properties of CNTs can be done in vacuum. Conversely, for biological applications, experiments should be carried out in liquids or in air/gas. In these cases, water and other molecules strongly affect the data. Several researchers focused on the effects of water molecules on physicochemical properties of CNTs [238–240]. If the samples include ions and other chemicals, which are commonly used for biological experiments, the effects can be markedly more complex.
8. Structures and Mechanical Properties Studied by AFM
AFM is the most convenient SPM for the standard characterization of CNT nanobioconjugates. Many researchers observed 3D structures of CNTs by AFM, and diverse lengths and widths of various types of CNT nanobioconjugates have been reported [69, 241–252]. Biochemical reactions, such as the interaction between protein and DNA molecules on CNT surfaces, were studied by AFM and other scanning techniques [232, 245, 253–264].
An impressive structural study using AFM described various ssDNA molecules on CNT surfaces [264]. Using the phase imaging mode, the authors found that the helical pitch of d(GT)30 was approximately 18 nm.
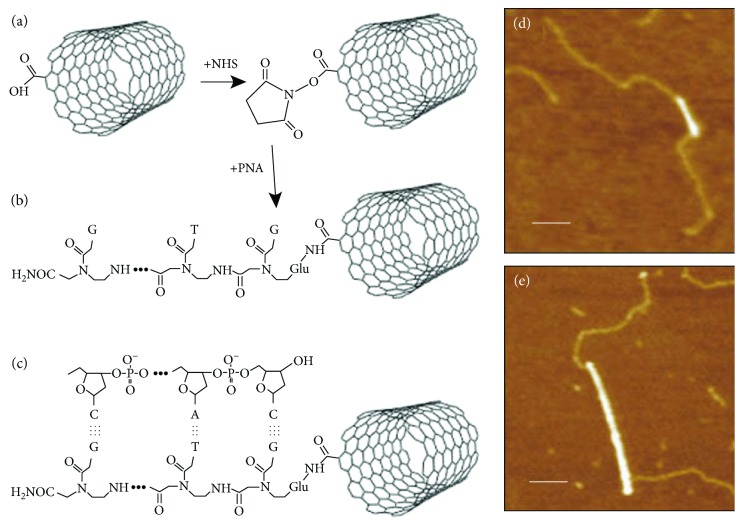
In another study, a peptide nucleic acid (PNA, NH2-Glu-GTGCTCATGGTG-CONH2) was fabricated and attached to the PNA at the ends of individual SWNTs. When ssDNA molecules having complementary sequences with DNA portions of PNA were reacted, the complementary DNA molecules avidly recognized the DNA regions. The hybridization was clearly confirmed by AFM observation (Figure 9) [263].
Figure 9.
Hybridization of PNA attached SWNTs and DNA. (a and b) PNA was attached to an end of SWNT with N-hydroxysuccinimide (NHS) esters. (c) Hybridization of DNA with PNA attached SWNT. (d and e) AFM images of PNA-SWNTs. Bright lines indicate SWNTs. The paler strands represent bound DNA. Scale bars are 100 nm. Diameters of SWNTs were 0.9 nm and 1.6 nm in (d) and (e), respectively (reprinted from Williams et al. [263] with permission).
Force spectroscopy using AFM is a unique method to directly measure interactions between organic molecules and CNTs [232, 265, 266]. In one study, a DNA molecule was inserted into the the smooth inner pores of CNTs and then retracted to measure the frictional force between the DNA and the CNT [265]. DNA extraction from CNT pores occurred at a nearly constant force. In another study, a DNA molecule was peeled from an SWNT surface [232]. The peeling force of ssDNA from SWNTs was much greater than that from flat graphite. A recent study provided dynamic observations of CNT nanobioconjugates using high-speed AFM [267].
Although there are numerous SPM studies that have provided routine AFM pictures of CNT nanobioconjugates, the use of specific functions of SPMs, such as force measurements, has been very limited. Future studies will hopefully utilize the full SPM functional repertoire to study CNT nanobioconjugates. For example, various types of forces, including friction, electrostatic, acoustic, and magnetic forces, can be measured by specific AFM options. Typical SPM options include scanning near-field optical microscopy, scanning thermal microscopy, scanning electrochemical microscopy, scanning Kelvin probe force microscopy, scanning chemical potential microscopy, scanning ion conductance microscopy, and scanning capacitance microscopy.
9. Manipulation of CNT Nanobioconjugates by Scanning Techniques
Manipulation of CNTs by SEM is a popular tool. Attachment of CNTs on SPM probes is usually carried out this way [136, 220–222, 224–230]. In one study, single CNT was attached between two AFM probes under SEM observation [230]. Then, the two AFM tips were separated to directly measure the breaking force of the CNT. The authors estimated that the breaking force was 1.3 μN.
AFM is advantageous to manipulate CNT nanobioconjugates in air or liquids. “Dragging” CNTs by an AFM tip was described. The CNTs were distorted and digested by manipulation with an AFM tip [268]. Other authors proposed DNA carriers using CNTs based on molecular dynamics calculation [269]. In their proposal, DNA molecules that are inserted into the inner pores of CNTs can be moved by CNT manipulation using an AFM tip.
The potential of single-cell surgery was mentioned earlier. Similarly, single-molecule surgery of nanobioconjugates with CNTs using the manipulation technique is also a challenging research target. Single-molecule surgery of CNT nanobioconjugates might be realized with a single SPM probe of CNT nanobioconjugates.
10. Approaches Using Mapping Methods Related Scanning Technique
One of the unique optical properties of CNTs is photoluminescence (PL) from CNTs. For example, Ito et al. combined PL measurements and SEM observation [270]. When (9, 4) SWNTs are excited with a laser wavelength of 730 nm, the SWNTs photoluminesce at 1130 nm. Excitation and emission wavelengths vary among CNTs with different chiralities. Furthermore, the emission wavelength and PL intensity fluctuate in a sensitive response to oxidation/reduction and other factors. By using PL measurements, various applications of CNT nanobioconjugates, such as nanobiosensors, are available. For PL measurements, a “PL map” can be obtained. Usually, the x- and y-axis of a PL map is the emission and excitation wavelength, respectively. Although SEM and SPM spatially scan the sample surface, PL reveals wavelength scanning. PL measurements by a variety of scanning techniques would provide rich information about CNT nanobioconjugates.
In a recent study, AFM infrared spectroscopy was used to study the morphological and optical properties of CNTs [271]. This new scanning technique provides spatial information of CNT nanobioconjugates.
Mapping techniques, such as PL mapping, and scanning techniques, such as SPM and SEM, have been independently used in various research fields. However, for the study of CNT nanobioconjugates, we believe that the combined use of the two techniques will be a boon to discovery. In particular, to understand the mechanisms of several unique responses of CNTs, the combined structural and physicochemical information would be valuable.
11. Conclusion
In this paper, the contributions of scanning techniques to studies of CNT nanobioconjugates have been summarized and future prospects discussed. Research subjects are categorized based on CNT applications not on the types of scanning methods. In addition, the possibility of a combination of mapping techniques and scanning techniques is also described. We hope that this review article informs future studies in this field.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Ajayan P. M. Nanotubes from carbon. Chemical Reviews. 1999;99(7):1787–1800. doi: 10.1021/cr970102g. [DOI] [PubMed] [Google Scholar]
- 2.Baughman R. H., Zakhidov A. A., de Heer W. A. Carbon nanotubes - the route toward applications. Science. 2002;297(5582):787–792. doi: 10.1126/science.1060928. [DOI] [PubMed] [Google Scholar]
- 3.Dai H. J. Carbon nanotubes: synthesis, integration, and properties. Accounts of Chemical Research. 2002;35(12):1035–1044. doi: 10.1021/ar0101640. [DOI] [PubMed] [Google Scholar]
- 4.Ebbesen T. W. Carbon nanotubes. Annual Review of Materials Science. 1994;24(1):235–264. doi: 10.1146/annurev.ms.24.080194.001315. [DOI] [Google Scholar]
- 5.Rao C. N. R., Satishkumar B. C., Govindaraj A., Nath M. Nanotubes. Chemphyschem. 2001;2(2):78–105. doi: 10.1002/1439-7641(20010216)2:2<78::AID-CPHC78>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Tasis D., Tagmatarchis N., Bianco A., Prato M. Chemistry of carbon nanotubes. Chemical Reviews. 2006;106(3):1105–1136. doi: 10.1021/cr050569o. [DOI] [PubMed] [Google Scholar]
- 7.Xia Y., Yang P., Sun Y., et al. One-dimensional nanostructures: synthesis, characterization, and applications. Advanced Materials. 2003;15(5):353–389. doi: 10.1002/adma.200390087. [DOI] [Google Scholar]
- 8.Anantram M. P., Leonard F. Physics of carbon nanotube electronic devices. Reports on Progress in Physics. 2006;69(3):507–561. doi: 10.1088/0034-4885/69/3/R01. [DOI] [Google Scholar]
- 9.Jiang J., Saito R., Samsonidze G. G., et al. Chirality dependence of exciton effects in single-wall carbon nanotubes: tight-binding model. Physical Review B. 2007;75(3, article 035407) doi: 10.1103/physrevb.75.035407. [DOI] [Google Scholar]
- 10.Ju S. Y., Doll J., Sharma I., Papadimitrakopoulos F. Selection of carbon nanotubes with specific chiralities using helical assemblies of flavin mononucleotide. Nature Nanotechnology. 2008;3(6):356–362. doi: 10.1038/nnano.2008.148. [DOI] [PubMed] [Google Scholar]
- 11.Telg H., Maultzsch J., Reich S., Hennrich F., Thomsen C. Chirality distribution and transition energies of carbon nanotubes. Physical Review Letters. 2004;93(17, article 177401) doi: 10.1103/physrevlett.93.177401. [DOI] [PubMed] [Google Scholar]
- 12.Yang F., Wang X., Zhang D. Q., et al. Chirality-specific growth of single-walled carbon nanotubes on solid alloy catalysts. Nature. 2014;510(7506):522–524. doi: 10.1038/nature13434. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J., Albelda M. T., Liu Y., Canary J. W. Chiral nanotechnology. Chirality. 2005;17(7):404–420. doi: 10.1002/chir.20178. [DOI] [PubMed] [Google Scholar]
- 14.Battigelli A., Menard-Moyon C., Da Ros T., Prato M., Bianco A. Endowing carbon nanotubes with biological and biomedical properties by chemical modifications. Advanced Drug Delivery Reviews. 2013;65(15):1899–1920. doi: 10.1016/j.addr.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Bekyarova E., Ni Y., Malarkey E. B., et al. Applications of carbon nanotubes in biotechnology and biomedicine. Journal of Biomedical Nanotechnology. 2005;1(1):3–17. doi: 10.1166/jbn.2005.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhattacharya K., Mukherjee S. P., Gallud A., et al. Biological interactions of carbon-based nanomaterials: from coronation to degradation. Nanomedicine-Nanotechnology Biology and Medicine. 2016;12(2):333–351. doi: 10.1016/j.nano.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du D., Wang J., Smith J. N., Timchalk C., Lin Y. H. Biomonitoring of organophosphorus agent exposure by reactivation of cholinesterase enzyme based on carbon nanotube-enhanced flow-injection amperometric detection. Analytical Chemistry. 2009;81(22):9314–9320. doi: 10.1021/ac901673a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frolov A. I., Rozhin A. G., Fedorov M. V. Ion interactions with the carbon nanotube surface in aqueous solutions: understanding the molecular mechanisms. Chemphyschem. 2010;11(12):2612–2616. doi: 10.1002/cphc.201000231. [DOI] [PubMed] [Google Scholar]
- 19.Gu F., Li C. Z., Wang S. F. Solution-chemical synthesis of carbon nanotube/ZnS nanoparticle core/shell heterostructures. Inorganic Chemistry. 2007;46(13):5343–5348. doi: 10.1021/ic7004858. [DOI] [PubMed] [Google Scholar]
- 20.Harrison B. S., Atala A. Carbon nanotube applications for tissue engineering. Biomaterials. 2007;28(2):344–353. doi: 10.1016/j.biomaterials.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 21.Herrero-Latorre C., Alvarez-Mendez J., Barciela-Garcia J., Garcia-Martin S., Pena-Crecente R. M. Characterization of carbon nanotubes and analytical methods for their determination in environmental and biological samples: a review. Analytica Chimica Acta. 2015;853:77–94. doi: 10.1016/j.aca.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Hui Z., Zhang X., Yu J., et al. Carbon nanotube-hybridized supramolecular hydrogel based on PEO-b-PPO-b-PEO/α-cyclodextrin as a potential biomaterial. Journal of Applied Polymer Science. 2010;116(4):1894–1901. doi: 10.1002/app.31729. [DOI] [Google Scholar]
- 23.Ishibashi A., Nakashima N. Individual dissolution of single-walled carbon nanotubes in aqueous solutions of steroid or sugar compounds and their Raman and near-IR spectral properties. Chemistry-a European Journal. 2006;12(29):7595–7602. doi: 10.1002/chem.200600326. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S., Rani R., Dilbaghi N., Tankeshwar K., Kim K. H. Carbon nanotubes: a novel material for multifaceted applications in human healthcare. Chemical Society Reviews. 2017;46(1):158–196. doi: 10.1039/C6CS00517A. [DOI] [PubMed] [Google Scholar]
- 25.Liu J. X., Ding S. N. Non-enzymatic amperometric determination of cellular hydrogen peroxide using dendrimer-encapsulated Pt nanoclusters/carbon nanotubes hybrid composites modified glassy carbon electrode. Sensors and Actuators B-Chemical. 2017;251:200–207. doi: 10.1016/j.snb.2017.05.043. [DOI] [Google Scholar]
- 26.Liu Z., Tabakman S. M., Chen Z., Dai H. J. Preparation of carbon nanotube bioconjugates for biomedical applications. Nature Protocols. 2009;4(9):1372–1381. doi: 10.1038/nprot.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu F. S., Gu L. R., Meziani M. J., et al. Advances in bioapplications of carbon nanotubes. Advanced Materials. 2009;21(2):139–152. doi: 10.1002/adma.200801491. [DOI] [Google Scholar]
- 28.Lv P. F., Feng Q., Wang Q. Q., Li G. H., Li D. W., Wei Q. F. Biosynthesis of bacterial cellulose/carboxylic multi-walled carbon nanotubes for enzymatic biofuel cell application. Materials. 2016;9(3):p. 183. doi: 10.3390/ma9030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paul A., Bhattacharya B. DNA functionalized carbon nanotubes for nonbiological applications. Materials and Manufacturing Processes. 2010;25(9):891–908. doi: 10.1080/10426911003720755. [DOI] [Google Scholar]
- 30.Pilla S., Kramschuster A., Gong S., Chandra A., Turng L. S. Solid and microcellular polylactide-carbon nanotube nanocomposites. International Polymer Processing. 2007;22(5):418–428. doi: 10.3139/217.2071. [DOI] [Google Scholar]
- 31.Sajid M. I., Jamshaid U., Jamshaid T., Zafar N., Fessi H., Elaissari A. Carbon nanotubes from synthesis to in vivo biomedical applications. International Journal of Pharmaceutics. 2016;501(1-2):278–299. doi: 10.1016/j.ijpharm.2016.01.064. [DOI] [PubMed] [Google Scholar]
- 32.Singhal R., Orynbayeva Z., Kalyana Sundaram R. V., et al. Multifunctional carbon-nanotube cellular endoscopes. Nature Nanotechnology. 2011;6(1):57–64. doi: 10.1038/nnano.2010.241. [DOI] [PubMed] [Google Scholar]
- 33.Umemura K. Hybrids of nucleic acids and carbon nanotubes for nanobiotechnology. Nanomaterials. 2015;5(1):321–350. doi: 10.3390/nano5010321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vardharajula S., Ali S. Z., Tiwari P. M., et al. Functionalized carbon nanotubes: biomedical applications. International Journal of Nanomedicine. 2012;7:5361–5374. doi: 10.2147/IJN.S35832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zamora-Ledezma C., Buisson L., Moulton S. E., et al. Carbon nanotubes induced gelation of unmodified hyaluronic acid. Langmuir. 2013;29(32):10247–10253. doi: 10.1021/la4016492. [DOI] [PubMed] [Google Scholar]
- 36.Dodziuk H., Ejchart A., Anczewski W., et al. Water solubilization, determination of the number of different types of single-wall carbon nanotubes and their partial separation with respect to diameters by complexation with eta-cyclodextrin. Chemical Communications. 2003;(8):986–987. doi: 10.1039/b211365a. [DOI] [PubMed] [Google Scholar]
- 37.Fu K., Huang W., Lin Y., et al. Functionalization of carbon nanotubes with bovine serum albumin in homogeneous aqueous solution. Journal of Nanoscience and Nanotechnology. 2002;2(5):457–461. doi: 10.1166/jnn.2002.135. [DOI] [PubMed] [Google Scholar]
- 38.Haggenmueller R., Rahatekar S. S., Fagan J. A., et al. Comparison of the quality of aqueous dispersions of single wall carbon nanotubes using surfactants and biomolecules. Langmuir. 2008;24(9):5070–5078. doi: 10.1021/la703008r. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa T., Fujisawa T., Numata M., et al. Single-walled carbon nanotubes acquire a specific lectin-affinity through supramolecular wrapping with lactose-appended schizophyllan. Chemical Communications. 2004;(19):2150–2151. doi: 10.1039/b407409b. [DOI] [PubMed] [Google Scholar]
- 40.Hobbie E. K., Fagan J. A., Becker M. L., Hudson S. D., Fakhri N., Pasquali M. Self-assembly of ordered nanowires in biological suspensions of single-wall carbon nanotubes. ACS Nano. 2009;3(1):189–196. doi: 10.1021/nn800609y. [DOI] [PubMed] [Google Scholar]
- 41.Jia Z., Zhao H., Bai Y., et al. Solvent processed conductive polymer with single-walled carbon nanotube composites. Journal of Materials Research. 2015;30(22):3403–3411. doi: 10.1557/jmr.2015.328. [DOI] [Google Scholar]
- 42.Jiang L. Q., Gao L., Sun J. Production of aqueous colloidal dispersions of carbon nanotubes. Journal of Colloid and Interface Science. 2003;260(1):89–94. doi: 10.1016/S0021-9797(02)00176-5. [DOI] [PubMed] [Google Scholar]
- 43.Liu P. Modifications of carbon nanotubes with polymers. European Polymer Journal. 2005;41(11):2693–2703. doi: 10.1016/j.eurpolymj.2005.05.017. [DOI] [Google Scholar]
- 44.Liu S. B., Wei L., Hao L., et al. Sharper and faster “nano darts” kill more bacteria: a study of antibacterial activity of individually dispersed pristine single-walled carbon nanotube. ACS Nano. 2009;3(12):3891–3902. doi: 10.1021/nn901252r. [DOI] [PubMed] [Google Scholar]
- 45.Najeeb C. K., Lee J. H., Kim J. H., Kim D. Highly efficient individual dispersion of single-walled carbon nanotubes using biocompatible dispersant. Colloids and Surfaces B-Biointerfaces. 2013;102:95–101. doi: 10.1016/j.colsurfb.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 46.O'Connell M. J., Boul P., Ericson L. M., et al. Reversible water-solubilization of single-walled carbon nanotubes by polymer wrapping. Chemical Physics Letters. 2001;342(3-4):265–271. doi: 10.1016/S0009-2614(01)00490-0. [DOI] [Google Scholar]
- 47.Seong M.-J., Park J. Dispersion efficiency of carbon nanotubes in deoxycholate sodium salts aqueous solutions. Journal of the Korean Physical Society. 2010;56:1391–1394. doi: 10.3938/jkps.56.1391. [DOI] [Google Scholar]
- 48.Sinani V. A., Gheith M. K., Yaroslavov A. A., et al. Aqueous dispersions of single-wall and multiwall carbon nanotubes with designed amphiphilic polycations. Journal of the American Chemical Society. 2005;127(10):3463–3472. doi: 10.1021/ja045670+. [DOI] [PubMed] [Google Scholar]
- 49.Star A., Stoddart J. F. Dispersion and solubilization of single-walled carbon nanotubes with a hyperbranched polymer. Macromolecules. 2002;35(19):7516–7520. doi: 10.1021/ma0204150. [DOI] [Google Scholar]
- 50.Wang J., Musameh M., Lin Y. H. Solubilization of carbon nanotubes by Nafion toward the preparation of amperometric biosensors. Journal of the American Chemical Society. 2003;125(9):2408–2409. doi: 10.1021/ja028951v. [DOI] [PubMed] [Google Scholar]
- 51.Wise A. J., Smith J. R., Bouropoulos N., Yannopoulos S. N., van der Merwe S. M., Fatouros D. G. Single-wall carbon nanotube dispersions stabilised with N-trimethyl-chitosan. Journal of Biomedical Nanotechnology. 2008;4(4):377–399. [Google Scholar]
- 52.Zorbas V., Smith A. L., Xie H., et al. Importance of aromatic content for peptide/single-walled carbon nanotube interactions. Journal of the American Chemical Society. 2005;127(35):12323–12328. doi: 10.1021/ja050747v. [DOI] [PubMed] [Google Scholar]
- 53.Albertorio F., Hughes M. E., Golovchenko J. A., Branton D. Base dependent DNA-carbon nanotube interactions: activation enthalpies and assembly-disassembly control. Nanotechnology. 2009;20(39):p. 395101. doi: 10.1088/0957-4484/20/39/395101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ao G. Y., Khripin C. Y., Zheng M. DNA-controlled partition of carbon nanotubes in polymer aqueous two-phase systems. Journal of the American Chemical Society. 2014;136(29):10383–10392. doi: 10.1021/ja504078b. [DOI] [PubMed] [Google Scholar]
- 55.Bae A. H., Hatano T., Nakashima N., Murakami H., Shinkai S. Electrochemical fabrication of single-walled carbon nanotubes DNA complexes by poly(ethylenedioxythiophene) and photocurrent generation by excitation of an intercalated chromophore. Organic & Biomolecular Chemistry. 2004;2(8):1139–1144. doi: 10.1039/B402044H. [DOI] [PubMed] [Google Scholar]
- 56.Cha T. G., Pan J., Chen H. R., et al. A synthetic DNA motor that transports nanoparticles along carbon nanotubes. Nature Nanotechnology. 2014;9(1):39–43. doi: 10.1038/nnano.2013.257. [DOI] [PubMed] [Google Scholar]
- 57.Dohi H., Kikuchi S., Kuwahara S., Sugai T., Shinohara H. Synthesis and spectroscopic characterization of single-wall carbon nanotubes wrapped by glycoconjugate polymer with bioactive sugars. Chemical Physics Letters. 2006;428(1–3):98–101. doi: 10.1016/j.cplett.2006.06.053. [DOI] [Google Scholar]
- 58.Gomez-Navarro C., De Pablo P. J., Gomez-Herrero J. Electrical properties of long molecules: single-walled carbon nanotubes and DNA. International Journal of Nanotechnology. 2005;2(1/2):p. 90. doi: 10.1504/IJNT.2005.006976. [DOI] [Google Scholar]
- 59.Gong M. J., Han T., Cai C. X., Lu T. H., Du J. Y. Fabrication and characterization of DNA-thionine-carbon nanotube nanocomposites. Journal of Electroanalytical Chemistry. 2008;623(1):8–14. doi: 10.1016/j.jelechem.2008.03.020. [DOI] [Google Scholar]
- 60.Li Z. Z., Wu Z. Y., Li K. The high dispersion of DNA-multiwalled carbon nanotubes and their properties. Analytical Biochemistry. 2009;387(2):267–270. doi: 10.1016/j.ab.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 61.McDonald T. J., Svedruzic D., Kim Y.-H., et al. Wiring-up hydrogenase with single-walled carbon nanotubes. Nano Letters. 2007;7(11):3528–3534. doi: 10.1021/nl072319o. [DOI] [PubMed] [Google Scholar]
- 62.Nakashima N., Okuzono S., Murakami H., Nakai T., Yoshikawa K. DNA dissolves single-walled carbon nanotubes in water. Chemistry Letters. 2003;32(5):456–457. doi: 10.1246/cl.2003.456. [DOI] [Google Scholar]
- 63.Ostojic G. N., Ireland J. R., Hersam M. C. Noncovalent functionalization of DNA-wrapped single-walled carbon nanotubes with platinum-based DNA cross-linkers. Langmuir. 2008;24(17):9784–9789. doi: 10.1021/la801311j. [DOI] [PubMed] [Google Scholar]
- 64.Palma M., Wang W., Penzo E., et al. Controlled formation of carbon nanotube junctions via linker-induced assembly in aqueous solution. Journal of the American Chemical Society. 2013;135(23):8440–8443. doi: 10.1021/ja4018072. [DOI] [PubMed] [Google Scholar]
- 65.Park J., Kim S., Seong M. J., Kim Y. J., Go H., Lee K. Functionalization of single-walled carbon nanotubes with ribonucleic acids. Journal of the Korean Physical Society. 2013;63(11):2199–2203. doi: 10.3938/jkps.63.2199. [DOI] [Google Scholar]
- 66.Tu X. M., Manohar S., Jagota A., Zheng M. DNA sequence motifs for structure-specific recognition and separation of carbon nanotubes. Nature. 2009;460(7252):250–253. doi: 10.1038/nature08116. [DOI] [PubMed] [Google Scholar]
- 67.Zheng M., Jagota A., Semke E. D., et al. DNA-assisted dispersion and separation of carbon nanotubes. Nature Materials. 2003;2(5):338–342. doi: 10.1038/nmat877. [DOI] [PubMed] [Google Scholar]
- 68.Umemura K., Izumi K., Oura S. Probe microscopic studies of DNA molecules on carbon nanotubes. Nanomaterials. 2016;6(10):p. 180. doi: 10.3390/nano6100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hayashida T., Umemura K. Atomic force microscopy of DNA-wrapped single-walled carbon nanotubes in aqueous solution. Colloids and Surfaces B: Biointerfaces. 2016;143:526–531. doi: 10.1016/j.colsurfb.2016.03.068. [DOI] [PubMed] [Google Scholar]
- 70.Nepal D., Sohn J. I., Aicher W. K., Lee S., Geckeler K. E. Supramolecular conjugates of carbon nanotubes and DNA by a solid-state reaction. Biomacromolecules. 2005;6(6):2919–2922. doi: 10.1021/bm050380m. [DOI] [PubMed] [Google Scholar]
- 71.Subbiah R. P., Lee H., Veerapandian M., Sadhasivam S., Seo S. W., Yun K. Structural and biological evaluation of a multifunctional SWCNT-AgNPs-DNA/PVA bio-nanofilm. Analytical and Bioanalytical Chemistry. 2011;400(2):547–560. doi: 10.1007/s00216-011-4757-1. [DOI] [PubMed] [Google Scholar]
- 72.Baccarin M., Cervini P., Cavalheiro E. T. G. Comparative performances of a bare graphite-polyurethane composite electrode unmodified and modified with graphene and carbon nanotubes in the electrochemical determination of escitalopram. Talanta. 2018;178:1024–1032. doi: 10.1016/j.talanta.2017.08.094. [DOI] [PubMed] [Google Scholar]
- 73.Ferancova A., Ovadekova R., Vanickova M., et al. DNA-modified screen-printed electrodes with nanostructured films of multiwall carbon nanotubes, hydroxyapatite and montmorillonite. Electroanalysis. 2006;18(2):163–168. doi: 10.1002/elan.200503383. [DOI] [Google Scholar]
- 74.Gu T. T., Wang J. L., Xia H. Q., Wang S., Yu X. T. Direct electrochemistry and electrocatalysis of horseradish peroxidase immobilized in a DNA/chitosan-Fe3O4 magnetic nanoparticle bio-complex film. Materials. 2014;7(2):1069–1083. doi: 10.3390/ma7021069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jung D. H., Kim B. H., Ko Y. K., et al. Covalent attachment and hybridization of DNA oligonucleotides on patterned single-walled carbon nanotube films. Langmuir. 2004;20(20):8886–8891. doi: 10.1021/la0485778. [DOI] [PubMed] [Google Scholar]
- 76.Khan A. A., Mirza E. H., Syed J., et al. Single and multi-walled carbon nanotube fillers in poly(methyl methacrylate)-based implant material. Journal of Biomaterials and Tissue Engineering. 2017;7(9):798–806. doi: 10.1166/jbt.2017.1638. [DOI] [Google Scholar]
- 77.Khan I., Pandit U. J., Wankar S., Limaye S. N. Design of electrochemical sensor based on fMWCNT-CPE decorated with Ti nanofilm and its electrocatalytic behavior towards aminotriazole. Electrocatalysis. 2017;8(3):196–213. doi: 10.1007/s12678-017-0358-x. [DOI] [Google Scholar]
- 78.Li H., Wang D. Q., Chen H. L., Liu B. L., Gao L. Z. A novel gelatin-carbon nanotubes hybrid hydrogel. Macromolecular Bioscience. 2003;3(12):720–724. doi: 10.1002/mabi.200300034. [DOI] [Google Scholar]
- 79.Muti M., Kuralay F., Erdem A. Single-walled carbon nanotubes-polymer modified graphite electrodes for DNA hybridization. Colloids and Surfaces B-Biointerfaces. 2012;91:77–83. doi: 10.1016/j.colsurfb.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 80.Paul A., Bhattacharya B., Bhattacharyya T. K. Fabrication and performance of solution-based micropatterned DNA functionalized carbon nanotube network as humidity sensors. IEEE Transactions on Nanotechnology. 2014;13(2):335–342. doi: 10.1109/TNANO.2014.2302843. [DOI] [Google Scholar]
- 81.Wang Z. M., Chen Y. M. Supramolecular hydrogels hybridized with single-walled carbon nanotubes. Macromolecules. 2007;40(9):3402–3407. doi: 10.1021/ma0702593. [DOI] [Google Scholar]
- 82.Zhang Q., Zhang L., Li J. H. DNA-hemoglobin-multiwalls carbon nanotube hybrid material with sandwich structure: preparation, characterization, and application in bioelectrochemistry. Journal of Physical Chemistry C. 2007;111(24):8655–8660. doi: 10.1021/jp071551f. [DOI] [Google Scholar]
- 83.Choi J. H., Nguyen F. T., Barone P. W., et al. Multimodal biomedical imaging with asymmetric single-walled carbon nanotube/iron oxide nanoparticle complexes. Nano Letters. 2007;7(4):861–867. doi: 10.1021/nl062306v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dubey P., Muthukumaran D., Dash S., Mukhopadhyay R., Sarkar S. Synthesis and characterization of water-soluble carbon nanotubes from mustard soot. Pramana-Journal of Physics. 2005;65(4):681–697. doi: 10.1007/BF03010456. [DOI] [Google Scholar]
- 85.Hao Y. Z., Xu P., He C., et al. Impact of carbondiimide crosslinker used for magnetic carbon nanotube mediated GFP plasmid delivery. Nanotechnology. 2011;22(28, article 285103) doi: 10.1088/0957-4484/22/28/285103. [DOI] [PubMed] [Google Scholar]
- 86.Lamprecht C., Liashkovich I., Neves V., et al. AFM imaging of functionalized carbon nanotubes on biological membranes. Nanotechnology. 2009;20(43, article 434001) doi: 10.1088/0957-4484/20/43/434001. [DOI] [PubMed] [Google Scholar]
- 87.Su J., Wang H. Y., Wu K. K., et al. Neutravidin-mediated extraction of isolated small diameter single walled carbon nanotubes for bio-recognition. Journal of Nanoscience and Nanotechnology. 2017;17(5):3588–3596. doi: 10.1166/jnn.2017.12860. [DOI] [Google Scholar]
- 88.Tasis D., Tagmatarchis N., Georgakilas V., Prato M. Soluble carbon nanotubes. Chemistry-a European Journal. 2003;9(17):4000–4008. doi: 10.1002/chem.200304800. [DOI] [PubMed] [Google Scholar]
- 89.Tibbetts G., Lake M., Strong K., Rice B. A review of the fabrication and properties of vapor-grown carbon nanofiber/polymer composites. Composites Science and Technology. 2007;67(7-8):1709–1718. doi: 10.1016/j.compscitech.2006.06.015. [DOI] [Google Scholar]
- 90.Wold D. J., Frisbie C. D. Fabrication and characterization of metal-molecule-metal junctions by conducting probe atomic force microscopy. Journal of the American Chemical Society. 2001;123(23):5549–5556. doi: 10.1021/ja0101532. [DOI] [PubMed] [Google Scholar]
- 91.Zhou R. J., Shi M. M., Chen X. Q., et al. Water-soluble and highly fluorescent hybrids of multi-walled carbon nanotubes with uniformly arranged gold nanoparticles. Nanotechnology. 2007;18(48):p. 7. doi: 10.1088/0957-4484/18/48/485603. [DOI] [Google Scholar]
- 92.Gholivand M. B., Solgi M. Sensitive warfarin sensor based on cobalt oxide nanoparticles electrodeposited at multi-walled carbon nanotubes modified glassy carbon electrode (Co(x)O(y)NPs/MWCNTs/GCE) Electrochimica Acta. 2017;246:689–698. doi: 10.1016/j.electacta.2017.06.105. [DOI] [Google Scholar]
- 93.Lobo A. O., Zanin H., Siqueira I. A. W. B., Leite N. C. S., Marciano F. R., Corat E. J. Effect of ultrasound irradiation on the production of nHAp/MWCNT nanocomposites. Materials Science & Engineering C-Materials for Biological Applications. 2013;33(7):4305–4312. doi: 10.1016/j.msec.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 94.Rahman M. M., Ahmed J., Asiri A. M. A glassy carbon electrode modified with γ-Ce2S3-decorated CNT nanocomposites for uric acid sensor development: a real sample analysis. RSC Advances. 2017;7(24):14649–14659. doi: 10.1039/C6RA27414E. [DOI] [Google Scholar]
- 95.Shahrokhian S., Salimian R., Rastgar S. Pd-Au nanoparticle decorated carbon nanotube as a sensing layer on the surface of glassy carbon electrode for electrochemical determination of ceftazidime. Materials Science & Engineering C-Materials for Biological Applications. 2014;34:318–325. doi: 10.1016/j.msec.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 96.Shahrokhian S., Salimian R., Kalhor H. R. A simple label-free electrochemical DNA biosensor based on carbon nanotube-DNA interaction. RSC Advances. 2016;6(19):15592–15598. doi: 10.1039/C5RA20907B. [DOI] [Google Scholar]
- 97.Wang J., Li S. P., Zhang Y. Z. A sensitive DNA biosensor fabricated from gold nanoparticles, carbon nanotubes, and zinc oxide nanowires on a glassy carbon electrode. Electrochimica Acta. 2010;55(15):4436–4440. doi: 10.1016/j.electacta.2010.02.078. [DOI] [Google Scholar]
- 98.Zhang Y. Z., Ma H. Y., Zhang K. Y., Zhang S. J., Wang J. An improved DNA biosensor built by layer-by-layer covalent attachment of multi-walled carbon nanotubes and gold nanoparticles. Electrochimica Acta. 2009;54(8):2385–2391. doi: 10.1016/j.electacta.2008.10.052. [DOI] [Google Scholar]
- 99.Zhang Y. Z., Wang J., Xu M. L. A sensitive DNA biosensor fabricated with gold nanoparticles/ploy (p-aminobenzoic acid)/carbon nanotubes modified electrode. Colloids and Surfaces B-Biointerfaces. 2010;75(1):179–185. doi: 10.1016/j.colsurfb.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 100.Cao C. F., Kim J. H., Yoon D., Hwang E. S., Kim Y. J., Baik S. Optical detection of DNA hybridization using absorption spectra of single-walled carbon nanotubes. Materials Chemistry and Physics. 2008;112(3):738–741. doi: 10.1016/j.matchemphys.2008.07.129. [DOI] [Google Scholar]
- 101.Huang C. Z., Liao Q. G., Li Y. F. Non-covalent anionic porphyrin functionalized multi-walled carbon nanotubes as an optical probe for specific DNA detection. Talanta. 2008;75(1):163–166. doi: 10.1016/j.talanta.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 102.Hwang E. S., Cao C. F., Hong S. H., et al. The DNA hybridization assay using single-walled carbon nanotubes as ultrasensitive, long-term optical labels. Nanotechnology. 2006;17(14):3442–3445. doi: 10.1088/0957-4484/17/14/016. [DOI] [PubMed] [Google Scholar]
- 103.Jeng E. S., Barone P. W., Nelson J. D., Strano M. S. Hybridization kinetics and thermodynamics of DNA adsorbed to individually dispersed single-walled carbon nanotubes. Small. 2007;3(9):1602–1609. doi: 10.1002/smll.200700141. [DOI] [PubMed] [Google Scholar]
- 104.Jeng E. S., Moll A. E., Roy A. C., Gastala J. B., Strano M. S. Detection of DNA hybridization using the near-infrared band-gap fluorescence of single-walled carbon nanotubes. Nano Letters. 2006;6(3):371–375. doi: 10.1021/nl051829k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martinez M. T., Tseng Y. C., Gonzalez M., Bokor J. Streptavidin as CNTs and DNA linker for the specific electronic and optical detection of DNA hybridization. Journal of Physical Chemistry C. 2012;116(42):22579–22586. doi: 10.1021/jp306535d. [DOI] [Google Scholar]
- 106.Shearer C. J., Yu L. P., Fenati R., et al. Adsorption and desorption of single-stranded DNA from single-walled carbon nanotubes. Chemistry-an Asian Journal. 2017;12(13):1625–1634. doi: 10.1002/asia.201700446. [DOI] [PubMed] [Google Scholar]
- 107.Zhang L., Huang C. Z., Li Y. F., Xiao S. J., Xie J. P. Label-free detection of sequence-specific DNA with multiwalled carbon nanotubes and their light scattering signals. Journal of Physical Chemistry B. 2008;112(23):7120–7122. doi: 10.1021/jp800092r. [DOI] [PubMed] [Google Scholar]
- 108.Binnig G., Rohrer H., Gerber C., Weibel E. Surface studies by scanning tunneling microscopy. Physical Review Letters. 1982;49(1):57–61. doi: 10.1103/PhysRevLett.49.57. [DOI] [Google Scholar]
- 109.Hansma P. K., Tersoff J. Scanning tunneling microscopy. Journal of Applied Physics. 1987;61(2):R1–R24. doi: 10.1063/1.338189. [DOI] [Google Scholar]
- 110.Cha T. G., Baker B. A., Sauffer M. D., et al. Optical Nanosensor architecture for cell-signaling molecules using DNA aptamer-coated carbon nanotubes. ACS Nano. 2011;5(5):4236–4244. doi: 10.1021/nn201323h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen X. M., Cai Z. M., Lin Z. J., et al. A novel non-enzymatic ECL sensor for glucose using palladium nanoparticles supported on functional carbon nanotubes. Biosensors & Bioelectronics. 2009;24(12):3475–3480. doi: 10.1016/j.bios.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 112.Dobrovolskaia M. A., McNeil S. E. Immunological properties of engineered nanomaterials. Nature Nanotechnology. 2007;2(8):469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 113.Jung D., Park H. W., Ma G. D., Lee C. Y., Kwon T., Han J. H. Optimal synthesis of horizontally aligned single-walled carbon nanotubes and their biofunctionalization for biosensing applications. Journal of Nanomaterials. 2016;2016:8. doi: 10.1155/2016/5140241.5140241 [DOI] [Google Scholar]
- 114.Karuwan C., Wisitsoraat A., Sappat A., Jaruwongrungsee K., Patthanasettakul V., Tuantranont A. Vertically aligned carbon nanotube based electrochemcial sensor for salbutamol detection. Sensor Letters. 2010;8(4):645–650. doi: 10.1166/sl.2010.1324. [DOI] [Google Scholar]
- 115.Khalaf A. L., Arasu P. T., Lim H. N., et al. Modified plastic optical fiber with CNT and graphene oxide nanostructured coatings for ethanol liquid sensing. Optics Express. 2017;25(5):5509–5520. doi: 10.1364/OE.25.005509. [DOI] [PubMed] [Google Scholar]
- 116.Khan I., Pandit U. J., Wankar S., Das R., Limaye S. N. Fabrication of electrochemical nanosensor based on polyaniline film-coated AgNP-MWCNT-modified GCE and its application for trace analysis of fenitrothion. Ionics. 2017;23(5):1293–1308. doi: 10.1007/s11581-016-1939-z. [DOI] [Google Scholar]
- 117.Kim S. N., Rusling J. F., Papadimitrakopoulos F. Carbon nanotubes for electronic and electrochemical detection of biomolecules. Advanced Materials. 2007;19(20):3214–3228. doi: 10.1002/adma.200700665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lei J. P., Ju H. X. Nanotubes in biosensing. Wiley Interdisciplinary Reviews-Nanomedicine and Nanobiotechnology. 2010;2(5):496–509. doi: 10.1002/wnan.94. [DOI] [PubMed] [Google Scholar]
- 119.Li J., Zhang Y. H., Yang T. Y., Zhang H., Yang Y. X., Xiao P. DNA biosensor by self-assembly of carbon nanotubes and DNA to detect riboflavin. Materials Science & Engineering C-Materials for Biological Applications. 2009;29(8):2360–2364. doi: 10.1016/j.msec.2009.06.006. [DOI] [Google Scholar]
- 120.Li N., Yuan R., Chai Y., Chen S., An H., Li W. New antibody immobilization strategy based on gold nanoparticles and azure I/multi-walled carbon nanotube composite membranes for an amperometric enzyme immunosensor. Journal of Physical Chemistry C. 2007;111(24):8443–8450. doi: 10.1021/jp068610u. [DOI] [Google Scholar]
- 121.Li Y., Yang C. Y., Chen S. M. Photoelectrocatalysis of hydrogen peroxide at functionalized multi-walled carbon nanotubes (f-MWCNT) with brilliant blue modified electrode. International Journal of Electrochemical Science. 2011;6(10):4829–4842. [Google Scholar]
- 122.Liu A. L., Zhong G. X., Chen J. Y., et al. A sandwich-type DNA biosensor based on electrochemical co-reduction synthesis of graphene-three dimensional nanostructure gold nanocomposite films. Analytica Chimica Acta. 2013;767:50–58. doi: 10.1016/j.aca.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 123.Netsuwan P., Sriwichai S., Phanichphant S., et al. Fabrication of carboxylated conducting polymer/CNTs composites thin films for immunosensor application. Molecular Crystals and Liquid Crystals. 2013;580(1):7–14. doi: 10.1080/15421406.2013.803890. [DOI] [Google Scholar]
- 124.Paul A., Bhattacharya B., Bhattacharyya T. K. Selective detection of Hg(II) over Cd(II) and Pb(II) ions by DNA functionalized CNT network. IEEE Sensors Journal. 2015;15(5):2774–2779. doi: 10.1109/jsen.2014.2382129. [DOI] [Google Scholar]
- 125.Periasamy A. P., Ho Y. H., Chen S. M. Multiwalled carbon nanotubes dispersed in carminic acid for the development of catalase based biosensor for selective amperometric determination of H2O2 and iodate. Biosensors & Bioelectronics. 2011;29(1):151–158. doi: 10.1016/j.bios.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 126.Rahman M. M., Shiddiky M. J. A., Rahman M. A., Shim Y. B. A lactate biosensor based on lactate dehydrogenase/nictotinamide adenine dinucleotide (oxidized form) immobilized on a conducting polymer/multiwall carbon nanotube composite film. Analytical Biochemistry. 2009;384(1):159–165. doi: 10.1016/j.ab.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 127.Ran X. Q., Yuan R., Chai Y. Q., Hong C. L., Qian X. Q. A sensitive amperometric immunosensor for alpha-fetoprotein based on carbon nanotube/DNA/Thi/nano-Au modified glassy carbon electrode. Colloids and Surfaces B-Biointerfaces. 2010;79(2):421–426. doi: 10.1016/j.colsurfb.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 128.Rao H. B., Liu Y. T., Zhong J., et al. Gold nanoparticle/chitosan@N,S co-doped multiwalled carbon nanotubes sensor: fabrication, characterization, and electrochemical detection of catechol and nitrite. ACS Sustainable Chemistry & Engineering. 2017;5(11):10926–10939. doi: 10.1021/acssuschemeng.7b02840. [DOI] [Google Scholar]
- 129.Shumyantseva V. V., Sigolaeva L. V., Agafonova L. E., et al. Facilitated biosensing via direct electron transfer of myoglobin integrated into diblock copolymer/multi-walled carbon nanotube nanocomposites. Journal of Materials Chemistry B. 2015;3(27):5467–5477. doi: 10.1039/C5TB00442J. [DOI] [PubMed] [Google Scholar]
- 130.Ti C. C., Umasankar Y., Chen S. M. Multiwalled carbon nanotubes encased in ruthenium oxide film as a hybrid material for neurotransmitters sensor. Electroanalysis. 2009;21(16):1855–1861. doi: 10.1002/elan.200904628. [DOI] [Google Scholar]
- 131.Hanna Varghese S., Nair R., Nair B. G., et al. Sensors based on carbon nanotubes and their applications: a review. Current Nanoscience. 2010;6(4):331–346. doi: 10.2174/157341310791659053. [DOI] [Google Scholar]
- 132.Wang J., Timchalk C., Lin Y. H. Carbon nanotube-based electrochemical sensor for assay of salivary cholinesterase enzyme activity: an exposure biomarker of organophosphate pesticides and nerve agents. Environmental Science & Technology. 2008;42(7):2688–2693. doi: 10.1021/es702335y. [DOI] [PubMed] [Google Scholar]
- 133.Zhao X. C., Hao F., Lu D. W., Liu W., Zhou Q. F., Jiang G. B. Influence of the surface functional group density on the carbon-nanotube-induced α-chymotrypsin structure and activity alterations. ACS Applied Materials & Interfaces. 2015;7(33):18880–18890. doi: 10.1021/acsami.5b05895. [DOI] [PubMed] [Google Scholar]
- 134.Al Faraj A., Fauvelle F., Luciani N., et al. In vivo biodistribution and biological impact of injected carbon nanotubes using magnetic resonance techniques. International Journal of Nanomedicine. 2011;6:351–361. doi: 10.2147/IJN.S16653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cavallo D., Fanizza C., Ursini C. L., et al. Multi-walled carbon nanotubes induce cytotoxicity and genotoxicity in human lung epithelial cells. Journal of Applied Toxicology. 2012;32(6):454–464. doi: 10.1002/jat.2711. [DOI] [PubMed] [Google Scholar]
- 136.Chen X., Kis A., Zettl A., Bertozzi C. R. A cell nanoinjector based on carbon nanotubes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(20):8218–8222. doi: 10.1073/pnas.0700567104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Deng X., Jia G., Wang H., et al. Translocation and fate of multi-walled carbon nanotubes in vivo. Carbon. 2007;45(7):1419–1424. doi: 10.1016/j.carbon.2007.03.035. [DOI] [Google Scholar]
- 138.di Giorgio M. L., Bucchianico S. D., Ragnelli A. M., Aimola P., Santucci S., Poma A. Effects of single and multi walled carbon nanotubes on macrophages: cyto and genotoxicity and electron microscopy. Mutation Research-Genetic Toxicology and Environmental Mutagenesis. 2011;722(1):20–31. doi: 10.1016/j.mrgentox.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 139.Dykas M. M., Poddar K., Yoong S. L., et al. Enhancing image contrast of carbon nanotubes on cellular background using helium ion microscope by varying helium ion fluence. Journal of Microscopy. 2018;269(1):14–22. doi: 10.1111/jmi.12604. [DOI] [PubMed] [Google Scholar]
- 140.Frame M. D., Dewar A. M., Mullick Chowdhury S., Sitharaman B. Vasoactive effects of stable aqueous suspensions of single walled carbon nanotubes in hamsters and mice. Nanotoxicology. 2013;8(8):867–875. doi: 10.3109/17435390.2013.837209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gannon C. J., Cherukuri P., Yakobson B. I., et al. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer. 2007;110(12):2654–2665. doi: 10.1002/cncr.23155. [DOI] [PubMed] [Google Scholar]
- 142.Han Z., Han X., Wang Z., Wu S., Zheng R. Thioaptamer conjugated single-wall carbon nanotubes in human breast cancer targeted photothermal therapy in-vivo and in-vitro. International Journal of Clinical and Experimental Medicine. 2016;9(1):58–64. [Google Scholar]
- 143.Huang N. N., Wang H. Q., Zhao J. H., Lui H., Korbelik M., Zeng H. S. Single-wall carbon nanotubes assisted photothermal cancer therapy: animal study with a murine model of squamous cell carcinoma. Lasers in Surgery and Medicine. 2010;42(9):638–648. doi: 10.1002/lsm.20968. [DOI] [PubMed] [Google Scholar]
- 144.Ji Z. F., Zhang D. Y., Li L., et al. The hepatotoxicity of multi-walled carbon nanotubes in mice. Nanotechnology. 2009;20(44):p. 445101. doi: 10.1088/0957-4484/20/44/445101. [DOI] [PubMed] [Google Scholar]
- 145.Kafa H., Wang J. T. W., Rubio N., et al. The interaction of carbon nanotubes with an in vitro blood-brain barrier model and mouse brain in vivo. Biomaterials. 2015;53:437–452. doi: 10.1016/j.biomaterials.2015.02.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liao J. L., Zhong S., Wang S. H., et al. Preparation and properties of a novel carbon nanotubes/poly(vinyl alcohol)/epidermal growth factor composite biological dressing. Experimental and Therapeutic Medicine. 2017;14(3):2341–2348. doi: 10.3892/etm.2017.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu X. W., Tao H. Q., Yang K., Zhang S. A., Lee S. T., Liu Z. A. Optimization of surface chemistry on single-walled carbon nanotubes for in vivo photothermal ablation of tumors. Biomaterials. 2011;32(1):144–151. doi: 10.1016/j.biomaterials.2010.08.096. [DOI] [PubMed] [Google Scholar]
- 148.Luo F., Pan L. L., Hong G., et al. In vitro and in vivo characterization of multi-walled carbon nanotubes/polycaprolactone composite scaffolds for bone tissue engineering applications. Journal of Biomaterials and Tissue Engineering. 2017;7(9):787–797. doi: 10.1166/jbt.2017.1629. [DOI] [Google Scholar]
- 149.Mahboobi S. H., Taheri A., Pishkenari H. N., Meghdari A., Hemmat M. Cellular injection using carbon nanotube: a molecular dynamics study. Nano. 2015;10(2):p. 1550025. doi: 10.1142/s1793292015500253. [DOI] [Google Scholar]
- 150.Moon H. K., Lee S. H., Choi H. C. In vivo near-infrared mediated tumor destruction by photothermal effect of carbon nanotubes. ACS Nano. 2009;3(11):3707–3713. doi: 10.1021/nn900904h. [DOI] [PubMed] [Google Scholar]
- 151.Muller J., Delos M., Panin N., Rabolli V., Huaux F., Lison D. Absence of carcinogenic response to multiwall carbon nanotubes in a 2-year bioassay in the peritoneal cavity of the rat. Toxicological Sciences. 2009;110(2):442–448. doi: 10.1093/toxsci/kfp100. [DOI] [PubMed] [Google Scholar]
- 152.Poulsen S. S., Saber A. T., Williams A., et al. MWCNTs of different physicochemical properties cause similar inflammatory responses, but differences in transcriptional and histological markers of fibrosis in mouse lungs. Toxicology and Applied Pharmacology. 2015;284(1):16–32. doi: 10.1016/j.taap.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 153.Robinson J. T., Welsher K., Tabakman S. M., et al. High performance in vivo near-ir (> 1 μm) imaging and photothermal cancer therapy with carbon nanotubes. Nano Research. 2010;3(11):779–793. doi: 10.1007/s12274-010-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rodriguez-Galvan A., Heredia A., Amelines-Sarria O., Rivera M., Medina L. A., Basiuk V. A. Non-covalent attachment of silver nanoclusters onto single-walled carbon nanotubes with human serum albumin as linking molecule. Applied Surface Science. 2015;331:271–277. doi: 10.1016/j.apsusc.2014.10.164. [DOI] [Google Scholar]
- 155.Singh R., Pantarotto D., McCarthy D., et al. Binding and condensation of plasmid DNA onto functionalized carbon nanotubes: toward the construction of nanotube-based gene delivery vectors. Journal of the American Chemical Society. 2005;127(12):4388–4396. doi: 10.1021/ja0441561. [DOI] [PubMed] [Google Scholar]
- 156.Smith B. R., Ghosn E. E. B., Rallapalli H., et al. Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nature Nanotechnology. 2014;9(6):481–487. doi: 10.1038/nnano.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Suehiro J., Ikeda N., Ohtsubo A., Imasaka K. Fabrication of bio/nano interfaces between biological cells and carbon nanotubes using dielectrophoresis. Microfluidics and Nanofluidics. 2008;5(6):741–747. doi: 10.1007/s10404-008-0276-6. [DOI] [Google Scholar]
- 158.Sun H. Y., Zhou J., Huang Z., et al. Carbon nanotube-incorporated collagen hydrogels improve cell alignment and the performance of cardiac constructs. International Journal of Nanomedicine. 2017;12:3109–3120. doi: 10.2147/IJN.S128030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tanaka M., Sato Y., Zhang M., et al. In vitro and in vivo evaluation of a three-dimensional porous multi-walled carbon nanotube scaffold for bone regeneration. Nanomaterials. 2017;7(2):p. 46. doi: 10.3390/nano7020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.VanHandel M., Alizadeh D., Zhang L. Y., et al. Selective uptake of multi-walled carbon nanotubes by tumor macrophages in a murine glioma model. Journal of Neuroimmunology. 2009;208(1-2):3–9. doi: 10.1016/j.jneuroim.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 161.Vittorio O., Quaranta P., Raffa V., et al. Magnetic carbon nanotubes: a new tool for shepherding mesenchymal stem cells by magnetic fields. Nanomedicine. 2011;6(1):43–54. doi: 10.2217/nnm.10.125. [DOI] [PubMed] [Google Scholar]
- 162.Wang X. J., Wang C., Cheng L., Lee S. T., Liu Z. Noble metal coated single-walled carbon nanotubes for applications in surface enhanced Raman scattering imaging and photothermal therapy. Journal of the American Chemical Society. 2012;134(17):7414–7422. doi: 10.1021/ja300140c. [DOI] [PubMed] [Google Scholar]
- 163.Welsher K., Liu Z., Sherlock S. P., et al. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nature Nanotechnology. 2009;4(11):773–780. doi: 10.1038/nnano.2009.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Welsher K., Sherlock S. P., Dai H. J. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(22):8943–8948. doi: 10.1073/pnas.1014501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wu H. X., Liu G., Zhuang Y. M., et al. The behavior after intravenous injection in mice of multiwalled carbon nanotube/Fe3O4 hybrid MRI contrast agents. Biomaterials. 2011;32(21):4867–4876. doi: 10.1016/j.biomaterials.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 166.Yang K. S., Yun J. S., Kim J. C., et al. Polydiacetylene single-walled carbon nanotubes nano-hybrid for cellular imaging applications. Journal of Nanoscience and Nanotechnology. 2012;12(1):377–385. doi: 10.1166/jnn.2012.5394. [DOI] [PubMed] [Google Scholar]
- 167.Zhao D. C., Alizadeh D., Zhang L. Y., et al. Carbon nanotubes enhance CpG uptake and potentiate antiglioma immunity. Clinical Cancer Research. 2011;17(4):771–782. doi: 10.1158/1078-0432.ccr-10-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bhirde A. A., Patel S., Sousa A. A., et al. Distribution and clearance of PEG-single-walled carbon nanotube cancer drug delivery vehicles in mice. Nanomedicine. 2010;5(10):1535–1546. doi: 10.2217/nnm.10.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bhirde A. A., Patel V., Gavard J., et al. Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano. 2009;3(2):307–316. doi: 10.1021/nn800551s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bianco A., Kostarelos K., Prato M. Applications of carbon nanotubes in drug delivery. Current Opinion in Chemical Biology. 2005;9(6):674–679. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 171.Chen J. Y., Chen S. Y., Zhao X. R., Kuznetsova L. V., Wong S. S., Ojima I. Functionalized single-walled carbon nanotubes as rationally designed vehicles for tumor-targeted drug delivery. Journal of the American Chemical Society. 2008;130(49):16778–16785. doi: 10.1021/ja805570f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Guo C., Al-Jamal W. T., Toma F. M., et al. Design of cationic multiwalled carbon nanotubes as efficient siRNA vectors for lung cancer xenograft eradication. Bioconjugate Chemistry. 2015;26(7):1370–1379. doi: 10.1021/acs.bioconjchem.5b00249. [DOI] [PubMed] [Google Scholar]
- 173.Kostarelos K., Bianco A., Prato M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nature Nanotechnology. 2009;4(10):627–633. doi: 10.1038/nnano.2009.241. [DOI] [PubMed] [Google Scholar]
- 174.Kostarelos K., Lacerda L., Pastorin G., et al. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nature Nanotechnology. 2007;2(2):108–113. doi: 10.1038/nnano.2006.209. [DOI] [PubMed] [Google Scholar]
- 175.Li J., Pant A., Chin C. F., et al. In vivo biodistribution of platinum-based drugs encapsulated into multi-walled carbon nanotubes. Nanomedicine-Nanotechnology Biology and Medicine. 2014;10(7):1465–1475. doi: 10.1016/j.nano.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 176.Liu Z., Fan A.. C., Rakhra K., et al. Supramolecular stacking of doxorubicin on carbon nanotubes for in vivo cancer therapy. Angewandte Chemie-International Edition. 2009;48(41):7668–7672. doi: 10.1002/anie.200902612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu Z., Sun X. M., Nakayama-Ratchford N., Dai H. J. Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano. 2007;1(1):50–56. doi: 10.1021/nn700040t. [DOI] [PubMed] [Google Scholar]
- 178.Liu Z., Tabakman S., Welsher K., Dai H. J. Carbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug delivery. Nano Research. 2009;2(2):85–120. doi: 10.1007/s12274-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lu Y., Aimetti A. A., Langer R., Gu Z. Bioresponsive materials. Nature Reviews Materials. 2016;2(1, article 16075) doi: 10.1038/natrevmats.2016.75. [DOI] [Google Scholar]
- 180.Lvov Y., Abdullayev E. Biosynthesis of bacterial cellulose/carboxylic multi-walled carbon nanotubes for enzymatic biofuel cell application. Progress in Polymer Science. 2016;9(3):p. 183. doi: 10.3390/ma9030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.McDevitt M. R., Chattopadhyay D., Kappel B. J., et al. Tumor targeting with antibody-functionalized, radiolabeled carbon nanotubes. Journal of Nuclear Medicine. 2007;48(7):1180–1189. doi: 10.2967/jnumed.106.039131. [DOI] [PubMed] [Google Scholar]
- 182.Meng L. J., Zhang X. K., Lu Q. H., Fei Z. F., Dyson P. J. Single walled carbon nanotubes as drug delivery vehicles: targeting doxorubicin to tumors. Biomaterials. 2012;33(6):1689–1698. doi: 10.1016/j.biomaterials.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 183.Prato M., Kostarelos K., Bianco A. Functionalized carbon nanotubes in drug design and discovery. Accounts of Chemical Research. 2008;41(1):60–68. doi: 10.1021/ar700089b. [DOI] [PubMed] [Google Scholar]
- 184.Ren J. F., Shen S., Wang D. G., et al. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials. 2012;33(11):3324–3333. doi: 10.1016/j.biomaterials.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 185.Sacchetti C., Liu-Bryan R., Magrini A., Rosato N., Bottini N., Bottini M. Polyethylene-glycol-modified single-walled carbon nanotubes for intra-articular delivery to chondrocytes. ACS Nano. 2014;8(12):12280–12291. doi: 10.1021/nn504537b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Siu K. S., Zheng X. F., Liu Y. L., et al. Single-walled carbon nanotubes noncovalently functionalized with lipid modified polyethylenimine for siRNA delivery in vitro and in Vivo. Bioconjugate Chemistry. 2014;25(10):1744–1751. doi: 10.1021/bc500280q. [DOI] [PubMed] [Google Scholar]
- 187.Tran P. A., Zhang L. J., Webster T. J. Carbon nanofibers and carbon nanotubes in regenerative medicine. Advanced Drug Delivery Reviews. 2009;61(12):1097–1114. doi: 10.1016/j.addr.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 188.Wong B. S., Yoong S. L., Jagusiak A., et al. Carbon nanotubes for delivery of small molecule drugs. Advanced Drug Delivery Reviews. 2013;65(15):1964–2015. doi: 10.1016/j.addr.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 189.Zhang X. K., Meng L. J., Lu Q. H., Fei Z. F., Dyson P. J. Targeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes. Biomaterials. 2009;30(30):6041–6047. doi: 10.1016/j.biomaterials.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 190.Azimirad V., Hosseinpour M., Shahabi P., Alimohammadi M., Sadighi M., Hatami H. Effects of injection of carbon nanotubes on EEG and results of a behavioral test in rats. Neurophysiology. 2015;47(3):198–204. doi: 10.1007/s11062-015-9521-2. [DOI] [Google Scholar]
- 191.Bardi G., Tognini P., Ciofani G., Raffa V., Costa M., Pizzorusso T. Pluronic-coated carbon nanotubes do not induce degeneration of cortical neurons in vivo and in vitro. Nanomedicine-Nanotechnology Biology and Medicine. 2009;5(1):96–104. doi: 10.1016/j.nano.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 192.Bellucci S., Chiaretti M., Cucina A., Carru G. A., Chiaretti A. I. Multiwalled carbon nanotube buckypaper: toxicology and biological effects in vitro and in vivo. Nanomedicine. 2009;4(5):531–540. doi: 10.2217/nnm.09.36. [DOI] [PubMed] [Google Scholar]
- 193.Cheng J. P., Chan C. M., Veca L. M., et al. Acute and long-term effects after single loading of functionalized multi-walled carbon nanotubes into zebrafish (Danio rerio) Toxicology and Applied Pharmacology. 2009;235(2):216–225. doi: 10.1016/j.taap.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 194.Chiaretti M., Mazzanti G., Bosco S., et al. Carbon nanotubes toxicology and effects on metabolism and immunological modification in vitro and in vivo. Journal of Physics: Condensed Matter. 2008;20(47):p. 10. [Google Scholar]
- 195.Clichici S., Biris A. R., Catoi C., Filip A., Tabaran F. Short-term splenic impact of single-strand DNA functionalized multi-walled carbon nanotubes intraperitoneally injected in rats. Journal of Applied Toxicology. 2014;34(4):332–344. doi: 10.1002/jat.2883. [DOI] [PubMed] [Google Scholar]
- 196.Clichici S., Mocan T., Filip A., et al. Blood oxidative stress generation after intraperitoneal administration of functionalized single-walled carbon nanotubes in rats. Acta Physiologica Hungarica. 2011;98(2):231–241. doi: 10.1556/APhysiol.98.2011.2.15. [DOI] [PubMed] [Google Scholar]
- 197.Gao J., Zhang X. C., Yu M., Ren G. G., Yang Z. Cognitive deficits induced by multi-walled carbon nanotubes via the autophagic pathway. Toxicology. 2015;337:21–29. doi: 10.1016/j.tox.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 198.Guo Y., Shi D. L., Cho H. S., et al. In vivo imaging and drug storage by quantum-dot-conjugated carbon nanotubes. Advanced Functional Materials. 2008;18(17):2489–2497. doi: 10.1002/adfm.200800406. [DOI] [Google Scholar]
- 199.Jain S., Thakare V. S., Das M., et al. Toxicity of multiwalled carbon nanotubes with end defects critically depends on their functionalization density. Chemical Research in Toxicology. 2011;24(11):2028–2039. doi: 10.1021/tx2003728. [DOI] [PubMed] [Google Scholar]
- 200.Jos A., Pichardo S., Puerto M., Sanchez E., Grilo A., Camean A. M. Cytotoxicity of carboxylic acid functionalized single wall carbon nanotubes on the human intestinal cell line Caco-2. Toxicology In Vitro. 2009;23(8):1491–1496. doi: 10.1016/j.tiv.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 201.Li H., Zhang T., Liang G. Y., Zhang Y. Q., Wang X. K. In vivo evaluation of acute toxicity of water-soluble carbon nanotubes. Toxicological and Environmental Chemistry. 2011;93(3):603–615. doi: 10.1080/02772248.2010.544472. [DOI] [Google Scholar]
- 202.Liu Z., Chen K., Davis C., et al. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Research. 2008;68(16):6652–6660. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Meng J., Yang M., Jia F. M., Xu Z., Kong H., Xu H. Y. Immune responses of BALB/c mice to subcutaneously injected multi-walled carbon nanotubes. Nanotoxicology. 2011;5(4):583–591. doi: 10.3109/17435390.2010.523483. [DOI] [PubMed] [Google Scholar]
- 204.Nunes A., Bussy C., Gherardini L., et al. In vivo degradation of functionalized carbon nanotubes after stereotactic administration in the brain cortex. Nanomedicine. 2012;7(10):1485–1494. doi: 10.2217/nnm.12.33. [DOI] [PubMed] [Google Scholar]
- 205.Pacurari M., Qian Y., Fu W., et al. Cell permeability, migration, and reactive oxygen species induced by multiwalled carbon nanotubes in human microvascular endothelial cells. Journal of Toxicology and Environmental Health-Part A: Current issues. 2012;75(2):112–128. doi: 10.1080/15287394.2011.615110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Patlolla A., McGinnis B., Tchounwou P. Biochemical and histopathological evaluation of functionalized single-walled carbon nanotubes in Swiss-Webster mice. Journal of Applied Toxicology. 2011;31(1):75–83. doi: 10.1002/jat.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Patlolla A. K., Berry A., Tchounwou P. B. Study of hepatotoxicity and oxidative stress in male Swiss-Webster mice exposed to functionalized multi-walled carbon nanotubes. Molecular and Cellular Biochemistry. 2011;358(1-2):189–199. doi: 10.1007/s11010-011-0934-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Schipper M. L., Nakayama-Ratchford N., Davis C. R., et al. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nature Nanotechnology. 2008;3(4):216–221. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]
- 209.Shen C. X., Zhang Q. F., Li J. A., Bi F. C., Yao N. Induction of programmed cell death in Arabidopsis and rice by single-wall carbon nanotubes. American Journal of Botany. 2010;97(10):1602–1609. doi: 10.3732/ajb.1000073. [DOI] [PubMed] [Google Scholar]
- 210.Shi X. F., Sitharaman B., Pham Q. P., et al. In vitro cytotoxicity of single‐walled carbon nanotube/biodegradable polymer nanocomposites. Journal of Biomedical Materials Research Part A. 2008;86A(3):813–823. doi: 10.1002/jbm.a.31671. [DOI] [PubMed] [Google Scholar]
- 211.Snyder-Talkington B. N., Dong C. L., Sargent L. M., et al. mRNAs and miRNAs in whole blood associated with lung hyperplasia, fibrosis, and bronchiolo-alveolar adenoma and adenocarcinoma after multi-walled carbon nanotube inhalation exposure in mice. Journal of Applied Toxicology. 2016;36(1):161–174. doi: 10.1002/jat.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yang S. T., Wang X., Jia G., et al. Long-term accumulation and low toxicity of single-walled carbon nanotubes in intravenously exposed mice. Toxicology Letters. 2008;181(3):182–189. doi: 10.1016/j.toxlet.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 213.Zhang D. Y., Deng X. Y., Ji Z. F., et al. Long-term hepatotoxicity of polyethylene-glycol functionalized multi-walled carbon nanotubes in mice. Nanotechnology. 2010;21(17):p. 175101. doi: 10.1088/0957-4484/21/17/175101. [DOI] [PubMed] [Google Scholar]
- 214.Zhang Y. B., Ali S. F., Dervishi E., et al. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived PC12 cells. ACS Nano. 2010;4(6):3181–3186. doi: 10.1021/nn1007176. [DOI] [PubMed] [Google Scholar]
- 215.Zhu L., Chang D. W., Dai L. M., Hong Y. L. DNA damage induced by multiwalled carbon nanotubes in mouse embryonic stem cells. Nano Letters. 2007;7(12):3592–3597. doi: 10.1021/nl071303v. [DOI] [PubMed] [Google Scholar]
- 216.Fazaeli Y., Gholibeikian M. Synthesis and biological evaluation of the toxicity of grafted 2-mercaptobenzimidazole multi-walled carbon nanotubes (MWCNTs) Iranian Journal of Science and Technology Transaction a-Science. 2017;41(1):145–150. doi: 10.1007/s40995-017-0197-x. [DOI] [Google Scholar]
- 217.Firme C. P., III, Bandaru P. R. Toxicity issues in the application of carbon nanotubes to biological systems. Nanomedicine-Nanotechnology Biology and Medicine. 2010;6(2):245–256. doi: 10.1016/j.nano.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 218.Yan L. A., Zhao F., Li S. J., Hu Z. B., Zhao Y. L. Low-toxic and safe nanomaterials by surface-chemical design, carbon nanotubes, fullerenes, metallofullerenes, and graphenes. Nanoscale. 2011;3(2):362–382. doi: 10.1039/C0NR00647E. [DOI] [PubMed] [Google Scholar]
- 219.Zhang X. Y., Hu W. B., Li J., Tao L., Wei Y. A comparative study of cellular uptake and cytotoxicity of multi-walled carbon nanotubes, graphene oxide, and nanodiamond. Toxicology Research. 2012;1(1):62–68. doi: 10.1039/c2tx20006f. [DOI] [Google Scholar]
- 220.Dai H. J., Hafner J. H., Rinzler A. G., Colbert D. T., Smalley R. E. Nanotubes as nanoprobes in scanning probe microscopy. Nature. 1996;384(6605):147–150. doi: 10.1038/384147a0. [DOI] [Google Scholar]
- 221.Hafner J. H., Cheung C. L., Lieber C. M. Growth of nanotubes for probe microscopy tips. Nature. 1999;398(6730):761–762. doi: 10.1038/19658. [DOI] [Google Scholar]
- 222.Hafner J. H., Cheung C. L., Oosterkamp T. H., Lieber C. M. High-yield assembly of individual single-walled carbon nanotube tips for scanning probe microscopies. Journal of Physical Chemistry B. 2001;105(4):743–746. doi: 10.1021/jp003948o. [DOI] [Google Scholar]
- 223.Kwon S., Woo Lee H., Park H., Kim S. Nanometer displacement measurement of a multiwalled carbon nanotube cantilever under aqueous conditions. Measurement Science and Technology. 2010;21(8):p. 085104. doi: 10.1088/0957-0233/21/8/085104. [DOI] [Google Scholar]
- 224.Nishijima H., Kamo S., Akita S., et al. Carbon-nanotube tips for scanning probe microscopy: preparation by a controlled process and observation of deoxyribonucleic acid. Applied Physics Letters. 1999;74(26):4061–4063. doi: 10.1063/1.123261. [DOI] [Google Scholar]
- 225.Stevens R. M., Nguyen C. V., Meyyappan M. Carbon nanotube scanning probe for imaging in aqueous environment. IEEE Transactions on Nanobioscience. 2004;3(1):56–60. doi: 10.1109/TNB.2004.824275. [DOI] [PubMed] [Google Scholar]
- 226.Uchihashi T., Choi N., Tanigawa M., et al. Carbon-nanotube tip for highly-reproducible imaging of deoxyribonucleic acid helical turns by noncontact atomic force microscopy. Japanese Journal of Applied Physics Part 2-Letters. 2000;39, 8B, Part 2:L887–L889. doi: 10.1143/JJAP.39.L887. [DOI] [Google Scholar]
- 227.Umemura K., Komatsu J., Uchihashi T., et al. Atomic force microscopy of RecA-DNA complexes using a carbon nanotube tip. Biochemical and Biophysical Research Communications. 2001;281(2):390–395. doi: 10.1006/bbrc.2001.4333. [DOI] [PubMed] [Google Scholar]
- 228.Wilson N. R., Macpherson J. V. Carbon nanotube tips for atomic force microscopy. Nature Nanotechnology. 2009;4(8):483–491. doi: 10.1038/nnano.2009.154. [DOI] [PubMed] [Google Scholar]
- 229.Wong S. S., Woolley A. T., Odom T. W., et al. Single-walled carbon nanotube probes for high-resolution nanostructure imaging. Applied Physics Letters. 1998;73(23):3465–3467. doi: 10.1063/1.122798. [DOI] [Google Scholar]
- 230.Yu M. F., Dyer M. J., Skidmore G. D., et al. Three-dimensional manipulation of carbon nanotubes under a scanning electron microscope. Nanotechnology. 1999;10(3):244–252. doi: 10.1088/0957-4484/10/3/304. [DOI] [Google Scholar]
- 231.Iijima M., Watabe T., Ishii S., et al. Fabrication and STM-characterization of novel hybrid materials of DNA/carbon nanotube. Chemical Physics Letters. 2005;414(4–6):520–524. doi: 10.1016/j.cplett.2005.09.032. [DOI] [Google Scholar]
- 232.Iliafar S., Mittal J., Vezenov D., Jagota A. Interaction of single-stranded DNA with curved carbon nanotube is much stronger than with flat graphite. Journal of the American Chemical Society. 2014;136(37):12947–12957. doi: 10.1021/ja5055498. [DOI] [PubMed] [Google Scholar]
- 233.Kilina S., Tretiak S., Yarotski D. A., et al. Electronic properties of DNA base molecules adsorbed on a metallic surface. Journal of Physical Chemistry C. 2007;111(39):14541–14551. doi: 10.1021/jp070805u. [DOI] [Google Scholar]
- 234.Wang X., Liu F., Andavan G.. T.. S., et al. Carbon nanotube-DNA nanoarchitectures and electronic functionality. Small. 2006;2(11):1356–1365. doi: 10.1002/smll.200600056. [DOI] [PubMed] [Google Scholar]
- 235.Lu W., Xiong Y., Hassanien A., Zhao W., Zheng M., Chen L. W. A scanning probe microscopy based assay for single-walled carbon nanotube metallicity. Nano Letters. 2009;9(4):1668–1672. doi: 10.1021/nl900194j. [DOI] [PubMed] [Google Scholar]
- 236.Toader M., Fiedler H., Hermann S., Schulz S. E., Gessner T., Hietschold M. Conductive AFM for CNT characterization. Nanoscale Research Letters. 2013;8(1):p. 24. doi: 10.1186/1556-276x-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhu J. Y., McMorrow J., Crespo-Otero R., et al. Solution-processable carbon nanoelectrodes for single-molecule investigations. Journal of the American Chemical Society. 2016;138(9):2905–2908. doi: 10.1021/jacs.5b12086. [DOI] [PubMed] [Google Scholar]
- 238.Chiashi S., Hanashima T., Mitobe R., Nagatsu K., Yamamoto T., Homma Y. Water encapsulation control in individual single-walled carbon nanotubes by laser irradiation. Journal of Physical Chemistry Letters. 2014;5(3):408–412. doi: 10.1021/jz402540v. [DOI] [PubMed] [Google Scholar]
- 239.Chiashi S., Kono K., Matsumoto D., et al. Adsorption effects on radial breathing mode of single-walled carbon nanotubes. Physical Review B. 2015;91(15):p. 5. [Google Scholar]
- 240.Homma Y., Chiashi S., Yamamoto T., et al. Photoluminescence measurements and molecular dynamics simulations of water adsorption on the hydrophobic surface of a carbon nanotube in water vapor. Physical Review Letters. 2013;110(15) doi: 10.1103/physrevlett.110.157402. [DOI] [PubMed] [Google Scholar]
- 241.Bauer B. J., Becker M. L., Bajpai V., et al. Measurement of single-wall nanotube dispersion by size exclusion chromatography. Journal of Physical Chemistry C. 2007;111(48):17914–17918. doi: 10.1021/jp071494q. [DOI] [Google Scholar]
- 242.Gao Z., Oudjedi L., Faes R., et al. Optical detection of individual ultra-short carbon nanotubes enables their length characterization down to 10 nm. Scientific Reports. 2015;5(1, article 17093):p. 10. doi: 10.1038/srep17093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gladchenko G. O., Lytvyn O. S., Karachevtsev M. V., Valeev V. A., Leontiev V. S., Karachevtsev V. A. Binding of polynucleotides with single-walled carbon nanotubes: effect of temperature. Materialwissenschaft und Werkstofftechnik. 2011;42(2):92–97. doi: 10.1002/mawe.201100738. [DOI] [Google Scholar]
- 244.Hayashida T., Kawashima T., Nii D., Ozasa K., Umemura K. Kelvin probe force microscopy of single-walled carbon nanotubes modified with DNA or poly(ethylene glycol) Chemistry Letters. 2013;42(6):666–668. doi: 10.1246/cl.130121. [DOI] [Google Scholar]
- 245.Hayashida T., Umemura K. Surface morphology of hybrids of double-stranded DNA and single-walled carbon nanotubes studied by atomic force microscopy. Colloids and Surfaces B: Biointerfaces. 2013;101:49–54. doi: 10.1016/j.colsurfb.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 246.Karachevtsev M. V., Lytvyn O. S., Stepanian S. G., Leontiev V. S., Adamowicz L., Karachevtsev V. A. SWNT-DNA and SWNT-polyC hybrids: AFM study and computer modeling. Journal of Nanoscience and Nanotechnology. 2008;8(3):1473–1480. [PubMed] [Google Scholar]
- 247.Khripin C. Y., Arnold-Medabalimi N., Zheng M. Molecular-crowding-induced clustering of DNA-wrapped carbon nanotubes for facile length fractionation. ACS Nano. 2011;5(10):8258–8266. doi: 10.1021/nn2029549. [DOI] [PubMed] [Google Scholar]
- 248.Koh B., Park J. B., Hou X. M., Cheng W. Comparative dispersion studies of single-walled carbon nanotubes in aqueous solution. Journal of Physical Chemistry B. 2011;115(11):2627–2633. doi: 10.1021/jp110376h. [DOI] [PubMed] [Google Scholar]
- 249.Lahiji R. R., Dolash B. D., Bergstrom D. E., Reifenberger R. Oligodeoxyribonucleotide association with single-walled carbon nanotubes studied by SPM. Small. 2007;3(11):1912–1920. doi: 10.1002/smll.200700184. [DOI] [PubMed] [Google Scholar]
- 250.Pease L. F., III, Tsai D.-H., Fagan J. A., et al. Length distribution of single-walled carbon nanotubes in aqueous suspension measured by electrospray differential mobility analysis. Small. 2009;5(24):2894–2901. doi: 10.1002/smll.200900928. [DOI] [PubMed] [Google Scholar]
- 251.Tsutsumi Y., Fujigaya T., Nakashima N. Size reduction of 3D-polymer-coated single-walled carbon nanotubes by ultracentrifugation. Nanoscale. 2015;7(46):19534–19539. doi: 10.1039/C5NR05066A. [DOI] [PubMed] [Google Scholar]
- 252.Vichchulada P., Shim J., Lay M. D. Non-oxidizing purification method for large volumes of long, undamaged single-walled carbon nanotubes. Journal of Physical Chemistry C. 2008;112(49):19186–19192. doi: 10.1021/jp803989d. [DOI] [Google Scholar]
- 253.Arnett C. M., Marsh C. P., Welch C. R., et al. Enzyme-mediated assimilation of DNA-functionalized single-walled carbon nanotubes. Langmuir. 2010;26(2):613–617. doi: 10.1021/la902564n. [DOI] [PubMed] [Google Scholar]
- 254.Ishibashi Y., Oura S., Umemura K. Adsorption of DNA binding proteins to functionalized carbon nanotube surfaces with and without DNA wrapping. European Biophysics Journal. 2017;46(6):541–547. doi: 10.1007/s00249-017-1200-3. [DOI] [PubMed] [Google Scholar]
- 255.Izumi K., Kumashiro Y., Oura S., Okano T., Umemura K. Removal of excess polymer from a suspension containing hybrids of thermoresponsive polymer and carbon nanotubes using aggregation phenomenon. Japanese Journal of Applied Physics. 2016;55(9):p. 5. [Google Scholar]
- 256.Nii D., Hayashida T., Umemura K. Controlling the adsorption and desorption of double-stranded DNA on functionalized carbon nanotube surface. Colloids and Surfaces B-Biointerfaces. 2013;106:234–239. doi: 10.1016/j.colsurfb.2013.01.054. [DOI] [PubMed] [Google Scholar]
- 257.Nii D., Hayashida T., Yamaguchi Y., Ikawa S., Shibata T., Umemura K. Selective binding of single-stranded DNA-binding proteins onto DNA molecules adsorbed on single-walled carbon nanotubes. Colloids and Surfaces B-Biointerfaces. 2014;121:325–330. doi: 10.1016/j.colsurfb.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 258.Oura S., Ito M., Nii D., Homma Y., Umemura K. Biomolecular recognition ability of RecA proteins for DNA on single-walled carbon nanotubes. Colloids and Surfaces B-Biointerfaces. 2015;126:496–501. doi: 10.1016/j.colsurfb.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 259.Oura S., Umemura K. Optimal conditions for decorating outer surface of single-walled carbon nanotubes with RecA proteins. Japanese Journal of Applied Physics. 2016;55(3S2):p. 03DF04. doi: 10.7567/jjap.55.03df04. [DOI] [Google Scholar]
- 260.Umemura K., Ishibashi Y., Oura S. Physisorption of DNA molecules on chemically modified single-walled carbon nanotubes with and without sonication. European Biophysics Journal. 2016;45(6):483–489. doi: 10.1007/s00249-016-1116-3. [DOI] [PubMed] [Google Scholar]
- 261.Umemura K., Ishizaka K., Nii D., Izumi K. Non-uniform binding of single-stranded DNA binding proteins to hybrids of single-stranded DNA and single-walled carbon nanotubes observed by atomic force microscopy in air and in liquid. Applied Surface Science. 2016;388:381–384. doi: 10.1016/j.apsusc.2015.12.144. [DOI] [Google Scholar]
- 262.Umemura K., Izumi K., Kumashiro Y., Oura S., Okano T. Protein adsorption on hybrids of thermoresponsive polymers and single-walled carbon nanotubes. International Journal of Polymer Science. 2016;2016:5. doi: 10.1155/2016/3539609.3539609 [DOI] [Google Scholar]
- 263.Williams K. A., Veenhuizen P. T. M., de la Torre B. G., Eritja R., Dekker C. Nanotechnology - carbon nanotubes with DNA recognition. Nature. 2002;420(6917):761–761. doi: 10.1038/420761a. [DOI] [PubMed] [Google Scholar]
- 264.Zheng M., Jagota A., Strano M. S., et al. Structure-based carbon nanotube sorting by sequence-dependent DNA assembly. Science. 2003;302(5650):1545–1548. doi: 10.1126/science.1091911. [DOI] [PubMed] [Google Scholar]
- 265.Lulevich V., Kim S., Grigoropoulos C. P., Noy A. Frictionless sliding of single-stranded DNA in a carbon nanotube pore observed by single molecule force spectroscopy. Nano Letters. 2011;11(3):1171–1176. doi: 10.1021/nl104116s. [DOI] [PubMed] [Google Scholar]
- 266.Wei G., Li Q., Steckbeck S., Ciacchi L. C. Direct force measurements on peeling heteropolymer ssDNA from a graphite surface using single-molecule force spectroscopy. Physical Chemistry Chemical Physics. 2014;16(9):3995–4001. doi: 10.1039/c3cp54121e. [DOI] [PubMed] [Google Scholar]
- 267.Zhang Y. L., Tunuguntla R. H., Choi P. O., Noy A. Real-time dynamics of carbon nanotube porins in supported lipid membranes visualized by high-speed atomic force microscopy. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1726):p. 20160226. doi: 10.1098/rstb.2016.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Postma H. W. C., Sellmeijer A., Dekker C. Manipulation and imaging of individual single-walled carbon nanotubes with an atomic force microscope. Advanced Materials. 2000;12(17):1299–1302. doi: 10.1002/1521-4095(200009)12:17<1299::AID-ADMA1299>3.0.CO;2-O. [DOI] [Google Scholar]
- 269.Korayem M. H., Estaji M., Homayooni A. Noncalssical multiscale modeling of ssDNA manipulation using a CNT-nanocarrier based on AFM. Colloids and Surfaces B-Biointerfaces. 2017;158:102–111. doi: 10.1016/j.colsurfb.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 270.Ito M., Ito Y., Nii D., Kato H., Umemura K., Homma Y. The effect of DNA adsorption on optical transitions in single walled carbon nanotubes. Journal of Physical Chemistry C. 2015;119(36):21141–21145. doi: 10.1021/acs.jpcc.5b05087. [DOI] [Google Scholar]
- 271.Rosenberger M. R., Wang M. C., Xie X., Rogers J. A., Nam S., King W. P. Measuring individual carbon nanotubes and single graphene sheets using atomic force microscope infrared spectroscopy. Nanotechnology. 2017;28(35, article 355707) doi: 10.1088/1361-6528/aa7c23. [DOI] [PubMed] [Google Scholar]