Abstract
When variably fatty acylated N-terminal amino acid sequences were appended to a green fluorescent reporter protein (GFP), chimeric GFPs were localized to different membranes in a fatty acylation-dependent manner. To explore the mechanism of localization, the properties of acceptor membranes and their interaction with acylated chimeric GFPs were analyzed in COS-7 cells. Myristoylated GFPs containing a palmitoylated or polybasic region colocalized with cholesterol and ganglioside GM1, but not with caveolin, at the plasma membrane and endosomes. A dipalmitoylated GFP chimera colocalized with cholesterol and GM1 at the plasma membrane and with caveolin in the Golgi region. Acylated GFP chimeras did not cofractionate with low-density caveolin-rich lipid rafts prepared with Triton X-100 or detergent-free methods. All GFP chimeras, but not full-length p62c-yes and caveolin, were readily solubilized from membranes with various detergents. These data suggest that, although N-terminal acylation can bring GFP to cholesterol and sphingolipid-enriched membranes, protein-protein interactions are required to localize a given protein to detergent-resistant membranes or caveolin-rich membranes. In addition to restricting acceptor membrane localization, N-terminal fatty acylation could represent an efficient means to enrich the concentration of signaling proteins in the vicinity of detergent-resistant membranes and facilitate protein-protein interactions mediating transfer to a detergent-resistant lipid raft core.
INTRODUCTION
Numerous signaling proteins contain combinations of covalently attached fatty acids (myristate and palmitate) at their N termini. Such proteins include several cellular Src-related protein tyrosine kinases (PTKs: Yes, Fyn, Lyn, Lck, Hck, Fgr, and Yrk), G protein α subunits (Gαi, Gαo, Gαz, and yeast GPA1), endothelial nitric oxide synthase (eNOS), and A-kinase–anchoring protein AKAP18 (Dunphy and Linder, 1998; Resh, 1999). In addition, several proteins are singly myristoylated and possess an adjacent or distant polybasic amino acid domain. These include the PTKs Src and Blk, MARCKS, and HIV-1 Nef and Gag proteins (Resh, 1999). Other proteins have been shown to contain two or more covalently linked palmitates at their N termini, as in GAP-43, Gαq, PSD-95, and regulator of G protein-signaling isoforms (Dunphy and Linder, 1998; Resh, 1999). In all cases, a cooperative interaction of at least two signals (including multiple N-terminal acylation or an acylation site juxtaposed to a polybasic domain) is required to promote efficient membrane association of the modified proteins (Dunphy and Linder, 1998; Johnson and Cornell, 1999; McCabe and Berthiaume, 1999; Resh, 1999). This cooperative mechanism is also used by Ras superfamily proteins (including H-, K-, and N-Ras) at the C terminus using different lipid anchors (farnesyl and geranylgeranyl) coupled with polybasic regions and/or palmitoylation (Choy et al., 1999; Magee and Marshall, 1999).
Recently, various combinations of lipids attached to proteins have been shown to specify lipidation-dependent enrichment of lipidated proteins to certain membranes (Choy et al., 1999; McCabe and Berthiaume, 1999). These recent advances provided a novel understanding of the existence of different lipid anchors attached to a variety of proteins. It appears that in the many cases studied to date differential lipidation allows for localization to different acceptor membranes. Thus, lipidated sequences seem to act as novel types of signal or retention sequences. We demonstrated that GFP chimeras containing myristoylation and palmitoylation signals or a myristoylation signal juxtaposed to an N-terminal polybasic region were localized similarly to the plasma membrane and endosomes in COS-7 cells. In contrast, a dipalmitoylated, nonmyristoylated GFP chimera was localized to the Golgi region and plasma membrane. Removal of the palmitoylation signal in myristoylated and palmitoylated proteins resulted in loss of localization specificity and led to relocalization to a variety of membranes. The fact that all myristoylated and palmitoylated GFP chimeras localized similarly to endosomes and plasma membrane ruled out the involvement of surrounding amino acids in subcellular localization. Thus, the combinatorial identity of N-terminal membrane-associating signals was likely important for subcellular distribution of these proteins.
Evidence has accumulated during the past few years supporting the existence of discrete membrane microdomains known as lipid rafts and also referred to as detergent-resistant membranes (DRMs), detergent-insoluble glycosphingolipid-rich fractions, and caveolin-enriched membranes (Parton and Simons, 1995; Simons and Ikonen, 1997; Anderson, 1998; Brown and London, 1998a, b; Kurzchalia and Parton, 1999; Resh, 1999; Galbiati et al., 1999b). These membrane subdomains have been reported to be enriched in glycosphingolipids, free cholesterol, and saturated phospholipids (Harder and Simons, 1997; Fridriksson et al., 1999), and in some cases several lipidated proteins including nonreceptor PTKs and caveolin (Kurzchalia and Parton, 1999; Oh and Schnitzer, 1999). These membrane rafts have been described as liquid-ordered (lo) phase lipid domains dispersed in a liquid crystalline (or liquid disordered, ld) lipid bilayer (Brown and London, 1998a, b). Membrane raft size has been shown to vary from 70–370 nm in diameter (Varma and Mayor, 1998; Hooper, 1999; Jacobson and Dietrich, 1999). A collection of data suggests that rafts are small and dynamic but can be stabilized into caveolae or larger domains by specific proteins as well as by antibody cross-linking or patching (Harder et al., 1998). Lipid rafts from different origins have been shown to be similar in many properties, but unique in others (e.g., constituent proteins or lipids), and support the existence of many kinds of lipid rafts (Parkin et al., 1996; Iwabuchi et al., 1998; Kurzchalia and Parton, 1999; Waugh et al., 1999).
Caveolin, a 21-kDa palmitoylated integral membrane cholesterol-binding protein (Murata et al., 1995), has been utilized as a marker for caveolae (Song et al., 1996, 1997; Orlandi and Fishman, 1998; Oh and Schnitzer, 1999). Because of the comparable properties (detergent insolubility, lipid constituents, and buoyancy) between caveolae and DRMs/lipid rafts, caveolin has also been utilized as a marker of DRMs/lipid rafts during isolation. However, selective isolation procedures indicated that caveolae do not equate with detergent-insoluble lipid rafts (Hooper, 1999) and that noncaveolar lipid rafts do exist as separate entities (Iwabuchi et al., 1998). Caveolin possesses a complex intracellular cycling pathway involving plasma membrane, caveolae, endoplasmic reticulum, ERGIC (endoplasmic reticulum Golgi intermediate compartment), and Golgi, and cytosol, and as such is found in a variety of membranes (Conrad et al., 1995; Uittenbogaard et al., 1998).
N-terminally acylated proteins, such as Yes, GAP-43, Gαi, Gαo, and Fyn, have been shown to be enriched in 4°C Triton X-100 (TX-100) insoluble structures (Arreaza et al., 1994; van't Hof and Resh, 1997; Arni et al., 1998; Galbiati et al., 1999b; Melkonian et al., 1999). In those proteins, loss of acylation resulted in the loss of functional fractionation to lipid rafts. In most cases, two saturated acyl chains are believed to be required for partitioning of the proteins into liquid-ordered cholesterol and glycosphingolipid-enriched membrane microdomains (Schroeder et al., 1994; Arni et al., 1998; Schroeder et al., 1998). Proteins modified by single acyl chains or bulky, branched hydrophobic lipids (farnesyl and geranylgeranyl) are not believed to partition favorably into liquid-ordered membrane rafts (Melkonian et al., 1999; Moffett et al., 2000). Other reports show that prenyl groups and myristate alone are sufficient to enrich in lipid rafts as defined by cofractionation with caveolin-enriched membranes (Song et al., 1997; Michaely et al., 1999).
Although several studies have demonstrated that mutations abolishing protein acylation resulted in a loss of function and fractionation to lipid rafts (reviewed by Dunphy and Linder, 1998; Resh, 1999), we are investigating whether the addition of acylated sequences to an otherwise cytosolic reporter protein would confer a gain of function and allow partitioning or fractionation into lipid rafts. Furthermore, to investigate the mechanism of lipidation-dependent localization of variably acylated GFPs, we characterized the biochemical composition of acceptor membranes. Myristoylated and palmitoylated or polybasic region-containing GFPs colocalized with cholesterol and sphingolipid-enriched membranes, but not with caveolin. A dipalmitoylated (but not myristoylated) GFP did colocalize with caveolin intracellularly. We also show that N-terminal acylation signals appended to GFP are not sufficient to enrich the resulting chimeric GFPs in lipid rafts prepared with the use of detergent and detergent-free methods. When the solubilization of chimeric GFPs was analyzed by a kinetic in situ extraction assay, acylated GFPs were found to be readily solubilized, as opposed to the detergen-resistant fatty acylated endogenous proteins caveolin and PTK p62c-yes. Our findings collectively support a model in which N-terminal acylation coupled with protein-protein interactions or perhaps additional protein-lipid interactions are required to bring these detergent-soluble proteins from cholesterol-, sphingolipid-enriched membranes to DRMs.
MATERIALS AND METHODS
Plasmids
The pCMV5 mammalian expression vector (Andersson et al., 1989) containing various acylated 11–16 amino acid sequences appended to the S65T mutant of GFP (YesGFP, Yes[C3S]GFP, Yes[G2A]GFP, GαoGFP, GAP-43GFP, Src16GFP, FynGFP, and LckGFP) and GFP alone were from a previous study (McCabe and Berthiaume, 1999) and are depicted in Table 1. The acylation status of these constructs has been previously determined and confirmed to conform to predicted modification based on metabolic labeling and subcellular fractionation studies in our previous report (McCabe and Berthiaume, 1999).
Table 1.
Colocalization of acylated proteins and lipid raft constituents in COS-7 cells
| Protein | Membrane associating signalsa | Cellular localizationb | Colocalizationc with
|
Lipid raft associationd | ||
|---|---|---|---|---|---|---|
| Caveolin | Cholesterol | GMa | ||||
| YesGFP | myr+palm (3,5)e | pm + endo | − | g+ | h+ | − |
| Yes(C3S)GFP | myr | endo + ER | − | + | + | − |
| Yes(G2A)GFP | none | cyto, nuc | − | − | − | − |
| FynGFP | myr+palm (3,6) | pm + endo | − | + | + | − |
| GαoGFP | myr+palm (3) | pm + endo | − | + | + | − |
| LckGFP | myr+palm (3,5) | pm + endo | − | + | + | − |
| Src16GFP | myr+pos | pm + endo | − | + | + | − |
| GAP-43GFP | palm+pos (3,4) | pm + Golgi | + (Golgi) | + | + | − |
| GFP | none | cyto, nuc | − | − | − | − |
| Caveolin | PTM+palm | pm/Golgi/ER/cytof | + | +g | +g | + |
| p62c-yes | myr+palm | pm + endof | + | +g | NDh | + |
Known cooperative signals responsible for membrane association. myr, myristoylation; pal, palmitoylation; pos, polybasic region; PTM, partial transmembrane domain.
As determined in this and a previous study (McCabe and Berthiaume, 1999). pm, plasma membrane; endo, endosomes; ER, endoplasmic reticulum; cyto, cytoplasm; Golgi, Golgi region.
Immunofluorescence using anti-GFP and anti-caveolin Abs. Cholesterol: free cholesterol as detected by filipin; GM1, ganglioside GM1 detection utilizing FITC-cholera toxin B subunit.
Lipid raft association as defined by 1% TX-100 and sodium carbonate sucrose gradient fractionation methods.
Numbers between parentheses refer to the position of palmitoylated cysteines.
As determined in Anderson (1998), Uittenbogaard et al. (1998), Luetterforst et al. (1999), and Luton et al. (1999).
Weak colocalization noted.
ND, Not determined.
Cell Lines, Antibodies, and Reagents
COS-7 cells were from the American Type Culture Collection (Manassas, VA) and were maintained in 10% fetal bovine serum in DMEM (Life Technologies, Rockville, MD) with 100 U/ml penicillin G sodium and 100 μg/ml streptomycin sulfate plus 2 mM l-glutamine (Sigma, St. Louis, MO) and passed twice per week with the use of a 0.25% trypsin/1 mM EDTA wash (Life Technologies). Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Rabbit polyclonal anti-GFP antibody (antibody) was developed in our laboratory with the use of highly purified recombinant GFP made in Escherichia coli as antigen; mouse monoclonal anti-GFP was from Chemicon (Temecula, CA). Mouse monoclonal anti-Yes PTK, anti–caveolin-1 (clone 2297), anti-syntaxin 6, anti-early endosome-associated protein-1 (EEA1) antibodies, and rabbit polyclonal anti-caveolin antibody were from Transduction Laboratories (Lexington, KY). Mouse monoclonal antibody to the cation-independent mannose 6-phosphate receptor (CI-MPR) was from Affinity Bioreagents (Golden, CO). Texas Red (TR) conjugated transferrin (Tf) (TR-Tf) and Prolong antifade mounting medium were from Molecular Probes (Eugene, OR). Mouse monoclonal antibodies to lysosome-associated membrane protein-1 (LAMP-1) and LAMP-2 were from Developmental Studies Hybridoma Bank (Iowa City, IA). Donkey anti-rabbit immunoglobulin (Ig) G-TR and IgG- fluorescein isothiocyanate (FITC), donkey anti-mouse IgG-TR and IgG-FITC secondary antibodies, and normal donkey serum (NDS) were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA). Enhanced chemiluminescence (ECL) Plus was from Amersham Pharmacia Biotech (Piscataway, NJ). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), as part of the CellTiter 96 AQueous assay kit, was from Promega (Madison, WI). Paraformaldehyde, filipin, FITC-cholera toxin B subunit (FITC-CTX), methyl β-cyclodextrin (MβCD), water-soluble cholesterol (cholesterol:MβCD complex), and TX-100 were from Sigma. n-octyl β–d-Glucopyranoside (n-octyl glucoside, OG) was from Boehringer Mannheim (Indianapolis, IN). All other reagents were of the highest grade commercially available.
Transfection and Immunofluorescence Microscopy
COS-7 cells were cultured and seeded on coverslips as described previously (McCabe and Berthiaume, 1999). All antibodies used were diluted in 4% NDS in phosphate-buffered saline (PBS) to prevent nonspecific binding and were optimized for fluorophore detection with the use of a laser scanning confocal microscope (model LSM 510, Zeiss, Oberkochen, Germany) mounted on a Zeiss Axiovert M100 inverted microscope with a 63× plan-apochromatic lens (1.40 NA; Cross Cancer Institute, University of Alberta, Edmonton, Canada). For fluorescent detection of GFP chimeras, mouse anti-GFP (1:200) or rabbit anti-GFP (1:2000) primary antibodies were used. Caveolin was detected with rabbit polyclonal anti-caveolin antibody (1:2000). The following antibody dilutions were used: late endosome marker anti-CI-MPR (1:200; Barr et al., 2000; Shisheva et al., 2001), early endosome marker anti-EEA-1 (1:100; Sorkina et al., 1999;), trans-Golgi network (TGN) marker anti-syntaxin 6 (3 μg/ml, 1:80; Vandenbulcke et al., 2000; Mills et al., 2001), and the late endosome/lysosome markers anti-LAMP-1 and anti-LAMP-2 (1:1000; Downey et al., 19990. For labeling of the endosomal recycling compartment, cells were incubated in the presence of 20 μg/ml TR-Tf for 15 min before processing to allow for accumulation in this compartment (Teter et al., 1998; Vandenbulcke et al., 2000). All final image analyses were done with Photoshop 5.5 (Adobe Systems, Mountain View, CA).
Filipin Detection of Cellular Free Cholesterol
Free cholesterol detection in COS-7 cells was achieved with the use of the methods of Cadigan et al. (1990) and Mukherjee et al. (1998), with slight modifications. Filipin, a fluorescent polyene antibiotic, was used to detect free cholesterol through interactions with a free 3β-hydroxyl group (Muller et al., 1984). Cells were washed four times with PBS and fixed with 1 ml of 2% paraformaldehyde in PBS at room temperature for 1 h, rinsed four times with PBS, and blocked/stained with 1 ml of filipin solution (50 μg/ml) containing 4% NDS in PBS for 1 h at 37°C. The filipin solution was prepared by dissolving 2.5 mg of filipin in 1 ml of dimethylformamide and adding this solution to 49 ml of PBS. When performing colocalization studies, the preceding steps were performed, followed by washes and incubation of the fixed cells with 80 μl of primary antibody in 4% NDS in PBS containing 0.1% saponin for 1 h. This was followed by a three PBST (PBS plus 0.1% Tween-20) washes and secondary antibody steps as above with the addition of 0.1% saponin to the antibody solutions to permeabilize the cells. Addition of 0.1% saponin under these conditions was previously demonstrated not to interfere with cholesterol detection (Cadigan et al., 1990; Abrami and van der Goot, 1999; Freeman Emmerson et al., 2001) Confocal microscopic detection of filipin was done with a UV laser 364-nm excitation line and detection with a 385- to 470-nm bandpass emission filter. Detection of FITC was done with an Argon laser 488-nm line for excitation and 505-nm longpass emission filter. No bleed through was observed between the FITC and UV filipin channels.
Cholesterol Depletion with the Use of MβCD
Rapid depletion of cellular cholesterol was performed based on the method of Subtil et al. (1999) with the following modifications. Cells were transfected with plasmids expressing various acylated chimeric GFPs and placed in DMEM containing lipoprotein-deficient serum in six-well plates for 18–20 h to reduce the late endosomal/lysosomal collection of free cholesterol derived from serum lipoproteins (Butler et al., 1992; Mukherjee et al., 1998). Cells were then acutely depleted of cholesterol by incubation for 1 h in 0 and 20 mM of MβCD dissolved in serum-free DMEM at 37°C. Coverslips were then washed, fixed, blocked, and processed for immunofluorescence and cholesterol fluorescent detection. To assess the viability of MβCD-treated cells, a modified single-cell viability assay based on that used by Racchi et al. (1997) was used. Briefly, transfected COS-7 cells expressing YesGFP were incubated with 20 mM MβCD for 1 h at 37°C. During the last 15 min of the incubation, 100 μl of MTT solution from the CellTiter 96 AQueous assay kit was added to 1 ml of media in six-well dishes, and formazan dye production was allowed to develop. To assess recovery after acute cholesterol reduction, transfected cells were incubated with 20 mM MβCD for 1 h at 37°C, followed by incubation of cells with 30 μg/ml cholesterol: MβCD complex for 1 h at 37°C. During the last 15 min, MTT solution was added to detect cell viability. After these treatments, cells lying on coverslips were processed for immunofluorescence and filipin fluorescence detection as described above. MTT formazan dye produced by viable cells creates initially punctate purple-black crystals observable with a light microscope, and continued or robust production of MTT formazan creates needle-like crystals in viable cells. Experiments were also done on living cells without immunofluorescence processing (i.e., live cell GFP detection and MTT formazan observation with the use of epifluorescence and light microscopy), and results obtained were identical to processed samples (McCabe and Berthiaume, unpublished results). Fixation and permeabilization of cells did not remove MTT formazan crystals produced by viable cells.
GM1 Glycosphingolipid Detection with the Use of FITC-CTX
COS-7 cells were transfected with various GFP chimeras as described above. Cells were washed with PBS and then incubated for 30 min at 37°C with 2 μg/ml (170 nM) FITC-CTX to label both plasma membrane and intracellular GM1-containing compartments (Joliot et al., 1997; Harder et al., 1998; Stulnig et al., 1998; Janes et al., 1999). A total of 100 μl of FITC-CTX solution (in 0.1% bovine serum albumin in serum-free DMEM) was added to each coverslip and immediately placed in a 5% CO2 incubator at 37°C for 20 min to allow living cell uptake. After incubation, coverslips were washed four times with serum-free DMEM and processed for normal immunofluorescence as above, with GFP chimeras detected with a TR secondary antibody. Control transfection/fixation experiments were carried out to ensure that GFP fluorescence was efficiently quenched under our fixation conditions to prevent bleed through into the TR and FITC/filipin channels.
Preparation of Caveolin-enriched Membrane Fractions with the Use of Sucrose Density Centrifugation
Transfected COS-7 cells grown to near confluence in 100-mm dishes were used to prepare caveolin-enriched membrane fractions. Two established methods, one using the nonionic detergent TX-100 and the other detergent-free (sodium carbonate), were used to prepare these fractions (Song et al., 1997; Galbiati et al., 1999b). After two washes with ice-cold PBS, two confluent dishes were scraped into either 2 ml of 500 mM sodium carbonate, pH 11.0, or 2 ml of MBS (2-(N-morpholino)ethanesulfonic acid-buffered saline, 25 mM 2-(N-morpholino)ethanesulfonic acid, pH 6.5, 0.15 M NaCl) containing 1% (wt/vol) TX-100 and solubilized for 20 min at 4°C. Homogenization was carried out with the use of 10 strokes of a tight-fitting Dounce homogenizer for TX-100–containing samples. For sodium carbonate-treated samples, the cells were homogenized with 10 strokes of a tight-fitting Dounce homogenizer followed by three 10-s bursts of a Polytron tissue grinder (Brinkmann Instruments, Westbury, NY), followed by three 20-s bursts of sonication (XL sonicator, Hert Systems, Farmingdale, NY) combined to more finely disrupt cellular membranes (Song et al., 1996). The homogenates were adjusted to 40% sucrose by addition of 2 ml of 80% sucrose prepared in MBS and placed at the bottom of an ultracentrifuge tube. A 5–40% discontinuous sucrose gradient was formed above and centrifuged at 39,000 rpm for 16–20 h, in an SW40Ti rotor (Beckmann Instruments, Palo Alto, CA). A light-scattering band was observed at the 5–30% sucrose interface that contained caveolin-rich membranes but excluded most other cellular proteins, as judged by Ponceau S staining of the polyvinylidene difluoride (PVDF) membranes. Twelve 1-ml fractions were removed from the top of the tubes, and a portion (32 μl) of each was analyzed by SDS-PAGE analysis followed by Western blot analysis.
In Situ Extraction of Transfected Cells with Nonionic Detergent
Separation of cells expressing various acylated GFP constructs into detergent-soluble and detergent-resistant fractions was performed as described by Wolven et al. (1997) and van't Hof and Resh (1997), with minor modifications. Confluent 100-mm plates of transfected cells were washed twice with ice-cold STE buffer (100 mM NaCl, 10 mM Tris, pH 7.4, 1 mM EDTA) and then incubated for 20 min with 2 ml of Csk buffer (10 mM 1,4-piperazinediethanesulfonic acid, pH 6.8, 100 mM KCl, 2.5 mM MgCl2, 1 mM CaCl2, 0.3 M sucrose, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride containing 1% TX-100 at 4°C). The resulting detergent-soluble fraction (S) was removed from the cells at different time intervals. The cellular detergent-resistant matrix (R) remaining on the tissue culture dish was incubated with 2.5 ml of ice-cold 1× lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 2 mM EDTA, 2 mM MgCl2, 5 mM NaF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride) for 10 min and scraped off the dish with a cell scraper. The content of the soluble fraction was then adjusted with 0.5 ml of 5× lysis buffer. R fractions were balanced with 1× lysis buffer solution and all tubes were clarified at 100,000 × g for 15 min at 4°C.
In additional experiments to assess the validity of the lipid rafts isolated in this study, 1% TX-100 in Csk buffer was replaced by a lower TX-100 concentration (0.1%), and 60 mM OG in Csk buffer was used to solubilize raft fractions (Melkonian et al., 1995). Supernatant fractions were removed from the centrifuge tubes without disturbing the pellet, and equivalent portions of both R and S fractions (64 μl) were added to 16 μl of 5× SDS sample buffer containing 100 mM dithiothreitol, boiled for 2 min, and analyzed by SDS-PAGE (12.5%). Gels were transferred onto Immobilon-P PVDF membranes (Millipore, Bedford, MA; 1 h, 120 V, 0.5 A). Western blot analyses were performed on these membranes with the use of 1× Blotto as a blocking and diluting solution. Membranes were probed with rabbit anti-GFP (1:2000), anti-Yes monoclonal (1:1000), and rabbit polyclonal anti-caveolin (1:4000) antibodies for 1 h at room temperature. Horseradish peroxidase-conjugated donkey anti-rabbit IgG and sheep anti-mouse IgGs (1:5000) were used as secondary antibodies and incubated with membranes for 1 h at room temperature. Next, six 5-min washes with PBST were done between steps. Enhanced chemiluminescence (ECL) detection was performed with the ECL Plus kit (Amersham-Pharmacia Biotech). Detection was from 5 s to 30 min with the use of X-OMAT AR film (Kodak, Rochester, NY). Control experiments were conducted to ensure no cross-reactivity between the anti-caveolin, anti-Yes, and anti-GFP antibodies was occurring. Membranes were stained post-ECL detection with Coomassie brilliant blue to demonstrate reproducibility, the relative distributions of cellular proteins, and the alterations in these distributions caused by detergent extraction.
RESULTS
Colocalization of Acylated Chimeric GFPs with Free Cholesterol
After the determination of the subcellular localization of N-terminally acylated GFP chimeras (McCabe and Berthiaume, 1999), we sought to further characterize the mechanisms leading to acylation-specific subcellular localization. In our previous study, we demonstrated that localization of myristoylated and variably palmitoylated chimeric GFPs was independent of amino acid sequence surrounding the dually acylated myr-GlyCys N-terminal motif. Therefore, we postulated that biophysical properties of both the acylated chimeric GFPs and the acceptor membranes would be at play in conferring proper localization. Lipid rafts/DRMs/caveolae are known to be enriched in cholesterol, glycosphingolipids, saturated glycerolipids, and caveolin. Because several N-terminally acylated signaling proteins have been found to be associated with these membrane domains (Kurzchalia and Parton, 1999), the rationale behind our investigation was to characterize the potential involvement of constituents of these lipid rafts in the location of our chimeric GFPs to a variety of membranes.
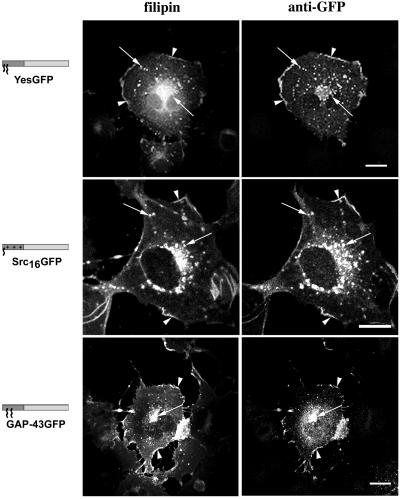
To evaluate the cholesterol requirements of the acceptor membranes, we localized intracellular pools of free cholesterol with the use of an established procedure and filipin (Cadigan et al., 1990). We also altered the cholesterol content of COS-7 cells with the use of the efficient cholesterol-depleting agent, MβCD. When fixed, transfected cells were stained with filipin (Figure 1), we found that myristoylated plus palmitoylated YesGFP and the filipin-derived free cholesterol signal showed very similar distributions and localization at the plasma membrane (Figure 1, arrowheads) and in vesicular structures previously identified as endosomes (Figure 1, arrows) (McCabe and Berthiaume, 1999). This pattern of plasma membrane and endosomal localization of dually acylated GFPs and cholesterol was consistent for all chimeric GFP constructs containing myristate and palmitate (YesGFP, GαoGFP, FynGFP, and LckGFP), despite the fact that these constructs vary in the number of palmitoylated cysteines and their position in the primary sequence (Table 1). Similarities in distribution and localization of signals corresponding to myristoylated nonpalmitoylated Yes(C3S)GFP or Fyn(C3,6S)GFP and filipin were also found (McCabe and Berthiaume, unpublished results). Signals corresponding to cytosolic nonacylated constructs, such as Yes(G2A)GFP and GFP alone, did not overlap with those of filipin (McCabe and Berthiaume, unpublished results). Myristoylated and polybasic region-containing Src16GFP also demonstrated plasma membrane (Figure 1, arrowheads) and endosomal (Figure 1, arrows) colocalization with filipin. Dually palmitoylated (nonmyristoylated) GAP-43GFP colocalized with filipin at the plasma membrane (Figure 1, arrowheads) and in a dense focal perinuclear area (Figure 1, arrow). This perinuclear area was previously identified as the Golgi apparatus based on giantin colocalization (McCabe and Berthiaume, 1999). Apparent colocalization between the weak endogenous p62c-yes signal and filipin was also demonstrated (McCabe and Berthiaume, unpublished results).
Figure 1.
Colocalization of chimeric GFPs with free cholesterol in COS-7 cells. Cells transfected with plasmids expressing various GFP chimeras were fixed, permeabilized, and incubated with filipin to detect free cholesterol and rabbit anti-GFP antibody followed by FITC-conjugated anti-rabbit antibody to detect acylated GFPs. In the models beside the images: dark gray/blue box, appended acylation sequence; light gray/green box, GFP; small acyl chain, myristate; large acyl chain, palmitate; plus (+) signs, polybasic region. Arrowheads indicate areas of plasma membrane colocalization, and arrows indicate endosomal/Golgi colocalization. Bars, 10 μm.
Cholesterol Depletion Affects Acylated GFP and Cholesterol Distribution
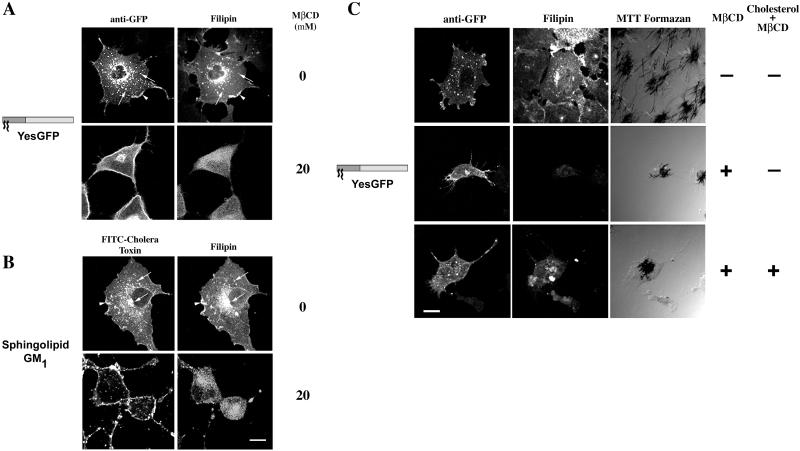
When we assessed the effects of cholesterol depletion on the structures harboring acylated GFPs, we found a significant relocalization of the fatty acylated GFPs as well as the sphingolipid GM1. As shown in Figure 2A, in the absence of MβCD, YesGFP was found at the plasma membrane, on peripheral endosomes, as well as clustered in a focal perinuclear area. Free cholesterol, as detected by filipin fluorescence, was seen concentrated in endosomal structures and at the plasma membrane. Colocalization of cholesterol and YesGFP in endosomal structures (Figure 2, arrows) and at the plasma membrane (Figure 2, arrowhead) was evident. In cells treated with 20 mM MβCD, a marked redistribution of YesGFP signal was seen reproducibly. The chimeric GFP signal appeared to be more concentrated at the plasma membrane of treated cells. The distribution of the fluorescent signal corresponding to GFP was also more homogeneous (less punctate) in treated versus control cells. Acylated GFPs were also found clustered in a perinuclear organelle, and a striking reduction in punctate endosomal structures normally decorated by myristoylated and palmitoylated GFPs was evident. Cells treated with MβCD showed a significant reduction in size consistent with a previous report (Ilangumaran and Hoessli, 1998). The efficiency of our MβCD treatment in free cholesterol removal can be appreciated by the reduced intensity of the filipin signal when cells were observed under identical conditions as compared with control cells. Our experimental conditions utilize concentrations of MβCD known to be efficient in cholesterol removal in COS-7 and other cells. The use of 10 mM MβCD for >30 min has been shown to cause a 50% reduction in quantifiable free cholesterol in Chinese hamster ovary cells (Subtil et al., 1999), and 5 mM MβCD reduced free cholesterol to 64% of control >2 h in COS-7 cells (Racchi et al., 1997). Results presented in Figure 2A with the use of YesGFP are typical of myristoylated and palmitoylated chimeric GFPs (FynGFP, LckGFP, and GαoGFP; McCabe and Berthiaume, unpublished results).
Figure 2.
Acute cholesterol depletion alters intracellular localization of acylated GFP chimeras and GM1. COS-7 cells, grown in lipoprotein-deficient serum for 18–20 h, were acutely depleted of cholesterol with the use of 20 mM MβCD for 1 h at 37°C. (A) GFP chimeras were detected with the use of rabbit anti-GFP and FITC-conjugated donkey anti-rabbit secondary antibody. Free cholesterol was detected with filipin. (B) Ganglioside GM1 distribution detected with FITC-CTX. Arrowheads indicate areas of plasma membrane colocalization, and arrows indicate endosomal colocalization. Model descriptions of Figures 2–4 are as in Figure 1. (C) Viability of MβCD-treated COS-7 cells expressing YesGFP. Expressing cells were treated with vehicle, 20 mM MβCD for 1 h at 37°C, or 20 mM MβCD for 1 h followed by repletion with 30 μg/ml cholesterol:MβCD for 1 h at 37°C. Cellular viability was determined by MTT formazan production. Bar, 10 μm.
To assess the effects of cholesterol depletion on another constituent of lipid rafts or DRMs, the ganglioside GM1, COS-7 cells were incubated in the presence of MβCD as described above and FITC-CTX was utilized to detect the ganglioside. As can be seen in Figure 2B, in the absence of MβCD, the FITC signal corresponding to GM1 was detected at the plasma membrane (Figure 2B, arrowhead) and in punctate structures clustered in a perinuclear area and in the periphery (Figure 2B, arrows). When compared with the distribution of the filipin signal, extensive similarity was observed and significant colocalization was evident at the plasma membrane (Figure 2B, arrowhead) and in intracellular endosomal structures (Figure 2B, arrows). In contrast, when cells were treated with 20 mM MβCD, a significant alteration in the GM1 distribution was observed. In the vast majority of cells, essentially no intracellular vesicular structures were visible, and the plasma membrane GM1 fraction became more prominent and displayed relatively large areas of GM1 clustering when compared with untreated cells.
To assess the effects of MβCD treatment on cellular viability, we utilized the tetrazolium dye, MTT, a well established marker of cellular viability. As can be seen in Figure 2C, treatment with 20 mM MβCD for 1 h at 37°C caused changes in cell morphology, altered distribution of acylated GFP chimeras, and reduced apparent number of endocytic structures, but MTT formazan crystals were still produced by treated cells, albeit at a lower level on average. On treatment with 20 mM MβCD followed by replenishment of cholesterol with 30 μg/ml cholesterol:MβCD complex, a restoration of more normal cellular morphology occurred, along with the distribution of acylated GFP-containing peripheral endocytic structures. In addition, robust production of MTT formazan crystals was seen in cholesterol-replenished cells, indicating apparently normal cellular function. Thus, acute cholesterol depletion did not affect cellular viability under these conditions and was completely reversible with supplementation of exogenous free cholesterol.
Attempts to lower cellular sphingolipids with the inhibitor of ceramide synthase, fumonisin B1, were carried out. Because of the toxicity and length of the treatment to achieve significant reduction in cellular sphingolipids, we were unfortunately unable to photograph viable cells with satisfactory results with the use of transiently transfected cells (McCabe and Berthiaume, unpublished results).
Colocalization of Acylated Chimeric GFPs with Organelle Markers
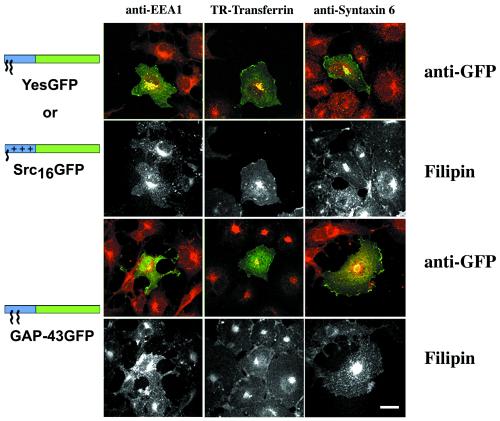
Acylated GFP chimeras have been previously shown to associate with intracellular structures identified as endosomes based on colocalization with the fluorescent lipoprotein particle, DiI-LDL, internalized via receptor-mediated endocytosis (McCabe and Berthiaume, 1999). To further assess the type of endocytic organelles where acylated chimeric GFPs are localized in comparison to free cholesterol localization, we utilized COS-7 established organelle markers in indirect triple immunofluorescence/filipin fluorescence protocols. Myristoylated/palmitoylated YesGFP and myristoylated/polybasic region-containing Src16GFP colocalized significantly with the early endosomal marker EEA1 and the endosomal recycling compartment marker TR-Tf (Figure 3). These chimeras also colocalized significantly with the late endosomal/lysosomal marker LAMP-2 (McCabe and Berthiaume, unpublished results). Only partial colocalization was seen between YesGFP or Src16GFP and the TGN marker, syntaxin 6. Similarly, little or no colocalization was seen between these two chimeric GFPs and the TGN/late endosomal marker CI-MPR (McCabe and Berthiaume, unpublished results). In contrast, the dually palmitoylated, nonmyristoylated GAP-43GFP showed little or very partial colocalization with EEA1 and TR-Tf (Figure 3) or with LAMP-2 (McCabe and Berthiaume, unpublished results) but demonstrated significant colocalization with syntaxin 6 (Figure 3) and CI-MPR (McCabe and Berthiaume, unpublished results). Thus, differentially acylated chimeric GFPs are found enriched in different endocytic organelle compartments under steady state. The specificity of association depends on the type of N-terminal acylation signal present in the GFP chimera. The corresponding free cholesterol distributions are shown below the merged immunofluorescence images in grayscale and demonstrate overlapping signals between filipin/free cholesterol and all the organelle marker signals studied in Figure 3.
Figure 3.
Triple colocalization of various chimeric GFPs with organelle markers and free cholesterol as detected by filipin in COS-7 cells. Colocalization of differentially acylated GFP chimeras (green) and organelle marker (red) distributions in transfected COS-7 cells can be seen in color. Filipin signal is shown in grayscale panels below corresponding color organelle colocalization panels. Bar, 10 μm.
Acylated Chimeric GFPs Colocalize with the Ganglioside GM1
The ganglioside GM1 is a known marker of caveolae but can also be found in other membrane domains (Parton, 1994; Iwabuchi et al., 1998). To compare the distribution of GM1 to that of our acylated GFP chimeras, COS-7 cells were fixed and permeabilized to abolish mature GFP epifluorescence (Pines, 1995), and FITC-CTX was utilized to detect GM1. Numerous reports regarding the usage of chromophore-conjugated CTX B subunit to detect GM1 exist (Joliot et al., 1997; Harder et al., 1998; Orlandi and Fishman, 1998; Janes et al., 1999). GFP was detected with the use of our rabbit polyclonal antibody followed by incubation with donkey anti-rabbit TR-conjugated secondary antibody (thus, in Figure 4, GFP staining is shown in red). Appropriate controls were done at the time of each experiment to ensure total inactivation of intrinsic GFP epifluorescence during our fixation conditions (McCabe and Berthiaume, unpublished results).
Figure 4.
Colocalization of various chimeric GFPs with the ganglioside GM1. Colocalization of GM1 as detected with FITC-CTX (green) with acylated chimeric GFPs YesGFP, Src16GFP, and GAP-43GFP, as detected with the use of rabbit anti-GFP antibody followed by TR-conjugated donkey anti-rabbit antibody. Arrowheads indicate areas of plasma membrane colocalization, and arrows indicate endosomal/Golgi colocalization. Bars, 10 μm.
We analyzed transfected COS-7 cells to determine whether our acylated chimeras would colocalize with GM1. As shown in Figure 4, similar distributions of the ganglioside GM1 and YesGFP were found in endosomes (arrows) and at the plasma membrane (arrowheads). Myristoylated Src16GFP, containing a polybasic membrane-associating signal, also displayed significant colocalization with GM1 in endosomes (Figure 4, arrows) and at the plasma membrane (Figure 4, arrowhead). In contrast to the above patterns of colocalization, dually palmitoylated GAP-43GFP, colocalized with GM1 in the Golgi/TGN (Figure 4, arrow) and at the plasma membrane (Figure 4, arrowhead) but not in peripheral endosomes. The distribution of endocytosed FITC-CTX (and hence GM1) has been recently assessed in COS-7 cells (Nichols et al., 2001) and found to accumulate in Tf-labeled compartments as well as the Golgi complex (previously shown for free cholesterol; Mukherjee et al., 1998). Thus, our results demonstrating the colocalization of acylated GFP chimeras, cholesterol, and GM1 in these compartments appear to be valid. The distributions of caveolin and the ganglioside GM1 overlapped at the plasma membrane and focal perinuclear structures, but this overlap was only partial (McCabe and Berthiaume, unpublished results).
Most Acylated Chimeric GFPs Do Not Colocalize with Caveolin
Several acylated signaling proteins have been documented to be present in caveolae (Oh and Schnitzer, 1999). To assess whether caveolin and acylated GFPs colocalized, we utilized indirect double immunofluorescence. As shown in Figure 5, the signals for acylated YesGFP and Src16GFP, when merged with the caveolin signal, displayed minimal colocalization, especially in peripheral endosomal vesicles. In the tightly clustered Golgi and endosomal recycling compartment area (Figure 5, arrows), some colocalization is apparent (yellow) but may represent the overlap of two intense signals in proximity of one another. In contrast to these results, the dually palmitoylated GAP-43GFP displayed significant colocalization with caveolin in a perinuclear focal compartment (Figure 5, arrow) previously identified as the Golgi apparatus. GAP-43GFP did not colocalize with caveolin significantly at any other sites. The distribution of endogenous p62c-yes was also compared with that of caveolin. When merged with the caveolin signal, minor colocalization was found intracellularly and at cell-cell contacts.
Figure 5.
Colocalization of chimeric GFPs and endogenous p62c-yes PTK with caveolin in COS-7 cells. COS-7 cells were transfected with plasmids encoding various acylated GFP chimeras and processed for indirect immunofluorescence analysis 24 h after transfection. The GFP chimeras were detected by incubation with mouse anti-GFP antibody followed by FITC-conjugated anti-mouse antibody. Endogenous p62c-yes was detected with mouse anti-Yes antibody and FITC-conjugated donkey anti-mouse secondary antibody. The same cells were incubated with rabbit anti-caveolin antibody followed by TR-conjugated anti-rabbit secondary antibody. Arrowheads point to caveolin-GAP-43GFP Golgi colocalization, and arrows point to clusters of acylated GFP-containing structures and caveolin-containing structures. Bars, 10 μm.
Acylated GFPs Do Not Cofractionate with Caveolin-enriched Lipid Rafts, but Full-Length p62c-yes Tyrosine Kinase Does
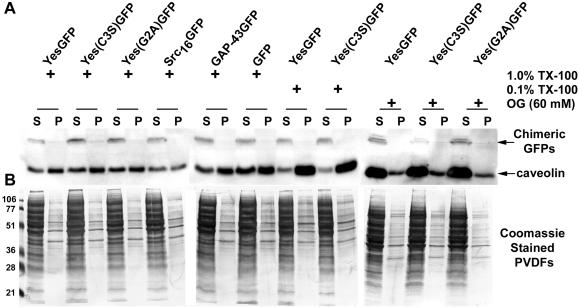
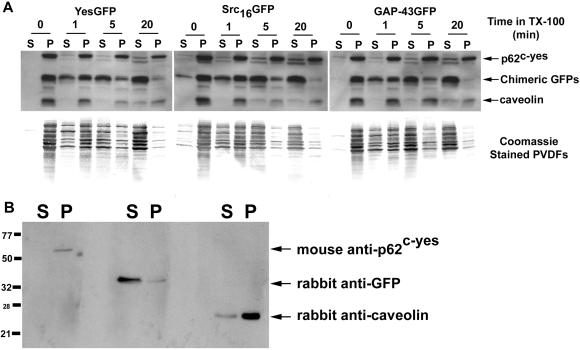
The presence of acylated signaling proteins in caveolae or lipid rafts has, in the past, been assessed with the use of acylated full-length signaling proteins with or without their acylation sites removed. We were interested in performing the converse experiment to assess whether addition of lipid anchors to a reporter protein could confer detergent resistance (enrichment in lipid rafts) to that reporter protein. To do so, COS-7 cells expressing various chimeric GFPs were grown to confluence in 100-mm dishes and used to prepare caveolin-enriched lipid rafts, with the use of two different established methods. With the use of the detergent-based isolation procedure of Song et al. (1997), in contrast to the presence of caveolin at the 5–30% sucrose interface (fractions 4 and 5), all GFP chimeras tested (cofractionate with caveolin in buoyant DRMs YesGFP, Yes[C3S]GFP, Yes[G2A ]) and GFP alone were present in the nonbuoyant fractions 9–12. In contrast, full-length p62c-yes did (Figure 6A). With the use of a cold 1% TX-100 sucrose density gradient fractionation method followed by Western blot analysis of aliquots for caveolin, we showed that caveolin was clearly enriched in the low-density raft fractions (lanes 4–5, at the 5–30% interface). In addition, caveolin was also present in higher density detergent-soluble fractions as well. This has also been seen by others in COS cells (Joliot et al., 1997). Overexposed blots did not show any chimeric GFP signal in the lipid raft region of the membrane. Coomassie-stained PVDF membranes demonstrated the presence of the vast majority of cellular proteins in the TX-100-soluble 40% sucrose fractions (McCabe and Berthiaume, unpublished results). When pellets found at the bottom of the ultracentrifuge tube were analyzed for chimeric GFPs or caveolin, only a small residual amount of both proteins was found, and likely represented trapped molecules present in large cytoskeletal aggregates (McCabe and Berthiaume, unpublished results).
Figure 6.
Caveolin-enriched lipid raft isolation with the use of detergent-based and detergent-free sucrose density gradient fractionation methods. (A) Caveolin-enriched lipid raft isolation with the use of a TX-100 at 4°C fractionation method. COS-7 cells transiently transfected with various acylated GFPs and GFP alone were subjected to subcellular fractionation after homogenization in buffer containing 1% TX-100. The distribution of acylated chimeric GFPs and endogenous caveolin are shown with the use of a discontinuous 5–40% sucrose gradient. Fractions were collected from the top of the gradient, separated by SDS-PAGE (12.5% acrylamide), and analyzed by immunoblotting and Coomassie staining of the immunoblot. Immunoblot analysis was done with rabbit anti-GFP antibody (1:2000) to detect GFP and the acylated chimeras, with anti-caveolin (1:4000) to detect endogenous caveolin, and with mouse anti-Yes antibody (1:1000) to detect endogenous p62c-yes PTK. (B) Caveolin enriched lipid raft isolation with the use of a sodium carbonate fractionation method. Transfected COS-7 cells were subjected to subcellular fractionation after homogenization in buffer containing 500 mM sodium carbonate, pH 11.0. The distribution of acylated chimeric GFPs and endogenous caveolin and p62c-yes PTK are shown. Fractions were collected and analyzed as above.
To avoid possible detergent-induced artifacts in the preparation of lipid rafts, we prepared lipid rafts with the use of an established detergent-free lipid raft isolation procedure (Song et al., 1997). As seen in Figure 6B, YesGFP, Yes(C3S)GFP, Yes(G2A)GFP, FynGFP, GαoGFP, GAP-43GFP, and GFP alone did not cofractionate with caveolin in the buoyant DRM fractions 4 and 5. Of note, the detergent-free sodium carbonate method restricted distribution of caveolin signal, with fractions 4 and 5 containing the majority of caveolin signal and minor amounts in fractions 6–8. To assess whether full-length Yes PTK also floated into raft membranes with the use of this detergent-free method, membranes were also blotted with monoclonal anti-Yes antibody. Full-length Yes PTK also cofractionated with caveolin-rich membranes fractions containing lipid rafts (4 and 5). We conclusively show that dual acylation or acylation combined with a polybasic domain are not sufficient to confer lipid raft localization, independent of the isolation procedure.
Acylated GFPs Are Readily Detergent Soluble
When transfected COS-7 cells were fractionated into S100 and P100 fractions in the absence of detergent, we found >90% of dually acylated chimeric GFPs in the P100 fraction (McCabe and Berthiaume, 1999). To further assess the membrane fractionation properties of the acylated chimeric GFPs analyzed in this study, a second procedure allowing separation of detergent-soluble and -resistant membrane fractions was utilized. With the use of the method of van't Hof and Resh (1997), which allows rapid cellular fractionation and separation of crude S100 and P100 fractions, we compared the solubilization of our chimeric GFPs and caveolin in a variety of detergent conditions. First, transiently transfected COS-7 cells expressing different chimeric GFP cDNAs were extracted with 1% TX-100, 0.1% TX-100, or 60 mM OG at 4°C for 20 min. The constructs analyzed in this experiment included a member of each of the three membrane association signal combination classes (i.e., YesGFP, Src16GFP, and GAP-43GFP). After 20 min of extraction, all chimeric GFPs were found exclusively in the TX-100-soluble fraction (Figure 7A). Thus, the presence of myristate and/or palmitate moieties with or without adjacent polybasic domains had no bearing on the detergent solubility in these experiments. In contrast to these results, the distribution of caveolin between S and resistant P fractions was markedly different. Caveolin was mostly found (∼60%) or largely found (80–90%) in the pellet fractions in the presence of 1% TX-100 or 0.1% TX-100, respectively. In contrast, and in agreement with previous reports, caveolin was readily solubilized in the presence of 60 mM OG (Melkonian et al., 1995). Coomassie-stained PVDF membranes demonstrate the efficiency of detergent extractions (0.1 or 1% TX-100 and 60 mM OG) which led to a near complete solubilization of total cellular proteins (Figure 7B).
Figure 7.
Solubilization of acylated chimeric GFPs expressed in COS-7 cells by 0.1 or 1% TX-100 and 60 mM OG at 4°C. COS-7 cells expressing various acylated and nonacylated GFP chimeras, and GFP alone, were solubilized in detergent at 4°C for 20 min and then separated into soluble (S) and resistant (P) fractions. Aliquots of soluble and resistant fractions were analyzed by immunoblotting with the use of rabbit anti-GFP and anti-caveolin antibodies. (A) Immunoblots show the distribution of the expressed proteins and endogenous caveolin in soluble and resistant fractions in the presence of indicated detergents. Plus signs indicate the type and concentration of detergent used to solubilize the cells. (B) Coomassie-stained PVDFs, corresponding to the immunoblots above, depict distribution of total cellular protein after detergent solubilization.
Acylated GFPs, but Not Caveolin and p62c-yes, Are Rapidly Solubilized from Cell Membranes
To further assess whether the various fatty acylated chimeric GFPs were present or not in DRMs and whether their presence in DRMs would be dependent on the time exposure of membranes to TX-100 detergent, we incubated cells expressing chimeric GFPs in the presence of a 1% TX-100 solution at 4°C for various durations (from 0–20 min). As shown in Figure 8A, at 0 min, essentially all GFP constructs, caveolin, and p62c-yes were found in the TX-100 resistant (“P”) fraction. All constructs tested (YesGFP, Src16GFP, and GAP-43GFP) showed a time-dependent solubilization that was essentially complete by 20 min. All constructs showed similar time-dependent extraction kinetics, suggesting that solubilization of acylated GFPs is very rapid and that all acylated GFPs are found in membrane domains that are TX-100 soluble at 4°C. Full-length caveolin (24 kDa) and Yes PTK (62 kDa) proteins were present on the PVDF membranes at their expected molecular weights, were much more resistant to TX-100 extraction, and were found largely in the pellet fraction. Coomassie-stained PVDFs below the Western blots are shown to demonstrate the reproducibility and efficiency of the extraction/solubilization procedure. Finally, in Figure 8B, the specificity of the three antibodies used in this study is shown with the use of aliquots of a fractionated cell lysate of transiently transfected COS-7 cells expressing YesGFP. The lysate was prepared with the use of 1% TX-100 at 4°C with 20 min of incubation as described above. In additional controls, antibodies were also combined pairwise and unequivocally demonstrated that no cross-reactivity was occurring between the antibodies (McCabe and Berthiaume, unpublished results).
Figure 8.
Solubilization kinetics of acylated GFPs, caveolin, and p62c-yes. COS-7 cells expressing various acylated and nonacylated GFP chimeras were separated into 4°C TX-100-soluble and -resistant fractions by incubating cell monolayers with 1% TX-100 for 0–20 min. The 1% TX-100 fractions containing solubilized total cellular proteins were rapidly removed from the tissue culture dishes and the detergent-resistant matrices solubilized with a 1× lysis buffer solution. These separated fractions were immunoblotted with rabbit anti-GFP (1:2000), anti-caveolin (1:4000), and mouse anti-Yes (1:1000) antibodies. (A) Immunoblots showing relative distributions of acylated GFPs, caveolin, and p62c-yes between TX-100-soluble and -resistant fractions. Markers, right, point to the position of migration of the indicated proteins. Below each immunoblot is a corresponding Coomassie-stained PVDF membrane showing the kinetic distribution of total cellular protein between TX-100-soluble and -resistant fractions. (B) Immunoblot showing the specificity of the three antibodies used in this analysis. YesGFP is being detected in the anti-GFP blot.
DISCUSSION
To investigate the mechanisms of lipidation-dependent subcellular localization, we analyzed the biochemical components and properties of both acylated proteins and acceptor membranes. We show that addition of a dual palmitoylation signal or N-terminal myristoylation signal combined with either a palmitoylation signal or polybasic region onto a GFP reporter protein conferred detergent-soluble localization to membranes enriched in free cholesterol and sphingolipids. Our data suggest that protein fatty acylation of an otherwise cytosolic reporter protein is not sufficient to confer fractionation to detergent-resistant membrane or partitioning to lipid rafts. Likely, protein-protein interactions are required for these processes to occur. Overall, our data are consistent with the possibility that fatty acylated GFPs are only loosely associated with lipid rafts and that multiple types of rafts exist inside the cell, caveolae representing only one of them.
All Myristoylated GFPs containing a Second Membrane Association Signal Colocalize with Cholesterol and Sphingolipids but Not Caveolin
Myristoylated GFPs containing two membrane association signals (myristoylation plus palmitoylation or myristoylation plus polybasic region) localize similarly to a variety of endocytic structures including early endosomes, endosomal recycling compartment, and late endosomes, as well as lysosomes and the plasma membrane, in COS-7 cells. Endosomes are heterogeneous and known to be enriched in cholesterol (Montesano et al., 1981). They also differ widely in structure, shape, lipid composition, function, and pH values (Teter et al., 1998). Consistent with our results, several variably lipidated proteins recently have been found to be associated with cholesterol-rich vesicles (Roy et al., 1999). We also show that a dually palmitoylated (but not myristoylated) GFP, that we previously found associated with the Golgi apparatus and the plasma membrane, colocalized primarily with membranes enriched in cholesterol in the perinuclear Golgi including the TGN compartment as well as the plasma membrane. Thus, we conclude that different combinations of acylation signals confer association with different types of membranes enriched in free cholesterol. This suggests that protein fatty acylation may provide a signal for recruitment or retention into cholesterol-rich membranes.
Cholesterol is a major component of the plasma membrane and endosomes and is enriched along with sphingolipids in DRMs (Brown and London, 1998a; Ilangumaran and Hoessli, 1998). Cholesterol has also been shown to be required for maintenance of caveolae structure and function (Hailstones et al., 1998; Orlandi and Fishman, 1998), endocytic retention/trafficking of glycosylphosphatidyl inositol proteins (Mayor et al., 1998), and clathrin-coated pit budding (Subtil et al., 1999). Of special interest is the fact that free cholesterol content is essential for endocytosis and maintenance of endosomal structure (Subtil et al., 1999). This stringent cholesterol requirement in endosomal biogenesis can certainly explain in part the significant accumulation of acylated GFPs at the plasma membrane and the loss of localization of chimeric GFPs to peripheral endosomal vesicular structures when transfected COS-7 cells are depleted of cholesterol. The absence of colocalization of acylated GFPs in peripheral endosomal vesicles in cholesterol-depleted cells suggest an alteration in recycling of acylated GFPs between the plasma membrane and various intracellular compartments, such as the endocytic recycling compartment or TGN (Kjersti Rodal et al., 1999). This possibility is further strengthened by the fact that active recycling of GM1 is apparently severely reduced in cholesterol-depleted cells, as seen by the absence of intracellular GM1 signal in comparison to untreated cells. The cholesterol content in membranes has been shown to be essential for proper localization of fatty acylated proteins. Cholesterol depletion experiments with MβCD significantly altered the subcellular localization of Fyn, Lck, Lyn, eNOS, and caveolin (Ilangumaran and Hoessli, 1998; Blair et al., 1999; Sheets et al., 1999). Thus, our results are consistent with cholesterol playing a key role in the proper localization of fatty acylated proteins. Also, combinations of myristoylation and palmitoylation or polybasic domain may represent a form of endosomal targeting signal. Because cholesterol is required for endosome formation, the presence of sphingolipids and cholesterol in these endosomal membranes may be incidental, and perhaps some other feature of these membranes are responsible for the targeting of lipidated GFPs to these structures.
The ganglioside GM1 has been reported to be enriched fourfold in caveolae as identified by the colocalization of gold-CTX B subunit with caveolin (Parton, 1994). Consequently, GM1 and caveolin have become common markers for the identification and purification of caveolae (Orlandi and Fishman, 1998). Despite this, the absence of colocalization of acylated GFPs (except for GAP-43GFP) with caveolin and localization of acylated GFPs to GM1-rich membranes suggest that other GM1-containing membranes exist. This conclusion is supported by several reports that suggest that GM1-containing membrane domains are believed to exist distributed randomly in coated pits or in clustered DRM rafts/glycolipid microdomains (Parton, 1994; Orlandi and Fishman, 1998; Sorice et al., 1999). The fact that all acylated GFP chimeras are found in membranes (plasma membrane domains and internal membranes) enriched in GM1 suggests that sphingolipids, in addition to cholesterol, may play a role in proper localization of acylated GFPs. From a mechanistic point of view, our localization data are consistent with biophysical partitioning of acylated GFPs into liquid-ordered membrane domains known to be enriched in cholesterol and sphingolipids (Brown and London, 1998a; Schroeder et al., 1998). Such simple biophysical partitioning of acylated GFPs to specific membranes or even membrane microdomains could represent an efficient means to simplify the sorting of variably lipidated proteins throughout the variety of cellular membranes.
All Myristoylated GFPs containing a Second Membrane Association Signal Do Not Cofractionate with Caveolin-rich Membranes or DRMs
All our myristoylated GFPs containing a second membrane association signal did not colocalize significantly with caveolin and did not cofractionate with caveolin-rich membranes or DRMs prepared by two different isolation procedures. This suggests that combination of myristoylation and palmitoylation or myristoylation and a polybasic domain are neither to confer apparent colocalization with caveolin nor sufficient to allow cofractionation with DRMs. As such, our results imply that our acylated GFPs are found in cholesterol-, sphingolipid-enriched membranes (based on colocalization data) and are TX-100 detergent soluble (based on biochemical fractionation data). These data are also consistent with the fact that only a proportion of cholesterol (26%) and gangliosides (67%) are TX-100 insoluble in MDCK cells (Brown and Rose, 1992). Thus, substantial amounts of these cellular lipids do exist in a detergent-soluble state (Janes et al., 1999).
To further characterize the membrane fractionation properties of our acylated GFPs in comparison to known lipid raft markers, we utilized endpoint and kinetic solubilization studies investigating the fractionation of known lipid rafts/DRM markers in COS cells in the presence of a variety of detergents. Our results showed proper fractionation of Yes PTK and caveolin into lipid rafts, corroborating the previous fractionation results of Melkonian et al. (1995). In contrast, acylated GFPs were readily soluble in all detergent types and conditions tested. However, these latter results appear to be in direct contradiction with those of Galbiati et al. (1999b), who studied the fate of a short myristoylated and palmitoylated 32-amino acid Gαi sequence appended to GFP. With the first 11 amino acids of Gαo and Gαi being identical (our GαoGFP chimera contains 11 amino acids from Gαo) the discrepancy may reside in the extra amino acids appended to GFP. Indeed, from the three-dimensional x-ray structure of several heterotrimeric Gα subunits, it appears that amino acids 9–32 are part of an α-helix (Lambright et al., 1996). Furthermore, Busconi and Denker (1997) demonstrated the importance of amino acids 11–14 of Gαo in membrane binding, with deletion leading to substantial reduction in membrane affinity. They also suggested that the N terminus of Gαo interacts with another protein in the acceptor membrane, independent of βγ binding. Perhaps this α-helix also forms independently when appended to GFP and contributes specific protein-protein interactions involved in colocalization and cofractionation with caveolin.
p62c-yes, which was shown to colocalize with YesGFP (McCabe and Berthiaume, 1999), minimally colocalized with caveolin but did cofractionate with caveolin-rich lipid rafts/DRMs independently of the isolation procedure used to prepare lipid rafts or DRMs (i.e., detergent-free and detergent-based methods). Our results confirmed those of others who showed that p62c-yes is found on recycling endosomes and plasma membrane and when expressed in MDCK cells is found in the TX-100-insoluble fraction (Arreaza et al., 1994; Melkonian et al., 1995; Luton et al., 1999). A minimal explanation for these results is that p62c-yes and YesGFP are both localized in cholesterol-, sphingolipid-enriched membranes of varying detergent solubility and that p62c-yes is localized in DRMs/lipid rafts that are different from those containing caveolin. These results are consistent with the prior demonstration of the existence of different types of DRMs/lipid rafts (Iwabuchi et al., 1998; Orlandi and Fishman, 1998; Hooper, 1999; Waugh et al., 1999). This observation is also consistent with the requirement of protein-protein interactions between p62c-yes and a given acceptor protein as a prerequisite to acquire detergent-resistance/lipid raft localization.
In direct contrast to the results obtained with p62c-yes, dually palmitoylated GAP-43GFP chimera colocalized with caveolin (in the Golgi area but not at the plasma membrane) but did not cofractionate with caveolin-rich DRMs. This may reflect common initial targeting and acylation of caveolin and GAP-43 at the level of the Golgi. Furthermore, caveolin and GAP-43 are known to have Golgi-targeting domains, and palmitoylation-deficient mutants of caveolin and GAP-43 showed negligible association with the Golgi complex. Interestingly, targeting to the plasma membrane is not required for palmitoylation of caveolin and GAP-43. (Liu et al., 1994; Galbiati et al., 1999a; Luetterforst et al., 1999; McLaughlin and Denny, 1999). Our findings support the requirement for protein-protein interactions as a prerequisite for localization to lipid rafts/DRMs, because full-length GAP-43 (but not our shortened GAP-43GFP chimera) was found in DRMs in COS-7 cells and neurons (Arni et al., 1998). In this report, Brown and coworkers found DRM association with a 20-amino acid sequence of GAP-43 appended to β-galactosidase. Because native β-gal is tetrameric, oligomerization of NM20–β-galactosidase would combine eight palmitate chains for membrane association, thereby indirectly promoting DRM association through multiple palmitoylation or aggregation. Also, in support of the protein-protein interaction requirement for caveolae localization are the recent results of Prabhakar et al. (2000). They showed that a nonacylated chimeric eNOS (normally myristoylated and dually palmitoylated) with a transmembrane domain directs eNOS to caveolae, suggesting that acylation is not required for selective targeting of eNOS to caveolae, but rather sequences within the protein specify the caveolar association.
Requirements for Fatty Acylation-dependent Subcellular Localization to Cholesterol-, Sphingolipid-rich Membranes and Lipid Rafts/DRMs
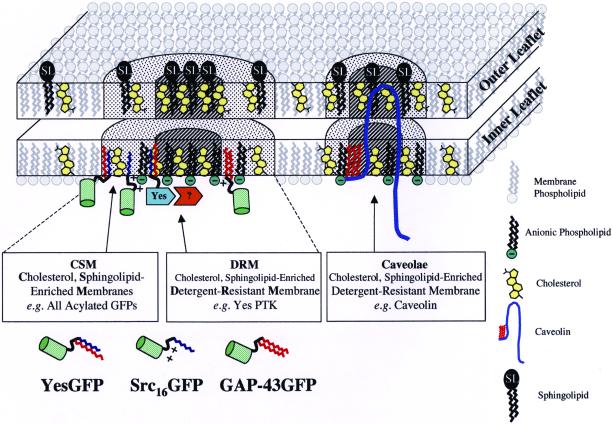
Figure 9 depicts a model integrating the facts that 1) all of our fatty acylated chimeric GFPs were detergent soluble and found in cholesterol-, sphingolipid-enriched membranes (based on our indirect fluorescence microscopic colocalization and TX-100 solubilization/fractionation results); 2) all of our acylated GFPs (except dually palmitoylated GAP-43) did not colocalize with caveolin (based on our indirect immunofluorescence results); 3) none of our acylated GFPs were found in lipid rafts prepared with standard detergent or detergent-free methods; and 4) in addition to proper fatty acylation, protein-protein interactions (e.g., perhaps via Src homology 2 protein-protein interaction or Src homology 3 protein-protein interaction domains) are required for p62c-yes to cofractionate with noncaveolar DRMs (based on the fact that p62c-yes did not colocalize with caveolin in our indirect immunofluorescence experiments but did cofractionate in caveolin enriched DRM/lipid rafts). Our model is similar to those of Ilangumaran and Hoessli (1998) and Madore et al. (1999), who also depict lipidated proteins in membrane domains of varying levels of liquid order state and detergent solubility.
Figure 9.
Model of fatty acylation-dependent membrane localization. The model depicts the localization of acylated GFPs with cholesterol and sphingolipid-enriched membrane microdomains (CSMs, dotted area). All three types of membrane association signal combinations (myristoylation plus palmitoylation: YesGFP; myristoylation plus polybasic region: Src16GFP; and dual palmitoylation plus polybasic region: GAP-43GFP) showed similar localization at the plasma membrane. Acylated full-length Yes PTK is shown in the cholesterol- and sphingolipid-enriched detergent-resistant membrane fraction (DRM, hatched area). Yes PTK is depicted in a DRM separate from DRMs containing caveolin, right (because Yes PTK and caveolin do not colocalize). Protein-protein interactions (represented by association with the unidentified orange protein) are believed to mediate Yes PTK association with the DRM core. For simplicity, lipid rafts present in endosomal or Golgi structures are not illustrated. Models of various lipid raft membrane constituents are shown in the legend at right.
In Figure 9, acylated GFPs, rather than associating with DRM core areas, lie juxtaposed in an annulus rich in cholesterol and sphingolipid and remain TX-100 soluble. This localization reconciles the fact that YesGFP and p62c-yes colocalize by immunofluorescence analysis, and only p62c-yes cofractionates with caveolin-rich DRMs/lipid rafts. Based on our previous observations demonstrating that combined addition of myristoylation and palmitoylation (e.g.,. YesGFP) or myristoylation plus polybasic domain (e.g., Src16GFP) onto GFP conferred similar localization to plasma membrane and endosomal membranes, we included negatively charged phospholipids on the inner leaflet of the plasma membrane (i.e., the outer leaflet of endosomes) in our model. The presence of negatively charged phospholipids in the inner leaflet of the plasma membrane is well documented (Devaux, 1991; Zwaal and Schroit, 1997). Furthermore, it is also consistent with the fact that a significant portion of these negatively charged phospholipids are known to contain two saturated acyl chains (Holub, 1980) and that this phospholipid population has been preferentially found in cholesterol-, sphingolipid-rich lipid rafts or DRM domains (Harder and Simons, 1997; Fridriksson et al., 1999). In addition, endosomal membranes, which can support a liquid-ordered membrane phase (Brown and London, 1998b), are known to be the most net negatively charged of all cellular membranes (Cavenaugh et al., 1996).
Also noted in our kinetic solubilization experiments was the difference in solubilization rates between p62c-yes and caveolin. These differences may reflect differences intrinsic to protein structures but could also be interpreted as p62c-yes and caveolin residing in different DRM membrane environments. This possibility would further confirm the absence of colocalization between the two proteins in our indirect immunofluorescence experiments. Thus, we depicted at least two types of DRMs or lipid rafts in our model. In various reports, several acylated signaling proteins were shown to be enriched in DRM fractions, but in those studies whether these signaling proteins actually colocalized with caveolin or not was not assessed (Arreaza et al., 1994; van't Hof and Resh, 1997; Arni et al., 1998; Galbiati et al., 1999b; Melkonian et al., 1999).
According to our data and those of others, we believe that N-terminal acylation acts primarily as a sensor/retention signal for cholesterol-, sphingolipid-enriched membranes. In a way, our results are in agreement with the kinetic bilayer-trapping mechanism of Shahinian and Silvius (1995) in which myristoylated GFPs would sample various cellular membranes rich in saturated lipids, sphingolipids, and cholesterol. Then, the presence of a polybasic domain adjacent to the myristate, through electrostatic attractions, would concentrate that myristoylated protein in membrane domains rich in sphingolipids, cholesterol, and saturated anionic phospholipids of endosomes and plasma membrane. Alternatively, myristoylated proteins could sample membranes until reaching a proper membrane environment containing a putative palmitoyltransferase enzyme. A productive interaction between the two could lead to the addition of a second saturated fatty acid on the myristoylated protein, which would further enhance its partitioning into liquid-ordered cholesterol-, sphingolipid-enriched membrane domains. Such partitioning would thus restrict the number of membranes containing dually acylated proteins, facilitate their intracellular sorting, and stimulate potential protein-protein interactions with other membrane bound proteins. On establishment of favorable protein-protein interactions, the complex could then move from the periphery (annulus) of cholesterol-, sphingolipid-enriched membrane domains to a core TX-100-insoluble membrane domain. We make a critical addition to the kinetic bilayer-trapping mechanism by including protein-protein interaction modules as part of the membrane “trapping” mechanism. The importance of protein-protein interactions in localization of proteins to DRMs/lipid rafts is also supported by work on G protein subunits, PSD-95, eNOS, and linker for activation of T cells (Busconi and Denker, 1997; Craven et al., 1999; Prabhakar et al., 2000; Fishburn et al., 2000; Harder and Kuhn, 2000). In these articles, the final destination or localization of a given fatty acylated protein was shown to be further altered or modified by protein modules (caveolin-binding domain or CBD, Src homology 2 protein-protein interaction domain, Src homology 3 protein-protein interaction domain, PDZ, βγ subunit-binding region, etc.) to combinatorially determine the ultimate steady-state destination of that protein.
ACKNOWLEDGMENTS
We thank E. Posse de Chaves for critical reading of the manuscript and X.J. Sun (Cross Cancer Institute) for help with the confocal analyses. The monoclonal antibodies H4A3 and H4B4 developed by J.T. August and J.E.K. Hildreth were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biological Sciences (Iowa City, IA). J. McCabe acknowledges Ph.D. and M.D./Ph.D. studentships from the Canadian Institutes of Health Research (CIHR) and Alberta Heritage Foundation for Medical Research (AHFMR), respectively. L.G. Berthiaume is a CIHR and AHFMR scholar. This study was supported by a CIHR grant.
Abbreviations used:
- CI-MPR
cation-independent mannose 6-phosphate receptor
- CTX
cholera toxin B subunit
- DRM
detergent-resistant membrane
- EEA1
early endosome antigen-1
- eNOS
endothelial nitric oxide synthase
- FITC
fluorescein isothiocyanate
- FITC-CTX
FITC-conjugated cholera toxin B subunit
- GFP
green fluorescent protein
- Ig
immunoglobulin
- LAMP
lysosome-associated membrane protein
- MβCD
methyl β-cyclodextrin
- MβCD
methyl β-cyclodextrin complex
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NDS
normal donkey serum
- OG
n-octyl β–d-glucopyranoside (n-octyl glucoside)
- PBS
phosphate-buffered saline
- PTK
protein tyrosine kinase
- PVDF
polyvinylidene difluoride
- TGN
trans-Golgi network
- TR
Texas Red
- TX-100
Triton X-100
REFERENCES
- Abrami L, van der Goot FG. Plasma membrane microdomains act as concentration platforms to facilitate intoxication by aerolysin. J Cell Biol. 1999;147:175–184. doi: 10.1083/jcb.147.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RGW. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- Andersson S, Davis DL, Dahlback H, Jornvall H, Russell DW. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989;264:8222–8229. [PubMed] [Google Scholar]
- Arni S, Keilbaugh SA, Ostermeyer AG, Brown DA. Association of GAP-43 with detergent-resistant membranes requires two palmitoylated cysteine residues. J Biol Chem. 1998;273:28478–28485. doi: 10.1074/jbc.273.43.28478. [DOI] [PubMed] [Google Scholar]
- Arreaza G, Melkonian KA, LaFevre-Bernt M, Brown DA. Triton X-100-resistant membrane complexes from cultured kidney epithelial cells contain the Src family protein tyrosine kinase p62yes. J Biol Chem. 1994;269:19123–19127. [PubMed] [Google Scholar]
- Barr VA, Phillips SA, Taylor SI, Renfrew Haft C. Overexpression of a novel sorting nexin, SNX15, affects endosome morphology and protein trafficking. Traffic. 2000;1:904–916. doi: 10.1034/j.1600-0854.2000.011109.x. [DOI] [PubMed] [Google Scholar]
- Blair A, Shaul PW, Yuhunna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem. 1999;274:32512–32519. doi: 10.1074/jbc.274.45.32512. [DOI] [PubMed] [Google Scholar]
- Brown D, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998a;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Structure and origin of ordered lipid domains in biological membranes. J Membr Biol. 1998b;164:103–114. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- Busconi L, Denker BM. Analysis of the N-terminal binding domain of Goα. Biochem J. 1997;328:23–31. doi: 10.1042/bj3280023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JD, Blanchette-Mackie J, Goldin E, O'Neill RR, Carstea G, Roff CF, Patterson MC, Patel S, Comly ME, Cooney A, Vanier MT, Brady RO, Pentchev PG. Progesterone blocks cholesterol translocation from lysosomes. J Biol Chem. 1992;267:23797–23805. [PubMed] [Google Scholar]
- Cadigan KM, Spillane DM, Chang TY. Isolation and characterization of Chinese hamster ovary cell mutants defective in intracellular low density lipoprotein-cholesterol trafficking. J Cell Biol. 1990;110:295–308. doi: 10.1083/jcb.110.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenaugh MM, Whitney JA, Carroll K, Zhang C, Boman AL, Rosenwald AG, Mellman I, Kahn RA. Intracellular distribution of Arf proteins in mammalian cells. J Biol Chem. 1996;271:21767–21774. doi: 10.1074/jbc.271.36.21767. [DOI] [PubMed] [Google Scholar]
- Choy E, Chiu VK, Silletti J, Feoktistov M, Morimoto T, Michaelson D, Ivanov IE, Philips MR. Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell. 1999;98:69–80. doi: 10.1016/S0092-8674(00)80607-8. [DOI] [PubMed] [Google Scholar]
- Conrad PA, Smart EJ, Ying Y, Anderson RGW, Bloom GS. Caveolin cycles between plasma membrane caveolae and the Golgi complex by microtubule-dependent and microtubule-independent steps. J Cell Biol. 1995;131:1421–1433. doi: 10.1083/jcb.131.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven SE, El-Husseini AE, Bredt DS. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron. 1999;22:497–509. doi: 10.1016/s0896-6273(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Devaux PF. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991;30:1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- Downey GP, Botelho RJ, Butler JR, Moltyaner Y, Chien P, Schrieber AD, Grinstein S. Phagosomal maturation, acidificatiion, and inhibition of bacterial growth in nonphagocytic cells transfected with FcγRIIA receptors. J Biol Chem. 1999;274:28436–28444. doi: 10.1074/jbc.274.40.28436. [DOI] [PubMed] [Google Scholar]
- Dunphy JT, Linder ME. Signaling functions of protein palmitoylation. Biochim Biophys Acta. 1998;1436:245–261. doi: 10.1016/s0005-2760(98)00130-1. [DOI] [PubMed] [Google Scholar]
- Fishburn CS, Pollitt SK, Bourne HR. Localization of a peripheral membrane protein: Gβγ targets Gαz. Proc Natl Acad Sci USA. 2000;97:1085–1090. doi: 10.1073/pnas.97.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman Emmerson, C., Brown, G.K., and Poulton, J. (2001). Synthesis of mitochondrial DNA in permeabilized human culture cells. Nucleic Acids Res. 29,e1. [DOI] [PMC free article] [PubMed]
- Fridriksson EK, Shipkova PA, Sheets ED, Holowka D, Baird B, McLafferty FW. Quantitative analysis of phospholipids in functionally important membrane domains from RBL-2H3 mast cells using tandem high-resolution mass spectrometry. Biochemistry. 1999;38:8056–8063. doi: 10.1021/bi9828324. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Meani D, Milligan G, Lublin DM, Lisanti MP, Parenti M. The dually acylated NH2-terminal domain of Gi1α is sufficient to target a green fluorescent protein reporter to caveolin-enriched plasma membrane domains. J Biol Chem. 1999a;274:5843–5850. doi: 10.1074/jbc.274.9.5843. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Minetti C, Chu JB, Lisanti MP. Phenotypic behavior of caveolin-3 mutations that cause autosomal dominant limb girdle muscular dystrophy (LGMD-1C): retention of LGMD-1C caveolin-3 mutants within the Golgi complex. J Biol Chem. 1999b;274:25632–25641. doi: 10.1074/jbc.274.36.25632. [DOI] [PubMed] [Google Scholar]
- Hailstones D, Steer LS, Parton RG, Stanley KK. Regulation of caveolin and caveolae by cholesterol in MDCK cells. J Lipid Res. 1998;39:369–379. [PubMed] [Google Scholar]
- Harder T, Kuhn M. Selective accumulation of raft-associated membrane protein LAT in T cell receptor signaling assemblies. J Cell Biol. 2000;151:199–207. doi: 10.1083/jcb.151.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder T, Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- Holub BJ. The biosynthesis of phosphatidylserines by acylation of 1-acyl-sn-glycero-3-phosphoserine in rat liver. Biochim Biophys Acta. 1980;618:255–262. doi: 10.1016/0005-2760(80)90031-4. [DOI] [PubMed] [Google Scholar]
- Hooper NM. Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae. Mol Membr Biol. 1999;16:145–156. doi: 10.1080/096876899294607. [DOI] [PubMed] [Google Scholar]
- Ilangumaran S, Hoessli DC. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem J. 1998;335:433–440. doi: 10.1042/bj3350433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi K, Handa K, Hakomori S. Separation of “glycosphingolipid signaling domain” from caveolin-containing membrane fraction in mouse melanoma B16 cells and its role in cell adhesion coupled with signaling. J Biol Chem. 1998;273:33766–33773. doi: 10.1074/jbc.273.50.33766. [DOI] [PubMed] [Google Scholar]
- Jacobson K, Dietrich C. Looking at lipid rafts? Trends Cell Biol. 1999;9:87–91. doi: 10.1016/s0962-8924(98)01495-0. [DOI] [PubMed] [Google Scholar]
- Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Cornell RB. Amphitropic proteins: regulation by reversible membrane interactions. Mol Membr Biol. 1999;16:217–235. doi: 10.1080/096876899294544. [DOI] [PubMed] [Google Scholar]
- Joliot A, Trembleau A, Raposo G, Calvet S, Volovitch M, Prochiantz A. Association of engrailed homeoproteins with vesicles presenting caveolae-like properties. Development. 1997;124:1865–1875. doi: 10.1242/dev.124.10.1865. [DOI] [PubMed] [Google Scholar]
- Kjersti Rodal S, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell. 1999;10:961–974. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia TV, Parton RG. Membrane microdomains and caveolae. Curr Opin Cell Biol. 1999;11:424–431. doi: 10.1016/s0955-0674(99)80061-1. [DOI] [PubMed] [Google Scholar]
- Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB. The 2.0 Å crystal structure of a heterotrimeric G protein. Nature. 1996;379:311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fisher DA, Storm DR. Intracellular sorting of neuromodulin (GAP-43) mutants modified in the membrane targeting domain. J Neurosci. 1994;14:5807–5817. doi: 10.1523/JNEUROSCI.14-10-05807.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetterforst R, Stang E, Zorzi N, Carozzi A, Way M, Parton RG. Molecular characterization of caveolin association with the Golgi complex: identification of a cis-Golgi targeting domain in the caveolin molecule. J Cell Biol. 1999;145:1443–1459. doi: 10.1083/jcb.145.7.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luton F, Verges M, Vaerman J, Sudol M, Mostov KE. The SRC family protein tyrosine kinase p62yes controls polymeric IgA transcytosis in vivo. Mol Cell. 1999;4:627–632. doi: 10.1016/s1097-2765(00)80213-0. [DOI] [PubMed] [Google Scholar]
- Madore N, Smith KL, Graham CH, Jen A, Brady K, Hall S, Morris R. Functionally different GPI proteins are organized in different domains on the neuronal surface. EMBO J. 1999;18:6917–6926. doi: 10.1093/emboj/18.24.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee T, Marshall C. New insights into the interaction of Ras with the plasma membrane. Cell. 1999;98:9–12. doi: 10.1016/S0092-8674(00)80601-7. [DOI] [PubMed] [Google Scholar]
- Mayor S, Sabharanjak S, Maxfield FR. Cholesterol-dependent retention of GPI-anchored proteins in endosomes. EMBO J. 1998;17:4626–4638. doi: 10.1093/emboj/17.16.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe JB, Berthiaume LG. Functional roles for fatty acylated amino-terminal domains in subcellular localization. Mol Biol Cell. 1999;10:3771–3786. doi: 10.1091/mbc.10.11.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin RE, Denny JB. Palmitoylation of GAP-43 by the ER-Golgi intermediate compartment and Golgi apparatus. Biochim Biophys Acta. 1999;1451:82–92. doi: 10.1016/s0167-4889(99)00074-9. [DOI] [PubMed] [Google Scholar]
- Melkonian KA, Chu T, Tortorella LB, Brown DA. Characterization of proteins in detergent-resistant membrane complexes from Madin-Darby canine kidney epithelial cells. Biochemistry. 1995;34:16161–16170. doi: 10.1021/bi00049a031. [DOI] [PubMed] [Google Scholar]
- Melkonian KA, Ostermeyer AG, Chen JZ, Roth MG, Brown DA. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. J Biol Chem. 1999;274:3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- Michaely PA, Mineo C, Ying Y, Anderson RGW. Polarized distribution of endogenous Rac1 and RhoA at the cell surface. J Biol Chem. 1999;274:21430–21436. doi: 10.1074/jbc.274.30.21430. [DOI] [PubMed] [Google Scholar]
- Mills IG, Urbe S, Clague MJ. Relationships bewteen EEA1 binding partners and their role in endosome fusion. J Cell Sci. 2001;114:1959–1965. doi: 10.1242/jcs.114.10.1959. [DOI] [PubMed] [Google Scholar]
- Moffett S, Brown DA, Linder ME. Lipid-dependent targeting of G proteins into rafts. J Biol Chem. 2000;275:2191–2198. doi: 10.1074/jbc.275.3.2191. [DOI] [PubMed] [Google Scholar]
- Montesano R, Vassalli P, Orci L. Structural heterogeneity of endocytic membranes in macrophages as revealed by the cholesterol probe, filipin. J Cell Sci. 1981;51:95–107. doi: 10.1242/jcs.51.1.95. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Zha X, Tabas I, Maxfield FR. Cholesterol distribution in living cells: fluorescence imaging using dehydroergosterol as a fluorescent cholesterol analog. Biophys J. 1998;75:1915–1925. doi: 10.1016/S0006-3495(98)77632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CP, Stephany DA, Winkler DF, Hoeg JM, Demosky SJ, Wunderlich JR. Filipin as a flow microfluorometry probe for cellular cholesterol. Cytometry. 1984;5:42–54. doi: 10.1002/cyto.990050108. [DOI] [PubMed] [Google Scholar]
- Murata M, Peränen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols BJ, Kenworthy AK, Polishchuk RS, Lodge R, Roberts TH, Hirschberg K, Phair RD, Lippincott-Schwartz J. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J Cell Biol. 2001;153:529–541. doi: 10.1083/jcb.153.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh P, Schnitzer JE. Immunoisolation of caveolae with high affinity antibody binding to the oligomeric cage. J Biol Chem. 1999;274:23144–23154. doi: 10.1074/jbc.274.33.23144. [DOI] [PubMed] [Google Scholar]
- Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol. 1998;141:905–915. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin ET, Turner AJ, Hooper NM. Isolation and characterization of two distinct low-density, Triton-insoluble, complexes from porcine lung membranes. Biochem J. 1996;319:887–896. doi: 10.1042/bj3190887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG. Ultrastructural localization of gangliosides: GM1 is concentrated in caveolae. J Histochem Cytochem. 1994;42:155–166. doi: 10.1177/42.2.8288861. [DOI] [PubMed] [Google Scholar]
- Parton RG, Simons K. Digging into caveolae. Science. 1995;269:1398–1399. doi: 10.1126/science.7660120. [DOI] [PubMed] [Google Scholar]
- Pines J. GFP in mammalian cells. Trends Genet. 1995;11:326–327. doi: 10.1016/s0168-9525(00)89092-7. [DOI] [PubMed] [Google Scholar]
- Prabhakar P, Cheng V, Michel T. A chimeric transmembrane domain directs endothelial nitric-oxide synthase palmitoylation and targeting to plasmalemmal caveolae. J Biol Chem. 2000;275:19416–19421. doi: 10.1074/jbc.M001952200. [DOI] [PubMed] [Google Scholar]
- Racchi M, Baetta R, Salvietti N, Ianna P, Franceschini G, Paoletti R, Fumagalli R, Govoni S, Trabucchi M, Soma M. Secretory processing of amyloid precursor protein is inhibited by increase in cellular cholesterol content. Biochem J. 1997;322:893–898. doi: 10.1042/bj3220893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh MD. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- Roy S, Luetterforst R, Harding A, Apolloni A, Etheridge M, Stang E, Rolls B, Hancock JF, Parton RG. Dominant-negative caveolin inhibits H-Ras function by disrupting cholesterol-rich plasma membrane domains. Nat Cell Biol. 1999;1:98–105. doi: 10.1038/10067. [DOI] [PubMed] [Google Scholar]
- Schroeder R, London E, Brown D. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc Natl Acad Sci USA. 1994;91:12130–12134. doi: 10.1073/pnas.91.25.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder RJ, Ahmed SN, Zhu Y, London E, Brown DA. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J Biol Chem. 1998;273:1150–1157. doi: 10.1074/jbc.273.2.1150. [DOI] [PubMed] [Google Scholar]
- Shahinian S, Silvius JR. Doubly-lipid-modified sequence motifs exhibit long-lived anchorage to lipid bilayer membranes. Biochemistry. 1995;34:3813–3822. doi: 10.1021/bi00011a039. [DOI] [PubMed] [Google Scholar]
- Sheets ED, Holowka D, Baird B. Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of FcεRI and their association with detergent-resistant membranes. J Cell Biol. 1999;145:877–887. doi: 10.1083/jcb.145.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shisheva A, Rusin B, Ikonomov OC, DeMarco C, Sbrissa D. Localization and insulin-regulated relocation of phosphoinositide 5-kinase PIKfyve in 3T3–L1 adipocytes. J Biol Chem. 2001;276:11859–11869. doi: 10.1074/jbc.M008437200. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. J Biol Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- Song KS, Sargiacomo M, Galbiati F, Parenti M, Lisanti MP. Targeting of a G alpha subunit (Gi1 alpha) and c-Src tyrosine kinase to caveolae membranes: clarifying the role of N-myristoylation. Cell Mol Biol. 1997;43:293–303. [PubMed] [Google Scholar]
- Sorice M, Garofalo T, Misasi R, Dolo V, Lucania G, Sansolini T, Parolini I, Sargiacomo M, Torrisi MR, Pavan A. Glycosphingolipid domains on cell plasma membrane. Biosci Rep. 1999;19:197–208. doi: 10.1023/a:1020277820120. [DOI] [PubMed] [Google Scholar]
- Sorkina T, Bild A, Tebar F, Sorkin A. Clathrin, adaptors, and eps15 in endosomes containing activated epidermal growth factor receptors. J Cell Sci. 1999;112:317–327. doi: 10.1242/jcs.112.3.317. [DOI] [PubMed] [Google Scholar]
- Stulnig TM, Berger M, Sigmund T, Raederstorff D, Stockinger H, Waldhausl W. Polyunsaturated fatty acids inhibit T cell signal transduction by modification of detergent-insoluble membrane domains. J Cell Biol. 1998;143:637–644. doi: 10.1083/jcb.143.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci USA. 1999;96:6775–6780. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter K, Chandy G, Quinones B, Pereyra K, Machen T, Moore HH. Cellubrevin-targeted fluorescence uncovers heterogeneity in the recycling endosomes. J Biol Chem. 1998;273:9625–9633. doi: 10.1074/jbc.273.31.19625. [DOI] [PubMed] [Google Scholar]
- Uittenbogaard A, Ying Y, Smart EJ. Characterization of a cytosolic heat-shock protein-caveolin chaperone complex. J Biol Chem. 1998;273:6525–6532. doi: 10.1074/jbc.273.11.6525. [DOI] [PubMed] [Google Scholar]
- Vandenbulcke F, Nouel D, Vincent JP, Mazella J, Beaudet A. Ligand-induced internalization of neurotensin in transfected COS-7 cells: differential intracellular trafficking of ligand and receptor. J Cell Sci. 2000;113:2963–2975. doi: 10.1242/jcs.113.17.2963. [DOI] [PubMed] [Google Scholar]
- van't Hof W, Resh MD. Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J Cell Biol. 1997;136:1023–1035. doi: 10.1083/jcb.136.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- Waugh MG, Lawson D, Hsuan JJ. Epidermal growth factor receptor activation is localized within low-buoyant density, non-caveolar membrane domains. Biochem J. 1999;337:591–597. [PMC free article] [PubMed] [Google Scholar]
- Wolven A, Okamura H, Rosenblatt Y, Resh MD. Palmitoylation of p59fyn is reversible and sufficient for plasma membrane association. Mol Biol Cell. 1997;8:1159–1173. doi: 10.1091/mbc.8.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaal RFA, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]