Abstract
Patients with schizophrenia show whole brain and cortical gray matter (GM) volume reductions which are progressive early in their illness. Microglia, the resident immune cells in the CNS, phagocytose neurons and synapses. Some post mortem and in vivo studies in schizophrenia show evidence for elevated microglial activation compared to matched controls. However, it is currently unclear how these results relate to changes in cortical structure.
Methods
Fourteen patients with schizophrenia and 14 ultra high risk for psychosis (UHR) subjects alongside two groups of age and genotype matched healthy controls received [11C]PBR28 PET scans to index TSPO expression, a marker of microglial activation and a 3T MRI scan. We investigated the relationship between the volume changes of cortical regions and microglial activation in cortical GM (as indexed by [11C]PBR28 distribution volume ratio (DVR).
Results
The total cortical GM volume was significantly lower in SCZ than the controls [mean (SD)/cm3: SCZ=448.83 (39.2) and controls=499.6 (59.2) (p=0.02) but not in UHR (mean (SD)=503.06 (57.9) and controls=524.46 (45.3) p=0.3). Regression model fitted the total cortical GM DVR values with the cortical regional volumes in SCZ (r=0.81; p<0.001) and in UHR (r=0.63; p=0.02). We found a significant negative correlation between the TSPO signal and total cortical GM volume in SCZ with the highest absolute correlation coefficient in the right superior-parietal cortex (r=-0.72; p=0.006).
Conclusions
These findings suggest that microglial activity is related to the altered cortical volume seen in schizophrenia. Longitudinal investigations are required to determine whether microglial activation leads to cortical gray matter loss.
Keywords: Schizophrenia, microglia, brain volume, PET, gray matter, psychosis
Introduction
Patients with schizophrenia have been consistently shown to have reduced whole brain and cortical gray matter volume relative to matched controls (Ellison-Wright et al., 2008; Haijma et al., 2013). Moreover, longitudinal brain morphometric studies show there are progressive reductions in gray and white matter volume in schizophrenia (Andreasen et al., 2011; Kahn and Sommer, 2015; van Haren et al., 2008). Similarly people at ultra high risk for psychosis (UHR), have reduced cortical and gray matter volumes (Dazzan et al., 2012), and demonstrate evidence for progressive loss during the transition to psychosis (Cannon et al., 2015; Pantelis et al., 2003; Sun et al., 2009; Ziermans et al., 2012). In contrast, elevations in white matter volume have been observed in schizophrenia, although less consistently (Walterfang et al., 2008; Witthaus et al., 2008). It is interesting to note that the change over time in cortical volume has been found to be related to change in symptoms and a transition from at risk symptoms to first episode psychosis (Borgwardt et al., 2007b). Baseline volumes have also been shown to act as a predictive marker for transition (Borgwardt et al., 2007a).
Though the exact pathophysiology of the brain morphometric changes in schizophrenia is not clear, immune dysregulation has been suggested as an underlying mechanism (Bergink et al., 2014; Fillman et al., 2016; Kirkpatrick and Miller, 2013; Laskaris et al., 2016). Supporting this, experimental animal studies of maternal immune activation show altered fetal brain development and behavioural and brain histopathological changes in offspring (Smith et al., 2007). Elevated levels of pro-inflammatory cytokines have been associated with the reductions in gray matter volume in patients with schizophrenia (Meisenzahl EM, 2001), and loss of gray matter in frontal cortex was associated with greater levels of pro-inflammatory cytokines in people at risk of psychosis who transitioned to psychosis (Cannon et al., 2015). Microglia, the brain’s resident immune cells, have a major role in synaptic pruning and phagocytosis within the central nervous system (Kreutzberg, 1996; Paolicelli et al., 2011). They are both activated by pro-inflammatory cytokines and release them (Hanisch, 2002; Kreutzberg, 1996). A recent rodent model based on immune loci identified in the largest genome-wide association study in schizophrenia to date showed that these genetic alterations lead to altered microglial pruning of synapses (Sekar et al., 2016). Moreover, post mortem studies show elevated markers of inflammation (Fillman et al., 2013; Foster et al., 2006; van Kesteren et al., 2017; Volk et al., 2015) and elevated microglial cell density (with a hypertrophic morphology) in the brains of schizophrenia patients compared with healthy controls (Bayer et al., 1999), particularly in the frontal and temporal lobes (Radewicz, 2000; van Kesteren et al., 2017). A recent systematic review and meta-analysis of post mortem brain studies in schizophrenia found significant increase in the density of microglia in patients compared to healthy controls (van Kesteren et al., 2017) but another review did not find any consistent difference in microglia markers in schizophrenia (Trépanier et al., 2016). Microglial activation might thus disrupt synaptic pruning to result in the gray matter volume loss seen in schizophrenia (Laskaris et al., 2016). Supporting this, a recent post-mortem study has shown that greater inflammatory brain markers are associated with smaller brain volumes in schizophrenia (Zhang et al., 2016).
Microglia express the 18kDa translocator specific protein (TSPO), a mitochondrial membrane protein, when they become activated, and can be indexed by PET tracers such as [11C]PBR28 that selectively bind to it (Brown et al., 2007; Owen et al., 2014; Owen et al., 2011). Thus [11C]PBR28 is an in vivo biomarker of microglial activation (Abourbeh et al., 2012; Dickens et al., 2014; Karlstetter et al., 2014; Lartey et al., 2014; Mattner et al., 2013; Rupprecht et al., 2010). We have recently reported increased [11C]PBR28 distribution volume ratio (DVR) in cortical gray matter in medicated patients with schizophrenia as well as UHR subjects when compared to healthy controls (Bloomfield et al., 2016b) using positron emission tomography (PET) imaging. This finding is consistent with some (Doorduin et al., 2009b; van Berckel et al., 2008), but not all PET studies, which either have reported no change (Collste et al., 2017; Coughlin et al., 2016; Hafizi et al., 2017; Holmes et al., 2016; Kenk et al., 2015; van der Doef et al., 2016) or a decrease in volume of distribution (VT) (Collste et al., 2017) in patients with psychosis or schizophrenia compared to healthy controls, suggesting there may be clinical or methodological differences between studies (Turkheimer et al., 2015b).
Microglial activation has been long associated with diaschisis: e.g. the phenomenon whereby local brain changes lead to functional alterations in areas distant from but connected to the initial area of damage (von Monakow in 1906)(Carrera and Tononi, 2014). As a result of diaschisis, secondary changes in the thalamus, substantia nigra pars reticulata, hippocampus and spinal cord have been well reported after focal ischemic or excitotoxic lesions of the cortex and/or striatum, and these are associated with inflammatory changes characterized by activation of microglia and astrocytes (Block et al., 2005). Notably, the facial nerve lesion model that Georg Kreutzberg introduced as the stereotypical preparation to study microglial response to injury in tissue with an intact blood-brain barrier, demonstrates microglial activation as a result of secondary injury (Blinzinger and Kreutzberg, 1968). PET imaging using [11C]-(R)-PK11195 has reported secondary thalamic and hippocampal increases in [11C]-(R)-PK11195 after stroke, traumatic brain injury, neurodegeneration and aging both in animal models and humans (Arlicot et al., 2010; Cagnin et al., 2001; Holmberg et al., 2009; Myers et al., 1991; Ouchi et al., 2005; Pappata et al., 2000; Ramlackhansingh et al., 2011; Schuitemaker et al., 2012a). The specificity of these findings has been elegantly demonstrated by Radlinska et al. (Radlinska et al., 2009) who showed [11C]-(R)-PK11195 increases in the spinal tract of patients with anterograde infarct as determined by diffusion tensor imaging. Notably, secondary microglia activation does not seem limited to anterograde and retrograde projections. Banati et al. (Banati et al., 2001) reported a trans-synaptic increase in [11C]-(R)-PK11195 binding in the thalamus of a male patient with limb denervation. Subsequently, Gerhard et al.(Gerhard et al., 2005) reported [11C]-(R)-PK11195 increases in the ipsilateral thalamic nuclei of six stroke patients. A meta-analysis of post-mortem studies of microglia in schizophrenia indicate that alterations are seen across a number of brain regions (van Kesteren et al., 2017), and there is no clear evidence for regional specificity of microglial or TSPO changes, albeit relatively few regions have been investigated and studies are probably underpowered to detect regional differences (Bloomfield et al., 2016b; Collste et al., 2017; Coughlin et al., 2016; Doorduin et al., 2009b; Hafizi et al., 2017; Holmes et al., 2016; Kenk et al., 2015; van Berckel et al., 2008; van der Doef et al., 2016; van Kesteren et al., 2017). Our prior [11C]PBR28 TSPO study found an alteration in cortical gray matter throughout the brains of patients with schizophrenia (Bloomfield et al., 2016b). Given the above, we predicted that alterations in microglial in schizophrenia would be linked to the generalized structural cortical reductions seen in schizophrenia (Ellison-Wright and Bullmore, 2010). In view of this, we modeled [11C]PBR28 TSPO alterations in schizophrenia with a multivariate statistical model to capture the cross-correlation between alterations in the TSPO measure and structural changes across the brain. As this involved a large number of regions, we used an elastic net approach because this controls for the multiple potential cross-correlations. To our knowledge, to-date, no studies that have investigated the link between microglial activation with brain volumetric changes in vivo in schizophrenia and subjects at risk of psychosis. Our a priori hypothesis was that microglial activity would inversely correlate with the total gray matter volume in schizophrenia patients and UHR subjects (increased [11C]PBR28 in patients with more gray matter volume deficits).
Methods
Subjects
Fourteen subjects meeting UHR criteria, as assessed on the comprehensive assessment of the at risk mental state (CAARMS) (Yung et al., 2005), were recruited from a London mental health clinic (Mean age ± SD: 24.3 ± 5.40; (M:F=7:7)). Fourteen subjects with schizophrenia (Mean age ± SD: 47.0 ± 9.31; (M:F=12:2)) were recruited from London mental health centres (South London and Maudsley NHS Foundation Trust). A pool of 22 healthy control subjects recruited through newspaper and poster adverts were used to provide two groups (n=14 and n=14) of age and genotype matched subjects for the two experimental cohorts (Table 1). The patients and controls were from the same study that was recently published(Bloomfield et al., 2016b). The study was approved by the local research ethics committee and was conducted in accordance with the Declaration of Helsinki. After complete description of the study to the subjects, written informed consent was obtained.
Table 1. Brain morphometric volume differences (cm3).
| SCZ Mean (SD) N=14 |
CTRL Mean (SD) N=14 |
P value FDR 0.05 |
UHR Mean (SD) N=14 |
CTRL Mean (SD) N=14 |
P value FDR 0.05 |
|
|---|---|---|---|---|---|---|
| Total Cortical GM | 448.83 (39.19) |
499.57 (59.21) |
0.02* | 503.06 (57.94) |
524.46 (45.27) |
0.30 |
| Frontal Cortical GM | 129.44 (11.84) |
138.62 (18.37) |
0.15 | 143.75 (16.06) |
148.67 (13.18) |
0.40 |
| Temporal Cortical GM | 101.32 (9.10) |
115.97 (13.94) |
0.01* | 116.068 (15.138) |
121.59 (12.80) |
0.32 |
| Brain regions significant at FDR <0.05 in whole brain volume analysis | ||||||
| Left cuneus GM | 2.80 (0.37) |
3.51 (0.45) |
0.0003 | 3.16 (0.40) |
3.58 (0.31) |
NS |
| Right superior parietal cortex GM | 12.22 (1.28) |
13.97 (1.01) |
0.001 | 13.81 (.40) |
14.36 (1.41) |
NS |
| Right transverse temporal cortex GM | 0.82 (0.16) |
1.07 (0.16)) |
0.001 | 1.016.9 (0.16) |
1.12 (.21) |
NS |
GM=gray matter; FDR=false discovery rate; SCZ=schizophrenia; CTRL=control
All subjects met the following inclusion criteria:
Aged 18+;
No significant physical or psychiatric health contraindications on assessment and medical examination by a trained physician.
Subjects were then screened based on the following exclusion criteria;
Exposure to any somatic medications, including anti-inflammatory medications, in the last 1 month.
History of substance abuse/dependence as determined by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) (SCID) (Spitzer et al., 1992).
Benzodiazepine use, whether prescribed or not (Rao and Butterworth, 1997). One subject was excluded due to a positive urine drug screening result for benzodiazepines on the scan day.
History of head injury resulting in unconsciousness, or any physical medical condition associated with inflammation.
At the time of screening, subjects were tested for TSPO genotype and subjects with a low affinity genotype were excluded as the PET radiotracer shows negligible specific binding(Owen et al., 2011).
In UHR and control subjects: antipsychotic medication exposure.
Significant prior exposure to radiation
Pregnancy or breast feeding.
Healthy control subjects with a personal history of a psychiatric disorder or a first degree relative with schizophrenia or a psychotic illness were excluded.
PET Imaging
All subjects then received a bolus injection of [11C]PBR28 (mean Mbq activity ±SD: 325.31 ± 27.03) followed by a 90-minute emission scan using a Siemens Biograph™ TruePoint™ PET-CT scanner (Siemens Medical Systems, Germany). A computer tomography (CT) scan was also acquired for attenuation and scatter correction. Images were reconstructed using filtered back projection and binned into 26 frames of increasing time length (durations: 8 x 15 s, 3 x 1 min, 5 x 2 min, 5 x 5 min, 5 x 10 min). During the PET acquisition, arterial blood data were sampled via the radial artery using a combined automatic-manual approach. A continuous (one sample per second) sampling system (ABSS Allogg, Mariefred, Sweden) measured whole blood activity for the first 15 minutes of each scan. Discrete blood samples manually withdrawn at 5, 10, 15, 20, 25, 30, 40, 50, 60, 70, 80, 90 minutes, were centrifuged to determine the plasma over blood activity ratio and filtered using HPLC to calculate the plasma fraction of authentic tracer free of metabolites (Tonietto et al., 2015; Tonietto et al., 2016). For further details please refer to original study and its supplementary material (Bloomfield et al., 2016b).
MRI imaging
Each subject underwent a MRI brain scan acquired with a 3T magnetic resonance imaging (MRI) scanner (Trio, Siemens Medical Systems, Germany). T1-weighted images were acquired with a 32-channel head coil (except for one subject where a 12-channel coil was used due to operator error) on a Siemens Tim Trio, 3-T MRI scanner (Siemens Healthcare, Erlangen, Germany). A magnetization-prepared rapid gradient echo (MPRAGE) sequence produced a 3D structural image with a 1 mm3 voxel size constructed from 160 slices (thickness 1mm) [time repetition (TR) = 2300 ms, time echo (TE) = 2.98 ms, flip angle of 9°, time to inversion (TI) = 900 ms, matrix coil mode = 24 channel Auto (Triple), FoV read = 256 mm, FoV phase = 93.8%].
Image analysis
MRI Cortical volume measures
Cortical reconstruction and volumetric segmentation on T1 weighted images were performed with the Freesurfer version 5.3 (http://surfer.nmr.mgh.harvard.edu) (Dale et al., 1999). The procedure involved intensity normalization, automated topology corrections and automatic segmentations of cortical and subcortical regions. The PET, but not MR, data for these subjects have been reported in full (Bloomfield et al., 2016b). The cortex was segmented into 68 regions (34 in each hemisphere) based on the Desikan-Killiany Atlas (Desikan et al., 2006), and the volume of each cortical region was calculated using the standard Freesurfer pipeline (Dale et al., 1999). The volumes of the cortical regions were used as input features to the regression model (see below). Widespread gray matter reductions are consistently reported in schizophrenia in total cortical gray matter, frontal cortex and temporal cortex and therefore these brain areas were selected as regions of interests (ROIs)(Andreasen et al., 2011; Haijma et al., 2013; Honea et al., 2005). In addition, we found increased [11C]PBR28 relative binding in total cortical gray matter, frontal cortex and temporal cortex in patients with schizophrenia and individuals at risk of psychosis (Bloomfield et al., 2016b). The frontal cortex included bilateral caudal middle frontal cortex, lateral orbitofrontal cortex, medial orbitofrontal cortex, pars opercularis, pars orbitalis, pars triangularis, rostral middle frontal cortex, superior frontal cortex, and frontal pole; the temporal cortex included bilateral banks of superior temporal sulcus, entorhinal cortex, fusiform cortex, inferior temporal cortex, middle temporal cortex, parahippocampal cortex, superior temporal cortex, temporal pole, and transverse temporal cortex.
PET imaging
All PET images were corrected for head movement using non-attenuation corrected images, as they include greater scalp signal, which improves re-alignment compared to attenuation-corrected images (Montgomery et al., 2006). Frames were realigned to a single ‘reference’ space identified by the individual T1 MRI scan. The transformation parameters were then applied to the corresponding attenuation-corrected PET frames, creating a movement-corrected dynamic image for analysis. Structural MRI images were used for gray/white matter segmentation and region of interest (ROI) definition. A neuroanatomical atlas (Tziortzi et al., 2011) was co-registered on each subject’s MRI scan first and then on the correspondent PET image using a combination of Statistical Parametric Mapping8 (http://www.fil.ion.ucl.ac.uk/spm) and FSL (http://www.fsl.fmrib.ox.ac.uk/fsl) functions, implemented in MIAKAT™ (http://www.miakat.org). Regional time-activity curves (TACs) were obtained by sampling the image with the coregistered atlas. The primary region of interest was total cortical gray matter. Secondary regions of interest were temporal and frontal lobe gray matter regions from the atlas (Radewicz, 2000).
Quantification of [11C]PBR28 tissue distribution was performed using the two tissue compartmental model accounting for endothelial vascular TSPO binding (2TCM-1K) (Rizzo et al., 2014; Veronese et al., 2017), as this has been shown to have improved performance compared with the two tissue compartmental model not accounting for endothelial binding (2TCM) (Rizzo et al., 2014). To account for variability of intersubject blood binding, typical of TSPO targeting tracers (Albrecht et al., 2016; Lockhart et al., 2003; Turkheimer et al., 2015b), regional distribution volume estimates were normalized for whole brain activity in agreement with previous studies (Loggia et al., 2015; Zürcher et al., 2015).
Our primary outcome measure therefore was the adjusted distribution volume ratio (DVR) calculated using an multiple analysis of covariance (MANCOVA) covarying for confounding factors that affect TSPO tracer binding (Turkheimer et al., 2015b). This approach produces estimated marginal means, in which the ROIs of interest are the dependent variables and genotype, age and whole brain signal are used as covariates. There are data to suggest that cortical microglial activation, hence TSPO binding, is elevated with aging (Schuitemaker et al., 2012b), which is also evident in our data (see (Bloomfield et al., 2016b), Supplemental Table 2). TSPO genotype was included as a co-variate in correlation analysis as there is evidence that a single nucleotide polymorphism affecting the TSPO gene significantly affects binding of the tracer (Owen et al., 2011). For this reason, we performed correlation analysis using age, genotype and whole brain signal as covariates. We also performed analysis using volume of distribution (VT) as secondary outcome measure. Volume of distribution (VT) is defined as the ratio of the radioligand concentration in target region to that in plasma at equilibrium (Innis et al., 2007).
Statistical analysis
Data, other than for gender and genotype, were shown to have a normal distribution following a Shapiro-Wilk test (Shapiro, 1965). Hence parametric tests were implemented for all but gender and affinity analyses, where a Mann-Whitney U test was used. Demographic data and tracer activity data were analysed using independent-samples t-tests.
Within each cohort, multiple analysis of covariance (MANCOVA) was used to determine whether there was an effect of the group on [11C]PBR28 adjusted DVR in the total cortical gray matter, frontal lobe, and temporal lobe, as well as on the total cortical gray matter volume and regional volumes within frontal and temporal lobes. These regions were chosen as regions of interest based on previous microglial activation studies (Doorduin et al., 2009a; van Berckel et al., 2008). For all statistical comparisons alpha was set at a 0.05 threshold (two-tailed) for significance. The results were reported in our recently published study (Bloomfield et al., 2016b) and presented in the supplementary table 1.
For cortical volume analysis, within each cohort, the volume of each cortical region was compared between the two groups with the t-test. A false discovery rate (FDR) at 0.05 was used to control the false positives across the multiple comparisons among the brain regions (Benjamini and Hochberg, 1995; Nichols and Hayasaka, 2003).
The main objective was to investigate the relationship between cortical microglial activation and the cortical gray matter volume decreases in patients. We therefore investigated the relationship between the differences in the volume of cortical regions and microglial activation in cortical gray matter (as indexed by [11C]PBR28 DVR). We performed a regression analysis of the total cortical gray matter [11C]PBR28 DVR value (dependent variable) against all the regional cortical gray matter volumes (independent variables) as described above. As the number of investigated regions was larger than the number of observations, we implemented a regulation of the parameters with the elastic net regularized general linear models (Zou and Hastie). Elastic net is a statistical regularization and variable selection method that prevents overfitting of the regression (Zou and Hastie). Because the parameter optimization is dependent on an internal cross-validation procedure, the performance of the regression may vary depending on sample stratification. To control the variability ‘r’, we performed 1000 iterations of the regression and used the mean correlative coefficients for the final regression model, and report the distribution of r and –logp values of the 1000 iterations (Carroll et al., 2009). The r values were between 0.7 and 1 and the –logp values were well beyond 2, indicating good fitting at a significance level of 0.01 or lower across all the iterations. The resulting regressed DVR and the DVR measured in our study was evaluated with Pearson’s correlation analysis to investigate the accuracy of the regression. To further investigate the localized relationship between the DVR and cortical region volumes, we also performed exploratory analyses using the same regression analysis within the DVR and regional volumes of the frontal cortex, as well as the DVR and regional volumes of the temporal cortex. The primary region of interest was total cortical gray matter. For the total cortical gray matter DVR, regression included all 68 regions of the cortex (i.e to identify which of the 68 cortical regional variables were most associated with cortical gray matter DVR). In the exploratory analyses, for the frontal and temporal lobe DVR, we included 18 regions within frontal and temporal cortex as secondary regions of interests, respectively. The regression analysis was performed with MATLAB (version R2013a, The Mathworks Inc., Natick, Massachusetts) with glmnet package (Qian et al., 2013).
Results
Cortical Gray Matter Volume Analysis (SCZ vs Controls)
The total cortical gray matter volume was significantly lower in SCZ than the healthy controls (p=0.02) (Table 1). Temporal cortical gray matter volume was also lower in Scz than controls but not frontal cortical gray matter volume (Table 1). Whole brain analysis with false discovery rate set at 0.05, revealed significantly lower gray matter volumes in schizophrenia patients compared to healthy control subjects in the following three regions; left cuneus, right superior parietal cortex and right transverse temporal cortex (Table 1).
Cortical Gray Matter Volume Analysis (UHR vs Controls)
The total cortical, frontal or temporal gray matter volume was not different between the UHR and the controls (Table 1). No significant volume difference was observed in the whole brain analysis at FDR 0.05.
Relationship between [11C]PBR28 DVR and brain volume in the SCZ
Total cortical gray matter [11C]PBR28 DVR and brain volume
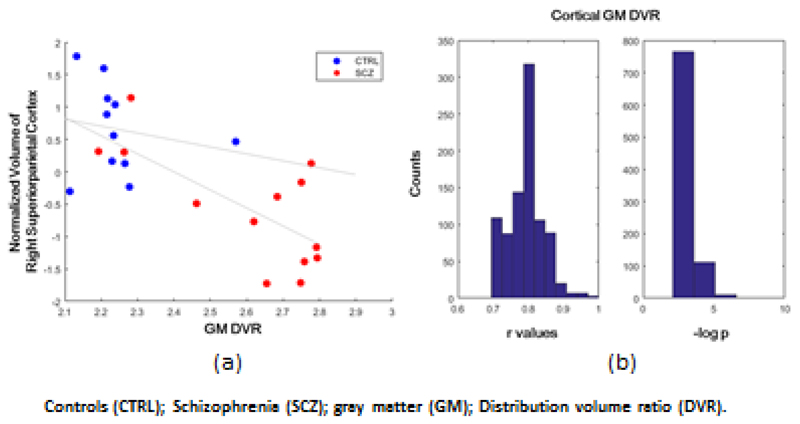
In the schizophrenia patients the volumes of cortical regions were significantly related to total cortical gray matter [11C]PBR28 DVR (r=-0.83; p<0.001) in the regression model. The top weighted regions were the right superior parietal cortex, left middle frontal cortex, left isthmus of the cingulate cortex, left temporal pole, and right lateral orbital frontal cortex. All these regions contributed negatively to the DVR values, indicating smaller volumes in these regions are related to greater total cortical [11C]PBR28 DVR. The region with the highest absolute correlation coefficient was the right superior-parietal cortex, and its volume was negatively correlated with the total cortical gray matter DVR (r=-0.70; p=0.008; Figure 1a). The regression found no significant relationship between total cortical gray matter DVR and cortical gray matter volumes in the matched healthy controls.
Figure 1. Negative correlation between normalized volume of right superior parietal cortex and [11C]PBR28 gray matter DVR (a) and the distribution of r and –logp for the 1000 regressions (b).
The distributions of the r and p values for the 1000 iterations indicate that the regression model using cortical gray matter volumes provided a good fit of the total cortical gray matter DVR as shown in Figure 1b. The lowest r value was 0.70, when the model used only one region volume (the right superior-parietal cortex) to fit the total cortical gray matter DVR.
b) Regional cortical gray matter [11C]PBR28 DVR and brain volume
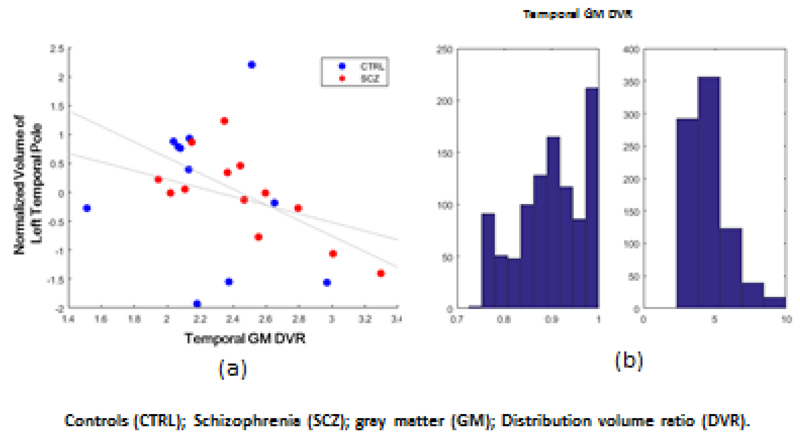
In this exploratory analyses, we also found that in the schizophrenia patients the gray matter DVR in the temporal cortex was significantly related to the volumes of left temporal pole, right superior temporal cortex, left and right entorhinal cortex, and right transverse temporal cortex (r=0.94; p<0.0001; Figure 4). The region with the highest absolute regression coefficient was the left temporal pole, which showed a negative correlation with the temporal cortical GM DVR (r=-0.72; p=0.005; Figure 5). The distributions of the r and p values for the 1000 iterations are shown in Figure 6. In contrast, there was no significant fit between the DVR values of the frontal lobe with the frontal volumes.
Relationship between [11C]PBR28 DVR and brain volume in the UHR
For the UHR group, the regression model found a significant fit between the total cortical gray matter DVR with the cortical region volumes (r=0.63; p=0.02). The regression model also fitted the DVR of the temporal lobe with its volumes (r=0.76; p=0.002). None of the regions used in the regression correlated with the DVR of the total cortical or temporal gray matter, indicating an additive effect from multiple regions. The regression model did not fit the DVR values of the frontal lobe with the corresponding volumes.
Finally, direct regional correlations between [11C]PBR28 DVR and MRI volume for cortical GM, frontal lobe, and temporal lobe in both schizophrenia patients and UHR subjects matched to their respective control groups were not significant (p>0.05).
Relationship between [11C]PBR28 VT and brain volume
In the schizophrenia patients, the regression model found a significant fit between the total cortical gray matter VT with the cortical region volumes (r=0.98; p<0.001) and temporal lobe (r=-0.88; p<0.001). However, none of the regions used in the regression correlated with the VT of the total cortical or temporal gray matter, which may indicate additive effect that otherwise were not predictive for VT individually. There was no gray matter volume ROI that was statistically significantly correlated with VT (Supplement Fig 1), though there was a trend towards a negative correlation between VT and gray matter volume in the temporal lobe (left transverse temporal volume r=-0.51; p=0.07; Supplement Fig 2). There was no significant fit between the frontal region VT values with the frontal volumes.
In the UHR subjects, the regression model found a significant fit between the total cortical gray matter VT with the cortical region volumes (r=0.90; p<0.001), temporal lobe (r=0.68; p<0.001) and frontal lobe (r=0.80; p<0.001). The region with the highest absolute correlation coefficient was the right pars orbitalis cortex, and its volume was positively correlated with the total cortical gray matter VT (r=0.76; p=0.002; Supplement Fig 3). For temporal gray matter VT, the highest absolute correlation coefficient was the left transverse temporal area (r=0.61; p=0.02; Supplement Fig 4) and for frontal lobe was right pars orbitalis cortex (r=0.71; p=0.004; Supplement Fig 5).
Discussion
In this experiment, we investigated the relationship between cortical volumes and TSPO signal from [11C]PBR28 PET scans in patients with schizophrenia, UHR subjects and respective matched control groups. We demonstrated that patients with schizophrenia exhibit a negative relationship between total cortical gray matter [11C]PBR-28 DVR and cortical gray matter volumes, and this was also seen for temporal cortex DVR and temporal cortical volumes. The lack of relationship in the healthy control subjects may suggest that relationship between total cortical gray matter [11C]PBR-28 DVR and cortical gray matter volumes is disorder specific. It is also possible lack of relationship in the healthy control subjects is due to the limited [11C]-PBR-28 DVR range in this cohort. In the UHR group, although the regression model fitted the DVR values of total cortical and temporal gray matter with the respective cortical region volumes, no individual regions showed any significant correlation.
To our knowledge this study is the first to investigate the relationship between microglia and cortical volumes in schizophrenia and UHR cohorts using multimodal imaging. Our findings are consistent with the hypothesis that microglial activation leads to GM volume reduction, but, as they are associations, a direct causal relationship cannot be inferred. Although the regression model indicated there is a relationship between DVR and gray matter volumes in the UHR group, there was no single region that showed a correlation between [11C]PBR28 DVR and cortical volumes. This difference with the schizophrenia results may reflect illness progression as UHR subjects are earlier in the development of disorder and many UHR subjects do not go on to develop schizophrenia. It would be interesting to follow up the UHR cohorts to examine the brain GM volume change and [11C]PBR28 signal during the course of the prodromal period to determine if there is a change with illness progression.
A similar significant inverse correlation between [11C]PBR28 binding and gray matter volume is also observed in patients with Alzheimer’s disease and mild cognitive impairment (Kreisl et al., 2013). Further experimental studies are required to determine the biological significance of elevated microglial activity in the context of brain volume changes in schizophrenia (Laskaris et al., 2016).
In our main [11C]PBR28 results in schizophrenia, we found increased [11C]PBR28 DVR in patients with schizophrenia compared to healthy controls but not with [11C]PBR28 VT between the two groups (Bloomfield et al., 2016b). We found >30% level of VT variability likely due in part to a small free fraction (fp) and DVR normalization reduced intersubject variability and thus possibly improved the signal to noise in our data (Bloomfield et al., 2016b). Therefore, it is not surprising that we did not find any significant correlation between [11C]PBR28 VT and cortical gray matter or regional (temporal or frontal) volume in patients with schizophrenia. These differences may be accounted for by methodological issues (see discussion below). Notwithstanding this, the direction of both the VT and DVR correlations is consistent with the direction of alteration seen in schizophrenia for the respective measure in temporal lobe (a trend level negative correlation between [11C]PBR28 VT and in temporal lobe). Finally, we observed a direct correlation between [11C]PBR28 VT and cortical volumes in UHR subjects. This is not what we expected and we think that this is likely due to noise in the data due to the fact that we did not find any significant differences in the gray matter volume or [11C]PBR28 VT between UHR subjects and healthy controls.
We used distribution volume ratio (DVR) as the main outcome measure and other groups used distribution volume (VT) or binding potential (BP). There is currently no universal consensus on the optimum analytical approaches for estimation of microglial activation using TSPO tracers (Bloomfield et al., 2016a; Narendran and Frankle, 2016; Turkheimer et al., 2015b). DVR as an outcome measure has been validated in animals, where inflammatory stimuli increase DVRs and microglial markers (Converse et al., 2011; Imaizumi et al., 2007) and also in other human studies using [11C]PBR28 (Dimber et al., 2016; Lyoo et al., 2015) and [11C]-(R)-PK11195 (Rissanen et al., 2014). VT is generally accepted to be the preferred outcome measure in the absence of a clear reference region. However, this is problematic for TSPO tracers for a number of reasons. Accurate VT estimation relies on two assumptions; first, the availability of reliable plasma input function measurement and second, that there are no systematic group differences in free parent radiotracer plasma levels. In our cohort of Schizophrenia patients, we observed a very low free parent radiotracer plasma levels (~2%) and the parent plasma levels were significantly different across group (patients vs controls)(Bloomfield et al., 2016a). Therefore accurate VT determination is not possible and potentially unreliable (Turkheimer et al., 2015a). Another issue is the confounding effect of plasma protein bound to plasma tracer. Binding of TSPO tracers to plasma proteins has been reported(Lockhart et al., 2003). which indicates this could confound VT estimation as studies show patients with schizophrenia show elevated acute phase protein in peripheral blood (Bloomfield et al., 2016a). Thus, in our study, we think DVR is less likely to be affected by differences in plasma proteins compared to VT. However, it should be noted that interpretation of DVR increases is not straightforward as there is no brain region devoid of TSPO, and further work is warranted to confirm the relationships observed. This issue is discussed in detail elsewhere (Bloomfield et al., 2016a; Turkheimer et al., 2015b).
Limitations
Except one patient with schizophrenia, all were taking antipsychotic medications (Supplementary Table 2) which may be a potential confounder moderating the relationship between microglial activity and brain GM volume. Antipsychotic medications are associated with brain volume reductions in schizophrenia (Ho et al., 2011) but volume change is related to illness progression after adjusting for antipsychotic effects (Van Haren et al., 2013). Some preclinical evidence suggests that antipsychotic medication may possess anti-inflammatory properties (Mondelli and Howes, 2014) by reducing microglial activity (Bian et al., 2008; Kato et al., 2008; Zhu F, 2014), although at higher doses they may increase microglial activity (Cotel et al., 2015). A recent study using [11C]-(R)-PK11195 to index TSPO availability, found significantly elevated [11C]-(R)-PK11195 in medicated patients (N=8) (taking monthly intramuscular injections of either Risperidone or Paliperidone) compared with healthy controls in prefrontal, anterior cingulate and parietal cortical regions (Holmes et al., 2016). However there were no significant differences in [11C]-(R)-PK11195 availability in antipsychotic-free patients (Holmes et al., 2016). Longitudinal clinical studies will be necessary to investigate change in brain volume and TSPO in patients with schizophrenia who were medication naïve to specifically look into the effects of antipsychotic medication on brain volume.
We did not correct for possible partial volume effects on [11C]PBR28 in this study. However gray matter reductions seen in schizophrenia patient would tend to underestimate the [11C]PBR28 DVR and thus the partial volume effect would be unlikely to explain the negative correlation between [11C]PBR28 DVR and GM observed in this study.
Due to modest sample size in this study, the results have to be interpreted with caution. To reduce the possibility that our results were acquired by chance, we also performed the regression 1000 times with random folding internal cross-validations to select the parameters. The distribution of r values (the correlation coefficient between the predicted DVR with gray matter brain region volumes and the actual DVR) was an indicator of the possible performances of the regression model. The values reported in our study were near the expected values of the r.
Future directions
The causal relationship between neuroinflammation and brain volume changes will likely require longitudinal clinical studies that specifically look into whether TSPO changes predict the onset or worsening of brain volume reduction in individuals prior to the onset of psychosis and are antipsychotic medication naïve.
Conclusions
In schizophrenia cortical volume reduction is associated with elevated microglial activation using a multimodal neuroimaging approach. This relationship was also observed at a marginal level in UHR subjects suggesting the relationship may become stronger with the development of the disorder.
Supplementary Material
Figure 2. Negative correlation between normalized volume of left temporal pole and temporal [11C]PBR28 gray matter DVR (a) and the distribution of r and –logp for the 1000 regressions (b).
Acknowledgements
Funding
This study was funded by Medical Research Council-UK (no. MC-A656-5QD30), Maudsley Charity (no. 666), Brain and Behavior Research Foundation, and Wellcome Trust (no. 094849/Z/10/Z) grants to Dr. Howes and the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London.
The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Conflicts of interest
Dr. Howes has received investigator-initiated research funding from and/or participated in advisory/ speaker meetings organised by Astra-Zeneca, Autifony, BMS, Eli Lilly, Heptares, Jansenn, Lundbeck, Lyden-Delta, Otsuka, Servier, Sunovion, Rand and Roche. Neither Dr. Howes or his family have been employed by or have holdings/ a financial stake in any biomedical company. Sudhakar Selvaraj, Peter S Bloomfield, Cao Bo, Mattia Veronese and Federico Turkheimer have no conflicts of interest for this work. The authors would like to thank all the clinical imaging staff at Imanova for their help with this study.
References
- Abourbeh G, Thézé B, Maroy R, Dubois A, Brulon V, Fontyn Y, Dollé F, Tavitian B, Boisgard R. Imaging microglial/macrophage activation in spinal cords of experimental autoimmune encephalomyelitis rats by positron emission tomography using the mitochondrial 18 kDa translocator protein radioligand [18F]DPA-714. J Neurosci. 2012;32(17):5728–5736. doi: 10.1523/JNEUROSCI.2900-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht DS, Granziera C, Hooker JM, Loggia ML. In Vivo Imaging of Human Neuroinflammation. ACS Chem Neurosci. 2016;7(4):470–483. doi: 10.1021/acschemneuro.6b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry. 2011;70(7):672–679. doi: 10.1016/j.biopsych.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlicot N, Petit E, Katsifis A, Toutain J, Divoux D, Bodard S, Roussel S, Guilloteau D, Bernaudin M, Chalon S. Detection and quantification of remote microglial activation in rodent models of focal ischaemia using the TSPO radioligand CLINDE. Eur J Nucl Med Mol Imaging. 2010;37(12):2371–2380. doi: 10.1007/s00259-010-1598-7. [DOI] [PubMed] [Google Scholar]
- Banati RB, Cagnin A, Brooks DJ, Gunn RN, Myers R, Jones T, Birch R, Anand P. Long-term trans-synaptic glial responses in the human thalamus after peripheral nerve injury. Neuroreport. 2001;12(16):3439–3442. doi: 10.1097/00001756-200111160-00012. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Buslei R, Havas L, Falkai P. Evidence for activation of microglia in patients with psychiatric illnesses. Neuroscience Letters. 1999;271(2):126–128. doi: 10.1016/s0304-3940(99)00545-5. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- Bergink V, Gibney SM, Drexhage HA. Autoimmunity, inflammation, and psychosis: a search for peripheral markers. Biol Psychiatry. 2014;75(4):324–331. doi: 10.1016/j.biopsych.2013.09.037. [DOI] [PubMed] [Google Scholar]
- Bian Q, Kato T, Monji A, Hashioka S, Mizoguchi Y, Horikawa H, Kanba S. The effect of atypical antipsychotics, perospirone, ziprasidone and quetiapine on microglial activation induced by interferon-Î3. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32(1):42–48. doi: 10.1016/j.pnpbp.2007.06.031. [DOI] [PubMed] [Google Scholar]
- Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat. 1968;85(2):145–157. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]
- Block F, Dihné M, Loos M. Inflammation in areas of remote changes following focal brain lesion. Prog Neurobiol. 2005;75(5):342–365. doi: 10.1016/j.pneurobio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Bloomfield PS, Howes OD, Turkheimer F, Selvaraj S, Veronese M. Response to Narendran and Frankle: The Interpretation of PET Microglial Imaging in Schizophrenia. Am J Psychiatry. 2016a;173(5):537–538. doi: 10.1176/appi.ajp.2016.15111417r. [DOI] [PubMed] [Google Scholar]
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, Bloomfield MA, Bonoldi I, Kalk N, Turkheimer F, McGuire P, et al. Microglial Activity in People at Ultra High Risk of Psychosis and in Schizophrenia: An [(11)C]PBR28 PET Brain Imaging Study. Am J Psychiatry. 2016b;173(1):44–52. doi: 10.1176/appi.ajp.2015.14101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgwardt SJ, McGuire PK, Aston J, Berger G, Dazzan P, Gschwandtner U, Pflüger M, D'Souza M, Radue EW, Riecher-Rössler A. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. Br J Psychiatry Suppl. 2007a;51:s69–75. doi: 10.1192/bjp.191.51.s69. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Riecher-Rössler A, Dazzan P, Chitnis X, Aston J, Drewe M, Gschwandtner U, Haller S, Pflüger M, Rechsteiner E, D'Souza M, et al. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007b;61(10):1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Brown AK, Fujita M, Fujimura Y, Liow JS, Stabin M, Ryu YH, Imaizumi M, Hong J, Pike VW, Innis RB. Radiation dosimetry and biodistribution in monkey and man of 11C-PBR28: a PET radioligand to image inflammation. J Nucl Med. 2007;48(12):2072–2079. doi: 10.2967/jnumed.107.044842. [DOI] [PubMed] [Google Scholar]
- Cagnin A, Myers R, Gunn RN, Lawrence AD, Stevens T, Kreutzberg GW, Jones T, Banati RB. In vivo visualization of activated glia by [11C] (R)-PK11195-PET following herpes encephalitis reveals projected neuronal damage beyond the primary focal lesion. Brain. 2001;124(Pt 10):2014–2027. doi: 10.1093/brain/124.10.2014. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TG, McEwen S, Addington J, Bearden CE, Cadenhead K, Cornblatt B, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77(2):147–157. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera E, Tononi G. Diaschisis: past, present, future. Brain. 2014;137(Pt 9):2408–2422. doi: 10.1093/brain/awu101. [DOI] [PubMed] [Google Scholar]
- Carroll MK, Cecchi GA, Rish I, Garg R, Rao AR. Prediction and interpretation of distributed neural activity with sparse models. Neuroimage. 2009;44(1):112–122. doi: 10.1016/j.neuroimage.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Collste K, Plavén-Sigray P, Fatouros-Bergman H, Victorsson P, Schain M, Forsberg A, Amini N, Aeinehband S, Erhardt S, Halldin C, Flyckt L, et al. Lower levels of the glial cell marker TSPO in drug-naive first-episode psychosis patients as measured using PET and [(11)C]PBR28. Mol Psychiatry. 2017 doi: 10.1038/mp.2016.247. [DOI] [PubMed] [Google Scholar]
- Converse AK, Larsen EC, Engle JW, Barnhart TE, Nickles RJ, Duncan ID. 11C-(R)-PK11195 PET imaging of microglial activation and response to minocycline in zymosan-treated rats. J Nucl Med. 2011;52(2):257–262. doi: 10.2967/jnumed.110.082743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotel MC, Lenartowicz EM, Natesan S, Modo MM, Cooper JD, Williams SC, Kapur S, Vernon AC. Microglial activation in the rat brain following chronic antipsychotic treatment at clinically relevant doses. Eur Neuropsychopharmacol. 2015;25(11):2098–2107. doi: 10.1016/j.euroneuro.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Ambinder EB, Ward RE, Minn I, Vranesic M, Kim PK, Ford CN, Higgs C, Hayes LN, Schretlen DJ, et al. In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [(11)C]DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry. 2016;6:e777. doi: 10.1038/tp.2016.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dazzan P, Soulsby B, Mechelli A, Wood SJ, Velakoulis D, Phillips LJ, Yung AR, Chitnis X, Lin A, Murray RM, McGorry PD, et al. Volumetric abnormalities predating the onset of schizophrenia and affective psychoses: an MRI study in subjects at ultrahigh risk of psychosis. Schizophr Bull. 2012;38(5):1083–1091. doi: 10.1093/schbul/sbr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickens AM, Vainio S, Marjamäki P, Johansson J, Lehtiniemi P, Rokka J, Rinne J, Solin O, Haaparanta-Solin M, Jones PA, Trigg W, et al. Detection of microglial activation in an acute model of neuroinflammation using PET and radiotracers 11C-(R)-PK11195 and 18F-GE-180. J Nucl Med. 2014;55(3):466–472. doi: 10.2967/jnumed.113.125625. [DOI] [PubMed] [Google Scholar]
- Dimber R, Guo Q, Bishop C, Adonis A, Buckley A, Kocsis A, Owen D, Kalk N, Newbould R, Gunn RN, Rabiner EA, et al. Evidence of Brain Inflammation in Patients with Human T-Lymphotropic Virus Type 1-Associated Myelopathy (HAM): A Pilot, Multimodal Imaging Study Using 11C-PBR28 PET, MR T1-Weighted, and Diffusion-Weighted Imaging. J Nucl Med. 2016;57(12):1905–1912. doi: 10.2967/jnumed.116.175083. [DOI] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009a;50(11):1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- Doorduin J, de Vries EFJ, Willemsen ATM, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in Schizophrenia-Related Psychosis: A PET Study. Journal of Nuclear Medicine. 2009b;50(11):1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res. 2010;117(1):1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165(8):1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, Cairns M, Weickert CS. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206–214. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- Fillman SG, Weickert TW, Lenroot RK, Catts SV, Bruggemann JM, Catts VS, Weickert CS. Elevated peripheral cytokines characterize a subgroup of people with schizophrenia displaying poor verbal fluency and reduced Broca's area volume. Mol Psychiatry. 2016;21(8):1090–1098. doi: 10.1038/mp.2015.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R, Kandanearatchi A, Beasley C, Williams B, Khan N, Fagerhol MK, Everall IP. Calprotectin in microglia from frontal cortex is up-regulated in schizophrenia: evidence for an inflammatory process? Eur J Neurosci. 2006;24(12):3561–3566. doi: 10.1111/j.1460-9568.2006.05219.x. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Schwarz J, Myers R, Wise R, Banati RB. Evolution of microglial activation in patients after ischemic stroke: a [11C](R)-PK11195 PET study. Neuroimage. 2005;24(2):591–595. doi: 10.1016/j.neuroimage.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Hafizi S, Tseng HH, Rao N, Selvanathan T, Kenk M, Bazinet RP, Suridjan I, Wilson AA, Meyer JH, Remington G, Houle S, et al. Imaging Microglial Activation in Untreated First-Episode Psychosis: A PET Study With [(18)F]FEPPA. Am J Psychiatry. 2017;174(2):118–124. doi: 10.1176/appi.ajp.2016.16020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39(5):1129–1138. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40(2):140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68(2):128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg P, Liljequist S, Wägner A. Secondary brain injuries in thalamus and hippocampus after focal ischemia caused by mild, transient extradural compression of the somatosensori cortex in the rat. Curr Neurovasc Res. 2009;6(1):1–11. doi: 10.2174/156720209787466073. [DOI] [PubMed] [Google Scholar]
- Holmes SE, Hinz R, Drake RJ, Gregory CJ, Conen S, Matthews JC, Anton-Rodriguez JM, Gerhard A, Talbot PS. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [(11)C](R)-PK11195 positron emission tomography study. Mol Psychiatry. 2016;21(12):1672–1679. doi: 10.1038/mp.2016.180. [DOI] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162(12):2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Imaizumi M, Kim H-J, Zoghbi SS, Briard E, Hong J, Musachio JL, Ruetzler C, Chuang D-M, Pike VW, Innis RB, Fujita M. PET imaging with [11C]PBR28 can localize and quantify upregulated peripheral benzodiazepine receptors associated with cerebral ischemia in rat. Neuroscience Letters. 2007;411(3):200–205. doi: 10.1016/j.neulet.2006.09.093. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Sommer IE. The neurobiology and treatment of first-episode schizophrenia. Mol Psychiatry. 2015;20(1):84–97. doi: 10.1038/mp.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlstetter M, Nothdurfter C, Aslanidis A, Moeller K, Horn F, Scholz R, Neumann H, Weber BH, Rupprecht R, Langmann T. Translocator protein (18 kDa) (TSPO) is expressed in reactive retinal microglia and modulates microglial inflammation and phagocytosis. J Neuroinflammation. 2014;11:3. doi: 10.1186/1742-2094-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Mizoguchi Y, Monji A, Horikawa H, Suzuki SO, Seki Y, Iwaki T, Hashioka S, Kanba S. Inhibitory effects of aripiprazole on interferon-γ-induced microglial activation via intracellular Ca2+ regulation in vitro. Journal of Neurochemistry. 2008;106(2):815–825. doi: 10.1111/j.1471-4159.2008.05435.x. [DOI] [PubMed] [Google Scholar]
- Kenk M, Selvanathan T, Rao N, Suridjan I, Rusjan P, Remington G, Meyer JH, Wilson AA, Houle S, Mizrahi R. Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET study with [18F]-FEPPA. Schizophr Bull. 2015;41(1):85–93. doi: 10.1093/schbul/sbu157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull. 2013;39(6):1174–1179. doi: 10.1093/schbul/sbt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N, Corona W, Morse CL, Zoghbi SS, Pike VW, McMahon FJ, et al. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer's disease. Brain. 2013;136(Pt 7):2228–2238. doi: 10.1093/brain/awt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lartey FM, Ahn GO, Shen B, Cord KT, Smith T, Chua JY, Rosenblum S, Liu H, James ML, Chernikova S, Lee SW, et al. PET imaging of stroke-induced neuroinflammation in mice using [18F]PBR06. Mol Imaging Biol. 2014;16(1):109–117. doi: 10.1007/s11307-013-0664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskaris LE, Di Biase MA, Everall I, Chana G, Christopoulos A, Skafidas E, Cropley VL, Pantelis C. Microglial activation and progressive brain changes in schizophrenia. Br J Pharmacol. 2016;173(4):666–680. doi: 10.1111/bph.13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart A, Davis B, Matthews JC, Rahmoune H, Hong G, Gee A, Earnshaw D, Brown J. The peripheral benzodiazepine receptor ligand PK11195 binds with high affinity to the acute phase reactant alpha1-acid glycoprotein: implications for the use of the ligand as a CNS inflammatory marker. Nucl Med Biol. 2003;30(2):199–206. doi: 10.1016/s0969-8051(02)00410-9. [DOI] [PubMed] [Google Scholar]
- Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, Hill E, Hsu S, Izquierdo-Garcia D, Ji RR, Riley M, et al. Evidence for brain glial activation in chronic pain patients. Brain. 2015;138(Pt 3):604–615. doi: 10.1093/brain/awu377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo CH, Ikawa M, Liow J-S, Zoghbi SS, Morse CL, Pike VW, Fujita M, Innis RB, Kreisl WC. Cerebellum Can Serve As a Pseudo-Reference Region in Alzheimer Disease to Detect Neuroinflammation Measured with PET Radioligand Binding to Translocator Protein. Journal of Nuclear Medicine. 2015;56(5):701–706. doi: 10.2967/jnumed.114.146027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner F, Staykova M, Berghofer P, Wong HJ, Fordham S, Callaghan P, Jackson T, Pham T, Gregoire MC, Zahra D, Rahardjo G, et al. Central nervous system expression and PET imaging of the translocator protein in relapsing-remitting experimental autoimmune encephalomyelitis. J Nucl Med. 2013;54(2):291–298. doi: 10.2967/jnumed.112.108894. [DOI] [PubMed] [Google Scholar]
- Meisenzahl EM, R D, Kirner A. Association of an interleukin-1β genetic polymorphism with altered brain structure in patients with schizophrenia. Am J Psychiatry. 2001;158:1316–1319. doi: 10.1176/appi.ajp.158.8.1316. [DOI] [PubMed] [Google Scholar]
- Mondelli V, Howes O. Inflammation: its role in schizophrenia and the potential anti-inflammatory effects of antipsychotics. Psychopharmacology (Berl) 2014;231(2):317–318. doi: 10.1007/s00213-013-3383-3. [DOI] [PubMed] [Google Scholar]
- Montgomery AJ, Thielemans K, Mehta MA, Turkheimer F, Mustafovic S, Grasby PM. Correction of Head Movement on PET Studies: Comparison of Methods. Journal of Nuclear Medicine. 2006;47(12):1936–1944. [PubMed] [Google Scholar]
- Myers R, Manjil LG, Frackowiak RS, Cremer JE. [3H]PK 11195 and the localisation of secondary thalamic lesions following focal ischaemia in rat motor cortex. Neurosci Lett. 1991;133(1):20–24. doi: 10.1016/0304-3940(91)90047-w. [DOI] [PubMed] [Google Scholar]
- Narendran R, Frankle WG. Comment on Analyses and Conclusions of "Microglial Activity in People at Ultra High Risk of Psychosis and in Schizophrenia: An [(11)C]PBR28 PET Brain Imaging Study". Am J Psychiatry. 2016;173(5):536–537. doi: 10.1176/appi.ajp.2016.15111417. [DOI] [PubMed] [Google Scholar]
- Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res. 2003;12(5):419–446. doi: 10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann Neurol. 2005;57(2):168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- Owen DR, Guo Q, Kalk NJ, Colasanti A, Kalogiannopoulou D, Dimber R, Lewis YL, Libri V, Barletta J, Ramada-Magalhaes J, Kamalakaran A, et al. Determination of [(11)C]PBR28 binding potential in vivo: a first human TSPO blocking study. J Cereb Blood Flow Metab. 2014;34(6):989–994. doi: 10.1038/jcbfm.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, Rhodes C, Pulford DJ, Bennacef I, Parker CA, StJean PL, et al. An 18-kDa Translocator Protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2011;32(1):1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361(9354):281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Pappata S, Levasseur M, Gunn RN, Myers R, Crouzel C, Syrota A, Jones T, Kreutzberg GW, Banati RB. Thalamic microglial activation in ischemic stroke detected in vivo by PET and [11C]PK1195. Neurology. 2000;55(7):1052–1054. doi: 10.1212/wnl.55.7.1052. [DOI] [PubMed] [Google Scholar]
- Qian J, Hastie T, Friedman J, Tibshirani R, Simon N. Glmnet for Matlab. 2013 [Google Scholar]
- Radewicz K, Garey LJ, Gentleman SM, Reynolds R. Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol. 2000;59:137–150. doi: 10.1093/jnen/59.2.137. [DOI] [PubMed] [Google Scholar]
- Radlinska BA, Ghinani SA, Lyon P, Jolly D, Soucy JP, Minuk J, Schirrmacher R, Thiel A. Multimodal microglia imaging of fiber tracts in acute subcortical stroke. Ann Neurol. 2009;66(6):825–832. doi: 10.1002/ana.21796. [DOI] [PubMed] [Google Scholar]
- Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G, Sharp DJ. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. 2011;70(3):374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- Rao VLR, Butterworth RF. Characterization of binding sites for the ω3 receptor ligands [3H]PK11195 and [3H]RO5-4864 in human brain. European Journal of Pharmacology. 1997;340(1):89–99. doi: 10.1016/s0014-2999(97)01395-2. [DOI] [PubMed] [Google Scholar]
- Rissanen E, Tuisku J, Rokka J, Paavilainen T, Parkkola R, Rinne JO, Airas L. In Vivo Detection of Diffuse Inflammation in Secondary Progressive Multiple Sclerosis Using PET Imaging and the Radioligand ¹¹C-PK11195. J Nucl Med. 2014;55(6):939–944. doi: 10.2967/jnumed.113.131698. [DOI] [PubMed] [Google Scholar]
- Rizzo G, Veronese M, Tonietto M, Zanotti-Fregonara P, Turkheimer FE, Bertoldo A. Kinetic modeling without accounting for the vascular component impairs the quantification of [lsqb]11C[rsqb]PBR28 brain PET data. J Cereb Blood Flow Metab. 2014 doi: 10.1038/jcbfm.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010;9(12):971–988. doi: 10.1038/nrd3295. [DOI] [PubMed] [Google Scholar]
- Schuitemaker A, van der Doef TF, Boellaard R, van der Flier WM, Yaqub M, Windhorst AD, Barkhof F, Jonker C, Kloet RW, Lammertsma AA, Scheltens P, et al. Microglial activation in healthy aging. Neurobiol Aging. 2012a;33(6):1067–1072. doi: 10.1016/j.neurobiolaging.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Schuitemaker A, van der Doef TF, Boellaard R, van der Flier WM, Yaqub M, Windhorst AD, Barkhof F, Jonker C, Kloet RW, Lammertsma AA, Scheltens P, et al. Microglial activation in healthy aging. Neurobiology of Aging. 2012b;33(6):1067–1072. doi: 10.1016/j.neurobiolaging.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, Genovese G, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530(7589):177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52(3–4):591–611. [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JW, Gibbon M, First MB. The structured clinical interview for dsm-iii-r (scid): I: history, rationale, and description. Archives of General Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Sun D, Phillips L, Velakoulis D, Yung A, McGorry PD, Wood SJ, van Erp TG, Thompson PM, Toga AW, Cannon TD, Pantelis C. Progressive brain structural changes mapped as psychosis develops in 'at risk' individuals. Schizophr Res. 2009;108(1–3):85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonietto M, Rizzo G, Veronese M, Bertoldo A. Modelling arterial input functions in positron emission tomography dynamic studies. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:2247–2250. doi: 10.1109/EMBC.2015.7318839. [DOI] [PubMed] [Google Scholar]
- Tonietto M, Rizzo G, Veronese M, Fujita M, Zoghbi SS, Zanotti-Fregonara P, Bertoldo A. Plasma radiometabolite correction in dynamic PET studies: Insights on the available modeling approaches. J Cereb Blood Flow Metab. 2016;36(2):326–339. doi: 10.1177/0271678X15610585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trépanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry. 2016;21(8):1009–1026. doi: 10.1038/mp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer Federico E, Rizzo G, Bloomfield Peter S, Howes O, Zanotti-Fregonara P, Bertoldo A, Veronese M. The methodology of TSPO imaging with positron emission tomography. Biochemical Society Transactions. 2015a;43(4):586–592. doi: 10.1042/BST20150058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer FE, Rizzo G, Bloomfield PS, Howes O, Zanotti-Fregonara P, Bertoldo A, Veronese M. The methodology of TSPO imaging with positron emission tomography. Biochem Soc Trans. 2015b;43(4):586–592. doi: 10.1042/BST20150058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, Laruelle M, Rabiner EA, Gunn RN. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: Dissection of D3 signal and anatomy. NeuroImage. 2011;54(1):264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, Luurtsema G, Windhorst AD, Cahn W, Lammertsma AA, Kahn RS. Microglia Activation in Recent-Onset Schizophrenia: A Quantitative (R)-[11C]PK11195 Positron Emission Tomography Study. Biological Psychiatry. 2008;64(9):820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- van der Doef TF, de Witte LD, Sutterland AL, Jobse E, Yaqub M, Boellaard R, de Haan L, Eriksson J, Lammertsma AA, Kahn RS, van Berckel BN. In vivo (R)-[(11)C]PK11195 PET imaging of 18kDa translocator protein in recent onset psychosis. NPJ Schizophr. 2016;2:16031. doi: 10.1038/npjschz.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haren NE, Cahn W, Hulshoff Pol HE, Kahn RS. Confounders of excessive brain volume loss in schizophrenia. Neurosci Biobehav Rev. 2013;37(10 Pt 1):2418–2423. doi: 10.1016/j.neubiorev.2012.09.006. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Hulshoff Pol HE, Schnack HG, Cahn W, Brans R, Carati I, Rais M, Kahn RS. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63(1):106–113. doi: 10.1016/j.biopsych.2007.01.004. [DOI] [PubMed] [Google Scholar]
- van Kesteren CF, Gremmels H, de Witte LD, Hol EM, Van Gool AR, Falkai PG, Kahn RS, Sommer IE. Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry. 2017;7(3):e1075. doi: 10.1038/tp.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese M, Reis Marques T, Bloomfield PS, Rizzo G, Singh N, Jones D, Agushi E, Mosses D, Bertoldo A, Howes O, Roncaroli F, et al. Kinetic modelling of [(11)C]PBR28 for 18 kDa translocator protein PET data: A validation study of vascular modelling in the brain using XBD173 and tissue analysis. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17712388. 271678X17712388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Chitrapu A, Edelson JR, Roman KM, Moroco AE, Lewis DA. Molecular mechanisms and timing of cortical immune activation in schizophrenia. Am J Psychiatry. 2015;172(11):1112–1121. doi: 10.1176/appi.ajp.2015.15010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterfang M, McGuire PK, Yung AR, Phillips LJ, Velakoulis D, Wood SJ, Suckling J, Bullmore ET, Brewer W, Soulsby B, Desmond P, et al. White matter volume changes in people who develop psychosis. Br J Psychiatry. 2008;193(3):210–215. doi: 10.1192/bjp.bp.107.043463. [DOI] [PubMed] [Google Scholar]
- Witthaus H, Brüne M, Kaufmann C, Bohner G, Ozgürdal S, Gudlowski Y, Heinz A, Klingebiel R, Juckel G. White matter abnormalities in subjects at ultra high-risk for schizophrenia and first-episode schizophrenic patients. Schizophr Res. 2008;102(1–3):141–149. doi: 10.1016/j.schres.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Yung AR, Pan Yuen H, McGorry PD, Phillips LJ, Kelly D, Dell'olio M, Francey SM, Cosgrave EM, Killackey E, Stanford C, Godfrey K, et al. Mapping the Onset of Psychosis: The Comprehensive Assessment of At-Risk Mental States. Australian and New Zealand Journal of Psychiatry. 2005;39(11–12):964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Catts VS, Sheedy D, McCrossin T, Kril JJ, Weickert CS. Cortical grey matter volume reduction in people with schizophrenia is associated with neuro-inflammation. Translational Psychiatry. 2016;6:e982. doi: 10.1038/tp.2016.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Z Y, Ding Y-q, Liu Y, Zhang X, et al. Minocycline and Risperidone Prevent Microglia Activation and Rescue Behavioral Deficits Induced by Neonatal Intrahippocampal Injection of Lipopolysaccharide in Rats. PLoS ONE. 2014;9(4):e93966. doi: 10.1371/journal.pone.0093966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziermans TB, Schothorst PF, Schnack HG, Koolschijn PC, Kahn RS, van Engeland H, Durston S. Progressive structural brain changes during development of psychosis. Schizophr Bull. 2012;38(3):519–530. doi: 10.1093/schbul/sbq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Hastie T. Regularization and variable selection via the elastic net [Google Scholar]
- Zürcher NR, Loggia ML, Lawson R, Chonde DB, Izquierdo-Garcia D, Yasek JE, Akeju O, Catana C, Rosen BR, Cudkowicz ME, Hooker JM, et al. Increased in vivo glial activation in patients with amyotrophic lateral sclerosis: assessed with [(11)C]-PBR28. Neuroimage Clin. 2015;7:409–414. doi: 10.1016/j.nicl.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.