Abstract
Purpose
Despite extensive research, mechanisms regulating postnatal eye growth and those responsible for ametropias are poorly understood. With the marked recent increases in myopia prevalence, robust and biologically-based clinical therapies to normalize refractive development in childhood are needed. Here, we review classic and contemporary literature about how circadian biology might provide clues to develop a framework to improve the understanding of myopia etiology, and possibly lead to rational approaches to ameliorate refractive errors developing in children.
Recent findings
Increasing evidence implicates diurnal and circadian rhythms in eye growth and refractive error development. In both humans and animals, ocular length and other anatomical and physiological features of the eye undergo diurnal oscillations. Systemically, such rhythms are primarily generated by the ‘master clock’ in the surpachiasmatic nucleus, which receives input from the intrinsically photosensitive retinal ganglion cells (ipRGCs) through the activation of the photopigment melanopsin. The retina also has an endogenous circadian clock. In laboratory animals developing experimental myopia, oscillations of ocular parameters are perturbed. Retinal signaling is now believed to influence refractive development; dopamine, an important neurotransmitter found in the retina, not only entrains intrinsic retinal rhythms to the light:dark cycle, but it also modulates refractive development. Circadian clocks comprise a transcription/translation feedback control mechanism utilizing so-called clock genes that have now been associated with experimental ametropias. Contemporary clinical research is also reviving ideas first proposed in the nineteenth century that light exposures might impact refraction in children. As a result, properties of ambient lighting are being investigated in refractive development. In other areas of medical science, circadian dysregulation is now thought to impact many non-ocular disorders, likely because the patterns of modern artificial lighting exert adverse physiological effects on circadian pacemakers. How, or if, such modern light exposures and circadian dysregulation contribute to refractive development is not known.
Summary
The premise of this review is that circadian biology could be a productive area worthy of increased investigation, which might lead to the improved understanding of refractive development and improved therapeutic interventions.
Keywords: circadian rhythms, clock genes, dopamine, melanopsin, myopia, refractive development
Introduction
Despite numerous investigative approaches, much speculation, and continuing controversy extending over several centuries,1 the etiology of refractive errors remains poorly understood. While clinicians and researchers have accumulated information, the field lacks a validated, broad biological framework to conceptualize the postnatal optical development of the eye. This review will concentrate on myopia (i.e., nearsightedness) because its prevalence is high, it is associated with significant ocular complications, and most recent research addresses mechanisms for myopia development as distinct from hyperopia (i.e., farsightedness). Importantly, considerable literature suggests that the mechanisms responsible for myopia vs hyperopia are not just reciprocal, but may be different.2–4
We shall discuss how advances in the understanding of circadian biology may relate to the puzzle of myopia etiology, reviewing ocular rhythms related to eye growth and refractive development and describing pertinent retinal biochemistry and molecular biology. We shall briefly summarize the expanding literature on how ambient lighting and modern behaviors contribute to circadian dysregulation in contemporary societies, and how circadian dysfunction is now increasingly recognized as contributing adversely to systemic health. We propose that applying concepts from modern circadian biology to eye development may provide the broad biological framework needed for a better understanding of myopia causality, and may lead to novel clinical therapies more efficacious than those currently available.
Clinical myopia results from the axial length of the eye exceeding the focusing power of the cornea and the lens, and thus the focal plane of distant objects is imaged anterior to the photoreceptors. Commonly, though not exclusively, myopia is due to excessive axial growth of the eye during childhood. Most distressingly, the incidence of myopia in children and young adults is increasing, especially in developed societies. Prevalence rates are particularly high in some urban areas of East Asia where 80%–90% or more of late adolescents have myopia. These high prevalences in East Asia developed relatively recently, often over just several decades.5–8 The prevalence of myopia in Western countries is somewhat lower but is still increasing in many areas.9–11 Current projections estimate that almost half of the world’s population may be myopic by 2050.12 Besides image defocus, clinicians have long recognized that myopia predisposes to many blinding conditions in adulthood, as reviewed elsewhere,1,13 including retinal detachment, various retinal and macular degenerations, open-angle glaucoma and forms of cataract. Besides visual blurring, myopia also ranks as a leading risk factor for acquired blindness later in life because of these associated diseases.
Complex interactions between genetics and the environment have long been hypothesized to contribute to the development and progression of myopia. Contemporary genetics offers the prospect of identifying specific regulatory pathways underlying the development of ametropias.14–16 To date, genetic linkage studies, genome-wide association studies, candidate gene studies, and meta-analyses have associated dozens of genes and gene variants with non-syndromic myopia.17,18 However, only some of these genes have been replicated in diverse population groups; furthermore, identified genes tend to exert only small effects, typically accounting for only a small proportion of myopia. It has proved difficult to implicate these genes in pathways identified in animal models, and much of the heritability of myopia is unexplained.14,19 Despite extensive efforts, the expanding, and at times, bewildering, gene lists emerging from contemporary analyses have not yet explained the rapidly increasing prevalence rates, and do not yet identify a path to develop approaches to lessen the adverse clinical impacts of myopia. Evidence best supports a role for inheritance in syndromic and high myopia (see Wojciechowski and Cheng,20 for review). For low and moderate myopia, as well as so-called ‘school myopia,’ however, it has been argued that features of modern environments, rather than genetics, may exert the major influence underlying myopia and its increasing prevalence in many areas of the world.15,21
The environmental parameters responsible for myopia, however, are far from clear. The non-genetic factors most consistently linked with myopia are higher socioeconomic status, higher scores on intelligence tests, higher levels of educational achievement, urban setting, and reduced time outdoors14,22–25; but an explanation of how these factors might lead to the biological effect of ocular enlargement remains obscure. Extensive use of the eyes for near tasks (e.g., reading), with the physiologic requirement for accommodation, has long been postulated to cause myopia. 26 However, modern epidemiological methods have yielded inconsistent and sometimes negative associations of near work with subsequent myopia.14,23–25 Topical pirenzepine, a muscarinic antagonist that does not block accommodation, slows myopia progression in children.27,28 Experimentally, an accommodative mechanism for myopia seems increasingly unlikely even though the mechanisms of myopia in animal models may not be identical to those occurring in children. As examples, experimental myopia develops after ciliary ganglionectomy to denervate the ciliary muscle,29,30 and myopia can be induced in squirrels that lack accommodative capacity.31 In contrast to the muscarinic pharmacology of the smooth intraocular muscles of mammals, the intraocular muscles of birds are striated, 32 and atropine is well-known to not block the nicotinic mechanism underlying accommodation in birds,33–35 which contrasts to the cycloplegic effects of atropine in children.36 Atropine blocks experimental myopia in chicks without affecting their intraocular muscles.37,38 The lack of cycloplegia with potent anti-myopia effects in birds supports a non-accommodative role in the mechanism of myopia, and provided the justification for the clinical pirenzepine studies discussed above.37 Finally, asymmetric vitreous cavity growth can be induced from local image distortions to specific retinal regions, but accommodation occurs symmetrically, and, therefore, would be unable to induce observed asymmetric vitreous cavity growth.39
The notion that light exposure might explain myopia etiology is now generating much interest.14,40 It was first postulated in the 19th century that inadequate lighting and/or reduced outdoor activities might be environmental factors contributing to myopia.41–45 Experimental myopia in several vertebrate species is reduced when animals are reared under bright laboratory lighting (e.g., 10 000–40 000 lux).46 In children, most, though not all, cross-sectional surveys and prospective studies find an anti-myopia effect of increasing outdoor exposure.47–49 Despite the statistical significance of the positive studies, however, the magnitudes of the favorable effects of increasing outdoor time in children are fairly modest overall, even if the protective effect is possibly meaningful in some children. For example, increasing outdoor exposure has been shown to exert a 9% reduction for myopia onset.48,49 Increased outdoor exposures may reduce myopia progression, but available estimates are approximately 0.2 diopters/year reduced progression.47–49 Outdoor sporting activities do not seem related to myopia inhibition.50 The protective mechanism of the outdoors is now assumed to be the intensity of outdoor lighting, compared to indoor lighting. Increased release of retinal dopamine, a retinal transmitter repeatedly linked to refractive development,4 is commonly hypothesized as a mechanism to explain the anti-myopia effects of the outdoors. In chicks, however, outdoor rearing resulted in only a transient inhibition in the progression of experimental myopia.51 The retinal and vitreous levels of dopamine and its principal metabolite DOPAC (3,4-dihydroxyphenylacetic acid) varied between tissues, eyes and cohorts; and this variability precluded identifying a consistent effect of the outdoors on dopamine or DOPAC. A robust marker, DOPAC was consistently depressed in the retina and vitreous of myopic eyes relative to their contralateral control eyes, similar to findings in myopia of chicks reared indoors. Thus, the effects of outdoor rearing on retinal dopamine metabolism were consistent with myopia, and not its inhibition,51 suggesting that a mechanism for any anti-myopia effects of outdoor exposure is more complicated than currently conceived.52 Vitamin D has been investigated as a potential mediator of the effects of time outdoors on myopia. The Avon Longitudinal Study of Parents and Children showed that vitamin D was a biomarker for time outdoors, but it was not independently associated with future myopia.53 Additionally, analysis of subjects with genetically determined low vitamin D levels uncovered no evidence of an association between serum vitamin D and refractive error.54 It is unknown if the duration or the timing of outdoor exposure in children, or some other feature of the complex nature of outdoor light,51 should be considered in future clinical investigations.
Circadian clocks and melanopsin
Circadian rhythms are biological variations with a period of approximately 24 h. They are generated by circadian clocks, which are autonomous cell-based molecular timing mechanisms. Circadian clocks regulate daily rhythms of sleep and alertness, blood pressure and heart rate, locomotor activity, hormone secretion, body temperature, metabolism, and many other physiological processes. Most of these rhythms are controlled directly or indirectly by the ‘master clock’ in suprachiasmatic nuclei (SCN) of the hypothalamus. 55 The clock mechanism generates daily rhythms of neuronal firing rate in the SCN and surrounding hypothalamic relay nuclei to control rhythmic physiology.56
Circadian rhythms and the molecular basis of circadian clocks
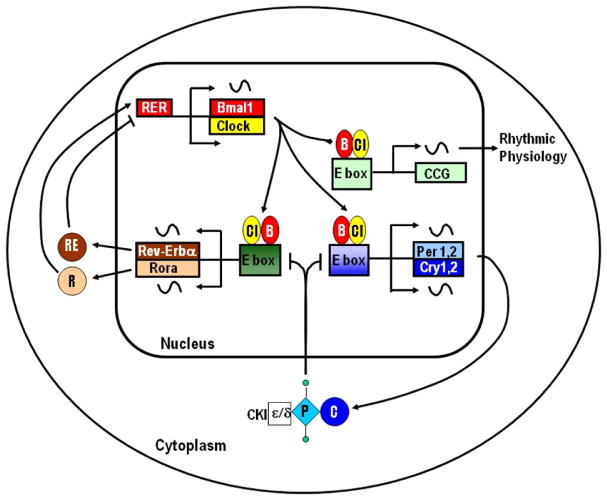
The molecular basis for circadian clocks consists of overlapping transcriptional–translational feedback loops (TTFL) involving a conserved set of ‘clock genes’ (Figure 1; for a recent review, see Takahashi57). Central to these feedback loops are two basic-helix-loop-helix PAS domain transcription factors Clock (Cl) and Bmal1 (B). Clock and Bmal1 heterodimerize and bind to E box elements in the promoters of the cryptochrome (Cry1 and Cry2) and period (Per1, Per2 and Per3) genes to activate their transcription. The protein products of the Cry and Per genes heterodimerize and complex with casein kinase 1 (CK1), which phosphorylates PER. The resulting complex translocates into the nucleus where it inhibits Clock/Bmal1-mediated transcription of the period and crytochrome genes. The temporal delay between transcription and translation combined phosphorylation, nuclear import, and proteasomal degradation results in daily rhythms of the transcripts and protein products of the period and cryptochrome genes. A second TTFL involves activation of the Rev-erb-a (RE) and Rora (R) genes by Clock/Bmal1. The protein products of these genes drive rhythmic Bmal1 expression through binding to the Rev-erb/Ror elements (RER) in the Bmal1 promoter. The output of clock is, in part, through clock-controlled genes (CCGs). Many CCGs have E box elements in their promoters that are rhythmically activated by Clock/Bmal1.
Figure 1.
Molecular mechanism of the circadian clock. Modified from Iuvone et al.,58 with permission from Elsevier, Ltd.
Npas2 is a paralog of Clock.59 Like Clock, Npas2 heterodimerizes with Bmal1 and activates circadian E boxes. Both Clock and Npas2 are expressed in the cells of SCN, where they have overlapping roles.60,61 Knocking out only one of the genes encoding these transcription factors does not disrupt circadian rhythms in the SCN; the double knockout is required. Coupled with the multiple period and crytochrome genes, these observations indicate that there is considerable redundancy built into the circadian molecular clock to ensure it keeps on ticking.
Peripheral circadian clocks
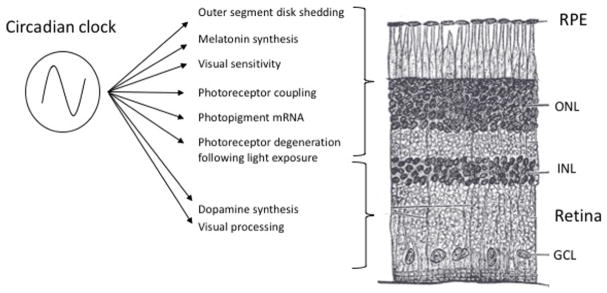
Prior to the early 1980s, vertebrate circadian rhythms were thought to be generated solely by the SCN.62 However, in 1983, evidence was presented demonstrating the presence of circadian clock(s) in the eye of Xenopus laevis that is/are independent of the SCN, generating a rhythm of the melatonin-synthesizing enzyme, serotonin N-acetyltransferase. This rhythm persists for several days in constant darkness and can be entrained by light in vitro in Xenopus eye cups maintained in organ culture.63 Subsequently, it was shown that isolated Xenopus photoreceptors generate rhythms of melatonin release that can be entrained by light or dopamine and persist for several days in constant darkness, providing definitive evidence for an autonomous circadian clock in photoreceptors.64,65 Circadian rhythms of melatonin release have also been observed in mammalian retina,66 as well as many non-mammalian species. It is now clear that many aspects of retinal physiology are under the influence of circadian clocks (Figure 2; for a recent review, see McMahon et al.67).
Figure 2.
Circadian processes in the retina and the retinal pigment epithelium, with their approximate location identified by retinal layer. RPE, retinal pigment epithelium; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Modified from McMahon et al.,67 with permission from Elsevier, Ltd.
Circadian clock genes are found in many retinal cell types, including RPE, bipolar cells, horizontal cells, amacrine cells, ganglion cells, and Müller glial cells, as well as in photoreceptor cells,68–74 particularly cones.75 The clocks in the retina use the same set of clock genes expressed in the SCN,76–79 although there appears to be less redundancy than in the SCN.80
Dopamine, a putative regulator of refractive development, 81–83 appears to play a particularly important role in the retinal circadian system. Clock genes are highly expressed in dopamine amacrine cells.68,84 Dopamine entrains circadian rhythms of gene expression in RPE, photoreceptors, and retinal ganglion cells,79,85–89 as well as the inner retinal rhythmic expression of the circadian reporter Per2 luciferase.90 Disruption of dopamine signaling disrupts circadian rhythms of cone ERG responses, contrast sensitivity,79,85 and protein phosphorylation in photoreceptor cells.91–93 Dopamine regulates connexin-36 gap junctions in the inner and outer retina.93,94 Interestingly, polymorphisms in the gene that encodes connexin-36, GJD2, have been associated with myopia in genome-wide association studies.95,96 With the presumed role of retinal dopamine in refractive development discussed below,81–83 findings suggest that interaction between dopamine and retinal circadian clocks may influence refractive development and myopia.
Melanopsin
In the 1990’s, a series of studies revealed that rod and cone photoreceptors were not required for photoentrainment of circadian rhythms of locomotor activity in mice,97–99 providing evidence for a non-rod, non-cone ocular photoreceptor. Subsequently, an opsin that regulates light-induced dermal melanophore migration in X. laevis was discovered and termed melanopsin.100 This opsin is more closely related to cephalopod opsins than to rod and cone opsins, suggesting an ancient evolutionary origin. It was found to be expressed in the Xenopus eye, as well as in the skin. In non-mammalian vertebrates, such as chicks, melanopsin is expressed in several retinal cell types. There are two forms of melanopsin, designated Opn4x and Opn4m due to sequence similarity to the Xenopus and mammalian genes, respectively.101 A human homolog of melanopsin has been cloned (gene symbol OPN4), and found to be exclusively expressed in a subpopulation of retinal ganglion cells,102 commonly referred to as intrinsically photosensitive retinal ganglion cells (ipRGCs).103 The ipRGCs are directly photosensitive, 104 through the activation of melanopsin, and some axons project directly to the SCN.105,106 Unlike rod and cone photoreceptors, which hyperpolarize in response to light, ipRGCs show a depolarizing response to light similar to photoreceptors of invertebrates. Melanopsin is a vitamin A-based photopigment, like the rod and cone opsins, but with a distinct action spectrum with maximum absorption at 484 nm (blue).
In addition to innervating the SCN to photoentrain circadian rhythms, ipRGCs influence retinal signal processing via retrograde signals. For example, the diurnal rhythm of the photopic ERG observed in wildtype mice is absent in melanopsin knockout (Opn4−/−) mice. ipRGCs appear to synapse with dopamine amacrine cells and increase their activity,107–109 providing a light-driven centrifugal signal to the inner retina. Furthermore, elimination of ipRGCs attenuates light adaptation in the cone ERG, and this effect is reversed by treatment with a dopamine receptor agonist. 109 The synapses between ipRGCs and dopamine amacrine cells may be reciprocal, as dopamine appears to regulate melanopsin expression.110 Collectively, these studies suggest that ipRGCs and melanopsin could influence the retinal pathways contributing to refractive development.
Another recently identified opsin, neuropsin (Opn5), is expressed in another subset of retinal ganglion cells.111 Neuropsin has been shown to play a role in the photoentrainment of retinal circadian clocks, but not the clocks in the SCN. Thus, neuropsin is another candidate for regulating rhythms of eye growth and refractive development.
Alterations of clock genes and clock-related genes in experimental myopia in chicks
A transcriptome analysis of the effects of 6 h of hyperopic defocus with a −15 D lens in chicks identified several classes of differentially expressed genes in combined retina and retinal pigment epithelium (RPE), including genes related to glutamate, GABA, glycine, and acetylcholine neurotransmission. 112 In addition, the expression of several circadian clock genes and clock-related genes was down-regulated. These included Clock (circadian locomotor output cycles kaput), Cry1 (cryptochrome 1), Npas2 (neuronal pas domain protein 2), Per3 (period homolog 3), as well as Mtnr1a, which encodes the type 1 melatonin receptor, and melanopsin (Opn4). These genes were unaffected by myopic defocus with a +15 D lens.
In another study, comparing the effects of form-deprivation in indoor-reared and outdoor-reared chicks revealed changes in the expression of clock genes in combined retina/RPE samples after 3 days of form-deprivation.51 As in other assays of experimental myopia,13 the magnitude of the altered retinal/RPE gene expression between goggled and non-goggled eyes was small in cohorts reared indoors or outdoors, regardless of chick age. Besides the small magnitude of the effects, there were also variabilities in the gene expression changes between cohorts, hampering generalized conclusions. Nonetheless, the expression of each of the assayed genes was affected under one or more conditions. These measurements were made at a single time of day, and it is unknown if rhythms of clock gene expression are affected by myopia-producing stimuli. Retina and RPE were not separated for analysis in these reports, and it is possible that the neurosensory retina and RPE contribute differently to the altered expression of clock or circadian rhythm-related genes, as recently seen for Opn4 in tree shrew eyes after minus lens wear.113
Potential involvement of the endogenous retinal clock in refractive development
Animal models of myopia are useful for isolating potential circadian rhythm effects from other factors modulating refractive development. Besides assaying clock and circadian rhythm-related genes just discussed, another approach is directly assaying the contributions of endogenous retinal clocks in refractive eye growth by investigating mice with genetic elimination of specific retinal clock genes. Due to the critical nature of clock genes and normal circadian rhythms, systemic knock-outs of intrinsic clock genes can reduce survival and cause other confounding metabolic abnormalities.114,115 Thus, retinal-specific knock-outs would be most advantageous to study. To date, we are only aware of results from retinal-specific Bmal1 knock-out mice with normal visual input in which the refractive error was significantly more myopic than wild-type mice across age, with longer axial length and vitreous chamber depth, but shallower anterior chamber depth.116 Interestingly, these changes are similar to those seen in chicks reared in constant light,117 a condition also known to exert intensity dependent effects on the circadian period,118 and conforming to a hypothesis that lighting effects on refractive development might relate to effects on the circadian clock.
Initial direct investigations of melanopsin in refraction
As mentioned above, time outdoors and increased light exposure have been shown to be protective against myopia in children,47,119 and increased light intensity inhibits form-deprivation myopia in animals.120,121 Studies have demonstrated that increased light exposure leads to an increase in retinal dopamine,122 which may, in part, be mediated by synaptic connections between ipRGCs and dopaminergic amacrine cells.123 Additionally, ipRGCs are spectrally tuned to short wavelength light with a peak sensitivity around 484 nm,124 which is a prominent wavelength in sunlight. Evidence from these studies suggest a potential role for melanopsin in refractive development.
To examine the contribution of melanopsin to normal refractive development and form-deprivation myopia in mice, a melanopsin knock out mouse (Opn4−/−) was utilized. 125 When raised under normal visual conditions, Opn4−/− mice were significantly more myopic at 4 weeks, then more hyperopic by 16 weeks, compared to wild type mice. Additionally, Opn4−/− mice undergoing form-deprivation were significantly more myopic than wild type animals. These results suggest that melanopsin signaling pathways contribute to both normal refractive development and form-deprivation myopia in mice. However, the mechanism by which melanopsin interacts with refractive development has yet to be elucidated.
The influence of monochromatic light on refractive status has been evaluated in guinea pigs to understand the role of melanopsin in myopia.126 Animals raised in short wavelength blue light (480 nm, peak sensitivity of melanopsin ganglion cells) were approximately 2 D less myopic than those raised in medium wavelength green light (530 nm, peak sensitivity of the guinea pig medium wavelength cone). Additionally, animals raised in blue light had decreased melatonin concentration in the pineal gland than those raised in medium wavelength light. The authors found that melanopsin-immunolabeled cells, melanopsin mRNA and protein were increased in animals that were raised in blue light compared to green light. While these results do not directly implicate melanopsin as having a role in the observed decrease in myopia with blue light, further investigations are required to understand the influence of ipRGCs in myopia.
A primary role for ipRGCs is photic regulation of melatonin release from the pineal gland.127 Recent studies have found that myopia may contribute to decreased sleep quality in humans.128,129 Jee et al., found an inverse relationship between sleep duration and myopia in Korean adolescents.129 Similarly, another study reported that children with high myopia exhibited the poorest scores on the Pittsburgh Sleep Quality Index (PSQI), a sleep quality questionnaire. 128 The authors attributed the observed decreased sleep quality to high demands in school, distress over poor vision, or decreased ipRGC function in myopic eyes. Sleep, circadian rhythm, and ipRGC activity have also been shown to be affected in diseases which affect the inner retina, such as glaucoma,130 and in outer retinal diseases, including age related macular degeneration.131 Another study reported a positive association between morning melatonin concentration and the magnitude of myopia, with myopes demonstrating up to three times greater melatonin concentration than non-myopes.132
Adhikari and colleagues evaluated the post-illumination pupil response in human subjects,133 and found no associations between refractive error and the ipRGC inputs to the pupil control pathway. However, another investigation found a significant positive correlation between a more hyperopic refractive error and a slower rate of pupil redilation to 0.1 Hz flashing stimuli, which varied as a function of light exposure over the previous 5 days.134 Conflicting results and current interest in light exposure and eye growth warrant further investigation into the role of melanopsin in refractive error development and potential relationships between light exposure, myopia, and the ipRGC-driven pupil responses.
Ocular diurnal rhythms and eye growth
Following initial observations by Jensen and Matson,135 Lauber and colleagues investigated the effects of constant darkness or constant light on ocular growth in chicks.136,137 Discovery of elongated eyes and flat corneas in chicks exposed to continuous light led to the hypothesis that the absence of normal diurnal cues (e.g., the light/dark cycle) may alter ocular diurnal rhythms, resulting in abnormal eye growth and refractive error development. Over the ensuing decades, some progress has been made towards understanding the role that ocular diurnal (or circadian) rhythms play in the process of emmetropization, and accordingly, in the development of ametropias.138 This section will focus on diurnal rhythms in axial length, choroidal thickness, scleral biosynthesis rates, and intraocular pressure (IOP), and discuss their potential influences on ocular growth and refractive error development in humans and animal models.
Natural diurnal variations in axial length and choroidal thickness
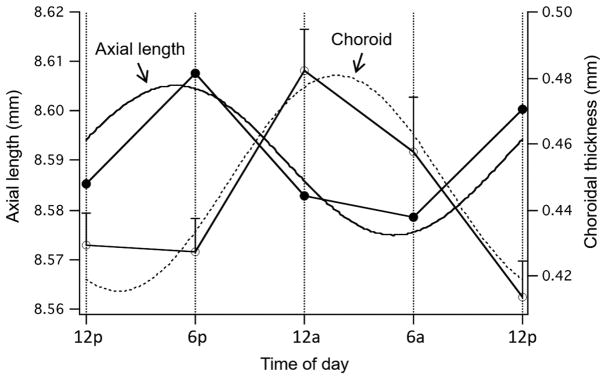
Diurnal variations in axial length of the eye were first reported by Weiss and Schaeffel,139 who found that eyes of young chicks grow during the day and ‘shrink’ at night. Axial length was measured as the distance from the anterior cornea to the RPE. This finding was corroborated and expanded upon by two later studies,140,141 one of which, by using more frequent measurements, showed that the acrophase (rhythm peak) occurred in the afternoon.140 Perhaps more importantly, the better resolution afforded by higher frequency ultrasonography140 and non-contact laser interferometry 141 made possible the discovery that the thickness of the choroid also showed diurnal oscillations, thickening during the night and thinning during the day, in approximate anti-phase to the rhythm in axial length (Figure 3). Thus, the night-time shortening of eye length, or at least part of it, was a result of the choroidal thickening. These rhythms persisted in constant darkness in chick for at least three cycles; hence, they are endogenous, or driven by a clock.142
Figure 3.
Mean rhythms in axial length (black circles; left axis) and choroidal thickness (white circles; right axis) in normal chick eyes measured at 6 h intervals over 24 h. For axial length, the slope of the data for each eye (i.e., the underlying eye growth rate) was subtracted out to yield the ‘cyclic component.’ The curves are the sine waves with a fixed 24 h period fit to the data. From Nickla,143 with permission from Springer.
These biometric rhythms were also found in a primate, the common marmoset.144 In young juvenile marmosets, the phases of the two rhythms were similar to those of chicks, with the axial rhythm peaking in the daytime, and the choroidal rhythm peaking at night, in approximate anti-phase. However, in older marmosets, the axial length rhythm peaked at night instead of day, resulting in the two rhythms being in-phase. This phase-relationship difference between the two age groups was hypothesized to be related to differing ocular growth rates; in slow-growing chick eyes that were responding to myopic defocus induced by positive spectacle lenses, the rhythms were in-phase as well, similar to that of the older, slower-growing monkey eyes.143
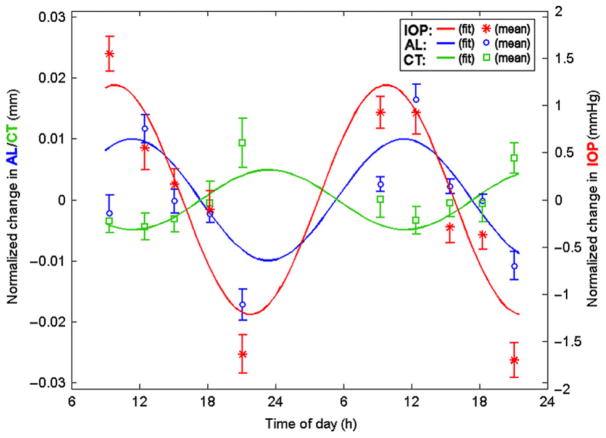
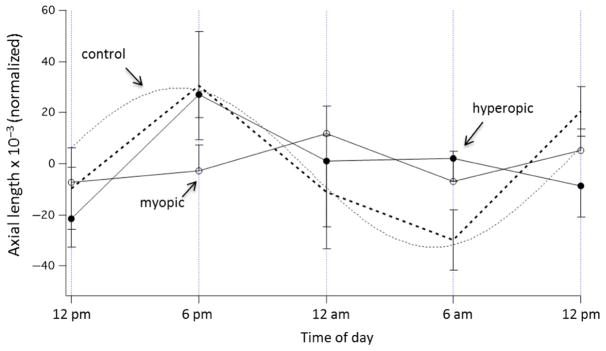
The evolution of precise, non-contact techniques, such as the partial coherence interferometry (PCI) and optical coherence tomography (OCT), has allowed changes in axial length and choroidal thickness of the human eye to be measured accurately at a micron level. Studies utilizing PCI have shown that the axial length of human eyes, across various ages (7–53 years), undergoes significant diurnal variations (Figure 4).145–148 Similar to findings in different animal eyes, the axial length of the human eye is typically longest during the midday and shortest at night, with an average magnitude of diurnal variation of around 25–45 μm.146,148 Furthermore, a study that measured ocular diurnal rhythms over two consecutive days (at 3 h intervals from 9:00 am to 9:00 pm each day) found the magnitude and timing of rhythms to be similar between age-matched adult myopic and emmetropic subjects (mean age, 25 years).148 The mean diurnal change in axial length equates to approximately 0.06 to 0.11 D in human eyes,149 which is a visually and clinically insignificant shift in focus. However, these findings demonstrate that rhythms are conserved across species, and thus may play an important role in regulating normal ocular growth in humans, similar to chicks and marmosets.138
Figure 4.
Rhythms in axial length (AL, blue), choroidal thickness (CT, green) and intraocular pressure (IOP, red) in human subjects measured at 10 different times over 2 consecutive days (symbols), fit with fixed 24 h period sine waves. Note that the rhythms in axial length and choroidal thickness are in approximate anti-phase to one another. From Chakraborty, et al.,148 with permission from Association for Research in Vision and Ophthalmology©.
There have been only limited investigations into diurnal variations of the choroid in human eyes. Brown et al., in a re-analysis of existing PCI data, reported the first evidence of diurnal variations in choroidal thickness of human subjects. 150 They found the diurnal fluctuations in choroidal thickness to be in approximate anti-phase to that of axial length. Since then, a number of studies using the PCI and OCT have confirmed diurnal variations in choroidal thickness, with the choroid being thickest during the night and thinnest during the day, having a mean diurnal amplitude of about 30 μm (Figure 4).148,151,152 With a mean subfoveal choroidal thickness of approximately 250, 30 μm corresponds to an approximately 12% change, resulting in a small refractive effect of about 0.18 D.148 Similar to animal eyes, the average phase of the longest axial length coincides with that of the thinnest choroid, and vice versa (i.e. peaks of the two rhythms are anti-phase by about 12 h)148; the magnitude and phase of these fluctuations are similar between adult myopes and emmetropes.148 Future studies are required to examine the phase associations in young progressing myopic and emmetropic children to better understand the relationship between ocular diurnal rhythms and refractive error development.
Effects of altered visual environment on diurnal variations
One of the crucial findings from Weiss & Schaeffel was that depriving the eye of form vision, which results in excessive elongation and myopia, altered the rhythm in axial growth so that instead of ‘shrinking’ at night like eyes with a normal visual experience, eyes grew at night as well as day, appearing to abolish the diurnal rhythm.139 This observation was the first concrete evidence since earlier constant light studies135–137 that ocular diurnal rhythms may be involved in eye growth regulation. However, while it was true that the deprived eyes were indeed growing faster at night, the rhythm in axial length was not abolished. Measurements at 6 h intervals showed that the rhythm was intact but phase-shifted by a few hours, bringing the axial length and choroid rhythms into exact anti-phase.140 Stronger evidence for an association between alterations in ocular growth rate and alterations in rhythm phase was that slowing growth by imposing myopic defocus with positive lenses caused a phase-delay in the axial rhythm and a phase-advance in the choroidal rhythm, thus bringing the two rhythms into phase.143 In faster-growing eyes wearing negative lenses, the two rhythms were similar to those of (faster-growing) form-deprived eyes. Data from eyes growing at different rates in response to form-deprivation or defocus showed a significant positive correlation between growth rate and the phase difference between the two rhythms, supporting a causal link between the two143; however, recent evidence regarding temporal integration properties (see below) has weakened this hypothesis.153
The molecular/cellular mechanisms underlying the diurnal changes in choroidal thickness, and how these might impinge on scleral growth, are unknown. It is likely that the choroid responds to upstream signals from the retina and/or RPE, but whether these choroidal responses involve thickness-dependent (and hence diurnal-dependent) release of growth factors, or whether the choroid acts as a barrier or facilitator to these growth factors depending on its thickness, requires further study. There is evidence that supports both hypotheses. For example, choroidal thickness predicts subsequent ocular growth rate in normal chick eyes, with thicker choroids predicting slower growth, and vice versa, in support of the ‘barrier’ hypothesis154 (however, see Guggenheim et al.155). Furthermore, thicker choroids synthesize more proteoglycans than thinner ones,156 hence the stiffness of the choroid, and thus the pressure (IOP) drop across it, might influence scleral proteoglycan synthesis in a diurnal manner.157 In support of the former ‘thickness-dependent release’ notion, thicker choroids of eyes responding to myopic defocus with decreased growth release greater quantities of retinoic acid than thinner choroids of eyes responding to negative lenses with increased eye growth. Furthermore, exogenous retinoic acid results in a decrease in scleral proteoglycan synthesis in vitro.158 Finally, co-culture of experimentally-thickened choroids with scleral punches results in decreased scleral proteoglycan synthesis.159 The precise signaling cascade, and exactly how diurnal rhythms in axial length (perhaps involving scleral proteoglycan synthesis), choroidal thickness and IOP interact to regulate ocular (i.e., scleral) growth clearly requires further study, but evidence strongly suggests that they play a crucial role in emmetropization.
The temporal integration dynamics of eye growth differ markedly for myopic vs hyperopic defocus: myopic defocus is more robust, such that the eye slows its growth in response to a few daily hours of exposure. The growth stimulating response to hyperopic defocus induced by negative lenses is thought to require almost the entire day of exposure. 160,161 However, recent evidence showed that a brief, 2 h exposure to hyperopic defocus in chicks given first thing in the morning resulted in a small but significant increase in eye growth.162 Conversely, if the eye is exposed to myopic defocus in the morning, the growth inhibition is significantly less than that resulting from defocus at midday. 153 Both ‘morning’ defocus conditions were associated with abnormal axial rhythms that did not exhibit a 24-h sinusoidal function (Figure 5). These results suggest that eye growth may be most responsive to ‘go’ signals in the first part of the light cycle, and more responsive to ‘stop’ signals in the mid to last half of the light cycle. This may be due to diurnal sensitivities of the retinal defocus ‘integrator,’ and/or a scleral growth regulator that are perhaps most susceptible to hyperopic defocus early in the day, and to myopic defocus later in the day, or to the potentially diurnally-dependent choroidal responses discussed above. Whether scheduling reading activities at specific times of the day could have a positive impact on school children at risk of developing myopia could be a novel approach to investigate interactions of near work with refractive development.
Figure 5.
Axial length (normalized to the mean) at 6 h intervals for chicks exposed to 2 h myopic (open symbols) or hyperopic (closed symbols) defocus in the morning (7 am–9 am), vs control eyes (dotted lines and sine wave fit). Note that data from both of the morning defocus conditions cannot be fit to a 24-h sine function (data from Nickla et al.).153,162
Defocus-induced phase shifts in axial length and choroidal thickness rhythms occur in humans as well.163,164 In two different studies, Chakraborty and colleagues investigated the effects of 12 h (9:00 am to 9:00 pm) monocular myopic163 and hyperopic164 defocus on ocular diurnal rhythms in adult human subjects. Consistent with observation in chicks, the introduction of +1.50 D of monocular myopic defocus for 12 h reduced the mean amplitude of the diurnal rhythm in axial length, and phase shifted both axial length (phase-delayed) and choroidal thickness (phase-advanced) rhythms of the defocused eye.163 Conversely, exposure to the same duration of −2.00 D of monocular hyperopic defocus results in a significant increase in the amplitude of diurnal change, but no change in the peak timing of diurnal rhythms in both parameters. 164 The effects of defocus were confined to the defocused eye with no significant crossover effects on the contralateral (untreated) eye. In both studies, the changes in axial length rhythms were approximately anti-phase to choroidal rhythms (peaks separated by approximately 12 h), suggesting that defocus induced changes in axial length may be associated with changes in choroidal thickness. These blur-induced bidirectional changes indicate that ocular diurnal rhythms of human subjects are sensitive to both the presence and sign of imposed defocus. These findings may help understand of the potential role of optical defocus in regulating longer-term ocular growth and refraction in human eyes.
The animal studies mentioned above provide definitive evidence that ‘abnormal’ visual input that causes changes in ocular growth rate also affects the phase, amplitude and form of the ocular diurnal rhythm curves, and thus support an association between altered rhythms and altered eye growth. Use of constant light135,136,165 or darkness166–168 also leads to abnormalities in eye growth, perhaps related to altered rhythms in ocular dimensions because constant light in particular can affect the circadian clock. However, the role of rhythms was not explicitly tested in early studies.
What about exposure to light at night, such that one might experience in modern society? The advent of artificial light, and resultant increase in the use of night-time illumination, including exposures to (predominantly) blue light from computer/video/TV screens, has become a recognized health concern,169–173 but its potential impact on vision and ocular health has largely been ignored. A retrospective study of 479 children published years before the recent surge of interest in ‘light at night’ reported a significant association between the use of nighttime lighting in the first 2 years of life and the development of myopia174 – a controversial finding that was not replicated by other studies published around the same time.175–178 Nevertheless, in chicks, exposure to a mere 2 h of light at mid-night caused an ‘acute’ growth stimulation over the 6 h following the illumination, which altered the rhythm in axial length, eventually resulting in longer eyes.179 Hence, fragmentary evidence from both humans and animals supports the notion that effects from light exposure, perhaps by influencing circadian rhythms, may underlie the development of ametropias. Future studies are needed to examine the interaction of contemporary patterns of light exposures and rhythms in ocular dimensions in young, progressing myopic children (or children at risk of developing myopia) to better understand the relationship between ocular diurnal rhythms and refractive error development, and to assess the potential of modifying the rhythms as a possible therapeutic target for inhibiting myopic ocular growth in children.
The rhythms in IOP and emmetropization: risk factors for myopia?
While ocular enlargement is an established consequence of elevated IOP in congenital glaucoma,180 a long-held, but at this point, mostly discredited hypothesis was that common myopia is a result of IOP stretching the sclera, but whether this was due to an abnormally-high IOP, or a weaker-than-normal sclera, was debatable (see Pruitt,181 for review). While some find higher daytime IOPs in myopes than nonmyopes, 182–186 other reports are contradictory. For example, a prospective study of 106 Chinese children found that in the 13 children who became myopic, IOP was higher after the development of myopia, but not prior to it.187 Another group followed emmetropic children over a 3 year period and reported that the IOP of children who developed myopia did not differ from those who remained emmetropic.188 It is notable that most of these studies were limited by non-controlled times of the day for IOP measurements, leaving unresolved the question of whether IOP measured at a single time point is a crucial risk factor for myopia, or whether the amplitude or phase of the rhythm in IOP specifically might impact myopia development. Several studies have addressed this issue by measuring IOP at different times of day in myopic and emmetopic subjects: most report that IOP peaks in the late night/early morning in both (2:00–4:00 am)189–191; however, it was inconclusive whether rhythm parameters differed between groups. Two studies found that myopes had a smaller IOP rhythm amplitude.192,193 By contrast, a prospective study of glaucoma patients with myopia reported no difference in IOP amplitude compared to emmetropes, but myopes had higher daytime IOPs and greater variability in phase.194 Determining diurnal IOP rhythms in humans using traditional IOP measurement techniques is fraught with confounding issues such as awakening participants for measures. Recent advances in technology have led to the development of novel sensors to measure IOP191,195 that may provide more comprehensive measurements of habitual diurnal IOP rhythms and further insight into relationships between IOP rhythms and myopia. To date, however, the bulk of evidence weighs against a role for IOP in the development of myopia.
Is there an association between the rhythms in IOP and rhythms in axial length?
All species studied exhibit diurnal fluctuations in IOP, however, their phases and amplitudes differ. In rabbits,196–198 rats,199,200 and mice,201 IOP is lower during the day than at night. By contrast, the rhythm in guinea pigs is more similar to that of humans191 and monkeys,202 with an acrophase (peak) in the early morning and a trough after lights off.203 In the chick, the IOP rhythm shows an acrophase at mid-day and persists in constant darkness, stablishing it as circadian.157 Notably, the phase and shape of the IOP rhythm in chick is similar to that of the rhythm in axial length (Figure 6), perhaps suggesting that it ‘drives’ the rhythm in length by inflating the eye during the day and relaxing it at night.140 In support of such a ‘mechanical’ mechanism, ocular compliance (change in length per change in exerted pressure) is consistent with IOP fluctuations accounting for the changes in length.
Figure 6.
The rhythms in IOP (black symbols) and axial length (open symbols), fit with fixed 24 h period sine waves. The data for the two parameters are from different sets of chicks (n = 10 in each group). Note that the rhythms in IOP are similar (almost in-phase) to the rhythms in axial length. Black bars indicate darkness. Nickla, et al.,157 with permission from Academic Press Limited.
However, observed dissociations between the IOP and axial length rhythms weaken this hypothesis. For instance, in form-deprived myopic eyes, the IOP rhythm becomes desynchronized from the light:dark cycle (i.e., show variable acrophases) and hence dissociated from the axial length rhythm which remains ‘normal’.157 Similarly, lesions of the superior cervical ganglion (sympathectomy) markedly reduce the amplitude of the IOP rhythm while having no effect on the rhythm in axial length.204 Therefore, while a purely ‘balloon-like’ inflation/deflation mechanism is not supported, an alternate way for the IOP rhythm to influence eye size is that the changing forces exerted on sclera could alter matrix (proteoglycan) synthesis which would eventually lead to changes in eye size.205 In support of this, the application of mechanical force changes the synthesis of matrix components in connective tissues (see van Kampen et al.,206 for review); furthermore, cyclic changes in force are the most effective at increasing biosynthesis rates.207 One hypothesis is that the rhythm in IOP influences the rhythm in scleral proteoglycan synthesis, which itself is rhythmic, with an efficacy that is dependent on the phase of the (rhythmic) scleral cell cycle. If so, then the phase at which IOP peaks with regard to one of these other rhythms might determine the effect of IOP on matrix synthesis and hence, ocular elongation, with myopia developing if its peak was coincident with an optimal phase of scleral biosynthethic ‘receptiveness’ to force. The evidence for a role for scleral biosynthesis in the development of myopia is discussed in the following section.
Scleral rhythms and emmetropization
Because ocular elongation is rhythmic,139–141 and because eye growth in chicks is influenced by the rate of synthesis of scleral proteoglycans (matrix molecules),156,208–210 it is plausible that a rhythm in scleral matrix synthesis underlies the rhythm in axial length. From in vitro explant cultures of chick sclera harvested at different times of the day, it was found that scleras from normal eyes synthesized more proteoglycans during the morning than at night,205,211 although scleras from myopic eyes had higher rates of synthesis at all times.205 More detailed data were obtained from experiments using a flow-through perfusion system that measured the secretion of radiolabeled proteoglycans from chick sclera at 2 h intervals205: scleras from normal eyes showed an endogenous 24 h (diurnal) rhythm in proteoglycan synthesis, chiefly chondroitin-6-sulfate, that persisted for at least 3 days in culture. Scleras from myopic eyes were also rhythmic, but Fourier analysis identified the major frequency component to be 1.875 cycles per day (i.e., ultradian), with a secondary diurnal frequency (i.e., 1 cycle per day). Because the phase was strongly re-set by the culture conditions, potential phase differences in synthesis rates between normal and myopic scleras could not be determined. Therefore, while an endogenous circadian rhythm in proteoglycan synthesis in chick sclera contributes to or may even underlie the oscillations in axial length, the lack of a large difference between normal vs myopic scleral rhythms suggests that the excessive elongation in myopic eyes is not a major result of alterations in rhythm parameters in scleral proteoglycan synthesis. However, the strong ultradian oscillations in scleras from myopic eyes might be a contributing factor, perhaps related to the altered (desynchronized) rhythms in IOP in these eyes. These speculations await further study.
In humans, a recent study using anterior segment OCT reported diurnal variations in the thickness of the temporal anterior sclera of young adults.212 The authors found significant thickening of the sclera in the early morning immediately after waking, and thinning of the sclera during the mid-day, with a mean amplitude of 21 μm. The total scleral thickness (668 μm) and the amplitude of diurnal variation was maximum at the scleral spur (52 μm), compared to regions up to 2.5 mm away from the scleral spur. If or how these diurnal fluctuations in scleral thickness relate to the onset or progression of myopia requires future research.
Retinal signaling in refractive development
Animal models of experimental myopia have revealed that local retinal processing is essential for the response to form deprivation or lens defocus, and presumably normal emmetropization.39,213 However, the specific retinal electrical and chemical signals that modulate refractive eye growth remain elusive. Here we review retinal circuitry and chemical signaling that may contribute to refractive development and how these are linked to circadian rhythms. Notably, retinal stimulation through the ON pathway of the retina stimulates two key regulators of circadian rhythms, dopamine and ipRGCs.
Retinal pathways relevant to refractive development
Light hyperpolizes the plasma membranes of both rods and cones to stimulate parallel vertical pathways in the retina that lead to activation of ON- and OFF-center ganglion cells (Figure 7). Visual information can travel from the rods to second and third order neurons via three different pathways: (1) Hyperpolarized rods synapse on rod ON bipolar cells causing them to depolarize and release glutamate. The rod bipolar cells synapse onto AII amacrine cells, which also depolarize, and form inhibitory synapses with cone OFF bipolar cells and excitatory electrical synapses with cone ON bipolar cells.214 (2) Rods form gap junctions with cones,215 which then activate cone pathways that stimulate ON and OFF pathways. (3) Rods form synapses to OFF bipolar cells.216 Cone activation by light leads to direct stimulation or inhibition of ON and OFF cone bipolar cells,214 which make excitatory synapses to ON- and OFF-center ganglion cells. Rods also regulate ON and OFF pathways, but indirectly through the AII amacrine cells. This complex neural network leads to several parallel retinal circuits that stimulate common ON and OFF pathways. Additionally, circadian rhythms modulate which pathways are being used through the opening and closing of gap junctions.217
Figure 7.
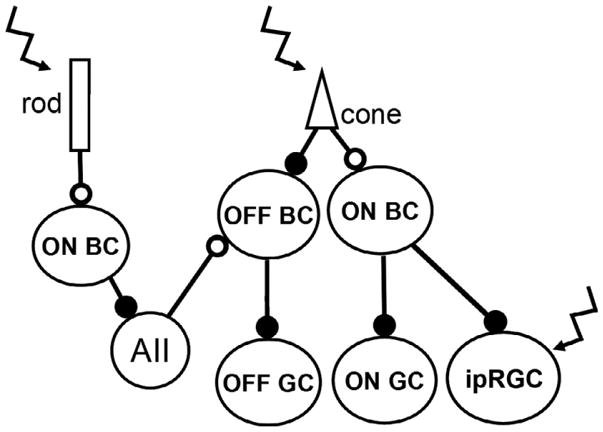
Schematic of the ON and OFF pathway circuits in the mammalian retina. Open circles indicate sign-inverting synapses, closed circles indicate sign-conserving synapses, and zig-zag arrows indicate photosensitive cells. BC: bipolar cells; AII: type II amacrine cell, GC: ganglion cell; ipRGC: intrinsically photosensitive retinal ganglion cell. Modified from Soucy et al., 1998.216
Additionally, ipRGCs can be stimulated directly by activation of the photopigment, melanopsin, or through the traditional rod and cone photoreceptor pathways (Figure 7).218 The five types of ipRGCs identified in mammals receive predominately ON pathway input.219 However, they are also unique in sending signals retrogradely from the ipRGCs to the dopaminergic amacrine cells, to potentially facilitate light-adapted vision.109 In addition to the role in imaging forming vision, ipRGCs also contribute to non-imaging forming behaviors, including the pupil light reflex and circadian photoentrainment.218,220
The contributions of these different retinal circuits to refractive development is complex.221 Evidence for photoreceptor-initiated pathways consists of studies showing that animal models with cone-dominated retinas have the most consistent and largest responses to experimental myopia (chick and tree shrew). Furthermore, in the chick, rod pathways may be suppressed diurnally,222 further implicating cone pathways in refractive development. However, another study suggests that the cone-rich fovea is not needed for emmetropization in non-human primates223: laser ablation of the fovea in rhesus monkeys did not prevent successful refractive development or the response to form-deprivation.223 This study suggests that the peripheral retina, which is composed of approximately 5% cones and 95% rods in humans,224 can control refractive development. However, these studies did not empirically test whether the rods or the cones are driving the response. Studies of mice with selective rod pathway dysfunction [rod transducin (Gnat1) mutation] fail to ‘emmetropize’; i.e., Gnat1−/− mice show no alterations in refractive error across age while wild-type mice shift towards more hyperopic refractions and then plateau at about 6 weeks of age.225 Furthermore, absence of rod pathway activation prevents the eye from responding to form-deprivation. Gnat1−/− mice showed no shift in refractive error even after 8 weeks of goggling while wild-type mice show a −2.54 ± 0.77 D shift by 3 weeks.225 Rod photoreceptors drive circadian photoentrainment across a wide range of intensities,226 thus, these effects could be due to loss of circadian rhythms rather than specific rod pathway stimulation. Additionally, cone dysfunction caused by elimination of cone transducin (Gnat2) did not alter refractive development with normal visual input, but did increase the susceptibility to myopia (2.5 D diopters greater).227
Studies on the contributions of ON and OFF pathways to refractive development and myopia have been relatively few. ON and OFF pathways have been examined in the chick model of form-deprivation myopia using pharmacological blocking agents228–231 and lens induced myopia using flicker stimulus (moving or stationary pattern stimuli). 232 However, no definitive conclusions have been reached. In a study using kittens, ON pathway blockage with D,L-2-amino-4-phosphonobutyric acid (APB) resulted in more hyperopic refractions and shorter axial lengths.233 Indirect clinical evidence of ON pathway involvement in modulating refractive eye growth comes from patients with retinopathy of prematurity in which the ON response, as recorded with multifocal ERGs, decreased as the amount of myopia increased (although myopia in retinopathy of prematurity is mainly due to changes in anterior chamber depth and corneal curvature rather than axial elongation). 234 Patients with the complete form of X-linked congenital stationary night blindness (CSNB1), who have a defect that results in loss of ON pathway transmission and not rod function, present with high myopia.235 In contrast, patients with the incomplete form of X-linked CSNB (CSNB2), in which a calcium channel defect decreases, but does not eliminate, both ON and OFF transmission, do not present with high myopia.235 More recently, a mouse model with a defect in the ON pathway between rods and ON bipolar cells due to an Nyx mutation has been examined. 236–238 Nob mice with this mutation have successful refractive development in a normal visual environment,239 suggesting that visual activation of intact retinal pathways provides feedback for a successful match between eye size and ocular power. However, form-deprivation leads to increased susceptibility to myopia in Nob mice, suggesting increased sensitivity to alterations in visual input.239 Increased susceptibility to form-deprivation was replicated in another ON pathway defect model with an mGluR6 mutation,240 suggesting that the ON pathway disruption is the important element for refractive development. Furthermore, the OFF pathway does not appear to be critical for refractive development, as disruption of the OFF pathway caused by Vsx1 mutation did not significantly alter refractive development or the response to form deprivation.241
Direct comparisons of the different experimental paradigms is difficult because it is unknown if the ON pathways is completely blocked or what compensatory mechanisms may have occurred for the loss of function. However, studies in mutant mice that are due to a functional defect in the retina (and not a retinal degeneration) suggest that defects in rod photoreceptor pathways and ON retinal pathways may have larger impacts on the refractive development and/or the susceptibility to experimental myopia (Table 1). However, it is notable that these mutations don’t affect refractive development under normal and visual deprived conditions equally, conforming to clinical hypotheses that complex interactions of genetic and environmental factors underlie myopic eye growth. The importance of ON pathway stimulation in refractive development may be due to its influence on circadian regulators, such as dopamine (discussed below) and melanopsin (see Section 2.3). Further work is needed to determine how circadian rhythms may alter these retinal pathways and therefore affect susceptibility of the eye to myopic eye growth.
Table 1.
Summary of the results on refractive development with normal visual input for form-deprivation in genetic mouse mutants with dysfunctional photoreceptor or ON/OFF pathways
Classic retinal neurotransmitters and dopamine in refractive development
Transmission of the visual signal through the retinal circuitry requires neurotransmitters and other neuromodulators and neuropeptides. Since transmission is regulated by gap junctions modulated by circadian rhythms and multiple neurotransmitters are modulated by dopamine, it is important to review which neurotransmitters could contribute to refractive eye growth through circadian entrainment, even if the direct evidence of this is currently sparse. Synaptic communication between the vertical pathways in the retina occurs through the release of glutamate. This is the main neurotransmitter between photoreceptor and bipolar cells in both the ON and OFF pathways, acting on metabotrophic and ionotropic glutamate receptors on the ON- and OFF-bipolar cells, respectively. Lateral communication and feedback occurs through GABA and glycine. GABA is the main inhibitory neurotransmitter in the retina and acts through GABA(A), GABA(B) and GABA(C) receptors, hyperpolarizing bipolar and amacrine cells, and decreasing action potentials in the ganglion cells,242 although GABA(B) receptor activation has also been shown to enhance excitatory signals.243 Glycine released from AII amacrine cells inhibits OFF cone bipolar cell neurotransmission. 244
Direct evidence that these classic neurotransmitters in the retina modulate refractive development is relatively sparse. Guoping et al. reported that the ratio of glutamate to GABA was upregulated in myopic guinea pig eyes,245 suggesting that the ratio of excitatory and inhibitory neurotransmitters may be important for myopic eye growth. Several studies in animals and humans implicate defects in glutamate receptors with myopia.95,240,246–248 However, since the glutamate receptors are so tightly coupled with ON and OFF pathway function it is difficult to determine if these associations are due to glutamate receptors or the loss of normal visual transmission through the retina.
Experiments with GABA(A), GABA(B), and GABA(C) agonists and antagonists in chicks identified complex eye growth effects, including myopia from a GABA(A) agonist in non-goggled eyes and reduced form-deprivation myopia from a number of agents.249 GABA(B) receptor antagonists inhibit form-deprivation myopia in guinea pigs.250–252 In mice with lens induced myopia, GABA transporter 1 levels are elevated in myopia retina.253 Studies on glycine and myopia are absent from the literature. Perhaps due to the ubiquitous nature of these neurotransmitters for inhibitory signals and the absence of methods to selectively isolate these critical neurotransmitters, the role of these potential signals has been difficult to elucidate.
Dopamine is an important retinal neuromodulator that regulates circadian rhythms,254 different aspects of visual function,255 retinal gap junctions for optimal retinal sensitivity under different light levels217 and light adaptation. 79,256 Dopamine synthesis and release occurs in a subset of dopaminergic amacrine and interplexiform cells257,258 and is stimulated by ON pathway visual transmission. 259–261 Tyrosine hydroxylase (TH), the rate-limiting enzyme for dopamine synthesis, and dopamine are found throughout the retina.262 The dopaminergic amacrine cells synapse onto the AII amacrine cells and have reciprocal synapses back to the rod ON bipolar cell.259 There is morphological evidence that a subtype of ON bipolar cell synapses onto dopaminergic amacrine cells.263,264
Dopamine is one of the few biochemical signals that has been implicated in form-deprivation myopia in both chick and mammalian models.81,82,265–273 Form-deprivation causes a decrease in dopamine levels which appears to be due to a decrease in dopamine biosynthesis81,274 and not due to a decrease in the number of dopaminergic amacrine cells.275 In addition, dopamine and the dopamine receptor agonist, apomorphine, reduce the expected axial elongation of experimental myopia in a dose-dependent fashion in chicks, rabbits, macaques, guinea pigs (only in form deprivation, not in lens defocus) and mice (although lower doses in mice appear to have the optimal affect).81,265,268,276–278 Reducing retinal dopamine in mice using pharmacology270 or genetic mutations271 produces myopic refractions under normal visual experience. Dopamine-mediated refractive changes appear to be due to activation of dopamine receptors since co-administration of dopamine D2 receptor antagonists abolish the effect of apomorphine in the chick,276 and genetic inactivation of D2 receptors reduces form-deprivation myopia in mice.269 Furthermore, D2-like receptor agonists (and selective D4 receptor agonists) reduce form deprivation in tree shrews.273 However, the role of dopamine signaling in refractive development and myopia is complex, since the depletion of retinal dopamine did not alter the response to form-deprivation,271 C57BL6 mice do not have a measureable decrease in retinal dopamine with form-deprivation,279 and the dopamine D1 receptor may also be involved.272 Further evidence of the complexity is demonstrated by studies in guinea pigs with spontaneously occurring myopia that suggest D1, and not D2 receptors are the mediators of the dopamine effects in slowing refractive eye growth.280
Our traditional view of two photoreceptor types with strict roles in scotopic and photopic conditions has been challenged by a number of findings, including the additional connections of rods to cones and rods to OFF bipolar cell connections216 and rods functioning effectively under daylight conditions.281 Likewise, viewing each neurotransmitter/neuromodulator as providing an isolated signal to retinal neurons for refractive eye growth is also likely simplistic. For instance, both GABA and dopamine are released by retinal dopaminergic cells,282 and interactions of dopamine and GABA may modulate the response to form-deprivation.283 Additionally, both GABA and dopamine can be released in response to light or depolarization of ON-bipolar cells. Since these two neurotransmitters/neuromodulators are so tightly linked and dopamine regulates circadian rhythms, it is difficult to determine which may be involved in signaling refractive eye growth.
Peptides, growth factors, and refractive eye growth
Other molecules also provide signaling in the retina, including glucagon, nitric oxide, growth factors, and retinoic acid, and they may also modulate refractive eye growth. Some of these molecules have also been linked to circadian rhythms or other neuromodulators known to control photoentrainment.284 For instance, vasoactive intestinal polypeptide (VIP) may signal eye growth, since blocking VIP in chicks reduced the development of form-deprivation myopia,285 and retinal levels of VIP increase with lid suture in primates.286 Increasing glucagon signaling via agonists or exogenous injections can inhibit form-deprivation in chicks.287,288 Although in a mouse model of form-deprivation myopia, expression of glucagon-related peptides did not change.289 Apolipoprotein A-1 may also act like a ‘stop’ signal for refractive eye growth,290 possibly by binding to retinoic acid.291
Growth factors have also been implicated as potential signals for refractive eye growth. Basic fibroblast growth factor (FGF2) and transforming growth factor beta (TGF-beta) appear to work in opposing directions in chick form-deprivation myopia, with FGF2 reducing myopia and TGF-beta having no effect alone, but blocking the effect of FGF2.292 Vitreal injections of FGF2 and insulin-like growth factor (IGF1) created excessive vitreous chamber growth and high myopia in chicks with normal visual input.293 FGF2 may also act downstream of dopaminergic amacrine cells,274 creating a signaling pathway for scleral growth. While FGF2 expression increased in myopic guinea pigs, a screen of Chinese individuals for single nucleotide polymorphisms in FGF2 and high myopia did not show any association,294 potentially revealing differences between avian myopia, mammalian models, and human myopia. However, a recent study showed that TGF-beta and FGF2 gene expression is increased in scleral desmocytes in the guinea pig after lens-induced myopia.295
Pathway from retina to optical structures in the eye
Regardless of which retinal circuits need to be stimulated for normal refractive development, the signaling molecule or signaling cascade needs to travel through the retina, RPE, choroid, and to the sclera to alter eye size. Thus, reviewing the receptor locations of the various signaling candidates and/or their effects on scleral cells may provide further insights into refractive eye growth mechanisms, keeping in mind that some of these signaling molecules directly or indirectly modulate circadian rhythms. In addition, we review potential signaling on the cornea that may alter corneal curvature and produce large changes in refractive power, particularly in the mouse model of myopia.
GABA receptors have been localized in chick cornea and sclera. GABA(C) receptor gene expression is found in chick scleral fibroblasts and chondrocytes,296 and GABA(A) and GABA(C) receptors are localized to corneal epithelium.297 GABAergic agents have been shown to alter DNA and glycosaminoglycan content of scleral fibroblast in culture.298 Glutamate and/or its receptors have not been reported in the sclera, but mGluR6 transcripts are found in corneal endothelium.299 Finally, dopamine acts by volume transmission in the retina which means that dopamine receptors are found on numerous retinal neurons as well as dopamine D2 and D5 receptors on the RPE.88,300–302 While the localization of dopamine receptors in isolated posterior sclera has not been reported, dopamine D1 receptors have been detected in the uveo-sclera.303 Additionally, dopamine receptors have been located on the corneal endothelial and epithelial cells.304,305 Finally, growth factors may influence scleral and/or corneal growth. TGF-beta and FGF2 genes are present in the sclera.295 TGF-beta acts to regulate growth of scleral fibroblasts.306 IGF2 receptor regulates cell differentiation in the cornea.307 Localization of these receptors to corneal, RPE, and sclera may underlie modulation of eye size/shape for refractive eye growth as shown by changes in gene expression in RPE after lens defocus of chicks308 and tree shrews,113 and by gene expression changes in sclera of tree shrews with myopia.309,310
Attenuated circadian signals in modern societies
For most vertebrates, environmental light is the most important Zeitgeber (German, for ‘time giver’) for synchronizing circadian activity. The 24-h light:dark cycle entrains almost all circadian rhythms in the body, including the eye, to environmental light. The technical evolution of artificial lighting has markedly affected how biological systems, both humans and other terrestrial animals, interact with light. The pronounced differences in spatial, temporal and spectral properties of artificial compared to natural lighting impact the environments of all developed societies.311,312 Indoor lighting is less intense and color-shifted compared to daytime outdoor lighting, depending on the characteristics of the illumination source.313,314 The intensity levels of typical indoor lighting measure 100–500 lux; that of outdoor lighting ranges from 1000 to over 100 000 lux, depending on atmospheric conditions.315,316 Sleep timing and melatonin rhythms, for instance, vary between time spent in a natural outdoor environment (e.g., camping) and in habitual indoors that include artificial light during both day and night.317
The situation during night arguably is even more complex than that during the day. The term ‘skyglow’ refers to diffuse luminance of the night sky from artificial sources, excluding light from the moon, stars and other natural lighting. Individual artificial lights at night illuminate directly the surrounding area. Broad areas of artificial lighting, as occurring in cities, illuminate the sky; and back scattering from atmospheric components, such as clouds or dust, create skyglow. Skyglow, or light pollution, is a major component of ambient illumination at night. The intensity of skyglow varies over 10 000-fold, hundreds of times more variation in night illumination than before the introduction of artificial lighting, and the radiance and extent of artificial light on the Earth’s surface is increasing.318 Because of reflections from clouds, skyglow that originates from ground lighting is actually greater on overcast than clear nights; further, the luminance can change very rapidly as the cloud cover shifts.319 Besides gross intensity effects, modern artificial lighting distorts the gradual and symmetrical light–dark transitions at the beginning and end of night; and skyglow may be greater in the evening than just before dawn because of human habits in the use of ground lighting.319 Many uncertainties and assumptions underlie efforts to estimate the distribution and intensity of ambient light at night. One worldwide atlas from satellite data shows marked differences in light intensity at night, with urban areas and economically developed regions far more affected than rural areas,320 both within and between countries. In addition to skyglow, artificial light exposures at night include increasing use of light emitting electronics, such as televisions, computers, and hand held devices, all of which have been shown to disrupt normal sleeping behavior in young children and adolescents,321,322 and can contribute to disruption of biological rhythms.
Of pertinence and concern here, increasingly literature points towards many adverse effects of artificial lighting on human health. Light synchronizes the circadian clock and, hence, circadian rhythms, to the light:dark cycle occurring not just daily but even seasonally; distortions in the ‘natural’ light:dark cycle act as major environmental stress factors. 323,324 The commonly hypothesized mechanism for adverse health effects from artificial patterns of lighting is disruption of endogenous circadian rhythms. Besides effects on sleep and mood, circadian disruptions in contemporary societies now seem to contribute to specific diseases, including some cancers, obesity, diabetes, and certain neurological diseases.170,323,325–327 Current research suggests that artificial light at night is a major factor for these circadian disruptions and increased disease risks.324,328
Could these circadian disruptions in modern societies also be contributing to the increasing prevalence of myopia? Though far from proven, this now seems a plausible hypothesis. The increasing myopia prevalence is a relatively modern phenomenon in developed and developing societies, occurring concurrently with the evolution of more efficient lighting over the past 200 years: from gas and early incandescent sources (carbon arc, lime, etc.) to more efficient incandescent vacuum bulbs and now to more advanced alternative light sources (fluorescence, LEDs, etc.). The retina is the main or exclusive light sensing organ across the vertebrate kingdom, providing the photic input to entrain the systemic circadian system and containing its own endogenous circadian clock. During daytime, the comparatively low intensity levels of indoor lighting have long been proposed as contributing to myopia development. 43–46 Based on the circadian hypothesis proposed here, the resulting reduced strength329 of the circadian signal from low intensity daytime lighting could provide a mechanism for myopia. The human eyelid behaves chiefly as a red-pass filter, with transmission estimates in the red of some 5%–20%330,331; thus even during sleep, light can reach the retina particularly from red-enriched sources like incandescent or specialized lighting, thus also dampening a circadian signal by confounding the dark phase.
Besides intensity, the color of light is increasingly recognized as influencing both circadian rhythms and refractive development. From available data in animals, blue–yellow opponency can entrain the circadian clock in fish332 and permits more dependable tracking of twilight progression in mice than intensity,333 phenomena that are likely more complex than melanopsin activation alone. Besides an influence of chromatic cues on the emmetropization mechanism, 334 the refractive impact of extended rearing under restricted chromaticity varies by species, with red light inducing myopia in chicks335 and guinea pigs,336 but inducing or shifting refraction towards hyperopia in tree shrews337 and Rhesus monkeys.338 These results raise questions about how chromaticity modulates refraction, including (1) the retinal pathways through which melanopsin and ipRGCs might potentially act to influence eye growth via blue light exposure (see report of violet light having protecting effects)339; (2) whether retinal cells expressing the circadian clock link to long wavelength cones to influence refraction; and (3) how the timing and intensity of chromatic stimuli govern eye growth. Resolving questions such as these will require future research.
Summary
Given the retina’s central role in entraining circadian rhythms to the light:dark cycle,67 as well as refractive development, a role for circadian retinal functions in regulating postnatal eye growth is conceivable and conforms to data reviewed here. For many decades, researchers have studied the influence of ambient lighting on refractive development in laboratory animals. In chick and monkeys, altering the daily light:dark cycle by varying the duration135,266,340,341 and intensity120,342–344 of light leads to significant changes in normal eye growth and in ocular responses to goggles and spectacle lenses. Diurnal and/or circadian changes in ocular dimensions seem linked to refractive development 138; and retinal neurotransmitters involved in refractive development, such as dopamine and GABA, also modulate retinal circadian functions.4,67,249 Finally, the anti-myopia effects of light exposure in both animals and children might be hinting towards a correction of some type of circadian disruption. In fact, sleep disturbances have now been identified in myopic children and adolescents, 128,129,345 and sleep disorders may imply an underlying circadian rhythm disturbance in myopic children.
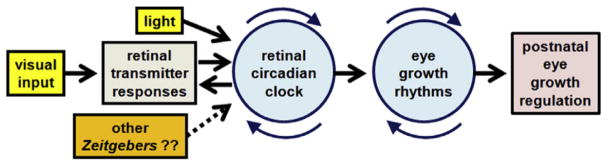
A proposed pathway that incorporates the retinal clock and eye growth rhythms in the regulation of postnatal eye growth and refraction is shown in Figure 8.346 This scheme suggests that retinal neurotransmitter responses to visual images interact reciprocally with the retinal circadian clock. Light and possibly other Zeitgebers, such as temperature and diet, synchronize the clock to the diurnal cycle, but also could desynchronize the clock if delivered in a non-physiologic manner. Presumably, the retinal clock regulates the diurnal or circadian rhythms in ocular growth and eye dimensions, which may comprise the mechanism through which the clock impacts the overall refractive development of the eye. This hypothetical framework conforms to the clinical and laboratory observations reviewed here; but much work is needed to identify the molecular components of this pathway in experimental animals and, ultimately, to learn if and how this framework might provide a means to favorably modify refractive development in children. Pathways independent of the circadian clock likely also modify refractive development, and how non-circadian mechanisms might interact with the retinal clock and growth rhythms requires future investigation.
Figure 8.
A proposed pathway that incorporates the retinal clock and eye growth rhythms in the regulation of postnatal eye growth and refraction. Modified from Stone, et al.,346 with permission from Elsevier.
Hypothesizing a role for circadian disruption as a myopia mechanism suggests approaches for developing novel, behaviorally based anti-myopia therapies. For example, circadian disruptions could result from either decreased amplitude or a phase shift of circadian rhythms, neither of which have been studied directly in relation to childhood refraction. Arguing against a mechanism involving a simple reduction in rhythm amplitude is the overall modest effect of outdoor exposures on protecting children against myopia. However, nighttime artificial lighting is now recognized as a major circadian disruptor for many non-ocular clinical conditions and diseases associated with altered rhythms. While previously proposed as a potential risk factor for myopia,174 nighttime lighting effects have been largely dismissed by researchers in this area because of disparate findings in subsequent research.175–178 Efforts to reduce ambient nighttime lighting, however, might increase the efficacy of outdoor exposures because reducing the intensity of nighttime lighting may enhance the circadian signal and augment the effects from elevated daytime lighting, as from outdoor exposures.
If the myopia risk mechanism in contemporary societies instead derives from a phase-shift of circadian rhythms in children, defining the nature of this may shift may lead to an effective therapy. The classic phase response curve, the relationship of bright light exposures to shifts of rhythms, is a basis for treating jet lag. The least effective times for using light exposures to shift circadian phases occur in the middle of the light phase, the so-called deadzone.347,348 Available anti-myopia studies typically increase outdoor exposures in the middle of the day, the time when any light-induced shift on biological rhythms would be expected to be minimal. Study of the timing of light exposures also could be a productive research direction for altering refractive development, but such investigations would be more efficiently and rationally designed after defining the nature of any circadian disorder in ametropic children, if it indeed occurs.
A very incomplete understanding the mechanistic basis of refractive disorders continues to hamper introduction of rational, effective therapies. This review brings together many recent findings suggesting that circadian biology may provide a direction that could move the field forward. How circadian rhythms or clock related genes might directly link to the regulation of eye growth is not known. Nonetheless, a circadian hypothesis could shift the research in refractive development from eye-specific and descriptive clinical hypotheses to an area of very active medical/biological investigation with rapidly advancing molecular and biochemical insights. The area of circadian biology, though, is quite complex. Hopefully, the ideas proposed here will stimulate novel research directions needed to understand the mechanisms underlying refractive errors.
Acknowledgments
This work was supported by National Institutes of Health R01 EY016435 (MTP), R01 EY004864 (PMI), R01 EY027711 (PMI), P30 EY006360 (PMI), R01 EY022342 (RAS/PMI/MTP), EY001583 (RAS), EY013636 (DLN) and EY025307 (DLN); the Department of Veterans Affairs Research Career Scientist Award (MTP), Research to Prevent Blindness (RAS, PMI), Paul and Evanina Bell Mackall Foundation Trust (RAS).
Footnotes
Disclosure
The authors report no conflicts of interest and have no proprietary interest in any of the materials mentioned in this article.
References
- 1.Curtin BJ. The Myopias: Basic Science and Clinical Management. Harper & Row; Philadelphia: 1985. [Google Scholar]
- 2.Schwahn HN, Schaeffel F. Flicker parameters are different for suppression of myopia and hyperopia. Vision Res. 1997;37:2661–2673. doi: 10.1016/s0042-6989(97)00114-4. [DOI] [PubMed] [Google Scholar]
- 3.Riddell N, Giummarra L, Hall NE, et al. Bidirectional expression of metabolic, structural, and immune pathways in early myopia and hyperopia. Front Neurosci. 2016;10:390. doi: 10.3389/fnins.2016.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013;114:106–119. doi: 10.1016/j.exer.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Lin LL, Tsai CB, Liu JC, et al. Correlation between ocular refractions with longitudinal study among schoolchildren in Taiwan. In: Tokoro T, editor. Myopia Updates Proceedings of the 6th International Conference on Myopia; Tokyo: Springer; 1998. pp. 53–57. [Google Scholar]
- 6.Pan CW, Saw SM, Wong TY. Epidemiology of myopia. In: Spaide RF, Ohno-Matsui K, Yannuzzi LA, editors. Pathologic Myopia. Springer; New York: 2014. pp. 25–38. [Google Scholar]
- 7.Xiang F, He M, Zeng Y, et al. Increases in the prevalence of reduced visual acuity and myopia in Chinese children in Guangzhou over the past 20 years. Eye (Lond) 2013;27:1353–1358. doi: 10.1038/eye.2013.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.You QS, Wu LJ, Duan JL, et al. Prevalence of myopia in school children in greater Beijing: the Beijing Childhood Eye Study. Acta Ophthalmol. 2014;92:e398–e406. doi: 10.1111/aos.12299. [DOI] [PubMed] [Google Scholar]
- 9.Parssinen O. The increased prevalence of myopia in Finland. Acta Ophthalmol. 2012;90:497–502. doi: 10.1111/j.1755-3768.2011.02210.x. [DOI] [PubMed] [Google Scholar]
- 10.Vitale S, Sperduto RD, Ferris FL., 3rd Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol. 2009;127:1632–1639. doi: 10.1001/archophthalmol.2009.303. [DOI] [PubMed] [Google Scholar]
- 11.Williams KM, Bertelsen G, Cumberland P, et al. Increasing prevalence of myopia in Europe and the impact of education. Ophthalmology. 2015;122:1489–1497. doi: 10.1016/j.ophtha.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Stone RA, Khurana TS. Gene profiling in experimental models of eye growth: clues to myopia pathogenesis. Vision Res. 2010;50:2322–2333. doi: 10.1016/j.visres.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherwin JC, Mackey DA. Update on the epidemiology and genetics of myopic refractive error. Expert Rev Ophthalmol. 2013;8:25. [Google Scholar]
- 15.Morgan IG, Rose KA. Gene-environmental interactions in the aetiology of myopia. In: Beuerman RW, Saw SM, Tan DTH, et al., editors. Myopia: Animal Models to Clinical Trials. World Scientific; New Jersey: 2010. pp. 45–61. [Google Scholar]
- 16.Wojciechowski R. Nature and nurture: the complex genetics of myopia and refractive error. Clin Genet. 2011;79:301–320. doi: 10.1111/j.1399-0004.2010.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan Q, Guo X, Tideman JW, et al. Childhood gene-environment interactions and age-dependent effects of genetic variants associated with refractive error and myopia: the CREAM Consortium. Sci Rep. 2016;6:25853. doi: 10.1038/srep25853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rong SS, Chen LJ, Pang CP. Myopia genetics – the Asia-pacific perspective. Asia Pac J Ophthalmol (Phila) 2016;5:236–244. doi: 10.1097/APO.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 19.Wojciechowski R, Hysi PG. Focusing in on the complex genetics of myopia. PLoS Genet. 2013;9:e1003442. doi: 10.1371/journal.pgen.1003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wojciechowski R, Cheng CY. Involvement of multiple molecular pathways in the genetics of ocular refraction and myopia. Retina. 2018;38:91–101. doi: 10.1097/IAE.0000000000001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan I, Rose K. How genetic is school myopia? Prog Retin Eye Res. 2005;24:1–38. doi: 10.1016/j.preteyeres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Rudnicka AR, Kapetanakis VV, Wathern AK, et al. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: implications for aetiology and early prevention. Br J Ophthalmol. 2016;100:882–890. doi: 10.1136/bjophthalmol-2015-307724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldschmidt E, Jacobsen N. Genetic and environmental effects on myopia development and progression. Eye (Lond) 2014;28:126–133. doi: 10.1038/eye.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012;32:3–16. doi: 10.1111/j.1475-1313.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 25.Zadnik K, Mutti DO. Incidence and distribution of refractive anomolies. In: Benjamin WJ, editor. Borish’s Clinical Refraction. Butterworth Heinemann Elsevier; St. Louis, MO: 2006. pp. 35–55. [Google Scholar]
- 26.Rosenfield M, Gilmartin B. Myopia and Nearwork. Butterworth-Heinemann; Oxford: 1998. [Google Scholar]
- 27.Siatkowski RM, Cotter S, Miller JM, et al. Safety and efficacy of 2% pirenzepine ophthalmic gel in children with myopia: a 1-year, multicenter, double-masked, placebo-controlled parallel study. Arch Ophthalmol. 2004;122:1667–1674. doi: 10.1001/archopht.122.11.1667. [DOI] [PubMed] [Google Scholar]
- 28.Tan DT, Lam DS, Chua WH, et al. One-year multicenter, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Ophthalmology. 2005;112:84–91. doi: 10.1016/j.ophtha.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 29.Lin T, Zhu X, Capehart C, et al. The ciliary ganglion and vitreous cavity shape. Curr Eye Res. 1996;15:453–460. doi: 10.3109/02713689609000756. [DOI] [PubMed] [Google Scholar]
- 30.Raviola E, Wiesel TN. An animal model of myopia. N Engl J Med. 1985;312:1609–1615. doi: 10.1056/NEJM198506203122505. [DOI] [PubMed] [Google Scholar]
- 31.McBrien NA, Moghaddam HO, New R, et al. Experimental myopia in a diurnal mammal (Sciurus carolinensis) with no accommodative ability. J Physiol. 1993;469:427–441. doi: 10.1113/jphysiol.1993.sp019821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walls GL. The Vertebrate Eye and Its Adaptive Radiation. Cranbrook Institute of Science; Bloomfield Hills, MI: 1942. [Google Scholar]
- 33.Gundlach RH, Chard RD, Skahen JR. The mechanism of accommodation in pigeons. J Comp Psychol. 1945;38:16. [Google Scholar]
- 34.Campbell HS, Smith JL. The pharmacology of the pigeon pupil. Arch Ophthalmol. 1962;67:4. doi: 10.1001/archopht.1962.00960020501020. [DOI] [PubMed] [Google Scholar]
- 35.Ostrin LA, Liu Y, Choh V, et al. The role of the iris in chick accommodation. Invest Ophthalmol Vis Sci. 2011;52:4710–4716. doi: 10.1167/iovs.10-6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennedy RH. Progression of myopia. Trans Am Ophthalmol Soc. 1995;93:755–800. [PMC free article] [PubMed] [Google Scholar]
- 37.Stone RA, Lin T, Laties AM. Muscarinic antagonist effects on experimental chick myopia. Exp Eye Res. 1991;52:755–758. doi: 10.1016/0014-4835(91)90027-c. [DOI] [PubMed] [Google Scholar]
- 38.McBrien NA, Moghaddam HO, Reeder AP. Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Invest Ophthalmol Vis Sci. 1993;34:205–215. [PubMed] [Google Scholar]
- 39.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Ramamurthy D, Lin Chua SY, Saw SM. A review of environmental risk factors for myopia during early life, childhood and adolescence. Clin Exp Optom. 2015;98:497–506. doi: 10.1111/cxo.12346. [DOI] [PubMed] [Google Scholar]
- 41.Carter RB. Eyesight: Good and Bad. 2. Macmillan and Co; London: 1880. [Google Scholar]
- 42.Cohn H. In: The Hygiene of the Eye in Schools. Turnbull WP, editor. Simpkin, Marshall and Co; London: 1886. [Google Scholar]
- 43.Harman NB. The control of school myopia. Sight Sav Rev. 1934;4:5. [Google Scholar]
- 44.Hobday R. Myopia and daylight in schools: a neglected aspect of public health? Perspect Public Health. 2016;136:50–55. doi: 10.1177/1757913915576679. [DOI] [PubMed] [Google Scholar]
- 45.Schaeffel F. Myopia – what is old and what is new? Optom Vis Sci. 2016;93:1022–1030. doi: 10.1097/OPX.0000000000000914. [DOI] [PubMed] [Google Scholar]
- 46.Norton TT, Siegwart JT., Jr Light levels, refractive development, and myopia – a speculative review. Exp Eye Res. 2013;114:48–57. doi: 10.1016/j.exer.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Read SA, Collins MJ, Vincent SJ. Light exposure and eye growth in childhood. Invest Ophthalmol Vis Sci. 2015;56:6779–6787. doi: 10.1167/iovs.14-15978. [DOI] [PubMed] [Google Scholar]
- 48.He M, Xiang F, Zeng Y, et al. Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. JAMA. 2015;314:1142–1148. doi: 10.1001/jama.2015.10803. [DOI] [PubMed] [Google Scholar]
- 49.Wu PC, Tsai CL, Wu HL, et al. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013;120:1080–1085. doi: 10.1016/j.ophtha.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Jones LA, Sinnott LT, Mutti DO, et al. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007;48:3524–3532. doi: 10.1167/iovs.06-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stone RA, Cohen Y, McGlinn AM, et al. Development of experimental myopia in chicks in a natural environment. Invest Ophthalmol Vis Sci. 2016;57:4779–4789. doi: 10.1167/iovs.16-19310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schaeffel F, Feldkaemper M. Myopia and outdoor exposures. Invest Ophthalmol Vis Sci. 2016;57:4790. doi: 10.1167/iovs.16-20540. [DOI] [PubMed] [Google Scholar]
- 53.Guggenheim JA, Williams C, Northstone K, et al. Does vitamin D mediate the protective effects of time outdoors on myopia? Findings from a prospective birth cohort. Invest Ophthalmol Vis Sci. 2014;55:8550–8558. doi: 10.1167/iovs.14-15839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuellar-Partida G, Williams KM, Yazar S, et al. Genetically low vitamin D concentrations and myopic refractive error: a Mendelian randomization study. Int J Epidemiol. 2017;46:1882–1890. doi: 10.1093/ije/dyx068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus: The Mind’s Clock. Oxford University Press; New York: 1991. [Google Scholar]
- 56.Gillette MU, Medanic M, McArthur AJ, et al. Intrinsic neuronal rhythms in the suprachiasmatic nuclei and their adjustment. Ciba Found Symp. 1995;183:134–144. doi: 10.1002/9780470514597.ch8. discussion 44–53. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18:164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iuvone PM, Tosini G, Pozdeyev N, et al. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog Retin Eye Res. 2005;24:433–456. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Reick M, Garcia JA, Dudley C, et al. NPAS2: an analog of clock operative in the mammalian forebrain. Science. 2001;293:506–509. doi: 10.1126/science.1060699. [DOI] [PubMed] [Google Scholar]
- 60.DeBruyne JP, Noton E, Lambert CM, et al. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron. 2006;50:465–477. doi: 10.1016/j.neuron.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 61.DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007;10:543–545. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nishino H, Kiyomi K, Brooks CM. The role of suprachiasmatic nuclei of the hypothalamus in the production of circadian rhythm. Brain Res. 1976;112:45–59. doi: 10.1016/0006-8993(76)90333-4. [DOI] [PubMed] [Google Scholar]
- 63.Besharse JC, Iuvone PM. Circadian clock in Xenopus eye controlling retinal serotonin N-acetyltransferase. Nature. 1983;305:133–135. doi: 10.1038/305133a0. [DOI] [PubMed] [Google Scholar]
- 64.Cahill GM, Besharse JC. Resetting the circadian clock in cultured Xenopus eyecups: regulation of retinal melatonin rhythms by light and D2 dopamine receptors. J Neurosci. 1991;11:2959–2971. doi: 10.1523/JNEUROSCI.11-10-02959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cahill GM, Besharse JC. Circadian clock functions localized in Xenopus retinal photoreceptors. Neuron. 1993;10:573–577. doi: 10.1016/0896-6273(93)90160-s. [DOI] [PubMed] [Google Scholar]
- 66.Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 67.McMahon DG, Iuvone PM, Tosini G. Circadian organization of the mammalian retina: from gene regulation to physiology and diseases. Prog Retin Eye Res. 2014;39:58–76. doi: 10.1016/j.preteyeres.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruan GX, Zhang DQ, Zhou T, Yamazaki S, McMahon DG. Circadian organization of the mammalian retina. Proc Natl Acad Sci. 2006;103:9703–9708. doi: 10.1073/pnas.0601940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tosini G, Davidson AJ, Fukuhara C, et al. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 2007;21:3866–3871. doi: 10.1096/fj.07-8371com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baba K, Sengupta A, Tosini M, et al. Circadian regulation of the PERIOD 2:LUCIFERASE bioluminescence rhythm in the mouse retinal pigment epithelium-choroid. Mol Vis. 2010;16:2605–2611. [PMC free article] [PubMed] [Google Scholar]
- 71.Sandu C, Hicks D, Felder-Schmittbuhl MP. Rat photoreceptor circadian oscillator strongly relies on lighting conditions. Eur J Neurosci. 2011;34:507–516. doi: 10.1111/j.1460-9568.2011.07772.x. [DOI] [PubMed] [Google Scholar]
- 72.Hiragaki S, Baba K, Coulson E, et al. Melatonin signaling modulates clock genes expression in the mouse retina. PLoS ONE. 2014;9:e106819. doi: 10.1371/journal.pone.0106819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dkhissi-Benyahya O, Coutanson C, Knoblauch K, et al. The absence of melanopsin alters retinal clock function and dopamine regulation by light. Cell Mol Life Sci. 2013;70:3435–3447. doi: 10.1007/s00018-013-1338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu L, Ruan G, Dai H, et al. Mammalian retinal Muller cells have circadian clock function. Mol Vis. 2016;22:275–283. [PMC free article] [PubMed] [Google Scholar]
- 75.Liu X, Zhang Z, Ribelayga CP. Heterogeneous expression of the core circadian clock proteins among neuronal cell types in mouse retina. PLoS ONE. 2012;7:e50602. doi: 10.1371/journal.pone.0050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chong NW, Chaurasia SS, Haque R, et al. Temporal-spatial characterization of chicken clock genes: circadian expression in retina, pineal gland, and peripheral tissues. J Neurochem. 2003;85:851–860. doi: 10.1046/j.1471-4159.2003.01723.x. [DOI] [PubMed] [Google Scholar]
- 77.Chaurasia SS, Pozdeyev N, Haque R, et al. Circadian clockwork machinery in neural retina: evidence for the presence of functional clock components in photoreceptor-enriched chick retinal cell cultures. Mol Vis. 2006;12:215–223. [PubMed] [Google Scholar]
- 78.Haque R, Ali FG, Biscoglia R, et al. CLOCK and NPAS2 have overlapping roles in the circadian oscillation of arylalkylamine N-acetyltransferase mRNA in chicken cone photoreceptors. J Neurochem. 2010;113:1296–1306. doi: 10.1111/j.1471-4159.2010.06698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hwang CK, Chaurasia SS, Jackson CR, et al. Circadian rhythm of contrast sensitivity is regulated by a dopamineneuronal PAS-domain protein 2-adenylyl cyclase 1 signaling pathway in retinal ganglion cells. J Neurosci. 2013;33:14989–14997. doi: 10.1523/JNEUROSCI.2039-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruan GX, Gamble KL, Risner ML, et al. Divergent roles of clock genes in retinal and suprachiasmatic nucleus circadian oscillators. PLoS ONE. 2012;7:e38985. doi: 10.1371/journal.pone.0038985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stone RA, Lin T, Laties AM, et al. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci U S A. 1989;86:704–706. doi: 10.1073/pnas.86.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iuvone PM, Tigges M, Fernandes A, et al. Dopamine synthesis and metabolism in rhesus monkey retina: development, aging, and the effects of monocular visual deprivation. Vis Neurosci. 1989;2:465–471. doi: 10.1017/s0952523800012360. [DOI] [PubMed] [Google Scholar]
- 83.Zhou X, Pardue MT, Iuvone PM, et al. Dopamine signaling and myopia development: what are the key challenges. Prog Retin Eye Res. 2017;61:60–71. doi: 10.1016/j.preteyeres.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dorenbos R, Contini M, Hirasawa H, et al. Expression of circadian clock genes in retinal dopaminergic cells. Vis Neurosci. 2007;24:1–8. doi: 10.1017/S0952523807070538. [DOI] [PubMed] [Google Scholar]
- 85.Jackson CR, Chaurasia SS, Hwang CR, et al. Dopamine D4 receptor activation controls circadian timing of the adenylyl cyclase 1/cyclic AMP signaling system in mouse retina. Eur J Neurosci. 2011;34:57–64. doi: 10.1111/j.1460-9568.2011.07734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kunst S, Wolloscheck T, Kelleher DK, et al. Pgc-1α and Nr4a1 are target genes of circadian melatonin and dopamine release in murine retina. Invest Ophthalmol Vis Sci. 2015;56:6084–6094. doi: 10.1167/iovs.15-17503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vancura P, Wolloscheck T, Baba K, et al. Circadian and dopaminergic regulation of fatty acid oxidation pathway genes in retina and photoreceptor cells. PLoS ONE. 2016;11:e0164665. doi: 10.1371/journal.pone.0164665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baba K, DeBruyne JP, Tosini G. Dopamine 2 receptor activation entrains circadian clocks in mouse retinal pigment epithelium. Sci Rep. 2017;7:5103. doi: 10.1038/s41598-017-05394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vancura P, Abdelhadi S, Csicsely E, et al. Gnaz couples the circadian and dopaminergic system to G protein-mediated signaling in mouse photoreceptors. PLoS ONE. 2017;12:e0187411. doi: 10.1371/journal.pone.0187411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruan GX, Allen GC, Yamazaki S, et al. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008;6:e249. doi: 10.1371/journal.pbio.0060249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pozdeyev N, Tosini G, Li L, et al. Dopamine modulates diurnal and circadian rhythms of protein phosphorylation in photoreceptor cells of mouse retina. Eur J Neurosci. 2008;27:2691–2700. doi: 10.1111/j.1460-9568.2008.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li H, Zhang Z, Blackburn MR, et al. Adenosine and dopamine receptors coregulate photoreceptor coupling via gap junction phosphorylation in mouse retina. J Neurosci. 2013;33:3135–3150. doi: 10.1523/JNEUROSCI.2807-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Z, Li H, Liu X, et al. Circadian clock control of connexin36 phosphorylation in retinal photoreceptors of the CBA/CaJ mouse strain. Vis Neurosci. 2015;32:E009. doi: 10.1017/S0952523815000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kothmann WW, Massey SC, O’Brien J. Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling. J Neurosci. 2009;29:14903–14911. doi: 10.1523/JNEUROSCI.3436-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Verhoeven VJ, Hysi PG, Wojciechowski R, et al. Genome-wide meta-analyses of multiancestry cohorts identify multiple new susceptibility loci for refractive error and myopia. Nat Genet. 2013;45:314–318. doi: 10.1038/ng.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoshikawa M, Yamashiro K, Miyake M, et al. Comprehensive replication of the relationship between myopia-related genes and refractive errors in a large Japanese cohort. Invest Ophthalmol Vis Sci. 2014;55:7343–7354. doi: 10.1167/iovs.14-15105. [DOI] [PubMed] [Google Scholar]
- 97.Foster RG, Provencio I, Hudson D, et al. Circadian photoreception in the retinally degenerate mouse (rd/rd) J Comp Physiol A. 1991;169:39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- 98.Provencio I, Wong S, Lederman AB, et al. Visual and circadian responses to light in aged retinally degenerate mice. Vision Res. 1994;34:1799–1806. doi: 10.1016/0042-6989(94)90304-2. [DOI] [PubMed] [Google Scholar]
- 99.Freedman MS, Lucas RJ, Soni B, et al. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 100.Provencio I, Jiang G, De Grip WJ, et al. Melanopsin: an opsin in melanophores, brain, and eye. Proc Natl Acad Sci U S A. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bellingham J, Chaurasia SS, Melyan Z, et al. Evolution of melanopsin photoreceptors: discovery and characterization of a new melanopsin in nonmammalian vertebrates. PLoS Biol. 2006;4:e254. doi: 10.1371/journal.pbio.0040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Provencio I, Rodriguez IR, Jiang G, et al. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berson DM. Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci. 2003;26:314–320. doi: 10.1016/S0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- 104.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 105.Hannibal J, Hindersson P, Knudsen SM, et al. The photo-pigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J Neurosci. 2002;22:RC191. doi: 10.1523/JNEUROSCI.22-01-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gooley JJ, Lu J, Chou TC, et al. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 107.Ostergaard J, Hannibal J, Fahrenkrug J. Synaptic contact between melanopsin-containing retinal ganglion cells and rod bipolar cells. Invest Ophthalmol Vis Sci. 2007;48:3812–3820. doi: 10.1167/iovs.06-1322. [DOI] [PubMed] [Google Scholar]
- 108.Zhang DQ, Wong KY, Sollars PJ, et al. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci U S A. 2008;105:14181–14186. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prigge CL, Yeh PT, Liou NF, et al. M1 IPRGCS influence visual function through retrograde signaling in the retina. J Neurosci. 2016;36:7184–7197. doi: 10.1523/JNEUROSCI.3500-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sakamoto K, Liu C, Kasamatsu M, et al. Dopamine regulates melanopsin mRNA expression in intrinsically photosensitive retinal ganglion cells. Eur J Neurosci. 2005;22:3129–3136. doi: 10.1111/j.1460-9568.2005.04512.x. [DOI] [PubMed] [Google Scholar]
- 111.Buhr ED, Yue WW, Ren X, et al. Neuropsin (OPN5)-mediated photoentrainment of local circadian oscillators in mammalian retina and cornea. Proc Natl Acad Sci U S A. 2015;112:13093–13098. doi: 10.1073/pnas.1516259112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stone RA, McGlinn AM, Baldwin DA, et al. Image defocus and altered retinal gene expression in chick: clues to the pathogenesis of ametropia. Invest Ophthalmol Vis Sci. 2011;52:5765–5777. doi: 10.1167/iovs.10-6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.He L, Frost MR, Siegwart JT, Jr, et al. Altered gene expression in tree shrew retina and retinal pigment epithelium produced by short periods of minus-lens wear. Exp Eye Res. 2018;168:77–88. doi: 10.1016/j.exer.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang G, Chen L, Grant GR, et al. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci Transl Med. 2016;8:324ra16. doi: 10.1126/scitranslmed.aad3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science (New York, NY) 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee DC, Chakraborty R, Iuvone PM, et al. Eliminating the master clock gene in the murine retina produces myopia. Invest Ophthalmol Vis Sci Supp. 2016;57:3616. [Google Scholar]
- 117.Stone RA, Lin T, Desai D, et al. Photoperiod, early postnatal eye growth, and visual deprivation. Vision Res. 1995;35:1195–1202. doi: 10.1016/0042-6989(94)00232-b. [DOI] [PubMed] [Google Scholar]
- 118.Aschoff J. Circadian rhythms: influences of internal and external factors on the period measured in constant conditions. Z Tierpsychol. 1979;49:225–249. doi: 10.1111/j.1439-0310.1979.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 119.Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279–1285. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 120.Smith EL, 3rd, Hung LF, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012;53:421–428. doi: 10.1167/iovs.11-8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ashby R, Ohlendorf A, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2009;50:5348–5354. doi: 10.1167/iovs.09-3419. [DOI] [PubMed] [Google Scholar]
- 122.Boatright JH, Hoel MJ, Iuvone PM. Stimulation of endogenous dopamine release and metabolism in amphibian retina by light- and K +-evoked depolarization. Brain Res. 1989;482:164–168. doi: 10.1016/0006-8993(89)90555-6. [DOI] [PubMed] [Google Scholar]
- 123.McCarthy CS, Megaw P, Devadas M, et al. Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia. Exp Eye Res. 2007;84:100–107. doi: 10.1016/j.exer.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 124.Dacey DM, Liao HW, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 125.Chakraborty R, Lee DC, Landis EG, et al. Melanopsin knock-out mice have abnormal refractive development and increased susceptibility to form-deprivation myopia. Invest Ophthalmol Vis Sci Supp. 2015;56:5843. [Google Scholar]
- 126.Wang F, Zhou J, Lu Y, et al. Effects of 530 nm green light on refractive status, melatonin, MT1 receptor, and melanopsin in the guinea pig. Curr Eye Res. 2011;36:103–111. doi: 10.3109/02713683.2010.526750. [DOI] [PubMed] [Google Scholar]
- 127.Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ayaki M, Torii H, Tsubota K, et al. Decreased sleep quality in high myopia children. Sci Rep. 2016;6:33902. doi: 10.1038/srep33902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jee D, Morgan IG, Kim EC. Inverse relationship between sleep duration and myopia. Acta Ophthalmol. 2016;94:e204–e210. doi: 10.1111/aos.12776. [DOI] [PubMed] [Google Scholar]
- 130.Gracitelli CP, Duque-Chica GL, Roizenblatt M, et al. Intrinsically photosensitive retinal ganglion cell activity is associated with decreased sleep quality in patients with glaucoma. Ophthalmology. 2015;122:1139–1148. doi: 10.1016/j.ophtha.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 131.Maynard ML, Zele AJ, Kwan AS, et al. Intrinsically photosensitive retinal ganglion cell function, sleep efficiency and depression in advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58:990–996. doi: 10.1167/iovs.16-20659. [DOI] [PubMed] [Google Scholar]
- 132.Kearney S, O’Donoghue L, Pourshahidi LK, et al. Myopes have significantly higher serum melatonin concentrations than non-myopes. Ophthalmic Physiol Opt. 2017;37:557–567. doi: 10.1111/opo.12396. [DOI] [PubMed] [Google Scholar]
- 133.Adhikari P, Pearson CA, Anderson AM, et al. Effect of age and refractive error on the melanopsin mediated post-illumination pupil response (PIPR) Sci Rep. 2015;5:17610. doi: 10.1038/srep17610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Orr D, Mutti DO, Shorter P, et al. The effect of light exposure on blue light-driven pupil responses in myopes and non-myopes. Optom Vis Sci. 2015;92 E-abstract 150035. [Google Scholar]
- 135.Jensen LS, Matson WE. Enlargement of avian eye by subjecting chicks to continuous incandescent illumination. Science. 1957;125:741. doi: 10.1126/science.125.3251.741. [DOI] [PubMed] [Google Scholar]
- 136.Lauber JK, McGinnis J. Eye lesions in domestic fowl reared under continuous light. Vision Res. 1966;6:619–626. doi: 10.1016/0042-6989(66)90073-3. [DOI] [PubMed] [Google Scholar]
- 137.Lauber JK, Shutze JV, McGinnis J. Effects of exposure to continuous light on the eye of the growing chick. Proc Soc Exp Biol Med. 1961;106:871–872. doi: 10.3181/00379727-106-26505. [DOI] [PubMed] [Google Scholar]
- 138.Nickla DL. Ocular diurnal rhythms and eye growth regulation: where we are 50 years after Lauber. Exp Eye Res. 2013;114:25–34. doi: 10.1016/j.exer.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weiss S, Schaeffel F. Diurnal growth rhythms in the chicken eye: relation to myopia development and retinal dopamine levels. J Comp Physiol A. 1993;172:263–270. doi: 10.1007/BF00216608. [DOI] [PubMed] [Google Scholar]
- 140.Nickla DL, Wildsoet C, Wallman J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp Eye Res. 1998;66:163–181. doi: 10.1006/exer.1997.0420. [DOI] [PubMed] [Google Scholar]
- 141.Papastergiou GI, Schmid GF, Riva CE, et al. Ocular axial length and choroidal thickness in newly hatched chicks and one-year-old chickens fluctuate in a diurnal pattern that is influenced by visual experience and intraocular pressure changes. Exp Eye Res. 1998;66:195–205. doi: 10.1006/exer.1997.0421. [DOI] [PubMed] [Google Scholar]
- 142.Nickla DL, Wildsoet CF, Troilo D. Endogenous rhythms in axial length and choroidal thickness in chicks: implications for ocular growth regulation. Invest Ophthalmol Vis Sci. 2001;42:584–588. [PubMed] [Google Scholar]
- 143.Nickla DL. The phase relationships between the diurnal rhythms in axial length and choroidal thickness and the association with ocular growth rate in chicks. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:399–407. doi: 10.1007/s00359-005-0077-2. [DOI] [PubMed] [Google Scholar]
- 144.Nickla DL, Wildsoet CF, Troilo D. Diurnal rhythms in intraocular pressure, axial length, and choroidal thickness in a primate model of eye growth, the common marmoset. Invest Ophthalmol Vis Sci. 2002;43:2519–2528. [PubMed] [Google Scholar]
- 145.Stone RA, Quinn GE, Francis EL, et al. Diurnal axial length fluctuations in human eyes. Invest Ophthalmol Vis Sci. 2004;45:63–70. doi: 10.1167/iovs.03-0294. [DOI] [PubMed] [Google Scholar]
- 146.Wilson LB, Quinn GE, Ying GS, et al. The relation of axial length and intraocular pressure fluctuations in human eyes. Invest Ophthalmol Vis Sci. 2006;47:1778–1784. doi: 10.1167/iovs.05-0869. [DOI] [PubMed] [Google Scholar]
- 147.Read SA, Collins MJ, Iskander DR. Diurnal variation of axial length, intraocular pressure, and anterior eye biometrics. Invest Ophthalmol Vis Sci. 2008;49:2911–2918. doi: 10.1167/iovs.08-1833. [DOI] [PubMed] [Google Scholar]
- 148.Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011;52:5121–5129. doi: 10.1167/iovs.11-7364. [DOI] [PubMed] [Google Scholar]
- 149.Bennett AG, Rabbetts RB. Bennett and Rabbetts’ Clinical Visual Optics. Elsevier Health Sciences; UK: 1998. [Google Scholar]
- 150.Brown JS, Flitcroft DI, Ying GS, et al. In vivo human choroidal thickness measurements: evidence for diurnal fluctuations. Invest Ophthalmol Vis Sci. 2009;50:5–12. doi: 10.1167/iovs.08-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Usui S, Ikuno Y, Akiba M, et al. Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjectscircadian changes in choroidal thickness. Invest Ophthalmol Vis Sci. 2012;53:2300–2307. doi: 10.1167/iovs.11-8383. [DOI] [PubMed] [Google Scholar]
- 152.Tan CS, Ouyang Y, Ruiz H, et al. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:261–266. doi: 10.1167/iovs.11-8782. [DOI] [PubMed] [Google Scholar]
- 153.Nickla DL, Thai P, Zanzerkia Trahan R, et al. Myopic defocus in the evening is more effective at inhibiting eye growth than defocus in the morning: effects on rhythms in axial length and choroid thickness in chicks. Exp Eye Res. 2017;154:104–115. doi: 10.1016/j.exer.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nickla D, Totonelly K. Choroidal thickness predicts ocular growith in normal chicks but not in eyes with experimentally altered growth. Clin Exp Optom. 2015;98:564–570. doi: 10.1111/cxo.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guggenheim J, Chen Y-P, Yip E, et al. Pre-treatment choroidal thickness is not predictive of susceptibility to form-deprivation myopia in chickens. Ophthalmic Physiol Opt. 2011;31:516–528. doi: 10.1111/j.1475-1313.2011.00827.x. [DOI] [PubMed] [Google Scholar]
- 156.Nickla DL, Wildsoet C, Wallman J. Compensation for spectacle lenses involves changes in proteoglycan synthesis in both the sclera and choroid. Curr Eye Res. 1997;16:320–326. doi: 10.1076/ceyr.16.4.320.10697. [DOI] [PubMed] [Google Scholar]
- 157.Nickla DL, Wildsoet C, Wallman J. The circadian rhythm in intraocular pressure and its relation to diurnal ocular growth changes in chicks. Exp Eye Res. 1998;66:183–193. doi: 10.1006/exer.1997.0425. [DOI] [PubMed] [Google Scholar]
- 158.Mertz JR, Wallman J. Choroidal retinoic acid synthesis: a possible mediator between refractive error and compensatory eye growth. Exp Eye Res. 2000;70:519–527. doi: 10.1006/exer.1999.0813. [DOI] [PubMed] [Google Scholar]
- 159.Marzani D, Wallman J. Growth of the two layers of the chick sclera is modulated reciprocally by visual conditions. Invest Ophthalmol Vis Sci. 1997;38:1726–1739. [PubMed] [Google Scholar]
- 160.Schmid KL, Wildsoet CF. Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Res. 1996;36:1023–1036. doi: 10.1016/0042-6989(95)00191-3. [DOI] [PubMed] [Google Scholar]
- 161.Zhu X, Park TW, Winawer J, et al. In a matter of minutes, the eye can know which way to grow. Invest Ophthalmol Vis Sci. 2005;46:2238–2241. doi: 10.1167/iovs.04-0956. [DOI] [PubMed] [Google Scholar]
- 162.Nickla DL, Jordan K, Yang J, et al. Brief hyperopic defocus or form deprivation have varying effects on eye growth and ocular rhythms depending on the time-of-day of exposure. Exp Eye Res. 2017;161:132–142. doi: 10.1016/j.exer.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chakraborty R, Read SA, Collins MJ. Monocular myopic defocus and daily changes in axial length and choroidal thickness of human eyes. Exp Eye Res. 2012;103:47–54. doi: 10.1016/j.exer.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 164.Chakraborty R, Read SA, Collins MJ. Hyperopic defocus and diurnal changes in human choroid and axial length. Optom Vis Sci. 2013;90:1187–1198. doi: 10.1097/OPX.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 165.Guo SS, Sivak JG, Callender MG, et al. Effects of continuous light on experimental refractive errors in chicks. Ophthalmic Physiol Opt. 1996;16:486–490. [PubMed] [Google Scholar]
- 166.Lauber JK, Kinnear A. Eye enlargement in birds induced by dim light. Can J Ophthalmol. 1979;14:265–269. [PubMed] [Google Scholar]
- 167.Gottlieb MD, Fugate-Wentzek LA, Wallman J. Different visual deprivations produce different ametropias and different eye shapes. Invest Ophthalmol Vis Sci. 1987;28:1225–1235. [PubMed] [Google Scholar]
- 168.Norton TT, Amedo AO, Siegwart JT., Jr Darkness causes myopia in visually experienced tree shrews. Invest Ophthalmol Vis Sci. 2006;47:4700–4707. doi: 10.1167/iovs.05-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Anisimov VN, Vinogradova IA, Panchenko AV, et al. Light-at-night-induced circadian disruption, cancer and aging. Curr Aging Sci. 2012;5:170–177. doi: 10.2174/1874609811205030002. [DOI] [PubMed] [Google Scholar]
- 170.Stevens RG, Brainard GC, Blask DE, et al. Adverse health effects of nighttime lighting: comments on American Medical Association policy statement. Am J Prev Med. 2013;45:343–346. doi: 10.1016/j.amepre.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 171.Chepesiuk R. Missing the dark: health effects of light pollution. Environ Health Perspect. 2009;117:A20–A27. doi: 10.1289/ehp.117-a20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Feillet C, van der Horst GT, Levi F, et al. Coupling between the circadian clock and cell cycle oscillators: implication for healthy cells and malignant growth. Front Neurol. 2015;6:96. doi: 10.3389/fneur.2015.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ostrin LA, Abbott KS, Queener HM. Attenuation of short wavelengths alters sleep and the ipRGC pupil response. Ophthalmic Physiol Opt. 2017;37:440–450. doi: 10.1111/opo.12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Quinn GE, Shin CH, Maguire MG, et al. Myopia and ambient lighting at night. Nature. 1999;399:113–114. doi: 10.1038/20094. [DOI] [PubMed] [Google Scholar]
- 175.Gwiazda J, Ong E, Held R, et al. Myopia and ambient night-time lighting. Nature. 2000;404:144. doi: 10.1038/35004663. [DOI] [PubMed] [Google Scholar]
- 176.Zadnik K, Jones LA, Irvin BC, et al. Myopia and ambient night-time lighting. CLEERE Study Group. Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error. Nature. 2000;404:143–144. doi: 10.1038/35004661. [DOI] [PubMed] [Google Scholar]
- 177.Guggenheim JA, Hill C, Yam TF. Myopia, genetics, and ambient lighting at night in a UK sample. Br J Ophthalmol. 2003;87:580–582. doi: 10.1136/bjo.87.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Saw SM, Wu HM, Hong CY, et al. Myopia and night lighting in children in Singapore. Br J Ophthalmol. 2001;85:527–528. doi: 10.1136/bjo.85.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nickla DL, Totonelly K. Brief light exposure at night disrupts the circadian rhythms in eye growth and choroidal thickness in chicks. Exp Eye Res. 2016;146:189–195. doi: 10.1016/j.exer.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dickens CJ, Hoskins HD., Jr . Epidemiology and pathophysiology of congenital glaucoma. In: Ritch R, Shields MB, Krupin T, editors. The Glaucomas. Mosby; St. Louise, MO: 1996. pp. 729–738. [Google Scholar]
- 181.Pruett RC. Progressive myopia and intraocular pressure: what is the linkage? A literature review. Acta Ophthalmol Suppl. 1988;185:117–127. doi: 10.1111/j.1755-3768.1988.tb02685.x. [DOI] [PubMed] [Google Scholar]
- 182.Quinn GE, Berlin JA, Young TL, et al. Association of intraocular pressure and myopia in children. Ophthalmology. 1995;102:180–185. doi: 10.1016/s0161-6420(95)31038-x. [DOI] [PubMed] [Google Scholar]
- 183.Parssinen O. Intraocular pressure in school myopia. Acta Ophthalmol (Copenh) 1990;68:559–563. doi: 10.1111/j.1755-3768.1990.tb04787.x. [DOI] [PubMed] [Google Scholar]
- 184.David R, Zangwill LM, Tessler Z, et al. The correlation between intraocular pressure and refractive status. Arch Ophthalmol. 1985;103:1812–1815. doi: 10.1001/archopht.1985.01050120046017. [DOI] [PubMed] [Google Scholar]
- 185.Tomlinson A, Phillips CI. Applanation tension and axial length of the eyeball. Br J Ophthalmol. 1970;54:548–553. doi: 10.1136/bjo.54.8.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Abdalla MI, Hamdi M. Applanation ocular tension in myopia and emmetropia. Br J Ophthalmol. 1970;54:122–125. doi: 10.1136/bjo.54.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Edwards MH, Brown B. IOP in myopic children: the relationship between increases in IOP and the development of myopia. Ophthalmic Physiol Opt. 1996;16:243–246. [PubMed] [Google Scholar]
- 188.Goss DA, Caffey TW. Clinical findings before the onset of myopia in youth: 5. Intraocular pressure. Optom Vis Sci. 1999;76:286–291. doi: 10.1097/00006324-199905000-00015. [DOI] [PubMed] [Google Scholar]
- 189.Quaranta L, Katsanos A, Russo A, et al. 24-hour intraocular pressure and ocular perfusion pressure in glaucoma. Surv Ophthalmol. 2013;58:26–41. doi: 10.1016/j.survophthal.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 190.Henkind P, Leitman M, Weitzman E. The diurnal curve in man: new observations. Invest Ophthalmol Vis Sci. 1973;12:705–707. [PubMed] [Google Scholar]
- 191.Liu JHK, Mansouri K, Weinreb RN. Estimation of 24-hour intraocular pressure peak timing and variation using a contact lens sensor. PLoS ONE. 2015;10:e0129529. doi: 10.1371/journal.pone.0129529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liu JH, Kripke DF, Twa MD, et al. Twenty-four-hour pattern of intraocular pressure in young adults with moderate to severe myopia. Invest Ophthalmol Vis Sci. 2002;43:2351–2355. [PubMed] [Google Scholar]
- 193.Loewen NA, Liu JH, Weinreb RN. Increased 24-hour variation of human intraocular pressure with short axial length. Invest Ophthalmol Vis Sci. 2010;51:933–937. doi: 10.1167/iovs.09-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jeong DW, Kook MS, Lee KS, et al. Circadian pattern of intraocular pressure fluctuations in young myopic eyes with open-angle glaucoma. Invest Ophthalmol Vis Sci. 2014;55:2148–2156. doi: 10.1167/iovs.13-13607. [DOI] [PubMed] [Google Scholar]
- 195.Pajic B, Pajic-Eggspuchler B, Haefliger I. Continuous IOP fluctuation recording in normal tension glaucoma patients. Curr Eye Res. 2011;36:1129–1136. doi: 10.3109/02713683.2011.608240. [DOI] [PubMed] [Google Scholar]
- 196.Lee TC, Kiuchi Y, Gregory DS. Light exposure decreases IOP in rabbits during the night. Curr Eye Res. 1995;14:443–448. doi: 10.3109/02713689509003754. [DOI] [PubMed] [Google Scholar]
- 197.Rowland JM, Potter DE, Reiter RJ. Circadian rhythm in intraocular pressure: a rabbit model. Curr Eye Res. 1981;1:169–173. doi: 10.3109/02713688109001822. [DOI] [PubMed] [Google Scholar]
- 198.McLaren JW, Brubaker RF, FitzSimon JS. Continuous measurement of intraocular pressure in rabbits by telemetry. Invest Ophthalmol Vis Sci. 1996;37:966–975. [PubMed] [Google Scholar]
- 199.Moore CG, Johnson EC, Morrison JC. Circadian rhythm of intraocular pressure in the rat. Curr Eye Res. 1996;15:185–191. doi: 10.3109/02713689608997412. [DOI] [PubMed] [Google Scholar]
- 200.Krishna R, Mermoud A, Baerveldt G, et al. Circadian rhythm of intraocular pressure: a rat model. Ophthalmic Res. 1995;27:163–167. doi: 10.1159/000267660. [DOI] [PubMed] [Google Scholar]
- 201.Dalvin LA, Fautsch MP. Analysis of circadian rhythm gene expression with reference to diurnal pattern of intraocular pressure in mice. Invest Ophthalmol Vis Sci. 2015;56:2657–2663. doi: 10.1167/iovs.15-16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bito LZ, Merritt SQ, DeRousseau CJ. Intraocular pressure of rhesus monkey (Macaca mulatta). I. An initial survey of two free-breeding colonies. Invest Ophthalmol Vis Sci. 1979;18:785–793. [PubMed] [Google Scholar]
- 203.Ostrin LA, Wildsoet CF. Optic nerve head and intraocular pressure in the guinea pig eye. Exp Eye Res. 2016;146:7–16. doi: 10.1016/j.exer.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Schmid GF, Papastergiou GI, Lin T, et al. Autonomic denervations influence ocular dimensions and intraocular pressure in chicks. Exp Eye Res. 1999;68:573–581. doi: 10.1006/exer.1998.0649. [DOI] [PubMed] [Google Scholar]
- 205.Nickla DL, Rada JA, Wallman J. Isolated chick sclera shows a circadian rhythm in proteoglycan synthesis perhaps associated with the rhythm in ocular elongation. J Comp Physiol [A] 1999;185:81–90. doi: 10.1007/s003590050368. [DOI] [PubMed] [Google Scholar]
- 206.van Kampen GPJ, van de Stadt RJ. Cartilage and chondrocyte responses to mechanical loadingin vitro. In: Helminen HJ, Kiviranta I, Tammi M, editors. Joint Loading: Biology and Health of Articular Structures. Butterworth & Co., Ltd; Kent, England: 1987. pp. 112–125. [Google Scholar]
- 207.Takano-Yamamoto T, Soma S, Nakagawa K, et al. Comparison of the effects of hydrostatic compressive force on glycosaminoglycan synthesis and proliferation in rabbit chondrocytes from mandibular condylar cartilage, nasal septum, and spheno-occipital synchondrosis in vitro. Am J Orthod Dentofacial Orthop. 1991;99:448–455. doi: 10.1016/S0889-5406(05)81578-1. [DOI] [PubMed] [Google Scholar]
- 208.Rada JA, McFarland AL, Cornuet PK, et al. Proteoglycan synthesis by scleral chondrocytes is modulated by a vision dependent mechanism. Curr Eye Res. 1992;11:767–782. doi: 10.3109/02713689209000750. [DOI] [PubMed] [Google Scholar]
- 209.Rada JA, Thoft RA, Hassell JR. Increased aggrecan (cartilage proteoglycan) production in the sclera of myopic chicks. Dev Biol. 1991;147:303–312. doi: 10.1016/0012-1606(91)90288-e. [DOI] [PubMed] [Google Scholar]
- 210.Rada JA, Matthews AL. Visual deprivation upregulates extracellular matrix synthesis by chick scleral chondrocytes. Invest Ophthalmol Vis Sci. 1994;35:2436–2447. [PubMed] [Google Scholar]
- 211.Devadas M, Morgan I. Light controls scleral precursor synthesis. NeuroReport. 1996;7:2010–2012. doi: 10.1097/00001756-199608120-00031. [DOI] [PubMed] [Google Scholar]
- 212.Read SA, Alonso-Caneiro D, Free KA, et al. Diurnal variation of anterior scleral and conjunctival thickness. Ophthalmic Physiol Opt. 2016;36:279–289. doi: 10.1111/opo.12288. [DOI] [PubMed] [Google Scholar]
- 213.Wallman J, Gottlieb MD, Rajaram V, et al. Local retinal regions control local eye growth and myopia. Science. 1987;237:73–77. doi: 10.1126/science.3603011. [DOI] [PubMed] [Google Scholar]
- 214.Kolb H, Nelson R, Ahnelt P, et al. Cellular organization of the vertebrate retina. Prog Brain Res. 2001;131:3–26. doi: 10.1016/s0079-6123(01)31005-1. [DOI] [PubMed] [Google Scholar]
- 215.Raviola E, Gilula NB. Gap junctions between photoreceptor cells in the vertebrate retina. Proc Natl Acad Sci U S A. 1973;70:1677–1681. doi: 10.1073/pnas.70.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Soucy E, Wang Y, Nirenberg S, et al. A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron. 1998;21:481–493. doi: 10.1016/s0896-6273(00)80560-7. [DOI] [PubMed] [Google Scholar]
- 217.Bloomfield SA, Volgyi B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat Rev Neurosci. 2009;10:495–506. doi: 10.1038/nrn2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sand A, Schmidt TM, Kofuji P. Diverse types of ganglion cell photoreceptors in the mammalian retina. Prog Retin Eye Res. 2012;31:287–302. doi: 10.1016/j.preteyeres.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011;34:572–580. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Schmidt TM, Alam NM, Chen S, et al. A role for melanopsin in alpha retinal ganglion cells and contrast detection. Neuron. 2014;82:781–788. doi: 10.1016/j.neuron.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chakraborty R, Pardue MT. Molecular and biochemical aspects of the retina on refraction. In: Hejtmancik JF, Nickerson JM, editors. Progress in Molecular Biology and Translational Science. Elsevier; Oxford: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Schaeffel F, Rohrer B, Lemmer T, et al. Diurnal control of rod function in the chicken. Vis Neurosci. 1991;6:641–653. doi: 10.1017/s0952523800002637. [DOI] [PubMed] [Google Scholar]
- 223.Smith EL, 3rd, Ramamirtham R, Qiao-Grider Y, et al. Effects of foveal ablation on emmetropization and form-deprivation myopia. Invest Ophthalmol Vis Sci. 2007;48:3914–3922. doi: 10.1167/iovs.06-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Osterberg G. Topography of the layer of rods and cones in the human retina. Acta Ophthal Suppl. 1935;6:1–103. [Google Scholar]
- 225.Park H, Jabbar SB, Tan CC, et al. Visually-driven ocular growth in mice requires functional rod photoreceptors. Invest Ophthalmol Vis Sci. 2014;55:6272–6279. doi: 10.1167/iovs.14-14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Altimus CM, Guler AD, Alam NM, et al. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat Neurosci. 2010;13:1107–1112. doi: 10.1038/nn.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chakraborty R, Park HN, Landis E, et al. Absence of cone mediated retinal pathways increases susceptibility to form-deprivation myopia in mice. Optom Vis Sci Suppl. 2016;93 doi: 10.1016/j.exer.2018.12.021. E-abstract 160074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Crewther DP, Crewther SG. Pharmacological modification of eye growth in normally reared and visually deprived chicks. Curr Eye Res. 1990;9:733–740. doi: 10.3109/02713689008999568. [DOI] [PubMed] [Google Scholar]
- 229.Crewther DP, Crewther SG, Xie RZ. Changes in eye growth produced by drugs which affect retinal ON or OFF responses to light. J Ocul Pharmacol Ther. 1996;12:193–208. doi: 10.1089/jop.1996.12.193. [DOI] [PubMed] [Google Scholar]
- 230.Morgan IG, Dvorak DR. A physiologically active kainic acid-preferring receptor in chicken retina. Neurosci Lett. 1984;44:299–304. doi: 10.1016/0304-3940(84)90039-9. [DOI] [PubMed] [Google Scholar]
- 231.Fujikado T. The effect of dopamine on the response to pattern stimulation – study of the chick ERG. Jpn J Ophthalmol. 1994;38:368–374. [PubMed] [Google Scholar]
- 232.Crewther DP, Crewther SG. Refractive compensation to optical defocus depends on the temporal profile of luminance modulation of the environment. NeuroReport. 2002;13:1029–1032. doi: 10.1097/00001756-200206120-00010. [DOI] [PubMed] [Google Scholar]
- 233.Smith EL, 3rd, Fox DA, Duncan GC. Refractive-error changes in kitten eyes produced by chronic on-channel blockade. Vision Res. 1991;31:833–844. doi: 10.1016/0042-6989(91)90150-4. [DOI] [PubMed] [Google Scholar]
- 234.Lu, Koh, Ling The ON/OFF response in retinopathy of prematurity subjects with myopia. Doc Ophthalmol. 2005;110:155–161. doi: 10.1007/s10633-005-3742-4. [DOI] [PubMed] [Google Scholar]
- 235.Miyake Y, Yagasaki K, Horiguchi M, et al. Congenital stationary night blindness with negative electroretinogram. A new classification. Arch Ophthalmol. 1986;104:1013–1020. doi: 10.1001/archopht.1986.01050190071042. [DOI] [PubMed] [Google Scholar]
- 236.Pardue MT, McCall MA, LaVail MM, et al. A naturally occurring mouse model of X-linked congenital stationary night blindness. Invest Ophthalmol Vis Sci. 1998;39:2443–2449. [PubMed] [Google Scholar]
- 237.Pusch CM, Zeitz C, Brandau O, et al. The complete form of X-linked congenital stationary night blindness is caused by mutations in a gene encoding a leucine-rich repeat protein. Nat Genet. 2000;26:324–327. doi: 10.1038/81627. [DOI] [PubMed] [Google Scholar]
- 238.Bech-Hansen NT, Naylor MJ, Maybaum TA, et al. Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat Genet. 2000;26:319–323. doi: 10.1038/81619. [DOI] [PubMed] [Google Scholar]
- 239.Pardue MT, Faulkner AE, Fernandes A, et al. High susceptibility to experimental myopia in a mouse model with a retinal on pathway defect. Invest Ophthalmol Vis Sci. 2008;49:706–712. doi: 10.1167/iovs.07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chakraborty R, Park HN, Hanif AM, et al. ON pathway mutations increase susceptibility to form-deprivation myopia. Exp Eye Res. 2015;137:79–83. doi: 10.1016/j.exer.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Chakraborty R, Park H, Aung MH, et al. Comparison of refractive development and retinal dopamine in OFF pathway mutant and C57BL/6J wild-type mice. Mol Vis. 2014;20:1318–1327. [PMC free article] [PubMed] [Google Scholar]
- 242.Lukasiewicz PD, Eggers ED, Sagdullaev BT, et al. GABAC receptor-mediated inhibition in the retina. Vision Res. 2004;44:3289–3296. doi: 10.1016/j.visres.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 243.Garaycochea J, Slaughter MM. GABAB receptors enhance excitatory responses in isolated rat retinal ganglion cells. J Physiol. 2016;594:5543–5554. doi: 10.1113/JP272374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ivanova E, Muller U, Wassle H. Characterization of the glycinergic input to bipolar cells of the mouse retina. Eur J Neurosci. 2006;23:350–364. doi: 10.1111/j.1460-9568.2005.04557.x. [DOI] [PubMed] [Google Scholar]
- 245.Guoping L, Xiang Y, Jianfeng W, et al. Alterations of glutamate and gamma-aminobutyric acid expressions in normal and myopic eye development in guinea pigs. Invest Ophthalmol Vis Sci. 2017;58:1256–1265. doi: 10.1167/iovs.16-21130. [DOI] [PubMed] [Google Scholar]
- 246.Wang H, Su S, Yang M, et al. Association of ZNF644, GRM6, and CTNND2 genes with high myopia in the Han Chinese population: Jiangsu Eye Study. Eye (London, England) 2016;30:1017–1022. doi: 10.1038/eye.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Audo I, Bujakowska K, Orhan E, et al. Whole-exome sequencing identifies mutations in GPR179 leading to autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2012;90:321–330. doi: 10.1016/j.ajhg.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Peachey NS, Ray TA, Florijn R, et al. GPR179 is required for depolarizing bipolar cell function and is mutated in autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet. 2012;90:331–339. doi: 10.1016/j.ajhg.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Stone RA, Liu J, Sugimoto R, et al. GABA, experimental myopia, and ocular growth in chick. Invest Ophthalmol Vis Sci. 2003;44:3933–3946. doi: 10.1167/iovs.02-0774. [DOI] [PubMed] [Google Scholar]
- 250.Cheng ZY, Wang XP, Schmid KL, et al. GABAB receptor antagonist CGP46381 inhibits form-deprivation myopia development in guinea pigs. Biomed Res Int. 2015;2015:207312. doi: 10.1155/2015/207312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Cheng ZY, Wang XP, Schmid KL, et al. Inhibition of form-deprivation myopia by a GABAAOr receptor antagonist (1,2,5,6-tetrahydropyridin-4-yl) methylphosphinic acid (TPMPA), in guinea pigs. Graefes Arch Clin Exp Ophthalmol. 2014;252:1939–1946. doi: 10.1007/s00417-014-2765-5. [DOI] [PubMed] [Google Scholar]
- 252.Chebib M, Hinton T, Schmid KL, et al. Novel, potent, and selective GABAC antagonists inhibit myopia development and facilitate learning and memory. J Pharmacol Exp Ther. 2009;328:448–457. doi: 10.1124/jpet.108.146464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Barathi VA, Chaurasia SS, Poidinger M, et al. Involvement of GABA transporters in atropine-treated myopic retina as revealed by iTRAQ quantitative proteomics. J Proteome Res. 2014;13:4647–4658. doi: 10.1021/pr500558y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Korshunov KS, Blakemore LJ, Trombley PQ. Dopamine: a modulator of circadian rhythms in the central nervous system. Front Cell Neurosci. 2017;11:91. doi: 10.3389/fncel.2017.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Jackson CR, Ruan GX, Aseem F, et al. Retinal dopamine mediates multiple dimensions of light-adapted vision. J Neurosci. 2012;32:9359–9368. doi: 10.1523/JNEUROSCI.0711-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ribelayga C, Mangel SC. Absence of circadian clock regulation of horizontal cell gap junctional coupling reveals two dopamine systems in the goldfish retina. J Comp Neurol. 2003;467:243–253. doi: 10.1002/cne.10927. [DOI] [PubMed] [Google Scholar]
- 257.Versaux-Botteri C, Martin-Martinelli E, Nguyen-Legros J, et al. Regional specialization of the rat retina: catecholamine-containing amacrine cell characterization and distribution. J Comp Neurol. 1986;243:422–433. doi: 10.1002/cne.902430311. [DOI] [PubMed] [Google Scholar]
- 258.Dowling JE, Ehinger B. The interplexiform cell system. I. Synapses of the dopaminergic neurons of the goldfish retina. Proc R Soc Lond B Biol Sci. 1978;201:7–26. doi: 10.1098/rspb.1978.0030. [DOI] [PubMed] [Google Scholar]
- 259.Voigt T, Wassle H. Dopaminergic innervation of A II amacrine cells in mammalian retina. J Neurosci. 1987;7:4115–4128. doi: 10.1523/JNEUROSCI.07-12-04115.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Boatright JH, Gordon JR, Iuvone PM. Inhibition of endogenous dopamine release in amphibian retina by L-2-amino-4-phosphonobutyric acid (L-AP4) and trans-2-aminocyclopentane-1,3-dicarboxylate (ACPD) Brain Res. 1994;649:339–342. doi: 10.1016/0006-8993(94)91084-7. [DOI] [PubMed] [Google Scholar]
- 261.Boelen MK, Boelen MG, Marshak DW. Light-stimulated release of dopamine from the primate retina is blocked by 1–2-amino-4-phosphonobutyric acid (APB) Vis Neurosci. 1998;15:97–103. doi: 10.1017/s0952523898151040. [DOI] [PubMed] [Google Scholar]
- 262.Dacey DM. The dopaminergic amacrine cell. J Comp Neurol. 1990;301:461–489. doi: 10.1002/cne.903010310. [DOI] [PubMed] [Google Scholar]
- 263.Hokoc JN, Mariani AP. Tyrosine hydroxylase immunoreactivity in the rhesus monkey retina reveals synapses from bipolar cells to dopaminergic amacrine cells. J Neurosci. 1987;7:2785–2793. doi: 10.1523/JNEUROSCI.07-09-02785.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Dumitrescu ON, Pucci FG, Wong KY, et al. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J Comp Neurol. 2009;517:226–244. doi: 10.1002/cne.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Iuvone PM, Tigges M, Stone RA, et al. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest Ophthalmol Vis Sci. 1991;32:1674–1677. [PubMed] [Google Scholar]
- 266.Bartmann M, Schaeffel F, Hagel G, et al. Constant light affects retinal dopamine levels and blocks deprivation myopia but not lens-induced refractive errors in chickens. Vis Neurosci. 1994;11:199–208. doi: 10.1017/s0952523800001565. [DOI] [PubMed] [Google Scholar]
- 267.Ohngemach S, Feldkaemper M, Schaeffel F. Pineal control of the dopamine D2-receptor gene and dopamine release in the retina of the chicken and their possible relation to growth rhythms of the eye. J Pineal Res. 2001;31:145–154. doi: 10.1034/j.1600-079x.2001.310208.x. [DOI] [PubMed] [Google Scholar]
- 268.Gao Q, Liu Q, Ma P, et al. Effects of direct intravitreal dopamine injections on the development of lid-suture induced myopia in rabbits. Graefes Arch Clin Exp Ophthalmol. 2006;244:1329–1335. doi: 10.1007/s00417-006-0254-1. [DOI] [PubMed] [Google Scholar]
- 269.Huang F, Yan T, Shi F, et al. Activation of dopamine D2 receptor is critical for the development of form-deprivation myopia in the C57BL/6 mouse. Invest Ophthalmol Vis Sci. 2014;55:5537–5544. doi: 10.1167/iovs.13-13211. [DOI] [PubMed] [Google Scholar]
- 270.Wu XH, Qian KW, Xu GZ, et al. The role of retinal dopamine in C57BL/6 mouse refractive development as revealed by intravitreal administration of 6-hydroxydopamine. Invest Ophthalmol Vis Sci. 2016;57:5393–5404. doi: 10.1167/iovs.16-19543. [DOI] [PubMed] [Google Scholar]
- 271.Bergen MA, Park HN, Chakraborty R, et al. Altered refractive development in mice with reduced levels of retinal dopamine. Invest Ophthalmol Vis Sci. 2016;57:4412–4419. doi: 10.1167/iovs.15-17784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Chen S, Zhi Z, Ruan Q, et al. Bright light suppresses form-deprivation myopia development with activation of dopamine D1 receptor signaling in the on pathway in retina. Invest Ophthalmol Vis Sci. 2017;58:2306–2316. doi: 10.1167/iovs.16-20402. [DOI] [PubMed] [Google Scholar]
- 273.Ward AH, Siegwart JT, Frost MR, et al. Intravitreally-administered dopamine D2-like (and D4), but not D1-like, receptor agonists reduce form-deprivation myopia in tree shrews. Vis Neurosci. 2017;34:E003. doi: 10.1017/S0952523816000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Rohrer B, Iuvone PM, Stell WK. Stimulation of dopaminergic amacrine cells by stroboscopic illumination or fibroblast growth factor (bFGF, FGF-2) injections: possible roles in prevention of form-deprivation myopia in the chick. Brain Res. 1995;686:169–181. doi: 10.1016/0006-8993(95)00370-6. [DOI] [PubMed] [Google Scholar]
- 275.Teakle EM, Wildsoet CF, Vaney DI. The spatial organization of tyrosine hydroxylase-immunoreactive amacrine cells in the chicken retina and the consequences of myopia. Vision Res. 1993;33:2383–2396. doi: 10.1016/0042-6989(93)90117-f. [DOI] [PubMed] [Google Scholar]
- 276.Rohrer B, Spira AW, Stell WK. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigmented epithelium. Vis Neurosci. 1993;10:447–453. doi: 10.1017/s0952523800004673. [DOI] [PubMed] [Google Scholar]
- 277.Yan T, Xiong W, Huang F, et al. Daily injection but not continuous infusion of apomorphine inhibits form-deprivation myopia in mice. Invest Ophthalmol Vis Sci. 2015;56:2475–2485. doi: 10.1167/iovs.13-12361. [DOI] [PubMed] [Google Scholar]
- 278.Dong F, Zhi Z, Pan M, et al. Inhibition of experimental myopia by a dopamine agonist: different effectiveness between form deprivation and hyperopic defocus in guinea pigs. Mol Vis. 2011;17:2824–2834. [PMC free article] [PubMed] [Google Scholar]
- 279.Wu XH, Li YY, Zhang PP, et al. Unaltered retinal dopamine levels in a C57BL/6 mouse model of form-deprivation myopia. Invest Ophthalmol Vis Sci. 2015;56:967–977. doi: 10.1167/iovs.13-13362. [DOI] [PubMed] [Google Scholar]
- 280.Jiang L, Long K, Schaeffel F, et al. Effects of dopaminergic agents on progression of naturally occurring myopia in albino guinea pigs (Cavia porcellus) Invest Ophthalmol Vis Sci. 2014;55:7508–7519. doi: 10.1167/iovs.14-14294. [DOI] [PubMed] [Google Scholar]
- 281.Tikidji-Hamburyan A, Reinhard K, Storchi R, et al. Rods progressively escape saturation to drive visual responses in daylight conditions. Nat Commun. 2017;8:1813. doi: 10.1038/s41467-017-01816-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Hirasawa H, Contini M, Raviola E. Extrasynaptic release of GABA and dopamine by retinal dopaminergic neurons. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140186. doi: 10.1098/rstb.2014.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Schmid KL, Strasberg G, Rayner CL, et al. The effects and interactions of GABAergic and dopaminergic agents in the prevention of form deprivation myopia by brief periods of normal vision. Exp Eye Res. 2013;110:88–95. doi: 10.1016/j.exer.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 284.Ko ML, Shi L, Huang CC, et al. Circadian phase-dependent effect of nitric oxide on L-type voltage-gated calcium channels in avian cone photoreceptors. J Neurochem. 2013;127:314–328. doi: 10.1111/jnc.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Seltner RL, Stell WK. The effect of vasoactive intestinal peptide on development of form deprivation myopia in the chick: a pharmacological and immunocytochemical study. Vision Res. 1995;35:1265–1270. doi: 10.1016/0042-6989(94)00244-g. [DOI] [PubMed] [Google Scholar]
- 286.Stone RA, Laties AM, Raviola E, et al. Increase in retinal vasoactive intestinal polypeptide after eyelid fusion in primates. Proc Natl Acad Sci U S A. 1988;85:257–260. doi: 10.1073/pnas.85.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Feldkaemper MP, Schaeffel F. Evidence for a potential role of glucagon during eye growth regulation in chicks. Vis Neurosci. 2002;19:755–766. doi: 10.1017/s0952523802196064. [DOI] [PubMed] [Google Scholar]
- 288.Vessey KA, Lencses KA, Rushforth DA, et al. Glucagon receptor agonists and antagonists affect the growth of the chick eye: a role for glucagonergic regulation of emmetropization? Invest Ophthalmol Vis Sci. 2005;46:3922–3931. doi: 10.1167/iovs.04-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Mathis U, Schaeffel F. Glucagon-related peptides in the mouse retina and the effects of deprivation of form vision. Graefes Arch Clin Exp Ophthalmol. 2007;245:267–275. doi: 10.1007/s00417-006-0282-x. [DOI] [PubMed] [Google Scholar]
- 290.Bertrand E, Fritsch C, Diether S, et al. Identification of apolipoprotein A1 as a “STOP” signal for myopia. Mol Cell Proteomics. 2006;5:2158–2166. doi: 10.1074/mcp.M600073-MCP200. [DOI] [PubMed] [Google Scholar]
- 291.Summers JA, Harper AR, Feasley CL, et al. Identification of apolipoprotein A-I as a retinoic acid-binding protein in the eye. J Biol Chem. 2016;291:18991–19005. doi: 10.1074/jbc.M116.725523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Rohrer B, Stell WK. Basic fibroblast growth factor (bFGF) and transforming growth factor beta (TGF-beta) act as stop and go signals to modulate postnatal ocular growth in the chick. Exp Eye Res. 1994;58:553–561. doi: 10.1006/exer.1994.1049. [DOI] [PubMed] [Google Scholar]
- 293.Ritchey ER, Zelinka CP, Tang J, et al. The combination of IGF1 and FGF2 and the induction of excessive ocular growth and extreme myopia. Exp Eye Res. 2012;99:1–16. doi: 10.1016/j.exer.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.An J, Hsi E, Zhou X, et al. The FGF2 gene in a myopia animal model and human subjects. Mol Vis. 2012;18:471–478. [PMC free article] [PubMed] [Google Scholar]
- 295.Chen BY, Wang CY, Chen WY, et al. Altered TGF-beta2 and bFGF expression in scleral desmocytes from an experimentally-induced myopia guinea pig model. Graefes Arch Clin Exp Ophthalmol. 2013;251:1133–1144. doi: 10.1007/s00417-013-2269-8. [DOI] [PubMed] [Google Scholar]
- 296.Cheng ZY, Chebib M, Schmid KL. rho1 GABAC receptors are expressed in fibrous and cartilaginous layers of chick sclera and located on sclera fibroblasts and chondrocytes. J Neurochem. 2011;118:281–287. doi: 10.1111/j.1471-4159.2011.07300.x. [DOI] [PubMed] [Google Scholar]
- 297.Cheng ZY, Chebib M, Schmid KL. Identification of GABA receptors in chick cornea. Mol Vis. 2012;18:1107–1114. [PMC free article] [PubMed] [Google Scholar]
- 298.Christian PG, Harkin DG, Schmid KL. GABAergic agents modify the response of chick scleral fibroblasts to myopic and hyperopic eye cup tissues. Curr Eye Res. 2014;39:172–187. doi: 10.3109/02713683.2013.834941. [DOI] [PubMed] [Google Scholar]
- 299.Vardi T, Fina M, Zhang L, et al. mGluR6 transcripts in non-neuronal tissues. J Histochem Cytochem. 2011;59:1076–1086. doi: 10.1369/0022155411425386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Rohrer B, Stell WK. Localization of putative dopamine D2-like receptors in the chick retina, using in situ hybridization and immunocytochemistry. Brain Res. 1995;695:110–116. doi: 10.1016/0006-8993(95)00700-z. [DOI] [PubMed] [Google Scholar]
- 301.Versaux-Botteri C, Gibert JM, Nguyen-Legros J, et al. Molecular identification of a dopamine D1b receptor in bovine retinal pigment epithelium. Neurosci Lett. 1997;237:9–12. doi: 10.1016/s0304-3940(97)00783-0. [DOI] [PubMed] [Google Scholar]
- 302.Guha S, Baltazar GC, Tu LA, et al. Stimulation of the D5 dopamine receptor acidifies the lysosomal pH of retinal pigmented epithelial cells and decreases accumulation of autofluorescent photoreceptor debris. J Neurochem. 2012;122:823–833. doi: 10.1111/j.1471-4159.2012.07804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Cavallotti C, Pescosolido N, Pescosolido V, et al. Determination of dopamine D1 receptors in the human uveo scleral tissue by light microscope autoradiography. Int Ophthalmol. 1999;23:171–179. doi: 10.1023/a:1010611419602. [DOI] [PubMed] [Google Scholar]
- 304.Cavallotti C, Pescosolido N, Artico M, et al. Localization of dopamine receptors in the rabbit cornea. Cornea. 1999;18:721–728. doi: 10.1097/00003226-199911000-00016. [DOI] [PubMed] [Google Scholar]
- 305.Grub M, Mielke J, Rohrbach M, et al. Dopamine receptors of the corneal epithelium and endothelium. Klin Monatsbl Augenheilkd. 2012;229:822–825. doi: 10.1055/s-0031-1299240. [DOI] [PubMed] [Google Scholar]
- 306.Seko Y, Tanaka Y, Tokoro T. Influence of bFGF as a potent growth stimulator and TGF-beta as a growth regulator on scleral chondrocytes and scleral fibroblasts in vitro. Ophthalmic Res. 1995;27:144–152. doi: 10.1159/000267651. [DOI] [PubMed] [Google Scholar]
- 307.Bohnsack RN, Warejcka DJ, Wang L, et al. Expression of insulin-like growth factor 2 receptor in corneal keratocytes during differentiation and in response to wound healing. Invest Ophthalmol Vis Sci. 2014;55:7697–7708. doi: 10.1167/iovs.14-15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Zhang Y, Raychaudhuri S, Wildsoet CF. Imposed optical defocus induces isoform-specific up-regulation of TGFβ gene expression in chick retinal pigment epithelium and choroid but not neural retina. PLoS ONE. 2016;11:e0155356. doi: 10.1371/journal.pone.0155356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Guo L, Frost MR, Siegwart JT, Jr, et al. Scleral gene expression during recovery from myopia compared with expression during myopia development in tree shrew. Mol Vis. 2014;20:1643–1659. [PMC free article] [PubMed] [Google Scholar]
- 310.Guo L, Frost MR, He L, et al. Gene expression signatures in tree shrew sclera in response to three myopiagenic conditions. Invest Ophthalmol Vis Sci. 2013;54:6806–6819. doi: 10.1167/iovs.13-12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Davies TW, Bennie J, Inger R, et al. Artificial light alters natural regimes of night-time sky brightness. Sci Rep. 2013;3:6. [Google Scholar]
- 312.Marshall J. Light in man’s environment. Eye (Lond) 2016;30:211–214. doi: 10.1038/eye.2015.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Henderson ST. Daylight and Its Spectrum. John Wiley & Sons; New York: 1977. Artificial daylight sources; pp. 271–301. [Google Scholar]
- 314.Savides TJ, Messin S, Senger C, et al. Natural light exposure of young adults. Physiol Behav. 1986;38:571–574. doi: 10.1016/0031-9384(86)90427-0. [DOI] [PubMed] [Google Scholar]
- 315. [accessed 02/06/2018];Recommended Light Levels. National Optical Astronomy Observatory (NOAO) https://www.noao.edu/
- 316.Ostrin LA. Objectively measured light exposure in emmetropic and myopic adults. Optom Vis Sci. 2017;94:229–238. doi: 10.1097/OPX.0000000000001013. [DOI] [PubMed] [Google Scholar]
- 317.Stothard ER, McHill AW, Depner CM, et al. Circadian entrainment to the natural light-dark cycle across seasons and the weekend. Curr Biol. 2017;27:508–513. doi: 10.1016/j.cub.2016.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Kyba CCM, Kuester T, Sanchez de Miguel A, et al. Artificially lit surface of Earth at night increasing in radiance and extent. Sci Adv. 2017;3:e1701528. doi: 10.1126/sciadv.1701528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Kyba CC, Tong KP, Bennie J, et al. Worldwide variations in artificial skyglow. Sci Rep. 2015;5:8409. doi: 10.1038/srep08409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Falchi F, Cinzano P, Duriscoe D, et al. The new world atlas of artificial night sky brightness. Sci Adv. 2016;2:e1600377. doi: 10.1126/sciadv.1600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Cain N, Gradisar M. Electronic media use and sleep in school-aged children and adolescents: a review. Sleep Med. 2010;11:735–742. doi: 10.1016/j.sleep.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 322.Lemola S, Perkinson-Gloor N, Brand S, et al. Adolescents’ electronic media use at night, sleep disturbance, and depressive symptoms in the smartphone age. J Youth Adolesc. 2015;44:405–418. doi: 10.1007/s10964-014-0176-x. [DOI] [PubMed] [Google Scholar]
- 323.LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15:443–454. doi: 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Stevens RG, Zhu Y. Electric light, particularly at night, disrupts human circadian rhythmicity: is that a problem? Philos Trans R Soc Lond B Biol Sci. 2015;370:20140120. doi: 10.1098/rstb.2014.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Cho Y, Ryu SH, Lee BR, et al. Effects of artificial light at night on human health: a literature review of observational and experimental studies applied to exposure assessment. Chronobiol Int. 2015;32:1294–1310. doi: 10.3109/07420528.2015.1073158. [DOI] [PubMed] [Google Scholar]
- 326.Fonken LK, Nelson RJ. The effects of light at night on circadian clocks and metabolism. Endocr Rev. 2014;35:648–670. doi: 10.1210/er.2013-1051. [DOI] [PubMed] [Google Scholar]
- 327.Zelinski EL, Deibel SH, McDonald RJ. The trouble with circadian clock dysfunction: multiple deleterious effects on the brain and body. Neurosci Biobehav Rev. 2014;40:80–101. doi: 10.1016/j.neubiorev.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 328.https://ntp.niehs.nih.gov/pubhealth/roc/candidates/meetings/workshop_alan.html.
- 329.Roenneberg T, Merrow M. Entrainment of the human circadian clock. Cold Spring Harb Symp Quant Biol. 2007;72:293–299. doi: 10.1101/sqb.2007.72.043. [DOI] [PubMed] [Google Scholar]
- 330.Ando K, Kripke DF. Light attenuation by the human eyelid. Biol Psychiat. 1996;39:22–25. doi: 10.1016/0006-3223(95)00109-3. [DOI] [PubMed] [Google Scholar]
- 331.Robinson J, Bayliss SC, Fielder AR. Transmission of light across the adult and neonatal eyelid in vivo. Vision Res. 1991;31:1837–1840. doi: 10.1016/0042-6989(91)90031-y. [DOI] [PubMed] [Google Scholar]
- 332.Pauers MJ, Kuchenbecker JA, Neitz M, et al. Changes in the colour of light cue circadian activity. Anim Behav. 2012;83:1143–1151. doi: 10.1016/j.anbehav.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Walmsley L, Hanna L, Mouland J, et al. Colour as a signal for entraining the mammalian circadian clock. PLoS Biol. 2015;13:e1002127. doi: 10.1371/journal.pbio.1002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Rucker FJ. The role of luminance and chromatic cues in emmetropisation. Ophthalmic Physiol Opt. 2013;33:196–214. doi: 10.1111/opo.12050. [DOI] [PubMed] [Google Scholar]
- 335.Foulds WS, Barathi VA, Luu CD. Progressive myopia or hyperopia can be induced in chicks and reversed by manipulation of the chromaticity of ambient light. Invest Ophthalmol Vis Sci. 2013;54:8004–8012. doi: 10.1167/iovs.13-12476. [DOI] [PubMed] [Google Scholar]
- 336.Jiang L, Zhang S, Schaeffel F, et al. Interactions of chromatic and lens-induced defocus during visual control of eye growth in guinea pigs (Cavia porcellus) Vision Res. 2014;94:24–32. doi: 10.1016/j.visres.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 337.Gawne TJ, Ward AH, Norton TT. Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vision Res. 2017;140:55–65. doi: 10.1016/j.visres.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Smith EL, 3rd, Hung LF, Arumugam B, et al. Effects of long-wavelength lighting on refractive development in infant rhesus monkeys. Invest Ophthalmol Vis Sci. 2015;56:6490–6500. doi: 10.1167/iovs.15-17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Torii H, Kurihara T, Seko Y, et al. Violet light exposure can be a preventive strategy against myopia progression. EBio-Medicine. 2017;15:210–219. doi: 10.1016/j.ebiom.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Li T, Howland HC, Troilo D. Diurnal illumination patterns affect the development of the chick eye. Vision Res. 2000;40:2387–2393. doi: 10.1016/s0042-6989(00)00098-5. [DOI] [PubMed] [Google Scholar]
- 341.Smith EL, 3rd, Bradley DV, Fernandes A, et al. Continuous ambient lighting and eye growth in primates. Invest Ophthalmol Vis Sci. 2001;42:1146–1152. [PubMed] [Google Scholar]
- 342.Ashby RS, Schaeffel F. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci. 2010;51:5247–5253. doi: 10.1167/iovs.09-4689. [DOI] [PubMed] [Google Scholar]
- 343.Karouta C, Ashby RS. Correlation between light levels and the development of deprivation myopia. Invest Ophthalmol Vis Sci. 2014;56:299–309. doi: 10.1167/iovs.14-15499. [DOI] [PubMed] [Google Scholar]
- 344.Lan W, Feldkaemper M, Schaeffel F. Intermittent episodes of bright light suppress myopia in the chicken more than continuous bright light. PLoS ONE. 2014;9:e110906. doi: 10.1371/journal.pone.0110906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Zhou Z, Morgan IG, Chen Q, et al. Disordered sleep and myopia risk among Chinese children. PLoS ONE. 2015;10:e0121796. doi: 10.1371/journal.pone.0121796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Stone RA, Pardue MT, Iuvone PM, et al. Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res. 2013;114:35–47. doi: 10.1016/j.exer.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Hughes S, Jagannath A, Hankins MW, et al. Photic regulation of clock systems. Methods Enzymol. 2015;552:125–143. doi: 10.1016/bs.mie.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 348.Johnson CH, Elliott JA, Foster R. Entrainment of circadian programs. Chronobiol Int. 2003;20:741–774. doi: 10.1081/cbi-120024211. [DOI] [PubMed] [Google Scholar]