Abstract
More than 500 abandoned uranium (U) mines within the Navajo Nation contribute U, arsenic (As) and other metals to groundwater, soil and potentially air through airborne transport. The adverse cardiovascular health effects attributed to cumulative exposure to these metals remains uncertain. The aim of this study was to examine whether environmental exposure to these metals may promote or exacerbate the oxidation of low-density lipoprotein (LDL) cholesterol in this Native American population. The correlation of cardiovascular biomarkers (oxidized LDL (oxLDL) and C-reactive protein (CRP)) from a Navajo cohort (n = 252) with mean annual As and U intakes from water and urine metals was estimated using linear regression. Proof-of-concept assays were performed to investigate whether As and U directly oxidize human LDL. Mean annual As intake from water was positively and significantly associated with oxLDL, but not CRP in this study population, while U intake estimates were negatively associated with oxLDL. In an acellular system, As, but not U, directly oxidized the apolipoprotein B-100 component of purified human LDL. Neither metal promoted lipid peroxidation of the LDL particle. Both the population and lab results are consistent with the hypothesis that As promotes oxidation of LDL, a crucial step in vascular inflammation and chronic vascular disease. Conversely, for outcomes related to U, negative associations were observed between U intake and oxLDL, and U only minimally altered human LDL in direct exposure experiments. Only urine U was correlated with CRP, whereas no other metals in water or urine were apparently reliable predictors of this inflammatory marker.
Introduction
Navajo Nation community members in the south-western United States (US), specifically in New Mexico, Utah, and Arizona, display significant risks for environmental exposure and potential adverse health effects from metals, including arsenic (As) and uranium (U), both from natural deposits and legacy of U mining (deLemos et al. 2009; Hund et al. 2015; Lewis et al 2017) which was extensive from the 1940s through the 1980s (Brugge and Goble 2002; US Environmental Protection Agency n.d.). Chronic exposure to several metals is associated with cardiovascular disease (CVD) (Lind, Olsén, and Lind 2012; Prozialeck et al. 2008; Shim et al. 2017; Solenkova et al. 2014). In recent decades, the Navajo population has experienced an increased prevalence of metabolic syndrome (Schumacher et al. 2008) and coronary heart disease (National Cholesterol Education Program 2002). Recently Harmon et al. (2017) found that proximity to abandoned mine sites may contribute to circulating inflammatory potential, defined as an inflammatory transcriptional response in cultured endothelial cells treated with serum from subjects in this community. While diet and lifestyle are important contributors to increasing rates of CVD, the contribution from exposure to metals in mining waste is unknown.
As often coexists with U deposits and is present in legacy U mining wastes (Blake et al. 2015; Katsoyiannis et al. 2007), contributing to contamination of soil, air, and water. Approximately 20% of unregulated water sources throughout the Navajo Nation exceed current US Environmental Protection Agency (EPA) maximal contaminant levels for U (US Army Corps of Engineers 2000). Recently Hoover et al. (2017) noted that little improvement has occurred, with estimates of 15% exceedances for As and 13% exceedances for U in tested water sources. Navajo Nation EPA estimated that more than 30% of the Navajo population lack access to public water supplies and concerns exist regarding As and U exposure through consumption of unregulated water (Navajo Access Workgroup 2010).
Chronic As exposure is associated with ischemic heart disease, hypertension, atherosclerosis, diabetes, and cardiovascular mortality (Medrano et al. 2010; Navas-Acien et al. 2006; Rahman et al. 1999; Tseng et al. 2003; Wang et al. 2002; Yang 2006). Accumulating epidemiological and mechanistic evidence suggests that the cardiovascular system is sensitive to environmentally relevant levels of As exposure (Howard et al. 1999; James et al. 2015; Lemaire et al. 2011; Moon et al. 2017, 2013; Wu, Molinaro, and Chen 2014). In contrast, little is known regarding cardiovascular-related effects of chronic environmental exposure to U. Limited evidence suggests a potential relationship between estimates of U exposure and circulatory systemic disease (Guseva Canu et al. 2012) and increased blood pressure (Kurttio et al. 2006). In addition, exposure to U mine waste on Navajo was linked to an enhanced likelihood of hypertension (Hund et al. 2015). Maximal containment levels for U were developed for outcomes related to cancer and kidney diseases, but not CVD, while maximal containment level for As takes cardiovascular outcomes into consideration (Hoover et al. 2017).
Oxidized low-density lipoprotein (oxLDL) is an emerging biomarker that is mechanistically associated with CVD (Gómez et al. 2014; Holvoet et al. 1998; Trpkovic et al. 2014) and predictive of cardiovascular outcomes including acute coronary artery disease (Meisinger et al. 2005) and myocardial infarction (Johnston et al. 2006). CRP is a well-established biomarker of inflammation associated with increased CVD risk (Danesh et al. 2004). Karim et al. (2013) reported that As exposure in Bangladesh residents was associated with oxLDL and CRP levels, but Moon et al. (2017) found no marked association between urine As and plasma CRP. Several metals are known to contribute indirectly to oxidation of LDL and lipids (Chisolm and Steinberg 2000; Jomova and Valko 2011). However, it is unknown whether As and U directly oxidize LDL cholesterol.
Few studies examined the relationship between chronic low-level As or U exposure and CVD in at-risk communities such as those on the Navajo Nation where CVD and diabetes are significant public health concerns (Moon et al. 2017, 2013). The aim of this investigation was to assess a potential association between these metals and plasma levels of oxLDL and CRP in several Navajo communities located in the southwestern United States with varying levels of As and U exposures. It was postulated that environmental exposure to As and U may be correlated with elevated plasma oxLDL and CRP concentrations. In addition, proof-of-concept acellular assays were performed to investigate whether As and U directly oxidize LDL cholesterol.
Methods
Community-derived biomarkers and exposure assessments: cohort recruitment and demographic information
Demographic, water use and clinical data were obtained in partnership with the Diné Network for Environmental Health (DiNEH) Project using an interviewer-administered survey. More detailed descriptions of the survey and population may be found elsewhere (deLemos et al. 2009; Harmon et al. 2016, 2017; Hund et al. 2015). Surveys were completed by 1,304 Navajo Nation adult members between 2005 and 2010. Data for the current study represent a subset of these participants (n = 252) who volunteered to provide non-fasting blood and urine samples between 2010 and 2011 at which time height and weight were measured to determine body mass index (BMI). These 252 participants were therefore not a randomly selected group. All participants provided informed consent, with oversight and approval from the UNM Human Research Review Committee and the Navajo Nation Human Research Review Board.
Water analysis and human exposure assessments
Study participants reported obtaining water from a total of 178 distinct sources, including 122 unregulated sources such as wells, springs, or livestock watering stations; 42 public water supply sources; and 14 sources that could not be classified. In order to evaluate metal exposure via drinking water, DiNEH staff collected water samples for 124 representative water sources (101 unregulated, 23 regulated) from 2003–2010 following US EPA Water Quality Standards Handbook, 2007 (https://www.epa.gov/wqs-tech/water-quality-standards-handbook). In US EPA region, nine labs conducted water quality analyses for unregulated sources using US EPA analysis method 200.8 (Inductively Coupled Plasma Mass Spectroscopy, ICP-MS). A small subset of samples (approximately 8%) was analyzed at the Carlsbad Environmental Monitoring and Research Center or at Stanford University. Navajo Nation EPA provided public water quality data. The compiled water data included As measurements for 113 sources (15 public water sources and 98 unregulated sources) and U measurements for 108 sources (16 public water sources and 92 unregulated sources).
Only unregulated sources with water quality information were included in the analyses and are representative of commonly used sources. A comprehensive description of As and U in unregulated water sources on the Navajo Nation may be found elsewhere (Hoover et al. 2017). Public, regulated water system sources, store purchased and bottled water without As or U measurements were presumed to be in compliance with Safe Drinking Water Act regulations for As (< 10 μg/L) and U (< 30 μg/L) (US Environmental Protection Agency 2015).
Annual As and U intakes from water were estimated from self-reported volume of water consumed and metal concentration for each water source used. Participants indicated how frequently they visited the source, the typical volume of hauled water and % used for cooking or drinking. This information was used to calculate the individual annual intake of As or U (mg/year) from drinking water using the associated As or U concentration for each separate water source.
Once the cumulative annual intake of water As and U from water was determined for each participant, a binary variable was employed to indicate low versus high metal consumption, based upon a threshold consumption of 1.4 mg/year in the distributions of both metals. This threshold represented a natural division in data distribution for both metals. In addition, subjects who utilized only regulated drinking water sources, were assigned to the low consumption groups for both As and U. Those considered to be in the high intake group were a small fraction of the 252 people in the cohort (10% for As and 15% for U).
Urine metals concentrations
Urine metals were determined by ICP-MS. Five-point standard curves were generated for each metal. Total As was measured without regard to specific species. Urinary concentrations of vanadium (V 51), nickel (Ni 60), As (AsO 91), and U (U 238) were available for 188 participants. Urinary copper (Cu) was available for a subset of participants (n = 94). Approximately 50% of urine U levels were below the level of detection (0.1 μg/L), therefore available U data were examined as a binary variable reflecting detect or non-detect as the threshold. The limit of detection (LOD) for urinary As was 0.2 μg/L.
Serum biomarker analysis
Plasma samples were tested for oxLDL by a sandwich ELISA that uses a monoclonal antibody specific for an epitope (monoclonal antibody 4E6) of the apolipoprotein B100 (ApoB 100) portion of the LDL particle, in accordance with manufacturer’s instructions (Mercodia, Uppsala, Sweden) (n = 252). All other biochemical analyses were performed by a Navajo Area Indian Health Service reference lab (LabCorp, Phoenix, AZ). CRP was assessed quantitatively by latex immunoturbidimetry (LabCorp, Phoenix, AZ) (n = 249). Glycated hemoglobin (HbA1c) (n = 249) was determined by the Roche Tinaquant assay (Roche Diagnostics, Indianapolis, IN).
Acellular assays of metal-induced LDL oxidation: materials
Acellular studies utilized copper sulfate (CuSO4) and sodium arsenite (NaAsO2) (Sigma-Aldrich Co., St. Louis, MO, USA); uranyl acetate (UA) (Electron Microscopy Sciences, Hatfield, PA, USA); and highly purified human LDL (Lee Biosolutions, St. Louis, MS, USA).
Determination of protein and lipid peroxidation of LDL
Direct ApoB 100 oxidation of the metal-treated LDL samples was determined by ELISA (as described earlier). Lipid peroxidation in the LDL-metal samples was determined using a thiobarbituric acid reactive substances (TBARS) assay kit, which normalized total lipid peroxides to a malondialdehyde (MDA) standard (Cayman Chemical Company, Ann Arbor, MI, USA) per manufacturer’s instructions.
Treatment of LDL by metals
Direct metal-induced oxidation of human LDL cholesterol was determined by performing individual dose responses of CuSO4, NaAsO2, and UA with purified human LDL. CuSO4 was used as a positive control since it is frequently utilized experimentally to oxidize LDL (Burkitt 2001). A concentration of 100 mg/dl human LDL was incubated with CuSO4 (20, 50, 150 and 450 μM for oxLDL ELISA; 15, 25, 45 and 75 μM for the TBARS assay), NaAsO2 (0.02, 0.07, 0.2 and 0.7 μM for oxLDL ELISA; and 0.2, 0.7, 2 and 7 μM for TBARS assay), and UA (0.3, 3, 30 and 300 nM). LDL was co-incubated with combinations of NaAsO2 and UA (0.02 μM NaAsO2 and 0.3 nM UA, 0.02 μM NaAsO2 and 3 nM UA, or 0.07 μM NaAsO2 and 0.3 nM UA). CuSO4 and NaAsO2 were dissolved in phosphate buffered saline (PBS) to achieve desired concentrations. UA was first dissolved in water at a concentration of 100 μM and then diluted to 300 nM in 4-(2-hydro-xyethyl)-1-piperazineethanesulfonic acid (HEPES) to obtain desired concentrations. For the experiments that tested the combined effect of NaAsO2 and UA, HEPES was used to dissolve both metals. After treatment, metal-treated purified human LDL samples were placed in a chamber with nitrogen flow for 10 min to reduce the contribution of atmospheric oxygen to LDL oxidation. Samples were then sealed and incubated at 37°C for 1–5 h. EDTA (4 mM) was immediately added to the final samples at each time point to chelate the reaction. Samples were immediately frozen at −80°C until further analysis. Lower concentrations of metals tested were physiologically relevant to normal or background levels of each metal in plasma (Agency for Toxic Substances and Disease Registry (ATSDR) 2007; Byrne and Benedik 1991; Ivanenko et al. 2013; McMillin, Travis, and Hunt 2009).
Statistical analyses
Summary statistics are reported as median and inter-quartile range (IQR) for continuous variables unless otherwise indicated. Logarithmic transformations were performed to normalize non-Gaussian distributions. Four linear regression models were constructed for each oxLDL and log CRP (Tables 2 and 3), all of which were derived from the full set of data and reduced by model selection using the Akaike Information Criterion (AIC) with stepwise selection allowed in both directions; models were successively revised so as to reduce AIC. All models include demographic (age, gender) and physiologic variables (BMI, HbA1c), and estimated annual water intakes of U and As as predictors. Models 1 and 2 include data from 249 participants. Models 3 and 4 additionally include continuous concentrations of urinary copper (Cu), vanadium (V), and nickel (Ni), As (log transformed) and U (binary as described before) as predictors for a subset of 94 participants with available data for urinary Cu. Second order interactions are included Models 2 and 4, and significant interactions reported. The majority (> 80%) of HbA1c levels in the participants were in prediabetic or diabetic categories, therefore, a binary variable with HbA1c >6.4% as the threshold used in regression models. Estimated annual intakes of As and U from drinking water are also included as binary variables (Water As bi, Water U bi) as previously described. Statistical analyses were performed using R version 2.12.1 (The R Foundation for Statistical Computing 2010, 64-bit). The time and dose-response relationships of purified human LDL and metal treatments were analyzed by two-way analysis of variance (ANOVA) followed by the Dunnett’s multiple comparison test using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA). The criterion for significance was set at p < 0.05.
Table 2.
Reduced regression models showing coefficient estimates for oxLDL.
| Coefficient | Estimate | Standard error | P-value | N |
|---|---|---|---|---|
| Model 1 | 249 | |||
| Water Asbi | 10.98 | 3.788 | 0.004 | |
| Adjusted R2 = 0.029, AIC = 2121, p = 0.004 | ||||
| Model 2 | 249 | |||
| Age (years) | −0.052 | 0.081 | 0.526 | |
| Female | −1.12 | 2.288 | 0.625 | |
| Water Asbi | 10.05 | 4.232 | 0.018 | |
| Water Ubi | −31.66 | 14.73 | 0.033 | |
| Adjusted R2 = 0.037,AIC = 2123, p = 0.02 | ||||
| Model 3 | 94 | |||
| Water Asbi | 15.9 | 7.086 | 0.027 | |
| Water Ubi | −11.66 | 5.305 | 0.031 | |
| Urine As | −3.889 | 1.916 | 0.045 | |
| Urine Ubi | 11.68 | 4.392 | 0.009 | |
| Adjusted R2 = 0.106,AIC = 800.7, p = 0.007 | ||||
| Model 4 | 94 | |||
| Age (years) | −1.568 | 0.823 | 0.06 | |
| Female | −54.19 | 24.07 | 0.027 | |
| Water Asbi | 16.09 | 6.978 | 0.024 | |
| Water Ubi | −35.28 | 17.89 | 0.052 | |
| Urine As | −6.023 | 2.143 | 0.006 | |
| Urine Ubi | 8.833 | 4.281 | 0.042 | |
| Urine Cu | −25.14 | 13.84 | 0.073 | |
| Female x Urine Cu | 17.42 | 7.427 | 0.021 | |
| Adjusted R2 = 0.203, AIC = 797, p = 0.002 | ||||
P < 0.05 was considered statistically significant.
Reduced models are shown. All full models included demographic (age, gender) and physiologic variables (BMI, HbA1c), and estimated annual water intakes of U and As as predictors.
Models 1 and 2 included data from 249 participants. Models 3 and 4 include a subset of 94 participants with available data for urinary Cu. Models 3 and 4 also included continuous concentrations of urinary copper (Cu), vanadium (V), and nickel (Ni), As (log transformed), and U (binary). Models 2 and 4 included second order interactions and significant interactions are reported.
Table 3.
Reduced regression models showing coefficient estimates for CRP.
| Coefficient | Estimate | Standard error | P-value | N* |
|---|---|---|---|---|
| Model 1 | 249 | |||
| Age (years) | −0.015 | 0.005 | 0.002 | |
| BMI, kg/m2 | 0.038 | 0.014 | 0.007 | |
| Adjusted R2 = 0.085, AIC = 750.4, p = < 0.0001 | ||||
| Model 2 | 249 | |||
| Age (years) | −0.016 | 0.005 | 0.001 | |
| Female x BMI | −0.059 | 0.03 | 0.049 | |
| Adjusted R2 = 0.092, AIC = 750.4, p = < 0.0001 | ||||
| Model 3 | 94 | |||
| Age (years) | −0.019 | 0.007 | 0.011 | |
| Female | −0.327 | 0.205 | 0.115 | |
| BMI, kg/m2 | 0.049 | 0.022 | 0.026 | |
| Adjusted R2 = 0.157, AIC = 263.1, p = 0.001 | ||||
| Model 4 | 94 | |||
| BMI, kg/m2 | 0.051 | 0.021 | 0.017 | |
| Age x HbA1c bi | −0.04 | 0.017 | 0.018 | |
| Female x Urine U bi | 1.296 | 0.589 | 0.031 | |
| Adjusted R2 = 0.246, AIC = 258.7, p = 0.0003 | ||||
P < 0.05 was considered statistically significant.
Reduced models are shown. All full models included demographic (age, gender) and physiologic variables (BMI, HbA1c), and estimated annual water intakes of U and As as predictors.
Models 1 and 2 included data from 249 participants. Models 3 and 4 include a subset of 94 participants with available data for urinary Cu. Models 3 and 4 also included continuous concentrations of urinary copper (Cu), vanadium (V), and nickel (Ni), As (log transformed), and U (binary). Models 2 and 4 included second order interactions and significant interactions are reported.
Results
Community-level association between mining metal exposure and LDL oxidation
This Navajo subset is known to exhibit a high prevalence of diabetes, hypertension and overweight/obesity compared to the general US population (Harmon et al. 2016; Hund et al. 2015). Table 1 shows clinical characteristics and biomarker levels by gender. Both genders were similar in age and displayed similar total cholesterol (TC), LDL cholesterol and non-fasting glucose levels. Women generally exhibited higher BMI, high-density lipoprotein (HDL) levels, lower triglycerides (TG), and lower blood pressures than men. Both genders displayed similar median HbA1c levels, although more women fell into prediabetic and diabetic categories of HbA1c classification. Women showed lower levels of oxLDL and higher CRP levels.
Table 1.
Navajo demographic and clinical characteristics by gender.
| Variable | Female (n = 145) | Male (n = 107) | Value above, below median for AS | Value above, below median for U |
|---|---|---|---|---|
| Age (years) | 55.5 ± 14 | 55.1 ± 14.7 | 54.0 ± 14.0, 56.2 ± 14.1 | 55.7 ± 14.1, 54.6 ± 14.4 |
| BMI, kg/m2 | 31.2 (27.7–34.9) | 28.2 (26.1–31.5) | 29.0 ± 5.5, 29.0 ± 5.0 | 28.7 ± 5.4, 29.3 ± 5.1 |
| Total Cholesterol, mg/dL | 182.5 (163.3–201.8) | 182 (159.5–204.5) | 184.1 ± 36.8, 186.9 ± 33.5 | 186.6 ± 36.2, 184.5 ± 34.6 |
| LDL, mg/dL | 105 (90.3–123.8) | 104 (88–122) | 109.0 ± 31.0, 107.1 ± 32.4 | 113.0 ± 28.9, 103.1 ± 33.2 |
| HDL, mg/dL | 47.5 (39–57) | 42 (34–49.5) | 47.6 ± 13.4, 47.8 ± 18.2 | 47.6 ± 14.0, 47.8 ± 17.8 |
| Triglycerides, mg/dL | 177 (127.5–247.3) | 191 (125–261.5) | 204.9 ± 141.7, 224.5 ± 136.8 | 198.6 ± 114.0, 231.0 ± 166.4 |
| Systolic BP (mmHg) | 126 (114–142) | 132 (121.5–145.5) | 128.8 ± 20.1, 134.0 ± 20.3 | 133.5 ± 21.0, 129.8 ± 19.6 |
| Diastolic BP (mmHg) | 76 (70–85) | 81 (73–88.5) | 77.6 ± 12.9, 80.6 ± 11.3 | 79.2 ± 13.0, 79.2 ± 11.3 |
| Glucose (non-fasting), mg/dL | 90 (77–122.8) | 92 (79–120) | 120.0 ± 75.4, 129.1 ± 89.2 | 119.9 ± 78.2, 129.3 ± 87.1 |
| Median HbA1c, % | 6.3 (5.9–7.3) | 6 (5.6–6.9) | 6.83 ± 1.97, 7.08 ± 2.10 | 6.74 ± 1.76, 7.17 ± 2.26 |
| % Normal (≤ 5.6%) | 7.7 | 26.4 | ||
| % Pre-diabetes (5.7–6.4%) | 50.3 | 39.6 | ||
| % Diabetes (≥ 6.5%) | 42 | 34 | ||
| Oxidized LDL, U/L | 45.7 (36.7–57) | 48.7 (36.7–57.2) | 48.8 ± 18.43, 48.1 ± 15.2 | 49.7 ± 18.3, 47.3 ± 15.5 |
| CRP, mg/L | 2.5 (0.9–5.2) | 1.8 (0.9–4.2) | 3.59 ± 3.83, 4.96 ± 12.69 | 4.02 ± 11.8, 4.54 ± 6.69 |
| CRP > 3.0 mg/L, % | 44 | 32 | ||
| Mean Water Arsenic, ppb (10–90%ile) | 2.48 (1.00–6.00) | 4.80 (1.00–4.85) | ||
| Mean Water Uranium, ppb (10–90%ile) | 5.35 (0.38–13.23) | 6.04 (0.38–13.30) |
Data are presented as median (IQR) or %; age was reported as mean (SD). BMI: body mass index; LDL: low-density lipoprotein; HDL: high-density lipoprotein; BP: blood pressure; HbA1c: glycated hemoglobin; CRP: C-reactive protein.
The median annual water intakes for As and U were similar, 0.49 mg/year (IQR 0–1.09) and 0.46 mg/year (IQR 0–1.13), respectively. Water As intake was not correlated with urine As, and similarly water U intake did not correlate with urine U (data not shown).
Median urine As [4.21 (IQR 2.25–6.78) μg/L] was below National Health and Nutrition Survey (NHANES) 50th percentile for total As [7.9 (IQR 7.00–9.10) μg/L] (non-creatinine corrected, adults >20 years old) (Centers for Disease Control and Prevention (CDC) 2017). It is noteworthy that seafood intake may contribute substantially to heavy metal intake in the NHANES population, while the Navajo consume relatively little seafood (Jones et al. 2016; Navas-Acien et al. 2011). However, 14.6% of participants exhibited urine U concentrations that exceeded NHANES 95th percentile (survey years 2003–2004; for all races, age, and gender) by 4.2 fold. Median values and IQR for other measured urine metals were: Cu, 12.45 (8.15–17.02) μg/L; Ni, 13.08 (6.67–25.24) μg/L; and V, 1.05 (0.61–1.56) μg/L. NHANES values for these urine metals are not presently available.
Results of the linear regression models for oxLDL are shown in Table 2. In Models 1 and 2, estimated annual intake of water As (water Asbi) was significantly associated with oxLDL. In Model 2, water Ubi was significantly, inversely associated with oxLDL. Including only individuals with available urine Cu (n = 94) in Models 3 and 4, model fit improved to R2 =0.11 (Model 3) and R2 =0.203 (Model 4). In Model 3, which included urinary metals concentrations, water intake estimates for As and U remained significant positive and negative predictors, respectively for oxLDL, and excreted U (urine U) was a positive predictor. Similar results were observed in Model 4, which included an interaction between female gender and urine Cu. BMI, HbA1c, and urine Ni and V did not appear as significant predictors in any model.
Results of the linear regression models for log CRP are presented in Table 3. In Models 1 and 3, age was a negative predictor and BMI was a positive predictor for log CRP. However, in Model 2 the interaction of gender and BMI was significantly, negatively associated with log CRP. Model fit improved in the subset analyses (Models 3 and 4), when urine metals data was included. In Model 4, BMI was again positively associated with for log CRP. However, significant interactions were only identified between gender and urine Ubi and between age and HbA1c suggesting that women with higher urine Ubi possessed higher levels of CRP. Water Asbi and Ubi and urine As, Cu, Ni, or V did not emerge as significant predictors in any of the models for CRP.
Acellular studies of LDL oxidation
Protein oxidation of LDL cholesterol by As and u
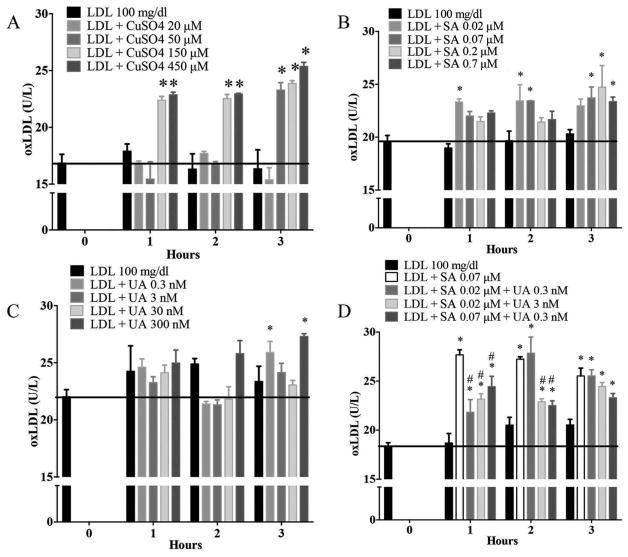
CuSO4 is a known oxidizer of LDL and was used as a positive control. CuSO4-treated LDL demonstrated a time and dose dependent response as expected, for CuSO4 concentrations greater than 50 μM as compared to untreated LDL (Figure 1A) as measured by ELISA for the ApoB 100 protein component of LDL. NaAsO2 appeared to directly oxidize LDL earlier at lower quantities and later at higher concentrations, but the level of oxLDL was similar at each time point (Figure 1B). UA only appeared to oxidize LDL at 3 hr, but inconsistently at only the lowest (0.3 nM) and highest (300 nM) concentrations (Figure 1C). Interestingly, in combined treatments, oxLDL levels were elevated at all time points when both NaAsO2 and UA were present in the reaction mixture (Figure 1D). Only the lowest combined concentrations of NaAsO2 (0.02 μM) and UA (0.3 nM) increased LDL oxidation to the same level as LDL treated with NaAsO2 alone (0.07 μM), however, LDL treated with the same concentration of NaAsO2 (0.07 μM) plus UA (0.3 nM) was significantly lower than LDL treated with NaAsO2 alone at 1 and 2 h but was similar at 3 h. LDL treated with 3 nM of UA alone was not significantly altered (Figure 1C), but when the same concentration was combined with NaAsO2, LDL oxidation was elevated at all time points. Data suggest that NaAsO2 directly oxidizes LDL, but when combined with UA, the interaction was unclear as measured by ELISA in this experimental system.
Figure 1.
Sodium arsenite directly oxidizes the apolipoprotein B component of LDL cholesterol. (A) CuSO4 was used as a positive control. Effects of different concentrations of (B) Sodium arsenite (SA); (C) Uranyl acetate (UA), or (D) the combination of SA and UA on the apoprotein B (ApoB) of human purified LDL as measured by the 4E6 ELISA antibody. * Indicates significant difference from LDL untreated. # represents significant difference from LDL treated with SA at 0.07 μM. Error bars show SEM. P < 0.05 was considered statistically significant.
Lipid peroxidation of LDL cholesterol by as and u
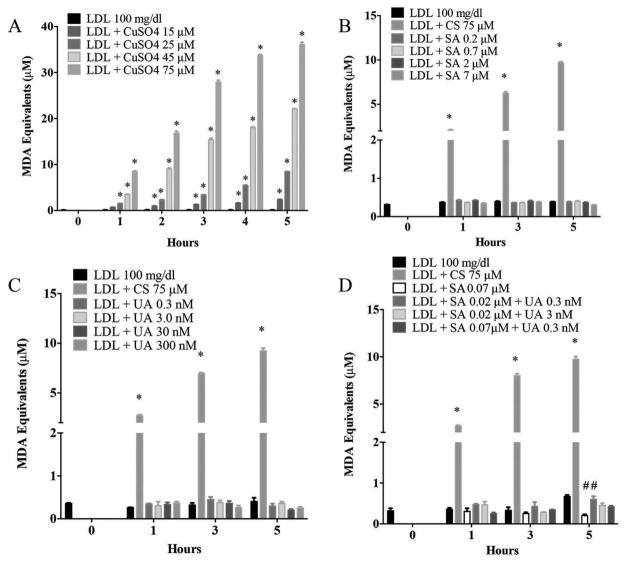
CuSO4-induced lipid peroxidation (MDA equivalents) of human LDL as expected with clear concentration- and time-dependent trends observed (Figure 2A). The level of lipid peroxides was not markedly affected by NaAsO2, UA, or combination of both metals (Figure 2B–D) especially in comparison to CuSO4-administered LDL, used repeatedly as a positive control (Figure 2A). Data suggest that NaAsO2 or UA failed to promote lipid peroxidation of LDL cholesterol under these conditions.
Figure 2.
Sodium arsenite and uranyl acetate do not increase lipid peroxidation of LDL as measured by TBARS (malondialdehyde equivalents). (A) CuSO4 (CS) was used as a positive control. Effects of different concentrations of (B) SA, (C) UA, or (D) the combination of SA and UA on lipid peroxidation of human purified LDL. * Indicates significant difference from LDL untreated; # represents significant difference from LDL treated with SA at 0.07 μM. Error bars show SEM. P < 0.05 was considered statistically significant.
Overall, these findings are coherent with our regression modeling data in that As may promote increased LDL oxidation, however, it appears that As may contribute directly to oxidation of the protein (Apo B 100) component and not the lipid components of the LDL particle. U does not appear to be associated with elevation in oxLDL levels.
Discussion
This study examined whether CVD biomarkers, oxLDL and CRP, were associated with annual estimated drinking water intake of As and U, as representative mining-related metal contaminants in this affected community. To establish proof-of-concept whether these metals might directly oxidize LDL was also determined. Both the population and lab results are consistent with the hypothesis that As promotes oxidation of LDL, a crucial step in vascular inflammation and chronic vascular diseases. Outcomes related to U demonstrated negative associations between U intake and oxLDL and U only minimally altered human LDL in direct exposure experiments. Thus cholesterol may not be considered a physiological or chemical target for U. Urine As and U were also associated with oxLDL, albeit in opposite directions from each other and from the water intakes of these metals. Only urine U as an interaction term with gender was correlated with CRP, whereas no other metals in water or urine were reliable predictors of this inflammatory marker.
In our studies, there was a limited correlation between our modeled estimates of metals uptake and measured urine levels in our cohort. Evidence indicates that this as an important contrast between a metric of chronic exposure (annual intake) and a more acute reflection of exposure (urine output). It is important to consider that our water intake calculations were based upon a survey tool wherein participants provided estimates of where they obtained water and for what purpose. Thus, while previously Calderon et al. (1999) studies found an immediate correlation between drinking water and urine levels of As, our estimate incorporates a longer history of water usage, albeit with likely increased error due to recall inaccuracies. Consistently, data demonstrated that annual estimate of As intake was best associated with oxLDL. Numerous epidemiological studies reported that As contributes to CVD development (Rahman et al. 1999; Tseng et al. 2003; Wang et al. 2002; Yang 2006), but limited evidence is available for As effects on the intermediate biochemical changes associated with atherogenesis. Chronic As exposure from drinking water was noted to be associated with biomarkers of inflammation and endothelial cell activation including oxLDL, the ratio of oxLDL with HDL, intercellular adhesion molecule-1 (Karim et al. 2013), and vascular cell adhesion molecule-1 (F. Wu et al. 2012). In contrast to other investigations, an association between As exposure and elevated plasma CRP levels was not found, although concentrations of As in the Eastern Agency region of Navajo Nation were lower than those detected in severely contaminated regions of Bangladesh (Karim et al. 2013; Peters et al. 2015).
OxLDL contains heterogeneous lipid and protein components that vary in degree and type of oxidation which induce a variety of pro-atherogenic effects (Reis et al. 2015). Data suggest that As, in direct contact with LDL particles, might modify the ApoB 100 component in a manner that makes it recognizable by the 4E6 antibody. While As was shown to promote chronic vascular remodeling, there is conflicting evidence whether As elevates lipid peroxide levels in ApoE−/− mice (Simeonova et al. 2003; Srivastava et al. 2009). In the present study, As exerted minimal acute effects on lipid peroxidation of purified human LDL. Minimally oxidized LDL is sufficient to promote vascular inflammation and pathological remodeling, and is typically characterized by ApoB oxidation with lower levels of TBARS (Itabe et al. 2003). However, the direct interaction of As with ApoB may be sufficient to drive minimally oxidized form of oxLDL and thereby play a role in systemic vascular disease. As the more chronic measure of annual As intake was found to be a more reliable predictor of oxLDL than the acute snapshot of urine As, the relevance of the ApoB100 target, rather than untargeted lipid peroxidation, becomes more important from a pathophysiologic standpoint.
It remains unknown how metals such as As and U may influence oxidative modifications of LDL. As is redox inactive but possesses an affinity for thiol-containing molecules (Jomova and Valko 2011). The ApoB-100 protein component of LDL contains cysteine residues (Segrest et al. 2001) that may potentially be modified by As. As is also known to bind hemoglobin on erythrocytes as well as other proteins (especially thiol-containing moieties) in the plasma, including thioredoxin and glutathione (Hughes et al. 2011; Schmidt, Fahlbusch, and Otto 2009), which may influence the bioavailability and pharmacokinetics of As as well as affect the behaviors of the bound proteins. Little is known regarding the effects of chronic low-level exposure to U, particularly in terms of CVD prevalence and pathogenesis. Limited studies on endothelial cell toxicity are generally negative (Dobson et al. 2006). Our results suggest that in an isolated acellular system, low concentrations of U do not directly modify either protein or lipid components of LDL. The concentrations of As and U that were employed reflect realistic levels that are detected in blood, albeit without addition of blood components that may reduce the overall bioavailability of the free metals. The interaction of U and NaAsO2 on ApoB-100 oxidation was unclear, and the level of oxidation was not greater than NaAsO2 treatment alone. Further, measures of U in drinking water were negatively associated with oxLDL and not at all with CRP levels in the Navajo population. This observed molecular antagonism in the acellular assays may help explain this association. An important consideration for mine wastes is the mixture of metal contaminants, not simply the residual ores of interest, which may be most toxic to exposed populations. Thus, while the vast majority of abandoned mine sites in this region were a result of U extraction, numerous other metals including As, V, Ni, and Cu may be omitted creating a legacy environmental health risk.
While our findings indicate the potential for direct effects between metal contaminants and LDL oxidation, the contribution of indirect effects should not be dismissed. Increased levels of reactive oxidants and reduced antioxidant capacity were noted in plasma from humans exposed to As in drinking water (Wu et al. 2001). Changes in redox status or enhanced free radical production (Lankin et al. 2014) and inflammatory factors may be induced in the presence of toxic metals in plasma or atherosclerotic lesions (Stadler, Lindner, and Davies 2004).
Limitations
The cross-sectional nature of our analysis does not allow for temporal relations between exposure and CVD outcomes to be established; however, this is the first population-based study to demonstrate a correlation between As and LDL oxidation in an exposed population in the U.S.A. The relatively high rate of hypertension and obesity in this cohort, along with other major effect modifiers such as socioeconomic status, education, smoking status, and access to health care, all are known contributors to CVD and affect many biomarkers (Harmon et al. 2016). Selection bias is a possibility in this volunteer population; however, no apparent biases in exposure risks were found (deLemos et al. 2009; Hund et al. 2015). Participants represented the full range of exposures including a significant proportion of unexposed volunteers, improving the generalizability of our results (Hund et al. 2015). Urine values of metals were measured without correction for urine dilution, which might increase the variability of this measure depending upon the hydration levels of participants. Blood samples were non-fasting, and medication use was not included in this initial analysis, which might influence LDL modifications. Because of the long storage times, it was not possible to assess TBARS in the banked serum from the DiNEH study as a parallel to the acellular assays. Finally, the lack of complete data for urine metals values and overall small size of the cohort limits the strength of conclusions from this community-based study.
To establish biological plausibility, proof-of-concept acellular assays were performed, which were selected to represent direct oxidation of LDL by As and U in the circulatory system, thereby contributing to the atherosclerotic process. However, this might also represent what may be occurring within developing lesions. It is postulated that most modifications to LDL probably occur in situ within the atherosclerotic lesion (Itabe, Obama, and Kato 2011), and more research is needed to assess whether As and/or U accumulate in vulnerable vascular lesions. Several metals including As were detected in atherosclerotic lesions and cardiovascular tissue (Shi, Shi, and Liu 2004; Simeonova et al. 2003). Importantly, our acellular data are in agreement with findings observed in human serum, in terms of ApoB modifications. Finally, although the buffers used in these experiments (PBS and HEPES) are stable and nonreactive, these reagents do not share the same milieu of factors that may contribute to the atherosclerotic process or affect metal-LDL interactions.
Conclusions
Our findings suggest that As intake from contaminated drinking water may influence oxidative modifications of LDL in the Navajo population. Direct oxidation of LDL by environmental metals such as U and As may be an overlooked contributing mechanism to explain chronic CVD health outcomes, although future research needs to explore such relationships in more physiologically relevant experimental systems. Specific exposure levels and temporal dynamics of oxidative effects of environmental metals require to be more comprehensively elucidated in future studies. For the Navajo community, there remains a dearth of literature exploring the potential adverse health effects of unremediated wastes and tailings from legacy and future mining operations in the region. Continued research into mining waste-related community health outcomes, and pathophysiological basis thereof, is essential to ensuring that mineral resources may be extracted with minimal negative impacts on public health. Exposure assessments and health outcomes need to be detailed in a more rigorous manner to permit such investigations.
Acknowledgments
The authors wish to sincerely thank all of the Navajo participants in this study, and Laurie Hudson and Karen Cooper for their technical assistance.
Funding
This study was funded by the National Institutes of Health (HL00773620, ES013208, ES014639, ES026673, ES025589)
Footnotes
Disclosure Statement
The authors declare that they do not have any competing financial interests to disclose.
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/uteh.
References
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for arsenic. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 2007. http://www.atsdr.cdc.gov/toxprofiles/tp2-c3.pdf. [PubMed] [Google Scholar]
- Blake JM, Avasarala S, Artyushkova K, Ali AS, Brearley AJ, Shuey C, Robinson WP, Nez C, Bill S, Lewis J, Hirani C, Pacheco JS, Cerrato JM. Elevated concentrations of U and co-occurring metals in abandoned mine wastes in a northeastern Arizona Native American community. Environmental Science & Technology. 2015;49:8506–14. doi: 10.1021/acs.est.5b01408. [DOI] [PubMed] [Google Scholar]
- Brugge D, Goble R. The history of uranium mining and the Navajo people. American Journal of Public Health. 2002;92:1410–19. doi: 10.2105/ajph.92.9.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkitt MJ. A critical overview of the chemistry of copper-dependent low density lipoprotein oxidation: Roles of lipid hydroperoxides, alpha-tocopherol, thiols, and ceruloplasmin. Archives of Biochemistry and Biophysics. 2001;394:117–35. doi: 10.1006/abbi.2001.2509. [DOI] [PubMed] [Google Scholar]
- Byrne AR, Benedik L. Uranium content of blood, urine and hair of exposed and non-exposed persons determined by radiochemical neutron activation analysis, with emphasis on quality control. The Science of the Total Environment. 1991;107:143–57. doi: 10.1016/0048-9697(91)90256-e. [DOI] [PubMed] [Google Scholar]
- Calderon RL, Hudgens E, Le XC, Schreinemachers D, Thomas DJ. Excretion of arsenic in urine as a function of exposure to arsenic in drinking water. Environment Health Perspective. 1999;107:663–67. doi: 10.1289/ehp.99107663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Fourth national report on human exposure to environmental chemicals updated tables, January 2017. Vol. 1. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2017. [Accessed January, 2017]. https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2017.pdf. [Google Scholar]
- Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: An overview. Free Radical Biology & Medicine. 2000;28:1815–26. doi: 10.1016/s0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GDO, Pepys MB, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engineering Journal Medica. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- De Lemos JL, Brugge D, Cajero M, Downs M, Durant JL, George CM, Henio-Adeky S, Nez T, Manning T, Rock T, Seschillie B, Shuey C, Lewis J. Development of risk maps to minimize uranium exposures in the Navajo Churchrock mining district. Environmental Health. 2009;8:29. doi: 10.1186/1476-069X-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson AW, Lack AK, Erikson KM, Aschner M. Depleted uranium is not toxic to rat brain endothelial (RBE4) cells. Biological Trace Element Research. 2006;110:61–72. doi: 10.1385/BTER:110:1:61. [DOI] [PubMed] [Google Scholar]
- Geomatics, TerraSpectra, United States Environmental Protection Agency Region IX, United States Environmental Protection Agency Region IX Superfund Records Center, and United States Army Corps of Engineers Los Angeles District. Abandoned Uranium Mines Project, Arizona, New Mexico, Utah –Navajo Lands, 1994–2000: Project Atlas. U.S. Environmental Protection Agency, Region 9 Superfund Records Center; 2000. https://books.google.com/books?id=6usEPwAACAAJ. [Google Scholar]
- Gómez M, Vila J, Elosua R, Molina L, Bruguera J, Sala J, Masià R, Covas MI, Marrugat J, Fitó M. Relationship of lipid oxidation with subclinical atherosclerosis and 10-year coronary events in general population. Atherosclerosis. 2014;232:134–40. doi: 10.1016/j.atherosclerosis.2013.10.026. [DOI] [PubMed] [Google Scholar]
- Guseva Canu I, Garsi JP, Caër-Lorho S, Jacob S, Collomb P, Acker A, Laurier D. Does uranium induce circulatory diseases? First results from a French cohort of uranium workers. Occupational and Environmental Medicine. 2012;69:404–09. doi: 10.1136/oemed-2011-100495. [DOI] [PubMed] [Google Scholar]
- Harmon ME, Campen MJ, Miller C, Shuey C, Cajero M, Lucas S, Pacheco B, Erdei E, Ramone S, Nez T, Lewis J. Associations of circulating oxidized LDL and conventional biomarkers of cardiovascular disease in a cross-sectional study of the Navajo population. PLoS One. 2016;11:e0143102. doi: 10.1371/journal.pone.0143102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon ME, Lewis J, Miller C, Hoover J, Ali AS, Shuey C, Cajero M, Lucas S, Zychowski K, Pacheco B, Erdei E, Ramone S, Nez T, Gonzales M, Campen MJ. Residential proximity to abandoned uranium mines and serum inflammatory potential in chronically exposed Navajo communities. Journal of Exposure Science & Environmental Epidemiology. 2017;27:365–71. doi: 10.1038/jes.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holvoet P, Vanhaecke J, Janssens S, Van De Werf F, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98:1487–94. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- Hoover J, Gonzales M, Shuey C, Barney Y, Lewis J. Elevated arsenic and uranium concentrations in unregulated water sources on the Navajo Nation, USA. Exposure Health. 2017;9:113–24. doi: 10.1007/s12403-016-0226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard BV, Lee ET, Cowan LD, Devereux RB, Galloway JM, Go OT, Howard WJ, Rhoades ER, Robbins DC, Sievers ML, Welty TK. Rising tide of cardiovascular disease in American Indians: The strong heart study. Circulation. 1999;99:2389–95. doi: 10.1161/01.CIR.99.18.2389. [DOI] [PubMed] [Google Scholar]
- Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: A historical perspective. Toxicological Sciences. 2011;123:305–32. doi: 10.1093/toxsci/kfr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hund L, Bedrick EJ, Miller C, Huerta G, Nez T, Ramone S, Shuey C, Cajero M, Lewis J. A Bayesian framework for estimating disease risk due to exposure to uranium mine and mill waste on the Navajo Nation. Journal Roy Statistical Soc: Series A. 2015;178:1069–91. doi: 10.1111/rssa.12099. [DOI] [Google Scholar]
- Itabe H, Mori M, Fujimoto Y, Higashi Y, Takano T. Minimally modified LDL is an oxidized LDL enriched with oxidized phosphatidylcholines. Journal of Biochemistry. 2003;134:459–65. doi: 10.1093/jb/mvg164. [DOI] [PubMed] [Google Scholar]
- Itabe H, Obama T, Kato R. The dynamics of oxidized LDL during atherogenesis. Journal of Lipids. 2011;2011:418313. doi: 10.1155/2011/418313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko NB, Ivanenko AA, Solovyev ND, Zeimal’ AE, Navolotskii DV, Drobyshev EJ. Biomonitoring of 20 trace elements in blood and urine of occupationally exposed workers by sector field inductively coupled plasma mass spectrometry. Talanta. 2013;116:764–69. doi: 10.1016/j.talanta.2013.07.079. [DOI] [PubMed] [Google Scholar]
- James KA, Byers T, Hokanson JE, Meliker JR, Zerbe GO, Marshall JA. Association between lifetime exposure to inorganic arsenic in drinking water and coronary heart disease in Colorado residents. Environment Health Perspective. 2015;123:128–34. doi: 10.1289/ehp.1307839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston N, Jernberg T, Lagerqvist B, Siegbahn A, Wallentin L. Improved identification of patients with coronary artery disease by the use of new lipid and lipoprotein biomarkers. The American Journal of Cardiology. 2006;97:640–45. doi: 10.1016/j.amjcard.2005.09.123. [DOI] [PubMed] [Google Scholar]
- Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Jones MR, Tellez-Plaza M, Vaidya D, Grau M, Francesconi KA, Goessler W, Guallar E, Post WS, Kaufman JD, Navas-Acien A. Estimation of inorganic arsenic exposure in populations with frequent seafood intake: Evidence from MESA and NHANES. American Journal of Epidemiology. 2016;184:590–602. doi: 10.1093/aje/kww097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim MR, Rahman M, Islam K, Al Mamun A, Hossain S, Hossain E, Aziz A, Yeasmin F, Agarwal S, Hossain MI, Saud ZA, Nikkon F, Hossain M, Mandal A, Jenkins RO, Haris PI, Miyataka H, Himeno S, Hossain K. Increases in oxidized low-density lipoprotein and other inflammatory and adhesion molecules with a concomitant decrease in high-density lipoprotein in the individuals exposed to arsenic in Bangladesh. Toxicological Sciences. 2013;135:17–25. doi: 10.1093/toxsci/kft130. [DOI] [PubMed] [Google Scholar]
- Katsoyiannis IA, Hug SJ, Ammann A, Zikoudi A, Hatziliontos C. Arsenic speciation and uranium concentrations in drinking water supply wells in northern Greece: Correlations with redox indicative parameters and implications for groundwater treatment. The Science of the Total Environment. 2007;383:128–40. doi: 10.1016/j.scitotenv.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Kurttio P, Harmoinen A, Saha H, Salonen L, Karpas Z, Komulainen H, Auvinen A. Kidney toxicity of ingested uranium from drinking water. American Journal of Kidney Diseases. 2006;47:972–82. doi: 10.1053/j.ajkd.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Lankin V, Konovalova G, Tikhaze A, Shumaev K, Kumskova E, Viigimaa M. The initiation of free radical peroxidation of low-density lipoproteins by glucose and its metabolite methylglyoxal: A common molecular mechanism of vascular wall injure in atherosclerosis and diabetes. Molecular and Cellular Biochemistry. 2014;395:241–52. doi: 10.1007/s11010-014-2131-2. [DOI] [PubMed] [Google Scholar]
- Lemaire M, Lemarié CA, Molina MF, Schiffrin EL, Lehoux S, Mann KK. Exposure to moderate arsenic concentrations increases atherosclerosis in ApoE−/− mouse model. Toxicological Sciences. 2011;122:211–21. doi: 10.1093/toxsci/kfr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, Hoover J, MacKenzie D. Mining and environmental health disparities in Native American communities. Current Environment Health Reports. 2017;4:130–41. doi: 10.1007/s40572-017-0140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PM, Olsén L, Lind L. Circulating levels of metals are related to carotid atherosclerosis in elderly. The Science of the Total Environment. 2012;416:80–88. doi: 10.1016/j.scitotenv.2011.11.064. [DOI] [PubMed] [Google Scholar]
- McMillin GA, Travis JJ, Hunt JW. Direct measurement of free copper in serum or plasma ultrafiltrate. American Journal of Clinical Pathology. 2009;131:160–65. doi: 10.1309/AJCP7Z9KBFINVGYF. [DOI] [PubMed] [Google Scholar]
- Medrano MAJ, Boix R, Pastor-Barriuso R, Palau M, Damián J, Ramis R, Del Barrio JL, Navas-Acien A. Arsenic in public water supplies and cardiovascular mortality in Spain. Environmental Research. 2010;110:448–54. doi: 10.1016/j.envres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Meisinger C, Baumert J, Khuseyinova N, Loewel H, Koenig W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation. 2005;112:651–57. doi: 10.1161/CIRCULATIONAHA.104.529297. [DOI] [PubMed] [Google Scholar]
- Moon KA, Ana Navas-Acien M, Grau-Pérez KA, Francesconi WG, Guallar E, Umans JG, Best LG, Newman JD. Low-moderate urine arsenic and biomarkers of thrombosis and inflammation in the strong heart study. PLoS One. 2017;12:e0182435. doi: 10.1371/journal.pone.0182435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, Goessler W, Pollak J, Silbergeld EK, Howard BV, Navas-Acien A. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease: A prospective cohort study. Annals of Internal Medicine. 2013;159:649–59. doi: 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cholesterol Education Program (NCEP) Third report of the (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3143. [PubMed] [Google Scholar]
- Navajo Access Workgroup. [Accessed October, 2010];Mapping of water infrastructure and homes without access to safe drinking water and basic sanitation on the Navajo Nation. 2010 https://www.epa.gov/sites/production/files/2015-07/documents/navaho-mapping-water-infrastructure-and-homes_0.pdf.
- Navas-Acien A, Francesconi KA, Silbergeld EK, Guallar E. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environmental Research. 2011;111:110–18. doi: 10.1016/j.envres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Silbergeld EK, Streeter RA, Clark JM, Burke TA, Guallar E. Arsenic exposure and type 2 diabetes: A systematic review of the experimental and epidemiological evidence. Environment Health Perspective. 2006;114:641–48. doi: 10.1289/ehp.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BA, Liu X, Hall MN, Ilievski V, Slavkovich V, Siddique AB, Alam S, Islam T, Graziano JH, Gamble MV. Arsenic exposure, inflammation, and renal function in Bangladeshi adults: Effect modification by plasma glutathione redox potential. Free Radical Biology & Medicine. 2015;85:174–82. doi: 10.1016/j.freeradbiomed.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck WC, Edwards JR, Nebert DW, Woods JM, Barchowsky A, Atchison WD. The vascular system as a target of metal toxicity. Toxicological Sciences. 2008;102:207–18. doi: 10.1093/toxsci/kfm263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing. Vienna, Austria: the R Foundation for Statistical Computing; 2010. R: A language and environment for statistical computing. http://www.R-project.org. [Google Scholar]
- Rahman M, Tondel M, Ahmad SA, Chowdhury IA, Faruquee MH, Axelson O. Hypertension and arsenic exposure in Bangladesh. Hypertension. 1999;33:74–78. doi: 10.1161/01.hyp.33.1.74. [DOI] [PubMed] [Google Scholar]
- Reis A, Rudnitskaya A, Chariyavilaskul P, Dhaun N, Melville V, Goddard J, Webb DJ, Pitt AR, Spickett CM. Top-down lipidomics of low density lipoprotein reveal altered lipid profiles in advanced chronic kidney disease. Journal of Lipid Research. 2015;56:413–22. doi: 10.1194/jlr.M055624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Fahlbusch B, Otto M. Size exclusion chromatography coupled to electrospray ionization mass spectrometry for analysis and quantitative characterization of arsenic interactions with peptides and proteins. Journal of Mass Spectrometry. 2009;44:898–910. doi: 10.1002/jms.1563. [DOI] [PubMed] [Google Scholar]
- Schumacher C, Ferucci ED, Lanier AP, Slattery ML, Schraer CD, Raymer TW, Dillard D, Murtaugh MA, Tom-Orme L. Metabolic syndrome: Prevalence among American Indian and Alaska native people living in the Southwestern United States and in Alaska. Metab Syndrome Relat Disorders. 2008;6:267–73. doi: 10.1089/met.2008.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest JP, Jones MK, De Loof H, Dashti N. Structure of apolipoprotein B-100 in low density lipoproteins. Journal of Lipid Research. 2001;42:1346–67. [PubMed] [Google Scholar]
- Shi H, Shi X, Liu KJ. Oxidative mechanism of arsenic toxicity and carcinogenesis. Molecular and Cellular Biochemistry. 2004;255:67–78. doi: 10.1023/b:mcbi.0000007262.26044.e8. [DOI] [PubMed] [Google Scholar]
- Shim YK, Lewin MD, Ruiz P, Eichner JE, Mumtaz MM. Prevalence and associated demographic characteristics of exposure to multiple metals and their species in human populations: The United States NHANES, 2007–2012. Journal of Toxicology and Environmental Health. Part A. 2017;80:502–12. doi: 10.1080/15287394.2017.1330581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeonova PP, Hulderman T, Harki D, Luster MI. Arsenic exposure accelerates atherogenesis in apolipoprotein E(−/−) mice. Environment Health Perspective. 2003;111:1744–48. doi: 10.1289/ehp.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solenkova NV, Newman JD, Berger JS, Thurston G, Hochman JS, Lamas GA. Metal pollutants and cardiovascular disease: Mechanisms and consequences of exposure. American Heart Journal. 2014;168:812–22. doi: 10.1016/j.ahj.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Vladykovskaya EN, Haberzettl P, Sithu SD, D’Souza SE, States JC. Arsenic exacerbates atherosclerotic lesion formation and inflammation in ApoE−/− mice. Toxicology and Applied Pharmacology. 2009;241:90–100. doi: 10.1016/j.taap.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler N, Lindner RA, Davies MJ. Direct detection and quantification of transition metal ions in human atherosclerotic plaques: Evidence for the presence of elevated levels of iron and copper. Arteriosclerosis Thrombosis Vascular Biologic. 2004;24:949–54. doi: 10.1161/01. ATV.0000124892.90999.cb. [DOI] [PubMed] [Google Scholar]
- Trpkovic A, Resanovic I, Stanimirovic J, Radak D, Mousa SA, Cenic-Milosevic D, Jevremovic D, Isenovic ER. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Critical Reviews in Clinical Laboratory Sciences. 2014;52:70–85. doi: 10.3109/10408363.2014.992063. [DOI] [PubMed] [Google Scholar]
- Tseng C, Chong C, Tseng C, Hsueh Y, Chiou H, Tseng C, Chen C. Long-term arsenic exposure and ischemic heart disease in arseniasis-hyperendemic villages in Taiwan. Toxicology Letters. 2003;137:15–21. doi: 10.1016/s0378-4274(02)00377-6. [DOI] [PubMed] [Google Scholar]
- US Environmental Protection Agency. Overviews and Factsheets. US EPA; 2015. [November 30, 2015]. National primary drinking water regulations. https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations. [Google Scholar]
- US Environmental Protection Agency. [Accessed August 16, 2017];Health and environmental impacts of uranium contamination in the Navajo Nation: Five-year plan. n.d https://www.epa.gov/sites/production/files/2016-06/documents/nn-5-year-plan-june-12.pdf.
- Wang C, Jeng J, Yip P, Chen C, Hsu L, Hsueh Y, Chiou H, Wu M, Chen C. Biological gradient between long-term arsenic exposure and carotid atherosclerosis. Circulation. 2002;105:1804–09. doi: 10.1161/01.cir.0000015862.64816.b2. [DOI] [PubMed] [Google Scholar]
- Wu F, Jasmine F, Kibriya MG, Liu M, Wójcik O, Parvez F, Rahaman R, Roy S, Paul-Brutus R, Segers S, Slavkovich V, Islam T, Levy D, Mey JL, Van Geen A, Graziano JH, Ahsan H, Chen Y. Association between arsenic exposure from drinking water and plasma levels of cardiovascular markers. American Journal of Epidemiology. 2012;175:1252–61. doi: 10.1093/aje/kwr464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Molinaro P, Chen Y. Arsenic exposure and subclinical endpoints of cardiovascular disease. Current Environment Health Reports. 2014;1:148–62. doi: 10.1007/s40572-014-0011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Chiou HY, Wang TW, Hsueh YM, Wang IH, Chen CJ, Lee TC. Association of blood arsenic levels with increased reactive oxidants and decreased antioxidant capacity in a human population of northeastern Taiwan. Environment Health Perspective. 2001;109:1011–17. doi: 10.1289/ehp.011091011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY. Does arsenic exposure increased the risk of development of peripheral vascular diseases in humans? Journal of Toxicology and Environmental Health Part A. 2006;69:1797–804. doi: 10.1080/15287390600630237. [DOI] [PubMed] [Google Scholar]