Abstract
There are many ways in which women experience sleep differently from men. Women contending with distinct sleep challenges respond differently to sleep disorders, as well as sleep deprivation and deficiency, and face particular health outcomes as a result of poor sleep. Idiosyncrasies, including changes that occur with the biological life cycles of menstruation, pregnancy, and menopause, make the understanding of sleep in women an important topic to study. Each phase of a woman’s life, from childhood to menopause, increases the risk of sleep disturbance in unique ways that may require distinct management. Indeed, new research is unraveling novel aspects of sleep pathology in women and the fundamental role that sex hormones play in influencing sleep regulation and arousals and possibly outcomes of sleep conditions. Moreover, studies indicate that during times of hormonal change, women are at an increased risk for sleep disturbances such as poor sleep quality and sleep deprivation, as well as sleep disorders such as OSA, restless legs syndrome, and insomnia. This article reviews sleep changes in female subjects from neonatal life to menopause.
Key Words: childhood, menopause, pregnancy, sex differences, sleep, women
Abbreviations: GnRH, gonadotropin-releasing hormone; NREM, non-rapid eye movement; PSQI, Pittsburgh Sleep Quality Index; REM, rapid eye movement; SDB, sleep-disordered breathing; SWS, slow wave sleep; TST, total sleep time; WASO, wake after sleep onset
Sleep and sleep disturbances in women have been gaining attention in recent years as scientists begin to understand the impact of sex as a biological variable on pathology. In 2014, the Society for Women’s Health Research convened a group of experts to identify the current state of the science and to identify areas for future research in sleep in women.1 Multiple gaps were identified in the current literature across sleep disorders. More recently, the Office of Research on Women’s Health of the National Institutes of Health, in collaboration with other institutes, organized a workshop entitled “Female Sex and Gender in Lung/Sleep Health and Disease,” with the goal of furthering the agenda of the study of sex and gender-specific differences across systems, including sleep. Hence, as sleep varies across the life span, understanding sleep in women and its implications is key. The present article reviews sleep in women across the life span and highlights some of the sex differences that are known to exist.
Sleep in Childhood
Sleep in Neonates and Infants
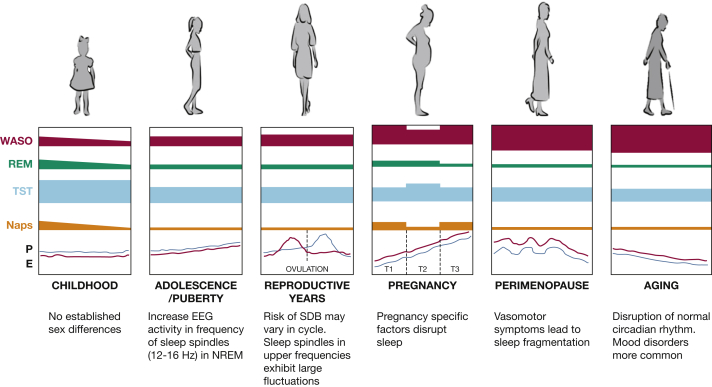
Although changes in sleep architecture within the first months of life are similar among male and female subjects, some differences in sleep quality and quantity exist, suggesting a delayed CNS maturation in male infants. In the neonate and young infant, sleep structure is immature and disorganized compared with children's sleep because underlying brain structures and sleep regulatory systems are not yet fully developed.2 Three sleep states can be identified in the normal term infant: active sleep, which is the precursor of rapid eye movement (REM) sleep; quiet sleep, which will, at a later time, differentiate into the three non-rapid eye movement (NREM) sleep stages; and indeterminate sleep. Neonates spend approximately 15 hours of the 24-hour period sleeping (Fig 1), one half of which is spent in active sleep.3
Figure 1.
Sleep in women across the life span. E = estrogen; NREM = non-rapid eye movement; P = progesterone; REM = rapid eye movement; SDB = sleep-disordered breathing; T = trimester; TST = total sleep time; WASO = wake after sleep onset.
In neonates, the existence of sex-related differences in sleep remains controversial: some studies did not show any difference, whereas others stress a more restless sleep in boys, with parents reporting excessive crying. Bach et al4 studied two groups of healthy preterm neonates (21 boys and 17 girls), and sleep was assessed by using EEG measurements. The authors showed that active sleep tended to be longer in boys (+7%), at the expense of quiet sleep (±6%). Although the differences in a 3-h period were relatively small in magnitude, a recurrence of such differences over 24 h can potentially be of great physiological relevance for the neonates. These differences also differed based on infants’ age. Hoppenbrouwers et al5 reported a longer duration of quiet sleep episodes in female infants during the first 6 months of life, whereas in older infants, Williams et al6 found a nonsignificant longer duration of quiet sleep in boys. This heterogeneity of findings can be explained by the significant number of confounders inconsistently considered in previous studies, such as thermal ambient parameters and other anthropometric characteristics.
During the first 3 months’ postterm, the sleep EEG gradually differentiates from the neonatal pattern to the infant pattern: the proportion of active sleep decreases and within quiet sleep, features of the three NREM sleep stages become visible. Only after the first 3 months does sleep start to consolidate with fewer but longer periods of sleep occurring mostly at night.
Studies suggest that maturation of the CNS and cortical function occurs more slowly in male infants. Thordstein et al7 showed that in 20 full-term neonates, the mean amount of infra-slow activity was 27% larger in boys compared with girls during sleep. Richardson et al8 studied 50 healthy infants by using daytime polysomnography. Arousability was assessed during both active sleep and quiet sleep by using a pulsatile air-jet to the nostrils at increasing pressures. The authors showed that at 2 to 4 weeks’ postnatal age, male infants were more easily aroused from quiet sleep (but not from active sleep) than female infants. However, this sex difference in arousal threshold was not observed at 2 to 3 months’ postnatal age, the age at which the risk of sudden infant death syndrome peaks, arguing against the hypothesis that this syndrome is caused by a preexisting inhibition of cortical arousal processes. Interestingly, an analysis of heart rate revealed that increases in heart rate during arousals tended to be larger in male subjects compared with their female counterparts.
To summarize, sleep in early life is a very dynamic process that changes every few weeks. Hence, there may be some sex differences in sleep of infants and neonates; however, these changes have not been fully explored as they seem to be influenced by age and confounders.
Sleep in Childhood and Puberty
The early years of childhood are characterized by rapid advances in growth, cognition, and behaviors, and in parallel, sleep changes reflect such developments. This scenario is not surprising given that the most relevant changes of the CNS in terms of both growth and differentiation occur during this time. The proportion of REM sleep continues to decline during childhood, reaching the adult level of 20% to 25% of total sleep time (TST) by 5 years of age. At this time, sleep schedules change continuously: between the ages of 1 and 4 years, children continue to take daytime naps to achieve their sleep requirements, while by 5 years of age, daytime napping ceases, and overnight sleep duration gradually declines.9
Sex differences in sleep at this age are very difficult to capture because sleep-wake patterns are influenced by a complex interplay between biological processes and environmental, behavioral, and social factors. In fact, a systematic review of observational studies analyzed within age-bands failed to show sex differences in sleep among children.3 Furthermore, Liu et al10 found no sex differences in sleep duration in children aged 5 to 12 years as measured by using actigraphy. However, sex differences in sleep quality are more evident once children reach puberty11 when sexual hormones and their related changes begin to affect sleep architecture. In addition, at this stage of life, the anatomy of the upper airway is well defined, and thus differences in upper airway collapsibility, the arousal response to increased inspiratory resistance, and ventilatory control contribute to explain the sex differences in sleep-disordered breathing (SDB).12
Sleep Changes With the First Menstrual Cycle
The main sex differences in sleep arise with the first menstrual cycle. With menarche, ovarian function increases and female hormones (estradiol and progesterone) are cyclically released in the bloodstream, and they regulate a large variety of homeostatic functions involving the cardio-circulatory, respiratory, and metabolic systems13 as well as the sleep-wake cycle regulation; hence the need to briefly review the role of these hormones.
Sex Hormones and Sleep
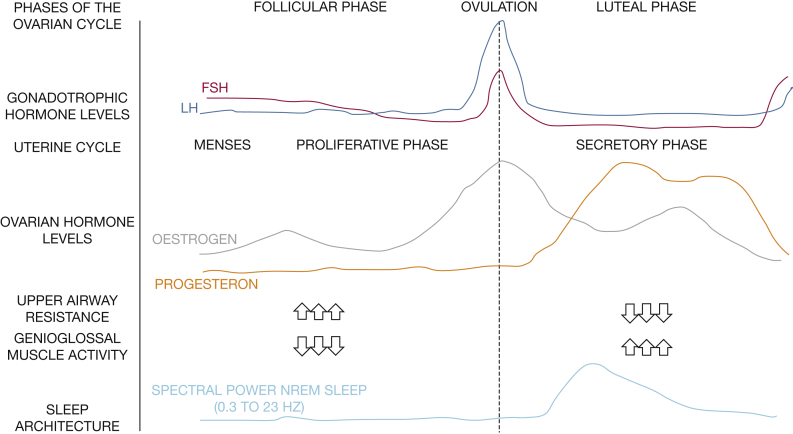
Animal studies have shown that compared with untreated ovariectomized rats, ovariectomized rats treated with estradiol, progesterone, or both spent less time in spontaneous NREM sleep and/or REM sleep during the dark phase. After being subjected to 6 h of sleep deprivation, the hormonally treated rats showed a larger increase in REM sleep amount from baseline but a less pronounced increase in NREM EEG delta power (a measure of sleep intensity/drive) compared with hormonally untreated ovariectomized rats.14 In addition, gonadectomy in female and male rats eliminates all sex differences in the sleep-wake cycle; adding back physiological levels of sex-specific steroids restores these differences.15 Although progesterone supplementation did not alter sleep architecture during normal undisturbed sleep in postmenopausal women, it seems to significantly improve environmentally related sleep disruptions.16 Progesterone administration seems to enhance sleep duration and sleep quality, mainly by improving slow wave sleep (SWS) and slow wave activity. However, this hormone is far more complex than initially thought, and the interaction of the various progesterone receptors is not fully understood. Progesterone is a ventilatory drive stimulant and has been known to increase the activity of the genioglossus muscle dilating the upper airway.17 In 11 healthy women without sleep issues, upper airway resistance was shown to be lower in the luteal phase compared with the follicular phase.18 These are properties that may protect against SDB. Conversely, because progesterone is a strong respiratory stimulant and has the potential to result in hypocapnia, it is theoretically possible that progesterone may lead to central apnea by causing a ventilatory overshoot. However, observational studies have not shown a high prevalence of central apneas in pregnant women suspected of having SDB19 and in population-based samples.20
Estrogens have also been proposed to be protective against SDB. Data from Bixler et al21 showed that, compared with premenopausal women, postmenopausal women not receiving hormone replacement therapy seem to be at an increased risk for SDB, whereas postmenopausal women receiving hormone replacement therapy were not. This protective effect of hormone replacement therapy has been debated in later studies as being a reflection of healthfulness rather than a physiological effect of sex steroid hormones.22 However, estradiol has been implicated in the pathogenesis of SDB, and animal studies show that estradiol likely prevents cardiorespiratory disorders and oxidative stress induced by chronic intermittent hypoxia,23 suggesting a complementary protective role against the known cardiovascular consequences of sleep apnea.24
Sleep at Menarche
Menstrual hormonal changes also seem to influence sleep architecture (Fig 2). The most dramatic change in sleep across the menstrual cycle is increased EEG activity in the frequency range of sleep spindles (12-16 Hz) in NREM sleep in the postovulatory luteal phase of the menstrual cycle, when progesterone and estradiol are high, compared with the follicular phase, when progesterone is low.25 This scenario is not unexpected given that estrogen and progesterone receptors are localized in many sleep/wake regulatory nuclei, including the basal forebrain, hypothalamus, dorsal raphe nucleus, and locus coeruleus.26
Figure 2.
Sleep and the menstrual cycle. FSH = follicular-stimulating hormone; LH = luteinizing hormone. See Figure 1 legend for expansion of other abbreviation.
Psychological factors may also influence women's sleep in relation to ovarian hormones. With the onset of menarche, women are twice as likely to have mood disorders than men.27 In addition, differences in the prevalence of insomnia have been observed in young men and women aged 15 to 30 years, with women having a 28% higher risk of reporting insomnia than their male counterparts.28 However, although young women report significantly more sleep problems than men, this perception of a poorer sleep quality in women is not reflected in objective polysomnographic measures, suggesting that other non-sleep-related conditions such as mood disorders play an important role.29
Sleep in Pregnancy
Pregnancy is associated with dynamic physiological changes that affect sleep and sleep disorders (Table 1).30, 31, 32, 33 These changes range from anatomic changes that have the potential to affect sleep duration, sleep fragmentation, and breathing during sleep to metabolic changes that increase the risk of restless legs syndrome. For instance, gastroesophageal reflux usually worsens with pregnancy progression, affecting 75% of gravidas in some populations,30 and may disrupt sleep. Nocturnal micturition related to an increase in overnight sodium excretion34 is another factor that leads to sleep fragmentation. The musculoskeletal system is also stressed due to the changes that occur to prepare for the growing uterus and the expected delivery,35 potentially disrupting sleep. Furthermore, nocturnal uterine contractions, caused by oxytocin’s nocturnal peak, may disrupt sleep.36 Changes in iron and folate metabolism in pregnancy37, 38 have also been proposed to explain the increased prevalence of restless legs syndrome among gravidas.39 Profound hormonal changes are also observed in pregnancy and affect sleep physiology and sleep architecture. Secretion of steroid sex hormones (estrogens and progesterone) increases exponentially in pregnancy, and these hormones influence sleep architecture by affecting both the circadian and homeostatic regulation of sleep.40
Table 1.
Sleep Characteristics and Physiological Physical Features Affecting Sleep in Pregnancy30, 31, 32, 33
| First Trimester | Second Trimester | Third Trimester | |
|---|---|---|---|
| Mechanical and physiological factors impacting sleep | Nocturia Musculoskeletal discomfort |
Fetal movement Uterine contractions Musculoskeletal discomfort Rhinitis and nasal congestion |
Nocturia Fetal movement Uterine contractions Heartburn Orthopnea Leg cramps Rhinitis and nasal congestion Sleeping position |
Both sleep duration and sleep quality41 are affected by pregnancy and seem to undergo dynamic changes with pregnancy progression. One study42 found that compared with nonpregnant women, pregnant women in the third trimester of gestation had 30 min less TST and were more likely to be short sleepers (≤ 6 h) and long sleepers (> 9 h). Self-reported sleep duration declines in the second trimester compared with the first trimester.43 Recently published data objectively evaluating sleep duration in the second trimester of pregnancy found that nearly 28% of women sleep < 7 h per night.44 Sleep duration differed according to age and race, with non-Hispanic black and Asian subjects having the shortest sleep duration, and younger pregnant women spending more time in bed compared with their older counterparts. A recent meta-analysis that included studies published through 2015 and pooling > 11,000 participants’ data found that the average Pittsburgh Sleep Quality Index (PSQI), a validated questionnaire based measure of sleep quality, score was 6.4 (95% CI, 5.3-6.85) and that 46% of all women experienced poor sleep.45 In addition, sleep quality seemed to worsen from the second to the third trimester by an average of 1.68 points (95% CI, 0.42-2.94).45
Poor sleep and short sleep duration have been associated with adverse outcomes in the general population. Pregnancy is no exception, and various sleep disturbances have been linked to adverse perinatal outcomes. In cross-sectional studies, self-reported poor sleep quality measured by using the PSQI and short sleep duration were independently associated with an increased risk of gestational diabetes.41, 46 These findings were confirmed in prospective longitudinal studies that assessed sleep duration (< 7 h)47, 48 and sleep midpoint later than 5:00 am by using actigraphy.48 It is likely, however, that body habitus is an important modifier of the associations. In a large study of > 2,500 women, short and long sleep duration seem to be significantly associated with a risk of developing gestational diabetes but only in nonobese women. Short sleep duration has also been linked to a higher risk of hypertensive disorders of pregnancy in large studies using self-report49; however, studies using objective measures of sleep duration in low-risk populations failed to show an association.48 Objectively measured short sleep duration has been associated with a higher risk of requiring a cesarean delivery50, 51, 52 and longer duration of labor.50, 53
SDB is another sleep condition that has been associated with adverse pregnancy outcomes. For instance, SDB increases the risk of gestational diabetes,20, 54, 55, 56 even after adjusting for body habitus. Although longitudinal studies showing associations of sleep disturbances with a diagnosis of gestational diabetes later in pregnancy suggest potential causality, it remains unclear whether women with a certain degree of insulin resistance at pregnancy onset would be more susceptible to the effects of sleep abnormalities with exposure to the physiological changes of pregnancy that enhance insulin resistance. SDB has also been associated with an increased risk of hypertensive disorders of pregnancy in numerous studies20, 55, 56, 57 even after adjusting for a comprehensive list of confounders.55, 56 Associations between both snoring and OSA and cesarean delivery have also been described.54, 56
Although the association differs according to type and gestational age at birth, insomnia,58 sleep apnea,58, 59, 60 and poor sleep61 are linked to preterm birth, even after adjusting for potential confounders. However, associations with growth restriction are much more controversial.55, 56, 62 Conflicting results are possibly related to outcome definition, maternal and paternal factors, and the cross-sectional nature of most studies in which growth is not measured longitudinally.
Data linking sleep disturbances to adverse outcomes such as placental abruption,63 stillbirths,56 and shorter telomere length64 and poor childhood outcomes65 are emerging and need to be investigated further.
Pathogenetic mechanisms behind these associations have been postulated but remain to be proven. The placenta has been proposed as a potential target organ in mediating adverse pregnancy outcomes in sleep disturbances66, 67, 68 as evidence of placental hypoxia and alterations in placenta-secreted markers have been shown in SDB. It is biologically plausible that other sleep disturbances may affect placental function as well, given associations of sleep deprivation with similar placenta-mediated outcomes. The hypothalamic-pituitary-adrenal axis has been postulated to play a potential role in the association between SDB and gestational diabetes.69, 70 However, preliminary data show flattening, rather than activation, of the cortisol awakening response.59 Other potential mechanisms include an enhanced inflammatory profile, endothelial dysfunction, and oxidative stress but remain to be proven; recent data, however, suggest that oxidative stress may not be implicated71 given potential downregulation by estradiol.23 The allostatic load hypothesis has also been proposed, suggesting that chronic sleep loss is both a precipitant of stress as well as a consequence of it, leading to a stress “overload” that may account for adverse pregnancy outcomes.72
In summary, sleep is significantly disturbed in pregnancy, and sleep disruptions have significant implications on perinatal health outcomes. Future research needs to focus on understanding the pathogenesis of these associations and to examine the impact of sleep-targeted interventions on perinatal outcomes.
Sleep in the Perimenopausal to Postmenopausal Stages
Sleep disturbances are common in older women, affecting > 40% to 60% of perimenopausal or postmenopausal women. In fact, the 2005 National Institutes of Health State-of-the-Science Conference Statement cites sleep disturbance as a core symptom of menopause.73 Many recent studies support subjective sleep quality deterioration starting in the perimenopausal period. Perceived sleep changes mostly relate to sleep fragmentation, increased awakenings, and poor sleep quality. In a longitudinal study observing premenopausal women over a span of 5 years, predictors of developing poor sleep during perimenopause included baseline depressive symptoms, daytime sleepiness, and CNS-active medications.74 Chronic insomnia may develop in as many as 31% to 42% of women by the end of their menopausal transition.75
Objective sleep findings, however, have been variable due to a small number of studies. Polysomnographic data have either found no differences or shown worse TST, sleep fragmentation, and sleep efficiency in perimenopausal/postmenopausal women compared with younger women.76, 77 In contrast, two longitudinal studies showed improved sleep architecture as women entered perimenopause, with greater TST and SWS compared with their premenopausal state.78, 79 There are many potential mechanisms by which sleep quality is affected during the latter part of a woman’s life, and they relate to vasomotor symptoms, hormonal changes, age-related changes, and increases in comorbid conditions such as depression and SDB.
Vasomotor Symptoms and Sleep
Hot flashes and night sweats are hallmarks of menopause, and they likely play a major role in sleep disturbances in menopausal women. Current evidence suggests a consistent coupling of vasomotor symptoms and poor self-reported sleep quality, especially when vasomotor symptoms are reported as severe, experienced during the nighttime, or are associated with night sweats.79, 80 When vasomotor symptoms have been induced in young women by using leuprolide (a gonadotropin-releasing hormone [GnRH] agonist), these women were more likely to report worse sleep quality, frequent awakenings, and have higher Insomnia Severity Index and PSQI scores.81 Moreover, the more severe or “bothersome” the reported vasomotor symptoms are, the more likely the occurrence of chronic insomnia.80, 82, 83, 84, 85 Hormonal and nonhormonal therapies directed at vasomotor symptoms have been shown to improve subjective sleep quality.85, 86, 87
Actigraphy and polysomnography confirm sleep fragmentation, increased wake after sleep onset (WASO), and poor sleep efficiency associated with vasomotor symptoms. When vasomotor activity is measured objectively by using skin conductance, similar findings of objective sleep disturbances are found. The duration of a vasomotor event, rather than the number of events during the night, correlated with sleep fragmentation, lighter stages of sleep, and delayed REM sleep onset.88 Moreover, the occurrence of objectively determined vasomotor events did not correlate with subjective sleep complaints, unless the vasomotor activity occurred in the first half of the night, suggesting the effect may be sleep stage-dependent.89
Hormonal Changes and Sleep
An important question remains about how much of poor sleep reported during menopause is directly related to hormonal changes independent of vasomotor symptoms. Although poorly understood, sleep regulation seems to be directly affected by endogenous estrogens. In the Study of Women’s Health Across the Nation (SWAN), a rapid rise in follicular-stimulating hormone, a marker of low estrogen levels, was associated with higher percentages of SWS and longer TST in postreproductive women.90 In contrast, another study found that higher follicular-stimulating hormone levels were associated with increased WASO even after adjusting for age, BMI, and hot flashes.91 Some have proposed that an increase in SWS may be observed as a compensatory response to sleep fragmentation and poor sleep associated with menopause.
It is likely that hormonal changes have both dependent and independent effects on sleep perception and architecture. In the Selective Estrogens, Menopause and Response to Therapy (SMART) study, hormone therapy improved subjective sleep quality by improving mild vasomotor symptoms.92 However, in women with high-intensity vasomotor symptoms (ie, > 7 severe symptoms per week, or > 50 events per week), hormonal therapy improved subjective sleep quality, independent of any effect on vasomotor symptoms.
Circadian disruption is also a prominent feature of aging in women and may be related directly to hormonal changes. At normal sleep onset, peripheral vasodilation with heat loss through the skin results from reduced activation of noradrenergic vasoconstrictor tone. A drop in core body temperature accompanies rapid onset of sleep and is strongly associated with melatonin secretion. In postmenopausal women, however, this drop in core body temperature is blunted, as are early morning cortisol levels. Postmenopausal women are also more likely to be phase-advanced by 1 h and are more likely to express a “morning” chronotype compared with premenopausal women.93
Age-Related Sleep Changes
Aging is independently associated with sleep fragmentation, insomnia symptoms, circadian derangements, and sleep architectural changes such as lighter sleep, reduced sleep efficiency, increased WASO, and decreased SWS and REM sleep.94 A 6-year longitudinal study of premenopausal women aged 46 years at baseline showed that both aging and hormonal changes independently influenced sleep architecture. Aging was related to decreased TST, greater awakenings, and poorer sleep efficiency regardless of menopausal state.
Nocturnal melatonin secretion generally decreases with age but also decreases specifically in relation to menopausal state.95 This observation has led to speculation that melatonin may play a direct role in menopausal transition. Indeed, the GnRH-luteinizing hormone-ovarian axis is influenced by diurnal neuronal stimuli, and thus the age-related disruption of normal circadian function may lead to menstrual irregularities or even amenorrhea. It has also been speculated that melatonin decrease may disinhibit the hypothalamic pulse generator, leading to an irregular release of GnRH and luteinizing hormone causing hot flashes. However, supplemental melatonin has not been shown to relieve hot flashes.96 Instead, exogenous melatonin in postmenopausal women improves mood, as well as perceived sleep symptoms and sleep quality.97
Sleep Changes Related to Mood Disorders
Comorbidities, in particular mood disorders, become more common in aging women and may affect sleep. Several longitudinal studies found that the rate of depression increases at least twofold during the menopausal transition independent of other known factors.98, 99, 100 Depression and anxiety are associated with poor sleep, as well as with vasomotor symptoms, in complex multidirectional interactions. For example, the SWAN study showed that perimenopausal women with anxiety had longer sleep latency and reduced sleep efficiency but only in those women also reporting vasomotor symptoms.101 This finding led to the postulation that vasomotor symptoms provoke sleep disturbances which instigate mood disorders in predisposed women. However, in midlife women aged 42 to 52 years who were followed up longitudinally for 3 years, reports of sleep problems, and not vasomotor symptoms, were predictive of negative mood the following day.102
More current is the “co-evolving system” hypothesis of sleep disturbances, vasomotor symptoms, and mood disorders. Evidence that supports this hypothesis emphasizes the fact that many women have their first onset of depression during the menopause transition when they are not experiencing vasomotor symptoms. Moreover, women with depressed mood and sleep disturbances do not have increased vasomotor symptoms compared with women without depressed mood. Finally, midlife and elderly women with depression more often have longer sleep latency, shorter TST, and disrupted REM sleep, whereas vasomotor-related sleep disturbances are more often characterized by frequent and prolonged awakenings.81
In summary, primary sleep disorders become more prevalent in older age, affecting > 53% of postmenopausal women.103, 104, 105 Moreover, poor sleep in perimenopausal/postmenopausal women is associated with inflammation,106 cardiovascular and metabolic disease,107, 108, 109 and mood disorders. Understanding the evolution of sleep across a woman’s life span may lead to effective therapies that affect women’s health and quality of life.
Conclusions
Sex differences and sleep changes in women across the life span are most prominent following puberty but remain understudied.1 The role of steroid sex hormones needs to be investigated further, and future research is needed to better understand the relationship between objective and subjective sleep changes in women undergoing the transition into the postreproductive stage, and to understand the interactions of the aging process, hormonal changes, and comorbid diseases. Studies are also needed to further examine the impact of sleep disturbances at various stages in life and especially in pregnancy where outcomes may act as precursors of long-term outcomes for mother and her offspring. Although pharmacogenomics were not covered in the present review, they are an important area for future research in sleep medicine as emerging data suggest that sex is one of the determinants of pharmacogenomics, and women may metabolize drugs differently than men, significantly affecting their therapeutic profile.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: G. B. has received research equipment support from Respironics. C. H. W. has received support from Jazz Pharmaceuticals and Avadel Pharmaceuticals. None declared (M. F. P.).
Role of sponsors: The sponsors had no role in the preparation of the manuscript.
Other contributions: The authors acknowledge Myriam Salameh, MD, at The Miriam Hospital for her assistance with preparation of the figure.
Footnotes
Drs Pengo and Won contributed equally to this work.
FUNDING/SUPPORT: Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grants R01HL-130702 and R01HD-078515 to Dr Bourjeily].
References
- 1.Mallampalli M.P., Carter C.L. Exploring sex and gender differences in sleep health: a Society for Women's Health Research Report. J Womens Health (Larchmt) 2014;23(7):553–562. doi: 10.1089/jwh.2014.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfson A, Montgomery-Downs H. The Oxford Handbook of Infant, Child, and Adolescent Sleep and Behavior. http://www.oxfordhandbooks.com/view/10.1093/oxfordhb/9780199873630.001.0001/oxfordhb-9780199873630. Accessed November 9, 2017.
- 3.Galland B.C., Taylor B.J., Elder D.E., Herbison P. Normal sleep patterns in infants and children: a systematic review of observational studies. Sleep Med Rev. 2012;16(3):213–222. doi: 10.1016/j.smrv.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Bach V., Telliez F., Leke A., Libert J.P. Gender-related sleep differences in neonates in thermoneutral and cool environments. J Sleep Res. 2000;9(3):249–254. doi: 10.1046/j.1365-2869.2000.00206.x. [DOI] [PubMed] [Google Scholar]
- 5.Hoppenbrouwers T., Hodgman J.E., Harper R.M., Sterman M.B. Respiration during the first six months of life in normal infants: IV. Gender differences. Early Hum Dev. 1980;4(2):167–177. doi: 10.1016/0378-3782(80)90020-1. [DOI] [PubMed] [Google Scholar]
- 6.Williams R.L., Karacan I., Hursch C.J. Wiley; New York, NY: 1974. Electroencephalography (EEG) of Human Sleep: Clinical Applications. [Google Scholar]
- 7.Thordstein M., Lofgren N., Flisberg A., Lindecrantz K., Kjellmer I. Sex differences in electrocortical activity in human neonates. Neuroreport. 2006;17(11):1165–1168. doi: 10.1097/01.wnr.0000227978.98389.43. [DOI] [PubMed] [Google Scholar]
- 8.Richardson H.L., Walker A.M., Horne R.S. Sleeping like a baby—does gender influence infant arousability? Sleep. 2010;33(8):1055–1060. doi: 10.1093/sleep/33.8.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iglowstein I., Jenni O.G., Molinari L., Largo R.H. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111(2):302–307. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- 10.Liu X., Liu L., Owens J.A., Kaplan D.L. Sleep patterns and sleep problems among schoolchildren in the United States and China. Pediatrics. 2005;115(suppl 1):241–249. doi: 10.1542/peds.2004-0815F. [DOI] [PubMed] [Google Scholar]
- 11.Roenneberg T., Kuehnle T., Pramstaller P.P. A marker for the end of adolescence. Curr Biol. 2004;14(24):R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 12.Lozo T., Komnenov D., Badr M.S., Mateika J.H. Sex differences in sleep disordered breathing in adults. Respir Physiol Neurobiol. 2017;245:65–75. doi: 10.1016/j.resp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Boukari R., Laouafa S., Ribon-Demars A., Bairam A., Joseph V. Ovarian steroids act as respiratory stimulant and antioxidant against the causes and consequences of sleep-apnea in women. Respir Physiol Neurobiol. 2017;239:46–54. doi: 10.1016/j.resp.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Deurveilher S., Rusak B., Semba K. Estradiol and progesterone modulate spontaneous sleep patterns and recovery from sleep deprivation in ovariectomized rats. Sleep. 2009;32(7):865–877. [PMC free article] [PubMed] [Google Scholar]
- 15.Cusmano D.M., Hadjimarkou M.M., Mong J.A. Gonadal steroid modulation of sleep and wakefulness in male and female rats is sexually differentiated and neonatally organized by steroid exposure. Endocrinology. 2014;155(1):204–214. doi: 10.1210/en.2013-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caufriez A., Leproult R., L'Hermite-Baleriaux M., Kerkhofs M., Copinschi G. Progesterone prevents sleep disturbances and modulates GH, TSH, and melatonin secretion in postmenopausal women. J Clin Endocrinol Metab. 2011;96(4):E614–E623. doi: 10.1210/jc.2010-2558. [DOI] [PubMed] [Google Scholar]
- 17.Popovic R.M., White D.P. Upper airway muscle activity in normal women: influence of hormonal status. J Appl Physiol. 1998;84(3):1055–1062. doi: 10.1152/jappl.1998.84.3.1055. [DOI] [PubMed] [Google Scholar]
- 18.Driver H.S., McLean H., Kumar D.V., Farr N., Day A.G., Fitzpatrick M.F. The influence of the menstrual cycle on upper airway resistance and breathing during sleep. Sleep. 2005;28(4):449–456. doi: 10.1093/sleep/28.4.449. [DOI] [PubMed] [Google Scholar]
- 19.Bourjeily G., Sharkey K.M., Mazer J., Moore R., Martin S., Millman R. Central sleep apnea in pregnant women with sleep disordered breathing. Sleep Breath. 2015;19(3):835–840. doi: 10.1007/s11325-014-1099-1. [DOI] [PubMed] [Google Scholar]
- 20.Facco F.L., Parker C.B., Reddy U.M. Association between sleep-disordered breathing and hypertensive disorders of pregnancy and gestational diabetes mellitus. Obstet Gynecol. 2017;129(1):31–41. doi: 10.1097/AOG.0000000000001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bixler E.O., Vgontzas A.N., Lin H.M. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 pt 1):608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 22.Mirer A.G., Peppard P.E., Palta M., Benca R.M., Rasmuson A., Young T. Menopausal hormone therapy and sleep-disordered breathing: evidence for a healthy user bias. Ann Epidemiol. 2015;25(10):779–784.e771. doi: 10.1016/j.annepidem.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laouafa S., Ribon-Demars A., Marcouiller F. Estradiol protects against cardiorespiratory dysfunctions and oxidative stress in intermittent hypoxia. Sleep. 2017;40(8) doi: 10.1093/sleep/zsx104. [DOI] [PubMed] [Google Scholar]
- 24.Bayliss D.A., Millhorn D.E., Gallman E.A., Cidlowski J.A. Progesterone stimulates respiration through a central nervous system steroid receptor-mediated mechanism in cat. Proc Natl Acad Sci U S A. 1987;84(21):7788–7792. doi: 10.1073/pnas.84.21.7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker F.C., Kahan T.L., Trinder J., Colrain I.M. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. Sleep. 2007;30(10):1283–1291. doi: 10.1093/sleep/30.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mong J.A., Baker F.C., Mahoney M.M. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci. 2011;31(45):16107–16116. doi: 10.1523/JNEUROSCI.4175-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kessler R.C., Walters E.E. Epidemiology of DSM-III-R major depression and minor depression among adolescents and young adults in the National Comorbidity Survey. Depress Anxiety. 1998;7(1):3–14. doi: 10.1002/(sici)1520-6394(1998)7:1<3::aid-da2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 28.Zhang B., Wing Y.K. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 29.Manber R., Gress J., Baker F. Sex differences in sleep and sleep disorders: a focus on women's sleep. Int J Sleep Disorders. 2006;1(1):7–15. [Google Scholar]
- 30.Habr F., Raker C., Lin C.L., Zouein E., Bourjeily G. Predictors of gastroesophageal reflux symptoms in pregnant women screened for sleep disordered breathing: a secondary analysis. Clin Res Hepatol Gastroenterol. 2013;37(1):93–99. doi: 10.1016/j.clinre.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 31.Bourjeily G., Mohsenin V. Sleep physiology in pregnancy. In: Rosene-Montella K., Bourjeily G., editors. Pulmonary Problems in Pregnancy. Humana Press; New York, NY: 2009. pp. 37–55. [Google Scholar]
- 32.Izci Balserak B., Lee K. Sleep and sleep disorders associated with pregnancy. In: Kryger M.H., Roth T., Dement W.C., editors. Principles and Practice of Sleep Medicine. Elsevier Health Sciences; Philadelphia, PA: 2017. [Google Scholar]
- 33.Driver H.S., Shapiro C.M. A longitudinal study of sleep stages in young women during pregnancy and postpartum. Sleep. 1992;15(5):449–453. doi: 10.1093/sleep/15.5.449. [DOI] [PubMed] [Google Scholar]
- 34.Parboosingh J., Doig A. Studies of nocturia in normal pregnancy. J Obstet Gynaecol Br Commonw. 1973;80(10):888–895. doi: 10.1111/j.1471-0528.1973.tb02147.x. [DOI] [PubMed] [Google Scholar]
- 35.Goldsmith L.T., Weiss G., Steinetz B.G. Relaxin and its role in pregnancy. Endocrinol Metab Clin North Am. 1995;24(1):171–186. [PubMed] [Google Scholar]
- 36.Hirst J.J., Haluska G.J., Cook M.J., Hess D.L., Novy M.J. Comparison of plasma oxytocin and catecholamine concentrations with uterine activity in pregnant rhesus monkeys. J Clin Endocrinol Metab. 1991;73(4):804–810. doi: 10.1210/jcem-73-4-804. [DOI] [PubMed] [Google Scholar]
- 37.Lee K.A., Zaffke M.E., Baratte-Beebe K. Restless legs syndrome and sleep disturbance during pregnancy: the role of folate and iron. J Womens Health Gend Based Med. 2001;10(4):335–341. doi: 10.1089/152460901750269652. [DOI] [PubMed] [Google Scholar]
- 38.Botez M.I., Lambert B. Folate deficiency and restless-legs syndrome in pregnancy. N Engl J Med. 1977;297(12):670. doi: 10.1056/NEJM197709222971220. [DOI] [PubMed] [Google Scholar]
- 39.Manconi M., Govoni V., De Vito A. Restless legs syndrome and pregnancy. Neurology. 2004;63(6):1065–1069. doi: 10.1212/01.wnl.0000138427.83574.a6. [DOI] [PubMed] [Google Scholar]
- 40.Deurveilher S., Rusak B., Semba K. Female reproductive hormones alter sleep architecture in ovariectomized rats. Sleep. 2011;34(4):519–530. doi: 10.1093/sleep/34.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai S., Tan S., Gluckman P.D. Sleep quality and nocturnal sleep duration in pregnancy and risk of gestational diabetes mellitus. Sleep. 2017;40(2) doi: 10.1093/sleep/zsw058. [DOI] [PubMed] [Google Scholar]
- 42.Signal T.L., Paine S.J., Sweeney B. Prevalence of abnormal sleep duration and excessive daytime sleepiness in pregnancy and the role of socio-demographic factors: comparing pregnant women with women in the general population. Sleep Med. 2014;15(12):1477–1483. doi: 10.1016/j.sleep.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Rawal S., Hinkle S.N., Zhu Y., Albert P.S., Zhang C. A longitudinal study of sleep duration in pregnancy and subsequent risk of gestational diabetes: findings from a prospective, multiracial cohort. Am J Obstet Gynecol. 2017;216(4):399.e1–399.e8. doi: 10.1016/j.ajog.2016.11.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reid K.J., Facco F.L., Grobman W.A. Sleep during pregnancy: the nuMoM2b Pregnancy and Sleep Duration and Continuity Study. Sleep. 2017;40(5) doi: 10.1093/sleep/zsx045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sedov I.D., Cameron E.E., Madigan S., Tomfohr-Madsen L.M. Sleep quality during pregnancy: a meta-analysis. Sleep Med Rev. 2018;38:168–176. doi: 10.1016/j.smrv.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Qiu C., Enquobahrie D., Frederick I.O., Abetew D., Williams M.A. Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: a pilot study. BMC Womens Health. 2010;10:17. doi: 10.1186/1472-6874-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herring S.J., Nelson D.B., Pien G.W. Objectively measured sleep duration and hyperglycemia in pregnancy. Sleep Med. 2014;15(1):51–55. doi: 10.1016/j.sleep.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Facco F.L., Grobman W.A., Reid K.J. Objectively measured short sleep duration and later sleep midpoint in pregnancy are associated with a higher risk of gestational diabetes. Am J Obstet Gynecol. 2017;217(4):447.e1–447.e13. doi: 10.1016/j.ajog.2017.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams M.A., Miller R.S., Qiu C., Cripe S.M., Gelaye B., Enquobahrie D. Associations of early pregnancy sleep duration with trimester-specific blood pressures and hypertensive disorders in pregnancy. Sleep. 2010;33(10):1363–1371. doi: 10.1093/sleep/33.10.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee K.A., Gay C.L. Sleep in late pregnancy predicts length of labor and type of delivery. Am J Obstet Gynecol. 2004;191(6):2041–2046. doi: 10.1016/j.ajog.2004.05.086. [DOI] [PubMed] [Google Scholar]
- 51.Naghi I., Keypour F., Ahari S.B., Tavalai S.A., Khak M. Sleep disturbance in late pregnancy and type and duration of labour. J Obstet Gynaecol. 2011;31(6):489–491. doi: 10.3109/01443615.2011.579196. [DOI] [PubMed] [Google Scholar]
- 52.Plancoulaine S., Flori S., Bat-Pitault F., Patural H., Lin J.S., Franco P. Sleep trajectories among pregnant women and the impact on outcomes: a population-based cohort study. Matern Child Health J. 2017;21(5):1139–1146. doi: 10.1007/s10995-016-2212-9. [DOI] [PubMed] [Google Scholar]
- 53.Tsai S.Y., Lin J.W., Kuo L.T., Lee C.N., Landis C.A. Nighttime sleep, daytime napping, and labor outcomes in healthy pregnant women in Taiwan. Res Nurs Health. 2013;36(6):612–622. doi: 10.1002/nur.21568. [DOI] [PubMed] [Google Scholar]
- 54.Bourjeily G., Raker C.A., Chalhoub M., Miller M.A. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;36(4):849–855. doi: 10.1183/09031936.00021810. [DOI] [PubMed] [Google Scholar]
- 55.Louis J.M., Mogos M.F., Salemi J.L., Redline S., Salihu H.M. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998-2009. Sleep. 2014;37(5):843–849. doi: 10.5665/sleep.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bourjeily G., Danilack V.A., Bublitz M.H. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med. 2017;38:50–57. doi: 10.1016/j.sleep.2017.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bin Y.S., Cistulli P.A., Ford J.B. Population-based study of sleep apnea in pregnancy and maternal and infant outcomes. J Clin Sleep Med. 2016;12(6):871–877. doi: 10.5664/jcsm.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Felder J.N., Baer R.J., Rand L., Jelliffe-Pawlowski L.L., Prather A.A. Sleep disorder diagnosis during pregnancy and risk of preterm birth. Obstet Gynecol. 2017;130(3):573–581. doi: 10.1097/AOG.0000000000002132. [DOI] [PubMed] [Google Scholar]
- 59.Bublitz M.H., Monteiro J., Caraganis A. Obstructive sleep apnea in gestational diabetes: a pilot study of the role of the hypothalamic pituitary adrenal axis. J Clin Sleep Med. 2018;14:87–93. doi: 10.5664/jcsm.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y.H., Kang J.H., Lin C.C., Wang I.T., Keller J.J., Lin H.C. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206(2):136.e1–136.e5. doi: 10.1016/j.ajog.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 61.Okun M.L., Schetter C.D., Glynn L.M. Poor sleep quality is associated with preterm birth. Sleep. 2011;34(11):1493–1498. doi: 10.5665/sleep.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Micheli K., Komninos I., Bagkeris E. Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology. 2011;22(5):738–744. doi: 10.1097/EDE.0b013e31822546fd. [DOI] [PubMed] [Google Scholar]
- 63.Qiu C., Sanchez S.E., Gelaye B., Enquobahrie D.A., Ananth C.V., Williams M.A. Maternal sleep duration and complaints of vital exhaustion during pregnancy is associated with placental abruption. J Matern Fetal Neonatal Med. 2015;28(3):350–355. doi: 10.3109/14767058.2014.916682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salihu H.M., King L., Patel P. Association between maternal symptoms of sleep disordered breathing and fetal telomere length. Sleep. 2015;38(4):559–566. doi: 10.5665/sleep.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bin Y.S., Cistulli P.A., Roberts C.L., Ford J.B. Childhood health and educational outcomes associated with maternal sleep apnea: a population record-linkage study. Sleep. 2017;40(11) doi: 10.1093/sleep/zsx158. [DOI] [PubMed] [Google Scholar]
- 66.Bourjeily G., Curran P., Butterfield K., Maredia H., Carpenter M., Lambert-Messerlian G. Placenta-secreted circulating markers in pregnant women with obstructive sleep apnea. J Perinat Med. 2015;43(1):81–87. doi: 10.1515/jpm-2014-0052. [DOI] [PubMed] [Google Scholar]
- 67.Bourjeily G., Butterfield K., Curran P., Lambert-Messerlian G. Obstructive sleep apnea is associated with alterations in markers of fetoplacental wellbeing. J Matern Fetal Neonatal Med. 2015;28(3):262–266. doi: 10.3109/14767058.2014.913131. [DOI] [PubMed] [Google Scholar]
- 68.Ravishankar S., Bourjeily G., Lambert-Messerlian G., He M., De Paepe M.E., Gundogan F. Evidence of placental hypoxia in maternal sleep disordered breathing. Pediatr Dev Pathol. 2015;18(5):380–386. doi: 10.2350/15-06-1647-OA.1. [DOI] [PubMed] [Google Scholar]
- 69.Izci-Balserak B., Pien G.W. The relationship and potential mechanistic pathways between sleep disturbances and maternal hyperglycemia. Curr Diab Rep. 2014;14(2):459. doi: 10.1007/s11892-013-0459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bourjeily G., Ankner G., Mohsenin V. Sleep-disordered breathing in pregnancy. Clin Chest Med. 2011;32(1):175–189. doi: 10.1016/j.ccm.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 71.Khan N., Lambert-Messerlian G., Monteiro J.F. Oxidative and carbonyl stress in pregnant women with obstructive sleep apnea. Sleep Breath. 2018;22:233–240. doi: 10.1007/s11325-017-1475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palagini L., Gemignani A., Banti S., Manconi M., Mauri M., Riemann D. Chronic sleep loss during pregnancy as a determinant of stress: impact on pregnancy outcome. Sleep Med. 2014;15(8):853–859. doi: 10.1016/j.sleep.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 73.NIH State-of-the-Science Conference Statement on management of menopause-related symptoms. NIH Consens State Sci Statements. 2005;22(1):1–38. https://consensus.nih.gov/2005/menopausestatement.htm Accessed December 14, 2017. [PubMed] [Google Scholar]
- 74.Lampio L., Saaresranta T., Engblom J., Polo O., Polo-Kantola P. Predictors of sleep disturbance in menopausal transition. Maturitas. 2016;94:137–142. doi: 10.1016/j.maturitas.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 75.Ciano C., King T.S., Wright R.R., Perlis M., Sawyer A.M. Longitudinal study of insomnia symptoms among women during perimenopause. J Obstet Gynecol Neonatal Nurs. 2017;46(6):804–813. doi: 10.1016/j.jogn.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu M., Belanger L., Ivers H., Guay B., Zhang J., Morin C.M. Comparison of subjective and objective sleep quality in menopausal and non-menopausal women with insomnia. Sleep Med. 2011;12(1):65–69. doi: 10.1016/j.sleep.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 77.Freedman R.R., Roehrs T.A. Lack of sleep disturbance from menopausal hot flashes. Fertil Steril. 2004;82(1):138–144. doi: 10.1016/j.fertnstert.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 78.Lampio L., Polo-Kantola P., Himanen S.L. Sleep during menopausal transition: a 6-year follow-up. Sleep. 2017;40(7) doi: 10.1093/sleep/zsx090. [DOI] [PubMed] [Google Scholar]
- 79.Joffe H., White D.P., Crawford S.L. Adverse effects of induced hot flashes on objectively recorded and subjectively reported sleep: results of a gonadotropin-releasing hormone agonist experimental protocol. Menopause. 2013;20(9):905–914. doi: 10.1097/GME.0b013e31828292d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hartz A., Ross J.J., Noyes R., Williams P. Somatic symptoms and psychological characteristics associated with insomnia in postmenopausal women. Sleep Med. 2013;14(1):71–78. doi: 10.1016/j.sleep.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 81.Joffe H., Crawford S., Economou N. A gonadotropin-releasing hormone agonist model demonstrates that nocturnal hot flashes interrupt objective sleep. Sleep. 2013;36(12):1977–1985. doi: 10.5665/sleep.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu Q., Lang C.P. Examining the relationship between subjective sleep disturbance and menopause: a systematic review and meta-analysis. Menopause. 2014;21(12):1301–1318. doi: 10.1097/GME.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 83.de Zambotti M., Colrain I.M., Javitz H.S., Baker F.C. Magnitude of the impact of hot flashes on sleep in perimenopausal women. Fertil Steril. 2014;102(6):1708–1715.e1. doi: 10.1016/j.fertnstert.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ayers B., Hunter M.S. Health-related quality of life of women with menopausal hot flushes and night sweats. Climacteric. 2013;16(2):235–239. doi: 10.3109/13697137.2012.688078. [DOI] [PubMed] [Google Scholar]
- 85.Pinkerton J.V., Abraham L., Bushmakin A.G., Cappelleri J.C., Komm B.S. Relationship between changes in vasomotor symptoms and changes in menopause-specific quality of life and sleep parameters. Menopause. 2016;23(10):1060–1066. doi: 10.1097/GME.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 86.Leeangkoonsathian E., Pantasri T., Chaovisitseree S., Morakot N. The effect of different progestogens on sleep in postmenopausal women: a randomized trial. Gynecol Endocrinol. 2017;33(12):933–936. doi: 10.1080/09513590.2017.1333094. [DOI] [PubMed] [Google Scholar]
- 87.Cintron D., Lahr B.D., Bailey K.R. Effects of oral versus transdermal menopausal hormone treatments on self-reported sleep domains and their association with vasomotor symptoms in recently menopausal women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS) Menopause. 2018;25:145–153. doi: 10.1097/GME.0000000000000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Savard M.H., Savard J., Caplette-Gingras A., Ivers H., Bastien C. Relationship between objectively recorded hot flashes and sleep disturbances among breast cancer patients: investigating hot flash characteristics other than frequency. Menopause. 2013;20(10):997–1005. doi: 10.1097/GME.0b013e3182885e31. [DOI] [PubMed] [Google Scholar]
- 89.Freedman R.R., Roehrs T.A. Sleep disturbance in menopause. Menopause. 2007;14(5):826–829. doi: 10.1097/GME.0b013e3180321a22. [DOI] [PubMed] [Google Scholar]
- 90.Sowers M.F., Zheng H., Kravitz H.M. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep. 2008;31(10):1339–1349. [PMC free article] [PubMed] [Google Scholar]
- 91.de Zambotti M., Colrain I.M., Baker F.C. Interaction between reproductive hormones and physiological sleep in women. J Clin Endocrinol Metab. 2015;100(4):1426–1433. doi: 10.1210/jc.2014-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stevenson J.C., Chines A., Pan K., Ryan K.A., Mirkin S. A pooled analysis of the effects of conjugated estrogens/bazedoxifene on lipid parameters in postmenopausal women from the Selective Estrogens, Menopause, and Response to Therapy (SMART) Trials. J Clin Endocrinol Metab. 2015;100(6):2329–2338. doi: 10.1210/jc.2014-2649. [DOI] [PubMed] [Google Scholar]
- 93.Gomez-Santos C., Saura C.B., Lucas J.A., Castell P., Madrid J.A., Garaulet M. Menopause status is associated with circadian- and sleep-related alterations. Menopause. 2016;23(6):682–690. doi: 10.1097/GME.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 94.Ohayon M.M., Carskadon M.A., Guilleminault C., Vitiello M.V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 95.Gursoy A.Y., Kiseli M., Caglar G.S. Melatonin in aging women. Climacteric. 2015;18(6):790–796. doi: 10.3109/13697137.2015.1052393. [DOI] [PubMed] [Google Scholar]
- 96.Chen W.Y., Giobbie-Hurder A., Gantman K. A randomized, placebo-controlled trial of melatonin on breast cancer survivors: impact on sleep, mood, and hot flashes. Breast Cancer Res Treat. 2014;145(2):381–388. doi: 10.1007/s10549-014-2944-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kotlarczyk M.P., Lassila H.C., O'Neil C.K. Melatonin Osteoporosis Prevention Study (MOPS): a randomized, double-blind, placebo-controlled study examining the effects of melatonin on bone health and quality of life in perimenopausal women. J Pineal Res. 2012;52(4):414–426. doi: 10.1111/j.1600-079X.2011.00956.x. [DOI] [PubMed] [Google Scholar]
- 98.Freeman E.W., Sammel M.D., Boorman D.W., Zhang R. Longitudinal pattern of depressive symptoms around natural menopause. JAMA Psychiatry. 2014;71(1):36–43. doi: 10.1001/jamapsychiatry.2013.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cohen L.S., Soares C.N., Vitonis A.F., Otto M.W., Harlow B.L. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 100.Bromberger J.T., Matthews K.A., Schott L.L. Depressive symptoms during the menopausal transition: the Study of Women's Health Across the Nation (SWAN) J Affect Disord. 2007;103(1-3):267–272. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bromberger J.T., Kravitz H.M., Chang Y.F., Cyranowski J.M., Brown C., Matthews K.A. Major depression during and after the menopausal transition: Study of Women's Health Across the Nation (SWAN) Psychol Med. 2011;41(9):1879–1888. doi: 10.1017/S003329171100016X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Burleson M.H., Todd M., Trevathan W.R. Daily vasomotor symptoms, sleep problems, and mood: using daily data to evaluate the domino hypothesis in middle-aged women. Menopause. 2010;17(1):87–95. doi: 10.1097/gme.0b013e3181b20b2d. [DOI] [PubMed] [Google Scholar]
- 103.Mirer A.G., Young T., Palta M., Benca R.M., Rasmuson A., Peppard P.E. Sleep-disordered breathing and the menopausal transition among participants in the Sleep in Midlife Women Study. Menopause. 2017;24(2):157–162. doi: 10.1097/GME.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Galvan T., Camuso J., Sullivan K. Association of estradiol with sleep apnea in depressed perimenopausal and postmenopausal women: a preliminary study. Menopause. 2017;24(1):112–117. doi: 10.1097/GME.0000000000000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Naufel M.F., Frange C., Andersen M.L. Association between obesity and sleep disorders in postmenopausal women. Menopause. 2018;25:139–144. doi: 10.1097/GME.0000000000000962. [DOI] [PubMed] [Google Scholar]
- 106.Huang W.Y., Huang C.C., Chang C.C., Kor C.T., Chen T.Y., Wu H.M. Associations of self-reported sleep quality with circulating interferon gamma-inducible protein 10, interleukin 6, and high-sensitivity C-reactive protein in healthy menopausal women. PLoS One. 2017;12(1):e0169216. doi: 10.1371/journal.pone.0169216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou Y., Yang R., Li C., Tao M. Sleep disorder, an independent risk associated with arterial stiffness in menopause. Sci Rep. 2017;7(1):1904. doi: 10.1038/s41598-017-01489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hita-Contreras F., Zagalaz-Anula N., Martinez-Amat A. Sleep quality and its association with postural stability and fear of falling among Spanish postmenopausal women. Menopause. 2018;25:62–69. doi: 10.1097/GME.0000000000000941. [DOI] [PubMed] [Google Scholar]
- 109.Chair S.Y., Wang Q., Cheng H.Y. Relationship between sleep quality and cardiovascular disease risk in Chinese post-menopausal women. BMC Womens Health. 2017;17(1):79. doi: 10.1186/s12905-017-0436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]