Abstract
Astrocytes are neural cells of ectodermal, neuroepithelial origin that provide for homeostasis and defense of the central nervous system (CNS). Astrocytes are highly heterogeneous in morphological appearance; they express a multitude of receptors, channels, and membrane transporters. This complement underlies their remarkable adaptive plasticity that defines the functional maintenance of the CNS in development and aging. Astrocytes are tightly integrated into neural networks and act within the context of neural tissue; astrocytes control homeostasis of the CNS at all levels of organization from molecular to the whole organ.
I. DEFINITION AND OVERVIEW OF FUNCTION
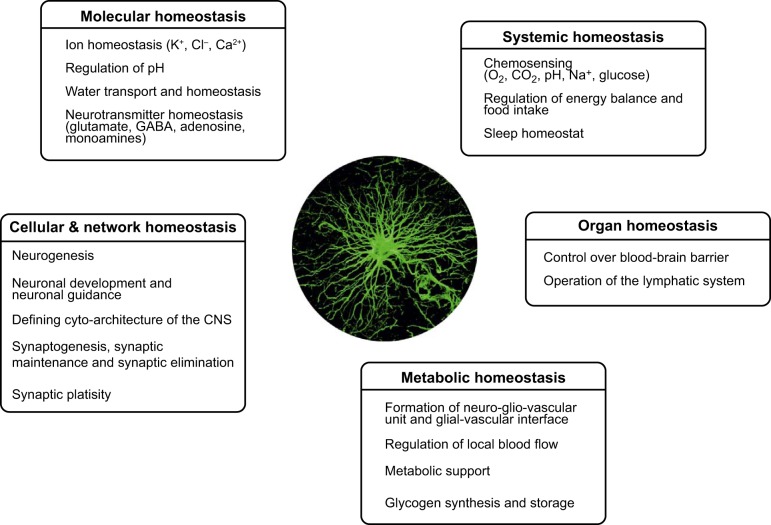
Astroglia are a class of neural cells (also known as astrocytes) of ectodermal, neuroepithelial origin that sustain homeostasis and provide for defense of the central nervous system (CNS) (FIGURE 1). Astrocytes are highly heterogeneous in form and function and demonstrate remarkable adaptive plasticity that defines the functional maintenance of the CNS in development and aging. Astrocytes are tightly integrated into neural networks and act within the context of neural tissue; astrocytes control homeostasis of the CNS at all levels of organization from molecular to the whole organ. Astrocytes maintain molecular homeostasis of the CNS by transporting major ions and protons, by removing and catabolizing neurotransmitters, and by releasing neurotransmitter precursors and scavengers of reactive oxygen species. Astrocytes sustain neurotransmission by supplying neurons with neurotransmitter precursors and control cellular homeostasis through embryonic neurogenesis (that occurs from radial glia) and adult neurogenesis (which involves stem astrocytes of neurogenic niches). Astrocytes regulate metabolic homeostasis through synthesizing glycogen and supplying neurons with energy substrates. Astrocytes define the cytoarchitecture of the grey matter by tiling the latter and by forming contacts with the vasculature by vascular endfeet and by glial sheets at all surfaces of the brain. The vascular endfeet, which plaster along the entire vasculature, release vasoactive substances thus contributing to functional hyperemia. Astrocytes in the guise of glia limitans form the pial cover of the CNS, control blood-brain barrier and act as chemosensors, thus contributing to systemic homeostasis (regulation of energy balance, blood pH and Na+ concentration). Finally, through mounting reactive response, astrocytes (together with microglia) represent the main defensive system of the CNS (we shall not discuss astrogliopathology in the present paper, instead recommending recent comprehensive reviews (257, 258, 1329, 1352, 1353, 1637, 1638, 1815, 1818). These numerous functions of astrocytes are of vital importance for all aspects of CNS operation, including its development, experience-dependent adaptation and aging.
FIGURE 1.
Homeostatic functions of astroglia.
II. HISTORIC PROLOGUE
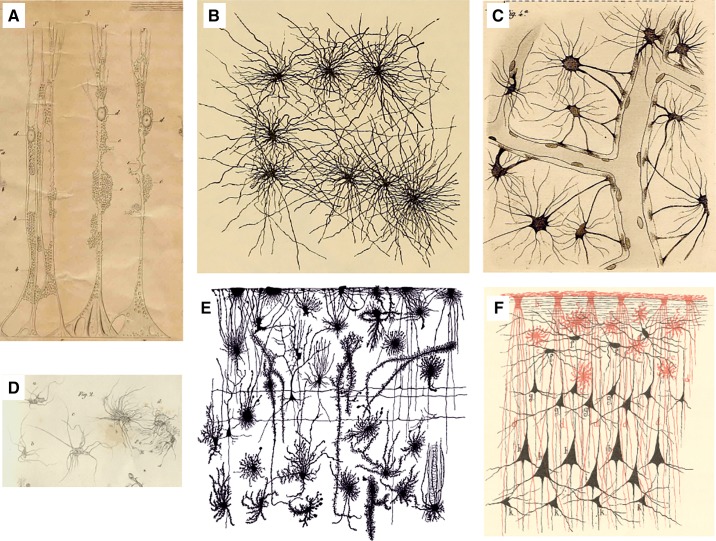
Rudolph Virchow introduced the concept of neuroglia1 (1826, 1827) as true connective tissue of the brain, with little considerations for its cellular nature. Virchow referred to neuroglia as a Zwischenmasse or in-between tissue, into “which the nervous system elements are embedded” (1827). The very first account of neural cell that was subsequently classified as glia was, however, produced some while before Virchow's seminal deliberation. This was a radial-like glial cell of the retina, the Müller cell, described by Heinrich Müller in 1851 (1165). These cells were thereafter characterized in most minute details by Max Schulze (1579). In 1857 Karl Bergmann (155) discovered radial-like glial cells of the cerebellum, today known as Bergmann glial cells. Parenchymal glia received much attention by 19th century neuroscientists and numerous detailed descriptions of these cells, under many different names, have been published (FIGURE 2). The parenchymal neuroglia were named Bindesubstanzzelle (binding substance cells or connective cells) by Otto Deiters (398) or Fasernetz sternförmiger Zellen (fiber network stellate cells) by Leopold Besser (164). Carl Frommann (536) was the first to introduce connotation of the glue by naming glia Leim erfüllten Interstitien (glue-filled interstitium); Albert von Kölliker (894) called glial cells Sternförmige Zellen (star-form cells), Eduard Rindfleisch (1469) called them Stützcelle or Neurogliazellen (supportive cells or neuroglial cells), Victor Butzke (271) called them Gliakörperchen (glial bodies), Moritz Jastrowitz (787, 788) called them spinnenähnliche Gliazellen or Spinnezellen (spider glial cells or spider cells), Carl Ludwig Schleich (1565) called them Mooszellen (moss cells), and Gustaf Magnus Retzius (1458) called them asteroide Gliäcyten or Sternzellen (starlike gliocytes or star cells). Camillo Golgi (who always used the term neuroglia) was the first to demonstrate that glia represent a cellular population distinct from nerve cells, although he also believed that glial cells and neurons may transform into each other. Golgi identified glia as round cells with numerous fine processes extended in all directions; many of these processes are directed towards blood vessels (585). Using the silver-chromate staining technique (reazione nera), Golgi described a remarkable diversity of glial cells in the brain, reported glial networks, and identified glial endfeet plastering blood vessels (583–585). The silver-chromate impregnation technique was also instrumental in visualizing and identifying radial glia (145).
FIGURE 2.
Historic images of astrocytes. A: Müller glial cell of the sheep retina drawn by Max Schulze using a microscope from Amici. y, Brushlike fibrils extending from the outer Müller fiber in the outer granular layer; x, internal limiting membrane; a, opening in the limiting membrane; b, very delicate network of fenestrated membranes similar in the ganglion cell layer; c, network in the so-called molecular layer; d, nuclei as part of the Müller fibers; ee, cavity in which the nuclei or the cells of the internal granular layer are located. [From Schulze (1579). Image has been kindly provided by Prof. Helmut Kettenmann, Max Delbruck Centre for Molecular Medicine, Berlin.] B: cortical astrocytes drawn by Albert von Kolliker (894). C: Camillo Golgi’s drawings of astrocytes contacting blood vessels (583). D: the “Spinnenzellen” of Moritz Jastrowitz (787). E: morphological diversity of neuroglia in human fetal cortex (1458). F: close interactions between neuroglial (red; both interlaminar and protoplasmic astrocytes are clearly presented) and neuronal (black) networks in human brain. [From Schleich (1565).]
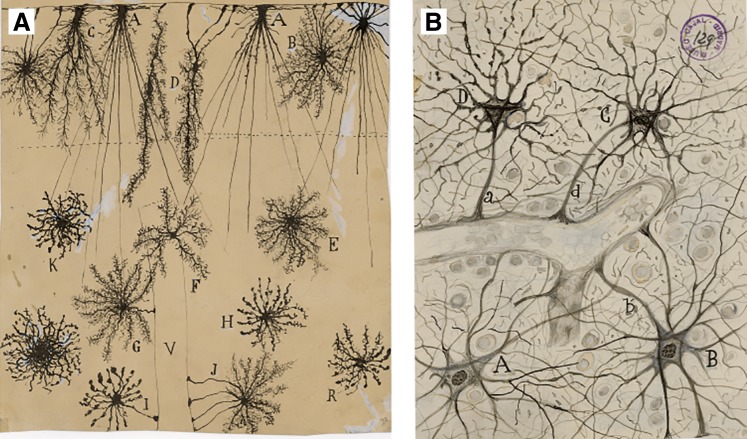
The term astrocyte (αστρον κψτοσ; astron, star and kytos, a hollow vessel, later cell, i.e., starlike cell) was introduced by Michael von Lenhossék in 1895 (969); prophetically Lenhossék proposed to call all parenchymal glia spongiocytes with astrocytes being a subtype.2 Slightly earlier Albert von Kölliker and William Lloyd Andriezen distinguished grey and white matter glia; Andriezen named glial cells in grey matter protoplasmic and those in white matter fibrous. Andriezen believed these two cell types had different ontogeny, the protoplasmic cells being of mesoblastic origin, while the fibrous cells being ectodermal. He also contemplated the complexity of protoplasmic processes, indicating that they have “shaggy granular contour, as if a fine moss constituted the protoplasmic processes” (44). The term astrocytes for denoting parenchymal neuroglia was much popularized by Santiago Ramón y Cajal (FIGURE 3), who developed an astroglia-specific gold and mercury chloride-sublimate staining technique (550), which labeled glial fibrillary acidic protein (GFAP); this staining allowed Cajal to confirm the origin of astrocytes from radial glia (1429, 1430). Most of 19th and early 20th century neuroscientists [with singular exception of Carl Weigert who thought that glia were needed only to fill the gaps between neurons (1871)], assigned numerous functions to astroglia. Golgi, for example, contemplated glia as distributors of nutritive materials (583, 584). Ernesto Lugaro envisaged thin glial processes that infiltrate the synapses and metabolize neuroactive substances (1019). The active role of astrocytes in controlling information flow in the brain was suggested by Carl Ludwig Schleich, who postulated that astroglial processes may (through swelling and shrinking) control synaptic transmission (1565). Similar ideas were entertained by Ramon y Cajal, who thought that retraction of astroglial processes allows information flow during wakefulness, whereas expansion of astroglial processes halts interneuronal connectivity, thus inducing sleep (1427). Cajal also suggested the central role of astrocytes in controlling the vasculature of the brain and mediating functional hyperemia: contraction/relaxation of astroglial perivascular processes could increase or decrease the diameter of brain capillaries, thus regulating the blood flow (1427). Fernando De Castro, a pupil of Cajal, proposed that neuroglial cells may release neuroactive substances and directly participate in neural transmission (389), whereas Robert Galambos considered neuroglia as a central element for higher brain functions while neurons “merely execute the instructions glia give them” (543). The theme of glia being the primary element of information processing, memory, cognition, and consciousness is regularly resurfacing (132, 299, 1368, 1369, 1473); this stimulating conjecture only lacks credible experimental support. Physiological examination of neuroglia began in late 1950s when these cells were probed with electrophysiological and radiotracer techniques applied to in situ and in vivo preparations from vertebrates and mammals, and the first data on dynamic interactions between neurons and glia have emerged (682, 697, 919, 1276, 1731, 1862). In the late 1980s, Jean de Villis established purified cultures of neuroglial cells, which allowed direct examination of physiology of astrocytes at the single-cell level (1155).
FIGURE 3.
Images of astroglia drawn by Santiago Ramon y Cajal. A: Golgi impregnated glia from human cortex (2-mo-old child) in the plexiform layer (A–D), second and third layers (E–H and K, R, respectively), and perivascular glia (I and J). V, blood vessel. B: perivascular astrocytes. [These images are part of the collection of the Cajal Legacy at the Cajal Institute of the Spanish Research Council (CSIC), Madrid, Spain. Images have been kindly provided by Professor Ricardo Martínez Murillo.]
III. EVOLUTION OF ASTROGLIA
The evolutionary emergence of glia (natural history of which is exceedingly difficult to follow because cells do not leave many trails in the fossils) most likely began with a transition from diffuse nervous system (present in Ctenophora and Cnidarians) to a centralized nervous system when first neuronal ganglia emerged. The diffuse nervous system (that evolved from the ectoderm) is composed from multipolar and unipolar neurons. These are integrated by chemical synapses into several semi-independent networks; there is no firm evidence for these networks containing any type of supportive or associated cells. Detection of glia in phylogenetically lower taxa is not a trivial task, primarily because of absence of any specific markers; rather, morphological criteria are used for cell type identification. Probably the main criterion is a close association with neurons and coverage of neuronal structures, i.e., the feature that is fundamental for glial cells. Despite remarkable similarity of glial functions between invertebrates and vertebrates, it is likely that neuroglia appeared in phylogeny on several occasions and evolved every time in a distinct way using disparate genetic associations. In insects, for example, development of glia is controlled by the gene glial cells missing (gsm; Refs. 736, 799); whereas in mammals the gsm homolog is not even expressed in the CNS (860).
Centralization of the nervous system emerged together with bilateral symmetry, and hence the origin of glia should be sought at the base of bilateralia. Indeed, some of the most ancient bilateralia, the Acoelomorpha (1181), already have a nervous system with the frontally localized “bilobed” (i.e., bilobar) brain with a cellular cortex and a dense internal neuropile (7). Electron microscopy of the brains of Symsagittifera roscoffensis, Convoluta psammophila, Amphiscolops sp., and Otocelis rubropunctata (free living Acoela worms) found non-neuronal cells with electron-dense cell bodies in which nuclei occupy most of the cytosol, and lamellar processes extend into neuropil and surround groups of neurites (120, 162). The compact anterior “brain” is also present in Platyzoa (Rotifera and platyhelmintes), although not all representatives of this superphylum possess glial cells. Neuroglia seem to be absent in Rotifera and in tubellarian flatworms such as Catenulida or Macrostomida or Rhabdocoela (653). Supportive glial cells however were identified in higher platyhelmintes, the polyclads and triclads, in which they insulate and support nerve cords (590). In round worms, glial cells are mostly associated with sensory organs, although several glial cells seem to be specialized for neuronal support in the CNS and can be therefore considered to be proto-astrocytes (see below). Neuroglia are well developed in molluscs, in Annelida, and even more developed and diverse in Arthropoda, in insects and crustaceans, with some cells being quite similar to astrocytes; some of these cells express typical markers such as GFAP (653). The radial glia replace parenchymal glia in Echinodermata (the sister phylum of chordata); similarly, radial glia are the main feature in the brains of lower chordata, and radial glia are associated with the emergence of a layered nervous system. An increase in the thickness of the brain triggered a wave of astroglia diversification, which progressed in vertebrates.
A. Proto-astrocytes in Caenorhabditis elegans
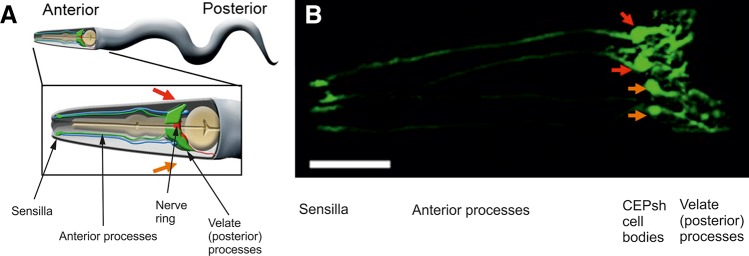
The nervous system of Caenorhabditis elegans is composed of 302 neurons, 50 supportive (glial) cells of ectodermal origin, and 6 supportive cells derived from mesoderm (1258, 1861, see also http://www.wormatlas.org/hermaphrodite/neuronalsupport/Neurosupportframeset.html). None of these glial cells expresses classical astroglial markers. The CNS of C. elegans is represented by the nerve ring located in the frontal part of the body; the nervous ring receives processes of sensory neurons that are located in the periphery. The central ring also contains cephalic and motor neurons, which send efferent signals through the ventral and dorsal nerve cords. Majority (46) of C. elegans glia are associated with the sensory system. These cells are subdivided into 26 socket cells and 20 sheath cells that (together with neuronal processes) compose the worm's sensory organs known as sensillas (1371). The remaining four glial cells known as cephalic sheath (CEPsh) cells are associated with the neural ring. These CEPsh cells are bipolar; the anterior processes enwrap cephalic neuronal dendrites and form the corresponding sensilla in the lips of the animal. Posterior processes of CEPsh cell have lamellar morphology; they ensheath the nerve ring and send processes to the neuropil, where they contact synapses (1258, 1683). Consequently, these CEPsh cells can be defined as proto-astrocytes (FIGURE 4). The C. elegans also contains six mesoderma-derived supportive cells (known as GLR cells), which are located around the nerve ring. These cells (rather uniquely) make gap junctions with neurons and muscle cells and possibly contribute to neuronal-muscular communications (1258). The function of proto-astrocytes in the round worms are yet to be fully characterized. Arguably they control ion homeostasis in perisynaptic regions and are involved in neuronal development and morphogenesis. Although artificial ablation of glial cells (by either exposure to laser beam, or by expressing the diphtheria toxin A gene under control of glia-specific promoter) renders a complex of morphological, developmental, sensory, and behavioral deficits, it is, nonetheless, compatible with survival of the worm (82). Incidentally, C. elegans glia display some intermediate neuronal/glial physiology; for example, they generate Ca2+ signals through activation of voltage-gated channels and do not have functional intracellular Ca2+ stores (1682).
FIGURE 4.
Proto-astrocytes (cephalic sheath, or CEPsh glial cells) of roundworm C. elegans. A: a cartoon of an adult worm showing the four CEPsh glial cells (green) positioned in the anterior of the worm (inset). The CEPsh cell bodies with their velate processes are positioned around the central nerve ring (red), which they enwrap along with the proximal section of the ventral nerve cord. Additionally, each CEPsh glial cell possesses a long anterior process, projecting to the anterior sensory tip, which closely interacts with the dendritic extension of a nearby cephalic neuron (blue). Arrows indicate the dorsal (red arrow) and ventral (orange arrow) side of the worm. B: a confocal image showing green fluorescent protein expression driven by the hlh-17 promoter to visualize the four CEPsh glial cells (worm strain VPR839). The anterior (head) of a juvenile (larval stage 4) worm is shown; the worm is turned ~45 degrees from “upright” such that all four CEP sheath cells are visible. The sheath portion of the cells that form a tube around the dendritic endings of the CEP neurons are seen at the left of the image. The dorsal (red arrow) and ventral (orange arrow) CEPsh cell bodies are seen. The thin sheetlike extensions that surround and invade the nerve ring are seen in the rightmost part of the image. Scale bar, 20 µm. [From Stout et al. (1683).]
B. Homeostatic Glia in Annelida
The medicinal leech Hirudo medicinalis was employed for studying glial physiology in pioneering experiments of Stephen Kuffler and David Potter in the mid 1960s (920). The nervous system of the leech is composed of the anterior and posterior brains and the chain of 21 ganglia that lie in between. The anterior brain comprises six ganglia fused into two neuronal masses, while seven fused ganglia in the aft form the posterior brain. Somatic ganglia innervate corresponding segments of the leech body (342, 403). Every ganglion is composed of 400 neurons (with exception of the 5th and 6th ganglia innervating the reproductive system, which have ~700 neurons) and 10 glial cells. Each ganglion contains two connective glial cells, which ensheath axons, six packet cells covering neuronal cell bodies, and two giant glial cells (403). All three types of glia are connected through gap junctions formed by innexins [of which leech expresses 21 types (819), with the Hm-inx2 type seemingly being specific for glia (463)], creating a panglial syncytium (1005). The nervous system of the leech additionally contains some amount of microglial cells that proliferate in response to lesions (954). Both packet glia and giant glial cells perform homeostatic functions and hence resemble astrocytes. The packet glial cells contribute to the regulation of extracellular K+, especially at high extracellular K+ concentrations (1214, 1547). Somata of giant glial cells are 80−100 μm in diameter and are localized in the center of the ganglion; extensively branched processes of these cells extend (for 300−350 μm) through the entire neuropil and contact neuronal dendrites (1172). Processes of giant glial cells partition neuropil, thus segregating neuronal ensembles into functional domains; sometimes glial processes invaginate into neuronal somata creating a structure known as “trophospongium“ (717). Giant glial cells are characterized by large K+ permeability and relatively hyperpolarized resting potential (approimately −75 mV). They express multiple types of neurotransmitter receptors including ionotropic glutamate, acetylcholine, and serotonin receptors as well as metabotropic receptors to glutamate, serotonin, myomodulin, and possibly P2Y-like purinoceptors and A1-like adenosine receptors (403, 1166). Giant glial cells participate in homeostatic responses, such as regulation of pH involving several plasmalemmal Na+-HCO3− cotransporter, Na+-H+ and Cl−-HCO3− exchangers (399, 404, 405), as well as in regulation of neurotransmitters turnover through Na+-dependent glutamate and Na+-dependent choline transporters (407, 705, 1898). Giant glial cells respond to neuronal activity and to evoked behaviors by changes in membrane potential (400) as well as by generation of cytosolic Ca2+ signals that occur in both somata and processes, often in compartmentalised manner (1004). In contrast to mammalian glia, the main source of Ca2+ signal generation in leech glia is through opening of plasmalemmal Ca2+ channels. Termination of Ca2+ signal termination is mediated by plasmalemmal Ca2+ pump and Na/Ca2+ exchanger; the intracellular Ca2+ stores, although present, seem to play a minor role (408, 1004).
C. Astroglia-like Cells in Arthropods: The Case of Drosophila
The Arthropods (of which insects and crustaceans are by far the most numerous representatives) have well-defined CNS. The brain of Arthropods is divided into protocerebrum (mainly receiving visual information), deuterocerebrum (receiving sensory input), and tritocerebrum (which acts as an integrating center). The neuroglia is elaborated and highly diversified; neuroglial cells account for ~10% of the total number of cells (~90,000 cells) in Drosophila CNS. Neuroglia of Drosophila are classified (for details and further reading, see Refs. 27, 467, 528, 652, 1325) into the following classes: 1) wrapping glia of the peripheral nervous system; 2) surface glia, which makes the brain-hemolymph barrier. It is subdivided into perineural glia (relatively small cells lying on the ganglionic surface) and subperineural or basal glia (represented by large sheetlike cells connected with septate junction that forms the actual barrier). 3) Cortex glia contact neuronal cells somata in the CNS; each glial cell establishes contacts with many neurons. 4) Neuropil glia are located in the neuropil and cover axons and synapses. The neuropil glia are further subdivided into ensheathing or fibrous and astrocyte-like glia, which forms a perisynaptic glial cover. 5) Finally, tract glial cells cover axonal tracts connecting different neuropils (1751). Within these classes glial cells could be further subdivided into multiple subtypes with distinct morphology and function (282, 467, 1751); for example, the glia of the lamina (neuropil) of the optic lobe is classified into fenestrated glia, pseudocartridge glia, distal and proximal satellite glia, epithelial glia, and marginal glia. In the deep optic lobe glial cells are represented by giant optic chiasm glia, small outer optic chiasm glia, medulla satellite glia, and medulla neuropil glia; similarly, specific glial types were described in olfactory system and in antennal lobes. Recent cell mapping using glia-specific GAL4 drivers allowed for precise numerical calculation of various cell types in the Drosophila CNS. The perineural glia accounted for ~17%, subperineural for 2%, cortex glia for 20%, astrocyte-like glia for 34%, and ensheathing glia for 27% of total glial cells (903).
A similar degree of complexity is also found in other insects, for example, in housefly Musca domestica or in tobacco hornworm Manduca sexta in which some other types of glial cells, such as the “chandelier glia,” have been described (288, 1528). Drosophila glial cells that belong to classes ii to iv (i.e., surface glia, cortex glia, and neuropil glia) serve functions characteristic for astrocytes in mammalian systems; however, the degree of specialization seems greater with distinct cell types responsible for a distinct set of functions. The coverage of neuronal bodies is provided by highly ramified cortex glia that do not have much semblance to mammalian astrocytes [although they originate from bipolar cells somewhat similar to radial glia (462)], whereas the brain-hemolymph barrier is provided by similarly distinct surface glial cells. The barrier is sealed by septate junctions composed from several proteins (567, 1578) of which the most important are gliotactin, neuroglian, and α and β subunits of Na+-K+-ATPase (also known as Nervana 2). The glial barrier in the insects is functionally analogous to the endothelial blood-brain barrier in vertebrates. It selectively limits the exchange between hemolymph and the CNS, for example, protecting the brain from substantial fluctuations in K+ that occur after feeding (467). Glial cells in the insect CNS also plaster tracheoles, which deliver oxygen to the nerve tissue (1367). The neuropil glia similarly demonstrate specialization: the flat ensheathing glial cells line the borders of the neuropil, thus isolating it from the surrounding cortex. In addition, ensheathing glia delineate glomeruli in the antennae lobe, whereas the astroglia-like cells extend complex arborizations into neuropil and provide for synaptic coverage (441). The neuropil glia are connected through gap junctions formed by innexins 2 and 3 and optic ganglion reduced (ogre) protein (1662).
Glial cells in insects provide for a multitude of homeostatic functions, such as regulation of ionic balance in the CNS fluids and regulating clearance, recycling, and metabolism of neurotransmitters. These functions are fulfilled by a complement of pumps, transporters, and specific enzymes. In the retina, glial cells are the key element for recycling the principal neurotransmitter histamine. After being released by activated photoreceptors, histamine is (partially) accumulated by glial cells closely covering synaptic contacts (1099). In the glial cell histamine is converted into β-alanyl-histamine or carcinine by ebony (N-β-alanyl-biogenic amine synthetase)-catalyzed reaction (216), the ebony activity is further associated with black protein in a yet unknown way (1966). Subsequently, carcinin is transported back to photoreceptors through the specific solute carrier transporter carT from SLC22A family (1669). After entering photoreceptors, carcinin is hydrolyzed to histamine with catalytic help of the so-called Tan (acyltransferase) protein (1760), thus concluding the histamine-carcinine shuttle. Mutations in the components of this shuttle disrupt fly vision (311, 1966). The ebony amine synthetase expressed in Drosophila glia seemingly also controls circadian clock (1696). Genetic modifications of Drosophila glia that interferes with vesicle trafficking (by specific expression of temperature-sensitive dynamin) and ionic transport (by glia-specific expression of bacterial Na+ channel) affects circadian rhythmicity (1213).
The neuropil glia in the insects express excitatory amino acid transporters dEAAT1 and dEAAT2 (847, 1586) and glutamine synthetase (392) responsible for transport and recycling of glutamate. Glutamate transporters are preferentially localized to glial perisynaptic processes (1472), and their loss instigates neurotoxicity leading to neuropil degeneration and decreased lifespan (985). Glutamate homeostasis mediated by glial transporters contributes to insect behaviors, in particular to sexual behavior and courtship. This seems to be associated with glial-specific cystine-glutamate transporter, which regulates ambient glutamate concentration and hence the strength of glutamatergic transmission. Loss of function mutation of these transporters (known as genderblind, gb) results in homosexual courtship (610). Insect glial cells have been also found to take up γ-aminobutyric acid (GABA), thus regulating GABA levels in the neuropil (1259).
Glia in the insects are imperative for trophic and metabolic support of neurons and for neuroprotection; loss of glial cells by targeted ablation results in massive neuronal death (212). Similarly, neurodegeneration and neuronal loss occur in several mutants with malfunctional glia (such as drop dead, swiss cheese, and repo) (251, 905, 1900). In the retina of the honeybee, glial cells supply photoreceptors with alanine, which subsequently is converted to pyruvate for use in the Krebs cycle (1761). Finally, glial cells in insects contribute to CNS defense by mounting reactive gliosis (1037) and phagocytosis both in development and in the adulthood (441, 928).
D. Astrocytes in Chordata and Low Vertebrates
In the CNS of early Deuterostomia (which include Chordata, Hemichordata, and Echinodermata), the parenchymal glia common to invertebrates are substituted by radial glial cells. The radial glia, although being present at some developmental stages in the insects (see above) and being identified in some protostomes (in Annelida and Scalidophora; A. Reichenbach, personal communication), are mainly associated with vertebrates (1424), with their neuroepithelial, layered CNS contrasting the fused ganglia brain in the majority of invertebrates. In the Echinodermata (represented by sea urchin, star fishes, or sea cucumber), which are placed at the very base of Chordata, radial glial cells are the only glia in the CNS (the latter appears in the form of a circumoral nerve ring connected to radial nerve cords). These radial glial cells produce and secrete the Reissner's substance (1076, 1821), mainly comprising the glycoprotein known as SCO-spondin, which probably acts as cell adhesion modulator (580). The Reissner's substance is found in radial glia throughout Chordata from cephalochordates to Homo sapiens. Glia of Echinodermata have a characteristic radial morphology with elongated shape, long processes spanning the whole thickness of the neural parenchyma, perpendicular orientation to the surface of the neuroepithelium, and high level of expression of intermediate filaments in the cytoplasm (1075, 1076). Functional properties of these glial cells remain unknown.
Similarly, in the CNS of many early vertebrates, radial glia represent the main type of neuroglia, with almost complete absence of parenchymal glial cells. This in particular is characteristic for the brains with thin parenchyma. In elasmobranchii (chondrichthian fish, such as sharks and rays), the brains are subclassified according to their gross morphology. In type I, or “laminar” brain, neurons mainly concentrate in the periventricular zone; the brain walls are thin and ventricles are large. In the type II or “elaborate” brains, neurons migrate away from the periventricular zone and form nuclei; these brains have thicker walls and the ventricles are relatively small (62, 266). In the type I brains, radial glia dominate, whereas the elaborated brains of type II contain numerous well-developed astrocyte-like parenchymal glia (62). Emergence of parenchymal astrocytes in elaborate brains probably reflects an increased homeostatic challenge of the enlarged nervous tissue that cannot be met by radial glia. This constrains homeostatic capabilities of the radial glia and hence prompts an increase in numbers and complexity of parenchymal astrocytes (1452). Another possible factor could be associated with an increase in complexity of vascularization, which requires perivascular glial support that cannot be provided by radial glia (1879). In low vertebrates, parenchymal glia are the main component of the blood-brain barrier; in sharks for example, astrocytes completely enwrap vessels making them endocellular capillaries (254, 255). The radial glia seem to be the predominant glial cell type in all anamniotes (that is in fishes and amphibians). In zebra fish (the teleost), radial glial cells express GFAP and possess glutamine synthetase (indicating their possible role in glutamate homeostasis and metabolism) and aquaporin-4 (indicating their role in water homeostasis) (611, 1025). In contrast to elasmobranchii, the blood-brain barrier in teleosts is made by vascular endothelial cells, the arrangement common to most of the vertebrates (254). Similarly to higher vertebrates in the zebra fish and in amphibians, radial glia endfeet enwrap blood vessels (1025). Radial glial cells in zebra fish do not mount reactive response to brain injury; instead, they increase neurogenesis thus closing the lesions without scar formation (113).
The radial glia dominate the brains of reptiles (8, 814), although parenchymal cells bearing astrocytic morphology have been identified in the CNS of some snakes and lizards (1148, 1271). Parenchymal astroglia-like cells frequently appear in the brains of Cayman crocodiles where they intermingle with radial glia, the latter still being the predominant type (815). In adult birds where the astrocytes proper became a predominant cell type, the radial glia remains, although radial glial presence diminishes in postnatal development (30, 816). In mammals, the radial glia disappear almost completely with the exception of radial-like cells in retina (Müller glia), in cerebellum (Bergmann glia), in hypothalamus (tanycytes), and in subventricular zone.
E. Recapitulation
The astroglia (i.e., the parenchymal homeostatic supportive cells of the CNS) evolved several times in independent manner into many distinct phenotypes. This very much agrees with our view on astrocytes as highly opportunistic supportive cells that tailor their form and function to match the demands of progressively changing nervous tissue. In this context, the CNS evolved through division of functions between cell types: the neurons become mostly responsible for rapid propagation of signals associated with action potential and chemical synapses, whereas neuroglia assumed the responsibility for homeostasis and defense (1813).
Although astroglial cells (or astroglia-like cells at the earlier evolutionary stages) generally perform similar functions, their appearance differs substantially at different phylogenetic stages. The most primitive astrocytes operational in C. elegans combine the features of peripheral and central glia by contributing to formation of sensillas with their centrifugal processes and covering neurons by their centripetal ones. Further evolution resulted in multiplying forms and diversification. In annelids, the astroglial functions are divided between packet cells, which support neuronal cell bodies, and highly idiosyncratic giant glial cells that control homeostasis of the neuropil. Even more diversification was achieved in insects in which specific cell types solely responsible for barrier function, for supporting neurons, for isolating neuropil, and for homeostatic control over neuropil have emerged. The evolution of astroglia erupted anew in Chordata with the ascendance of radial glia, which became universal neural precursors, provided scaffold for neuronal migration, and in anamniotes assumed general control over CNS homeostasis. Increase in the size and thickness of the brain instigated an appearance of true astrocytes that combined all supportive functions in one cell type, which however is remarkably plastic and can modify the phenotype according to tissue demands. At the same time, many key homeostatic molecules such as ion pumps and neurotransmitter transporters are conserved through phylogeny. Despite the independent and multiple evolution, the main trend in astroglia development remains the same, and it is represented by gradual yet unabating shift of homeostatic and defensive functions from neurons to neuroglia. Together, increased intricacy of astroglial process-synaptic complexes contributed to a progressive increase in the computation power of the mammalian brain.
IV. ASTROGLIAL ORIGINS AND DEVELOPMENT
A. Astrogliogenesis in Mammals
Astrocytes are classical neural cells which (as neurons and oligodendroglial cells) originate from the neuroepithelium-derived radial glia, that are the universal neural progenitor (906). Development of astrocytes, however, differs from that for neurons. The latter originate from dedicated progenitor cells which derive from radial glial through asymmetric division. Astrocytes, in contrast, can develop from glial intermediate progenitors, through transformation of radial glia, from proliferation of differentiated astrocytes and even from NG2 glial cells (1140, 1564). The first stage of development of the nervous system is solely neuronogenic; the asymmetric division of radial glia produces either neuron-restricted intermediate progenitors or immature neurons. The neuronogenic period is promoted by pro-neuronogenic factors such as neurogenin 1, which simultaneously suppresses gliogenic route. The gliogenic switch in rodents occurs around embryonic day 12.5 in the spinal cord and around E16–18 in the cortex; the onset is controlled by the transcription factors nuclear factor I-A (NFIA) (410) and Sox 9 that acts in association with NFIA (827, 1675), by Notch signaling, or by the transcriptional repressor N-CoR. Many of these pathways are converging on JAK/STAT signaling cascade (527, 665). Activated STAT3 together with the co-activator complex p300/CBP bind to promoter of astroglial genes, thus triggering expression of astrocyte-specific genes, such as for example the gene of GFAP (830, 1779). Inhibition of this cascade is critical for suppression of the gliogenesis, and this is the mechanism of action of neurogenin 1, which binds to the p300/CBP complex and thus stops gliogenesis; other proneuronal factors such as Ngn2, NeuroD1, and Mash1 may also act in the same way. This inhibition is an important part of neuronal developmental sequence, allowing massive wave of neuronogenesis to populate neuronal layers and create early connectome. The gliogenic switch is regulated by several factors, most notably by cytokines of IL-6 family, which include ciliary neurotrophic factor (CNTF), leukemia inhibitor factor (LIF), and cardiotrophin-1 (CT-1) that all activate JAK/STAT cascade through signal-transducing co-receptors LIFRβ and gp130 (1191). In the in vivo context the relevant agonist of LIFRβ and gp130 is cardioytropin-1, which is secreted by newborn neurons. This arguably represents the timing mechanism that coordinates neurono- and gliogenesis (101, 1634). It seems that subpopulations of neuronogenic and gliogenic radial glia operate together, although it is impossible to exclude the third population of multipurpose radial glial cells (114). Asymmetric division of radial glia (which occurs in the subventricular zone) produces intermediate glial progenitor cells, which further develop to immature proliferative astrocytes; these latter migrate through cortical layers while continuing to proliferate. A distinct class of neural progenitors (which produce neurons, oligodendrocytes, and astrocytes) has been also identified in the layer 1 on embryonic and neonatal cortex (also known as marginal zone); these progenitors apparently colonize ventral and dorsal telencephalic ventricular zones (231, 364). The marginal zone progenitors may give rise to astrocytes of superficial layers (1–4), which could be one of the explanations for morphological differences between superficial and deep astroglia.
The embryonic astrogliogenesis, however, is responsible for only a fraction of astrocytes populating the CNS: the major peak of glial generation occurs during the second and third postnatal weeks (in rodents) when the number of nonneuronal (i.e., mostly glial) cells increases from ~4 million to over 140 million (96). In the cat, gliogenesis seems to be more prolonged and glia-to-neuron ratio increases from ~0.8 in young kittens (weighting 0.5 kg and being ~60 days old) to ~1.48 in mature animals of 3 kg weight (236). We may therefore assume that in humans the glial development similarly spans well into the adulthood.
The main part of postnatal astrogenesis, which accounts for ~50% of all astrocytes, is associated with symmetric division of differentiated astrocytes that occurs throughout the CNS (561). Incidentally this mechanism was proposed by Cajal a century ago, when he found pairs of astrocytes joined by their soma; these pairs Cajal defined as twin astrocytes or “astrocitos gemelos” (1428). Another source of astrocytes is associated with direct transformation of radial glia, which, at around birth, lose their apical processes and redesign themselves into protoplasmic astrocytes (395, 1566, 1830); this metamorphosis may account for 10–15% of mature astroglia (1140, 1511). Conceivably, astrocytes directly transformed from radial glial cells become stem cells of neurogenic niches in adulthood (1108). Thus astrogliogenesis proceeds through two distinct phases: in the early embryogenesis it occurs through intermediate glial progenitors that originate, through asymmetric division, from radial glia. These precursors migrate through the neural tissue, proliferate, and transform into astrocytes. The postnatal wave of astrogliogenesis (which is substantially larger) occurs through direct transformation of radial glia and symmetric division of differentiated astrocytes. Another possible source of astroglia may be associated with NG2 glial cells (1227), which can generate protoplasmic astrocytes that stay strictly in the ventral forebrain and do not migrate to other areas; furthermore, this astrogenic route seems to be temporally limited (748, 1959, 1960).
Morphological and functional heterogeneity of astrocytes, at least in part, is associated with their place of birth. It seems that diverse astroglial phenotypes are somehow linked to the site of their origin in the ventricular zone and are associated with the subfamily of progenitors, which were born from the spatially restricted set of radial glia and which migrated together along the processes of their parental cells (781, 1140, 1762). This regionalization of astrocytes proceeds together with regionalization of neurons, thus creating columnar structures that contain both types of cells (547, 1045). Recently developed optogenetic cell fate tracing technique (551), which uses GFAP promoter controlled expression of six fluorescent proteins, showed that astroglial clones disperse radially and remain within the confines of a single column (very much like neurons originated from the same radial glia; see Refs. 547, 1045). Thus neurons and astrocytes that are born together do grow together and acquire regional specificity. Furthermore, it seems that regional maturation of neurons and astrocytes is regulated by similar molecular factors. In the spinal cord, both cell types are under control of the bHLH factor scl/tal1 that regulates development of V2b interneurons and ventral astrocytes (410, 1176). At the same time astroglial phenotypes are shaped by their neuronal neighbors. In the cerebellum, for example, this involves the sonic hedgehog system that differentially regulates gene expression in Bergmann glia and velate astrocytes (499). In the adult brain, astroglial proliferation was found in many regions including cerebral cortex, corpus callosum, striatum, hypothalamus, and septum, although the rate of division is very low ranging between 0.05 and 0.45% (560).
B. Recapitulation
Astroglial development is fundamentally different from that of neurons: at birth neurons are generally postmitotic, whereas astrocytes retain proliferative capacity. Moreover, epigenetic regulation of astroglia seems to be similarly different: the glial methylome remains more fetal and hence labile during adulthood (989). In summary, a combination of genetic, developmental, and epigenetic factors make astrocytes truly opportunistic cells and define life-long adaptive plasticity of astroglia.
V. IDENTIFICATION AND MORPHOLOGY OF ASTROGLIA
A. Identification
Visualization and identification of astrocytes, especially in the in situ preparations and in the in vivo brain, are tasks far from trivial. The difficulties are in remarkable morphological heterogeneity and in the absence of a universal marker that may label all cells of astroglial lineage. Existing techniques include classical histological staining and immunocytochemistry (performed on fixed tissues), genetically controlled expression of astroglia-specific fluorescent markers, incubation with fluorescent probes with some preferential glial affinity, or intraglial injection of fluorescent dyes. Classical histological techniques (95) are represented by 1) Golgi staining (the silver nitrate impregnation technique) that exists in several modifications and in skillful hands may deliver detailed images of astrocytes with primary and secondary and even fine processes, when used in combination with electron microscopy (32, 1251, 1270); 2) a sublimated gold-chloride staining of Cajal, which labels astroglial filaments and endfeet (550, 1193); and 3) Hortega's silver impregnation method, which, with some modifications, has been occasionally used to label astrocytes for light and electron microscopy (885).
1. Genetic profiling of astrocytes
The mRNA expression levels of genes characterize the transcriptional state of the cell, thus providing insight into its function, activity, and developmental state as well as (in the case of disease) degree of pathological remodeling. The transcriptomic profile of human and mouse brain astrocytes have been characterized by microarray and RNA sequencing technologies in combination with cell sorting techniques such as fluorescence-activated cell sorting (FACS) or immunopanning of immuno- or transgenically labeled astrocytes. These studies have revealed novel functions of astrocytes, identified new cell specific markers, and characterized the molecular profile of reactive astrocyte in disease models, as well as highlighted differences and similarities between mouse and human astrocytes and between developing and mature astroglia.
a) major findings from transcriptional studies.
The first transcriptional study of astroglial cells isolated in vivo from adult mice cortex used FACS of astrocytes expressing green fluorescent protein (GFP) under control of either GFAP or glutamate transporter 1 (GLT-1) promoters (1010). It has been found that the majority of astrocyte-enriched genes (~34%) are involved in cellular metabolism and that genes encoding enzymes of the tricarboxylic acid cycle were expressed to a higher degree in astrocytes compared with neurons. Moreover, subsequent functional assays indicated that astrocytes exhibit prominent oxidative metabolism in the intact adult brain and hence can significantly contribute to functional brain imaging, including blood-oxygen level-dependent (BOLD) MRI signals (1010).
The first study to characterize the transcriptional profile of astrocytes isolated from the developing postnatal mouse cortex was performed by Ben A. Barres group (275). Cell suspensions from the cortices of 1- to 8-day-old and 17- to 30-day-old transgenic mice expressing EGFP under control of S100B promoter were first depleted of oligodendrocytes by immunopanning, and subsequently S100B/EGFP positive astrocytes were isolated by FACS. A comparison between immature (days 7–8) and mature (days 17–30) astrocytes showed that the genes highly expressed in the immature astrocytes are involved in general cell proliferation and development (e.g., cell cycle genes) and are not astrocyte specific. Conversely, most genes enriched in mature astrocytes are astroglia specific (the same result was found for oligodendrocytes). Astrocyte-enriched genes, which increase their expression during maturation, included genes encoding secreted proteins such as ApoE, ApoJ/clusterin, Pla2g7, Sparc, Sparcl1, and Mfge8 and genes implicated in psychiatric diseases such as Npas3, Mcl1, Lgi1/4, and Gpr56. Because expression of phagocytic genes in astrocytes increased with the brain maturation, the authors suggested that an important role for astrocytes in the mature brain is to clear apoptotic cells and amyloid deposits and to eliminate synapses. Consequently, aberrant regulation of synaptic formation by astrocytes during development and in the mature brain may contribute to neurodevelopmental, psychiatric, and neurological diseases (275). That astrocytes are capable of synapse elimination in vitro and in vivo was confirmed by the same group in a later study (334), and it has been hypothesized that disruption of the ability of astrocytes and microglia to eliminate and regulate synapse formation contributes to the pathogenesis of psychiatric disorders (335).
Reactive astrogliosis is a common response of astrocytes to brain injury and disease. The transcriptional profile of reactive astroglia was studied on FACS-isolated astrocytes from adult Aldh1l1-EGFP transgenic mice following brain injury (either ischemic stroke by MCAO causing cell death, or LPS injection causing neuroinflammation) (1933). Reactive astrocytes were found to markedly alter their transcriptional profile. Major changes have been detected in the genes encoding extracellular matrix genes, reflecting the ability of reactive astrocytes to modify the extracellular matrix when forming glial scar. Similarly, genes encoding intermediate filament proteins (GFAP, vimentin, and nestin) were highly upregulated in reactive astrocytes, reflecting morphological changes that occur upon activation. Other groups of genes, expression of which increased in the reactive astrocytes, encoded cytokines, proteins of the antigen presentation, and complement pathways, suggesting a role for astrocytes in communicating with the immune system upon injury. Even though reactive astrocytes induced by the two models of injury had similarities in their expression profiles, there was still a large number of genes that differed, highlighting that the response of astrocytes to injury is not uniform; instead, it is more complex and depends on pathology. Finally, this study found that the transcriptional profile of reactive astrocytes is highly similar to the transcriptional profile of cultured neonatal astrocytes produced by the McCarthy-de-Vellis method (1933). This finding was important since it stresses the significance of working with in vivo models instead of astrocytes in the dish when studying their physiological functions.
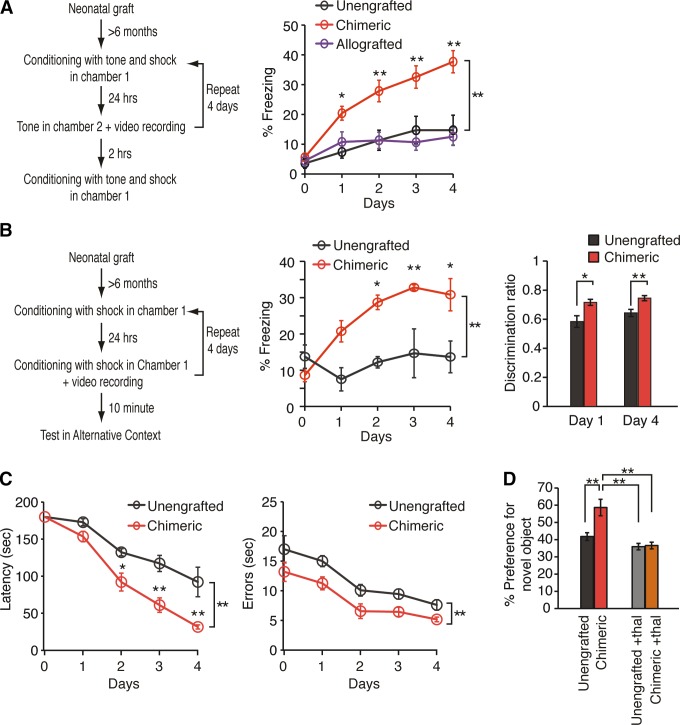
Morphological studies have shown that human astrocytes are much larger and more complex than mouse astrocytes (1248, 1249), and that mice transplanted with human astrocyte progenitor cells perform better in learning and memory tests (637). The first RNA-sequencing study of the transcriptional differences between cortical human and mouse astrocytes was published recently (1946). The purified fetal and mature/adult astrocyte populations from mice and human were obtained by immunopanning with an antibody against the astrocyte-enriched HepaCam after depletion of other cell populations. Comparing the fetal and mature human astrocytes, the authors found that fetal astrocytes had higher expression of proliferative and cell-cycle genes, which is similar to the astrocytes of the developing mice discussed above. With maturation, human astrocytes showed increased expression of genes involved in nerve impulse-transmission, cell-cell signaling, fatty acid metabolism, cell adhesion, and ion homeostasis. When human and mice astrocytes were compared, it appeared that 52% of mouse astrocyte-associated genes were enriched in human astrocytes, whereas only 30% of the human astrocyte-associated genes were enriched in mouse astrocytes. The same differences were found for the other cell types analyzed (1946). This suggests that in general human cells are more specialized than cells in mice, which makes sense evolutionarily, and that the transcriptional differences between human and mice astrocytes are similar to the species differences for other cell types. Unfortunately, the authors do not have a more detailed analysis and discussion of the differences between human and mouse astrocytes; however, their data are available online (http://web.stanford.edu/group/barres_lab/brainseq2/brainseq2.html) being thus available for all interested to do their own analysis.
b) molecular heterogeneity of astrocytes.
To date, no genomic studies have characterized the expected molecular heterogeneity of astrocytes because most studies were performed on cell populations of astrocytes identified by a marker gene or protein. Despite several comparisons of transcriptomes from astrocyte populations expressing specific cells markers, e.g., GFAP versus GLT1 positive astrocytes (1010), ALDH1L1 versus GLT1 positive astrocytes (1916), and ALDH1L1 versus HEPACAM positive astrocytes (1946), no major differences between these cell populations have been found, indicating that the markers are coexpressed in the majority of astrocytes. One way to identify the molecular heterogeneity of astrocytes would be to use unbiased single-cell transcriptomics. A few studies have collected transcriptome information of single astrocytes (384, 1732, 1938). However, these studies do not include further description of astrocyte heterogeneity possibly because this information could not be extracted from the data due to a relatively small sample size [only Zeisel et al. (1938) were able to identify two astrocyte subclasses, GFAP (type 1) vs. MFGE8 (type 2) expressing astrocytes].
2. Immunocytochemistry
The universal marker that may stain and reveal all astrocytes in the CNS does not exist. Remarkable morphological heterogeneity of astrocytes coincides with a substantial diversity in expression of different molecules and hence antibodies against them label subpopulations of astroglial cells with substantial regional differences (TABLE 1).
Table 1.
Markers of astrocytes
| Molecule/Antigen | Detection Agent/Technique | Properties and Functional Relevance | Reference Nos. |
|---|---|---|---|
| Glial fibrillary acidic protein, GFAP | Monoclonal and polyclonal antibodies | Intermediate filament protein, expressed in many cells outside the nervous system; in the CNS expressed in a subpopulation of astrocytes with substantial region variability. Generally, GFAP expression is upregulated in reactive astroglia. | 477, 715, 1355, 1880 |
| Vimentin | Monoclonal and polyclonal antibodies | Intermediate filament protein; expressed in immature astrocytes, in subpopulations of protoplasmic and fibrous astrocytes, in Bergmann glia, and in tanycytes. Vimentin expression is upregulated in reactive astrocytes. | 394, 597, 1350, 1351, 1568 |
| S100B protein | Monoclonal antibodies | Ca2+-binding proteins, which act as Ca2+ buffers as well as Ca2+ sensors. Antibodies against S100B stain more astrocytes than GFAP in the grey as well as in the white matter. | 444, 1251, 1549 |
| Glutamate transporters: EAAT-1 (GLAST), EAAT-2 (GLT-1) | Monoclonal antibodies | Astroglia-specific glutamate transporters; show regional variability: EAAT1 is predominantly expresed in cerebellum; in other regfions EAAT2 is the main transporter type. | 110, 807, 1570, 1606, 1882 |
| Glutamine synthetase | Monoclonal and polyclonal antibodies | Astroglia-specific enzyme converting ammonia and glutamate into glutamine. Expressed in the majority of astrocytes. Immunostaining reveals full structure of the cell due to cytosolic localization of the enzyme. | 49, 417, 1237, 1920 |
| Aldehyde dehydrogenase 1 family, member L1 (ALDH1L1) | ALDH1L11-specific polyclonal antibody | ALDH1L1 is a key enzyme in folate metabolism contributing to nucleotide biosynthesis and cell division. Proposed as a specific astroglial markers with a reach substantially broader than GFAP. ALDH1L1 expression however changes with age, and it was also detected in a subpopulation of oligodendrocytes. | 275, 1916 |
| Connexins: Cx43, Cx30 | Monoclonal and polyclonal antibodies | Both Cx43 and Cx30 are expressed exclusively in astrocytes; the Cx30 is expressed mostly in grey matter (being particularly concentrated in astroglial endfeet) and is absent in astrocytes from white matter. | 413, 1189 |
| Aquaporin: AQP4 | Monoclonal antibodies | AQP4 in the CNS is expressed exclusively in astrocytes and ependymocytes. In healthy astrocytes, AQP4 is preferentially located in the endfeet and hence stains this structure. | 1184 |
| Transcriptional factor SOX9 | Polyclonal antibodies | Specifically labels nuclei of astrocytes outside the neurogenic niches. | 1701 |
a) GFAP.
GFAP was discovered in early 1970s (478, 1781); its exclusive expression in astrocytes in the CNS was soon noted (180, 1018) and has been well documented since (715). The GFAP, of which astrocytes express 10 different isoforms, belongs to an extended family of intermediate filaments and, together with vimentin, nestin and, occasionally, synemin, forms astroglial cytoskeleton (715, 1355). Genetic deletion of GFAP produces rather subtle physiological phenotypes; in GFAP−/− mice, however, reactive astrogliosis is substantially impaired, whereas double deletion of GFAP and vimentin disrupts reactivity even more, which in turn exacerbates neuropathology (1351, 1880).
Staining with GFAP antibodies visualizes only a fraction of astrocytes with a substantial regional (and probably developmental) heterogeneity. This applies to mammals and birds, with some regions (striatum and tectum in mammals and neostriatum, paleostriatum augmentatum, and the superficial zone of tectum in birds) almost completely devoid of GFAP immunoreactivity (813). Astrocytes in cell cultures are almost invariably GFAP positive, whereas subpopulations of GFAP-labeled cells in situ and in vivo are substantially smaller (1842). The largest subpopulation of GFAP-positive astrocytes is present in juvenile hippocampus, with ~80% (or even more) of all cells being labeled with appropriate antibodies (264, 1251); similarly all Bergmann glia in cerebellum are GFAP immunoreactive (46, 1236). At the same time, the majority of astrocytes in other regions of healthy brain are not stained with GFAP antibodies (866, 1549, 1847). Morphology of GFAP-positive profiles is somewhat limited (FIGURE 5, A–C), because the immunolabeling of the cytoskeleton reveals only major processes, with finer parts of the cell remaining unstained (353). Thus GFAP reveals neither peripheral and perisynaptic processes (1451), nor endfeet plastering small (<8 μm) blood vessels (1622). Out of 47 cell-specific molecular signatures identified with single-cell mRNA sequencing of juvenile primary somato-sensory cortex and hippocampus of mice (1938), only 2 belonged to astrocytes. The gfap was being mainly limited to layer 1 astrocytes and glia limitans, and Mfge8 (encoding globule-EGF factor 8 protein or lactadherin) was expressed in the remaining parenchymal astrocytes, again pointing to the limited presence of GFAP in a substantial population of astroglial cells. Despite all these limitations, GFAP labeling is considered, by some, as the best for laser capture microdissection approach (1836, 1837).
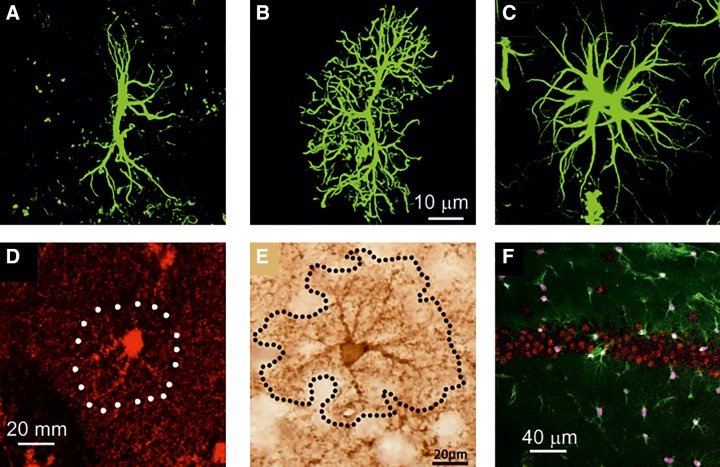
FIGURE 5.
Visualization of rodent astrocytes with immunostaining against canonic markers GFAP, S100B, and glutamine synthetase. A–C: GFAP-stained astrocytes in entorhinal cortex (A), prefrontal cortex (B), and CA1 area of hippocampus (C). [A–C from Rodriguez et al. (1483). Reprinted with permission from Eureka Science Ltd.] D: astrocyte stained with antibody against S100B in the dentate gyrus of hippocampus. E: hippocampal astrcytes stained with anti-glutamine synthetase antibody. [D and E from Rodriguez-Arellano et al. (1480), with permission from Elsevier; and Rodriguez et al. (1486), with permission from Elsevier.] F: cortical tissue preparation with astrocytes labeled in green (EGFP expressed under EAAT2 promoter), astroglial nuclei labeled in pink were stained with antibodies against SOX9, and neurons (in red) were stained with antibodies against NeuroN. (From Sun and Nedergaard, unpublished observation.)
b) S100B protein.
The glycoprotein S100B is one of 24 S100 Ca2+-binding proteins, which are expressed only in vertebrates and act as Ca2+ buffers as well as transducers (Ca2+ sensors) for the intracellular Ca2+ signaling (444). In the CNS, S100B regulates various aspects of cell proliferation and differentiations, and it is known as an inhibitor of apoptosis (444, 622, 1436). There is some evidence that in astrocytes S100B may also contribute to shaping Ca2+ signals (1901). The S100B is also linked to regulation of the assembly of intermediate filaments by inhibiting GFAP polymerization in the presence of micromolar Ca2+ (172). Astrocytes produce and secrete (by a yet unidentified mechanism) S100B, which has (depending on concentration) either neurotrophic/neuroprotective or neurotoxic effects, stimulates astroglial proliferation and contributes (in higher concentrations) to astroglial reactivity and positively regulates microglial activation (10, 171, 956, 1788, 1824). There are also some indications that S100B acts as a regulator of synaptic plasticity and long-term potentiation (1229). Overall, S100B is engaged in intercellular signaling and may act as an extracellular messenger (445). In pathological conditions, expression of S100B substantially changes, and increased levels of this protein in serum and cerebrospinal fluids may have certain diagnostic relevance (444).
Because of high level of expression, S100B is universally used as a marker for astrocytes (FIGURE 5D), both in physiology and in pathology; astrogliotic response being associated with upregulation of S100B. In rodent hippocampi, S100B as a rule stains more astrocytes than GFAP; only ~80% of cells stained with S100B were also GFAP positive (1251). In the whole rat brain, the antibody against S100B stained approximately three times more astrocytes than GFAP; incidentally, the GFAP stained more cells in white versus grey matter, and very few GFAP-positive profiles were visualized in the cortex and in the brain stem (1549). The cell specificity of the S100B is, however, substantially less than that for GFAP. In the CNS it is expressed not only in astroglia but also in oligodendrocytes, in ependymal cells, in the choroid plexus epithelium, in vascular endothelial cells, in lymphocytes, and in some neurons (1664), in particular in neurons in the brain stem, cerebellum, forebrain, and the limbic system (1466).
c) glutamate transporters and glutamine synthetase.
Glutamate transporters and glutamine synthetase are key molecules behind glutamate turnover in the CNS (see sect. XIID). Astroglial glutamate transporters EAAT-1 (GLAST) and EAAT2 (GLT-1) are expressed almost exclusively in astrocytes (1570). The EAAT-1 is the most widespread, and respective antibodies label radial glia, fibrous and protoplasmic astrocytes, cerebellar Bergmann glia, retinal Müller glia, radial stem glia in the dentate gyrus and subventricular zone in both developing and adult CNS (110, 1606, 1882). The specific monoclonal antibody ACSA-1 against extracellular epitopes of EAAT-1 labeled most of protoplasmic and fibrous astrocytes as well as Bergmann and Müller glia (807). There are some issues with EAAT-1/2 specificity; the splice variant of EAAT-1 (which is rarely expressed in astrocytes) has been found in some neurons, in oligodendrocytes, ependymal cells, and epithelial cells of the plexus choroideus (1569). The EAAT2, in its turn, shows transient neuronal expression (including cerebral cortex and basal ganglia) during fetal development (1241).
Glutamine synthetase (GS) stains virtually all types of astrocytes (FIGURE 5E), including radial glia, Bergmann glia, retinal Müller glia, tanycytes, and ependymal cells; importantly, anti-GS antibodies label astrocytes in many regions with weak GFAP immunoreactivity (49). In the mouse entorhinal cortex, double stained with anti-GS and anti-GFAP antibodies, 78% of all labeled glial cells were solely GS positive, 12% GFAP positive, and only 10% were positive for both GS and GFAP (1920). Similarly, in the hippocampus the separate population of GS-positive astrocytes was identified; double staining showed that only 60% of these cells were positive for GFAP (1847). Glutamine synthetase is a cytosolic enzyme and hence staining with appropriate antibodies reveals the whole extent of the cytoplasm including fine perisynaptic processes (417, 1237). There are some indications that GS in astrocytes in vitro may be associated with vesicular structures (50). Several sporadic reports about GS expression in oligodendrocytes (281, 372, 1132) and in neurons (1476) remain debatable and unconfirmed. Arguably, GS may be considered as the most inclusive astrocyte marker.
d) other markers.
Several proteins, which are more or less exclusively expressed in astrocytes, have been identified and can be used as markers with varying success (TABLE 1). Vimentin (similarly to GFAP) is a member of the extended family of intermediate filaments present in mesenchymal cells. It is involved in many cellular functions and in particular in regulation of cell differentiation, adhesion, migration, regeneration stress, and cellular signaling (774). In the CNS, vimentin is primarily expressed in the astroglia, and particularly in the immature astrocytes. After birth, expression of vimentin steadily decreases, although it is still present at immunocytochemically detectable levels in protoplasmic and fibrous astrocytes in hippocampus and corpus callosum as well as in Bergmann glia and in tanycytes where it is coexpressed with GFAP (394, 1351). Vimentin also seems to be present in adult neural stem cells with astroglia-like phenotype in the neurogenic niches, and vimentin expression is upregulated in reactive astroglia (440, 597).
The water channel aquaporin 4 (AQP4) in the CNS is present in astrocytes and ependymocytes (534, 1217). In astrocytes, expression of AQP4 is highly polarized with the highest concentration in the endfeet (1184); as a result, labeling with AQP4 antibodies preferentially reveals endfeet structures. Astrocytes throughout the brain express connexins, with predominant expression of Cx43 and, to a lesser extent, of Cx30 (413, 1189). Staining the brain tissues with antibodies against both connexins shows punctate patterns; the Cx30 is expressed mostly in grey matter astrocytes (being particularly concentrated in their endfeet) and is absent in astrocytes from white matter (1189). The key enzyme of foliate metabolism, aldehyde dehydrogenase 1 family, member L1 (ALDH1L1) was found to be specifically expressed in astrocytes (1212) and was recently suggested as a specific antigenic marker. Polyclonal antibodies against ALDH1L1 stained more astrocytes than GFAP; at the cellular level ALDH1L1-staining revealed soma and fine processes (275). However, later analysis showed that ALDH1L1 expression changes with age, and ALDH1L1 is also expressed in a subpopulation of oligodendrocytes (1916). Furthermore, ALDH1L1 was reported to label mainly cortical astroglia with rather little staining of white matter astrocytes (1837). Another enzyme, the brain-specific form of fructose-1,6-bisphosphate aldolase, known also as aldolase C, was found to be expressed preferentially in astrocytes, although it was also detected in Purkinje neurons (1841). Astrocytes in mouse and human brain are enriched with transcription factor SOX9. Immunostaining with specific antibodies further identified an overlap between SOX9 nuclear staining and EAAT2 immunoreactivity, indicating specific astroglial labeling (1701). Antibodies against SOX9 stain the nucleus and hence do not reveal full astroglial profiles (FIGURE 5F), although they were successfully used for FACS isolation of astrocytes and for isotopic fractionation (1701).
Interlaminar astrocytes as well as fibrous astrocytes in the human brain are readily labeled with antibodies against CD44, a receptor for extracellular matrix molecules (179, 1655). Protoplasmic astrocytes are, as a rule, CD44 negative, although they may acquire this protein at later ages and in pathology (1655).
3. Injection of fluorescent dyes
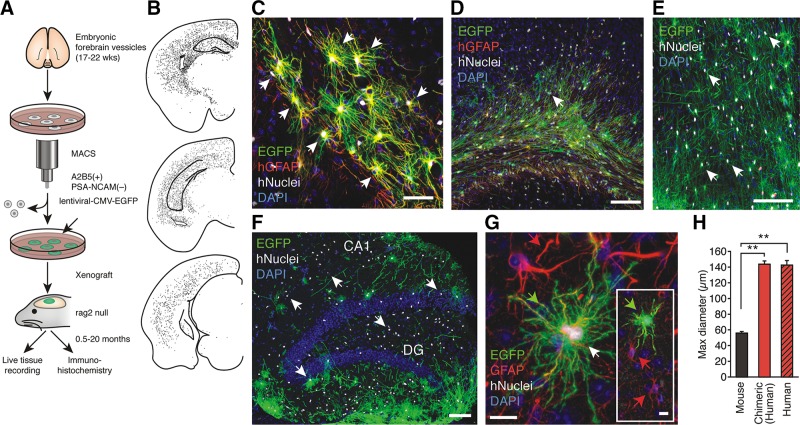
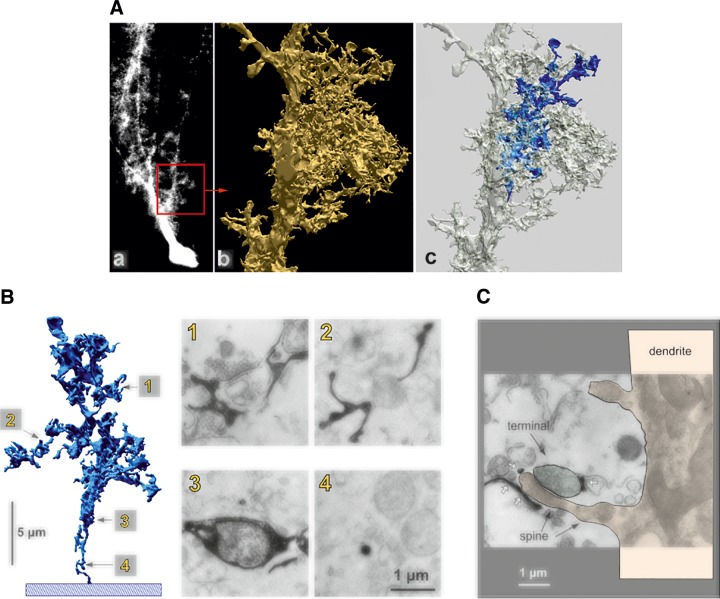
Astrocytes can be visualized by fluorescent dyes, which can be injected (by microelectrodes) or perfused into (through the patch pipette) cells of interest (FIGURE 6A). For such labeling membrane impermeable probes, such as Lucifer yellow, Alexa dyes or biocytin are usually employed (264, 1251, 1839). Alternatively, astrocytes can be loaded with fluorescent probes by diolistic labeling in which Gene Gun technology is used to deliver gold or tungsten particles coated with lipophilic dyes into cells in slices (FIGURE 6B). Combining probes with different spectral properties (for example, red DiI and green DiD) allows selective visualization of adjacent cells (1248).
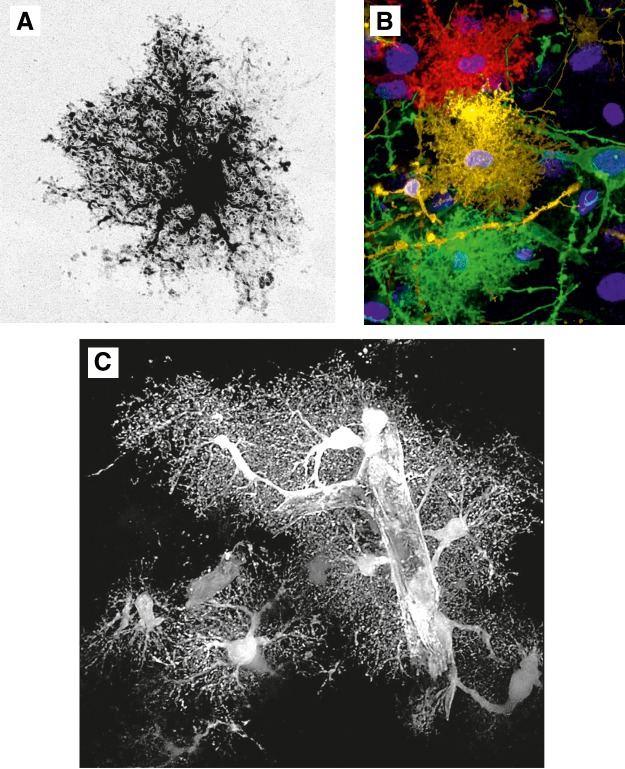
FIGURE 6.
Protoplasmic astrocytes. A: cortical protoplasmic astrocytes filled/injected with fluorescent dye. [Image courtesy of Prof. Milos Pekny and Dr. Ulrika Wilhelmsson (University of Göteborg).] B: protoplasmic astrocytes diolistically labeled with spectrally distinct probes. C: the EGFP-expressing cortical protoplasmic astrocytes with endfeet plastering the blood vessel.
4. Gliophilic fluorescent probes
The ability of astrocytes to preferentially accumulate fluorescent Ca2+ probes in acetoxymethyl (AM) form was recognized in early experiments on brain slices (883, 1599) and was often utilized to monitor Ca2+ signals specifically in astroglia (158, 1003). Subsequently, high propensity of astrocytes to accumulate AM calcium probes was noted during in vivo brain imaging when bolus loading was implemented to load neural cells with the indicator (FIGURE 7). As a rule, intracellular concentration of the Ca2+ indicator was much (~4−5 times) higher in astrocytes when compared with their neighboring neurons (701, 1680). Specific glial accumulation of Ca2+ probes is possibly associated with low glial expression of ABC cassette transporters that mediate extrusion of these dyes (1061). Of note, the bulk loading with Ca2+ indicators stains mainly astroglial soma, and hence it does not allow dynamic Ca2+ recordings from peripheral processes (1445).
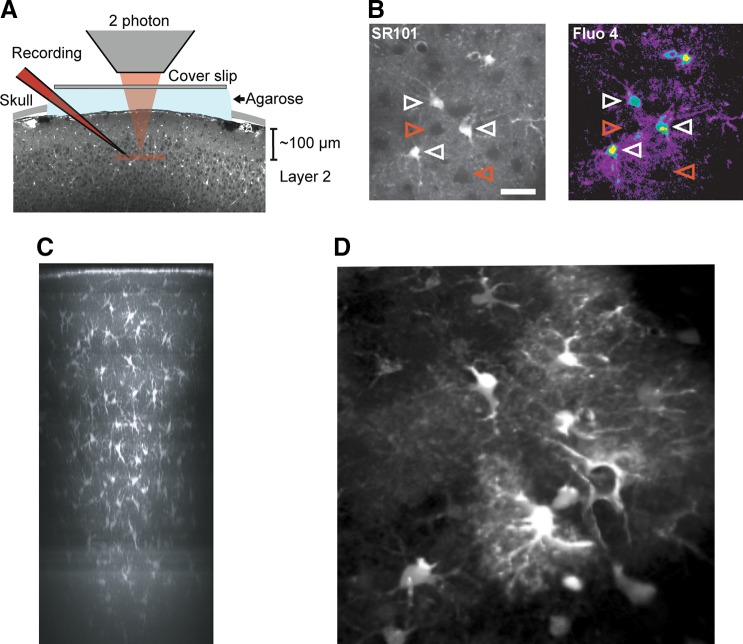
FIGURE 7.
Two photon imaging of astrocytes in vivo. A: experimental setup. Exposed somatosensory cortex was loaded with the specific astrocyte marker sulforhodamine 101 (SR101) and Ca2+ indicator fluo 4-AM. Coverslip and 1% agarose were mounted on top of cranial window to minimize brain pulsation. Recording electrode was loaded with Texas red-dextran (red) and inserted into cortical layer 2 (100–150 μm below pial surface). B: example images showing cortical layer 2 astrocytes double labeled with SR101 and Fluo 4-AM. Only SR101-positive astrocytes also labeled with Fluo 4-AM (white arrowhead). Neurons appeared as dark round shape area (red arrowhead). Scale bar, 30 μm. [A and B from Tian et al. (1750).] C: overview side projection of an SR101-stained area (revealing astrocytes) in mouse neocortex ~30 min after dye application. The image is a maximum-intensity side-projection from a stack of fluorescence images taken through cranial window on an anesthetized mouse. [C from Nimmerjahn et al. (1224). Reprinted by permission from Macmillan Publishers Inc.] D: cortical astrocytes loaded with SR101 and imaged (using two photon confocal system) through the cranial window on an anesthetized mouse. (Image kindly provided by Dr. Hajime Hirose, RIKEN, Japan.)
Another gliophilic fluorescent probe that received much popularity in the imaging experiments is a cationic dye sulforhodamine 101 and its analogs sulforhodamine B or G (1224). Sulforhodamine is selectively taken up by astrocytes and, because of cytoplasmic localization, it reveals detailed cellular structure (FIGURE 7). The sulforhodamines readily penetrate blood-brain barrier (1803) and hence astroglial staining could be achieved by intravenous injections of sulforhodamine B (20 mg/kg). Astrocytes become fluorescent in ~40 min after injection, and the staining persists for up to 5 h (54). The accumulation of sulforhodamine 101 into astrocytes seems to be mediated by organic anion transporters, which are differentially expressed in astrocytes from different brain regions. As a result, sulforhodamine 101 readily stains hippocampal astrocytes but does not accumulate in astrocytes in the ventrolateral medulla (1572). Furthermore, sulforhodamine 101 labels only a subpopulation of astrocytes, which increases in postnatal development and generally coincides with a subpopulation of GFAP-positive mature astroglia (811). Of note, sulforhodamine 101 (used in the concentrations commonly utilized for astroglia staining) affects neuronal excitability; in particular, it induces seizure activity in slices (824) and in vivo (1440).
5. Astroglia-specific expression of genetically encoded markers
Astrocytes can be visualized in tissue preparations and in the in vivo CNS with the aid of fluorescent proteinacious probes selectively expressed in astroglia under the control of cell-specific promoters (FIGURE 6C). The first animal models of “fluorescent” astrocytes employed GFP or its enhanced analog (GFP or EGFP) expressed under the control of human GFAP promoter (1236, 1707, 1964) or by murine S100β gene promoter (1829, 1972). The pool of available astroglial promoters is continuously increasing, and several fluorescent protein probes with distinct spectral characteristics are now in use (703, 999). Astrocytes can also be visualized through expression of genetically encoded Ca2+ indicators (976) such as yellow Cameleon-Nano 50 (YC-Nano50) (821) or green GCaMP (fused GFP, calmodulin and a peptide sequence from myosin light chain kinase) (1190). Several variants of GCaMP have been tested in astroglia, including GCaMP2 (722), GCaMP 3 (1609), GCaMP5 (16), and red RCaMPs (16). Alternatively, astrocytes in vivo or in situ can be transfected with fluorescent markers using the lentiviral system. With the use of this approach, neurons and astrocytes were spectrally separated through neuronal expression of the red fluorescent protein tdTomato and astroglial expression of EGFP (1580).
B. Heterogeneity and Main Types
The class of astroglia embraces many cell subpopulations with radically distinct morphology and function; hence, the matter of astrocytic classification and definition according to structure and function has been always under debate (866). In this respect, we take a rather simplistic approach by recognizing all the cells of neuroepithelial origin that are responsible for regulation of any aspect of CNS homeostasis as astrocytes. According to this logic, the neural cells, the main function of which is myelination, belong to oligodendroglia (including mature oligodendrocytes and their precursors also known as NG2 glia), while microglia are clearly distinct in their myeloid origin.
1. Astroglial numbers
For a long time, there has been a significant confusion about the total number of glial cells and of astrocytes in the CNS of various species, most notably in the human brain. Estimates of (total) glia to neuron ratio in the humans varied wildly from 1:1 to 50:1 (116, 698, 1832), while the belief that neuroglia outnumber neurons by a factor of 10 remains popular in the literature. From this reckoning, another, even more popular, presumption that astrocytes are the most numerous cells in the brain is repeatedly proclaimed (e.g., Refs. 744, 1352, 1491, 1762).3 The morphometry, however, comes with rather different numbers. In rodents, astrocytes account for 10–20% of total cells in the brain (1701). A recently developed method of isotopic fractionation [which was validated by unbiased stereological techniques for humans and macaque monkeys (85)]shows that total numbers of neurons and glia in the human brain are roughly the same, with substantial variations between different brain regions (79, 676, 970). The ratio between nonneuronal cells and neurons varied between 11:1 for brain stem, 3.7:1 in the cortical regions including corpus callosum, and 0.2:1 in the cerebellum (for details of controversial history of glial numbers, see Ref. 1832). Similar figures were obtained with stereology: for example, in the neocortex the average number of neurons was 21.4 billion in females and 26.3 billion in males, whereas the mean number of glial cells was 27.9 billion in females and 38.9 billion in males, which gives an overall glia-to-neuron ratio of ~1.3 (1361). The glial-neuronal ratio (excluding microglia) in the grey matter of the human cortex was estimated at 1.65 (1603). How many of those glial cells are astrocytes? Again, the precise numbers are difficult to obtain due to the limitations of the universality of glial markers. On the basis of morphological criteria, in the neocortex astrocytes accounted for ~20−40%, oligodendrocytes for 50−75% and microglia for 5−10% of the total glial population (197, 1361). Other numerical estimations set microglia at ~10−15% of all glia in the human brain (1130), whereas NG-2 glia account for another 5−10% (386), so both oligodendrocytes and astrocytes account for ~75% of all neuroglia. Assuming substantial predominance of oligodendroglia in the white matter, which occupies more than a half of the human brain (1835), we have to conclude that astrocytes are not, in all likelihood, the major glial population and possibly account for 20−40% of all glial cells.
There is a well-documented increase in the glial density and hence in the number of astrocytes throughout the evolutionary ladder, with glia-to-neuron ratio increasing from ~0.05/0.1 in invertebrates to much higher numbers in mammals. In the cortex the glia-to-neuron ratio is 0.3−0.4 in rodents and rabbit, ~1.1 in cat; ~1.2 in horse, 0.5−1.0 in rhesus monkey, somewhere between 1.5 and >2 in humans, and 4−7.5 in elephants and whales (331, 443, 482, 531, 664, 984, 1300, 1448). Incidentally in singing birds with their remarkable intelligence and computing abilities reflected by an extreme packing density of neurons, the glia-to-neuron ratio is similar to rodents ranging between 0.4 and 0.6 (1262). In primates, the steady increase in astrocyte-to-neuron ratio from 0.6 for Saki monkey to 1.2 in Gorilla gorilla and to 1.65 in Homo sapiens was quantified (1603). An increase in glial-neuron ratio in mammalian evolution most likely reflects an increase in neuronal energy expenditure and hence a need for more support provided by glia; increased synaptic transmission similarly accounts for higher demand for homeostatic clearance as well as maintenance of balance of neurotransmitters and ions.
2. Main types of astrocytes in mammalian brain
a) protoplasmic astrocytes.
Protoplasmic astroglia represent the major population of astrocytes in the grey matter of the brain and of the spinal cord. Rodent protoplasmic astrocytes (FIGURES 6 AND 7) are characterized by a small round somata (~10 μm in diameter) from which 5−10 primary processes (~50 μm long) emanate; these processes branch to form highly elaborated and dense peripheral arborization that underlies distinctive spongiform morphology (264). The overall morphological appearances of protoplasmic astrocytes differ not only between but also within anatomical structure. In the CA1 hippocampal region, fusiform, elongated, and spherical cells were distinguished (264, 1232); many more morphological appearances are observed throughout the brain (476, 1270). Similarly, there is a degree of functional heterogeneity. For example, hippocampal astrocytes have been categorized into two subtypes as GluR cells (which express ionotropic glutamate receptors) and GluT cells (which lack glutamate receptors, but express glutamate transporters) (1088). These subtypes have distinct morphology and membrane properties (1665, 1839). It should be noted, however, that hippocampal GluT-astrocytes express metabotropic GluRs (168, 169, 277, 1335), and there is also evidence that they do express some ionotropic GluRs (154, 1952). Protoplasmic astrocytes in the grey matter contact each other only by their finest processes, thus occupying nonoverlapping territorial domains (264, 1251).
The density of protoplasmic astrocytes ranges between 10,000 and 30,000 per mm3 in different brain areas of rodents, whereas the volume of a single protoplasmic astrocyte lies within a range of 50,000−80,000 μm3. The surface area of protoplasmic astrocyte could be as large as 80,000 μm2 with absolutely the major part of this surface area being associated with peripheral processes (264, 1251, 1451). These peripheral processes are rather short (2−10 μm) and are ultrathin (with thickness of perisynaptic processes being in a range of 100−200 nm). These fine processes exhibit morphological plasticity (672). At least one of the processes of the protoplasmic astrocyte contacts the blood vessel where it forms perivascular endfeet. Protoplasmic astrocytes also send processes to the pial surface, where they form subpial endfeet, which contribute to glia limitans. A single protoplasmic astrocyte in the rodent cortex contacts 4−8 neurons, surrounds ~300−600 neuronal dendrites, and provides cover for up to 20,000−120,000 synapses residing within its domain (264, 627).
b) fibrous astrocytes.
These cells populate white matter of the brain and of the spinal cord, the optic nerve, and the nerve fiber layer of the retina. The somata of fibrous astrocytes are organized in rows between the axonal bundles. Fibrous astrocytes project long (up to 100 μm) processes, which are radially oriented in the direction of the axon bundles (1022, 1248). Fibrous astrocytes have much fewer terminal fine processes as compared with protoplasmic ones; in addition, processes of fibrous astrocytes overlap reflecting the absence of domain organization characteristic for protoplasmic cells. The processes of fibrous astrocytes establish several perivascular or subpial endfeet and send numerous extensions (perinodal processes) that contact axons at nodes of Ranvier. Fibrous astrocytes show diverse morphology; for example, in rodent optic nerve, fibrous astrocytes are subdivided into transverse, random, and longitudinal depending on the orientation of processes with respect to the long axis of the nerve (268). The monoclonal antibody Mab 6.17 raised against homogenates of embryonic rat muscle (which recognizes yet unknown proteins possibly synthesized by Schwann cells and concentrated in the cleft of neuromuscular junctions) was reported to specifically stain fibrous but not protoplasmic astrocytes in the brains of rats (1467).
c) surface-associated astrocytes.
These are represented by a specific subpopulation of astrocytes associated with the cortical surface in the posterior prefrontal and amygdaloid cortex. The somata of these cells are positioned at the cortical surface. The surface-associated astrocytes send two types of processes: one descending to the layer I and the other extending to surround pial vessels (500).
d) velate astrocytes.
Velate astrocytes represent a variation of protoplasmic astrocytes dwelling in the regions of the brain that are densely packed with small neurons, for example, in the olfactory bulb or in granular layer of cerebellar cortex (305). Velate astrocytes have a small soma and relatively short leaflike processes with a very high surface-to-volume ratio (20−30 μm−1). In cerebellum, these processes ensheath several granule neurons (as if forming envelopes of vellum, hence their name). These processes envelop groups of granule neurons and the glomeruli comprising mossy fiber rosettes, Golgi neuron boutons, and granule cell dendrites. Possibly this morphological arrangement allows for isolating synaptic structures and separate groups of mossy fibers conveying different types of information (252, 721, 1301). Cerebellar velate astrocytes show GFAP immunoreactivity (884).
e) pituicytes.
The major type of astroglia of the neurohypophysis is known as pituicytes (661). They express canonical astroglial markers GFAP and S100B. Pituicytes show a remarkable heterogeneity and are subclassified into major, dark, ependymal, oncocytic, and granular pituicytes based on morphological criteria (1723). The neurohypophysis does not contain neuronal cell bodies, but only terminals of magnocellular neurons. These terminals are surrounded by pituicytes, which provide for osmosensitivity and regulate neuropeptides secretion through release of taurine and through dynamic modulation of terminal coverage (754, 1260, 1506). In contrast to other astroglia, pituicytes receive synaptic contacts, and they are sensitive to several neurotransmitters and neurohormones (660).
f) gomori astrocytes.
In the arcuate nucleus of the hypothalamus, the specific set of astrocytes rich in iron and positive for Gomori's chrome alum hematoxylin staining has been recognized (1926). Some Gomori astrocytes have been also identified in the hippocampus (1924). It has been suggested that Gomori astrocytes may supply heme to neurons for the synthesis of heme enzymes (1563). These astrocytes have unusually high glucose metabolism and express high-capacity GLUT2 glucose transporters, which make them comply with the specific metabolic needs of hypothalamic neurons (1925).
g) perivascular and marginal astrocytes.
Perivascular astrocytes are localized close to the pia mater, where they form numerous endfeet with blood vessels. As a rule, they do not contact neurons, and their main function is in establishing the pial and perivascular glia limitans barrier, which assists in isolating the brain parenchyma from the vascular and subarachnoid compartments (996).
h) ependymocytes, choroid plexus cells, and retinal pigment epithelial cells.
These cells line up the ventricles and the subretinal space. The choroid plexus cells produce the cerebrospinal fluid, which fills the brain ventricles, spinal canal, and the subarachnoid space. Ependymocytes are endowed with numerous very small movable processes (microvilli and kinocilia), which by rhythmic movements produce a stream of cerebrospinal fluid (CSF) (1449).
i) radial glia.
Radial glia can be defined as bipolar cells that extend through the whole thickness of the neural tube. These cells form one basal endfoot and one or several pial endfeet. The radial glia was first described by Camillo Golgi as epithelial cells (584) and named radial glia by Giuseppe Magini (1046). The neuroblast potential of these cells was suggested by von Lenhossék and Ramon y Cajal (969, 1426; for more historic details, see Refs. 1424, 1619). The radial glia are thus universal neural precursor cells.
J) radial astrocytes.
The true (primary) radial glia exist only in the developing brain. In the adult mammalian brain several cell types with radial-like morphology populate different regions. These cells are referred to as adult radial glia, radial glialike cells, or radial astrocytes. Considering that their function is invariably associated with local homeostasis we believe that the latter term is more appropriate. In addition, it clearly distinguishes between true radial glia (which primarily have neurogenic function) from adult parenchymal glia with radial morphology.
Retinal Müller cells combine radial morphology with fine processes characteristic of protoplasmic astrocytes (1449). The cell bodies of Müller glia are located in the inner nuclear layer of the retina, and their main processes extend to the subretinal space (these processes are equipped with microvilli) and to the vitreal space (the terminal part of these processes is packed with multivesicular bodies related to secretion). The side processes of Müller cells contact and enwrap neurons (some of these processes have velate morphology), cover synapses (by fine perisynaptic processes), and form endfeet, which plaster blood vessels (1447, 1450). In the human retina, a single Müller cell covers and supports ~16 neurons.
The cerebellar radial astrocytes are represented by Bergmann glia, which have small cell bodies (~15 μm in diameter), are located in the Purkinje cell layer, and extend three to six processes to the pia. These processes send highly elaborated branches with high surface to volume ratio (~20 μm−1) that cover synapses formed by terminals of granule neurons. A single Bergmann glial cell ensheathes 6,000–8,000 synapses (608, 609). Several (~8 in rodents) Bergmann glial cells surround a single Purkinje neuron with Bergmann glial processes forming a “tunnel” around the dendritic arborisation of Purkinje neurons.
Radial astrocytes present in the supraoptic nucleus have cell bodies located at the ventral borders of the nuclei in the ventral glia lamina. These cells send several thick processes in dorsoventral direction often spanning the entire nucleus (211, 773).
The neurogenic niche of the subventricular zone contains a subpopulation of radial glia-like neural stem cells, which express markers of astroglia and of stem cells. These radial stem astrocytes are not only participating in adult neurogenesis but provide for local homeostasis by extending processes to plaster blood vessels and by forming fine perisynaptic processes. These latter mostly cover asymmetric synapses localized within territorial reach of these astrocytes (1124, 1158). Some radial astrocytes were also found in the olfactory bulb and are possibly associated with neurogenesis and migration of young neurons.
An extended class of radial astrocytes found in the periventricular organs, in the hypothalamus, in the hypophysis, and in the raphe part of the spinal cord is represented by tanycytes (1482). Tanycytes [their name coming from Greek verb tanyo (τανυω), which means to stretch or to elongate] are bipolar cells. Their processes contact the ventricular wall and portal capillaries. In the periventricular organs, tanycytes establish the brain-blood barrier by forming tight junctions between their somata. Tanycytes show a degree of heterogeneity in expression of various markers, morphological appearance, and function. Tanycytes localized near to dorsomedial and ventromedial hypothalamic nuclei are classified as α1 and α2 tanycytes, while those localized close to the arcuate nucleus and median eminence are defined as β1 and β2 tanycytes (18, 362, 1474, 1482). Hypothalamic tanycytes can, potentially, be stem cells underlying adult neurogenesis; tanycytes have been found to express several markers of stem cells including nestin (108), doublecortin-like proteins (1523), bromodeoxyuridine (BrdU), and Sox2 (957).
3. Human astroglia
a) protoplasmic and fibrous astrocytes.
Human parenchymal astrocytes are much larger and far more complex than astroglial cells in lesser vertebrates (FIGURE 8). The average diameter of the domain belonging to a human protoplasmic astrocyte is ~2.5 times larger than the domain formed by a rat astrocyte (142 vs. 56 μm). The volume of the human protoplasmic astrocyte domain is ~16.5 times larger than that of the corresponding domain in a rat brain. Furthermore, human protoplasmic astrocytes have ~10 times more primary processes emanating from their somata, and correspondingly much more complex processes arborisation (1248). As a result, human protoplasmic astrocytes contact and integrate around 2 million of synapses residing in their territorial domains, whereas rodent astrocytes cover ~20,000–120,000 synaptic contacts (264, 1248). Likewise, the fibrous astrocytes, populating the white matter, are ~2.2 times larger in humans than in rodents.
FIGURE 8.
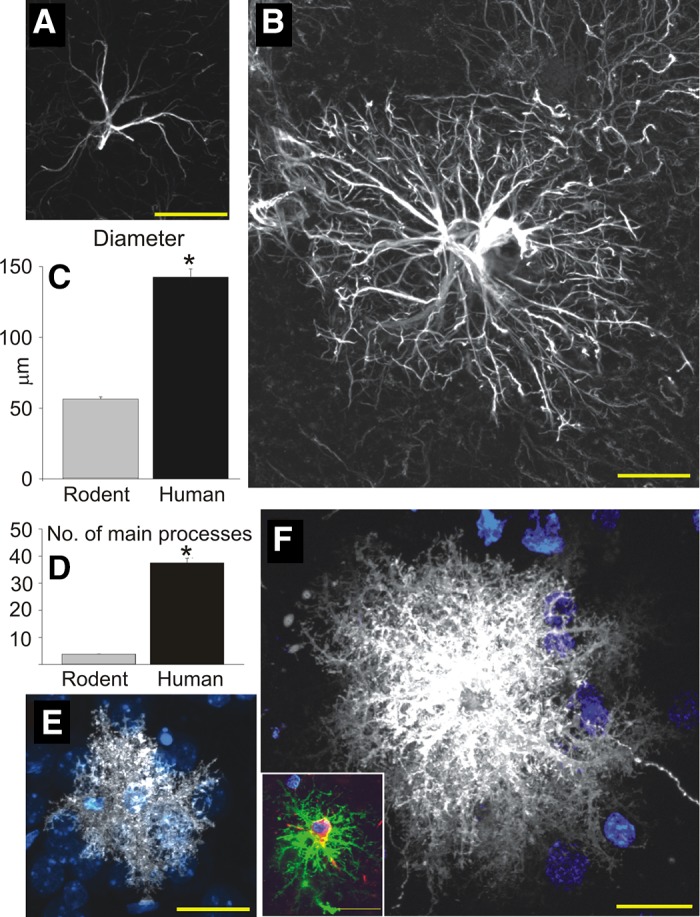
Comparison of rodent and human protoplasmic astrocytes. A: typical mouse protoplasmic astrocyte. GFAP, white. Scale bar, 20 μm. B: typical human protoplasmic astrocyte in the same scale. Scale bar, 20 μm. C and D: human protoplasmic astrocytes are 2.55-fold larger and have 10-fold more main GFAP processes than mouse astrocytes (human, n = 50 cells from 7 patients; mouse, n = 65 cells from 6 mice; means ± SE; *P < 0.005, t-test). E: mouse protoplasmic astrocyte diolistically labeled with DiI (white) and sytox (blue) revealing the full structure of the astrocyte including its numerous fine processes. Scale bar, 20 μm. F: human astrocyte diolistically labeled demonstrates the highly complicated network of fine process that defines the human protoplasmic astrocyte. Scale bar, 20 μm. Inset: human protoplasmic astrocyte diolistically labeled as well as immunolabelled for GFAP (green) demonstrating colocalization. Scale bar, 20 μm. [From Oberheim et al. (1248).]
b) interlaminar astrocytes.
Interlaminar astrocytes [the name introduced by Jorge Colombo (350)] have been originally described by Carlo Martinotti, William Lloyd Andriezen (who named them “caudate neuroglia fibre cells”), and Gustav Retzius as cells with small cell bodies, residing in the upper cortical layer and sending long processes towards deeper layers (44, 1074, 1458). Many decades later it was found that these astroglial cells exist only in the brains of higher primates including old world monkeys, apes, and humans (347, 349, 350). Human interlaminar astrocytes (FIGURE 9) have a small spheroid cell body (~10 μm) localized in the supragranular layer (or layer I) of the cortex and several short and one or two very long (up to 1 mm) processes, which penetrate through the cortex, and end in layers II to IV (1248). The density of interlaminar astrocytes in human brain is quite high and is higher than in chimpanzee (1248). Processes of interlaminar astrocytes often demonstrate a spiriform or corkscrew pattern. The processes terminate in the neuropil and occasionally on the vasculature (1248); terminal portions of these processes end up with specific boutonlike structures also known as terminal masses or end bulbs, which usually contain mitochondria. The processes of interlaminar astrocytes run parallel to each other forming a “palisade.” The phylogenetic origin of interlaminar astrocytes remains unknown, although they were found in brown lemur, Eulemur fulvus (348), indicating that they could have appeared ~30 million years ago in prosimians. The interlaminar astrocytes appear postnatally and are thought to originate from some astroglial precursors and not directly from radial glia (349). The interlaminar astrocytes are specifically stained with CD44 antibodies; they have high immunoreactivity for GFAP and S100B and have low expression of glutamine synthetase and glutamate transporters (1655). Long processes of interlaminar astrocytes contact several blood vessels as well as the pia and traverse territorial domains of protoplasmic astrocytes. Electrophysiologically interlaminar astrocytes displayed passive currents similar to other types of astroglia (1655). The specific function of these cells remains unknown, although they might be instrumental in connecting distant cells and integrating cellular groups into wider structures.
FIGURE 9.
Interlaminar and varicose projection astrocytes in human cortex. A: interlaminar astrocytes as seen by William Lloyd Andriezen (44). B: pial surface and layers 1–2 of human cortex. GFAP staining is in white; DAPI is in blue. Scale bar, 100 μm. Yellow line indicates border between layer 1 and 2. C: interlaminar astrocyte processes. Scale bar, 10 μm. [B and C from Oberheim et al. (1247), with permission of Springer.] D: varicose projection astrocytes reside in layers 5–6 and extend long processes characterized by evenly spaced varicosities. Inset: varicose projection astrocyte from chimpanzee cortex. GFAP, white; MAP2, red; DAPI, blue. Yellow arrowheads indicate varicose projections. Scale bar, 50 μm. E: diolistic labeling (white) of a varicose projection astrocyte whose long process terminates in the neuropil. Sytox, blue. Scale bar, 20 μm. F: high-power image of the yellow box in B, highlighting the varicosities seen along the processes. Scale bar, 10 μm. G–I: individual z-sections of the astrocyte in E, demonstrating long processes, straighter fine processes, and association with the vasculature. [D–I from Oberheim et al. (1248).]
c) polarized astrocytes.
These cells are similarly present only in the brains of primates. Their somata are positioned in the deep cortical layers very near to the white matter. These cells have one or two long (up to 1 mm in length) processes that penetrate into superficial cortical layers (1248).
d) varicose projection astrocytes.
The varicose projection astrocytes exist only in the brains of humans (FIGURE 9). These cells are characterized by several (up to 5) very long (up to 1 mm) unbranched processes that extend in all directions through the deep cortical layers. These processes are endowed with evenly spaced varicosities (1248). These cells are similar to atypical protoplasmic astrocytes, described in adult human brains. These cells acquire immunopositivity to CD44, while their numbers vary considerably between individual samples; they are never observed in neonatal brains (1655). Morphologically approximately one-third of these CD44-positive cells were similar to regular protoplasmic astrocytes; some (~10%) had decreased arborization, while ~60% had long and thin processes (1655). The atypical astrocytes phenotype was suggested to reflect individual age-dependent, possibly adaptive, changes.
e) human astrocytes and intelligence.
The possible contribution of astrocytes to the information processing remains a debatable question. It has been hypothesized that astrocytes represent the smallest computing unit of the brain, the microchip, for integration of synaptic input. This hypothesis is based on the observation that astrocytes in visual cortex generate Ca2+ signals to a shorter tuning range of light than neurons. This indicates that astrocytes are more selective than neurons to the visual input and that astrocytic Ca2+ spikes represent processed second level information (1581). Since the volume of human astrocytes is 15- to 20-fold larger than in the rodents, a single astrocytic domain covers many more synapses in the human brain. It is therefore tantalizing to ask whether integration of information by astrocytes constitutes an essential part of processing power and in part explains human intelligence. To start addressing this question, the humanized mice pups were developed by intra-brain implantation of human glial cell progenitors at postnatal day 1 (637). The human glial cells expanded, populated large parts of the chimerized mice brain, and replaced mouse glial progenitor cells (FIGURE 10). Further analysis demonstrated that the threshold for induction of long-term potentiation (LTP) in hippocampus was reduced, in a pathway partly driven by tumor necrosis factor (TNF)-α, but not by release of d-serine or adenosine. The humanized chimeric mice displayed enhanced memory and ability to learn in cognitive tests, including novel object recognition or auditory fear conditioning. Several parallel lines of work have similarly shown that human glial progenitors, independently of whether they are generated from embryonic tissue or from induced stem cells, exhibit a growth advantage and slowly replace the host murine glial progenitor cells and mature astrocytes (582, 1885).
FIGURE 10.
Human astrocytes replace host glia in mice engrafted with human glial progenitors. A: schematic outlining the procedure for magnetic cell sort-based isolation (MACS) of human glial progenitors, tagging with EGFP and xenografting at P1. The chimeric mice brains were analyzed in 0.5- to 20-mo-old chimeric mice. B: representative dot map showing the distribution of human nuclear antigen (hNuclei)+ cells in 3 coronal sections from a 10-mo-old human chimeric mouse. C: the complex fine structure of human astrocytes in chimeric brain replicates the classical star-shaped appearance of human astrocytes labeled with hGFAP in situ. Most cells in the field are EGFP+/hNuclei+/hGFAP+ (hGFAP, red). D: at 5 mo, EGFP+ cells typically infiltrated corpus callosum and cortical layers V and VI. All EGFP+ cells labeled with an antibody directed against human nuclear antigen (hNuclei) and most of the human cells were also labeled with an antibody directed against human GFAP (hGFAP, red). E: at 11 mo, many areas of cortex were infiltrated by evenly distributed EGFP+ /hNuclei+ cells. F: the hippocampus was also populated with EGFP+/hNuclei+ cells in a 14-mo-old animal, with the highest density in the dentate. G: human EGFP+/hNuclei+/GFAP+ cells (green arrow) were significantly larger than host murine astrocytes (red arrow). The anti-GFAP antibody cross-reacted with both human and mouse GFAP (red). Inset shows same field in lower magnification. H: histogram compares the diameter of mouse cortical astrocytes to human cortical astrocytes in situ (freshly resected surgical samples) and xenografted human astrocytes in cortex of chimeric mouse brain. The maximal diameter of mouse and human astrocytes (in situ and in chimeric mice) was determined in sections stained with an anti-GFAP antibody that labels both human and mouse GFAP. In B–F, n = 50–65; **P < 0.01, Bonferroni t-test. EGFP, green; hNuclei, white and white arrow; DAPI, blue. Scale bars: 50 μm (C); 100 μm (D–F); 10 μm (G). Data graphed as means ± SE. [From Han et al. (637).]
C. Gender Differences
There are multiple indications for sexual dimorphism of astroglia (6). Gonadal hormones regulate the expression of GFAP, astroglial numbers, and morphology. Steroid deficiency causes astroglial atrophy in hippocampus, whereas treatment of organotypic cultures with estradiol and testosterone increases astrocyte complexity and extension of their processes (409). In young rats for example, the total number of GFAP-positive hippocampal astrocytes was higher in males, whereas feminization or masculinization of these animals reversed this difference (351). In adult rats, the GFAP immunoreactivity and astroglial numbers were highest in proestrus females compared with males or diestrus females; moreover, male astrocytes displayed more stellate, whereas female astrocytes had more reactive morphological appearance (63). In the somatosensory cortex, GFAP immunoreactivity and astroglial processes density are maximal in both proestrus and diestrus, while they decrease significantly during estrus (1685). In medial amygdala of adult rats, the number and complexity of astrocytes were higher in males, when compared with females or with testicular feminized mutant males lacking functional androgen receptors (798). Similarly, male astrocytes are more complex and have longer and more numerous processes in preoptic area (33), in nucleus arcuatus (1143), and in supraoptic nucleus (1692). In the cerebellum, Bergmann glial cells in males demonstrated more immunoreactivity for GFAP, whereas in females they showed more immunoreactivity for vimentin (1693). Sexual dimorphism was also extended to cellular functions; for example, female astrocytes in culture demonstrated more efficient glutamate uptake (1153).
D. Astroglial Networks or Syncytia
Macroglia (that is astrocytes and oligodendrocytes) represent the reticular portion of the brain being intracellularly connected into functional syncytia by gap junctions. The latter are ubiquitous structures responsible for intercellular integration in many tissues connecting, for example, epithelial cells of the gastrointestinal tract and kidney, providing metabolic coupling in the liver, electrical coupling in the heart, intercellular signaling in endocrine tissues, and defining cochlear physiology and hence hearing (217, 638, 1216, 1488). Gap junctions are specialized areas where two apposing membranes of adjacent cells come very close together so that the intercellular cleft is reduced to a width of ~2–3 nm (493). Within these areas, each gap junction is made of many hundreds of intercellular channels or connexons, which provide for intercellular transport of ions, second messengers, and other biologically active molecules with smaller than 1,000 Da (see sect. VIIH). In the grey matter pairs of astrocytes are connected with ~230 gap junctions on average, and injection of Lucifer yellow or biocytin into a single astrocyte results in staining of ~50–100 adjacent astroglial cells.
The earlier concept of panglial syncytium that connects all macroglia into single functional network, although being confirmed for invertebrates (1163), does not fully apply to mammalian CNS. First, the gap junctions between oligodendrocytes are rather restricted (1438). Second, in many brain regions anatomically segregated astroglial networks follow anatomical structures; for example, astroglial syncytia are confined to individual barrels of somatosensory cortex or to individual glomeruli in the olfactory bulb (571, 739, 1509). Coupling between adjacent astrocytes is also not ubiquitous; some (probably up to 15–20%) of the neighboring astrocytes are uncoupled as judged by either dye diffusion or by double patch-clamp recordings from the pairs of closely apposed cells (740, 1103). Thus astroglial coupling is defined not only by spatial proximity but also by some other factors, and astroglial networks may represent a wiring system parallel to that formed by neurons. The gap junctional connectivity may also represent a specialized “analog” intercellular signaling system (alternative to “binary” interneuronal communication), which by utilizing intercellular diffusion of multiple molecules, can provide a second level of information processing in the CNS.
Panglial syncytia, which connect astrocytes and oligodendrocytes, have been visualized in thalamus, neocortex, and hippocampus (604). Gap junction plaques between oligodendrocytes and astrocytes, localized on oligodendroglial somata, processes and outer (abaxonal) layer of the myelin sheath have been visualized with freeze-fracture electron microscopy (1079, 1437). Coupling between astrocytes and oligodendrocytes was also confirmed electrophysiologically (in pair recordings) and by the dye coupling (270, 856, 1337, 1477). The functional role of panglial connectivity remains largely unknown; an obvious suggestion links this to K+ homeostasis (1105). Mutations in oligodendroglial/astroglial connexins cause severe deficits in myelination in white matter (1281). Mutation in the gene GJB1, which encodes Cx32, underlies Charcot-Marie-Tooth disease associated with CNS white matter lesions (963, 1736), whereas mutations in GJC2 (encoding Cx47) are linked to leucoencephalopathies (253) and hypomyelinating leukodystrophies known as Pelizaeus-Merzbacher disease (1774). In mice, genetic deletion of either Cx32 or Cx47 does not result in an obvious phenotype; elimination of both genes however causes severe white matter demyelination (1104).
E. Recapitulation
Astrocytes display a morphological heterogeneity comparable to that of neurons; therefore, detailed mapping of gene expression and functional idiosyncrasies of astrocytes in different parts of the brain is of paramount importance. This task is far from being trivial as no universal marker of astroglial cells has yet been discovered; neither the ideal astroglia-specific promoter allowing expression of reporter proteins has been identified. Astroglial syncytia with their anatomical segregation add another level of complexity providing anatomically predetermined communication channels. Most intriguing is the evolution of astrocytes in higher primates and in humans; these astrocytes are distinguished by their complexity, size, and specific cell types. The idiosyncracies of human glia are cell autonomous and they persist even after grafting human fetal astrocytes into a non-human host brain; moreover, human cells outcompete astrocytes of the host, populate the brain, and confer improved cognitive performance.
VI. ASTROGLIAL MEMBRANE PHYSIOLOGY: ION DISTRIBUTION AND MEMBRANE POTENTIAL
A. Ion Distribution
Similarly to all living cells there is a disparity between cytosolic and extracellular ion concentrations in astrocytes (FIGURE 11). Conceptually cytosolic ion concentrations are defined by respective membrane permeability, by energy-dependent active transport and, in case of Ca2+, by cytosolic specific buffers. Intra-astrocytic concentration of K+ lies between 120 and 140 mM, while K+ concentration in the CSF and in the interstitial fluid (ISF) is ~3 mM, being substantially lower than in the plasma (639, 801). This sets the equilibrium potential for K+ (EK) at −98 mV (at 37°C). Concentration of cytosolic Na+ in astrocytes (15−20 mM) is generally higher than in the majority of neurons (8−10 mM), although in some neurons [Na+]i may also be as high as ~15 mM (1499). Only few actual measurements of resting [Na+]i in astroglia exist, and they show substantial variability ranging from 9−19 mM in cultured cells (851, 1461, 1496, 1498, 1500) to 15−20 mM in brain slices (940, 1777); in vivo recordings are still pending. With Na+ concentration in the CSF around145−155 mM, the corresponding ENa ranges between +55 and +60 mV. It has to be noted that [Na+]CSF may undergo rhythmic and substantial (by 10−40 mM) fluctuations (648). Concentration of ionized Ca2+ in the cytoplasm of astrocytes ranges between 50 and 150 nM, being somewhat higher than in neurons (1950). With the assumption of [Ca2+]CSF in adult rodents around 1.4 mM (800), the ECa lies between +120 and +140 mV. Astroglial distribution of Mg2+, the second major divalent cation controlling multiple cellular functions, has been poorly investigated. Cytosolic concentration of free Mg2+ in cultured astrocytes measured with fluorescent probe Mag-fura 2 was around 125 μM (81); the CSF Mg2+ has been determined at ~0.9 mM [thus being substantially less than in serum (1699)] and hence EMg is ~25 mV.
FIGURE 11.
Ion distribution and corresponding values of equilibrium potentials for different ions between the cerebrospinal fluid, interstitial space, and cytoplasm of astrocytes and neurons.
It is generally believed that astrocytes contain relatively high concentration of Cl− ions (when compared with 5 mM in neurons), which varies between 30 and 60 mM, with ECl being set around −35 mV (the [Cl−]o is ~120 mM). High [Cl−]i was deduced from membrane potential recordings, in which activation of GABAA receptors (that are essentially Cl− channels) always depolarized cultured astrocytes (857), and the apparent ECl was calculated at approximately −35 mV. With the use of gramicidin perforated patch clamp with different Cl− concentrations in the pipette the [Cl−]i was estimated at ~29 mM (126). The radioactive Cl− extrusion assay estimated resting [Cl−]i between 31 and 43 mM (865), whereas ion-sensitive microelectrode measurements found [Cl−]i to be between 20 and 40 mM (854). In Bergmann glial cells in situ, [Cl−]i (measured with chloride-sensitive probe MQAE) was around 50 mM in young (P5 to P6) mice and ~35 mM in animals aged between P13 and P100 (1778). High astroglial Cl− is supposedly maintained by activity of various Cl− transporters, such as for example Na+/K+/2Cl− (NKCC1) cotransporters that move 2 Cl− into the cell in exchange for 1 K+ and 1 Na+. It has to be noted, however, that high NKCC1 activity in cultured astrocytes contrasts to a rather low performance of this transporter in the in situ hipocampal preparation (942). Hence, direct measurements of astroglial [Cl−]i in situ or in vivo in different types of astroglia are of paramount importance; these measurements, however, are yet to be done. The concentration of protons in astroglial cytoplasm is ~63 nM (pH 7.2), which, assuming the extracellular H+ concentration to be ~40 nM (pH 7.4) sets the EH at approximately −12 mV. The cytoplasm of astrocytes is rich in CO2 (~1.2 mM) and HCO3− (~17 mM).
B. Membrane Potential
The most characteristic electrophysiological signature of mature astrocytes is their hyperpolarized resting potential that is set close to EK (approximately −80 mV) and low input resistance (5−20 MΩ) indicative of high resting membrane permeability for K+ (379, 1125, 1126). This is also reflected by nearly linear current to voltage relationship, which is the most characteristic electrophysiological signature of astroglia (FIGURE 12; Refs. 11, 337, 436, 455, 772, 811, 1012, 1265). Incidentally neonatal astrocytes are even more hyperpolarized (Vm around −85 mV) (1951). Some variability of resting Vm (between −85 and −25 mV) reported for astrocytes in culture, in organotypic and acute slices, and in the optic nerve (208, 917, 1096), most likely reflect preparation artifact. These membrane properties are intrinsic for astrocytes as isolated cells are very similar to syncytially connected cells in the nervous tissue (454); astrocytes in the syncytia are isopotential (1027). Fluctuations of astroglial Vm generally reflect changes in extracellular K+ concentration (36, 379).
FIGURE 12.
Passive membrane properties of astrocytes. A: voltage-clamp recordings from astrocytes freshly isolated from the cortex of transgenic mice expressing EGFP under control of the GFAP promoter. Astrocytes were identified by specific fluorescence; whole-cell currents were recorded in response to hyperpolarizing and depolarizing steps from −120 to +60 mV (step interval, 20 mV). To construct the current-voltage relationship, amplitudes of currents were normalized to the value measured at 0 mV; every point is mean ± SD for 20 cells. [From Lalo et al. (935).] B: voltage-clamp recordings from astrocytes in acute slices obtained from 3- and 20-mo-old GFAP-EGFP mice; astrocytes were identified by fluorescence. [From Lalo et al. (932).] C: voltage-clamp recordings from human astrocytes grafted into mouse brain. [From Han et al. (637).]
C. Recapitulation
Despite prominent morphological heterogeneity, astrocytes show remarkably similar electrophysiological signatures with hyperpolarized membrane potential and predominant passive K+ permeability. The negative and stable resting membrane potential of astrocytes influences their homeostatic capabilities through defining the electrodriving force for numerous membrane transporters.
VII. ION CHANNELS
A. Potassium Channels
1. Inward rectifier potassium channels, Kir
Inward rectifying K+ channels, which pass K+ ions more easily into the cell (the inward direction) than out of the cell (the outward direction) have two transmembrane domain topology, assemble as homo- or heteromeric tetramers, and are represented by 16 subtypes encoded by distinct genes (KCNJ1−KCNJ18) classified into 7 families from Kir1.x to Kir7.x (695, 914). The Kir1.x, Kir4.x, Kir5.x, and Kir7.x channels are weakly rectifying channels; Kir2.x are strongly rectifying constitutively active channels; Kir3.x are G protein-coupled channels; whereas Kir6 subunits assemble with sulfonylurea receptors (SUR, of which 4 isoforms exist) to form ATP-gated K+ channels. Channel rectification reflects a voltage-dependent block of these channels by Mg2+ and polyamines (1016). Inward rectifying channels in general have high open probability at potentials more negative than resting Vm; this open probability decreases with depolarization.
The Kir4.1 (encoded by KCNJ10 gene) is the main type of astroglial inwardly rectifying K+ channel. It is functionally expressed in parenchymal astrocytes and radial astrocytes including retinal Müller cells and cerebellar Bergmann glia (TABLE 2 and Refs. 269, 696, 770, 817, 1646). Inward rectifying K+ currents were recorded from cultured and freshly isolated astrocytes from hippocampus, neocortex, optic nerve, cerebellum, spinal cord, and retina; the early experiments revealed their characteristic voltage dependence, sensitivity to extracellular K+, and inhibitory effects of extracellular Cs+ and Ba2+ (107, 1242, 1434, 1647, 1763). Astroglial inwardly rectifying currents are also modulated by extracellular Na+: reducing the latter significantly diminished Kir amplitude (1435). The Kir4.1 channels are present in forebrain, midbrain, and hindbrain with predominant astroglial localization (696), although distribution of Kir4.1 channels has regional variability; for example, immunoreactivity for Kir4.1 is prominent in many hippocampal astrocytes, in astroglial cells in the cerebral cortex, in the deep cerebellar nuclei, in Bergmann glia, and in Müller cells but not in astrocytes in white matter (1397). This latter finding is somewhat controversial as Kir4.1 immunoreactivity was detected in white matter astrocytes in the optic nerve (817). In the spinal cord, expression of Kir4.1 channels and density of inward rectifier K+ currents are the highest in astrocytes from ventral horn and the lowest in astrocytes from the apex of the dorsal horn (1264). At the cellular level, Kir4.1 channels are usually concentrated in perivascular endfeet, in perisynaptic processes of astrocytes (696), and in the radial processes of Bergmann glia (229). The anatomical segregation of Kir4.1 was also demonstrated by double staining with dystrophin (specifically localized in the membranes of perivascular endfeet) and with tomato lectin, which labels vascular endothelial cells (1154). Not all perisynaptic processes express Kir4.1 however: in olfactory bulb for example more than a half of astroglial processes surrounding excitatory synapses were immunopositive for Kir4.1 (696). Concentration of Kir4.1 channels in the processes is developmentally regulated and gradual shift of Kir4.1 from the soma to the periphery has been observed postnatally between P0 and P60 (1154). The overall density of Kir4.1 channels substantially (severalfold) increases during postnatal development (1588). Studies on cellular and subcellular localization of Kir4.1 channels have to be treated with care, however, because of the lack of perfectly specific antibodies. The Kir4.1 channels significantly contribute to the resting astroglial membrane conductance; inhibition of Kir currents with Ba2+ increases input resistance up to 20-fold and depolarizes astroglia (1434). Genetic deletion of Kir4.1 channels in vivo or inhibition of their transcription in vitro increases the input resistance and depolarizes resting potential by 15−20 mV in protoplasmic astrocytes (1265, 1588) and in Müller glia (891). In uncoupled (by 30 min incubation with gap junction blocker carbenoxolone), astrocytes in slices 100 μM Ba2+ reduced the membrane conductance by ~50% and depolarized the membrane from −70 to −60 mV (1588). The degree of rectification of Kir4.1 channels can be regulated by endogenous polyamines such as spermine and spermidine (1627). An elevation in extracellular K+ concentration increases the inward K+ currents proportionally to the square root of [K+]o (1269, 1434).
Table 2.
Channels in astrocytes
| Current or Channel Type/Subunit | Experimental Preparation/Technique | Pharmacology | Biophysical Properties and Functional Relevance | Reference Nos. |
|---|---|---|---|---|
| Voltage-gated sodium channels | ||||
| INa | Rat/cultured primary astroglia/whole cell voltage clamp | TTX (KD ~0.5 mM), STX (KD ~30 nM) | First detection of fast voltage-activated Na+ currents in astrocytes; INa amplitudes were ~1 nA. Authors suggested that astrocytes synthesize Na+ (and K+) channels for subsequent insertion into the neighboring axon. | 166 |
| INa | Rat/cultured primary astroglia/optic nerve/whole cell voltage clamp | TTX (KD ~2.8 nM) | INa was detected in the majority of type 2 astrocytes and in many type 1 cells; respective current amplitudes were 400–3,100 pA and 10–300 pA. Glial INa had slower kinetics when compared with neuronal currents. In type 1 astrocytes, the steady-state inactivation curve was significantly shifted into hyperpolarizing direction with h∞ ~-80 mV. | 105, 106 |
| INa fast, INa slow | Rat/cultured primary astroglia/optic nerve, hippocampus/whole cell voltage clamp | Two types of INa with distinct (fast and slow) kinetics and steady-state inactivation were dissected. With increasing time in culture, slow current became dominant in hippocampal cultures. | 1649, 1650 | |
| INa TTX sensitive, INa TTX resistant | Rat/cultured primary astroglia/optic nerve, hippocampus/whole cell voltage clamp | TTX (KD ~5.7 nM), TTX (KD ~1,007 nM) | The TTX-sensitive INa was confined to stellate astrocytes, whereas TTX-resistant INa dominated in fibroblast-like astrocytes. | 1647, 1652 |
| INa slow | Rat/tissue prints/optic nerve, hippocampus/whole cell voltage clamp | TTX (10 μM) | In tissue prints of astrocytes at P2, INa was not detected; in contrast, at P10, all astrocytes demonstrated slow INa. | 107 |
| INa | Rat/acute slices/hippocampus/whole cell voltage clamp | INa was recorded from a minor subpopulation (5 of 40) of GFAP-positive astrocytes. | 1651 | |
| INa | Rat/acute slices/spinal cord/whole cell voltage clamp | INa with amplitudes of 20–55 pA was recorded from a subpopulation of cells with small somata and long processes with faint GFAP staining. | 337 | |
| Nav1.5, Nav1.2, Nav1.3 | Rat/cultured primary astroglia/spinal cord/RT-PCR, in situ hybridization, immunocytochemistry | The Nav1.5 identified as the predominant type of astroglial Na+ channels. | 185, 189 | |
| Nav1.6 | Rat/cultured primary astroglia/spinal cord/immunocytochemistry | The Nav1.6 expression was detected in embryonic cultures; immunoreactivity was confined to stellate cells only. | 1444 | |
| Non-voltage-gated sodium channels | ||||
| Nax | Rat | The Nax channel is expressed in astrocytes and ependymocytes in circumventricular organs. The channel is activated by an increase in [Na+]o above 150 mM; the activation threshold is reduced to ~140 mM in the presence of endothelin-3 acting through ETBRs. | 1234, 1613, 1864 | |
| ENaC | Rat/tissue section/immunocytochemistry | The ENaC γ-subunits were found in GFAP-positive astrocytes using specific antibodies. | 1116 | |
| Voltage-gated calcium channels | ||||
| L-type Ca2+ currents | Rat/cultured primary astroglia/cortex/two-electrode patch clamp | Co2+ (2–10 mM), Cd2+ (1 mM), nifedipine (10 μM) | Ba2+-dependent action potentials or Ba2+ current were measured from individual astrocytes; at 10 mM Ba2+, maximal amplitudes were at nA range. Norepinephrine or dbt-cAMP enhanced or induced Ba2+ currents. | 1038, 1041 |
| L-type Ca2+ currents | Rat/cultured primary astroglia/cortex/whole cell patch clamp | Nifedipine (100 μM) | Ca2+ currents with maximal amplitudes ~100–200 pA were recorded only after cell preincubation for 1–2 h with forskolin or 8-bromo-cAMP. | 104 |
| L- and T-type of Ca2+ currents | Rat/cultured primary astroglia/acutely isolated astrocytes/tissue prints/optic nerve/whole cell patch clamp | High (L) and low (T) Ca2+ currents with maximal amplitudes of 50–200 pA were recorded from the majority of neonatal astrocytes. With age (at P30), T currents disappeared and L currents were detected only in 37% of cells. | 106, 107 | |
| L-type Ca2+ currents | Rat/cultured primary astroglia/acutely isolated astrocytes/hippocampus/ [Ca2+]i microfluorimetry | Co2+ (1 mM), verapamil (>30 μM), nifedipine (100 μM), BAY K 8644 (10 μM) | High K+ depolarization triggered transient elevations in [Ca2+]i reaching ~1 mM; these were strictly dependent on [Ca2+]o, where inhibited by Ca2+ blockers and potentiated by BAY K 8644. | 460, 1040 |
| L-, N-, and R-type Ca2+ currents | Rat/cultured primary astroglia/cortex/whole cell or perforated patch clamp | Nifedipine (5 μM), ω-conotoxin GVIA (3 μM), SNX-482 (100 nM) | Ba2+ whole cell and single-channel currents showed complex pharmacological behavior. Whole cell currents were partially sensitive to L-, N-, and R-type Ca2+ blockers. RT-PCR revealed expression of relevant α1 subunits. | 374 |
| L-type Ca2+ currents | Rat/cultured primary astroglia/hippocampus/ [Ca2+]i microfluorimetry | Cd2+ (100 μM) | High K+ depolarization triggered transient elevations in [Ca2+]i, which instigated vesicular release of glutamate. RT-PCR revealed expression of mRNAs for α1B, α1C, α1D, and α1E VGCC subunits. | 1909 |
| Cav1.2, Cv1.3 channels | Mouse/primary culture/cortex/[Ca2+]i microfluorimetry | Verapamil (5 μM), nifedipine (5 μM) | High K+ depolarization induced [Ca2+]i transients, which disappeared after removal of extracellular Ca2+. Expression of mRNA for Cav1.2 and Cav1.3 was detected at mRNA and protein levels. In Cav1.2 KO mice, [Ca2+]i transients were reduced by 80%. | 314 |
| L-type Ca2+ channels | Rat/acute slices/ventrobasal thalamus/[Ca2+]i microfluorimetry | Nifedipine (1 μM), BAY K 8644 | Spontaneous astroglial [Ca2+]i oscillations were potentiated by BAY K 8644 and inhibited by nifedipine. | 1331, 1332 |
| Ca2+ release-activated Ca2+ channels (ICRAC/ORAI) | ||||
| ORAI1 | Rat/primary culture/cortex/[Ca2+]i microfluorimetry, RT-PCR, immunocytochemistry | Expression of ORAI1 and STIM1 was detected at mRNA and protein level; overexpression of ORA1/STIM1 increased, whereas siRNA KO decreased thrombin-induced SOCE. | 1150 | |
| ORAI3, ORAI2 | Rat/primary culture/hippocampus/[Ca2+]i microfluorimetry, RT-PCR | Pyr6 (5μM) | Expression of ORAI3 mRNA was predominant, exceeding expression of ORAI2 6 times; ORAI1 was not detected at all. | 1492 |
| TRP channels | ||||
| TRPA1 | Rat/tissue sections/trigeminal caudal nucleus/immunogold electron microscopy | TRPA1 immunoreactivity was detected in the somata and processes of astrocytes; immunoreactivity increased significantly in the fine process of astrocyte in rats with experimental inflammation of the temporomandibular joint. | 965 | |
| TRPA1 | Rat/primary cultures, acute slices/hippocampus/[Ca2+]i microfluorimetry, whole cell voltage clamp | Gd3+, Li3+, TRP antagonist HC030031 (40 μM), TRPA1 agonist AITC (1 μM) | Constitutive activity of TRPA1 channels was found to trigger “spotty” near-membrane Ca2+ microdomains and contributed to resting [Ca2+]i. | 1611 |
| TRPV1 | Rat/brain, spinal cord/immunocytochemistry, immuno-EM | TRPV1 channels were identified in astrocytes throughout the CNS. TRPV1 protein was preferentially localized in astroglial processes. | 442, 1755 | |
| TRPV1 | Rat/primary culture/whole cell patch clamp, immunocytochemistry | Capsazepine (30 μM) | TRPV1 channels were detected immunocytochemically; ~90% of all cell demonstrated TRPV1-mediated currents. | 746 |
| TRPV4 | Rat/primary culture/whole cell patch clamp/[Ca2+]i microfluorimetry, immunocytochemistry, RT-PCR | TRPV4 agonist 4α-phorbol 12,13-didecanoate, 4αPDD (3 μM) | TPPV4 were detected at transcript and protein levels. Astrocytes generated TRPV4-mediated currents and [Ca2+]i transients. | 135 |
| TRPV4 | Rat/hippocampus/acute slices, primary cultures/whole cell patch clamp/[Ca2+]i microfluorimetry, immunocytochemistry, Q-PCR | TRPV4 agonist 4αPDD (5 μM) | Postischemic astrogliosis was associated with an increase in TRPV4 expression, TRPV4-mediated currents, and [Ca2+]i transients | 265 |
| TRPC1, TRPC4, TRPC5, | Mouse embryo/primary culture, acutely isolated astrocytes/cortex/[Ca2+]i microfluorimetry, immunocytochemistry, RT-PCR | Members of TRPC family were detected at transcript and protein level. Treatment with anti-TRPC1 inhibiting antibody suppressed astroglial [Ca2+]i transients, likely due to an inhibition of SOCE | 587, 606, 1055, 1393, 1462 | |
| TRPC6 | Mouse/primary culture/[Ca2+]i microfluorimetry | In vitro knockdown of TPRC6 reduced receptor-induced Ca2+ entry. Treatment of astrocytes with IL-1β for 24–48 h increased TRPC6 expression and disrupted Ca2+ homeostasis. | 163 | |
| Acid-sensitive ion channels, ASICs | ||||
| ASIC1, ASIC 2a, ASIC3 | Rat/primary culture/cortex/[Ca2+]i microfluorimetry, whole cell voltage clamp | ASICs expression was confirmed with immunocytochemistry and Western blotting; ASICs were mainly localized to the nuclei. | 746 | |
| Inward rectifying potassium channels | ||||
| Inward rectifying K+ current | Rat/acutely isolated astrocytes/hippocampus/whole cell patch clamp | Ba2+ (10 mM) | The inwardly rectifying K+ currents were isolated after inhibiting other K+ conductances with a mixture of TEA and 4-AP. | 1763 |
| Inward rectifying K+ current | Rat/primary cultures/spinal cord/whole cell patch clamp, cell-attached patch clamp | Cs+ (KD 189 μM), Ba2+ (KD 3.5 μM), TEA (90% block at 10 mM) | Two types of single-channel currents with conductances of 28 and 80 pS were detected. The open probability was largest at EK. | 1434 |
| Kir4.1 channels | Rat/primary culture/acute slices/whole cell patch clamp; RT-PCR, immunocytochemistry | Ba2+ (100 μM) | The Kir4.1 channels (detected at mRNA and protein levels) were principally responsible for resting K+ conductance. Genetic deletion of Kir4.1 protein resulted in depolarizing shift in resting Vm and in a 4.5 times increase in the input resistance. | 1265 |
| Kir4.1 homomeric, Kir4.1/Kir5.1 heteromeric channels | Mouse/brain sections/cortex, hippocampus, olfactory bulb, thalamus/immunocytochemistry/immunoelectron microscopy | Both Kir4.1 and Kir5.1 are expressed specifically in astrocytes; the Kir4.1/Kir5.1 colocalization was detected in cortex, hippocampus, and pontine nucleus. The Kir4.1/Kir5.1 heteromers were mainly confined to perivascular and perisynaptic processes. | 694 | |
| Kir2.1, Kir2.2, Kir 2.3 | Mouse/acute slices/hippocampus/whole cell patch clamp, RT-PCR | Ba2+ (100 μM) | Strongly rectifying Kir currents reflected the expression (at mRNA level) of Kir2.1, Kir2.2, Kir2.3 channel isoforms. These current were inhibited following activation of AMPA receptors; inhibition was due to an increased Na+ influx. | 1576 |
| Kir6.1 | Rat, mouse/brain sections, acute slices/cerebellum | Tolbutamide (100 μM) | Kir6.1 expression was found in astrocytes from hippocampus, cortex, and cerebellum; Kir6.2 was detected in neurons only. Whole cell recordings revealed tolbutamide-sensitive KATP currents in Bergmann glial cells. | 1745 |
| Voltage-independent two-pore domain (K2P) K+ channels | ||||
| TREK-1,TWIK-1 | Rat/Acute slices/Hippocampus/Whole-cell patch clamp, Immunocytochemistry | Quinine (200 μM) | TREK-1,TWIK-1 channels were detected immunocytochemically and whole-cell currents sensitive to quinine were considered to reflect their operation. | 1955 |
| TREK-1,TREK-2,TWIK-1 | Mouse/freshly isolated astrocytes/hippocampus/whole cell voltage clamp, singe-cell RT-PCR | Quinine (200 μM), bupivacaine (200 μM) | Transcripts for TREK-1,TREK-2, and TWIK-1 were identified in single astrocytes. K2P currents (with almost linear I–V relationship) were isolated as quinine- or bupivacaine-sensitive components after inhibition of Kir and A-currents with Ba2+ and 4-AP. | 1588 |
| TWIK-1/TREK-1 heterodimer | Mouse/primary cultures/brain sections/whole cell voltage clamp, immunocytochemistry | The TREK1/K2P2.1(KCNK2) and TWIK1/K2P1.1(KCNK1) channels were localized in hippocampal astrocytes by immunocytochemistry. Acidification increased currents mediated by TREK1 channels. Further analysis in cultured cortical astrocytes and in astrocytes in hippocampal slices identified TWIK-1/TREK-1 heterodimer (formed by disulfide bridge between cysteine-cysteine residuals of both subunits) as the predominant form of astroglial channel. | 756, 1874, 1955 | |
| Voltage-gated delayed rectifying K+ currents | ||||
| KSI (slow inactivating), KD, KA | Rat/primary cultures/spinal cord/whole cell patch clamp | 4-AP (100 μM to 8 mM) | At 100 μM, 4-AP inhibited slowly inactivating outward current KSI; at 2 mM, KA and KD; at 8 mM, KIR. | 214 |
| Kv1.5 | Rat/primary cultures, acute slices/hippocampus, cerebellum, spinal cord/immunocytochemistry, whole cell patch clamp | TEA (0.4–40 mM) | Specific Kv1.5 antibodies revealed widespread staining of GFAP-positive astrocytes, with particularly high immunoreactivity in perivascular endfeet. Treatment of astrocytes with Kv1.5 siRNA halved the amplitudes of delayed rectifying whole cell currents. | 1513 |
| Kv1.4 | Rat/tissue sections/spinal cord/immunocytochemistry, Western blot, in situ hybridization | Both mRNA and protein for Kv1.4 were identified in astrocytes; Kv1.4 expression increased 6 wk after spinal cord injury | 466 | |
| Kv11.1, or ERG1 (ether-à-go-go-related gene) | Rat/acute slices/hippocampus/immunocytochemistry, whole cell patch clamp | Dofetilide (100–1,000 nM), E4031 (100 nM) | ERG1 channels were localized to astrocytes by immunocytochemistry and characterized electrophysiologically | 475 |
| Rapidly inactivated K+ current (A-current) | ||||
| A-current | Rat/hippocampus/acutely isolated astrocytes/whole cell patch clamp | 4-AP (1 mM) | The A-current has a characteristic fast kinetics and steady-state inactivation with V0.5 at −60 mV | 1763 |
| Kv4.3, Kv3.2, Kv1 | Rat/primary cultures/hippocampus/whole cell patch clamp, RT-PCR, immunocytochemistry | 4-AP (4 mM), TEA (10 mM), ETYA (nonhydrolyzable analog of arachidonic acid, 10 μM) | Pharmacological analysis indicated that Kv4, Kv3, and Kv1 account for 70, 10, and <5% of A-current, respectively. | 124 |
| Ca2+-activated K+ channels | ||||
| BK, IK | Rat/primary culture/cortex, hippocampus/cell-attached and whole cell patch clamp | TEA (1 mM) | Two types of unitary KCa currents with conductances of 71 ± 6 and 161 ± 11 pS were detected. KCa currents were potentiated by activation of mGluRs. | 562 |
| SK3 (KCa2.3) | Rat, mice/supraoptic nucleus/sections/immunocytochemistry | Immunoreactivity for SK3(KCa2.3) was mainly confined to astroglial processes. | 65 | |
| BK | Rat/cortex/acute slices/perforated whole cell patch clamp, cell-attached patch clamp | Iberiotoxin, IbTX (200 nM), TEA (1 mM) | In astroglial endfeet, both whole cell and single-channel (unitary conductance 225 pS) BK currents were observed. | 511 |
| KCa3.1 | Mouse/acute slices/cortex, whole cell patch clamp, immunocytochemistry, RT-PCR | KCa3.1 blocker TRAM-34 (1 mM), KCa2.1–2.3 and KCa3.1 agonist NS309 | Astrocytes express KCa2.3 and KCa3.1 mRNA; immunoreactivity of KCa3.1 was restricted to processes. KCa2.1/KCa3/1 opener increased, whereas KCa3.1 inhibitor decreased whole cell currents. | 1006 |
| Intracellular Ca2+ channels | ||||
| InsP3R type 2 | Mouse, rat/primary cultures, brain sections/RT-PCR, immunocytochemistry, transgenic techniques | The InsP3R2 are considered to be the main type of InsP3 receptors in astroglia. Genetic deletion of InsP3 R2 leads to an almost complete disappearance of global astrocytic Ca2+ signals. | 678, 718, 820, 1381, 1598, 1602, 1817 | |
| InsP3R type 1, InsP3R type 2 | Mouse/primary cultures/organotypic and freshly isolated hipocampal slices/RT-PCR, immunocytochemistry, /[Ca2+]i microfluorimetry | InsP3 R1 transcripts and proteins were detected in astrocytes cultured from hippocampus and entorhinal cortex. Evidence for InsP3R1/2-mediated Ca2+ release was obtained in imaging experiments. | 607, 986, 1604 | |
| RyR3 | Mouse/primary cultures/[Ca2+]i microfluorimetry | Ryanodine (200 mM), chloro-m-cresol (4 mM) | RYR agonist chloro-m-cresol triggered [Ca2+]i elevation. | 1089 |
| TPC1, TPC2 | Rat/primary culture/cortex/[Ca2+]i microfluorimetry, RT-PCR | NED-19 (TPC channel blocker, 10 μM), bafilomycin 1A (100 nM) | Injection of 100 nM NAADP triggered [Ca2+]i transients, sensitive to NED-19 and preincubation with bafilomycin 1A. RT-PCR revealed transcripts for TPC1 and TPC2 channels. | 1370 |
| Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels | ||||
| Hyperpolarization-activated cyclic nucleotide-gated channels, HCN1–4 | Mouse/freshly isolated, FACS sorted astrocytes, acute slices/RT-qPCR, whole cell patch clamp | NCN 1–4 were detected at mRNA level; the whole cell HNC-mediated currents have been characterized. | 719, 1520 | |
| Anion channels | ||||
| Voltage-gated Cl− channels, ClC-1, ClC-2, ClC3 | Mouse/primary cultures, acute slices/whole cell voltage clamp | ClC channels were detected at mRNA and protein levels. Inwardly rectifying Cl− currents (i.e., promoting Cl− efflux) have been characterized. | 456, 1054, 1326, 1489 | |
| Best1 | Mouse/primary culture, acute slices/cortex, hippocampus/[Ca2+]i microfluorimetry, whole cell perforated patch clamp, RT-PCR, immunocytochemistry | Niflumic acid (100 μM), flufenamic acid (100 μM), NPPB (100 μM) | Best1 was detected at mRNA (single-cell RT-PCR) and protein levels. Whole cell currents were characterized in isolated astrocytes and in astrocytes in slices. Silencing of Best1 gene with shRNA significantly suppressed Cl− currents. | 1324 |
| Volume-regulated anion channels, VRAC/SWELL1 | Mouse/primary culture, acute slices/whole cell voltage clamp | Channels have been mainly characterized in vitro; VRAC channels have been shown to mediate release of excitatory amino acids, which have been detected in vitro and in situ. Increase in [Na+]i suppressed VRAC activity. | 5, 138, 867, 868, 1122, 1333 | |
| Aquaporins | ||||
| Aquaporin: AQP1, AQP4, AQP9 | Mouse, rat/primary culture, brain sections/RT-PCR, immunocytochemostry | The main astroglial type is AQP4, which is primarily concentrated in the endfeet; are important water pathways, form the molecular basis for the glymphatic system. | 1184, 1749 | |
| Connexons and pannexons | ||||
| Cx43 | Mouse/primary culture/Northern blot, immunocytochemistry, double-cell patch clamp | The Cx43 expression was found at cRNA and protein level with immunoreactivity; mainly concentrated at intercellular contact areas. Single-channel conductance was determined at ~56 pS. | 570 | |
| Cx26 | Rat, mouse/brain/tissue sections/Northern blot, Western blot, immunocytochemistry, EM | Expression of Cx26 was detected in astroglial gap junctions. | 1187 | |
| Cx30 | Rat/whole brain/primary cultures, tissue sections, acute brain slices/immunocytochemistry, EM | Cx30 protein was detected in astrocytes in situ and in vitro; Cx30 expression increased postnatally attaining maximum at 4 wk after birth. Cx30 was colocalized with Cx43 at gap junctions. | 923, 1189 | |
| Cx26-Cx32 or Cx43/32-Cx47 | Mouse/whole brain/brain sections/immunocytochemistry | Heteromeric Cx26-Cx32 or Cx43/32-Cx47 gap junctional channels were found to connect astrocytes with oligodendrocytes. | 28 | |
| Panx1 | Mouse/primary cultures/RT-PCR, whole cell perforated patch clamp | CBX (50 mM), MFQ (100 nM) | Detected at mRNA level and by voltage clamp on cultured embryonic astrocytes. | 749, 758, 1443 |
The Kir4.1 may coassemble with Kir5.1 subunits forming a heteromeric channels with high sensitivity to pH (1728). These Kir4.1./Kir5.1 heteromers were identified in astrocytes in the retrotrapezoid nucleus and were suggested to contribute to chemoception (1164). In the Müller glia, Kir4.1/Kir5.1 heteromeric channels are concentrated in the cell body and in the distal part of the cell, whereas Kir4.1 homomers are localized in the endfeet and in the perivascular processes; this distribution possibly reflects specific roles played by each channel type (769). In protoplasmic astrocytes, Kir4.1/Kir5.1 heteromeric channels were identified in the olfactory bulb and neocortex, whereas hippocampal astrocytes and cerebellar Bergmann glial cells expressed solely Kir4.1 homomers (229, 694). At the cellular level, Kir4.1/Kir5.1 heteromeric channels mostly occur in perisynaptic and perivascular processes and in the processes close to pia mater (694). In the optic nerve, the Kir4.1/Kir5.1 heteromers were rather homogeneously distributed between somata and processes (229).
Some hippocampal astrocytes also express mRNA for Kir2.1 (KCNJ2), Kir2.2 (KCNJ12), and Kir2.3 (KCNJ4) channels, thus generating strongly rectifying whole cell currents, compatible with Kir2.x functional expression. These currents were inhibited by Na+ influx through AMPA receptors (1576). The Kir2.1 immunoreactivity was also detected in some hippocampal astrocytes (1677). The Kir2.2 channels were localized (by immunostaining) in Bergmann glial cells and in cerebellar astrocytes (971). Transcripts for Kir2.1, Kir2.2, and Kir2.4 were detected in Müller glia (1419), while Kir2.1 protein was also identified in these cells by immunocytochemistry (890). In the Müller glia, the Kir2.1 channels are localized in membranes facing neuronal structures, whereas Kir4.1 channels are mostly associated with perivascular endfeet; it was therefore hypothesized that (in the context of K+ siphoning) the former channels mediate K+ influx, while the latter mediate K+ efflux (890).
Little is known about astroglial expression of Kir3.x (encoded by KCNJ3/5/6/9 genes) subunits that assemble into G protein-activated K+ channels. The mRNA specific for Kir3.1 and Kir3.2 was detected in Müller glia (1419), although this was not confirmed by immunocytochemistry (1629), suggesting the absence of functional protein. There is sporadic evidence about expression of Kir3.1 protein (detected by Western blot) in cultured astrocytes from cortex and spinal cord (1268). In contrast, astroglial expression of ATP-sensitive K+ channels assembled from Kir6.x and SUR1/2 is well documented. The K+-ATP channels open when ATP concentration decreases, and hence they are possibly involved in cell protection in conditions of metabolic stress; additionally, K+-ATP channels were suggested to regulate gap junctional permeability in astroglial syncytia (1745, 1897). Müller glial cells express mRNA for Kir6.1 and Kir6.2 (1419) and are immunopositive for Kir6.1 and SUR1 (465, 1628, 1629). The GFAP-positive astrocytes from the brain stem express high levels of mRNA specific for Kir6.1, and much lower levels for SUR1 (837), whereas Kir6.2 mRNA immunoreactivity has been found in astrocytes in the corpus callosum and in cerebellar white matter (1954). In the adult rat brain, however, only Kir6.1 (with Kir6.2 being confined to neurons) were detected by immunocytochemistry in hippocampal, cortical, and cerebellar astrocytes as well as in Bergmann glia; in the latter, the K+-ATP currents (isolated as a tolbutamide-sensitive component of whole cell K+ conductance) were recorded (1745). The K+-ATP currents (defined as diazoxide-activated and tolbutamide-inhibited currents) were also detected in astrocytes from hippocampus and brain stem (237, 1936); the proportion of astrocytes expressing these currents substantially decreased during postnatal development [from 57% at P8−11 to only 8% at P16−19 (237)]. Ultrastructurally, Kir6.1 channels in the cerebellum are concentrated in perisynaptic and peridendritic processes (1745).
2. Voltage-independent K+ channels
The two-pore-domain potassium channels (K2P) family is encoded by 15 KCNK genes (480). The K2P channels are open at (and hence contribute to) the resting membrane potential. Single-cell PCR performed on freshly isolated hippocampal astrocytes revealed a somewhat mosaic expression of TREK1/K2P2.1(KCNK2), TREK2/K2P10.1(KCNK10), and TWIK1/K2P1.1(KCNK1) channels, with some cells expressing all three subtypes (1588). Electrophysiological isolation of K2P current employing quinine and bupivacaine was not exactly straightforward because these agents also inhibit Kir channels and A-currents. Hence, quinine- and bupivacaine-sensitive currents were recorded in the presence of Ba2+ and 4-aminopyridine (4-AP). These currents reversed at EK and had almost linear current-voltage relationships (1588). The TREK1/K2P2.1(KCNK2) and TWIK1/K2P1.1(KCNK1) channels were also localized in hippocampal astrocytes by immunocytochemistry (1955). Acidification was shown to increase astroglial currents mediated by TREK1 channels (1874). Further analysis of cultured cortical astrocytes and astrocytes in hippocampal slices identified TWIK-1/TREK-1 heterodimer (formed by disulfide bridge between cysteine-cysteine residuals of both subunits) as the predominant channel type (756).
Evidence for the contribution of K2P channels to astroglial resting conductance remains controversial; in hippocampal astrocytes it was reported to be quite considerable [~58% in astrocytes in hippocampal slices (1955); and even ~70% in cultured astroglia (756)]. Conversely, a rather low (~10%) contribution of K2P channels to astroglial K+ permeability was found in freshly isolated hippocampal astrocytes (1588). In hippocampal slices isolated from TWIK-1/TREK-1 single and double knockout mice, neither astrocytic resting membrane potential nor resting conductance were affected (454). Subcellular protein fractionation of hippocampal tissue (using GFAP as a marker) demonstrated predominant retention of TWIK-1 channels in the cytosol with a rather minor (~5%) plasmalemmal presence (1857), further indicating a relatively minor role of these channels in astroglial K+ conductance. The TWIK-1 channels were shown to poorly discriminate between cations (308); furthermore, TWIK-1 channels have been shown to be permeable to NH4+ over K+ (1029), and hence they may provide for astroglial accumulation of ammonium (1266) released during physiological synaptic transmission. When [K+]o decreases below 3 mM, TWIK-1 channels become permeable for Na+ (1030) and, hypothetically, can generate Na+ influx into astrocytes even in physiological conditions when [K+]o may transiently fall below 2 mM following bursts of neuronal activity (668).
3. Voltage-gated K+ channels, Kv
Voltage-gated K+ channels are represented by 12 subfamilies (Kv1 to Kv12) of which delayed rectifying (KD) and transient (KA) channels are operative in astroglia. These channels are inactive at the resting membrane potential, and their opening requires cell depolarization.
a) delayed rectifier.
Delayed rectifying K+ currents have been recorded from astrocytes in culture and in situ; these currents were monitored from astroglial cells in various regions of the CNS including cortex, hippocampus, cerebellum, and spinal cord (215). The threshold for KD activation was at about −20 mV, and current amplitudes varied between 2 and 6 nA. Not much is known about the molecular diversity of astroglial delayed rectifier channels. The Kv1.5 (KCNA5) channel immunoreactivity has been identified in primary cultured rat spinal cord astrocytes as well as in astrocytes in adult rat hippocampal and cerebellar slices; immunoreactivity was particularly high in perivascular astrocytes and their endfeet. Treatment of these astrocytes with Kv1.4 antisense mRNA reduced current amplitude by ~50% (1513). Specific antibodies and in situ hybridization also revealed transcripts and proteins of Kv1.4 (KCNA4) in rat spinal cord astrocytes (466). Finally, the Kv11.1/ERG1 (KCNH2) channels were identified in hippocampal astrocytes by immunocytochemistry and characterized electrophysiologically (475).
b) A-type currents.
The rapidly activating and inactivating A-type currents have been recorded from cultured astrocytes and astrocytes in situ; their full activation requires conditioning hyperpolarization to −110 or −120 mV to remove strong steady-state inactivation characteristic for A channels. Their activation threshold lies around −70 to −50 mV, and these currents demonstrate rapid time-dependent inactivation (124, 215). In primary hippocampal astrocytes from rats, pharmacological, RT-PCR, and immunocytochemical analysis showed that 70, 10, and < 5% of total A currents are mediated by Kv4, Kv3, and Kv1 channels, respectively (124).
4. Ca2+-dependent K+ channels, KCa
The Ca2+-dependent K+ channels (KCa), which are structurally similar to voltage-gated K+ channels, are represented by three types (1869), designated as channels with big conductance (BK, or KCa1.1/KCNMA1), intermediate conductance (IK, or KCa3.1/KCNN4), and small conductance (SK, or KCa2.1−2.3/KCNN1−KCNN3). The KCa channels have a dual gating mechanism being controlled by cytosolic Ca2+ concentration as well as by Vm: without Ca2+ binding, the channel cannot be activated by membrane depolarization (1869).
Astrocytes express several types of KCa channels. In rat cultured cortical and hippocampal astrocytes, two types of unitary KCa currents with conductances of 70 pS and 160 pS (reflecting IK and BK channels) were detected; these channels, however, were blocked only by TEA and were insensitive to classical KCa blockers such as charybdotoxin, iberiotoxin, or apamin (562). Transcripts for SK (KCa 2.3) and IK (KCa3.1) channels were identified in mouse cortical astrocytes in acutely isolated slices (1006). The KCa3.1 immunoreactivity was confined to astroglial perivascular processes and endfeet; electrophysiology revealed only currents mediated by KCa3.1 [as judged by their sensitivity to the selective KCa3.1 blocker TRAM-34 and insensitivity to the KCa2.3 blocker apamin (1006)]. Similarly, the KCa2.3 protein has been immunocytochemically identified in astrocytic processes in the rat supraoptic nucleus (65). The BK channels (KCa1.1) proteins were identified by immunocytochemistry in the perivascular astroglial endfeet in hippocampus and cerebellum (1409), whereas patch-clamping of endfeet revealed large-conductance (225 pS) BK-single channel currents (511).
5. Multiple K+ channels underlie passive conductance of astroglial cells
Simultaneous expression of several types of K+ channels in astroglial cells ensures their passive properties across wide range of membrane potentials: at all levels of Vm, current-to-voltage (I–V) relation of the mature astroglial membrane is linear in vitro and in situ in all species (FIGURE 12) (455, 637, 1937, 1953). Passive membrane conductance of astrocytes is not associated with gap junctional coupling: neither pharmacological inhibition nor genetic deletion of connexins affect I–V linearity (455, 935, 1573, 1838), with the exception of the olfactory bulb (1453). At least three types of K+ channels, the inwardly rectifiers, the two-pore domain channels, and possibly Ca2+-activated K+ channels are responsible for hyperpolarized resting Vm. The relative contribution of each type of K+ channel probably varies in different astrocytes; in hippocampus, Kir4.1 channels account for ~50% of overall K+ conductance (1028, 1588). A high resting K+ conductance and highly negative Vm define the homeostatic functions of astrocytes (1244) assisting transmembrane movement of ions and providing electrical driving force for membrane transporters.
B. Sodium Channels
1. Voltage-gated sodium channels
Physiological diversity of voltage-gated Na+ channels (Nav) arises from nine isoforms (Nav1.1 to Nav1.9) of pore-forming α-subunit (encoded by SCN1A−11A genes) and four isoforms of auxiliary β-subunits encoded by SCN1B−4B genes (225, 297). Sodium channel proteins and sodium currents have been detected in astroglia in vitro and in situ (TABLE 2). The very first description of voltage-activated Na+ currents in cultured astrocytes was made in 1985 (166); in these experiments relatively large (~1 nA) tetrodotoxin (TTX)- and saxitoxin (STX)-sensitive Na+ currents were recorded. The relatively high densities of Na+ channels were somewhat incompatible with the nonexcitable nature of astrocytes, and hence the hypothesis of glial cells as donors of Na+ channels for neighboring neuronal elements was considered. Subsequently, Na+ currents were characterized in cultured astroglial cells (105, 106, 1647–1649, 1652). These currents were rather substantial varying between 100 and 300 pA in amplitude in the so-called type-1 astrocytes4 (105) and reaching 3 nA in type 2 astrocytes from the optic nerve; these latter astrocytes could even be forced to generate an action potential. Two types of Na+ currents with different kinetics (fast and slow) were subsequently described in cultured astrocytes from the optic nerve (1649) and hippocampus (1650). In astrocytes from the spinal cord, two types of INa distinct in their sensitivity to TTX (TTX sensitive and TTX resistant) were also characterized (1647, 1652).
Sodium currents were also found in more physiological preparations; first they were detected in nonenzymatically (“tissue prints”) isolated astrocytes from the optic nerve (107), and subsequently in astrocytes from hippocampal (1651) and spinal cord (187, 337) slices. In the slice preparations, INa were never found in all astrocytes: in the hippocampus (at P5 to P24) only 5 of 40 GFAP-positive cells had relatively small (~300 pA) Na+ current, whereas in the spinal cord (at P1 to P19) INa was confined to a population of cells with small somata and longish processes, with some faint GFAP staining. No Na+ currents were detected in neonatal astrocytes from stratum radiatum in acute hippocampal slices (1951). In contrast, Na+ currents were recorded from sulforhodamin101-negative astrocytes in juvenile (P3 to P15) hippocampal slices and were absent in more mature astroglial cells (811). At the molecular level, the predominate type of astroglial Na+ channels is represented by Nav1.5 (which corresponds to the TTX-resistant or cardiac channel type) detected both in vitro and in situ at mRNA and protein levels (185, 186, 1316), although Nav1.2, Nav1.3 (189), and Nav1.6 (1444, 1556) were also identified. Expression of Nav1.6 channels was found to be substantially upregulated in reactive astroglia (1957).
Despite substantial amount of data identifying voltage-gated Na channels in astrocytes from different regions of the brain, the exact localization and mechanisms of activation of these channels by physiological stimuli remain to be elucidated. Indeed, direct electrophysiological recordings of Na+ currents from mature astrocytes in situ remain scarce, while recordings from astrocytes in vivo are yet to be obtained. Nevertheless, the Nav channels may carry several physiological roles, and they seem to contribute to pathological responses of astroglia (see Refs. 188, 1315, 1317 for recent review and literature). Strategic localization of Nav channels in perisynaptic processes, which can possibly experience depolarization following activation of ionotropic receptors or Na+-dependent glutamate transporters, may contribute to generation of local Na+ signals (see sect. XB), this may in turn regulate numerous glial homeostatic cascades (881, 1499). It was also suggested that Nav-mediated influx of Na+ is needed for sustained activity of Na+/K+ pumps, and inhibition of Nav channels with TTX instigates cell death because of the pump failure (1648).
2. Na+o-regulated Na+ channels, Nax
Astroglia (astrocytes and ependymocytes) of the subfornical organ and organum vasculosum of the lamina terminalis (both are parts of the circumventricular organs surrounding ventricles) express a peculiar type of Na+ channel that is activated by an increase in extracellular Na+ concentration ([Na+]o) (1235, 1865). These channels are known as Nax and they were originally cloned from rat astrocytes (558) and from human cardiac muscle (568). The Nax primary sequence is distinct from the Nav channel family; likewise, the Nax is very different from voltage-gated Na+ channels in its voltage dependence as well as kinetic and gating mechanism. Most likely, the Nax channel belongs to a distinct class of membrane channels (1234). The Nax channels in vitro are activated by an increase in concentration of extracellular Na+ above 150 mM; in vivo, in the presence of endothelin-3, which activates ETB receptors selectively expressed in astroglia, the threshold for Nax activation is shifted to 140 mM of [Na+]o (706). Peak amplitudes of Nax currents reached ~1.5 pA/pF. The Nax channels act as molecular sensors for [Na+] in the circulation (see sect. XIIJ).
3. Epithelial sodium channel, ENaC
Epithelial Na+ channels constitute, together with acid-sensitive ion channels, an ENaC/degenerin superfamily; the ENaCs are non-voltage-gated, amiloride-sensitive Na+ channels encoded by four genes classified (in humans) as SCNN1A, SCNN1B, SCNN1G, and SCNN1D (643). The ENaCs are expressed in the CNS, and they have been being identified in neurons, ependymal cells of the choroid plexus, endothelial cells, and astrocytes (34, 1117, 1834). Strong immunoreactivity for γ-subunit of ENaC was detected in GFAP-positive astrocytes in circumventricular organs, white matter, and pia mater (1116); incidentally, no α- or β- ENaC immunoreactivity was detected in astrocytes, possibly indicating a unique homotrimeric channel assembly. Specific expression of ENaC in astrocytes from circumventricular organs may indicate their role in regulation of systemic Na+ homeostasis.
C. Calcium Channels
1. Voltage-gated Ca2+ channels
Three main families of voltage-gated calcium channels (VGCCs) are present in the nervous system; they are classified as Cav1.1–1.4 or L-type Ca2+ channels; Cav2.1–2.3 or P/Q/N/R-type channels, and Cav3.1–3.3 or T-type Ca2+ channels (296). Evidence for functional expression of VGCCs in astrocytes in living brain remains, however, controversial. Voltage-activated calcium currents were recorded in cultured astroglia (TABLE 2); the L-type currents were first contemplated from the recordings of Ba2+-dependent action potentials that can be generated after inhibition of K+ conductances in the presence of elevated intracellular cAMP (1038). Subsequently Ba2+ currents were detected in voltage-clamp experiments on cultured cortical astrocytes; these currents were blocked by Ca2+ channel blockers Co2+, Cd2+, and nifedipine and were much enhanced (or even induced) by norepinephrine and dibutyryl-cAMP (104, 333, 1041). Depolarization-evoked elevations in [Ca2+]i which were inhibited by Co2+, verapamil, and nifedipine, while being potentiated by BAY K 8644, have been monitored in primary cultured and freshly isolated astrocytes (460, 1040).
Expression of various VGCCs in astrocytes has been documented at the molecular level. The Cav1.2 and Cav1.3 channels were found in the transcriptome of rodent cortical astrocytes (275, 1945). In neonatal cultured cortical astrocytes, expression of α1B (N-type), α1C (L-type), α1D (L-type), α1E (R-type), and α1G (T-type) VGCCs was detected at mRNA and protein levels (948). Expression of transcripts for α1C (L-type), α1B (N-type), and α1E (R-type) was further corroborated by electrophysiological recordings of ion currents with relevant pharmacology (374). The Cav2.2 N-type and Cav2.3 R-type VGCCs were immunocytochemically detected in pituicytes in situ, whereas the same cells in vitro expressed (at mRNA and protein levels) Cav1.2 L-type, Cav2.1 P/Q-type, Cav2.2 N-type, Cav2.3 R-type, and Cav3.1 T-type subunits (1851).
Much less evidence for functional Ca2+ channels originate from experiments in situ. Occasional recordings of T- and L-type Ca2+ currents from cells in young hippocampal slices (19) were, most likely, obtained from NG2 glia, which, in contrast to astrocytes, has more “excitable” phenotype (1228). Spontaneous [Ca2+]i oscillations in astrocytes from the ventrobasal thalamus slices were somewhat potentiated by Bay K 8644 (1331) and were markedly blocked by 1 μM nifedipine (1332), these being indirect evidence for functional VGCCs. Another indirect evidence for functional astroglial VGCCs derives from the analysis of the presynaptic plasticity; in these experiments intracellular perfusion of astrocytes with membrane-impermeable VGCC inhibitor D890 had certain effects on synaptic strength (972). At the same time, experiments specifically dedicated to detecting voltage-gated Ca2+ currents or [Ca2+]i transients in slices failed to find them (289, 1848).
There are however some indications of upregulation of astrocytic VGCCs expression and their specific role in pathological conditions. For example, expression of L-type Ca2+ channels increased in reactive astrocytes following mechanic or ischemic brain injury (1876). Activation of astrocytes by LPS induced upregulation of Cav1.2 channels, whereas genetic deletion of these channels prevented astrogliotic response (314). Similarly, expression of Cav1.2 channels in astrocytes in vitro and in the brain is substantially upregulated by exposure to ammonium (in culture) or in animal models of hyperammonemia (1852).
2. Ca2+ release activated Ca2+ channels of Orai family
The Ca2+-release activated Ca2+ current (ICRAC) is the principal component of the store-operated Ca2+ entry (SOCE) in the majority of nonexcitable cells (1320). The molecular substrate of ICRAC is represented by a family of three Orai1,2,35 proteins, gating of which is controlled by the stromal interacting molecules, STIM1 and STIM2 localized in the membrane of the endoplasmic reticulum (1636). The nature of SOCE in astrocytes is complex with a documented role for TRPC1 channels (see below and sect. XA), while the astroglial expression and function of ORAI channels is yet to be fully characterized.
Orai proteins have been found only in astrocytes in vitro, in primary cultures. The Orai1 and STIM1 were detected by immunocytochemistry in rat cortical astroglia; overexpression of Orai1 increased the amplitude of SOCE in response to activation of PAR receptors with thrombin, whereas it was decreased by siRNA knockout (1150). In hippocampal rat astrocytes, the mRNA expression profile was different: the predominant isoform was Orai3; Orai 2 expression was six times lower, whereas Orai1 was not detected at all (1492). At the same time siRNA silencing of ORAI1 reduced SOCE in primary cultured cortical astrocytes. The only electrophysiological recording of ICRAC was hitherto obtained from acutely dissociated Müller cells; the contribution of Orai channels was deduced from sensitivity to the specific blocker Synta 66 (1139).
3. Intracellular Ca2+ channels
Intracellular Ca2+ channels are represented by three distinct families of 1) inositol trisphosphate receptors, or InsP3Rs (1052); 2) Ca2+-gated Ca2+ release channels, generally known as ryanodine receptors, or RyRs (1931); and 3) two-pore channels or TPCs activated by nicotinic acid adenine dinucleotide phosphate or NAADP (1339). The first two types of intracellular channels dwell in the endomembrane of the endoplasmic reticulum and mediate Ca2+ mobilization from the latter (1805), whereas TPCs are localized to the acidic Ca2+ stores (278).
The InsP3 receptor family comprises three members: the InsP3R1, InsP3R2, and InsP3R3, which all are activated by InsP3 and modulated by ionized Ca2+. The InsP3R1 shows specific “bell-shaped” regulation by [Ca2+]i: channel open probability increases with an increase in [Ca2+]i between the resting level (~50−100 nM) and ~1 μM, and inhibited at higher [Ca2+]i. Activity of InsP3R2 and InsP3R3 linearly increases with an increase in [Ca2+]i. The InsP3R2 seems to be a predominant intracellular Ca2+ channel in astrocytes (678, 718, 1598, 1602, 1817). Genetic deletion of this receptor completely abolishes or strongly reduces endoplasmic reticulum Ca2+ release and Ca2+ signaling in hippocampal and cortical astrocytes (820, 1381). This, however, is not a universal finding, and several experiments in vivo demonstrated residual Ca2+ signals in astrocytes from InsP3 R2−/− mice (e.g., Ref. 663; for details, see sect. XA). These may indicate functional expression of other InsP3 receptors, and indeed, the InsP3R1 receptors were detected in hippocampal and cortical astrocytes at mRNA and protein levels (607, 1492). Direct comparison of [Ca2+]i dynamics in astrocytes from InsP3R2 and InsP3R2/3 knockouts revealed functional Ca2+ release mediated by both InsP3R1 and InsP3R2 receptors (1604).
The RyRs are also represented by three types (633): the RyR1 (also known as “skeletal”), RyR2 (or “heart”), and RyR3 (or “brain”). The RyRs are activated by cytosolic Ca2+ and therefore act as an amplifier of Ca2+ signals; they also can be stimulated by the naturally occurring intracellular second messenger cyclic ADP-ribose. Expression of ryanodine receptors in cultured astrocytes was visualized by using fluorescent ryanodine (1819) and with immunocytochemistry (1623). In primary cultured and acutely dissociated cerebellar astrocytes, RyR3 mRNA and protein have been detected (1089), whereas immunocytochemistry revealed RyR3 in hippocampal astrocytes in situ (678). The functional role for RyRs in astrocytes remains unclear; caffeine-induced Ca2+ signals (presumably associated with RyR-mediated Ca2+ release) were observed in astroglial cultures (1623) and in astrocytes from thalamus (1331), but not from hippocampus (118). In primary astroglial cultures another agonist of RyRs, chloro-m-cresol (with EC50 1.5 mM) also triggered a [Ca2+]i increase (1089).
Not much is known about expression of TPCs in astroglia; intracellular injection of 100 nM NAADP triggered Ca2+ release from acidic organelles in cultured rat cortical astrocytes. These NAADP-induced Ca2+ transients were sensitive to TPC antagonist NED-19 and bafilomycin 1A, known to disrupt endolysosomal system (1370). The RT-PCR demonstrated the expression of mRNA for TPC1 and TPC2; when overexpressed both channel subtypes were colocalized with endosomal and lysosomal organelles. Overexpression of TPC1 and TPC2 potentiated NAADP-induced [Ca2+]i transients and facilitated autophagy (1370). The NAADP receptors were also visualized in cortical astrocytes in vitro with fluorescent probe NED-19, which revealed punctate staining with a high degree of colocalization with lysotraker (lysosomal fluorescent marker). Treatment of these cells with membrane-permeable NAADP-AM evoked [Ca2+]i transients (99). Furthermore, TPCs were also suggested to contribute to astrocytic Ca2+ signaling induced by ATP and endothelin-1 (99).
4. Transient receptor potential or TRP channels
The extended family of transient receptor potential6 (TRP) channels with 27 members present in humans (1221), contributes to numerous and widely diverse physiological functions, being particularly important for all types of sensing including thermal sensation, nociception, chemoception, equilibrioception, and taste sensing (1219). The TRP channels (which are subclassified into 6 groups) are cation channels permeable to Na+, Ca2+, and K+ with great heterogeneity of permeation properties (1291). In the CNS, all TRPs members are expressed at different levels, with particularly high expression of TRPV, TRPC, and TRPM channels (1218). Several types of TRP channels are operational in astroglia (1816).
a) trpa1 channels.
The TRPA1 (where “A” stands for ankyrin) channels have high unitary conductance (~110 pS) and substantial Ca2+ permeability [PCa/Pmonovalent ~5.9−7.9, with fractional Ca2+ current up to 23% (1221)]. These channels are known to be activated by noxious cold (below 17°C), by pungent substances derived from plants, by growth factors (via G protein-coupled receptors), and by proinflammatory agents (1220, 1221). The TRPA1 channels were found in somata and processes of astrocytes in the brain stem, in the rat trigeminal caudal nucleus using immunogold electron microscopy (965). Transcripts for TRPA1 were also detected in hippocampal astrocytes (1608). Functional expression of TRPA1 was monitored in a subpopulation of hippocampal astrocytes cocultured with neurons and in astrocytes in acute slices (1611). The operation of TRPA1 was deduced from monitoring spontaneous near-membrane (“spotty”) Ca2+ transients, which were suppressed by Gd3+, La3+, broad-spectrum TRP channel antagonist HC 030031, and following treatment with anti-TRPA1 siRNA. At the same time, TRPA1 specific agonist allyl isothiocyanate (AITC) potentiated near-membrane [Ca2+]i transients and induced whole cell currents (1611). Inhibition of TRPA1 channels with Gd3+, La3+, and HC 030031 resulted in a significant (from ~120 to ~50 nM) decrease in basal [Ca2+]i, this decrease was completely absent in TRPA1−/− mice (1608). Cytosolic [Ca2+]i dynamics controlled by TRPA1 was suggested to regulate astroglial uptake of GABA (1611) and release of d-serine (1608).
b) trpc channels.
The TRPC (where “C” stands for “canonical”) channels were detected in freshly isolated and in primary cultured astrocytes. Embryonic astrocytes in culture expressed mRNA encoding TRPC1 to TRPC6 proteins; these TRP channels were implicated in the generation of [Ca2+]i oscillations induced by diacylglycerol analog oleyl-acetyl-glycerol, stimulation of metabotropic glutamate receptors or depletion of endoplasmic reticulum Ca2+ store (606, 1393). Transcripts for TRPC1, 2, 3, 4, and 6 were detected in astrocytes cultured from the spinal cord (1133). At the protein level, TRPC1, TRPC4, TRPC5, and TRPC6 were identified in cultured and freshly isolated embryonic mouse astrocytes (163). In the same embryonic cultured astrocytes, TRPC1 channels coimmunoprecipitated with InsP3 receptors and SERCA2b endoplasmic reticulum Ca2+-ATPases (587), indicating intimate functional relations between the endoplasmic reticulum and plasmalemmal TRPC1-containing channels, compatible with the role of TRPC channels in astroglial store-operated Ca2+ entry (see sect. XA). Similar coimmunoprecipitation of TRPC1 channels, InsP3 receptors, and Homer proteins was found in cortical astrocytes cultured from 3- to 5-day-old rats (1868), whereas colocalization of TRPC4 channels with ZO-1 scaffolding proteins was detected in cultured fetal human astrocytes (1644). In primary astrocytes cultured from visual cortices of newborn rats or freshly isolated from the same region of 1-, 8-, and 55-days old rats, expression of TRPC1, TRPC4, and TRPC5 channels was detected in Western blots and their cellular localization was mapped with immune labeling showing that TRPC1 channels were predominantly localized to the plasma membrane (1055). Expression of TRPC channels increased with age: at 1 day of age 47, 7, and 70% of astrocytes studied expressed TRPC1, TRPC4, and TRPC5 proteins, respectively, whereas at 55 days of age, all astrocytes expressed all three isoforms (1055).
Coassembly of TRPC1, which is a channel forming subunit, with ancillary TRPC4 and TRPC5 subunits forms a functional cationic channel (713, 1684) with substantial (PCa/Pmonovalent varying between 1 and 9) Ca2+ permeability (1291). Activation of astroglial TRPC channels contributes to Ca2+ signals evoked by purinergic and glutamatergic (1055), as well as by mechanical (1055, 1461, 1462) stimulation. Treatment of astrocytes with an anti-TRPC blocking antibody substantially reduced [Ca2+]i transients (1055, 1461, 1816). In primary cultured cortical astrocytes TRPC channels were claimed to be activated by hyposmotic shock, with ensuing Ca2+ signaling reportedly instigating trafficking of aquaporin-1 water channels to the plasma membrane, hence increasing water transport (352). The Na+/Ca2+ selectivity of astrocytic TRPC channels seem to be controlled independently: treatment with anti-TRPC1 antibodies increased Na+ influx while decreasing Ca2+ entry (1461). The TRPC6 channels have been shown to mediate Ca2+ influx into embryonic astrocytes acutely treated with interleukin-1β (163).
c) trpv channels.
The family of TRPV (“V” for vanilloid) channels incorporates six members, which are activated by various chemical (e.g., pungent agents such as capsaicin and allyl isothiocyanate), thermal and noxious stimuli; TRPV4 channels are also sensitive to osmotic pressure. All TRPV channels are Ca2+ permeable with PCa/Pmonovalent between 1 and 10 for TRPV1–4, and PCa/Pmonovalent >100 for TRPV5,6 (1221, 1291).
Immunoelectron microscopy and immunocytochemistry revealed wide expression of TRPV1 channels in rodent astrocytes in several brain regions and in the spinal cord, with preferential localization in the astrocytic endfeet (442, 1755). In cultured astrocytes, TRPV1 channels carried cationic currents activated by extracellular acidification (746). The TRPV1 channels may contribute to regulation or initiation of astrogliosis in the spinal cord (319), whereas activation of TRPV1 apparently contributes to Ca2+-dependent rearrangement of the cytoskeleton (707). The TRPV1 channels were also suggested to regulate production of ciliary neurotrophic factor with neuroprotective features (1192). Expression of TRPV1 channels was detected at transcript and protein levels in astrocytes in circumventricular organs including the organum vasculosum of the lamina terminalis, subfornical organ, and area postrema, which all represent chemosensitive areas of the brain. These channels were concentrated in the thick perivascular astroglial processes and were suggested to contribute to chemosensing. The blood-borne signals indeed may be recognized by astroglial processes: blood infusion of the TRPV1-selective agonist resiniferatoxin triggered expression of immediate early gene c-Fos in astrocytes from circumventricular organs (1059).
Another member of the TRPV family, TRPV4 channel, was detected in cortical and hippocampal astrocytes (86, 135, 265, 994); the TRPV4 channels also showed the tendency to be concentrated mainly in the processes. In primary cortical astrocytes, TRPV4 channels can be activated by hyposmotic stress and by cell swelling; their activation triggers cytosolic Ca2+ signals sensitive to the TRPV inhibitor ruthenium red, while specific agonist 4α-phorbol 12,13-didecanoate induces cationic currents (135). Similarly, TRPV4-mediated currents and [Ca2+]i transients (sensitive to ruthenium red and the TRPV4 selective inhibitor RN1734) were recorded from astrocytes in hippocampal slices (265). It has been postulated that TRPV4 acting in concert with AQP-4 contributes to the development of regulatory volume decrease induced by hyposmotic shock (136, 137).
D. Hyperpolarization-Activated Cyclic Nucleotide-Gated (HCN) Channels
The Na+/K+ HCN channels (HCN1−4, with Na+/K+ permeability ratio of 1: 4) have been detected in healthy astrocytes in situ; however, their expression increased significantly after ischemia (719, 1520). At transcript level, all four mRNA for HCN 1−4 were identified, whereas only HCN1−3 proteins have been detected in reactive post-stroke astrocytes; in these cells, the whole cell HNC-mediated currents have been characterized (719, 1520).
E. Acid-Sensitive Ion Channels, or ASICs
The acid-sensitive ion channels are rather ubiquitously expressed in the nervous system; ASIC1, ASIC2a, and ASIC3 were localized with immunocytochemistry in cultured rat cortical astrocytes. All three isoforms were predominantly localized in the nuclei and were not associated with membrane currents (746). In freshly isolated mouse hippocampal astrocytes, however, single-cell RT-PCR revealed transcripts neither for ASICs, nor for TRPV1 channels (1874). Expression of ASIC1a was reported to be increased in reactive astrocytes in the context of chronic epilepsy, and their activity may contribute to seizure generation (1911). To conclude, the functional importance of ASICs for astroglial physiology in vivo remains unknown.
F. Anion Channels
The whole cell outwardly rectifying voltage-dependent Cl− currents were initially observed in cultured astrocytes depolarized to potentials more positive than −50 mV (166, 602). These findings, however, were not replicated in other studies, which demonstrated almost complete absence of anion conductance in astroglia at rest (868). Changes of astrocytic morphology (due to osmotic stress or treatment with cAMP, which induced cell stellation), however, activated the dormant Cl− conductance (945). Apparently, Cl− channels in astroglia are controlled by direct link to cytoskeletal actin (946). The actin connection may also explain earlier findings that single-channel Cl− currents could be detected only in excised patches (106).
Several types of chloride (anion) channels [represented by the ClC channel family, by cystic fibrosis transmembrane conductance regulator (CFTR) channels, by voltage-dependent anion-selective channels (VDAC), by Ca2+-dependent Cl− channels, and by volume-regulated anion channels (VRAC)] have been identified in astroglia (868). In cultured astrocytes, ClC-1, -2, and -3 channels were detected at transcript and protein levels (1326, 1944), while ClC-mediated currents were characterized in astrocytes in vitro as well as in hippocampal slices (1054, 1326). The immunoreactivity for ClC-2 was also found in Bergmann glia in cerebellum (192). Of note, ClC-2 channels were clustered in astroglial processes in the vicinity of GABAergic synapses, which prompted the hypothesis of astroglia-dependent regulation of intracleft Cl− concentration and hence of GABAergic transmission (1618). The ClC channels are inwardly rectifying (i.e., promoting Cl− efflux at potentials below ECl) and hence they mediate Cl− secretion from the astrocyte. Furthermore, ClC-2 channel open probability is high at astroglial resting potential and is modulated by change in the cell volume. Thus these channels facilitate release of Cl− in response to cell swelling (456, 1489).
The VRAC are fundamental for cell volume control and contribute to regulation of cell proliferation, cell motility, and apoptosis. Molecular identity of VRAC was discovered recently after identification of a plasma membrane spanning protein leucine-rich repeat containing 8A (LRRC8A) that was dubbed SWELL1 (1416, 1833). In astrocytes, VRAC are specifically important for regulatory volume decrease that invariably follows astroglial swelling due to osmotic disbalance (868). The VRAC channel currents induced by osmotic stress have been characterized in astrocytes in vitro (5, 138, 1326). The VRAC channels were also reported to form a conduit for excitatory amino acids; this was initially suggested by monitoring release of radioalbeled taurine, glutamate, and aspartate (867, 1333). Glutamate release, mediated by VRACs, has been subsequently observed in astroglial primary cultures (506, 991) and in astrocytes in situ (111, 1717). The activity of VRAC is claimed to be regulated by cytosolic Na+: increase in [Na+]i suppressed VRAC activity (1122). The LRRC8A/SWELL1 are critical for swelling-activated release of glutamate and taurine from cultured rat astrocytes (757).
Astrocytes also express an anion channel of Bestrophin (Best) family. The Best gene, responsible for a dominantly inherited, juvenile-onset form of macular degeneration called Best vitelliform macular dystrophy, encodes a Ca2+-activated anion channel (655). In the brain, Best1 channels were reported to be largely expressed in astrocytes (1255, 1324), being concentrated in perisynaptic regions (1322, 1893). These Best1 channels were activated by relatively moderate increases in [Ca2+]i at Vm −70 mV (i.e., close to astroglial resting potential), while Best1-mediated Cl− currents could be blocked by broad-spectrum antagonists of anion channels such as niflumic acid, NPPB, and flufenamic acid (1324). Astroglial Best1 channels are suggested to form a conduit for secretion of glutamate and GABA (see sect. XIE).
G. Aquaporins
Astrocytes express three members of aquaporin family, the AQP1, AQP4, and AQP9 (84, 1546), although AQP4 has by far the largest presence (1184). The AQP4 is composed from eight intramembrane helical segments M1−M8 and two translation initiation sites designated as Met1 and Met23, which in turn define two AQP4 isoforms, M1 and M23 (1314). Hypothalamic tanycytes express solely AQP9 (474). The highest expression of AQP4 has been indentified in the cerebellum with lower AQP4 presence in the hippocampus, diencephalons, and cortex (750). Astroglial AQP4 channels are concentrated in the perivascular and subpial endfeet, where their density is at least 10 times higher than in other parts of the cell. In the endfoot membrane, the AQP4 channels are assembled into orthogonal arrays of particles (also known as square arrays) that are observed under electron microscopy of freeze-fracture replicas (411, 1439, 1889). High densities of AQP4 in the endfeet mediate direct interactions with pericytes (618), with endothelial cells and with extracellular matrix with a specific role for proteoglycan agrin (280).
Physiological roles of AQP4 are subject of intense investigations, as it seems to contribute to a wide variety of CNS functions. Rather surprisingly, at the time of generation, the AQP4 knockout mice did not demonstrate any obvious phenotype (1031). Subsequently, the gross alterations of nervous function have been revealed as AQP4−/− mice displayed seriously impaired olfaction (1014), and even more seriously affected hearing: nearly all mice lacking AQP4 are deaf (1014, 1111). More detailed studies revealed a sevenfold decrease in astroglial water permeability (1641), deficient K+ buffering, compromised regulation of extracellular space (181), impaired hippocampal LTP/LTD without any changes in background synaptic transmission (1557, 1632), and altered spatial memory (1632, 1941). The AQP4 channels were found to be linked to astroglial Ca2+ signaling induced by osmotic stress (1749). Astroglial AQP4 channels also seem critically important for operation of the CNS glymphatic system (see sect. XIIH). Finally, genetic deletion of AQP4 significantly modifies various neuropathologies (1184).
H. Connexons
Connexons are fundamental for integrating astrocytes into interconnected syncytia. They form gap junctional channels spanning through the membranes of adjacent cells. Gap junctional channels are composed from two precisely aligned connexons from the two adjacent coupled cells (414, 1524). Assemblies of 100s of connexons into large cristalline-like structures make gap junction plaques between adjacent cells. When connexons fail to align with a congruent proteins from a neighboring cell, they may act as a stand-alone gated pores, known as hemichannels (490).
Connexons are assembled from six subunits, known as connexins (Cx), that represent a multigene family (with 21 members in human genome) which differ in molecular mass between 26 and 62 kDa; this underlies the nomenclature (e.g., Cx26 or Cx43). All connexins are similar in topology with four membrane-spanning domains. Functional connexons have a high conductance reflecting the pore with diameter between 6.5 and 15 Å; this pore is permeable not only for ions but also for hydrophilic molecules with molecular mass <1 kDa, including second messengers, nucleotides, glucose, various metabolites (397, 650, 1714), and even siRNA (1783). Of note, connexins have a high turnover rate with a half-life of several hours; degradation of connexins involves internalization into a specific double-membrane vacuoles known as annular junctions or connexosomes (888). Generally, connexons may assemble into homo- or heteromers, and gap junction channels can be made from identical connexons (making homotypic channel) or different (heterotypic channels). When the channel is composed from heteromeric connexons, it is classified as heteromeric. Finally, the gap junctions can connect the same type of cells (homocellular gap junction) or different type of cells (heterocellular gap junction). In astrocytes, connexons may also connect parts of the same cell through reflexive gap junctions (1890); these may create signaling and diffusional shortcuts in the highly arborized astroglia.
Astroglial expression of connexins is complex, and hence, astrocytes may contain multiple types of gap junctional channels. Astrocyte-astrocyte homocellular gap junctions are composed from Cx26, Cx30, and Cx43 (570, 923, 1187), of which Cx43 is the most abundant (1186). It seems that Cx30 and Cx43 may assemble as both homo- and heteromers, whereas Cx26 seems to make homomers only (28). Expression of Cx43 is ubiquitous, while the highest expression of Cx30 has been determined in thalamus and leptomeninges. Substantially lower expression of Cx30 is found in cortex and hippocampus, and no expression at all was detected in white matter (1186, 1640). Expression of Cx26 is restricted to astrocytes in subcortical areas, such as hypothalamus as well as reticular thalamic and the subtalamic nuclei (1188). Astrocyte-oligodendrocyte heterocellular gap junctions are composed from heterotypic channels which, in vitro, can be represented by four operational complexes: Cx47/Cx43, Cx47/Cx30, Cx32/Cx30, or Cx32/Cx26 (1049), although in situ the Cx32/Cx30, Cx47/Cx43 appear to dominate functional intercellular connectivity (28, 1282). Pairing Cx43 and Cx32 does not produce functional gap junctional channel (1878). There are some sporadic reports of astrocyte-neuronal heterocellular contacts (31, 1182, 1299), which are probably limited to developing brain and could not be considered as a significant pathway for glial-neuronal communication.
In cultured astrocytes obtained from Cx43 knockouts, expression of Cx26, Cx30, Cx40, Cx45, and Cx46 was detected (412, 1741), whereas Cx30 expression was almost doubled in Cx43-deficient animals (1742). These changes in expression explain why genetic deletion of Cx43 results only in partial (~50%) suppression of gap junctional astroglial connectivity (1742). Expression of connexins may be regulated by neuronal factors: coculturing neurons with astrocytes upregulates Cx43 and initiates Cx30 expression in the latter (899). Connexon-based gap junction channels are voltage dependent (being sensitive to trans-junctional potential, although some channels are also regulated by Vm); this voltage gating depends on the subunit composition (691). Conductance of Cx43-formed gap junctional channel in cultured astrocytes was around 50−60 pS (413, 570). Biophysical properties of connexons are regulated by multiple factors including pH, [Ca2+]i, or phosphorylation state, which is controlled by protein kinases A, C, and G and by mitogen-activated protein kinases, etc. (473, 691). Connexons are also positively modulated by intracellular spermine and spermidine (134).
Unpaired connexons, the hemichannels, have been identified in astrocytes in vitro and in vivo; all three connexons expressed in astrocytes (Cx26, Cx30, and Cx43) can operate as hemichannels (572). As a rule, these hemichannels are closed in healthy resting cells, but can be activated by low external calcium concentration, by substantial depolarization, by some specific intracellular Ca2+ signals, or by exposure to proinflammatory agents (1273, 1274). The hemichannels are considered to provide conduits for astroglial secretion of various substances, including neurotransmitters and neuromodulators (see sect. XIC). Depolarization activates unpaired connexons, which generate voltage- and time-dependent transmembrane currents, biophysical parameters of which depend on channel subunit composition. In physiological conditions (i.e., at normal concentration of divalent cations), open probability of Cx channels is very low at resting membrane potential. Mouse Cx30 channels show prominent voltage dependence (1784). This is not the case for Cx43 (357), while human Cx26 hemichannels show bell-shaped voltage dependence (593). The unitary conductance for Cx26 hemichannels is ~320 pS (593), for Cx32 hemichannels ~90 pS (1535), and for Cx43 hemichannels ~220 pS (1457). Of note, the conductance of Cx43 gap junctional channel is about four times lower than that of unpaired one (413, 570), although theoretically it should be only half. This probably indicates some additional regulation of the channel in gap junctional configuration.
I. Pannexons
Pannexons have no homology with connexons and are classified as gap junction proteins because of some homology with innexons; pannexons never form intercellular channels and hence exist as stand-alone plasmalemmal gated pores (490).7 At the same time the overall topology (four transmembrane domains) and pharmacological properties of pannexins and connexins are rather similar, suggesting some common functional evolution and gating mechanisms (1002). Depending of the stimulation mode, pannexon opens as a large-conductance (~500 pS) pore or operates as ~50 pS anion channel (1032, 1856). Pannexon channels are assembled from six subunits, of which three subtypes pannexin1, -2, and -3 (or Panx1, -2,-3) are known. Transcripts for Panx1 were identified in astroglia in vitro and in situ (749, 1443), and Panx1 currents were electrophysiologically characterized in primary cortical astrocytes (758). Astroglial pannexons were activated by voltage and by stimulation of P2X7 receptors; they were blocked by gap junctions blockers carbenoxolone (CBF) and mefloquine (MFQ) and were permeable to fluorescent tracer YoPro (758). The Panx1 channels in astrocytes could also be activated by an elevation of extracellular K+ concentration, which shifts activation potential of pannexons to more hyperpolarized values (1620). Astroglial pannexons were reported to be activated by fibroblast growth factor 1 (554). Pannexons, in high conductance state, can be permeable to molecules with molecular mass up to 1.5 kDa and hence may contribute to astroglial secretion (see sect. XIC). Panx1 in particular is considered as transmembrane conduit for ATP (375). The P2X7-pannexon complex was also implicated in Ca2+-independent release of d-serine from cultured astrocytes (1306). The evidence for functional pannexons and their physiological role in astrocytes in vivo remain, at best, circumstantial.
J. Recapitulation
Astrocytes express a remarkable variety of ion channels, including several types of voltage-gated channels usually associated with excitable cells. Whether some of these channels (most notably voltage-gated Na+ and Ca2+ channels) contribute to astroglial physiology, or their expression is induced in special circumstances, remains to be clarified. The backbone of astroglial membrane permeability is formed by K+ channels and connexons. Connexons underlie functional integration of astrocytes into syncytia, fundamental for intercellular signaling and long-range diffusion of metabolites and second messengers. Several types of K+ channels underlie ohmic conductance at the wide range of membrane potentials essentially defining the passive electrophysiological signature of astrocyte. Large K+ permeability confers stability on astroglial membrane potential and hence secures many homeostatic functions; the variable complement of other channels allows for versatility and flexibility, hence contributing to astroglial adaptive capabilities.
VIII. RECEPTORS
Astrocytes are capable of expressing virtually any type of receptor found in the CNS (TABLE 3), which allows astroglia to perceive the neurochemical landscape of the nervous tissue. The first hints for astroglial expression of functional neurotransmitter receptors came from intracellular microelectrode recordings from pericruciate cortical cells of anesthetized cats. The cells were impaled blindly, and neurons and glia were distinguished by their excitability. Iontophoretic injections of GABA or acetylcholine (ACh) evoked glial depolarization, which was considered to reflect modulation of membrane ion pumps (908). Glial depolarization in response to GABA, glycine, β-alanine, and taurine was also observed in organotypic preparations of medulla oblongata, pons, and spinal cord and was considered to reflect K+ release from neurons (731). Direct electrophysiological recordings from cultured astrocytes, devoid of neuronal contamination, demonstrated functional expression of glutamate, GABA, and glycine receptors (220, 853). Subsequent experiments on astroglia in vitro demonstrated that astrocytes indeed express multiple receptor types.
Table 3.
Astroglial receptors to neurotransmitters and neuromodulators
| Receptor Type/Subunits | Properties, Function, and Localization | Reference Nos. |
|---|---|---|
| Ionotropic receptors | ||
| AMPA glutamate receptors: GluA1, GluA2, GluA3, GluA4 | Detected in hippocampus, cortex, cerebellum, white matter, Bergmann glial cells, immature astrocytes. Cationic Na+/K+ channels or Na+/K+/Ca2+ channels. Receptors lacking GluA2 subunit (predominantly localized in Bergmann glial cells) have moderate Ca2+ permeability (PCa/Pmonovalent ~1). Activation triggers cell depolarization and Ca2+ influx. | 777, 932, 935, 1169, 1589 |
| Glutamate NMDA receptors: GluN1, GluN2C, GluN2D, GluN3 | Detected at mRNA and protein level in cortex, hippocampus, spinal cord, amygdala, locus coeruleus, and retinal Müller glia. NMDA receptor-mediated currents were characterized in astrocytes from cortex, spinal cord, and subpopulation of hippocampal astrocytes. Na+/K+/Ca2+ channels. Astroglial receptors display weak Mg2+ block and intermediate Ca2+ permeability (PCa/Pmonovalent ~3). Activation triggers inward current and Ca2+ entry. | 52, 354, 464, 935, 972, 1304, 1305, 1562, 1593, 1965 |
| P2X purinoceptors: P2X1/5, P2X7 | At mRNA and protein levels, all seven subunits of P2X receptors were identified (in various combinations) in astrocytes from cortex, hippocampus, cerebellum, spinal cord, brain stem and retina. P2X1/5-mediated currents were recorded in cortical astrocytes; functional P2X7 receptors were found in cortex, hippocampus, and retina. Na+/K+/Ca2+ channels. Ca2+ permeability of P2X1/5 receptors is: PCa/Pmonovalent ~2. Ca2+ permeability of P2X7 receptors depends on the pore formation and can be very high (PCa/Pmonovalent >10). Activation triggers cationic current and Ca2+ entry. | 435, 516, 520, 541, 763, 776, 829, 937, 1001, 1305, 1313 |
| GABAA receptors: α2, γ1 | Detected at mRNA, protein, and functional levels in hippocampus, cortex, cerebellum, optic nerve, spinal cord, and pituitary gland. Cl– channel. Activation triggers Cl− efflux and cell depolarization. Possibly play fundamental role in regulation of [Cl−] in the synaptic cleft to maintain inhibitory transmission. | 339, 468, 773, 853, 854, 857, 1042, 1167, 1336, 1800 |
| Glycine receptors: α1, β | Mainly present in the spinal cord, where they were detected at transcript, protein, and functional levels. Cl– channel. Activation triggers Cl− efflux and cell depolarization. | 874, 1336 |
| Nicotinic cholinoreceptors: α3, α4, α7; β2, β4 | Detected in astrocytes in vitro and in hippocampal astrocytes in situ. Na+/K+/Ca2+ channels. Receptors containing α7 subunit display high Ca2+ permeability (PCa/Pmonovalent ~6). | 601, 1257, 1597, 1726, 1737 |
| Metabotropic receptors | ||
| Glutamate receptors: mGluR3, mGluR5 | Detected in astrocytes throughout the CNS. The most abundant is mGluR3 receptor subtype which inhibits adenylyl cyclase; mGluR5 receptors associated with Ca2+ signaling are downregulated in first postnatal weeks. | 277, 720, 879, 947, 1308, 1400, 1401, 1702 |
| GABAB receptors: GABAB1a, GABAB1b, GABAB2 | Detected in hippocampus, cortex, and spinal cord. Linked to PLC through Gi/o proteins; activation of GABAB receptors triggers Ca2+ release from the ER with associated Ca2+ signals and [Ca2+]i oscillations in vitro, in situ and in vivo. | 822, 1008, 1066, 1800 |
| Adenosine receptors: A1, A2A, A2B, A3 | Detected at mRNA, protein, and functional levels in hippocampus, cortex, cerebellum, and spinal cord. All receptors were found to be linked to Ca2+ signaling and to cAMP cascades. A1 and A2 receptors regulate expression of glutamate and GABA transporters receptors; A2A receptors regulate Na+/K+ pump while A3 receptors mediate neuroprotection. | 177, 178, 317, 338, 369, 438, 504, 796, 1085, 1230, 1399 |
| P2Y purinoceptors: P2Y1,2,4,6,12,13,14 | Detected at mRNA, protein, and functional levels throughout the CNS, in hippocampus, cortex, cerebellum, brain stem, retina, and spinal cord; the P2Y1,2,4 are being dominating types. Generally are linked to PLC/InsP3/Ca2+ signaling cascade. P2Y receptors are also coupled to various signaling pathways, including MAP kinases, extracellular signal-regulated kinase (ERKs), the stress-activated protein kinases (SAPKs), the JNKs, p38/MAPK, glycogen synthase kinase, and the Akt kinase. | 159, 226, 248, 519, 541, 771, 795, 842, 880, 1202, 1347, 1348, 1390, 1808 |
| Muscarinic cholinoreceptors, mAChR: M1, M2, M3 | Detected in hippocampus and amygdala. Control PLC, InsP3 production, and Ca2+ release from the ER. M3 receptors are linked to neurogenesis by regulating astroglial expression and release of fibronectin and laminin-1. | 56, 617, 1599 |
| Adrenergic receptors: α1AR, α2AR; β1AR, β2AR, β3AR | Detected at mRNA, protein, and functional levels in hippocampus, cortex, cerebellum, optic nerve, and spinal cord. α-ARs are mainly linked to PLC/InsP3/Ca2+ signaling, whereas β-ARs mainly control cAMP production and glial glucose metabolism. | 123, 431, 459, 684, 882, 1596 |
| Serotonin receptors: 5-HT1A, 5-HT2A, 5-HT2B, 5-HT5A, 5-HT7 | Detected at mRNA and protein level throughout the brain. The 5-HT2B is the predominant type; mainly 5-HT receptors are linked to PLC/InsP3/Ca2+ signaling. 5-HT2B receptors are also connected to ERK1/2, andcPLA2. 5-HT7 receptors (found in suprachiasmatic nucleus) stimulate adenylyl cyclase. | 129, 651, 688, 895, 1536, 1561, 1943 |
| Dopamine receptors: D1, D2, D3, D5 | Detected at mRNA and protein levels in basal ganglia and in substantia nigra. Astroglial D2 receptors are found in neocortex where they account for 30% of all D2 binding sites; D2 receptors are also present in fibrous astrocytes of white matter. Mainly linked to PLC/InsP3/Ca2+ signaling. | 88, 858, 1134, 1135, 1455, 1459, 1782, 1934 |
| Histamine receptors: H1, H2, H3 | Have been detected at mRNA and protein level and functionally characterized in hippocampus and cerebellum. Linked to PLC/InsP3/Ca2+ signaling, adenylyl cyclase, glucose metabolism, and regulation of expression of glutamate transporters. Regulate synthesis of cAMP. | 60, 764, 809, 882, 987, 1100, 1599 |
| Cannabinoid receptors: CB1 | Detected in hippocampus in situ. Linked to Ca2+ signaling and to synaptic plasticity. | 592, 634, 1197, 1198 |
| Oxytocin and vasopressin (V1b) receptors | Detected in hypothalamus. Linked to PLC/InsP3/Ca2+ signaling. V1b receptors also control PKC, CaMKII, and ERK1/2 signaling cascades. | 424, 662, 1709, 1949 |
| PACAP/VIP receptors: PAC1, VPAC1, VPAC2 | Have been detected throughout the brain. All receptors stimulate adenylyl cyclase; PAC1 receptors are linked to PLC/InsP3/Ca2+ signaling. May also regulate energy metabolism and glycogenolysis. | 70, 605, 656, 803, 1077 |
| Bradykinin receptors: B2 | Identified in cultured astrocytes; linked to PLC/InsP3/Ca2+ signaling and Ca2+-activated Cl− currents. | 17, 330, 990 |
| Opioid receptors: μ, δ, κ | Mainly detected in astrocytes in vitro. Linked to various signaling cascades, regulate expression of glutamate transporters, and may affect growth and development. | 128, 983, 1673 |
The complement of receptors expressed by astrocytes in situ and in vivo is restrictive and depends on the brain region. The modality of neurotransmitter receptors expressed by astroglia matches that of their neuronal neighbors and is most likely controlled by the local neurotransmitter environment (1814). For example, receptors expressed by Bergmann glial cells and its neuronal neighbor the Purkinje neuron are optimized to sense neurotransmitters released by neuronal afferents, which form synapses accessible by these cells. Similarly, astrocytes express glycine receptors in the spinal cord, where glycine acts as a main inhibitory mediator. Astroglial expression of dopamine receptors is prominent in basal ganglia, which utilize dopaminergic transmission, while astroglial expression of serotonin receptors is restricted to areas contacting serotonergic terminals (1806). Therefore, expression of astroglial receptors in vivo is regulated by neurochemical input, which makes astrocytes perceptive to signals specific for each particular region of the brain.
A. Purinoceptors
Purinergic signaling system that utilizes purines and pyrimidines as extracellular signaling molecules is arguably the most ubiquitous being present in every organ and system without any obvious anatomical segregation. Correspondingly most of the living cells possess at least one (and often several) type(s) of purinoceptors (263). These latter are classified into adenine (P0) receptors, adenosine (A or P1) receptors, and nucleotide (ATP, ADP, UTP) receptors of the P2X (ionotropic) and P2Y (metabotropic) varieties (262, 1239, 1807). In the CNS, purines and pyrimidines are released from neurons (mainly from their terminals) and from neuroglia, with Ca2+-regulated exocytosis being probably the most widespread (and certainly the most studied) mechanism (2). After being released ATP is rapidly degraded by specific extracellular enzymes, known as ectonucleotidases (1968) to ADP, AMP, and adenosine that all act as purinergic agonists, which often can have opposite effects on target cells. The absolute majority of neuroglial cells studied so far express some purinoceptors (except P0 receptors, astroglial presence of which has not been studied yet). Neuroglial cells are also capable of releasing ATP and adenosine, which makes them an important source of physiologically released purines in the CNS and places purinergic transmission at the fore of gliotransmission (267, 1810). Purinergic signaling system also contributes to neuropathology; in particular, ATP released from damaged cells acts as a universal “danger” signal (often defined as damage-associated molecular pattern, DAMP) that controls glial defensive reactions such as reactive astrogliosis and activation of microglia (520).
1. Adenosine receptors
Adenosine receptors, represented by four subtypes (A1, A2A, A2B, and A3) with distinct pharmacological and functional properties (525), are classical G protein-coupled seven-transmembrane-spanning metabotropic receptors. Different receptors subtypes may assemble as heteromers and even oligomerize with other metabotropic receptors (263). As a rule the A1 and A3 receptors exert an inhibitory effect on adenylyl cyclase (mediated through Gi/o proteins), whereas A2A and A2B receptors activate cAMP production via Gs proteins. All adenosine receptors may regulate phospholipase C (PLC) and thus inositol 1,4,5-trisphosphate (InsP3) synthesis. In some cells A1 receptors were reported to activate K+ and/or Ca2+ channels (3, 204, 383, 525).
All four types of adenosine receptors have been identified in astrocytes (TABLE 3) using various functional assays applied to both in vitro and in situ preparations (383). Transcriptome analysis reveals high expression of A2B receptors in cortical astrocytes (275, 1010). The first report of adenosine receptors in glia described adenosine-induced hyperpolarization in a subpopulation of cultured spinal cord and cerebellar astrocytes; this effect was blocked by broad antagonist 8-phenyltheophylline (734). Shortly thereafter, adenosine receptors with pharmacological profiles corresponding to A1 and A2 types were identified in human fetal astrocytes (1894). The A1 receptors suppressed, whereas A2 receptors potentiated synthesis of cAMP. In astroglial cultures prepared from 2-day-old rats, the cAMP production was stimulated by activation of A2B receptors in a dose-dependent manner (1345). In the same cultures stimulation of A1 receptors in type 1 (but not in type 2) astrocytes inhibited production of cAMP (1344).
Expression of A1 receptor-specific mRNA was demonstrated in rat cultured astroglia (176). Activation of A1 receptors in rat cultured astrocytes was linked to PLC. Incidentally, PLC activation was observed only in cultures with high levels of A1 receptor expression (177) and upregulation of A1 receptors synthesis potentiated A1-dependent PLC stimulation (176, 178). Activation of PLC is directly linked to Ca2+ mobilization from the endoplasmic reticulum, and indeed, stimulation of A1 receptors in cultured neonatal astrocytes initiated intracellular Ca2+ release as well as Ca2+ entry and potentiated histamine induced Ca2+ mobilization (1343). Similarly, adenosine induced [Ca2+]i elevation in the majority of astrocytes in acute rat hippocampal slices (1399). Other types of adenosine receptors are also connected to Ca2+ signaling. In astrocytes in olfactory bulb slices, adenosine, which occurred following enzymatic degradation of ATP released from olfactory nerve terminals, induced [Ca2+]i elevation via activation of A2A receptors (438). In acutely isolated cortical astrocytes, A2B receptors (as judged by sensitivity to the selective A2B antagonist alloxazine) triggered Ca2+ signals (1391). The sensitivity of acutely isolated cells to adenosine was much higher when compared with cultured cells, thus indicating modified adenosine receptor expression in the in vitro conditions (1391). The A3 receptors mediated adenosine- and guanosine-evoked [Ca2+]i transients in cultured mouse astrocytes (317). Adenosine, acting through A1 or A2B receptors, also modulates astroglial Ca2+ signals originated from stimulation of other metabotropic receptors and endoplasmic reticulum Ca2+ release (25, 504, 796, 1252, 1253, 1753). Similarly, activation of A1 receptors suppressed sustained Ca2+ influx following opening of P2X7 receptors in cultured cortical astrocytes (1233).
Adenosine receptors modulate astroglial uptake of neurotransmitters through A1-dependent regulation of expression of EAAT2/GLT1 (1896) or regulation of GABA transport by A1-A2 heteromers; in this latter case, A1 receptors signal through Gs proteins to increase, while A2 receptors signal through Gi/o proteins to inhibit GAT1/3 GABA transporters (369). In hippocampal astrocytes, A2A receptors inhibit EAAT2/GLT1 transporter (1085, 1230) and evoke glutamate release from astrocytes through [Ca2+]i and a protein kinase A-dependent pathway (981, 1230). This in turn potentiated neuronal activity due to an increase in glutamate concentration in the synaptic zones (1230). In astrocytes (it has been argued) A2A receptors directly (through physical association) interact with Na+-K+-ATPase providing negative modulation. Suppression of the activity of the Na+/K+ pump following A2A receptor activation has been considered as a mechanism for subsequent decrease in glutamate transporter-dependent uptake due to decrease in the transmembrane Na+ gradient (1084). Selective deletion of A2A receptors from astrocytes (using Gfa2 promoter) decreases glutamate uptake and triggers psychomotor and cognitive deficits that have some semblance to schizophrenic phenotype (1086). Adenosine receptors also exert trophic effects; for example, A1 and A3 receptors contribute to neuroprotection probably through stimulating the PI3K and ERK1/2 MAPK pathways (184, 338).
2. Ionotropic P2X purinoceptors
Ionotropic P2X purinoceptors are archetypal ligand-gated cationic (Na+/K+/Ca2+) channels (FIGURE 13), assembled as homo- or heterotrimers from seven different subunits encoded by distinct genes that are classified P2X1 to P2X7 according to historical order of cloning (1239). Receptors formed through homo- or heteromeric assembly of P2X1 to P2X6 subunits (hitherto homomeric composition was shown for P2X1–5 subunits, P2X6 subunits apparently cannot olygomerize; heteromeric compositions are represented by P2X1/2, P2X1/4, P2X1/5, P2X2/3, P2X2/6, and P2X4/6 channels) are activated by low (submicromolar and micromolar) ATP concentrations, while homomeric P2X7 receptors require millimolar ATP for activation (1240, 1705).
FIGURE 13.
Main properties of ionotropic receptors functionally expressed in astroglia.
There is a substantial discrepancy between evidence for P2X subunits expression in astroglia and data on their functional manifestation. Primary cultured astrocytes contain transcripts for P2X1–5 and P2X7 receptors (435, 541). In tissue extracts from rat nucleus accumbens, RT-PCR revealed expression of all seven P2X mRNAs (516). Cortical astrocytes contained transcripts for P2X1 and P2X5 subunits (937). In acutely isolated rat retinal Müller cells, P2X3, P2X4, P2X5 but not P2X7 mRNAs were identified (776), whereas P2X7 mRNA was detected in human Müller glia (1313). Immunocytochemistry also demonstrated expression of P2X subunits. In astrocytes from healthy nucleus accumbens, P2X2,3,4 receptors proteins were detected (although all 7 subunits were present at mRNA level); mechanical lesion however triggered additional expression of P2X1 and P2X7 subunits (516). Immunoreactivity for P2X1 and P2X2 receptors was detected in astroglial cells in cerebellum (829, 1001), and P2X2 receptors were found in spinal cord astrocytes (828), P2X4 receptors were identified in astrocytes from the brain stem (69) and in Müller glia (708), whereas hippocampal astrocytes were immunoreactive for P2X1–4, P2X6, and P2X7 receptors (922).
Electrophysiologically, P2X-mediated currents are much less characterized. In primary cultured astrocytes, ATP evoked inward currents and [Ca2+]i rise, although subunit composition of the underlying receptors was not identified (1050, 1844). In hippocampal astrocytes, both acutely isolated or in slices, no P2X-mediated currents were found, contrasting RT-PCR and immunocytochemistry findings indicative of P2X subunits expression (778). No ATP-induced currents have been found in Bergmann glial cells in acute cerebellar slices (880). In astrocytes from acutely isolated optic nerves, ATP triggered large [Ca2+]i transients sensitive to the P2X receptor antagonist NF023 (used at 100 μM concentration at which it blocks all P2X1–4 receptors); the [Ca2+]i response could be also mimicked by the broad P2X agonist α,β-methylene ATP (α,β-meATP) (631, 786). In cortical mouse astrocytes, expression of P2X1/5 heteromeric receptor was reported (FIGURE 14) (937). These receptors are highly sensitive to ATP (EC50 ~50 nM); they have specific biophysical properties represented by biphasic kinetics with distinct peak and steady-state components, activation of “rebound” tail current following the washout of the agonist, and a very little desensitization in response to the repetitive agonist applications (936, 937). The P2X1/5 receptors contribute to “glial synaptic currents” triggered in astrocytes by stimulation of neuronal afferents in cortical slices (932, 936). The P2X1/5 receptors also mediate spontaneous “miniature” postsynaptic currents in astrocytes in cortical slices (936). Astroglial P2X1/5 have intermediate Ca2+ permeability (PCa/Pmonovalent ~2.2), and their activation by endogenous agonists or by synaptically released ATP triggered transient cytoplasmic Ca2+ signals (1305). It is of course important to mention that most humans carry a single-nucleotide polymorphism at the 3′ splice site of exon 10 of the human P2X5 gene, which renders the P2X5 subunit nonfunctional (898).
FIGURE 14.
P2X1/5 receptor-mediated currents in cortical astrocytes. A: the family of ATP currents evoked by repetitive applications of the agonist. The currents show no apparent desensitization. Current traces have a complex kinetics comprising the peak, the steady-state component, and the “rebound” inward current recorded upon ATP washout as indicated on the graph. B: concentration dependence of ATP-induced currents in cortical astrocytes. Membrane currents recorded from a single cell in response to different ATP concentrations are shown on the left. The right panel shows the concentration-response curves constructed from 9 similar experiments; current amplitudes were measured at the initial peak and at the end of the current, as indicated on the graph. C: inhibition of ATP-induced currents by PPADS. Currents recorded at various concentrations of PPADS are shown on the left, and the concentration dependence of inhibition for peak and steady-state components constructed for 7 individual experiments is presented on the right. The peak component of the response was more sensitive to PPADS. Application of PPADS started 2 min before application of ATP. All recordings were made at holding potential of −80 mV. [From Lalo et al. (937).]
Astroglial P2X7 receptors have received much attention, mostly because of their pathological significance (i.e., initiation of apoptosis and cell lysis, regulation of astrogliosis or controlling processing and release of cytokines; see Refs. 520, 763 for relevant literature). The P2X7 receptors are unique in their low ATP sensitivity (in all probability it is ATP4- that acts as a true agonist), almost complete absence of desensitization, and ability to produce large transmembrane pores upon intense stimulation (1239). The very first in situ hybridization mapping (346) failed to detect P2X7 receptors in the brain. Subsequent studies revealed P2X7 transcripts and proteins in many areas including cortex, hippocampus, medulla oblongata, cerebellum, thalamus, and amygdala (1658). The specificity of antibodies used for labeling P2X7 receptors is, however, far from ideal (1621), thus making many observations contentious.
In primary cultured astroglia, expression of P2X7 receptors was frequently documented at both the transcriptional and protein levels (435, 457, 541, 752, 797, 1194, 1309, 1850). Freshly isolated astrocytes as well as astrocytes in slice preparations similarly demonstrated immunoreactivity for P2X7 receptors (922, 1261, 1309). At the same time, in-depth analysis of the cellular distribution of P2X7 mRNA in the rat brain using isotopic in situ hybridization detected these receptors in neurons, oligodendrocytes, and microglia but failed to identify P2X7 receptors transcripts in astrocytes (1930). Astroglial expression of P2X7 receptors, as a rule, increases after brain injury of various etiology (516, 517, 1194).
In cultured astrocytes, both P2X7-mediated (as judged by pharmacology, activation by Bz-ATP, and inhibition by oxATP) [Ca2+]i transients (93, 541, 1233) and membrane currents (457, 1238, 1515) were characterized. The P2X7 currents were also detected in Müller cells freshly isolated from human retina (1313). In isolated optic nerve P2X7 receptors mediated Ca2+ signaling, which was absent in P2X7−/− mice (631). The P2X7 membrane currents were recorded from rat and mouse cortical astrocytes in acute slices. These currents demonstrated all characteristic properties of P2X7 receptors; they were activated by millimolar ATP and 100 μM Bz-ATP; the agonist sensitivity was increased by removal of extracellular divalent cations; currents were blocked by Brilliant Blue G, as well as by a rather specific agonist A 438079, and they were absent in P2X7 receptor knockout animals (1261).
Contribution of P2X7 receptors to astroglial physiology is multifaceted, and seemingly, it may involve pathways and mechanisms not related to opening of the cationic channel. Indeed, activation of P2X7 receptors in cultured astrocytes is linked to release of glutamate, GABA, ATP, and other purines by exocytosis, through P2X7-associated transmembrane pore or through Cl−/HCO3−-dependent mechanism of an unidentified nature (93, 457, 458, 1690). Sustained release of glutamate was also observed in astrocytes in situ in hippocampal slices following prolonged activation of P2X7 receptors (503). Activation of P2X7 receptors increased (by ~60 times) synthesis of endocannabinoid 2-arachidonoylglycerol in cultured astrocytes (1840), modulated release of TNF-α (915), stimulated nitric oxide (NO) production (1175, 1194), induced phosphorylation of AKT (783) and p38MAPK/ERK1/ERK2 (565), prompted transmembrane transport of NADH (1015), increased production of lipid mediators of inflammation cysteinyl leukotrienes (91), and regulated NF-κB signaling (797). Evidence also exists indicating P2X7-mediated regulation of expression of P2Y2 receptors (370) and aquaporin-4 (960) in cultured rat astrocytes.
All these functional outcomes of P2X7 receptors activation are mediated through diverse intracellular signaling pathways. The P2X7 receptor has a uniquely extended COOH terminus that interacts with several proteins and hence activates intracellular signaling pathways such as extracellular signal-regulated protein kinases (ERKs), serine-threonine kinase Akt (Akt), c-Jun NH2-terminal kinases (JNKs), mitogen-activated protein kinase (MAPK), and p38 kinase (520). This P2X7-dependent signaling may be activated by low physiological concentrations of ATP, reflecting non-channel mode of receptor function (426). There are also occasional data on heterogeneity of P2X7 signaling. Stimulation of P2X7 receptors led to pore formation in cortical but not in hippocampal astrocytes (173). This heterogeneity may reflect, for instance, expression of different splice variants of the receptor: the splice variant P2X7A is linked to pore formation and cell death, whereas the P2X7B variant does not form the pore and stimulates cell growth (12).
3. Metabotropic P2Y receptors
Metabotropic P2Y purinoceptors are subclassified into the P2Y1,2,4,6,11 and P2Y12,13,14 groups based on phylogenetic similarity and G protein preference (1), with P2Y1,2,4,6,11 receptors being coupled through Gq/G11 proteins to PLC and hence to InsP3-mediated Ca2+ release, while P2Y12,13,14 receptors modulate ion channels and inhibit adenylyl cyclase via Gi/o proteins.
Metabotropic P2Y purinoceptors are widely expressed in astroglia (1810). Cortical astrocytes in culture express mRNA for P2Y1,2,4,6,12,13 and UDP-glucose P2Y14 receptor (4, 141, 435, 541), whereas spinal cord astrocytes predominantly express transcripts for P2Y1,2 receptors (495). In acutely isolated hippocampal CA1 astrocytes, P2Y1 receptors were identified in ~50% of cells at both transcriptional and protein levels although some cells expressed P2Y2,4 receptors. Proportion of cells bearing P2Y2 receptors increased from ~5% at P8−P12 to ~38% at P25 (1961). Isolated rat and human Müller retinal glial cells expressed mRNA and proteins of P2Y1,2,4,6 receptors (532, 533). Immunoreactivity of P2Y1,4 receptors was detected in the nucleus accumbens; in cortex, astrocytes were positively stained for P2Y1,2,4,6 receptors (518).
Stimulation of P2Y receptors of cultured astrocytes, as a rule, leads to PLC-dependent InsP3 production and Ca2+ signals originating from InsP3-induced endoplasmic reticulum Ca2+ release (159, 248, 771, 795, 842, 1347, 1348, 1808). The P2Y-mediated InsP3-induced intracellular Ca2+ release was characterized in cultured spinal cord astrocytes (1532, 1533) and in retinal Müller glia (233). Activation of SOCE often contributes to P2Y-mediated astroglial Ca2+ signaling (893). Stimulation of P2Y receptors evoked [Ca2+]i transients in pituitary glia and in rat neurohypophysial astrocytes (1758, 1773). Pharmacological profiling of astrocytic [Ca2+]i dynamics in vitro and in situ reveals the leading role for P2Y1 and P2Y2 receptors (141, 489), although other receptors (especially P2Y14) may also contribute (541). Propagating astroglial Ca2+ waves require P2Y1 and P2Y2 receptors (142, 223). The P2Y/PLC/InsP3 cascade and resulting Ca2+ signaling were also characterized in detail in astrocytes in situ, in Bergmann glia (880, 1390), in stratum radiatum (221), in olfactory bulb (438), and in the optic nerve (631).
In addition to being closely linked to Ca2+ signaling, P2Y receptors are coupled with numerous intracellular signaling pathways, including MAP kinases [ERKs, the stress-activated protein kinases (SAPKs), the JNKs, and p38/MAPKs, (226, 1201, 1202)]. The P2Y receptors induce Akt phosphorylation in astrocytes in vitro (519), while P2Y1,2,4 receptors are linked to glycogen synthase kinase (GSK)-3, a signaling molecule involved in cell survival, cell cycle regulation, proliferation, and differentiation (1200). The P2Y receptors in astrocytes are also coupled with αvβ3/β5 integrin signaling pathways, which control cytoskeletal remodeling and motility (1872), while P2Y2,4 receptors regulate ERK1/2 and the signal transducer and activator of transcription 3 (STAT3) signaling cascade (1863). Stimulation of P2Y2 receptors induces the phosphorylation and hence transactivation of the epidermal growth factor receptor (EGFR), which regulates astroglial proliferation (1380).
B. Glutamate Receptors
1. Ionotropic glutamate receptors in astroglia
a) ampa receptors.
Functional expression of ionotropic glutamate receptors of the AMPA type in astroglia has been well documented in vitro and in situ. In particular, these receptors were electrophysiologically characterized in astrocytes in hippocampal (777, 1589) and cortical slices (FIGURE 15; Refs. 932, 935), as well as in cerebellar Bergmann glia (1169, 1522). All four main subunits of AMPA receptors (GluA1–GluA4) have been identified in astrocytes, although combinations may vary in different brain regions. In hippocampus, for example, GluA2 and GluA4 subunits are predominantly expressed, which is reflected by linear I–V relation and low Ca2+ permeability (1589). In cortical astrocytes, GluA1 and GluA4 subunits are the most abundant (355). The AMPA receptor complex in Bergmann glia is devoid of GluA2 subunit which stipulates double-rectifying I–V relationship and some (PCa/Pmonovalent ~1) Ca2+ permeability (563, 1169). In the spinal cord, astroglia immunoreactivity for GluA4 subunit was concentrated in the perivascular processes, whereas somata were stained with GluR2/3 antibodies (228). Conditional deletion of AMPA 1/4 receptors from Bergmann glia led to a retraction of glial perisynaptic processes and deficient fine motor coordination (1522).
FIGURE 15.
AMPA and NMDA receptor-mediated currents in cortical astrocytes. A: NBQX inhibits the fast component of glutamate-induced current. Representative traces illustrate the current before, during, and after application of 30 μM NBQX (left panel) as well as the NBQX-sensitive current obtained by subtraction (right panel). The concentration dependence of the block of the fast component for four cells (IC50 = 2.2 ± 0.4 μM, Hill coefficient = 1.9) is shown in the inset. B: d-AP5 inhibits the slow component of glutamate-induced current. Representative traces demonstrating the effect of 1 μM d-AP5 (left panel) and the d-AP5-sensitive component were obtained by subtraction (right panel). The concentration dependence of the block for five cells (IC50 = 0.64 ± 0.1 μM, Hill coefficient = 1.6) is shown in the inset. C: NMDA-induced (2-s application) currents in a single astrocyte and concentration-response curve constructed from six such experiments (EC50 0.34 ± 0.06 μM, Hill coefficient = 1.5). D: glycine-dependent potentiation of astrocyte NMDA response. NMDA-induced currents in glycine-free normal extracellular solution are shown on the top; NMDA-induced currents in the presence of different glycine concentrations (30 nM and 1, 10, and 30 μM) are displayed on the bottom. The concentration-response curve (ΔInorm represents the amplitudes of current increase normalized to the maximal increase at 30 μM glycine) constructed from seven experiments is shown on the right (EC50 1.1 ± 0.07 μM, Hill coefficient = 1.2). [From Lalo et al. (935).]
b) kainate receptors.
Functional expression of kainate receptors in astrocytes is yet to be identified, although the relevant subunits were detected at the mRNA and protein levels (228, 548). Expression of GluK1–5 was also found in reactive (but not in healthy) astrocytes in a model of temporal lobe epilepsy (1798).
c) nmda receptors.
Astroglial expression of the N-methyl-d-aspartate (NMDA) receptors was initially detected by EM immunocytochemistry of cortical preparations: the GluN1 and GluN2A/B labeling was found in peripheral astrocytic processes (52, 354). Transcripts for GluN1 and GluN2A/B subunits were identified in Bergmann glia (1023) and in cortical astroglia (1562). In human astrocytes, transcripts for all seven NMDA receptors subunits (i.e., GluN1, GluN2/A-D, and GluN3A,B) have been found (962). The GluN1 immunoreactivity was detected in adult rat astrocytes from the lateral and basal nuclei of the amygdala in the basolateral amygdala (497), in the bed nucleus of the stria terminalis (600), and in the locus coeruleus (1785). In humans, GluN1, GluN2A, and GluN2B proteins were detected in cortical astrocytes [where they predominantly concentrate in the processes (354)], in fetal astroglia (962), and in Müller glial cells (1414).
Exposure of slices to NMDA induced membrane currents and [Ca2+]i transients in the cortical (935, 1261, 1562), in the spinal cord (1965), and in a subpopulation of hippocampal astrocytes (1400, 1593, 1666), whereas small NMDA-induced Na+ currents were observed in cerebellar Bergmann glial cells (1168). Evidence for functional NMDA receptors in hippocampal astrocytes remains controversial; several studies on slices or on acutely isolated cells failed to identify any NMDA receptor-mediated currents or [Ca2+]i responses (276, 1589, 1600; see also Refs. 714, 1809). Cationic currents mediated by NMDA receptors were characterized in astrocytes acutely isolated (with nonenzymatic vibro-dissection procedure) from mice somatosensory cortex (FIGURE 15). These NMDA-induced currents were positively modulated by glycine and blocked by specific NMDA receptor antagonists MK-801 and d-2-amino-phosphonopentanoic acid (d-AP5) (935, 1809).
Astroglial NMDA receptors are assembled as heterotetramers from GluN1, GluN2 C or D, and GluN3 subunits. This composition defines their specific biophysical properties represented by weak Mg2+ block (which develops at about −120 mV) and relatively low Ca2+ permeability (PCa/Pmonovalent ~3), as well as idiosyncratic pharmacology reflected by sensitivity to memantine and GluN2C/D subunit-selective antagonist UBP141 (464, 936, 1304, 1305). In cortical astrocytes NMDA receptors generate miniature spontaneous currents or they can be activated by neuronal synaptic inputs (932). Opening of astroglial NMDA receptors (in response to endogenous agonist application or synaptic stimulation) results in Ca2+ influx and in generation of Ca2+ signals (1305). In primary cultured adult rat astrocytes, NMDA receptors were found to be activated by mechanical stimulation delivered as fluid shear stress in the absence of agonists (1057). In cocultures of hippocampal astrocytes and neurons, application of NMDA-glycine mixture caused substantial (~30 mV) depolarization of astroglia; this depolarization was sensitive to MK 801 (972). In acute slices from astroglia-specific NR1 subunit knockout mice, application of NMDA with glycine caused much smaller astroglial depolarizations when compared with wild-type controls (972). Activation of astroglial NMDA receptors was also linked to antioxidant transcriptional activation nuclear factor-erythroid 2-related factor-2 (Nrf2), which stimulates release of glutathione precursors, thus boosting neuronal glutathione biosynthesis and providing for antioxidant protection (794).
2. Metabotropic glutamate receptors
The seven-transmembrane-spanning domain G protein-coupled metabotropic glutamate receptors are divided into three groups, according to their G protein coupling and signal transduction. Group I comprises mGluRs 1 and 5, group II includes mGluRs 2 and 3, and group III includes mGluRs 4, 6, 7, and 8. In general, group I receptors are coupled to PLC/InsP3/Ca2+ release cascade, whereas receptors from groups II and III are coupled to adenylyl cyclase (1231).
The mGluR3 that inhibits adenylyl cyclase is the most abundant astroglial receptor throughout life span (1702). In early postnatal development astrocytes almost universally express mGluR5 receptors which, when activated, trigger Ca2+ signals and [Ca2+]i oscillations both in vitro and in situ (361, 879, 947, 1400, 1401). The mGluR-mediated Ca2+ astroglial signals were claimed to accompany hippocampal synaptic transmission, hence providing astrocytes with a detector of the latter (720, 1308); these observations however were not universally confirmed (1700). Astroglial expression of mGluR5 was shown to be downregulated in postnatal development, and these receptors almost completely disappear in adult brain (277, 1702). In the developing brain mGluR5 receptors contribute to functional maturation of astrocytes; ablation of these receptors led to serious deficits in arborization of astroglial processes and expression of glutamate transporters (1149).
C. GABA and Glycine Receptors
1. GABAA receptors
Ionic currents mediated by an opening of GABAA receptors have been widely recorded and characterized in astrocytes in vitro, in acutely isolated cells, and in astrocytes in slices from various brain regions, including hippocampus, cerebellum, retina, hypothalamus, supraoptic nucleus, and spinal cord (339, 773, 853, 854, 857, 1042, 1167, 1336, 1800). These GABAA currents were invariably inward (Cl− efflux) at resting membrane potentials, with Erev around −40 mV reflecting high [Cl−]i (see sect. VIA). Unitary GABAA currents have 30-pS single-channel conductance. The subunit composition of astroglial GABAA receptors remains unknown; α1 and β1 subunits were detected in hippocampal astrocytes (523) and α2 and γ1 in Bergmann glia (1470). The pharmacology of astrocytic GABAA receptors is complex: in Bergmann glia benzodiazepine was inactive [reflecting expression of γ1 subunit (1167)]; in cerebral cortical and fibrous astrocytes, benzodiazepine potentiated GABAA currents, whereas methyl-4-ethyl-6,7-dimethoxy-beta-carboline-3-carboxylate (DMCM) reduced GABAA currents in fibrous astrocytes while potentiating these currents in protoplasmic astroglia (1504). Astroglial GABAA receptors are linked to shaping cell morphology, and their sustained activation (in vitro) leads to an increased complexity of astroglial processes (1087). Astroglial GABAA receptors, however, may have a much more important role in regulation of extracellular Cl− concentration (see sect. XIIA). In freshly isolated hippocampal astrocytes (523) and in astrocytes in hippocampal slices (1098), activation of GABAA receptors triggers Ca2+ influx through voltage-gated Ca2+ channels and hence evokes Ca2+ signaling.
2. GABAB receptors
Metabotropic GABAB receptors are widespread in astrocytes, and their activation primarily results in endoplasmic reticulum Ca2+ release (822, 1098, 1222). Astrocytes express all three types (GABAB1a, GABAB1b, and GABAB2) of GABAB receptors, which, in the hippocampus, tend to be clustered in perisynaptic processes (307). Rather unexpectedly, astroglial GABAB receptors are linked to the PLC/InsP3 cascade through Gi/o proteins (1066), not through Gq/G11 proteins typically required. Activation of GABAB receptors triggers Ca2+ signals and [Ca2+]i oscillations in astrocytes in vitro, in situ, and in vivo (822, 1008, 1066, 1800).
3. Glycine receptors
Glycine receptors, which, similarly to GABAA receptors, belong to pentameric receptors and similarly to GABAA receptors, mediate Cl− fluxes, have been detected in astrocytes in spinal cord slices (1336). Single-cell RT-PCR performed on these astrocytes revealed expression of α1 and (in ~50% of cells) β-subunits of the receptor (874). Glycine is the major inhibitory neurotransmitter in the spinal cord, and, predictably, not only astrocytes but also oligodendroglia and oligodendroglial precursors in this part of the CNS express glycine receptors (1336).
D. Acetylcholine Receptors
1. Ionotropic (nicotinic) ACh receptors
Ionotropic ACh receptors, historically known as nicotinic receptors (nAChRs), are widely expressed throughout the CNS; the nAChRs are archetypal pentameric ligand-gated channels permeable to Na+, K+, and Ca2+. The first evidence for astroglial expression of nAChRs was obtained in autoradiographic studies (726); subsequently, nicotine was found to hyperpolarize cultured astroglia derived from several regions of the brain and the spinal cord (732, 735). Operational nAChRs were found in astroglial cells in cultures and in situ; in both types of preparations, their activation triggers [Ca2+]i elevation resulting from Ca2+ influx through the receptor (1257, 1726) as well as from further amplification by Ca2+-induced Ca2+ release (1597). Precise subunit composition of astroglial nAChRs remains unknown, although receptor Ca2+ permeability infers the presence of α7 subunit [for α7 homomeric receptors PCa/Pmonovalent is ~6 (1312)]. Analysis of mRNA expression in cortical astrocytes found α4, α7, and β2 subunits, whereas in human astrocytes from hippocampus and entorhinal cortex, the α3, α7, and β4 subunits were revealed by immunocytochemistry (601, 1737). Astroglial α7-nAChRs are glio- and neuroprotective. This neuroprotection is possibly mediated through the release of glial cell-derived neurotrophic factor, GDNF (998, 1719). The density of α7 nAChRs is claimed to greatly increase in Alzheimer's disease (1929); furthermore, β-amyloid may either activate or inhibit α7 receptors [depending on concentration (1293)] which in turn may affect astroglial pathological responses and even trigger release of neurotoxic factors (1726).
2. Metabotropic (muscarinic) ACh receptors
Functional M1 and M2 receptors linked to InsP3 production were identified in cultured astroglia, whereas M3 receptors were identified at mRNA level (42, 1179). In hippocampal slices, carbachol (100 μM) triggered [Ca2+]i transients in ~35% of GFAP or S100B positive astrocytes; these responses were blocked by atropine and selective M1 receptor inhibitor pirenzepine (1599). Furthermore, muscarinic ACh receptor-mediated Ca2+ responses were elicited by synaptically released ACh in astrocytes in hippocampal slices (56); arguably, these Ca2+ responses contribute to ACh-dependent synaptic plasticity in the hippocampus. In cortical astrocytes cocultured with hippocampal neurons, carbachol, acting through M3 receptors, induced expression and release of fibronectin and laminin-1, which in turn accelerated neuritogenesis (616); incidentally, release of these factors (and consequently neuritogenesis) was inhibited by alcohol (617).
E. Receptors for Monoamines
1. Adrenergic receptors
The noradrenergic innervation of the brain is provided by numerous projections of neurons, the somata of which are localized in locus coeruleus. These projections do not form localized synaptic contacts; rather, norepinephrine is released from axonal varicosities into the neuropil and hence exerts neurohormonal action through volume transmission. Neurons from rostral and medium portions of locus coeruleus innervate the cerebral hemispheres and cerebellum; projections from the caudal part are sent to the spinal cord. Astrocytes throughout the brain abundantly express adrenergic receptors, which regulate metabolism, morphological plasticity, and physiological responses. The very first observation of catecholamine-mediated increase in cAMP in fetal rat cultured astrocytes was made in 1972 (577). The notion that astrocytes can be an important, if not the main, target of adrenergic innervation (FIGURE 16) appeared in late 1980s (1676) after discoveries that astroglial adrenoceptors control many aspects of cellular metabolism and trophic responses (for early studies, see Refs. 644, 689, 1530, 1757, 1786, 1787).
FIGURE 16.
Direct stimulation of locus coeruleus elicits Ca2+ transients in cortical astrocytes. A: the scheme of experimental setup. B: average fluorescence of the image field in C over time showing Ca2+ response to locus coeruleus stimulation. C: fluo 4-labeled astrocytes taken at time points indicated by numbers in B. Insets are blown-up view of boxed area in pictures. Scale bar, 50 μm. D: images from coronal sections through somatosensory cortex illustrating the effect of N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) on locus coeruleus projection neurons as labeled by antibodies to TH (in red) and MAP-2 (in green) with DAPI labeling of nuclei for orientation. Scale bar, 100 μm (20 μm in inset). E: consistent with the significant reduction in locus coeruleus projection neurons and despite almost twice the stimulation intensity (216 ± 33 vs. 400 ± 39 μA; P = 0.004, t-test), there is a significant reduction in the cortical Ca2+ response to locus coeruleus stimulation in DSP-4-treated animals (P = 0.013, t-test). [From Bekar et al. (123).]
Both α- and β-adrenoceptors have been found in astroglia in vitro, in slices, and in vivo at mRNA, protein, and functional levels (687). Ultrastructural analysis revealed α- and β-adrenergic receptors in astrocytic processes (51, 53), which are often closely associated with varicosities and terminals of axons of noradrenergic neurons (53, 344, 949). Freshly dissociated and FACS sorted murine cortical astrocytes were found to express mRNA for α1A-, α2A-, β1-, and (albeit at much lower level) β2-adrenoceptors (687).
The α1-adrenoceptors are linked to PLC and InsP3 cascades, and their activation elicits Ca2+ release from the endoplasmic reticulum, an effect well documented in astrocytes in culture (247, 1531, 1596), in freshly dissociated cells (1747), in slices (459, 882), and in vivo (FIGURE 17) (431). These receptors are activated by endogenous adrenergic agonists as well as by norepinephrine released from neuronal varicosities following stimulation of locus coeruleus (431). In neocortical slices from 8- to 12-wk-old mice, as well as in freshly isolated astrocytes from the same slices, norepinephrine caused robust [Ca2+]i increase in all astrocytes; on the contrary, the effect on neurons was rather minor. This signaling was mediated by α1 adrenoceptors (Ca2+ responses were mimicked by specific agonist A61603 and inhibited by specific blocker terazosin); the sensitivity of astroglial receptors was high, with KD for norepinephrine of ~360 nM (1311).
FIGURE 17.
Stimulation of α1- and β-adrenoceptors triggers Ca2+ signaling in astrocytes in vivo. A: cortical astrocytes loaded with rhod 2-AM were imaged in layers I/II to detect changes in [Ca2+]i. Agonists were injected using a microelectrode loaded with an artificail cerebrospinal fluid (aCSF) and the tracer Alexa 488. B: representative images of astrocytic [Ca2+]i responses to the α1-AR agonist methoxamine in awake Glt-1-EGFP transgenic mice. Astrocyte-specific loading of rhod 2-AM was confirmed by colocalization with Glt-1-EGFP. Scale bar = 100 μm. C: rhod 2-AM ΔF/F0 traces from B, normalized to Glt-1-EGFP fluorescence. Representative aCSF-microinjection trace is shown at the bottom. D: astrocytic [Ca2+]i responses to adrenergic and acetylcholinergic receptor agonists, measured by rhod 2-AM ΔF/F0. Bar graph shows the percentage of astrocytes within the puff radius responding with a [Ca2+]i increase. ***P < 0.001, one-way ANOVA, Bonferroni correction [n = 25 trials in 6 animals (methoxamine); 14 trials in 4 animals (isoproterenol); 24 trials in 5 animals (dexmedetomidine); 23 trials in 5 animals (carbachol)]. E and F: bar graphs showing average rhod 2-AM ΔF/F0 and response duration from trials with responding cells. **P < 0.01; *P < 0.05, one-way ANOVA, Bonferroni correction [n = 25 trials (methoxamine), 13 trials (isoproterenol), 17 trials (dexmedetomidine), 20 trials (carbachol)]. Data are means ± SE. [From Ding et al. (431).]
The α2-adrenoceptors in astrocytic processes have been identified immunochemically in the intact brain (53, 1118). Their functional expression has been corroborated in primary cultures of astrocytes by α2-adrenoceptor binding (684), by mRNA and protein localization (1118, 1460), and by Ca2+ imaging (123, 1223, 1531).
All three types of β-adrenoceptors, i.e., β1-, β2-, and β3-receptors have been identified in astrocytes in vitro and in vivo using radioligand binding, mRNA, and protein detection as well as immunocytochemistry (298, 997, 1060, 1596). The β1-adrenoceptors are linked to regulation of glycogen synthesis and mediate cAMP-dependent inhibition of astrocytic K+ channels; β2-adrenoceptors are mostly connected to adenylyl cyclase through Gs proteins and are implicated (together with β3-adrenoceptors) in regulation of glucose uptake through modulation of GLUT1 glucose transporter and glucose metabolism through PKA signaling pathways (448, 684, 755, 1514). Individual astrocytes simultaneously express α- and β-adrenoceptors, and stimulation of both receptors triggers temporally distinct cellular events represented by Ca2+ oscillations developing within seconds and much slower tonic increase in cAMP, which saturates within 100s of seconds (723).
2. Serotonin receptors
Serotonin (or 5-hydroxytryptamine) is one of the ancient signaling molecules, being produced and released by bacteria, in which it regulates swimming behavior and growth (1215). Astroglial cells express several types of metabotropic 5-HT receptors, including 5-HT1A, 5-HT2A, 5-HT2B, and 5-HT5A subtypes, which all have been identified at transcript and protein level in glial cultures, in freshly isolated cells, and in the brain tissue (80, 293, 1536). These receptors tend to concentrate at the astroglial processes (293). In general, 5-HT2 receptors are coupled to PLC with subsequent production of InsP3 and DAG and Ca2+ signaling; serotonin-induced [Ca2+]i transients were characterized in astroglia in vitro (1536) and in situ (651).
In astrocytes in vitro, the 5-HT2B receptor seems to be predominant (895). Expression of mRNA of this receptor in astrocytes, freshly isolated and FACS sorted from the mouse brain, is about two times larger than in neurons from the same preparation (1943). There is mounting evidence that serotonin-specific reuptake inhibitors (major antidepressant drugs such as fluoxetine or sertraline) interact with and activate astroglial 5-HT2B receptors (688, 1943) with subsequent Ca2+ signaling (1561) or phosphorylation of extracellular regulated kinases 1/2 (ERK1/2) or upregulation of calcium-dependent phospholipase A2 (cPLA2) (688, 1943). Interactions between these antidepressants and astroglial 5-HT2B receptors may represent a pharmacologically relevant mechanism, while the receptor per se can be a legitimate target for new therapies (1364).
The 5-HT1 and 5-HT5 receptors are connected (negatively for the former and positively for the latter) with adenylyl cyclase. There is also evidence for astroglial expression of 5-HT7 receptor that stimulates adenylyl cyclase: it was found in primary cultures of rat (704, 1614) and human (343) astrocytes and detected immunocytochemically in the mouse suprachiasmatic nucleus (129).
3. Dopamine receptors
Astroglial expression of dopamine receptors was discovered by autoradiography in the striatum (729). Using cell cultures and slices of basal ganglia, D1, D2, D4, and D5 receptors were identified at transcript and protein levels (1134). Similar experiments on striatal astroglial cultures revealed more restricted expression of D1 (1934) or D2 receptors (88). Astrocytic D1 receptors were found in monkey and human cultured striatal astrocytes, with pharmacology somewhat different from rats (1820). Striatal astrocytes were also reported to express D5 receptors (235). Subsequently, dopamine receptors were detected and characterized in cortical structures. Strong expression of D2 receptors was found, at the ultrastructural level, in astroglial processes surrounding cortical interneurons; astroglial D2 expression accounted for one-third of all D2 binding sites in the neocortex from rodents, monkeys, and humans (858). Strong immunoreactivity for D2 receptors was identified in human fibrous astrocytes in the white matter (1135). The D1 receptor subtype was detected in mouse cortical astrocytes in vitro and in situ (1456).
Signaling cascades regulated by dopamine receptors are diverse; generally, activation of D1 and D2 receptors by dopamine or by specific agonists in astrocytes triggers [Ca2+]i transients of variable amplitude and kinetics, and it is universally conceded that these Ca2+ responses originate from InsP3-induced endoplasmic reticulum Ca2+ release (858, 1455, 1459, 1782). An alternative mechanism of dopamine-induced Ca2+ signaling, described in astrocytes cultured from midbrain, cortex, and hippocampus, proceeds through dopamine uptake, oxidation of dopamine by monoamine oxidase B, and release of reactive oxygen species, which induce lipid peroxidation and activation of PLC with subsequent opening of InsP3Rs (1782). In hippocampal slices, dopamine induced a biphasic Ca2+ response comprising initial [Ca2+]i elevation associated with Ca2+ release from the endoplasmic reticulum, which was followed by [Ca2+]i dropping below the resting baseline. The [Ca2+]i increase was mediated by D1 and D2 receptors, whereas [Ca2+]i undershoot by D2 receptors (792). In addition, D1 receptors regulate glycolytic activity [manifested by an increase in cytosolic NADH (1455)]. The D2 receptors were shown to link with αB-crystallin (1595), whereas D5 receptors with phosphoinositide 3-kinase (235). Chronic stimulation of D2 receptors in cultured astroglia with ropinirole stimulated NGF and GDNF secretion from cultured rat cortical astrocytes (1256), whereas in rat hippocampal slices D2 receptors activated by apomorphine (a nonspecific dopamine receptor agonist) induced S100B secretion (1195).
4. Histamine receptors
Astrocytes express H1, H2, and H3 histamine receptors, which have been identified by binding, expression, and functional assays. The first evidence for functional histamine receptors in astroglia derived from autoradiographic studies [which showed binding of H1 and H2 antagonists (725)] and electrophysiology (733): at high concentrations histamine caused depolarization (sensitive to H1 antagonist pyrilamine), while at low concentrations it caused hyperpolarization (sensitive to H2 antagonist cimetidine). These initial observations have been subsequently confirmed by binding and pharmacological assays (see Ref. 808 for references). Transcripts for H1, H2, and H3 receptors have been detected in astrocytes in vitro (809), while H3 receptors were also detected by immunocytochemistry in situ (1100); of note, astroglial H3 receptors expression is rather minor. Functionally, H1 receptors are linked to several signaling cascades including 1) Ca2+ signaling mediated by PLC/InsP3 with subsequent Ca2+ release [this has been characterized both in vitro and in situ (764, 882, 1599)]; 2) regulation of glucose metabolism, the underlying mechanism possibly associated with histamine-induced Ca2+ signaling (60); 3) upregulation of EAAT2/GLT-1 glutamate transporter (496); and 4) stimulation of the release of neurotrophic factors GNGF and neurotropin-3 (809, 987). Activation of H2 receptors stimulates cAMP production and glycogen breakdown (60, 913).
F. Bradykinin Receptors
Bradykinin receptors of the B2 type (the G protein 7-transmembrane metabotropic receptors) have been identified in cultured astrocytes (330); activation of these receptors results in InsP3 production and Ca2+ release from the endoplasmic reticulum (1328, 1672); the [Ca2+]i increase was also concomitant with the activation of an inward current (578). This inward current likely reflects Cl− efflux through Ca2+-activated anion channels, possibly of the VRAC variety (17, 990).
G. Cannabinoid Receptors
Cannabinoid receptors of CB1 and CB2 types are metabotropic receptors coupled to Gi/o proteins. The CB1 receptors were identified in rat cultured astroglia (1667). They were also detected in brain tissue, in astrocytes, in the caudate putamen nucleus (1484), in the dorsal horn of the spinal cord (1529), and in olfactory and limbic structures where CB1 immunoreactivity was concentrated in perivascular glia (1136).
Astroglial CB1 receptors are linked to regulation of metabolism: their activation increases glucose oxidation and ketogenesis in vitro (196, 1534). In astroglial cultures stimulation of CB1 receptors inhibits NO production (1137) and release of TNF-α and chemokines in LPS-activated astrocytes (1601). In astrocytes from mouse stratum radiatum pressure, application of 2-arachidonylglycerol (2AG), arachidonylethanolamide (AEA), (R)-(+)-methanandamide (MAEA), or (R)-(+)-WIN 55,212–2 (WIN) triggered [Ca2+]i transients that were inhibited by 2 μM of the selective antagonist of CB1 receptors AM251. The CB1-mediated astroglial Ca2+ signaling was also reportedly triggered by depolarization-induced neuronal release of endocannabinoids (1197). Subsequently, it was shown that glutamate released from astrocytes acts on presynaptic mGluRs to increase neurotransmitter release in the CA1 and CA3 hippocampal regions (1198). A similar mechanism may also induce presynaptic LTP (592). At the same time, acute stimulation of the CB1 receptors triggered LTD and impaired working memory in mice; these effects were eliminated in conditional astroglia-specific CB1 knockout animals (634).
H. Neuropeptide Receptors
1. Vasopressin and oxytocin receptors
Vasopressin induced [Ca2+]i transients in about two-thirds of cultured astroglia from circumventricular organs; these were sensitive to the broad V1 receptor antagonist d(CH2)5[Tyr(Me)2]8-arginine-vasopressin (810). Similarly, V1 receptors mediated vasopressin-induced Ca2+ signaling in pituicytes (662). There is some regional heterogeneity in astroglial receptors expression: hippocampal astrocytes in vitro were reported to predominantly express V1b receptors, whereas cortical astrocytes express mainly V1a receptor; activation of both subtypes resulted in [Ca2+]i elevation and stimulated glutamate release (1709). Activation of V1a receptors in embryonic cortical astrocytes also caused activation of PKC, CaMKII, and ERK1/2 signaling cascades (1949), while significantly decreasing gene expression of cytokines including IL-1β and TNF-α (1948). These effects were mediated through cAMP response element-binding protein (CREB) activation (1948).
Oxytocin receptors were initially detected in cultured astrocytes using autoradiography (425); subsequently, the functional link of these receptors to PLC/InsP3/endoplasmic reticulum Ca2+ release has been directly demonstrated in rat embryonic hippocampal astrocytes in vitro (424). Similar oxytocin-induced Ca2+ signaling was demonstrated in cultures of female postpubertal hypothalamic astrocytes; incidentally, oxytocin-induced [Ca2+]i transients were blocked by mGluR1 antagonist and potentiated by mGluR1 agonist, which led the authors to suggest that oxytocin receptors are linked to Ca2+ signaling through mGluRs (926). Expression of astroglial oxytocin receptors was controlled by neuronal factors in a rather peculiar way: neuronal conditioned medium increased (probably through transforming growth factor-β, TGF-β), whereas neuronal membranes fraction decreased the level of oxytocin receptors mRNA and number of oxytocin binding sites (1129).
2. Endothelin receptors
There are three endothelins (ET-1, ET-2, and ET-3) and two types of endothelin receptors, ETA and ETB, both belonging to a metabotropic 7-transmembrane domain G protein superfamily. The ET-1 is the preferential ligand for ETA receptors, while all endothelins are equipotent at ETB receptors. In the healthy brain, overall astroglial expression of endothelin receptors is relatively low (812, 1376, 1487). In primary astroglial cultures, ETA and ETB receptors have been identified by autoradiography (724, 1801). Activation of ETA/B receptors triggered [Ca2+]i transients in astroglia (200, 1068). In mouse cerebellar Bergmann glial cells, application of 100 nM of all three isoforms of endothelin triggered [Ca2+]i transients originating from endoplasmic reticulum Ca2+ release; these [Ca2+]i transients were inhibited by the selective ETB receptor antagonist BQ-788 (1767).
Stimulation of ETA/B receptors inhibits astroglial gap junctions due to dephosphorylation of Cx43. This inhibition was found in culture (200, 569) and in hippocampal slices (201), using the dye transfer technique as well as paired patch voltage-clamp recordings (1102). Blockade of gap junctions led to a subsequent inhibition of intercellular Ca2+ waves and intercellular diffusion of glucose (199). Inhibition of gap junctional connectivity occurred rapidly, with complete block of dye transfer in minutes; however, the connectivity recovered within ~1.5 h in the presence of ET-1 reflecting desensitization of ETA receptors. Chronic activation of ETA/B receptors with ET-1 downregulated Cx43 expression, increased astroglial glucose uptake, and boosted astroglial proliferation. Furthermore, activated endothelin receptors increased protein synthesis and changed astroglial morphology in vitro (658). It should be noted that in pathology astrocytes (which normally do not produce endothelin) start to express and release ET-1 and upregulate expression of endothelin receptors (737).
3. Pituitary adenylyl cyclase activating polypeptide and vasoactive intestinal peptide receptors
Pituitary adenylyl cyclase activating polypeptide (PACAP) is a member of the secretin/glucagon/vasoactive intestinal peptide (VIP) family acting through specific receptors classified as PACAP-selective PAC1, VPAC1, and VPAC2 receptors (286). All three types of receptors were immunolocalized in astrocytes throughout the brain (803); in cultured astrocytes, however, predominate expression of mRNA for PAC1 and VPAC2 receptor was detected (70, 605; see also Ref. 1077) for additional references). All three receptor types are linked to multiple signaling cascades; all three activate adenylyl cyclase (656, 1077). PAC1 receptors are, in addition, coupled to PLC and hence stimulate InsP3 production and Ca2+ release from the endoplasmic reticulum (1733, 1734). Activation of PAC1 receptors is also linked to phosphorylation of ERK1/2 and possibly to activation of PKA and PKC (1077). Exposure of cultured astrocytes to picomolar concentrations of PACAP significantly increases their proliferation (656), whereas inhibition of PACAP/VIP receptors suppressed neocortical astrogenesis (1973). Both PACAP and VIP stimulate astroglial glycogenolysis (1653) and induce morphological plasticity as manifested by increased stellation of cultured astroglia (1373).
4. Natriuretic peptide receptors
Atrial natriuretic peptide (ANP), brain natriuretic peptide, and C type natriuretic peptide (CNP) are present in the CNS (1405). They act through binding to natriuretic peptide receptors (NPRs). ANP binds preferentially to NPR-A, while brain natriuretic peptide and CNP bind to NPR-B receptors; all NPs bind with equal affinity to NPR-C (1017). The ANP receptors were first identified in cultured mouse astrocytes by measuring 125I-ANP binding (1739). Subsequent experiments revealed astroglial expression of all three types of natriuretic peptide receptors: the NPR-A (213, 1698), NPR-B (1698, 1967), and NPR-C (1631, 1698). NPR-A and NPR-B are plasmalemma-bound guanylyl cyclase receptors, which mediate intracellular signaling by increasing intracellular cGMP. The NPR-C is a “clearance receptor” that removes peptides from the extracellular space, but does not itself possess guanylyl cyclase activity (1405).
5. Angiotensin II receptors
In primary cultures astrocytes mainly express AT1 angiotensin II receptors (1698); albeit in the healthy brain the expression levels are quite low (1245). The AT1 is a G protein-linked receptor that induces STAT1 and STAT2 phosphorylation with further recruitment of activation and mobilization of the transcription factor NF-κB. Signaling to astrocytes through AT1 receptors seems to contribute to the regulation of permeability of the blood-brain barrier (538). Expression of AT1 receptors is upregulated in various forms of pathology, such as heart failure (768) or lesioning of afferent axons with subsequent synaptic degeneration (538).
6. Somatostatin receptors
Somatostatin receptors have been identified in astrocytes in situ in the hippocampus, amygdala, and hypothalamus by colocalization of a somatostatin-gold conjugate and GFAP immunoreactivity (907, 1107). In the hippocampus, both parenchymal and perivascular astrocytes in the stratum oriens and stratum radiatum of CA1 and the dentate gyrus bind the somatostatin conjugate (1107), with receptors being present in both astrocytic processes and cell bodies. Expression of somatostatin transmembrane receptors (SSTR) was analyzed at the mRNA level, and it appeared that rat and human cortical astrocytes express SST1, SST2, and SST4 receptor subtypes (501); in hypothalamic astroglial cultures only the SST2 subtype was detected (1825).
7. Opioid receptors
First evidence for opioid receptors in astroglia derived from analyzing binding of the opioid ligand [3H]etorphine and κ-receptor ligand [3H]ethylketocyclazocine in cryosections of the pituitary gland; this binding increased significantly after sectioning the pituitary stalk that resulted in gliotic response. On the basis of this observation, the predominant localization of κ-opioid receptors in astrocytes (pituicytes) was advocated (256). Astroglial expression of opioid receptors was also suggested in experiments demonstrating that morphine inhibited norepinephrine-induced accumulation of glucose into rat cortical astrocytes in vitro (1346). Slightly later autoradiography (with [3H]naloxone) demonstrated opioid receptors in astrocytes cultured from embryonic chick forebrain (1043) and operational δ- and κ-opioid receptors were contemplated for cultured rat astrocytes from cortex, striatum, and brain stem on a basis of specific agonists/antagonists effects on cAMP production (483, 484). Selective stimulation of κ-opioid receptors induced [Ca2+]i transients, which were blocked by nifedipine, although the exact mechanism remains unclear (485). Activation of astroglial δ-receptors, however, triggered [Ca2+]i elevation associated with endoplasmic reticulum Ca2+ release (1746). Transcripts of δ-, κ-, and μ-opioid receptors were found in astroglial cultures from cortex, striatum, cerebellum, hippocampus, and hypothalamus, with overall prevalence of δ- and κ-receptors and minor expression of μ-type (1521). Similarly μ-receptors were not detected in astrocytes in situ in rat spinal cord (831). At the same time μ-receptors in immortalized rat cortical astrocytes regulate expression of isoforms of thrombospondins (1387). Activation of opioid receptors exerts various effects on astroglial physiology; for example, by regulating expression of glutamate transporters (983), or affecting astroglial growth (1673) or activating ERK/MAPK phosphorylation signaling cascade (128).
8. Tachykinin receptors
All three metabotropic tachykinin receptors, the NK1 sensitive to substance P, NK2, activated by neurokinin A and NK3, activated by neurokinin B are expressed in astroglia. In cultured astrocytes activation of NK1 receptors by substance P inhibits K+ channels while activating Cl− channels, which leads to depolarization (83). In astrocytes in situ activation of NK1 receptors with 1 μM substance P triggered [Ca2+]i transients in ~15% of all cells tested (651).
9. Bombesin receptors
Bombesin, originally discovered in the frog skin (38), is represented in mammals by neuromedin B, a gastrin-releasing peptide, and neuromedin C. These peptides and their receptors are found across the CNS. Bombesin binding sites were found in astrocytes in rat explant cultures from cortex, cerebellum, brain stem, and spinal cord (728), whereas bombesin BB2 receptors transcripts were identified in cultured human astrocytes (1078). Stimulation of these receptors with neuromedin C and neuromedin B (the latter being 50 times less potent than the former) induced [Ca2+]i transients, membrane hyperpolarization, and (under voltage-clamp) outward (presumed K+) currents with Erev approximately −80 mV (1078).
I. Receptors for Leptin and Insulin
Leptin is the “anorexic adipokine,” the cytokine produced by adipose cells and released into the circulation. Leptin acts on hypothalamic neural circuitry to curb food intake. The leptin receptors [LepR or Ob(ese)R; obese protein being another name for leptin] are membrane spanning receptors of class 1 cytokine receptors family linked to JAK/STAT, PI3K-Akt, or ERK signaling cascades (1730). Probably the first observation of astrocyte-associated Lep-Rs was obtained by double anti-GFAP and anti-LepR immunostaining of the arcuate nucleus and median eminence (322). Subsequently, leptin receptors were immunolocalized in GFAP-positive astrocytes in the subcommissural organ of rabbits (377) and in GFAP-positive glia of the nucleus tractus solitarius (378). Strong astrocytic presence of leptin receptors (again revealed by specific immunoreactivity) was found in mice living on a high-fat diet (742) or in mutant mice predisposed to obesity (1307). The LepR mRNA was localized in astroglia in healthy rat hypothalamus with double-labeling fluorescent in situ hybridization; the LepR protein was colocalized with GFAP-positive astrocytic profiles (743). Incidentally, in adult-onset obesity hypothalamic astrocytes and not neurons show the most prominent increase in LepR expression (742, 1307), which possibly indicate a special role for astrocytes in regulation of food-related behaviors (see sect. XIIM). Leptin receptors contribute to regulation of astroglial glutamate transport and glucose uptake (539).
Insulin receptors (IRs) have been detected in rodent and human astrocytes in primary cultures (673, 1958). Astrocyte-specific (using GFAP and EAAT1 promoters) conditional deletion of astroglial IRs impaired brain glucose sensing, changed astroglial morphology, decreased astroglial coverage of hypothalamic neurons, and suppressed glucose transport into the brain (549).
J. Complement Receptors
Complement, which consists of ~20 plasma proteins and another ~10 membrane proteins, represents an important part of the humoral immune system. Complement activation produces C3 convertase, which in turn activates the third complement component (C3). Subsequent processes result in generation of small fragments with signaling properties, represented by C3a, C3b, and C5a molecules (1354). Receptors for C3a (557, 767, 1615) and C5a (555, 1196) have been detected in human fetal astrocytes and in rodent astroglia; the C5a-like receptor was also identified in rat astrocytes (559). The immunoreactivity for CR1 receptors, which regulate phagocytosis of various particles tagged with C3b, C4b, andC1q, has been found to preferentially colocalize with astroglia in the human brain (514, 556).
K. Platelet-Activating Factor Receptor
Platelet-activating factor (PAF) (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) is a potent biologically active molecule, discovered initially as an activator of platelets; it is operational in various tissues including CNS. Receptors for PAF were, for the first time, detected in cultured astrocytes using binding to specific ligand [3H]WEB 2086 (239). Subsequently, it was found that picomolar concentrations of PAF stimulate formation of InsP3 in astroglia in vitro (1180, 1384) as well as promote concentration-dependent astroglial secretion of NGF (240) and release of prostaglandin E2 from cortical cultured astroglia (1738).
L. Protease-Activated Receptors
Protease-activated receptors are G protein-coupled metabotropic receptors activated following cleavage of a part of extracellular domain by serine proteases; three members of this family (PAR-1, PAR-3, and PAR-4) are activated by thrombin, whereas PAR-2 is a trypsin receptor (1639). Existence of receptors for thrombin in astroglia was suggested following early observation of thrombin-induced stimulation of proliferation and morphological remodeling (599, 1374). Thrombin-activated PAR-1 and trypsin-activated PAR-2 receptors have been initially detected at mRNA level in primary cultured rat newborn astrocytes; activation of these receptors induced [Ca2+]i transients reflecting endoplasmic reticulum Ca2+ release (1770, 1772). The astroglial PAR-1 receptor demonstrated a peculiar mode of desensitization, which involved cleavage of the NH2 terminus (1771). Subsequent analysis revealed expression of transcripts and proteins for all four (PAR1−4) receptors in cultured astroglia, and all four receptors were linked to generation of Ca2+ signals (1855). Astroglial PAR receptors are linked to several intracellular signaling pathways, which include production of ROS and stabilization of hypoxia inducible factor-1α through ERK, JNK, and PI3K/Akt cascades (1962). Activation of PAR-1 receptors (for example, by selective peptide agonist TFLLR) is often used for selective stimulation of astroglia in situ (933, 1607), despite the fact that the very same TFLLR-sensitive receptors are known to trigger neuronal Ca2+ signaling (635).
M. Ephrin Receptors
Ephrins and their receptors (EphA and EphB) contribute to remodeling synaptic contacts (1174); ephrin receptors are tyrosine kinases activated by membrane-associated ephrin ligands. These Eph receptors (as well as ephrins) have been found in perisynaptic astrocyte processes (290, 510). Activation of EphA receptors induced formation of phylopodia and release of glutamate from astroglial cells in organotypic hippocampal slices (1207), while in cultured astrocytes activation of EphB3 and EphA4 receptors triggered secretion of d-serine (1963).
N. Succinate Receptors
The citric acid cycle intermediate succinic acid may rise quite high [from basal level of 5 μM up to 125 μM (709)] in tissues in physiology (for example during exercise) and in disease (for example in hypertension and metabolic diseases). Receptors for succinate known as SUCNR1 (GPCR91) are classical G protein-coupled metabotropic receptors, and they have been localized (at transcript level) in astrocytes (630). Stimulation of these receptors with succinate (with EC50 ~50−60 μM) triggered [Ca2+]i transients in ~15% of astrocytes from acute slices of nucleus accumbens (1138).
O. Toll-like Receptors
Toll-like receptors (TLRs), deeply involved in various defensive mechanisms associated with neuroglia, are type 1 transmembrane glycoproteins containing a leucine-rich repeat (LRR) domain and a Toll/IL-1 receptor (TIR) domain. These receptors are linked to multiple intracellular signaling systems through five intracellular “adaptor” signal transmitting proteins, which, for example, include myeloid differentiation factor 88 (MyD88) that links TLRs to NF-κB, TNF-α, or interleukin cascades (391, 1759). Although expression of TLRs 1−9 was found in cultured mouse astrocytes, the TLR3 (at least in physiological conditions) seems to be the most abundant (292, 1097). Astroglial expression of TLR3 was detected in vitro, in situ, and in vivo (498). In human astrocytes expression of a TLR1−5 and TLR9 has been reported (250, 779); of these TLR2−4 were identified also in vivo, especially in pathological conditions (250, 779, 1695). In general, expression of TLRs remarkably increases in diseased CNS and in reactive astroglia (1759). In the latter, TLR3 mediate production and secretion of numerous neuroprotective factors such as ciliary neurotrophic factor, neurotrophin-4, and vascular endothelial growth factor, consequently, activation of TLR-3 boosts neuronal survival in organotypic brain cultures (250).
P. Steroid Receptors
Classical nuclear estrogen receptors of α- and β-types (ERα or ERβ) have been found in astrocytes in primary and explant cultures from several brain regions (303, 730, 1545); ERβ receptors were localized by immunostaining in the processes and perikarya of astrocytes of hippocampal tissue (78). Estradiol triggered Ca2+ signaling in cultured astroglia (303) through transactivation of mGluR1 and subsequent InsP3-induced Ca2+ release from the endoplasmic reticulum (925). The ERα and ERβ may localize not only intracellularly, but also at the plasma membrane; in addition specific membrane ERs, classified as G protein-coupled estrogen receptor (GPER or GPR30), the Gq-membrane ER (the Gq-mER) and the ER-X have been characterized (6). The GPER and Gq-mER have been detected in astrocytes (26, 924), where they were linked to generation of cytoplasmic Ca2+ signals (924) or modulation of expression of EAAT2 glutamate transporters (836). In cultured astrocytes activation of membrane α-ER receptors by estradiol and related compounds was shown to either inhibit glutamate uptake (1545) or stimulate it by increasing EAAT1/2 expression (836). Astrocytes also express nuclear progesterone receptors (PR), membrane progesterone receptors of the PRA type, and progesterone receptor membrane component-1 (Pgrmc1) (930, 1866) as well as androgen receptors, which were found in primary cultured astroglia (806) and in minor populations of astrocytes in situ (446).
Q. Recapitulation
Numerous receptors expressed in astrocytes safeguard their ability to monitor neurochemical environment and tune homeostatic responses to the requirements of the nervous tissue. Astroglial receptors are linked to intracellular ionic signaling and astroglial metabolism through both ionotropic and metabotropic signaling cascades. Astrocytes follow synaptic transmission by a wide assortment of receptors to neurotransmitters and neuromodulators. The modality and types of these receptors vary between brain regions and show developmental plasticity; the immediate neurotransmitter environment most likely regulates expression of receptors. Notably in the adult awake behaving brain, astroglial calcium signaling is almost exclusively governed by the noradrenergic input from the locus coeruleus.
IX. ASTROGLIAL MEMBRANE TRANSPORTERS
A. ATP-Dependent Transporters
1. P-pumps
a) the Na+-K+-ATPase, or NKA.
The NKA is arguably the most prominent astroglial P-pump, which catalyzes transmembrane movement of Na+ and K+ with stoichiometry of 3Na+ (expelled from the cell):2K+ (imported into the cell). Unequal charge transfer across the membrane stipulates electrogeneity of NKA; as the pump removes 1 positive charge from the cell, the net outward current is generated resulting in a moderate hyperpolarization. The NKA is composed from several subunits: the principal catalytic α subunit, which binds ATP and the selective antagonist ouabain, assembles with β-subunit required for pump operation; the associated γ subunit may regulate the affinity of NKA to K+ and Na+ (832, 1697). The NKA subunits exist in several isoforms (α1−α4 and β1−β3) with distinct tissue and cell localization (191); in the CNS, the α1 subunit is expressed in all cell types, the α2 subunit is present in astrocytes, whereas the α3 subunit is preferentially localized in neurons (690, 805).
Cell specific expression of α isoforms of NKA defines the functional properties of neuronal and astroglial NKA. The affinity of the α2-containing pump to K+ is substantially lower compared with NKA composed of α1 and α3 isoforms (the [K+]0.5 for α2β1 composition is ~3.6 mM, whereas [K+]0.5 for α1β1, α1β2, α3β1, and α3β2 assemblies vary between 0.25 and 0.65 mM; Ref. 942). Consequently, astroglial NKA is activated by physiological rises in [K+]o, whereas neuronal NKA, being fully saturated at physiological [K+]o, is activated solely by an increase in [Na+]i (625, 674, 686, 942, 1496). This distinct sensitivity to extracellular K+ defines the contribution of astroglial NKA to K+ buffering (see sect. XIIA). The astroglial NKA is stimulated by norepinephrine through β-adrenoceptors (625) and regulated by endogenous ouabain-like molecules (690). Loss of function mutation of α2 subunit of astroglial NKA causes deficits in K+ buffering and (through downregulation of glutamate transporters expression) in glutamate uptake, which underlies increased susceptibility to spreading depression and a subtype of migraine with aura, known as familial hemiplegic migraine type 2 (283).
b) Ca2+-ATPases.
Plasmalemmal Ca2+-ATPases (PMCAs) or Ca2+ pumps extrude excess of Ca2+ into the extracellular space thus contributing to the cell Ca2+ homeostasis (1814). The pump operates as a Ca2+-H+ exchanger with stoichiometry 1Ca2+:1/2H+; each transportation cycle is associated with a hydrolysis of one ATP molecule (234). Cultured cerebellar astrocytes were found to express (at both mRNA and protein levels) PMCA1, PMCA4 and to a lesser extend PMCA2 (530). Another Ca2+ pump, the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) is operational in the membrane of the endoplasmic reticulum; it is solely responsible for maintaining high intra-endoplasmic reticulum Ca2+ concentration. Astrocytes predominantly express the SERCA2b subtype of the pump (1152).
2. Astroglial F- and V-type pumps
The F-type and V-type ion pumps are involved in proton transport. The V (vacuolar)-type H+ pumps are present in astrocytes in the plasmalemma (~35% of total amount), in lysosomes, and in secretory vesicles where they provide for an acidic intraluminal environment driving vesicular neurotransmitter transporters (1318, 1388). The F-type pumps are ubiquitously present in mitochondria being the fundamental part of ATP synthesis.
3. ABC binding cassette transporters
The ABC transporters represent an extended superfamily (in excess of 100 members) of ATP-dependent membrane transporters (654) subclassified into five groups (from A to G), which are expressed throughout the CNS. Astrocytes express several members of ATP cassette transporters including, for example, ABCA1, ABCA2, and ABCA3 transporters (863), P-glycoprotein (mdr1a, mdr1b), multidrug resistance-associated-protein (mrp1, mrp4, mrp5), etc. (92).
B. Secondary Transporters of Solute Carrier Family
1. Glutamate transporters
a) Na+-dependent glutamate transporters.
Astroglial glutamate transporters (known as excitatory amino acid transporters or EAATs) are members of SLC1 family of high-affinity glutamate and neutral amino acid transporters (see TABLE 4). Astrocytes specifically express EAAT1/SLC1A6 and EAAT2/SLC1A2, rodent versions of which (respectively) are often referred to as GLAST1 (glutamate-aspartate transporter 1; Ref. 1678) and GLT-1 (glutamate transporter 1; Ref. 1392). The EAAT2 transporter was the first to be identified in the rat brain (381); shortly after the EAAT1 was discovered (1727), and characterization of human variants followed (68). Both EAAT1 and EAAT2 are assembled as homotrimers, and several functional splice variants have been described (for a comprehensive account of the molecular structure and structure-function relations of glutamate transporters, see Ref. 1792). Astroglial glutamate transporters show differential expression in various brain regions: the EAAT1 is predominant in cerebellum (966), in the retina (1442), and in circumventricular organs (148); whereas EAAT2 prevails in all other parts of the brain. Expression of both transporters is rather high: the EAAT1 and EAAT2 account for 0.3 and 1.3% of total tissue protein in hippocampus and 1.8 and 0.3% for all tissue protein in cerebellum. The average density of EAAT1 is ~4,700/μm2 in Bergmann glia and ~2,300/μm2 in the CA1 region, whereas the density of EAAT2 is ~8,500/μm2 in hippocampus and ~740/μm2 in the cerebellum (966). The actual densities of both transporters are certainly much higher in perisynaptic membranes. The EM immunogold experiments clearly demonstrated that EAAT1 as well as EAAT2 are preferentially concentrated in astroglial membranes facing neuropil and their density is lower in the membrane portions facing neuronal somata or other astrocytes (313). Of note, ~10% of total EAAT2 is expressed in neurons (1956).
Table 4.
Astroglial SLC transporters
| Transporter Type | SLC Classification | Stoichiometry | Properties and Function | Reference Nos. |
|---|---|---|---|---|
| Excitatory amino-acid transporters 1 and 2: EAAT1, EAAT2; in rodents known as GLAST and GLT-1 | SLC1A3, SLC1A2 | 3Na+: 1H+: 1Glu (In): 1K+ (Out). Electrogenic | EAAT1/2 provide for ~80% of glutamate uptake in the CNS. Erev >10 mV; do not reverse in physiological conditions. Operation of EAATs results in substantial increase of [Na+]i. | 380, 966, 1290, 1494, 1792, 1939 |
| Sxc− cystine/glutamate antiporter | SCL7A11 | 1 Glu (Out): 1 Cys (In). Electroneutral | Exchanges glutamate for cystine; the latter is a main precursor for glutathione. Regulates extracellular glutamate in extrasynaptic regions. Electroneutral | 23, 326, 844, 1162 |
| Na+-coupled neutral amino acid transporters: SNAT 3, SNAT 5 | SLC38A3, SLC38A5 | 1Na+ (In): 1Gln: 1 H+ (Out). Electroneutral | Exports glutamine from astrocytes. | 680, 1552 |
| GABA transporter: GAT-1/GAT-3 | SLC6A12 | 2Na+:1Cl−: 1GABA (In), (?) 3Na+:1Cl−: 1GABA (In). Electrogenic | Normally removes extracellular GABA. Erev is close to resting Vm, can reverse in physiological conditions. | 121, 356, 1044, 1121 |
| Glycine transporter: GlyT1 | SLC6A9 | 2Na+: 1Cl−: 1Gly (In). Electrogenic | Removes extracellular glycine. Erev is close to resting Vm, can reverse in physiological conditions. | 491, 1932 |
| Cationic amino acid transporter: Acs-1 | SLC7A10 | Electroneutral | Na+ -independent, high-affinity transport of small neutral d- and l-amino acids. In physiological conditions may transport glycine, l- and d-serine and l-cysteine. | 470 |
| Sodium-calcium exchanger: NCX1, NCX2, NCX3 | SLC8A1, SLC8A2, SLC8A3 | 3Na+ (In): 1Ca2+ (Out). Electrogenic | Electrogenic exchanger; Erev close to Vm, operates in both forward and reverse modes. | 195, 588, 878, 1303, 1461, 1725 |
| Sodium-proton exchanger: NHE1 | SLC9A1 | 1Na+ (In): 1H+ (Out). Electroneutral | Removes H+ from astrocytes; Erev >60 mV, hence cannot reverse in physiological conditions. | 321, 402 |
| Sodium-bicarbonate transporter: NBC | SLC4A4 | 1Na+: 2/3 HCO3− (In). Electrogenic | Erev close to Vm, operates in both forward and reverse modes. | 244, 321, 402, 603, 1743 |
| Sodium-potassium-chloride co-transporter: NKCC1 | SLC12A2 | 1Na+: 1K+: 2Cl− (In). Electroneutral | Erev is determined by [Cl−]i, in physiological context; transport is always inward. May participate in clearing large K+ loads. | 789, 818, 942, 1039 |
| Mitochondrial sodium-lithium-calcium exchanger: NCLX | SLC8B1 | 3Na+ (In): 1Ca2+ (Out) | Main pathway for Ca2+ extrusion from mitochondria. In cultured astrocytes interacts with store-operated Ca2+ entry. | 1327 |
| Na+-dependent ascorbic acid transporter: SVCT2 | SLC23A2 | 2Na+: 1Asc− (In). Electrogenic | Accumulates ascorbic acid into astrocytes; part of antioxidant defense. | 149, 553, 896 |
| Concentrative nucleoside transporter: CNT2, CNT3 | SLC28A2, SLC28/A3 | 1 Na+: 1 nucleoside (In). Electrogenic | Accumulates adenosine into astrocytes. Part of adenosine homeostatic cycle. | 975, 1363 |
| Equilibrative nucleoside transporters: ENT-1, ENT-2, ENT-3, ENT-4 | SLC29A1, SLC29A2, SLC29A3, SLC29A4 | Electroneutral | Transports adenosine across plasmalemma according to its concentration gradient. | 205 |
| Na+-dependent dopamine transporter: DAT1 | SLC6A3 | 1/2 Na+:1 DA: 1Cl− (In) | Uptake of dopamine; data on astroglial functional expression are controversial. | 834, 886, 1722 |
| Neutral amino acid transporter subtype ASCT2 | SLC1A5 | 1Na+: 1d-Ser (In) | Transmembrane transport of d-serine. | 1070 |
| Norepinephrine transporter: NET/SLC6A2 | SLC6A2 | 1NA: 2Na+ and 1 Cl−(In). Electrogenic | Uptake of norepinephrine and dopamine; has higher activity for dopamine. | 765, 1577, 1722 |
| Vesicular glutamate transporters: VGLUT1, VGLUT2, VGLUT3 | SLC17A6, SLC17A7, SLC17A8 | Unknown; is H+ and Cl− dependent | Glutamate accumulation in secretory vesicles. | 153, 169, 368, 529, 902, 1144, 1279 |
| Vesicular nucleotide transporter: VNUT | SLC17A9 | Unknown | ATP accumulation in secretory vesicles. | 1550 |
| Vesicular polyamine transporter: VPAT | SLC18B1 | Unknown | Spermine and spermidine accumulation in secretory vesicles. | 693 |
| Glucose transporters: GLUT1, GLUT2 | SLC2A1, SLC2A2 | Electroneutral | GLUT1 is the main astroglial glucose transporter. | 22, 967, 1151 |
| Sodium-dependent glucose transporter | 1Glucose: 2 Na+ (In) | Detected only in astrocytes in vitro. | 1799 | |
| Monocarboxylate transporters: MCT1, MCT4 | SLC16A1, SLC16A3 | Electroneutral | Lactate-exporting transporters. | 151, 1422 |
| Monocarboxylate transporter: MCT2 | SLC16A7 | Lactate-importing transporter. Was identified in astroglial endfeet in the brain stem and in the corpus callosum. | 1356, 1422 | |
| Zink transporter: ZnT | SLC30 | Main Zn2+ transporter. | 1590 |
Astroglial glutamate transporters utilize the transmembrane Na+ gradient as the driving force. Translocation of a single glutamate molecule is coupled with cotransport of 3 Na+ and 1 H+ and with countertransport of 1 K+ (FIGURE 18; Refs. 1290, 1939). With the use of this stoichiometry, both the equilibrium potential for the transporter and glutamate concentrating capacity could be calculated using modified Nernst equation:
FIGURE 18.
Stoichiometry of astroglial solute carrier (SLC) neurotransmitter transporters. EAAT1,2 , excitatory amino acid transporters 1 and 2; Sxc, cystine-glutamate exchanger; GAT1,3, GABA transporters 1 and 3; GlyT1, glycine transporter; ENT, equilibrative nucleotide transporters; CNT, concentrative nucleotide transporters; NET, norepinephrine transporter.
The EEAAT is defined by transmembrane ion gradients and by cytosolic and extracellular glutamate concentrations (877, 1939). The extracellular concentration of glutamate in resting conditions is around 25 nM (677), the cytosolic concentration of glutamate in neurons is usually assumed to be in a range of 1−10 mM. In astrocytes, however, glutamine synthetase catalyzed conversion of glutamate into glutamine ascertains much lower glutamate levels determined at around 0.3 mM (227). Hence, at the resting state, the EEAAT lies at a positive membrane potential around ~9 mV, and reversal of the transporter is possible only in conditions of substantial increase in extracellular K+ and intracellular Na+. Such a reversal can be achieved in experimental conditions (1713), which however reflect a severe pathology associated with a loss of cellular ion homeostasis. In physiological conditions, the stoichiometry of the transporter is capable of supporting ~106 transmembrane glutamate gradient and can operate as an uptake system even when transmembrane ion gradients are seriously perturbed. Transmembrane Na+ gradient contributes the most to setting EEAAT: 10-fold changes in [Na+]o shift the equilibrium potential by 100 mV, whereas 10-fold changes in [Glu−]o, [H+]o, and [K+]o shift EEAAT by 31, 25, and −31 mV, respectively (1939). Nonetheless, in the physiological context, the major role belongs to glutamate, the concentration of which in the synaptic cleft may increase by 5−6 orders in magnitude during synaptic activity. Increase in extracellular glutamate shifts EEAAT toward positive values; at 1 mM of glutamate in the cleft, the transporter reverses at 145 mV. This is a strong safety factor that ensures sustained glutamate uptake regardless of ionic perturbations. Transporter stoichiometry also confers electrogeneity to the EAATs, which generate transmembrane current mainly carried by Na+ ions (877, 1792). This Na+ influx may elevate [Na+]i by 10−20 mM (1777), thus contributing to cytoplasmic Na+ signals (see sect. XB).
Operation of astroglial glutamate transporters is also associated with thermodynamically uncoupled Cl− flux through an aqueous pore (similar to a classical ion channel) that is an intrinsic part of the transporter molecule (1541, 1791). The Cl− current reverses at ECl, and Cl− flux is relatively minor for astroglial transporters, in contrast to EAAT4/5 which have a substantial Cl− permeability. Nonetheless, activation of glutamate uptake was described as the major pathway for Cl− efflux in cerebellar Bergmann glia (1778). The Cl− flux pathway also allows movement of water that is accompanying operation of the transporter to compensate for redistribution of osmolytes (1790). It has been estimated that water flux through EAATs attains ~10% of that through aquaporins (1034, 1036).
Kinetics of the glutamate translocation by EAATs is relatively slow; both EAAT1 and EAAT2 transport ~30 molecules of glutamate per second (1286, 1956). The glutamate binding to the transporters (Km ~20 μM) is however much faster, and hence glutamate transporters concentrated at the perisynaptic processes act as almost instant buffers for glutamate. The higher is the density of transporters, the higher is their buffering capacity (1792). The EAAT2 in cultured hippocampal astrocytes has a remarkable lateral mobility regulated by glutamate, possibly allowing a continuous exchange of glutamate-bound and unbound transporters to maintain high buffer capacity of perisynaptic zones (1177).
b) sxc− cystine/glutamate antiporter.
The cystine/glutamate antiporter, in contrast to Na+-dependent glutamate transporters, exports glutamate from the cell in exchange for cystine (FIGURE 18). This exchanger, known as Sxc−, is a heteromeric amino acid transporter composed of xCT/SCL7A11 and 4F2hc/SLC3A2 proteins (243). In the CNS, Sxc− is mainly expressed in astroglia (23, 326, 844), although it was also detected in some immature neurons, in microglia, in endothelial cells, in ependymal cells, in the choroid plexus, and in the leptomeninges (232). Expression of Sxc− in cultured astrocytes is substantially (up to 7 times) upregulated by dibutyryl-cAMP (581, 1587) and by depletion of intracellular glutathione (1587). The Sxc− antiporter provides cystine required for production of glutathione and contributes to regulation of extrasynaptic level of glutamate (see sect. XIID).
2. Glutamine transporters
Main astroglial transporters for glutamine belong to the SLC38 sodium-coupled neutral amino acid transporter (SNAT) family. Astroglial complement of transporters (also known as N system) is represented by SNAT3/SLC38A3 and SNAT5/SLC38A5; glutamine transport is coupled with cotransport of 1Na+ and countertransport of 1H+ being thus electroneutral (1552). Increase in [Na+]i directly stimulates glutamine efflux (1752).These transporters are optimized for glutamine efflux; the neuronal system A (represented by SNAT1/SLC38A1, SNAT2/SLC38A2, and SNAT4/SLC38A4) is in contrast specialized for glutamine uptake, and it is electrogenic because it cotransports a single molecule of uncharged glutamine with 1Na+ (241). Incidentally, these electrogenic transporters depolarize neurons and hence glutamine may act as an extracellular messenger (1517). Interaction of N and A transporting systems underlie the operation of glutamine-glutamate(GABA) shuttle (see sect. XIID).
3. GABA transporters
Transporters for GABA belong to an extended family (~20 members) of SLC6 sodium- and chloride-dependent neurotransmitter transporters. The GAT1/SLC6A1, GAT3/SLC6A11, and betaine/GABA transporter-1 BGT-1/SLC6A12 have been identified in the CNS, with the first two members being the most important (1585, 1956). The GAT3 is considered to be the more or less specific astrocytic transporter; the GAT1 is predominantly neuronal, although it is also present in astroglia (356, 1044). There is evidence for expression of BGT1 transporter in cultured astrocytes (182, 1263); however, expression in situ has not been unequivocally confirmed. The GAT3 are almost exclusively (as judged by immunocytochemistry) concentrated in astroglial processes oriented towards neuropil which covers symmetric and asymmetric synapses close to neuronal somata, as well as basal and apical dendrites (1121, 1463). There are also some species differences: in rats, for example, GAT3 is localized to astrocytes, whereas in cats, monkeys, and humans substantial expression was found also in oligodendroglia (1406). In the cerebellum GAT3 is highly expressed in the perisynaptic processes of Bergmann glia, which are the main sink for GABA because Purkinje neurons lack GABA transporters (1121). Similarly, in the thalamus, GABA transporters (GAT1 and GAT3) are expressed only in astroglia (387); GAT1 is concentrated in membranes close to synapses, whereas GAT3 is localized more distantly, being thus responsible for extrasynaptic GABA transport (121).
Transport of GABA is thermodynamically coupled with symport of Na+ and Cl− (FIGURE 18) with stoichiometry 1GABA:2Na+:1Cl− (GABA being a zwitterion does not carry any charge); as the result, GABA translocation is associated with net influx of a positive charge (846, 1013, 1420). There is some evidence that Cl− fluxes can be equilibrated, hence reducing the effective stoichiometry to 1GABA:2Na+, although this matter remains unresolved (1007). With the assumption of high cytosolic Cl− content in astroglia, the contribution of Cl− to transporter equilibrium may however be relevant. Very recently, the classical stoichiometry of GABA transporters was challenged by experiments on Xenopus oocytes expressing GAT1 and GAT3; shifts in reversal potentials evoked by 10-fold changes in [Na]o, [Cl]o, and [GABA]o hinted for 1GABA:3Na+:1Cl− coupling stoichiometry (1881).
The reversal potential of GABA transporter (as calculated by Nernst equation) is
EGAT lies around −50 mV for 1GABA:2Na+:1Cl− coupling; the extra Na+ in stoichiometry obviously shifts EGAT to more positive (about −10 mV) values. This is functionally important because EGAT at −50 mV implies that relatively small depolarization and/or an increase in [Na+]i can turn the transporter into the reverse mode, which have been experimentally demonstrated in neocortical slices (1777). Extracellular concentration of GABA also substantially affects the EGAT; increase in [GABA]o shifts the equilibrium potential to positive values thus favoring GABA uptake. Conceptually, however, the probability for astroglial GABA release in vivo in physiological conditions remains questionable (see sect. XIE). To conclude, precise determination of stoichiometry of astroglial GABA transporter is ultimately needed. There is some evidence for lateral mobility of GABA transporters; however, whether it occurs in astrocytes and what is its functional significance remains a matter for speculation and modeling (1584). Finally, membrane presence of GABA transporters can be regulated by internalization (1585).
4. Glycine transporters
Sodium-chloride-dependent plasmalemmal glycine transporters are represented by two varieties, GlyT1/SLC6A9 and GlyT2/SLC6A5. It is generally accepted that the GlyT1 belongs to astroglia (1932), whereas GlyT2 operates in neurons, in glycinergic nerve terminals (491), and in glutamatergic neurons where it possibly regulates extracellular glycine needed for modulation of NMDA receptors (165). Cellular discrimination of receptors reflects different functional roles, with glial GlyT1 being mainly responsible for removal of glycine and termination of transmission and neuronal GlyT2 being responsible for replenishment of releasable pool of glycine in the terminal. There are, however, some indications for GlyT2 expression in astroglia: these transporters were found in the so-called gliosomes (subcellular particles) obtained from spinal cord astrocytes (1423), in cultured rat cortical cultured astrocytes (at mRNA and protein levels), and in brain slices by double immunostaining with anti-GlyT2 and anti-GFAP antibodies (67). Incidentally, there are also some hints for GlyT2 compensating the GlyT1, after the latter was specifically and conditionally knocked out in astroglia (492).
Glycine transporters have a distinct stoichiometry: the GlyT1 cotransports a single molecule of glycine together with 2Na+ and 1Cl− (FIGURE 18), whereas GlyT2 has the stoichiometry 1Gly:3Na+:1Cl− (1510). The equilibrium potential for GlyT1 is defined by extracellular concentration of glycine and intracellular concentration of Na+, and hence can be reversed at physiological membrane potentials. This reversal was demonstrated electrophysiologically in heterologous expression system (76). Release of glycine through reversed transporter was also shown on cultured cortical astrocytes (1605). This release was stimulated by dopamine which rapidly increased glycine synthesis; subsequent increase in cytoplasmic glycine concentration arguably led to a transporter reversal. Release of glycine following reversal of GlyT1 induced by 20-mV depolarization of Bergmann glial cells was found in cerebellar slices (747). The GlyT2 in contrast has a very positive reversal potential; in addition, having an extra Na+ molecule for coupled transport makes the driving force for glycine uptake for GlyT2 two orders of magnitude larger, which allows neuronal transporter to maintain intraterminal glycine concentration ~20−40 mM needed for low-affinity (KD ~20 mM) vesicular glycine transporter (1704). Astrocytes in the brain stem and spinal cord also express high densities of Acs-1/ SLC7A10 amino acid transporters, which mediate efflux of glycine (470).
5. Adenosine transporters
Adenosine transport in astrocytes is mediated by two classes of transporters (FIGURE 18): the plasmalemmal equilibrative [i.e., controlled by adenosine transmembrane gradient (870)], nucleoside transporters ENT-1/SLC29A1, ENT-2/SLC29A2, ENT-3/SLC29A3, and ENT-4/SLC29A4 and Na+-dependent concentrative nucleoside transporters CNT2/SLC28A2 and CNT3/SLC28A3, which cotransport adenosine together with 1 molecule of Na+. These latter transporters have been indentified in cultured (1363) and freshly isolated astrocytes (975).
6. Transporters for monoamines
There are numerous reports indicating that astrocytes in vitro accumulate norepinephrine (642, 727), which uptake (as well as uptake of dopamine) occurs in Na+-dependent manner (1360, 1591), indicating the role for dedicated transporter. This most likely is represented by norepinephrine transporter NET/SLC6A2 that couples monoamine transport with 2Na+ and 1Cl− (FIGURE 18). The NET transports both monoamines, with higher affinity for dopamine than norepinephrine (1294). This transporter was identified in astrocytes at mRNA, protein, immunostaining, and functional levels (765, 1577, 1722). Data on expression of dedicated dopamine transporter DAT/SLC6A3 remain controversial with evidence for (834, 1722) and against (886) its presence in astrocytes.
7. d-Serine transporters
Transmembrane transport of d-serine in astrocytes is mainly mediated by a neutral amino acid transporter subtype ASCT2 (SLC1A5), which is an alanine-, serine-, cysteine-preferring neutral amino acid transporter (1070). The ASCT2 is Na+ dependent, with Na+ to amino acid stoichiometry of 1:1 (1091).
8. Sodium-calcium exchangers
a) plasmalemmal sodium-calcium exchanger, or ncx.
Plasmalemmal Na+-Ca2+ exchanger (NCX) is a fundamental component of cellular Ca2+ homeostasis. The NCX utilizes transmembrane Na+ gradient for export of Ca2+ from the cells against concentration gradient; these exchangers are expressed in the majority of cell types (human erythrocytes being an exception). An important feature of the NCX mechanism is its ability to operate in both forward (Ca2+ extrusion) and reverse (Ca2+ influx) modes, the switch between these modes being determined by membrane potential and transmembrane Na+ gradient (195). It was indeed the reverse mode of NCX operation that was initially discovered in cultured astrocytes using cellular imaging (193) and 45Ca2+ uptake (1724).
The NCX belongs to an SLC8 family (1026) represented by three gene products NCX1/SLC8A1, NCX2/ SLC8A2, and NCX3/SLC8A3, all of which are expressed in astroglia, with some evidence indicating higher expression of NCX1 (1317). Another type of the Na+-Ca2+ exchanger, the K+-dependent NCKX/SLC24, is expressed exclusively in neurons and has not been detected in astrocytes (29). The NCX proteins are mainly concentrated in astroglial peripheral and perisynaptic processes, where they are often colocalized with glutamate transporters and possibly with glutamate ionotropic receptors (194, 1120).
The stoichiometry of astroglial NCX is 3Na+:1Ca2+, and hence the equilibrium potential of the exchanger could be calculated from Nernst equation, which can be reduced to the simple formula ENCX = (nENa – 2ECa)/(n − 2), where n is a stoichiometry of Na+, while ENa and ECa are equilibrium potentials of Na+ and Ca2+, respectively. Assuming [Ca2+]i of 50−80 nM and [Na+]i of 15 mM, the ENCX could be as negative as about −85 to −90 mV, being thus very close (or even slightly more negative) to the resting membrane potential of astrocytes. The ENCX of course is profoundly influenced by fluctuations in [Ca2+]i and [Na+] in the cytoplasm and in the extracellular space, and hence the operational mode of NCX seems to dynamically fluctuate between forward and reverse transport. Conceptually, depolarization and increase in intracellular Na+ concentration favor NCX operation in the reverse mode, whereas increase in [Ca2+]i promotes the forward mode of the exchanger. The role of NCX in Ca2+ extrusion and shaping [Ca2+]i transients has been directly demonstrated in vitro (588, 1725) and in situ (878); inhibition of NCX transport increased the amplitude and prolonged the recovery of [Ca2+]i responses to metabotropic agonists. The reverse mode of NCX has been similarly documented (878, 1303, 1461); Ca2+ influx through the NCX could attain excitotoxic proportion and trigger astroglial death (209, 873). Finally, the reversal of NCX by artificial increase in [Na+]i was demonstrated in astrocytes expressing channelrhodopsin2; activation of the latter by light increased cytosolic Na+, which in turn led to Ca2+ influx through the NCX (1912).
b) mitochondrial sodium-calcium exchanger, or nclx.
Movements of Ca2+ across mitochondrial membrane are fundamental for excitation-energy coupling because an increase in intramitochondrial Ca2+ stimulates energy production by regulating Krebs cycle enzymes (1712). During cellular activity accompanied with [Ca2+]i increase, Ca2+ enters mitochondria through mitochondrial Ca2+ uniporter (MCU), which is, in essence, a highly selective Ca2+ channel (875). Termination of mitochondrial Ca2+ is supported by mitochondrial Na+/Ca2+ and sometimes H+/Ca2+ exchangers. The mitochondrial Na+-Ca2+ exchanger has been isolated and cloned and named NCLX/SLC8B1 [NCLX stands for Na+-Ca2+-Li+ because the latter can also be transported (1302)]. The NCLX is abundantly expressed in astroglial mitochondria and contributes to shaping of cytosolic and mitochondrial Ca2+ and Na+ signals (1327).
9. Sodium-proton exchanger, NHE
The Na+-H+ exchanger expressed in astrocytes is represented by NHE1/SLC9A1 transporter; this is the main system expelling H+ from astroglia (321, 402). The electroneutral stoichiometry [1Na+ in exchange for 1H+ (1278)] is reflected by a positive reversal potential, which ascertains that NHE1 operates as H+ extruder across the whole range of physiological membrane potentials. An important task for NHE1 (FIGURE 19) is to export protons generated by metabolism and acquired by astrocytes in the process of glutamate uptake (each glutamate brings a single H+ ion) and Ca2+ extrusion (PMCA exchanges 1 or 2 H+ for each Ca2+ ion expelled). Activity of astroglial NHE1 is positively modulated by phosphorylation mediated by several protein kinases associated with metabotropic signaling cascades (1454). Acidification of the cytosol by metabolic inhibition triggers substantial influx of Na+, which supports extrusion of protons; this in turn triggers Na+ elevation, Ca2+ entry through the reversed Na+/Ca2+ exchanger, aberrant signaling, abnormal release of glutamate, and cell death (209, 301, 872).
FIGURE 19.
Astrocytes and pH homeostasis. Astrocytes regulate extracellular pH by supplying H+ through operation of NHE, EAAT1/2, MCT, and possibly V-H+ pump, by removing H+ by PMCA and by supplying extracellular space with HCO3− through NBC. EAAT1,2, excitatory amino acid transporters 1 and 2; NHE, Na+-H+ exchanger, PMCA, plasmalemmal Ca2+-ATPase; VH+ pump, vacuolar H+ pump localized in plasmalemma; MCT, monocarboxylate transporter; NBC, Na+-bicarbonate exchanger.
10. Sodium-bicarbonate cotransporter, NBC
Astroglial sodium-bicarbonate transporter NBCe1/SLC4A4 [member of SLC4 family of bicarbonate transporters (1490)] provides for transmembrane flux of bicarbonate being, together with NHE, the main component of astroglial pH homeostatic system (321, 402). Operational NBCe1 was discovered in leech glial cells (405) and subsequently in mammalian cultured astroglia (1246) as well as in astrocytes in hippocampal slices (603). The NBC cotransports Na+ and bicarbonate with a stoichiometry 1Na+: 2HCO3− or 1Na+:3HCO3− [this latter stoichiometry for example was described in retinal Müller cells (1210)], resulting thus in the net influx of one or two negative charge(s), which defines the electrogenic effect of NBCe1 (FIGURE 19). In astrocytes, the NBCe1 operates in both forward mode (which results in an increase in [Na+]i and [HCO3−]i) and reverse mode in which both Na+ and HCO3− are exported from the cell. The ENBCe1 lies around −70 mV, and hence minor changes in astroglial membrane potential or transmembrane gradients of bicarbonate to sodium rapidly change the mode of transporter operation (244, 1743).
11. Sodium-potassium-chloride cotransporter, NKCC1
The Na+-K+-Cl− cotransporter 1 NKCC1/SLC12A2 moves Na+, K+, and Cl− into the cell with electroneutral stoichiometry of 1Na+:1K+:2Cl− (1035). In physiological context, NKCC1 always operates in the forward (i.e., importing all three ions) mode and hence the NKCC1 is implicated in K+ buffering (see sect. XIA). In pathological conditions, excessive increase in [Na+]i may reverse the transporter (850). Astroglial expression of NKCC1 was shown at mRNA level in Bergmann glia (818); NKCC1 was also immunolocalized in astrocytes from optic nerve (1039) and spinal cord (1410). Function of NKCC1 was mainly studied in astrocytes in vitro (1687, 1689) or in the isolated optic nerve (1039). In cultured astrocytes, NKCC1 was responsible for setting high resting Cl− concentration, for cell swelling (789), for K+ uptake, and for the release of excitatory amino acids (1688). Similarly, functional NKCC1 responsible for high [Cl−]i was described in Bergmann glial cells (1778). At the same time, experiments in situ in hippocampal slices questioned a functional role of NKCC1 in protoplasmic astrocytes (942).
12. Vesicular neurotransmitter transporters
Accumulation of neurotransmitters in specific secretory vesicles (see sect. XIB) is a task for three families of vesicular transporters. The SLC17 family contains vesicular transporters for glutamate (VGLUT) and nucleotides (VNUT); SLC 18 family embraces vesicular transporters for acetylcholine (VAChT), monoamines (VMAT), and polyamines (VPAT); while GABA/glycine vesicular transporter (VIAAT or VGAT) is the sole member of SLC32 family (190, 312, 782, 953, 1550). All vesicular transporters use the energy of trans(vesicular)membrane H+ gradient that is created by vacuolar H+-ATPases. The stoichiometry and precise mechanism of vesicular transporters operation is not yet entirely clear; for VGLUT for example, intravesicular H+ and Cl− allosterically activate transport of glutamate (481); VNUT are similarly Cl− dependent (1789).
All three types of vesicular glutamate transporters (VGLUT1–2/SLC17A6–8) have been identified in astrocytes in culture (169, 368, 529, 902, 1144). The data obtained in situ are less uniform. Western blotting, single vesicle imaging, and immunostaining of cortical, hippocampal, and cerebellar slices with subsequent quantitative image analysis failed to identify astroglial expression of all three VGLUTs (977). Similarly, mRNA for VGLUT1–3 was not detected in the RNA-sequencing transcriptome of mouse cortical astrocytes (1945). At the same time, immunogold electron microscopy has detected VGLUT1 associated with glutamate-containing synaptic-like microvesicles located in rat hippocampal astroglial processes (153); immunostaining and confocal microscopy revealed astroglial expression of VGLUT1 in the dentate-molecular layers, in the stratum radiatum of CA1 hippocampal area, in the frontal cortex, and in the striatum (1279). Immunostaining in combination with confocal microscopy as well as immunogold labeling in combination with electron microscopy identified expression of VGLUT3 in astrocytes in hippocampus, in frontal cortex, and in Bergmann glia (1280). Astrocytic expression of VNUT/SLC17A9 transporter has been discovered recently (1550); immunoreactivity for VNUT colocalized with vesicular markers in astrocytes freshly isolated from somatosensory cortex of adult mice (933). Astrocytes were found to express VPAT/SLC18B1 polyamine transporter that concentrates spermine and spermidine in secretory vesicles (693). There are sporadic data on astroglial expression (in vitro) of VMAT as well (1867), whereas VGATs so far have not been found in astroglia. Finally, there are predictions for specific d-serine vesicular transporter VSerT (1071), the molecular nature of which, however, remains unknown.
13. Glucose transporters
The main astroglial glucose transporter is GLUT1/SLC2A1 (22). Immunostaining revealed the presence of this transporter in grey matter astroglia (1151) with preferential localization in endfeet and perisynaptic processes. Exposure of astrocytes to glutamate rapidly (within seconds) and potently (up to 14 times) stimulates glucose uptake into astrocytes acting most likely through GLUT1 (1000). There is some evidence for astroglial expression (probably limited to some brain areas, for example, to hypothalamus) of low-affinity GLUT2/SLC2A2 glucose transporter (967). Astrocytes in culture were also reported to express Na+-dependent glucose transporter SGLT1/SLC5A1, which imports glucose together with 2 Na+ molecules (1799).
14. Monocarboxylate transporters, MCT
The main pathway for astroglial secretion of lactate is provided by plasmalemmal monocarboxylate transporters 1 and 4 (MCT1/SLC16A1, MCT4/SLC16A3) which are specialized on H+-linked transport of lactate, although they have different affinities (Km for MCT1 ~5 mM, Km for MCT4 ~34 mM; Ref. 629). Astroglial localization of MCT1 was confirmed with immunocytochemistry in situ; usually MCT1 immunoreactivity colocalizes with S100B (but not with GFAP) indicating their presence in distal processes (1389). Astrocytes in culture were found to specifically express only MCT1 at transcript and protein levels (396, 1356). Astrocytes in vivo, however, express both MCT1 and MCT4 (151, 1422); apparently MCT4 expression requires higher oxygen tension (1493). In the brain stem and in the corpus callosum of rats (but not mice), some perivascular endfeet of astrocytes were reported to express MCT2/SLC16A7 transporter (1356, 1422). The MCT transporters are equilibrative and may mediate either export or import of lactate depending on concentration gradeints for monocarboxylate and H+ (629).
15. Ascorbic acid transporters
l-Ascorbic acid is a powerful scavenger of reactive oxygen species and part of antioxidant system. Plasmalemmal Na+-dependent vitamin C transporter SVCT2/SLC23A2, which translocates the reduced form of the ascorbic acid (with a stoichiometry of 2 Na+ together with 1 ascorbate), has been identified in cultured astrocytes (149, 896), although it was not detected in situ (149). Focal ischemia following unilateral middle cerebral artery occlusion induced astroglial expression of SVCT2 (150). The SVCT2 transporters have been detected in hypothalamic tanycytes in vitro and in situ at transcript and protein levels (553).
16. Zinc transporter
In the CNS, Zn2+ released at glutamatergic synapses interacts with plasmalemmal receptors, in particular with NMDA receptors, and contributes to synaptic plasticity. Some astrocytes (for example, Bergmann glial cells; Ref. 1860) express high densities of several types of Zn2+ transporter ZnT (belonging to SLC30 family), although the precise role of astroglia in Zn2+ homeostasis and role of Zn2+ in glial signaling and glial-neuronal interactions remains to be characterized (1590).
C. Recapitulation
Membrane transporters are critical elements of astroglial homeostatic system. The genes for transporters are the most represented in the astroglial transcriptome, and similarly their expression level is the highest (275, 1010). The energy-dependent Na+/K+ pump maintains concentration gradients for these two ions, which is critical to sustain membrane potential and to generate the electrochemical driving force for operation of SLC transports. These latter translocate multiple molecules, from ions and neurotransmitters to energy substrates, across the membrane to maintain stable molecular environment of the brain and to support vital neuronal functions.
X. IONIC SIGNALING IN ASTROGLIA
Transient fluctuations in cytosolic ionic content represent the most ancient signaling system. The existence of transmembrane ionic gradients is imperative for life, and the very first cells already were in possession of these gradients. What defined the composition of the ancestral cytosol remains largely unknown, hypothetically, for example, the low [Ca2+]i reflected similarly low Ca2+ concentration in the primeval ocean (848, 1395). Transmembrane ion gradients also developed by following Donnan forces (449) and of course ultimately required selective permeability of the cellular membrane. The resulting ionic disparity and cytosolic fluctuations in ion concentrations have been used for cell signaling needs ever since. Conceptually, changes in cytosolic concentration of any ion, di- or monovalent, can contribute to regulation of cellular events, and hence any ion can act as a second messenger (1277). In astrocytes, the intracellular signaling role for Ca2+ and Na+ is well documented, for Cl− it has been proposed (125), and for K+ it has not yet been considered.
A. Calcium Signaling in Astroglia
The concept of glial Ca2+ excitability was instigated by a series of observations on primary cultures which demonstrated that chemical or mechanical stimulation of astrocytes almost invariably triggers changes in [Ca2+]i either in the form of [Ca2+]i transients or [Ca2+]i oscillations (385, 479, 842, 864, 1093). Discovery of [Ca2+]i waves traveling over astroglial syncytium (306, 360, 361, 382) following focal stimuli bestowed long-range communication capabilities on Ca2+ signals. Studying astroglial [Ca2+]i become popular among gliobiologists with a host of papers published; for comprehensive list of references, we direct the reader to relevant reviews covering 25 years of glial calcium signaling (15, 115, 408, 512, 1518, 1610, 1808, 1814, 1817, 1831).
Calcium ions are arguably the most widespread and ubiquitous signaling molecules operating in all types of living cells (160, 284, 666, 1378, 1394). Calcium homeostasis and calcium signaling in astroglia (simialrly to other eukariotic cells) are maintained by coordinated activity of Ca2+ fluxes created by plasmalemmal and organellar channels, pumps, and transporters and Ca2+ binding to intracellular proteins which serve as Ca2+ buffers and Ca2+ sensors (FIGURE 20; for extended account on Ca2+ signaling, see Refs. 161, 260, 285, 929, 1377, 1379, 1661).
FIGURE 20.
Molecular pathways of astroglial Ca2+ signaling. Cytoplasmic calcium signaling is driven by the concentration gradients for Ca2+ ions between extracellular space and the cytosol and between the lumen of intracellular organelles (mainly endoplasmic reticulum) and the cytosol. Physiological stimulation opens plasmalemmal or intracellular Ca2+ channels, thus creating Ca2+ fluxes which translate into [Ca2+]i dynamical changes in intracellular compartments. In astroglia, Ca2+ signals originate from both InsP3-induced Ca2+ release from the ER calcium store (which can be also amplified by Ca2+ release through ryanodine receptors) and Ca2+ entry via plasmalemmal Ca2+ channels (see sect. VIIC for detailed description), ionotropic receptors (see sect. VIII), or reversed Na+-Ca2+ exchanger. In the cytosol, Ca2+ interacts with Ca2+-bindng proteins, which are represneted by Ca2+ buffers and Ca2+ sensors. The latter are Ca2+-regulated enzymes, which initiate various cellular reactions. Cytosolic Ca2+ signals are terminated by Ca2+ extrusion into extracellular space by Ca2+ pumps and Na+-Ca2+ exchanger and Ca2+ uptake into the ER (by SERCA pumps) and mitochondria (by mitochondrial uniporter). Mitochondrial Ca2+ entry acts as the main link between cell activity and energy production by regulating mitochondrial electron transport and ATP synthesis. CBP, Ca2+ binding proteins; ER, endoplasmic reticulum; GPCR, G protein-coupled metabotropic receptors; InsP3R2, InsP3 receptor type 2; NCX, Na+-Ca2+ exchanger; ORAI, Ca2+ release-activated Ca2+ channels; PMCA, plasmalemmal Ca2+-ATPase; RyR, ryanodine receptors; SERCA, sarco(endo)plasmic reticulum Ca2+-ATPase; SOCE, store-operated Ca2+ entry; TRP, transient receptor potential channels; VGCCs, voltage-gated calcium channels.
Recent advances in Ca2+ imaging revealed a highly variable spatiotemporal organization of Ca2+ signals across complex morphological landscape of astroglial cell. Introduction of genetically encoded Ca2+ indicators and new microscopic techniques demonstrated that astroglial compartments have specific mechanisms controlling [Ca2+]i and hence are capable of generating distinct Ca2+ signals. In physiological (in situ or in vivo) settings, Ca2+ signals in astroglial processes often develop independently from somatic Ca2+ signals and demonstrate singular kinetic profiles and underlying mechanisms. In the distal processes, Ca2+ signals occur as localized microdomains either spontaneously without obvious link to neuronal activity (821, 1208, 1609) or in response to synaptic stimulation (423, 609, 821, 1225, 1660). Local Ca2+ signals in astroglial processes may be linked to specific morphological structures such as “appendages” in Bergmann glial cells (FIGURE 21; Ref. 609) or perisynaptic leaflets (1610). As a rule, local Ca2+ signals in astroglial processes developed independently from the somatic [Ca2+]i transients and had distinct kinetics. Usually [Ca2+]i transients were larger and longer in the soma; in the processes both frequent and relatively fast [Ca2+]i fluctuations (423, 1308, 1831) as well as very slow (~40 s) events, defined as “Ca2+ twinkles” (821) were reported.
FIGURE 21.
Ca2+ microdomains in Bergmann glia. Stimulation of parallel fibers triggers local calcium signals in Bergmann glial cells. A: experimental protocol. Parallel fibers (PF) were stimulated via a pipette connected to a stimulator (STIM) while calcium-dependent fluorescence responses were recorded in a Bergmann glial cell (BG). PCL, Purkinje cell layer. B: confocal fluorescence intensity image of a patch-clamped Bergmann glial cell dialyzed with the calcium indicator Oregon green 488 BAPTA-1 (right). Two processes were distinguished (indicated as 1, 2). C: calcium signals in response to PF stimulation were measured independently for each process (left). Time of PF stimulation is marked by arrows. D: the responding process (1) was further subdivided into regions of interest, in which calcium signals were measured separately (right). Time of PF stimulation is marked by an arrow and a dotted line. (From Matyiash, Grosche, Reichenbach, Verkhratsky, and Kettenmann, unpublished data.)
Spatial heterogeneity of astroglial [Ca2+]i dynamics reflects different molecular mechanisms of Ca2+ signal generation. Genetic deletion of InsP3R type 2 (InsP3R2−/−) led to an almost complete disappearance of spontaneous and evoked somatic [Ca2+]i responses, (14, 820, 1381, 1382), indicative of the predominant role for metabotropic receptors/PLC/InsP3 signaling cascade. In hippocampal astroglial processes, the fastest (half time of recovery ~700 ms) and most localized (microdomain size ~4 μm) [Ca2+]i transients coexisted with longer (recovery half time >3 s) and larger (~12 μm in size) [Ca2+]i microdomains. Both types of localized Ca2+ dynamics in astroglial processes were associated with InsP3R-mediated Ca2+ release; they were inhibited by intracellular injection of heparin and were almost completely eliminated in InsP3R2−/− mice (423). This observation was not universally confirmed, and in many experiments local evoked and spontaneous Ca2+ signals in distal astroglial processes were only partially reduced in the type 2 InsP3R−/− mice (663, 821, 1660). In hippocampal astrocytes studied in situ, spontaneous Ca2+ tranients confined to fine processes persisted in InsP3R2−/− KO mice and were entirely dependent on plasmalemmal Ca2+ influx (1516). Moreover, in type 2 InsP3R−/− animals, astroglial [Ca2+]i transients evoked by metabotropic agonist endothelin were almost completely inhibited in the soma, while they were left unchanged in the processes (1660). Anatomical segregation of Ca2+ signaling suggests alternative routes for Ca2+ mobilization in different parts of the astrocyte. These alternative molecular cascades may involve Ca2+ entry through ionotropic receptors and plasmalemmal channels, or Ca2+ influx mediated by reverse mode of NCX (115, 1610, 1817, 1831). Reversal of NCX may readily occur in response to rise in [Na+]i accompanying operation of major neurotransmitter transporters; such NCX-mediated [Ca2+]i events have been, for example, observed in astrocytes in slices of the olfactory bulb (439). Heterogeneity of Ca2+ mobilization may also arise from alternative intracellular signaling associated, for example, with cAMP (663).
In depth analysis of resting [Ca2+]i in hippocampal astrocytes in situ and cortical astrocytes in vivo with fluorescence-lifetime imaging microscopy revealed a [Ca2+]i gradient between peripheral processes and soma (1950). Basal [Ca2+]i levels in the distal processes of 21-day-old mice were ~125 nM, whereas [Ca2+]i in the soma was ~100 nM. These gradients were even higher in cells from younger animals ([Ca2+]i in processes and soma were ~200 and 130 nM, respectively; Ref. 1950). Incidentally, these experiments also pointed out that astroglial resting [Ca2+]i was ~1.5−2 times higher compared with their neighboring neurons (1950). The gradient of basal [Ca2+]i aimed from the distal processes to the soma highlights regional differences in [Ca2+]i regulation and possibly further corroborates the importance of transmembrane Ca2+ entry in peripheral structures of astrocytes. Compartmentalization of Ca2+ signaling in fine astroglial processes may involve specific positioning of mitochondria which tend to immobilize themselves in sites of focal [Ca2+]i spikes (423). Similarly, functional compartmentalization can occur around plasmalemma-endoplasmic reticulum junctions characterized in astrocytes in vitro; it appeared that key plasmalemmal (NCX1, NKA) and endoplasmic reticulum (SERCA2 and InsP3Rs) molecules coimmunoprecipitated, indicating the link between plasmalemma and endomembrane (968). Finally, localized [Ca2+]i dynamics may reflect specific distribution of various types of receptors or channels at different cellular locations (1831). The physiological relevance for heterogeneous basal [Ca2+]i in astroglia remains to be explored; local differences of [Ca2+]i homeostasis may reflect functional specialization of subcellular compartments.
Further probing of local Ca2+ signaling in astrocytes using membrane-tethered Ca2+ indicator revealed even smaller submembrane [Ca2+]i transients (“spotty Ca2+ signals”) associated with Ca2+ influx through TRPA1 channels (1608). Importantly, Ca2+ mobilizing mechanisms differ between astrocytes from different brain regions: Ca2+ microdomains in Bergmann glia and in the main processes of hippocampal astrocytes were mediated solely by InsP3 receptors (423, 879). The near-membrane [Ca2+]i transients were mediated by TRPA1 in astrocytes in CA1 hippocampal area, whereas TRPA1 contribution was less prominent in stratum radiatum and absent in CA3 region (1608). In neocortical astrocytes, [Ca2+]i transients may be amplified by RyR-mediated [Ca2+]i-induced Ca2+ release (1311), which however seems to be absent in hippocampal astroglia (118). Sporadic evidence even suggests the role for voltage-dependent Ca2+ channels in generating astroglial [Ca2+]i responses (972, 1332). In the in vivo settings, sensory stimulation triggered global [Ca2+]i transients in astrocytes in somatosensory cortex. These Ca2+ signals were initiated in the processes and then spread towards the soma with a speed of 15 μm/s; genetic deletion of type 2 InsP3R eliminated this type of signaling (821). In the olfactory bulb, however, physiological stimulation of the olfactory sensory neuron terminals triggered [Ca2+]i transients only in the astroglial processes, and left the soma idle (1288). In cortical astrocytes from adult (7−20 wk) mice examined in culture, in slices, and in vivo, spontaneous Ca2+ microdomains in fine processes were linked to Ca2+ release from mitochondria mediated by brief openings of mitochondrial permeability transition pore (13).
Further adding to the complexity, astroglial [Ca2+]i dynamics and Ca2+ signaling mechanism undergo developmental regulation (1702, 1950). In the brain of adult nonanesthetized animals, astrocytes in the CNS are under control of almost ubiquitous noradrenergic innervation; sensory stimulation, arousal, and locomotion are associated with release of norepinephrine from locus coeruleus projections which trigger large global [Ca2+]i signals in astrocytes in multiple brain regions (FIGURE 22, Refs. 431, 1341). These global Ca2+ signals were mediated by α1-adrenoreceptors, and inhibition of α1-adrenoceptrs almost completely blocked all spontaneous Ca2+ activity in awake behaving mice (431). A similar role for monoaminergic input has been recently described in Drosophila glia, where Ca2+ signals in vivo were driven by norepinephrine analogs tyramine or octopamine (1033). Similar global astroglial signaling is observed in attention and vigilance state, when widespread astrocytic responses are evoked by acetylcholine release from projection of the nucleus basalis of Meynert and are mediated through mAChRs-InsP3 signaling cascade (316, 1720). Finally, global astroglial [Ca2+]i signaling that occurs throughout the entire cortex was observed in response to transcranial direct current stimulation; this type of Ca2+ signaling was again mediated solely through α1-adrenoceptors (1141). How all these globalized Ca2+ signals interact with Ca2+ microdomains remains hitherto unknown.
FIGURE 22.
Startle stimulation triggers widespread astroglial Ca2+ signaling. A: astrocytic Ca2+ transients measured by rhod 2-AM were detected in response to 30 s of 3-Hz whisker stimulation. Representative local field potential LFP and electromyogram (EMG) recordings are shown. B: representative images of rhod 2-AM fluorescence increases during whisker stimulation in a Glt-1-EGFP animal. Scale bar = 100 μM. C: selected cells from B. Rhod 2 ΔF/F0 was normalized to Glt-1-EGFP fluorescence. Electrocorticogram (ECoG) traces corresponding to rhod 2 ΔF/F0 are shown below. D: air pulses were directed at the face or tail of the animal to elicit a startle response. Representative ECoG and EMG traces are shown with no apparent evoked ECoG response and strong EMG activity, indicative of a startle response. E and F: representative images and corresponding rhod 2 ΔF/F0 traces show stable and repeatable astrocytic [Ca2+]i transients after startle stimulations. Bottom: representative ECoG traces from startle stimulation are shown. G: bar graph showing the response rate of cortical astrocytes to whisker and startle stimulation, averaged across animals. *** P < 0.001, one-way ANOVA, Bonferroni correction [n = 11 animals (parietal cortex startle and whisker stimulation), 4 animals (frontal cortex startle)]. H: scatter diagram with superimposed mean and SE for the delay from the onset of whisker/startle stimulation to the beginning of astrocytic rhod 2 ΔF/F0 responses. Average delay from each successful trial is shown. *** P < 0.001, one-way ANOVA, Bonferroni correction [n = 28 trials in 14 animals (whisker stimulation), 38 trials in 13 animals (parietal cortex startle), and 9 trials in 4 animals (frontal cortex startle)]. Data are means ± SE. [From Ding et al. (431).]
Another fundamental aspect of astroglial [Ca2+]i signaling is linked to the propagating intercellular Ca2+ waves that have been widely considered as the substrate for long-range information transfer in astrocytic syncytia (388, 1553, 1741). Propagating Ca2+ waves have been characterized in detail in astrocytes in vitro (360, 361, 619, 1635, 1935) and in situ (649, 1560, 1622, 1873, 1883), and the mechanisms of propagation have been discerned. The spread of Ca2+ wave was found to be mediated either by intercellular diffusion of InsP3 (or possibly also cyclic ADP-ribose, cADPR) through gap junctions (20, 712, 974) or through regenerative paracrine ATP-mediated signaling (40, 61, 366, 659) or through the combination of both (1553). Operation of these mechanisms seems to be anatomically segregated: for example, ATP-mediated propagation dominates in corpus callosum, whereas in the neocortex Ca2+ waves depend entirely on gap junctions (620).
Appearance, physiological role, and mechanisms of propagating astroglial Ca2+ waves in vivo are yet to be investigated and apprehended. Propagating Ca2+ waves were detected in cerebellum in syncytia of velate astrocytes; they were sensitive to purinoceptor antagonists and spread for ~50 μm at a speed of 4−11 μm/s (722). Spontaneous Ca2+ waves were also observed in Bergmann glia (1081). In contrast, Ca2+ waves have not been detected in the in vivo cortex in both awake and anesthetized animals. In cortical astrocytes Ca2+ signals seem to be restricted to individual cells or to a limited number of adjacent cells, without much spreading to neighboring regions. For example, stimulation of sensory inputs by mechanical displacement of whiskers in mice induced [Ca2+]i transients in astrocytes in the barrel cortex; these Ca2+ signals were confined to individual cells and did not produce propagating Ca2+ waves (1858). Sensory stimulation of astrocytes in the visual cortex of ferrets evoked [Ca2+]i transients in single astrocytes but did not trigger propagating Ca2+ waves (1581). Synchronized somatic [Ca2+]i transients were similarly observed in cortical astrocytes imaged in vivo in response to focal application of α/β-adrenoceptors agonists (123). All in all, the extent of propagation and the velocity of astroglial Ca2+ waves seem to be substantially limited in vivo compared with experiments in the dish.
Propagating fast (at 60 μm/s) Ca2+ waves were found to spread through thousands of astrocytes in mouse hippocampus; these waves were called somewhat romantically “glissandi” from a musical term denoting a rapid series of ascending or descending notes on the musical scale (921). These Ca2+ waves were sensitive to inhibitors of gap junctions and purinoceptors, and somewhat unexpectedly, they were blocked by 2 μM TTX (921). These observations, however, were performed on mice heavily anaesthetised with urethane, while cortices were removed by aspiration to gain the access to hippocampi; consequently these unusually fast Ca2+ waves might reflect the traumatic or toxic injury of the brain tissue.
To conclude, hierarchy of astroglial Ca2+ signals ranges from local microdomains to propagating Ca2+ waves and synchronized global Ca2+ signals (FIGURE 23). Characterization of physiological targets and coding principles of astroglial Ca2+ signaling remain the ultimate and yet unanswered question. Fluctuations in [Ca2+]i obviously impact on gene expression and on astroglial energy metabolism (through accumulation in mitochondria); these however are generic targets of Ca2+ signals in all eukaryotic life forms. Which astroglia-specific processes are governed by [Ca2+]i? How do intracellular proteins decipher incoming Ca2+ signals? The Ca2+-regulated secretion is the most popular target, yet [Ca2+]i may regulate many other astroglial mechanisms from expression of membrane transporters to regulation of K+ uptake (1853). The remarkable heterogeneity in parameters and mechanisms of astroglial Ca2+ signaling most likely reflects an extensive adaptive potential of astrocytes, which depending on the context may employ distinct Ca2+ signaling toolkits or rapidly remodel the existing ones to meet the homeostatic requirements of their immediate environment.
FIGURE 23.
Hierarchy of astroglial Ca2+ signaling.
B. Sodium Signaling in Astrocytes
Stimulation of astrocytes, either mechanical or chemical or synaptic, triggers [Na+]i transients with distinct spatiotemporal parameters; these transients are the substrate of the cytosolic Na+ signaling (881, 1499). The [Na+]i transients in response to physiological stimulations have been observed in astrocytes both in vitro (851, 1496, 1498) and in situ (877, 878, 940, 1495). Regulation of [Na+]i has its own idiosyncrasies: 1) it relies solely on plasmalemmal Na+ transport because of absence of intracellular storage compartments and 2) cells do not have (or we are not yet aware) specific Na+ buffers, which may spatially organize cytosolic Na+ dynamics. The signaling function of Na+ is mediated through the transmembrane concentration gradients which drive multiple plasmalemmal transporters and through “Na+ sensors” represented by Na+-sensitive enzymes. These are by far less characterized compared with Ca2+ sensors, although some of them have been identified. Several metabotropic receptors including class A G protein-coupled receptors (to which adenosine or dopamine receptors belong) have a conserved binding site for Na+, which negatively modulates their affinity to agonists (845). Changes in Na+ promote the dissociation of trimeric G proteins, thus affecting the activation or inhibition of Gβγ-gated ion channels (202, 1471). The Na+-dependent K+ channels, which mediate slow afterhyperpolarization, operate in neurons (170), whereas in astrocytes increases in [Na+]i facilitate spermine-induced inhibition of inward rectifier Kir4.1 channels (916).
Astroglial Na+ homeostasis and Na+ signals are governed solely by transmembrane Na+ fluxes (FIGURE 24); Na+ ions enter the cytosol through plasmalemmal channels and Na+-coupled SLC transporters and are extruded mainly by NKA and possibly by NCX operating in the reverse mode (881, 1499). Resting [Na+]i in astrocytes is relatively high (15−20 mM, see sect. VIA); it defines driving force and sets the equilibrium for many Na+-dependent transporters critical for homeostatic activity of astroglia. Inhibition of NKA with ouabain or by decreasing extracellular K+ results in rapid increase in [Na+]i (586, 762, 869, 1496, 1645, 1846), which indicates significant constitutive Na+ influx at rest. This influx may reflect background channel activity or Na+ entry mediated by plasmalemmal transporters. The NKCC1 seems to contribute to setting resting [Na+]i in astrocytes in vitro, as its inhibition by the diuretic bumetanide decreases resting [Na+]i by several millimolar (851, 1689). The role of NKCC1 in regulation of astroglial ionic fluxes in vivo remains, however, questionable (942). Resting Na+ influx may be also mediated by Na+/bicarbonate (NBC) transporter (851).
FIGURE 24.
Na+ entry pathways. Astroglial Na+ signaling is generated by Na+ entry through plasmalemma; the Na+ influx occurs through Na+ channels, ionotropic receptors, and TRP channels. Na+ entry also accompanies operation of plasmalemmal SLC transporters and Na+-Ca2+ exchanger. Termination of Na+ signals is mainly mediated by Na+ extrusion via Na+-K+ pump. The TRPC channels are the molecular substrate of astroglial store-operated Ca2+ entry; as these channels pass both Na+ and Ca2+, they establish the link between metabotropic activation of Ca2+ release and Na+ signaling. AMPAR, AMPA receptors; EAAT1,2, excitatory amino acid transporters 1 and 2; ER, endoplasmic reticulum; GAT1,3, GABA transporters 1 and 3; GlyT1, glycine transporter; GPCR, G protein-coupled metabotropic receptors; NCX, Na+-Ca2+ exchanger; Nav, voltage-gated Na+ channels; Nax, Na channels gated by extracellular concentration of Na+; NKA, Na+-K+-ATPase; NMDAR, NMDA receptors; P2XR, P2X puriunoceptors; SOCE, store-operated Ca2+ entry; TRP, transient receptor potential channels.
Operation of glutamate transporters (which cotransport 3 Na+ with 1 glutamate) creates substantial Na+ entry, which may elevate [Na+] by 10−20 mM, both in response to endogenous glutamate or to synaptic stimulation (FIGURE 25). Glutamate transporter-associated [Na+]i transients have been observed in cultured astrocytes (310), in situ in Bergmann glia (140, 877), in astrocytes from neocortex (938, 1776), and from calyx of Held (in these [Na+]i increased by ~3.5 mM; Ref. 1780). Uptake of GABA produces smaller Na+ entry (reflecting the 2Na+: 1GABA stoichiometry of GAT) and may increase [Na+]i by 4−6 mM (310, 1777). Sodium entry can also be mediated by ionotropic receptors (878, 940), by TRP channels (1461), or by NCX operating in the forward mode (878). Relative contribution of Na+ entry pathways differs between different astrocytes: in hippocampal astrocytes Na+ influx occurs solely through glutamate transporter, whereas in Bergmann glia the contributions of transporter and AMPA glutamate receptors are almost equal (940). In response to synaptic stimulation, [Na+]i transients are directly proportional to the stimulation strength. At low stimulation intensity, [Na+]i increased only in astroglial processes; increase in the intensity of stimulation resulted in larger [Na+]i transients occupying the whole cell from processes to soma (940).
FIGURE 25.
Astroglial Na+ signaling associated with glutamate uptake in cerebellar Bergmann glial cells. A: voltage dependence of glutamate-transporter current as compared with kainate-induced (AMPA-mediated) currents. The current traces are shown on the top (transporter current on the left and receptor-mediated current on the right), while current-voltage curves for glutamate- and kainite-induced currents are displayed at the bottom. For each cell membrane, currents at different voltages were normalized to the amplitude of current recorded at the holding potential of −70 mV. Note that glutamate-induced responses do not reverse at +20 mV, whereas kainate-induced currents show a reversal potential close to 0 mV. *P < 0.05 and **P < 0.01, significant difference between glutamate- and kainite-induced currents. B: simultaneous recordings of glutamate-induced inward current and [Na+]i elevation in normal conditions in Na+-free (Na+ substituted by NMDG+) solution. C: stimulation of parallel fibers activates membrane currents and transient [Na+]i elevation in Bergmann glial cell. The [Na+]i transient is not affected by inhibitors of ionotropic glutamate receptors. [From Kirischuk et al. (877).]
Astroglial [Na+] transients evoked by either chemical or synaptic stimulation are quite long-lasting with recovery times in a range of tens of seconds (310, 878, 940, 1780), which argues against free Na+ diffusion in the cytosol. Mechanisms shaping kinetics of [Na+]i transients are enigmatic; possibly long-lasting [Na+]i responses may reflect prolonged periods of Na+ entry or Na+ binding/unbinding to plasmalemmal transporters (1494). Similarly unknown is the possibility for generation of [Na+]i microdomains, especially in small subcellular compartments; these diffusion-limiting regions may be associated with perisynaptic processes or with astroglial endfeet (1243). Astrocytic Na+ signals can propagate through individual cells and through astroglial syncytia in the form of spreading [Na+]i waves with a velocity ~60 μm/s; in hippocampal slices, these Na+ waves expand through all neighboring astrocytes (941). Astroglial Na+ waves propagate mainly through Cx30 or Cx43 gap junctions (941).
The existence of spatially restricted localized [Na+]i microdomains is indirectly supported by specific colocalization of Na+-dependent transporters, pumps, and channels in astroglial processes. Electron microscopy of cortical and hippocampal astrocytes revealed concentration of NCX1–3 in distal processes enwrapping excitatory synapses (1120), which coincided with the localization of NMDA receptors (354). In cultured astrocytes, NCX was colocalized with NKA at specific domains of plasma membrane directly opposing outposts of the endoplasmic reticulum (194); local Na+ signaling may occur in these compartments (589). The NKA are also spatially linked to glutamate transporters as indicated by colocalization and coimmunoprecipitation of α2 NKA subunit and EAAT1/2 in cortical astrocytes (329, 1475, 1501). Local Na+ signals associated with glutamate transport in astroglial perivascular endfeet in hippocampal slices increase ATP consumption possibly reflecting local homeostatic adaptation (939).
Astroglial Na+ executes the signaling function through several mechanisms (FIGURE 26). Changes in transmembrane Na+ gradient control multiple transporters responsible for neurotransmitter homeostasis, and relatively small increases in [Na+]i can reverse for instance GABA and glycine transport. Cytosolic Na+ also regulates glutamine-glutamate(GABA) shuttle through direct action on glutamine synthetase (139) and regulation of glutamine transporters (1752). These regulatory mechanisms are of great potential importance in perisynaptic processes, where local [Na+]i transients may control local homeostatic responses. Fluctuations of [Na+]i contribute to regulation of K+ buffering through affecting NKA transport; similarly, [Na+]i is fundamentally important for pH homeostasis by regulating NBC and NHE. By controlling reversal potential of NCX, astroglial [Na+]i transients may contribute to Ca2+ signaling, for example, by initiating local Ca2+ influx in distal processes. Changes in [Na+]i are directly linked to astroglial metabolism, through controlling glycolysis and lactate production and possibly regulating ATP synthesis (309). Cytosolic Na+ signals can invade mitochondria (156); moreover, astroglial mitochondria can generate spontaneous Na+ spikes (77). This mitochondrial connection may further implicate Na+ into regulation of astroglial energy metabolism, which however remains hypothetical (1494).
FIGURE 26.
Na+ signals regulate multiple molecules and multiple homeostatic functions (see text for further details). ASCT2, alanine-serine-cysteine transporter 2; ASIC, acid sensing ion channels; CNT2, concentrative nucleoside transporters; EAAT, excitatory amino acid transporters; ENaC, epithelial sodium channels; GAT, GABA transporters; GS, glutamine synthetase; GlyT1, glycine transporter; iGluRs, ionotropic glutamate receptors; Nax, Na+ channels activated by extracellular Na+; NAAT, Na+-dependent ascorbic acid transporter; NBC, Na+/HCO3− (sodium-bicarbonate) cotransporter; NCX, Na+/Ca2+ exchanger; NCLX, mitochondrial Na+/Ca2+ exchanger; NHE, Na+/H+ exchanger; NKCC1, Na+/K+/Cl− cotransporter; NET, norepinephrine transporter; MCT1, monocarboxylase transporter 1; P2XRs, ionotropic purinoceptors; SN1,2, sodium-coupled neutral amino acid transporters which underlie exit of glutamine; TRP, transient receptor potential channels; ROS, reactive oxygen species; VRAC, volume-regulated anion channels.
In summary, the sodium signaling system may be considered as the pathway for fast coordination of neuronal activity with “homeostatic” response of astroglia mediated through Na+-dependent transporters, concentrated in perisynaptic processes. Influx of Na+ into these perisynaptic processes, characterized by exceedingly high surface-to-volume ratio, may also trigger local depolarization, which will further regulate plasmalemmal transporters.
C. Recapitulation
Intracellular ionic signaling provides the substrate for astroglial excitability coupling external stimulation with functional cellular response. Spatiotemporal fluctuations in cytosolic concentration of two ions, Ca2+ and Na+, emerge in response to physiological stimulation. Astroglial calcium signaling has complex hierarchical organization ranging from highly localized near-membrane Ca2+ microdomains to global Ca2+ spikes and oscillations engulfing whole astrocyte; these global signals further develop into propagating Ca2+ waves in astroglial syncytia. Importantly, in the in vivo awake brain, Ca2+ waves seem to be much more restricted than in the ex vivo preparations. Complex organization of Ca2+ signals (FIGURE 23) reflects distinct mechanisms of generation associated either with production of InsP3 and Ca2+ release from the endoplasmic reticulum (global Ca2+ signals and propagating Ca2+ waves) or with Ca2+ entry through plasmalemmal channels and reversed Na+/Ca2+ exchanger. Astroglial Na+ signaling relies on Na+ entry through plasmalemmal channels and transporters. The two systems of ionic signaling are mechanistically connected as many membrane channels are permeable to both Ca2+ and Na+, while depletion of endoplasmic reticulum stores triggers store-operated activation of TRPC channels that generate fluxes of both ions. Physiological outcome of ionic signals is defined either by direct interaction of Ca2+ with enzymatic cascades or by dynamic changes in transmembrane Na+ gradients that regulate the majority of SLC transporters. Ionic signals regulate several known astroglial functions (see sect. X), although precise pathways are still in need of in depth experimental analysis.
XI. ASTROCYTES AS SECRETORY CELLS OF THE CNS
A. The Concept of Astroglia as Gliocrine Cells
Astroglial secretion has been postulated at the beginning of 20th century. In 1909, Hans Held observed, using the molybdenum hematoxylin stain, granular inclusions in neuroglial processes, which he interpreted as a sign of active secretion (671). A year later, Jean Nageotte visualized secretory granules in parenchymal grey matter glia using the Altmann method of fucsin labeling (1185). These secretory granules (also known as gliosomes) have been often described afterwards, and astrocytes were linked to an endocrine role, secreting substances from endfeet to the blood (579, 1362). This endocrine role has never been confirmed; however, astrocytes were found to be capable of secreting a remarkable assortment of molecules [see TABLE 5; of note, astroglial secretome contains ~180 proteins (450, 849)] that contribute to the regulation of CNS development and homeostasis, synaptogenesis, and cognitive function. This stimulated the concept of glycrine system in which neuroglial cells (as microglia and to some extend oligodendrocytes are also capable of releasing biologically active agents) are regarded as elements of the brain-wide secretory network (1797, 1811). Astroglia-derived secretory substances include (TABLE 5) 1) classical neurotransmitters; 2) neuromodulators; 3) neurotransmitter precursors; 4) hormones and peptides; 5) eicosanoids; 6) metabolic substrates; 7) scavengers of ROS; 8) growth factors; 9) various “trophic” factors that, for example, regulate synaptogenesis and synaptic connectivity; and 10) pathologically relevant molecules such as inflammatory factors. Apart from these, astrocytes constantly secrete ions (most notably Cl−) and water. Secretion pathways operational in astrocytes include 1) vesicle-based exocytosis, 2) diffusion through plasmalemmal pores/channels, and 3) extrusion through plasmalemmal transporters (FIGURE 27). The same molecule can be released through different pathways.
Table 5.
Astroglial signaling molecules and mechanisms of their secretion
| Secreted Substance | Function | Secretion Mechanisms |
|---|---|---|
| Neurotransmitters | ||
| Glutamate | Activates glutamate ionotropic (AMPA, KA, and NMDA) and metabotropic receptors in neurons and neuroglia. May regulate synaptic strength and neuronal excitability and affect synaptic plasticity. | Exocytosis (368, 745, 993, 1065, 1145, 1328, 1906, 1942); connexons (572, 1919); P2X7Rs (457, 503); Best-1 channels (1893); VRAC: (111, 506, 867, 991, 1717); cystine-glutamate antiporter Scx− (1080, 1162); reversed EAAT1/2, only in severe pathological conditions (1290, 1713). |
| [H+]i-dependent glutamate release in Bergmann glia. | ||
| ATP | Activates neuronal and glial ionotropic P2X receptors (excitatory action) and metabotropic P2Y receptors (Ca2+ signaling; astroglial Ca2+ waves, trophic effects). Rapidly degrades to ADP, AMP, and adenosine by ectonucleotidases, adenosine acts through A1 (inhibitory effects), A2A, A2B, and A3 (metabotropic effects) receptors in neurons and neuroglia. | Exocytosis (513, 628, 933, 1334, 1795); lysosomes (785, 979, 1947); connexons (61, 324, 365, 823, 1663, 1681, 1754); Panx-1 (1691); P2X7R (1690) |
| GABA | Inhibitory neurotransmitter acting on neurons and on neuroglia. | Reversed GABA transporters, GAT1 or GAT3 (670, 1776, 1777); Best-1 chloride channels (964); VRAC (900) |
| Glycine | Inhibitory neurotransmitter in the spinal cord. Coagonist for NMDA receptors. | Reversed glycine transporters GlyT1 (492); VRAC (327) |
| Precursors for neurotransmitters and neuromodulators | ||
| Glutamine | Precursor for neuronal glutamate and GABA. | Glutamine transporters: sodium-coupled neutral amino acid transporters SNAT3/SLC38A3 and SNAT5/SLC38A5 (1552, 1752) |
| Pro-enkephalin | Precursor for enkephalins (112). | Unknown |
| l-Serine | Precursor for neuronal d-serine (1913). | Alanine-serine-cysteine transporter 1, ASCT1 and ASCT2 transporters |
| Neuromodulators | ||
| Taurine | Agonist to both glycine and of GABAA receptors. In supraoptic nucleus astroglia-derived taurine regulates inhibitory glycine receptor tone (327). | Connexins (1918); VRAC (327, 867, 1918) |
| l-Aspartate | Positive modulator of NMDA receptors. | VRAC (867); connexons (1919) |
| Kynurenic acid | Inhibitor of NMDA and acetylcholine receptors; astroglia-derived kynurenic acid affects glutamatergic (291, 1396), GABAergic (122), cholinergic (1969), and dopaminergic (1441) transmission. Aberrant production and synthesis can be associated with schizophrenia (1918). | Unknown |
| Lipoprotein receptor-related protein 4 (Lrp4) | Regulates presynaptic glutamate release (1703). | Unknown |
| Endozepines | Expressed at high levels in astrocytes in thalamus and in astrocytes and tanycytes in hypothalamus. Astroglia-derived endozepine acts as a positive modulator of synaptic inhibition in the thalamic reticular nucleus (332). | Unknown |
| Polyamines, spermine, and spermidine | Spermine and spermidine are predominantly localized in astrocytes; they can be released through Ca2+ dependent process and regulate various cellular functions, including modification, stimulation, or inhibition of AMPA, NMDA receptors, K+ channels, and gap junction. | Exocytosis (693) |
| Hormones and neuropeptides | ||
| Atrial natriuretic peptide (ANP) | Local vasodilatator. Contributes to control of systemic salt intake. | Exocytosis (911) |
| Endothelin-3 | Local vasoactive hormone (471). | Unknown |
| Sphingosine 1-phosphate | Regulates cell proliferation and immune response. | ATP-binding cassette transporter A1 (1544) |
| Neuropeptide Y | Metabotropic neurotransmitter. | Exocytosis (1407, 1425) |
| Thyroid hormones thyroxine (T4) and triiodothyronine (T3) | Astrocytes exclusively express type 2 deiodinase (D2) that converts T4 to T3. Astrocytes accumulate T4 by organic anion transporting polypeptide 1C1 (OATP1C1/SLCO1C1), convert it to T3, which is then released to brain parenchyma. | L-type amino acid transporter 2 LAT2/SLC7A8 (1156) |
| Sphingosine 1-phosphate | Regulation of cell proliferation and immune response. | ATP-binding cassette transporter A1 (1544) |
| Eicosanoids | ||
| Arachidonic acid, prostanoids | Multiple; including intercellular signaling and control of innate immunity. | Direct release from membranes (1178, 1905, 1907) |
| Metabolic substrates | ||
| Lactate | Possible energy substrate in neurons. Possible activator of specific metabotropic receptors. | Monocarboxylate transporters MCT1, MCT4 (151, 1422); pannexons (?) (833); lactate-permeable channel (1656) |
| Citrate | Astrocyte synthesize and release citrate in physiological context (1877). In the extracellular space, citrate may possibly regulate (through chelation) Ca2+ and Mg2+. | NaDC/SLC13A (Na+-dependent dicarboxylate transporter) (1297); VRAC (?) |
| Growth factors | ||
| Neurotrophins: NGF, NT-3, BDNF | Multiple trophic effects including regulation of neuronal survival, growth, and regeneration. | Largely unknown. Pro-BDNF (secreted by neurons) is taken up into astrocytes by endocytosis, and after conversion into mature form is released by exocytosis (147) |
| Pro-inflammatory factors | ||
| TNF-α | Mediates AMPA (117) and GABA receptor trafficking (1408); affects and may even induce synaptic plasticity; impairs learning and memory (621). | Unknown |
| IL-1β, IL-6 | Control of neuroinflammatory response; regulation of learning and memory, trophic effects (328, 488). | Unknown |
| C3a complement factor | Modulation of neuronal morphology and function (through C3a receptor); control of neuroinflammatory response. | Exocytosis, lysosomes(?) (931, 982, 1674) |
Reference numbers are given in parentheses.
FIGURE 27.
Multiple astroglial secretory pathways.
B. Exocytosis
Exocytosis utilizes specific membranous organelles carrying heterogeneous cargo and capable of fusing with plasmalemma, hence providing the release of their luminal content. Exocytosis is an evolutionary conserved (appearing probably in Archea) and ubiquitous property of most eukaryotic cells (1657). Exocytotic vesicular release (that underlies both constitutive and regulated secretion) is regulated by an extended family of SNARE (soluble N-ethyl maleimide-sensitive fusion protein attachment protein receptor) proteins. Of these, R(arginine)-SNAREs (also known as vesicle-associated membrane proteins or VAMPs) are associated with the membranes of secretory vesicles, while Q(glutamine)-SNAREs are associated with plasmalemma (784, 839). In the process of excitation-secretion coupling, high [Ca2+]i instigates formation of a ternary SNARE complex from R/Q-SNAREs, which mediates fusion of vesicles with plasma membrane (1694).
1. Astroglial secretory organelles
Astrocytes possess intracellular (synaptic-like microvesicles, dense-core vesicles, and lysosomes) and extracellular (ecto- and endosomes) secretory organelles (FIGURE 28). Intracellular vesicles are cellular organelles that may completely fuse with cellular membranes, whereas extracellular vesicles are membranous compartments released into the surrounding environment.
FIGURE 28.
Astroglial secretory organelles.
a) synaptic-like microvesicles, or SLMVs.
Astroglial SLMVs have a diameter of 30−100 nm; they usually appear in pairs or in groups (up to 15) of vesicles, thus being much less numerous than neuronal synaptic vesicles, 100−1,000 of which are clustered in presynaptic terminals (153, 169, 804). The SLMVs were identified in processes and somata of astrocytes from various brain regions (including hippocampus, cortex, and cerebellum) both in culture and in situ (153, 169, 1145, 1280). The role for large (1−3 μm) SLMVs identified in astroglial processes in hippocampal slices (825) remains unclear. Astroglial SLMVs contain vesicular neurotransmitter transporters VGLUT1–3 and VNUT (see sect. IXB12). In astrocytes in culture, SLMVs were found to store glutamate or costore glutamate and d-serine (1071, 1160). In contrast, analysis of astrocytes in situ revealed distinct populations of glutamate and d-serine-containing vesicles (153). Also, intracellular mobility of astroglial vesicles has its peculiarities: for example, glutamate-containing vesicle movement accelerates with an increase in [Ca2+]i (1671), whereas peptidergic vesicles and endolysosomes slow down (1402, 1404); such differences have never been seen in neurons.
b) dense-core vesicles, or DCVs.
Astroglial DCVs usually have a diameter of 100−600 nm, and their population is substantially smaller than SLMVs (279, 368, 753, 1407), even smaller DCVs (~50 nm) were also reported (1404). In astrocytes in culture, DCVs were found to carry secretory proteins secretogranin II (279, 1295, 1407) and secretogranin III (1296), chromogranins (753), ANP (902, 1295), neuropeptide Y (1407, 1425), and ATP (341, 1310). The chromogranin and secretogranin II-containing DCVs were also found in human astrocytes from tissues obtained during surgery (753).
c) secretory lysosomes.
Lysosomes are the essential intracellular organelle of eukaryotes generally responsible for degradation and recycling of macromolecules and autophagy (909), although lysosomes may also participate in secretion. There are several reports indicating the role of lysosomes in astroglial secretion, in particular in Ca2+-dependent release of ATP in astrocytes in culture (785, 979, 1947). Astrocytic secretory lysosomes have diameters of 300−500 nm; they can be tagged with various fluorescent conjugated dextrans (1793), with FM-dyes or with [2'/3′-O- (N'-methylanthraniloyl)adenosine-5′-O-triphosphate], or MANT-ATP (1947); they coexist (and can be coreleased) with other types of secretory vesicles in the same cell (993). Astroglial secretory lysosomes are in possession of VNUT, which mediate accumulation of ATP (1292), and they express lysosomal specific markers such as cathepsin D, lysosomal-associated membrane protein 1 (LAMP1), and ras-related protein Rab7 (1947). Lysosomal exocytosis is mediated by tetanus toxin-insensitive vesicle-associated membrane protein VAMP7 (304), and downregulation of VAMP7 suppresses ATP release (1804). Lysosomal fusion was also reported to be triggered by distinct slow and localized Ca2+ signals (1804). Similarly to other cells, secretory lysosomes in astrocytes are likely to play a role in membrane repair (43).
d) extracellular vesicles.
The extracellular vesicles represented by exosomes and ectosomes carry a wide spectrum of bioactive substances including cytokines, signaling proteins, mRNA, and microRNA. Exosomes are formed intracellularly from multivesicular bodies (1083), whereas ectosomes are produced through direct budding of the plasma membrane (1744). Astroglial exosomal secretion has been observed in response to oxidative and heat stress (1735) or in pathology, for example, in astrocytes surrounding amyloid plaques in a mouse model of familial Alzheimer's disease (1854). Some astroglia-released exosomes were also containing mitochondrial DNA (613). Despite high expectations of exosome-mediated intercellular signaling in the CNS (537), the astroglial component of it remains largely uncharacterized.
Shedding of ectosomes from astroglial membrane is triggered following activation of P2X7 purinoceptors. It occurs because of rapid activation of acid sphingomyelinase that moves to the plasma membrane outer leaflet and modifies membrane structure/fluidity leading to vesicle blebbing and shedding (174, 175). Astroglial ectosomes are heterogeneous with diameters between 100 and 1,000 nm (174, 1411). These ectosomes were reported to carry fibroblast growth factor-2 and vascular endothelial growth factor (1411), IL-1β (174), nucleoside triphosphate diphosphohydrolases (302), matrix metalloproteinases (1551), and acid sphingomyelinase and high levels of phosphatidylserine on their membrane outer leaflet (174).
2. Slow time course of astroglial exocytosis: molecular determinants
Kinetics of exocytosis differ very much between different cells, being determined by 1) number and spatial clustering of vesicles, 2) the nature of SNARE proteins, 3) number of R-SNAREs associated with the vesicle, and 4) the spatio-temporal organization of “trigger” [Ca2+]i nanodomain (469, 839, 1716). Kinetics of astroglial exocytosis are fundamentally different that of neurons. In the latter, fusion is extremely fast, being typically fully accomplished in <0.5−1 ms (1694), whereas in astrocytes an exocytotic event develops in a time scale of seconds (1811). Indeed, time lapse video monitoring of fluorescently labeled VGLUT1/2-containing vesicles in single astrocytes showed the development of fusion within hundreds of milliseconds after the onset of [Ca2+]i increase (1065, 1537). Even slower exocytosis (developing in seconds) was observed when imaging synapto-pHluorin-labeled vesicles (222, 993). Similar slow kinetics were observed using fluorescent synaptobrevin 2-labeled vesicles (1056). Exocytosis of peptidergic vesicles in astrocytes unfolded with about a minute delay after stimulation (1295, 1407, 1425); similarly slow was the lysosomal release of ATP (1310, 1413, 1947). Capacitance measurements further corroborated the slowness of astroglial exocytosis, finding it about two orders of magnitude slower than in neurons (901, 902).
This sluggish (when compared with neurons) exocytosis reflects several idiosyncrasies of astroglial excitation-secretion coupling. First, astrocytes contain fewer vesicles, and these vesicles are not clustered as it is typical for active zones of neuronal terminals (153, 169, 804). Second, the spatiotemporal evolution of Ca2+ signals in astroglia, which are often associated with Ca2+ release from the stores, is slower than in neuronal terminal where “trigger” Ca2+ signals originate solely from Ca2+ entry through voltage-gated channels. Third, astroglial SNARE complexes as well as their vesicular densities differ from their neuronal counterparts: in neurons exocytosis is supported by the ternary fusion complex formed by VAMP2, SNAP25, and syntaxin, whereas in astrocytes the ternary fusion complex assembles from VAMP2/3 or VAMP7, SNAP23, and syntaxin (1811). Substitution of SNAP25 with SNAP23 in the ternary complex halves its stability, arguably impeding the tethering/docking/fusion process. Further delays in exocytotic kinetics are associated with the densities of R-SNAREs associated with a single vesicle: there are ~25 VAMP2 molecules linked to a single vesicle in astrocyte versus ~70 molecules per vesicle in neurons (1625, 1716).
3. Nonexocytotic functions of vesicles in astrocytes
Intracellular vesicles contribute to the delivery of various molecular components to the plasmalemma. In particular, the VAMP2 labeled vesicles in astrocytes in vitro were immunopositive for the AMPA receptor subunits GluA1, 2, 3, which can be incorporated into the plasma membrane upon vesicular fusion (368). Similar mechanisms may regulate the density of the astroglial glutamate transporter EAAT2 (978, 1670), and disruption of this delivery pathway compromises astroglial glutamate uptake in pathology (1505). Vesicles in astrocytes in vitro also have been reported to carry CB1 cannabinoid receptors (1284) as well as AQP4 water channels (1403). Vesicles also deliver membranes, and hence exocytosis is involved in membrane repair (43, 1659) and possibly in astroglial morphological plasticity (1796).
C. Diffusion Through Membrane Channels
Secretion by diffusion through plasmalemmal pores is driven by transmembrane concentration gradient, which for many biologically active molecules could be rather high. For example, cytosolic ATP concentration (~5 mM) exceeds ambient extracellular concentration (low nM) by ~107 times, whereas glutamate ([Glu]i ~0.3 mM, [Glu]o ~25 nM) exceeds by 104 times. Any conductive pore large enough to accommodate the secreting molecule can therefore act as a conduit, and nonvesicular release of various signaling molecules have been identified in different tissues [for instance in taste buds (871) or erythrocytes (1415)]. Connexin hemichannels (see sect. VIIH) were probably the first (711) and are certainly the most studied (572) diffusion secretion pathway in astrocytes. Activation of connexin hemichannels occurred in response to lowering extracellular Ca2+ (711) or metabolic inhibition (358), or treatment of cell cultures with proinflammatory cytokines (1457). Opening of these hemichannels was associated with the release of glutamate or ATP (143, 1681, 1919), and hence, the role of unpaired connexons in release of astroglial signaling molecules was considered (61, 365, 1553). In particular, connexon-mediated release of ATP has been recognized as a paracrine signal critical for the generation of intercellular Ca2+ waves in cultured astroglial cells (365, 1681). Pannexons also have been implicated in glutamate and ATP release from astroglia (572) and were shown to mediate release of d-serine (1306).
The repertoire of hemichannel-mediated secretion could be much wider. There are indications that numerous signaling molecules such as NAD+, adenosine, InsP3, cyclic ADP-ribose, nicotinic acid adenine dinucleotide phosphate, lactate, and NO (249, 509, 596, 833, 1147, 1643) all can pass through Cx hemichannels in either direction (i.e., serving for secretion or uptake depending on concentration gradients). In addition, hemichannels could support uptake or release of several metabolites such as glucose, ascorbate, or glutathione (572, 1431). Both connexons and pannexons are also permeable to several synthetic molecules including Lucifer yellow, ethidium, calcein, propidium, 5 (6)-carboxyfluorescein and 2-(4-amidinophenyl)-1H-indole-6-carboxamide, and DAPI, which are often used for monitoring the activity of these channels (1525). Connexons are densely expressed at perivascular astroglial endfeet and may therefore contribute to astroglia-vasculature signaling. Release of ATP (with subsequent degradation to adenosine) from glia limitans endfeet through Cx43 hemichannels was claimed to induce dilatation of pial arterioles (1904). Release of ATP from astrocytes through Cx43 gap junctions was also shown to affect synaptic activity in hippocampal slices; Cx43 were activated by local drop in [Ca2+] following photoactivation of exogenous extracellular Ca2+ buffer (1754). The Cx43 hemichannels seem to be active in resting conditions when they provide constitutive ATP secretion to regulate basal excitatory transmission (324). Astroglial connexons (and possible pannexons) have been also implicated in release of lactate in acute slices from the brain stem, hippocampus, hypothalamus, and cortex (833). Inhibition of astroglial Cx43 channels in vivo by injection of selective inhibitor TAT-L2 peptide into basolateral amygdala impaired memory consolidation and resulted in amnesia for auditory fear conditioning. This can be rescued by coinjection of a somewhat eclectic mixture of neuroactive molecules (glutamate, glutamine, lactate, d-serine, glycine, and ATP) (1663). Opening of astroglial hemichannels is caused and regulated by many factors, which include membrane depolarization, mechanical stimulation, decrease in extracellular or increase in cytosolic [Ca2+], fluctuations in pH, reactive oxygen species, or intracellular phosphorylation of connexins (572). Activity of connexons is also regulated by growth factors, proinflammatory cytokines, or CB1 cannabinoid receptors (535, 1275), whereas in tanycytes Cx43 channels are activated by extracellular glucose (1273).
A second route for secretion by diffusion is associated with plasmalemmal ion channels. The most characterized of these channels are P2X7 purinoceptors operating in dilated (pore-forming) mode (see sect. VIIIA). The P2X7 receptors have been implicated in the release of ATP and excitatory amino acids (457, 503, 1690). There is also evidence for glutamate release through TREK-1 channels following activation of metabotropic receptors. Apparently, G protein βγ-subunits directly activate TREK-1 channels by interaction with the NH2 terminus of the latter (1893). Glutamate and GABA may also diffuse through anion channels, including volume-regulated anion channels and Best-1 chloride channels (1893). Glutamate released from astrocytes through these channels was claimed to activate synaptic NMDA receptors in CA1 pyramidal neurons (636), thus modulating synaptic plasticity (1323). Evidence about Best-1-mediated secretory pathway, however inspiring, is yet in need of independent confirmation.
D. Transporter-Mediated Release
Plasmalemmal transporters widely contribute to astroglial secretion; they are involved in release of many of the neuroactive molecules originating from astroglia. Specific roles of plasmalemmal transporters are summarized in TABLE 5 and discussed below.
E. Signaling Molecules Secreted by Astrocytes
1. Neurotransmitters
Experimental evidence accumulated over the last decades demonstrated that astrocytes are capable of releasing major neurotransmitters:8 glutamate, ATP, GABA, and glycine (see Refs. 55, 1383, 1526, 1922 for extensive reference lists). Astroglial secretion of ATP is probably the best documented, and it has been observed in cell cultures, in slices and, to some extent, in vivo and may occur through both exocytosis (933, 934, 1183, 1971) and diffusion through membrane channels (324, 1663). ATP released from astrocytes serves as a source of adenosine, which, in turn, provides for brain-wide inhibition and contributes to sleep homeostat (628, see also sect. XII).
Astroglial release of glutamate also received much attention starting from the first observation on cultured astroglia that demonstrated Ca2+-dependent glutamate release from astrocytes stimulated with metabotropic agonist bradykinin (1328). This initial finding was followed by numerous confirming experiments in vitro and some in situ, which further strengthened the concept of exocytotic release of glutamate from astroglial cells. Data for glutamate diffusion through plasmalemmal channels were also mounted (see Refs. 55, 1144, 1330, 1971 for references and also refer to the previous section).
Proving exocytotic astroglial release of neurotransmitters in vivo turned out to be a challenging task. The majority of the in vivo data were derived from a transgenic mouse model expressing a dominant negative (dn)SNARE (which is the cytosolic tail of VAMP2) in astrocytes (628, 1334). The cytosolic tail of VAMP2 competes with VAMP2 in the ternary complexes, thus suppressing exocytosis. The (dn)SNARE mice displayed changes in behavior and alterations in synaptic transmission which were thought to reflect occluded astroglial exocytosis (700, 933, 1183, 1765). At the same time there is some evidence for (dn)SNARE penetrating to neurons, thus raising the possibility that the impairment of neuronal, rather than astroglial, exocytosis may account for the phenotype observed (540, 1633). Glutamate may also be released from astrocytes via connexons and pannexons as well as through anion channels as has been discussed in the previous section. Recently a [H+]i-dependent glutamate release pathway was detected in Bergmann glia, although the molecular nature of this pathway remains to be clarified (146).
Astrocytes in the neonatal optic nerve (1250), in the brain stem (198), and in the cerebellum (133, 1072) were claimed to contain GABA and express GABA synthesizing enzyme glutamic acid decarboxylase or GAD67. Immunoreactivity for GABA was also observed in ~80% of hippocampal astrocytes (955), although another report (1921) found only ~20% of GABA-positive astrocytes in hippocampus. In addition to GAD67-mediated synthesis, GABA may also be produced from putrescine degradation by monoaminoxidase B (MAOB) which is also predominantly an astroglial enzyme; this pathway was documented for Bergmann glia (1923).
Astrocytes do not express vesicular GABA transporter and hence are incapable of exocytotic GABA release. Nevertheless, Ca2+-independent GABA release was detected in cultured rodent (544, 545) and human (961) astroglia and confirmed in observations in situ on cerebellar Bergmann glia (98) and on astrocytes from olfactory bulb (900) as well as from hippocampus and cortex (670, 876, 955).
Astroglial release of GABA can be mediated either by the reverse mode of the GABA transporter (48, 544, 670, 876) or by diffusion through plasmalemmal anion channels, such as volume-regulated anion channels (900) or Best-1 anion channels. The latter pathway received much promotion recently (964, 1922), although the role for Best-1 had been already met with criticism (429). Whether astrocytes can indeed release appreciable quantities of GABA in physiological context remains doubtful. Uptake provides the only realistic source for GABA in astrocytes. Accumulated GABA is readily metabolized by being fed into tricarboxylic acid cycle by GABA transaminase (1575), and hence, cytosolic GABA concentration must be rather low. Cytosolic concentration of GABA, in turn, is critical for both transporter-mediated and for diffusional release. Surprisingly, information on intracellular GABA concentration in astrocytes in vivo is unavailable, and therefore, the probability and role of physiological astroglial GABA release is yet to be judged. Astrocytes are also reported to release glycine, either by reversed glycine transport (492) or through VRAC (327). In the brain stem and in the spinal cord, glycine is, arguably, released from astrocytes by the Na+-independent alanine-serine-cysteine 1 (Asc-1) transporter, and this release is critical for maintaining the glycine content in neuronal presynaptic terminal and hence for tonic inhibition of motor neurons (470).
2. Neuromodulators
a) l- and d-serine.
The concept of d-serine as a specific astroglial neuromodulator received much popularity in recent years following initial observations claiming the exclusive astrocytic presence of d-serine and its synthesizing enzyme serine racemase (1558, 1559, 1892). Physiological experiments were to follow; these demonstrated exocytosis of d-serine-containing vesicles from astrocytes in vitro (1071) and found that manipulating with astroglial Ca2+ signaling or with astroglial metabolism affects NMDA-mediated synaptic transmission in brain slices arguably through the lack of astroglia-derived d-serine (515, 675, 1161). Furthermore, inhibition of resting Ca2+ entry through genetic deletion of TRPA1 channels was found to decrease constitutive secretion of d-serine and reduce long-term potentiation (1608). Subsequent analysis, however, questioned all these results, when astroglial immunoreactivity for d-serine and serine racemase appeared to be largely an artifact (see Ref. 1891 for comprehensive account). More selective antibodies revealed predominantly neuronal expression of both d-serine and serine racemase [forebrain neurons (838, 1131), Purkinje neurons (1131) and magnocellular neurons (1859)]. Selective transgenic suppression of serine racemase in neurons caused its marked (~65%) decrease in cortical and hippocampal tissues, whereas selective interference with astrocytes produced only marginal effects (144). Release of d-serine from cortical slices was found to be Ca2+ independent, with the bulk of this release mediated by neuronal alanine-serine-cysteine transporter 1 (Asc-1/SLC7A10) (1502). Pharmacological inhibition or genetic deletion of Asc-1 reduced LTP at the hippocampal Schaffer collateral-CA1 synapse because of decreased d-serine release (1543). Finally, a conditional knockout of serine racemase from neurons but not from astrocytes impaired LTP at the same synapses (144); neuronal deletion of the enzyme also reduced dendritic remodelling and plasticity (94).
The astrocytic role in d-serine neuromodulation is, most likely, confined to the supply of the l-serine, the substrate for serine racemase. In the absence of l-serine, neuronal d-serine production cannot proceed. The l-serine is synthesized almost exclusively in astrocytes endowed with specific enzymes, such as for example 3-phosphoglycerate dehydrogenase, or Phgdh (1910, 1913). Genetic deletion of the latter specifically from astrocytes severely depressed (by ~80%) neuronal synthesis of d-serine (1913). Mutations of the Phgdh cause profound neurodevelopmental deficits, with patients showing low levels of d- and l-serine in the cerebrospinal fluid (889). Hence, astrocytes are indispensable for d-serine neuromodulation through production of l-serine and its shuttling to neurons.
b) kynurenic acid.
Kynurenic acid (or KYNA) is an endogenous inhibitor of NMDA receptors (by blocking the glycine binding site) and even more potent noncompetitive and allosteric inhibitor of α7 nicotinic receptors (699). KYNA is a product of tryptophan metabolism; de novo synthesis of KYNA occurs through transmutation of bioprecursor kynurenine catalyzed by kynurenine aminotransferases (KAT), of which KATII is the predominant brain enzyme present almost exclusively in astrocytes (614, 615). Astroglial secretion of KYNA contributes to regulation of glutamatergic (291, 1396), GABAergic (122), cholinergic (1969), and dopaminergic (1441) neurotransmission and hence is involved in regulation of various cognitive processes (1895). Impairments of astroglial KYNA production and secretion are connected to various pathologies, of which the most notable is the link to pathophysiology of schizophrenia (1583).
c) lactate.
In addition to being an energy substrate, lactate, produced in astrocytes through aerobic glycolysis and released by MCT1/4 transporters and/or hemichannels (833), may act as a signaling molecule mediating astroglial-neuronal communication (152, 433). Lactate directly modulates neuronal excitability by suppressing firing of hippocampal neurons in vivo (576) and of cerebral neurons in culture (224). In locus coeruleus, lactate release from optogenetically activated astrocytes excites neighboring neurons and triggers norepinephrine release both in slices and in vivo (1729). Molecular substrates of lactate signaling are yet to be fully identified; the hydroxycarboxylic acid (HCA)/GRP81 G protein-coupled receptor linked to the cAMP signaling cascade is one candidate; these receptors are expressed in many neurons and also in endfeet of astrocytes (951).
3. Hormones
a) thyroid hormones.
Astrocytes are an indispensable element of the thyroid regulation of the CNS. The l-thyroxine (or T4) secreted by the thyroid gland represents a prohormone that needs to be converted (through de-iodination) into the biologically active triiodothyronine (or T3). The T4 crosses the blood-brain barrier by several dedicated transporters (1887), and the conversion into T3 occurs in astrocytes, which specifically express the converting enzyme type 2 deiodinase (D2) (612). Uptake of T4 into astrocytes is mediated by organic anion-transporting polypeptides 1c (OATP14/SLCO1C1) (421), and T3 is released into extracellular space (for subsequent uptake into neurons and other glial cells) by L-type amino acid transporter 2 (LAT2/SLC7A8) (859).
b) neurosteroids.
Although all steroid hormones readily penetrate the blood-brain barrier, they are additionally synthesized within the CNS, which is considered to be steroidogenic tissue. Neurosteroids are produced in both neurons and neuroglia and are involved in local autocrine or paracrine signaling (6, 437). Neurosteroids, being lipophilic, do not require specific transmembrane translocation pathways. In hypothalamus, astrocytes synthesize progesterone, the synthesis of which is regulated by estradiol acting through membrane endoplasmic reticulum α receptors, transactivation of mGluR1, and Ca2+ release from the endoplasmic reticulum with subsequent activation of protein kinase A that stimulates progesterone synthesis from cholesterol (315, 1112). Estradiol (which derives through aromatase-induced conversion of testosterone) was not found to be produced in healthy astrocytes, which lack aromatase; however, the latter is upregulated following injury (294).
4. Growth factors
Astrocytes synthesize and release several growth factors, including fibroblast growth factor type 2 (FGF) (1756), epidermal growth factor (EGF) (1073), insulin-like growth factor (IGF) (320), and neurotrophins such as nerve growth factor (NGF), neurotropin-23 (NT-3), and brain-derived growth factor (BDNF) (453). Mechanisms of growth factor secretion remain largely unknown. In hippocampus, astrocytes provide for maturation of pro-BDNF secreted by neurons. Pro-BDNF is taken up into astroglial cells by endocytosis, and after conversion into the mature form, it is released by VAMP2-mediated exocytosis (147).
5. Neuropeptides
Astrocytes synthesize several neuropeptides in a region specific manner (1769). The main neuropeptides produced in astroglia include angiotensinogen (766, 1679), substance P (1113), somatostatin (1616), proenkephalin and enkephalin (1101, 1616), neuropeptide Y (NPY) (102), nociceptin (272), and atrial natriuretic peptide (ANP) (1095). Secretion of NPY is Ca2+ dependent and involves exocytosis of DCVs (1425). Similarly, astroglial ANP is also stored in vesicles and secreted by regulated exocytosis (911).
6. Polyamines
Polyamines, spermine and spermidine, influence various cellular processes, from gene expression and protein synthesis to protection (1349). In particular, polyamines act as endogenous modifiers of AMPA (447) and NMDA (1254) receptors, Kir4.1 channels (1627), and connexons (134). In the CNS spermine and spermidine are predominantly found in astrocytes (950), whereas polyamine synthesizing enzymes mainly show neuronal localization. The pathway for polyamines shuttling to astroglia is unknown. Depolarization induces Ca2+-dependent release of polyamines from brain slices which may reflect exocytosis from astrocytes that express polyamine vesicular transporter VPAT/SLC18B1 (693).
F. Recapitulation
Astrocytes are dedicated secretory cells in the CNS and release ~200 bioactive molecules ranging from neurotransmitters and neuromodulators to growth factors and hormones. These molecules are released by several mechanisms, and quite often the same molecule can be secreted by different pathways. This, arguably, increases the versatility and flexibility of astroglial secretion. The detailed description of astroglial vesicular release (and in particular the molecular machinery responsible) in vivo remains to be accomplished. In contrast to neurons, where secretion is restricted (with some exceptions) to presynaptic terminals, astrocytes provide for humoral signaling by releasing bioactive agents into extracellular space, where they approach multiple targets. Gliosecretion regulates multiple brain functions from synaptic transmission to morphological plasticity and metabolism.
XII. PHYSIOLOGICAL FUNCTIONS OF ASTROGLIA
A. Ion Homeostasis in the CNS, or Ionostasis
Ionic homeostasis of the CNS is of paramount importance for nervous function, because it defines overall excitability and major signaling processes. Ion concentrations in the nervous tissue, however, are not static, and ion fluctuations regulate major systemic processes such as memory (683) or sleep (432). Astrocytes through numerous ion transporters are fundamental for ionostasis.
1. Potassium homeostasis
Control of interstitial K+ concentration is the most canonical function of astroglia. The critical role for astrocytes in maintaining extracellular K+ homeostasis in the CNS was proposed in the mid-1960s by Leif Hertz, Steven Kuffler, and Richard Orkand. Both energy-dependent NKA-mediated pathway (682) and diffusion through K+ channels (1276) have been suggested as underlying mechanisms. Rises of [K+]o during neuronal activity are associated with repolarizing K+ efflux (710), with a single action potential increasing local [K+]o by ~1 mM (1433). In addition, K+ exit is linked to an activation of postsynaptic ionotropic glutamate receptors (1465), with particularly large K+ efflux through NMDA receptors (1612) or with K+ release through K+-Cl− cotransporter activated during GABAergic transmission (1822). Extracellular K+ may also rise following K+ efflux from astrocytes associated with operation of EAAT1/2 glutamate transporters. Arguably, most of K+ released in synaptic compartments is associated with dendritic arborizations, while axonal action potentials account for a smaller fraction of K+ entering the interstitial space (679, 741). Physiological increases in [K+]o in the nervous tissue occurring in relation to neuronal activity are rather moderate; for example, mechanical stimulation of skin results in 0.4 mM [K+]o increase in the spinal cord (669), while [K+]o rises by 0.5 mM in occipital cortex in response to visual stimulation (1624). Maximal increase of 3 mM was recorded in the spinal cord in response to noxious cutaneous stimulation (1708). Direct repetitive electrical stimulation of the cortical surface or thalamic nuclei may increase [K+]o in the somatosensory cortex by 8–12 mM (667), and even higher levels can be observed in pathology (683). It needs to be remembered, of course, that experimental techniques for ion concentration measurements have serious spatial limitations, and K+ concentration could be higher in tiny extracellular compartments. Astroglial regulation of extracellular K+ is mediated through several overlapping mechanisms, which can be generally classified into 1) diffusion through K+ channels, 2) active transport by NKA, and 3) transport by SLC transporters. All three mechanisms have been studied, and numerous experiments in vitro and in situ found evidence corroborating their contribution (683, 892, 1267, 1642).
The initial concept (892, 1276, 1843) of astroglial spatial K+ buffering postulated that K+ enters glia through plasmalemmal K+ channels; subsequently, K+ ions are redistributed through glial syncytium via gap junctions and released distantly. This concept has been found to operate also at a single-cell level in Müller glia. Here, K+ enters these cells though Kir4.1 channels densely populating perisynaptic processes in the inner plexiform layer of the retina. Subsequently K+ is equilibrated through the cell and is released (again through Kir4.1 channels) from the endfoot or from perivascular processes. This type of K+ buffering was defined as K+ siphoning (892, 1211). The concept of spatial K+ buffering rests on the ability of Kir4.1 channels to accumulate K+ locally and on gap junctions, which provide for K+ redistribution. Experiments in vitro have demonstrated that Kir4.1 channels indeed are mainly responsible for resting K+ permeability of astrocytes and could be considered as main players in K+ buffering (90, 436, 917, 1209). Nonetheless, analysis of conditional astroglia-specific Kir4.1 knockout mice revealed neither the expected phenotype (neuronal hyperexcitability) nor significant alterations in the kinetics of [K+]o following neuronal stimulation. Changes in [K+]o dynamics were limited to moderate decrease in its recovery kinetics and appearance of pronounced undershoot following [K+]o recovery (323). At the same time, genetic deletion of Kir4.1 channels caused a significant (~20 mV) depolarizing shift in the resting membrane potential and decreased the resting K+ permeability. These data therefore argue against a dominant role of Kir4.1 channels in K+ buffering. The role for Kir4.1 channels in K+ clearance, however, remains debatable (943, 1244). Densities of Kir4.1 channels markedly increase in postnatal development, and hence, experiments on younger animals could be somewhat misleading. Moreover, expression of Kir4.1 channels differs between different brain regions, and therefore, the relative contribution of these channels to K+ buffering can have regional variability. The Kir4.1 channels may be specifically important for K+ uptake from narrow spaces such as synaptic clefts, this role being in agreement with Kir4.1 densely populating perisynaptic processes (1244).
The energy-dependent K+ buffering mediated through NKA has been discovered in experiments in cultured astrocytes (681). Numerous subsequent experiments performed in the in situ preparations revealed the leading role of NKA in extracellular K+ clearance associated with physiological neuronal activity (371, 942, 1433, 1902). In this scenario, K+ accumulated by astrocytes is subsequently released into the extracellular space to be taken up by neurons, thus fully restoring ionic gradients. Astrocytes therefore rapidly buffer excess K+ released during neuronal activity; when neurons stop firing, K+ is shuttled to the neuronal compartment (683, 944). Release of K+ is mediated through Kir4.1 channels [deficient release may explain the increased undershoot in Kir4.1 astroglial knockout mice (323)] and possibly through other channels or SLC transporters. The Na+/K+/Cl− transporter NKCC1 was also suggested to participate in K+ buffering, and indeed, it can do so in astroglial cultures (942), although its role in situ and in vivo was not confirmed (942). The NKCC1 is activated by K+ increases above 10 mM (1845) or by extracellular hypertonicity (1418) and may therefore contribute to K+ clearance in pathological context. All in all, several astroglial systems are working in concert to ensure reliability and versatility of K+ homeostasis.
2. Chloride homeostasis
Strong stimulation of neuronal GABA receptors (induced for example by intense firing of activity of GABAergic interneurons) may deplete Cl− from the synaptic cleft. Astrocytes, however, respond to GABA by Cl− efflux, and hence, astroglial Cl− secretion can therefore counteract Cl− entry into neurons thus maintaining [Cl−]o and preserving the driving force for Cl− to sustain inhibitory neurotransmission (854). This hypothesis has been directly corroborated by recent experiments, which demonstrated an astroglial role in maintaining GABAergic neurotransmission. Moreover, these experiments revealed the role of gap junctions and astroglial syncytium for Cl− homeostasis by showing that inhibition of connexons during intense stimulation of GABAergic transmission to CA1 neurons resulted in a collapse of the Cl− gradient in these neurons (468).
3. Regulation of extracellular Ca2+
Astrocytes may also contribute to regulation of extracellular Ca2+ concentration. In the course of neuronal activity, Ca2+ concentration in narrow extracellular compartments and particularly in the perisynaptic cleft undergoes substantial fluctuations due to massive Ca2+ influx into neurons upon activation of Ca2+ channels. The extracellular Ca2+ concentration may decrease below 1 mM, which in turn can affect Ca2+ signaling both in the presynaptic terminal and in the postsynaptic compartment with obvious consequences for synaptic transmission (1519). In addition, lowering of [Ca2+]o causes astroglial release of ATP, which excites hippocampal interneurons (1754). Lowering of extracellular Ca2+ concentration to ~0.5 mM was shown to trigger InsP3-induced Ca2+ release from the endoplasmic reticulum in astrocytes (probably mediated by autocrine release of ATP) (1935). This may, at least hypothetically, aid in restoring [Ca2+]o because Ca2+ can leave the astrocyte through either plasmalemmal Ca2+ pumps or Na+/Ca2+ exchangers.
4. Regulation of pH
Homeostatic control over cytosolic and extracellular pH is of a paramount importance for brain function, because even mild acidification or alkalinization significantly affects neuronal excitability and synaptic transmission. In resting conditions, the electrochemical gradient for H+ is aimed into the cytosol. Neuronal metabolism is the main source of extracellular protons, which are extruded by the Na+/H+ (NHE) transporter. The second major source of protons is associated with exocytosis of neurotransmitters because the lumen of vesicles is highly acidic (pH ~5.2–5.7; Ref. 1114). Protons may also be released from astrocytes together with lactate. Astrocytes regulate extracellular pH by 1) removing H+ in the course of operation of EAAT1/2-mediated glutamate uptake with a single H+ cotransported together with a glutamate molecule, and 2) by supplying extracellular space with HCO3− through the Na+/HCO3− (NBC) transporter, which can operate in both forward and reverse modes depending on membrane potential and [Na+]i (401, 1497). The NBC is very sensitive to bicarbonate levels being active at [HCO3−]o as low as 0.3 mM (406). Astrocytes also release H+ by NHE and possibly by plasmalemmal V-type H+ pumps, which apparently play a dominant role in astroglial cells in the in situ rat optic nerve compared with the same cells in culture (640). This coincides with a substantially greater astroglial pH buffering capacity in situ versus in vitro (167, 640).
B. Water Homeostasis and Regulation of the Extracellular Space Volume
Astrocytes control water movements through several pathways, of which most notable are aquaporins and membrane transporters. In astroglial endfeet, aquaporin 4 (AQP4) channels are colocalized with Kir4.1, with both channels being anchored to the endfeet membrane via dystrophin/α-syntrophin complexes containing a PDZ domain. This close colocalization and possible cooperation of AQP and Kir4.1 channels links K+ and water movements (35). Functional coupling between Kir4.1 and AQP4, however, remains debatable, with some evidence showing independent operation of these two channels (1940).
Astroglial water fluxes also contribute to regulation of extracellular volume (1184). Genetic deletion of AQP4 led to ~25% increase in the extracellular volume (1917), while animals lacking AQP4 in astrocytes had an increased brain water content (624). Astrocytes also contribute to dynamic changes in the extracellular volume that are associated with neuronal activity. Synaptic transmission evokes rapid and considerable (5–30%) reduction in the extracellular volume (430, 1710). This reduction is generally believed to reflect swelling of astroglial perisynaptic processes resulting from cotransport of water with K+, glutamate, bicarbonate, and other molecules (623, 1036). In these settings an important pathway for water transport is represented by plasmalemmal SLCs, such as EAAT1, GAT-1, MCT1, GLUT1, etc. with 40–500 molecules of water being cotransported along with the substrate against osmotic gradient. Some of these transporters (for example, EAAT1 or GAT-1) also possess a pore for passive water transport (1036). The AQP4 provides a conduit for water exit from astrocytes, and deletion of AQP4 enhances shrinkage of extracellular space (623).
C. Astrocytes and Homeostasis of Reactive Oxygen Species
Astrocytes are the key element of the CNS antioxidative system. Neuronal energy metabolism, which relies entirely on oxidative phosphorylation (127), constantly generates a high amount of reactive oxygen species (ROS), which need to be scavenged to avoid cellular damage. The antioxidant system of the CNS mainly relies on glutathione and ascorbic acid (1053). Glutathione scavenges ROS in a nonenzymatic direct reaction or else it acts as an electron donor for glutathione peroxidase. Astrocytes as a rule contain more glutathione compared with neurons (451). Neuronal synthesis of glutathione requires either cysteine or glutamylcysteine as obligatory precursors, both of which are provided by astroglia. Astrocytes accumulate cystine through the Sxc− glutamate/cystine exchanger (232); subsequently, cystine is reduced to cysteine, which is further converted to glutamylcysteine (CysGlu). Astrocytes release cysteine and glutathione, the latter being converted into CysGlu by ectoenzyme γ-glutamyl transpeptidase (γGT). Both cysteine and CysGlu are accumulated into neurons for glutathione synthesis, with cysteine being transported by neuronal EAAT2/3 transporters (318). Neurons by themselves are unable to accumulate cystine for further conversion because they express very little of the Sxc− transporter. In the presence of astrocytes in vitro, neurons sustain high levels of glutathione. Removal of astroglia from culture or inhibition of γGT prevents neuronal synthesis of glutathione and facilitates ROS toxicity (452). In coculture, a single astrocyte is able to protect up to 20 neurons (420).
Astroglial antioxidant capacity is also mediated by ascorbic acid, the reduced form of vitamin C. Astrocytes represent the reservoir for ascorbic acid (367, 1053); moreover, they are able to accumulate the dehydroascorbic acid derived from ROS oxidation and released by neurons and reduce it (using glutathione) to ascorbic acid (1464, 1886). Neuronal activity (probably through release of glutamate) stimulates astroglial release of ascorbic acid (1884). This release can be mediated through anion channels such as VRAC (1626) or even through hemichannels (363), although this latter mechanism has not yet been identified in astrocytes.
D. Neurotransmitters Homeostasis
Astroglia are fundamental for neurotransmitter turnover in the brain. Astrocytes remove and inactivate, through accumulation and metabolic conversion, glutamate, GABA, adenosine, and norepinephrine (FIGURE 29). In addition, astrocytes produce glutamine, an obligatory precursor for neuronal glutamate and GABA, which is indispensible for both excitatory and inhibitory neurotransmission.
FIGURE 29.
Astrocytes and neurotransmitter homeostasis. Astrocytes take up glutamate, GABA, adenosine, and monoamines. Glutamate is converted to glutamine (by glutamine synthetase, GS), which is shuttled to neurons for subsequent conversion into glutamate and GABA. GABA accumulated by astrocytes is mainly consumed by tricarboxylic acid cycle; adenosine is converted to AMP by adenosine kinase (ADK) while monoamines are degraded by astroglial monoamine oxidase B (MAO-B).
1. Glutamate and GABA
Glutamate, or glutamic acid, is ubiquitously present in all types of cells, and it acts as the major excitatory neurotransmitter in the CNS. During elementary acts of synaptic transmission, glutamate, released from neuronal terminals, can reach millimolar levels in the synaptic cleft (1092). The glutamate homeostatic system is responsible for 1) rapid removal of glutamate from the synaptic cleft, 2) control of glutamate spillover to neighboring synapses, and 3) rapid replenishment of the glutamate-releasable pool in the neuronal terminals. In addition, this system needs to keep ambient glutamate concentration rather low to prevent excitotoxicity. These multiple functions are mainly performed by astroglia.
First and foremost, astrocytes are the sole de novo synthesizers of glutamate from glucose in the CNS (685). This synthesis requires tricarboxylic acid cycle intermediate α-ketoglutarate (1574), which is produced by the enzyme pyruvate carboxylase exclusively expressed in astrocytes (1594, 1927). Astroglial cells express another enzyme fundamental for glutamate turnover, the glutamine synthetase. The exclusive astroglial expression of glutamine synthetase was discovered by Michael Norenberg and Antonio Martinez-Hernandes (1237). Glutamine synthetase catalyzes conversion of glutamate to glutamine. Glutamine in turn is a direct precursor for glutamate; neurons express phosphate-activated glutaminase that deaminates glutamine to glutamate. Glutamine synthetase is also a central cytosolic enzyme for detoxifying ammonium (NH4+), which is converted into glutamine (359). In physiological settings, NH4+ is released by active neurons and accumulated by astrocytes via diffusion through channels as well as by dedicated transporter (1064).
Second, astroglia are the main sink for glutamate released during neurotransmission. About 80% of extracellular glutamate in the CNS is taken up by astrocytes (380) using EAAT1/2 transporters (see sect. IXB1). Astroglial glutamate transport shapes glutamate dynamics in the synaptic cleft and prevents (or allows) spillover, thus contributing to the time course, efficacy, and outcome of synaptic transmission (1062, 1768). Knockout of astroglial glutamate transporters results in excitotoxicity and paralysis associated with increased glutamate level in the interstitium (1508). Third, astrocytes provide for the replenishment of glutamate in neuronal terminals. Glutamate entering into astrocytes undergoes conversion to glutamine (an energy-dependent reaction requiring one molecule ATP per one molecule of glutamate), which is subsequently shuttled to neurons through coordinated A and N systems of glutamine transporters (see sect. IXB2). Sodium entry, associated with EAAT1/2-mediated uptake of gltutamate, stimulates glutamine efflux thus coordinating glutamate/glutamine fluxes: increase in glutamate uptake increases glutamine release (1752, 1780). After entering neuronal terminals, glutamine is converted into glutamate in excitatory neurons; in the inhibitory neurons, glutamate is further converted into GABA. This sequence of transporting and biochemical events represents the glutamine-glutamate(GABA) shuttle (FIGURE 27), which is indispensible for maintenance of excitatory and inhibitory neurotransmission (242, 680). Part of the glutamate accumulated into astrocytes is metabolized to α-ketoglutarate by glutamate-dehydrogenase (GDH) or aspartate-glutamate transferase (AAT) and used for energy production; it is estimated that ~85% of glutamate is converted into glutamine and returned to neurons, whereas ~15% is oxidized (1507), thus contributing to cover energy costs of glutamate handling (1094). Astroglial glutamate transporters have been reported to colocalize with glycolytic enzymes (glutamate dehydrogenase and hexokinase) and with mitochondria (564), suggesting the existence of an “energy hub” supporting glutamine-glutamate (GABA) shuttle.
Postnatal development of the brain is associated with an increase in astroglial glutamate uptake (427) because of an increase in the expression of EAAT1/2 (1775). In neonatal neocortex, glutamate uptake is substantially lower than in hippocampus, which probably allows for glutamate spillover and activation of extrasynaptic receptors. Cortical maturation is accompanied by more stringent control of extracellular glutamate (641). In hippocampus there is also a developmental switch in transporters expression; in the neonatal tissue, the EAAT1 and (neuronal) EAAT3 predominate, whereas in adulthood the EAAT2 becomes the major form (97). Impairment of the glutamine-glutamate (GABA) shuttle affects neurotransmission. In the retina inhibition of glutamine synthetase rapidly (in 2 min) reduced the b-wave indicative of glutamatergic light response (103). Inhibition of astroglial Krebs cycle and hence inhibition of glutamate synthesis and thus conversion to glutamine suppresses glutamate-dependent memory consolidation in chicks; this can be rescued by injecting exogenous glutamine (574). Likewise, inhibition of glutamine synthetase rapidly depletes synaptic GABA content and reduces inhibitory neurotransmission in mouse hippocampus (1283). Astrocytes also remove extracellular GABA, which is catabolized through tricarboxylic acid cycle.
Astrocytes may also dynamically regulate extrasynaptic concentration of glutamate, not through its uptake but rather through its release via the cystine/glutamate antiporter Sxc−(1162). Increases in the extrasynaptic glutamate may affect neurons through both NMDA and metabotropic glutamate receptors. Genetic deletion of the catalytic subunit of the Sxc− led to ∼50% decrease in extracellular glutamate measured by microdialysis (1080); a similar decrease was observed following chronic administration of cocaine or nicotine known to reduce expression of Sxc− (87, 1162).
2. Adenosine homeostasis
Adenosine is ubiquitously present throughout the CNS, regulating numerous functions, of which the most notable is an inhibitory neuromodulation mediated through presynaptic A1 receptors, stimulation of which suppresses release of major excitatory neurotransmitters, including glutamate, acetylcholine, dopamine, and serotonin (526). The sources of adenosine in the CNS are 1) ATP released from neural cells and degraded by ectonucleotidases (263) and 2) adenosine released from cells by equilibrative transporters; such release often accompanies cellular or metabolic stress. Adenosine, released by neurons, mediates synaptic depression and represents an autonomic feedback mechanism by which neurons downregulate excitatory transmission during episodes of prolonged activity (1011).
Adenosine is accumulated into astrocytes by equilibrating and concentrating transporters (see sect. IXB5), after which it is rapidly phosphorylated to AMP by adenosine kinase (ADK), which represents the key enzyme of adenosine metabolism (204). In the adult CNS, astrocytes express high levels of ADK (1686), which makes them important element of purines homeostasis and metabolism. Manipulations with adenosine kinase immediately affect presynaptic inhibition: overexpression of ADK results in reduction of adenosine level and promotes seizures (980); pharmacological inhibition of ADK increases neuronal firing (1298), whereas genetic deletion of ADK is incompatible with life (205).
3. Monoamines
Monoamines (norepinephrine, dopamine, and serotonin) in the CNS are catabolized through deamination or methylation, the former process being catalyzed by monoamine oxidase A and B (MAO A/B) and the latter by catecholamine O-methyl transferase (COMT). The newborn brain contains both MAO A and MAO B; however, in the postnatal development MAO B activity increases ~15 times, whereas MAO A only doubles (1928). Astrocytes in vitro were reported to express both MAO A and MAO B (684), whereas astroglia in situ express MAO B; moreover, astrocytes are the main containers of this enzyme in the CNS (973, 1468, 1548). The MAO B is inhibited by l-deprenyl, also known as selegiline (1468). The 11C-deuterium-l-deprenyl is a popular (and the only) astroglial tracer for PET brain imaging (1481). The second monoamine catabolizing enzyme, the COMT, has been detected both in astrocytes and in processes of neurons (642, 835).
E. Lactate Production and Turnover
The concept of astroglia-mediated supply of neurons with the energy substrate rests, conceptually, on a disproportionally high uptake of glucose by astrocytes. It has been postulated that neurons utilize ~90–95% (75) of energy consumed by the brain, although later estimates found neuronal share exaggerated (21). In the resting brain, glucose, however, is shared roughly equally between neurons and astrocytes (336, 1206). Glucose metabolism, consequently, differs between neurons and astrocytes; the former rely preferentially on oxidative metabolism with high ATP production (218), whereas the latter utilize glucose mainly through aerobic glycolysis which yields relatively low amounts of ATP but produces substantial amount of lactate (109, 127, 183).
The astrocyte-neuron lactate shuttle hypothesis, ANLSH (1357, 1358), assumes that neuronal metabolism relies, during rest and in particular during activity, on lactate secreted by astrocytes. Astrocytic lactate production is linked to synaptic glutamate release, with subsequent Na+-dependent glutamate uptake into astrocytes. An increase in astrocytic [Na+]i activates the NKA and increases aerobic glycolysis, thus producing lactate, which is subsequently transported to neurons to support their metabolism. Astrocytes are equipped with export lactate MCT1 and MCT4 transporters (see sect. IXB14) and hence secrete lactate produced by aerobic glycolysis. Astrocytes (in culture, in slices, and in vivo) were also reported to express lactate-permeable channels, which may rapidly release large quantities of lactate (1656). Lactate (which is present in the brain interstitium at ~1–1.5 mM concentration) is taken up and utilized by neurons for ATP production; moreover, neurons even seem to prefer lactate over glucose (127, 218, 1417). Neuronal mitochondria contain a dedicated lactate oxidation complex that facilitates lactate entry into the mitochondrial oxidative cycle (657). Finally, in vivo brain activity leads to a rapid increase in lactate levels; when activity terminates, lactate returns to the basal levels (274, 1058).
Several experimental arguments corroborated the ANLSH. For example, electrical activity can be sustained in cultures of neurons, in slice preparations, and in the isolated brain based on oxidation of lactate (775, 1582). Astrocytes in culture have been found to release higher quantities of lactate than cultured neurons and hence can be the lactate source. It has been argued, however, that this observation reflects predominant neuronal expression of lactate dehydrogenase 1 that is strongly inhibited by pyruvate and that the increase in pyruvate concentration that occurs during neural activity directs pyruvate towards the tricarboxylic acid cycle rather than towards conversion to lactate (325). This argument is further supported by the observation that cultured neurons release as much lactate as astrocytes when oxidative metabolism is blocked (1849). In the cerebellum, Bergmann glial cells take up several times more fluorescent glucose than Purkinje neurons (109). Neuronal activity in barrel cortex in vivo stimulated uptake of fluorescent glucose preferentially into astrocytes rather than neurons (336). However, this analysis was based on experiments using 6-deoxy-N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-aminoglucose (6-NBDG), which cannot be phosphorylated in the cytosol by the hexokinase. In contrast, in vivo studies based on analysis of a near-infrared 2-deoxyglucose analog showed that neuronal uptake of the tracer is approximately two- to threefold higher than neighboring astrocytes across all regions studied (1020). Expression of hexokinase, which correlates directly with the rate of glucose metabolism in different regions, is consistently higher in neurons than astrocytes in mouse and human brain (1020). Moreover, an analysis of phosphorylated 2-fluoro-2-deoxy-d-glucose (FDG6P) in vivo found high concentrations of FDG6P in nerve terminals, suggesting that neuronal glucose-derived pyruvate (and not lactate derived from astrocytes) is the major oxidative fuel for active neurons, (1338). On the other hand, monitoring NADH levels in hippocampal slices indicated a clear separation between an increase in oxidative neuronal and glycolytic astroglial glucose metabolism (841), yet when these experiments were repeated in vivo, no clear separation was observed (840). In summary, although it is well documented that high neuronal activity is coupled with lactate production, definitive in vivo studies defining which, neuronal or astrocytic, lactate production predominates are yet to be done.
At the same time, the overall picture could become even more complicated, after it was found that up to 60% of lactate increase in the active brain derives from the blood (219). At the same time, it turned out that astrocytes are able not only to produce and release, but also to take-up and buffer extracellular lactate loads (546). Thus astroglia may have a dual role in metabolic support of neurons; it may produce and release lactate or else take lactate up from the blood and commute it to neurons (508).
In addition to producing and redistributing lactate, astrocytes are the sole possessors of brain glycogen. The glycogen granules in the brain were discovered in 1926 (716). In the mature brain, glycogen is present mostly in astrocytes, although it has been also detected in small sub-population of neurons in the brain stem and in some ependymal and choroid plexus cells (245, 295, 1048). Incidentally neurons do express glycogen synthase, which however is kept inactive by proteasomal-dependent mechanisms; when artificially activated it causes neuronal apoptosis (1823). Physiological activity affects glycogen levels in astrocytes; it is controlled by norepinephrine, serotonin, and VIP. Glycogen increases in sleep and anesthesia and decreases in awake and active brain, while glycogen mobilization helps to sustain neuronal activity (127, 246, 1047). Glycogenolysis is also critically important for maintenance of LTP (1706) and for memory consolidation (573). Are these physiological effects associated only with the glycogen function as an energy reserve? Total amount of glycogen in the brain is very low, being in a range of 0.5–1.5 g, which is 100 times less than in the liver (245). This amount of glycogen cannot sustain the brain activity for any reasonable time, and it might exert its physiological effects through yet unknown mechanisms, for example, through local signaling of undefined nature.
The metabolic role of astroglia and distribution of energy consumption between neurons and glia are far form being resolved. Futhermore, the metabolic plasticity of the brain may dynamically affect neuronal-glial energetic balance and lactate turnover.
F. Regulation of Synaptic Connectivity and Synaptic Transmission
1. Astroglial synaptic coverage
At least half of all synaptic contacts within the CNS are covered with perisynaptic astroglial membranous sheaths, which represent terminal extensions of peripheral astroglial processes (known as PAPs). The PAPs and perisynaptic processes are immunopositive for glutamate synthetase and astroglial glutamate transporters, and they do not express detectable levels of GFAP (415, 416, 1451). Perisynaptic glial compartments are generally devoid of organelles (1375, 1451), including endoplasmic reticulum (1340), although they may contain very small (0.2–0.4 μm) spherical mitochondria (419). The perisynaptic processes specifically express ezrin and radixin (415, 418, 952), which associate with actin and may contribute to morphological plasticity of astroglial processes. Rapid filopodial movements of astroglial processes have been characterized in vitro and in organotypic cultures (702, 952). This form of morphological remodeling may participate in synaptic plasticity (672), although it has yet to be confirmed in the in vivo brain.
The degree of astroglial coverage differs between brain regions and seems to reflect the nature of the synapse. In the neocortex 29–56% of excitatory synapses are enwrapped by glial processes; in layer IV of the somatosensory cortex, ~90% of synapses are covered with glial membrane (157). In the hippocampus the number of enwrapped synapses varies between 60 and 90% (1802, 1888). In the cerebellum, complex appendages emanating from the processes of Bergmann glial cells (FIGURE 30) cover almost 90% of synapses formed by climbing fibers and ~65% of synapses formed by parallel fibers on the Purkinje neuron (609, 1903). Degree of coverage also varies between different types of synapses. In the hippocampus, astrocytes cover ~50% of small macular synapses, while ~90% of large mushroom spines and perforated synapses are enwrapped with astrocytic processes (1888). In organotypic slices, 97% of complex synapses and 78% of simple synapses have astroglial coverage (1024). At the synapses formed by mossy fibers on granule neurons, coverage of individual synapses is ~15% (1128, 1711); however, astrocytes also cover whole glomeruli thus isolating them (1451).
FIGURE 30.
Microdomain organization of Bergmann glia and coverage of parallel fibers to Purkinje neuron synapses. A: reconstruction of an appendage based on electron microscopic data. a: Fluorescence light micrograph of a dye-injected Bergmann glial cell is shown; the red square (20 × 20 mm) corresponds to the portion that was reconstructed from consecutive ultrathin sections. b: One of the lateral appendages, arising directly from fiber with all the other side branches omitted for clarity. c: The same appendage as shown in b, but with one of the appendages marked by blue. This labeled structure is shown in isolation and at higher magnification in B. B: fine structure of appendages and relation to synapses. a: A small lateral appendage, arising from the reconstructed part of the glial fiber (blue in Ac), is shown as a slightly turned, isolated three-dimensional reconstruction (left). Electron micrographs of four sections contributing to the reconstruction (designated 1–4) are shown on the right; glial compartments appear black after conversion of the injected dye. The location of these sections in the reconstruction is indicated by the labeled arrows: 1, region directly contacting synapses; 2, glial compartments without direct synaptic contacts; 3, bulging glial structure containing a mitochondrion; 4, the stalk of the appendage. C: another example of a synapse contacted by glial compartments, appearing as black structures (white arrows); in this case, the postsynaptic element can be traced back along the spine (black arrows) to the Purkinje cell dendrite (reddish overlay; the presynaptic terminal is labeled by a green overlay). [From Grosche et al. (609).]
The perisynaptic astroglial processes covering synapses are exceedingly thin, their profiles being on average <200 nm (and often <100 nm) in diameter, although they have an exceedingly large surface (1451). The perisynaptic astroglial membrane represents the major part of the cell surface area, accounting for ~80% of the total astroglial plasmalemma, while contributing only a minor fraction (~4–10%) to the cellular volume; as a result, astroglial perisynaptic processes have an extremely high surface to volume ratio (~25 μm−1; Ref. 608). Perisynaptic astroglial membrane is densely packed with receptors, ion channels, and multiple transporters; ion fluxes are particularly important for generation of local Ca2+ and Na+ signals, which couple homeostatic astroglial cascades with neuronal activity (1813). The perisynaptic processes are plastic structures; for example, they rapidly ensheath dendrites and synapses in neurons newly generated in dentate gyrus and integrated into adult hippocampus (912).
2. Tripartite synapse and synaptic cradle
Intimate morphological relations between astroglial membranes and synaptic structures, as well as wide expression of neurotransmitter receptors in astrocytes, led to a concept of a synaptic triad (855), which subsequently developed into the tripartite synapse model (58, 626). The tripartite synapse (FIGURE 31) highlights glial contribution to the regulation of synaptic transmission; astroglial processes become a legitimate part of synaptology together with pre- and postsynaptic neuronal compartments. The tripartite model further developed into a multipartite synapse, composed of the following components: 1) the presynaptic terminal; 2) the postsynaptic compartment, represented, for example, by the dendritic spine; 3) the perisynaptic process of the astrocyte; 4) the process of neighboring microglial cell that periodically contacts the synaptic structure; and 5) the extracellular matrix (ECM), which is present in the synaptic cleft and also extends extrasynaptically (390, 434, 494, 1205, 1812).
FIGURE 31.
Tripartite synapse and synaptic cradle. A: the original concept of tripartite synapse. “During synaptic neurotransmission, neurons release neurotransmitters from nerve terminals into the synaptic cleft to communicate with other neurons (1). The neurotransmitter released from the synapse (or other co-released neurotransmitter) can, under certain circumstances, “spill over” from the synaptic cleft and reach neurotransmitter receptors in adjacent astrocytes, eliciting intracellular increases in Ca2+ concentrations in the glial cells (2). The increase in the glial-cell Ca2+ concentration causes it to release a chemical neurotransmitter from the glial cell, which in the case of astrocytes is glutamate (3), that feeds back to the presynaptic nerve terminal to modulate synaptic neurotransmission.” [From Araque et al. (58). The figure from original submission (although left unpublished) was kindly provided by Prof. V. Parpura (Birmingham University, Birmingham, AL).] B: astroglial cradle embraces and fosters multi-partite synapse in the CNS. The majority of synapses in the brain and in the spinal cord are composed of several components that include the presynaptic terminal, the postsynaptic compartment, the perisynaptic process of the astrocyte, the process of neighboring microglial cell that periodically contacts the synaptic structure, and the extracellular matrix (ECM) present in the synaptic cleft and also extended extrasynaptically. Astroglial perisynaptic sheath enwraps synaptic structures and regulate, influence, and assist synaptogenesis, synaptic maturation, synaptic maintenance, and synaptic extinction. [From Verkhratsky and Nedergaard (1812).]
The tripartite synapse model principally focuses on a bidirectional rapid neuronal-glial communications; neurotransmitter-induced glial Ca2+ signaling with subsequent exocytotic release of neurotransmitters from the astroglia (the act of “gliotransmission”) lies at its core. The role of astroglia in regulation of synaptic connectivity is, however, immensely wider: astrocytes control emergence and shaping of synaptic networks, they regulate ionic homeostasis of the synaptic cleft, control neurotransmitters dynamics, prevent or allow neurotransmitter spillover, and contribute to synaptic extinction. These multiple roles of astroglia in synaptic physiology were synthesized in the concept of the astroglial cradle (FIGURE 31, Refs. 1205, 1812). This model regards astroglial coverage as a multipurpose tool, responsible for multiple functions mandatory for physiological neurotransmission.
3. Synaptogenesis, synaptic maturation, and elimination
Throughout life, synapses emerge, remodel, and disappear, which is an important part of neuroplasticity, learning and memory. Synaptic connections progress through several stages that include 1) formation of an initial contact between the terminal and postsynaptic neuron, 2) maturation, 3) stabilization and maintenance, and 4) elimination. Embryonic synaptogenesis proceeds mainly without glia. The main wave of genesis of excitatory glutamatergic synapses in mammals occurs in the first postnatal weeks; this massive emergence of synapses immediately follows the wave of astrogliogenesis (1115). The critical role for astrocytes in synaptogenesis was discovered in vitro: addition of astrocytes to pure neuronal cultures increased the number of synapses sevenfold (1386). This link between astroglia and synaptogenesis has been subsequently confirmed (487). Astrocytes support synaptogenesis through secreting multiple factors of which the most important are cholesterol (1090) and thrombospondins (486), although other factors [such as estradiol, protocadherins, or integrins (1385)] are also involved. Cholesterol, in particular, provides building material for new membranes, which appear during synaptogenesis, and cholesterol may also be locally converted into steroid hormones, which in turn can act as synaptogenic signals (486, 1385). Astrocytes also secrete hevin, which potentiate genesis of excitatory synapses and secreted protein acidic and rich in cysteine (SPARC), which inhibits hevin-induced synaptogenesis (918). Astrocytes remain indispensable for synapse formation throughout life; astroglial deficiency affects synaptogenesis in adult CNS and prevents regeneration after injury (1762). Astroglial factors are similarly important for maturation of synapses. These factors include activity-dependent neurotrophic factor and TNF-α, which regulate the trafficking of glutamate receptors into postsynaptic membranes, whereas cholesterol may enhance neurotransmitter release from presynaptic terminals (487). Astroglia-derived glypicans 4 and 6 facilitate synapse maturation by increasing the number of glutamatergic α-amino-3-hydroxy-5-methyl-isoxazole propionate (AMPA) receptors at postsynaptic sites (24).
Astroglial synaptic coverage may also exert dynamic control on the synaptic density. In hippocampus, for example, in the absence of astroglial perisynaptic processes, or in the conditions of disrupted ezrin signaling affecting their motility, dendritic spines disappeared faster (1226). Synapse stabilization in Purkinje neurons similarly requires astroglial coverage, which also regulates synaptic density (988). Astrocytes may regulate synaptic density through dynamic remodeling of coverage of postsynaptic membranes. In the lateral amygdala, for example, retraction of astroglial processes from synapses is a prerequisite for synaptic remodeling associated with implicit memory consolidation of Pavlovian threat conditioning (1285).
Astroglia contribute to synaptic elimination and regulate synaptic morphology. For example, astrocytes in vitro were reported to label synaptic terminals with complement factor C1q. This tag is subsequently recognized by microglia, which eliminate these tagged synapses by selective phagocytosis (1555); furthermore, astrocytes by themselves can engulf and eliminate synapses in the developing brain (334).
4. Astroglial release of neurotransmitters
Emergence of the concept of the tripartite synapse had stimulated great interest in astroglial secretion of neurotransmitters. Numerous studies, in vitro (in astroglial-neuronal cocultures), in situ, and in vivo have addressed this matter; many sets of data have been generated, and heated debates ensued (55, 632, 1205, 1526). As has been discussed above (see sect. XIE), astrocytes contain neurotransmitters and do possess several pathways for releasing glutamate, ATP, and GABA. Conceptually, however, these neurotransmitters are released from astrocytes slower than from neuronal terminals, and astroglial release seems to be more diffused in space, as astrocytes lack any kind of presynaptic active zones, where vesicles concentrate.
How does astroglial neurotransmitter release affect neurons? The spectrum of neuronal responses is quite wide. Astroglial release of glutamate, for example, has been shown to result in 1) inhibition of evoked and spontaneous excitatory or inhibitory postsynaptic currents (EPSCs or IPSCs) in hippocampus (57, 992); 2) potentiation of spontaneous excitatory postsynaptic currents (EPSCs) or inhibitory postsynaptic currents (IPSCs) in hippocampus (804, 822, 992, 1537,1538); 3) synaptic potentiation (1198, 1199, 1366); 4) increase in neuronal excitability (168); 5) generation of slow inward currents, or SICs, in hippocampus, cortex, brain stem, and spinal cord, with this being probably the most frequent observation (47, 100, 316, 502, 591, 826, 1539, 1542, 1607, 1906); 6) modulation of long-term potentiation or long-term depression (634, 1119, 1199); and 7) heterosynaptic depression (41). Astroglia-derived ATP and/or adenosine caused 1) suppression of EPSCs (238, 1069, 1334, 1592), 2) potentiation of EPSCs (594, 595), 3) inhibition of NMDA receptors through P2X-PCD95 signaling cascade (934), 4) generation of SICs (373), 5) increase in neuronal excitability (959), and 6) modulation of LTP (959, 1334). There is also some limited information about modulation of neuronal function with astroglia-released GABA (900, 964) or TNF-α (1668); the latter was even reported to induce “gliogenic” LTP in the spinal cord (910).
These multiple effects, which can be exerted presynaptically, postsynaptically, or extrasynaptically, modulate distinct neuronal mechanisms that most likely differ between brain regions, reflecting complexity of astroglial-neuronal communications. Astrocytes can release neurotransmitters by several mechanisms, which can prevail in different contexts. There are several arguments against Ca2+-dependent vesicular neurotransmitter release from astrocytes. First, artificial obliteration or enhancement of astroglial Ca2+ signaling does not produce any apparent effects on neuronal activity (14, 507, 1382). Second, extracellular levels of glutamate are not affected in astroglia-expressing dnSNARE (suppressor of exocytosis) mice (340). Third, the astroglial origin of glutamate that induces SICs remains to be unequivocally confirmed; similar effects could reflect, for example, somatodendritic release from neurons (see Ref. 1205 for details). Finally, stimulation protocols used to activate astrocytes in situ may not necessarily reflect physiological situation in vivo. Overall, astrocytes probably do release neurotransmitters, which, however, act in a slow and long-lasting manner; they target extrasynaptic low-affinity receptors and modulate multiple synapses, rather than affecting individual synaptic events.
5. Control of neurotransmitter dynamic in the synaptic cleft
Astrocytes, through their dedicated transporters, define dynamics of neurotransmitter concentration in the synaptic cleft and hence shape postsynaptic responses (1062, 1768). This is particularly prominent for glutamatergic transmission. Glutamate released from the presynaptic terminal, while diffusing to the postsynaptic membrane, faces rapid buffering by astroglial glutamate transporters densely populating the perisynaptic astroglial membranes. It has been estimated (1062) that glutamate, exocytosed from presynaptic terminal, encounters, within the diffusion distance of 0.5 μm, ~7,000 glutamate transporters, which substantially exceeds the number of AMPA receptors (~25) available for postsynaptic activation. Astroglial glutamate buffering reduces the amplitude and shortens NMDA receptor-mediated EPSCs in hippocampal (72, 428, 1106, 1764) and cerebellar (1289) synapses. Inhibition of glutamate uptake more than doubled the half time of the decay of the NMDA receptor-mediated EPSCs in CA1 hippocampal synapses (66). The AMPA receptor-mediated EPSCs, because of their fast desensitization, are generally insensitive to astroglial glutamate uptake (692, 1540), although in the auditory nerve synapse in the nucleus magnocellularis as well as in cerebellar parallel and climbing fiber synapses astroglial glutamate transport accelerates AMPA receptor-mediated EPSCs (1287, 1289, 1715). Removal of AMPA receptor desensitization with cyclothiazide renders AMPA receptor-mediated EPSCs sensitive to glutamate buffering (1764), again indicating a significant role of the latter in defining glutamate intracleft dynamics. Glutamate transporters similarly limit activation of neuronal metabotropic glutamate receptors: pharmacological inhibition (230, 1446) or genetic deletion (1062) of transporters resulted in severalfold potentiation of mGluR-mediated synaptic currents. Astroglial glutamate uptake seems to be regulated by neuronal activity. In mouse cortex, bursts of action potentials led to a rapid and short-lived (~50 ms) threefold suppression of glutamate uptake confined only to active synapses (64), which may reflect another mechanism of glia-dependent modulation of synaptic transmission.
Astroglial GABA transporters also have a role in controlling concentration of GABA in the synaptic cleft. The astrocytic GAT-3 for example was reported to regulate extracellular GABA and hence tonic GABAergic transmission in hippocampus (852). In thalamus, astrocytes express both GAT-1 and GAT-3 (387, 1828), activity of which affects kinetics of GABAB receptor-mediated postsynaptic currents, with GAT-1 mainly affecting peak of IPSCs, while GAT-3 prevents neurotransmitter spillover (121).
6. Synaptic isolation
Astroglia, through perisynaptic processes and glutamate transporters, are instrumental for preserving spatial isolation of synaptic transmission, thus maintaining signaling specificity. Glutamate buffering prevents diffusion of neurotransmitters between adjacent synapses. Pharmacological inhibition of glutamate uptake with dl-threo-β-benzyloxyaspartic acid (dl-TBOA) significantly prolonged EPSCs in response to stimulation of multiple inputs in organotypic acute hippocampal slices, this being indicative of glutamate spillover (66). Similar results were obtained in cerebellum in parallel fibers to Purkinje neuron synapses of transgenic mice lacking EAAT1 transporters (1063). The degree of synaptic isolation is heterogeneous between CNS regions, and it changes during development. As a result, glutamate diffusion and synaptic interconnectivity may also change at different developmental stages and in different physiological context. Moreover, morphological plasticity of astroglial processes can rapidly affect synapse to synapse crosstalk.
7. Morphological plasticity of perisynaptic glial coverage
Dynamic changes in astroglial synaptic coverage represent another mechanism of regulation of synaptic transmission. Morphological plasticity of perisynaptic processes potentially may affect synaptic isolation, volume of the synaptic cleft, and availability of neurotransmitter transporters. Astroglial morphological plasticity is well known from in vitro studies in which various stimuli (such as norepinephrine or dibutyryl cAMP) result is rapid stellation (1479, 1794). Morphological remodeling of astroglial processes that affects neurotransmission has been subsequently described in the supraoptic nucleus in situ: in lactating rats, astroglial processes retracted thus permitting diffusion of glutamate and increasing presynaptic inhibition mediated by mGluRs (1260). In transgenic mice expressing Ca2+-impermeable AMPA receptors, Bergmann glial cells undergo retraction of perisynaptic processes with consequent potentiation of excitatory transmission (759). Astroglial morphological plasticity is activity dependent, and 24 h of whisker stimulation increases astroglial coverage (paralleled with an increase in EAAT expression) of synapses in the barrel cortex (566). Induction of LTP in hippocampus enhances motility of perisynaptic astroglial processes, which depends on glial Ca2+ signaling (1372). Astroglial synaptic coverage is highly dynamic and depends on the brain status. In sleep, for example, synaptic coverage decreases, while in wakefulness and sleep deprivation perisynaptic processes come closer to the dendrites and increase synaptic coverage (130).
G. Astrocytes Regulate Brain Microcirculation: The Concept of Neurovascular Unit
Activity of the brain is linked to local circulation, and increase in neuronal firing rapidly trigger vasodilatation of small vessels localized within ~200–250 μm from the site of increased neuronal activity. This is known as functional hyperemia that was discovered by Angelo Mosso (1159) and by Charles Roy and Charles Sherrington (1512), who postulated that “. . . the brain possesses an intrinsic mechanism by which its vascular supply can be varied locally in correspondence with local variations of functional activity” (1512).
Coupling of neuronal activity with local circulation is controlled by multiple mechanisms; neurons, for example, are known to release vasoactive agents such as NO or prostaglandins, and brain vasculature is richly innervated by neuronal projections that exocytose vasoactive neurotransmitters including acetylcholine, norepinephrine, serotonin, or dopamine (74, 300, 738). Compelling evidence indicates a specific role of astrocytes in neurovascular coupling and regulation of local blood flow. First, astrocytes were proposed to release arachidonic acid in response to stimulation with glutamate; subsequently, this arachidonic acid was suggested to be converted to a potent vasodilator epoxyeicosatrienoic acids, or EETs, by cytochrome P-450 (645).
This initial hypothesis was soon tested in experiments in brain slices, which revealed even more complex astroglial roles: glutamate stimulation of astrocytes and astroglial Ca2+ signals may evoke both vasodilatation and vasoconstriction. The vasodilatation was attributed to astroglia-derived prostaglandin E2 (PGE2) (1970), whereas vasoconstriction was found to be mediated by 20-hydroxyeicosatetraenoic acid (20-HETE) (1171). In the retina, both effects could be observed with vasodilatation being mediated by PGE2 and EETs and vasoconstriction by 20-HETE (1110, 1127). Astroglia-dependent functional hyperemia mediated by prostaglandins (1718) as well as by EETs (995) was subsequently detected in vivo in the cortex. Astrocytes were also found to regulate resting vascular tone in similar bidirectional manner. In the in situ and in vivo cortex, the pressure-evoked vasomotor tone was controlled by astroglia-derived ATP; the latter was released following TRPV4-mediated [Ca2+]i elevation (862). Similarly ATP released from astrocytes constricted arterioles in the retina (927). The tonic dilatation was also regulated by astrocytes, but in a COX1-dependent way (1503). The Ca2+ dependence of astroglia-vascular coupling has been questioned recently, when it appeared that type 2 InsP3R−/− mice (that are unable to generate global Ca2+ signals) functional hyperemia was preserved (210, 791, 1721).
An alternative mechanism associates vasoconstriction with the release of K+ from astroglial endfeet; increase in extracellular K+ supposedly hyperpolarizes vascular smooth muscle, hence causing vasodilatation. Initially the Kir4.1 channels were suggested to mediate this K+ efflux (1342); however, this was negated by experiments on Kir4.1−/− mice which have fully preserved functional hyperemia (1109). The hypothesis, however, has resurfaced recently, and the Ca2+-dependent K+ channels were found to be responsible for K+ efflux from astroglial endfeet that subsequently hyperpolarized smooth muscle cells (511).
Accumulation of experimental evidence for astroglia-controlled neurovascular coupling strengthen the concept of neurovascular (or neuro-glio-vascular) unit as an assembly of neurons, interneurons, astrocytes, microglia, basal lamina, smooth muscle cells, pericytes, endothelial cells, and extracellular matrix, which by coordinated and reciprocal signaling, provides for local control of cerebral blood flow (646, 1173, 1870). Astroglia represent the central element of the neurovascular unit through integrating all other components that reside in its structural domain; moreover, the astroglial endfoot appears as a key element for neurovascular communication.
H. The Glymphatic System and the Unique Perivascular Space of the CNS
Any type of biological activity is linked to production of potential toxic waste products. In peripheral tissues, the lymphatic system plays an essential role in the removal of excess fluid and metabolic waste. The density of lymphatic capillaries matches the local rate of metabolism across multiple tissues and organs (1567). However, in spite of neural tissue being highly metabolically active, no conventional lymphatic system is present in the eye, in the brain, or in the spinal cord (9). Recent work has documented the existence of lymphatic vessels in close proximity to, but outside of, the brain in the dural and meningeal membrane (71, 1009). A characteristic feature of the CNS is the existence of a unique perivascular space created by astrocytic endfeet, which encase ~99% of the entire vasculature (1082). Although this observation has been questioned by the finding of a less complete coverage using cryofixation (897), it is clear that astrocytic endfeet form a donut-shaped tunnel system that surrounds arteries, capillaries, and veins. The separation of the vasculature from remaining tissue by vascular endfeet is unique to the CNS, as in peripheral organs blood vessels are either directly embedded into the tissue or are surrounded by loose fibrous structures. A series of recent studies have identified an organized pathway for interstitial solute clearance from the brain that utilizes the perivascular space as a highway for fast fluid transport. It was designated the “glymphatic system” (FIGURE 32) because it operates similarly to the peripheral lymphatic system and depends on astroglial AQP4 water channels (761). This system consists of a periarterial CSF influx path and a perivenous interstitial fluid (ISF) clearance route, which are coupled through convective interstitial bulk flow supported by astrocytic AQP4 channels (1204).
FIGURE 32.
Schematic outline of the glymphatic system. Convective glymphatic fluxes of cerebrospinal fluid (CSF) and interstitial fluid (ISF) propel the waste products of neuron metabolism into the paravenous space, from which they are directed into lymphatic vessels and ultimately return to the general circulation for clearance by the kidney and liver. [From Nedergaard (1204).]
The brain-wide glymphatic pathway serves as a waste disposal system for fluid, as well as for larger solutes and proteins, such as β-amyloid and tau, from the CNS. Quantitative analysis shows that 65% of β-amyloid is cleared by the glymphatic system (761). Glymphatic transport declines significantly as a function of aging and more rapidly in rodent models of Alzheimer's disease, stroke, and diabetes (542, 793, 904, 1365). With the use of magnetic resonance imaging (MRI) in rats (760), it was shown that clinically relevant lumbar intrathecal administration of CSF tracers permits the capture of all the key components necessary to assess glymphatic function (1914). It has been also demonstrated that the lateral and supine positions, which are the most popular sleep positions among humans and most animal species, are superior for β-amyloid clearance compared with the prone position (958). Most surprisingly, the sleep-wake cycle regulates glymphatic activity, which is primarily active during sleep or anesthesia (1899). The glymphatic pathway is also involved in brain metabolism, delivers both lipids and glucose, and plays a key role in the export of lactate (1020, 1021, 1432). Additionally, very recent work has documented the existence of glymphatic fluid transport in the human brain (472, 887). Overall, brain-wide astrocytic fluid transport may therefore serve as a critical pathway for removal of neurotoxic proteins that accumulate in neurodegenerative diseases.
I. Astroglia in Central Chemoreception of Oxygen, pH, and CO2 and in Regulation of Respiration
Astrocytes throughout the brain possess an oxygen sensor linked to Ca2+ signaling. Decrease in Po2 triggered [Ca2+]i increase in cortical astrocytes in vivo, in astrocytes from brain slices, and in astrocytes in primary culture (isolated from cortex, hippocampus, midbrain, and brain stem) (45). The threshold for triggering astroglial [Ca2+]i responses was determined at ~17 mmHg, being thus substantially lower than Po2 threshold (~37 mmHg) in glomus cells of the carotid body (45). The actual oxygen sensor is associated with mitochondria; apparently hypoxia-induced reduction in respiration triggered production of ROS with subsequent activation of PLC and InsP3-induced Ca2+ release from the endoplasmic reticulum. Hypoxia-induced Ca2+ signaling was claimed to initiate tetanus-toxin-sensitive (i.e., vesicular) ATP release from astrocytes; in the brain stem, this ATP in turn activated neuronal circuits responsible for respiration. Inhibition of astroglial ATP secretion suppressed hypoxia-induced increases in respiratory rate (45).
Astrocytes in the brain stem (in retrotrapezoid nucleus) act as central chemosensors for CO2 and pH. Decrease in pH by 0.2–0.4 units triggers Ca2+ signals in brain stem astroglia with subsequent ATP release (598). This pH sensitivity is seemingly specific for brain stem astrocytes; cortical astroglia appear insensitive to pH fluctuations (843). In the brain stem, astroglia-derived ATP signals to respiratory neuronal network, thus initiating ventilatory response (598, 1875). The mechanism of pH-induced Ca2+ signaling involves Na+ entry through NBC1 with subsequent reversal of NCX and Ca2+ influx (1766). The ATP release pathway is seemingly Ca2+ dependent (implying a role for exocytosis), although evidence exists suggesting connexin hemichannels as a conduit (751).
J. Astrocytes in Regulation of Systemic Sodium Homeostasis
Brain chemosensors for the body sodium homeostatic system are localized in the circumventricular organs, which surround the ventricles. Changes in Na+ concentration in blood plasma or in cerebrospinal fluid modulate circumventricular neuronal activity and result in physiological (changes in kidney Na+ excretion) or behavioral (avoidance of dietary NaCl) responses (1630). Astrocytes in circumventricular organs and in particular in the subfornical organ act as sensors of systemic [Na+]. This is mediated by [Na+]o-sensitive Nax channels (see sect. VIIB2) specifically expressed by astrocytes in this part of the brain. Increase in plasma [Na+] above 140 mM activates Nax, which results in an increase in [Na+]i, activation of the Na+/K+ pump, and stimulation of aerobic glycolysis. Aerobic glycolysis produces lactate that is transferred to neighboring GABAergic neurons; consequently, neurons increase ATP production with subsequent closure of ATP-sensitive K+ channels, which causes neuronal depolarization and initiation of a systemic Na+-homeostatic response (1234, 1235, 1613). The role for astrocytes was further corroborated by the observation that genetic deletion of Nax from astrocytes altered Na+-aversive behaviors following Na+ overload (1613).
K. Astroglia and Circadian Rhythms
The circadian clock is localized in the suprachiasmatic nuclei (SCN) within the anterior hypothalamus; this structure generates rhythmical activity is driven by transcription-translation feedback loops of ubiquitous canonical clock genes (1617). Neurons from SCN are capable to oscillate in isolation; in vivo however circadian rhythms are coordinated and synchronized by intercellular communications. Astroglial cells contain circadian genes that undergo rhythmic expression (1412, 1908). Astrocytes isolated from SCN are similarly capable of circadian activity, which results in circadian ATP release triggered by InsP3-induced Ca2+ signaling (1067). This circadian ATP signaling is also controlled by mitochondria (261). Astroglia in the SCN display rhythmic morphological changes from stellate to more protoplasmic shape during the day/night cycle. This morphological remodeling is accompanied by changes in expression of GFAP and glutamate transporters, as well as with day/night changes in glial synaptic coverage which is higher at night (119, 780). Astrocytes have been also suggested to modulate SCN neurons by releasing TNF-α (461).
L. Astroglia and Sleep
Conceptually, sleep is regulated by the circadian clock (discussed above) and by the sleep homeostat, which controls the pressure to sleep (521). The sleep/wake cycle is associated with complex changes in astroglia (203). The astroglial transcriptome undergoes significant remodeling, with 1.4% of all transcripts showing changes in expression in sleep versus wakefulness (130). Astrocytes express 396 unique genes associated with wakefulness and 55 with sleep (130); at the same time, sleep does not affect the oligodendroglial transcriptome (131). Changes in astroglial transcriptome parallel morphological plasticity: sleep deprivation increases astroglial synaptic coverage in prefrontal cortex (130). To the contrary during normal sleep, the extracellular volume increases, which is likely to be mediated by changes in astroglial morphology (1899). Further evidence links astrocytes to fundamental elements of the sleep homeostat associated with an elevation of brain adenosine content in the wake state (1398, 1740). Conditional expression of dnSNARE in astrocytes (which apparently suppresses vesicular release of ATP and neurotransmitters; see sect. XIB) resulted in a reduced sleep pressure as well as prevented compensatory increases in sleep time following sleep deprivation in healthy wild-type animals (628). Very similar changes in sleep/wake behavior were observed in animals subjected to the intracerebroventricular infusion of adenosine A1 receptor antagonist, 8-cyclopentyl-1,3-dimethylxanthine (CPT) (628). These observations have not been universally confirmed because the interstitial level of adenosine has been found unchanged in dnSNARE mice (540). The adenosine A1 receptor pathways were also found to underlie increased sleep pressure under acute (induced by peripheral administration of lipopolysaccharide, LPS) inflammatory conditions (1183). It was further found, by using biosensor-based monitoring, that adenosine concentration indeed increases during wakefulness and sleep deprivation (1571). The astrocyte-sleep connection was further corroborated in experiments using astroglia-expressed channelrhodopsin-2: optical stimulation of hypothalamic astrocytes was found to induce sleep (1359). Finally, astrocytes may regulate sleep-wake cycle through a completely different mechanism, associated with dynamic ionostatic control of extracellular ionic composition (432).
M. Systemic Energy Homeostasis
A complex cellular network localized in the hypothalamic nuclei represented by the arcuate nucleus, the ventromedial hypothalamic nucleus, and the paraventricular nucleus (PVN) regulates body energy balance. These hypothalamic structures integrate numerous signals including major metabolites (glucose, free fatty acids, and amino acids) as well as dedicated hormones (leptin, ghrelin and insulin) and regulate appetite and food intake, deposition of fat, and energy expenditure (1157). Specialized hypothalamic astrocytes contribute to systemic energy homeostasis by glucose sensing, by providing the conduit for hormones to the hypothalamic neurons, and by directly affecting activity of these neurons.
1. Glucose sensing
The link between glucose concentration in the blood and stimulation of appetite (at low concentrations) or feeling of satiation has been proposed almost a century ago (287). Hypothalamus contains several population of “glucose-sensing” neurons, which respond to elevated glucose concentration with either increase (glucose-excited cells) or decrease (glucose-inhibited cells) in their firing rate (37, 1272). The sensitivity of these neurons to physiological glucose fluctuations remains debatable, although a subpopulation of glucose-inhibited orexin-producing neurons readily respond to physiologically relevant increases in glucose concentration (259). Tanycytes represent the second glucose-sensing cellular population of the hypothalamus. In the medial eminence, β2 tanycytes constitute the blood-brain barrier sealed by tight junctions between the somata of these glial cells; this barrier prevents diffusion of blood-originated molecules to the brain parenchyma. At the same time processes of β2 tanycytes establish direct contact with the fenestrated vessels of the pituitary portal blood system, thus being able to inspect the molecular composition of the blood, including glucose and hormones (1170, 1482, 1554).
These hypothalamic tanycytes have complex signaling: they express P2X and P2Y1 purinoceptors, ACh receptors, and histamine receptors all linked to the generation of intracellular Ca2+ signals (376, 524). Furthermore, β2 tanycytes are functionally connected to propagating Ca2+ waves and are capable of releasing ATP (376, 524). Application of glucose or its nonmetabolizable analogs to the somata of tanycytes in acute slices triggered [Ca2+]i elevation and Ca2+ waves; this signaling was mediated by ATP acting through P2Y1 receptors to evoke Ca2+ release from the endoplasmic reticulum (206, 524). This glucose-induced Ca2+ signaling was cell autonomous and was replicated in the in vitro experiments: increases in extracellular glucose from 2 to 10 mM triggered Ca2+ signaling in cultured tanycytes (1273). These Ca2+ responses were similarly ATP dependent and required functional Cx43 hemichannels through which ATP was presumably released (1273). Mechanisms of glucose-induced Ca2+ signaling remain unclear; they may involve [Na+]i rise (generated by glucose-Na+ cotransporter SGLT1) with subsequent reversal of NCX or operation of yet unknown glucose-sensitive metabotropic receptor coupled to PLC/InsP3/Ca2+ release cascade (206). This astroglial signaling potentially may influence hypothalamic neurons; some initial data indicate that optogenetic stimulation of tanycytes through Ca2+-permeable channelrhodopsin2 resulted in excitation of neurons in the arcuate nucleus (207). Hypothalamic parenchymal astrocytes may also employ the GLUT2-glucokinase2 pathway for glucose-sensing via K-ATP channels (967), although this pathway requires physiological confirmation. Finally, conditional genetic deletion of astroglial insulin receptors affects glucose-sensing and systemic glucose metabolism (549).
2. Tanycytes provide a conduit for peripheral hormones to the brain
As mentioned above, tanycytes are capable of sensing circulating hormones, and they are the first to respond to intraperitoneal injection of leptin by leptin receptor (LepR)-mediated stimulation of STAT3 phosphorylation that occurs (as visualized by phosphorylated STAT3 immunoreactivity) in the processes contacting fenestrated capillaries (89). Moreover, leptin, which freely diffuses through these fenestra, is taken up by tanycytes (89, 1554), the process of which is somehow mediated by LepRs (89). It is hypothesized that captured leptin is transported through tanycytes and released into the parenchyma of hypothalamus from which it may further disseminate through the brain; this hypothesis has been further extended to ghrelin and possibly other peptide hormones (273, 345).
3. Astrocytes may regulate food intake
Food intake is controlled by two populations of hypothalamic neurons: agouti-related protein (AGRP) expressing neurons that stimulate food intake and pro-opiomelanocortin (POMC) neurons that inhibit food-seeking behavior (73). The role of astrocytes in regulation of food intake was first suggested based on the analysis of transgenic animals in which LepRs were specifically deleted from astrocytes. This manipulation reduced the number and complexity of hypothalamic (but not hippocampal) astroglia processes and decreased astroglial synaptic coverage on both AGRP and POMC neurons, which arguably led to an aberrant synaptic transmission and aberrant feeding behavior (861). Incidentally, astroglial deletion of LepRs prevented the development of morbid obesity in the modified mice (790). Stimulation of medial basal hypothalamic astrocytes carrying muscarinic receptor variant hM3Dq (using the Designer Receptor Exclusively Activated by Designer Drug,or DREADD technology) inhibited both basal feeding and feeding evoked by ghrelin. These effects were arguably mediated through stimulation of A1 adenosine receptors located on AGRP neurons (1915). Such an artificial stimulation, however, may not necessarily reflect physiological context, and hence more research is needed.
N. Astroglia and Control of Reproduction
The gonadotropin-releasing hormone (GnRH), secreted by specific gonadotropin-releasing hormone neurons in the hypothalamus, controls coordinated release of luteinizing hormone and follicle-stimulating hormone, which both regulate reproductive function. There is some evidence for astrocytes influencing these events. Astroglia show hormone-dependent phasic morphological plasticity in the estrus cycle, which may regulate the density of synapses (e.g., GABAergic; Ref. 1319). Increase in astroglial membrane coverage at high estrogen levels prevents formation of inhibitory synaptic inputs in arcuate neurons (552) Steroid-induced remodeling of astroglia was also reported to reduce the number of dendritic spines in rat hypothalamus (1142). Thus astroglial morphological plasticity may represent a mechanistic link between plasma levels of 17β-estradiol and pulsatile secretion of gonadotropin hormones (422). Astrocytes also secrete several factors, which simulate GnRH release; these factors include transforming growth factor-β1, astrocyte-derived transforming growth factor-α, or neuroactive steroid metabolites (1051). It was also reported that oxytocin-induced PGE2 secretion from astrocytes regulates pulsatile release of GnRH from neurons (1321).
O. Müller Glial Cells as Light Guides in Retina
The most exotic astroglial function belongs to Müller retinal glial cells. Müller cells perform all archetypal astroglial functions such as K+ homeostasis, glutamate uptake, regulation of extracellular pH, control of synaptogenesis, etc. (1450). In addition, Müller cells, which span the whole thickness of retina and are oriented along the direction of the light path, act as single-cell optical fibers that convey light directly to photoreceptors with minimal distortion or loss of photons (FIGURE 33). In the human retina, every Müller cell is coupled to one cone photoreceptor cell (responsible for sharp sight under daylight conditions, i.e., photopic vision) and several (~10) rod photoreceptor cells. The endfoot of the Müller cell is shaped as a funnel, and apparently acts as a light collector at the vitreal surface of the retina (522).
FIGURE 33.
Müller cells as light guides. Shown as an artistic impression, the light penetrates the retina using Müller glial cells as guides. (Artwork courtesy of Dr. Jens Grosche, Leipzig University.)
P. Recapitulation
The main and most fundamental function of astroglia is sustaining homeostasis at all levels of CNS organization. Employing a variety of mechanisms, astrocytes provide for molecular, cellular, structural, organ, and systemic homeostatic control. Through a synaptic cradle that embraces CNS synapses, astrocytes control the birth, maturation, and extinction of synapses; through releasing numerous neuroactive substances and regulating ionostasis, astrocytes affect neuronal excitability and synaptic connectivity. Astrocytes orchestrate homeostasis of the brain as an organ, particularly through establishing the glymphatic system that removes accumulated waste. Astrocytes are chemosensing elements of the brain contributing to systemic homeostasis of ions, metabolites, and energy.
Surprisingly limited efforts have been made into understanding how astrocytes participate in brain function on a macroscopic scale. The majority of functional studies of astrocytes have focused on local signaling. Even the archetypal supportive functions, such as astrocytic K+ buffering or glutamate uptake, have been generally studied at a cellular level. However, the brain is an organ assembled, on a macroscopic scale, to support the basic needs for energy metabolites and export of waste products. Since astrocytes play pivotal roles in both of these processes, a higher order of organization of astrocytes must exist to fulfil brain-wide tasks, in addition to the cellular specialization needed to meet local demands. The best example of a macroscopic function of astrocytes is the glymphatic system. Astrocytes create, by their vascular endfeet, the perivascular space which provides a low-resistance pathway for fast influx of cerebrospinal fluid. The highly polarized expression of the water channel in astrocytic endfeet facing the perivascular space allow direct influx of CSF which intermix with the extracellular fluid before leaving the brain via transport along the perivenous space and meningeal lymph vessels. Thus astrocytes provide the structural and functional background for the brain-wide fluid transport system.
XIII. ASTROCYTES AND HIGHER COGNITIVE FUNCTIONS
Do astrocytes directly contribute to higher cognitive functions of the brain? Are astroglial cells contributing to emotions, learning, memory, and generation of thoughts? The finding that electrically silent astrocytes can instigate Ca2+ signals in neighboring cocultured neurons (1203, 1328) sparked an interest in studies of glial-neuronal signaling. Initial findings prompted the provoking question of whether astrocytes can modulate or even contribute to synaptic transmission. The answer, generally, is positive, as first documented by multiple groups in 1998 (59, 822, 1478). A myriad of studies, using a variety of techniques, has since expanded this concept to almost all regions of the brain and the spinal cord. It is now universally acknowledged that astrocytes can modulate both the intrinsic neuronal excitability and the strength of synaptic transmission (55, 393). Many ex vivo studies, however, suffer from technical limitations, such as the use of slices prepared from newborn animals with immature astrocytes, or use of nonphysiological manipulations. Suprathreshold stimulation of astrocytes may compromise their housekeeping function or directly evoke nonphysiological release of neurotransmitters present in the cytosol, thus creating artificial astrocyte-to-neuron signaling. Hence, despite considerable experimental effort, the question of whether astrocytes directly contribute to cognitive functions, or allow these cognitive functions through their multifaceted support remains open. Are astrocytes merely supportive cells that beyond their servile functions have little or no influence on classical measures of information processing, such as learning or working memory? The answer is yet to be procured.
Several indirect observations obtained in vivo indicate that astrocytic activity is associated with the state of arousal. For example, astrocytic Ca2+ signals are strongly suppressed by anesthesia in intact animals (1581, 1748). Conversely, most, if not all, astroglial Ca2+ signaling is mediated by norepinephrine in awake behaving mice (431, 1341, 1660). Norepinephrine is regarded as the “fight-or-flight” transmitter, which is released in response to environmental clues that require a reorientation in focus (575). It is presently accepted that activation of astrocytic α1-adrenoceptors account for >90% of spontaneous Ca2+ signaling in awake behaving mice and that most, if not all, evoked Ca2+ increases in response to startle responses or locomotion are similarly eliminated by the α1-adrenoceptors antagonists. However, the functional significance of the increases in astrocytic Ca2+ that occur simultaneously across most brain regions in response to locus coeruleus activation has not been established. Possibly these signals prepare the brain for a surge in the metabolic rate and are coupled to glycogenolysis and vascular response (123, 575). However, the lack of mice with conditional deletion of astrocytic adrenergic receptors has for now prevented an analysis of the functional importance of astrocytic Ca2+ signaling evoked by norepinephrine release from locus coeruleus projections.
There are indications that process of learning is associated with astroglial changes. Exposure to enriched environment, for example, increases complexity and extension of astroglial processes and enhances astroglial synaptic coverage (802, 1485), with hypertrophic astrocytes boosting synaptogenesis during motor skill learning (39). Physical exercise similarly enhances astroglial profiles (1485) and increases expression of glucose transporters (22).
Only sporadic studies have approached the question of the role of astrocytes in cognitive function in vivo. One of these studies analyzed the impairment of working memory induced by cannabinoid or marijuana. Mice with conditional astrocytic deletion of CB1 cannabinoid receptors did not display deficits in working memory in response to cannabinoid. In contrast, mice with conditional deletion of CB1 receptors in glutamatergic or inhibitory neurons exhibited the expected detrimental effects of cannabinoid (634). Thus excessive stimulation of astrocytic CB1 receptors may mediate cannabinoid-related impairment of cognitive function. Another study found that exogenous TNF-α acting through astrocytic TNF receptor type 1 in mice induced a persistent change in hippocampal excitatory synapses and suppressed contextual memory and learning in the experimental autoimmune encephalitis (621). Yet, a broad behavioral screening of cognitive function in mice with astrocytes deficient in InsP3R type 2 failed to identify anxiety or depressive behaviors. Neither Morris water maze performance nor sensory and motor functions were affected in these animals (1381).
From an evolutionary perspective, human astrocytes are 15- to 20-fold larger than their rodent counterpartners, while genomic analysis show a much more complex set of intracellular signaling pathways in human compared with mice astrocytes (see sect. VB3). Even more significantly, implantation of human glial cells progenitors into mouse brain revealed that humanized chimeric mice outperform their control littermates which received intraventricular engraftment of murine glial cells progenitors in several cognitive tests (FIGURE 34; Ref. 637).
FIGURE 34.
Humanized chimeric mice learn faster than wild-type controls. A: auditory fear conditioning assessed in a cohort of human chimeric, mouse chimeric, and unengrafted control rag1 mice. Chimeric mice exhibit prolonged freezing behavior in test chamber 2, during exposure to the tonal conditioned stimulus when compared with unengrafted mice and allografted mice (n = 5–20; *P < 0.05; **P < 0.01; two-way repeated measures ANOVA with Bonferroni test; means ± SE). This difference persisted throughout all 4 days. B: contextual fear conditioning in human glial-chimeric mice and littermate controls. Freezing behavior was quantified for chimeric and unengrafted littermate controls during the 2 min of acclimatization period (n = 6; *P < 0.05; **P < 0.01; two-way repeated measures ANOVA with Bonferroni test). In addition, the mean discrimination ratio for each day was obtained from freezing scores in the training chamber and the alternative chamber (freezing in training chamber/total freezing time). Chimeric mice demonstrated significantly higher abilities to discriminate the chambers (n = 8–13; *P < 0.05; **P < 0.01; two-way repeated measures ANOVA with Bonferroni test). C: Barnes maze testing in chimeric and unengrafted littermate controls. Chimeric mice demonstrated a significant learning advantage, as reflected in a shorter latency and fewer errors in solving the maze (n = 6; *P < 0.05; **P < 0.01; two-way repeated measures ANOVA with Bonferroni test). D: object-location memory task (OLT) in chimeric mice and their unengrafted littermate controls demonstrated a learning advantage in chimeric mice via enhanced recognition of the novel displaced object. Thalidomide eliminated the learning advantage of chimeric mice, suggesting that the learning enhancement was TNF-α mediated (n = 7; **P < 0.01; one-way ANOVA with Bonferroni test). All data plotted as means ± SE. [From Han et al. (637).]
None of these studies however brings us closer to answering the question of whether astrocytes directly participate in complex cognitive function. The effect of prolonged exposure to high concentrations of cannabinoid or TNF-α does not reflect normal information processing, and both compounds may impair the supportive functions of astrocytes and thereby indirectly depress information processing. Deletion of InsP3 receptors may induce adaptive changes during development that fully compensate for astrocytic functions. Similarly, remodeling of synapses in response to neurotrophic factors released by human astrocytes may boost the plasticity of host neuronal network and thereby enhance cognition by mechanisms that do not involve direct participation of astrocytes. Thus none of these studies can be taken as a definitive proof that astrocytes are a partaker in higher brain function. Wherever the uncertainty exists, there is always room for conjecture. Emergence of new experimental techniques, such as DREADD or optogenetics, opens exciting new possibilities. These new techniques, however, have to be treated with great care, because introduction of artificial signaling pathways may result in nonphysiological responses and hence lead to erroneous conclusions. A major task for the future is to define the importance of neuroglia signaling in awake behaving animals in the absence of pathology concurrently with use of physiologically relevant manipulations of astrocytes.
XIV. THE EPILOGUE
Astrocytes are the supportive cells of the CNS, and they provide this support on all levels of organization of the nervous tissue. Multiple functions of astroglia reflect evolutionary division of tasks that bestowed electrical excitability and fast information transfer on neurons, while shifting homeostatic tasks to astroglia. Somehow the definition of being “supportive” scares the gliobiological community, which for years has tried to present astrocytes as worthy competitors of neurons in tasks (such as fast neurotransmitter release) of which astrocytes are not terribly capable. To be an omnipotent supportive cell is however not less important than to be able to fire action potentials. To the very best of our knowledge, astrocytes are the primary supportive, homeostatic, and defensive cells of the CNS, which, without this support, simply cannot exist.
GRANTS
This work was supported by the National Institutes of Health (NIH). M. Nedergaard was supported by NIH, Novo Nordisk, and the Adelson Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We are very grateful to Prof. Helmut Kettenmann (Berlin) and Dr. Alexandr Chvatal (Prague) for providing us with materials on glial history and to Dr. Nadia Alling for helpful discussions. We are also grateful to Andrea Grostøl Dietz, Stephanie von Holstein-Rathlou, Kristian Nygaard Mortensen, Andrew James Samson, Simon Sanggaard, Mauro DiNuzzo, Camilla Dall, Celia Johanne Kjærby Palner, Benjamin Travis Kress, Harlan Albert Mullins, and Pia Crine Christensen for critically reading the manuscript.
Address for reprint requests and other correspondence: A. Verkhratsky, Faculty of Life Sciences, The University of Manchester, Manchester, UK (e-mail: Alexej.Verkhratsky@manchester.ac.uk) or M. Nedergaard, Center for Translational Neuromedicine, University of Rochester Medical Center, Rochester, NY 14642 (e-mail: nedergaard@urmc.rochester.edu or nedergaard@sund.ku.dk).
Footnotes
The term glia does not mean glue; glue in Greek is “κολλα.” Virchow defined glia as “connective substance, which forms in the brain, in the spinal cord, and in the higher sensory nerves a sort of Nervenkitt (neuroglia)”; the word “kitt” (German) as well as “glia” (Greek) refers to putty or gum; the root “glia” also means slippery (in both physical and moral terms).
“I would suggest that all supporting cells be named spongiocytes. And the most common form in vertebrates be named spider cells or astrocytes, and use the term neuroglia only cum grano salis (with a grain of salt), at least until we have a clearer view.” p. 180 (969).
These references were randomly chosen from more than 100 papers that start with this statement.
Classification introduced by Martin Ruff for cultured astroglia with type 1 cells having largely fibroblast morphology and type 2 cells being stellate (1421).
Named after three Greek sister-goddesses Orai, the keepers of the Heaven’s gate (505).
Rather popular definition of pannexons as hemichannels is therefore erroneous; they are bona fide plasmalemmal channels (see also Ref. 1654). Reports claiming Panx1 gap junctions in the artificial expression system (1527) cannot be as yet considered fully relevant.
These are often called gliotransmitters, which in our view is somewhat misleading as chemically these molecules are the same regardless of their cellular (neurons vs. glia) source.
REFERENCES
- 1.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58: 281–341, 2006. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci 32: 19–29, 2009. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Abbracchio MP, Cattabeni F. Brain adenosine receptors as targets for therapeutic intervention in neurodegenerative diseases. Ann N Y Acad Sci 890: 79–92, 1999. doi: 10.1111/j.1749-6632.1999.tb07983.x. [DOI] [PubMed] [Google Scholar]
- 4.Abbracchio MP, Ceruti S. Roles of P2 receptors in glial cells: focus on astrocytes. Purinergic Signal 2: 595–604, 2006. doi: 10.1007/s11302-006-9016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, Mongin AA. Pharmacological comparison of swelling-activated excitatory amino acid release and Cl− currents in cultured rat astrocytes. J Physiol 572: 677–689, 2006. doi: 10.1113/jphysiol.2005.103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acaz-Fonseca E, Avila-Rodriguez M, Garcia-Segura LM, Barreto GE. Regulation of astroglia by gonadal steroid hormones under physiological and pathological conditions. Prog Neurobiol 144: 5–26, 2016. doi: 10.1016/j.pneurobio.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Achatz JG, Martinez P. The nervous system of Isodiametra pulchra (Acoela) with a discussion on the neuroanatomy of the Xenacoelomorpha and its evolutionary implications. Front Zool 9: 27, 2012. doi: 10.1186/1742-9994-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Achucarro N. De l’évulotion de la névroglie, et spécialement de ses relations avec l’appareil vasculaire. Trab Lab Invest Biol (Madrid) 13: 169–212, 1915. [Google Scholar]
- 9.Adamczyk LA, Gordon K, Kholová I, Meijer-Jorna LB, Telinius N, Gallagher PJ, van der Wal AC, Baandrup U. Lymph vessels: the forgotten second circulation in health and disease. Virchows Arch 469: 3–17, 2016. doi: 10.1007/s00428-016-1945-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adami C, Sorci G, Blasi E, Agneletti AL, Bistoni F, Donato R. S100B expression in and effects on microglia. Glia 33: 131–142, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 11.Adermark L, Lovinger DM. Electrophysiological properties and gap junction coupling of striatal astrocytes. Neurochem Int 52: 1365–1372, 2008. doi: 10.1016/j.neuint.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adinolfi E, Cirillo M, Woltersdorf R, Falzoni S, Chiozzi P, Pellegatti P, Callegari MG, Sandonà D, Markwardt F, Schmalzing G, Di Virgilio F. Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. FASEB J 24: 3393–3404, 2010. doi: 10.1096/fj.09-153601. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Wu PH, Hughes EG, Fukaya M, Tischfield MA, Langseth AJ, Wirtz D, Bergles DE. Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 93: 587–605.e7, 2017. doi: 10.1016/j.neuron.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327: 1250–1254, 2010. doi: 10.1126/science.1184821. [DOI] [PubMed] [Google Scholar]
- 15.Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD. What is the role of astrocyte calcium in neurophysiology? Neuron 59: 932–946, 2008. doi: 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akerboom J, Carreras Calderón N, Tian L, Wabnig S, Prigge M, Tolö J, Gordus A, Orger MB, Severi KE, Macklin JJ, Patel R, Pulver SR, Wardill TJ, Fischer E, Schüler C, Chen TW, Sarkisyan KS, Marvin JS, Bargmann CI, Kim DS, Kügler S, Lagnado L, Hegemann P, Gottschalk A, Schreiter ER, Looger LL. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front Mol Neurosci 6: 2, 2013. doi: 10.3389/fnmol.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akita T, Okada Y. Regulation of bradykinin-induced activation of volume-sensitive outwardly rectifying anion channels by Ca2+ nanodomains in mouse astrocytes. J Physiol 589: 3909–3927, 2011. doi: 10.1113/jphysiol.2011.208173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akmayev IG, Fidelina OV. Morphological aspects of the hypothalamic-hypophyseal system. VI. The tanycytes: their relation to the sexual differentiation of the hypothalamus. An enzyme-histochemical study. Cell Tissue Res 173: 407–416, 1976. [DOI] [PubMed] [Google Scholar]
- 19.Akopian G, Kressin K, Derouiche A, Steinhäuser C. Identified glial cells in the early postnatal mouse hippocampus display different types of Ca2+ currents. Glia 17: 181–194, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 20.Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science 258: 1812–1815, 1992. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- 21.Alle H, Roth A, Geiger JR. Energy-efficient action potentials in hippocampal mossy fibers. Science 325: 1405–1408, 2009. doi: 10.1126/science.1174331. [DOI] [PubMed] [Google Scholar]
- 22.Allen A, Messier C. Plastic changes in the astrocyte GLUT1 glucose transporter and beta-tubulin microtubule protein following voluntary exercise in mice. Behav Brain Res 240: 95–102, 2013. doi: 10.1016/j.bbr.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Allen JW, Shanker G, Aschner M. Methylmercury inhibits the in vitro uptake of the glutathione precursor, cystine, in astrocytes, but not in neurons. Brain Res 894: 131–140, 2001. doi: 10.1016/S0006-8993(01)01988-6. [DOI] [PubMed] [Google Scholar]
- 24.Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, Barres BA. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 486: 410–414, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alloisio S, Cugnoli C, Ferroni S, Nobile M. Differential modulation of ATP-induced calcium signalling by A1 and A2 adenosine receptors in cultured cortical astrocytes. Br J Pharmacol 141: 935–942, 2004. doi: 10.1038/sj.bjp.0705707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almey A, Filardo EJ, Milner TA, Brake WG. Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology 153: 5373–5383, 2012. doi: 10.1210/en.2012-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altenhein B, Cattenoz PB, Giangrande A. The early life of a fly glial cell. Wiley Interdiscip Rev Dev Biol 5: 67–84, 2016. doi: 10.1002/wdev.200. [DOI] [PubMed] [Google Scholar]
- 28.Altevogt BM, Paul DL. Four classes of intercellular channels between glial cells in the CNS. J Neurosci 24: 4313–4323, 2004. doi: 10.1523/JNEUROSCI.3303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altimimi HF, Schnetkamp PP. Na+/Ca2+-K+ exchangers (NCKX): functional properties and physiological roles. Channels (Austin) 1: 62–69, 2007. doi: 10.4161/chan.4366. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez-Buylla A, Theelen M, Nottebohm F. Mapping of radial glia and of a new cell type in adult canary brain. J Neurosci 8: 2707–2712, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvarez-Maubecin V, Garcia-Hernandez F, Williams JT, Van Bockstaele EJ. Functional coupling between neurons and glia. J Neurosci 20: 4091–4098, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez MI, Rivas L, Lacruz C, Toledano A. Astroglial cell subtypes in the cerebella of normal adults, elderly adults, and patients with Alzheimer’s disease: a histological and immunohistochemical comparison. Glia 63: 287–312, 2015. doi: 10.1002/glia.22751. [DOI] [PubMed] [Google Scholar]
- 33.Amateau SK, McCarthy MM. Sexual differentiation of astrocyte morphology in the developing rat preoptic area. J Neuroendocrinol 14: 904–910, 2002. doi: 10.1046/j.1365-2826.2002.00858.x. [DOI] [PubMed] [Google Scholar]
- 34.Amin MS, Wang HW, Reza E, Whitman SC, Tuana BS, Leenen FH. Distribution of epithelial sodium channels and mineralocorticoid receptors in cardiovascular regulatory centers in rat brain. Am J Physiol Regul Integr Comp Physiol 289: R1787–R1797, 2005. doi: 10.1152/ajpregu.00063.2005. [DOI] [PubMed] [Google Scholar]
- 35.Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci 4: 991–1001, 2003. doi: 10.1038/nrn1252. [DOI] [PubMed] [Google Scholar]
- 36.Amzica F, Massimini M. Glial and neuronal interactions during slow wave and paroxysmal activities in the neocortex. Cereb Cortex 12: 1101–1113, 2002. doi: 10.1093/cercor/12.10.1101. [DOI] [PubMed] [Google Scholar]
- 37.Anand BK, Chhina GS, Sharma KN, Dua S, Singh B. Activity of single neurons in the hypothalamic feeding centers: Effect of glucose. Am J Physiol 207: 1146–1154, 1964. [DOI] [PubMed] [Google Scholar]
- 38.Anastasi A, Erspamer V, Bucci M. Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia 27: 166–167, 1971. doi: 10.1007/BF02145873. [DOI] [PubMed] [Google Scholar]
- 39.Anderson BJ, Li X, Alcantara AA, Isaacs KR, Black JE, Greenough WT. Glial hypertrophy is associated with synaptogenesis following motor-skill learning, but not with angiogenesis following exercise. Glia 11: 73–80, 1994. doi: 10.1002/glia.440110110. [DOI] [PubMed] [Google Scholar]
- 40.Anderson CM, Bergher JP, Swanson RA. ATP-induced ATP release from astrocytes. J Neurochem 88: 246–256, 2004. doi: 10.1111/j.1471-4159.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- 41.Andersson M, Blomstrand F, Hanse E. Astrocytes play a critical role in transient heterosynaptic depression in the rat hippocampal CA1 region. J Physiol 585: 843–852, 2007. doi: 10.1113/jphysiol.2007.142737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.André C, Dos Santos G, Koulakoff A. Muscarinic receptor profiles of mouse brain astrocytes in culture vary with their tissue of origin but differ from those of neurons. Eur J Neurosci 6: 1702–1709, 1994. doi: 10.1111/j.1460-9568.1994.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 43.Andrews NW, Chakrabarti S. There’s more to life than neurotransmission: the regulation of exocytosis by synaptotagmin VII. Trends Cell Biol 15: 626–631, 2005. doi: 10.1016/j.tcb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Andriezen WL. The neuroglia elements of the human brain. BMJ 2: 227–230, 1893. doi: 10.1136/bmj.2.1700.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angelova PR, Kasymov V, Christie I, Sheikhbahaei S, Turovsky E, Marina N, Korsak A, Zwicker J, Teschemacher AG, Ackland GL, Funk GD, Kasparov S, Abramov AY, Gourine AV. Functional oxygen sensitivity of astrocytes. J Neurosci 35: 10460–10473, 2015. doi: 10.1523/JNEUROSCI.0045-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ango F, Wu C, Van der Want JJ, Wu P, Schachner M, Huang ZJ. Bergmann glia and the recognition molecule CHL1 organize GABAergic axons and direct innervation of Purkinje cell dendrites. PLoS Biol 6: e103, 2008. doi: 10.1371/journal.pbio.0060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci 24: 6920–6927, 2004. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Angulo MC, Le Meur K, Kozlov AS, Charpak S, Audinat E. GABA, a forgotten gliotransmitter. Prog Neurobiol 86: 297–303, 2008. doi: 10.1016/j.pneurobio.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Anlauf E, Derouiche A. Glutamine synthetase as an astrocytic marker: its cell type and vesicle localization. Front Endocrinol (Lausanne) 4: 144, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anlauf E, Derouiche A. A practical calibration procedure for fluorescence colocalization at the single organelle level. J Microsc 233: 225–233, 2009. doi: 10.1111/j.1365-2818.2009.03112.x. [DOI] [PubMed] [Google Scholar]
- 51.Aoki C. β-Adrenergic receptors: astrocytic localization in the adult visual cortex and their relation to catecholamine axon terminals as revealed by electron microscopic immunocytochemistry. J Neurosci 12: 781–792, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aoki C, Venkatesan C, Go CG, Mong JA, Dawson TM. Cellular and subcellular localization of NMDA-R1 subunit immunoreactivity in the visual cortex of adult and neonatal rats. J Neurosci 14: 5202–5222, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aoki C, Venkatesan C, Kurose H. Noradrenergic modulation of the prefrontal cortex as revealed by electron microscopic immunocytochemistry. Adv Pharmacol 42: 777–780, 1998. doi: 10.1016/S1054-3589(08)60862-5. [DOI] [PubMed] [Google Scholar]
- 54.Appaix F, Girod S, Boisseau S, Römer J, Vial JC, Albrieux M, Maurin M, Depaulis A, Guillemain I, van der Sanden B. Specific in vivo staining of astrocytes in the whole brain after intravenous injection of sulforhodamine dyes. PLoS One 7: e35169, 2012. doi: 10.1371/journal.pone.0035169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron 81: 728–739, 2014. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Araque A, Martín ED, Perea G, Arellano JI, Buño W. Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. J Neurosci 22: 2443–2450, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci 10: 2129–2142, 1998. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- 58.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22: 208–215, 1999. doi: 10.1016/S0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 59.Araque A, Sanzgiri RP, Parpura V, Haydon PG. Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J Neurosci 18: 6822–6829, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arbonés L, Picatoste F, García A. Histamine stimulates glycogen breakdown and increases 45Ca2+ permeability in rat astrocytes in primary culture. Mol Pharmacol 37: 921–927, 1990. [PubMed] [Google Scholar]
- 61.Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci USA 99: 9840–9845, 2002. doi: 10.1073/pnas.152588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ari C, Kálmán M. Evolutionary changes of astroglia in Elasmobranchii comparing to amniotes: a study based on three immunohistochemical markers (GFAP, S-100, and glutamine synthetase). Brain Behav Evol 71: 305–324, 2008. doi: 10.1159/000129654. [DOI] [PubMed] [Google Scholar]
- 63.Arias C, Zepeda A, Hernández-Ortega K, Leal-Galicia P, Lojero C, Camacho-Arroyo I. Sex and estrous cycle-dependent differences in glial fibrillary acidic protein immunoreactivity in the adult rat hippocampus. Horm Behav 55: 257–263, 2009. doi: 10.1016/j.yhbeh.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 64.Armbruster M, Hanson E, Dulla CG. Glutamate clearance is locally modulated by presynaptic neuronal activity in the cerebral cortex. J Neurosci 36: 10404–10415, 2016. doi: 10.1523/JNEUROSCI.2066-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Armstrong WE, Rubrum A, Teruyama R, Bond CT, Adelman JP. Immunocytochemical localization of small-conductance, calcium-dependent potassium channels in astrocytes of the rat supraoptic nucleus. J Comp Neurol 491: 175–185, 2005. doi: 10.1002/cne.20679. [DOI] [PubMed] [Google Scholar]
- 66.Arnth-Jensen N, Jabaudon D, Scanziani M. Cooperation between independent hippocampal synapses is controlled by glutamate uptake. Nat Neurosci 5: 325–331, 2002. doi: 10.1038/nn825. [DOI] [PubMed] [Google Scholar]
- 67.Aroeira RI, Sebastião AM, Valente CA. GlyT1 and GlyT2 in brain astrocytes: expression, distribution and function. Brain Struct Funct 219: 817–830, 2014. doi: 10.1007/s00429-013-0537-3. [DOI] [PubMed] [Google Scholar]
- 68.Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci 14: 5559–5569, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ashour F, Deuchars J. Electron microscopic localisation of P2X4 receptor subunit immunoreactivity to pre- and post-synaptic neuronal elements and glial processes in the dorsal vagal complex of the rat. Brain Res 1026: 44–55, 2004. doi: 10.1016/j.brainres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 70.Ashur-Fabian O, Giladi E, Brenneman DE, Gozes I. Identification of VIP/PACAP receptors on rat astrocytes using antisense oligodeoxynucleotides. J Mol Neurosci 9: 211–222, 1997. doi: 10.1007/BF02800503. [DOI] [PubMed] [Google Scholar]
- 71.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212: 991–999, 2015. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Asztely F, Erdemli G, Kullmann DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron 18: 281–293, 1997. doi: 10.1016/S0896-6273(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 73.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature 488: 172–177, 2012. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature 468: 232–243, 2010. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 21: 1133–1145, 2001. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 76.Aubrey KR, Vandenberg RJ, Clements JD. Dynamics of forward and reverse transport by the glial glycine transporter, glyt1b. Biophys J 89: 1657–1668, 2005. doi: 10.1529/biophysj.105.061572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Azarias G, Van de Ville D, Unser M, Chatton JY. Spontaneous Na+ transients in individual mitochondria of intact astrocytes. Glia 56: 342–353, 2008. doi: 10.1002/glia.20619. [DOI] [PubMed] [Google Scholar]
- 78.Azcoitia I, Sierra A, Garcia-Segura LM. Localization of estrogen receptor β-immunoreactivity in astrocytes of the adult rat brain. Glia 26: 260–267, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 79.Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 513: 532–541, 2009. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 80.Azmitia EC, Gannon PJ, Kheck NM, Whitaker-Azmitia PM. Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology 14: 35–46, 1996. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- 81.Babu AN, Cheng TP, Zhang A, Altura BT, Altura BM. Low concentrations of ethanol deplete type-2 astrocytes of intracellular free magnesium. Brain Res Bull 50: 59–62, 1999. doi: 10.1016/S0361-9230(99)00091-X. [DOI] [PubMed] [Google Scholar]
- 82.Bacaj T, Tevlin M, Lu Y, Shaham S. Glia are essential for sensory organ function in C. elegans. Science 322: 744–747, 2008. doi: 10.1126/science.1163074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Backus KH, Berger T, Kettenmann H. Activation of neurokinin receptors modulates K+ and Cl− channel activity in cultured astrocytes from rat cortex. Brain Res 541: 103–109, 1991. doi: 10.1016/0006-8993(91)91081-B. [DOI] [PubMed] [Google Scholar]
- 84.Badaut J, Hirt L, Granziera C, Bogousslavsky J, Magistretti PJ, Regli L. Astrocyte-specific expression of aquaporin-9 in mouse brain is increased after transient focal cerebral ischemia. J Cereb Blood Flow Metab 21: 477–482, 2001. doi: 10.1097/00004647-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 85.Bahney J, von Bartheld CS. Validation of the isotropic fractionator: comparison with unbiased stereology and DNA extraction for quantification of glial cells. J Neurosci Methods 222: 165–174, 2014. doi: 10.1016/j.jneumeth.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bai JZ, Lipski J. Differential expression of TRPM2 and TRPV4 channels and their potential role in oxidative stress-induced cell death in organotypic hippocampal culture. Neurotoxicology 31: 204–214, 2010. doi: 10.1016/j.neuro.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 87.Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci 6: 743–749, 2003. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 88.Bal A, Bachelot T, Savasta M, Manier M, Verna JM, Benabid AL, Feuerstein C. Evidence for dopamine D2 receptor mRNA expression by striatal astrocytes in culture: in situ hybridization and polymerase chain reaction studies. Brain Res Mol Brain Res 23: 204–212, 1994. doi: 10.1016/0169-328X(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 89.Balland E, Dam J, Langlet F, Caron E, Steculorum S, Messina A, Rasika S, Falluel-Morel A, Anouar Y, Dehouck B, Trinquet E, Jockers R, Bouret SG, Prévot V. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab 19: 293–301, 2014. doi: 10.1016/j.cmet.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ballanyi K, Grafe P, ten Bruggencate G. Ion activities and potassium uptake mechanisms of glial cells in guinea-pig olfactory cortex slices. J Physiol 382: 159–174, 1987. doi: 10.1113/jphysiol.1987.sp016361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ballerini P, Ciccarelli R, Caciagli F, Rathbone MP, Werstiuk ES, Traversa U, Buccella S, Giuliani P, Jang S, Nargi E, Visini D, Santavenere C, Di Iorio P. P2X7 receptor activation in rat brain cultured astrocytes increases the biosynthetic release of cysteinyl leukotrienes. Int J Immunopathol Pharmacol 18: 417–430, 2005. doi: 10.1177/039463200501800303. [DOI] [PubMed] [Google Scholar]
- 92.Ballerini P, Di Iorio P, Ciccarelli R, Nargi E, D’Alimonte I, Traversa U, Rathbone MP, Caciagli F. Glial cells express multiple ATP binding cassette proteins which are involved in ATP release. Neuroreport 13: 1789–1792, 2002. doi: 10.1097/00001756-200210070-00019. [DOI] [PubMed] [Google Scholar]
- 93.Ballerini P, Rathbone MP, Di Iorio P, Renzetti A, Giuliani P, D’Alimonte I, Trubiani O, Caciagli F, Ciccarelli R. Rat astroglial P2Z (P2X7) receptors regulate intracellular calcium and purine release. Neuroreport 7: 2533–2537, 1996. doi: 10.1097/00001756-199611040-00026. [DOI] [PubMed] [Google Scholar]
- 94.Balu DT, Coyle JT. Neuronal D-serine regulates dendritic architecture in the somatosensory cortex. Neurosci Lett 517: 77–81, 2012. doi: 10.1016/j.neulet.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bancroft JD, Gamble M (Editors). Theory and Practice of Histological Techniques (6th ed.). New York: Churchill Livingstone/Elsevier, 2008, p. 744. [Google Scholar]
- 96.Bandeira F, Lent R, Herculano-Houzel S. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc Natl Acad Sci USA 106: 14108–14113, 2009. doi: 10.1073/pnas.0804650106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bar-Peled O, Ben-Hur H, Biegon A, Groner Y, Dewhurst S, Furuta A, Rothstein JD. Distribution of glutamate transporter subtypes during human brain development. J Neurochem 69: 2571–2580, 1997. doi: 10.1046/j.1471-4159.1997.69062571.x. [DOI] [PubMed] [Google Scholar]
- 98.Barakat L, Bordey A. GAT-1 and reversible GABA transport in Bergmann glia in slices. J Neurophysiol 88: 1407–1419, 2002. [DOI] [PubMed] [Google Scholar]
- 99.Barceló-Torns M, Lewis AM, Gubern A, Barneda D, Bloor-Young D, Picatoste F, Churchill GC, Claro E, Masgrau R. NAADP mediates ATP-induced Ca2+ signals in astrocytes. FEBS Lett 585: 2300–2306, 2011. doi: 10.1016/j.febslet.2011.05.062. [DOI] [PubMed] [Google Scholar]
- 100.Bardoni R, Ghirri A, Zonta M, Betelli C, Vitale G, Ruggieri V, Sandrini M, Carmignoto G. Glutamate-mediated astrocyte-to-neuron signalling in the rat dorsal horn. J Physiol 588: 831–846, 2010. doi: 10.1113/jphysiol.2009.180570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barnabé-Heider F, Wasylnka JA, Fernandes KJ, Porsche C, Sendtner M, Kaplan DR, Miller FD. Evidence that embryonic neurons regulate the onset of cortical gliogenesis via cardiotrophin-1. Neuron 48: 253–265, 2005. doi: 10.1016/j.neuron.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 102.Barnea A, Aguila-Mansilla N, Bigio EH, Worby C, Roberts J. Evidence for regulated expression of neuropeptide Y gene by rat and human cultured astrocytes. Regul Pept 75-76: 293–300, 1998. doi: 10.1016/S0167-0115(98)00081-0. [DOI] [PubMed] [Google Scholar]
- 103.Barnett NL, Pow DV, Robinson SR. Inhibition of Müller cell glutamine synthetase rapidly impairs the retinal response to light. Glia 30: 64–73, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 104.Barres BA, Chun LL, Corey DP. Calcium current in cortical astrocytes: induction by cAMP and neurotransmitters and permissive effect of serum factors. J Neurosci 9: 3169–3175, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barres BA, Chun LL, Corey DP. Glial and neuronal forms of the voltage-dependent sodium channel: characteristics and cell-type distribution. Neuron 2: 1375–1388, 1989. doi: 10.1016/0896-6273(89)90076-7. [DOI] [PubMed] [Google Scholar]
- 106.Barres BA, Chun LL, Corey DP. Ion channel expression by white matter glia. I. Type 2 astrocytes and oligodendrocytes. Glia 1: 10–30, 1988. doi: 10.1002/glia.440010104. [DOI] [PubMed] [Google Scholar]
- 107.Barres BA, Koroshetz WJ, Chun LL, Corey DP. Ion channel expression by white matter glia: the type-1 astrocyte. Neuron 5: 527–544, 1990. doi: 10.1016/0896-6273(90)90091-S. [DOI] [PubMed] [Google Scholar]
- 108.Barrett P, Ivanova E, Graham ES, Ross AW, Wilson D, Plé H, Mercer JG, Ebling FJ, Schuhler S, Dupré SM, Loudon A, Morgan PJ. Photoperiodic regulation of cellular retinol binding protein, CRBP1 [corrected] and nestin in tanycytes of the third ventricle ependymal layer of the Siberian hamster. J Endocrinol 191: 687–698, 2006. doi: 10.1677/joe.1.06929. [DOI] [PubMed] [Google Scholar]
- 109.Barros LF, Courjaret R, Jakoby P, Loaiza A, Lohr C, Deitmer JW. Preferential transport and metabolism of glucose in Bergmann glia over Purkinje cells: a multiphoton study of cerebellar slices. Glia 57: 962–970, 2009. doi: 10.1002/glia.20820. [DOI] [PubMed] [Google Scholar]
- 110.Barry D, McDermott K. Differentiation of radial glia from radial precursor cells and transformation into astrocytes in the developing rat spinal cord. Glia 50: 187–197, 2005. doi: 10.1002/glia.20166. [DOI] [PubMed] [Google Scholar]
- 111.Basarsky TA, Feighan D, MacVicar BA. Glutamate release through volume-activated channels during spreading depression. J Neurosci 19: 6439–6445, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Batter DK, Vilijn MH, Kessler J. Cultured astrocytes release proenkephalin. Brain Res 563: 28–32, 1991. doi: 10.1016/0006-8993(91)91510-8. [DOI] [PubMed] [Google Scholar]
- 113.Baumgart EV, Barbosa JS, Bally-Cuif L, Götz M, Ninkovic J. Stab wound injury of the zebrafish telencephalon: a model for comparative analysis of reactive gliosis. Glia 60: 343–357, 2012. doi: 10.1002/glia.22269. [DOI] [PubMed] [Google Scholar]
- 114.Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, Rowitch DH. Astrocyte development and heterogeneity. Cold Spring Harb Perspect Biol 7: a020362, 2014. doi: 10.1101/cshperspect.a020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci 19: 182–189, 2016. doi: 10.1038/nn.4201. [DOI] [PubMed] [Google Scholar]
- 116.Bear MF, Connors B, Paradiso M. Neuroscience-Exploring the Brain (3rd ed.). Philadelphia, PA: Lippincott Williams & Wilkins, 2006. [Google Scholar]
- 117.Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science 295: 2282–2285, 2002. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 118.Beck A, Nieden RZ, Schneider HP, Deitmer JW. Calcium release from intracellular stores in rodent astrocytes and neurons in situ. Cell Calcium 35: 47–58, 2004. doi: 10.1016/S0143-4160(03)00171-4. [DOI] [PubMed] [Google Scholar]
- 119.Becquet D, Girardet C, Guillaumond F, François-Bellan AM, Bosler O. Ultrastructural plasticity in the rat suprachiasmatic nucleus. Possible involvement in clock entrainment. Glia 56: 294–305, 2008. doi: 10.1002/glia.20613. [DOI] [PubMed] [Google Scholar]
- 120.Bedini C, Lanfranchi A. The central and peripheral nervous system of Acoela (plathelminthes). An electron microscopical study. Acta Zoologica (Stockholm) 72: 101–106, 1991. doi: 10.1111/j.1463-6395.1991.tb00322.x. [DOI] [Google Scholar]
- 121.Beenhakker MP, Huguenard JR. Astrocytes as gatekeepers of GABAB receptor function. J Neurosci 30: 15262–15276, 2010. doi: 10.1523/JNEUROSCI.3243-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beggiato S, Antonelli T, Tomasini MC, Tanganelli S, Fuxe K, Schwarcz R, Ferraro L. Kynurenic acid, by targeting α7 nicotinic acetylcholine receptors, modulates extracellular GABA levels in the rat striatum in vivo. Eur J Neurosci 37: 1470–1477, 2013. doi: 10.1111/ejn.12160. [DOI] [PubMed] [Google Scholar]
- 123.Bekar LK, He W, Nedergaard M. Locus coeruleus α-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex 18: 2789–2795, 2008. doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bekar LK, Loewen ME, Cao K, Sun X, Leis J, Wang R, Forsyth GW, Walz W. Complex expression and localization of inactivating Kv channels in cultured hippocampal astrocytes. J Neurophysiol 93: 1699–1709, 2005. doi: 10.1152/jn.00850.2004. [DOI] [PubMed] [Google Scholar]
- 125.Bekar LK, Walz W. Evidence for chloride ions as intracellular messenger substances in astrocytes. J Neurophysiol 82: 248–254, 1999. [DOI] [PubMed] [Google Scholar]
- 126.Bekar LK, Walz W. Intracellular chloride modulates A-type potassium currents in astrocytes. Glia 39: 207–216, 2002. doi: 10.1002/glia.10096. [DOI] [PubMed] [Google Scholar]
- 127.Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14: 724–738, 2011. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 128.Belcheva MM, Clark AL, Haas PD, Serna JS, Hahn JW, Kiss A, Coscia CJ. Mu and kappa opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes. J Biol Chem 280: 27662–27669, 2005. doi: 10.1074/jbc.M502593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Belenky MA, Pickard GE. Subcellular distribution of 5-HT(1B) and 5-HT(7) receptors in the mouse suprachiasmatic nucleus. J Comp Neurol 432: 371–388, 2001. doi: 10.1002/cne.1109. [DOI] [PubMed] [Google Scholar]
- 130.Bellesi M, de Vivo L, Tononi G, Cirelli C. Effects of sleep and wake on astrocytes: clues from molecular and ultrastructural studies. BMC Biol 13: 66, 2015. doi: 10.1186/s12915-015-0176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bellesi M, Pfister-Genskow M, Maret S, Keles S, Tononi G, Cirelli C. Effects of sleep and wake on oligodendrocytes and their precursors. J Neurosci 33: 14288–14300, 2013. doi: 10.1523/JNEUROSCI.5102-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bellini-Leite S, Pereira AJ. Is global workspace a cartesian theater? How the neuro-astroglial interaction model solves conceptual issues. J Cogn Sci 14: 335–360, 2013. doi: 10.17791/jcs.2013.14.4.335. [DOI] [Google Scholar]
- 133.Benagiano V, Virgintino D, Rizzi A, Flace P, Troccoli V, Bormann J, Monaghan P, Robertson D, Roncali L, Ambrosi G. Glutamic acid decarboxylase-positive neuronal cell bodies and terminals in the human cerebellar cortex. Histochem J 32: 557–564, 2000. doi: 10.1023/A:1004106428844. [DOI] [PubMed] [Google Scholar]
- 134.Benedikt J, Inyushin M, Kucheryavykh YV, Rivera Y, Kucheryavykh LY, Nichols CG, Eaton MJ, Skatchkov SN. Intracellular polyamines enhance astrocytic coupling. Neuroreport 23: 1021–1025, 2012. doi: 10.1097/WNR.0b013e32835aa04b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Benfenati V, Amiry-Moghaddam M, Caprini M, Mylonakou MN, Rapisarda C, Ottersen OP, Ferroni S. Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience 148: 876–892, 2007. doi: 10.1016/j.neuroscience.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 136.Benfenati V, Caprini M, Dovizio M, Mylonakou MN, Ferroni S, Ottersen OP, Amiry-Moghaddam M. An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc Natl Acad Sci USA 108: 2563–2568, 2011. doi: 10.1073/pnas.1012867108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Benfenati V, Ferroni S. Water transport between CNS compartments: functional and molecular interactions between aquaporins and ion channels. Neuroscience 168: 926–940, 2010. doi: 10.1016/j.neuroscience.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 138.Benfenati V, Nicchia GP, Svelto M, Rapisarda C, Frigeri A, Ferroni S. Functional down-regulation of volume-regulated anion channels in AQP4 knockdown cultured rat cortical astrocytes. J Neurochem 100: 87–104, 2007. doi: 10.1111/j.1471-4159.2006.04164.x. [DOI] [PubMed] [Google Scholar]
- 139.Benjamin AM. Influence of Na+, K+, and Ca2+ on glutamine synthesis and distribution in rat brain cortex slices: a possible linkage of glutamine synthetase with cerebral transport processes and energetics in the astrocytes. J Neurochem 48: 1157–1164, 1987. doi: 10.1111/j.1471-4159.1987.tb05641.x. [DOI] [PubMed] [Google Scholar]
- 140.Bennay M, Langer J, Meier SD, Kafitz KW, Rose CR. Sodium signals in cerebellar Purkinje neurons and Bergmann glial cells evoked by glutamatergic synaptic transmission. Glia 56: 1138–1149, 2008. doi: 10.1002/glia.20685. [DOI] [PubMed] [Google Scholar]
- 141.Bennett GC, Ford AP, Smith JA, Emmett CJ, Webb TE, Boarder MR. P2Y receptor regulation of cultured rat cerebral cortical cells: calcium responses and mRNA expression in neurons and glia. Br J Pharmacol 139: 279–288, 2003. doi: 10.1038/sj.bjp.0705242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bennett MR, Buljan V, Farnell L, Gibson WG. Purinergic junctional transmission and propagation of calcium waves in spinal cord astrocyte networks. Biophys J 91: 3560–3571, 2006. doi: 10.1529/biophysj.106.082073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bennett MV, Contreras JE, Bukauskas FF, Sáez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci 26: 610–617, 2003. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Benneyworth MA, Li Y, Basu AC, Bolshakov VY, Coyle JT. Cell selective conditional null mutations of serine racemase demonstrate a predominate localization in cortical glutamatergic neurons. Cell Mol Neurobiol 32: 613–624, 2012. doi: 10.1007/s10571-012-9808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bentivoglio M, Mazzarello P. The history of radial glia. Brain Res Bull 49: 305–315, 1999. doi: 10.1016/S0361-9230(99)00065-9. [DOI] [PubMed] [Google Scholar]
- 146.Beppu K, Sasaki T, Tanaka KF, Yamanaka A, Fukazawa Y, Shigemoto R, Matsui K. Optogenetic countering of glial acidosis suppresses glial glutamate release and ischemic brain damage. Neuron 81: 314–320, 2014. doi: 10.1016/j.neuron.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 147.Bergami M, Santi S, Formaggio E, Cagnoli C, Verderio C, Blum R, Berninger B, Matteoli M, Canossa M. Uptake and recycling of pro-BDNF for transmitter-induced secretion by cortical astrocytes. J Cell Biol 183: 213–221, 2008. doi: 10.1083/jcb.200806137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Berger UV, Hediger MA. Distribution of the glutamate transporters GLAST and GLT-1 in rat circumventricular organs, meninges, and dorsal root ganglia. J Comp Neurol 421: 385–399, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 149.Berger UV, Hediger MA. The vitamin C transporter SVCT2 is expressed by astrocytes in culture but not in situ. Neuroreport 11: 1395–1399, 2000. doi: 10.1097/00001756-200005150-00009. [DOI] [PubMed] [Google Scholar]
- 150.Berger UV, Lu XC, Liu W, Tang Z, Slusher BS, Hediger MA. Effect of middle cerebral artery occlusion on mRNA expression for the sodium-coupled vitamin C transporter SVCT2 in rat brain. J Neurochem 86: 896–906, 2003. doi: 10.1046/j.1471-4159.2003.01891.x. [DOI] [PubMed] [Google Scholar]
- 151.Bergersen L, Rafiki A, Ottersen OP. Immunogold cytochemistry identifies specialized membrane domains for monocarboxylate transport in the central nervous system. Neurochem Res 27: 89–96, 2002. doi: 10.1023/A:1014806723147. [DOI] [PubMed] [Google Scholar]
- 152.Bergersen LH. Lactate transport and signaling in the brain: potential therapeutic targets and roles in body-brain interaction. J Cereb Blood Flow Metab 35: 176–185, 2015. doi: 10.1038/jcbfm.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bergersen LH, Morland C, Ormel L, Rinholm JE, Larsson M, Wold JF, Røe AT, Stranna A, Santello M, Bouvier D, Ottersen OP, Volterra A, Gundersen V. Immunogold detection of L-glutamate and D-serine in small synaptic-like microvesicles in adult hippocampal astrocytes. Cereb Cortex 22: 1690–1697, 2012. doi: 10.1093/cercor/bhr254. [DOI] [PubMed] [Google Scholar]
- 154.Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron 19: 1297–1308, 1997. doi: 10.1016/S0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 155.Bergmann K. Notiz über einige Strukturverhältnisse des Cerebellums und Rükenmarks. Z Med 8: 360–363, 1857. [Google Scholar]
- 156.Bernardinelli Y, Azarias G, Chatton JY. In situ fluorescence imaging of glutamate-evoked mitochondrial Na+ responses in astrocytes. Glia 54: 460–470, 2006. doi: 10.1002/glia.20387. [DOI] [PubMed] [Google Scholar]
- 157.Bernardinelli Y, Muller D, Nikonenko I. Astrocyte-synapse structural plasticity. Neural Plast 2014: 232105, 2014. doi: 10.1155/2014/232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bernardinelli Y, Salmon C, Jones EV, Farmer WT, Stellwagen D, Murai KK. Astrocytes display complex and localized calcium responses to single-neuron stimulation in the hippocampus. J Neurosci 31: 8905–8919, 2011. doi: 10.1523/JNEUROSCI.6341-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bernstein M, Behnisch T, Balschun D, Reymann KG, Reiser G. Pharmacological characterisation of metabotropic glutamatergic and purinergic receptors linked to Ca2+ signalling in hippocampal astrocytes. Neuropharmacology 37: 169–178, 1998. doi: 10.1016/S0028-3908(98)00012-4. [DOI] [PubMed] [Google Scholar]
- 160.Berridge MJ. Unlocking the secrets of cell signaling. Annu Rev Physiol 67: 1–21, 2005. doi: 10.1146/annurev.physiol.67.040103.152647. [DOI] [PubMed] [Google Scholar]
- 161.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21, 2000. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 162.Bery A, Cardona A, Martinez P, Hartenstein V. Structure of the central nervous system of a juvenile acoel, Symsagittifera roscoffensis. Dev Genes Evol 220: 61–76, 2010. doi: 10.1007/s00427-010-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Beskina O, Miller A, Mazzocco-Spezzia A, Pulina MV, Golovina VA. Mechanisms of interleukin-1β-induced Ca2+ signals in mouse cortical astrocytes: roles of store- and receptor-operated Ca2+ entry. Am J Physiol Cell Physiol 293: C1103–C1111, 2007. doi: 10.1152/ajpcell.00249.2007. [DOI] [PubMed] [Google Scholar]
- 164.Besser L. Zur Histiogenese der nervösen Elementarteile in den Centralorganen der neugeborenen Menschen. Arch Pathol Anat Physiol Klin Med 36: 305–333, 1866. doi: 10.1007/BF02042748. [DOI] [Google Scholar]
- 165.Betz H, Gomeza J, Armsen W, Scholze P, Eulenburg V. Glycine transporters: essential regulators of synaptic transmission. Biochem Soc Trans 34: 55–58, 2006. doi: 10.1042/BST0340055. [DOI] [PubMed] [Google Scholar]
- 166.Bevan S, Chiu SY, Gray PT, Ritchie JM. The presence of voltage-gated sodium, potassium and chloride channels in rat cultured astrocytes. Proc R Soc Lond B Biol Sci 225: 299–313, 1985. doi: 10.1098/rspb.1985.0063. [DOI] [PubMed] [Google Scholar]
- 167.Bevensee MO, Weed RA, Boron WF. Intracellular pH regulation in cultured astrocytes from rat hippocampus. I. Role Of HCO3−. J Gen Physiol 110: 453–465, 1997. doi: 10.1085/jgp.110.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391: 281–285, 1998. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 169.Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhäuser C, Pilati E, Volterra A. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci 7: 613–620, 2004. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- 170.Bhattacharjee A, Kaczmarek LK. For K+ channels, Na+ is the new Ca2+. Trends Neurosci 28: 422–428, 2005. doi: 10.1016/j.tins.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 171.Bianchi R, Giambanco I, Donato R. S100B/RAGE-dependent activation of microglia via NF-kappaB and AP-1 Co-regulation of COX-2 expression by S100B, IL-1β and TNF-α. Neurobiol Aging 31: 665–677, 2010. doi: 10.1016/j.neurobiolaging.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 172.Bianchi R, Verzini M, Garbuglia M, Giambanco I, Donato R. Mechanism of S100 protein-dependent inhibition of glial fibrillary acidic protein (GFAP) polymerization. Biochim Biophys Acta 1223: 354–360, 1994. doi: 10.1016/0167-4889(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 173.Bianco F, Colombo A, Saglietti L, Lecca D, Abbracchio MP, Matteoli M, Verderio C. Different properties of P2X(7) receptor in hippocampal and cortical astrocytes. Purinergic Signal 5: 233–240, 2009. doi: 10.1007/s11302-009-9137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, Clementi E, Matteoli M, Verderio C. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J 28: 1043–1054, 2009. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bianco F, Pravettoni E, Colombo A, Schenk U, Möller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1β release from microglia. J Immunol 174: 7268–7277, 2005. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 176.Biber K, Fiebich BL, Gebicke-Härter P, van Calker D. Carbamazepine-induced upregulation of adenosine A1-receptors in astrocyte cultures affects coupling to the phosphoinositol signaling pathway. Neuropsychopharmacology 20: 271–278, 1999. doi: 10.1016/S0893-133X(98)00059-1. [DOI] [PubMed] [Google Scholar]
- 177.Biber K, Klotz KN, Berger M, Gebicke-Härter PJ, van Calker D. Adenosine A1 receptor-mediated activation of phospholipase C in cultured astrocytes depends on the level of receptor expression. J Neurosci 17: 4956–4964, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Biber K, Lubrich B, Fiebich BL, Boddeke HW, van Calker D. Interleukin-6 enhances expression of adenosine A(1) receptor mRNA and signaling in cultured rat cortical astrocytes and brain slices. Neuropsychopharmacology 24: 86–96, 2001. doi: 10.1016/S0893-133X(00)00169-X. [DOI] [PubMed] [Google Scholar]
- 179.Bignami A, Dahl D. Brain-specific hyaluronate-binding protein. A product of white matter astrocytes? J Neurocytol 15: 671–679, 1986. doi: 10.1007/BF01611865. [DOI] [PubMed] [Google Scholar]
- 180.Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res 43: 429–435, 1972. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- 181.Binder DK, Yao X, Zador Z, Sick TJ, Verkman AS, Manley GT. Increased seizure duration and slowed potassium kinetics in mice lacking aquaporin-4 water channels. Glia 53: 631–636, 2006. doi: 10.1002/glia.20318. [DOI] [PubMed] [Google Scholar]
- 182.Bitoun M, Tappaz M. Gene expression of the transporters and biosynthetic enzymes of the osmolytes in astrocyte primary cultures exposed to hyperosmotic conditions. Glia 32: 165–176, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 183.Bittner CX, Loaiza A, Ruminot I, Larenas V, Sotelo-Hitschfeld T, Gutiérrez R, Córdova A, Valdebenito R, Frommer WB, Barros LF. High resolution measurement of the glycolytic rate. Front Neuroenergetics 2: 26, 2010. doi: 10.3389/fnene.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Björklund O, Shang M, Tonazzini I, Daré E, Fredholm BB. Adenosine A1 and A3 receptors protect astrocytes from hypoxic damage. Eur J Pharmacol 596: 6–13, 2008. doi: 10.1016/j.ejphar.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 185.Black JA, Dib-Hajj S, Cohen S, Hinson AW, Waxman SG. Glial cells have heart: rH1 Na+ channel mRNA and protein in spinal cord astrocytes. Glia 23: 200–208, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 186.Black JA, Newcombe J, Waxman SG. Astrocytes within multiple sclerosis lesions upregulate sodium channel Nav1.5. Brain 133: 835–846, 2010. doi: 10.1093/brain/awq003. [DOI] [PubMed] [Google Scholar]
- 187.Black JA, Sontheimer H, Minturn JE, Ransom BR, Waxman SG. The expression of sodium channels in astrocytes in situ and in vitro. Prog Brain Res 94: 89–107, 1992. doi: 10.1016/S0079-6123(08)61742-2. [DOI] [PubMed] [Google Scholar]
- 188.Black JA, Waxman SG. Noncanonical roles of voltage-gated sodium channels. Neuron 80: 280–291, 2013. doi: 10.1016/j.neuron.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 189.Black JA, Westenbroek R, Minturn JE, Ransom BR, Catterall WA, Waxman SG. Isoform-specific expression of sodium channels in astrocytes in vitro: immunocytochemical observations. Glia 14: 133–144, 1995. doi: 10.1002/glia.440140208. [DOI] [PubMed] [Google Scholar]
- 190.Blakely RD, Edwards RH. Vesicular and plasma membrane transporters for neurotransmitters. Cold Spring Harb Perspect Biol 4: a005595, 2012. doi: 10.1101/cshperspect.a005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol Renal Fluid Electrolyte Physiol 275: F633–F650, 1998. [DOI] [PubMed] [Google Scholar]
- 192.Blanz J, Schweizer M, Auberson M, Maier H, Muenscher A, Hübner CA, Jentsch TJ. Leukoencephalopathy upon disruption of the chloride channel ClC-2. J Neurosci 27: 6581–6589, 2007. doi: 10.1523/JNEUROSCI.0338-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Blaustein MP, Goldman WF, Fontana G, Krueger BK, Santiago EM, Steele TD, Weiss DN, Yarowsky PJ. Physiological roles of the sodium-calcium exchanger in nerve and muscle. Ann N Y Acad Sci 639: 254–274, 1991. doi: 10.1111/j.1749-6632.1991.tb17315.x. [DOI] [PubMed] [Google Scholar]
- 194.Blaustein MP, Juhaszova M, Golovina VA, Church PJ, Stanley EF. Na/Ca exchanger and PMCA localization in neurons and astrocytes: functional implications. Ann N Y Acad Sci 976: 356–366, 2002. doi: 10.1111/j.1749-6632.2002.tb04762.x. [DOI] [PubMed] [Google Scholar]
- 195.Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev 79: 763–854, 1999. [DOI] [PubMed] [Google Scholar]
- 196.Blázquez C, Sánchez C, Daza A, Galve-Roperh I, Guzmán M. The stimulation of ketogenesis by cannabinoids in cultured astrocytes defines carnitine palmitoyltransferase I as a new ceramide-activated enzyme. J Neurochem 72: 1759–1768, 1999. doi: 10.1046/j.1471-4159.1999.721759.x. [DOI] [PubMed] [Google Scholar]
- 197.Blinkow S, Glezer I. The neuroglia, in The Human Brain in Figures and Tables; A quantitative handbook, edited by Blinkow SM, Gleser II. New York: Plenum, 1968, p. 237–253. [Google Scholar]
- 198.Blomqvist A, Broman J. Light and electron microscopic immunohistochemical demonstration of GABA-immunoreactive astrocytes in the brain stem of the rat. J Neurocytol 17: 629–637, 1988. doi: 10.1007/BF01260990. [DOI] [PubMed] [Google Scholar]
- 199.Blomstrand F, Giaume C. Kinetics of endothelin-induced inhibition and glucose permeability of astrocyte gap junctions. J Neurosci Res 83: 996–1003, 2006. doi: 10.1002/jnr.20801. [DOI] [PubMed] [Google Scholar]
- 200.Blomstrand F, Giaume C, Hansson E, Rönnbäck L. Distinct pharmacological properties of ET-1 and ET-3 on astroglial gap junctions and Ca2+ signaling. Am J Physiol Cell Physiol 277: C616–C627, 1999. [DOI] [PubMed] [Google Scholar]
- 201.Blomstrand F, Venance L, Sirén AL, Ezan P, Hanse E, Glowinski J, Ehrenreich H, Giaume C. Endothelins regulate astrocyte gap junctions in rat hippocampal slices. Eur J Neurosci 19: 1005–1015, 2004. doi: 10.1111/j.0953-816X.2004.03197.x. [DOI] [PubMed] [Google Scholar]
- 202.Blumenstein Y, Maximyuk OP, Lozovaya N, Yatsenko NM, Kanevsky N, Krishtal O, Dascal N. Intracellular Na+ inhibits voltage-dependent N-type Ca2+ channels by a G protein betagamma subunit-dependent mechanism. J Physiol 556: 121–134, 2004. doi: 10.1113/jphysiol.2003.056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Blutstein T, Haydon PG. The importance of astrocyte-derived purines in the modulation of sleep. Glia 61: 129–139, 2013. doi: 10.1002/glia.22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Boison D. Adenosine as a neuromodulator in neurological diseases. Curr Opin Pharmacol 8: 2–7, 2008. doi: 10.1016/j.coph.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Boison D, Chen JF, Fredholm BB. Adenosine signaling and function in glial cells. Cell Death Differ 17: 1071–1082, 2010. doi: 10.1038/cdd.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bolborea M, Dale N. Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci 36: 91–100, 2013. doi: 10.1016/j.tins.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bolborea M, Dale N. Tanycytes excite neurons of the hypothalamic arcuate nucleus in mice. Proc Physiol Soc 34: SA049, 2015. [Google Scholar]
- 208.Bolton S, Greenwood K, Hamilton N, Butt AM. Regulation of the astrocyte resting membrane potential by cyclic AMP and protein kinase A. Glia 54: 316–328, 2006. doi: 10.1002/glia.20384. [DOI] [PubMed] [Google Scholar]
- 209.Bondarenko A, Svichar N, Chesler M. Role of Na+-H+ and Na+-Ca2+ exchange in hypoxia-related acute astrocyte death. Glia 49: 143–152, 2005. doi: 10.1002/glia.20107. [DOI] [PubMed] [Google Scholar]
- 210.Bonder DE, McCarthy KD. Astrocytic Gq-GPCR-linked IP3R-dependent Ca2+ signaling does not mediate neurovascular coupling in mouse visual cortex in vivo. J Neurosci 34: 13139–13150, 2014. doi: 10.1523/JNEUROSCI.2591-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bonfanti L, Poulain DA, Theodosis DT. Radial glia-like cells in the supraoptic nucleus of the adult rat. J Neuroendocrinol 5: 1–5, 1993. doi: 10.1111/j.1365-2826.1993.tb00357.x. [DOI] [PubMed] [Google Scholar]
- 212.Booth GE, Kinrade EF, Hidalgo A. Glia maintain follower neuron survival during Drosophila CNS development. Development 127: 237–244, 2000. [DOI] [PubMed] [Google Scholar]
- 213.Borán MS, García A. The cyclic GMP-protein kinase G pathway regulates cytoskeleton dynamics and motility in astrocytes. J Neurochem 102: 216–230, 2007. doi: 10.1111/j.1471-4159.2007.04464.x. [DOI] [PubMed] [Google Scholar]
- 214.Bordey A, Sontheimer H. Differential inhibition of glial K(+) currents by 4-AP. J Neurophysiol 82: 3476–3487, 1999. [DOI] [PubMed] [Google Scholar]
- 215.Bordey A, Sontheimer H. Ion channel expression by astrocytes in situ: comparison of different CNS regions. Glia 30: 27–38, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 216.Borycz J, Borycz JA, Loubani M, Meinertzhagen IA. Tan and ebony genes regulate a novel pathway for transmitter metabolism at fly photoreceptor terminals. J Neurosci 22: 10549–10557, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bosco D, Haefliger JA, Meda P. Connexins: key mediators of endocrine function. Physiol Rev 91: 1393–1445, 2011. doi: 10.1152/physrev.00027.2010. [DOI] [PubMed] [Google Scholar]
- 218.Boumezbeur F, Mason GF, de Graaf RA, Behar KL, Cline GW, Shulman GI, Rothman DL, Petersen KF. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J Cereb Blood Flow Metab 30: 211–221, 2010. doi: 10.1038/jcbfm.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Boumezbeur F, Petersen KF, Cline GW, Mason GF, Behar KL, Shulman GI, Rothman DL. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J Neurosci 30: 13983–13991, 2010. doi: 10.1523/JNEUROSCI.2040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bowman CL, Kimelberg HK. Excitatory amino acids directly depolarize rat brain astrocytes in primary culture. Nature 311: 656–659, 1984. doi: 10.1038/311656a0. [DOI] [PubMed] [Google Scholar]
- 221.Bowser DN, Khakh BS. ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J Neurosci 24: 8606–8620, 2004. doi: 10.1523/JNEUROSCI.2660-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bowser DN, Khakh BS. Two forms of single-vesicle astrocyte exocytosis imaged with total internal reflection fluorescence microscopy. Proc Natl Acad Sci USA 104: 4212–4217, 2007. doi: 10.1073/pnas.0607625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bowser DN, Khakh BS. Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J Gen Physiol 129: 485–491, 2007. doi: 10.1085/jgp.200709780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bozzo L, Puyal J, Chatton JY. Lactate modulates the activity of primary cortical neurons through a receptor-mediated pathway. PLoS One 8: e71721, 2013. doi: 10.1371/journal.pone.0071721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Brackenbury WJ, Isom LL. Na channel β subunits: Overachievers of the ion channel family. Front Pharmacol 2: 53, 2011. doi: 10.3389/fphar.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Brambilla R, Neary JT, Cattabeni F, Cottini L, D’Ippolito G, Schiller PC, Abbracchio MP. Induction of COX-2 and reactive gliosis by P2Y receptors in rat cortical astrocytes is dependent on ERK1/2 but independent of calcium signalling. J Neurochem 83: 1285–1296, 2002. doi: 10.1046/j.1471-4159.2002.01239.x. [DOI] [PubMed] [Google Scholar]
- 227.Bramham CR, Torp R, Zhang N, Storm-Mathisen J, Ottersen OP. Distribution of glutamate-like immunoreactivity in excitatory hippocampal pathways: a semiquantitative electron microscopic study in rats. Neuroscience 39: 405–417, 1990. doi: 10.1016/0306-4522(90)90277-B. [DOI] [PubMed] [Google Scholar]
- 228.Brand-Schieber E, Lowery SL, Werner P. Select ionotropic glutamate AMPA/kainate receptors are expressed at the astrocyte-vessel interface. Brain Res 1007: 178–182, 2004. doi: 10.1016/j.brainres.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 229.Brasko C, Hawkins V, De La Rocha IC, Butt AM. Expression of Kir4.1 and Kir5.1 inwardly rectifying potassium channels in oligodendrocytes, the myelinating cells of the CNS. Brain Struct Funct 222: 41–59, 2017. doi: 10.1007/s00429-016-1199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Brasnjo G, Otis TS. Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron 31: 607–616, 2001. doi: 10.1016/S0896-6273(01)00377-4. [DOI] [PubMed] [Google Scholar]
- 231.Breunig JJ, Gate D, Levy R, Rodriguez J Jr, Kim GB, Danielpour M, Svendsen CN, Town T. Rapid genetic targeting of pial surface neural progenitors and immature neurons by neonatal electroporation. Neural Dev 7: 26, 2012. doi: 10.1186/1749-8104-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bridges RJ, Natale NR, Patel SA. System xc− cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol 165: 20–34, 2012. doi: 10.1111/j.1476-5381.2011.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bringmann A, Pannicke T, Weick M, Biedermann B, Uhlmann S, Kohen L, Wiedemann P, Reichenbach A. Activation of P2Y receptors stimulates potassium and cation currents in acutely isolated human Müller (glial) cells. Glia 37: 139–152, 2002. doi: 10.1002/glia.10025. [DOI] [PubMed] [Google Scholar]
- 234.Brini M, Carafoli E. The plasma membrane Ca2+ ATPase and the plasma membrane sodium calcium exchanger cooperate in the regulation of cell calcium. Cold Spring Harb Perspect Biol 3: a004168, 2011. doi: 10.1101/cshperspect.a004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Brito V, Beyer C, Küppers E. BDNF-dependent stimulation of dopamine D5 receptor expression in developing striatal astrocytes involves PI3-kinase signaling. Glia 46: 284–295, 2004. doi: 10.1002/glia.10356. [DOI] [PubMed] [Google Scholar]
- 236.Brizzee KR, Jacobs LA. The glia/neuron index in the submolecular layers of the motor cortex in the cat. Anat Rec 134: 97–105, 1959. doi: 10.1002/ar.1091340110. [DOI] [PubMed] [Google Scholar]
- 237.Brockhaus J, Deitmer JW. Developmental downregulation of ATP-sensitive potassium conductance in astrocytes in situ. Glia 32: 205–213, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 238.Brockhaus J, Deitmer JW. Long-lasting modulation of synaptic input to Purkinje neurons by Bergmann glia stimulation in rat brain slices. J Physiol 545: 581–593, 2002. doi: 10.1113/jphysiol.2002.028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Brodie C. Functional PAF receptors in glia cells: binding parameters and regulation of expression. Int J Dev Neurosci 12: 631–640, 1994. doi: 10.1016/0736-5748(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 240.Brodie C. Platelet activating factor induces nerve growth factor production by rat astrocytes. Neurosci Lett 186: 5–8, 1995. doi: 10.1016/0304-3940(95)11267-Z. [DOI] [PubMed] [Google Scholar]
- 241.Bröer S. The SLC38 family of sodium-amino acid co-transporters. Pflugers Arch 466: 155–172, 2014. doi: 10.1007/s00424-013-1393-y. [DOI] [PubMed] [Google Scholar]
- 242.Bröer S, Brookes N. Transfer of glutamine between astrocytes and neurons. J Neurochem 77: 705–719, 2001. doi: 10.1046/j.1471-4159.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- 243.Bröer S, Wagner CA. Structure-function relationships of heterodimeric amino acid transporters. Cell Biochem Biophys 36: 155–168, 2002. doi: 10.1385/CBB:36:2-3:155. [DOI] [PubMed] [Google Scholar]
- 244.Brookes N, Turner RJ. K+-induced alkalinization in mouse cerebral astrocytes mediated by reversal of electrogenic Na+-HCO3− cotransport. Am J Physiol Cell Physiol 267: C1633–C1640, 1994. [DOI] [PubMed] [Google Scholar]
- 245.Brown AM. Brain glycogen re-awakened. J Neurochem 89: 537–552, 2004. doi: 10.1111/j.1471-4159.2004.02421.x. [DOI] [PubMed] [Google Scholar]
- 246.Brown AM, Sickmann HM, Fosgerau K, Lund TM, Schousboe A, Waagepetersen HS, Ransom BR. Astrocyte glycogen metabolism is required for neural activity during aglycemia or intense stimulation in mouse white matter. J Neurosci Res 79: 74–80, 2005. doi: 10.1002/jnr.20335. [DOI] [PubMed] [Google Scholar]
- 247.Brune T, Deitmer JW. Intracellular acidification and Ca2+ transients in cultured rat cerebellar astrocytes evoked by glutamate agonists and noradrenaline. Glia 14: 153–161, 1995. doi: 10.1002/glia.440140210. [DOI] [PubMed] [Google Scholar]
- 248.Bruner G, Murphy S. UTP activates multiple second messenger systems in cultured rat astrocytes. Neurosci Lett 162: 105–108, 1993. doi: 10.1016/0304-3940(93)90571-2. [DOI] [PubMed] [Google Scholar]
- 249.Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J 15: 10–12, 2001. [DOI] [PubMed] [Google Scholar]
- 250.Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia 53: 688–695, 2006. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- 251.Buchanan RL, Benzer S. Defective glia in the Drosophila brain degeneration mutant drop-dead. Neuron 10: 839–850, 1993. doi: 10.1016/0896-6273(93)90200-B. [DOI] [PubMed] [Google Scholar]
- 252.Buffo A, Rossi F. Origin, lineage and function of cerebellar glia. Prog Neurobiol 109: 42–63, 2013. doi: 10.1016/j.pneurobio.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 253.Bugiani M, Al Shahwan S, Lamantea E, Bizzi A, Bakhsh E, Moroni I, Balestrini MR, Uziel G, Zeviani M. GJA12 mutations in children with recessive hypomyelinating leukoencephalopathy. Neurology 67: 273–279, 2006. doi: 10.1212/01.wnl.0000223832.66286.e4. [DOI] [PubMed] [Google Scholar]
- 254.Bundgaard M, Abbott NJ. All vertebrates started out with a glial blood-brain barrier 4-500 million years ago. Glia 56: 699–708, 2008. doi: 10.1002/glia.20642. [DOI] [PubMed] [Google Scholar]
- 255.Bundgaard M, Cserr H. A glial blood-brain barrier in elasmobranchs. Brain Res 226: 61–73, 1981. doi: 10.1016/0006-8993(81)91083-0. [DOI] [PubMed] [Google Scholar]
- 256.Bunn SJ, Hanley MR, Wilkin GP. Evidence for a κ-opioid receptor on pituitary astrocytes: an autoradiographic study. Neurosci Lett 55: 317–323, 1985. doi: 10.1016/0304-3940(85)90455-0. [DOI] [PubMed] [Google Scholar]
- 257.Burda JE, Bernstein AM, Sofroniew MV. Astrocyte roles in traumatic brain injury. Exp Neurol 275: 305–315, 2016. doi: 10.1016/j.expneurol.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81: 229–248, 2014. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O’Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron 50: 711–722, 2006. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 260.Burdakov D, Petersen OH, Verkhratsky A. Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium 38: 303–310, 2005. doi: 10.1016/j.ceca.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 261.Burkeen JF, Womac AD, Earnest DJ, Zoran MJ. Mitochondrial calcium signaling mediates rhythmic extracellular ATP accumulation in suprachiasmatic nucleus astrocytes. J Neurosci 31: 8432–8440, 2011. doi: 10.1523/JNEUROSCI.6576-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Burnstock G, Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol 16: 433–440, 1985. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- 263.Burnstock G, Verkhratsky A. Purinergic Signalling and the Nervous System. Heidelberg: Springer, 2012. doi: 10.1007/978-3-642-28863-0. [DOI] [Google Scholar]
- 264.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22: 183–192, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Butenko O, Dzamba D, Benesova J, Honsa P, Benfenati V, Rusnakova V, Ferroni S, Anderova M. The increased activity of TRPV4 channel in the astrocytes of the adult rat hippocampus after cerebral hypoxia/ischemia. PLoS One 7: e39959, 2012. doi: 10.1371/journal.pone.0039959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Butler AB, Hodos W. Vertebrate Neuroanatomy: Evolution and Adaptation (2nd ed.). New York: Wiley, 2005. doi: 10.1002/0471733849. [DOI] [Google Scholar]
- 267.Butt AM. ATP: a ubiquitous gliotransmitter integrating neuron-glial networks. Semin Cell Dev Biol 22: 205–213, 2011. doi: 10.1016/j.semcdb.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 268.Butt AM, Colquhoun K, Tutton M, Berry M. Three-dimensional morphology of astrocytes and oligodendrocytes in the intact mouse optic nerve. J Neurocytol 23: 469–485, 1994. doi: 10.1007/BF01184071. [DOI] [PubMed] [Google Scholar]
- 269.Butt AM, Kalsi A. Inwardly rectifying potassium channels (Kir) in central nervous system glia: a special role for Kir4.1 in glial functions. J Cell Mol Med 10: 33–44, 2006. doi: 10.1111/j.1582-4934.2006.tb00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Butt AM, Ransom BR. Visualization of oligodendrocytes and astrocytes in the intact rat optic nerve by intracellular injection of lucifer yellow and horseradish peroxidase. Glia 2: 470–475, 1989. doi: 10.1002/glia.440020609. [DOI] [PubMed] [Google Scholar]
- 271.Butzke V. Studien über den feineren Bau der Grosshirnrinde. Arch Psychiatr Nervenkr 3: 575–601, 1871. doi: 10.1007/BF02166452. [DOI] [Google Scholar]
- 272.Buzas B. Regulation of nociceptin/orphanin FQ gene expression in astrocytes by ceramide. Neuroreport 13: 1707–1710, 2002. doi: 10.1097/00001756-200210070-00003. [DOI] [PubMed] [Google Scholar]
- 273.Cabral A, Valdivia S, Fernandez G, Reynaldo M, Perello M. Divergent neuronal circuitries underlying acute orexigenic effects of peripheral or central ghrelin: critical role of brain accessibility. J Neuroendocrinol 26: 542–554, 2014. doi: 10.1111/jne.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Caesar K, Hashemi P, Douhou A, Bonvento G, Boutelle MG, Walls AB, Lauritzen M. Glutamate receptor-dependent increments in lactate, glucose and oxygen metabolism evoked in rat cerebellum in vivo. J Physiol 586: 1337–1349, 2008. doi: 10.1113/jphysiol.2007.144154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28: 264–278, 2008. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Cai Z, Kimelberg HK. Glutamate receptor-mediated calcium responses in acutely isolated hippocampal astrocytes. Glia 21: 380–389, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 277.Cai Z, Schools GP, Kimelberg HK. Metabotropic glutamate receptors in acutely isolated hippocampal astrocytes: developmental changes of mGluR5 mRNA and functional expression. Glia 29: 70–80, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 278.Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, Lin P, Xiao R, Wang C, Zhu Y, Lin Y, Wyatt CN, Parrington J, Ma J, Evans AM, Galione A, Zhu MX. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459: 596–600, 2009. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Calegari F, Coco S, Taverna E, Bassetti M, Verderio C, Corradi N, Matteoli M, Rosa P. A regulated secretory pathway in cultured hippocampal astrocytes. J Biol Chem 274: 22539–22547, 1999. doi: 10.1074/jbc.274.32.22539. [DOI] [PubMed] [Google Scholar]
- 280.Camassa LM, Lunde LK, Hoddevik EH, Stensland M, Boldt HB, De Souza GA, Ottersen OP, Amiry-Moghaddam M. Mechanisms underlying AQP4 accumulation in astrocyte endfeet. Glia 63: 2073–2091, 2015. doi: 10.1002/glia.22878. [DOI] [PubMed] [Google Scholar]
- 281.Cammer W. Glutamine synthetase in the central nervous system is not confined to astrocytes. J Neuroimmunol 26: 173–178, 1990. doi: 10.1016/0165-5728(90)90088-5. [DOI] [PubMed] [Google Scholar]
- 282.Cantera R, Trujillo-Cenoz O. Glial cells in insect ganglia. Microsc Res Tech 35: 285–293, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 283.Capuani C, Melone M, Tottene A, Bragina L, Crivellaro G, Santello M, Casari G, Conti F, Pietrobon D. Defective glutamate and K+ clearance by cortical astrocytes in familial hemiplegic migraine type 2. EMBO Mol Med 8: 967–986, 2016. doi: 10.15252/emmm.201505944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Carafoli E. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci USA 99: 1115–1122, 2002. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Carafoli E, Krebs J. Why Calcium? How calcium became the best communicator. J Biol Chem 291: 20849–20857, 2016. doi: 10.1074/jbc.R116.735894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Cardoso JC, Vieira FA, Gomes AS, Power DM. PACAP, VIP and their receptors in the metazoa: insights about the origin and evolution of the ligand-receptor pair. Peptides 28: 1902–1919, 2007. doi: 10.1016/j.peptides.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 287.Carlson AJ. The Control of Hunger in Health and Disease. Chicago, IL: Univ. of Chicago Press, 1919. [Google Scholar]
- 288.Carlson SD, Saint Marie RL. Structure and function of insect glia. Annu Rev Entomol 35: 597–621, 1990. doi: 10.1146/annurev.en.35.010190.003121. [DOI] [Google Scholar]
- 289.Carmignoto G, Pasti L, Pozzan T. On the role of voltage-dependent calcium channels in calcium signaling of astrocytes in situ. J Neurosci 18: 4637–4645, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Carmona MA, Murai KK, Wang L, Roberts AJ, Pasquale EB. Glial ephrin-A3 regulates hippocampal dendritic spine morphology and glutamate transport. Proc Natl Acad Sci USA 106: 12524–12529, 2009. doi: 10.1073/pnas.0903328106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Carpenedo R, Pittaluga A, Cozzi A, Attucci S, Galli A, Raiteri M, Moroni F. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Eur J Neurosci 13: 2141–2147, 2001. doi: 10.1046/j.0953-816x.2001.01592.x. [DOI] [PubMed] [Google Scholar]
- 292.Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia 49: 360–374, 2005. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- 293.Carson MJ, Thomas EA, Danielson PE, Sutcliffe JG. The 5HT5A serotonin receptor is expressed predominantly by astrocytes in which it inhibits cAMP accumulation: a mechanism for neuronal suppression of reactive astrocytes. Glia 17: 317–326, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 294.Carswell HV, Dominiczak AF, Garcia-Segura LM, Harada N, Hutchison JB, Macrae IM. Brain aromatase expression after experimental stroke: topography and time course. J Steroid Biochem Mol Biol 96: 89–91, 2005. doi: 10.1016/j.jsbmb.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 295.Cataldo AM, Broadwell RD. Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. I. Neurons and glia. J Elec Micro Techn 3: 413–437, 1986. doi: 10.1002/jemt.1060030406. [DOI] [PubMed] [Google Scholar]
- 296.Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol 3: a003947, 2011. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Catterall WA. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol 590: 2577–2589, 2012. doi: 10.1113/jphysiol.2011.224204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Catus SL, Gibbs ME, Sato M, Summers RJ, Hutchinson DS. Role of β-adrenoceptors in glucose uptake in astrocytes using β-adrenoceptor knockout mice. Br J Pharmacol 162: 1700–1715, 2011. doi: 10.1111/j.1476-5381.2010.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Caudle RM. Memory in astrocytes: a hypothesis. Theor Biol Med Model 3: 2, 2006. doi: 10.1186/1742-4682-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Cauli B, Hamel E. Revisiting the role of neurons in neurovascular coupling. Front Neuroenergetics 2: 9, 2010. doi: 10.3389/fnene.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Cengiz P, Kintner DB, Chanana V, Yuan H, Akture E, Kendigelen P, Begum G, Fidan E, Uluc K, Ferrazzano P, Sun D. Sustained Na+/H+ exchanger activation promotes gliotransmitter release from reactive hippocampal astrocytes following oxygen-glucose deprivation. PLoS One 9: e84294, 2014. doi: 10.1371/journal.pone.0084294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ceruti S, Colombo L, Magni G, Viganò F, Boccazzi M, Deli MA, Sperlágh B, Abbracchio MP, Kittel A. Oxygen-glucose deprivation increases the enzymatic activity and the microvesicle-mediated release of ectonucleotidases in the cells composing the blood-brain barrier. Neurochem Int 59: 259–271, 2011. doi: 10.1016/j.neuint.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 303.Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology 145: 3788–3795, 2004. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- 304.Chaineau M, Danglot L, Galli T. Multiple roles of the vesicular-SNARE TI-VAMP in post-Golgi and endosomal trafficking. FEBS Lett 583: 3817–3826, 2009. doi: 10.1016/j.febslet.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 305.Chan-Palay V, Palay SL. The form of velate astrocytes in the cerebellar cortex of monkey and rat: high voltage electron microscopy of rapid Golgi preparations. Z Anat Entwicklungsgesch 138: 1–19, 1972. doi: 10.1007/BF00519921. [DOI] [PubMed] [Google Scholar]
- 306.Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron 6: 983–992, 1991. doi: 10.1016/0896-6273(91)90238-U. [DOI] [PubMed] [Google Scholar]
- 307.Charles KJ, Calver AR, Jourdain S, Pangalos MN. Distribution of a GABAB-like receptor protein in the rat central nervous system. Brain Res 989: 135–146, 2003. doi: 10.1016/S0006-8993(03)03163-9. [DOI] [PubMed] [Google Scholar]
- 308.Chatelain FC, Bichet D, Douguet D, Feliciangeli S, Bendahhou S, Reichold M, Warth R, Barhanin J, Lesage F. TWIK1, a unique background channel with variable ion selectivity. Proc Natl Acad Sci USA 109: 5499–5504, 2012. doi: 10.1073/pnas.1201132109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Chatton JY, Magistretti PJ, Barros LF. Sodium signaling and astrocyte energy metabolism. Glia 64: 1667–1676, 2016. doi: 10.1002/glia.22971. [DOI] [PubMed] [Google Scholar]
- 310.Chatton JY, Pellerin L, Magistretti PJ. GABA uptake into astrocytes is not associated with significant metabolic cost: implications for brain imaging of inhibitory transmission. Proc Natl Acad Sci USA 100: 12456–12461, 2003. doi: 10.1073/pnas.2132096100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Chaturvedi R, Reddig K, Li HS. Long-distance mechanism of neurotransmitter recycling mediated by glial network facilitates visual function in Drosophila. Proc Natl Acad Sci USA 111: 2812–2817, 2014. doi: 10.1073/pnas.1323714111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Chaudhry FA, Boulland JL, Jenstad M, Bredahl MK, Edwards RH. Pharmacology of neurotransmitter transport into secretory vesicles. Handb Exp Pharmacol 184: 77–106, 2008. doi: 10.1007/978-3-540-74805-2_4. [DOI] [PubMed] [Google Scholar]
- 313.Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron 15: 711–720, 1995. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 314.Cheli VT, Santiago González DA, Smith J, Spreuer V, Murphy GG, Paez PM. L-type voltage-operated calcium channels contribute to astrocyte activation in vitro. Glia 64: 1396–1415, 2016. doi: 10.1002/glia.23013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Chen C, Kuo J, Wong A, Micevych P. Estradiol modulates translocator protein (TSPO) and steroid acute regulatory protein (StAR) via protein kinase A (PKA) signaling in hypothalamic astrocytes. Endocrinology 155: 2976–2985, 2014. doi: 10.1210/en.2013-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Chen N, Sugihara H, Sharma J, Perea G, Petravicz J, Le C, Sur M. Nucleus basalis-enabled stimulus-specific plasticity in the visual cortex is mediated by astrocytes. Proc Natl Acad Sci USA 109: E2832–E2841, 2012. doi: 10.1073/pnas.1206557109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Chen Y, Rathbone MP, Hertz L. Guanosine-induced increase in free cytosolic calcium concentration in mouse astrocytes in primary cultures: does it act on an A3 adenosine receptor? J Neurosci Res 65: 184–189, 2001. doi: 10.1002/jnr.1141. [DOI] [PubMed] [Google Scholar]
- 318.Chen Y, Swanson RA. The glutamate transporters EAAT2 and EAAT3 mediate cysteine uptake in cortical neuron cultures. J Neurochem 84: 1332–1339, 2003. doi: 10.1046/j.1471-4159.2003.01630.x. [DOI] [PubMed] [Google Scholar]
- 319.Chen Y, Willcockson HH, Valtschanoff JG. Influence of the vanilloid receptor TRPV1 on the activation of spinal cord glia in mouse models of pain. Exp Neurol 220: 383–390, 2009. doi: 10.1016/j.expneurol.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Chesik D, De Keyser J. Progesterone and dexamethasone differentially regulate the IGF-system in glial cells. Neurosci Lett 468: 178–182, 2010. doi: 10.1016/j.neulet.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 321.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev 83: 1183–1221, 2003. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 322.Cheunsuang O, Morris R. Astrocytes in the arcuate nucleus and median eminence that take up a fluorescent dye from the circulation express leptin receptors and neuropeptide Y Y1 receptors. Glia 52: 228–233, 2005. doi: 10.1002/glia.20239. [DOI] [PubMed] [Google Scholar]
- 323.Chever O, Djukic B, McCarthy KD, Amzica F. Implication of Kir4.1 channel in excess potassium clearance: an in vivo study on anesthetized glial-conditional Kir4.1 knock-out mice. J Neurosci 30: 15769–15777, 2010. doi: 10.1523/JNEUROSCI.2078-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Chever O, Lee CY, Rouach N. Astroglial connexin43 hemichannels tune basal excitatory synaptic transmission. J Neurosci 34: 11228–11232, 2014. doi: 10.1523/JNEUROSCI.0015-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Chih CP, Roberts EL Jr. Energy substrates for neurons during neural activity: a critical review of the astrocyte-neuron lactate shuttle hypothesis. J Cereb Blood Flow Metab 23: 1263–1281, 2003. doi: 10.1097/01.WCB.0000081369.51727.6F. [DOI] [PubMed] [Google Scholar]
- 326.Cho Y, Bannai S. Uptake of glutamate and cysteine in C-6 glioma cells and in cultured astrocytes. J Neurochem 55: 2091–2097, 1990. doi: 10.1111/j.1471-4159.1990.tb05800.x. [DOI] [PubMed] [Google Scholar]
- 327.Choe KY, Olson JE, Bourque CW. Taurine release by astrocytes modulates osmosensitive glycine receptor tone and excitability in the adult supraoptic nucleus. J Neurosci 32: 12518–12527, 2012. doi: 10.1523/JNEUROSCI.1380-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Choi SS, Lee HJ, Lim I, Satoh J, Kim SU. Human astrocytes: secretome profiles of cytokines and chemokines. PLoS One 9: e92325, 2014. doi: 10.1371/journal.pone.0092325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Cholet N, Pellerin L, Magistretti PJ, Hamel E. Similar perisynaptic glial localization for the Na+,K+-ATPase α 2 subunit and the glutamate transporters GLAST and GLT-1 in the rat somatosensory cortex. Cereb Cortex 12: 515–525, 2002. doi: 10.1093/cercor/12.5.515. [DOI] [PubMed] [Google Scholar]
- 330.Cholewinski AJ, Stevens G, McDermott AM, Wilkin GP. Identification of B2 bradykinin binding sites on cultured cortical astrocytes. J Neurochem 57: 1456–1458, 1991. doi: 10.1111/j.1471-4159.1991.tb08314.x. [DOI] [PubMed] [Google Scholar]
- 331.Christensen JR, Larsen KB, Lisanby SH, Scalia J, Arango V, Dwork AJ, Pakkenberg B. Neocortical and hippocampal neuron and glial cell numbers in the rhesus monkey. Anat Rec (Hoboken) 290: 330–340, 2007. doi: 10.1002/ar.20504. [DOI] [PubMed] [Google Scholar]
- 332.Christian CA, Huguenard JR. Astrocytes potentiate GABAergic transmission in the thalamic reticular nucleus via endozepine signaling. Proc Natl Acad Sci USA 110: 20278–20283, 2013. doi: 10.1073/pnas.1318031110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Chun LLY, Barres BA, Corey DP. Induction of a calcium channel in astrocytes by cAMP. Soc Neurosci Abstracts 12: 1346, 1986. [Google Scholar]
- 334.Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504: 394–400, 2013. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Chung WS, Welsh CA, Barres BA, Stevens B. Do glia drive synaptic and cognitive impairment in disease? Nat Neurosci 18: 1539–1545, 2015. doi: 10.1038/nn.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Chuquet J, Quilichini P, Nimchinsky EA, Buzsáki G. Predominant enhancement of glucose uptake in astrocytes versus neurons during activation of the somatosensory cortex. J Neurosci 30: 15298–15303, 2010. doi: 10.1523/JNEUROSCI.0762-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Chvátal A, Pastor A, Mauch M, Syková E, Kettenmann H. Distinct populations of identified glial cells in the developing rat spinal cord slice: ion channel properties and cell morphology. Eur J Neurosci 7: 129–142, 1995. doi: 10.1111/j.1460-9568.1995.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 338.Ciccarelli R, D’Alimonte I, Ballerini P, D’Auro M, Nargi E, Buccella S, Di Iorio P, Bruno V, Nicoletti F, Caciagli F. Molecular signalling mediating the protective effect of A1 adenosine and mGlu3 metabotropic glutamate receptor activation against apoptosis by oxygen/glucose deprivation in cultured astrocytes. Mol Pharmacol 71: 1369–1380, 2007. doi: 10.1124/mol.106.031617. [DOI] [PubMed] [Google Scholar]
- 339.Clark B, Mobbs P. Transmitter-operated channels in rabbit retinal astrocytes studied in situ by whole-cell patch clamping. J Neurosci 12: 664–673, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Clasadonte J, Dong J, Hines DJ, Haydon PG. Astrocyte control of synaptic NMDA receptors contributes to the progressive development of temporal lobe epilepsy. Proc Natl Acad Sci USA 110: 17540–17545, 2013. doi: 10.1073/pnas.1311967110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem 278: 1354–1362, 2003. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- 342.Coggeshall RE, Fawcett DW. The fine structure of the central nervous system of the leech, Hirudo Medicinalis. J Neurophysiol 27: 229–289, 1964. [DOI] [PubMed] [Google Scholar]
- 343.Cohen Z, Bouchelet I, Olivier A, Villemure JG, Ball R, Stanimirovic DB, Hamel E. Multiple microvascular and astroglial 5-hydroxytryptamine receptor subtypes in human brain: molecular and pharmacologic characterization. J Cereb Blood Flow Metab 19: 908–917, 1999. doi: 10.1097/00004647-199908000-00010. [DOI] [PubMed] [Google Scholar]
- 344.Cohen Z, Molinatti G, Hamel E. Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. J Cereb Blood Flow Metab 17: 894–904, 1997. doi: 10.1097/00004647-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 345.Collden G, Balland E, Parkash J, Caron E, Langlet F, Prevot V, Bouret SG. Neonatal overnutrition causes early alterations in the central response to peripheral ghrelin. Mol Metab 4: 15–24, 2014. doi: 10.1016/j.molmet.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology 36: 1277–1283, 1997. doi: 10.1016/S0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- 347.Colombo JA. The interlaminar glia: from serendipity to hypothesis. Brain Struct Funct 222: 1109–1129, 2017. doi: 10.1007/s00429-016-1332-8. [DOI] [PubMed] [Google Scholar]
- 348.Colombo JA, Fuchs E, Härtig W, Marotte LR, Puissant V. “Rodent-like” and “primate-like” types of astroglial architecture in the adult cerebral cortex of mammals: a comparative study. Anat Embryol (Berl) 201: 111–120, 2000. doi: 10.1007/PL00008231. [DOI] [PubMed] [Google Scholar]
- 349.Colombo JA, Reisin HD. Interlaminar astroglia of the cerebral cortex: a marker of the primate brain. Brain Res 1006: 126–131, 2004. doi: 10.1016/j.brainres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 350.Colombo JA, Yáñez A, Puissant V, Lipina S. Long, interlaminar astroglial cell processes in the cortex of adult monkeys. J Neurosci Res 40: 551–556, 1995. doi: 10.1002/jnr.490400414. [DOI] [PubMed] [Google Scholar]
- 351.Conejo NM, González-Pardo H, Cimadevilla JM, Argüelles JA, Díaz F, Vallejo-Seco G, Arias JL. Influence of gonadal steroids on the glial fibrillary acidic protein-immunoreactive astrocyte population in young rat hippocampus. J Neurosci Res 79: 488–494, 2005. doi: 10.1002/jnr.20372. [DOI] [PubMed] [Google Scholar]
- 352.Conner MT, Conner AC, Bland CE, Taylor LH, Brown JE, Parri HR, Bill RM. Rapid aquaporin translocation regulates cellular water flow: mechanism of hypotonicity-induced subcellular localization of aquaporin 1 water channel. J Biol Chem 287: 11516–11525, 2012. doi: 10.1074/jbc.M111.329219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Connor JR, Berkowitz EM. A demonstration of glial filament distribution in astrocytes isolated from rat cerebral cortex. Neuroscience 16: 33–44, 1985. doi: 10.1016/0306-4522(85)90044-2. [DOI] [PubMed] [Google Scholar]
- 354.Conti F, Barbaresi P, Melone M, Ducati A. Neuronal and glial localization of NR1 and NR2A/B subunits of the NMDA receptor in the human cerebral cortex. Cereb Cortex 9: 110–120, 1999. doi: 10.1093/cercor/9.2.110. [DOI] [PubMed] [Google Scholar]
- 355.Conti F, Minelli A, Brecha NC. Cellular localization and laminar distribution of AMPA glutamate receptor subunits mRNAs and proteins in the rat cerebral cortex. J Comp Neurol 350: 241–259, 1994. doi: 10.1002/cne.903500208. [DOI] [PubMed] [Google Scholar]
- 356.Conti F, Minelli A, Melone M. GABA transporters in the mammalian cerebral cortex: localization, development and pathological implications. Brain Res Brain Res Rev 45: 196–212, 2004. doi: 10.1016/j.brainresrev.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 357.Contreras JE, Sáez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci USA 100: 11388–11393, 2003. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Contreras JE, Sánchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Sáez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA 99: 495–500, 2002. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Cooper AJ, Plum F. Biochemistry and physiology of brain ammonia. Physiol Rev 67: 440–519, 1987. [DOI] [PubMed] [Google Scholar]
- 360.Cornell-Bell AH, Finkbeiner SM. Ca2+ waves in astrocytes. Cell Calcium 12: 185–204, 1991. doi: 10.1016/0143-4160(91)90020-F. [DOI] [PubMed] [Google Scholar]
- 361.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247: 470–473, 1990. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 362.Cortés-Campos C, Elizondo R, Llanos P, Uranga RM, Nualart F, García MA. MCT expression and lactate influx/efflux in tanycytes involved in glia-neuron metabolic interaction. PLoS One 6: e16411, 2011. doi: 10.1371/journal.pone.0016411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Corti A, Casini AF, Pompella A. Cellular pathways for transport and efflux of ascorbate and dehydroascorbate. Arch Biochem Biophys 500: 107–115, 2010. doi: 10.1016/j.abb.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 364.Costa MR, Kessaris N, Richardson WD, Götz M, Hedin-Pereira C. The marginal zone/layer I as a novel niche for neurogenesis and gliogenesis in developing cerebral cortex. J Neurosci 27: 11376–11388, 2007. doi: 10.1523/JNEUROSCI.2418-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA 95: 15735–15740, 1998. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Cotrina ML, Lin JH, López-García JC, Naus CC, Nedergaard M. ATP-mediated glia signaling. J Neurosci 20: 2835–2844, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Covarrubias-Pinto A, Acuña AI, Beltrán FA, Torres-Díaz L, Castro MA. Old things new view: ascorbic acid protects the brain in neurodegenerative disorders. Int J Mol Sci 16: 28194–28217, 2015. doi: 10.3390/ijms161226095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Crippa D, Schenk U, Francolini M, Rosa P, Verderio C, Zonta M, Pozzan T, Matteoli M, Carmignoto G. Synaptobrevin2-expressing vesicles in rat astrocytes: insights into molecular characterization, dynamics and exocytosis. J Physiol 570: 567–582, 2006. doi: 10.1113/jphysiol.2005.094052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Cristóvão-Ferreira S, Navarro G, Brugarolas M, Pérez-Capote K, Vaz SH, Fattorini G, Conti F, Lluis C, Ribeiro JA, McCormick PJ, Casadó V, Franco R, Sebastião AM. A1R-A2AR heteromers coupled to Gs and G i/0 proteins modulate GABA transport into astrocytes. Purinergic Signal 9: 433–449, 2013. doi: 10.1007/s11302-013-9364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.D’Alimonte I, Ciccarelli R, Di Iorio P, Nargi E, Buccella S, Giuliani P, Rathbone MP, Jiang S, Caciagli F, Ballerini P. Activation of P2X(7) receptors stimulates the expression of P2Y(2) receptor mRNA in astrocytes cultured from rat brain. Int J Immunopathol Pharmacol 20: 301–316, 2007. doi: 10.1177/039463200702000210. [DOI] [PubMed] [Google Scholar]
- 371.D’Ambrosio R, Gordon DS, Winn HR. Differential role of KIR channel and Na+/K+-pump in the regulation of extracellular K+ in rat hippocampus. J Neurophysiol 87: 87–102, 2002. doi: 10.1152/jn.00240.2001. [DOI] [PubMed] [Google Scholar]
- 372.D’Amelio F, Eng LF, Gibbs MA. Glutamine synthetase immunoreactivity is present in oligodendroglia of various regions of the central nervous system. Glia 3: 335–341, 1990. doi: 10.1002/glia.440030504. [DOI] [PubMed] [Google Scholar]
- 373.D’Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PG. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci USA 104: 1995–2000, 2007. doi: 10.1073/pnas.0609408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.D’Ascenzo M, Vairano M, Andreassi C, Navarra P, Azzena GB, Grassi C. Electrophysiological and molecular evidence of L-(Cav1), N- (Cav2.2), and R- (Cav2.3) type Ca2+ channels in rat cortical astrocytes. Glia 45: 354–363, 2004. doi: 10.1002/glia.10336. [DOI] [PubMed] [Google Scholar]
- 375.Dahl G. ATP release through pannexon channels. Philos Trans R Soc Lond B Biol Sci 370: 20140191, 2015. doi: 10.1098/rstb.2014.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Dale N. Purinergic signaling in hypothalamic tanycytes: potential roles in chemosensing. Semin Cell Dev Biol 22: 237–244, 2011. doi: 10.1016/j.semcdb.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 377.Dall’Aglio C, Ceccarelli P, Pascucci L, Brecchia G, Boiti C. Receptors for leptin and estrogen in the subcommissural organ of rabbits are differentially modulated by fasting. Brain Res 1124: 62–69, 2006. doi: 10.1016/j.brainres.2006.09.091. [DOI] [PubMed] [Google Scholar]
- 378.Dallaporta M, Pecchi E, Pio J, Jean A, Horner KC, Troadec JD. Expression of leptin receptor by glial cells of the nucleus tractus solitarius: possible involvement in energy homeostasis. J Neuroendocrinol 21: 57–67, 2009. doi: 10.1111/j.1365-2826.2008.01799.x. [DOI] [PubMed] [Google Scholar]
- 379.Dallérac G, Chever O, Rouach N. How do astrocytes shape synaptic transmission? Insights from electrophysiology. Front Cell Neurosci 7: 159, 2013. doi: 10.3389/fncel.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Danbolt NC. Glutamate uptake. Prog Neurobiol 65: 1–105, 2001. doi: 10.1016/S0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 381.Danbolt NC, Pines G, Kanner BI. Purification and reconstitution of the sodium- and potassium-coupled glutamate transport glycoprotein from rat brain. Biochemistry 29: 6734–6740, 1990. doi: 10.1021/bi00480a025. [DOI] [PubMed] [Google Scholar]
- 382.Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron 8: 429–440, 1992. doi: 10.1016/0896-6273(92)90271-E. [DOI] [PubMed] [Google Scholar]
- 383.Daré E, Schulte G, Karovic O, Hammarberg C, Fredholm BB. Modulation of glial cell functions by adenosine receptors. Physiol Behav 92: 15–20, 2007. doi: 10.1016/j.physbeh.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 384.Darmanis S, Sloan SA, Zhang Y, Enge M, Caneda C, Shuer LM, Hayden Gephart MG, Barres BA, Quake SR. A survey of human brain transcriptome diversity at the single cell level. Proc Natl Acad Sci USA 112: 7285–7290, 2015. doi: 10.1073/pnas.1507125112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Dave V, Gordon GW, McCarthy KD. Cerebral type 2 astroglia are heterogeneous with respect to their ability to respond to neuroligands linked to calcium mobilization. Glia 4: 440–447, 1991. doi: 10.1002/glia.440040503. [DOI] [PubMed] [Google Scholar]
- 386.Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci 24: 476–488, 2003. doi: 10.1016/S1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 387.De Biasi S, Vitellaro-Zuccarello L, Brecha NC. Immunoreactivity for the GABA transporter-1 and GABA transporter-3 is restricted to astrocytes in the rat thalamus. A light and electron-microscopic immunolocalization. Neuroscience 83: 815–828, 1998. doi: 10.1016/S0306-4522(97)00414-4. [DOI] [PubMed] [Google Scholar]
- 388.De Bock M, Decrock E, Wang N, Bol M, Vinken M, Bultynck G, Leybaert L. The dual face of connexin-based astroglial Ca2+ communication: a key player in brain physiology and a prime target in pathology. Biochim Biophys Acta 1843: 2211–2232, 2014. doi: 10.1016/j.bbamcr.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 389.De Castro F. [The anatomical aspects of the ganglionic synaptic transmission in mammals]. Arch Int Physiol 59: 479–525, 1951. [DOI] [PubMed] [Google Scholar]
- 390.De Leo JA, Tawfik VL, LaCroix-Fralish ML. The tetrapartite synapse: path to CNS sensitization and chronic pain. Pain 122: 17–21, 2006. doi: 10.1016/j.pain.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 391.De Nardo D. Toll-like receptors: activation, signalling and transcriptional modulation. Cytokine 74: 181–189, 2015. doi: 10.1016/j.cyto.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 392.De Pinto V, Caggese C, Prezioso G, Ritossa F. Purification of the glutamine synthetase II isozyme of Drosophila melanogaster and structural and functional comparison of glutamine synthetases I and II. Biochem Genet 25: 821–836, 1987. doi: 10.1007/BF00502602. [DOI] [PubMed] [Google Scholar]
- 393.De Pittà M, Brunel N, Volterra A. Astrocytes: Orchestrating synaptic plasticity? Neuroscience 323: 43–61, 2016. doi: 10.1016/j.neuroscience.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 394.De Vitry F, Picart R, Jacque C, Tixier-Vidal A. Glial fibrillary acidic protein. A cellular marker of tanycytes in the mouse hypothalamus. Dev Neurosci 4: 457–460, 1981. doi: 10.1159/000112813. [DOI] [PubMed] [Google Scholar]
- 395.DeAzevedo LC, Fallet C, Moura-Neto V, Daumas-Duport C, Hedin-Pereira C, Lent R. Cortical radial glial cells in human fetuses: depth-correlated transformation into astrocytes. J Neurobiol 55: 288–298, 2003. doi: 10.1002/neu.10205. [DOI] [PubMed] [Google Scholar]
- 396.Debernardi R, Pierre K, Lengacher S, Magistretti PJ, Pellerin L. Cell-specific expression pattern of monocarboxylate transporters in astrocytes and neurons observed in different mouse brain cortical cell cultures. J Neurosci Res 73: 141–155, 2003. doi: 10.1002/jnr.10660. [DOI] [PubMed] [Google Scholar]
- 397.Decrock E, De Bock M, Wang N, Bultynck G, Giaume C, Naus CC, Green CR, Leybaert L. Connexin and pannexin signaling pathways, an architectural blueprint for CNS physiology and pathology? Cell Mol Life Sci 72: 2823–2851, 2015. doi: 10.1007/s00018-015-1962-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Deiters O. Untersuchungen über Gehirn und Rückenmark des Menschen und der Säugethiere. Braunschweig: Vieweg, 1865. doi: 10.5962/bhl.title.61884 [DOI] [Google Scholar]
- 399.Deitmer JW. Electrogenic sodium-dependent bicarbonate secretion by glial cells of the leech central nervous system. J Gen Physiol 98: 637–655, 1991. doi: 10.1085/jgp.98.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Deitmer JW, Kristan WB Jr. Glial responses during evoked behaviors in the leech. Glia 26: 186–189, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 401.Deitmer JW, Rose CR. Ion changes and signalling in perisynaptic glia. Brain Res Brain Res Rev 63: 113–129, 2010. doi: 10.1016/j.brainresrev.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 402.Deitmer JW, Rose CR. pH regulation and proton signalling by glial cells. Prog Neurobiol 48: 73–103, 1996. doi: 10.1016/0301-0082(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 403.Deitmer JW, Rose CR, Munsch T, Schmidt J, Nett W, Schneider HP, Lohr C. Leech giant glial cell: functional role in a simple nervous system. Glia 28: 175–182, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 404.Deitmer JW, Schlue WR. An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cells. J Physiol 411: 179–194, 1989. doi: 10.1113/jphysiol.1989.sp017567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Deitmer JW, Schlue WR. The regulation of intracellular pH by identified glial cells and neurones in the central nervous system of the leech. J Physiol 388: 261–283, 1987. doi: 10.1113/jphysiol.1987.sp016614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Deitmer JW, Schneider HP. Acid/base transport across the leech giant glial cell membrane at low external bicarbonate concentration. J Physiol 512: 459–469, 1998. doi: 10.1111/j.1469-7793.1998.459be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Deitmer JW, Schneider HP. Intracellular acidification of the leech giant glial cell evoked by glutamate and aspartate. Glia 19: 111–122, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 408.Deitmer JW, Verkhratsky AJ, Lohr C. Calcium signalling in glial cells. Cell Calcium 24: 405–416, 1998. doi: 10.1016/S0143-4160(98)90063-X. [DOI] [PubMed] [Google Scholar]
- 409.Del Cerro S, Garcia-Estrada J, Garcia-Segura LM. Neuroactive steroids regulate astroglia morphology in hippocampal cultures from adult rats. Glia 14: 65–71, 1995. doi: 10.1002/glia.440140109. [DOI] [PubMed] [Google Scholar]
- 410.Deneen B, Ho R, Lukaszewicz A, Hochstim CJ, Gronostajski RM, Anderson DJ. The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron 52: 953–968, 2006. doi: 10.1016/j.neuron.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 411.Dermietzel R. Visualization by freeze-fracturing of regular structures in glial cell membranes. Naturwissenschaften 60: 208, 1973. doi: 10.1007/BF00599446. [DOI] [PubMed] [Google Scholar]
- 412.Dermietzel R, Gao Y, Scemes E, Vieira D, Urban M, Kremer M, Bennett MV, Spray DC. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res Brain Res Rev 32: 45–56, 2000. doi: 10.1016/S0165-0173(99)00067-3. [DOI] [PubMed] [Google Scholar]
- 413.Dermietzel R, Hertberg EL, Kessler JA, Spray DC. Gap junctions between cultured astrocytes: immunocytochemical, molecular, and electrophysiological analysis. J Neurosci 11: 1421–1432, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Dermietzel R, Hwang TK, Spray DS. The gap junction family: structure, function and chemistry. Anat Embryol (Berl) 182: 517–528, 1990. doi: 10.1007/BF00186458. [DOI] [PubMed] [Google Scholar]
- 415.Derouiche A. The perisynaptic astrocyte process as a glial compartment-immunolabeling for glutamine synthetase and other glial markers. Adv Mol Cell Biol 31: 147–163, 2003. doi: 10.1016/S1569-2558(03)31006-9. [DOI] [Google Scholar]
- 416.Derouiche A, Anlauf E, Aumann G, Mühlstädt B, Lavialle M. Anatomical aspects of glia-synapse interaction: the perisynaptic glial sheath consists of a specialized astrocyte compartment. J Physiol Paris 96: 177–182, 2002. doi: 10.1016/S0928-4257(02)00004-9. [DOI] [PubMed] [Google Scholar]
- 417.Derouiche A, Frotscher M. Astroglial processes around identified glutamatergic synapses contain glutamine synthetase: evidence for transmitter degradation. Brain Res 552: 346–350, 1991. doi: 10.1016/0006-8993(91)90103-3. [DOI] [PubMed] [Google Scholar]
- 418.Derouiche A, Frotscher M. Peripheral astrocyte processes: monitoring by selective immunostaining for the actin-binding ERM proteins. Glia 36: 330–341, 2001. doi: 10.1002/glia.1120. [DOI] [PubMed] [Google Scholar]
- 419.Derouiche A, Haseleu J, Korf HW. Fine astrocyte processes contain very small mitochondria: Glial oxidative capability may fuel transmitter metabolism. Neurochem Res 40: 2402–2413, 2015. doi: 10.1007/s11064-015-1563-8. [DOI] [PubMed] [Google Scholar]
- 420.Desagher S, Glowinski J, Premont J. Astrocytes protect neurons from hydrogen peroxide toxicity. J Neurosci 16: 2553–2562, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Dezonne RS, Lima FR, Trentin AG, Gomes FC. Thyroid hormone and astroglia: endocrine control of the neural environment. J Neuroendocrinol 27: 435–445, 2015. doi: 10.1111/jne.12283. [DOI] [PubMed] [Google Scholar]
- 422.Dhandapani KM, Mahesh VB, Brann DW. Astrocytes and brain function: implications for reproduction. Exp Biol Med (Maywood) 228: 253–260, 2003. doi: 10.1177/153537020322800303. [DOI] [PubMed] [Google Scholar]
- 423.Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, Tiret P, Volterra A. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci 14: 1276–1284, 2011. doi: 10.1038/nn.2929. [DOI] [PubMed] [Google Scholar]
- 424.Di Scala-Guenot D, Mouginot D, Strosser MT. Increase of intracellular calcium induced by oxytocin in hypothalamic cultured astrocytes. Glia 11: 269–276, 1994. doi: 10.1002/glia.440110308. [DOI] [PubMed] [Google Scholar]
- 425.Di Scala-Guenot D, Strosser MT. Oxytocin receptors on cultured astroglial cells. Kinetic and pharmacological characterization of oxytocin-binding sites on intact hypothalamic and hippocampic cells from foetal rat brain. Biochem J 284: 491–497, 1992. doi: 10.1042/bj2840491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Di Virgilio F, Ferrari D, Adinolfi E. P2X(7): a growth-promoting receptor-implications for cancer. Purinergic Signal 5: 251–256, 2009. doi: 10.1007/s11302-009-9145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Diamond JS. Deriving the glutamate clearance time course from transporter currents in CA1 hippocampal astrocytes: transmitter uptake gets faster during development. J Neurosci 25: 2906–2916, 2005. doi: 10.1523/JNEUROSCI.5125-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Diamond JS. Neuronal glutamate transporters limit activation of NMDA receptors by neurotransmitter spillover on CA1 pyramidal cells. J Neurosci 21: 8328–8338, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Diaz MR, Wadleigh A, Hughes BA, Woodward JJ, Valenzuela CF. Bestrophin1 channels are insensitive to ethanol and do not mediate tonic GABAergic currents in cerebellar granule cells. Front Neurosci 5: 148, 2012. doi: 10.3389/fnins.2011.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Dietzel I, Heinemann U, Hofmeier G, Lux HD. Transient changes in the size of the extracellular space in the sensorimotor cortex of cats in relation to stimulus-induced changes in potassium concentration. Exp Brain Res 40: 432–439, 1980. doi: 10.1007/BF00236151. [DOI] [PubMed] [Google Scholar]
- 431.Ding F, O’Donnell J, Thrane AS, Zeppenfeld D, Kang H, Xie L, Wang F, Nedergaard M. α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 54: 387–394, 2013. doi: 10.1016/j.ceca.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Ding F, O’Donnell J, Xu Q, Kang N, Goldman N, Nedergaard M. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 352: 550–555, 2016. doi: 10.1126/science.aad4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.DiNuzzo M. Astrocyte-neuron interactions during learning may occur by lactate signaling rather than metabolism. Front Integr Neurosci 10: 2, 2016. doi: 10.3389/fnint.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Dityatev A, Rusakov DA. Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol 21: 353–359, 2011. doi: 10.1016/j.conb.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Dixon SJ, Yu R, Panupinthu N, Wilson JX. Activation of P2 nucleotide receptors stimulates acid efflux from astrocytes. Glia 47: 367–376, 2004. doi: 10.1002/glia.20048. [DOI] [PubMed] [Google Scholar]
- 436.Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci 27: 11354–11365, 2007. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Do Rego JL, Vaudry H. Comparative aspects of neurosteroidogenesis: from fish to mammals. Gen Comp Endocrinol 227: 120–129, 2016. doi: 10.1016/j.ygcen.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 438.Doengi M, Deitmer JW, Lohr C. New evidence for purinergic signaling in the olfactory bulb: A2A and P2Y1 receptors mediate intracellular calcium release in astrocytes. FASEB J 22: 2368–2378, 2008. doi: 10.1096/fj.07-101782. [DOI] [PubMed] [Google Scholar]
- 439.Doengi M, Hirnet D, Coulon P, Pape HC, Deitmer JW, Lohr C. GABA uptake-dependent Ca2+ signaling in developing olfactory bulb astrocytes. Proc Natl Acad Sci USA 106: 17570–17575, 2009. doi: 10.1073/pnas.0809513106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97: 703–716, 1999. doi: 10.1016/S0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 441.Doherty J, Logan MA, Taşdemir OE, Freeman MR. Ensheathing glia function as phagocytes in the adult Drosophila brain. J Neurosci 29: 4768–4781, 2009. doi: 10.1523/JNEUROSCI.5951-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Doly S, Fischer J, Salio C, Conrath M. The vanilloid receptor-1 is expressed in rat spinal dorsal horn astrocytes. Neurosci Lett 357: 123–126, 2004. doi: 10.1016/j.neulet.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 443.Dombrowski SM, Hilgetag CC, Barbas H. Quantitative architecture distinguishes prefrontal cortical systems in the rhesus monkey. Cereb Cortex 11: 975–988, 2001. doi: 10.1093/cercor/11.10.975. [DOI] [PubMed] [Google Scholar]
- 444.Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, Geczy CL. Functions of S100 proteins. Curr Mol Med 13: 24–57, 2013. doi: 10.2174/156652413804486214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Donato R, Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, Tubaro C, Giambanco I. S100B’s double life: intracellular regulator and extracellular signal. Biochim Biophys Acta 1793: 1008–1022, 2009. doi: 10.1016/j.bbamcr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 446.DonCarlos LL, Sarkey S, Lorenz B, Azcoitia I, Garcia-Ovejero D, Huppenbauer C, Garcia-Segura LM. Novel cellular phenotypes and subcellular sites for androgen action in the forebrain. Neuroscience 138: 801–807, 2006. doi: 10.1016/j.neuroscience.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 447.Donevan SD, Rogawski MA. Intracellular polyamines mediate inward rectification of Ca2+-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Proc Natl Acad Sci USA 92: 9298–9302, 1995. doi: 10.1073/pnas.92.20.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Dong JH, Chen X, Cui M, Yu X, Pang Q, Sun JP. β2-Adrenergic receptor and astrocyte glucose metabolism. J Mol Neurosci 48: 456–463, 2012. doi: 10.1007/s12031-012-9742-4. [DOI] [PubMed] [Google Scholar]
- 449.Donnan F. Theorie der Membrangleichgewichte und Membranpotentiale bei Vorhandensein von nicht dialysierenden Elektrolyten. Ein Beitrag zur physikalisch-chemischen Physiologie. Zeitschrift Für Elektrochemie Angew Phys Chem 17: 572–581, 1911. [Google Scholar]
- 450.Dowell JA, Johnson JA, Li L. Identification of astrocyte secreted proteins with a combination of shotgun proteomics and bioinformatics. J Proteome Res 8: 4135–4143, 2009. doi: 10.1021/pr900248y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem 267: 4912–4916, 2000. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- 452.Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci 19: 562–569, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Du Y, Dreyfus CF. Oligodendrocytes as providers of growth factors. J Neurosci Res 68: 647–654, 2002. doi: 10.1002/jnr.10245. [DOI] [PubMed] [Google Scholar]
- 454.Du Y, Kiyoshi CM, Wang Q, Wang W, Ma B, Alford CC, Zhong S, Wan Q, Chen H, Lloyd EE, Bryan RM Jr, Zhou M. Genetic deletion of TREK-1 or TWIK-1/TREK-1 potassium channels does not alter the basic electrophysiological properties of mature hippocampal astrocytes in situ. Front Cell Neurosci 10: 13, 2016. doi: 10.3389/fncel.2016.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Du Y, Ma B, Kiyoshi CM, Alford CC, Wang W, Zhou M. Freshly dissociated mature hippocampal astrocytes exhibit passive membrane conductance and low membrane resistance similarly to syncytial coupled astrocytes. J Neurophysiol 113: 3744–3750, 2015. doi: 10.1152/jn.00206.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Duan D, Ye L, Britton F, Horowitz B, Hume JR. A novel anionic inward rectifier in native cardiac myocytes. Circ Res 86: E63–E71, 2000. doi: 10.1161/01.RES.86.4.e63. [DOI] [PubMed] [Google Scholar]
- 457.Duan S, Anderson CM, Keung EC, Chen Y, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci 23: 1320–1328, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Duan S, Neary JT. P2X(7) receptors: properties and relevance to CNS function. Glia 54: 738–746, 2006. doi: 10.1002/glia.20397. [DOI] [PubMed] [Google Scholar]
- 459.Duffy S, MacVicar BA. Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J Neurosci 15: 5535–5550, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Duffy S, MacVicar BA. Potassium-dependent calcium influx in acutely isolated hippocampal astrocytes. Neuroscience 61: 51–61, 1994. doi: 10.1016/0306-4522(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 461.Duhart JM, Leone MJ, Paladino N, Evans JA, Castanon-Cervantes O, Davidson AJ, Golombek DA. Suprachiasmatic astrocytes modulate the circadian clock in response to TNF-α. J Immunol 191: 4656–4664, 2013. doi: 10.4049/jimmunol.1300450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Dumstrei K, Wang F, Hartenstein V. Role of DE-cadherin in neuroblast proliferation, neural morphogenesis, and axon tract formation in Drosophila larval brain development. J Neurosci 23: 3325–3335, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Dykes IM, Freeman FM, Bacon JP, Davies JA. Molecular basis of gap junctional communication in the CNS of the leech Hirudo medicinalis. J Neurosci 24: 886–894, 2004. doi: 10.1523/JNEUROSCI.3676-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Dzamba D, Honsa P, Valny M, Kriska J, Valihrach L, Novosadova V, Kubista M, Anderova M. Quantitative analysis of glutamate receptors in glial cells from the cortex of GFAP/EGFP mice following ischemic injury: focus on NMDA receptors. Cell Mol Neurobiol 35: 1187–1202, 2015. doi: 10.1007/s10571-015-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Eaton MJ, Skatchkov SN, Brune A, Biedermann B, Veh RW, Reichenbach A. SURI and Kir6.1 subunits of K(ATP)-channels are co-localized in retinal glial (Müller) cells. Neuroreport 13: 57–60, 2002. doi: 10.1097/00001756-200201210-00016. [DOI] [PubMed] [Google Scholar]
- 466.Edwards L, Nashmi R, Jones O, Backx P, Ackerley C, Becker L, Fehlings MG. Upregulation of Kv 1.4 protein and gene expression after chronic spinal cord injury. J Comp Neurol 443: 154–167, 2002. doi: 10.1002/cne.10115. [DOI] [PubMed] [Google Scholar]
- 467.Edwards TN, Meinertzhagen IA. The functional organisation of glia in the adult brain of Drosophila and other insects. Prog Neurobiol 90: 471–497, 2010. doi: 10.1016/j.pneurobio.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Egawa K, Yamada J, Furukawa T, Yanagawa Y, Fukuda A. Cl− homeodynamics in gap junction-coupled astrocytic networks on activation of GABAergic synapses. J Physiol 591: 3901–3917, 2013. doi: 10.1113/jphysiol.2013.257162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Eggermann E, Bucurenciu I, Goswami SP, Jonas P. Nanodomain coupling between Ca2+ channels and sensors of exocytosis at fast mammalian synapses. Nat Rev Neurosci 13: 7–21, 2011. doi: 10.1038/nrn3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Ehmsen JT, Liu Y, Wang Y, Paladugu N, Johnson AE, Rothstein JD, du Lac S, Mattson MP, Höke A. The astrocytic transporter SLC7A10 (Asc-1) mediates glycinergic inhibition of spinal cord motor neurons. Sci Rep 6: 35592, 2016. doi: 10.1038/srep35592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Ehrenreich H, Kehrl JH, Anderson RW, Rieckmann P, Vitkovic L, Coligan JE, Fauci AS. A vasoactive peptide, endothelin-3, is produced by and specifically binds to primary astrocytes. Brain Res 538: 54–58, 1991. doi: 10.1016/0006-8993(91)90375-6. [DOI] [PubMed] [Google Scholar]
- 472.Eide PK, Ringstad G. MRI with intrathecal MRI gadolinium contrast medium administration: a possible method to assess glymphatic function in human brain. Acta Radiol Open 4: 2058460115609635, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Ek-Vitorin JF, King TJ, Heyman NS, Lampe PD, Burt JM. Selectivity of connexin 43 channels is regulated through protein kinase C-dependent phosphorylation. Circ Res 98: 1498–1505, 2006. doi: 10.1161/01.RES.0000227572.45891.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Elkjaer M, Vajda Z, Nejsum LN, Kwon T, Jensen UB, Amiry-Moghaddam M, Frøkiaer J, Nielsen S. Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochem Biophys Res Commun 276: 1118–1128, 2000. doi: 10.1006/bbrc.2000.3505. [DOI] [PubMed] [Google Scholar]
- 475.Emmi A, Wenzel HJ, Schwartzkroin PA, Taglialatela M, Castaldo P, Bianchi L, Nerbonne J, Robertson GA, Janigro D. Do glia have heart? Expression and functional role for ether-a-go-go currents in hippocampal astrocytes. J Neurosci 20: 3915–3925, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Emsley JG, Macklis JD. Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol 2: 175–186, 2006. doi: 10.1017/S1740925X06000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem Res 25: 1439–1451, 2000. doi: 10.1023/A:1007677003387. [DOI] [PubMed] [Google Scholar]
- 478.Eng LF, Vanderhaeghen JJ, Bignami A, Gerstl B. An acidic protein isolated from fibrous astrocytes. Brain Res 28: 351–354, 1971. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- 479.Enkvist MO, Holopainen I, Akerman KE. Glutamate receptor-linked changes in membrane potential and intracellular Ca2+ in primary rat astrocytes. Glia 2: 397–402, 1989. doi: 10.1002/glia.440020602. [DOI] [PubMed] [Google Scholar]
- 480.Enyedi P, Czirják G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev 90: 559–605, 2010. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 481.Eriksen J, Chang R, McGregor M, Silm K, Suzuki T, Edwards RH. Protons regulate vesicular glutamate transporters through an allosteric mechanism. Neuron 90: 768–780, 2016. doi: 10.1016/j.neuron.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Eriksen N, Pakkenberg B. Total neocortical cell number in the mysticete brain. Anat Rec (Hoboken) 290: 83–95, 2007. doi: 10.1002/ar.20404. [DOI] [PubMed] [Google Scholar]
- 483.Eriksson PS, Hansson E, Rönnbäck L. δ and κ opiate receptors in primary astroglial cultures from rat cerebral cortex. Neurochem Res 15: 1123–1126, 1990. doi: 10.1007/BF01101714. [DOI] [PubMed] [Google Scholar]
- 484.Eriksson PS, Hansson E, Rönnbäck L. δ and κ opiate receptors in primary astroglial cultures. Part II: Receptor sets in cultures from various brain regions and interactions with beta-receptor activated cyclic AMP. Neurochem Res 17: 545–551, 1992. doi: 10.1007/BF00968781. [DOI] [PubMed] [Google Scholar]
- 485.Eriksson PS, Nilsson M, Wågberg M, Hansson E, Rönnbäck L. κ-Opioid receptors on astrocytes stimulate L-type Ca2+ channels. Neuroscience 54: 401–407, 1993. doi: 10.1016/0306-4522(93)90261-D. [DOI] [PubMed] [Google Scholar]
- 486.Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, Green EM, Lawler J, Dolmetsch R, Garcia KC, Smith SJ, Luo ZD, Rosenthal A, Mosher DF, Barres BA. Gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 139: 380–392, 2009. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature 468: 223–231, 2010. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Erta M, Giralt M, Esposito FL, Fernandez-Gayol O, Hidalgo J. Astrocytic IL-6 mediates locomotor activity, exploration, anxiety, learning and social behavior. Horm Behav 73: 64–74, 2015. doi: 10.1016/j.yhbeh.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 489.Espallergues J, Solovieva O, Técher V, Bauer K, Alonso G, Vincent A, Hussy N. Synergistic activation of astrocytes by ATP and norepinephrine in the rat supraoptic nucleus. Neuroscience 148: 712–723, 2007. doi: 10.1016/j.neuroscience.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 490.Esseltine JL, Laird DW. Next-generation connexin and pannexin cell biology. Trends Cell Biol 26: 944–955, 2016. doi: 10.1016/j.tcb.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 491.Eulenburg V, Armsen W, Betz H, Gomeza J. Glycine transporters: essential regulators of neurotransmission. Trends Biochem Sci 30: 325–333, 2005. doi: 10.1016/j.tibs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 492.Eulenburg V, Retiounskaia M, Papadopoulos T, Gomeza J, Betz H. Glial glycine transporter 1 function is essential for early postnatal survival but dispensable in adult mice. Glia 58: 1066–1073, 2010. doi: 10.1002/glia.20987. [DOI] [PubMed] [Google Scholar]
- 493.Evans WH, Martin PE. Gap junctions: structure and function (Review) Mol Membr Biol 19: 121–136, 2002. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- 494.Faissner A, Pyka M, Geissler M, Sobik T, Frischknecht R, Gundelfinger ED, Seidenbecher C. Contributions of astrocytes to synapse formation and maturation. Potential functions of the perisynaptic extracellular matrix. Brain Res Brain Res Rev 63: 26–38, 2010. doi: 10.1016/j.brainresrev.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 495.Fam SR, Gallagher CJ, Salter MW. P2Y1 purinoceptor-mediated Ca2+ signaling and Ca2+ wave propagation in dorsal spinal cord astrocytes. J Neurosci 20: 2800–2808, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Fang Q, Hu WW, Wang XF, Yang Y, Lou GD, Jin MM, Yan HJ, Zeng WZ, Shen Y, Zhang SH, Xu TL, Chen Z. Histamine up-regulates astrocytic glutamate transporter 1 and protects neurons against ischemic injury. Neuropharmacology 77: 156–166, 2014. doi: 10.1016/j.neuropharm.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 497.Farb CR, Aoki C, Ledoux JE. Differential localization of NMDA and AMPA receptor subunits in the lateral and basal nuclei of the amygdala: a light and electron microscopic study. J Comp Neurol 362: 86–108, 1995. doi: 10.1002/cne.903620106. [DOI] [PubMed] [Google Scholar]
- 498.Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol 28: 138–145, 2007. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 499.Farmer WT, Abrahamsson T, Chierzi S, Lui C, Zaelzer C, Jones EV, Bally BP, Chen GG, Théroux JF, Peng J, Bourque CW, Charron F, Ernst C, Sjöström PJ, Murai KK. Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Science 351: 849–854, 2016. doi: 10.1126/science.aab3103. [DOI] [PubMed] [Google Scholar]
- 500.Feig SL, Haberly LB. Surface-associated astrocytes, not endfeet, form the glia limitans in posterior piriform cortex and have a spatially distributed, not a domain, organization. J Comp Neurol 519: 1952–1969, 2011. doi: 10.1002/cne.22615. [DOI] [PubMed] [Google Scholar]
- 501.Feindt J, Becker I, Blömer U, Hugo HH, Mehdorn HM, Krisch B, Mentlein R. Expression of somatostatin receptor subtypes in cultured astrocytes and gliomas. J Neurochem 65: 1997–2005, 1995. doi: 10.1046/j.1471-4159.1995.65051997.x. [DOI] [PubMed] [Google Scholar]
- 502.Fellin T, Carmignoto G. Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit. J Physiol 559: 3–15, 2004. doi: 10.1113/jphysiol.2004.063214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Fellin T, Pozzan T, Carmignoto G. Purinergic receptors mediate two distinct glutamate release pathways in hippocampal astrocytes. J Biol Chem 281: 4274–4284, 2006. doi: 10.1074/jbc.M510679200. [DOI] [PubMed] [Google Scholar]
- 504.Ferroni S, Marchini C, Ogata T, Schubert P. Recovery of deficient cholinergic calcium signaling by adenosine in cultured rat cortical astrocytes. J Neurosci Res 68: 615–621, 2002. doi: 10.1002/jnr.10248. [DOI] [PubMed] [Google Scholar]
- 505.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185, 2006. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 506.Feustel PJ, Jin Y, Kimelberg HK. Volume-regulated anion channels are the predominant contributors to release of excitatory amino acids in the ischemic cortical penumbra. Stroke 35: 1164–1168, 2004. doi: 10.1161/01.STR.0000124127.57946.a1. [DOI] [PubMed] [Google Scholar]
- 507.Fiacco TA, Agulhon C, Taves SR, Petravicz J, Casper KB, Dong X, Chen J, McCarthy KD. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron 54: 611–626, 2007. doi: 10.1016/j.neuron.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 508.Figley CR. Lactate transport and metabolism in the human brain: implications for the astrocyte-neuron lactate shuttle hypothesis. J Neurosci 31: 4768–4770, 2011. doi: 10.1523/JNEUROSCI.6612-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Figueroa XF, Lillo MA, Gaete PS, Riquelme MA, Sáez JC. Diffusion of nitric oxide across cell membranes of the vascular wall requires specific connexin-based channels. Neuropharmacology 75: 471–478, 2013. doi: 10.1016/j.neuropharm.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 510.Filosa A, Paixão S, Honsek SD, Carmona MA, Becker L, Feddersen B, Gaitanos L, Rudhard Y, Schoepfer R, Klopstock T, Kullander K, Rose CR, Pasquale EB, Klein R. Neuron-glia communication via EphA4/ephrin-A3 modulates LTP through glial glutamate transport. Nat Neurosci 12: 1285–1292, 2009. doi: 10.1038/nn.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci 9: 1397–1403, 2006. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 512.Finkbeiner S. Calcium waves in astrocytes-filling in the gaps. Neuron 8: 1101–1108, 1992. doi: 10.1016/0896-6273(92)90131-V. [DOI] [PubMed] [Google Scholar]
- 513.Florian C, Vecsey CG, Halassa MM, Haydon PG, Abel T. Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. J Neurosci 31: 6956–6962, 2011. doi: 10.1523/JNEUROSCI.5761-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Fonseca MI, Chu S, Pierce AL, Brubaker WD, Hauhart RE, Mastroeni D, Clarke EV, Rogers J, Atkinson JP, Tenner AJ. Analysis of the putative role of CR1 in Alzheimer’s disease: genetic association, expression and function. PLoS One 11: e0149792, 2016. doi: 10.1371/journal.pone.0149792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Fossat P, Turpin FR, Sacchi S, Dulong J, Shi T, Rivet JM, Sweedler JV, Pollegioni L, Millan MJ, Oliet SH, Mothet JP. Glial d-serine gates NMDA receptors at excitatory synapses in prefrontal cortex. Cereb Cortex 22: 595–606, 2012. doi: 10.1093/cercor/bhr130. [DOI] [PubMed] [Google Scholar]
- 516.Franke H, Grosche J, Schädlich H, Krügel U, Allgaier C, Illes P. P2X receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience 108: 421–429, 2001. doi: 10.1016/S0306-4522(01)00416-X. [DOI] [PubMed] [Google Scholar]
- 517.Franke H, Günther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D, Illes P. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol 63: 686–699, 2004. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- 518.Franke H, Krügel U, Grosche J, Heine C, Härtig W, Allgaier C, Illes P. P2Y receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience 127: 431–441, 2004. doi: 10.1016/j.neuroscience.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 519.Franke H, Sauer C, Rudolph C, Krügel U, Hengstler JG, Illes P. P2 receptor-mediated stimulation of the PI3-K/Akt-pathway in vivo. Glia 57: 1031–1045, 2009. doi: 10.1002/glia.20827. [DOI] [PubMed] [Google Scholar]
- 520.Franke H, Verkhratsky A, Burnstock G, Illes P. Pathophysiology of astroglial purinergic signalling. Purinergic Signal 8: 629–657, 2012. doi: 10.1007/s11302-012-9300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Franken P, Dijk DJ. Circadian clock genes and sleep homeostasis. Eur J Neurosci 29: 1820–1829, 2009. doi: 10.1111/j.1460-9568.2009.06723.x. [DOI] [PubMed] [Google Scholar]
- 522.Franze K, Grosche J, Skatchkov SN, Schinkinger S, Foja C, Schild D, Uckermann O, Travis K, Reichenbach A, Guck J. Muller cells are living optical fibers in the vertebrate retina. Proc Natl Acad Sci USA 104: 8287–8292, 2007. doi: 10.1073/pnas.0611180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Fraser DD, Duffy S, Angelides KJ, Perez-Velazquez JL, Kettenmann H, MacVicar BA. GABAA/benzodiazepine receptors in acutely isolated hippocampal astrocytes. J Neurosci 15: 2720–2732, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Frayling C, Britton R, Dale N. ATP-mediated glucosensing by hypothalamic tanycytes. J Physiol 589: 2275–2286, 2011. doi: 10.1113/jphysiol.2010.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53: 527–552, 2001. [PMC free article] [PubMed] [Google Scholar]
- 526.Fredholm BB, Chen JF, Masino SA, Vaugeois JM. Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu Rev Pharmacol Toxicol 45: 385–412, 2005. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- 527.Freeman MR. Specification and morphogenesis of astrocytes. Science 330: 774–778, 2010. doi: 10.1126/science.1190928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Freeman MR, Doherty J. Glial cell biology in Drosophila and vertebrates. Trends Neurosci 29: 82–90, 2006. doi: 10.1016/j.tins.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 529.Fremeau RT Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, Reimer RJ, Chaudhry FA, Edwards RH. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci USA 99: 14488–14493, 2002. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Fresu L, Dehpour A, Genazzani AA, Carafoli E, Guerini D. Plasma membrane calcium ATPase isoforms in astrocytes. Glia 28: 150–155, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 531.Friede R. Der quantitative Anteil der Glia an der Cortexentwicklung. Acta Anat (Basel) 20: 290–296, 1954. doi: 10.1159/000140905. [DOI] [PubMed] [Google Scholar]
- 532.Fries JE, Goczalik IM, Wheeler-Schilling TH, Kohler K, Guenther E, Wolf S, Wiedemann P, Bringmann A, Reichenbach A, Francke M, Pannicke T. Identification of P2Y receptor subtypes in human muller glial cells by physiology, single cell RT-PCR, and immunohistochemistry. Invest Ophthalmol Vis Sci 46: 3000–3007, 2005. doi: 10.1167/iovs.05-0043. [DOI] [PubMed] [Google Scholar]
- 533.Fries JE, Wheeler-Schilling TH, Kohler K, Guenther E. Distribution of metabotropic P2Y receptors in the rat retina: a single-cell RT-PCR study. Brain Res Mol Brain Res 130: 1–6, 2004. doi: 10.1016/j.molbrainres.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 534.Frigeri A, Gropper MA, Umenishi F, Kawashima M, Brown D, Verkman AS. Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J Cell Sci 108: 2993–3002, 1995. [DOI] [PubMed] [Google Scholar]
- 535.Froger N, Orellana JA, Cohen-Salmon M, Ezan P, Amigou E, Sáez JC, Giaume C. Cannabinoids prevent the opposite regulation of astroglial connexin43 hemichannels and gap junction channels induced by pro-inflammatory treatments. J Neurochem 111: 1383–1397, 2009. doi: 10.1111/j.1471-4159.2009.06407.x. [DOI] [PubMed] [Google Scholar]
- 536.Frommann C. Untersuchungen über die normale und pathologische Anatomie der Rückenmarks. Jena: Verlag Friedrisc Frommann, 1867. [Google Scholar]
- 537.Frühbeis C, Fröhlich D, Krämer-Albers EM. Emerging roles of exosomes in neuron-glia communication. Front Physiol 3: 119, 2012. doi: 10.3389/fphys.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Füchtbauer L, Groth-Rasmussen M, Holm TH, Løbner M, Toft-Hansen H, Khorooshi R, Owens T. Angiotensin II type 1 receptor (AT1) signaling in astrocytes regulates synaptic degeneration-induced leukocyte entry to the central nervous system. Brain Behav Immun 25: 897–904, 2011. doi: 10.1016/j.bbi.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 539.Fuente-Martín E, García-Cáceres C, Granado M, de Ceballos ML, Sánchez-Garrido MA, Sarman B, Liu ZW, Dietrich MO, Tena-Sempere M, Argente-Arizón P, Díaz F, Argente J, Horvath TL, Chowen JA. Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J Clin Invest 122: 3900–3913, 2012. doi: 10.1172/JCI64102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Fujita T, Chen MJ, Li B, Smith NA, Peng W, Sun W, Toner MJ, Kress BT, Wang L, Benraiss A, Takano T, Wang S, Nedergaard M. Neuronal transgene expression in dominant-negative SNARE mice. J Neurosci 34: 16594–16604, 2014. doi: 10.1523/JNEUROSCI.2585-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Fumagalli M, Brambilla R, D’Ambrosi N, Volonté C, Matteoli M, Verderio C, Abbracchio MP. Nucleotide-mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia 43: 218–03, 2003. doi: 10.1002/glia.10248. [DOI] [PubMed] [Google Scholar]
- 542.Gaberel T, Gakuba C, Goulay R, Martinez De Lizarrondo S, Hanouz JL, Emery E, Touze E, Vivien D, Gauberti M. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis? Stroke 45: 3092–3096, 2014. doi: 10.1161/STROKEAHA.114.006617. [DOI] [PubMed] [Google Scholar]
- 543.Galambos R. A glia-neural theory of brain function. Proc Natl Acad Sci USA 47: 129–136, 1961. doi: 10.1073/pnas.47.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Gallo V, Patrizio M, Levi G. GABA release triggered by the activation of neuron-like non-NMDA receptors in cultured type 2 astrocytes is carrier-mediated. Glia 4: 245–255, 1991. doi: 10.1002/glia.440040302. [DOI] [PubMed] [Google Scholar]
- 545.Gallo V, Suergiu R, Levi G. Kainic acid stimulates GABA release from a subpopulation of cerebellar astrocytes. Eur J Pharmacol 132: 319–322, 1986. doi: 10.1016/0014-2999(86)90624-2. [DOI] [PubMed] [Google Scholar]
- 546.Gandhi GK, Cruz NF, Ball KK, Dienel GA. Astrocytes are poised for lactate trafficking and release from activated brain and for supply of glucose to neurons. J Neurochem 111: 522–536, 2009. doi: 10.1111/j.1471-4159.2009.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Gao P, Postiglione MP, Krieger TG, Hernandez L, Wang C, Han Z, Streicher C, Papusheva E, Insolera R, Chugh K, Kodish O, Huang K, Simons BD, Luo L, Hippenmeyer S, Shi SH. Deterministic progenitor behavior and unitary production of neurons in the neocortex. Cell 159: 775–788, 2014. doi: 10.1016/j.cell.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.García-Barcina JM, Matute C. Expression of kainate-selective glutamate receptor subunits in glial cells of the adult bovine white matter. Eur J Neurosci 8: 2379–2387, 1996. doi: 10.1111/j.1460-9568.1996.tb01201.x. [DOI] [PubMed] [Google Scholar]
- 549.García-Cáceres C, Quarta C, Varela L, Gao Y, Gruber T, Legutko B, Jastroch M, Johansson P, Ninkovic J, Yi CX, Le Thuc O, Szigeti-Buck K, Cai W, Meyer CW, Pfluger PT, Fernandez AM, Luquet S, Woods SC, Torres-Alemán I, Kahn CR, Götz M, Horvath TL, Tschöp MH. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell 166: 867–880, 2016. doi: 10.1016/j.cell.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.García-Marín V, García-López P, Freire M. Cajal’s contributions to glia research. Trends Neurosci 30: 479–487, 2007. doi: 10.1016/j.tins.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 551.García-Marqués J, López-Mascaraque L. Clonal identity determines astrocyte cortical heterogeneity. Cereb Cortex 23: 1463–1472, 2013. doi: 10.1093/cercor/bhs134. [DOI] [PubMed] [Google Scholar]
- 552.García-Segura LM, Chowen JA, Párducz A, Naftolin F. Gonadal hormones as promoters of structural synaptic plasticity: cellular mechanisms. Prog Neurobiol 44: 279–307, 1994. doi: 10.1016/0301-0082(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 553.García ML, Salazar K, Millán C, Rodríguez F, Montecinos H, Caprile T, Silva C, Cortes C, Reinicke K, Vera JC, Aguayo LG, Olate J, Molina B, Nualart F. Sodium vitamin C cotransporter SVCT2 is expressed in hypothalamic glial cells. Glia 50: 32–47, 2005. doi: 10.1002/glia.20133. [DOI] [PubMed] [Google Scholar]
- 554.Garré JM, Retamal MA, Cassina P, Barbeito L, Bukauskas FF, Sáez JC, Bennett MV, Abudara V. FGF-1 induces ATP release from spinal astrocytes in culture and opens pannexin and connexin hemichannels. Proc Natl Acad Sci USA 107: 22659–22664, 2010. doi: 10.1073/pnas.1013793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Gasque P, Chan P, Fontaine M, Ischenko A, Lamacz M, Götze O, Morgan BP. Identification and characterization of the complement C5a anaphylatoxin receptor on human astrocytes. J Immunol 155: 4882–4889, 1995. [PubMed] [Google Scholar]
- 556.Gasque P, Chan P, Mauger C, Schouft MT, Singhrao S, Dierich MP, Morgan BP, Fontaine M. Identification and characterization of complement C3 receptors on human astrocytes. J Immunol 156: 2247–2255, 1996. [PubMed] [Google Scholar]
- 557.Gasque P, Singhrao SK, Neal JW, Wang P, Sayah S, Fontaine M, Morgan BP. The receptor for complement anaphylatoxin C3a is expressed by myeloid cells and nonmyeloid cells in inflamed human central nervous system: analysis in multiple sclerosis and bacterial meningitis. J Immunol 160: 3543–3554, 1998. [PubMed] [Google Scholar]
- 558.Gautron S, Dos Santos G, Pinto-Henrique D, Koulakoff A, Gros F, Berwald-Netter Y. The glial voltage-gated sodium channel: cell- and tissue-specific mRNA expression. Proc Natl Acad Sci USA 89: 7272–7276, 1992. doi: 10.1073/pnas.89.15.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Gavrilyuk V, Kalinin S, Hilbush BS, Middlecamp A, McGuire S, Pelligrino D, Weinberg G, Feinstein DL. Identification of complement 5a-like receptor (C5L2) from astrocytes: characterization of anti-inflammatory properties. J Neurochem 92: 1140–1149, 2005. doi: 10.1111/j.1471-4159.2004.02942.x. [DOI] [PubMed] [Google Scholar]
- 560.Ge WP, Jia JM. Local production of astrocytes in the cerebral cortex. Neuroscience 323: 3–9, 2016. doi: 10.1016/j.neuroscience.2015.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Ge WP, Miyawaki A, Gage FH, Jan YN, Jan LY. Local generation of glia is a major astrocyte source in postnatal cortex. Nature 484: 376–380, 2012. doi: 10.1038/nature10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Gebremedhin D, Yamaura K, Zhang C, Bylund J, Koehler RC, Harder DR. Metabotropic glutamate receptor activation enhances the activities of two types of Ca2+-activated K+ channels in rat hippocampal astrocytes. J Neurosci 23: 1678–1687, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15: 193–204, 1995. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 564.Genda EN, Jackson JG, Sheldon AL, Locke SF, Greco TM, O’Donnell JC, Spruce LA, Xiao R, Guo W, Putt M, Seeholzer S, Ischiropoulos H, Robinson MB. Co-compartmentalization of the astroglial glutamate transporter, GLT-1, with glycolytic enzymes and mitochondria. J Neurosci 31: 18275–18288, 2011. doi: 10.1523/JNEUROSCI.3305-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Gendron FP, Neary JT, Theiss PM, Sun GY, Gonzalez FA, Weisman GA. Mechanisms of P2X7 receptor-mediated ERK1/2 phosphorylation in human astrocytoma cells. Am J Physiol Cell Physiol 284: C571–C581, 2003. doi: 10.1152/ajpcell.00286.2002. [DOI] [PubMed] [Google Scholar]
- 566.Genoud C, Quairiaux C, Steiner P, Hirling H, Welker E, Knott GW. Plasticity of astrocytic coverage and glutamate transporter expression in adult mouse cortex. PLoS Biol 4: e343, 2006. doi: 10.1371/journal.pbio.0040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Genova JL, Fehon RG. Neuroglian, gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila. J Cell Biol 161: 979–989, 2003. doi: 10.1083/jcb.200212054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.George AL Jr, Knittle TJ, Tamkun MM. Molecular cloning of an atypical voltage-gated sodium channel expressed in human heart and uterus: evidence for a distinct gene family. Proc Natl Acad Sci USA 89: 4893–4897, 1992. doi: 10.1073/pnas.89.11.4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Giaume C, Cordier J, Glowinski J. Endothelins Inhibit Junctional Permeability in Cultured Mouse Astrocytes. Eur J Neurosci 4: 877–881, 1992. doi: 10.1111/j.1460-9568.1992.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 570.Giaume C, Fromaget C, el Aoumari A, Cordier J, Glowinski J, Gros D. Gap junctions in cultured astrocytes: single-channel currents and characterization of channel-forming protein. Neuron 6: 133–143, 1991. doi: 10.1016/0896-6273(91)90128-M. [DOI] [PubMed] [Google Scholar]
- 571.Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci 11: 87–99, 2010. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- 572.Giaume C, Leybaert L, Naus CC, Sáez JC. Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and roles. Front Pharmacol 4: 88, 2013. doi: 10.3389/fphar.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Gibbs ME, Anderson DG, Hertz L. Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia 54: 214–222, 2006. doi: 10.1002/glia.20377. [DOI] [PubMed] [Google Scholar]
- 574.Gibbs ME, Hertz L. Importance of glutamate-generating metabolic pathways for memory consolidation in chicks. J Neurosci Res 81: 293–300, 2005. doi: 10.1002/jnr.20548. [DOI] [PubMed] [Google Scholar]
- 575.Gibbs ME, Hutchinson DS, Summers RJ. Role of β-adrenoceptors in memory consolidation: β3-adrenoceptors act on glucose uptake and β2-adrenoceptors on glycogenolysis. Neuropsychopharmacology 33: 2384–2397, 2008. doi: 10.1038/sj.npp.1301629. [DOI] [PubMed] [Google Scholar]
- 576.Gilbert E, Tang JM, Ludvig N, Bergold PJ. Elevated lactate suppresses neuronal firing in vivo and inhibits glucose metabolism in hippocampal slice cultures. Brain Res 1117: 213–223, 2006. doi: 10.1016/j.brainres.2006.07.107. [DOI] [PubMed] [Google Scholar]
- 577.Gilman AG, Schrier BK. Adenosine cyclic 3′,5′-monophosphate in fetal rat brain cell cultures. I. Effect of catecholamines. Mol Pharmacol 8: 410–416, 1972. [PubMed] [Google Scholar]
- 578.Gimpl G, Walz W, Ohlemeyer C, Kettenmann H. Bradykinin receptors in cultured astrocytes from neonatal rat brain are linked to physiological responses. Neurosci Lett 144: 139–142, 1992. doi: 10.1016/0304-3940(92)90735-P. [DOI] [PubMed] [Google Scholar]
- 579.Glees P. Neuroglia Morphology and Function. Oxford: Blackwell, 1955. [Google Scholar]
- 580.Gobron S, Monnerie H, Meiniel R, Creveaux I, Lehmann W, Lamalle D, Dastugue B, Meiniel A. SCO-spondin: a new member of the thrombospondin family secreted by the subcommissural organ is a candidate in the modulation of neuronal aggregation. J Cell Sci 109: 1053–1061, 1996. [DOI] [PubMed] [Google Scholar]
- 581.Gochenauer GE, Robinson MB. Dibutyryl-cAMP (dbcAMP) up-regulates astrocytic chloride-dependent L-[3H]glutamate transport and expression of both system xc(-) subunits. J Neurochem 78: 276–286, 2001. doi: 10.1046/j.1471-4159.2001.00385.x. [DOI] [PubMed] [Google Scholar]
- 582.Goldman SA, Nedergaard M, Windrem MS. Glial progenitor cell-based treatment and modeling of neurological disease. Science 338: 491–495, 2012. doi: 10.1126/science.1218071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Golgi C. Opera Omnia. Milano: Hoepli, 1903. [Google Scholar]
- 584.Golgi C. Sulla fina anatomia degli organi centrali del systema nervoso.Studi di Camillo Golgi Professore di Pathologia Generale e Istologia nell’Universita di Pavia (con 24 tavole). Reggio Emilia: Tip. S. Calderini e Figlio, 1885. [Google Scholar]
- 585.Golgi C. Sulla sostanza connettiva del cervello (nevroglia). Rendiconti del R Instituto Lombardo di Scienze e Lettere 3: 275–277, 1870. [Google Scholar]
- 586.Golovina V, Song H, James P, Lingrel J, Blaustein M. Regulation of Ca2+ signaling by Na+ pump α-2 subunit expression. Ann N Y Acad Sci 986: 509–513, 2003. doi: 10.1111/j.1749-6632.2003.tb07236.x. [DOI] [PubMed] [Google Scholar]
- 587.Golovina VA. Visualization of localized store-operated calcium entry in mouse astrocytes. Close proximity to the endoplasmic reticulum. J Physiol 564: 737–749, 2005. doi: 10.1113/jphysiol.2005.085035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Golovina VA, Bambrick LL, Yarowsky PJ, Krueger BK, Blaustein MP. Modulation of two functionally distinct Ca2+ stores in astrocytes: role of the plasmalemmal Na/Ca exchanger. Glia 16: 296–305, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 589.Golovina VA, Song H, James PF, Lingrel JB, Blaustein MP. Na+ pump α 2-subunit expression modulates Ca2+ signaling. Am J Physiol Cell Physiol 284: C475–C486, 2003. doi: 10.1152/ajpcell.00383.2002. [DOI] [PubMed] [Google Scholar]
- 590.Golubev AI. Glia and neuroglia relationships in the cerebral nervous system of the Turbellaria (electron microscopic data). Fortschr Zool 36: 31–37, 1988. [Google Scholar]
- 591.Gómez-Gonzalo M, Losi G, Chiavegato A, Zonta M, Cammarota M, Brondi M, Vetri F, Uva L, Pozzan T, de Curtis M, Ratto GM, Carmignoto G. An excitatory loop with astrocytes contributes to drive neurons to seizure threshold. PLoS Biol 8: e1000352, 2010. doi: 10.1371/journal.pbio.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Gómez-Gonzalo M, Navarrete M, Perea G, Covelo A, Martín-Fernández M, Shigemoto R, Luján R, Araque A. Endocannabinoids induce lateral long-term potentiation of transmitter release by stimulation of gliotransmission. Cereb Cortex 25: 3699–3712, 2015. doi: 10.1093/cercor/bhu231. [DOI] [PubMed] [Google Scholar]
- 593.González D, Gómez-Hernández JM, Barrio LC. Species specificity of mammalian connexin-26 to form open voltage-gated hemichannels. FASEB J 20: 2329–2338, 2006. doi: 10.1096/fj.06-5828com. [DOI] [PubMed] [Google Scholar]
- 594.Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci 8: 1078–1086, 2005. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- 595.Gordon GR, Iremonger KJ, Kantevari S, Ellis-Davies GC, MacVicar BA, Bains JS. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron 64: 391–403, 2009. doi: 10.1016/j.neuron.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Gossman DG, Zhao HB. Hemichannel-mediated inositol 1,4,5-trisphosphate (IP3) release in the cochlea: a novel mechanism of IP3 intercellular signaling. Cell Commun Adhes 15: 305–315, 2008. doi: 10.1080/15419060802357217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Götz M, Sirko S, Beckers J, Irmler M. Reactive astrocytes as neural stem or progenitor cells: in vivo lineage, in vitro potential, and genome-wide expression analysis. Glia 63: 1452–1468, 2015. doi: 10.1002/glia.22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science 329: 571–575, 2010. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Grabham P, Cunningham DD. Thrombin receptor activation stimulates astrocyte proliferation and reversal of stellation by distinct pathways: involvement of tyrosine phosphorylation. J Neurochem 64: 583–591, 1995. doi: 10.1046/j.1471-4159.1995.64020583.x. [DOI] [PubMed] [Google Scholar]
- 600.Gracy KN, Pickel VM. Comparative ultrastructural localization of the NMDAR1 glutamate receptor in the rat basolateral amygdala and bed nucleus of the stria terminalis. J Comp Neurol 362: 71–85, 1995. doi: 10.1002/cne.903620105. [DOI] [PubMed] [Google Scholar]
- 601.Graham AJ, Ray MA, Perry EK, Jaros E, Perry RH, Volsen SG, Bose S, Evans N, Lindstrom J, Court JA. Differential nicotinic acetylcholine receptor subunit expression in the human hippocampus. J Chem Neuroanat 25: 97–113, 2003. doi: 10.1016/S0891-0618(02)00100-X. [DOI] [PubMed] [Google Scholar]
- 602.Gray PT, Ritchie JM. A voltage-gated chloride conductance in rat cultured astrocytes. Proc R Soc Lond B Biol Sci 228: 267–288, 1986. doi: 10.1098/rspb.1986.0055. [DOI] [PubMed] [Google Scholar]
- 603.Grichtchenko II, Chesler M. Depolarization-induced alkalinization of astrocytes in gliotic hippocampal slices. Neuroscience 62: 1071–1078, 1994. doi: 10.1016/0306-4522(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 604.Griemsmann S, Höft SP, Bedner P, Zhang J, von Staden E, Beinhauer A, Degen J, Dublin P, Cope DW, Richter N, Crunelli V, Jabs R, Willecke K, Theis M, Seifert G, Kettenmann H, Steinhäuser C. Characterization of panglial gap junction networks in the thalamus, neocortex, and hippocampus reveals a unique population of glial cells. Cereb Cortex 25: 3420–3433, 2015. doi: 10.1093/cercor/bhu157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Grimaldi M, Cavallaro S. Functional and molecular diversity of PACAP/VIP receptors in cortical neurons and type I astrocytes. Eur J Neurosci 11: 2767–2772, 1999. doi: 10.1046/j.1460-9568.1999.00693.x. [DOI] [PubMed] [Google Scholar]
- 606.Grimaldi M, Maratos M, Verma A. Transient receptor potential channel activation causes a novel form of [Ca 2+]i oscillations and is not involved in capacitative Ca 2+ entry in glial cells. J Neurosci 23: 4737–4745, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Grolla AA, Sim JA, Lim D, Rodriguez JJ, Genazzani AA, Verkhratsky A. Amyloid-β and Alzheimer’s disease type pathology differentially affects the calcium signalling toolkit in astrocytes from different brain regions. Cell Death Dis 4: e623, 2013. doi: 10.1038/cddis.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Grosche J, Kettenmann H, Reichenbach A. Bergmann glial cells form distinct morphological structures to interact with cerebellar neurons. J Neurosci Res 68: 138–149, 2002. doi: 10.1002/jnr.10197. [DOI] [PubMed] [Google Scholar]
- 609.Grosche J, Matyash V, Möller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci 2: 139–143, 1999. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- 610.Grosjean Y, Grillet M, Augustin H, Ferveur JF, Featherstone DE. A glial amino-acid transporter controls synapse strength and courtship in Drosophila. Nat Neurosci 11: 54–61, 2008. doi: 10.1038/nn2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Grupp L, Wolburg H, Mack AF. Astroglial structures in the zebrafish brain. J Comp Neurol 518: 4277–4287, 2010. doi: 10.1002/cne.22481. [DOI] [PubMed] [Google Scholar]
- 612.Guadaño-Ferraz A, Obregón MJ, St Germain DL, Bernal J. The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain. Proc Natl Acad Sci USA 94: 10391–10396, 1997. doi: 10.1073/pnas.94.19.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and glioblastoma cells release exosomes carrying mtDNA. J Neural Transm (Vienna) 117: 1–4, 2010. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 614.Guidetti P, Hoffman GE, Melendez-Ferro M, Albuquerque EX, Schwarcz R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia 55: 78–92, 2007. doi: 10.1002/glia.20432. [DOI] [PubMed] [Google Scholar]
- 615.Guidetti P, Okuno E, Schwarcz R. Characterization of rat brain kynurenine aminotransferases I and II. J Neurosci Res 50: 457–465, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 616.Guizzetti M, Moore NH, Giordano G, Costa LG. Modulation of neuritogenesis by astrocyte muscarinic receptors. J Biol Chem 283: 31884–31897, 2008. doi: 10.1074/jbc.M801316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Guizzetti M, Moore NH, Giordano G, VanDeMark KL, Costa LG. Ethanol inhibits neuritogenesis induced by astrocyte muscarinic receptors. Glia 58: 1395–1406, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Gundersen GA, Vindedal GF, Skare O, Nagelhus EA. Evidence that pericytes regulate aquaporin-4 polarization in mouse cortical astrocytes. Brain Struct Funct 219: 2181–2186, 2014. doi: 10.1007/s00429-013-0629-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci 19: 520–528, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Haas B, Schipke CG, Peters O, Söhl G, Willecke K, Kettenmann H. Activity-dependent ATP-waves in the mouse neocortex are independent from astrocytic calcium waves. Cereb Cortex 16: 237–246, 2006. doi: 10.1093/cercor/bhi101. [DOI] [PubMed] [Google Scholar]
- 621.Habbas S, Santello M, Becker D, Stubbe H, Zappia G, Liaudet N, Klaus FR, Kollias G, Fontana A, Pryce CR, Suter T, Volterra A. Neuroinflammatory TNFα impairs memory via astrocyte signaling. Cell 163: 1730–1741, 2015. doi: 10.1016/j.cell.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 622.Hachem S, Laurenson AS, Hugnot JP, Legraverend C. Expression of S100B during embryonic development of the mouse cerebellum. BMC Dev Biol 7: 17, 2007. doi: 10.1186/1471-213X-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Haj-Yasein NN, Jensen V, Østby I, Omholt SW, Voipio J, Kaila K, Ottersen OP, Hvalby Ø, Nagelhus EA. Aquaporin-4 regulates extracellular space volume dynamics during high-frequency synaptic stimulation: a gene deletion study in mouse hippocampus. Glia 60: 867–874, 2012. doi: 10.1002/glia.22319. [DOI] [PubMed] [Google Scholar]
- 624.Haj-Yasein NN, Vindedal GF, Eilert-Olsen M, Gundersen GA, Skare Ø, Laake P, Klungland A, Thorén AE, Burkhardt JM, Ottersen OP, Nagelhus EA. Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc Natl Acad Sci USA 108: 17815–17820, 2011. doi: 10.1073/pnas.1110655108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Hajek I, Subbarao KV, Hertz L. Acute and chronic effects of potassium and noradrenaline on Na+,K+-ATPase activity in cultured mouse neurons and astrocytes. Neurochem Int 28: 335–342, 1996. doi: 10.1016/0197-0186(95)00081-X. [DOI] [PubMed] [Google Scholar]
- 626.Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 13: 54–63, 2007. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 627.Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J Neurosci 27: 6473–6477, 2007. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61: 213–219, 2009. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Halestrap AP. The monocarboxylate transporter family–structure and functional characterization. IUBMB Life 64: 1–9, 2012. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- 630.Hamel D, Sanchez M, Duhamel F, Roy O, Honoré JC, Noueihed B, Zhou T, Nadeau-Vallée M, Hou X, Lavoie JC, Mitchell G, Mamer OA, Chemtob S. G-protein-coupled receptor 91 and succinate are key contributors in neonatal postcerebral hypoxia-ischemia recovery. Arterioscler Thromb Vasc Biol 34: 285–293, 2014. doi: 10.1161/ATVBAHA.113.302131. [DOI] [PubMed] [Google Scholar]
- 631.Hamilton N, Vayro S, Kirchhoff F, Verkhratsky A, Robbins J, Gorecki DC, Butt AM. Mechanisms of ATP- and glutamate-mediated calcium signaling in white matter astrocytes. Glia 56: 734–749, 2008. doi: 10.1002/glia.20649. [DOI] [PubMed] [Google Scholar]
- 632.Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci 11: 227–238, 2010. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- 633.Hamilton SL, Serysheva II. Ryanodine receptor structure: progress and challenges. J Biol Chem 284: 4047–4051, 2009. doi: 10.1074/jbc.R800054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, Koehl M, Abrous DN, Mendizabal-Zubiaga J, Grandes P, Liu Q, Bai G, Wang W, Xiong L, Ren W, Marsicano G, Zhang X. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148: 1039–1050, 2012. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 635.Han KS, Mannaioni G, Hamill CE, Lee J, Junge CE, Lee CJ, Traynelis SF. Activation of protease activated receptor 1 increases the excitability of the dentate granule neurons of hippocampus. Mol Brain 4: 32, 2011. doi: 10.1186/1756-6606-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.Han KS, Woo J, Park H, Yoon BJ, Choi S, Lee CJ. Channel-mediated astrocytic glutamate release via Bestrophin-1 targets synaptic NMDARs. Mol Brain 6: 4, 2013. doi: 10.1186/1756-6606-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, Silva AJ, Takano T, Goldman SA, Nedergaard M. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12: 342–353, 2013. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Hanner F, Sorensen CM, Holstein-Rathlou NH, Peti-Peterdi J. Connexins and the kidney. Am J Physiol Regul Integr Comp Physiol 298: R1143–R1155, 2010. doi: 10.1152/ajpregu.00808.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev 65: 101–148, 1985. [DOI] [PubMed] [Google Scholar]
- 640.Hansen DB, Garrido-Comas N, Salter M, Fern R. HCO3−-independent pH regulation in astrocytes in situ is dominated by V-ATPase. J Biol Chem 290: 8039–8047, 2015. doi: 10.1074/jbc.M115.636597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Hanson E, Armbruster M, Cantu D, Andresen L, Taylor A, Danbolt NC, Dulla CG. Astrocytic glutamate uptake is slow and does not limit neuronal NMDA receptor activation in the neonatal neocortex. Glia 63: 1784–1796, 2015. doi: 10.1002/glia.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Hansson E. Transport of monoamine and amino acid neurotransmitters by primary astroglial cultures. Neurochem Res 10: 667–675, 1985. doi: 10.1007/BF00964405. [DOI] [PubMed] [Google Scholar]
- 643.Hanukoglu I, Hanukoglu A. Epithelial sodium channel (ENaC) family: phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene 579: 95–132, 2016. doi: 10.1016/j.gene.2015.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Harden TK, McCarthy KD. Identification of the beta adrenergic receptor subtype on astroglia purified from rat brain. J Pharmacol Exp Ther 222: 600–605, 1982. [PubMed] [Google Scholar]
- 645.Harder DR, Alkayed NJ, Lange AR, Gebremedhin D, Roman RJ. Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke 29: 229–234, 1998. doi: 10.1161/01.STR.29.1.229. [DOI] [PubMed] [Google Scholar]
- 646.Harder DR, Zhang C, Gebremedhin D. Astrocytes function in matching blood flow to metabolic activity. News Physiol Sci 17: 27–31, 2002. [DOI] [PubMed] [Google Scholar]
- 647.Hardie RC. A brief history of trp: commentary and personal perspective. Pflugers Arch 461: 493–498, 2011. doi: 10.1007/s00424-011-0922-9. [DOI] [PubMed] [Google Scholar]
- 648.Harrington MG, Salomon RM, Pogoda JM, Oborina E, Okey N, Johnson B, Schmidt D, Fonteh AN, Dalleska NF. Cerebrospinal fluid sodium rhythms. Cerebrospinal Fluid Res 7: 3, 2010. doi: 10.1186/1743-8454-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Harris-White ME, Zanotti SA, Frautschy SA, Charles AC. Spiral intercellular calcium waves in hippocampal slice cultures. J Neurophysiol 79: 1045–1052, 1998. [DOI] [PubMed] [Google Scholar]
- 650.Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol 94: 120–143, 2007. doi: 10.1016/j.pbiomolbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Härtel K, Schnell C, Hülsmann S. Astrocytic calcium signals induced by neuromodulators via functional metabotropic receptors in the ventral respiratory group of neonatal mice. Glia 57: 815–827, 2009. doi: 10.1002/glia.20808. [DOI] [PubMed] [Google Scholar]
- 652.Hartenstein V. Morphological diversity and development of glia in Drosophila. Glia 59: 1237–1252, 2011. doi: 10.1002/glia.21162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Hartline DK. The evolutionary origins of glia. Glia 59: 1215–1236, 2011. doi: 10.1002/glia.21149. [DOI] [PubMed] [Google Scholar]
- 654.Hartz AM, Bauer B. ABC transporters in the CNS - an inventory. Curr Pharm Biotechnol 12: 656–673, 2011. doi: 10.2174/138920111795164020. [DOI] [PubMed] [Google Scholar]
- 655.Hartzell HC, Qu Z, Yu K, Xiao Q, Chien LT. Molecular physiology of bestrophins: multifunctional membrane proteins linked to best disease and other retinopathies. Physiol Rev 88: 639–672, 2008. doi: 10.1152/physrev.00022.2007. [DOI] [PubMed] [Google Scholar]
- 656.Hashimoto H, Kunugi A, Arakawa N, Shintani N, Fujita T, Kasai A, Kawaguchi C, Morita Y, Hirose M, Sakai Y, Baba A. Possible involvement of a cyclic AMP-dependent mechanism in PACAP-induced proliferation and ERK activation in astrocytes. Biochem Biophys Res Commun 311: 337–343, 2003. doi: 10.1016/j.bbrc.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 657.Hashimoto T, Hussien R, Cho HS, Kaufer D, Brooks GA. Evidence for the mitochondrial lactate oxidation complex in rat neurons: demonstration of an essential component of brain lactate shuttles. PLoS One 3: e2915, 2008. doi: 10.1371/journal.pone.0002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.Hasselblatt M, Bunte M, Dringen R, Tabernero A, Medina JM, Giaume C, Sirén AL, Ehrenreich H. Effect of endothelin-1 on astrocytic protein content. Glia 42: 390–397, 2003. doi: 10.1002/glia.10224. [DOI] [PubMed] [Google Scholar]
- 659.Hassinger TD, Guthrie PB, Atkinson PB, Bennett MV, Kater SB. An extracellular signaling component in propagation of astrocytic calcium waves. Proc Natl Acad Sci USA 93: 13268–13273, 1996. doi: 10.1073/pnas.93.23.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Hatton GI. Astroglial modulation of neurotransmitter/peptide release from the neurohypophysis: present status. J Chem Neuroanat 16: 203–221, 1999. doi: 10.1016/S0891-0618(98)00067-2. [DOI] [PubMed] [Google Scholar]
- 661.Hatton GI. Pituicytes, glia and control of terminal secretion. J Exp Biol 139: 67–79, 1988. [DOI] [PubMed] [Google Scholar]
- 662.Hatton GI, Bicknell RJ, Hoyland J, Bunting R, Mason WT. Arginine vasopressin mobilises intracellular calcium via V1-receptor activation in astrocytes (pituicytes) cultured from adult rat neural lobes. Brain Res 588: 75–83, 1992. doi: 10.1016/0006-8993(92)91346-G. [DOI] [PubMed] [Google Scholar]
- 663.Haustein MD, Kracun S, Lu XH, Shih T, Jackson-Weaver O, Tong X, Xu J, Yang XW, O’Dell TJ, Marvin JS, Ellisman MH, Bushong EA, Looger LL, Khakh BS. Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron 82: 413–429, 2014. doi: 10.1016/j.neuron.2014.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Hawkins A, Olszewski J. Glia/nerve cell index for cortex of the whale. Science 126: 76–77, 1957. doi: 10.1126/science.126.3263.76. [DOI] [PubMed] [Google Scholar]
- 665.He F, Ge W, Martinowich K, Becker-Catania S, Coskun V, Zhu W, Wu H, Castro D, Guillemot F, Fan G, de Vellis J, Sun YE. A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat Neurosci 8: 616–625, 2005. doi: 10.1038/nn1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 666.Heilbrunn LV. An Outline of General Physiology. Philadelphia: Saunders, 1943. [Google Scholar]
- 667.Heinemann U, Lux HD. Ceiling of stimulus induced rises in extracellular potassium concentration in the cerebral cortex of cat. Brain Res 120: 231–249, 1977. doi: 10.1016/0006-8993(77)90903-9. [DOI] [PubMed] [Google Scholar]
- 668.Heinemann U, Lux HD. Undershoots following stimulus-induced rises of extracellular potassium concentration in cerebral cortex of cat. Brain Res 93: 63–76, 1975. doi: 10.1016/0006-8993(75)90286-3. [DOI] [PubMed] [Google Scholar]
- 669.Heinemann U, Schaible HG, Schmidt RF. Changes in extracellular potassium concentration in cat spinal cord in response to innocuous and noxious stimulation of legs with healthy and inflamed knee joints. Exp Brain Res 79: 283–292, 1990. doi: 10.1007/BF00608237. [DOI] [PubMed] [Google Scholar]
- 670.Héja L, Barabás P, Nyitrai G, Kékesi KA, Lasztóczi B, Toke O, Tárkányi G, Madsen K, Schousboe A, Dobolyi A, Palkovits M, Kardos J. Glutamate uptake triggers transporter-mediated GABA release from astrocytes. PLoS One 4: e7153, 2009. doi: 10.1371/journal.pone.0007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Held H. Über die Neuroglia marginalis der menschlichen Grosshirnrinde. Monatschr f Psychol u Neurol 26: 360–416, 1909. doi: 10.1159/000209823. [DOI] [Google Scholar]
- 672.Heller JP, Rusakov DA. Morphological plasticity of astroglia: understanding synaptic microenvironment. Glia 63: 2133–2151, 2015. doi: 10.1002/glia.22821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Heni M, Hennige AM, Peter A, Siegel-Axel D, Ordelheide AM, Krebs N, Machicao F, Fritsche A, Häring HU, Staiger H. Insulin promotes glycogen storage and cell proliferation in primary human astrocytes. PLoS One 6: e21594, 2011. doi: 10.1371/journal.pone.0021594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674.Henn FA, Haljamäe H, Hamberger A. Glial cell function: active control of extracellular K+ concentration. Brain Res 43: 437–443, 1972. doi: 10.1016/0006-8993(72)90399-X. [DOI] [PubMed] [Google Scholar]
- 675.Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of d-serine from astrocytes. Nature 463: 232–236, 2010. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Herculano-Houzel S. The glia/neuron ratio: how it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia 62: 1377–1391, 2014. doi: 10.1002/glia.22683. [DOI] [PubMed] [Google Scholar]
- 677.Herman MA, Jahr CE. Extracellular glutamate concentration in hippocampal slice. J Neurosci 27: 9736–9741, 2007. doi: 10.1523/JNEUROSCI.3009-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Hertle DN, Yeckel MF. Distribution of inositol-1,4,5-trisphosphate receptor isotypes and ryanodine receptor isotypes during maturation of the rat hippocampus. Neuroscience 150: 625–638, 2007. doi: 10.1016/j.neuroscience.2007.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Hertz L. Astrocytic energy metabolism and glutamate formation–relevance for 13C-NMR spectroscopy and importance of cytosolic/mitochondrial trafficking. Magn Reson Imaging 29: 1319–1329, 2011. doi: 10.1016/j.mri.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 680.Hertz L. The glutamate-glutamine (GABA) cycle: importance of late postnatal development and potential reciprocal interactions between biosynthesis and degradation. Front Endocrinol (Lausanne) 4: 59, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Hertz L. Inhibition by barbiturates of an intense net uptake of potassium into astrocytes. Neuropharmacology 18: 629–632, 1979. doi: 10.1016/0028-3908(79)90116-3. [DOI] [PubMed] [Google Scholar]
- 682.Hertz L. Possible role of neuroglia: a potassium-mediated neuronal-neuroglial-neuronal impulse transmission system. Nature 206: 1091–1094, 1965. doi: 10.1038/2061091a0. [DOI] [PubMed] [Google Scholar]
- 683.Hertz L, Chen Y. Importance of astrocytes for potassium ion (K+) homeostasis in brain and glial effects of K+ and its transporters on learning. Neurosci Biobehav Rev 71: 484–505, 2016. doi: 10.1016/j.neubiorev.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 684.Hertz L, Chen Y, Gibbs ME, Zang P, Peng L. Astrocytic adrenoceptors: a major drug target in neurological and psychiatric disorders? Curr Drug Targets CNS Neurol Disord 3: 239–267, 2004. doi: 10.2174/1568007043337535. [DOI] [PubMed] [Google Scholar]
- 685.Hertz L, Dringen R, Schousboe A, Robinson SR. Astrocytes: glutamate producers for neurons. J Neurosci Res 57: 417–428, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 686.Hertz L, Gerkau NJ, Xu J, Durry S, Song D, Rose CR, Peng L. Roles of astrocytic Na+,K+-ATPase and glycogenolysis for K+ homeostasis in mammalian brain. J Neurosci Res 93: 1019–1030, 2015. doi: 10.1002/jnr.23499. [DOI] [PubMed] [Google Scholar]
- 687.Hertz L, Lovatt D, Goldman SA, Nedergaard M. Adrenoceptors in brain: cellular gene expression and effects on astrocytic metabolism and [Ca2+]i. Neurochem Int 57: 411–420, 2010. doi: 10.1016/j.neuint.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Hertz L, Rothman DL, Li B, Peng L. Chronic SSRI stimulation of astrocytic 5-HT2B receptors change multiple gene expressions/editings and metabolism of glutamate, glucose and glycogen: a potential paradigm shift. Front Behav Neurosci 9: 25, 2015. doi: 10.3389/fnbeh.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Hertz L, Schousboe I, Hertz L, Schousboe A. Receptor expression in primary cultures of neurons or astrocytes. Prog Neuropsychopharmacol Biol Psychiatry 8: 521–527, 1984. doi: 10.1016/0278-5846(84)90010-1. [DOI] [PubMed] [Google Scholar]
- 690.Hertz L, Song D, Xu J, Peng L, Gibbs ME. Role of the astrocytic Na+, K+-ATPase in K+ homeostasis in brain: K+ uptake, signaling pathways and substrate utilization. Neurochem Res 40: 2505–2516, 2015. doi: 10.1007/s11064-014-1505-x. [DOI] [PubMed] [Google Scholar]
- 691.Hervé JC, Derangeon M. Gap-junction-mediated cell-to-cell communication. Cell Tissue Res 352: 21–31, 2013. doi: 10.1007/s00441-012-1485-6. [DOI] [PubMed] [Google Scholar]
- 692.Hestrin S, Sah P, Nicoll RA. Mechanisms generating the time course of dual component excitatory synaptic currents recorded in hippocampal slices. Neuron 5: 247–253, 1990. doi: 10.1016/0896-6273(90)90162-9. [DOI] [PubMed] [Google Scholar]
- 693.Hiasa M, Miyaji T, Haruna Y, Takeuchi T, Harada Y, Moriyama S, Yamamoto A, Omote H, Moriyama Y. Identification of a mammalian vesicular polyamine transporter. Sci Rep 4: 6836, 2014. doi: 10.1038/srep06836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Hibino H, Fujita A, Iwai K, Yamada M, Kurachi Y. Differential assembly of inwardly rectifying K+ channel subunits, Kir4.1 and Kir5.1, in brain astrocytes. J Biol Chem 279: 44065–44073, 2004. doi: 10.1074/jbc.M405985200. [DOI] [PubMed] [Google Scholar]
- 695.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90: 291–366, 2010. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 696.Higashi K, Fujita A, Inanobe A, Tanemoto M, Doi K, Kubo T, Kurachi Y. An inwardly rectifying K+ channel, Kir4.1, expressed in astrocytes surrounds synapses and blood vessels in brain. Am J Physiol Cell Physiol 281: C922–C931, 2001. [DOI] [PubMed] [Google Scholar]
- 697.Hild W, Chang JJ, Tasaki I. Electrical responses of astrocytic glia from the mammalian central nervous system cultivated in vitro. Experientia 14: 220–221, 1958. doi: 10.1007/BF02159099. [DOI] [PubMed] [Google Scholar]
- 698.Hilgetag CC, Barbas H. Are there ten times more glia than neurons in the brain? Brain Struct Funct 213: 365–366, 2009. doi: 10.1007/s00429-009-0202-z. [DOI] [PubMed] [Google Scholar]
- 699.Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits α7 nicotinic receptor activity and increases non-α7 nicotinic receptor expression: physiopathological implications. J Neurosci 21: 7463–7473, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Hines DJ, Haydon PG. Inhibition of a SNARE-sensitive pathway in astrocytes attenuates damage following stroke. J Neurosci 33: 4234–4240, 2013. doi: 10.1523/JNEUROSCI.5495-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Hirase H, Qian L, Barthó P, Buzsáki G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol 2: E96, 2004. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Hirrlinger J, Hülsmann S, Kirchhoff F. Astroglial processes show spontaneous motility at active synaptic terminals in situ. Eur J Neurosci 20: 2235–2239, 2004. doi: 10.1111/j.1460-9568.2004.03689.x. [DOI] [PubMed] [Google Scholar]
- 703.Hirrlinger PG, Scheller A, Braun C, Quintela-Schneider M, Fuss B, Hirrlinger J, Kirchhoff F. Expression of reef coral fluorescent proteins in the central nervous system of transgenic mice. Mol Cell Neurosci 30: 291–303, 2005. doi: 10.1016/j.mcn.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 704.Hirst WD, Price GW, Rattray M, Wilkin GP. Identification of 5-hydroxytryptamine receptors positively coupled to adenylyl cyclase in rat cultured astrocytes. Br J Pharmacol 120: 509–515, 1997. doi: 10.1038/sj.bjp.0700921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Hirth IC, Deitmer JW. 5-Hydroxytryptamine-mediated increase in glutamate uptake by the leech giant glial cell. Glia 54: 786–794, 2006. doi: 10.1002/glia.20417. [DOI] [PubMed] [Google Scholar]
- 706.Hiyama TY, Yoshida M, Matsumoto M, Suzuki R, Matsuda T, Watanabe E, Noda M. Endothelin-3 expression in the subfornical organ enhances the sensitivity of Nax, the brain sodium-level sensor, to suppress salt intake. Cell Metab 17: 507–519, 2013. doi: 10.1016/j.cmet.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 707.Ho KW, Lambert WS, Calkins DJ. Activation of the TRPV1 cation channel contributes to stress-induced astrocyte migration. Glia 62: 1435–1451, 2014. doi: 10.1002/glia.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Ho T, Vessey KA, Fletcher EL. Immunolocalization of the P2X4 receptor on neurons and glia in the mammalian retina. Neuroscience 277: 55–71, 2014. doi: 10.1016/j.neuroscience.2014.06.055. [DOI] [PubMed] [Google Scholar]
- 709.Hochachka PW, Dressendorfer RH. Succinate accumulation in man during exercise. Eur J Appl Physiol Occup Physiol 35: 235–242, 1976. doi: 10.1007/BF00423282. [DOI] [PubMed] [Google Scholar]
- 710.Hodgkin AL, Huxley AF. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol 116: 449–472, 1952. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Hofer A, Dermietzel R. Visualization and functional blocking of gap junction hemichannels (connexons) with antibodies against external loop domains in astrocytes. Glia 24: 141–154, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 712.Höfer T, Venance L, Giaume C. Control and plasticity of intercellular calcium waves in astrocytes: a modeling approach. J Neurosci 22: 4850–4859, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci USA 99: 7461–7466, 2002. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Hogan-Cann AD, Anderson CM. Physiological roles of non-neuronal NMDA receptors. Trends Pharmacol Sci 37: 750–767, 2016. doi: 10.1016/j.tips.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 715.Hol EM, Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol 32: 121–130, 2015. doi: 10.1016/j.ceb.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 716.Holmes EG, Holmes BE. Contributions to the study of brain metabolism: carbohydrate metabolism relationship of glycogen and lactic acid. Biochem J 20: 1196–1203, 1926. doi: 10.1042/bj0201196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717.Holmgren E. Beiträge zur Morphologie der Zelle: I. Nervenzellen. Anat Hefte 18: 267–326, 1901. doi: 10.1007/BF02246377. [DOI] [Google Scholar]
- 718.Holtzclaw LA, Pandhit S, Bare DJ, Mignery GA, Russell JT. Astrocytes in adult rat brain express type 2 inositol 1,4,5-trisphosphate receptors. Glia 39: 69–84, 2002. doi: 10.1002/glia.10085. [DOI] [PubMed] [Google Scholar]
- 719.Honsa P, Pivonkova H, Harantova L, Butenko O, Kriska J, Dzamba D, Rusnakova V, Valihrach L, Kubista M, Anderova M. Increased expression of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in reactive astrocytes following ischemia. Glia 62: 2004–2021, 2014. doi: 10.1002/glia.22721. [DOI] [PubMed] [Google Scholar]
- 720.Honsek SD, Walz C, Kafitz KW, Rose CR. Astrocyte calcium signals at Schaffer collateral to CA1 pyramidal cell synapses correlate with the number of activated synapses but not with synaptic strength. Hippocampus 22: 29–42, 2012. doi: 10.1002/hipo.20843. [DOI] [PubMed] [Google Scholar]
- 721.Hoogland TM, Kuhn B. Recent developments in the understanding of astrocyte function in the cerebellum in vivo. Cerebellum 9: 264–271, 2010. doi: 10.1007/s12311-009-0139-z. [DOI] [PubMed] [Google Scholar]
- 722.Hoogland TM, Kuhn B, Göbel W, Huang W, Nakai J, Helmchen F, Flint J, Wang SS. Radially expanding transglial calcium waves in the intact cerebellum. Proc Natl Acad Sci USA 106: 3496–3501, 2009. doi: 10.1073/pnas.0809269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Horvat A, Zorec R, Vardjan N. Adrenergic stimulation of single rat astrocytes results in distinct temporal changes in intracellular Ca2+ and cAMP-dependent PKA responses. Cell Calcium 59: 156–163, 2016. doi: 10.1016/j.ceca.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 724.Hösli E, Hösli L. Autoradiographic evidence for endothelin receptors on astrocytes in cultures of rat cerebellum, brainstem and spinal cord. Neurosci Lett 129: 55–58, 1991. doi: 10.1016/0304-3940(91)90719-A. [DOI] [PubMed] [Google Scholar]
- 725.Hösli E, Hösli L. Autoradiographic localization of binding sites for [3H]histamine and H1- and H2-antagonists on cultured neurones and glial cells. Neuroscience 13: 863–870, 1984. doi: 10.1016/0306-4522(84)90101-5. [DOI] [PubMed] [Google Scholar]
- 726.Hösli E, Hösli L. Autoradiographic localization of binding sites for muscarinic and nicotinic agonists and antagonists on cultured astrocytes. Exp Brain Res 71: 450–454, 1988. doi: 10.1007/BF00247507. [DOI] [PubMed] [Google Scholar]
- 727.Hösli E, Hösli L. Autoradiographic studies on the uptake of 3H-noradrenaline and 3H-GABA in cultured rat cerebellum. Exp Brain Res 26: 319–324, 1976. doi: 10.1007/BF00234935. [DOI] [PubMed] [Google Scholar]
- 728.Hösli E, Hösli L. Binding of cholecystokinin, bombesin and muscarine to neurons and astrocytes in explant cultures of rat central nervous system: autoradiographic and immunohistochemical studies. Neuroscience 61: 63–72, 1994. doi: 10.1016/0306-4522(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 729.Hösli E, Hösli L. Binding sites for [3H]dopamine and dopamine-antagonists on cultured astrocytes of rat striatum and spinal cord: an autoradiographic study. Neurosci Lett 65: 177–182, 1986. doi: 10.1016/0304-3940(86)90300-9. [DOI] [PubMed] [Google Scholar]
- 730.Hösli E, Jurasin K, Rühl W, Lüthy R, Hösli L. Colocalization of androgen, estrogen and cholinergic receptors on cultured astrocytes of rat central nervous system. Int J Dev Neurosci 19: 11–19, 2001. doi: 10.1016/S0736-5748(00)00082-4. [DOI] [PubMed] [Google Scholar]
- 731.Hösli L, Hösli E, Andrès PF, Landolt H. Evidence that the depolarization of glial cells by inhibitory amino acids is caused by an efflux of K+ from neurones. Exp Brain Res 42: 43–48, 1981. doi: 10.1007/BF00235727. [DOI] [PubMed] [Google Scholar]
- 732.Hösli L, Hösli E, Della Briotta G, Quadri L, Heuss L. Action of acetylcholine, muscarine, nicotine and antagonists on the membrane potential of astrocytes in cultured rat brainstem and spinal cord. Neurosci Lett 92: 165–170, 1988. doi: 10.1016/0304-3940(88)90054-7. [DOI] [PubMed] [Google Scholar]
- 733.Hösli L, Hösli E, Schneider U, Wiget W. Evidence for the existence of histamine H1- and H2-receptors on astrocytes of cultured rat central nervous system. Neurosci Lett 48: 287–291, 1984. doi: 10.1016/0304-3940(84)90052-1. [DOI] [PubMed] [Google Scholar]
- 734.Hösli L, Hösli E, Uhr M, Della Briotta G. Electrophysiological evidence for adenosine receptors on astrocytes of cultured rat central nervous system. Neurosci Lett 79: 108–112, 1987. doi: 10.1016/0304-3940(87)90680-X. [DOI] [PubMed] [Google Scholar]
- 735.Hösli L, Hösli E, Winter T, Stauffer S. Coexistence of cholinergic and somatostatin receptors on astrocytes of rat CNS. Neuroreport 5: 1469–1472, 1994. doi: 10.1097/00001756-199407000-00015. [DOI] [PubMed] [Google Scholar]
- 736.Hosoya T, Takizawa K, Nitta K, Hotta Y. Glial cells missing: a binary switch between neuronal and glial determination in Drosophila. Cell 82: 1025–1036, 1995. doi: 10.1016/0092-8674(95)90281-3. [DOI] [PubMed] [Google Scholar]
- 737.Hostenbach S, D’haeseleer M, Kooijman R, De Keyser J. The pathophysiological role of astrocytic endothelin-1. Prog Neurobiol 144: 88–102, 2016. doi: 10.1016/j.pneurobio.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 738.Hotta H. Neurogenic control of parenchymal arterioles in the cerebral cortex. Prog Brain Res 225: 3–39, 2016. doi: 10.1016/bs.pbr.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 739.Houades V, Koulakoff A, Ezan P, Seif I, Giaume C. Gap junction-mediated astrocytic networks in the mouse barrel cortex. J Neurosci 28: 5207–5217, 2008. doi: 10.1523/JNEUROSCI.5100-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 740.Houades V, Rouach N, Ezan P, Kirchhoff F, Koulakoff A, Giaume C. Shapes of astrocyte networks in the juvenile brain. Neuron Glia Biol 2: 3–14, 2006. doi: 10.1017/S1740925X06000081. [DOI] [PubMed] [Google Scholar]
- 741.Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab 32: 1222–1232, 2012. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Hsuchou H, He Y, Kastin AJ, Tu H, Markadakis EN, Rogers RC, Fossier PB, Pan W. Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain 132: 889–902, 2009. doi: 10.1093/brain/awp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743.Hsuchou H, Pan W, Barnes MJ, Kastin AJ. Leptin receptor mRNA in rat brain astrocytes. Peptides 30: 2275–2280, 2009. doi: 10.1016/j.peptides.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 744.Hu X, Yuan Y, Wang D, Su Z. Heterogeneous astrocytes: active players in CNS. Brain Res Bull 125: 1–18, 2016. doi: 10.1016/j.brainresbull.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 745.Hua X, Malarkey EB, Sunjara V, Rosenwald SE, Li WH, Parpura V. Ca2+-dependent glutamate release involves two classes of endoplasmic reticulum Ca2+ stores in astrocytes. J Neurosci Res 76: 86–97, 2004. doi: 10.1002/jnr.20061. [DOI] [PubMed] [Google Scholar]
- 746.Huang C, Hu ZL, Wu WN, Yu DF, Xiong QJ, Song JR, Shu Q, Fu H, Wang F, Chen JG. Existence and distinction of acid-evoked currents in rat astrocytes. Glia 58: 1415–1424, 2010. [DOI] [PubMed] [Google Scholar]
- 747.Huang H, Barakat L, Wang D, Bordey A. Bergmann glial GlyT1 mediates glycine uptake and release in mouse cerebellar slices. J Physiol 560: 721–736, 2004. doi: 10.1113/jphysiol.2004.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Huang W, Zhao N, Bai X, Karram K, Trotter J, Goebbels S, Scheller A, Kirchhoff F. Novel NG2-CreERT2 knock-in mice demonstrate heterogeneous differentiation potential of NG2 glia during development. Glia 62: 896–913, 2014. doi: 10.1002/glia.22648. [DOI] [PubMed] [Google Scholar]
- 749.Huang Y, Grinspan JB, Abrams CK, Scherer SS. Pannexin1 is expressed by neurons and glia but does not form functional gap junctions. Glia 55: 46–56, 2007. doi: 10.1002/glia.20435. [DOI] [PubMed] [Google Scholar]
- 750.Hubbard JA, Hsu MS, Seldin MM, Binder DK. Expression of the astrocyte water channel aquaporin-4 in the mouse brain. ASN Neuro 7: 1759091415605486, 2015. doi: 10.1177/1759091415605486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751.Huckstepp RT, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol 588: 3901–3920, 2010. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Hung AC, Sun SH. The P2X(7) receptor-mediated phospholipase D activation is regulated by both PKC-dependent and PKC-independent pathways in a rat brain-derived Type-2 astrocyte cell line, RBA-2. Cell Signal 14: 83–92, 2002. doi: 10.1016/S0898-6568(01)00230-3. [DOI] [PubMed] [Google Scholar]
- 753.Hur YS, Kim KD, Paek SH, Yoo SH. Evidence for the existence of secretory granule (dense-core vesicle)-based inositol 1,4,5-trisphosphate-dependent Ca2+ signaling system in astrocytes. PLoS One 5: e11973, 2010. doi: 10.1371/journal.pone.0011973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Hussy N, Brès V, Rochette M, Duvoid A, Alonso G, Dayanithi G, Moos FC. Osmoregulation of vasopressin secretion via activation of neurohypophysial nerve terminals glycine receptors by glial taurine. J Neurosci 21: 7110–7116, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 755.Hutchinson DS, Summers RJ, Gibbs ME. β2- and β3-adrenoceptors activate glucose uptake in chick astrocytes by distinct mechanisms: a mechanism for memory enhancement? J Neurochem 103: 997–1008, 2007. doi: 10.1111/j.1471-4159.2007.04789.x. [DOI] [PubMed] [Google Scholar]
- 756.Hwang EM, Kim E, Yarishkin O, Woo DH, Han KS, Park N, Bae Y, Woo J, Kim D, Park M, Lee CJ, Park JY. A disulphide-linked heterodimer of TWIK-1 and TREK-1 mediates passive conductance in astrocytes. Nat Commun 5: 3227, 2014. doi: 10.1038/ncomms4227. [DOI] [PubMed] [Google Scholar]
- 757.Hyzinski-García MC, Rudkouskaya A, Mongin AA. LRRC8A protein is indispensable for swelling-activated and ATP-induced release of excitatory amino acids in rat astrocytes. J Physiol 592: 4855–4862, 2014. doi: 10.1113/jphysiol.2014.278887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 758.Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci 29: 7092–7097, 2009. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 759.Iino M, Goto K, Kakegawa W, Okado H, Sudo M, Ishiuchi S, Miwa A, Takayasu Y, Saito I, Tsuzuki K, Ozawa S. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science 292: 926–929, 2001. doi: 10.1126/science.1058827. [DOI] [PubMed] [Google Scholar]
- 760.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 123: 1299–1309, 2013. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 761.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4: 147ra111, 2012. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Illarionova NB, Brismar H, Aperia A, Gunnarson E. Role of Na,K-ATPase α1 and α2 isoforms in the support of astrocyte glutamate uptake. PLoS One 9: e98469, 2014. doi: 10.1371/journal.pone.0098469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 763.Illes P, Verkhratsky A. Purinergic neurone-glia signalling in cognitive-related pathologies. Neuropharmacology 104: 62–75, 2016. doi: 10.1016/j.neuropharm.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 764.Inagaki N, Fukui H, Ito S, Yamatodani A, Wada H. Single type-2 astrocytes show multiple independent sites of Ca2+ signaling in response to histamine. Proc Natl Acad Sci USA 88: 4215–4219, 1991. doi: 10.1073/pnas.88.10.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 765.Inazu M, Takeda H, Matsumiya T. Functional expression of the norepinephrine transporter in cultured rat astrocytes. J Neurochem 84: 136–144, 2003. doi: 10.1046/j.1471-4159.2003.01514.x. [DOI] [PubMed] [Google Scholar]
- 766.Intebi AD, Flaxman MS, Ganong WF, Deschepper CF. Angiotensinogen production by rat astroglial cells in vitro and in vivo. Neuroscience 34: 545–554, 1990. doi: 10.1016/0306-4522(90)90163-X. [DOI] [PubMed] [Google Scholar]
- 767.Ischenko A, Sayah S, Patte C, Andreev S, Gasque P, Schouft MT, Vaudry H, Fontaine M. Expression of a functional anaphylatoxin C3a receptor by astrocytes. J Neurochem 71: 2487–2496, 1998. doi: 10.1046/j.1471-4159.1998.71062487.x. [DOI] [PubMed] [Google Scholar]
- 768.Isegawa K, Hirooka Y, Katsuki M, Kishi T, Sunagawa K. Angiotensin II type 1 receptor expression in astrocytes is upregulated leading to increased mortality in mice with myocardial infarction-induced heart failure. Am J Physiol Heart Circ Physiol 307: H1448–H1455, 2014. doi: 10.1152/ajpheart.00462.2014. [DOI] [PubMed] [Google Scholar]
- 769.Ishii M, Fujita A, Iwai K, Kusaka S, Higashi K, Inanobe A, Hibino H, Kurachi Y. Differential expression and distribution of Kir5.1 and Kir4.1 inwardly rectifying K+ channels in retina. Am J Physiol Cell Physiol 285: C260–C267, 2003. doi: 10.1152/ajpcell.00560.2002. [DOI] [PubMed] [Google Scholar]
- 770.Ishii M, Horio Y, Tada Y, Hibino H, Inanobe A, Ito M, Yamada M, Gotow T, Uchiyama Y, Kurachi Y. Expression and clustered distribution of an inwardly rectifying potassium channel, KAB-2/Kir4.1, on mammalian retinal Müller cell membrane: their regulation by insulin and laminin signals. J Neurosci 17: 7725–7735, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 771.Ishimoto H, Nakahata N, Matsuoka I, Nakanishi H. Effects of ATP on phosphoinositide hydrolysis and prostaglandin E2 generation in rabbit astrocytes. J Pharm Pharmacol 49: 520–524, 1997. doi: 10.1111/j.2042-7158.1997.tb06835.x. [DOI] [PubMed] [Google Scholar]
- 772.Isokawa M, McKhann GM II. Electrophysiological and morphological characterization of dentate astrocytes in the hippocampus. J Neurobiol 65: 125–134, 2005. doi: 10.1002/neu.20186. [DOI] [PubMed] [Google Scholar]
- 773.Israel JM, Schipke CG, Ohlemeyer C, Theodosis DT, Kettenmann H. GABAA receptor-expressing astrocytes in the supraoptic nucleus lack glutamate uptake and receptor currents. Glia 44: 102–110, 2003. doi: 10.1002/glia.10272. [DOI] [PubMed] [Google Scholar]
- 774.Ivaska J, Pallari HM, Nevo J, Eriksson JE. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res 313: 2050–2062, 2007. doi: 10.1016/j.yexcr.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 775.Izumi Y, Benz AM, Katsuki H, Zorumski CF. Endogenous monocarboxylates sustain hippocampal synaptic function and morphological integrity during energy deprivation. J Neurosci 17: 9448–9457, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 776.Jabs R, Guenther E, Marquordt K, Wheeler-Schilling TH. Evidence for P2X3, P2X4, P2X5 but not for P2X7 containing purinergic receptors in Müller cells of the rat retina. Brain Res Mol Brain Res 76: 205–210, 2000. doi: 10.1016/S0169-328X(99)00339-3. [DOI] [PubMed] [Google Scholar]
- 777.Jabs R, Kirchhoff F, Kettenmann H, Steinhäuser C. Kainate activates Ca2+-permeable glutamate receptors and blocks voltage-gated K+ currents in glial cells of mouse hippocampal slices. Pflugers Arch 426: 310–319, 1994. doi: 10.1007/BF00374787. [DOI] [PubMed] [Google Scholar]
- 778.Jabs R, Matthias K, Grote A, Grauer M, Seifert G, Steinhäuser C. Lack of P2X receptor mediated currents in astrocytes and GluR type glial cells of the hippocampal CA1 region. Glia 55: 1648–1655, 2007. doi: 10.1002/glia.20580. [DOI] [PubMed] [Google Scholar]
- 779.Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol 175: 4320–4330, 2005. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- 780.Jackson FR. Glial cell modulation of circadian rhythms. Glia 59: 1341–1350, 2011. doi: 10.1002/glia.21097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 781.Jacobsen CT, Miller RH. Control of astrocyte migration in the developing cerebral cortex. Dev Neurosci 25: 207–216, 2003. doi: 10.1159/000072269. [DOI] [PubMed] [Google Scholar]
- 782.Jacobsson JA, Stephansson O, Fredriksson R. C6ORF192 forms a unique evolutionary branch among solute carriers (SLC16, SLC17, and SLC18) and is abundantly expressed in several brain regions. J Mol Neurosci 41: 230–242, 2010. doi: 10.1007/s12031-009-9222-7. [DOI] [PubMed] [Google Scholar]
- 783.Jacques-Silva MC, Rodnight R, Lenz G, Liao Z, Kong Q, Tran M, Kang Y, Gonzalez FA, Weisman GA, Neary JT. P2X7 receptors stimulate AKT phosphorylation in astrocytes. Br J Pharmacol 141: 1106–1117, 2004. doi: 10.1038/sj.bjp.0705685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 784.Jahn R, Scheller RH. SNAREs–engines for membrane fusion. Nat Rev Mol Cell Biol 7: 631–643, 2006. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 785.Jaiswal JK, Andrews NW, Simon SM. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J Cell Biol 159: 625–635, 2002. doi: 10.1083/jcb.200208154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 786.James G, Butt AM. Adenosine 5′ triphosphate evoked mobilization of intracellular calcium in central nervous system white matter of adult mouse optic nerve. Neurosci Lett 268: 53–56, 1999. doi: 10.1016/S0304-3940(99)00370-5. [DOI] [PubMed] [Google Scholar]
- 787.Jastrowitz M. Encephalitis und Myelitis des ersten Kindersalters. Arch f Psychiat 2: 389–414, 1870. doi: 10.1007/BF02046645. [DOI] [Google Scholar]
- 788.Jastrowitz M. Studien über die Encephalitis und Myelitis des ersten Kindesalters. Arch f Psychiat 3: 162–213, 1872. doi: 10.1007/BF02156041. [DOI] [Google Scholar]
- 789.Jayakumar AR, Norenberg MD. The Na-K-Cl Co-transporter in astrocyte swelling. Metab Brain Dis 25: 31–38, 2010. doi: 10.1007/s11011-010-9180-3. [DOI] [PubMed] [Google Scholar]
- 790.Jayaram B, Pan W, Wang Y, Hsuchou H, Mace A, Cornelissen-Guillaume GG, Mishra PK, Koza RA, Kastin AJ. Astrocytic leptin-receptor knockout mice show partial rescue of leptin resistance in diet-induced obesity. J Appl Physiol (1985) 114: 734–741, 2013. doi: 10.1152/japplphysiol.01499.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 791.Jego P, Pacheco-Torres J, Araque A, Canals S. Functional MRI in mice lacking IP3-dependent calcium signaling in astrocytes. J Cereb Blood Flow Metab 34: 1599–1603, 2014. doi: 10.1038/jcbfm.2014.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 792.Jennings A, Tyurikova O, Bard L, Zheng K, Semyanov A, Henneberger C, Rusakov DA. Dopamine elevates and lowers astroglial Ca2+ through distinct pathways depending on local synaptic circuitry. Glia 65: 447–459, 2017. doi: 10.1002/glia.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 793.Jiang Q, Zhang L, Ding G, Davoodi-Bojd E, Li Q, Li L, Sadry N, Nedergaard M, Chopp M, Zhang Z. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab 37: 1326–1337, 2017. doi: 10.1177/0271678X16654702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794.Jimenez-Blasco D, Santofimia-Castaño P, Gonzalez A, Almeida A, Bolaños JP. Astrocyte NMDA receptors’ activity sustains neuronal survival through a Cdk5-Nrf2 pathway. Cell Death Differ 22: 1877–1889, 2015. doi: 10.1038/cdd.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 795.Jiménez AI, Castro E, Communi D, Boeynaems JM, Delicado EG, Miras-Portugal MT. Coexpression of several types of metabotropic nucleotide receptors in single cerebellar astrocytes. J Neurochem 75: 2071–2079, 2000. doi: 10.1046/j.1471-4159.2000.0752071.x. [DOI] [PubMed] [Google Scholar]
- 796.Jiménez AI, Castro E, Mirabet M, Franco R, Delicado EG, Miras-Portugal MT. Potentiation of ATP calcium responses by A2B receptor stimulation and other signals coupled to Gs proteins in type-1 cerebellar astrocytes. Glia 26: 119–128, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 797.John GR, Simpson JE, Woodroofe MN, Lee SC, Brosnan CF. Extracellular nucleotides differentially regulate interleukin-1beta signaling in primary human astrocytes: implications for inflammatory gene expression. J Neurosci 21: 4134–4142, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 798.Johnson RT, Breedlove SM, Jordan CL. Sex differences and laterality in astrocyte number and complexity in the adult rat medial amygdala. J Comp Neurol 511: 599–609, 2008. doi: 10.1002/cne.21859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 799.Jones BW, Fetter RD, Tear G, Goodman CS. Glial cells missing: a genetic switch that controls glial versus neuronal fate. Cell 82: 1013–1023, 1995. doi: 10.1016/0092-8674(95)90280-5. [DOI] [PubMed] [Google Scholar]
- 800.Jones HC, Keep RF. Brain fluid calcium concentration and response to acute hypercalcaemia during development in the rat. J Physiol 402: 579–593, 1988. doi: 10.1113/jphysiol.1988.sp017223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Jones HC, Keep RF. The control of potassium concentration in the cerebrospinal fluid and brain interstitial fluid of developing rats. J Physiol 383: 441–453, 1987. doi: 10.1113/jphysiol.1987.sp016419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 802.Jones TA, Greenough WT. Ultrastructural evidence for increased contact between astrocytes and synapses in rats reared in a complex environment. Neurobiol Learn Mem 65: 48–56, 1996. doi: 10.1006/nlme.1996.0005. [DOI] [PubMed] [Google Scholar]
- 803.Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, Cha CI. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J Comp Neurol 476: 388–413, 2004. doi: 10.1002/cne.20231. [DOI] [PubMed] [Google Scholar]
- 804.Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci 10: 331–339, 2007. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- 805.Juhaszova M, Blaustein MP. Na+ pump low and high ouabain affinity alpha subunit isoforms are differently distributed in cells. Proc Natl Acad Sci USA 94: 1800–1805, 1997. doi: 10.1073/pnas.94.5.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 806.Jung-Testas I, Baulieu EE. Steroid hormone receptors and steroid action in rat glial cells of the central and peripheral nervous system. J Steroid Biochem Mol Biol 65: 243–251, 1998. doi: 10.1016/S0960-0760(97)00191-X. [DOI] [PubMed] [Google Scholar]
- 807.Jungblut M, Tiveron MC, Barral S, Abrahamsen B, Knöbel S, Pennartz S, Schmitz J, Perraut M, Pfrieger FW, Stoffel W, Cremer H, Bosio A. Isolation and characterization of living primary astroglial cells using the new GLAST-specific monoclonal antibody ACSA-1. Glia 60: 894–907, 2012. doi: 10.1002/glia.22322. [DOI] [PubMed] [Google Scholar]
- 808.Juric DM, Kržan M, Lipnik-Stangelj M. Histamine and astrocyte function. Pharmacol Res 111: 774–783, 2016. doi: 10.1016/j.phrs.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 809.Jurič DM, Mele T, Carman-Kržan M. Involvement of histaminergic receptor mechanisms in the stimulation of NT-3 synthesis in astrocytes. Neuropharmacology 60: 1309–1317, 2011. doi: 10.1016/j.neuropharm.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 810.Jurzak M, Müller AR, Gerstberger R. Characterization of vasopressin receptors in cultured cells derived from the region of rat brain circumventricular organs. Neuroscience 65: 1145–1159, 1995. doi: 10.1016/0306-4522(94)00539-H. [DOI] [PubMed] [Google Scholar]
- 811.Kafitz KW, Meier SD, Stephan J, Rose CR. Developmental profile and properties of sulforhodamine 101–labeled glial cells in acute brain slices of rat hippocampus. J Neurosci Methods 169: 84–92, 2008. doi: 10.1016/j.jneumeth.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 812.Kallakuri S, Kreipke CW, Schafer PC, Schafer SM, Rafols JA. Brain cellular localization of endothelin receptors A and B in a rodent model of diffuse traumatic brain injury. Neuroscience 168: 820–830, 2010. doi: 10.1016/j.neuroscience.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 813.Kálmán M. GFAP expression withdraws–a trend of glial evolution? Brain Res Bull 57: 509–511, 2002. doi: 10.1016/S0361-9230(01)00713-4. [DOI] [PubMed] [Google Scholar]
- 814.Kálmán M, Martin-Partido G, Hidalgo-Sanchez M, Majorossy K. Distribution of glial fibrillary acidic protein-immunopositive structures in the developing brain of the turtle Mauremys leprosa. Anat Embryol (Berl) 196: 47–65, 1997. doi: 10.1007/s004290050079. [DOI] [PubMed] [Google Scholar]
- 815.Kálmán M, Pritz MB. Glial fibrillary acidic protein-immunopositive structures in the brain of a Crocodilian, Caiman crocodilus, and its bearing on the evolution of astroglia. J Comp Neurol 431: 460–480, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 816.Kálmán M, Székely AD, Csillag A. Distribution of glial fibrillary acidic protein and vimentin-immunopositive elements in the developing chicken brain from hatch to adulthood. Anat Embryol (Berl) 198: 213–235, 1998. doi: 10.1007/s004290050179. [DOI] [PubMed] [Google Scholar]
- 817.Kalsi AS, Greenwood K, Wilkin G, Butt AM. Kir4.1 expression by astrocytes and oligodendrocytes in CNS white matter: a developmental study in the rat optic nerve. J Anat 204: 475–485, 2004. doi: 10.1111/j.0021-8782.2004.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 818.Kanaka C, Ohno K, Okabe A, Kuriyama K, Itoh T, Fukuda A, Sato K. The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience 104: 933–946, 2001. doi: 10.1016/S0306-4522(01)00149-X. [DOI] [PubMed] [Google Scholar]
- 819.Kandarian B, Sethi J, Wu A, Baker M, Yazdani N, Kym E, Sanchez A, Edsall L, Gaasterland T, Macagno E. The medicinal leech genome encodes 21 innexin genes: different combinations are expressed by identified central neurons. Dev Genes Evol 222: 29–44, 2012. doi: 10.1007/s00427-011-0387-z. [DOI] [PubMed] [Google Scholar]
- 820.Kanemaru K, Kubota J, Sekiya H, Hirose K, Okubo Y, Iino M. Calcium-dependent N-cadherin up-regulation mediates reactive astrogliosis and neuroprotection after brain injury. Proc Natl Acad Sci USA 110: 11612–11617, 2013. doi: 10.1073/pnas.1300378110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 821.Kanemaru K, Sekiya H, Xu M, Satoh K, Kitajima N, Yoshida K, Okubo Y, Sasaki T, Moritoh S, Hasuwa H, Mimura M, Horikawa K, Matsui K, Nagai T, Iino M, Tanaka KF. In vivo visualization of subtle, transient, and local activity of astrocytes using an ultrasensitive Ca2+ indicator. Cell Reports 8: 311–318, 2014. doi: 10.1016/j.celrep.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 822.Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci 1: 683–692, 1998. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- 823.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci 28: 4702–4711, 2008. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 824.Kang J, Kang N, Yu Y, Zhang J, Petersen N, Tian GF, Nedergaard M. Sulforhodamine 101 induces long-term potentiation of intrinsic excitability and synaptic efficacy in hippocampal CA1 pyramidal neurons. Neuroscience 169: 1601–1609, 2010. doi: 10.1016/j.neuroscience.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 825.Kang N, Peng H, Yu Y, Stanton PK, Guilarte TR, Kang J. Astrocytes release d-serine by a large vesicle. Neuroscience 240: 243–257, 2013. doi: 10.1016/j.neuroscience.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 826.Kang N, Xu J, Xu Q, Nedergaard M, Kang J. Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J Neurophysiol 94: 4121–4130, 2005. doi: 10.1152/jn.00448.2005. [DOI] [PubMed] [Google Scholar]
- 827.Kang P, Lee HK, Glasgow SM, Finley M, Donti T, Gaber ZB, Graham BH, Foster AE, Novitch BG, Gronostajski RM, Deneen B. Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron 74: 79–94, 2012. doi: 10.1016/j.neuron.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 828.Kanjhan R, Housley GD, Burton LD, Christie DL, Kippenberger A, Thorne PR, Luo L, Ryan AF. Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. J Comp Neurol 407: 11–32, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 829.Kanjhan R, Housley GD, Thorne PR, Christie DL, Palmer DJ, Luo L, Ryan AF. Localization of ATP-gated ion channels in cerebellum using P2x2R subunit-specific antisera. Neuroreport 7: 2665–2669, 1996. doi: 10.1097/00001756-199611040-00051. [DOI] [PubMed] [Google Scholar]
- 830.Kanski R, van Strien ME, van Tijn P, Hol EM. A star is born: new insights into the mechanism of astrogenesis. Cell Mol Life Sci 71: 433–447, 2014. doi: 10.1007/s00018-013-1435-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 831.Kao SC, Zhao X, Lee CY, Atianjoh FE, Gauda EB, Yaster M, Tao YX. Absence of μ opioid receptor mRNA expression in astrocytes and microglia of rat spinal cord. Neuroreport 23: 378–384, 2012. doi: 10.1097/WNR.0b013e3283522e1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 832.Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem 71: 511–535, 2002. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 833.Karagiannis A, Sylantyev S, Hadjihambi A, Hosford PS, Kasparov S, Gourine AV. Hemichannel-mediated release of lactate. J Cereb Blood Flow Metab 36: 1202–1211, 2016. doi: 10.1177/0271678X15611912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 834.Karakaya S, Kipp M, Beyer C. Oestrogen regulates the expression and function of dopamine transporters in astrocytes of the nigrostriatal system. J Neuroendocrinol 19: 682–690, 2007. doi: 10.1111/j.1365-2826.2007.01575.x. [DOI] [PubMed] [Google Scholar]
- 835.Karhunen T, Tilgmann C, Ulmanen I, Panula P. Catechol-O-methyltransferase (COMT) in rat brain: immunoelectron microscopic study with an antiserum against rat recombinant COMT protein. Neurosci Lett 187: 57–60, 1995. doi: 10.1016/0304-3940(95)11337-V. [DOI] [PubMed] [Google Scholar]
- 836.Karki P, Webb A, Zerguine A, Choi J, Son DS, Lee E. Mechanism of raloxifene-induced upregulation of glutamate transporters in rat primary astrocytes. Glia 62: 1270–1283, 2014. doi: 10.1002/glia.22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837.Karschin A, Brockhaus J, Ballanyi K. KATP channel formation by the sulphonylurea receptors SUR1 with Kir6.2 subunits in rat dorsal vagal neurons in situ. J Physiol 509: 339–346, 1998. doi: 10.1111/j.1469-7793.1998.339bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 838.Kartvelishvily E, Shleper M, Balan L, Dumin E, Wolosker H. Neuron-derived d-serine release provides a novel means to activate N-methyl-d-aspartate receptors. J Biol Chem 281: 14151–14162, 2006. doi: 10.1074/jbc.M512927200. [DOI] [PubMed] [Google Scholar]
- 839.Kasai H, Takahashi N, Tokumaru H. Distinct initial SNARE configurations underlying the diversity of exocytosis. Physiol Rev 92: 1915–1964, 2012. doi: 10.1152/physrev.00007.2012. [DOI] [PubMed] [Google Scholar]
- 840.Kasischke KA, Lambert EM, Panepento B, Sun A, Gelbard HA, Burgess RW, Foster TH, Nedergaard M. Two-photon NADH imaging exposes boundaries of oxygen diffusion in cortical vascular supply regions. J Cereb Blood Flow Metab 31: 68–81, 2011. doi: 10.1038/jcbfm.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 841.Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science 305: 99–103, 2004. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- 842.Kastritsis CH, Salm AK, McCarthy K. Stimulation of the P2Y purinergic receptor on type 1 astroglia results in inositol phosphate formation and calcium mobilization. J Neurochem 58: 1277–1284, 1992. doi: 10.1111/j.1471-4159.1992.tb11339.x. [DOI] [PubMed] [Google Scholar]
- 843.Kasymov V, Larina O, Castaldo C, Marina N, Patrushev M, Kasparov S, Gourine AV. Differential sensitivity of brainstem versus cortical astrocytes to changes in pH reveals functional regional specialization of astroglia. J Neurosci 33: 435–441, 2013. doi: 10.1523/JNEUROSCI.2813-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 844.Kato S, Ishita S, Sugawara K, Mawatari K. Cystine/glutamate antiporter expression in retinal Müller glial cells: implications for dl-alpha-aminoadipate toxicity. Neuroscience 57: 473–482, 1993. doi: 10.1016/0306-4522(93)90080-Y. [DOI] [PubMed] [Google Scholar]
- 845.Katritch V, Fenalti G, Abola EE, Roth BL, Cherezov V, Stevens RC. Allosteric sodium in class A GPCR signaling. Trends Biochem Sci 39: 233–244, 2014. doi: 10.1016/j.tibs.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 846.Kavanaugh MP, Arriza JL, North RA, Amara SG. Electrogenic uptake of γ-aminobutyric acid by a cloned transporter expressed in Xenopus oocytes. J Biol Chem 267: 22007–22009, 1992. [PubMed] [Google Scholar]
- 847.Kawano T, Takuwa K, Kuniyoshi H, Juni N, Nakajima T, Yamamoto D, Kimura Y. Cloning and characterization of a Drosophila melanogaster cDNA encoding a glutamate transporter. Biosci Biotechnol Biochem 63: 2042–2044, 1999. doi: 10.1271/bbb.63.2042. [DOI] [PubMed] [Google Scholar]
- 848.Kazmierczak J, Kempe S, Kremer B. Calcium in the early evolution of living systems: a biohistorical approach. Curr Org Chem 17: 1738–1750, 2013. doi: 10.2174/13852728113179990081. [DOI] [Google Scholar]
- 849.Keene SD, Greco TM, Parastatidis I, Lee SH, Hughes EG, Balice-Gordon RJ, Speicher DW, Ischiropoulos H. Mass spectrometric and computational analysis of cytokine-induced alterations in the astrocyte secretome. Proteomics 9: 768–782, 2009. doi: 10.1002/pmic.200800385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 850.Keep RF, Xiang J, Betz AL. Potassium cotransport at the rat choroid plexus. Am J Physiol Cell Physiol 267: C1616–C1622, 1994. [DOI] [PubMed] [Google Scholar]
- 851.Kelly T, Kafitz KW, Roderigo C, Rose CR. Ammonium-evoked alterations in intracellular sodium and pH reduce glial glutamate transport activity. Glia 57: 921–934, 2009. doi: 10.1002/glia.20817. [DOI] [PubMed] [Google Scholar]
- 852.Kersanté F, Rowley SC, Pavlov I, Gutièrrez-Mecinas M, Semyanov A, Reul JM, Walker MC, Linthorst AC. A functional role for both γ-aminobutyric acid (GABA) transporter-1 and GABA transporter-3 in the modulation of extracellular GABA and GABAergic tonic conductances in the rat hippocampus. J Physiol 591: 2429–2441, 2013. doi: 10.1113/jphysiol.2012.246298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 853.Kettenmann H, Backus KH, Schachner M. Aspartate, glutamate and gamma-aminobutyric acid depolarize cultured astrocytes. Neurosci Lett 52: 25–29, 1984. doi: 10.1016/0304-3940(84)90345-8. [DOI] [PubMed] [Google Scholar]
- 854.Kettenmann H, Backus KH, Schachner M. γ-Aminobutyric acid opens Cl− channels in cultured astrocytes. Brain Res 404: 1–9, 1987. doi: 10.1016/0006-8993(87)91349-7. [DOI] [PubMed] [Google Scholar]
- 855.Kettenmann H, Faissner A, Trotter J. Neuron-glia interactions in homeostasis and degeneration. In: Comprehensive Human Physiology edited by Greger R, Windhort U. Berlin: Springer Verlag, 1996, p. 533–543. doi: 10.1007/978-3-642-60946-6_27 [DOI] [Google Scholar]
- 856.Kettenmann H, Ransom BR. Electrical coupling between astrocytes and between oligodendrocytes studied in mammalian cell cultures. Glia 1: 64–73, 1988. doi: 10.1002/glia.440010108. [DOI] [PubMed] [Google Scholar]
- 857.Kettenmann H, Schachner M. Pharmacological properties of gamma-aminobutyric acid-, glutamate-, and aspartate-induced depolarizations in cultured astrocytes. J Neurosci 5: 3295–3301, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 858.Khan ZU, Koulen P, Rubinstein M, Grandy DK, Goldman-Rakic PS. An astroglia-linked dopamine D2-receptor action in prefrontal cortex. Proc Natl Acad Sci USA 98: 1964–1969, 2001. doi: 10.1073/pnas.98.4.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 859.Kim DK, Kim IJ, Hwang S, Kook JH, Lee MC, Shin BA, Bae CS, Yoon JH, Ahn SG, Kim SA, Kanai Y, Endou H, Kim JK. System L-amino acid transporters are differently expressed in rat astrocyte and C6 glioma cells. Neurosci Res 50: 437–446, 2004. doi: 10.1016/j.neures.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 860.Kim J, Jones BW, Zock C, Chen Z, Wang H, Goodman CS, Anderson DJ. Isolation and characterization of mammalian homologs of the Drosophila gene glial cells missing. Proc Natl Acad Sci USA 95: 12364–12369, 1998. doi: 10.1073/pnas.95.21.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 861.Kim JG, Suyama S, Koch M, Jin S, Argente-Arizon P, Argente J, Liu ZW, Zimmer MR, Jeong JK, Szigeti-Buck K, Gao Y, Garcia-Caceres C, Yi CX, Salmaso N, Vaccarino FM, Chowen J, Diano S, Dietrich MO, Tschöp MH, Horvath TL. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat Neurosci 17: 908–910, 2014. doi: 10.1038/nn.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 862.Kim KJ, Iddings JA, Stern JE, Blanco VM, Croom D, Kirov SA, Filosa JA. Astrocyte contributions to flow/pressure-evoked parenchymal arteriole vasoconstriction. J Neurosci 35: 8245–8257, 2015. doi: 10.1523/JNEUROSCI.4486-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 863.Kim WS, Guillemin GJ, Glaros EN, Lim CK, Garner B. Quantitation of ATP-binding cassette subfamily-A transporter gene expression in primary human brain cells. Neuroreport 17: 891–896, 2006. doi: 10.1097/01.wnr.0000221833.41340.cd. [DOI] [PubMed] [Google Scholar]
- 864.Kim WT, Rioult MG, Cornell-Bell AH. Glutamate-induced calcium signaling in astrocytes. Glia 11: 173–184, 1994. doi: 10.1002/glia.440110211. [DOI] [PubMed] [Google Scholar]
- 865.Kimelberg HK. Active accumulation and exchange transport of chloride in astroglial cells in culture. Biochim Biophys Acta 646: 179–184, 1981. doi: 10.1016/0005-2736(81)90285-6. [DOI] [PubMed] [Google Scholar]
- 866.Kimelberg HK. The problem of astrocyte identity. Neurochem Int 45: 191–202, 2004. doi: 10.1016/j.neuint.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 867.Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci 10: 1583–1591, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 868.Kimelberg HK, Macvicar BA, Sontheimer H. Anion channels in astrocytes: biophysics, pharmacology, and function. Glia 54: 747–757, 2006. doi: 10.1002/glia.20423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 869.Kimelberg HK, Pang S, Treble DH. Excitatory amino acid-stimulated uptake of 22Na+ in primary astrocyte cultures. J Neurosci 9: 1141–1149, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 870.King AE, Ackley MA, Cass CE, Young JD, Baldwin SA. Nucleoside transporters: from scavengers to novel therapeutic targets. Trends Pharmacol Sci 27: 416–425, 2006. doi: 10.1016/j.tips.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 871.Kinnamon SC, Finger TE. A taste for ATP: neurotransmission in taste buds. Front Cell Neurosci 7: 264, 2013. doi: 10.3389/fncel.2013.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 872.Kintner DB, Look A, Shull GE, Sun D. Stimulation of astrocyte Na+/H+ exchange activity in response to in vitro ischemia depends in part on activation of ERK1/2. Am J Physiol Cell Physiol 289: C934–C945, 2005. doi: 10.1152/ajpcell.00092.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 873.Kintner DB, Luo J, Gerdts J, Ballard AJ, Shull GE, Sun D. Role of Na+-K+-Cl− cotransport and Na+/Ca2+ exchange in mitochondrial dysfunction in astrocytes following in vitro ischemia. Am J Physiol Cell Physiol 292: C1113–C1122, 2007. doi: 10.1152/ajpcell.00412.2006. [DOI] [PubMed] [Google Scholar]
- 874.Kirchhoff F, Mülhardt C, Pastor A, Becker CM, Kettenmann H. Expression of glycine receptor subunits in glial cells of the rat spinal cord. J Neurochem 66: 1383–1390, 1996. doi: 10.1046/j.1471-4159.1996.66041383.x. [DOI] [PubMed] [Google Scholar]
- 875.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427: 360–364, 2004. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 876.Kirischuk S, Héja L, Kardos J, Billups B. Astrocyte sodium signaling and the regulation of neurotransmission. Glia 64: 1655–1666, 2016. doi: 10.1002/glia.22943. [DOI] [PubMed] [Google Scholar]
- 877.Kirischuk S, Kettenmann H, Verkhratsky A. Membrane currents and cytoplasmic sodium transients generated by glutamate transport in Bergmann glial cells. Pflugers Arch 454: 245–252, 2007. doi: 10.1007/s00424-007-0207-5. [DOI] [PubMed] [Google Scholar]
- 878.Kirischuk S, Kettenmann H, Verkhratsky A. Na+/Ca2+ exchanger modulates kainate-triggered Ca2+ signaling in Bergmann glial cells in situ. FASEB J 11: 566–572, 1997. [DOI] [PubMed] [Google Scholar]
- 879.Kirischuk S, Kirchhoff F, Matyash V, Kettenmann H, Verkhratsky A. Glutamate-triggered calcium signalling in mouse bergmann glial cells in situ: role of inositol-1,4,5-trisphosphate-mediated intracellular calcium release. Neuroscience 92: 1051–1059, 1999. doi: 10.1016/S0306-4522(99)00067-6. [DOI] [PubMed] [Google Scholar]
- 880.Kirischuk S, Möller T, Voitenko N, Kettenmann H, Verkhratsky A. ATP-induced cytoplasmic calcium mobilization in Bergmann glial cells. J Neurosci 15: 7861–7871, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 881.Kirischuk S, Parpura V, Verkhratsky A. Sodium dynamics: another key to astroglial excitability? Trends Neurosci 35: 497–506, 2012. doi: 10.1016/j.tins.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 882.Kirischuk S, Tuschick S, Verkhratsky A, Kettenmann H. Calcium signalling in mouse Bergmann glial cells mediated by α1-adrenoreceptors and H1 histamine receptors. Eur J Neurosci 8: 1198–1208, 1996. doi: 10.1111/j.1460-9568.1996.tb01288.x. [DOI] [PubMed] [Google Scholar]
- 883.Kirischuk S, Verkhratsky A. [Ca2+]i recordings from neural cells in acutely isolated cerebellar slices employing differential loading of the membrane-permeant form of the calcium indicator fura-2. Pflugers Arch 431: 977–983, 1996. doi: 10.1007/s004240050094. [DOI] [PubMed] [Google Scholar]
- 884.Kita Y, Kawakami K, Takahashi Y, Murakami F. Development of cerebellar neurons and glias revealed by in utero electroporation: Golgi-like labeling of cerebellar neurons and glias. PLoS One 8: e70091, 2013. doi: 10.1371/journal.pone.0070091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 885.Kitoh T, Matsushita M. A new staining method of astrocytes for paraffin section. Acta Neuropathol 49: 67–69, 1980. doi: 10.1007/BF00692222. [DOI] [PubMed] [Google Scholar]
- 886.Kittel-Schneider S, Kenis G, Schek J, van den Hove D, Prickaerts J, Lesch KP, Steinbusch H, Reif A. Expression of monoamine transporters, nitric oxide synthase 3, and neurotrophin genes in antidepressant-stimulated astrocytes. Front Psychiatry 3: 33, 2012. doi: 10.3389/fpsyt.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 887.Kiviniemi V, Wang X, Korhonen V, Keinänen T, Tuovinen T, Autio J, LeVan P, Keilholz S, Zang YF, Hennig J, Nedergaard M. Ultra-fast magnetic resonance encephalography of physiological brain activity: glymphatic pulsation mechanisms? J Cereb Blood Flow Metab 36: 1033–1045, 2016. doi: 10.1177/0271678X15622047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 888.Kjenseth A, Fykerud T, Rivedal E, Leithe E. Regulation of gap junction intercellular communication by the ubiquitin system. Cell Signal 22: 1267–1273, 2010. doi: 10.1016/j.cellsig.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 889.Klomp LW, de Koning TJ, Malingré HE, van Beurden EA, Brink M, Opdam FL, Duran M, Jaeken J, Pineda M, Van Maldergem L, Poll-The BT, van den Berg IE, Berger R. Molecular characterization of 3-phosphoglycerate dehydrogenase deficiency–a neurometabolic disorder associated with reduced l-serine biosynthesis. Am J Hum Genet 67: 1389–1399, 2000. doi: 10.1086/316886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 890.Kofuji P, Biedermann B, Siddharthan V, Raap M, Iandiev I, Milenkovic I, Thomzig A, Veh RW, Bringmann A, Reichenbach A. Kir potassium channel subunit expression in retinal glial cells: implications for spatial potassium buffering. Glia 39: 292–303, 2002. doi: 10.1002/glia.10112. [DOI] [PubMed] [Google Scholar]
- 891.Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci 20: 5733–5740, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 892.Kofuji P, Newman EA. Potassium buffering in the central nervous system. Neuroscience 129: 1045–1056, 2004. doi: 10.1016/j.neuroscience.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 893.Koizumi S, Saito Y, Nakazawa K, Nakajima K, Sawada JI, Kohsaka S, Illes P, Inoue K. Spatial and temporal aspects of Ca2+ signaling mediated by P2Y receptors in cultured rat hippocampal astrocytes. Life Sci 72: 431–442, 2002. doi: 10.1016/S0024-3205(02)02273-7. [DOI] [PubMed] [Google Scholar]
- 894.Kölliker A. Handbuch der Gewebelehre des Menschen (6th ed.). Leipzig: Engelmann, 1896. [Google Scholar]
- 895.Kong EK, Peng L, Chen Y, Yu AC, Hertz L. Up-regulation of 5-HT2B receptor density and receptor-mediated glycogenolysis in mouse astrocytes by long-term fluoxetine administration. Neurochem Res 27: 113–120, 2002. doi: 10.1023/A:1014862808126. [DOI] [PubMed] [Google Scholar]
- 896.Korcok J, Yan R, Siushansian R, Dixon SJ, Wilson JX. Sodium-ascorbate cotransport controls intracellular ascorbate concentration in primary astrocyte cultures expressing the SVCT2 transporter. Brain Res 881: 144–151, 2000. doi: 10.1016/S0006-8993(00)02829-8. [DOI] [PubMed] [Google Scholar]
- 897.Korogod N, Petersen CC, Knott GW. Ultrastructural analysis of adult mouse neocortex comparing aldehyde perfusion with cryo fixation. eLife 4: e05793, 2015. doi: 10.7554/eLife.05793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 898.Kotnis S, Bingham B, Vasilyev DV, Miller SW, Bai Y, Yeola S, Chanda PK, Bowlby MR, Kaftan EJ, Samad TA, Whiteside GT. Genetic and functional analysis of human P2X5 reveals a distinct pattern of exon 10 polymorphism with predominant expression of the nonfunctional receptor isoform. Mol Pharmacol 77: 953–960, 2010. doi: 10.1124/mol.110.063636. [DOI] [PubMed] [Google Scholar]
- 899.Koulakoff A, Ezan P, Giaume C. Neurons control the expression of connexin 30 and connexin 43 in mouse cortical astrocytes. Glia 56: 1299–1311, 2008. doi: 10.1002/glia.20698. [DOI] [PubMed] [Google Scholar]
- 900.Kozlov AS, Angulo MC, Audinat E, Charpak S. Target cell-specific modulation of neuronal activity by astrocytes. Proc Natl Acad Sci USA 103: 10058–10063, 2006. doi: 10.1073/pnas.0603741103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 901.Kreft M, Krizaj D, Grilc S, Zorec R. Properties of exocytotic response in vertebrate photoreceptors. J Neurophysiol 90: 218–225, 2003. doi: 10.1152/jn.01025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 902.Kreft M, Stenovec M, Rupnik M, Grilc S, Krzan M, Potokar M, Pangrsic T, Haydon PG, Zorec R. Properties of Ca(2+)-dependent exocytosis in cultured astrocytes. Glia 46: 437–445, 2004. doi: 10.1002/glia.20018. [DOI] [PubMed] [Google Scholar]
- 903.Kremer MC, Jung C, Batelli S, Rubin GM, Gaul U. The glia of the adult Drosophila nervous system. Glia 65: 606–638, 2017. doi: 10.1002/glia.23115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 904.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76: 845–861, 2014. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 905.Kretzschmar D, Hasan G, Sharma S, Heisenberg M, Benzer S. The swiss cheese mutant causes glial hyperwrapping and brain degeneration in Drosophila. J Neurosci 17: 7425–7432, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 906.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32: 149–184, 2009. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 907.Krisch B, Buchholz C, Mentlein R. Somatostatin binding sites on rat diencephalic astrocytes. Light-microscopic study in vitro and in vivo. Cell Tissue Res 263: 253–263, 1991. doi: 10.1007/BF00318767. [DOI] [PubMed] [Google Scholar]
- 908.Krnjević K, Schwartz S. Some properties of unresponsive cells in the cerebral cortex. Exp Brain Res 3: 306–319, 1967. doi: 10.1007/BF00237557. [DOI] [PubMed] [Google Scholar]
- 909.Kroemer G, Jäättelä M. Lysosomes and autophagy in cell death control. Nat Rev Cancer 5: 886–897, 2005. doi: 10.1038/nrc1738. [DOI] [PubMed] [Google Scholar]
- 910.Kronschläger MT, Drdla-Schutting R, Gassner M, Honsek SD, Teuchmann HL, Sandkühler J. Gliogenic LTP spreads widely in nociceptive pathways. Science 354: 1144–1148, 2016. doi: 10.1126/science.aah5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 911.Krzan M, Stenovec M, Kreft M, Pangrsic T, Grilc S, Haydon PG, Zorec R. Calcium-dependent exocytosis of atrial natriuretic peptide from astrocytes. J Neurosci 23: 1580–1583, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 912.Krzisch M, Temprana SG, Mongiat LA, Armida J, Schmutz V, Virtanen MA, Kocher-Braissant J, Kraftsik R, Vutskits L, Conzelmann KK, Bergami M, Gage FH, Schinder AF, Toni N. Pre-existing astrocytes form functional perisynaptic processes on neurons generated in the adult hippocampus. Brain Struct Funct 220: 2027–2042, 2015. doi: 10.1007/s00429-014-0768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 913.Kubo A, Fukui H, Inagaki N, Kanamura A, Wada H. Histamine-induced cyclic AMP accumulation in type-1 and type-2 astrocytes in primary culture. Eur J Pharmacol 208: 249–253, 1991. doi: 10.1016/0922-4106(91)90102-N. [DOI] [PubMed] [Google Scholar]
- 914.Kubo Y, Adelman JP, Clapham DE, Jan LY, Karschin A, Kurachi Y, Lazdunski M, Nichols CG, Seino S, Vandenberg CA. International Union of Pharmacology. LIV. Nomenclature and molecular relationships of inwardly rectifying potassium channels. Pharmacol Rev 57: 509–526, 2005. doi: 10.1124/pr.57.4.11. [DOI] [PubMed] [Google Scholar]
- 915.Kucher BM, Neary JT. Bi-functional effects of ATP/P2 receptor activation on tumor necrosis factor-alpha release in lipopolysaccharide-stimulated astrocytes. J Neurochem 92: 525–535, 2005. doi: 10.1111/j.1471-4159.2004.02885.x. [DOI] [PubMed] [Google Scholar]
- 916.Kucheryavykh YV, Antonov SM, Shuba YM, Rivera Y, Inyushin MY, Veh RW, Verkhratsky A, Nichols CG, Eaton MJ, Skatchkov SN. Sodium Accumulated in Glia During Glutamate Transport Increases Polyamine Dependent Block of Kir4.1 Channels. Programme No 23605/ C15 2012. New Orleans, LA: Society for Neuroscience, 2012. [Google Scholar]
- 917.Kucheryavykh YV, Kucheryavykh LY, Nichols CG, Maldonado HM, Baksi K, Reichenbach A, Skatchkov SN, Eaton MJ. Downregulation of Kir4.1 inward rectifying potassium channel subunits by RNAi impairs potassium transfer and glutamate uptake by cultured cortical astrocytes. Glia 55: 274–281, 2007. doi: 10.1002/glia.20455. [DOI] [PubMed] [Google Scholar]
- 918.Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, Chakraborty C, Workman G, Weaver M, Sage EH, Barres BA, Eroglu C. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci USA 108: E440–E449, 2011. doi: 10.1073/pnas.1104977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 919.Kuffler SW, Nicholls JG. The physiology of neuroglial cells. Ergeb Physiol 57: 1–90, 1966. doi: 10.1007/BF02259903. [DOI] [PubMed] [Google Scholar]
- 920.Kuffler SW, Potter DD. Glia in the leech central nervous system: physiological properties and neuron-glia relationship. J Neurophysiol 27: 290–320, 1964. [DOI] [PubMed] [Google Scholar]
- 921.Kuga N, Sasaki T, Takahara Y, Matsuki N, Ikegaya Y. Large-scale calcium waves traveling through astrocytic networks in vivo. J Neurosci 31: 2607–2614, 2011. doi: 10.1523/JNEUROSCI.5319-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 922.Kukley M, Barden JA, Steinhäuser C, Jabs R. Distribution of P2X receptors on astrocytes in juvenile rat hippocampus. Glia 36: 11–21, 2001. doi: 10.1002/glia.1091. [DOI] [PubMed] [Google Scholar]
- 923.Kunzelmann P, Schröder W, Traub O, Steinhäuser C, Dermietzel R, Willecke K. Late onset and increasing expression of the gap junction protein connexin30 in adult murine brain and long-term cultured astrocytes. Glia 25: 111–119, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 924.Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci 30: 12950–12957, 2010. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 925.Kuo J, Hariri OR, Bondar G, Ogi J, Micevych P. Membrane estrogen receptor-alpha interacts with metabotropic glutamate receptor type 1a to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology 150: 1369–1376, 2009. doi: 10.1210/en.2008-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 926.Kuo J, Hariri OR, Micevych P. An interaction of oxytocin receptors with metabotropic glutamate receptors in hypothalamic astrocytes. J Neuroendocrinol 21: 1001–1006, 2009. doi: 10.1111/j.1365-2826.2009.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 927.Kur J, Newman EA. Purinergic control of vascular tone in the retina. J Physiol 592: 491–504, 2014. doi: 10.1113/jphysiol.2013.267294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 928.Kurant E. Keeping the CNS clear: glial phagocytic functions in Drosophila. Glia 59: 1304–1311, 2011. doi: 10.1002/glia.21098. [DOI] [PubMed] [Google Scholar]
- 929.La Rovere RM, Roest G, Bultynck G, Parys JB. Intracellular Ca(2+) signaling and Ca(2+) microdomains in the control of cell survival, apoptosis and autophagy. Cell Calcium 60: 74–87, 2016. doi: 10.1016/j.ceca.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 930.Labombarda F, Meffre D, Delespierre B, Krivokapic-Blondiaux S, Chastre A, Thomas P, Pang Y, Lydon JP, Gonzalez SL, De Nicola AF, Schumacher M, Guennoun R. Membrane progesterone receptors localization in the mouse spinal cord. Neuroscience 166: 94–106, 2010. doi: 10.1016/j.neuroscience.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 931.Lafon-Cazal M, Adjali O, Galéotti N, Poncet J, Jouin P, Homburger V, Bockaert J, Marin P. Proteomic analysis of astrocytic secretion in the mouse. Comparison with the cerebrospinal fluid proteome. J Biol Chem 278: 24438–24448, 2003. doi: 10.1074/jbc.M211980200. [DOI] [PubMed] [Google Scholar]
- 932.Lalo U, Palygin O, North RA, Verkhratsky A, Pankratov Y. Age-dependent remodelling of ionotropic signalling in cortical astroglia. Aging Cell 10: 392–402, 2011. doi: 10.1111/j.1474-9726.2011.00682.x. [DOI] [PubMed] [Google Scholar]
- 933.Lalo U, Palygin O, Rasooli-Nejad S, Andrew J, Haydon PG, Pankratov Y. Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol 12: e1001747, 2014. doi: 10.1371/journal.pbio.1001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 934.Lalo U, Palygin O, Verkhratsky A, Grant SG, Pankratov Y. ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD-95 multi-protein complex. Sci Rep 6: 33609, 2016. doi: 10.1038/srep33609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 935.Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci 26: 2673–2683, 2006. doi: 10.1523/JNEUROSCI.4689-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 936.Lalo U, Pankratov Y, Parpura V, Verkhratsky A. Ionotropic receptors in neuronal-astroglial signalling: what is the role of “excitable” molecules in non-excitable cells. Biochim Biophys Acta 1813: 992–1002, 2011. doi: 10.1016/j.bbamcr.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 937.Lalo U, Pankratov Y, Wichert SP, Rossner MJ, North RA, Kirchhoff F, Verkhratsky A. P2X1 and P2X5 subunits form the functional P2X receptor in mouse cortical astrocytes. J Neurosci 28: 5473–5480, 2008. doi: 10.1523/JNEUROSCI.1149-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 938.Lamy CM, Chatton JY. Optical probing of sodium dynamics in neurons and astrocytes. Neuroimage 58: 572–578, 2011. doi: 10.1016/j.neuroimage.2011.06.074. [DOI] [PubMed] [Google Scholar]
- 939.Langer J, Gerkau NJ, Derouiche A, Kleinhans C, Moshrefi-Ravasdjani B, Fredrich M, Kafitz KW, Seifert G, Steinhäuser C, Rose CR. Rapid sodium signaling couples glutamate uptake to breakdown of ATP in perivascular astrocyte endfeet. Glia 65: 293–308, 2017. doi: 10.1002/glia.23092. [DOI] [PubMed] [Google Scholar]
- 940.Langer J, Rose CR. Synaptically induced sodium signals in hippocampal astrocytes in situ. J Physiol 587: 5859–5877, 2009. doi: 10.1113/jphysiol.2009.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 941.Langer J, Stephan J, Theis M, Rose CR. Gap junctions mediate intercellular spread of sodium between hippocampal astrocytes in situ. Glia 60: 239–252, 2012. doi: 10.1002/glia.21259. [DOI] [PubMed] [Google Scholar]
- 942.Larsen BR, Assentoft M, Cotrina ML, Hua SZ, Nedergaard M, Kaila K, Voipio J, MacAulay N. Contributions of the Na+/K+-ATPase, NKCC1, and Kir4.1 to hippocampal K+ clearance and volume responses. Glia 62: 608–622, 2014. doi: 10.1002/glia.22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 943.Larsen BR, MacAulay N. Kir4.1-mediated spatial buffering of K+: experimental challenges in determination of its temporal and quantitative contribution to K+ clearance in the brain. Channels (Austin) 8: 544–550, 2014. doi: 10.4161/19336950.2014.970448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 944.Larsen BR, Stoica A, MacAulay N. Managing brain extracellular K+ during neuronal activity: the physiological role of the Na+/K+-ATPase subunit isoforms. Front Physiol 7: 141, 2016. doi: 10.3389/fphys.2016.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 945.Lascola CD, Kraig RP. Whole-cell chloride currents in rat astrocytes accompany changes in cell morphology. J Neurosci 16: 2532–2545, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 946.Lascola CD, Nelson DJ, Kraig RP. Cytoskeletal actin gates a Cl− channel in neocortical astrocytes. J Neurosci 18: 1679–1692, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 947.Latour I, Gee CE, Robitaille R, Lacaille JC. Differential mechanisms of Ca2+ responses in glial cells evoked by exogenous and endogenous glutamate in rat hippocampus. Hippocampus 11: 132–145, 2001. doi: 10.1002/hipo.1031. [DOI] [PubMed] [Google Scholar]
- 948.Latour I, Hamid J, Beedle AM, Zamponi GW, Macvicar BA. Expression of voltage-gated Ca2+ channel subtypes in cultured astrocytes. Glia 41: 347–353, 2003. doi: 10.1002/glia.10162. [DOI] [PubMed] [Google Scholar]
- 949.Latsari M, Dori I, Antonopoulos J, Chiotelli M, Dinopoulos A. Noradrenergic innervation of the developing and mature visual and motor cortex of the rat brain: a light and electron microscopic immunocytochemical analysis. J Comp Neurol 445: 145–158, 2002. doi: 10.1002/cne.10156. [DOI] [PubMed] [Google Scholar]
- 950.Laube G, Veh RW. Astrocytes, not neurons, show most prominent staining for spermidine/spermine-like immunoreactivity in adult rat brain. Glia 19: 171–179, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 951.Lauritzen KH, Morland C, Puchades M, Holm-Hansen S, Hagelin EM, Lauritzen F, Attramadal H, Storm-Mathisen J, Gjedde A, Bergersen LH. Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex 24: 2784–2795, 2014. doi: 10.1093/cercor/bht136. [DOI] [PubMed] [Google Scholar]
- 952.Lavialle M, Aumann G, Anlauf E, Pröls F, Arpin M, Derouiche A. Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc Natl Acad Sci USA 108: 12915–12919, 2011. doi: 10.1073/pnas.1100957108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 953.Lawal HO, Krantz DE. SLC18: vesicular neurotransmitter transporters for monoamines and acetylcholine. Mol Aspects Med 34: 360–372, 2013. doi: 10.1016/j.mam.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 954.Le Marrec-Croq F, Drago F, Vizioli J, Sautière PE, Lefebvre C. The leech nervous system: a valuable model to study the microglia involvement in regenerative processes. Clin Dev Immunol 2013: 274019, 2013. doi: 10.1155/2013/274019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 955.Le Meur K, Mendizabal-Zubiaga J, Grandes P, Audinat E. GABA release by hippocampal astrocytes. Front Comput Neurosci 6: 59, 2012. doi: 10.3389/fncom.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 956.Leclerc E, Fritz G, Weibel M, Heizmann CW, Galichet A. S100B and S100A6 differentially modulate cell survival by interacting with distinct RAGE (receptor for advanced glycation end products) immunoglobulin domains. J Biol Chem 282: 31317–31331, 2007. doi: 10.1074/jbc.M703951200. [DOI] [PubMed] [Google Scholar]
- 957.Lee DA, Bedont JL, Pak T, Wang H, Song J, Miranda-Angulo A, Takiar V, Charubhumi V, Balordi F, Takebayashi H, Aja S, Ford E, Fishell G, Blackshaw S. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci 15: 700–702, 2012. doi: 10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 958.Lee H, Xie L, Yu M, Kang H, Feng T, Deane R, Logan J, Nedergaard M, Benveniste H. The effect of body posture on brain glymphatic transport. J Neurosci 35: 11034–11044, 2015. doi: 10.1523/JNEUROSCI.1625-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 959.Lee HU, Yamazaki Y, Tanaka KF, Furuya K, Sokabe M, Hida H, Takao K, Miyakawa T, Fujii S, Ikenaka K. Increased astrocytic ATP release results in enhanced excitability of the hippocampus. Glia 61: 210–224, 2013. doi: 10.1002/glia.22427. [DOI] [PubMed] [Google Scholar]
- 960.Lee M, Lee SJ, Choi HJ, Jung YW, Frøkiaer J, Nielsen S, Kwon TH. Regulation of AQP4 protein expression in rat brain astrocytes: role of P2X7 receptor activation. Brain Res 1195: 1–11, 2008. doi: 10.1016/j.brainres.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 961.Lee M, Schwab C, McGeer PL. Astrocytes are GABAergic cells that modulate microglial activity. Glia 59: 152–165, 2011. doi: 10.1002/glia.21087. [DOI] [PubMed] [Google Scholar]
- 962.Lee MC, Ting KK, Adams S, Brew BJ, Chung R, Guillemin GJ. Characterisation of the expression of NMDA receptors in human astrocytes. PLoS One 5: e14123, 2010. doi: 10.1371/journal.pone.0014123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 963.Lee MJ, Nelson I, Houlden H, Sweeney MG, Hilton-Jones D, Blake J, Wood NW, Reilly MM. Six novel connexin32 (GJB1) mutations in X-linked Charcot-Marie-Tooth disease. J Neurol Neurosurg Psychiatry 73: 304–306, 2002. doi: 10.1136/jnnp.73.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 964.Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ. Channel-mediated tonic GABA release from glia. Science 330: 790–796, 2010. doi: 10.1126/science.1184334. [DOI] [PubMed] [Google Scholar]
- 965.Lee SM, Cho YS, Kim TH, Jin MU, Ahn DK, Noguchi K, Bae YC. An ultrastructural evidence for the expression of transient receptor potential ankyrin 1 (TRPA1) in astrocytes in the rat trigeminal caudal nucleus. J Chem Neuroanat 45: 45–49, 2012. doi: 10.1016/j.jchemneu.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 966.Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci 18: 8751–8757, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 967.Leloup C, Allard C, Carneiro L, Fioramonti X, Collins S, Pénicaud L. Glucose and hypothalamic astrocytes: more than a fueling role? Neuroscience 323: 110–120, 2016. doi: 10.1016/j.neuroscience.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 968.Lencesova L, O’Neill A, Resneck WG, Bloch RJ, Blaustein MP. Plasma membrane-cytoskeleton-endoplasmic reticulum complexes in neurons and astrocytes. J Biol Chem 279: 2885–2893, 2004. doi: 10.1074/jbc.M310365200. [DOI] [PubMed] [Google Scholar]
- 969.Lenhossék M. Der feinere Bau des Nervensystems im Lichte neuester Forschung (2nd ed.). Berlin: Fischer's Medicinische Buchhandlung H. Kornfield, 1895. [Google Scholar]
- 970.Lent R, Azevedo FA, Andrade-Moraes CH, Pinto AV. How many neurons do you have? Some dogmas of quantitative neuroscience under revision. Eur J Neurosci 35: 1–9, 2012. doi: 10.1111/j.1460-9568.2011.07923.x. [DOI] [PubMed] [Google Scholar]
- 971.Leonoudakis D, Mailliard W, Wingerd K, Clegg D, Vandenberg C. Inward rectifier potassium channel Kir2.2 is associated with synapse-associated protein SAP97. J Cell Sci 114: 987–998, 2001. [DOI] [PubMed] [Google Scholar]
- 972.Letellier M, Park YK, Chater TE, Chipman PH, Gautam SG, Oshima-Takago T, Goda Y. Astrocytes regulate heterogeneity of presynaptic strengths in hippocampal networks. Proc Natl Acad Sci USA 113: E2685–E2694, 2016. doi: 10.1073/pnas.1523717113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 973.Levitt P, Pintar JE, Breakefield XO. Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc Natl Acad Sci USA 79: 6385–6389, 1982. doi: 10.1073/pnas.79.20.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 974.Leybaert L, Sanderson MJ. Intercellular calcium signaling and flash photolysis of caged compounds. A sensitive method to evaluate gap junctional coupling. Methods Mol Biol 154: 407–430, 2001. [DOI] [PubMed] [Google Scholar]
- 975.Li B, Gu L, Hertz L, Peng L. Expression of nucleoside transporter in freshly isolated neurons and astrocytes from mouse brain. Neurochem Res 38: 2351–2358, 2013. doi: 10.1007/s11064-013-1146-5. [DOI] [PubMed] [Google Scholar]
- 976.Li D, Agulhon C, Schmidt E, Oheim M, Ropert N. New tools for investigating astrocyte-to-neuron communication. Front Cell Neurosci 7: 193, 2013. doi: 10.3389/fncel.2013.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 977.Li D, Hérault K, Silm K, Evrard A, Wojcik S, Oheim M, Herzog E, Ropert N. Lack of evidence for vesicular glutamate transporter expression in mouse astrocytes. J Neurosci 33: 4434–4455, 2013. doi: 10.1523/JNEUROSCI.3667-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 978.Li D, Hérault K, Zylbersztejn K, Lauterbach MA, Guillon M, Oheim M, Ropert N. Astrocyte VAMP3 vesicles undergo Ca2+-independent cycling and modulate glutamate transporter trafficking. J Physiol 593: 2807–2832, 2015. doi: 10.1113/JP270362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 979.Li D, Ropert N, Koulakoff A, Giaume C, Oheim M. Lysosomes are the major vesicular compartment undergoing Ca2+-regulated exocytosis from cortical astrocytes. J Neurosci 28: 7648–7658, 2008. doi: 10.1523/JNEUROSCI.0744-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 980.Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Invest 118: 571–582, 2008. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 981.Li XX, Nomura T, Aihara H, Nishizaki T. Adenosine enhances glial glutamate efflux via A2a adenosine receptors. Life Sci 68: 1343–1350, 2001. doi: 10.1016/S0024-3205(00)01036-5. [DOI] [PubMed] [Google Scholar]
- 982.Lian H, Yang L, Cole A, Sun L, Chiang AC, Fowler SW, Shim DJ, Rodriguez-Rivera J, Taglialatela G, Jankowsky JL, Lu HC, Zheng H. NFκB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron 85: 101–115, 2015. doi: 10.1016/j.neuron.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 983.Liang J, Chao D, Sandhu HK, Yu Y, Zhang L, Balboni G, Kim DH, Xia Y. δ-Opioid receptors up-regulate excitatory amino acid transporters in mouse astrocytes. Br J Pharmacol 171: 5417–5430, 2014. doi: 10.1111/bph.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 984.Lidow MS, Song ZM. Primates exposed to cocaine in utero display reduced density and number of cerebral cortical neurons. J Comp Neurol 435: 263–275, 2001. doi: 10.1002/cne.1028. [DOI] [PubMed] [Google Scholar]
- 985.Liévens JC, Rival T, Iché M, Chneiweiss H, Birman S. Expanded polyglutamine peptides disrupt EGF receptor signaling and glutamate transporter expression in Drosophila. Hum Mol Genet 14: 713–724, 2005. doi: 10.1093/hmg/ddi067. [DOI] [PubMed] [Google Scholar]
- 986.Lim D, Iyer A, Ronco V, Grolla AA, Canonico PL, Aronica E, Genazzani AA. Amyloid β deregulates astroglial mGluR5-mediated calcium signaling via calcineurin and Nf-kB. Glia 61: 1134–1145, 2013. doi: 10.1002/glia.22502. [DOI] [PubMed] [Google Scholar]
- 987.Lipnik-Stangelj M, Carman-Krzan M. Activation of histamine H1-receptor enhances neurotrophic factor secretion from cultured astrocytes. Inflamm Res 53: 245–252, 2004. doi: 10.1007/s00011-004-1247-3. [DOI] [PubMed] [Google Scholar]
- 988.Lippman Bell JJ, Lordkipanidze T, Cobb N, Dunaevsky A. Bergmann glial ensheathment of dendritic spines regulates synapse number without affecting spine motility. Neuron Glia Biol 6: 193–200, 2010. doi: 10.1017/S1740925X10000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 989.Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND, Lucero J, Huang Y, Dwork AJ, Schultz MD, Yu M, Tonti-Filippini J, Heyn H, Hu S, Wu JC, Rao A, Esteller M, He C, Haghighi FG, Sejnowski TJ, Behrens MM, Ecker JR. Global epigenomic reconfiguration during mammalian brain development. Science 341: 1237905, 2013. doi: 10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 990.Liu HT, Akita T, Shimizu T, Sabirov RZ, Okada Y. Bradykinin-induced astrocyte-neuron signalling: glutamate release is mediated by ROS-activated volume-sensitive outwardly rectifying anion channels. J Physiol 587: 2197–2209, 2009. doi: 10.1113/jphysiol.2008.165084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 991.Liu HT, Tashmukhamedov BA, Inoue H, Okada Y, Sabirov RZ. Roles of two types of anion channels in glutamate release from mouse astrocytes under ischemic or osmotic stress. Glia 54: 343–357, 2006. doi: 10.1002/glia.20400. [DOI] [PubMed] [Google Scholar]
- 992.Liu QS, Xu Q, Arcuino G, Kang J, Nedergaard M. Astrocyte-mediated activation of neuronal kainate receptors. Proc Natl Acad Sci USA 101: 3172–3177, 2004. doi: 10.1073/pnas.0306731101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 993.Liu T, Sun L, Xiong Y, Shang S, Guo N, Teng S, Wang Y, Liu B, Wang C, Wang L, Zheng L, Zhang CX, Han W, Zhou Z. Calcium triggers exocytosis from two types of organelles in a single astrocyte. J Neurosci 31: 10593–10601, 2011. doi: 10.1523/JNEUROSCI.6401-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 994.Liu X, Bandyopadhyay BC, Nakamoto T, Singh B, Liedtke W, Melvin JE, Ambudkar I. A role for AQP5 in activation of TRPV4 by hypotonicity: concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J Biol Chem 281: 15485–15495, 2006. doi: 10.1074/jbc.M600549200. [DOI] [PubMed] [Google Scholar]
- 995.Liu X, Li C, Gebremedhin D, Hwang SH, Hammock BD, Falck JR, Roman RJ, Harder DR, Koehler RC. Epoxyeicosatrienoic acid-dependent cerebral vasodilation evoked by metabotropic glutamate receptor activation in vivo. Am J Physiol Heart Circ Physiol 301: H373–H381, 2011. doi: 10.1152/ajpheart.00745.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 996.Liu X, Zhang Z, Guo W, Burnstock G, He C, Xiang Z. The superficial glia limitans of mouse and monkey brain and spinal cord. Anat Rec (Hoboken) 296: 995–1007, 2013. doi: 10.1002/ar.22717. [DOI] [PubMed] [Google Scholar]
- 997.Liu Y, Jia WG, Strosberg AD, Cynader M. Morphology and distribution of neurons and glial cells expressing β-adrenergic receptors in developing kitten visual cortex. Brain Res Dev Brain Res 65: 269–273, 1992. doi: 10.1016/0165-3806(92)90188-3. [DOI] [PubMed] [Google Scholar]
- 998.Liu Y, Zeng X, Hui Y, Zhu C, Wu J, Taylor DH, Ji J, Fan W, Huang Z, Hu J. Activation of α7 nicotinic acetylcholine receptors protects astrocytes against oxidative stress-induced apoptosis: implications for Parkinson’s disease. Neuropharmacology 91: 87–96, 2015. doi: 10.1016/j.neuropharm.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 999.Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450: 56–62, 2007. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- 1000.Loaiza A, Porras OH, Barros LF. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J Neurosci 23: 7337–7342, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1001.Loesch A, Burnstock G. Electron-immunocytochemical localization of P2X1 receptors in the rat cerebellum. Cell Tissue Res 294: 253–260, 1998. doi: 10.1007/s004410051175. [DOI] [PubMed] [Google Scholar]
- 1002.Lohman AW, Isakson BE. Differentiating connexin hemichannels and pannexin channels in cellular ATP release. FEBS Lett 588: 1379–1388, 2014. doi: 10.1016/j.febslet.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1003.Lohr C, Deitmer JW. Ca2+ imaging of glia. In: Calcium Measurement Methods, edited by Verkhratsky A, Petersen OH. New York: Humana Press/Springer, 2010, p. 221–249. doi: 10.1007/978-1-60761-476-0_12. [DOI] [Google Scholar]
- 1004.Lohr C, Deitmer JW. Calcium signaling in invertebrate glial cells. Glia 54: 642–649, 2006. doi: 10.1002/glia.20368. [DOI] [PubMed] [Google Scholar]
- 1005.Lohr C, Deitmer JW. Structural and physiological properties of leech giant glial cells as studied by confocal microscopy. Exp Biol Online 2: 8, 1997. doi: 10.1007/s00898-997-0008-5. [DOI] [Google Scholar]
- 1006.Longden TA, Dunn KM, Draheim HJ, Nelson MT, Weston AH, Edwards G. Intermediate-conductance calcium-activated potassium channels participate in neurovascular coupling. Br J Pharmacol 164: 922–933, 2011. doi: 10.1111/j.1476-5381.2011.01447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1007.Loo DD, Eskandari S, Boorer KJ, Sarkar HK, Wright EM. Role of Cl− in electrogenic Na+-coupled cotransporters GAT1 and SGLT1. J Biol Chem 275: 37414–37422, 2000. doi: 10.1074/jbc.M007241200. [DOI] [PubMed] [Google Scholar]
- 1008.Losi G, Mariotti L, Carmignoto G. GABAergic interneuron to astrocyte signalling: a neglected form of cell communication in the brain. Philos Trans R Soc Lond B Biol Sci 369: 20130609, 2014. doi: 10.1098/rstb.2013.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1009.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature 523: 337–341, 2015. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1010.Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci 27: 12255–12266, 2007. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1011.Lovatt D, Xu Q, Liu W, Takano T, Smith NA, Schnermann J, Tieu K, Nedergaard M. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc Natl Acad Sci USA 109: 6265–6270, 2012. doi: 10.1073/pnas.1120997109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1012.Lovinger DM, White G. Ethanol potentiation of 5-hydroxytryptamine3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol Pharmacol 40: 263–270, 1991. [PubMed] [Google Scholar]
- 1013.Lu CC, Hilgemann DW. GAT1 (GABA:Na+:Cl−) cotransport function. Steady state studies in giant Xenopus oocyte membrane patches. J Gen Physiol 114: 429–444, 1999. doi: 10.1085/jgp.114.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1014.Lu DC, Zhang H, Zador Z, Verkman AS. Impaired olfaction in mice lacking aquaporin-4 water channels. FASEB J 22: 3216–3223, 2008. doi: 10.1096/fj.07-104836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1015.Lu H, Burns D, Garnier P, Wei G, Zhu K, Ying W. P2X7 receptors mediate NADH transport across the plasma membranes of astrocytes. Biochem Biophys Res Commun 362: 946–950, 2007. doi: 10.1016/j.bbrc.2007.08.095. [DOI] [PubMed] [Google Scholar]
- 1016.Lu Z. Mechanism of rectification in inward-rectifier K+ channels. Annu Rev Physiol 66: 103–129, 2004. doi: 10.1146/annurev.physiol.66.032102.150822. [DOI] [PubMed] [Google Scholar]
- 1017.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev 52: 375–414, 2000. [PubMed] [Google Scholar]
- 1018.Ludwin SK, Kosek JC, Eng LF. The topographical distribution of S-100 and GFA proteins in the adult rat brain: an immunohistochemical study using horseradish peroxidase-labelled antibodies. J Comp Neurol 165: 197–207, 1976. doi: 10.1002/cne.901650206. [DOI] [PubMed] [Google Scholar]
- 1019.Lugaro E. Sulle funzioni della nevroglia. Riv Patol Nerv Ment 12: 225–233, 1907. [Google Scholar]
- 1020.Lundgaard I, Li B, Xie L, Kang H, Sanggaard S, Haswell JD, Sun W, Goldman S, Blekot S, Nielsen M, Takano T, Deane R, Nedergaard M. Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nat Commun 6: 6807, 2015. doi: 10.1038/ncomms7807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1021.Lundgaard I, Lu ML, Yang E, Peng W, Mestre H, Hitomi E, Deane R, Nedergaard M. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J Cereb Blood Flow Metab 37: 2112–2124, 2017. doi: 10.1177/0271678X16661202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1022.Lundgaard I, Osório MJ, Kress BT, Sanggaard S, Nedergaard M. White matter astrocytes in health and disease. Neuroscience 276: 161–173, 2014. doi: 10.1016/j.neuroscience.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1023.Luque JM, Richards JG. Expression of NMDA 2B receptor subunit mRNA in Bergmann glia. Glia 13: 228–232, 1995. doi: 10.1002/glia.440130309. [DOI] [PubMed] [Google Scholar]
- 1024.Lushnikova I, Skibo G, Muller D, Nikonenko I. Synaptic potentiation induces increased glial coverage of excitatory synapses in CA1 hippocampus. Hippocampus 19: 753–762, 2009. doi: 10.1002/hipo.20551. [DOI] [PubMed] [Google Scholar]
- 1025.Lyons DA, Talbot WS. Glial cell development and function in zebrafish. Cold Spring Harb Perspect Biol 7: a020586, 2014. doi: 10.1101/cshperspect.a020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1026.Lytton J. Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem J 406: 365–382, 2007. doi: 10.1042/BJ20070619. [DOI] [PubMed] [Google Scholar]
- 1027.Ma B, Buckalew R, Du Y, Kiyoshi CM, Alford CC, Wang W, McTigue DM, Enyeart JJ, Terman D, Zhou M. Gap junction coupling confers isopotentiality on astrocyte syncytium. Glia 64: 214–226, 2016. doi: 10.1002/glia.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1028.Ma B, Xu G, Wang W, Enyeart JJ, Zhou M. Dual patch voltage clamp study of low membrane resistance astrocytes in situ. Mol Brain 7: 18, 2014. doi: 10.1186/1756-6606-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1029.Ma L, Xie YP, Zhou M, Chen H. Silent TWIK-1 potassium channels conduct monovalent cation currents. Biophys J 102: L34–L36, 2012. doi: 10.1016/j.bpj.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1030.Ma L, Zhang X, Chen H. TWIK-1 two-pore domain potassium channels change ion selectivity and conduct inward leak sodium currents in hypokalemia. Sci Signal 4: ra37, 2011. doi: 10.1126/scisignal.2001726. [DOI] [PubMed] [Google Scholar]
- 1031.Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J Clin Invest 100: 957–962, 1997. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1032.Ma W, Compan V, Zheng W, Martin E, North RA, Verkhratsky A, Surprenant A. Pannexin 1 forms an anion-selective channel. Pflugers Arch 463: 585–592, 2012. doi: 10.1007/s00424-012-1077-z. [DOI] [PubMed] [Google Scholar]
- 1033.Ma Z, Stork T, Bergles DE, Freeman MR. Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature 539: 428–432, 2016. doi: 10.1038/nature20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1034.MacAulay N, Gether U, Klaeke DA, Zeuthen T. Passive water and urea permeability of a human Na+-glutamate cotransporter expressed in Xenopus oocytes. J Physiol 542: 817–828, 2002. doi: 10.1113/jphysiol.2002.020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1035.Macaulay N, Zeuthen T. Glial K+ clearance and cell swelling: key roles for cotransporters and pumps. Neurochem Res 37: 2299–2309, 2012. doi: 10.1007/s11064-012-0731-3. [DOI] [PubMed] [Google Scholar]
- 1036.MacAulay N, Zeuthen T. Water transport between CNS compartments: contributions of aquaporins and cotransporters. Neuroscience 168: 941–956, 2010. doi: 10.1016/j.neuroscience.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 1037.MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron 50: 869–881, 2006. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 1038.MacVicar BA. Voltage-dependent calcium channels in glial cells. Science 226: 1345–1347, 1984. doi: 10.1126/science.6095454. [DOI] [PubMed] [Google Scholar]
- 1039.MacVicar BA, Feighan D, Brown A, Ransom B. Intrinsic optical signals in the rat optic nerve: role for K+ uptake via NKCC1 and swelling of astrocytes. Glia 37: 114–123, 2002. doi: 10.1002/glia.10023. [DOI] [PubMed] [Google Scholar]
- 1040.MacVicar BA, Hochman D, Delay MJ, Weiss S. Modulation of intracellular Ca2+ in cultured astrocytes by influx through voltage-activated Ca2+ channels. Glia 4: 448–455, 1991. doi: 10.1002/glia.440040504. [DOI] [PubMed] [Google Scholar]
- 1041.MacVicar BA, Tse FW. Norepinephrine and cyclic adenosine 3′:5′-cyclic monophosphate enhance a nifedipine-sensitive calcium current in cultured rat astrocytes. Glia 1: 359–365, 1988. doi: 10.1002/glia.440010602. [DOI] [PubMed] [Google Scholar]
- 1042.MacVicar BA, Tse FW, Crichton SA, Kettenmann H. GABA-activated Cl− channels in astrocytes of hippocampal slices. J Neurosci 9: 3577–3583, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1043.Maderspach K, Solomonia R. Glial and neuronal opioid receptors: apparent positive cooperativity observed in intact cultured cells. Brain Res 441: 41–47, 1988. doi: 10.1016/0006-8993(88)91381-9. [DOI] [PubMed] [Google Scholar]
- 1044.Madsen KK, White HS, Schousboe A. Neuronal and non-neuronal GABA transporters as targets for antiepileptic drugs. Pharmacol Ther 125: 394–401, 2010. doi: 10.1016/j.pharmthera.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 1045.Magavi S, Friedmann D, Banks G, Stolfi A, Lois C. Coincident generation of pyramidal neurons and protoplasmic astrocytes in neocortical columns. J Neurosci 32: 4762–4772, 2012. doi: 10.1523/JNEUROSCI.3560-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1046.Magini G. Nervoglia e cellule nervose cerebrali nei feti. Pavia: Tipografia Fratelli Fusi, 1888, p. 281–291. [Google Scholar]
- 1047.Magistretti PJ, Morrison JH, Shoemaker WJ, Sapin V, Bloom FE. Vasoactive intestinal polypeptide induces glycogenolysis in mouse cortical slices: a possible regulatory mechanism for the local control of energy metabolism. Proc Natl Acad Sci USA 78: 6535–6539, 1981. doi: 10.1073/pnas.78.10.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1048.Magistretti PJ, Sorg O, Martin J-L. Regulation of glycogen metabolism in astrocytes: physiological, pharmacological, and pathological aspects. In: Astrocytes: Pharmacology and Function edited by Murphy S. San Diego, CA: Academic, 1993, p. 243–265. doi: 10.1016/B978-0-12-511370-0.50015-1. [DOI] [Google Scholar]
- 1049.Magnotti LM, Goodenough DA, Paul DL. Functional heterotypic interactions between astrocyte and oligodendrocyte connexins. Glia 59: 26–34, 2011. doi: 10.1002/glia.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1050.Magoski NS, Walz W. Ionic dependence of a P2-purinoceptor mediated depolarization of cultured astrocytes. J Neurosci Res 32: 530–538, 1992. doi: 10.1002/jnr.490320408. [DOI] [PubMed] [Google Scholar]
- 1051.Mahesh VB, Dhandapani KM, Brann DW. Role of astrocytes in reproduction and neuroprotection. Mol Cell Endocrinol 246: 1–9, 2006. doi: 10.1016/j.mce.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 1052.Mak DO, Foskett JK. Inositol 1,4,5-trisphosphate receptors in the endoplasmic reticulum: a single-channel point of view. Cell Calcium 58: 67–78, 2015. doi: 10.1016/j.ceca.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1053.Makar TK, Nedergaard M, Preuss A, Gelbard AS, Perumal AS, Cooper AJ. Vitamin E, ascorbate, glutathione, glutathione disulfide, and enzymes of glutathione metabolism in cultures of chick astrocytes and neurons: evidence that astrocytes play an important role in antioxidative processes in the brain. J Neurochem 62: 45–53, 1994. doi: 10.1046/j.1471-4159.1994.62010045.x. [DOI] [PubMed] [Google Scholar]
- 1054.Makara JK, Rappert A, Matthias K, Steinhäuser C, Spät A, Kettenmann H. Astrocytes from mouse brain slices express ClC-2-mediated Cl− currents regulated during development and after injury. Mol Cell Neurosci 23: 521–530, 2003. doi: 10.1016/S1044-7431(03)00080-0. [DOI] [PubMed] [Google Scholar]
- 1055.Malarkey EB, Ni Y, Parpura V. Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia 56: 821–835, 2008. doi: 10.1002/glia.20656. [DOI] [PubMed] [Google Scholar]
- 1056.Malarkey EB, Parpura V. Temporal characteristics of vesicular fusion in astrocytes: examination of synaptobrevin 2-laden vesicles at single vesicle resolution. J Physiol 589: 4271–4300, 2011. doi: 10.1113/jphysiol.2011.210435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1057.Maneshi MM, Maki B, Gnanasambandam R, Belin S, Popescu GK, Sachs F, Hua SZ. Mechanical stress activates NMDA receptors in the absence of agonists. Sci Rep 7: 39610, 2017. doi: 10.1038/srep39610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1058.Mangia S, Giove F, Tkác I, Logothetis NK, Henry PG, Olman CA, Maraviglia B, Di Salle F, Uğurbil K. Metabolic and hemodynamic events after changes in neuronal activity: current hypotheses, theoretical predictions and in vivo NMR experimental findings. J Cereb Blood Flow Metab 29: 441–463, 2009. doi: 10.1038/jcbfm.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1059.Mannari T, Morita S, Furube E, Tominaga M, Miyata S. Astrocytic TRPV1 ion channels detect blood-borne signals in the sensory circumventricular organs of adult mouse brains. Glia 61: 957–971, 2013. doi: 10.1002/glia.22488. [DOI] [PubMed] [Google Scholar]
- 1060.Mantyh PW, Rogers SD, Allen CJ, Catton MD, Ghilardi JR, Levin LA, Maggio JE, Vigna SR. β2-Adrenergic receptors are expressed by glia in vivo in the normal and injured central nervous system in the rat, rabbit, and human. J Neurosci 15: 152–164, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1061.Manzini I, Schweer TS, Schild D. Improved fluorescent (calcium indicator) dye uptake in brain slices by blocking multidrug resistance transporters. J Neurosci Methods 167: 140–147, 2008. doi: 10.1016/j.jneumeth.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 1062.Marcaggi P, Attwell D. Role of glial amino acid transporters in synaptic transmission and brain energetics. Glia 47: 217–225, 2004. doi: 10.1002/glia.20027. [DOI] [PubMed] [Google Scholar]
- 1063.Marcaggi P, Billups D, Attwell D. The role of glial glutamate transporters in maintaining the independent operation of juvenile mouse cerebellar parallel fibre synapses. J Physiol 552: 89–107, 2003. doi: 10.1113/jphysiol.2003.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1064.Marcaggi P, Jeanne M, Coles JA. Neuron-glial trafficking of NH4+ and K+: separate routes of uptake into glial cells of bee retina. Eur J Neurosci 19: 966–976, 2004. doi: 10.1111/j.0953-816X.2004.03165.x. [DOI] [PubMed] [Google Scholar]
- 1065.Marchaland J, Calì C, Voglmaier SM, Li H, Regazzi R, Edwards RH, Bezzi P. Fast subplasma membrane Ca2+ transients control exo-endocytosis of synaptic-like microvesicles in astrocytes. J Neurosci 28: 9122–9132, 2008. doi: 10.1523/JNEUROSCI.0040-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1066.Mariotti L, Losi G, Sessolo M, Marcon I, Carmignoto G. The inhibitory neurotransmitter GABA evokes long-lasting Ca2+ oscillations in cortical astrocytes. Glia 64: 363–373, 2016. doi: 10.1002/glia.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1067.Marpegan L, Swanstrom AE, Chung K, Simon T, Haydon PG, Khan SK, Liu AC, Herzog ED, Beaulé C. Circadian regulation of ATP release in astrocytes. J Neurosci 31: 8342–8350, 2011. doi: 10.1523/JNEUROSCI.6537-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1068.Marsault R, Vigne P, Breittmayer JP, Frelin C. Astrocytes are target cells for endothelins and sarafotoxin. J Neurochem 54: 2142–2144, 1990. doi: 10.1111/j.1471-4159.1990.tb04921.x. [DOI] [PubMed] [Google Scholar]
- 1069.Martín ED, Fernández M, Perea G, Pascual O, Haydon PG, Araque A, Ceña V. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia 55: 36–45, 2007. doi: 10.1002/glia.20431. [DOI] [PubMed] [Google Scholar]
- 1070.Martineau M, Parpura V, Mothet JP. Cell-type specific mechanisms of d-serine uptake and release in the brain. Front Synaptic Neurosci 6: 12, 2014. doi: 10.3389/fnsyn.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1071.Martineau M, Shi T, Puyal J, Knolhoff AM, Dulong J, Gasnier B, Klingauf J, Sweedler JV, Jahn R, Mothet JP. Storage and uptake of d-serine into astrocytic synaptic-like vesicles specify gliotransmission. J Neurosci 33: 3413–3423, 2013. doi: 10.1523/JNEUROSCI.3497-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1072.Martínez-Rodríguez R, Tonda A, Gragera RR, Paz-Doel R, García-Cordovilla R, Fernández-Fernández E, Fernández AM, González-Romero F, López-Bravo A. Synaptic and non-synaptic immunolocalization of GABA and glutamate acid decarboxylase (GAD) in cerebellar cortex of rat. Cell Mol Biol (Noisy-le-grand) 39: 115–123, 1993. [PubMed] [Google Scholar]
- 1073.Martinez R, Gomes FC. Neuritogenesis induced by thyroid hormone-treated astrocytes is mediated by epidermal growth factor/mitogen-activated protein kinase-phosphatidylinositol 3-kinase pathways and involves modulation of extracellular matrix proteins. J Biol Chem 277: 49311–49318, 2002. doi: 10.1074/jbc.M209284200. [DOI] [PubMed] [Google Scholar]
- 1074.Martinotti C. Contributo allo studio della corteccia cerebrale, ed all’origine centrale dei nervi. Ann Fren Sci Affin 1: 314–332, 1889. [Google Scholar]
- 1075.Mashanov VS, Zueva OR, Garcia-Arraras JE. Organization of glial cells in the adult sea cucumber central nervous system. Glia 58: 1581–1593, 2010. [DOI] [PubMed] [Google Scholar]
- 1076.Mashanov VS, Zueva OR, Heinzeller T, Aschauer B, Naumann WW, Grondona JM, Cifuentes M, Garcia-Arraras JE. The central nervous system of sea cucumbers (Echinodermata: Holothuroidea) shows positive immunostaining for a chordate glial secretion. Front Zool 6: 11, 2009. doi: 10.1186/1742-9994-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1077.Masmoudi-Kouki O, Gandolfo P, Castel H, Leprince J, Fournier A, Dejda A, Vaudry H, Tonon MC. Role of PACAP and VIP in astroglial functions. Peptides 28: 1753–1760, 2007. doi: 10.1016/j.peptides.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 1078.Mason S, Smart D, Marshall IC, McKnight A, Skepper JN, McNulty S. Identification and characterisation of functional bombesin receptors in human astrocytes. Eur J Pharmacol 438: 25–34, 2002. doi: 10.1016/S0014-2999(02)01268-2. [DOI] [PubMed] [Google Scholar]
- 1079.Massa PT, Mugnaini E. Cell junctions and intramembrane particles of astrocytes and oligodendrocytes: a freeze-fracture study. Neuroscience 7: 523–538, 1982. doi: 10.1016/0306-4522(82)90285-8. [DOI] [PubMed] [Google Scholar]
- 1080.Massie A, Schallier A, Kim SW, Fernando R, Kobayashi S, Beck H, De Bundel D, Vermoesen K, Bannai S, Smolders I, Conrad M, Plesnila N, Sato H, Michotte Y. Dopaminergic neurons of system x(c)−-deficient mice are highly protected against 6-hydroxydopamine-induced toxicity. FASEB J 25: 1359–1369, 2011. doi: 10.1096/fj.10-177212. [DOI] [PubMed] [Google Scholar]
- 1081.Mathiesen C, Brazhe A, Thomsen K, Lauritzen M. Spontaneous calcium waves in Bergman glia increase with age and hypoxia and may reduce tissue oxygen. J Cereb Blood Flow Metab 33: 161–169, 2013. doi: 10.1038/jcbfm.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1082.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58: 1094–1103, 2010. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 1083.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics 73: 1907–1920, 2010. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 1084.Matos M, Augusto E, Agostinho P, Cunha RA, Chen JF. Antagonistic interaction between adenosine A2A receptors and Na+/K+-ATPase-α2 controlling glutamate uptake in astrocytes. J Neurosci 33: 18492–18502, 2013. doi: 10.1523/JNEUROSCI.1828-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1085.Matos M, Augusto E, Santos-Rodrigues AD, Schwarzschild MA, Chen JF, Cunha RA, Agostinho P. Adenosine A2A receptors modulate glutamate uptake in cultured astrocytes and gliosomes. Glia 60: 702–716, 2012. doi: 10.1002/glia.22290. [DOI] [PubMed] [Google Scholar]
- 1086.Matos M, Shen HY, Augusto E, Wang Y, Wei CJ, Wang YT, Agostinho P, Boison D, Cunha RA, Chen JF. Deletion of adenosine A2A receptors from astrocytes disrupts glutamate homeostasis leading to psychomotor and cognitive impairment: relevance to schizophrenia. Biol Psychiatry 78: 763–774, 2015. doi: 10.1016/j.biopsych.2015.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1087.Matsutani S, Yamamoto N. Neuronal regulation of astrocyte morphology in vitro is mediated by GABAergic signaling. Glia 20: 1–9, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 1088.Matthias K, Kirchhoff F, Seifert G, Hüttmann K, Matyash M, Kettenmann H, Steinhäuser C. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci 23: 1750–1758, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1089.Matyash M, Matyash V, Nolte C, Sorrentino V, Kettenmann H. Requirement of functional ryanodine receptor type 3 for astrocyte migration. FASEB J 16: 84–86, 2002. doi: 10.1096/fj.01-0380fje. [DOI] [PubMed] [Google Scholar]
- 1090.Mauch DH, Nägler K, Schumacher S, Göritz C, Müller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science 294: 1354–1357, 2001. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 1091.Maucler C, Pernot P, Vasylieva N, Pollegioni L, Marinesco S. In vivo d-serine hetero-exchange through alanine-serine-cysteine (ASC) transporters detected by microelectrode biosensors. ACS Chem Neurosci 4: 772–781, 2013. doi: 10.1021/cn4000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1092.Mayer ML. Structure and mechanism of glutamate receptor ion channel assembly, activation and modulation. Curr Opin Neurobiol 21: 283–290, 2011. doi: 10.1016/j.conb.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1093.McCarthy KD, Salm AK. Pharmacologically-distinct subsets of astroglia can be identified by their calcium response to neuroligands. Neuroscience 41: 325–333, 1991. doi: 10.1016/0306-4522(91)90330-Q. [DOI] [PubMed] [Google Scholar]
- 1094.McKenna MC. Glutamate pays its own way in astrocytes. Front Endocrinol (Lausanne) 4: 191, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1095.McKenzie JC, Berman NE, Thomas CR, Young JK, Compton LY, Cothran LN, Liu WL, Klein RM. Atrial natriuretic peptide-like (ANP-LIR) and ANP prohormone immunoreactive astrocytes and neurons of human cerebral cortex. Glia 12: 228–243, 1994. doi: 10.1002/glia.440120308. [DOI] [PubMed] [Google Scholar]
- 1096.McKhann GM II, D’Ambrosio R, Janigro D. Heterogeneity of astrocyte resting membrane potentials and intercellular coupling revealed by whole-cell and gramicidin-perforated patch recordings from cultured neocortical and hippocampal slice astrocytes. J Neurosci 17: 6850–6863, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1097.McKimmie CS, Fazakerley JK. In response to pathogens, glial cells dynamically and differentially regulate Toll-like receptor gene expression. J Neuroimmunol 169: 116–125, 2005. doi: 10.1016/j.jneuroim.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 1098.Meier SD, Kafitz KW, Rose CR. Developmental profile and mechanisms of GABA-induced calcium signaling in hippocampal astrocytes. Glia 56: 1127–1137, 2008. doi: 10.1002/glia.20684. [DOI] [PubMed] [Google Scholar]
- 1099.Meinertzhagen IA, O’Neil SD. Synaptic organization of columnar elements in the lamina of the wild type in Drosophila melanogaster. J Comp Neurol 305: 232–263, 1991. doi: 10.1002/cne.903050206. [DOI] [PubMed] [Google Scholar]
- 1100.Mele T, Jurič DM. Identification and pharmacological characterization of the histamine H3 receptor in cultured rat astrocytes. Eur J Pharmacol 720: 198–204, 2013. doi: 10.1016/j.ejphar.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 1101.Melner MH, Low KG, Allen RG, Nielsen CP, Young SL, Saneto RP. The regulation of proenkephalin expression in a distinct population of glial cells. EMBO J 9: 791–796, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1102.Même W, Ezan P, Venance L, Glowinski J, Giaume C. ATP-induced inhibition of gap junctional communication is enhanced by interleukin-1 β treatment in cultured astrocytes. Neuroscience 126: 95–104, 2004. doi: 10.1016/j.neuroscience.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 1103.Meme W, Vandecasteele M, Giaume C, Venance L. Electrical coupling between hippocampal astrocytes in rat brain slices. Neurosci Res 63: 236–243, 2009. doi: 10.1016/j.neures.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 1104.Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Connexins are critical for normal myelination in the CNS. J Neurosci 23: 5963–5973, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1105.Menichella DM, Majdan M, Awatramani R, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Genetic and physiological evidence that oligodendrocyte gap junctions contribute to spatial buffering of potassium released during neuronal activity. J Neurosci 26: 10984–10991, 2006. doi: 10.1523/JNEUROSCI.0304-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1106.Mennerick S, Zorumski CF. Glial contributions to excitatory neurotransmission in cultured hippocampal cells. Nature 368: 59–62, 1994. doi: 10.1038/368059a0. [DOI] [PubMed] [Google Scholar]
- 1107.Mentlein R, Buchholz C, Krisch B. Somatostatin-binding sites on rat telencephalic astrocytes. Light- and electron-microscopic studies in vitro and in vivo. Cell Tissue Res 262: 431–443, 1990. doi: 10.1007/BF00305240. [DOI] [PubMed] [Google Scholar]
- 1108.Merkle FT, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci USA 101: 17528–17532, 2004. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1109.Metea MR, Kofuji P, Newman EA. Neurovascular coupling is not mediated by potassium siphoning from glial cells. J Neurosci 27: 2468–2471, 2007. doi: 10.1523/JNEUROSCI.3204-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1110.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci 26: 2862–2870, 2006. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1111.Mhatre AN, Stern RE, Li J, Lalwani AK. Aquaporin 4 expression in the mammalian inner ear and its role in hearing. Biochem Biophys Res Commun 297: 987–996, 2002. doi: 10.1016/S0006-291X(02)02296-9. [DOI] [PubMed] [Google Scholar]
- 1112.Micevych PE, Chaban V, Ogi J, Dewing P, Lu JK, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology 148: 782–789, 2007. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- 1113.Michel JP, Sakamoto N, Bouvier R, Tommasi M, Pearson J. Substance P-immunoreactive astrocytes related to deep white matter and striatal blood vessels in human brain. Brain Res 377: 383–387, 1986. doi: 10.1016/0006-8993(86)90886-3. [DOI] [PubMed] [Google Scholar]
- 1114.Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394: 192–195, 1998. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 1115.Miller FD, Gauthier AS. Timing is everything: making neurons versus glia in the developing cortex. Neuron 54: 357–369, 2007. doi: 10.1016/j.neuron.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 1116.Miller RL, Loewy AD. ENaC γ-expressing astrocytes in the circumventricular organs, white matter, and ventral medullary surface: sites for Na+ regulation by glial cells. J Chem Neuroanat 53: 72–80, 2013. doi: 10.1016/j.jchemneu.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1117.Miller RL, Wang MH, Gray PA, Salkoff LB, Loewy AD. ENaC-expressing neurons in the sensory circumventricular organs become c-Fos activated following systemic sodium changes. Am J Physiol Regul Integr Comp Physiol 305: R1141–R1152, 2013. doi: 10.1152/ajpregu.00242.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1118.Milner TA, Lee A, Aicher SA, Rosin DL. Hippocampal α2a-adrenergic receptors are located predominantly presynaptically but are also found postsynaptically and in selective astrocytes. J Comp Neurol 395: 310–327, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 1119.Min R, Nevian T. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat Neurosci 15: 746–753, 2012. doi: 10.1038/nn.3075. [DOI] [PubMed] [Google Scholar]
- 1120.Minelli A, Castaldo P, Gobbi P, Salucci S, Magi S, Amoroso S. Cellular and subcellular localization of Na+-Ca2+ exchanger protein isoforms, NCX1, NCX2, and NCX3 in cerebral cortex and hippocampus of adult rat. Cell Calcium 41: 221–234, 2007. doi: 10.1016/j.ceca.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 1121.Minelli A, DeBiasi S, Brecha NC, Zuccarello LV, Conti F. GAT-3, a high-affinity GABA plasma membrane transporter, is localized to astrocytic processes, and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J Neurosci 16: 6255–6264, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1122.Minieri L, Pivonkova H, Harantova L, Anderova M, Ferroni S. Intracellular Na(+) inhibits volume-regulated anion channel in rat cortical astrocytes. J Neurochem 132: 286–300, 2015. doi: 10.1111/jnc.12962. [DOI] [PubMed] [Google Scholar]
- 1123.Minke B. The history of the Drosophila TRP channel: the birth of a new channel superfamily. J Neurogenet 24: 216–233, 2010. doi: 10.3109/01677063.2010.514369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1124.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 3: 265–278, 2008. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1125.Mishima T, Hirase H. In vivo intracellular recording suggests that gray matter astrocytes in mature cerebral cortex and hippocampus are electrophysiologically homogeneous. J Neurosci 30: 3093–3100, 2010. doi: 10.1523/JNEUROSCI.5065-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1126.Mishima T, Sakatani S, Hirase H. Intracellular labeling of single cortical astrocytes in vivo. J Neurosci Methods 166: 32–40, 2007. doi: 10.1016/j.jneumeth.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 1127.Mishra A, Newman EA. Inhibition of inducible nitric oxide synthase reverses the loss of functional hyperemia in diabetic retinopathy. Glia 58: 1996–2004, 2010. doi: 10.1002/glia.21068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1128.Mitchell SJ, Silver RA. GABA spillover from single inhibitory axons suppresses low-frequency excitatory transmission at the cerebellar glomerulus. J Neurosci 20: 8651–8658, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1129.Mittaud P, Labourdette G, Zingg H, Guenot-Di Scala D. Neurons modulate oxytocin receptor expression in rat cultured astrocytes: involvement of TGF-β and membrane components. Glia 37: 169–177, 2002. doi: 10.1002/glia.10029. [DOI] [PubMed] [Google Scholar]
- 1130.Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol 101: 249–255, 2001. [DOI] [PubMed] [Google Scholar]
- 1131.Miya K, Inoue R, Takata Y, Abe M, Natsume R, Sakimura K, Hongou K, Miyawaki T, Mori H. Serine racemase is predominantly localized in neurons in mouse brain. J Comp Neurol 510: 641–654, 2008. doi: 10.1002/cne.21822. [DOI] [PubMed] [Google Scholar]
- 1132.Miyake T, Kitamura T. Glutamine synthetase immunoreactivity in two types of mouse brain glial cells. Brain Res 586: 53–60, 1992. doi: 10.1016/0006-8993(92)91370-T. [DOI] [PubMed] [Google Scholar]
- 1133.Miyano K, Morioka N, Sugimoto T, Shiraishi S, Uezono Y, Nakata Y. Activation of the neurokinin-1 receptor in rat spinal astrocytes induces Ca2+ release from IP3-sensitive Ca2+ stores and extracellular Ca2+ influx through TRPC3. Neurochem Int 57: 923–934, 2010. doi: 10.1016/j.neuint.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 1134.Miyazaki I, Asanuma M, Diaz-Corrales FJ, Miyoshi K, Ogawa N. Direct evidence for expression of dopamine receptors in astrocytes from basal ganglia. Brain Res 1029: 120–123, 2004. doi: 10.1016/j.brainres.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 1135.Mladinov M, Mayer D, Brčić L, Wolstencroft E, thi Man N, Holt I, Hof PR, Morris GE, Šimić G. Astrocyte expression of D2-like dopamine receptors in the prefrontal cortex. Transl Neurosci 1: 238–242, 2010. doi: 10.2478/v10134-010-0035-6. [DOI] [Google Scholar]
- 1136.Moldrich G, Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides 21: 1735–1742, 2000. doi: 10.1016/S0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- 1137.Molina-Holgado F, Molina-Holgado E, Guaza C, Rothwell NJ. Role of CB1 and CB2 receptors in the inhibitory effects of cannabinoids on lipopolysaccharide-induced nitric oxide release in astrocyte cultures. J Neurosci Res 67: 829–836, 2002. doi: 10.1002/jnr.10165. [DOI] [PubMed] [Google Scholar]
- 1138.Molnár T, Dobolyi A, Nyitrai G, Barabás P, Héja L, Emri Z, Palkovits M, Kardos J. Calcium signals in the nucleus accumbens: activation of astrocytes by ATP and succinate. BMC Neurosci 12: 96, 2011. doi: 10.1186/1471-2202-12-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1139.Molnár T, Yarishkin O, Iuso A, Barabas P, Jones B, Marc RE, Phuong TT, Križaj D. Store-operated calcium entry in Müller glia is controlled by synergistic activation of TRPC and orai channels. J Neurosci 36: 3184–3198, 2016. doi: 10.1523/JNEUROSCI.4069-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1140.Molofsky AV, Deneen B. Astrocyte development: a guide for the perplexed. Glia 63: 1320–1329, 2015. doi: 10.1002/glia.22836. [DOI] [PubMed] [Google Scholar]
- 1141.Monai H, Ohkura M, Tanaka M, Oe Y, Konno A, Hirai H, Mikoshiba K, Itohara S, Nakai J, Iwai Y, Hirase H. Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat Commun 7: 11100, 2016. doi: 10.1038/ncomms11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1142.Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci 19: 1464–1472, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1143.Mong JA, McCarthy MM. Ontogeny of sexually dimorphic astrocytes in the neonatal rat arcuate. Brain Res Dev Brain Res 139: 151–158, 2002. doi: 10.1016/S0165-3806(02)00541-2. [DOI] [PubMed] [Google Scholar]
- 1144.Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. Glia 54: 700–715, 2006. doi: 10.1002/glia.20367. [DOI] [PubMed] [Google Scholar]
- 1145.Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci 24: 2633–2642, 2004. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1146.Montell C. The history of TRP channels, a commentary and reflection. Pflugers Arch 461: 499–506, 2011. doi: 10.1007/s00424-010-0920-3. [DOI] [PubMed] [Google Scholar]
- 1147.Montero TD, Orellana JA. Hemichannels: new pathways for gliotransmitter release. Neuroscience 286: 45–59, 2015. doi: 10.1016/j.neuroscience.2014.11.048. [DOI] [PubMed] [Google Scholar]
- 1148.Monzon-Mayor M, Yanes C, Ghandour MS, de Barry J, Gombos G. Glial fibrillary acidic protein and vimentin immunohistochemistry in the developing and adult midbrain of the lizard Gallotia galloti. J Comp Neurol 295: 569–579, 1990. doi: 10.1002/cne.902950406. [DOI] [PubMed] [Google Scholar]
- 1149.Morel L, Higashimori H, Tolman M, Yang Y. VGluT1+ neuronal glutamatergic signaling regulates postnatal developmental maturation of cortical protoplasmic astroglia. J Neurosci 34: 10950–10962, 2014. doi: 10.1523/JNEUROSCI.1167-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1150.Moreno C, Sampieri A, Vivas O, Peña-Segura C, Vaca L. STIM1 and Orai1 mediate thrombin-induced Ca2+ influx in rat cortical astrocytes. Cell Calcium 52: 457–467, 2012. doi: 10.1016/j.ceca.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 1151.Morgello S, Uson RR, Schwartz EJ, Haber RS. The human blood-brain barrier glucose transporter (GLUT1) is a glucose transporter of gray matter astrocytes. Glia 14: 43–54, 1995. doi: 10.1002/glia.440140107. [DOI] [PubMed] [Google Scholar]
- 1152.Morita M, Kudo Y. Growth factors upregulate astrocyte [Ca2+]i oscillation by increasing SERCA2b expression. Glia 58: 1988–1995, 2010. doi: 10.1002/glia.21067. [DOI] [PubMed] [Google Scholar]
- 1153.Morizawa Y, Sato K, Takaki J, Kawasaki A, Shibata K, Suzuki T, Ohta S, Koizumi S. Cell-autonomous enhancement of glutamate-uptake by female astrocytes. Cell Mol Neurobiol 32: 953–956, 2012. doi: 10.1007/s10571-012-9829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1154.Moroni RF, Inverardi F, Regondi MC, Pennacchio P, Frassoni C. Developmental expression of Kir4.1 in astrocytes and oligodendrocytes of rat somatosensory cortex and hippocampus. Int J Dev Neurosci 47, Pt B: 198–205, 2015. doi: 10.1016/j.ijdevneu.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 1155.Morrison RS, de Vellis J. Growth of purified astrocytes in a chemically defined medium. Proc Natl Acad Sci USA 78: 7205–7209, 1981. doi: 10.1073/pnas.78.11.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1156.Morte B, Bernal J. Thyroid hormone action: astrocyte-neuron communication. Front Endocrinol (Lausanne) 5: 82, 2014. doi: 10.3389/fendo.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1157.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature 443: 289–295, 2006. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 1158.Moss J, Gebara E, Bushong EA, Sánchez-Pascual I, O’Laoi R, El M’Ghari I, Kocher-Braissant J, Ellisman MH, Toni N. Fine processes of Nestin-GFP-positive radial glia-like stem cells in the adult dentate gyrus ensheathe local synapses and vasculature. Proc Natl Acad Sci USA 113: E2536–E2545, 2016. doi: 10.1073/pnas.1514652113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1159.Mosso A. Sulla circolazione del sangue nel cervello dell’uomo. Mem Real Acc Lincei 5: 237–358, 1880. [Google Scholar]
- 1160.Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter d-serine. Proc Natl Acad Sci USA 102: 5606–5611, 2005. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1161.Mothet JP, Rouaud E, Sinet PM, Potier B, Jouvenceau A, Dutar P, Videau C, Epelbaum J, Billard JM. A critical role for the glial-derived neuromodulator d-serine in the age-related deficits of cellular mechanisms of learning and memory. Aging Cell 5: 267–274, 2006. doi: 10.1111/j.1474-9726.2006.00216.x. [DOI] [PubMed] [Google Scholar]
- 1162.Moussawi K, Riegel A, Nair S, Kalivas PW. Extracellular glutamate: functional compartments operate in different concentration ranges. Front Syst Neurosci 5: 94, 2011. doi: 10.3389/fnsys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1163.Mugnaini E. Cell junctions of astrocytes, ependymal and related cells in the mammal central nervous system, with emphasis on the hypothesis of a generalized syncytium of supporting cells. In: Astrocytes, edited by Federoff S, Vernadakis A. New York: Academic, 1986, p. 329–371. doi: 10.1016/B978-0-12-250451-8.50018-7 [DOI] [Google Scholar]
- 1164.Mulkey DK, Wenker IC. Astrocyte chemoreceptors: mechanisms of H+ sensing by astrocytes in the retrotrapezoid nucleus and their possible contribution to respiratory drive. Exp Physiol 96: 400–406, 2011. doi: 10.1113/expphysiol.2010.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1165.Müller H. Zur Histologie der Netzhaut. Z Wiss Zool 3: 234–237, 1851. [Google Scholar]
- 1166.Müller M, Henrich A, Klockenhoff J, Dierkes PW, Schlue WR. Effects of ATP and derivatives on neuropile glial cells of the leech central nervous system. Glia 29: 191–201, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 1167.Müller T, Fritschy JM, Grosche J, Pratt GD, Möhler H, Kettenmann H. Developmental regulation of voltage-gated K+ channel and GABAA receptor expression in Bergmann glial cells. J Neurosci 14: 2503–2514, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1168.Müller T, Grosche J, Ohlemeyer C, Kettenmann H. NMDA-activated currents in Bergmann glial cells. Neuroreport 4: 671–674, 1993. doi: 10.1097/00001756-199306000-00017. [DOI] [PubMed] [Google Scholar]
- 1169.Müller T, Möller T, Berger T, Schnitzer J, Kettenmann H. Calcium entry through kainate receptors and resulting potassium-channel blockade in Bergmann glial cells. Science 256: 1563–1566, 1992. doi: 10.1126/science.1317969. [DOI] [PubMed] [Google Scholar]
- 1170.Mullier A, Bouret SG, Prevot V, Dehouck B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol 518: 943–962, 2010. doi: 10.1002/cne.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1171.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431: 195–199, 2004. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 1172.Munsch T, Deitmer JW. Calcium transients in identified leech glial cells in situ evoked by high potassium concentrations and 5-hydroxytryptamine. J Exp Biol 167: 251–265, 1992. [DOI] [PubMed] [Google Scholar]
- 1173.Muoio V, Persson PB, Sendeski MM. The neurovascular unit - concept review. Acta Physiol (Oxf) 210: 790–798, 2014. doi: 10.1111/apha.12250. [DOI] [PubMed] [Google Scholar]
- 1174.Murai KK, Pasquale EB. Eph receptors and ephrins in neuron-astrocyte communication at synapses. Glia 59: 1567–1578, 2011. doi: 10.1002/glia.21226. [DOI] [PubMed] [Google Scholar]
- 1175.Murakami K, Nakamura Y, Yoneda Y. Potentiation by ATP of lipopolysaccharide-stimulated nitric oxide production in cultured astrocytes. Neuroscience 117: 37–42, 2003. doi: 10.1016/S0306-4522(02)00804-7. [DOI] [PubMed] [Google Scholar]
- 1176.Muroyama Y, Fujiwara Y, Orkin SH, Rowitch DH. Specification of astrocytes by bHLH protein SCL in a restricted region of the neural tube. Nature 438: 360–363, 2005. doi: 10.1038/nature04139. [DOI] [PubMed] [Google Scholar]
- 1177.Murphy-Royal C, Dupuis JP, Varela JA, Panatier A, Pinson B, Baufreton J, Groc L, Oliet SH. Surface diffusion of astrocytic glutamate transporters shapes synaptic transmission. Nat Neurosci 18: 219–226, 2015. doi: 10.1038/nn.3901. [DOI] [PubMed] [Google Scholar]
- 1178.Murphy S, Pearce B, Jeremy J, Dandona P. Astrocytes as eicosanoid-producing cells. Glia 1: 241–245, 1988. doi: 10.1002/glia.440010402. [DOI] [PubMed] [Google Scholar]
- 1179.Murphy S, Pearce B, Morrow C. Astrocytes have both M1 and M2 muscarinic receptor subtypes. Brain Res 364: 177–180, 1986. doi: 10.1016/0006-8993(86)91000-0. [DOI] [PubMed] [Google Scholar]
- 1180.Murphy S, Welk G. Hydrolysis of polyphosphoinositides in astrocytes by platelet-activating factor. Eur J Pharmacol 188: 399–401, 1990. doi: 10.1016/0922-4106(90)90200-H. [DOI] [PubMed] [Google Scholar]
- 1181.Mwinyi A, Bailly X, Bourlat SJ, Jondelius U, Littlewood DT, Podsiadlowski L. The phylogenetic position of Acoela as revealed by the complete mitochondrial genome of Symsagittifera roscoffensis. BMC Evol Biol 10: 309, 2010. doi: 10.1186/1471-2148-10-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1182.Nadarajah B, Thomaidou D, Evans WH, Parnavelas JG. Gap junctions in the adult cerebral cortex: regional differences in their distribution and cellular expression of connexins. J Comp Neurol 376: 326–342, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 1183.Nadjar A, Blutstein T, Aubert A, Laye S, Haydon PG. Astrocyte-derived adenosine modulates increased sleep pressure during inflammatory response. Glia 61: 724–731, 2013. doi: 10.1002/glia.22465. [DOI] [PubMed] [Google Scholar]
- 1184.Nagelhus EA, Ottersen OP. Physiological roles of aquaporin-4 in brain. Physiol Rev 93: 1543–1562, 2013. doi: 10.1152/physrev.00011.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1185.Nageotte J. Phenomenes de secretion dans le protoplasma des cellules nevrogliques de la substance grise. C R Soc Biol (Paris) 68: 1068–1069, 1910. [Google Scholar]
- 1186.Nagy JI, Dudek FE, Rash JE. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res Brain Res Rev 47: 191–215, 2004. doi: 10.1016/j.brainresrev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 1187.Nagy JI, Li X, Rempel J, Stelmack G, Patel D, Staines WA, Yasumura T, Rash JE. Connexin26 in adult rodent central nervous system: demonstration at astrocytic gap junctions and colocalization with connexin30 and connexin43. J Comp Neurol 441: 302–323, 2001. doi: 10.1002/cne.1414. [DOI] [PubMed] [Google Scholar]
- 1188.Nagy JI, Lynn BD, Tress O, Willecke K, Rash JE. Connexin26 expression in brain parenchymal cells demonstrated by targeted connexin ablation in transgenic mice. Eur J Neurosci 34: 263–271, 2011. doi: 10.1111/j.1460-9568.2011.07741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1189.Nagy JI, Patel D, Ochalski PA, Stelmack GL. Connexin30 in rodent, cat and human brain: selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience 88: 447–468, 1999. doi: 10.1016/S0306-4522(98)00191-2. [DOI] [PubMed] [Google Scholar]
- 1190.Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat Biotechnol 19: 137–141, 2001. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- 1191.Nakashima K, Wiese S, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Yoshida K, Kishimoto T, Sendtner M, Taga T. Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J Neurosci 19: 5429–5434, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1192.Nam JH, Park ES, Won SY, Lee YA, Kim KI, Jeong JY, Baek JY, Cho EJ, Jin M, Chung YC, Lee BD, Kim SH, Kim EG, Byun K, Lee B, Woo DH, Lee CJ, Kim SR, Bok E, Kim YS, Ahn TB, Ko HW, Brahmachari S, Pletinkova O, Troconso JC, Dawson VL, Dawson TM, Jin BK. TRPV1 on astrocytes rescues nigral dopamine neurons in Parkinson’s disease via CNTF. Brain 138: 3610–3622, 2015. doi: 10.1093/brain/awv297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1193.Naoumenko J, Feigin I. A modification for paraffin sections of the Cajal gold-sublimate stain for astrocytes. J Neuropathol Exp Neurol 20: 602–604, 1961. doi: 10.1097/00005072-196120040-00011. [DOI] [PubMed] [Google Scholar]
- 1194.Narcisse L, Scemes E, Zhao Y, Lee SC, Brosnan CF. The cytokine IL-1β transiently enhances P2X7 receptor expression and function in human astrocytes. Glia 49: 245–258, 2005. doi: 10.1002/glia.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1195.Nardin P, Tramontina AC, Quincozes-Santos A, Tortorelli LS, Lunardi P, Klein PR, Wartchow KM, Bobermin LD, Gottfried C, Elisabetsky E, Gonçalves CA. In vitro S100B secretion is reduced by apomorphine: effects of antipsychotics and antioxidants. Prog Neuropsychopharmacol Biol Psychiatry 35: 1291–1296, 2011. doi: 10.1016/j.pnpbp.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 1196.Nataf S, Davoust N, Barnum SR. Kinetics of anaphylatoxin C5a receptor expression during experimental allergic encephalomyelitis. J Neuroimmunol 91: 147–155, 1998. doi: 10.1016/S0165-5728(98)00169-6. [DOI] [PubMed] [Google Scholar]
- 1197.Navarrete M, Araque A. Endocannabinoids mediate neuron-astrocyte communication. Neuron 57: 883–893, 2008. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 1198.Navarrete M, Araque A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68: 113–126, 2010. doi: 10.1016/j.neuron.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 1199.Navarrete M, Perea G, Fernandez de Sevilla D, Gómez-Gonzalo M, Núñez A, Martín ED, Araque A. Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol 10: e1001259, 2012. doi: 10.1371/journal.pbio.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1200.Neary JT, Kang Y. P2 purinergic receptors signal to glycogen synthase kinase-3beta in astrocytes. J Neurosci Res 84: 515–524, 2006. doi: 10.1002/jnr.20969. [DOI] [PubMed] [Google Scholar]
- 1201.Neary JT, Kang Y, Bu Y, Yu E, Akong K, Peters CM. Mitogenic signaling by ATP/P2Y purinergic receptors in astrocytes: involvement of a calcium-independent protein kinase C, extracellular signal-regulated protein kinase pathway distinct from the phosphatidylinositol-specific phospholipase C/calcium pathway. J Neurosci 19: 4211–4220, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1202.Neary JT, Kang Y, Willoughby KA, Ellis EF. Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J Neurosci 23: 2348–2356, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1203.Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 263: 1768–1771, 1994. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- 1204.Nedergaard M. Neuroscience. Garbage truck of the brain. Science 340: 1529–1530, 2013. doi: 10.1126/science.1240514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1205.Nedergaard M, Verkhratsky A. Artifact versus reality–how astrocytes contribute to synaptic events. Glia 60: 1013–1023, 2012. doi: 10.1002/glia.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1206.Nehlig A, Wittendorp-Rechenmann E, Lam CD. Selective uptake of [14C]2-deoxyglucose by neurons and astrocytes: high-resolution microautoradiographic imaging by cellular 14C-trajectography combined with immunohistochemistry. J Cereb Blood Flow Metab 24: 1004–1014, 2004. doi: 10.1097/01.WCB.0000128533.84196.D8. [DOI] [PubMed] [Google Scholar]
- 1207.Nestor MW, Mok LP, Tulapurkar ME, Thompson SM. Plasticity of neuron-glial interactions mediated by astrocytic EphARs. J Neurosci 27: 12817–12828, 2007. doi: 10.1523/JNEUROSCI.2442-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1208.Nett WJ, Oloff SH, McCarthy KD. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J Neurophysiol 87: 528–537, 2002. doi: 10.1152/jn.00268.2001. [DOI] [PubMed] [Google Scholar]
- 1209.Neusch C, Rozengurt N, Jacobs RE, Lester HA, Kofuji P. Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J Neurosci 21: 5429–5438, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1210.Newman EA. Acid efflux from retinal glial cells generated by sodium bicarbonate cotransport. J Neurosci 16: 159–168, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1211.Newman EA, Frambach DA, Odette LL. Control of extracellular potassium levels by retinal glial cell K+ siphoning. Science 225: 1174–1175, 1984. doi: 10.1126/science.6474173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1212.Neymeyer V, Tephly TR, Miller MW. Folate and 10-formyltetrahydrofolate dehydrogenase (FDH) expression in the central nervous system of the mature rat. Brain Res 766: 195–204, 1997. doi: 10.1016/S0006-8993(97)00528-3. [DOI] [PubMed] [Google Scholar]
- 1213.Ng FS, Tangredi MM, Jackson FR. Glial cells physiologically modulate clock neurons and circadian behavior in a calcium-dependent manner. Curr Biol 21: 625–634, 2011. doi: 10.1016/j.cub.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1214.Nicholls JG, Kuffler SW. Extracellular space as a pathway for exchange between blood and neurons in the central nervous system of the leech: ionic composition of glial cells and neurons. J Neurophysiol 27: 645–671, 1964. [DOI] [PubMed] [Google Scholar]
- 1215.Nichols DE, Nichols CD. Serotonin receptors. Chem Rev 108: 1614–1641, 2008. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 1216.Nickel R, Forge A. Gap junctions and connexins in the inner ear: their roles in homeostasis and deafness. Curr Opin Otolaryngol Head Neck Surg 16: 452–457, 2008. doi: 10.1097/MOO.0b013e32830e20b0. [DOI] [PubMed] [Google Scholar]
- 1217.Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci 17: 171–180, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1218.Nilius B. Transient receptor potential (TRP) channels in the brain: the good and the ugly. Eur Rev 20: 343–355, 2012. doi: 10.1017/S1062798711000597. [DOI] [Google Scholar]
- 1219.Nilius B, Appendino G. Spices: the savory and beneficial science of pungency. Rev Physiol Biochem Pharmacol 164: 1–76, 2013. doi: 10.1007/112_2013_11. [DOI] [PubMed] [Google Scholar]
- 1220.Nilius B, Appendino G, Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch 464: 425–458, 2012. doi: 10.1007/s00424-012-1158-z. [DOI] [PubMed] [Google Scholar]
- 1221.Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol 12: 218, 2011. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1222.Nilsson M, Eriksson PS, Rönnbäck L, Hansson E. GABA induces Ca2+ transients in astrocytes. Neuroscience 54: 605–614, 1993. doi: 10.1016/0306-4522(93)90232-5. [DOI] [PubMed] [Google Scholar]
- 1223.Nilsson M, Hansson E, Rönnbäck L. Adrenergic and 5-HT2 receptors on the same astroglial cell. A microspectrofluorimetric study on cytosolic Ca2+ responses in single cells in primary culture. Brain Res Dev Brain Res 63: 33–41, 1991. doi: 10.1016/0165-3806(91)90064-P. [DOI] [PubMed] [Google Scholar]
- 1224.Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods 1: 31–37, 2004. doi: 10.1038/nmeth706. [DOI] [PubMed] [Google Scholar]
- 1225.Nimmerjahn A, Mukamel EA, Schnitzer MJ. Motor behavior activates Bergmann glial networks. Neuron 62: 400–412, 2009. doi: 10.1016/j.neuron.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1226.Nishida H, Okabe S. Direct astrocytic contacts regulate local maturation of dendritic spines. J Neurosci 27: 331–340, 2007. doi: 10.1523/JNEUROSCI.4466-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1227.Nishiyama A, Boshans L, Goncalves CM, Wegrzyn J, Patel KD. Lineage, fate, and fate potential of NG2-glia. Brain Res 1638, Pt B: 116–128, 2016. doi: 10.1016/j.brainres.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1228.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci 10: 9–22, 2009. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 1229.Nishiyama H, Knopfel T, Endo S, Itohara S. Glial protein S100B modulates long-term neuronal synaptic plasticity. Proc Natl Acad Sci USA 99: 4037–4042, 2002. doi: 10.1073/pnas.052020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1230.Nishizaki T, Nagai K, Nomura T, Tada H, Kanno T, Tozaki H, Li XX, Kondoh T, Kodama N, Takahashi E, Sakai N, Tanaka K, Saito N. A new neuromodulatory pathway with a glial contribution mediated via A2a adenosine receptors. Glia 39: 133–147, 2002. doi: 10.1002/glia.10100. [DOI] [PubMed] [Google Scholar]
- 1231.Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50: 295–322, 2010. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1232.Nixdorf-Bergweiler BE, Albrecht D, Heinemann U. Developmental changes in the number, size, and orientation of GFAP-positive cells in the CA1 region of rat hippocampus. Glia 12: 180–195, 1994. doi: 10.1002/glia.440120304. [DOI] [PubMed] [Google Scholar]
- 1233.Nobile M, Monaldi I, Alloisio S, Cugnoli C, Ferroni S. ATP-induced, sustained calcium signalling in cultured rat cortical astrocytes: evidence for a non-capacitative, P2X7-like-mediated calcium entry. FEBS Lett 538: 71–76, 2003. doi: 10.1016/S0014-5793(03)00129-7. [DOI] [PubMed] [Google Scholar]
- 1234.Noda M, Hiyama TY. The Nax channel: What it is and what it does. Neuroscientist 21: 399–412, 2015. doi: 10.1177/1073858414541009. [DOI] [PubMed] [Google Scholar]
- 1235.Noda M, Sakuta H. Central regulation of body-fluid homeostasis. Trends Neurosci 36: 661–673, 2013. doi: 10.1016/j.tins.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 1236.Nolte C, Matyash M, Pivneva T, Schipke CG, Ohlemeyer C, Hanisch UK, Kirchhoff F, Kettenmann H. GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia 33: 72–86, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 1237.Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res 161: 303–310, 1979. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- 1238.Nörenberg W, Schunk J, Fischer W, Sobottka H, Riedel T, Oliveira JF, Franke H, Illes P. Electrophysiological classification of P2X7 receptors in rat cultured neocortical astroglia. Br J Pharmacol 160: 1941–1952, 2010. doi: 10.1111/j.1476-5381.2010.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1239.North RA. Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 1240.North RA, Verkhratsky A. Purinergic transmission in the central nervous system. Pflugers Arch 452: 479–485, 2006. doi: 10.1007/s00424-006-0060-y. [DOI] [PubMed] [Google Scholar]
- 1241.Northington FJ, Traystman RJ, Koehler RC, Rothstein JD, Martin LJ. Regional and cellular expression of glial (GLT1) and neuronal (EAAC1) glutamate transporter proteins in ovine fetal brain. Neuroscience 85: 1183–1194, 1998. doi: 10.1016/S0306-4522(97)00673-8. [DOI] [PubMed] [Google Scholar]
- 1242.Nowak L, Ascher P, Berwald-Netter Y. Ionic channels in mouse astrocytes in culture. J Neurosci 7: 101–109, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1243.Nuriya M, Yasui M. Endfeet serve as diffusion-limited subcellular compartments in astrocytes. J Neurosci 33: 3692–3698, 2013. doi: 10.1523/JNEUROSCI.3050-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1244.Nwaobi SE, Cuddapah VA, Patterson KC, Randolph AC, Olsen ML. The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol 132: 1–21, 2016. doi: 10.1007/s00401-016-1553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1245.O’Callaghan EL, Bassi JK, Porrello ER, Delbridge LM, Thomas WG, Allen AM. Regulation of angiotensinogen by angiotensin II in mouse primary astrocyte cultures. J Neurochem 119: 18–26, 2011. doi: 10.1111/j.1471-4159.2011.07406.x. [DOI] [PubMed] [Google Scholar]
- 1246.O’Connor ER, Sontheimer H, Ransom BR. Rat hippocampal astrocytes exhibit electrogenic sodium-bicarbonate co-transport. J Neurophysiol 72: 2580–2589, 1994. [DOI] [PubMed] [Google Scholar]
- 1247.Oberheim NA, Goldman SA, Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol Biol 814: 23–45, 2012. doi: 10.1007/978-1-61779-452-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1248.Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. Uniquely hominid features of adult human astrocytes. J Neurosci 29: 3276–3287, 2009. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1249.Oberheim NA, Wang X, Goldman S, Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci 29: 547–553, 2006. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 1250.Ochi S, Lim JY, Rand MN, During MJ, Sakatani K, Kocsis JD. Transient presence of GABA in astrocytes of the developing optic nerve. Glia 9: 188–198, 1993. doi: 10.1002/glia.440090304. [DOI] [PubMed] [Google Scholar]
- 1251.Ogata K, Kosaka T. Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience 113: 221–233, 2002. doi: 10.1016/S0306-4522(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 1252.Ogata T, Nakamura Y, Schubert P. Potentiated cAMP rise in metabotropically stimulated rat cultured astrocytes by a Ca2+-related A1/A2 adenosine receptor cooperation. Eur J Neurosci 8: 1124–1131, 1996. doi: 10.1111/j.1460-9568.1996.tb01280.x. [DOI] [PubMed] [Google Scholar]
- 1253.Ogata T, Nakamura Y, Tsuji K, Shibata T, Kataoka K, Schubert P. Adenosine enhances intracellular Ca2+ mobilization in conjunction with metabotropic glutamate receptor activation by t-ACPD in cultured hippocampal astrocytes. Neurosci Lett 170: 5–8, 1994. doi: 10.1016/0304-3940(94)90225-9. [DOI] [PubMed] [Google Scholar]
- 1254.Ogden KK, Traynelis SF. New advances in NMDA receptor pharmacology. Trends Pharmacol Sci 32: 726–733, 2011. doi: 10.1016/j.tips.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1255.Oh SJ, Han KS, Park H, Woo DH, Kim HY, Traynelis SF, Lee CJ. Protease activated receptor 1-induced glutamate release in cultured astrocytes is mediated by Bestrophin-1 channel but not by vesicular exocytosis. Mol Brain 5: 38, 2012. doi: 10.1186/1756-6606-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1256.Ohta K, Kuno S, Inoue S, Ikeda E, Fujinami A, Ohta M. The effect of dopamine agonists: the expression of GDNF, NGF, and BDNF in cultured mouse astrocytes. J Neurol Sci 291: 12–16, 2010. doi: 10.1016/j.jns.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 1257.Oikawa H, Nakamichi N, Kambe Y, Ogura M, Yoneda Y. An increase in intracellular free calcium ions by nicotinic acetylcholine receptors in a single cultured rat cortical astrocyte. J Neurosci Res 79: 535–544, 2005. doi: 10.1002/jnr.20398. [DOI] [PubMed] [Google Scholar]
- 1258.Oikonomou G, Shaham S. The glia of Caenorhabditis elegans. Glia 59: 1253–1263, 2011. doi: 10.1002/glia.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1259.Oland LA, Gibson NJ, Tolbert LP. Localization of a GABA transporter to glial cells in the developing and adult olfactory pathway of the moth Manduca sexta. J Comp Neurol 518: 815–838, 2010. doi: 10.1002/cne.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1260.Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science 292: 923–926, 2001. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- 1261.Oliveira JF, Riedel T, Leichsenring A, Heine C, Franke H, Krügel U, Nörenberg W, Illes P. Rodent cortical astroglia express in situ functional P2X7 receptors sensing pathologically high ATP concentrations. Cereb Cortex 21: 806–820, 2011. doi: 10.1093/cercor/bhq154. [DOI] [PubMed] [Google Scholar]
- 1262.Olkowicz S, Kocourek M, Lučan RK, Porteš M, Fitch WT, Herculano-Houzel S, Němec P. Birds have primate-like numbers of neurons in the forebrain. Proc Natl Acad Sci USA 113: 7255–7260, 2016. doi: 10.1073/pnas.1517131113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1263.Olsen M, Sarup A, Larsson OM, Schousboe A. Effect of hyperosmotic conditions on the expression of the betaine-GABA-transporter (BGT-1) in cultured mouse astrocytes. Neurochem Res 30: 855–865, 2005. doi: 10.1007/s11064-005-6879-3. [DOI] [PubMed] [Google Scholar]
- 1264.Olsen ML, Campbell SL, Sontheimer H. Differential distribution of Kir4.1 in spinal cord astrocytes suggests regional differences in K+ homeostasis. J Neurophysiol 98: 786–793, 2007. doi: 10.1152/jn.00340.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1265.Olsen ML, Higashimori H, Campbell SL, Hablitz JJ, Sontheimer H. Functional expression of Kir4.1 channels in spinal cord astrocytes. Glia 53: 516–528, 2006. doi: 10.1002/glia.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1266.Olsen ML, Khakh BS, Skatchkov SN, Zhou M, Lee CJ, Rouach N. New insights on astrocyte ion channels: critical for homeostasis and neuron-glia signaling. J Neurosci 35: 13827–13835, 2015. doi: 10.1523/JNEUROSCI.2603-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1267.Olsen ML, Sontheimer H. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem 107: 589–601, 2008. doi: 10.1111/j.1471-4159.2008.05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1268.Olsen ML, Sontheimer H. Mislocalization of Kir channels in malignant glia. Glia 46: 63–73, 2004. doi: 10.1002/glia.10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1269.Olsen ML, Sontheimer H. Voltage-activated ion channels in glial cells. In: Neuroglia, edited by Kettenmann H, Ransom BR. Oxford, UK: Oxford Univ. Press, 2005, p. 112–130. [Google Scholar]
- 1270.Olude MA, Mustapha OA, Aderounmu OA, Olopade JO, Ihunwo AO. Astrocyte morphology, heterogeneity, and density in the developing African giant rat (Cricetomys gambianus). Front Neuroanat 9: 67, 2015. doi: 10.3389/fnana.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1271.Onteniente B, Kimura H, Maeda T. Comparative study of the glial fibrillary acidic protein in vertebrates by PAP immunohistochemistry. J Comp Neurol 215: 427–436, 1983. doi: 10.1002/cne.902150407. [DOI] [PubMed] [Google Scholar]
- 1272.Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature 222: 282–284, 1969. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- 1273.Orellana JA, Sáez PJ, Cortés-Campos C, Elizondo RJ, Shoji KF, Contreras-Duarte S, Figueroa V, Velarde V, Jiang JX, Nualart F, Sáez JC, García MA. Glucose increases intracellular free Ca2+ in tanycytes via ATP released through connexin 43 hemichannels. Glia 60: 53–68, 2012. doi: 10.1002/glia.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1274.Orellana JA, Sáez PJ, Shoji KF, Schalper KA, Palacios-Prado N, Velarde V, Giaume C, Bennett MV, Sáez JC. Modulation of brain hemichannels and gap junction channels by pro-inflammatory agents and their possible role in neurodegeneration. Antioxid Redox Signal 11: 369–399, 2009. doi: 10.1089/ars.2008.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1275.Orellana JA, Shoji KF, Abudara V, Ezan P, Amigou E, Sáez PJ, Jiang JX, Naus CC, Sáez JC, Giaume C. Amyloid β-induced death in neurons involves glial and neuronal hemichannels. J Neurosci 31: 4962–4977, 2011. doi: 10.1523/JNEUROSCI.6417-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1276.Orkand RK, Nicholls JG, Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol 29: 788–806, 1966. [DOI] [PubMed] [Google Scholar]
- 1277.Orlov SN, Hamet P. Intracellular monovalent ions as second messengers. J Membr Biol 210: 161–172, 2006. doi: 10.1007/s00232-006-0857-9. [DOI] [PubMed] [Google Scholar]
- 1278.Orlowski J, Grinstein S. Na+/H+ exchangers of mammalian cells. J Biol Chem 272: 22373–22376, 1997. doi: 10.1074/jbc.272.36.22373. [DOI] [PubMed] [Google Scholar]
- 1279.Ormel L, Stensrud MJ, Bergersen LH, Gundersen V. VGLUT1 is localized in astrocytic processes in several brain regions. Glia 60: 229–238, 2012. doi: 10.1002/glia.21258. [DOI] [PubMed] [Google Scholar]
- 1280.Ormel L, Stensrud MJ, Chaudhry FA, Gundersen V. A distinct set of synaptic-like microvesicles in atroglial cells contain VGLUT3. Glia 60: 1289–1300, 2012. doi: 10.1002/glia.22348. [DOI] [PubMed] [Google Scholar]
- 1281.Orthmann-Murphy JL, Abrams CK, Scherer SS. Gap junctions couple astrocytes and oligodendrocytes. J Mol Neurosci 35: 101–116, 2008. doi: 10.1007/s12031-007-9027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1282.Orthmann-Murphy JL, Freidin M, Fischer E, Scherer SS, Abrams CK. Two distinct heterotypic channels mediate gap junction coupling between astrocyte and oligodendrocyte connexins. J Neurosci 27: 13949–13957, 2007. doi: 10.1523/JNEUROSCI.3395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1283.Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, Haydon PG, Coulter DA. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci 13: 584–591, 2010. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1284.Osborne KD, Lee W, Malarkey EB, Irving AJ, Parpura V. Dynamic imaging of cannabinoid receptor 1 vesicular trafficking in cultured astrocytes. ASN Neuro 1: AN20090040, 2009. doi: 10.1042/AN20090040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1285.Ostroff LE, Manzur MK, Cain CK, Ledoux JE. Synapses lacking astrocyte appear in the amygdala during consolidation of Pavlovian threat conditioning. J Comp Neurol 522: 2152–2163, 2014. doi: 10.1002/cne.23523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1286.Otis TS, Kavanaugh MP. Isolation of current components and partial reaction cycles in the glial glutamate transporter EAAT2. J Neurosci 20: 2749–2757, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1287.Otis TS, Wu YC, Trussell LO. Delayed clearance of transmitter and the role of glutamate transporters at synapses with multiple release sites. J Neurosci 16: 1634–1644, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1288.Otsu Y, Couchman K, Lyons DG, Collot M, Agarwal A, Mallet JM, Pfrieger FW, Bergles DE, Charpak S. Calcium dynamics in astrocyte processes during neurovascular coupling. Nat Neurosci 18: 210–218, 2015. doi: 10.1038/nn.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1289.Overstreet LS, Kinney GA, Liu YB, Billups D, Slater NT. Glutamate transporters contribute to the time course of synaptic transmission in cerebellar granule cells. J Neurosci 19: 9663–9673, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1290.Owe SG, Marcaggi P, Attwell D. The ionic stoichiometry of the GLAST glutamate transporter in salamander retinal glia. J Physiol 577: 591–599, 2006. doi: 10.1113/jphysiol.2006.116830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1291.Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol 68: 685–717, 2006. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- 1292.Oya M, Kitaguchi T, Yanagihara Y, Numano R, Kakeyama M, Ikematsu K, Tsuboi T. Vesicular nucleotide transporter is involved in ATP storage of secretory lysosomes in astrocytes. Biochem Biophys Res Commun 438: 145–151, 2013. doi: 10.1016/j.bbrc.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 1293.Oz M, Lorke DE, Yang KH, Petroianu G. On the interaction of β-amyloid peptides and α7-nicotinic acetylcholine receptors in Alzheimer’s disease. Curr Alzheimer Res 10: 618–630, 2013. doi: 10.2174/15672050113109990132. [DOI] [PubMed] [Google Scholar]
- 1294.Pacholczyk T, Blakely RD, Amara SG. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature 350: 350–354, 1991. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- 1295.Paco S, Margelí MA, Olkkonen VM, Imai A, Blasi J, Fischer-Colbrie R, Aguado F. Regulation of exocytotic protein expression and Ca2+-dependent peptide secretion in astrocytes. J Neurochem 110: 143–156, 2009. doi: 10.1111/j.1471-4159.2009.06116.x. [DOI] [PubMed] [Google Scholar]
- 1296.Paco S, Pozas E, Aguado F. Secretogranin III is an astrocyte granin that is overexpressed in reactive glia. Cereb Cortex 20: 1386–1397, 2010. doi: 10.1093/cercor/bhp202. [DOI] [PubMed] [Google Scholar]
- 1297.Pajor AM. Sodium-coupled dicarboxylate and citrate transporters from the SLC13 family. Pflugers Arch 466: 119–130, 2014. doi: 10.1007/s00424-013-1369-y. [DOI] [PubMed] [Google Scholar]
- 1298.Pak MA, Haas HL, Decking UK, Schrader J. Inhibition of adenosine kinase increases endogenous adenosine and depresses neuronal activity in hippocampal slices. Neuropharmacology 33: 1049–1053, 1994. doi: 10.1016/0028-3908(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 1299.Pakhotin P, Verkhratsky A. Electrical synapses between Bergmann glial cells and Purkinje neurones in rat cerebellar slices. Mol Cell Neurosci 28: 79–84, 2005. doi: 10.1016/j.mcn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 1300.Pakkenberg H. The number of nerve cells in the cerebral cortex of man. J Comp Neurol 128: 17–20, 1966. doi: 10.1002/cne.901280103. [DOI] [PubMed] [Google Scholar]
- 1301.Palay S, Chan-Palay V. Cerebellar Cortex. Berlin: Springer-Verlag, 1974. doi: 10.1007/978-3-642-65581-4 [DOI] [Google Scholar]
- 1302.Palty R, Sekler I. The mitochondrial Na+/Ca2+ exchanger. Cell Calcium 52: 9–15, 2012. doi: 10.1016/j.ceca.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 1303.Paluzzi S, Alloisio S, Zappettini S, Milanese M, Raiteri L, Nobile M, Bonanno G. Adult astroglia is competent for Na+/Ca2+ exchanger-operated exocytotic glutamate release triggered by mild depolarization. J Neurochem 103: 1196–1207, 2007. doi: 10.1111/j.1471-4159.2007.04826.x. [DOI] [PubMed] [Google Scholar]
- 1304.Palygin O, Lalo U, Pankratov Y. Distinct pharmacological and functional properties of NMDA receptors in mouse cortical astrocytes. Br J Pharmacol 163: 1755–1766, 2011. doi: 10.1111/j.1476-5381.2011.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1305.Palygin O, Lalo U, Verkhratsky A, Pankratov Y. Ionotropic NMDA and P2X1/5 receptors mediate synaptically induced Ca2+ signalling in cortical astrocytes. Cell Calcium 48: 225–231, 2010. doi: 10.1016/j.ceca.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 1306.Pan HC, Chou YC, Sun SH. P2X7 R-mediated Ca2+-independent d-serine release via pannexin-1 of the P2X7 R-pannexin-1 complex in astrocytes. Glia 63: 877–893, 2015. doi: 10.1002/glia.22790. [DOI] [PubMed] [Google Scholar]
- 1307.Pan W, Hsuchou H, He Y, Sakharkar A, Cain C, Yu C, Kastin AJ. Astrocyte leptin receptor (ObR) and leptin transport in adult-onset obese mice. Endocrinology 149: 2798–2806, 2008. doi: 10.1210/en.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1308.Panatier A, Vallée J, Haber M, Murai KK, Lacaille JC, Robitaille R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146: 785–798, 2011. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 1309.Panenka W, Jijon H, Herx LM, Armstrong JN, Feighan D, Wei T, Yong VW, Ransohoff RM, MacVicar BA. P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. J Neurosci 21: 7135–7142, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1310.Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, Pryazhnikov E, Khiroug L, Giniatullin R, Zorec R. Exocytotic release of ATP from cultured astrocytes. J Biol Chem 282: 28749–28758, 2007. doi: 10.1074/jbc.M700290200. [DOI] [PubMed] [Google Scholar]
- 1311.Pankratov Y, Lalo U. Role for astroglial α1-adrenoreceptors in gliotransmission and control of synaptic plasticity in the neocortex. Front Cell Neurosci 9: 230, 2015. doi: 10.3389/fncel.2015.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1312.Pankratov Y, Lalo U, Krishtal OA, Verkhratsky A. P2X receptors and synaptic plasticity. Neuroscience 158: 137–148, 2009. doi: 10.1016/j.neuroscience.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 1313.Pannicke T, Fischer W, Biedermann B, Schädlich H, Grosche J, Faude F, Wiedemann P, Allgaier C, Illes P, Burnstock G, Reichenbach A. P2X7 receptors in Müller glial cells from the human retina. J Neurosci 20: 5965–5972, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1314.Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci 14: 265–277, 2013. doi: 10.1038/nrn3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1315.Pappalardo LW, Black JA, Waxman SG. Sodium channels in astroglia and microglia. Glia 64: 1628–1645, 2016. doi: 10.1002/glia.22967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1316.Pappalardo LW, Liu S, Black JA, Waxman SG. Dynamics of sodium channel Nav1.5 expression in astrocytes in mouse models of multiple sclerosis. Neuroreport 25: 1208–1215, 2014. doi: 10.1097/WNR.0000000000000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1317.Pappalardo LW, Samad OA, Black JA, Waxman SG. Voltage-gated sodium channel Nav 1.5 contributes to astrogliosis in an in vitro model of glial injury via reverse Na+/Ca2+ exchange. Glia 62: 1162–1175, 2014. doi: 10.1002/glia.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1318.Pappas CA, Ransom BR. A depolarization-stimulated, bafilomycin-inhibitable H+ pump in hippocampal astrocytes. Glia 9: 280–291, 1993. doi: 10.1002/glia.440090406. [DOI] [PubMed] [Google Scholar]
- 1319.Párducz A, Perez J, Garcia-Segura LM. Estradiol induces plasticity of GABAergic synapses in the hypothalamus. Neuroscience 53: 395–401, 1993. doi: 10.1016/0306-4522(93)90203-R. [DOI] [PubMed] [Google Scholar]
- 1320.Parekh AB, Putney JW Jr. Store-operated calcium channels. Physiol Rev 85: 757–810, 2005. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 1321.Parent AS, Rasier G, Matagne V, Lomniczi A, Lebrethon MC, Gérard A, Ojeda SR, Bourguignon JP. Oxytocin facilitates female sexual maturation through a glia-to-neuron signaling pathway. Endocrinology 149: 1358–1365, 2008. doi: 10.1210/en.2007-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1322.Park H, Han KS, Oh SJ, Jo S, Woo J, Yoon BE, Lee CJ. High glutamate permeability and distal localization of Best1 channel in CA1 hippocampal astrocyte. Mol Brain 6: 54, 2013. doi: 10.1186/1756-6606-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1323.Park H, Han KS, Seo J, Lee J, Dravid SM, Woo J, Chun H, Cho S, Bae JY, An H, Koh W, Yoon BE, Berlinguer-Palmini R, Mannaioni G, Traynelis SF, Bae YC, Choi SY, Lee CJ. Channel-mediated astrocytic glutamate modulates hippocampal synaptic plasticity by activating postsynaptic NMDA receptors. Mol Brain 8: 7, 2015. doi: 10.1186/s13041-015-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1324.Park H, Oh SJ, Han KS, Woo DH, Park H, Mannaioni G, Traynelis SF, Lee CJ. Bestrophin-1 encodes for the Ca2+-activated anion channel in hippocampal astrocytes. J Neurosci 29: 13063–13073, 2009. doi: 10.1523/JNEUROSCI.3193-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1325.Parker RJ, Auld VJ. Roles of glia in the Drosophila nervous system. Semin Cell Dev Biol 17: 66–77, 2006. doi: 10.1016/j.semcdb.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 1326.Parkerson KA, Sontheimer H. Biophysical and pharmacological characterization of hypotonically activated chloride currents in cortical astrocytes. Glia 46: 419–436, 2004. doi: 10.1002/glia.10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1327.Parnis J, Montana V, Delgado-Martinez I, Matyash V, Parpura V, Kettenmann H, Sekler I, Nolte C. Mitochondrial exchanger NCLX plays a major role in the intracellular Ca2+ signaling, gliotransmission, and proliferation of astrocytes. J Neurosci 33: 7206–7219, 2013. doi: 10.1523/JNEUROSCI.5721-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1328.Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature 369: 744–747, 1994. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 1329.Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A. Glial cells in (patho)physiology. J Neurochem 121: 4–27, 2012. doi: 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1330.Parpura V, Zorec R. Gliotransmission: Exocytotic release from astrocytes. Brain Res Brain Res Rev 63: 83–92, 2010. doi: 10.1016/j.brainresrev.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1331.Parri HR, Crunelli V. The role of Ca2+ in the generation of spontaneous astrocytic Ca2+ oscillations. Neuroscience 120: 979–992, 2003. doi: 10.1016/S0306-4522(03)00379-8. [DOI] [PubMed] [Google Scholar]
- 1332.Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci 4: 803–812, 2001. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- 1333.Pasantes-Morales H, Schousboe A. Release of taurine from astrocytes during potassium-evoked swelling. Glia 2: 45–50, 1989. doi: 10.1002/glia.440020105. [DOI] [PubMed] [Google Scholar]
- 1334.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science 310: 113–116, 2005. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 1335.Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci 17: 7817–7830, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1336.Pastor A, Chvátal A, Syková E, Kettenmann H. Glycine- and GABA-activated currents in identified glial cells of the developing rat spinal cord slice. Eur J Neurosci 7: 1188–1198, 1995. doi: 10.1111/j.1460-9568.1995.tb01109.x. [DOI] [PubMed] [Google Scholar]
- 1337.Pastor A, Kremer M, Möller T, Kettenmann H, Dermietzel R. Dye coupling between spinal cord oligodendrocytes: differences in coupling efficiency between gray and white matter. Glia 24: 108–120, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 1338.Patel AB, Lai JC, Chowdhury GM, Hyder F, Rothman DL, Shulman RG, Behar KL. Direct evidence for activity-dependent glucose phosphorylation in neurons with implications for the astrocyte-to-neuron lactate shuttle. Proc Natl Acad Sci USA 111: 5385–5390, 2014. doi: 10.1073/pnas.1403576111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1339.Patel S. Function and dysfunction of two-pore channels. Sci Signal 8: re7, 2015. doi: 10.1126/scisignal.aab3314. [DOI] [PubMed] [Google Scholar]
- 1340.Patrushev I, Gavrilov N, Turlapov V, Semyanov A. Subcellular location of astrocytic calcium stores favors extrasynaptic neuron-astrocyte communication. Cell Calcium 54: 343–349, 2013. doi: 10.1016/j.ceca.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 1341.Paukert M, Agarwal A, Cha J, Doze VA, Kang JU, Bergles DE. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82: 1263–1270, 2014. doi: 10.1016/j.neuron.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1342.Paulson OB, Newman EA. Does the release of potassium from astrocyte endfeet regulate cerebral blood flow? Science 237: 896–898, 1987. doi: 10.1126/science.3616619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1343.Peakman MC, Hill SJ. Adenosine A1 receptor-mediated changes in basal and histamine-stimulated levels of intracellular calcium in primary rat astrocytes. Br J Pharmacol 115: 801–810, 1995. doi: 10.1111/j.1476-5381.1995.tb15004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1344.Peakman MC, Hill SJ. Adenosine A1 receptor-mediated inhibition of cyclic AMP accumulation in type-2 but not type-1 rat astrocytes. Eur J Pharmacol 306: 281–289, 1996. doi: 10.1016/0014-2999(96)00202-6. [DOI] [PubMed] [Google Scholar]
- 1345.Peakman MC, Hill SJ. Adenosine A2B-receptor-mediated cyclic AMP accumulation in primary rat astrocytes. Br J Pharmacol 111: 191–198, 1994. doi: 10.1111/j.1476-5381.1994.tb14043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1346.Pearce B, Cambray-Deakin M, Murphy S. Astrocyte opioid receptors: activation modifies the noradrenaline-evoked increase in 2-[14C]deoxyglucose incorporation into glycogen. Neurosci Lett 55: 157–160, 1985. doi: 10.1016/0304-3940(85)90012-6. [DOI] [PubMed] [Google Scholar]
- 1347.Pearce B, Langley D. Purine- and pyrimidine-stimulated phosphoinositide breakdown and intracellular calcium mobilisation in astrocytes. Brain Res 660: 329–332, 1994. doi: 10.1016/0006-8993(94)91307-2. [DOI] [PubMed] [Google Scholar]
- 1348.Pearce B, Murphy S, Jeremy J, Morrow C, Dandona P. ATP-evoked Ca2+ mobilisation and prostanoid release from astrocytes: P2-purinergic receptors linked to phosphoinositide hydrolysis. J Neurochem 52: 971–977, 1989. doi: 10.1111/j.1471-4159.1989.tb02549.x. [DOI] [PubMed] [Google Scholar]
- 1349.Pegg AE. The function of spermine. IUBMB Life 66: 8–18, 2014. doi: 10.1002/iub.1237. [DOI] [PubMed] [Google Scholar]
- 1350.Pekny M. Astrocytic intermediate filaments: lessons from GFAP and vimentin knock-out mice. Prog Brain Res 132: 23–30, 2001. doi: 10.1016/S0079-6123(01)32062-9. [DOI] [PubMed] [Google Scholar]
- 1351.Pekny M, Johansson CB, Eliasson C, Stakeberg J, Wallén A, Perlmann T, Lendahl U, Betsholtz C, Berthold CH, Frisén J. Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J Cell Biol 145: 503–514, 1999. doi: 10.1083/jcb.145.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1352.Pekny M, Pekna M. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev 94: 1077–1098, 2014. doi: 10.1152/physrev.00041.2013. [DOI] [PubMed] [Google Scholar]
- 1353.Pekny M, Pekna M, Messing A, Steinhäuser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A. Astrocytes: a central element in neurological diseases. Acta Neuropathol 131: 323–345, 2016. doi: 10.1007/s00401-015-1513-1. [DOI] [PubMed] [Google Scholar]
- 1354.Pekny M, Wilhelmsson U, Bogestål YR, Pekna M. The role of astrocytes and complement system in neural plasticity. Int Rev Neurobiol 82: 95–111, 2007. doi: 10.1016/S0074-7742(07)82005-8. [DOI] [PubMed] [Google Scholar]
- 1355.Pekny T, Faiz M, Wilhelmsson U, Curtis MA, Matej R, Skalli O, Pekny M. Synemin is expressed in reactive astrocytes and Rosenthal fibers in Alexander disease. APMIS 122: 76–80, 2014. doi: 10.1111/apm.12088. [DOI] [PubMed] [Google Scholar]
- 1356.Pellerin L, Bergersen LH, Halestrap AP, Pierre K. Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J Neurosci Res 79: 55–64, 2005. doi: 10.1002/jnr.20307. [DOI] [PubMed] [Google Scholar]
- 1357.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91: 10625–10629, 1994. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1358.Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab 32: 1152–1166, 2012. doi: 10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1359.Pelluru D, Konadhode RR, Bhat NR, Shiromani PJ. Optogenetic stimulation of astrocytes in the posterior hypothalamus increases sleep at night in C57BL/6J mice. Eur J Neurosci 43: 1298–1306, 2016. doi: 10.1111/ejn.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1360.Pelton EW II, Kimelberg HK, Shipherd SV, Bourke RS. Dopamine and norepinephrine uptake and metabolism by astroglial cells in culture. Life Sci 28: 1655–1663, 1981. doi: 10.1016/0024-3205(81)90322-2. [DOI] [PubMed] [Google Scholar]
- 1361.Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B. Neocortical glial cell numbers in human brains. Neurobiol Aging 29: 1754–1762, 2008. doi: 10.1016/j.neurobiolaging.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 1362.Penfield W. Neuroglia, normal and pathological. In: Cytology and Cellular Pathology of the Nervous System. New York: Paul B. Hoeber, 1932, p. 421–479. [Google Scholar]
- 1363.Peng L, Huang R, Yu AC, Fung KY, Rathbone MP, Hertz L. Nucleoside transporter expression and function in cultured mouse astrocytes. Glia 52: 25–35, 2005. doi: 10.1002/glia.20216. [DOI] [PubMed] [Google Scholar]
- 1364.Peng L, Verkhratsky A, Gu L, Li B. Targeting astrocytes in major depression. Expert Rev Neurother 15: 1299–1306, 2015. doi: 10.1586/14737175.2015.1095094. [DOI] [PubMed] [Google Scholar]
- 1365.Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, Regan S, Kasper T, Peng S, Ding F, Benveniste H, Nedergaard M, Deane R. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis 93: 215–225, 2016. doi: 10.1016/j.nbd.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1366.Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317: 1083–1086, 2007. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- 1367.Pereanu W, Spindler S, Cruz L, Hartenstein V. Tracheal development in the Drosophila brain is constrained by glial cells. Dev Biol 302: 169–180, 2007. doi: 10.1016/j.ydbio.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1368.Pereira A Jr, Dos Santos RP, Barros RF. The calcium wave model of the perception-action cycle: evidence from semantic relevance in memory experiments. Front Psychol 4: 252, 2013. doi: 10.3389/fpsyg.2013.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1369.Pereira A Jr, Furlan FA. Astrocytes and human cognition: modeling information integration and modulation of neuronal activity. Prog Neurobiol 92: 405–420, 2010. doi: 10.1016/j.pneurobio.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 1370.Pereira GJ, Hirata H, Fimia GM, do Carmo LG, Bincoletto C, Han SW, Stilhano RS, Ureshino RP, Bloor-Young D, Churchill G, Piacentini M, Patel S, Smaili SS. Nicotinic acid adenine dinucleotide phosphate (NAADP) regulates autophagy in cultured astrocytes. J Biol Chem 286: 27875–27881, 2011. doi: 10.1074/jbc.C110.216580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1371.Perens EA, Shaham S. C. elegans daf-6 encodes a patched-related protein required for lumen formation. Dev Cell 8: 893–906, 2005. doi: 10.1016/j.devcel.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 1372.Perez-Alvarez A, Navarrete M, Covelo A, Martin ED, Araque A. Structural and functional plasticity of astrocyte processes and dendritic spine interactions. J Neurosci 34: 12738–12744, 2014. doi: 10.1523/JNEUROSCI.2401-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1373.Perez V, Bouschet T, Fernandez C, Bockaert J, Journot L. Dynamic reorganization of the astrocyte actin cytoskeleton elicited by cAMP and PACAP: a role for phosphatidylinositol 3-kinase inhibition. Eur J Neurosci 21: 26–32, 2005. doi: 10.1111/j.1460-9568.2004.03845.x. [DOI] [PubMed] [Google Scholar]
- 1374.Perraud F, Besnard F, Sensenbrenner M, Labourdette G. Thrombin is a potent mitogen for rat astroblasts but not for oligodendroblasts and neuroblasts in primary culture. Int J Dev Neurosci 5: 181–188, 1987. doi: 10.1016/0736-5748(87)90028-1. [DOI] [PubMed] [Google Scholar]
- 1375.Peters A, Palay SL, Webster HF. The Fine Structure of the Nervous System: The Neurons and Supporting Cells (3rd ed.). New York: Oxford Univ. Press, 1991. [Google Scholar]
- 1376.Peters CM, Rogers SD, Pomonis JD, Egnaczyk GF, Keyser CP, Schmidt JA, Ghilardi JR, Maggio JE, Mantyh PW. Endothelin receptor expression in the normal and injured spinal cord: potential involvement in injury-induced ischemia and gliosis. Exp Neurol 180: 1–13, 2003. doi: 10.1016/S0014-4886(02)00023-7. [DOI] [PubMed] [Google Scholar]
- 1377.Petersen OH. Ca2+ signalling in the endoplasmic reticulum/secretory granule microdomain. Cell Calcium 58: 397–404, 2015. doi: 10.1016/j.ceca.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 1378.Petersen OH, Michalak M, Verkhratsky A. Calcium signalling: past, present and future. Cell Calcium 38: 161–169, 2005. doi: 10.1016/j.ceca.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 1379.Petersen OH, Petersen CC, Kasai H. Calcium and hormone action. Annu Rev Physiol 56: 297–319, 1994. doi: 10.1146/annurev.ph.56.030194.001501. [DOI] [PubMed] [Google Scholar]
- 1380.Peterson TS, Camden JM, Wang Y, Seye CI, Wood WG, Sun GY, Erb L, Petris MJ, Weisman GA. P2Y2 nucleotide receptor-mediated responses in brain cells. Mol Neurobiol 41: 356–366, 2010. doi: 10.1007/s12035-010-8115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1381.Petravicz J, Boyt KM, McCarthy KD. Astrocyte IP3R2-dependent Ca2+ signaling is not a major modulator of neuronal pathways governing behavior. Front Behav Neurosci 8: 384, 2014. doi: 10.3389/fnbeh.2014.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1382.Petravicz J, Fiacco TA, McCarthy KD. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci 28: 4967–4973, 2008. doi: 10.1523/JNEUROSCI.5572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1383.Petrelli F, Bezzi P. Novel insights into gliotransmitters. Curr Opin Pharmacol 26: 138–145, 2016. doi: 10.1016/j.coph.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 1384.Petroni A, Salami M, Blasevich M, Papini N, Galella G, Colombo C, Galli C. Eicosanoid and inositol phosphate response to platelet-activating factor (PAF) and to a PAF antagonist in rat astroglial cells. Brain Res Dev Brain Res 78: 169–174, 1994. doi: 10.1016/0165-3806(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 1385.Pfrieger FW. Role of glial cells in the formation and maintenance of synapses. Brain Res Brain Res Rev 63: 39–46, 2010. doi: 10.1016/j.brainresrev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 1386.Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science 277: 1684–1687, 1997. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- 1387.Phamduong E, Rathore MK, Crews NR, D’Angelo AS, Leinweber AL, Kappera P, Krenning TM, Rendell VR, Belcheva MM, Coscia CJ. Acute and chronic mu opioids differentially regulate thrombospondins 1 and 2 isoforms in astrocytes. ACS Chem Neurosci 5: 106–114, 2014. doi: 10.1021/cn400172n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1388.Philippe JM, Dubois JM, Rouzaire-Dubois B, Cartron PF, Vallette F, Morel N. Functional expression of V-ATPases in the plasma membrane of glial cells. Glia 37: 365–373, 2002. doi: 10.1002/glia.10041. [DOI] [PubMed] [Google Scholar]
- 1389.Pierre K, Pellerin L, Debernardi R, Riederer BM, Magistretti PJ. Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience 100: 617–627, 2000. doi: 10.1016/S0306-4522(00)00294-3. [DOI] [PubMed] [Google Scholar]
- 1390.Piet R, Jahr CE. Glutamatergic and purinergic receptor-mediated calcium transients in Bergmann glial cells. J Neurosci 27: 4027–4035, 2007. doi: 10.1523/JNEUROSCI.0462-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1391.Pilitsis JG, Kimelberg HK. Adenosine receptor mediated stimulation of intracellular calcium in acutely isolated astrocytes. Brain Res 798: 294–303, 1998. doi: 10.1016/S0006-8993(98)00430-2. [DOI] [PubMed] [Google Scholar]
- 1392.Pines G, Danbolt NC, Bjørås M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI. Cloning and expression of a rat brain l-glutamate transporter. Nature 360: 464–467, 1992. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- 1393.Pizzo P, Burgo A, Pozzan T, Fasolato C. Role of capacitative calcium entry on glutamate-induced calcium influx in type-I rat cortical astrocytes. J Neurochem 79: 98–109, 2001. doi: 10.1046/j.1471-4159.2001.00539.x. [DOI] [PubMed] [Google Scholar]
- 1394.Plattner H, Verkhratsky A. The ancient roots of calcium signalling evolutionary tree. Cell Calcium 57: 123–132, 2015. doi: 10.1016/j.ceca.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 1395.Plattner H, Verkhratsky A. Inseparable tandem: evolution chooses ATP and Ca2+ to control life, death and cellular signalling. Philos Trans R Soc Lond B Biol Sci 371: 20150419, 2016. doi: 10.1098/rstb.2015.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1396.Pocivavsek A, Wu HQ, Potter MC, Elmer GI, Pellicciari R, Schwarcz R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology 36: 2357–2367, 2011. doi: 10.1038/npp.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1397.Poopalasundaram S, Knott C, Shamotienko OG, Foran PG, Dolly JO, Ghiani CA, Gallo V, Wilkin GP. Glial heterogeneity in expression of the inwardly rectifying K+ channel, Kir4.1, in adult rat CNS. Glia 30: 362–372, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 1398.Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience 99: 507–517, 2000. doi: 10.1016/S0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 1399.Porter JT, McCarthy KD. Adenosine receptors modulate [Ca2+]i in hippocampal astrocytes in situ. J Neurochem 65: 1515–1523, 1995. doi: 10.1046/j.1471-4159.1995.65041515.x. [DOI] [PubMed] [Google Scholar]
- 1400.Porter JT, McCarthy KD. GFAP-positive hippocampal astrocytes in situ respond to glutamatergic neuroligands with increases in [Ca2+]i. Glia 13: 101–112, 1995. doi: 10.1002/glia.440130204. [DOI] [PubMed] [Google Scholar]
- 1401.Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci 16: 5073–5081, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1402.Potokar M, Stenovec M, Gabrijel M, Li L, Kreft M, Grilc S, Pekny M, Zorec R. Intermediate filaments attenuate stimulation-dependent mobility of endosomes/lysosomes in astrocytes. Glia 58: 1208–1219, 2010. [DOI] [PubMed] [Google Scholar]
- 1403.Potokar M, Stenovec M, Jorgačevski J, Holen T, Kreft M, Ottersen OP, Zorec R. Regulation of AQP4 surface expression via vesicle mobility in astrocytes. Glia 61: 917–928, 2013. doi: 10.1002/glia.22485. [DOI] [PubMed] [Google Scholar]
- 1404.Potokar M, Stenovec M, Kreft M, Kreft ME, Zorec R. Stimulation inhibits the mobility of recycling peptidergic vesicles in astrocytes. Glia 56: 135–144, 2008. doi: 10.1002/glia.20597. [DOI] [PubMed] [Google Scholar]
- 1405.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev 27: 47–72, 2006. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 1406.Pow DV, Sullivan RK, Williams SM, Scott HL, Dodd PR, Finkelstein D. Differential expression of the GABA transporters GAT-1 and GAT-3 in brains of rats, cats, monkeys and humans. Cell Tissue Res 320: 379–392, 2005. doi: 10.1007/s00441-004-0928-0. [DOI] [PubMed] [Google Scholar]
- 1407.Prada I, Marchaland J, Podini P, Magrassi L, D’Alessandro R, Bezzi P, Meldolesi J. REST/NRSF governs the expression of dense-core vesicle gliosecretion in astrocytes. J Cell Biol 193: 537–549, 2011. doi: 10.1083/jcb.201010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1408.Pribiag H, Stellwagen D. TNF-α downregulates inhibitory neurotransmission through protein phosphatase 1-dependent trafficking of GABA(A) receptors. J Neurosci 33: 15879–15893, 2013. doi: 10.1523/JNEUROSCI.0530-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1409.Price DL, Ludwig JW, Mi H, Schwarz TL, Ellisman MH. Distribution of rSlo Ca2+-activated K+ channels in rat astrocyte perivascular endfeet. Brain Res 956: 183–193, 2002. doi: 10.1016/S0006-8993(02)03266-3. [DOI] [PubMed] [Google Scholar]
- 1410.Price TJ, Hargreaves KM, Cervero F. Protein expression and mRNA cellular distribution of the NKCC1 cotransporter in the dorsal root and trigeminal ganglia of the rat. Brain Res 1112: 146–158, 2006. doi: 10.1016/j.brainres.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1411.Proia P, Schiera G, Mineo M, Ingrassia AM, Santoro G, Savettieri G, Di Liegro I. Astrocytes shed extracellular vesicles that contain fibroblast growth factor-2 and vascular endothelial growth factor. Int J Mol Med 21: 63–67, 2008. [PubMed] [Google Scholar]
- 1412.Prolo LM, Takahashi JS, Herzog ED. Circadian rhythm generation and entrainment in astrocytes. J Neurosci 25: 404–408, 2005. doi: 10.1523/JNEUROSCI.4133-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1413.Pryazhnikov E, Khiroug L. Sub-micromolar increase in [Ca2+]i triggers delayed exocytosis of ATP in cultured astrocytes. Glia 56: 38–49, 2008. doi: 10.1002/glia.20590. [DOI] [PubMed] [Google Scholar]
- 1414.Puro DG, Yuan JP, Sucher NJ. Activation of NMDA receptor-channels in human retinal Müller glial cells inhibits inward-rectifying potassium currents. Vis Neurosci 13: 319–326, 1996. doi: 10.1017/S0952523800007562. [DOI] [PubMed] [Google Scholar]
- 1415.Qiu F, Wang J, Spray DC, Scemes E, Dahl G. Two non-vesicular ATP release pathways in the mouse erythrocyte membrane. FEBS Lett 585: 3430–3435, 2011. doi: 10.1016/j.febslet.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1416.Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 157: 447–458, 2014. doi: 10.1016/j.cell.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1417.Qu H, Håberg A, Haraldseth O, Unsgård G, Sonnewald U. 13C MR spectroscopy study of lactate as substrate for rat brain. Dev Neurosci 22: 429–436, 2000. doi: 10.1159/000017472. [DOI] [PubMed] [Google Scholar]
- 1418.Qusous A, Geewan CS, Greenwell P, Kerrigan MJ. siRNA-mediated inhibition of Na+-K+-2Cl- cotransporter (NKCC1) and regulatory volume increase in the chondrocyte cell line C-20/A4. J Membr Biol 243: 25–34, 2011. doi: 10.1007/s00232-011-9389-z. [DOI] [PubMed] [Google Scholar]
- 1419.Raap M, Biedermann B, Braun P, Milenkovic I, Skatchkov SN, Bringmann A, Reichenbach A. Diversity of Kir channel subunit mRNA expressed by retinal glial cells of the guinea-pig. Neuroreport 13: 1037–1040, 2002. doi: 10.1097/00001756-200206120-00012. [DOI] [PubMed] [Google Scholar]
- 1420.Radian R, Kanner BI. Stoichiometry of sodium- and chloride-coupled gamma-aminobutyric acid transport by synaptic plasma membrane vesicles isolated from rat brain. Biochemistry 22: 1236–1241, 1983. doi: 10.1021/bi00274a038. [DOI] [PubMed] [Google Scholar]
- 1421.Raff MC, Abney ER, Cohen J, Lindsay R, Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci 3: 1289–1300, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1422.Rafiki A, Boulland JL, Halestrap AP, Ottersen OP, Bergersen L. Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience 122: 677–688, 2003. doi: 10.1016/j.neuroscience.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 1423.Raiteri L, Stigliani S, Usai C, Diaspro A, Paluzzi S, Milanese M, Raiteri M, Bonanno G. Functional expression of release-regulating glycine transporters GLYT1 on GABAergic neurons and GLYT2 on astrocytes in mouse spinal cord. Neurochem Int 52: 103–112, 2008. doi: 10.1016/j.neuint.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 1424.Rakic P. Elusive radial glial cells: historical and evolutionary perspective. Glia 43: 19–32, 2003. doi: 10.1002/glia.10244. [DOI] [PubMed] [Google Scholar]
- 1425.Ramamoorthy P, Whim MD. Trafficking and fusion of neuropeptide Y-containing dense-core granules in astrocytes. J Neurosci 28: 13815–13827, 2008. doi: 10.1523/JNEUROSCI.5361-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1426.Ramon y Cajal S. Histologie du système nerveux de l’homme et des vertébrés. Paris: Maloine, 1909. [Google Scholar]
- 1427.Ramón y Cajal S. Algunas conjeturas sobre el mechanismoanatomico de la ideacion, asociacion y atencion. Madrid: Imprenta y Libreria de Nicolas Moya, 1895. [Google Scholar]
- 1428.Ramón y Cajal S. Contribucion al conocimiento de la neuroglia del cerebro humano. Trab Lab Invest Biol Univ Madrid 11: 255–315, 1913. [Google Scholar]
- 1429.Ramón y Cajal S. El proceder del oro-sublimado para la coloracion de la neuroglia. Trab Lab Invest Biol Univ Madrid 14: 155–162, 1916. [Google Scholar]
- 1430.Ramón y Cajal S. Un nuevo proceder para la impregnación de la neuroglía. Bol Soc Esp Biol II: 104–108, 1913. [Google Scholar]
- 1431.Rana S, Dringen R. Gap junction hemichannel-mediated release of glutathione from cultured rat astrocytes. Neurosci Lett 415: 45–48, 2007. doi: 10.1016/j.neulet.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 1432.Rangroo Thrane V, Thrane AS, Plog BA, Thiyagarajan M, Iliff JJ, Deane R, Nagelhus EA, Nedergaard M. Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Sci Rep 3: 2582, 2013. doi: 10.1038/srep02582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1433.Ransom CB, Ransom BR, Sontheimer H. Activity-dependent extracellular K+ accumulation in rat optic nerve: the role of glial and axonal Na+ pumps. J Physiol 522: 427–442, 2000. doi: 10.1111/j.1469-7793.2000.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1434.Ransom CB, Sontheimer H. Biophysical and pharmacological characterization of inwardly rectifying K+ currents in rat spinal cord astrocytes. J Neurophysiol 73: 333–346, 1995. [DOI] [PubMed] [Google Scholar]
- 1435.Ransom CB, Sontheimer H, Janigro D. Astrocytic inwardly rectifying potassium currents are dependent on external sodium ions. J Neurophysiol 76: 626–630, 1996. [DOI] [PubMed] [Google Scholar]
- 1436.Raponi E, Agenes F, Delphin C, Assard N, Baudier J, Legraverend C, Deloulme JC. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia 55: 165–177, 2007. doi: 10.1002/glia.20445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1437.Rash JE, Duffy HS, Dudek FE, Bilhartz BL, Whalen LR, Yasumura T. Grid-mapped freeze-fracture analysis of gap junctions in gray and white matter of adult rat central nervous system, with evidence for a “panglial syncytium” that is not coupled to neurons. J Comp Neurol 388: 265–292, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 1438.Rash JE, Yasumura T, Dudek FE, Nagy JI. Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J Neurosci 21: 1983–2000, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1439.Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc Natl Acad Sci USA 95: 11981–11986, 1998. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1440.Rasmussen R, Nedergaard M, Petersen NC. Sulforhodamine 101, a widely used astrocyte marker, can induce cortical seizure-like activity at concentrations commonly used. Sci Rep 6: 30433, 2016. doi: 10.1038/srep30433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1441.Rassoulpour A, Wu HQ, Ferre S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J Neurochem 93: 762–765, 2005. doi: 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]
- 1442.Rauen T, Rothstein JD, Wässle H. Differential expression of three glutamate transporter subtypes in the rat retina. Cell Tissue Res 286: 325–336, 1996. doi: 10.1007/s004410050702. [DOI] [PubMed] [Google Scholar]
- 1443.Ray A, Zoidl G, Weickert S, Wahle P, Dermietzel R. Site-specific and developmental expression of pannexin1 in the mouse nervous system. Eur J Neurosci 21: 3277–3290, 2005. doi: 10.1111/j.1460-9568.2005.04139.x. [DOI] [PubMed] [Google Scholar]
- 1444.Reese KA, Caldwell JH. Immunocytochemical localization of NaCh6 in cultured spinal cord astrocytes. Glia 26: 92–96, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 1445.Reeves AM, Shigetomi E, Khakh BS. Bulk loading of calcium indicator dyes to study astrocyte physiology: key limitations and improvements using morphological maps. J Neurosci 31: 9353–9358, 2011. doi: 10.1523/JNEUROSCI.0127-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1446.Reichelt W, Knöpfel T. Glutamate uptake controls expression of a slow postsynaptic current mediated by mGluRs in cerebellar Purkinje cells. J Neurophysiol 87: 1974–1980, 2002. doi: 10.1152/jn.00704.2001. [DOI] [PubMed] [Google Scholar]
- 1447.Reichenbach A. Attempt to classify glial cells by means of their process specialization using the rabbit retinal Müller cell as an example of cytotopographic specialization of glial cells. Glia 2: 250–259, 1989. doi: 10.1002/glia.440020406. [DOI] [PubMed] [Google Scholar]
- 1448.Reichenbach A. Glia:neuron index: review and hypothesis to account for different values in various mammals. Glia 2: 71–77, 1989. doi: 10.1002/glia.440020202. [DOI] [PubMed] [Google Scholar]
- 1449.Reichenbach A, Bringmann A. Comparative anatomy of glial cells in mammals. In: Evolution of Nervous Systems (2nd ed.), edited by Kaas J. New York: Academic Press/Elsevier, 2017, p. 309–348. doi: 10.1016/B978-0-12-804042-3.00050-6. [DOI] [Google Scholar]
- 1450.Reichenbach A, Bringmann A. Müller Cells in the Healthy and Diseased Retina. New York: Springer, 2010, p. 432. doi: 10.1007/978-1-4419-1672-3. [DOI] [PubMed] [Google Scholar]
- 1451.Reichenbach A, Derouiche A, Kirchhoff F. Morphology and dynamics of perisynaptic glia. Brain Res Brain Res Rev 63: 11–25, 2010. doi: 10.1016/j.brainresrev.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 1452.Reichenbach A, Neumann M, Brückner G. Cell length to diameter relation of rat fetal radial glia–does impaired K+ transport capacity of long thin cells cause their perinatal transformation into multipolar astrocytes? Neurosci Lett 73: 95–100, 1987. doi: 10.1016/0304-3940(87)90038-3. [DOI] [PubMed] [Google Scholar]
- 1453.Rela L, Bordey A, Greer CA. Olfactory ensheathing cell membrane properties are shaped by connectivity. Glia 58: 665–678, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1454.Ren J, Song D, Bai Q, Verkhratsky A, Peng L. Fluoxetine induces alkalinization of astroglial cytosol through stimulation of sodium-hydrogen exchanger 1: dissection of intracellular signaling pathways. Front Cell Neurosci 9: 61, 2015. doi: 10.3389/fncel.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1455.Requardt RP, Hirrlinger PG, Wilhelm F, Winkler U, Besser S, Hirrlinger J. Ca2+ signals of astrocytes are modulated by the NAD+/NADH redox state. J Neurochem 120: 1014–1025, 2012. [DOI] [PubMed] [Google Scholar]
- 1456.Requardt RP, Wilhelm F, Rillich J, Winkler U, Hirrlinger J. The biphasic NAD(P)H fluorescence response of astrocytes to dopamine reflects the metabolic actions of oxidative phosphorylation and glycolysis. J Neurochem 115: 483–492, 2010. doi: 10.1111/j.1471-4159.2010.06940.x. [DOI] [PubMed] [Google Scholar]
- 1457.Retamal MA, Froger N, Palacios-Prado N, Ezan P, Sáez PJ, Sáez JC, Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci 27: 13781–13792, 2007. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1458.Retzius G. Mit 32 Tafeln. Stockholm: Von Gustav Fischer, 1894, p. 87 Biologishe Untersuchungen. Neue Folge, vol VI. [Google Scholar]
- 1459.Reuss B, Unsicker K. Atypical neuroleptic drugs downregulate dopamine sensitivity in rat cortical and striatal astrocytes. Mol Cell Neurosci 18: 197–209, 2001. doi: 10.1006/mcne.2001.1017. [DOI] [PubMed] [Google Scholar]
- 1460.Reutter MA, Richards EM, Sumners C. Regulation of α2A-adrenergic receptor expression by epinephrine in cultured astroglia from rat brain. J Neurochem 70: 86–95, 1998. doi: 10.1046/j.1471-4159.1998.70010086.x. [DOI] [PubMed] [Google Scholar]
- 1461.Reyes RC, Verkhratsky A, Parpura V. Plasmalemmal Na+/Ca2+ exchanger modulates Ca2+-dependent exocytotic release of glutamate from rat cortical astrocytes. ASN Neuro 4: e00075, 2012. doi: 10.1042/AN20110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1462.Reyes RC, Verkhratsky A, Parpura V. TRPC1-mediated Ca2+ and Na+ signalling in astroglia: differential filtering of extracellular cations. Cell Calcium 54: 120–125, 2013. doi: 10.1016/j.ceca.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1463.Ribak CE, Tong WM, Brecha NC. GABA plasma membrane transporters, GAT-1 and GAT-3, display different distributions in the rat hippocampus. J Comp Neurol 367: 595–606, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 1464.Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci 23: 209–216, 2000. doi: 10.1016/S0166-2236(99)01543-X. [DOI] [PubMed] [Google Scholar]
- 1465.Rice ME, Nicholson C. Glutamate- and aspartate-induced extracellular potassium and calcium shifts and their relation to those of kainate, quisqualate and N-methyl-d-aspartate in the isolated turtle cerebellum. Neuroscience 38: 295–310, 1990. doi: 10.1016/0306-4522(90)90029-4. [DOI] [PubMed] [Google Scholar]
- 1466.Rickmann M, Wolff JR. S100 protein expression in subpopulations of neurons of rat brain. Neuroscience 67: 977–991, 1995. doi: 10.1016/0306-4522(94)00615-C. [DOI] [PubMed] [Google Scholar]
- 1467.Ridet JL, Alonso G, Chauvet N, Chapron J, Koenig J, Privat A. Immunocytochemical characterization of a new marker of fibrous and reactive astrocytes. Cell Tissue Res 283: 39–49, 1996. doi: 10.1007/s004410050510. [DOI] [PubMed] [Google Scholar]
- 1468.Riederer P, Konradi C, Schay V, Kienzl E, Birkmayer G, Danielczyk W, Sofic E, Youdim MB. Localization of MAO-A and MAO-B in human brain: a step in understanding the therapeutic action of l-deprenyl. Adv Neurol 45: 111–118, 1987. [PubMed] [Google Scholar]
- 1469.Rindfleisch E. Handbuch der pathologischen Gewebelehre (3rd ed.). Leipzig: Engelmann, 1873. [Google Scholar]
- 1470.Riquelme R, Miralles CP, De Blas AL. Bergmann glia GABAA receptors concentrate on the glial processes that wrap inhibitory synapses. J Neurosci 22: 10720–10730, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1471.Rishal I, Keren-Raifman T, Yakubovich D, Ivanina T, Dessauer CW, Slepak VZ, Dascal N. Na+ promotes the dissociation between Galpha GDP and Gbeta gamma, activating G protein-gated K+ channels. J Biol Chem 278: 3840–3845, 2003. doi: 10.1074/jbc.C200605200. [DOI] [PubMed] [Google Scholar]
- 1472.Rival T, Soustelle L, Strambi C, Besson MT, Iché M, Birman S. Decreasing glutamate buffering capacity triggers oxidative stress and neuropil degeneration in the Drosophila brain. Curr Biol 14: 599–605, 2004. doi: 10.1016/j.cub.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 1473.Robertson JM. Astrocyte domains and the three-dimensional and seamless expression of consciousness and explicit memories. Med Hypotheses 81: 1017–1024, 2013. doi: 10.1016/j.mehy.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 1474.Robins SC, Stewart I, McNay DE, Taylor V, Giachino C, Goetz M, Ninkovic J, Briancon N, Maratos-Flier E, Flier JS, Kokoeva MV, Placzek M. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat Commun 4: 2049, 2013. doi: 10.1038/ncomms3049. [DOI] [PubMed] [Google Scholar]
- 1475.Robinson MB, Jackson JG. Astroglial glutamate transporters coordinate excitatory signaling and brain energetics. Neurochem Int 98: 56–71, 2016. doi: 10.1016/j.neuint.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1476.Robinson SR. Changes in the cellular distribution of glutamine synthetase in Alzheimer’s disease. J Neurosci Res 66: 972–980, 2001. doi: 10.1002/jnr.10057. [DOI] [PubMed] [Google Scholar]
- 1477.Robinson SR, Hampson EC, Munro MN, Vaney DI. Unidirectional coupling of gap junctions between neuroglia. Science 262: 1072–1074, 1993. doi: 10.1126/science.8093125. [DOI] [PubMed] [Google Scholar]
- 1478.Robitaille R. Modulation of synaptic efficacy and synaptic depression by glial cells at the frog neuromuscular junction. Neuron 21: 847–855, 1998. doi: 10.1016/S0896-6273(00)80600-5. [DOI] [PubMed] [Google Scholar]
- 1479.Rodnight RB, Gottfried C. Morphological plasticity of rodent astroglia. J Neurochem 124: 263–275, 2013. doi: 10.1111/jnc.12087. [DOI] [PubMed] [Google Scholar]
- 1480.Rodríguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A. Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience 323: 170–182, 2016. doi: 10.1016/j.neuroscience.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 1481.Rodriguez-Vieitez E, Saint-Aubert L, Carter SF, Almkvist O, Farid K, Schöll M, Chiotis K, Thordardottir S, Graff C, Wall A, Långström B, Nordberg A. Diverging longitudinal changes in astrocytosis and amyloid PET in autosomal dominant Alzheimer’s disease. Brain 139: 922–936, 2016. doi: 10.1093/brain/awv404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1482.Rodríguez EM, Blázquez JL, Pastor FE, Peláez B, Peña P, Peruzzo B, Amat P. Hypothalamic tanycytes: a key component of brain-endocrine interaction. Int Rev Cytol 247: 89–164, 2005. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- 1483.Rodríguez JJ, Butt AM, Gardenal E, Parpura V, Verkhratsky A. Complex and differential glial responses in Alzheimer’s disease and ageing. Curr Alzheimer Res 13: 343–358, 2016. doi: 10.2174/1567205013666160229112911. [DOI] [PubMed] [Google Scholar]
- 1484.Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat caudate putamen nucleus. J Neurosci 21: 823–833, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1485.Rodríguez JJ, Terzieva S, Olabarria M, Lanza RG, Verkhratsky A. Enriched environment and physical activity reverse astrogliodegeneration in the hippocampus of AD transgenic mice. Cell Death Dis 4: e678, 2013. doi: 10.1038/cddis.2013.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1486.Rodríguez JJ, Yeh CY, Terzieva S, Olabarria M, Kulijewicz-Nawrot M, Verkhratsky A. Complex and region-specific changes in astroglial markers in the aging brain. Neurobiol Aging 35: 15–23, 2014. doi: 10.1016/j.neurobiolaging.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 1487.Rogers SD, Peters CM, Pomonis JD, Hagiwara H, Ghilardi JR, Mantyh PW. Endothelin B receptors are expressed by astrocytes and regulate astrocyte hypertrophy in the normal and injured CNS. Glia 41: 180–190, 2003. doi: 10.1002/glia.10173. [DOI] [PubMed] [Google Scholar]
- 1488.Rohr S. Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc Res 62: 309–322, 2004. doi: 10.1016/j.cardiores.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 1489.Roman RM, Smith RL, Feranchak AP, Clayton GH, Doctor RB, Fitz JG. ClC-2 chloride channels contribute to HTC cell volume homeostasis. Am J Physiol Gastrointest Liver Physiol 280: G344–G353, 2001. [DOI] [PubMed] [Google Scholar]
- 1490.Romero MF, Chen AP, Parker MD, Boron WF. The SLC4 family of bicarbonate (HCO3−) transporters. Mol Aspects Med 34: 159–182, 2013. doi: 10.1016/j.mam.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1491.Ronaldson PT, Bendayan R. HIV-1 viral envelope glycoprotein gp120 produces oxidative stress and regulates the functional expression of multidrug resistance protein-1 (Mrp1) in glial cells. J Neurochem 106: 1298–1313, 2008. doi: 10.1111/j.1471-4159.2008.05479.x. [DOI] [PubMed] [Google Scholar]
- 1492.Ronco V, Grolla AA, Glasnov TN, Canonico PL, Verkhratsky A, Genazzani AA, Lim D. Differential deregulation of astrocytic calcium signalling by amyloid-β, TNFα, IL-1β and LPS. Cell Calcium 55: 219–229, 2014. doi: 10.1016/j.ceca.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 1493.Rosafio K, Pellerin L. Oxygen tension controls the expression of the monocarboxylate transporter MCT4 in cultured mouse cortical astrocytes via a hypoxia-inducible factor-1α-mediated transcriptional regulation. Glia 62: 477–490, 2014. doi: 10.1002/glia.22618. [DOI] [PubMed] [Google Scholar]
- 1494.Rose CR, Chatton JY. Astrocyte sodium signaling and neuro-metabolic coupling in the brain. Neuroscience 323: 121–134, 2016. doi: 10.1016/j.neuroscience.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 1495.Rose CR, Karus C. Two sides of the same coin: sodium homeostasis and signaling in astrocytes under physiological and pathophysiological conditions. Glia 61: 1191–1205, 2013. doi: 10.1002/glia.22492. [DOI] [PubMed] [Google Scholar]
- 1496.Rose CR, Ransom BR. Intracellular sodium homeostasis in rat hippocampal astrocytes. J Physiol 491: 291–305, 1996. doi: 10.1113/jphysiol.1996.sp021216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1497.Rose CR, Ransom BR. Mechanisms of H+ and Na+ changes induced by glutamate, kainate, and d-aspartate in rat hippocampal astrocytes. J Neurosci 16: 5393–5404, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1498.Rose CR, Ransom BR. Regulation of intracellular sodium in cultured rat hippocampal neurones. J Physiol 499: 573–587, 1997. doi: 10.1113/jphysiol.1997.sp021951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1499.Rose CR, Verkhratsky A. Principles of sodium homeostasis and sodium signalling in astroglia. Glia 64: 1611–1627, 2016. doi: 10.1002/glia.22964. [DOI] [PubMed] [Google Scholar]
- 1500.Rose CR, Waxman SG, Ransom BR. Effects of glucose deprivation, chemical hypoxia, and simulated ischemia on Na+ homeostasis in rat spinal cord astrocytes. J Neurosci 18: 3554–3562, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1501.Rose EM, Koo JC, Antflick JE, Ahmed SM, Angers S, Hampson DR. Glutamate transporter coupling to Na,K-ATPase. J Neurosci 29: 8143–8155, 2009. doi: 10.1523/JNEUROSCI.1081-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1502.Rosenberg D, Artoul S, Segal AC, Kolodney G, Radzishevsky I, Dikopoltsev E, Foltyn VN, Inoue R, Mori H, Billard JM, Wolosker H. Neuronal d-serine and glycine release via the Asc-1 transporter regulates NMDA receptor-dependent synaptic activity. J Neurosci 33: 3533–3544, 2013. doi: 10.1523/JNEUROSCI.3836-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1503.Rosenegger DG, Tran CH, Wamsteeker Cusulin JI, Gordon GR. Tonic local brain blood flow control by astrocytes independent of phasic neurovascular coupling. J Neurosci 35: 13463–13474, 2015. doi: 10.1523/JNEUROSCI.1780-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1504.Rosewater K, Sontheimer H. Fibrous and protoplasmic astrocytes express GABAA receptors that differ in benzodiazepine pharmacology. Brain Res 636: 73–80, 1994. doi: 10.1016/0006-8993(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 1505.Rossi D. Astrocyte physiopathology: At the crossroads of intercellular networking, inflammation and cell death. Prog Neurobiol 130: 86–120, 2015. doi: 10.1016/j.pneurobio.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 1506.Rosso L, Mienville JM. Pituicyte modulation of neurohormone output. Glia 57: 235–243, 2009. doi: 10.1002/glia.20760. [DOI] [PubMed] [Google Scholar]
- 1507.Rothman DL, De Feyter HM, de Graaf RA, Mason GF, Behar KL. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed 24: 943–957, 2011. doi: 10.1002/nbm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1508.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16: 675–686, 1996. doi: 10.1016/S0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 1509.Roux L, Benchenane K, Rothstein JD, Bonvento G, Giaume C. Plasticity of astroglial networks in olfactory glomeruli. Proc Natl Acad Sci USA 108: 18442–18446, 2011. doi: 10.1073/pnas.1107386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1510.Roux MJ, Supplisson S. Neuronal and glial glycine transporters have different stoichiometries. Neuron 25: 373–383, 2000. doi: 10.1016/S0896-6273(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 1511.Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. Nature 468: 214–222, 2010. doi: 10.1038/nature09611. [DOI] [PubMed] [Google Scholar]
- 1512.Roy CS, Sherrington CS. On the regulation of the blood-supply of the brain. J Physiol 11: 85–108, 1890. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1513.Roy ML, Saal D, Perney T, Sontheimer H, Waxman SG, Kaczmarek LK. Manipulation of the delayed rectifier Kv1.5 potassium channel in glial cells by antisense oligodeoxynucleotides. Glia 18: 177–184, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 1514.Roy ML, Sontheimer H. β-adrenergic modulation of glial inwardly rectifying potassium channels. J Neurochem 64: 1576–1584, 1995. doi: 10.1046/j.1471-4159.1995.64041576.x. [DOI] [PubMed] [Google Scholar]
- 1515.Rubini P, Pagel G, Mehri S, Marquardt P, Riedel T, Illes P. Functional P2X7 receptors at cultured hippocampal astrocytes but not neurons. Naunyn-Schmiedebergs Arch Pharmacol 387: 943–954, 2014. doi: 10.1007/s00210-014-1005-1. [DOI] [PubMed] [Google Scholar]
- 1516.Rungta RL, Bernier LP, Dissing-Olesen L, Groten CJ, LeDue JM, Ko R, Drissler S, MacVicar BA. Ca2+ transients in astrocyte fine processes occur via Ca2+ influx in the adult mouse hippocampus. Glia 64: 2093–2103, 2016. doi: 10.1002/glia.23042. [DOI] [PubMed] [Google Scholar]
- 1517.Rusakov DA. Astroglial glutamate transporters trigger glutaminergic gliotransmission. J Physiol 590: 2187–2188, 2012. doi: 10.1113/jphysiol.2012.233577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1518.Rusakov DA. Disentangling calcium-driven astrocyte physiology. Nat Rev Neurosci 16: 226–233, 2015. doi: 10.1038/nrn3878. [DOI] [PubMed] [Google Scholar]
- 1519.Rusakov DA, Fine A. Extracellular Ca2+ depletion contributes to fast activity-dependent modulation of synaptic transmission in the brain. Neuron 37: 287–297, 2003. doi: 10.1016/S0896-6273(03)00025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1520.Rusnakova V, Honsa P, Dzamba D, Ståhlberg A, Kubista M, Anderova M. Heterogeneity of astrocytes: from development to injury - single cell gene expression. PLoS One 8: e69734, 2013. doi: 10.1371/journal.pone.0069734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1521.Ruzicka BB, Fox CA, Thompson RC, Meng F, Watson SJ, Akil H. Primary astroglial cultures derived from several rat brain regions differentially express μ, δ and κ opioid receptor mRNA. Brain Res Mol Brain Res 34: 209–220, 1995. doi: 10.1016/0169-328X(95)00165-O. [DOI] [PubMed] [Google Scholar]
- 1522.Saab AS, Neumeyer A, Jahn HM, Cupido A, Šimek AA, Boele HJ, Scheller A, Le Meur K, Götz M, Monyer H, Sprengel R, Rubio ME, Deitmer JW, De Zeeuw CI, Kirchhoff F. Bergmann glial AMPA receptors are required for fine motor coordination. Science 337: 749–753, 2012. doi: 10.1126/science.1221140. [DOI] [PubMed] [Google Scholar]
- 1523.Saaltink DJ, Håvik B, Verissimo CS, Lucassen PJ, Vreugdenhil E. Doublecortin and doublecortin-like are expressed in overlapping and non-overlapping neuronal cell population: implications for neurogenesis. J Comp Neurol 520: 2805–2823, 2012. doi: 10.1002/cne.23144. [DOI] [PubMed] [Google Scholar]
- 1524.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 83: 1359–1400, 2003. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 1525.Sáez JC, Schalper KA, Retamal MA, Orellana JA, Shoji KF, Bennett MV. Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp Cell Res 316: 2377–2389, 2010. doi: 10.1016/j.yexcr.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 1526.Sahlender DA, Savtchouk I, Volterra A. What do we know about gliotransmitter release from astrocytes? Philos Trans R Soc Lond B Biol Sci 369: 20130592, 2014. doi: 10.1098/rstb.2013.0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1527.Sahu G, Sukumaran S, Bera AK. Pannexins form gap junctions with electrophysiological and pharmacological properties distinct from connexins. Sci Rep 4: 4955, 2014. doi: 10.1038/srep04955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1528.Saint Marie RL, Carlson SD. The fine structure of neuroglia in the lamina ganglionaris of the housefly, Musca domestica L. J Neurocytol 12: 213–241, 1983. doi: 10.1007/BF01148463. [DOI] [PubMed] [Google Scholar]
- 1529.Salio C, Doly S, Fischer J, Franzoni MF, Conrath M. Neuronal and astrocytic localization of the cannabinoid receptor-1 in the dorsal horn of the rat spinal cord. Neurosci Lett 329: 13–16, 2002. doi: 10.1016/S0304-3940(02)00549-9. [DOI] [PubMed] [Google Scholar]
- 1530.Salm AK, McCarthy KD. The evidence for astrocytes as a target for central noradrenergic activity: expression of adrenergic receptors. Brain Res Bull 29: 265–275, 1992. doi: 10.1016/0361-9230(92)90056-4. [DOI] [PubMed] [Google Scholar]
- 1531.Salm AK, McCarthy KD. Norepinephrine-evoked calcium transients in cultured cerebral type 1 astroglia. Glia 3: 529–538, 1990. doi: 10.1002/glia.440030612. [DOI] [PubMed] [Google Scholar]
- 1532.Salter MW, Hicks JL. ATP-evoked increases in intracellular calcium in neurons and glia from the dorsal spinal cord. J Neurosci 14: 1563–1575, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1533.Salter MW, Hicks JL. ATP causes release of intracellular Ca2+ via the phospholipase C β/IP3 pathway in astrocytes from the dorsal spinal cord. J Neurosci 15: 2961–2971, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1534.Sánchez C, Galve-Roperh I, Rueda D, Guzmán M. Involvement of sphingomyelin hydrolysis and the mitogen-activated protein kinase cascade in the Delta9-tetrahydrocannabinol-induced stimulation of glucose metabolism in primary astrocytes. Mol Pharmacol 54: 834–843, 1998. [DOI] [PubMed] [Google Scholar]
- 1535.Sánchez HA, Orellana JA, Verselis VK, Sáez JC. Metabolic inhibition increases activity of connexin-32 hemichannels permeable to Ca2+ in transfected HeLa cells. Am J Physiol Cell Physiol 297: C665–C678, 2009. doi: 10.1152/ajpcell.00200.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1536.Sandén N, Thorlin T, Blomstrand F, Persson PA, Hansson E. 5-Hydroxytryptamine2B receptors stimulate Ca2+ increases in cultured astrocytes from three different brain regions. Neurochem Int 36: 427–434, 2000. doi: 10.1016/S0197-0186(99)00134-5. [DOI] [PubMed] [Google Scholar]
- 1537.Santello M, Bezzi P, Volterra A. TNFα controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 69: 988–1001, 2011. doi: 10.1016/j.neuron.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 1538.Santello M, Calì C, Bezzi P. Gliotransmission and the tripartite synapse. Adv Exp Med Biol 970: 307–331, 2012. doi: 10.1007/978-3-7091-0932-8_14. [DOI] [PubMed] [Google Scholar]
- 1539.Sanzgiri RP, Araque A, Haydon PG. Prostaglandin E2 stimulates glutamate receptor-dependent astrocyte neuromodulation in cultured hippocampal cells. J Neurobiol 41: 221–229, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 1540.Sarantis M, Ballerini L, Miller B, Silver RA, Edwards M, Attwell D. Glutamate uptake from the synaptic cleft does not shape the decay of the non-NMDA component of the synaptic current. Neuron 11: 541–549, 1993. doi: 10.1016/0896-6273(93)90158-N. [DOI] [PubMed] [Google Scholar]
- 1541.Sarantis M, Everett K, Attwell D. A presynaptic action of glutamate at the cone output synapse. Nature 332: 451–453, 1988. doi: 10.1038/332451a0. [DOI] [PubMed] [Google Scholar]
- 1542.Sasaki T, Kuga N, Namiki S, Matsuki N, Ikegaya Y. Locally synchronized astrocytes. Cereb Cortex 21: 1889–1900, 2011. doi: 10.1093/cercor/bhq256. [DOI] [PubMed] [Google Scholar]
- 1543.Sason H, Billard JM, Smith GP, Safory H, Neame S, Kaplan E, Rosenberg D, Zubedat S, Foltyn VN, Christoffersen CT, Bundgaard C, Thomsen C, Avital A, Christensen KV, Wolosker H. Asc-1 transporter regulation of synaptic activity via the tonic release of d-serine in the forebrain. Cereb Cortex 27: 1573–1587, 2017. doi: 10.1093/cercor/bhv350. [DOI] [PubMed] [Google Scholar]
- 1544.Sato K, Malchinkhuu E, Horiuchi Y, Mogi C, Tomura H, Tosaka M, Yoshimoto Y, Kuwabara A, Okajima F. Critical role of ABCA1 transporter in sphingosine 1-phosphate release from astrocytes. J Neurochem 103: 2610–2619, 2007. doi: 10.1111/j.1471-4159.2007.04958.x. [DOI] [PubMed] [Google Scholar]
- 1545.Sato K, Matsuki N, Ohno Y, Nakazawa K. Estrogens inhibit l-glutamate uptake activity of astrocytes via membrane estrogen receptor alpha. J Neurochem 86: 1498–1505, 2003. doi: 10.1046/j.1471-4159.2003.01953.x. [DOI] [PubMed] [Google Scholar]
- 1546.Satoh J, Tabunoki H, Yamamura T, Arima K, Konno H. Human astrocytes express aquaporin-1 and aquaporin-4 in vitro and in vivo. Neuropathology 27: 245–256, 2007. doi: 10.1111/j.1440-1789.2007.00774.x. [DOI] [PubMed] [Google Scholar]
- 1547.Saubermann AJ, Castiglia CM, Foster MC. Preferential uptake of rubidium from extracellular space by glial cells compared to neurons in leech ganglia. Brain Res 577: 64–72, 1992. doi: 10.1016/0006-8993(92)90538-K. [DOI] [PubMed] [Google Scholar]
- 1548.Saura J, Kettler R, Da Prada M, Richards JG. Quantitative enzyme radioautography with 3H-Ro 41-1049 and 3H-Ro 19-6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci 12: 1977–1999, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1549.Savchenko VL, McKanna JA, Nikonenko IR, Skibo GG. Microglia and astrocytes in the adult rat brain: comparative immunocytochemical analysis demonstrates the efficacy of lipocortin 1 immunoreactivity. Neuroscience 96: 195–203, 2000. doi: 10.1016/S0306-4522(99)00538-2. [DOI] [PubMed] [Google Scholar]
- 1550.Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, Yamamoto A, Moriyama Y. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci USA 105: 5683–5686, 2008. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1551.Sbai O, Ould-Yahoui A, Ferhat L, Gueye Y, Bernard A, Charrat E, Mehanna A, Risso JJ, Chauvin JP, Fenouillet E, Rivera S, Khrestchatisky M. Differential vesicular distribution and trafficking of MMP-2, MMP-9, and their inhibitors in astrocytes. Glia 58: 344–366, 2010. [DOI] [PubMed] [Google Scholar]
- 1552.Scalise M, Pochini L, Galluccio M, Indiveri C. Glutamine transport. From energy supply to sensing and beyond. Biochim Biophys Acta 1857: 1147–1157, 2016. doi: 10.1016/j.bbabio.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 1553.Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia 54: 716–725, 2006. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1554.Schaeffer M, Langlet F, Lafont C, Molino F, Hodson DJ, Roux T, Lamarque L, Verdié P, Bourrier E, Dehouck B, Banères JL, Martinez J, Méry PF, Marie J, Trinquet E, Fehrentz JA, Prévot V, Mollard P. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc Natl Acad Sci USA 110: 1512–1517, 2013. doi: 10.1073/pnas.1212137110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1555.Schafer DP, Stevens B. Phagocytic glial cells: sculpting synaptic circuits in the developing nervous system. Curr Opin Neurobiol 23: 1034–1040, 2013. doi: 10.1016/j.conb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1556.Schaller KL, Krzemien DM, Yarowsky PJ, Krueger BK, Caldwell JH. A novel, abundant sodium channel expressed in neurons and glia. J Neurosci 15: 3231–3242, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1557.Scharfman HE, Binder DK. Aquaporin-4 water channels and synaptic plasticity in the hippocampus. Neurochem Int 63: 702–711, 2013. doi: 10.1016/j.neuint.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1558.Schell MJ, Brady RO Jr, Molliver ME, Snyder SH. d-Serine as a neuromodulator: regional and developmental localizations in rat brain glia resemble NMDA receptors. J Neurosci 17: 1604–1615, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1559.Schell MJ, Molliver ME, Snyder SH. d-Serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci USA 92: 3948–3952, 1995. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1560.Schipke CG, Haas B, Kettenmann H. Astrocytes discriminate and selectively respond to the activity of a subpopulation of neurons within the barrel cortex. Cereb Cortex 18: 2450–2459, 2008. doi: 10.1093/cercor/bhn009. [DOI] [PubMed] [Google Scholar]
- 1561.Schipke CG, Heuser I, Peters O. Antidepressants act on glial cells: SSRIs and serotonin elicit astrocyte calcium signaling in the mouse prefrontal cortex. J Psychiatr Res 45: 242–248, 2011. doi: 10.1016/j.jpsychires.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 1562.Schipke CG, Ohlemeyer C, Matyash M, Nolte C, Kettenmann H, Kirchhoff F. Astrocytes of the mouse neocortex express functional N-methyl-d-aspartate receptors. FASEB J 15: 1270–1272, 2001. [DOI] [PubMed] [Google Scholar]
- 1563.Schipper HM. Gomori-positive astrocytes: biological properties and implications for neurologic and neuroendocrine disorders. Glia 4: 365–377, 1991. doi: 10.1002/glia.440040404. [DOI] [PubMed] [Google Scholar]
- 1564.Schitine C, Nogaroli L, Costa MR, Hedin-Pereira C. Astrocyte heterogeneity in the brain: from development to disease. Front Cell Neurosci 9: 76, 2015. doi: 10.3389/fncel.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1565.Schleich CL. Schmerzlose Operationen: Örtliche Betäubung mit indiffrenten Flüssigkeiten. Psychophysik des natürlichen und künstlichen Schlafes. Berlin: Julius Springer, 1894, p. 256. [Google Scholar]
- 1566.Schmechel DE, Rakic P. A Golgi study of radial glial cells in developing monkey telencephalon: morphogenesis and transformation into astrocytes. Anat Embryol (Berl) 156: 115–152, 1979. doi: 10.1007/BF00300010. [DOI] [PubMed] [Google Scholar]
- 1567.Schmid-Schönbein GW. Microlymphatics and lymph flow. Physiol Rev 70: 987–1028, 1990. [DOI] [PubMed] [Google Scholar]
- 1568.Schmidt-Kastner R, Szymas J. Immunohistochemistry of glial fibrillary acidic protein, vimentin and S-100 protein for study of astrocytes in hippocampus of rat. J Chem Neuroanat 3: 179–192, 1990. [PubMed] [Google Scholar]
- 1569.Schmitt A, Asan E, Lesch KP, Kugler P. A splice variant of glutamate transporter GLT1/EAAT2 expressed in neurons: cloning and localization in rat nervous system. Neuroscience 109: 45–61, 2002. doi: 10.1016/S0306-4522(01)00451-1. [DOI] [PubMed] [Google Scholar]
- 1570.Schmitt A, Asan E, Püschel B, Kugler P. Cellular and regional distribution of the glutamate transporter GLAST in the CNS of rats: nonradioactive in situ hybridization and comparative immunocytochemistry. J Neurosci 17: 1–10, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1571.Schmitt LI, Sims RE, Dale N, Haydon PG. Wakefulness affects synaptic and network activity by increasing extracellular astrocyte-derived adenosine. J Neurosci 32: 4417–4425, 2012. doi: 10.1523/JNEUROSCI.5689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1572.Schnell C, Hagos Y, Hülsmann S. Active sulforhodamine 101 uptake into hippocampal astrocytes. PLoS One 7: e49398, 2012. doi: 10.1371/journal.pone.0049398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1573.Schools GP, Zhou M, Kimelberg HK. Development of gap junctions in hippocampal astrocytes: evidence that whole cell electrophysiological phenotype is an intrinsic property of the individual cell. J Neurophysiol 96: 1383–1392, 2006. doi: 10.1152/jn.00449.2006. [DOI] [PubMed] [Google Scholar]
- 1574.Schousboe A, Scafidi S, Bak LK, Waagepetersen HS, McKenna MC. Glutamate metabolism in the brain focusing on astrocytes. Adv Neurobiol 11: 13–30, 2014. doi: 10.1007/978-3-319-08894-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1575.Schousboe A, Waagepetersen HS. GABA: homeostatic and pharmacological aspects. Prog Brain Res 160: 9–19, 2007. doi: 10.1016/S0079-6123(06)60002-2. [DOI] [PubMed] [Google Scholar]
- 1576.Schröder W, Seifert G, Hüttmann K, Hinterkeuser S, Steinhäuser C. AMPA receptor-mediated modulation of inward rectifier K+ channels in astrocytes of mouse hippocampus. Mol Cell Neurosci 19: 447–458, 2002. doi: 10.1006/mcne.2001.1080. [DOI] [PubMed] [Google Scholar]
- 1577.Schroeter S, Apparsundaram S, Wiley RG, Miner LH, Sesack SR, Blakely RD. Immunolocalization of the cocaine- and antidepressant-sensitive l-norepinephrine transporter. J Comp Neurol 420: 211–232, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 1578.Schulte J, Tepass U, Auld VJ. Gliotactin, a novel marker of tricellular junctions, is necessary for septate junction development in Drosophila. J Cell Biol 161: 991–1000, 2003. doi: 10.1083/jcb.200303192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1579.Schulze M. Observationes de Retinae Structura Penitiori . Bonn, Germany: Univ. of Bonn, 1859. [Google Scholar]
- 1580.Schulze W, Hayata-Takano A, Kamo T, Nakazawa T, Nagayasu K, Kasai A, Seiriki K, Shintani N, Ago Y, Farfan C, Hashimoto R, Baba A, Hashimoto H. Simultaneous neuron- and astrocyte-specific fluorescent marking. Biochem Biophys Res Commun 459: 81–86, 2015. doi: 10.1016/j.bbrc.2015.02.073. [DOI] [PubMed] [Google Scholar]
- 1581.Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 320: 1638–1643, 2008. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- 1582.Schurr A, West CA, Rigor BM. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science 240: 1326–1328, 1988. doi: 10.1126/science.3375817. [DOI] [PubMed] [Google Scholar]
- 1583.Schwarcz R, Hunter CA. Toxoplasma gondii and schizophrenia: linkage through astrocyte-derived kynurenic acid? Schizophr Bull 33: 652–653, 2007. doi: 10.1093/schbul/sbm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1584.Scimemi A. Plasticity of GABA transporters: an unconventional route to shape inhibitory synaptic transmission. Front Cell Neurosci 8: 128, 2014. doi: 10.3389/fncel.2014.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1585.Scimemi A. Structure, function, and plasticity of GABA transporters. Front Cell Neurosci 8: 161, 2014. doi: 10.3389/fncel.2014.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1586.Seal RP, Daniels GM, Wolfgang WJ, Forte MA, Amara SG. Identification and characterization of a cDNA encoding a neuronal glutamate transporter from Drosophila melanogaster. Receptors Channels 6: 51–64, 1998. [PubMed] [Google Scholar]
- 1587.Seib TM, Patel SA, Bridges RJ. Regulation of the system x(C)- cystine/glutamate exchanger by intracellular glutathione levels in rat astrocyte primary cultures. Glia 59: 1387–1401, 2011. doi: 10.1002/glia.21176. [DOI] [PubMed] [Google Scholar]
- 1588.Seifert G, Hüttmann K, Binder DK, Hartmann C, Wyczynski A, Neusch C, Steinhäuser C. Analysis of astroglial K+ channel expression in the developing hippocampus reveals a predominant role of the Kir4.1 subunit. J Neurosci 29: 7474–7488, 2009. doi: 10.1523/JNEUROSCI.3790-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1589.Seifert G, Steinhäuser C. Glial cells in the mouse hippocampus express AMPA receptors with an intermediate Ca2+ permeability. Eur J Neurosci 7: 1872–1881, 1995. doi: 10.1111/j.1460-9568.1995.tb00708.x. [DOI] [PubMed] [Google Scholar]
- 1590.Sekler I, Silverman WF. Zinc homeostasis and signaling in glia. Glia 60: 843–850, 2012. doi: 10.1002/glia.22286. [DOI] [PubMed] [Google Scholar]
- 1591.Semenoff D, Kimelberg HK. Autoradiography of high affinity uptake of catecholamines by primary astrocyte cultures. Brain Res 348: 125–136, 1985. doi: 10.1016/0006-8993(85)90368-3. [DOI] [PubMed] [Google Scholar]
- 1592.Serrano A, Haddjeri N, Lacaille JC, Robitaille R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci 26: 5370–5382, 2006. doi: 10.1523/JNEUROSCI.5255-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1593.Serrano A, Robitaille R, Lacaille JC. Differential NMDA-dependent activation of glial cells in mouse hippocampus. Glia 56: 1648–1663, 2008. doi: 10.1002/glia.20717. [DOI] [PubMed] [Google Scholar]
- 1594.Shank RP, Bennett GS, Freytag SO, Campbell GL. Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain Res 329: 364–367, 1985. doi: 10.1016/0006-8993(85)90552-9. [DOI] [PubMed] [Google Scholar]
- 1595.Shao W, Zhang SZ, Tang M, Zhang XH, Zhou Z, Yin YQ, Zhou QB, Huang YY, Liu YJ, Wawrousek E, Chen T, Li SB, Xu M, Zhou JN, Hu G, Zhou JW. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via αB-crystallin. Nature 494: 90–94, 2013. doi: 10.1038/nature11748. [DOI] [PubMed] [Google Scholar]
- 1596.Shao Y, Sutin J. Expression of adrenergic receptors in individual astrocytes and motor neurons isolated from the adult rat brain. Glia 6: 108–117, 1992. doi: 10.1002/glia.440060205. [DOI] [PubMed] [Google Scholar]
- 1597.Sharma G, Vijayaraghavan S. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc Natl Acad Sci USA 98: 4148–4153, 2001. doi: 10.1073/pnas.071540198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1598.Sharp AH, Nucifora FC Jr, Blondel O, Sheppard CA, Zhang C, Snyder SH, Russell JT, Ryugo DK, Ross CA. Differential cellular expression of isoforms of inositol 1,4,5-triphosphate receptors in neurons and glia in brain. J Comp Neurol 406: 207–220, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 1599.Shelton MK, McCarthy KD. Hippocampal astrocytes exhibit Ca2+-elevating muscarinic cholinergic and histaminergic receptors in situ. J Neurochem 74: 555–563, 2000. doi: 10.1046/j.1471-4159.2000.740555.x. [DOI] [PubMed] [Google Scholar]
- 1600.Shelton MK, McCarthy KD. Mature hippocampal astrocytes exhibit functional metabotropic and ionotropic glutamate receptors in situ. Glia 26: 1–11, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 1601.Sheng WS, Hu S, Min X, Cabral GA, Lokensgard JR, Peterson PK. Synthetic cannabinoid WIN55,212-2 inhibits generation of inflammatory mediators by IL-1β-stimulated human astrocytes. Glia 49: 211–219, 2005. doi: 10.1002/glia.20108. [DOI] [PubMed] [Google Scholar]
- 1602.Sheppard CA, Simpson PB, Sharp AH, Nucifora FC, Ross CA, Lange GD, Russell JT. Comparison of type 2 inositol 1,4,5-trisphosphate receptor distribution and subcellular Ca2+ release sites that support Ca2+ waves in cultured astrocytes. J Neurochem 68: 2317–2327, 1997. doi: 10.1046/j.1471-4159.1997.68062317.x. [DOI] [PubMed] [Google Scholar]
- 1603.Sherwood CC, Stimpson CD, Raghanti MA, Wildman DE, Uddin M, Grossman LI, Goodman M, Redmond JC, Bonar CJ, Erwin JM, Hof PR. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc Natl Acad Sci USA 103: 13606–13611, 2006. doi: 10.1073/pnas.0605843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1604.Sherwood MW, Arizono M, Hisatsune C, Bannai H, Ebisui E, Sherwood JL, Panatier A, Oliet SH, Mikoshiba K. Astrocytic IP3 Rs: Contribution to Ca2+ signalling and hippocampal LTP. Glia 65: 502–513, 2017. doi: 10.1002/glia.23107. [DOI] [PubMed] [Google Scholar]
- 1605.Shibasaki K, Hosoi N, Kaneko R, Tominaga M, Yamada K. Glycine release from astrocytes via functional reversal of GlyT1. J Neurochem 140: 395–403, 2017. doi: 10.1111/jnc.13741. [DOI] [PubMed] [Google Scholar]
- 1606.Shibata T, Yamada K, Watanabe M, Ikenaka K, Wada K, Tanaka K, Inoue Y. Glutamate transporter GLAST is expressed in the radial glia-astrocyte lineage of developing mouse spinal cord. J Neurosci 17: 9212–9219, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1607.Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J Neurosci 28: 6659–6663, 2008. doi: 10.1523/JNEUROSCI.1717-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1608.Shigetomi E, Jackson-Weaver O, Huckstepp RT, O’Dell TJ, Khakh BS. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive d-serine release. J Neurosci 33: 10143–10153, 2013. doi: 10.1523/JNEUROSCI.5779-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1609.Shigetomi E, Kracun S, Sofroniew MV, Khakh BS. A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nat Neurosci 13: 759–766, 2010. doi: 10.1038/nn.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1610.Shigetomi E, Patel S, Khakh BS. Probing the complexities of astrocyte calcium signaling. Trends Cell Biol 26: 300–312, 2016. doi: 10.1016/j.tcb.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1611.Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci 15: 70–80, 2011. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1612.Shih PY, Savtchenko LP, Kamasawa N, Dembitskaya Y, McHugh TJ, Rusakov DA, Shigemoto R, Semyanov A. Retrograde synaptic signaling mediated by K+ efflux through postsynaptic NMDA receptors. Cell Reports 5: 941–951, 2013. doi: 10.1016/j.celrep.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 1613.Shimizu H, Watanabe E, Hiyama TY, Nagakura A, Fujikawa A, Okado H, Yanagawa Y, Obata K, Noda M. Glial Nax channels control lactate signaling to neurons for brain [Na+] sensing. Neuron 54: 59–72, 2007. doi: 10.1016/j.neuron.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 1614.Shimizu M, Nishida A, Zensho H, Yamawaki S. Chronic antidepressant exposure enhances 5-hydroxytryptamine7 receptor-mediated cyclic adenosine monophosphate accumulation in rat frontocortical astrocytes. J Pharmacol Exp Ther 279: 1551–1558, 1996. [PubMed] [Google Scholar]
- 1615.Shinjyo N, de Pablo Y, Pekny M, Pekna M. Complement Peptide C3a Promotes Astrocyte Survival in Response to Ischemic Stress. Mol Neurobiol 53: 3076–3087, 2016. doi: 10.1007/s12035-015-9204-4. [DOI] [PubMed] [Google Scholar]
- 1616.Shinoda H, Marini AM, Cosi C, Schwartz JP. Brain region and gene specificity of neuropeptide gene expression in cultured astrocytes. Science 245: 415–417, 1989. doi: 10.1126/science.2569236. [DOI] [PubMed] [Google Scholar]
- 1617.Siepka SM, Yoo SH, Park J, Lee C, Takahashi JS. Genetics and neurobiology of circadian clocks in mammals. Cold Spring Harb Symp Quant Biol 72: 251–259, 2007. doi: 10.1101/sqb.2007.72.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1618.Sík A, Smith RL, Freund TF. Distribution of chloride channel-2-immunoreactive neuronal and astrocytic processes in the hippocampus. Neuroscience 101: 51–65, 2000. doi: 10.1016/S0306-4522(00)00360-2. [DOI] [PubMed] [Google Scholar]
- 1619.Sild M, Ruthazer ES. Radial glia: progenitor, pathway, and partner. Neuroscientist 17: 288–302, 2011. doi: 10.1177/1073858410385870. [DOI] [PubMed] [Google Scholar]
- 1620.Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem 284: 18143–18151, 2009. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1621.Sim JA, Young MT, Sung HY, North RA, Surprenant A. Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci 24: 6307–6314, 2004. doi: 10.1523/JNEUROSCI.1469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1622.Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci 23: 9254–9262, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1623.Simpson PB, Holtzclaw LA, Langley DB, Russell JT. Characterization of ryanodine receptors in oligodendrocytes, type 2 astrocytes, and O-2A progenitors. J Neurosci Res 52: 468–482, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 1624.Singer W, Lux HD. Extracellular potassium gradients and visual receptive fields in the cat striate cortex. Brain Res 96: 378–383, 1975. doi: 10.1016/0006-8993(75)90751-9. [DOI] [PubMed] [Google Scholar]
- 1625.Singh P, Jorgačevski J, Kreft M, Grubišić V, Stout RF Jr, Potokar M, Parpura V, Zorec R. Single-vesicle architecture of synaptobrevin2 in astrocytes. Nat Commun 5: 3780, 2014. doi: 10.1038/ncomms4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1626.Siushansian R, Dixon SJ, Wilson JX. Osmotic swelling stimulates ascorbate efflux from cerebral astrocytes. J Neurochem 66: 1227–1233, 1996. doi: 10.1046/j.1471-4159.1996.66031227.x. [DOI] [PubMed] [Google Scholar]
- 1627.Skatchkov SN, Eaton MJ, Krusek J, Veh RW, Biedermann B, Bringmann A, Pannicke T, Orkand RK, Reichenbach A. Spatial distribution of spermine/spermidine content and K+-current rectification in frog retinal glial (Müller) cells. Glia 31: 84–90, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 1628.Skatchkov SN, Rojas L, Eaton MJ, Orkand RK, Biedermann B, Bringmann A, Pannicke T, Veh RW, Reichenbach A. Functional expression of Kir 6.1/SUR1-KATP channels in frog retinal Müller glial cells. Glia 38: 256–267, 2002. doi: 10.1002/glia.10073. [DOI] [PubMed] [Google Scholar]
- 1629.Skatchkov SN, Thomzig A, Eaton MJ, Biedermann B, Eulitz D, Bringmann A, Pannicke T, Veh RW, Reichenbach A. Kir subfamily in frog retina: specific spatial distribution of Kir 6.1 in glial (Müller) cells. Neuroreport 12: 1437–1441, 2001. doi: 10.1097/00001756-200105250-00028. [DOI] [PubMed] [Google Scholar]
- 1630.Skøtt O. Body sodium and volume homeostasis. Am J Physiol Regul Integr Comp Physiol 285: R14–R18, 2003. doi: 10.1152/ajpregu.00100.2003. [DOI] [PubMed] [Google Scholar]
- 1631.Skowrońska M, Zielińska M, Albrecht J. Stimulation of natriuretic peptide receptor C attenuates accumulation of reactive oxygen species and nitric oxide synthesis in ammonia-treated astrocytes. J Neurochem 115: 1068–1076, 2010. doi: 10.1111/j.1471-4159.2010.07008.x. [DOI] [PubMed] [Google Scholar]
- 1632.Skucas VA, Mathews IB, Yang J, Cheng Q, Treister A, Duffy AM, Verkman AS, Hempstead BL, Wood MA, Binder DK, Scharfman HE. Impairment of select forms of spatial memory and neurotrophin-dependent synaptic plasticity by deletion of glial aquaporin-4. J Neurosci 31: 6392–6397, 2011. doi: 10.1523/JNEUROSCI.6249-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1633.Sloan SA, Barres BA. Looks can be deceiving: reconsidering the evidence for gliotransmission. Neuron 84: 1112–1115, 2014. doi: 10.1016/j.neuron.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1634.Sloan SA, Barres BA. Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr Opin Neurobiol 27: 75–81, 2014. doi: 10.1016/j.conb.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1635.Smith SJ. Do astrocytes process neural information? Prog Brain Res 94: 119–136, 1992. doi: 10.1016/S0079-6123(08)61744-6. [DOI] [PubMed] [Google Scholar]
- 1636.Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, Putney JW Jr. Emerging perspectives in store-operated Ca2+ entry: roles of Orai, Stim and TRP. Biochim Biophys Acta 1763: 1147–1160, 2006. doi: 10.1016/j.bbamcr.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 1637.Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci 16: 249–263, 2015. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1638.Sofroniew MV. Astrogliosis. Cold Spring Harb Perspect Biol 7: a020420, 2014. doi: 10.1101/cshperspect.a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1639.Soh UJ, Dores MR, Chen B, Trejo J. Signal transduction by protease-activated receptors. Br J Pharmacol 160: 191–203, 2010. doi: 10.1111/j.1476-5381.2010.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1640.Söhl G, Odermatt B, Maxeiner S, Degen J, Willecke K. New insights into the expression and function of neural connexins with transgenic mouse mutants. Brain Res Brain Res Rev 47: 245–259, 2004. doi: 10.1016/j.brainresrev.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 1641.Solenov E, Watanabe H, Manley GT, Verkman AS. Sevenfold-reduced osmotic water permeability in primary astrocyte cultures from AQP-4-deficient mice, measured by a fluorescence quenching method. Am J Physiol Cell Physiol 286: C426–C432, 2004. doi: 10.1152/ajpcell.00298.2003. [DOI] [PubMed] [Google Scholar]
- 1642.Somjen GG. Ion regulation in the brain: implications for pathophysiology. Neuroscientist 8: 254–267, 2002. doi: 10.1177/1073858402008003011. [DOI] [PubMed] [Google Scholar]
- 1643.Song EK, Rah SY, Lee YR, Yoo CH, Kim YR, Yeom JH, Park KH, Kim JS, Kim UH, Han MK. Connexin-43 hemichannels mediate cyclic ADP-ribose generation and its Ca2+-mobilizing activity by NAD+/cyclic ADP-ribose transport. J Biol Chem 286: 44480–44490, 2011. doi: 10.1074/jbc.M111.307645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1644.Song X, Zhao Y, Narcisse L, Duffy H, Kress Y, Lee S, Brosnan CF. Canonical transient receptor potential channel 4 (TRPC4) co-localizes with the scaffolding protein ZO-1 in human fetal astrocytes in culture. Glia 49: 418–429, 2005. doi: 10.1002/glia.20128. [DOI] [PubMed] [Google Scholar]
- 1645.Sonnewald U, Hertz L, Schousboe A. Mitochondrial heterogeneity in the brain at the cellular level. J Cereb Blood Flow Metab 18: 231–237, 1998. doi: 10.1097/00004647-199803000-00001. [DOI] [PubMed] [Google Scholar]
- 1646.Sontheimer H. Voltage-dependent ion channels in glial cells. Glia 11: 156–172, 1994. doi: 10.1002/glia.440110210. [DOI] [PubMed] [Google Scholar]
- 1647.Sontheimer H, Black JA, Ransom BR, Waxman SG. Ion channels in spinal cord astrocytes in vitro. I. Transient expression of high levels of Na+ and K+ channels. J Neurophysiol 68: 985–1000, 1992. [DOI] [PubMed] [Google Scholar]
- 1648.Sontheimer H, Fernandez-Marques E, Ullrich N, Pappas CA, Waxman SG. Astrocyte Na+ channels are required for maintenance of Na+/K+-ATPase activity. J Neurosci 14: 2464–2475, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1649.Sontheimer H, Minturn JE, Black JA, Ransom BR, Waxman SG. Two types of Na+-currents in cultured rat optic nerve astrocytes: changes with time in culture and with age of culture derivation. J Neurosci Res 30: 275–287, 1991. doi: 10.1002/jnr.490300202. [DOI] [PubMed] [Google Scholar]
- 1650.Sontheimer H, Ransom BR, Cornell-Bell AH, Black JA, Waxman SG. Na+-current expression in rat hippocampal astrocytes in vitro: alterations during development. J Neurophysiol 65: 3–19, 1991. [DOI] [PubMed] [Google Scholar]
- 1651.Sontheimer H, Waxman SG. Expression of voltage-activated ion channels by astrocytes and oligodendrocytes in the hippocampal slice. J Neurophysiol 70: 1863–1873, 1993. [DOI] [PubMed] [Google Scholar]
- 1652.Sontheimer H, Waxman SG. Ion channels in spinal cord astrocytes in vitro. II. Biophysical and pharmacological analysis of two Na+ current types. J Neurophysiol 68: 1001–1011, 1992. [DOI] [PubMed] [Google Scholar]
- 1653.Sorg O, Magistretti PJ. Characterization of the glycogenolysis elicited by vasoactive intestinal peptide, noradrenaline and adenosine in primary cultures of mouse cerebral cortical astrocytes. Brain Res 563: 227–233, 1991. doi: 10.1016/0006-8993(91)91538-C. [DOI] [PubMed] [Google Scholar]
- 1654.Sosinsky GE, Boassa D, Dermietzel R, Duffy HS, Laird DW, MacVicar B, Naus CC, Penuela S, Scemes E, Spray DC, Thompson RJ, Zhao HB, Dahl G. Pannexin channels are not gap junction hemichannels. Channels (Austin) 5: 193–197, 2011. doi: 10.4161/chan.5.3.15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1655.Sosunov AA, Wu X, Tsankova NM, Guilfoyle E, McKhann GM II, Goldman JE. Phenotypic heterogeneity and plasticity of isocortical and hippocampal astrocytes in the human brain. J Neurosci 34: 2285–2298, 2014. doi: 10.1523/JNEUROSCI.4037-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1656.Sotelo-Hitschfeld T, Niemeyer MI, Mächler P, Ruminot I, Lerchundi R, Wyss MT, Stobart J, Fernández-Moncada I, Valdebenito R, Garrido-Gerter P, Contreras-Baeza Y, Schneider BL, Aebischer P, Lengacher S, San Martín A, Le Douce J, Bonvento G, Magistretti PJ, Sepúlveda FV, Weber B, Barros LF. Channel-mediated lactate release by K+-stimulated astrocytes. J Neurosci 35: 4168–4178, 2015. doi: 10.1523/JNEUROSCI.5036-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1657.Spang A, Saw JH, Jørgensen SL, Zaremba-Niedzwiedzka K, Martijn J, Lind AE, van Eijk R, Schleper C, Guy L, Ettema TJG. Complex archaea that bridge the gap between prokaryotes and eukaryotes. Nature 521: 173–179, 2015. doi: 10.1038/nature14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1658.Sperlágh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Prog Neurobiol 78: 327–346, 2006. doi: 10.1016/j.pneurobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 1659.Sreetama SC, Takano T, Nedergaard M, Simon SM, Jaiswal JK. Injured astrocytes are repaired by Synaptotagmin XI-regulated lysosome exocytosis. Cell Death Differ 23: 596–607, 2016. doi: 10.1038/cdd.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1660.Srinivasan R, Huang BS, Venugopal S, Johnston AD, Chai H, Zeng H, Golshani P, Khakh BS. Ca2+ signaling in astrocytes from Ip3r2-/- mice in brain slices and during startle responses in vivo. Nat Neurosci 18: 708–717, 2015. doi: 10.1038/nn.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1661.Stathopulos PB, Ikura M. Store operated calcium entry: From concept to structural mechanisms. Cell Calcium 63: 3–7, 2017. doi: 10.1016/j.ceca.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 1662.Stebbings LA, Todman MG, Phillips R, Greer CE, Tam J, Phelan P, Jacobs K, Bacon JP, Davies JA. Gap junctions in Drosophila: developmental expression of the entire innexin gene family. Mech Dev 113: 197–205, 2002. doi: 10.1016/S0925-4773(02)00025-4. [DOI] [PubMed] [Google Scholar]
- 1663.Stehberg J, Moraga-Amaro R, Salazar C, Becerra A, Echeverría C, Orellana JA, Bultynck G, Ponsaerts R, Leybaert L, Simon F, Sáez JC, Retamal MA. Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. FASEB J 26: 3649–3657, 2012. doi: 10.1096/fj.11-198416. [DOI] [PubMed] [Google Scholar]
- 1664.Steiner J, Bernstein HG, Bielau H, Berndt A, Brisch R, Mawrin C, Keilhoff G, Bogerts B. Evidence for a wide extra-astrocytic distribution of S100B in human brain. BMC Neurosci 8: 2, 2007. doi: 10.1186/1471-2202-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1665.Steinhäuser C, Berger T, Frotscher M, Kettenmann H. Heterogeneity in the membrane current pattern of identified glial cells in the hippocampal slice. Eur J Neurosci 4: 472–484, 1992. doi: 10.1111/j.1460-9568.1992.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 1666.Steinhäuser C, Jabs R, Kettenmann H. Properties of GABA and glutamate responses in identified glial cells of the mouse hippocampal slice. Hippocampus 4: 19–35, 1994. doi: 10.1002/hipo.450040105. [DOI] [PubMed] [Google Scholar]
- 1667.Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 58: 1017–1030, 2010. doi: 10.1002/glia.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1668.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-α. Nature 440: 1054–1059, 2006. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 1669.Stenesen D, Moehlman AT, Krämer H. The carcinine transporter CarT is required in Drosophila photoreceptor neurons to sustain histamine recycling. eLife 4: e10972, 2015. doi: 10.7554/eLife.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1670.Stenovec M, Kreft M, Grilc S, Pangrsic T, Zorec R. EAAT2 density at the astrocyte plasma membrane and Ca2 +-regulated exocytosis. Mol Membr Biol 25: 203–215, 2008. doi: 10.1080/09687680701790925. [DOI] [PubMed] [Google Scholar]
- 1671.Stenovec M, Kreft M, Grilc S, Potokar M, Kreft ME, Pangrsic T, Zorec R. Ca2+-dependent mobility of vesicles capturing anti-VGLUT1 antibodies. Exp Cell Res 313: 3809–3818, 2007. doi: 10.1016/j.yexcr.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 1672.Stephens GJ, Cholewinski AJ, Wilkin GP, Djamgoz MB. Calcium-mobilizing and electrophysiological effects of bradykinin on cortical astrocyte subtypes in culture. Glia 9: 269–279, 1993. doi: 10.1002/glia.440090405. [DOI] [PubMed] [Google Scholar]
- 1673.Stiene-Martin A, Gurwell JA, Hauser KF. Morphine alters astrocyte growth in primary cultures of mouse glial cells: evidence for a direct effect of opiates on neural maturation. Brain Res Dev Brain Res 60: 1–7, 1991. doi: 10.1016/0165-3806(91)90149-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1674.Stokowska A, Atkins AL, Morán J, Pekny T, Bulmer L, Pascoe MC, Barnum SR, Wetsel RA, Nilsson JA, Dragunow M, Pekna M. Complement peptide C3a stimulates neural plasticity after experimental brain ischaemia. Brain 140: 353–369, 2017. doi: 10.1093/brain/aww314. [DOI] [PubMed] [Google Scholar]
- 1675.Stolt CC, Lommes P, Sock E, Chaboissier MC, Schedl A, Wegner M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev 17: 1677–1689, 2003. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1676.Stone EA, Ariano MA. Are glial cells targets of the central noradrenergic system? A review of the evidence. Brain Res Brain Res Rev 14: 297–309, 1989. doi: 10.1016/0165-0173(89)90015-5. [DOI] [PubMed] [Google Scholar]
- 1677.Stonehouse AH, Pringle JH, Norman RI, Stanfield PR, Conley EC, Brammar WJ. Characterisation of Kir2.0 proteins in the rat cerebellum and hippocampus by polyclonal antibodies. Histochem Cell Biol 112: 457–465, 1999. doi: 10.1007/s004180050429. [DOI] [PubMed] [Google Scholar]
- 1678.Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na+-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci USA 89: 10955–10959, 1992. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1679.Stornetta RL, Hawelu-Johnson CL, Guyenet PG, Lynch KR. Astrocytes synthesize angiotensinogen in brain. Science 242: 1444–1446, 1988. doi: 10.1126/science.3201232. [DOI] [PubMed] [Google Scholar]
- 1680.Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci USA 100: 7319–7324, 2003. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1681.Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem 277: 10482–10488, 2002. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 1682.Stout RF Jr, Parpura V. Voltage-gated calcium channel types in cultured C. elegans CEPsh glial cells. Cell Calcium 50: 98–108, 2011. doi: 10.1016/j.ceca.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 1683.Stout RF Jr, Verkhratsky A, Parpura V. Caenorhabditis elegans glia modulate neuronal activity and behavior. Front Cell Neurosci 8: 67, 2014. doi: 10.3389/fncel.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1684.Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29: 645–655, 2001. doi: 10.1016/S0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 1685.Struble RG, Afridi S, Beckman-Randall S, Li M, Cady C, Nathan B, McAsey ME. Neocortical and hippocampal glial fibrillary acidic protein immunoreactivity shows region-specific variation during the mouse estrous cycle. Neuroendocrinology 83: 325–335, 2006. doi: 10.1159/000095340. [DOI] [PubMed] [Google Scholar]
- 1686.Studer FE, Fedele DE, Marowsky A, Schwerdel C, Wernli K, Vogt K, Fritschy JM, Boison D. Shift of adenosine kinase expression from neurons to astrocytes during postnatal development suggests dual functionality of the enzyme. Neuroscience 142: 125–137, 2006. doi: 10.1016/j.neuroscience.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 1687.Su G, Haworth RA, Dempsey RJ, Sun D. Regulation of Na+-K+-Cl− cotransporter in primary astrocytes by dibutyryl cAMP and high [K+]o. Am J Physiol Cell Physiol 279: C1710–C1721, 2000. [DOI] [PubMed] [Google Scholar]
- 1688.Su G, Kintner DB, Flagella M, Shull GE, Sun D. Astrocytes from Na+-K+-Cl− cotransporter-null mice exhibit absence of swelling and decrease in EAA release. Am J Physiol Cell Physiol 282: C1147–C1160, 2002. doi: 10.1152/ajpcell.00538.2001. [DOI] [PubMed] [Google Scholar]
- 1689.Su G, Kintner DB, Sun D. Contribution of Na+-K+-Cl− cotransporter to high-[K+]o- induced swelling and EAA release in astrocytes. Am J Physiol Cell Physiol 282: C1136–C1146, 2002. doi: 10.1152/ajpcell.00478.2001. [DOI] [PubMed] [Google Scholar]
- 1690.Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci 26: 1378–1385, 2006. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1691.Suadicani SO, Iglesias R, Wang J, Dahl G, Spray DC, Scemes E. ATP signaling is deficient in cultured Pannexin1-null mouse astrocytes. Glia 60: 1106–1116, 2012. doi: 10.1002/glia.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1692.Suárez I, Bodega G, Rubio M, Fernandez B. Sexual dimorphism in the distribution of glial fibrillary acidic protein in the supraoptic nucleus of the hamster. J Anat 178: 79–82, 1991. [PMC free article] [PubMed] [Google Scholar]
- 1693.Suárez I, Bodega G, Rubio M, Fernández B. Sexual dimorphism in the hamster cerebellum demonstrated by glial fibrillary acidic protein (GFAP) and vimentin immunoreactivity. Glia 5: 10–16, 1992. doi: 10.1002/glia.440050103. [DOI] [PubMed] [Google Scholar]
- 1694.Südhof TC. Calcium control of neurotransmitter release. Cold Spring Harb Perspect Biol 4: a011353, 2012. doi: 10.1101/cshperspect.a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1695.Suh HS, Zhao ML, Rivieccio M, Choi S, Connolly E, Zhao Y, Takikawa O, Brosnan CF, Lee SC. Astrocyte indoleamine 2,3-dioxygenase is induced by the TLR3 ligand poly(I:C): mechanism of induction and role in antiviral response. J Virol 81: 9838–9850, 2007. doi: 10.1128/JVI.00792-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1696.Suh J, Jackson FR. Drosophila ebony activity is required in glia for the circadian regulation of locomotor activity. Neuron 55: 435–447, 2007. doi: 10.1016/j.neuron.2007.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1697.Suhail M. Na, K-ATPase: Ubiquitous Multifunctional Transmembrane Protein and its Relevance to Various Pathophysiological Conditions. J Clin Med Res 2: 1–17, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1698.Sumners C, Tang W, Paulding W, Raizada MK. Peptide receptors in astroglia: focus on angiotensin II and atrial natriuretic peptide. Glia 11: 110–116, 1994. doi: 10.1002/glia.440110206. [DOI] [PubMed] [Google Scholar]
- 1699.Sun L, Kosugi Y, Kawakami E, Piao YS, Hashimoto T, Oyanagi K. Magnesium concentration in the cerebrospinal fluid of mice and its response to changes in serum magnesium concentration. Magnes Res 22: 266–272, 2009. [DOI] [PubMed] [Google Scholar]
- 1700.Sun MY, Devaraju P, Xie AX, Holman I, Samones E, Murphy TR, Fiacco TA. Astrocyte calcium microdomains are inhibited by bafilomycin A1 and cannot be replicated by low-level Schaffer collateral stimulation in situ. Cell Calcium 55: 1–16, 2014. doi: 10.1016/j.ceca.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 1701.Sun W, Cornwell A, Li J, Peng S, Osorio MJ, Aalling N, Wang S, Benraiss A, Lou N, Goldman SA, Nedergaard M. SOX9 is an astrocyte-specific nuclear marker in the adult brain outside the neurogenic regions. J Neurosci 37: 4493–4507, 2017. doi: 10.1523/JNEUROSCI.3199-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1702.Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, Nedergaard M. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339: 197–200, 2013. doi: 10.1126/science.1226740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1703.Sun XD, Li L, Liu F, Huang ZH, Bean JC, Jiao HF, Barik A, Kim SM, Wu H, Shen C, Tian Y, Lin TW, Bates R, Sathyamurthy A, Chen YJ, Yin DM, Xiong L, Lin HP, Hu JX, Li BM, Gao TM, Xiong WC, Mei L. Lrp4 in astrocytes modulates glutamatergic transmission. Nat Neurosci 19: 1010–1018, 2016. doi: 10.1038/nn.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1704.Supplisson S, Roux MJ. Why glycine transporters have different stoichiometries. FEBS Lett 529: 93–101, 2002. doi: 10.1016/S0014-5793(02)03251-9. [DOI] [PubMed] [Google Scholar]
- 1705.Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol 71: 333–359, 2009. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- 1706.Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144: 810–823, 2011. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1707.Suzuki R, Watanabe J, Arata S, Funahashi H, Kikuyama S, Shioda S. A transgenic mouse model for the detailed morphological study of astrocytes. Neurosci Res 47: 451–454, 2003. doi: 10.1016/j.neures.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 1708.Svoboda J, Motin V, Hájek I, Syková E. Increase in extracellular potassium level in rat spinal dorsal horn induced by noxious stimulation and peripheral injury. Brain Res 458: 97–105, 1988. doi: 10.1016/0006-8993(88)90500-8. [DOI] [PubMed] [Google Scholar]
- 1709.Syed N, Martens CA, Hsu WH. Arginine vasopressin increases glutamate release and intracellular Ca2+ concentration in hippocampal and cortical astrocytes through two distinct receptors. J Neurochem 103: 229–237, 2007. [DOI] [PubMed] [Google Scholar]
- 1710.Syková E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev 88: 1277–1340, 2008. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1711.Sylantyev S, Savtchenko LP, Ermolyuk Y, Michaluk P, Rusakov DA. Spike-driven glutamate electrodiffusion triggers synaptic potentiation via a homer-dependent mGluR-NMDAR link. Neuron 77: 528–541, 2013. doi: 10.1016/j.neuron.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1712.Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 23: 84–94, 2008. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- 1713.Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature 348: 443–446, 1990. doi: 10.1038/348443a0. [DOI] [PubMed] [Google Scholar]
- 1714.Tabernero A, Medina JM, Giaume C. Glucose metabolism and proliferation in glia: role of astrocytic gap junctions. J Neurochem 99: 1049–1061, 2006. doi: 10.1111/j.1471-4159.2006.04088.x. [DOI] [PubMed] [Google Scholar]
- 1715.Takahashi M, Sarantis M, Attwell D. Postsynaptic glutamate uptake in rat cerebellar Purkinje cells. J Physiol 497: 523–530, 1996. doi: 10.1113/jphysiol.1996.sp021785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1716.Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, Müller SA, Rammner B, Gräter F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmüller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell 127: 831–846, 2006. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 1717.Takano T, Kang J, Jaiswal JK, Simon SM, Lin JH, Yu Y, Li Y, Yang J, Dienel G, Zielke HR, Nedergaard M. Receptor-mediated glutamate release from volume sensitive channels in astrocytes. Proc Natl Acad Sci USA 102: 16466–16471, 2005. doi: 10.1073/pnas.0506382102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1718.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci 9: 260–267, 2006. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 1719.Takarada T, Nakamichi N, Kawagoe H, Ogura M, Fukumori R, Nakazato R, Fujikawa K, Kou M, Yoneda Y. Possible neuroprotective property of nicotinic acetylcholine receptors in association with predominant upregulation of glial cell line-derived neurotrophic factor in astrocytes. J Neurosci Res 90: 2074–2085, 2012. doi: 10.1002/jnr.23101. [DOI] [PubMed] [Google Scholar]
- 1720.Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, Mikoshiba K, Hirase H. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci 31: 18155–18165, 2011. doi: 10.1523/JNEUROSCI.5289-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1721.Takata N, Nagai T, Ozawa K, Oe Y, Mikoshiba K, Hirase H. Cerebral blood flow modulation by Basal forebrain or whisker stimulation can occur independently of large cytosolic Ca2+ signaling in astrocytes. PLoS One 8: e66525, 2013. doi: 10.1371/journal.pone.0066525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1722.Takeda H, Inazu M, Matsumiya T. Astroglial dopamine transport is mediated by norepinephrine transporter. Naunyn-Schmiedebergs Arch Pharmacol 366: 620–623, 2002. doi: 10.1007/s00210-002-0640-0. [DOI] [PubMed] [Google Scholar]
- 1723.Takey Y, Pearl GS. Ultrastructure of the human neurohypophysis. In: Ultrastructure of Endocrine Cells and Tissues, edited by Motta PM. Boston: Martinus Nijjhof Publishers, 1984, p. 77–88. doi: 10.1007/978-1-4613-3861-1_7. [DOI] [Google Scholar]
- 1724.Takuma K, Matsuda T, Hashimoto H, Asano S, Baba A. Cultured rat astrocytes possess Na+-Ca2+ exchanger. Glia 12: 336–342, 1994. doi: 10.1002/glia.440120410. [DOI] [PubMed] [Google Scholar]
- 1725.Takuma K, Matsuda T, Hashimoto H, Kitanaka J, Asano S, Kishida Y, Baba A. Role of Na+-Ca2+ exchanger in agonist-induced Ca2+ signaling in cultured rat astrocytes. J Neurochem 67: 1840–1845, 1996. doi: 10.1046/j.1471-4159.1996.67051840.x. [DOI] [PubMed] [Google Scholar]
- 1726.Talantova M, Sanz-Blasco S, Zhang X, Xia P, Akhtar MW, Okamoto S, Dziewczapolski G, Nakamura T, Cao G, Pratt AE, Kang YJ, Tu S, Molokanova E, McKercher SR, Hires SA, Sason H, Stouffer DG, Buczynski MW, Solomon JP, Michael S, Powers ET, Kelly JW, Roberts A, Tong G, Fang-Newmeyer T, Parker J, Holland EA, Zhang D, Nakanishi N, Chen HS, Wolosker H, Wang Y, Parsons LH, Ambasudhan R, Masliah E, Heinemann SF, Piña-Crespo JC, Lipton SA. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci USA 110: E2518–E2527, 2013. doi: 10.1073/pnas.1306832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1727.Tanaka K. Expression cloning of a rat glutamate transporter. Neurosci Res 16: 149–153, 1993. doi: 10.1016/0168-0102(93)90082-2. [DOI] [PubMed] [Google Scholar]
- 1728.Tanemoto M, Kittaka N, Inanobe A, Kurachi Y. In vivo formation of a proton-sensitive K+ channel by heteromeric subunit assembly of Kir5.1 with Kir4.1. J Physiol 525: 587–592, 2000. doi: 10.1111/j.1469-7793.2000.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1729.Tang F, Lane S, Korsak A, Paton JF, Gourine AV, Kasparov S, Teschemacher AG. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat Commun 5: 3284, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1730.Tartaglia LA. The leptin receptor. J Biol Chem 272: 6093–6096, 1997. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 1731.Tasaki I, Chang JJ. Electric response of glia cells in cat brain. Science 128: 1209–1210, 1958. doi: 10.1126/science.128.3333.1209. [DOI] [PubMed] [Google Scholar]
- 1732.Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, Yao Z, Levi B, Gray LT, Sorensen SA, Dolbeare T, Bertagnolli D, Goldy J, Shapovalova N, Parry S, Lee C, Smith K, Bernard A, Madisen L, Sunkin SM, Hawrylycz M, Koch C, Zeng H. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci 19: 335–346, 2016. doi: 10.1038/nn.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1733.Tatsuno I, Arimura A. Pituitary adenylate cyclase-activating polypeptide (PACAP) mobilizes intracellular free calcium in cultured rat type-2, but not type-1, astrocytes. Brain Res 662: 1–10, 1994. doi: 10.1016/0006-8993(94)90790-0. [DOI] [PubMed] [Google Scholar]
- 1734.Tatsuno I, Morio H, Tanaka T, Hirai A, Tamura Y, Saito Y, Arimura A. Astrocytes are one of the main target cells for pituitary adenylate cyclase-activating polypeptide in the central nervous system. Astrocytes are very heterogeneous regarding both basal movement of intracellular free calcium ([Ca2+]i) and the [Ca2+]i response to PACAP at a single cell level. Ann N Y Acad Sci 805: 613–619, 1996. doi: 10.1111/j.1749-6632.1996.tb17529.x. [DOI] [PubMed] [Google Scholar]
- 1735.Taylor AR, Robinson MB, Gifondorwa DJ, Tytell M, Milligan CE. Regulation of heat shock protein 70 release in astrocytes: role of signaling kinases. Dev Neurobiol 67: 1815–1829, 2007. doi: 10.1002/dneu.20559. [DOI] [PubMed] [Google Scholar]
- 1736.Taylor RA, Simon EM, Marks HG, Scherer SS. The CNS phenotype of X-linked Charcot-Marie-Tooth disease: more than a peripheral problem. Neurology 61: 1475–1478, 2003. doi: 10.1212/01.WNL.0000095960.48964.25. [DOI] [PubMed] [Google Scholar]
- 1737.Teaktong T, Graham A, Court J, Perry R, Jaros E, Johnson M, Hall R, Perry E. Alzheimer’s disease is associated with a selective increase in α7 nicotinic acetylcholine receptor immunoreactivity in astrocytes. Glia 41: 207–211, 2003. doi: 10.1002/glia.10132. [DOI] [PubMed] [Google Scholar]
- 1738.Teather LA, Lee RK, Wurtman RJ. Platelet-activating factor increases prostaglandin E2 release from astrocyte-enriched cortical cell cultures. Brain Res 946: 87–95, 2002. doi: 10.1016/S0006-8993(02)02866-4. [DOI] [PubMed] [Google Scholar]
- 1739.Teoh R, Kum W, Cockram CS, Young JD, Nicholls MG. Mouse astrocytes possess specific ANP receptors which are linked to cGMP production. Clin Exp Pharmacol Physiol 16: 323–327, 1989. doi: 10.1111/j.1440-1681.1989.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 1740.Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. J Neurosci 23: 4278–4287, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1741.Theis M, Giaume C. Connexin-based intercellular communication and astrocyte heterogeneity. Brain Res 1487: 88–98, 2012. doi: 10.1016/j.brainres.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 1742.Theis M, Jauch R, Zhuo L, Speidel D, Wallraff A, Döring B, Frisch C, Söhl G, Teubner B, Euwens C, Huston J, Steinhäuser C, Messing A, Heinemann U, Willecke K. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J Neurosci 23: 766–776, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1743.Theparambil SM, Naoshin Z, Thyssen A, Deitmer JW. Reversed electrogenic sodium bicarbonate cotransporter 1 is the major acid loader during recovery from cytosolic alkalosis in mouse cortical astrocytes. J Physiol 593: 3533–3547, 2015. doi: 10.1113/JP270086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1744.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9: 581–593, 2009. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 1745.Thomzig A, Wenzel M, Karschin C, Eaton MJ, Skatchkov SN, Karschin A, Veh RW. Kir6.1 is the principal pore-forming subunit of astrocyte but not neuronal plasma membrane K-ATP channels. Mol Cell Neurosci 18: 671–690, 2001. doi: 10.1006/mcne.2001.1048. [DOI] [PubMed] [Google Scholar]
- 1746.Thorlin T, Eriksson PS, Persson PA, Aberg ND, Hansson E, Rönnbäck L. δ-Opioid receptors on astroglial cells in primary culture: mobilization of intracellular free calcium via a pertussis sensitive G protein. Neuropharmacology 37: 299–311, 1998. doi: 10.1016/S0028-3908(98)00026-4. [DOI] [PubMed] [Google Scholar]
- 1747.Thorlin T, Eriksson PS, Rönnbäck L, Hansson E. Receptor-activated Ca2+ increases in vibrodissociated cortical astrocytes: a nonenzymatic method for acute isolation of astrocytes. J Neurosci Res 54: 390–401, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 1748.Thrane AS, Rangroo Thrane V, Zeppenfeld D, Lou N, Xu Q, Nagelhus EA, Nedergaard M. General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. Proc Natl Acad Sci USA 109: 18974–18979, 2012. doi: 10.1073/pnas.1209448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1749.Thrane AS, Rappold PM, Fujita T, Torres A, Bekar LK, Takano T, Peng W, Wang F, Rangroo Thrane V, Enger R, Haj-Yasein NN, Skare Ø, Holen T, Klungland A, Ottersen OP, Nedergaard M, Nagelhus EA. Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc Natl Acad Sci USA 108: 846–851, 2011. doi: 10.1073/pnas.1015217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1750.Tian GF, Takano T, Lin JH, Wang X, Bekar L, Nedergaard M. Imaging of cortical astrocytes using 2-photon laser scanning microscopy in the intact mouse brain. Adv Drug Deliv Rev 58: 773–787, 2006. doi: 10.1016/j.addr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 1751.Tix S, Eule E, Fischbach KF, Benzer S. Glia in the chiasms and medulla of the Drosophila melanogaster optic lobes. Cell Tissue Res 289: 397–409, 1997. doi: 10.1007/s004410050886. [DOI] [PubMed] [Google Scholar]
- 1752.Todd AC, Marx MC, Hulme SR, Bröer S, Billups B. SNAT3-mediated glutamine transport in perisynaptic astrocytes in situ is regulated by intracellular sodium. Glia 65: 900–916, 2017. doi: 10.1002/glia.23133. [DOI] [PubMed] [Google Scholar]
- 1753.Toms NJ, Roberts PJ. Group 1 mGlu receptors elevate [Ca2+]i in rat cultured cortical type 2 astrocytes: [Ca2+]i synergy with adenosine A1 receptors. Neuropharmacology 38: 1511–1517, 1999. doi: 10.1016/S0028-3908(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 1754.Torres A, Wang F, Xu Q, Fujita T, Dobrowolski R, Willecke K, Takano T, Nedergaard M. Extracellular Ca2+ acts as a mediator of communication from neurons to glia. Sci Signal 5: ra8, 2012. doi: 10.1126/scisignal.2002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1755.Tóth A, Boczán J, Kedei N, Lizanecz E, Bagi Z, Papp Z, Edes I, Csiba L, Blumberg PM. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res Mol Brain Res 135: 162–168, 2005. doi: 10.1016/j.molbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 1756.Trentin AG, Alvarez-Silva M, Moura Neto V. Thyroid hormone induces cerebellar astrocytes and C6 glioma cells to secrete mitogenic growth factors. Am J Physiol Endocrinol Metab 281: E1088–E1094, 2001. [DOI] [PubMed] [Google Scholar]
- 1757.Trimmer PA, McCarthy KD. Immunocytochemically defined astroglia from fetal, newborn and young adult rats express beta-adrenergic receptors in vitro. Brain Res 392: 151–165, 1986. doi: 10.1016/0165-3806(86)90241-5. [DOI] [PubMed] [Google Scholar]
- 1758.Troadec JD, Thirion S, Petturiti D, Bohn MT, Poujeol P. ATP acting on P2Y receptors triggers calcium mobilization in primary cultures of rat neurohypophysial astrocytes (pituicytes). Pflugers Arch 437: 745–753, 1999. doi: 10.1007/s004240050841. [DOI] [PubMed] [Google Scholar]
- 1759.Trudler D, Farfara D, Frenkel D. Toll-like receptors expression and signaling in glia cells in neuro-amyloidogenic diseases: towards future therapeutic application. Mediators Inflamm 2010: 497987, 2010. doi: 10.1155/2010/497987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1760.True JR, Yeh SD, Hovemann BT, Kemme T, Meinertzhagen IA, Edwards TN, Liou SR, Han Q, Li J. Drosophila tan encodes a novel hydrolase required in pigmentation and vision. PLoS Genet 1: e63, 2005. doi: 10.1371/journal.pgen.0010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1761.Tsacopoulos M, Veuthey AL, Saravelos SG, Perrottet P, Tsoupras G. Glial cells transform glucose to alanine, which fuels the neurons in the honeybee retina. J Neurosci 14: 1339–1351, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1762.Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SP, Merkle F, Kessaris N, Alvarez-Buylla A, Richardson WD, Rowitch DH. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 337: 358–362, 2012. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1763.Tse FW, Fraser DD, Duffy S, MacVicar BA. Voltage-activated K+ currents in acutely isolated hippocampal astrocytes. J Neurosci 12: 1781–1788, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1764.Tsukada S, Iino M, Takayasu Y, Shimamoto K, Ozawa S. Effects of a novel glutamate transporter blocker, (2S, 3S)-3-[3-[4-(trifluoromethyl)benzoylamino]benzyloxy]aspartate (TFB-TBOA), on activities of hippocampal neurons. Neuropharmacology 48: 479–491, 2005. doi: 10.1016/j.neuropharm.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 1765.Turner JR, Ecke LE, Briand LA, Haydon PG, Blendy JA. Cocaine-related behaviors in mice with deficient gliotransmission. Psychopharmacology (Berl) 226: 167–176, 2013. doi: 10.1007/s00213-012-2897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1766.Turovsky E, Theparambil SM, Kasymov V, Deitmer JW, Del Arroyo AG, Ackland GL, Corneveaux JJ, Allen AN, Huentelman MJ, Kasparov S, Marina N, Gourine AV. Mechanisms of CO2/H+ sensitivity of astrocytes. J Neurosci 36: 10750–10758, 2016. doi: 10.1523/JNEUROSCI.1281-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1767.Tuschick S, Kirischuk S, Kirchhoff F, Liefeldt L, Paul M, Verkhratsky A, Kettenmann H. Bergmann glial cells in situ express endothelinB receptors linked to cytoplasmic calcium signals. Cell Calcium 21: 409–419, 1997. doi: 10.1016/S0143-4160(97)90052-X. [DOI] [PubMed] [Google Scholar]
- 1768.Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci 8: 935–947, 2007. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- 1769.Ubink R, Calza L, Hökfelt T. ‘Neuro’-peptides in glia: focus on NPY and galanin. Trends Neurosci 26: 604–609, 2003. doi: 10.1016/j.tins.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 1770.Ubl JJ, Reiser G. Characteristics of thrombin-induced calcium signals in rat astrocytes. Glia 21: 361–369, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 1771.Ubl JJ, Sergeeva M, Reiser G. Desensitisation of protease-activated receptor-1 (PAR-1) in rat astrocytes: evidence for a novel mechanism for terminating Ca2+ signalling evoked by the tethered ligand. J Physiol 525: 319–330, 2000. doi: 10.1111/j.1469-7793.2000.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1772.Ubl JJ, Vöhringer C, Reiser G. Co-existence of two types of [Ca2+]i-inducing protease-activated receptors (PAR-1 and PAR-2) in rat astrocytes and C6 glioma cells. Neuroscience 86: 597–609, 1998. doi: 10.1016/S0306-4522(97)00686-6. [DOI] [PubMed] [Google Scholar]
- 1773.Uchiyama M, Nakajima Y, Sakuma Y, Kato M. Purinergic regulation of intracellular Ca2+ concentration of rat pituitary folliculo-stellate cells in primary culture. J Neuroendocrinol 13: 378–385, 2001. doi: 10.1046/j.1365-2826.2001.00639.x. [DOI] [PubMed] [Google Scholar]
- 1774.Uhlenberg B, Schuelke M, Rüschendorf F, Ruf N, Kaindl AM, Henneke M, Thiele H, Stoltenburg-Didinger G, Aksu F, Topaloğlu H, Nürnberg P, Hübner C, Weschke B, Gärtner J. Mutations in the gene encoding gap junction protein α 12 (connexin 46.6) cause Pelizaeus-Merzbacher-like disease. Am J Hum Genet 75: 251–260, 2004. doi: 10.1086/422763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1775.Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. Differential developmental expression of the two rat brain glutamate transporter proteins GLAST and GLT. Eur J Neurosci 9: 1646–1655, 1997. doi: 10.1111/j.1460-9568.1997.tb01522.x. [DOI] [PubMed] [Google Scholar]
- 1776.Unichenko P, Dvorzhak A, Kirischuk S. Transporter-mediated replacement of extracellular glutamate for GABA in the developing murine neocortex. Eur J Neurosci 38: 3580–3588, 2013. doi: 10.1111/ejn.12380. [DOI] [PubMed] [Google Scholar]
- 1777.Unichenko P, Myakhar O, Kirischuk S. Intracellular Na+ concentration influences short-term plasticity of glutamate transporter-mediated currents in neocortical astrocytes. Glia 60: 605–614, 2012. doi: 10.1002/glia.22294. [DOI] [PubMed] [Google Scholar]
- 1778.Untiet V, Kovermann P, Gerkau NJ, Gensch T, Rose CR, Fahlke C. Glutamate transporter-associated anion channels adjust intracellular chloride concentrations during glial maturation. Glia 65: 388–400, 2017. doi: 10.1002/glia.23098. [DOI] [PubMed] [Google Scholar]
- 1779.Urayama S, Semi K, Sanosaka T, Hori Y, Namihira M, Kohyama J, Takizawa T, Nakashima K. Chromatin accessibility at a STAT3 target site is altered prior to astrocyte differentiation. Cell Struct Funct 38: 55–66, 2013. doi: 10.1247/csf.12034. [DOI] [PubMed] [Google Scholar]
- 1780.Uwechue NM, Marx MC, Chevy Q, Billups B. Activation of glutamate transport evokes rapid glutamine release from perisynaptic astrocytes. J Physiol 590: 2317–2331, 2012. doi: 10.1113/jphysiol.2011.226605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1781.Uyeda CT, Eng LF, Bignami A. Immunological study of the glial fibrillary acidic protein. Brain Res 37: 81–89, 1972. doi: 10.1016/0006-8993(72)90347-2. [DOI] [PubMed] [Google Scholar]
- 1782.Vaarmann A, Gandhi S, Abramov AY. Dopamine induces Ca2+ signaling in astrocytes through reactive oxygen species generated by monoamine oxidase. J Biol Chem 285: 25018–25023, 2010. doi: 10.1074/jbc.M110.111450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1783.Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, Mathias RT, Robinson RB, Rosen MR, Cohen IS, Brink PR. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol 568: 459–468, 2005. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1784.Valiunas V, Weingart R. Electrical properties of gap junction hemichannels identified in transfected HeLa cells. Pflugers Arch 440: 366–379, 2000. doi: 10.1007/s004240000294. [DOI] [PubMed] [Google Scholar]
- 1785.Van Bockstaele EJ, Colago EE. Selective distribution of the NMDA-R1 glutamate receptor in astrocytes and presynaptic axon terminals in the nucleus locus coeruleus of the rat brain: an immunoelectron microscopic study. J Comp Neurol 369: 483–496, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 1786.Van Calker D, Hamprecht B. Effects of neurohormones on glial cells. Adv Cell Neurobiol 1: 31–67, 1980. doi: 10.1016/B978-0-12-008301-5.50006-6. [DOI] [Google Scholar]
- 1787.Van Calker D, Müller M, Hamprecht B. Adrenergic α- and β-receptors expressed by the same cell type in primary culture of perinatal mouse brain. J Neurochem 30: 713–718, 1978. doi: 10.1111/j.1471-4159.1978.tb10776.x. [DOI] [PubMed] [Google Scholar]
- 1788.Van Eldik LJ, Wainwright MS. The Janus face of glial-derived S100B: beneficial and detrimental functions in the brain. Restor Neurol Neurosci 21: 97–108, 2003. [PubMed] [Google Scholar]
- 1789.Van Liefferinge J, Massie A, Portelli J, Di Giovanni G, Smolders I. Are vesicular neurotransmitter transporters potential treatment targets for temporal lobe epilepsy? Front Cell Neurosci 7: 139, 2013. doi: 10.3389/fncel.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1790.Vandenberg RJ, Handford CA, Campbell EM, Ryan RM, Yool AJ. Water and urea permeation pathways of the human excitatory amino acid transporter EAAT1. Biochem J 439: 333–340, 2011. doi: 10.1042/BJ20110905. [DOI] [PubMed] [Google Scholar]
- 1791.Vandenberg RJ, Huang S, Ryan RM. Slips, leaks and channels in glutamate transporters. Channels (Austin) 2: 51–58, 2008. doi: 10.4161/chan.2.1.6047. [DOI] [PubMed] [Google Scholar]
- 1792.Vandenberg RJ, Ryan RM. Mechanisms of glutamate transport. Physiol Rev 93: 1621–1657, 2013. doi: 10.1152/physrev.00007.2013. [DOI] [PubMed] [Google Scholar]
- 1793.Vardjan N, Gabrijel M, Potokar M, Svajger U, Kreft M, Jeras M, de Pablo Y, Faiz M, Pekny M, Zorec R. IFN-γ-induced increase in the mobility of MHC class II compartments in astrocytes depends on intermediate filaments. J Neuroinflammation 9: 144, 2012. doi: 10.1186/1742-2094-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1794.Vardjan N, Kreft M, Zorec R. Dynamics of β-adrenergic/cAMP signaling and morphological changes in cultured astrocytes. Glia 62: 566–579, 2014. doi: 10.1002/glia.22626. [DOI] [PubMed] [Google Scholar]
- 1795.Vardjan N, Kreft M, Zorec R. Regulated Exocytosis in Astrocytes is as Slow as the Metabolic Availability of Gliotransmitters: Focus on Glutamate and ATP. Adv Neurobiol 11: 81–101, 2014. doi: 10.1007/978-3-319-08894-5_5. [DOI] [PubMed] [Google Scholar]
- 1796.Vardjan N, Verkhratsky A, Zorec R. Pathologic potential of astrocytic vesicle traffic: new targets to treat neurologic diseases? Cell Transplant 24: 599–612, 2015. doi: 10.3727/096368915X687750. [DOI] [PubMed] [Google Scholar]
- 1797.Vardjan N, Zorec R. Excitable astrocytes: Ca- and cAMP-regulated exocytosis. Neurochem Res 40: 2414–2424, 2015. doi: 10.1007/s11064-015-1545-x. [DOI] [PubMed] [Google Scholar]
- 1798.Vargas JR, Takahashi DK, Thomson KE, Wilcox KS. The expression of kainate receptor subunits in hippocampal astrocytes after experimentally induced status epilepticus. J Neuropathol Exp Neurol 72: 919–932, 2013. doi: 10.1097/NEN.0b013e3182a4b266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1799.Véga C, R Sachleben L Jr, Gozal D, Gozal E. Differential metabolic adaptation to acute and long-term hypoxia in rat primary cortical astrocytes. J Neurochem 97: 872–883, 2006. doi: 10.1111/j.1471-4159.2006.03790.x. [DOI] [PubMed] [Google Scholar]
- 1800.Vélez-Fort M, Audinat E, Angulo MC. Central role of GABA in neuron-glia interactions. Neuroscientist 18: 237–250, 2012. doi: 10.1177/1073858411403317. [DOI] [PubMed] [Google Scholar]
- 1801.Venance L, Prémont J, Glowinski J, Giaume C. Gap junctional communication and pharmacological heterogeneity in astrocytes cultured from the rat striatum. J Physiol 510: 429–440, 1998. doi: 10.1111/j.1469-7793.1998.429bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1802.Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci 19: 6897–6906, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1803.Vérant P, Ricard C, Serduc R, Vial JC, van der Sanden B. In vivo staining of neocortical astrocytes via the cerebral microcirculation using sulforhodamine B. J Biomed Opt 13: 064028, 2008. doi: 10.1117/1.3041163. [DOI] [PubMed] [Google Scholar]
- 1804.Verderio C, Cagnoli C, Bergami M, Francolini M, Schenk U, Colombo A, Riganti L, Frassoni C, Zuccaro E, Danglot L, Wilhelm C, Galli T, Canossa M, Matteoli M. TI-VAMP/VAMP7 is the SNARE of secretory lysosomes contributing to ATP secretion from astrocytes. Biol Cell 104: 213–228, 2012. doi: 10.1111/boc.201100070. [DOI] [PubMed] [Google Scholar]
- 1805.Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev 85: 201–279, 2005. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- 1806.Verkhratsky A. Physiology of neuronal-glial networking. Neurochem Int 57: 332–343, 2010. doi: 10.1016/j.neuint.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 1807.Verkhratsky A, Burnstock G. Biology of purinergic signalling: its ancient evolutionary roots, its omnipresence and its multiple functional significance. BioEssays 36: 697–705, 2014. doi: 10.1002/bies.201400024. [DOI] [PubMed] [Google Scholar]
- 1808.Verkhratsky A, Kettenmann H. Calcium signalling in glial cells. Trends Neurosci 19: 346–352, 1996. doi: 10.1016/0166-2236(96)10048-5. [DOI] [PubMed] [Google Scholar]
- 1809.Verkhratsky A, Kirchhoff F. NMDA receptors in glia. Neuroscientist 13: 28–37, 2007. doi: 10.1177/1073858406294270. [DOI] [PubMed] [Google Scholar]
- 1810.Verkhratsky A, Krishtal OA, Burnstock G. Purinoceptors on neuroglia. Mol Neurobiol 39: 190–208, 2009. doi: 10.1007/s12035-009-8063-2. [DOI] [PubMed] [Google Scholar]
- 1811.Verkhratsky A, Matteoli M, Parpura V, Mothet JP, Zorec R. Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion. EMBO J 35: 239–257, 2016. doi: 10.15252/embj.201592705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1812.Verkhratsky A, Nedergaard M. Astroglial cradle in the life of the synapse. Philos Trans R Soc Lond B Biol Sci 369: 20130595, 2014. doi: 10.1098/rstb.2013.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1813.Verkhratsky A, Nedergaard M. The homeostatic astroglia emerges from evolutionary specialization of neural cells. Philos Trans R Soc Lond B Biol Sci 371: 20150428, 2016. doi: 10.1098/rstb.2015.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1814.Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev 78: 99–141, 1998. [DOI] [PubMed] [Google Scholar]
- 1815.Verkhratsky A, Parpura V, Pekna M, Pekny M, Sofroniew M. Glia in the pathogenesis of neurodegenerative diseases. Biochem Soc Trans 42: 1291–1301, 2014. doi: 10.1042/BST20140107. [DOI] [PubMed] [Google Scholar]
- 1816.Verkhratsky A, Reyes RC, Parpura V. TRP channels coordinate ion signalling in astroglia. Rev Physiol Biochem Pharmacol 166: 1–22, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1817.Verkhratsky A, Rodríguez JJ, Parpura V. Calcium signalling in astroglia. Mol Cell Endocrinol 353: 45–56, 2012. doi: 10.1016/j.mce.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 1818.Verkhratsky A, Sofroniew MV, Messing A, deLanerolle NC, Rempe D, Rodríguez JJ, Nedergaard M. Neurological diseases as primary gliopathies: a reassessment of neurocentrism. ASN Neuro 4: e00082, 2012. doi: 10.1042/AN20120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1819.Verkhratsky A, Solovyova N, Toescu EC. Calcium excitability of glial cells. In: Glia in Synaptic Transmission , edited by Volterra A, Haydon P, Magistretti P. Oxford, UK: OUP, 2002, p. 99–109. [Google Scholar]
- 1820.Vermeulen RJ, Jongenelen CA, Langeveld CH, Wolters EC, Stoof JC, Drukarch B. Dopamine D1 receptor agonists display a different intrinsic activity in rat, monkey and human astrocytes. Eur J Pharmacol 269: 121–125, 1994. doi: 10.1016/0922-4106(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 1821.Viehweger E, Robitail S, Rohon MA, Jacquemier M, Jouve JL, Bollini G, Simeoni MC. Measuring quality of life in cerebral palsy children. Ann Readapt Med Phys 51: 119–137, 2008. doi: 10.1016/j.annrmp.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 1822.Viitanen T, Ruusuvuori E, Kaila K, Voipio J. The K+-Cl cotransporter KCC2 promotes GABAergic excitation in the mature rat hippocampus. J Physiol 588: 1527–1540, 2010. doi: 10.1113/jphysiol.2009.181826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1823.Vilchez D, Ros S, Cifuentes D, Pujadas L, Vallès J, García-Fojeda B, Criado-García O, Fernández-Sánchez E, Medraño-Fernández I, Domínguez J, García-Rocha M, Soriano E, Rodríguez de Córdoba S, Guinovart JJ. Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat Neurosci 10: 1407–1413, 2007. doi: 10.1038/nn1998. [DOI] [PubMed] [Google Scholar]
- 1824.Villarreal A, Seoane R, González Torres A, Rosciszewski G, Angelo MF, Rossi A, Barker PA, Ramos AJ. S100B protein activates a RAGE-dependent autocrine loop in astrocytes: implications for its role in the propagation of reactive gliosis. J Neurochem 131: 190–205, 2014. doi: 10.1111/jnc.12790. [DOI] [PubMed] [Google Scholar]
- 1825.Viollet C, Lanneau C, Faivre-Bauman A, Zhang J, Djordjijevic D, Loudes C, Gardette R, Kordon C, Epelbaum J. Distinct patterns of expression and physiological effects of sst1 and sst2 receptor subtypes in mouse hypothalamic neurons and astrocytes in culture. J Neurochem 68: 2273–2280, 1997. doi: 10.1046/j.1471-4159.1997.68062273.x. [DOI] [PubMed] [Google Scholar]
- 1826.Virchow R. Die Cellularpathologie in ihrer Begründung auf physiologische and pathologische Gewebelehre. Zwanzig Vorlesungen gehalten während der Monate Februar, März und April 1858 im pathologischen Institut zu Berlin. Berlin: August Hirschwald, 1858, p. 440. [Google Scholar]
- 1827.Virchow R. Gesammelte Abhandlungen zyr wissenschaftlischen Medizin. Frankfurt, Germany: Verlag von Meidinger Sohn & Comp, 1856, p. 1024. [Google Scholar]
- 1828.Vitellaro-Zuccarello L, Calvaresi N, De Biasi S. Expression of GABA transporters, GAT-1 and GAT-3, in the cerebral cortex and thalamus of the rat during postnatal development. Cell Tissue Res 313: 245–257, 2003. doi: 10.1007/s00441-003-0746-9. [DOI] [PubMed] [Google Scholar]
- 1829.Vives V, Alonso G, Solal AC, Joubert D, Legraverend C. Visualization of S100B-positive neurons and glia in the central nervous system of EGFP transgenic mice. J Comp Neurol 457: 404–419, 2003. doi: 10.1002/cne.10552. [DOI] [PubMed] [Google Scholar]
- 1830.Voigt T. Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes. J Comp Neurol 289: 74–88, 1989. doi: 10.1002/cne.902890106. [DOI] [PubMed] [Google Scholar]
- 1831.Volterra A, Liaudet N, Savtchouk I. Astrocyte Ca2+ signalling: an unexpected complexity. Nat Rev Neurosci 15: 327–335, 2014. doi: 10.1038/nrn3725. [DOI] [PubMed] [Google Scholar]
- 1832.Von Bartheld CS, Bahney J, Herculano-Houzel S. The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J Comp Neurol 524: 3865–3895, 2016. doi: 10.1002/cne.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1833.Voss FK, Ullrich F, Münch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 344: 634–638, 2014. doi: 10.1126/science.1252826. [DOI] [PubMed] [Google Scholar]
- 1834.Waldmann R, Champigny G, Bassilana F, Voilley N, Lazdunski M. Molecular cloning and functional expression of a novel amiloride-sensitive Na+ channel. J Biol Chem 270: 27411–27414, 1995. doi: 10.1074/jbc.270.46.27411. [DOI] [PubMed] [Google Scholar]
- 1835.Walhovd KB, Johansen-Berg H, Káradóttir RT. Unraveling the secrets of white matter–bridging the gap between cellular, animal and human imaging studies. Neuroscience 276: 2–13, 2014. doi: 10.1016/j.neuroscience.2014.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1836.Waller R, Woodroofe MN, Francese S, Heath PR, Wharton SB, Ince PG, Sharrack B, Simpson JE. Isolation of enriched glial populations from post-mortem human CNS material by immuno-laser capture microdissection. J Neurosci Methods 208: 108–113, 2012. doi: 10.1016/j.jneumeth.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 1837.Waller R, Woodroofe MN, Wharton SB, Ince PG, Francese S, Heath PR, Cudzich-Madry A, Thomas RH, Rounding N, Sharrack B, Simpson JE. Gene expression profiling of the astrocyte transcriptome in multiple sclerosis normal appearing white matter reveals a neuroprotective role. J Neuroimmunol 299: 139–146, 2016. doi: 10.1016/j.jneuroim.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 1838.Wallraff A, Köhling R, Heinemann U, Theis M, Willecke K, Steinhäuser C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci 26: 5438–5447, 2006. doi: 10.1523/JNEUROSCI.0037-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1839.Wallraff A, Odermatt B, Willecke K, Steinhäuser C. Distinct types of astroglial cells in the hippocampus differ in gap junction coupling. Glia 48: 36–43, 2004. doi: 10.1002/glia.20040. [DOI] [PubMed] [Google Scholar]
- 1840.Walter L, Dinh T, Stella N. ATP induces a rapid and pronounced increase in 2-arachidonoylglycerol production by astrocytes, a response limited by monoacylglycerol lipase. J Neurosci 24: 8068–8074, 2004. doi: 10.1523/JNEUROSCI.2419-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1841.Walther EU, Dichgans M, Maricich SM, Romito RR, Yang F, Dziennis S, Zackson S, Hawkes R, Herrup K. Genomic sequences of aldolase C (Zebrin II) direct lacZ expression exclusively in non-neuronal cells of transgenic mice. Proc Natl Acad Sci USA 95: 2615–2620, 1998. doi: 10.1073/pnas.95.5.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1842.Walz W. Controversy surrounding the existence of discrete functional classes of astrocytes in adult gray matter. Glia 31: 95–103, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 1843.Walz W. Do neuronal signals regulate potassium flow in glial cells? Evidence from an invertebrate central nervous system. J Neurosci Res 7: 71–79, 1982. doi: 10.1002/jnr.490070108. [DOI] [PubMed] [Google Scholar]
- 1844.Walz W, Gimpl G, Ohlemeyer C, Kettenmann H. Extracellular ATP-induced currents in astrocytes: involvement of a cation channel. J Neurosci Res 38: 12–18, 1994. doi: 10.1002/jnr.490380104. [DOI] [PubMed] [Google Scholar]
- 1845.Walz W, Hertz L. Intense furosemide-sensitive potassium accumulation in astrocytes in the presence of pathologically high extracellular potassium levels. J Cereb Blood Flow Metab 4: 301–304, 1984. doi: 10.1038/jcbfm.1984.42. [DOI] [PubMed] [Google Scholar]
- 1846.Walz W, Hertz L. Sodium transport in astrocytes. J Neurosci Res 11: 231–239, 1984. doi: 10.1002/jnr.490110303. [DOI] [PubMed] [Google Scholar]
- 1847.Walz W, Lang MK. Immunocytochemical evidence for a distinct GFAP-negative subpopulation of astrocytes in the adult rat hippocampus. Neurosci Lett 257: 127–130, 1998. doi: 10.1016/S0304-3940(98)00813-1. [DOI] [PubMed] [Google Scholar]
- 1848.Walz W, MacVicar B. Electrophysiological properties of glial cells: comparison of brain slices with primary cultures. Brain Res 443: 321–324, 1988. doi: 10.1016/0006-8993(88)91626-5. [DOI] [PubMed] [Google Scholar]
- 1849.Walz W, Mukerji S. Lactate release from cultured astrocytes and neurons: a comparison. Glia 1: 366–370, 1988. doi: 10.1002/glia.440010603. [DOI] [PubMed] [Google Scholar]
- 1850.Wang CM, Chang YY, Sun SH. Activation of P2X7 purinoceptor-stimulated TGF-β 1 mRNA expression involves PKC/MAPK signalling pathway in a rat brain-derived type-2 astrocyte cell line, RBA-2. Cell Signal 15: 1129–1137, 2003. doi: 10.1016/S0898-6568(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 1851.Wang D, Yan B, Rajapaksha WR, Fisher TE. The expression of voltage-gated Ca2+ channels in pituicytes and the up-regulation of L-type Ca2+ channels during water deprivation. J Neuroendocrinol 21: 858–866, 2009. doi: 10.1111/j.1365-2826.2009.01906.x. [DOI] [PubMed] [Google Scholar]
- 1852.Wang F, Du T, Liang C, Verkhratsky A, Peng L. Ammonium increases Ca2+ signalling and upregulates expression of Cav1.2 gene in astrocytes in primary cultures and in the in vivo brain. Acta Physiol (Oxf) 214: 261–274, 2015. doi: 10.1111/apha.12500. [DOI] [PubMed] [Google Scholar]
- 1853.Wang F, Smith NA, Xu Q, Fujita T, Baba A, Matsuda T, Takano T, Bekar L, Nedergaard M. Astrocytes modulate neural network activity by Ca2+-dependent uptake of extracellular K+. Sci Signal 5: ra26, 2012. doi: 10.1126/scisignal.2002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1854.Wang G, Dinkins M, He Q, Zhu G, Poirier C, Campbell A, Mayer-Proschel M, Bieberich E. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD). J Biol Chem 287: 21384–21395, 2012. doi: 10.1074/jbc.M112.340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1855.Wang H, Ubl JJ, Reiser G. Four subtypes of protease-activated receptors, co-expressed in rat astrocytes, evoke different physiological signaling. Glia 37: 53–63, 2002. doi: 10.1002/glia.10012. [DOI] [PubMed] [Google Scholar]
- 1856.Wang J, Ambrosi C, Qiu F, Jackson DG, Sosinsky G, Dahl G. The membrane protein Pannexin1 forms two open-channel conformations depending on the mode of activation. Sci Signal 7: ra69, 2014. doi: 10.1126/scisignal.2005431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1857.Wang W, Putra A, Schools GP, Ma B, Chen H, Kaczmarek LK, Barhanin J, Lesage F, Zhou M. The contribution of TWIK-1 channels to astrocyte K+ current is limited by retention in intracellular compartments. Front Cell Neurosci 7: 246, 2013. doi: 10.3389/fncel.2013.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1858.Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci 9: 816–823, 2006. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- 1859.Wang YF, Sun MY, Hou Q, Parpura V. Hyposmolality differentially and spatiotemporally modulates levels of glutamine synthetase and serine racemase in rat supraoptic nucleus. Glia 61: 529–538, 2013. doi: 10.1002/glia.22453. [DOI] [PubMed] [Google Scholar]
- 1860.Wang ZY, Stoltenberg M, Huang L, Danscher G, Dahlström A, Shi Y, Li JY. Abundant expression of zinc transporters in Bergman glia of mouse cerebellum. Brain Res Bull 64: 441–448, 2005. doi: 10.1016/j.brainresbull.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 1861.Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol 160: 313–337, 1975. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- 1862.Wardell WM. Electrical and pharmacological properties of mammalian neuroglial cells in tissue-culture. Proc R Soc Lond B Biol Sci 165: 326–361, 1966. doi: 10.1098/rspb.1966.0071. [DOI] [PubMed] [Google Scholar]
- 1863.Washburn KB, Neary JT. P2 purinergic receptors signal to STAT3 in astrocytes: difference in STAT3 responses to P2Y and P2X receptor activation. Neuroscience 142: 411–423, 2006. doi: 10.1016/j.neuroscience.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 1864.Watanabe E, Fujikawa A, Matsunaga H, Yasoshima Y, Sako N, Yamamoto T, Saegusa C, Noda M. Nav2/NaG channel is involved in control of salt-intake behavior in the CNS. J Neurosci 20: 7743–7751, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1865.Watanabe E, Hiyama TY, Shimizu H, Kodama R, Hayashi N, Miyata S, Yanagawa Y, Obata K, Noda M. Sodium-level-sensitive sodium channel Nax is expressed in glial laminate processes in the sensory circumventricular organs. Am J Physiol Regul Integr Comp Physiol 290: R568–R576, 2006. doi: 10.1152/ajpregu.00618.2005. [DOI] [PubMed] [Google Scholar]
- 1866.Waters EM, Torres-Reveron A, McEwen BS, Milner TA. Ultrastructural localization of extranuclear progestin receptors in the rat hippocampal formation. J Comp Neurol 511: 34–46, 2008. doi: 10.1002/cne.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1867.Watson C, Bates J, Franczak R. Serotonin regulation by astrocytes. FASEB J 23: 790–792, 2009. [Google Scholar]
- 1868.Weerth SH, Holtzclaw LA, Russell JT. Signaling proteins in raft-like microdomains are essential for Ca2+ wave propagation in glial cells. Cell Calcium 41: 155–167, 2007. doi: 10.1016/j.ceca.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 1869.Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev 57: 463–472, 2005. doi: 10.1124/pr.57.4.9. [DOI] [PubMed] [Google Scholar]
- 1870.Wei HS, Kang H, Rasheed ID, Zhou S, Lou N, Gershteyn A, McConnell ED, Wang Y, Richardson KE, Palmer AF, Xu C, Wan J, Nedergaard M. Erythrocytes are oxygen-sensing regulators of the cerebral microcirculation. Neuron 91: 851–862, 2016. doi: 10.1016/j.neuron.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1871.Weigert C. Kenntnis der normalen menschlichen Neuroglia. Frankfurt, Germany: Moritz Diesterweg, 1895, p. 213. [Google Scholar]
- 1872.Weisman GA, Wang M, Kong Q, Chorna NE, Neary JT, Sun GY, González FA, Seye CI, Erb L. Molecular determinants of P2Y2 nucleotide receptor function: implications for proliferative and inflammatory pathways in astrocytes. Mol Neurobiol 31: 169–183, 2005. doi: 10.1385/MN:31:1-3:169. [DOI] [PubMed] [Google Scholar]
- 1873.Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron 43: 647–661, 2004. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 1874.Weller J, Steinhäuser C, Seifert G. pH-Sensitive K+ Currents and Properties of K2P Channels in Murine Hippocampal Astrocytes. Adv Protein Chem Struct Biol 103: 263–294, 2016. doi: 10.1016/bs.apcsb.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 1875.Wenker IC, Kréneisz O, Nishiyama A, Mulkey DK. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol 104: 3042–3052, 2010. doi: 10.1152/jn.00544.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1876.Westenbroek RE, Bausch SB, Lin RC, Franck JE, Noebels JL, Catterall WA. Upregulation of L-type Ca2+ channels in reactive astrocytes after brain injury, hypomyelination, and ischemia. J Neurosci 18: 2321–2334, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1877.Westergaard N, Sonnewald U, Unsgård G, Peng L, Hertz L, Schousboe A. Uptake, release, and metabolism of citrate in neurons and astrocytes in primary cultures. J Neurochem 62: 1727–1733, 1994. doi: 10.1046/j.1471-4159.1994.62051727.x. [DOI] [PubMed] [Google Scholar]
- 1878.White TW, Paul DL, Goodenough DA, Bruzzone R. Functional analysis of selective interactions among rodent connexins. Mol Biol Cell 6: 459–470, 1995. doi: 10.1091/mbc.6.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1879.Wicht H, Derouiche A, Korf HW. An immunocytochemical investigation of glial morphology in the Pacific hagfish: radial and astrocyte-like glia have the same phylogenetic age. J Neurocytol 23: 565–576, 1994. doi: 10.1007/BF01262057. [DOI] [PubMed] [Google Scholar]
- 1880.Wilhelmsson U, Li L, Pekna M, Berthold CH, Blom S, Eliasson C, Renner O, Bushong E, Ellisman M, Morgan TE, Pekny M. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci 24: 5016–5021, 2004. doi: 10.1523/JNEUROSCI.0820-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1881.Willford SL, Anderson CM, Spencer SR, Eskandari S. Evidence for a revised ion/substrate coupling stoichiometry of GABA transporters. J Membr Biol 248: 795–810, 2015. doi: 10.1007/s00232-015-9797-6. [DOI] [PubMed] [Google Scholar]
- 1882.Williams SM, Sullivan RK, Scott HL, Finkelstein DI, Colditz PB, Lingwood BE, Dodd PR, Pow DV. Glial glutamate transporter expression patterns in brains from multiple mammalian species. Glia 49: 520–541, 2005. doi: 10.1002/glia.20139. [DOI] [PubMed] [Google Scholar]
- 1883.Willmott NJ, Wong K, Strong AJ. Intercellular Ca2+ waves in rat hippocampal slice and dissociated glial-neuron cultures mediated by nitric oxide. FEBS Lett 487: 239–247, 2000. doi: 10.1016/S0014-5793(00)02359-0. [DOI] [PubMed] [Google Scholar]
- 1884.Wilson JX, Peters CE, Sitar SM, Daoust P, Gelb AW. Glutamate stimulates ascorbate transport by astrocytes. Brain Res 858: 61–66, 2000. doi: 10.1016/S0006-8993(99)02433-6. [DOI] [PubMed] [Google Scholar]
- 1885.Windrem MS, Schanz SJ, Morrow C, Munir J, Chandler-Militello D, Wang S, Goldman SA. A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brains are chimeric for human glia. J Neurosci 34: 16153–16161, 2014. doi: 10.1523/JNEUROSCI.1510-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1886.Winkler BS, Orselli SM, Rex TS. The redox couple between glutathione and ascorbic acid: a chemical and physiological perspective. Free Radic Biol Med 17: 333–349, 1994. doi: 10.1016/0891-5849(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 1887.Wirth EK, Schweizer U, Köhrle J. Transport of thyroid hormone in brain. Front Endocrinol (Lausanne) 5: 98, 2014. doi: 10.3389/fendo.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1888.Witcher MR, Kirov SA, Harris KM. Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia 55: 13–23, 2007. doi: 10.1002/glia.20415. [DOI] [PubMed] [Google Scholar]
- 1889.Wolburg H. Orthogonal arrays of intramembranous particles: a review with special reference to astrocytes. J Hirnforsch 36: 239–258, 1995. [PubMed] [Google Scholar]
- 1890.Wolff JR, Stuke K, Missler M, Tytko H, Schwarz P, Rohlmann A, Chao TI. Autocellular coupling by gap junctions in cultured astrocytes: a new view on cellular autoregulation during process formation. Glia 24: 121–140, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 1891.Wolosker H, Balu DT, Coyle JT. The rise and fall of the d-serine-mediated gliotransmission hypothesis. Trends Neurosci 39: 712–721, 2016. doi: 10.1016/j.tins.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1892.Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-d-aspartate neurotransmission. Proc Natl Acad Sci USA 96: 13409–13414, 1999. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1893.Woo DH, Han KS, Shim JW, Yoon BE, Kim E, Bae JY, Oh SJ, Hwang EM, Marmorstein AD, Bae YC, Park JY, Lee CJ. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 151: 25–40, 2012. doi: 10.1016/j.cell.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 1894.Woods MD, Freshney RI, Ball SG, Vaughan PF. Regulation of cyclic AMP formation in cultures of human foetal astrocytes by β2-adrenergic and adenosine receptors. J Neurochem 53: 864–869, 1989. doi: 10.1111/j.1471-4159.1989.tb11784.x. [DOI] [PubMed] [Google Scholar]
- 1895.Wu HQ, Pereira EF, Bruno JP, Pellicciari R, Albuquerque EX, Schwarcz R. The astrocyte-derived α7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J Mol Neurosci 40: 204–210, 2010. doi: 10.1007/s12031-009-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1896.Wu J, Lee MR, Kim T, Johng S, Rohrback S, Kang N, Choi DS. Regulation of ethanol-sensitive EAAT2 expression through adenosine A1 receptor in astrocytes. Biochem Biophys Res Commun 406: 47–52, 2011. doi: 10.1016/j.bbrc.2011.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1897.Wu XF, Liu WT, Liu YP, Huang ZJ, Zhang YK, Song XJ. Reopening of ATP-sensitive potassium channels reduces neuropathic pain and regulates astroglial gap junctions in the rat spinal cord. Pain 152: 2605–2615, 2011. doi: 10.1016/j.pain.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 1898.Wuttke WA, Pentreath VW. Evidence for the uptake of neuronally derived choline by glial cells in the leech central nervous system. J Physiol 420: 387–408, 1990. doi: 10.1113/jphysiol.1990.sp017919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1899.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science 342: 373–377, 2013. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1900.Xiong WC, Montell C. Defective glia induce neuronal apoptosis in the repo visual system of Drosophila. Neuron 14: 581–590, 1995. doi: 10.1016/0896-6273(95)90314-3. [DOI] [PubMed] [Google Scholar]
- 1901.Xiong Z, O’Hanlon D, Becker LE, Roder J, MacDonald JF, Marks A. Enhanced calcium transients in glial cells in neonatal cerebellar cultures derived from S100B null mice. Exp Cell Res 257: 281–289, 2000. doi: 10.1006/excr.2000.4902. [DOI] [PubMed] [Google Scholar]
- 1902.Xiong ZQ, Stringer JL. Sodium pump activity, not glial spatial buffering, clears potassium after epileptiform activity induced in the dentate gyrus. J Neurophysiol 83: 1443–1451, 2000. [DOI] [PubMed] [Google Scholar]
- 1903.Xu-Friedman MA, Harris KM, Regehr WG. Three-dimensional comparison of ultrastructural characteristics at depressing and facilitating synapses onto cerebellar Purkinje cells. J Neurosci 21: 6666–6672, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1904.Xu HL, Pelligrino DA. ATP release and hydrolysis contribute to rat pial arteriolar dilatation elicited by neuronal activation. Exp Physiol 92: 647–651, 2007. doi: 10.1113/expphysiol.2006.036863. [DOI] [PubMed] [Google Scholar]
- 1905.Xu J, Chalimoniuk M, Shu Y, Simonyi A, Sun AY, Gonzalez FA, Weisman GA, Wood WG, Sun GY. Prostaglandin E2 production in astrocytes: regulation by cytokines, extracellular ATP, and oxidative agents. Prostaglandins Leukot Essent Fatty Acids 69: 437–448, 2003. doi: 10.1016/j.plefa.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 1906.Xu J, Peng H, Kang N, Zhao Z, Lin JH, Stanton PK, Kang J. Glutamate-induced exocytosis of glutamate from astrocytes. J Biol Chem 282: 24185–24197, 2007. doi: 10.1074/jbc.M700452200. [DOI] [PubMed] [Google Scholar]
- 1907.Xu J, Yu S, Sun AY, Sun GY. Oxidant-mediated AA release from astrocytes involves cPLA2 and iPLA2. Free Radic Biol Med 34: 1531–1543, 2003. doi: 10.1016/S0891-5849(03)00152-7. [DOI] [PubMed] [Google Scholar]
- 1908.Yagita K, Yamanaka I, Emoto N, Kawakami K, Shimada S. Real-time monitoring of circadian clock oscillations in primary cultures of mammalian cells using Tol2 transposon-mediated gene transfer strategy. BMC Biotechnol 10: 3, 2010. doi: 10.1186/1472-6750-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1909.Yaguchi T, Nishizaki T. Extracellular high K+ stimulates vesicular glutamate release from astrocytes by activating voltage-dependent calcium channels. J Cell Physiol 225: 512–518, 2010. doi: 10.1002/jcp.22231. [DOI] [PubMed] [Google Scholar]
- 1910.Yamasaki M, Yamada K, Furuya S, Mitoma J, Hirabayashi Y, Watanabe M. 3-Phosphoglycerate dehydrogenase, a key enzyme for l-serine biosynthesis, is preferentially expressed in the radial glia/astrocyte lineage and olfactory ensheathing glia in the mouse brain. J Neurosci 21: 7691–7704, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1911.Yang F, Sun X, Ding Y, Ma H, Yang TO, Ma Y, Wei D, Li W, Xu T, Jiang W. Astrocytic acid-sensing ion channel 1a contributes to the development of chronic epileptogenesis. Sci Rep 6: 31581, 2016. doi: 10.1038/srep31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1912.Yang J, Yu H, Zhou D, Zhu K, Lou H, Duan S, Wang H. Na+-Ca2+ exchanger mediates ChR2-induced [Ca2+]i elevation in astrocytes. Cell Calcium 58: 307–316, 2015. doi: 10.1016/j.ceca.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 1913.Yang JH, Wada A, Yoshida K, Miyoshi Y, Sayano T, Esaki K, Kinoshita MO, Tomonaga S, Azuma N, Watanabe M, Hamase K, Zaitsu K, Machida T, Messing A, Itohara S, Hirabayashi Y, Furuya S. Brain-specific Phgdh deletion reveals a pivotal role for l-serine biosynthesis in controlling the level of d-serine, an N-methyl-d-aspartate receptor co-agonist, in adult brain. J Biol Chem 285: 41380–41390, 2010. doi: 10.1074/jbc.M110.187443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1914.Yang L, Kress BT, Weber HJ, Thiyagarajan M, Wang B, Deane R, Benveniste H, Iliff JJ, Nedergaard M. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J Transl Med 11: 107, 2013. doi: 10.1186/1479-5876-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1915.Yang L, Qi Y, Yang Y. Astrocytes control food intake by inhibiting AGRP neuron activity via adenosine A1 receptors. Cell Reports 11: 798–807, 2015. doi: 10.1016/j.celrep.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 1916.Yang Y, Vidensky S, Jin L, Jie C, Lorenzini I, Frankl M, Rothstein JD. Molecular comparison of GLT1+ and ALDH1L1+ astrocytes in vivo in astroglial reporter mice. Glia 59: 200–207, 2011. doi: 10.1002/glia.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1917.Yao X, Hrabetová S, Nicholson C, Manley GT. Aquaporin-4-deficient mice have increased extracellular space without tortuosity change. J Neurosci 28: 5460–5464, 2008. doi: 10.1523/JNEUROSCI.0257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1918.Ye ZC, Oberheim N, Kettenmann H, Ransom BR. Pharmacological “cross-inhibition” of connexin hemichannels and swelling activated anion channels. Glia 57: 258–269, 2009. doi: 10.1002/glia.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1919.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci 23: 3588–3596, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1920.Yeh CY, Verkhratsky A, Terzieva S, Rodríguez JJ. Glutamine synthetase in astrocytes from entorhinal cortex of the triple transgenic animal model of Alzheimer’s disease is not affected by pathological progression. Biogerontology 14: 777–787, 2013. doi: 10.1007/s10522-013-9456-1. [DOI] [PubMed] [Google Scholar]
- 1921.Yoon BE, Jo S, Woo J, Lee JH, Kim T, Kim D, Lee CJ. The amount of astrocytic GABA positively correlates with the degree of tonic inhibition in hippocampal CA1 and cerebellum. Mol Brain 4: 42, 2011. doi: 10.1186/1756-6606-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1922.Yoon BE, Lee CJ. GABA as a rising gliotransmitter. Front Neural Circuits 8: 141, 2014. doi: 10.3389/fncir.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1923.Yoon BE, Woo J, Chun YE, Chun H, Jo S, Bae JY, An H, Min JO, Oh SJ, Han KS, Kim HY, Kim T, Kim YS, Bae YC, Lee CJ. Glial GABA, synthesized by monoamine oxidase B, mediates tonic inhibition. J Physiol 592: 4951–4968, 2014. doi: 10.1113/jphysiol.2014.278754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1924.Young JK, Baker JH, Muller T. Immunoreactivity for brain-fatty acid binding protein in gomori-positive astrocytes. Glia 16: 218–226, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 1925.Young JK, McKenzie JC. GLUT2 immunoreactivity in Gomori-positive astrocytes of the hypothalamus. J Histochem Cytochem 52: 1519–1524, 2004. doi: 10.1369/jhc.4A6375.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1926.Young JK, McKenzie JC, Baker JH. Association of iron-containing astrocytes with dopaminergic neurons of the arcuate nucleus. J Neurosci Res 25: 204–213, 1990. doi: 10.1002/jnr.490250208. [DOI] [PubMed] [Google Scholar]
- 1927.Yu AC, Schousboe A, Hertz L. Metabolic fate of 14C-labeled glutamate in astrocytes in primary cultures. J Neurochem 39: 954–960, 1982. doi: 10.1111/j.1471-4159.1982.tb11482.x. [DOI] [PubMed] [Google Scholar]
- 1928.Yu PH, Hertz L. Differential expression of type A and type B monoamine oxidase of mouse astrocytes in primary cultures. J Neurochem 39: 1492–1495, 1982. doi: 10.1111/j.1471-4159.1982.tb12598.x. [DOI] [PubMed] [Google Scholar]
- 1929.Yu WF, Guan ZZ, Bogdanovic N, Nordberg A. High selective expression of α7 nicotinic receptors on astrocytes in the brains of patients with sporadic Alzheimer’s disease and patients carrying Swedish APP 670/671 mutation: a possible association with neuritic plaques. Exp Neurol 192: 215–225, 2005. doi: 10.1016/j.expneurol.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 1930.Yu Y, Ugawa S, Ueda T, Ishida Y, Inoue K, Kyaw Nyunt A, Umemura A, Mase M, Yamada K, Shimada S. Cellular localization of P2X7 receptor mRNA in the rat brain. Brain Res 1194: 45–55, 2008. doi: 10.1016/j.brainres.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 1931.Yuchi Z, Van Petegem F. Ryanodine receptors under the magnifying lens: insights and limitations of cryo-electron microscopy and X-ray crystallography studies. Cell Calcium 59: 209–227, 2016. doi: 10.1016/j.ceca.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 1932.Zafra F, Aragón C, Olivares L, Danbolt NC, Giménez C, Storm-Mathisen J. Glycine transporters are differentially expressed among CNS cells. J Neurosci 15: 3952–3969, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1933.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci 32: 6391–6410, 2012. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1934.Zanassi P, Paolillo M, Montecucco A, Avvedimento EV, Schinelli S. Pharmacological and molecular evidence for dopamine D(1) receptor expression by striatal astrocytes in culture. J Neurosci Res 58: 544–552, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 1935.Zanotti S, Charles A. Extracellular calcium sensing by glial cells: low extracellular calcium induces intracellular calcium release and intercellular signaling. J Neurochem 69: 594–602, 1997. doi: 10.1046/j.1471-4159.1997.69020594.x. [DOI] [PubMed] [Google Scholar]
- 1936.Zawar C, Plant TD, Schirra C, Konnerth A, Neumcke B. Cell-type specific expression of ATP-sensitive potassium channels in the rat hippocampus. J Physiol 514: 327–341, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1937.Zayas-Santiago A, Agte S, Rivera Y, Benedikt J, Ulbricht E, Karl A, Dávila J, Savvinov A, Kucheryavykh Y, Inyushin M, Cubano LA, Pannicke T, Veh RW, Francke M, Verkhratsky A, Eaton MJ, Reichenbach A, Skatchkov SN. Unidirectional photoreceptor-to-Müller glia coupling and unique K+ channel expression in Caiman retina. PLoS One 9: e97155, 2014. doi: 10.1371/journal.pone.0097155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1938.Zeisel A, Muñoz-Manchado AB, Codeluppi S, Lönnerberg P, La Manno G, Juréus A, Marques S, Munguba H, He L, Betsholtz C, Rolny C, Castelo-Branco G, Hjerling-Leffler J, Linnarsson S. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347: 1138–1142, 2015. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 1939.Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature 383: 634–637, 1996. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]
- 1940.Zhang H, Verkman AS. Aquaporin-4 independent Kir4.1 K+ channel function in brain glial cells. Mol Cell Neurosci 37: 1–10, 2008. doi: 10.1016/j.mcn.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1941.Zhang J, Li Y, Chen ZG, Dang H, Ding JH, Fan Y, Hu G. Glia protein aquaporin-4 regulates aversive motivation of spatial memory in Morris water maze. CNS Neurosci Ther 19: 937–944, 2013. doi: 10.1111/cns.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1942.Zhang Q, Pangrsic T, Kreft M, Krzan M, Li N, Sul JY, Halassa M, Van Bockstaele E, Zorec R, Haydon PG. Fusion-related release of glutamate from astrocytes. J Biol Chem 279: 12724–12733, 2004. doi: 10.1074/jbc.M312845200. [DOI] [PubMed] [Google Scholar]
- 1943.Zhang S, Li B, Lovatt D, Xu J, Song D, Goldman SA, Nedergaard M, Hertz L, Peng L. 5-HT2B receptors are expressed on astrocytes from brain and in culture and are a chronic target for all five conventional ‘serotonin-specific reuptake inhibitors’. Neuron Glia Biol 6: 113–125, 2010. doi: 10.1017/S1740925X10000141. [DOI] [PubMed] [Google Scholar]
- 1944.Zhang XD, Morishima S, Ando-Akatsuka Y, Takahashi N, Nabekura T, Inoue H, Shimizu T, Okada Y. Expression of novel isoforms of the CIC-1 chloride channel in astrocytic glial cells in vitro. Glia 47: 46–57, 2004. doi: 10.1002/glia.20024. [DOI] [PubMed] [Google Scholar]
- 1945.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34: 11929–11947, 2014. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1946.Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, Duncan JA III, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MG, Barres BA. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89: 37–53, 2016. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1947.Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu XS, Duan S. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol 9: 945–953, 2007. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]
- 1948.Zhao L, Brinton RD. Suppression of proinflammatory cytokines interleukin-1β and tumor necrosis factor-α in astrocytes by a V1 vasopressin receptor agonist: a cAMP response element-binding protein-dependent mechanism. J Neurosci 24: 2226–2235, 2004. doi: 10.1523/JNEUROSCI.4922-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1949.Zhao L, Brinton RD. Vasopressin-induced cytoplasmic and nuclear calcium signaling in embryonic cortical astrocytes: dynamics of calcium and calcium-dependent kinase translocation. J Neurosci 23: 4228–4239, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1950.Zheng K, Bard L, Reynolds JP, King C, Jensen TP, Gourine AV, Rusakov DA. Time-resolved imaging reveals heterogeneous landscapes of nanomolar Ca2+ in neurons and astroglia. Neuron 88: 277–288, 2015. doi: 10.1016/j.neuron.2015.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1951.Zhong S, Du Y, Kiyoshi CM, Ma B, Alford CC, Wang Q, Yang Y, Liu X, Zhou M. Electrophysiological behavior of neonatal astrocytes in hippocampal stratum radiatum. Mol Brain 9: 34, 2016. doi: 10.1186/s13041-016-0213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1952.Zhou M, Kimelberg HK. Freshly isolated hippocampal CA1 astrocytes comprise two populations differing in glutamate transporter and AMPA receptor expression. J Neurosci 21: 7901–7908, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1953.Zhou M, Schools GP, Kimelberg HK. Development of GLAST(+) astrocytes and NG2(+) glia in rat hippocampus CA1: mature astrocytes are electrophysiologically passive. J Neurophysiol 95: 134–143, 2006. doi: 10.1152/jn.00570.2005. [DOI] [PubMed] [Google Scholar]
- 1954.Zhou M, Tanaka O, Suzuki M, Sekiguchi M, Takata K, Kawahara K, Abe H. Localization of pore-forming subunit of the ATP-sensitive K+-channel, Kir6.2, in rat brain neurons and glial cells. Brain Res Mol Brain Res 101: 23–32, 2002. doi: 10.1016/S0169-328X(02)00137-7. [DOI] [PubMed] [Google Scholar]
- 1955.Zhou M, Xu G, Xie M, Zhang X, Schools GP, Ma L, Kimelberg HK, Chen H. TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J Neurosci 29: 8551–8564, 2009. doi: 10.1523/JNEUROSCI.5784-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1956.Zhou Y, Danbolt NC. GABA and glutamate transporters in brain. Front Endocrinol (Lausanne) 4: 165, 2013. doi: 10.3389/fendo.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1957.Zhu H, Zhao Y, Wu H, Jiang N, Wang Z, Lin W, Jin J, Ji Y. Remarkable alterations of Nav1.6 in reactive astrogliosis during epileptogenesis. Sci Rep 6: 38108, 2016. doi: 10.1038/srep38108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1958.Zhu SQ, Kum W, Ho SK, Young JD, Cockram CS. Structure-function relationships of insulin receptor interactions in cultured mouse astrocytes. Brain Res 529: 329–332, 1990. doi: 10.1016/0006-8993(90)90846-4. [DOI] [PubMed] [Google Scholar]
- 1959.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 135: 145–157, 2008. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- 1960.Zhu X, Zuo H, Maher BJ, Serwanski DR, LoTurco JJ, Lu QR, Nishiyama A. Olig2-dependent developmental fate switch of NG2 cells. Development 139: 2299–2307, 2012. doi: 10.1242/dev.078873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1961.Zhu Y, Kimelberg HK. Developmental expression of metabotropic P2Y(1) and P2Y(2) receptors in freshly isolated astrocytes from rat hippocampus. J Neurochem 77: 530–541, 2001. doi: 10.1046/j.1471-4159.2001.00241.x. [DOI] [PubMed] [Google Scholar]
- 1962.Zhu Z, Reiser G. Signaling mechanism of protease activated receptor 1-induced proliferation of astrocytes: stabilization of hypoxia inducible factor-1α triggers glucose metabolism and accumulation of cyclin D1. Neurochem Int 79: 20–32, 2014. doi: 10.1016/j.neuint.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 1963.Zhuang Z, Huang J, Cepero ML, Liebl DJ. Eph signaling regulates gliotransmitter release. Commun Integr Biol 4: 223–226, 2011. doi: 10.4161/cib.4.2.14507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1964.Zhuo L, Sun B, Zhang CL, Fine A, Chiu SY, Messing A. Live astrocytes visualized by green fluorescent protein in transgenic mice. Dev Biol 187: 36–42, 1997. doi: 10.1006/dbio.1997.8601. [DOI] [PubMed] [Google Scholar]
- 1965.Ziak D, Chvátal A, Syková E. Glutamate-, kainate- and NMDA-evoked membrane currents in identified glial cells in rat spinal cord slice. Physiol Res 47: 365–375, 1998. [PubMed] [Google Scholar]
- 1966.Ziegler AB, Brüsselbach F, Hovemann BT. Activity and coexpression of Drosophila black with ebony in fly optic lobes reveals putative cooperative tasks in vision that evade electroretinographic detection. J Comp Neurol 521: 1207–1224, 2013. doi: 10.1002/cne.23247. [DOI] [PubMed] [Google Scholar]
- 1967.Zielińska M, Fresko I, Konopacka A, Felipo V, Albrecht J. Hyperammonemia inhibits the natriuretic peptide receptor 2 (NPR-2)-mediated cyclic GMP synthesis in the astrocytic compartment of rat cerebral cortex slices. Neurotoxicology 28: 1260–1263, 2007. doi: 10.1016/j.neuro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 1968.Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8: 437–502, 2012. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1969.Zmarowski A, Wu HQ, Brooks JM, Potter MC, Pellicciari R, Schwarcz R, Bruno JP. Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. Eur J Neurosci 29: 529–538, 2009. doi: 10.1111/j.1460-9568.2008.06594.x. [DOI] [PubMed] [Google Scholar]
- 1970.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 6: 43–50, 2003. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 1971.Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, Parpura V. Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN Neuro 4: e00080, 2012. doi: 10.1042/AN20110061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1972.Zuo Y, Lubischer JL, Kang H, Tian L, Mikesh M, Marks A, Scofield VL, Maika S, Newman C, Krieg P, Thompson WJ. Fluorescent proteins expressed in mouse transgenic lines mark subsets of glia, neurons, macrophages, and dendritic cells for vital examination. J Neurosci 24: 10999–11009, 2004. doi: 10.1523/JNEUROSCI.3934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1973.Zupan V, Hill JM, Brenneman DE, Gozes I, Fridkin M, Robberecht P, Evrard P, Gressens P. Involvement of pituitary adenylate cyclase-activating polypeptide II vasoactive intestinal peptide 2 receptor in mouse neocortical astrocytogenesis. J Neurochem 70: 2165–2173, 1998. doi: 10.1046/j.1471-4159.1998.70052165.x. [DOI] [PubMed] [Google Scholar]