Cation/H+ antiporters affect signaling, reproduction, and stress tolerance, indicating critical roles of pH and cation balance on trafficking in the endomembrane system.
Abstract
Plants remodel their cells through the dynamic endomembrane system. Intracellular pH is important for membrane trafficking, but the determinants of pH homeostasis are poorly defined in plants. Electrogenic proton (H+) pumps depend on counter-ion fluxes to establish transmembrane pH gradients at the plasma membrane and endomembranes. Vacuolar-type H+-ATPase-mediated acidification of the trans-Golgi network is crucial for secretion and membrane recycling. Pump and counter-ion fluxes are unlikely to fine-tune pH; rather, alkali cation/H+ antiporters, which can alter pH and/or cation homeostasis locally and transiently, are prime candidates. Plants have a large family of predicted cation/H+ exchangers (CHX) of obscure function, in addition to the well-studied K+(Na+)/H+ exchangers (NHX). Here, we review the regulation of cytosolic and vacuolar pH, highlighting the similarities and distinctions of NHX and CHX members. In planta, alkalinization of the trans-Golgi network or vacuole by NHXs promotes membrane trafficking, endocytosis, cell expansion, and growth. CHXs localize to endomembranes and/or the plasma membrane and contribute to male fertility, pollen tube guidance, pollen wall construction, stomatal opening, and, in soybean (Glycine max), tolerance to salt stress. Three-dimensional structural models and mutagenesis of Arabidopsis (Arabidopsis thaliana) genes have allowed us to infer that AtCHX17 and AtNHX1 share a global architecture and a translocation core like bacterial Na+/H+ antiporters. Yet, the presence of distinct residues suggests that some CHXs differ from NHXs in pH sensing and electrogenicity. How H+ pumps, counter-ion fluxes, and cation/H+ antiporters are linked with signaling and membrane trafficking to remodel membranes and cell walls awaits further investigation.
BACKGROUND
Multicellular and unicellular organisms have evolved mechanisms to regulate ion and pH homeostasis in response to developmental cues and to a changing environment. Such pH and ion homeostasis depends on the coordinated action of multiple transporters at the plasma membrane (PM) and in subcellular organelles. Together, proton (H+) and ion pumps, cotransporters, and channels determine ionic cellular distribution, which is critical for the maintenance of membrane potential, pH control, osmolality, transport of nutrients, and protein activity. A central theme has emerged in plant cell biology that cells respond and adapt to diverse cues through a dynamic endomembrane system (Adamowski and Friml, 2015; Gu et al., 2017). The cytoplasmic pH of mammalian or plant cells is regulated within narrow limits, usually at about pH 7 to 7.5. Remarkably, the luminal pH of the secretory system of mammalian, yeast, or plant cells is not homogenous but appears to be finely regulated, as they have steady-state pH values ranging from weakly acidic in cis-Golgi to acidic in lysosomes or vacuoles (Wu et al., 2001; Orij et al., 2011; Martinière et al., 2013; Shen et al., 2013). A specific pH value in separate organelles suggests that the luminal pH may be optimized for processes associated with each compartment. The endomembrane and endocytic system of eukaryote cells serves several major functions, including (1) to sort cargo (e.g. enzymes, transporters or receptors, and secreted factors) to specific destinations and establish cell polarity, (2) to modulate the protein and lipid composition of membrane domains through remodeling, and (3) to modify properties of the cell wall of plants or fungi through synthesis and remodeling. In spite of these vital functions, the molecular determinants of pH and cation homeostasis and their impact on plant biology are poorly understood.
Here, we briefly review cytosolic pH (pHcyt) and vacuolar pH (pHvac) regulation mediated by PM transporters and vacuolar/endomembrane transporters. The biophysical concepts and electrophysiological tools that laid the foundation for pH and ion homeostasis are especially crucial in order to understand how the molecular inventory of pumps, channels, and cotransporters is integrated in plant life. Although many transporter genes have been characterized in the last two to three decades, the molecular identities of additional determinants of pH have yet to be defined. In the second part, we focus on a superfamily of cation/H+ antiporters (CPA) that likely fine-tune pH and cation levels in diverse intracellular compartments. The views presented here are based on bioenergetic concepts and, in part, on functional studies using yeast and analyses of plant mutants. By highlighting the similarities and differences between two types of CPA (CPA1 and CPA2), this review provides new insights linking pH and cation homeostasis with the operation of the dynamic endomembrane system, which impinges on all aspects of plant life.
TRANSPORTERS INVOLVED IN pH HOMEOSTASIS INCLUDE H+ PUMPS, ION CHANNELS, AND K+ COTRANSPORTERS
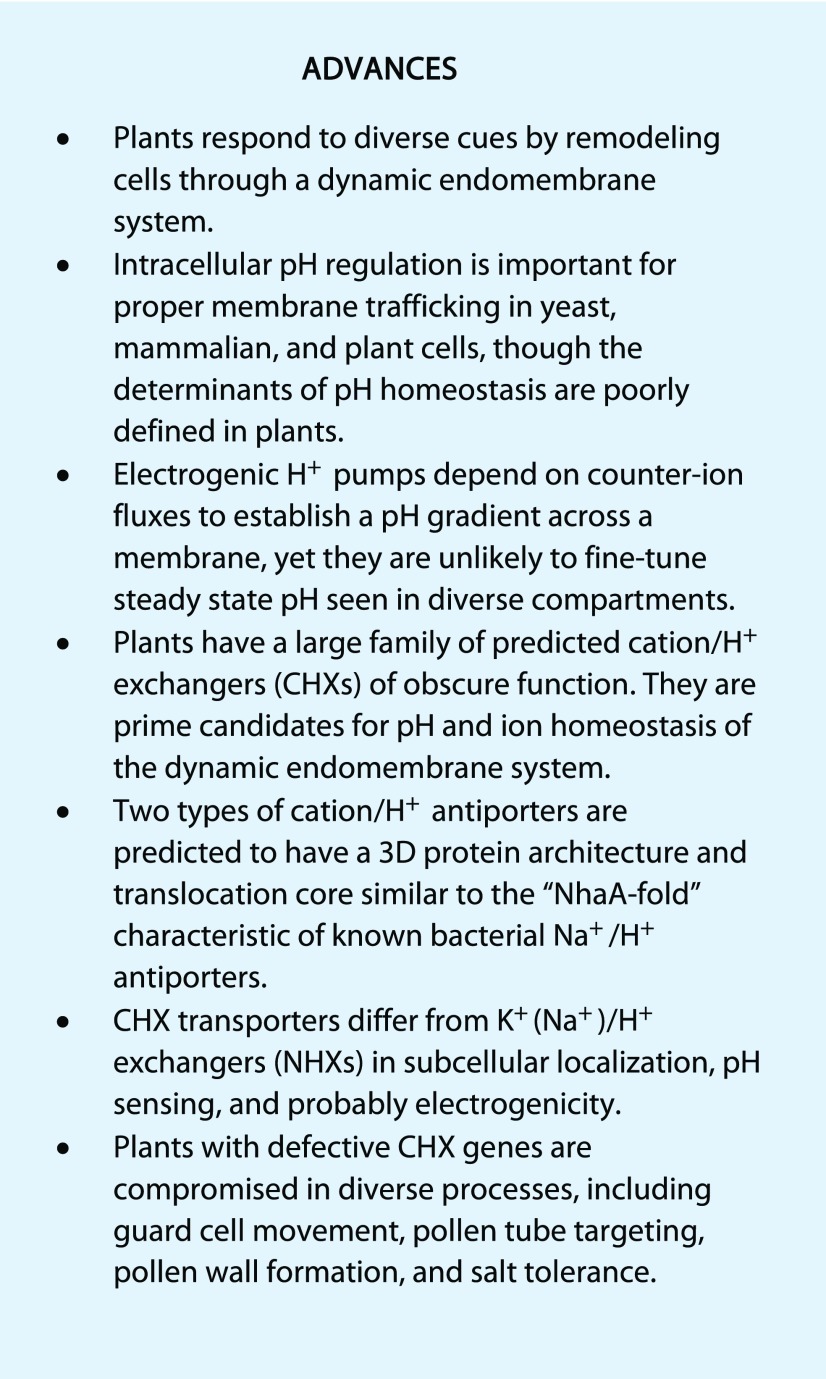
Fungal and algal cells have been used as powerful models to understand pH and membrane potential regulation in flowering plants. Both fungal and plant cells have two types of H+ pumps. The P-type ATPase at the PM pumps H+ to the external medium, and the V-type ATPase associated with vacuolar membranes or endomembranes acidifies intracellular compartments (Fig. 1). Plant cells have an additional H+-pumping PPase (AVP; Lin et al., 2012; Martinoia et al., 2012) that energizes the vacuole and endomembrane. Plant as well as fungal cells have a steep electrical gradient (Δψ) of about −150 to −250 mV (negative inside) and a pH gradient (ΔpH) of 1.5 to 2 (acidic outside) across the PM. Similarly, there is a ΔpH of 1.5 (acidic inside the lumen) across the vacuolar membrane and a small Δψ (+30 mV, positive inside). The H+ electrochemical gradient generated by H+ pumps provides energy to drive the transport of most ions and metabolites across membranes. A long-standing question has been how plant or fungal cells maintain the pHcyt at neutrality, ranging from pH 7.1 to 7.5, and an acidic vacuolar lumen.
Figure 1.
H+ pumps, ion channels, and cation/H+ antiporters contribute to pH and K+ homeostasis in a model plant (A) or yeast (B) cell. A, At left, a model plant cell. H+-ATPases (AHA and VHA) or PPase (AVP), depicted as wide arrows, pump H+ outside the cell or into the lumen. At the vacuolar membrane, a ClC-type transporter (green) mediates anion influx and a K+/H+ antiporter (NHX1/2) mediates H+ efflux for K+ uptake. Post-Golgi compartments or the trans-Golgi network (TGN) are sorting compartments of biosynthetic or secretory cargo destined for the PM or destined via prevacuolar compartment (PVC) to the vacuole. Proteins/cargo can be recycled via endocytosis in early endosomes (EE) or recycling vesicles (RE) and then sorted at the TGN or PVC. Cargo is marked for degradation transit via the PVC to the vacuole. The pH of the cytosol is 7.3, and other compartments are color coded: ER, pH 7.1 to 7.5; cis-Golgi to TGN, pH 6.8 to 6.3/6.1; PVC, pH 6.2 to 6.7; and vacuole, pH 5.2 to 6 (Martinière et al., 2013; Shen et al., 2013). At right, magnified TGN and PVC compartments. Acidification of the lumen by an electrogenic vacuolar H+-ATPase (AtVHA) and anion (A− or Cl−) influx via a ClCa-type antiporter can be alkalinized by a K+/H+ antiporter, such as AtNHX5/6 or AtCHX17 Box 1. ER, Endoplasmic reticulum; GA, Golgi apparatus; LE, late endosome; MVB, multivesicular body; Nuc, nucleus. H+ pumps, shown as wide arrows, include the following: VHA, V-H+-pumping ATPase (red); AHA, PM-H+-pumping ATPase (blue); and AVP, H+-pumping pyrophosphatase (purple). Antiporters, depicted as ovals, are SOS1 (PM Na/H+ exchanger); NHX, K(Na)/H+ exchanger (red purple); CHX17, K+/H+ exchanger (blue); and ClC, anion/H+ exchanger (green). CHX (light blue) members also are found at the ER and the PM. B, A model yeast cell. H+ extrusion by Pma1 (PM-H+-ATPase) generates an H+ electrochemical gradient that is modulated by K+ influx (Trk1/2) and efflux (Tok1) pathways and by the Nha1 Na+(K+)/H+ exchanger. The vacuole (Vac) is acidified by Vma, vacuolar-type H+-ATPase. Golgi or PVC (magnified at right) are acidified by the Vma H+ pump and by counter-ion flux of anion (A−) via Gef1. Compartment pH is alkalinized by Nhx1 in the PVC and by Kha1 in the Golgi. Ena1-4, PM Na+-pumping ATPase; Nha1, PM Na+(K+)/H+ antiporter; Trk1/2, K+ uptake transporter; Tok1, K+ efflux; Kha1, Golgi K+/H+ antiporter; Nhx1, K+(Na+)/H+ exchanger; and Gef1, anion channel like.
Interplay of ΔpH and Δψ across the PM
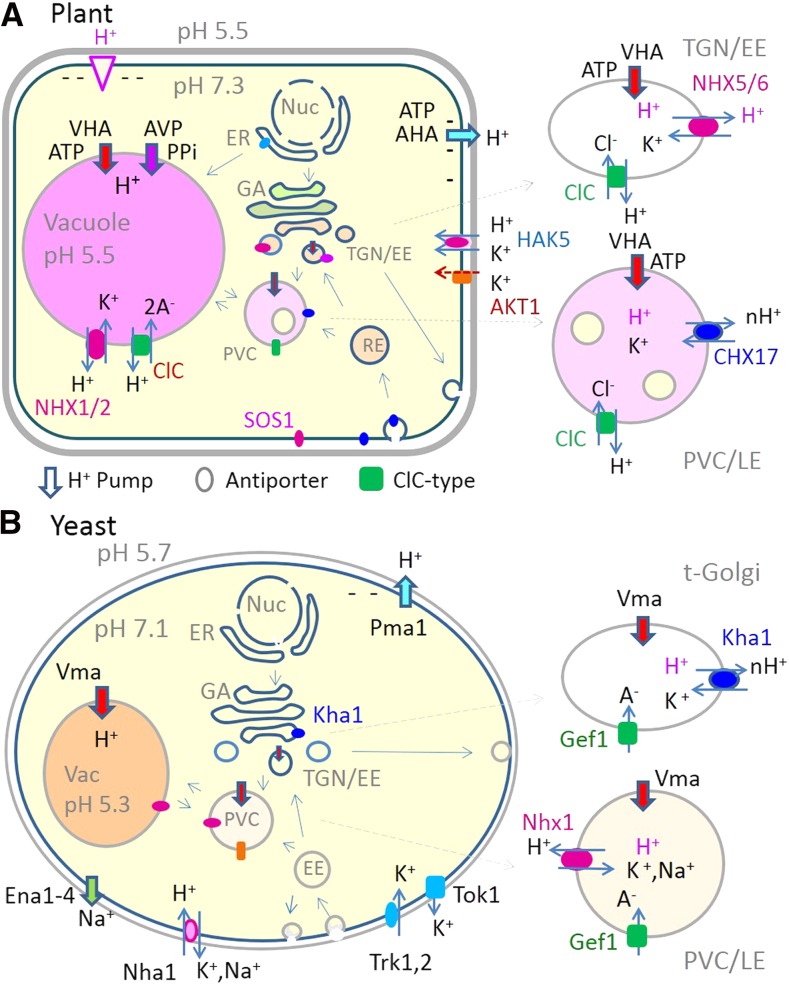
A few classic experiments demonstrate the role of the PM H+ pump and additional ion fluxes on pH regulation in Neurospora crassa. When acid stress is imposed, a decrease in pHcyt (pH 6.6) causes an increase in outward pumping of H+ (Sanders et al., 1981). H+ pumping has been detected as a transient hyperpolarization (−250 mV) using intracellular microelectrodes. Surprisingly, the hyperpolarization is followed by a sustained depolarization to a membrane potential of −155 mV. Current-voltage analysis based on a kinetic model shows that pump velocity (current) increases when pHcyt decreases, but pump velocity also increases as the cell depolarizes (Fig. 2A). Furthermore, pHcyt alkalinization depends on micromolar K+ provided to K+-starved cells (Blatt et al., 1987). Thus, depolarization is caused by another ion flux, later shown to be K+ inward movement in cotransport with H+ (Rodriguez-Navarro et al., 1986). The entry of 1K+ with 1H+ would electrically balance the extrusion of 2H+ per ATP hydrolyzed by PM ATPase. Figure 2A shows a diagram of how an N. crassa cell adjusts its pHcyt after acid stress.
Figure 2.
Cytosolic pH homeostasis depends on the PM H+ pump, K+/H+ cotransport, and K+ channels. A, Response to acid stress in N. crassa. a, When acid stress is imposed on a K+-starved cell, pHcyt decreases to ∼6.9, which causes an increase in outward pumping of H+. H+ pumping is detected as a transient hyperpolarization (−280 mV). b, Addition of 50 μm K+ causes a depolarization to −190 mV. Current-voltage analysis shows that pump velocity increases when the cell depolarizes. K+ inward movement in cotransport with H+ depolarizes the cell and alkalinizes pHcyt. c, At 1 mm Kext, voltage-dependent K+ entry via channel(s) further depolarizes the cell, and transporters together maintain pHcyt at 7.3. Pump (Pma1), symporter (HAK1), and uptake pathway (Trk) have been identified in N. crassa, and comparable transporters (parentheses) are confirmed in Arabidopsis (Fig. 1A). B, Alkaline stress in plants: a model. d, A plant cell in pH 7.5 medium has little or no pH gradient across the PM. H+ extrusion by the PM-ATPase generates an electrical gradient when the cell is K+ starved. e, When K+ext is low, the electrical gradient drives H+ uptake via an electrogenic K+/2H+ antiporter (e.g. CHX17). Net accumulation of H+ into the cytosol would acidify and maintain pHcyt near neutral. Such an antiporter would be activated or induced by basic pH and inactived at pH 7. K+ lost from the cell is taken up and recycled by the voltage-sensitive K+ channel (e.g. AtAKT1 complex). C, E. coli at alkaline pH. In the absence of a pH gradient, the H+ pump of the respiratory chain complexes generates a PMF of mostly Δψ. An Na+/2H+ electrogenic antiporter (NhaA) mediates H+ accumulation inside to maintain cytoplasmic neutrality (Padan et al., 2005).
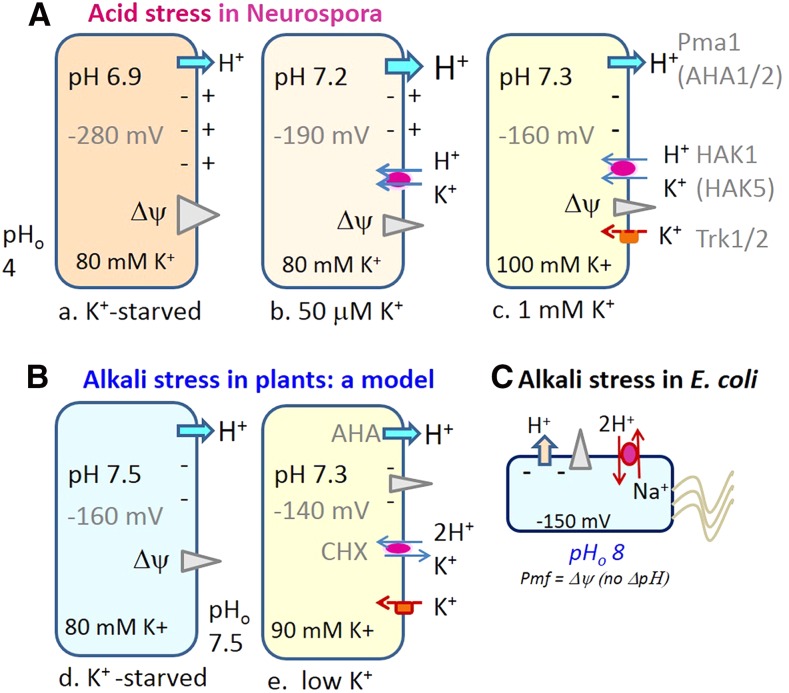
The example illustrates an important concept in intracellular pH regulation. An electrogenic H+ pump generates an H+ electrochemical gradient (or proton motive force [PMF]) with two interconvertible components, the Δψ and the ΔpH Box 1; Sze, 1985). A charge imbalance or Δψ is generated with only a minute difference in charge across a membrane (Rodríguez-Navarro, 2000). However, as emphasized before, the concentration of H+ cannot change unless the Δψ is lowered (Smith and Raven, 1979). Cytosolic acidification activates the PM H+ pump, although pump velocity depends on the Δψ. A steep Δψ antagonizes further H+ extrusion, whereas depolarization of the membrane potential by K+/H+ symport increases H+ pump velocity. Thus, an H+ extrusion pump alone is insufficient to generate and maintain a constant pHcyt. The example seen in N. crassa has been verified by physiological, molecular, and genetic studies in plant cells (Maathuis and Sanders, 1994; Walker et al., 1996; Rubio et al., 2008). In Arabidopsis (Arabidopsis thaliana) roots, K+ entry via a K+/H+ symporter (HAK5) is predominant when [K+]ext is very low (10–100 μm) and K+ entry mediated by K+ channel from the AKT1 complex dominates when [K+]ext ranges from 0.1 to 1 mm (Fig. 2A; Rubio et al., 2008; Nieves-Cordones et al., 2014). The Km of HAK5 is reported to be 10 to 30 μm K+, and the Km of the AKT1 complex is around 0.8 mm K+ (Gierth and Mäser, 2007). In-depth reviews on K+ transporters and their regulation are available (Gierth and Mäser, 2007; Wang and Wu, 2013; Véry et al., 2014). Thus, pH and Δψ homeostasis result from a collaboration mainly between H+ pumps, H+-coupled K+ transporters, and K+ channels at the PM.
Less is known about pH homeostasis after alkaline stress in plant cells, although studies using prokaryotes (Padan et al., 2005; Krulwich et al., 2011) and yeast (see below) provide valuable insights. Neutraliphiles are bacteria that can grow at external pH (pHext) ranging from about ∼5 to 9 and maintain a pHcyt of 7.5 to 7.7. A major strategy for bacterial pH homeostasis is the use of primary H+ pumps of the respiratory chain complexes, H+-coupled ATP synthase, and H+-coupled cation antiporters. At alkaline conditions, active inward transport of H+ is crucial for adaptation (Fig. 2C). Escherichia coli, a neutraliphile, pumps H+ out to generate a Δψ (negative inside) and a pH gradient (acid outside), except that the PMF is formed by the respiratory chain. Under alkaline conditions when pHext is 7.5, the transmembrane (TM) ΔpH would be negligible and the Δψ becomes relatively high (e.g. −150 mV). To avoid alkalinization of the cytosol, NhaA is induced in E. coli under alkaline stress. NhaA catalyzes the entry of 2H+ for 1Na+; thus, H+ enters the cell down the Δψ component of the PMF Box 1. Extruded Na is taken up into the cell to be recycled for additional H+ entry. H+ accumulation against its gradient restores cytosol pH to near neutrality (Padan et al., 2005).
Could plant cells respond in a similar manner under alkaline stress? In theory, a similar electrogenic cation/H+ antiporter, K+/2H+, could operate to maintain pHcyt near neutrality. For instance, a weak alkaline medium at pHext 7.5 eliminates any ΔpH across the PM. Assuming that the PM-ATPase continues to extrude H+, a Δψ is maintained even if pump activity is suboptimal at pHo 7.5. The Δψ is the major driving force for net active influx of H+, which could be achieved by electrogenic K+/2H+ exchange (Fig. 2B) and K+/H+ symport. K+ extruded by an antiporter is likely recycled by K+ uptake pathways, resulting in a net gain of H+ into the cell without a net loss of K+. Although there is no experimental evidence in plants yet, a K+/H+ antiport activity is inferred in alkali-stressed Chlorella fusca. The pHi remained constant, as a weak base loading up to 20 mm was accompanied by increased K+ efflux (Tromballa, 1987). These results suggest cation/H+ antiport at the PM.
ΔpH and Δψ across Endomembranes and Luminal pH Homeostasis
Unlike the nearly constant pH of the cytosol, pHvac varies depending on the cell type and function. In most cells, pHvac ranges from pH 4 to 6 and the TM ΔpH is 1.5 to 2.5 (Kurkdjian and Guern, 1989). Curiously, the luminal pH of many endomembrane organelles, such as the Golgi, TGN, and PVC, is weakly acidic, as determined by pH-sensitive fluorescent protein targeted to each organelle (Martinière et al., 2013; Shen et al., 2013). The pH in endoluminal compartments is largely determined by V-type H+-ATPase or H+-PPase with a similar principle to that described above Box 1, although the natures of the counter-ion pathways and cotransporters differ.
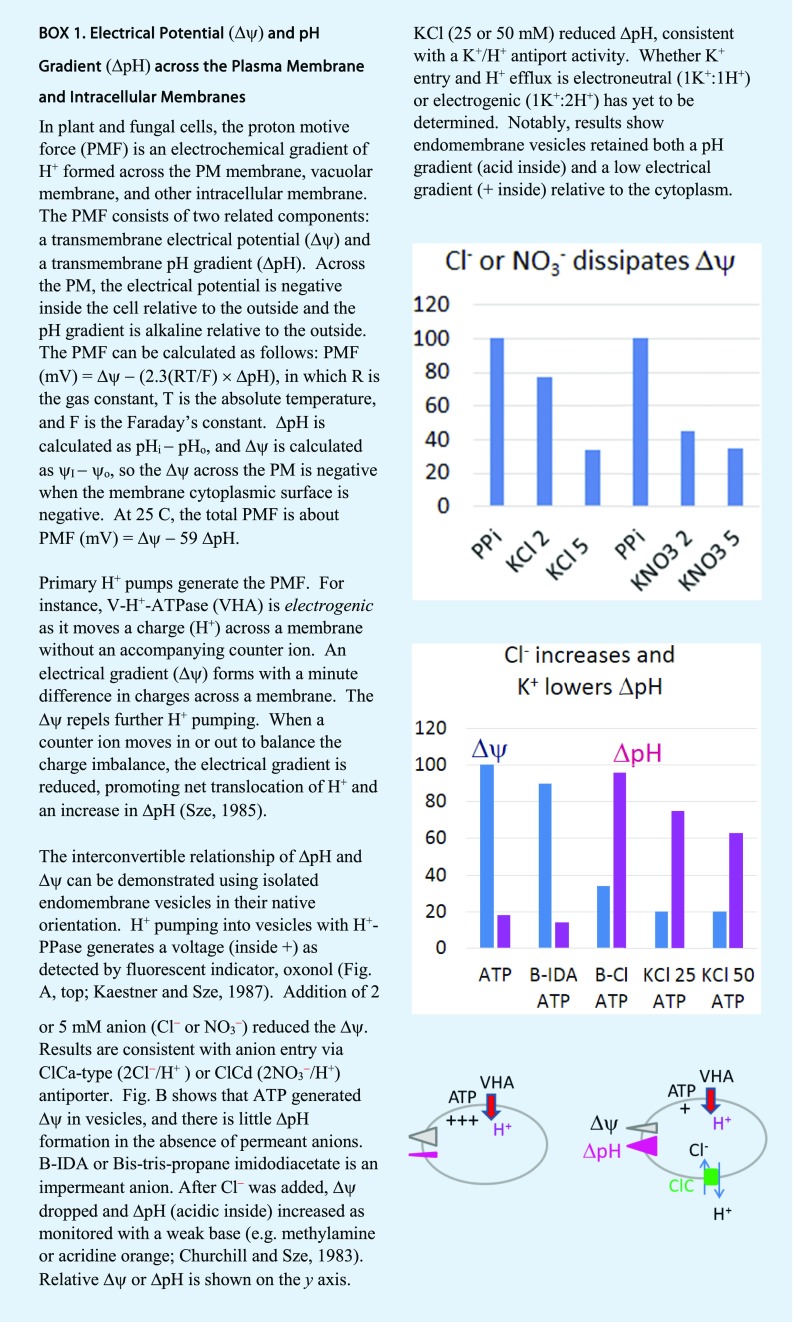
In plant intracellular membranes, the electrogenic V-ATPase (VHA) or PPase (AVP) pumps naked H+ into compartments, unaccompanied by other ions; thus, the pump generates an electrical gradient (positive inside). When anions enter the lumen, the Δψ drops and a pH gradient (acidic inside) develops Box 1; Churchill and Sze, 1983; Kaestner and Sze, 1987). In theory, the H+ pumps could generate a maximum ΔpH gradient of about 3 or an acidic lumen of pH 4, although that is rarely seen in endoluminal compartments. The steady-state pH ranges from neutral in the ER to pH 6.8 in cis-Golgi, pH 5.6 to 6.3 in TGN, pH 6.2 to 6.7 in PVC, and pH 5.2 to 6 in vacuoles (Martinière et al., 2013; Shen et al., 2013; Luo et al., 2015). H+ pumps (V-ATPase or H+-PPase) and the counter-ion flux establish a pH gradient, but they are unlikely to modulate the fine differences in steady-state pH (Casey et al., 2010) observed in the lumen. A steady-state pH is maintained when the rate of H+ pumping is balanced by the rate of H+ leak.
What are the molecular identities of H+ leak pathways in plant endomembranes? Although various H+-coupled transporters could serve this purpose (Schumacher, 2014), we suggest that K+-coupled H+ flux provides a key leak pathway for modulating pH and K+ homeostasis, as K+ is the most abundant and available ion in cells. The major contributors of acidic pH in the endomembrane system are attributed to the electrogenic V-ATPase or H+-PPase pumps and to counter-ion flux from anions (Fig. 1A; Schumacher, 2014). In isolated endomembrane vesicles, anion influx is likely mediated by ClC-type (e.g. 2NO3−/H+; De Angeli et al., 2006) antiporters, as chloride or nitrate is most effective in reducing Δψ and raising ΔpH Box 1. Although some ClCs are anion channels, endomembrane ClCs in animals and plants are electrogenic exchangers, 2A−/1H+ (Jentsch, 2008). Plant ClC-type antiporters have been localized to vacuoles (ClCa to ClCc or ClCg) and PVC as well as Golgi (ClCf) and TGNs (ClCd; von der Fecht-Bartenbach et al., 2007; Barbier-Brygoo et al., 2011). Vacuoles serve as a storage or recycling reservoir of many ions and metabolites, so the tonoplast contains multiple H+-coupled transporters with the ability to alkalinize vacuoles (Schumacher, 2014). However, endomembrane compartments (like PVC, Golgi, and TGN) specialized for other functions may be limited in H+-coupled transporters. Thus, K+ coupled to H+ flux provides the most likely leak pathway for modulating pH and K+ homeostasis in intracellular organelles. This idea has been proposed before (Pittman, 2012). Box 1 shows an example where KCl decreased ΔpH in endomembrane vesicles consistent with the presence of K+ influx in exchange for H+ export (Churchill and Sze, 1983, 1984).
In addition to pH homeostasis, cation/H+ antiporters could load K+ into endomembrane vesicles to support cation-dependent activities as seen in yeast (see below). It is known that plants deprived of K+ nutrition show reduced [K+] in vacuoles (Walker et al., 1996) and perhaps other endomembrane compartments. The effect of reduced endoluminal [K+] on the operation of the endomembrane system and on intracellular pH homeostasis is unclear.
In Vivo Roles of PM and Vacuolar H+ Pumps
Based on genetic experiments in yeast, the PM and vacuolar H+ pumps collaborate, yet how the two pumps sense and respond to pHcyt or signaling cues is not well understood. In Glc-deprived wild-type yeast, Glc stimulated cytosol alkalinization and vacuole acidification, consistent with Glc stimulation of ScPma1 (PM-H+-ATPase) and ScVma (V-H+-ATPase; Kane, 2016). In cells subjected to acid stress, vma mutants show a relatively acidic pHcyt compared with that of wild-type cells, indicating that Vma contributes to pHcyt homeostasis.
ScPma1 is an essential gene, as it is rate limiting for growth. Point mutations with lowered Pma1 activity reduced yeast growth in acidic medium (Serrano et al., 1986). In contrast, mutants of Vma subunits are able to grow between pHext 4 and 7 but fail to grow below pH 3 or above pH 7 (Orij et al., 2011; Kane, 2016). Conditional growth of vma mutants at pHo 5.5 (Nelson and Nelson, 1990) indicates that an acidic pHext in some way causes sufficient acidification of endomembrane compartments to support cell proliferation. However, at external alkaline pH, growth ceases unless the Vma H+ pump is active. These results clearly show that pHext influences pHvac or the pH of diverse endoluminal compartments/vesicles, although the nature of the communication between the PM and vacuolar membranes (e.g. endocytosis) is unclear. This example underscores the need to maintain an acidic pH in the lumen of the endomembrane system for cell proliferation.
In plants, the PMF generated by two specific PM H+-ATPases is essential for growth, although the essentiality of H+ pumps can be masked by the overlapping expression of multiple Arabidopsis AHA genes, which encode PM-autoinhibited H+-ATPases. AHA1 and AHA2 are highly expressed and make up 80% of all AHA transcripts. Double knockdown mutants (aha1 aha2) are embryo lethal under optimal growth conditions, yet the transmission of male or female gametes is unaffected (Haruta et al., 2010). Although single knockdown mutants (aha2) complete their life cycle, mutants secrete fewer H+. Moreover, growth is retarded in the aha2 mutant relative to the wild type when ΔpH (at pHo 8) or membrane potential is reduced (with 100 mm [K+]ext). Thus, the PMF generated by both AHA1 and AHA2 is essential for cell functions and embryo growth. Furthermore, the aha2 mutant shows an up-regulation of the K+ transporters HAK5 and CHX17, whereas reduced membrane potential suppresses their expression in the wild type or the aha2 mutant. Thus, genetic studies in plants indicate a close link between PMF and K+ fluxes at the PM, especially at low [K+]ext (Haruta and Sussman, 2012).
Determining the roles of H+ pumps in the endomembrane system has been challenging due to multiple pumps, V- and P-type H+-ATPases, and H+-PPases (Schumacher, 2014). The plant V-ATPase complex consists of over 10 different subunits, each encoded by one or up to five genes (Sze et al., 2002). Loss of the only VHA-A subunit gene in Arabidopsis results in male gametophytic lethality, indicating that the V-ATPase activity is essential for pollen development and cannot be replaced by H+-PPase (Dettmer et al., 2005). Another vha mutant, lacking subunit E1, shows defective embryo development. Localization to vacuoles and endosomes of VHA-E1 suggests a role in maintaining a functional secretory system (Strompen et al., 2005). To address the specific role of vacuolar membrane V-ATPase, a double mutant, vha-a2 vha-a3, has been analyzed. VHA-a2 and VHA-a3 are isoforms of a large integral subunit localized to the tonoplast. Although the double mutant is reduced in size and the pHvac is less acidic than in wild-type plants, the vha-a2 vha-a3 mutant is viable (Krebs et al., 2010). This finding suggested that the loss of V-ATPase function in vacuoles can be replaced partially by H+-PPase. These results also indicate that the V-ATPase complex with the remaining VHA-a1 subunit associates with TGN/EE, and the endomembrane system is actively involved in secretory and endocytic processes essential for growing and developing cells. Even mutants lacking both the vacuolar H+-ATPase and H+-PPase (AVP1) are conditionally viable and have acidic vacuoles. Furthermore, pHvac in the mutant is increased by concanamycin A, an inhibitor of V-ATPase at the TGN. Together, these results indicate that TGN/EE V-ATPase contributes to vacuole acidification (Kriegel et al., 2015). Furthermore, a weak V-ATPase mutant, det3, has a TGN/EE lumen pH of 6.7 instead of 5.6, delayed endocytosis of a PM receptor, BRASSINOSTEROID INSENSITIVE1, and a reduced cellulose synthase A (CesA) complex at the PM (Luo et al., 2015). Thus, V-ATPase is essential for growth, and pump-mediated acidification of TGN/EE is crucial for the secretion of an enzyme required for wall synthesis and for membrane recycling of a brassinosteroid receptor.
Surprisingly, a P-type H+-ATPase acidifies vacuoles of petunia (Petunia hybrida) flowers. A ph5 mutant defective in a gene encoding a P-type ATPase has altered petal color that depends on anthocyanin and pHvac. PH5 localizes to the tonoplast and is an H+ pump homologous to AHA10 of Arabidopsis (Verweij et al., 2008). Intriguingly, PH5 activity is enhanced by a partner protein, PH1, which resembles a P-type ATPase but lacks H+ pump activity due to the loss of a conserved Asp (Eisenach et al., 2014). Coexpression of PH5 and PH1 forms an H+ pump that restores the red flower color and acidifies pHvac of a mutant to 5.3 instead of greater than 6 (Faraco et al., 2014). Patch-clamp experiments of petunia leaf vacuoles demonstrated that the ATP-dependent outward current is partially sensitive to an inhibitor of V-ATPase and largely sensitive to vanadate, a specific inhibitor of P-type ATPases. If PH5/PH1 has a coupling ratio of 1H+/ATP, then it can generate a steeper pH gradient than V-ATPase, which has a coupling ratio of 2H+/ATP (Eisenach et al., 2014). Thus, pHvac can be hyperacidified by the collaboration of different H+ pumps in plant cells with particular functions.
CPA: A MAJOR H+ LEAK PATHWAY?
Transporters that exchange cation flux for H+ transport in the opposite direction hold the potential to modulate pH, electrical, and cation balance locally (Fig. 1A, right). Higher plant genomes predict a superfamily of monovalent CPAs (Chanroj et al., 2012) that are separated into two families. The CPA1 subfamily includes Arabidopsis (At) NHX1 to NHX6, SOS1/NHX7, and NHX8 and yeast ScNhx1 and ScNha1 (Chanroj et al., 2012; Table II). The CPA2 family includes yeast ScKha1, 28 AtCHX (predicted cation/H+ exchanger), and six AtKEA (K+-efflux antiporter) isoforms. Why do plants need so many CPAs? What do they do in planta?
Table II. Functions of CHX and NHX in Arabidopsis and other plants.
In or It, Morning glory; Pp, Physcomitrella patens. Reference groups are as follows: (1) Yokoi et al. (2002); Venema et al. (2002); Leidi et al. (2010); Bassil et al. (2011b); Barragán et al. (2012); Andrés et al. (2014). (2) Venema et al. (2003); Bassil et al. (2011a). (3) Wu et al. (1996); Shi et al. (2002). (4) An et al. (2007). (5) Fukada-Tanaka et al. (2000); Yamaguchi et al. (2001); Yoshida et al. (2005). (6) Zhao et al. (2008, 2015. (7) Chanroj (2011); Chanroj et al. (2013). (8) Chanroj et al. (2011, 2013,Padmanaban et al. (2017). (9) Chen et al. (2016). (10) Padmanaban et al. (2007). (11a) Guan et al. (2014). (11b) Qi et al. (2014). (12) Mottaleb et al. (2013). (13) Lu et al. (2011); Evans et al. (2012).
| Plant Transporter | Tissue Expression | Membrane Location | Transport Mode | Main Function or Mutant Phenotype | References |
|---|---|---|---|---|---|
| NHX | |||||
| AtNHX1, NHX2 | Ubiquitous | Tonoplast | K+(Na)/H+ | K+ uptake into vacuolar compartments for cell expansion; vacuole biogenesis; vacuole alkalinization; guard cell movement | 1 |
| AtNHX5, NHX6, LeNHX2 | Ubiquitous | Golgi, TGN | K+(Na)/H+ | pH homeostasis in TGN/PVC affects cargo sorting to vacuole and vacuole biogenesis | 2 |
| AtSOS1/NHX7 | Root, stem, and leaf xylem | PM | Na+/H+ | sos1 mutant is hypersensitive to Na+ and Li+, cannot grow on low K+; extrudes Na+ out of the cell | 3 |
| AtNHX8 | Ubiquitous | PM | Li+/H+ | nhx8 mutant is hypersensitive to Li; extrudes Li+ out of the cell. | 4 |
| InNHX1 or ItNHX1 | Flower petal | Tonoplast | K+(Na)/H+ | Vacuole alkalinization turns morning glory petals blue | 5 |
| CHX | |||||
| AtCHX13 | Pollen, root tip | PM | K+ | chx13 mutant is sensitive to K+ deficiency | 6 |
| AtCHX14 | Pollen, root, shoot vascular | PM | K+ efflux | Overexpressing plant is sensitive to low K+ | |
| AtCHX16 | Root | ER, PVC, PM | K+ | chx16 chx17 chx18 chx19 mutant shows lower seed set; poor plant growth on hydroponic medium | 7 |
| AtCHX17 | Root, pollen | PVC, PM | K+/nH+ (predicted from core residues) K+ (Rb) | Triple chx17 chx18 chx19 mutant shows disorganized pollen wall; reduced male fertility; impaired fertilization, reduced seed set | 8 |
| AtCHX18 | Hydathode, vascular, sperm | PVC, PM | |||
| AtCHX19 | Pollen | PVC, PM | |||
| OsCHX14 | Flower | Endomembrane, ER | K+ (Rb) | Jasmonic acid (JA) signaling and flower opening; K+, Rb transport in oocyte | 9 |
| AtCHX20 | Guard cell, root cap | Reticulate, ER | K+ | Participates in stomatal opening, guard cell movement | 10 |
| GmSALT3 | Root, vascular | ER | Confers salt tolerance in wild soybean | 11a | |
| Gm/GsCHX1 | K+ | 11b | |||
| PpCHX1,2 | Protonema | Punctate, PM | K+ efflux? | No phenotype yet | 12 |
| AtCHX23 (CHX21) | Pollen, tube | ER | K+ | Involved in pollen tube targeting of ovule | 13 |
Less is known about CPA2 than CPA1 activity. Vacuolar-localized AtNHX1 catalyzes K+/H+ as well as Na+/H+ exchange with low affinity using purified AtNHX1 reconstituted in a proteoliposome (Venema et al., 2002) or AtNHX1 expressed in yeast vacuoles (Hernández et al., 2009). Similarly, tomato (Solanum lycopersicum) LeNHX2, an ortholog of Golgi/TGN-localized AtNHX5/6, mediates K+/H+ exchange in proteoliposomes (Venema et al., 2003). PM-associated AtNHX7/SOS1 and NHX8 mediate Na+(Li+)/H+ exchange (Chanroj et al., 2012; Table II). AtCHXs studied so far have been localized to the ER, endosomes, or PM (Table II), although the mode of transport of any plant CHX or yeast Kha1 has not been demonstrated.
Plant CHX17 and NHX1 Share a Conserved Transport Core But Differ in pH Dependence and Perhaps Electrogenicity
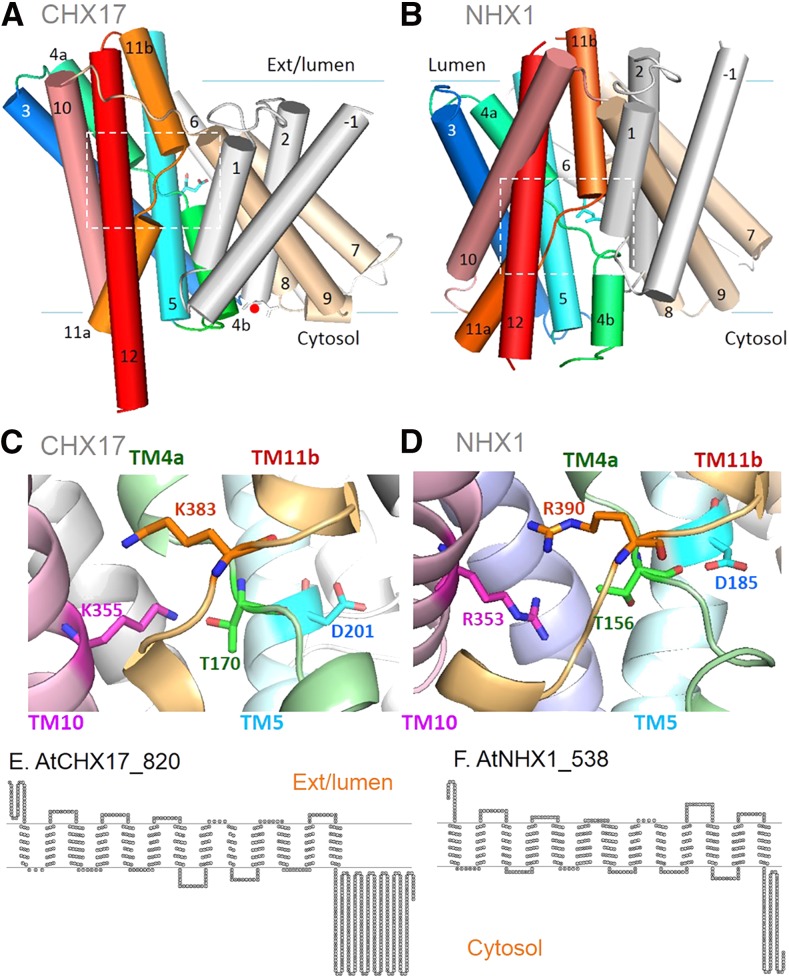
If both NHX and CHX are predicted as cation/H+ antiporters, based on the Pfam0999 domain of 12 to 13 TM helices, what is the molecular basis for the separation of CPA1 from CPA2? The availability of crystal structures from bacterial transporters allows one to model the protein architecture of poorly characterized transporters and gain insights to their mode of transport through model-based mutagenesis.
Using prediction tools like I-Tasser (Yang et al., 2015) or PHYRE2 (Kelley et al., 2015), the first 3D models of plant PeNHX3 or AtCHX17 (Wang et al., 2014; Czerny et al., 2016; Fig. 3, A and B) have been generated with high confidence based on the structures of bacterial Na+/H+ antiporters. The four best templates are E. coli NhaA (Padan, 2014), Methanocaldococcus jannichii NhaP (Paulino et al., 2014), Pyrococcus abysinnia NhaP (Wöhlert et al., 2014), and Thermus thermophilus NapA (Lee et al., 2013; Coincon et al., 2016). Although a 3D model is not a substitute for a structure determined experimentally, the quality of the first 3D model of AtCHX17 based on TtNapA (pdb 4bwz) is sufficiently high to identify critical core residues within a global architecture.
Figure 3.
AtCHX17 and AtNHX1 share similar protein architecture and transport core. A and B, NhaA protein fold. AtCHX17 (A) and AtNHX1 (B) models are predicted to have an NhaA fold based on Na+/H+ antiporter structures, TtNapA (pdb 5bz3) and MjNhaP (pdb 4czb), respectively. TM α-helices are shown as cylinders with the external/lumen face at the top. The dimerization domain is shown in gray and beige. The transport domain of six helices is shown in color. Helices TM4 (green) and TM11 (orange) cross over at the unwound regions in the middle of the membrane (boxes), and TM5 (cyan) and TM12 (red) helices face one another across the intersection. Two additional helices are TM3 (blue) and TM10 (salmon). Conserved Asp, D201 (A) or D185 (B), is shown as a stick (cyan) protruding from the TM5 helix. A Glu (red circle) at the cytosolic face of the TM2 helix is conserved in CHX17 (E111) and other CPA2, but not in NHX members. C and D, Core residues critical for activity. The regions located in the boxes in A and B are magnified, and TM α-helices are represented as ribbons. TM1 and TM12 are hidden for clarity. C, AtCHX17. Residues corresponding to N200 (TM5), D201 (TM5), K355 (TM10), and T170 and K383 (at unwound helices of TM4 and TM11) are critical for activity, as shown by mutagenesis (Czerny et al., 2016). (TM helix colors correspond to A and B.) D, AtNHX1. Residues equivalent to N184, D185* (TM5), and R353* (TM10) are critical in poplar NHX3 (Wang et al., 2014). T156 and R390 (in the unwound region of TM4 and TM11) are predicted to be core residues. N200 (CHX17) and N184 (NHX1) are not shown. Lys K355 in CHX17 is replaced by Arg R353 in NHX1. E and F, AtCHX17 and AtNHX1 show a similar transport domain (residues 1–440) but have distinct hydrophilic tails of unknown function. Topology was predicted by TOPO2 (http://www.sacs.ucsf.edu/cgi-bin/open-topo2.py) based on TMHMM2, with slight modification according to the PHYRE2 model.
The 3D model of AtCHX17 is predicted have a unique structure like crystallized TtNapA or EcNhaA Na+/H+ antiporters. The NhaA fold (Padan, 2014) refers to a unique architecture, unlike that of other secondary active transporters (Forrest et al., 2011). This fold consists of two TM helices, TM4 and TM11, that cross over at the unwound region in the middle of the membrane (box in Fig. 3A). In the center of the AtCHX17 model, the discontinuous regions of TM4 and TM11 intersect in close proximity to an Asn (N200) and an Asp (D201), both of which are conserved in TM5 of nearly all eukaryote CPA members. Mutagenesis of N200, D201, and residues (T170 and K383) in the unwound helices eliminates or reduces CHX17 activity (Fig. 3C) monitored as yeast tolerance to hygromycin B (HygB; Czerny et al., 2016). These results support the idea that AtCHX17 has a transport core similar to bacterial CHXs (Padan, 2014). D201 of AtCHX17 is postulated to bind and translocate alternatively an H+ and a K+ (or Na+) in the transport cycle of an antiporter
Similarly, plant NHX1 is predicted to have an NhaA fold as in MjNhaP (Fig. 3B), and several residues needed for AtCHX17 activity are conserved in AtNHX1. These include Asn N184 and Asp D185 in the TM5 helix, a basic residue R353 in the TM10 helix, T156 in the unwound helix of TM4, and R390 within the broken helix TM11 of AtNHX1 (Fig. 3D; H. Sze, unpublished observation). Residues N187 and D188 in TM5 of poplar (Populus euphratica) NHX3 are essential for activity (Wang et al., 2014). Thus, the transport domains of CHX17 and NHX1 appear highly conserved to monovalent cation/H+ antiporters (Lee et al., 2013; Paulino et al., 2014). AtNHX1 or a tomato ortholog (LeNHX2) of AtNHX5/6 has been verified previously by reconstitution in liposomes to mediate K+(Na+)/H+ exchange (Venema et al., 2002, 2003). Although the transport mode of CHX17 has not been verified, modeling and mutagenesis of conserved core residues are consistent with it being a cation/H+ antiporter (Fig. 3, C and D). For simplicity in this review, we consider CHX protein as a K+/H+ antiporter, although alternative modes are not eliminated.
Notably, several residues conserved in CPA2 members are absent from NHXs. First, a Glu at the cytosolic end of the TM2 helix is conserved in nine AtCHXs and in fungal or bacterial CPA2 members (Czerny et al., 2016). E111 in AtCHX17 is located at the rim of the funnel that provides access of the substrate to the transport core (D201) at the center of the protein (Fig. 3A, red circle). The mutation E111C inhibits CHX17 activity, suggesting that residues in this region likely contribute to pH sensing, as shown in EcNhaA (Krulwich et al., 2011). Protonation or deprotonation of titratable side chains facing the cytosol may cause conformational changes. Second, a Lys (K355) needed for activity in AtCHX17 is absolutely conserved in all 28 CHX sequences from Arabidopsis (Sze et al., 2004), six AtKEA, yeast Kha1, and bacterial CPA2 members (Chanroj et al., 2012; Czerny et al., 2016). In contrast, AtNHX1 shows an Arg (R353) that is strictly conserved in CPA1 members (Fig. 3, C and D).
The significance of a switch from R to K in TM10 was not understood until recently. Amazingly, TtNapA with K305 (wild type) or K305H, but not K305R, mediated electrogenic transport, possibly as Na+/2H+ exchange (Uzdavinys et al., 2017). K305 and D157 of TtNapA, which are conserved as K355 and D201 in AtCHX17, are thought to be two dominant H+ carriers. These findings indicate that plant CHXs and NHXs share similar transport modes but differ in their activation by pH, as shown in yeast (see below). Furthermore, CHXs may exchange K+ for H+ electrogenically, whereas plant NHXs are electroneutral. Future mutagenesis combined with direct transport assays of CHXs and NHXs are needed to determine the mode of transport, electrogenicity, and residues in the pH sensor/regulatory region.
Insights from Yeast: Plant NHX1 and CHX17 Play Differential Roles in pH and Cation Homeostasis and Affect Membrane Trafficking
Budding yeast (Saccharomyces cerevisiae) is an excellent model in which to decipher the roles of plant CPA genes, as yeast has only one ScNhx1 and one ScKha1 gene. Although the role of ScKha1 is not well understood, ScNhx1 and ScKha1 are functional orthologs of AtNHX1 and AtCHX17, respectively (Table I). Yeast mutants with loss of function in either ScNhx1 or ScKha1 (Fig. 1B) and additional PM cation-handling transporters are sensitive to pHext and/or extreme alkali cation levels, indicating that K+/H+ exchangers and K+ and Na+ transporters play roles in pH and cation homeostasis. Table I summarizes key findings: (1) plant NHX1 or ScNhx1 confers yeast tolerance to high (0.5 m) K+ or Na+ at acidic pHext, whereas AtCHX17 and ScKha1 are incompetent; (2) AtCHX17 or ScKha1 confers tolerance to growth on alkaline medium with low (8 mm) K+ levels, although AtNHX1 is ineffective; and (3) both AtNHX1 and AtCHX17 confer resistance to HygB at pH 5.6, although AtNHX1 is more effective than AtCHX17. In addition, yeast mutants containing a wild-type ScNhx1 but lacking ScKha1 and PM K+ transporters, trk1 trk2 and tok1, fail to grow on medium containing low K+, although growth is restored with AtCHX17 or ScKha1 alone. These findings clearly underscore different roles of AtCHX17 and AtNHX1 in a unicellular model. Notably, both participate in pH homeostasis: AtNHX1 restores growth at acidic pH, whereas AtCHX17 is effective at alkaline pH. These results are mimicked by yeast ScNhx1 and ScKha1, which promote growth best at pH 5 (Nass et al., 1997) and at weak alkaline pH (Wu et al., 2016), respectively.
Table I. Plant NHX and CHX members show distinct roles in yeast growth.
Plant (At for Arabidopsis and Le for tomato) or yeast (Sc for S. cerevisiae) genes or vector only (None) were expressed in various mutants that were unable to grow under limiting conditions I to IV. The yeast strain is shown in parentheses. Growth was scored as none (−), no or low (−/+), low (+), medium (++), or high (+++). Blank spaces, Not determined. Localization refers to GFP-tagged protein in yeast cells only. Results are taken from Chanroj et al. (2011) unless indicated otherwise in the footnotes. Note that, in experiment I, AtNHX activity was assayed using the AXT3 (ena1-4, nha1, nhx1) strain (Yokoi et al., 2002) or with nhx1 (Gaxiola et al., 1999). CHX activity was tested in the KTA40-2 strain at pH 5. High K+/Na+ varied from 50 to 400 mm. The KTA40-2 (ena1-4, nha1, nhx1, kha1) strain was used to test conditions II and IV (Chanroj et al., 2011). LMM04 (ena1-4, nha1, kha1, trk1,2, tok1) was used to test condition III. LMB01 (ena1-4, nha1, kha1) has wild-type ScNhx1. (See Fig. 1B legend for gene names.)
| Transporter Gene | Localization | Relative Yeast Growth | |||
|---|---|---|---|---|---|
| I. High K+/Na+, pH ∼ 5 | II. Low K+ at pH 7.5 | III. Low K+ at pH 7.5 | IV. HygB at pH 5.6 | ||
| Plant (At or Le) or yeast (Sc) | In yeast only | (AXT3) | (KTA40-2) | (LMM04) | (KTA40-2) |
| None | − | −/+ | −/+ | − | |
| AtNHX1 and NHX2a | Vacuole, endosome | +++ | + | − | +++ |
| AtNHX5, NHX6, and LeNHX2b | Endomembrane, post-Golgi | ++ | +++b | ||
| ScNhx1 | PVC, endosomef | +++c (KTA40-2) | − (LMB01) | +++ (LMB01) | |
| AtCHX20d | Golgi, ERESe | − | ++ | −/+ | − |
| AtCHX17, AtCHX18, and AtCHX19 | Golgi | − | +++ | +++ | + |
| ScKha1 | Golgie | − (AXT3) | +++ (AXT3) | ++ (AXT3) | |
References are as follows: Gaxiola et al. (1999); Quintero et al. (2000); Yokoi et al. (2002).
References are as follows: Yokoi et al. (2002), AtNHX5; Venema et al. (2003), LeNhx2.
Brett et al. (2005) tested the wild type and nhx1 with 0.6 m KCl at pH 4.
AtCHX20-GFP fluorescence in yeast resembled ER exit site (ERES) markers. Fluorescence patterns of CHX17-GFP and ScKHA1-GFP in yeast were similar (Chanroj et al., 2011; Wu et al., 2016).
The conditional growth phenotypes, such as tolerance to alkaline pH at low K+ and resistance to hygromycin, are challenging to interpret. Thus, we review studies that probe the genetic bases of these phenotypes in yeast to provide a cellular framework for the roles of cation/H+ antiporters. Collectively, these reports link intracellular cation and pH balance to membrane trafficking, protein/cargo sorting, and vacuole biogenesis.
Tolerance to Alkaline pH in Yeast Is Associated with Vacuolar Biogenesis and Protein Sorting
To reveal processes that confer tolerance of wild-type yeast to alkaline pH, a genome-wide screen of mutants has identified genes involved in (1) H+ pumping by the V-ATPase complex, (2) vacuole biogenesis and protein sorting, (3) Fe and Cu metal homeostasis, and (4) cell polarity and cell wall organization and biogenesis (Serrano et al., 2004). These results indicate that the acidification of endosomal and vacuole compartments by the V-ATPase, as well as processes in membrane trafficking and vacuole biogenesis, are critical for cell proliferation. One vacuolar protein-sorting (Vps) gene is Vps44 or ScNhx1, which encodes an Na+/H+ antiporter at the PVC (Bowers et al., 2000; Bowers and Stevens, 2005). The vps44 mutants secrete 35% of a vacuole-destined carboxypeptidase Y (CPY) and missort markers that associate with PVC, Golgi, or vacuolar membrane in wild-type cells. This result provides the first evidence that Na+(K+)/H+ exchange-mediated ion balance is critical for endosomal function and protein sorting in a eukaryote cell.
pHvac of an nhx1 mutant (pH 4–4.7) is more acidic than that of the wild type (pH 5.3), consistent with the idea that NHX1 catalyzes K+ uptake and H+ efflux from compartments acidified by the vacuolar H+-pumping ATPase (Brett et al., 2005). These results suggest that proper pH homeostasis in LE/PVC is critical for the sorting of proteins, like CPY, to the vacuole. In one model, CPY binds to the CPY receptor (Vps10p) in the late Golgi, and after the complex moves to the LE/MVB, a change in pH causes dissociation of the ligand, and the empty receptor is returned to the late Golgi. As a weak base can alleviate the growth inhibition in the nhx1 mutant under low-pH stress and restore endosomal trafficking, Brett et al. (2005) concluded that the pH, rather than K+ homeostasis, is critical for cargo trafficking out of the endosome (PVC).
Curiously, yeast carrying mutations in Vps genes also are hypersensitive to HygB (Wagner et al., 2006; Banuelos et al., 2010). Sensitivity to HygB of the nhx1 mutant is rescued by wild-type AtNHX1 (Table I). Similarly, AtCHX17 confers HygB tolerance to yeast mutants lacking several cation/H+ transporters and also reduces the missorting of vacuolar CPY to the exterior (Chanroj et al., 2011). The reduction in CPY secretion by CHX17 is more effective when pHext is weakly alkaline, whereas AtNHX1 is more effective at acidic pHext. In yeast, ScNhx1 and ScKha1 are localized to the PVC and Golgi (Fig. 1B), respectively. Thus, like yeast orthologs, plant CHX17 and NHX1 influence endosomal trafficking in a differential manner.
HygB Sensitivity Is Related to Impaired Budding and/or Fusion in Membrane Trafficking
A long-standing question is, How is HygB resistance related to pH and ion balance in yeast endomembranes? HygB inhibits eukaryote protein translation at greater than 100 μg mL−1. Below 50 μg mL−1 HygB, wild-type yeast is resistant, although the growth of vacuolar trafficking mutants is sensitive (Banuelos et al., 2010; Cyert and Philpott, 2013). Gentamycin, a related aminoglycoside, inhibits growth drastically, especially in yeast mutants lacking Nhx1, Vps16, Vps51 to Vps54, Vps41, or Sac1 (Wagner et al., 2006). Labeled gentamycin is taken up into kidney cells by endocytosis and then delivered by retrograde traffic to the lysosome (Sandoval and Molitoris, 2004). Intriguingly, a high level of gentamycin also is found in yeast vacuoles of gentamycin-sensitive mutants, although a lower level of gentamycin is seen in wild-type yeast. Many luminal and membrane vacuolar proteins are synthesized at the ER, transit through the Golgi, and are delivered from the TGN to the vacuole via vesicular transport. Very low levels (10 μg mL−1) of HygB disrupt vacuole morphology, localization of an LE marker (Pep12), and vacuole localization of Tor1p in mutants, like sac1, vps1, vps34, vps52, and vps54 (Ejzykowicz et al., 2017). The Vps gene products affected are involved in either budding or fusion events at the Golgi and LE interface. These results indicate that sensitivity to low levels of HygB is due to defects at the interface of the trans-Golgi and LE and is independent of translation inhibition. Thus, these findings from mammalian and yeast cells indicate that ion and pH homeostasis as well as the fusion of endosomes with vacuoles or with the trans-Golgi are critical for drug detoxification.
A working idea is that pH and ion balance in endosomes (e.g. PVC or TGN) results in proper vacuole biogenesis and cargo sorting, leading to inactivation of the aminoglycoside in the vacuole. Sensitivity results when endocytosed toxin is either not delivered into an appropriate compartment and/or not inactivated, so the toxin leaks into the cytosol, perhaps to perturb membrane microdomains involved in fusion or fission events (Ejzykowicz et al., 2017). If so, resistance to HygB conferred by yeast or plant NHX1 or by AtCHX17 supports the idea that active cation/H+ transport and pH balance in particular endomembrane compartments (PVC or LE) are paramount to the operation of retrograde and anterograde trafficking and vacuole biogenesis.
Is K+ Cycling Needed at the PM and Endomembrane?
Another challenging result is the rescue of yeast mutants lacking Kha1 and PM cation transporters (Ena1-4, Nha1, Trk1,2, and Tok1) to grow at low K+ and alkaline pH by ScKha1 or AtCHX17 (Fig. 1B; Table I). Yet, 58 mm [K+]ext alone restores growth in these mutants (Chanroj et al., 2011), suggesting that high [K+]ext compensates for CHX17 or ScKha1 function. A simple interpretation is that AtCHX17 promotes K+ acquisition, although the lack of 86Rb (K+) uptake into yeast expressing AtCHX17 does not support this idea (Chanroj, 2011). Perhaps CHX17 promotes K+ uptake and efflux in mutants lacking other PM K+ transporters, as K+ needs to be continually taken up and extruded to maintain an electrical potential across the PM (Rodríguez-Navarro, 2000). Interestingly, yeast expressing rice (Oryza sativa) OsCHX14 (ortholog of AtCHX17) also show tolerance to low K+ at alkaline pHext. Yet, OsCHX14 enhances Rb+ content in frog oocytes (Chen et al., 2016), supporting a role in K+ acquisition. Two possible scenarios are as follows: (1) AtCHX17-associated vesicles traffic among Golgi and PM and facilitate K+ cycling into and out of yeast when [K+]ext is low; and (2) CHX17 actively fills particular compartments (Golgi) with sufficient K+ required for K+-dependent processes. When [K+]ext is high, perhaps K+ uptake and loss occur via nonspecific cation pathways or recycling vesicles in the absence of Kha1.
Compartment [K+] Affects Metal Homeostasis in Yeast
Although the bases of metal homeostasis within a eukaryotic cell are incompletely understood, intracellular compartments are involved in maintaining a balance between supply and demand. For example, yeast or plant vacuoles contain metal transporters that load and unload metals (Cyert and Philpott, 2013; Blaby-Haas and Merchant, 2014). In addition, the post-Golgi compartment is a storage site of Mn (Li et al., 2008) and Cu, which are assembled to form PM- or apoplast-metalloproteins. Cu(I) taken up by a PM Cu transporter, Ctr1, in yeast can be sequestered into a post-Golgi compartment via a Cu-pumping ATPase, CCC2. Luminal Cu then binds to Cu-binding enzymes, such as the apoferroxidase Fet3p. The mature enzyme is then sorted to the PM to oxidize external ferrous to ferric iron, which is taken up by the iron transporter Ftr1 (Cyert and Philpott, 2013).
Several genes involved in metal homeostasis are important for yeast survival on alkaline medium (Serrano et al., 2004). Recently Sckha1 was identified in a screen for mutants unable to grow on medium depleted of Cu (Wu et al., 2016). The kha1 mutant had reduced Fe levels and reduced Fet3 ferroxidase activity, although Cu levels were unchanged. ApoFet3 becomes active after it binds Cu, only in the presence of high K+. These results indicate that Kha1 loads K+ into the Golgi and high [K+] promotes Cu binding to apoFet3 to form the active ferroxidase. Ferroxidase then forms a heterodimer with the iron transporter, Ftr1, and the complex is sorted to the PM to mediate Fe uptake (Singh et al., 2006). K+ is apparently not bound to the enzyme but is thought to form an ion atmosphere via electrostatic interactions to optimize Cu binding around the ferroxidase. These results illustrate a role of subcellular compartmentalized K+ in Fet3 maturation. Although no pH changes were detected in the cytosol or Golgi of kha1 mutants, it is possible that a PMF indirectly promotes Fe uptake (Davis-Kaplan et al., 2004) in part to drive K+ into the lumen via Kha1.
Cu binding to apoproteins in post-Golgi compartments is likely conserved in plants. Cu is transported into and out of endomembrane compartments through AtHMA5, a Cu pump, and other Cu transporters (Blaby-Haas and Merchant, 2014; Printz et al., 2016). Plants possess multicopper oxidases, like extracellular laccases (Turlapati et al., 2011) and apoplastic ascorbate oxidases. Whether transporters, like AtCHX17, maintain a suitable pH and cation balance for Cu binding to apoproteins in plants is not yet known.
Environmental Stress Affects Cell Wall Properties in Yeast
Alkaline pH has long been thought to cause cell wall damage in S. cerevisiae, as the transcription profile induced by alkaline pH stress overlaps with that seen after cell wall damage (Serrano et al., 2006). The wall gives the cell osmotic integrity and defines cell shape during growth, budding, mating, and sporulation. The wall consists of a load-bearing polysaccharide of β-1,3-glucan, chitin, and β-1,6-glucan, which cross-links wall components. Some wall proteins are glycosylphosphatidylinositol modified and linked to β-1,6-glucan, whereas other proteins are connected by alkali-sensitive linkages to β-1,3-glucan. Moreover, the wall status is thought to be monitored by cell surface proteins, like the wall sensor Wsc1 (Levin, 2011). Thus, the wall is dynamic, as its composition and organization are altered by environmental stresses and signals regulating the cell cycle (Klis et al., 2006; Orlean, 2012).
In a genome-wide screen for cell wall-related phenotypes, kha1 is one of 145 mutants sensitive to agents that perturb cell walls. Compared with wild-type cells, the kha1 mutants secrete more β-1,3-glucan, a cell wall mannoprotein, Cwp1, and a covalently linked cell wall glycoprotein, Ssr1 or CCW14 (de Groot et al., 2001). Although the molecular basis has not been determined, the results indicate that the Golgi K+/H+ antiporter, Kha1, has some role in the modification and/or delivery of wall components in yeast.
Summary
In yeast, endosomal ScNhx1 is important for maintaining pHcyt and pHvac balance (Brett et al., 2005), and luminal pH of PVC/LE alkalinized by Nhx1 confers HygB resistance and is critical for membrane trafficking, protein sorting, and vacuole biogenesis. Like yeast Kha1, AtCHX17 also confers HygB resistance to mutants and reduces missorting of the vacuolar protein CPY, supporting the idea that cation and pH balance in particular endomembrane compartments is paramount to the operation of retrograde and anterograde trafficking. Distinct roles of ScNhx1 and ScKha1 may result in part from differential localization at endosome/PVC and Golgi, respectively (Table I). In yeast, plant NHX1 and CHX17 perform distinct, yet cooperative, roles similar to ScNhx1 and ScKha1, respectively. Whether CHX17-mediated pH and [K+] in compartments influence membrane trafficking, metal homeostasis, or cell wall remodeling in plants (Wolf et al., 2012), as Kha1 does in yeast, has yet to be determined.
DISTINCT FUNCTIONS OF NHX, CHX, AND KEA IN PLANTA
NHX functions have been reviewed previously (Bassil and Blumwald, 2014), so major points are summarized here (Table II). Although only a fraction of AtCHX or AtKEA genes have been characterized, the emerging picture indicates that CPA2 function is integrated with multiple aspects of plant biology, including signaling and development.
Endomembrane AtNHX Loads K+ in the Lumen and Alkalinizes Compartment pH
Vacuolar AtNHX1/2
The first indication that plant vacuoles take up Na+ in exchange for downhill H+ efflux was based on Na+ dissipation of a pH gradient (acidic inside) generated artificially in isolated vacuoles (Blumwald and Poole, 1985). The molecular identities of cation/H+ antiport were verified later in yeast. The heterologous expression of AtNHX1 or AtNHX2 confers tolerance to high Na+ and high K+ (Table I) in yeast nhx1 mutants. Furthermore, purified AtNHX1 reconstituted in proteoliposomes shows specificity for K+ and Na+ followed by Cs+ and Li+. Thus, AtNHX1 has been established as a K+(Na+)/H+ antiporter (Venema et al., 2002). Plants overexpressing AtNHX1 have higher vacuolar [K+] content than Na+ even under moderate Na stress (Leidi et al., 2010). Thus, the ability to retain more intracellular K+ than Na+ helps plants withstand stress from Na+ shock.
AtNHX1 and AtNHX2 share redundant or overlapping roles in vivo, as single mutants, Atnhx1 and Atnhx2, show weak or no change in plant growth relative to the wild type. AtNHX2 and AtNHX1 share similar protein sequences, tissue expression, membrane localization, and transport activities (Yokoi et al., 2002; Barragán et al., 2012). A double nhx1 nhx2 mutant shows reduced growth and small cell size as well as reduced K+(Na+)/H+ exchange in isolated tonoplast vesicles (Bassil et al., 2011b; Barragán et al., 2012). The vacuolar K+ level is lower, and the lumen is more acidic at pH 5.8 instead of pH 6.2 in the wild type (Bassil et al., 2011b). Similarly, pHvac in guard cells is more acidic at pH 5.5 in nhx1 nhx2 mutants than at pH 5.9 of wild-type plants under light (Andrés et al., 2014). Together, these results support the idea that NHX1 and NHX2 regulate intravacuolar [K+] and pH by catalyzing K+ influx into the lumen coupled to H+ efflux. Moreover, high vacuolar K+ content confers tolerance to salt or osmotic stress in Arabidopsis. The antiporters are important in cells where cell expansion depends on an increase in vacuole volume as ions and water enter.
These results extend a previous study in which InNHX1-mediated alkalinization of vacuoles (pH ∼ 7) turned morning glory (Ipomoea nil) petals blue, whereas an Innhx1 mutant had purple petals and acidic (pH ∼ 6.4) vacuoles (Yamaguchi et al., 2001). Localized to vacuoles of petals, ItNHX1 protein was enriched in open morning glory (Ipomoea tricolor) flowers (Yoshida et al., 2005).
Endosomal NHX5/NHX6
Based on homology to tomato NHX2 (Venema et al., 2003), AtNHX5 or AtNHX6 likely mediates K+/H+ exchange. They are localized to mobile, punctate spots corresponding to Golgi/TGN and endosomes (Bassil et al., 2011a). Single mutants are unchanged from the wild type, but nhx5 nhx6 plants are dwarfed and have reduced cell size. The endocytosis of FM4-64 is not altered, although its trafficking to the vacuole is delayed (Bassil et al., 2011a). Furthermore, protein sorting to the vacuole is disrupted, as a vacuole-destined CPY is secreted, or storage proteins are sorted to the apoplast. The endosomal pH of nhx5 nhx6 mutants is decreased by 0.3, which could reduce the interaction of a vacuolar sorting receptor with its cargo (Reguera et al., 2015). These results support the idea that altered pH homeostasis in TGN/PVC impaired cargo sorting and trafficking to the vacuole and compromised vacuole biogenesis and function, causing reduced growth. Thus, the cellular role of AtNHX5 or AtNHX6 is similar to that of the yeast ScNhx1p, which is needed for proper vacuolar protein sorting from the endosome (LE/PVC) to the vacuole (Bowers et al., 2000; Brett et al., 2005).
Diverse Roles of Plant CHX Transporters: Signaling, Development, and More
The in planta roles of CHX genes based largely on mutant analyses are intriguingly diverse and seem disconnected (Table II). The cellular and molecular bases are largely unknown at present, although a unifying theme is emerging (see below).
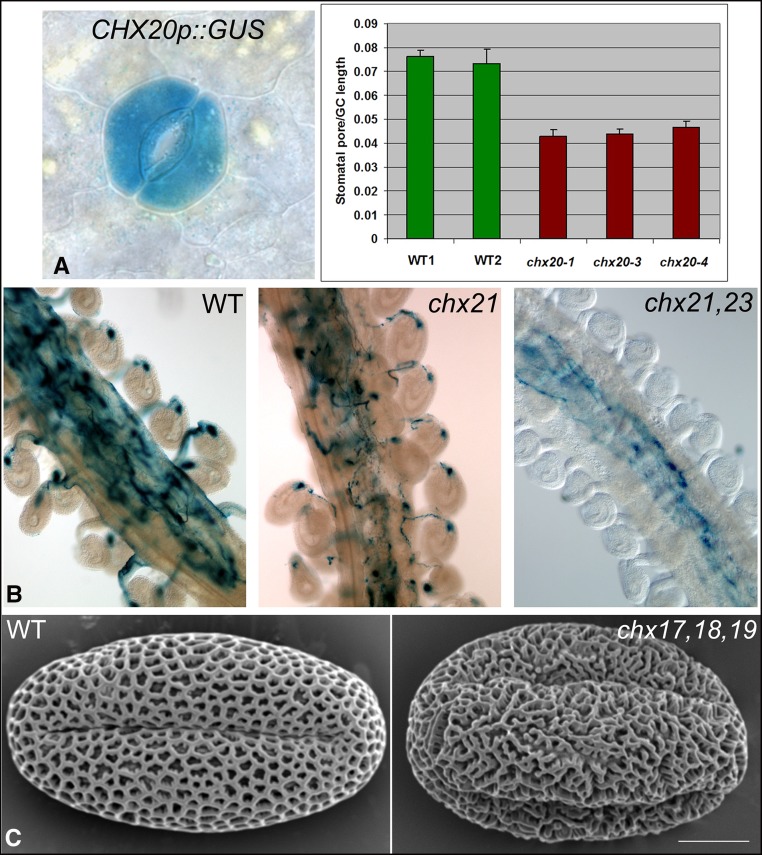
Guard Cell Movement
Only one Arabidopsis CHX gene is expressed preferentially in guard cells. Single chx20 mutants show no obvious morphological or growth differences, although three independent alleles of the mutant are impaired by 35% in light-induced opening of the stomatal aperture (Padmanaban et al., 2007; Fig. 4A). AtCHX20 localizes to perinuclear and reticulate endomembranes, resembling the ER of guard cells. As all inward and outward K+ channels and K+ cotransporters are functional (Jezek and Blatt, 2017), impaired guard cell movement is independent of K+ availability as an osmoticum. Assuming that AtNHX1/2 and AtNHX5/6 associate with the tonoplast and that the TGN is functional in guard cells of the chx20 mutant, these results indicate that K+ loading into vacuoles to increase cell turgor and cell expansion is not limiting (Bassil et al., 2011b; Andrés et al., 2014). Thus, CHX20 occupies a distinct functional niche.
Figure 4.
Phenotypes of chx mutants in Arabidopsis. A, Guard cell movement. Promoter::GUS shows CHX20 expression in guard cells (left). Light-dependent stomatal aperture is reduced in three alleles of chx20 mutants (right) compared with the wild type (WT). Aperture size is expressed as a ratio of maximal aperture size per length of guard cell (GC) pair. Twenty apertures were measured per treatment. Error bars indicate se (Padmanaban et al., 2007). B, Pollen tubes target ovules. GUS-expressing (blue) pollen tubes of the wild type or the single chx21 mutant grow in the transmitting tract of pistils and target ovules on either side. Blue dots inside ovules indicate pollen tube burst and release of contents. Tubes of double chx21 chx23 mutants grow in the transmitting tract but fail to target ovules (Lu et al., 2011). C, Wall construction. The reticulate pattern of wild-type pollen grains is disorganized and spongy in the chx17 chx18 chx19 triple mutant (Padmanaban et al., 2017). Bar = 5 μm.
The shape and size of vacuoles undergo dramatic conversion, from multiple small compartments in guard cells of closed stomata to a few large vacuoles in open stomata (Meckel et al., 2004; Gao et al., 2005). 3D reconstruction in wild-type guard cells shows that one or a few vacuoles of open stomata look swollen and form a continuous structure, whereas vacuoles of closed stomata appear shrunken and convoluted (Andrés et al., 2014). Like chx20, nhx1 nhx2 double mutants fail to open stomata fully in response to light. Imaging shows that mutant vacuoles appear small and separate, suggesting that the fusion of compartments to form large vacuoles is defective. Whether changes in intraluminal pH and/or cation homeostasis compromise membrane fusion has yet to be established. What is clear is that cation/H+ antiport by NHX1/2 provides an osmoticum and an H+ leak to sustain vacuolar dynamics.
Less clear is the role of ER-associated CHX20. Whether CHX20 is involved in vacuolar biogenesis from part of the ER independent of the post-Golgi (Marty, 1999; Viotti et al., 2013) or in cell wall pectin-mediated mechanics of guard cells (Amsbury et al., 2016) is considered. Intriguingly, AtCHX20-GFP signal occurs at the border of two guard cell poles (Padmanaban et al., 2007), where wall stiffening is proposed to affect the degree of stomatal opening (Carter et al., 2017).
Adaptation to Stress: K+ Starvation or High Salt
In plants, CHX17 expression in roots is induced by salt stress, K+ starvation, acidic pHext, and abscisic acid treatment (Cellier et al., 2004), suggesting a role in the adaption to stress. Although single chx17 mutants show no obvious morphological differences, mutants contain less K+ in roots than the wild type when K+ is depleted. These results suggest that CHX17 has a role in K+ acquisition and K+ homeostasis in plants (Cellier et al., 2004) as in yeast, where the expression of AtCHX17, AtCHX18, or AtCHX19 in K+-transport mutants restores growth on low-K+ medium (Chanroj et al., 2011). Whether CHX17 contributes to K+ uptake and its distribution directly or indirectly (via endocytosis and membrane trafficking) awaits experimentation.
Intriguingly, mapping of a quantitative trait locus related to salt tolerance in a wild soybean (Glycine max) uncovered a CHX gene similar to AtCHX20 (Guan et al., 2014). GmSALT3 (salt-tolerant-associated gene on chromosome3) is expressed in vascular tissues of soybean roots, and the protein colocalizes with an ER marker. The Na+ content of the salt-tolerant haplotype is reduced in stems and in leaves compared with that of salt-sensitive cultivars. A salt-sensitive cultivar contains an insertion mutation that truncates the transcript of GmSALT3. Analyses of over 200 haplotypes, including landraces and wild soybeans, support the idea that the haplotype H1 (GmSALT3) is associated with salt tolerance and is the ancestral allele. Although its transport activity has yet to be determined, the results suggest that GmSALT3 reduces the movement of root Na+ into shoots and leaves and provide the first genetic evidence that a CHX gene promotes salt tolerance in plants. GmSALT3 (Glyma03g32900) is identical to wild soybean Gm/GsCHX1 (Glysoja01g005509 in W05), which conferred salt tolerance in tobacco (Nicotiana tabacum) BY2 cells or in hairy roots of salt-sensitive C08 soybean (Qi et al., 2014). The basis of GmSALT3/CHX1 in conferring salt tolerance in soybean plants has yet to be determined.
JA Signaling and Floret Closing in Rice
A homolog of AtCHX17 in rice might mediate K+ homeostasis in lodicules that control floret closing. A JA signaling mutant shows delayed floret closing, increases [K+] in the lodicules, and enhances OsCHX14 expression (Chen et al., 2016). In yeast, OsCHX14 confers weak tolerance to alkaline pH at low K+ and also to 0.5 m K+, suggesting a role in K+ homeostasis. In oocytes, OsCHX14 enhances Rb+ contents, indicating that it mediates K+ transport. OsCHX14 localizes to the ER in protoplasts, yet its association with other endomembranes also is considered. Although the bases of OsCHX14 action remain obscure, it participates in JA signaling in programmed cell death at the lodicules, resulting in floret closure.
Male Fertility, Pollen Wall Formation, and Embryogenesis
Three CHXs (AtCHX17, AtCHX18, and AtCHX19) share similarities in protein sequence, localization to the PVC and PM in plants (Table II), and activities in yeast (Table I). Single or double mutants appear normal; however, the triple chx17 chx18 chx19 mutant (Chanroj et al., 2013) shows 50% reduced seed set. In addition, the highly reticulate pattern of walls is disorganized in mutant pollen grains (Padmanaban et al., 2017; Fig. 4C). As walls begin to form in the haploid microspore after meiosis when AtCHX17 is expressed (Bock et al., 2006), we propose that the scaffold of inner walls formed in chx17 chx18 chx19 mutant microspores is defective, causing deformation of the outer wall (exine).
Moreover, mutant pollen tubes grow, target, and enter ovules, although many targeted ovules fail to develop into seeds. Live-cell imaging suggests that failed fertilization could be due to impaired function of pollen tube and/or sperm (Padmanaban et al., 2017), where AtCHX19 and AtCHX18, respectively, are expressed. We reason that a disorganized pollen grain wall alone is unlikely to cause reduced fertility, although defects in cargo sorting and secretion that alter the vacuole, PM, and cell walls would likely hamper fertilization. Successful fertilization depends on multiple steps, including pollen tube burst at the right time and place and fusion of the released sperms with the egg and the central cell (Dresselhaus et al., 2016; Ge et al., 2017). All these events depend on signaling that leads to rapid remodeling of the PM and walls (Gu et al., 2017). In cases when fertilization is successful, a delay in embryo development is observed in the chx17 chx18 chx19 mutant, suggesting that CHX17 and CHX18 expression in micropylar endosperm has a crucial role in embryogenesis (Belmonte et al., 2013; Padmanaban et al., 2017).
Pollen Tube Signaling and Navigation
The molecular framework of pollen tube sensing and navigation in response to female signals is emerging (Higashiyama and Takeuchi, 2015), although many questions remain. After pollen grains land on a receptive stigma, pollen tubes grow toward ovules in response to secreted cues from the embryo sac (female gametophyte). An arabinogalactan-derived factor, AMOR, from ovular sporophytic tissues enables Torenia fournieri pollen tubes to sense guidance cues (Mizukami et al., 2016). Primed tubes are then attracted to ovules by LURE peptides, secreted by synergid cells. LUREs are sensed by receptor-like kinases at the PM of pollen tubes (Takeuchi and Higashiyama, 2016; Wang et al., 2016). How the receptors transduce the signals to bring about a shift in directional growth is mostly unknown. Curiously, two K+ transporters, AtCHX21 and AtCHX23, are essential for pollen tube targeting to ovules.
Male fertility is severely impaired in chx21 chx23 double mutants but not in single chx21 and chx23 mutants. Double mutant pollen grains germinate, and pollen tubes grow through the entire length of the transmitting tract. Oddly, chx21 chx23 mutant tubes fail to turn toward the ovules and do not enter the micropyle (Lu et al., 2011; Fig. 4B). CHX23 can mediate K+ transport, as shown by its ability to restore the growth of E. coli lacking three K+ uptake systems (Trk, Kup, and Kdp). The fluorescent protein-tagged CHX23 was localized at reticulate membranes and with ER markers in transfected pollen tubes. The possibility that CHX23-associated ER affects endosome or PM dynamics and function (Bayer et al., 2017; Stefano and Brandizzi, 2018) is considered. One model is that CHX21- and CHX23-mediated cation and pH balance is critical for signal reception or transduction, which includes trafficking via the cytoskeleton and processes that lead to a shift in directional tube growth (Lu et al., 2011).
Two studies show different results from Lu et al. (2011). One concludes that reduced seed set in plants homozygous for chx21−/− and heterozygous for chx23−/+ is due to defects in female (Evans et al., 2012) rather than male transmission. Another study localizes AtCHX23 to the chloroplast (Song et al., 2004) and should be viewed with caution (Lu et al., 2011).
Other: A Potential Protein-Interacting Domain at the C-Tail of CHX
The function of the long hydrophilic tail in CHX proteins (Fig. 3E) remains a mystery. Most NHX members have a hydrophilic tail of ∼100 residues (Fig. 3F), except for the PM AtNHX8 and AtNHX7/SOS1, which have tails of ∼300 and 680 residues, respectively. Little to no sequence similarity is observed between tails of CHXs and those of AtNHX7 and NHX8. The AtSOS1 C-terminal tail has a regulatory role, including an autoinhibitory domain that is deactivated by the phosphorylation of Ser-1136 (Quintero et al., 2011). In contrast, truncation of the C tail does not affect AtCHX17 activity, although its sorting and localization in plant cells are altered (Chanroj et al., 2013). Structural modeling of the tail domain predicts an architecture similar to that of bacteria universal stress proteins (Czerny et al., 2016), a potential protein interaction domain. Experiments are needed to determine the roles of plant CHX C tails and to identify interacting partners that may participate in membrane trafficking, protein activity, and/or signal transduction.
Additional AtCHX genes are developmentally expressed in the male gametophyte (Bock et al., 2006), although their roles are mostly unexplored. Not all AtCHXs can be expressed functionally in yeast mutants so far. Yet all CHXs expressed in E. coli defective in three K+ uptake systems (Trk, Kup, and Kdp) restore growth on low K+ (Chanroj et al., 2011; Mottaleb et al., 2013). Whether K+ transport into E. coli is relevant in eukaryotes has been questioned, as mutations at core residues that eliminate CHX17 activity in yeast fail to inhibit CHX17-dependent growth in the E. coli mutant (Czerny et al., 2016).
AtKEA1 to AtKEA3 Mediate pH, Cation, or Osmotic Homeostasis in Plastids
Three of six Arabidopsis AtKEAs function in the plastid. All KEA proteins possess a conserved Pfam 00999 domain characteristic of a CHX, although only clade 1 possesses a KTN domain at the C terminus (Chanroj et al., 2012). AtKEA3 is targeted to the thylakoid membrane and likely modulates pH homeostasis in the lumen and stroma, as single kea3 mutants show increased ΔpH (Kunz et al., 2014). AtKEA1 and a close homolog, AtKEA2, are targeted to the inner envelop of chloroplasts. A kea1 kea2 double mutant has stunted growth, pale green leaves, and swollen chloroplasts with reduced thylakoids (Kunz et al., 2014). Although reduced PMF and photosynthetic efficiency could compromise growth in kea1 kea2 mutants, AtKEA1 and AtKEA2 are localized at the two poles of dividing plastids early in leaf development, before chloroplast differentiation (Aranda-Sicilia et al., 2016). Furthermore, double mutants contain 30% fewer chloroplasts per cell. Thus, AtKEA1 and AtKEA2 might alter the pH, ionic, or osmotic environment locally to influence plastid fission and thylakoid formation from the inner envelope during chloroplast development. Similarly, a rice ortholog of AtKEA1 affects chloroplast development, as mutants show albino midribs and altered chloroplast ultrastructure (Sheng et al., 2014).
AtKEA2 is thought to exchange K+ or Na+ for H+ based on the rescue of the yeast Δnhx1 mutant by an N-terminal truncated AtKEA2 with an intact antiport domain (Aranda-Sicilia et al., 2012). When reconstituted in proteoliposomes, monovalent cations reduce an imposed pH gradient (acidic inside), indicating that AtKEA2 mediated cation/H+ exchange with a preference for K+ over Na+. The 3D model of AtKEA2 has an NhaA fold, and its transport core shares identical and similar residues with that of AtCHX17 (Czerny et al., 2016). As ΔpH across the thylakoid membrane increases in the single kea3 mutant, we suggest that AtKEA3 mediates 2H+ out of the lumen in exchange for 1K+. At the inner envelope, we propose that AtKEA2 (or AtKEA1) catalyzes the efflux of 1K+ from the plastid for the uptake of 2H+. In either case, antiport would be driven by the PMF across the inner envelope or the thylakoid membrane. Although these ideas need verification, it is clear that AtKEA1, AtKEA2, and AtKEA3 in some way maintain pH, osmotic, and K+ balance for plastid development and chloroplast function. Nearly nothing is known about AtKEA4 to AtKEA6.
MODEL: THE ION AND pH MICROENVIRONMENT OF THE ENDOMEMBRANE SYSTEM INFLUENCES CARGO SORTING AS WELL AS MEMBRANE AND CELL WALL REMODELING
In spite of the diverse processes affected by NHX, CHX, and KEA transporters, a common theme is emerging. First, cells respond to developmental and environmental cues by remodeling themselves using a dynamic membrane system. Examples show that CHXs are involved in processes such as light-induced guard cell movement, male-female interactions that result in fertilization and seed set, and adaptation to environmental stress. Second, the association of cation/H+ antiporters at different parts of the endomembrane system and in organelles indicates that a regulated pH, ionic, or osmotic balance could affect protein conformation, protein-protein or ligand binding, and dissociation, which are essential for membrane trafficking. Membrane dynamics include membrane biogenesis, endocytosis, recycling, autophagy, exocytosis, and exosomes. Trafficking depends on (1) membrane fission/budding, (2) directional vesicle transport via the cytoskeleton, and (3) membrane fusion at the destination. Each process may rely on a pH (and Δψ) range and cation microenvironment. An imbalance could disrupt the binding or dissociation between a protein and its sorting receptor, the binding between tethering complexes and fusion proteins, and pH-dependent activities, any of which will lead to missorted or modified cargo/protein.
Insights from structural models and mutant phenotypes have highlighted different roles of NHX and CHX transporters: (1) subclades of CPA1 or CPA2 are localized on distinct endomembranes (Table II); (2) AtCHX17 and AtNHX1 are differentially activated at basic and acidic pH values in yeast, respectively, and distinct residues at the pH sensor region (e.g. E111) and ion-binding sites in AtCHX17 support this idea; and (3) the possibility that CPA2 antiporters, like plant CHXs and KEAs, which contain a conserved Lys in TM10, mediate electrogenic (K+/nH+) exchange is considered not settled (Călinescu et al., 2017; Uzdavinys et al., 2017).
We favor a model where multiple CHXs are involved in distinct membrane-trafficking events, some of which remodel membranes, cell walls, or both in particular cell types. This idea stems from several observations: (1) the diversification of CHX genes (Chanroj et al., 2012) coincides with the evolution of walls in land plants, particularly pectins (Popper et al., 2011); (2) the wall pattern of chx17 chx18 chx19 mutant pollen grains was severely disorganized (Padmanaban et al., 2017); (3) the expression of CHXs is observed in highly secretory cells/tissues, including root caps (Padmanaban et al., 2007), hydathodes, and vasculature (H. Sze, unpublished results); and (4) many CHX genes are expressed in pollen, where specific cargo, targeted sorting, and pectin remodeling are important for polar tip growth and reorientation. Besides cellulose, hemicellulose, and callose, the pollen tube wall contains pectin, which is predominant at the growing tip (Chebli et al., 2012; Mollet et al., 2013). The pectin is secreted as methyl esterified polymers and then partially deesterified in a spatial and temporal manner to allow Ca2+ to cross-link two neighboring polymers. Together with several pectin-degrading enzymes that are delivered to specific locations, the cell can regulate its extensibility and growth. Moreover, the growing tube senses female cues and turns to target an ovule, then finally bursts to release two sperms in a matter of hours. Tube growth, reorientation, and burst are consequences of membrane and wall remodeling. Enzymes needed for pectin modification (Willats et al., 2001; Pelloux et al., 2007) are delivered to the wall in a spatial and temporal manner, as shown for pectin methylesterase (PME) and PMEI (PME inhibitor; Röckel et al., 2008), perhaps via the exosome (Prado et al., 2014). As multiple AtPMEs and PMEIs have varied pH optima, ranging from 5 to 9 (Pelloux et al., 2007), CHXs may be critical for male fertility, as they influence pH and K+ homeostasis and provide a microenvironment optimal for pectin remodeling in space and time (Chanroj, 2011).
CONCLUSION
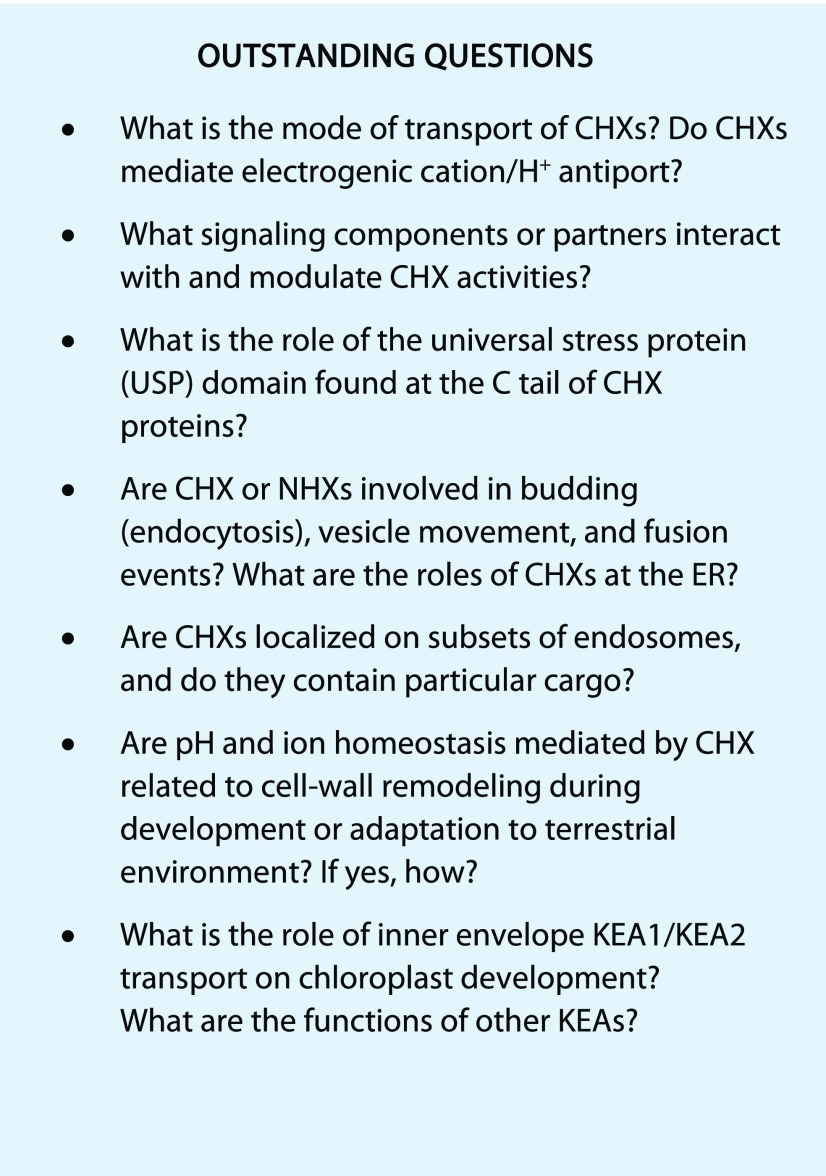
K+(Na+)/H+ antiporters are emerging as crucial players in pH and cation homeostasis that alter growth, signaling, development, and stress adaptation. Both AtCHX17 and AtNHX1 are modeled to share a protein architecture and translocation core similar to bacterial Na+/H+ antiporters. Yet, analyses of mutants indicate that CHX-type and NHX-type transporters occupy distinct functional niches, possibly due to differential endomembrane distribution, pH set point, and electrogenic versus electroneutral exchange (see Outstanding Questions). Future efforts need to focus on the following: (1) determining the transport mode and electrogenicity of CHX and KEA transporters; (2) identifying cargo contained in CHX-associated compartments; and (3) finding interacting partners of CPAs and links with membrane-trafficking and signal transduction networks. Studies to integrate components of the endomembrane system with determinants of pH and ion balance will shed light on plant strategies to develop, reproduce, and survive.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Lucy Forrest (National Institutes of Health) as well as Andriy Anishkin, Erwan Michard, Jose Feijo, and Robert T. Su (University of Maryland) for discussions.
Footnotes
Research in the laboratory of H.S. was supported by the U.S. Department of Energy, Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences (BES DEFG-0207ER15883). H.S. is supported by a Quishi Chair Professorship at the College of Life Sciences at Zhejiang University. S.C. is supported by the National Science and Technology Development Agency of Thailand (SCH-NR2015-253). Work was also supported in part by National Science Foundation (IBN209788) to H.S.
Articles can be viewed without a subscription.
References
- Adamowski M, Friml J (2015) PIN-dependent auxin transport: action, regulation, and evolution. Plant Cell 27: 20–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsbury S, Hunt L, Elhaddad N, Baillie A, Lundgren M, Verhertbruggen Y, Scheller HV, Knox JP, Fleming AJ, Gray JE (2016) Stomatal function requires pectin de-methyl-esterification of the guard cell wall. Curr Biol 26: 2899–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An R, Chen QJ, Chai MF, Lu PL, Su Z, Qin ZX, Chen J, Wang XC (2007) AtNHX8, a member of the monovalent cation:proton antiporter-1 family in Arabidopsis thaliana, encodes a putative Li/H antiporter. Plant J 49: 718–728 [DOI] [PubMed] [Google Scholar]
- Andrés Z, Pérez-Hormaeche J, Leidi EO, Schlücking K, Steinhorst L, McLachlan DH, Schumacher K, Hetherington AM, Kudla J, Cubero B, et al. (2014) Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proc Natl Acad Sci USA 111: E1806–E1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda-Sicilia MN, Cagnac O, Chanroj S, Sze H, Rodríguez-Rosales MP, Venema K (2012) Arabidopsis KEA2, a homolog of bacterial KefC, encodes a K+/H+ antiporter with a chloroplast transit peptide. Biochim Biophys Acta 1818: 2362–2371 [DOI] [PubMed] [Google Scholar]
- Aranda-Sicilia MN, Aboukila A, Armbruster U, Cagnac O, Schumann T, Kunz HH, Jahns P, Rodríguez-Rosales MP, Sze H, Venema K (2016) Envelope K+/H+ antiporters AtKEA1 and AtKEA2 function in plastid development. Plant Physiol 172: 441–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuelos MG, Moreno DE, Olson DK, Nguyen Q, Ricarte F, Aguilera-Sandoval CR, Gharakhanian E (2010) Genomic analysis of severe hypersensitivity to hygromycin B reveals linkage to vacuolar defects and new vacuolar gene functions in Saccharomyces cerevisiae. Curr Genet 56: 121–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier-Brygoo H, De Angeli A, Filleur S, Frachisse JM, Gambale F, Thomine S, Wege S (2011) Anion channels/transporters in plants: from molecular bases to regulatory networks. Annu Rev Plant Biol 62: 25–51 [DOI] [PubMed] [Google Scholar]
- Barragán V, Leidi EO, Andrés Z, Rubio L, De Luca A, Fernández JA, Cubero B, Pardo JM (2012) Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 24: 1127–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil E, Blumwald E (2014) The ins and outs of intracellular ion homeostasis: NHX-type cation/H+ transporters. Curr Opin Plant Biol 22: 1–6 [DOI] [PubMed] [Google Scholar]
- Bassil E, Ohto MA, Esumi T, Tajima H, Zhu Z, Cagnac O, Belmonte M, Peleg Z, Yamaguchi T, Blumwald E (2011a) The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell 23: 224–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil E, Tajima H, Liang YC, Ohto MA, Ushijima K, Nakano R, Esumi T, Coku A, Belmonte M, Blumwald E (2011b) The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 23: 3482–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer EM, Sparkes I, Vanneste S, Rosado A (2017) From shaping organelles to signalling platforms: the emerging functions of plant ER-PM contact sites. Curr Opin Plant Biol 40: 89–96 [DOI] [PubMed] [Google Scholar]
- Belmonte MF, Kirkbride RC, Stone SL, Pelletier JM, Bui AQ, Yeung EC, Hashimoto M, Fei J, Harada CM, Munoz MD, et al. (2013) Comprehensive developmental profiles of gene activity in regions and subregions of the Arabidopsis seed. Proc Natl Acad Sci USA 110: E435–E444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaby-Haas CE, Merchant SS (2014) Lysosome-related organelles as mediators of metal homeostasis. J Biol Chem 289: 28129–28136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt MR, Rodriguez-Navarro A, Slayman CL (1987) Potassium-proton symport in Neurospora: kinetic control by pH and membrane potential. J Membr Biol 98: 169–189 [DOI] [PubMed] [Google Scholar]
- Blumwald E, Poole RJ (1985) Na/H antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris. Plant Physiol 78: 163–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock KW, Honys D, Ward JM, Padmanaban S, Nawrocki EP, Hirschi KD, Twell D, Sze H (2006) Integrating membrane transport with male gametophyte development and function through transcriptomics. Plant Physiol 140: 1151–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers K, Stevens TH (2005) Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta 1744: 438–454 [DOI] [PubMed] [Google Scholar]
- Bowers K, Levi BP, Patel FI, Stevens TH (2000) The sodium/proton exchanger Nhx1p is required for endosomal protein trafficking in the yeast Saccharomyces cerevisiae. Mol Biol Cell 11: 4277–4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett CL, Tukaye DN, Mukherjee S, Rao R (2005) The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol Biol Cell 16: 1396–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Călinescu O, Dwivedi M, Patiño-Ruiz M, Padan E, Fendler K (2017) Lysine 300 is essential for stability but not for electrogenic transport of the Escherichia coli NhaA Na+/H+ antiporter. J Biol Chem 292: 7932–7941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R, Woolfenden H, Baillie A, Amsbury S, Carroll S, Healicon E, Sovatzoglou S, Braybrook S, Gray JE, Hobbs J, et al. (2017) Stomatal opening involves polar, not radial, stiffening of guard cells. Curr Biol 27: 2974–2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JR, Grinstein S, Orlowski J (2010) Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 11: 50–61 [DOI] [PubMed] [Google Scholar]
- Cellier F, Conéjéro G, Ricaud L, Luu DT, Lepetit M, Gosti F, Casse F (2004) Characterization of AtCHX17, a member of the cation/H+ exchangers, CHX family, from Arabidopsis thaliana suggests a role in K+ homeostasis. Plant J 39: 834–846 [DOI] [PubMed] [Google Scholar]
- Chanroj S. (2011) Plant-specific K+ transporters with distinct properties and their emerging roles in endomembrane trafficking. PhD dissertation.University of Maryland, College Park, MD [Google Scholar]
- Chanroj S, Lu Y, Padmanaban S, Nanatani K, Uozumi N, Rao R, Sze H (2011) Plant-specific cation/H+ exchanger 17 and its homologs are endomembrane K+ transporters with roles in protein sorting. J Biol Chem 286: 33931–33941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanroj S, Wang G, Venema K, Zhang MW, Delwiche CF, Sze H (2012) Conserved and diversified gene families of monovalent cation/H+ antiporters from algae to flowering plants. Front Plant Sci 3: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanroj S, Padmanaban S, Czerny DD, Jauh GY, Sze H (2013) K+ transporter AtCHX17 with its hydrophilic C tail localizes to membranes of the secretory/endocytic system: role in reproduction and seed set. Mol Plant 6: 1226–1246 [DOI] [PubMed] [Google Scholar]
- Chebli Y, Kaneda M, Zerzour R, Geitmann A (2012) The cell wall of the Arabidopsis pollen tube--spatial distribution, recycling, and network formation of polysaccharides. Plant Physiol 160: 1940–1955 10.1104/pp.112.199729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ma J, Miller AJ, Luo B, Wang M, Zhu Z, Ouwerkerk PB (2016) OsCHX14 is involved in the K+ homeostasis in rice (Oryza sativa) flowers. Plant Cell Physiol 57: 1530–1543 [DOI] [PubMed] [Google Scholar]
- Churchill KA, Sze H (1983) Anion-sensitive, H-pumping ATPase in membrane vesicles from oat roots. Plant Physiol 71: 610–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill KA, Sze H (1984) Anion-sensitive, H-pumping ATPase of oat roots: direct effects of Cl, NO3, and a disulfonic stilbene. Plant Physiol 76: 490–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coincon M, Uzdavinys P, Nji E, Dotson DL, Winkelmann I, Abdul-Hussein S, Cameron AD, Beckstein O, Drew D (2016) Crystal structures reveal the molecular basis of ion translocation in sodium/proton antiporters. Nat Struct Mol Biol 23: 248–255 [DOI] [PubMed] [Google Scholar]
- Cyert MS, Philpott CC (2013) Regulation of cation balance in Saccharomyces cerevisiae. Genetics 193: 677–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerny DD, Padmanaban S, Anishkin A, Venema K, Riaz Z, Sze H (2016) Protein architecture and core residues in unwound α-helices provide insights to the transport function of plant AtCHX17. Biochim Biophys Acta 1858: 1983–1998 [DOI] [PubMed] [Google Scholar]
- Davis-Kaplan SR, Ward DM, Shiflett SL, Kaplan J (2004) Genome-wide analysis of iron-dependent growth reveals a novel yeast gene required for vacuolar acidification. J Biol Chem 279: 4322–4329 [DOI] [PubMed] [Google Scholar]
- De Angeli A, Monachello D, Ephritikhine G, Frachisse JM, Thomine S, Gambale F, Barbier-Brygoo H (2006) The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 442: 939–942 [DOI] [PubMed] [Google Scholar]
- de Groot PW, Ruiz C, Vázquez de Aldana CR, Duenas E, Cid VJ, Del Rey F, Rodríquez-Peña JM, Pérez P, Andel A, Caubín J, et al. (2001) A genomic approach for the identification and classification of genes involved in cell wall formation and its regulation in Saccharomyces cerevisiae. Comp Funct Genomics 2: 124–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J, Schubert D, Calvo-Weimar O, Stierhof YD, Schmidt R, Schumacher K (2005) Essential role of the V-ATPase in male gametophyte development. Plant J 41: 117–124 [DOI] [PubMed] [Google Scholar]
- Dresselhaus T, Sprunck S, Wessel GM (2016) Fertilization mechanisms in flowering plants. Curr Biol 26: R125–R139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach C, Baetz U, Martinoia E (2014) Vacuolar proton pumping: more than the sum of its parts? Trends Plant Sci 19: 344–346 [DOI] [PubMed] [Google Scholar]
- Ejzykowicz DE, Locken KM, Ruiz FJ, Manandhar SP, Olson DK, Gharakhanian E (2017) Hygromycin B hypersensitive (hhy) mutants implicate an intact trans-Golgi and late endosome interface in efficient Tor1 vacuolar localization and TORC1 function. Curr Genet 63: 531–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AR, Hall D, Pritchard J, Newbury HJ (2012) The roles of the cation transporters CHX21 and CHX23 in the development of Arabidopsis thaliana. J Exp Bot 63: 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Faraco M, Spelt C, Bliek M, Verweij W, Hoshino A, Espen L, Prinsi B, Jaarsma R, Tarhan E, de Boer AH, et al. (2014) Hyperacidification of vacuoles by the combined action of two different P-ATPases in the tonoplast determines flower color. Cell Rep 6: 32–43 [DOI] [PubMed] [Google Scholar]
- Forrest LR, Krämer R, Ziegler C (2011) The structural basis of secondary active transport mechanisms. Biochim Biophys Acta 1807: 167–188 [DOI] [PubMed] [Google Scholar]
- Fukada-Tanaka S, Inagaki Y, Yamaguchi T, Saito N, Iida S (2000) Colour-enhancing protein in blue petals. Nature 407: 581. [DOI] [PubMed] [Google Scholar]
- Gao XQ, Li CG, Wei PC, Zhang XY, Chen J, Wang XC (2005) The dynamic changes of tonoplasts in guard cells are important for stomatal movement in Vicia faba. Plant Physiol 139: 1207–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaxiola RA, Rao R, Sherman A, Grisafi P, Alper SL, Fink GR (1999) The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc Natl Acad Sci USA 96: 1480–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Bergonci T, Zhao Y, Zou Y, Du S, Liu MC, Luo X, Ruan H, García-Valencia LE, Zhong S, et al. (2017) Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science 358: 1596–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierth M, Mäser P (2007) Potassium transporters in plants: involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett 581: 2348–2356 [DOI] [PubMed] [Google Scholar]
- Gu Y, Zavaliev R, Dong X (2017) Membrane trafficking in plant immunity. Mol Plant 10: 1026–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan R, Qu Y, Guo Y, Yu L, Liu Y, Jiang J, Chen J, Ren Y, Liu G, Tian L, et al. (2014) Salinity tolerance in soybean is modulated by natural variation in GmSALT3. Plant J 80: 937–950 [DOI] [PubMed] [Google Scholar]
- Haruta M, Sussman MR (2012) The effect of a genetically reduced plasma membrane protonmotive force on vegetative growth of Arabidopsis. Plant Physiol 158: 1158–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M, Burch HL, Nelson RB, Barrett-Wilt G, Kline KG, Mohsin SB, Young JC, Otegui MS, Sussman MR (2010) Molecular characterization of mutant Arabidopsis plants with reduced plasma membrane proton pump activity. J Biol Chem 285: 17918–17929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández A, Jiang X, Cubero B, Nieto PM, Bressan RA, Hasegawa PM, Pardo JM (2009) Mutants of the Arabidopsis thaliana cation/H+ antiporter AtNHX1 conferring increased salt tolerance in yeast: the endosome/prevacuolar compartment is a target for salt toxicity. J Biol Chem 284: 14276–14285 10.1074/jbc.M806203200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama T, Takeuchi H (2015) The mechanism and key molecules involved in pollen tube guidance. Annu Rev Plant Biol 66: 393–413 [DOI] [PubMed] [Google Scholar]
- Jentsch TJ. (2008) CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit Rev Biochem Mol Biol 43: 3–36 10.1080/10409230701829110 [DOI] [PubMed] [Google Scholar]
- Jezek M, Blatt MR (2017) The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol 174: 487–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner KH, Sze H (1987) Potential-dependent anion transport in tonoplast vesicles from oat roots. Plant Physiol 83: 483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane PM. (2016) Proton transport and pH control in fungi. Adv Exp Med Biol 892: 33–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ (2015) The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10: 845–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klis FM, Boorsma A, De Groot PW (2006) Cell wall construction in Saccharomyces cerevisiae. Yeast 23: 185–202 [DOI] [PubMed] [Google Scholar]
- Krebs M, Beyhl D, Görlich E, Al-Rasheid KA, Marten I, Stierhof YD, Hedrich R, Schumacher K (2010) Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc Natl Acad Sci USA 107: 3251–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel A, Andrés Z, Medzihradszky A, Krüger F, Scholl S, Delang S, Patir-Nebioglu MG, Gute G, Yang H, Murphy AS, et al. (2015) Job sharing in the endomembrane system: vacuolar acidification requires the combined activity of V-ATPase and V-PPase. Plant Cell 27: 3383–3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich TA, Sachs G, Padan E (2011) Molecular aspects of bacterial pH sensing and homeostasis. Nat Rev Microbiol 9: 330–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz HH, Gierth M, Herdean A, Satoh-Cruz M, Kramer DM, Spetea C, Schroeder JI (2014) Plastidial transporters KEA1, -2, and -3 are essential for chloroplast osmoregulation, integrity, and pH regulation in Arabidopsis. Proc Natl Acad Sci USA 111: 7480–7485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkdjian A, Guern J (1989) Intracellular pH: measurement and importance in cell activity. Annu Rev Plant Physiol Plant Mol Biol 40: 271–303 [Google Scholar]
- Lee C, Kang HJ, von Ballmoos C, Newstead S, Uzdavinys P, Dotson DL, Iwata S, Beckstein O, Cameron AD, Drew D (2013) A two-domain elevator mechanism for sodium/proton antiport. Nature 501: 573–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidi EO, Barragán V, Rubio L, El-Hamdaoui A, Ruiz MT, Cubero B, Fernández JA, Bressan RA, Hasegawa PM, Quintero FJ, et al. (2010) The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J 61: 495–506 [DOI] [PubMed] [Google Scholar]
- Levin DE. (2011) Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics 189: 1145–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Chanroj S, Wu Z, Romanowsky SM, Harper JF, Sze H (2008) A distinct endosomal Ca2+/Mn2+ pump affects root growth through the secretory process. Plant Physiol 147: 1675–1689 10.1104/pp.108.119909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SM, Tsai JY, Hsiao CD, Huang YT, Chiu CL, Liu MH, Tung JY, Liu TH, Pan RL, Sun YJ (2012) Crystal structure of a membrane-embedded H+-translocating pyrophosphatase. Nature 484: 399–403 [DOI] [PubMed] [Google Scholar]
- Lu Y, Chanroj S, Zulkifli L, Johnson MA, Uozumi N, Cheung A, Sze H (2011) Pollen tubes lacking a pair of K+ transporters fail to target ovules in Arabidopsis. Plant Cell 23: 81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Scholl S, Doering A, Zhang Y, Irani NG, Rubbo SD, Neumetzler L, Krishnamoorthy P, Van Houtte I, Mylle E, et al. (2015) V-ATPase activity in the TGN/EE is required for exocytosis and recycling in Arabidopsis. Nat Plants 1: 15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJ, Sanders D (1994) Mechanism of high-affinity potassium uptake in roots of Arabidopsis thaliana. Proc Natl Acad Sci USA 91: 9272–9276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinière A, Bassil E, Jublanc E, Alcon C, Reguera M, Sentenac H, Blumwald E, Paris N (2013) In vivo intracellular pH measurements in tobacco and Arabidopsis reveal an unexpected pH gradient in the endomembrane system. Plant Cell 25: 4028–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E, Meyer S, De Angeli A, Nagy R (2012) Vacuolar transporters in their physiological context. Annu Rev Plant Biol 63: 183–213 [DOI] [PubMed] [Google Scholar]
- Marty F. (1999) Plant vacuoles. Plant Cell 11: 587–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckel T, Hurst AC, Thiel G, Homann U (2004) Endocytosis against high turgor: intact guard cells of Vicia faba constitutively endocytose fluorescently labelled plasma membrane and GFP-tagged K-channel KAT1. Plant J 39: 182–193 [DOI] [PubMed] [Google Scholar]
- Mizukami AG, Inatsugi R, Jiao J, Kotake T, Kuwata K, Ootani K, Okuda S, Sankaranarayanan S, Sato Y, Maruyama D, et al. (2016) The AMOR arabinogalactan sugar chain induces pollen-tube competency to respond to ovular guidance. Curr Biol 26: 1091–1097 [DOI] [PubMed] [Google Scholar]
- Mollet JC, Leroux C, Dardelle F, Lehner A (2013) Cell wall composition, biosynthesis and remodeling during pollen tube growth. Plants (Basel) 2: 107–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottaleb SA, Rodríguez-Navarro A, Haro R (2013) Knockouts of Physcomitrella patens CHX1 and CHX2 transporters reveal high complexity of potassium homeostasis. Plant Cell Physiol 54: 1455–1468 [DOI] [PubMed] [Google Scholar]
- Nass R, Rao R (1998) Novel localization of a Na+/H+ exchanger in a late endosomal compartment of yeast: implications for vacuole biogenesis. J Biol Chem 273: 21054–21060 [DOI] [PubMed] [Google Scholar]
- Nass R, Cunningham KW, Rao R (1997) Intracellular sequestration of sodium by a novel Na+/H+ exchanger in yeast is enhanced by mutations in the plasma membrane H+-ATPase: insights into mechanisms of sodium tolerance. J Biol Chem 272: 26145–26152 [DOI] [PubMed] [Google Scholar]
- Nelson H, Nelson N (1990) Disruption of genes encoding subunits of yeast vacuolar H(+)-ATPase causes conditional lethality. Proc Natl Acad Sci USA 87: 3503–3507 10.1073/pnas.87.9.3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieves-Cordones M, Alemán F, Martínez V, Rubio F (2014) K+ uptake in plant roots: the systems involved, their regulation and parallels in other organisms. J Plant Physiol 171: 688–695 [DOI] [PubMed] [Google Scholar]
- Orij R, Brul S, Smits GJ (2011) Intracellular pH is a tightly controlled signal in yeast. Biochim Biophys Acta 1810: 933–944 [DOI] [PubMed] [Google Scholar]
- Orlean P. (2012) Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics 192: 775–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E. (2014) Functional and structural dynamics of NhaA, a prototype for Na+ and H+ antiporters, which are responsible for Na+ and H+ homeostasis in cells. Biochim Biophys Acta 1837: 1047–1062 [DOI] [PubMed] [Google Scholar]
- Padan E, Bibi E, Ito M, Krulwich TA (2005) Alkaline pH homeostasis in bacteria: new insights. Biochim Biophys Acta 1717: 67–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanaban S, Chanroj S, Kwak JM, Li X, Ward JM, Sze H (2007) Participation of endomembrane cation/H+ exchanger AtCHX20 in osmoregulation of guard cells. Plant Physiol 144: 82–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanaban S, Czerny DD, Levin KA, Leydon AR, Su RT, Maugel TK, Zou Y, Chanroj S, Cheung AY, Johnson MA, et al. (2017) Transporters involved in pH and K+ homeostasis affect pollen wall formation, male fertility, and embryo development. J Exp Bot 68: 3165–3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulino C, Wöhlert D, Kapotova E, Yildiz Ö, Kühlbrandt W (2014) Structure and transport mechanism of the sodium/proton antiporter MjNhaP1. eLife 3: e03583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux J, Rustérucci C, Mellerowicz EJ (2007) New insights into pectin methylesterase structure and function. Trends Plant Sci 12: 267–277 [DOI] [PubMed] [Google Scholar]
- Pittman JK. (2012) Multiple transport pathways for mediating intracellular pH homeostasis: the contribution of H+/ion exchangers. Front Plant Sci 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA, Michel G, Hervé C, Domozych DS, Willats WG, Tuohy MG, Kloareg B, Stengel DB (2011) Evolution and diversity of plant cell walls: from algae to flowering plants. Annu Rev Plant Biol 62: 567–590 [DOI] [PubMed] [Google Scholar]
- Prado N, Alché JdeD, Casado-Vela J, Mas S, Villalba M, Rodríguez R, Batanero E (2014) Nanovesicles are secreted during pollen germination and pollen tube growth: a possible role in fertilization. Mol Plant 7: 573–577 [DOI] [PubMed] [Google Scholar]
- Printz B, Lutts S, Hausman JF, Sergeant K (2016) Copper trafficking in plants and its implication on cell wall dynamics. Front Plant Sci 7: 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Li MW, Xie M, Liu X, Ni M, Shao G, Song C, Kay-Yuen Yim A, Tao Y, Wong FL, et al. (2014) Identification of a novel salt tolerance gene in wild soybean by whole-genome sequencing. Nat Commun 5: 4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero FJ, Blatt MR, Pardo JM (2000) Functional conservation between yeast and plant endosomal Na+/H+ antiporters. FEBS Lett 471: 224–228 [DOI] [PubMed] [Google Scholar]
- Quintero FJ, Martinez-Atienza J, Villalta I, Jiang X, Kim WY, Ali Z, Fujii H, Mendoza I, Yun DJ, Zhu JK, et al. (2011) Activation of the plasma membrane Na/H antiporter Salt-Overly-Sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proc Natl Acad Sci USA 108: 2611–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera M, Bassil E, Tajima H, Wimmer M, Chanoca A, Otegui MS, Paris N, Blumwald E (2015) pH regulation by NHX-type antiporters is required for receptor-mediated protein trafficking to the vacuole in Arabidopsis. Plant Cell 27: 1200–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röckel N, Wolf S, Kost B, Rausch T, Greiner S (2008) Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis, and reflects the distribution of esterified and de-esterified pectins. Plant J 53: 133–143 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Navarro A, Blatt MR, Slayman CL (1986) A potassium-proton symport in Neurospora crassa. J Gen Physiol 87: 649–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Navarro A. (2000) Potassium transport in fungi and plants. Biochim Biophys Acta 1469: 1–30 [DOI] [PubMed] [Google Scholar]
- Rubio F, Nieves-Cordones M, Alemán F, Martínez V (2008) Relative contribution of AtHAK5 and AtAKT1 to K+ uptake in the high-affinity range of concentrations. Physiol Plant 134: 598–608 [DOI] [PubMed] [Google Scholar]
- Sanders D, Hansen UP, Slayman CL (1981) Role of the plasma membrane proton pump in pH regulation in non-animal cells. Proc Natl Acad Sci USA 78: 5903–5907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval RM, Molitoris BA (2004) Gentamicin traffics retrograde through the secretory pathway and is released in the cytosol via the endoplasmic reticulum. Am J Physiol Renal Physiol 286: F617–F624 [DOI] [PubMed] [Google Scholar]
- Schumacher K. (2014) pH in the plant endomembrane system: an import and export business. Curr Opin Plant Biol 22: 71–76 [DOI] [PubMed] [Google Scholar]
- Serrano R, Kielland-Brandt MC, Fink GR (1986) Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature 319: 689–693 [DOI] [PubMed] [Google Scholar]
- Serrano R, Bernal D, Simón E, Ariño J (2004) Copper and iron are the limiting factors for growth of the yeast Saccharomyces cerevisiae in an alkaline environment. J Biol Chem 279: 19698–19704 [DOI] [PubMed] [Google Scholar]
- Serrano R, Martín H, Casamayor A, Ariño J (2006) Signaling alkaline pH stress in the yeast Saccharomyces cerevisiae through the Wsc1 cell surface sensor and the Slt2 MAPK pathway. J Biol Chem 281: 39785–39795 [DOI] [PubMed] [Google Scholar]
- Shen J, Zeng Y, Zhuang X, Sun L, Yao X, Pimpl P, Jiang L (2013) Organelle pH in the Arabidopsis endomembrane system. Mol Plant 6: 1419–1437 [DOI] [PubMed] [Google Scholar]
- Sheng P, Tan J, Jin M, Wu F, Zhou K, Ma W, Heng Y, Wang J, Guo X, Zhang X, et al. (2014) Albino midrib 1, encoding a putative potassium efflux antiporter, affects chloroplast development and drought tolerance in rice. Plant Cell Rep 33: 1581–1594 [DOI] [PubMed] [Google Scholar]
- Shi H, Quintero FJ, Pardo JM, Zhu JK (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 14: 465–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Severance S, Kaur N, Wiltsie W, Kosman DJ (2006) Assembly, activation, and trafficking of the Fet3p.Ftr1p high affinity iron permease complex in Saccharomyces cerevisiae. J Biol Chem 281: 13355–13364 [DOI] [PubMed] [Google Scholar]
- Smith FA, Raven JA (1979) Intracellular pH and its regulation. Annu Rev Plant Physiol 30: 289–311 [Google Scholar]
- Song CP, Guo Y, Qiu Q, Lambert G, Galbraith DW, Jagendorf A, Zhu JK (2004) A probable Na+(K+)/H+ exchanger on the chloroplast envelope functions in pH homeostasis and chloroplast development in Arabidopsis thaliana. Proc Natl Acad Sci USA 101: 10211–10216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano G, Brandizzi F (2018) Advances in Plant ER Architecture and Dynamics. Plant Physiol 176: 178–186 10.1104/pp.17.01261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strompen G, Dettmer J, Stierhof YD, Schumacher K, Jürgens G, Mayer U (2005) Arabidopsis vacuolar H-ATPase subunit E isoform 1 is required for Golgi organization and vacuole function in embryogenesis. Plant J 41: 125–132 [DOI] [PubMed] [Google Scholar]
- Sze H. (1985) H+-translocating ATPases: advances using membrane vesicles. Annu Rev Plant Physiol 36: 175–208 [Google Scholar]
- Sze H, Schumacher K, Müller ML, Padmanaban S, Taiz L (2002) A simple nomenclature for a complex proton pump: VHA genes encode the vacuolar H(+)-ATPase. Trends Plant Sci 7: 157–161 10.1016/S1360-1385(02)02240-9 [DOI] [PubMed] [Google Scholar]
- Sze H, Padmanaban S, Cellier F, Honys D, Cheng NH, Bock KW, Conéjéro G, Li X, Twell D, Ward JM, et al. (2004) Expression patterns of a novel AtCHX gene family highlight potential roles in osmotic adjustment and K+ homeostasis in pollen development. Plant Physiol 136: 2532–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Higashiyama T (2016) Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 531: 245–248 [DOI] [PubMed] [Google Scholar]
- Tromballa H. (1987) Base uptake, K+ transport and intracellular pH regulation by the green alga Chlorella fusca. Biochim Biophys Acta 904: 216–226 [Google Scholar]
- Turlapati PV, Kim KW, Davin LB, Lewis NG (2011) The laccase multigene family in Arabidopsis thaliana: towards addressing the mystery of their gene function(s). Planta 233: 439–470 [DOI] [PubMed] [Google Scholar]
- Uzdavinys P, Coinçon M, Nji E, Ndi M, Winkelmann I, von Ballmoos C, Drew D (2017) Dissecting the proton transport pathway in electrogenic Na+/H+ antiporters. Proc Natl Acad Sci USA 114: E1101–E1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema K, Quintero FJ, Pardo JM, Donaire JP (2002) The Arabidopsis Na+/H+ exchanger AtNHX1 catalyzes low affinity Na+ and K+ transport in reconstituted liposomes. J Biol Chem 277: 2413–2418 [DOI] [PubMed] [Google Scholar]
- Venema K, Belver A, Marin-Manzano MC, Rodríguez-Rosales MP, Donaire JP (2003) A novel intracellular K+/H+ antiporter related to Na+/H+ antiporters is important for K+ ion homeostasis in plants. J Biol Chem 278: 22453–22459 [DOI] [PubMed] [Google Scholar]
- Verweij W, Spelt C, Di Sansebastiano GP, Vermeer J, Reale L, Ferranti F, Koes R, Quattrocchio F (2008) An H+ P-ATPase on the tonoplast determines vacuolar pH and flower colour. Nat Cell Biol 10: 1456–1462 [DOI] [PubMed] [Google Scholar]
- Véry AA, Nieves-Cordones M, Daly M, Khan I, Fizames C, Sentenac H (2014) Molecular biology of K+ transport across the plant cell membrane: what do we learn from comparison between plant species? J Plant Physiol 171: 748–769 [DOI] [PubMed] [Google Scholar]
- Viotti C, Krüger F, Krebs M, Neubert C, Fink F, Lupanga U, Scheuring D, Boutté Y, Frescatada-Rosa M, Wolfenstetter S, et al. (2013) The endoplasmic reticulum is the main membrane source for biogenesis of the lytic vacuole in Arabidopsis. Plant Cell 25: 3434–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Fecht-Bartenbach J, Bogner M, Krebs M, Stierhof YD, Schumacher K, Ludewig U (2007) Function of the anion transporter AtCLC-d in the trans-Golgi network. Plant J 50: 466–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner MC, Molnar EE, Molitoris BA, Goebl MG (2006) Loss of the homotypic fusion and vacuole protein sorting or Golgi-associated retrograde protein vesicle tethering complexes results in gentamicin sensitivity in the yeast Saccharomyces cerevisiae. Antimicrob Agents Chemother 50: 587–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DJ, Leigh RA, Miller AJ (1996) Potassium homeostasis in vacuolate plant cells. Proc Natl Acad Sci USA 93: 10510–10514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu WH (2013) Potassium transport and signaling in higher plants. Annu Rev Plant Biol 64: 451–476 [DOI] [PubMed] [Google Scholar]
- Wang L, Feng X, Zhao H, Wang L, An L, Qiu QS (2014) Functional analysis of the Na+,K+/H+ antiporter PeNHX3 from the tree halophyte Populus euphratica in yeast by model-guided mutagenesis. PLoS ONE 9: e104147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Liang L, Xue Y, Jia PF, Chen W, Zhang MX, Wang YC, Li HJ, Yang WC (2016) A receptor heteromer mediates the male perception of female attractants in plants. Nature 531: 241–244 [DOI] [PubMed] [Google Scholar]
- Willats WG, McCartney L, Mackie W, Knox JP (2001) Pectin: cell biology and prospects for functional analysis. Plant Mol Biol 47: 9–27 [PubMed] [Google Scholar]
- Wöhlert D, Kühlbrandt W, Yildiz O (2014) Structure and substrate ion binding in the sodium/proton antiporter PaNhaP. eLife 3: e03579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S, Hématy K, Höfte H (2012) Growth control and cell wall signaling in plants. Annu Rev Plant Biol 63: 381–407 [DOI] [PubMed] [Google Scholar]
- Wu MM, Grabe M, Adams S, Tsien RY, Moore HP, Machen TE (2001) Mechanisms of pH regulation in the regulated secretory pathway. J Biol Chem 276: 33027–33035 [DOI] [PubMed] [Google Scholar]
- Wu SJ, Ding L, Zhu JK (1996) SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell 8: 617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Kim H, Seravalli J, Barycki JJ, Hart PJ, Gohara DW, Di Cera E, Jung WH, Kosman DJ, Lee J (2016) Potassium and the K+/H+ exchanger Kha1p promote binding of copper to ApoFet3p multi-copper ferroxidase. J Biol Chem 291: 9796–9806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Fukada-Tanaka S, Inagaki Y, Saito N, Yonekura-Sakakibara K, Tanaka Y, Kusumi T, Iida S (2001) Genes encoding the vacuolar Na+/H+ exchanger and flower coloration. Plant Cell Physiol 42: 451–461 [DOI] [PubMed] [Google Scholar]
- Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y (2015) The I-TASSER suite: protein structure and function prediction. Nat Methods 12: 7–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi S, Quintero FJ, Cubero B, Ruiz MT, Bressan RA, Hasegawa PM, Pardo JM (2002) Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J 30: 529–539 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Kawachi M, Mori M, Maeshima M, Kondo M, Nishimura M, Kondo T (2005) The involvement of tonoplast proton pumps and Na+(K+)/H+ exchangers in the change of petal color during flower opening of morning glory, Ipomoea tricolor cv. Heavenly Blue. Plant Cell Physiol 46: 407–415 [DOI] [PubMed] [Google Scholar]
- Zhao J, Cheng NH, Motes CM, Blancaflor EB, Moore M, Gonzales N, Padmanaban S, Sze H, Ward JM, Hirschi KD (2008) AtCHX13 is a plasma membrane K+ transporter. Plant Physiol 148: 796–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Li P, Motes CM, Park S, Hirschi KD (2015) CHX14 is a plasma membrane K-efflux transporter that regulates K+ redistribution in Arabidopsis thaliana. Plant Cell Environ 38: 2223–2238 [DOI] [PubMed] [Google Scholar]