Tumor progression locus 2 (TPL2), a serine/threonine protein kinase, is a major inflammatory mediator in immune cells. The predominant inflammatory actions of TPL2 depend on the activation of mitogen-activated protein kinases (MAPK) and the upregulated production of the cytokines tumor necrosis factor alpha (TNF-α) and interleukin 1β (IL-1β) in macrophages and dendritic cells in response to lipopolysaccharide (LPS).
KEYWORDS: Clostridium difficile, TPL2, inflammation
ABSTRACT
Tumor progression locus 2 (TPL2), a serine/threonine protein kinase, is a major inflammatory mediator in immune cells. The predominant inflammatory actions of TPL2 depend on the activation of mitogen-activated protein kinases (MAPK) and the upregulated production of the cytokines tumor necrosis factor alpha (TNF-α) and interleukin 1β (IL-1β) in macrophages and dendritic cells in response to lipopolysaccharide (LPS). Significant increases in TNF-α, IL-6, IL-β, and IL-8 levels in patients with Clostridium difficile infection (CDI) have been reported. Both TNF-α and IL-6 have been postulated to play key roles in the systemic inflammatory response in CDI, and IL-8 is essential for the development of local intestinal inflammatory responses in CDI. The objective of this study was to elucidate the role of TPL2 in the pathogenesis of CDI. We found that TPL2 was significantly activated in human and mouse intestinal tissues upon C. difficile toxin exposure or CDI. We further demonstrated that TPL2 knockout (TPL2-KO) mice were significantly more resistant to CDI than wild-type mice, with significantly reduced production of TNF-α, IL-6, IL-1β, KC (a mouse homologue of IL-8), and myeloperoxidase (MPO) in the ceca and colons of TPL2-KO mice. Finally, we found that TPL2 inhibition by a specific inhibitor or TPL2 gene ablation significantly reduced TcdB-induced production of TNF-α, IL-6, IL-β, and KC by inhibiting the activation of p38, extracellular signal-regulated kinase (ERK), and c-Jun NH2-terminal kinase (JNK). Taken together, our data suggest that TPL2 represents a potential therapeutic target for CDI treatment.
INTRODUCTION
Clostridium difficile infection (CDI) is primarily a toxin-mediated disease. The major virulence factors of C. difficile are two exotoxins, toxin A (TcdA) and toxin B (TcdB), which glucosylate Rho GTPases, leading to disruption of the cytoskeleton and tight junctions, while stimulating intestinal epithelial or immune cells to produce a storm of proinflammatory cytokines and chemokines (1–3). These cytokines and chemokines have been proposed to promote the recruitment of neutrophils and possibly other immune cells to the intestines, leading to a profound inflammatory immune response (4–9). Therefore, controlling/reducing the production of proinflammatory cytokines/chemokines represents an attractive approach to the treatment of major CDI symptoms. However, our knowledge of several aspects of the cytokine/chemokine storms in CDI is limited. How do toxins induce the production of these cytokines/chemokines in vitro and, most importantly in vivo?
The course of CDI is characterized by an initial intestinal inflammatory process followed by a systemic inflammatory response (10). Significant increases in the concentrations of tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and IL-1β in CDI patients have been reported (10). Both TNF-α and IL-6 have been postulated to play key roles in the systemic inflammatory response in CDI (10), and IL-8/CXCL1 are indicated to be essential to the development of local intestinal inflammatory responses in CDI in humans (10–12) or animal models (13). An excessive inflammatory process can be detrimental.
Tumor progression locus 2 (TPL2) functions as a serine/threonine kinase in the mitogen-activated protein kinase (MAPK) signal transduction cascade known to regulate both innate and adaptive immunity and plays a critical role in the response to inflammatory signals. The predominant proinflammatory actions of TPL2 depend on the activation of MAPKs, including extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK), and p38 (14–16). Both TcdA and TcdB are proinflammatory toxins capable of inducing proinflammatory cytokines, including TNF-α, IL-1β, and IL-6, which are implicated in the development and progression of CDI (17–19). However, the precise cellular mechanism and signaling pathways of TPL2 in C. difficile toxin-induced proinflammatory cytokine production are unknown.
Here we report that the TPL2/MAPK pathway plays a key role in C. difficile-mediated intestinal inflammation by regulating the production of major proinflammatory cytokines/chemokines, which have been reported to be the major drivers of inflammation in CDI patients.
RESULTS
CDI induces TPL2 activation in vivo and ex vivo.
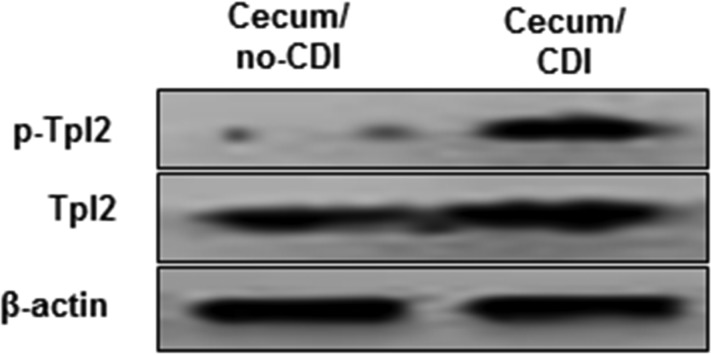
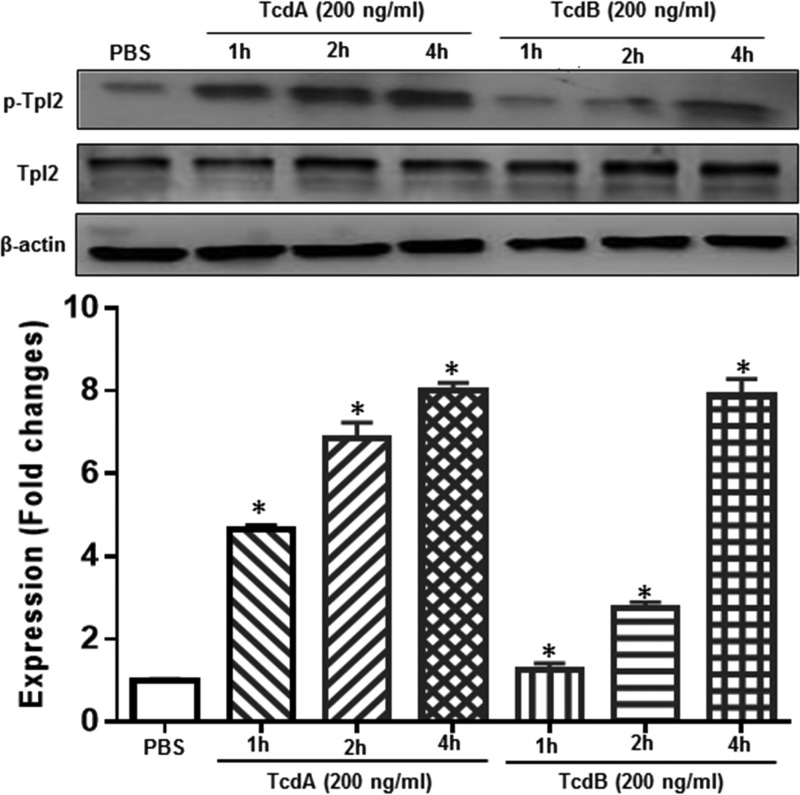
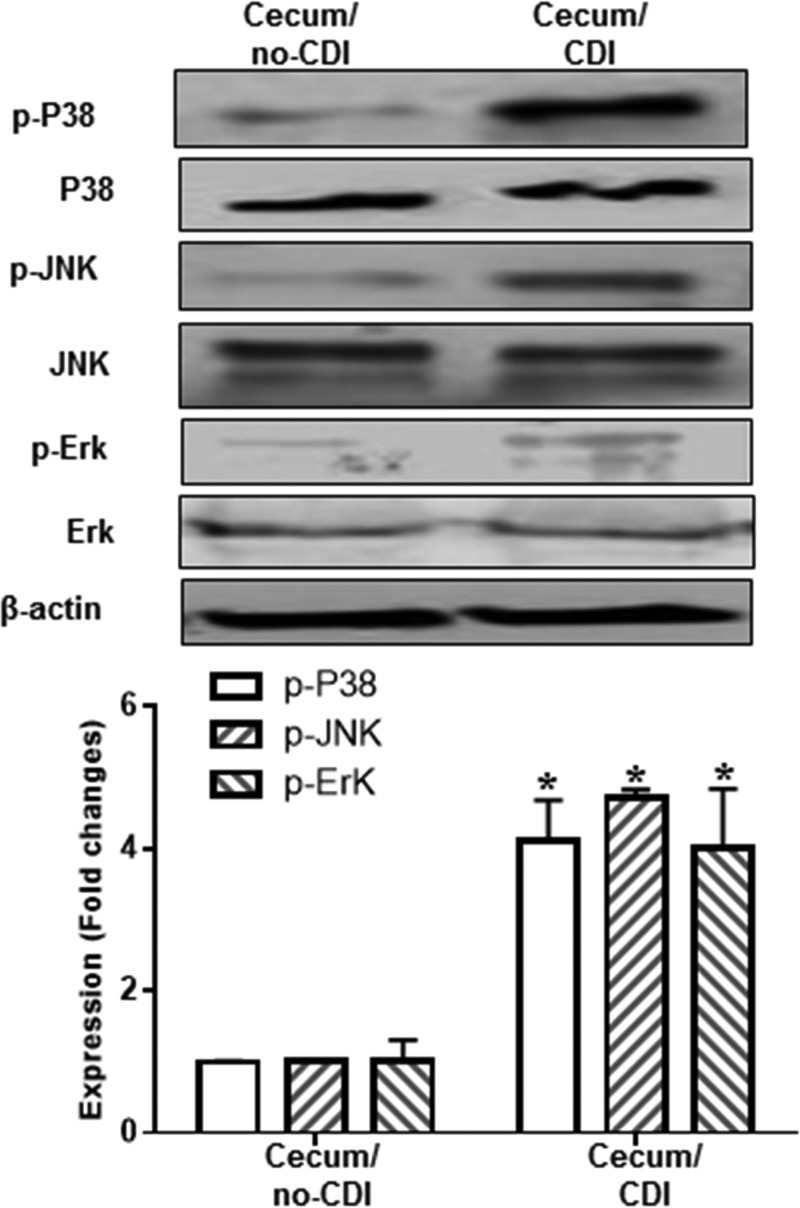
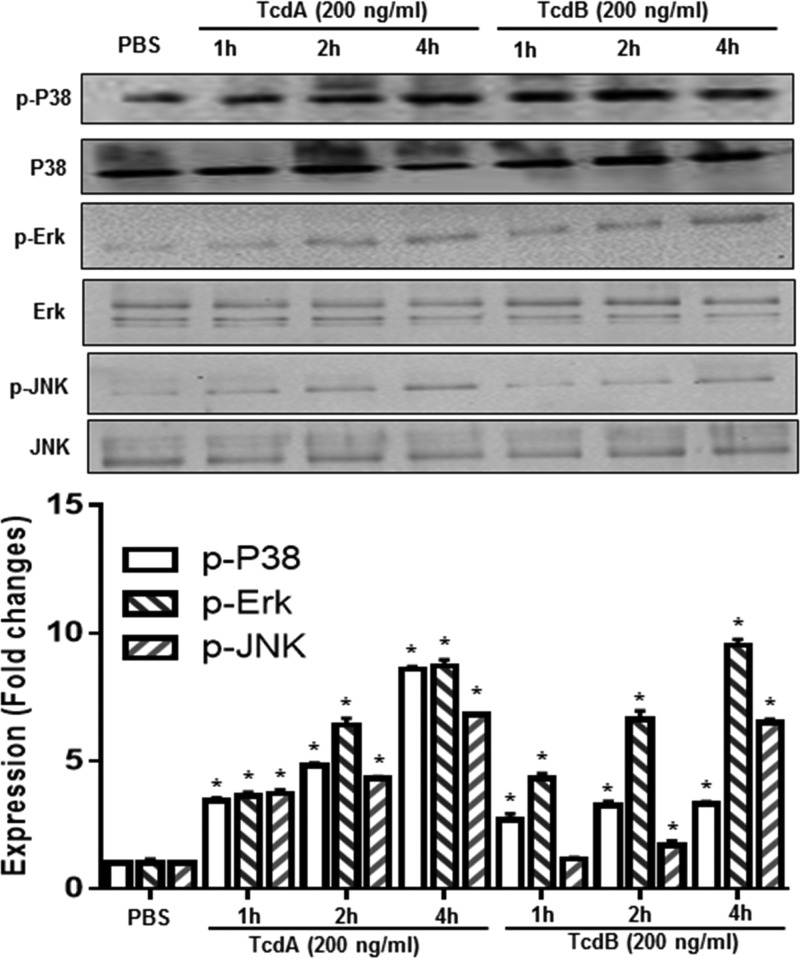
To investigate whether TPL2 is involved in C. difficile toxin-mediated intestinal inflammation, we first studied whether TPL2 is activated in vivo in a mouse model of C. difficile infection (CDI). To this end, cecal tissues from noninfected mice or C. difficile-infected mice that were moribund (n = 3) were analyzed for activation of TPL2 by Western blot analysis. As shown in Fig. 1, C. difficile infection significantly induced TPL2 phosphorylation. Most importantly, TcdA and TcdB also induced TPL2 phosphorylation in human colon tissues (mixture of three tissue samples) in a time-dependent manner (Fig. 2).
FIG 1.
C. difficile infection (CDI)-induced activation of TPL2. Cecal tissues from noninfected or C. difficile-infected C57/BL6 mice were homogenized, and supernatants were used for the analysis of phosphorylated TPL2 (p-TPL2) and total TPL2 by Western blot analysis. Cecal tissues were collected from three mice in each group.
FIG 2.
TcdA and TcdB induce TPL2 activation in human colon tissue. Human colonic tissues (mixture of 3 samples) were exposed to TcdA or TcdB at 200 ng/ml for the indicated times. Tissues were lysed for 30 min and were centrifuged, and supernatants were used for Western blot analysis. Asterisks indicate significant differences (*, P < 0.01) from expression in the control.
TPL2-KO mice are more resistant to C. difficile infection than wild-type (WT) mice.
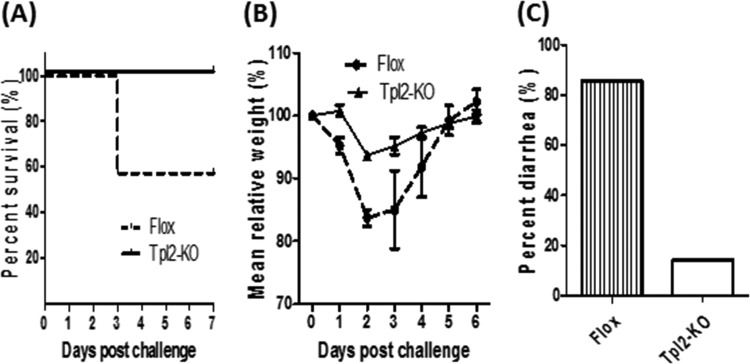
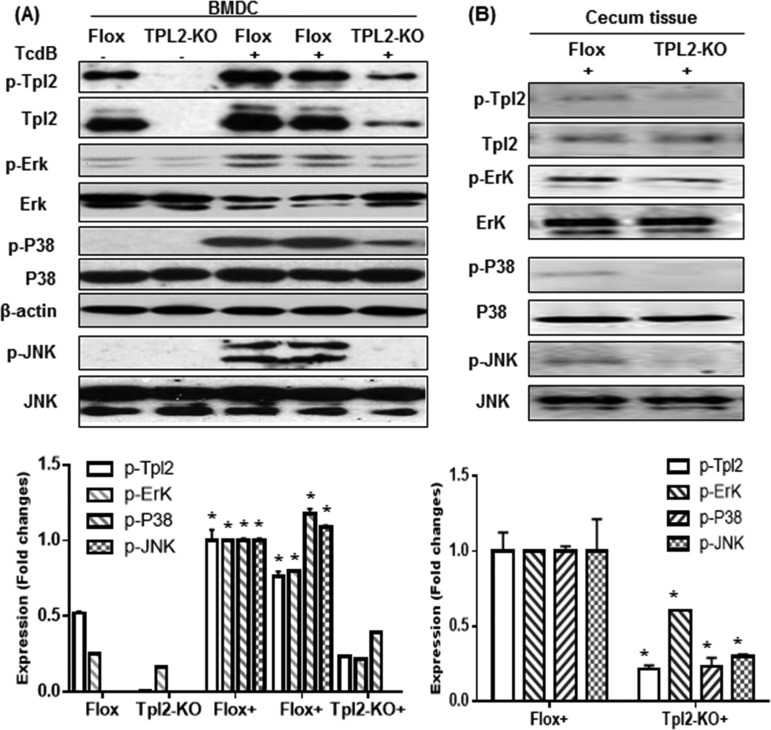
We then asked whether TPL2 plays an important role in vivo during CDI. To address this question, age- and genetic-background-matched control TPL2-floxed (Flox) and TPL2 knockout (TPL2-KO) mice (n, 10 in each group) were orally inoculated with C. difficile UK1 spores (106 CFU/mouse) after antibiotic pretreatment. Mice were monitored for a week after infection. Mice in the TPL2-KO group were significantly more resistant to CDI than those in the Flox group, as shown by the percentages of surviving mice (Fig. 3A), weight changes (Fig. 3B), and percentages of mice with diarrhea (Fig. 3C).
FIG 3.
TPL2-KO mice are more resistant to C. difficile infection than wild-type mice. TPL2-KO or TPL2-floxed (Flox) mice (n, 10 per group) were challenged with 106 C. difficile UK1 spores after treatment with a mixture of antibiotics. Mice were monitored for survival (P, <0.05 for the difference between the two groups) (A), weight changes (P, <0.0001 for the difference between the two groups) (B), and the percentage with diarrhea in the 7-day monitoring period (C).
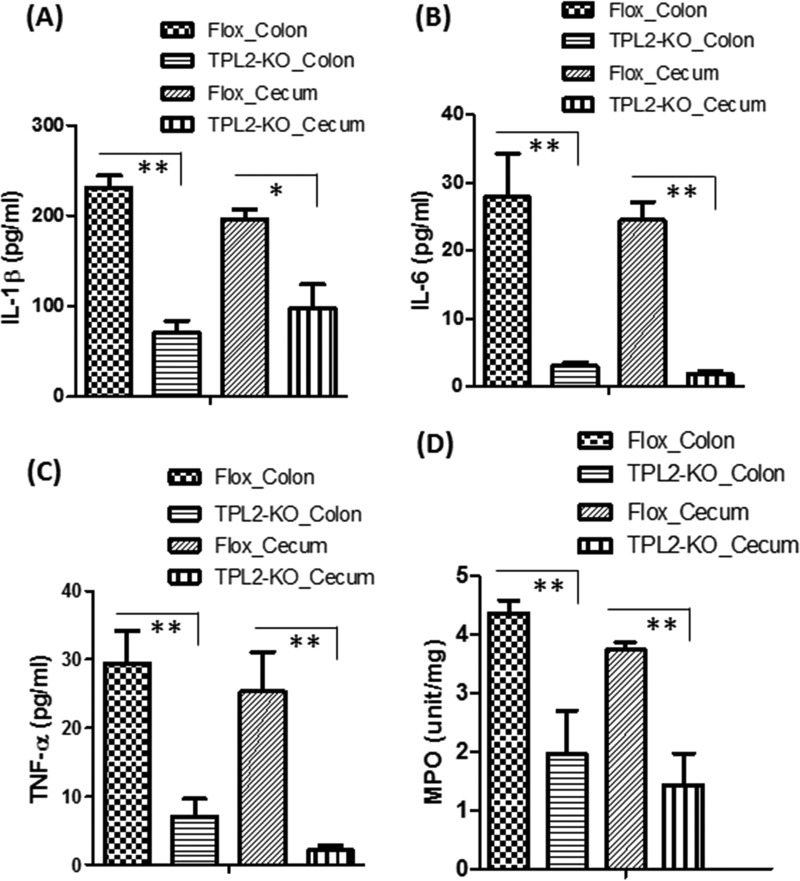
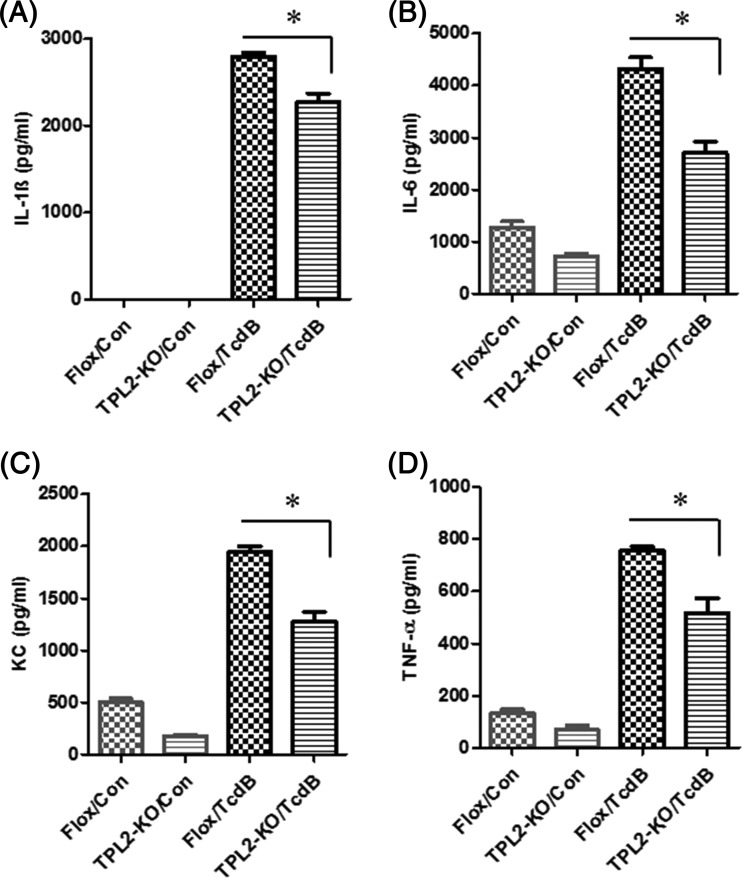
Intestinal tissues from the TPL2-KO group produced significantly smaller amounts of IL-1β, IL-6, and TNF-α, as well as myeloperoxidase (MPO), a marker of neutrophil infiltration, than intestinal tissues from the TPL2-floxed group (Fig. 4).
FIG 4.
C. difficile-infected TPL2-KO mice produce significantly lower levels of proinflammatory markers in intestines than WT mice. TPL2-KO or TPL2-floxed (Flox) mice were challenged with 106 C. difficile UK1 spores after pretreatment with a mixture of antibiotics. On day 3 postinfection, three mice from each group were euthanized, and colon and cecum tissues were collected. Levels of IL-1β (A), IL-6 (B), TNF-α (C), and MPO (in units per milligram) (D) were determined. Bars stand for means; error bars, standard deviations. Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01) between the Flox and TPL2-KO groups.
TPL2 inhibition or knockout abolishes C. difficile toxin-induced production of proinflammatory cytokines/chemokines.
Having demonstrated that TPL2-KO mice exhibited significantly reduced intestinal inflammation and production of proinflammatory cytokines, we wondered how TPL2 regulates the production of proinflammatory cytokines in the context of CDI.
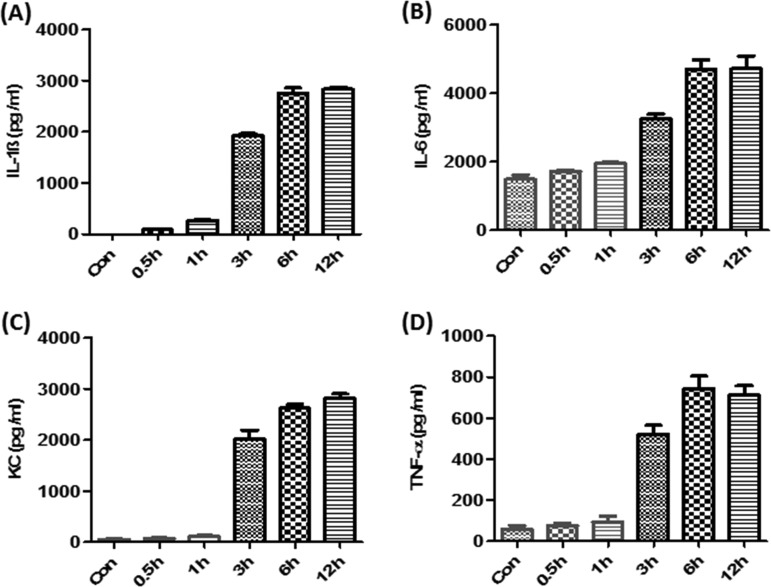
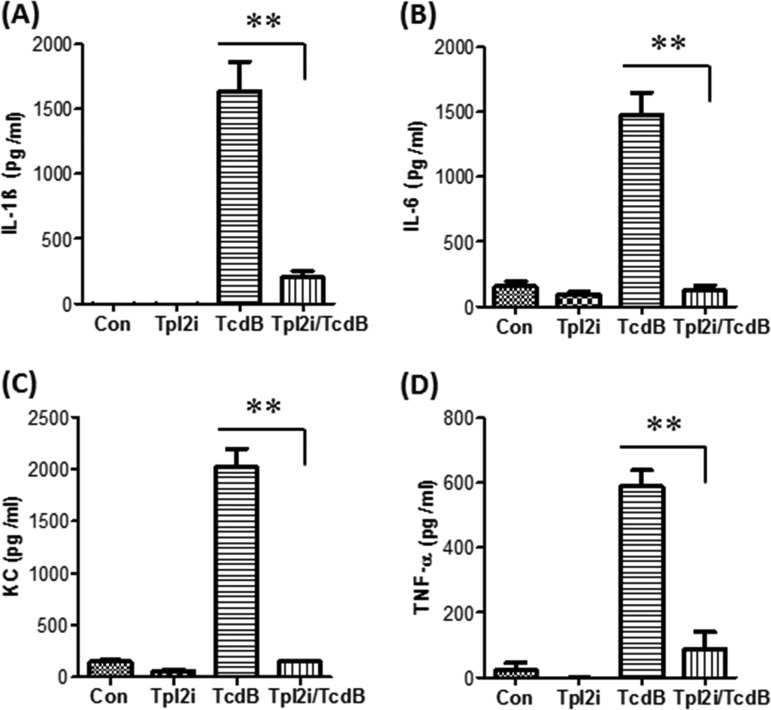
We and other groups reported previously that TcdA and TcdB induced the production of proinflammatory cytokines/chemokines by macrophages or dendritic cells (20, 21). In addition, recent studies have shown that TcdB plays more-important roles than TcdA in the pathogenesis of CDI. Therefore, we investigated the role of TPL2 in the production of proinflammatory cytokines/chemokines by bone marrow-derived dendritic cells (BMDCs) or bone marrow-derived macrophages (BMMPs) exposed to TcdB. As shown in Fig. 5 and Fig. S3 in the supplemental material, TcdB induced time-dependent production of IL-1β, IL-6, TNF-α, and CXCL1 (KC) by BMDCs (Fig. 5) or BMMPs (Fig. S3). Interestingly, a TPL2-specific inhibitor (TPL2i) dramatically reduced the production of IL-1β, IL-6, TNF-α, and CXCL1 (KC) by BMDCs (Fig. 6) or BMMPs (see Fig. S4 in the supplemental material). To further verify the positive regulation of proinflammatory cytokines/chemokines by TPL2, BMDCs or BMMPs derived from TPL2-KO or Flox mice were exposed to TcdB at 200 ng/ml for 6 h, and supernatants were collected for the detection of cytokines/chemokines by enzyme-linked immunosorbent assays (ELISA). As shown in Fig. 7 and Fig. S5 in the supplemental material, TPL2 deficiency significantly mitigated the production of IL-1β, IL-6, TNF-α, and CXCL1 (KC) by BMDCs (Fig. 7) or BMMPs (Fig. S5), though to different extents.
FIG 5.
TcdB induces the production of proinflammatory cytokines/chemokines by BMDCs. BMDCs derived from wild-type mice were exposed to TcdB for the indicated times, and supernatants were collected for the determination of IL-1β, IL-6, KC, and TNF-α levels by ELISA. The results presented are representative of three independent experiments (n = 3). Bars stand for means; error bars, standard deviations.
FIG 6.
A TPL2-specific inhibitor significantly inhibits the production of proinflammatory cytokines/chemokines by BMDCs. BMDCs derived from wild-type mice were pretreated with a TPL2 inhibitor (TPL2i) at 20 μM for 30 min, followed by exposure to TcdB (200 ng/ml) at 37°C for 3 h. Controls include cells treated with TPL2i, TcdB, or PBS (Con). Supernatants were collected, and IL-1β (A), IL-6 (B), KC (C), and TNF-α (D) levels were determined. The results presented are representative of three independent experiments (n = 3). Bars stand for means; error bars, standard deviations. Asterisks indicate significant differences (**, P < 0.01) between the TcdB and TcdB-plus-TPL2i groups.
FIG 7.
TPL2 knockout reduces TcdB-induced production of proinflammatory cytokines/chemokines by BMDCs. BMDCs from TPL2-floxed (Flox) or knockout (TPL2-KO) mice in 24-well plates were exposed to TcdB (200 ng/ml) for 6 h, after which supernatants were collected for determination of the production of IL-1β (A), IL-6 (B), KC (C), and TNF-α (D) by ELISA. The results presented are representative of three independent experiments (n = 3). Bars stand for means; error bars, standard deviations. Asterisks indicate significant differences (*, P < 0.05) between the Flox/TcdB and TPL2-KO/TcdB groups. Con, control.
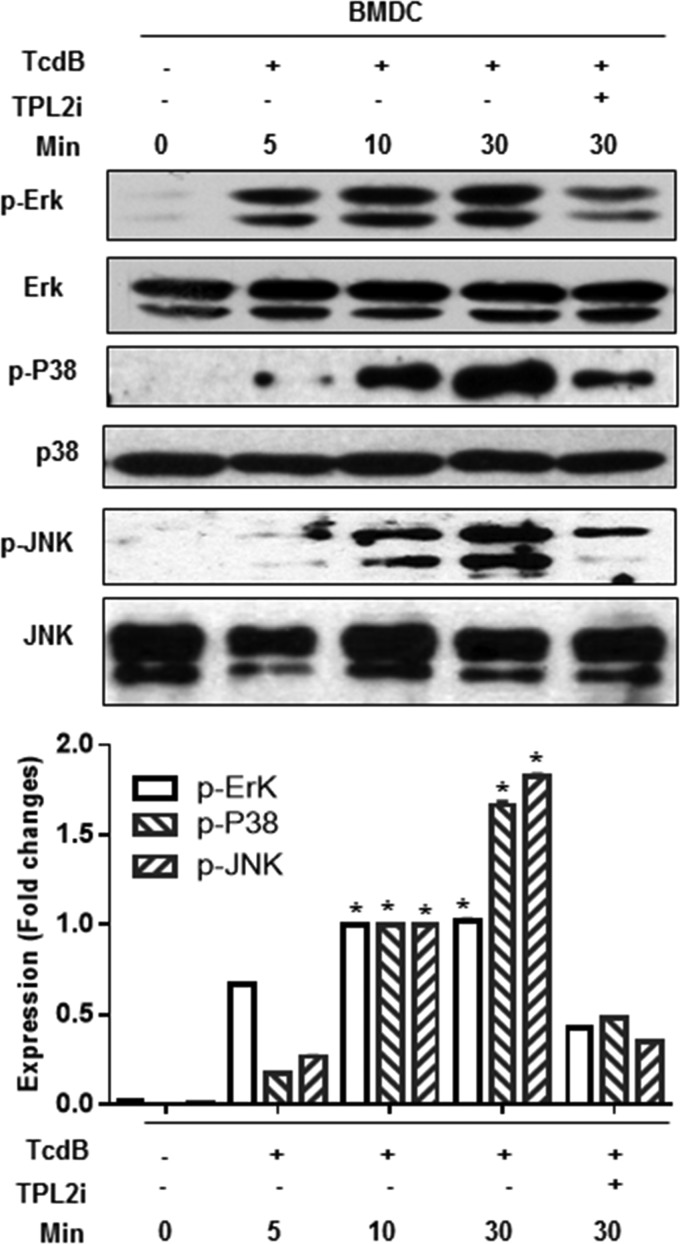
TPL2 inhibition or knockout reduces TcdB-mediated MAPK activation.
TPL2 is a serine/threonine kinase. Previous studies showed that TPL2 is critical for the production of TNF because of its role in ERK activation by macrophages in response to lipopolysaccharide (LPS) (22). In addition, the role of p38 signaling cascades in inflammatory disease has been well established. To investigate whether mitogen-activated protein kinases (MAPKs) are involved in TPL2-mediated positive regulation of the production of proinflammatory cytokines/chemokines, we studied whether TcdB induces the activation of MAPKs and TPL2, and whether TPL2 deficiency or inhibition affects the activation of MAPKs and TPL2. We found that TcdB activated p38, ERK, and JNK in a time-dependent manner both in BMDCs (Fig. 8) and in BMMPs (see Fig. S6 in the supplemental material). Furthermore, TPL2i effectively inhibited the activation of p38, ERK, and JNK (Fig. 8). These phenomena were further confirmed using BMDCs, cecal tissues (Fig. 9), or BMMPs (see Fig. S7 in the supplemental material) derived from Flox or TPL2-KO mice.
FIG 8.
TPL2 inhibition reduces MAPK activation in BMDCs. BMDCs from wild-type mice were exposed to TcdB (200 ng/ml) in the absence or presence of TPL2i (20 μm) in 24-well plates for the indicated times. Cells were harvested, lysed, and used for determination of the activation of ERK, p38, and JNK by Western blot analysis. Experiments were repeated 3 times, and representative results are shown. Asterisks indicate significant differences (*, P < 0.01) from results for the control.
FIG 9.
TPL2 knockout reduces MAPK activation. (A) BMDCs from TPL2-floxed (Flox) or TPL2 knockout (TPL2-KO) mice were exposed to TcdB (200 ng/ml) in 24-well plates for 30 min. (B) Cecal tissues from Flox or TPL2-KO mice infected with C. difficile were harvested, lysed, and used for determination of the activation of TPL2, ERK, p38, and JNK by Western blot analysis. Experiments were repeated 3 times, and representative results are shown. Asterisks indicate significant differences (*, P < 0.01) from results for the control.
More importantly, p38, ERK, and JNK were also activated in cecal tissues collected from C. difficile-infected mice (Fig. 10). Ex vivo studies showed that TcdA and TcdB also induced the activation of p38, ERK, and JNK in human colonic tissue (Fig. 11).
FIG 10.
C. difficile infection (CDI)-induced activation of p38, JNK, and ERK. Cecal tissues from noninfected and C. difficile-infected C57/BL6 mice were homogenized, and supernatants were used for analysis of phosphorylated (p-) and total p38, JNK, and ERK by Western blot analysis. β-Actin served as a loading control. Asterisks indicate significant differences (*, P < 0.01) from results for noninfected mice.
FIG 11.
TcdA- and TcdB-induced activation of MAPKs in human colonic tissue. Human colonic tissues were exposed to TcdA or TcdB at 200 ng/ml for the indicated times. Tissues were lysed for 30 min and were then centrifuged, and supernatants were used for Western blot analysis to detect phosphorylated and total p38, ERK, or JNK. Experiments were repeated 3 times, and representative results are shown. Asterisks indicate significant differences (*, P < 0.01) from results for the control (PBS).
MAPK inhibitors inhibit the production of proinflammatory cytokines by BMDCs and BMMPs.
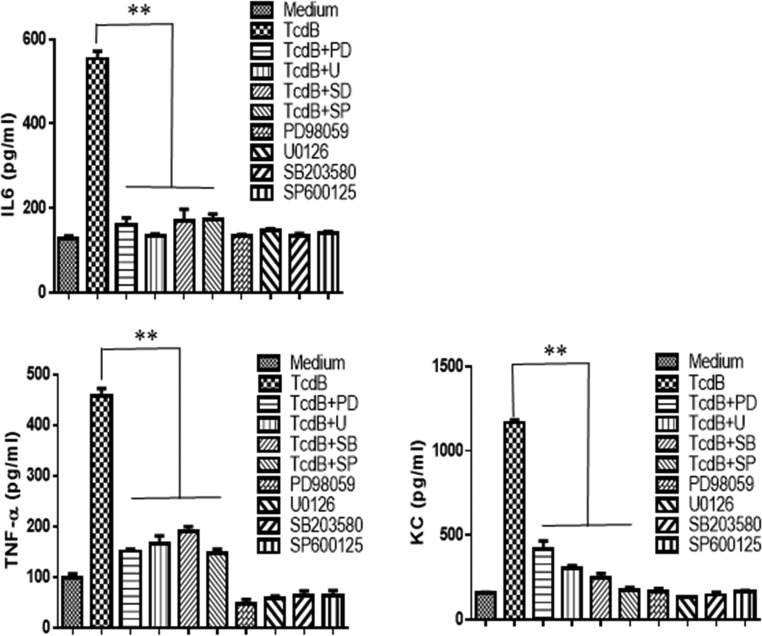
To further investigate whether MAPKs are involved in the production of cytokines, MAPK-specific inhibitors were used. As shown in Fig. 12 and Fig. S8 in the supplemental material, inhibitors of p38, ERK, or JNK dramatically reduced the production of IL-6, TNF-α, and CXCL1 (KC) by BMDCs (Fig. 12) or BMMPs (Fig. S8) exposed to TcdB at 200 ng/ml. Therefore, we concluded that TcdB activated TPL2, which further activated p38, ERK, and JNK for the production of proinflammatory cytokines/chemokines.
FIG 12.
MAPK-specific inhibitors significantly inhibit the production of proinflammatory cytokines/chemokines by BMDCs. BMDCs derived from wild-type mice were pretreated with SB203580 at 10 μM (inhibitor for p38), PD98059 at 20 μM (inhibitor for Erk), U0126 at 20 μM (inhibitor for MEK1/2), or SP600125 at 40 μM (inhibitor for JNK) for 30 min, followed by exposure to TcdB (200 ng/ml) for 5 h. Controls included cells treated with inhibitors only, TcdB only, or PBS (medium). Supernatants were collected, and levels of IL-6, KC, and TNF-α were determined. The results presented are representative of three independent experiments (n = 3). Bars stand for means; error bars, standard deviations. Asterisks indicate significant differences (**, P < 0.01) between results with TcdB alone and results with TcdB plus inhibitors.
DISCUSSION
TPL2/Cot (tumor progression locus 2, cancer Osaka thyroid; also known as MAP3k8) was originally identified as a proto-oncogene in a human thyroid carcinoma cell line (23). Later, TPL2 as a kinase was shown to have important functions in immune cells in regulating tumor necrosis factor alpha, Toll-like receptor (TLR), and G protein-coupled receptor signaling (22, 24, 25). TPL2 is expressed mainly in the immune cells but can also be detected in other cells, such as fibroblasts (26, 27). TPL2 is highly expressed in the spleen, thymus, and lungs but can be detected in the brain, testes, and liver at low levels (28, 29). Many studies have focused on the role of TPL2 in response to TLR ligands (22, 30, 31). Several reports have shown that TPL2 is important for host defense against infections in mouse models, including Listeria monocytogenes (24), Toxoplasma gondii (32), Mycobacterium tuberculosis (33), and influenza virus (34) infections. However, all these infectious agents are intracellular pathogens, and they respond to host immune defense differently from extracellular pathogens.
For the first time, we showed that TPL2 was activated, in vitro and in vivo, in response to C. difficile toxins or CDI. Our data showed that TPL2 positively regulated the production of major proinflammatory cytokines and chemokines, which have been postulated to be key drivers of intestinal inflammation as well as systemic inflammatory responses during CDI (10, 35). TcdA has been reported to activate p38, ERK, and JNK, leading to the production of IL-8 in intestinal cells (HT29) (36). p38 is activated by CDI in hamsters (37). p38, ERK, and TPL2 are also involved in inflammation in inflammatory bowel diseases (IBD) (38, 39), as well as in obesity-associated inflammation (40). Our report is the first to show that C. difficile toxins can activate p38, ERK, and JNK in human colon tissues.
C. difficile is a spore-forming, toxin-producing enteric pathogen. The major virulence factors of C. difficile are two large secreted protein toxins, TcdA and TcdB, which mainly target the Rho GTPases of host cells, while other intracellular targets, such as mitochondria, have been reported (41). Once secreted from C. difficile in the colon, TcdA and TcdB first attack intestinal epithelial cells, leading to the production of proinflammatory chemokines, disruption of tight junctions, and induction of cell apoptosis or necrosis (42), resulting, in turn, in a compromised intestinal barrier, where toxins can get access to the submucosa (43). Once in the submucosa, toxins can activate immune cells, including dendritic cells, macrophages, and mast cells, to produce proinflammatory cytokines/chemokines such as IL-6, TNF, IL-1β, and IL-17, while causing necrosis or apoptosis (44) of immune cells. It has been reported that neurons in intestine could be activated as well to produce neuron peptides, which further induce immune cells to produce proinflammatory cytokines/chemokines (45, 46). The proinflammatory cytokines/chemokines produced in the intestine further facilitate the infiltration of monocytes, especially neutrophils, into the site of infection, leading to a robust inflammatory response, which is the hallmark of CDI pathogenesis (43). In severe cases, toxins can gain access to blood, amplifying the systemic inflammatory responses and further spread to organs, causing organ failure (47). Therefore, the initial major symptoms of CDI are intestinal inflammation and diarrhea. Uncontrolled inflammation can cause tissue damage (megacolon). Control of CDI-induced inflammation would be an effective adjuvant therapy once the disease symptoms start.
The roles of TPL2 in inflammatory diseases, such as IBD (38), periodontitis (15, 48), and obesity-associated inflammation (40), have been investigated in mouse models. These studies show that TPL2 is predominantly proinflammatory. TPL2 inhibition or deficiency in mice significantly reduced inflammation symptoms. Our data further support the proinflammatory roles of TPL2 in inflammation during CDI manifestation. In another model of inflammation, TPL2-KO mice challenged with an endotoxin (LPS) produced dramatically reduced levels of TNF-α and IL-1β (40). Intraperitoneal treatment of mice with the TPL2 kinase inhibitor ameliorated dextran sulfate sodium (DSS)-induced colitis (38). These results clearly argue for targeting TPL2 kinase for the treatment of IBD and other diseases associated with increased TNF-α and IL-1β production. Our data indicate that TPL2 inhibition could be a potentially effective therapy for CDI.
Neutrophil infiltration is a hallmark event in CDI-mediated intestinal inflammation (49). TPL2 has important roles in neutrophil infiltration (26). In the future, we will investigate the role of TPL2 in neutrophil infiltration in the context of CDI. In addition, we will further investigate the connection between Rho GTPases, which are intracellular targets of C. difficile toxins, and TPL2 in regulating signaling pathways involved in the production of proinflammatory mediators in CDI.
There are a couple of potential caveats to targeting the inflammatory response during acute CDI. For example, there is evidence that adaptive immunity is important for the prevention of recurrent infection. It is difficult to predict how TLP2 inhibition will affect those beneficial responses. In addition, p38 is involved in the “nonclassical” lymphocyte activation cascade and may contribute to the adaptive response. It would be interesting to know how TLP2 impacts anti-toxin immunity; this will be one of the subjects of a follow-up study.
MATERIALS AND METHODS
Animals.
All studies followed the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (50) and were approved by the Institutional Animal Care and Use Committee (IACUC) at Tufts University. Wild-type (WT) C57BL/6 mice were purchased from Charles River Laboratories. TPL2-floxed (Flox) or TPL2 knockout (TPL2-KO) C57BL/6 mice were generated by genOway (see Fig. S1A in the supplemental material). The mice were bred, and all the offspring were screened by DNA genotyping (Fig. S1B).
For genotyping, mouse genomic DNA was isolated from tail biopsy specimens following overnight digestion at 55°C in a buffer containing 50 mM Tris-HCl (pH 8.0), 10 mM EDTA, 100 mM NaCl, 0.1% SDS, and 1 mg/ml proteinase K, followed by heat inactivation. The WT and TPL2-floxed alleles were distinguished with primers F1/R1 (F1, GAGTAACAGCTAGGCAGACAAACAGGTTAGC; R1, AGCCT CCAAG GAACA AGGAG AACAT CC), which should yield a 421-bp product for the WT and a 523-bp product for the Flox allele (Fig. S1B). The TPL2-KO and WT mice were distinguished with primers F2/R2 (F2, AAACAGCAATCTGGATGTGAGACTAGATGG; R2, AGTTCCAAGCAGAGCAGAAGGAAACG), which yielded a 3,168-bp product from TPL2-KO mice (Fig. S1C) and no reliable band from WT mice.
To further characterize the TPL2-KO phenotype, bone marrow-derived macrophages (BMMPs) from TPL2-floxed or knockout mice were treated with LPS (100 ng/ml) for 4 h. mRNA was extracted, and reverse transcription-PCR (RT-PCR) was performed. TPL2-KO mice displayed significantly reduced synthesis of the TPL2 and IL-1β genes (see Fig. S2 in the supplemental material).
Mouse model of C. difficile infection.
A mouse model of C. difficile infection was established as described previously (32, 51). Groups of 10 C57BL/6 mice each (wild type, TPL2-KO, or TPL2-floxed) were given a mixture of five antibiotics, including kanamycin (0.4 mg/ml), gentamicin (0.035 mg/ml), colistin (850 U/ml), metronidazole (0.215 mg/ml), and vancomycin (0.045 mg/ml), in their drinking water for 4 days. After 4 days of antibiotic treatment, all mice were given autoclaved water for 2 days, followed by a single dose of clindamycin (10 mg/kg) given intraperitoneally 1 day before challenge with 106 C. difficile UK1 spores/mouse by gavage. On day 3 postinfection, 3 mice from each group were euthanized, and colonic and cecal tissues were harvested and used for analysis of the activation/induction of TPL2 or the production of TNF-α, IL-1β, IL-6, KC, and MPO as described below. The animals were monitored daily for weight changes, diarrhea and survival, and moribund animals were euthanized.
Determination of TPL2 activation/expression or MPO activity in the intestines of mice infected with C. difficile.
Cecal and colonic tissues from mice were dissected and were immersed in liquid nitrogen to snap-freeze. All the samples were stored at −80°C for later use or were kept on ice for immediate homogenization. The tissue samples (30 mg) were homogenized in 300 μl radioimmunoprecipitation assay (RIPA) lysis buffer with 1% protease inhibitor cocktail and were centrifuged at 13,000 rpm for 15 min, and the supernatants were collected and stored at −20°C prior to analysis of activation/expression by Western blot analysis. Concentration of cytokines/chemokines (TNF-α, IL-1β, IL-6, and KC) in supernatants were determined by an ELISA kit from R&D Systems.
MPO activity in the tissue supernatants was determined according to the instructions with the MPO kit (catalog no. ab105136; Abcam). Briefly, 50 μl of the reaction mixture was added to the tissue samples, which were incubated at room temperature for 30 to 120 min, followed by the addition of a stop mixture and incubation at room temperature for another 10 min. Finally, trinitrobenzene (TNB) reagents were added and incubated at room temperature for 5 min. The optical density was measured at 412 nm. Results were expressed as MPO units per milligram (wet weight) of tissue. Here, 1 U of MPO is the amount of MPO that hydrolyzes the substrate and generates taurine chloramine to consume 1.0 μmol of TNB per min at 25°C.
TPL2 activation/expression in human colonic tissues treated with TcdA or TcdB.
Human colonic tissues were purchased from the National Disease Research Interchange (NDRI), Philadelphia, PA. Minced tissues were treated with 200 ng/ml TcdA or TcdB at 37°C for 1, 2, or 4 h. After toxin treatments, the tissues were homogenized at 4°C in RIPA buffer with 1% protease inhibitor and were centrifuged at 13,000 rpm for 15 min, and the supernatants were collected and stored at −20°C prior to analysis of TPL2 activation/expression by Western blot analysis.
Generation of BMDCs.
Primary mouse bone marrow-derived dendritic cells (BMDCs) were obtained and cultured as described previously (52). BMDCs were isolated from bone marrow of the tibias and femurs of WT or TPL2-KO C57BL/6 mice. The cells were cultured in RPMI 1640 medium containing 10% fetal calf serum (FCS) and supplemented with 100 IU/ml penicillin, 100 mg/ml streptomycin, and 10 μM β-mercaptoethanol, with 10% conditioned medium from granulocyte-macrophage colony-stimulating factor-producing B16 cells for BMDC differentiation. Cells (3 × 107/2 ml/well) were seeded in 24-well plates. Half of the medium (1 ml) was replaced with fresh medium every other day. On day 6, cells were harvested and were used in all subsequent experiments.
Generation of BMMPs.
Primary mouse BMMPs were generated and cultured as described previously (53). BMMPs were isolated from the bone marrow of the tibias and femurs of WT or TPL2-KO C57BL/6 mice. The cells were cultured in RPMI 1640 medium containing 10% FCS, supplemented with 100 IU/ml penicillin, 100 mg/ml streptomycin, and 10 μM β-mercaptoethanol, with 10% conditioned medium (L929) for BMMP differentiation. A total of 5 × 107 cells/ml/well were seeded in 24-well plates. The cells were washed twice with phosphate-buffered saline (PBS) every 2 to 3 days and were incubated with fresh medium. BMMPs were fully differentiated at day 6.
Production of cytokines/chemokines by MAPKs or activation of MAPKs in mouse BMDCs or BMMPs treated with TcdB.
BMDCs or BMMPs from WT or TPL2-KO mice were exposed to a TPL2 inhibitor (TPL2i; catalog no. sc-204351; Santa Cruz Biotechnologies) at 20 μM, SP600125 at 40 μM (catalog no. 8177; Cell Signaling Technology), SB203580 at 10 μM (catalog no. 5633; Cell Signaling Technology), PD98059 at 20 μM (catalog no. 9900; Cell Signaling Technology), U0126 at 20 μM (catalog no. 9903; Cell Signaling Technology), or nothing, followed by exposure to TcdB at 200 ng/ml for various times. The levels of the cytokines/chemokines (IL-1β, IL-6, CXCL1/KC, TNF-α) in the supernatants were determined by ELISA according to the manufacturer's instructions (R&D Systems).
Western blot analysis.
Protein extracts from tissues or cells were subjected to 12% SDS-PAGE separation. Then the proteins were transferred to a nylon membrane. After blocking for 1 h at room temperature with 5% skim milk, the membrane was incubated overnight at 4°C with a TPL2 antibody (dilution, 1:250; catalog no. sc373677; Santa Cruz Biotechnologies, Santa Cruz, CA) or a phospho-TPL2 (Ser400) antibody (dilution, 1:500; catalog no. 4491; Cell Signaling, Beverly, MA), a phospho-p38 MAPK antibody (dilution, 1:500; catalog no. CAS 9211; Cell Signaling), a p38 MAPK antibody (dilution, 1:500; catalog no. CAS 9212; Cell Signaling), a phospho-p44/42 MAPK (ERK1/2) antibody (dilution, 1:500; catalog no. CAS 9101; Cell Signaling), a p44/42 MAPK (ERK1/2) antibody (dilution, 1:500; catalog no. CAS 9102; Cell Signaling), a phospho-SAPK (stress-activated protein kinase)/JNK antibody (dilution, 1:500; catalog no. CAS 9251; Cell Signaling), or a SAPK/JNK antibody (dilution, 1:500; catalog no. CAS 9252; Cell Signaling). After a wash with PBST (PBS with 0.05% Tween), the membrane was incubated with a horseradish peroxidase-conjugated goat anti-rabbit (catalog no. ab97051; goat anti-rabbit IgG; dilution, 1:3,000) or goat anti-mouse (catalog no. ab97023; goat anti-mouse IgG; dilution, 1:3,000) secondary antibody (both from Abcam, Cambridge, MA), and the antibody-reactive bands were revealed by enhanced chemiluminescence detection on Hyperfilm (Thermo Fisher Scientific, Waltham, MA).
Statistical analysis.
Data were analyzed by Kaplan-Meier survival analysis with a log rank test of significance, by analysis of variance (ANOVA), and by one-way or two-way ANOVA followed by Bonferroni posttests using the Prism statistical software program. Results are expressed as means ± standard errors of means. Differences were considered statistically significant if the P value was <0.05.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants K01-DK092352, R21-AI113470, R03-DK112004, and R01-AI132711 to X. Sun and by ARS/USDA grants 1950-51000-071-02S and 1950-51000-071-02S, NIH grants P30 DK046200 and DK108722 01A1, and the Robert C. and Veronica Atkins Foundation to A. Greenberg.
All the authors contributed to the writing of this article. X.S. designed the project and participated in data analysis. Y.W. and S.W. performed experiments and data analysis. All authors read and approved the final manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00095-18.
REFERENCES
- 1.Elliott B, Chang BJ, Golledge CL, Riley TV. 2007. Clostridium difficile-associated diarrhoea. Intern Med J 37:561–568. doi: 10.1111/j.1445-5994.2007.01403.x. [DOI] [PubMed] [Google Scholar]
- 2.Kelly CP, Pothoulakis C, LaMont JT. 1994. Clostridium difficile colitis. N Engl J Med 330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 3.Voth DE, Ballard JD. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev 18:247–263. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarchum I, Liu M, Shi C, Equinda M, Pamer EG. 2012. Critical role for MyD88-mediated neutrophil recruitment during Clostridium difficile colitis. Infect Immun 80:2989–2996. doi: 10.1128/IAI.00448-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasegawa M, Yamazaki T, Kamada N, Tawaratsumida K, Kim YG, Nunez G, Inohara N. 2011. Nucleotide-binding oligomerization domain 1 mediates recognition of Clostridium difficile and induces neutrophil recruitment and protection against the pathogen. J Immunol 186:4872–4880. doi: 10.4049/jimmunol.1003761. [DOI] [PubMed] [Google Scholar]
- 6.McDermott AJ, Frank CR, Falkowski NR, McDonald RA, Young VB, Huffnagle GB. 2014. Role of GM-CSF in the inflammatory cytokine network that regulates neutrophil influx into the colonic mucosa during Clostridium difficile infection in mice. Gut Microbes 5:476–484. doi: 10.4161/gmic.29964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linevsky JK, Pothoulakis C, Keates S, Warny M, Keates AC, Lamont JT, Kelly CP. 1997. IL-8 release and neutrophil activation by Clostridium difficile toxin-exposed human monocytes. Am J Physiol 273:G1333–G1340. [DOI] [PubMed] [Google Scholar]
- 8.De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, Gunzer M, Roers A, Hogg N. 2013. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 121:4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 9.Rowley SM, Kuriakose T, Dockery LM, Tran-Ngyuen T, Gingerich AD, Wei L, Watford WT. 2014. Tumor progression locus 2 (Tpl2) kinase promotes chemokine receptor expression and macrophage migration during acute inflammation. J Biol Chem 289:15788–15797. doi: 10.1074/jbc.M114.559344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czepiel J, Biesiada G, Brzozowski T, Ptak-Belowska A, Perucki W, Birczynska M, Jurczyszyn A, Strzalka M, Targosz A, Garlicki A. 2014. The role of local and systemic cytokines in patients infected with Clostridium difficile. J Physiol Pharmacol 65:695–703. [PubMed] [Google Scholar]
- 11.Hansen A, Alston L, Tulk SE, Schenck LP, Grassie ME, Alhassan BF, Veermalla AT, Al-Bashir S, Gendron FP, Altier C, MacDonald JA, Beck PL, Hirota SA. 2013. The P2Y6 receptor mediates Clostridium difficile toxin-induced CXCL8/IL-8 production and intestinal epithelial barrier dysfunction. PLoS One 8:e81491. doi: 10.1371/journal.pone.0081491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jose S, Madan R. 2016. Neutrophil-mediated inflammation in the pathogenesis of Clostridium difficile infections. Anaerobe 41:85–90. doi: 10.1016/j.anaerobe.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steele J, Chen K, Sun X, Zhang Y, Wang H, Tzipori S, Feng H. 2012. Systemic dissemination of Clostridium difficile toxins A and B is associated with severe, fatal disease in animal models. J Infect Dis 205:384–391. doi: 10.1093/infdis/jir748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong JH, Bhatia A, Toth Z, Oh S, Inn KS, Liao CP, Roy-Burman P, Melamed J, Coetzee GA, Jung JU. 2011. TPL2/COT/MAP3K8 (TPL2) activation promotes androgen depletion-independent (ADI) prostate cancer growth. PLoS One 6:e16205. doi: 10.1371/journal.pone.0016205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vougioukalaki M, Kanellis DC, Gkouskou K, Eliopoulos AG. 2011. Tpl2 kinase signal transduction in inflammation and cancer. Cancer Lett 304:80–89. doi: 10.1016/j.canlet.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Martel G, Rousseau S. 2014. TPL2 signalling: from Toll-like receptors-mediated ERK1/ERK2 activation to cystic fibrosis lung disease. Int J Biochem Cell Biol 52:146–151. doi: 10.1016/j.biocel.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Sun X, Savidge T, Feng H. 2010. The enterotoxicity of Clostridium difficile toxins. Toxins (Basel) 2:1848–1880. doi: 10.3390/toxins2071848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vohra P, Poxton IR. 2012. Induction of cytokines in a macrophage cell line by proteins of Clostridium difficile. FEMS Immunol Med Microbiol 65:96–104. doi: 10.1111/j.1574-695X.2012.00952.x. [DOI] [PubMed] [Google Scholar]
- 19.Péchiné S, Gleizes A, Janoir C, Gorges-Kergot R, Barc MC, Delmee M, Collignon A. 2005. Immunological properties of surface proteins of Clostridium difficile. J Med Microbiol 54:193–196. doi: 10.1099/jmm.0.45800-0. [DOI] [PubMed] [Google Scholar]
- 20.Sun X, He X, Tzipori S, Gerhard R, Feng H. 2009. Essential role of the glucosyltransferase activity in Clostridium difficile toxin-induced secretion of TNF-α by macrophages. Microb Pathog 46:298–305. doi: 10.1016/j.micpath.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng J, Hirota SA, Gross O, Li Y, Ulke-Lemee A, Potentier MS, Schenck LP, Vilaysane A, Seamone ME, Feng H, Armstrong GD, Tschopp J, Macdonald JA, Muruve DA, Beck PL. 2010. Clostridium difficile toxin-induced inflammation and intestinal injury are mediated by the inflammasome. Gastroenterology 139:542–552, 552.e1–552.e3. doi: 10.1053/j.gastro.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN. 2000. TNF-α induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 103:1071–1083. doi: 10.1016/S0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 23.Miyoshi J, Higashi T, Mukai H, Ohuchi T, Kakunaga T. 1991. Structure and transforming potential of the human cot oncogene encoding a putative protein kinase. Mol Cell Biol 11:4088–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mielke LA, Elkins KL, Wei L, Starr R, Tsichlis PN, O'Shea JJ, Watford WT. 2009. Tumor progression locus 2 (Map3k8) is critical for host defense against Listeria monocytogenes and IL-1β production. J Immunol 183:7984–7993. doi: 10.4049/jimmunol.0901336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaiser F, Cook D, Papoutsopoulou S, Rajsbaum R, Wu X, Yang HT, Grant S, Ricciardi-Castagnoli P, Tsichlis PN, Ley SC, O'Garra A. 2009. TPL-2 negatively regulates interferon-beta production in macrophages and myeloid dendritic cells. J Exp Med 206:1863–1871. doi: 10.1084/jem.20091059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu D, Matsumoto ML, McKenzie BS, Zarrin AA. 26 November 2017. TPL2 kinase action and control of inflammation. Pharmacol Res doi: 10.1016/j.phrs.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 27.Koliaraki V, Roulis M, Kollias G. 2012. Tpl2 regulates intestinal myofibroblast HGF release to suppress colitis-associated tumorigenesis. J Clin Invest 122:4231–4242. doi: 10.1172/JCI63917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gantke T, Sriskantharajah S, Ley SC. 2011. Regulation and function of TPL-2, an IκB kinase-regulated MAP kinase kinase kinase. Cell Res 21:131–145. doi: 10.1038/cr.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patriotis C, Makris A, Bear SE, Tsichlis PN. 1993. Tumor progression locus 2 (Tpl-2) encodes a protein kinase involved in the progression of rodent T-cell lymphomas and in T-cell activation. Proc Natl Acad Sci U S A 90:2251–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugimoto K, Ohata M, Miyoshi J, Ishizaki H, Tsuboi N, Masuda A, Yoshikai Y, Takamoto M, Sugane K, Matsuo S, Shimada Y, Matsuguchi T. 2004. A serine/threonine kinase, Cot/Tpl2, modulates bacterial DNA-induced IL-12 production and Th cell differentiation. J Clin Invest 114:857–866. doi: 10.1172/JCI20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerjee A, Gugasyan R, McMahon M, Gerondakis S. 2006. Diverse Toll-like receptors utilize Tpl2 to activate extracellular signal-regulated kinase (ERK) in hemopoietic cells. Proc Natl Acad Sci U S A 103:3274–3279. doi: 10.1073/pnas.0511113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, Kelly CP. 2008. A mouse model of Clostridium difficile-associated disease. Gastroenterology 135:1984–1992. doi: 10.1053/j.gastro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 33.McNab FW, Ewbank J, Rajsbaum R, Stavropoulos E, Martirosyan A, Redford PS, Wu X, Graham CM, Saraiva M, Tsichlis P, Chaussabel D, Ley SC, O'Garra A. 2013. TPL-2-ERK1/2 signaling promotes host resistance against intracellular bacterial infection by negative regulation of type I IFN production. J Immunol 191:1732–1743. doi: 10.4049/jimmunol.1300146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuriakose T, Tripp RA, Watford WT. 2015. Tumor progression locus 2 promotes induction of IFNλ, interferon stimulated genes and antigen-specific CD8+ T cell responses and protects against influenza virus. PLoS Pathog 11:e1005038. doi: 10.1371/journal.ppat.1005038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu H, Chen K, Sun Y, Carter M, Garey KW, Savidge TC, Devaraj S, Tessier ME, von Rosenvinge EC, Kelly CP, Pasetti MF, Feng H. 2017. Cytokines are markers of the Clostridium difficile-induced inflammatory response and predict disease severity. Clin Vaccine Immunol 24:e00037-17. doi: 10.1128/CVI.00037-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JY, Park HR, Oh YK, Kim YJ, Youn J, Han JS, Kim JM. 2007. Effects of transcription factor activator protein-1 on interleukin-8 expression and enteritis in response to Clostridium difficile toxin A. J Mol Med (Berl) 85:1393–1404. doi: 10.1007/s00109-007-0237-7. [DOI] [PubMed] [Google Scholar]
- 37.Bobo LD, El Feghaly RE, Chen YS, Dubberke ER, Han Z, Baker AH, Li J, Burnham CA, Haslam DB. 2013. MAPK-activated protein kinase 2 contributes to Clostridium difficile-associated inflammation. Infect Immun 81:713–722. doi: 10.1128/IAI.00186-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawrenz M, Visekruna A, Kuhl A, Schmidt N, Kaufmann SH, Steinhoff U. 2012. Genetic and pharmacological targeting of TPL-2 kinase ameliorates experimental colitis: a potential target for the treatment of Crohn's disease? Mucosal Immunol 5:129–139. doi: 10.1038/mi.2011.57. [DOI] [PubMed] [Google Scholar]
- 39.Herlaar E, Brown Z. 1999. p38 MAPK signalling cascades in inflammatory disease. Mol Med Today 5:439–447. [DOI] [PubMed] [Google Scholar]
- 40.Perfield JW II, Lee Y, Shulman GI, Samuel VT, Jurczak MJ, Chang E, Xie C, Tsichlis PN, Obin MS, Greenberg AS. 2011. Tumor progression locus 2 (TPL2) regulates obesity-associated inflammation and insulin resistance. Diabetes 60:1168–1176. doi: 10.2337/db10-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He D, Sougioultzis S, Hagen S, Liu J, Keates S, Keates AC, Pothoulakis C, Lamont JT. 2002. Clostridium difficile toxin A triggers human colonocyte IL-8 release via mitochondrial oxygen radical generation. Gastroenterology 122:1048–1057. doi: 10.1053/gast.2002.32386. [DOI] [PubMed] [Google Scholar]
- 42.Chumbler NM, Farrow MA, Lapierre LA, Franklin JL, Lacy DB. 2016. Clostridium difficile toxins TcdA and TcdB cause colonic tissue damage by distinct mechanisms. Infect Immun 84:2871–2877. doi: 10.1128/IAI.00583-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aktories K, Barbieri JT. 2005. Bacterial cytotoxins: targeting eukaryotic switches. Nat Rev Microbiol 3:397–410. doi: 10.1038/nrmicro1150. [DOI] [PubMed] [Google Scholar]
- 44.Warny M, Keates AC, Keates S, Castagliuolo I, Zacks JK, Aboudola S, Qamar A, Pothoulakis C, LaMont JT, Kelly CP. 2000. p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J Clin Invest 105:1147–1156. doi: 10.1172/JCI7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anton PM, Gay J, Mykoniatis A, Pan A, O'Brien M, Brown D, Karalis K, Pothoulakis C. 2004. Corticotropin-releasing hormone (CRH) requirement in Clostridium difficile toxin A-mediated intestinal inflammation. Proc Natl Acad Sci U S A 101:8503–8508. doi: 10.1073/pnas.0402693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pothoulakis C, Castagliuolo I, LaMont JT, Jaffer A, O'Keane JC, Snider RM, Leeman SE. 1994. CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc Natl Acad Sci U S A 91:947–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Bella S, Ascenzi P, Siarakas S, Petrosillo N, di Masi A. 2016. Clostridium difficile toxins A and B: insights into pathogenic properties and extraintestinal effects. Toxins (Basel) 8:E134. doi: 10.3390/toxins8050134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohnishi T, Okamoto A, Kakimoto K, Bandow K, Chiba N, Matsuguchi T. 2010. Involvement of Cot/Tp12 in bone loss during periodontitis. J Dent Res 89:192–197. doi: 10.1177/0022034509353405. [DOI] [PubMed] [Google Scholar]
- 49.Abt MC, McKenney PT, Pamer EG. 2016. Clostridium difficile colitis: pathogenesis and host defence. Nat Rev Microbiol 14:609–620. doi: 10.1038/nrmicro.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 51.Sun X, Wang H, Zhang Y, Chen K, Davis B, Feng H. 2011. Mouse relapse model of Clostridium difficile infection. Infect Immun 79:2856–2864. doi: 10.1128/IAI.01336-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matheu MP, Sen D, Cahalan MD, Parker I. 2008. Generation of bone marrow derived murine dendritic cells for use in 2-photon imaging. J Vis Exp doi: 10.3791/773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weischenfeldt J, Porse B. 2008. Bone marrow-derived macrophages (BMM): isolation and applications. CSH Protoc 2008:pdb.prot5080. doi: 10.1101/pdb.prot5080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.