Abstract
Rationale
Despite increasing prevalence and incidence of heart failure (HF), therapeutic options remain limited. In early stages of HF, sudden cardiac death (SCD) from ventricular arrhythmias claims many lives. Reactive oxygen species (ROS) have been implicated in both arrhythmias and contractile dysfunction. However, little is known about how ROS in specific subcellular compartments contribute to HF or SCD pathophysiology. The role of ROS in chronic proteome remodeling has not been explored.
Objective
We will test the hypothesis that elevated mitochondrial ROS (mROS) is a principal source of oxidative stress in HF and in vivo reduction of mROS mitigates SCD.
Methods and Results
Using a unique guinea pig model of non-ischemic HF that recapitulates important features of human HF, including prolonged QT interval and high incidence of spontaneous arrhythmic SCD, compartment-specific ROS sensors revealed increased mROS in resting and contracting left ventricular (LV) myocytes in failing hearts. Importantly, mitochondrially-targeted antioxidant (MitoTEMPO) normalized global cellular ROS. Further, in vivo MitoTEMPO treatment of HF animals prevented and reversed HF; eliminated SCD by decreasing dispersion of repolarization and ventricular arrhythmias; suppressed chronic HF-induced remodeling of the expression proteome; and prevented specific phosphoproteome alterations. Pathway analysis of mROS-sensitive networks indicated that increased mROS in HF disrupts the normal coupling between cytosolic signals and nuclear gene programs driving mitochondrial function, antioxidant enzymes, Ca2+ handling and action potential repolarization, suggesting new targets for therapeutic intervention.
Conclusion
mROS drive both acute emergent events, such as electrical instability responsibly for SCD, and those that mediate chronic HF remodeling, characterized by suppression or altered phosphorylation of metabolic, antioxidant and ion transport protein networks. In vivo reduction of mROS prevents and reverses electrical instability, SCD and HF. Our findings support the feasibility of targeting the mitochondria as a potential new therapy for HF and SCD while identifying new mROS-sensitive protein modifications.
Keywords: Reactive oxygen species, mitochondria, heart failure, cardiac arrhythmia, proteomics, phosphorylation, ventricular fibrillation, antioxidant, sudden cardiac death
Subject Terms: Arrhythmias, Basic Science Research, Heart Failure, Oxidant Stress, Sudden Cardiac Death
INTRODUCTION
Heart failure (HF) is a clinical syndrome of progressive functional degradation that occurs secondary to various insults including hypertension, myocardial infarction, tachycardia, or toxic stimuli. It affects an estimated 26 million people worldwide1 and in the United States alone, there are ~300,000 HF-related deaths per year with an estimated economic impact of $39 billion2. While conventional treatments forestall cardiac pump failure, 2–5% of heart failure patients still die of sudden cardiac death (SCD) each year3. There are few effective treatments to prevent SCD, although implantable cardiac defibrillators (ICDs) can decrease fatalities from such events. However, many HF patients die before meeting the criteria for ICD implantation (cardiac ejection fraction less than 35%), and in a community-wide study, only one-third of SCD cases analyzed had LV dysfunction severe enough to qualify for a prophylactic ICD 4. Moreover, in a more selective group of patients with non-ischemic systolic HF, ICD therapy decreased SCD but did not significantly improve long-term survival5. Thus, novel therapies for HF/SCD are urgently needed.
Mitochondrial dysfunction is increasingly recognized as a significant factor in the progression of HF6, 7 and as a mechanism of arrhythmogenesis 8, 9. Impairment of several mitochondrial functions has been noted in HF, including defects in network organization, fission/fusion, mitophagy, biogenesis, substrate selection, oxidative phosphorylation, the unfolded protein response, and redox signaling. Defective mROS handling has emerged as a central factor10, underscored by data in transgenic mice showing that overexpression of a ROS scavenging enzyme (catalase) in the mitochondria, but not the cytoplasm, blunts HF11. The need to target a specific source of ROS, in a specific compartment, may partly explain why clinical trials of more general antioxidants, such as vitamins E12 or C13, have failed to improve, or even worsened, outcomes in HF patients. Targeting mitochondrial oxidative stress with a peptide (e.g., Bendavia, a.k.a. SS-31, MTP-131, or elamipretide) that prevents cardiolipin oxidation14 improves outcomes in animal models of ischemia-reperfusion injury15 or pressure-overload HF16, but it is still unclear whether it is effective in humans17–19. The clinical effectiveness of MitoQ, a targeted form of ubiquinone, in heart failure also remains to be determined20.
It is unknown if mitochondrial antioxidant therapy is beneficial for HF-associated SCD, in part, due to a paucity of appropriate animal models. In this regard, we previously reported that preserving mitochondrial ROS balance by enhancing coupling between Ca2+ and the Krebs cycle abrogated oxidative stress during increased energy demand and prevented both SCD and HF progression in a guinea pig model of acquired long QT and spontaneous SCD, which has electrophysiological and Ca2+ handling characteristics close to that of humans8. All of these studies provide a strong rationale for targeting mROS to prevent both acute adverse events and chronic remodeling in HF; however, it is not known what mechanisms are involved in coupling mROS to HF signaling, or if mitochondrial antioxidant intervention has the potential to rescue cardiac function and prevent SCD when HF is already present. Moreover, how mROS intervention impacts the signal transduction pathways and protein post-translational modifications implicated in cardiac hypertrophy and failure has not been determined.
Here, we establish mROS as the key driver of HF remodeling and SCD from the subcellular level to the whole organism by utilizing novel tools to assess: i) compartment-specific ROS responses in ventricular myocytes from normal and failing hearts, ii) the ability of a targeted mROS scavenger to prevent or reverse HF and SCD in vivo, and iii) the impact of mitochondrial antioxidant intervention on changes in the proteome and phosphoproteome that occur during the development of HF. We show, for the first time, that baseline and stimulated H2O2 levels are elevated in the mitochondrial matrix compartment in HF and that SCD can be eliminated and contractile function rescued by an mROS scavenger. We describe the HF-induced changes in the proteome and phosphoproteome that are mROS-dependent, opening the door to novel protein targets and pathways for therapeutic intervention for HF/SCD.
METHODS
All data and materials supporting the findings of this study are available within the article and in the Online Data Supplement or from the corresponding authors on reasonable request.
RESULTS
Spontaneous non-fatal (PVC) and fatal (SCD) arrhythmia incidence during cardiac hypertrophy and failure
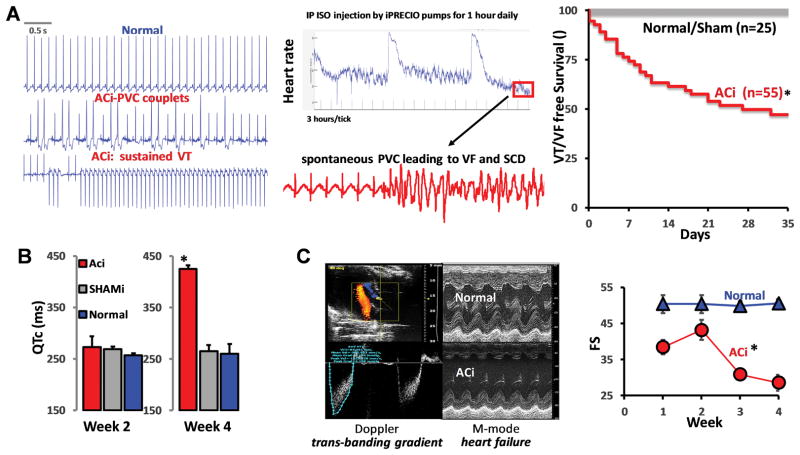
The guinea pig HF/SCD model combines ascending aortic constriction (AC) with daily administration of the β-adrenergic agonist, isoproterenol (Iso, 2mg/kg/day) (ACi model) and progresses from a brief compensated hypertrophy phase (<2 weeks post banding) to HF by 4 weeks post banding8. The model displays typical hallmarks of HF, including prolonged QT interval, cellular action potential prolongation, downregulation of repolarizing K+ channels (e.g., KCNJ2, KCNH4, KCNQ1), depressed Ca2+ handling proteins (CACNA1C, ATP2A2, RYR2), and decreases in mitochondrial metabolic and antioxidant proteins21. Pathways of inflammation and extracellular matrix remodeling are also markedly increased in this model21. A unique feature of the model is the high incidence of spontaneous SCD (~60% over 4 weeks) due to ventricular tachycardia/ fibrillation (VT/VF) (Fig. 1A), usually occurring in the recovery periods in between the daily β-adrenergic challenge delivered by an implanted programmable pump. While QT prolongation (Fig. 1B) and SCD occurs quite early after pressure-overload, prior to overt failure, the rate of non-fatal premature ventricular complexes (PVCs) increases over time in parallel with the cardiac contractile decompensation (Fig. 1C).
FIGURE 1. Phenoytpe of ACi guinea pig model of non-ischemic heart failure (HF) leading to sudden cardiac death (SCD).
A) Representative telemetric electrocardiogram recordings showing spontaneous arrhythmias including SCD. Top trace shows normal sinus rhythm from a control animal. Middle and lower traces show premature ventricular complexes (PVC) and sustained ventricular tachycardia (VT) from an ACi animal. All recordings were collected at 10 PM, 9 hours post β-adrenergic stimulus (see methods for detailed ACi protocol). Middle panel shows heart rate response over 4 days (96 hours) to intraperitoneal infusion of isoproterenol (Iso) via implanted programmable pump (0.7mg/kg), given once daily for 1 hour at 1PM. Fatal arrhythmias typical occurred well after heart rate recovery, 5–6 hours post isoproterenol injection. Right panel displays Kaplan Meier survival curve estimating VT/VF-free survival. More than 50% of animals in the ACi (N=55) group experienced SCD (Normal, N=25). B) Average measurements of corrected QT intervals at week 2 and week 4 showing that long QT was induced by pressure overload (Normal (N=5): no aortic constriction, no daily Iso; Shami (N=5): Sham-operated + daily Iso; ACi (N=12): aortic constriction + daily Iso. C) Serial echo Doppler studies were performed at baseline, 2 weeks and 4 weeks to verify model consistency, i.e., specific transaortic and transbanding pressure gradients and peak velocities. Right panel M-mode echo in ACi group (N=9) as compared to Normal controls (N=5). By week 4, cardiac function (Fractional Shortening: FS%) declined in ACi group to 28%, as compared to Normal controls (~51%), as shown in the righthand panel.
H2O2 levels are elevated in the mitochondrial matrix and cytoplasm of adult cardiac myocytes from failing hearts and are strongly attenuated by a mitochondrial ROS scavenger
We incorporated the genetically-encoded, compartment-specific H2O2 sensors, cytoORP and mitoORP22 into adenoviral gene transfer vectors23 and expressed them in vivo via injection of the ventricular free wall during the open chest aortic banding procedure. Expression persisted throughout the protocol, allowing us to study compartment-specific ROS levels in freshly isolated myocytes from normal and failing hearts (Fig. 2A). The ratiometric nature of the probes (opposite redox responses of 405nm versus 488nm excitation wavelength signals at 520nm emission), together with the ability to calibrate each experiment in terms of the max and min 405/488nm ratio, permitted us to calculate the fractional oxidation of the sensor (Fox), as described in 23, which facilitated quantitative comparisons of basal and stimulated H2O2 levels in both compartments for each experimental group.
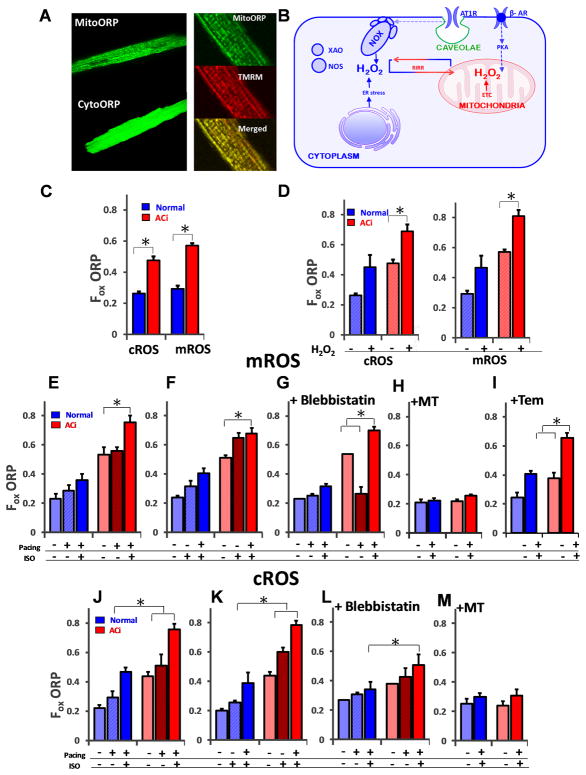
FIGURE 2. Mitochondrial ROS imbalance revealed by compartment-specific H2O2 probes.
A) Genetically-encoded H2O2 sensors targeting the cytosol (cytoORP) or mitochondrial matrix (mitoORP) were expressed in vivo by viral gene transfer to quantify ROS in guinea pig left ventricular (LV) myocytes isolated from normal or failing hearts. The enlarged images in the right-hand panel show representative images of a myocyte expressing mitoORP (top, 128x128 pixels, scale bar- 0.14μm/pixel ) together with the mitochondrial membrane potential sensor tetramethylrhodamine methyl ester (TMRM;middle) and their co-localization indicated in the merged image (bottom, 256x256 pixels, scale bar- 0.268μm/pixel ) B) Schematic showing the major sources of ROS in a cardiomyocyte leading to oxidative stress in the pathogenesis of heart failure including NADPH oxidase (NOX), xanthine oxidase (XAO), monoamine oxidase (MAO) and the mitochondrial electron transport chain (ETC). RIRR: mitochondrial ROS-induced ROS release. C) Baseline ROS levels (37oC; 1 Hz field stimulation), represented by Fractional oxidation of the ORP probes, (Fox) were similar across intracellular cytoplasmic and mitochondrial compartments in normal hearts, while both cROS and mROS were significantly higher in failing LV myocytes (red bars), as compared normal controls (blue bars). mROS levels were higher than cROS levels in ACi group. D) The response to an oxidative perturbation was tested by exposure to exogenous H2O2 (1 mM). Scavenging of exogenous ROS in failing LV myocytes was impaired in the ACi group (red bars) as compared to Normal myocytes (blue bars). E) In a sequential protocol (left panel); pacing alone (1 Hz) did not significantly increase mROS in either normal controls or ACi myocytes unless paired with β-AR stimulation with Iso (100nM; left panel). F) In a second protocol, Iso alone increased intracellular mROS production without pacing in the ACi group Baseline and stimulated mROS levels were higher in ACi compared to the normal group. G) Uncoupling myofilament activation from EC coupling with blebbistatin treatment (right panel) prevented the mROS increase from field stimulation alone (the level was even decreased), but did not suppress mROS accumulation induced by Iso + Pacing in ACi myocytes. H) Treatment with MitoTEMPO inhibited ROS generation in the presence of Iso+Pacing. On the contrary, I) Tempol was not able to prevent ROS generation. J) cROS response for sequential application of Pacing (1Hz) + Iso or K) Iso + Pacing (middle) in normal or ACi groups. cROS was maximally elevated in both groups when Pacing and Iso were combined, while L) Blebbistatin markedly suppressed the exaggerated cROS response in ACi. M) MitoTEMPO also effectively inhibited cROS generation in presence of Iso+Pacing. N≥5 replicates for all experiments, >20 myocytes for each group; Error bars show SEM; *p<0.05 vs. control group.
Possible sources of ROS contributing to oxidative stress in heart failure include the mitochondrial electron transport chain, NADPH oxidases, monoamine oxidases, xanthine oxidase or uncoupled nitric oxide synthase24 (Fig. 2B). Crosstalk between compartments can occur in several ways, i) by mitochondrial ROS-induced ROS release (RIRR)25, whereby cytoplasmic ROS (cROS) is amplified by a regenerative mitochondrial ROS response, ii) by mitochondrial ROS-dependent activation of NADPH oxidase26, or iii) by the scavenger system of one compartment (e.g., the mitochondria) contributing to ROS scavenging in another27, given the membrane permeability of H2O2. Measurement of baseline ROS levels in mitochondria (mROS) and cytoplasm (cROS) of quiescent LV myocytes from normal and failing hearts (Fig. 2C) revealed that H2O2 in the failing group was significantly higher in both compartments compared to normal non-failing heart cells (cROS Normal: 0.26±0.01; cROS HF: 0.47±0.02; p<0.05; mROS Normal: 0.29±.020; mROS HF: 0.57±.02; p<0.05). The dynamic response to a fixed ROS exposure (1mM tert-butyl hydroperoxide) was tested and markedly higher H2O2 levels were observed in HF myocytes compared to normal, in both the cytoplasm and mitochondrial matrix compartments under exogenous ROS stress (Fig. 2D). These findings are consistent with the significant downregulation of mitochondrial antioxidant proteins in this model21 (and in the proteomics data described below).
We previously demonstrated that increased work by electrical stimulation in the presence of β-adrenergic activation greatly enhances ROS accumulation measured with an untargeted ROS probe (carboxymethyl dihydrodichlorofluorescein) in HF/SCD myocytes8. Interestingly, using the targeted probes, here we show that mROS increased significantly during 1 Hz pacing in the ACi group, but only in the presence of β-adrenergic stimulation (Fig. 2E) – when the order was reversed, we discovered that β-adrenergic stimulation alone sufficed to induce near maximal mitochondrial oxidative stress (Fig. 2F). This effect was not reversed by uncoupling contraction from excitation/Ca2+ using blebbistatin (Fig. 2G). Indeed, in the presence of blebbistatin, pacing without β-adrenergic activation lowered mROS in HF/SCD cells down to the normal levels, presumably by increasing NADPH availability through Ca2+-stimulated activation of the Krebs cycle 8, 28. This effect on mROS was overcome when a higher energy demand (through increased Ca2+ cycling) was imposed by β-adrenergic receptor activation. The cROS responses for the same protocols described above showed an additive effect of Iso and pacing (Fig. 2J, K), as well as suppression of the cROS response by blebbistatin treatment with no anomalous effects (Fig. 2L). MitoTEMPO is composed of a heterocyclic nitroxyl moiety linked to a triphenylphosphonium ion that facilitates its accumulation in mitochondria29, conferring functionality as a superoxide dismutase mimetic and peroxyl scavenger. MitoTEMPO attenuated both mROS and cROS generation in the presence of β-adrenergic stimulation with pacing (Fig 2H, M). The untargeted antioxidant analog Tempol, however, did not prevent mROS generation in the presence of β-adrenergic stimulation (Fig 2I). These observations indicate that the mROS response to both energy demand and β-adrenergic stimulation are enhanced in HF.
We next evaluated the impact of mitochondrial versus non-mitochondrial ROS sources on compartment-specific redox responses. MitoTEMPO (25 nM) effectively normalized both cROS and mROS in ACi myocytes, while minimally impacting ROS in Normal control myocytes. Tempol also normalized both mROS and cROS in ACi myocytes at baseline, but, as described above, was less effective than MitoTEMPO under conditions of high oxidative stress. In contrast, the NADPH oxidase (NOX) inhibitor apocynin, (100μM) only partially inhibited cROS and decreased mROS marginally in ACi myocytes (Fig. 3A–B). GP91-ds-tat (3μM), a specific inhibitor of NOX2, similarly had a marginal effect on mROS and cROS in myocytes from failing hearts. These data indicate that basal, receptor independent, NOX activity, in the absence of exogenous angiotensin II (Ang II), contributes to oxidative stress in HF myocytes, but to a lesser extent than do mitochondria. Moreover, mitochondria are essential for scavenging ROS in both the mitochondrial matrix and the cytosol compartments.
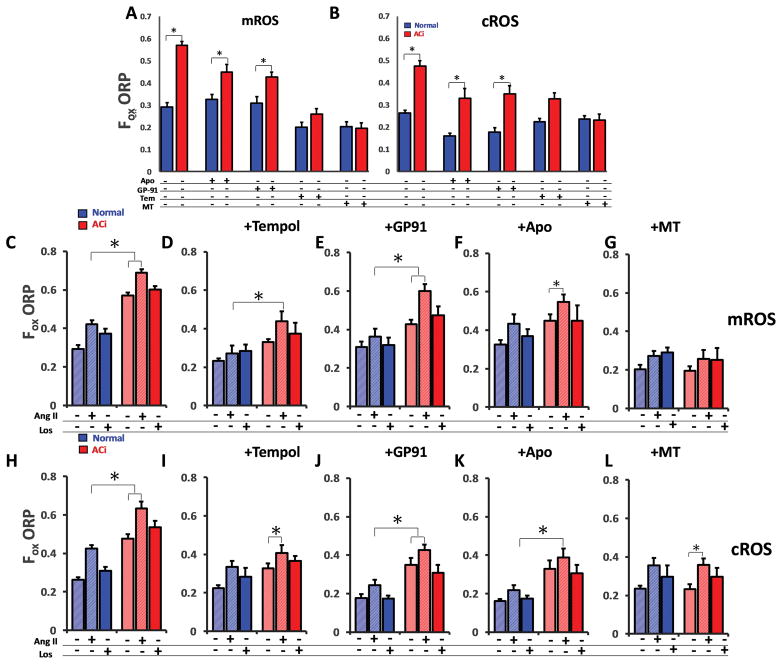
FIGURE 3. Targeted antioxidant effects on mROS and cROS in HF.
A–B) MitoTEMPO, the mROS specific scavenger, markedly decreased basal mROS (left panel) and cROS (right panel) in LV myocytes from ACi hearts (red bars) and partly (N.S.) suppressed basal mROS in normal myocytes (blue bars). Tempol, a general antioxidant, decreased mROS and cROS Apocynin, an NOX inhibitor and GP91-ds-tat (peptide inhibitor for NOX2), decreased cROS in both normal and ACi significantly, but had only a modest (but significant) effect on mROS in ACi. Both apocynin and GP91-ds-tat had no effect on mROS levels in the Normal group. C) Angiotensin II (Ang II) increased mROS in both normal and ACi groups. Reversal by the Angiotensin receptor blocker Losartan (Los) confirmed that effects are mediated by the Angiotensin type 1 receptor. High baseline levels contributed to higher overall ROS levels in ACi but the Ang II-activated/Los-sensitive mROS responses were quantitatively similar in normal and ACi groups. Los did not decrease the high baseline ROS levels in ACi. D, I) treatment with tempol blunted Ang II responses in both mROS and cROS. E–F, J–K) Treatment with Apocynin and GP91-ds-tat only marginally changed the Ang II-induced ROS in the mitochondrial compartment but basal and Ang II-stimulated cROS were sensitive to Apocynin and GP91-ds-tat. G,L) MitoTEMPO lowered the baseline ROS levels and effectively prevented Ang II-mediated increase in mROS but did not abolish cROS generation upon Ang II stimulation;. N≥5 replicates for all experiments, >20 myocytes for each group; Error bars show SEM; *p<0.05 vs. control group, ANOVA; p < .001.
Compartment-specific ROS responses to Angiotensin II receptor activation
Activation of the renin-angiotensin system leads to elevated Ang II in HF and contributes to LV structural remodeling. Ang II receptor-mediated NOX activation triggers ROS-dependent signal cascades that contribute to cardiac hypertrophy and fibrosis and NOX-derived ROS might also directly impact excitation-contraction coupling and increase arrhythmias through redox effects on Ca2+ handling proteins and ion channels30. To date, Ang II hormone-stimulated H2O2 responses in the cytoplasmic and mitochondrial compartments have not been selectively measured in cardiac myocytes. Therefore, we examined the H2O2 response to Ang II (100 pM), and its inhibition by the antagonist losartan (100 nM), in normal and HF myocytes in the cytoplasm (cytoORP) and in the mitochondrial matrix (mitoORP), where the NOX signal could propagate through RIRR9. Ang II significantly increased both mROS (Fig. 3C) and cROS (Fig. 3H) in HF and Normal groups (p<0.05) and this effect was inhibited by Ang II receptor blockade with losartan (p<0.01). Notably, the absolute increase in ROS level attributable to Ang II stimulation was similar in normal and HF groups, although the latter took place on top of the higher baseline noted above. In addition, unlike apocynin, losartan had no effect on basal ROS, indicating that the high baseline was not due to auto activation of Ang II receptors by endogenous Ang II, known to be present in heart cells31, but rather, was likely due to a constitutively active, yet undetermined, ROS source. Tempol normalized baseline mROS and cROS in ACi (p<.01), but only partially blocked mROS generation in the presence of Ang II (mROS vs cROS, p<0.05) (Fig 3D, I). This partial suppression could reflect the contribution of RIRR to mitochondrial oxidative stress during Ang II receptor activation. Like Tempol, Apocynin had a greater effect on Ang II-induced cROS (Fig. 3K) than mROS (mROS vs cROS, p<0.05) (Fig. 3F) in addition to its baseline cROS-lowering effect.
Consistent with this, specifically targeting NOX2 inhibition with GP91-ds-tat only marginally alleviated mROS in the presence of Ang II (mROS vs cROS, p<0.01) (Fig 3E, J). The effect of Ang II on cROS in the presence of GP91-ds-tat was modest but significant (p<0.01). Interestingly, in the presence of either the NOX2-specific inhibitor (GP91-ds-tat) or the nonselective oxidase inhibitor (apocynin), mROS generation was still not suppressed during Ang II activation, suggesting an alternative pathway besides NOX2 was activated by receptor stimulation leading to mitochondrial oxidative stress. Most importantly, MitoTEMPO blocked the increase in mROS signal in the presence of Ang II (p<0.01) (Fig. 3G, L) but did not suppress the cROS response, demonstrating that it does preferentially scavenge mROS over extramitochondrial sources.
Mitochondrially-Targeted Antioxidant Therapy Prevents or Reverses HF and SCD
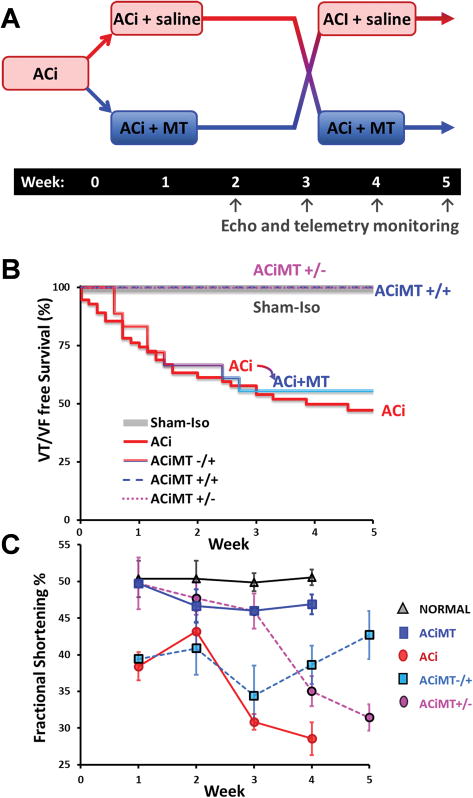
The cellular data described above indicated that oxidative stress in HF/SCD myocytes could be normalized by MitoTEMPO. To determine if this intervention could be used therapeutically to mitigate HF or SCD in vivo, we designed a crossover study in which MitoTEMPO (delivered via implanted osmotic pump, dose: 0.7mg/kg/day) was present i) from the onset of the ACi protocol through the development of HF (4 weeks; ACiMT+/+ group), ii) from the time of aortic banding until overt contractile dysfunction is usually evident in the untreated ACi group (3 weeks post banding; ACiMT+/− group), or iii) after HF had already developed in the ACi group (3 weeks post banding; ACiMT−/+ group) (Fig. 4A).
FIGURE 4. Cross-over study with MitoTEMPO.
A) Schematic of a randomized 2x2 crossover study of vehicle (ACi) and MitoTEMPO (MT) treatments. The protocols were as follows: i) ACi, ii) ACi treated with MT for 5-weeks (ACiMT+/+), iii) ACi for 3 weeks and MT treatment discontinued at 3 weeks (ACiMT+/−), iv) ACi for 3 weeks and MT treatment started at 3 weeks (ACiMT−/+), v) normal controls. Cardiac function (echo) and ECG recordings were sampled at weeks 2/3/4/5. B) Survival curve shows than more than 50% of animals died by week 3 in ACi (N=55) but survival was 100% at week 5 in the ACiMT+/+ (N=10) group. Survival was significantly improved with MT treatment at week 3 (ACiMT−/+, N=18)). Discontinuation of MT treatment in ACiMT+/− (N=16) did not result in an increase in mortality. C) Cardiac function was preserved by MitoTEMPO treatment as compared to ACi (N=10). Plot shows the time series of fractional shortening (FS%) for all groups over a 5-week period. While MT therapy preserved cardiac function in ACiMT+/+ (N=7, blue symbols), FS% decreased within 1 week when MT was discontinued (ACiMT+/−; N=12, magenta symbols), although SCD was not observed (above). Conversely, treatment with MT after the development of HF at week 3 restored cardiac function in ACiMT−/+ (N=7, Cyan symbols) in parallel with SCD prevention. Error bars show SEM; *p<0.05 vs. control group.
When MitoTEMPO was present during the entire protocol, no mortality was observed (Fig. 4B) and cardiac function did not decline over 4 weeks, similar to the normal controls (Fig. 4C). Moreover, MitoTEMPO treated animals showed no cardiac dilation (Online Figure IA). Interestingly, in the ACiMT+/− group, although HF and SCD were prevented initially, cessation of MitoTEMPO treatment at 3-week post banding resulted in a rapid descent into HF (decline in FS% from 46% to 31%; Fig. 4C). Surprisingly, removing supplemental mROS scavenging quickly led to HF, but did not increase SCD incidence (Fig. 4B). Most important from the therapeutic perspective, the ACiMT−/+ group demonstrated that HF could be reversed, and SCD eliminated, by MitoTEMPO treatment after HF/SCD is already present (Fig. 4B). Cardiac function improved (FS% from 30% to 42%) (Fig. 4C) and 100% of the animals survived after the introduction of MitoTEMPO at week 3 (Fig. 4B).
To extend the validity of our findings to a different HF model that was not subjected to daily isoproterenol stress, we repeated the MitoTEMPO treatment protocol in a guinea pig model of aortic constriction alone (AC)8. Observations were made over a period of 5 weeks. The AC group does not experience SCD, and demonstrates a slow progression of HF over a period of 6–8 weeks. Death occurs at end stage due to pump failure. Whereas cardiac function in AC starts declining by week 2 post banding, treatment with MitoTEMPO preserved the cardiac function and prevented the onset of HF (Online Figure IV).
Effects of MitoTEMPO on in vivo electrophysiological properties of HF/SCD model
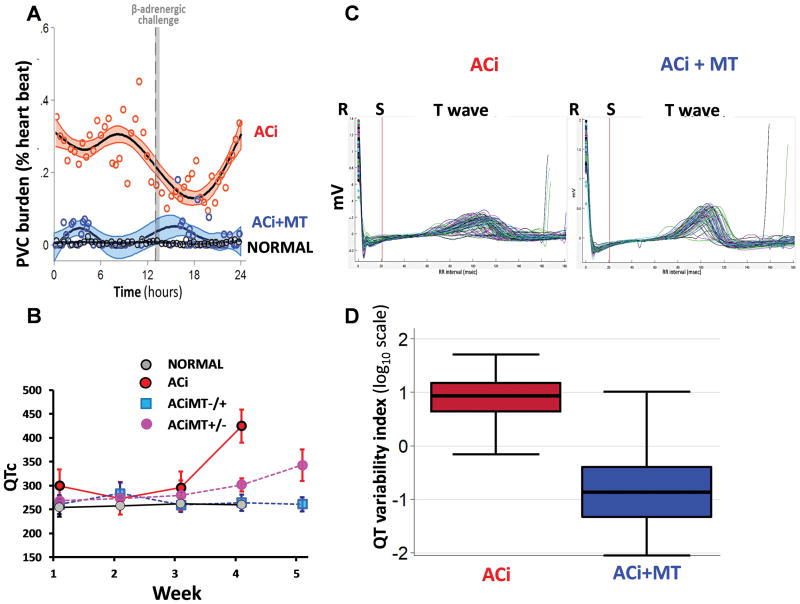
Analysis of the electrophysiological phenotype showed that MitoTEMPO had no effect on basal heart rate or the response to daily isoproterenol challenge (Online Figure IB). The daily Iso infusion rapidly increased heart rate to a maximum of ~390–400 bpm in all groups and then it slowly declined back to the baseline rate in ~4 hours. Continuous telemetry recordings indicated a high frequency of PVCs (Fig. 5A), and spontaneous VT/VF in the ACi group (Fig. 1A), as well as in the ACiMT−/+ group prior to initiating MitoTEMPO treatment. MitoTEMPO treatment markedly suppressed arrhythmias (Fig. 5A) and blunted QT prolongation evident by 4 weeks post banding (Fig. 5B). The ACi and ACiMT−/+ groups (prior to treatment) demonstrated increased dispersion of repolarization, as indicated by a variable amplitude and duration of the T wave (Fig. 5C), which is considered prognostic of arrhythmia risk and is frequently increased in patients with prolonged QT interval. MitoTEMPO treatment of the ACi animals reduced the dispersion of repolarization (Fig. 5D), as indexed by the QTVI32, 33, in parallel with the elimination of SCD.
FIGURE 5. Electrophysiological characteristics of ACi, ACiMT animals.
A) Plot shows % of heart beats with premature ventricular complexes (PVCs) over a period of 24 hours. MT therapy (blue) reduced the PVC burden as compared to ACi (red). B) QT prolongation was observed in ACi with the progression of heart failure over 4 weeks and was prevented by MT treatment (Blue squares). When MT therapy was discontinued, prolongation of the QT was observed (red-blue gradient circles). C) Dispersion of repolarization, evidenced by variability of the T wave, was observed in the ACi group after HF development, which was mitigated by MT treatment. D) The Box and whisker plots show the QT variability index (QTVI) for the ACi animals before (0.87±0.55; in red) and after (−0.80±0.66; p<0.05; blue) MT treatment. N=5 animals in each group. Error bars show SEM; *p<0.05 vs. control group.
MitoTEMPO prevents HF proteome and phosphoproteome remodeling
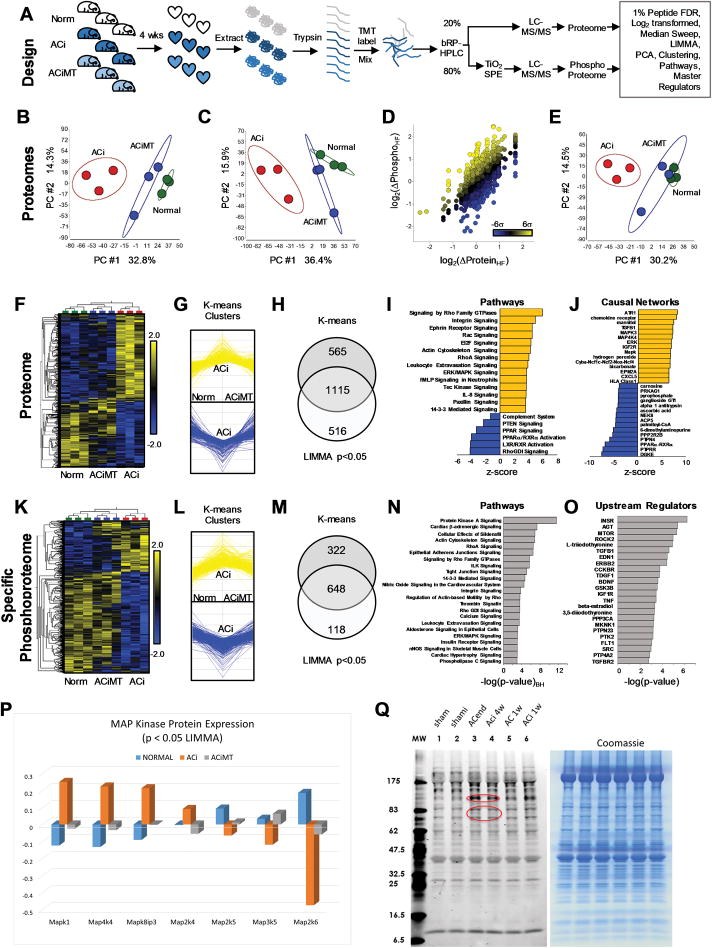
Next, we sought to define the impact of mitochondrial antioxidant therapy on i) HF/SCD-associated proteome remodeling, ii) changes in the specific phosphorylation of proteins altered in HF, and iii) the signal transduction pathways underlying HF/SCD that were normalized by MitoTEMPO treatment. Hearts from Normal, ACi, or ACiMT groups were excised and the LV free wall was immediately subjected to rapid heat stabilization to halt biological activity (Denator AB, Sweden). This method has been shown to be superior to snap freezing for the preservation of phosphorylation-dependent post-translational modifications34. Proteins were extracted from the hearts and digested for labeling with Tandem Mass Tags (TMT) (Fig. 6A). Samples were then mixed and divided into unenriched and titanium dioxide (TiO2) - enriched fractions for multiplex LC-MS/MS analysis of the expression- and phospho- proteomes, respectively. Spectral normalization and statistical methods were as described previously21 and principal components analysis (PCA), hierarchical clustering, pathway, and causal network analyses were carried out using commercial software packages, as described in the Supplemental Methods. Peptide identification/quantification, statistical and pathway analyses and specific phosphosite annotations are available in the Supplemental Proteomics Data. The internal structure of the variance of the data, as illustrated by PCA, for both the expression proteome (Fig. 6B) and the phosphoproteome (Fig. 6C), revealed that MitoTEMPO-treated ACi hearts resembled normal non-failing hearts with respect to the largest principal component (PC1), whereas no groupwise distinction was evident in the second component (PC2). We detected 4195 phosphoforms (on 1550 proteins) and were able to quantify specific phosphorylation for 2951 phosphoforms (that is, we had corresponding expression data). As expected, there was an overall correlation between the change in phosphoform abundance and protein abundance in HF, but many individual phosphoforms changed more dramatically than the underlying protein (Fig. 6D; colored yellow and blue respectively). To extract bona fide differential phosphoform regulation between the Normal, ACi and ACiMT groups, irrespective of changes in protein abundance, specific phosphorylation was calculated by subtracting the protein abundance from phosphoform abundance on the log2 scale (equivalent to dividing on the linear scale) for each of the samples. Once normalized, the specific phosphoproteome from ACi hearts remained distinct from that of Normal or ACiMT hearts with respect to PC1 (Fig. 6E).
FIGURE 6. Impact of MitoTEMPO on the Heart Failure (HF) Proteome and Phosphoproteome.
A) Proteins from 3 normal, 3 failing (ACi) and 3 MitoTEMPO-treated ACi (ACiMT) guinea pig hearts were extracted, digested and analyzed as detailed in “Methods”. B) Unsupervised principal component analysis (PCA) indicates that the experimental groups primarily segregate along the first principal component (PC #1), with the ACi group (red) the most distinct of the three proteomes, whereas the ACiMT (blue) and Normal (green) heart proteomes differed marginally from each other. C) PCA of the phosphoproteome. D) Plot of HF-induced changes in phosphoproteins as a function of their changes in expression level, as compared to normal controls; used to determine extent of hyper- or hypo-phosphorylation (colored according to the binned distribution of log2(Δphos/Δprot)HF). E) After accounting for protein expression, ACi phosphoproteome was still distinct as assessed by PCA. F–I) Identification of biological pathways in HF influenced by MitoTEMPO, we examined subset of proteins whose levels differed significantly between groups and whose changes were mitigated by MitoTEMPO. F) A heatmap was used to visualize the supervised hierarchical correlation clustering of proteins with p<0.05 by LIMMA multigroup comparison. G) K-means clustering to parse the proteome by signal intensity into groups whose expression changed in ACi relative to the normal group, but were also mitigated by MitoTEMPO. H) Proteins meeting both criteria (the intersection of sets from F & G; 1115 proteins) were subjected to further Pathway Analysis. I) Ingenuity Pathway Analysis of the proteins changed in ACi but mitigated by MitoTEMPO (Benjamini-Hochberg-corrected p values <0.05) were ranked by z-score. Orange represents pathways inferred to be activated in ACi but not in ACiMT group and blue represents pathways inferred to be inhibited in ACi but not in ACiMT group. Cytoskeletal remodeling and MAP Kinase pathways exemplify the former and ligand-activated metabolic transcription factor pathways (i.e. LXR/RXR, PPAR) exemplify the latter. J) Ranking of causal regulators (by z-score, see supplement for full list) associated with ACi but not changed in ACiMT group. These represent master regulators whose activation or inhibition are consistent with expression or activation states of other subordinate regulators within the dataset. Note that hydrogen peroxide is an inferred primary regulator identified by the MitoTEMPO-influenced proteome. K, L, M) LIMMA and K-means clustering analysis for the specific Phosphoproteome. 648 Phosphoforms met the aforementioned 2 criteria, corresponding to 334 phosphoproteins. N) Over-representation analysis of the phosphoproteins indicates they fall into pathways that include β-adrenergic signaling, integrin signaling, nitric oxide signaling, ERK/MAPK signaling and calcium signaling, among others (activation state undeterminable). O) Upstream regulators that impact the phosphoprotein levels irrespective of phosphorylation status. P) ACi-associated alterations in expression of MAP kinase pathway proteins that were prevented by MitoTEMPO treatment. Q) Increase in canonical (proline-directed) MAP kinase target phosphorylation sites in end stage heart failure with aortic constriction alone (AC) or in the ACi model compared to non-failing sham-operated controls (each lane is a sample from 5–6 pooled hearts per group). Right panel shows equivalence of sample loading by Coomassie blue staining.
Heatmaps of hierarchically- clustered up- or down-regulated proteins in the proteome (Fig. 6F, p<0.05) and the specific phosphoproteome (Fig. 6K, p<0.05) illustrate how MitoTEMPO largely prevented HF-induced protein remodeling, as well as the posttranslational modifications induced by the ACi protocol, in accord with the protective functional effects described above. Moreover, K-means cluster analysis identified 1680 proteins whose abundance increased or decreased in HF in the expression proteome that were prevented by MitoTEMPO (Fig. 6G). 66% (1115) of these reached statistical significance (p<0.05) by LIMMA multigroup test35 (Venn diagram intersection in Fig. 6H). Similarly, 970 phosphoform changes altered in HF were prevented by MitoTEMPO treatment (Fig. 6L and 6M) and 67% (648) of these reached significance (p< 0.05 by LIMMA) (Fig. 6M).
The HF protein changes prevented by MitoTEMPO (i.e., those modulated by mROS) were subjected to pathway analysis (IPA, Qiagen). Significantly overrepresented pathways (Benjamini-Hochberg corrected p<0.05) are tabulated in the Supplemental Proteomics Data, with Z-scores indicating which pathways are inferred activated or inhibited in HF that were specifically responsive to mitochondrial antioxidant treatment. Top mROS-activated pathways in the HF expression proteome (orange bars in Fig. 6I) included Rho GTPase, integrin signaling, cytoskeletal remodeling and ERK/MAPK signaling, while HF pathways inhibited by mROS prominently featured nuclear receptors regulating metabolic protein expression, i.e., PPAR, RXR and LXR, as well as the Rho GDP dissociation inhibitor (RhoGDI) pathway (blue bars in Fig. 6I).
We next carried out a causal network analysis to identify master upstream regulators that might contribute directly, or through other regulators, to the HF phenotype that were specifically prevented by MitoTEMPO treatment. Validating this approach, hydrogen peroxide was identified as a putative regulator with a high positive Z-score, along with MAP kinases, angiotensin receptor, TGFβ1 and NOX. The putative regulators inhibited in HF and prevented by MitoTEMPO included the negative regulator of MAP kinases, protein tyrosine phosphatase receptor type r (PTPRR), PPARα-RXRα and diacylglycerol kinases (DGKE) (Fig. 6J).
Pathway and causal network analysis was also performed on the phosphoproteome data. In this case, activation or inhibition of activity cannot be predicted, as it would depend on the specific functional impact of hypo- or hyper-phosphorylation on each individual target. Nevertheless, significant changes in phosphorylation state of proteins residing in PKA/β-adrenergic, RhoA signaling, PKG (Effects of Sildenafil), nitric oxide, integrin, angiotensin, TGFβ and MAP kinase signaling pathways were observed (Fig. 6L–O). Significant downregulation of mitochondrial antioxidant, tricarboxylic acid cycle, and electron transport chain proteins were observed in the ACi group that were abrogated by MitoTEMPO treatment (Online Figure II). Of special relevance to the SCD phenotype, changes in the expression and phosphorylation profile of proteins essential for excitation-contraction coupling (ion channels and transporters) were in evidence (Online Figure III).
Pathway analysis strongly implicated the 3 main MAP kinase pathways (ERK1/2, p38, and JNK) as upstream causal regulators of the gene networks altered in HF that were prevented by MitoTEMPO treatment. Direct evidence of changes in the mROS-sensitive expression of MAPK cascade proteins with HF was present in the proteome (Fig. 6P). Further confirmation was obtained by kinome analysis (Cell Signaling, Inc.) employing an antibody recognizing canonical proline-directed (sequence PXsP) MAP kinase substrate phosphosites (Fig. 6Q), indicating activation of MAPK target phosphorylation in the ACi model at end-stage HF (as well as in AC without concomitant isoproterenol treatment), with modest activation also observed at the compensated hypertrophy stage, when there are far fewer changes in protein expression21.
DISCUSSION
The major finding of this study was that mROS scavenging can prevent the onset of HF, or reverse established heart failure, while eliminating SCD, although the former effect persisted only if the treatment was sustained. Mitochondrial ROS was shown to be a major contributor to chronic HF-induced protein remodeling, as demonstrated by the beneficial effects cardiac of MitoTEMPO on protein expression and post-translational modification (phosphoproteome). Importantly, this study is the first to characterize the global subset of protein phospho-targets implicated in HF that are mROS-dependent. In addition, using novel compartment-specific ROS probes, it was shown that these effects were consistent with the ability of MitoTEMPO to normalize ROS levels in the mitochondrial matrix and cytosolic compartments of failing heart cells. Reversing the deficit of mROS scavenging in HF was sufficient to reverse the HF phenotype and prevent sudden death even though other apocynin-sensitive sources of ROS were present, contributing to elevated basal and Ang II-stimulated ROS in the cytoplasm. The observation that contractile function was preserved only as long as the exogenous scavenger was present indicates that cardiac decompensation is an mROS-dependent process. As MitoTEMPO was delivered as a systemic drug, extra-cardiac effects of MitoTEMPO cannot be discounted. But the agreement between the effects in myocytes and effects on cardiac physiology are consistent with the conclusions in this study. The ability of MitoTEMPO to impact the ventricular proteome and phosphoproteome, to decrease arrhythmia incidence and to restore contractility support cardiac muscle as a primary target.
Antioxidant therapy for HF has had a long, and somewhat disappointing, history. Various antioxidants have been shown to prevent hypertrophy and/or failure in animal models and attenuate cardiomyocyte growth in cell models of agonist - induced hypertrophy. For example, vitamin E or catalase treatment inhibited the hypertrophic response to TNFα or Ang II in neonatal rat cardiomyocytes 36 and chronic vitamin E treatment of blunted pressure-overload hypertrophy and HF in guinea pigs subjected to aortic constriction for 20 weeks37. In mice subjected to transverse aortic constriction, N-2-mercaptopropionylglycine treatment attenuated the increase in the HF biomarker atrial natriuretic peptide and slightly decreased cardiac hypertrophy without effects on hemodynamics38, while the superoxide dismutase mimetic, M40401 39 or TEMPOL40 attenuated markers of oxidative stress but did not prevent HF. Examples of positive effects of antioxidants on post-MI injury and coronary artery disease are also well-represented in the literature (reviewed in41), with several small clinical trials showing beneficial outcomes in humans42, 43 Nevertheless, larger prospective clinical trials for several nutritional (Vitamin E and C42, 44–47) or general antioxidants (NAC 48, oxopurinol49 have either shown no benefit or detrimental outcomes. Speculation about the reasons for the failure to demonstrate efficacy in humans include a failure of the antioxidant to reach sufficient levels in the appropriate compartment, insufficient ability of the tissues to regenerate the antioxidant after electron transfer, thus rendering it an oxidant50, or too much ROS scavenging suppressing physiological signals that may protect the heart. In an attempt to overcome one or more of these problems, the focus has recently shifted towards antioxidants targeted to the mitochondria20. This approach was spurred on by results in mouse models of hypertrophy51 or cardiac aging52 showing that transgenic overexpression of a mitochondrial, but not a cytoplasmic, catalase (an enzyme that scavenges H2O2) prevented cardiac dysfunction. Interestingly, six-fold cardiac-specific overexpression of mitochondrial superoxide dismutase (SOD) in transgenic mice caused physiological hypertrophy and supranormal function, which was attributed to improved myocardial blood flow due to increased H2O2 efflux into the vasculature53.
Antioxidant molecules linked to a triphenylphosphonium ion (TPP+), such as SkQ149, 54, 55, mitoQ49, and MitoTEMPO49 facilitate partitioning of the antioxidant into the mitochondrial matrix compartment according to the Nernst potential of the lipophilic cation (in cardiac mitochondria, this would result in approximately a 300-fold increase in the level of the compound in the matrix compared to the extracellular space). Lifelong treatment of mice with the targeted plastiquinone SkQ1 decreased age-related cardiac fibrosis and hypertrophy55 with minimal effects on gene expression, although significant changes in gene pathways involved in cell-cell contact, leukocyte extravasation and inflammation were noted. MitoQ10, a targeted form of coenzyme Q49, decreased systemic blood pressure and the associated cardiac hypertrophy in spontaneously hypertensive rats56. Long-term (28 week) treatment of normal mice with MitoQ also had minimal effects on metabolism or gene expression57. While MitoQ treatment appears to be protective in many situations involving oxidative damage, such as ischemia-reperfusion injury58, it is still unclear whether it is effective in preventing non-ischemic heart failure. A central role of mROS in cardiac pathology is also supported by studies employing the Szeto-Schiller peptide SS-3159 (alternatively known as Bendavia, MTP-131 or Elamipretide). This peptide is thought to act by decreasing mROS production and preventing oxidation of cardiolipin in the mitochondrial membrane60, 61. It has been shown to be effective against myocardial infarction-induced ventricular remodeling62 and improved function of Gq overexpressing or Ang II-treated mice63. Recently, Elamipretide was also shown to improve mitochondrial and cardiac function in canine ischemic HF induced by microembolization16. Several phase 2 clinical trials with Elamipretide in HF patients are underway (clinicaltrials.gov accessions: NCT02814097, NCT02914665, NCT02788747).
MitoTEMPO is a TPP+-linked nitroxide molecule capable of scavenging superoxide and peroxyl radicals64 with a structure distinct from the targeted quinones. The inherent stability of the oxidized form of the nitroxyl moiety65 of MitoTEMPO may be less toxic than other redox cycling antioxidant/oxidants66 including mitoQ50, which could explain the unequivocal beneficial effects observed in the present study. In a mouse model of cardiomyopathy induced by cardiac-specific overexpression of angiotensin converting enzyme, MitoTEMPO was shown to prevented ventricular arrhythmias and to mitigate mitochondrial dysfunction, but its untargeted form, TEMPOL, or other antioxidants (the NO synthase inhibitor L-NIO, precursor of tetrahydrobiopterin sepiapterin, apocynin, xanthine oxidase inhibitor allopurinol) did not9. MitoTEMPO was also shown to attenuate cardiomyopathy secondary to diabetes in db/db67 or high-fat fed C57BL/6J68 mice and prevented the oxidation of calmodulin-dependent kinase II in a streptozotocin-treated diabetic mice69. While many interventions have been shown to inhibit the progression of heart failure, the demonstration in the present study that MitoTEMPO can rescue contractile function of failing hearts has therapeutic relevance. Moreover, the efficiency of MitoTEMPO to suppress arrhythmias (premature ventricular complexes and reentrant tachyarrhythmias) in the guinea pig model is likely to be relevant to humans, given the similar role of the rapid (IKr) and slow components (IKs) of the delayed rectifier K+ current in cardiac repolarization, as well as the long cellular action potential plateau morphology that contributes to the prolonged QT interval of human heart failure70. Notably, MitoTEMPO withdrawal resulted in a manifest decline in contractile function without a return of SCD incidence, indicating that the pathophysiological regulation of myofilament responses may be isolated from the electrophysiological abnormalities. Further detailed investigation of this differential response is warranted to identify which mROS-dependent processes are involved in contractile versus electrical dysfunction in HF.
NOX also contributed to higher basal ROS in the ACi model, as evidenced by the partial inhibition of both mROS and cROS by apocynin or the GP91-ds-tat inhibitor of NOX2. These findings also indicate a hormone insensitive NOX component that is responsible for the receptor independent basal NOX activity. Notably, MitoTEMPO was much more effective than the untargeted TEMPOL at preventing energy demand-related oxidative stress, while NOX inhibition was more effective at eliminating cROS increases induced by Ang II receptor stimulation.
In this study, proteome analysis yielded key insights into the pathways and causal networks triggered by mROS during cardiac decompensation. Importantly, the canonical pathways linked to heart failure in our previous multi-omic study of the ACi model21 were among those significantly impacted by MitoTEMPO treatment. By inference, pathway z-scores (Supplemental Data file; tab 7) indicate that, during HF, ROS positively influence cytoskeletal remodeling (Rho GTPases, Integrin Signaling, Actin Cytoskeletal Remodeling), stress signals (ERK/MAPK), and inflammation (NF-κB Activation), while negatively influencing mitochondrial/metabolic pathways (LXR/RXR Activation, PPARα/RXRα Activation, PTEN signaling), antioxidant (Antioxidant Action of Vitamin C), and cyclic AMP signaling (Protein Kinase A Signaling, Cardiac β-adrenergic Signaling). Based on the present results and our prior work, we suggest a working hypothesis whereby mROS disrupts the nuclear integration of cytoplasmic signals (e.g., Ca2+, energy state, 2nd messengers) to negatively impact the inherent ability of the myocyte to adapt to changing conditions and demands (schema in Fig. 7). This provides a framework for further exploration of the causal chain of events linked to oxidative stress in HF.
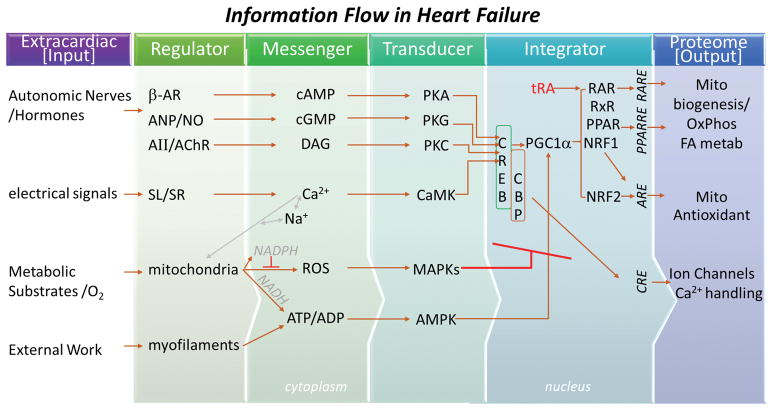
FIGURE 7. Role of mROS in the disruption of information flow in heart failure.
Scheme describing role of mROS in HF and SCD. Analysis of HF-associated proteome remodeling indicates depressed β-adrenergic and Nitric oxide signaling, Na+- and Ca2+-handling, mitochondrial function, and antioxidant activity, which would impact corresponding second messengers (cyclic nucleotides, diacylglycerol - DAG, Ca2+, ROS, ATP:ADP), whose signals are transduced by corresponding kinase networks, i.e., protein kinases A, G, C, calmodulin dependent kinases, mitogen activated protein kinases, AMP-activated protein kinase (PKA, PKG, PKC, CaMK, MAPK, AMPK). Nuclear integration (via the cyclic-AMP response element binding protein complex; CREB/CBP) of the kinase inputs normally couples cellular activity directly to ion transport proteins through CREB response elements (CRE) or indirectly to the metabolic/antioxidant proteome through coactivators, transcription factors and their corresponding response elements (PGC1α, transretinoic acid - tRA, RAR, RxR, NRF1, NRF2). Elevated mROS (through MAP kinase activation) disrupts coupling between input signals and protein expression to induce chronic proteome remodeling in HF in parallel with markedly altering the phosphoproteome. The effects of mROS on HF/SCD can be prevented or reversed by the mitochondrially-targeted antioxidant, MitoTEMPO.
MAP kinase pathways are known to be activated by ROS, so we further confirmed altered MAPK signaling in the ACi model in multiple ways: 1) significant MAPK activation was inferred from the subset of proteins altered in the expression proteome of the failing heart that were prevented by MitoTEMPO treatment, 2) MAPK was identified as a causal network regulator in the phosphoproteome alterations in HF that were prevented by MitoTEMPO, and 3) MAPK target phosphorylation sites were increased in end-stage heart failure, as detected by a MAPK phosphosite-directed antibody specific against proline directed Ser phosphorylation (PXsP). The role of stress-activated kinase pathways in cardiac hypertrophy and failure has been well-studied using genetically-engineered mice, but the net effect of MAP kinase inhibition on outcomes is difficult to predict, specific to the targeted subfamily, and thus far, unproven as a clinical intervention71. Double knockout of Erk1 and Erk2 results in eccentric cardiomyocyte growth, while activation of the Erk pathway by Mek1 overexpression promotes concentric hypertrophy72, however, suppression of Erk signaling did not diminish the hypertrophic response to pressure overload in the same study. Increased activation of MAPK signaling by knockout of the dual specificity phosphatase DUSP6 increased hypertrophic cardiac growth throughout the lifetime of the mouse and protected against cardiac decompensation after myocardial infarction or pressure overload73, in keeping with the protective anti-apoptotic effect of Erk1/2 noted in other studies74, 75. Enhanced cardiac hypertrophy and myopathy in response to pressure overload, Ang II infusion, or isoproterenol treatment was reported with dominant negative inhibition of p38 MAP kinase signaling, through a calcineurin-dependent mechanism76, while in normal myocytes, the p38 pathway was shown to be a negative regulator of excitation-contraction coupling through modulation of phosphatase activity77. The apoptosis signal-regulating kinase 1, Ask1, is potentially the most direct connection between cellular ROS and MAPK activation. An upstream activator of p38 MAP kinase and JNK, Ask1 activity is held in check by interaction with reduced thioredoxin. Transgenic overexpression of Ask1 did not affect the hypertrophic response to pressure overload or isoproterenol treatment but promoted apoptotic cell death and cardiomyopathy78. This effect was associated with JNK1/2, but not p38 MAP kinase, activation. Ask1 knockout was reported to inhibit ventricular hypertrophy after pressure overload or myocardial infarction while decreasing markers of apoptotic cell death79. The present results establishing a relationship between mROS and MAP kinase activation bears further scrutiny in follow up studies to define which downstream actions of mROS involve specific MAP kinases and whether selective subproteomes within the cell respond differentially. For example, it will be interesting to determine if the ROS-dependent pathways modifying ion channels/excitability are the same as those impacting the observed chronic metabolic gene/protein remodeling.
In summary, this study highlights mROS as a major upstream factor driving both acute electrophysiological instability (leading to SCD) and chronic proteome remodeling (protein expression and phosphorylation) during cardiac decompensation. Detailed analysis of the mROS-dependent contribution to heart failure suggests that ROS-dependent signal transduction pathways, probably in the MAP kinase family, interrupt the coupling of cytosolic signals to the metabolic, antioxidant and ion transport subproteomes as heart failure develops. The broad and significant impact of mROS scavenging to prevent or reverse the HF/SCD phenotype provides a tool to identify other control nodes for therapeutic intervention and warrants further testing to establish clinical utility.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Sudden cardiac death (SCD) from ventricular arrhythmias accounts for the majority of deaths in the early stages of heart failure (HF).
The mechanisms of SCD are poorly understood, precluding the development of new, more effective therapies.
Reactive oxygen species (ROS), sources of oxidative stress, have been implicated in the pathogenesis of both HF and SCD, but little is known about the role of compartment-specific ROS dynamics.
The response to antioxidant therapies in clinical trials has been mixed at best, but the effect of targeted compartment-specific antioxidant therapy remains to be established.
What New Information Does This Article Contribute?
We quantified compartment-specific ROS dynamics and elucidated the acute and chronic roles of mitochondrial ROS (mROS) in the context of HF, ventricular arrhythmias and SCD, in a guinea pig model that uniquely recapitulates key features of human HF, including acquired long QT, progression from hypertrophic to dilated cardiomyopathy, and a high incidence of spontaneous SCD.
In vivo scavenging of mROS not only prevented the progression of HF and eliminated SCD, but also reversed the impaired contractility of failing hearts.
The beneficial effects of mROS scavenging include prevention of HF-associated chronic proteome and phosphoproteome remodeling through mitigation of ROS-sensitive signaling pathways.
While HF confers a higher risk for SCD, the mechanisms linking HF and SCD remain unclear. Understanding the mechanisms is essential for improved identification of individuals at high risk for SCD and for the design of new, more effective therapies. Implantable cardioverter-defibrillators (ICDs) are the only effective therapy for SCD, but this is palliative and carries many risks. Importantly, ICDs do not prevent or reverse the disease process. Multiple sources of ROS in specific subcellular compartments may contribute to HF, but these have not been selectively quantified before. Herein, we show that impaired mROS balance is the major contributor to high basal and demand-stimulated ROS levels in HF, with NADPH oxidase contributing to cytoplasmic ROS (cROS) primarily during angiotensin II receptor stimulation. Enhancing mROS scavenging with a targeted superoxide scavenger prevents and reverses electrical instability, contractile failure and chronic HF-induced proteome/phosphoproteome remodeling, and prevents SCD. Our results support the feasibility of targeting the mitochondria for therapeutic intervention in HF and SCD while identifying new mROS-sensitive protein modifications.
Acknowledgments
The authors would like to thank Dr. Robert N. Cole and Robert O’Meally of the JHU Mass Spectrometry Core and Dr. Genaro Ramirez-Correa for technical assistance.
SOURCES OF FUNDING
This work was supported by NHLBI/National Institutes of Health Grants NIH R01HL137259 and R01HLHL134821 (to BO’R), American Heart Association Postdoctoral Fellowship 14POST20380749 and DoD PRMRP PR162017 (to S.D.) and NIH K99HL130662 (to D.D.).
Nonstandard Abbreviations and Acronyms
- AC
Aortic constriction
- ACi
Aortic constriction + Isoproterenol
- ACiMT
ACi + MitoTEMPO
- Ang II
angiotensin II
- cROS
cytoplasmic ROS
- ECG
electrocardiography
- FS
fractional shortening
- HF
heart failure
- HR
heart rate
- Iso
isoproterenol
- mROS
mitochondrial ROS
- NOX
NADPH oxidase
- PVC
premature ventricular complexes
- ROS
reactive oxygen species
- SCD
sudden cardiac death
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
AUTHOR CONTRIBUTIONS
S.D. and D.D. designed and performed the experiments, analyzed the data, interpreted the results, and wrote the manuscript. D.B.F. performed the proteomic experiments, analyzed the data and interpreted the results. A.S. prepared the adenoviral vectors. B.O’R. designed the experiments, interpreted the results, and revised the manuscript.
References
- 1.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CS, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 2.Writing Group M; Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 3.Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, Dargie HJ, Granger CB, Kjekshus J, Kober L, Latini R, Maggioni AP, Packer M, Pitt B, Solomon SD, Swedberg K, Tavazzi L, Wikstrand J, Zannad F, Zile MR, McMurray JJV. Declining risk of sudden death in heart failure. N Engl J Med. 2017;377:41–51. doi: 10.1056/NEJMoa1609758. [DOI] [PubMed] [Google Scholar]
- 4.Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: Two-year findings from the oregon sudden unexpected death study. J Am Coll Cardiol. 2006;47:1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 5.Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, Eiskjaer H, Brandes A, Thogersen AM, Gustafsson F, Egstrup K, Videbaek R, Hassager C, Svendsen JH, Hofsten DE, Torp-Pedersen C, Pehrson S Investigators D. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 6.Marin-Garcia J, Akhmedov AT, Moe GW. Mitochondria in heart failure: The emerging role of mitochondrial dynamics. Heart Fail Rev. 2013;18:439–456. doi: 10.1007/s10741-012-9330-2. [DOI] [PubMed] [Google Scholar]
- 7.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: Ischemia--reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 8.Liu T, Takimoto E, Dimaano VL, DeMazumder D, Kettlewell S, Smith G, Sidor A, Abraham TP, O’Rourke B. Inhibiting mitochondrial na+/ca2+ exchange prevents sudden death in a guinea pig model of heart failure. Circulation research. 2014;115:44–54. doi: 10.1161/CIRCRESAHA.115.303062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sovari AA, Rutledge CA, Jeong EM, Dolmatova E, Arasu D, Liu H, Vahdani N, Gu L, Zandieh S, Xiao L, Bonini MG, Duffy HS, Dudley SC., Jr Mitochondria oxidative stress, connexin43 remodeling, and sudden arrhythmic death. Circ Arrhythm Electrophysiol. 2013;6:623–631. doi: 10.1161/CIRCEP.112.976787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai DF, Hsieh EJ, Liu Y, Chen T, Beyer RP, Chin MT, MacCoss MJ, Rabinovitch PS. Mitochondrial proteome remodelling in pressure overload-induced heart failure: The role of mitochondrial oxidative stress. Cardiovascular research. 2012;93:79–88. doi: 10.1093/cvr/cvr274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR Hope, Investigators H-TT. Effects of long-term vitamin e supplementation on cardiovascular events and cancer: A randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 13.Nightingale AK, Crilley JG, Pegge NC, Boehm EA, Mumford C, Taylor DJ, Styles P, Clarke K, Frenneaux MP. Chronic oral ascorbic acid therapy worsens skeletal muscle metabolism in patients with chronic heart failure. Eur J Heart Fail. 2007;9:287–291. doi: 10.1016/j.ejheart.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, Szeto HH. The mitochondrial-targeted compound ss-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol. 2013;24:1250–1261. doi: 10.1681/ASN.2012121216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown DA, Hale SL, Baines CP, del Rio CL, Hamlin RL, Yueyama Y, Kijtawornrat A, Yeh ST, Frasier CR, Stewart LM, Moukdar F, Shaikh SR, Fisher-Wellman KH, Neufer PD, Kloner RA. Reduction of early reperfusion injury with the mitochondria-targeting peptide bendavia. J Cardiovasc Pharmacol Ther. 2014;19:121–132. doi: 10.1177/1074248413508003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabbah HN, Gupta RC, Kohli S, Wang M, Hachem S, Zhang K. Chronic therapy with elamipretide (mtp-131), a novel mitochondria-targeting peptide, improves left ventricular and mitochondrial function in dogs with advanced heart failure. Circ Heart Fail. 2016;9:e002206. doi: 10.1161/CIRCHEARTFAILURE.115.002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hortmann M, Robinson S, Mohr M, Mauler M, Stallmann D, Reinohl J, Duerschmied D, Peter K, Carr J, Gibson CM, Bode C, Ahrens I. The mitochondria-targeting peptide elamipretide diminishes circulating htra2 in st-segment elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2017 doi: 10.1177/2048872617710789. 2048872617710789. [DOI] [PubMed] [Google Scholar]
- 18.Daaboul Y, Korjian S, Weaver WD, Kloner RA, Giugliano RP, Carr J, Neal BJ, Chi G, Cochet M, Goodell L, Michalak N, Rusowicz-Orazem L, Alkathery T, Allaham H, Routray S, Szlosek D, Jain P, Gibson CM. Relation of left ventricular mass and infarct size in anterior wall st-segment elevation acute myocardial infarction (from the embrace stemi clinical trial) Am J Cardiol. 2016;118:625–631. doi: 10.1016/j.amjcard.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 19.Chakrabarti AK, Feeney K, Abueg C, Brown DA, Czyz E, Tendera M, Janosi A, Giugliano RP, Kloner RA, Weaver WD, Bode C, Godlewski J, Merkely B, Gibson CM. Rationale and design of the embrace stemi study: A phase 2a, randomized, double-blind, placebo-controlled trial to evaluate the safety, tolerability and efficacy of intravenous bendavia on reperfusion injury in patients treated with standard therapy including primary percutaneous coronary intervention and stenting for st-segment elevation myocardial infarction. Am Heart J. 2013;165:509–514. e507. doi: 10.1016/j.ahj.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Dietl A, Maack C. Targeting mitochondrial calcium handling and reactive oxygen species in heart failure. Curr Heart Fail Rep. 2017;14:338–349. doi: 10.1007/s11897-017-0347-7. [DOI] [PubMed] [Google Scholar]
- 21.Foster DB, Liu T, Kammers K, O’Meally R, Yang N, Papanicolaou KN, Talbot CC, Jr, Cole RN, O’Rourke B. Integrated omic analysis of a guinea pig model of heart failure and sudden cardiac death. J Proteome Res. 2016;15:3009–3028. doi: 10.1021/acs.jproteome.6b00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer AJ, Dick TP. Fluorescent protein-based redox probes. Antioxidants & redox signaling. 2010;13:621–650. doi: 10.1089/ars.2009.2948. [DOI] [PubMed] [Google Scholar]
- 23.Dey S, Sidor A, O’Rourke B. Compartment-specific control of reactive oxygen species scavenging by antioxidant pathway enzymes. J Biol Chem. 2016;291:11185–11197. doi: 10.1074/jbc.M116.726968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93:903–907. doi: 10.1136/hrt.2005.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ros) and ros-induced ros release. Physiol Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dikalov S. Cross talk between mitochondria and nadph oxidases. Free Radic Biol Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 28.Kohlhaas M, Liu T, Knopp A, Zeller T, Ong MF, Bohm M, O’Rourke B, Maack C. Elevated cytosolic na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation. 2010;121:1606–1613. doi: 10.1161/CIRCULATIONAHA.109.914911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dikalova AE, Kirilyuk IA, Dikalov SI. Antihypertensive effect of mitochondria-targeted proxyl nitroxides. Redox Biol. 2015;4:355–362. doi: 10.1016/j.redox.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. Nadph oxidases in cardiovascular health and disease. Antioxidants & redox signaling. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 31.Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin ii mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 32.Berger RD. Qt variability. Journal of Electrocardiology. 2003;36:83–87. doi: 10.1016/j.jelectrocard.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 33.DeMazumder D, Limpitikul WB, Dorante M, Dey S, Mukhopadhyay B, Zhang Y, Moorman JR, Cheng A, Berger RD, Guallar E, Jones SR, Tomaselli GF. Entropy of cardiac repolarization predicts ventricular arrhythmias and mortality in patients receiving an implantable cardioverter-defibrillator for primary prevention of sudden death. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2016;18:1818–1828. doi: 10.1093/europace/euv399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rountree CB, Van Kirk CA, You H, Ding W, Dang H, VanGuilder HD, Freeman WM. Clinical application for the preservation of phospho-proteins through in-situ tissue stabilization. Proteome Sci. 2010;8:61. doi: 10.1186/1477-5956-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kammers K, Cole RN, Tiengwe C, Ruczinski I. Detecting significant changes in protein abundance. EuPA Open Proteom. 2015;7:11–19. doi: 10.1016/j.euprot.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, Namba M. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin ii. Circulation. 1998;98:794–799. doi: 10.1161/01.cir.98.8.794. [DOI] [PubMed] [Google Scholar]
- 37.Dhalla AK, Hill MF, Singal PK. Role of oxidative stress in transition of hypertrophy to heart failure. J Am Coll Cardiol. 1996;28:506–514. doi: 10.1016/0735-1097(96)00140-4. [DOI] [PubMed] [Google Scholar]
- 38.Date MO, Morita T, Yamashita N, Nishida K, Yamaguchi O, Higuchi Y, Hirotani S, Matsumura Y, Hori M, Tada M, Otsu K. The antioxidant n-2-mercaptopropionyl glycine attenuates left ventricular hypertrophy in in vivo murine pressure-overload model. J Am Coll Cardiol. 2002;39:907–912. doi: 10.1016/s0735-1097(01)01826-5. [DOI] [PubMed] [Google Scholar]
- 39.Lu Z, Xu X, Hu X, Lee S, Traverse JH, Zhu G, Fassett J, Tao Y, Zhang P, dos Remedios C, Pritzker M, Hall JL, Garry DJ, Chen Y. Oxidative stress regulates left ventricular pde5 expression in the failing heart. Circulation. 2010;121:1474–1483. doi: 10.1161/CIRCULATIONAHA.109.906818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moens AL, Takimoto E, Tocchetti CG, Chakir K, Bedja D, Cormaci G, Ketner EA, Majmudar M, Gabrielson K, Halushka MK, Mitchell JB, Biswal S, Channon KM, Wolin MS, Alp NJ, Paolocci N, Champion HC, Kass DA. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin: Efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation. 2008;117:2626–2636. doi: 10.1161/CIRCULATIONAHA.107.737031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meagher E, Rader DJ. Antioxidant therapy and atherosclerosis: Animal and human studies. Trends in Cardiovascular Medicine. 2001;11:162–165. doi: 10.1016/s1050-1738(01)00105-0. [DOI] [PubMed] [Google Scholar]
- 42.Pasupathy S, Tavella R, Grover S, Raman B, Procter NEK, Du YT, Mahadavan G, Stafford I, Heresztyn T, Holmes A, Zeitz C, Arstall M, Selvanayagam J, Horowitz JD, Beltrame JF. Early use of n-acetylcysteine with nitrate therapy in patients undergoing primary percutaneous coronary intervention for st-segment-elevation myocardial infarction reduces myocardial infarct size (the naciam trial [n-acetylcysteine in acute myocardial infarction]) Circulation. 2017;136:894–903. doi: 10.1161/CIRCULATIONAHA.117.027575. [DOI] [PubMed] [Google Scholar]
- 43.Pasupathy S, Tavella R, Beltrame JF. Myocardial infarction with nonobstructive coronary arteries (minoca): The past, present, and future management. Circulation. 2017;135:1490–1493. doi: 10.1161/CIRCULATIONAHA.117.027666. [DOI] [PubMed] [Google Scholar]
- 44.Shekelle P, Morton S, Hardy ML. Effect of supplemental antioxidants vitamin c, vitamin e, and coenzyme q10 for the prevention and treatment of cardiovascular disease. Evid Rep Technol Assess (Summ) 2003:1–3. [PMC free article] [PubMed] [Google Scholar]
- 45.Sethi R, Takeda N, Nagano M, Dhalla NS. Beneficial effects of vitamin e treatment in acute myocardial infarction. J Cardiovasc Pharmacol Ther. 2000;5:51–58. doi: 10.1177/107424840000500107. [DOI] [PubMed] [Google Scholar]
- 46.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins e and c in the prevention of cardiovascular disease in men: The physicians’ health study ii randomized controlled trial. JAMA. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marchioli R, Schweiger C, Levantesi G, Tavazzi L, Valagussa F. Antioxidant vitamins and prevention of cardiovascular disease: Epidemiological and clinical trial data. Lipids. 2001;36:S53–63. doi: 10.1007/s11745-001-0683-y. [DOI] [PubMed] [Google Scholar]
- 48.Durham JD, Caputo C, Dokko J, Zaharakis T, Pahlavan M, Keltz J, Dutka P, Marzo K, Maesaka JK, Fishbane S. A randomized controlled trial of n-acetylcysteine to prevent contrast nephropathy in cardiac angiography. Kidney Int. 2002;62:2202–2207. doi: 10.1046/j.1523-1755.2002.00673.x. [DOI] [PubMed] [Google Scholar]
- 49.Reyes AJ, Leary WP. Allopurinol or oxypurinol in heart failure therapy - a promising new development or end of story? Cardiovasc Drugs Ther. 2005;19:311–313. doi: 10.1007/s10557-005-4971-1. [DOI] [PubMed] [Google Scholar]
- 50.Doughan AK, Dikalov SI. Mitochondrial redox cycling of mitoquinone leads to superoxide production and cellular apoptosis. Antioxidants & redox signaling. 2007;9:1825–1836. doi: 10.1089/ars.2007.1693. [DOI] [PubMed] [Google Scholar]
- 51.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW, 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin ii-induced cardiac hypertrophy and galphaq overexpression-induced heart failure. Circulation research. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang PT, Chen CL, Ohanyan V, Luther DJ, Meszaros JG, Chilian WM, Chen YR. Overexpressing superoxide dismutase 2 induces a supernormal cardiac function by enhancing redox-dependent mitochondrial function and metabolic dilation. J Mol Cell Cardiol. 2015;88:14–28. doi: 10.1016/j.yjmcc.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anisimov VN, Egorov MV, Krasilshchikova MS, Lyamzaev KG, Manskikh VN, Moshkin MP, Novikov EA, Popovich IG, Rogovin KA, Shabalina IG, Shekarova ON, Skulachev MV, Titova TV, Vygodin VA, Vyssokikh MY, Yurova MN, Zabezhinsky MA, Skulachev VP. Effects of the mitochondria-targeted antioxidant skq1 on lifespan of rodents. Aging. 2011;3:1110–1119. doi: 10.18632/aging.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manskikh VN, Gancharova OS, Nikiforova AI, Krasilshchikova MS, Shabalina IG, Egorov MV, Karger EM, Milanovsky GE, Galkin II, Skulachev VP, Zinovkin RA. Age-associated murine cardiac lesions are attenuated by the mitochondria-targeted antioxidant skq1. Histol Histopathol. 2015;30:353–360. doi: 10.14670/HH-30.353. [DOI] [PubMed] [Google Scholar]
- 56.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cocheme HM, Murphy MP, Dominiczak AF. Mitochondria-targeted antioxidant mitoq10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez-Cuenca S, Cocheme HM, Logan A, Abakumova I, Prime TA, Rose C, Vidal-Puig A, Smith AC, Rubinsztein DC, Fearnley IM, Jones BA, Pope S, Heales SJ, Lam BY, Neogi SG, McFarlane I, James AM, Smith RA, Murphy MP. Consequences of long-term oral administration of the mitochondria-targeted antioxidant mitoq to wild-type mice. Free Radic Biol Med. 2010;48:161–172. doi: 10.1016/j.freeradbiomed.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 58.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 59.Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. British journal of pharmacology. 2014;171:2029–2050. doi: 10.1111/bph.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu S, Soong Y, Seshan SV, Szeto HH. Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. American journal of physiology. Renal physiology. 2014;306:F970–980. doi: 10.1152/ajprenal.00697.2013. [DOI] [PubMed] [Google Scholar]
- 61.Birk AV, Chao WM, Bracken C, Warren JD, Szeto HH. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial atp synthesis. British journal of pharmacology. 2014;171:2017–2028. doi: 10.1111/bph.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dai W, Shi J, Gupta RC, Sabbah HN, Hale SL, Kloner RA. Bendavia, a mitochondria-targeting peptide, improves postinfarction cardiac function, prevents adverse left ventricular remodeling, and restores mitochondria-related gene expression in rats. J Cardiovasc Pharmacol. 2014;64:543–553. doi: 10.1097/FJC.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 63.Dai DF, Chen T, Szeto H, Nieves-Cintron M, Kutyavin V, Santana LF, Rabinovitch PS. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circulation research. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Namiecinski M, Pulaski L, Kochman A, Skolimowski J, Bartosz G, Metodiewa D. Cytotoxicity, cytoprotection and neurotoxicity of novel deprenyl-related propargylamines, stable nitroxide free radicals, in vitro and in vivo. In Vivo. 2004;18:171–180. [PubMed] [Google Scholar]
- 66.Kagan VE, Tyurina YY. Recycling and redox cycling of phenolic antioxidants. Ann N Y Acad Sci. 1998;854:425–434. doi: 10.1111/j.1749-6632.1998.tb09921.x. [DOI] [PubMed] [Google Scholar]
- 67.Ni R, Cao T, Xiong S, Ma J, Fan GC, Lacefield JC, Lu Y, Le Tissier S, Peng T. Therapeutic inhibition of mitochondrial reactive oxygen species with mito-tempo reduces diabetic cardiomyopathy. Free Radic Biol Med. 2016;90:12–23. doi: 10.1016/j.freeradbiomed.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jeong EM, Chung J, Liu H, Go Y, Gladstein S, Farzaneh-Far A, Lewandowski ED, Dudley SC., Jr Role of mitochondrial oxidative stress in glucose tolerance, insulin resistance, and cardiac diastolic dysfunction. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.115.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z, Yang J, Glynn P, Sossalla S, Swaminathan PD, Weiss RM, Yang B, Rokita AG, Maier LS, Efimov IR, Hund TJ, Anderson ME. Diabetes increases mortality after myocardial infarction by oxidizing camkii. J Clin Invest. 2013;123:1262–1274. doi: 10.1172/JCI65268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holzem KM, Gomez JF, Glukhov AV, Madden EJ, Koppel AC, Ewald GA, Trenor B, Efimov IR. Reduced response to ikr blockade and altered herg1a/1b stoichiometry in human heart failure. J Mol Cell Cardiol. 2016;96:82–92. doi: 10.1016/j.yjmcc.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu R, Molkentin JD. Regulation of cardiac hypertrophy and remodeling through the dual-specificity mapk phosphatases (dusps) Journal of Molecular and Cellular Cardiology. 101:44–49. doi: 10.1016/j.yjmcc.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kehat I, Davis J, Tiburcy M, Accornero F, Saba-El-Leil MK, Maillet M, York AJ, Lorenz JN, Zimmermann WH, Meloche S, Molkentin JD. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circulation research. 2011;108:176–183. doi: 10.1161/CIRCRESAHA.110.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maillet M, Purcell NH, Sargent MA, York AJ, Bueno OF, Molkentin JD. Dusp6 (mkp3) null mice show enhanced erk1/2 phosphorylation at baseline and increased myocyte proliferation in the heart affecting disease susceptibility. J Biol Chem. 2008;283:31246–31255. doi: 10.1074/jbc.M806085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, Kitsis RN, Molkentin JD. The mek1-erk1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Purcell NH, Wilkins BJ, York A, Saba-El-Leil MK, Meloche S, Robbins J, Molkentin JD. Genetic inhibition of cardiac erk1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci U S A. 2007;104:14074–14079. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, Braunwart J, Glascock BJ, Klevitsky R, Kimball TF, Hewett TE, Molkentin JD. Targeted inhibition of p38 mapk promotes hypertrophic cardiomyopathy through upregulation of calcineurin-nfat signaling. J Clin Invest. 2003;111:1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaikkonen L, Magga J, Ronkainen VP, Koivisto E, Perjes A, Chuprun JK, Vinge LE, Kilpio T, Aro J, Ulvila J, Alakoski T, Bibb JA, Szokodi I, Koch WJ, Ruskoaho H, Kerkela R. P38alpha regulates serca2a function. J Mol Cell Cardiol. 2014;67:86–93. doi: 10.1016/j.yjmcc.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Q, Sargent MA, York AJ, Molkentin JD. Ask1 regulates cardiomyocyte death but not hypertrophy in transgenic mice. Circulation research. 2009;105:1110–1117. doi: 10.1161/CIRCRESAHA.109.200741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Hikoso S, Takeda T, Watanabe T, Asahi M, Taniike M, Matsumura Y, Tsujimoto I, Hongo K, Kusakari Y, Kurihara S, Nishida K, Ichijo H, Hori M, Otsu K. Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci U S A. 2003;100:15883–15888. doi: 10.1073/pnas.2136717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.