Abstract
Peroxisome proliferator-activated receptor γ (PPARγ) is a member of the nuclear receptor family and plays an important role in adipocyte differentiation, glucose homeostasis, and insulin sensitivity. Thiazolidinediones (TZDs), synthetic ligands of PPARγ, have been used for the treatment of diabetes mellitus for two decades. TZDs were expected to be amazing drugs not only for type 2 diabetes but also for metabolic syndrome and atherosclerotic vascular disease because they can reduce both insulin resistance and inflammation in experimental studies. However, serious unwanted effects pushed TZDs back to an optional second-tier drug for type 2 diabetes. Nevertheless, PPARγ is still one of the most important targets for the treatment of insulin resistance and diabetes mellitus, and novel strategies to modulate PPARγ activity to enhance its beneficial effects and reduce unwanted adverse effects are anticipated. Recent studies showed that post-translational modification (PTM) of PPARγ regulates PPARγ activity or stability and may be a novel way to optimize PPARγ activity with reduced adverse effects. In this review, we will focus on recent advances in PTM of PPARγ and the mechanisms regulating PPARγ function as well as in the development of PPARγ modulators or agonists.
Keywords: PPARgamma, post-translational modification, metabolic disease
Introduction
Insulin resistance is the key pathophysiologic abnormality of many metabolic diseases such as type 2 diabetes mellitus, obesity, dyslipidemia, and cardiovascular diseases 1. Therefore, reducing insulin resistance is the most important strategy for improving metabolic deterioration. Thiazolidinediones (TZDs), peroxisome proliferator-activated receptor γ (PPARγ) agonists, have shown many beneficial effects not only by enhancing insulin sensitivity but also by demonstrating anti-inflammatory and antioxidant properties, whose actions are related to anti-atherosclerosis 2, 3. Thus, TZDs were considered a magic bullet for the treatment of type 2 diabetes and atherosclerosis. Indeed, TZDs demonstrated a preventive role for recurrent ischemic stroke in several clinical trials 4 and for restenosis after percutaneous coronary intervention (PCI) 5– 7. However, TZDs increased the risk of peripheral edema, bone loss, and congestive heart failure 8– 10. A meta-analysis of clinical trials showed that rosiglitazone significantly increased the risk of myocardial infarction 11. Although later studies revealed that rosiglitazone did not increase the risk of heart attack and the US Food and Drug Administration (FDA) removed the warning labels from rosiglitazone-containing drugs regarding the issue of increasing heart attack in 2013, rosiglitazone’s cardiovascular safety issue alongside the above-mentioned adverse effects still lead to many physicians hesitating to prescribe TZDs in their clinical practice. Nevertheless, PPARγ is still one of the most important targets for the treatment of insulin resistance and type 2 diabetes, and novel strategies to modulate PPARγ activity to enhance its beneficial effects and reduce unwanted adverse effects are strongly anticipated. Recent studies showed that post-translational modification (PTM) of PPARγ regulates PPARγ activity or stability and may be a novel way to optimize PPARγ activity with reduced adverse effects. In addition, selective PPARγ modulators (sPPAR γMs), dual or pan PPAR agonists, have been developed and tested for their metabolic effects in animal studies and in some clinical trials.
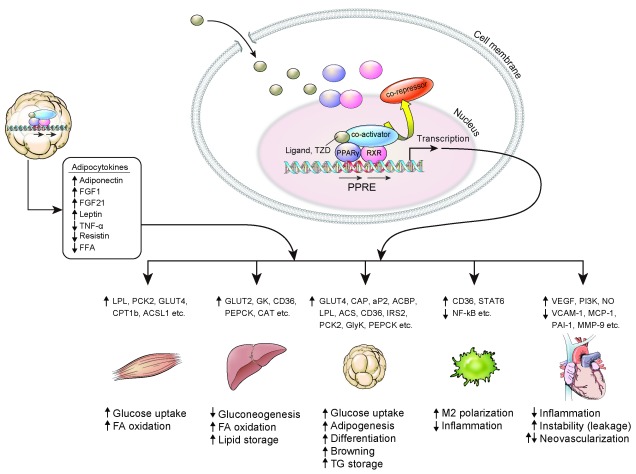
PPARγ, a therapeutic target for insulin resistance ( Figure 1)
PPARγ is a master regulator of adipocyte differentiation. It is also involved in glucose homeostasis and insulin sensitivity. The expression of PPARγ is most abundant in adipose tissue 12. Evidence has shown that the primary target of TZDs is adipose tissue, where it increases the expression of Glut4 and CAP 13, and an animal model lacking PPARγ in adipose tissue had a significantly lower response to TZDs 14, 15. TZDs inhibit the expression of TNF-α, IL-6, and resistin in adipose tissue, which promote insulin resistance and chronic inflammation 16, 17, while TZDs increased the production of adiponectin and fibroblast growth factor 21 (FGF21), which enhance fatty acid oxidation and insulin sensitivity 18, 19. TZDs increase lipogenesis by aP2, LPL, CD36, fatty acid transport protein, PEPCK, and the glycerol transporter aquaporin 7 2 and make adipose tissue store more lipid, while TZDs remove lipid accumulation in other tissues such as muscle and liver 20.
Figure 1. Effect of PPARγ activation on various tissues.
ACSL1, acyl-CoA synthetase long chain family member 1; CD36, cluster of differentiation 36; CPT1b, carnitine palmitoyltransferase 1B; FA, fatty acid; FFA, free fatty acid; FGF, fibroblast growth factor; GK, glucokinase; GLUT, glucose transporter; GlyK, glycerol kinase; IRS2, insulin receptor substrate 1; LPL, lipoprotein lipase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; MCP-1, monocyte chemoattractant protein 1; MMP-9, matrix metalloproteinase 9; NO, nitric oxide; PAI-1, plasminogen activator inhibitor type 1; PCK2, peroxisome proliferator-activated receptor gamma 2 binding site; PEPCK, phosphoenolpyruvate carboxykinase; PI3K, phosphoinositide 3-kinase; PPARγ, peroxisome proliferator-activated receptor γ; PPRE, peroxisome proliferator-activated receptor response element; RXR, retinoid X receptor; STAT6, signal transducer and activator of transcription 6; TG, triglyceride; TNF-α, tumor necrosis factor α; TZD, thiazolidinedione; VCAM-1, vascular cell adhesion molecule-1; VEGF, vascular endothelial growth factor.
From these studies, it seems that improvement of insulin sensitivity in liver and muscle might be secondary to the effects of TZDs in adipose tissue. However, there is also evidence showing that TZDs have an insulin-sensitizing effect on other peripheral organs. It has been demonstrated that ablation of liver PPARγ in mice reduced hepatic steatosis but worsened hyperlipidemia, triglyceride clearance, and muscle insulin resistance 21. The expression of PPARγ in skeletal muscle is relatively low compared to adipose tissue, and the physiological significance of PPARγ in skeletal muscle has been shown to work indirectly in previous studies 22. However, selective activation of PPARγ in skeletal muscle showed significant protection from high-fat diet-induced insulin resistance and associated changes in muscle phenotype, such as decreasing the quantity of lipid in myocytes and increasing the number of oxidative muscle fiber types 23. It suggests that the activation of PPARγ can act directly on muscle tissue to improve insulin sensitivity. Macrophage PPARγ is also implicated in anti-inflammation and lipid metabolism 24, and mice lacking macrophage PPARγ are more prone to whole-body insulin resistance 25, 26.
PPARγ agonists and their effects on the vascular system: friend or foe?
PPARγ is expressed in the endothelium and vascular smooth muscle in the blood vessel wall 27, 28. Despite controversial cardiovascular effects of TZDs in humans, most experimental studies showed beneficial effects on vascular systems. TZDs inhibit the proliferation and migration of vascular smooth muscle cells (VSMCs), with potential favorable effects on atherosclerosis 29, 30. Smooth muscle-specific dominant-negative PPARγ transgenic mice showed a loss of nitric oxide responsiveness and high contractility 31, which resulted in systolic hypertension. In humans, dominant-negative mutations of PPARγ are associated with early hypertension and insulin resistance 32. Activation of PPARγ inhibits CCAAT/enhancer-binding protein-δ (C/EBPδ), which is a well-known mediator of the proinflammatory response in vascular cells 33.
TZDs also reduce activation and inflammation in endothelial cells by suppressing the expression of inflammation-associated genes 34– 37. On the other hand, TZDs induce vascular endothelial growth factor (VEGF) in endothelial cells and increase endothelial cell proliferation and migration by the Akt-dependent pathway 38– 40. In recent data, rosiglitazone significantly increased endothelial cell migration and vascular leakage in an animal study with increased VEGF expression and suppressed tight junction proteins, which caused instability of the endothelial membrane 41. This result could be related to vascular permeability, peripheral edema, and congestive heart failure associated with the use of TZDs, contrary to their beneficial effect on vascular cells. We still need more concrete evidence to understand the role of TZDs in the whole vascular system under various conditions.
Regulation of PPARγ by PTMs to reduce the side effects of TZDs
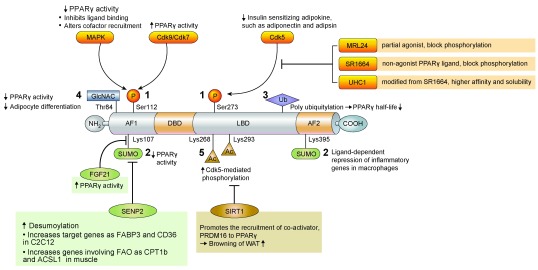
The PTM of PPARγ involves several pathways, including phosphorylation, SUMOylation, ubiquitination, β-O-linked N-acetylglucosamine modification (O-GlcNAcylation), and acetylation. These PTMs are known to regulate both PPARγ expression and its transcriptional activity 42 and have been recently suggested as a good modality for reducing the side effects of PPARγ activation by TZDs 43 ( Figure 2).
Figure 2. Regulation of PPARγ by post translational modification.
Ac, acetyl; ACSL1, acyl-CoA synthetase long chain family member 1; AF1, activation function 1; AF2, activation function 2; CD36, cluster of differentiation 36; Cdk, cyclin-dependent kinase; CPT1b, carnitine palmitoyltransferase 1B; DBD, DNA-binding domain; FABP3, fatty-acid-binding protein 3, muscle and heart; FAO, fatty acid oxidation; FGF, fibroblast growth factor; GlcNAC, N-acetylglucosamine; LBD, ligand-binding domain; Lys, lysine; MAPK, mitogen-activated protein kinase; P, phosphate; PPARγ, peroxisome proliferator-activated receptor γ; PRDM16, PR domain containing 16; SENP2, small ubiquitin-like modifier-specific protease 2; Ser, serine; SIRT1, sirtuin 1; SUMO, small ubiquitin-like modifier; Thr, threonine; Ub, ubiquitin; WAT, white adipose tissue.
Phosphorylation
Phosphorylation at serine 112 (S112) in the N-terminal AF-1 domain was first identified, and various studies revealed that net results of PPARγ phosphorylation may inhibit or stimulate its transcriptional activity depending on the cellular contexts and kinases involved 44– 48. Phosphorylation at S273 in the ligand-binding domain is mediated by cyclin-dependent kinase 5 (Cdk5), which is activated by pro-inflammatory stimuli and free fatty acids 49. S273 phosphorylation affects the expression of insulin-sensitizing adipokines such as adiponectin and adipsin but not those affecting adipogenesis. PPARγ partial agonist MRL24 specifically blocks the phosphorylation of PPARγ at S273 and has higher anti-diabetic activity and fewer side effects than does rosiglitazone 49. SR1664 and similar non-agonist PPARγ ligands were also developed for blocking cdk5-mediated phosphorylation and showed improved insulin sensitivity in high-fat diet-fed mice without causing side effects such as fluid retention and weight gain 43, 50. More recently, it has been reported that phosphorylation at S273 is also facilitated by MEK/ERK, and inhibition of MEK and ERK improves insulin resistance, suggesting that MEK and ERK inhibitors can be therapeutic targets for diabetes through the modulation of PPARγ function 51.
SUMOylation
Small ubiquitin-like modifier (SUMO) modification is a reversible process and may affect protein stability, transcriptional activity, and protein–protein interaction. PPARγ is known as a target of SUMOylation. Lysine 107 (K107) of PPARγ2 is the major SUMOylation site, and deSUMOylation of this site increases the transcriptional activity of PPARγ 52. The K107R mutant form of PPARγ stimulates adipogenesis and suppresses neointimal formation after balloon injury more effectively than does the PPARγ wild-type form 53, 54. SUMOylation at K107 of PPARγ may be linked to S112 phosphorylation 53. PPARγ SUMOylation at K107 is markedly increased in FGF21-knockout mice, suggesting that FGF21 regulates PPARγ SUMOylation by an unknown mechanism 19. SUMOylation of PPARγ at K395 (K365 of PPARγ1) is stimulated by PPARγ agonists, and this modification inhibits the transcription of inflammatory response genes, such as iNOS, through recruiting transcriptional repressors to the NFkB complex in macrophages 55.
SUMO-specific protease 2 (SENP2) is the major deSUMOylation enzyme of PPARγ 56. Overexpression of SENP2 in C2C12 cells effectively induces PPARγ target genes such as Fabp3 and Cd36 but not Adrp; thus, SENP2 can induce the expression of PPARγ target genes in a selective manner 56. SENP2 deSUMOylates PPARγ and PPARδ and activates genes involved in fatty acid oxidation such as Cpt1b and Acsl1, which results in an increase of fatty acid oxidation in muscle. Interestingly, palmitate increases SENP2 expression via the TLR4-MyD88-NFkB pathway. These results suggest that SENP2 is an important regulator of fatty acid metabolism in skeletal muscle 57.
Ubiquitination
Ubiquitination is the covalent attachment of ubiquitin, a 76-amino-acid peptide, to lysine residues in the substrate protein. PPARγ has a short half-life and is degraded by the polyubiquitin-proteasome pathway 58. Inhibition of proteasome activity by proteasome inhibitors increases PPARγ stability, suggesting ubiquitin modification of PPARγ is an important determinant of PPARγ activity 58. Several ubiquitin ligases, such as FBOX9 and Cul4B, and an ubiquitin-specific protease (HAUSP) targeting PPARγ have been identified, and an increase in PPARγ stability generally promotes PPARγ activity and adipogenesis 59– 61. Interestingly, PPARγ agonists, TZDs, stimulate the ubiquitination of PPARγ, which can be mediated by an ubiquitin ligase, Siah1 58, 62. Therefore, PPARγ ubiquitination may be differently regulated by several ubiquitin E3 ligases or proteases upon various conditions.
O-GlcNAcylation
O-GlcNAcylation is the post-translational cycling of a single β-O-linked N-acetylglucosamine (O-GlcNAc) on the hydroxyl groups of serine or threonine residues of target proteins. A major O-GlcNAc site in PPARγ is T84 in the AF-1 domain in PPARγ2, and increased O-GlcNAcylation reduces its transcriptional activity and adipocyte differentiation 63.
Acetylation
Deacetylation at K268 and K293 by the NAD-dependent deacetylase sirtuin 1 (SIRT1) is necessary for the interaction of PPARγ with PRDM16, a transcriptional co-activator for the browning of WAT 64. Therefore, SIRT1-dependent PPARγ deacetylation selectively regulates PPARγ activity.
Other PPARγ modulators and agonists
Considering that patients with insulin resistance show many conjugated metabolic problems such as atherosclerosis, obesity, fatty liver, etc., there have been many efforts to develop sPPAR γMs, dual or pan PPAR agonists, with potent efficacy but less-deleterious side effects.
sPPAR γMs bind to the ligand-binding domain of PPARγ in many ways, which leads to different receptor conformations and cofactor functions 65. INT131, a potent non-TZD sPPARγM now in clinical trials, showed excellent glucose lowering with significantly less weight gain, edema with fluid retention, and cardiomegaly than do current TZDs 66. Balaglitazone also showed positive effects in the treatment of patients with type 2 diabetes compared to placebo and pioglitazone (phase III clinical study, n = 409), better glycemic control, and less edema compared to pioglitazone 45 mg 67. CMHX008 was also tested for its effects in in vitro and in vivo models, showing excellent results by far 68.
PPARα/γ dual activation has been the focus of new targets from many pharmaceutical companies, and several clinical trials have been performed in potential treatments such as muraglitazar, tesaglitazar, and aleglitazar. However, owing to unpredictable side effects, the studies were all stopped for further development. Unfortunately, muraglitazar increased cardiovascular events, tesaglitazar increased renal toxicity, and aleglitazar showed bone fractures, heart failure, and gastrointestinal side effects 69– 71. Recently, (E)-N-(4-(3-(5-bromo-4-hydroxy-2-methoxyphenyl)acryloyl)phenyl)-4- tert-butylbenzamide (SN158) showed anti-diabetic effects through PPARα/γ dual activation. SN158 increased adipogenic differentiation of 3T3-L1 preadipocytes, enhanced fatty acid oxidation in hepatocytes, and increased glucose uptake in myotubes. It lowered plasma glucose and lipid levels in ob/ob mice without severe weight gain. Thus, it represents another candidate PPARα/γ agonist to enhance many metabolic profiles in obesity-related diseases 72.
There are some natural products which activate PPARγ and PPARα simultaneously or activate the PPARγ dimer partner retinoid X receptor. Compared to full TZDs, these natural products usually show fewer side effects and comparable anti-diabetic effects 73. Honokiol, amorfrutin 1, amorfrutin B, amorphastilbol, genistein, biochanin A, sargaquinoic acid, sargahydroquinoic acid, resveratrol, etc. were tested for their efficacy in in vitro and in vivo studies 73.
The development of PPARα/γ/δ pan agonists as anti-diabetic, anti-obesity, or hypolipidemic drugs is still actively ongoing 74, 75. For example, IVA 337 is a potent and well-balanced pan PPAR agonist which showed promising results in vitro and in vivo and is expected to be used to treat patients with metabolic syndrome and non-alcoholic steatohepatitis 76.
Conclusion
PPARγ is still one of the most important targets for the treatment of insulin resistance and diabetes mellitus, even though current use of TZDs in clinical practice is limited because of undesirable adverse effects. Thus, novel strategies to modulate PPARγ activity to enhance its beneficial effects and reduce unwanted side effects have been strongly anticipated. Recent advances in understanding how PTM of PPARγ modulates PPARγ activity provide novel ways to optimize PPARγ activity with reduced adverse effects. In addition, selective PPARγ modulators, dual or pan PPAR agonists, have been developed and tested for their metabolic effects in animal studies and in some clinical trials.
We hope safer PPARγ agonists or modulators with excellent efficacy and fewer adverse effects will be available for treating metabolic diseases and insulin resistance in the near future.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Alexander Orekhov, Institute for Atherosclerosis Research, Skolkovo Innovative Center, Moscow, Russian Federation
Laszlo Nagy, SBP Medical Discovery Institute, Florida, USA; Department of Biochemistry and Molecular Biology, University of Debrecen, Debrecen, Hungary
Attila Pap, Department of Biochemistry and Molecular Biology, University of Debrecen, Debrecen, Hungary
Funding Statement
This work was supported by National Research Foundation Grant by Ministry of Science and ICT, Republic of Korea (NRF-2016R1A2B3010373).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Laakso M, Kuusisto J: Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293–302. 10.1038/nrendo.2014.29 [DOI] [PubMed] [Google Scholar]
- 2. Tontonoz P, Spiegelman BM: Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. 10.1146/annurev.biochem.77.061307.091829 [DOI] [PubMed] [Google Scholar]
- 3. Ceriello A: Thiazolidinediones as anti-inflammatory and anti-atherogenic agents. Diabetes Metab Res Rev. 2008;24(1):14–26. 10.1002/dmrr.790 [DOI] [PubMed] [Google Scholar]
- 4. Lee M, Saver JL, Liao HW, et al. : Pioglitazone for Secondary Stroke Prevention: A Systematic Review and Meta-Analysis. Stroke. 2017;48(2):388–93. 10.1161/STROKEAHA.116.013977 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Patel D, Walitt B, Lindsay J, et al. : Role of pioglitazone in the prevention of restenosis and need for revascularization after bare-metal stent implantation: a meta-analysis. JACC Cardiovasc Interv. 2011;4(3):353–60. 10.1016/j.jcin.2010.10.016 [DOI] [PubMed] [Google Scholar]
- 6. Wang Z, Zhang T, Sun L, et al. : Pioglitazone Attenuates Drug-Eluting Stent-Induced Proinflammatory State in Patients by Blocking Ubiquitination of PPAR. PPAR Res. 2016;2016: 7407153. 10.1155/2016/7407153 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Choi D, Kim SK, Choi SH, et al. : Preventative effects of rosiglitazone on restenosis after coronary stent implantation in patients with type 2 diabetes. Diabetes Care. 2004;27(11):2654–60. 10.2337/diacare.27.11.2654 [DOI] [PubMed] [Google Scholar]
- 8. Berlie HD, Kalus JS, Jaber LA: Thiazolidinediones and the risk of edema: a meta-analysis. Diabetes Res Clin Pract. 2007;76(2):279–89. 10.1016/j.diabres.2006.09.010 [DOI] [PubMed] [Google Scholar]
- 9. Lago RM, Singh PP, Nesto RW: Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370(9593):1129–36. 10.1016/S0140-6736(07)61514-1 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Billington EO, Grey A, Bolland MJ: The effect of thiazolidinediones on bone mineral density and bone turnover: systematic review and meta-analysis. Diabetologia. 2015;58(10):2238–46. 10.1007/s00125-015-3660-2 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Nissen SE, Wolski K: Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–71. 10.1056/NEJMoa072761 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Fajas L, Auboeuf D, Raspé E, et al. : The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem. 1997;272(30):18779–89. 10.1074/jbc.272.30.18779 [DOI] [PubMed] [Google Scholar]
- 13. Ribon V, Johnson JH, Camp HS, et al. : Thiazolidinediones and insulin resistance: peroxisome proliferatoractivated receptor gamma activation stimulates expression of the CAP gene. Proc Natl Acad Sci U S A. 1998;95(25):14751–6. 10.1073/pnas.95.25.14751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He W, Barak Y, Hevener A, et al. : Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100(26):15712–7. 10.1073/pnas.2536828100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chao L, Marcus-Samuels B, Mason MM, et al. : Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. J Clin Invest. 2000;106(10):1221–8. 10.1172/JCI11245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peraldi P, Xu M, Spiegelman BM: Thiazolidinediones block tumor necrosis factor-alpha-induced inhibition of insulin signaling. J Clin Invest. 1997;100(7):1863–9. 10.1172/JCI119715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steppan CM, Bailey ST, Bhat S, et al. : The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–12. 10.1038/35053000 [DOI] [PubMed] [Google Scholar]
- 18. Berg AH, Combs TP, Du X, et al. : The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7(8):947–53. 10.1038/90992 [DOI] [PubMed] [Google Scholar]
- 19. Dutchak PA, Katafuchi T, Bookout AL, et al. : Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148(3):556–67. 10.1016/j.cell.2011.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Ye JM, Dzamko N, Cleasby ME, et al. : Direct demonstration of lipid sequestration as a mechanism by which rosiglitazone prevents fatty-acid-induced insulin resistance in the rat: comparison with metformin. Diabetologia. 2004;47(7):1306–13. 10.1007/s00125-004-1436-1 [DOI] [PubMed] [Google Scholar]
- 21. Gavrilova O, Haluzik M, Matsusue K, et al. : Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278(36):34268–76. 10.1074/jbc.M300043200 [DOI] [PubMed] [Google Scholar]
- 22. Norris AW, Chen L, Fisher SJ, et al. : Muscle-specific PPARgamma-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest. 2003;112(4):608–18. 10.1172/JCI17305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amin RH, Mathews ST, Camp HS, et al. : Selective activation of PPARgamma in skeletal muscle induces endogenous production of adiponectin and protects mice from diet-induced insulin resistance. Am J Physiol Endocrinol Metab. 2010;298(1):E28–37. 10.1152/ajpendo.00446.2009 [DOI] [PubMed] [Google Scholar]
- 24. Wahli W, Michalik L: PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol Metab. 2012;23(7):351–63. 10.1016/j.tem.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 25. Hevener AL, Olefsky JM, Reichart D, et al. : Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117(6):1658–69. 10.1172/JCI31561 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. : Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–20. 10.1038/nature05894 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Marx N, Schönbeck U, Lazar MA, et al. : Peroxisome proliferator-activated receptor gamma activators inhibit gene expression and migration in human vascular smooth muscle cells. Circ Res. 1998;83(11):1097–103. 10.1161/01.RES.83.11.1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xin X, Yang S, Kowalski J, et al. : Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem. 1999;274(13):9116–21. 10.1074/jbc.274.13.9116 [DOI] [PubMed] [Google Scholar]
- 29. Li AC, Brown KK, Silvestre MJ, et al. : Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000;106(4):523–31. 10.1172/JCI10370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lim S, Lee KS, Lee JE, et al. : Effect of a new PPAR-gamma agonist, lobeglitazone, on neointimal formation after balloon injury in rats and the development of atherosclerosis. Atherosclerosis. 2015;243(1):107–19. 10.1016/j.atherosclerosis.2015.08.037 [DOI] [PubMed] [Google Scholar]
- 31. Halabi CM, Beyer AM, de Lange WJ, et al. : Interference with PPAR gamma function in smooth muscle causes vascular dysfunction and hypertension. Cell Metab. 2008;7(3):215–26. 10.1016/j.cmet.2007.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barroso I, Gurnell M, Crowley VE, et al. : Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402(6764):880–3. 10.1038/47254 [DOI] [PubMed] [Google Scholar]
- 33. Takata Y, Kitami Y, Yang ZH, et al. : Vascular inflammation is negatively autoregulated by interaction between CCAAT/enhancer-binding protein-delta and peroxisome proliferator-activated receptor-gamma. Circ Res. 2002;91(5):427–33. 10.1161/01.RES.0000031271.20771.4F [DOI] [PubMed] [Google Scholar]
- 34. Marx N, Mach F, Sauty A, et al. : Peroxisome proliferator-activated receptor-gamma activators inhibit IFN-gamma-induced expression of the T cell-active CXC chemokines IP-10, Mig, and I-TAC in human endothelial cells. J Immunol. 2000;164(12):6503–8. 10.4049/jimmunol.164.12.6503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jackson SM, Parhami F, Xi XP, et al. : Peroxisome proliferator-activated receptor activators target human endothelial cells to inhibit leukocyte-endothelial cell interaction. Arterioscler Thromb Vasc Biol. 1999;19(9):2094–104. 10.1161/01.ATV.19.9.2094 [DOI] [PubMed] [Google Scholar]
- 36. Pasceri V, Wu HD, Willerson JT, et al. : Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation. 2000;101(3):235–8. 10.1161/01.CIR.101.3.235 [DOI] [PubMed] [Google Scholar]
- 37. Hong HK, Cho YM, Park KH, et al. : Peroxisome proliferator-activated receptor gamma mediated inhibition of plasminogen activator inhibitor type 1 production and proliferation of human umbilical vein endothelial cells. Diabetes Res Clin Pract. 2003;62(1):1–8. 10.1016/S0168-8227(03)00142-6 [DOI] [PubMed] [Google Scholar]
- 38. Dimmeler S, Dernbach E, Zeiher AM: Phosphorylation of the endothelial nitric oxide synthase at ser-1177 is required for VEGF-induced endothelial cell migration. FEBS Lett. 2000;477(3):258–62. 10.1016/S0014-5793(00)01657-4 [DOI] [PubMed] [Google Scholar]
- 39. Rikitake Y, Kawashima S, Yamashita T, et al. : Lysophosphatidylcholine inhibits endothelial cell migration and proliferation via inhibition of the extracellular signal-regulated kinase pathway. Arterioscler Thromb Vasc Biol. 2000;20(4):1006–12. 10.1161/01.ATV.20.4.1006 [DOI] [PubMed] [Google Scholar]
- 40. Dimmeler S, Zeiher AM: Akt takes center stage in angiogenesis signaling. Circ Res. 2000;86(1):4–5. 10.1161/01.RES.86.1.4 [DOI] [PubMed] [Google Scholar]
- 41. Ku YH, Cho BJ, Kim MJ, et al. : Rosiglitazone increases endothelial cell migration and vascular permeability through Akt phosphorylation. BMC Pharmacol Toxicol. 2017;18(1):62. 10.1186/s40360-017-0169-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Beekum O, Fleskens V, Kalkhoven E: Posttranslational modifications of PPAR-gamma: fine-tuning the metabolic master regulator. Obesity (Silver Spring). 2009;17(2):213–9. 10.1038/oby.2008.473 [DOI] [PubMed] [Google Scholar]
- 43. Choi SS, Kim ES, Koh M, et al. : A novel non-agonist peroxisome proliferator-activated receptor γ (PPARγ) ligand UHC1 blocks PPARγ phosphorylation by cyclin-dependent kinase 5 (CDK5) and improves insulin sensitivity. J Biol Chem. 2014;289(38):26618–29. 10.1074/jbc.M114.566794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hu E, Kim JB, Sarraf P, et al. : Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274(5295):2100–3. [DOI] [PubMed] [Google Scholar]
- 45. Adams M, Reginato MJ, Shao D, et al. : Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem. 1997;272(8):5128–32. 10.1074/jbc.272.8.5128 [DOI] [PubMed] [Google Scholar]
- 46. Shao D, Rangwala SM, Bailey ST, et al. : Interdomain communication regulating ligand binding by PPAR-gamma. Nature. 1998;396(6709):377–80. 10.1038/24634 [DOI] [PubMed] [Google Scholar]
- 47. Rangwala SM, Rhoades B, Shapiro JS, et al. : Genetic modulation of PPARgamma phosphorylation regulates insulin sensitivity. Dev Cell. 2003;5(4):657–63. 10.1016/S1534-5807(03)00274-0 [DOI] [PubMed] [Google Scholar]
- 48. Compe E, Drané P, Laurent C, et al. : Dysregulation of the peroxisome proliferator-activated receptor target genes by XPD mutations. Mol Cell Biol. 2005;25(14):6065–76. 10.1128/MCB.25.14.6065-6076.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Choi JH, Banks AS, Estall JL, et al. : Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466(7305):451–6. 10.1038/nature09291 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Choi JH, Banks AS, Kamenecka TM, et al. : Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature. 2011;477(7365):477–81. 10.1038/nature10383 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Banks AS, McAllister FE, Camporez JP, et al. : An ERK/Cdk5 axis controls the diabetogenic actions of PPARγ. Nature. 2015;517(7534):391–5. 10.1038/nature13887 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Ohshima T, Koga H, Shimotohno K: Transcriptional activity of peroxisome proliferator-activated receptor gamma is modulated by SUMO-1 modification. J Biol Chem. 2004;279(28):29551–7. 10.1074/jbc.M403866200 [DOI] [PubMed] [Google Scholar]
- 53. Yamashita D, Yamaguchi T, Shimizu M, et al. : The transactivating function of peroxisome proliferator-activated receptor gamma is negatively regulated by SUMO conjugation in the amino-terminal domain. Genes Cells. 2004;9(11):1017–29. 10.1111/j.1365-2443.2004.00786.x [DOI] [PubMed] [Google Scholar]
- 54. Lim S, Ahn BY, Chung SS, et al. : Effect of a peroxisome proliferator-activated receptor gamma sumoylation mutant on neointimal formation after balloon injury in rats. Atherosclerosis. 2009;206(2):411–7. 10.1016/j.atherosclerosis.2009.02.031 [DOI] [PubMed] [Google Scholar]
- 55. Pascual G, Fong AL, Ogawa S, et al. : A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437(7059):759–63. 10.1038/nature03988 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Chung SS, Ahn BY, Kim M, et al. : SUMO modification selectively regulates transcriptional activity of peroxisome-proliferator-activated receptor γ in C2C12 myotubes. Biochem J. 2011;433(1):155–61. 10.1042/BJ20100749 [DOI] [PubMed] [Google Scholar]
- 57. Koo YD, Choi JW, Kim M, et al. : SUMO-Specific Protease 2 (SENP2) Is an Important Regulator of Fatty Acid Metabolism in Skeletal Muscle. Diabetes. 2015;64(7):2420–31. 10.2337/db15-0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hauser S, Adelmant G, Sarraf P, et al. : Degradation of the peroxisome proliferator-activated receptor gamma is linked to ligand-dependent activation. J Biol Chem. 2000;275(24):18527–33. 10.1074/jbc.M001297200 [DOI] [PubMed] [Google Scholar]
- 59. Lee KW, Kwak SH, Koo YD, et al. : F-box only protein 9 is an E3 ubiquitin ligase of PPARγ. Exp Mol Med. 2016;48:e234. 10.1038/emm.2016.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li P, Song Y, Zan W, et al. : Lack of CUL4B in Adipocytes Promotes PPARγ-Mediated Adipose Tissue Expansion and Insulin Sensitivity. Diabetes. 2017;66:300–13. 10.2337/db16-0743 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Lee KW, Cho JG, Kim CM, et al. : Herpesvirus-associated ubiquitin-specific protease (HAUSP) modulates peroxisome proliferator-activated receptor γ (PPARγ) stability through its deubiquitinating activity. J Biol Chem. 2013;288(46):32886–96. 10.1074/jbc.M113.496331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kilroy G, Kirk-Ballard H, Carter LE, et al. : The ubiquitin ligase Siah2 regulates PPARγ activity in adipocytes. Endocrinology. 2012;153(3):1206–18. 10.1210/en.2011-1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ji S, Park SY, Roth J, et al. : O-GlcNAc modification of PPARγ reduces its transcriptional activity. Biochem Biophys Res Commun. 2012;417(4):1158–63. 10.1016/j.bbrc.2011.12.086 [DOI] [PubMed] [Google Scholar]
- 64. Qiang L, Wang L, Kon N, et al. : Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell. 2012;150(3):620–32. 10.1016/j.cell.2012.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Higgins LS, Depaoli AM: Selective peroxisome proliferator-activated receptor gamma (PPARgamma) modulation as a strategy for safer therapeutic PPARgamma activation. Am J Clin Nutr. 2010;91(1):267S–72. 10.3945/ajcn.2009.28449E [DOI] [PubMed] [Google Scholar]
- 66. Depaoli AM, Higgins LS, Henry RR, et al. : Can a selective PPARγ modulator improve glycemic control in patients with type 2 diabetes with fewer side effects compared with pioglitazone? Diabetes Care. 2014;37(7):1918–23. 10.2337/dc13-2480 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Henriksen K, Byrjalsen I, Qvist P, et al. : Efficacy and safety of the PPARγ partial agonist balaglitazone compared with pioglitazone and placebo: a phase III, randomized, parallel-group study in patients with type 2 diabetes on stable insulin therapy. Diabetes Metab Res Rev. 2011;27(4):392–401. 10.1002/dmrr.1187 [DOI] [PubMed] [Google Scholar]
- 68. Ming Y, Hu X, Song Y, et al. : CMHX008, a novel peroxisome proliferator-activated receptor γ partial agonist, enhances insulin sensitivity in vitro and in vivo. PLoS One. 2014;9(7):e102102. 10.1371/journal.pone.0102102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nissen SE, Wolski K, Topol EJ: Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294(20):2581–6. 10.1001/jama.294.20.joc50147 [DOI] [PubMed] [Google Scholar]
- 70. Goldstein BJ, Rosenstock J, Anzalone D, et al. : Effect of tesaglitazar, a dual PPAR alpha/gamma agonist, on glucose and lipid abnormalities in patients with type 2 diabetes: a 12-week dose-ranging trial. Curr Med Res Opin. 2006;22(12):2575–90. 10.1185/030079906X154169 [DOI] [PubMed] [Google Scholar]
- 71. Lincoff AM, Tardif JC, Schwartz GG, et al. : Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: the AleCardio randomized clinical trial. JAMA. 2014;311(5):1515–25. 10.1001/jama.2014.3321 [DOI] [PubMed] [Google Scholar]
- 72. Jung Y, Cao Y, Paudel S, et al. : Antidiabetic effect of SN158 through PPARα/γ dual activation in ob/ob mice. Chem Biol Interact. 2017;268:24–30. 10.1016/j.cbi.2017.02.014 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Wang L, Waltenberger B, Pferschy-Wenzig EM, et al. : Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): a review. Biochem Pharmacol. 2014;92(1):73–89. 10.1016/j.bcp.2014.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. An HJ, Lee B, Kim SM, et al. : A PPAR Pan Agonist, MHY2013 Alleviates Age-Related Hepatic Lipid Accumulation by Promoting Fatty Acid Oxidation and Suppressing Inflammation. Biol Pharm Bull. 2018;41(1):29–35. 10.1248/bpb.b17-00371 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Huang Y, Powers C, Moore V, et al. : The PPAR pan-agonist bezafibrate ameliorates cardiomyopathy in a mouse model of Barth syndrome. Orphanet J Rare Dis. 2017;12(1):49. 10.1186/s13023-017-0605-5 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Boubia B, Poupardin O, Barth M, et al. : Design, Synthesis, and Evaluation of a Novel Series of Indole Sulfonamide Peroxisome Proliferator Activated Receptor (PPAR) α/γ/δ Triple Activators: Discovery of Lanifibranor, a New Antifibrotic Clinical Candidate. J Med Chem. 2018;61(6):2246–65. 10.1021/acs.jmedchem.7b01285 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation