See Schiff (doi:10.1093/awx209) for a scientific commentary on this article.
Bedside examination does not reliably detect consciousness in patients with acute severe traumatic brain injury. Edlow, Chatelle et al. report that stimulus-based functional MRI and EEG techniques enhance early detection of consciousness and cortical responses to language in these patients, which could alter time-sensitive decisions about withdrawal of life-sustaining therapies.
Keywords: traumatic brain injury, consciousness, functional MRI, EEG, intensive care unit
Abstract
See Schiff (doi:10.1093/awx209) for a scientific commentary on this article.
Patients with acute severe traumatic brain injury may recover consciousness before self-expression. Without behavioural evidence of consciousness at the bedside, clinicians may render an inaccurate prognosis, increasing the likelihood of withholding life-sustaining therapies or denying rehabilitative services. Task-based functional magnetic resonance imaging and electroencephalography techniques have revealed covert consciousness in the chronic setting, but these techniques have not been tested in the intensive care unit. We prospectively enrolled 16 patients admitted to the intensive care unit for acute severe traumatic brain injury to test two hypotheses: (i) in patients who lack behavioural evidence of language expression and comprehension, functional magnetic resonance imaging and electroencephalography detect command-following during a motor imagery task (i.e. cognitive motor dissociation) and association cortex responses during language and music stimuli (i.e. higher-order cortex motor dissociation); and (ii) early responses to these paradigms are associated with better 6-month outcomes on the Glasgow Outcome Scale-Extended. Patients underwent functional magnetic resonance imaging on post-injury Day 9.2 ± 5.0 and electroencephalography on Day 9.8 ± 4.6. At the time of imaging, behavioural evaluation with the Coma Recovery Scale-Revised indicated coma (n = 2), vegetative state (n = 3), minimally conscious state without language (n = 3), minimally conscious state with language (n = 4) or post-traumatic confusional state (n = 4). Cognitive motor dissociation was identified in four patients, including three whose behavioural diagnosis suggested a vegetative state. Higher-order cortex motor dissociation was identified in two additional patients. Complete absence of responses to language, music and motor imagery was only observed in coma patients. In patients with behavioural evidence of language function, responses to language and music were more frequently observed than responses to motor imagery (62.5–80% versus 33.3–42.9%). Similarly, in 16 matched healthy subjects, responses to language and music were more frequently observed than responses to motor imagery (87.5–100% versus 68.8–75.0%). Except for one patient who died in the intensive care unit, all patients with cognitive motor dissociation and higher-order cortex motor dissociation recovered beyond a confusional state by 6 months. However, 6-month outcomes were not associated with early functional magnetic resonance imaging and electroencephalography responses for the entire cohort. These observations suggest that functional magnetic resonance imaging and electroencephalography can detect command-following and higher-order cortical function in patients with acute severe traumatic brain injury. Early detection of covert consciousness and cortical responses in the intensive care unit could alter time-sensitive decisions about withholding life-sustaining therapies.
Introduction
Current clinical tools are not reliable for detecting consciousness or predicting recovery in patients with severe traumatic brain injury (TBI). Bedside behavioural examination, the gold standard for clinical assessment of consciousness, may be limited by a patient’s neurological deficits (e.g. aphasia, quadriparesis), fluctuating state (e.g. related to arousal, pain, medical complications) or an examiner’s subjective interpretation of ambiguous responses (Gill-Thwaites, 2006). These limitations lead to an approximately 40% rate of misclassifying conscious patients as unconscious (Childs et al., 1993; Andrews et al., 1996; Schnakers et al., 2009). Given that early recovery of consciousness is associated with better long-term functional outcomes (Giacino and Kalmar, 1997; Whyte et al., 2001), the absence of a reliable diagnostic tool for detecting consciousness in the intensive care unit (ICU) creates uncertainty for families facing decisions about continuation of life-sustaining treatments and limits access to rehabilitative care for patients who are given inaccurate poor prognoses.
Since a landmark study in 2006 demonstrated that functional MRI can detect evidence of consciousness in a patient whose chronic behavioural examination suggested a vegetative state (also referred to as unresponsive wakefulness syndrome) (Owen et al., 2006), there has been growing interest in using functional imaging techniques to identify covert consciousness, or cognitive motor dissociation (CMD) (Schiff, 2015). Recent functional MRI and EEG studies using active motor imagery tasks have demonstrated that a small but important minority of patients with chronic post-traumatic disorders of consciousness shows signs of CMD, as evidenced by command-following during the task (Monti et al., 2010; Cruse et al., 2011). In addition, studies using passive language and music stimuli have shown that some patients with chronic post-traumatic disorders of consciousness demonstrate association cortex responses despite absent behavioural evidence of language expression and comprehension (Coleman et al., 2009; Okumura et al., 2014), a state defined here as higher-order cortex motor dissociation (HMD). Yet, despite emerging evidence that functional MRI and EEG can detect CMD and HMD in patients with chronic post-traumatic disorders of consciousness, no studies have focused on ICU patients with acute severe TBI. Early detection of consciousness and higher-order cortical function in this population could not only inform the diagnosis of level of consciousness but could also predict subsequent recovery of meaningful neurological function (Giacino and Kalmar, 1997; Whyte et al., 2001; Coleman et al., 2009; Stender et al., 2014). Furthermore, early, reliable, and objective information about a patient’s level of consciousness may assist caregiver and family decision-making in the ICU.
In this prospective observational study, we hypothesized that: (i) stimulus-based functional MRI and EEG detect CMD and HMD in ICU patients with acute severe TBI; and (ii) better 6-month outcomes on the Glasgow Outcome Scale-Extended (GOSE) are associated with early functional MRI and EEG responses.
Materials and methods
Experimental design
We prospectively screened all patients with TBI admitted to the Neurosciences ICU, Multidisciplinary ICU, and Surgical ICU at a single academic hospital between June 2012 and November 2014. Inclusion criteria were: (i) age 18 to 65 years; and (ii) head trauma with Glasgow Coma Scale score of 3–8 with no eye opening for at least 24 h. Exclusion criteria were: (i) life expectancy <6 months, as estimated by a treating physician; (ii) prior severe brain injury or neurodegenerative disease; (iii) penetrating TBI with intracranial metal or other body metal precluding MRI; and (iv) no fluency in English prior to the injury (because the functional MRI and EEG paradigms were administered in English).
Surrogate decision-makers were approached for consent ≥24 h after injury, and written informed consent was obtained in accordance with a research protocol approved by our Institutional Review Board. Functional MRI was performed as soon as the patient was clinically stable for transport to the MRI scanner, as determined by the treating ICU physicians and nurses. Whenever possible, the EEG was scheduled within 24 h of the functional MRI scan to enable comparison of the functional MRI and EEG data. Administration of sedative, anxiolytic, and/or analgesic medications was allowed for patient safety or comfort during the functional MRI and/or the EEG.
A cohort of age- and sex-matched healthy subjects was enrolled to compare their functional MRI and EEG responses to those of the patient sample. Healthy subjects had no history of neurological, psychiatric, cardiovascular, pulmonary, renal or endocrinological disease. They provided written informed consent and underwent the same functional MRI and EEG protocols as the patients. All patient and healthy subject MRI scans were performed on the same scanner, and EEGs were performed using the same equipment.
Neurobehavioural and outcome assessments
Demographic and clinical data were collected at the time of enrolment in accordance with the National Institutes of Health Common Data Element Guidelines for TBI (Maas et al., 2010). Immediately prior to functional MRI and EEG, each patient’s level of consciousness was characterized via behavioural evaluation with the Coma Recovery Scale-Revised (CRS-R) (Giacino et al., 2004) as coma, vegetative state, minimally conscious state without language function (MCS−; i.e. at least one of the following: visual fixation, visual pursuit, object localization, localization to noxious stimulation, object manipulation, automatic motor responses, or non-functional communication), minimally conscious state with language function (MCS+; i.e. at least one of the following: command-following, object recognition, or intelligible verbalization) (Giacino et al., 2002; Bruno et al., 2012b; Schnakers et al., 2015a), or emergence from the MCS (i.e. functional object use and/or functional communication). If a patient emerged from MCS, the diagnosis of post-traumatic confusional state (PTCS; Stuss et al., 1999) was confirmed based on criteria derived from the Confusion Assessment Protocol (CAP) (Sherer et al., 2005).
Functional outcome at 6 months was measured with the GOSE (Wilson et al., 1998). Patients and their surrogates were assessed either in-person at the study site or, if this was not feasible, through a validated GOSE phone questionnaire (Pettigrew et al., 2003). Six-month level of consciousness was also recorded for patients who returned for in-person CRS-R and CAP evaluation and for patients whose medical records from an affiliated rehabilitation hospital included clinical assessments with the CRS-R and CAP. All behavioural evaluations and outcome assessments were conducted by a single investigator (B.L.E). Additional details regarding characteristics of the CRS-R, CAP, and GOSE are provided in the Supplementary material.
Functional MRI data acquisition
MRI data were acquired with a 32-channel head coil on a 3 T Skyra MRI scanner (Siemens Medical Solutions) located in the Neurosciences ICU. Auditory stimuli were presented to all subjects via MRI-compatible earphones (Newmatic Medical) connected to the scanner’s sound system. The blood oxygen level-dependent (BOLD) functional MRI sequence used the following parameters: echo time = 30 ms, repetition time = 4000 ms, in-plane resolution = 2.0 × 2.0 mm, slice thickness = 2 mm, interslice gap = 2.5 mm, matrix = 94 × 94, field of view = 192 × 192 mm2, 49 slices, 2× GRAPPA acceleration. High-spatial resolution 3D T1-weighted multi-echo magnetization prepared gradient echo (MEMPRAGE) anatomical images were acquired for registration purposes (van der Kouwe et al., 2008): field of view = 256 × 256 mm2, acquisition matrix = 256 × 256, 176 sagittal slices (thickness 1 mm), 3× GRAPPA acceleration, echo time = 1.69, 3.55, 5.41, and 7.27 ms, repetition time = 2530 ms, inversion time = 1200–1300 ms, 1.0 mm3 isotropic resolution, flip angle = 7°.
Stimulus-based functional MRI paradigms
Each functional MRI paradigm utilized a block design and was comprised of two runs, with each run containing three 24-s rest blocks and two 24-s stimulation blocks (Supplementary Fig. 1). In total, 144 s of rest data and 96 s of stimulation data were analysed for each paradigm. Prior to the first rest block, 36 s of data were acquired to obtain a stable baseline BOLD signal. These data were excluded from analysis.
The language paradigm consisted of alternating 24-s blocks of rest, forwards language, rest, backwards language, and rest. The forwards language stimulus was a clip from John F. Kennedy’s Inaugural Address. This 24-s clip was time-reversed to create the backwards language stimulus. Backwards language functional MRI data were acquired as part of a preplanned secondary analysis, based on findings from a previous study showing that differential cortical activation patterns during forwards versus backwards language may reveal linguistic processing as compared with non-linguistic processing (Fernandez-Espejo et al., 2008). The backwards language acoustically matched the forwards language but violated several phonological properties of language. These characteristics make backwards language a potential control condition for non-linguistic aspects of speech (Fernandez-Espejo et al., 2008).
The music paradigm used the same block design as the language paradigm. The music stimulus was a 24-s clip from Aaron Copland’s ‘Rodeo – Four Dance Episodes’ with frequent changes in tempo, to increase the probability of observing a brain response to music (Danielsen et al., 2014), and no lyrics, to investigate cortical processing of prosody and rhythm without processing linguistic content (Danielsen et al., 2014). This paradigm was included based on prior studies suggesting that music alters cortical activity and connectivity in healthy subjects (Kovacs et al., 2006; Brattico et al., 2011; Wu et al., 2013) and patients with impaired consciousness (O'Kelly et al., 2013; Okumura et al., 2014).
The motor imagery paradigm involved a right hand squeeze imagery task previously used in patients with chronic disorders of consciousness (Cruse et al., 2011, 2012). This paradigm used the same block design as the language and music paradigms, except that instructions were repeated at 6-s intervals (e.g. ‘keep squeezing’ or ‘keep resting’). Instructions administered before and during the functional MRI scan are detailed in the Supplementary material and in Supplementary Table 1.
Functional MRI data analysis
In a first-level analysis of the individual runs, functional MRI data processing was performed using the FMRI Expert Analysis Tool (FEAT) version 6.00 in FSL 5.0.7 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). Forwards language, music and motor imagery stimuli were contrasted against rest and forwards language was also contrasted against backwards language. Z-statistic images were thresholded (Z > 3.1) and a corrected cluster significance threshold of P = 0.05 was used. Higher-level analysis was carried out using a fixed effects model (FLAME in FSL) (Beckmann et al., 2003; Woolrich et al., 2004). The statistical threshold for cluster significance (Z > 3.1) and the size of the Gaussian kernel (full-width at half-maximum = 10 mm) were both selected to decrease false positive cluster activations (Eklund et al., 2016). Additional details on analysis are provided in the Supplementary material.
We then used FEATQuery in FSL to quantify the percentage of voxels activated within each stimulus-specific region of interest. For healthy subjects, we defined a positive response by the criterion that >0% of region of interest voxels met the aforementioned statistical threshold. For patients, we defined a positive response by two criteria: (i) >0% of region of interest voxels met the statistical threshold; and (ii) the percentage of activated region of interest voxels was above the 2.5th percentile of a normal range (2.5th to 97.5th percentile) derived from the age- and sex-matched healthy subjects’ data for each region of interest in each paradigm. Although a single investigator performed the behavioural assessments and the functional MRI analyses (B.L.E.), the acute behavioural evaluation always preceded the functional MRI analysis, and our quantitative approach to functional MRI analysis reduced the likelihood that bias was inadvertently introduced due to knowledge of a patient’s behavioural diagnosis.
Functional MRI regions of interest
We selected a priori regions of interest based upon functional MRI studies of language, music and motor imagery in patients with chronic traumatic disorders of consciousness and healthy subjects. For the language and music stimuli, we used the bilateral Heschl’s gyrus and superior temporal gyrus regions of interest distributed by the Harvard-Oxford Cortical Structural Atlas (Makris et al., 2006) (Fig. 1). To reduce the false positive rate (FPR), we only considered superior temporal gyrus activation in subjects who also demonstrated activation within Heschl’s gyrus primary auditory cortex. For the motor imagery task, the bilateral supplementary motor areas from the Harvard-Oxford Cortical Structural Atlas and premotor cortices from the Juelich Histological Atlas (Eickhoff et al., 2005) were combined as a single region of interest (Fig. 1 and Supplementary Fig. 2). All regions of interest were transformed from standard atlas space into patient native functional MRI space for analysis, consistent with prior functional MRI studies of patients with disorders of consciousness (Fernandez-Espejo et al., 2008; Coleman et al., 2009; Monti et al., 2010; Bardin et al., 2011). See Supplementary material for additional details.
Figure 1.
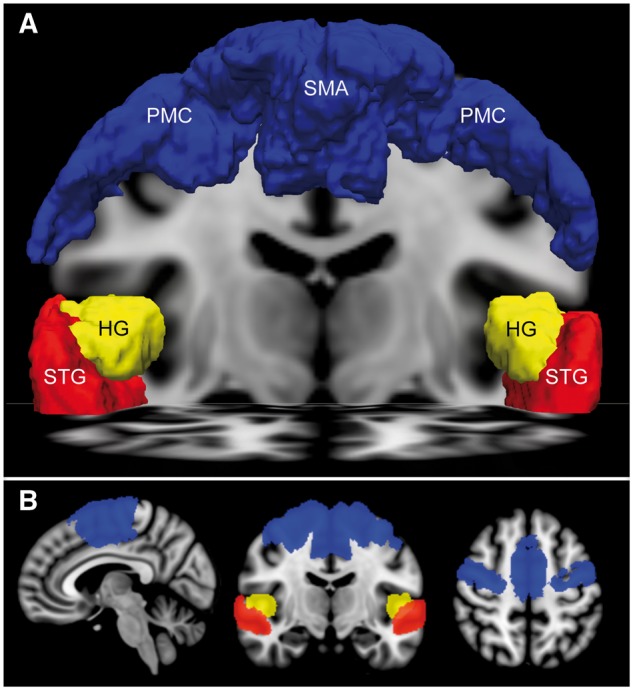
Regions of interest for functional MRI analysis. (A) Anterior view of the supplementary motor area (SMA) and premotor cortex (PMC) region of interest (blue) used to assess motor imagery fMRI responses, as well as the Heschl’s gyrus (HG, yellow) and superior temporal gyrus (STG, red) regions of interest used to assess language and music functional MRI responses. All regions of interest are rendered in MNI152 space and superimposed upon a coronal image at the level of the mid-thalamus and an axial image at the level of the STG. (B) Sagittal (left), coronal (middle), and axial (right) images of the supplementary motor areas/premotor cortices, Heschl’s gyrus, and superior temporal gyrus regions of interest.
EEG data acquisition and preprocessing
EEG data were acquired using a 19-electrode clinical XLTEK EEG system (Natus Medical Inc.) at a 200- or 256-Hz sampling rate and analysed using EEGlab (Delorme and Makeig, 2004) and customized MATLAB code (MathWorks, Natick, MA). All recordings were filtered (third-order Butterworth, zero-phase shift digital filter, 1–30 Hz) and re-referenced using the Hjorth Laplacian transform to optimize spatial localization and avoid contaminating activity at the reference (Lepage et al., 2014). Artefact rejection was performed with EEGlab using independent component analysis by a research neuropsychologist (C.C.). For patients, trials with large artefacts were discarded by consensus review with a fellowship-trained clinical neurophysiologist/epileptologist (E.S.R., C.C.). EEG recordings were also reviewed post hoc for evidence of epileptiform activity (E.S.R.) using the American Clinical Neurophysiology Society criteria (Hirsch et al., 2013). Both EEG analysts were blind to the behavioural diagnosis and functional MRI results.
Stimulus-based EEG paradigms
Auditory stimuli were administered via a portable speaker system (Scosche Inc.) placed on a table next to the subject’s bed. The EEG language, music, and motor imagery paradigms were the same as the functional MRI paradigms, except that the EEG paradigms used 12 24-s blocks of stimulus and rest (Supplementary Fig. 3). Also, the backwards language stimulus was not administered during EEG.
EEG classification using power spectral density
Following artefact rejection, we estimated the power spectral density of the voltage activity recorded at each electrode. Absolute power estimates were averaged within four frequency bands [delta (1–3 Hz), theta (4–7 Hz), alpha (8–13 Hz), beta (14–30 Hz)], resulting in a matrix (76 features × 576 s for music and language or 388 s for motor imagery) that was used for classifier analysis. Any segments where data had been removed following artefact rejection were padded to preserve indexing between trials.
For each patient and paradigm (language, music, and motor imagery), we used a support vector machine with a linear kernel to classify the data matrices as corresponding to either stimulation blocks (on) or rest blocks (off) (Burges, 1998). We used a 20-fold cross-validation procedure repeated 10 times to ensure a stable classifier accuracy estimate and used the average accuracy of these 10 iterations for analysis. We chose 20-fold as it provided the most stable results in our healthy subject cohort. For each iteration of the cross-validation, we randomly generated 20 disjoint partitions of each subject’s data matrix such that 19 folds were used for training and the last fold was used for evaluation. This process was repeated 20 times for a given partition. To test for significance, we performed a permutation test (Good, 2004) based on 500 permutations (Ojala and Garriga, 2010). To do so, we randomly exchanged the labels for the data (on or off) and used the shuffled labels to train and evaluate a classifier following the same procedure we used on the original data. The P-value was calculated as the sum of all accuracies obtained from the permuted data that were equal to or higher than the accuracies obtained from the original (i.e. non-permuted) data, divided by the number of permutations (Noirhomme et al., 2014). We considered a patient to have a positive EEG response if P < 0.05 (see Supplementary material for additional details).
Definitions of cognitive and higher-order cortex motor dissociation
We applied Schiff’s definition of CMD (Schiff, 2015) to ICU patients with acute severe TBI. Specifically, a patient with a behavioural diagnosis of coma, vegetative state, or MCS− who demonstrates command-following on functional MRI or EEG meets criteria for CMD. However, based on unique ICU challenges (e.g. pharmacological sedation and arousal fluctuations due to metabolic abnormalities, infections, endocrinological derangements, and/or medication effects) (Posner et al., 2007), and based on prior functional MRI and EEG studies suggesting that responses to passive stimuli have prognostic relevance in patients with subacute (Fischer et al., 2004) and chronic disorders of consciousness (Di et al., 2007; Coleman et al., 2009), we also aimed to identify patients with acute disorders of consciousness who demonstrated concordant EEG and association cortex functional MRI responses during passive stimulation, despite absent language function on behavioural evaluation. Importantly, association cortex responses to passive stimuli are not interpreted as evidence of covert consciousness, or CMD (see ‘Discussion’ section). Nevertheless, an association cortex response to language or music stimuli in a patient whose CRS-R exam indicates coma, vegetative state, or MCS− still represents a dissociation between the functional MRI/EEG findings and behavioural findings. Thus, we classify these patients as having HMD. See Fig. 2 for a schematic overview of the prespecified criteria for CMD and HMD.
Figure 2.
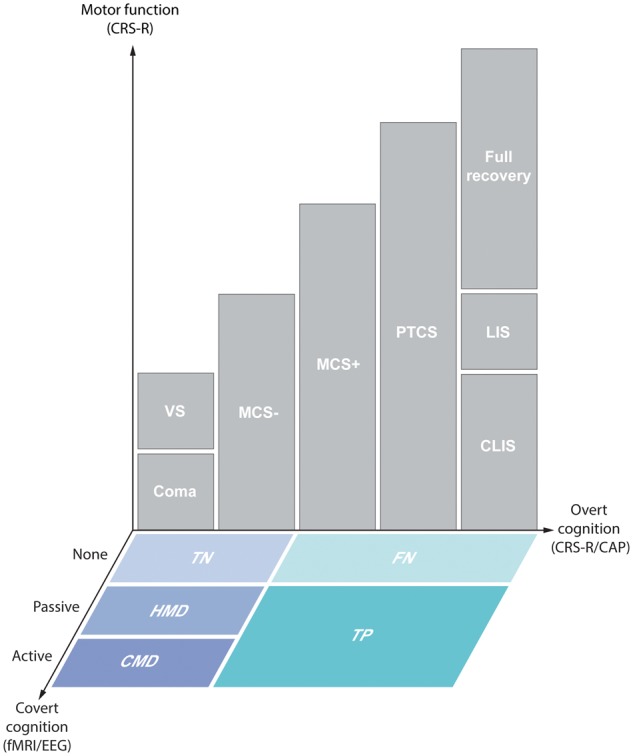
Schematic of the three dimensions of detecting consciousness. Patients were assessed for motor function and overt cognitive function via bedside behavioural evaluation with the CRS-R and CAP. Covert cognition that evades detection by behavioural evaluation was assessed with functional MRI (fMRI) and EEG. Levels of consciousness indicated by overt cognition are defined as coma, vegetative state (VS), minimally conscious state without language function (MCS−), minimally conscious state with language function (MCS+), post-traumatic confusional state (PTCS), complete locked-in syndrome (CLIS), locked-in syndrome with preservation of minimal motor function (LIS), and full recovery. Cognitive motor dissociation (CMD) is defined by functional MRI or EEG responses demonstrating command-following on an active motor imagery task despite absence of behavioural evidence of language function. Higher-order cortex motor dissociation (HMD) is defined as functional MRI and EEG responses within association cortex (e.g. Wernicke’s area) during passive language or music stimuli despite absence of behavioural evidence of language. Using the behavioural diagnosis as the reference standard, patients without behavioural evidence of language (coma, vegetative state, and MCS−) are classified as true negatives (TN) if there are no functional MRI or EEG responses. Patients with behavioural evidence of language [MCS+, PTCS, CLIS (with assistive communication devices), LIS, and full recovery] are classified as false negatives (FN) if there are no functional MRI or EEG responses, and true positives (TP) if there are functional MRI and EEG responses.
We implemented several procedures to reduce the FPR of CMD and HMD detection. First, the binary categorization of functional MRI responses was based on an objective marker (i.e. presence or absence of suprathreshold voxels) rather than subjective visual inspection of the data. Likewise, EEG responses were categorized using an objective, data-driven classifier method. Second, for the diagnosis of HMD, we required that a functional MRI response occur within association cortices (e.g. Wernicke’s area), not just primary sensory cortices (Heschl’s gyrus). Third, for a diagnosis of HMD we required the presence of both functional MRI and EEG responses to language or music. Collectively, these methods ensured that patients who met the prespecified criteria have a high likelihood of consciousness (i.e. CMD) or higher-order cortical responses to environmental stimuli (i.e. HMD).
Statistical analyses
To assess the reliability of each paradigm to detect behavioural evidence of language function, we calculated the true positive rate (TPR; i.e. sensitivity), true negative rate (TNR; i.e. specificity), false negative rate (FNR) and the FPR in the patient cohort. The CRS-R-derived behavioural diagnosis was the reference standard for language function and the stimulus-based functional MRI or EEG responses were the test criterion. Notably, the FPR may include both false positives (i.e. patients wrongly diagnosed by the functional MRI/EEG tests) and cases of dissociation between behavioural responses and functional MRI/EEG responses (i.e. CMD or HMD). We also calculated the TPR and FNR in the healthy subject cohort. TNR and FPR values were not calculated because all healthy subjects exhibited behavioural evidence of language function.
To test our hypothesis pertaining to the association between 6-month ordinal GOSE scores and early functional MRI and EEG responses, Mann-Whitney statistics were calculated to investigate the difference between GOSE scores of patients with and without responses to each paradigm and considered significant at P < 0.05 (two-sided). Several post hoc analyses were conducted to assess for potential confounding. To test the relationship between time from injury to functional MRI/EEG and responses to the paradigms, we calculated Mann-Whitney statistics with time post-injury as a continuous predictor and response as a dichotomous outcome. We assessed the impact of sedation on functional MRI and EEG responses by classifying sedation in four categories: (i) no sedation; (ii) intermittent doses of non-anaesthetic sedatives; (iii) continuous infusion of low-dose anaesthetic sedatives (e.g. propofol drip rate <150 mg/h); and (iv) continuous infusion of high-dose anaesthetic sedatives (e.g. propofol drip rate ≥150 mg/h). We tested for an association between sedation category and functional MRI/EEG responses, as well as between sedation category and level of consciousness at the time of functional MRI/EEG (dichotomized as presence or absence of language function), using a 4 × 2 Fisher’s exact test (one-sided). Statistical analyses were performed in SPSS v24.0.
Results
Demographics and clinical characteristics
Of 399 patients consecutively screened for eligibility, 28 patients with severe TBI met all eligibility criteria and 16 [12 males, mean ± standard deviation (SD) age = 28.9 ± 9.2 years] were enrolled (Table 1 and Supplementary Fig. 4). At the time of functional MRI (mean ± SD post-injury Day 9.2 ± 5.0), behavioural examination indicated coma (n = 2), vegetative state (n = 3), MCS− (n = 3), MCS+ (n = 4) or PTCS (n = 4). EEG was performed within 24 h of functional MRI in 11 patients. However, scheduling and ICU issues (e.g. urgent therapeutic interventions) led to an increased time between the two assessments for five patients (range: −7 to +3 days between functional MRI and EEG). There was no association between functional MRI/EEG responses and time post-injury (U = 10–11.5, P = 0.08–0.88). Sedation category was not associated with functional MRI/EEG responses or level of consciousness at the time of functional MRI/EEG (Fisher’s exact test, df = 3; P = 0.30–0.99 for both analyses). The types and doses of sedative, anxiolytic, and analgesic medications administered at the time of functional MRI and EEG are reported in Supplementary Table 4. Patient-specific data regarding epileptiform activity are reported in the Supplementary material and Supplementary Table 5. There were no adverse events. The healthy subject cohort was composed of 16 healthy subjects (12 males, mean ± SD age 28.5 ± 7.8 years).
Table 1.
Patient demographics and clinical characteristics
| ID | Age (years) | Sex | TBI mechanism | iGCS | Day of fMRI | CRS-R at fMRI | CRS-R subscale scores at fMRI | LoC at fMRI | Day of EEG | CRS-R at EEG | CRS-R subscale scores at EEG | LoC at EEG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 27 | M | MVA | 5 T | 16 | 23 | A4V5M6O3C2Ar3 | PTCS | 17 | 23 | A4V5M6O3C2Ar3 | PTCS |
| P2 | 21 | M | Ped versus car | 4–8 T | 1 | 4 | A0V0M3O1C0Ar0 | MCS− | 2 | 4 | A0V0M3O1C0Ar0 | MCS− |
| P3 | 19 | F | MVA | 5 T | 3 | 1 | A0V0M1O0C0Ar0 | Coma | 4 | 1 | A0V0M1O0C0Ar0 | Coma |
| P4 | 19 | M | Fall | 3–7 T | 17 | 23 | A4V5M6O3C2Ar3 | PTCS | 10 | 22 | A4V5M6O3C2Ar2 | PTCS |
| P5 | 34 | M | Fall | 5 T | 15 | 3 | A0V0M0O2C0Ar1 | VS | 16 | 3 | A0V0M0O2C0Ar1 | VS |
| P6 | 28 | F | MVA | 3 | 7 | 6 | A0V1M2O1C0Ar2 | VS | 10 | 11 | A3V2M3O1C0Ar2 | MCS+ |
| P7 | 45 | M | MVA | 5 T | 13 | 18 | A3V5M5O3C1Ar1 | MCS+ | 14 | 18 | A3V5M5O3C1Ar1 | MCS+ |
| P8 | 33 | M | Fall | 5–7 T | 8 | 20 | A4V5M5O2C2Ar2 | PTCS | 8 | 20 | A4V5M5O2C2Ar2 | PTCS |
| P9 | 32 | M | Ped versus car | 5–7 T | 11 | 9 | A3V2M2O1C0Ar1 | MCS+ | 13 | 15 | A3V3M5O1C1Ar2 | MCS+ |
| P10 | 24 | M | Assault | 3–7 T | 12 | 10 | A1V1M5O1C0Ar2 | MCS− | 13 | 10 | A1V1M5O1C0Ar2 | MCS− |
| P11 | 22 | F | Ped versus car | 6 T | 14 | 22 | A4V5M6O3C1Ar3 | PTCS | 14 | 22 | A4V5M6O3C1Ar3 | PTCS |
| P12 | 27 | F | Fall | 3 | 8 | 1 | A0V0M1O0C0Ar0 | Coma | 8 | 1 | A0V0M1O0C0Ar0 | Coma |
| P13 | 18 | M | Fall | 3–7 | 4 | 12 | A3V2M5O1C0Ar1 | MCS+ | 6 | 21 | A4V5M6O3C2Ar1 | PTCS |
| P14 | 51 | M | Ped versus car | 3 | 8 | 3 | A0V0M1O0C1Ar1 | VS | N/Aa | N/Aa | N/Aa | N/Aa |
| P15 | 29 | M | Ped versus car | 4–7 | 7 | 3 | A0V0M3O0C0Ar0 | MCS− | 8 | 7 | A3V0M3O0C0Ar1 | MCS+ |
| P16 | 33 | M | Fall | 3–4 | 3 | 12 | A4V2M5O0C0Ar1 | MCS+ | 4 | 14 | A4V2M6O0C0Ar2 | PTCS |
The initial Glasgow Coma Scale (iGCS) is defined as the best (i.e. highest) and worst (i.e. lowest) post-resuscitation GCS score assessed by a qualified clinician who performed a reliable examination (not confounded by sedation and/or paralytics) prior to ICU admission. Level of consciousness (LoC) is assessed via behavioural evaluation with the CRS-R as coma, vegetative state (VS), MCS−, MCS+, or PTCS (emerged from MCS but disoriented). The subscales for the CRS-R are Auditory Function (A), Visual Function (V), Motor Function (M), Oromotor Function (O), Communication (C), and Arousal (Ar).
F = female; fMRI = functional MRI; M = male; MVA = motor vehicle accident; N/A = not applicable; Ped = pedestrian.
aPatient died before EEG due to withholding of life-sustaining treatment.
Stimulus-based functional MRI assessment of brain responses
Functional MRI was completed in 93.7% (music and motor imagery) to 100% (language) of patients. The normal range (2.5th to 97.5th percentile) in the healthy subject cohort for the percentage of activated voxels within each region of interest for each paradigm is reported in Supplementary Table 3 and subject-specific functional MRI responses are shown in Supplementary Fig. 5. Patients’ individual functional MRI responses are reported in Table 2 and Supplementary Table 6, and shown in Supplementary Fig. 6. For all patients with region of interest-specific functional MRI activation, the percentage of activated voxels was above the lower limit of the normal range in controls.
Table 2.
Patient functional MRI and EEG responses to language, music, and motor imagery
| ID | LoC at fMRI/EEG | Language | Music | Motor imagery | CMD or HMD | GOSE at follow-up | |||
|---|---|---|---|---|---|---|---|---|---|
| fMRI | EEG | fMRI | EEG | fMRI | EEG | ||||
| P1 | PTCS/PTCS | + | + | + | + | + | + | N/A | 3 |
| P2 | MCS−/MCS− | + | + | + | + | − | − | HMD | 7 |
| P3 | Coma/Coma | − | − | − | − | − | − | No | 7 |
| P4 | PTCS/PTCS | − | + | N/Aa | N/Ab | N/Aa | N/Ab | N/A | 7 |
| P5 | VS/VS | − | − | − | − | + | − | CMD | 3 |
| P6 | VS/MCS+ | + | + | + | + | + | − | CMD | 3 |
| P7 | MCS+/MCS+ | + | − | + | − | − | − | N/A | 5 |
| P8 | PTCS/PTCS | − | + | − | − | − | − | N/A | 3 |
| P9 | MCS+/MCS+ | + | + | + | + | − | + | N/A | 4 |
| P10 | MCS−/MCS− | + | − | − | + | + | − | CMD | 5 |
| P11 | PTCS/PTCS | − | − | − | + | − | + | N/A | 7 |
| P12 | Coma/Coma | − | N/Ac | − | N/Ac | − | N/Ac | No | 1e |
| P13 | MCS+/PTCS | + | + | + | + | + | − | N/A | 7 |
| P14 | VS/N/Ae | −d | N/Ae | + | N/Ae | + | N/Ae | CMD | 1e |
| P15 | MCS−/MCS+ | + | + | − | + | − | − | HMD | 5 |
| P16 | MCS+/PTCS | + | + | + | − | + | − | N/A | 5 |
CMD is defined by functional MRI or EEG evidence of command-following on the active motor imagery task despite absence of language function on behavioural evaluation. HMD is defined as functional MRI and EEG evidence of cortical responses to passive language or music stimuli despite behavioural absence of language.
aUnable to complete functional MRI due to severe agitation.
bEEG data unusable due to motion artefacts.
cEEG data unusable due to myogenic artefacts.
dPatient 14 had functional MRI activation within the superior temporal gyrus, but because there was no activation in primary auditory cortex (i.e. Heschl’s gyrus), Patient 14 did not meet the prespecified criteria for a positive response to language.
ePatient died due to withholding of life-sustaining treatment.
fMRI = functional MRI; LoC = level of consciousness; N/A = not applicable; VS = vegetative state.
Language
All 16 healthy subjects [100% (95% exact confidence interval (CI): 79.4–100%)] demonstrated responses within Heschl’s gyrus and superior temporal gyrus. Of the eight patients with behavioural evidence of language function, five had a functional MRI response to language (TPR = 5/8; FNR = 3/8). Of the eight patients without behavioural signs of language, three had no functional MRI response to language (TNR = 3/8; FPR = 5/8). The sensitivity and specificity of language functional MRI for behavioural evidence of language in patients was thus 62.5% (95% CI: 24.5–91.5%) and 37.5% (95% CI: 8.5–75.5%), respectively. In the analysis of forwards versus backwards language responses, 9 of 16 healthy subjects and 6 of 16 patients demonstrated more superior temporal gyrus activation to forwards language (see Supplementary Tables 3 and 6 for subject-specific data). Greater superior temporal gyrus activation to forwards language was 50% (95% CI: 15.7–84.3%) sensitive and 62.5% (95% CI: 24.5–91.5%) specific for behavioural evidence of language in patients.
Music
Fifteen of 16 healthy subjects [93.8% (95% CI: 69.8–99.8%)] demonstrated responses within Heschl’s gyrus and superior temporal gyrus. Of the seven patients with behavioural evidence of language function, five displayed a functional MRI response to music (TPR = 5/7; FNR = 2/7). Of the eight patients without behavioural evidence of language, three responded to music (TNR = 5/8; FPR = 3/8). In patients, functional MRI response to music was thus 71.4% (95% CI: 29.0–96.3%) sensitive and 62.5% (95% CI: 24.5–91.5%) specific for behavioural evidence of language.
Motor imagery
Eleven of 16 healthy subjects [68.8% (95% CI: 41.3–89.0%)] demonstrated responses within supplementary motor areas/premotor cortices. Of the seven patients with behavioural evidence of language, three showed functional MRI evidence of command-following (TPR = 3/7; FNR = 4/7). Of the eight patients without behavioural evidence of language, four showed functional MRI evidence of command-following (TNR = 4/8; FPR = 4/8). Functional MRI responses to motor imagery were thus 42.9% (95% CI: 9.9–81.6%) sensitive and 50% (95% CI: 15.7–84.3%) specific for behavioural evidence of language in patients.
Stimulus-based EEG assessment of brain responses
EEG was completed in 86.6% (music and motor imagery) to 93.3% (language) of patients. The percentage of EEG data discarded due to artefact was 1.2 ± 1.7% for healthy subjects and 1.7 ± 3.8% for patients. Patient and healthy subject group-level EEG responses are summarized below. Single-subject EEG responses are reported in Table 2 and Supplementary Table 6 for patients and Supplementary Table 3 for healthy subjects.
Language
Fourteen of 16 healthy subjects had EEG responses to language [87.5% (95% CI: 61.7–98.4%)]. Of the 10 patients with behavioural evidence of language function, eight had EEG responses to language (TPR = 8/10; FNR = 2/10). Of the four patients without behavioural signs of language, three had no EEG response to language (TNR = 3/4; FPR = 1/4). EEG response to language was thus 80% (95% CI: 44.4–97.5%) sensitive and 75% (95% CI: 19.4–99.4%) specific for detecting behavioural signs of language in patients.
Music
Fourteen of 16 healthy subjects had EEG responses to music [87.5% (95% CI: 61.7–98.4%)]. Of the nine patients with behavioural evidence of language function, six had EEG responses to music (TPR = 6/9; FNR = 3/9). Of the four patients without behavioural evidence of language, two had no EEG response to music (TNR = 2/4; FPR = 2/4). EEG response to music was thus 66.7% (95% CI: 29.9–92.5%) sensitive and 50% (95% CI: 6.8–93.2%) specific for detecting behavioural evidence of language in patients.
Motor imagery
Twelve of 16 healthy subjects had EEG responses to motor imagery [75.0% (95% CI: 47.6–92.7%)]. Of the nine patients with behavioural evidence of language function, three had EEG responses to motor imagery (TPR = 3/9; FNR = 6/9). Of the four patients without behavioural evidence of language, none had EEG response to motor imagery (TNR = 4/4; FPR = 0/4). EEG response to motor imagery was thus 33.3% (95% CI: 7.5–70.1%) sensitive and 100% (95% CI: 39.8–100%) specific for detecting behavioural signs of language in patients.
Detection of covert command-following and higher-order cortical responses
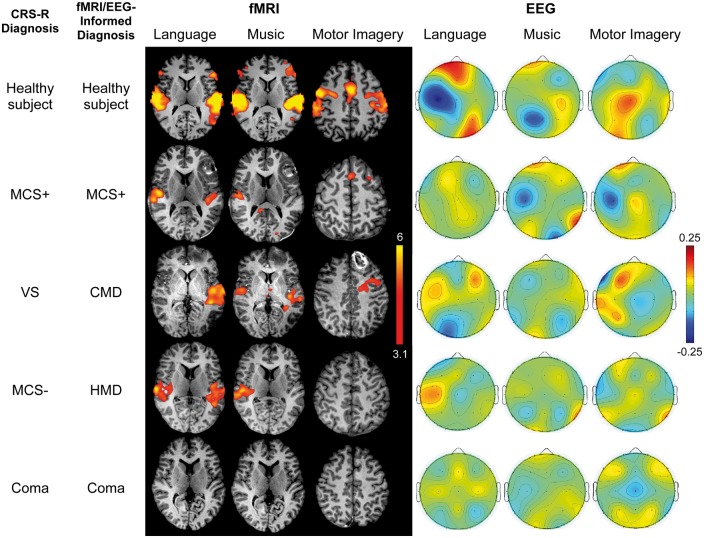
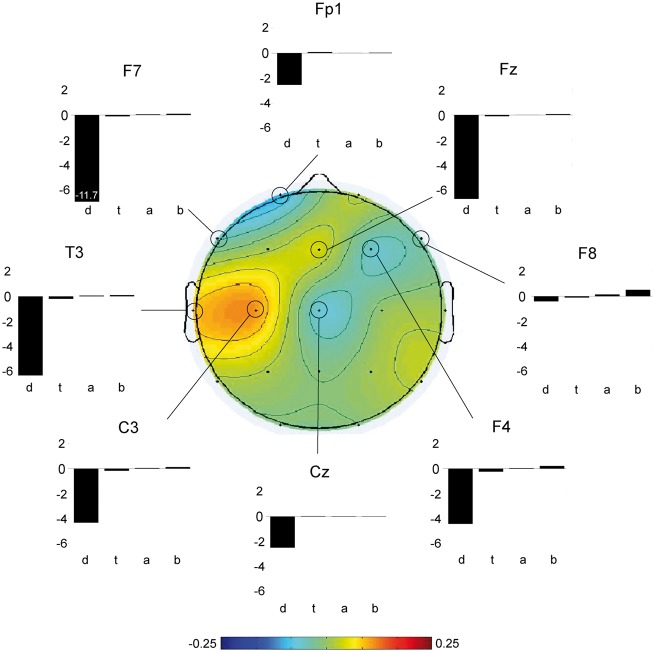
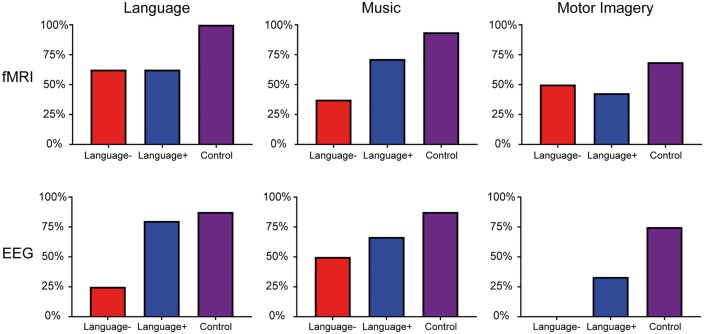
CMD was identified by functional MRI in four of eight patients who did not have behavioural evidence of language function (three vegetative state, one MCS−). HMD was identified in two additional patients who had both functional MRI and EEG responses to passive stimuli (both MCS−). Figure 3 shows representative functional MRI and EEG responses for a healthy subject, a patient with behavioural and functional MRI/EEG evidence of language function, a patient with CMD, a patient with HMD, and a patient without behavioural or functional MRI/EEG evidence of language function. Figure 4 shows an EEG spectral topography plot for an HMD patient, with stimulus-based frequency changes at individual electrodes. Figure 5 shows functional MRI responses for a CMD patient whose behavioural evaluation indicated vegetative state, but whose functional MRI responses revealed evidence of command-following. Figure 6 shows the percentage of functional MRI and EEG responders in patients with and without behavioural evidence of language function, as well as in healthy subjects.
Figure 3.
Stimulus-based functional MRI responses and EEG topographic plots. Functional MRI (fMRI) and EEG results are shown for the language, music and motor imagery paradigms for representative subjects: a healthy subject (C2), a patient with behavioural and functional MRI/EEG evidence of language function (Patient P9), a patient with no behavioural evidence of language but functional MRI evidence of command-following (CMD; Patient P6), a patient with no behavioural evidence of language but functional MRI/EEG evidence of cortical activation to passive stimuli (HMD; Patient P2), and a patient without behavioural or functional MRI/EEG evidence of language (Patient P3). Functional MRI data are shown as Z-statistic images to demonstrate stimulus-specific responses. Z-statistic images are thresholded at cluster-corrected Z scores of 3.1 (inset colour bar) and superimposed on T1-weighted axial images. All EEG data are shown as topographic plots illustrating the averaged weights attributed to each electrode by the classifier, based on their ability to differentiate between the two conditions for each paradigm (e.g. language versus rest). Red colours show coefficient values > 0. Blue colours show values < 0 (inset colour bar). The larger the absolute value of a feature weight (either positive or negative), the more important it was for discriminating between stimulus and rest conditions. Functional MRI data are in radiological convention; EEG data are in anatomical convention.
Figure 4.
EEG classifier results in HMD. For a patient who met the prespecified criteria for HMD (P2), we show spectral power changes during the language paradigm for the eight electrodes with the largest weights (i.e. the electrodes that best discriminated between language and rest; see inset colour bar). A decrement in delta power was observed at each electrode [units = 10log(µV2/Hz)], with a more pronounced change in the left hemisphere in the temporal region known to be involved in language processing (electrode T3). For electrode F7, the decrement in delta power was 11.7 microvolts. d = delta; t = theta; a = alpha; b = beta. The overall P-value in this analysis was P = 0.01.
Figure 5.
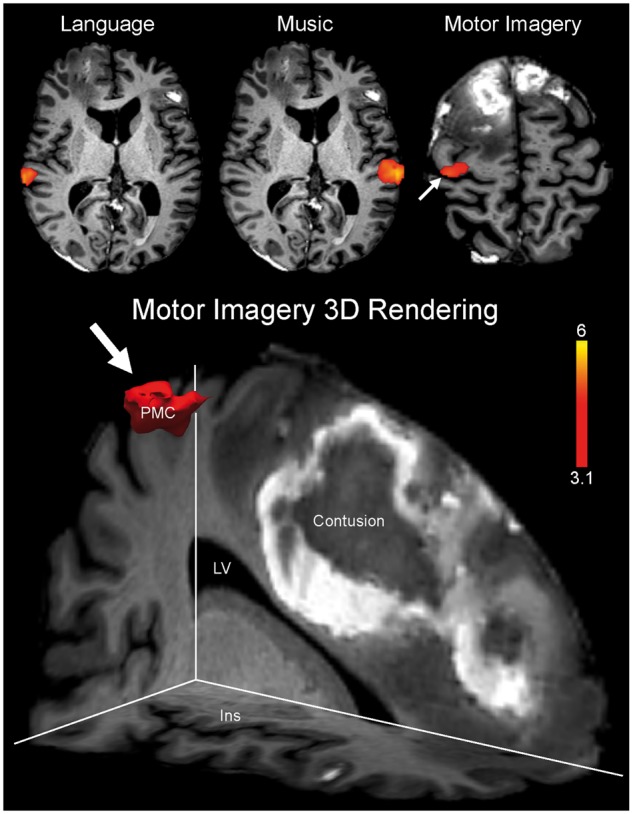
Functional MRI evidence of command-following in CMD. Functional MRI data are shown as Z-statistic images to demonstrate stimulus-specific responses in a patient whose behavioural evaluation suggested a vegetative state (Patient P14). Z-statistic images are thresholded at cluster-corrected Z scores of 3.1 (inset colour bar) and superimposed on T1-weighted axial images. There is functional MRI evidence of command-following on the motor imagery task (arrow), indicating CMD. In the bottom panel, a 3D rendering of the functional MRI response to the motor imagery task is shown (arrow). This response is located within the prespecified supplementary motor area/premotor cortex region of interest. Specifically, the response is located within the premotor cortex (PMC) in close neuroanatomic proximity to a right frontal contusion. The images in the top row are shown in radiological convention. Notably, despite functional MRI activation within the superior temporal gyrus during the language stimulus (top left), the patient was classified as having an absent response to language because of the absence of a response within Heschl’s gyrus. Ins = insula; LV = lateral ventricle.
Figure 6.
Percentage of functional MRI and EEG responders in patients and healthy subjects. Results for patients without behavioural evidence of language function (Language−; i.e. CRS-R/CAP-based behavioural diagnosis indicates coma, vegetative state, or MCS−) are represented as red bars. Results for patients with behavioural evidence of language function (Language+; i.e. CRS-R/CAP-based behavioural diagnosis indicates MCS+ or post-traumatic confusional state) are represented as blue bars. Results for healthy subjects (Control) are represented as purple bars.
Stimulus-based functional MRI and EEG response correlations with functional outcome
Two patients died in the ICU (GOSE = 1) after withholding life-sustaining therapy. One patient (Patient P12) died 5 days after functional MRI and EEG. One patient (Patient P14) died 2 days after functional MRI, before EEG data could be acquired. For the 14 patients who survived, follow-up GOSE evaluation was performed in-person (n = 11) or via phone interview (n = 3) at mean ± SD 6.2 ± 0.9 months post-injury. GOSE scores ranged from 1 (death) to 7 (lower good recovery) (median 5.0; see Table 2). Follow-up level of consciousness data were available for all 14 survivors (eight via in-person CRS-R/CAP assessment and six via CRS-R/CAP data obtained from clinical records). All survivors, including the three survivors of acute CMD and both patients with acute HMD, recovered beyond PTCS by 6 months. Highest acute level of consciousness based on the CRS-R score at the time of functional MRI or EEG was 64% (95% CI: 35.1–87.2%) sensitive for detecting recovery beyond PTCS by 6 months. In contrast, adding CMD and HMD to acute level of consciousness improves sensitivity to 93% (95% CI: 66.1–99.8%) for detecting recovery beyond PTCS by 6 months.
GOSE scores were not associated with acute functional MRI responses to language (Mann-Whitney U = 28.5, P = 0.87), music (U = 27.5, P = 0.95), or motor imagery (U = 13.0, P = 0.37), or with acute EEG responses to language (U = 18.5, P = 0.61), music (U = 23.5, P = 0.62) or motor imagery (U = 13.0, P = 0.81). Similarly, GOSE scores were not higher for patients who had more functional MRI activation to forwards language versus backwards language (U = 27.0, P = 0.68).
Discussion
In this prospective study of patients with acute severe TBI, we demonstrate that it is feasible to use stimulus-based functional MRI and EEG in the ICU to identify covert consciousness that evades detection on bedside behavioural examination. We identified a substantial proportion of ICU patients whose functional MRI and EEG responses revealed either command-following (CMD) or higher-order cortical responses to passive stimuli (HMD) despite a behavioural diagnosis of vegetative state or MCS−. Of the eight patients who lacked behavioural evidence of language function (two coma, three vegetative state, and three MCS−), four demonstrated functional MRI responses consistent with command following (three vegetative state, one MCS−) and two patients without evidence of command following showed higher-order cortical responses to language and/or music on both functional MRI and EEG (two MCS−).
Our observations in ICU patients with acute severe TBI are consistent with and build upon those of prior functional MRI and EEG studies in patients with chronic disorders of consciousness that reported CMD rates ranging from 8% (Lule et al., 2013) to 45% (Hauger et al., 2015). Similarly, the proportion of ICU patients in this study that met criteria for HMD is consistent with proportions of patients with chronic disorders of consciousness who respond to passive event-related potential paradigms (14–33%) (Faugeras et al., 2011; King et al., 2013; Sitt et al., 2014). Yet while all three acute CMD survivors and both acute HMD patients in our study recovered beyond PTCS by 6 months post-injury, we did not detect an association between 6-month functional outcomes on the GOSE and early functional MRI and EEG responses.
Collectively, these findings suggest that (i) stimulus-based functional MRI can detect CMD in the ICU when an active motor imagery task is used; and (ii) functional MRI and EEG techniques using passive language and music stimuli enable detection of higher-order cortex responses in patients without behavioural evidence of language function. Confirmation of the clinical relevance of a response to a passive stimulus awaits corroborating diagnostic and/or prognostic evidence. Below we discuss our findings in terms of their clinical, scientific, and ethical significance for the ICU population.
Rationale for stimulus-based functional MRI and EEG in the ICU
The motivation to develop quantitative, stimulus-based functional MRI and EEG biomarkers in the ICU is based on several challenges that currently limit the accuracy of diagnosis and prognosis in patients with acute brain injuries. First, the alarmingly high rate of misdiagnosis observed in the vegetative state population (∼40%) when using clinical consensus rather than a CRS-R evaluation (Schnakers et al., 2009) is particularly concerning in the ICU, because patients often cannot tolerate being off pharmacological sedation for the 15–35 min required to perform the CRS-R. Additionally, the CRS-R behavioural examination can be limited by motor deficits, aphasia (Majerus et al., 2009), fluctuating vigilance (Piarulli et al., 2016), or sensory impairment that may be unrecognized during the acute stage of injury in the ICU. Examiner bias inherent to interpreting behavioural responses further contributes to the potential failure to detect consciousness.
Given prior evidence that conscious patients (i.e. MCS) have a better prognosis than unconscious patients (i.e. coma and vegetative state) (Giacino and Kalmar, 1997; Katz et al., 2009; Luaute et al., 2010; Bruno et al., 2012a; Whyte et al., 2013), early diagnosis (and its corresponding prognosis) can drive decisions regarding discontinuation of life-sustaining treatment (Truog et al., 2008; Turgeon et al., 2011). Challenges in detecting consciousness in the ICU can potentially lead to early withdrawal of care in patients who retain sufficient cortical function to support recovery. Even for patients who are provided chronic life-sustaining treatment, an inaccurate poor prognosis may limit a patient’s access to intensive rehabilitative care, leading to a self-fulfilling prophecy whereby the early prognosis contributes to a patient’s poor outcome.
Defining covert consciousness in ICU patients
There is ongoing debate about how to define consciousness in patients who cannot express themselves at the bedside but who show signs of covert consciousness with functional MRI or EEG (Ropper, 2010). This debate is underscored by the many terms, including ‘covert cognition’ (Schnakers et al., 2015b), ‘functional locked-in syndrome’ (Bruno et al., 2011), ‘minimally conscious star (*)’ (Gosseries et al., 2014), and ‘CMD’ (Schiff, 2015) that have been used to classify these patients. It is now accepted that patients with CMD should be distinguished from vegetative state patients, as covert consciousness may carry a more favourable prognosis (Di et al., 2008; Stender et al., 2014; Wang et al., 2015). However, consensus criteria for defining consciousness based upon the integrated results of behavioural, functional MRI and EEG data currently do not exist.
Recently, it was proposed that a diagnosis of CMD requires functional MRI or EEG-based evidence of command-following during a motor imagery task (Schiff, 2015). Here, we implemented Schiff’s CMD definition in the ICU population and used a motor imagery task that was previously used to detect CMD in patients with chronic disorders of consciousness (Cruse et al., 2011). In addition to detecting CMD patients in the ICU, we also identified a separate group of patients whose responses to passive stimuli on functional MRI and EEG are greater than those expected based on behavioural evaluation. Our motivation for classifying this group as HMD is based upon diagnostic and prognostic considerations that are unique to the ICU environment. First, command-following may not be the only type of brain activity that is relevant to the diagnosis and prognosis of ICU patients with acute severe TBI. Rather, higher-order cortex responses to language and music suggest a residual capacity to process environmental stimuli and, if corroborated, might also inform diagnosis and prognosis. Second, requiring command-following during a motor imagery task may be too high a bar for defining a clinically meaningful functional MRI/EEG response in patients with acute severe TBI. Compared to patients with subacute or chronic disorders of consciousness, patients with acute disorders of consciousness are more likely to have poor or fluctuating arousal, which decreases the likelihood that a patient can perform a functional MRI- or EEG-based command-following task. This concern about the poor reliability of functional MRI- and EEG-based command-following tasks is highlighted by their limited sensitivity in healthy populations (Hauger et al., 2015), which ranges from 71% to 100% in prior studies (Boly et al., 2007; Bardin et al., 2012; Hauger et al., 2017). In our sample of 16 healthy subjects, the rates of functional MRI- and EEG-based command-following during the motor imagery task were 69% and 75%, respectively. These findings underscore the need for caution in interpreting negative findings on functional MRI and EEG motor imagery tasks.
Importantly, we do not to assert that HMD is indicative of covert consciousness. Current conceptual models of consciousness, such as the global neuronal workspace theory (Dehaene et al., 2006) and the information integration theory (Tononi, 2004), propose that consciousness requires the integrated activity of association cortices. However, such activation is likely necessary but not sufficient for consciousness. Indeed, prior functional MRI studies have shown that association cortices respond to passive language stimuli in healthy humans who are sleeping (Portas et al., 2000) or undergoing propofol sedation (Davis et al., 2007; Liu et al., 2012). Moreover, one could argue that an association cortex response is not only insufficient for consciousness, but also insufficient proof of a potential for consciousness. A previous study identified focal regions of association cortex activity that could represent isolated ‘islands of cortex’ in a patient with vegetative state (Schiff et al., 1999). This intriguing possibility of ‘words without mind’ suggests that association cortex responses may not be a proxy for higher-level cortical function but rather may be associated with reflexive, non-purposeful behaviour (Fischer and Truog, 2015). Nevertheless, the potential clinical relevance of identifying ICU patients with HMD is supported by previous studies reporting recovery of consciousness in patients with chronic disorders of consciousness who had higher-order cortex responses (e.g. Coleman et al., 2009). Ultimately, we expect that the HMD criteria proposed here will be refined in future studies. While our multimodal functional MRI and EEG-based approach strengthens the reliability of an HMD diagnosis, demonstration of its clinical validity will require larger longitudinal studies that test its diagnostic and prognostic relevance.
Accuracy of stimulus-based functional MRI and EEG in the ICU
We observed variable responses to language, music, and motor imagery stimuli in healthy subjects and in patients with behavioural evidence of language function (i.e. MCS+ and PTCS). In healthy subjects, functional MRI and EEG sensitivity was highest for the language paradigm, lower for music, and lowest for motor imagery. Similar results were observed in patients, with the highest functional MRI and EEG sensitivity observed for language, followed by music and motor imagery. The low sensitivity of our right-hand squeeze motor imagery paradigm for detecting behavioural evidence of language supports the need for development of motor imagery tasks that have lower cognitive demands and/or tasks whose responses are more robustly detected by functional MRI and EEG. Passive paradigms that identify HMD also require further investigation, as they may provide valuable information when responses to active tasks are not evident.
Importantly, it is difficult to identify false positive functional MRI and EEG results in this population of patients for whom there is no definitive gold standard diagnostic test to define the level of consciousness. Future studies are needed to develop robust methods for identification of false positive results, as well as false negative results in patients receiving sedation. The optimal timing for functional MRI and EEG data acquisition also remains to be determined, as the level of consciousness and corresponding cortical function may fluctuate substantially in acutely brain-injured patients due to dynamic pathophysiological processes such as intracranial hypertension, cerebral oedema, and mass effect.
Prognostic value of stimulus-based functional MRI and EEG in the ICU
GOSE scores did not differ between patients with or without functional MRI and EEG responses in the ICU. This result does not support findings from previous studies showing that patients with subacute or chronic disorders of consciousness who have functional MRI evidence of covert consciousness had better long-term recovery (Di et al., 2008; Coleman et al., 2009). Although recovery from PTCS was evident in all 14 survivors at 6 months post-injury, it is notable that this group included three patients with acute CMD and two with HMD. Many factors influence recovery in patients with acute severe TBI, but it is possible that early cortical responses detected by functional MRI and EEG in these five patients contributed to the emergence of behavioural signs of consciousness. Even if cortical responses to passive stimuli in HMD patients are conservatively interpreted as disconnected islands of cortex, the subsequent recovery of self-expression and comprehension suggests that part of the neuronal architecture capable of supporting consciousness may have been present acutely. On the other hand, the observation that one comatose subject with no evidence of CMD or HMD also recovered from PTCS supports the notion that prognosis should not be based upon negative findings from these techniques.
Limitations and methodological considerations
Although our 16-patient sample was the largest to date that has been studied with stimulus-based functional MRI and EEG in the ICU (Edlow et al., 2013), because our study was designed to determine the feasibility of detecting CMD and HMD in an ICU population, the sample size was likely too small to demonstrate the prognostic utility of these techniques. In addition, our findings were likely confounded by variables inherent to the ICU environment. First, individual patient responses may have been affected by sedation, even if there was no statistically significant association between level of sedation (analysed as an ordinal variable) and functional MRI or EEG responses for the entire cohort. It was not possible to use a more granular approach to assess the effect of sedatives on cortical responses (i.e. analysing sedation as a continuous variable), because many patients received multiple sedatives for which equivalencies have not been established, the effect of sedatives on cortical responses may vary with hepatic and renal metabolism, and standardized sedation rating scales that have been validated in non-brain injured ICU patients (Sessler et al., 2002) are not necessarily applicable to brain-injured patients (Roberts et al., 2011). Second, acute fluctuations in arousal that are common in the ICU may have caused inconsistent responses (Piarulli et al., 2016). For example, functional MRI responses to the active motor imagery task but not the passive language stimulus were observed in Patients P5 and P14. Third, while we chose the GOSE score as our outcome measure based on its widespread use in severe TBI studies (Bagiella et al., 2010), the GOSE lacks sensitivity for targeting brain injury-related impairments. In addition, all surviving patients recovered beyond PTCS, making specificity analysis of functional MRI/EEG combined with CRS-R/CAP for predicting 6-month level of consciousness not possible in this study. Future studies investigating the prognostic utility of functional MRI and EEG in the ICU will therefore require larger sample sizes and will need to consider alternative functional outcomes measures.
It should also be noted that because of feasibility considerations, the early behavioural evaluations, follow-up assessments, and functional MRI analyses were not performed by independent investigators in this study. Nevertheless, our implementation of quantitative, unbiased methods for functional MRI analysis and CMD/HMD detection mitigates this limitation. To reduce the FPR, we also required that positive functional MRI responses in patients were above the lower limit of a normal range of functional MRI activation derived from matched healthy subjects, and that both functional MRI and EEG evidence of higher-order cortical processing were evident for HMD classification. With regard to generalizability, our EEG classifier is built on single-subject data, making it ideally suited for severe TBI patients with variable types and locations of brain lesions, but potentially limiting its generalizability across subjects.
Ethical considerations
Diagnostic studies of consciousness that use advanced, investigational techniques in the acute disorders of consciousness population raise challenging ethical questions. First, the field has not developed a systematic way to interpret negative findings. The absence of functional MRI and EEG responses does not necessarily indicate absence of consciousness, especially considering that ∼25% of healthy subjects do not demonstrate responses in the expected brain regions when performing motor imagery tasks. Second, despite extensive efforts to reduce false positives, functional MRI and EEG may suggest preserved consciousness in patients who are actually unconscious. Should these advanced methods be incorporated into clinical practice, clinicians will require training on how to interpret negative and positive findings in the context of the limited sensitivity and specificity of these methods.
Ultimately, we anticipate that this study and others like it will encourage open dialogue between clinicians and families about the uncertainty of prognostication and the limitations of different assessment tools (Fins, 2015). We further emphasize that the methods described here are investigational and will need to be tested across multiple sites and settings before becoming integrated into clinical care. Finally, we acknowledge that access to advanced techniques, such as those presented here, is currently limited to hospitals with specific technical expertise and infrastructure. However, we are confident that, if these tools are validated, their potential benefit to patients and families will encourage dissemination of standardized acquisition protocols and analysis pipelines that meet the needs of community medical centres. Stimulus-based functional MRI and EEG methods should be refined to produce streamlined, timely, and intuitive outputs that can be interpreted by a clinician.
Conclusions
Stimulus-based functional MRI can provide evidence of consciousness that evades detection by bedside examination in ICU patients with acute severe TBI. In addition, a subset of ICU patients exists whose higher-order cortex responses on functional MRI and EEG are greater than those suggested by the bedside examination. Multimodal assessment with the CRS-R, stimulus-based functional MRI and EEG may provide a more robust evaluation of consciousness and higher-order cortical function than bedside examination alone. If these findings are validated in future studies, stimulus-based functional MRI and EEG may enable ICU clinicians to render more accurate prognoses and help families make informed decisions about continuation of life-sustaining therapies.
Supplementary Material
Acknowledgements
The authors thank the nursing staffs of the Massachusetts General Hospital Neurosciences ICU, Multidisciplinary ICU, and the Surgical ICU. We also thank Joseph Cohen and the EEG technologists, as well as Kellie Cahill and the MRI technologists for their assistance with data acquisition. We thank Kimberly Main Knoper for assistance with artwork in Figure 2. We would like to acknowledge Dylan Tisdall and Andre van der Kouwe (Athinoula A. Martinos Center for Biomedical Imaging) and Himanshu Bhat (Siemens Medical Center) for the provision of WIP711D (vNav Motion-Corrected Multiecho MPRAGE) used to acquire MEMPRAGE data. We are grateful to the patients and families in this study for their participation and support.
Funding
This work was supported by grants from the National Institutes of Health (K23NS094538), the Center for Integration of Medicine & Innovative Technology (Boston, MA, USA), the American Academy of Neurology/American Brain Foundation, the James S. McDonnell Foundation, and the Massachusetts General Hospital Department of Neurology and Division of Neurocritical Care and Emergency Neurology.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- CAP
Confusion Assessment Protocol
- CMD
cognitive motor dissociation
- CRS-R
Coma Recovery Scale-Revised
- FNR
false negative rate
- FPR
false positive rate
- GOSE
Glasgow Outcome Scale-Extended
- HMD
higher-order cortex motor dissociation
- ICU
intensive care unit
- MCS+/−
minimally conscious state with/without language
- PTCS
post-traumatic confusional state
- TBI
traumatic brain injury
- TNR
true negative rate
- TPR
true positive rate
References
- Andrews K, Murphy L, Munday R, Littlewood C. Misdiagnosis of the vegetative state: retrospective study in a rehabilitation unit. BMJ 1996; 313: 13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagiella E, Novack TA, Ansel B, Diaz-Arrastia R, Dikmen S, Hart T, et al. Measuring outcome in traumatic brain injury treatment trials: recommendations from the traumatic brain injury clinical trials network. J Head Trauma Rehabil 2010; 25: 375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin JC, Fins JJ, Katz DI, Hersh J, Heier LA, Tabelow K, et al. Dissociations between behavioural and functional magnetic resonance imaging-based evaluations of cognitive function after brain injury. Brain 2011; 134: 769–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin JC, Schiff ND, Voss HU. Pattern classification of volitional functional magnetic resonance imaging responses in patients with severe brain injury. Arch Neurol 2012; 69: 176–81. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage 2003; 20: 1052–63. [DOI] [PubMed] [Google Scholar]
- Boly M, Coleman MR, Davis MH, Hampshire A, Bor D, Moonen G, et al. When thoughts become action: an fMRI paradigm to study volitional brain activity in non-communicative brain injured patients. Neuroimage 2007; 36: 979–92. [DOI] [PubMed] [Google Scholar]
- Brattico E, Alluri V, Bogert B, Jacobsen T, Vartiainen N, Nieminen S, et al. A Functional MRI study of happy and sad emotions in music with and without lyrics. Front Psychol 2011; 2: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno MA, Ledoux D, Vanhaudenhuyse A, Gosseries O, Thibaut A, Laureys S. Prognosis of patients with altered state of consciousness. In: Schnakers C, Laureys S, editors. Coma and disorders of consciousness. London: Springer; 2012a. p. 11–23. [Google Scholar]
- Bruno MA, Majerus S, Boly M, Vanhaudenhuyse A, Schnakers C, Gosseries O, et al. Functional neuroanatomy underlying the clinical subcategorization of minimally conscious state patients. J Neurol 2012b; 259: 1087–98. [DOI] [PubMed] [Google Scholar]
- Bruno MA, Vanhaudenhuyse A, Thibaut A, Moonen G, Laureys S. From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: recent advances in our understanding of disorders of consciousness. J Neurol 2011; 258: 1373–84. [DOI] [PubMed] [Google Scholar]
- Burges CJC. A tutorial on support vector machines for pattern recognition. Data Min Knowl Discov 1998; 2: 121–67. [Google Scholar]
- Childs NL, Mercer WN, Childs HW. Accuracy of diagnosis of persistent vegetative state. Neurology 1993; 43: 1465–7. [DOI] [PubMed] [Google Scholar]
- Coleman MR, Davis MH, Rodd JM, Robson T, Ali A, Owen AM, et al. Towards the routine use of brain imaging to aid the clinical diagnosis of disorders of consciousness. Brain 2009; 132: 2541–52. [DOI] [PubMed] [Google Scholar]
- Cruse D, Chennu S, Chatelle C, Bekinschtein TA, Fernandez-Espejo D, Pickard JD, et al. Bedside detection of awareness in the vegetative state: a cohort study. Lancet 2011; 378: 2088–94. [DOI] [PubMed] [Google Scholar]
- Cruse D, Chennu S, Chatelle C, Fernandez-Espejo D, Bekinschtein TA, Pickard JD, et al. Relationship between etiology and covert cognition in the minimally conscious state. Neurology 2012; 78: 816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen A, Otnaess MK, Jensen J, Williams SC, Ostberg BC. Investigating repetition and change in musical rhythm by functional MRI. Neuroscience 2014; 275: 469–76. [DOI] [PubMed] [Google Scholar]
- Davis MH, Coleman MR, Absalom AR, Rodd JM, Johnsrude IS, Matta BF, et al. Dissociating speech perception and comprehension at reduced levels of awareness. Proc Natl Acad Sci USA 2007; 104: 16032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn Sci 2006; 10: 204–11. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 2004; 134: 9–21. [DOI] [PubMed] [Google Scholar]
- Di H, Boly M, Weng X, Ledoux D, Laureys S. Neuroimaging activation studies in the vegetative state: predictors of recovery? Clin Med 2008; 8: 502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di HB, Yu SM, Weng XC, Laureys S, Yu D, Li JQ, et al. Cerebral response to patient's own name in the vegetative and minimally conscious states. Neurology 2007; 68: 895–9. [DOI] [PubMed] [Google Scholar]
- Edlow BL, Giacino JT, Wu O. Functional MRI and outcome in traumatic coma. Curr Neurol Neurosci Rep 2013; 13: 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 2005; 25: 1325–35. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci USA 2016; 113: 7900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faugeras F, Rohaut B, Weiss N, Bekinschtein TA, Galanaud D, Puybasset L, et al. Probing consciousness with event-related potentials in the vegetative state. Neurology 2011; 77: 264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Espejo D, Junque C, Vendrell P, Bernabeu M, Roig T, Bargallo N, et al. Cerebral response to speech in vegetative and minimally conscious states after traumatic brain injury. Brain Inj 2008; 22: 882–90. [DOI] [PubMed] [Google Scholar]
- Fins JJ. Rights come to mind: brain injury, ethics, and the struggle for consciousness. New York, NY: Cambridge University Press; 2015. [Google Scholar]
- Fischer C, Luaute J, Adeleine P, Morlet D. Predictive value of sensory and cognitive evoked potentials for awakening from coma. Neurology 2004; 63: 669–73. [DOI] [PubMed] [Google Scholar]
- Fischer DB, Truog RD. What is a reflex? A guide for understanding disorders of consciousness. Neurology 2015; 85: 543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, et al. The minimally conscious state: definition and diagnostic criteria. Neurology 2002; 58: 349–53. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Kalmar K. The vegetative and minimally conscious states: a comparison of clinical features and functional outcome. J Head Trauma Rehabil 1997; 12: 36–51. [Google Scholar]
- Giacino JT, Kalmar K, Whyte J. The JFK coma recovery scale-revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 2004; 85: 2020–9. [DOI] [PubMed] [Google Scholar]
- Gill-Thwaites H. Lotteries, loopholes and luck: misdiagnosis in the vegetative state patient. Brain Inj 2006; 20: 1321–8. [DOI] [PubMed] [Google Scholar]
- Good PI. Permutation, parametric and bootstrap tests of hypotheses. 3rd ednNew York, NY: Springer; 2004. [Google Scholar]
- Gosseries O, Zasler ND, Laureys S. Recent advances in disorders of consciousness: focus on the diagnosis. Brain Inj 2014; 28: 1141–50. [DOI] [PubMed] [Google Scholar]
- Hauger SL, Schanke AK, Andersson S, Chatelle C, Schnakers C, Lovstad M. The clinical diagnostic utility of electrophysiological techniques in assessment of patients with disorders of consciousness following acquired brain injury: a systematic review. J Head Trauma Rehabil 2017; 32: 185–96. [DOI] [PubMed] [Google Scholar]
- Hauger SL, Schnakers C, Andersson S, Becker F, Moberget T, Giacino JT, et al. Neurophysiological indicators of residual cognitive capacity in the minimally conscious state. Behav Neurol 2015; 2015: 145913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, et al. American Clinical Neurophysiology Society's standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol 2013; 30: 1–27. [DOI] [PubMed] [Google Scholar]
- Katz DI, Polyak M, Coughlan D, Nichols M, Roche A. Natural history of recovery from brain injury after prolonged disorders of consciousness: outcome of patients admitted to inpatient rehabilitation with 1-4 year follow-up. Prog Brain Res 2009; 177: 73–88. [DOI] [PubMed] [Google Scholar]
- King JR, Faugeras F, Gramfort A, Schurger A, El Karoui I, Sitt JD, et al. Single-trial decoding of auditory novelty responses facilitates the detection of residual consciousness. Neuroimage 2013; 83: 726–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs S, Peeters R, Smits M, De Ridder D, Van Hecke P, Sunaert S. Activation of cortical and subcortical auditory structures at 3 T by means of a functional magnetic resonance imaging paradigm suitable for clinical use. Invest Radiol 2006; 41: 87–96. [DOI] [PubMed] [Google Scholar]
- Lepage KQ, Kramer MA, Chu CJ. A statistically robust EEG re-referencing procedure to mitigate reference effect. J Neurosci Methods 2014; 235: 101–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lauer KK, Ward BD, Rao SM, Li SJ, Hudetz AG. Propofol disrupts functional interactions between sensory and high-order processing of auditory verbal memory. Hum Brain Mapp 2012; 33: 2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luaute J, Maucort-Boulch D, Tell L, Quelard F, Sarraf T, Iwaz J, et al. Long-term outcomes of chronic minimally conscious and vegetative states. Neurology 2010; 75: 246–52. [DOI] [PubMed] [Google Scholar]
- Lule D, Noirhomme Q, Kleih SC, Chatelle C, Halder S, Demertzi A, et al. Probing command following in patients with disorders of consciousness using a brain-computer interface. Clin Neurophysiol 2013; 124: 101–6. [DOI] [PubMed] [Google Scholar]
- Maas AI, Harrison-Felix CL, Menon D, Adelson PD, Balkin T, Bullock R, et al. Common data elements for traumatic brain injury: recommendations from the interagency working group on demographics and clinical assessment. Arch Phys Med Rehabil 2010; 91: 1641–9. [DOI] [PubMed] [Google Scholar]
- Majerus S, Bruno MA, Schnakers C, Giacino JT, Laureys S. The problem of aphasia in the assessment of consciousness in brain-damaged patients. Prog Brain Res 2009; 177: 49–61. [DOI] [PubMed] [Google Scholar]
- Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, et al. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res 2006; 83: 155–71. [DOI] [PubMed] [Google Scholar]
- Monti MM, Vanhaudenhuyse A, Coleman MR, Boly M, Pickard JD, Tshibanda L, et al. Willful modulation of brain activity in disorders of consciousness. N Engl J Med 2010; 362: 579–89. [DOI] [PubMed] [Google Scholar]
- Noirhomme Q, Lesenfants D, Gomez F, Soddu A, Schrouff J, Garraux G, et al. Biased binomial assessment of cross-validated estimation of classification accuracies illustrated in diagnosis predictions. Neuroimage Clin 2014; 4: 687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Kelly J, James L, Palaniappan R, Taborin J, Fachner J, Magee WL. Neurophysiological and behavioral responses to music therapy in vegetative and minimally conscious states. Front Hum Neurosci 2013; 7: 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala M, Garriga GC. Permutation tests for studying classifier performance. J Mach Learn Res 2010; 11: 1833–63. [Google Scholar]
- Okumura Y, Asano Y, Takenaka S, Fukuyama S, Yonezawa S, Kasuya Y, et al. Brain activation by music in patients in a vegetative or minimally conscious state following diffuse brain injury. Brain Inj 2014; 28: 944–50. [DOI] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science 2006; 313: 1402. [DOI] [PubMed] [Google Scholar]
- Pettigrew LE, Wilson JT, Teasdale GM. Reliability of ratings on the Glasgow outcome scales from in-person and telephone structured interviews. J Head Trauma Rehabil 2003; 18: 252–8. [DOI] [PubMed] [Google Scholar]
- Piarulli A, Bergamasco M, Thibaut A, Cologan V, Gosseries O, Laureys S. EEG ultradian rhythmicity differences in disorders of consciousness during wakefulness. J Neurol 2016; 263: 1746–60. [DOI] [PubMed] [Google Scholar]
- Portas CM, Krakow K, Allen P, Josephs O, Armony JL, Frith CD. Auditory processing across the sleep-wake cycle: simultaneous EEG and fMRI monitoring in humans. Neuron 2000; 28: 991–9. [DOI] [PubMed] [Google Scholar]
- Posner JB, Saper CB, Schiff ND, Plum F. Plum and Posner's diagnosis of stupor and coma 4th edn. New York: Oxford University Press; 2007. [Google Scholar]
- Roberts DJ, Hall RI, Kramer AH, Robertson HL, Gallagher CN, Zygun DA. Sedation for critically ill adults with severe traumatic brain injury: a systematic review of randomized controlled trials. Crit Care Med 2011; 39: 2743–51. [DOI] [PubMed] [Google Scholar]
- Ropper AH. Cogito ergo sum by MRI. N Engl J Med 2010; 362: 648–9. [DOI] [PubMed] [Google Scholar]
- Schiff N, Ribary U, Plum F, Llinas R. Words without mind. J Cogn Neurosci 1999; 11: 650–6. [DOI] [PubMed] [Google Scholar]
- Schiff ND. Cognitive motor dissociation following severe brain injuries. JAMA Neurol 2015; 72: 1413–5. [DOI] [PubMed] [Google Scholar]
- Schnakers C, Edlow BL, Chatelle C, Giacino J. Minimally conscious state. In: Laureys S, Gosseries O, Tononi G, editors. The neurology of consciousness. 2nd ednSan Diego, CA: Academic Press; 2015a. [Google Scholar]
- Schnakers C, Giacino JT, Lovstad M, Habbal D, Boly M, Di H, et al. Preserved covert cognition in noncommunicative patients with severe brain injury? Neurorehabil Neural Repair 2015b; 29: 308–17. [DOI] [PubMed] [Google Scholar]
- Schnakers C, Vanhaudenhuyse A, Giacino J, Ventura M, Boly M, Majerus S, et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol 2009; 9: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002; 166: 1338–44. [DOI] [PubMed] [Google Scholar]
- Sherer M, Nakase-Thompson R, Yablon SA, Gontkovsky ST. Multidimensional assessment of acute confusion after traumatic brain injury. Arch Phys Med Rehabil 2005; 86: 896–904. [DOI] [PubMed] [Google Scholar]
- Sitt JD, King JR, El Karoui I, Rohaut B, Faugeras F, Gramfort A, et al. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain 2014; 137: 2258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stender J, Gosseries O, Bruno MA, Charland-Verville V, Vanhaudenhuyse A, Demertzi A, et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study. Lancet 2014; 384: 514–22. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Binns MA, Carruth FG, Levine B, Brandys CE, Moulton RJ, et al. The acute period of recovery from traumatic brain injury: posttraumatic amnesia or posttraumatic confusional state? J Neurosurg 1999; 90: 635–43. [DOI] [PubMed] [Google Scholar]
- Tononi G. An information integration theory of consciousness. BMC Neurosci 2004; 5: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truog RD, Campbell ML, Curtis JR, Haas CE, Luce JM, Rubenfeld GD, et al. Recommendations for end-of-life care in the intensive care unit: a consensus statement by the American College [corrected] of Critical Care Medicine. Crit Care Med 2008; 36: 953–63. [DOI] [PubMed] [Google Scholar]
- Turgeon AF, Lauzier F, Simard JF, Scales DC, Burns KE, Moore L, et al. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ 2011; 183: 1581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kouwe AJ, Benner T, Salat DH, Fischl B. Brain morphometry with multiecho MPRAGE. Neuroimage 2008; 40: 559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Di H, Hu X, Jing S, Thibaut A, Di Perri C, et al. Cerebral response to subject's own name showed high prognostic value in traumatic vegetative state. BMC Med 2015; 13: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte J, Cifu D, Dikmen S, Temkin N. Prediction of functional outcomes after traumatic brain injury: a comparison of 2 measures of duration of unconsciousness. Arch Phys Med Rehabil 2001; 82: 1355–9. [DOI] [PubMed] [Google Scholar]
- Whyte J, Nakase-Richardson R, Hammond FM, McNamee S, Giacino JT, Kalmar K, et al. Functional outcomes in traumatic disorders of consciousness: 5-year outcomes from the National Institute on Disability and Rehabilitation Research Traumatic Brain Injury Model Systems. Arch Phys Med Rehabil 2013; 94: 1855–60. [DOI] [PubMed] [Google Scholar]
- Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow outcome scale and the extended Glasgow outcome scale: guidelines for their use. J Neurotrauma 1998; 15: 573–85. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 2004; 21: 1732–47. [DOI] [PubMed] [Google Scholar]
- Wu J, Zhang J, Ding X, Li R, Zhou C. The effects of music on brain functional networks: a network analysis. Neuroscience 2013; 250: 49–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.