Abstract
Context
Genome-wide association studies (GWASs) have been successful in identifying loci associated with osteoporosis and obesity. However, the findings explain only a small fraction of the total genetic variance.
Objective
The aim of this study was to identify novel pleiotropic genes important in osteoporosis and obesity.
Design and Setting
A pleiotropic conditional false discovery rate method was applied to three independent GWAS summary statistics of femoral neck bone mineral density, body mass index, and waist-to-hip ratio. Next, differential expression analysis was performed for the potentially pleiotropic genes, and weighted genes coexpression network analysis (WGCNA) was conducted to identify functional connections between the suggested pleiotropic genes and known osteoporosis/obesity genes using transcriptomic expression data sets in osteoporosis/obesity-related cells.
Results
We identified seven potentially pleiotropic loci—rs3759579 (MARK3), rs2178950 (TRPS1), rs1473 (PUM1), rs9825174 (XXYLT1), rs2047937 (ZNF423), rs17277372 (DNM3), and rs335170 (PRDM6)—associated with osteoporosis and obesity. Of these loci, the PUM1 gene was differentially expressed in osteoporosis-related cells (B lymphocytes) and obesity-related cells (adipocytes). WGCNA showed that PUM1 positively interacted with several known osteoporosis genes (AKAP11, JAG1, and SPTBN1). ZNF423 was the highly connected intramodular hub gene and interconnected with 21 known osteoporosis-related genes, including JAG1, EN1, and FAM3C.
Conclusions
Our study identified seven potentially pleiotropic genes associated with osteoporosis and obesity. The findings may provide new insights into a potential genetic determination and codetermination mechanism of osteoporosis and obesity.
Pleiotropic analysis of osteoporosis and obesity was performed by cFDR. Strong pleiotropic enrichment and seven potentially pleiotropic SNPs were found for osteoporosis and obesity.
Recently, genome-wide association studies (GWASs) have identified thousands of variants for many human complex diseases, but these single-nucleotide polymorphisms (SNPs) failed to explain a substantial proportion of the heritability of most complex diseases studied, such as osteoporosis and obesity. Because of polygenic architecture, a large number of SNPs with small to modest effect sizes (but still important) have not been identified (1, 2). Currently, an important task and challenge is to take full advantage of existing GWAS data to explore more novel genetic variants underlying complex diseases, especially for the SNPs that do not meet the conservative Bonferroni-corrected significance level. Previous studies estimated that about 4.6% of SNPs and 16.9% of genes are involved in pleiotropic effects (3). The presence of pleiotropy in the genome indicates that correlated traits may have overlapping genetic determinants.
Osteoporosis is the most common metabolic bone disease that is characterized by reduced bone mineral density (BMD), deficiencies in the structure of bone tissue, and increased risk of low-trauma fractures (4). The most widely accepted measurement for predicting the risk of osteoporosis is BMD (4). BMD is a highly heritable trait, and its heritability ranges from 0.5 to 0.9 (5). To date, previous GWASs had identified ∼200 genes associated with osteoporosis-related traits (6–9), which explained <10% of the total genetic variance in any single study population. Therefore, novel statistical approaches to identify novel genes/variants important for osteoporosis-related traits are needed.
Obesity is a disease of excessive storage of body fat because of chronic imbalance of energy intake and consumption (10). It becomes a common public health problem because it can increase the risk of developing other serious diseases, such as type 2 diabetes, hypertension, cardiovascular disease, and coronary heart diseases (11, 12). A widely used measurement for obesity research is body mass index (BMI), defined as body weight divided by the square of height, which has a strong genetic determination, with heritability of 0.4 to 0.7 (13–15). Body fat distribution is also a heritable trait, with heritability ranging from 0.3 to 0.6 (16, 17).
Osteoporosis and obesity are closely related diseases (18). Obesity has long been viewed as a protective factor to osteoporosis (19, 20), whereas other studies suggested that fat accumulation had a negative effect on bone mass (18, 21, 22). Adipocyte tissue secretes the estrogen synthesis enzyme, leptin, adiponectin, and various proinflammatory cytokines, which are important to bone remodeling (23, 24). Adipocytes and osteoblasts originate from a common progenitor, bone marrow mesenchymal stem cells (25). Some studies suggested that bone-derived factors, such as osteopontin and osteocalcin, exert an endocrine regulation on body weight and glucose homeostasis (26). In addition, many reports have proved overlapping genetic susceptibility in osteoporosis and obesity. The genetic correlation between BMD and BMI is about 0.4 in Chinese Han ethnicity, suggesting that the two traits share pleiotropic genes (27). For the currently reported osteoporosis and obesity GWAS SNPs/genes, 12 genes or loci are shared between osteoporosis and obesity, such as DNM3, CDKAL1, and MPP7. Several obesity and osteoporosis shared genomic regions have been found through a bivariate whole-genome linkage scan, which include interleukin 6 and tumor necrosis factor α that influence both osteoporosis and obesity (28). A bivariate genome-wide association analysis discovered that SOX6 played a pleiotropic role in both bone and fat, providing further support for the existence of pleiotropic genes for osteoporosis and obesity (29). However, despite these interesting yet limited findings of the pleiotropic loci underlying osteoporosis and obesity, there are many other shared genetic loci influencing these two diseases awaiting identification.
As far as we know, Andreassen et al. (30) recently proposed a novel genetic pleiotropy-informed conditional false discovery rate (cFDR) method to identify shared genetic variants using existing GWAS summary statistics data. This method exploits the idea that a variant with statistically significant effects on two related traits is more likely to be a true effect and therefore has a higher probability of being detected in multiple independent studies. This method incorporates the summary statistics from two independent GWASs to test variants for association with the principal trait (the trait that is being tested for the association) conditional on different strengths of association with the second trait (conditional trait). The cFDR method allows us to focus specifically on the subset of variants with a given strength of association in the conditional trait (30). Andreassen et al. (30) have applied this method and successfully discovered novel pleiotropic loci that are associated with schizophrenia and bipolar disorder/cardiovascular disease risk factors (30, 31). Our group also has applied this method in a number of other pairs of correlated traits/diseases (32–38).
In this study, we applied the cFDR method with GWAS summary statistics data from three large independent studies to identify novel variants with pleiotropic effects on the two related traits, femoral neck (FN) BMD and BMI or FN BMD and waist-to-hip ratio (WHR). With this approach, we identified seven pleiotropic variants implicating a shared genetic mechanism for FN BMD and BMI or FN BMD and WHR. The results of this study enable us to better characterize the potentially genetic mechanisms underlying the correlations of FN BMD and BMI or FN BMD and WHR, as well as better understand the potentially mechanistic relationships of osteoporosis and obesity.
Materials and Methods
GWAS data sets
The GWAS data sets we applied in this study were all downloaded from publicly available online database. These data sets contained summary statistics, including the P values of association and direction of effect for each variant. To our knowledge, the data sets we used are among the largest and/or the latest meta-analysis results in the osteoporosis and obesity fields, respectively.
The data set containing the summary statistics results for association with FN BMD was derived from the study of the Genetic Factors for Osteoporosis Consortium in 2015 (http://www.gefos.org/?q=content/data-release-2015) (9). This GWAS meta-analysis used whole-genome sequencing, whole-exome sequencing, and deep imputation of previous genotype data to identify variants associated with FN BMD, lumbar spine BMD, and forearm BMD. The GWAS meta-analysis was performed on >10 million genotyped or imputed SNPs in 33 individual studies consisting of 53,236 Caucasian participants. Because hip fractures are the most severe type of osteoporotic fractures and directly associated with high morbidity and mortality (39), FN and total hip BMD are the most important risk traits for the study of osteoporosis.
The data set with summary statistics results of BMI was taken from a GWAS meta-analysis of 339,224 (322,154 European descent individuals and 17,070 non-European descent individuals) individuals performed by the Genetic Investigation of Anthropometric Traits Consortium (http://www.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium_data_files) (40). The meta-analysis that combined 125 studies and contained >2 million genotyped or imputed SNPs was to identify the variants association with BMI. The WHR association data set was downloaded from a GWAS meta-analysis in up to 224,459 individuals (210,088 European ancestry individuals and 14,371 non-European ancestry individuals) analyzed by the Genetic Investigation of Anthropometric Traits Consortium (41). The WHR association meta-analysis combined 57 GWAS studies and 52 Metabochip studies, which included ∼2.5 million genotyped and imputed SNPs. An easily accessible and widely used measurement of body fat distribution is WHR, a ratio of waist and hip circumferences. WHR is a better index of abdominal fat and fat distribution than waist circumference, where a ratio of waist and hip circumferences can better distinguish abdominal or gluteal-femoral adiposity as waist circumference divided by hip circumferences.
Statistical analysis
SNP pruning and annotation
The original GWAS results were adjusted by using genomic control to ensure that the variance estimates for each SNP are not inflated due to potential population structure. First, we removed the SNPs that passed the Bonferroni-corrected significance threshold in the original GWAS and annotated SNPs to genes in the GWAS studies (FN BMD, BMI, and WHR). Next, we removed variants with large correlations between pairs of variants based on linkage disequilibrium. The pruning algorithm started at a window of 50 SNPs, where linkage disequilibrium was calculated between each pair of SNPs by using the HapMap III genotypes. If the pairs have an r2 value >0.2, the SNP with the smaller minor allele frequency of that pair will be removed. After the initial removal of SNPs, the window slid five SNPs forward and repeated the process until there were no pairs of SNPs with r2 > 0.2. After the pruning process, 122,152 variants for FN BMD and BMI and 120,573 variants for FN BMD and WHR were used in the cFDR analysis.
Conditional Q-Q plots for assessing pleiotropic enrichment
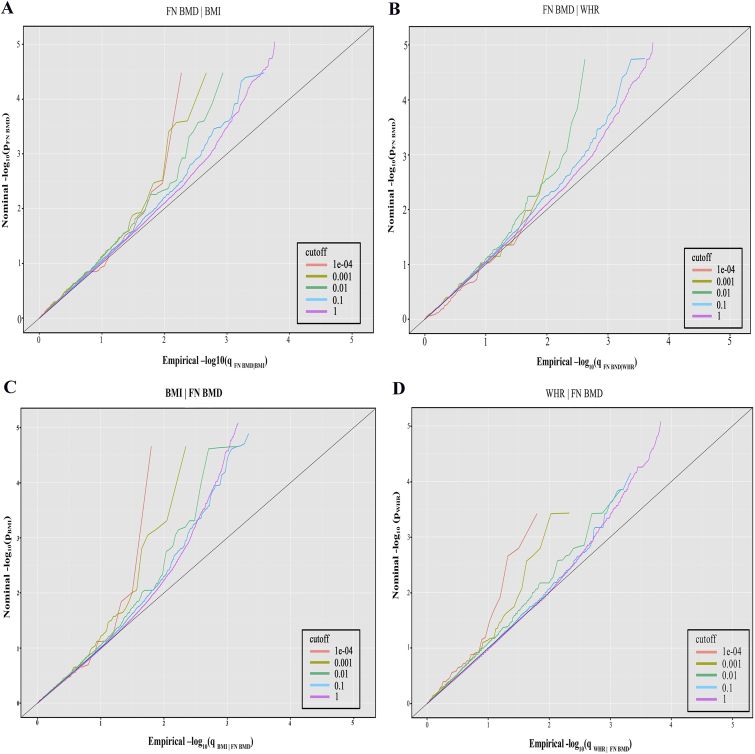
To assess the enrichment of association with the principal trait, we constructed conditional Q-Q plots based on varying levels of significance with the conditional trait. The Q-Q plots present a graph of the observed distribution of P values plotted against the expected distribution of P values under the null hypothesis. We plotted the Q-Q curve for the empirical quantiles of nominal –log10(P) values for SNPs’ association with the principal trait for the subset of SNPs with significance levels below the varying thresholds with the conditional trait. The nominal P values are plotted on the y-axis, and q values are plotted on the x-axis. The q values are the quantiles of the nominal P values for SNPs’ association with the principal phenotype for the subset of SNPs with significance levels below the varying thresholds with the conditional phenotype. The q values can be calculated as the number of SNPs with P values less than or equal to the P value divided by the total number of SNPs in the group. Pleiotropic enrichment can be observed when the Q-Q curve is leftward deflected from the expected null line. Pleiotropic enrichment means an increase in the number of loci that are associated with the principal trait when gradually restricting the subset of SNPs at stronger significance levels of association with the conditional trait. Larger spacing between conditional Q-Q plots stands for a greater extent of pleiotropic genes shared between traits, and the earlier departure from the null line suggests a greater proportion of true associations for the nominal P value of the given phenotype.
Conditional statistics for one trait
The unconditional false discovery rate for a set of variants is defined as the probability of a false-positive association for a random SNP. For a set of observed association P values, the unconditional false discovery rate is estimated as the ratio of the expected quantile of the P value under the null hypothesis with the observed quantile.
The cFDR extends this idea to the two-phenotype case, which is characterized as the probability that a random SNP is not associated with the principal phenotype given that the observed P values for each phenotype are smaller than predefined disease-specific significance thresholds. The cFDR is given by cFDR(pi|pj), where represents the null hypothesis that the particular SNP is not associated with the principal trait i, pi represents the association for the particular variant with the principal trait, and pj represents the association of the same SNP with the conditional trait.
We computed the cFDR of each variant for the BMI or WHR conditioned on the association with FN BMD (BMI|FN BMD or WHR|FN BMD) and vice versa (FN BMD|BMI or FN BMD|WHR). To estimate whether the cFDR method results in the enrichment of associated SNPs, we controlled the subset of SNPs being tested using the following criteria for Pj ≤ pj; Pj ≤ 1 (all SNPs), Pj ≤ 0.1, Pj ≤ 0.01, Pj ≤ 0.001, and Pj ≤ 0.0001, based on significance for the association with the conditional trait. SNPs with a cFDR value <0.05 were deemed to be statistically significantly associated with the principal phenotype.
Conjunction statistics for both traits
Next, we computed the conjunction cFDR (ccFDR), which is defined as the probability that a given SNP has a false-positive association with both the principal and conditional traits. The maximum cFDR value of the two traits was deemed equal to the ccFDR value of each variant.
All SNPs were considered to be statistically significantly associated with both traits when the ccFDR was <0.05.
Manhattan plots for conditional statistics and conjunction statistics
To illustrate the localization of the independent loci associated with BMI or WHR conditional on FN BMD and vice versa, as well as independent loci with a pleiotropic effect on FN BMD and BMI or FN BMD and WHR, we present conditional or conjunction Manhattan plots, which display all the SNPs analyzed in the study and their chromosomal location in the genome. Any variant with a –log10(cFDR)/–log10(ccFDR) value >1.3 (equivalent to cFDR or ccFDR <0.05) was determined to be statistically significantly associated with the principal trait or both traits.
Functional prediction for SNPs
For the potentially pleiotropic SNPs identified in FN BMD and BMI or FN BMD and WHR, we applied the F-SNP program (http://compbio.cs.queensu.ca/F-SNP/) to analyze and predict their potential functions. The F-SNP program provides the functional effects at splicing, transcriptional, translational, and posttranslational levels (42).
Differential expression analysis
We performed differential expression analysis in two expression data sets for the identified potentially pleiotropic genes to explore their functions in relationship to the related phenotypes (individually or pleiotropically). We downloaded two normalized publicly available expression data sets from GEO (https://www.ncbi.nlm.nih.gov/gds/). An experiment for the data set GSE7429 was performed to identify genes associated with bone using a design of high vs low BMD in circulating B lymphocytes. B lymphocytes, an important cell type of the immune system that is related to bone metabolism, express/secrete factors involved in osteoclastogenesis, such as receptor tumor necrosis factor superfamily member 11 and osteoprotegerin (43). In addition, B cells may serve as precursors of osteoclasts, and the precursors have the ability to differentiate into osteoclasts after estrogen deficiency (44, 45). GSE2508, which contains data on transcriptomic expression of 20 lean children and 19 obese children, was designed to detect obesity-related genes in adipocytes. t Tests were performed to identify differential expression genes through comparing means of the gene expression signals in two groups. The nominal significance threshold was set as P values <0.05.
Weighted gene coexpression network analysis
To explore potential cofunctions of putative pleiotropic genes identified in our study, we used weighted genes coexpression network analysis (WGCNA) to construct interaction networks of pleiotropic genes with known osteoporosis or obesity genes, respectively (46). First, we searched osteoporosis or obesity GWAS references with keywords BMD, WHR, BMI, and GWAS in PubMed and selected osteoporosis/obesity-associated SNPs with a P value ≤5.0 × 10−8. A total of 203 osteoporosis and 379 obesity association genes were selected. Next, combining osteoporosis/obesity GWAS association genes and cFDR suggested genes, we identified probes representing 152 osteoporosis-related genes (14 novel and 138 known genes) and 303 obesity-related genes (25 novel and 278 known genes) and applied WGCNA (R package) to generate networks in gene expression data sets E-MEXP-1618 (http://www.ebi.ac.uk/arrayexpress/experiments/E-MEXP-1618/) and GSE2508, respectively. Expression data set E-MEXP1618 was generated from transiliac bone biopsy specimens from 84 postmenopausal white women in which most cells were osteocytes, with some very small proportions being osteoclasts and osteoblasts, with 41 low FN BMD women (T score ≤–1.0) and 43 normal or high FN BMD women (T score >–1.0). Finally, the module network was visualized by the software Cytoscape (47), including nodes and module edges with topological overlap measurement (TOM) larger than 0.10 with pleiotropic genes. TOM is a parameter ranging from 0 to 1, where a TOM of 0 means that two genes are not connected at all and two genes with a high TOM are considered to highly interconnect with the same sets of genes (48).
Results
Assessment of pleiotropic enrichment
Conditional Q-Q plots for FN BMD given nominal P values of association with BMI (Fig. 1A) or WHR (Fig. 1B) showed general polygenic pleiotropic enrichment across different significance thresholds for BMI or WHR. The gradual leftward shift when restricting the P value thresholds of associations with BMI or WHR indicated an increase in the number of true associations with FN BMD conditioned on BMI or WHR. A stronger enrichment can be observed for BMI or WHR conditional on FN BMD association nominal P values (Fig. 1C and 1D), as there appears to be a greater amount of separation between the different curves.
Figure 1.
Stratified Q-Q plots of nominal vs empirical –log10P values in principal trait below the standard GWAS threshold of P < 5.0 × 10−8 as a function of significance of the association with conditional trait at the level of P < 1, P < 0.1, P < 0.01, P < 0.001, and P < 0.0001, respectively. (A) FN BMD|BMI (FN BMD conditional on BMI), (B) FN BMD|WHR, (C) BMI|FN BMD, and (D) WHR|FN BMD. The diagonal indicates the null hypothesis.
FN BMD–associated gene loci identified with cFDR
We identified a total of 27 SNPs associated with FN BMD when conditional on BMI and 32 loci given the association with WHR with a significance threshold of cFDR <0.05 (Supplemental Tables 2 and 4). Of the 27 SNPs for FN BMD|WHR, several SNPs are located at or near BMD-related genes, such as MARK3, ZBTB40, OPG, SOX6, and GALNT3 (Supplemental Table 2). Some SNPs are located at or near noncoding RNA, such as rs944082, rs11209223, and rs11952384 (GNG12-AS1 and MEF2C-AS1). Some SNPs found by FN BMD|WHR are located at known BMD-related genes, such as MARK3, SMAD3, and DNM3, and four SNPs are located at a noncoding RNA (GNG12-AS1) (Supplemental Table 4). Of these identified SNPs associated with FN BMD conditional on BMI or WHR, 20 SNPs overlapped; therefore, a total of 39 SNPs (44 genes) were associated with FN BMD, in which 19 genes were not identified in any previous GWASs.
BMI- or WHR-associated gene loci identified with cFDR
We detected a total of 147 SNPs statistically significantly (cFDR <0.05) associated with BMI and 19 SNPs statistically significantly associated with WHR given their association with FN BMD (Supplemental Tables 1 and 3). There were 87 SNPs with P values smaller than 10−5, which were not detected in the original meta-analysis. Of these SNPs, we found six SNPs located at FTO, five at MC4R, five at SEC16B, and four at TFAP2B, which were known candidate genes related to BMI and obesity. Our analysis also confirmed several loci associated with WHR, such as TBX15 and DNM3, which were associated with WHR in a previous meta-analysis (41). Of these SNPs, we identified 72 novel statistically significant loci, such as DNMT3A and POC5, which need further experimental and statistical validation. There were three SNPs (rs11079813, rs6723710, rs751008) located at SKAP1, LINC01122, and DOCK1 identified in the analyses of BMI|FN BMD and WHR|FN BMD.
Pleiotropic gene loci for FN BMD and BMI or FN BMD and WHR
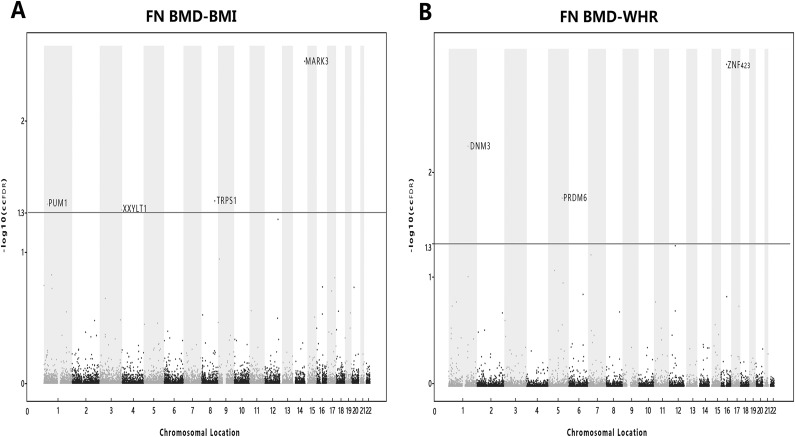
We detected seven independent pleiotropic variants—rs3759579 (MARK3), rs2178950 (TRPS1), rs1473 (PUM1), rs9828174 (XXYLT1), rs2047937 (ZNF423), rs17277372 (DNM3), and rs335170 (PRDM6)—which were statistically significantly associated with FN BMD and BMI or FN BMD and WHR (ccFDR <0.05). Of these loci, rs3759579 (MARK3), rs2178950 (TRPS1), rs1473 (PUM1), and rs9828174 (XXYLT1) were associated with FN BMD and BMI and rs2047937 (ZNF423), rs17277372 (DNM3), and rs335170 (PRDM6) with FN BMD and WHR (Fig. 2 and Table 1). Conforming to the positive relationship between BMD and BMI found in most epidemiological studies, three (MARK3, PUM1, and XXYLT1) of four potentially pleiotropic SNPs of FN BMD and BMI have the same effect direction on both FN BMD and BMI (Table 1). Interestingly, all of FN BMD and WHR association loci (ZNF423, DNM3, and PRDM6) have an opposite influence on both FN BMD and WHR, which is relatively consistent with a negative relationship of osteoporosis and body fat distribution in previous studies (49, 50). Taken together, the relationship and mechanism between obesity and osteoporosis may be complex. Four potentially pleiotropic SNPs (rs3759579, rs2178950, rs17277372, and rs2047937) located at MARK3, TRPS1, DNM3, and ZNF423 genes, respectively, were known to be related with bone or fat metabolism. Three SNPs (rs1473, rs9828174, rs335170) located at or close to genes (PUM1, XXYLT1 and PRDM6) were novel.
Figure 2.
Conjunction Manhattan plot of conjunction –log10 ccFDR values for FN BMD and BMI or FN BMD and WHR, and the solid line marking the –log10 ccFDR value of 1.3 corresponds to a ccFDR of 0.05. (A) FN BMD and BMI and (B) FN BMD and WHR. The figure shows the genomic locations of pleiotropic loci.
Table 1.
Pleiotropic Loci Associated With Both FN BMD and BMI/WHR With cFDR <0.05
| Characteristic | rs3759579 | rs2178950 | rs1473 | rs9825174 | rs2047937 | rs17277372 | rs335170 |
|---|---|---|---|---|---|---|---|
| Gene | MARK3 | TRPS1 | PUM1 | XXYLT1 | ZNF423 | DNM3 | PRDM6 |
| Chromosome | 14q32.32 | 8q24.12 | 1p35.2 | 3q29 | 16q12 | 1q24.3 | 5q23.2 |
| A1 | A | C | A | C | C | A | A |
| A2 | G | G | G | T | T | G | C |
| P value BMI | 2.18E-05 | 8.86E-04 | 4.89E-04 | 7.13E-04 | / | / | / |
| P value WHR | / | / | / | / | 3.80E-04 | 1.60E-03 | 2.20E-03 |
| P value FN BMD | 3.31E-05 | 3.95E-04 | 2.67E-04 | 2.50E-04 | 2.10E-07 | 7.80E-07 | 1.80E-05 |
| Effect for BMI | − | − | + | − | / | / | / |
| Effect for WHR | / | / | / | / | − | + | + |
| Effect for FN BMD | − | + | + | − | + | − | − |
| cFDR BMI|FN BMD | 7.43E-04 | 3.06E-02 | 2.66E-02 | 3.64E-02 | / | / | / |
| cFDR FN BMD|BMI | 3.54E-03 | 4.08E-02 | 4.33E-02 | 4.78E-02 | / | / | / |
| cFDR WHR|FN BMD | / | / | / | / | 1.14E-03 | 6.40E-03 | 1.91E-02 |
| cFDR FN BMD|WHR | / | / | / | / | 2.21E-05 | 1.01E-04 | 1.96E-03 |
| ccFDR | 3.54E-03 | 4.08E-02 | 4.33E-02 | 4.78E-02 | 1.14E-03 | 6.40E-03 | 1.91E-02 |
Independent gene loci (r2 < 0.2) with SNPs that have ccFDR <0.05 in FN BMD and BMI/FN BMD and WHR. All SNPs are listed with their nearest gene(s), chromosomal location, effect allele A1, noneffect allele A2, raw P values for univariate GWAS for each trait, cFDR values for each trait, and ccFDR values. The effect direction of the potentially pleiotropic SNP on FN BMD and BMI/WHR was obtained from the original GWAS data. The effect represents the summary of effect directions (“+” indicates positive effect of A1 allele, “−” indicates negative effect of the A1 allele, and “/” indicates there is no value for the column).
Using the F-SNP program, we investigated the potential functions of these seven SNPs. The SNP rs3759579, within the intron of MARK3, is located at potential transcription factor–binding sites and may have a role in transcriptional regulation. A G-A change at rs3759579 may produce binding sites of CP2 and p300. Previous studies suggested the protein encoded by CP2 may be involved in osteoblast differentiation (51). P300, a histone acetyltransferase, has been demonstrated to be involved in adipogenesis through regulation of peroxisome proliferator activated receptor γ (PPARγ) and C/EBPβ expression (52) and osteoclastogenesis (53). The XXYLT1 SNP rs9825174 is located at a transcription factor–binding site, and the C-T transition may generate a binding site of C/EBPβ, which plays important roles in inducing expression of PPARγ (54). The G-C change in TRPS1 rs2178950 was found to produce a new exonic splicing enhancer motif—ACTGAA, which may regulate splicing of its pre-messenger RNA (mRNA). The other four SNPs did not show any known transcriptional regulation functions or splicing regulation functions according to the F-SNP program.
By performing t tests for differential expression analysis for the pleiotropic genes in the transcriptomic expression data of GSE2508, we found that TRPS1, MARK3, PUM1, and PRDM6 were differentially expressed between the lean and obese participants (TRPS1, P = 0.002; MARK3, P = 0.004; PUM1, P = 0.033; and PRDM6, P = 0.018). Furthermore, t tests of the transcriptomic expression data of GSE7249 showed that two genes (PUM1 and DNM3) were differentially expressed between the high BMD and low BMD participants with P = 0.042 and P = 0.014, respectively (Tables 2 and 3). The results of differential expression analysis for the seven potentially pleiotropic genes in the E-MEXP1618 were not statistically significant. A possible reason is that bone biopsy specimens were collected from several bone cells, potentially leading to the differential expression of these genes confounded by heterogeneous cell proportions (thus with larger noise) of these cells in different participants.
Table 2.
Basic Characteristics of the Studied GEO Data Sets
| Characteristic | GSE7429 | E-MEXP-1618 | GSE2508 |
|---|---|---|---|
| Design | High BMD vs low BMD | Normal or high FN BMD vs low BMD | Obese vs lean |
| Disease | Osteoporosis | Osteoporosis | Obesity |
| Target cells | Circulating B lymphocytes | Transiliac bone biopsies | Adipocytes |
| Sample size | (10 high vs 10 low) | (43 normal or high vs 41 low) | (19 obese vs 20 lean) |
| Platform | Affymetrix Human Genome U133A Array | Affymetrix GeneChip Human Genome U133 Plus 2.0 | Affymetrix Human Genome U95 Array |
Table 3.
Differential Expression Analysis for the Pleiotropic Genes in Osteoporosis- or Obesity-Related Cells
| Gene Symbol | Probe ID | t Test P Value |
|---|---|---|
| MARK3 | 202568_s_at | 4.02E-01 |
| MARK3 | 232537_x_at | 5.00E-02 |
| MARK3 | 49762_at | 4.00E-03 |
| TRPS1 | 218502_s_at | 5.22E-01 |
| TRPS1 | 234351_x_at | 1.05E-01 |
| TRPS1 | 55443_at | 2.00E-03 |
| PUM1 | 201166_s_at | 4.20E-02 |
| PUM1 | 201164_s_at | 5.50E-02 |
| PUM1 | 40048_at | 3.30E-02 |
| XXYLT1 | NA | NA |
| XXYLT1 | 226891_at | 2.49E-01 |
| XXYLT1 | 78532_at | 8.53E-01 |
| ZNF423 | 214761_at | 5.43E-01 |
| ZNF423 | 214761_at | 2.61E-01 |
| ZNF423 | 83639_at | 6.57E-01 |
| DNM3 | 209839_at | 1.40E-02 |
| DNM3 | 1558502_s_at | 4.07E-01 |
| DNM3 | 51761_at | 5.40E-02 |
| PRDM6 | NA | NA |
| PRDM6 | 230311_s_at | 8.00E-02 |
| PRDM6 | 71140_at | 1.81E-02 |
We only listed the most statistically significant expression results of probes with one gene with multiple detected probes. Bold values indicate nominally significant (P ≤ 0.05) of t-test.
Abbreviation: NA, not available.
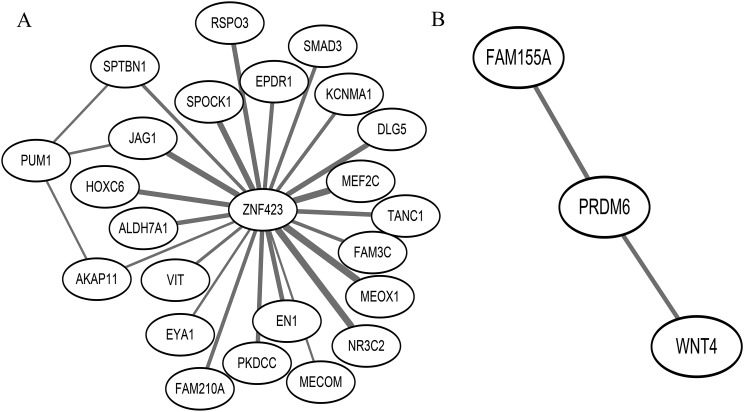
Using WGCNA for the 152 osteoporosis genes (138 known and 14 novel genes) in bone biopsy specimens, 137 genes were parsed into two gene modules. Potentially pleiotropic genes MARK3, TRPS1, PRDM6, and XXYLT1 were in the blue module, whereas potentially pleiotropic genes DNM3, PUM1, and ZNF423 were in the turquoise module. In the turquoise module, ZNF423 is one of three top network hub genes (JAG1, ZNF423, and HOXC6). With the TOM ≥0.10, there were 21 known osteoporosis-related genes interconnected with ZNF423 (Fig. 3A), and ZNF423 was positively correlated with all of the 21 known osteoporosis-associated genes, such as JAG1, EN1, and SMAD3. PUM1 was interconnected with AKAP11, JAG1, and SPTBN1 in the turquoise module with TOM ≥0.10 (Fig. 3A). Furthermore, PRDM6 interacted with FAM155A and WNT4 in the blue module (Fig. 3B). There were three pleiotropic genes identified in two modules, MARK3 and TPRS1 in the turquoise module and ZNF423 in the blue module, when applying network analysis for 303 obesity-related genes (278 known and 25 novel genes) in adipocytes. However, the interconnection between ZNF423, MARK3, TRPS1, and obesity-related genes was relatively weak.
Figure 3.
(A) ZNF423 and PUM1 centered network view, which characterizes the coexpression relationships for ZNF423 and PUM1 with BMD-associated genes in the osteoporosis turquoise module. This network view includes all edges and their corresponding nodes connected to ZNF423 and PUM1 with a TOM ≥0.10. The width of edges presents the value of TOM; the larger the TOM, the larger the width of the edge. All of the correlations between nodes are positive. (B) PRDM6 centered network view in the osteoporosis blue module, which presents the edges and nodes connected with PRDM6 with TOM ≥0.10.
Discussion
In the current study, we applied the cFDR method that combined the summary statistics from independent GWAS meta-analyses to identify the variants that are associated with FN BMD and BMI or FN BMD and WHR, as well as successfully identified seven pleiotropic variants (rs3759579, rs2178950, rs1473, rs9825174, rs2047937, rs17277372, rs335170) that were associated with FN BMD-BMI or FN BMD-WHR.
Although previous studies have found the four genes (MARK3, TRPS1, DNM3, and ZNF423) playing an important role in osteoporosis or obesity individually, our study for the first time discovered the pleiotropic effects of these genes in both osteoporosis and obesity. MARK3 was previously found to associate with BMD (55). Lennerz et al. (56) reported that the mice loss of Par-1a/MARK3/C-TAK1 kinase showed increased energy expenditure and reduced adiposity. DNM3 was associated with BMD (55) or WHR (57) in previous GWASs. Our results, together with previous studies, suggested that MARK3 and DNM3 play pleiotropic roles in osteoporosis and obesity.
The SNP rs2047937, located at the intron of the ZNF423 (also called ZFP423) gene, encodes a nuclear protein that is a DNA-binding transcription factor by using distinct zinc fingers in different signaling pathways. Multipotent mesenchymal precursor cells can differentiate into several cell types, such as osteoblasts and adipocytes (58). Numerous studies have demonstrated that the bone morphogenetic protein (BMP) and small mothers against decapentaplegia (SMAD) signal pathway not only induced the recruitment and differentiation of mesenchymal precursors into osteoblasts (59) but also controlled the preadipocyte commitment (60) and the differentiation of committed preadipocytes to adipocytes (61). ZNF423 was identified as a DNA-binding cofactor associated with SMADs in response to BMPs, leading to activate transcription of BMP target genes (62). Gupta et al. (60) reported that ZNF423 acted as a prominent preadipocyte commitment factor and regulated the expression of PPARγ through amplification of the BMP signaling pathway, and they discovered that Znf423 knockout mice exhibited noticeable defects in adipose mass. In the osteoporosis turquoise module, ZNF423 was found to highly interact with reported osteoporosis-associated genes, such as JAG1 and EN1. JAG1 participated in normal trabecular bone formation and inhibited periosteal expansion through affecting osteoprogenitor cells and their progeny but not mature osteoblasts (63). Recently, Zfp423 was found to act as a hinge regulated by Zfp521 coordinating regulation of adipocyte and osteoblast differentiation (64). Statistical evidence obtained in our study, together with previously reported biological functions, suggested that ZNF423 may play a role in both bone and fat metabolism through progenitor cells regulating cell differentiation between osteoblasts and adipocytes.
The TRPS1 gene encodes a zinc finger protein that has several different zinc finger motifs, including a GATA-type and IKAROS-like zinc fingers (65). Mutation of the human TRPS1 gene leads to trichorhinophalangeal syndrome, which exhibits abnormal development of various organs, including craniofacial and skeletal malformation due to abnormal differentiation of cartilage and bone (66). Numerous studies demonstrated that TRPS1 not only modulated chondrocyte proliferation, differentiation, and ossification but also controlled mitotic progression in chondrocytes (67). In addition, TRPS1 can directly bind the osteocalcin promoter in the presence or absence of RUNX2, mediating the osteocalcin transcription to regulate the osteoblast differentiation and deposition of mineralized matrix (68). TRPS1 mRNA was downregulated in obese adipocytes compared with nonobese adipocytes, suggesting that it might contribute to obesity pathology. Combining with biological functions, our study implicates that TRPS1 may play a pleiotropic role in bone and obesity metabolism.
Importantly, three novel SNPs located at 1p35.2 (PUM1), 5q23.2 (PRDM6), and 3q29 (XXYLT1) have not been reported in previous osteoporosis- or obesity-related research. PUM1 encodes a member of the PUF family that is implicated in various physiological processes and serves as a translational regulator of specific mRNAs by binding to their 3′ untranslated regions (69). Many studies indicated that PUM1 represses gene expression by binding to an eight-nucleotide RNA consensus sequence (the pumilio response element, 5-UGUANAUA-3), resulting in mRNA decay and translation inhibition (70, 71). By using the RNA-binding protein immunoprecipitation chip method, >1000 PUM1 target mRNAs have been identified (71, 72). In the list of mRNA targets, we found several genes related to osteoporosis or obesity, such as LRP5, WNT5A, BMP2, LMX1B, RQCD1, and ETS2 (40, 55, 73). PUM1 can recruit CCR4-NOT deadenylase subunits to cause translational inhibition and mRNA degradation (74). Several studies found that subunits of CCR4-NOT complex were involved in bone mass regulation (75) and adipocyte differentiation (76), suggesting that PUM1 can recruit CCR4-NOT deadenylase subunits and subunits of CCR4-NOT complex were involved in bone mass regulation. Therefore, we speculated that PUM1 may recruit CCR4–NOT complex controlling gene expression and involved in osteoporosis and obesity pathology. PUM1, meanwhile, was differentially expressed in two groups of B lymphocytes and adipocytes, suggesting its pleiotropic effect in the two phenotypes. PUM1 serves as a translational regulator and interacts with several osteoporosis GWAS-associated genes (AKAP11, JAG1, and SPTBN1), suggesting that it might regulate osteoporosis association genes and participate in osteoporosis pathology. Taken together, our study suggests that PUM1 probably plays an important role in regulating posttranscriptional translation of osteoporosis- and obesity-related genes. However, its functions in osteoporosis and obesity pathology need further functional studies.
Compared with a standard single phenotype analysis, cFDR simultaneously analyzing multiple related traits allows for an increased discovery of trait-associated variants using GWAS summary data (30). In addition, it allows us to take advantage of polygenic pleiotropy for further exploring common loci or genes that may influence multiple related phenotypes. Furthermore, through restricting the subset of SNPs at a different significance level, it can greatly lessen the burden of multiple testing and subsequently improve the detection of trait-associated variants. It is worth noting that the study samples for the cFDR method should be independent under the assumption so that maximum power can be achieved, because the overlap in the GWAS samples may reduce the information used for detection of potentially pleiotropic loci. In our study, a small proportion of cohorts overlapped in each pair of the summary statistics (FN BMD and BMI or FN BMD and WHR). Although the power of cFDR may be reduced owing to the overlapping samples, the potentially genetic pleiotropic variants identified in our study were robust and reliable.
In conclusion, we detected seven pleiotropic loci (MARK3, DNM3, ZNF423, TRPS1, PUM1, PRDM6, and XXYLT1) for FN BMD and BMI or FN BMD and WHR by using the cFDR method with existing individual GWAS summary statistics data. Of the seven genes, four of them (MARK3, DNM3, ZNF423, and TRPS1) were reported as potential candidate genes in osteoporosis or obesity in individual GWAS, and our study for the first time suggested their potentially pleiotropic roles. The other three novel potentially pleiotropic genes (PUM1, PRDM6, and XXYLT1) were not found in previous bone or obesity research. The findings in the current study enhance our knowledge of the shared genetic influences between osteoporosis and obesity and suggest a possible research direction for subsequent functional studies of these potentially implicated genes in the joint pathophysiology of both disorders.
Supplementary Material
Acknowledgments
Financial Support: This study was partially supported by Natural Science Foundation of China (81570807, 30900810, 31271344, and 31071097), Hunan Provincial Construct Program of the Key Discipline in Ecology (0713), and the Cooperative Innovation Center of Engineering and New Products for Developmental Biology of Hunan Province (20134486). H.W.D. was partially supported by grants from the National Institutes of Health (D43TW009107, R01AR069055, U19AG055373, R01MH104680, and R01AR059781) and the Edward G. Schlieder Endowment fund to Tulane University.
Author Contributions: Y.H., L.-J.T., and H.-W.D. designed the study design; Y.H. collected data, performed data analysis, and drafted the manuscript; Y.H., X.-D.C., L.Z., S.-S.M., Q.Z., L.-J.T., and H.-W.D. interpreted the data; Y.H., L.-J.T., H.S., and H.-W.D. revised the manuscript; L.-J.T. and H.-W.D. approved the final version of manuscript.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations:
- BMD
bone mineral density
- BMI
body mass index
- BMP
bone morphogenetic protein
- ccFDR
conjunction conditional false discovery rate
- cFDR
conditional false discovery rate
- FN
femoral neck
- GWAS
genome-wide association study
- mRNA
messenger RNA
- PPARγ
peroxisome proliferator activated receptor γ
- SNP
single-nucleotide polymorphism
- TOM
topological overlap measurement
- WGCNA
weighted genes coexpression network analysis
- WHR
waist-to-hip ratio.
References
- 1. Stahl EA, Wegmann D, Trynka G, Gutierrez-Achury J, Do R, Voight BF, Kraft P, Chen R, Kallberg HJ, Kurreeman FA, Kathiresan S, Wijmenga C, Gregersen PK, Alfredsson L, Siminovitch KA, Worthington J, de Bakker PI, Raychaudhuri S, Plenge RM; Diabetes Genetics Replication and Meta-analysis Consortium Myocardial Infarction Genetics Consortium . Bayesian inference analyses of the polygenic architecture of rheumatoid arthritis. Nat Genet. 2012;44(5):483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM. Finding the missing heritability of complex diseases. Nature. 2009;461(7265):747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sivakumaran S, Agakov F, Theodoratou E, Prendergast JG, Zgaga L, Manolio T, Rudan I, McKeigue P, Wilson JF, Campbell H. Abundant pleiotropy in human complex diseases and traits. Am J Hum Genet. 2011;89(5):607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14(Suppl 3):S13–S18. [DOI] [PubMed] [Google Scholar]
- 5. Deng HW, Chen WM, Conway T, Zhou Y, Davies KM, Stegman MR, Deng H, Recker RR. Determination of bone mineral density of the hip and spine in human pedigrees by genetic and life-style factors. Genet Epidemiol. 2000;19(2):160–177. [DOI] [PubMed] [Google Scholar]
- 6. Richards JB, Zheng HF, Spector TD. Genetics of osteoporosis from genome-wide association studies: advances and challenges. Nat Rev Genet. 2012;13(8):576–588. [DOI] [PubMed] [Google Scholar]
- 7. Zhang L, Choi HJ, Estrada K, Leo PJ, Li J, Pei YF, Zhang Y, Lin Y, Shen H, Liu YZ, Liu Y, Zhao Y, Zhang JG, Tian Q, Wang YP, Han Y, Ran S, Hai R, Zhu XZ, Wu S, Yan H, Liu X, Yang TL, Guo Y, Zhang F, Guo YF, Chen Y, Chen X, Tan L, Zhang L, Deng FY, Deng H, Rivadeneira F, Duncan EL, Lee JY, Han BG, Cho NH, Nicholson GC, McCloskey E, Eastell R, Prince RL, Eisman JA, Jones G, Reid IR, Sambrook PN, Dennison EM, Danoy P, Yerges-Armstrong LM, Streeten EA, Hu T, Xiang S, Papasian CJ, Brown MA, Shin CS, Uitterlinden AG, Deng HW. Multistage genome-wide association meta-analyses identified two new loci for bone mineral density. Hum Mol Genet. 2013;23(7):1923–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qin L, Liu Y, Wang Y, Wu G, Chen J, Ye W, Yang J, Huang Q. Computational characterization of osteoporosis associated SNPs and genes identified by genome-wide association studies. PLoS One. 2016;11(3):e0150070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zheng HF, Forgetta V, Hsu YH, Estrada K, Rosello-Diez A, Leo PJ, Dahia CL, Park-Min KH, Tobias JH, Kooperberg C, Kleinman A, Styrkarsdottir U, Liu CT, Uggla C, Evans DS, Nielson CM, Walter K, Pettersson-Kymmer U, McCarthy S, Eriksson J, Kwan T, Jhamai M, Trajanoska K, Memari Y, Min J, Huang J, Danecek P, Wilmot B, Li R, Chou WC, Mokry LE, Moayyeri A, Claussnitzer M, Cheng CH, Cheung W, Medina-Gómez C, Ge B, Chen SH, Choi K, Oei L, Fraser J, Kraaij R, Hibbs MA, Gregson CL, Paquette D, Hofman A, Wibom C, Tranah GJ, Marshall M, Gardiner BB, Cremin K, Auer P, Hsu L, Ring S, Tung JY, Thorleifsson G, Enneman AW, van Schoor NM, de Groot LC, van der Velde N, Melin B, Kemp JP, Christiansen C, Sayers A, Zhou Y, Calderari S, van Rooij J, Carlson C, Peters U, Berlivet S, Dostie J, Uitterlinden AG, Williams SR, Farber C, Grinberg D, LaCroix AZ, Haessler J, Chasman DI, Giulianini F, Rose LM, Ridker PM, Eisman JA, Nguyen TV, Center JR, Nogues X, Garcia-Giralt N, Launer LL, Gudnason V, Mellström D, Vandenput L, Amin N, van Duijn CM, Karlsson MK, Ljunggren Ö, Svensson O, Hallmans G, Rousseau F, Giroux S, Bussière J, Arp PP, Koromani F, Prince RL, Lewis JR, Langdahl BL, Hermann AP, Jensen JE, Kaptoge S, Khaw KT, Reeve J, Formosa MM, Xuereb-Anastasi A, Åkesson K, McGuigan FE, Garg G, Olmos JM, Zarrabeitia MT, Riancho JA, Ralston SH, Alonso N, Jiang X, Goltzman D, Pastinen T, Grundberg E, Gauguier D, Orwoll ES, Karasik D, Davey-Smith G, Smith AV, Siggeirsdottir K, Harris TB, Zillikens MC, van Meurs JB, Thorsteinsdottir U, Maurano MT, Timpson NJ, Soranzo N, Durbin R, Wilson SG, Ntzani EE, Brown MA, Stefansson K, Hinds DA, Spector T, Cupples LA, Ohlsson C, Greenwood CM, Jackson RD, Rowe DW, Loomis CA, Evans DM, Ackert-Bicknell CL, Joyner AL, Duncan EL, Kiel DP, Rivadeneira F, Richards JB; AOGC ConsortiumUK10K Consortium . Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature. 2015;526(7571):112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khaodhiar L, McCowen KC, Blackburn GL. Obesity and its comorbid conditions. Clin Cornerstone. 1999;2(3):17–31. [DOI] [PubMed] [Google Scholar]
- 11. Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–643. [DOI] [PubMed] [Google Scholar]
- 12. Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. [DOI] [PubMed] [Google Scholar]
- 13. Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27(4):325–351. [DOI] [PubMed] [Google Scholar]
- 14. Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am J Hum Genet. 2012;90(1):7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zaitlen N, Kraft P, Patterson N, Pasaniuc B, Bhatia G, Pollack S, Price AL. Using extended genealogy to estimate components of heritability for 23 quantitative and dichotomous traits. PLoS Genet. 2013;9(5):e1003520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Selby JV, Newman B, Quesenberry CP Jr, Fabsitz RR, Carmelli D, Meaney FJ, Slemenda C. Genetic and behavioral influences on body fat distribution. Int J Obes. 1990;14(7):593–602. [PubMed] [Google Scholar]
- 17. Poveda A, Ibáñez ME, Rebato E. Heritability and genetic correlations of obesity-related phenotypes among Roma people. Ann Hum Biol. 2012;39(3):183–189. [DOI] [PubMed] [Google Scholar]
- 18. Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab. 2007;92(5):1640–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reid IR, Ames R, Evans MC, Sharpe S, Gamble G, France JT, Lim TM, Cundy TF. Determinants of total body and regional bone mineral density in normal postmenopausal women—a key role for fat mass. J Clin Endocrinol Metab. 1992;75(1):45–51. [DOI] [PubMed] [Google Scholar]
- 20. Ravn P, Cizza G, Bjarnason NH, Thompson D, Daley M, Wasnich RD, McClung M, Hosking D, Yates AJ, Christiansen C. Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group. J Bone Miner Res. 1999;14(9):1622–1627. [DOI] [PubMed] [Google Scholar]
- 21. Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg. 2011;6(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao LJ, Jiang H, Papasian CJ, Maulik D, Drees B, Hamilton J, Deng HW. Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res. 2007;23(1):17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Magni P, Dozio E, Galliera E, Ruscica M, Corsi MM. Molecular aspects of adipokine-bone interactions. Curr Mol Med. 2010;10(6):522–532. [DOI] [PubMed] [Google Scholar]
- 24. Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14(3-4):222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113(6):846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gómez-Ambrosi J, Rodríguez A, Catalán V, Frühbeck G. The bone-adipose axis in obesity and weight loss. Obes Surg. 2008;18(9):1134–1143. [DOI] [PubMed] [Google Scholar]
- 27. Deng FY, Lei SF, Li MX, Jiang C, Dvornyk V, Deng HW. Genetic determination and correlation of body mass index and bone mineral density at the spine and hip in Chinese Han ethnicity. Osteoporos Int. 2005;17(1):119–124. [DOI] [PubMed] [Google Scholar]
- 28. Tang ZH, Xiao P, Lei SF, Deng FY, Zhao LJ, Deng HY, Tan LJ, Shen H, Xiong DH, Recker RR, Deng HW. A bivariate whole-genome linkage scan suggests several shared genomic regions for obesity and osteoporosis. J Clin Endocrinol Metab. 2007;92(7):2751–2757. [DOI] [PubMed] [Google Scholar]
- 29. Liu YZ, Pei YF, Liu JF, Yang F, Guo Y, Zhang L, Liu XG, Yan H, Wang L, Zhang YP, Levy S, Recker RR, Deng HW. Powerful bivariate genome-wide association analyses suggest the SOX6 gene influencing both obesity and osteoporosis phenotypes in males. PLoS One. 2009;4(8):e6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andreassen OA, Djurovic S, Thompson WK, Schork AJ, Kendler KS, O’Donovan MC, Rujescu D, Werge T, van de Bunt M, Morris AP, McCarthy MI, Roddey JC, McEvoy LK, Desikan RS, Dale AM; International Consortium for Blood Pressure GWASDiabetes Genetics Replication and Meta-analysis ConsortiumPsychiatric Genomics Consortium Schizophrenia Working Group . Improved detection of common variants associated with schizophrenia by leveraging pleiotropy with cardiovascular-disease risk factors. Am J Hum Genet. 2013;92(2):197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andreassen OA, Thompson WK, Schork AJ, Ripke S, Mattingsdal M, Kelsoe JR, Kendler KS, O’Donovan MC, Rujescu D, Werge T, Sklar P, Roddey JC, Chen CH, McEvoy L, Desikan RS, Djurovic S, Dale AM; Psychiatric Genomics Consortium (PGC)Bipolar Disorder and Schizophrenia Working Groups . Improved detection of common variants associated with schizophrenia and bipolar disorder using pleiotropy-informed conditional false discovery rate. PLoS Genet. 2013;9(4):e1003455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Greenbaum J, Wu K, Zhang L, Shen H, Zhang J, Deng HW. Increased detection of genetic loci associated with risk predictors of osteoporotic fracture using a pleiotropic cFDR method. Bone. 2017;99:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lv WQ, Zhang X, Zhang Q, He JY, Liu HM, Xia X, Fan K, Zhao Q, Shi XZ, Zhang WD, Sun CQ, Deng HW. Novel common variants associated with body mass index and coronary artery disease detected using a pleiotropic cFDR method. J Mol Cell Cardiol. 2017;112:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peng C, Lou HL, Liu F, Shen J, Lin X, Zeng CP, Long JR, Su KJ, Zhang L, Greenbaum J, Deng WF, Li YM, Deng HW. Enhanced identification of potential pleiotropic genetic variants for bone mineral density and breast cancer. Calcif Tissue Int. 2017;101(5):489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peng C, Shen J, Lin X, Su KJ, Greenbaum J, Zhu W, Lou HL, Liu F, Zeng CP, Deng WF, Deng HW. Genetic sharing with coronary artery disease identifies potential novel loci for bone mineral density. Bone. 2017;103:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang XF, Lin X, Li DY, Zhou R, Greenbaum J, Chen YC, Zeng CP, Peng LP, Wu KH, Ao ZX, Lu JM, Guo YF, Shen J, Deng HW. Linking Alzheimer’s disease and type 2 diabetes: novel shared susceptibility genes detected by cFDR approach. J Neurol Sci. 2017;380:262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou R, Lin X, Li DY, Wang XF, Greenbaum J, Chen YC, Zeng CP, Lu JM, Ao ZX, Peng LP, Bai XC, Shen J, Deng HW. Identification of novel genetic loci for osteoporosis and/or rheumatoid arthritis using cFDR approach. PLoS ONE. 2017;12:e0183842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zeng CP, Chen YC, Lin X, Greenbaum J, Chen YP, Peng C, Wang XF, Zhou R, Deng WM, Shen J, Deng HW. Increased identification of novel variants in type 2 diabetes, birth weight and their pleiotropic loci. J Diabetes. 2017;9(10):898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359(9319):1761–1767. [DOI] [PubMed] [Google Scholar]
- 40. Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, Ferreira T, Gustafsson S, Kutalik Z, Luan J, Mägi R, Randall JC, Winkler TW, Wood AR, Workalemahu T, Faul JD, Smith JA, Zhao JH, Zhao W, Chen J, Fehrmann R, Hedman AK, Karjalainen J, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bolton JL, Bragg-Gresham JL, Buyske S, Demirkan A, Deng G, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Goel A, Gong J, Jackson AU, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Mangino M, Leach IM, Medina-Gomez C, Medland SE, Nalls MA, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Shungin D, Stančáková A, Strawbridge RJ, Sung YJ, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Isaacs A, Albrecht E, Ärnlöv J, Arscott GM, Attwood AP, Bandinelli S, Barrett A, Bas IN, Bellis C, Bennett AJ, Berne C, Blagieva R, Blüher M, Böhringer S, Bonnycastle LL, Böttcher Y, Boyd HA, Bruinenberg M, Caspersen IH, Chen YI, Clarke R, Daw EW, de Craen AJM, Delgado G, Dimitriou M, Doney ASF, Eklund N, Estrada K, Eury E, Folkersen L, Fraser RM, Garcia ME, Geller F, Giedraitis V, Gigante B, Go AS, Golay A, Goodall AH, Gordon SD, Gorski M, Grabe HJ, Grallert H, Grammer TB, Gräßler J, Grönberg H, Groves CJ, Gusto G, Haessler J, Hall P, Haller T, Hallmans G, Hartman CA, Hassinen M, Hayward C, Heard-Costa NL, Helmer Q, Hengstenberg C, Holmen O, Hottenga JJ, James AL, Jeff JM, Johansson Å, Jolley J, Juliusdottir T, Kinnunen L, Koenig W, Koskenvuo M, Kratzer W, Laitinen J, Lamina C, Leander K, Lee NR, Lichtner P, Lind L, Lindström J, Lo KS, Lobbens S, Lorbeer R, Lu Y, Mach F, Magnusson PKE, Mahajan A, McArdle WL, McLachlan S, Menni C, Merger S, Mihailov E, Milani L, Moayyeri A, Monda KL, Morken MA, Mulas A, Müller G, Müller-Nurasyid M, Musk AW, Nagaraja R, Nöthen MM, Nolte IM, Pilz S, Rayner NW, Renstrom F, Rettig R, Ried JS, Ripke S, Robertson NR, Rose LM, Sanna S, Scharnagl H, Scholtens S, Schumacher FR, Scott WR, Seufferlein T, Shi J, Smith AV, Smolonska J, Stanton AV, Steinthorsdottir V, Stirrups K, Stringham HM, Sundström J, Swertz MA, Swift AJ, Syvänen AC, Tan ST, Tayo BO, Thorand B, Thorleifsson G, Tyrer JP, Uh HW, Vandenput L, Verhulst FC, Vermeulen SH, Verweij N, Vonk JM, Waite LL, Warren HR, Waterworth D, Weedon MN, Wilkens LR, Willenborg C, Wilsgaard T, Wojczynski MK, Wong A, Wright AF, Zhang Q, Brennan EP, Choi M, Dastani Z, Drong AW, Eriksson P, Franco-Cereceda A, Gådin JR, Gharavi AG, Goddard ME, Handsaker RE, Huang J, Karpe F, Kathiresan S, Keildson S, Kiryluk K, Kubo M, Lee JY, Liang L, Lifton RP, Ma B, McCarroll SA, McKnight AJ, Min JL, Moffatt MF, Montgomery GW, Murabito JM, Nicholson G, Nyholt DR, Okada Y, Perry JRB, Dorajoo R, Reinmaa E, Salem RM, Sandholm N, Scott RA, Stolk L, Takahashi A, Tanaka T, van ’t Hooft FM, Vinkhuyzen AAE, Westra HJ, Zheng W, Zondervan KT, Heath AC, Arveiler D, Bakker SJL, Beilby J, Bergman RN, Blangero J, Bovet P, Campbell H, Caulfield MJ, Cesana G, Chakravarti A, Chasman DI, Chines PS, Collins FS, Crawford DC, Cupples LA, Cusi D, Danesh J, de Faire U, den Ruijter HM, Dominiczak AF, Erbel R, Erdmann J, Eriksson JG, Farrall M, Felix SB, Ferrannini E, Ferrières J, Ford I, Forouhi NG, Forrester T, Franco OH, Gansevoort RT, Gejman PV, Gieger C, Gottesman O, Gudnason V, Gyllensten U, Hall AS, Harris TB, Hattersley AT, Hicks AA, Hindorff LA, Hingorani AD, Hofman A, Homuth G, Hovingh GK, Humphries SE, Hunt SC, Hyppönen E, Illig T, Jacobs KB, Jarvelin MR, Jöckel KH, Johansen B, Jousilahti P, Jukema JW, Jula AM, Kaprio J, Kastelein JJP, Keinanen-Kiukaanniemi SM, Kiemeney LA, Knekt P, Kooner JS, Kooperberg C, Kovacs P, Kraja AT, Kumari M, Kuusisto J, Lakka TA, Langenberg C, Marchand LL, Lehtimäki T, Lyssenko V, Männistö S, Marette A, Matise TC, McKenzie CA, McKnight B, Moll FL, Morris AD, Morris AP, Murray JC, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Madden PAF, Pasterkamp G, Peden JF, Peters A, Postma DS, Pramstaller PP, Price JF, Qi L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ridker PM, Rioux JD, Ritchie MD, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schunkert H, Schwarz PEH, Sever P, Shuldiner AR, Sinisalo J, Stolk RP, Strauch K, Tönjes A, Trégouët DA, Tremblay A, Tremoli E, Virtamo J, Vohl MC, Völker U, Waeber G, Willemsen G, Witteman JC, Zillikens MC, Adair LS, Amouyel P, Asselbergs FW, Assimes TL, Bochud M, Boehm BO, Boerwinkle E, Bornstein SR, Bottinger EP, Bouchard C, Cauchi S, Chambers JC, Chanock SJ, Cooper RS, de Bakker PIW, Dedoussis G, Ferrucci L, Franks PW, Froguel P, Groop LC, Haiman CA, Hamsten A, Hui J, Hunter DJ, Hveem K, Kaplan RC, Kivimaki M, Kuh D, Laakso M, Liu Y, Martin NG, März W, Melbye M, Metspalu A, Moebus S, Munroe PB, Njølstad I, Oostra BA, Palmer CNA, Pedersen NL, Perola M, Pérusse L, Peters U, Power C, Quertermous T, Rauramaa R, Rivadeneira F, Saaristo TE, Saleheen D, Sattar N, Schadt EE, Schlessinger D, Slagboom PE, Snieder H, Spector TD, Thorsteinsdottir U, Stumvoll M, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Walker M, Wallaschofski H, Wareham NJ, Watkins H, Weir DR, Wichmann HE, Wilson JF, Zanen P, Borecki IB, Deloukas P, Fox CS, Heid IM, O’Connell JR, Strachan DP, Stefansson K, van Duijn CM, Abecasis GR, Franke L, Frayling TM, McCarthy MI, Visscher PM, Scherag A, Willer CJ, Boehnke M, Mohlke KL, Lindgren CM, Beckmann JS, Barroso I, North KE, Ingelsson E, Hirschhorn JN, Loos RJF, Speliotes EK; LifeLines Cohort Study; ADIPOGen Consortium; AGEN-BMI Working Group; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GLGCICBPMAGIC Investigators; MuTHER Consortium; MIGen Consortium; PAGE Consortium; ReproGen Consortium; GENIE Consortium; International Endogene Consortium . Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, Strawbridge RJ, Pers TH, Fischer K, Justice AE, Workalemahu T, Wu JMW, Buchkovich ML, Heard-Costa NL, Roman TS, Drong AW, Song C, Gustafsson S, Day FR, Esko T, Fall T, Kutalik Z, Luan J, Randall JC, Scherag A, Vedantam S, Wood AR, Chen J, Fehrmann R, Karjalainen J, Kahali B, Liu CT, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bragg-Gresham JL, Buyske S, Demirkan A, Ehret GB, Feitosa MF, Goel A, Jackson AU, Johnson T, Kleber ME, Kristiansson K, Mangino M, Leach IM, Medina-Gomez C, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Stančáková A, Sung YJ, Tanaka T, Teumer A, Van Vliet-Ostaptchouk JV, Yengo L, Zhang W, Albrecht E, Ärnlöv J, Arscott GM, Bandinelli S, Barrett A, Bellis C, Bennett AJ, Berne C, Blüher M, Böhringer S, Bonnet F, Böttcher Y, Bruinenberg M, Carba DB, Caspersen IH, Clarke R, Daw EW, Deelen J, Deelman E, Delgado G, Doney AS, Eklund N, Erdos MR, Estrada K, Eury E, Friedrich N, Garcia ME, Giedraitis V, Gigante B, Go AS, Golay A, Grallert H, Grammer TB, Gräßler J, Grewal J, Groves CJ, Haller T, Hallmans G, Hartman CA, Hassinen M, Hayward C, Heikkilä K, Herzig KH, Helmer Q, Hillege HL, Holmen O, Hunt SC, Isaacs A, Ittermann T, James AL, Johansson I, Juliusdottir T, Kalafati IP, Kinnunen L, Koenig W, Kooner IK, Kratzer W, Lamina C, Leander K, Lee NR, Lichtner P, Lind L, Lindström J, Lobbens S, Lorentzon M, Mach F, Magnusson PK, Mahajan A, McArdle WL, Menni C, Merger S, Mihailov E, Milani L, Mills R, Moayyeri A, Monda KL, Mooijaart SP, Mühleisen TW, Mulas A, Müller G, Müller-Nurasyid M, Nagaraja R, Nalls MA, Narisu N, Glorioso N, Nolte IM, Olden M, Rayner NW, Renstrom F, Ried JS, Robertson NR, Rose LM, Sanna S, Scharnagl H, Scholtens S, Sennblad B, Seufferlein T, Sitlani CM, Smith AV, Stirrups K, Stringham HM, Sundström J, Swertz MA, Swift AJ, Syvänen AC, Tayo BO, Thorand B, Thorleifsson G, Tomaschitz A, Troffa C, van Oort FV, Verweij N, Vonk JM, Waite LL, Wennauer R, Wilsgaard T, Wojczynski MK, Wong A, Zhang Q, Zhao JH, Brennan EP, Choi M, Eriksson P, Folkersen L, Franco-Cereceda A, Gharavi AG, Hedman AK, Hivert MF, Huang J, Kanoni S, Karpe F, Keildson S, Kiryluk K, Liang L, Lifton RP, Ma B, McKnight AJ, McPherson R, Metspalu A, Min JL, Moffatt MF, Montgomery GW, Murabito JM, Nicholson G, Nyholt DR, Olsson C, Perry JR, Reinmaa E, Salem RM, Sandholm N, Schadt EE, Scott RA, Stolk L, Vallejo EE, Westra HJ, Zondervan KT, Amouyel P, Arveiler D, Bakker SJ, Beilby J, Bergman RN, Blangero J, Brown MJ, Burnier M, Campbell H, Chakravarti A, Chines PS, Claudi-Boehm S, Collins FS, Crawford DC, Danesh J, de Faire U, de Geus EJ, Dörr M, Erbel R, Eriksson JG, Farrall M, Ferrannini E, Ferrières J, Forouhi NG, Forrester T, Franco OH, Gansevoort RT, Gieger C, Gudnason V, Haiman CA, Harris TB, Hattersley AT, Heliövaara M, Hicks AA, Hingorani AD, Hoffmann W, Hofman A, Homuth G, Humphries SE, Hyppönen E, Illig T, Jarvelin MR, Johansen B, Jousilahti P, Jula AM, Kaprio J, Kee F, Keinanen-Kiukaanniemi SM, Kooner JS, Kooperberg C, Kovacs P, Kraja AT, Kumari M, Kuulasmaa K, Kuusisto J, Lakka TA, Langenberg C, Le Marchand L, Lehtimäki T, Lyssenko V, Männistö S, Marette A, Matise TC, McKenzie CA, McKnight B, Musk AW, Möhlenkamp S, Morris AD, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Palmer LJ, Penninx BW, Peters A, Pramstaller PP, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ridker PM, Ritchie MD, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schwarz PE, Shuldiner AR, Staessen JA, Steinthorsdottir V, Stolk RP, Strauch K, Tönjes A, Tremblay A, Tremoli E, Vohl MC, Völker U, Vollenweider P, Wilson JF, Witteman JC, Adair LS, Bochud M, Boehm BO, Bornstein SR, Bouchard C, Cauchi S, Caulfield MJ, Chambers JC, Chasman DI, Cooper RS, Dedoussis G, Ferrucci L, Froguel P, Grabe HJ, Hamsten A, Hui J, Hveem K, Jöckel KH, Kivimaki M, Kuh D, Laakso M, Liu Y, März W, Munroe PB, Njølstad I, Oostra BA, Palmer CN, Pedersen NL, Perola M, Pérusse L, Peters U, Power C, Quertermous T, Rauramaa R, Rivadeneira F, Saaristo TE, Saleheen D, Sinisalo J, Slagboom PE, Snieder H, Spector TD, Stefansson K, Stumvoll M, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Veronesi G, Walker M, Wareham NJ, Watkins H, Wichmann HE, Abecasis GR, Assimes TL, Berndt SI, Boehnke M, Borecki IB, Deloukas P, Franke L, Frayling TM, Groop LC, Hunter DJ, Kaplan RC, O’Connell JR, Qi L, Schlessinger D, Strachan DP, Thorsteinsdottir U, van Duijn CM, Willer CJ, Visscher PM, Yang J, Hirschhorn JN, Zillikens MC, McCarthy MI, Speliotes EK, North KE, Fox CS, Barroso I, Franks PW, Ingelsson E, Heid IM, Loos RJ, Cupples LA, Morris AP, Lindgren CM, Mohlke KL; ADIPOGen Consortium; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GEFOS Consortium; GENIE Consortium; GLGCICBPInternational Endogene Consortium; LifeLines Cohort Study; MAGIC Investigators; MuTHER Consortium; PAGE ConsortiumReproGen Consortium . New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee PH, Shatkay H. F-SNP: computationally predicted functional SNPs for disease association studies. Nucleic Acids Res. 2008;36(Database issue):D820–D824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xiao P, Chen Y, Jiang H, Liu YZ, Pan F, Yang TL, Tang ZH, Larsen JA, Lappe JM, Recker RR, Deng HW. In vivo genome-wide expression study on human circulating B cells suggests a novel ESR1 and MAPK3 network for postmenopausal osteoporosis. J Bone Miner Res. 2008;23(5):644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sato T, Shibata T, Ikeda K, Watanabe K. Generation of bone-resorbing osteoclasts from B220+ cells: its role in accelerated osteoclastogenesis due to estrogen deficiency. J Bone Miner Res. 2001;16(12):2215–2221. [DOI] [PubMed] [Google Scholar]
- 45. Pugliese LS, Gonçalves TO, Popi AF, Mariano M, Pesquero JB, Lopes JD. B-1 lymphocytes differentiate into functional osteoclast-like cells. Immunobiology. 2012;217(3):336–344. [DOI] [PubMed] [Google Scholar]
- 46. Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9(1):559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol. 2005;4:Article17.10.2202/1544-6115.1128. [DOI] [PubMed] [Google Scholar]
- 49. Kim CJ, Oh KW, Rhee EJ, Kim KH, Jo SK, Jung CH, Won JC, Park CY, Lee WY, Park SW, Kim SW. Relationship between body composition and bone mineral density (BMD) in perimenopausal Korean women. Clin Endocrinol (Oxf). 2009;71(1):18–26. [DOI] [PubMed] [Google Scholar]
- 50. Arazi H, Eghbali E, Saeedi T, Moghadam R. The relationship of physical activity and anthropometric and physiological characteristics to bone mineral density in postmenopausal women. J Clin Densitom. 2016;19(3):382–388. [DOI] [PubMed] [Google Scholar]
- 51. Kang HC, Chae JH, Kim BS, Han SY, Kim SH, Auh CK, Yang SI, Kim CG. Transcription factor CP2 is involved in activating mBMP4 in mouse mesenchymal stem cells. Mol Cells. 2004;17(3):454–461. [PubMed] [Google Scholar]
- 52. Qi C, Surapureddi S, Zhu YJ, Yu S, Kashireddy P, Rao MS, Reddy JK. Transcriptional coactivator PRIP, the peroxisome proliferator-activated receptor gamma (PPARgamma)-interacting protein, is required for PPARgamma-mediated adipogenesis. J Biol Chem. 2003;278(28):25281–25284. [DOI] [PubMed] [Google Scholar]
- 53. Shakibaei M, Buhrmann C, Mobasheri A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J Biol Chem. 2011;286(13):11492–11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hamm JK, Park BH, Farmer SR. A role for C/EBPbeta in regulating peroxisome proliferator-activated receptor gamma activity during adipogenesis in 3T3-L1 preadipocytes. J Biol Chem. 2001;276(21):18464–18471. [DOI] [PubMed] [Google Scholar]
- 55. Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, Oei L, Albagha OM, Amin N, Kemp JP, Koller DL, Li G, Liu CT, Minster RL, Moayyeri A, Vandenput L, Willner D, Xiao SM, Yerges-Armstrong LM, Zheng HF, Alonso N, Eriksson J, Kammerer CM, Kaptoge SK, Leo PJ, Thorleifsson G, Wilson SG, Wilson JF, Aalto V, Alen M, Aragaki AK, Aspelund T, Center JR, Dailiana Z, Duggan DJ, Garcia M, Garcia-Giralt N, Giroux S, Hallmans G, Hocking LJ, Husted LB, Jameson KA, Khusainova R, Kim GS, Kooperberg C, Koromila T, Kruk M, Laaksonen M, Lacroix AZ, Lee SH, Leung PC, Lewis JR, Masi L, Mencej-Bedrac S, Nguyen TV, Nogues X, Patel MS, Prezelj J, Rose LM, Scollen S, Siggeirsdottir K, Smith AV, Svensson O, Trompet S, Trummer O, van Schoor NM, Woo J, Zhu K, Balcells S, Brandi ML, Buckley BM, Cheng S, Christiansen C, Cooper C, Dedoussis G, Ford I, Frost M, Goltzman D, González-Macías J, Kähönen M, Karlsson M, Khusnutdinova E, Koh JM, Kollia P, Langdahl BL, Leslie WD, Lips P, Ljunggren Ö, Lorenc RS, Marc J, Mellström D, Obermayer-Pietsch B, Olmos JM, Pettersson-Kymmer U, Reid DM, Riancho JA, Ridker PM, Rousseau F, Slagboom PE, Tang NL, Urreizti R, Van Hul W, Viikari J, Zarrabeitia MT, Aulchenko YS, Castano-Betancourt M, Grundberg E, Herrera L, Ingvarsson T, Johannsdottir H, Kwan T, Li R, Luben R, Medina-Gómez C, Palsson ST, Reppe S, Rotter JI, Sigurdsson G, van Meurs JB, Verlaan D, Williams FM, Wood AR, Zhou Y, Gautvik KM, Pastinen T, Raychaudhuri S, Cauley JA, Chasman DI, Clark GR, Cummings SR, Danoy P, Dennison EM, Eastell R, Eisman JA, Gudnason V, Hofman A, Jackson RD, Jones G, Jukema JW, Khaw KT, Lehtimäki T, Liu Y, Lorentzon M, McCloskey E, Mitchell BD, Nandakumar K, Nicholson GC, Oostra BA, Peacock M, Pols HA, Prince RL, Raitakari O, Reid IR, Robbins J, Sambrook PN, Sham PC, Shuldiner AR, Tylavsky FA, van Duijn CM, Wareham NJ, Cupples LA, Econs MJ, Evans DM, Harris TB, Kung AW, Psaty BM, Reeve J, Spector TD, Streeten EA, Zillikens MC, Thorsteinsdottir U, Ohlsson C, Karasik D, Richards JB, Brown MA, Stefansson K, Uitterlinden AG, Ralston SH, Ioannidis JP, Kiel DP, Rivadeneira F. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44(5):491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lennerz JK, Hurov JB, White LS, Lewandowski KT, Prior JL, Planer GJ, Gereau RW IV, Piwnica-Worms D, Schmidt RE, Piwnica-Worms H. Loss of Par-1a/MARK3/C-TAK1 kinase leads to reduced adiposity, resistance to hepatic steatosis, and defective gluconeogenesis. Mol Cell Biol. 2010;30(21):5043–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Heid IM, Jackson AU, Randall JC, Winkler TW, Qi L, Steinthorsdottir V, Thorleifsson G, Zillikens MC, Speliotes EK, Mägi R, Workalemahu T, White CC, Bouatia-Naji N, Harris TB, Berndt SI, Ingelsson E, Willer CJ, Weedon MN, Luan J, Vedantam S, Esko T, Kilpeläinen TO, Kutalik Z, Li S, Monda KL, Dixon AL, Holmes CC, Kaplan LM, Liang L, Min JL, Moffatt MF, Molony C, Nicholson G, Schadt EE, Zondervan KT, Feitosa MF, Ferreira T, Lango Allen H, Weyant RJ, Wheeler E, Wood AR, Estrada K, Goddard ME, Lettre G, Mangino M, Nyholt DR, Purcell S, Smith AV, Visscher PM, Yang J, McCarroll SA, Nemesh J, Voight BF, Absher D, Amin N, Aspelund T, Coin L, Glazer NL, Hayward C, Heard-Costa NL, Hottenga JJ, Johansson A, Johnson T, Kaakinen M, Kapur K, Ketkar S, Knowles JW, Kraft P, Kraja AT, Lamina C, Leitzmann MF, McKnight B, Morris AP, Ong KK, Perry JR, Peters MJ, Polasek O, Prokopenko I, Rayner NW, Ripatti S, Rivadeneira F, Robertson NR, Sanna S, Sovio U, Surakka I, Teumer A, van Wingerden S, Vitart V, Zhao JH, Cavalcanti-Proença C, Chines PS, Fisher E, Kulzer JR, Lecoeur C, Narisu N, Sandholt C, Scott LJ, Silander K, Stark K, Tammesoo ML, Teslovich TM, Timpson NJ, Watanabe RM, Welch R, Chasman DI, Cooper MN, Jansson JO, Kettunen J, Lawrence RW, Pellikka N, Perola M, Vandenput L, Alavere H, Almgren P, Atwood LD, Bennett AJ, Biffar R, Bonnycastle LL, Bornstein SR, Buchanan TA, Campbell H, Day IN, Dei M, Dörr M, Elliott P, Erdos MR, Eriksson JG, Freimer NB, Fu M, Gaget S, Geus EJ, Gjesing AP, Grallert H, Grässler J, Groves CJ, Guiducci C, Hartikainen AL, Hassanali N, Havulinna AS, Herzig KH, Hicks AA, Hui J, Igl W, Jousilahti P, Jula A, Kajantie E, Kinnunen L, Kolcic I, Koskinen S, Kovacs P, Kroemer HK, Krzelj V, Kuusisto J, Kvaloy K, Laitinen J, Lantieri O, Lathrop GM, Lokki ML, Luben RN, Ludwig B, McArdle WL, McCarthy A, Morken MA, Nelis M, Neville MJ, Paré G, Parker AN, Peden JF, Pichler I, Pietiläinen KH, Platou CG, Pouta A, Ridderstråle M, Samani NJ, Saramies J, Sinisalo J, Smit JH, Strawbridge RJ, Stringham HM, Swift AJ, Teder-Laving M, Thomson B, Usala G, van Meurs JB, van Ommen GJ, Vatin V, Volpato CB, Wallaschofski H, Walters GB, Widen E, Wild SH, Willemsen G, Witte DR, Zgaga L, Zitting P, Beilby JP, James AL, Kähönen M, Lehtimäki T, Nieminen MS, Ohlsson C, Palmer LJ, Raitakari O, Ridker PM, Stumvoll M, Tönjes A, Viikari J, Balkau B, Ben-Shlomo Y, Bergman RN, Boeing H, Smith GD, Ebrahim S, Froguel P, Hansen T, Hengstenberg C, Hveem K, Isomaa B, Jørgensen T, Karpe F, Khaw KT, Laakso M, Lawlor DA, Marre M, Meitinger T, Metspalu A, Midthjell K, Pedersen O, Salomaa V, Schwarz PE, Tuomi T, Tuomilehto J, Valle TT, Wareham NJ, Arnold AM, Beckmann JS, Bergmann S, Boerwinkle E, Boomsma DI, Caulfield MJ, Collins FS, Eiriksdottir G, Gudnason V, Gyllensten U, Hamsten A, Hattersley AT, Hofman A, Hu FB, Illig T, Iribarren C, Jarvelin MR, Kao WH, Kaprio J, Launer LJ, Munroe PB, Oostra B, Penninx BW, Pramstaller PP, Psaty BM, Quertermous T, Rissanen A, Rudan I, Shuldiner AR, Soranzo N, Spector TD, Syvanen AC, Uda M, Uitterlinden A, Völzke H, Vollenweider P, Wilson JF, Witteman JC, Wright AF, Abecasis GR, Boehnke M, Borecki IB, Deloukas P, Frayling TM, Groop LC, Haritunians T, Hunter DJ, Kaplan RC, North KE, O’Connell JR, Peltonen L, Schlessinger D, Strachan DP, Hirschhorn JN, Assimes TL, Wichmann HE, Thorsteinsdottir U, van Duijn CM, Stefansson K, Cupples LA, Loos RJ, Barroso I, McCarthy MI, Fox CS, Mohlke KL, Lindgren CM; MAGIC . Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet. 2010;42(11):949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2(1):35–43. [DOI] [PubMed] [Google Scholar]
- 59. Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2009;22(4):233–241. [DOI] [PubMed] [Google Scholar]
- 60. Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, Spiegelman BM. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464(7288):619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Huang H, Song TJ, Li X, Hu L, He Q, Liu M, Lane MD, Tang QQ. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci USA. 2009;106(31):12670–12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hata A, Seoane J, Lagna G, Montalvo E, Hemmati-Brivanlou A, Massagué J. OAZ uses distinct DNA- and protein-binding zinc fingers in separate BMP-Smad and Olf signaling pathways. Cell. 2000;100(2):229–240. [DOI] [PubMed] [Google Scholar]
- 63. Youngstrom DW, Dishowitz MI, Bales CB, Carr E, Mutyaba PL, Kozloff KM, Shitaye H, Hankenson KD, Loomes KM. Jagged1 expression by osteoblast-lineage cells regulates trabecular bone mass and periosteal expansion in mice. Bone. 2016;91:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Addison WN, Fu MM, Yang HX, Lin Z, Nagano K, Gori F, Baron R. Direct transcriptional repression of Zfp423 by Zfp521 mediates a bone morphogenic protein-dependent osteoblast versus adipocyte lineage commitment switch. Mol Cell Biol. 2014;34(16):3076–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kaiser FJ, Möröy T, Chang GT, Horsthemke B, Lüdecke HJ. The RING finger protein RNF4, a co-regulator of transcription, interacts with the TRPS1 transcription factor. J Biol Chem. 2003;278(40):38780–38785. [DOI] [PubMed] [Google Scholar]
- 66. Momeni P, Glöckner G, Schmidt O, von Holtum D, Albrecht B, Gillessen-Kaesbach G, Hennekam R, Meinecke P, Zabel B, Rosenthal A, Horsthemke B, Lüdecke HJ. Mutations in a new gene, encoding a zinc-finger protein, cause tricho-rhino-phalangeal syndrome type I. Nat Genet. 2000;24(1):71–74. [DOI] [PubMed] [Google Scholar]
- 67. Wuelling M, Pasdziernik M, Moll CN, Thiesen AM, Schneider S, Johannes C, Vortkamp A. The multi zinc-finger protein Trps1 acts as a regulator of histone deacetylation during mitosis. Cell Cycle. 2014;12(14):2219–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Piscopo DM, Johansen EB, Derynck R. Identification of the GATA factor TRPS1 as a repressor of the osteocalcin promoter. J Biol Chem. 2009;284(46):31690–31703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Spassov DS, Jurecic R. Cloning and comparative sequence analysis of PUM1 and PUM2 genes, human members of the Pumilio family of RNA-binding proteins. Gene. 2002;299(1-2):195–204. [DOI] [PubMed] [Google Scholar]
- 70. Cheong CG, Hall TM. Engineering RNA sequence specificity of Pumilio repeats. Proc Natl Acad Sci USA. 2006;103(37):13635–13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Galgano A, Forrer M, Jaskiewicz L, Kanitz A, Zavolan M, Gerber AP. Comparative analysis of mRNA targets for human PUF-family proteins suggests extensive interaction with the miRNA regulatory system. PLoS One. 2008;3(9):e3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Morris AR, Mukherjee N, Keene JD. Ribonomic analysis of human Pum1 reveals cis-trans conservation across species despite evolution of diverse mRNA target sets. Mol Cell Biol. 2008;28(12):4093–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang T, Zhang J, Cao Y, Zhang M, Jing L, Jiao K, Yu S, Chang W, Chen D, Wang M. Wnt5a/Ror2 mediates temporomandibular joint subchondral bone remodeling. J Dent Res. 2015;94(6):803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Van Etten J, Schagat TL, Hrit J, Weidmann CA, Brumbaugh J, Coon JJ, Goldstrohm AC. Human Pumilio proteins recruit multiple deadenylases to efficiently repress messenger RNAs. J Biol Chem. 2012;287(43):36370–36383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Washio-Oikawa K, Nakamura T, Usui M, Yoneda M, Ezura Y, Ishikawa I, Nakashima K, Noda T, Yamamoto T, Noda M. Cnot7-null mice exhibit high bone mass phenotype and modulation of BMP actions. J Bone Miner Res. 2007;22(8):1217–1223. [DOI] [PubMed] [Google Scholar]
- 76. Sohn EJ, Jung DB, Lee J, Yoon SW, Won GH, Ko HS, Kim SH. CCR4-NOT2 promotes the differentiation and lipogenesis of 3T3-L1 adipocytes via upregulation of PPARx03B3; CEBPα and inhibition of P-GSK3α/β and β-catenin. Cell Physiol Biochem. 2015;37(5):1881–1889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.