The controlled production of granulocytes must be able to fulfill different needs of the organism throughout its lifetime. The maintenance of a stable basal level of mature granulocytes is ensured by steady-state granulopoiesis,1 whereas in stress situations, such as severe infection, large numbers of neutrophils are required and a program called emergency granulopoiesis is activated.2 These two programs are differentially regulated at the transcriptional level,3 and require appropriate expression of cell- and stage-specific transcription factors in order to serve particular demands for granulocyte production. It has been shown that the CCAAT/enhancer-binding protein (C/EBP) family of transcription factors plays a critical role in these processes,3–5 however, the function of C/EBPγ in normal and emergency granulopoiesis remains elusive. C/EBPγ is ubiquitously expressed in hematopoietic cells,6,7 and despite lacking transactivation domains, it has been reported that C/EBPγ controls gene regulation by dimerizing with other transcription factors.8,9 Herein, we generated a C/EBPγ conditional knockout (KO) mouse model in which Cebpg is depleted in the hematopoietic system, and demonstrated that the transcription factor C/EBPγ, despite being expressed at high levels in all hematopoietic cells, is dispensable for steady-state and emergency granulopoiesis.
To study the role of C/EBPγ in hematopoiesis we generated a Cebpg conditional KO murine model (Cebpgfl/fl) (Online Supplementary Figure S1) which we crossed with Vav-iCre transgenic mice, generating Cebpgfl/fl Vav-iCre− (wild-type, WT) and Cebpgfl/fl Vav-iCre+ (KO) animals. We validated our model and demonstrated efficient Cre-mediated recombination resulting in controlled deletion of this transcription factor in the hematopoietic system (Online Supplementary Figure S2). Presence of a tdTomato reporter demonstrated expression of C/EBPγ in all hematopoietic cells (Online Supplementary Figure S2H and data not shown). C/EBPγ lacks transactivation domains and it has been proposed that it acts by heterodimerizing with other factors, such as C/EBPβ and Activating Transcription Factor 4 (ATF4).8,9 Using electrophoretic mobility shift assays (EMSAs) we demonstrated the presence of C/EBPγ heterodimers in murine WT bone marrow (BM) and spleen (SP) cells; complexes which were not present in Cebpg KO cells (Online Supplementary Figure S3). Of note, despite the fact that granulocytes represent a high percentage of the total BM population, our EMSAs do not specifically addressed whether these complexes are present in the granulocytic lineage cells. In summary, we generated Cebpgfl mice which report for C/EBPγ expression in all hematopoietic cells, we demonstrated the presence of C/EBPγ heterodimers in adult hematopoietic cells, and induced a controlled deletion of this transcription factor in the hematopoietic system.
Surprisingly, Cebpg KO mice were viable and healthy, and showed no signs of disease during their life-span (mice were observed until the age of 70 weeks). Since granulopoiesis takes place in the BM from multipotent hematopoietic stem cells (HSCs) and C/EBPγ is expressed in long-term (LT)-HSCs,6 we investigated whether C/EBPγ is involved in HSC function. Phenotypically defined LT-HSCs (LKS SLAM; LKS: lineage−, c-kit+, Sca-1+; signaling lymphocyte activation molecule (SLAM): CD48−, CD150+) were sorted, and gene expression profiling was performed (E-MTAB-6245). Using LIMMA package to assess differential gene expression, we detected 426 probesets identifying dysregulated genes in Cebpg KO versus WT LT-HSC (P<0.05 and fold change >1) (Online Supplementary Figure S4A and Online Supplementary Table S1 and Table S2). However, gene set enrichment analysis demonstrated scarce differentially regulated pathways in the Cebpg KO LT-HSC in comparison to WT LT-HSC (Online Supplementary Table S3). To determine whether the changes in gene expression would have an impact on the functionality of Cebpg KO LT-HSC, competitive limiting-dilution repopulating assays were performed, revealing no significant differences in WT and Cebpg KO LT-HSC numbers and repopulating abilities (Online Supplementary Figure S4B-D). To corroborate our results in vitro, we performed serial replating assays, which demonstrated no differences between WT and Cebpg KO cells (Online Supplementary Figure S4E,F). Together, our in vitro and in vivo assays indicate that Cebpg is dispensable for HSC functions. Nevertheless, future studies should determine whether C/EBPγ may play a role in LT-HSC self-renewal.
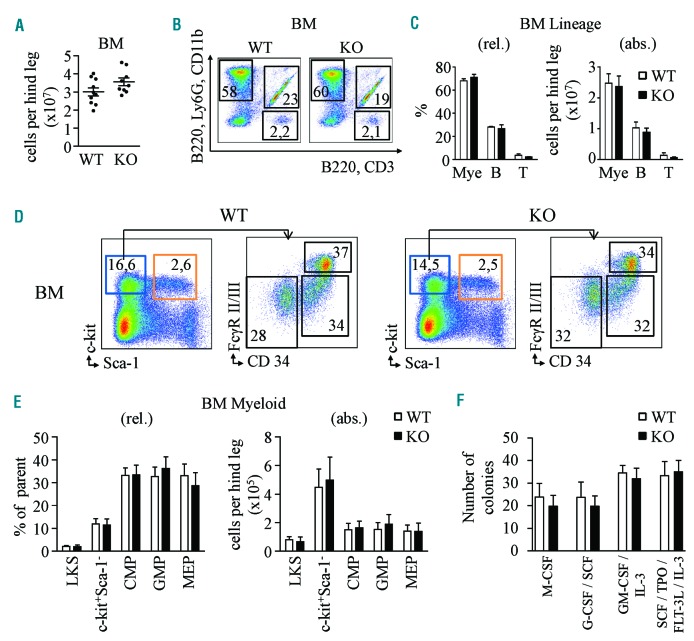
Next, we investigated whether Cebpg ablation affects steady-state granulopoiesis. We demonstrated that the deletion of Cebpg did not affect total cell numbers in BM, and that Cebpg KO mice presented a similar percentage and numbers of mature cells as their WT littermates (Figure 1A–C). In addition, BM and blood neutrophils presented similar morphology in Cebpg KO and WT mice, and no differences in granule content were detected (Online Supplementary Figure S5). Furthermore, flow cytometric analysis showed similar numbers of bone marrow progenitors in both WT and KO mice (Figure 1D,E). Similarly, later stages of granulocytic development4 demonstrated no differences between WT and Cebpg KO mice (Online Supplementary Figure S6). Since it was previously reported that straight Cebpg KO BM cells show reduced colony formation in vitro,8 we performed colony culture assays using several combinations of hematopoietic growth factors (Figure 1F). Unexpectedly, our model did not recapitulate the changes reported previously,8 possibly due to the distinct approaches employed to induce Cebpg ablation (straight versus conditional deletion) or the different murine background (C57BL/6-129v versus pure C57BL/6NCrl). Taken together these data suggest that in our murine model, C/EBPγ is dispensable for basal granulopoiesis.
Figure 1.
Steady-state granulopoiesis is not affected by genetic deletion of Cebpg gene. (A) BM cellularity in WT and Cebpg KO mice. Y axis indicates absolute number of cells per hind leg. (B) Representative flow cytometry plots from WT and Cebpg KO BM cells stained for myeloid (Ly6G, CD11b), B-cell (B220), and T-cell (CD3) markers. Upper left quadrant indicates percentage of myeloid cells, upper right the percentage of B cells, and lower right the percentage of T cells. (C) Quantification of myeloid cells (Mye), B cells (B), and T cells (T) based on flow cytometry data illustrated in panel B. Y axes indicate relative percentage (rel., left panel) and absolute number (abs., right panel) of cells. (D) Representative flow cytometry plots from WT and Cebpg KO BM cells. Left plots were gated from lineage negative cells and stained for c-kit and Sca-1 markers. Blue box indicates percentage of myeloid progenitor cells (lineage−, c-kit+, Sca-1+), and orange box indicates percentage of LKS (lineage−, c-kit+, Sca-1+) cells. Right plots represent expression of FcγRII/III and CD34 in myeloid progenitor cells. Lower left gate indicates percentage of megakaryocyte erythroid progenitor cells (MEP), lower right indicates percentage of common myeloid progenitor cells (CMP), and upper right percentage of granulocyte macrophage progenitor cells (GMP). (E) Quantification of panel D. (C–E) Each group contains 12 mice from three independent experiments. (F) Colony forming assays using WT (white bars) and Cebpg KO (black bars) BM cells. 7.5×103 cells were plated per well. Y axis indicates total number of colonies per well. X axis indicate the cytokines included in each condition. Two-tailed Student’s t-tests were used to assess statistical significance. BM: bone marrow; WT: wild-type; KO: knockout; LKS: lineage−, c-kit+, Sca-1+ cells; SCF: stem cell factor; IL: interleukin; TPO: thrombopoietin; FLT-3L: FMS-like tyrosin kinase 3 ligand.
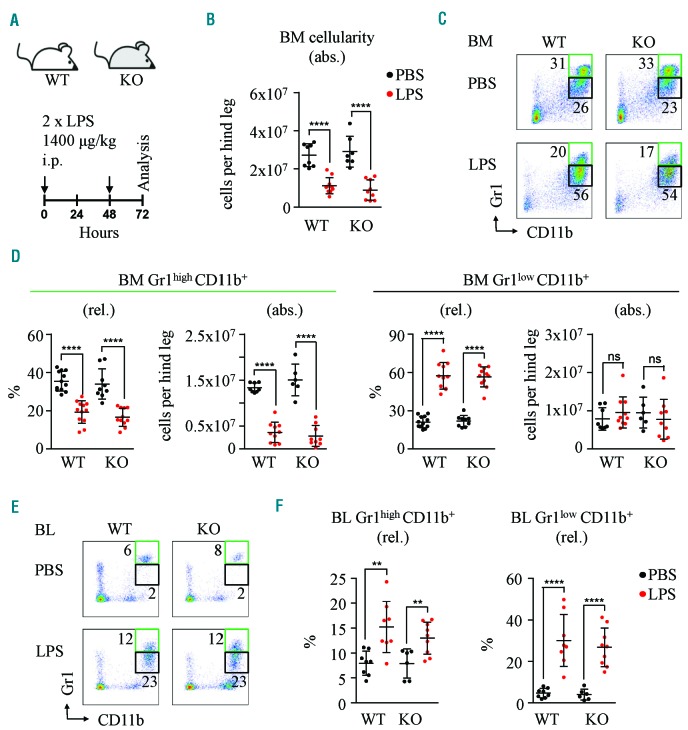
Since Cebpg deficient mice showed no defects in steady-state conditions, we subsequently examined whether C/EBPγ is required during lipopolysaccharide (LPS)-induced emergency granulopoiesis. We challenged WT and Cebpg KO mice with high doses of LPS to mimic a severe systemic infection (Figure 2A), and analyzed typical surrogate hallmarks of emergency granulopoiesis.10,11 Strikingly, BM cellularity, and the frequency and absolute numbers of mature Gr1high CD11b+ and immature Gr1low CD11b+ BM neutrophils after LPS treatment was equal in both Cebpg KO and WT controls (Figure 2B–D). Furthermore, we demonstrated that the increase in mature and immature blood neutrophils was similar in WT and Cebpg KO mice (Figure 2E,F). In addition, LPS treatment resulted in a general increase in SP cellularity and an increase in both mature and immature granulocytes, which was comparable in WT and Cebpg KO mice (Online Supplementary Figure S7). These experiments demonstrate that Cebpg deficient mice present a similar LPS-induced emergency granulopoiesis as WT mice, indicating that this transcription factor is dispensable for the response to this type of challenge.
Figure 2.
Cebpg KO mice respond normally to LPS injection. (A) Schematic representation of the experimental setup. WT and Cebpg KO mice were treated intraperitoneally (i.p.) with 1400μg/kg LPS at 0 and 48 hours. Analysis was performed 24 hours after last injection. (B) BM cellularity in WT and Cebpg KO mice treated with PBS vehicle control (black) or LPS (red). Y axis indicates the number of cells per hind leg. (C) Representative flow cytometry plots from WT and Cebpg KO BM cells 24 hours after last PBS or LPS injection. Cells were stained for Gr1 and CD11b cell surface markers. Green boxes indicate percentage of Gr1high CD11b+ cells and black boxes indicate percentage of Gr1low CD11b+cells. (D) Quantification of panel C. Graphs indicate relative (rel.) and absolute (abs.) number of Gr1high CD11b+ cells (left) and Gr1low CD11b+ cells (right). (E) Flow cytometric analysis of blood (BL) from WT and Cebpg KO mice 24 hours after last PBS or LPS injection. Green boxes indicate percentage of Gr1high CD11b+ cells and black boxes indicate percentage of Gr1low CD11b+ cells. (F) Quantification of panel E. Left graph indicates relative number of Gr1high CD11b+ cells and right graph indicates relative number of Gr1low CD11b+ cells. PBS vehicle control treatment indicated in black and LPS in red. Y axes indicate percentages (%). At least eight animals were included in each group. All data represent mean ±s.d. from three independent experiments. Two-tailed Student’s t-tests were used to assess statistical significance (ns: non-significant, **P<0.01, ****P<0.0001). BM: bone marrow; WT: wild-type; KO: knockout; LPS: lipopolysaccharide; PBS: phosphate-buffered saline.
Since we did not observe an effect of Cebpg deletion during LPS-induced emergency granulopoiesis, which is dependent on toll-like receptor 4,11,12 we proceeded to challenge mice with granulocyte-colony stimulating factor (G-CSF), a more general emergency granulopoiesis inducer (Online Supplementary Figure S8A). In agreement with published data,3,11 we observed that G-CSF alone is sufficient to accurately mimic emergency granulopoiesis. Of note, this chronic G-CSF treatment protocol did not result in significant changes in total BM cellularity, since G-CSF-induced granulopoiesis in the BM is accompanied by the mobilization of granulocytes to blood13(Online Supplementary Figure S8B). We showed similar responses in WT and Cebpg KO mice, as demonstrated by a decrease in relative and absolute number of mature Gr1high CD11b+ cells and an increase in immature Gr1low CD11b+ neutrophils in BM (Online Supplementary Figure S8C,D), whereas in SP we observed an increase in both mature and immature granulocytic populations (Online Supplementary Figure S8E-G). Thus, our results demonstrate that Cebpg KO mice respond to G-CSF-induced emergency granulopoiesis in a similar fashion as WT mice.
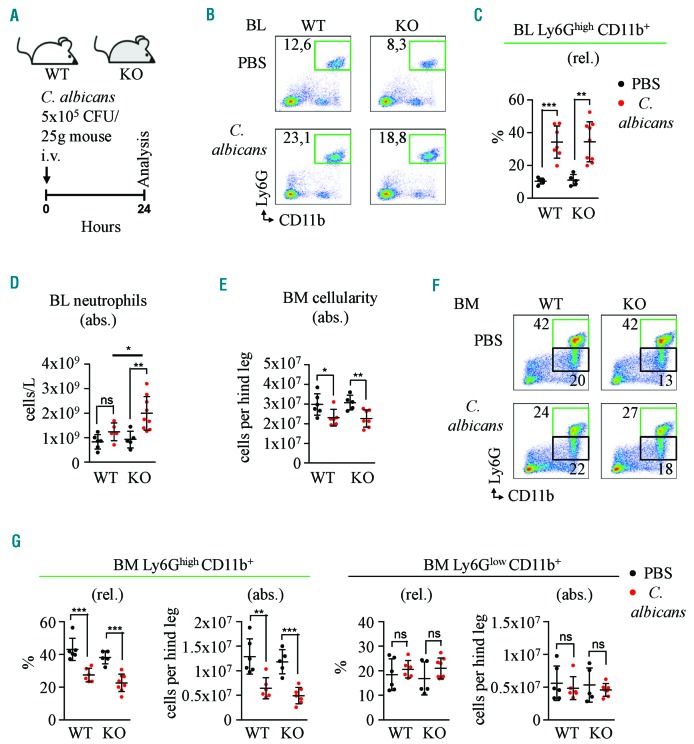
The presence of C/EBPγ:C/EBPβ heterodimers in BM and SP (Online Supplementary Figure S3) suggests that C/EBPγ may modulate C/EBPβ function. Since C/EBPβ plays a critical role in C. albicans-induced emergency granulopoiesis,4 we investigated whether ablation of Cebpg alters the response to fungal infection (Figure 3A). As expected, we observed an increase in the percentage of mature neutrophils in blood (Figure 3B,C) upon C. albicans administration, which was similar in WT and Cebpg KO mice. Interestingly, we detected a significant increase in absolute blood neutrophil cell counts in KO mice in comparison to control littermates (∼2-fold in KO vs. ∼1.5-fold in WT) (Figure 3D). This difference was not limited to neutrophils because higher cell counts were measured for the remaining leukocyte subsets as well (data not shown). Since higher egress of the BM cells could explain higher absolute cell counts in blood, we compared the reduction in BM cellularity between WT and KO mice following C. albicans treatment. However, no significant differences were observed (Figure 3E). Further, we showed that both WT and Cebpg KO mice presented typical hallmarks of emergency granulopoiesis (Figure 3F,G). Since it was reported that C/EBPβ is crucial for the amplification of immature hematopoietic cells after induction of systemic candidemia,4 we next assessed early granulocytic developmental stages in control mice treated with phosphate-buffered saline (PBS) and C. albicans infected mice. Although small differences were observed in the relative number of LKS upon treatment, detailed analysis of myeloid progenitors and distinct granulocytic differentiation stages revealed identical dynamics in relative and absolute cell counts in WT and Cebpg KO mice (Online Supplementary Figure S9 and Figure S10A,B). We then assessed whether the minor differences mentioned above are functionally relevant. However, we observed no significant differences in the microbiological outcome and survival rate in Cebpg KO mice compared to WT animals (Online Supplementary Figure S10C,D). These data suggest that C/EBPγ is not required for efficient emergency granulopoiesis induced by systemic candidemia.
Figure 3.
Cebpg deficient and WT mice respond to C. albicans infection in the same fashion. (A) Graphical representation of C. albicans infection in WT and Cebpg KO mice. Animals were treated once (t=0) intravenously (i.v.) with 5×105 CFU/25g body weight. Analysis was performed 24h after infection. (B–C) Representative flow cytometry plots (B) and corresponding quantification (C) from WT and Cebpg KO blood (BL) from mice treated with PBS or C. albicans. Green boxes indicate percentage of Ly6Ghigh CD11b+ cells. Graphic indicates relative number of Ly6Ghigh CD11b+ cells. Y axis represents percentage (%). (D) Number of neutrophils per L in peripheral BL determined by Auto Hematology Analyzer. WT and Cebpg KO mice were treated with PBS (black) or C. albicans (red). (E) BM cellularity in WT and Cebpg KO mice treated with PBS (black) or C. albicans (red). Y axis indicates the number of cells per hind leg. (F) Representative flow cytometry plots from WT and Cebpg KO BM cells. Cells were stained for Ly6G and CD11b cell surface markers. Green boxes indicate percentage of Ly6Ghigh CD11b+ cells and black boxes indicate percentage of Ly6Glow CD11b+ cells. (G) Quantification of panel F. Left graphs indicate relative (rel.) and absolute (abs.) number of Ly6Ghigh CD11b+ cells. Right graphs indicate rel. and abs. number of Ly6Glow CD11b+ cells. Each group contains at least five mice. All data represent mean ±s.d. from two independent experiments. Two-tailed Student’s t-tests were used to assess statistical significance (ns: non-significant, *P<0.05, **P<0.01, ***P<0.001). BM: bone marrow; WT: wild-type; KO: knockout; BL: blood.
Taken together, our data reveal that C/EBPγ is dispensable for steady-state and emergency granulopoiesis. This is in sharp contrast to the results obtained with a previously reported Cebpg KO murine model generated by Kaisho et al.,14 in which mice exhibited a high mortality rate within 48h after birth. However, Cebpg ablation in this model was not limited to the hematopoietic system, and it was suggested that the early neonatal death could be caused by lung lesions.14 On the contrary, in our conditional KO mice, C/EBPγ expression is abolished mainly in hematopoietic cells, which makes these animals optimal for studying C/EBPγ function in the hematopoietic system.
In summary, the data presented herein demonstrate that specific deletion of Cebpg in the murine hematopoietic system did not alter hematopoietic stem and progenitor cell properties, their ability to commit to the myeloid lineage, and produce granulocytes in normal and stress conditions. As a whole, these surprising observations point to a transcription factor redundancy responsible for controlled production of granulocytes in steady-state and emergency granulopoiesis.
Supplementary Material
Acknowledgements:
The authors would like to thank the Flow Cytometry Core Facility, IMG AS CR, Prague, Czech Republic for their support with the flow cytometry and sorting presented herein. We would like to thank Dr. P. Johnson and Dr. J. Salotti for advice in EMSA assays and Dr. V. Korinek for his technical support.
Footnotes
Funding: the study was supported by a GACR grant (18-08577S) and institutional funding from the IMG AS CR (RVO 68378050) to MA-J, a FAPESP grant (2015/21866-1) to LLF-P, and by a GA UK fellowship (project No. 341015) from Charles University in Prague to MK. DGT was supported by a STaR Investigator Award, an RCE Core grant, a Tier 3 RNA Biology Center grant MOE2014-T3-1-006 from the NRF and MOE, Singapore; and NIH/NCI grant P01 CA66996. This work used instruments provided by C4Sys infrastructure and it was also supported by the Czech Centre for Phenogenomics (CCP, project no. LM2015040), and OP RDI CZ.1.05/2.1.00/19.0395 (Higher quality and capacity for transgenic models).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Bugl S, Wirths S, Radsak MP, et al. Steady-state neutrophil homeostasis is dependent on TLR4/TRIF signaling. Blood. 2013;121(5):723–733. [DOI] [PubMed] [Google Scholar]
- 2.Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14(5):302–314. [DOI] [PubMed] [Google Scholar]
- 3.Hirai H, Zhang P, Dayaram T, et al. C/EBPbeta is required for ‘emergency’ granulopoiesis. Nat Immunol. 2006;7(7):732–739. [DOI] [PubMed] [Google Scholar]
- 4.Satake S, Hirai H, Hayashi Y, et al. C/EBPbeta is involved in the amplification of early granulocyte precursors during candidemia-induced “emergency” granulopoiesis. J Immunol. 2012;189(9):4546–4555. [DOI] [PubMed] [Google Scholar]
- 5.Zhang DE, Zhang P, Wang ND, Hetherington CJ, Darlington GJ, Tenen DG. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc Natl Acad Sci U S A. 1997;94(2):569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alberich-Jorda M, Wouters B, Balastik M, et al. C/EBPgamma dereg ulation results in differentiation arrest in acute myeloid leukemia. J Clin Invest. 2012;122(12):4490–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roman C, Platero JS, Shuman J, Calame K. Ig/EBP-1: a ubiquitously expressed immunoglobulin enhancer binding protein that is similar to C/EBP and heterodimerizes with C/EBP. Genes Dev. 1990;4(8):1404–1415. [DOI] [PubMed] [Google Scholar]
- 8.Huggins CJ, Malik R, Lee S, et al. C/EBPgamma suppresses senescence and inflammatory gene expression by heterodimerizing with C/EBPbeta. Mol Cell Biol. 2013;33(16):3242–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huggins CJ, Mayekar MK, Martin N, et al. C/EBPgamma as a critical regulator of cellular stress response networks through heterodimerization with ATF4. Mol Cell Biol. 2015;36(5):693–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boettcher S, Gerosa RC, Radpour R, et al. Endothelial cells translate pathogen signals into G-CSF-driven emergency granulopoiesis. Blood. 2014;124(9):1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boettcher S, Ziegler P, Schmid MA, et al. Cutting edge: LPS-induced emergency myelopoiesis depends on TLR4-expressing non-hematopoietic cells. J Immunol. 2012;188(12):5824–5828. [DOI] [PubMed] [Google Scholar]
- 12.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–2088. [DOI] [PubMed] [Google Scholar]
- 13.Lieschke GJ, Grail D, Hodgson G, et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84(6):1737–1746. [PubMed] [Google Scholar]
- 14.Kaisho T, Tsutsui H, Tanaka T, et al. Impairment of natural killer cytotoxic activity and interferon gamma production in CCAAT/enhancer binding protein gamma-deficient mice. J Exp Med. 1999;190(11):1573–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.