Abstract
Blood erythropoietin (EPO) increases primarily to hypoxia. In sickle cell anaemia (homozygous HBBE6V; HbSS), plasma EPO is elevated due to hemolytic anaemia-related hypoxia. Hydroxyurea treatment reduces haemolysis and anaemia by increasing foetal haemoglobin, which leads to lower hypoxic transcriptional responses in blood mononuclear cells but paradoxically further increases EPO. To investigate this apparent hypoxia-independent EPO regulation, we assessed two sickle cell disease (SCD) cohorts for genetic associations with plasma EPO, by prioritizing 237,079 quantitative trait loci for expression level and/or transcript isoform variations of 12,727 genes derived from SCD blood mononuclear cells. We found an association between the T allele of SNP rs60684937 and increased plasma EPO (n = 567, combined P = 5.5 × 10 − 8 adjusted for haemoglobin and hydroxyurea) and validated it in independent SCD patients (n = 183, P = 0.018). The T allele of rs60684937 was associated with a relatively increased expression of a non-coding transcript of PRKAR1A (cAMP-dependent protein kinase type I-alpha regulatory subunit) in 58 SCD patients (P = 7.9 × 10 − 7) and 58 HapMap Yoruba samples (P = 0.0011). In conclusion, we demonstrate that plasma EPO elevation with hydroxyurea in SCD is independent of hypoxic responses and that genetic variation at SNP rs60684937 may contribute to EPO regulation through a cAMP-dependent protein kinase A pathway.
Introduction
Circulating erythropoietin (EPO) binds to its high affinity receptor (EPOR) on the cell surface of erythroid progenitors in the bone marrow to initiate cellular signalling that retards apoptosis of erythroid progenitors and enables their proliferation and differentiation into reticulocytes (1,2). During foetal development EPO is produced in the liver and, after birth, primarily in the kidneys (3) in a subset of peritubular fibroblast-like interstitial cells in the renal cortex (4,5). The regulation of EPO expression, which spatially and temporally parallels protein synthesis, is mediated by tissue-specific factors and hypoxia (6). Low oxygen tension stabilizes hypoxia inducible factor (HIF)-α subunits, which associate with constitutive HIF-β to form heterodimers that bind to the HIF response elements of target genes including EPO (7,8). Hypoxia dependent EPO gene expression primarily requires HIF-2α rather than HIF-1α (9) in both kidney and liver (10–13).
Sickle cell disease (SCD) is due to homozygosity for a mutation in the HBB gene (HBBGlu6Val; haemoglobin S) or to compound heterozygosity for HBBGlu6Val and other HBB point mutations or β thalassaemia. Polymerization of haemoglobin S in sickle cell anaemia (SCA or HbSS) (14) causes rigid, distorted erythrocytes that have a short intravasular life-span and are prone to promote microvascular occlusion through a variety of mechanisms (15). EPO is elevated in SCD due to anaemia- and microvascular occlusion-associated hypoxia (16). Hydroxyurea treatment in SCA increases the production of foetal haemoglobin or haemoglobin F, which interferes with haemoglobin S polymerization and ameliorates clinical complications (17,18). A number of investigators have observed paradoxically higher serum EPO levels with hydroxyurea treatment in SCD despite higher haemoglobin concentrations (19–22). In this study, we demonstrated an association of hydroxyurea treatment with lower hypoxic transcriptional responses in SCD, excluding the possibility that increased haemoglobin F may exacerbate tissue hypoxia due to its high affinity for oxygen (23). We further investigated the genetic basis of the inferred, hypoxia-independent EPO regulation.
Results
Hydroxyurea treatment is associated with lower hypoxic transcriptional responses in HbSS individuals
The overall study design is illustrated in Figure 1. We profiled gene expression in blood mononuclear cells using Affymetrix Human Exon 1.0 ST Array. To assess gene expression difference in HbSS individuals that was hypoxia-induced relative to HbAA control individuals, we used as reference the gene expression difference detected in Chuvash Polycythemia patients with homozygous VHLR200W, a germ line mutation that causes constantly elevated hypoxic responses (24,25) (see Supplementary Material, Table S1 for sample information). In the absence of hydroxyurea treatment, gene expression variation in HbSS correlated closely with variation associated with homozygous VHLR200W (Pearson’s r = 0.79, Fig. 2A). The strong correlation does not appear to be caused by stochasticity in the sample structure (Supplementary Material, Fig. S1). For 377 genes detected at a false discovery rate (FDR) of 5% with >1.2 fold-change in both VHLR200W homozygotes and HbSS individuals, the correlation of expression variations between the two mutations reached r = 0.97. Hydroxyurea treatment in general moderated the expression difference caused by HbSS (r=-0.85, Fig. 2B), especially for the 377 genes that share altered regulation with VHLR200W homozygotes (r=-0.95), suggesting that hydroxyurea treatment decreases hypoxic transcriptional responses in mononuclear cells in sickle cell patients.
Figure 1.
An illustration of the study design.
Figure 2.
Hydroxyurea therapy is associated with lower global hypoxic transcriptional responses induced by HbSS. (A) Predominant hypoxic transcription in the HbSS patients. The log2 fold changes of gene expression in HbSS were plotted against those in VHLR200W homozygotes. (B) Hydroxyurea treatment is associated with lower hypoxic transcription. The log2 fold changes of gene expression associated with hydroxyurea treatment among HbSS patients were plotted against those induced by HbSS relative to HbAA control individuals in the absence of hydroxyurea treatment. (C) The same as (B) except that the log2 fold changes of gene expression were obtained by adjusting for covariates and cell type counts of blood mononuclear cells. (D) Increased haemoglobin F with hydroxyurea treatment is associated with lower hypoxic transcription in SCD, tested in the UIC expression cohort that has haemoglobin F measurements available. In (A)–(D) the blackcolored genes were those detected at 5% FDR and showed >1.2 fold change in both HbSS and VHLR200W homozygotes.
Blood mononuclear cells are largely comprised of lymphocytes and monocytes with very small proportions of hematopoietic progenitors and in sickle patients with small proportions of nucleated red blood cells (NRBCs) that are absent in healthy individuals. To assess the expression difference in HbSS individuals accounting for cell type variations of blood mononuclear cells, we further analysed gene expression data by adjusting for cell counts of lymphocytes, monocytes and NRBCs using a linear regression model. The correction toned down the expression increase in HbSS for several genes with erythroid function, such as SLC4A1 and AHSP, which is consistent with the presence of NRBCs in HbSS but not control individuals. The overall expression difference in HbSS and the relatively reduced expression difference associated with hydroxyurea treatment remained (r=-0.77 across all analysed genes and r=-0.86 for the 377 genes that share altered regulation with VHLR200W homozygotes, Fig. 2C).
To further assess the responsiveness of gene expression in mononuclear cells to hypoxia status of HbSS individuals, the correlation between gene expression and haemoglobin concentration was examined in HbSS individuals without hydroxyurea treatment, for whom haemoglobin level serves as an indicator of anaemia and hypoxia. The analysis was carried out in an independent cohort (Supplementary Material, Table S2) for more robust conclusions. Among the 377 hypoxic genes, those that showed greater increase in HbSS relative to HbAA also showed greater increase with lower haemoglobin concentrations in non hydroxyurea-treated HbSS individuals (r=-0.77, Supplementary Material, Fig. S2). As decreasing haemoglobin concentration indicates increasing hypoxia, the observation suggests that expression in mononuclear cells effectively reflects hypoxia status of HbSS individuals. Hydroxyurea treatment is associated with increased haemoglobin F production, increased haemoglobin concentration and decreased white blood cell counts (18). We further examined the correlation between expression levels of the 377 hypoxic genes and variation of haemoglobin F in HbSS individuals regardless of hydroxyurea treatment, adjusting for age, gender, haemoglobin concentration, and white blood cell counts. Genes up-regulated by hypoxia were in general expressed at lower levels with haemoglobin F and vice versa (r=−0.90, Fig. 2D). The observation excludes the possibility of haemoglobin F causing tissue hypoxia due to its high oxygen affinity (23) and instead suggests a release from hypoxic stress with hydroxyurea treatment in SCD.
Hydroxyurea treatment is paradoxically associated with higher plasma EPO level
As hydroxyurea treatment ameliorates hypoxia while EPO is a hypoxia-induced gene, hydroxyurea treatment is expected to suppress EPO. Several previous studies (19,21) observed, however, that plasma EPO levels are higher in hydroxyurea-treated SCD patients. This is confirmed by our analysis in the Walk-PHaSST (eβ = 1.48 IU/l, P = 4.4 × 10 − 10, n = 591) and PUSH (eβ = 1.33 IU/l, P = 2.0 × 10 − 4, n = 396) SCD cohorts. We further analysed the data by adjusting for variation in haemoglobin concentration that serves as an indicator of variation in oxygen delivery. The higher plasma EPO levels with hydroxyurea treatment persisted and were strengthened after the adjustment (P = 2.5 × 10 − 15 in Walk-PHaSST and P = 2.5 × 10 − 7 in PUSH, see Table 1), suggesting that the effect of hydroxyurea treatment on blood EPO level is independent of the magnitude of hypoxic responses. As EPO is not stored, elevated plasma EPO levels correlate with an increase of mRNA through increased transcription or transcript stability.
Table 1.
Hydroxyurea treatment increases EPO levels in SCD. Using a linear model, log(EPO) was regressed on age (year), gender, square root of haemoglobin concentration (g/dl), hydroxyurea treatment and clinical sites. Exponent of βs and P values are shown
| Walk-PHaSST (n = 586) |
PUSH (n = 387) |
|||
|---|---|---|---|---|
| eβ (IU/l) | P | eβ (IU/l) | P | |
| age | 1.01 | 0.0018 | 1.00 | 1 |
| female gender | 0.79 | 2.7 × 10−5 | 0.93 | 0.2 |
| haemoglobin | 0.44 | <2.0 × 10−16 | 0.16 | <2.0 × 10−16 |
| hydroxyurea | 1.62 | 2.5 × 10−15 | 1.41 | 2.5 × 10−7 |
Hypoxia-independent genetic regulation of plasma EPO levels in SCD
To identify potential factors controlling plasma EPO levels in SCD, we investigated the genetic regulation in 326 SCD patients from the Walk-PHaSST cohort and 241 SCD patients from the PUSH cohort (Supplementary Material, Table S3). Genotypes of single nucleotide polymorphisms (SNPs) were imputed to 1000 genomes phase 1 data for both cohorts, with the average imputation concordance rate estimated to be 0.95 (Supplementary Material, Fig. S3). Genetic dosages of common SNPs with minor allele frequency (MAF) >0.1 and imputation dosage r2 >0.8 were tested for association with plasma EPO levels by linear regression, adjusting for age, gender, clinical severity of HBB genotype (HbSS and HbSβ0-thalassaemia versus HbSC and HbSβ+-thalassaemia), hydroxyurea treatment, clinical sites, population stratification, and concentrations of haemoglobin subtypes (haemoglobin S, haemoglobin F, haemoglobin A and haemoglobin C) if available to count for variation in the hypoxic responses. Patients treated with recombinant EPO products were excluded from the analysis. Patients with serum creatinine level ≥1.1 mg/dL were also excluded from analysis, as kidney dysfunction is known to affect EPO production (26). The association P values from the two cohorts were combined using the inverse variance approach.
To leverage our cohorts and to facilitate biological interpretation, we prioritized SNPs by focusing on local expression quantitative trait loci (eQTL), defined as SNPs that associate with the expression levels of genes located <1Mb away at genome-wide FDR of 5%. We mapped eQTL of overall gene expression levels and transcript isoform variations (Materials and Methods) in 58 SCD patients (Supplementary Material, Table S4). We identified 12,915 unique SNPs associated with overall expression levels of 638 genes, and 863,175 unique SNPs associated with transcript isoform variations of 12,606 genes. Pruning the associations at r2 >0.8 resulted in 1,453 and 265,010 unique SNPs for overall gene expression level and transcript isoform variations, respectively. Combining the two lists of eQTL, 237,079 unique SNPs passed the imputation quality threshold (dosage r2 > 0.8) across the Walk-PHaSST and PUSH cohorts, which were tested for association with plasma EPO levels as described above. The association results are included in Supplemental Information.
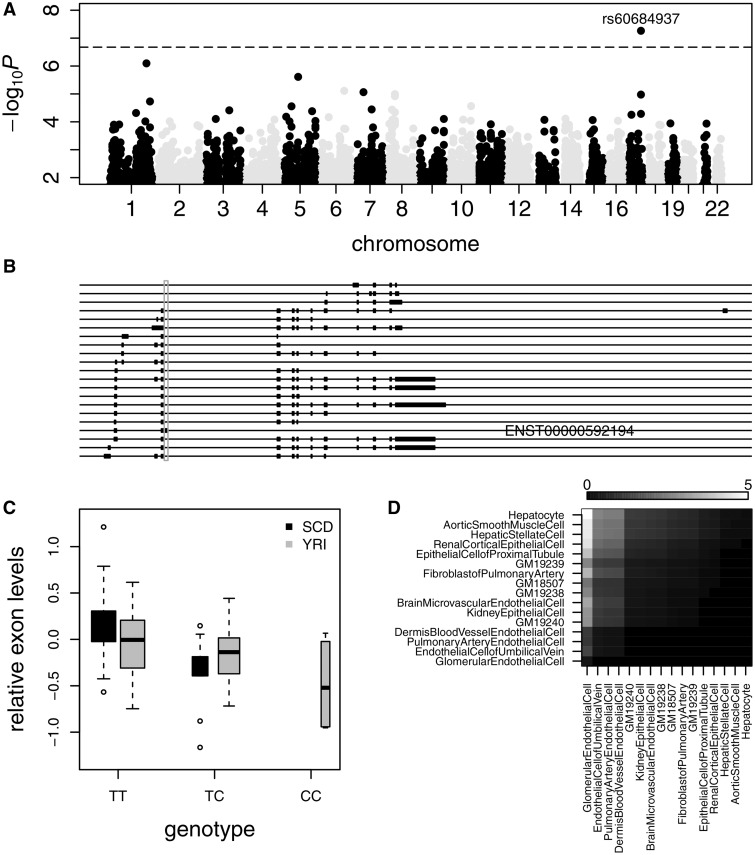
We found that SNP rs60684937, located at 67,419,130 bp on chromosome 17, was significantly associated with plasma EPO levels at Bonferroni corrected P <0.05 (combined P = 5.5 × 10 − 8, Fig. 3A). The T allele of the SNP was associated with increased EPO levels in both Walk-PHaSST (eβ = 1.34 IU/l, P= 1.2 × 10 − 5) and PUSH (eβ = 1.26 IU/l, P= 1.7 × 10 − 3) cohorts (Table 2). Between patients with and those without hydroxyurea treatment, the genetic effect appeared to be consistent (Supplementary Material, Table S5). The association was validated in additional adult SCD patients from the Howard cohort (eβ = 1.41 IU/l, P = 0.029, n = 79) and the UIC cohort (eβ = 1.18 IU/l, P = 0.25, n = 104) (Table 2). We experimentally validated 21/22 TT, 5/6 TC and 0/1 CC imputed genotypes at rs60684937 for 29 randomly chosen individuals from the Walk-PHaSST cohort (Materials and Methods). The T allele of rs60684937 is the ancestral allele and has a frequency of 0.83 and 0.85 in the Walk-PHaSST and PUSH cohorts, respectively. In the 1000 genomes phase 1 data the frequency of the T allele is 0.85 among the Americans of African Ancestry in South West USA data and variable across other population samples (0.93 Europeans, 0.86 Americans, 0.83 Africans, 0.56 Asians).
Figure 3.
SNP rs60684937 is associated with plasma EPO levels in SCD and the relative expression levels of a non-coding transcript of PRKAR1A. (A) The -log10 P-values of plasma EPO association were plotted along the chromosomes. Horizontal line represents Bonferroni corrected P = 0.05. (B) The transcript isoforms of PRKAR1A annotated by gencode version 19. The transcribed region associated with rs60684937 was marked by grey box. (C) The relative expression levels of the PRKAR1A exon, log2(exon/gene), were plotted against the genotypes of rs60684937, for SCD (black box) and YRI (grey box) samples. The expression levels were adjusted by age, gender, Hb genotype and hydroxyurea treatment in SCD or the first 13 principal components in YRI samples. (D) The relative enrichment of DNase hypersensitivity peaks derived from various cell types in the LD block of rs60684937 (r2 > 0.3). For each column, the corresponding cell type was compared with each of other cell types by one-sided binomial test, for the alternative hypothesis that the other cell type had less enrichment. Cell types were ordered to the level of significance and –log10P from the pair-wise comparisons are shown with colour key. From the most to the least enriched cell types were glomerular endothelial cell, endothelial cell of the umbilical vein, pulmonary artery endothelial cell, dermis blood vessel endothelial cell, kidney epithelial cell, brain microvascular endothelial cell, fibroblast of pulmonary artery, epithelial cell of the proximal tubule, renal cortical epithelial cell, hepatic stellate cell, aortic smooth muscle cell, and hepatocyte. Four HapMap lymphoblastoid cell lines from YRI samples (GM18507, GM19238-GM19240) are also included for comparison.
Table 2.
Association of the effect allele (T) of rs60684937, located at chr 17 at 67419130 bp, with plasma EPO levels in GWAS. dr2: imputation dose r2. eβ: exponent of β
| effect allele frequency | dr2 | eβ (IU/l) | P | exponent of combined β (IU/l) | combined P | |
|---|---|---|---|---|---|---|
| Walk-PHaSST (n = 326) | 0.83 | 0.88 | 1.34 | 1.2 × 10−5 | 1.31 | 5.5 × 10−8 |
| PUSH (n = 241) | 0.85 | 0.9 | 1.26 | 0.0017 | ||
| Howard (n=79) | 0.84 | 0.88 | 1.41 | 0.029 | 1.28 | 0.018 |
| UIC (n = 104) | 0.86 | 0.91 | 1.18 | 0.25 |
rs60684937 is associated with a relatively increased level of a non-coding transcript of PRKAR1A
The linkage disequilibrium (LD) block of rs60684937 (r2 = 0.5) spans a 7.3kb region within the first intron of MAP2K6 gene that encodes mitogen-activated protein kinase kinase 6. The SNP showed no association with either the overall expression level (nominal P = 0.7) or transcript isoform variation (nominal P range 0.1–0.9) of MAP2K6 in blood mononuclear cells. Instead, the SNP was significantly associated with the expression level of a transcribed region of PRKAR1A (Protein Kinase, cAMP-Dependent, Regulatory, Type I, Alpha) gene, specific to the non-coding transcript ENST00000592194 (Ensembl transcript ID, Fig. 3B). The T allele of rs60684937 is associated with increased expression of the non-coding transcript relative to other PRKAR1A transcripts (β = 0.47, P = 7.9 × 10 − 7) in the 58 SCD eQTL mapping samples (Fig. 3C). The association between the T allele of rs60684937 and the relatively increased expression of the non-coding transcript among PRKAR1A transcripts was validated in lymphoblastoid cell lines of 58 Yoruba in Ibadan, Nigeria (YRI) population samples from HapMap (β = 0.13, P = 0.0011) (Fig. 3C).
The association of the T allele of rs60684937 with isoform variation in PRKAR1A and with plasma EPO suggests a correlation between the relative expression of the non-coding transcript and EPO levels. Relative to the other exons in PRKAR1A, the expression levels of the rs60684937-associated exon were higher with higher plasma EPO concentrations (exon by plasma EPO interaction effect: β = 0.094, P = 0.037, n = 42 in VHLR200W homozygotes; β = 0.073, P = 0.2, n = 32 in SCD patients) and with hydroxyurea treatment (exon by hydroxyurea interaction effect: β = 0.11, P = 0.2, n = 32 in SCD patients) (Materials and Methods), complying with the effect of rs60684937 on PRKAR1A isoform variation and plasma EPO levels.rs60684937 is located ∼900 kb downstream of PRKAR1A, while the extended LD block (r2 > 0.1) of the SNP only covers an ∼200 kb region, implying potential long-range regulation of the causal variant on PRKAR1A isoform expression. We reasoned that a genetic locus enriched with active regulatory elements derived from a specific cell type is likely to have an effect in that cell type (27). To explore the target cell types additional to blood mononuclear cells for the detected genetic locus, we tested the relative enrichment in the ∼30 kb LD block (r2 > 0.3) of rs60684937 for DNase hypersensitivity peaks derived from a variety of cell types in the Encyclopedia of DNA Elements (ENCODE) project, including cell types originating from kidney, liver, lung, brain, muscle, skin and umbilical vein. Relative to lymphoblastoid cell lines, the 30 kb region was more enriched with DNase hypersensitivity peaks derived from endothelial cells, particularly glomerular endothelial cells (Fig. 3D). Since there are no DNase hypersensitivity data from peritubular fibroblast-like interstitial cells, it is unclear whether active regulatory regions from these renal EPO-producing cells overlap with the detected genetic locus.
Discussion
We found that hydroxyurea treatment in SCD is associated with lower hypoxic transcriptional responses, but paradoxically this treatment is associated with higher plasma EPO levels. We then hypothesized that factors additional to hypoxia may regulate EPO transcription and/or transcript stability in the setting of hydroxyurea therapy. We found and validated a genetic variation distant to the EPO gene that associates with the plasma EPO level and that locally regulates the isoform variation of PRKAR1A.
We observed an apparent suppressive effect of hydroxyurea treatment on hypoxic transcriptional responses in blood mononuclear cells, consistent with known effect of hydroxyurea treatment in reducing anaemia and improving red blood cell rheology and oxygen delivery (28). The suppressive effect of hydroxyurea treatment on hypoxic gene expression remained after adjustment for inter-individual heterogeneity in blood mononuclear cell type composition, demonstrating the robustness of the hypoxic gene expressions derived from blood mononuclear cells. The hydroxyurea treatment-associated higher plasma EPO levels, occurring with lesser degree of anaemia and coexistent lower HIF activity, implies a distinct mechanism of EPO transcriptional regulation independent of, and other than, HIF.
Previous studies have demonstrated that eQTL are more likely to be associated with complex traits and diseases compared to random SNPs (29,30). Our comparison of genetic associations of EPO using genome-wide SNPs and using eQTL derived from blood mononuclear cells of SCD patients appears to support this conclusion (Supplementary Material, Fig. S4). In principle, eQTL derived from cell types and environmental conditions as relevant as possible to the studied phenotype are preferred, which is however often compromised by the availability of such eQTL data. Significant proportions of both shared and tissue/cell type specific eQTL were observed in previous studies (27,31,32), with the definition of the two being more or less arbitrary considering that eQTL exhibit quantitative variation in effect size among distinct tissue/cell types (33). As demonstrated in this study, gene expression in blood mononuclear cells showed robust transcriptional responses to the anaemic/hypoxic condition that characterizes SCD. We expect that a substantial proportion of eQTL derived from blood mononuclear cells may affect gene regulation in diverse physiological processes in SCD, including EPO production.
EPO gene expression depends on a suite of cis regulatory elements, for example the 50 bp downstream enhancer (34) that contains several elements including an hepatocyte nuclear factor 4 (HNF-4) element (35–37), and trans factors including HNF-4 to augment hypoxia inducibility (38,39). Here, we show that the ancestral T allele of rs60684937 is associated with increased plasma EPO and relatively increased level of a non-coding transcript of PRKAR1A gene that encodes the type 1a regulatory subunit of cAMP-dependent protein kinase A (PKA). A relative increase in the non-coding transcript may reflect a shift among PKA isoforms with different activities, thereby modulating HNF-4 binding to HNF-4 element (40) in the EPO downstream enhancer. Altered PKA activity may affect other transcription factors as well, for example retinoic acid receptor-alpha (41) that was shown to stimulate EPO transcription through binding to the HNF-4 element in mouse cell lines (42). Under hydroxyurea treatment, intracellular cAMP levels may be altered (43) which in turn may modulate PKA signalling and plasma EPO concentration. Although it is unknown whether chemotherapy can suppress PRKAR1A expression, a variety of malignancies display over-expression of PRKAR1A (44) and cancer chemotherapy reportedly induces a transient increase in serum EPO (45–47).
Genetic association using eQTL directly related rs60684937 to transcript isoform variation in PRKAR1A. Although no significant association was found between rs60684937 and the expression of its most proximate gene, MAP2K6, in mononuclear cells, we could not exclude the possibility that the causal variant may affect MAP2K6 regulation or function. MAP2K6 activates p38 mitogen-activated protein kinase in response to environmental stress (48), which is essential for HIF-1 activation under hypoxic conditions (49). Selective activation among p38 isoforms by MAP2K6 appeared to regulate some downstream gene expression (50). Functional studies are needed to elucidate the molecular mechanisms underlying the identified genetic association with plasma EPO.
Materials and Methods
Study samples
The study was approved by the IRBs of the participating institutions and all subjects provided written informed consent. The study included three GWAS (Supplementary Material, Table S3) and four expression (Supplementary Materials, Tables S1, S2, S4) cohorts.
Gene expression analysis
Messenger RNA from peripheral blood mononuclear cells was profiled using Affymetrix Human Exon 1.0 ST Array (51) or Affymetrix Human gene 2.0 ST array (52) as described in previous studies. Probe sequences were aligned to human genome assembly GRCh37 allowing ≤1 mismatches (53) to select those with a perfect unique match. Probes that interrogated multiple genes or that contained single nucleotide polymorphisms (SNPs) with ≥1% minor allele frequency (MAF) in 1000 genomes data of European or African population were removed. Annotation was based on Gencode release 19. Probe level intensities were log2 transformed, background corrected (54) and quantile normalized (55). Probe intensity was subtracted by the corresponding probe mean across samples. Gene-level expression intensities were summarized as mean probe intensity within each gene.
Hypoxic gene expression was analysed by comparing 15 Chuvash Polycythemia patients, characterized by a homozygous VHLR200W mutation that causes constitutive hypoxic responses, versus 17 Chuvash control individuals. Transcriptional alteration in HbSS was determined in 13 HbSS subjects without hydroxyurea treatment versus 16 African American control individuals, and validated by quantitative RT-PCR (51). Gene expression in 19 HbSS subjects with hydroxyurea treatment was compared with 13 subjects without treatment. Age and gender were matched in all comparisons (see Supplementary Material, Table S1 for more information about the samples). Analysed samples were restricted to those with serum ferritin concentration ≥21 μg/L to avoid a confounding effect of iron deficiency that may cause hypoxic responses. To detect differential expression, for each gene a d-statistic was calculated. The d-statistic is a modified t-statistic that stabilizes statistical variance for low expression genes thereby improving across-gene comparison (56). For cell-count adjusted analysis in sickle cell disease (SCD) patients, gene expression levels were regressed on HbSS genotype or hydroxyurea treatment, together with variations in age, gender, absolute cell counts of nucleated red blood cells, lymphocytes and monocytes. FDR was estimated by 100 permutations (57). Multiple test correction was performed using the Benjamini and Hochberg method (58).
Genotype data processing and imputation
Genomic DNA isolated from peripheral blood mononuclear cells was labelled and hybridized to the Illumina Human 610-Quad SNP array (Walk-PHaSST, PUSH, Howard) or Affymetrix Axiom genome-wide Pan-African GeneChip array (UIC). Samples having a genotype rate <95% were removed. SNPs deviating from Hardy Weinberg equilibrium (P <0.0001) or with a minor allele frequency (MAF) <0.01 were removed. Population outliers were identified based on principal components analysis (59) of the genotype data. Proportion of identity-by-descent was calculated pair-wise using PLINK (60) to identify related individuals ( ≥0.125) and potentially contaminated DNA samples (mean ≥0.033). SNP genotypes were imputed to 1000 genomes project phase 1 data, with European and African reference panels, using Beagle (61) version 4. To provide an empirical estimation of the imputation quality for each array platform, we randomly masked 5% of array-genotyped SNPs on chromosome 22, and re-imputed chromosome 22 with the same parameters we used for whole genome imputation (Supplementary Material, Fig. S3). SNPs with dosage r2 ≤0.8 or MAF ≤0.1 within each cohort were excluded from association analysis. Walk-PHaSST patients from the Howard University site were selected out for validation, referred to as a Howard University cohort, while the remaining Walk-PHaSST patients were referred to as Walk-PHaSST cohort.
Experimental validation of rs60684937genotypes
We randomly selected 29 frozen DNA samples from the Walk-PHaSTT cohort. A 480bp fragment spanning rs60684937 site was amplified from 10-100 ng of DNA with primers (5’-CACT CC CAAGGCTGTTGTCA-3’ and 5’-CCTGGCCAA CATG GTG AA AC-3’) at a concentration of 200 nM each in a 20ul reaction, using Platinum Taq DNA polymerase (life technologies). PCR conditions were as follows: one cycle of 95 °C for 3 min, 40 cycles of 95 °C for 30 s, 63 °C for 30 s, and 72 °C for 30 s, one cycle of 72 °C for 15 min. PCR products were cleaved with 5 units of DpnII (New England Biolabs) at 37 °C for 2 h and were resolved on a 3% Agarose gel. DpnII cuts the amplified fragments differentially at the rs60684937 site, with TT genotype showing bands at 110 and 40 bp while CC genotype showing band at 150 bp.
Meta-analysis
A linear regression model was applied adjusting for variations in age, gender, haemoglobin concentrations, hydroxyurea treatment and clinical sites. The proportion of European ancestry using the first principal component of genotype data was also adjusted, since it was the primary source of population structure. Combined P values were estimated using an inverse variance approach (62), and adjusted by Bonferroni correction. The genomic inflation factor is 1.0, suggesting no significant population stratification.
Expression quantitative traits
Expression data of 35 samples from a Howard University cohort were pooled with 23 samples from University of Chicago cohort. Batch effect of gene expression levels were adjusted by ComBat (63). Genotype processing and imputation were carried out as described above. To map eQTL for overall gene expression levels, SNPs <1Mb away from target genes were tested for association with expression levels. The top 16 principal components were regressed out from gene expression levels to account for potential covariates. P values were adjusted by BH approach (58). To map eQTL for transcript isoform variations, SNP <1Mb away from target genes were tested for SNP by exon interaction effects, using a previously published approach (64). Within each gene, exon expression levels across exons and samples were fitted by a linear model:
Here denotes length ( exon expression levels. denotes by exon identity matrix. denotes exon main effects. denotes alternative allele dosages across samples at a given SNP. denotes SNP main effect. denotes identity for the exon. denotes the interaction effect between the exon and the tested SNP. denotes random errors. P value for the interaction effect was estimated by likelihood ratio test comparing with a reduced model without the SNP by exon interaction effect. The top 17 principal components estimated from exon-level data were regressed out to account of potential covariates. P values were adjusted by BH approach. For 58 of the Yoruba in Ibadan, Nigeria (YRI) samples (65), eleven principal components derived from exon expression levels were regressed out before testing for exon-specific SNP associations. To test for exon by EPO interaction effect in homozygous VHLR200W individuals and HbSS patients or for exon by hydroxyurea treatment interaction effect in HbSS patients, the above mentioned linear model was fitted to the exon level data, with the genetic dosage being replaced by EPO or hydroxyurea treatment.
Enrichment of Detected Genetic Locus with Encode DNAse Hypersensitivity Peak
The LD block (r2 > 0.3) of rs60684937 covers ∼30kb region. For each cell line, DNase hypersensitivity peaks were classified as either within or outside of the 30kb region. To assess the relative enrichment of DNase hypersensitivity peaks in the 30kb region among cell lines, each cell line was treated as a reference and compared with each of the others using one-sided binomial test, for the alternative hypothesis that the peaks derived from the other cell line were less overlapped with the 30kb region.
Supplementary Material
Supplementary Material is available at HMG online.
Conflict of Interest statement. None declared.
Funding
This work is supported in part by National Institutes of Health (NIH) grants R01 HL079912-04, 2 R25-HL03679-08, and 1P30HL107253 (V.R.G.); 1P50HL118006 (S.N.); R01HL111656 and R01HL127342 (R.F.M.); KL2TR000048 and K23HL125984 (S.L.S); UL1TR000050 (UIC Center for Clinical and Translational Science).
Supplementary Material
References
- 1. Bunn H.F. (2013) Erythropoietin. Cold Spring Harb. Perspect. Med., 3, a011619.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koury M.J., Bondurant M.C. (1990) Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science, 248, 378–381. [DOI] [PubMed] [Google Scholar]
- 3. Erslev A.J., Caro J., Besarab A. (1985) Why the kidney?. Nephron, 41, 213–216. [DOI] [PubMed] [Google Scholar]
- 4. Maxwell P.H., Osmond M.K., Pugh C.W., Heryet A., Nicholls L.G., Tan C.C., Doe B.G., Ferguson D.J., Johnson M.H., Ratcliffe P.J. (1993) Identification of the renal erythropoietin-producing cells using transgenic mice. Kidney Int., 44, 1149–1162. [DOI] [PubMed] [Google Scholar]
- 5. Bachmann S., Le Hir M., Eckardt K.U. (1993) Co-localization of erythropoietin mRNA and ecto-5'-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietin. J. Histochem. Cytochem., 41, 335–341. [DOI] [PubMed] [Google Scholar]
- 6. Semenza G.L., Koury S.T., Nejfelt M.K., Gearhart J.D., Antonarakis S.E. (1991) Cell-type-specific and hypoxia-inducible expression of the human erythropoietin gene in transgenic mice. Proc. Natl. Acad. Sci. U. S. A, 88, 8725–8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiang B.H., Rue E., Wang G.L., Roe R., Semenza G.L. (1996) Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J. Biol. Chem., 271, 17771–17778. [DOI] [PubMed] [Google Scholar]
- 8. Huang L.E., Arany Z., Livingston D.M., Bunn H.F. (1996) Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J. Biol. Chem, 271, 32253–32259. [DOI] [PubMed] [Google Scholar]
- 9. Gruber M., Hu C.J., Johnson R.S., Brown E.J., Keith B., Simon M.C. (2007) Acute postnatal ablation of Hif-2alpha results in anemia. Proc. Natl. Acad. Sci. U. S. A, 104, 2301–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scortegagna M., Ding K., Zhang Q., Oktay Y., Bennett M.J., Bennett M., Shelton J.M., Richardson J.A., Moe O., Garcia J.A. (2005) HIF-2alpha regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood, 105, 3133–3140. [DOI] [PubMed] [Google Scholar]
- 11. Kapitsinou P.P., Liu Q., Unger T.L., Rha J., Davidoff O., Keith B., Epstein J.A., Moores S.L., Erickson-Miller C.L., Haase V.H. (2010) Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood, 116, 3039–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paliege A., Rosenberger C., Bondke A., Sciesielski L., Shina A., Heyman S.N., Flippin L.A., Arend M., Klaus S.J., Bachmann S. (2010) Hypoxia-inducible factor-2alpha-expressing interstitial fibroblasts are the only renal cells that express erythropoietin under hypoxia-inducible factor stabilization. Kidney Int., 77, 312–318. [DOI] [PubMed] [Google Scholar]
- 13. Rankin E.B., Biju M.P., Liu Q., Unger T.L., Rha J., Johnson R.S., Simon M.C., Keith B., Haase V.H. (2007) Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J. Clin. Invest., 117, 1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pauling L., Itano H.A., and., et al. (1949) Sickle cell anemia a molecular disease. Science, 110, 543–548. [DOI] [PubMed] [Google Scholar]
- 15. Rees D.C., Williams T.N., Gladwin M.T. (2010) Sickle-cell disease. Lancet, 376, 2018–2031. [DOI] [PubMed] [Google Scholar]
- 16. Jelkmann W. (2007) Erythropoietin after a century of research: younger than ever. Eur. J. Haematol., 78, 183–205. [DOI] [PubMed] [Google Scholar]
- 17. Platt O.S., Orkin S.H., Dover G., Beardsley G.P., Miller B., Nathan D.G. (1984) Hydroxyurea enhances fetal hemoglobin production in sickle cell anemia. J. Clin. Invest., 74, 652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steinberg M.H., Barton F., Castro O., Pegelow C.H., Ballas S.K., Kutlar A., Orringer E., Bellevue R., Olivieri N., Eckman J., et al. (2003) Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA, 289, 1645–1651. [DOI] [PubMed] [Google Scholar]
- 19. Gordeuk V.R., Campbell A., Rana S., Nouraie M., Niu X., Minniti C.P., Sable C., Darbari D., Dham N., Onyekwere O., et al. (2009) Relationship of erythropoietin, fetal hemoglobin, and hydroxyurea treatment to tricuspid regurgitation velocity in children with sickle cell disease. Blood, 114, 4639–4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pulte E.D., McKenzie S.E., Caro J., Ballas S.K. (2014) Erythropoietin levels in patients with sickle cell disease do not correlate with known inducers of erythropoietin. Hemoglobin, 38, 385–389. [DOI] [PubMed] [Google Scholar]
- 21. Papassotiriou I., Voskaridou E., Stamoulakatou A., Loukopoulos D. (2000) Increased erythropoietin level induced by hydroxyurea treatment of sickle cell patients. Hematol. J., 1, 295–300. [DOI] [PubMed] [Google Scholar]
- 22. Charache S., Dover G.J., Moore R.D., Eckert S., Ballas S.K., Koshy M., Milner P.F., Orringer E.P., Phillips G., Jr., Platt O.S., et al. (1992) Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood, 79, 2555–2565. [PubMed] [Google Scholar]
- 23. Papassotiriou I., Kister J., Griffon N., Stamoulakatou A., Abraham D.J., Marden M.C., Loukopoulos D., Poyart C. (1998) Modulating the oxygen affinity of human fetal haemoglobin with synthetic allosteric modulators. Br. J. Haematol., 102, 1165–1171. [DOI] [PubMed] [Google Scholar]
- 24. Ang S.O., Chen H., Hirota K., Gordeuk V.R., Jelinek J., Guan Y., Liu E., Sergueeva A.I., Miasnikova G.Y., Mole D., et al. (2002) Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat. Genet., 32, 614–621. [DOI] [PubMed] [Google Scholar]
- 25. Zhang X., Zhang W., Ma S.F., Miasniakova G., Sergueeva A., Ammosova T., Xu M., Nekhai S., Nourai M., Wade M.S., et al. (2014) Iron deficiency modifies gene expression variation induced by augmented hypoxia sensing. Blood Cells Mol. Dis., 52, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacobson L.O., Goldwasser E., Fried W., Plzak L. (1957) Role of the kidney in erythropoiesis. Nature, 179, 633–634. [DOI] [PubMed] [Google Scholar]
- 27. Brown C.D., Mangravite L.M., Engelhardt B.E. (2013) Integrative modeling of eQTLs and cis-regulatory elements suggests mechanisms underlying cell type specificity of eQTLs. PLoS Genet., 9, e1003649.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lemonne N., Charlot K., Waltz X., Ballas S.K., Lamarre Y., Lee K., Hierso R., Connes C., Etienne-Julan M., Romana M., et al. (2015) Hydroxyurea treatment does not increase blood viscosity and improves red blood cell rheology in sickle cell anemia. Haematologica, 100, e383–e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nicolae D.L., Gamazon E., Zhang W., Duan S., Dolan M.E., Cox N.J. (2010) Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet., 6, e1000888.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fraser H.B., Xie X. (2009) Common polymorphic transcript variation in human disease. Genome Res., 19, 567–575. [DOI] [PubMed] [Google Scholar]
- 31. Ding J., Gudjonsson J.E., Liang L., Stuart P.E., Li Y., Chen W., Weichenthal M., Ellinghaus E., Franke A., Cookson W., et al. (2010) Gene expression in skin and lymphoblastoid cells: Refined statistical method reveals extensive overlap in cis-eQTL signals. Am. J. Hum. Genet., 87, 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Nas A., Ingram-Drake L., Sinsheimer J.S., Wang S.S., Schadt E.E., Drake T., Lusis A.J. (2010) Expression quantitative trait loci: replication, tissue- and sex-specificity in mice. Genetics, 185, 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nica A.C., Parts L., Glass D., Nisbet J., Barrett A., Sekowska M., Travers M., Potter S., Grundberg E., Small K., et al. (2011) The architecture of gene regulatory variation across multiple human tissues: the MuTHER study. PLoS Genet., 7, e1002003.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Semenza G.L., Wang G.L. (1992) A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol., 12, 5447–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blanchard K.L., Acquaviva A.M., Galson D.L., Bunn H.F. (1992) Hypoxic induction of the human erythropoietin gene: cooperation between the promoter and enhancer, each of which contains steroid receptor response elements. Mol. Cell. Biol., 12, 5373–5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang G.L., Semenza G.L. (1996) Molecular basis of hypoxia-induced erythropoietin expression. Curr. Opin. Hematol., 3, 156–162. [DOI] [PubMed] [Google Scholar]
- 37. Semenza G.L., Dureza R.C., Traystman M.D., Gearhart J.D., Antonarakis S.E. (1990) Human erythropoietin gene expression in transgenic mice: multiple transcription initiation sites and cis-acting regulatory elements. Mol. Cell. Biol., 10, 930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Galson D.L., Tsuchiya T., Tendler D.S., Huang L.E., Ren Y., Ogura T., Bunn H.F. (1995) The orphan receptor hepatic nuclear factor 4 functions as a transcriptional activator for tissue-specific and hypoxia-specific erythropoietin gene expression and is antagonized by EAR3/COUP-TF1. Mol. Cell. Biol., 15, 2135–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang W., Tsuchiya T., Yasukochi Y. (1999) Transitional change in interaction between HIF-1 and HNF-4 in response to hypoxia. J. Hum. Genet., 44, 293–299. [DOI] [PubMed] [Google Scholar]
- 40. Viollet B., Kahn A., Raymondjean M. (1997) Protein kinase A-dependent phosphorylation modulates DNA-binding activity of hepatocyte nuclear factor 4. Mol. Cell. Biol., 17, 4208–4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rochette-Egly C., Oulad-Abdelghani M., Staub A., Pfister V., Scheuer I., Chambon P., Gaub M.P. (1995) Phosphorylation of the retinoic acid receptor-alpha by protein kinase A. Mol. Endocrinol., 9, 860–871. [DOI] [PubMed] [Google Scholar]
- 42. Kambe T., Tada-Kambe J., Kuge Y., Yamaguchi-Iwai Y., Nagao M., Sasaki R. (2000) Retinoic acid stimulates erythropoietin gene transcription in embryonal carcinoma cells through the direct repeat of a steroid/thyroid hormone receptor response element half-site in the hypoxia-response enhancer. Blood, 96, 3265–3271. [PubMed] [Google Scholar]
- 43. Cokic V.P., Andric S.A., Stojilkovic S.S., Noguchi C.T., Schechter A.N. (2008) Hydroxyurea nitrosylates and activates soluble guanylyl cyclase in human erythroid cells. Blood, 111, 1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Loilome W., Juntana S., Namwat N., Bhudhisawasdi V., Puapairoj A., Sripa B., Miwa M., Saya H., Riggins G.J., Yongvanit P. (2011) PRKAR1A is overexpressed and represents a possible therapeutic target in human cholangiocarcinoma. Int. J. Cancer, 129, 34–44. [DOI] [PubMed] [Google Scholar]
- 45. Birgegard G., Wide L., Simonsson B. (1989) Marked erythropoietin increase before fall in Hb after treatment with cytostatic drugs suggests mechanism other than anaemia for stimulation. Br. J. Haematol., 72, 462–466. [DOI] [PubMed] [Google Scholar]
- 46. Saijo Y., Nakai Y., Saito J., Sugawara S., Suzuki S., Numata Y., Motomiya M. (1992) Changes in serum erythropoietin levels during chemotherapy for lung cancer. Chemotherapy, 38, 281–285. [DOI] [PubMed] [Google Scholar]
- 47. Sawabe Y., Takiguchi Y., Kikuno K., Iseki T., Ito J., Iida S., Kuriyama T., Yonemitsu H. (1998) Changes in levels of serum erythropoietin, serum iron and unsaturated iron binding capacity during chemotherapy for lung cancer. Jpn. J. Clin. Oncol., 28, 182–186. [DOI] [PubMed] [Google Scholar]
- 48. Stein B., Brady H., Yang M.X., Young D.B., Barbosa M.S. (1996) Cloning and characterization of MEK6, a novel member of the mitogen-activated protein kinase kinase cascade. J. Biol. Chem., 271, 11427–11433. [DOI] [PubMed] [Google Scholar]
- 49. Emerling B.M., Platanias L.C., Black E., Nebreda A.R., Davis R.J., Chandel N.S. (2005) Mitochondrial reactive oxygen species activation of p38 mitogen-activated protein kinase is required for hypoxia signaling. Mol. Cell. Biol., 25, 4853–4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dashti S.R., Efimova T., Eckert R.L. (2001) MEK6 regulates human involucrin gene expression via a p38alpha - and p38delta -dependent mechanism. J. Biol. Chem., 276, 27214–27220. [DOI] [PubMed] [Google Scholar]
- 51. Zhang X., Zhang W., Ma S.F., Desai A.A., Saraf S., Miasniakova G., Sergueeva A., Ammosova T., Xu M., Nekhai S., et al. (2014) Hypoxic response contributes to altered gene expression and precapillary pulmonary hypertension in patients with sickle cell disease. Circulation, 129, 1650–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang X., Zhang W., Saraf S.L., Nouraie M., Han J., Gowhari M., Hassan J., Miasnikova G., Sergueeva A., Nekhai S., et al. (2015) Genetic polymorphism of APOB is associated with diabetes mellitus in sickle cell disease. Hum. Genet., 134, 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Langmead B., Salzberg S.L. (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods, 9, 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Borevitz J.O., Liang D., Plouffe D., Chang H.S., Zhu T., Weigel D., Berry C.C., Winzeler E., Chory J. (2003) Large-scale identification of single-feature polymorphisms in complex genomes. Genome Res., 13, 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Irizarry R.A., Hobbs B., Collin F., Beazer-Barclay Y.D., Antonellis K.J., Scherf U., Speed T.P. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics, 4, 249–264. [DOI] [PubMed] [Google Scholar]
- 56. Tusher V.G., Tibshirani R., Chu G. (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U. S. A, 98, 5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Anderson M.J., Ter Braak C.J.F. (2003) Permutation tests for multi-factorial analysis of variance. J. Statist. Comput. Simulat., 73, 85–113. [Google Scholar]
- 58. Benjamini Y., Hochberg Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Statist. Soc. Ser. B, 57, 289–300. [Google Scholar]
- 59. Yang J., Lee S.H., Goddard M.E., Visscher P.M. (2011) GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet., 88, 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A., Bender D., Maller J., Sklar P., de Bakker P.I., Daly M.J., et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet., 81, 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Browning S.R., Browning B.L. (2007) Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet., 81, 1084–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Willer C.J., Li Y., Abecasis G.R. (2010) METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics, 26, 2190–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Johnson W.E., Li C., Rabinovic A. (2007) Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics, 8, 118–127. [DOI] [PubMed] [Google Scholar]
- 64. Zhang X., Zhang W. (2016) Transcript Iso form Variation Associated with Cytosine Modifi cation in Human Lymphoblastoid Cell Lines. Genetics, 203, 985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang W., Duan S., Kistner E.O., Bleibel W.K., Huang R.S., Clark T.A., Chen T.X., Schweitzer A.C., Blume J.E., Cox N.J., et al. (2008) Evaluation of genetic variation contributing to differences in gene expression between populations. Am. J. Hum. Genet., 82, 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.