Abstract
Insulin resistance (IR), a key component of the metabolic syndrome, precedes the development of diabetes, cardiovascular disease, and Alzheimer's disease. Its etiological pathways are not well defined, although many contributory mechanisms have been established. This article summarizes such mechanisms into the hypothesis that factors like nutrient overload, physical inactivity, hypoxia, psychological stress, and environmental pollutants induce a network of cellular stresses, stress responses, and stress response dysregulations that jointly inhibit insulin signaling in insulin target cells including endothelial cells, hepatocytes, myocytes, hypothalamic neurons, and adipocytes. The insulin resistance-inducing cellular stresses include oxidative, nitrosative, carbonyl/electrophilic, genotoxic, and endoplasmic reticulum stresses; the stress responses include the ubiquitin-proteasome pathway, the DNA damage response, the unfolded protein response, apoptosis, inflammasome activation, and pyroptosis, while the dysregulated responses include the heat shock response, autophagy, and nuclear factor erythroid-2-related factor 2 signaling. Insulin target cells also produce metabolites that exacerbate cellular stress generation both locally and systemically, partly through recruitment and activation of myeloid cells which sustain a state of chronic inflammation. Thus, insulin resistance may be prevented or attenuated by multiple approaches targeting the different cellular stresses and stress responses.
1. Introduction
The hormone insulin plays an important role in maintaining physiological levels of blood glucose, through various effects on insulin target cells. In endothelial cells, it promotes the release of nitric oxide and endothelin, which, respectively, promote vasodilation and vasoconstriction, and the combined vasodilatory and vasoconstrictive effects improve the distribution of blood glucose to target organs such as skeletal muscles [1]. It promotes glycogen synthesis in hepatocytes, skeletal myocytes, and adipocytes [2, 3], downregulates the expression of gluconeogenetic enzymes in hepatocytes, and promotes glucose uptake through the GLUT 4 receptor in skeletal myocytes and adipocytes [2, 3]. In specific types of hypothalamic neurons, it inhibits the expression of orexigenic neuropeptides such as neuropeptide Y (NYP) or agouti-related peptide (AgRP) and thereby contributes to decreased food intake [4–8]. Insulin also inhibits food intake by promoting expression of anorexigenic neuropeptides such as proopiomelanocorticotropin (POMC) and cocaine- and amphetamine-regulated transcript (CaRT) in the arcuate nucleus, which together promote the activity of α-melanocyte-stimulating hormone in neurons in the paraventricular nucleus (4–8). Besides inhibiting AgRP synthesis, insulin-induced hyperpolarization of the AgRP-expressing arcuate neurons reduces the firing rate of these neurons and results in the generation and transmission of signals from the motor nucleus of the vagus nerve to the liver, resulting in increased hepatic interleukin 6 (IL-6) production, IL-6-mediated activation of signal transducer and activator of transcription 3 (STAT-3), and STAT-3-mediated decrease in the expression of gluconeogenic genes such as glucose-6-phosphatase and phosphoenol pyruvate carboxykinase (PEPCK) [9–12].
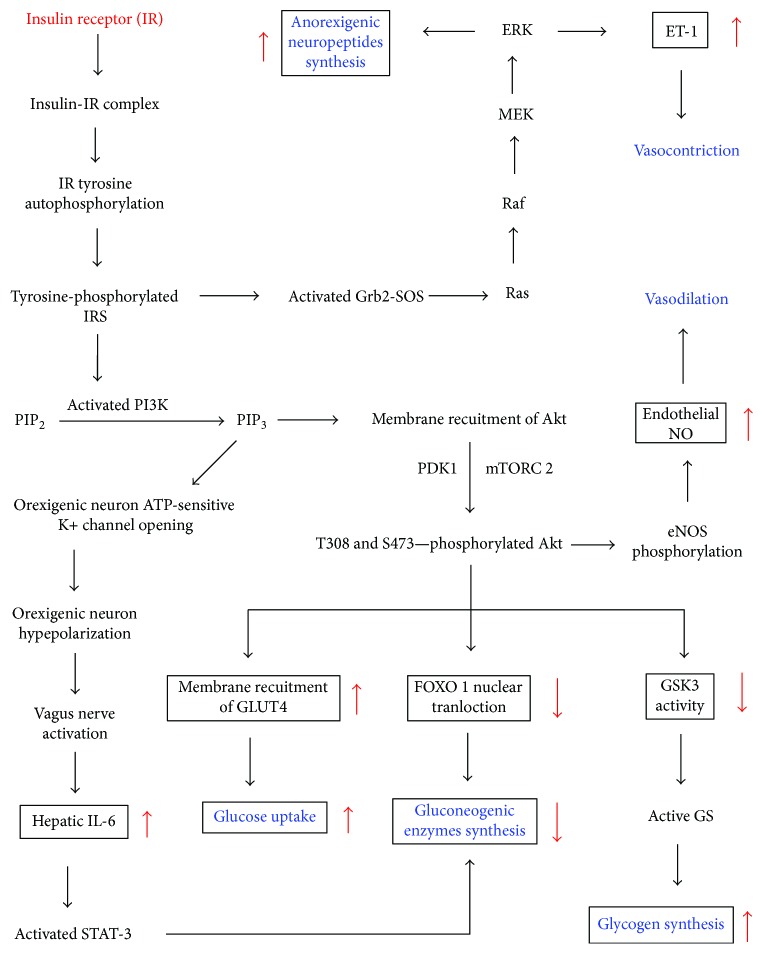
Insulin resistance refers to a condition in which insulin-responsive cells undergo a less than normal response to insulin, such as a reduced activation of endothelial nitric oxide synthase in endothelial cells [13]. It involves disruption of specific events in the insulin signaling pathways. Insulin signaling begins with insulin binding to the insulin receptor (IR), a receptor tyrosine kinase, which then undergoes autophosphorylation of various intracellular tyrosine residues, resulting in the recruitment and tyrosine phosphorylation of adaptor proteins including insulin receptor substrates (IRS) such as IRS1 and IRS2 (Figure 1).
Figure 1.
Insulin signaling pathways via the insulin receptor (IR) and insulin receptor substrates (IRS) [1–4, 9–12]. ERK: extracellular signal-regulated kinase; eNOS: endothelial nitric oxide synthase; FOXO: forkhead box O transcription factor; GLUT 4: glucose transporter 4; Grb2-SOS: growth factor receptor-bound 2- (Grb2-) son of sevenless (Sos) protein complex; GS: glycogen synthase; GSK3: glycogen synthase kinase 3; IL-6: interleukin 6; MEK: MAPK (mitogen-activated protein kinase)/ERK kinase; mTORC 2: mammalian target of rapamycin 2; NO: nitric oxide; PDK 1: 3-phosphoinositide-dependent kinase-1; PIP2: phosphatidyl inositol 4,5-biphosphate; PIP3: phosphatidyl inositol 3,4,5-triphosphate; STAT-3: signal transducer and activator of transcription 3.
Signaling downstream of IRS occurs by several pathways (Figure 1). One such pathway sequentially involves activation of phosphatidyl inositol 3-kinase (PI3K); conversion of phosphatidyl inositol 4,5-biphosphate (PIP2) to phosphatidyl inositol 3,4,5-triphosphate (PIP3); recruitment of Akt (protein kinase B (PKB)) to the plasma membrane; phosphorylation of Akt by 3-phosphoinositide-dependent kinase-1 (PDK 1) and mammalian target of rapamycin complex 2 (mTORC 2); and Akt-mediated phosphorylation of a number of downstream protein substrates that induce effects such as activation of glycogen synthase (GS) in adipocytes, skeletal myocytes, and hepatocytes, translocation of glucose transporter 4 (GLUT 4) to the plasma membrane of adipocytes and skeletal myocytes, phosphorylation of the forkhead transcription factor (FOXO 1) to inhibit expression of gluconeogenic enzymes in hepatocytes, or activation of endothelial nitric oxide synthase in endothelial cells [1–3, 13] (Figure 1). Akt also activates mTORC 1 which not only is involved in feedback inhibition of IRS but also inhibits synthesis of orexigenic neuropeptides by hypothalamic neurons (not shown) [14].
In another pathway which involves orexigenic (AgRP-producing) hypothalamic neurons, PI3K promotes opening of ATP-sensitive K+ channels, resulting in sequential hyperpolarization of these neurons, transmission of signals from the vagus nerve to the liver, increased hepatic IL-6 synthesis, activation of STAT-3, and decreased expression of gluconeogenic enzymes (Figure 1) [9–12]. On the other hand, insulin-mediated upregulation of the production of anorexigenic neuropeptides by hypothalamic neurons proceeds through IRS-mediated activation of the growth factor receptor-bound 2- (Grb2-) son of sevenless (Sos) protein complex (Grb2-Sos) and downstream activation of the Ras-Raf-MEK-ERK pathway [4]. In endothelial cells, ERK promotes the synthesis of endothelin-1 [1].
Because insulin resistance contributes to the development of noncommunicable diseases such as diabetes, cardiovascular disease, fatty liver disease, Alzheimer's disease, and impaired lung function [12, 13, 15–18], much effort has been directed toward understanding the mechanisms of its pathogenesis through studies involving cell cultures, animal models, and clinical studies. Cell cultures of hepatocytes, adipocytes, skeletal muscle cells, endothelial cells, or neurons incubated with palmitate or high sugar concentrations develop insulin resistance [19–26]. Some of the cellular events and mechanisms that have been shown to be involved in the development of insulin resistance in these cells both in vitro and in vivo include (i) toll-like receptor 4 (TLR4) and associated inhibitor of kappa B kinase- (IKK-) nuclear factor kappa B (NF-κB) signaling [27–31]; (ii) advanced glycation end products (AGEs) or uric acid-induced receptor for AGE (RAGE) signaling via NF-κB [30, 32–34]; (iii) oxidized low-density lipoprotein- (oxLDL-) mediated RAGE or Lox-1 signaling and the resultant activation of NF-κB and formation of peroxynitrite [30, 35, 36]; (iv) upregulation of NADPH oxidase (Nox) expression and activity [20, 21, 30, 37–39]; (v) increased mitochondrial reactive oxygen species (ROS) generation [30, 40]; (vi) upregulation of inducible nitric oxide synthase (INOS) [30, 41–43]; (vii) increased diacylglycerol synthesis [30, 44]; (viii) increased ceramide synthesis [30, 45, 46]; (ix) activation of protein kinase C (PKC) isoforms [30, 37, 47]; (x) activation of mitogen-activated protein kinases (MAPKs) such as c-Jun N-terminal kinase (JNK), p38 MAPK, and extracellular signal-regulated kinase (ERK) [20, 28, 30]; (xi) endoplasmic reticulum stress and the unfolded protein response [30, 43, 48–50]; (xii) dysregulation of the heat shock response [51–53]; (xiii) autophagy dysregulation [54]; (xiv) apoptosis [55]; (xv) p53 activation [56]; and (xvi) inflammasome activation [57, 58]. Thus, insulin resistance is regarded as a complex disorder that defies a single etiological pathway [59].
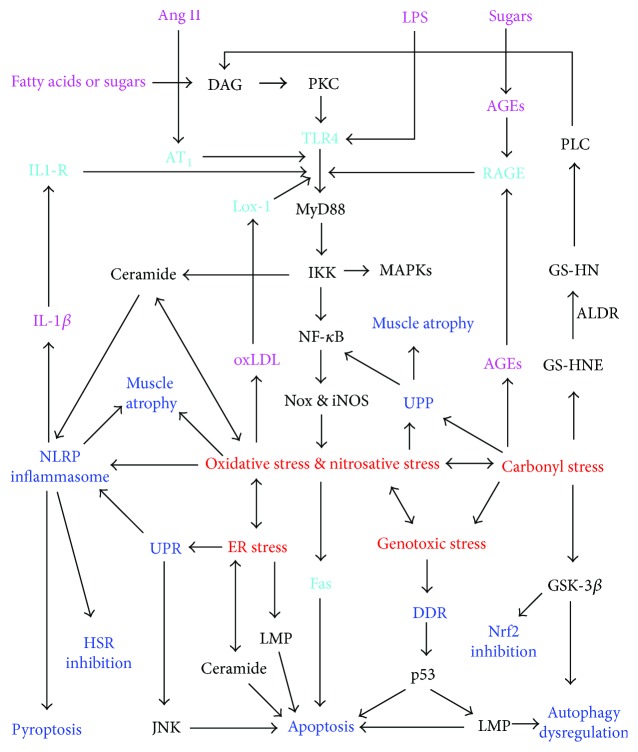
This review summarizes the above mechanisms into a unifying hypothesis that the pathogenesis of insulin resistance involves generation of oxidative stress, nitrosative stress, carbonyl stress, endoplasmic reticulum stress, and genotoxic stress through interconnected pathways; induction of various responses to these stresses, such as the unfolded protein response (UPR), the ubiquitin proteasome pathway (UPP), DNA damage response (DDR), the NRLP3 inflammasome, and apoptosis; and the dysregulation of stress responses such as autophagy, heat shock response, and nuclear factor erythroid-2-related factor 2 (Nrf2) signaling in insulin target cells (as exemplified in Figure 2). Each of the stresses, stress responses, and stress response dysregulations contributes to insulin resistance in multiple ways.
Figure 2.
Pathways to interconnected cellular stresses, stress responses, and stress response dysregulations that contribute to insulin resistance in insulin target cells exposed to excess nutrients (sugars or fatty acids such as palmitate), angiotensin II (Ang II), or bacterial lipopolysaccharide (LPS). ALDR: aldose reductase; AGE: advanced glycation end product; AT1: angiotensin receptor type 1; DAG: diacylglycerol; DDR: DNA damage response; GS-HNE: glutathione-HNE adduct; GS-HN: glutathionyl-1,4-dihydroxynonene; GSK: glycogen synthase kinase; HSR: heat shock response; IL-1β: interleukin 1β; IL1-R: interleukin 1 receptor; iNOS: inducible nitric oxide synthase; IKK: inhibitor of kappa B kinase; LMP: lysosomal membrane permeabilization; MAPK: mitogen-activated protein kinase; NF-κB: nuclear factor kappa B; Nox: NADPH oxidase; PKC: protein kinase C; PLC: phospholipase C; TLR4: toll-like receptor 4; UPP: ubiquitin-proteosome pathway; UPR: unfolded protein response. The stresses, stress responses, and signaling pathways generating them contribute to insulin resistance by multiple mechanisms as described in the text. Insulin resistance may occur because of the combined effects of different components of the system, and these components may promote IR to different extents in different cell types.
2. Pathways to Cellular Stresses in Insulin Target Cells
2.1. Pathways to Oxidative and Nitrosative Stresses in Response to Overnutrition, Physical Inactivity, Hypoxia, Psychological Stress, or Environmental Pollutants
As illustrated in Figure 2, cell surface receptors such as the TLR4, RAGE, Lox-1, and angiotensin receptor type 1 (AT1) are involved in signaling pathways that generate oxidative stress and nitrosative stress.
A high-fat or high-fructose diet promotes the growth of gram-negative bacteria in the colon, resulting in endotoxemia and the release of enteric lipopolysaccharide (LPS) into blood plasma [60, 61]. LPS is a direct ligand for TLR4 and induces TLR4-dependent oxidative stress and inhibition of insulin signaling in both peripheral insulin target cells and hypothalamic neurons [28, 30, 62, 63]. TLR4 signaling via MyD88 and IRAK 4 leads to the activation of IKK [28, 30, 64]. One of the most important targets of IKK activation is NF-κB, which, for example, was found to be essential for palmitate-induced insulin resistance in C2C12 skeletal muscle cells [65]. NF-κB induces expression of protein tyrosine phosphatase B (PTPB), a negative regulator of the insulin receptor [66] and proinflammatory genes such as tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), and interleukin 6 (IL-6) [67]. It also upregulates the expression of Nox and iNOS, which produce superoxide anions (O2−) and nitric oxide (NO), respectively [28–30, 68, 69]. Superoxide anions are rapidly converted to hydrogen peroxide (H2O2) by superoxide dismutase (SOD) [70]. Superoxide anions also rapidly react with NO to form peroxynitrite (OONO−) [70], which reacts with hydrogen peroxide (H2O2) to form singlet oxygen (1O2) [30, 71], which in turn reacts with biomolecules such as lipids and proteins to form organic hydroperoxides (ROOH) [30]. Excessive production of reactive oxygen species (ROS) such as superoxide anions, hydrogen peroxide, organic hydroperoxides, and singlet oxygen results in oxidative stress when the ROS outweigh the cellular antioxidant capacity [72]. Likewise, excessive formation of peroxynitrite results in nitrosative stress. NF-κB-dependent induction of iNOS and Nox may further contribute to mitochondrial oxidative and nitrosative stresses. This is because, even when H2O2 and NO are generated extramitochondrially, they readily enter the mitochondria and induce electron leakage from the electron transport chain (ETC), thus promoting the generation of mitochondrial superoxide anions, H2O2, peroxynitrite, singlet oxygen, and lipid hydroperoxides [30, 70].
The nonenzymatic reaction of sugars with proteins (Maillard reaction) leads to the formation of hydrogen peroxide, singlet oxygen, and advanced glycation end products (AGEs) such as glyoxal lysine and methylglyoxal lysine [73, 74]. AGEs accumulate in plasma and tissues of animals and humans on diets rich in fructose or preformed AGEs [75]. AGEs signal via the RAGE receptor to induce activation of NF-κB via some components of the TLR4 pathway and thus produce similar effects as LPS, including the induction of oxidative and nitrosative stresses (Figure 2) [30, 32, 76].
Oversupply of fatty acids to insulin target cells occurs because of excessive dietary intake, obesity, or muscle inactivity-associated decrease in beta oxidation of fatty acids [59, 77, 78]. Palmitate and laurate can induce the activation of IKK, NF-κB, and oxidative stress independently of LPS [79–84]. This at least partly involves increased synthesis of diacylglycerol (DAG), a cofactor of protein kinase C (PKC) isoforms which activate Nox isoforms and NF-κB [30, 59, 80, 82–85]. Similarly, exposure of endothelial cells to high glucose levels results in DAG formation and subsequent activation of PKC and Nox [80]. The activation of Nox and NF-κB by PKC isoforms may involve PKC-induced TLR4 signaling as shown in Figure 2 [30, 64, 81]. However, DAG-PKC-induced insulin resistance without TLR4 activation has also been reported [84].
Palmitate is a substrate of serine palmitoyl transferase (SPT) in the first step of the de novo biosynthesis of the sphingolipid ceramide, an inhibitor of insulin signaling by multiple mechanisms including activation of protein phosphatase 2A (PP2A) and PKC-ζ, which promote dephosphorylation of Akt or serine phosphorylation of IRS, respectively [45, 81, 86, 87]. Long-term ceramide action also promotes serine phosphorylation of IRS via sequential activation of the double-stranded RNA-activated protein kinase (PKR) and JNK [88]. Palmitate-induced TLR4-IKK signaling promotes ceramide biosynthesis by upregulating the synthesis of SPT and ceramide synthases (Figure 2) [81, 86]. Ceramide is an important contributor to oxidative stress. It induces mitochondrial superoxide anion and H2O2 generation by blocking the electron transport system at complex III [89]. Mitochondrial superoxide anions generated in this manner induce opening of the mitochondrial permeability transition pore, allowing mitochondrial ROS to move into the cytoplasm [90]. Some mechanisms by which ceramide induces insulin resistance, such as apoptosis induction, JNK activation, and mitochondrial fission, depend on such ceramide-induced oxidative stress [91–95]. ROS and NO promote mitochondrial fission, which in turn promotes ROS formation through cytochrome c oxidase [96, 97].
In a recent clinical trial, a high-saturated-fat diet increased the serum concentrations of angiotensin-converting enzyme (ACE) independently of weight gain [98]. In the classical renin-angiotensin system (RAS), ACE converts angiotensin I to the active angiotensin II which signals via angiotensin receptors 1 and 2 (AT1 and AT2) [99] and TLR4 to induce NF-κB activation, mitochondrial fission, and insulin resistance in skeletal muscle cells, vascular smooth muscle cells, and endothelial cells [31, 99–104]. Angiotensin II signaling upregulates xanthine oxidase protein expression and activity in a Nox-dependent manner in endothelial cells [105]. Furthermore, it activates 12-lipoxygenase, whose product, 12-hydroxyeicosatetraenoic acid (12-HETE), induces NF-κB in endothelial cells and aldosterone in adrenal glomerulosa cells [106, 107]. Aldosterone levels increase in humans during obesity, and this hormone correlates with insulin resistance independently of the body mass index [108, 109]. Aldosterone increases superoxide production in endothelial cells though mineralocorticoid receptor- (MR-) mediated activation of Nox and Rac 1 [110]. It also promotes MR-induced de novo ceramide synthesis in these cells [111]. This adrenal hormone readily enters the brain, such that its levels in the brain are directly proportional to its plasma levels in rats [112]. It activates the hypothalamic renin-angiotensin system and associated oxidative stress in hypothalamic neurons [112, 113].
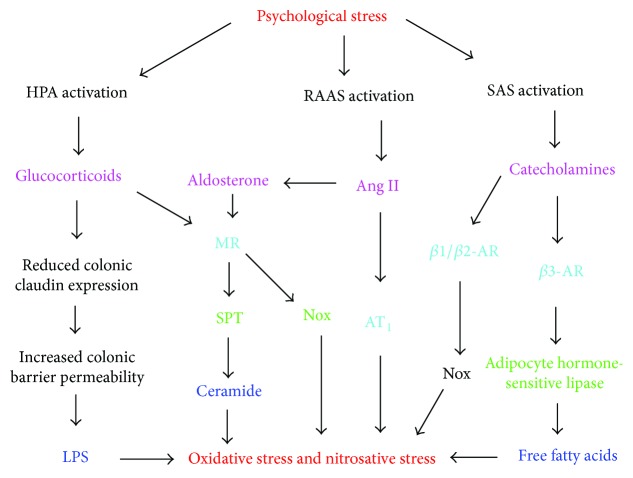
Psychological stress (PS) is another major inducer of oxidative stress and insulin resistance [114–116]. This is partly through increased production of aldosterone [109], angiotensin II [117], and glucocorticoids such as corticosterone and cortisol (Figure 3) [118–120]. Glucocorticoids upregulate the expression of SPT and ceramide synthases and thus contribute to ceramide-mediated oxidative stress and insulin resistance [121, 122]. They have a higher affinity for the mineralocorticoid receptor than for the glucocorticoid receptor, and their binding to the former increases Nox expression in adipocytes [123]. PS also contributes to LPS-induced oxidative stress and insulin resistance by promoting colonic barrier permeability and the translocation of bacteria and LPS from the intestinal lumen to the blood [124]. Chronic peripheral administration of corticotropin-releasing factor was demonstrated to cause such colonic barrier dysfunction in rats [125]. This involves glucocorticoid-mediated downregulation of the intestinal epithelial tight junction protein, claudin 1 [126].
Figure 3.
Psychological stress-dependent pathways to oxidative and nitrosative stresses. Psychological stress activates the renin-angiotensin-aldosterone system (RAAS), the hypothalamic-pituitary-adrenal axis (HPA), and the sympathetic adrenomedullary system (SAS), leading to increased availability of angiotensin II (Ang II), aldosterone, glucocorticoids, catecholamines, and free fatty acids which induce oxidative and nitrosative stresses in insulin target cells [109, 117–120, 127, 128]. Glucocorticoids and aldosterone promote de novo ceramide synthesis in endothelial cells and may thereby contribute to plasma ceramides [111, 121–123]. Glucocorticoids also increase colon epithelial barrier permeability and thus increase circulating LPS [124–126]. Angiotensin II, glucocorticoids, aldosterone, and catecholamines upregulate Nox activity in various insulin target cells [105, 110, 130, 132].
Chronic activation of the sympathetic nervous system and the associated increase in catecholamines such as epinephrine and norepinephrine are another hallmark of PS [127]. These catecholamines contribute to insulin resistance in the heart by activating β-adrenergic receptors (β-AR) [128]. Activation of β-AR induces oxidative stress in cardiomyocytes, adipocytes, and endothelial cells, at least partly by β2-AR-mediated upregulation of Nox [128–132]. The β3-AR activates hormone-sensitive lipase in adipocytes and thus promotes accumulation of free fatty acids and the associated increase in ceramide synthesis and MAPK activation [133]. AR stimulation inhibits adiponectin gene expression in adipocytes via protein kinase A [134], and this should further promote ceramide accumulation and ceramide-dependent oxidative stress because adiponectin increases ceramidase activity [135].
Mountain climbing or obstructive sleep apnea (OSA) induces insulin resistance, and this is associated with hypoxia-induced oxidative and nitrosative stresses [136, 137]. OSA worsens during periods of rapid weight gain [138]. Chronic asthma also induces intermittent hypoxia [139], and an association between asthma and insulin resistance was demonstrated in children and adolescents [18, 140, 141]. Many mechanisms have been reported to be involved in hypoxia-induced oxidative and nitrosative stresses. During the switch from normoxia to hypoxia, a burst of superoxide formation occurs at mitochondrial complex 1 due to deactivation of this complex in cells such as endothelial cells and neurons [142]. Hypoxia also increases superoxide formation at mitochondrial complex III [143]. In human umbilical endothelial cells, hypoxia was found to induce expression of the human circadian locomotor output cycle protein kaput (hCLOCK), which promoted the production of ROS, which in turn promoted Rhoa and NF-κB signaling [144]. Hypoxia upregulates 12/15-lipoxygenase, whose metabolites, namely, 13-hydroperoxyoctadecadienoc acid (13-HPODE), 12-hydroxyeicosatetraenoic acid (12-HETE), and 15-hydroxy-eicosatetraenoic acid (15-HETE), activate NF-κB, iNOS, and mitochondrial oxidative stress in endothelial cells, cardiomyocytes, smooth muscle cells, neurons, and adipocytes [145–151]. Since 13-HPODE, 12-HETE, and 15-HETE activate PKC isoforms [149, 152, 153], the 12/15-lipoxygenase-dependent, hypoxia-induced oxidative and nitrosative stresses may follow the pathway outlined in Figure 4.
Figure 4.
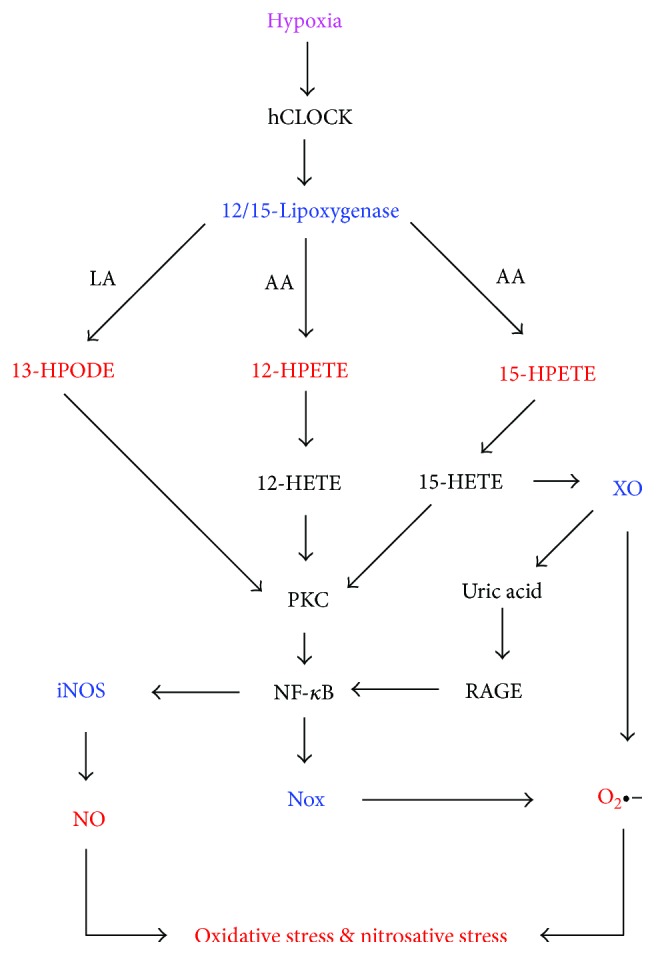
Pathways for the hypoxia-induced generation of oxidative and nitrosative stresses through the human circadian locomotor output cycle protein kaput- (hCLOCK-) mediated 12/15-lipoxygenase activation [143–150]. 12/15-Lipoxygenase catalyses the production of ROS in the form of 13-hydroperoxy-octadecadienoic acid (13-HPODE) from linoleic acid (LA) or 12-hydroperoxy-eicosatetraenoic acid (12-HPETE) and 15-hydroperoxy-eicosatetraenoic acid (15-HPETE) from arachidonic acid (AA). 13-HPODE, 12-HPETE, and 15-HPETE are reduced to the corresponding hydroxy derivatives 13-HODE, 12-HETE, and 15-HETE, respectively. 13-HPODE, 12-HETE, and 15-HETE activate PKC isoforms [148, 151, 152], which promote activation of NF-κB, NADPH oxidase (Nox) isoforms, and inducible nitric oxide synthase (iNOS) [30, 144–150]. iNOS and Nox produce nitric oxide (NO) and superoxide anions (O2·−), respectively, which undergo enzymatic and nonenzymatic reactions that lead to the formation of other ROS such as hydrogen peroxide and singlet oxygen, as well as the RNS, peroxynitrite [30]. 15-HETE activates xanthine oxidase (XO) [154], which catalyses the formation of both superoxide anions and uric acid, and the latter signals via the receptor for advanced glycation end products (RAGE) to activate NF-κB [34].
As indicated in this figure, 15-S-HETE also activates xanthine oxidase (XO) in endothelial cells [154]. Such increase in XO activity occurs during hypoxia [155] and leads to increased uric acid (UA) formation in lowlanders at high altitude [156]. Uric acid is a promoter of oxidative stress via the RAGE receptor in endothelial cells [34]. During intermittent hypoxia, XO-derived ROS activate Nox2 [157]. In addition, hypoxia increases catecholamine production [158] and, like psychological stress, has been reported to induce mucosal barrier failure and endotoxemia in rats and primates [159, 160]. However, a recent study in humans found that hypoxia increased gut inflammation but not gut permeability [161].
Long-term exposure to traffic-related air pollution was found to be positively associated with insulin resistance in children [162]. Particulate matter, ozone, nitrogen oxides, and transition metals are among the potent oxidants in polluted air that induce endogenous ROS formation and oxidative stress [163]. Cadmium, a heavy metal pollutant from industrial plants, which makes its way into the food chain and induces oxidative stress was recently found to be positively associated with insulin resistance [164].
2.2. Oxidative Stress Produces Carbonyl Stress and Vice Versa
Decomposition of lipid hydroperoxides produces reactive carbonyl compounds including acrolein, glyoxal, methylglyoxal, malondialdehyde, 4-hydroperoxy-2-nonenal, 4-hydroxy-2-nonenal (HNE), 4-oxo-2-nonenal, 2,4-decadienal, and 9-oxo-nonanic acid [165]. Elevated formation of such products constitutes carbonyl stress. Thus, oxidative stress, through increased production of lipid hydroperoxides, promotes carbonyl stress. As mentioned in the previous section, methylglyoxal- and glyoxal-derived AGEs promote oxidative stress via the RAGE receptor. Likewise, 4-HNE, one of the predominant lipid-derived aldehydes formed in insulin-responsive cells during high-fat or high-glucose diets, promotes the formation of reactive oxygen and nitrogen species [166]. Cholesterol secosterol aldehydes, which are produced via the reaction of cholesterol with singlet oxygen or ozone [30, 167], increase oxidative stress by inactivating catalase and thus promoting the accumulation of hydrogen peroxide and lipid hydroperoxides [168].
2.3. Oxidative and Carbonyl Stresses Promote Endoplasmic Reticulum Stress and Vice Versa
Noncytoplasmic and nonmembrane proteins synthesized at the rough endoplasmic reticulum (ER) undergo translocation into the ER lumen, where calcium-dependent molecular chaperones assist their folding into the correct tertiary structures [169]. The ER calcium transporter, sarco- (endo-) plasmic reticulum Ca2+ ATPase (SERCA), pumps calcium ions into this organelle and thereby promotes the activity of the molecular chaperones [170, 171]. The reversible S-glutathionylation of SERCA thiols by NO and peroxynitrite increases SERCA activity, but the irreversible sulfonation of these thiols by ROS such as hydrogen peroxide and singlet oxygen causes its inactivation [30, 170–172]. The ensuing accumulation of unfolded or misfolded proteins in the ER constitutes ER stress [173]. Carbonylation by aldehydes such as acrolein, methylglyoxal, glyoxal, and HNE also reduces SERCA activity [174, 175]. ER stress leads to enhanced Nox 4 activity in the ER, resulting in increased hydrogen peroxide formation and oxidative stress [176]. Such increased ER oxidative stress promotes calcium efflux from the ER and calcium influx into the mitochondria, which induces mitochondrial ROS production and oxidative stress [30, 176]. Thus, there is a vicious cycle between ER stress and oxidative stress [30, 177].
2.4. Oxidative, Carbonyl, and Nitrosative Stresses Generate Genotoxic Stress
Oxidative stress, carbonyl stress, and nitrosative stress contribute to genotoxic stress by availing genotoxic reactive oxygen, carbonyl, and nitrogen species that modify DNA. ROS react with the nitrogenous bases in DNA to induce a variety of base modifications. One of the most common of such modifications is the conversion of guanine to 8-oxo-7,8-dihydroguanine (8-oxoG), whose levels in urine have been suggested to be a marker of whole-body oxidative stress [178, 179]. 8-oxoG is most readily formed by singlet oxygen, although the hydroxyl radical also contributes to its formation [180, 181]. Mitochondrial DNA is exposed to singlet oxygen generated through mechanisms such as the reaction of peroxynitrite with hydrogen peroxide or cytochrome c-mediated conversion of cardiolipin hydroperoxide to triplet carbonyls in the mitochondria [30]. DNA-damaging hydroxyl radicals may be generated by the Fenton reaction between DNA-bound Fe2+ and hydrogen peroxide [182]. 8-oxoG undergoes further oxidative modifications, as well as crosslinking with lysine to generate protein-DNA adducts [183]. The reaction of singlet oxygen or hydroxyl radicals with deoxyribose in DNA generates single-strand breaks, but double-strand breaks can be generated when the single-strand breaks occur in close proximity [178, 184]. Peroxynitrite induces single-strand breaks in DNA through deoxyribose oxidation or via the formation of 8-nitroguanine [185]. Reactive carbonyl compounds derived from the decomposition of lipid hydroperoxides react with DNA bases to form exocyclic propano- and etheno-DNA adducts, as recently reviewed [186]. The glycoxidation of histone proteins by glyoxal and methylglyoxal promotes the oxidative generation of DNA strand breaks [187]. The formation of hydrogen peroxide and singlet oxygen during protein glycoxidation [74] may explain this phenomenon. Genotoxic stress in turn promotes oxidative stress (Section 3.4).
3. Mechanisms of the Inhibition of Insulin Signaling by Cellular Stresses
3.1. Oxidative Stress
The oxidative modifications of biomolecules including lipids, nucleic acids, and proteins contribute to insulin resistance. Some common types of oxidative protein modifications include hydroperoxidation, glutathionylation, and sulfonation. As shown in Figure 5, proteins (Pr) react with singlet oxygen to form protein hydroperoxides (Pr-OOH). The latter is relatively long lived and inactivates enzymes even when singlet oxygen is no longer in the system [188, 189]. Pr-OOHs react with thiol (-SH) groups in other proteins to form hydroxy proteins (Pr-OH) and protein sulfenic acids (Pr-S-OH), and the latter readily reacts with glutathione (GSH) to form glutathionylated proteins (Pr-S-SG) [190–192]. Hydrogen peroxide also induces protein glutathionylation, analogously to protein hydroperoxides, but the latter is more reactive [189]. Sulfenic acids (Pr-SOH) react further with H2O2 to form sulfinic acids (Pr-SO2H), which react with H2O2 to form sulfonic acids (Pr-SO3H) [190]. The reaction of superoxide radicals with thiols may also lead to the conversion of the latter to sulfonates via persulphenyl derivatives [193]. Ozone or ozone-like oxidants have been suggested to be formed in biological systems [74, 167]. Ozone largely converts thiolate ions to sulfonates [194] and was postulated to be an important contributor to the conversion of methionine sulfoxide to methionine sulfonate [195].
Figure 5.
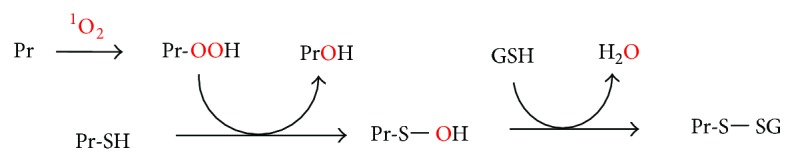
Mechanism of singlet oxygen-mediated protein glutathionylation. Reaction of 1O2 with a protein (Pr) on residues such as tryptophan and histidine generates a protein hydroperoxide (Pr-OOH). Reaction of Pr-OOH with cysteine residues in other proteins (Pr-SH) converts the latter to sulfenic acids (Pr-SOH) [189], which readily react with glutathione (GSH) to form glutathionylated proteins (Pr-S-SG) [190, 191].
At low levels, ROS including H2O2 and singlet oxygen stimulate insulin signaling by the PI3K-Akt pathway through inhibition of protein tyrosine phosphatase 1B (PTP1B) which dephosphorylates the insulin receptor [196, 197]. On the other hand, a high concentration of H2O2 was found to induce insulin resistance in hepatocytes, and systemic removal of hydrogen peroxide improved insulin resistance in obese mice [196, 198]. ROS activate stress-sensitive kinases which reduce insulin signaling [199], and it was proposed that at high H2O2 concentrations, JNK activation outweighs PTP1B inactivation [196].
Activation of JNK and p38 by ROS occurs through the modification of their regulatory proteins. For example, MAPK phosphatase 1 deactivates JNK and p38 MAPK by dephosphorylation, but glutathionylation targets this phosphatase for proteosomal degradation [199].
Protein-protein interaction between glutathione S-transferase P (GSTP) and JNK keeps the latter in an inactive state, but oxidative modification of the former breaks this interaction and activates JNK [200, 201]. Another protein whose oxidative modification promotes insulin resistance is thioredoxin which, in the native state, binds to and inactivates apoptotic signaling kinase 1 (ASK1), an upstream activator of both JNK and p38 pathways [202, 203]. While the release of thioredoxin from ASK would also allow the former to act as a thiol-reducing antioxidant, oxidative stress promotes the p38 MAPK- and FOXO-dependent expression of thioredoxin-interacting protein (TXNIP) [204] and transfer of the latter from the nucleus to the cytoplasm and mitochondria, where it binds to thioredoxin 1 and thioredoxin 2, respectively, and this aggravates oxidative stress [204, 205]. Increased oxidative stress also favors the activatory binding of TXNIP to the NLRP3 inflammasome [205], a key contributor to insulin resistance as described in Section 4.5.
Several studies have reported that the inhibition of glycolysis in muscle cells induces insulin resistance [206–208], for example, through a compensatory increase in lipid uptake [208]. Protein peroxides generated by singlet oxygen inhibit the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GPD) [209]. Besides reduced glycolysis, GPD inhibition enhances the conversion of dihydroxyacetone phosphate to methylglyoxal [210], which is one of the major reactive carbonyls contributing to insulin resistance (Section 3.3).
Reactive oxygen species such as H2O2 promote Ser637 dephosphorylation of the GTPase, dynamin-related protein 1 (Drp1), resulting in translocation of the latter to the mitochondria, where its polymerization into a ring-like structure induces mitochondrial fission [211], an important contributor to insulin resistance and oxidative stress [94, 95].
Perhaps one of the greatest contributions of oxidative stress to insulin resistance is that it generates metabolites that create positive feedback loops for potentiation of TLR4, RAGE, and other signaling pathways associated with activation of NF-κB and insulin signal-inhibiting serine kinases such as PKC, IKK, JNK, and p38 MAPK. For example, oxidative stress leads to the oxidation of low-density lipoproteins (LDL), and oxidized LDL (oxLDL) signals via Lox-1, RAGE, and Fas receptors to activate NF-κB and MAPKs as recently reviewed [30], and the plasma concentration of oxLDL is an independent risk factor for insulin resistance [212].
p38 MAPK promotes the expression of aldose reductase (ALDR) [213], which is subsequently activated by oxidative modification [214], and makes a major contribution to insulin resistance [215]. ALDR reduces HNE-glutathione adduct (GS-HNE) to glutathionyl-1,4-dihydroxynonene (GS-HN), which activates phospholipase C, leading to DAG formation and the activation of PKC, MAPKs, and NF-κB (Figure 2) [166, 216]. DAG oxidation also contributes to PKC-dependent signaling, since DAG hydroperoxide is a more potent activator of PKC than unoxidized DAG [217]. In the presence of excess glucose, ALDR catalyses the first reaction of the polyol pathway, which leads to the production of both DAG and AGES, thus contributing to signaling via both TLR4 and RAGE [218]. The role of ALDR in potentiating LPS-TLR4 signaling via PKC is evidenced by findings that its inhibition alleviates endotoxin-induced inflammatory diseases [216, 219, 220].
Mammalian xanthine dehydrogenase (XDH) is reversibly converted to xanthine oxidase (XO) by oxidative modification of specific cysteine residues [221]. As already mentioned, lipoxygenase-mediated 15-HETE formation during hypoxia activates XO. Fructose metabolism in hepatocytes is associated with increased XO-mediated conversion of AMP to uric acid [85, 222–224], which promotes insulin resistance through RAGE, TLR4, NF-κB, Nox, mitochondrial oxidative stress, ER stress, and skeletal muscle atrophy [30, 34, 223–227].
Oxidative stress promotes ceramide synthesis even independently of SPT and ceramide synthases. Singlet oxygen converts sphingomyelin to ceramide, even in protein-free liposomes [228]. In glioma cells, superoxide promotes ceramide formation through activation of neutral sphingomyelinase [229]. Sphingomyelinase inhibition reduces intramyocellular ceramide and protects muscle cells from insulin resistance [230, 231].
H2O2 downregulates the expression of carnitine palmitoyl transferase 1 (CPT1), acyl COA oxidase (ACOX), and peroxisome proliferator-activated receptor-alpha (PPAR-α) in hepatocytes [232] and PPAR-γ in endothelial cells [233]. CPT1 and ACOX are involved in fatty acid oxidation and reduction of DAG and ceramide levels [232, 234, 235]. PPAR-α reduces oxidative stress by upregulating superoxide dismutase and catalase expression and inhibiting NF-κB activity [232, 236, 237]. PPAR-γ inhibits NF-κB and upregulates the expression of adiponectin, an adipokine that improves insulin sensitivity [238–240].
3.2. Nitrosative Stress
The importance of nitrosative stress in insulin resistance is evidenced by reports that inhibition of iNOS or peroxynitrite in various cell types prevents insulin resistance [41–43]. Peroxynitrite decomposes into radicals that cause inhibitory tyrosine nitration of proteins in the insulin signaling pathway [70]. The reaction of peroxynitrite with proteins generates thyl radicals and sulfenates, leading to protein glutathionylation [192]. Nitrosoglutathione causes glutathionylation and inhibition of GADPH [241]. Peroxynitrite contributes to palmitate-induced DNA damage and inflammasome activation [23, 242]. It also induces ceramide formation in endothelial cells [243]. Nevertheless, the effects of peroxynitrite are mediated to some extent by ROS since peroxynitrite-derived radicals can initiate free radical lipid peroxidation [244], and the reaction of peroxynitrite with hydrogen peroxide generates singlet oxygen [30, 71]. Thus, iNOS and NO donor-induced IRS-1 degradation in skeletal muscle cells was accentuated by concomitant oxidative stress [245].
3.3. Carbonyl/Electrophilic Stress
Reactive carbonyl species contribute to insulin resistance in various ways. Methylglyoxal, HNE, and cholesterol secosterol aldehydes participate in the generation of oxidative stress and associated NF-κB and MAPK activation (Sections 2.1, 2.2, and 3.1). In addition, methylglyoxal adducts insulin and inhibits the latter's proper interaction with the insulin receptor [246]. Protein-HNE adducts correlate with intramyocellular lipid content and the severity of insulin resistance in humans [247]. HNE forms Michael adducts with His196 and Cys311 of Akt2 and thus inhibits downstream phosphorylation of Akt substrates such as glycogen synthase kinase 3β (GSK3β) and MDM2, resulting in the activation of the former and inhibition of the latter [247]. GSK3β inhibits glycogen synthase and IRS and thus prevents both glycogen synthesis and glucose transport [248–250]. It also promotes hepatic gluconeogenesis by an unknown mechanism [251] and contributes to the dysregulation of the Nrf2 antioxidant response [252]. MDM2 is a negative regulator of the p53 protein, which promotes insulin resistance as discussed in Section 3.4.
Human adipocytes and white adipose tissue express the full enzymatic machinery required for the synthesis and metabolism of asymmetric NGNGdimethylarginine (ADMA) which uncouples NOS and thus promotes ROS formation, increases TLR4 expression, decreases IRS-1 and GLUT-4 expression, and inhibits IRS-1 tyrosine phosphorylation and GLUT-4 translocation [253–256]. Plasma levels of ADMA increase during oxidative stress, mainly due to decreased expression and activity of the ADMA-degrading enzyme, dimethylarginine dimethylaminohydrolase (DDAH) [255–259]. HNE downregulates DDAH-1 expression through an miR-21-dependent mechanism [259].
3.4. Genotoxic Stress
Increased oxidative DNA damage determined as serum 8-hydroxy-2-deoxy-guanosine (8-OHdG) was found in lean normoglycemic offspring of type 2 diabetics, who are more predisposed to insulin resistance [260]. Similarly, serum level of 8-OHdG was found to be increased in prediabetes [261]. Mice deficient in 8-oxoguanine DNA glycosylase (the enzyme that performs base excision repair of DNA by cleaving 8-oxoG and other modified bases) were found to be prone to insulin resistance upon high-fat feeding [262]. Mitochondrial DNA damage promotes palmitate-induced insulin resistance mainly by increasing mitochondrial oxidative stress, ER stress, JNK activation, and apoptosis, since overexpression of DNA glycosylase/apurinic/apyrimidinic lyase (hOGG1) in the mitochondria of skeletal muscle cells abrogated these effects [263, 264]. Interestingly, prevention of mitochondrial DNA damage in cardiomyocytes exposed to angiotensin II prevented mitochondrial superoxide production in these cells [265]. In the latter study, mtDNA damage was found to cause impairments in mitochondrial protein expression, cellular respiration, and complex 1 activity prior to enhanced mitochondrial superoxide production. In addition, oxidized mitochondrial DNA released into the cytoplasm during apoptotic signaling activates the NLRP3 inflammasome [266].
3.5. Endoplasmic Reticulum Stress
Endoplasmic reticulum stress contributes to insulin resistance by promoting oxidative stress, especially mitochondrial oxidative stress and the resultant carbonyl and genotoxic stresses (Section 2.3) and ceramide synthesis [46], as well as by triggering the unfolded protein response [267], inflammasome activation [268], and apoptosis (Section 4.4).
4. Inhibition of Insulin Signaling by Cellular Stress Responses
4.1. The Ubiquitin-Proteosome Pathway
The ubiquitin-proteosome pathway (UPP) is the major cytosolic mechanism for the selective degradation of damaged proteins, such as oxidatively modified proteins, whereby the damaged proteins are conjugated to multiple ubiquitin molecules and then degraded by the 26S proteasome [269]. This system is upregulated by mild and moderate oxidative stress and is required for cells to cope with oxidative stress [269]. On the other hand, UPP-mediated degradation of the NF-κB inhibitor, iKB, causes activation of NF-κB [270]. NF-κB promotes oxidative stress and induces expression of proinflammatory cytokines including TNF-α and IL-6 which, via the JAK-STAT pathway, upregulate expression of suppressors of cytokine signaling (SOCS) proteins such as SOCS1 and SOCS3 [271–273]. Association of the SOCS proteins with IRS targets the latter for degradation by the ubiquitin-proteosome pathway in multiple cell types [271–273]. Accordingly, palmitate-induced insulin resistance in L6 myotubes was found to be dependent on constitutive phosphorylation of STAT 3 and the associated increase in protein expression of SOCS 3 [274], and the ubiquitination and proteosomal degradation of IRS-1 and Akt was demonstrated to contribute to palmitate or NO donor-induced insulin resistance in HepG2 cells and skeletal muscle cells, respectively [245, 275]. Moreover, increased SOCS1/SOCS3 expression during uveitis induces insulin resistance in neuroretina [276], and SOCS3 overexpression is responsible for the induction of insulin resistance in mice infected with hepatitis C virus [277].
Several factors contribute to increased UPP during the pathogenesis of insulin resistance. For example, the 15-lipoxygenase product, 15-HETE, induces the expression of key enzymes of the UPP pathway [150]. Inhibition of the adipose tissue ERK1/2 pathway during a high-fat diet was reported to enhance the UPP-mediated degradation of adiponectin [278], although contradictory results that ERK activity increases in hypertrophic adipocytes have also been obtained [279]. Increased HNE activity during a high-fat diet enhances the ubiquitin-proteosome-mediated degradation of adiponectin [280]. This is detrimental since adiponectin improves insulin sensitivity by (i) upregulating ceramidase activity to decrease ceramide levels [135]; (ii) increasing the levels of tetrahydrobiopterin (BH4), which lowers hepatocyte gluconeogenesis by activating AMP-activated kinase (AMPK) in an eNOS-dependent process [281]; and (iii) upregulating the silent information regulator 1 (SIRT1), a nicotinamide adenine dinucleotide-dependent histone deacetylase [282].
SIRT1 plays a role in the reduction of oxidative stress through increased expression of superoxide dismutase and catalase subsequently to FOXO4 deacetylation [283]. It also activates AMP-activated kinase (AMPK), which promotes insulin sensitivity by inhibiting PKC isoforms and the associated NF-κB activation, oxidative stress, ER stress, and apoptosis [284–291]. AMPK also induces mitochondrial biogenesis, which limits endothelial cell dysfunction, for example, in response to angiotensin II signaling [292]. Phosphorylation of SIRT1 by JNK1 primes SIRT1 for ubiquitination and degradation, and persistent JNK1 activation in obesity causes severe hepatic SIRT1 degradation [293]. SIRT1 reduction is detrimental to insulin signaling in various tissues including liver, skeletal muscle, and adipose tissues [294–296]. AMPK1 is also diminished in insulin-resistant individuals, and pharmacological agents that activate it, such as metformin, improve insulin signaling [290].
Increased mitochondrial DNA methylation in NADH dehydrogenase 6 (ND6) and displacement loop (D-loop) regions significantly correlates with insulin resistance, and SIRT1 deregulation was suggested to be involved in such epigenetic changes [297]. Since AMPK-mediated phosphorylation results in inhibition of DNA methyl transferase 1 (DNMT1) [298], UPP-mediated SIRT1 downregulation may induce such epigenetic changes through AMPK inhibition. Inflammasome activation might also contribute to this process by promoting DNMT1 expression (see Section 4.5). On the other hand, one study recently found that oxidative stress downregulated DNMT1 isoform 3, the isoform that is responsible for mitochondrial DNA methylation [299]. Further studies are necessary to resolve this apparent contradiction.
The UPP may especially be relevant in skeletal muscle insulin resistance by contributing to skeletal muscle atrophy, which occurs in two steps, namely, (i) the release of myofilaments from the sarcomere by cysteine proteases such as calpain and caspases and (ii) UPP-mediated degradation of the myofilament fragments [300, 301]. Calpain activation may in turn rely on the release of calcium from the ER during ER stress. Calpain activation in the skeletal muscle results in inhibited Akt activity, which in turn results in the activation of Foxo transcription factors that activate expression of components of the ubiquitin-proteosome system involved in muscle protein degradation [301]. Muscle atrophy per se has been associated with insulin resistance due to a decline in muscle oxidative capacity [302]. Exposure of C2C12 myotubes to 25 μM H2O2 induced calpain-dependent atrophy without cell death [300]. Exposure of C2 myotubes to peroxynitrite induced degradation of the myosin heavy chain muscle through activation of p38 MAPK and upregulation of the muscle-specific E3 ubiquitin ligases atrogin-1 and MuRF1 [303]. Increased expression of the transforming growth factor-β (TGF-β) and myostatin, via NF-κB, induces proteosomal degradation of cellular proteins [304, 305], and muscle myostatin mRNA correlates with HOMA2-IR in nondiabetic individuals [306].
While mild or moderate oxidative stress upregulates the ubiquitin-proteosome pathway, severe or sustained oxidative stress inactivates this system, especially the 26S proteasome [269, 307]. This also contributes to insulin resistance since proteosomal dysfunction, characterized by increased levels of carbonylated and ubiquitinated proteins, aggravates oxidative stress and ER stress [308].
4.2. The Unfolded Protein Response
ER stress triggers a transcriptional and translational response referred to as the unfolded protein response (UPR), aimed at reducing the translation of global proteins, enhancing the degradation of unfolded proteins, and increasing the transcription of genes that enhance the protein folding capacity of the ER [267]. The double-stranded RNA-dependent protein kinase- (PKR-) like ER kinase (PERK), the inositol requiring kinase 1 (IRE 1), and activating transcription factor 6 (ATF 6) are ER transmembrane proteins that are key components of three different UPR signaling pathways [267]. Details of the signaling events that occur after activation of PERK, IRE1, and ATF 6 have been described elsewhere [267, 309].
All three UPR pathways promote NF-κB activity and oxidative stress [310, 311]. Besides, IRE-1 stimulates ASK-1 and thus activates JNK and p38 MAPK [312]. PERK promotes insulin resistance by (i) activating JNK and p38 MAPK [49, 313]; (ii) phosphorylating FOXO on S298, a site which is not phosphorylated by Akt and whose phosphorylation counteracts the effects of Akt [49]; (iii) downregulating expression of the serine protease prostatin (PRSS8), which regulatorily degrades TLR4 [314]; (iv) inducing the pseudokinase tribble 3 (TRB3), which is increased in the liver of obese mice and humans and contributes to hepatic insulin resistance [49, 315]. TRB3 binds to Akt and prevents insulin-mediated Akt phosphorylation [49, 173, 315].
Low-grade hypothalamic inflammation induced by TNF-α was found to reduce oxygen consumption and the expression of thermogenic proteins in adipose tissue and skeletal muscles, and this was associated with insulin resistance in a rat model [316]. PERK, to a lesser extent, ATF, and IRE upregulate the C/EBP homologous protein (CHOP) [317], which downregulates cAMP-induced upregulation of uncoupling protein 1 in adipocytes and thus prevents adaptive thermogenesis [318].
4.3. The DNA Damage Response
The DNA damage response is a complex mechanism to detect DNA damage, including strand breaks or base modifications, promote their repair, and in case of excessive damage to activate cell death pathways [319]. Sensing of double-strand breaks by the Mre11-Rad50-Nbs1 (MRN) complex leads to the activation of ataxia telangiectasia mutated (ATM) and subsequent activation of DNA damage checkpoints [319]. ATM activates NF-κB following DNA damage [320]. Checkpoints induce changes in telomeric chromatin, recruit DNA repair proteins to sites of DNA damage, and induce cell death by apoptosis [321]. One of the checkpoints, Chk2, promotes transcription of genes involved in DNA repair and phosphorylates tumor suppressor p53, thereby reversing inhibition of the latter by MDM2 [319]. Chk2 also phosphorylates MDM4 and thereby reduces p53 degradation [319]. Such activation of the p53 pathway is upregulated in adipose, endothelial, hepatic, and skeletal muscle tissues of obese rodents and humans [322–326]. Although this protein exhibits antioxidant activity at low levels of oxidative stress, it becomes prooxidant at higher oxidative stress levels, through activation of NF-κB [327] and promotion of ceramide synthesis by upregulating ceramide synthases [328]. p53 promotes cellular senescence in adipose tissue, and this is associated with increased production of proinflammatory cytokines, which promote adipose tissue infiltration by neutrophils and macrophages, and systemic insulin resistance [322–326]. Many other metabolic effects of p53 are opposed to insulin signaling, as has been exhaustively reviewed [322, 327], including but not limited to JNK activation; apoptosis; repressed expression of the insulin receptor and glucose transporters 1, 3, and 4; enhanced transcription of phosphatase and tensin homologue (PTEN) which reduces phosphorylation of P13K and Akt; Akt degradation; glycolysis inhibition; and downregulated expression of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) in the skeletal muscle, leading to the reduction in mitochondrial biogenesis and energy consumption. Reduced mitochondrial biogenesis leads to a lower capacity for fatty acid metabolism in skeletal muscle cells and accumulation of intramyocellular lipids including DAG which is a major contributor to skeletal muscle insulin resistance [329, 330].
4.4. Apoptosis
Apoptosis is a type of programmed cell death in response to cellular damage or other physiological cues, characterized by controlled autodigestion of the cell by caspases [331, 332]. It is regarded as extrinsic when it involves death receptors such as CD95 (Fas) or intrinsic if it occurs independently of such receptors, and both forms of apoptosis were found to be involved in insulin resistance in a mouse model [55]. Caspase-8 and caspase-9, respectively, act as initiator caspases for extrinsic and intrinsic apoptosis, and each initiator caspase starts off a cascade for the activation of executer caspases, which degrade key cellular proteins [333].
Oxidative stress promotes Fas ligand expression in various cell types and thus promotes extrinsic apoptosis [334]. Activation of the Fas receptor by metabolites such as IL-1β, oxLDL, singlet oxygen, HNE, or JNK leads to its intracellular recruitment of the adaptor protein FADD to form a death-inducing complex (DISC) which activates initiator caspase-8, while the autocatalytic activation of procaspase-9, the initiator of intrinsic apoptosis, requires the assembly of a multiprotein complex, the apoptosome, which comprises seven copies of heterodimers between apoptotic protease-activating factor 1 (Apaf-1) and cytochrome c [331–335]. Thus, the release of the latter from the mitochondrial membrane into the cytoplasm is a key event in intrinsic apoptosis.
During unresolved ER stress, ER calcium efflux promotes lysosomal membrane permeabilization (LMP) and the release of lysosomal cathepsins, which promote mitochondrial outer membrane permeabilization (MOMP) and the release of cytochrome c from the mitochondrial intermembrane space [331, 332]. Localization of p53 protein on the lysosomal membrane upon sustained DNA damage also contributes to LMP [336]. Activated JNK contributes to apoptosis in various ways including (i) inducing the expression of proapoptotic genes through transactivation of c-jun or p53, (ii) phosphorylating the BH3-only family of Bcl2 proteins which antagonize the antiapoptotic activity of Bcl2 or Bcl Xl, and (iii) activating Bim, a BH3-domain-only protein which activates Bax, which in turn promotes MOMP and cytochrome c release [337].
In humans, the progression of nonalcoholic fatty liver disease is associated with increasing apoptosis and insulin resistance in the muscle, liver, and adipose tissue [338]. The link between hepatocyte apoptosis and insulin resistance was demonstrated in a mouse model [56]. Adipocytes of obese mice were found to display a proapoptotic phenotype, and genetic inactivation of the key proapoptotic protein Bid protected against adipose tissue macrophage infiltration and systemic insulin resistance [55]. Prevention of apoptosis prevents palmitate-induced insulin resistance in hypothalamic neurons [339]. Palmitate induces apoptosis and insulin resistance in skeletal muscle myotubes, and cell-permeable effector caspase inhibitors reverse the insulin resistance, indicating that cellular remodeling associated with apoptotic signaling induces insulin resistance [340]. In these cells, caspases inhibit glycolysis, in particular the glycolysis-limiting enzymes phosphofructokinase and pyruvate kinase [340, 341]. In adipocytes, the proapoptotic caspase-3 and caspase-6, which participate in both intrinsic and extrinsic apoptosis, cleave peroxisome proliferator-activated receptor-γ (PPAR-γ), which results in the nuclear export and cytoplasmic degradation of this transcription factor [342]. Inactivation of PPAR-γ in adipocytes leads to downregulation of some genes that are important for insulin sensitivity not only in adipose tissue but also in other tissues such as those of the skeletal muscle. For example, PPAR-γ inactivation results in decreased expression of GLUT 4 and decreased secretion of adiponectin [343, 344]. Extensive apoptosis of adipocytes, hepatocytes, and skeletal muscle cells is also expected to contribute to systemic hyperglycemia and hyperglycemia-induced stresses that lead to insulin resistance.
4.5. NRLP3 Inflammasome Activation
Interleukin-1β (IL-1β) is an inflammatory cytokine which activates both myeloid and nonmyeloid cells to produce other inflammatory cytokines and chemokines [345, 346]. Processing of the inactive pro-IL-1β into the active IL-1β requires the formation and activation of a cytoplasmic multiprotein complex called the inflammasome [58, 345]. One of the most intensively studied inflammasomes is the NLRP3 inflammasome which is expressed by myeloid cells and some nonmyeloid cells such as adipocytes, hepatocytes, endothelial cells, skeletal muscle cells, and aortic smooth muscle cells [345, 347–350]. Components of the NLRP3 inflammasome include the NLRP3 sensor, ASC adaptor, and caspase-1 [345]. Signaling pathways through NF-κB, p38, and ERK1 are involved in the expression of both NLRP3 and pro-IL-1β [345, 351–353]. Assembly of the NLRP3 components into the active inflammasome complex occurs in response to “danger signals” including increased intracellular ceramide; RAGE- or IREα-dependent increased expression of thioredoxin-binding protein (TXNIP); oxidation of thioredoxin and its dissociation from TXNIP, allowing the latter to bind to the inflammasome; release of lysosomal cathepsin B as a result of LMP; IRE1-α- and PERK-dependent activation of CHOP; and release of oxidized mitochondrial DNA as a result of MOMP [58, 204, 205, 265, 267, 353–359].
IL-1β is a ligand for the IL-1 receptor which, like TLR4, signals via MyD88, IL-1 receptor-activated kinases (IRAK1 to 4), IKK, and NF-κB (Figure 2) [57, 58, 360] and should thus potentiate the whole model for insulin resistance in insulin target cells (Figure 2). It downregulates IRS-1 expression and reduces tyrosine phosphorylation of IRS-1 in adipocytes [58, 361]. Preadipocytes release IL-1β, which both controls adipocyte differentiation and promotes adipocyte insulin resistance even in the absence of macrophages [58]. IL-1β also induces epigenetic changes that promote insulin resistance. For example, it stimulates the expression of DNA methyl transferase 1, which hypermethylates the adiponectin promoter and thereby suppresses the expression of this proinsulin signaling adipokine [362].
While inflammasome assembly, caspase-1 activation, and IL-1β processing occur and promote insulin resistance in hepatocytes and mature adipocytes, secretion of IL-1β by these cells is controversial [58, 268, 363]. Nevertheless, caspase-1 induces the highly inflammatory pyroptotic death of these cells, and this could contribute to the recruitment and activation of inflammatory myeloid cells such as macrophages that secrete IL-1β [268, 363]. In the skeletal muscle, activation of the inflammasome contributes to muscle atrophy through activation of atrogenic genes such as MuRF1 and atrogin 1 [350].
NLRP inflammasome activation in endothelial cells leads to increased IL-1β in serum and C-reactive protein (CRP) production by endothelial cells [349]. IL-1β stimulates endothelial cell production of chemokines such as monocyte chemoattractant protein-1 (MCP-1) and vascular cell adhesion molecule-1 (VCAM-1) which promote leukocyte-endothelium interactions [349, 364], and this may contribute to the transient migration of neutrophils into adipose tissue that occurs at an early stage of high-fat feeding [365]. This process may be further facilitated by the chemotactic effects of H2O2 and IL-8 produced by adipocytes [30, 366]. Once in adipose tissue, neutrophils may produce large quantities of chemokines and cytokines including IL-1β and IL8, resulting in the recruitment of other immune system cells such as macrophages which sustain chronic inflammation [367, 368].
5. Inhibition of Insulin Signaling through the Dysregulation of Cellular Stress Responses
5.1. Dysregulation of the Heat Shock Response
The heat shock response, which relies on heat shock proteins such as HSP70, is important for physiological resolution of inflammation [369]. Cellular HSP70, HSP72, and HSP25 protect against insulin resistance in humans by mechanisms involving prevention of JNK phosphorylation and apoptosis [51–53]. Obese, insulin-resistant individuals have reduced expression of HSP72 [51]. In adipocytes, downregulation of HSP expression follows sustained NLRP3 inflammasome activation and the associated caspase-1-mediated cleavage of HuR, an mRNA-binding protein that enhances the expression of SIRT1 [369]. This results in reduced SIRT1-dependent upregulation of the transcription and activity of heat shock factor 1 (HSF1), the transcription factor of heat shock proteins [369].
5.2. Autophagy Dysregulation
(Macro)autophagy is a homoeostatic process for the bulk degradation of cytoplasmic components including damaged organelles, misfolded proteins, and oxidized lipids, whereby such components are enclosed into double-membraned vesicles called autophagosomes that subsequently fuse with lysosomes [54, 370–372]. Autophagy-related proteins (Atg) are involved in autophagosome formation, and these are functionally categorized into several units, namely, the Atg1/ULK complex (mammals express Ulk 1/2), the class III phosphatidyl inositol 3-kinase (PI3K) complex, the Atg2-Atg18/WIPI complex, the Atg12 conjugation system, the Atg8/LC3 conjugation system, and Atg9 vesicles [373].
Low levels of ROS induce autophagy [373–375], but higher ROS levels inhibit this response [376]. Obesity, which is associated with oxidative stress, is characterized by inhibited autophagy [377], even in adipose tissue that has elevated expression of autophagy genes [378]. Autophagy inhibition occurs partly due to (i) degradation of autophagy proteins by cell death proteases including calpain 1 and caspases such as caspase-3, caspase-6, and caspase-8 [379], (ii) LMP and the release of cathepsins [379, 380], (iii) SIRT downregulation [381, 382], (vi) inhibition of PPAR-α [383], and (vii) increased expression of GSK3β [384].
Severe hepatic downregulation of the autophagy gene Atg7 was found to occur in genetic and dietary models of obesity, and this caused insulin resistance through enhanced ER stress [54]. Paradoxically, muscle- or liver-specific knockout of the Atg7 gene protected mice from obesity and insulin resistance by upregulating the expression of fibroblast growth factor (FGF21) [385]. FGF21 improves insulin sensitivity by inhibiting mTORC 1 [385, 386], activating NRf2 antioxidant signaling, suppressing the NF-κB pathway, enhancing adiponectin production, and promoting thermogenesis [387–391]. The apparently contradictory effects of obesity-associated downregulation of Atg7 and genetic knockout of Atg7 on hepatocyte insulin resistance [54, 385] may be better understood by considering that Atg7 knockout prevents obesity [385]. In obesity, but not in the lean state, there is resistance to FGF21, because of downregulation of its receptor machinery, including β-klotho protein levels [392–394]. Although klotho is critical for FGF21 function [395], it was recently reported that factors beyond β-klotho downregulation are important contributors to adipose tissue FGF21 resistance [396].
5.3. Dysregulation of the Nrf2 Antioxidant Response
The nuclear factor erythroid-2-related factor-2 (Nrf2) is the master regulator of antioxidant responses, attenuating both oxidative and electrophilic stresses [397, 398]. Under basal conditions, Nrf2 localizes on the cytoskeleton, where its activity is limited through interaction with Kelch-like ECH-associated protein 1 (Keap1), which targets it for ubiquitination and proteosomal degradation [399]. Modification of cysteine residues in Keap1 by ROS, RNS, or RCCs frees Nrf2 to translocate to the nucleus and induce transcription of antioxidant genes having the antioxidant response element in the promoter region [397, 400]. Nevertheless, Nrf2 levels were found to be lower in prediabetic and diabetic patients than in healthy subjects [401], and short-term treatment of high-fat diet-fed mice with curcumin was found to improve insulin sensitivity through attenuating Nrf2 signaling defect [402].
Suppression of Nrf2 activity may be partly due to the direct interaction of p53 with ARE-containing promoters [403]. ERK activation was reported to induce Nrf2 suppression and insulin resistance in cardiomyocytes exposed to hydrogen peroxide [404], but an opposite effect of ERK activation was reported in HepG2 cells exposed to methylglyoxal [405]. In neuronal cells exposed to H2O2, GSK3β activation was shown to be responsible for the cytoplasmic accumulation of Nrf2 [251]. This may involve H2O2-mediated activatory phosphorylation of tyrosine 216 of GSK3β, and the latter phosphorylates the tyrosine kinase Fyn, which then translocates to the nucleus, and phosphorylates tyrosine 568 of Nrf2, leading to Nrf2 export from the nucleus [406].
Nrf2 antioxidant response is also downregulated by cortisol [407], whose production is increased during psychological stress [117]. Obesity is associated with higher adipose tissue expression of 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1), an enzyme that converts cortisone to active cortisol [408, 409]. Cortisol is a ligand for the mineralocorticoid receptor, whose expression increases in obesity [122].
Notably, Nrf2 is Janus-faced, and its overexpression was found to worsen insulin resistance in mice [410]. Nrf2-knockout mice on a long-term high-fat diet had increased FGF21 expression and better insulin sensitivity than wild-type mice on the same diet, and overexpression of Nrf2 in ST-2 cells was found to decrease insulin sensitivity associated with decreased FGF21 mRNA levels and activity [411]. Nrf2 overexpression may occur during autophagy blockade, which is associated with an increase in the cellular levels of p62 [412, 413]. p62 normally participates in autophagosome formation and undergoes lysosomal degradation with the contents of the autophagosome [412, 413]. However, during autophagy blockade, it sequesters Keap1 into autophagosomes, leading to stabilization and overactivation of Nrf2 by the so called noncanonical pathway [412]. Thus, for beneficial effects, the level of Nrf2 activation needs tight regulation.
6. Conclusion
There is substantial literature in support of the hypothesis that insulin resistance develops from a coordinated interplay between various cellular stresses and stress responses that develop upon the exposure of insulin-responsive cells to hypoxia, excess sugars or certain types of fatty acids, environmental pollutants, or hormones released during psychological stress and obesity. This knowledge will help in the design of better strategies for the prevention and management of insulin resistance.
Conflicts of Interest
The author declares that there are no conflicts of interests regarding the publication of this paper.
References
- 1.Kolka C. M., Bergman R. N. The endothelium in diabetes: its role in insulin access and diabetic complications. Reviews in Endocrine and Metabolic Disorders. 2013;14(1):13–19. doi: 10.1007/s11154-012-9233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddle K. Signalling by insulin and IGF receptors: supporting acts and new players. Journal of Molecular Endocrinology. 2011;47(1):R1–R10. doi: 10.1530/JME-11-0022. [DOI] [PubMed] [Google Scholar]
- 3.Dimitriadis G., Mitrou P., Lambadiari V., Maratou E., Raptis S. A. Insulin effects in muscle and adipose tissue. Diabetes Research and Clinical Practice. 2011;93(Supplement 1):S52–S59. doi: 10.1016/S0168-8227(11)70014-6. [DOI] [PubMed] [Google Scholar]
- 4.Fick L. J., Belsham D. D. Nutrient sensing and insulin signaling in neuropeptide-expressing immortalized, hypothalamic neurons: a cellular model of insulin resistance. Cell Cycle. 2010;9(16):3186–3193. doi: 10.4161/cc.9.16.12601. [DOI] [PubMed] [Google Scholar]
- 5.Plum L., Belgardt B. F., Bruning J. C. Central insulin action in energy and glucose homeostasis. Journal of Clinical Investigation. 2006;116(7):1761–1766. doi: 10.1172/JCI29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinridders A., Ferris H. A., Cai W., Kahn C. R. Insulin action in brain regulates systemic metabolism and brain function. Diabetes. 2014;63(7):2232–2243. doi: 10.2337/db14-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Posey K. A., Clegg D. J., Printz R. L., et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. American Journal of Physiology-Endocrinology and Metabolism. 2009;296(5):E1003–E1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clegg D. J., Gotoh K., Kemp C., et al. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiology & Behavior. 2011;103(1):10–16. doi: 10.1016/j.physbeh.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Könner A. C., Janoschek R., Plum L., et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metabolism. 2007;5(6):438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Obici S., Zhang B. B., Karkanias G., Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nature Medicine. 2002;8(12):1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 11.Pocai A., Lam T. K. T., Gutierrez-Juarez R., et al. Hypothalamic KATP channels control hepatic glucose production. Nature. 2005;434(7036):1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- 12.Blázquez E., Velázquez E., Hurtado-Carneiro V., Ruiz-Albusac J. M. Insulin in the brain: its pathophysiological implications for states related with central insulin resistance, type 2 diabetes and Alzheimer’s disease. Frontiers in Endocrinology. 2014;5:p. 161. doi: 10.3389/fendo.2014.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shanik M. H., Xu Y., Skrha J., Dankner R., Zick Y., Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31(Supplement 2):S262–S268. doi: 10.2337/dc08-s264. [DOI] [PubMed] [Google Scholar]
- 14.Watterson K. R., Bestow D., Gallagher J., et al. Anorexigenic and orexigenic hormone modulation of mammalian target of rapamycin complex 1 activity and the regulation of hypothalamic agouti-related protein mRNA expression. Neurosignals. 2013;21(1-2):28–41. doi: 10.1159/000334144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milanski M., Arruda A. P., Coope A., et al. Inhibition of hypothalamic inflammation reverses diet-induced insulin resistance in the liver. Diabetes. 2012;61(6):1455–1462. doi: 10.2337/db11-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin C.-L., Huang C.-N. The neuroprotective effects of the anti-diabetic drug linagliptin against Aß-induced neurotoxicity. Neural Regeneration Research. 2016;11(2):236–237. doi: 10.4103/1673-5374.177724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de la Monte S. M. Insulin resistance and neurodegeneration: progress towards the development of new therapeutics for Alzheimer’s disease. Drugs. 2017;77(1):47–65. doi: 10.1007/s40265-016-0674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forno E., Han Y.-Y., Muzumdar R. H., Celedón J. C. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. Journal of Allergy and Clinical Immunology. 2015;136(2):304–311.e8. doi: 10.1016/j.jaci.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q., Cheng X. L., Zhang D. Y., et al. Tectorigenin attenuates palmitate-induced endothelial insulin resistance via targeting ROS-associated inflammation and IRS-1 pathway. PLoS One. 2013;8(6, article e66417) doi: 10.1371/journal.pone.0066417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao D., Nong S., Huang X., et al. The effects of palmitate on hepatic insulin resistance are mediated by NADPH oxidase 3-derived reactive oxygen species through JNK and p38MAPK pathways. Journal of Biological Chemistry. 2010;285(39):29965–29973. doi: 10.1074/jbc.M110.128694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Figueiredo A. S. P., Salmon A. B., Bruno F., et al. Nox2 mediates skeletal muscle insulin resistance induced by a high fat diet. Journal of Biological Chemistry. 2015;290(21):13427–13439. doi: 10.1074/jbc.M114.626077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuzefovych L., Wilson G., Rachek L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. American Journal of Physiology-Endocrinology and Metabolism. 2010;299(6):E1096–E1105. doi: 10.1152/ajpendo.00238.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rachek L. I., Musiyenko S. I., LeDoux S. P., Wilson G. L. Palmitate induced mitochondrial deoxyribonucleic acid damage and apoptosis in l6 rat skeletal muscle cells. Endocrinology. 2007;148(1):293–299. doi: 10.1210/en.2006-0998. [DOI] [PubMed] [Google Scholar]
- 24.Renström F., Burén J., Svensson M., Eriksson J. W. Insulin resistance induced by high glucose and high insulin precedes insulin receptor substrate 1 protein depletion in human adipocytes. Metabolism. 2007;56(2):190–198. doi: 10.1016/j.metabol.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Jaiswal N., Maurya C. K., Pandey J., Rai A. K., Tamrakar A. K. Fructose-induced ROS generation impairs glucose utilization in L6 skeletal muscle cells. Free Radical Research. 2015;49(9):1055–1068. doi: 10.3109/10715762.2015.1031662. [DOI] [PubMed] [Google Scholar]
- 26.Kwon B., Querfurth H. W. Opposite effects of saturated and unsaturated free fatty acids on intracellular signaling and metabolism in neuronal cells. Inflammation and Cell Signaling. 2014;1(2) doi: 10.14800/ics.200. [DOI] [Google Scholar]
- 27.Moraes J. C., Coope A., Morari J., et al. High-fat diet induces apoptosis of hypothalamic neurons. PLoS One. 2009;4(4, article e5045) doi: 10.1371/journal.pone.0005045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J. J., Sears D. D. TLR4 and insulin resistance. Gastroenterology Research and Practice. 2010;2010:11. doi: 10.1155/2010/212563.212563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Velloso L. A., Folli F., Saad M. J. TLR4 at the crossroads of nutrients, gut microbiota, and metabolic inflammation. Endocrine Reviews. 2015;36(3):245–271. doi: 10.1210/er.2014-1100. [DOI] [PubMed] [Google Scholar]
- 30.Onyango A. N. The contribution of singlet oxygen to insulin resistance. Oxidative Medicine and Cellular Longevity. 2017;2017:14. doi: 10.1155/2017/8765972.8765972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakashima T., Umemoto S., Yoshimura K., et al. TLR4 is a critical regulator of angiotensin II-induced vascular remodeling: the roles of extracellular SOD and NADPH oxidase. Hypertension Research. 2015;38(10):649–655. doi: 10.1038/hr.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakaguchi M., Murata H., Yamamoto K., et al. TIRAP, an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding. PLoS One. 2011;6(8, article e23132) doi: 10.1371/journal.pone.0023132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaens K. H. J., Goossens G. H., Niessen P. M., et al. Nε-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34(6):1199–1208. doi: 10.1161/ATVBAHA.113.302281. [DOI] [PubMed] [Google Scholar]
- 34.Cai W., Duan X.-M., Liu Y., et al. Uric acid induces endothelial dysfunction by activating the HMGB1/RAGE signaling pathway. BioMed Research International. 2017;2017:11. doi: 10.1155/2017/4391920.4391920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scazzocchio B., Varì R., D'Archivio M., et al. Oxidized LDL impair adipocyte response to insulin by activating serine/threonine kinases. Journal of Lipid Research. 2009;50(5):832–845. doi: 10.1194/jlr.M800402-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clavreul N., Bachschmid M. M., Hou X., et al. S-Glutathiolation of p21ras by peroxynitrite mediates endothelial insulin resistance caused by oxidized low-density lipoprotein. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26(11):2454–2461. doi: 10.1161/01.ATV.0000242791.28953.4c. [DOI] [PubMed] [Google Scholar]
- 37.Pereira S., Park E., Mori Y., et al. FFA-induced hepatic insulin resistance in vivo is mediated by PKCδ, NADPH oxidase, and oxidative stress. American Journal of Physiology-Endocrinology and Metabolism. 2014;307(1):E34–E46. doi: 10.1152/ajpendo.00436.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sukumar P., Viswambharan H., Imrie H., et al. Nox2 NADPH oxidase has a critical role in insulin resistance–related endothelial cell dysfunction. Diabetes. 2013;62(6):2130–2134. doi: 10.2337/db12-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.den Hartigh L. J., Omer M., Goodspeed L., et al. Adipocyte-specific deficiency of NADPH oxidase 4 delays the onset of insulin resistance and attenuates adipose tissue inflammation in obesity. Arteriosclerosis, Thrombosis, and Vascular Biology. 2017;37(3):466–475. doi: 10.1161/ATVBAHA.116.308749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson E. J., Lustig M. E., Boyle K. E., et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. The Journal of Clinical Investigation. 2009;119(3):573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cha H.-N., Song S. E., Kim Y.-W., Kim J.-Y., Won K.-C., Park S.-Y. Lack of inducible nitric oxide synthase prevents lipid-induced skeletal muscle insulin resistance without attenuating cytokine level. Journal of Pharmacological Sciences. 2011;117(2):77–86. doi: 10.1254/jphs.11093FP. [DOI] [PubMed] [Google Scholar]
- 42.Charbonneau A., Marette A. Inducible nitric oxide synthase induction underlies lipid-induced hepatic insulin resistance in mice: potential role of tyrosine nitration of insulin signaling proteins. Diabetes. 2010;59(4):861–871. doi: 10.2337/db09-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zanotto T. M., Quaresma P. G. F., Guadagnini D., et al. Blocking iNOS and endoplasmic reticulum stress synergistically improves insulin resistance in mice. Molecular Metabolism. 2017;6(2):206–218. doi: 10.1016/j.molmet.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erion D. M., Shulman G. I. Diacylglycerol-mediated insulin resistance. Nature Medicine. 2010;16(4):400–402. doi: 10.1038/nm0410-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campana M., Bellini L., Rouch C., et al. Inhibition of central de novo ceramide synthesis restores insulin signaling in hypothalamus and enhances β-cell function of obese Zucker rats. Molecular Metabolism. 2018;8:23–36. doi: 10.1016/j.molmet.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chavez J. A., Summers S. A. A ceramide-centric view of insulin resistance. Cell Metabolism. 2012;15(5):585–594. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Turban S., Hajduch E. Protein kinase C isoforms: mediators of reactive lipid metabolites in the development of insulin resistance. FEBS Letters. 2011;585(2):269–274. doi: 10.1016/j.febslet.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 48.Panzhinskiy E., Ren J., Nair S. Protein tyrosine phosphatase 1B and insulin resistance: role of endoplasmic reticulum stress/reactive oxygen species/nuclear factor kappa B axis. PLoS One. 2013;8(10, article e77228) doi: 10.1371/journal.pone.0077228. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Zhang W., Hietakangas V., Wee S., Lim S. C., Gunaratne J., Cohen S. M. ER stress potentiates insulin resistance through PERK-mediated FOXO phosphorylation. Genes & Development. 2013;27(4):441–449. doi: 10.1101/gad.201731.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diaz B., Fuentes-Mera L., Tovar A., et al. Saturated lipids decrease mitofusin 2 leading to endoplasmic reticulum stress activation and insulin resistance in hypothalamic cells. Brain Research. 2015;1627:80–89. doi: 10.1016/j.brainres.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 51.Chung J., Nguyen A. K., Henstridge D. C., et al. HSP72 protects against obesity-induced insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(5):1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geiger P. C., Gupte A. A. Heat shock proteins are important mediators of skeletal muscle insulin sensitivity. Exercise and Sport Sciences Reviews. 2011;39(1):34–42. doi: 10.1097/JES.0b013e318201f236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chichester L., Wylie A. T., Craft S., Kavanagh K. Muscle heat shock protein 70 predicts insulin resistance with aging. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2015;70(2):155–162. doi: 10.1093/gerona/glu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang L., Li P., Fu S., Calay E. S., Hotamisligil G. S. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metabolism. 2010;11(6):467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alkhouri N., Gornicka A., Berk M. P., et al. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. Journal of Biological Chemistry. 2010;285(5):3428–3438. doi: 10.1074/jbc.M109.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Derdak Z., Lang C. H., Villegas K. A., et al. Activation of p53 enhances apoptosis and insulin resistance in a rat model of alcoholic liver disease. Journal of Hepatology. 2011;54(1):164–172. doi: 10.1016/j.jhep.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nov O., Kohl A., Lewis E. C., et al. Interleukin-1β may mediate insulin resistance in liver-derived cells in response to adipocyte inflammation. Endocrinology. 2010;151(9):4247–4256. doi: 10.1210/en.2010-0340. [DOI] [PubMed] [Google Scholar]
- 58.Stienstra R., Tack C. J., Kanneganti T.-D., Joosten L. A. B., Netea M. G. The inflammasome puts obesity in the danger zone. Cell Metabolism. 2012;15(1):10–18. doi: 10.1016/j.cmet.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 59.Samuel V. T., Shulman G. I. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148(5):852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pendyala S., Walker J. M., Holt P. R. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. 2012;142(5):1100–1101.e2. doi: 10.1053/j.gastro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.di Luccia B., Crescenzo R., Mazzoli A., et al. Rescue of fructose-induced metabolic syndrome by antibiotics or faecal transplantation in a rat model of obesity. PLoS One. 2015;10(8, article e0134893) doi: 10.1371/journal.pone.0134893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jia L., Vianna C. R., Fukuda M., et al. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nature Communications. 2014;5:p. 3778. doi: 10.1038/ncomms4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rorato R., Borges B. d. C., Uchoa E. T., Antunes-Rodrigues J., Elias C. F., Elias L. L. K. LPS-induced low-grade inflammation increases hypothalamic JNK expression and causes central insulin resistance irrespective of body weight changes. International Journal of Molecular Sciences. 2017;18(12):p. 1431. doi: 10.3390/ijms18071431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loegering D. J., Lennartz M. R. Protein kinase C and toll-like receptor signaling. Enzyme Research. 2011;2011:7. doi: 10.4061/2011/537821.537821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J., Wu W., Li D., Guo Y., Ding H. Overactivation of NF-κB impairs insulin sensitivity and mediates palmitate-induced insulin resistance in C2C12 skeletal muscle cells. Endocrine. 2010;37(1):157–166. doi: 10.1007/s12020-009-9283-y. [DOI] [PubMed] [Google Scholar]
- 66.Yu I.-C., Lin H.-Y., Liu N.-C., et al. Neuronal androgen receptor regulates insulin sensitivity via suppression of hypothalamic NF-κB–mediated PTP1B expression. Diabetes. 2013;62(2):411–423. doi: 10.2337/db12-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen L., Chen R., Wang H., Liang F. Mechanisms linking inflammation to insulin resistance. International Journal of Endocrinology. 2015;2015:9. doi: 10.1155/2015/508409.508409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Frontiers in Immunology. 2014;5:p. 461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morgan M. J., Liu Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Research. 2011;21(1):103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Radi R. Protein tyrosine nitration: biochemical mechanisms and structural basis of functional effects. Accounts of Chemical Research. 2013;46(2):550–559. doi: 10.1021/ar300234c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.di Mascio P., Bechara E. J. H., Medeiros M. H. G., Briviba K., Sies H. Singlet molecular oxygen production in the reaction of peroxynitrite with hydrogen peroxide. FEBS Letters. 1994;355(3):287–289. doi: 10.1016/0014-5793(94)01224-5. [DOI] [PubMed] [Google Scholar]
- 72.Lugrin J., Rosenblatt-Velin N., Parapanov R., Liaudet L. The role of oxidative stress during inflammatory processes. Biological Chemistry. 2014;395(2):203–230. doi: 10.1515/hsz-2013-0241. [DOI] [PubMed] [Google Scholar]
- 73.Elgawish A., Glomb M., Friedlander M., Monnier V. M. Involvement of hydrogen peroxide in collagen cross-linking by high glucose in vitro and in vivo. Journal of Biological Chemistry. 1996;271(22):12964–12971. doi: 10.1074/jbc.271.22.12964. [DOI] [PubMed] [Google Scholar]
- 74.Onyango A. N. Endogenous generation of singlet oxygen and ozone in human and animal tissues: mechanisms, biological significance, and influence of dietary components. Oxidative Medicine and Cellular Longevity. 2016;2016:22. doi: 10.1155/2016/2398573.2398573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aragno M., Mastrocola R. Dietary sugars and endogenous formation of advanced glycation endproducts: emerging mechanisms of disease. Nutrients. 2017;9(12):p. 385. doi: 10.3390/nu9040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Silva C. O., da Silva O. A., Duarte G. P., Descomps B., La S. Apocynin decreases AGEs-induced stimulation of NF-κB protein expression in vascular smooth muscle cells from GK rats. Pharmaceutical Biology. 2015;53(4):488–493. doi: 10.3109/13880209.2014.924150. [DOI] [PubMed] [Google Scholar]
- 77.Eriksson J. W. Metabolic stress in insulin’s target cells leads to ROS accumulation – a hypothetical common pathway causing insulin resistance. FEBS Letters. 2007;581(19):3734–3742. doi: 10.1016/j.febslet.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 78.Salaun E., Lefeuvre-Orfila L., Cavey T., et al. Myriocin prevents muscle ceramide accumulation but not muscle fiber atrophy during short-term mechanical unloading. Journal of Applied Physiology. 2016;120(2):178–187. doi: 10.1152/japplphysiol.00720.2015. [DOI] [PubMed] [Google Scholar]
- 79.Han C. Y., Kargi A. Y., Omer M., et al. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: dissociation of adipocyte hypertrophy from inflammation. Diabetes. 2010;59(2):386–396. doi: 10.2337/db09-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Inoguchi T., Li P., Umeda F., et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49(11):1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 81.Holland W. L., Bikman B. T., Wang L.-P., et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid–induced ceramide biosynthesis in mice. The Journal of Clinical Investigation. 2011;121(5):1858–1870. doi: 10.1172/jci43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barma P., Bhattacharya S., Bhattacharya A., et al. Lipid induced overexpression of NF-κB in skeletal muscle cells is linked to insulin resistance. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2009;1792(3):190–200. doi: 10.1016/j.bbadis.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 83.Joseph L. C., Barca E., Subramanyam P., et al. Inhibition of NAPDH oxidase 2 (NOX2) prevents oxidative stress and mitochondrial abnormalities caused by saturated fat in cardiomyocytes. PLoS One. 2016;11(1, article e0145750) doi: 10.1371/journal.pone.0145750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Galbo T., Perry R. J., Jurczak M. J., et al. Saturated and unsaturated fat induce hepatic insulin resistance independently of TLR-4 signaling and ceramide synthesis in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(31):12780–12785. doi: 10.1073/pnas.1311176110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cosentino-Gomes D., Rocco-Machado N., Meyer-Fernandes J. R. Cell signaling through protein kinase C oxidation and activation. International Journal of Molecular Sciences. 2012;13(12):10697–10721. doi: 10.3390/ijms130910697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maceyka M., Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510(7503):58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Powell D. J., Turban S., Gray A., Hajduch E., Hundal H. S. Intracellular ceramide synthesis and protein kinase Cζ activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochemical Journal. 2004;382(2):619–629. doi: 10.1042/bj20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hage Hassan R., Pacheco de Sousa A. C., Mahfouz R., et al. Sustained action of ceramide on the insulin signaling pathway in muscle cells: implication of the double-stranded RNA-activated protein kinase. Journal of Biological Chemistry. 2016;291(6):3019–3029. doi: 10.1074/jbc.M115.686949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.García-Ruiz C., Colell A., Marí M., Morales A., Fernández-Checa J. C. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Journal of Biological Chemistry. 1997;272(17):11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 90.Taddeo E. P., Laker R. C., Breen D. S., et al. Opening of the mitochondrial permeability transition pore links mitochondrial dysfunction to insulin resistance in skeletal muscle. Molecular Metabolism. 2014;3(2):124–134. doi: 10.1016/j.molmet.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matsunaga T., Kotamraju S., Kalivendi S. V., Dhanasekaran A., Joseph J., Kalyanaraman B. Ceramide-induced intracellular oxidant formation, iron signaling, and apoptosis in endothelial cells: protective role of endogenous nitric oxide. Journal of Biological Chemistry. 2004;279(27):28614–28624. doi: 10.1074/jbc.m400977200. [DOI] [PubMed] [Google Scholar]
- 92.Symons J. D., Abel E. D. Lipotoxicity contributes to endothelial dysfunction: a focus on the contribution from ceramide. Reviews in Endocrine and Metabolic Disorders. 2013;14(1):59–68. doi: 10.1007/s11154-012-9235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Didion S. P., Faraci F. M. Ceramide-induced impairment of endothelial function is prevented by CuZn superoxide dismutase overexpression. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;25:90–95. doi: 10.1161/01.atv.0000149868.74075.5d. [DOI] [PubMed] [Google Scholar]
- 94.Smith M. E., Tippetts T. S., Brassfield E. S., et al. Mitochondrial fission mediates ceramide-induced metabolic disruption in skeletal muscle. Biochemical Journal. 2013;456(3):427–439. doi: 10.1042/bj20130807. [DOI] [PubMed] [Google Scholar]
- 95.Jheng H. F., Tsai P. J., Guo S. M., et al. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Molecular and Cellular Biology. 2012;32(2):309–319. doi: 10.1128/mcb.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yuan H., Gerencser A. A., Liot G., et al. Mitochondrial fission is an upstream and required event for bax foci formation in response to nitric oxide in cortical neurons. Cell Death & Differentiation. 2007;14(3):462–471. doi: 10.1038/sj.cdd.4402046. [DOI] [PubMed] [Google Scholar]
- 97.Zhao G., Cao K., Xu C., et al. Crosstalk between mitochondrial fission and oxidative stress in paraquat-induced apoptosis in mouse alveolar type II cells. International Journal of Biological Sciences. 2017;13(7):888–900. doi: 10.7150/ijbs.18468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schüler R., Osterhoff M. A., Frahnow T., et al. High‐saturated‐fat diet increases circulating angiotensin‐converting enzyme, which is enhanced by the rs4343 polymorphism defining persons at risk of nutrient‐dependent increases of blood pressure. Journal of the American Heart Association. 2017;6(1, article e004465) doi: 10.1161/jaha.116.004465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cabello-Verrugio C., Morales M. G., Rivera J. C., Cabrera D., Simon F. Renin-angiotensin system: an old player with novel functions in skeletal muscle. Medicinal Research Reviews. 2015;35(3):437–463. doi: 10.1002/med.21343. [DOI] [PubMed] [Google Scholar]
- 100.Ruiz-Ortega M., Lorenzo O., Ruperez M., Konig S., Wittig B., Egido J. Angiotensin II activates nuclear transcription factor κB through AT1 and AT2 in vascular smooth muscle cells: molecular mechanisms. Circulation Research. 2000;86(12):1266–1272. doi: 10.1161/01.RES.86.12.1266. [DOI] [PubMed] [Google Scholar]
- 101.Ji Y., Liu J., Wang Z., Liu N. Angiotensin II induces inflammatory response partly via toll-like receptor 4-dependent signaling pathway in vascular smooth muscle cells. Cellular Physiology and Biochemistry. 2009;23(4-6):265–276. doi: 10.1159/000218173. [DOI] [PubMed] [Google Scholar]
- 102.Zhou M.-S., Liu C., Tian R., Nishiyama A., Raij L. Skeletal muscle insulin resistance in salt-sensitive hypertension: role of angiotensin II activation of NFκB. Cardiovascular Diabetology. 2015;14(1):p. 45. doi: 10.1186/s12933-015-0211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mitsuishi M., Miyashita K., Muraki A., Itoh H. Angiotensin II reduces mitochondrial content in skeletal muscle and affects glycemic control. Diabetes. 2009;58(3):710–717. doi: 10.2337/db08-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen Y., Lin J.-R., Gao P.-J. Mitochondrial division inhibitor Mdivi-1 ameliorates angiotensin II-induced endothelial dysfunction. Acta Physiologica Sinica. 2016;68(5):669–676. doi: 10.13294/j.aps.2016.0079. [DOI] [PubMed] [Google Scholar]
- 105.Landmesser U., Spiekermann S., Preuss C., et al. Angiotensin II induces endothelial xanthine oxidase activation: role for endothelial dysfunction in patients with coronary disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(4):943–948. doi: 10.1161/01.atv.0000258415.32883.bf. [DOI] [PubMed] [Google Scholar]
- 106.Vonach C., Viola K., Giessrigl B., et al. NF-κB mediates the 12(S)-HETE-induced endothelial to mesenchymal transition of lymphendothelial cells during the intravasation of breast carcinoma cells. British Journal of Cancer. 2011;105(2):263–271. doi: 10.1038/bjc.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nadler J. L., Natarajan R., Stern N. Specific action of the lipoxygenase pathway in mediating angiotensin II-induced aldosterone synthesis in isolated adrenal glomerulosa cells. The Journal of Clinical Investigation. 1987;80(6):1763–1769. doi: 10.1172/jci113269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bruder-Nascimento T., da Silva M. A. B., Tostes R. C. The involvement of aldosterone on vascular insulin resistance: implications in obesity and type 2 diabetes. Diabetology & Metabolic Syndrome. 2014;6(1):p. 90. doi: 10.1186/1758-5996-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kubzansky L. D., Adler G. K. Aldosterone: a forgotten mediator of the relationship between psychological stress and heart disease. Neuroscience & Biobehavioral Reviews. 2010;34(1):80–86. doi: 10.1016/j.neubiorev.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Iwashima F., Yoshimoto T., Minami I., Sakurada M., Hirono Y., Hirata Y. Aldosterone induces superoxide generation via Rac1 activation in endothelial cells. Endocrinology. 2008;149(3):1009–1014. doi: 10.1210/en.2007-0864. [DOI] [PubMed] [Google Scholar]
- 111.Zhang Y., Pan Y., Bian Z., et al. Ceramide production mediates aldosterone-induced human umbilical vein endothelial cell (HUVEC) damages. PLoS One. 2016;11(1, article e0146944) doi: 10.1371/journal.pone.0146944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Z.-H., Yu Y., Kang Y.-M., Wei S.-G., Felder R. B. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. American Journal of Physiology-Heart and Circulatory Physiology. 2008;294(2):H1067–H1074. doi: 10.1152/ajpheart.01131.2007. [DOI] [PubMed] [Google Scholar]
- 113.Huang B. S., Zheng H., Tan J., Patel K. P., Leenen F. H. H. Regulation of hypothalamic renin-angiotensin system and oxidative stress by aldosterone. Experimental Physiology. 2011;96(10):1028–1038. doi: 10.1113/expphysiol.2011.059840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang L., Muxin G., Nishida H., Shirakawa C., Sato S., Konishi T. Psychological stress-induced oxidative stress as a model of sub-healthy condition and the effect of TCM. Evidence-Based Complementary and Alternative Medicine. 2007;4(2):202. doi: 10.1093/ecam/nel080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li L., Li X., Zhou W., Messina J. L. Acute psychological stress results in the rapid development of insulin resistance. Journal of Endocrinology. 2013;217(2):175–184. doi: 10.1530/joe-12-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aschbacher K., O’Donovan A., Wolkowitz O. M., Dhabhar F. S., Su Y., Epel E. Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology. 2013;38(9):1698–1708. doi: 10.1016/j.psyneuen.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hayashi M., Takeshita K., Uchida Y., et al. Angiotensin II receptor blocker ameliorates stress-induced adipose tissue inflammation and insulin resistance. PLoS One. 2014;9(12, article e116163) doi: 10.1371/journal.pone.0116163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hasan K. M. M., Rahman M. S., Arif K. M. T., Sobhani M. E. Psychological stress and aging: role of glucocorticoids (GCs) Age. 2012;34(6):1421–1433. doi: 10.1007/s11357-011-9319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zafir A., Banu N. Modulation of in vivo oxidative status by exogenous corticosterone and restraint stress in rats. Stress. 2009;12(2):167–177. doi: 10.1080/10253890802234168. [DOI] [PubMed] [Google Scholar]
- 120.Joergensen A., Broedbaek K., Weimann A., et al. Association between urinary excretion of cortisol and markers of oxidatively damaged DNA and RNA in humans. PLoS One. 2011;6(6, article e20795) doi: 10.1371/journal.pone.0020795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Holland W. L., Brozinick J. T., Wang L.-P., et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metabolism. 2007;5(3):167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 122.Chen T.-C., Benjamin D. I., Kuo T., et al. The glucocorticoid-Angptl4-ceramide axis induces insulin resistance through PP2A and PKCζ. Science Signaling. 2017;10(489, article eaai7905) doi: 10.1126/scisignal.aai7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hirata A., Maeda N., Nakatsuji H., et al. Contribution of glucocorticoid–mineralocorticoid receptor pathway on the obesity-related adipocyte dysfunction. Biochemical and Biophysical Research Communications. 2012;419(2):182–187. doi: 10.1016/j.bbrc.2012.01.139. [DOI] [PubMed] [Google Scholar]
- 124.de Punder K., Pruimboom L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Frontiers in Immunology. 2015;6:p. 223. doi: 10.3389/fimmu.2015.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Teitelbaum A. A., Gareau M. G., Jury J., Yang P. C., Perdue M. H. Chronic peripheral administration of corticotropin-releasing factor causes colonic barrier dysfunction similar to psychological stress. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2008;295(3):G452–G459. doi: 10.1152/ajpgi.90210.2008. [DOI] [PubMed] [Google Scholar]
- 126.Zheng G., Victor Fon G., Meixner W., et al. Chronic stress and intestinal barrier dysfunction: glucocorticoid receptor and transcription repressor HES1 regulate tight junction protein claudin-1 promoter. Scientific Reports. 2017;7(1):p. 4502. doi: 10.1038/s41598-017-04755-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ranabir S., Reetu K. Stress and hormones. Indian Journal of Endocrinology and Metabolism. 2011;15(1):18–22. doi: 10.4103/2230-8210.77573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mangmool S., Denkaew T., Parichatikanond W., Kurose H. β-Adrenergic receptor and insulin resistance in the heart. Biomolecules & Therapeutics. 2017;25(1):44–56. doi: 10.4062/biomolther.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fu Y.-C., Yin S.-C., Chi C.-S., Hwang B., Hsu S.-L. Norepinephrine induces apoptosis in neonatal rat endothelial cells via a ROS-dependent JNK activation pathway. Apoptosis. 2006;11(11):2053–2063. doi: 10.1007/s10495-006-0192-8. [DOI] [PubMed] [Google Scholar]
- 130.Theccanat T., Philip J. L., Razzaque A. M., et al. Regulation of cellular oxidative stress and apoptosis by G protein-coupled receptor kinase-2; the role of NADPH oxidase 4. Cellular Signalling. 2016;28(3):190–203. doi: 10.1016/j.cellsig.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Andersson D. C., Fauconnier J., Yamada T., et al. Mitochondrial production of reactive oxygen species contributes to the β-adrenergic stimulation of mouse cardiomycytes. The Journal of Physiology. 2011;589(7):1791–1801. doi: 10.1113/jphysiol.2010.202838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu Q., Dalic A., Fang L., et al. Myocardial oxidative stress contributes to transgenic β2-adrenoceptor activation-induced cardiomyopathy and heart failure. British Journal of Pharmacology. 2011;162(5):1012–1028. doi: 10.1111/j.1476-5381.2010.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mottillo E. P., Shen X. J., Granneman J. G. β3-adrenergic receptor induction of adipocyte inflammation requires lipolytic activation of stress kinases p38 and JNK. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2010;1801(9):1048–1055. doi: 10.1016/j.bbalip.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fasshauer M., Klein J., Neumann S., Eszlinger M., Paschke R. Adiponectin gene expression is inhibited by β-adrenergic stimulation via protein kinase A in 3T3-L1 adipocytes. FEBS Letters. 2001;507(2):142–146. doi: 10.1016/s0014-5793(01)02960-x. [DOI] [PubMed] [Google Scholar]
- 135.Holland W. L., Xia J. Y., Johnson J. A., et al. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Molecular Metabolism. 2017;6(3):267–275. doi: 10.1016/j.molmet.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Siervo M., Riley H. L., Fernandez B. O., et al. Effects of prolonged exposure to hypobaric hypoxia on oxidative stress, inflammation and gluco-insular regulation: the not-so-sweet price for good regulation. PLoS One. 2014;9(4, article e94915) doi: 10.1371/journal.pone.0094915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lavie L. Oxidative stress—a unifying paradigm in obstructive sleep apnea and comorbidities. Progress in Cardiovascular Diseases. 2009;51(4):303–312. doi: 10.1016/j.pcad.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 138.Hamilton G. S., Joosten S. A. Obstructive sleep apnoea and obesity. Australian Family Physician. 2017;46(7):460–463. [PubMed] [Google Scholar]
- 139.Guo R.-B., Sun P. L., Zhao A. P., et al. Chronic asthma results in cognitive dysfunction in immature mice. Experimental Neurology. 2013;247:209–217. doi: 10.1016/j.expneurol.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 140.Arshi M., Cardinal J., Hill R. J., Davies P. S. W., Wainwright C. Asthma and insulin resistance in children. Respirology. 2010;15(5):779–784. doi: 10.1111/j.1440-1843.2010.01767.x. [DOI] [PubMed] [Google Scholar]
- 141.Cottrell L., Neal W. A., Ice C., Perez M. K., Piedimonte G. Metabolic abnormalities in children with asthma. American Journal of Respiratory and Critical Care Medicine. 2011;183(4):441–448. doi: 10.1164/rccm.201004-0603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hernansanz-Agustín P., Ramos E., Navarro E., et al. Mitochondrial complex I deactivation is related to superoxide production in acute hypoxia. Redox Biology. 2017;12:1040–1051. doi: 10.1016/j.redox.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chandel N. S., McClintock D. S., Feliciano C. E., et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia. Journal of Biological Chemistry. 2000;275(33):25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 144.Tang X., Guo D., Lin C., et al. hCLOCK causes Rho-kinase-mediated endothelial dysfunction and NF-κB-mediated inflammatory responses. Oxidative Medicine and Cellular Longevity. 2015;2015:9. doi: 10.1155/2015/671839.671839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Song L., Yang H., Wang H. X., et al. Inhibition of 12/15 lipoxygenase by baicalein reduces myocardial ischemia/reperfusion injury via modulation of multiple signaling pathways. Apoptosis. 2014;19(4):567–580. doi: 10.1007/s10495-013-0946-z. [DOI] [PubMed] [Google Scholar]
- 146.Choudhary R., Malairaman U., Katyal A. Inhibition of 12/15 LOX ameliorates cognitive and cholinergic dysfunction in mouse model of hypobaric hypoxia via. attenuation of oxidative/nitrosative stress. Neuroscience. 2017;359:308–324. doi: 10.1016/j.neuroscience.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 147.Pallast S., Arai K., Wang X., Lo E. H., van Leyen K. 12/15-Lipoxygenase targets neuronal mitochondria under oxidative stress. Journal of Neurochemistry. 2009;111(3):882–889. doi: 10.1111/j.1471-4159.2009.06379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sears D. D., Miles P. D., Chapman J., et al. 12/15-Lipoxygenase is required for the early onset of high fat diet-induced adipose tissue inflammation and insulin resistance in mice. PLoS One. 2009;4(9, article e7250) doi: 10.1371/journal.pone.0007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Natarajan R., Reddy M. A., Malik K. U., Fatima S., Khan B. V. Signaling mechanisms of nuclear factor-κB-mediated activation of inflammatory genes by 13-hydroperoxyoctadecadienoic acid in cultured vascular smooth muscle cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21(9):1408–1413. doi: 10.1161/hq0901.095278. [DOI] [PubMed] [Google Scholar]
- 150.Whitehouse A. S., Khal J., Tisdale M. J. Induction of protein catabolism in myotubes by 15(S)-hydroxyeicosatetraenoic acid through increased expression of the ubiquitin–proteasome pathway. British Journal of Cancer. 2003;89(4):737–745. doi: 10.1038/sj.bjc.6601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li J., Rao J., Liu Y., et al. 15-Lipoxygenase promotes chronic hypoxia–induced pulmonary artery inflammation via positive interaction with nuclear factor-κB. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33(5):971–979. doi: 10.1161/ATVBAHA.113.301335. [DOI] [PubMed] [Google Scholar]
- 152.Guo L., Tang X., Chu X., et al. Role of protein kinase C in 15-HETE-induced hypoxic pulmonary vasoconstriction. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2009;80(2-3):115–123. doi: 10.1016/j.plefa.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 153.Tang D. G., Diglio C. A., Honn K. V. 12(S)-HETE-induced microvascular endothelial cell retraction results from PKC-dependent rearrangement of cytoskeletal elements and αVβ3 integrins. Prostaglandins. 1993;45(3):249–267. doi: 10.1016/0090-6980(93)90051-8. [DOI] [PubMed] [Google Scholar]
- 154.Chattopadhyay R., Tinnikov A., Dyukova E., et al. 12/15-Lipoxygenase-dependent ROS production is required for diet-induced endothelial barrier dysfunction. Journal of Lipid Research. 2015;56(3):562–577. doi: 10.1194/jlr.M055566. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 155.Terada L. S., Guidot D. M., Leff J. A., et al. Hypoxia injures endothelial cells by increasing endogenous xanthine oxidase activity. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(8):3362–3366. doi: 10.1073/pnas.89.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sinha S., Singh S. N., Ray U. S. Total antioxidant status at high altitude in lowlanders and native highlanders: role of uric acid. High Altitude Medicine & Biology. 2009;10(3):269–274. doi: 10.1089/ham.2008.1082. [DOI] [PubMed] [Google Scholar]
- 157.Nanduri J., Vaddi D. R., Khan S. A., et al. HIF-1α activation by intermittent hypoxia requires NADPH oxidase stimulation by xanthine oxidase. PLoS One. 2015;10(3, article e0119762) doi: 10.1371/journal.pone.0119762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kumar G. K., Rai V., Sharma S. D., et al. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. Journal of Physiology. 2006;575(1):229–239. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang F., Wu W., Deng Z., et al. High altitude increases the expression of hypoxia-inducible factor 1α and inducible nitric oxide synthase with intest-inal mucosal barrier failure in rats. Interantional Journal of Clinical and Experimental Pathology. 2015;8:5189–5195. [PMC free article] [PubMed] [Google Scholar]
- 160.Gaffin S. L., Brock-Utne J., Zanotti A., Wells M. T. Hypoxia-induced endotoxemia in primates: role of reticuloendothelial system function and anti-lipopolysaccharide plasma. Aviation, Space, and Environmental Medicine. 1986;57:1044–1049. [PubMed] [Google Scholar]
- 161.Šket R., Treichel N., Debevec T., et al. Hypoxia and inactivity related physiological changes (constipation, inflammation) are not reflected at the level of gut metabolites and butyrate producing microbial community: the PlanHab study. Frontiers in Physiology. 2017;8:p. 250. doi: 10.3389/fphys.2017.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thiering E., Cyrys J., Kratzsch J., et al. Long-term exposure to traffic-related air pollution and insulin resistance in children: results from the GINIplus and LISAplus birth cohorts. Diabetologia. 2013;56(8):1696–1704. doi: 10.1007/s00125-013-2925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lodovici M., Bigagli E. Oxidative stress and air pollution exposure. Journal of Toxicology. 2011;2011:9. doi: 10.1155/2011/487074.487074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pizzino G., Irrera N., Bitto A., et al. Cadmium-induced oxidative stress impairs glycemic control in adolescents. Oxidative Medicine and Cellular Longevity. 2017;2017:6. doi: 10.1155/2017/6341671.6341671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Onyango A. N. Small reactive carbonyl compounds as tissue lipid oxidation products; and the mechanisms of their formation thereby. Chemistry and Physics of Lipids. 2012;165(7):777–786. doi: 10.1016/j.chemphyslip.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 166.Ramana K. V., Bhatnagar A., Srivastava S., et al. Mitogenic responses of vascular smooth muscle cells to lipid peroxidation-derived aldehyde 4-hydroxy-trans-2-nonenal (HNE): role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth. Journal of Biological Chemistry. 2006;281(26):17652–17660. doi: 10.1074/jbc.M600270200. [DOI] [PubMed] [Google Scholar]
- 167.Wentworth P., Jr., Nieva J., Takeuchi C., et al. Evidence for ozone formation in human atherosclerotic arteries. Science. 2003;302(5647):1053–1056. doi: 10.1126/science.1089525. [DOI] [PubMed] [Google Scholar]
- 168.Laynes L., Raghavamenon A. C., D’Auvergne O., Achuthan V., Uppu R. M. MAPK signaling in H9c2 cardiomyoblasts exposed to cholesterol secoaldehyde – role of hydrogen peroxide. Biochemical and Biophysical Research Communications. 2011;404(1):90–95. doi: 10.1016/j.bbrc.2010.11.070. [DOI] [PubMed] [Google Scholar]
- 169.Braakman I., Hebert D. N. Protein folding in the endoplasmic reticulum. Cold Spring Harbor Perspectives in Biology. 2013;5(5, article a013201) doi: 10.1101/cshperspect.a013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Adachi T., Weisbrod R. M., Pimentel D. R., et al. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nature Medicine. 2004;10(11):1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 171.Pastore A., Piemonte F. Protein glutathionylation in cardiovascular diseases. International Journal of Molecular Sciences. 2013;14(12):20845–20876. doi: 10.3390/ijms141020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Qin F., Siwik D. A., Lancel S., et al. Hydrogen peroxide–mediated SERCA cysteine 674 oxidation contributes to impaired cardiac myocyte relaxation in senescent mouse heart. Journal of the American Heart Association. 2013;2(4, article e000184) doi: 10.1161/JAHA.113.000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ozcan U., Cao Q., Yilmaz E., et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 174.Haberzettl P., Vladykovskaya E., Srivastava S., Bhatnagar A. Role of endoplasmic reticulum stress in acrolein-induced endothelial activation. Toxicology and Applied Pharmacology. 2009;234(1):14–24. doi: 10.1016/j.taap.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shao C. H., Capek H. L., Patel K. P., et al. Carbonylation contributes to SERCA2a activity loss and diastolic dysfunction in a rat model of type 1 diabetes. Diabetes. 2011;60(3):947–959. doi: 10.2337/db10-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zeeshan H., Lee G., Kim H.-R., Chae H.-J. Endoplasmic reticulum stress and associated ROS. International Journal of Molecular Sciences. 2016;17(12):p. 327. doi: 10.3390/ijms17030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chaube R., Werstuck G. H. Mitochondrial ROS versus ER ROS: which comes first in myocardial calcium dysregulation? Frontiers in Cardiovascular Medicine. 2016;3:p. 36. doi: 10.3389/fcvm.2016.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Osipov A. N., Smetanina N. M., Pustovalova M. V., Arkhangelskaya E., Klokov D. The formation of DNA single-strand breaks and alkali-labile sites in human blood lymphocytes exposed to 365-nm UVA radiation. Free Radical Biology & Medicine. 2014;73:34–40. doi: 10.1016/j.freeradbiomed.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 179.Valavanidis A., Vlachogianni T., Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. Journal of Environmental Science and Health, Part C. 2009;27(2):120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 180.Cadet J., Wagner J. R. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harbor Perspectives in Biology. 2013;5, article a012559 doi: 10.1101/cshperspect.a012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Frelon S., Douki T., Favier A., Cadet J. Hydroxyl radical is not the main reactive species involved in the degradation of DNA bases by copper in the presence of hydrogen peroxide. Chemical Research in Toxicology. 2003;16(2):191–197. doi: 10.1021/tx025650q. [DOI] [PubMed] [Google Scholar]
- 182.Henle E. S., Han Z., Tang N., Rai P., Luo Y., Linn S. Sequence-specific DNA cleavage by Fe2+-mediated Fenton reactions has possible biological implications. Journal of Biological Chemistry. 1999;274(2):962–971. doi: 10.1074/jbc.274.2.962. [DOI] [PubMed] [Google Scholar]
- 183.Thapa B., Munk B. H., Burrows C. J., Schlegel H. B. Computational study of oxidation of guanine by singlet oxygen (1Δg) and formation of guanine:lysine cross-links. Chemistry - A European Journal. 2017;23(24):5804–5813. doi: 10.1002/chem.201700231. [DOI] [PubMed] [Google Scholar]
- 184.Woodbine L., Brunton H., Goodarzi A. A., Shibata A., Jeggo P. A. Endogenously induced DNA double strand breaks arise in heterochromatic DNA regions and require ataxia telangiectasia mutated and Artemis for their repair. Nucleic Acids Research. 2011;39(16):6986–6997. doi: 10.1093/nar/gkr331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.ul Islam B., Habib S., Ahmad P., Allarakha S., Moinuddin, Ali A. Pathophysiological role of peroxynitrite induced DNA damage in human diseases: a special focus on poly(ADP-ribose) polymerase (PARP) Indian Journal of Clinical Biochemistry. 2015;30(4):368–385. doi: 10.1007/s12291-014-0475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gentile F., Arcaro A., Pizzimenti S., et al. DNA damage by lipid peroxidation products: implications in cancer, inflammation and autoimmunity. AIMS Genetics. 2017;4(2):103–137. doi: 10.3934/genet.2017.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Roberts M. J., Wondrak G. T., Laurean D. C., Jacobson M. K., Jacobson E. L. DNA damage by carbonyl stress in human skin cells. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2003;522(1-2):45–56. doi: 10.1016/S0027-5107(02)00232-4. [DOI] [PubMed] [Google Scholar]
- 188.Gracanin M., Davies M. J. Inhibition of protein tyrosine phosphatases by amino acid, peptide, and protein hydroperoxides: potential modulation of cell signaling by protein oxidation products. Free Radical Biology & Medicine. 2007;42(10):1543–1551. doi: 10.1016/j.freeradbiomed.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 189.Rahmanto A. S., Morgan P. E., Hawkins C. L., Davies M. J. Cellular effects of photogenerated oxidants and long-lived, reactive, hydroperoxide photoproducts. Free Radical Biology & Medicine. 2010;49(10):1505–1515. doi: 10.1016/j.freeradbiomed.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 190.Gupta V., Carroll K. S. Sulfenic acid chemistry, detection and cellular lifetime. Biochimica et Biophysica Acta (BBA) - General Subjects. 2014;1840(2):847–875. doi: 10.1016/j.bbagen.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dalle-Donne I., Rossi R., Giustarini D., Colombo R., Milzani A. S-Glutathionylation in protein redox regulation. Free Radical Biology & Medicine. 2007;43(6):883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 192.Grek C. L., Zhang J., Manevich Y., Townsend D. M., Tew K. D. Causes and consequences of cysteine S-glutathionylation. The Journal of Biological Chemistry. 2013;288(37):26497–26504. doi: 10.1074/jbc.R113.461368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wefers H., Sies H. Oxidation of glutathione by the superoxide radical to the disulfide and the sulfonate yielding singlet oxygen. European Journal of Biochemistry. 1983;137(1-2):29–36. doi: 10.1111/j.1432-1033.1983.tb07791.x. [DOI] [PubMed] [Google Scholar]
- 194.Enami S., Hoffmann M. R., Colussi A. J. Ozone oxidizes glutathione to a sulfonic acid. Chemical Research in Toxicology. 2009;22(1):35–40. doi: 10.1021/tx800298j. [DOI] [PubMed] [Google Scholar]
- 195.Onyango A. N. Alternatives to the ‘water oxidation pathway’ of biological ozone formation. Journal of Chemical Biology. 2016;9(1):1–8. doi: 10.1007/s12154-015-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Iwakami S., Misu H., Takeda T., et al. Concentration-dependent dual effects of hydrogen peroxide on insulin signal transduction in H4IIEC hepatocytes. PLoS One. 2011;6(11, article e27401) doi: 10.1371/journal.pone.0027401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhuang S., Kochevar I. E. Singlet oxygen–induced activation of Akt/protein kinase B is independent of growth factor receptors. Photochemistry and Photobiology. 2003;78(4):361–371. doi: 10.1562/0031-8655(2003)0780361SOAOPK2.0.CO2. [DOI] [PubMed] [Google Scholar]
- 198.Ikemura M., Nishikawa M., Hyoudou K., Kobayashi Y., Yamashita F., Hashida M. Improvement of insulin resistance by removal of systemic hydrogen peroxide by PEGylated catalase in obese mice. Molecular Pharmaceutics. 2010;7(6):2069–2076. doi: 10.1021/mp100110c. [DOI] [PubMed] [Google Scholar]
- 199.Son Y., Cheong Y.-K., Kim N.-H., Chung H.-T., Kang D. G., Pae H.-O. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? Journal of Signal Transduction. 2011;2011:6. doi: 10.1155/2011/792639.792639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Castro-Caldas M., Carvalho A. N., Rodrigues E., Henderson C., Wolf C. R., Gama M. J. Glutathione S-transferase pi mediates MPTP-induced c-Jun N-terminal kinase activation in the nigrostriatal pathway. Molecular Neurobiology. 2012;45(3):466–477. doi: 10.1007/s12035-012-8266-9. [DOI] [PubMed] [Google Scholar]
- 201.Tew K. D., Manevich Y., Grek C., Xiong Y., Uys J., Townsend D. M. The role of glutathione S-transferase P in signaling pathways and S-glutathionylation in cancer. Free Radical Biology & Medicine. 2011;51(2):299–313. doi: 10.1016/j.freeradbiomed.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Klotz L.-O., Kröncke K.-D., Sies H. Singlet oxygen-induced signaling effects in mammalian cells. Photochemical & Photobiological Sciences. 2003;2(2):88–94. doi: 10.1039/B210750C. [DOI] [PubMed] [Google Scholar]
- 203.Matsukawa J., Matsuzawa A., Takeda K., Ichijo H. The ASK1-MAP kinase cascades in mammalian stress response. Journal of Biochemistry. 2004;136(3):261–265. doi: 10.1093/jb/mvh134. [DOI] [PubMed] [Google Scholar]
- 204.Li X., Rong Y., Zhang M., et al. Up-regulation of thioredoxin interacting protein (Txnip) by p38 MAPK and FOXO1 contributes to the impaired thioredoxin activity and increased ROS in glucose-treated endothelial cells. Biochemical and Biophysical Research Communications. 2009;381(4):660–665. doi: 10.1016/j.bbrc.2009.02.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lane T., Flam B., Lockey R., Kolliputi N. TXNIP shuttling: missing link between oxidative stress and inflammasome activation. Frontiers in Physiology. 2013;4(50) doi: 10.3389/fphys.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Choi C. S., Kim Y.-B., Lee F. N., Zabolotny J. M., Kahn B. B., Youn J. H. Lactate induces insulin resistance in skeletal muscle by suppressing glycolysis and impairing insulin signaling. American Journal of Physiology-Endocrinology and Metabolism. 2002;283(2):E233–E240. doi: 10.1152/ajpendo.00557.2001. [DOI] [PubMed] [Google Scholar]
- 207.Kleinert M., Sylow L., Fazakerley D. J., et al. Acute mTOR inhibition induces insulin resistance and alters substrate utilization in vivo. Molecular Metabolism. 2014;3(6):630–641. doi: 10.1016/j.molmet.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chao L. C., Wroblewski K., Zhang Z., et al. Insulin resistance and altered systemic glucose metabolism in mice lacking nur77. Diabetes. 2009;58(12):2788–2796. doi: 10.2337/db09-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Morgan P. E., Dean R. T., Davies M. J. Inhibition of glyceraldehyde-3-phosphate dehydrogenase by peptide and protein peroxides generated by singlet oxygen attack. European Journal of Biochemistry. 2002;269(7):1916–1925. doi: 10.1046/j.1432-1033.2002.02845.x. [DOI] [PubMed] [Google Scholar]
- 210.Beisswenger P. J., Howell S. K., Smith K., Szwergold B. S. Glyceraldehyde-3-phosphate dehydrogenase activity as an independent modifier of methylglyoxal levels in diabetes. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2003;1637(1):98–106. doi: 10.1016/S09254439(02)00219-3. [DOI] [PubMed] [Google Scholar]
- 211.Iqbal S., Hood D. A. Oxidative stress-induced mitochondrial fragmentation and movement in skeletal muscle myoblasts. American Journal of Physiology-Cell Physiology. 2014;306(12):C1176–C1183. doi: 10.1152/ajpcell.00017.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Park K., Gross M., Lee D.-H., et al. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care. 2009;32(7):1302–1307. doi: 10.2337/dc09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Huang Z., Hong Q., Zhang X., et al. Aldose reductase mediates endothelial cell dysfunction induced by high uric acid concentrations. Cell Communication and Signaling. 2017;15(1):p. 3. doi: 10.1186/s12964-016-0158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ramana K. V., Friedrich B., Tammali R., West M. B., Bhatnagar A., Srivastava S. K. Requirement of aldose reductase for the hyperglycemic activation of protein kinase C and formation of diacylglycerol in vascular smooth muscle cells. Diabetes. 2005;54(3):818–829. doi: 10.2337/diabetes.54.3.818. [DOI] [PubMed] [Google Scholar]
- 215.Lanaspa M. A., Ishimoto T., Li N., et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nature Communications. 2013;4:p. 2434. doi: 10.1038/ncomms3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zeng K.-W., Li J., Dong X., et al. Anti-neuroinflammatory efficacy of the aldose reductase inhibitor FMHM via phospholipase C/protein kinase C-dependent NF-κB and MAPK pathways. Toxicology and Applied Pharmacology. 2013;273(1):159–171. doi: 10.1016/j.taap.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 217.Toriumi K., Horikoshi Y., Yoshiyuki Osamura R., Yamamoto Y., Nakamura N., Takekoshi S. Carbon tetrachloride-induced hepatic injury through formation of oxidized diacylglycerol and activation of the PKC/NF-κB pathway. Laboratory Investigation. 2013;93(2):218–229. doi: 10.1038/labinvest.2012.145. [DOI] [PubMed] [Google Scholar]
- 218.Srivastava S. K., Yadav U. C. S., Reddy A. B. M., et al. Aldose reductase inhibition suppresses oxidative stress-induced inflammatory disorders. Chemico-Biological Interactions. 2011;191(1-3):330–338. doi: 10.1016/j.cbi.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pandey S., Srivastava S. K., Ramana K. V. A potential therapeutic role for aldose reductase inhibitors in the treatment of endotoxin-related inflammatory diseases. Expert Opinion on Investigational Drugs. 2012;21(3):329–339. doi: 10.1517/13543784.2012.656198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Song X.-M., Yu Q., Dong X., et al. Aldose reductase inhibitors attenuate β-amyloid-induced TNF-α production in microlgia via ROS-PKC-mediated NF-κB and MAPK pathways. International Immunopharmacology. 2017;50:30–37. doi: 10.1016/j.intimp.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 221.Nishino T., Okamoto K., Eger B. T., Pai E. F., Nishino T. Mammalian xanthine oxidoreductase – mechanism of transition from xanthine dehydrogenase to xanthine oxidase. FEBS Journal. 2008;275(13):3278–3289. doi: 10.1111/j.1742-4658.2008.06489.x. [DOI] [PubMed] [Google Scholar]
- 222.Abdelmalek M. F., Lazo M., Horska A., et al. Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology. 2012;56(3):952–960. doi: 10.1002/hep.25741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lanaspa M. A., Sanchez-Lozada L. G., Choi Y. J., et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. Journal of Biological Chemistry. 2012;287(48):40732–40744. doi: 10.1074/jbc.M112.399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Spiga R., Marini M. A., Mancuso E., et al. Uric acid is associated with inflammatory biomarkers and induces inflammation via activating the NF-κB signaling pathway in HepG2 cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2017;37(6):1241–1249. doi: 10.1161/ATVBAHA.117.309128. [DOI] [PubMed] [Google Scholar]
- 225.Choi Y. J., Shin H. S., Choi H. S., et al. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Laboratory Investigation. 2014;94(10):1114–1125. doi: 10.1038/labinvest.2014.98. [DOI] [PubMed] [Google Scholar]
- 226.Kanbay M., Jensen T., Solak Y., et al. Uric acid in metabolic syndrome: from an innocent bystander to a central player. European Journal of Internal Medicine. 2016;29:3–8. doi: 10.1016/j.ejim.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Derbre F., Ferrando B., Gomez-Cabrera M. C., et al. Inhibition of xanthine oxidase by allopurinol prevents skeletal muscle atrophy: role of p38 MAPkinase and E3 ubiquitin ligases. PLoS One. 2012;7(10, article e46668) doi: 10.1371/journal.pone.0046668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Grether-Beck S., Bonizzi G., Schmitt-Brenden H., et al. Non-enzymatic triggering of the ceramide signalling cascade by solar UVA radiation. The EMBO Journal. 2000;19(21):5793–5800. doi: 10.1093/emboj/19.21.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sawada M., Nakashima S., Kiyono T., et al. p53 regulates ceramide formation by neutral sphingomyelinase through reactive oxygen species in human glioma cells. Oncogene. 2001;20(11):1368–1378. doi: 10.1038/sj.onc.1204207. [DOI] [PubMed] [Google Scholar]
- 230.Ussher J. R., Koves T. R., Cadete V. J. J., et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes. 2010;59(10):2453–2464. doi: 10.2337/db09-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Verma M., Yateesh A., Neelima K., et al. Inhibition of neutral sphingomyelinases in skeletal muscle attenuates fatty-acid induced defects in metabolism and stress. SpringerPlus. 2014;3(1):p. 255. doi: 10.1186/2193-1801-3-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Li J.-L., Wang Q.-Y., Luan H.-Y., Kang Z.-C., Wang C.-B. Effects of L-carnitine against oxidative stress in human hepatocytes: involvement of peroxisome proliferator-activated receptor alpha. Journal of Biomedical Science. 2012;19(1):p. 32. doi: 10.1186/1423-0127-19-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Blanquicett C., Kang B.-Y., Ritzenthaler J. D., Jones D. P., Hart C. M. Oxidative stress modulates PPARγ in vascular endothelial cells. Free Radical Biology & Medicine. 2010;48(12):1618–1625. doi: 10.1016/j.freeradbiomed.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Paumen M. B., Ishida Y., Muramatsu M., Yamamoto M., Honjo T. Inhibition of carnitine palmitoyltransferase I augments sphingolipid synthesis and palmitate-induced apoptosis. Journal of Biological Chemistry. 1997;272(6):3324–3329. doi: 10.1074/jbc.272.6.3324. [DOI] [PubMed] [Google Scholar]
- 235.Chan S. M. H., Sun R.-Q., Zeng X.-Y., et al. Activation of PPARα ameliorates hepatic insulin resistance and steatosis in high fructose–fed mice despite increased endoplasmic reticulum stress. Diabetes. 2013;62(6):2095–2105. doi: 10.2337/db12-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhang N., Chu E. S. H., Zhang J., et al. Peroxisome proliferator activated receptor alpha inhibits hepatocarcinogenesis through mediating NF-κB signaling pathway. Oncotarget. 2014;5(18):8330–8340. doi: 10.18632/oncotarget.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pawlak M., Lefebvre P., Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. Journal of Hepatology. 2015;62(3):720–733. doi: 10.1016/j.jhep.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 238.Liu X., Yu Z., Huang X., et al. Peroxisome proliferator-activated receptor γ (PPARγ) mediates the protective effect of quercetin against myocardial ischemia-reperfusion injury via suppressing the NF-κB pathway. American Journal of Translational Research. 2016;8(12):5169–5186. [PMC free article] [PubMed] [Google Scholar]
- 239.Zhang Y., Zhan R.-X., Chen J.-Q., et al. Pharmacological activation of PPAR gamma ameliorates vascular endothelial insulin resistance via a non-canonical PPAR gamma-dependent nuclear factor-kappa B trans-repression pathway. European Journal of Pharmacology. 2015;754:41–51. doi: 10.1016/j.ejphar.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 240.Bouskila M., Pajvani U. B., Scherer P. E. Adiponectin: a relevant player in PPARγ-agonist-mediated improvements in hepatic insulin sensitivity? International Journal of Obesity. 2005;29(S1):S17–S23. doi: 10.1038/sj.ijo.0802908. [DOI] [PubMed] [Google Scholar]
- 241.Mohr S., Hallak H., de Boitte A., Lapetina E. G., Brüne B. Nitric oxide-induced S-glutathionylation and inactivation of glyceraldehyde-3-phosphate dehydrogenase. Journal of Biological Chemistry. 1999;274(14):9427–9430. doi: 10.1074/jbc.274.14.9427. [DOI] [PubMed] [Google Scholar]
- 242.Bellezza I., Grottelli S., Costanzi E., et al. Peroxynitrite activates the NLRP3 inflammasome cascade in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Molecular Neurobiology. 2017 doi: 10.1007/s12035-017-0502-x. [DOI] [PubMed] [Google Scholar]
- 243.Pautz A., Franzen R., Dorsch S., et al. Cross-talk between nitric oxide and superoxide determines ceramide formation and apoptosis in glomerular cells. Kidney International. 2002;61(3):790–796. doi: 10.1046/j.1523-1755.2002.00222.x. [DOI] [PubMed] [Google Scholar]
- 244.Morita M., Naito Y., Yoshikawa T., Niki E. Plasma lipid oxidation induced by peroxynitrite, hypochlorite, lipoxygenase and peroxyl radicals and its inhibition by antioxidants as assessed by diphenyl-1-pyrenylphosphine. Redox Biology. 2016;8:127–135. doi: 10.1016/j.redox.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sugita H., Fujimoto M., Yasukawa T., et al. Inducible nitric-oxide synthase and NO donor induce insulin receptor substrate-1 degradation in skeletal muscle cells. Journal of Biological Chemistry. 2005;280(14):14203–14211. doi: 10.1074/jbc.M411226200. [DOI] [PubMed] [Google Scholar]
- 246.Nigro C., Raciti G. A., Leone A., et al. Methylglyoxal impairs endothelial insulin sensitivity both in vitro and in vivo. Diabetologia. 2014;57(7):1485–1494. doi: 10.1007/s00125-014-3243-7. [DOI] [PubMed] [Google Scholar]
- 247.Ingram K. H., Hill H., Moellering D. R., et al. Skeletal muscle lipid peroxidation and insulin resistance in humans. The Journal of Clinical Endocrinology & Metabolism. 2012;97(7):E1182–E1186. doi: 10.1210/jc.2011-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Shearn C. T., Fritz K. S., Reigan P., Petersen D. R. Modification of Akt2 by 4-hydroxynonenal inhibits insulin-dependent Akt signaling in HepG2 cells. Biochemistry. 2011;50(19):3984–3996. doi: 10.1021/bi200029w. [DOI] [PubMed] [Google Scholar]
- 249.Orena S. J., Torchia A. J., Garofalo R. S. Inhibition of glycogen-synthase kinase 3 stimulates glycogen synthase and glucose transport by distinct mechanisms in 3T3-L1 adipocytes. Journal of Biological Chemistry. 2000;275(21):15765–15772. doi: 10.1074/jbc.M910002199. [DOI] [PubMed] [Google Scholar]
- 250.MacAulay K., Woodgett J. R. Targeting glycogen synthase kinase-3 (GSK-3) in the treatment of type 2 diabetes. Expert Opinion on Therapeutic Targets. 2008;12(10):1265–1274. doi: 10.1517/14728222.12.10.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sutherland C. What are the bona fide GSK3 substrates? International Journal of Alzheimer's Disease. 2011;2011:23. doi: 10.4061/2011/505607.505607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Rojo A. I., Sagarra M. R. ., Cuadrado A. GSK-3β down-regulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. Journal of Neurochemistry. 2008;105(1):192–202. doi: 10.1111/j.1471-4159.2007.05124.x. [DOI] [PubMed] [Google Scholar]
- 253.Spoto B., Parlongo R. M., Parlongo G., Sgro' E., Zoccali C. The enzymatic machinery for ADMA synthesis and degradation is fully expressed in human adipocytes. Journal of Nephrology. 2007;20(5):554–559. [PubMed] [Google Scholar]
- 254.Iwasaki H. Activities of asymmetric dimethylarginine-related enzymes in white adipose tissue are associated with circulating lipid biomarkers. Diabetology & Metabolic Syndrome. 2012;4(1):p. 17. doi: 10.1186/1758-5996-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Yang Z.-C., Wang K. S., Wu Y., et al. Asymmetric dimethylarginine impairs glucose utilization via ROS/TLR4 pathway in adipocytes: an effect prevented by vitamin E. Cellular Physiology and Biochemistry. 2009;24(1-2):115–124. doi: 10.1159/000227819. [DOI] [PubMed] [Google Scholar]
- 256.Zheng J., Wang K., Jin P., et al. The association of adipose-derived dimethylarginine dimethylaminohydrolase-2 with insulin sensitivity in experimental type 2 diabetes mellitus. Acta Biochimica et Biophysica Sinica. 2013;45(8):641–648. doi: 10.1093/abbs/gmt058. [DOI] [PubMed] [Google Scholar]
- 257.Trocha M., Merwid-Lad A., Szuba A., Sozanski T., Magdalan J., Szelag A. Asymmetric dimethylarginine synthesis and degradation under physiological and pathological conditions. Advanced Clinical and Experimental Medicine. 2010;19:233–243. [Google Scholar]
- 258.Chen P., Xia K., Zhao Z., Deng X., Yang T. Atorvastatin modulates the DDAH1/ADMA system in high-fat diet-induced insulin-resistant rats with endothelial dysfunction. Vascular Medicine. 2012;17(6):416–423. doi: 10.1177/1358863X12467492. [DOI] [PubMed] [Google Scholar]
- 259.Chen L., Zhou J.-P., Kuang D.-B., Tang J., Li Y.-J., Chen X.-P. 4-HNE increases intracellular ADMA levels in cultured HUVECs: evidence for miR-21-dependent mechanisms. PLoS One. 2013;8(5, article e64148) doi: 10.1371/journal.pone.0064148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zengi A., Ercan G., Caglayan O., et al. Increased oxidative DNA damage in lean normoglycemic offspring of type 2 diabetic patients. Experimental and Clinical Endocrinology & Diabetes. 2011;119(8):467–471. doi: 10.1055/s-0031-1275289. [DOI] [PubMed] [Google Scholar]
- 261.Al-Aubaidy H. A., Jelinek H. F. 8-Hydroxy-2-deoxy-guanosine identifies oxidative DNA damage in a rural prediabetes cohort. Redox Report. 2010;15(4):155–160. doi: 10.1179/174329210X12650506623681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Sampath H., Vartanian V., Rollins M. R., Sakumi K., Nakabeppu Y., Lloyd R. S. 8-Oxoguanine DNA glycosylase (OGG1) deficiency increases susceptibility to obesity and metabolic dysfunction. PLoS One. 2012;7(12, article e51697) doi: 10.1371/journal.pone.0051697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yuzefovych L. V., Solodushko V. A., Wilson G. L., Rachek L. I. Protection from palmitate-induced mitochondrial DNA damage prevents from mitochondrial oxidative stress, mitochondrial dysfunction, apoptosis, and impaired insulin signaling in rat L6 skeletal muscle cells. Endocrinology. 2012;153(1):92–100. doi: 10.1210/en.2011-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Yuzefovych L. V., LeDoux S. P., Wilson G. L., Rachek L. I. Mitochondrial DNA damage via augmented oxidative stress regulates endoplasmic reticulum stress and autophagy: crosstalk, links and signaling. PLoS One. 2013;8(12, article e83349) doi: 10.1371/journal.pone.0083349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ricci C., Pastukh V., Leonard J., et al. Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. American Journal of Physiology-Cell Physiology. 2008;294(2):C413–C422. doi: 10.1152/ajpcell.00362.2007. [DOI] [PubMed] [Google Scholar]
- 266.Shimada K., Crother T. R., Karlin J., et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kim I., Xu W., Reed J. C. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nature Reviews Drug Discovery. 2008;7(12):1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 268.Lebeaupin C., Proics E., de Bieville C. H. D., et al. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death & Disease. 2015;6(9, article e1879) doi: 10.1038/cddis.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Shang F., Taylor A. Ubiquitin–proteasome pathway and cellular responses to oxidative stress. Free Radical Biology & Medicine. 2011;51(1):5–16. doi: 10.1016/j.freeradbiomed.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Chen Z. J. Ubiquitin signalling in the NF-κB pathway. Nature Cell Biology. 2005;7(8):758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Emanuelli B., Peraldi P., Filloux C., et al. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-α in the adipose tissue of obese mice. Journal of Biological Chemistry. 2001;276(51):47944–47949. doi: 10.1074/jbc.M104602200. [DOI] [PubMed] [Google Scholar]
- 272.Rui L., Yuan M., Frantz D., Shoelson S., White M. F. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. Journal of Biological Chemistry. 2002;277(44):42394–42398. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 273.Senn J. J., Klover P. J., Nowak I. A., et al. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. Journal of Biological Chemistry. 2003;278(16):13740–13746. doi: 10.1074/jbc.M210689200. [DOI] [PubMed] [Google Scholar]
- 274.Mashili F., Chibalin A. V., Krook A., Zierath J. R. Constitutive STAT3 phosphorylation contributes to skeletal muscle insulin resistance in type 2 diabetes. Diabetes. 2013;62(2):457–465. doi: 10.2337/db12-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ishii M., Maeda A., Tani S., Akagawa M. Palmitate induces insulin resistance in human HepG2 hepatocytes by enhancing ubiquitination and proteasomal degradation of key insulin signaling molecules. Archives of Biochemistry and Biophysics. 2015;566:26–35. doi: 10.1016/j.abb.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 276.Liu X., Mameza M. G., Lee Y. S., et al. Suppressors of cytokine-signaling proteins induce insulin resistance in the retina and promote survival of retinal cells. Diabetes. 2008;57(6):1651–1658. doi: 10.2337/db07-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lerat H., Imache M. R., Polyte J., et al. Hepatitis C virus induces a prediabetic state by directly impairing hepatic glucose metabolism in mice. Journal of Biological Chemistry. 2017;292(31):12860–12873. doi: 10.1074/jbc.M117.785030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Gu D., Wang Z., Dou X., et al. Inhibition of ERK1/2 pathway suppresses adiponectin secretion via accelerating protein degradation by ubiquitin–proteasome system: relevance to obesity-related adiponectin decline. Metabolism. 2013;62(8):1137–1148. doi: 10.1016/j.metabol.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ozaki K.-I., Awazu M., Tamiya M., et al. Targeting the ERK signaling pathway as a potential treatment for insulin resistance and type 2 diabetes. American Journal of Physiology-Endocrinology and Metabolism. 2016;310(8):E643–E651. doi: 10.1152/ajpendo.00445.2015. [DOI] [PubMed] [Google Scholar]
- 280.Wang Z., Dou X., Gu D., et al. 4-Hydroxynonenal differentially regulates adiponectin gene expression and secretion via activating PPARγ and accelerating ubiquitin–proteasome degradation. Molecular and Cellular Endocrinology. 2012;349(2):222–231. doi: 10.1016/j.mce.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Abudukadier A., Fujita Y., Obara A., et al. Tetrahydrobiopterin has a glucose-lowering effect by suppressing hepatic gluconeogenesis in an endothelial nitric oxide synthase–dependent manner in diabetic mice. Diabetes. 2013;62(9):3033–3043. doi: 10.2337/db12-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Shen Z., Liang X., Rogers C. Q., Rideout D., You M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2010;298(3):G364–G374. doi: 10.1152/ajpgi.00456.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Cheng Y., Takeuchi H., Sonobe Y., et al. Sirtuin 1 attenuates oxidative stress via upregulation of superoxide dismutase 2 and catalase in astrocytes. Journal of Neuroimmunology. 2014;269(1-2):38–43. doi: 10.1016/j.jneuroim.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 284.Ceolotto G., Gallo A., Papparella I., et al. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(12):2627–2633. doi: 10.1161/ATVBAHA.107.155762. [DOI] [PubMed] [Google Scholar]
- 285.Balteau M., van Steenbergen A., Timmermans A. D., et al. AMPK activation by glucagon-like peptide-1 prevents NADPH oxidase activation induced by hyperglycemia in adult cardiomyocytes. American Journal of Physiology-Heart and Circulatory Physiology. 2014;307(8):H1120–H1133. doi: 10.1152/ajpheart.00210.2014. [DOI] [PubMed] [Google Scholar]
- 286.Cacicedo J. M., Yagihashi N., Keaney J. F., Jr, Ruderman N. B., Ido Y. AMPK inhibits fatty acid-induced increases in NF-κB transactivation in cultured human umbilical vein endothelial cells. Biochemical and Biophysical Research Communications. 2004;324(4):1204–1209. doi: 10.1016/j.bbrc.2004.09.177. [DOI] [PubMed] [Google Scholar]
- 287.Salminen A., Hyttinen J. M. T., Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. Journal of Molecular Medicine. 2011;89(7):667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Yang W. M., Lee W. CTRP5 ameliorates palmitate-induced apoptosis and insulin resistance through activation of AMPK and fatty acid oxidation. Biochemical and Biophysical Research Communications. 2014;452(3):715–721. doi: 10.1016/j.bbrc.2014.08.145. [DOI] [PubMed] [Google Scholar]
- 289.Tsai K.-L., Chen L.-H., Chiou S.-H., et al. Coenzyme Q10 suppresses oxLDL-induced endothelial oxidative injuries by the modulation of LOX-1-mediated ROS generation via the AMPK/PKC/NADPH oxidase signaling pathway. Molecular Nutrition & Food Research. 2011;55(S2):S227–S240. doi: 10.1002/mnfr.201100147. [DOI] [PubMed] [Google Scholar]
- 290.Ruderman N. B., Carling D., Prentki M., Cacicedo J. M. AMPK, insulin resistance, and the metabolic syndrome. Journal of Clinical Investigation. 2013;123(7):2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Cheang W. S., Tian X. Y., Wong W. T., et al. Metformin protects endothelial function in diet-induced obese mice by inhibition of endoplasmic reticulum stress through 5′ adenosine monophosphate–activated protein kinase–peroxisome proliferator–activated receptor δ pathway. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34(4):830–836. doi: 10.1161/ATVBAHA.113.301938. [DOI] [PubMed] [Google Scholar]
- 292.Li C., Reif M. M., Craige S. M., Kant S., Keaney J. F., Jr Endothelial AMPK activation induces mitochondrial biogenesis and stress adaptation via eNOS-dependent mTORC1 signaling. Nitric Oxide. 2016;55-56:45–53. doi: 10.1016/j.niox.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Gao Z., Zhang J., Kheterpal I., Kennedy N., Davis R. J., Ye J. Sirtuin 1 (SIRT1) protein degradation in response to persistent c-Jun N-terminal kinase 1 (JNK1) activation contributes to hepatic steatosis in obesity. Journal of Biological Chemistry. 2011;286(25):22227–22234. doi: 10.1074/jbc.M111.228874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Cao Y., Jiang X., Ma H., Wang Y., Xue P., Liu Y. SIRT1 and insulin resistance. Journal of Diabetes and Its Complications. 2016;30(1):178–183. doi: 10.1016/j.jdiacomp.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 295.Salminen A., Kaarniranta K., Kauppinen A. Crosstalk between oxidative stress and SIRT1: impact on the aging process. International Journal of Molecular Sciences. 2013;14(2):3834–3859. doi: 10.3390/ijms14023834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Zhang H.-H., Ma X.-J., Wu L.-N., et al. SIRT1 attenuates high glucose-induced insulin resistance via reducing mitochondrial dysfunction in skeletal muscle cells. Experimental Biology and Medicine. 2015;240(5):557–565. doi: 10.1177/1535370214557218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Zheng L. D., Linarelli L. E., Brooke J., et al. Mitochondrial epigenetic changes link to increased diabetes risk and early-stage prediabetes indicator. Oxidative Medicine and Cellular Longevity. 2016;2016:10. doi: 10.1155/2016/5290638.5290638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Marin T. L., Gongol B., Zhang F., et al. AMPK promotes mitochondrial biogenesis and function by phosphorylating the epigenetic factors DNMT1, RBBP7, and HAT1. Science Signaling. 2017;10(464, article eaaf7478) doi: 10.1126/scisignal.aaf7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Saini S. K., Mangalhara K. C., Prakasam G., Bamezai R. N. K. DNA methyltransferase1 (DNMT1) isoform3 methylates mitochondrial genome and modulates its biology. Scientific Reports. 2017;7(1):p. 1525. doi: 10.1038/s41598-017-01743-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.McClung J. M., Judge A. R., Talbert E. E., Powers S. K. Calpain-1 is required for hydrogen peroxide-induced myotube atrophy. American Journal of Physiology-Cell Physiology. 2009;296(2):C363–C371. doi: 10.1152/ajpcell.00497.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Smith I. J., Lecker S. H., Hasselgren P.-O. Calpain activity and muscle wasting in sepsis. American Journal of Physiology-Endocrinology and Metabolism. 2008;295(4):E762–E771. doi: 10.1152/ajpendo.90226.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Dirks M. L., Wall B. T., van de Valk B., et al. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes. 2016;65(10):2862–2875. doi: 10.2337/db15-1661. [DOI] [PubMed] [Google Scholar]
- 303.Rom O., Kaisari S., Reznick A. Z., Aizenbud D. Peroxynitrite induces degradation of myosin heavy chain via p38 MAPK and muscle-specific E3 ubiquitin ligases in C2 skeletal myotubes. Advances in Experimental Medicine and Biology. 2014;832:1–8. doi: 10.1007/5584_2014_9. [DOI] [PubMed] [Google Scholar]
- 304.Carnac G., Vernus B., Bonnieu A. Myostatin in the pathophysiology of skeletal muscle. Current Genomics. 2007;8(7):415–422. doi: 10.2174/138920207783591672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Sriram S., Subramanian S., Sathiakumar D., et al. Modulation of reactive oxygen species in skeletal muscle by myostatin is mediated through NF-κB. Aging Cell. 2011;10(6):931–948. doi: 10.1111/j.1474-9726.2011.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Brandt C., Nielsen A. R., Fischer C. P., Hansen J., Pedersen B. K., Plomgaard P. Plasma and muscle myostatin in relation to type 2 diabetes. PLoS One. 2012;7(5, article e37236) doi: 10.1371/journal.pone.0037236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Catalgol B., Ziaja I., Breusing N., et al. The proteasome is an integral part of solar ultraviolet a radiation-induced gene expression. Journal of Biological Chemistry. 2009;284(44):30076–30086. doi: 10.1074/jbc.M109.044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Díaz-Ruiz A., Guzmán-Ruiz R., Moreno N. R., et al. Proteasome dysfunction associated to oxidative stress and proteotoxicity in adipocytes compromises insulin sensitivity in human obesity. Antioxidants & Redox Signaling. 2015;23(7):597–612. doi: 10.1089/ars.2014.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Mei Y., Thompson M. D., Cohen R. A., Tong X. Y. Endoplasmic reticulum stress and related pathological processes. Journal of Pharmacological & Biomedical Analysis. 2013;1(2) [PMC free article] [PubMed] [Google Scholar]
- 310.Tam A. B., Mercado E. L., Hoffmann A., Niwa M. ER stress activates NF-κB by integrating functions of basal IKK activity, IRE1 and PERK. PLoS One. 2012;7(10, article e45078) doi: 10.1371/journal.pone.0045078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Willy J. A., Young S. K., Stevens J. L., Masuoka H. C., Wek R. C. CHOP links endoplasmic reticulum stress to NF-κB activation in the pathogenesis of nonalcoholic steatohepatitis. Molecular Biology of the Cell. 2015;26(12):2190–2204. doi: 10.1091/mbc.E15-01-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Sano R., Reed J. C. ER stress-induced cell death mechanisms. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2013;1833(12):3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Cao S. S., Luo K. L., Shi L. Endoplasmic reticulum stress interacts with inflammation in human diseases. Journal of Cellular Physiology. 2016;231(2):288–294. doi: 10.1002/jcp.25098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Uchimura K., Hayata M., Mizumoto T., et al. The serine protease prostasin regulates hepatic insulin sensitivity by modulating TLR4 signalling. Nature Communications. 2014;5:p. 3428. doi: 10.1038/ncomms4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Du K., Herzig S., Kulkarni R. N., Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300(5625):1574–1577. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- 316.Arruda A. P., Milanski M., Coope A., et al. Low-grade hypothalamic inflammation leads to defective thermogenesis, insulin resistance, and impaired insulin secretion. Endocrinology. 2011;152(4):1314–1326. doi: 10.1210/en.2010-0659. [DOI] [PubMed] [Google Scholar]
- 317.Li Y., Guo Y., Tang J., Jiang J., Chen Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochimica et Biophysica Sinica. 2014;46(8):629–640. doi: 10.1093/abbs/gmu048. [DOI] [PubMed] [Google Scholar]
- 318.Okla M., Wang W., Kang I., Pashaj A., Carr T., Chung S. Activation of Toll-like receptor 4 (TLR4) attenuates adaptive thermogenesis via endoplasmic reticulum stress. Journal of Biological Chemistry. 2015;290(44):26476–26490. doi: 10.1074/jbc.M115.677724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Nowsheen S., Yang E. S. The intersection between DNA damage response and cell death pathways. Experimental Oncology. 2013;34(3):243–254. [PMC free article] [PubMed] [Google Scholar]
- 320.Piret B., Schoonbroodt S., Piette J. The ATM protein is required for sustained activation of NF-κB following DNA damage. Oncogene. 1999;18(13):2261–2271. doi: 10.1038/sj.onc.1202541. [DOI] [PubMed] [Google Scholar]
- 321.Zhou B. B. S., Elledge S. J. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408(6811):433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 322.Strycharz J., Drzewoski J., Szemraj J., Sliwinska A. Is p53 involved in tissue-specific insulin resistance formation? Oxidative Medicine and Cellular Longevity. 2017;2017:23. doi: 10.1155/2017/9270549.9270549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Shimizu I., Yoshida Y., Suda M., Minamino T. DNA damage response and metabolic disease. Cell Metabolism. 2014;20(6):967–977. doi: 10.1016/j.cmet.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 324.Minamino T., Orimo M., Shimizu I., et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nature Medicine. 2009;15(9):1082–1087. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- 325.Shimizu I., Yoshida Y., Katsuno T., et al. p53-induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure. Cell Metabolism. 2012;15(1):51–64. doi: 10.1016/j.cmet.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 326.Vergoni B., Cornejo P. J., Gilleron J., et al. DNA damage and the activation of the p53 pathway mediate alterations in metabolic and secretory functions of adipocytes. Diabetes. 2016;65(10):3062–3074. doi: 10.2337/db16-0014. [DOI] [PubMed] [Google Scholar]
- 327.Picco V., Pagès G. Linking JNK activity to the DNA damage response. Genes & Cancer. 2013;4(9-10):360–368. doi: 10.1177/1947601913486347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Heffernan-Stroud L. A., Obeid L. M. p53 and regulation of bioactive sphingolipids. Advances in Enzyme Regulation. 2011;51(1):219–228. doi: 10.1016/j.advenzreg.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Petersen K. F., Dufour S., Befroy D., Garcia R., Shulman G. I. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. New England Journal of Medicine. 2004;350(7):664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Szendroedi J., Yoshimura T., Phielix E., et al. Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(26):9597–9602. doi: 10.1073/pnas.1409229111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Matés J. M., Segura J. A., Alonso F. J., Márquez J. Oxidative stress in apoptosis and cancer: an update. Archives of Toxicology. 2012;86(11):1649–1665. doi: 10.1007/s00204-012-0906-3. [DOI] [PubMed] [Google Scholar]
- 332.Zhou M., Li Y., Hu Q., et al. Atomic structure of the apoptosome: mechanism of cytochrome c- and dATP-mediated activation of Apaf-1. Genes & Development. 2015;29(22):2349–2361. doi: 10.1101/gad.272278.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Cullen S. P., Martin S. J. Caspase activation pathways: some recent progress. Cell Death & Differentiation. 2009;16(7):935–938. doi: 10.1038/cdd.2009.59. [DOI] [PubMed] [Google Scholar]
- 334.Suzuki M., Aoshiba K., Nagai A. Oxidative stress increases Fas ligand expression in endothelial cells. Journal of Inflammation. 2006;3(1):p. 11. doi: 10.1186/1476-9255-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Sommerfeld A., Reinehr R., Häussinger D. Free fatty acids shift insulin-induced hepatocyte proliferation towards CD95-dependent apoptosis. Journal of Biological Chemistry. 2015;290(7):4398–4409. doi: 10.1074/jbc.M114.617035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Johansson A.-C., Appelqvist H., Nilsson C., Kågedal K., Roberg K., Öllinger K. Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis. 2010;15(5):527–540. doi: 10.1007/s10495-009-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Malhi H., Bronk S. F., Werneburg N. W., Gores G. J. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. Journal of Biological Chemistry. 2006;281(17):12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 338.Ferreira D. M. S., Castro R. E., Machado M. V., et al. Apoptosis and insulin resistance in liver and peripheral tissues of morbidly obese patients is associated with different stages of non-alcoholic fatty liver disease. Diabetologia. 2011;54(7):1788–1798. doi: 10.1007/s00125-011-2130-8. [DOI] [PubMed] [Google Scholar]
- 339.Mayer C. M., Belsham D. D. Palmitate attenuates insulin signaling and induces endoplasmic reticulum stress and apoptosis in hypothalamic neurons: rescue of resistance and apoptosis through adenosine 5′ monophosphate-activated protein kinase activation. Endocrinology. 2010;151(2):576–585. doi: 10.1210/en.2009-1122. [DOI] [PubMed] [Google Scholar]
- 340.Turpin S. M., Lancaster G. I., Darby I., Febbraio M. A., Watt M. J. Apoptosis in skeletal muscle myotubes is induced by ceramides and is positively related to insulin resistance. American Journal of Physiology-Endocrinology and Metabolism. 2006;291(6):E1341–E1350. doi: 10.1152/ajpendo.00095.2006. [DOI] [PubMed] [Google Scholar]
- 341.Pradelli L. A., Villa E., Zunino B., Marchetti S., Ricci J.-E. Glucose metabolism is inhibited by caspases upon the induction of apoptosis. Cell Death & Disease. 2014;5(9, article e1406) doi: 10.1038/cddis.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Guilherme A., Tesz G. J., Guntur K. V. P., Czech M. P. Tumor necrosis factor-α induces caspase-mediated cleavage of peroxisome proliferator-activated receptor γ in adipocytes. Journal of Biological Chemistry. 2009;284(25):17082–17091. doi: 10.1074/jbc.M809042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Keuper M., Wernstedt Asterholm I., Scherer P. E., et al. TRAIL (TNF-related apoptosis-inducing ligand) regulates adipocyte metabolism by caspase-mediated cleavage of PPARgamma. Cell Death & Disease. 2013;4(1, article e474) doi: 10.1038/cddis.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.El Akoum S. PPAR gamma at the crossroads of health and disease: a masterchef in metabolic homeostasis. Endocrinology & Metabolic Syndrome. 2014;3(1):p. 126. doi: 10.4172/2161-1017.1000126. [DOI] [Google Scholar]
- 345.Tőzsér J., Benkő S. Natural compounds as regulators of NLRP3 inflammasome-mediated IL-1β production. Mediators of Inflammation. 2016;2016:16. doi: 10.1155/2016/5460302.5460302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Meng G., Zhang F., Fuss I., Kitani A., Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30(6):860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Libby P., Ordovas J. M., Auger K. R., Robbins A. H., Birinyi L. K., Dinarello C. A. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. The American Journal of Pathology. 1986;124(2):179–185. [PMC free article] [PubMed] [Google Scholar]
- 348.Tangi T. N., Elmabsout A. A., Bengtsson T., Sirsjö A., Fransén K. Role of NLRP3 and CARD8 in the regulation of TNF-α induced IL-1β release in vascular smooth muscle cells. International Journal of Molecular Medicine. 2012;30(3):697–702. doi: 10.3892/ijmm.2012.1026. [DOI] [PubMed] [Google Scholar]
- 349.Li Y., Wang P., Yang X., et al. SIRT1 inhibits inflammatory response partly through regulation of NLRP3 inflammasome in vascular endothelial cells. Molecular Immunology. 2016;77:148–156. doi: 10.1016/j.molimm.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 350.Huang N., Kny M., Riediger F., et al. Deletion of Nlrp3 protects from inflammation-induced skeletal muscle atrophy. Intensive Care Medicine Experimental. 2017;5(1):p. 3. doi: 10.1186/s40635-016-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Bauernfeind F. G., Horvath G., Stutz A., et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. Journal of Immunology. 2009;183(2):787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Ghonime M. G., Shamaa O. R., Das S., et al. Inflammasome priming by lipopolysaccharide is dependent upon ERK signaling and proteasome function. Journal of Immunology. 2014;192(8):3881–3888. doi: 10.4049/jimmunol.1301974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.He Q., You H., Li X.-M., Liu T.-H., Wang P., Wang B.-E. HMGB1 promotes the synthesis of pro-IL-1β and pro-IL-18 by activation of p38 MAPK and NF-κB through receptors for advanced glycation end-products in macrophages. Asian Pacific Journal of Cancer Prevention. 2012;13(4):1365–1370. doi: 10.7314/APJCP.2012.13.4.1365. [DOI] [PubMed] [Google Scholar]
- 354.Vandanmagsar B., Youm Y.-H., Ravussin A., et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nature Medicine. 2011;17(2):179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Scheiblich H., Schlütter A., Golenbock D. T., Latz E., Martinez-Martinez P., Heneka M. T. Activation of the NLRP3 inflammasome in microglia: the role of ceramide. Journal of Neurochemistry. 2017;143(5):534–550. doi: 10.1111/jnc.14225. [DOI] [PubMed] [Google Scholar]
- 356.Gross O., Thomas C. J., Guarda G., Tschopp J. The inflammasome: an integrated view. Immunological Reviews. 2011;243(1):136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 357.Zhou R., Yazdi A. S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 358.Lerner A. G., Upton J. P., Praveen P. V. K., et al. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metabolism. 2012;16(2):250–264. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.de Zoete M. R., Palm N. W., Zhu S., Flavell R. A. Inflammasomes. Cold Spring Harbor Perspectives in Biology. 2014;6(12) doi: 10.1101/cshperspect.a016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Li C., Zienkiewicz J., Hawiger J. Interactive sites in the MyD88 toll/interleukin (IL) 1 receptor domain responsible for coupling to the IL1β signaling pathway. Journal of Biological Chemistry. 2005;280(28):26152–26159. doi: 10.1074/jbc.M503262200. [DOI] [PubMed] [Google Scholar]
- 361.Jager J., Grémeaux T., Cormont M., Le Marchand-Brustel Y., Tanti J. F. Interleukin-1β-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148(1):241–251. doi: 10.1210/en.2006-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Kim A. Y., Park Y. J., Pan X., et al. Obesity-induced DNA hypermethylation of the adiponectin gene mediates insulin resistance. Nature Communications. 2015;6(1):p. 7585. doi: 10.1038/ncomms8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Wree A., Eguchi A., McGeough M. D., et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59(3):898–910. doi: 10.1002/hep.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Xiao H., Lu M., Lin T. Y., et al. Sterol regulatory element binding p1rotein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation. 2013;128(6):632–642. doi: 10.1161/CIRCULATIONAHA.113.002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Elgazar-Carmon V., Rudich A., Hadad N., Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. Journal of Lipid Research. 2008;49(9):1894–1903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 366.Makki K., Froguel P., Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflammation. 2013;2013:12. doi: 10.1155/2013/139239.139239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Mantovani A., Cassatella M. A., Costantini C., Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nature Reviews Immunology. 2011;11(8):519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 368.Mraz M., Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. The Journal of Endocrinology. 2014;222(3):R113–R127. doi: 10.1530/JOE-14-0283. [DOI] [PubMed] [Google Scholar]
- 369.Newsholme P., de Bittencourt P. I. H., Jr. The fat cell senescence hypothesis: a mechanism responsible for abrogating the resolution of inflammation in chronic disease. Current Opinion in Clinical Nutrition and Metabolic Care. 2014;17(4):295–305. doi: 10.1097/MCO.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 370.Codogno P., Meijer A. J. Autophagy: a potential link between obesity and insulin resistance. Cell Metabolism. 2010;11(6):449–451. doi: 10.1016/j.cmet.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 371.Hotamisligil G. S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Zhao Y., Zhang C.-F., Rossiter H., et al. Autophagy is induced by UVA and promotes removal of oxidized phospholipids and protein aggregates in epidermal keratinocytes. Journal of Investigative Dermatology. 2013;133(6):1629–1637. doi: 10.1038/jid.2013.26. [DOI] [PubMed] [Google Scholar]
- 373.Shibutani S. T., Yoshimori T. A current perspective of autophagosome biogenesis. Cell Research. 2014;24(1):58–68. doi: 10.1038/cr.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Rahman M., Mofarrahi M., Kristof A. S., Nkengfac B., Harel S., Hussain S. N. A. Reactive oxygen species regulation of autophagy in skeletal muscles. Antioxidants & Redox Signaling. 2014;20(3):443–459. doi: 10.1089/ars.2013.5410. [DOI] [PubMed] [Google Scholar]
- 375.Sasnauskiene A., Kadziauskas J., Vezelyte N., Jonusiene V., Kirveliene V. Damage targeted to the mitochondrial interior induces autophagy, cell cycle arrest and, only at high doses, apoptosis. Autophagy. 2009;5(5):743–744. doi: 10.4161/auto.5.5.8701. [DOI] [PubMed] [Google Scholar]
- 376.Luo C., Li Y., Wang H., et al. Mitochondrial accumulation under oxidative stress is due to defects in autophagy. Journal of Cellular Biochemistry. 2013;114(1):212–219. doi: 10.1002/jcb.24356. [DOI] [PubMed] [Google Scholar]
- 377.Portovedo M., Ignacio-Souza L. M., Bombassaro B., et al. Saturated fatty acids modulate autophagy’s proteins in the hypothalamus. PLoS One. 2015;10(3, article e0119850) doi: 10.1371/journal.pone.0119850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Soussi H., Clément K., Dugail I. Adipose tissue autophagy status in obesity: expression and flux—two faces of the picture. Autophagy. 2016;12(3):588–589. doi: 10.1080/15548627.2015.1106667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Norman J. M., Cohen G. M., Bampton E. T. W. The in vitro cleavage of the hAtg proteins by cell death proteases. Autophagy. 2010;6(8):1042–1056. doi: 10.4161/auto.6.8.13337. [DOI] [PubMed] [Google Scholar]
- 380.Rodríguez-Muela N., Hernández-Pinto A. M., Serrano-Puebla A., et al. Lysosomal membrane permeabilization and autophagy blockade contribute to photoreceptor cell death in a mouse model of retinitis pigmentosa. Cell Death & Differentiation. 2015;22(3):476–487. doi: 10.1038/cdd.2014.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Goligorsky M. S. SIRTing out the link between autophagy and ageing. Nephrology Dialysis Transplantation. 2010;25(8):2434–2436. doi: 10.1093/ndt/gfq348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Cho J.-H., Kim G.-Y., Pan C.-J., et al. Downregulation of SIRT1 signaling underlies hepatic autophagy impairment in glycogen storage disease type Ia. PLoS Genetics. 2017;13(5, article e1006819) doi: 10.1371/journal.pgen.1006819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Jiao M., Ren F., Zhou L., et al. Peroxisome proliferator-activated receptor α activation attenuates the inflammatory response to protect the liver from acute failure by promoting the autophagy pathway. Cell Death & Disease. 2014;5(8, article e1397) doi: 10.1038/cddis.2014.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Ren F., Zhang L., Zhang X., et al. Inhibition of glycogen synthase kinase 3β promotes autophagy to protect mice from acute liver failure mediated by peroxisome proliferator-activated receptor α. Cell Death & Disease. 2016;7(3, article e2151) doi: 10.1038/cddis.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Kim K. H., Jeong Y. T., Oh H., et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nature Medicine. 2013;19(1):83–92. doi: 10.1038/nm.3014. [DOI] [PubMed] [Google Scholar]
- 386.Gong Q., Hu Z., Zhang F., et al. Fibroblast growth factor 21 improves hepatic insulin sensitivity by inhibiting mammalian target of rapamycin complex 1 in mice. Hepatology. 2016;64(2):425–438. doi: 10.1002/hep.28523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Yu Y., He J., Li S., et al. Fibroblast growth factor 21 (FGF21) inhibits macrophage-mediated inflammation by activating Nrf2 and suppressing the NF-κB signaling pathway. International Immunopharmacology. 2016;38:144–152. doi: 10.1016/j.intimp.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 388.Lin Z., Tian H., Lam K. S. L., et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metabolism. 2013;17(5):779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 389.Holland W. L., Adams A. C., Brozinick J. T., et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metabolism. 2013;17(5):790–797. doi: 10.1016/j.cmet.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Xu P., Zhang Y., Wang W., et al. Long-term administration of fibroblast growth factor 21 prevents chemically-induced hepatocarcinogenesis in mice. Digestive Diseases and Sciences. 2015;60(10):3032–3043. doi: 10.1007/s10620-015-3711-z. [DOI] [PubMed] [Google Scholar]
- 391.Zhang J., Li Y. Fibroblast growth factor 21 analogs for treating metabolic disorders. Frontiers in Endocrinology. 2015;6:p. 168. doi: 10.3389/fendo.2015.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Fisher F. M., Chui P. C., Antonellis P. J., et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;59(11):2781–2789. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Jeon J. Y., Choi S.-E., Ha E. S., et al. Association between insulin resistance and impairment of FGF21 signal transduction in skeletal muscles. Endocrine. 2016;53(1):97–106. doi: 10.1007/s12020-015-0845-x. [DOI] [PubMed] [Google Scholar]
- 394.Gallego-Escuredo J. M., Gomez-Ambrosi J., Catalan V., et al. Opposite alterations in FGF21 and FGF19 levels and disturbed expression of the receptor machinery for endocrine FGFs in obese patients. International Journal of Obesity. 2015;39(1):121–129. doi: 10.1038/ijo.2014.76. [DOI] [PubMed] [Google Scholar]
- 395.Adams A. C., Cheng C. C., Coskun T., Kharitonenkov A. FGF21 requires βklotho to act in vivo. PLoS One. 2012;7(11, article e49977) doi: 10.1371/journal.pone.0049977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Markan K. R., Naber M. C., Small S. M., Peltekian L., Kessler R. L., Potthoff M. J. FGF21 resistance is not mediated by downregulation of beta-klotho expression in white adipose tissue. Molecular Metabolism. 2017;6(6):602–610. doi: 10.1016/j.molmet.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Kim S., Lee H.-G., Park S.-A., et al. Keap1 cysteine 288 as a potential target for diallyl trisulfide-induced Nrf2 activation. PLoS One. 2014;9(1, article e85984) doi: 10.1371/journal.pone.0085984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.David J. A., Rifkin W. J., Rabbani P. S., Ceradini D. J. The Nrf2/Keap1/ARE pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. Journal of Diabetes Research. 2017;2017:15. doi: 10.1155/2017/4826724.4826724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Chen B., Lu Y., Chen Y., Cheng J. The role of Nrf2 in oxidative stress-induced endothelial injuries. Journal of Endocrinology. 2015;225(3):R83–R99. doi: 10.1530/JOE-14-0662. [DOI] [PubMed] [Google Scholar]
- 400.Fourquet S., Guerois R., Biard D., Toledano M. B. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. Journal of Biological Chemistry. 2010;285(11):8463–8471. doi: 10.1074/jbc.M109.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Jiménez-Osorio A., Picazo A., González-Reyes S., Barrera-Oviedo D., Rodríguez-Arellano M., Pedraza-Chaverri J. Nrf2 and redox status in prediabetic and diabetic patients. International Journal of Molecular Sciences. 2014;15(12):20290–20305. doi: 10.3390/ijms151120290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.He H.-J., Wang G.-Y., Gao Y., Ling W.-H., Yu Z.-W., Jin T.-R. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World Journal of Diabetes. 2012;3(5):94–104. doi: 10.4239/wjd.v3.i5.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Faraonio R., Vergara P., di Marzo D., et al. p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. Journal of Biological Chemistry. 2006;281(52):39776–39784. doi: 10.1074/jbc.M605707200. [DOI] [PubMed] [Google Scholar]
- 404.Tan Y., Ichikawa T., Li J., et al. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress–induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes. 2011;60(2):625–633. doi: 10.2337/db10-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Cheng A.-S., Cheng Y.-H., Chiou C.-H., Chang T.-L. Resveratrol upregulates Nrf2 expression to attenuate methylglyoxal-induced insulin resistance in Hep G2 cells. Journal of Agricultural and Food Chemistry. 2012;60(36):9180–9187. doi: 10.1021/jf302831d. [DOI] [PubMed] [Google Scholar]
- 406.Jain A. K., Jaiswal A. K. GSK-3β acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. Journal of Biological Chemistry. 2007;282(22):16502–16510. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- 407.Kratschmar D. V., Calabrese D., Walsh J., et al. Suppression of the Nrf2-dependent antioxidant response by glucocorticoids and 11β-HSD1-mediated glucocorticoid activation in hepatic cells. PLoS One. 2012;7(5, article e36774) doi: 10.1371/journal.pone.0036774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Matsuda M., Shimomura I. Roles of oxidative stress, adiponectin, and nuclear hormone receptors in obesity-associated insulin resistance and cardiovascular risk. Hormone Molecular Biology and Clinical Investigation. 2014;19(2):75–88. doi: 10.1515/hmbci-2014-0001. [DOI] [PubMed] [Google Scholar]
- 409.Wake D. J., Rask E., Livingstone D. E. W., Söderberg S., Olsson T., Walker B. R. Local and systemic impact of transcriptional up-regulation of 11β-hydroxysteroid dehydrogenase type 1 in adipose tissue in human obesity. The Journal of Clinical Endocrinology & Metabolism. 2003;88(8):3983–3988. doi: 10.1210/jc.2003-030286. [DOI] [PubMed] [Google Scholar]
- 410.Xu J., Kulkarni S. R., Donepudi A. C., More V. R., Slitt A. L. Enhanced Nrf2 activity worsens insulin resistance, impairs lipid accumulation in adipose tissue, and increases hepatic steatosis in leptin-deficient mice. Diabetes. 2012;61(12):3208–3218. doi: 10.2337/db11-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Chartoumpekis D. V., Ziros P. G., Psyrogiannis A. I., et al. Nrf2 represses FGF21 during long-term high-fat diet–induced obesity in mice. Diabetes. 2011;60(10):2465–2473. doi: 10.2337/db11-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Lau A., Wang X. J., Zhao F., et al. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Molecular and Cellular Biology. 2010;30(13):3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Lau A., Zheng Y., Tao S., et al. Arsenic inhibits autophagic flux, activating the Nrf2-Keap1 pathway in a p62-dependent manner. Molecular and Cellular Biology. 2013;33(12):2436–2446. doi: 10.1128/MCB.01748-12. [DOI] [PMC free article] [PubMed] [Google Scholar]