Abstract
The mitogen activated protein kinase/extracellular signal-related kinase (MAPK/ERK) signaling pathway serves an integral role in growth, proliferation, differentiation, migration, and survival of all mammalian cells. Aberrant signaling of this pathway is often observed in several types of hematologic and solid malignancies. The most frequent insult to this signaling cascade, leading to its constitutive activation, is to the serine/threonine kinase Rapidly Accelerating Fibrosarcoma (RAF). Considering this, the development and approval of various small-molecule inhibitors targeting the MAPK/ERK pathway has become a mainstay of treatment as either mono- or combination therapy in these cancers. Although effective initially, a major clinical barrier with these inhibitors is the relapse of patients due to drug resistance. Knowledge of the mechanisms of resistance to these drugs is still premature highlighting the need for a more in depth understanding of how patients become insensitive to these pharmacologic interventions. Herein, we will succinctly summarize the milestones in the approval of select MAPK/ERK pathway inhibitors, their use in patients, and major modes of resistance.
1 Introduction
The MAPK/ERK signaling pathway is evolutionarily conserved and critical in regulating cell growth, proliferation, differentiation, migration, and survival [1]. Unfortunately, in many cancers including melanoma, thyroid, colorectal, non-small cell lung cancer (NSCLC), and hairy cell leukemia (HCL) this signaling pathway is altered leading to uncontrolled cellular processes, constitutive activation and cancer cell growth. Given the strong link of aberrations in this pathway and cancer cell growth, targeted inhibition of this pathway has become a central focus of cancer therapeutic development in recent years [2]. This effort has led to the development and Food & Drug Administration (FDA) approval of several small molecule inhibitors targeting mitogen-activated protein kinase/extracellular-signal regulated kinase (MAPK/ERK) pathway tyrosine kinase proteins such as BRAF and MEK [3,4]. The MAPK/ERK pathway is activated by extracellular signaling molecule(s) that bind to their respective trans-membrane receptor tyrosine kinases (RTKs), such as epidermal growth factor and its receptor – EGF and EGFR respectively. Upon receptor activation, the extracellular signal is transduced inside the cell, subsequently activating the GTPase, Rat Sarcoma protein (RAS), via guanine nucleotide exchange from GDP to GTP. The active GTP-bound-RAS can then initiate activation of the three-tier MAPK cascade – RAF→MEK→ERK (RAF, rapidly accelerated fibrosarcoma; MEK, mitogen activated protein kinase kinase ). As the final protein kinase in the pathway, activated ERK is then able to phosphorylate downstream cytoplasmic and/or nuclear effectors that regulate and ultimately alter cell growth, proliferation, differentiation, migration, and survival [1–3].
As aforementioned, the MAPK/ERK pathway is frequently altered at its early stages. This includes overexpression/amplification of RTKs, activating mutations to RTKs, sustained production of signaling molecules, and point mutations of RAS or RAF. Although the MAPK/ERK signaling pathway can be altered at various places, many cancers present with mutations to RAF [5,6]. In melanoma, more than 50% of patients present with a point mutation to the BRAF isoform at codon 600, where a valine is substituted for glutamate (V600E) in almost 90% of cases [7]. A recent genomic analysis of melanomas through next-generation sequencing and the cancer genome atlas project demonstrated that BRAF hot-spot mutations are the most common mutation identified, with 52% harboring BRAF somatic mutations; the most common being V600E (75%), followed by V600K (11%), K601 (3%), and V600R (2%). Both BRAF V600 and K601 hot-spot mutations were anti-correlated with hot-spot NRAS mutations while BRAF non-hot-spot mutations co-occurred with RAS (N/H/K) hot-spot and NF1 mutations [8]. Similar to melanoma, approximately 45% of papillary thyroid cancers and 10% of colorectal cancer patients harbor similar BRAF mutations [9,10,11]. Interestingly, this same mutation has been found in 100% of HCL patients [12]. It has become apparent in the last few years that a large subset of patients with these cancers are effected by aberrations to the MAPK/ERK pathway, specifically BRAF, which has led to the development and approval of small molecule BRAF inhibitors for the treatment of these malignancies. While BRAF has been shown as an effective target in the MAPK/ERK pathway for several cancers where this pathway is aberrant, other selective small molecule inhibitors of MEK, the downstream effector of RAF, have also been recently developed and are now FDA approved for use in cancer patients [13]. Despite their initial efficacy, however, the biggest barrier faced in the clinic with these selective small molecule inhibitors is the emergence of resistance following treatment in a majority of patients. This resistance can come from a myriad of escape mechanisms that cancer cells have developed to overcome this targeted drug effect. Given the multiple mechanisms of resistance that exist with these inhibitors, overcoming this resistance does not have a simple solution and is the ongoing work of many researchers and clinicians.
In this review, we will discuss landmark clinical trials investigating the use of MAPK/ERK pathway inhibitors as monotherapy and in combination for the treatment of melanoma, thyroid cancer, colorectal cancer, NSCLC, and HCL. Further discussion will also describe a detailed overview of the mechanisms of resistance to these inhibitors and the current clinical application of these inhibitors including their application in standard of care treatment regimens, costs, and side effects.
2 MAPK/ERK Pathway Inhibitors as Monotherapies
2.1 BRAF Inhibitors
2.1.1
Vemurafenib (formerly PLX4032; brand name Zelboraf; developed by Plexxikon and Genentech) was approved by the FDA in 2011 and by the European Commission in 2012 for the treatment of BRAFV600E-mutated late-stage melanoma. This reversible, small molecule inhibitor is orally administered and causes apoptosis of melanoma cells by selectively targeting BRAFV600 mutations.
In 2009, a Phase I multi-center study was conducted in patients with metastatic cancer to identify the maximum tolerated dose and safety and pharmacokinetic profiles of the selective BRAF inhibitor [14]. Of the fifty-five patients enrolled in the dose-escalation study, 49 patients had metastatic melanoma and 16 patients in this subset harbored the BRAFV600E mutation. Newly enrolled patients were administered PLX4032 at 160mg twice daily followed by escalation. During the study, dose-limiting toxicities were first observed at 720mg twice daily of PLX4032 with minor adverse effects (AEs) such as grade 2 or 3 rash, fatigue, and arthralgia. These AEs were also observed in patients given doses up to 1120mg twice daily. Of the 16 patients with BRAFV600E mutant melanomas, partial and complete responses were seen in 11 patients (10 and 1, respectively). Following the dose-escalation phase of the study, an extension phase was completed in an additional 32 patients with BRAFV600E-mutated metastatic melanoma. Patients were administered 960mg twice daily and showed partial and complete responses in 24 and 2 patients, respectively. From this, the recommended Phase II dose (RP2D) was established as 960mg twice daily. Overall, this study demonstrated that the use of vemurafenib as a targeted therapy in humans with metastatic melanoma harboring the activating BRAFV600E mutant is safe. Additionally, dosing for further clinical trials was determined.
In 2011, the Phase II clinical trial (BRIM-2) conducted by Ribas and colleagues was pivotal in showing the efficacy of vemurafenib in previously treated metastatic melanoma patients with the BRAFV600E mutation [15]. The primary endpoint of this open-label, multi-center study was overall response rate (ORR) to vemurafenib in stage IV melanoma patients who had previously received either one or more systemic therapies. Secondary endpoints included the duration of patient response to vemurafenib, progression free survival (PFS), overall survival (OS), and safety. In BRIM-2, 53% of patients demonstrated response overall, and the median duration of response was 6.8 months. The AEs observed in the study were similar to the Phase I trial of PLX4032 which included rash, fatigue, and arthralgia. In addition, investigators noted liver function abnormalities and the development of secondary skin tumors. These skin lesions were the most common Grade 3 AEs and are thought to be the result of paradoxical MAPK/ERK pathway activation [16]. Cells that harbor oncogenic RAS or upregulated receptor tyrosine kinases are able to evade BRAF inhibition through feedback activation of the pathway [17,18]. In vemurafenib treated patients, it is suggested that oncogenic RAS has a major role in bypassing direct pathway inhibition [17,18]. In these cells, despite BRAF inhibition, cell proliferation and growth is achieved through upstream activation by RAS. Most AEs were manageable and could be reversed with a simple dose reduction. Results from the BRIM-2 study, including the ORR of 53%, demonstrated the effectiveness of vemurafenib in BRAFV600E melanoma patients previously treated with other systemic agents.
Following these early Phase I and II clinical trials, a randomized Phase III clinical trial was performed directly comparing the efficacy of vemurafenib to dacarbazine in previously untreated metastatic melanoma patients with the BRAFV600E mutation [19]. The co-primary endpoints of the study were OS and PFS, whereas secondary endpoints were response rate, duration, and safety. In this trial, 675 patients were randomly assigned to receive either vemurafenib (960mg orally twice daily) or dacarbazine (1000mg per square meter of body surface area intravenously every 3 weeks). After 6 months of treatment, the OS was found to be higher in vemurafenib treated patients (84%) compared to dacarbazine treated patients (64%). Due to the significant benefit observed in the vemurafenib group, crossover from dacarbazine to vemurafenib was recommended in patients not responding to dacarbazine. Common minor AEs to vemurafenib again included rash, fatigue, arthralgia, alopecia, photosensitivity, and nausea, and were typically managed with dose reduction. 18% of patients treated with vemurafenib developed secondary skin tumors which were treated with simple excision. In addition to the increased OS, vemurafenib also improved median PFS in previously untreated BRAFV600E metastatic melanoma patients to 5.3 months versus 1.6 months in dacarbazine treated patients.
Vemurafenib is FDA approved (August 2011) for use in unresectable metastatic melanoma harboring the BRAFV600E mutation, but on-going clinical trials are now testing this selective, small inhibitor in other cancer models, specifically those with the same activating BRAF mutation (V600E or V600K), such as thyroid cancer and HCL. In an on-going pilot study, the efficacy of vemurafenib used alone or in combination with radioiodine in radioiodine refractory BRAF mutant thyroid cancers (ClinicalTrials.gov Identifier: NCT02145143) is being tested to determine treatment effects. Similarly, there are on-going clinical trials testing the efficacy of vemurafenib in HCL. Notably, there is one completed multi-center Phase II trial that reports greater than 96% response rates in patients and a median of 23 months for response duration [20]. In 2017, the FDA approved vemurafenib in the treatment of Erdheim Chester Disease. As a whole, these studies show promising results for the possibility of vemurafenib approval in other cancers harboring activating BRAF mutations.
The approval for use of vemurafenib in metastatic melanoma has changed the current standard of care and patient prognosis. Both preclinical and clinical studies have shown the efficacy of selectively targeting a protein that plays a major role in a pathway contributing to tumor progression. Although vemurafenib has dramatically altered the breadth of treatment options for patients, it is important to highlight that vemurafenib is rarely used as monotherapy and is instead more efficacious in combination therapy with MEK inhibitors. This is in large part due to the feedback activation of the MAPK/ERK pathway observed with single agent treatment with vemurafenib. Combination strategies and why they are more effective than the use of vemurafenib alone will be discussed in a later section.
2.1.2
Dabrafenib (formerly GSK2118438; brand name Tafinlar; developed by GlaxoSmithKline) was approved by the FDA and the European Union for use in unresectable or metastatic BRAFV600E or BRAFV600K melanoma in 2013. Like vemurafenib, dabrafenib is a reversible, small molecule inhibitor of the kinase BRAF harboring the V600E or V600K mutation. The use of dabrafenib is contraindicated in patients with wild-type BRAF (BRAFWT) due to the risk of tumor progression in these patients. Melanoma patients with BRAFWT have a unique response to selective BRAF inhibitors by demonstrating hyperactivation of the MAPK/ERK pathway. Although the exact mechanism has yet to be elucidated, there is evidence to suggest that selective BRAF inhibitors drive the dimerization of other RAF isoforms, like CRAF [21, 22]. Alternatively, Holderfield and colleagues proposed a mechanism that interrupts inhibitory autophosphorylation of the protein [23]. This allows for paradoxical pathway activation independent of BRAF ultimately leading to cell proliferation.
During the initial Phase I study from 2009 to 2012 the tolerability and safety of dabrafenib was tested at multiple centers in Australia and the USA to ultimately establish a standard recommended dose to be used in Phase II trials [24]. In this study, patients with the BRAFV600E mutation were treated with dabrafenib in a dose-escalation protocol. Dabrafenib was well-tolerated in patients with an activating BRAFV600E mutation, with most AEs as minor and these included fatigue, pyrexia, and cutaneous squamous cell carcinoma. Although dabrafenib administration was increased to 300mg twice daily in this study, no maximum tolerated recorded dose was noted and 150mg twice daily was established as the RP2D.
The clinical efficacy and safety of dabrafenib was tested in a multi-center Phase II (BREAK-2) trial in patients with metastatic BRAF-mutated melanoma [25]. Patients enrolled in the study received dabrafenib 150mg twice daily orally until they experienced dose-limiting AEs, disease progression, or death. The primary endpoint of the BREAK-2 study was ORR, while PFS and OS were secondary endpoints. The study enrolled patients with BRAFV600E (76 patients) and BRAFV600K mutations (16 patients) with a confirmed response rate of 59% and 13% respectively. Results from the trial supported that dabrafenib was an active agent in both BRAFV600E and BRAFV600K mutations, but more effective in patients with the V600E mutation. The median PFS was 6.8 months in BRAFV600E patients and 4.5 months in BRAFV600K patients. The most common AEs observed during the Phase II study were arthralgia, pyrexia, and hyperkeratosis; 10% of patients developed secondary skin tumors. Overall, BREAK-2 confirmed the safety and activity of dabrafenib in metastatic melanoma patients with BRAFV600E mutations.
FDA approval of dabrafenib in 2013 for unresectable or metastatic melanoma was based on results from a multi-center international, open-label randomized, active control Phase III trial [26]. In this study, the improvement in PFS was observed in patients treated with dabrafenib compared to systemic treatment with dacarbazine. In this study, 250 patients with previously untreated disease and BRAFV600E mutations were enrolled. The median PFS of dabrafenib treated patients was 5.1 months compared to only 2.7 months with dacarbazine; patients from the dacarbazine treatment group were allowed to cross over to the dabrafenib treatment group. The AEs of dabrafenib in this study were similar to those observed in earlier Phase I and II trials, but also included erythrodysethesia palmar plantar syndrome and papilloma. At the end of this study, dabrafenib was recommended for use in BRAFV600E positive unresectable or metastatic melanoma at a dose of 150mg twice daily. Ongoing clinical efficacy trials of dabrafenib are currently enrolling in both papillary thyroid cancer (ClinicalTrials.gov Identifier: NCT01723202) and refractory leukemia (ClinicalTrials.gov Identifier: NCT03091257; NCT02551718).
Similar to vemurafenib, dabrafenib has improved treatment options for patients but has been shown to be most effective when used in combination with other therapies that target the MAPK/ERK pathway or immune response. These combinations will be discussed in Section 3.
2.1.3
Encorafenib (also known as LGX818; developed by Novartis), is a selective BRAFV600E inhibitor currently in Phase III trials to be approved for use in combination with a MEK inhibitor in BRAF-mutated melanoma. Despite sharing the same selectivity for mutant BRAF with the inhibitors mentioned in 2.1.1 and 2.1.2, encorafenib has some unique characteristics that distinguish it from other BRAF inhibitors, providing potential therapeutic benefit. In September 2017, Deloard and colleagues published preclinical and clinical (Phase I) data on the potent small molecule inhibitor [27]. The preclinical data shows that encorafenib is not only more potent than its counterparts (vemurafenib and dabrafenib), but also has a longer dissociation half-life. Moreover, encorafenib treatment results in less severe AEs than observed in vemurafenib and dabrafenib treated patients, including secondary skin tumors. The finding that encorafenib is more potent, has a longer dissociation half-life, and less incidence of secondary skin tumors makes it a promising drug in the pipeline for approval. In the Phase I clinical trial, the primary objective was to determine the maximum tolerated dose (MTD) and RP2D. BRAF inhibitor naïve and previously treated patients were enrolled in the study, where the former group of patients responded better. The ORR in BRAF inhibitor naïve patients was 60% and minimal in patients previously treated with a BRAF inhibitor. The MTD was determined to be 450mg twice daily, but due to dose limiting toxicities in the extension phase of the study the PR2D was declared as 300mg twice daily. During Phase I of the trial, the standard of care for the metastatic melanoma shifted greatly to the use of BRAF and MEK inhibitors in combination. As a result, encorafenib clinical trials progressed forward to test its use in combination with the MEK inhibitor binimetinib. This combination treatment strategy will be discussed in subsequent sections.
2.1.4
Sorafenib (brand name Nexavar; co-developed by Bayer and Onyx Pharmaceuticals), is a multi-targeted tyrosine kinase inhibitor (TKI) that is FDA and European Union approved for use in renal cell carcinoma (RCC), hepatocellular carcinoma (HCC), and radioactive-iodine refractive differentiated thyroid cancer (RAIR DTC) [28,29]. Although originally discovered as a Raf-1 inhibitor, sorafenib has since been found to be a potent inhibitor of several other kinases including BRAF. In 2005 and 2007, the FDA approved the use of sorafenib in RCC and HCC, respectively. Its indication in RCC was based on the Phase III clinical trial, TARGET (Treatment Approaches in Renal Cancer Global Evaluation Trial), which determined the effect of sorafenib or placebo on OS and PFS in patients with advanced RCC [30]. In this study, the median OS was 19.3 months and 15.9 months and PFS was 5.5 months and 2.8 months in sorafenib and placebo treated groups, respectively. Sorafenib’s approval for use in HCC was based on the Phase III clinical trial, SHARP (Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol), which compared the administration of sorafenib or placebo in previously untreated patients with unresectable disease [31]. In this international multi-center study, OS and time to symptomatic progression were the primary endpoints. Pharmacological intervention showed a significant increase in median OS from 7.9 months in placebo versus 10.7 months and sorafenib treated patients. There was no significant difference observed in time to symptomatic progression.
The 2013 FDA approval of sorafenib in RAIR DTC was based on the Phase III randomized control trial, DECISION [32]. In this study, 417 patients with RAIR DTC were randomized to undergo treatment with sorafenib or placebo; patients treated with sorafenib demonstrated median PFS of 10.8 months versus 5.8 months with placebo (Hazard Ratio was 0.59; 95% CI, 0.45 – 0.76; p < 0.0001), with a 41% decrease in risk of disease progression or death. There are numerous on-going clinical trials testing sorafenib in solid and hematological tumors including breast cancer, glioma, and acute myeloid leukemia. In these trials, the efficacy of sorafenib is being tested alone or in combination with the current standard of care, whether it be chemotherapy or targeted therapy, in each respective malignancy. In addition to these on-going trials, there is work in chronic myelomonocytic leukemia (CMML) using sorafenib (ClinicalTrials.gov; Identifier: NCT01620216). In 2014 Zhang and colleagues showed that a subset of CMML patients with wild-type RAS harbor mutations in its downstream effector, BRAF [33]. This gives premise for the use of targeting BRAF in CMML since it is mutated and contributes towards pathway activation and subsequent tumor progression. Taken together, future work is warranted evaluating the use of selective BRAF inhibitors.
2.2 MEK Inhibitors
2.2.1
Trametinib (brand name Mekinist, GlaxoSmithKline Pharmaceuticals) was approved by the FDA in 2013 and by the European Union in 2014 for unresectable or metastatic melanoma with BRAF (V600E or V600K) mutations. Trametinib is an orally administered, reversible selective allosteric inhibitor of MEK1 and MEK2 [34]. MEK is a kinase downstream of both RAS and RAF in the MAP kinase pathway. Although approved for monotherapy, more recent treatment strategies recommend combining trametinib with dabrafenib for improved effect.
Initial Phase I studies conducted from 2008–2010 recommended an oral dose of 2mg daily for trametinib which was well-tolerated in patients with minor AEs observed [34]. FDA approval was ultimately based on a multi-center Phase III trial of over 300 patients enrolled from 2010–2011 [35]. In this trial, patients were randomized to receive either 2mg daily of oral trametinib or standard intravenous chemotherapy with dacarbazine or paclitaxel. The results of the trial showed a disease PFS of 4.8 months in patients receiving trametinib versus only 1.5 months in patients receiving standard chemotherapy and a 56% risk reduction in mortality [35].
Notably, a 2012 Phase II study was conducted to determine whether there is a role for trametinib in patients with metastatic BRAF-mutated melanoma who have previously been treated with a BRAF inhibitor based on the hypothesis that inhibition of MEK, which is downstream from BRAF, may be a useful adjunct in patients who have developed resistance to BRAF inhibition [36]. In this study, patients were divided into two cohorts, those previously treated with a BRAF inhibitor (either dabrafenib or vemurafenib) and those previously treated with other systemic chemo- or immunotherapy. In the group of patients previously treated with a BRAF inhibitor, there was a significant decrease in disease response; no patients showed an objective response versus 25% of patients who were naïve to BRAF inhibitor therapy who demonstrated either complete or partial responses. There was a median PFS period of only 1.8 months compared to 4.0 months in patients who had not previously been treated with a BRAF inhibitor. This study suggested that the mechanisms of resistance from BRAF inhibitor treatment confer similar resistance to treatment with MEK inhibitors.
In comparing trametinib with vemurafenib, a major finding of multiple studies has been the lack of development of cutaneous SCC with treatment of trametinib [35]. While dermatological side effects such as rashes are common in patients treated with both trametinib and vemurafenib, trametinib appears to avoid the concerning development of SCC found in up to a quarter of patients who receive vemurafenib [37].
While currently only approved for treatment in patients with metastatic BRAFV600E or BRAFV600K mutated melanoma, there are some studies that suggest that trametinib may have a role in the treatment of patients with rarer BRAF-mutations. Currently a Phase II study is in progress to determine the efficacy of trametinib in patients with non-BRAFV600E mutations (ClinicalTrials.gov; Identifier: NCT02296112).
The possible role of trametinib in the treatment of KRAS-mutated NSCLC treatment was investigated in a 2015 Phase II study which enrolled 129 patients and randomized them to receive treatment with either trametinib or docetaxel [38]. The study was terminated early due to failure to demonstrate any difference in PFS between the groups.
2.2.2
Cobimetinib (GDC-0973 or brand-name Cotellic, developed by Exelixis and Genentech) was first introduced as a novel MEK inhibitor in 2012. In vitro studies indicated that this drug was particularly potent in BRAF or KRAS mutant cancer cell lines, a finding that was confirmed with in vivo human mutant xenograft tumor models [39]. In 2014, a Phase Ib study was performed evaluating cobimetinib in conjunction with vemurafenib in patients with BRAF-mutated melanoma. The 129 patients who were enrolled in the trial had either progressed on vemurafenib or had never been trialed on a BRAF inhibitor. The study was able to achieve its primary endpoint of demonstrating that cobimetinib was safe for use in conjunction with vemurafenib. Additionally, 15% of patients who had previously progressed on vemurafenib prior to being enrolled in the study and 87% of patients who had never been trialed on a BRAF-inhibitor demonstrated objective responses to therapy [40]. Based on promising trial results, in 2015 the FDA and the European Union approved cobimetinib in conjunction with vemurafenib for the treatment of advanced stage BRAFV600E or BRAFV600K mutated melanoma. Cobimetinib is not approved for single-drug use [41].
2.2.3
Selumetinib (study name AZD6244, ARRY-142886) was granted orphan drug status by the FDA in May 2016 for the treatment of patients with stage III or IV differentiated thyroid cancer. Similar to trametinib, selumetinib is an orally administered selective, allosteric inhibitor of MEK1 and MEK2. Initial Phase I trials recommended a dosing of 75mg twice daily [42]. While studied in a number of different tumors, the best-supported application for selumetinib has been in patients with metastatic differentiated thyroid cancers that are refractory to radioiodine concentration. The inability of certain thyroid cancers to concentrate iodine has been linked to mutations in BRAF and RAS, both of which lead to activation of MAPK signaling [43]. A 2013 study demonstrated that selumetinib was able to sensitize patients with differentiated thyroid cancer who were previously immune to radioiodine concentration in order to allow for successful radioiodine treatment [44]. In this study, 20 patients with metastatic, radioiodine-resistant differentiated thyroid cancer were treated with selumetinib for 4 weeks. After 4 weeks of treatment, they underwent iodine-124 PET imaging to determine whether the tumor had improved iodine uptake. Of the 20 patients enrolled, selumetinib was able to increase the uptake of iodine in 12/20 patients [44]. The most notable response was in patients with NRAS mutations; 5/5 patients received radioiodine treatment with 4 patients demonstrating partial responses and 1 patient showing stable disease. A randomized, large-scale Phase III study with a goal recruitment of 400 patients is currently in progress to validate these results (ClinicalTrials.gov; Identifier: NCT01843062]. This study, expected to be completed in 2019, aims to compare the response of patients with differentiated thyroid cancer receiving selumetinib and radioactive iodine with those receiving a placebo and radioactive iodine treatment.
Selumetinib has been studied extensively for the treatment of KRAS-mutated non-small cell lung cancer. There have been mixed findings supporting its efficacy in this disease. A 2010 Phase II study comparing selumetinib to standard-of-care treatment did not show any significant difference in outcomes [45]. However, a 2013 Phase II study showed that the combination of selumetinib and docetaxel acted synergistically, leading to an improved OS and PFS compared to placebo and docetaxel [46]. Based on these results, the large Phase III (SELECT-1) trial was conducted randomizing 500 patients with KRAS-mutated NSCLC to receive either selumetinib and docetaxel or placebo and docetaxel. The results, which were published in 2017, showed no significant difference in OS or PFS between both treatment groups [47].
2.2.4
Pimasertib (AS-703026), from Santhera Pharmaceuticals and licensed by Merck Serono and Sanofi, is a selective, oral small-molecule MEK inhibitor which was first introduced in 2009 with promising anti-tumor activity in in vitro models. A 2012 Phase I study established its safety and recommended a dose of 60mg twice daily [48]. Combination therapy studies have been performed, including a 2015 Phase I study which evaluated the combination of pimasertib plus FOLFIRI in patients with KRAS-mutated metastatic colorectal cancer; however, the study was limited, as patients were only able to tolerate a maximum dose of 45 mg daily due to adverse events of mucositis [49]. More promisingly, results were recently published in 2016 of a multi-center Phase II clinical trial evaluating pimasertib compared to dacarbazine in the treatment of cutaneous NRAS melanoma. 194 patients were randomized to pimasertib 60mg twice daily or dacarbazine infusion; the trial found that median progression free survival was significantly longer in patients treated with pimasertib (13.0 versus 6.9 weeks) [50].
2.2.5
Binimetinib (ARRY-162, MEK-162) by Array BioPharma is a novel orally available small molecule MEK inhibitor that was accepted by the FDA in September 2017 for review of its New Drug Application [51]. Preliminary data published in 2010 indicated that binimetinib is a potent inhibitor of cell proliferation in mutant B-Raf and Ras cell lines and has anti-tumor activity in xenograft tumor models across a variety of cancer types including colorectal cancer and pancreatic cancer [52]. A Phase I trial was published in 2017 demonstrating safety and efficacy of binimetinib in 93 patients with biliary cancer, KRAS-mutant colorectal cancer, and BRAF-mutant colorectal cancer. The recommended treatment dose was 45mg twice daily. The trial noted dose-limiting adverse events of dermatitis acneiform and ocular toxicity, including chorioretinopathy [53]. Further phase I and II trials have evaluated binimetinib with a variety of targeted and chemotherapy agents; for example, a 2017 study demonstrated the safety of combined binimetinib and FOLFOX in 26 patients with colorectal cancer who previously progressed on standard therapies [54]. Currently, the COLUMBUS (encorafenib in combination with binimetinib in BRAF-mutant melanoma) and BEACON CRC (encorafenib, binimetinib and cetuximab in BRAF-mutant colorectal cancer) are major Phase III trials in progress to evaluate the role of binimetinib in advanced cancers [51].
3 MAPK/ERK Pathway Inhibitors as Combination Therapy by Malignancy
3.1 Melanoma
Although the use of a BRAF inhibitor as monotherapy in patients with BRAF-mutant unresectable or metastatic melanoma has been shown to improve OS and PSF compared to standard chemotherapy, there are major barriers in clinical use of these agents including the development of resistance and/or secondary skin tumors. Resistance to these inhibitors is likely due to reactivation of the MAPK/ERK pathway [55, 56, 57], while the development of secondary skin tumors is the result of BRAF inhibitor-induced paradoxical MAPK/ERK pathway activation [22, 27, 58]. Similar to BRAF inhibitors, MEK inhibitors have also improved OS of these patients, but do not lead to same pitfalls. As a result, the use of MAPK/ERK pathway inhibitors in combination has overcome many of the limitations associated with the use of BRAF inhibitors as monotherapy.
Within the past half-decade, the use of BRAF and MEK inhibitors in combination therapy has been FDA and European Union approved for the treatment of metastatic melanoma patients. The advantage of using the two MAPK/ERK pathway inhibitors in combination was first seen with dabrafenib and trametinib in 2014. The approval of this combination treatment was based on the multi-center open label randomized Phase III clinical trial enrolling patients with advanced (stage III or IV) BRAF mutant (V600E or V600K) melanoma [59]. Patients were randomly selected to receive one of three treatment regimens – trametinib 2mg daily with dabrafenib 150mg twice daily, trametinib 1mg once daily with dabrafenib 150mg twice daily, or dabrafenib as a single agent 150mg twice daily. The patients treated with BRAF/MEK inhibitor combination therapy experienced a higher ORR and PFS compared to the BRAF inhibitor alone. The combination treated group had an ORR of 67% (complete 10%; partial 56%) compared to 51% (complete 9%; partial 43%) in the dabrafenib-alone group. The median PFS was 9.3 vs 8.8 months in the combination and monotherapy groups, respectively.
In 2015, vemurafenib was approved by the FDA and the European Union for use in combination with the MEK inhibitor cobimetinib. The approval of this combination therapy was based on the Phase III randomized clinical trial testing the combination treatment on previously untreated patients with metastatic melanoma patients [60]. In this study, all patients first received vemurafenib and were then randomly selected to either receive cobimetinib, or placebo. The group receiving combination therapy experienced a prolonged progression free survival (PFS) compared to the vemurafenib alone group – 9.9 months and 6.2 months after starting treatment, respectively. Additionally, patients that were treated with combination therapy lived longer and had complete or partial response to treatment compared to the patients treated with BRAF inhibitor monotherapy. The overall rate of response was 68% in vemurafenib-cobimetinib treated patients, with a completed response in 10% of patients. In vemurafenib-placebo treated patients, there was a ORR of 45%, with a complete response in 4% of patients. Additionally, there was an improvement in the survival rate at 9 months from 73% in the control group to 81% in the combination therapy group. From this study, it was determined that the administration of the BRAF and MEK inhibitors vemurafenib and cobimetinib in combination provides metastatic melanoma patients with increased efficacy over a BRAF inhibitor alone.
Since the approval of these MAPK/ERK pathway inhibitor combinations, the landscape for treatment of metastatic melanoma has shifted drastically. It is important to emphasize that these trials not only show that BRAF and MEK inhibitors used together are effective, but also mitigate the AEs of these agents when used alone – specifically secondary skin tumors. In the 2014 Phase III trial, only 2% of patients given the dabrafenib-trametinib combination developed cutaneous squamous cell carcinomas compared to 9% of dabrafenib-only patients [59]. Similarly, the vemurafenib-cobimetinib combination resulted in less occurrences (2%) than vemurafenib alone (11%) [60].
In addition to these approved BRAF and MEK inhibitor combinations, another BRAF and MEK inhibitor combination in the pipeline for FDA approval is encorafenib and binimetinib. The two-part Phase III trial of these agents (COLUMBUS) demonstrated that the combination of encorafenib and binimetinib improves ORR and PFS of metastatic melanoma patients, as well as lowers the incidence of severe AEs. The first part of the study measured various endpoints (e.g. ORR, PFS, AEs) of encorafenib-binimetinib, encorafenib alone, and vemurafenib alone [61]. The ORR was highest in the combination group at 63% (complete 8%; partial 55%), followed by encorafenib alone at 51% (complete 5%; partial 45%), and lowest in vemurafenib alone at 40% (complete 6%; partial 35%). The PFS was 14.9 months in the combination group, whereas the BRAF inhibitors alone were 9.6 and 7.3 months in encorafenib and vemurafenib, respectively. In addition to improved outcomes with BRAF/MEK inhibitor combination therapy, exposure time to the targeted agents was also extended (combination 51 weeks, encorafenib 31 weeks, and vemurafenib 27 weeks). Part 2 of the study focused on the contribution of binimetinib to the combination therapy [62]. At the end of the trial PFS was extended from 7.4 months in encorafenib-alone to 12.9 months in encorafenib-binimetinib combination. The ORR was also improved from 50% to 66% in encorafenib alone and encorafenib-binimetinib combination, respectively. With the recent publishing of the Part 2 results in September 2017, it is without question that BRAF/MEK inhibitor combinations are advantageous alternatives to MAPK/ERK pathway inhibitors as monotherapies. Additionally, the use of BRAF and MEK inhibitors in combination with immunotherapies has gained a substantial amount of support in the field [63].
Immunotherapy, such as anti-PD1 (programmed cell death protein 1) and CTLA-4 (cytotoxic T-lymphocyte associated antigen 4) antibodies, and BRAF/MEK inhibitors are currently considered first line agents in the treatment of metastatic/advanced stage melanoma. Consequently, there is interest in investigating whether these agents can be used safely and efficaciously in combination therapy. Unfortunately, a 2013 Phase I study on the combination of vemurafenib and the CTLA-1 antibody ipilimumab in 12 BRAF-mutated melanoma patients was terminated prior to Phase II investigations due to dose limiting toxic elevations in hepatic enzymes in more than 50% of patients at both the full approved doses and at a lower dose of vemurafenib [64]. However, a more recent Phase I study (KEYNOTE-022 study) was released in 2017 evaluating the combination of the PD-L1 inhibitor pembrolizumab, dabrafenib, and trametinib in 15 patients with metastatic BRAF-mutated melanoma. In these patients, there was a 20% rate of dose limiting toxicities related to elevations in hepatic enzymes; in all patients, these elevations normalized with pauses in therapy. Overall, patients demonstrated promising responses, with 1 patient showing a complete response, 9 patients showing partial responses, and 2 patients with stable disease. The trial is currently in Phase II [65]. An additional Phase II study evaluating the combination of cobimetinib, vemurafenib, and the PD-L1 inhibitor atezoliumab and a Phase III study evaluating the combination of dabrafenib, trametinib, and the novel PD-1 inhibitor PDR001 are currently underway. If successful, these studies have the potential to dramatically alter the current paradigm of care in patients with advanced stage melanoma (ClinicalTrials.gov Identifier: NCT02902029; NCT02967692).
3.2 Non-Small Cell Lung Cancer
In the last few years, many studies have been completed to establish the possible role of BRAF inhibitors in the treatment of NSCLC [66–68]. An estimated 2.6% of NSCLCs have BRAF mutations [66]. Key in vitro experiments first demonstrated that treatment with dabrafenib and trametinib significantly inhibited cell growth in BRAF-mutated NSCLC cells. This lead to a 2016 Phase II multicenter international study to determine the efficacy of this combination therapy (dabrafenib and trametinib) in the treatment of patients with BRAF-mutated stage IV NSCLC [67]. In this study, 57 patients with BRAFV600E-mutant metastatic NSCLC previously treated with platinum-based systemic therapy were enrolled and treated with the combination of dabrafenib (150mg twice daily) and trametinib (2mg once daily) for multiple 21 day cycles until disease progression was noted. The results of the study indicated that 63.2% of patients demonstrated complete or partial response to treatment with a median PFS of 9.7 months [67]. By comparison, literature evaluating the prior standard of care estimated the response rate and median PFS for this chemotherapy regimen in the treatment of BRAF-mutated NSCLC patients to be 9% and 3.1 months, respectively [68]. Of note however, in this combination trial more than half of patients (56%) experienced a more serious grade 3 or 4 adverse event (SAE) and nearly all patients experienced some adverse event. Despite these SAEs, only 12% of patients required discontinuation of the trial due to these toxicities [67]. Accordingly, in 2017, the FDA and the European Union approved the combination of dabrafenib and trametinib for treatment of patients with metastatic NSCLC with BRAFV600E mutations [69].
3.3 Colorectal Cancer
The use of BRAF inhibitors in the treatment of colorectal cancer has a long history of investigation. Unlike treatment of BRAF-mutated melanomas, very rapid resistance and reactivation of the downstream MAPK/ERK pathway occurs in BRAF-mutated colorectal cancer cells, rendering single agent treatment with BRAF inhibitors virtually ineffective [70]. However, some evidence exists supporting the utility of combining vemurafenib with other chemotherapy agents. A 2015 study suggested some benefit of combination therapy with vemurafenib and panitumumab, an EGFR inhibitor approved by the FDA in 2006 for patients with metastatic colorectal cancer with disease progression despite prior treatment [71]. In this study, 15 metastatic colorectal cancer patients with BRAFV600E mutations were enrolled; all patients had previously failed standard chemotherapy treatment. The combination of vemurafenib and panitumumab initially resulted in tumor regression in 10 out of 12 patients; however, the authors noted that this response was modest with all patients having disease progression within 1 year [71].
A chemotherapy regimen that has shown more promise however in the treatment of metastatic BRAF-mutated colorectal cancer is combining vemurafenib, irinotecan, and cetuximab. Similar to panitumumab, cetuximab is a EGFR inhibitor approved by the FDA for treatment of metastatic colorectal cancer in conjunction with standard chemotherapy such as irinotecan [72]. In preliminary in vitro studies, the combination of vemurafenib, cetuximab, and effect augmentation with irinotecan was more effective in the treatment of colorectal tumor cells with BRAF mutations that any of the individual drugs alone [73]. Based on these findings, a 2016 study enrolled 19 patients with mutated BRAFV600E metastatic colorectal cancer and treated them with the combination of cetuximab, irinotecan, and vemurafenib [74]. Results from this study demonstrated tumor regression radiographically in over a third (35%) of patients. Based on these outcomes, a large Phase II study enrolling 106 patients randomized patients with BRAF mutated metastatic colorectal cancer to receive irinotecan and cetuximab with or without vemurafenib (ClinicalTrials.gov; NCT02164916). Interim results of the study released in 2017 demonstrated that the addition of vemurafenib significantly increased progression-free survival (PFS) from 2.0 to 4.4 months and improved disease control rate from 22% to 67% [75].
3.4 Thyroid Cancer
To date, there are no approved MAPK/ERK pathway combination therapies for use in thyroid carcinomas. Despite this, there are some on-going Phase I clinical trials testing the therapeutic potential of a MAPK/ERK pathway inhibitor used in combination with another therapeutic intervention. For example, a pilot study is testing the effect of vemurafenib plus the biological agent KTN3379 in BRAF mutant radioiodine refractory thyroid carcinoma patients. The primary endpoint of this study will measure if the combination can increase tumoral radioiodine incorporation. In addition, standard safety and tolerability are outcomes that are being assessed in this early study (ClinicalTrials.gov Identifier: NCT02456701). Additionally, there is another on-going Phase I study testing the use of dabrafenib and lapatinib, the dual HER2/neu and EGFR inhibitor, in unresectable radioiodine refractory thyroid cancer patients (ClinicalTrials.gov Identifier: NCT01947023).
4 Mechanisms of Resistance to MAPK/ERK Pathway Inhibitors
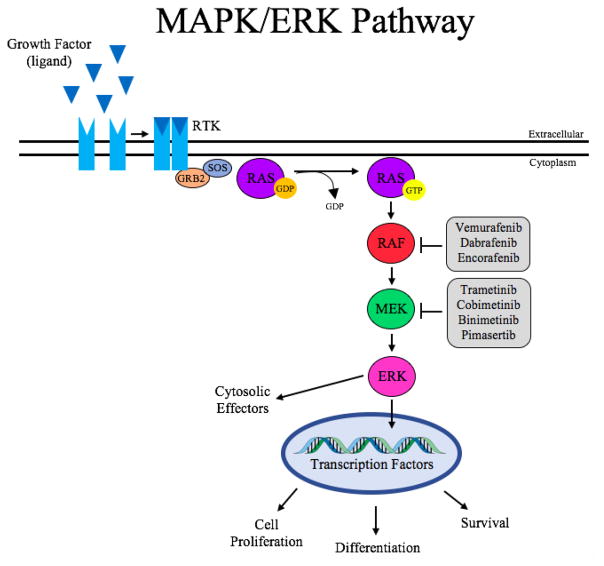
MAPK/ERK pathway inhibitors have proven to be effective treatments in various cancer types, but the major clinical problem faced with these targeted therapies is the emergence of resistance soon after the start of treatment. An initial strategy to overcome resistance to BRAF inhibitors involved adding another MAPK/ERK pathway inhibitor targeting the serine/threonine kinase, MEK. The rationale behind adding another MAPK/ERK pathway inhibitor to the treatment strategy is based on pathway reactivation [16, 21–23]. When given alone, BRAF inhibitors can induce the pathway to become activated evading the blockade. By adding in an inhibitor of a downstream effector (MEK) you are targeting two nodes of the pathway increasing its inhibition. Since the preceding section focused extensively on the use of MAPK/ERK pathway inhibitor combination therapies in various cancers, we will review the postulated mechanisms of resistance to MAPK/ERK pathway inhibitors, namely in inhibitors of BRAF. It is important to note that mechanisms of BRAF inhibitor resistance can vary within an individual type of cancer, as well as between different malignancies. For example, it cannot be assumed that all melanoma patients that develop resistance to a given BRAF inhibitor have the same underlying mechanism. There are various modes that can attribute towards resistance, but one thing that is shared amongst these patients is the median time to resistance – 6–8 months after the start of treatment [76, 77]. As with any type of resistance, BRAF inhibitor resistance can be primary (intrinsic) and/or acquired. See Figure 1.
4.1 Primary (Intrinsic) Resistance
Despite the large majority of metastatic melanoma patients that present with the activating BRAF mutation (V600E or V600K), 20% of these patients are initially refractory to selective BRAF inhibitors and do not respond [76]. Similarly, there are a subset of metastatic colorectal cancer patients that have the BRAF mutant (<10%) and do not respond to BRAF inhibitors [78]. In melanoma, mechanisms of intrinsic resistance can include, but not limited to: RAC1 mutations, loss of PTEN, dysregulation of cell cycle proteins, and changes to the microenvironment. In colorectal cancers harboring the same BRAF mutation, intrinsic resistance to these inhibitors is primarily due to feedback activation of EGFR [79].
The RAC1P29S mutation is the third most common hotspot mutation present in metastatic melanomas patients, following behind the first and second most common BRAFV600 and NRASQ61, respectively [80]. RAC1 is a member of the RAS GTPase superfamily and has an integral role in cell motility and growth. Its exact role in melanogenesis is not fully understood, but evidence supports that RAC1 is a key player in epithelial mesenchymal transition (EMT) [81–84]. Unlike BRAF and NRAS mutations, the RAC1 hotspot mutation is recognized as a UVB (sunlight) induced DNA damage (e.g. C → T transition in dipyrimidine). This mutation keeps the GTPase in a mostly active state – compared to mutant isoforms of RAS which are constitutively active – through increased GDP → GTP exchange [80]. Moreover, melanoma cell lines that possess the RAC1P29S somatic mutation have been shown to be resistant to BRAF inhibitors [85]. In this study, Watson and colleagues showed that the expression of mutant RAC1 in melanoma cell lines lead to increased cell viability and decreased apoptosis in vitro and enhanced tumor growth in vivo when treated with BRAF inhibitors constituting resistance. Interestingly, when the RAC1 mutant was silenced and cells still expressed mutant BRAF, sensitivity to the kinase inhibitors was increased [85].
Additionally, the loss of PTEN in melanoma not only contributes to tumorigenesis in melanoma patients, but can contribute to BRAF inhibitor resistance [88, 89]. PTEN gene is a tumor suppressor gene that encodes a phosphatase responsible for dephosphorylating products of PI3K. Loss of this protein results in decreased apoptotic and increased mitogen pathway signaling due to increased phosphorylation of AKT. This can be reversed with the addition of PTEN to cells deficient of the protein [88, 89]. In 2011, Paraiso and colleagues showed that the loss of PTEN contributed to BRAF inhibitor resistance through changes in expression of BIM, a pro-apoptotic regulator [90, 91]. When a BRAF inhibitor was used treat melanoma cells without PTEN there is an increase in AKT signaling compared to cells that do express the phosphatase. This increase led to decreased apoptosis which was determined to be mediated by expression of BIM. The increased expression of BIM correlated to cells that expressed PTEN, whereas BIM expression was suppressed in cells not expressing PTEN. To confirm the contribution of BIM knockdown studies of the protein were conducted and in cells expressing PTEN, the siRNA of BIM led to abrogated apoptosis [90]. Not only can loss of PTEN contribute to intrinsic BRAF inhibitor resistance, but it has also been shown that loss-of-function mutations can also be a mechanism of resistance [92].
The dysregulation of a critical cell cycle regulator, CDK4, is suggested to be another intrinsic mode of resistance to BRAF inhibitors [93]. More specifically this positive regulator of cell cycle progression is found to have activating mutations in a large subset of metastatic melanoma patients, therefore rendering them nonresponsive to targeted treatment. The active CDK4 protein phosphorylates RB (retinoblastoma protein) which promotes the transition of a cell from the G1 phase to S phase of the cell cycle. CDK4 is also responsible for phosphorylating other protein critical in cell cycle progression and inhibition of apoptosis and/or cell senescence [92]. Interestingly, when another cell-cycle regulator, cyclin D1, is overexpressed in cells also expressing mutated CDK4, BRAF inhibitor resistance is seen [94].
A unique feature of CRC patients with BRAF mutants is their primary resistance to BRAF inhibitors. Despite these patients carrying the activing BRAFV600 mutation, the selective inhibitors are not effective as they are in melanoma patients carrying the same mutant [95]. The general mechanism is the feedback activation of the MAPK/ERK pathway through EGFR. Studies using a BRAF inhibitor in combination with an antibody or small-molecule inhibitor targeting this growth factor receptor have shown optimal synergism compared to the BRAF inhibitor alone in CRC patients [54]. It is also important to emphasize that CRC patients generally have higher expression levels of EGFR than melanoma patients lending some reasoning for the difference in response to BRAF inhibitors. Since melanoma patients express a smaller amount of EGFR than CRC patients, feedback activation through a growth factor receptor is not a mode of resistance to the BRAF inhibitor [95, 79].
4.2 Secondary (Acquired) Resistance
For the patients that do initially respond to a BRAF inhibitor, but show disease progression a few months after the start of treatment the resistance mechanisms can be generally classified as MAPK/ERK pathway dependent or independent. In the two following subsections, we will discuss various modes of resistance that fall into each of these categories focusing on those most prevalent in melanomas.
4.2.1 MAPK/ERK Pathway Dependent
Resistance mechanisms that are dependent on this signaling pathway include somatic activating mutations of NRAS, an isoform of the GTPase upstream of BRAF. As mentioned previously, activating mutations of NRAS contribute to paradoxical MAPK/ERK pathway activation through dimerization of CRAF [2, 3, 6]. Additionally, there are alterations made directly to BRAF itself such as truncation, amplification, or fusion of the protein kinase. In the instance of the latter, it has been reported the AGAP3-BRAF or various other fusion genes are strong contributors to acquired resistance in melanoma patients [96, 97]. Moreover, it has been shown that despite having BRAF fusion genes, these melanomas are still sensitive to MEK inhibitors in combination with a PI3K inhibitor or CDK4/6 inhibitor [97]. When changes are made to BRAF it develops the ability to “escape” inhibition by selective small molecules such as vemurafenib, dabrafenib, and encorafenib. Downstream of BRAF, modifications can be made to the MAPK/ERK pathway that ultimately lead to resistance against BRAF inhibitors. In some instances, MEK mutations can occur that render the kinase constitutively active and can subsequently activate ERK [98]. There have been some reported cases that show the activation of ERK in a MEK-independent manner by another protein kinase, COT (MAP3K8; mitogen activated protein kinase kinase kinase 8) [99]. In the MAPK/ERK pathway-dependent modes of resistance, cellular processes like immune response, cell cycle regulation, and angiogenesis are affected in a manner that promotes tumor cell maintenance. Although this drug-resistance favors cancer progression, it also provides researchers with the opportunity to develop therapies that target these aberrant cellular processes. Examples of this include application of cutting-edge immunotherapies such as the immune checkpoint inhibitors of PD-L1 and CTLA-4, as well as cyclin dependent kinase 4 (CDK4) and vascular endothelial growth factor receptor (VEGFR) inhibitors to antagonize cell cycle progression and angiogenesis, respectively [100–102].
4.2.2 MAPK/ERK Pathway Independent
Alternatively, resistance to BRAF inhibitors can occur in a MAPK/ERK pathway-independent manner. In such instances, RTKs can be overexpressed or other signaling pathways can be upregulated [103]. Overexpression of RTKs and their respective RTK ligands (e.g. EGF and EGFR, PDGF and PDGFR) are commonly observed [104]. The PI3K/AKT signaling pathway is most often upregulated to compensate for the blockade of the MAPK/ERK pathway [105, 106]. In this pathway, various insults can be made that promote its increased signaling. The activity of AKT is tightly regulated by the phosphatase, PTEN. Upon phosphorylation by PI3K, AKT becomes activated and acts on its downstream effectors such as mTOR and GSK. Activity of Akt can be reversed via phosphate removal by PTEN. In a resistant state, PTEN has been reported to harbor inactivating mutations that lead to the phosphorylation of AKT and subsequent pathway activation [107]. This signaling cascade can also be altered to promote BRAF inhibitor resistance with the amplification of AKT. Due to the crosstalk to the MAPK/ERK and PI3K/AKT pathways, one approach to overcome resistance is the combination of inhibitors of each pathway. There is a breadth of preclinical and clinical data supporting this approach [108, 109].
4.3 Novel Approach to Overcoming BRAF Resistance
As resistance to BRAF inhibitors has developed into a more clinically relevant problem, deciphering these resistance mechanisms has become a critically important strategy to identify novel methods to overcome them. Given the many pathways for BRAF inhibitor resistance, simultaneously targeting of these multiple pathways at once to ultimately overcome resistance would be an ideal strategy. A representative example of this multi-pathway approach is the use of novel heat shock protein 90 (Hsp90) inhibitors. Hsp90 is a molecular chaperone that functions as the hub of proteostasis for many cellular protein. The activity of this chaperone assures proper protein folding and stability, therefore playing a major role in their function [110]. The substrates of Hsp90 are referred to as “clients” and are involved in a vast range of cellular processes. Interestingly, many of the proteins involved not only in cancer development and maintenance, but also BRAF inhibitor resistance are all clients of Hsp90 chaperone function [111]. This highlights the prospect of simultaneously targeting multiple signaling pathways through the inhibition of this molecular chaperone. While early Hsp90 inhibitors such as geldanamycin and 17-AAG were trialed in melanoma [112] and thyroid cancer (ClinicalTrials.gov Identifier: 00087386) patients in Phase II they never progressed to Phase III given the hepatotoxicity observed with treatment. This toxicity was believed to be due to a dose-escalation effect resulting from upregulation of Hsp70 as a secondary effect of the heat shock response induced by Hsp90 inhibition. Given the pro-survival processes Hsp70 regulates, it counteracted the inhibitory effects of Hsp90 inhibition requiring higher doses to maintain the inhibitory effect until a dose-limiting toxicity had occurred. Novel Hsp90 inhibitors in more advanced clinical and preclinical studies that do not induce this significant heat shock effect may be on the horizon as a novel strategy to overcome BRAF resistance in several tumors [113, 114]. Some of these have moved into Phase III trials such as retaspimycin and ganetespib as combination therapies, but have not been FDA approved. Other preclinical and early clinical strategies in development involve combinations of immunotherapy agents with BRAF or MEK inhibitors, histone deacetylase inhibitors with BRAF inhibitors, and check point inhibitors combined with BRAF/MEK inhibition [115, 116]. Further translational and clinical testing will be needed to define which of these strategies may be the most successful in overcoming this resistance problem.
5 Clinical Applications of BRAF and MEK Inhibitors
5.1 Standard of Care
5.1.1 Melanoma
The current NCCN guidelines for the treatment of unresectable metastatic melanoma offer patients and clinicians a wide range of options. For patients with brain metastases, treatment historically involved radiation therapy with or without palliative resection. However, this paradigm has been rapidly shifting due to the development of BRAF inhibitors and immunotherapy. Emerging evidence suggests that BRAF inhibitors may have an important role in the treatment of patients with brain metastases as an adjunct to radiation. A 2012 Phase II study treated 172 patients with metastatic BRAF mutated melanoma and at least one brain metastases with dabrafenib; of patients with BRAFV600E mutations who had never received local treatment for brain metastases, 40% achieved and overall intracranial response. Of the patients who had previously received local treatment (surgery, whole-brain radiotherapy, or stereotactic radiosurgery), the overall intracranial response rate was 31% [117]. To further investigate this potential therapeutic strategy, an upcoming Phase II trial will aim to investigate the concurrent roles of dabrafenib and trametinib with stereotactic radiation (ClinicalTrials.gov Identifier: NCT02974803). For patients without brain metastases, options include systemic therapy, intralesional injections, palliative surgical resection, radiation, or palliative supportive care. First line systemic therapies for non-resectable metastatic melanoma include immunotherapy, clinical trials, and for the large subset of patients with BRAFV600E mutations, treatment includes either the combination of dabrafenib and trametinib or vemurafenib and cobimetinib. Other systemic therapies are broader and include traditional cytotoxic agents such as dacarbazine and paclitaxel, high dose IL-2, imatinib (if a c-Kit mutation is present), temozolomide, vinblastine, nitrosourea, and interferon alfa-2b; all of these options are very rarely used in current clinical practice and only if no other options including clinical trials are available to the patient. In general, patients are nearly always treated with immunotherapy or targeted therapy [118].
5.1.1 Differentiated Thyroid Cancer
The current NCCN guidelines for the treatment of metastatic differentiated thyroid cancer relies heavily on radioactive iodine treatment. However, there are a subset of patients with persistent disease, locoregional recurrence, or distant metastases that do not uptake iodine. In these patients, the recommendation is to suppress TSH levels with levothyroxine treatment. Additionally, options include resection or treatment with radiation therapy, surveillance only in asymptomatic patients with slow disease progression, treatment with non-FDA approved small molecule inhibitors, or treatment with lenvatinib or sorafenib for disease that has failed these other options. Cost and side effect profiles of these inhibitors must be weighed as part of the risk/benefit analysis and treatment should always be performed on an individual basis [119].
5.1.1 Metastatic Colorectal Cancer
The current NCCN guidelines offer many chemotherapy options for the treatment of metastatic colorectal cancer. First line or initial therapy includes FOLFOX, CAPEOX, FOLFIRI, FOLFOXIRI, 5FU/leucovorin, or capecitabine, all plus or minus bevacizumab. In patients with KRAS/NRAS wild-type and left-sided tumors, treatment options expand to include FOLFOX or FOLFIRI plus cetuximab or panitumumab. There are to date no NCCN-recommended chemotherapy regimens that include BRAF or MEK inhibitors [120].
5.1.1 NSCLC
The current guidelines for the treatment of stage IV metastatic non-small cell lung cancer depend on the initial determination of EGFR, ALK, ROS1, and PD-L1 tumor markers. If the patient is found to have a sensitizing EGFR mutation, first line treatment is with erlotinib, afatinib, or gefitinib. If the patient has ALK rearrangement, first line treatment is with crizotinib or ceritinib, and if the patient has ROS1 rearrangement, first line therapy is with crizotinib. If the patient is PD-L1 expression positive, first line therapy is with pembrolizumab. For all other patients or those who demonstrate disease progression with the above therapies, first line therapy is with doublet chemotherapy (typically cisplatin or carboplatin-based). Very recently in 2017, the combination therapy of dabrafenib and trametinib has been approved by the FDA for the treatment of BRAF-mutated NSCLC [121].
5.1.1 HCL
The current NCCN guidelines for the treatment of patients with hairy cell leukemia recommend initial chemotherapy with the purine analogs cladribine or pentostatin. If there is less than complete response or relapse at less than a year, options include treatment with a purine analog plus or minus rituximab, interferon alpha, or rituximab alone. The current recommendation for any progression despite these therapies is treatment with vemurafenib plus or minus rituximab, or ibrutinib [122].
5.2 Common or Noted Side Effects of BRAF and MEK inhibitors
5.2.1 Vemurafenib
The most common side effects are dermatological, including skin rash and photosensitivity [123]. Cutaneous squamous cell carcinoma or keratoacanthomas develop in 15–30% of patients; in the 2011 Phase III trial conducted by Chapman et al, 18% of patients developed SCC or keratoacanthomas or both; and all could be treated by simple excision [19]. Other adverse events include arthralgia, fatigue, nausea, vomiting, diarrhea, neutropenia, peripheral edema, and alopecia [123].
5.2.2 Dabrafenib
The most common side effects are dermatological, including hyperkeratosis, papillomas, or palmar-plantar erythrodysesthesia. Between 5–20% of patients develop cutaneous SCC or keratoacanthoma. Other adverse events include fatigue, headache, arthralgia, peripheral edema, nausea, vomiting, diarrhea, and hyperglycemia [124].
5.2.3 Sorafenib
The most common side effects are hypertension, headache, peripheral neuropathy, palmar-plantar erythrodysesthesia, alopecia, rash with desquamation, hypocalcemia, fatigue, and weight loss [125]. In the 2014 Phase III study by Brose, et al, serious adverse effects included secondary malignancy in 4.3% of patients, dyspnea, and pleural effusion [32].
5.2.4 Trametinib
The most common side effects are hypertension, cardiomyopathy (7–11%), skin rash (8% with grade 3 or 4 rash), dermatitis acneiform, diarrhea, minor bleeding (<1% grade 3–4), and peripheral edema. Rare events include ocular events such as a blurred vision, reversible chorioretinopathy, and retinal-vein occlusion/retinal detachment. Of note, there are no findings of cutaneous SCC or keratoacanthomas as seen with vemurafenib and dabrafenib [35, 126].
5.2.5 Cobimetinib
The adverse effects of cobimetinib are reported in conjunction with vemurafenib treatment. The most common side effects are diarrhea, nausea, vomiting, elevated liver enzymes, elevated creatine kinase levels, and central serous retinopathy, as reported in the 2014 study by Larkin, et al [60]. Other adverse events include decreased EF, hypertension, dermatologic side effects (rare cutaneous SCC or keratoacanthomas), minor hemorrhage (<1% grades 3 or 4), and visual impairment, including blurred vision, chorioretinopathy, and retinal detachment (12% overall, 2% grades 3 or 4) [127].
5.2.6 Selumetinib
The most common side effects are fatigue, rash, and elevated liver enzymes. Other side effects events include nausea, diarrhea, peripheral edema, oral mucositis, electrolyte abnormalities, and dyspnea. Most adverse events are grade 1 or 2 [42].
5.3 Average Monthly Costs for Selective Inhibitors (Medicare Data)
| 1.1.1.1.1.1.1 DRUG | 1.1.1.1.1.1.2 COST |
|---|---|
| 1.1.1.1.1.1.3 Vemurafenib | 1.1.1.1.1.1.4 $13,021 for 30 days [123] |
| 1.1.1.1.1.1.5 Dabrafenib | 1.1.1.1.1.1.6 $11581 for 30 days [124] |
| 1.1.1.1.1.1.7 Sorafenib | 1.1.1.1.1.1.8 $19775 for 30 days [125] |
| 1.1.1.1.1.1.9 Trametinib | 1.1.1.1.1.1.10 $12573 for 30 days [126] |
| 1.1.1.1.1.1.11 Cobimetinib | 1.1.1.1.1.1.12 $7856 for 28 days [127] |
| 1.1.1.1.1.1.13 Selumetinib | 1.1.1.1.1.1.14 Average wholesale price unavailable |
6 Conclusion
The development and approval of BRAF and MEK inhibitors in BRAF-mutant cancers has dramatically changed the landscape of treatment options and clinical outcomes of cancer patients, especially those with metastatic melanoma. While the initial response to these inhibitors is robust, long term complete responses are rare and most patients will develop resistance, leading to disease progression. Additionally, the side effect profiles to BRAF inhibitors are of concern due to the risk of developing secondary skin tumors, such as cutaneous squamous cell carcinomas. The advent of combination therapy with BRAF and MEK inhibitors has eliminated many of the initial concerns associated with BRAF inhibitor monotherapy; evidence demonstrates both improved side effect profiles and prolonged clinical endpoints like ORR and PFS. Future treatment strategies involving BRAF and MEK inhibitors will aim to overcome currently recognized mechanisms of resistance and provide synergistic anti-tumor activity. Excitingly, HSP90 small-molecule inhibitors – especially C-terminal targeted – are on the horizon of being an alternative to overcome resistance, and new research supports the role of MAPK/ERK pathway inhibitors in combination with other protein or system targets, like CDK4/6 and PI3K or immunotherapy, respectively.
Key Points.
There have been many advances in the development and approval of small-molecule inhibitors targeting the MAPK/ERK pathway for the treatment of many cancers.
An understanding of the clinical indications to use these inhibitors and the mechanisms of subsequent drug resistance is key to successful implementation in cancer treatment.
Acknowledgments
Funding: The authors would like to acknowledge the following sources of funding that supported in part the work and effort completed on the manuscript. These include funding from the Department of Surgery at the University of Michigan (MSC and TW) as well as grant funding from the National Institute of Health (R01CA120458 and R01CA216919 – MSC) and (T32GM007767 – JNS).
APPENDIX
Figure 1. MAPK/ERK Pathway.
This signaling pathway is activated when a growth factor (ligand) binds to its respective growth factor receptor. Once activated, the RTK transmits the extracellular signal inside the cell via the adaptor protein GRB2. The membrane bound GTPase, RAS, then becomes activated by the exchange of GDP for GTP. This is facilitated by the nucleotide exchange factor, SOS. Activated RAS then initiates the signaling cascade of MAP kinases. Finally, activated ERK can phosphorylate its cytosolic or nuclear effectors. The latter results in changes to transcription and ultimately cell proliferation, differentiation, and survival. GRB2 – growth factor receptor bound protein 2; ERK (MAPK) – extracellular-signal related kinase; MEK – MAPK/ERK kinase; RAF (MAPKKK) – rapidly accelerated fibrosarcoma; RAS (MAPKK) – rat sarcoma protein; RTK – receptor tyrosine kinase; SOS – sons of sevenless
Footnotes
Compliance with Ethical Standards
Conflict of Interest: MSC has received consulting fees from EEPI and Acousys Biomedical Devices LLC in the last year – unrelated to the current manuscript. He is cofounder of NanoPharm LLC, and MedGuider LLC and currently serves as CEO of MedGuider. He has ownership interest in both companies but no royalties have been generated from either company and neither company is related at all to the topic in the manuscript. The other authors declare no conflict of interest.
References
- 1.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Research. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 2.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signaling pathways in cancer. Oncogene. 2007;26:3279–90. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 3.Robert PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 4.Seshacharyulu P, Ponnusamy MP, Haridas D, et al. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Thargets. 2012;16(1):15–31. doi: 10.1517/14728222.2011.648617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2022;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 6.Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72(10):2457–67. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85. doi: 10.1186/1479-5876-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akbani, et al. the Cancer Genome Atlas Network. Genomic Classification of Cutaneous Melanoma. Cell. 2015;161:1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang KT, Lee CH. BRAF mutation in papillary thyroid carcinoma: pathogenic role and clinical implications. J Clin Med Assoc. 2010;73(3):113–28. doi: 10.1016/S1726-4901(10)70025-3. [DOI] [PubMed] [Google Scholar]
- 10.Clarke CN, Kopetz ES. BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: clinical characteristics, clinical behavior, and response to targeted therapies. J Gastrointest Oncol. 2015;6(6):660–67. doi: 10.3978/j.issn.2078-6891.2015.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yarchoan M, LiVolsi VA, Brose MS. BRAF mutation and thyroid cancer recurrence. Clinical Oncology. 2015;33(1):7–8. doi: 10.1200/JCO.2014.59.3657. [DOI] [PubMed] [Google Scholar]
- 12.Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305–15. doi: 10.1056/NEJMoa1014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ragad T. Targeting RTK signaling pathways in cancer. Cancers (Basel) 2015;7(3):1758–84. doi: 10.3390/cancers7030860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribas A, Kim KB, Schuchter LM, et al. BRIM-2: an open-label, multicenter phase II study of vemurafenib in previously treated patients with BRAFV600E mutation-positive melanoma. J Clin Oncol. 2011;29(Suppl):8509–8509. [Google Scholar]
- 16.Zhang C, Spevak W, et al. RAF inhibitors that evade paradoxical MAPK pathway activation. Nature. 2015;526:583–586. doi: 10.1038/nature14982. [DOI] [PubMed] [Google Scholar]
- 17.Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366(3):207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberholzer PA, et al. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol. 2012;30:316–321. doi: 10.1200/JCO.2011.36.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiacci E, Park JH, De Carolis L, et al. Targeting mutant BRAF in relapsed or refractory hairy-cell leukemia. N Engl J Med. 2015;373:1733–47. doi: 10.1056/NEJMoa1506583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140(2):209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatzivassilious G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to active the MAPK pathway and enhance growth. Nature. 2010;464:431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 23.Holderfield M, Merritt H, Chan J, et al. RAF inhibitors active the MAPK pathway by relieving inhibitory autophosphorylation. Cell. 2013;23(5):594–602. doi: 10.1016/j.ccr.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 24.Falchoook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumors: a phase 1 dose-escalation trial. Lancet. 2012;379(9829):1893–901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ascierto PA, Minor D, Ribas A, et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol. 2013;31(26):3205–11. doi: 10.1200/JCO.2013.49.8691. [DOI] [PubMed] [Google Scholar]
- 26.Hauschild A, Grob JJ, Demidov LV, et al. Debrafenib in BRAF-mutated metastatic melanoma: a multicenter, open-label phase 3 randomized controlled trial. Lancet. 2012;380(9839):358–65. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 27.Delord JP, Robert C, Nyakas M, et al. Phase I dose-escalation and –expansion study of the BRAF inhibitor encorafenib (LGX818) in metastatic BRAF-mutant melanoma. Clin Can Res. 2017;23(18):5339–5348. doi: 10.1158/1078-0432.CCR-16-2923. [DOI] [PubMed] [Google Scholar]
- 28. [Accessed Sept 2016];US FDA hematology/oncology (cancer) approvals and safety notifications. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm279174.htm.
- 29.Ali SM, He J, Carson W, et al. Extended antitumor response of a BRAF V600E papillary thyroid carcinoma to vemurafenib. Case Rep Oncol. 2014;7(2):343–8. doi: 10.1159/000363377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 31.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 32.Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomized, double-blind, phase 3 trial. Lancet. 2014;384(9940):319–28. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Singh RR, Stingo F, et al. BRAAF kinase domain mutation are present in a subset of chronic myelomonocytic leukemia with wild-type RAS. Am J Hematol. 2014;89(5):499–504. doi: 10.1002/ajh.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:782–789. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 36.Kim KB, Kefford R, Pavlick AC, et al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol. 2013;31:482–489. doi: 10.1200/JCO.2012.43.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gencler B, Gonul M. Cutaneous Side Effects of BRAF Inhibitors in Advanced Melanoma: Review of the Literature. Dermatol Res Pract. 2016;2016:5361569. doi: 10.1155/2016/5361569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blumenschein GR, Jr, Smit EF, Planchard D, et al. A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC)dagger. Ann Oncol. 2015;26:894–901. doi: 10.1093/annonc/mdv072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoeflich KP, Merchant M, Orr C, et al. Intermittent administration of MEK inhibitor GDC-0973 plus PI3K inhibitor GDC-0941 triggers robust apoptosis and tumor growth inhibition. Cancer Res. 2012;72(1):210–9. doi: 10.1158/0008-5472.CAN-11-1515. [DOI] [PubMed] [Google Scholar]
- 40.Ribas A, Gonzalez R, Pavlick A, et al. Combination of vemurafenib and cobimetinib in patients with advanced BRAF(V600)-mutated melanoma: a phase 1b study. Lancet Oncol. 2014;15(9):954–65. doi: 10.1016/S1470-2045(14)70301-8. [DOI] [PubMed] [Google Scholar]
- 41.FDA approves Cotellic as part of combination treatment for advanced melanoma. [Accessed 17 Dec 2017];fda.gov. 2015 https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm471934.htm.
- 42.Banerji U, Camidge DR, Verheul HM, et al. The first-in-human study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1/2 inhibitor AZD6244 (ARRY-142886): a phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res. 2010;16:1613–1623. doi: 10.1158/1078-0432.CCR-09-2483. [DOI] [PubMed] [Google Scholar]
- 43.Chakravarty D, Santos E, Ryder M, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011;121:4700–4711. doi: 10.1172/JCI46382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623–632. doi: 10.1056/NEJMoa1209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hainsworth JD, Cebotaru CL, Kanarev V, et al. A phase II, open-label, randomized study to assess the efficacy and safety of AZD6244 (ARRY-142886) versus pemetrexed in patients with non-small cell lung cancer who have failed one or two prior chemotherapeutic regimens. J Thorac Oncol. 2010;5:1630–1636. doi: 10.1097/JTO.0b013e3181e8b3a3. [DOI] [PubMed] [Google Scholar]
- 46.Janne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013;14:38–47. doi: 10.1016/S1470-2045(12)70489-8. [DOI] [PubMed] [Google Scholar]
- 47.Janne PA, van den Heuvel MM, Barlesi F, et al. Selumetinib Plus Docetaxel Compared With Docetaxel Alone and Progression-Free Survival in Patients With KRAS-Mutant Advanced Non-Small Cell Lung Cancer: The SELECT-1 Randomized Clinical Trial. JAMA. 2017;317:1844–1853. doi: 10.1001/jama.2017.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Awada A, Delord JP, Houede N, et al. Safety and recommended phase II dose (RP2D) of the selective oral MEK1/2 inhibitor pimasertib (MSC1936369B/AS703026): results of a phase I trial. Eur J Cancer. 2012;48:6185–186. (abstract 604) [Google Scholar]
- 49.Macarulla T, Cervantes A, Tabernero J, et al. Phase I study of FOLFIRI plus pimasertib as second-line treatment for KRAS-mutated metastatic colorectal cancer. Br J Cancer. 2015;112(12):1874–81. doi: 10.1038/bjc.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lebbe C, Lesimple T, Kruit W, et al. Pimasertib (PIM) versus dacarbazine (DTIC) in patients (pts) with cutaneous NRAS melanoma: a controlled, open-label phase II trial with crossover. Ann Oncol. 2016;27(6):1136. [Google Scholar]
- 51.Binimetinib (MEK 162) arraybiopharma.com; 2017. [Accessed 17 Dec 2017]. http://www.arraybiopharma.com/product-pipeline/binimetinib/ [Google Scholar]
- 52.Lee PA, Wallace E, Marlow A, et al. Preclinical development of ARRY-162, a potent and selective MEK 1/2 inhibitor. Cancer Res. 2010;70:2515. (abstract) [Google Scholar]
- 53.Bendell JC, Javle M, Bekaii-Sabb TS, et al. A phase 1 dose-escalation and expansion study of binimetinib (MEK162), a potent and selective oral MEK1/2 inhibitor. Br J Cancer. 2017;116(5):575–583. doi: 10.1038/bjc.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cho M, Gong J, Frankel P, et al. A phase I clinical trial of binimetinib in combination with FOLFOX in patients with advanced metastatic colorectal cancer who failed prior standard therapy. Oncotarget. 2017;8(45):79750–79760. doi: 10.18632/oncotarget.19336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rizos H, Menzies AM, Pupo GM, et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res. 2014;20:1965–1977. doi: 10.1158/1078-0432.CCR-13-3122. [DOI] [PubMed] [Google Scholar]
- 56.Van Allen EM, Wagle N, Sucker A, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4:94–109. doi: 10.1158/2159-8290.CD-13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4:80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition verus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877–88. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 60.Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–76. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 61.Dummer R, Ascierto PA, Gogas HJ, et al. Results of COLUMBUS Part 1: A phase 3 trial of encorafenib (enco) plus binimetinib (bini) versus vemurafenib (vem) or enco in BRAF-mutant melanoma. Array BioPharma Inc. Society for Melanoma Research 2016 Congress; Nov 2011; Boston, MA. [Google Scholar]
- 62.Dummer R, Ascierto PA, Gogas HJ, et al. Results of COLUMBUS Part 2: a phase 3 trial of encorafenib plus binimetinib versus encorafenib in BRAF-mutant melanoma. Array BioPharma Inc. ESMO Congress; 2017; Madrid, Spain. [Google Scholar]
- 63.Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Onc. 2017;14:463–482. doi: 10.1038/nrclinonc.2017.43. [DOI] [PubMed] [Google Scholar]
- 64.Ribas A, Hodi FS, Callahan M, et al. Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med. 2013;368(14):1365–6. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]
- 65.Ribas A, Hodi FS, Lawrence D, et al. KEYNOTE -022 Update: Phase 1 Study of First of First -Line Pembrolizumab Plus Dabrafenib and Trametinib for BRAF-Mutant Advanced Melanoma. ESMO Congress; 2017; Abstract 1216O. [Google Scholar]
- 66.Cui G, Liu D, Li W, et al. A meta-analysis of the association between BRAF mutation and nonsmall cell lung cancer. Medicine (Baltimore) 2017;96:e6552. doi: 10.1097/MD.0000000000006552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Planchard D, Besse B, Groen HJ, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016;17:984–993. doi: 10.1016/S1470-2045(16)30146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT) Lancet. 2016;387:1415–1426. doi: 10.1016/S0140-6736(16)00004-0. [DOI] [PubMed] [Google Scholar]
- 69.FDA grants regular approval to dabrafenib and trametinib combination for metastatic NSCLC with BRAF V600E mutation. [Accessed 17 Dec 2017];fda.gov. 2017 https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm564331.htm.
- 70.Cohen R, Cervera P, Svrcek M, et al. BRAF-Mutated Colorectal Cancer: What Is the Optimal Strategy for Treatment? Curr Treat Options Oncol. 2017;18:9. doi: 10.1007/s11864-017-0453-5. [DOI] [PubMed] [Google Scholar]
- 71.Yaeger R, Cercek A, O’Reilly EM, et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin Cancer Res. 2015;21:1313–1320. doi: 10.1158/1078-0432.CCR-14-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Geng F, Wang Z, Yin H, et al. Molecular Targeted Drugs and Treatment of Colorectal Cancer: Recent Progress and Future Perspectives. Cancer Biother Radiopharm. 2017;32:149–160. doi: 10.1089/cbr.2017.2210. [DOI] [PubMed] [Google Scholar]
- 73.Yang H, Higgins B, Kolinsky K, et al. Antitumor activity of BRAF inhibitor vemurafenib in preclinical models of BRAF-mutant colorectal cancer. Cancer Res. 2012;72:779–789. doi: 10.1158/0008-5472.CAN-11-2941. [DOI] [PubMed] [Google Scholar]
- 74.Hong DS, Morris VK, El Osta B, et al. Phase IB Study of Vemurafenib in Combination with Irinotecan and Cetuximab in Patients with Metastatic Colorectal Cancer with BRAFV600E Mutation. Cancer Discov. 2016;6:1352–1365. doi: 10.1158/2159-8290.CD-16-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kopetz S. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406) [Accessed 24 Jul 2017];ascopubs.org. 2017 doi: 10.1200/JCO.20.01994. http://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.3505. [DOI] [PMC free article] [PubMed]
- 76.Manzano JL, Layos L, Buges C, et al. Resistant mechanisms to BRAF inhibitors in melanoma. Ann Transl Med. 2016;4(12):237. doi: 10.21037/atm.2016.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Solit DB, Rosen N. Resistance to BRAF inhibitors in melanomas. N Engl J Med. 2011;362:772–74. doi: 10.1056/NEJMcibr1013704. [DOI] [PubMed] [Google Scholar]
- 78.Mao M, Feng T, Mariadason JM, Tsao CC, et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can ber overcome with PI3K inhibition or demethylating agents. Clin Cancer Res. 2012;19(3):657–667. doi: 10.1158/1078-0432.CCR-11-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prahallad A, Sun C, Huang S. Unresponsiveness of colon cancer to BRAF (V600E) inhibition through feedback activation of EGFR. Nature. 2012;483(7387):100–3. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 80.Halaban R. RAC1 and melanoma. Clin Ther. 2015;37(3):682–685. doi: 10.1016/j.clinthera.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rajendran V, Gopalakrishnan C, Purohit R. Impact of point mutation P29S in RAC1 on tumorigenesis. Tumour Biol. 2016;37(11):15293–15304. doi: 10.1007/s13277-016-5329-y. [DOI] [PubMed] [Google Scholar]
- 82.Zhou Y, Liao Q, Han Y, et al. Rac1 overexpression is correlated with epithelial mesenchymal transition and predicts poor prognosis in non-small lung cancer. J Cancer. 2016;7(14):2100–2109. doi: 10.7150/jca.16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu B, Xiong J, Liu G, et al. High expression of Rac1 is correlated with partial reversed cell polarity and poor prognosis in invasive ductal carcinoma of the breast. Tumor Biol. 2017;39(7):1010428317710908. doi: 10.1177/1010428317710908. [DOI] [PubMed] [Google Scholar]
- 84.Watson IR, Li L, Cabeceiras PK, et al. The RAC1 P29S hotspot mutation in melanoma confers resistance to pharmacological inhibition of RAF. Cancer Research. 2014 doi: 10.1158/0008-5472.CAN-14-1232-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stahl JM, Cheung M, Sharma A, et al. Loss of PTEN promotes tumor development in malignant melanoma. Cancer Research. 2003;63(11):2881–2890. [PubMed] [Google Scholar]
- 86.Wu H, Giel V, Haluska FG. PTEN signaling pathways in melanoma. Oncogene. 2003;22:3113–3122. doi: 10.1038/sj.onc.1206451. [DOI] [PubMed] [Google Scholar]
- 87.Kennedy SG, Wagner AJ, Conzen SD, et al. The PI3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 88.Cheney IW, Johnson DE, Vaillancourt MT, et al. Suppression of tumorigenicity of glioblastoma cells by adenovirus-mediated MMAC1/PTEN gene transfer. Cancer Res. 1998;58:2331–2334. [PubMed] [Google Scholar]
- 89.Paraiso KH, Xiang Y, Rebecca VW, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through suppression of BIM expression. Cancer Research. 2011;71(7):2750–60. doi: 10.1158/0008-5472.CAN-10-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gogada R, Yadav N, Liu J, et al. BIM, a proapoptotic protein, upregulated via transcription factor E2F1-deendet mechanism, functions as a prosurvival molecule in cancer. JBC. 2012 doi: 10.1074/jbc.M112.386102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Catalanotti F, Cheng DT, Shoushtari AN, et al. PTEN loss-of-function alterations are associated with intrinsic resistance to BRAF inhibitors in metastatic melanoma. JCO Precision Oncology. 2017 doi: 10.1200/PO.1600054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sheppard KE, McArthur GA. The cell-cycle regulator CDK4: an emerging therapeutic target in melanoma. Clinical Cancer Research. 2013 doi: 10.1158/1078-0432.CCR-13-0259. [DOI] [PubMed] [Google Scholar]
- 93.Smalley KS, Lioni M, Dalla M, et al. Increased cyclin D1 expression can mediate BRAF inhibitor resistance in BRAF V600E-mutated melanomas. Mol Cancer Ther. 2008;7(9):2876–83. doi: 10.1158/1535-7163.MCT-08-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. N Engl J Med. 2009;361(1):98–99. doi: 10.1056/NEJMc0904160. [DOI] [PubMed] [Google Scholar]
- 95.Kopetz S, Desai J, Chan E, et al. PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors. Journal of Clinical Oncology. 2010;28(10):3534–3434. [Google Scholar]
- 96.Kulkarni A, Al-Hraishawi H, Simhadri S, et al. BRAF fusion as a novel mechanism of acquired resistance to vemurafenib in BRAFv600E mutant melanomas. Biology of Human Tumors. 2017 doi: 10.1158/1078-0432.CCR-16-0758. [DOI] [PubMed] [Google Scholar]
- 97.Kim HS, Jung M, Kang HN, et al. Oncogenic BRAF fusions in mucosal melanomas active the MAPK pathway and are sensitive to MEK/PI3K inhibition or MEK/CDK4/6 inhibition. doi: 10.1038/onc.2016.486. [DOI] [PubMed] [Google Scholar]
- 98.Emery CM, Vijayendran KG, Zipser MC. MEK1 mutations confer resistance to MEK and BRAF inhibition. PNAS. 2009 doi: 10.1073/pnas.0905833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johannessen CM, Boehm JS, Kim SY. COT/MAP3K8 drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468(7326):968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–82. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13:417–30. doi: 10.1038/nrclinonc.2016.26. [DOI] [PubMed] [Google Scholar]
- 102.Goel HL, Mercurio AM. VEGF targets the tumor cell. Nat Rev Cancer. 2013;13:871–82. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to BRAF(V600E) inhibition by RTK or NRAS upregulation. Nature. 2010;468(7326):973–7. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shi H, Kong X, Ribas A, et al. Combinatorial treatments that overcome PDGFRβ-driven resistance of melanoma cell to BRAF(V600E) inhibition. Cancer Res. 2011;71:5067–74. doi: 10.1158/0008-5472.CAN-11-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perna D, Karreth FA, Rust AG. BRAF inhibitor resistance mediated by the AKT pathway in an oncogenic BRAF mouse melanoma model. PNAS. 2015 doi: 10.1073/pnas.1418163112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 107.Paraiso KHT, Xiang Y, Rebecca VW, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 2011;71(7):2750–60. doi: 10.1158/0008-5472.CAN-10-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sweetlove M, Wrightson E, Kolekar S, et al. Inhibitors of pan-PI3K signaling synergize with BRAF or MEK inhibitors to prevent BRAF-mutant melanoma cell growth. Front Oncol. 2015;5:135. doi: 10.3389/fonc.2015.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Temraz S, Mukerji D, Shamseddine A. Dual inhibition of MEK and PI3K pathway in KRAS and BRAF mutated colorectal cancers. Int J Mol Sci. 2015;16(9):22976–22988. doi: 10.3390/ijms160922976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schopf FH, Biebl MM, Buchner J. The HSP90 chaperone machinery. Nat Rev Molecular Cell Biology. 2017;18:345–60. doi: 10.1038/nrm.2017.20. [DOI] [PubMed] [Google Scholar]
- 111.Miyata Y, Nakamoto H, Necker L. The therapeutic target Hsp90 and cancer hallmarks. Curr Pharm Des. 2013;19(3):347–65. doi: 10.2174/138161213804143725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pacey S, Gore M, Chao D, et al. A phase II trial of 17-allylamino, 17-demthoxygeldanamycin (17-AAG, tanespimycin) in patients with metastatic melanoma. Invest New Drugs. 2012;30(1):341–9. doi: 10.1007/s10637-010-9493-4. [DOI] [PubMed] [Google Scholar]
- 113.Byrd KM, Subramanian C, Sanchez J, et al. Synthesis and biological evaluation of Novobiocin core analogues as Hsp90 inhibitors. Chem Eur J. 2016;22:6921–31. doi: 10.1002/chem.201504955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.White PT, Subramanian C, Zhu Q, et al. Novel Hsp90 inhibitors effectively target functions of thyroid cancer stem cell preventing migration and invasion. Surgery. 2016;159(1):142–51. doi: 10.1016/j.surg.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Raveendran S, Rao A, Storkus W. Combination immunotherapy of melanoma by inhibiting HSP90 and targeting its client proteins (TUM7P.934) J Immunol. 2014;192(1 Supplemental):203.16. [Google Scholar]
- 116.Chai RC, Vieusseux JL, Lang BJ, et al. Histone deacteylase activity mediates acquired resistance towards structurally diverse hsp90 inhibitors. Molecular Oncology. 2017;11(5):567–83. doi: 10.1002/1878-0261.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–95. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- 118.National Comprehensive Cancer Network. Melanoma NCCN Guidelines with NCCN Evidence Blocks. [Accessed 24 Jul 2017];nccn.org. 2017 https://www.nccn.org/professionals/physician_gls/pdf/melanoma_blocks.pdf.
- 119.National Comprehensive Cancer Network. Thyroid Carcinoma NCCN Guidelines with NCCN Evidence Blocks. [Accessed 24 Jul 2017];nccn.org. 2017 https://www.nccn.org/professionals/physician_gls/pdf/thyroid_blocks.pdf.
- 120.National Comprehensive Cancer Network. Colon Cancer NCCN Guidelines with NCCN Evidence Blocks. [Accessed 24 Jul 2017];nccn.org. 2017 https://www.nccn.org/professionals/physician_gls/pdf/colon_blocks.pdf.
- 121.National Comprehensive Cancer Network. Non-Small Cell Lung Cancer NCCN Guidelines with NCCN Evidence Blocks. [Accessed 24 Jul 2017];nccn.org. 2017 https://www.nccn.org/professionals/physician_gls/pdf/nscl_blocks.pdf.
- 122.National Comprehensive Cancer Network. Hairy Cell Leukemia NCCN Guidelines with NCCN Evidence Blocks. [Accessed 24 Jul 2017];nccn.org. 2017 https://www.nccn.org/professionals/physician_gls/pdf/hairy_cell.pdf.
- 123.Lexicomp. Vemurafenib: Drug Information. [Accessed 24 Jul 2017];UpToDate.com. 2017 https://www.uptodate.com/contents/vemurafenib-drug-information?source=search_result&search=Vemurafenib&selectedTitle=1~42.
- 124.Lexicomp. Dabrafenib: Drug Information. [Accessed 24 Jul 2017];UpToDate.com. 2017 https://www.uptodate.com/contents/dabrafenib-drug-information?source=search_result&search=dabrafenib&selectedTitle=1~33.
- 125.Lexicomp. Sorafenib: Drug Information. [Accessed 24 Jul 2017];UpToDate.com. 2017 https://www.uptodate.com/contents/sorafenib-drug-information?source=search_result&search=sorafenib&selectedTitle=1~107.
- 126.Lexicomp. Trametinib: Drug Information. [Accessed 24 Jul 2017];UpToDate.com. 2017 https://www.uptodate.com/contents/trametinib-drug-information?source=search_result&search=trametinib&selectedTitle=1~28.
- 127.Lexicomp. Cobimetinib: Drug Information. [Accessed 24 Jul 2017];UpToDate.com. 2017 https://www.uptodate.com/contents/cobimetinib-drug-information?source=search_result&search=cobimetinib&selectedTitle=1~18.