Abstract
The plant-derived steroid, digoxin, a specific inhibitor of Na,K-ATPase, has been used for centuries in the treatment of heart disease. Recent studies demonstrate the presence of a digoxin analog, ouabain, in mammalian tissue, but its biological role has not been elucidated. Here, we show in renal epithelial cells that ouabain, in doses causing only partial Na,K-ATPase inhibition, acts as a biological inducer of regular, low-frequency intracellular calcium ([Ca2+]i) oscillations that elicit activation of the transcription factor, NF-κB. Partial inhibition of Na,K-ATPase using low extracellular K+ and depolarization of cells did not have these effects. Incubation of cells in Ca2+-free media, inhibition of voltage-gated calcium channels, inositol triphosphate receptor antagonism, and redistribution of actin to a thick layer adjacent to the plasma membrane abolished [Ca2+]i oscillations, indicating that they were caused by a concerted action of inositol triphosphate receptors and capacitative calcium entry via plasma membrane channels. Blockade of ouabain-induced [Ca2+]i oscillations prevented activation of NF-κB. The results demonstrate a new mechanism for steroid signaling via plasma membrane receptors and underline a novel role for the steroid hormone, ouabain, as a physiological inducer of [Ca2+]i oscillations involved in transcriptional regulation in mammalian cells.
Ionized calcium is a key carrier of information in the cell (1). To prevent the toxic effects of a sustained increase in calcium, both excitable and nonexcitable cells use trains of calcium spikes for transmitting information (2, 3). This signaling system has a high level of specificity as cells can decode differences in intracellular calcium ([Ca2+]i) oscillation frequency and amplitude (4–6). Recent studies suggest that signaling via [Ca2+]i oscillations triggers the activation of NF-κB and other calcium-dependent transcription factors (7–9).
The Na,K-ATPase is an integral plasma membrane protein that establishes the electrochemical gradient across the plasma membrane and is of vital importance for all mammalian cells. Several recent studies suggest that this pump may also act as a signal transducer and transcription activator involved in cell growth and differentiation (10–14). Ouabain, the ligand of Na,K-ATPase, is a steroid derivative (15) that, unlike other steroid hormones, binds specifically to an integral plasma membrane protein. Ouabain derivatives have been used for centuries in the treatment of heart disease. Recent studies demonstrate the presence of endogenous ouabain in mammalian tissues (16–18). The physiological role of the ouabain/Na,K-ATPase complex has, despite extensive research, remained controversial. Here, we report that binding of ouabain to Na,K-ATPase activates a [Ca2+]i oscillatory signaling pathway that triggers the activation of NF-κB in nonexcitable renal epithelial cells.
Materials and Methods
Preparation of Primary Proximal Tubule Cells.
Rat proximal tubule (RPT) cells were prepared from kidneys of 20-day-old male Sprague–Dawley rats (19). Cells were cultured in supplemented DMEM (20 mM Hepes/24 mM NaHCO3/10 μg/ml penicillin/10 μg/ml streptomycin/10% FBS) on glass coverslips or culture dishes for 48 h in 5% CO2 at 37°C. Cells were starved for serum (1% FBS) and cultured in the absence of antibiotics for 24 h before the experiment.
Ratiometric Imaging.
Cells were incubated with 3 μM Fura-2/AM (Molecular Probes) for 1 h in DMEM at 37°C for [Ca2+]i measurements and 10 μM SBFI/AM (Molecular Probes) for 2 h in DMEM at 32°C for intracellular sodium concentration ([Na+]i) measurements. Ratiometric imaging was performed by using a heated chamber (FCS2, Bioptechs, Butler, PA) mounted on a Zeiss Axiovert 135 microscope using a 40×/1.4 epifluorescence oil-immersion objective. Fura-2/AM and SBFI/AM-loaded cells were excited at wavelength 340/10 nm and 380/10 nm, and emission fluorescence was collected with a 510/30-nm band pass filter. Data were recorded with a GenIISys image intensifier system connected to a CCD camera (MTI CCD72; Dage–MTI, Michigan City, IN) and analyzed by using acquisition software from Inovision. Cells were excited every 30 s, which corresponds to a Nyquist frequency of 16.7 mHz. All experiments were performed by using P medium (100 mM NaCl/4 mM KCl/20 mM Hepes/25 mM NaHCO3/1 mM CaCl2/1.2 mM MgCl2/1 mM NaH2PO4⋅H2O/10 mM D-glucose). Nifedipine (Sigma) was used at 50 μM, 2-aminoethoxydiphenyl borate (Sigma) was used at 50 μM, jasplakinolide (JP, Molecular Probes) was used at 6 μM, 4-aminopyridine (Sigma) was used at 0.5 mM, and Bay K 8644 (Sigma) was used at 10 μM.
Power Spectrum Analysis.
A power spectrum of a signal is the squared magnitude of its Fourier transform and describes the contribution to a signal by each of its sine wave components. By using MATLAB, the oscillating section of a single cell measurement was filtered, centered, and trend corrected by computing Gauss least-square approximation and subtracting the trend from the original data. Fast Fourier Transform was used to calculate the discrete Fourier transform. This produces a spectrum where the peaks correspond to the different frequencies present in the original data. The dominant peak was determined by comparing the relative power of the peaks in the spectrum. The relative power was calculated by determining the area between the two extremes closest to the peak, divided by the total area of the power spectrum.
Actin-Green Fluorescent Protein (GFP) Transfection.
RPT cells were transiently transfected on culture day 2 by using CLONfectin (CLONTECH) with plasmid containing 1 μg/ml actin-GFP DNA construct according to manufacturer's specifications. Cells were examined 24 h after transfection with a Leica TCS SP inverted confocal scanning laser microscope. GFP fluorescence was excited at 488 nm and detected with a 510–570-nm band pass filter.
Immunocytochemistry and Confocal Microscopy.
Immunocytochemistry was performed according to standard protocol. NF-κB was probed with rabbit anti-human polyclonal NF-κB p65 antibody (1:200) (Santa Cruz Biotechnology); inositol triphosphate (IP3) receptors were probed with mouse anti-rat IP3 receptor antibody (3 μg/ml) (gift from K. Mikoshiba, Tokyo University, Tokyo). Fluorescent secondary antibodies were goat anti-rabbit Alexa 546 and goat anti-mouse Alexa 546 (1:200) (Molecular Probes). The immunolabeled cells were recorded with a Leica TCS SP inverted confocal scanning laser microscope by using 40×/1.4 numerical aperture objective. Red fluorescence was excited at 543 nm and detected with a 560–620-nm band pass filter. Preparations where the primary antibody was omitted from the staining protocol were used as negative controls, and IL-1β-treated cells were used as a positive control. NF-κB activation was semiquantitatively calculated as the ratio between the mean fluorescent signal intensity in a given comparable area in the nucleus and cytosol. Two persons that were blind to the protocol performed the calculations on a software package from Scion Image. In some sets of experiments, [Ca2+]i measurements and NF-κB immunocytochemistry were performed on the same cell population.
Western Blot and Subcellular Fractionation.
Western blot and subcellular fractionation were performed as described (7). Briefly, after incubation with ouabain, cells were placed on ice and lysed. Nuclear and cytosolic extracts were obtained. NF-κB was probed with rabbit anti-human polyclonal NF-κB p65 (1:5,000) (Santa Cruz Biotechnology); IκBα was probed with rabbit anti-human antibody (1:1,000) (Santa Cruz Biotechnology) in a whole cell lysate.
Results
Low-Dose Ouabain Induces [Ca2+]i Oscillations.
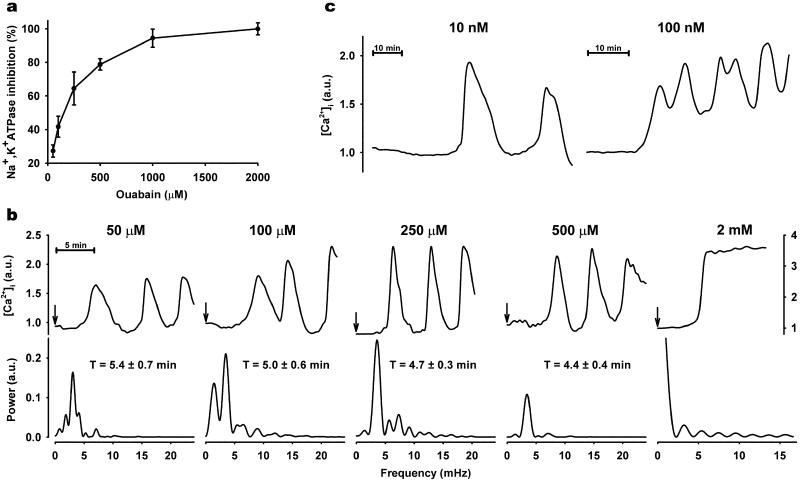
To record changes in intracellular calcium, we used single RPT cells from primary cultures. These cells have a high expression of Na,K-ATPase, are inert to the calcium-sensitive dye, Fura-2/AM, and as previously described, respond to biological stimuli with [Ca2+]i oscillations (19). Rat Na,K-ATPase has relatively low ouabain sensitivity, and full inhibition of the enzyme requires millimolar concentrations of the drug. In the present study, we found that ouabain, only in doses resulting in partial Na,K-ATPase inhibition (Fig. 1a), gave rise to [Ca2+]i oscillations of low frequency (Fig. 1b). This response was detected in approximately one-third of the cells and was generally initiated in one cell at the periphery of a cell cluster before propagating to neighboring cells. To determine whether the oscillations were a random process or whether they possessed an intrinsic regularity, we performed power spectrum analysis (19). Results of these calculations revealed a consistent periodicity that ranged between 5.4 ± 0.7 min for 50 μM ouabain and 4.4 ± 0.4 min for 250 μM ouabain. Full Na,K-ATPase inhibition with 2 mM ouabain did not cause oscillations but, rather, resulted in a sustained increase in [Ca2+]i (Fig. 1b). The magnitude of the sustained [Ca2+]i response to 2 mM ouabain was approximately 2-fold higher than the amplitude of the oscillatory response evoked by low-dose ouabain. Amplitude of the oscillations for all partial inhibitory ouabain doses was in the same range. The concentrations of ouabain used in Fig. 1b are much higher than those reportedly observed in the blood (20–22). Ouabain binding to Na,K-ATPase may, under physiological conditions, be expected to increase as a function of time (23). To test this possibility, RPT cells were superfused with solutions containing 10 or 100 nM ouabain at a slow rate for 3 h. Oscillations were then detected in a few cells in each cluster (Fig. 1c).
Figure 1.
Effect of ouabain on [Ca2+]i in primary culture of RPT cells. (a) Na,K-ATPase activity measured as ouabain-sensitive 86Rb+ uptake (mean ± SE). (b Upper) Representative single cell [Ca2+]i tracings in response to indicated ouabain concentrations. At time 0 (indicated by arrow), cells were exposed to ouabain concentrations ranging from 50 μM to 2 mM, and recordings were made every 30 s. Arbitrary units (a.u.) represent ratio values corresponding to [Ca2+]i changes. (Lower) Spectral analysis of ouabain-induced [Ca2+]i oscillations. Each plot corresponds to the single-cell recording above. [Ca2+]i oscillation periodicity (T) of each ouabain concentration was calculated as mean ± SE from ≈50 cells from at least three separate experiments. (c) Representative single-cell [Ca2+]i tracings observed in cells superfused for 3 h at a slow rate (100 μl/min) with nanomolar ouabain.
Contribution of Various Calcium Transporters to the Oscillatory Response.
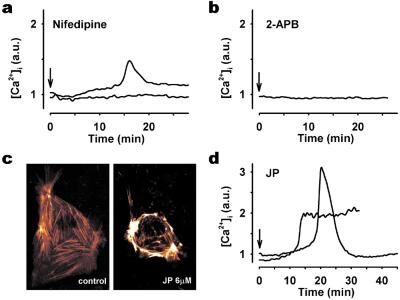
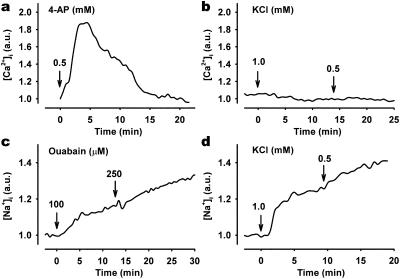
Calcium-free media completely abolished [Ca2+]i oscillations. Nifedipine, an inhibitor of L-type voltage-gated calcium (LVGC) channels, also abolished [Ca2+]i oscillations. The majority of nifedipine-exposed cells exhibited no measurable effect on [Ca2+]i, whereas a few cells displayed a single, low-magnitude [Ca2+]i transient (Fig. 2a). This indicates that LVGC channels are involved in the generation of [Ca2+]i oscillations. Incubation of cells with BayK 8644, an activator of LVGC channels, did not elicit [Ca2+]i oscillations (data not shown). Because Na,K-ATPase is an electrogenic pump, partial inhibition will lead to depolarization of the cell. Exposure of cells to the depolarizing agent, 4-aminopyridine, caused either transient (Fig. 4a) or sustained (data not shown) [Ca2+]i changes depending on the dose, but it did not bring about [Ca2+]i oscillations. Because neither depolarization nor BayK 8644 could initiate [Ca2+]i oscillations, we find it unlikely that activation of LVGC channels by Na,K-ATPase inhibition is the only cause of the oscillations.
Figure 2.
Characterization of ouabain-induced [Ca2+]i response in RPT cells. Typical [Ca2+]i response in cells preincubated with 50 μM nifedipine for 5 min (a), 50 μM 2-aminoethoxydiphenyl borate for 5 min (b), and 6 μM JP for 45 min (d) before 250 μM ouabain treatment. In studies using nifedipine (a) and JP (d), two different types of responses were observed. Each experiment was repeated at least four times by using individual cell preparations. (c) Confocal microscopy images of cellular F-actin distribution in actin-GFP transiently transfected cells treated without (Left) or with (Right) 6 μM JP for 30 min. JP caused polymerization and reorganization of F-actin subjacent to the cell membrane.
Figure 4.
Effect of membrane depolarization and low K+ on ouabain-mediated changes in [Ca2+]i and [Na+]i. [Ca2+]i response in cells exposed (arrow) to 5 mM 4-aminopyridine (a) or reductions in extracellular K+ (b) from 4.0 mM to 1.0 or 0.5 mM. Effect of ouabain (c) and low extracellular K+ (d) on representative single-cell [Na+]i measurements. SBFI/AM-loaded cells were treated with 100 μM and 250 μM ouabain or low extracellular K+, and ratio images were recorded every 30 s. Arbitrary units (a.u.) represent ratio values corresponding to changes in [Ca2+]i (a and b) and [Na+]i (c and d).
When cells were exposed to 5 μM ryanodine, a concentration known to activate ryanodine receptors (24), no changes in [Ca2+]i were detected. Inhibition of ryanodine receptors by 100 μM ryanodine (24) did not prevent ouabain-induced [Ca2+]i oscillations (data not shown). In contrast, IP3 receptor inhibition with 2-aminoethoxydiphenyl borate abolished the oscillations (Fig. 2b). IP3 receptors in RPT cells were identified with immunocytochemistry (data not shown). Depletion of calcium from intracellular stores is generally followed by an influx of calcium from extracellular space via Ca2+ release-activated Ca2+ (CRAC) channels (25, 26). According to current models, slow [Ca2+]i oscillations are dependent on the interaction between calcium stores in the endoplasmic reticulum and CRAC (26). Hyperpolymerization and reorganization of the actin cytoskeleton network can interrupt this interaction (27). JP, a drug that binds directly to actin and causes actin hyperpolymerization subjacent to the plasma membrane, has been shown to interrupt the communication between the endoplasmic reticulum (ER) and CRAC (27). Exposure of RPT cells to JP caused typical actin reorganization (Fig. 2c). When JP-treated cells were challenged with ouabain, no [Ca2+]i oscillations were observed (Fig. 2d). Ouabain-mediated inhibition of Na,K-ATPase is typically accompanied by an increase of [Na+]i. A nonspecific effect of JP on ion flux via the plasma membrane was not likely as measurement of [Na+]i after ouabain incubation revealed an intact response in JP-treated cells (data not shown).
Oscillations of [Ca2+]i Activate NF-κB.
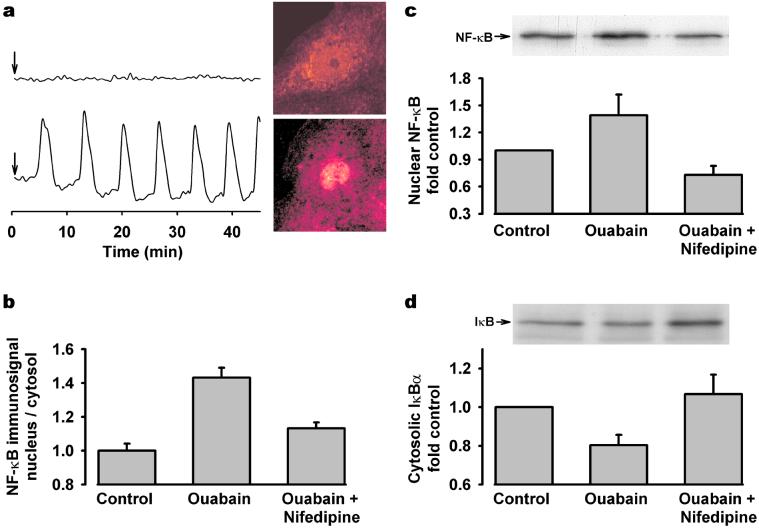
The transcription factor NF-κB is a pleiotropic regulator of the inducible expression of many genes. Its activation has been shown to be sensitive to, and preferentially activated by, low-frequency [Ca2+]i oscillations (8, 9). Previous studies using endothelial and cardiac cells suggest that ouabain may activate NF-κB (28, 29). As depicted in Fig. 3a, individual cells that responded to ouabain with typical, slow [Ca2+]i oscillations showed increased NF-κB nuclear staining compared with cells in which no changes in [Ca2+]i were detected. The ratio between NF-κB immunosignal localized to the nucleus and cytosol was semiquantitatively estimated in the entire cell population following ouabain exposure and was significantly higher than the response observed in control cells (Fig. 3b). NF-κB nuclear translocation was also confirmed by subcellular fractionation and Western blotting (Fig. 3c). In its inactive form, NF-κB is associated with IκB, a cytoplasmic repressor protein that masks the nuclear localization signal of NF-κB (30). Treatment of RPT cells with ouabain caused a significant reduction in IκBα content and is consistent with the observed NF-κB activation (Fig. 3d). Prevention of ouabain-induced [Ca2+]i oscillations with nifedipine abrogated NF-κB activation (Fig. 3 b–d). Interestingly, when cells were exposed to high ouabain concentrations, a lesser degree of NF-κB activation, compared with low ouabain challenge, was observed (data not shown). Such a finding strengthens the proposed involvement of low-frequency [Ca2+]i oscillations, rather than global Ca2+ elevations, in regulating NF-κB activation.
Figure 3.
Effect of [Ca2+]i oscillations on ouabain-induced NF-κB activation. (a) A cell cluster was treated with 250 μM ouabain (indicated by arrow), and individual cells were analyzed for both [Ca2+]i and NF-κB immunofluorescence. (Upper) Typical nonoscillating [Ca2+]i response (Left) and its corresponding cellular NF-κB localization (Right). (Lower) Typical oscillating [Ca2+]i response (Left) and its corresponding cellular NF-κB localization (Right). (b) Semiquantitative analysis of NF-κB immunofluorescence signal showing translocation from cytosol to nucleus in cells exposed to 250 μM ouabain, in the absence or presence of 50 μM nifedipine. Values are mean ± SE 50–150 cells. Representative Western blot and densitometric analysis of three to five experiments showing changes in (c) nuclear NF-κB and (d) cytosolic IκBα protein in cells exposed to 250 μM ouabain in the presence or absence of nifedipine.
Inhibition of Na,K-ATPase by low K+ Does Not Induce [Ca2+]i Oscillations.
Lowering extracellular K+ concentration will inhibit Na,K-ATPase. Both ouabain and graded reduction of extracellular K+ evoked similar, dose-dependent [Na+]i increases in all cells (Fig. 4 c and d). Lowering extracellular K+ did not, however, have any measurable effect on [Ca2+]i (Fig. 4b) or NF-κB nuclear translocation. The ratio between NF-κB immunosignals in nucleus and cytosol was not significantly different in control and in cells exposed to low (1 mM) extracellular K+ (data not shown).
Discussion
Calcium is a universal signaling system that modulates gene activation in many biological processes (1, 5, 6, 31). Oscillations reduce the threshold for activation of calcium-dependent transcription factors and provide, by their level of frequency, specificity to the response (5, 7–9). The present study identifies ouabain, a steroid hormone, as a physiological inducer of this extraordinarily versatile second messenger event and indicates a novel and important role for the ouabain/Na,K-ATPase complex as a regulator of gene transcription.
Generation and propagation of [Ca2+]i oscillations require an interplay between calcium transporters located in the plasma membrane and internal stores (32). The key events in this process are release of Ca2+ from ER and Ca2+ influx via plasma membrane channels, such as voltage-gated calcium channels and CRAC (1, 26). These transporters communicate with each other in various ways. Calcium itself is an important messenger that can stimulate calcium release from intracellular stores in a process called Ca2+-induced Ca2+ release (CICR). The CICR process is of fundamental importance in [Ca2+]i oscillations, and elevated levels of Ca2+ increase the sensitivity of the IP3 receptor (33). Our experiments with nifedipine and 2-aminoethoxydiphenyl borate show that both LVGC channels and CICR pathways are necessary to elicit [Ca2+]i oscillations. Na,K-ATPase establishes plasma membrane potential, and pump inhibition leads to depolarization and subsequent activation of voltage-gated Ca2+ channels that initiate Ca2+ influx and CICR. Based on our results, we conclude that although LVGC channels are involved in the generation of [Ca2+]i oscillations, membrane depolarization alone cannot explain this phenomenon. Because low K+-mediated Na,K-ATPase inhibition did not bring about [Ca2+]i oscillations, it seems that ouabain-induced [Ca2+]i oscillations are not a primary result of pump inhibition. Accordingly, these observations suggest a more direct, ligand-specific effect of ouabain on Na,K-ATPase-dependent Ca2+ signaling through calcium transporter function. Although our results implicate IP3 receptor in ouabain-induced Ca2+ signaling, the mechanism by which Na,K-ATPase and IP3 receptor communicate is unclear. Because of the close spatial location of IP3 receptor in ER and Na,K-ATPase in plasma membrane, one plausible scenario could be a physical interaction through the cytoskeleton. A message pathway via physical interaction between ER and CRAC has recently been reported (27). Depletion of ER serves as a trigger for CRAC channel-mediated Ca2+ influx and ER refilling (3, 25, 26). This signaling pathway is of vital importance for Ca2+ oscillations, as was confirmed in our experiments where this interaction was disrupted. A similar physical interaction between ER and Na,K-ATPase cannot be excluded. Another possibility is that ouabain binding to Na,K-ATPase stimulates a signal cascade that leads to activation of phospholipase C and subsequent generation of IP3. Such a scenario has been suggested to occur in ouabain-treated cardiac myocytes (11).
The regular, low-frequency intracellular [Ca2+]i oscillations induced by ouabain triggered the activation of the transcription factor NF-κB. Inactive NF-κB is present in the cytosol, complexed with the inhibitory protein, IκB (30). Disinhibition of NF-κB occurs when it is released from IκB inhibition, resulting in the translocation of NF-κB to the nucleus and the binding of NF-κB to the κ motif of the target gene. NF-κB is a pleiotropic regulator of many genes. It is well known that NF-κB is involved in inflammatory responses, but more recent studies implicate this transcription factor in processes related to growth and differentiation (34–36). Activation of NF-κB is a Ca2+-dependent process. In studies where Ca2+ oscillations were artificially induced in lymphocytes, NF-κB was preferentially activated by low-frequency oscillations (8). The present study identifies ouabain as a physiological inducer of slow [Ca2+]i oscillations that activate active NF-κB.
Recently, it has been shown that ouabain is present in mammalian tissues. Endogenous ouabain is, like other steroid hormones, synthesized and released from the adrenals (21). It is also locally produced in other tissues, such as the hypothalamus (16, 18). Its physiological role has not been clarified (37). It has been suggested that ouabain may act as a natriuretic hormone (22, 38), but unlike most other natriuretic factors, it may also have a vasoconstrictive effect (39). According to recent studies, ouabain plays a role in growth, differentiation, and apoptosis (10, 11, 14, 40). Consistent with these findings, high circulating levels of ouabain are found in pregnancy (41) and postnatally (42). Our studies were performed on RPT cells that express a high level of Na,K-ATPase. Interestingly, it has been reported that endogenous ouabain levels are increased following nephrectomy (43), a condition that is associated with compensatory growth of the remaining kidney. The results from the present study indicate that the ouabain/Na,K-ATPase complex may, via a calcium signaling pathway, trigger the activation of NF-κB and, likely, also other calcium-dependent transcriptional factors. In pilot studies, it was found that the cAMP response element, CREB, was phosphorylated at site Ser-133 in RPT cells exposed to doses of ouabain that induce [Ca2+]i oscillations. Ser-133 phosphorylation is one of the earliest steps in CREB activation (44).
Rapid plasma membrane-initiated steroid signaling events have been observed in mammalian cells, yet little is known about the specific membrane-bound steroid receptors responsible for initiating signal transduction (45). Recently, a rapid nongenomic response to steroid was characterized in Arabidopsis and shown to occur via a specific plasma membrane receptor (46). Such a mechanism for steroid-mediated mammalian cell signaling has not been established. Based, however, on the specificity of the ouabain response observed here, we propose a novel role for Na,K-ATPase as a plasma membrane-bound steroid receptor involved in signal transduction. Evidence suggests that other mammalian steroids, such as aldosterone, progesterone, and estrogen, may in addition to their well established intracellular receptor-dependent effects evoke specific cellular responses by binding to plasma membrane receptors (47). The ability of ouabain, a well recognized ligand of Na,K-ATPase, to induce second-messenger [Ca2+]i oscillations represents an excellent model for continued study of this steroid effect.
Acknowledgments
We thank Eivor Zettergren, Louise Gustafsson, and Ann-Christine Eklöf for experimental assistance. This work was supported by grants from the Swedish Research Council, the Märta and Gunnar V. Philipson Foundation, the Foundation of Axel Tielman's Memory (A.A.), and by the Stiftelsen Frimurare Barnhuset Stockholm (to O.A., P.U., and M.L.).
Abbreviations
- [Ca2+]i
intracellular calcium concentration
- CRAC
calcium release-activated calcium channel
- JP
jasplakinolide
- [Na+]i
intracellular sodium concentration
- LVGC
L-type voltage-gated calcium
- CICR
Ca2+-induced Ca2+ release
- RPT
rat proximal tubule
- GFP
green fluorescent protein
- ER
endoplasmic reticulum
- IP3
inositol triphosphate
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Berridge M J, Bootman M D, Lipp P. Nature (London) 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 2.Gomez T M, Spitzer N C. Nature (London) 1999;397:350–355. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- 3.Putney J W. Science. 1998;279:191–192. doi: 10.1126/science.279.5348.191. [DOI] [PubMed] [Google Scholar]
- 4.Berridge M J. Nature (London) 1997;386:759–760. doi: 10.1038/386759a0. [DOI] [PubMed] [Google Scholar]
- 5.De Koninck P, Schulman H. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 6.Meldolesi J. Nature (London) 1998;392:863. doi: 10.1038/31804. ., 865–866. [DOI] [PubMed] [Google Scholar]
- 7.Dolmetsch R E, Lewis R S, Goodnow C C, Healy J I. Nature (London) 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 8.Dolmetsch R E, Xu K, Lewis R S. Nature (London) 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 9.Hu Q, Deshpande S, Irani K, Ziegelstein R C. J Biol Chem. 1999;274:33995–33998. doi: 10.1074/jbc.274.48.33995. [DOI] [PubMed] [Google Scholar]
- 10.Lichtstein D, McGowan M H, Russell P, Carper D A. Hypertens Res. 2000;23:S51–S53. doi: 10.1291/hypres.23.supplement_s51. [DOI] [PubMed] [Google Scholar]
- 11.Kometiani P, Li J, Gnudi L, Kahn B B, Askari A, Xie Z. J Biol Chem. 1998;273:15249–15256. doi: 10.1074/jbc.273.24.15249. [DOI] [PubMed] [Google Scholar]
- 12.Haas M, Askari A, Xie Z. J Biol Chem. 2000;275:27832–27837. doi: 10.1074/jbc.M002951200. [DOI] [PubMed] [Google Scholar]
- 13.Peng M, Huang L, Xie Z, Huang W H, Askari A. J Biol Chem. 1996;271:10372–10378. doi: 10.1074/jbc.271.17.10372. [DOI] [PubMed] [Google Scholar]
- 14.Huang L, Li H, Xie Z. J Mol Cell Cardiol. 1997;29:429–437. doi: 10.1006/jmcc.1996.0320. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura A, Abrell L M, Maggiali F, Berova N, Nakanishi K, Labutti J, Magil S, Haupert G T, Jr, Hamlyn J M. Biochemistry. 2001;40:5835–5844. doi: 10.1021/bi0101751. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura A, Guo J, Itagaki Y, Bell C, Wang Y, Haupert G T, Jr, Magil S, Gallagher R T, Berova N, Nakanishi K. Proc Natl Acad Sci USA. 1999;96:6654–6659. doi: 10.1073/pnas.96.12.6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoner W. Cell Mol Biol. 2001;47:273–280. [PubMed] [Google Scholar]
- 18.Schoner W. Exp Clin Endocrinol Diabetes. 2000;108:449–454. doi: 10.1055/s-2000-8140. [DOI] [PubMed] [Google Scholar]
- 19.Uhlen P, Laestadius A, Jahnukainen T, Soderblom T, Backhed F, Celsi G, Brismar H, Normark S, Aperia A, Richter-Dahlfors A. Nature (London) 2000;405:694–697. doi: 10.1038/35015091. [DOI] [PubMed] [Google Scholar]
- 20.Fedorova O V, Anderson D E, Bagrov A Y. Am J Hypertens. 1998;11:796–802. doi: 10.1016/s0895-7061(98)00042-9. [DOI] [PubMed] [Google Scholar]
- 21.Hamlyn J M, Blaustein M P, Bova S, DuCharme D W, Harris D W, Mandel F, Mathews W R, Ludens J H. Proc Natl Acad Sci USA. 1991;88:6259–6263. doi: 10.1073/pnas.88.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrandi M, Manunta P, Balzan S, Hamlyn J M, Bianchi G, Ferrari P. Hypertension. 1997;30:886–896. doi: 10.1161/01.hyp.30.4.886. [DOI] [PubMed] [Google Scholar]
- 23.Mernissi G E, Doucet A. Am J Physiol. 1984;247:F485–F490. doi: 10.1152/ajprenal.1984.247.3.F485. [DOI] [PubMed] [Google Scholar]
- 24.Llano I, Gonzalez J, Caputo C, Lai F A, Blayney L M, Tan Y P, Marty A. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- 25.Ma H T, Patterson R L, van Rossum D B, Birnbaumer L, Mikoshiba K, Gill D L. Science. 2000;287:1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- 26.Putney J W. Cell. 1999;99:5–8. doi: 10.1016/s0092-8674(00)80056-2. [DOI] [PubMed] [Google Scholar]
- 27.Patterson R L, van Rossum D B, Gill D L. Cell. 1999;98:487–499. doi: 10.1016/s0092-8674(00)81977-7. [DOI] [PubMed] [Google Scholar]
- 28.Bereta J, Cohen M C, Bereta M. FEBS Lett. 1995;377:21–25. doi: 10.1016/0014-5793(95)01301-6. [DOI] [PubMed] [Google Scholar]
- 29.Xie Z, Kometiani P, Liu J, Li J, Shapiro J I, Askari A. J Biol Chem. 1999;274:19323–19328. doi: 10.1074/jbc.274.27.19323. [DOI] [PubMed] [Google Scholar]
- 30.Baeuerle P A, Baltimore D. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 31.Li W, Llopis J, Whitney M, Zlokarnik G, Tsien R Y. Nature (London) 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 32.Berridge M J. Nature (London) 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 33.Mak D O, McBride S, Foskett J K. Proc Natl Acad Sci USA. 1998;95:15821–15825. doi: 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattson M P, Camandola S. J Clin Invest. 2001;107:247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jobin C, Sartor R B. Am J Physiol Cell Physiol. 2000;278:C451–C462. doi: 10.1152/ajpcell.2000.278.3.C451. [DOI] [PubMed] [Google Scholar]
- 36.Baldwin A S., Jr J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi H. Hypertens Res. 2000;23:S1–S5. doi: 10.1291/hypres.23.supplement_s1. [DOI] [PubMed] [Google Scholar]
- 38.Blaustein M P. Am J Physiol. 1977;232:C165–C173. doi: 10.1152/ajpcell.1977.232.5.C165. [DOI] [PubMed] [Google Scholar]
- 39.Blaustein M P. Kidney Int. 1996;49:1748–1753. doi: 10.1038/ki.1996.260. [DOI] [PubMed] [Google Scholar]
- 40.Isaev N K, Stelmashook E V, Halle A, Harms C, Lautenschlager M, Weih M, Dirnagl U, Victorov I V, Zorov D B. Neurosci Lett. 2000;283:41–44. doi: 10.1016/s0304-3940(00)00903-4. [DOI] [PubMed] [Google Scholar]
- 41.Vakkuri O, Arnason S S, Pouta A, Vuolteenaho O, Leppaluoto J. J Endocrinol. 2000;165:669–677. doi: 10.1677/joe.0.1650669. [DOI] [PubMed] [Google Scholar]
- 42.Di Bartolo V, Balzan S, Pieraccini L, Ghione S, Pegoraro S, Biver P, Revoltella R, Montali U. Life Sci. 1995;57:1417–1425. doi: 10.1016/0024-3205(95)02104-q. [DOI] [PubMed] [Google Scholar]
- 43.Yamada K, Goto A, Hui C, Yagi N, Nagoshi H, Sasabe M, Sugimoto T. Am J Physiol. 1994;266:H1357–H1362. doi: 10.1152/ajpheart.1994.266.4.H1357. [DOI] [PubMed] [Google Scholar]
- 44.Shaywitz A J, Greenberg M E. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt B M, Gerdes D, Feuring M, Falkenstein E, Christ M, Wehling M. Front Neuroendocrinol. 2000;21:57–94. doi: 10.1006/frne.1999.0189. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z Y, Seto H, Fujioka S, Yoshida S, Chory J. Nature (London) 2001;410:380–383. doi: 10.1038/35066597. [DOI] [PubMed] [Google Scholar]
- 47.Wehling M. Annu Rev Physiol. 1997;59:365–393. doi: 10.1146/annurev.physiol.59.1.365. [DOI] [PubMed] [Google Scholar]