Abstract
Neuroinflammation is a complex process involving both the peripheral circulation and the Central Nervous System (CNS) and is considered to underlie many CNS disorders including depression, anxiety, schizophrenia, and pain. Stressors including early-life adversity, psychosocial stress, and infection appear to prime microglia toward a pro-inflammatory phenotype. Subsequent inflammatory challenges then drive an exaggerated neuroinflammatory response involving the upregulation of pro-inflammatory mediators that is associated with CNS dysfunction. Several pharmacologic inhibitors of pro-inflammatory cytokines including TNF-α and IL-1β show good clinical efficacy in terms of ameliorating neuroinflammatory processes. Mind/body and plant-based interventions such as yoga, breathing exercises, meditation, and herbs/spices have also been demonstrated to reduce pro-inflammatory cytokines and have a positive impact on depression, anxiety, cognition, and pain. As the intricate connections between the immune system and the nervous system continue to be elucidated, successful therapies for reducing neuroinflammation will likely involve an integrated approach combining drug therapy with nonpharmacologic interventions.
Keywords: Tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), interleukin 1β (IL-1β), microglia, stress, mind/body
Overview of Neuroinflammation
Inflammation is increasingly recognized as playing an important role in the disease process. The loss of immune regulation leading to chronic inflammation results in increased levels of inflammatory markers in the peripheral circulation that includes cytokines and the acute-phase reactant C-reactive protein (CRP). Elevation of these markers appear to predict not only disease risk but also the acceleration of the progression of diseases involving inflammation including cardiovascular disease, cancer, asthma, HIV (human immunodeficiency virus), and rheumatoid arthritis (RA).1 In fact, in a recent review, Fullerton and Gilroy2 suggest that inflammation is “a new therapeutic frontier” and it is clear that inflammation that does not resolve contributes to many chronic diseases. The inflammatory process can follow many different cellular cascades and its resolution is likely equally complex and dependent on tissue and stimulus-specific processes.2 Nowhere is this more evident than in the nervous system. Inflammation of the nervous system (termed neuroinflammation) has been shown to play a pivotal role in neurodegenerative diseases such as Alzheimer disease, Parkinson disease, traumatic brain injury, and stroke.3–5 What has emerged more recently is that diseases that were largely thought to be based on dysregulation of neurotransmitter systems such as mood disorders (including depression and anxiety), schizophrenia, and chronic pain now appear to have neuroinflammation as a key element (reviewed in Skaper et al4).
Although recent studies are now focusing on the role of neuroinflammation in what have typically been considered to be psychiatric disorders, it is important to note that the concept of the integration of the immune system and the nervous system is not new. For example, in Ayurveda (the traditional system of medicine of India that dates back thousands of years), there is a tissue described as “Majja Dhatu” which is defined as that which fills the empty spaces in bone. Its main function is communication and it relates to both the bone marrow (source of red and white blood cells) and the nervous system.6 Thus, Majja Dhatu represents an integrated immune and nervous system because the ancient Ayurvedic physicians considered them to be one functional unit.
This is in contrast to the evolution of Western medicine, where until very recently, the immune system was considered to be entirely separate from the nervous system. Indeed, the Central Nervous System (CNS) was long considered to be immune privileged, meaning that the introduction of antigens into the CNS did not elicit a typical inflammatory immune response. Inflammation was originally described as “calor (heat), dolor (pain), rubor (redness), and tumor (swelling)” by the Roman scholar Celsus in the first century Alzheimer disease, and these cardinal signs of inflammation were not thought to be present within the CNS. However, the concept of inflammation has recently expanded to include diseases that do not display any of Celsus’ cardinal signs. Within the CNS, this includes the concept of neuroinflammation where none of the 4 cardinal signs are exhibited: conventional inflammatory cells are not present and changes to brain tissue resemble degeneration rather than classical inflammatory pathways.7 We now know that neuroinflammation is largely mediated by microglia, and to some extent astrocytes and mast cells within the brain, and plays an important role in neurodegenerative diseases as well as mood disorders, pain, and other CNS disorders. Many excellent reviews on this have been published within the past 5 years.3,4,7–9
One of the earliest observations in the 20th century that correlated inflammation, neurodegeneration, and mood disorders involved the sequela of events that occurred following the Influenza A (Spanish flu) outbreak of 1918. This illness was associated with postencephalic Parkinson disease and it has been suggested that the Spanish flu was a neurovirulent strain that has since been implicated in manic-depressive disorder, schizophrenia, and Parkinson disease.10 Recent studies have demonstrated that inflammatory cytokines including IL-6, TNF-α, and CRP are elevated in the blood of patients with Parkinson disease.11 The severity of depression and anxiety in these patients has also been positively correlated with levels of TNF-α and CRP.12 These findings support the hypothesis that a peripheral inflammatory challenge, whether it is infection or an immune challenge, can induce a neuroinflammatory response in the brain.13
One of the first papers that described the interaction of the immune system with the nervous system in terms of mood disorders was published in 1991 by Dr RS Smith where he presented the “Macrophage theory of depression.”14 He wrote that “excessive secretion of the macrophage monokines IL-1, interferon alpha (IFN-α) and tumor necrosis factor (TNF) is proposed as the major cause of depression.” This theory was based on 7 observations including (a) volunteers given monokines develop all of the symptoms of major clinical depressive disorder, (b) diseases where there is high macrophage activation are associated with high rates of depression, (c) microglia can secrete monokines, and he noted a large body of epidemiologic studies supported his hypothesis, even in 1991.14 Smith believed that the cytokine IL-1 was responsible for the hormonal abnormalities observed in major depression and proposed that this cytokine could be a mediator for depression. He also suggested that IFN-α and TNF-α were important cytokines in the evolution of depression as patients given these cytokines displayed many symptoms of depression. He noted that patients with RA had a significantly greater incidence of depression (as did women) and additionally suggested that his macrophage theory of depression would predict the higher rates of depression found with coronary heart disease and stroke. Finally, in a fascinating observation, Smith noted that the “food-gut-allergy axis” may play a role in macrophage activation. He postulated that food itself could affect behavior and noted that dairy was the most frequent food that resulted in behavior dysfunction. He also suggested that fish oil could be prophylactic against depression.14 Smith was particularly prescient when he linked the gut and mood disorders as we now know the importance of the microbiome in health and disease.
Almost 3 decades after Smith published this seminal paper, the biomedical community has embraced the concept of inflammation influencing CNS dysfunction from neurodegeneration to mental health disorders, to pain syndromes and opioid addiction. Microglia, and to some extent astrocytes, appear to be the key regulators of neuroinflammation and a diverse range of stimuli can drive these cells into an inflammatory phenotype.
There is a large body of literature examining the role of neuroinflammation in neurodegenerative diseases, but this review will focus on the most well-studied neuroinflammatory mediators (IL-1β, TNF-α, IL-6, CRP) and examine how these proteins modulate psychiatric diseases and pain. We will then examine nonpharmacologic interventions, that have been shown to be efficacious in psychiatric dysfunction and also result in a decrease in these peripherally circulating inflammatory mediators.
Microglia are the principle modulators of neuroinflammation in the CNS
Microglia are the main immune effectors in the brain and perform multiple functions. They are considered to be resident macrophages, and once activated, they migrate to the site of injury or disease when pathogens, disease, or other inflammatory signals affect the CNS. Activated microglia then differentiate to either a neurotoxic (sometimes called M1) or a neuroprotective phenotype (termed M2), although it is important to recognize that microglia can differentiate along a spectrum between neurotoxic and neuroprotective functions. When in a neurotoxic state, microglia secrete pro-inflammatory cytokines and chemokines including interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), and interleukin 6 (IL-6) as well as other pro-inflammatory molecules such as nitric oxide and superoxide anion. The neuroprotective phenotype releases anti-inflammatory cytokines such as IL-10 and downregulates the pro-inflammatory response.3,15,16 Pro-inflammatory microglia defend against invading pathogens and clear cellular debris to promote tissue repair, and this response is ultimately positive for CNS recovery following injury or some other short-term challenge. Chronic inflammation occurs when microglia remain in the pro-inflammatory state, leading to upregulation of pro-inflammatory cytokines, and ultimately neuronal cell death. It has been suggested that when acute inflammation is unable to resolve, ie, “frustrated resolution,” then a maladaptive immunity evolves where there is ongoing acute inflammation that is accompanied by the inability to develop adaptive immunity, and this may underlie many of the diseases that are driven by chronic inflammation.2
Microglia have also been shown to contact synaptic elements (synaptic clefts, axon terminals, dendritic spines) and microglial surveillance has been linked to synaptic maturation and pruning during development.17,18 There is evidence that the engulfment of synapses by microglia can provide neuroprotection during increased neural activity or excitotoxicity but that chronic stress or other pathologic conditions can result in microglial-mediated synaptic loss.9,19 Recently, a subset of microglia called “dark microglia” have been identified in a rodent model. These microglia are rarely found under normal conditions in the brain but become abundant with chronic stress, normal aging, Alzheimer pathology, and in fractalkine-signaling knockout mice.20 The dark microglia are usually found in clusters and are often associated with the vasculature in brain regions such as the hippocampus, cerebral cortex, amygdala, and hypothalamus. They appear to be extremely active, more so than normally activated microglia, and can engulf entire synapses between axon terminals and synaptic spines.20 These highly phagocytic cells show signs of oxidative stress that includes an electron-dense, condensed cytoplasm, hence their name of “dark microglia.” Interestingly, an electron micrographic study that was published more than 50 years ago also described microglia that were very electron dense.20,21 It has yet to be determined whether the dark microglia represent a subset of hyperactive/hyperinflammatory microglia or whether they are a novel myeloid cell that infiltrates the brain. Either way, they could play a major role in synaptic remodeling and/or stripping under pathologic conditions and may contribute to diseases such as depression where synaptic loss has been documented.
Cellular pathways that lead to activation of microglia are a target for the development of drugs to combat neuroinflammation. For example, activation of the nuclear factor κB (NF-κB) pathway has been shown to play a key role in neuroinflammation, is linked to depression-like symptoms, and is the focus of studies examining factors that can activate this pathway.22,23 This area of investigation has seen explosive growth in the past few years and there are many intriguing hypotheses and compounds that are being developed and tested on this topic but are beyond the scope of the current review.
Stress can prime neuroinflammatory pathways
There is a growing body of literature documenting that life stressors can predispose an individual to the development of psychiatric disorders (reviewed in Frank et al24). These stressors appear to prime the neuroinflammatory response, resulting in an exaggerated response to subsequent pro-inflammatory challenges. Both acute stressors (eg, meeting a deadline, a “fender bender” auto accident, or taking an exam) and chronic stressors (such as loss of a spouse, caring for an ill family member, divorce, or natural disasters) are all associated with elevated peripheral markers of inflammation. The most common markers that have been measured in the peripheral circulation include the pro-inflammatory cytokines IL-1β, TNF-α, IL-6, and the acute-phase CRP. A recent meta-analysis examining alterations in inflammatory cytokines to acute laboratory stress found that this type of stress-induced significant increases in peripheral IL-1β, IL-6, and TNF-α but not CRP.1 Although it is well established that stress has an overall effect on immune function, there is a great deal of variability in how each individual responds to stress. Some individuals show a large immune response to stress, whereas others show little or no response. In addition, early-life experiences affect the immune system, and these early alterations can result in a heightened immune response to stressors during young adulthood and adulthood.25 A recent study in rodents showed that lipopolysaccharide (LPS)-induced maternal immune activation at E12 (embryonic day 12) significantly upregulated the fetal brain expression of IL-1β, TNF-α, and IL-6 and this upregulation was sustained through P40 (40 days postpartum).26 Epidemiologic studies have shown that prenatal or neonatal activation of the immune system by infection, stress, or malnutrition can predispose an individual to diseases such as schizophrenia and autism.27 We will further explore the link between inflammation and schizophrenia later in this review. It is important to note that a peripheral inflammatory challenge, whether it is infection or an immune challenge, can induce a neuroinflammatory response in the brain.13
It has been suggested that individuals who respond to everyday stresses with a large increase in inflammatory mediators may be less susceptible to acute infections but are much more prone to chronic inflammation and diseases related to inflammation.1 Frank et al24 have proposed a model of microglial priming where an initial stress results in increased levels of glucocorticoids within the CNS, which subsequently induces the extracellular release of the high mobility group box 1 protein (HMGB1) forming a danger-associated molecular pattern (DAMP) in the brain. The DAMPs are a group of endogenous molecules that are released in response to conditions such as a broken bone that do not involve the entry of pathogens into the body. HMGB1 is present in the brain and is thought to signal innate immune cells such as microglia to a number of different conditions including stress.28 HMGB1 release then induces the upregulation of the NLRP3 inflammasome in microglia, which cleaves IL-1β into its active form, and the microglia are then considered to be primed. If a subsequent immune challenge occurs, then these “primed” microglia respond with a potentiated neuroinflammatory response.24 Microglial priming may occur more readily in some individuals, and these people could be particularly vulnerable to stress-induced psychiatric disorders.
In addition, psychosocial stress results in microglia shifting toward a pro-inflammatory phenotype, presumably through glucocorticoid release.29,30 A recent rodent study used a model of repeated social defeat to examine the development of glial activation under psychosocial stress.31 Male rats were introduced into the cage of the resident, dominant male who then attacked the intruder for a duration of 10 minutes or shorter, depending on when the intruder displayed submission. The intruders were then placed for 60 minutes in a wire mesh cage that prevented further physical contact but still allowed visual, olfactory, and auditory interactions. This protocol was repeated for 5 consecutive days, using different residents but the same intruder. The intruders showed transient increased corticosterone levels that were correlated with increased expression of the translocator protein (TSPO; a biomarker for activated microglia and astrocytes) as measured by positron emission tomography using 11C-PK11195 as the tracer. This increase in microglial activation was also accompanied by depressive and anxiety-like behavior. However, in this model of very short term social defeat, all physiological and psychological parameters induced by the repeated social defeat resolved within 3 to 6 months.31 It is not known whether a social defeat paradigm that continued for months would have a more long-lasting effect.
Based on these findings, one can easily imagine how chronic psychosocial stress can lead to chronic neuroinflammation and subsequent mood disorders. It is important to note that women appear to be more susceptible to mood disorders following both short-term and long-term inflammation. Women respond to stressors in a more pro-inflammatory way than do men, with increased mobilization of various types of immune cells and decreased sensitivity to glucocorticoids.32
Taken together, these studies demonstrate that stress, including early childhood adversity and psychosocial stress, can prime the neuroinflammatory response to subsequent inflammatory challenges, and that chronic stress results in chronic inflammation that is associated with mood disorders.
Neuroinflammation in Psychiatric Disorders and Pain
Depression
Smith’s original macrophage theory of depression has been validated by an extensive and detailed body of work that has examined the bidirectional interaction between the immune system and the nervous system in mood disorders. Chronic inflammatory conditions such as multiple sclerosis, RA, inflammatory bowel disease, obesity, and even spinal cord injury are often comorbid with depression.33–36 In fact, patients with RA suffer depression rates of 9.5% to 41.5% which is significantly higher compared with the general population (6.7%).37 These diseases result in increased levels of circulating pro-inflammatory cytokines and CRP which are thought to contribute to the development of depression. The reverse situation is also true; a large number of patients with major depression or bipolar disorder have elevated peripheral inflammatory mediators including cytokines and acute phase proteins such as CRP in their blood or cerebrospinal fluid (CSF) compared with healthy controls.33,38,39 It is worth noting that in adolescents and young adults, multiple depressive episodes predicts a an increase in CRP levels later in adulthood.40 Many of these cytokines return to normal levels following treatment and recovery from depression.41
Finally, artificially inducing an increase in peripheral pro-inflammatory cytokines by administration of endotoxin or with cytokine therapy for the treatment of cancer leads to the development of depressive symptoms.33,42 This acute systemic inflammation results in what is commonly referred to as sickness behavior (anhedonia, mood deterioration, fatigue, cognitive deficits).43 For example, a low-dose LPS injection in humans results in a significant increase in serum CRP, TNF-α, and IL-6 as well as a significant increase in IL-6 in the CSF within 0 to 4 hours following the injection. This is correlated with an increase in dysthymia scores (persistent depressive disorder).33
Human studies have shown that the TNF-α inhibitor infliximab, used to treat many inflammatory diseases including RA, Crohn disease, and psoriasis, is able to reduce depression. A randomized controlled trial of infliximab suggested that although infliximab was not generally effective in reducing treatment-resistant depression, it did appear to be efficacious in patients with high baseline markers for inflammation such as CRP.44 In addition, high levels of CRP in patients were associated with worse outcomes with SSRIs (serotonin selective reuptake inhibitors such as Lexapro/escitalopram) that are used to treat depression.45 The SSRI monotherapy appears to be more effective in patients with low CRP levels, whereas combination therapy (bupropion/SSRI) is more effective in patients with high CRP levels.45 Serum CRP levels could potentially be used as a biomarker to guide pharmacologic therapy in the treatment of depression.
Inflammatory cytokines such as IL-6 appear to predict depression during aging46 and might also be used as a therapeutic guide for treating depression in the elderly. The incidence of depression increases with age and is often associated with chronic pain.47 Not only is there an elevation of peripheral pro-inflammatory mediators during aging but age also appears to prime microglia to a subsequent neuroinflammatory challenge47 as described earlier in this review. In addition, aging also results in an increase in very pro-inflammatory dark microglia in rodents.20 Abnormal activation of microglia and increased microglial cell numbers are observed in patients with depression and anxiety, independent of age, although age certainly exacerbates this process.8,48
So how do peripheral pro-inflammatory cytokines influence the brain? There are several routes by which these mediators enter the brain and activate neuroinflammatory processes. They can reach the brain through the vagus nerve that projects to the nucleus of the solitary tract which then communicates with the hypothalamus and the amygdala.13 The blood-brain barrier (BBB) itself is disrupted by LPS and pro-inflammatory cytokines including TNF-α, and they can cross the BBB via upregulation of influx carriers.49 Pro-inflammatory mediators can also enter the brain in areas that do not have a BBB such as the circumventricular organs and the choroid plexus.50 Once these peripheral mediators enter the brain, they drive neuroinflammatory processes including microglial activation, and this can result in mood disorders such as depression.
Anxiety
Depression is often linked to anxiety and increased blood levels of TNF-α induce anxiety behavior in a rodent model of chronic mild stress.51 Inhibiting TNF-α by administering infliximab reduces anxiety in both the rodent model of mild stress and a model of persistent pain.51,52 In addition, mild chronic stress also results in microglia activation as well as upregulation of cytokines including IL-1β and IL-6 in the hippocampus.53 Additional data confirming the role of pro-inflammatory mediators in the development of anxiety come from experiments where mice that are genetically engineered to overexpress IL-6 or TNF-α show anxiogenic behavior.54
People with anxiety that is independent of depression also have higher levels of IL-6.55 Healthy medical students that take a high-stakes nationwide examination for promotion have increased anxiety levels and show significantly increased levels of a number of different pro-inflammatory cytokines including IL-1β and TNF-α. The anxiety states of the students peaked 1 day before the exam, although the increased levels of the pro-inflammatory cytokines were highest immediately after the exam, and then significantly decreased 1 week later.56 Interestingly, a randomized controlled trial found that omega-3 supplementation lowered both inflammation and anxiety in a different cohort of anxious medical students.57 Thus, human studies have found a correlation between anxiety and increased levels of pro-inflammatory mediators such as IL-1β, IL-6, and TNF-α.
Schizophrenia
Inflammation has also been shown to play an important role in schizophrenia. Chronic systemic increases in pro-inflammatory cytokines including IL-1β, IL-6, IL-8, and TNF-α have been well-documented in people with schizophrenia and first episode psychosis compared with healthy controls.58,59 Cytokine serum levels are correlated with exacerbation/remission of symptoms as well as with antipsychotic treatment.58–60 In particular, patients with schizophrenia have elevated levels of IL-6 in both serum and cerebral spinal fluid and this elevation appears to increase the risk for cognitive decline.61 Polymorphisms in both the IL-1β and IL-6 genes that increase blood levels for both of these cytokines are associated with schizophrenia.59 Maternal infection, including influenza, Toxoplasma gondii, and herpes simplex virus type 2 are also well recognized as risk factors for schizophrenia in the offspring.27 These infections are associated with an increase in pro-inflammatory cytokines, and elevated maternal levels of TNF-α at the time of birth are correlated with offspring who have psychosis compared with control offspsring.27
In addition, there appears to be a lower level of messenger RNA (mRNA) for the anti-inflammatory cytokine IL-2 in the blood of people with schizophrenia.58 It is hypothesized that people having schizophrenia might also have a blunted ability to dampen the inflammatory response because they produce less IL-2. Results supporting these findings show levels of soluble TNF-α receptor 1 (sTNFR1) are significantly higher in patients with acute-stage schizophrenia compared with healthy controls. Significantly, high levels of sTNFR1 are a good indicator of patients that will not respond well to drug therapy and exhibit deterioration during the course of in-patient care.62
Patients with schizophrenia have changes in microglia along with increases in pro-inflammatory cytokines. Postmortem studies of brain tissue from schizophrenic patients have found an increase in microglial density and activation, as well as increased degeneration compared with controls.63 Although dysregulation of the dopaminergic system is thought to underlie the development of psychosis in schizophrenia, there is also a loss of cortical synapses that may be mediated by activated microglia.63 Interestingly, many of the maternal risk factors for developing schizophrenia, including infection, can also prime microglia toward a pro-inflammatory phenotype following a subsequent challenge.
Taken together, these studies suggest that inflammation plays an important role in the development of schizophrenia. Pre- or perinatal immunologic challenges can prime microglia, resulting in structural changes and the release of pro-inflammatory mediators that accumulate as patients’ age.60 Current treatments for schizophrenia all involve inhibiting dopamine neurotransmission. However, there is a wide variability in how patients respond to these medications. Some patients respond well, some are delayed responders, and some are resistant to treatment. Elevation in IL-6 is associated with delayed responders and treatment resistance is associated with increased levels of IL-6 and IL-6r, and sTNFR1.59 It may be possible in the future to use these pro-inflammatory mediators as trait markers to predict treatment effectiveness or to develop co-therapeutics that target specific cytokines as adjuncts to dopaminergic therapies.
Pain
Neuroinflammation has recently been identified as playing an important role in the establishment and maintenance of both neuropathic pain and chronic pain. Pro-inflammatory cytokines and chemokines and microglial activation have been shown to contribute to the pain process.64,65 Neuropathic pain is complex and results from peripheral nerve damage that includes damage by injury, infection, diabetes, and other diseases. Chronic pain occurs when individuals have pain at sites that were previously injured but are now apparently healthy.65 Patients with neuropathic pain have been shown to have higher IL-2 and TNF-α mRNA and protein levels compared with patients with a painless neuropathy or healthy controls.66 Pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α have been demonstrated to accelerate pain sensitization, whereas inhibitors of these cytokines reduce neuropathic pain.65 Chronic pain increases the risk for depression by as much 4 times and neuroinflammation appears to be a common pathway that links pain and depression.47 In addition, chronic inflammation is now thought to be an important factor in fibromyalgia (FM). Fibromyalgia is a musculoskeletal pain condition that is characterized by widespread pain, muscle tenderness, and fatigue/sleep disturbances and is most common in women.67 A recent study that analyzed pro-inflammatory mediators in the cerebral spinal fluid and plasma of patients using a multiplex protein panel revealed that the pro-inflammatory cytokines IL-8, IL-6, and some chemokines are elevated in patients with FM, highlighting the importance of chronic inflammation in FM.67
Microglia have also been implicated in pain conditions and it has been suggested that chronic pain is the result of dysregulated glial activation.68 The pro-inflammatory mediators that are released by activated microglia are thought to contribute to pain hypersensitivity, and the microglial inhibitor minocycline has been shown to reduce pain hypersensitivity in a number of different models including burn injuries, spinal cord injury, and chronic constriction injury.68 For example, spinal microglia play an important role in the pathogenesis of neuropathic pain following nerve injury in a rodent model where minocycline reduced the neuropathic pain. However, only males showed a reduction in pain, suggesting that the microglia signaling is sex dependent in this context.69 Treatment of pain with cytokine inhibitors also shows encouraging results in patients.70 A single intrathecal treatment of the TNF-α inhibitor etanercept provided up to 8 weeks of relief for patients having intractable discogenic back pain, whereas 2 consecutive injections of etanercept provided 26 weeks of relief for lumbar herniated disc patients. Treatment with rilonacept, an IL-1 inhibitor, was well tolerated and reduced pain in a small group of patients with chronic refractory gouty arthritis (reviewed in Ji et al70).
Finally, neuroinflammation may be an important link between chronic pain and opioid abuse. Chronic pain patients with comorbid depression are more likely to develop opioid abuse than pain patients without depression.71 Persistent inflammatory pain results in animals self-administering more heroin than nonpain animals, suggesting that chronic pain increases opioid-addictive behavior.72 Chronic opioid use itself results in neuroinflammation with increases in IL-1β and TNF-α.71 It is hypothesized that the inflammation induced by opioids renders them less effective, thus more drug is needed leading to increased dependence. Opioid drugs activate microglia in brain regions including the limbic system that regulates reward and emotion, and morphine has also been shown to activate the inflammasome, leading to increased production of inflammatory mediators.71,73 A recent study has demonstrated a key role for TNF-α in opiate dependence and has identified solTNF-α (soluble TNF-α) as a critical mediator for the development of opioid tolerance.74 In addition, genetic polymorphisms in the IL-1β gene are associated with an increased risk for opioid dependence, although it is not clear what the functional consequences of this polymorphism might be.75 Thus, opioid use appears to initiate a vicious cycle of neuroinflammation that is exacerbated by pain and/or depression, leading to reduced efficacy and tolerance that can result in opioid dependence. It has been suggested that inhibiting neuroinflammation during opioid use could enhance the analgesic efficacy of opioids and might decrease the risk for addiction in the chronic pain population.71
Nonpharmacologic Interventions that Lower Pro-inflammatory Mediators
Developing novel therapeutics that target neuroinflammatory pathways is a very active area of research,76,77 and, as mentioned above, there are already several inhibitors of TNF-α and IL-1 that are on the market for clinical use. However, these drugs are not without potentially serious side effects and a more holistic approach combined with drug therapy might ultimately prove to be the most effective way to lower neuroinflammation. Traditional systems of medicine, including Ayurveda and Traditional Chinese Medicine, take a holistic approach to managing health and disease that includes diet, lifestyle, exercise, rest, diet, herbs, and bodywork. The emerging field of Integrative Medicine is embracing these holistic principles, and many allopathic medical centers are now incorporating mind/body practices and dietary programs into their treatment protocols. Evidence is emerging that nonpharmacologic interventions including yoga, meditation, acupuncture, and diet/herbs can successfully lower circulating pro-inflammatory cytokines.
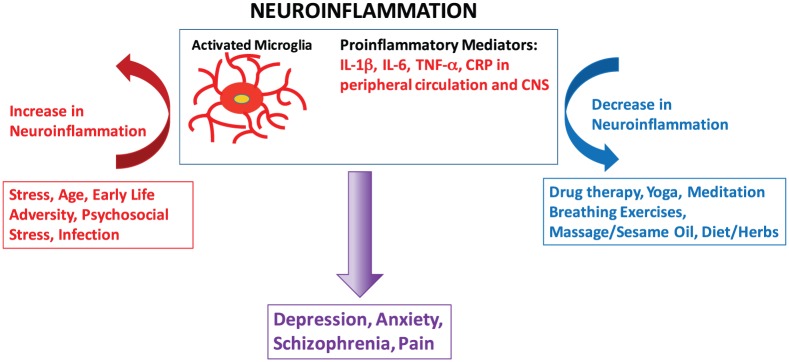
We will focus on the practices incorporated into Ayurveda, the traditional system of medicine of India. Ayurveda has been practiced in India for thousands of years. It uses diet, daily routines, bodywork (including therapeutic massage), herbs, spices, and exercise to both maintain health and treat disease. Ayurveda has a unique approach where each individual is classified according to their individual physiology and psychology (known as Dosha). Each treatment plan is then tailored to the genetics and individual constitution of the person and also to the unique nature of a particular disease. This is very similar to the approach now being adopted by modern Western medicine where “personalized medicine” based on an individual’s genetic profile is increasingly being used to guide therapeutics. In India, Ayurvedic physicians receive rigorous training that includes modern biomedical physiology and pharmacology. Ayurveda is becoming increasingly popular in the West and is currently undergoing a licensing process in the Unites States. There is a growing body of work evaluating the reduction in pro-inflammatory cytokines in response to many of the therapies that are used by Ayurveda, including mind/body interventions (MBIs) and herbal therapies. Although there is variability in this emerging field, some general observations can be made (Figure 1).
Figure 1.
Stress, early-life adversity, psychosocial stress, age, and infection can prime microglia and activate neuroinflammatory processes that include increases in pro-inflammatory mediators in both the peripheral circulation and in the CNS. Neuroinflammation is now thought to underlie many psychiatric disorders including depression, anxiety, and schizophrenia as well as pain. Integrated therapies that include drug therapy as well as mind/body and plant-based therapies will likely be the most successful approach for reducing neuroinflammation and ameliorating CNS dysfunction. CNS indicates Central Nervous System.
Yoga
There is a large body of literature documenting that yoga reduces stress, anxiety, and depression, and studies are now emerging that show that the regular practice of yoga can also lower inflammatory mediators. For example, a recent study examined the relationship between inflammatory cytokines and yoga in 218 volunteers.78 The yoga group practiced yoga daily for 1 hour for a period of 5 years. The control group did not practice yoga but did exercise. Both groups performed a session of moderate and strenuous exercise by performing a standardized shuttle walk. In the shuttle walk, the subjects had to walk between 2 markers in a given amount of time, and this time was gradually decreased. When subjects had more time to walk between the markers, they engaged in moderate exercise and when they had a short amount of time to walk between the markers they engaged in more strenuous exercise. Blood samples were collected before and after the shuttle walk test, and TNF-α and IL-6 were measured. At rest, the non-yoga group had significantly higher levels of TNF-α than the yoga group indicating that the yoga group had lower baseline inflammation compared with the controls.
There was a significant increase in both IL-6 and TNF-α in the non-yoga group compared with the yoga group following the shuttle walk, suggesting that yoga reduces the inflammatory response to moderate exercise. The authors concluded that the regular practice of yoga reduces inflammatory cytokines in general and might protect the individual from inflammatory diseases.78 This study is particularly important because it examined how yoga influences the body’s response to a stressor, in this case exercise, and found that it effectively dampened the pro-inflammatory response. It would be very interesting to evaluate how yoga effects the inflammatory response to a short-term nonphysical stressor such as taking a high stakes exam. Note that “yoga” in this context refers to a relaxing, moderate form of exercise and breathwork versus the high stress yoga sometimes found in some intense workouts.
Another group examined the effect of a short-term yoga-based lifestyle intervention on stress and inflammation in patients with chronic diseases.79 Eighty-six subjects participated in 10 days of yoga (2 hours per day, theory and practice) over a 2-week period. A significant decrease in cortisol (13%), IL-6 (10%), and TNF-α (32%) from baseline to the end of study was observed, suggesting that yoga can lower pro-inflammatory mediators in people with chronic diseases. However, it is important to note that there is a great deal of variability in studies of yoga and inflammatory cytokines, with some studies finding significant decreases in pro-inflammatory cytokines, whereas other studies find little to no effect. This is due, at least in part, to differences in the type of yoga practiced and the study design. Several recent meta-analysis examining yoga and pro-inflammatory mediators in both healthy practitioners and in people with disease have concluded that there is an overall pattern of downregulation of pro-inflammatory markers with the practice of yoga.80,81 In particular, yoga decreases levels of IL-1β, although the effect sizes are small to medium. Studies measuring both IL-6 and TNF-α show mixed results, although overall, yoga appears to have the potential for reducing both cytokines.81 A reason for this variability may lie in the duration of the yoga intervention. Most studies with yoga last only 8 to 12 weeks which is a relatively short period of time. For populations that have inflammatory diseases such as chronic heart failure, an 8-week yoga intervention appears to be sufficient to reduce pro-inflammatory mediators.82 In contrast, inflammatory markers are not reduced in a breast cancer survivor population immediately after a yoga intervention, but significant decreases were found at a 3-month follow-up.83 Taken together, it appears as if a long-term, sustained practice is ideal for producing consistent decreases in peripheral pro-inflammatory mediators.81
Meditation and breathing exercises
Breathing practices alone have been found to be an effective intervention for many stress-related disorders including anxiety, posttraumatic stress disorder, depression, and attention deficit hyperactivity disorder.84 Breathing exercises also appear to be particularly beneficial in ameliorating pain. Breathing exercises performed for 30 minutes a day, 7 days a week for 12 weeks resulted in significant improvements of 27% to 63% in pain thresholds on various tender points for women with FM.85 In another study of pain in FM, slow breathing rates reduced pain intensity and feelings of unpleasantness in response to thermal stimuli in women with FM.86 Unfortunately, neither of these studies measured cytokine levels, but they did establish that breathing practices could be used to modulate the sensation of pain.
A recent study of yogic breathing (YB) found that this practice decreased inflammatory cytokine levels. The small study randomized 20 volunteers into 2 groups, YB (2 breathing exercises for 10 minutes each) and AC (attention control; read a text of their choice for 20 minutes).87 The YB consisted of 10 minutes of OM chanting and 10 minutes of alternate nostril breathing. Inflammatory cytokines were then measured in the saliva following a single session of either YB or AC. No differences between the groups were observed for IL-6 or TNF-α in the saliva, but there was a significant decrease in IL-8 and IL-1β.87 Peripheral circulating cytokines were not measured so it is not known whether there was a decrease in pro-inflammatory cytokines in the blood. This appears to be the only study to date that examined cytokine levels following a breathing practice that was not coupled with meditation. Although these results are encouraging, additional studies are needed to confirm that a breathing practice alone can reduce pro-inflammatory mediators in the peripheral circulation.
Breathing practices are often part of mindfulness meditation, and there is a fairly extensive literature that has examined the effect of meditation on pro-inflammatory mediators. There are many forms of meditation that use various different techniques, but overall, meditation seems to reduce blood cortisol, CRP, TNF-α, IL-6, and the activity of the transcription factor NF-κB in a number of different types of meditation practices.80,88–90 In addition, a recent meta-analysis found that MBIs that included breathing, meditation, yoga, and Tai Chi downregulated pro-inflammatory genes and pathways and identified the NF-κB pathway as being consistently downregulated. The authors concluded that “the various psychological and physiological benefits of MBIs may be mediated through the downregulation of proinflammatory genes and pathways.”91
Therapeutic massage/sesame oil
Body massage with herbalized oils is a cornerstone of Ayurvedic therapies. Most of the herbalized oils used in treatments have sesame oil as their base, and sesame oil itself has been shown to have anti-inflammatory properties in a number of different in vitro and animal models. Sesame oil has been shown to inhibit microglial activation and the release of pro-inflammatory cytokines involved in neuroinflammation. For example, sesamin, a bioactive component of sesame oil, inhibits the production of IL-6, TNF-α, and IL-1β in a microglial cell line activated by MPP+ (1-methyl-4-phenylpyridinium), the active metabolite of the neurotoxin MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine).92 In a rodent model of intracerebral hemorrhage (ICH), intracerebroventricular administration of sesamin prevented the ICH-induced increase in microglial cells, and many of these microglia in the sesamin-treated brains exhibited a resting morphology rather than a pro-inflammatory phenotype.93 Both sesamol (another bioactive compound in sesame oil) and sesamin given orally reduced circulating cytokines including IL-6, TNF-α, IL-1β, and CRP in a rodent model of inflammation induced by LPS injection.94 Note that sesame oil was also frequently used by pharmacies in the United States during the 18th century to compound many drugs and tonics, a practice that was later discontinued.
Although there are no studies to date examining whether massage with sesame oil reduces peripheral inflammatory mediators, there are several studies demonstrating that sesame oil massage can reduce pain. A triple-blind randomized control trial found that massage with topical sesame oil was effective in significantly reducing pain severity in patients with limb trauma.95 In another randomized clinical study of 150 patients with limb trauma, topical application of sesame oil was shown to lower the severity of perceived pain and reduce the frequency of nonsteroidal anti-inflammatory drug use in these patients.96 Clinical studies are needed to evaluate the effect of topical application of sesame oil on circulating cytokines, but sesame oil offers the potential to be useful as an adjunct therapy for treating neuroinflammation.
Diet/herbs
Inflammation related to food is currently a very popular topic, and there are many diets that claim to curb inflammation to improve health and ameliorate disease. Some studies have shown that diets that are high in saturated fats are linked to the expression of pro-inflammatory genes of adipose tissue in individuals at risk for metabolic syndrome.97 In contrast, the Mediterranean Diet, consisting of fruit, nuts, vegetables, legumes, whole grains, seafood, red wine, and olive oil, has been proposed to reduce inflammation, in part by altering the gut microbiome.98 However, the interactions between diet and inflammation are complex and difficult to quantify in clinical studies. For example, as dietary intake across the globe has become more westernized, the rates of obesity and inflammatory diseases including type 2 diabetes have increased.99 However, in India, although the incidence of type 2 diabetes is increasing as the diet becomes more westernized in urban centers, the lowest rates of Alzheimer in the world are found in rural India.100 Diet certainly plays a role, and the use of a wide variety of spices in traditional South Asian cuisine could make a positive contribution to health status. Most spices have been shown to have some type of anti-inflammatory activity, and black and red pepper, clove, garlic, licorice, ginger, cinnamon, coriander, and turmeric have all been shown to modulate inflammatory pathways.101
Turmeric is a staple of Indian cuisine and is the most well-studied spice. There are more than 1300 PubMed references to curcumin (the active component of turmeric) and inflammation. Curcumin supplementation (1 g per day for 8 weeks) has recently been shown to significantly reduce serum concentrations of IL-6, TNF-α, and MCP-1 in individuals with metabolic syndrome who were not taking lipid-lowering drugs.102 Supporting these findings, a meta-analysis found that curcumin lowered circulating IL-6 concentrations in 9 randomized clinical trials.103 Not only has curcumin consumption found to be of benefit in diabetes, metabolic disease, and obesity but it is also beneficial in diseases involving neuroinflammation such as depression.104 Based on its ability to lower circulating pro-inflammatory cytokines in people, supplementation with curcumin or turmeric might be an effective adjunct therapy for treating neuroinflammatory diseases.
It is important to note that most plants exhibit some type of anti-inflammatory activity, particularly in vitro but it is difficult to determine whether this activity has any clinical consequences. Assessing neuroinflammatory properties of herbs that are traditionally used to treat diseases such as memory loss (neurodegeneration), depression, and anxiety is an important approach to determine which plants might have clinical efficacy in a biomedical context. Our laboratory is studying the medicinal herb Bacopa monnieri. Bacopa (sometimes called Brahmi) is an herb that is used in Ayurveda to improve memory and cognition. Bacopa is an important ingredient in many herbal formulas that are designed to manage a wide range of CNS conditions including memory loss, anxiety, lack of concentration, and poor cognition. Importantly, Bacopa is also used to treat inflammatory conditions such as arthritis, asthma, stress, depression, and neurodegenerative disorders including Alzheimer disease and Parkinson disease.105 This anti-inflammatory activity has been well-documented in animal models of arthritis where treatment with Bacopa inhibited the release of both TNF-α and IL-6.105,106 In addition, LPS-induced TNF-α release from whole blood drawn from healthy volunteers was significantly downregulated in a dose-dependent manner by different fractions of Bacopa.107
We have created both water and alkaloid fractions from the Bacopa plant and have examined their anti-inflammatory activity using LPS-activated N9 microglial cells. We have found that several of these fractions significantly inhibit the release of both TNF-α and IL-6 from activated microglia. Interestingly, Bacoside A is the most abundant dammarane saponin triterpenoid in Bacopa and is considered to be primarily responsible for its biological activity. But in our studies, purified Bacoside A did not inhibit the release of the pro-inflammatory cytokines.108 Given that our fractions of Bacopa contained multiple phytochemical constituents and were able to inhibit the pro-inflammatory microglial response, this study highlights the complexity of working with plant material. There are more than a dozen of less abundant bacosides in Bacopa that alone or in concert with other phytochemical constituents may be responsible for the ability of Bacopa to inhibit neuroinflammation and positively affect CNS function.
Conclusions
Neuroinflammation is a complicated process involving both the peripheral circulation and the CNS. Neuroinflammation is now thought to underlie many CNS disorders including depression, anxiety, schizophrenia, and pain. In addition, both acute and chronic stressors such as early-life adversity, psychosocial stress, infection, and viruses appear to prime microglia toward a pro-inflammatory phenotype in susceptible individuals. Subsequent inflammatory challenges then drive an exaggerated neuroinflammatory response. Several pharmacologic inhibitors of pro-inflammatory cytokines including TNF-α and IL-1β are now on the market and show good clinical efficacy in terms of ameliorating neuroinflammatory processes. There are also a number of mind/body and plant-based interventions including yoga, breathwork, meditation, and herbs/spices that have also been shown to reduce pro-inflammatory cytokines and have a positive impact on CNS dysfunction including depression, anxiety, cognitive dysfunction, and pain. As we continue to elucidate the intricate connections between the immune system and the nervous system, successful therapies for reducing neuroinflammation will almost certainly involve an integrated approach combining drug therapy with nonpharmacologic interventions.
Acknowledgments
The author thanks Dr Douglas Coffin for his careful review and editing of this manuscript.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The author received funding from the University of Montana Small grants program for the research described in this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: DIL wrote the manuscript.
ORCID iD: Diana I Lurie  https://orcid.org/0000-0002-4486-4869
https://orcid.org/0000-0002-4486-4869
References
- 1. Marsland AL, Walsh C, Lockwood K, John-Henderson NA. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain Behav Immun. 2017;64:208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15:551–567. [DOI] [PubMed] [Google Scholar]
- 3. González H, Elgueta D, Montoya A, Pacheco R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J Neuroimmunol. 2014;274:1–13. [DOI] [PubMed] [Google Scholar]
- 4. Skaper SD, Facci L, Zusso M, Giusti P. An inflammation-centric view of neurological disease: beyond the neuron. Front Cell Neurosci. 2018;12:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martín A, Domercq M, Matute C. Inflammation in stroke: the role of cholinergic, purinergic and glutamatergic signaling [published online ahead of print May 4, 2018]. Ther Adv Neurol Disord. doi: 10.1177/1756286418774267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lad V. Textbook of Ayurveda: Fundamental Principles (Vol. 1). Albuquerque, New Mexico: Ayurvedic Press; 2002. [Google Scholar]
- 7. Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singhal G, Baune BT. Microglia: an interface between the loss of neuroplasticity and depression. Front Cell Neurosci. 2017;11:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wohleb ES. Neuron-microglia interactions in mental health disorders: “for better, and for worse”. Front Immunol. 2016;7:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayase Y, Tobita K. Influenza virus and neurological diseases. Psychiatry Clin Neurosci. 1997;51:181–184. [DOI] [PubMed] [Google Scholar]
- 11. Boyko AA, Troyanova NI, Kovalenko EI, Sapozhnikov AM. Similarity and differences in inflammation-related characteristics of the peripheral immune system of patients with Parkinson’s and Alzheimer’s diseases. Int J Mol Sci. 2017;18:E2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang XM, Zhang YG, Li AL, et al. Relationship between levels of inflammatory cytokines in the peripheral blood and the severity of depression and anxiety in patients with Parkinson’s disease. Eur Rev Med Pharmacol Sci. 2016;20:3853–3856. [PubMed] [Google Scholar]
- 13. McCusker RH, Kelley KW. Immune-neural connections: how the immune system’s response to infectious agents influences behavior. J Exp Biol. 2013;216:84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298–306. [DOI] [PubMed] [Google Scholar]
- 15. Nakagawa Y, Chiba K. Diversity and plasticity of microglial cells in psychiatric and neurological disorders. Pharmacol Therapeut. 2015;154:21–35. [DOI] [PubMed] [Google Scholar]
- 16. Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nature Reviews Immunology. 2014;14:463–477. [DOI] [PubMed] [Google Scholar]
- 17. Schafer DP, Lehrman EK, Kautzman AG, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691– 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31:16064–16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Milior G, Lecours C, Samson L, et al. Fractalkine receptor deficiency impairs microglial and neuronal responsiveness to chronic stress. Brain Behav Immun. 2016;55:114–125. [DOI] [PubMed] [Google Scholar]
- 20. Bisht K, Sharma KP, Lecours C, et al. Dark microglia: a new phenotype predominantly associated with pathological states. Glia. 2016;64:826–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schultz RL, Maynard EA, Pease DC. Electron microscopy of neurons and neuroglia of cerebral cortex and corpus callosum. Am J Anat. 1957;100:369–407. [DOI] [PubMed] [Google Scholar]
- 22. Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010;107:2669–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fulenwider HD, Smith BM, Nichenko AS, et al. Cellular and behavioral effects of lipopolysaccharide treatment are dependent upon neurokinin-1 receptor activation. J Neuroinflammation. 2018;15:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frank MG, Weber MD, Watkins LR, Maier SF. Stress-induced neuroinflammatory priming: a liability factor in the etiology of psychiatric disorders. Neurobiol Stress. 2016;4:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ganguly P, Brenhouse HC. Broken or maladaptive? altered trajectories in neuroinflammation and behavior after early life adversity. Dev Cogn Neurosci. 2015;11:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O’Loughlin E, Pakan JMP, Yilmazer-Hanke D, McDermott KW. Acute in utero exposure to lipopolysaccharide induces inflammation in the pre- and postnatal brain and alters the glial cytoarchitecture in the developing amygdala. J Neuroinflammation. 2017;14:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown AS. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol. 2012;72:1272–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. [DOI] [PubMed] [Google Scholar]
- 29. Frank MG, Watkins LR, Maier SF. The permissive role of glucocorticoids in neuroinflammatory priming: mechanisms and insights. Curr Opin Endocrinol Diabetes Obes. 2015;22:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chijiwa T, Oka T, Lkhagvasuren B, Yoshihara K, Sudo N. Prior chronic stress induces persistent polyi: C-induced allodynia and depressive-like behavior in rats: possible involvement of glucocorticoids and microglia. Physiol Behav. 2015;147:264–273. [DOI] [PubMed] [Google Scholar]
- 31. Kopschina Feltes P, de Vries EF, Juarez-Orozco LE, et al. Repeated social defeat induces transient glial activation and brain hypometabolism: a positron emission tomography imaging study [published online ahead of print January 1, 2017]. J Cereb Blood Flow Metab. doi: 10.1177/271678X17747189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bekhbat M, Neigh GN. Sex differences in the neuro-immune consequences of stress: focus on depression and anxiety. Brain Behav Immun. 2018;67:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Engler H, Brendt P, Wischermann J, et al. Selective increase of cerebrospinal fluid IL-6 during experimental systemic inflammation in humans: association with depressive symptoms. Mol Psychiatry. 2017;22:1448–1454. [DOI] [PubMed] [Google Scholar]
- 34. Allison DJ, Ditor DS. Targeting inflammation to influence mood following spinal cord injury: a randomized clinical trial. J Neuroinflammation. 2015;12:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morris G, Reiche EMV, Murru A, et al. Multiple immune-inflammatory and oxidative and nitrosative stress pathways explain the frequent presence of depression in multiple sclerosis. Mol Neurobiol. 2018;55:6282–6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Decarie-Spain L, Sharma S, Hryhorczuk C, et al. Nucleus accumbens inflammation mediates anxiodepressive behavior and compulsive sucrose seeking elicited by saturated dietary fat. Mol Metab. 2018;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Withers MH, Gonzalez LT, Karpouzas GA. Identification and treatment optimization of comorbid depression in rheumatoid arthritis. Rheumatol Ther. 2017;4:281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kohler CA, Freitas TH, Maes M, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135:373–387. [DOI] [PubMed] [Google Scholar]
- 39. Ye G, Yin GZ, Tang Z, et al. Association between increased serum interleukin-6 levels and sustained attention deficits in patients with major depressive disorder [published online ahead of print February 8, 2018]. Psychol Med. doi: 10.1017/S0033291718000090. [DOI] [PubMed] [Google Scholar]
- 40. Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ. Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol Psychiatry. 2012;71:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dahl J, Ormstad H, Aass HC, et al. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology. 2014;45:77–86. [DOI] [PubMed] [Google Scholar]
- 42. Bollen J, Trick L, Llewellyn D, Dickens C. The effects of acute inflammation on cognitive functioning and emotional processing in humans: a systematic review of experimental studies. J Psychosom Res. 2017;94:47–55. [DOI] [PubMed] [Google Scholar]
- 43. Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jha MK, Minhajuddin A, Gadad BS, et al. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? findings from the CO-MED trial. Psychoneuroendocrinology. 2017;78:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baune BT, Smith E, Reppermund S, et al. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: the prospective Sydney Memory and Aging Study. Psychoneuroendocrinology. 2012;37:1521–1530. [DOI] [PubMed] [Google Scholar]
- 47. Zis P, Daskalaki A, Bountouni I, Sykioti P, Varrassi G, Paladini A. Depression and chronic pain in the elderly: links and management challenges. Clin Interv Aging. 2017;12:709–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Beumer W, Gibney SM, Drexhage RC, et al. The immune theory of psychiatric diseases: a key role for activated microglia and circulating monocytes. J Leukoc Biol. 2012;92:959–975. [DOI] [PubMed] [Google Scholar]
- 49. Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. 2017;60:1–12. [DOI] [PubMed] [Google Scholar]
- 50. Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, Jacque C. Cytokine signals propagate through the brain. Mol Psychiatry. 2000;5:604–615. [DOI] [PubMed] [Google Scholar]
- 51. Karson A, Demirtas T, Bayramgurler D, Balci F, Utkan T. Chronic administration of infliximab (TNF-α inhibitor) decreases depression and anxiety-like behaviour in rat model of chronic mild stress. Basic Clin Pharmacol Toxicol. 2013;112:335– 340. [DOI] [PubMed] [Google Scholar]
- 52. Chen J, Song Y, Yang J, et al. The contribution of TNF-α in the amygdala to anxiety in mice with persistent inflammatory pain. Neurosci Lett. 2013;541:275–280. [DOI] [PubMed] [Google Scholar]
- 53. Wang YL, Han QQ, Gong WQ, et al. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J Neuroinflammation. 2018;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Salim S, Chugh G, Asghar M. Inflammation in anxiety. Adv Protein Chem Struct Biol. 2012;88:1–25. [DOI] [PubMed] [Google Scholar]
- 55. O’Donovan A, Hughes BM, Slavich GM, et al. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain Behav Immun. 2010;24:1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kamezaki Y, Katsuura S, Kuwano Y, Tanahashi T, Rokutan K. Circulating cytokine signatures in healthy medical students exposed to academic examination stress. Psychophysiology. 2012;49:991–997. [DOI] [PubMed] [Google Scholar]
- 57. Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. 2011;25:1725–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Boerrigter D, Weickert TW, Lenroot R, et al. Using blood cytokine measures to define high inflammatory biotype of schizophrenia and schizoaffective disorder. J Neuroinflammation. 2017;14:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rodrigues-Amorim D, Rivera-Baltanas T, Spuch C, et al. Cytokines dysregulation in schizophrenia: a systematic review of psychoneuroimmune relationship [published online ahead of print November 24, 2017]. Schizophr Res. doi: 10.1016/j.schres.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 60. De Picker LJ, Morrens M, Chance SA, Boche D. Microglia and brain plasticity in acute psychosis and schizophrenia illness course: a meta-review. Front Psychiatry. 2017;8:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Frydecka D, Misiak B, Pawlak-Adamska E, et al. Interleukin-6: the missing element of the neurocognitive deterioration in schizophrenia? the focus on genetic underpinnings, cognitive impairment and clinical manifestation. Eur Arch Psychiatry Clin Neurosci. 2015;265:449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nishimon S, Ohnuma T, Takebayashi Y, et al. High serum soluble tumor necrosis factor receptor 1 predicts poor treatment response in acute-stage schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:145–154. [DOI] [PubMed] [Google Scholar]
- 63. Howes OD, McCutcheon R. Inflammation and the neural diathesis-stress hypothesis of schizophrenia: a reconceptualization. Transl Psychiatry. 2017;7:e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang ZJ, Jiang BC, Gao YJ. Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain. Cell Mol Life Sci. 2017;74:3275–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2013;2013:480739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Uceyler N, Rogausch JP, Toyka KV, Sommer C. Differential expression of cytokines in painful and painless neuropathies. Neurology. 2007;69:42–49. [DOI] [PubMed] [Google Scholar]
- 67. Backryd E, Tanum L, Lind AL, Larsson A, Gordh T. Evidence of both systemic inflammation and neuroinflammation in fibromyalgia patients, as assessed by a multiplex protein panel applied to the cerebrospinal fluid and to plasma. J Pain Res. 2017;10:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Carniglia L, Ramirez D, Durand D, et al. Neuropeptides and microglial activation in inflammation, pain, and neurodegenerative diseases. Mediators Inflamm. 2017;2017:5048616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sorge RE, Mapplebeck JC, Rosen S, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology. 2018;129:343–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cahill CM, Taylor AM. Neuroinflammation-a co-occurring phenomenon linking chronic pain and opioid dependence. Curr Opin Behav Sci. 2017;13:171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hipolito L, Wilson-Poe A, Campos-Jurado Y, et al. Inflammatory pain promotes increased opioid self-administration: role of dysregulated ventral tegmental area μ opioid receptors. J Neurosci. 2015;35:12217–12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Xu E, Liu J, Wang X, Xiong H. Inflammasome in drug abuse. Int J Physiol Pathophysiol Pharmacol. 2017;9:165–177. [PMC free article] [PubMed] [Google Scholar]
- 74. Eidson LN, Inoue K, Young LJ, Tansey MG, Murphy AZ. Toll-like receptor 4 mediates morphine-induced neuroinflammation and tolerance via soluble tumor necrosis factor signaling. Neuropsychopharmacology. 2017;42:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu L, Hutchinson MR, White JM, Somogyi AA, Coller JK. Association of IL-1B genetic polymorphisms with an increased risk of opioid and alcohol dependence. Pharmacogenet Genomics. 2009;19:869–876. [DOI] [PubMed] [Google Scholar]
- 76. Rahimifard M, Maqbool F, Moeini-Nodeh S, et al. Targeting the TLR4 signaling pathway by polyphenols: a novel therapeutic strategy for neuroinflammation. Ageing Res Rev. 2017;36:11–19. [DOI] [PubMed] [Google Scholar]
- 77. Song L, Pei L, Yao S, Wu Y, Shang Y. NLRP3 inflammasome in neurological diseases, from functions to therapies. Front Cell Neurosci. 2017;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vijayaraghava A, Doreswamy V, Narasipur OS, Kunnavil R, Srinivasamurthy N. Effect of yoga practice on levels of inflammatory markers after moderate and strenuous exercise. J Clin Diagn Res. 2015;9:CC08-CC12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yadav RK, Magan D, Mehta N, Sharma R, Mahapatra SC. Efficacy of a short-term yoga-based lifestyle intervention in reducing stress and inflammation: preliminary results. J Altern Complement Med. 2012;18:662–667. [DOI] [PubMed] [Google Scholar]
- 80. Moraes LJ, Miranda MB, Loures LF, Mainieri AG, Marmora CHC. A systematic review of psychoneuroimmunology-based interventions. Psychol Health Med. 2018;23:635–652. [DOI] [PubMed] [Google Scholar]
- 81. Falkenberg RI, Eising C, Peters ML. Yoga and immune system functioning: a systematic review of randomized controlled trials. J Behav Med. 2018;41:467–482. [DOI] [PubMed] [Google Scholar]
- 82. Pullen PR, Thompson WR, Benardot D, et al. Benefits of yoga for African American heart failure patients. Med Sci Sports Exerc. 2010;42:651–657. [DOI] [PubMed] [Google Scholar]
- 83. Kiecolt-Glaser JK, Bennett JM, Andridge R, et al. Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2014;32:1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brown RP, Gerbarg PL, Muench F. Breathing practices for treatment of psychiatric and stress-related medical conditions. Psychiatr Clin North Am. 2013;36:121–140. [DOI] [PubMed] [Google Scholar]
- 85. Tomas-Carus P, Branco JC, Raimundo A, Parraca JA, Batalha N, Biehl-Printes C. Breathing exercises must be a real and effective intervention to consider in women with fibromyalgia: a pilot randomized controlled trial [published online ahead of print April 13, 2018]. J Altern Complement Med. doi: 10.1089/acm.2017.0335. [DOI] [PubMed] [Google Scholar]
- 86. Zautra AJ, Fasman R, Davis MC, Craig AD. The effects of slow breathing on affective responses to pain stimuli: an experimental study. Pain. 2010;149:12–18. [DOI] [PubMed] [Google Scholar]
- 87. Twal WO, Wahlquist AE, Balasubramanian S. Yogic breathing when compared to attention control reduces the levels of pro-inflammatory biomarkers in saliva: a pilot randomized controlled trial. BMC Complement Altern Med. 2016;16:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Creswell JD, Taren AA, Lindsay EK, et al. Alterations in resting-state functional connectivity link mindfulness meditation with reduced interleukin-6: a randomized controlled trial. Biol Psychiatry. 2016;80:53–61. [DOI] [PubMed] [Google Scholar]
- 89. Black DS, Slavich GM. Mindfulness meditation and the immune system: a systematic review of randomized controlled trials. Ann N Y Acad Sci. 2016;1373:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pascoe MC, Thompson DR, Jenkins ZM, Ski CF. Mindfulness mediates the physiological markers of stress: systematic review and meta-analysis. J Psychiatr Res. 2017;95:156–178. [DOI] [PubMed] [Google Scholar]
- 91. Buric I, Farias M, Jong J, Mee C, Brazil IA. What is the molecular signature of mind-body interventions? a systematic review of gene expression changes induced by meditation and related practices. Front Immunol. 2017;8:670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bournival J, Plouffe M, Renaud J, Provencher C, Martinoli MG. Quercetin and sesamin protect dopaminergic cells from MPP+-induced neuroinflammation in a microglial (N9)-neuronal (PC12) coculture system. Oxid Med Cell Longev. 2012;2012:921941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ohnishi M, Monda A, Takemoto R, et al. Sesamin suppresses activation of microglia and p44/42 MAPK pathway, which confers neuroprotection in rat intracerebral hemorrhage. Neuroscience 2013;232:45–52. [DOI] [PubMed] [Google Scholar]
- 94. Yashaswini PS, Sadashivaiah B, Ramaprasad TR, Singh SA. In vivo modulation of LPS induced leukotrienes generation and oxidative stress by sesame lignans. J Nutr Biochem. 2017;41:151–157. [DOI] [PubMed] [Google Scholar]
- 95. Nasiri M, Farsi Z. Effect of light pressure stroking massage with sesame (Sesamum indicum L.) oil on alleviating acute traumatic limbs pain: a triple-blind controlled trial in emergency department. Complement Ther Med. 2017;32:41–48. [DOI] [PubMed] [Google Scholar]
- 96. Bigdeli Shamloo MB, Nasiri M, Dabirian A, Bakhtiyari A, Mojab F, Alavi Majd H. The effects of topical sesame (Sesamum indicum) oil on pain severity and amount of received non-steroid anti-inflammatory drugs in patients with upper or lower extremities trauma. Anesth Pain Med. 2015;5:e25085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Van Dijk SJ, Feskens EJ, Bos MB, et al. A saturated fatty acid-rich diet induces an obesity-linked proinflammatory gene expression profile in adipose tissue of subjects at risk of metabolic syndrome. Am J Clin Nutr. 2009;90:1656–1664. [DOI] [PubMed] [Google Scholar]
- 98. Bailey MA, Holscher HD. Microbiome-mediated effects of the Mediterranean diet on inflammation. Adv Nutr. 2018;9:193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34:1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. World Alzheimer Report 2015, 2015. www.alz.co.uk/research/WorldAlzheimerReport2015.pdf. Accessed June 11, 2018.
- 101. Kannappan R, Gupta SC, Kim JH, Reuter S, Aggarwal BB. Neuroprotection by spice-derived nutraceuticals: you are what you eat! Mol Neurobiol. 2011;44:142–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Panahi Y, Hosseini MS, Khalili N, et al. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: a post-hoc analysis of a randomized controlled trial. Biomed Pharmacother. 2016;82:578–582. [DOI] [PubMed] [Google Scholar]
- 103. Derosa G, Maffioli P, Simental-Mendia LE, Bo S, Sahebkar A. Effect of curcumin on circulating interleukin-6 concentrations: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2016;111:394–404. [DOI] [PubMed] [Google Scholar]
- 104. Mantzorou M, Pavlidou E, Vasios G, Tsagalioti E, Giaginis C. Effects of curcumin consumption on human chronic diseases: a narrative review of the most recent clinical data. Phytother Res. 2018;32:957–975. [DOI] [PubMed] [Google Scholar]
- 105. Lurie D, Coffin J. The role of Bacopa monnieri in inflammatory and neurodegenerative diseases. In: Motohashi N, ed. Occurrences, Structure, Biosynthesis, and Health Benefits Based on Their Evidences of Medicinal Phytochemicals in Vegetables and Fruits (Vol. 3). New York, NY: Nova Science Publishers; 2015:27–61. [Google Scholar]
- 106. Viji V, Shobha B, Kavitha SK, Ratheesh M, Kripa K, Helen A. Betulinic acid isolated from Bacopa monniera (L.) Wettst suppresses lipopolysaccharide stimulated interleukin-6 production through modulation of nuclear factor-kappaB in peripheral blood mononuclear cells. Int Immunopharmacol. 2010;10:843–849. [DOI] [PubMed] [Google Scholar]
- 107. Viji V, Helen A. Inhibition of pro-inflammatory mediators: role of Bacopa monniera (L.) Wettst. Inflammopharmacology. 2011;19:283–291. [DOI] [PubMed] [Google Scholar]
- 108. Nemetchek MD, Stierle AA, Stierle DB, Lurie DI. The Ayurvedic plant Bacopa monnieri inhibits inflammatory pathways in the brain. J Ethnopharmacol. 2017;197:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]