Abstract
Obesity and metabolic syndrome are considered as responsible for a condition known as the non-alcoholic fatty liver disease that goes from simple accumulation of triglycerides to hepatic inflammation and may progress to cirrhosis. Patients with obesity also have an increased risk of primary liver malignancies and increased body mass index is a predictor of decompensation of liver cirrhosis. Sarcopenic obesity confers a risk of physical impairment and disability that is significantly higher than the risk induced by each of the two conditions alone as it has been shown to be an independent risk factor for chronic liver disease in patients with obesity and a prognostic negative marker for the evolution of liver cirrhosis and the results of liver transplantation. Cirrhotic patients with obesity are at high risk for depletion of various fat-soluble, water-soluble vitamins and trace elements and should be supplemented appropriately. Diet, physical activity and protein intake should be carefully monitored in these fragile patients according to recent recommendations. Bariatric surgery is sporadically used in patients with morbid obesity and cirrhosis also in the setting of liver transplantation. The risk of sarcopenia, micronutrient status, and the recommended supplementation in patients with obesity and cirrhosis are discussed in this review. Furthermore, the indications and contraindications of bariatric surgery-induced weight loss in the cirrhotic patient with obesity are discussed.
Keywords: Obesity, Cirrhosis, Sarcopenia, Malnutrition, Bariatric surgery
Core tip: Obesity is a frequent cause of chronic liver disease that can progress to cirrhosis. Cirrhotic patients with obesity frequently have alterations in specific aspects of nutritional status, such as poor protein intake and micronutrient deficiencies. Diet, physical activity and protein intake should be carefully monitored. Bariatric surgery may be an option in the management of patients with morbid obesity and cirrhosis also in the setting of liver transplantation but scientific evidence is still scarce.
INTRODUCTION
Liver cirrhosis and obesity: Definitions and epidemiology
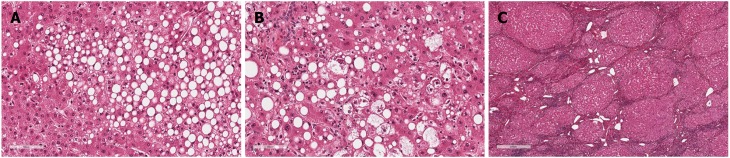
Obesity is associated with many adverse consequences for health and is closely related to insulin resistance, dyslipidemia, and hypertension. Non-alcoholic fatty liver disease (NAFLD) represents the hepatic manifestation of metabolic syndrome and is intimately related to the chronic intrahepatic inflammation that, in turn, is linked to adiposity and insulin resistance. Defined as a fatty infiltration of the liver exceeding 5% at histology in the absence of previous or ongoing significant alcohol consumption[1] and/or drug and/or virus infection, NAFLD includes a wide spectrum of histopathological alterations ranging from simple steatosis (Figure 1A) to non-alcoholic steatohepatitis (NASH) (Figure 1B) and cirrhosis (Figure 1C) with end-stage liver disease. Furthermore, NAFLD has been also shown to increase the risk of primary liver malignancies such as hepatocellular carcinoma (HCC)[2-4]. However, the vast majority of patients with NAFLD will not progress as only a minority of those with NASH (3%-5%)[5] are at a high risk of developing chronic liver disease complications. In patients with NASH, 11% develop cirrhosis and approximately 40% of patients die within 15 years from any cause (of which 7.3% are due to liver-related complications, especially in those with advanced fibrosis or cirrhosis)[6]. Obesity and the chronic inflammation associated with it also have a negative effect on health. Excessive adipose tissue leads to an increased production of adipokines (such as IL-6, TNFa, monocyte chemoattracting protein-1, and plasminogen activator inhibitor-1) with proinflammatory, pro-fibrogenic, pro-angiogenic, and pro-oxidant effects on several tissues. Few data exist regarding the epidemiological and clinical impact of obesity in patients with pre-existent liver disease, however, obesity is considered an independent risk factor for the presence of severe fibrosis, fibrosis progression, and cirrhosis[7,8]. Several population-based studies have identified obesity as an independent risk factor for alcohol-induced liver damage. Indeed, ethanol influences the adipose tissue production of hormones and cytokines, and excess adiposity induces an exacerbation of the proinflammatory state. However, it is still unclear whether the hepatotoxic consequences of obesity and ethanol ingestion are additive or synergistic. The DIONYSOS study clearly showed a synergistic effect between alcohol consumption and elevated body mass index (BMI) on hepatic steatosis in a large cohort of subjects in Northern Italy[9]. The prevalence of hepatic steatosis determined by ultrasonography was increased to 46% in subjects with a daily intake of > 60 g of alcohol and to 76% in patients with obesity compared to lean controls who only revealed hepatic steatosis in 16% of cases. Moreover, in individuals with obesity drinking > 60 g of alcohol per day, steatosis was found at an even higher level of 95% and the relative risk of cirrhosis increased more than six-fold in women with obesity and alcohol consumption (> 150 g/wk) vs normal weight and drinking < 70 g/wk women. Ekstedt et al[10] found an accelerated progression of fibrosis in patients with NAFLD who drank moderate amounts of alcohol (up to 140 g/wk). Liu et al[11] analyzed more than 1 million middle-aged women in the UK and reported that in women with obesity who drank > 150 g of alcohol/week, the relative risk of cirrhosis increased more than six fold.
Figure 1.
Non-alcoholic fatty liver disease and hepatic histopathological alterations. NAFLD includes a wide spectrum of histopathological alterations ranging from simple steatosis (A) to non-alcoholic steatohepatitis (B) and cirrhosis (C). NAFLD: Non-alcoholic fatty liver disease.
In patients with chronic hepatitis C virus (HCV)-related liver disease and severe liver fibrosis or compensated cirrhosis, up to 43% had obesity, and 32% were overweight. Each quartile increase in BMI is associated with a 14% increase in the risk of clinical events in the follow-up (worsening of liver fibrosis or decompensation of cirrhosis over 3.5 years of follow-up)[12]. Among obesity-related variables, the severity of insulin resistance and the histological grade of steatosis appeared as the factors more tightly associated with the progression of liver disease. Contrarily, weight loss is indeed beneficial in patients with advanced chronic liver disease, reducing the progression of fibrosis and cirrhosis[13,14].
Berzigotti et al[13,14] demonstrate that increased BMI is a strong predictor of decompensation in patients with compensated cirrhosis of various etiologies, independent of other previously described predictors such as albumin and portal hypertension. More specifically, clinical decompensation of cirrhosis (ascites, encephalopathy, or jaundice) developed in 14% of patients with normal weight, in 31% of overweight patients, and in 43% of patients with obesity. Obesity also negatively impacts portal hypertension. Indeed, when comparing the results after a 1-year course of timolol or a placebo, portal hypertension was reduced only in patients who were of normal weight or overweight, whereas patients with obesity showed a significant increase.
Even in patients with end-stage liver disease and, therefore, awaiting liver transplantation (LT), obesity could worsen the prognosis. Recent data suggest that in these patients, obesity increases substantially (HR = 13.1), independently and significantly (P = 0.016) the risk of portal vein thrombosis[15], which may render the transplantation technically more difficult and increase the risk of portal thrombosis recurrence. Indeed, obesity is considered as a risk factor for venous thromboembolism as well as thrombosis of the hepatic artery[16]. The proinflammatory, prothrombotic, and hypofibrinolytic milieu patients with obesity may be responsible for the local thrombophilia that favors portal vein thrombosis. Concerning perioperative LT complications, operative time, blood product usage, length of stay in the intensive care unit, infectious complications, and biliary complications requiring intervention have been shown to be higher in recipients with obesity[17]. Despite the increased technical operative challenges and medical complexities associated with recipients with obesity, morbid obesity in itself should not be an absolute contraindication to LT as these patients have reasonable long-term outcomes. Although the complex interplay between obesity and cirrhosis is far from elucidated, undoubtedly alcohol, viral hepatitis, and obesity appear to be a dangerous combination. General impact of obesity on liver pathophysiology are summarized in Table 1.
Table 1.
Impact of obesity on liver pathophysiology
| Pathologies | Obesity |
| Hepatic steatosis[9] | Increased1 |
| Cirrhosis[11] | Increased1 |
| Hepatoxicity[9] | Increased1 |
| Liver primary tumors (as hepatocarcinoma)[2-4] | Increased2 |
| Chronic hepatitis C progression[12] | Increased |
| Decompensation of cirrhotic patients[13,14] | Increased |
| Portal hypertension[13,14] | Increased |
| 3Risk of post-operative complications after LT[15-17] | Increased |
Mostly if associated to alcohol ingestion;
Mostly if associated to non-alcoholic fatty liver disease (NAFLD);
Thromboembolism, infectious and biliary complications. LT: Liver transplantation.
RISK OF SARCOPENIA IN PATIENTS WITH OBESITY AND CIRRHOSIS
Sarcopenia is a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength with a risk of adverse outcomes such as physical disability, poor quality of life, and death[18]. The profound negative clinical impact of low skeletal muscle mass and function was originally described in the elderly, but its role is now unequivocally emerging in many chronic progressive diseases, such as chronic kidney disease, chronic heart failure, chronic pulmonary insufficiency, and type 2 diabetes[18]. Recent evidence suggests that low muscle mass and function may have a similar negative impact in obesity despite the potential difficulties in identifying and defining muscle changes within the obese phenotype[19]. Whatever the definition, the coexistence of sarcopenia and obesity in the same patient, now indicated as “sarcopenic obesity”, confers a risk of physical impairment and disability that is significantly higher than the risk induced by each of the two conditions alone[19-21]. The precise determination of body muscle and body mass fat requires the use of dual energy X-ray absorptiometry (DXA), but an estimation can be obtained at the clinical level with the use of bioimpedance analysis (BIA) or anthropometric measurements[18]. In this section, the role of sarcopenic obesity as a prognostic factor in patients with liver chronic disease will be briefly elucidated. The problem will be analyzed according to two different perspectives. First, the possible role played by sarcopenia as an independent risk factor for the occurrence of liver diseases in patients with obesity will be discussed and, second, the significance of sarcopenia as a negative prognostic marker for disease progression and death in patients with obesity and advanced liver disease will be addressed.
Sarcopenia as an independent risk factor for chronic liver disease in patients with obesity
As already mentioned above, NAFLD is today the most common liver disorder in Western countries and it is becoming the leading cause of chronic liver disease and cirrhosis[22]. Visceral obesity and related metabolic disorders, insulin resistance, in particular, are considered the most relevant risk factors for NAFLD occurrence and progression[22]. Recent evidence suggests, however, that the prevalence of NAFLD and its severity could be independently and negatively affected also by the coexistence of sarcopenia in the clinical picture, with sarcopenic obesity being both an independent risk factor for NAFLD and a marker for its progression to the more advanced stages and cirrhosis[23].
Significant information about the role of sarcopenia in chronic liver disease was produced in an analysis of data from the Korea National Health and Nutrition Examination Survey (KNHANES). KNHANES includes a nationwide cross-sectional cohort with a representative sample of the Korean population and is conducted annually to assess health and nutritional status. Hong et al[24], by analyzing 452 apparently healthy adults in the Korean Obesity Sarcopenic Study, demonstrated a higher risk of NAFLD in individuals with lower muscle mass compared to a control group. Individuals with sarcopenia had more body fat mass, more components of the metabolic syndrome, and higher levels of low-grade systemic inflammation compared with those of people with normal muscle mass. Moreover, the association between sarcopenia retained its significance also after adjustment for all these potential confounding factors[24]. More recently, Lee et al[25] tested the association between sarcopenia and progression of NALFD by analyzing the degree of liver fibrosis in the KNHANES population. NAFLD was identified in 2761 (28.5%) of 9676 subjects and sarcopenia was identified in 337 of the subjects with NAFLD (12.2%). In subjects with NAFLD, sarcopenia was significantly and independently associated with a higher level of liver fibrosis. The authors concluded that sarcopenia was associated with significant liver fibrosis in subjects with NAFLD, and this association was independent of obesity and insulin resistance[25].
The independent and additive role of reduced skeletal muscle mass and increased visceral fat mass in increasing the risk of the onset of NAFLD, and in serving as important factors involved in the progression from simple steatosis to liver fibrosis, has been recently confirmed in an independent Japanese sample[26]. Taking into consideration the limitation of these studies and the caveats in extrapolating these data to the Caucasian population, the association of sarcopenia with visceral obesity and insulin resistance is now considered an important risk factor for NAFLD, which further accelerates its progression to more advanced life-threatening stages.
Sarcopenia as a prognostic negative marker in the cirrhotic patient with obesity
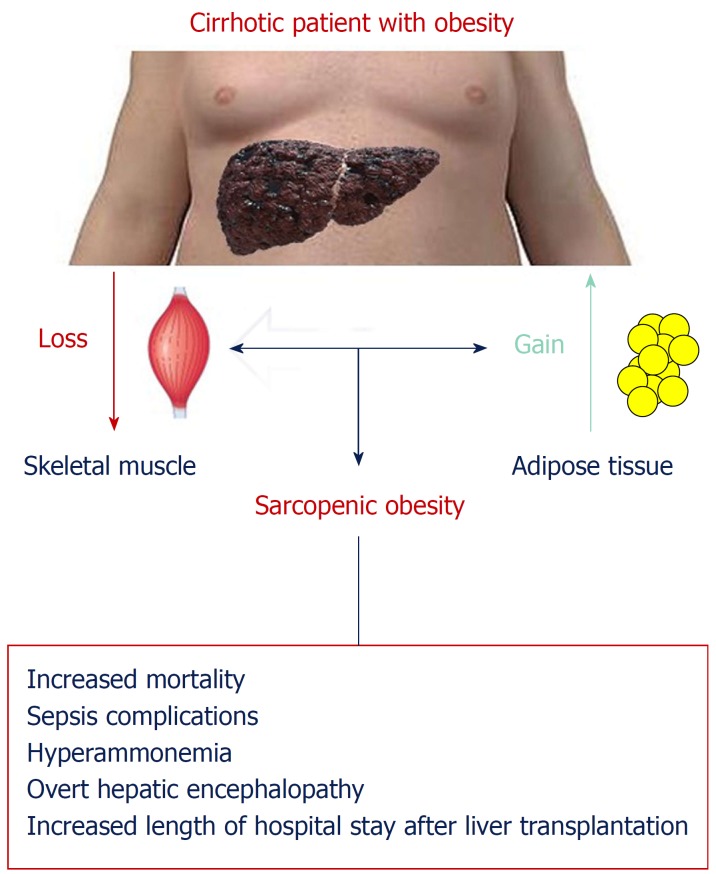
As shown in Figure 2, cirrhotic patients with obesity frequently have a combined loss of skeletal muscle and gain of adipose tissue, culminating in the condition known as “sarcopenic obesity.” Sarcopenia in cirrhotic patients has been associated with increased mortality, sepsis complications, hyperammonemia, overt hepatic encephalopathy (HE), and an increased length of hospital stay after LT[27]. The prognostic impact of the coexistence of sarcopenia and obesity in patients with chronic liver disease has been analyzed recently in a few studies. Montano-Loza et al[28] evaluated the frequencies of sarcopenia and sarcopenic obesity in a cohort of 457 cirrhotic patients evaluated for LT, aiming to establish the impact of these muscular abnormalities on the prognosis of cirrhotic patients. In this sample, sarcopenia was present in 43% and sarcopenic obesity in 20% of patients. Both patients with sarcopenia and with sarcopenic obesity had worse median survival than patients without muscular abnormalities[28]. Hara et al[29] evaluated the independent prognostic impact of skeletal muscle mass and visceral fat accumulation in 161 patients with cirrhosis. Patients with sarcopenia or sarcopenic obesity both had a poor prognosis, and this difference was pronounced in the subset of patients classified as Child-Pugh class A. However, the group with the worst prognosis was represented by patients having sarcopenic obesity[29]. This latter observation seems to confirm that the coexistence of visceral obesity and sarcopenia could be considered the worst clinical situation for a patient with cirrhosis. Muscle wasting and fatty muscle infiltration in cirrhotic patients are part of the frailty complex present in these patients, characterized as decreased reserve and resistance to stressors, resulting from cumulative declines across multiple physiologic systems and predisposition to poor outcomes[28].
Figure 2.
Sarcopenia as a prognostic negative marker in the cirrhotic patient with obesity. Cirrhotic patients with obesity frequently have a combined loss of skeletal muscle and gain of adipose tissue, culminating in the condition known as “sarcopenic obesity”. Sarcopenia in cirrhotic patients has been associated with increased mortality, sepsis complications, hyperammonemia, overt hepatic encephalopathy, and an increased length of hospital stay after liver transplantation.
The prognostic negative role of sarcopenia and obesity could have an impact also in LT. Sarcopenia and sarcopenic obesity are seen in a significant number of patients with cirrhosis undergoing liver transplant evaluation, particularly in patients with NASH[30]. Pre-transplant sarcopenia is widely recognized as associated with short-term survival after living donor LT[31]. The question of whether the coexistence of obesity and sarcopenia could impose additional risk after LT over that imposed by sarcopenia alone is still debated. Hammad et al[32] evaluated 200 patients undergoing adult-to-adult living donor LT and classified them into four subgroups: sarcopenic overweight, sarcopenic non-overweight, non-sarcopenic overweight, and non-sarcopenic non-overweight. Sarcopenic patients had a higher incidence of postoperative bacteremia and major postoperative complications, and poorer overall post-transplant survival than non-sarcopenic patients. Overweight recipients had a significantly higher overall survival rate than non-overweight patients. In contrast, sarcopenic non-overweight subjects had a higher incidence of postoperative bacteremia, and major postoperative complications compared with the sarcopenic overweight subgroup and possessed the poorest overall survival among the four recipient subgroups. Thus, this study indicates that a preoperative sarcopenic overweight status does not confer additional significant morbidity or mortality risks than the stand-alone sarcopenia in living donor LT. The study was however mostly confined to patients who were overweight and does not address specifically the impact of obesity itself[32]. On the other hand, obesity presents important medical and surgical challenges during and after a liver transplant. Specifically, obesity is associated with an increased incidence of wound infections, wound dehiscence, biliary complications, and overall infection[33].
Sarcopenic obesity could represent an important clinical problem also for long-term survival after LT. Sarcopenia tends to persist after transplantation[30,31], possibly as the consequence of insufficient physical exercise or use of immunosuppressive agents that impair skeletal muscle growth and protein accretion[30], whereas body fat normally increased as a result of better nutrition and the regression of the hypermetabolic state related to the pre-transplant chronic disease state[34]. This double phenomenon paves the way to sarcopenic obesity. Schütz et al[35] analyzed body composition in patients after liver and kidney transplantation and patients with liver cirrhosis or on chronic hemodialysis and demonstrated that, despite excellent graft function, many long-term liver or kidney transplant survivors exhibit a phenotype of sarcopenic obesity with increased fat but low muscle mass[35]. Choudhary et al[36] evaluated 82 living donor liver transplant recipients with at least 12 mo of follow-up. Post-transplant sarcopenic obesity was present in 88%, and metabolic syndrome was present in 52% of recipients with no significant difference among liver failure etiologies. Patients with sarcopenic obesity had a significantly higher BMI and significantly higher prevalence of metabolic syndrome when compared to patients without sarcopenic obesity[36]. Thus, the weight gain frequently observed after LT could contribute to the creation of a novel group of patients with sarcopenic obesity and associated metabolic derangements.
MICRONUTRIENT STATUS AND RECOMMENDED SUPPLEMENTATION IN PATIENTS WITH OBESITY AND CIRRHOSIS
Micronutrient deficiencies of fat-soluble and water-soluble vitamins as well as minerals are highly common in end-stage liver disease. Therefore, a periodical nutritional and clinical assessment should be performed to test micronutrient serum levels, estimating adequate nutritional intake and paying attention to clinical signs of vitamins and minerals deficiencies. Fat-soluble vitamin (A, D, E, and K) deficiencies and other free radical scavengers, such as carotenoids are likely to develop in liver disease due to reduced oral intake, malabsorption, and/or hepatic effects involving the reduced synthesis of carrier and transfer proteins, cholestasis and bacterial overgrowth[37,38].
Deficiencies in water-soluble vitamins, leading to neuropsychiatric symptoms, may stem from diminished hepatic storage, inadequate diet, alcoholism, medications, and chronic renal failure[37]. The most prevalent and significant deficiencies are discussed below, beginning with vitamin D deficiency, which is almost universal and also related with obesity.
Vitamin D deficiency (VDD, < 50 nmol/L; < 20 ng/mL) is a worldwide pandemic, reported in more than half of the general adult population[39-42]. Specifically, VDD is widespread among patients with chronic liver disease, with prevalence described between 70%-90%[40,43-49], while the prevalence of vitamin D insufficiency (< 75 nmol/L; < 30 ng/mL) is almost universal[49]. For example, among 202 adults before LT, 84% were 25(OH)D deficient and 77% had low 1,25(OH)2D, whereas 3 mo following transplantation, both increased significantly but to a lesser extent for 1,25(OH)2D[50]. Moreover, the severity of VDD is positively correlated with the severity of liver disease[44-47]. In cirrhotic patients, significant correlations were found between vitamin D levels and the degree of liver dysfunction, whether expressed as Child-Pugh stages A-C, MELD score, or liver stiffness evaluated by transient elastography[44,45]. In epidemiologic studies, VDD has been demonstrated to be a predictor of liver disease morbidity and mortality. In a Danish population-based sample of over 2500 middle-aged subjects followed prospectively for a median follow-up period of 16.5 years, lower serum 25(OH)D levels were independently associated with higher incidence of fatal and non-fatal liver disease[51]. Similarly, in another prospective study with 22 years follow-up[52], those with a higher serum 25(OH) vitamin D had lower chronic liver disease mortality. Among cirrhotic patients, VDD was found to be an independent risk factor for mortality[43,45]. With the increasing prevalence of obesity and NAFLD-related cirrhosis, attention should be paid to the cirrhotic patient with obesity. An association between VDD and obesity has also been suggested. Since vitamin D is a lipophilic molecule it is selectively deposited in subcutaneous and visceral adipose tissue. This sequestration leads to a reduction of its availability for hydroxylation[40,41,53,54]. In addition, an independent association was also observed between VDD and insulin resistance[55,56], the metabolic syndrome as a whole[57], and type 2 diabetes or prediabetes, after adjusting for multiple confounders including BMI[55,56,58,59]. In contrast, some studies failed to show such associations once adiposity is adjusted for[60,61] or any significant association between markers of insulin resistance and 25(OH)D serum levels[62,63]. NAFLD by itself has been suggested to be associated with VDD, but the independence of this association from body fat mass is controversial. The association between a diagnosis of NAFLD and lower serum 25(OH)D levels compared to healthy controls was demonstrated in several studies[64-67], however, NAFLD was biopsy-proven in only two of the studies[64,65]. In addition, significantly higher levels of 25(OH)D were observed among patients with steatosis compared to patients with NASH[64,65]. In partial agreement, another study, describing a sub-analysis of the NASH Clinical Research Network (CRN) cohort, found that VDD was independently associated with NASH and the presence of fibrosis, but not with the degree of steatosis[68]. However, while most studies adjusted for BMI or waist circumference, a study carefully adjusting for adiposity (evaluated by dual-energy X-ray absorptiometry), suggests that there is no relationship between vitamin D levels and the amount of liver fat (by Magnetic Resonance Spectroscopy and liver biopsy) or the severity of NASH[69].
To summarize, a few possible mechanisms could explain VDD among cirrhotic patients with obesity: (1) NAFLD as the undelaying cause of cirrhosis; (2) Sedentary lifestyle or long standing chronic illness leading to reduced exposure to sunlight; (3) Consumption of high-caloric “empty” foods, low in mineral and vitamin content; (4) Increased fat mass which enables sequestration of vitamin D in the adipose tissue compartment thus lowering plasma concentration; and (5) Hepatocellular dysfunction in chronic liver disease which may disturb the formation of the active metabolite of vitamin D as well as vitamin D-binding proteins.
Due to the high prevalence of VDD among cirrhotic patients, especially those with advanced disease, NAFLD, or cholestatic liver diseases[70], it is reasonable to assess serum 25(OH)D levels[71]. The main sources of vitamin D are sunlight exposure, food naturally enriched or artificially fortified with vitamin D, and oral supplements. The adequate sunlight exposure is 5-15 min during the daytime (10:00 AM-15:00 PM) on a daily basis[40]. However, after exposing healthy individuals with obesity (BMI > 30 kg/m2) and matched lean control subjects (BMI < 25 kg/m2) to whole-body ultraviolet radiation, the subjects with obesity showed a 57% lower increase in circulating concentrations of vitamin D3, 24 h after irradiation[53]. Vitamin D3 can be found abundantly in egg yolk and oily fish (e.g. salmon, mackerel, tuna, sardines) and vitamin D2 mainly in mushrooms[40,49]. Industrial products such as bread, margarine, cereals, or milk may be fortified with either vitamin D2 or vitamin D3. Oral supplements may contain vitamin D2 or D3 depending on the manufacturer and brand[40,49]. However, most studies that have evaluated NAFLD and high-dose vitamin D supplementation, failed to show improvement in liver fat, NASH histology, or liver enzymes[72,73]. Oral high-dose vitamin D supplementation administrated to subjects with NAFLD and type-2 diabetes for 24 wk, did not lead to significant change in hepatic steatosis, HOMA-IR, or liver enzymes[74]. Similarly, in a pilot prospective study, administration of high-dose oral vitamin D3 supplementation (25000 IU/wk) for 24 wk to 12 non-cirrhotic patients with biopsy-proven NASH resulted in no significant impact on liver histology, liver enzymes, insulin resistance, or adipocytokine profile[73]. Conversely, some studies, not specific for NAFLD but mostly pre-diabetic patients, demonstrated improvement in insulin sensitivity with vitamin D supplementation[75-77]. Findings are also conflicting with regard to the effect of vitamin D supplementation in the case of advanced liver disease. In a cohort of advanced decompensated cirrhotic patients (Child-Pugh C, score of ≥ 10) randomly assigned to vitamin D treatment or a control receiving standard care and followed for 6 mo, the survival, as well as Child-Pugh and MELD scores, were comparable between the groups[48]. Nevertheless, supplementation of high-dose oral vitamin D resulted in several clinical improvements such as an anti-depressant effect among patients with chronic liver disease (mainly women)[78], and 60% risk reduction for acute rejection among patients after LT[79]. In a Cochrane review of vitamin D supplementation for chronic liver diseases in adults, including 15 randomized clinical trials (RCT) with 1034 participants[80], vitamin D supplementation had no beneficial or harmful effects on all-cause mortality (OR = 0.69; 95%CI: 0.09 to 5.40), and the authors concluded that there is no convincing evidence that vitamin D supplementation has a therapeutic impact in chronic liver disease.
There are no guidelines or clear recommendations regarding vitamin D supplementation for cirrhotic patients with obesity. One authoritative paper[49] recommended vitamin D status assessment in all patients with chronic liver disease and initiation of supplementation with 1000-4000 IU/d of vitamin D3 when 25(OH)D levels are under < 50 nmol/L or < 20 ng/mL. The guidelines of a consensus workshop supported by the British Association for the Study of the Liver and the British Liver Trust, recommends supplementation of 1 g calcium and 800 IU/D vitamin D to patients with chronic liver disease (especially patients with cirrhosis or severe cholestasis) as a general preventive treatment for osteoporosis along with adequate nutrition monitoring[81]. The European Association for the Study of the Liver (EASL) recommendation includes supplementation with calcium (1000-1200 mg/d) and vitamin D (400-800 IU/d) in all patients with cholestatic liver disease for bone loss reduction, although the evidence to support this is low[70]. Interestingly, in a small study of NAFLD patients, daily supplementation with 2000 IU cholecalciferol for 6 mo did not correct hypovitaminosis D in the majority of patients with NASH compared to those with steatosis, thus a higher dose may be needed in NASH or NASH-cirrhosis patients but this finding needs further confirmation[82].
Vitamin E (tocopherol) is an inexpensive and well-tolerated molecule with antioxidant properties. In vitro and animal studies have shown that vitamin E ameliorates liver necrosis and fibrosis[83], and prevents hepatic stellate cell activation[84]. Relevantly for patients with obesity and liver disease, high-dose (800 IU/d) long-term (24 mo) vitamin E treatment among nondiabetic NASH patients led to improvement in NASH versus placebo but not fibrosis. However, cirrhotic patients were excluded from this RCT[85], and thus vitamin E cannot be recommended for NASH-cirrhosis or cryptogenic cirrhosis patients[86]. Vitamin E status, expressed as serum vitamin E/total serum cholesterol ratio, is lower among patients with primary biliary cholangitis and primary sclerosing cholangitis compared to patients with either cryptogenic or alcoholic cirrhosis, probably due to reduced gastrointestinal absorption[87]. Indeed, a reduced concentration of vitamin E and low vitamin E/total cholesterol ratio has been demonstrated in 44% and 64% of patients with primary biliary cholangitis and 32% and 43% of patients with other chronic cholestatic liver diseases, respectively. Serum vitamin E concentration and vitamin E/total cholesterol ratio were restored to normal by oral or intramuscular supplements of the vitamin, but not in patients with severe deficiency of vitamin E (less than 5 μmol/L and less than 1 μmol/mmol total cholesterol)[88]. Generally, carotenoids, as well as tocopherols, are major natural protective agents against free radical-mediated liver damage. Interestingly, it has been demonstrated that patients with cirrhosis (of mixed etiologies) have extremely low hepatic levels of carotenoids and tocopherols, compared with controls, even in the presence of normal serum levels[89]. Similarly, retinol, a-tocopherol, and carotenoid plasma levels in patients with chronic cholestatic liver disease (primary biliary cholangitis and primary sclerosing cholangitis) were demonstrated to be significantly lower compared to the general population, despite comparable nutritional intake, suggesting a role for the malabsorption of fat-soluble vitamins[38]. Clinical manifestations of vitamin E deficiency may include increased platelet aggregation, decreased red blood cell survival, hemolytic anemia, decreased serum creatinine with creatinuria, and neuronal degeneration[37], but these are not common[38,87,88].
Zinc is the second most prevalent trace element in the body, playing a central role in several metabolic, anti-inflammatory and immune response pathways, with more than 300 enzymes having zinc ions within their catalytic domains[90]. Dietary zinc is prevalent in foods which are rich in protein (i.e. red meat, lamb, pork, oysters, etc.) and its absorption can be inhibited by high consumption of foods rich in phytate, certain dietary fibers, and calcium[91,92]. The recommended dietary allowance (RDA) of zinc is 8 mg/d for women and 13 mg/d for men over 19 years old[93]. The liver is the main organ of zinc metabolism, however, in chronic liver disease, metabolism is altered by inadequate dietary intake, protein and amino acid metabolism alterations, diminished hepatic extraction, portosystemic shunts, impaired absorption (mostly in advanced liver disease), and an increase in various inflammatory cytokines[90,94]. Moreover, cirrhosis is a catabolic state resulting in muscle catabolism leading to increased zinc loss in the urine. This urinary loss of zinc is aggravated by the prevalent use of diuretics in order to treat edema and ascites[95]. Zinc deficiency has many clinical implications in cirrhotic patients. One of the major complications in cirrhotic patients is HE, presented in 30%-40% of the patients as overt encephalopathy (OHE) and in 60%-80% as mild cognitive dysfunction (minimal hepatic encephalopathy, MHE)[96]. Zinc deficiency results in impaired nitrogen metabolism due to the reduced enzymatic activity of urea cycle enzymes and decreased muscle glutamine synthesis. Furthermore, zinc levels were found to be negatively correlated with ammonia serum levels[97,98]. These impaired metabolic pathways can be corrected by supplementing zinc. In a small preliminary double-blind, placebo-controlled trial among patients with cirrhosis, hyperammonemia, and hypozincemia, zinc acetate preparation at a dose of 150 mg/d for 3 mo decreased blood ammonia levels[99]. Indeed, Marchesini et al[100] demonstrated in patients with advanced cirrhosis, that long-term treatment with zinc supplementation accelerated and improved hepatic conversion of amino acids to urea compared with matched controls receiving standard treatment. This biochemical improvement was associated with a clinical improvement measured by the performance in psychometric tests and Child-Pugh score. Similar results were observed in an RCT comparing zinc supplementation on top of standard treatment vs. standard treatment alone (protein-restricted diet, branched-chain amino acids, and lactulose) for 6 mo. Zinc supplementation improved the physical component scale and neuropsychological tests and decreased HE grade, Child-Pugh score and blood ammonia levels[101]. A significant improvement in neuropsychological tests and Child-Pugh score along with ammonia was also reported in patients with only MHE after 3 mo of lactulose, antioxidant and zinc therapy vs lactulose therapy alone[102]. Additionally, zinc is a crucial co-factor in the process of wound healing since it activates the synthesis of collagen and metabolism of nucleic acids. Thus, zinc is an essential factor in the restoration of liver parenchyma after liver injury or resection and is required in large amounts over a short period of time[90,103]. In terms of clinical signs for zinc deficiency, skin lesions are very prominent, usually an erythematous rash or scaly plaques. A unique manifestation is necrolytic acral erythema (NAE), associated with zinc deficiency in patients with HCV infection[104], expressed on the dorsal aspects of the feet and extending to the toes. The treatment of NAE must combine oral zinc supplementation and HCV eradication. For more than 70 years, hypozincemia has also been associated with impaired night vision, initially in alcoholic cirrhosis and later on in various liver cirrhosis etiologies[103]. Importantly, one of the disturbing manifestations of zinc deficiency is an alteration in taste and smell. This may further exacerbate malnutrition due to reduced intake in the cirrhotic patient[37,105,106]. According to expert opinion, zinc supplementation can be administrated as a long-term treatment or at least until zinc blood level is within normal range with a recommended dose of 50 mg elemental zinc (220 mg zinc sulfate) once daily, in order to avoid copper malabsorption[37,103].
Magnesium is one of the most prevalent intracellular cations, secondary only to potassium. It has been demonstrated that chronic alcohol consumption impairs magnesium homeostasis affecting the brain, skeletal muscles, heart, and liver[107]. In addition, alcohol abuse can decrease nutritional intake and increase excretion of magnesium by an indirect effect on renal tubules[107]. Other factors that might lead to hypomagnesemia in cirrhotic patients are the poor absorption of magnesium in the distal jejunum, exacerbation of magnesium urinary excretion due to elevated aldosterone, growth hormone, and glucagon blood levels, and chronic administration of loop diuretics[108]. The association between magnesium serum levels and the presence of cirrhosis or its severity is questionable. Two studies have found that magnesium concentration was not significantly different between patients with and without liver cirrhosis and no significant correlation was found with the Child-Pugh score[109,110]. One study found magnesium levels were significantly lower in cirrhotic patients compared to controls, but there was no difference between compensated and decompensated patients[111]. Conversely, another study demonstrated a negative correlation between serum magnesium and Child-Pugh score, and lower levels of magnesium among patients with cirrhosis compared to controls[112]. This disagreement can result from the different disparity of etiologies for the liver disease. Nangliya et al[112] reported that the prevalence of alcoholic liver disease was over 40%, however, Agarwal et al[110] excluded chronic alcohol abuse. Another explanation is that magnesium is a cellular cation and perhaps its serum level does not reflect its true concentration. Koivisto et al[113] argued that serum magnesium concentrations may be normal despite intracellular depletion and that a true estimation of magnesium depletion is through a magnesium loading test, since magnesium uptake is increased in the condition of magnesium depletion. In their study, 10 cirrhotic patients were compared to six healthy controls. No difference was found between pre-loading magnesium levels, but the uptake of magnesium (calculated as the delta between the IV administered dose and the amount excreted in 24-h urine) was more than four-fold higher among cirrhotics compared to controls[113]. Due to scarce data and conflicting evidence, there are no clear clinical practice recommendations regarding magnesium supplementation in cirrhotic patients.
While all patients with chronic liver disease are at risk for depletion of various fat-soluble, water-soluble vitamins and trace elements, the most well-recognized micronutrient deficiencies related to alcohol overuse are vitamin B12 (cyanocobalamin), vitamin A, vitamin D, thiamine (B1), folate (B9), pyridoxine (B6), and zinc[114,115]. Following supplementation, when needed, an annual check-up once stable levels are achieved may be recommended[116]. Administration of B-complex vitamins such as thiamine, folate, and pyridoxine is needed to prevent Wernicke encephalopathy[114], Korsakoff’s syndrome, megaloblastic anaemia and neuropathies[116]. Moreover, and regardless of the etiology of cirrhosis, a consensus paper by the International Society for Hepatic Encephalopathy and Nitrogen Metabolism stated that since vitamin status is not easily assessed and since multivitamin supplementation is cheap and generally safe, use of short-term (2-wk course) oral vitamin supplements could be justified in patients with decompensated cirrhosis or at risk of malnutrition, and clinically apparent vitamin deficiencies should be treated specifically[117].
PROTEIN AND CALORIE INTAKE IN PATIENTS WITH OBESITY AND CIRRHOSIS
Decreased muscle mass and function are more prevalent among cirrhotic patients with either MHE or OHE compared to no HE, and protein malnutrition is an independent risk factor for both OHE (OR = 3.4; 95%CI: 1.4-6.9; P < 0.001) and MHE (OR = 2.15; 95%CI: 1.1-4.1; P = 0.002)[118]. In the late 90s, the recommendations for caloric intake were near-normal for patients with well-compensated liver cirrhosis [25-35 kcal/(kg•d)] and up to 30-40 kcal/(kg•d) in malnourished or critically ill cirrhotic patients[119]. The ESEPEN currently recommends a daily caloric intake of 35-40 kcal/(kg•d) for all cirrhotic patients regardless of their liver disease etiology or degree of liver function compensation[120], without a specific reference for cirrhotic patients with obesity, which may still be malnourished. However, weight loss should be encouraged in patients with obesity and well-compensated cirrhosis, nevertheless, over-restriction will result in endogenous muscle breakdown. Indeed, in later guidelines, Amodio et al[117] recommend careful monitoring alongside increased physical activity and caloric intake of 25-35 kcal/(kg•d) in patients with obesity (30-40 kg/m2) and not less than 20-25 kcal/(kg•d) in patients with morbid obesity (> 40 kg/m2). The recommended dietary pattern is for small, frequent meals evenly distributed throughout the day (every 3-6 h) with a late evening snack containing at least 50 g of complex carbohydrate[117].
The attitude toward daily protein intake has also changed. In the past, the daily protein intake of cirrhotic patients was tightly restricted to less than 0.8 g/(kg•d) due to concerns about the increased risk for HE. However, the tolerance of proteins in cirrhotic patients has been shown to be higher than previously believed[117,121], and the importance of substantial daily protein intake for sustaining adequate muscle mass is becoming more and more clear[117]. Córdoba et al[121] showed that administration of a low-protein diet [0.5 g/(kg•d)] worsened HE and exacerbated protein breakdown compared to a daily protein intake of 1.2 g/(kg•d).
Currently, the recommended daily protein intake is 1.2-1.5 g/(kg•d)[120]. For cirrhotic patients with obesity, a moderately hypocaloric diet must include an adequate amount of proteins [1.2-1.5 g/(kg•d)] in order to accomplish weight loss without muscle or lean mass depletion[117]. In cirrhotic patients, there is variable tolerance to different dietary proteins according to their source. Some uncontrolled studies have shown a better tolerance to vegetable proteins over meat proteins and to dairy proteins over mixed source proteins[122-124]. Interestingly, a 14-d casein-vegetable, high-protein, high-calorie diet was shown to improve mental performance and to decrease ammonia levels in 150 patients with overt HE[125]. The advantages of vegetable proteins may stem from the fact that they are rich in dietary fiber with prebiotic properties, which result in decreased transit time and intraluminal pH leading to increased fecal ammonia excretion. Moreover, vegetable proteins are rich in ornithine and arginine, that can facilitate clearance of ammonia through the urea cycle[126,127]. Vegetable-protein based diets can also be beneficial in terms of caloric and fiber intake among overweight patients with cirrhosis who are attempting to lose weight. In addition, plant-based proteins are rich in branched-chain amino acids (BCAA)[117,128,129]. In hepatic decompensation, there is a shift toward aromatic amino acids (AAA) (phenylalanine, tyrosine, and tryptophan) rather than BCAAs (isoleucine, leucine, and valine), however, AAA are assumed to cross the blood-brain barrier, act as false neurotransmitters, and induce HE[130,131]. BCAA supplementation can induce tolerability to meat protein and enable adequate protein intake[132]. Moreover, substituting meat with dairy or vegetable proteins along with BCAA supplements is better than reducing total proteins intake[117]. In a meta-analysis of 16 RCTs, BCAAs were found to have a beneficial effect on HE compared to placebo or best supportive care (diet, lactulose, or neomycin), however, no conclusions could be drawn regarding nutritional effects[133]. General nutritional recommendations in the cirrhotic patients with obesity are summarized in Table 2.
Table 2.
Nutritional recommendations for cirrhotic patients with obesity
| Nutrient | Recommendation |
| 1Daily energy intake[117] | 25-35 kcal/(kg•d) in patients with BMI 30-40 kg/m2 20-25 kcal/(kg•d) in patients with BMI > 40 kg/m2 |
| 2Protein intake[119] | 1.2-1.5 g/(kg•d) |
| 3Micronutrients | Identify and correct micronutrient deficiencies |
| Fiber | 25-45 g/d |
The recommended dietary pattern is for small, frequent meals evenly distributed throughout the day (every 3-6 h) with a late evening snack containing at least 50 g of complex carbohydrate.
Vegetable-protein based diets can also be beneficial in terms of caloric and fiber intake among overweight patients with cirrhosis who are attempting to lose weight. In addition, plant-based proteins are rich in branched-chain amino acids (BCAA)[117,127,128].
Cirrhotic patients with obesity are at high risk for depletion of various fat-soluble, water-soluble vitamins and trace elements and should be supplemented appropriately. BMI: Body mass index.
BARIATRIC SURGERY-INDUCED WEIGHT LOSS IN PATIENTS WITH OBESITY AND CIRRHOSIS: INDICATIONS AND CONTRAINDICATIONS
In recent years, parallel to the rapid and sharp increase in the prevalence of obesity[134], bariatric surgery has reached a rapid and deep penetration, as it is the only therapeutic means leading to long-term weight loss with consequent improvement of obesity-related comorbidities and patients’ quality of life[135]. NAFLD has become an extremely frequent condition in recent years due to the obesity epidemic[136]. While NAFLD includes a spectrum of histologic features ranging from simple liver steatosis to steatohepatitis (NASH), liver fibrosis has been shown to be the main determinant of mortality linked to NASH in the long-term[137,138]. Obesity has been shown to be associated with an increased risk of primary liver cancer in several large epidemiological studies. Potential mechanisms responsible for this increased risk include the occurrence of NAFLD and type 2 diabetes[139]. Furthermore, there is evidence that the probability of clinical decompensation of liver cirrhosis is significantly increased in the presence of obesity compared to overweight and lean subjects[140].
As a consequence, LT surgeons are faced more and more frequently with candidates for LT with morbid obesity[141]. Traditionally obesity has been considered a main risk factor for postoperative morbidity and mortality in the context of major and complex surgical procedures[142,143]. However, obesity is a heterogeneous disease including different conditions sharing a common denominator represented by an increased BMI. Indeed, there is clear epidemiological evidence that the presence of obesity has a protective effect against postoperative mortality and morbidity in the range of BMI below 35 kg/m2. This phenomenon, known as the obesity paradox, has been attributed to the increased reserve of energy due to obesity that may confer an advantage in a condition of stress such as that after a major surgical procedure[144]. Furthermore, the presence of metabolic syndrome, which is associated with obesity but not obesity alone, is significantly associated with an increased risk of postoperative morbidity and mortality after major abdominal surgery. In spite of this, in many LT centers the presence of obesity, defined only on the basis of a BMI above 35 or 40 kg/m2, is considered as a contraindication to LT[145]. Interestingly, the use of simple diagnostic tools such as anthropometric measures that include the psoas muscle diameter and visceral fat measure coupled with the presence of metabolic comorbidities may help to identify suitable candidates for LT among individuals with obesity without the need for massive weight loss[146,147].
Losing weight before LT remains an important goal but there is no consensus on how best to achieve it, especially in patients with obesity and cirrhosis. Many non-surgical methods can be used to allow patients to reach the desired BMI and be finally listed for LT, including increased physical activity, diet, and behavioral therapy as well as intragastric balloon[33]. Spengler et al[33] recently suggested the use of diet and lifestyle modifications in patients with compensated cirrhosis. For patients with compensated cirrhosis, bariatric surgery has also been proposed in preparation for LT[145-147]. Although this strategy may seem legitimate, bariatric surgery may lead to severe postoperative complications that a patient with a compromised liver function may not tolerate. Indeed, only patients with compensated cirrhosis are potential candidates for bariatric surgery as mortality in patients with decompensated cirrhosis would be inacceptable[148]. There is also evidence that bariatric surgery is associated with a significantly lower morbidity and mortality if an LT program is also present in the same hospital. This underlines the complexity of the patients that need multidisciplinary care from specialists in both hepatology and bariatrics.
The timing of surgery
The timing of bariatric surgery in LT candidates is imperative. While it has been suggested that bariatric surgery-induced weight loss may increase the candidacy for LT, only patients with compensated liver cirrhosis are potential candidates to bariatric surgery because the risk of mortality is too high in the setting of decompensated cirrhosis[148]. The primary indication for LT in patients with a conserved liver function is represented by HCC. While international guidelines for access to bariatric surgery include a disease-free interval of at least five years after the radical treatment of any malignancy, a different strategy to access a curative treatment such as LT for this specific category of patients seems to be legitimate. Only patients with the best chances of long-term survival should then be selected for this ambitious therapeutic strategy, which includes bariatric surgery to lose weight and control metabolic comorbidities followed by LT. Endovascular and/or percutaneous interventional radiology techniques should be concomitantly used to obtain temporary local control of HCC. Several preoperative scores such as the alpha-fetoprotein score[149], the Milan criteria, and Metroticket 2.0 may be useful tools to guide the selection of patients in order to obtain long-term survival rates after LT comparable to those obtained for patients with non-tumoral disease[150].
The option of performing bariatric surgery after LT offers several advantages including normal liver function with normal prothrombin time, the restoration of normal portal pressure, the possibility to optimize the nutritional status and the choice of procedure in relation to the presence or absence of metabolic syndrome. The presence of adhesions linked to transplantation is not a formal contraindication to the laparoscopic approach nor is the use of immunosuppressive drugs[151].
As obesity recurs often after LT, affecting the graft with NASH recurrence and patient survival with metabolic comorbidities, some authors evoked the seducing possibility of performing a bariatric procedure, namely sleeve gastrectomy (SG), at the same time as the LT[152]. However, a panel of argument still stands against this policy, these include: the potentially disastrous consequences of the most feared complication of SG, a leak at the top of the staple line in the setting of immunosuppressive treatment in a freshly transplanted patient; the reduced amount of calories that the transplanted patient would be able to assume with the diet spontaneously for several months after surgery; the impact of the two procedures on the body composition with the risk of excessive fatty free mass loss.
The choice of procedure
The presence of portal hypertension may render any bariatric procedure difficult and lead to life-threatening bleeding. The choice of the procedure should then take into account the risk of bleeding. A transjugular intrahepatic portosystemic shunt (TIPS) may be considered in patients with signs of portal hypertension such as platelets below 100000, a large spleen on CT scan, the presence of collateral circulation on the abdomen or on CT scan and esophageal varices. In case of doubt, a direct measure of the portal pressure should be obtained. It should be considered that in the case of large spontaneous portosystemic shunts (larger than 1 cm), the TIPS might be inefficacious. The possibility to access the biliary tree endoscopically after LT should also be considered. Procedures with an intestinal bypass definitely limit the endoscopic access to the bile duct. Furthermore, some patients may need a biliodigestive reconstruction on a Roux-en-Y loop that should be then fashioned in addition to the intestinal bypass of the bariatric procedure. There is a risk of portal vein thrombosis in any abdominal procedure and bariatric surgery has been shown to increase this risk. The potential risk of liver complications due to the intestinal bypass is another point that needs attention. It is well recognized that bariatric procedures including an intestinal bypass may lead to liver failure. Although the mechanisms underlying this complication have not been completely elucidated, the abnormal intestinal microbiota derived from bacterial overgrowth may cause intestinal permeability defects that expose hosts to noxious gut-derived factors such as bacterial lipopolysaccharide, other toll-like receptor ligands, and toxic bile acids[153]. Interestingly, most, if not all, cases of liver failure reported in the literature include patients with a biliopancreatic diversion, in which the length of the bypassed intestine is very long, and the jejuno-ileal bypass, whereas only a few cases of liver failure in patients with Roux-en-Y gastric bypass (RYGP) have been reported[154]. The jejuno-ileal bypass was proscribed several decades ago and nowadays the Scopinaro’s procedure is rarely performed. This is in line with the hypothesis that an altered microbiota may be a major determinant of increased intestinal permeability as the more distal is the bypass the more altered is the intestinal flora.
The absorption of immunosuppressive drugs should also be considered when choosing the most appropriate bariatric procedure. The absorption of drugs may not only be altered by the presence of an intestinal bypass but also by the changes in the intestinal pH linked to the suppression of hydrochloric acid secretion[155]. Finally, the etiology of liver disease (namely NASH vs viral infections and alcohol abuse), the presence of metabolic syndrome, and NASH recurrence on the graft in cases of patients that have already been transplanted are key factors to consider when choosing the type of bariatric procedure.
The three most common bariatric procedures currently performed worldwide are laparoscopic adjustable gastric banding (LAGB), the SG, and RYGP. Several factors are involved in the choice of the bariatric procedure in the setting of LT (Table 3). Although the LAGB has the advantage of being the easiest procedure with the lowest risk of bleeding, it also has the worse results in terms of weight loss with the highest rate of failure. Furthermore, the presence of foreign material around the mesogastric junction, especially in patients with esophageal varices, may cause problems. The SG is currently the most performed procedure[156,157] that offers several advantages, including the fact that no intestinal bypass is done, leaving the whole digestive tract accessible to endoscopic exploration, avoiding intestinal bypass related interference with the absorption of immunosuppressive drugs, and leaving the possibility to fashion a Roux-en-Y loop in the event of biliary complications after LT or in cases where a biliodigestive reconstruction is indicated such as in primary biliary cholangitis. However, in cases of portal hypertension, the division of the greater curvature vessels may expose to the risk of bleeding especially if portal pressure is increased and no TIPS has been put in place. Bleeding may be then difficult to control and should be feared as a life-threatening complication. The use of buttressing material for the division of the stomach may reduce significantly the risk of bleeding in these patients and should be used[158].
Table 3.
Main factors involved in the choice of the bariatric procedure in the setting of liver transplantation
| Factors to consider | SG | RYGB |
| Bleeding risk of bleeding1 | Increased | Low |
| Endoscopic access to the biliary tree | Conserved | Impossible |
| Risk of portal vein thrombosis | Increased | Not affected |
| Risk of bariatric surgery induced liver failure | Absent | Low |
| Absorption of immunosuppressive drugs | Poorly affected | Decreased |
| Etiology of liver cirrhosis (NASH vs others) | Effective | Very Effective |
Consider measuring of portal pressure and use of transjugular intrahepatic portosystemic shunt in case of bariatric surgery before liver transplantation. SG: Sleeve gastrectomy; RYGP: Roux-en-Y gastric bypass; NASH: Non-alcoholic steato hepatitis.
Of note is also the fact that the SG is followed by an extreme decrease in hydrochloric acid secretion, resulting in an altered pH, which may consequently interfere with immunosuppressive drug absorption[155]. Although short and medium-term results of SG on weight loss and control of metabolic syndrome indicate equivalent efficacy between the SG and the RYGP[159], long-term results of SG are still too scarce to drive definite conclusions on the equivalence of efficacy between the two procedures, especially in a NASH setting[160].
The RYGP has the advantage of a reduced risk of bleeding, as only the lesser curvature of the stomach, where bleeding may occur, needs to be dissected before gastric division. It is also more effective against metabolic complications of obesity that may be either present before or after LT[161]. Strong evidence in support of the preventive effect of RYGP against the occurrence of type 2 diabetes has come from several studies[162,163]. Recently Safwan et al[164] reported a series of 11 patients with a previous history of bariatric surgery that underwent LT and showed that biliary complications could be successfully addressed with a second Roux-en-Y loop in patients with an RYGP. One additional technical point concerns the use of the endoluminal tube to guide the shaping of the gastric pouch or sleeve. If the preoperative endoscopy has shown the presence of esophageal varices, in spite of appropriate endoscopic eradication, the orogastric tube may eventually injure the varices causing bleeding. For this reason, it should be better avoided as far as possible especially in patients with a low prothrombin time and platelet count. SG is also associated with an increased risk of portal thrombosis compared to RYGP in non-cirrhotic patients[165]. This may be a further concern, as liver cirrhosis is a risk factor for portal thrombosis in itself. The occurrence of portal thrombosis may further complicate the access to LT. The risk of dumping syndrome and hyperinsulinemic hypoglycemia may be further concerns of the RYGP.
In conclusion, with all of the potential that bariatric surgery has candidates to LT with morbid obesity, well-designed studies are still necessary to gain widespread acceptance from clinicians. Several points need to be clarified, including the most appropriate methods for accurate patient evaluation and selection, the optimal surgical procedure and its timing to maximize the efficiency of bariatric surgery in liver recipients with obesity. Only when this research is furthered to the point where the effectiveness of bariatric surgery is no longer in doubt, the latter will be included in the care of liver recipients with obesity for mainstream use. In the meantime, the policy of a case-by-case discussion involving a multidisciplinary team, including hepatologists and both LT and bariatric surgeons, seems to be justified.
CONCLUSION
With the recent epidemic of obesity, the coexistence of liver cirrhosis and obesity has become very frequent. The complex interplay between obesity and the liver especially in the setting of liver cirrhosis is a formidable challenge for current medicine that goes from the prevention of liver complications of obesity, screening for these complications in patients at risk to the management of patients with sarcopenia and end-stage liver disease. Bariatric surgery has shown promising results although evidence is still scarce.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest. No financial support.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 4, 2018
First decision: May 30, 2018
Article in press: June 25, 2018
P- Reviewer: Giorgio A, Grassi A, Karatapanis S, Manesis EK, McMillin MA, Ocker M, Romanelli RG S- Editor: Gong ZM L- Editor: A E- Editor: Yin SY
Contributor Information
Luigi Schiavo, Department of Translational Medical Science, University of Campania “Luigi Vanvitelli”, Naples 80131, Italy; IX Division of General Surgery, Vascular Surgery and Applied Biotechnology, Naples University Policlinic, Naples 80131, Italy.
Luca Busetto, Department of Medicine, University of Padua, Padua 35128, Italy; Center for the Study and the Integrated Management of Obesity, University Hospital of Padua, Padua 35128, Italy.
Manuela Cesaretti, Department of HPB Surgery and Liver Transplantation, Hôpital Beaujon, AP-HP, Clichy 92110, France; Department of Nanophysics, Italian Institute of Technology, Genova 16163, Italy.
Shira Zelber-Sagi, School of Public Health, University of Haifa, Haifa 3498838, Israel; Department of Gastroenterology and Liver disease, Tel Aviv Medical Center, 62431, Tel-Aviv 62431, Israel.
Liat Deutsch, Department of Gastroenterology and Liver disease, Tel Aviv Medical Center, 62431, Tel-Aviv 62431, Israel; The Sackler Faculty of Medicine, Tel-Aviv University, Tel-Aviv 62431, Israel.
Antonio Iannelli, Digestive Unit, Archet 2 Hospital, University Hospital of Nice, F-06202, Nice, France; Inserm, U1065, Team 8 “Hepatic complications of obesity”, Nice F-06204, France; University of Nice Sophia-Antipolis, Nice F-06107, France. iannelli.a@chu-nice.fr.
References
- 1.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 2.Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463–471. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 4.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 5.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 6.Angulo P. Long-term mortality in nonalcoholic fatty liver disease: is liver histology of any prognostic significance? Hepatology. 2010;51:373–375. doi: 10.1002/hep.23521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, Naveau S. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635–638. doi: 10.1053/jhep.2002.31782. [DOI] [PubMed] [Google Scholar]
- 8.Ortiz V, Berenguer M, Rayón JM, Carrasco D, Berenguer J. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97:2408–2414. doi: 10.1111/j.1572-0241.2002.05995.x. [DOI] [PubMed] [Google Scholar]
- 9.Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 10.Ekstedt M, Franzén LE, Holmqvist M, Bendtsen P, Mathiesen UL, Bodemar G, Kechagias S. Alcohol consumption is associated with progression of hepatic fibrosis in non-alcoholic fatty liver disease. Scand J Gastroenterol. 2009;44:366–374. doi: 10.1080/00365520802555991. [DOI] [PubMed] [Google Scholar]
- 11.Liu B, Balkwill A, Reeves G, Beral V; Million Women Study Collaborators. Body mass index and risk of liver cirrhosis in middle aged UK women: prospective study. BMJ. 2010;340:c912. doi: 10.1136/bmj.c912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everhart JE, Lok AS, Kim HY, Morgan TR, Lindsay KL, Chung RT, Bonkovsky HL, Ghany MG; HALT-C Trial Group. Weight-related effects on disease progression in the hepatitis C antiviral long-term treatment against cirrhosis trial. Gastroenterology. 2009;137:549–557. doi: 10.1053/j.gastro.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berzigotti A, Abraldes JG. Impact of obesity and insulin-resistance on cirrhosis and portal hypertension. Gastroenterol Hepatol. 2013;36:527–533. doi: 10.1016/j.gastrohep.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Berzigotti A, Albillos A, Villanueva C, Genescá J, Ardevol A, Augustín S, Calleja JL, Bañares R, García-Pagán JC, Mesonero F, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology. 2017;65:1293–1305. doi: 10.1002/hep.28992. [DOI] [PubMed] [Google Scholar]
- 15.Ayala R, Grande S, Bustelos R, Ribera C, García-Sesma A, Jimenez C, Moreno E, Martínez-López J. Obesity is an independent risk factor for pre-transplant portal vein thrombosis in liver recipients. BMC Gastroenterol. 2012;12:114. doi: 10.1186/1471-230X-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allman-Farinelli MA. Obesity and venous thrombosis: a review. Semin Thromb Hemost. 2011;37:903–907. doi: 10.1055/s-0031-1297369. [DOI] [PubMed] [Google Scholar]
- 17.LaMattina JC, Foley DP, Fernandez LA, Pirsch JD, Musat AI, D’Alessandro AM, Mezrich JD. Complications associated with liver transplantation in the obese recipient. Clin Transplant. 2012;26:910–918. doi: 10.1111/j.1399-0012.2012.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolland Y, Lauwers-Cances V, Cristini C, Abellan van Kan G, Janssen I, Morley JE, Vellas B. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l’OSteoporose) Study. Am J Clin Nutr. 2009;89:1895–1900. doi: 10.3945/ajcn.2008.26950. [DOI] [PubMed] [Google Scholar]
- 20.Koster A, Ding J, Stenholm S, Caserotti P, Houston DK, Nicklas BJ, You T, Lee JS, Visser M, Newman AB, et al. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci. 2011;66:888–895. doi: 10.1093/gerona/glr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beavers KM, Hsu FC, Houston DK, Beavers DP, Harris TB, Hue TF, Kim LJ, Koster A, Penninx BW, Simonsick EM, et al. The role of metabolic syndrome, adiposity, and inflammation in physical performance in the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2013;68:617–623. doi: 10.1093/gerona/gls213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Obes Facts. 2016;9:65–90. doi: 10.1159/000443344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tovo CV, Fernandes SA, Buss C, de Mattos AA. Sarcopenia and non-alcoholic fatty liver disease: Is there a relationship? A systematic review. World J Hepatol. 2017;9:326–332. doi: 10.4254/wjh.v9.i6.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology. 2014;59:1772–1778. doi: 10.1002/hep.26716. [DOI] [PubMed] [Google Scholar]
- 25.Lee YH, Kim SU, Song K, Park JY, Kim DY, Ahn SH, Lee BW, Kang ES, Cha BS, Han KH. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008-2011) Hepatology. 2016;63:776–786. doi: 10.1002/hep.28376. [DOI] [PubMed] [Google Scholar]
- 26.Shida T, Akiyama K, Oh S, Sawai A, Isobe T, Okamoto Y, Ishige K, Mizokami Y, Yamagata K, Onizawa K, et al. Skeletal muscle mass to visceral fat area ratio is an important determinant affecting hepatic conditions of non-alcoholic fatty liver disease. J Gastroenterol. 2018;53:535–547. doi: 10.1007/s00535-017-1377-3. [DOI] [PubMed] [Google Scholar]
- 27.Anand AC. Nutrition and Muscle in Cirrhosis. J Clin Exp Hepatol. 2017;7:340–357. doi: 10.1016/j.jceh.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montano-Loza AJ, Angulo P, Meza-Junco J, Prado CM, Sawyer MB, Beaumont C, Esfandiari N, Ma M, Baracos VE. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7:126–135. doi: 10.1002/jcsm.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hara N, Iwasa M, Sugimoto R, Mifuji-Moroka R, Yoshikawa K, Terasaka E, Hattori A, Ishidome M, Kobayashi Y, Hasegawa H, et al. Sarcopenia and Sarcopenic Obesity Are Prognostic Factors for Overall Survival in Patients with Cirrhosis. Intern Med. 2016;55:863–870. doi: 10.2169/internalmedicine.55.5676. [DOI] [PubMed] [Google Scholar]
- 30.Carias S, Castellanos AL, Vilchez V, Nair R, Dela Cruz AC, Watkins J, Barrett T, Trushar P, Esser K, Gedaly R. Nonalcoholic steatohepatitis is strongly associated with sarcopenic obesity in patients with cirrhosis undergoing liver transplant evaluation. J Gastroenterol Hepatol. 2016;31:628–633. doi: 10.1111/jgh.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaido T, Tamai Y, Hamaguchi Y, Okumura S, Kobayashi A, Shirai H, Yagi S, Kamo N, Hammad A, Inagaki N, et al. Effects of pretransplant sarcopenia and sequential changes in sarcopenic parameters after living donor liver transplantation. Nutrition. 2017;33:195–198. doi: 10.1016/j.nut.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Hammad A, Kaido T, Hamaguchi Y, Okumura S, Kobayashi A, Shirai H, Kamo N, Yagi S, Uemoto S. Impact of sarcopenic overweight on the outcomes after living donor liver transplantation. Hepatobiliary Surg Nutr. 2017;6:367–378. doi: 10.21037/hbsn.2017.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spengler EK, O’Leary JG, Te HS, Rogal S, Pillai AA, Al-Osaimi A, Desai A, Fleming JN, Ganger D, Seetharam A, et al. Liver Transplantation in the Obese Cirrhotic Patient. Transplantation. 2017;101:2288–2296. doi: 10.1097/TP.0000000000001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richards J, Gunson B, Johnson J, Neuberger J. Weight gain and obesity after liver transplantation. Transpl Int. 2005;18:461–466. doi: 10.1111/j.1432-2277.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 35.Schütz T, Hudjetz H, Roske AE, Katzorke C, Kreymann G, Budde K, Fritsche L, Neumayer HH, Lochs H, Plauth M. Weight gain in long-term survivors of kidney or liver transplantation--another paradigm of sarcopenic obesity? Nutrition. 2012;28:378–383. doi: 10.1016/j.nut.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Choudhary NS, Saigal S, Saraf N, Mohanka R, Rastogi A, Goja S, Menon PB, Mishra S, Mittal A, Soin AS. Sarcopenic obesity with metabolic syndrome: a newly recognized entity following living donor liver transplantation. Clin Transplant. 2015;29:211–215. doi: 10.1111/ctr.12505. [DOI] [PubMed] [Google Scholar]
- 37.Johnson TM, Overgard EB, Cohen AE, DiBaise JK. Nutrition assessment and management in advanced liver disease. Nutr Clin Pract. 2013;28:15–29. doi: 10.1177/0884533612469027. [DOI] [PubMed] [Google Scholar]
- 38.Floreani A, Baragiotta A, Martines D, Naccarato R, D’odorico A. Plasma antioxidant levels in chronic cholestatic liver diseases. Aliment Pharmacol Ther. 2000;14:353–358. doi: 10.1046/j.1365-2036.2000.00729.x. [DOI] [PubMed] [Google Scholar]
- 39.Hansen KE, Johnson MG. An update on vitamin D for clinicians. Curr Opin Endocrinol Diabetes Obes. 2016;23:440–444. doi: 10.1097/MED.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stokes CS, Volmer DA, Grünhage F, Lammert F. Vitamin D in chronic liver disease. Liver Int. 2013;33:338–352. doi: 10.1111/liv.12106. [DOI] [PubMed] [Google Scholar]
- 41.Lim LY, Chalasani N. Vitamin d deficiency in patients with chronic liver disease and cirrhosis. Curr Gastroenterol Rep. 2012;14:67–73. doi: 10.1007/s11894-011-0231-7. [DOI] [PubMed] [Google Scholar]
- 42.Targher G, Byrne CD. Lower 25-hydroxyvitamin D3 levels and increased risk of liver diseases: is there a causal link? Endocrine. 2014;47:3–4. doi: 10.1007/s12020-014-0220-3. [DOI] [PubMed] [Google Scholar]
- 43.Stokes CS, Krawczyk M, Reichel C, Lammert F, Grünhage F. Vitamin D deficiency is associated with mortality in patients with advanced liver cirrhosis. Eur J Clin Invest. 2014;44:176–183. doi: 10.1111/eci.12205. [DOI] [PubMed] [Google Scholar]
- 44.Paternostro R, Wagner D, Reiberger T, Mandorfer M, Schwarzer R, Ferlitsch M, Trauner M, Peck-Radosavljevic M, Ferlitsch A. Low 25-OH-vitamin D levels reflect hepatic dysfunction and are associated with mortality in patients with liver cirrhosis. Wien Klin Wochenschr. 2017;129:8–15. doi: 10.1007/s00508-016-1127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Putz-Bankuti C, Pilz S, Stojakovic T, Scharnagl H, Pieber TR, Trauner M, Obermayer-Pietsch B, Stauber RE. Association of 25-hydroxyvitamin D levels with liver dysfunction and mortality in chronic liver disease. Liver Int. 2012;32:845–851. doi: 10.1111/j.1478-3231.2011.02735.x. [DOI] [PubMed] [Google Scholar]
- 46.Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010;55:2624–2628. doi: 10.1007/s10620-009-1069-9. [DOI] [PubMed] [Google Scholar]
- 47.Fisher L, Fisher A. Vitamin D and parathyroid hormone in outpatients with noncholestatic chronic liver disease. Clin Gastroenterol Hepatol. 2007;5:513–520. doi: 10.1016/j.cgh.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 48.Jha AK, Jha SK, Kumar A, Dayal VM, Jha SK. Effect of replenishment of vitamin D on survival in patients with decompensated liver cirrhosis: A prospective study. World J Gastrointest Pathophysiol. 2017;8:133–141. doi: 10.4291/wjgp.v8.i3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitson MT, Roberts SK. D-livering the message: the importance of vitamin D status in chronic liver disease. J Hepatol. 2012;57:897–909. doi: 10.1016/j.jhep.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 50.Reese PP, Bloom RD, Feldman HI, Huverserian A, Thomasson A, Shults J, Hamano T, Goral S, Shaked A, Olthoff K, et al. Changes in vitamin D binding protein and vitamin D concentrations associated with liver transplantation. Liver Int. 2012;32:287–296. doi: 10.1111/j.1478-3231.2011.02638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skaaby T, Husemoen LL, Borglykke A, Jørgensen T, Thuesen BH, Pisinger C, Schmidt LE, Linneberg A. Vitamin D status, liver enzymes, and incident liver disease and mortality: a general population study. Endocrine. 2014;47:213–220. doi: 10.1007/s12020-013-0107-8. [DOI] [PubMed] [Google Scholar]
- 52.Wang JB, Abnet CC, Chen W, Dawsey SM, Fan JH, Yin LY, Yin J, Major JM, Taylor PR, Qiao YL, et al. Association between serum 25(OH) vitamin D, incident liver cancer and chronic liver disease mortality in the Linxian Nutrition Intervention Trials: a nested case-control study. Br J Cancer. 2013;109:1997–2004. doi: 10.1038/bjc.2013.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 54.Stein EM, Strain G, Sinha N, Ortiz D, Pomp A, Dakin G, McMahon DJ, Bockman R, Silverberg SJ. Vitamin D insufficiency prior to bariatric surgery: risk factors and a pilot treatment study. Clin Endocrinol (Oxf) 2009;71:176–183. doi: 10.1111/j.1365-2265.2008.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 56.Ganji V, Zhang X, Shaikh N, Tangpricha V. Serum 25-hydroxyvitamin D concentrations are associated with prevalence of metabolic syndrome and various cardiometabolic risk factors in US children and adolescents based on assay-adjusted serum 25-hydroxyvitamin D data from NHANES 2001-2006. Am J Clin Nutr. 2011;94:225–233. doi: 10.3945/ajcn.111.013516. [DOI] [PubMed] [Google Scholar]
- 57.Barchetta I, De Bernardinis M, Capoccia D, Baroni MG, Fontana M, Fraioli A, Morini S, Leonetti F, Cavallo MG. Hypovitaminosis D is independently associated with metabolic syndrome in obese patients. PLoS One. 2013;8:e68689. doi: 10.1371/journal.pone.0068689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shankar A, Sabanayagam C, Kalidindi S. Serum 25-hydroxyvitamin d levels and prediabetes among subjects free of diabetes. Diabetes Care. 2011;34:1114–1119. doi: 10.2337/dc10-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knekt P, Laaksonen M, Mattila C, Härkänen T, Marniemi J, Heliövaara M, Rissanen H, Montonen J, Reunanen A. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology. 2008;19:666–671. doi: 10.1097/EDE.0b013e318176b8ad. [DOI] [PubMed] [Google Scholar]
- 60.Kayaniyil S, Retnakaran R, Harris SB, Vieth R, Knight JA, Gerstein HC, Perkins BA, Zinman B, Hanley AJ. Prospective associations of vitamin D with β-cell function and glycemia: the PROspective Metabolism and ISlet cell Evaluation (PROMISE) cohort study. Diabetes. 2011;60:2947–2953. doi: 10.2337/db11-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grimnes G, Emaus N, Joakimsen RM, Figenschau Y, Jenssen T, Njølstad I, Schirmer H, Jorde R. Baseline serum 25-hydroxyvitamin D concentrations in the Tromsø Study 1994-95 and risk of developing type 2 diabetes mellitus during 11 years of follow-up. Diabet Med. 2010;27:1107–1115. doi: 10.1111/j.1464-5491.2010.03092.x. [DOI] [PubMed] [Google Scholar]
- 62.Lamendola CA, Ariel D, Feldman D, Reaven GM. Relations between obesity, insulin resistance, and 25-hydroxyvitamin D. Am J Clin Nutr. 2012;95:1055–1059. doi: 10.3945/ajcn.111.032060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rajakumar K, de las Heras J, Lee S, Holick MF, Arslanian SA. 25-hydroxyvitamin D concentrations and in vivo insulin sensitivity and β-cell function relative to insulin sensitivity in black and white youth. Diabetes Care. 2012;35:627–633. doi: 10.2337/dc11-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, Arcaro G. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007;17:517–524. doi: 10.1016/j.numecd.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 65.Dasarathy J, Periyalwar P, Allampati S, Bhinder V, Hawkins C, Brandt P, Khiyami A, McCullough AJ, Dasarathy S. Hypovitaminosis D is associated with increased whole body fat mass and greater severity of non-alcoholic fatty liver disease. Liver Int. 2014;34:e118–e127. doi: 10.1111/liv.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jablonski KL, Jovanovich A, Holmen J, Targher G, McFann K, Kendrick J, Chonchol M. Low 25-hydroxyvitamin D level is independently associated with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2013;23:792–798. doi: 10.1016/j.numecd.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barchetta I, Angelico F, Del Ben M, Baroni MG, Pozzilli P, Morini S, Cavallo MG. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med. 2011;9:85. doi: 10.1186/1741-7015-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nelson JE, Roth CL, Wilson LA, Yates KP, Aouizerat B, Morgan-Stevenson V, Whalen E, Hoofnagle A, Mason M, Gersuk V, et al. Vitamin D Deficiency Is Associated With Increased Risk of Non-alcoholic Steatohepatitis in Adults With Non-alcoholic Fatty Liver Disease: Possible Role for MAPK and NF-κB? Am J Gastroenterol. 2016;111:852–863. doi: 10.1038/ajg.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bril F, Maximos M, Portillo-Sanchez P, Biernacki D, Lomonaco R, Subbarayan S, Correa M, Lo M, Suman A, Cusi K. Relationship of vitamin D with insulin resistance and disease severity in non-alcoholic steatohepatitis. J Hepatol. 2015;62:405–411. doi: 10.1016/j.jhep.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 70.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 71.Leslie WD, Bernstein CN, Leboff MS; American Gastroenterological Association Clinical Practice Commitee. AGA technical review on osteoporosis in hepatic disorders. Gastroenterology. 2003;125:941–966. doi: 10.1016/s0016-5085(03)01062-x. [DOI] [PubMed] [Google Scholar]
- 72.Barchetta I, Cimini FA, Cavallo MG. Vitamin D Supplementation and Non-Alcoholic Fatty Liver Disease: Present and Future. Nutrients. 2017;9 doi: 10.3390/nu9091015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kitson MT, Pham A, Gordon A, Kemp W, Roberts SK. High-dose vitamin D supplementation and liver histology in NASH. Gut. 2016;65:717–718. doi: 10.1136/gutjnl-2015-310417. [DOI] [PubMed] [Google Scholar]
- 74.Barchetta I, Del Ben M, Angelico F, Di Martino M, Fraioli A, La Torre G, Saulle R, Perri L, Morini S, Tiberti C, et al. No effects of oral vitamin D supplementation on non-alcoholic fatty liver disease in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. BMC Med. 2016;14:92. doi: 10.1186/s12916-016-0638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med. 2009;26:19–27. doi: 10.1111/j.1464-5491.2008.02636.x. [DOI] [PubMed] [Google Scholar]
- 76.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient - a randomised, placebo-controlled trial. Br J Nutr. 2010;103:549–555. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 77.Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, Manson JE, Hu FB. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29:650–656. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- 78.Stokes CS, Grünhage F, Baus C, Volmer DA, Wagenpfeil S, Riemenschneider M, Lammert F. Vitamin D supplementation reduces depressive symptoms in patients with chronic liver disease. Clin Nutr. 2016;35:950–957. doi: 10.1016/j.clnu.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 79.Bitetto D, Fabris C, Falleti E, Fornasiere E, Fumolo E, Fontanini E, Cussigh A, Occhino G, Baccarani U, Pirisi M, et al. Vitamin D and the risk of acute allograft rejection following human liver transplantation. Liver Int. 2010;30:417–444. doi: 10.1111/j.1478-3231.2009.02154.x. [DOI] [PubMed] [Google Scholar]
- 80.Bjelakovic G, Nikolova D, Bjelakovic M, Gluud C. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2017;11:CD011564. doi: 10.1002/14651858.CD011564.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Collier JD, Ninkovic M, Compston JE. Guidelines on the management of osteoporosis associated with chronic liver disease. Gut. 2002;50 Suppl 1:i1–i9. doi: 10.1136/gut.50.suppl_1.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dasarathy J, Varghese R, Feldman A, Khiyami A, McCullough AJ, Dasarathy S. Patients with Nonalcoholic Fatty Liver Disease Have a Low Response Rate to Vitamin D Supplementation. J Nutr. 2017;147:1938–1946. doi: 10.3945/jn.117.254292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parola M, Leonarduzzi G, Biasi F, Albano E, Biocca ME, Poli G, Dianzani MU. Vitamin E dietary supplementation protects against carbon tetrachloride-induced chronic liver damage and cirrhosis. Hepatology. 1992;16:1014–1021. doi: 10.1002/hep.1840160426. [DOI] [PubMed] [Google Scholar]
- 84.Houglum K, Venkataramani A, Lyche K, Chojkier M. A pilot study of the effects of d-alpha-tocopherol on hepatic stellate cell activation in chronic hepatitis C. Gastroenterology. 1997;113:1069–1073. doi: 10.1053/gast.1997.v113.pm9322499. [DOI] [PubMed] [Google Scholar]
- 85.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 87.Muñoz SJ, Heubi JE, Balistreri WF, Maddrey WC. Vitamin E deficiency in primary biliary cirrhosis: gastrointestinal malabsorption, frequency and relationship to other lipid-soluble vitamins. Hepatology. 1989;9:525–531. doi: 10.1002/hep.1840090403. [DOI] [PubMed] [Google Scholar]
- 88.Jeffrey GP, Muller DP, Burroughs AK, Matthews S, Kemp C, Epstein O, Metcalfe TA, Southam E, Tazir-Melboucy M, Thomas PK. Vitamin E deficiency and its clinical significance in adults with primary biliary cirrhosis and other forms of chronic liver disease. J Hepatol. 1987;4:307–317. doi: 10.1016/s0168-8278(87)80539-1. [DOI] [PubMed] [Google Scholar]
- 89.Leo MA, Rosman AS, Lieber CS. Differential depletion of carotenoids and tocopherol in liver disease. Hepatology. 1993;17:977–986. [PubMed] [Google Scholar]
- 90.Grüngreiff K, Reinhold D, Wedemeyer H. The role of zinc in liver cirrhosis. Ann Hepatol. 2016;15:7–16. doi: 10.5604/16652681.1184191. [DOI] [PubMed] [Google Scholar]
- 91.Ma J, Betts NM. Zinc and copper intakes and their major food sources for older adults in the 1994-96 continuing survey of food intakes by individuals (CSFII) J Nutr. 2000;130:2838–2843. doi: 10.1093/jn/130.11.2838. [DOI] [PubMed] [Google Scholar]
- 92.Huskisson E, Maggini S, Ruf M. The role of vitamins and minerals in energy metabolism and well-being. J Int Med Res. 2007;35:277–289. doi: 10.1177/147323000703500301. [DOI] [PubMed] [Google Scholar]
- 93.Gibson RS, King JC, Lowe N. A Review of Dietary Zinc Recommendations. Food Nutr Bull. 2016;37:443–460. doi: 10.1177/0379572116652252. [DOI] [PubMed] [Google Scholar]
- 94.Himoto T, Masaki T. Associations between Zinc Deficiency and Metabolic Abnormalities in Patients with Chronic Liver Disease. Nutrients. 2018;10 doi: 10.3390/nu10010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chiba M, Katayama K, Takeda R, Morita R, Iwahashi K, Onishi Y, Kita H, Nishio A, Kanno T, Saito T, et al. Diuretics aggravate zinc deficiency in patients with liver cirrhosis by increasing zinc excretion in urine. Hepatol Res. 2013;43:365–373. doi: 10.1111/j.1872-034X.2012.01093.x. [DOI] [PubMed] [Google Scholar]
- 96.Nusrat S, Khan MS, Fazili J, Madhoun MF. Cirrhosis and its complications: evidence based treatment. World J Gastroenterol. 2014;20:5442–5460. doi: 10.3748/wjg.v20.i18.5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Van der Rijt CC, Schalm SW, Schat H, Foeken K, De Jong G. Overt hepatic encephalopathy precipitated by zinc deficiency. Gastroenterology. 1991;100:1114–1118. doi: 10.1016/0016-5085(91)90290-2. [DOI] [PubMed] [Google Scholar]
- 98.Rabbani P, Prasad AS. Plasma ammonia and liver ornithine transcarbamoylase activity in zinc-deficient rats. Am J Physiol. 1978;235:E203–E206. doi: 10.1152/ajpendo.1978.235.2.E203. [DOI] [PubMed] [Google Scholar]
- 99.Katayama K, Saito M, Kawaguchi T, Endo R, Sawara K, Nishiguchi S, Kato A, Kohgo H, Suzuki K, Sakaida I, et al. Effect of zinc on liver cirrhosis with hyperammonemia: a preliminary randomized, placebo-controlled double-blind trial. Nutrition. 2014;30:1409–1414. doi: 10.1016/j.nut.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 100.Marchesini G, Fabbri A, Bianchi G, Brizi M, Zoli M. Zinc supplementation and amino acid-nitrogen metabolism in patients with advanced cirrhosis. Hepatology. 1996;23:1084–1092. doi: 10.1053/jhep.1996.v23.pm0008621138. [DOI] [PubMed] [Google Scholar]
- 101.Takuma Y, Nouso K, Makino Y, Hayashi M, Takahashi H. Clinical trial: oral zinc in hepatic encephalopathy. Aliment Pharmacol Ther. 2010;32:1080–1090. doi: 10.1111/j.1365-2036.2010.04448.x. [DOI] [PubMed] [Google Scholar]
- 102.Mousa N, Abdel-Razik A, Zaher A, Hamed M, Shiha G, Effat N, Elbaz S, Elhelaly R, Hafez M, El-Wakeel N, et al. The role of antioxidants and zinc in minimal hepatic encephalopathy: a randomized trial. Therap Adv Gastroenterol. 2016;9:684–691. doi: 10.1177/1756283X16645049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mohammad MK, Zhou Z, Cave M, Barve A, McClain CJ. Zinc and liver disease. Nutr Clin Pract. 2012;27:8–20. doi: 10.1177/0884533611433534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tabibian JH, Gerstenblith MR, Tedford RJ, Junkins-Hopkins JM, Abuav R. Necrolytic acral erythema as a cutaneous marker of hepatitis C: report of two cases and review. Dig Dis Sci. 2010;55:2735–2743. doi: 10.1007/s10620-010-1273-7. [DOI] [PubMed] [Google Scholar]
- 105.Pimentel CF, Lai M. Nutrition Interventions for Chronic Liver Diseases and Nonalcoholic Fatty Liver Disease. Med Clin North Am. 2016;100:1303–1327. doi: 10.1016/j.mcna.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 106.Tandon P, Raman M, Mourtzakis M, Merli M. A practical approach to nutritional screening and assessment in cirrhosis. Hepatology. 2017;65:1044–1057. doi: 10.1002/hep.29003. [DOI] [PubMed] [Google Scholar]
- 107.Romani AM. Magnesium homeostasis and alcohol consumption. Magnes Res. 2008;21:197–204. [PubMed] [Google Scholar]
- 108.Cohen L. Magnesium and liver cirrhosis: a hypothesis. Magnesium. 1985;4:1–4. [PubMed] [Google Scholar]
- 109.Rahelić D, Kujundzić M, Romić Z, Brkić K, Petrovecki M. Serum concentration of zinc, copper, manganese and magnesium in patients with liver cirrhosis. Coll Antropol. 2006;30:523–528. [PubMed] [Google Scholar]
- 110.Agarwal A, Avarebeel S, Choudhary NS, Goudar M, Tejaswini CJ. Correlation of Trace Elements in Patients of Chronic Liver Disease with Respect to Child- Turcotte- Pugh Scoring System. J Clin Diagn Res. 2017;11:OC25–OC28. doi: 10.7860/JCDR/2017/26519.10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kar K, Dasgupta A, Vijaya Bhaskar M, Sudhakar K. Alteration of micronutrient status in compensated and decompensated liver cirrhosis. Indian J Clin Biochem. 2014;29:232–237. doi: 10.1007/s12291-013-0349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nangliya V, Sharma A, Yadav D, Sunder S, Nijhawan S, Mishra S. Study of trace elements in liver cirrhosis patients and their role in prognosis of disease. Biol Trace Elem Res. 2015;165:35–40. doi: 10.1007/s12011-015-0237-3. [DOI] [PubMed] [Google Scholar]
- 113.Koivisto M, Valta P, Höckerstedt K, Lindgren L. Magnesium depletion in chronic terminal liver cirrhosis. Clin Transplant. 2002;16:325–328. doi: 10.1034/j.1399-0012.2002.01141.x. [DOI] [PubMed] [Google Scholar]
- 114.Chao A, Waitzberg D, de Jesus RP, Bueno AA, Kha V, Allen K, Kappus M, Medici V. Malnutrition and Nutritional Support in Alcoholic Liver Disease: a Review. Curr Gastroenterol Rep. 2016;18:65. doi: 10.1007/s11894-016-0539-4. [DOI] [PubMed] [Google Scholar]
- 115.O'Shea RS, Dasarathy S, McCullough AJ; Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 116.Rossi RE, Conte D, Massironi S. Diagnosis and treatment of nutritional deficiencies in alcoholic liver disease: Overview of available evidence and open issues. Dig Liver Dis. 2015;47:819–825. doi: 10.1016/j.dld.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 117.Amodio P, Bemeur C, Butterworth R, Cordoba J, Kato A, Montagnese S, Uribe M, Vilstrup H, Morgan MY. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology. 2013;58:325–336. doi: 10.1002/hep.26370. [DOI] [PubMed] [Google Scholar]
- 118.Merli M, Giusto M, Lucidi C, Giannelli V, Pentassuglio I, Di Gregorio V, Lattanzi B, Riggio O. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis. 2013;28:281–284. doi: 10.1007/s11011-012-9365-z. [DOI] [PubMed] [Google Scholar]
- 119.Plauth M, Merli M, Kondrup J, Weimann A, Ferenci P, Müller MJ; ESPEN Consensus Group. ESPEN guidelines for nutrition in liver disease and transplantation. Clin Nutr. 1997;16:43–55. doi: 10.1016/s0261-5614(97)80022-2. [DOI] [PubMed] [Google Scholar]
- 120.Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J; DGEM (German Society for Nutritional Medicine), Ferenci P, Holm E, Vom Dahl S, Müller MJ, Nolte W; ESPEN (European Society for Parenteral and Enteral Nutrition) ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr. 2006;25:285–294. doi: 10.1016/j.clnu.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 121.Córdoba J, López-Hellín J, Planas M, Sabín P, Sanpedro F, Castro F, Esteban R, Guardia J. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41:38–43. doi: 10.1016/j.jhep.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 122.BESSMAN AN, MIRICK GS. Blood ammonia levels following the ingestion of casein and whole blood. J Clin Invest. 1958;37:990–998. doi: 10.1172/JCI103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fenton JC, Knight EJ, Humpherson PL. Milk-and-cheese diet in portal-systemic encephalopathy. Lancet. 1966;1:164–166. doi: 10.1016/s0140-6736(66)90696-9. [DOI] [PubMed] [Google Scholar]
- 124.Greenberger NJ, Carley J, Schenker S, Bettinger I, Stamnes C, Beyer P. Effect of vegetable and animal protein diets in chronic hepatic encephalopathy. Am J Dig Dis. 1977;22:845–855. doi: 10.1007/BF01076158. [DOI] [PubMed] [Google Scholar]
- 125.Gheorghe L, Iacob R, Vădan R, Iacob S, Gheorghe C. Improvement of hepatic encephalopathy using a modified high-calorie high-protein diet. Rom J Gastroenterol. 2005;14:231–238. [PubMed] [Google Scholar]
- 126.Uribe M, Dibildox M, Malpica S, Guillermo E, Villallobos A, Nieto L, Vargas F, Garcia Ramos G. Beneficial effect of vegetable protein diet supplemented with psyllium plantago in patients with hepatic encephalopathy and diabetes mellitus. Gastroenterology. 1985;88:901–907. doi: 10.1016/s0016-5085(85)80006-8. [DOI] [PubMed] [Google Scholar]
- 127.Weber FL Jr, Minco D, Fresard KM, Banwell JG. Effects of vegetable diets on nitrogen metabolism in cirrhotic subjects. Gastroenterology. 1985;89:538–544. doi: 10.1016/0016-5085(85)90448-2. [DOI] [PubMed] [Google Scholar]
- 128.Uribe M, Márquez MA, Garcia Ramos G, Ramos-Uribe MH, Vargas F, Villalobos A, Ramos C. Treatment of chronic portal--systemic encephalopathy with vegetable and animal protein diets. A controlled crossover study. Dig Dis Sci. 1982;27:1109–1116. doi: 10.1007/BF01391449. [DOI] [PubMed] [Google Scholar]
- 129.Shekib LA, Zoueil ME, Youssef MM, Mohamed SW. Amino acid composition and In Vitro digestibility of lentil and rice proteins and their mixture (Koshary) Food Chem. 1986;20:61–67. [Google Scholar]
- 130.Silk DB. Branched chain amino acids in liver disease: fact or fantasy? Gut. 1986;27 Suppl 1:103–110. doi: 10.1136/gut.27.suppl_1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fischer JE, Baldessarini RJ. False neurotransmitters and hepatic failure. Lancet. 1971;2:75–80. doi: 10.1016/s0140-6736(71)92048-4. [DOI] [PubMed] [Google Scholar]
- 132.Dam G, Ott P, Aagaard NK, Vilstrup H. Branched-chain amino acids and muscle ammonia detoxification in cirrhosis. Metab Brain Dis. 2013;28:217–220. doi: 10.1007/s11011-013-9377-3. [DOI] [PubMed] [Google Scholar]
- 133.Gluud LL, Dam G, Les I, Marchesini G, Borre M, Aagaard NK, Vilstrup H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;5:CD001939. doi: 10.1002/14651858.CD001939.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric Surgery Worldwide 2013. Obes Surg. 2015;25:1822–1832. doi: 10.1007/s11695-015-1657-z. [DOI] [PubMed] [Google Scholar]
- 136.Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377:2063–2072. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- 137.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389–97.e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 139.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. Lancet. 2014;384:755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Watt KD. Reducing the load: the evolution and management of obesity and nonalcoholic steatohepatitis before liver transplantation. Liver Transpl. 2012;18 Suppl 2:S52–S58. doi: 10.1002/lt.23515. [DOI] [PubMed] [Google Scholar]
- 141.Iannelli A. Bariatric surgery and liver transplant. Liver Transpl. 2017;23:1369–1370. doi: 10.1002/lt.24948. [DOI] [PubMed] [Google Scholar]
- 142.Saab S, Lalezari D, Pruthi P, Alper T, Tong MJ. The impact of obesity on patient survival in liver transplant recipients: a meta-analysis. Liver Int. 2015;35:164–170. doi: 10.1111/liv.12431. [DOI] [PubMed] [Google Scholar]
- 143.Perez-Protto SE, Quintini C, Reynolds LF, You J, Cywinski JB, Sessler DI, Miller C. Comparable graft and patient survival in lean and obese liver transplant recipients. Liver Transpl. 2013;19:907–915. doi: 10.1002/lt.23680. [DOI] [PubMed] [Google Scholar]
- 144.Karagozian R, Bhardwaj G, Wakefield DB, Baffy G. Obesity paradox in advanced liver disease: obesity is associated with lower mortality in hospitalized patients with cirrhosis. Liver Int. 2016;36:1450–1456. doi: 10.1111/liv.13137. [DOI] [PubMed] [Google Scholar]
- 145.Terjimanian MN, Harbaugh CM, Hussain A, Olugbade KO Jr, Waits SA, Wang SC, Sonnenday CJ, Englesbe MJ. Abdominal adiposity, body composition and survival after liver transplantation. Clin Transplant. 2016;30:289–294. doi: 10.1111/ctr.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Shirai H, Yagi S, Kamo N, Okajima H, Uemoto S. Impact of Skeletal Muscle Mass Index, Intramuscular Adipose Tissue Content, and Visceral to Subcutaneous Adipose Tissue Area Ratio on Early Mortality of Living Donor Liver Transplantation. Transplantation. 2017;101:565–574. doi: 10.1097/TP.0000000000001587. [DOI] [PubMed] [Google Scholar]
- 147.Lazzati A, Iannelli A, Schneck AS, Nelson AC, Katsahian S, Gugenheim J, Azoulay D. Bariatric surgery and liver transplantation: a systematic review a new frontier for bariatric surgery. Obes Surg. 2015;25:134–142. doi: 10.1007/s11695-014-1430-8. [DOI] [PubMed] [Google Scholar]
- 148.Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:897–901. doi: 10.1016/j.cgh.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 149.Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, Compagnon P, Vanlemmens C, Dumortier J, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986–94.e3; quiz e14-15. doi: 10.1053/j.gastro.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 150.Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, Cescon M, Di Sandro S, Yi-Feng H, Lauterio A, et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology. 2018;154:128–139. doi: 10.1053/j.gastro.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 151.Al-Nowaylati AR, Al-Haddad BJ, Dorman RB, Alsaied OA, Lake JR, Chinnakotla S, Slusarek BM, Sampson BK, Ikramuddin S, Buchwald H, et al. Gastric bypass after liver transplantation. Liver Transpl. 2013;19:1324–1329. doi: 10.1002/lt.23734. [DOI] [PubMed] [Google Scholar]
- 152.Heimbach JK, Watt KD, Poterucha JJ, Ziller NF, Cecco SD, Charlton MR, Hay JE, Wiesner RH, Sanchez W, Rosen CB, et al. Combined liver transplantation and gastric sleeve resection for patients with medically complicated obesity and end-stage liver disease. Am J Transplant. 2013;13:363–368. doi: 10.1111/j.1600-6143.2012.04318.x. [DOI] [PubMed] [Google Scholar]
- 153.Prakash G, Drenick EJ, Wexler H, DeLucia L, Finegold SM. Microbial flora in the bypassed jejunum of patients with biliopancreatic bypass for obesity. Am J Clin Nutr. 1987;46:273–276. doi: 10.1093/ajcn/46.2.273. [DOI] [PubMed] [Google Scholar]
- 154.D'Albuquerque LA, Gonzalez AM, Wahle RC, de Oliveira Souza E, Mancero JM, de Oliveira e Silva A. Liver transplantation for subacute hepatocellular failure due to massive steatohepatitis after bariatric surgery. Liver Transpl. 2008;14:881–885. doi: 10.1002/lt.21472. [DOI] [PubMed] [Google Scholar]
- 155.Rogers CC, Alloway RR, Alexander JW, Cardi M, Trofe J, Vinks AA. Pharmacokinetics of mycophenolic acid, tacrolimus and sirolimus after gastric bypass surgery in end-stage renal disease and transplant patients: a pilot study. Clin Transplant. 2008;22:281–291. doi: 10.1111/j.1399-0012.2007.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Clapp B, Wynn M, Martyn C, Foster C, O’Dell M, Tyroch A. Long term (7 or more years) outcomes of the sleeve gastrectomy: a meta-analysis. Surg Obes Relat Dis. 2018;14:741–747. doi: 10.1016/j.soard.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 157.Noel P, Nedelcu M, Eddbali I, Manos T, Gagner M. What are the long-term results 8 years after sleeve gastrectomy? Surg Obes Relat Dis. 2017;13:1110–1115. doi: 10.1016/j.soard.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 158.Berger ER, Clements RH, Morton JM, Huffman KM, Wolfe BM, Nguyen NT, Ko CY, Hutter MM. The Impact of Different Surgical Techniques on Outcomes in Laparoscopic Sleeve Gastrectomies: The First Report from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) Ann Surg. 2016;264:464–473. doi: 10.1097/SLA.0000000000001851. [DOI] [PubMed] [Google Scholar]
- 159.Peterli R, Wölnerhanssen BK, Vetter D, Nett P, Gass M, Borbély Y, Peters T, Schiesser M, Schultes B, Beglinger C, et al. Laparoscopic Sleeve Gastrectomy Versus Roux-Y-Gastric Bypass for Morbid Obesity-3-Year Outcomes of the Prospective Randomized Swiss Multicenter Bypass Or Sleeve Study (SM-BOSS) Ann Surg. 2017;265:466–473. doi: 10.1097/SLA.0000000000001929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schneck AS, Anty R, Patouraux S, Bonnafous S, Rousseau D, Lebeaupin C, Bailly-Maitre B, Sans A, Tran A, Gugenheim J, et al. Roux-En Y Gastric Bypass Results in Long-Term Remission of Hepatocyte Apoptosis and Hepatic Histological Features of Non-alcoholic Steatohepatitis. Front Physiol. 2016;7:344. doi: 10.3389/fphys.2016.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Geerts A, Darius T, Chapelle T, Roeyen G, Francque S, Libbrecht L, Nevens F, Pirenne J, Troisi R. The multicenter Belgian survey on liver transplantation for hepatocellular failure after bariatric surgery. Transplant Proc. 2010;42:4395–4398. doi: 10.1016/j.transproceed.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 162.Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219–234. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 163.Adams TD, Davidson LE, Litwin SE, Kim J, Kolotkin RL, Nanjee MN, Gutierrez JM, Frogley SJ, Ibele AR, Brinton EA, et al. Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N Engl J Med. 2017;377:1143–1155. doi: 10.1056/NEJMoa1700459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Safwan M, Collins KM, Abouljoud MS, Salgia R. Outcome of liver transplantation in patients with prior bariatric surgery. Liver Transpl. 2017;23:1415–1421. doi: 10.1002/lt.24832. [DOI] [PubMed] [Google Scholar]
- 165.Shaheen O, Siejka J, Thatigotla B, Pham DT. A systematic review of portomesenteric vein thrombosis after sleeve gastrectomy. Surg Obes Relat Dis. 2017;13:1422–1431. doi: 10.1016/j.soard.2017.03.015. [DOI] [PubMed] [Google Scholar]