Abstract
Neurological disorders, such as spinal cord injury, stroke, traumatic brain injury, cerebral palsy, and multiple sclerosis cause motor impairments that are a huge burden at the individual, family, and societal levels. Spinal reflex abnormalities contribute to these impairments. Spinal reflex measurements play important roles in characterizing and monitoring neurological disorders and their associated motor impairments, such as spasticity, which affects nearly half of those with neurological disorders. Spinal reflexes can also serve as therapeutic targets themselves. Operant conditioning protocols can target beneficial plasticity to key reflex pathways; they can thereby trigger wider plasticity that improves impaired motor skills, such as locomotion. These protocols may complement standard therapies such as locomotor training and enhance functional recovery. This paper reviews the value of spinal reflexes and the therapeutic promise of spinal reflex operant conditioning protocols; it also considers the complex process of translating this promise into clinical reality.
Electronic supplementary material
The online version of this article (10.1007/s13311-018-0643-2) contains supplementary material, which is available to authorized users.
Key Words: Spinal reflex, H-reflex, clinical translation, operant conditioning, plasticity, rehabilitation, neurological disorders.
Introduction
Neurological disorders—including spinal cord injury (SCI), stroke, traumatic brain injury (TBI), cerebral palsy, and multiple sclerosis (MS)—affect many millions of people in the USA and throughout the world (e.g., Table 1). These disorders disrupt the brain’s influence on the spinal cord, producing abnormal spinal reflexes that impair motor control. Reflex abnormalities limit mobility (e.g., walking), disrupt sleep, and can cause pain and fatigue [3, 7, 8]. They contribute to spasticity and contractures. Spasticity is one of the most common sequelae of neurological disorders, and a major contributor to functional loss [9–13].
Table 1.
Estimated prevalence and incidence of some neurological disorders in the USA. Note that prevalence and incidence vary across studies [1]
Currently, the treatment of motor impairments, such as spasticity, includes rehabilitation (physical and occupational therapy [14]) and pharmacological interventions [15–20]. Examples of treatments based on rehabilitation are therapeutic exercise [21], stretching [21, 22], and mobility and gait training [22, 23]. These functional training regimes may include devising new strategies to accommodate motor impairments; and assistive devices may be prescribed to support achievement of patients’ functional goals. Pharmacological solutions, such as antispasmodics (e.g., baclofen) and neurotoxins (e.g., botulinum toxin) aim to relieve the symptoms of spasticity by reducing muscle activity [15–20].
However, despite treatment functional mobility and quality of life (QoL) often do not return to pre-injury states or even to functionally useful levels (e.g., a walking speed that enables participation in the community [24–26]). This begs the question: what is the current barrier to effective treatment of spasticity and other motor abnormalities associated with many neurological disorders? The answer, in part, is that current rehabilitation does not target abnormal reflexes.
Reflexes play a crucial role in the control of muscle tone at rest and during movement [27–30]; thus, reflex abnormalities are an important factor in motor impairments [31]. Current understanding of the role of reflexes and the consequences of their abnormalities is based on more than a century of research, much of which has focused on the H-reflex (or Hoffmann reflex [32, 33]). The H-reflex is a spinally mediated response to nerve stimulation. It is commonly described as the electrical analog of the spinal stretch reflex (e.g., the knee jerk reflex), which is produced by a wholly spinal and largely monosynaptic pathway [28]. H-reflex size indicates the excitability of this reflex pathway. The pathway’s excitability reflects the current state of the spinal motoneurons and of the afferent synapses on the motoneurons that are activated by the nerve stimulation [28]. Thus, the H-reflex has long been, and continues to be, a valuable tool for investigating mechanisms of neuromotor control and for elucidating neuromotor impairments [27–30, 34, 35]. For example, in people with SCI, brain control of reflex pathways is impaired [31, 36–38] (i.e., supraspinal connections to the spinal cord are disrupted). This often results in exaggerated stretch reflexes (i.e., hyperreflexia) [31, 36] that impair motor behaviors such as walking. For example, the normal modulation of reflex excitability during walking (e.g., reduced excitability during the swing phase) may disappear, resulting in foot drop, clonus, and other abnormalities [31, 36].
Over the last several decades, the H-reflex has acquired further scientific and clinical importance with the development and exploitation of operant conditioning protocols that can modify spinal reflex pathways. These protocols are among the first of a powerful new class of noninvasive therapies that can target beneficial plasticity (i.e., neuronal and/or synaptic changes that improve important functions such as locomotion) to critical sites in the central nervous system (CNS) (e.g., [39]). Using immediate visual feedback to guide brain activity, they can, for example, change spinal reflex pathways so as to decrease spasticity and restore more normal motor function [37, 40–42]. Spinal reflex operant conditioning has been demonstrated to improve locomotion in studies of animals with incomplete SCI [39, 43] and in several small studies of people with incomplete SCI [41, 44]; other applications are being explored. By targeting beneficial plasticity to an important reflex pathway, operant conditioning protocols trigger wider plasticity that markedly improves important motor functions such as locomotion [39, 41]. Thus, these protocols have the potential to improve the treatment of motor impairments due to neurological disorders; they should be able to complement other therapies and enhance functional recovery.
The therapeutic impact of spinal reflex conditioning on people with neurological disorders hinges on aligning research to support clinical translation [45]. Clinical translation is the process of transitioning from theory, basic science, and mechanistic studies to (in the case of spinal reflex conditioning) a robust, clinically practical, operant conditioning system and protocol. Clinical research oriented toward clinical translation and potentially commercialization is critical to bridging this gap. The key questions for clinical translation reflect many factors, including particularly the market requirements—the needs and wants of those who will buy and use it: rehabilitation clinics, therapists, payors, and the patients themselves. Attention to these factors, coupled with technology development to support clinical research, leads to product development, a business plan, and, ideally, to eventual commercialization and widespread clinical use. This whole process is iterative: clinical research drives further market research and vice versa; and these, in turn, drive further business model and technology development, which can lead to further clinical evaluation.
In this paper, we review current and potential future uses of H-reflex operant conditioning for treatment of motor impairments related to neurological injury or disease. To begin, we first describe the H-reflex in more detail. Specifically, we summarize the procedure for its elicitation, discuss the measurement of the reflex, and summarize insights into nervous system control of muscular function derived from its measurement. This sets the stage for reviewing H-reflex operant conditioning protocols, the steps needed for their successful clinical translation, and the work necessary to accomplish these steps.
The H-Reflex
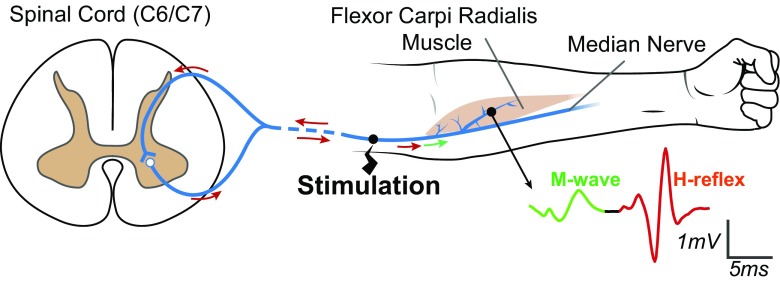
The H-reflex is a spinally mediated, largely monosynaptic, response to nerve stimulation that was discovered a century ago by Hoffmann [32, 33]. Its size (usually measured by electromyography (EMG)) reflects the excitability of a spinal stretch reflex pathway. The pathway itself consists of group I (and large group II) afferents from muscle spindles (and Golgi tendon organs) that project monosynaptically (and to some extent di- and tri-synaptically) to spinal α-motoneurons, and the motoneurons, which activate the muscle [27–30, 42, 46] (Fig. 1). The H-reflex is elicited in a muscle by electrical stimulation of the nerve that innervates it. In humans, this is achieved noninvasively using surface skin electrodes (i.e., transcutaneous). The size of the H-reflex changes with the parameters of stimulation. As stimulus strength increases, the H-reflex increases to a maximum (Hmax) and then declines as the recruitment of more and more (and eventually all) efferent axons into the M-wave prevents them from participating in the H-reflex (i.e., recruitment curves; see [28, 30, 34]).
Fig. 1.
H-reflex and M-wave pathways for the wrist flexor muscle flexor carpi radialis (FCR). The median nerve is stimulated by a short (0.5-ms) pulse at a current just above M-wave threshold, resulting in two electromyographic (EMG) responses in the FCR. The first is the direct muscle response (M-wave), produced by excitation of a few large α-motoneuron axons (green). The second is the spinally mediated H-reflex, produced by excitation of the largest proprioceptive afferent axons (red)
The H-reflexes of a variety of different muscles have been elicited and studied [28, 30] (Table 2). The ability to elicit the H-reflex is affected by the current level of muscle contraction, the surface accessibility of the nerve, neuromotor pathology, and other factors. For example, an H-reflex can be elicited from the wrist flexor, flexor carpi radialis (FCR), when the muscle is at rest; in contrast, the forearm flexor, brachoradialis, produces an H-reflex only when the muscle is active [28, 30].
Table 2.
A sample of peripheral nerves, the muscles they innervate, and selected studies of their H-reflexes. (See also [27, 28, 30, 34])
| Nerve | Muscle |
|---|---|
| Tibial (posterior) | Soleus*, gastrocnemius [47–50], flexor digitorum brevis [51], semitendinosus [50, 52], abductor hallucis [53, 54] |
| Femoral | Quadriceps (vastus lateralis, rectus femoris, vastus medialis) [55–59] |
| Sciatic | Biceps femoris [50, 60, 61] |
| Peroneal | Peroneus longus [62–66], tibialis anterior [50, 67] |
| Median | Abductor pollicis brevis [68–71], flexor carpi radialis [50, 71–79], flexor digitorum superficialis [50, 68, 80] |
| Ulnar | Abductor digiti minimi [50, 69, 81], flexor carpi ulnaris [50, 81] |
| Radial | Extensor carpi radialis [71, 82], brachioradialis [50, 83, 84], extensor digitorum communis [67, 81] |
| Cervical (C3/C4) | Trapezius [85–87] |
| Musculocutaneous | Biceps brachii [81, 88] |
*Many studies have examined the soleus H-reflex ([27] for review)
H-reflex measures capture the task-dependent nature of reflex pathways [27]. Reflex gain (i.e., H-reflex size at a specific level of ongoing muscle contraction) is lower in standing than in sitting [29, 89–92], lower still during running [89, 93–95]; athletes, such as dancers, show significantly lower H-reflexes during standing but not during sitting [92, 96]. Thus, H-reflex measures help to understand the role reflexes play in movement, and to quantify the effects of reflex abnormalities due to neurological disorders. H-reflex size and latency are recommended (e.g., by Cigna Medical Coverage [97]) for clinical assessments of neuropathies [28, 77, 98] and radiculopathies [28, 61, 99–103]. For example, unilateral radiculopathies can be detected by comparing the H-reflex sizes and latencies on the asymptomatic and symptomatic sides [28, 101–103]. In bilateral radiculopathies, normative values can be used to identify abnormalities [28, 77]. In Fisher’s and in Guillain-Barre syndrome, the H-reflex is typically absent [104]. Thus, the H-reflex is part of an ensemble of methods, including the F-wave, imaging (e.g., CT scan), and patient history, that, together, aid in the diagnosis and ongoing assessment of these conditions (see [61] for example).
Decades of research has revealed the value of H-reflex measures to characterize reflex pathways in stroke [27, 82, 105–110], dystonia [111, 112], periodic movement disorders [113, 114], Parkinson’s disease [115], and cerebral palsy [116–120]. In people with SCI, for example, changes in H-reflex size and latency evolve from early injury (i.e., the period of spinal shock) to the onset of chronic hyperreflexia [121, 122]. People with spastic hyperreflexia due to SCI exhibit less H-reflex decrease from sitting to standing [93], and the H-reflex is inappropriately elevated during the swing phase of locomotion [31, 36, 37, 41]. The latter pattern, also observed in stroke, contributes to locomotor impairments such as clonus and foot drop [123].
H-reflex measures have also been used in defining the effects of therapeutic interventions on reflex pathways (e.g., baclofen [124–127], botulinum toxin [128, 129], locomotor training [130–133], cycling [134, 135], muscle stretching [110, 136–139], therapeutic massage [140, 141], whole body vibration [21, 142], spinal cord stimulation [114, 143–146], transcranial direct current stimulation [147] and transcranial magnetic stimulation [27, 148–151]).
One of the features of the H-reflex is that its reliable measurement across sessions can be achieved with attention to key components including the setup (e.g., electrode locations), environment (e.g., temperature), posture/movement of the patient, and time of day [30, 72, 152–155]. For example, muscle and stimulation sites can be kept consistent across days by attending to skin landmarks, such as in the SENIAM project [156]. Grid arrays are promising options for automation of the electrode placement process [157–160]; further work is needed to validate and optimize them.
The H-reflex, however, changes with temperature [161, 162], age [163], caffeine intake [164], muscle circumpressure [165], time of day [155, 166], medication (e.g., [124, 125, 129]), and muscle activation or movement in another limb [27]. For example, hand/arm cycling (i.e., with a cycle ergometer) decreases the soleus H-reflex [167], and ankle plantarflexion (i.e., ankle extension) reduces the FCR H-reflex [168, 169]. Due to these influential variables, care must be used in H-reflex elicitation and interpretation [28, 35]. For example, a stable posture and environment (temperature, comfort) are crucial. In addition, it is important to monitor the background EMG of agonist and antagonist muscles [41]. Joint angle and muscle contraction can also affect nerve stimulation and EMG measurement of the M-wave and H-reflex (e.g., [170]). Thus, it is important to use controls, such as the maximum M-wave, in any study in which limb position is a potential variable or confound. All these considerations are important for any effective effort to translate H-reflex measurement and/or operant conditioning systems and protocols into clinical use.
Evidence that the H-reflex tracks functional outcomes begs the question, why not change the H-reflex directly? Evidence to date supports the hypothesis that appropriate H-reflex operant conditioning can improve function, without adverse side effects. The next sections describe this new therapy, its potential value, and what it will take to translate it into widespread clinical use.
Spinal Reflexes as Therapeutic Targets
Over the past 35 years, many studies have shown that people and animals can gradually increase or decrease the spinal stretch reflex or its electrical analog the H-reflex when they are exposed to an operant conditioning protocol that rewards them when reflex size satisfies a criterion [37, 171–174]. The reflex changes gradually over days and weeks due to plasticity in both the brain and the spinal cord. This plasticity appears to comprise a hierarchy in which the plasticity in the brain induces and maintains the plasticity in the spinal cord [43, 175, 176] (reviewed in [42, 177, 178]).
Figure 2 illustrates the operant conditioning protocol for the human soleus H-reflex [33]. This protocol enables investigators to separate change in the H-reflex due to plasticity in the brain from change due to plasticity in the spinal cord [37]. Furthermore, it enables analysis of the developmental time courses of the brain and spinal plasticity (i.e., neuronal and/or synaptic changes) and of their persistence after conditioning ends [37, 41].
Fig. 2.

The spinal reflex operant conditioning protocol. Left—subject with EMG electrodes above the right soleus muscle and nerve stimulation electrodes behind the knee. Subject faces monitor in standard study posture. Monitor displays visual feedback to the subject. In all trials, a background EMG graph shows the correct range and its current value. If the soleus EMG stays in the range for at least 2 s, an H-reflex is elicited. In control trials, there is no feedback as to the size of the H-reflex. In conditioning trials, an H-reflex graph is also shown with a shaded target area. If H-reflex size for the trial falls in the shaded area, the bar is green and the trial is a success; otherwise, the bar is red and the trial is not a success. The screen also shows the success rate for the current 75-trial run. (Modified from [37])
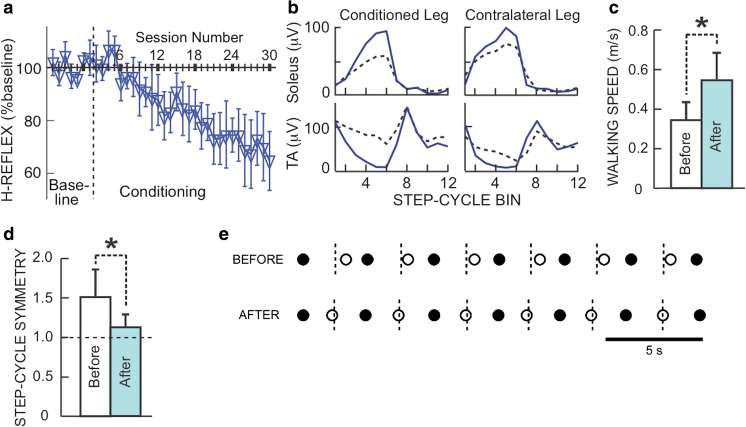
Because the operant conditioning protocol changes the spinal reflex pathway, it affects behaviors, such as locomotion, that use the pathway [179, 180]. This suggested that reflex conditioning could be used therapeutically; the results are very encouraging. In both rats and humans with incomplete SCI, H-reflex conditioning that targets beneficial plasticity (i.e., changes that produce beneficial functional outcomes) to a specific reflex pathway can improve locomotion (Fig. 3) [41, 43, 44]. Furthermore, and most importantly, these initial human studies, and studies in spinal cord-injured rats, indicate that, by producing beneficial plasticity in a key reflex pathway, the operant conditioning protocol triggers wider beneficial plasticity that markedly improves locomotion and persists after conditioning ends. Thus, in humans with incomplete SCI, down-conditioning of the soleus H-reflex in one leg improves locomotor activity in the muscles of both legs; this accounts for the marked improvement in walking speed and symmetry.
Fig. 3.
H-reflex change and walking improvement in people with incomplete SCI who decreased the H-reflex. (a) Average (±SE) H-reflexes for baseline and conditioning sessions. (b) Rectified locomotor EMG in soleus and tibialis anterior (TA) of both legs before (dashed) and after (solid) H-reflex decrease in a subject. The step cycle is divided into 12 equal bins, starting from foot contact (bins 1–7 are stance, bins 8–12 are swing). After H-reflex decrease, EMG modulation is better in both legs. This helps explain why walking is faster and more symmetrical. (c) Average (±SE) 10-m walking speeds before and after H-reflex decrease (*p < 0.05, paired t test). (d) Average step-cycle symmetry before and after H-reflex decrease (ratio of time between the nonconditioned leg’s foot contact (nFC) and the conditioned leg’s foot contact (cFC) to time between cFC and nFC). (In each person, the soleus H-reflex of the more impaired leg was down-conditioned.) A ratio of 1 is perfect symmetry. Initially, the ratio is > 1. After conditioning, it has decreased toward 1 in every subject (*p = 0.05). (e) Successive step cycles in a subject before and after HR decrease. Each nFC (solid) and cFC (open) are shown. Short vertical dashed lines mark the midpoints between nFCs, which is when cFC should occur. Before HR decrease, cFC occurs too late; afterward, it occurs on time. (Adapted from [41])
Operant conditioning of reflexes is an appealing therapeutic approach to the rehabilitation of locomotion for multiple reasons. First, it is uniquely targeted; it is possible to operantly condition a specific abnormal reflex pathway and strengthen or weaken it as appropriate for an individual person’s disability. Second, while continued testing is prudent, there are no known side effects to the conditioning protocol, and it does not affect locomotion in healthy participants [37, 181]. Furthermore, in rats with incomplete spinal cord injury, inappropriate conditioning (i.e., down-conditioning the soleus H-reflex to further weaken stance) did not further impair locomotion [39]. The absence of deleterious effects is likely to reflect appropriate compensatory plasticity; and it is in accord with the negotiated equilibrium model of spinal cord function [177, 178, 182]. Third, in a person with impaired motor function, appropriate reflex conditioning can trigger wider beneficial plasticity [41]. Fourth, evidence suggests that the beneficial effects of conditioning are persistent. In rats with SCI, the beneficial effects of appropriate reflex conditioning continue to increase after conditioning ends [183]. And in people with SCI, follow-up sessions showed that decreases in the amplitude of the H-reflex caused by down-conditioning were still apparent three months later [41]. Fifth, reflex conditioning should be able to complement other therapies and enhance recovery. By reducing hyperreflexia, soleus H-reflex down-conditioning can enable more normal locomotion and thereby enhance the effectiveness of locomotor training (e.g., [184]).
Similarly, in people in whom a stroke has produced a disabling flexor synergy in the arm, down-conditioning of a flexor muscle (e.g., FCR) H-reflex might reduce FCR activation by afferent input and thereby enable more effective reach-and-grasp practice. Studies are underway to determine if the effects shown with the lower-limb reflex conditioning protocol translate to the upper limb.
Current research on the therapeutic use of spinal reflex conditioning is focused on extending the results of Thompson et al. [41] to additional populations of people with motor deficits, such as people who have experienced stroke [185] or TBI [186]. Concurrent animal research is exploring its value for improving function after peripheral nerve transection and regeneration (e.g., [187, 188]). Ongoing investigations are exploring the combination of spinal reflex conditioning with other rehabilitation therapies, such as locomotor training [189]. Finally, scientific inquiries continue into the mechanisms of the plasticity in the brain and spinal cord that underlies spinal reflex conditioning (see [42, 177, 178] for review).
Clinical Translation of Spinal Reflex Operant Conditioning
This section addresses what must happen for spinal reflex conditioning to be effectively translated to clinical care of patients with motor impairments associated with reflex abnormalities. Drawing on the material reviewed and the requirements of clinical translation, we identify the scientific, clinical, and translational issues that must be addressed for spinal reflex conditioning to achieve widespread use as a new rehabilitation therapy.
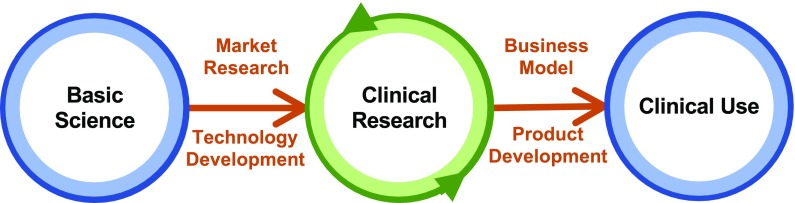
As Fig. 4 indicates, clinical translation proceeds from basic science to clinical research studies to clinical use. In addition to basic and clinical research, it involves market, regulatory, and reimbursement research, and business model and technology development, all informing each other along the way. One of the first steps in the process is identifying and interviewing, as part of market research, key stakeholders to understand their needs and desires [190–192]. For example, clinicians need intervention protocols that are efficient in terms of setup and execution, results that are clinically important in terms of patient function, and a reimbursement model that fits within current policies.
Fig. 4.
The process of translation to clinical use following the steps that define the market and the needs of customers/stakeholders within it
Interviewing stakeholders (users, buyers, and payees) also explores their costs, resources, buying decisions, and regulatory requirements. This initial step in the process of translation helps shape an understanding of the tasks involved in properly positioning the technology for widespread use in the rehabilitation market. These market requirements are used to inform the product development and overall translation plan. For example, understanding how clinicians weigh the benefits of spinal reflex operant conditioning, with its attendant procedural requirements, against the time demands and the reimbursement challenges [193] is important for designing an effective and feasible clinical system, efficient therapist training, and a viable reimbursement model. How these issues are addressed will help shape the business model for operant conditioning.
In addition, it is necessary to consider the buyer’s purchasing threshold. To disseminate spinal reflex conditioning, its cost must be reasonable and justified; i.e., it must provide value to payers, including hospitals and clinics, or to consumers directly, if paid out of pocket. The $30-billion rehabilitation market that spinal reflex conditioning will enter is fragmented; nearly half of the 16,000 rehabilitation clinics are independently owned [194]. Thus, device cost is an important barrier. Extra technological features (e.g., a camera or forceplates to ensure appropriate posture) might introduce insurmountable costs that are a barrier to sales and may not add significant value.
Table 3 provides a broad summary of issues for stakeholders and the role these issues are likely to play in the translation of spinal reflex conditioning. Clearly, the goals differ substantially across stakeholders. Thus, successful clinical translation of spinal reflex conditioning requires three key items, each forming a part of the overall steps highlighted in Fig. 4:
-
(i)
Strong clinical evidence that a clinically feasible protocol can produce clinically significant functional improvements in key clinical populations (e.g., people with stroke, TBI, SCI, MS, cerebral palsy)
-
(ii)
A cost-effective and implementable reimbursement model
-
(iii)
A robust, easy-to-use, and affordable operant conditioning system for clinical use
Table 3.
Key stakeholders in the clinical translation of spinal reflex conditioning. Their roles, goals, and challenges are indicated
| Stakeholder | Role in translation | Needs, wants, goals | Challenges |
|---|---|---|---|
| Patients | Participate in operant conditioning treatment; judge effectiveness; pay portion of costs | Improved function (e.g., walking); reduced need for drugs (e.g., baclofen, botulinum toxin); decreased need for assistive devices; improved ability to function in the community | Time commitment; potential cost of device and co-pays |
| Clinician/therapist | Decide whom will benefit; provide operant conditioning | Clinically important improvement in symptoms and function | Setup and implementation time; deliver outcomes within reimbursement constraints |
| Clinical administrator | Buy operant conditioning device | High-quality outcomes; marketability and branding/name recognition for state-of-the-art device/therapy | FDA approval; meets clinical need and sustains itself financially; capital investment |
| Payers | Approve payment for operant conditioning or pay within existing coverage/reimbursement guidelines | Improved outcomes; value; satisfy patient needs | High-quality RCT trials; serve aging population; decrease disability burdens |
| Researcher | Optimize methods and outcomes of spinal reflex conditioning | Good study outcomes; research papers; knowledge contribution; presentations; patents | Grant funding; tech support; facilities; sufficient time to complete studies |
Clinical Research Studies
Studies are needed to determine 1) who spinal reflex conditioning will work for and 2) how effectively it works in combination with other therapies. Both are critical in supporting a therapist’s decision concerning what therapies to administer.
For example, neurological disorders in which spasticity contributes significantly to functional impairment may be particularly amenable to reflex operant conditioning therapies [38]. On the other hand, people with disorders associated with substantial cognitive impairments may not be able to participate effectively in a reflex conditioning protocol. It is therefore critical to understand in which disorders and/or for which patient populations reflex operant conditioning protocols are most likely to be effective. In addition, conditioning protocols should be developed for an appropriately broad range of muscle groups.
Furthermore, clinical studies need to determine the number and length of sessions necessary to elicit significant functional improvements that persist. For example, the current operant conditioning protocol, which entails 30 1-h sessions, may not be clinically feasible (due to limits on physical therapy visits) and/or might preclude combining reflex conditioning with other therapies (e.g., locomotor training). Thus, clinical research aimed at reducing the number of sessions required is important. Ideally, these studies should establish dose-response curves for different disorders and different motor impairments. In addition, they should enhance the reliability of reflex conditioning (e.g., at present, conditioning is successful in about 70% of people with incomplete SCI [41]).
Clinical studies of spinal reflex conditioning should also evaluate the impact of spinal reflex conditioning in combination with other therapies. The limited evidence to date supports the hypothesis that the combination of spinal reflex conditioning with another effective less targeted therapy, such as locomotor training, will be more effective than either therapy alone [45].
These studies would support clinical uptake and reimbursement by defining the clinical value of the system. It will also be important to address the cost-effectiveness of spinal reflex conditioning and safety [195]. This requires both class-1 (randomized clinical trials (RCT)) and class-2 (e.g., prospective, longitudinal, observational) studies. Class-2 studies often lack the strict controls and randomization of class-1 studies. Real-world class-2 evidence is often more useful and less costly for determining cost-effectiveness, long-term benefits in a variety of patient populations, and other effects of a therapy [195–197]. RCTs are typically conducted in narrowly defined populations, within specialized and very controlled environments by highly trained personnel; thus, they do not reproduce or relate closely to the complex and continually varying realities of a clinical setting [197]. While class-1 evidence is invaluable for defining relationships between a treatment and its effects, class-2 evidence illuminates a treatment’s effectiveness in diverse clinical settings and patient populations, and in the presence of other treatments. Additionally, as medical device development is often incremental, real-world data can provide less costly and a more practical process for continuing to evaluate its safety and effectiveness as its clinical implementation progresses [196].
A Reimbursement Model
A model that defines how a therapy is paid for is a critical step in clinical translation; it is essential for shaping an effective business model for a new therapy. Clinical evidence is critical to determine how clinicians (therapists, physicians, nurses) are reimbursed by insurance companies. Many insurers base their decisions on Centers for Medicare and Medicaid Services (CMS) guidelines [195]. To obtain CMS coverage and payment codes requires substantial supportive class-1 evidence. Payment codes specify the procedures, diagnostic tests, and treatments for which insurance companies and other payers reimburse. However, a payment code alone does not necessarily result in actual payment [195]. In this regard, other evidence (e.g., concerning cost-effectiveness, length of stay, readmission rates, efficacy in a variety of disorders, etc.) is also important and is becoming more so. The clinical evidence needed to ensure actual reimbursement for new medical device treatments is substantial (see [198, 199] for examples of the assessment of functional electrical stimulation devices). The landscape for reimbursement is a large and complicated one. It is therefore important to engage with therapists, insurers, and other stakeholders, including CMS, early on in the clinical translation process.
Technology and Product Development
In its present form, spinal reflex operant conditioning is a complex procedure that uses a cumbersome software/hardware system and requires extensive operator (i.e., therapist) training. Thus, its use is currently limited to laboratory environments and highly skilled personnel. This constraint inhibits its wider clinical use. A robust and easy-to-use system would enable clinical therapists to participate in the further evaluation and eventual dissemination of spinal reflex operant conditioning. This section covers considerations for such a system, including cost and regulations.
To be effective, this clinical system, its accompanying protocol, and associated documentation must ensure appropriate procedures, including maintenance of body and limb posture, appropriate concurrent activation of relevant muscles, electrode placements, stimulation parameters, measurement specifics, and adequate numbers of trials and sessions. In short, standardization and rigorous maintenance of appropriate methods is extremely important. Furthermore, these methods need to be straightforward and convenient so that they can be easily mastered and followed by conscientious therapists. It is essential that the whole process of setup and treatment fit within the work-flow of a busy clinic and conform to the prevailing reimbursement rules for session times and types.
The realization of this practical clinical system is a daunting enterprise. It requires, along with much else, automated algorithms to determine appropriate background EMG activity for agonist and antagonist muscles, detect the M-wave and H-reflex, build M and H recruitment curves, select from them appropriate stimulus intensities, define reward criteria, and update stimulation and reward parameters as needed over the course of treatment. Furthermore, development and validation of each part of the new system entails extensive, highly iterative testing by representative therapists. This guides the development of the product requirements (i.e., what a product should do), including all the technical, usability, and functional requirements of the system.
For example, the selection of recording and stimulation sites seeks to identify electrode locations that 1) are sensitive and specific to the targeted muscle’s EMG activity and 2) provide a soleus M-wave/H-reflex recruitment curve that enables stimulation at a level that elicits a small M-wave and an H-reflex on the rising phase of H-reflex recruitment. This site selection task is time-consuming and requires significant training. In a clinically practical system, this onerous task could be avoided by multi-electrode grid arrays [157–160] and an automated procedure that selects the most appropriate electrodes. Figure 5 illustrates the use of such an array for automatic selection of stimulation sites for operant conditioning of the FCR H-reflex. One of the many steps in developing this array is to define the minimum number of candidate electrodes needed to identify the best stimulation sites and to ensure that this identification can be performed within a clinically practical and reimbursable setup time.
Fig. 5.
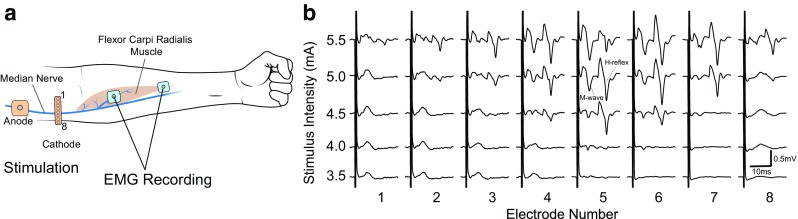
Automated stimulation-site selection. (a) The stimulation cathode electrode is an 8-electrode linear array (1–8; 5 mm between electrodes) (OT Bioelettronica) placed across the cubital fossa to stimulate the median nerve. The anode is placed 2–3 cm proximally, avoiding the biceps muscle (to reduce muscle artifacts). The flexor carpi radialis (FCR) is recorded differentially using two standard 2.2 × 2.2-cm self-adhesive electrodes (Vermed). One recording electrode is placed over the FCR muscle belly, and the other distal to this, on the muscle/tendon junction. An algorithm cycles through each stimulation electrode using a Digitimer DS5 isolated stimulator and a digitally controlled multiplexer (Digitimer D188). (b) This produces a series of recruitment curves for each stimulation electrode. The electrode that requires the least current to elicit an H-reflex is identified (i.e., electrode 5)
Once this initial technological development is complete, it is necessary to finalize product specifications and requirements to initiate a more formal product development cycle. This formal medical device development cycle requires strict adherence to detailed product design and manufacturing principles (e.g., quality assurance). Such adherence and its full documentation are essential for Food and Drug Administration (FDA) approval. FDA classification and regulation is the final step in medical device product development. The FDA defines three classes of devices (Classes I, II, III). Devices are classified according to their risk. For example, a stethoscope is Class I, an ultrasound imager Class II, and an implanted pacemaker Class III.
As a putative Class-II device, a spinal reflex conditioning system has two options for FDA approval. If its intended use and its technical specifications match those of an existing FDA-approved device (i.e., a predicate device), its safety and effectiveness can be determined to be equivalent to those of the predicate device, and it can thereby be approved. If not, it needs to pursue a de novo pathway, a classification path for novel, low to medium risk, medical devices that do not have existing predicate devices. A de novo pathway requires safety and effectiveness data for FDA classification and approval. FDA approval is needed for establishing payment and coverage. The regulation landscape is continually evolving. It is therefore, in general, extremely worthwhile to meet with FDA officials early on to discuss the appropriate regulatory pathway and to ensure that clinical studies and product development are shaped to fulfill FDA requirements.
Conclusions
Spinal reflexes, in particular the H-reflex, are useful biomarkers for evaluating neurological disabilities, for guiding therapeutic interventions, and for assessing the functional effects of these interventions. In addition, the H-reflex is itself a valuable therapeutic target. Noninvasive H-reflex operant conditioning protocols can target beneficial plasticity to critical spinal sites; they can thereby initiate much wider beneficial plasticity that markedly improves important motor functions such as locomotion. These targeted plasticity protocols could complement less specific rehabilitation therapies and enhance functional recovery. The successful translation of this exciting new therapeutic approach into widespread clinical practice requires further clinical studies and hardware/software development, market research, a realistic business model, a viable reimbursement strategy, and regulatory approval. This complex and arduous process has just begun.
Electronic Supplementary Material
(PDF 513 kb)
Acknowledgments
The National Center for Adaptive Neurotechnologies (NCAN) of the Wadsworth Center is supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (NIH) (Grant 1P41EB018783 (JRW)). The authors’ work at the Wadsworth Center has also been supported by NIH grants NS22189 (JRW), HD36020 (XYChen), NS061823 (JRW and XYChen), HD32571 (AWEnglish), VA Merit Award 1 I01 BX002550 (JRW), the New York State Spinal Cord Injury Research Board (SCIRB), and the the National Center of Neuromodulation for Rehabilitation (NC NM4R) NIH grant P2CHD086844 (SAKautz). In addition, we would like to acknowledge the invaluable assistance and guidance of the Center for Translation of Rehabilitation Advances and Technology (TREAT), a national rehabilitation research resource funded by the National Center for Medical Rehabilitation Research (NCMRR) of the NIH (Grants P2CHD086841 (RMGreenwald) and R24HD065703 (RMGreenwald)).
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Brashear A. Spasticity: Diagnosis and Management. Demos Medical Publishing; 2nd ed; 2016.
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics’2017 Update: A report from the American Heart Association. Circulation. 2017. 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed]
- 3.Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: Stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil [online]. Elsevier Ltd; 2014;95:986–995.e1. 10.1016/j.apmr.2013.10.032 [DOI] [PMC free article] [PubMed]
- 4.Bose P, Hou J. Chapter 14 Traumatic Brain Injury (TBI) -Induced Spasticity. 2015;1–15. [PubMed]
- 5.Maenner MJ, Blumberg SJ, Kogan MD, Christensen D, Yeargin-Allsopp M, Schieve LA. Prevalence of cerebral palsy and intellectual disability among children identified in two U.S. National Surveys, 2011-2013. Ann Epidemiol [online]. Elsevier Inc; 2016;26:222–6. 10.1016/j.annepidem.2016.01.001 [DOI] [PMC free article] [PubMed]
- 6.Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–31. 10.2147/CLEP.S68889 [DOI] [PMC free article] [PubMed]
- 7.Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord. 2005;43:577–86. doi: 10.1038/sj.sc.3101757. [DOI] [PubMed] [Google Scholar]
- 8.Simpson LA, Eng JJ, Hsieh JTC, Wolfe and the Spinal Cord Injury Re DL. The health and life priorities of individuals with spinal cord injury: A Systematic Review. J Neurotrauma [online]. 2012;29:1548–55. 10.1089/neu.2011.2226 [DOI] [PMC free article] [PubMed]
- 9.Thibaut A, Chatelle C, Ziegler E, Bruno MA, Laureys S, Gosseries O. Spasticity after stroke: Physiology, assessment and treatment. Brain Inj. 2013;27:1093–105. doi: 10.3109/02699052.2013.804202. [DOI] [PubMed] [Google Scholar]
- 10.Rizzo MA, Hadjimichael OC, Preiningerova J, Vollmer TL. Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult Scler J [online] 2004;10:589–95. doi: 10.1191/1352458504ms1085oa. [DOI] [PubMed] [Google Scholar]
- 11.Wedekind C, Lippert-Grüner M. Long-term outcome in severe traumatic brain injury is significantly influenced by brainstem involvement. Brain Inj [online]. Taylor & Francis. 2005;19:681–4. doi: 10.1080/02699050400025182. [DOI] [PubMed] [Google Scholar]
- 12.Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, Benedict RE, Kirby RS, Durkin MS. Prevalence of Cerebral Palsy in 8-year-old children in three areas of the United States in 2002: A Multisite Collaboration. Pediatrics [online]. 2008;121:547–54. 10.1542/peds.2007-1270 [DOI] [PubMed]
- 13.Holtz KA, Lipson R, Noonan VK, Kwon BK, Mills PB. Prevalence and effect of problematic spasticity after traumatic spinal cord injury. Arch Phys Med Rehabil [online]. Elsevier Inc; 2017;98:1132–8. 10.1016/j.apmr.2016.09.124 [DOI] [PubMed]
- 14.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol [online]. Elsevier Ltd. 2009;8:741–54. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- 15.Domingo A, Al-Yahya AA, Asiri Y, Eng JJ, Lam T. A systematic review of the effects of pharmacological agents on walking function in people with spinal cord injury. J Neurotrauma [online]. 2012;29:865–79. 10.1089/neu.2011.2052 [DOI] [PMC free article] [PubMed]
- 16.Rekand T. Clinical assessment and management of spasticity: A review. Acta Neurol Scand. 2010;122:62–6. doi: 10.1111/j.1600-0404.2010.01378.x. [DOI] [PubMed] [Google Scholar]
- 17.Gupta AD, Chu WH, Howell S, et al. A systematic review: efficacy of botulinum toxin in walking and quality of life in post-stroke lower limb spasticity. Syst Rev [online]. Systematic Reviews. 2018;7:1. doi: 10.1186/s13643-017-0670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheean G. Botulinum toxin treatment of adult spasticity. Drug Saf [online]. 2006;29:31–48. 10.2165/00002018-200629010-00003 [DOI] [PubMed]
- 19.Ward AB. Spasticity treatment with botulinum toxins. J Neural Transm. 2008;115:607–16. 10.1007/s00702-007-0833-2 [DOI] [PubMed]
- 20.Dario A, Tomei G. A benefit-risk assessment of baclofen in severe spinal spasticity. Drug Saf [online]. 2004;27:799–818. 10.2165/00002018-200427110-00004 [DOI] [PubMed]
- 21.Martins A. The role of spasticity in functional neurorehabilitation-part II: Non- pharmacological and pharmacological management: A multidisciplinary approach. Arch Med. 2016;8:1–7.
- 22.Rekand T, Hagen E, Gronning M. Spasticity following spinal cord injury. Tidsskr Nor Legeforen [online]. 2012;132:970–3. doi: 10.4045/tidsskr.10.0872. [DOI] [PubMed] [Google Scholar]
- 23.Reier PJ, Howland DR, Mitchell G, Wolpaw JR, Hoh D, Lane MA. Spinal cord injury: Repair, plasticity and rehabilitation. eLS [online]. 2017;1–12. 10.1002/9780470015902.a0021403.pub2
- 24.Harkema SJ, Schmidt-Read M, Lorenz DJ, Edgerton VR, Behrman AL. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor trainingbased rehabilitation. Arch Phys Med Rehabil [online]. Elsevier Inc. 2012;93:1508–17. doi: 10.1016/j.apmr.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 25.Peurala SH, Karttunen AH, Sjigren T, Paltamaa J, Heinonen A. Evidence for the effectiveness of walking training on walking and self-care after stroke: A systematic review and meta-analysis of randomized controlled trials. J Rehabil Med. 2014;46:387–99. doi: 10.2340/16501977-1805. [DOI] [PubMed] [Google Scholar]
- 26.Fritz S, Lusardi M. White paper: “Walking speed: the sixth vital sign.” J Geriatr Phys Ther. 2016;32:1. 10.1519/00139143-200932020-00002 [PubMed]
- 27.Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord of Movement. Pierrot-deseilligny E, Burke D, editors. Cambridge University Press; 2012.
- 28.Burke D. Clinical uses of H reflexes of upper and lower limb muscles. Clin Neurophysiol Pract [online]. International Federation of Clinical Neurophysiology; 2016;1:9–17. doi:10.1016/j.cnp.2016.02.003 [DOI] [PMC free article] [PubMed]
- 29.Knikou M. The H-reflex as a probe: Pathways and pitfalls. J Neurosci Methods. 2008;171:1–12. doi: 10.1016/j.jneumeth.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Zehr EP. Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol. 2002;86:455–68. doi: 10.1007/s00421-002-0577-5. [DOI] [PubMed] [Google Scholar]
- 31.Yang JF, Fung J, Edamura M, Blunt R, Stein RB, Barbeau H. H-Reflex modulation during walking in spastic paretic subjects. Can J Neurol Sci / J Can des Sci Neurol. 1991;18:443–52. 10.1017/S0317167100032133 [DOI] [PubMed]
- 32.Hoffmann P. Über die Beziehungen der Sehnenreflexe zur willkür liehen Bewegung und zum Tonus. Z Biol. 1918;68:351–70. [Google Scholar]
- 33.Hoffmann P. Beiträge zur Kenntnis der menschlichen Reflexe mit besonderer Berücksichtigung. Arch Anat Physiol. 1910;1:223–46. [Google Scholar]
- 34.Palmieri RM, Ingersoll CD, Hoffman MA. The Hoffmann reflex: Methodologic considerations and applications for use in sports medicine and athletic training research. J Athl Train. 2004;39:268–77. doi: 10.1007/s00421-003-0967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misiaszek JE. The H-reflex as a tool in neurophysiology: Its limitations and uses in understanding nervous system function. Muscle and Nerve. 2003;28:144–60. doi: 10.1002/mus.10372. [DOI] [PubMed] [Google Scholar]
- 36.Boorman GI, Lee RG, Becker WJ, Windhorst UR. Impaired “natural reciprocal inhibition” in patients with spasticity due to incomplete spinal cord injury. Electroencephalogr Clin Neurophysiol - Electromyogr Mot Control. 1996;101:84–92. doi: 10.1016/0924-980X(95)00262-J. [DOI] [PubMed] [Google Scholar]
- 37.Thompson AK, Chen XY, Wolpaw JR. Acquisition of a simple motor skill: Task-dependent adaptation plus long-term change in the human soleus H-reflex. J Neurosci [online]. 2009;29:5784–92. 10.1523/JNEUROSCI.4326-08.2009 [DOI] [PMC free article] [PubMed]
- 38.Mukherjee A, Chakravarty A. Spasticity mechanisms - for the clinician. Front Neurol. 2010;MAR:1–10. doi:10.3389/fneur.2010.00149 [DOI] [PMC free article] [PubMed]
- 39.Chen Y, Chen L, Liu R, Wang Y, Chen XY, Wolpaw JR. Locomotor impact of beneficial or nonbeneficial H-reflex conditioning after spinal cord injury. J Neurophysiol [online]. 2014;111:1249–58. doi: 10.1152/jn.00756.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson AK, Chen XY, Wolpaw JR. Soleus H-reflex operant conditioning changes the H-reflex recruitment curve. Muscle and Nerve. 2013;47:539–44. doi: 10.1002/mus.23620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson AK, Pomerantz FR, Wolpaw JR. Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans. J Neurosci [online]. 2013;33:2365–75. 10.1523/JNEUROSCI.3968-12.2013 [DOI] [PMC free article] [PubMed]
- 42.Thompson AK, Wolpaw JR. Operant conditioning of spinal reflexes: from basic science to clinical therapy. Front Integr Neurosci [online]. 2014;8:1–8. doi: 10.3389/fnint.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Chen XY, Jakeman LB, Chen L, Stokes BT, Wolpaw JR. Operant conditioning of H-reflex can correct a locomotor abnormality after spinal cord injury in rats. J Neurosci [online]. 2006;26:12537–43. 10.1523/JNEUROSCI.2198-06.2006 [DOI] [PMC free article] [PubMed]
- 44.Manella KJ, Roach KE, Field-Fote EC. Operant conditioning to increase ankle control or decrease reflex excitability improves reflex modulation and walking function in chronic spinal cord injury. J Neurophysiol [online]. 2013;109:2666–79. doi: 10.1152/jn.01039.2011. [DOI] [PubMed] [Google Scholar]
- 45.Frontera WR, Bean JF, Damiano D, et al. Rehabilitation research at the national institutes of health moving the field forward (executive summary). Phys Ther. 2017;97:387–96. 10.1093/ptj/pzx027 [DOI] [PMC free article] [PubMed]
- 46.Thompson AK, Wolpaw JR. Targeted neuroplasticity for rehabilitation. Prog Brain Res. 2015;218:157–72. doi: 10.1016/bs.pbr.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morelli M, Sullivan SJ, Chapman CE. Inhibitory influence of soleus massage onto the medial gastrocnemius H-reflex. Electromyogr Clin Neurophysiol [online]. 1998;38:87–93. [PubMed] [Google Scholar]
- 48.Thompson CS, Schabrun S, Marshall PW. H-reflex excitability is inhibited in soleus, but not gastrocnemius, at the short-latency response of a horizontal jump-landing task. Hum Mov Sci [online]. Elsevier B.V.; 2016;47:1–8. 10.1016/j.humov.2016.01.007 [DOI] [PubMed]
- 49.Friesenbichler B, Lepers R, Maffiuletti NA. Soleus and lateral gastrocnemius H-reflexes during standing with unstable footwear. Muscle and Nerve. 2015;51:764–6. doi: 10.1002/mus.24601. [DOI] [PubMed] [Google Scholar]
- 50.Jusic A, Baraba R, Bogunovic A. H-reflex and F-wave potentials in leg and arm muscles. Electromyogr Clin Neurophysiol. Belgium. 1995;35:471–8. [PubMed] [Google Scholar]
- 51.Abbruzzese M, Rubino V, Schieppati M. Task-dependent effects evoked by foot muscle afferents on leg muscle activity in humans. Electroencephalogr Clin Neurophysiol - Electromyogr Mot Control. 1996;101:339–48. doi: 10.1016/0924-980X(96)95682-9. [DOI] [PubMed] [Google Scholar]
- 52.Roujeau T, Decq P, Lefaucheur JP. Surface EMG recording of heteronymous reflex excitation of semitendinosus motoneurones by group II afferents. Clin Neurophysiol. 2004;115:1313–9. doi: 10.1016/j.clinph.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 53.Ellrich J, Steffens H, Treede RD, Schomburg ED. The Hoffmann reflex of human plantar foot muscles. Muscle Nerve. United States. 1998;21:732–8. doi: 10.1002/(SICI)1097-4598(199806)21:6<732::AID-MUS4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 54.Versino M, Candeloro E, Tavazzi E, Moglia A, Sandrini G, Alfonsi E. The H reflex from the abductor brevis hallucis muscle in healthy subjects. Muscle and Nerve. 2007;36:39–46. doi: 10.1002/mus.20775. [DOI] [PubMed] [Google Scholar]
- 55.Hopkins JT, Ingersoll CD, Krause BA, Edwards JE, Cordova ML. Effect of knee joint effusion on quadriceps and soleus motoneuron pool excitability. Med Sci Sports Exerc [online] 2001;33:123–6. doi: 10.1097/00005768-200101000-00019. [DOI] [PubMed] [Google Scholar]
- 56.Fahrer H, Rentsch HU, Gerber NJ, Beyeler C, Hess CW, Grünig B. Knee effusion and reflex inhibition of the quadriceps. A bar to effective retraining. J Bone Joint Surg Br. 1988;70:635–8. doi: 10.1302/0301-620X.70B4.3403614. [DOI] [PubMed] [Google Scholar]
- 57.Dietz V, Faist M, Pierrot-Deseilligny E. Amplitude modulation of the quadriceps H-reflex in the human during the early stance phase of gait. Exp Brain Res [online]. 1990;79:221–4. doi: 10.1007/BF00228893. [DOI] [PubMed] [Google Scholar]
- 58.Pazzinatto MF, de Oliveira Silva D, Pappas E, Magalhães FH, de Azevedo FM. Is quadriceps H-reflex excitability a risk factor for patellofemoral pain? Med Hypotheses [online]. Elsevier; 2017;108:124–7. 10.1016/j.mehy.2017.08.019 [DOI] [PubMed]
- 59.de Oliveira Silva D, Magalhães FH, Faria NC, et al. Vastus medialis Hoffmann reflex excitability is associated with pain level, self-reported function, and chronicity in women with patellofemoral pain. Arch Phys Med Rehabil. 2017;98:114–9. 10.1016/j.apmr.2016.06.011 [DOI] [PubMed]
- 60.Pierrot-Deseilligny E, Morin C, Bergego C, Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Exp brain Res. Germany; 1981;42:337–50. [DOI] [PubMed]
- 61.Zheng CJ, Zhu Y, Jin X, et al. Potential advantages of the H-reflex of the biceps femoris-long head in documenting S1 radiculopathy. J Clin Neurophysiol [online]. 2014;31. [DOI] [PubMed]
- 62.Hall RC, Nyland J, Nitz AJ, Pinerola J, Johnson DL. Relationship between ankle invertor H-reflexes and acute swelling induced by inversion ankle sprain. J Orthop Sport Phys Ther [online]. 1999;29:339–44. 10.2519/jospt.1999.29.6.339 [DOI] [PubMed]
- 63.Kim KM, Hart JM, Saliba SA, Hertel J. Modulation of the fibularis longus hoffmann reflex and postural instability associated with chronic ankle instability. J Athl Train. 2016;51:637–43. doi: 10.4085/1062-6050-51.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishikawa T, Ozaki T, Mizuno K, Grabiner MD. Increased reflex activation of the peroneus longus following application of an ankle brace declines over time. J Orthop Res. 2002;20:1323–6. doi: 10.1016/S0736-0266(02)00054-2. [DOI] [PubMed] [Google Scholar]
- 65.Sefton JM, Hicks-Little CA, Koceja DM, Cordova ML. Effect of inversion and ankle bracing on peroneus longus Hoffmann reflex. Scand J Med Sci Sport. 2007;17:539–46. doi: 10.1111/j.1600-0838.2006.00593.x. [DOI] [PubMed] [Google Scholar]
- 66.Nishikawa T, Grabiner MD. Peroneal motoneuron excitability increases immediately following application of a semirigid ankle brace. J Orthop Sports Phys Ther [online]. 1999;29:168–73. doi: 10.2519/jospt.1999.29.3.168. [DOI] [PubMed] [Google Scholar]
- 67.Garcia HA, Fisher MA, Gilai A. H reflex analysis of segmental reflex excitability in flexor and extensor muscles. Neurology. United States. 1979;29:984–91. doi: 10.1212/WNL.29.7.984. [DOI] [PubMed] [Google Scholar]
- 68.Burke D, Adams RW, Skuse NF. The effects of voluntary contraction on the H reflex of human limb muscles. Brain. England. 1989;112:417–33. doi: 10.1093/brain/112.2.417. [DOI] [PubMed] [Google Scholar]
- 69.Miyama S, Arimoto K, Kimiya S. H reflex in patients with spastic quadriplegia. No To Hattatsu. Japan. 2009;41:21–6. [PubMed] [Google Scholar]
- 70.Köster B, Lauk M, Timmer J, et al. Central mechanisms in human enhanced physiological tremor. Neurosci Lett. 1998;241:135–8. doi: 10.1016/S0304-3940(98)00015-9. [DOI] [PubMed] [Google Scholar]
- 71.Miller T, Newall AR, Jackson DA. H-reflexes in the upper extremity and the effects of voluntary contraction. Electromyogr Clin Neurophysiol. 1995;35:121–8. [PubMed]
- 72.Hopkins JT, Wagie NC. Intrasession and intersession reliability of the quadriceps Hoffmann reflex. Electromyogr Clin Neurophysiol [online]. 2003;43:85–89. [PubMed] [Google Scholar]
- 73.Eliaspour D, Sanati E, Hedayati-Moghaddam MR, Rayegani SM, Bahrami MH. Utility of flexor carpi radialis H-reflex in diagnosis of cervical radiculopathy. J Clin Neurophysiol. 2009;26:458–60. 10.1097/WNP.0b013e3181c2bb00 [DOI] [PubMed]
- 74.Domingo A, Klimstra M, Nakajima T, Lam T, Hundza SR. Walking phase modulates H-reflex amplitude in flexor carpi radialis. J Mot Behav [online]. Routledge; 2014;46:49–57. 10.1080/00222895.2013.854731 [DOI] [PubMed]
- 75.Skills M. Inhibitory effects of circumferential pressure on flexor carpi radialis h-reflex in adults with neurological deficits 1. 2010;89–103. 10.2466/PMS.110.1.89-103 [DOI] [PubMed]
- 76.Cavallari P, Lalli S. Changes in excitability of the flexor carpi radialis H-reflex following tactile stimulation of the index fingertip. Exp Brain Res. 1998;120:345–51. doi: 10.1007/s002210050408. [DOI] [PubMed] [Google Scholar]
- 77.Schimsheimer RJ, Ongerboer de Visser BW, Kemp B, Bour LJ. The flexor carpi radialis H-reflex in polyneuropathy: relations to conduction velocities of the median nerve and the soleus H-reflex latency. J Neurol Neurosurg Psychiatry [online] 1987;50:447–52. doi: 10.1136/jnnp.50.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stowe AM, Hughes-Zahner L, Stylianou AP, Schindler-Ivens S, Quaney BM. Between-day reliability of upper extremity H-reflexes. J Neurosci Methods. 2008;170:317–23. doi: 10.1016/j.jneumeth.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 79.Cincotta M, Ziemann U. Neurophysiology of unimanual motor control and mirror movements. Clin Neurophysiol. 2008;119:744–62. doi: 10.1016/j.clinph.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 80.Abbruzzese G, Trompetto C, Schieppati M. The excitability of the human motor cortex increases during execution and mental imagination of sequential but not repetitive finger movements. Exp Brain Res [online]. 1996;111:465–72. doi: 10.1007/BF00228736. [DOI] [PubMed] [Google Scholar]
- 81.Bodofsky EB. Contraction-induced upper extremity H reflexes: Normative values. Arch Phys Med Rehabil. 1999;80:562–5. doi: 10.1016/S0003-9993(99)90200-9. [DOI] [PubMed] [Google Scholar]
- 82.Phadke CP, Robertson CT, Condliffe EG, Patten C. Upper-extremity H-reflex measurement post-stroke: Reliability and inter-limb differences. Clin Neurophysiol [online]. International Federation of Clinical Neurophysiology. 2012;123:1606–15. doi: 10.1016/j.clinph.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 83.Kao JT, Sharma S, Curtis CG, Clarke HM. The role of the brachioradialis H Reflex in the management and prognosis of obstetrical brachial plexus palsy. Handchir Mikrochir plast Chir. 2003;35:106–11. 10.1055/s-2003-40768 [DOI] [PubMed]
- 84.Lee J, Park GJ, Doo HC, Park SG, Jeong YS, Hah JS. Utility of H-reflex in the diagnosis cervical radiculopathy. Yeungnam Univ J Med [online]. Yeungnam University College of Medicine; 1997;14:111–22.
- 85.Alexander CM, Harrison PJ. The bilateral reflex control of the trapezius muscle in humans. Exp Brain Res. 2002;142:418–24. doi: 10.1007/s00221-001-0951-2. [DOI] [PubMed] [Google Scholar]
- 86.Alexander CM, Stynes S, Thomas A, Lewis J, Harrison PJ. Does tape facilitate or inhibit the lower fibres of trapezius? Man Ther [online]. 2003;8:37–41. 10.1054/math.2002.0485 [DOI] [PubMed]
- 87.Vangsgaard S, Taylor JL, Hansen EA, Madeleine P. Changes in H reflex and neuromechanical properties of the trapezius muscle after 5 weeks of eccentric training: a randomized controlled trial. J Appl Physiol [online]. 2014;116:1623–31. 10.1152/japplphysiol.00164.2014 [DOI] [PubMed]
- 88.Miller TA, Mogyoros I, Kiernan M, Burke D. Reproducibility of a heteronymous monosynaptic reflex in biceps brachii. Electroencephalogr Clin Neurophysiol Electromyogr. 1995;97:318–25. doi: 10.1016/0924-980X(95)00121-Z. [DOI] [PubMed] [Google Scholar]
- 89.Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci. 1986;6:1308–13. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Larsen B, Voigt M. Changes in the gain of the soleus H-reflex with changes in the motor recruitment level and/or movement speed. Eur J Appl Physiol. 2004;93:19–29. doi: 10.1007/s00421-004-1152-z. [DOI] [PubMed] [Google Scholar]
- 91.Funase K, Imanaka K, Nishihira Y. Excitability of the soleus motoneuron pool revealed by the developmental slope of the H-reflex as reflex gain. Electromyogr Clin Neurophysiol [online] 1994;34:477–489. [PubMed] [Google Scholar]
- 92.Mynark RG, Koceja DM. Comparison of soleus H-reflex gain from prone to standing in dancers and controls. Electroencephalogr Clin Neurophysiol - Electromyogr Mot Control. 1997;105:135–40. doi: 10.1016/S0924-980X(96)96096-8. [DOI] [PubMed] [Google Scholar]
- 93.Field-Fote EC, Brown KM, Lindley SD. Influence of posture and stimulus parameters on post-activation depression of the soleus H-reflex in individuals with chronic spinal cord injury. Neurosci Lett. 2006;410:37–41. doi: 10.1016/j.neulet.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goulart F, Valls-Solé J, Alvarez R. Posture-related changes of soleus H-reflex excitability. Muscle Nerve [online] 2000;23:925–32. doi: 10.1002/(SICI)1097-4598(200006)23:6<925::AID-MUS13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 95.Angulo-Kinzler RM, Mynark RG, Koceja DM. Soleus H-reflex gain in elderly and young adults: modulation due to body position. J Gerontol A Biol Sci Med Sci. 1998;53:M120–5. doi: 10.1093/gerona/53A.2.M120. [DOI] [PubMed] [Google Scholar]
- 96.Nielsen J, Crone C, Hultborn H. H-reflexes are smaller in dancers from The Royal Danish Ballet than in well-trained athletes. Eur J Appl Physiol Occup Physiol [online]. 1993;66:116–21. doi:10.1007/BF01427051 [DOI] [PubMed]
- 97.CMS. Billing and Coding Guidelines: NEURO-005 Nerve Conduction Studies and Electromyography. 2012.
- 98.Millan-Guerrero RO, Trujillo-Hernandez B, Isais-Millan S, et al. H-reflex and clinical examination in the diagnosis of diabetic polyneuropathy. JIntMedRes [online]. 2012;40:694–700. doi: 10.1177/147323001204000233. [DOI] [PubMed] [Google Scholar]
- 99.Dillingham TR, Marin R, Belandres PV, Chang A. Extensor digitorum brevis reflex in normals and patients with radiculopathies. Muscle Nerve [online]. Wiley Subscription Services, Inc. A Wiley Company. 1995;18:52–9. doi: 10.1002/mus.880180108. [DOI] [PubMed] [Google Scholar]
- 100.Mazzocchio R, Scarfò GB, Mariottini A, Muzii VF, Palma L. Recruitment curve of the soleus H-reflex in chronic back pain and lumbosacral radiculopathy. BMC Musculoskelet Disord [online] 2001;2:4. doi: 10.1186/1471-2474-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alrowayeh HN, Sabbahi MA. H-reflex amplitude asymmetry is an earlier sign of nerve root involvement than latency in patients with S1 radiculopathy. BMC Res Notes [online]. BioMed Central Ltd. 2011;4:102. doi: 10.1186/1756-0500-4-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jin X, Zhu Y, Lu FZ, et al. H-reflex to S1-root stimulation improves utility for diagnosing S1 radiculopathy. Clin Neurophysiol [online]. International Federation of Clinical Neurophysiology. 2010;121:1329–35. doi: 10.1016/j.clinph.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 103.Zheng C, Zhu Y, Lu F, et al. Diagnostic advantage of S1 foramen-evoked H-reflex for S1 radiculopathy in patients with diabetes mellitus. Int J Neurosci [online]. Taylor & Francis; 2013;123:770–5. 10.3109/00207454.2013.801843 [DOI] [PubMed]
- 104.Gordon PH, Wilbourn a J. Early electrodiagnostic findings in Guillain-Barré syndrome. Arch Neurol. 2001;58:913–7. doi: 10.1001/archneur.58.6.913. [DOI] [PubMed] [Google Scholar]
- 105.Yang Y, Xiao J, Song W. Post-activation depression of the lower extremities in stroke patients with spasticity and spastic equinovarus deformity. Arq Neuropsiquiatr [online]. 2015;73:493–8. doi: 10.1590/0004-282X20150052. [DOI] [PubMed] [Google Scholar]
- 106.Kawaishi Y, Matsumoto N, Nishiwaki T, Hirano T. Postactivation depression of soleus H-reflex increase with recovery of lower extremities motor functions in patients with subacute stroke. J Phys Ther Sci [online]. 2017;29:1539–42. doi: 10.1589/jpts.29.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Okuyama K, Kawakami M, Hiramoto M, Muraoka K, Fujiwara T, Liu M. Relationship between spasticity and spinal neural circuits in patients with chronic hemiparetic stroke. Exp Brain Res [online]. Springer Berlin Heidelberg; 2017;0:1–7. 10.1007/s00221-017-5119-9 [DOI] [PubMed]
- 108.Çakır T, Evcik FD, Subaşı V, Demirdal ÜS, Kavuncu V. Investigation of the H reflexes, F waves and sympathetic skin response with electromyography (EMG) in patients with stroke and the determination of the relationship with functional capacity. Acta Neurol Belg. 2015;115:295–301. doi: 10.1007/s13760-014-0397-5. [DOI] [PubMed] [Google Scholar]
- 109.Higashi T, Funase K, Kusano K, et al. Motoneuron pool excitability of hemiplegic patients: Assessing recovery stages by using H-reflex and M response. Arch Phys Med Rehabil. 2001;82:1604–10. doi: 10.1053/apmr.2001.25081. [DOI] [PubMed] [Google Scholar]
- 110.Nielsen J, Petersen N, Ballegaard M, Biering-Sørensen F, Kiehn O. H-reflexes are less depressed following muscle stretch in spastic spinal cord injured patients than in healthy subjects. Exp Brain Res [online]. 1993;97:173–6. doi: 10.1007/BF00228827. [DOI] [PubMed] [Google Scholar]
- 111.Koelman JHTM, Willemse RB, Bour LJ, Hilgevoord AAJ, Speelman JD, Ongerboer de Visser BW. Soleus H-reflex tests in dystonia. Mov Disord [online]. Wiley Subscription Services, Inc., A Wiley Company; 1995;10:44–50. 10.1002/mds.870100109 [DOI] [PubMed]
- 112.Marchand-Pauvert V, Iglesias C. Properties of human spinal interneurones: normal and dystonic control. J Physiol. 2008;586:1247–56. doi: 10.1113/jphysiol.2007.145904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rijsman RM, Stam CJ, De Weerd AW. Abnormal H-reflexes in periodic limb movement disorder; Impact on understanding the pathophysiology of the disorder. Clin Neurophysiol. 2005;116:204–10. doi: 10.1016/j.clinph.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 114.Heide AC, Winkler T, Helms HJ, et al. Effects of transcutaneous spinal direct current stimulation in idiopathic restless legs patients. Brain Stimul [online]. Elsevier Ltd; 2014;7:636–42. 10.1016/j.brs.2014.06.008 [DOI] [PubMed]
- 115.Sabbahi M, Etnyre B, Al-Jawayed IA, Hasson S, Jankovic J. Methods of H-reflex evaluation in the early stages of Parkinson’s disease. J Clin Neurophysiol. United States. 2002;19:67–72. doi: 10.1097/00004691-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 116.Mahmud AS, Alwan BM, Mezaal MA. H-reflex excitability in children with spastic cerebral palsy. J Fac Med. 2011;53:11–4. [Google Scholar]
- 117.Hodapp M, Klisch C, Berger W, Mall V, Faist M. Modulation of soleus H-reflexes during gait in healthy children. Exp Brain Res. 2007;178:252–60. doi: 10.1007/s00221-006-0730-1. [DOI] [PubMed] [Google Scholar]
- 118.Futagi Y, Abe J. H-reflex study in normal children and patients with cerebral palsy. Brain Dev [online]. 1985;7:414–20. doi: 10.1016/S0387-7604(85)80139-X. [DOI] [PubMed] [Google Scholar]
- 119.Leonard CT, Moritani T, Hirschfeld H, Forssberg H. Deficits in reciprocal inhibition of children with cerebral palsy as revealed by h reflex testing. Dev Med Child Neurol [online]. Blackwell Publishing Ltd; 1990;32:974–84. 10.1111/j.1469-8749.1990.tb08120.x [DOI] [PubMed]
- 120.Parvin S, Mansouri M, Amiri S, Marzbani H, Kharazi MR, Mirbagheri MM. Contribution of reflex hyper-excitability to muscle stiffness in children with cerebral palsy. 2016 23rd Iranian Conference on Biomedical Engineering and 2016 1st International Iranian Conference on Biomedical Engineering (ICBME) 2016. Online. p. 89–92. 10.1109/ICBME.2016.7890935
- 121.Ditunno JF, Little JW, Tessler A, Burns AS. Spinal shock revisited: A four-phase model. Spinal Cord. 2004;42:383–95. doi: 10.1038/sj.sc.3101603. [DOI] [PubMed] [Google Scholar]
- 122.Ko HY, Ditunno JF, Graziani V, Little JW. The pattern of reflex recovery during spinal shock. Spinal cord Off J Int Med Soc Paraplegia. 1999;37:402–9. doi: 10.1038/sj.sc.3100840. [DOI] [PubMed] [Google Scholar]
- 123.Cho SH, Lee JH. Comparison of the amplitudes of the H-reflex of post-stroke hemiplegia patients and normal adults during walking. J Phys Ther Sci. 2013;25:729–32. doi: 10.1589/jpts.25.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ørsnes G, Crone C, Krarup C, Petersen N, Nielsen J. The effect of baclofen on the transmission in spinal pathways in spastic multiple sclerosis patients. Clin Neurophysiol. 2000;111:1372–9. doi: 10.1016/S1388-2457(00)00352-7. [DOI] [PubMed] [Google Scholar]
- 125.Macdonell RA, Talalla A, Swash M, Grundy D. Intrathecal baclofen and the H-reflex. J Neurol Neurosurg Psychiatry [online]. 1989;52:1110–2. 10.1136/jnnp.52.9.1110 [DOI] [PMC free article] [PubMed]
- 126.Hoving MA, van Kranen-Mastenbroek VH, van Raak EP, et al. Placebo controlled utility and feasibility study of the H-reflex and flexor reflex in spastic children treated with intrathecal baclofen. Clin Neurophysiol. 2006;117:1508–17. 10.1016/j.clinph.2006.04.014 [DOI] [PubMed]
- 127.Stokic DS, Yablon SA, Hayes A, Vesovic-Potic V, Olivier J. Dose-response relationship between the H-reflex and continuous intrathecal baclofen administration for management of spasticity. Clin Neurophysiol. 2006;117:1283–9. doi: 10.1016/j.clinph.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 128.Marchand-Pauvert V, Aymard C, Giboin LS, Dominici F, Rossi A, Mazzocchio R. Beyond muscular effects: Depression of spinal recurrent inhibition after botulinum neurotoxin A. J Physiol. 2013;591:1017–29. doi: 10.1113/jphysiol.2012.239178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kerzoncuf M, Bensoussan L, Delarque A, Durand J, Viton JM, Rossi-Durand C. Plastic changes in spinal synaptic transmission following botulinum toxin a in patients with post-stroke spasticity. J Rehabil Med. 2015;47:910–6. doi: 10.2340/16501977-2014. [DOI] [PubMed] [Google Scholar]
- 130.Knikou M, Smith AC, Mummidisetty CK. Locomotor training improves reciprocal and nonreciprocal inhibitory control of soleus motoneurons in human spinal cord injury. J Neurophysiol [online]. 2015;113:2447–60. doi: 10.1152/jn.00872.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smith AC, Rymer WZ, Knikou M. Locomotor training modifies soleus monosynaptic motoneuron responses in human spinal cord injury. Exp brain Res [online]. 2015;233:89–103. doi: 10.1007/s00221-014-4094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Trimble MH, Kukulka CG, Behrman AL. The effect of treadmill gait training on low-frequency depression of the soleus H-reflex: Comparison of a spinal cord injured man to normal subjects. Neurosci Lett. 1998;246:186–8. doi: 10.1016/S0304-3940(98)00259-6. [DOI] [PubMed] [Google Scholar]
- 133.Caron G, Marqueste T, Decherchi P. Restoration of post-activation depression of the H-reflex by treadmill exercise in aged rats. Neurobiol Aging [online] Elsevier Inc. 2016;42:61–8. doi: 10.1016/j.neurobiolaging.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 134.Phadke CP, Flynn SM, Thompson FJ, Behrman AL, Trimble MH, Kukulka CG. Comparison of single bout effects of bicycle training versus locomotor training on paired reflex depression of the soleus H-reflex after motor incomplete spinal cord injury. Arch Phys Med Rehabil [online]. 2009;90:1218–28. 10.1016/j.apmr.2009.01.022 [DOI] [PubMed]
- 135.Sosnoff JJ, Motl RW. Effect of acute unloaded arm versus leg cycling exercise on the soleus H-reflex in adults with multiple sclerosis. Neurosci Lett [online]. Elsevier Ireland Ltd. 2010;479:307–11. doi: 10.1016/j.neulet.2010.05.086. [DOI] [PubMed] [Google Scholar]
- 136.Mirbagheri MM, Alibiglou L, Thajchayapong M, Rymer WZ. Muscle and reflex changes with varying joint angle in hemiparetic stroke. J Neuroeng Rehabil [online]. 2008;5:6. doi: 10.1186/1743-0003-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Budini F, Tilp M. Changes in H-reflex amplitude to muscle stretch and lengthening in humans. Rev Neurosci. 2016;27:511–22. doi: 10.1515/revneuro-2016-0001. [DOI] [PubMed] [Google Scholar]
- 138.Mezzarane RA, Nakajima T, Zehr EP. After stroke bidirectional modulation of soleus stretch reflex amplitude emerges during rhythmic arm cycling. Front Hum Neurosci [online]. 2014;8:1–9. doi: 10.3389/fnhum.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jessop T, Depaola A, Casaletto L, Englard C, Knikou M. Short-term plasticity of human spinal inhibitory circuits after isometric and isotonic ankle training. Eur J Appl Physiol. 2013;113:273–84. doi: 10.1007/s00421-012-2438-1. [DOI] [PubMed] [Google Scholar]
- 140.Sefton JEM, Yarar C, Carpenter DM, Berry JW. Physiological and clinical changes after therapeutic massage of the neck and shoulders. Man Ther [online]. Elsevier Ltd; 2011;16:487–94. 10.1016/j.math.2011.04.002 [DOI] [PubMed]
- 141.Goldberg J, Seaborne DE, Sullivan SJ, Leduc BE. The effect of therapeutic massage on H-reflex amplitude in persons with a spinal cord injury. Phys Ther. United States. 1994;74:728–37. doi: 10.1093/ptj/74.8.728. [DOI] [PubMed] [Google Scholar]
- 142.Ji Q, He H, Zhang C, et al. Effects of whole-body vibration on neuromuscular performance in individuals with spinal cord injury: A systematic review. Clin Rehabil [online]. 2016;026921551667101. 10.1177/0269215516671014 [DOI] [PubMed]
- 143.Winkler T, Hering P, Straube A. Spinal DC stimulation in humans modulates post-activation depression of the H-reflex depending on current polarity. Clin Neurophysiol [online]. International Federation of Clinical Neurophysiology. 2010;121:957–61. doi: 10.1016/j.clinph.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 144.Hofstoetter US, McKay WB, Tansey KE, Mayr W, Kern H, Minassian K. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J Spinal Cord Med [online]. 2014;37:202–11. doi: 10.1179/2045772313Y.0000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Murray LM, Tahayori B, Knikou M. Transspinal direct current stimulation produces persistent plasticity in human motor pathways. Sci Rep [online]. Springer US; 2018;8. 10.1038/s41598-017-18872-z [DOI] [PMC free article] [PubMed]
- 146.Ievins A, Moritz CT. Therapeutic stimulation for restoration of function after spinal cord injury. Physiology [online]. 2017;32:391–8. 10.1152/physiol.00010.2017 [DOI] [PubMed]
- 147.Del Felice A, Daloli V, Masiero S, Manganotti P. Contralesional cathodal versus dual transcranial direct current stimulation for decreasing upper limb spasticity in chronic stroke individuals: A Clinical and Neurophysiological Study. J Stroke Cerebrovasc Dis [online]. Elsevier Inc.; 2016;25:2932–41. 10.1016/j.jstrokecerebrovasdis.2016.08.008 [DOI] [PubMed]
- 148.Chieffo R, Comi G, Leocani L. Noninvasive neuromodulation in poststroke gait disorders: Rationale, feasibility, and state of the art. Neurorehabil Neural Repair. 2016;30:71–82. 10.1177/1545968315586464 [DOI] [PubMed]
- 149.Palm U, Ayache SS, Padberg F, Lefaucheur JP. Non-invasive brain stimulation therapy in multiple sclerosis: A review of tDCS, rTMS and ECT results. Brain Stimul [online]. Elsevier Inc. 2014;7:849–54. doi: 10.1016/j.brs.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 150.Tazoe T, Perez MA. Effects of repetitive transcranial magnetic stimulation on recovery of function after spinal cord injury. Arch Phys Med Rehabil [online]. Elsevier Ltd. 2015;96:S145–55. doi: 10.1016/j.apmr.2014.07.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim DH, Shin JC, Jung S, Jung TM, Kim DY. Effects of intermittent theta burst stimulation on spasticity after stroke. Neuroreport. 2015;26:561–6. 10.1097/WNR.0000000000000388 [DOI] [PMC free article] [PubMed]
- 152.Christie AD, Inglis JG, Boucher JP, Gabriel DA. Reliability of the FCR H-Reflex. J Clin Neurophysiol [online]. 2005;22:204–9. [PubMed] [Google Scholar]
- 153.Hopkins JT, Ingersoll CD, Cordova ML, Edwards JE. Intrasession and intersession reliability of the soleus H-reflex in supine and standing positions. Electromyogr Clin Neurophysiol [online]. 2000;40:89–94. [PubMed] [Google Scholar]
- 154.Palmierie R, Hoffman M. Ingersoll C. Intersession reliability for h-reflex measurements arising from the soleus, peroneal, and tibialis anterior musculature. Int J Neurosci [online]. Taylor & Francis. 2002;112:841–50. doi: 10.1080/00207450290025851. [DOI] [PubMed] [Google Scholar]
- 155.Carp JS, Tennissen AM, Xiang YC, Wolpaw JR. Diurnal H-reflex variation in mice. Exp. Brain Res. 2006;168:517–28. doi: 10.1007/s00221-005-0106-y. [DOI] [PubMed] [Google Scholar]
- 156.Stegeman D, Hermens H. Standards for surface electromyography: The European project Surface EMG for non-invasive assessment of muscles (SENIAM) [online] 2007. [Google Scholar]
- 157.Miljković N, Malešević N, Kojić V, Bijelić G, Keller T, Popović DB. Recording and assessment of evoked potentials with electrode arrays. Med Biol Eng Comput. 2015;53:857–67. doi: 10.1007/s11517-015-1292-9. [DOI] [PubMed] [Google Scholar]
- 158.Andersen RE, Ranieri A. A novel sciatic nerve stimulation technique for assessing phase modulation of the H-reflex in the hamstrings during human gait [online]. Aalborg University; 2015.
- 159.Botter A, Vieira TM. Optimization of surface electrodes location for H-reflex recordings in soleus muscle. J Electromyogr Kinesiol [online]. Elsevier Ltd. 2017;34:14–23. doi: 10.1016/j.jelekin.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 160.Kojic V, Miljkovic N, Maleševic N, Popovic DB. H-reflex recorded by multi-pad EMG electrodes. 11th Symposium on Neural Network Applications in Electrical Engineering (NEUREL) 2012. Online. p. 119–22. 10.1109/NEUREL.2012.6419981
- 161.Racinais S, Cresswell AG. Temperature affects maximum H-reflex amplitude but not homosynaptic postactivation depression. Physiol Rep. 2013;1:1–7. doi: 10.1002/phy2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Krause BA, Hopkins JT, Ingersoll CD, Cordova ML, Edwards JE. The Relationship of Ankle Temperature During Cooling and Rewarming to the Human Soleus H Reflex. Sport Rehabil. 2000;9:253–62. doi: 10.1123/jsr.9.3.253. [DOI] [Google Scholar]
- 163.Dewhurst S, Riches PE, Nimmo MA, De Vito G. Temperature dependence of soleus H-reflex and M wave in young and older women. Eur J Appl Physiol. 2005;94:491–9. doi: 10.1007/s00421-005-1384-6. [DOI] [PubMed] [Google Scholar]
- 164.Walton C, Kalmar J, Cafarelli E. Caffeine increases spinal excitability in humans. Muscle and Nerve. 2003;28:359–64. doi: 10.1002/mus.10457. [DOI] [PubMed] [Google Scholar]
- 165.Mori N, Horino H, Matsugi A, Kamata N, Hiraoka K. Tonic suppression of the soleus H-reflex during rhythmic movement of the contralateral ankle. J Phys Ther Sci [online]. 2015;27:1287–90. doi: 10.1589/jpts.27.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lagerquist O, Zehr EP, Baldwin ERL, Klakowicz PM, Collins DF. Diurnal changes in the amplitude of the Hoffmann reflex in the human soleus but not in the flexor carpi radialis muscle. Exp Brain Res. 2006;170:1–6. doi: 10.1007/s00221-005-0172-1. [DOI] [PubMed] [Google Scholar]
- 167.Hundza SR, Zehr EP. Suppression of soleus H-reflex amplitude is graded with frequency of rhythmic arm cycling. Exp Brain Res. 2009;193:297–306. doi: 10.1007/s00221-008-1625-0. [DOI] [PubMed] [Google Scholar]
- 168.Zehr EP, Klimstra M, Johnson EA, Carroll TJ. Rhythmic leg cycling modulates forearm muscle H-reflex amplitude and corticospinal tract excitability. Neurosci Lett. 2007;419:10–4. doi: 10.1016/j.neulet.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 169.Baldissera F, Cavallari P, Leocani L. Cyclic modulation of the H-reflex in a wrist flexor during rhythmic flexion-extension movements of the ipsilateral foot. Exp Brain Res. 1998;118:427–30. doi: 10.1007/s002210050297. [DOI] [PubMed] [Google Scholar]
- 170.Frigon A, Carroll TJ, Jones KE, Zehr EP, Collins DF. Ankle position and voluntary contraction alter maximal M waves in soleus and tibialis anterior. Muscle and Nerve. 2007;35:756–66. doi: 10.1002/mus.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Evatt ML, Wolf SL, Segal RL. Modification of human spinal stretch reflexes: Preliminary studies. Neurosci Lett. 1989;105:350–5. doi: 10.1016/0304-3940(89)90646-0. [DOI] [PubMed] [Google Scholar]
- 172.Wolpaw JR, Braitman DJ, Seegal RF. Adaptive plasticity in primate spinal stretch reflex: initial development. J Neurophysiol. 1983;50:1296–311. doi: 10.1152/jn.1983.50.6.1296. [DOI] [PubMed] [Google Scholar]
- 173.Carp JS. H-Reflex Operant conditioning in mice. J Neurophysiol [online]. 2006;96:1718–27. 10.1152/jn.00470.2006 [DOI] [PubMed]
- 174.Chen XY, Wolpaw JR. Operant conditioning of H-reflex in freely moving rats. J Neurophysiol. 1995;73:411–5. [DOI] [PubMed]
- 175.Wolpaw JR. The cerebellum in maintenance of a motor skill: A hierarchy of brain and spinal cord plasticity underlies H-reflex conditioning. Learn Mem [online]. 2006;13:208–15. doi: 10.1101/lm.92706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen XY, Wolpaw JR. Ablation of cerebellar nuclei prevents H-reflex down-conditioning in rats. Learn Mem [online]. 2005;12:248–54. doi: 10.1101/lm.91305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wolpaw JR. What can the spinal cord teach us about learning and memory? Neuroscientist. 2010;16:532–49. doi: 10.1177/1073858410368314. [DOI] [PubMed] [Google Scholar]
- 178.Norton JJ, Wolpaw JR. Acquisition, maintenance, and therapeutic use of a simple motor skill. Curr Opin Behav Sci [online]. Elsevier; 2018;20:138–44. 10.1016/j.cobeha.2017.12.021 [DOI] [PMC free article] [PubMed]
- 179.Chen Y. The interaction of a new motor skill and an old one: H-reflex conditioning and locomotion in rats. J Neurosci [online]. 2005;25:6898–906. 10.1523/JNEUROSCI.1684-05.2005 [DOI] [PMC free article] [PubMed]
- 180.Chen Y, Chen L, Wang Y, Wolpaw JR, Chen XY. Operant conditioning of rat soleus H-reflex oppositely affects another H-reflex and changes locomotor kinematics. J Neurosci [online]. 2011;31:11370–5. 10.1523/JNEUROSCI.1526-11.2011 [DOI] [PMC free article] [PubMed]
- 181.Makihara Y, Segal RL, Wolpaw JR, Thompson AK. Operant conditioning of the soleus H-reflex does not induce long-term changes in the gastrocnemius H-reflexes and does not disturb normal locomotion in humans. J Neurophysiol [online]. 2014;112:1439–46. doi: 10.1152/jn.00225.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wolpaw JR. The negotiated equilibrium model of spinal cord function. J Physiol. 2018;in press. [DOI] [PMC free article] [PubMed]
- 183.Chen Y, Chen L, Wang Y, Wolpaw JR, Chen XY. Persistent beneficial impact of H-reflex conditioning in spinal cord-injured rats. J Neurophysiol [online]. 2014;112:2374–81. doi: 10.1152/jn.00422.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Thompson AK, Gill CR, Cote RH, Wolpaw JR. A strategy to enhance the plasticity in the targeted pathway: Operant down-conditioning of the soleus H-reflex during walking in people with chronic incomplete spinal cord injury. Program No. 780.14. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience 2017. Online.
- 185.Gill CR, Segal RL, Feng WW, Thompson AK. Operant down-conditioning of the soleus H-reflex in people after stroke. Program No. 780.15. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience 2017. Online.
- 186.McCane LM, Heckman SM, Sullivan B, et al. Operant conditioning the soleus H-reflex in a person with traumatic brain injury improves mobility. Program No. 780.06. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience 2017. Online.
- 187.Chen L, Chen Y, Yang X, Wang Y, Wolpaw JR, Chen XY. Impact of soleus H-reflex conditioning on soleus H-reflex and locomotor function in rats with sciatic nerve transection and regeneration. Program No. 780.04. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience 2017. Online.
- 188.English AW, Chen Y, Carp JS, Wolpaw JR, Chen Y, Chen XY. Recovery of electromyographic activity after transection and surgical repair of the rat sciatic nerve recovery of electromyographic activity after transection and surgical repair of the rat Sciatic Nerve. J Neurophysiol. 2007;1127–34. 10.1152/jn.01035.2006 [DOI] [PubMed]
- 189.Chen Y, Chen L, Yang X, Wang Y, Wolpaw JR, Chen XY. Effects of combining H-reflex conditioning and locomotor training on locomotor recovery in rats with lateral column transection: Initial study. Program No. 780.09. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience 2017. Online.
- 190.Hani S, de Marcellis-warin N. Open innovation and involvement of end-users in the medical device technologies’ design & development process: End-users’ perspectives. Technol Invest [online]. 2016;7:73–85. 10.4236/ti.2016.73010
- 191.Martin JL, Clark DJ, Morgan SP, Crowe JA, Murphy E. A user-centred approach to requirements elicitation in medical device development: A case study from an industry perspective. Appl Ergon [online]. Elsevier Ltd; 2012;43:184–90. 10.1016/j.apergo.2011.05.002 [DOI] [PubMed]
- 192.Money AG, Barnett J, Kuljis J, Craven MP, Martin JL, Young T. The role of the user within the medical device design and development process: Medical device manufacturers’ perspectives. BMC Med Inform Decis Mak [online]. BioMed Central Ltd; 2011;11:15. 10.1186/1472-6947-11-15 [DOI] [PMC free article] [PubMed]
- 193.WebPT. The State of Rehab Therapy in 2017 [online]. 2017. Available at: https://www.webpt.com/resources/webinars/the-state-of-rehab-therapy-in-2017
- 194.Harris W and Co. Physical Therapy Market Overview [online]. 2014. Available at: https://www.harriswilliams.com/system/files/industry_update/2014.2.17_physical_therapy_overview.pdf
- 195.Diage T. Planning for Successful Medical Device Reimbursement: [online]. 2013. Available at: https://www.namsa.com/expertise/library/planning-for-successful-medical-device-reimbursement-so-your-device-is-cleared-now-what/
- 196.Tarricone R, Boscolo PR, Armeni P. What type of clinical evidence is needed to assess medical devices? Eur Respir Rev [online]. 2016;25:259–65. doi: 10.1183/16000617.0016-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence—What is it and what can it tell us? N Engl J Med [online]. 2016;375:2293–7. 10.1056/NEJMsb1609216 [DOI] [PubMed]
- 198.Cigna. Medical Coverage Policy (0160): Electrical Stimulation Therapy and Devices [online]. 2014. Available at: https://cignaforhcp.cigna.com/
- 199.Capital Blue. Neuromuscular and functional neuromuscular electrical stimulation [online]. 2017. Available at: https://www.capbluecross.com/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 513 kb)