Abstract
Human performance, endurance, and resilience have biological limits that are genetically and epigenetically predetermined but perhaps not yet optimized. There are few systematic, rigorous studies on how to raise these limits and reach the true maxima. Achieving this goal might accelerate translation of the theoretical concepts of conditioning, hormesis, and stress adaptation into technological advancements. In 2017, an Air Force-sponsored conference was held at the University of Massachusetts for discipline experts to display data showing that the amplitude and duration of biological performance might be magnified and to discuss whether there might be harmful consequences of exceeding typical maxima. The charge of the workshop was “to examine and discuss and, if possible, recommend approaches to control and exploit endogenous defense mechanisms to enhance the structure and function of biological tissues.” The goal of this white paper is to fulfill and extend this workshop charge. First, a few of the established methods to exploit endogenous defense mechanisms are described, based on workshop presentations. Next, the white paper accomplishes the following goals to provide: (1) synthesis and critical analysis of concepts across some of the published work on endogenous defenses, (2) generation of new ideas on augmenting biological performance and resilience, and (3) specific recommendations for researchers to not only examine a wider range of stimulus doses but to also systematically modify the temporal dimension in stimulus inputs (timing, number, frequency, and duration of exposures) and in measurement outputs (interval until assay end point, and lifespan). Thus, a path forward is proposed for researchers hoping to optimize protocols that support human health and longevity, whether in civilians, soldiers, athletes, or the elderly patients. The long-term goal of these specific recommendations is to accelerate the discovery of practical methods to conquer what were once considered intractable constraints on performance maxima.
Keywords: preconditioning, conditioning, adaptation, hormesis, stress, tolerance, biphasic, dose–response, U-shaped, J-shaped, fitness, endurance, epigenetics, dietary restriction, caloric restriction
Introduction and Historical Overview
Over the last century and a half, a large body of scientific research has demonstrated the existence of biological defense mechanisms that are triggered by stressful but subtoxic stimuli and culminate in the protection, repair, and enhancement of biological systems at the molecular, cellular, and organismal levels. There are a number of terms with overlapping meanings that have been used to define these natural phenomena, including but not limited to hormesis, conditioning, tolerance, adaptive homeostasis, priming, nonmonotonicity, biphasic, rebound effect, overcompensation, plasticity, and stress adaptation.1–7 As discussed below, researchers have identified many classes of stimuli that trigger endogenous defense mechanisms—ranging from chemical and radiation exposures to intermittent fasting, regular exercise, and phytochemical intake. However, translation into specific clinical recommendations has been sluggish. One explanation for this gap in the field is the relative scarcity of rigorous animal studies that systematically (1) optimize the dose, duration, frequency, and timing of the stimulus; (2) coadminister potentially synergistic stimuli; (3) measure the temporal kinetics of the biological response; (4) assess the influence of modifiers such as age, gender, and comorbidities; and (5) rigorously confirm a lack of adverse effects. Perhaps due to some of these deficiencies, the amplitude and duration of the defensive response to mildly stressful stimuli have been disappointingly modest.
The key problems listed above were addressed collaboratively among experts in the field of endogenous defenses at an Air Force-sponsored conference organized by Dr Edward Calabrese and hosted at the University of Massachusetts on October 25 and 26, 2017. The specific charge of the workshop was “to examine, discuss, and if possible, recommend approaches to control and exploit endogenous defense mechanisms to enhance the structure and function of biological tissues.” The goal of this review is not to abridge the entire presentations but to describe key findings of relevance to the workshop charge, to expand upon some of the discussion, and to integrate the current state of knowledge into a broad, if speculative, bird’s-eye view. As there was no open discussion or consensus on specific research guidelines at the conference itself, the recommendations of this white paper were formulated and written afterward to address the critical need for a systematic approach to hormesis. The article was then approved by all the workshop participants. It is not our intent to make specific clinical recommendations but, rather, to propose specific research guidelines on how to systematically optimize protocols that raise biological fitness in at least two in vivo organisms, across the dimensions of response amplitude and response duration, in order to reach the genetically and epigenetically determined maxima.
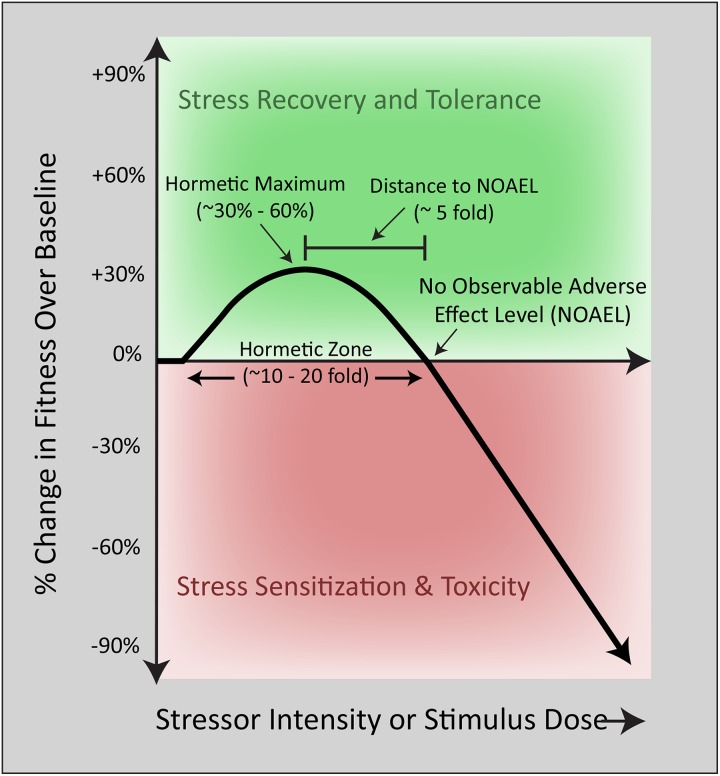
The first scientific study of a biphasic dose–response phenomenon was conducted in the 1880s by the German pharmacologist Hugo Schulz, who reported that disinfectants stimulated yeast metabolism at low concentrations but inhibited the same process at higher concentrations.1,5,8 Subsequent work in 1943 at the University of Idaho by forestry researchers Ehrlich and Southam corroborated the existence of nonlinear dose–response curves in studies of the effects of red cedar tree extracts on fungi metabolism.1,5,8,9 Southam and Ehrlich were the first to define this phenomenon as “hormesis,” from the Greek word “hormaein” (to excite, stimulate, or spur into action). Since these seminal reports, biphasic dose–response curves have been repeatedly observed in response to pharmacological and environmental stimuli otherwise known as harmful, particularly in the fields of toxicology and ionizing radiation.5,10,11 Modest stimulatory effects on fitness measures such as hyperplasia or cell metabolism are often reported at low doses, whereas sufficiently high doses overwhelm and inhibit natural defense mechanisms and may lead to the atrophy or demise of irreparably injured cells. These nonlinear responses almost invariably result in J-shaped curves (or inverted J-shaped or β curves, depending on the outcome measure), with reproducible, quantitative features, such as a typically modest stimulation amplitude and placement of the stimulatory portion of the curve immediately adjacent to the “no observable adverse effect level” (Figure 1).
Figure 1.
The traditional biphasic dose–response curve. Biological fitness as a function of stimulus intensity. The mathematical features of a typical biphasic dose–response curve in the literature are displayed. At low doses, a single exposure to a stimulus elicits a modest increase in cellular fitness according to the principles of hormesis. With increasing stressor intensity, cellular defense systems are overwhelmed, and frank toxicity emerges at stimulus levels greater than the no observable adverse effect level (NOAEL).
Hormesis shares striking dose–response similarities with a number of other biological phenomena, such as preconditioning, which was reported in influential ischemia studies of the 1980s.12–14 In those studies, preconditioning was defined as an adaptive response to transient, sublethal ischemic episodes. Exposure to brief ischemic episodes improved the ability of stressed tissues to survive and recover from subsequent, longer enduring ischemic attacks. Recent studies also support the existence of postconditioning, in which exposure to brief ischemic events after the severe ischemic challenge similarly stimulates endogenous defenses and mitigates ischemia/reperfusion injury.15,16
Both hormesis and preconditioning share similarities with drug tolerance in pharmacology, in that exposure to drugs also elicits compensatory biological effects (eg, increased detoxification by the liver) that temper the subsequent biological impact of the drug and elicit the need for dose escalation to generate the same response as before. Similarly, the father of stress research, Hans Selye, coined the term “general adaptation syndrome” in 1936 to describe a stage of resistance observed after an initial alarm reaction in animals exposed to a wide variety of stressors.17,18 If the stressors were chronic and unremitting, the resistance stage would be followed by an exhaustion stage. Selye defined stimulatory and beneficial stress as “eustress,” whereas negative, unyielding stress was defined by him as “distress” and reported to elicit immunosuppression, ulcer formation, and perhaps untimely death. Selye presciently wrote of “conditioning factors,” such as preexisting disease states and dietary factors, which he predicted modify the course of general adaptation syndrome.17 Finally, biphasic effects of psychological stress on cognitive function have been studied in humans for many decades, beginning with the 1908 report of Yerkes and Dodson on the nonlinear relationship between task complexity and human performance and its revival by Broadhurst in the 1950s.2,19,20 In sum, the biphasic nature of the dose–response curve has been reported by many researchers in entirely independent fields of study; it is a generalizable, common evolutionary strategy displayed at multiple levels of cellular and tissue organization across all phyla—in plants, microbes, invertebrates, and vertebrates.
Attempts to Unify the Terminology and Identify Shared Mechanisms
Although we intend to focus on the beneficial effects of the stimulatory portion of the biphasic dose-response curve, the hormetic response is not construed a priori as either beneficial or harmful but is more straightforwardly defined as low-dose stimulation and high-dose inhibition.21 Hormesis per se may or may not produce a desirable outcome. Two examples of undesirable outcomes would be stimulation of bacterial growth at low antibiotic doses and increased growth of weeds at low doses of herbicides. In contrast, terms such as preconditioning and postconditioning imply a favorable boost in natural resilience. Furthermore, the term hormesis is typically used to encompass responses to single-stimulus exposures, whereas pre- and postconditioning are defined as the response to two or more stimulus exposures, with one “stimulatory” and one “challenge” exposure. On the other hand, the dose–response profiles of conditioning, adaptation, tolerance, and hormesis all assume the basic U- or J-shaped profile.6,22,23
Given the discipline-specific contexts in which these terms are employed, it has been difficult to unify the terminology across fields, although the phenotypic expression of resilience is similar across various levels of biological organization. For example, one common response to various classes of stressful stimuli is the activation and nuclear translocation of master transcription factors (eg, nuclear factor erythroid 2-related factor 2 or Nrf2) after engagement of key stress-sensitive signal transduction pathways. Stress-sensitive transcription factors often induce the expression of genes involved in survival, repair, redox equilibrium, protein quality control, and mitochondrial bioenergetics. The subsequent changes in phenotype marshal the structural and functional improvements observed in stimulated, stress-resistant cells. Although low levels of reactive oxygen species lie at the apex of many stress-sensitive signal transduction cascades,24,25 the biological responses elicited by hormetic and conditioning stimuli are remarkably variegated, involving activation or induction of hundreds of signaling pathways and genes, as discussed further below. In addition, the classes of stimuli that elicit beneficial stress responses are highly varied, ranging from exercise, dietary fasting, drug exposure, light, electricity (eg, transcranial direct current stimulation), to ionizing radiation. For partly these reasons, it has been difficult to pinpoint one master stress-sensing switch common to all forms of expression of biological resilience. Therefore, it might be more productive to view the biological mechanisms underlying natural resilience as inherently variable, permitting remarkable flexibility and redundancy in biological systems. Despite this natural diversity, biphasic dose–response curves are relatively uniform in profile and quantitative features, perhaps reflecting the convergence of a wide array of stress response pathways on the elaboration of an archetypal resistant phenotype. Indeed, this funneling of distinct stress-sensitive pathways may explain why the potency of hormetic stimuli and their specific mechanisms of action do not necessarily change the quantitative features of the biphasic dose–response curve.26
Constraints on Biological Performance Maxima
Although changes in receptor activation, signal transduction cascades, posttranslational modifications, and gene expression are frequently summoned to explain the biphasic nature of the hormetic curve, the specific reasons underlying the complete switch in direction of the curve (ie, from stimulation to inhibition) remain poorly understood at a mechanistic level. Related to the switch in direction is the limit in amplitude or “ceiling” of the stimulatory phase of the typical hormetic dose–response curve, often described as ∼30% to 60% of control values3,5,6,27–29 (Figure 1). Potential explanations for these constraints may be availability of nutrients and resources, saturation in enzyme kinetics and metabolic pathways, or the eventual corruption of biological information, such as with protein oxidation and misfolding in chronically stimulated cells. The stimulatory maxima likely depend on both the existing bioenergetic state of the perturbed cell and the subsequent energy needs of the hormetic response. Prolonged maintenance of a primed state might be too energetically demanding, and dramatic intracellular changes may be too disruptive to homeostasis, leading to instability. However, these answers do not satisfactorily explain why the amplitude of the biphasic dose–response curve might be constrained under conditions of nutrient repletion. In addition, they fail to explain why cells with sufficient access to energy resources do not generally maintain the heightened state of resilience for extended periods but revert to baseline conditions once the stimulus has waned, usually within a week or two. In response to stress, a sustained increase in prosurvival factors may increase the probability of malignant transformation and may therefore not have been favored by evolution; the proteins that orchestrate cell survival (eg, Bcl2) commonly antagonize the effects of tumor suppressor proteins (eg, p53). It is also not known whether a dramatic increase in the stimulatory portion of the biphasic dose-response curve will elicit a net loss in other variables that are equally important for long-term health. For example, the fitness advantage of stress adaptation in plants under conditions of injury or disease may be disadvantageous under disease-free conditions.30
One possible reason for the low stimulatory maximum or hormetic ceiling in Figure 1 may be that the stress-induced increase in biological performance has been evolutionarily shaped by the intermittent nature of many stressors, which would not demand a chronic response to a single stimulus. Almost all biological processes are designed to wax and wane, as best exemplified by circadian rhythms driven by cyclical expressions of genes that are entrained to the environmental photoperiod. Another example of biological rhythmicity is the fortification of muscle and bone during the recovery interval between exercise bouts, rather than during the period of physical exertion itself. It may not be energetically feasible to upregulate endogenous defenses and fortify injured cells during an episode of ischemic conditioning, as oxygen and nutrient delivery to tissue is a prerequisite for metabolic recovery and robust generation of ATP. Thus, rest periods appear critical for stress recovery, as most repair processes are engaged during the periods of reprieve, unless perhaps the stimulus is extremely low in dose.31 Thus, if cells are exposed to “conditioning” stimuli at repeated, rhythmic intervals and not only once, the hormetic responses might be all the more profound and closer to the true biological maxima (discussed further below).
Intermittent and rhythmic stressors of low intensity may elicit serial mini-responses that progressively build layers of a strong foundation for the maximal expression of biological fitness. In contrast, chronic and severe stressors elicit pathological disorders, perhaps including progressive neurodegenerative conditions with cumulative and inexorable neuron loss.32 Neurodegenerative diseases often display an initial compensatory phase with a U-shaped profile during which symptoms are held at bay until a threshold of neuron loss is exceeded.33–42 Over many years of progression, the degree of protein deposition, the severity of neuron loss, and the intensity of the neurodegenerative disease rise incrementally, supporting the view that the duration of the disease lies in rough proportion to “disease dose.” This concept supports the importance of the temporal dimension of biphasic stress–response curves and is consistent with Selye’s temporal distinctions between acute eustress and chronic distress (see “Introduction and Historical Overview” section). Indeed, the correlation between disease duration and disease severity/dose may underlie the U-shaped profile of the early compensatory changes in neurodegenerative disorders when plotted as a function of time from disease onset.32 Another example of the critical importance of the temporal dimension is that the duration of the recovery interval between two stressors also determines the direction of stress response (see discussion in43). In other words, stimuli applied without a sufficient recovery interval might potentiate lethality or toxicity instead of conditioning the organism.44 In the field of neurodegeneration, the stress potentiation phenomenon is often cited as support of the “two-hit hypothesis,” in which dual hits that are either too close in time or too intense in concentration elicit greater than additive toxicity rather than any conditioning effect.32,45
Can the Typical Constraints on Biological Performance be Circumvented?
Despite some of the restrictions alluded to above, biological performance and resilience are likely more plastic than anticipated and the dynamic range (or duration) of the stimulatory portion of the average biphasic dose–response curve may be enhanced by a number of modifications and/or vary with the nature of the stimuli. For example, the enhancements in cognition with hormetic stimuli such as transcranial direct current stimulation and low-level light therapy have been shown to endure for months.46–49 In order to raise the ceiling of the stimulatory portion of the biphasic dose–response curve, it is important to consider the lifestyles of hominid and other predatory ancestors.50 Prehistoric hominids had intermittent access to food relative to modern, nutrient-replete conditions, and yet, the food-deprived brain and musculoskeletal system had to continue to function well in order to tackle the problem of food scarcity and secure additional nutrient resources. This evolutionary requirement might explain why hominids and other mammals engage in greater physical activity and exhibit superior cognitive function after short periods of dietary restriction, which activate a series of beneficial metabolic changes centering on increased production of ketones.50–54 Modern eating habits and sedentary lifestyles rarely deplete glycogen stores or raise performance-enhancing ketones, effectively short-circuiting the biological processes favored by evolution. As argued below, intermittent periods of dietary restriction can even extend lifespan (see “Dietary Restriction as a Hormetic or Conditioning Stimulus” and “Lifespan Extension as an Enduring Hormetic Response” sections), suggesting that repeated exposures to hormetic stimuli can continue to have profound and enduring beneficial effects. From a clinical perspective, a major advantage of implementing the recommended changes in eating and exercise habits is that they have few, if any negative side effects compared to medications and activate a wider array of biological targets, increasing the likelihood of sizable and enduring beneficial effects. In contrast, the traditional strategy of designing selective drugs to preferentially bind a single or few targets has often met with disappointment in the clinic.
As stated above, mitochondrial biogenesis and other endogenous defenses might not be increased during the stress period, but during the rest period, when protein synthesis is boosted and cellular structures and organelles such as synapses and mitochondria are formed. The intermittent nature of conditioning stimuli (such as food deprivation) and the subsequent recovery period may be important for optimizing health, similar to the periodicity of rest–wake cycles. Perhaps for this very reason, the long-term health consequences of prolonged circadian disruption are grim.55–57 The importance of biological rhythmicity is well supported by recent studies, showing that prevention of an increase in sleep time in preconditioned animals abolished ischemic tolerance in preclinical stroke models.58 In short, strengthening circadian control by increasing the amplitude of biological rhythms—such as increasing the degree of physical exertion during the day and the duration and quality of nighttime sleep—may further optimize biological performance. By extension, limiting meals to a 7-hour period during the day to raise the amplitude of the circadian rhythm of food intake might benefit patients with metabolic disorders, and limiting tube-feeding times in critically ill patients to restricted diurnal hours could speed their recovery.59,60
Dietary Restriction as a Hormetic or Conditioning Stimulus
Avoidance of nutrient repletion may be a simple and economical strategy to accelerate a safe recovery from planned stressors, including elective surgeries. Approximately 80 years ago, caloric restriction without malnutrition, when compared to ad libitum feeding, was found to extend the lifespan of rats.61 Dietary restriction has two highly evolutionarily conserved properties: (1) It increases multiple forms of stress resistance, and (2) it produces a rapid onset of beneficial effects. Many studies confirm the beneficial effects of dietary restriction in protecting against radiation, inflammation, and ischemia–reperfusion injury.51,62,63 For example, within a few weeks of onset, dietary restriction can reduce mortality and precondition the liver or kidney against injury in response to ischemia, leading to superior redox equilibria and insulin sensitivity.64 Dietary restriction can also protect against infections such as cerebral malaria65—an example of “cross-tolerance,” whereby pre-exposure to one type of mild stimulus can condition against another entirely different type of harmful stimulus.
As previously mentioned, dietary restriction acts to switch liver metabolism from the breakdown of hepatic glycogen into glucose to the breakdown of adipose fatty acids into ketones, which are important not only as the metabolic substrates for ATP synthesis but also serve as signaling molecules. These metabolic changes increase mitochondrial numbers, angiogenesis, exercise capacity, and longevity.66–68 Studies in vertebrates, including nonhuman primates and human patients, demonstrate that dietary restriction accomplishes the following: (1) reductions in disease burden, markers of oxidative stress, and inflammation; (2) improvements in cholesterol, triglycerides, and blood pressure; and (3) delays in mortality.68–71 In humans, the benefits of dietary restriction are not limited to those who struggle with weight or shoulder the burden of disease. Even in nonobese 21- to 51-year-old humans, caloric restriction is not only feasible but also mitigates cardiac and metabolic risk factors and inflammation—although it may also reduce bone density.68,72,73 In nonoverweight, healthy middle-aged adults, a simple reduction in meal frequency without any change in calories reduces cortisol levels and body fat mass without overall weight changes, whereas blood pressure and total cholesterol values are increased.74
Dietary restriction can be achieved with a number of methods, although most dietary restriction studies have tested the effects of 20% to 40% reductions in caloric intake. Alternative approaches include restricting the time of feeding to 7 hours during the day without reducing total caloric intake or eliminating specific nutrients, such as methionine, from the diet.75,76 Dietary administration of exogenous ketones may also exert benefits52 and is sometimes practiced to enhance performance in elite athletes. On the other hand, no clear benefits of boosting dietary ketone intake in military Special Operations Forces have been observed.77
Our closest living relatives, the chimpanzees, engage in tribal warfare and patrol their territorial boundaries for considerable distances. During these times, the males restrict feeding and social behavior.78,79 However, not all behaviors observed in great apes are adaptive, and it may be difficult to make sound recommendations on caloric restriction for soldiers, as military personnel generally require a wide range of kilocalories per day during training missions (3109-7131 kcal).80 Glucocorticoid secretion during periods of stress is known to increase caloric intake and dictate energy balance.81 The rigor of basing a military diet on research data derived exclusively from caged animals living in relatively stress-free, nonenriched, constant temperature environments remains in question. Although studies are warranted to determine the effects of dietary modifications on modern-day soldiers during periods of high stress and simulated combat, there is no assurance that they will accurately depict the complex relationships existing between dietary patterns, cortisol levels, anxiety, and measures of health and vigor during the starker privations of wartime.
Based on 17 years of experience in Iraq and Afghanistan, the US Army is revamping its physical fitness test to emphasize strength training. The average infantryman carries ∼80 pounds of equipment for a marching load, and in an emergency their load may be considerably heavier. As an example, casualty evacuation requires carrying or dragging a fellow, body-armored soldier to safety, translating to perhaps 200 pounds or more of total extra weight. Low muscle mass is associated with frailty and mortality,82 and soldiers losing sufficient muscle mass from protein or calorie restriction may lower their probabilities of survival; caution must therefore be exercised with recommendations of fasting or limiting protein intake immediately, before, and during wartime. Rigorous retrospective studies of dietary habits, combat readiness, and battle success probability are recommended and should be followed by prospective studies if the data are unambiguously positive.
Physical Exertion as a Hormetic or Conditioning Stimulus
Aside from being hungry more often, hominid ancestors likely existed in a better state of physical conditioning due to higher activity profiles than modern humans. Exercise is a primordial preconditioning stimulus shown to benefit brain and musculoskeletal health in manifold ways, including improvements in mitochondrial metabolism and biogenesis.25,51,83 Exercise has long been known to be effective in both the young and fit and the old and infirm.84 A recent meta-analysis of the exercise literature on young human patients (6-35 years of age) concluded that physical activity significantly boosts executive function.85 Exercise is also known to improve sleep quality,86 which would strengthen circadian control and produce salutary effects on a number of physiological variables. Indeed, engagement in previous occupational and physical activities is thought of as a preconditioning stimulus that reduces the risk of subsequent injury, such as during the intense basic training of the Army infantry.87
Studies conducted with experimental models demonstrate that exercise activates 5′ adenosine monophosphate-activated protein kinase (AMPK) and reduces myocardial infarct sizes by 50%.88 Furthermore, the combination of exercise and caloric restriction elicits additive increases in measures such as dendritic spine densities and neurotrophic factors in rodent models of diabetes.89,90 These enhancements may be explained by observations that exercise combined with dietary fasting further encourages the liver to switch its metabolism from glucose to ketones.31,91–93 Integrative interventions combining changes in diet, exercise, and mental training have similarly been shown to improve or maintain cognitive function in elderly human patients.94,95 The combined benefits of exercise and dietary restriction support the view that the limits of biological resilience may be extended by simultaneous application of two or more hormetic stimuli as well as by repeated application over time.
In summary, regularly scheduled, intermittent physical training (exercise) is a conditioning stimulus widely known to improve health and performance indices. Military commanders, athletic coaches, and family physicians recommend exercise to enhance the stamina and performance of almost everyone. Compared to, for example, dietary restrictions, nutraceuticals, or pharmaceuticals, physical exercise is easier to implement without developing strict guidelines or identifying therapeutic indices. Its practitioners might also enjoy the relative lack of deleterious side effects compared to classical medical interventions, although a failure to recognize its J- or U-shaped dose responsive nature can result in accidental injury or even death.
Divergence and Convergence of Biological Mediators of Stress Resistance
The mechanisms underlying the benefits of exercise and caloric restriction have been reviewed in detail elsewhere.53,91,96–98 Briefly, physical exertion increases a number of markers of cellular fitness, including but not limited to increases in AMPK, superoxide dismutases, brain-derived neurotrophic factor (BDNF), cyclic adenosine monophosphate response element-binding protein (CREB), peroxisome proliferator-activated receptor γ coactivator-1α (PGC1α), sirtuin 3 (SIRT3), calcium influx, and mitochondrial biogenesis.51,62,99,100 For example, dietary restriction and exercise stimulate increased levels of PGC1α and circulating ketones (such as 3-β-hydroxybutyrate), which then induce the expression of BDNF to help maintain dendritic spines.54,101,102 Exercise may also induce neuronal expression of SIRT3, which is known to mediate hormetic responses to oxidative, excitotoxic, and bioenergetic stressors through the hyperacetylation of mitochondrial superoxide dismutase 2 and cyclophilin D.103
Benefits of a hormetic stimulus, such as dietary restriction, can be blocked by antioxidant supplementation, such as with the thiol N-acetyl cysteine, suggesting a mechanistic role for reactive oxygen species in the hormetic response.25,104,105 The “mitohormesis” hypothesis specifically asserts that reactive oxygen species escape from the mitochondrial electron transport chain and act as signaling molecules to promote cellular fitness and extend lifespan.24,25,106 These stress-induced increases in reactive oxygen species are thought to activate Nrf2 and elicit phase II enzyme-directed improvements in redox equilibrium. During the Nrf2 activation process, reactive cysteine residues on the Nrf2-inhibitory protein Kelch-like ECH associated protein 1 (Keap1) react with reactive oxygen species or electrophiles to induce conformational changes in Keap1, causing the Nrf2–Keap1 complex to dissociate.107 Once Nrf2 is released from the Nrf2–Keap1 complex, the Keap1-dependent ubiquitination and degradation of Nrf2 are halted. The activated form of Nrf2 then translocates into the nucleus and binds to antioxidant response elements that promote the transcription of more than 200 genes involved in detoxification and redox equilibrium, including glutathione-S-transferase, superoxide dismutase, nicotinamide adenine dinucleotide phosphate (NAD(P)H): quinone oxidoreductase thioredoxins, peroxiredoxins, glutathione synthetase, and so on.107 For example, exposure to low concentrations of thiol-reactive agents, such as the cigarette component acrolein, preconditions cells and protects them against subsequent exposures to higher acrolein doses through the Nrf2-mediated induction of γ-glutamylcysteine synthetase and the subsequent production of glutathione.108
Although molecules involved in redox equilibrium, such as glutathione, heme oxygenase 1, and γ-glutamylcysteine synthetase, are often espoused as biomarkers for stress adaptation, they may not mediate cross-tolerance or underlie all cases of hormesis or preconditioning.109 For example, the ability of dietary restriction to precondition against ischemia–reperfusion injury is not Nrf2 dependent but may be mediated by a natural increase in the production of hydrogen sulfide,110 a molecule that acts as an endogenous neuromodulator of long-term potentiation in the hippocampus and as a vasodilator, among other phylogenetically ancient roles.111–113 Hydrogen sulfide production is controlled by expression of the cystathionine γ-lyase (cgl) gene, without which dietary restriction has no positive effects and hepatic stress resistance mediated by dietary restriction is abolished.110 Animals without cgl exhibit hypertension, consistent with the vasodilatory properties of hydrogen sulfide.114 Hydrogen sulfide can also act as a mimetic of dietary restriction and extend lifespan.110,115 Conversely, low levels of hydrogen sulfide are a biomarker of vascular dysfunction in humans.116 These findings are consistent with the view that increases in cellular resilience are not necessarily mediated by common biological pathways from beginning to end but can be initiated by many distinct pathways that converge upon mitochondria to supply the extra energy for building resilience.
In plants, an initial environmental stress tends to produce broad, nonspecific responses involving numerous signaling systems, whereas repeated exposures may elicit more specific and focused responses.117,118 Some plant genes are “trainable” with repeated stress exposure and display transcription rates that are higher than in other “nontrainable” genes. In mammals, ischemic preconditioning is associated with near-global downregulation of gene expression at the RNA level, whereas focal ischemia in the absence of preconditioning leads to a widespread and dramatic increase in the expression of a large number of genes.119,120 These findings have been interpreted to suggest that preconditioning “reprograms” and blunts the transcriptional response to ischemia–reperfusion injury.120 Similarly, epileptic preconditioning of the hippocampus elicits downregulation of 73% of the responding genes in the stress-tolerant group, including genes across different functional categories.121 However, blocking protein translation prevents the development of ischemic tolerance, suggesting that preconditioning-induced tolerance depends not only on the downregulation of many important genes but also on the upregulation of others.122,123
During the state of ischemic tolerance, groups of nuclear genes are often expressed. One such group of genes codes for the evolutionarily conserved polycomb proteins and is important in the development and regulation of cell identity.124 The knockdown of two polycomb proteins attenuates ischemic tolerance, whereas polycomb overexpression attenuates ischemic injury.125 These collective results suggest that the induction of key proteins to elicit the broad silencing of genes might be leveraged as a therapeutic approach, with the caveat that long-term, chronic induction of prosurvival genes combined with near-global transcriptional suppression might also drive oncogenesis. Nevertheless, the future translation of these ideas into practical applications is supported by clinical reports that gene expression profiles in peripheral immune cells predict stroke prognosis in African-American patients.126 Somewhat analogously, psychological resilience against posttraumatic stress disorder has been associated with a robust reduction in the conserved transcriptional response to adversity, which is characterized by increases in proinflammatory proteins and decreases in antiviral- and antibody-related expressions of genes many years after war service.127
An effective method of achieving global gene silencing is through epigenetic changes that may be heritable without changing the specific DNA sequence, such as by covalent modifications of DNA and histones, chromatin folding, DNA packaging around nucleosomes, and expression of noncoding RNA.128 Epigenetic changes were succinctly defined by Conrad Waddington in the 1940s as “the interactions of genes with their environment which [sic] bring the phenotype into being.”128 The best-studied example of epigenetic control of phenotype is the viable yellow Agouti mouse. As the first biosensor of maternal nutritional effects, studies of the Agouti mouse ushered in the era of environmental epigenomics.129,130 In the Agouti mouse model, genetically identical mice exhibit strikingly different coat color, body mass, and susceptibility to cancer or diabetes, depending entirely on the dietary supplementation of pregnant mothers with various methyl donors, such as folic acid, vitamin B12, choline, and betaine.131–133 The viable yellow mouse is obese and easily distinguished from the mottled or brown mice that have been exposed in utero to dietary supplementation with methyl donors or hypermethylating phytoestrogens. Supplementation of pregnant mothers with either folic acid or phytoestrogen similarly counteracts the hypomethylating effects of the endocrine disruptor bisphenol A in the Agouti model.134 The impact of gestational diet on hypermethylation, Agouti expression, and obesity persist into adulthood, suggesting stable, enduring effects on the epigenome.132 It is worth emphasizing that the hormetic stimulus of methyl donor food intake in the Agouti studies is probably intermittent or rhythmic, as it is likely to be entrained to the light–dark cycle of the animal facility.
The effects of low-dose X-ray exposure on the Agouti mouse model conform to the U-shaped profile of hormesis. Rather than observing an increase in yellow-coated, obese, illness-prone animals after exposure to low doses of radiation (similar to levels employed in the clinic), the incidence of brown-coated, healthier Agouti animals increased by 30%. As expected, this was associated with increased DNA methylation.135 Antioxidants administered prior to and during low-dose radiation exposures abolished their salutary effects and prevented the increase in DNA methylation after radiation, suggesting again that adaptive effects are mediated by increases in reactive oxygen species.135 These observations are consistent with the mitohormesis hypothesis introduced above.24,25,106
In summary, the Agouti mouse studies indicate that enduring, heritable effects on the epigenome and fitness might be evoked not only through dietary supplementation with methyl donors but also through hormetic application of low-dose X-rays. Furthermore, the abrogation of the epigenetic response to low-dose radiation by antioxidant supplementation strongly suggests mechanistic roles for reactive oxygen species and epigenetics in mediating a hormetic response. While reactive oxygen species may serve as a mechanism for cellular systems to sense, initiate, and signal hormetic responses, repetitive hormetic conditioning may reconfigure the 3-dimensional structure of the genome and reprogram its gene expression toward a longer lasting favorable state. If epigenetic mechanisms are partially responsible for hormetic reprogramming and repetitive conditioning reprograms gene expression to produce a longer lasting, healthier phenotype, then beneficial effects of repetitive conditioning—such as bouts of exercise—may also be enduring and heritable.
Lifespan Extension as an Enduring Hormetic Response
The extension of lifespan by hormetic stimuli is an excellent example of an enduring stress response. Lifespan has been extended with dietary restriction of organisms ranging from yeast, flies, and nematodes to rodents and primates.61,67,69 Not surprisingly, the degree of resistance to stress is a predictor of lifespan, in that increased stress resistance correlates with increased life expectancy in single-gene mutant nematodes, and mild stressors, such as heat, can extend nematode lifespan.136–139 Multiple exposures to mild heat shock increase lifespan by almost 50%, whereas single exposures to heat shock early in life only increase lifespan by ∼20%,140 consistent with the recommendations made below. However, the positive effects of heat exposures on nematode lifespan are greater if the stimuli are applied early in life, perhaps because lifespan-predictive proteins such as heat shock protein 16 become less responsive as the worms age.141,142 If heat is applied repeatedly to induce thermotolerance and longevity, it must be carefully titrated. Failure to systematically reduce the heat stimulus during successive exposures may abrogate the beneficial response. Heat application therefore seems to have a lower therapeutic index than exercise, which can be dramatically increased in duration and intensity until biological maxima in strength and endurance are reached and maintained for long periods. On the other hand, a mild increase in body temperature as a result of exercise might have safer hormetic properties. Notably, higher temperatures within the United States and Germany are correlated with lower mortality rates, although these studies do not establish any causal links.143,144 However, because all stress–response curves are biphasic, temperature extremes may lead to higher death rates.145
Given the short life cycle and tractability of genetic manipulations in Caenorhabditis elegans—the first eukaryotic metazoan to be sequenced—the biological mechanisms underlying hormesis, fitness, and longevity can be rigorously and rapidly demonstrated in vivo in this versatile model organism. AGE1 is the nematode homologue of PI3 kinase, a protein known to activate the Akt signal transduction pathway. Lifespan-extending mutations in the age1 gene of C elegans were discovered many years ago.146,147 Furthermore, thermotolerance can be elicited by mutations in age1, whereas thermotolerance is suppressed with loss-of-function mutations in genes of the dauer formation pathway, part of which lies downstream of AGE-1–mediated Akt activation.148,149 Expression of daf-18, a gene of the dauer formation pathway, is essential for the display of thermotolerance,148 whereas DAF-16 (homologous to mammalian FoxO3) regulates lifespan and stress reactions to toxins after it translocates to the nucleus and integrates developmental and environmental inputs.150,151 Given the relative ease and affordability of in vivo genetic and pharmacological manipulations of C elegans and studies of its aging and lifespan, the nematode model seems a logical choice for the systematic studies of hormesis discussed in the section entitled “Identification of Knowledge Gaps and Recommendations for Systematic Studies.”
Infection as a Hormetic Stressor
Many stressors of biological systems elicit adaptive responses, provided the dosimetry is rigorously investigated. The stressors most frequently associated with biphasic dose–response curves include ionizing radiation, chemicals (manmade or natural), physical exercise, oxygen free radicals, oxygen reduction or oxygen/substrate restriction (hypoxic or ischemic conditioning, respectively), and calorie restriction. However, the realm of hormetic stressors is likely to extend beyond physical and chemical agents to infectious biological pathogens (eg, viruses, bacteria, and mold). Exposure of higher organisms to low “doses” of microbes is essential for a properly functioning immune system, comparable to the impact of regular physical exercise on muscle and bone conditioning. However, high pathogen loads that overcome the epidermal barrier and other biological shields can have severely damaging effects, consistent with the biphasic nature of the stress–response curve. Recent data support differential responses of the innate immune system to infectious stress; low pathogen loads are more likely to elicit immune tolerance and resistance and to repair tissue damage, whereas higher pathogen loads may shut down these energy-intensive responses.152–155
In 1947, Paul Beeson reported that repeated injections of the archetypal pathogen-associated molecular pattern (PAMP) lipopolysaccharide elicited endotoxin tolerance in rabbits.156,157 Subsequent studies confirmed that the process of preconditioning cells or animals with PAMPs mitigates hyperinflammation.153,155 For example, pretreatment of macrophages with lipopolysaccharides was found to (1) reprogram their gene expression profiling through Toll-like receptor-induced chromatin modifications, (2) blunt the increase in proinflammatory tumor necrosis factor-α, (3) increase anti-inflammatory molecules such as interleukin-10, and (4) boost phagocytic and bactericidal properties.153,158–160 In contrast to protolerance and anti-inflammatory responses induced by lipopolysaccharides, a fungal cell wall component (β-glucan) was shown to elicit “trained immunity,” which sensitizes or primes immune and cytokine responses via mitogen activated protein kinase (MAPK) signal transduction pathways and epigenome modification.152–155,161,162 However, the concentration-dependent impact of both lipopolysaccharides and β-glucan on tumor necrosis factor-α and the secretion of interleukin-6 is biphasic. Lipopolysaccharides also induce U-shaped responses in both the proliferative and the phagocytic activities of microglia. The signal transduction processes mediating these activities involve reactive oxygen species and PI3 kinase activation,153,163 consistent with the principles of mitohormesis and the thermal stress-induced tolerance in nematodes.
The History of Ischemic Conditioning and Its Clinical Translation
In 1943, Noble reported that whole-body trauma elicited resistance against further trauma that was otherwise fatal in both rats and guinea pigs.164 Later that decade, Noell and Chinn discovered that exposure of the rabbit brain to anoxia delayed mortality in response to anoxia re-exposure. Similar effects were reported in the 1960s by Dahl and Balfour in rats and were attributed to increases in pyruvate and glycolysis.165–167 This old literature has not been associated with the appellation of biological “preconditioning” and “tolerance.” Instead, these terms are often attributed to studies by Murry and colleagues in the 1980s,168 although they were employed earlier—including in a 1964 article on lysosome shock by Janoff.169 Myocardial adaptations to sublethal ischemic events were described in influential studies of Murry and others in the 1980s, which demonstrated that (1) four 5-minute episodes of circumflex occlusions, with intervals of 5 minutes of reperfusion between each episode, protected myocardial tissue against a sustained 40 minutes of occlusion and reduced infarct size by an impressive 75%168 and (2) four intermittent 10-minute episodes of myocardial ischemia led to no loss of ATP or tissue necrosis, unlike continuous coronary artery occlusion for 40 consecutive minutes.170 These studies were swiftly followed by an explosion of work on ischemic preconditioning, including reports of marked protection of the rat retina from ischemia and robust protection of the brain against global ischemia and stroke injury.171–179
The different mechanisms underlying ischemic tolerance are the subject of numerous reviews and will not be elaborated in detail here.12–14,180–183 Briefly, ischemic tolerance was divided into an early phase (manifested within hours) and a delayed phase (manifested within days) early in the history of preconditioning research. The former was hypothesized to be mediated by ion channel openings, rapid posttranslational modifications, and autocoid secretion (eg, adenosine, bradykinin, nitric oxide, and adrenergics) and the latter by delayed changes in gene expression and protein synthesis, culminating in increased resilience, inflammation resolution, and the mitigation of reactive oxygen species and ischemia/reperfusion injury.183–185 The early studies of preconditioning also revealed the existence of a window between these two phases during which the system was refractory, and no protection could be provoked although reintroducing a brief ischemic episode defeated this noncompliance186,187 and proved consistent with the benefits of repetitive conditioning.
Aside from the groundbreaking work on ischemic tolerance in the heart and brain, ischemic preconditioning in one vascular bed (via brief circumflex branch occlusion) was reported to protect remote myocardium from sustained coronary artery occlusion.188 This discovery of remote preconditioning against myocardial infarction was extended in subsequent experiments employing brief occlusion of the anterior mesenteric artery and left renal artery189 as well as brief occlusion of the femoral artery or tourniquet occlusion of a limb.180,184,190,191 For example, Birnbaum and colleagues reported that partial femoral artery occlusion in rabbits protected the “distant” myocardium from coronary artery occlusion.190 Remote preconditioning was also shown to extend to difficult-to-reach organs, such as the brain and spinal cord.192–204 Studies in mice revealed that the mass of the tissue exposed to remote conditioning was less important than the optimal number of remote conditioning cycles (4 cycles with 5 minutes of reperfusion) and that cycles lasting 2 minutes offered similar protection as 5-minute cycles, whereas 10-minute cycles abolished protection,205 as predicted by the biphasic nature of hormesis and the critical nature of its temporal dimension. These findings confirm that dose, frequency, and duration are all equally important features of hormesis that need to be systematically tested to identify the optimal regimens.
In humans as well as other mammals, diffusible plasma factors appear to harbor protective properties after remote limb preconditioning.206–208 Similarly, exercise elicits cardioprotection through a humoral factor in human plasma that can be transferred to ex vivo heart preparations harvested from lower species.209 Exercising only one side of the body also makes the opposite side stronger in humans, perhaps through diffusible factors.210 Somewhat analogously, unilateral dopaminergic neurotoxicity elicits “cross-hemispheric” conditioning and completely prevents behavioral deficits and cell loss in response to a second hit in the opposite hemisphere, which may explain the asymmetrical nature of dopaminergic cell loss and motor deficits in early Parkinson disease.43 Aside from nitrite, adenosine, or inflammatory factors such as potential diffusible mediators of remote preconditioning,204,211,212 extracellular vesicles such as exosomes, microvesicles, and apoptotic bodies containing peptides, proteins, microRNA (eg, miR-144), and DNA may also convey long-distance communication in remote conditioning.213–215 Perhaps because remote conditioning is dependent on freely diffusible factors, its benefits are independent of the ischemic region.211,216 However, there also appear to be direct neuron-dependent effects, as a ganglionic blocker may prevent the benefits of remote preconditioning, and patients with diabetic neuropathy exhibit lower amplitude conditioning responses.189,208,217,218 This body of work reveals the critical importance of in vivo studies of preconditioning, in which diffusible plasma factors and their long-distance effects can be scrutinized. For partly these reasons, our recommendation for systematic studies of hormesis is to find and use the appropriate in vivo models.
Based on a large body of preclinical work, remote preconditioning has been translated with at least some degree of success to the clinic. For example, it may benefit patients with cardiovascular disease, in whom it has been shown to salvage myocardium and reduce stroke recurrence.219–226 Specifically, remote limb preconditioning reduced stroke recurrence from 23% to 5% at 90 days and 27% to 8% at 300 days in patients with intracranial arterial stenosis.220 The safe, twice-daily application of the conditioning stimulus for almost 1 year in the latter studies raises the possibility of enduring hormetic effects in humans with regularly repeating (ie, rhythmic) hormetic stimulation. Ischemic tolerance in humans may be associated with the release of tumor necrosis factor-α and appears to be induced by transient ischemic attacks and preinfarction angina.227–232 However, the neuroprotective potential of transient ischemic attacks is generally believed to recede in elderly patients,233 although the available data are contradicted by Meng and colleagues’ body of work, in which beneficial effects of remote conditioning were still evident in patients in their 80s and 90s.234
If hypoxia and acidosis limit athletic performance and ischemic preconditioning increases both stimuli, then repeated bouts of remote preconditioning should logically increase athletic performance. Consistent with these assumptions, remote preconditioning has the ability to amplify maximal athletic performance in elite swimmers,206 enhance power output in cyclists,235–237 and preserve blood flow in treadmill runners.238 However, men appear to profit from remote ischemic preconditioning with greater gains in strength and peripheral oxygen extraction than women,239 and not all studies have invariably shown benefits of ischemic conditioning on athleticism.240 Nevertheless, a number of strategies for leveraging remote preconditioning to boost athletic performance have been reviewed,241 and ethical considerations of ischemic preconditioning in high-stake competitions have been raised.242
In preclinical animal studies, experimental conditions are tightly controlled, and the population of animals is genetically uniform compared to the conspicuous heterogeneity of human populations enrolled in clinical trials. As a result, the capacity to detect small changes—including hormesis—under conditions of high background noise is greatly hindered in the latter. This creates a serious, expensive problem for the pharmaceutical industry and has likely contributed to many trial failures, including those that reported no efficacy of remote conditioning.243 One strategy to overcome the variance inherent in human patients might be to combine end points for an integrated value for health assessment, as discussed in the section entitled “How Do We Define Health and Can We Boost It in Both the Sick and Healthy?”
Repetitive Conditioning for Extension of Biological Resilience
The initial studies on ischemic preconditioning in the brain often employed a single preconditioning stimulus and reported that the protection waned within a week or so. However, the well-established phenomenon of long-term facilitation in the Aplysia sea slug with intermittent, repeated stimulation, and the widespread recognition that repeated stimuli cultivate superior memory consolidation, have prompted researchers to test repetitive conditioning stimuli as a means of augmenting the size and duration of the hormetic stress response.244–246 For example, episodic exposure to hypoxia, sometimes even postinjury, has been shown to induce long-lasting tolerance in models of retinal ischemia, myocardial infarction, spinal cord injury, glaucoma, and stroke—enduring effect associated with an anti-inflammatory phenotype.247–253 Moreover, reductions in infarct size as well as in adverse cardiac remodeling with repetitive remote conditioning can be enhanced by repetitive remote conditioning in a dose-dependent manner.254 Similarly, multiple exposures of plants to dehydration in a short period of time primes them to survive subsequent periods of drought better than prolonged stress exposure and is thought to “train” future transcriptional responses.118 The concept that repetitive presentations of a conditioning stimulus prolong the period of induced resilience has recently been reviewed in detail.255
It is important to note that the traditional remote ischemic preconditioning protocols involve repetitive tightening of the cuff and not a single ischemia/reperfusion event, suggesting that repeated bouts of hypoxia conditioning are effective in humans. In patients with stage 1 arterial hypertension, intermittent hypoxia conditioning can mitigate high blood pressure.256 Similarly, patients with spinal cord injury demonstrate superior locomotor recovery257,258 and improved hand dexterity259 after intermittent hypoxic postconditioning. Furthermore, sustained improvements in endothelial function (ie, flow-mediated dilation and resting skin microcirculation) have been reported in the clinic following repetitive remote conditioning with a cuff.260 Repetitive conditioning of the arm has also been shown to accelerate the healing of leg ulcers in human patients.261 The benefits of repetitive, intermittent hypoxia as a conditioning stimulus may be analogous to the advantages of high-intensity, interval training in humans relative to moderately intense endurance exercise.262–264
Some limitations of repetitive conditioning are important to recognize. After chronic treatments, some degree of habituation may be observed, suggesting that the conditioning stimulus may need to be modified frequently to prevent desensitization.265 Another concern is the potentially toxic effect of stress overstimulation, perhaps because the stimulus is too high in intensity or administered for too long a duration or at the wrong time. For example, although animals raised in environmentally enriched conditions typically fare better after subsequent traumatic brain injury compared to animals raised in standard housing conditions, the standard housing elicits better outcomes than enriched housing if applied during the acute recovery period immediately following the trauma.266 Similarly, initiation of exercise immediately after traumatic brain injury increases signs of inflammation and fails to mitigate the lesion or its neurological sequelae, in contrast to exercise initiated following a 5-week delay posttrauma.267 These findings further confirm the importance of optimizing hormesis in the temporal dimension. Indeed, the most serious challenge for the translation of hormesis and conditioning to the clinic is the identification of the hormetic “sweet spot,” especially in diverse populations of the old (or even young) with comorbidities that likely shift the dose–response and duration–response curves. For example, some researchers have proposed that the physically “untrained” populations might profit more from remote ischemic conditioning than highly trained athletes who may already be maximally preconditioned by their prior vigorous training, thereby shrinking the dynamic hormetic range for the latter.242 In other words, the closer the athletes are to their genetically and epigenetically predetermined maxima, the lower may be the size of any additional hormetic response.
How Do We Define Health and Can We Boost It in Both the Sick and Healthy?
The definition of health according to the Constitution of the World Health Organization in 1948 was “a state of complete physical, mental, and social well-being, and not merely the absence of disease or infirmity.”268 More recently, health has been defined more plainly and less stringently as the “ability to adapt.”269 Acute adaptive responses are, by definition, those that provide a survival advantage, not unlike long-term adaptations on an evolutionary timescale. It might be useful to think of the “ability to adapt” as analogous to pulling a spring—such as cocking a weapon—and to acknowledge that the beneficial effects of conditioning stimuli may not be evident in measurable biomarkers until there is a toxic trigger and the weapon is fired, which only then elicits perceptible changes in cellular defense systems. The ability to rapidly transition to this state of heightened tension or stress resistance might distinguish the healthy from the frail, but its dynamic range might recede with aging and comorbidities.233,270 On the other hand, as mentioned earlier, remote limb conditioning has the capacity to reduce the risk of stroke recurrence or white matter hyperintensities in patients with symptomatic intracranial atherosclerotic stenosis, including in patients in their 80s and 90s or in patients with cerebral small-vessel disease.220,234,271 Furthermore, remote conditioning is still feasible in critically ill patients after subarachnoid hemorrhage, a condition otherwise associated with significant mortality and morbidity.272,273 As stated in the “Repetitive Conditioning for Extension of Biological Resilience” section, the view that highly fit or elite athletes might be more difficult to precondition than untrained, less physically fit patients has also been expressed.242 Collectively, these arguments presage the challenges of accurately predicting who will benefit from a hormetic challenge, the extent of such benefit, and how and when it should be applied in the clinic.
Aside from lifestyle modifications in exercise habits and restriction of caloric intake, the specific content of the diet also influences overall health measures and stress resistance. Phytochemicals in food, such as polyphenols and catechins, have long been proposed to serve as hormetic stimuli.274–276 The biphasic nature of the response to phytochemicals has been defined as xenohormesis and is not unexpected given that plants often produce these compounds to discourage predation and that many phytochemicals harbor electrophilic properties.277 Phytochemicals, such as polyphenols, have the ability to exert both antioxidant and prooxidant actions, and the latter effects may be mediated by oxidized, thiol-reactive derivatives.278 Electrophiles and oxidized phytochemicals encourage Nrf2 translocation to the nucleus by inhibitory action on Keap1, as described above.
The distinct advantage of bioactive food components, as opposed to selective drugs, is that they impact a multitude of targets. For example, flavanols have pleiotropic effects on health, including boosts in glutathione and anti-inflammatory activities.279 However, not all of the effects of flavanols, such as on vascular function and plasma antioxidant capacity, are sizable in amplitude when measured individually, but when integrated across a number of health measures, the effects of flavanols have been reported as statistically significant and designated as an integrative biomarker—“the index of vascular health.”279 A similarly integrative approach might be used to characterize a human hormetic biomarker of practical significance in both clinical and nonclinical settings.280 Similarly, the effects of nutritional interventions on inflammation and metabolism need to be examined across the dosing and temporal dimensions, while applying a “systems biology” approach at the molecular, cellular, and organ levels.281 Other clear advantages of a plant-rich diet replete with xenohormetic phytochemicals are the high therapeutic index, wide therapeutic window, safety of repeated, lifelong exposure, and ease of implementation, including in the infirm and frail (and maybe even young adults) who cannot or will not exercise or restrict calories. On the other hand, delivering fresh, uncontaminated sources of phytochemicals to soldiers in battle and citizens of underdeveloped countries poses a logistical problem. Furthermore, it is unknown the extent to which hormetic phytochemicals in processed military food (Meals-Ready-to-Eat) with years-long shelf lives, or in canned goods, remain bioactive.
The Implications of Exceptional Hormesis in Plants
As discussed above, hormesis has been described in organisms ranging from primates to nematodes. Similarly, plants also have the potential to exhibit growth stimulation in response to many types of environmental stressors, such as herbicides and natural plant toxins.5,27,282,283 Repetitive pre-exposures to dehydration stress, for example, have been shown to activate epigenetic and chromatin-based mechanisms in plants to produce a type of “transcriptional memory” that affords protection against subsequent periods of severe drought.30,284 During times of severely limited growth, hormesis is difficult to elicit in plants. However, when plants exhibit a growth rate between an optimal and a genetically maximal rate, stimulation of growth between 10% and 60% is possible with hormetic stimulation.285,286 Furthermore, exceptionally high growth rates may be observed when hormetic stimuli are applied during periods of suboptimal and optimal rates of growth, as the distance to maximal growth rate is then the greatest, effectively expanding the potential dynamic range. For these reasons, mature plants seem to exhibit less hormetic growth capacity.
The observations in plants have important implications for the practice of assessing hormesis in mammalian systems, as laboratory animals are generally permitted ad libitum access to food and are examined during young adulthood, rather than under suboptimal conditions, which may shrink the dynamic range and preclude the emergence of “exceptional” hormesis. Similarly, the section entitled “Repetitive Conditioning for Extension of Biological Resilience” highlights the possibility that highly trained athletes display lower preconditioning capacity than untrained populations. Given the potential significance of dynamic range, humans with preexisting diseases might also display a reduction in the amplitude of hormesis if endogenous defenses are already stimulated to some degree by the disease itself or by comorbidities.32 Finally, the “transcriptional memory” induced in plants by hormetic stress may compromise some aspects of plant growth and, therefore, may have grave implications for translating mammalian stress responses to the clinic.284
Identification of Knowledge Gaps and Recommendations for Systematic Studies
More than 10 000 studies with U-shaped dose–response curves have been found in the literature.287 This collective body of work demonstrates that hormesis is a fundamental property of biological systems and is displayed at all levels of tissue organization. The typical amplitude of the stimulatory portion of the hormetic dose–response curve and the duration of the response may be evolutionarily constrained for reasons that have been speculated upon in previous sections of this review. However, most hormesis studies involve single-stimulus exposures and have not systematically varied experimental inputs or outputs in the temporal dimension, such as the timing, frequency, number, and duration of exposures. Instead, hormesis studies typically only systematically vary the dose (ie, the amplitude) of a single-stimulus exposure and measure outcomes at one time point only. Second, most subcellular “mechanistic” studies on hormesis tend to focus on a single molecular pathway at a time and therefore fail to portray the interactive and dynamic nature of intracellular signal transduction. For example, divergent sensory signals may converge upon a few common pathways (for signal processing) before diverging again into multiple pathways that initiate many distinct but coordinated responses in an “hourglass” shape. Third, researchers have not satisfactorily explained at a mechanistic level how a biphasic dose–response curve abruptly switches direction at the threshold dose. Fourth, researchers have not settled on a valid biomarker(s) of hormesis, which seems essential before hormesis can be tested and optimized in humans. Fifth, too few hormesis studies are conducted in whole animals and most fail to examine neurological or functional outcomes, which seem important to address from the perspective of evolutionarily adaptive behavior. Sixth, the potential for systemic toxicity is not typically measured by examining liver markers, inflammatory molecules, or a shortening of lifespan.
Many studies on hormesis have employed tumor cell lines grown in culture dishes. Tumor cell lines have accumulated numerous antiapoptosis mutations and might therefore display a greater propensity toward proliferative, stimulatory responses than differentiated, postmitotic cells examined in vitro or in vivo (discussed in32). Even for undifferentiated cells with proliferative capacity in vivo, the immune system might keep cell division under check to reduce the risk of oncogenic transformation. The immune system, with its orchestration of cell and organ crosstalk, and the hypothalamic–pituitary–adrenal axis are two key components of the stress response that are missing from most of the in vitro studies of hormesis. Therefore, in vivo models should be employed in future studies, such as C elegans and Mus musculus, whose genotypes and phenotypes are well defined. In both organisms, the salubrious effects of intermittent fasting or restricted feeding have been shown to increase lifespan—perhaps the best indicator to date of an enduring hormetic effect. Another advantage of in vivo models is that they can be examined at the behavioral level, with, for example, functional tests of spatial learning or memory, affect, and sensorimotor coordination. Further, at least two model organisms should be employed before proposing the clinical trials necessary to translate this hormetic therapy to humans, because validating key findings in two independent model systems increase the potential for generalizability. Other well-studied alternatives to nematodes might be fruit flies and zebrafish. Although high-throughput assays are easier and cheaper to implement in smaller sized creatures than rodents, mice are phylogenetically closer to Homo sapiens than flies, fish, or worms, and can be examined for important behaviors such as anxiety and depression-like mood.
Table 1 lists a series of recommendations to accelerate the discovery and validation of interventions that both enhance the amplitude and extend the duration of biological performance. Although the underlying assumption is that hormesis can eventually be leveraged in humans, there is no intent to make specific human health recommendations without validation of experimental work in clinical trials. The field of hormesis is not sufficiently mature for all-encompassing recommendations on timing, dose, duration, frequency, and so on. Based on the success of intermittent fasting, regular bouts of exercise, and repetitive ischemic conditioning, as well as the rhythmicity imposed by circadian clocks and the photoperiod, it appears that the development of hormetic responses may be optimized by application of rhythmic stressors. Stress rhythmicity permits anabolic changes during a critical period of recovery and quiescence, when the cell is relatively free of homeostatic disruptions. Thus, the intermittent reapplication of one type of hormetic stimulus might build additional layers of a robust biological foundation that reaches for the genetically predetermined maximum in both amplitude and endurance. Figure 2 illustrates this concept.
Table 1.
Systematic Approach for Optimal Hormetic Dose and Frequency.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
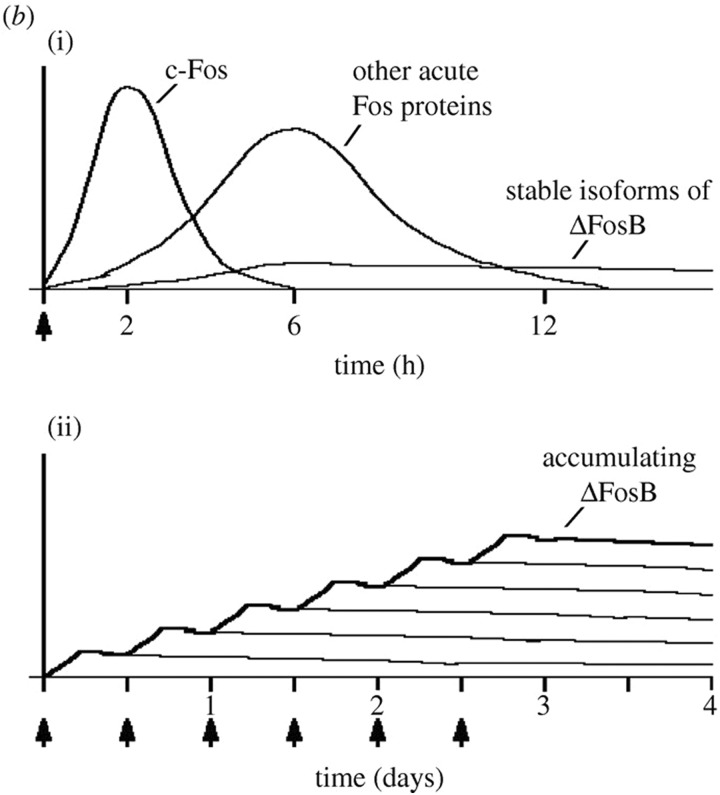
Figure 2.
Kinetics of stress protein accumulation after repetitive exposure to cocaine. Repeated exposure to the biochemical stress of cocaine leads to chronic changes in activator protein -1 (AP-1) complex protein ΔFosB in the lower panel. This “staircase” pattern in induced gene expression is analogous to the proposed “layered” hormetic effects of repetitive conditioning and might be measured at the level of the “hormesis proteome.” The accumulated level of ΔFosB isoform expression continues after the cessation of the stimulus (arrows at bottom of figure). Adapted from Nestler et al. (2008).295
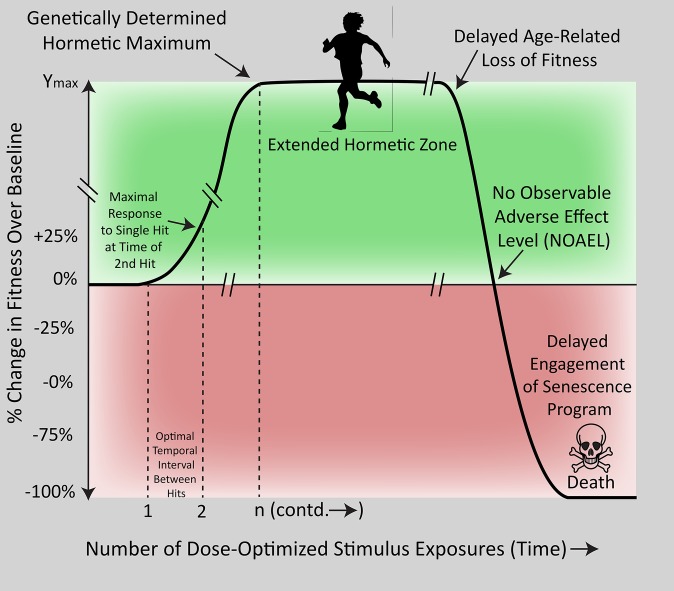
Figure 3.
Idealized hormesis enhancement/extension curve. Biological fitness (ie, integrated indices of health) as a function of repeated exposures to optimized hormetic stimuli applied at rhythmic intervals. The amplitude of the hormetic maximum is extended to the genetically determined peak until age-related loss of fitness overwhelms natural defense systems and the inevitable (but perhaps delayed) engagement of senescence programs culminates in death. Note that death should result because of the passage of time rather than stimulus exposures per se. A single hormetic stimulus exposure results in a modest amplitude effect and a time-limited response, but repeated exposures build, in stepwise manner, the layers of a strong foundation for an extended, high-amplitude response. Other assumptions of this idealized graph include that environmental stimuli such as dietary/lifestyle factors are optimized so that the maximal epigenetic/genetic potential can be reached. Hormetic maxima may vary with gender, genetic vulnerabilities, geographic region (eg, altitude), age, comorbidities, etc.
To accomplish the goal of the workshop, a systematic approach (Table 1) would be valuable in optimizing studies of optimal hormesis/conditioning. There are many alternative approaches to achieve rigor that are not listed above and the idealized guidelines in the table may have to be adjusted for “real” experiments and considerations of the costs. Researchers can build upon these recommendations and vary them in some systematic manner depending on their specific models and hypotheses. Note that the optimal doses and temporal features identified with this approach may not translate from rodents or nematodes to humans due to the pharmacodynamic and pharmacokinetic differences among species, but the nature of the stimulus, the biological pathways, and the integrated biomarker indices might be expected to translate. Finally, there are other possible confounding factors such as gender, age, and comorbidities that may influence the dosimetry and temporal kinetics of the hormetic “sweet spot.”
Conclusion
Hormesis is a “stress-modulated, enhanced metabolic state of the cell involving energy, material, and information processing” (definition by Dr Walter Kozumbo) and is mediated by reactive oxygen species, various signal transduction cascades, mitochondrial bioenergetics, epigenetic modifications, and changes in gene expression—revealing coactivation of multiple biological targets. The typically modest amplitude of hormesis in response to single-stimulus exposure appears to be highly evolutionarily conserved, suggestive of some adaptive value to its ceiling when the environmental conditions are only transient. It might be bioenergetically wasteful to build a dramatic response to a fleeting environmental shift when conditions promptly return to the status quo.
The collective body of work reviewed here suggests that the amplitude of hormesis may be augmented by implementing multiple lifestyle changes, such as dietary restriction, physical exertion, improving diet quality with hormetic phytochemicals, and applying conditioning stimuli in a repetitive, but carefully calibrated manner. A series of recommendations were made to systematically test the temporal features of the hormetic stimulus. It will be important not only to examine potentially synergistic interactions (ie, the cumulative effects of simultaneous conditioning with multiple stimuli) but also to avoid driving the hormetic response into the inhibitory or toxic zone of the biphasic dose-response curve. Furthermore, hormetic doses that are maximally effective in the young and fit might prove too potent in healthy older patients and, as with synergistic stimuli, convert a beneficial response into a toxic one. For example, older patients with diabetes and obesity might expect a fate worse than young and athletic patients. In the case of severely diseased or aged organisms and trained athletes, however, it remains unsettled whether they can always be preconditioned. As they are already frequently exposed to stress-conditioning episodes and may be near full conditioning capacity, they may display lower dynamic ranges or conditioning potential relative to untrained healthy young adults. On the other hand, even modest improvements in health indices might make a profound difference in quality of life in severely ill patients or in the athletic prowess and mental stamina of the otherwise physically fit. Finally, fluctuations in hormetic capacity should be considered in the context of the temporal dimension, including that of circadian cycling,288 as application of stressful stimuli at the “wrong” time of day might thwart any improvements in health indices.
The goals of eventual clinical application would be far less challenging if we could identify a reliable biomarker of resilience—loosely defined as “any characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.”289 However, the identification of a single, master biomarker, if it is even possible, is seriously hampered by the polymorphic nature of stress responses and their ephemeral temporal dynamics. For these and other reasons, an integrated health index might be of greater value as a biomarker and much more robust when individual factors are known to fluctuate widely across patients. It is important to note that a biomarker of hormesis is not necessarily the same as the biochemical mechanism underlying the hormetic effect.
Leon Megginson’s interpretation of Charles Darwin’s Origin of Species led him to write that
“. . . it is not the most intellectual of the species that survives; it is not the strongest that survives; but the species that survives is the one that is able best to adapt and adjust to the changing environment in which it finds itself.”290
Fleeting/transient or repetitive/rhythmic shifts in environmental stressors might influence the shape of the hormesis curve more profoundly than previously imagined, given that the temporal dimension is poorly explored. Furthermore, the heterogeneity of hormetic sensors, mechanisms, and effectors may provide a survival advantage in conferring adaptability to a greater diversity of challenges, fulfilling the very definition of evolutionary fitness under shifting environmental conditions.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funded by the Air Force (AFOSR D9550-13-1-0047 to EJC). RKL is supported by the National Institutes of Health (R15NS093539 to RKL, 1R21NS107960-01 and 1R15NS093539-01 to PI RKL).
References
- 1. Calabrese EJ. Hormesis: a revolution in toxicology, risk assessment and medicine. EMBO Rep. 2004;5:S37–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calabrese EJ. Converging concepts: adaptive response, preconditioning, and the Yerkes-Dodson Law are manifestations of hormesis. Ageing Res Rev. 2008;7(1):8–20. [DOI] [PubMed] [Google Scholar]
- 3. Calabrese EJ. Hormesis is central to toxicology, pharmacology and risk assessment. Hum Exp Toxicol. 2010;29(4):249–261. [DOI] [PubMed] [Google Scholar]
- 4. Calabrese EJ. Biphasic dose responses in biology, toxicology and medicine: accounting for their generalizability and quantitative features. Environ Pollut. 2013;182:452–460. [DOI] [PubMed] [Google Scholar]
- 5. Calabrese EJ. Hormesis: a fundamental concept in biology. Microb Cell. 2014;1(5):145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calabrese EJ, Bachmann KA, Bailer AJ, et al. Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose–response framework. Toxicol Appl Pharmacol. 2007;222(1):122–128. [DOI] [PubMed] [Google Scholar]
- 7. Calabrese EJ, Baldwin LA. The frequency of U-shaped dose responses in the toxicological literature. Toxicol Sci. 2001;62(2):330–338. [DOI] [PubMed] [Google Scholar]
- 8. Calabrese EJ, Baldwin LA. Chemical hormesis: its historical foundations as a biological hypothesis. Hum Exp Toxicol. 2000;19(1):2–31. [DOI] [PubMed] [Google Scholar]
- 9. Southam CM, Ehrlich J. Effects of extracts of Western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathology. 1943;33:517–524. [Google Scholar]
- 10. Calabrese EJ, Baldwin LA. Hormesis: the dose–response revolution. Annu Rev Pharmacol Toxicol. 2003;43:175–197. [DOI] [PubMed] [Google Scholar]
- 11. Mattson MP. Awareness of hormesis will enhance future research in basic and applied neuroscience. Crit Rev Toxicol. 2008;38(7):633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8(4):398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7(6):437–448. [DOI] [PubMed] [Google Scholar]
- 14. Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26(5):248–254. [DOI] [PubMed] [Google Scholar]
- 15. Zhao H. The protective effect of ischemic postconditioning against ischemic injury: from the heart to the brain. J Neuroimmune Pharmacol. 2007;2(4):313–318. [DOI] [PubMed] [Google Scholar]
- 16. Pan J, Li X, Peng Y. Remote ischemic conditioning for acute ischemic stroke: dawn in the darkness. Rev Neurosci. 2016;27(5):501–510. [DOI] [PubMed] [Google Scholar]
- 17. Selye H. Stress and the general adaptation syndrome. Br Med J. 1950;1(4667):1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Selye H. Stress Without Distress. Philadelphia, PA: Signet; 1975. [Google Scholar]
- 19. Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol. 1908;18:459–482. [Google Scholar]
- 20. Broadhurst PL. Emotionality and the Yerkes-Dodson Law. J Exp Psychol. 1957;54(5):345–352. [DOI] [PubMed] [Google Scholar]
- 21. Calabrese EJ, Baldwin LA. Defining hormesis. Hum Exp Toxicol. 2002;21(2):91–97. [DOI] [PubMed] [Google Scholar]
- 22. Calabrese EJ. Preconditioning is hormesis, part II: how the conditioning dose mediates protection: dose optimization within temporal and mechanistic frameworks. Pharmacol Res. 2016;110:265–275. [DOI] [PubMed] [Google Scholar]
- 23. Calabrese EJ. Preconditioning is hormesis, part I: documentation, dose–response features and mechanistic foundations. Pharmacol Res. 2016;110:242–264. [DOI] [PubMed] [Google Scholar]
- 24. Tapia PC. Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients: “mitohormesis” for health and vitality. Med Hypotheses. 2006;66(4):832–843. [DOI] [PubMed] [Google Scholar]
- 25. Ristow M, Schmeisser K. Mitohormesis: promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose Response. 2014;12(2):288–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Calabrese EJ. Hormesis within a mechanistic context. Homeopathy. 2015;104(2):90–96. [DOI] [PubMed] [Google Scholar]
- 27. Calabrese EJ, Blain R. The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview. Toxicol Appl Pharmacol. 2005;202(3):289–301. [DOI] [PubMed] [Google Scholar]
- 28. Calabrese EJ, Blain RB. The hormesis database: the occurrence of hormetic dose responses in the toxicological literature. Regul Toxicol Pharmacol. 2011;61(1):73–81. [DOI] [PubMed] [Google Scholar]
- 29. Calabrese EJ, Mattson MP. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech Dis. 2017;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lamke J, Baurle I. Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol. 2017;18(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mattson MP, Moehl K, Ghena N, Schmaedick M, Cheng A. Intermittent metabolic switching, neuroplasticity and brain health. Nat Rev Neurosci. 2018;19(2):63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leak RK. Conditioning against the pathology of Parkinson’s disease. Conditioning Medicine. 2018;1(3):85–104. [PMC free article] [PubMed] [Google Scholar]
- 33. Nandhagopal R, Kuramoto L, Schulzer M, et al. Longitudinal evolution of compensatory changes in striatal dopamine processing in Parkinson’s disease. Brain. 2011;134(pt 11):3290–3298. [DOI] [PubMed] [Google Scholar]
- 34. Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13(7):290–296. [DOI] [PubMed] [Google Scholar]
- 35. Appel-Cresswell S, de la Fuente-Fernandez R, Galley S, McKeown MJ. Imaging of compensatory mechanisms in Parkinson’s disease. Curr Opin Neurol. 2010;23(4):407–412. [DOI] [PubMed] [Google Scholar]
- 36. Brotchie J, Fitzer-Attas C. Mechanisms compensating for dopamine loss in early Parkinson disease. Neurology. 2009;72(suppl 7):S32–S38. [DOI] [PubMed] [Google Scholar]
- 37. Obeso JA, Schapira AH. Compensatory mechanisms in Parkinson’s disease. Mov Disord. 2009;24(1):153–154. [DOI] [PubMed] [Google Scholar]
- 38. Blesa J, Trigo-Damas I, Dileone M, Del Rey NL, Hernandez LF, Obeso JA. Compensatory mechanisms in Parkinson’s disease: circuits adaptations and role in disease modification. Exp Neurol. 2017;298(pt B):148–161. [DOI] [PubMed] [Google Scholar]
- 39. Bezard E, Gross CE, Brotchie JM. Presymptomatic compensation in Parkinson’s disease is not dopamine-mediated. Trends Neurosci. 2003;26(4):215–221. [DOI] [PubMed] [Google Scholar]
- 40. Zigmond MJ. Do compensatory processes underlie the preclinical phase of neurodegenerative disease? Insights from an animal model of Parkinsonism. Neurobiol Dis. 1997;4(3-4):247–253. [DOI] [PubMed] [Google Scholar]
- 41. Bezard E, Dovero S, Prunier C, et al. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J Neurosci. 2001;21(17):6853–6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Navntoft CA, Dreyer JK. How compensation breaks down in Parkinson’s disease: insights from modeling of denervated striatum. Mov Disord. 2016;31(3):280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weilnau JN, Carcella MA, Miner KM, et al. Evidence for cross-hemispheric preconditioning in experimental Parkinson’s disease. Brain Struct Funct. 2018;223(3):1255–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roedter A, Winkler C, Samii M, Walter GF, Brandis A, Nikkhah G. Comparison of unilateral and bilateral intrastriatal 6-hydroxydopamine-induced axon terminal lesions: evidence for interhemispheric functional coupling of the two nigrostriatal pathways. J Comp Neurol. 2001;432(2):217–229. [DOI] [PubMed] [Google Scholar]
- 45. Leak RK. Adaptation and sensitization to proteotoxic stress. Dose Response. 2014;12(1):24–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kadosh RC. Using transcranial electrical stimulation to enhance cognitive functions in the typical and atypical brain. Translat Neurosci. 2013;4:20–33. [Google Scholar]
- 47. Giordano J, Bikson M, Kappenman ES, et al. Mechanisms and effects of transcranial direct current stimulation. Dose Response. 2017; Jan-May 2017:1–22 doi:10.1177/1559325816685467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cotler HB, Chow RT, Hamblin MR, Carroll J. The use of low level laser therapy (LLLT) for musculoskeletal pain. MOJ Orthop Rheumatol. 2015;2(5):00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang YY, Sharma SK, Carroll J, Hamblin MR. Biphasic dose response in low level light therapy – an update. Dose Response. 2011;9(4):602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mattson MP. Lifelong brain health is a lifelong challenge: from evolutionary principles to empirical evidence. Ageing Res Rev. 2015;20:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Camandola S, Mattson MP. Brain metabolism in health, aging, and neurodegeneration. EMBO J. 2017;36(11):1474–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kashiwaya Y, Bergman C, Lee JH, et al. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2013;34(6):1530–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59(2):293–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marosi K, Kim SW, Moehl K, et al. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J Neurochem. 2016;139(5):769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science. 2016;354(6315):994–999. [DOI] [PubMed] [Google Scholar]
- 56. Lunn RM, Blask DE, Coogan AN, et al. Health consequences of electric lighting practices in the modern world: a report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci Total Environ. 2017;607-608:1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mattson MP, Allison DB, Fontana L, et al. Meal frequency and timing in health and disease. Proc Natl Acad Sci U S A. 2014;111(47):16647–16653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brager AJ, Yang T, Ehlen JC, Simon RP, Meller R, Paul KN. Sleep is critical for remote preconditioning-induced neuroprotection. Sleep. 2016;39(11):2033–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gazendam JAC, Van Dongen HPA, Grant DA, Freedman NS, Zwaveling JH, Schwab RJ. Altered circadian rhythmicity in patients in the ICU. Chest. 2013;144(2):483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sunderram J, Sofou S, Kamisoglu K, Karantza V, Androulakis IP. Time-restricted feeding and the realignment of biological rhythms: translational opportunities and challenges. J Transl Med. 2014;12:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McDonald RB, Ramsey JJ. Honoring Clive McCay and 75 years of calorie restriction research. J Nutr. 2010;140(7):1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16(6):706–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mattson MP, Duan W, Wan R, Guo Z. Prophylactic activation of neuroprotective stress response pathways by dietary and behavioral manipulations. NeuroRx. 2004;1(1):111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mitchell JR, Verweij M, Brand K, et al. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell. 2010;9(1):40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mejia P, Trevino-Villarreal JH, Hine C, et al. Dietary restriction protects against experimental cerebral malaria via leptin modulation and T-cell mTORC1 suppression. Nat Commun. 2015;6:6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mager DE, Wan R, Brown M, et al. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 2006;20(6):631–637. [DOI] [PubMed] [Google Scholar]
- 67. Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161(1):106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ravussin E, Redman LM, Rochon J, et al. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci. 2015;70(9):1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Johnson JB, Summer W, Cutler RG, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42(5):665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35(5):714–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Villareal DT, Fontana L, Weiss EP, et al. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med. 2006;166(22):2502–2510. [DOI] [PubMed] [Google Scholar]
- 73. Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42(8):709–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stote KS, Baer DJ, Spears K, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85(4):981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lee BC, Kaya A, Gladyshev VN. Methionine restriction and lifespan control. Ann N Y Acad Sci. 2016;1363:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hine C, Mitchell JR. Calorie restriction and methionine restriction in control of endogenous hydrogen sulfide production by the transsulfuration pathway. Exp Gerontol. 2015;68:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Scott JM, Deuster PA. Ketones and human performance. J Spec Oper Med. 2017;17(2):112–116. [DOI] [PubMed] [Google Scholar]
- 78. Mitani JC, Watts DP, Amsler SJ. Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr Biol. 2010;20(12):R507–R508. [DOI] [PubMed] [Google Scholar]
- 79. Wilson M, Wrangham R. Intergroup relations in chimpanzees. Annu Rev Anthropol. 2003;32:363–393. [Google Scholar]
- 80. Tharion WJ, Lieberman HR, Montain SJ, et al. Energy requirements of military personnel. Appetite. 2005;44(1):47–65. [DOI] [PubMed] [Google Scholar]
- 81. Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview: glucocorticoids – food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. 2004;145(6):2633–2638. [DOI] [PubMed] [Google Scholar]
- 82. Srikanthan P, Karlamangla AS. Muscle mass index as a predictor of longevity in older adults. Am J Med. 2014;127(6):547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Raefsky SM, Mattson MP. Adaptive responses of neuronal mitochondria to bioenergetic challenges: roles in neuroplasticity and disease resistance. Free Radic Biol Med. 2017;102:203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mitchell T, Barlow CE. Review of the role of exercise in improving quality of life in healthy individuals and in those with chronic diseases. Curr Sports Med Rep. 2011;10(4):211–216. [DOI] [PubMed] [Google Scholar]
- 85. Verburgh L, Konigs M, Scherder EJ, Oosterlaan J. Physical exercise and executive functions in preadolescent children, adolescents and young adults: a meta-analysis. Br J Sports Med. 2014;48(12):973–979. [DOI] [PubMed] [Google Scholar]
- 86. Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW. The effects of physical activity on sleep: a meta-analytic review. J Behav Med. 2015;38(3):427–449. [DOI] [PubMed] [Google Scholar]
- 87. Jones BH, Cowan DN, Tomlinson JP, Robinson JR, Polly DW, Frykman PN. Epidemiology of injuries associated with physical training among young men in the army. Med Sci Sports Exerc. 1993;25(2):197–203. [PubMed] [Google Scholar]
- 88. Kristiansen SB, Solskov L, Jessen N, et al. 5-Aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside increases myocardial glucose uptake during reperfusion and induces late pre-conditioning: potential role of AMP-activated protein kinase. Basic Clin Pharmacol Toxicol. 2009;105(1):10–16. [DOI] [PubMed] [Google Scholar]
- 89. Stranahan AM, Lee K, Martin B, et al. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19(10):951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11(3):309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mattson MP. Challenging oneself intermittently to improve health. Dose Response. 2014;12(4):600–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4(1):177–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Murray AJ, Knight NS, Cole MA, et al. Novel ketone diet enhances physical and cognitive performance. FASEB J. 2016;30(12):4021–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–2263. [DOI] [PubMed] [Google Scholar]
- 95. Schneider N, Yvon C. A review of multidomain interventions to support healthy cognitive ageing. J Nutr Health Aging. 2013;17(3):252–257. [DOI] [PubMed] [Google Scholar]
- 96. O’Flanagan CH, Smith LA, McDonell SB, Hursting SD. When less may be more: calorie restriction and response to cancer therapy. BMC Med. 2017;15(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ribaric S. Diet and aging. Oxid Med Cell Longev. 2012;2012:741468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011;10(2):187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Summermatter S, Handschin C. PGC-1alpha and exercise in the control of body weight. Int J Obes (Lond). 2012;36(11):1428–1435. [DOI] [PubMed] [Google Scholar]
- 100. Marosi K, Bori Z, Hart N, et al. Long-term exercise treatment reduces oxidative stress in the hippocampus of aging rats. Neuroscience. 2012;226:21–28. [DOI] [PubMed] [Google Scholar]
- 101. Cheng A, Wan R, Yang JL, et al. Involvement of PGC-1alpha in the formation and maintenance of neuronal dendritic spines. Nat Commun. 2012;3:1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sleiman SF, Henry J, Al-Haddad R, et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. Elife. 2016;5 e15092. 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Cheng A, Yang Y, Zhou Y, et al. Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab. 2016;23(1):128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sanchez-Roman I, Gomez A, Perez I, et al. Effects of aging and methionine restriction applied at old age on ROS generation and oxidative damage in rat liver mitochondria. Biogerontology. 2012;13(4):399–411. [DOI] [PubMed] [Google Scholar]
- 105. Elshorbagy AK, Valdivia-Garcia M, Mattocks DA, et al. Effect of taurine and N-acetylcysteine on methionine restriction-mediated adiposity resistance. Metabolism. 2013;62(4):509–517. [DOI] [PubMed] [Google Scholar]
- 106. Yun J, Finkel T. Mitohormesis. Cell Metab. 2014;19(5):757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol. 2013;100:30–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sthijns MM, Randall MJ, Bast A, Haenen GR. Adaptation to acrolein through upregulating the protection by glutathione in human bronchial epithelial cells: the materialization of the hormesis concept. Biochem Biophys Res Commun. 2014;446(4):1029–1034. [DOI] [PubMed] [Google Scholar]
- 109. Sthijns MM, Thongkam W, Albrecht C, et al. Silver nanoparticles induce hormesis in A549 human epithelial cells. Toxicol Vitro. 2017;40:223–233. [DOI] [PubMed] [Google Scholar]
- 110. Hine C, Harputlugil E, Zhang Y, et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160(1-2):132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92(2):791–896. [DOI] [PubMed] [Google Scholar]
- 112. Predmore BL, Alendy MJ, Ahmed KI, Leeuwenburgh C, Julian D. The hydrogen sulfide signaling system: changes during aging and the benefits of caloric restriction. Age (Dordr). 2010;32(4):467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16(3):1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yang G, Wu L, Jiang B, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322(5901):587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Miller DL, Roth MB. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans . Proc Natl Acad Sci U S A. 2007;104(51):20618–20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rajpal S, Katikaneni P, Deshotels M, et al. Total sulfane sulfur bioavailability reflects ethnic and gender disparities in cardiovascular disease. Redox Biol. 2018;15:480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Avramova Z. The jasmonic acid-signalling and abscisic acid-signalling pathways cross talk during one, but not repeated, dehydration stress: a non-specific ‘panicky’ or a meaningful response? Plant Cell Environ. 2017;40(9):1704–1710. [DOI] [PubMed] [Google Scholar]
- 118. Ding Y, Fromm M, Avramova Z. Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis . Nat Commun. 2012;3:740. [DOI] [PubMed] [Google Scholar]
- 119. Stenzel-Poore MP, Stevens SL, King JS, Simon RP. Preconditioning reprograms the response to ischemic injury and primes the emergence of unique endogenous neuroprotective phenotypes: a speculative synthesis. Stroke. 2007;38(suppl 2):680–685. [DOI] [PubMed] [Google Scholar]
- 120. Stenzel-Poore MP, Stevens SL, Xiong Z, et al. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362(9389):1028–1037. [DOI] [PubMed] [Google Scholar]
- 121. Jimenez-Mateos EM, Hatazaki S, Johnson MB, et al. Hippocampal transcriptome after status epilepticus in mice rendered seizure damage-tolerant by epileptic preconditioning features suppressed calcium and neuronal excitability pathways. Neurobiol Dis. 2008;32(3):442–453. [DOI] [PubMed] [Google Scholar]
- 122. Matsuyama N, Leavens JE, McKinnon D, Gaudette GR, Aksehirli TO, Krukenkamp IB. Ischemic but not pharmacological preconditioning requires protein synthesis. Circulation. 2000;102(19 suppl 3):III312–III318. [DOI] [PubMed] [Google Scholar]
- 123. Barone FC, White RF, Spera PA, et al. Ischemic preconditioning and brain tolerance: temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke. 1998;29(9):1937–1950; discussion 1950-1931. [DOI] [PubMed] [Google Scholar]
- 124. Xiao J, Jin R, Yu X, et al. Cis and trans determinants of epigenetic silencing by polycomb repressive complex 2 in Arabidopsis . Nat Genet. 2017;49(10):1546–1552. [DOI] [PubMed] [Google Scholar]
- 125. Stapels M, Piper C, Yang T, et al. Polycomb group proteins as epigenetic mediators of neuroprotection in ischemic tolerance. Sci Signal. 2010;3(111):ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Meller R, Pearson AN, Hardy JJ, et al. Blood transcriptome changes after stroke in an African American population. Ann Clin Transl Neurol. 2016;3(2):70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kohrt BA, Worthman CM, Adhikari RP, et al. Psychological resilience and the gene regulatory impact of posttraumatic stress in Nepali child soldiers. Proc Natl Acad Sci U S A. 2016;113(29):8156–8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Jirtle RL. Epigenetics: How Genes and Environment Interact. Berlin, Germany: Springer-Verlag; 2013. [Google Scholar]
- 129. Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8(4):253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Dolinoy DC, Jirtle RL. Environmental epigenomics in human health and disease. Environ Mol Mutagen. 2008;49(1):4–8. [DOI] [PubMed] [Google Scholar]
- 131. Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23(15):5293–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114(4):567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kaminen-Ahola N, Ahola A, Maga M, et al. Maternal ethanol consumption alters the epigenotype and the phenotype of offspring in a mouse model. PLoS Genet. 2010;6(1):e1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104(32):13056–13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Bernal AJ, Dolinoy DC, Huang D, Skaar DA, Weinhouse C, Jirtle RL. Adaptive radiation-induced epigenetic alterations mitigated by antioxidants. FASEB J. 2013;27(2):665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Cypser JR, Johnson TE. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J Gerontol A Biol Sci Med Sci. 2002;57(3):B109–B114. [DOI] [PubMed] [Google Scholar]
- 137. Cypser JR, Tedesco P, Johnson TE. Hormesis and aging in Caenorhabditis elegans . Exp Gerontol. 2006;41(10):935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Cypser J, Johnson TE. Hormesis extends the correlation between stress resistance and lifespan in long-lived mutants of Caenorhabditis elegans . Hum Exp Toxicol. 2001;20(6):295–296; discussion 319-220. [DOI] [PubMed] [Google Scholar]
- 139. Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended lifespan conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci U S A. 1995;92(16):7540–7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wu D, Cypser JR, Yashin AI, Johnson TE. Multiple mild heat-shocks decrease the Gompertz component of mortality in Caenorhabditis elegans . Exp Gerontol. 2009;44(9):607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Olsen A, Vantipalli MC, Lithgow GJ. Lifespan extension of Caenorhabditis elegans following repeated mild hormetic heat treatments. Biogerontology. 2006;7(4):221–230. [DOI] [PubMed] [Google Scholar]
- 142. Panzer S, Penner E, Nelson PJ, Prochazka E, Benda H, Saurugger PN. Identification of the platelet glycoprotein IIb/IIIa complex as a target antigen in primary biliary cirrhosis-associated autoimmune thrombocytopenia. Evidence that platelet-reactive autoantibodies can also bind to the mitochondrial antigen M2. J Autoimmun. 1990;3(4):473–483. [DOI] [PubMed] [Google Scholar]
- 143. Bentham CG. Health. In Palutikoff JP, Subak S, Agnew MD. (eds) Economic Impacts of the Hot Summer and Unusally Warm Year of 1995. London, UK: Department of the Environment; 1997. [Google Scholar]
- 144. Lerchl A. Changes in the seasonality of mortality in Germany from 1946 to 1995: the role of temperature. Int J Biometeorol. 1998;42(2):84–88. [DOI] [PubMed] [Google Scholar]
- 145. Lichtman JH, Leifheit-Limson EC, Jones SB, Wang Y, Goldstein LB. Average temperature, diurnal temperature variation, and stroke hospitalizations. J Stroke Cerebrovasc Dis. 2016;25(6):1489–1494. [DOI] [PubMed] [Google Scholar]
- 146. Johnson TE. Increased lifespan of age-1 mutants in Caenorhabditis elegans and lower Gompertz rate of aging. Science. 1990;249(4971):908–912. [DOI] [PubMed] [Google Scholar]
- 147. Kirkwood TB, Finch CE. Ageing: the old worm turns more slowly. Nature. 2002;419(6909):794–795. [DOI] [PubMed] [Google Scholar]
- 148. Cypser JR, Johnson TE. Hormesis in Caenorhabditis elegans dauer-defective mutants. Biogerontology. 2003;4(4):203–214. [DOI] [PubMed] [Google Scholar]
- 149. Lithgow GJ, White TM, Hinerfeld DA, Johnson TE. Thermotolerance of a long-lived mutant of Caenorhabditis elegans . J Gerontol. 1994;49(6):B270–B276. [DOI] [PubMed] [Google Scholar]
- 150. Henderson ST, Johnson TE. . daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans . Curr Biol. 2001;11(24):1975–1980. [DOI] [PubMed] [Google Scholar]
- 151. Murphy CT, McCarroll SA, Bargmann CI, et al. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans . Nature. 2003;424(6946):277–283. [DOI] [PubMed] [Google Scholar]
- 152. Ifrim DC, Quintin J, Joosten LA, et al. Trained immunity or tolerance: opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clin Vaccine Immunol. 2014;21(4):534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Schmidt C, Schneble N, Wetzker R. The fifth dimension of innate immunity. J Cell Commun Signal. 2014;8(4):363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Quintin J, Saeed S, Martens JHA, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12(2):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Bauer M, Weis S, Netea MG, Wetzker R. Remembering pathogen dose: long-term adaptation in innate immunity. Trends Immunol. 2018;39(6):438–445. [DOI] [PubMed] [Google Scholar]
- 156. Beeson PB, Technical Assistance of Elizabeth Roberts. Tolerance to bacterial pyrogens: I. factors influencing its development. J Exp Med. 1947;86(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Beeson PB, Elizabeth R. Tolerance to bacterial pyrogens: II. Role of the reticulo-endothelial system. J Exp Med. 1947;86(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9(10):692–703. [DOI] [PubMed] [Google Scholar]
- 159. West MA, Heagy W. Endotoxin tolerance: a review. Crit Care Med. 2002;30(suppl 1):S64–S73. [PubMed] [Google Scholar]
- 160. Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447(7147):972–978. [DOI] [PubMed] [Google Scholar]
- 161. Quintin J, Cheng SC, van der Meer JW, Netea MG. Innate immune memory: towards a better understanding of host defense mechanisms. Curr Opin Immunol. 2014;29:1–7. [DOI] [PubMed] [Google Scholar]
- 162. Saeed S, Quintin J, Kerstens HH, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345(6204):1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Lehmann K, Muller JP, Schlott B, et al. PI3Kgamma controls oxidative bursts in neutrophils via interactions with PKCalpha and p47phox. Biochem J. 2009;419(3):603–610. [DOI] [PubMed] [Google Scholar]
- 164. Noble RL. The development of resistance by rats and guinea pigs to amounts of trauma usually fatal. Am J Physiol. 1943;138(2):346–351. [Google Scholar]
- 165. Noell WK, Chinn HI. The cerebral survival time of rabbits in anoxia; effects of previous oxygenation. Q Res Rep. 1948;60:8. [PubMed] [Google Scholar]
- 166. Simon RP. Tolerance, Historical Overview. New York, NY: Springer; 2013. [Google Scholar]
- 167. Dahl NA, Balfour WM. Prolonged anoxic survival due to anoxia pre-exposure: brain ATP, lactate, and pyruvate. Am J Physiol. 1964;207:452–456. [DOI] [PubMed] [Google Scholar]
- 168. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. [DOI] [PubMed] [Google Scholar]
- 169. Janoff A. Alterations in lysosomes (intracellular enzymes) during shock; effects of preconditioning (tolerance) and protective drugs. Int Anesthesiol Clin. 1964;2:251–269. [DOI] [PubMed] [Google Scholar]
- 170. Reimer KA, Murry CE, Yamasawa I, Hill ML, Jennings RB. Four brief periods of myocardial ischemia cause no cumulative ATP loss or necrosis. Am J Physiol. 1986;251(6 pt 2):H1306–H1315. [DOI] [PubMed] [Google Scholar]
- 171. Roth S, Li B, Rosenbaum PS, et al. Preconditioning provides complete protection against retinal ischemic injury in rats. Invest Ophthalmol Vis Sci. 1998;39(5):777–785. [PubMed] [Google Scholar]
- 172. Gidday JM, Fitzgibbons JC, Shah AR, Park TS. Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat. Neurosci Lett. 1994;168(1-2):221–224. [DOI] [PubMed] [Google Scholar]
- 173. Miller BA, Perez RS, Shah AR, Gonzales ER, Park TS, Gidday JM. Cerebral protection by hypoxic preconditioning in a murine model of focal ischemia–reperfusion. Neuroreport. 2001;12(8):1663–1669. [DOI] [PubMed] [Google Scholar]
- 174. Kitagawa K. Ischemic tolerance in the brain: endogenous adaptive machinery against ischemic stress. J Neurosci Res. 2012;90(5):1043–1054. [DOI] [PubMed] [Google Scholar]
- 175. Kitagawa K, Matsumoto M, Mabuchi T, et al. Ischemic tolerance in hippocampal CA1 neurons studied using contralateral controls. Neuroscience. 1997;81(4):989–998. [DOI] [PubMed] [Google Scholar]
- 176. Kitagawa K, Matsumoto M, Tagaya M, et al. ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res. 1990;528(1):21–24. [DOI] [PubMed] [Google Scholar]
- 177. Chen J, Graham SH, Zhu RL, Simon RP. Stress proteins and tolerance to focal cerebral ischemia. J Cereb Blood Flow Metab. 1996;16(4):566–577. [DOI] [PubMed] [Google Scholar]
- 178. Chen J, Simon R. Ischemic tolerance in the brain. Neurology. 1997;48(2):306–311. [DOI] [PubMed] [Google Scholar]
- 179. Simon RP, Niiro M, Gwinn R. Prior ischemic stress protects against experimental stroke. Neurosci Lett. 1993;163(2):135–137. [DOI] [PubMed] [Google Scholar]
- 180. Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res. 2008;79(3):377–386. [DOI] [PubMed] [Google Scholar]
- 181. Dirnagl U. Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann N Y Acad Sci. 2012;1268:21–25. [DOI] [PubMed] [Google Scholar]
- 182. Dirnagl U, Meisel A. Endogenous neuroprotection: mitochondria as gateways to cerebral preconditioning? Neuropharmacology. 2008;55(3):334–344. [DOI] [PubMed] [Google Scholar]
- 183. Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83(4):1113–1151. [DOI] [PubMed] [Google Scholar]
- 184. Koch S, Della-Morte D, Dave KR, Sacco RL, Perez-Pinzon MA. Biomarkers for ischemic preconditioning: finding the responders. J Cereb Blood Flow Metab. 2014;34(6):933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol. 1998;275(4 pt 2):H1375–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Li Y, Kloner RA. Cardioprotective effects of ischemic preconditioning can be recaptured after they are lost. J Am Coll Cardiol. 1994;23(2):470–474. [DOI] [PubMed] [Google Scholar]
- 187. Yang XM, Arnoult S, Tsuchida A, et al. The protection of ischaemic preconditioning can be reinstated in the rabbit heart after the initial protection has waned. Cardiovasc Res. 1993;27(4):556–558. [DOI] [PubMed] [Google Scholar]
- 188. Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87(3):893–899. [DOI] [PubMed] [Google Scholar]
- 189. Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94(9):2193–2200. [DOI] [PubMed] [Google Scholar]
- 190. Birnbaum Y, Hale SL, Kloner RA. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997;96(5):1641–1646. [DOI] [PubMed] [Google Scholar]
- 191. Kharbanda RK, Mortensen UM, White PA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo . Circulation. 2002;106(23):2881–2883. [DOI] [PubMed] [Google Scholar]
- 192. Vlasov TD, Korzhevskii DE, Polyakova EA. Ischemic preconditioning of the rat brain as a method of endothelial protection from ischemic/repercussion injury. Neurosci Behav Physiol. 2005;35(6):567–572. [DOI] [PubMed] [Google Scholar]
- 193. Dave KR, Saul I, Prado R, Busto R, Perez-Pinzon MA. Remote organ ischemic preconditioning protect brain from ischemic damage following asphyxial cardiac arrest. Neurosci Lett. 2006;404(1-2):170–175. [DOI] [PubMed] [Google Scholar]
- 194. Gurcun U, Discigil B, Boga M, et al. Is remote preconditioning as effective as direct ischemic preconditioning in preventing spinal cord ischemic injury? J Surg Res. 2006;135(2):385–393. [DOI] [PubMed] [Google Scholar]
- 195. Sun XC, Li WB, Li QJ, et al. Limb ischemic preconditioning induces brain ischemic tolerance via p38 MAPK. Brain Res. 2006;1084(1):165–174. [DOI] [PubMed] [Google Scholar]
- 196. Rehni AK, Shri R, Singh M. Remote ischaemic preconditioning and prevention of cerebral injury. Indian J Exp Biol. 2007;45(3):247–252. [PubMed] [Google Scholar]
- 197. Ren C, Gao X, Steinberg GK, Zhao H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience. 2008;151(4):1099–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Malhotra S, Naggar I, Stewart M, Rosenbaum DM. Neurogenic pathway mediated remote preconditioning protects the brain from transient focal ischemic injury. Brain Res. 2011;1386:184–190. [DOI] [PubMed] [Google Scholar]
- 199. Hahn CD, Manlhiot C, Schmidt MR, Nielsen TT, Redington AN. Remote ischemic per-conditioning: a novel therapy for acute stroke? Stroke. 2011;42(10):2960–2962. [DOI] [PubMed] [Google Scholar]
- 200. Jensen HA, Loukogeorgakis S, Yannopoulos F, et al. Remote ischemic preconditioning protects the brain against injury after hypothermic circulatory arrest. Circulation. 2011;123(7):714–721. [DOI] [PubMed] [Google Scholar]
- 201. Xu T, Gong Z, Zhu WZ, et al. Remote ischemic preconditioning protects neurocognitive function of rats following cerebral hypoperfusion. Med Sci Monit. 2011;17(11):BR299–BR304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Hu S, Dong H, Zhang H, et al. Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Res. 2012;1459:81–90. [DOI] [PubMed] [Google Scholar]
- 203. Wei D, Ren C, Chen X, Zhao H. The chronic protective effects of limb remote preconditioning and the underlying mechanisms involved in inflammatory factors in rat stroke. PLoS One. 2012;7(2):e30892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Liu ZJ, Chen C, Li XR, et al. Remote Ischemic preconditioning-mediated neuroprotection against stroke is associated with significant alterations in peripheral immune responses. CNS Neurosci Ther. 2016;22(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Johnsen J, Pryds K, Salman R, Lofgren B, Kristiansen SB, Botker HE. The remote ischemic preconditioning algorithm: effect of number of cycles, cycle duration and effector organ mass on efficacy of protection. Basic Res Cardiol. 2016;111(2):10. [DOI] [PubMed] [Google Scholar]
- 206. Jean-St-Michel E, Manlhiot C, Li J, et al. Remote preconditioning improves maximal performance in highly trained athletes. Med Sci Sports Exerc. 2011;43(7):1280–1286. [DOI] [PubMed] [Google Scholar]
- 207. Shimizu M, Tropak M, Diaz RJ, et al. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci (Lond). 2009;117(5):191–200. [DOI] [PubMed] [Google Scholar]
- 208. Jensen RV, Stottrup NB, Kristiansen SB, Botker HE. Release of a humoral circulating cardioprotective factor by remote ischemic preconditioning is dependent on preserved neural pathways in diabetic patients. Basic Res Cardiol. 2012;107(5):285. [DOI] [PubMed] [Google Scholar]
- 209. Michelsen MM, Stottrup NB, Schmidt MR, et al. Exercise-induced cardioprotection is mediated by a bloodborne, transferable factor. Basic Res Cardiol. 2012;107(3):260. [DOI] [PubMed] [Google Scholar]
- 210. Lee M, Carroll TJ. Cross education: possible mechanisms for the contralateral effects of unilateral resistance training. Sports Med. 2007;37(1):1–14. [DOI] [PubMed] [Google Scholar]
- 211. Dezfulian C, Taft M, Corey C, et al. Biochemical signaling by remote ischemic conditioning of the arm versus thigh: is one raise of the cuff enough? Redox Biol. 2017;12:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114(10):1601–1610. [DOI] [PubMed] [Google Scholar]
- 213. Giricz Z, Varga ZV, Baranyai T, et al. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol. 2014;68:75–78. [DOI] [PubMed] [Google Scholar]
- 214. Lawson C, Vicencio JM, Yellon DM, Davidson SM. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol. 2016;228(2): R57–71. [DOI] [PubMed] [Google Scholar]
- 215. Li J, Rohailla S, Gelber N, et al. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol. 2014;109(5):423. [DOI] [PubMed] [Google Scholar]
- 216. Rassaf T, Ferdinandy P, Schulz R. Nitrite in organ protection. Br J Pharmacol. 2014;171(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374(9700):1557–1565. [DOI] [PubMed] [Google Scholar]
- 218. Lang SC, Elsasser A, Scheler C, et al. Myocardial preconditioning and remote renal preconditioning – identifying a protective factor using proteomic methods? Basic Res Cardiol. 2006;101(2):149–158. [DOI] [PubMed] [Google Scholar]
- 219. Munk K, Andersen NH, Schmidt MR, et al. Remote ischemic conditioning in patients with myocardial infarction treated with primary angioplasty: impact on left ventricular function assessed by comprehensive echocardiography and gated single-photon emission CT. Circ Cardiovasc Imaging. 2010;3(6):656–662. [DOI] [PubMed] [Google Scholar]
- 220. Meng R, Asmaro K, Meng L, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79(18):1853–1861. [DOI] [PubMed] [Google Scholar]
- 221. Wang S, Li H, He N, et al. Impact of remote ischaemic preconditioning on major clinical outcomes in patients undergoing cardiovascular surgery: a meta-analysis with trial sequential analysis of 32 randomised controlled trials. Int J Cardiol. 2017;227:882–891. [DOI] [PubMed] [Google Scholar]
- 222. Botker HE, Kharbanda R, Schmidt MR, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375(9716):727–734. [DOI] [PubMed] [Google Scholar]
- 223. Sloth AD, Schmidt MR, Munk K, et al. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2014;35(3):168–175. [DOI] [PubMed] [Google Scholar]
- 224. Schmidt MR, Rasmussen ME, Botker HE. Remote ischemic conditioning for patients with STEMI. J Cardiovasc Pharmacol Ther. 2017;22(4):302–309. [DOI] [PubMed] [Google Scholar]
- 225. Hausenloy DJ, Kharbanda R, Rahbek Schmidt M, et al. Effect of remote ischaemic conditioning on clinical outcomes in patients presenting with an ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur Heart J. 2015;36(29):1846–1848. [PubMed] [Google Scholar]
- 226. Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65(2):177–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Weih M, Kallenberg K, Bergk A, et al. Attenuated stroke severity after prodromal TIA: a role for ischemic tolerance in the brain? Stroke. 1999;30(9):1851–1854. [DOI] [PubMed] [Google Scholar]
- 228. Wegener S, Gottschalk B, Jovanovic V, et al. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004;35(3):616–621. [DOI] [PubMed] [Google Scholar]
- 229. Reiter R, Henry TD, Traverse JH. Preinfarction angina reduces infarct size in ST-elevation myocardial infarction treated with percutaneous coronary intervention. Circ Cardiovasc Interv. 2013;6(1):52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Castillo J, Moro MA, Blanco M, et al. The release of tumor necrosis factor-alpha is associated with ischemic tolerance in human stroke. Ann Neurol. 2003;54(6):811–819. [DOI] [PubMed] [Google Scholar]
- 231. Herrett E, Bhaskaran K, Timmis A, Denaxas S, Hemingway H, Smeeth L. Association between clinical presentations before myocardial infarction and coronary mortality: a prospective population-based study using linked electronic records. Eur Heart J. 2014;35(35):2363–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Lonborg J, Kelbaek H, Vejlstrup N, et al. Influence of pre-infarction angina, collateral flow, and pre-procedural TIMI flow on myocardial salvage index by cardiac magnetic resonance in patients with ST-segment elevation myocardial infarction. Eur Heart J Cardiovasc Imaging. 2012;13(5):433–443. [DOI] [PubMed] [Google Scholar]
- 233. Della Morte D, Abete P, Gallucci F, et al. Transient ischemic attack before nonlacunar ischemic stroke in the elderly. J Stroke Cerebrovasc Dis. 2008;17(5):257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Meng R, Ding Y, Asmaro K, et al. Ischemic conditioning is safe and effective for octo- and nonagenarians in stroke prevention and treatment. Neurotherapeutics. 2015;12(3):667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Crisafulli A, Tangianu F, Tocco F, et al. Ischemic preconditioning of the muscle improves maximal exercise performance but not maximal oxygen uptake in humans. J Appl Physiol (1985). 2011;111(2):530–536. [DOI] [PubMed] [Google Scholar]
- 236. de Groot PC, Thijssen DH, Sanchez M, Ellenkamp R, Hopman MT. Ischemic preconditioning improves maximal performance in humans. Eur J Appl Physiol. 2010;108(1):141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Patterson SD, Bezodis NE, Glaister M, Pattison JR. The effect of ischemic preconditioning on repeated sprint cycling performance. Med Sci Sports Exerc. 2015;47(8):1652–1658. [DOI] [PubMed] [Google Scholar]
- 238. Bailey TG, Birk GK, Cable NT, et al. Remote ischemic preconditioning prevents reduction in brachial artery flow-mediated dilation after strenuous exercise. Am J Physiol Heart Circ Physiol. 2012;303(5): H533–538. [DOI] [PubMed] [Google Scholar]
- 239. Paradis-Deschenes P, Joanisse DR, Billaut F. Sex-specific impact of ischemic preconditioning on tissue oxygenation and maximal concentric force. Front Physiol. 2016;7:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Gibson N, White J, Neish M, Murray A. Effect of ischemic preconditioning on land-based sprinting in team-sport athletes. Int J Sports Physiol Perform. 2013;8(6):671–676. [DOI] [PubMed] [Google Scholar]
- 241. Kilduff LP, Finn CV, Baker JS, Cook CJ, West DJ. Preconditioning strategies to enhance physical performance on the day of competition. Int J Sports Physiol Perform. 2013;8(6):677–681. [DOI] [PubMed] [Google Scholar]
- 242. Sharma V, Marsh R, Cunniffe B, Cardinale M, Yellon DM, Davidson SM. From protecting the heart to improving athletic performance – the benefits of local and remote ischaemic preconditioning. Cardiovasc Drugs Ther. 2015;29(6):573–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Schmidt MR, Sloth AD, Johnsen J, Botker HE. Remote ischemic conditioning: the cardiologist’s perspective. J Cardiovasc Med (Hagerstown). 2012;13(11):667–674. [DOI] [PubMed] [Google Scholar]
- 244. Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol. 2000;529(Pt 1):215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Mauelshagen J, Sherff CM, Carew TJ. Differential induction of long-term synaptic facilitation by spaced and massed applications of serotonin at sensory neuron synapses of Aplysia californica . Learn Mem. 1998;5(3):246–256. [PMC free article] [PubMed] [Google Scholar]
- 246. Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila . Cell. 1994;79(1):35–47. [DOI] [PubMed] [Google Scholar]
- 247. Gidday JM, Zhang L, Chiang CW, Zhu Y. Enhanced retinal ganglion cell survival in glaucoma by hypoxic postconditioning after disease onset. Neurotherapeutics. 2015;12(2):502–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Zhu Y, Zhang L, Schmidt JF, Gidday JM. Glaucoma-induced degeneration of retinal ganglion cells prevented by hypoxic preconditioning: a model of glaucoma tolerance. Mol Med. 2012;18:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Chen T, Kato H, Liu XH, Araki T, Itoyama Y, Kogure K. Ischemic tolerance can be induced repeatedly in the gerbil hippocampal neurons. Neurosci Lett. 1994;177(1-2):159–161. [DOI] [PubMed] [Google Scholar]
- 250. Zhu Y, Zhang Y, Ojwang BA, Brantley MA, Jr, Gidday JM. Long-term tolerance to retinal ischemia by repetitive hypoxic preconditioning: role of HIF-1alpha and heme oxygenase-1. Invest Ophthalmol Vis Sci. 2007;48(4):1735–1743. [DOI] [PubMed] [Google Scholar]
- 251. Stowe AM, Altay T, Freie AB, Gidday JM. Repetitive hypoxia extends endogenous neurovascular protection for stroke. Ann Neurol. 2011;69(6):975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Neckar J, Ostadal B, Kolar F. Myocardial infarct size-limiting effect of chronic hypoxia persists for five weeks of normoxic recovery. Physiol Res. 2004;53(6):621–628. [PubMed] [Google Scholar]
- 253. Lovett-Barr MR, Satriotomo I, Muir GD, et al. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci. 2012;32(11):3591–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Wei M, Xin P, Li S, et al. Repeated remote ischemic postconditioning protects against adverse left ventricular remodeling and improves survival in a rat model of myocardial infarction. Circ Res. 2011;108(10):1220–1225. [DOI] [PubMed] [Google Scholar]
- 255. Gidday JM. Extending injury- and disease-resistant CNS phenotypes by repetitive epigenetic conditioning. Front Neurol. 2015;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Lyamina NP, Lyamina SV, Senchiknin VN, Mallet RT, Downey HF, Manukhina EB. Normobaric hypoxia conditioning reduces blood pressure and normalizes nitric oxide synthesis in patients with arterial hypertension. J Hypertens. 2011;29(11):2265–2272. [DOI] [PubMed] [Google Scholar]
- 257. Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology. 2014;82(2):104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Navarrete-Opazo A, Alcayaga J, Sepulveda O, Rojas E, Astudillo C. Repetitive intermittent hypoxia and locomotor training enhances walking function in incomplete spinal cord injury subjects: a randomized, triple-blind, placebo-controlled clinical trial. J Neurotrauma. 2017;34(9):1803–1812. [DOI] [PubMed] [Google Scholar]
- 259. Trumbower RD, Hayes HB, Mitchell GS, Wolf SL, Stahl VA. Effects of acute intermittent hypoxia on hand use after spinal cord trauma: a preliminary study. Neurology. 2017;89(18):1904–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Jones H, Hopkins N, Bailey TG, Green DJ, Cable NT, Thijssen DH. Seven-day remote ischemic preconditioning improves local and systemic endothelial function and microcirculation in healthy humans. Am J Hypertens. 2014;27(7):918–925. [DOI] [PubMed] [Google Scholar]
- 261. Shaked G, Czeiger D, Abu Arar A, Katz T, Harman-Boehm I, Sebbag G. Intermittent cycles of remote ischemic preconditioning augment diabetic foot ulcer healing. Wound Repair Regen. 2015;23(2):191–196. [DOI] [PubMed] [Google Scholar]
- 262. Ramos JS, Dalleck LC, Tjonna AE, Beetham KS, Coombes JS. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis. Sports Med. 2015;45(5):679–692. [DOI] [PubMed] [Google Scholar]
- 263. Weston KS, Wisloff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med. 2014;48(16):1227–1234. [DOI] [PubMed] [Google Scholar]
- 264. Seeger JP, Lenting CJ, Schreuder TH, et al. Interval exercise, but not endurance exercise, prevents endothelial ischemia-reperfusion injury in healthy subjects. Am J Physiol Heart Circ Physiol. 2015;308(4): H351–H357. [DOI] [PubMed] [Google Scholar]
- 265. McLaughlin K, Lytvyn Y, Luca MC, Liuni A, Gori T, Parker JD. Repeated daily dosing with sildenafil provides sustained protection from endothelial dysfunction caused by ischemia and reperfusion: a human in vivo study. Am J Physiol Heart Circ Physiol. 2014;307(6):H888–H894. [DOI] [PubMed] [Google Scholar]
- 266. Jacqmain J, Nudi ET, Fluharty S, Smith JS. Pre and post-injury environmental enrichment effects functional recovery following medial frontal cortical contusion injury in rats. Behav Brain Res. 2014;275:201–211. [DOI] [PubMed] [Google Scholar]
- 267. Piao CS, Stoica BA, Wu J, et al. Late exercise reduces neuroinflammation and cognitive dysfunction after traumatic brain injury. Neurobiol Dis. 2013;54:252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Sartorius N. The meanings of health and its promotion. Croat Med J. 2006;47(4):662–664. [PMC free article] [PubMed] [Google Scholar]
- 269. No authors listed (Editorial). What is health? The ability to adapt. Lancet. 2009;373(9666):781. [DOI] [PubMed] [Google Scholar]
- 270. Kocsis GF, Pipis J, Fekete V, et al. Lovastatin interferes with the infarct size-limiting effect of ischemic preconditioning and postconditioning in rat hearts. Am J Physiol Heart Circ Physiol. 2008;294(5):H2406–H2409. [DOI] [PubMed] [Google Scholar]
- 271. Wang Y, Meng R, Song H, et al. Remote ischemic conditioning may improve outcomes of patients with cerebral small-vessel disease. Stroke. 2017;48(11):3064–3072. [DOI] [PubMed] [Google Scholar]
- 272. Koch S, Katsnelson M, Dong C, Perez-Pinzon M. Remote ischemic limb preconditioning after subarachnoid hemorrhage: a phase Ib study of safety and feasibility. Stroke. 2011;42(5):1387–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Koch S, Gonzalez N. Preconditioning the human brain: proving the principle in subarachnoid hemorrhage. Stroke. 2013;44(6):1748–1753. [DOI] [PubMed] [Google Scholar]
- 274. Son TG, Camandola S, Mattson MP. Hormetic dietary phytochemicals. Neuromolecular medicine. 2008;10(4):236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Lee J, Jo DG, Park D, Chung HY, Mattson MP. Adaptive cellular stress pathways as therapeutic targets of dietary phytochemicals: focus on the nervous system. Pharmacol Rev. 2014;66(3):815–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Lindsay DG. Nutrition, hormetic stress and health. Nutr Res Rev. 2005;18(2):249–258. [DOI] [PubMed] [Google Scholar]
- 277. Baur JA, Sinclair DA. What is xenohormesis? Am J Pharmacol Toxicol. 2008;3(1):152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Lemmens KJA, Sthijns MMJPE, van der Vijgh WJF, Bast A, Haenen GRMM. The antioxidant flavonoid monoHER provides efficient protection and includes the innate Nrf2 mediated adaptation in endothelial cells subjected to oxidative stress. PharmaNutrition. 2014;2(3):69–74. [Google Scholar]
- 279. Weseler AR, Ruijters EJ, Drittij-Reijnders MJ, Reesink KD, Haenen GR, Bast A. Pleiotropic benefit of monomeric and oligomeric flavanols on vascular health – a randomized controlled clinical pilot study. PLoS One. 2011;6(12): e28460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Weseler AR, Bast A. Pleiotropic-acting nutrients require integrative investigational approaches: the example of flavonoids. J Agric Food Chem. 2012;60(36):8941–8946. [DOI] [PubMed] [Google Scholar]
- 281. Calcada D, Vianello D, Giampieri E, et al. The role of low-grade inflammation and metabolic flexibility in aging and nutritional modulation thereof: a systems biology approach. Mech Ageing Dev. 2014;136-137:138–147. [DOI] [PubMed] [Google Scholar]
- 282. Rodriguez-Salus M, Bektas Y, Schroeder M, et al. The synthetic elicitor 2-(5-bromo-2-hydroxy-phenyl)-thiazolidine-4-carboxylic acid links plant immunity to hormesis. Plant Physiol. 2016;170(1):444–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Vargas-Hernandez M, Macias-Bobadilla I, Guevara-Gonzalez RG, et al. Plant hormesis management with biostimulants of biotic origin in agriculture. Front Plant Sci. 2017;8:1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Avramova Z. Transcriptional ‘memory’ of a stress: transient chromatin and memory (epigenetic) marks at stress-response genes. Plant J. 2015;83(1):149–159. [DOI] [PubMed] [Google Scholar]
- 285. Belz R, Cedergreen N. Parthenin hormesis in plants depends on growth conditions. Environ Exp Bot. 2010;69:293–301. [Google Scholar]
- 286. Belz RG, Duke SO. Herbicides and plant hormesis. Pest Manag Sci. 2014;70(5):698–707. [DOI] [PubMed] [Google Scholar]
- 287. Calabrese E, Baldwin LA. The dose determines the stimulation (and poison): development of a chemical hormesis database. Int J Toxicol. 1997;16(6):545–559. [Google Scholar]
- 288. Gnocchi D, Pedrelli M, Hurt-Camejo E, Parini P. Lipids around the clock: focus on circadian rhythms and lipid metabolism. Biology (Basel). 2015;4(1):104–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5(6):463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Megginson L. Lessons from Europe for American business. Southwestern Soc Sci Q 1963;44(1):3–13. [Google Scholar]
- 291. Kirino T, Tsujita Y, Tamura A. Induced tolerance to ischemia in gerbil hippocampal neurons. J Cereb Blood Flow Metab. 1991;11(2):299–307. [DOI] [PubMed] [Google Scholar]
- 292. Lusardi TA, Farr CD, Faulkner CL, et al. Ischemic preconditioning regulates expression of microRNAs and a predicted target, MeCP2, in mouse cortex. J Cereb Blood Flow Metab. 2010;30(4):744–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Zhang J, Yang ZJ, Klaus JA, Koehler RC, Huang J. Delayed tolerance with repetitive transient focal ischemic preconditioning in the mouse. Stroke. 2008;39(3):967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Radak Z, Ishihara K, Tekus E, et al. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol. 2017;12:285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Nestler EJ. Transcriptional mechanisms of addiction: role ofdelta fosB. Phil Trans R Soc B. 2008; 363:3245–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]