Abstract
Sporulosol (1), a new ketal, together with four known compounds, has been isolated from the liquid fermentation cultures of a wetland-soil-derived fungus, Paraconiothyrium sporulosum. Its structure was elucidated primarily by NMR experiments, and was further confirmed by X-ray crystallography. Sporulosol was obtained as a racemic mixture and the resolved two enantiomers racemized immediately after chiral separation. Sporulosol appears to be the first ketal derived from a 6H-benzo[c]chromen-6-one and a benzofuranone unit. The compound showed modest cytotoxicity toward the human tumor cell line T24, with an IC50 value of 18.2 µM.
Keywords: Paraconiothyrium sporulosum, ketal, racemate, cytotoxicity
1. Introduction
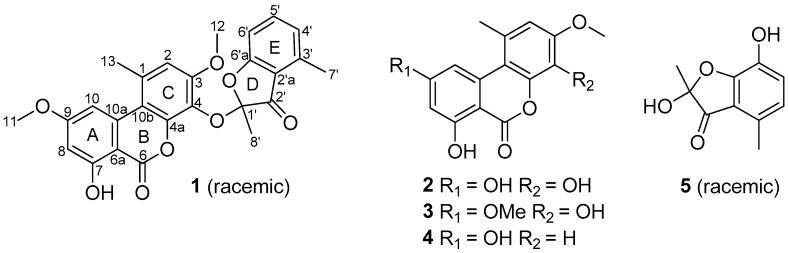
Fungi are one of the most important sources of new drugs or lead compounds; many important medicines have been discovered directly from fungi or developed based on their secondary metabolites [1], such as the antibiotic penicillin, anti-cholesteremic agent lovastatin, immuno-suppressant cyclosporin A, and the anti-multiple sclerosis fingolimod. Fungal species isolated from the wetland environments have attracted increasing attention due to their ability to produce a variety of bioactive secondary metabolites. Examples include stachybisbins A and B [2], flaviphenalenones A–C, aspulvinones P and Q, methybutyrolactone III [3,4], 5-hydroxymethylasterric acid, 3,5-dichlorosulochrin [4], and isocoumarin glycosides [5]. Based on this consideration, we initiated chemical studies of the wetland-soil-derived fungal species in the genus of Paraconiothyrium, which was reclassified as a separate genus as Paraconiothyrium by Verkley in 2004 [6]. Prior to our chemical studies, a variety of bioactive secondary metabolites were reported from this genus [7,8,9,10,11,12,13,14,15,16,17]. Our chemical investigations of P. brasiliense, and P. hawaiiense grown in solid-substrate fermentation cultures have also resulted in the isolation of structurally diverse and biologically active natural products including brasilamides A–N and hawaiinolides A–G [18,19,20,21,22]. During an ongoing search for new bioactive secondary metabolites from the species of this genus, a strain of Paraconiothyrium sporulosum, which was collected at Poyang Lake, Jiangxi Province, People’s Republic of China, was subjected to our chemical investigation. A new ketal, named sporulosol (1), was isolated from the liquid fermentation cultures of the fungus, together with four known compounds (2–5; Figure 1). Details of the isolation, structure elucidation, and cytotoxicity of these compounds are reported herein.
Figure 1.
Structures of compounds 1–5.
2. Results and Discussion
Sporulosol (1) was assigned a molecular formula of C26H22O8 (16 degrees of unsaturation) on the basis of high-resolution electrospray ionization mass spectrometry (HRESIMS). Analysis of its nuclear magnetic resonance (NMR) data (Table 1) revealed the presence of an exchangeable proton (δH 11.89), five methyl groups including two O-methyls, one ketal carbon (δC 105.4), 18 aromatic/olefinic carbons with six protonated, one carboxylic carbon (δC 164.4), and one α,β-unsaturated ketone carbon (δC 195.9). These data accounted for all the NMR resonances and suggested that 1 was a pentacyclic metabolite. The 1H and 13C NMR data of 1 (Table 1) revealed structural features that were closely related to the known compounds graphislactone A (3) [23] and 2-hydroxy-2,4-dimethyl-3(2H)-benzofuranone [24], but some chemical shifts for 1 were significantly different from those reported for the known compounds, warranting detailed 2D NMR analysis. Interpretation of the NMR data revealed a tetrasubstituted aryl ring (A), with a hydroxy and an O-methyl group attached to C-7 and C-9, respectively, which was confirmed by the HMBC correlations from H-8 to C-6a, C-7, and C-10a; H-10 to C-6a, C-9, and C-10a; OH-7 to C-7 and C-8; and from H3-11 to C-9. Correlations from H-2 to C-1, C-3, C-4, C-4a, and C-10b; H3-13 to C-1 and C-2; and from H3-12 to C-3 established the pentasubstituted aryl ring (C). In turn, the HMBC cross peaks from H-4′ to C-5′, C-6′, and C-7′; H-5′ to C-4′ and C-6′a; H-6′ to C-2′a and C-5′; and from H3-7′ to C-2′a, C-3′, and C-4′ enabled junction of the trisubstituted aryl ring (E) with a methyl group attached to C-3′. Additional correlations from H3-8′ to C-1′ and C-2′, plus the 13C NMR chemical shifts for C-2′a (δC 117.4) and C-6′a (δC 170.0), indicated that these three carbons form an α,β-unsaturated ketone with C-1′ connected to C-6′a via the C-1′/C-6′a ether linkage. Considering the 1H NMR chemical shift of OH-7 (δH 11.89) and the unsaturation requirement of 1, the carboxylic carbon (δC 164.4) was connected to C-6, and acylated the C-4a oxygen to form ring B, while C-4 and C-1′ were attached to the only remaining oxygen atom to form an ester linkage. Based on these data, the planar structure of 1 was established.
Table 1.
NMR data for 1 and 2.
| Pos. | 1 | 2 | ||||
|---|---|---|---|---|---|---|
| δCa, Mult. | δHb (J in Hz) | HMBC | δCc Mult. | δHd (J in Hz) | HMBC | |
| 1 | 134.0, qC | 126.6, qC | ||||
| 2 | 112.8, CH | 6.90, s | 1, 3, 4, 4a, 10b | 112.8, CH | 6.93, s | 1, 3, 4, 10a, 10b, 12 |
| 3 | 153.8, qC | 147.8, qC | ||||
| 4 | 128.0, qC | 140.6, qC | ||||
| 4a | 147.0, qC | 138.8, qC | ||||
| 6 | 164.4, qC | 164.8, qC | ||||
| 6a | 98.9, qC | 98.2, qC | ||||
| 7 | 165.0, qC | 165.0, qC | ||||
| 8 | 99.5, CH | 6.60, d (2.0) | 7, 6a, 10a | 101.3, CH | 6.46, d (2.0) | 6a, 9, 10 |
| 9 | 166.7, qC | 165.3, qC | ||||
| 10 | 104.2, CH | 7.28, d (2.0) | 6a, 9, 10a | 104.9, CH | 7.37, d (2.0) | 6a, 8, 9, 10b |
| 10a | 137.9, qC | 132.8, qC | ||||
| 10b | 111.0, qC | 111.2, qC | ||||
| 11 | 55.4, CH3 | 3.96, s | 9 | 55.7, CH3 | 3.67, s | 3 |
| 12 | 55.2, CH3 | 3.67, s | 3 | 24.5, CH3 | 2.75, s | 1, 2, 10, 10a |
| 13 | 24.8, CH3 | 2.78, s | 1, 2 | |||
| 1′ | 105.4, qC | |||||
| 2′ | 195.9, qC | |||||
| 2′a | 117.4, qC | |||||
| 3′ | 139.8, qC | |||||
| 4′ | 123.8, CH | 6.88, d (7.8) | 5′, 6′, 7′ | |||
| 5′ | 137.7, CH | 7.45, t (7.8) | 4′, 6′a | |||
| 6′ | 109.8, CH | 6.72, d (7.8) | 2′a, 5′ | |||
| 6′a | 170.0, qC | |||||
| 7′ | 16.7, CH3 | 2.53, s | 2′a, 3′, 4′ | |||
| 8′ | 19.4, CH3 | 1.80, s | 1′, 2′ | |||
| OH-7 | 11.89, s | 7, 8 | 11.95, s | 6a, 7, 8 | ||
a Recorded at 100 MHz in acetone-d6; b Recorded at 400 MHz in acetone-d6; c Recorded at 150 MHz in acetone-d6; d Recorded at 600 MHz in acetone-d6.
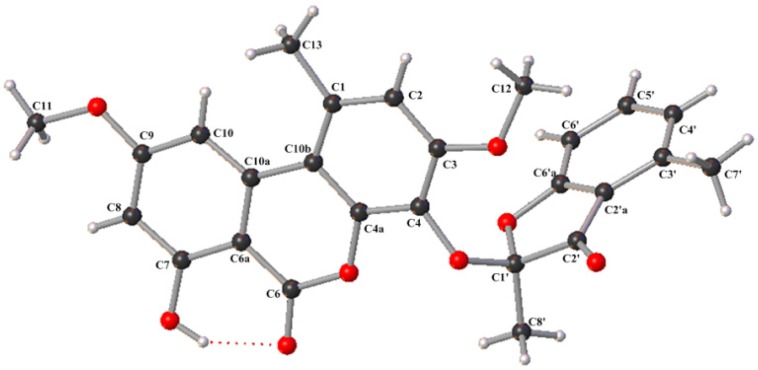
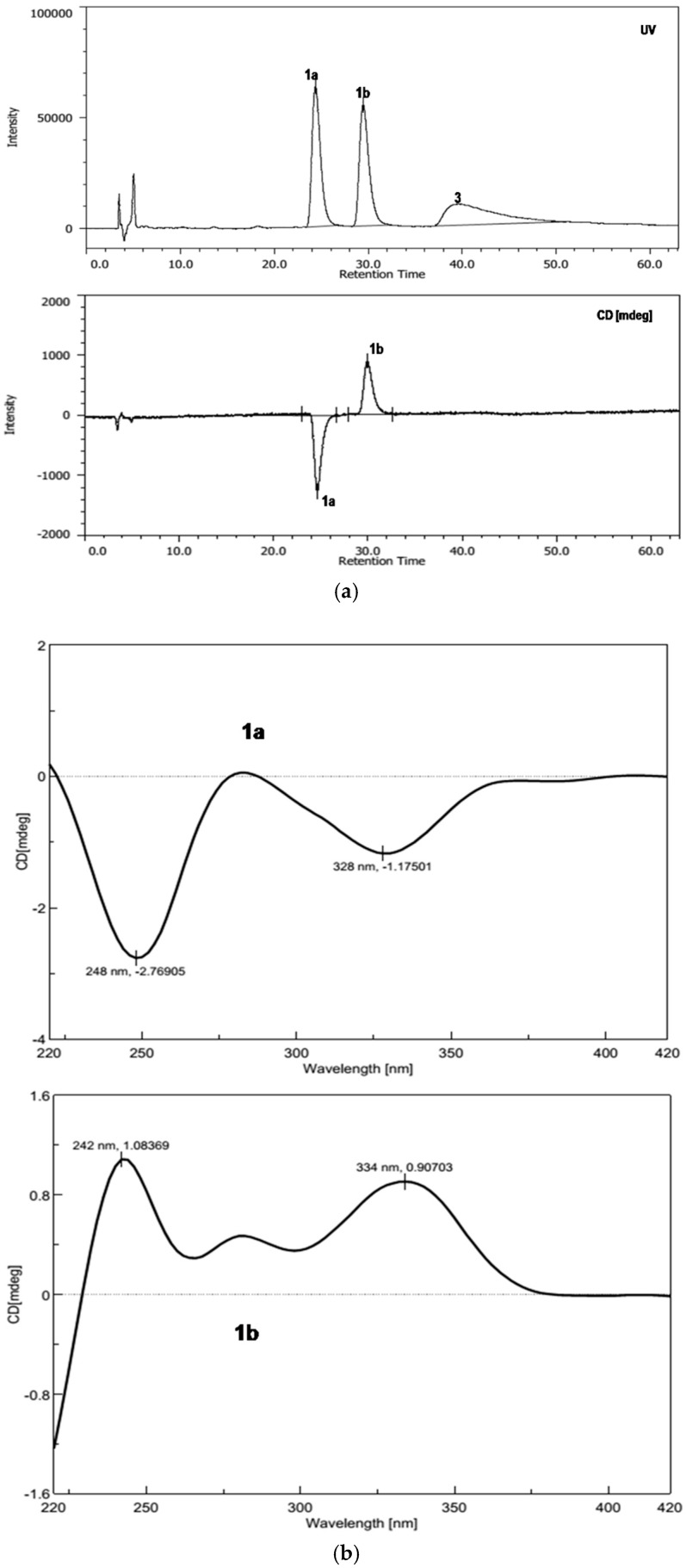
The proposed structure was confirmed by single-crystal X-ray diffraction analysis using Cu Kα radiation, and a perspective ORTEP plot is shown in Figure 2. Compound 1 was found to crystallize as a mixture of 1′S and 1′R enantiomers, which explained the low optical rotation value of +1.0 ([α]; c 0.10, MeOH) measured for 1. Subsequently, the racemic mixture was resolved into two enantiomers (1a and 1b; 1:1) and decomposition product graphislactone A (3) using chiral stationary phase (4.6 × 250 mm; 4% 2-propanol in hexanes for 60 min; 0.8 mL/min) (Figure S7). However, the other decomposition product 2-hydroxy-2,4-dimethyl-3(2H)-benzofuranone was not detected during resolution. The absolute configuration of C-1′ was assigned by the application of Snatzke’s chirality rule for cyclopentenones [25], through which the negative or positive Cotton effect for n → π * transition in the 330–365 nm region of the CD spectrum was used to assign the 1′S (1a) and 1′R (1b) absolute configurations by HPLC-CD analysis (Figure 3). Analysis of each collected peak for 1′S (1a) and 1′R (1b) revealed the presence of both enantiomers, suggesting the occurrence of spontaneous equilibration. Similarly, the co-isolated known compound, enalin A (5) [26], was also separated into two enantiomers 5a and 5b in a ratio of 1:1, and the resolved enantiomers again racemized immediately after chiral separation (Figure S8). However, subsequent HPLC-CD analysis of 5 was unsuccessful, possibly due to the poor HPLC and CD behavior of the compound (Figure S9). To our knowledge, fungal natural products including isopestacin, pestacin, pestalachloride A, fimetarone A, and arugosins K−M, have been reported as racemic mixtures of the S and R enantiomers [27,28,29,30,31].
Figure 2.
Thermal ellipsoid representation of 1. (Note: A different numbering system is used for the structural data deposited with the CCDC.).
Figure 3.
(a) HPLC-CD chromatogram of sporulosol (1) using a CHIRALPAK AD-H column (4.6 × 250 mm; 10% 2-Propanol in Hexane for 63 min; 1.0 mL/min); (b) HPLC-CD spectra of (1′S)-sporulosol (1a) and (1′R)-sporulosol (1b).
The molecular formula of 2 was determined to be C15H12O6 by HRESIMS. Analysis of the 1H and 13C NMR data of 2 (Table 1) revealed structural features similar to the known compound graphislactone A (3) [23], except that the 9-OMe signal disappeared, which was verified by interpretation of the 2D NMR data. Compound 2 was the first naturally occurring 4,7,9-trihydroxy-3-methoxy-1-methyl-6H-benzo[c]chromen-6-one [32], and its NMR data have yet to be reported. Compounds 3–5 were readily identified as graphislactone A (3) [23], 7,9-dihydroxy-3-methoxy-1-methyl-6H-benzo[c]chromen-6-one (4) [33], and enalin A (5) [26] by comparison of their NMR and MS data with those reported.
To verify that 1 was authentic natural product, a portion of the EtOAc extract freshly prepared from the freeze-dried fermented liquid substrate was subjected to HPLC-MS analysis using HPLC-grade H2O and CH3CN as solvents. Compound 1 was identified on the HPLC-MS chromatogram of the crude extract by comparison of its retention time and ESIMS data with an authentic sample (Figure S10), indicating that 1 is indeed a naturally occurring metabolite.
Compounds 1–4 were tested for cytotoxicity against a panel of five human tumor cell lines: HeLa, T24, A549, HCT116, and SH-SY5Y (Table 2). Compounds 1 and 4 showed modest cytotoxicity to T24 cells, with IC50 values of 18.2 and 4.5 µM, respectively (the positive control cisplatin showed an IC50 value of 10.9 µM). Compound 4 was also cytotoxic to Hela cells, showing an IC50 value of 5.4 µM (the positive control cisplatin showed an IC50 value of 8.7 µM).
Table 2.
Cytotoxicity of compounds 1–4.
| Compound | IC50 (μM) | ||||
|---|---|---|---|---|---|
| HeLa | T24 | A549 | HCT116 | SH-SY5Y | |
| 1 | 31.8 ± 0.2 | 18.2 ±5.3 | 23.1 ± 2.9 | 50.1 ± 7.0 | >50 |
| 2 | 11.7 ± 0.7 | 22.8 ± 1.7 | 25.1 ± 1.8 | 81.5 ± 3.4 | 41.5 ± 2.0 |
| 3 | >50 | 77.8 ± 8.0 | >50 | >50 | 71.7 ± 3.2 |
| 4 | 5.4 ± 0.1 | 4.5 ± 0.4 | 93.7 ± 5.6 | 20.9 ± 2.6 | 38.8 ± 4.9 |
| cisplatin | 8.7 ± 0.3 | 10.9 ± 0.7 | 17.0 ± 0.5 | 19.9 ± 0.7 | 11.2 ± 2.0 |
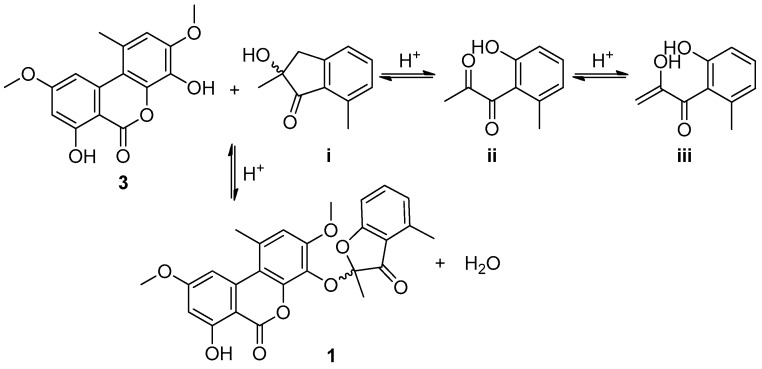
Although sporulosol (1) is a ketal derived from two known metabolites, it is the first example of such a ketal originating from the 6H-benzo[c]chromen-6-one and benzofuranone moieties, representing a new type of chemical structure. The presence of such a ketal moiety generated two enantiomers racemized constantly even right after chiral resolution. In addition, the plausible biosynthetic precursor was identified as a racemic mixture, too. The proposed precursors and the reaction cascades leading to the generation of 1 are illustrated in Scheme 1.
Scheme 1.
Plausible biosynthetic pathways for sporulosol (1).
3. Materials and Methods
3.1. General Experimental Procedures
An Analytical Automatic Polarimeter (Rodolph Research) was used to record optical rotations of the isolated compounds, and a Shimadzu Biospec-1601 spectrophotometer was used to measure the ultraviolet (UV) spectra. A Nicolet Magna-IR 750 spectrophotometer was used to record the infrared (IR) spectra. Nuclear magnetic resonance (NMR) spectra were recorded on Inova-400 and Inova-600 spectrometers using TMS as an internal standard. The HMBC and HMQC experiments were optimized for 8.0 and 145.0 Hz, respectively. Electrospray ionization mass spectrometry (ESIMS) and high-resolution mass spectrometry (HRMS) data were measured on an Agilent accurate mass quadrupole time-of-flight LC/MS G6550 instrument. High-performance liquid chromatography–circular dichroism (HPLC-CD) chromatograms were recorded on a JASCO LC 2000-CD 2095 instrument. All HPLC analysis and separation were performed using an Agilent 1260 instrument (Agilent, Santa Clara, CA, USA) equipped with a variable-wavelength UV detector.
3.2. Fungal Material
The strain P. sporulosum Verkley was isolated from the soil samples that were collected at Poyang Lake, Jiangxi Province, P. R. China, in December 2010. The fungus was identified by morphological observation and sequence (Genbank Accession No. JX077030) analyses of the ITS region of the rDNA. The identified P. sporulosum strain was cultured on Potato Dextrose Agar (PDA) at room temperature for 10 days, and the resulting agar plugs were cut into small pieces (0.5 × 0.5 × 0.5 cm3) under aseptic conditions. Fifteen pieces were inoculated into three 250 mL Erlenmeyer flasks, each containing 50 mL medium (0.4% glucose, 1% malt extract, and 0.4% yeast extract; pH 6.5), which were then incubated at room temperature on an orbital shaker at 170 rpm for 5 days to prepare the seed culture. The fermentation was carried out in 24 Fernbach flasks of 500 mL, each containing 5.0 mL seed culture and 200 mL synthetic dropout medium (2% malt extract, 6% dextrin, 0.7% peptone form fish, 0.7% cottonseed flour, 0.25% MgSO4·7H2O, 0.25% CaCO3, 0.1% FeSO4·7H2O, and 0.001% ZnSO4·7H2O), and incubated at 25 °C on a rotary shaker at 170 rpm for 30 days.
3.3. Extraction and Isolation
The fermented culture was extracted repeatedly with ethyl acetate (EtOAc; 4 × 4.8 L), yielding 5.0 g crude extract upon removal of the organic solvent under vacuum. Subsequently, the crude extract was fractionated by vacuum liquid chromatography on silica gel with gradient elution of petroleum ether (PE)–EtOAc. The fractions eluted with 88:12–82:18 PE–EtOAc were combined (338.4 mg) and separated by Sephadex LH-20 column chromatography (CC; 1:1 MeOH–CH2Cl2). The subfraction (119.5 mg) was purified by reversed-phase HPLC (Agilent Zorbax SB-C18 column; 5 μm; 9.4 × 250 mm; 45% MeOH in H2O for 38 min; 2 mL/min) to afford 5 (4.0 mg, tR 21.0 min), and the resulting fraction eluted with 100% MeOH was further purified by RP HPLC (74% MeOH in H2O for 25 min; 2 mL/min) to afford 4 (1.0 mg, tR 22.0 min). The fractions eluted with 80:20–78:22 PE–EtOAc were combined (445.0 mg) and separated by medium-pressure C18 RP silica gel CC using MeOH–H2O gradient elution (20–100%). Purification of the subfraction (68.9 mg) eluted with 80:20 MeOH–H2O by HPLC (70% MeOH in H2O for 15 min, followed by 82% MeOH in H2O for 30 min; 2 mL/min) yielded 3 (3.0 mg, tR 16.5 min) and 1 (4.0 mg, tR 41.0 min). The fractions eluted with 67:33–60:40 PE–EtOAc were combined (1.0 g) and further separated by C18 RP silica gel CC eluting with 20–100% MeOH–H2O. The subfractions eluted with 25:75–35:65 MeOH–H2O were combined (430.2 mg), separated by Sephadex LH-20 CC (1:1 MeOH–CH2Cl2), and purified by RP HPLC (55% MeOH in H2O for 40 min; 2 mL/min) to afford 2 (3.0 mg, tR 37.0 min).
3.4. Sporulosol (1)
Sporulosol (1), colorless needles; [α] +1.0 (c 0.10, MeOH); m.p. 130–132 °C; UV (MeOH) λmax (log ε) 340 (3.57) nm; IR (neat) νmax 3555, 2997, 2848, 1726, 1673, 1444, 1233, 1203, 1134 1078, 929, 795 cm−1; for 1H, 13C, and HMBC NMR data, see Table 1; HRESIMS m/z 463.1385 [M + H]+ (calcd. for C26H22O8, 463.1387).
X-ray Structure Analysis of 1 [34]. X-ray diffraction intensities were recorded with an Oxford Diffraction Gemini E diffractometer using Cu Kα radiation, λ = 1.5418, Ǻ at 99(6) K. All calculations were carried out using SHELXL-97 [35] and refined using full-matrix least-squares difference Fourier techniques. The Siemens Area Detector Absorption Program (SADABS) [36] was used to determine absorption corrections. The colorless crystal of 1 was obtained in acetone–H2O (30:1). Altogether, 4128 independent reflections were collected from the 10,139 measurements, yielding R1 = 0.0482 and wR2 = 0.1208 [I > 2σ(I)]. Crystal data: colorless crystal (0.20 × 0.18 × 0.04 mm); C26H22O8, M = 471.00, space group C2/c; monoclinic crystal; unit cell dimensions a = 32.3086(15) Ǻ, b = 8.9000(4) Ǻ, c = 15.4083(7) Ǻ, V = 4350.5(3) Ǻ3, Z = 8, Dcalcd = 1.438 mg/mm3, µ = 0.906 mm−1, F(000) = 1974.
3.5. 4,7,9-Trihydroxy-3-methoxy-1-methyl-6H-benzo[c]chromen-6-one (2)
4,7,9-trihydroxy-3-methoxy-1-methyl-6H-benzo[c]chromen-6-one (2), white powder; UV (acetone) λmax (log ε) 340 (2.20) nm; IR (neat) νmax 3458, 2980, 2848, 2158, 1673, 1604, 1397, 1230, 1197, 1120, 798 cm−1; for 1H and 13C NMR data, see Table 1; HRESIMS m/z 289.0705 [M + H]+ (calcd. for C15H12O6, 289.0707).
3.6. MTT Assay
The cytotoxicity of compounds 1–4 was evaluated with the MTT assay [37]. The cell lines at a density of (2–5) × 103 cells/well were seeded in 96-well plates and allowed to adhere for 24 h. Subsequently, compounds 1–4 and cisplatin were added at appropriate concentrations and incubated with cells at 37 °C for 48 h in a 5% CO2-containing incubator. Finally, 20 μL of MTS (Promega) was added to each well in the dark to assess the proliferation after 90 min incubation at 37 °C. The optical density was recorded on a microplate reader at 490 nm. All tests were run in triplicate.
Acknowledgments
We gratefully acknowledge financial support from the National Natural Science Foundation of China (81402832) and the National Program of Drug Research and Development (2012ZX09301-003).
Supplementary Materials
The supplementary materials are available online. NMR spectra of Compounds 1 and 2 (Figures S1–S6), Figure S7: HPLC chromatogram of sporulosol (1) using a CHIRALPAK AD-H column; Figure S8: HPLC chromatogram of enalin A (5) using a CHIRALPAK AD-H column title; Figure S9: HPLC-CD chromatogram of enalin A (5) using a CHIRALPAK AD-H column title; Figure S10: HPLC-MS analysis of the crude extract.
Author Contributions
Y.C. and F.R. conceived and designed the experiments; C.Z., P.F., Y.Z. performed the experiments and analyzed the data; X.L. contributed materials; C.Z. and F.R. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1–5 are available from the authors.
References and Notes
- 1.Schueffler A., Anke T. Fungal natural products in research and development. Nat. Prod. Rep. 2014;31:1425–1448. doi: 10.1039/C4NP00060A. [DOI] [PubMed] [Google Scholar]
- 2.Bao Y., Chen G., Wu Y., Li X., Hu D., Liu X., Li X., Yao X., Gao H. Stachybisbins A and B, the first cases of seco-bisabosquals from Stachybotrys bisbyi. Fitoterapia. 2015;105:151–155. doi: 10.1016/j.fitote.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L., Feng B., Sun Y., Wu H., Li S., Liu B., Liu F., Zhang W., Chen G., Bai J., et al. Flaviphenalenones A–C, three new phenalenone derivatives from the fungus Aspergillus flavipes PJ03-11. Tetrahedron Lett. 2016;57:645–649. doi: 10.1016/j.tetlet.2015.12.099. [DOI] [Google Scholar]
- 4.Zhang L., Feng B., Zhao Y., Sun Y., Liu B., Liu F., Chen G., Bai J., Hua H., Wang H., et al. Polyketide butenolide, diphenyl ether, and benzophenone derivatives from the fungus Aspergillus flavipes PJ03-11. Bioorg. Med. Chem. Lett. 2016;26:346–350. doi: 10.1016/j.bmcl.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Tian J., Li P., Li X., Sun P., Gao H., Liu X., Huang P., Tang J., Yao X. New antibacterial isocoumarin glycosides from a wetland soil derived fungal strain Metarhizium anisopliae. Bioorg. Med. Chem. Lett. 2016;26:1391–1396. doi: 10.1016/j.bmcl.2016.01.074. [DOI] [PubMed] [Google Scholar]
- 6.Verkley G.J., da Silva M., Wicklow D.T., Crous P.W. Paraconiothyrium, a new genus to accommodate the mycoparasite Coniothyrium minitans, anamorphs of Paraphaeosphaeria, and four new species. Stud. Mycol. 2004;50:323–335. [Google Scholar]
- 7.Tsuda M., Mugishima T., Komatsu K., Sone T., Tanaka M., Mikami Y., Kobayashi J. Modiolides A and B, two new 10-membered macrolides from a marine-derived fungus. J. Nat. Prod. 2003;66:412–415. doi: 10.1021/np0203943. [DOI] [PubMed] [Google Scholar]
- 8.Mohamed I.E., Kehraus S., Krick A., König G.M., Kelter G., Maier A., Fiebig H.H., Kalesse M., Malek N.P., Gross H. Mode of action of epoxyphomalins A and B and characterization of related metabolites from the marine-derived fungus Paraconiothyrium sp. J. Nat. Prod. 2010;73:2053–2056. doi: 10.1021/np100310k. [DOI] [PubMed] [Google Scholar]
- 9.Shiono Y., Kikuchi M., Koseki T., Murayama T., Kwon E., Aburai N., Kimura K. Isopimarane diterpene glycosides, isolated from endophytic fungus Paraconiothyrium sp. MY-42. Phytochemistry. 2011;72:1400–1405. doi: 10.1016/j.phytochem.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Almeida C., Aouad N.E., Martín J., Pérez-Victoria I., González-Menéndez V., Platas G., de la Cruz M., Monteiro M.C., de Pedro N., Bills G.F., et al. Graminin B, a furanone from the fungus Paraconiothyrium sp. J. Antibiot. 2014;67:421–423. doi: 10.1038/ja.2014.11. [DOI] [PubMed] [Google Scholar]
- 11.Amand S., Vallet M., Guedon L., Genta-Jouve G., Wien F., Mann S., Dupont J., Prado S., Nay B. A reactive eremophilane and its antibacterial 2(1H)-naphthalenone rearrangement product, witnesses of a microbial chemical warfare. Org. Lett. 2017;19:4038–4041. doi: 10.1021/acs.orglett.7b01788. [DOI] [PubMed] [Google Scholar]
- 12.Soliman S.S., Tsao R., Raizada M.N. Chemical inhibitors suggest endophytic fungal paclitaxel is derived from both mevalonate and non-mevalonate-like pathways. J. Nat. Prod. 2011;74:2497–2504. doi: 10.1021/np200303v. [DOI] [PubMed] [Google Scholar]
- 13.Somjaipeng S., Medina A., Kwaśna H., Ordaz Ortiz J., Magan N. Isolation, identification, and ecology of growth and taxol production by an endophytic strain of Paraconithyrium variabile from English yew trees (Taxus baccata) Fungal Biol. 2015;119:1022–1031. doi: 10.1016/j.funbio.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 14.De Gusmño N.B., Kaouadji M., Steiman R., Seigle-murandi F., Ulrichc J. Coniothyriol, an uncommon polyketide from Coniothyrium sporulosum. Nat. Prod. Lett. 1993;2:287–292. doi: 10.1080/10575639308043824. [DOI] [Google Scholar]
- 15.Guiraud P., Steiman R., Seigle-murandi F., de Gusmño N.B. Antimicrobial and antitumor activities of mycosporulone. J. Nat. Prod. 1999;62:1222–1224. doi: 10.1021/np9805084. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L., Feng B., Chen G., Li S., Sun Y., Wu H., Bai J., Hua H., Wang H., Pei Y. Sporulaminals A and B: A pair of unusual epimeric spiroaminal derivatives from a marine-derived fungus Paraconiothyrium sporulosum YK-03. RSC Adv. 2016;6:42361–42366. doi: 10.1039/C6RA01401A. [DOI] [Google Scholar]
- 17.Zhang L., Li S., Wu H., Chen G., Li L., Bai J., Hua H., Wang H., Pei Y. 3,4-Dihydroisocoumarin derivatives from the marine-derived fungus Paraconiothyrium sporulosum YK-03. Phytochem. Lett. 2017;20:200–203. doi: 10.1016/j.phytol.2017.04.039. [DOI] [Google Scholar]
- 18.Liu L., Gao H., Chen X., Cai X., Yang L., Guo L., Yao X., Che Y. Brasilamides A–D: Sesquiterpenoids from the plant endophytic fungus Paraconiothyrium brasiliense. Eur. J. Org. Chem. 2010:3302–3306. doi: 10.1002/ejoc.201000284. [DOI] [Google Scholar]
- 19.Liu L., Chen X., Li D., Zhang Y., Li L., Guo L., Cao Y., Che Y. Bisabolane sesquiterpenoids from the plant endophytic fungus Paraconiothyrium brasiliense. J. Nat. Prod. 2015;78:746–753. doi: 10.1021/np5009569. [DOI] [PubMed] [Google Scholar]
- 20.Guo Z., Ren F., Che Y., Liu G., Liu L. New bergamotane sesquiterpenoids from the plant endothytic fungus Paraconiothyrium brasiliense. Molecules. 2015;20:14611–14620. doi: 10.3390/molecules200814611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S., Zhang Y., Niu S., Liu X., Che Y. Cytotoxic cleistanthane and cassane diterpenoids from the entomogenous fungus Paraconiothyrium hawaiiense. J. Nat. Prod. 2014;77:1513–1518. doi: 10.1021/np500302e. [DOI] [PubMed] [Google Scholar]
- 22.Chen S., Zhang Y., Zhao C., Ren F., Liu X., Che Y. Hawaiinolides E–G, cytotoxic cassane and cleistanthane diterpenoids from the entomogenous fungus Paraconiothyrium hawaiiense. Fitoterapia. 2014;99:236–242. doi: 10.1016/j.fitote.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Tanahashi T., Kuroishi M., Kuwahara A., Nagakura N., Hamada N. Four phenolics from the cultured lichen mycobiont of Graphis scripta var. pulverulenta. Chem. Pharm. Bull. 1997;45:1183–1185. doi: 10.1248/cpb.45.1183. [DOI] [Google Scholar]
- 24.Machida K., Trifonov L.S., Ayer W.A., Lu Z., Laroche A., Huang H.C., Cheng K.J., Zantige J.L. 3(2H)-Benzofuranones and chromanes from liquid cultures of the mycoparasitic fungus Coniothyrium minitans. Phytochemistry. 2001;58:173–177. doi: 10.1016/S0031-9422(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 25.Bekker R., Li X., ElSohly H.N., Clark A.M., Brandt E.V., Ferreira D. Resolution and absolute configuration of naturally occurring auronols. J. Nat. Prod. 2001;64:345–347. doi: 10.1021/np000463i. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y., Wu X., Deng Z., Wang J., Zhou S., Vrijmoed L.L., Jones E.B. The metabolites of the mangrove fungus Verruculina enalia No. 2606 from a salt lake in the Bahamas. Phytochemistry. 2002;59:469–471. doi: 10.1016/S0031-9422(01)00470-8. [DOI] [PubMed] [Google Scholar]
- 27.Strobel G., Ford E., Worapong J., Harper J.K., Arif A.M., Grant D.M., Fung P.C., Chau R.M. Isopestacin, an isobenzofuranone from Pestalotiopsis microspora, possessing antifungal and antioxidant activities. Phytochemistry. 2002;60:179–183. doi: 10.1016/S0031-9422(02)00062-6. [DOI] [PubMed] [Google Scholar]
- 28.Harper J.K., Arif A.M., Ford E.J., Strobel G.A., Porco J.A., Jr., Tomer D.P., Oneill K.L., Heider E.M., Grant D.M. Pestacin: A, 1,3-dihydro isobenzofuran from Pestalotiopsis microspora possessing antioxidant and antimycotic activities. Tetrahedron. 2003;59:2471–2476. doi: 10.1016/S0040-4020(03)00255-2. [DOI] [Google Scholar]
- 29.Li E., Jiang L., Guo L., Zhang H., Che Y. Pestalachlorides A–C, antifungal metabolites from the plant endophytic fungus Pestalotiopsis adusta. Bioorg. Med. Chem. 2008;16:7894–7899. doi: 10.1016/j.bmc.2008.07.075. [DOI] [PubMed] [Google Scholar]
- 30.Li E., Zhang F., Niu S., Liu X., Liu G., Che Y. A spiro[chroman-3,7′-isochromene]-4,6′(8′H)-dione from the Cordyceps-colonizing fungus Fimetariella sp. Org. Lett. 2012;14:3320–3323. doi: 10.1021/ol3012919. [DOI] [PubMed] [Google Scholar]
- 31.Sun T., Kuang R., Chen G., Qin S., Wang C., Hu D., Wu B., Liu X., Yao X., Gao H. Three pairs of new isopentenyl dibenzo[b,e]oxepinone enantiomers from Talaromyces flavus, a wetland soil-derived fungus. Molecules. 2016;21:1184–1195. doi: 10.3390/molecules21091184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dellafiora L., Dall’Asta C., Cruciani G., Galaverna G., Cozzini P. Molecular modelling approach to evaluate poisoning of topoisomerase I by alternariol derivatives. Food Chem. 2015;189:93–101. doi: 10.1016/j.foodchem.2015.02.083. [DOI] [PubMed] [Google Scholar]
- 33.Harris T.M., Hay J.V. Biogenetically modeled syntheses of heptaacetate metabolites alternariol and lichexanthone. J. Am. Chem. Soc. 1977;99:1631–1637. doi: 10.1021/ja00447a058. [DOI] [Google Scholar]
- 34.Crystallographic data for 1 have been deposited with the Cambridge Crystallographic Data Centre (deposition number CCDC 1585447, accessed on 14/11/2017). Copies of the data can be obtained, free of charge, on application to the director, CCDC 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44 1223 336033 or email: deposit@ccdc.cam.ac.uk).
- 35.Sheldrick G.M. SHELXL-97, Program for X-ray Crystal Structure Solution and Refinement. University of Göttingen; Göttingen, Germany: 1997. [Google Scholar]
- 36.Sheldrick G.M. SADABS, Program for Empirical Absorption Correction of Area Detector Data. University of Göttingen; Göttingen, Germany: 1999. [Google Scholar]
- 37.Zhang N., Chen Y., Jiang R., Li E., Chen X., Xi Z., Guo Y., Liu X., Zhou Y., Che Y., et al. PARP and RIP 1 are required for autophagy induced by 11′-deoxyverticillin A, which precedes caspase-dependent apoptosis. Autophagy. 2011;7:598–612. doi: 10.4161/auto.7.6.15103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.