Abstract
Background
Pelvic floor muscle exercises are a widely used and well-established form of stress incontinence treatment, with success rates varying from 21% to 84%, although with a better subjective than objective outcome.
Material/Methods
“Incontinence Impact Questionnaire” (IIQ), PFM EMG assessment was done at the beginning and after the 6-week training program.
Results
Statistically significant differences appeared in the BASE and R values. In the symptomatic group (with SUI symptoms), the value of BASE was 3.26 μV, and after training it was 3.95 μV. The R values before and after training were 4.55 μV and 4.25 μV. In the symptomatic group (without SUI symptoms), the value of BASE was 2.88 μV and 3.52 μV and R values were 7.16 μV and 3.92 μV. In the control group, BASE was 3.05 μV and 4.11 μV and R was 7.82 μV and 4.39 μV.
Conclusions
The results indicate that a 6-week training process influences PFM EMG activity in pregnant women. During Q, the value of PFM activity after a training session tended to increase in the symptomatic and control groups, but in the symptomatic group it remains practically unchanged. Our results show the probable process of decreasing control of PFM activity during long-lasting contractions in symptomatic and control women. The comparison of BASE before and after training averaged the values of R after five 10-s contractions and showed an increase in the Base and decrease in the R.
MeSH Keywords: Electromyography, Female Urogenital Diseases and Pregnancy Complications, Urinary Incontinence
Background
Urinary incontinence is defined as involuntary urine loss, which has such a severe social and negative hygienic influence that an undesirable situation develops’ [1]. The official definition of stress urinary incontinence (SUI) proposed by the International Continence Society reads: ‘Involuntary loss of urine when the vesical pressure (the pressure inside the bladder) exceeds the maximal urethral pressure in the absence of detrusor muscle activity’ [2].
SUI is the most common type of urinary dysfunction in younger women. Vaginal delivery is considered as the predominant cause but not all women develop this condition. Some of them develop it many years after childbearing has been completed. Others experience SUI before pregnancy, and its occurrence during pregnancy can be partially correlated with the hormones relaxin and progesterone [3].
Pelvic floor muscle (PFM) exercises are a widely used and well-established form of stress incontinence treatment, with success rates between 21% and 84% [4,5]. The aim of the PFM training process (PMFT) is to teach and to bring back control over the peri-vaginal muscles, by learning to contract the pelvic-floor muscles [6]. PFM rehabilitation should begin with tonic activity, as this is often deficient [7]. This differs from many other programs for SUI, which focus on strong PFM holds sustained for up to 10 s. Retraining of a tonic PFM activity involves very delicate and prolonged muscle holds [8]. Some patients need to learn to relax abdominal muscle activity prior to commencing this approach. According to the Cochrane Database of Systematic Review, pelvic floor muscle exercises are widely prescribed for pregnant women, as a prevention and therapy specifically for those with SUI before pregnancy.
Nevertheless, to make the PFMT effectiveness significant, especially the long-lasting one, further testing is necessary [9,10].
Pre-pregnancy, antepartum, and intrapartum factors are connected with pelvic floor strength. This type of exercise exerts a protective effect against both the rate of spontaneous leakage and the need for episiotomy [11]. The use of sEMG to evaluate the neuromuscular function of the PFM using electrodes placed on vaginal probes is widely used to increase our knowledge of pelvic floor function [12–14]. Collected epidemiological data show that women after pregnancy are at high risk of developing urinary incontinence, and pregnancy and delivery appear to be risk factors [3,15]. These women may be at higher risk of (FI) [16,17]. Almost 30% of women have urinary incontinence and many of them have fecal incontinence after delivery. A literature review showed that [18,19] PFMT given by professional physiotherapists is an efficient intervention approach for urinary incontinence. In women without incontinence incidents, PFMT received during pregnancy [20,21] and after delivery [22,23] can also have a positive preventive influence. The effect of PFMT in the high-risk group of women with SUI during pregnancy is not known. In view of the application of intervention, after a 4-session training program, we can see some interesting effects. Various PFMT protocols in non-pregnant women have been reported in the literature; however, the numbers of repetitions and the duration of contraction and rest have not been precisely described. The recommended frequency of PFMT ranges from 2 to 3 times a week for up to a 3-month period, an length of time necessary to achieve minimum muscle strength [24]. PFMT during pregnancy in preventing urinary incontinence is still open to question. The Cochrane Review recommends that further interventions using pelvic floor muscles exercises during pregnancy should gather information about urinary incontinence, and highlights the need for large-scale research of population-based approaches, using intensive PFMT and enrolling antenatal women, disregarding the continence condition or consistency. There has been a lack of studies on the effect of implementing PFMT in a more general training program for pregnant women [10]. However, PFMT should be implemented before, during, and after pregnancy. Mørkved and Bø [25] found that PFMT needs to be incorporated as a basic part of women’s physical activity in general. Early intervention with PFMT in pregnancy may prevent the onset of UI in late pregnancy and postpartum [26]. The surface electromyography method is used in pregnant women for many reasons. The non-invasive electrodes were used, for example, for the prediction of preterm delivery [27]. As a highly diagnostic and informative method, it is appropriate to use in evaluating muscle activation of the pelvic floor during pregnancy.
The aim of this study was to show the differences in the level of pelvic floor muscle sEMG determined in 3 measurement protocol tasks (Q – quick flicks; STA – static contraction; and BASE/REST baselines before measurement and after 10-s contractions) among 3 test groups (with, without urinary incontinence problems, and a control group) before and after a 6-week training process.
Material and Methods
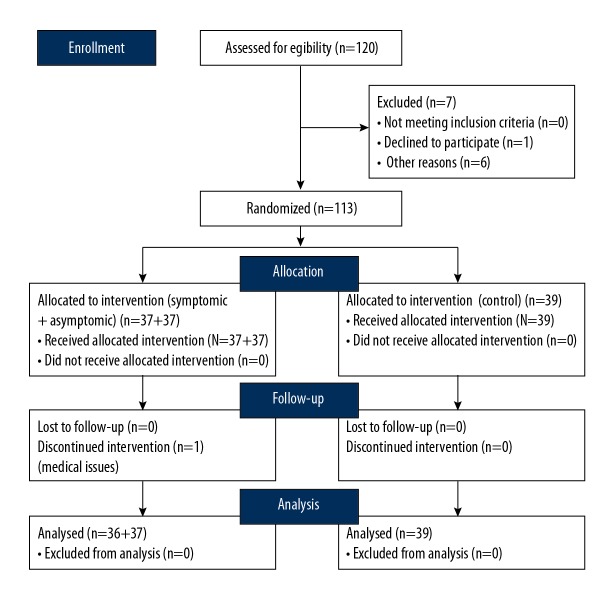
The RTC was designed among 113 healthy pregnant women (age 30.01±3.56 y, symptomatic – 30.17 y, asymptomatic – 30.22 y, and control – 30.18 y.) who volunteered for the study (Figure 1).
Figure 1.
SMART Trial CONSORT diagram.
The average week of pregnancy was 21.41 and all pregnancies were single-fetus. All the subjects were divided into 3 groups: 1 – the experimental group: symptomatic women with training (symptomatic); 2 – the control group of asymptomatic women with training (asymptomatic); and 3 – the control group of asymptomatic women without training (control). The division was based on the questionnaire – “Incontinence Impact Questionnaire (IIQ)” results. The questionnaire was executed before and after the experiment. The study was conducted in the Laboratory of Physical Effort and Genetics in Sport, Gdansk University of Physical Education and Sport (AWFiS) in Poland. The principle of the Helsinki Declaration was upheld and the project received the approval of the Bioethics Commission in Gdansk. At the beginning of the trail, pelvic-floor muscle EMG assessment was conducted. Next, a 6-week training program was implemented. The second EMG assessment took place after 6 weeks, according to a predetermined schedule. All participants were assessed for the electrical activity of the pelvic-floor muscle contraction before and after 6 weeks using the TeleMyo™ 2400T Direct Transmission System (DTS), Noraxon EMG and Sensors System (Scottsdale, AZ, USA) TeleMyo™ DTS. For the pelvic floor, we used vaginal probes (Lifecare PR-02, Everyway Medical Instruments Co., Ltd., Taiwan). Women were asked to insert the probe into the vagina ensuring each electrode surface pointed towards each hip [28]. The study was conducted based on SENIAM standards on electromyography [29] deviating from them insofar as the participants were not taught how to correctly perform pelvic-floor muscle contraction before the EMG assessment. In the test, women received the following instruction: On the command “Contract” – immediately contract your pelvic-floor muscles as much as you can, keeping your abdominals, legs, and buttocks relaxed. During the study, the participants lay in a supine position with hips flexed and knees bent to approximately 90º. The evaluation of pelvic-floor muscles with surface electromyography is painless and not invasive [30]. During the test, the participants from the 3 groups performed 5 quick flick (Q) repetitions with five 10-s contractions, after which the average value of the resting electrical potential was analyzed - rest (R); continuous static pelvic-floor muscle tension in 1 min - static hold (STA). The value of the pelvic-floor muscle electrical potential was also analyzed before the baseline study (BASE).
Six-week training program
In the experiment, a 6-week structured exercise program was employed, designed according to the available guidelines [31,32]. Group exercise sessions were held 3 times a week (18 sessions) at the sport facilities of Gdansk University of Physical Education and Sport. Each session consisted of: a warm up, an aerobic part in the form of aerobics choreography with music (25 min), strength conditioning exercises (25 min), and stretching and breathing exercises and relaxation (10 min). In the strengthening part, the women performed 9 exercises for each muscle group in 2 sets of 12–16 repetitions, with a break of 30 s between sets. The women were instructed to perform the repetitions until they felt uncomfortable. They were also trained to contract pelvic floor muscles together with other muscle groups to improve coordination and to consciously relax them between the strengthening exercise repetitions and after the set of exercises and also during the stretching part. Only the resistance of their own body was applied. At the end of this part, the women performed isolated pelvic floor muscle exercises (keeping abdominals, buttocks, and tights relaxed) according to the modified graduated strength training program by Miller [33].
The training process was conducted by a certified Pregnancy and Postnatal Exercise Specialist whose competences met the European educational standard for this profession [34,35]. Once every 2 weeks, the principal researcher would check the quality of the exercise program implementation. In the individual activities, the women were supposed to perform the same exercise technique (activate pelvic floor muscles during the increase of the intra-abdominal pressure).
STATISTCA v.10.0 was used for the statistical analysis of the results (variation analysis with post hoc Bonferroni test). Expected differences in pelvic-floor electrical activity are due to the 6-week training process.
Results
Analysis of the results achieved from the questionnaire concerning the influence of SUI on the quality of life showed that the examined symptomatic pregnant women achieved in most of the measured parameters the lowest values of electrical activity of PFM when compared to the rest of the examined subjects. During Q (responsible for urine continence in sudden increase of intra-abdominal pressure: e.g., laughing, coughing, jumping) the value of electrical activity of PFM after a 6-week training session tends to increase in the symptomatic and control groups; in the second group (asymptomatic), it remains practically unchanged. The symptomatic group before the training session achieved values of PFM activity at the level of 12.36±6.01 μV, and after the 6 weeks training this value increased to 14.03±6.78 μV. The asymptomatic group achieved 12.43±6.29 μV and after training 12.88±5.64 μV. Results obtained by pregnant women from the control group showed a similar tendency as in the symptomatic group. The values of electrical activity from the first measurement were 12.49±7.38 μV in the initial test and 13.74±7.2 μV after 6 weeks. The highest increase in the level of PFM activity was observed in the symptomatic group. Similar results were found in the control group. These women had not been informed about the training procedure before the experiment to avoid external influence (Figure 2).
Figure 2.
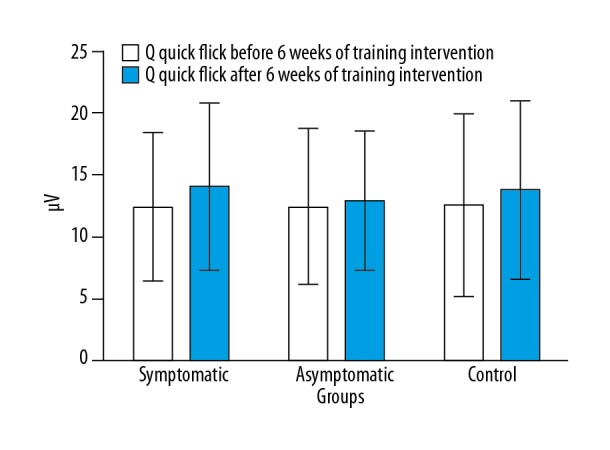
Comparison of EMG activity level of pelvic floor muscles during Q, before and after the 6-week training session in symptomatic, asymptomatic, and control pregnant women groups.
During STA, the examined symptomatic women achieved in the first measurement 29.04±89.11 μV and in 10 s 14.12±7.83 μV. The asymptomatic women revealed an increase in PFM activity similarly to the symptomatic group. In the first measurement, they obtained 12.24±7.49 μV. After the 6-week training process, these values were 13.93±5.02 μV. In the control women, the tendency was similar to the symptomatic women and they obtained lower results in the second measurement. The values were 20.37±60.35 μV and 13.7±6.22 μV. The results allow one to see the probable process of decreasing the control of PFM activity during long-lasting contractions in the symptomatic and control women. This may point to the effect of an increased level of PFM fatigue caused by increased intra-abdominal pressure after the 6-week period and the trimester of pregnancy. In the symptomatic women, the results differed and were statistically significant (Figure 3).
Figure 3.
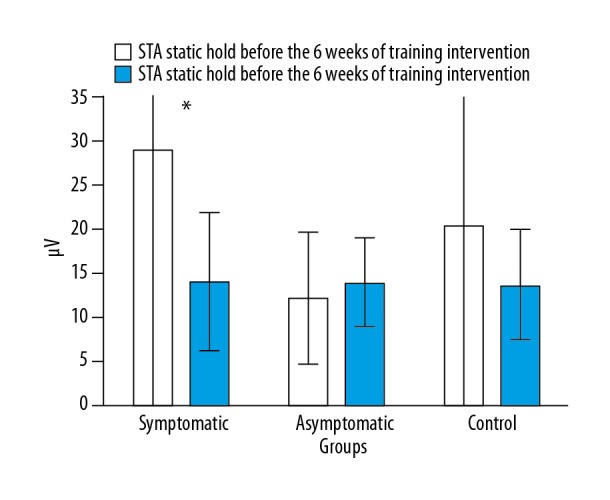
Comparison of EMG activity level of pelvic floor muscles during STA before and after the 6-week training session in the symptomatic, asymptomatic, and control pregnant women groups.
The comparison of BASE activity before and after the 6-week training process and the averaged values of R (relaxation) after five 10-s contractions showed an increase in the Base level and a decrease in the R level. In the symptomatic women, the value of BASE before training was 3.26±2.48 μV, and after 3.95±4.53 μV. The R values before and after training were 4.55±2.51 μV and 4.25±2.93 μV. In the asymptomatic women, the BASE values of PFM activity before and after the training process were 2.88±1.52 μV and 3.52±3.34 μV, and the R values were 7.16 ± 18.56 μV and 3.92±3.17 μV. In the control women, the measured values before and after the training process were 3.05±2.32 μV and 4.11±4.94 μV in BASE and 7.82±19.45 μV and 4.39±3.89 μV in R (Figure 4).
Figure 4.
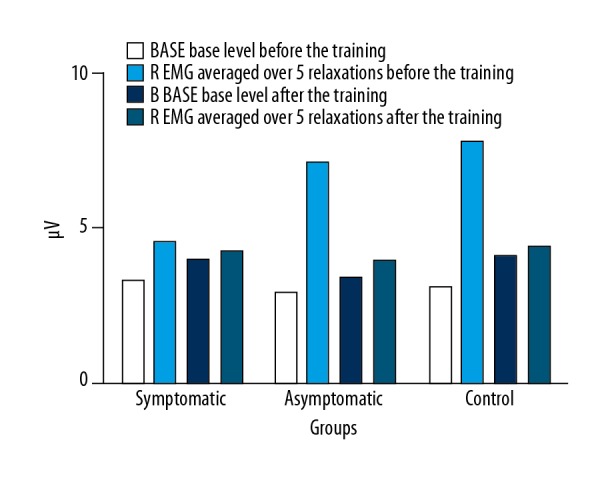
Comparison of the pelvic floor muscles EMG-activity level in BASE and R stages before and after the 6-week training session (* P<0.05).
Discussion
Patient awareness and actual practice of floor exercises did not meet guidelines, calling for strategies to improve awareness and adherence [36]. A PFMT program is an effective method for primigravid pregnant and primiparous postpartum women, with a concomitant decrease in urinary symptoms [37].
The study compared the level of PFM values sEMG determined in different conditions of muscle contraction in 3 groups of women (exercising with urinary incontinence problems, exercising without problems, and a control group) before and after a 6-week training procedure. The obtained results showed differences in the results within the 3 test groups. The symptomatic and control groups achieved higher results in Q after the 6-week training and the sEMG values remained unchanged in the asymptomatic group. Despite the lack of statistical significance in the differences, there was an upward trend in the symptomatic group, which might suggest that the training made it possible to increase the efficiency of muscle contraction, which is responsible for holding urine and/or stopping urine flow during laughter, coughing, and sneezing. Our results agree with a previous study [34] that found an increase of PFM activity after training sessions during short 10-s contractions. Tonic activation in PFM rehabilitation should begin with tonic activity, as this is often incomplete [7]. This is different from many other programs for SUI which focus on strong PFM holds (up to 10 s) [38], and retraining of a tonic PFM action involves very gentle and prolonged muscle holds.
Some patients need to learn to relax abdominal muscle activity prior to commencing this approach. Using an independent TrA contraction to get a PFM co-contraction helps to ensure the very low level of PFM activation required. The use of tactile input medial to ASIS, by both patient and therapist, teaches and confirms the correct abdominal activation. According to this study, it can be assumed that in this aspect, the quality of life of the examined women has improved [39,40]. It may be also confirmed according to the values of the answers given in the IIQ questionnaire; after the experiment period they were enhanced by 16.38%. No significant increase in the sEMG values of pelvic floor muscles was observed in the asymptomatic group. It can be expected that in the group of women without stress urinary incontinence, the training of pelvic floor muscles was a supportive exercise for the correct functioning of these muscles. The findings obtained in the control group are puzzling.
The results in the second measurement showed a slight increase in the electrical activity of the pelvic floor muscles. The women in this group did not exhibit the symptoms of stress urinary incontinence prior to the experiment. The standard daily activities, including physical activity (if performed) could have such an effect (an increase in the sEMG value), i.e., maintain the effective functioning of the pelvic floor muscles. Finally, for some reason, they were in the group without WNM symptoms. The women in the control group did not receive information about pelvic floor muscle training and, perhaps for this reason, knowing the purpose of the project, they themselves performed physical activity directed at improving the pelvic floor muscles.
Pelvic floor muscle strength improved significantly after intensive pelvic floor muscle training [20]. Pelvic floor muscle training during pregnancy results in improved muscle coordination and flexibility. The effect may be on the central nervous system and the muscles, and training seems to facilitate rather than block this [41]. In our study in STA, similar results were obtained by the symptomatic and control women, where decreases in PFM activity values were observed. In the symptomatic women, the difference between the results achieved before and after the training session was statistically significant. The asymptomatic women showed an increase in PFM activity after the 6-week training. The PFMT programs of 2 tests were more challenging to systematize and were characterized rather by strength and endurance [42,43]. Their programs consist of fast and slow contractions, a rather high numbers of sets (8–10 daily) with few repeats in a set but many contractions (80–100 daily).
The programs could influence strength or endurance, or both, according to the number of repetitions performed daily and the amount of spontaneous effort with each contraction. In all the tests, the control group was given standard service which could consist of advice on PFMT. At 1 year postpartum, the average number of daily VPFMCs in the PFMT and control groups was 20 vs. 5 [42], and 86 vs. 35, respectively. A study [26] found that after 6 and 12 sessions of PFMT with EMG biofeedback, there were increased EMG amplitudes and improved function using vaginal palpation, perineometry, and the Brink Scale. They found that EMG amplitudes were higher in fast contractions and lower in contractions of 10 and 20 s, respectively. Our study also found a greater increase in EMG activity in the fast contractions, possibly due to the nature of the type II muscle fibers, which can generate a higher level of strength and power, but are easily fatigued [44]. Analysis of the results showed an increase of BASE values in all the examined groups after the 6-week training session and a decrease in the R values in all the examined subjects [45] showed that the EMG activity not only increased during the contractions, but also during the rest period at the beginning of the training session, although not significantly. It should be stressed that by evaluating the pelvic diaphragm in patients with pelvic organ prolapse in the resting state, it was preliminarily confirmed that the consistency of ultrasound and MRI was only moderate. The comparison of these 2 diagnostic methods under the dynamic muscle contraction state needs to be further explored [46]. Our research, however, draws attention to the importance of the measurement of sEMG, and thus it fills a gap in the functional diagnosis of muscles in this area. Our results might be interpreted according to microgenetic theory, which emphasizes the importance of the specific pattern of neuronal networks formed during the course of the training, which promotes, among others, automatic control and an increase in the level of strength and pelvic muscles strength [47], prevents stress during urinary incontinence, and therefore improves the quality of life of the examined women.
Conclusions
During Q (responsible for urine continence in a sudden increase of intra-abdominal pressure: e.g., laughing, coughing, jumping) the value of electrical activity of PFM after 6 weeks of training tended to increase in the symptomatic training group and the control group; in the training asymptomatic group it remained statistically unchanged. The results allow one to see the probable process of decreasing the control of PFM activity during long-lasting contractions in the symptomatic group and control group. The comparison of BASE activity before and after the 6-week training process and the averaged values of R (relaxation) after five 10-s contractions showed an increase in the Base level and a decrease in the R level.
Footnotes
Conflicts of interest
None.
Source of support: This work was supported by scientific funding from Gdańsk University of Physical Education and Sports, Gdańsk, Poland
References
- 1.Nygaard I, Barber MD, Burgio KL, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311–16. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGuire EJ. Pathophysiology of stress urinary incontinence. Rev Urol. 2004;6(Suppl 5):S11–17. [PMC free article] [PubMed] [Google Scholar]
- 3.Viktrup L. The risk of lower urinary tract symptoms five years after the first delivery. Neurourol Urodyn. 2002;21(1):2–29. doi: 10.1002/nau.2198. [DOI] [PubMed] [Google Scholar]
- 4.Marques A, Stothers L, Macnab A. The status of pelvic floor muscle training for women. Can Urol Assoc J. 2010;4(6):419–24. doi: 10.5489/cuaj.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szumilewicz A, Worska A, Piernicka M, et al. The exercise-induced irisin is associated with improved levels of glucose homeostasis markers in pregnant women participating in 8-week prenatal group fitness program: A pilot study. Biomed Res Int. 2017;2017:9414525. doi: 10.1155/2017/9414525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batista RLA, Franco MM, Naldoni LMV, et al. Biofeedback and the electromyographic activity of pelvic floor muscles in pregnant women. Rev Bras Fisioter. 2011;15:386–92. doi: 10.1590/s1413-35552011005000026. [DOI] [PubMed] [Google Scholar]
- 7.Sapsford R. Rehabilitation of pelvic floor muscles utilizing trunk stabilization. Man Ther. 2004;9:3–12. doi: 10.1016/s1356-689x(03)00131-0. [DOI] [PubMed] [Google Scholar]
- 8.Sapsford RR, Hodges PW. Contraction of the pelvic floor muscles during abdominal maneuvers. Arch Phy Med Rehabil. 2001;82:1081–88. doi: 10.1053/apmr.2001.24297. [DOI] [PubMed] [Google Scholar]
- 9.Anderson J, Shroff D, Curtis A, et al. The veterans affairs shift change physician-to-physician handoff project. Jt Comm J Qual Patient Saf. 2010;36(2):62–71. doi: 10.1016/s1553-7250(10)36012-0. [DOI] [PubMed] [Google Scholar]
- 10.Hay-Smith J, Morkved S, Fairbrother KA, Herbison GP. Pelvic floor muscle training for prevention and treatment of urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2008;(4):CD007471. doi: 10.1002/14651858.CD007471. [DOI] [PubMed] [Google Scholar]
- 11.Riva D, Minini G. Risk factors, prevention, evaluation, and treatment. Springer Cham Heidelberg New York Dordrecht London; © Springer International Publishing Switzerland: 2016. Childbirth-related pelvic floor dysfunction. [Google Scholar]
- 12.Aukee P, Penttinen J, Airaksinen O. The effect of aging on the electromyographic activity of pelvic floor muscles. A comparative study among stress incontinent patients and asymptomatic women. Maturitas. 2003;44(4):253–57. doi: 10.1016/s0378-5122(03)00044-6. [DOI] [PubMed] [Google Scholar]
- 13.Madill SJ, McLean L. Quantification of abdominal and pelvic floor muscle synergies in response to voluntary pelvic floor muscle contractions. J Electromyogr Kinesiol. 2008;18(6):955–64. doi: 10.1016/j.jelekin.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Thompson JA, O’Sullivan PB, Briffa NK, Neumann P. Assessment of voluntary pelvic floor muscle contraction in continent and incontinent women using transperineal ultrasound, manual muscle testing and vaginal squeeze pressure measurements. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(6):624–30. doi: 10.1007/s00192-006-0081-2. [DOI] [PubMed] [Google Scholar]
- 15.Rortveit G, Daltveit AK, Hannestad YS, et al. Urinary incontinence after vaginal delivery or cesarean section. N Engl J Med. 2003;348(10):900–7. doi: 10.1056/NEJMoa021788. [DOI] [PubMed] [Google Scholar]
- 16.Eason E, Labrecque M, Marcoux S, Mondor M. Anal incontinence after childbirth. CMAJ. 2002;166(3):326–30. [PMC free article] [PubMed] [Google Scholar]
- 17.Pollack J, Nordenstam J, Brismar S, et al. Anal incontinence after vaginal delivery: A five-year prospective cohort study. Obstet Gynecol. 2004;104(6):1397–402. doi: 10.1097/01.AOG.0000147597.45349.e8. [DOI] [PubMed] [Google Scholar]
- 18.Boyle R, Hay-Smith EJ, Cody JD, Morkved S. Pelvic floor muscle training for prevention and treatment of urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2012;10:CD007471. doi: 10.1002/14651858.CD007471.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Hay-Smith EJ, Bo K, Berghmans LC, et al. WITHDRAWN: Pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev. 2007;(1):CD001407. doi: 10.1002/14651858.CD001407.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Morkved S, Bo K, Schei B, Salvesen KA. Pelvic floor muscle training during pregnancy to prevent urinary incontinence: a single-blind randomized controlled trial. Obstet Gynecol. 2003;101(2):313–19. doi: 10.1016/s0029-7844(02)02711-4. [DOI] [PubMed] [Google Scholar]
- 21.Sangsawang B, Sangsawang N. Stress urinary incontinence in pregnant women: A review of prevalence, pathophysiology, and treatment. Int Urogynecol J. 2013;24(6):901–12. doi: 10.1007/s00192-013-2061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiarelli P, Cockburn J. Promoting urinary continence in women after delivery: Randomised controlled trial. BMJ. 2002;324(7348):1241. doi: 10.1136/bmj.324.7348.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woldringh C, Albers-Heitner CP, van den Wijngaart M, Lagro-Janssen ALM. Pelvic floor muscle training is not effective in women with UI in pregnancy: A randomised controlled trial. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):383–90. doi: 10.1007/s00192-006-0175-x. [DOI] [PubMed] [Google Scholar]
- 24.de Oliveira C, Lopes MAB, e Pereira LCL, Zugaib M. Effects of pelvic floor muscle training during pregnancy. Clinics (Sao Paulo) 2007;62(4):439–46. doi: 10.1590/s1807-59322007000400011. [DOI] [PubMed] [Google Scholar]
- 25.Mørkved S, Bø K. Effect of pelvic floor muscle training during pregnancy and after childbirth on prevention and treatment of urinary incontinence: A systematic review. Br J Sports Med. 2014;48(4):299–310. doi: 10.1136/bjsports-2012-091758. [DOI] [PubMed] [Google Scholar]
- 26.Woodley SJ, Boyle R, Cody JD, et al. Pelvic floor muscle training for prevention and treatment of urinary and fecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2017;12:CD007471. doi: 10.1002/14651858.CD007471.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucovnik M, Maner WL, Chambliss LR, et al. Noninvasive uterine electromyography for prediction of preterm delivery. Presented orally at the 57th Annual Scientific Meeting of the Society for Gynecologic Investigation; Orlando, FL. March 24–27, 2010. [Google Scholar]
- 28.Halski T, Ptaszkowski K, Slupska L, Dymarek R. The evaluation of bioelectrical activity of pelvic floor muscles depending on probe location: A pilot study. Biomed Res Int. 2013;2013:238312. doi: 10.1155/2013/238312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rett MT, Simoes JA, Herrmann V, et al. Management of stress urinary incontinence with surface electromyography-assisted biofeedback in women of reproductive age. Phys Ther. 2007;87(2):136–42. doi: 10.2522/ptj.20050318. [DOI] [PubMed] [Google Scholar]
- 30.Glazer HI, Marinoff SC, Sleight IJ. Web-enabled Glazer surface electromyographic protocol for the remote, real-time assessment and rehabilitation of pelvic floor dysfunction in vulvar vestibulitis syndrome. A case report. J Reprod Med. 2002;47(9):728–30. [PubMed] [Google Scholar]
- 31.The American College of Obstetricians and Gynecologists (ACOG) Committee Opinion No. 650. 2015. Physical Activity and Exercise During Pregnancy and the Postpartum Period. [Google Scholar]
- 32.Szumilewicz A, Worska A, Rajkowska N, Santos-Rocha R. Summary of guidelines for exercise in pregnancy – are they comprehensive enough for designing the contents of a prenatal exercise program? Current Women’s Health Reviews. 2015;11(1):3–12. [Google Scholar]
- 33.Miller JM. Dissertation [Thesis] University of Michigan, Horace H. Rackham School of Graduate Studies; 1996. On pelvic floor muscle function and stress urinary incontinence: Effects of posture, parity and volitional control; pp. 145–57. [Google Scholar]
- 34.Santos-Rocha R, Szumilewicz A, Perales M, Pajaujiene S. EuropeActive Standards EQF Level 5 – Pregnancy and Postnatal Exercise Specialist. EuropeActive. 2016 [Google Scholar]
- 35.Miller JM. On pelvic floor muscle function and stress urinary incontinence: Effects of posture, parity and volitional control. University of Michigan; 2012. [Google Scholar]
- 36.Ismail SI. An audit of NICE guidelines on antenatal pelvic floor exercises. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(12):1417–22. doi: 10.1007/s00192-009-0967-x. [DOI] [PubMed] [Google Scholar]
- 37.Marques J, Botelho S, Pereira LC, et al. Pelvic floor muscle training program increases muscular contractility during first pregnancy and postpartum: electromyographic study. Neurourol Urodyn. 2013;32(7):998–1003. doi: 10.1002/nau.22346. [DOI] [PubMed] [Google Scholar]
- 38.de Souza Abreu N, de Castro Villas Boas B, Netto JMB, Figueiredo AA. Dynamic lumbopelvic stabilization for treatment of stress urinary incontinence in women: Controlled and randomized clinical trial. Neurourol Urodyn. 2017;36(8):2160–68. doi: 10.1002/nau.23261. [DOI] [PubMed] [Google Scholar]
- 39.Aukee P, Immonen P, Penttinen J, et al. Increase in pelvic floor muscle activity after 12 weeks’ training: A randomized prospective pilot study. Urology. 2002;60(6):1020–23. doi: 10.1016/s0090-4295(02)02125-8. discussion 1023–24. [DOI] [PubMed] [Google Scholar]
- 40.Chamochumbi CC, Nunes FR, Guirro RR, Guirro EC. Comparison of active and passive forces of the pelvic floor muscles in women with and without stress urinary incontinence. Rev Bras Fisioter. 2012;16(4):314–19. doi: 10.1590/s1413-35552012005000020. [DOI] [PubMed] [Google Scholar]
- 41.Salvesen KA, Morkved S. Randomised controlled trial of pelvic floor muscle training during pregnancy. BMJ. 2004;329(7462):378–80. doi: 10.1136/bmj.38163.724306.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glazener CM, Herbison GP, Wilson PD, et al. Conservative management of persistent postnatal urinary and faecal incontinence: Randomised controlled trial. BMJ. 2001;323(7313):593–96. doi: 10.1136/bmj.323.7313.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dumoulin C, Hay-Smith EJC, Mac Habée-Séguin G. The Cochrane Collaboration. John Wiley & Sons, Ltd; 2014. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. [DOI] [PubMed] [Google Scholar]
- 44.Johnson VY. How the principles of exercise physiology influence pelvic floor muscle training. J Wound Ostomy Continence Nurs. 2001;28(3):150–55. doi: 10.1067/mjw.2001.113245. [DOI] [PubMed] [Google Scholar]
- 45.Batista RL, Franco MM, Naldoni LM, et al. Biofeedback and the electromyographic activity of pelvic floor muscles in pregnant women. Rev Bras Fisioter. 2011;15(5):386–92. doi: 10.1590/s1413-35552011005000026. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Ren M, Liu Y, et al. Perineal ultrasound versus magnetic resonance imaging (MRI) detection for valuation of pelvic diaphragm in resting state. Med Sci Monit. 2018;24:4449–54. doi: 10.12659/MSM.906648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pąchalska M, Góral-Pólrola J, Mueller A, Kropotov JD. Neuropsychology and the neurophysiology of perceptual microgenesis. Acta Neuropsychologica. 2017;15(4):365–89. [Google Scholar]