Abstract
Microglia respond to environmental changes by releasing cytokines that beneficially or detrimentally affect surrounding cells in addition to functioning as the resident CNS macrophages. Interactions between glia and neurons participate in many critical brain functions and diseases. We previous demonstrated that activation of microglia facilitates hypothalamic CRF neuronal activity and pain precipitation in rats. The intricate CNS environment complicates studying crosstalk between microglia and hypothalamic neurons in vivo. BV2 cells derived from raf/myc-immortalised murine neonatal microglia are the most frequently used substitute for primary cultures of microglia. In this study, we used BV2 cells and primary cultures of glia from neonatal rats to explore the interaction between microglia and hypothalamic neurons in vitro. Lipopolysaccharide (LPS) stimulated BV2 cells to adopt a microglia-like phenotype including an amoebae-like shape, Iba-1 positive staining and IL-1β secretion. Primary cultures of hypothalamic neurons treated with culture medium from LPS-treated BV2 cells increased CRF, CRFR, pCREB and cAMP levels compared to untreated neurons. Primary cultures of hypothalamic neurons incubated with culture medium from LPS-treated primary cultures of glia or exogenous IL-1β also increased CRF levels. Importantly, this increase in protein expression appeared to be IL-1β mediated and treatment with an anti-IL-1β antibody blocked the increased expression. Our data provide direct evidence that microglia can modulate hypothalamic neuronal activity and IL-1β may play a critical role in bridging the communication between microglia and neurons.
Keyword: Neuroscience
1. Introduction
Microglial activation plays a broad role in the brain's innate immunity and in inflammatory neuropathologies and has served as a causative factor for a range of neurological disorders (Gehrmann et al., 1995). In addition to their role as the resident macrophages in the central nervous system, microglia can also detect changes in the local environment and release pro-inflammatory cytokines that affect the surrounding cells in positive or negative ways (Eyo and Wu, 2013; Kettenmann et al., 2011). A growing body of evidence suggests that microglia-to-neuron signaling is implicated in a plethora of neurological diseases and psychological disorders (Eyo and Wu, 2013). Inflammatory cytokines released from microglia have been found to be mediators of neurodegeneration and neuropathic pain (Milligan and Watkins, 2009; Sommer, 2003). We previously demonstrated that visceral hypersensitivity was associated with microglial activation and accumulation of cytokine IL-1β and TNF-α in the spinal cord (Chen et al., 2015) and hypothalamus (Zhang et al., 2016) of rats.
The hypothalamus integrates multiple sources of afferent inputs and sculpts integrated autonomic outputs for metabolic homeostasis and modulates autonomic output. Corticotrophin-releasing factor (CRF) originating from the hypothalamic paraventricular nucleus (PVN) elevates adrenocorticotropin hormone (ACTH) and corticosteroid levels via the hypothalamic-pituitary-adrenal (HPA) axis. CRF signaling and HPA axis activation regulate neuronal development, stress response, autonomic functions and pain sensitivity (Zhang et al., 2016). In vivo rodent studies suggest that microglia facilitate stress and visceral pain by upregulating CRFR expression in neighboring CRF neurons (Du et al., 2010).
Microglial activation has served as a causative factor for a range of neurological disorders (Watkins et al., 2001). Microglia release a number of effectors to modulate CRF release in the PVN including proinflammatory factors (e.g. IL-1β, TNF-α), growth factors, NO and its reactive oxygen species (ROS) derivatives (Zhang et al., 2016). The intricate central nervous system (CNS) environment complicates studying crosstalk between microglia and hypothalamic neurons in vivo. BV2 cells derived from raf/myc-immortalised murine neonatal microglia are the most frequently used substitute for microglia due to their high fidelity and suitability (Henn et al., 2009). In this study, we stimulated BV2 cells with lipopolysaccharide (LPS) and explored the crosstalk between microglia and hypothalamic neurons. The interaction between cultured hypothalamic neurons and microglia was further validated with primary cultures of glia. Our data indicate that microglia release IL-1β, which activates hypothalamic neurons in vitro.
2. Materials and methods
2.1. Animals
Adult male and female Sprague-Dawley rats were provided by the Experimental Animal Center of the Southern Medical University of China [license #: SCXK (Su) 2011-0003] for breeding. Rats were housed in a vivarium maintained on a standard 12 h light–dark cycle (lights on at 07:00 AM), with constant temperature and humidity (22 °C and 50%) and ad libitum access to food and water. After vivarium habituation for 7 days after delivery, 1 male and 2 female rats were mated to produce the litters. All procedures were conducted in accordance with the guidelines as described in the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1978) and were approved by the Institutional Animal Care and Use Committee at Anhui University of Science and Technology and Xuzhou Medical University.
2.2. Reagents
Polymyxin B sulfate (PMBS) and LPS were purchased from Sigma Aldrich (P1004, L2880, St Louis, MO). Recombinant rat IL-1β and anti-rat IL-1β were bought from Peprotech (400-01B, 500-P80, Rocky Hill, NJ). Primary antibody for NeuN came for Millipore (MAB377, Burlington, MA). Primary antibodies for CRF (ab8901), Iba-1 (ab5076) and p-CREB (ab32096) were purchased from Abcam (Cambridge, United Kingdom). Primary antibody for CRFR came from Santa Cruz Biotechnology (sc-12383, Dallas, TX). Alkaline phosphatase-conjugated rabbit anti-goat IgG (2B-2311) and horse anti-mouse second antibody (2B-2310) came from ZSGB-BIO (Beijing, China). Secondary antibody TRITC-conjugated donkey anti-goat IgG H&L (ab6882), donkey anti-rabbit IgG H&L (ab6799), donkey anti-mouse IgG H&L (ab6817) were purchased from Abcam (Cambridge, United Kingdom). IL-1β, CRF and cAMP ELISA kits were purchased from R&D (RLB00, MBS703710, KGE012B, Minneapolis, MN).
2.3. BV2 cell culture, primary cultures of microglia and hypothalamic neuron culture
BV2 cells were cultured as described previously (Dai et al., 2015). Briefly, BV2 cells were cultured in DMEM-HG media containing 10% fetal bovine serum, 100 IU/mL penicillin, and 100 mg/mL streptomycin. Plates were incubated at 5% CO2, 37 °C incubator. Microglia isolation and purification follows Tamashiro's report (Tamashiro et al., 2012) with minor optimization. Briefly, rat pups aged 1–2-days were used for primary cultures of microglia. Whole brain was removed and transferred into a new Petri dish with 5 mL of L-15 solution (Leibowitz L-15 + 0.1% Bovine serum albumin (BSA) + 1% Pen/Strep, Gibco) on ice. Mixed glial cultures were prepared from cortex. Brain tissues were pipetted up and down 10 times following soft and mechanical dissociation before cells were treated with 0.05% trypsin for 30 min at 37 °C. The reaction was stopped with 10% fetal bovine serum (FBS). Mixed glia cells in the supernatant were collected and cultured in high glucose DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, and 2 mM of L-glutamine (D-10 medium). Mixed glia become confluence after 14–21 days in culture media. Loosely attached primary cultures of microglia were obtained after shaking at 100 rps (Dragon Lab, #SK-D 1807-E, China) for 2 hours at 37 °C. The cell pellet was re-suspended and re-plated with D-10 medium for the following experiment.
Primary cultures of hypothalamic cells were derived from the hypothalamus of 3-day-old pups. All plates and chamber slides were coated with 150 μg/mL poly-D-lysine (Sigma-Aldrich) and rinsed with sterile distilled water 2 days before plating. The border of excised hypothalamuses was delineated by the optic chiasm and the mammillary bodies, and laterally by the hypothalamic sulci, approximately 2 mm deep. Hypothalamus from 10–12 neonatal rats were pooled in one 15 mL tube. 0.05% trypsin was added to the tissue, and tissue/trypsin mixture was incubated in a 37 °C water bath for 15 minutes. The tissue/trypsin was inverted 3 times every 5 minutes. Digestion was stopped by adding DMEM containing 10% FBS, followed by triturating the tissue with a 10 mL pipette up and down (10 times). The tissue was allowed to settle for 5 minutes and the supernatant was collected and transferred to a new conical tube. The supernatant was centrifuged at 1000 rpm for 5 minutes. The supernatant was then removed and the cell pellet was re-suspended in D-10 medium. Cells were diluted to 5 × 105 cells/mL, and 500 μL was plated in 24-well plates or 400 μL 8-well chamber slides. The culture medium was changed (serum free SM1 neuronal culture kit, 05712, StemCell Technologies) completely and carefully without disturbing the cells after 6 hours. Half of the culture media was removed and replaced with fresh media every 3 days. The cells were cultured for 14 days before treatment.
Anti-Rat IL-1β was used to block IL-1β in the conditional medium (CM) or recombinant rat IL-1β. Anti-rat IL-1β was added to CM at 1.25 μg/mL and recombinant rat IL-1β was added at 250 pg/ml.
2.4. Immunofluorescent staining
Immunofluorescent staining was performed to verify the cell types. Cultured cells were fixed in 4% paraformaldehyde for 30 min at room temperature and washed with PBS 3 times. The cells were treated with 0.3% Triton X-100 for 15 min, blocking serum for 30 min and incubated with primary polyclonal rabbit anti-CRF (1:200), and mouse anti-NeuN antibody (1:200), rabbit anti-Iba-1 (1:1000) or rabbit anti-GFAP (1:400) overnight. The cells were then washed and incubated with TRITC-conjugated donkey anti-rabbit IgG (1:200, bright orange) and FITC-conjugated donkey anti-mouse IgG (1:200, green) for 1 h. 4′,6-diamidino-2-phenylindole (DAPI) was used to stain the nucleus of cells (blue). The slide was mounted for fluorescence microscope examination.
2.5. Western blot
Hypothalamic cell lysates were prepared as described previously (Dai et al., 2015). Briefly, cell lysates were collected from cultured hypothalamic cells that had been treated for 24 hours. Cultured hypothalamic neurons were homogenized in buffer A (5 mM HEPES, 5 mM EDTA, 1 mM DTT, 2 μg/mL aprotinin, 1 mM NaF, 10 mM KCl, 5 mM EGTA, 1 mM PMSF, 2 μg/mL leupeptin, and 1 mM Na3VO4) on ice and centrifuged at 12,000 rpm for 15 min at 4 °C. The pellet was resuspended in 50 μL of cold buffer C (5 mM HEPES, 5 mM EDTA, 1 mM DTT, 2 μg/mL aprotinin, 1 mM NaF, 50 mM NaCl, 5 mM EGTA, 1 mM PMSF, 1 mM leupeptin, and 1 mM Na3VO4), homogenized for 1 min, and incubated on ice for 50 min to allow for high salt extraction. Cellular debris was removed by centrifugation for 15 min at 4 °C. The supernatant containing nuclear proteins was collected and stored in aliquots at -80 °C for further immunoblotting analysis.
Equal amounts of protein were separated with SDS-PAGE, and transferred to a PVDF membrane. After BSA blocking, the membrane was incubated with p-CREB (1:500), CRFR (1:500), Tubulin (1:500) or β-actin (1:1000) antibodies at 4 °C overnight. Membranes were washed and probed with alkaline phosphatase conjugated secondary antibody for 2 hours at room temperature. Proteins were visualized by BCIP/NBT Alkaline Phosphatase Color Development Kit (Beyotime Biotechnology, Shanghai, China). Image Proplus (Media Cybernetics, Rockville, MD) was used for analysis of protein bands.
2.6. Enzyme-linked immunosorbent assay (ELISA) and Ca2+ imaging
IL-1β, cAMP, and CRF levels were determined by ELISA following the manufacturer's instructions. Intracellular Ca2+ levels were determined with a Ca2+-sensitive fluorochrome (Fluo-3 AM, Beyotime Biotechnology, Shanghai China). After treatment, cells were collected in serum-free DMEM andincubated at 37 °C for 1 h in the dark. Ca2+ signals were determined by Becton Dickinson FACS Calibur flow cytometer (for Fluo-3).
2.7. Statistical analysis
All data are presented as means ± SEM. Statistical analyses were performed using one-way analysis of variance followed by Post hoc Bonferroni's multiple comparison (SPSS 13.0 for Windows). p < 0.05 was considered statistically significant.
3. Results
3.1. Primary cultures of microglia and hypothalamic neurons from rat brain
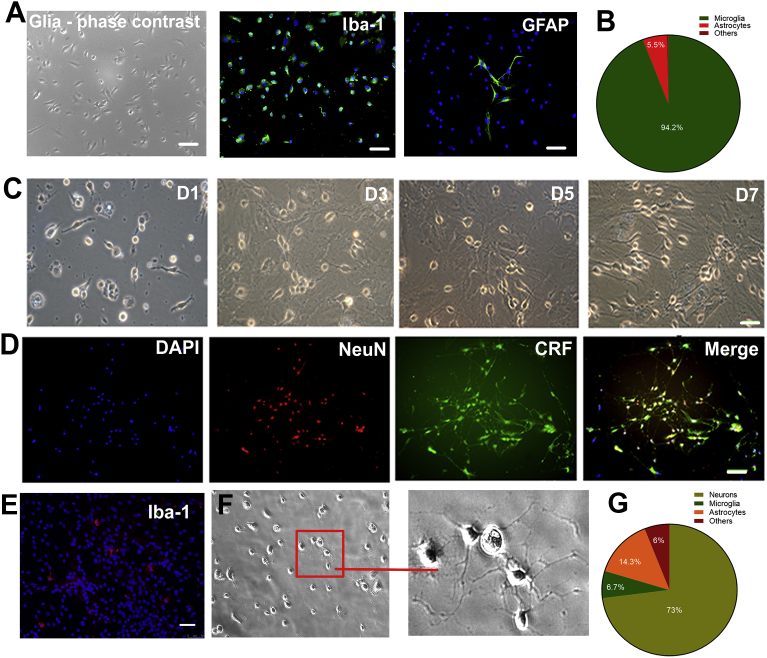
Primary cultures of microglia were obtained from rat cortex. Immunofluorescence staining showed that 94.2% of cells stained positive for Iba-1, suggesting that they are microglia, and 5.5% stained positive for GFAP (an astrocyte marker). No NeuN (a neuronal marker) positive staining was observed in the cell culture (Fig. 1A and B). Fig. 1C shows phase contrast views of the primary cultures of hypothalamic neurons on days 1, 3, 5 and 7 post-plating. Immunocytochemistry staining revealed extensive CRF- and NeuN-positive staining in the primary cultures of hypothalamic neurons on day 7 (Fig. 1D). High resolution cultured neurons can be observed in Fig. 1F. A very low density of Iba-1 positive cells presented in cultured hypothalamic neurons (Fig. 1E). The percentage of cells staining positively for NeuN, Iba-1 and GFAP was 73%, 6.7% and 14.3%, respectively. Six percent of cells did not show any positive staining (Fig. 1G). Hypothalamic neurons were predominated in culture dish and could survive for at least 3 weeks.
Fig. 1.
Primary cultures of microglia and hypothalamic neurons from rat brain. Primary cultures of microglia were obtained from rat cortex. (A) Cultured cells presented a high density of Iba-1 positive staining and very few GFAP positive staining. (B) Immunofluorescent stain analysis on that 94.2% cells presents Iba-1 positive staining, 5.5% of cells presents GFAP staining, and no NeuN positive staining was visualized. (C) Phase contrast views of the primary cultures of hypothalamic neuron culture on days 1, 3, 5 and 7 post-plating. (D) Immunocytochemistry staining revealed extensive CRF- and NeuN-positive staining in the primary cultured hypothalamic neurons on day 7. (E) A very low density of Iba-1 positive cells presented in cultured hypothalamic neurons. (F) Low and high resolution of cultured neurons. (G) Percentage of NeuN, Iba-1 and GFAP positive stainings was 73%, 6.7% and 14.3%, respectively. 6% cells did not show any positive staining. Scale bar = 100 μm.
3.2. LPS induces morphological change, increases cellular Iba-1 expression and IL-1β level in culture medium in cultured BV2 cells
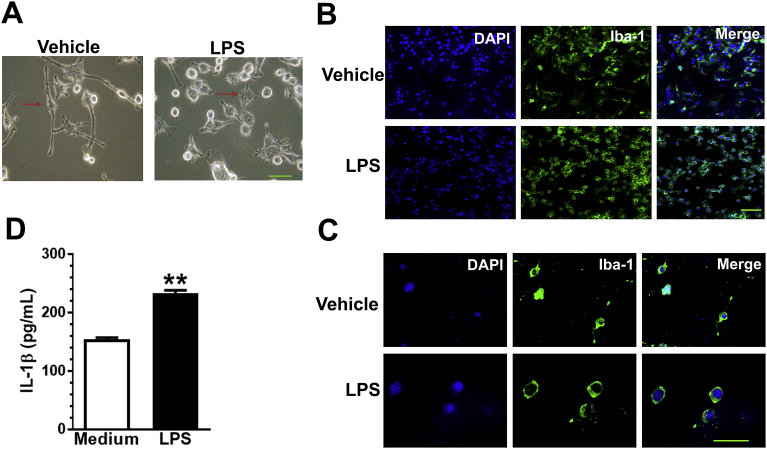
BV2 cells without LPS treatment presented ramified cell morphology with fine processes extending tens of microns away from the soma (Fig. 2A, left). BV2 cells treated with LPS (10 μg/mL) presented a rounded amoeboid-like appearance with fine processes extending from the soma (Fig. 2A, right). LPS-treated BV2 cells had increased Iba-1 staining compared with those without LPS treatment (Fig. 2B, low magnification, and Fig. 2C, high magnification). BV2 cells were treated with LPS (10 μg/mL) for 30 min. IL-1β level in the supernatant elevated in treated cultures (t(4) = 13.42, p = 0.0002).
Fig. 2.
LPS induces morphological change, increases cellular Iba-1 expression and IL-1β level in culture medium of BV2 cells. (A) BV2 cells without LPS treatment presented ramified cell morphology with fine processes extending tens of microns away from the soma (left). BV2 cells treated with LPS (10 μg/mL) presented a rounded amoeboid-like appearance with fine processes from soma (right). (B and C) LPS-treated BV2 cells present higher Iba-1-positive staining compared with the non LPS-treated BV2 cells (B, low magnification, and C, high magnification). LPS incubation induced IL-1β level in culture medium compare with non-LPS treated group (D). All data are presented as means ± SEM; **p < 0.01; Scale bar = 100 μm.
3.3. LPS-treated BV2 culture medium increases the levels of CRF, CRFR, p-CREB and cAMP in primary cultures of hypothalamic neurons
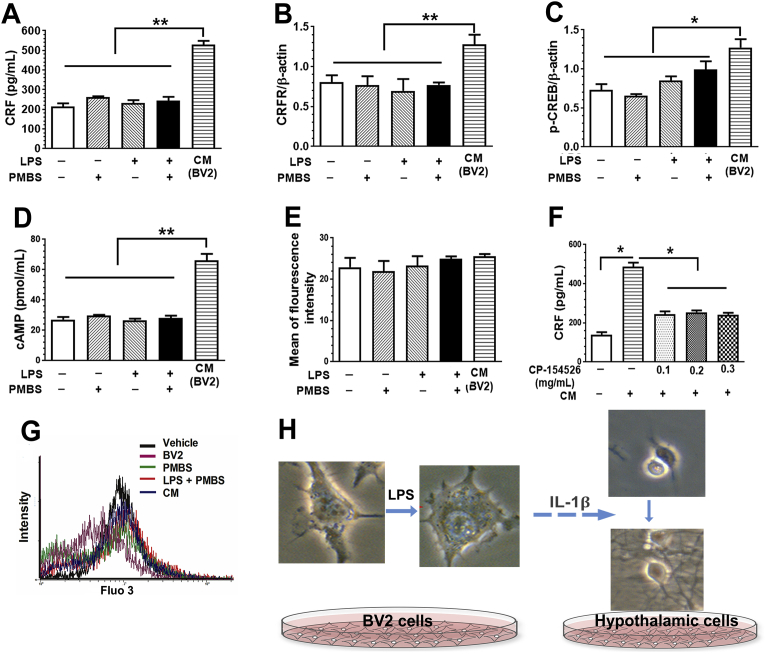
Cultured rat hypothalamic neurons incubated with supernatant from BV2 cells treated with LPS (conditioned medium, CM) had increased CRF levels compared to cultured hypothalamic neurons incubated with LPS, PMBS, or both (p < 0.01). Treatment with LPS, PMBS (a LPS antagonist), or both did not affect the CRF level in culture media of primary cultures of hypothalamic neurons (F(3,8) = 1.89, p = 0.209) (Fig. 3A).
Fig. 3.
LPS-treated BV2 culture medium increase the level of CRF, CRFR1, CREB and cAMP in cultured hypothalamic cells. Culture media (CM) from LPS-treated increased the levels of CRF (A), CRFR (B), p-CREB (C) and intracellular cAMP (D) in primary cultures of hypothalamic neurons. LPS, PMBS, or their combination did not present any effect. (E) There was no difference in intracellular Ca2+ level. G is the intracellular Ca2+ measurement. (F) CP-154526, a selective antagonist of the CRFR, at 0.1, 0.2, and 0.3 mg/mL, prevented CM-induced increase in CRF level. H is experimental flow chart. Data were presented as means ± SEM; *, p < 0.05; **, p < 0.01.
To assess the effect of activated BV2 cells on the CRFR and p-CREB expression in cultured hypothalamic cells, we cultured of rat hypothalamic cells for 24 h in CM and analyzed cell nuclear lysates for CRFR and p-CREB expression. Even though LPS and PMBS treatment did not alter CRFR levels (F(3,8) = 0.194, p = 0.898), CM treatment increased CRFR levels compared to LPS, PMBS or both (p < 0.01; Fig. 3B). LPS and PMBS treatment altered p-CREB levels (F(3,8) = 4.38, p = 0.042). CM treatment increased the p-CREB level compared to LPS, PMBS or both (p < 0.05; Fig. 3C). Consistently, CM treatment increased the cAMP level compared to LPS, PMBS or both (P < 0.01, Fig. 3D), but not the intracellular Ca2+ level (F(4,10) = 0.645, p = 0.643; Fig. 3E). CP-154526, a selective antagonist of CRFR, prevented CM-induced increase in CRF level at 0.1, 0.2, and 0.3 mg/mL, (F(4,10) = 85.31, p < 0.01; Fig. 3F). The calcium Fluo 3 reading is shown in Figure G while a representative example of experimental flow is shown in Fig. 3H.
3.4. LPS stimulates primary cultures of glia to release IL-1β and facilitates CRF release from primary cultures of hypothalamic neurons
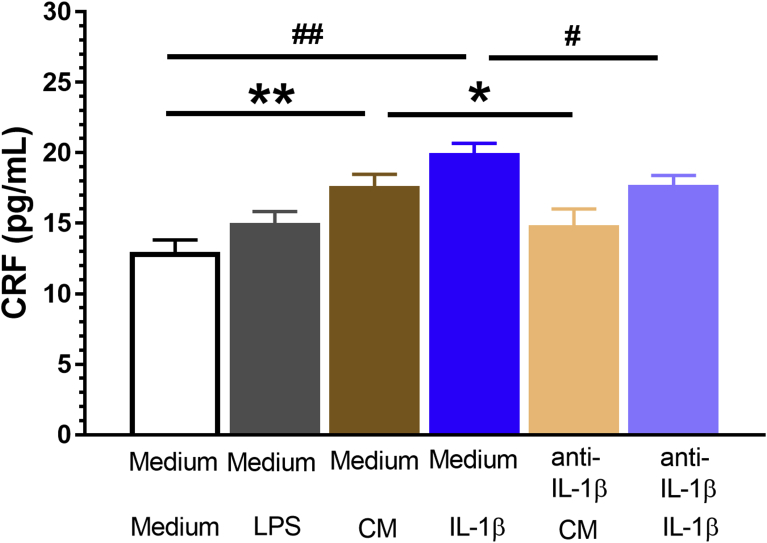
Primary cultures of hypothalamic neurons incubated with CM from cultures of glial cells had an increase in CRF levels when compared to regular medium (t(4) = -5.29, p = 0.006), which can be forestalled by pretreatment with an anti-IL-1β antibody (5 μg/mL) (regular medium + CM vs. anti-IL-1β + CM; t(4) = 3.438, p = 0.026). Consistently, cultured hypothalamic neurons incubated with IL-1β (250 pg/mL) displayed an increase in CRF levels when compared to regular medium (t(4) = -9.94, p = 0.001), which can also be prevented by pretreatment with an anti-IL-1β antibody (regular medium + IL-1β vs. anti-IL-1β + IL-1β; t(4) = 4.08, p = 0.02). LPS alone did not affect the CRF release from cultured hypothalamic neurons. Exogenous IL-1β also induced CRF release from cultured hypothalamic neurons. The effect of CM and IL-1β on CRF release can be preempted by pretreatment with anti- IL-1β antibody (Fig. 4). Preliminary experiments did not reveal effect of the antibody against IL-1β on CRF protein levels in cells incubated with culture medium (data not shown).
Fig. 4.
LPS stimulates primary cultured glial cells to release IL-1β and facilitates CRF release by primary cultured hypothalamic neurons. Primary cultures of hypothalamic neurons incubated with CM from cultured glial cells presented an increase in CRF level as compared to regular medium, which can be prevented by anti-IL-1β antibody (5 μg/mL) pretreatment (regular medium + CM vs. anti-IL-1β + CM). Consistently, cultured hypothalamic neurons incubated with exogenous IL-1β (250 pg/mL) presented an increase in CRF level as compared to regular medium, which can be neutralized by anti-IL-1β antibody pretreatment (regular medium + IL-1β vs. anti-IL-1β + IL-1β). Data were presented as means ± SEM; *, #p < 0.05; **, ##p < 0.01.
4. Discussion
In this study, we evaluated microglia-neuron crosstalk in vitro with BV2 cells, primary cultures of microglia and hypothalamic neurons. Our data demonstrate that BV2 cells and cultured glial cells treated with LPS can release IL-1β into the culture medium and that this media stiumulates cultured hypothalamic neurons. Moreover, activated hypothalamic cells presented an increase in CRF, CRFR, p-CREB and cAMP, but not intracellular Ca2+ signaling. These findings provide direct evidence of microglia-neuron communucation connected by IL-1β.
The in vivo study of microglia in neurology, toxicology and immunology may need a large number of animals. Using a microglia-like cell line can simulate an in vivo context at a lower cost and higher efficiency. The immortalised murine microglial cell line BV2 has been used frequently as a substitute for primary cultures of microglia due to its high fidelity and suitability (Henn et al., 2009). As described previously (Henn et al., 2009), we demonstrated that LPS can stimulate BV2 cells into micorglia-like state demonstrated by characteristic morphorlogical changes and increase in Iba-1 expression. Morphological change indicates the cell motility for microglia activation, and increase in Iba-1 expression evidences the microglia differentiation and activation. LPS is a specific activator of the TLR4 receptor. TLR4 signaling in microglia plays an important role in sensing environmental change (Akira et al., 2006). Converging evidence suggests that LPS-treated BV2 microglia constitutively release various neurotrophic factors and pro-inflammatory cytokines, which can modulate neighboring neuronal function (Kettenmann et al., 2013).
As reported previously (Schmidt et al., 1995; Tilders and Schmidt, 1998), we found LPS treatment increased the level of IL-1β in the BV2 cell and primacy glia culture medium. IL-1β is a critical pro-inflammatory factor that bridges the communication between glia and neurons. The hypothalamus receives information from many sources, then processes this information and generates output to control metabolism, homeostasis, and autonomic system. CRF originating from the hypothalamic PVN elevates ACTH and corticosteroid levels via the HPA axis (Ferguson et al., 2008). Microglia facilitate stress and visceral pain by upregulating CRFR expression in neighboring CRF neurons (Du et al., 2010). In this study, we examined the communication between microglia and neurons with BV2 cells and primary cultures of hypothalamic cells.
We successfully isolated and cultured rat glia and hypothalamic neurons. Primary cultures of hypothalamic neurons were positively stained for NeuN+ and CRF+. LPS, PMBS or both did not alter the levels of CRF, CRFR, p-CREB, cAMP, or intracellular Ca2+ in cultured hypothalamic cells. However, conditioned medium from the LPS treated BV2 cells or cultured glial cells significantly increased the levels of CRF, CRFR, p-CREB and cAMP in cultured hypothalamic cells. These suggest that chemicals released from LPS-treated BV2 cells modulate the activities of hypothalamic cells.
Our data revealed that the IL-1β level was elevated in LPS-treated BV2 cells. Pro-inflammatory cytokine IL-1β, released from activated BV2 microglia, can enhance the activity of the HPA axis (Bale and Vale, 2004). Schmidt et al. (1995) reported that IL-1β (5 pg/kg, i.p.) could induce a long lasting increase in hypothalamic CRF in adult male rats. Microglia is the major resource of brain IL-1β (Dheen et al., 2007), and IL-1β stimulates CRF and ACTH Release (Mazzocchi et al., 1993). Consistently, we found that CRF levels were elevated in hypothalamic cells treated with conditioned medium from BV2 cells. LPS treated primary cultures of glia can release IL-1β, which could be simulated by exogenous IL-1β and neutralized by anti-IL-1β antibody, which further demonstrated the participation of IL-1β in glia-neuron crosstalk.
Our data also indicated that LPS-treated BV2 cell culture media promoted CRFR and p-CREB expression in cultured hypothalamic cells. CRF is a key factor in the stress response and regulates the development of acute and chronic pain, including visceral pain (Zhang et al., 2016). CRFR, a class B/secretin-like GPCR, has five different subunits (Gs, Gi, Gq, Go, and Gz). Discrete microdomains within the cell mediate precise spatiotemporal signaling and regulate cAMP production by using nine G protein–regulated transmembrane adenylyl cyclases. Upon IL-1β stimulation, CRF binds to CRFR, and in turn increases CRFR expression. This short, positive feedback stimulates more CRF release into the hypophyseal portal system, which causes ACTH release, initiates HPA axis, and maintains homeostasis. Gs is the main coupling subunit of CRFR, leading to an increase in cyclic AMP (cAMP) production and activation of multiple signaling cascades, such as PKA and pCREB (Bonfiglio et al., 2011). CRF, a key modulator in stress response, takes part in various kinds of acute and chronic pain and contributes the development of functional visceralgia. Moreover, You et al. (2012) found that CRF acted on CRFR1, activating Gq and Gi. Gq and Gi activation leads to IP3 production and a subsequent increase of Ca2+ in human pregnant myometrium at term (37–42 weeks) labor. In line with this, we found that CRF, CRFR, p-CREB and cAMP levels were elevated with conditioned medium stimulation.
In this study, we used an in vitro approach to evaluate microglia-neuron crosstalk. Our data revealed that LPS-treated BV2 cells and primary cultures of microglia could release IL-1β into culture medium that had a stimulatory effect on primary cultures of hypothalamic neurons. These findings provide direct evidence of microglia-neuron communication in the hypothalamus.
Declarations
Author contribution statement
Xinrong Tao, Na Li, Fei Liu, Yuting Hu, Jing Liu: Performed the experiments; Analyzed and interpreted the data.
Zhang Ym: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was supported by grants from the National Natural Science Foundation of China (81471161, 81771203, 81772065), the Natural Science Foundation of Jiangsu Province (BK20161171) and Top Talent Project of Anhui Department of Education (gxbjZD16).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Michael Wormald for proofreading this manuscript.
References
- Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Bale T.L., Vale W.W. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bonfiglio J.J., Inda C., Refojo D., Holsboer F., Arzt E., Silberstein S. The corticotropin-releasing hormone network and the hypothalamic-pituitary-adrenal axis: molecular and cellular mechanisms involved. Neuroendocrinology. 2011;94:12–20. doi: 10.1159/000328226. [DOI] [PubMed] [Google Scholar]
- Chen Z.Y., Zhang X.W., Yu L., Hua R., Zhao X.P., Qin X., Zhang Y.M. Spinal toll-like receptor 4-mediated signalling pathway contributes to visceral hypersensitivity induced by neonatal colonic irritation in rats. Eur. J. Pain. 2015;19:176–186. doi: 10.1002/ejp.534. [DOI] [PubMed] [Google Scholar]
- Dai X.J., Li N., Yu L., Chen Z.Y., Hua R., Qin X., Zhang Y.M. Activation of BV2 microglia by lipopolysaccharide triggers an inflammatory reaction in PC12 cell apoptosis through a toll-like receptor 4-dependent pathway. Cell Stress Chaperones. 2015;20:321–331. doi: 10.1007/s12192-014-0552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheen S.T., Kaur C., Ling E.A. Microglial activation and its implications in the brain diseases. Curr. Med. Chem. 2007;14:1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- Du F., Yin L., Shi M., Cheng H., Xu X., Liu Z., Zhang G., Wu Z. Involvement of microglial cells in infrasonic noise-induced stress via upregulated expression of corticotrophin releasing hormone type 1 receptor. Neuroscience. 2010;167:909–919. doi: 10.1016/j.neuroscience.2010.02.060. [DOI] [PubMed] [Google Scholar]
- Eyo U.B., Wu L.J. Bidirectional microglia-neuron communication in the healthy brain. Neural Plast. 2013;2013 doi: 10.1155/2013/456857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A.V., Latchford K.J., Samson W.K. The paraventricular nucleus of the hypothalamus - a potential target for integrative treatment of autonomic dysfunction. Expert Opin. Ther. Targets. 2008;12:717–727. doi: 10.1517/14728222.12.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann J., Matsumoto Y., Kreutzberg G.W. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- Henn A., Lund S., Hedtjärn M., Schrattenholz A., Pörzgen P., Leist M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX. 2009;26:83–94. doi: 10.14573/altex.2009.2.83. [DOI] [PubMed] [Google Scholar]
- Kettenmann H., Hanisch U.K., Noda M., Verkhratsky A. Physiology of microglia. Physiol. Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kettenmann H., Kirchhoff F., Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Mazzocchi G., Musajo F.G., Malendowicz L.K., Andreis P.G., Nussdorfer G.G. Interleukin-1beta stimulates corticotropin-releasing hormone (CRH) and adrenocorticotropin (ACTH) release by rat adrenal gland in vitro. Mol. Cell. Neurosci. 1993;4:267–270. doi: 10.1006/mcne.1993.1034. [DOI] [PubMed] [Google Scholar]
- Milligan E.D., Watkins L.R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E.D., Janszen A.W., Wouterlood F.G., Tilders F.J. Interleukin-1-induced long-lasting changes in hypothalamic corticotropin-releasing hormone (CRH)--neurons and hyperresponsiveness of the hypothalamus-pituitary-adrenal axis. J. Neurosci.: Off. J. Soc. Neurosci. 1995;15:7417–7426. doi: 10.1523/JNEUROSCI.15-11-07417.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C. Painful neuropathies. Curr. Opin. Neurol. 2003;16:623–628. doi: 10.1097/01.wco.0000093106.34793.06. [DOI] [PubMed] [Google Scholar]
- Tamashiro T.T., Dalgard C.L., Byrnes K.R. Primary microglia isolation from mixed glial cell cultures of neonatal rat brain tissue. J. Vis. Exp.: JoVE. 2012 doi: 10.3791/3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilders F.J., Schmidt E.D. Interleukin-1-induced plasticity of hypothalamic CRH neurons and long-term stress hyperresponsiveness. Ann. N. Y. Acad. Sci. 1998;840:65–73. doi: 10.1111/j.1749-6632.1998.tb09550.x. [DOI] [PubMed] [Google Scholar]
- Watkins L.R., Milligan E.D., Maier S.F. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- You X., Gao L., Liu J., Xu C., Liu C., Li Y., Hui N., Gu H. CRH activation of different signaling pathways results in differential calcium signaling in human pregnant myometrium before and during labor. J. Clin. Endocrinol. Metabol. 2012;97:E1851–E1861. doi: 10.1210/jc.2011-3383. [DOI] [PubMed] [Google Scholar]
- Zhang G., Yu L., Chen Z.Y., Zhu J.S., Hua R., Qin X., Cao J.L., Zhang Y.M. Activation of corticotropin-releasing factor neurons and microglia in paraventricular nucleus precipitates visceral hypersensitivity induced by colorectal distension in rats. Brain Behav. Immun. 2016;55:93–104. doi: 10.1016/j.bbi.2015.12.022. [DOI] [PubMed] [Google Scholar]