Abstract
The global obesity epidemic is fueling alarming rates of diabetes, associated with increased risk of cardiovascular disease and cancer. Leptin is a hormone secreted by adipose tissue that is a key regulator of body weight (BW) and energy expenditure. Leptin-deficient humans and mice are obese, diabetic, and infertile and have hepatic steatosis. Although leptin replacement therapy can alleviate the pathologies seen in leptin-deficient patients and mouse models, treatment is costly and requires daily injections. Because adipocytes are the source of leptin secretion, we investigated whether mouse embryonic fibroblasts (MEFs), capable of forming adipocytes, could be injected into ob/ob mice and prevent the metabolic phenotype seen in these leptin-deficient mice. We performed a single subcutaneous injection of MEFs into leptin-deficient ob/ob mice. The MEF injection formed a single fat pad that is histologically similar to white adipose tissue. The ob/ob mice receiving MEFs (obRs) had significantly lower BW compared with nontreated ob/ob mice, primarily because of decreased adipose tissue mass. Additionally, obR mice had significantly less liver steatosis and greater glucose tolerance and insulin sensitivity. obR mice also manifested lower food intake and greater energy expenditure than ob/ob mice, providing a mechanism underlying their metabolic improvement. Furthermore, obRs have sustained metabolic protection and restoration of fertility. Collectively, our studies show the importance of functional adipocytes in preventing metabolic abnormalities seen in leptin deficiency and point to the possibility of cell-based therapies for the treatment of leptin-deficient states.
Leptin-deficient ob/ob mice were injected with mouse embryonic fibroblasts, which formed adipose tissue in vivo and prevented the metabolic abnormalities of leptin deficiency.
Metabolic syndrome is a global epidemic that consists of multiple risk factors including central obesity and insulin resistance. Approximately 35% of adults in the United States meet the criteria for metabolic syndrome, which is associated with an elevated risk of developing type 2 diabetes and cardiovascular disease (CVD) (1, 2). CVD is the leading cause of death in the United States, and the combined annual cost associated with diabetes and CVD is >$500 billion (2). A contributing factor to the obesity epidemic is the recent large rises in energy intake, coupled with an increasingly sedentary lifestyle (3, 4). Numerous contributing factors play a role in body composition, yet hormones facilitate many processes regulating energy balance. A key hormone involved in the regulation of energy balance is leptin.
Leptin is a principal component in the determination of adiposity. One of the main functions of leptin is to communicate the body’s nutritional status to centers of the brain, primarily through the hypothalamus, to regulate many processes that control food consumption and energy expenditure (5). Evidence indicates that multiple tissues are capable of leptin expression, but the predominant source of leptin is adipose (5–9). Leptin levels are positively correlated with body mass index and percentage body fat in patients and are highly associated with total body triglyceride (TG) storage (10–12). The amount of leptin closely reflects the body’s nutritional status, and a deficiency can have profound metabolic consequences. Human leptin deficiency, caused by a mutation in the leptin gene, presents with hyperphagia, early-onset obesity, and hyperinsulinemia (13–15). Leptin-deficient ob/ob mice are a well-described model for metabolic studies that display hyperphagia and early-onset obesity (16). Originally described in 1949, ob/ob mice have a mutation in the leptin gene (Lep) resulting in the production of a nonfunctioning protein and are a valuable tool for the study of metabolic defects and therapies for leptin deficiency (5, 16). Furthermore, ob/ob mice have increased fatty acid synthesis in both adipose and liver in the setting of increased adiposity and hepatic steatosis (17). Additionally, ob/ob mice have impaired glucose tolerance and insulin sensitivity, along with elevated levels of other hormones associated with insulin resistance, including insulin and gastric inhibitory polypeptide (GIP) (18–20). Importantly, leptin-deficient humans and leptin-deficient ob/ob mice have been successfully treated with recombinant leptin to circumvent hyperphagia, obesity, and infertility (15, 21, 22). Unfortunately, exogenous leptin therapy is expensive, has side effects, and can require multiple daily injections. It has been postulated that an ideal therapy would provide leptin endogenously (23–25).
The production of endogenous leptin occurs primarily in white adipose tissue (WAT) (5). Previous studies have shown that adipose from wild-type (WT) mice can be surgically implanted into ob/ob mice to circumvent metabolic consequences, but this method required multiple adipose donor mice and several adipose implants (26). Recent work has shown that 3T3-L1 adipocytes overexpressing leptin can be encapsulated and injected into ob/ob mice (27). Although ob/ob mice receiving overexpressing adipocytes did have an improvement in energy expenditure and glucose tolerance, there was no significant change in body weight (BW), fat mass, or insulin sensitivity (27). Previous work has shown that 3T3-F442A preadipocytes can form fat pads similar to an epididymal WAT that is able to produce leptin in vivo. However, these fat pads expressed only 10% to 15% of leptin mRNA when compared with epididymal WAT. These studies were performed in immunodeficient athymic nude mice that produce leptin, and it was not possible to verify leptin secretion by these ectopic fat pads (28). We and others have used mouse embryonic fibroblasts (MEFs) to generate adipocytes in vitro. These MEFs must be treated with an adipogenic cocktail consisting of the glucocorticoid dexamethasone, insulin, and 1-methyl-3-isobutylxanthine (a phosphodiesterase inhibitor that raises cAMP levels). As an alternative, we investigated whether a single injection of MEFs, which are capable of forming adipocytes, could improve the pathological metabolic phenotypes seen in leptin-deficient ob/ob mice in vivo.
Materials and Methods
Animals
Mice were purchased from Jackson Laboratories (B6.Cg-Lepob/J) as either homozygous (ob/ob) or heterozygous (ob/+) for use in studies or breeding, respectively. For prevention experiments, injection of WT C57Bl/6J MEFs was performed in male ob/ob mice at 2 to 3 weeks of age. Initial studies characterized mice 8 to 10 weeks after MEF injection, and long-term studies were performed in a separate cohort ~7 months after MEF injections. For the final rescue experiment, injection of MEFs was performed in male ob/ob mice at 12 weeks of age and characterized 8 weeks after MEF injection. Animals were maintained on a 12-hour light, 12-hour dark cycle, in a temperature-controlled room (~22°C). All mice and procedures were on a protocol approved by the Institutional Animal Care and Use Committee of Washington University in St. Louis. Animals were given appropriate care while undergoing research that complies with the standards in the Guide for the Care and Use of Laboratory Animals (29).
MEF isolation and injection
MEFs were prepared from E14 C57BL/6J mouse embryos and delivered via subcutaneous injection, as previously described (30, 31). Briefly, mouse embryos were carefully collected after cervical dislocation in females on embryonic day 14. The embryonic head and internal organs were removed, and the remaining embryo was minced then added to a tube containing 1.25 mL of 0.05% trypsin/EDTA and incubated in a water bath for 45 minutes at 37°C. Next, 5 mL DMEM with 10% fetal bovine serum was added and pipetted vigorously 25 to 30 times, then passed through a 70-μm nylon mesh cell strainer into a new tube. The cell suspension was then centrifuged at 1000 rpm for 5 minutes and suspended in 150 μL PBS. The equivalent of MEFs from one embryo was injected into each mouse with a 25-gauge needle. MEFs were delivered by subcutaneous injection into the tissue overlying the sternum in mice under isoflurane anesthetization. Our group has previously shown that WT MEF-derived fat pads have similar expression of adipocyte-specific genes when compared with WAT (31).
Body composition
Composition of total body adiposity and lean mass were performed with EchoMRI-100H (EchoMRI LLC), as previously described (32).
Histology
Hematoxylin and eosin (H&E) staining was performed on tissues fixed in 10% neutral buffered formalin. Slides were imaged with NanoZoomer (Hamamatsu NanoZoomer HT model) at ×20 magnification. Adipocyte size was measured and calculated in Adiposoft software (ImageJ) (33).
Liver TGs
Liver TGs were extracted and measured via the Folch method, as previously described (34, 35). Briefly, ~50 to 80 mg of liver was minced, weighed, and then extracted overnight in 2:1 chloroform/methanol. After phase separation, an aliquot of the bottom phase was removed, followed by the addition of 1% Triton X-100 in chloroform. The solvent was evaporated, then deionized water was added and TGs were quantified with Infinity™ Triglyceride Reagent (Thermo Scientific).
Glucose tolerance and insulin sensitivity
A glucose tolerance test (GTT) was performed in mice ~8 to 10 weeks after MEF transplantation by fasting mice for 4 hours, followed by an IP injection of 10% dextrose: 2% volume/kg lean mass (as determined by EchoMRI) or 1% volume/kg BW, as indicated. Blood glucose was measured by tail bleed immediately before injection, and serial measurements were taken at 20, 40, 60, and 120 minutes after injection with a glucometer (Contour®, Bayer). The next week, an insulin tolerance test (ITT) was performed after a 4-hour fast and an IP injection of insulin (Humulin R): 1 U/kg lean mass (as determined by EchoMRI) or 1 U/kg BW, as indicated. Blood glucose was measured by tail bleed immediately before injection, and serial measurements were taken at 20, 40, 60, and 120 minutes after injection. Glucose measurements were performed with a blood glucose monitoring system (Contour®, Bayer).
Metabolic parameters
Indirect calorimetry and movement were determined with the TSE Systems Phenomaster automated metabolic caging system. Food and water intake was measured daily, for 1 week, in individually housed mice.
Plasma assays
Plasma analyses were performed commercially with a mouse metabolic array routine discovery assay (Eve Technologies).
Statistical analysis
Graphs were plotted in GraphPad Prism 7.0e (GraphPad Software, La Jolla, CA), and data are expressed as mean ± SD. Data were analyzed with either a one-way or two-way ANOVA followed by post hoc analysis unless otherwise indicated. P < 0.05 was considered significant. A rescue index for ob/ob mice receiving MEFs (obRs) was quantified for several metabolic parameters as (ob/ob – obR)/(ob/ob – WT).
Results
Transplantation of MEFs prevents obesity and hepatic steatosis
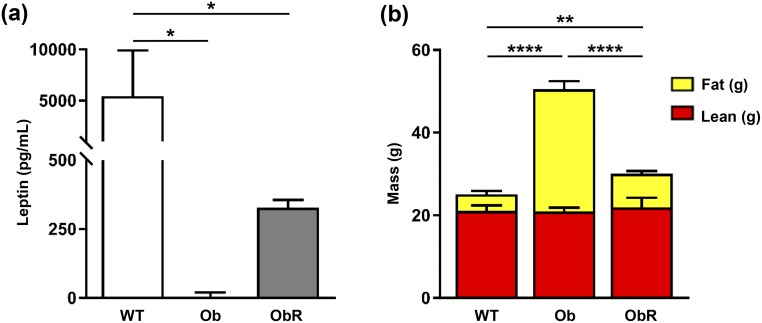
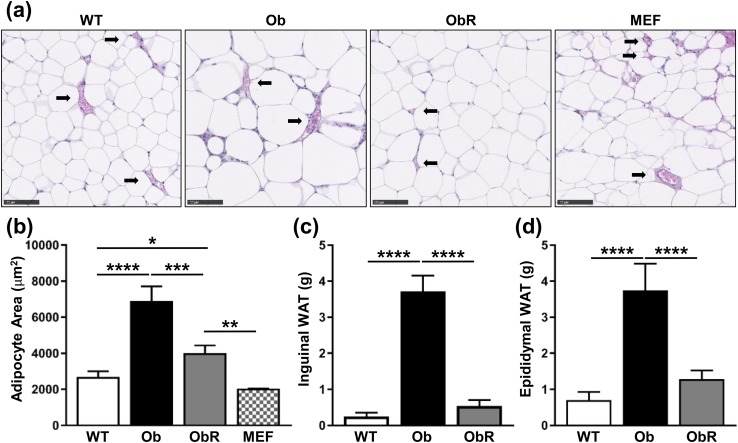
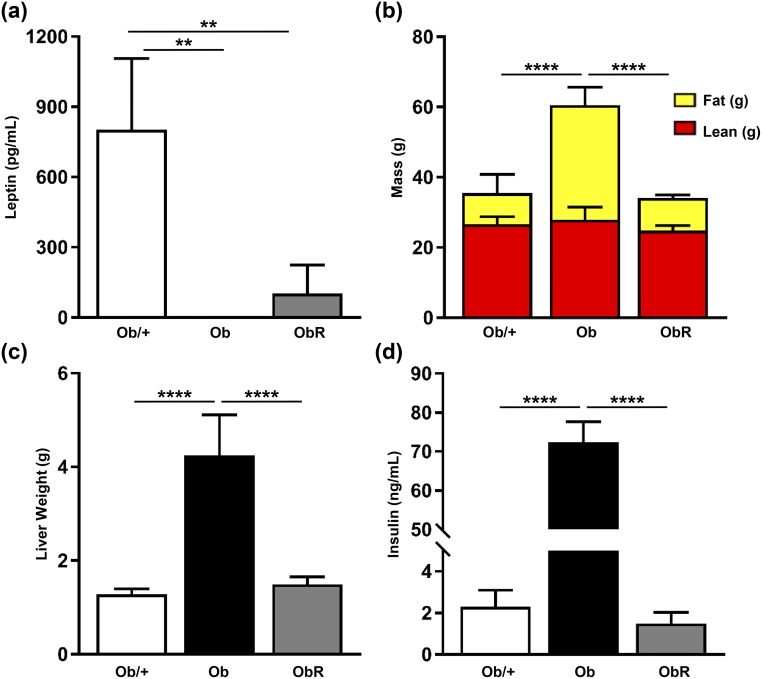
To investigate whether the pathological metabolic phenotypes seen in leptin-deficient ob/ob mice could be prevented by a MEF-derived fat pad, we performed a single subcutaneous injection of MEFs, isolated from WT C57Bl/6J mice, overlying the sternum of 2- to 3-week-old male ob/ob mice. After 10 weeks, we found that ob/ob mice rescued with MEF implantation (obR) had a measurable amount of plasma leptin, yet it was only ~6% of that found in WT mice [Fig. 1(a)]. Despite low levels of plasma leptin, obR mice had a 40% lower BW than ob/ob mice, as evidenced predominantly by a decrease in fat mass of 72%, with no difference in lean mass between groups [Fig. 1(b)]. This is equivalent to a rescue of 84% with regard to fat mass. To more closely examine WAT, we performed H&E staining on sections of epididymal WAT and MEF-derived fat pads [Fig. 2(a)]. Importantly, vascularization was observed in all groups, including in the MEF-derived fat pad [Fig. 2(a)]. Quantification of adipocyte area from H&E sections revealed that obR mice had a 42% smaller adipocyte area compared with ob/ob adipocytes; however, obR adipocytes were still 49% greater compared with WT adipocytes [Fig. 2(b)]. This represents a 69% rescue of the ob/ob phenotype. Notably, adipocytes from the ectopic MEF fat pad in obR mice were statistically similar in size to epididymal WAT of WT mice [Fig. 2(b)]. When examining WAT depots we found that obR mice had 86% lower inguinal (subcutaneous) [Fig. 2(c)] and 66% lower epididymal (visceral) WAT [Fig. 2(d)] adipose mass compared with ob/ob mice, equal to rescues of 92% and 81% of the ob/ob phenotype, respectively. MEF fat pads (0.55 ± 0.13 g) made up 1.6% of obR BW. Remarkably, obR mice had statistically similar amounts of inguinal and epididymal WAT relative to WT mice.
Figure 1.
Decreased BW and adipose mass in Ob/Ob rescue mice. (a) Leptin levels in plasma of mice taken at terminal necropsy. (b) Body composition, determined by EchoMRI. Data represented as mean ± SD (n = 4 to 5). Significance determined by one-way ANOVA. *P < 0.05; **P < 0.01; ****P < 0.0001.
Figure 2.
Adipose mass and size decreased in Ob/Ob rescue mice. (a) Representative H&E staining performed on epididymal WAT and MEF fat pad taken at necropsy (20× objective; scale bar, 100 μm). (b) Quantification of adipocyte area from H&E-stained sections of epididymal WAT and MEF fat pad. Terminal weights of (c) subcutaneous inguinal and (d) visceral epididymal WAT. Data represented as mean ± SD (n = 4 to 5). Significance determined by one-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
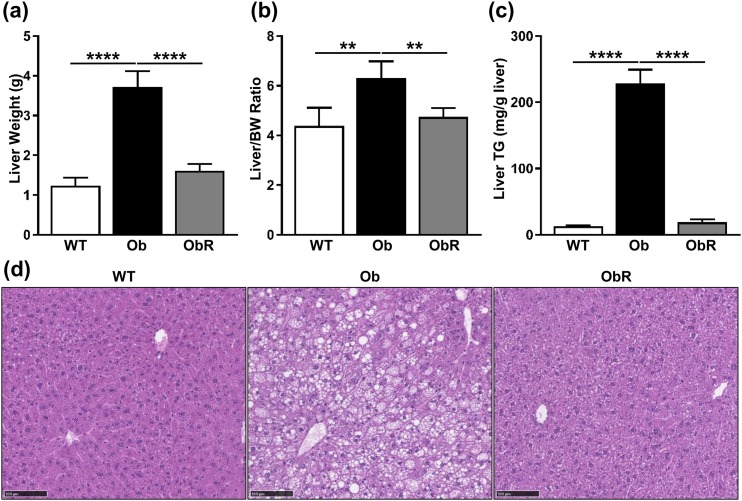
Because transplantation of MEFs clearly reduced obesity due to leptin deficiency, we next investigated the effect of MEFs on hepatic steatosis in leptin deficiency. First, we saw that obR mice had a dramatic 57% lower liver weight [Fig. 3(a)] and 25% lower liver/BW ratio when compared with ob/ob mice [Fig. 3(b)], representing rescues of 85% and 81%, respectively. Next, liver TGs were determined biochemically, and we saw that ob/ob mice had ~12-fold higher liver TGs compared with obR liver TGs, which were similar to those of WT mice [Fig. 3(c)] and resulted in a rescue of 97% of the ob/ob phenotype. These results were confirmed histologically, as evidenced by fewer lipid droplets in liver sections of obR mice compared with ob/ob mice, indicating decreased steatosis [Fig. 3(d)].
Figure 3.
Hepatomegaly and steatosis diminished in Ob/Ob rescue mice. Terminal measurements of (a) total liver mass, (b) liver/BW ratio, and (c) liver TGs. (d) Representative H&E staining performed on liver sections (20× objective; scale bar, 100 μm). Data represented as mean ± SD (n = 4 to 5). Significance determined by one-way ANOVA. **P < 0.01; ****P < 0.0001.
Glucose tolerance and insulin sensitivity restored by MEF transplantation
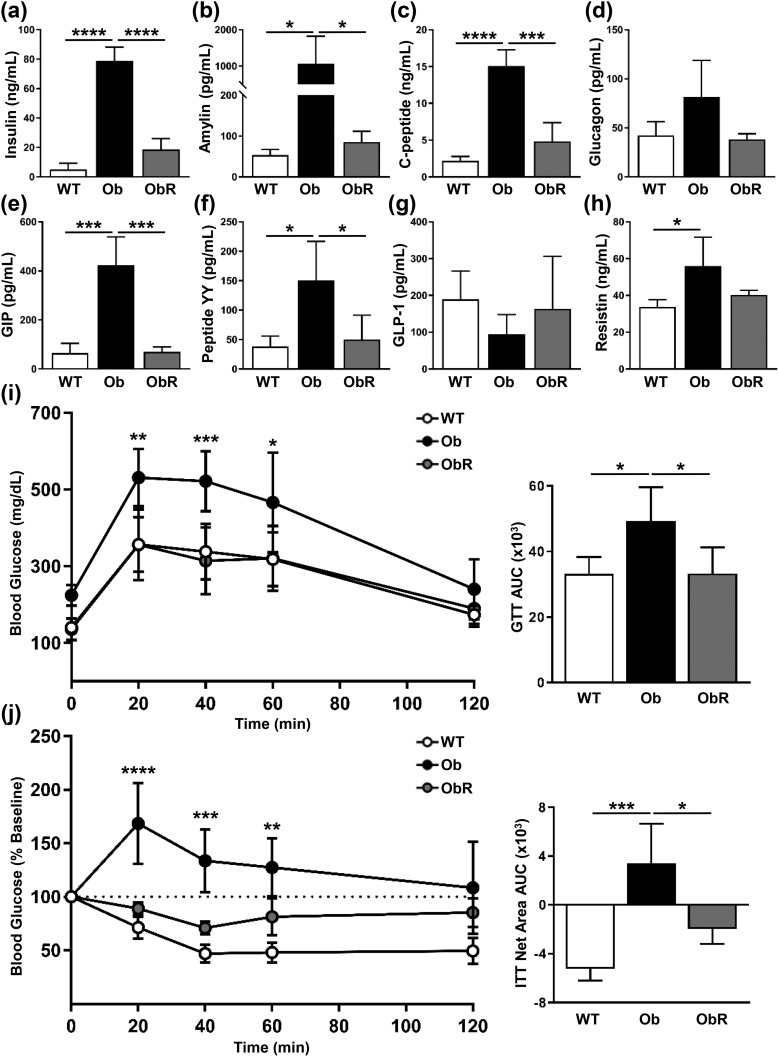
Next, we examined the effect of MEF transplantation on glucose tolerance and insulin sensitivity in leptin-deficient mice. We found that obR mice had fasting plasma levels of insulin 76% lower than those of ob/ob mice [Fig. 4(a)], which matches a rescue value of 82%. In addition, we saw improvements in other hormones associated with insulin resistance and obesity, including amylin, C-peptide, and GIP, and a trend toward decreased resistin [Fig. 4(b)–4(h)]. A GTT was performed and revealed that obR mice had better glucose handling than leptin-deficient mice, with a 32% reduction in area under the curve (AUC) [Fig. 4(i)], consistent with a 100% rescue. Furthermore, obR mice had greater insulin sensitivity than ob/ob mice, as evidenced by ITT, where obR mice had a substantially lower AUC than ob/ob mice [Fig. 4(j)], with a rescue value of 62%. Importantly, obR mice had similar values to WT mice at all time points for the GTT and ITT, and similar AUC values.
Figure 4.
Glucose and insulin tolerance improved in Ob/Ob rescue mice. Measurements of plasma (a) insulin, (b) amylin, (c) C-peptide, (d) glucagon, (e) GIP, (f) peptide yy, (g) glucagon-like peptide-1 (GLP-1), and (h) resistin from blood taken at terminal necropsy. (i) GTT performed after IP injection of glucose (2 mg/kg lean weight), with calculation of area under the curve (AUC) (right). (j) ITT performed after IP injection of insulin (1 U/kg lean weight) and expressed as percentage change from baseline, with calculation of AUC net area from baseline (right). Data represented as mean ± SD (n = 4 to 5). Significance determined by either two-way (GTT and ITT) or one-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Increased energy expenditure and decreased food intake in obR mice
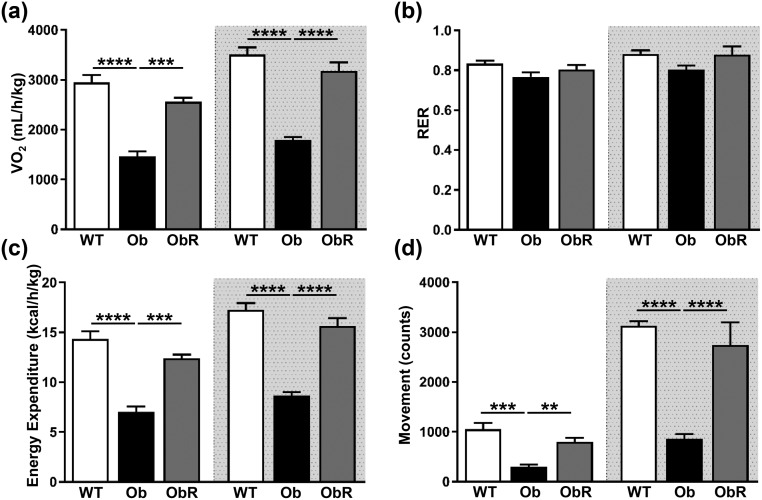
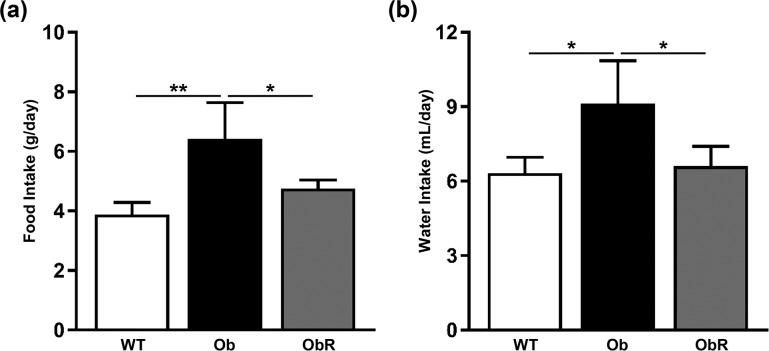
To explore mechanisms that could explain the improvements in metabolic endpoints in obR mice, we placed mice in metabolic chambers. Oxygen consumption was increased by 75% to 77% (as the mean of light and dark cycle measurements) in obR relative to ob/ob mice [Fig. 5(a)], which correspond to rescues of 74% and 81%, respectively. There was no change in the source of energy burned (i.e., carbohydrate vs fat), as evidenced by a similar respiratory exchange ratio between all groups [Fig. 5(b)]. As expected, obR mice had greater energy expenditure than ob/ob mice, with a 76% to 80% rise during light and dark cycles [Fig. 5(c)], which reflects rescues of 73% and 81%, respectively. Lastly, obR mice movement was 2.7 and 3.2 times higher than that of ob/ob mice in the light and dark cycle, consistent with rescues of 67% and 83%, respectively [Fig. 5(d)]. In addition, obR mice had a 26% lower food intake [Fig. 6(a)] and 27% lower water intake [Fig. 6(b)] in obR compared with ob/ob mice, corresponding to a 66% and 87% rescue, respectively.
Figure 5.
Ob/Ob rescue mice are more metabolically active. At ~12 weeks of age, mice were placed in metabolic chambers for indirect calorimetry. (a) Oxygen consumption, (b) respiratory exchange ratio (RER), (c) energy expenditure, and (d) movement. Data represented as the average per 12 hours and expressed as mean ± SEM (n = 4 to 5). Significance determined by one-way ANOVA. **P < 0.01; ***P < 0.001; ****P < 0.0001.
Figure 6.
Food intake decreased in rescue mice. Intake of (a) food and (b) water was measured daily for 1 week and averaged for each mouse. Data are expressed as group mean ± SEM (n = 4 to 5). Significance determined by one-way ANOVA. *P < 0.05; **P < 0.01.
MEF transplantation prevents metabolic defects in ob/ob mice in the long term
Next, to determine whether MEF transplantation could sustain protection in leptin-deficient mice, we characterized mice ~7 months after MEF transplantation rather than after only 10 weeks, as performed in the first cohort. Again, we observed that obR mice had a measurable amount of plasma leptin, yet this was only ~13% of that found in ob/+ mice [Fig. 7(a)]. obR mice had substantially lower BW than ob/ob mice, chiefly because of a 72% decrease in adipose tissue mass, corresponding to a rescue of 98%, with no difference in lean mass [Fig. 7(b)]. MEF fat pads (0.79 ± 0.21 g) made up 2.2% of obR BW. Furthermore, liver weight was 65% lower in obR mice than in ob/ob mice, equivalent to a rescue of 93% [Fig. 7(c)]. Although hyperglycemia is largely absent in ob/ob mice of this age, we did observe a decrease in plasma insulin by 98% in obR relative to ob/ob [Fig. 7(d)]. Lastly, MEF transplantation into leptin-deficient ob/ob mice also restored fertility. Four male obR mice, at 3 to 4 months of age, were bred with WT females, resulting in litters within 1 month in all four cages. In addition, 3- to 4-month-old obR females produced litters when bred with WT male mice within a month of breeding.
Figure 7.
MEF transplants sustain protection over the long term. (a) At ~7 months of age, plasma leptin levels measured from blood taken from mice at terminal necropsy. (b) Body composition determined by EchoMRI. (c) Liver weight and (d) plasma insulin levels measured from blood taken from mice at terminal necropsy. Data represented as mean ± SD (n = 3 to 4). Significance determined by one-way ANOVA. **P < 0.01; ****P < 0.0001.
Rescue of obese mice with MEF injection
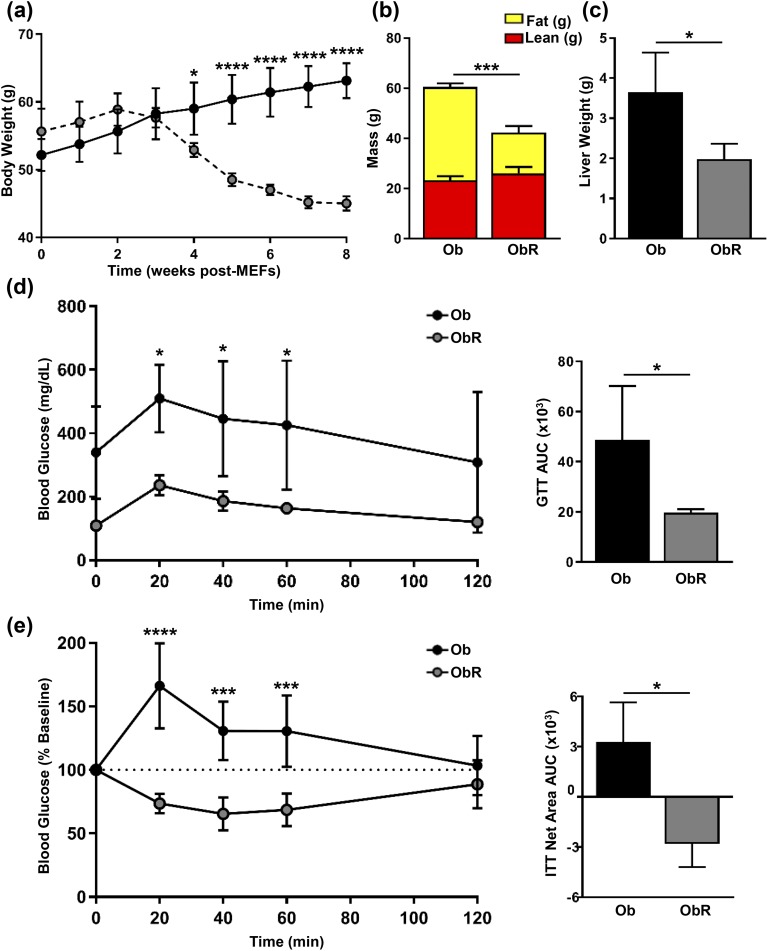
Because our previous experiments demonstrated that MEF implants prevent the onset of obesity and other metabolic consequences associated with leptin deficiency in ob/ob mice, we next investigated whether the injection of MEFs could rescue ob/ob mice that were already morbidly obese. We injected MEFs into 12-week-old ob/ob mice that were obese and hyperglycemic. Both ob/ob and obR mice continued to gain BW in the first 2 weeks, yet the BW in obR mice peaked at 2 weeks, then declined, reaching statistical significance 4 weeks after MEF implantation [Fig. 8(a)]. After a total of 8 weeks after MEFs, obR mice had a 30% lower BW than ob/ob mice [Fig. 8(a)]. Additionally, fat mass in obR mice was decreased by 2.3-fold relative to ob/ob mice, and there was no significant difference in lean mass [Fig. 8(b)]. MEF fat pads (1.0 ± 0.07 g) made up ~2.4% of obR BW. As with our previous experiments, we found that obR liver weight was 46% less than that in ob/ob mice [Fig. 8(c)]. Glucose tolerance was also improved in obR mice, with an 2.5-fold lower AUC compared with ob/ob mice [Fig. 8(d)]. Lastly, insulin sensitivity was also improved in obR mice, as evidenced by an ITT AUC that was much lower than that of ob/ob mice [Fig. 8(e)].
Figure 8.
Obese ob/ob mice are rescued with MEF implantation. At 12 weeks of age ob/ob mice were treated with or without subcutaneous injection of WT MEFs. (a) BWs were determined weekly and represented as weeks after MEF implantation. (b) After 8 weeks post-MEFs, body composition determined by EchoMRI. (c) Liver weight measured at necropsy. (d) GTT performed after IP injection of glucose (1 mg/kg BW), with calculation of AUC (right). (e) ITT performed after IP injection of insulin (1 U/kg BW) and expressed as percentage change from baseline, with calculation of AUC net area from baseline (right). Data represented as mean ± SD (n = 4). Significance determined by either two-way (GTT and ITT) or one-way ANOVA. *P < 0.05; ***P < 0.001; ****P < 0.0001.
Discussion
Leptin is produced by WAT and plays a critical role in the regulation of nutritional status, body composition, metabolism, and reproduction. Leptin deficiency manifests itself as severe obesity, hepatic steatosis, and insulin resistance in humans and rodent models. This condition can be successfully treated with the use of recombinant leptin injections (15, 23). In this work, we aimed to determine whether leptin production by WT MEF-derived fat pads is capable of preventing and rescuing the metabolic derangements observed in leptin-deficient ob/ob mice. Our results confirm that MEFs injected subcutaneously into ob/ob mice develop into fat pads that are histologically similar to epididymal WAT and are capable of producing measurable amounts of leptin in the plasma. Importantly, before the onset of obesity, obR mice were protected from fat mass accumulation and hepatic steatosis. These results correlated with increased energy expenditure and decreased food intake as well as substantial improvement in glucose tolerance and insulin sensitivity. The protection from obesity was sustained in the long term, as phenotyping in a separate cohort of mice at 7 months yielded similar results. In our final assessment, we determined that obese ob/ob mice injected with MEFs later in life (at 12 weeks of age) demonstrated loss of fat mass, as well as a resolution of hepatic steatosis and improvements in glucose homeostasis. Our study demonstrates a role of in vivo development of WT MEF-derived fat pads in preventing and rescuing the development and progression of long-term metabolic disease in the leptin-deficient ob/ob mouse model.
A plausible explanation for the effects seen in obR mice is that WT MEF-derived fat pads resulted in sufficient production of functional leptin to compensate for the lack of leptin in the ob/ob mouse, thereby ameliorating the metabolic derangements observed in the model. Although one could argue that other adipokines produced by the fat pad could be contributing to the improvement in metabolic parameters, this possibility seems less likely because ob/ob mice are only deficient in leptin and retain their sensitivity to leptin signaling. The possibility of leptin as a driving factor in obR protection is further substantiated by past studies in lipodystrophic mice. Lipodystrophy, a condition characterized by the degenerative loss of adipose tissue, leads to severely low leptin levels and development of insulin resistance and hepatic steatosis; however, it can be effectively treated with leptin replacement therapies (36, 37). Earlier work in a mouse model of lipodystrophy showed that transplantation of WAT from WT mice remedies metabolic complications in lipodystrophic mice (38). Conversely, adipose transplanted from leptin-deficient ob/ob mice has no effect (39). Remarkably, leptin levels observed in our obR mice were only 5% to 15% of baseline levels seen in control mice. Previous studies have shown that plasma leptin as low as 0.5 ng/mL (relative to background) is associated with decreased BW and increased satiety (40), which is similar to what we observed in our studies. Unexpectedly, younger WT mice had leptin levels approximately six times higher [Fig. 1(a)] than those of 7-month-old ob/+ mice [Fig. 7(a)]. Although we cannot fully explain the variation, it could be caused by heterozygosity for the leptin allele. Previous evidence has suggested that ob/+ mice produce less leptin protein per mass of body fat than normal +/+ mice (41).
Our studies suggest that leptin is acting in obR mice by promoting satiety and stimulating increased energy expenditure. Leptin achieves these actions primarily at the level of the central nervous system and is a key regulator in food intake in humans and mouse models (42, 43). Evidence has shown that decreased food intake is only partially responsible for the effects of leptin therapy in improving ob/ob mice, and increased energy expenditure in obR mice probably plays a large role as leptin administration leads to activation of sympathetic input and increased catecholamine production (44–45). Although satiety and increased energy expenditure are important driving factors in restoring metabolic homeostasis in obR mice, we cannot exclude the effect of leptin in peripheral tissues, and additional studies are needed to determine the exact role this effect plays in improving metabolic regulation in obR mice. The leptin receptor is expressed in multiple peripheral tissues, and leptin has been shown to have beneficial effects on glucose homeostasis (47–50). In adipocytes and WAT ex vivo, lipolysis is increased upon leptin administration, in addition to an increase of glucose utilization by brown adipose (51). Studies have also shown that fatty acid oxidation is increased in skeletal muscle, and insulin sensitivity is improved in the liver (52, 53). Furthermore, leptin has been found to decrease insulin transcription and secretion in pancreatic islet cells (54–56).
In this report, we demonstrate a simple, effective, and sustainable method of resolving the phenotypic effects of leptin deficiency in the ob/ob mouse model. Leptin gene therapy via adenoviral transduction has been attempted in ob/ob mice, yet success was limited by low expression levels, rodent death after therapy, and lack of sustained improvement in phenotype (25, 57). In our study, all mice treated with MEFs survived and were protected from obesity for up to 7 months. Additionally, compared with adenoviral leptin production, daily treatment with recombinant leptin achieved a far greater decrease in BW over time (25), which showed a similar trend to what we observed in our obR mice 3 weeks after MEF implantation (Fig. 8). Interestingly, weight loss occurred between 2 and 3 weeks after transplantation into adult ob/ob mice, and this weight loss coincided with in vitro differentiation of MEFs into adipocytes, which takes ~2 weeks with an adipogenic cocktail (30). Implantation of gut-derived cells, engineered with a mifepristone-inducible leptin expression construct, has also been used to help improve metabolic effects in ob/ob mice. Although these mice demonstrated a decrease in BW and food intake, the observed effect depended on mifepristone treatment, and animals regained fat mass and food intake after therapy cessation (58). These interpretations are further complicated by the use of mifepristone, which is a glucocorticoid receptor antagonist, and glucocorticoid levels can be associated with the development of metabolic syndrome (59). Another group attempted to rescue ob/ob mice with encapsulated, leptin-overexpressing 3T3-L1 cells. Although improvements were noted in glucose tolerance and insulin sensitivity, adipose tissue formation was not documented, and ob/ob mice were not protected from obesity or hyperphagia (27). These findings are in contrast to our study and may be related to inefficient fat pad formation in the 3T3-L1 model.
Our work shows that leptin-deficient ob/ob mice provide an adequate host environment for MEF-derived fat pad development in vivo. Our findings differ from those of previous studies that used diet-induced obesity (DIO) models and showed that DIO mice were not suitable hosts for adipogenesis when injected with purified preadipocytes (60). A likely explanation for this finding is that DIO mice tend to be leptin resistant, whereas ob/ob mice retain sensitivity to leptin signaling. It is also worth considering that ob/ob mice are hyperinsulinemic, providing another possible requirement for fat pad development. Hyperinsulinemia is also present in lipodystrophy, and several studies have shown that fat pads are capable of developing in these settings (31, 60, 61). Insulin has been shown to play an important role in the development of adipocytes, and it is possible that high insulin levels are necessary for the development of fat pads in vivo in the setting of obesity. Although hyperinsulinemia is present in all the previously mentioned models, earlier evidence has shown that ob/ob and lipodystrophic mice have insulin levels that are many times greater than those of DIO mice (62–65). Additionally, ob/ob and lipodystrophic mice are characterized by elevations in circulating glucocorticoids (65–68), known facilitators of adipogenesis. Although DIO mice display hyperinsulinemia, it is possible that the combination of elevated glucocorticoids and insulin seen in ob/ob and lipodystrophic mice provides an opportunistic niche that hyperinsulinemia itself does not provide.
Other groups have used a purified cell population subset isolated by flow cytometry based on the expression or lack of expression of certain cell surface markers (60, 69, 70). In contrast, our study used unpurified fibroblasts from E14 mouse embryos, which sufficiently formed WAT in vivo. Alternatively, adipose tissue transplants have also been used to counteract the phenotype of ob/ob mice. Ashwell et al. (71) successfully transplanted fat tissue from C57BL/6 into ob/ob mice; however, the metabolic effects of the fat transplants in obese mice were not examined. Work in lipodystrophic mice has revealed that the amount of fat transplanted is proportional to the amount of leptin secretion (38). Later studies found that transplantation of WAT from C57BL/6 mice into ob/ob mice resulted in increased plasma leptin and reductions in BW, food intake, and hyperinsulinemia. Unfortunately, this effect required transplants from four to eight donors, requiring ≤30 injections (26). Notably, WT MEF-derived fat pads were the result of a single injection, and fat pad mass was ~0.5 to 1 g in size, which was sufficient for ameliorating effects on BW and was sustained over time.
Finally, it is important to mention the practical implications of our work in relation to the restoration of fertility in ob/ob mice. Under normal circumstances, obtaining sufficient ob/ob homozygous progeny is expensive and time-consuming because successful breeding occurs only with heterozygous animals. Treatment with recombinant leptin is sufficient to restore fertility in ob/ob mice, yet this requires daily injections (72). Recent reports indicate that adipose transplants effectively restore fertility in ob/ob mice and improve the generation of homozygous offspring, but this procedure requires skilled survival surgery and up to eight transplantations (73). In contrast, our method successfully restores fertility to ob/ob mice after a single injection of MEFs in mice under light sedation. Furthermore, a single pregnant dam can provide embryos for MEFs to sufficiently rescue up to eight ob/ob mice.
Collectively, our studies show that fat pads derived from WT MEFs can ameliorate the negative effects on whole-body energy homeostasis and infertility in leptin-deficient ob/ob mice. Importantly, MEF-derived fat pads are capable of sustaining benefits in the long term and may be applied to other types of leptin deficiency, including lipodystrophy. Future studies may provide a better understanding of in vivo fat development and aid in the discovery of treatments for metabolic diseases. Our findings suggest the possibility of future cell-based therapies for patients with leptin deficiency, lipodystrophy, or other mutations in adipose-specific genes.
Acknowledgments
Financial Support: This work was funded by National Institutes of Health (NIH) Grant DK106083 (to C.A.H.) and T32 Grant DK007120 (to D.F.). Imaging work was supported by the Alafi Neuroimaging Laboratory, the Hope Center for Neurological Disorders, and NIH Shared Instrumentation Grant S10 RR 0227552 to Washington University. Metabolic studies were supported by Diabetes Research Center Grant P30 DK020579.
Author Contributions: D.F. designed and performed the experiments, interpreted data, assembled the figures, and drafted the manuscript. M.B. performed initial experiments, interpreted data, assembled figures, and prepared the manuscript. I.H. and Y.D. assisted with performing experiments. C.A.H. conceived of the study, performed initial experiments, and directed the research. The manuscript was reviewed and approved by all authors.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AUC
area under the curve
- BW
body weight
- CVD
cardiovascular disease
- DIO
diet-induced obesity
- GIP
gastric inhibitory polypeptide
- GTT
glucose tolerance test
- H&E
hematoxylin and eosin
- ITT
insulin tolerance test
- MEF
mouse embryonic fibroblast
- obR
ob/ob mouse receiving mouse embryonic fibroblasts
- TG
triglyceride
- WAT
white adipose tissue
- WT
wild-type
References
- 1. Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313(19):1973–1974. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2017 update: a report from the American Heart Association [published corrections appear in Circulation. 2017;135:e646 and Circulation. 2017;136:e196] Circulation. 2017;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cutler DM, Glaeser EL, Shapiro JM. Why have Americans become more obese? J Econ Perspect. 2003;17(3):93–118. [Google Scholar]
- 4. Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring). 2008;16(10):2323–2330. [DOI] [PubMed] [Google Scholar]
- 5. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. [DOI] [PubMed] [Google Scholar]
- 6. Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, Yoshimasa Y, Tanaka I, Mori T, Nakao K. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997;3(9):1029–1033. [DOI] [PubMed] [Google Scholar]
- 7. Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140(12):5995–5998. [DOI] [PubMed] [Google Scholar]
- 8. Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393(6686):684–688. [DOI] [PubMed] [Google Scholar]
- 9. Odle AK, Haney A, Allensworth-James M, Akhter N, Childs GV. Adipocyte versus pituitary leptin in the regulation of pituitary hormones: somatotropes develop normally in the absence of circulating leptin. Endocrinology. 2014;155(11):4316–4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–295. [DOI] [PubMed] [Google Scholar]
- 11. Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155–1161. [DOI] [PubMed] [Google Scholar]
- 12. Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1(12):1311–1314. [DOI] [PubMed] [Google Scholar]
- 13. Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O’Rahilly S. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903–908. [DOI] [PubMed] [Google Scholar]
- 14. Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet. 1998;18(3):213–215. [DOI] [PubMed] [Google Scholar]
- 15. Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341(12):879–884. [DOI] [PubMed] [Google Scholar]
- 16. Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41(12):317–318. [DOI] [PubMed] [Google Scholar]
- 17. Hems DA, Rath EA, Verrinder TR. Fatty acid synthesis in liver and adipose tissue of normal and genetically obese (ob/ob) mice during the 24-hour cycle. Biochem J. 1975;150(2):167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flatt PR, Bailey CJ, Kwasowski P, Swanston-Flatt SK, Marks V. Abnormalities of GIP in spontaneous syndromes of obesity and diabetes in mice. Diabetes. 1983;32(5):433–435. [DOI] [PubMed] [Google Scholar]
- 19. Flatt PR, Bailey CJ, Kwasowski P, Page T, Marks V. Plasma immunoreactive gastric inhibitory polypeptide in obese hyperglycaemic (ob/ob) mice. J Endocrinol. 1984;101(3):249–256. [DOI] [PubMed] [Google Scholar]
- 20. Flatt PR, Bailey CJ. Development of glucose intolerance and impaired plasma insulin response to glucose in obese hyperglycaemic (ob/ob) mice. Horm Metab Res. 1981;13(10):556–560. [DOI] [PubMed] [Google Scholar]
- 21. Weigle DS, Bukowski TR, Foster DC, Holderman S, Kramer JM, Lasser G, Lofton-Day CE, Prunkard DE, Raymond C, Kuijper JL. Recombinant ob protein reduces feeding and body weight in the ob/ob mouse. J Clin Invest. 1995;96(4):2065–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137(7):3144–3147. [DOI] [PubMed] [Google Scholar]
- 23. Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540–543. [DOI] [PubMed] [Google Scholar]
- 24. Baldo BA. Side effects of cytokines approved for therapy. Drug Saf. 2014;37(11):921–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morsy MA, Gu MC, Zhao JZ, Holder DJ, Rogers IT, Pouch WJ, Motzel SL, Klein HJ, Gupta SK, Liang X, Tota MR, Rosenblum CI, Caskey CT. Leptin gene therapy and daily protein administration: a comparative study in the ob/ob mouse. Gene Ther. 1998;5(1):8–18. [DOI] [PubMed] [Google Scholar]
- 26. Klebanov S, Astle CM, DeSimone O, Ablamunits V, Harrison DE. Adipose tissue transplantation protects ob/ob mice from obesity, normalizes insulin sensitivity and restores fertility. J Endocrinol. 2005;186(1):203–211. [DOI] [PubMed] [Google Scholar]
- 27. DiSilvestro DJ, Melgar-Bermudez E, Yasmeen R, Fadda P, Lee LJ, Kalyanasundaram A, Gilor CL, Ziouzenkova O. Leptin production by encapsulated adipocytes increases brown fat, decreases resistin, and improves glucose intolerance in obese mice. PLoS One. 2016;11(4):e0153198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mandrup S, Loftus TM, MacDougald OA, Kuhajda FP, Lane MD. Obese gene expression at in vivo levels by fat pads derived from s.c. implanted 3T3-F442A preadipocytes. Proc Natl Acad Sci USA. 1997;94(9):4300–4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Research Council Guide for the Care and Use of Laboratory Animals.8th ed. Washington, DC: National Academies Press; 2011.
- 30. Harris CA, Haas JT, Streeper RS, Stone SJ, Kumari M, Yang K, Han X, Brownell N, Gross RW, Zechner R, Farese RV Jr. DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. J Lipid Res. 2011;52(4):657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bauerle KT, Hutson I, Scheller EL, Harris CA. Glucocorticoid receptor signaling is not required for in vivo adipogenesis. Endocrinology. 2018;159(5):2050–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bose SK, Hutson I, Harris CA. Hepatic glucocorticoid receptor plays a greater role than adipose GR in metabolic syndrome despite renal compensation. Endocrinology. 2016;157(12):4943–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Galarraga M, Campión J, Muñoz-Barrutia A, Boqué N, Moreno H, Martínez JA, Milagro F, Ortiz-de-Solórzano C. Adiposoft: automated software for the analysis of white adipose tissue cellularity in histological sections. J Lipid Res. 2012;53(12):2791–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 35. Ferguson D, Zhang J, Davis MA, Helsley RN, Vedin LL, Lee RG, Crooke RM, Graham MJ, Allende DS, Parini P, Brown JM. The lipid droplet-associated protein perilipin 3 facilitates hepatitis C virus–driven hepatic steatosis. J Lipid Res. 2017;58(2):420–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moon HS, Dalamaga M, Kim SY, Polyzos SA, Hamnvik OP, Magkos F, Paruthi J, Mantzoros CS. Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr Rev. 2013;34(3):377–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109(10):1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, Vinson C, Eckhaus M, Reitman ML. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest. 2000;105(3):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Colombo C, Cutson JJ, Yamauchi T, Vinson C, Kadowaki T, Gavrilova O, Reitman ML. Transplantation of adipose tissue lacking leptin is unable to reverse the metabolic abnormalities associated with lipoatrophy. Diabetes. 2002;51(9):2727–2733. [DOI] [PubMed] [Google Scholar]
- 40. Murphy JE, Zhou S, Giese K, Williams LT, Escobedo JA, Dwarki VJ. Long-term correction of obesity and diabetes in genetically obese mice by a single intramuscular injection of recombinant adeno-associated virus encoding mouse leptin. Proc Natl Acad Sci USA. 1997;94(25):13921–13926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chung WK, Belfi K, Chua M, Wiley J, Mackintosh R, Nicolson M, Boozer CN, Leibel RL. Heterozygosity for Lep(ob) or Lep(rdb) affects body composition and leptin homeostasis in adult mice. Am J Physiol. 1998;274(4 Pt 2):R985–R990. [DOI] [PubMed] [Google Scholar]
- 42. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–770. [DOI] [PubMed] [Google Scholar]
- 43. Caron A, Lee S, Elmquist JK, Gautron L. Leptin and brain-adipose crosstalks. Nat Rev Neurosci. 2018;19(3):153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zeng W, Pirzgalska RM, Pereira MM, Kubasova N, Barateiro A, Seixas E, Lu YH, Kozlova A, Voss H, Martins GG, Friedman JM, Domingos AI. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell. 2015;163(1):84–94. [DOI] [PubMed] [Google Scholar]
- 45. Satoh N, Ogawa Y, Katsuura G, Numata Y, Tsuji T, Hayase M, Ebihara K, Masuzaki H, Hosoda K, Yoshimasa Y, Nakao K. Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretion. Diabetes. 1999;48(9):1787–1793. [DOI] [PubMed] [Google Scholar]
- 46. Levin N, Nelson C, Gurney A, Vandlen R, de Sauvage F. Decreased food intake does not completely account for adiposity reduction after ob protein infusion. Proc Natl Acad Sci USA. 1996;93(4):1726–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83(7):1263–1271. [DOI] [PubMed] [Google Scholar]
- 48. Lancha A, Frühbeck G, Gómez-Ambrosi J. Peripheral signalling involved in energy homeostasis control. Nutr Res Rev. 2012;25(2):223–248. [DOI] [PubMed] [Google Scholar]
- 49. De Matteis R, Dashtipour K, Ognibene A, Cinti S. Localization of leptin receptor splice variants in mouse peripheral tissues by immunohistochemistry. Proc Nutr Soc. 1998;57(3):441–448. [DOI] [PubMed] [Google Scholar]
- 50. Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26(11):1407–1433. [DOI] [PubMed] [Google Scholar]
- 51. Siegrist-Kaiser CA, Pauli V, Juge-Aubry CE, Boss O, Pernin A, Chin WW, Cusin I, Rohner-Jeanrenaud F, Burger AG, Zapf J, Meier CA. Direct effects of leptin on brown and white adipose tissue. J Clin Invest. 1997;100(11):2858–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lam NT, Lewis JT, Cheung AT, Luk CT, Tse J, Wang J, Bryer-Ash M, Kolls JK, Kieffer TJ. Leptin increases hepatic insulin sensitivity and protein tyrosine phosphatase 1B expression. Mol Endocrinol. 2004;18(6):1333–1345. [DOI] [PubMed] [Google Scholar]
- 53. Andreev VP, Paz-Filho G, Wong ML, Licinio J. Deconvolution of insulin secretion, insulin hepatic extraction post-hepatic delivery rates and sensitivity during 24-hour standardized meals: time course of glucose homeostasis in leptin replacement treatment. Horm Metab Res. 2009;41(2):142–151. [DOI] [PubMed] [Google Scholar]
- 54. Kulkarni RN, Wang ZL, Wang RM, Hurley JD, Smith DM, Ghatei MA, Withers DJ, Gardiner JV, Bailey CJ, Bloom SR. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J Clin Invest. 1997;100(11):2729–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Emilsson V, Liu YL, Cawthorne MA, Morton NM, Davenport M. Expression of the functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion. Diabetes. 1997;46(2):313–316. [DOI] [PubMed] [Google Scholar]
- 56. Seufert J, Kieffer TJ, Habener JF. Leptin inhibits insulin gene transcription and reverses hyperinsulinemia in leptin-deficient ob/ob mice. Proc Natl Acad Sci USA. 1999;96(2):674–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Muzzin P, Eisensmith RC, Copeland KC, Woo SL. Correction of obesity and diabetes in genetically obese mice by leptin gene therapy. Proc Natl Acad Sci USA. 1996;93(25):14804–14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Oosman SN, Lam AW, Harb G, Unniappan S, Lam NT, Webber T, Bruch D, Zhang QX, Korbutt GS, Kieffer TJ. Treatment of obesity and diabetes in mice by transplant of gut cells engineered to produce leptin. Mol Ther. 2008;16(6):1138–1145. [DOI] [PubMed] [Google Scholar]
- 59. Fleseriu M, Biller BMK, Findling JW, Molitch ME, Schteingart DE, Gross C; SEISMIC Study Investigators . Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J Clin Endocrinol Metab. 2012;97(6):2039–2049. [DOI] [PubMed] [Google Scholar]
- 60. Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135(2):240–249. [DOI] [PubMed] [Google Scholar]
- 61. Wu X, Hutson I, Akk AM, Mascharak S, Pham CTN, Hourcade DE, Brown R, Atkinson JP, Harris CA. Contribution of adipose-derived factor D/adipsin to complement alternative pathway activation: lessons from lipodystrophy. J Immunol. 2018;200(8):2786–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kang JS, Pilkington JD, Ferguson D, Kim HK, Romsos DR. Dietary glucose and fat attenuate effects of adrenalectomy on energy balance in ob/ob mice. J Nutr. 1992;122(4):895–905. [DOI] [PubMed] [Google Scholar]
- 63. Garthwaite TL, Martinson DR, Tseng LF, Hagen TC, Menahan LA. A longitudinal hormonal profile of the genetically obese mouse. Endocrinology. 1980;107(3):671–676. [DOI] [PubMed] [Google Scholar]
- 64. Colombo C, Haluzik M, Cutson JJ, Dietz KR, Marcus-Samuels B, Vinson C, Gavrilova O, Reitman ML. Opposite effects of background genotype on muscle and liver insulin sensitivity of lipoatrophic mice. Role of triglyceride clearance. J Biol Chem. 2003;278(6):3992–3999. [DOI] [PubMed] [Google Scholar]
- 65. Luque RM, Kineman RD. Impact of obesity on the growth hormone axis: evidence for a direct inhibitory effect of hyperinsulinemia on pituitary function. Endocrinology. 2006;147(6):2754–2763. [DOI] [PubMed] [Google Scholar]
- 66. Naeser P. Function of the adrenal cortex in obese-hyperglycemic mice (gene symbol ob). Diabetologia. 1974;10(5):449–453. [DOI] [PubMed] [Google Scholar]
- 67. Haluzik M, Dietz KR, Kim JK, Marcus-Samuels B, Shulman GI, Gavrilova O, Reitman ML. Adrenalectomy improves diabetes in A-ZIP/F-1 lipoatrophic mice by increasing both liver and muscle insulin sensitivity. Diabetes. 2002;51(7):2113–2118. [DOI] [PubMed] [Google Scholar]
- 68. Altirriba J, Poher AL, Caillon A, Arsenijevic D, Veyrat-Durebex C, Lyautey J, Dulloo A, Rohner-Jeanrenaud F. Divergent effects of oxytocin treatment of obese diabetic mice on adiposity and diabetes. Endocrinology. 2014;155(11):4189–4201. [DOI] [PubMed] [Google Scholar]
- 69. Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322(5901):583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Joe AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27(10):2563–2570. [DOI] [PubMed] [Google Scholar]
- 71. Ashwell M, Meade CJ, Medawar P, Sowter C. Adipose tissue: contributions of nature and nurture to the obesity of an obese mutant mouse (ob/ob). Proc R Soc Lond B Biol Sci. 1977;195(1120):343–353. [DOI] [PubMed] [Google Scholar]
- 72. Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology. 1997;138(3):1190–1193. [DOI] [PubMed] [Google Scholar]
- 73. Barros CC, Almeida SS, Mori MA, Valero VB, Haro AS, Batista EC, Rosa TS, Bacurau RF, Würtele M, Araújo RC. Efficient method for obtaining Lep(ob)/Lep(ob)-derived animal models using adipose tissue transplantations. Int J Obes. 2009;33(8):938–944. [DOI] [PubMed] [Google Scholar]