Abstract
The neuropeptide galanin has been shown to suppress epileptic seizures. In cortical and hippocampal areas, galanin is normally mainly expressed in noradrenergic afferents. We have generated a mouse overexpressing galanin in neurons under the platelet-derived growth factor B promoter. RIA and HPLC analysis revealed up to 8-fold higher levels of galanin in transgenic as compared with wild-type mice. Ectopic galanin overexpression was detected especially in dentate granule cells and hippocampal and cortical pyramidal neurons. Galanin-overexpressing mice showed retardation of seizure generalization during hippocampal kindling, a model for human complex partial epilepsy. The high levels of galanin in mossy fibers found in the transgenic mice were further increased after seizures. Frequency facilitation of field excitatory postsynaptic potentials, a form of short-term synaptic plasticity assessed in hippocampal slices, was reduced in mossy fiber–CA3 cell synapses of galanin-overexpressing mice, indicating suppressed glutamate release. This effect was reversed by application of the putative galanin receptor antagonist M35. These data provide evidence that ectopically overexpressed galanin can be released and dampen the development of epilepsy by means of receptor-mediated action, at least partly by reducing glutamate release from mossy fibers.
Galanin, a 29-aa neuropeptide (1), is widely distributed throughout the rat brain (2, 3) and coexists with classic transmitters in several systems (4), with particularly high levels in noradrenergic locus coeruleus neurons (5, 6). Galanin-binding sites have been mapped (7, 8), and three receptor subtypes, GalR1 (9–11), GalR2 (12–14, 49), and GalR3 (15), have been cloned. There is evidence for involvement of galanin in multiple neuronal functions (16, 17), as also revealed in recent studies on transgenic mice (18–20).
Many studies support an important role of neuropeptides in seizure activity (21–23). This role is true also for galanin. Thus, intrahippocampal injection of galanin during status epilepticus (SE) in rats has powerful anticonvulsant effects (24). In accordance with these data, mice overexpressing the galanin gene under the dopamine-β-hydroxylase promoter, presumably in noradrenergic fibers, show increased resistance to SE and kainate- and pentylenetetrazol-evoked seizures (25). Conversely, administration of galanin antagonists, such as M35 (26), and targeted disruption of the galanin gene increase seizure susceptibility in these models (24, 25). Taken together, available data indicate that galanin released from noradrenergic fibers (3, 5, 27) can suppress epileptic seizures. Although the underlying mechanisms are not known, it has been shown that galanin can inhibit glutamate release by means of activation of presynaptic ATP-dependent K+ channels (28).
We speculated that galanin release from neurons not normally containing this neuropeptide, e.g., in the epileptic focus, might be necessary to optimize the seizure-suppressant action of galanin. To explore the functional consequences of its ectopic localization, we generated a mouse with the aim to overexpress galanin in widespread areas of the nervous system by using the platelet-derived growth factor B (PDGF-B) promoter. Here we report on the cellular distribution of galanin in this transgenic, galanin-overexpressing (GalOE) mouse. We sought to determine whether ectopically expressed galanin can suppress the development of epilepsy, as well as to explore the possible underlying electrophysiological mechanisms. We used kindling, an animal model for complex partial seizures with secondary generalization, the most common type of human epilepsy (29, 30). In kindling, repetitive daily electrical stimulations of limbic forebrain areas result in generalization of initially focal seizures.
Materials and Methods
Generation and Genotyping of GalOE Mice.
A galanin/galanin message-associated peptide (GMAP) gene construct, including the second endogenous intron of the galanin gene, using a 1.3-kb PDGF-B promoter (31), was injected into pronuclei from fertilized mouse oocytes (32) from a cross between F1 (C57BL/6 × CBA) mice.‡‡ Mouse-tail biopsies were used for genotyping and DNA was amplified by using PCR with specific primers for galanin 5′-TGCCTCCCTAGAGTCGACGAGGGATCCTCGTGCGCT-3′ and 5′-AGGCATCCCAAGTCCCAGAGTGGCTGA-3′ and Taq DNA polymerase (2.5 units; Sigma); incubations were at 95°C for 45 s, at 54°C for 1 min, and at 72°C for 1 min for 30 cycles, finally, at 72°C for 10 min. The PCR product showed a 445-bp wild-type (WT) band or a 248-bp GalOE band.
Tissue Extractions.
Tissues from frontal, parietal, and piriform cortices and from hippocampus were cut into small pieces on ice, and 1 M acetic acid (Merck) (10 ml/g of tissue) was added, and then the sample boiled for 10 min. Samples were homogenized (Scientific Industries, New York) and centrifuged at 1,500 × g for 10 min at 8°C. Supernatants were collected, and a second extraction was performed with 10 ml of distilled water per 1 g of tissue. The combined supernatants were lyophilized and stored at −70°C until analysis.
RIA.
Lyophilized samples were reconstituted in 1 ml of phosphate buffer (0.05 M, pH 7.4), and 100 μl of each sample, antibody, and standard were used. Galanin content was measured by using a rat galanin antiserum (33). HPLC-purified (HPLC pump 3500, Amersham Pharmacia) 125I-labeled rat galanin prepared with chloramine-T was used as radioligand, and rat galanin was used as standard (Neosystem, Strasbourg, France). Samples and standards were measured on a GammaMaster 1277 (LKB Wallac, Gaithersburg, MD).
Protein concentration was measured by using the Lowry method with Folin phenol reagent (34) and optimized for samples with low concentrations of protein (E.T., unpublished). As standards, 30 μl of the calibrator (BSA, Sigma), controls, samples, and blanks were incubated with 150 μl of reagent containing alkaline copper sulfate solution (Merck) for exactly 10 min, and then 20 μl per sample Folin phenol reagent was added. After 30-min incubation, samples were placed in equal volumes on microtiter plates and analyzed at 690 nm on a Vmax photometer (Molecular Devices).
HPLC.
Reverse-phase HPLC was performed by using LKB Ultrapac TSK ODS-120T (5 μm, 4.6 × 250 mm), eluted with a 40-min linear gradient of acetonitrile (20–40%) in water containing 0.1% trifluoroacetic acid. Samples were passed through Millipore GS filters (0.22 μm) before chromatography. Samples of 200 μl were injected. Fractions (0.5 ml) were collected at an elution rate of 1.0 ml/min. Each fraction was lyophilized and redissolved in 100 μl of distilled water before analysis. The fractions were assayed for immunoreactivity in the tubes used for their collection.
Kindling.
Studies were performed according to National Institutes of Health guidelines and approved by the local Ethical Committee. Eight GalOE and seven WT male mice, weighing 22–25 g, were subjected to kindling stimulations. In addition, six GalOE and six WT animals served as electrode-implanted, nonstimulated controls. Animals were anesthetized with sodium pentobarbital (60 mg/kg i.p.) and mounted in a Kopf stereotaxic frame. Bipolar stainless-steel stimulating/recording electrodes were implanted into the left ventral hippocampus according to atlas of Franklin and Paxinos (35) (tooth bar at 0; 2.9 mm caudal to bregma; 3.0 mm lateral to midline; 3.0 mm ventral to dura). After 10 days of recovery, kindling stimulations (1 ms square pulses of 100 Hz for 1 s) were given once a day. On the first day, stimulation threshold was determined by increasing current intensity by 10 μA steps, starting from 10 μA, until focal epileptiform activity (afterdischarge, AD) of at least 5 s duration was elicited. The electroencephalogram was recorded on a MacLab system before, during, and at least 1 min after AD had ceased, and was stored on a Power PC computer for further analysis. Behavioral convulsions were scored according to the modified scale of Racine (36): grade 1, facial twitches; grade 2, chewing and nodding; grade 3, forelimb clonus; grade 4, rearing, body jerks, tail rising; grade 5, imbalance, hind-limb clonus, vocalization. Experimenter was unaware of the group identity of the individual animals when evaluating seizure grades. Four to six weeks after the mice had exhibited five grade 5 seizures, AD threshold was redetermined and mice were rekindled by using the same stimulation parameters as during initial kindling, until they had experienced three grade 5 seizures. Animals were killed 4 or 24 h after last rekindling stimulation for in situ hybridization or immunocytochemistry, respectively.
In Situ Hybridization.
A 48-mer oligonucleotide galanin probe (nucleotides 152–199; Life Technologies; ref. 37) was labeled with deoxyadenosine 5′-[γ-[35S]thio]triphosphate (New England Nuclear) at the 3′-end by using terminal deoxynucleotidyltransferase (Amersham Pharmacia) and purified by using QIAquick Nucleotide Removal Kit (Qiagen, Chatsworth, CA). The brains were rapidly taken out, frozen on dry ice, cut on a cryostat into 14-μm-thick sections, and mounted on Probe On slides (Fisher). Then, sections were air-dried overnight, incubated with the oligonucleotide (0.5 ng) for 16–18 h at 42°C, rinsed in 1× SSC (0.15 M sodium chloride, 0.015 M sodium citrate, pH 7), rapidly dehydrated, air-dried, and opposed to β-max x-ray films (Amersham Pharmacia) for 3 weeks. The films were developed, and resulting images were digitized by using a charge-coupled device camera (Sony) and image grabber software. Analysis of optical densities was made by image analysis program National Institute of Mental Health IMAGE 1.57 (Wayne Rasband, Bethesda, MD). Gray levels for standards were used in third-degree polynomial calibration to obtain equivalent values of tissue radioactivity (nCi/g). Subsequently, sections were dipped in liquid photo emulsion NTB2 (Kodak), developed in Kodak D19, fixed in Kodak 3000, counterstained with toluidine blue, examined in a Nikon Microphot-FX microscope equipped with a dark-field condenser and epipolarization illumination, and photographed with Kodak Tri-X black-and-white film.
Immunocytochemistry.
Animals were perfused by means of the ascending aorta with Tyrode's solution followed by 4% paraformaldehyde and 14% (wt/vol) saturated picric acid in 0.16 M sodium phosphate buffer, pH 7.0. The brains were taken out and postfixed for 2 h at 4°C, transferred to 0.01 M PBS containing 10% (wt/vol) sucrose and stored overnight at 4°C, cut on a cryostat (Microm) at 14-μm thickness and mounted on gelatin-coated slides. Sections were incubated overnight at 4°C with galanin antiserum (33) followed by secondary antibodies conjugated to FITC (Jackson ImmunoResearch) for 30 min at 37°C, rinsed in PBS, mounted in PBS/glycerol (1:10) containing 0.1% para-phenylenediamine, and analyzed by using a Nikon Microphot-FX microscope equipped with epifluorescence (Nikon) and appropriate filter combinations, and photographed on Kodak T-max black-and-white film (400 ASA).
Electrophysiology.
Under halothane anesthesia, eight GalOE and eight WT mice were decapitated, and their brains were removed and put into ice-cold artificial cerebrospinal fluid (aCSF) with the following composition: 119 mM NaCl, 2.5 mM KCl, 1.3 mM MgSO4, 2.5 mM CaCl2, 26.2 mM NaHCO3, 1 mM NaH2PO4 and 11 mM glucose, bubbled with a mixture of 95% O2 and 5% CO2. Hippocampi were dissected out and transverse slices (450 μm thick) were cut on a Vibratome (Ted Pella, Inc., Redding, CA). Slices were maintained at room temperature in a submerged chamber filled with gassed aCSF. For recordings, slices were transferred to a submerged chamber continuously perfused (at a speed of 3–4 ml/min) with gassed aCSF at room temperature. Stimulations were delivered at the dentate granule cell layer with alternating frequencies of 0.1 or 1 Hz (10 square pulses of 0.1-ms duration), and field excitatory postsynaptic potentials (fEPSPs) were recorded from the proximal part of the stratum lucidum of the CA3 area. To discriminate mossy fiber fEPSPs from possible contamination of associational–commissural synapses, the selective metabotropic glutamate receptor group II (mGluR II) agonist (2S,1′S,2′S)-2-(carboxycyclopropyl)glycine (L-CCG-I; 10 μM) was added to the perfusion medium at the end of the experiments (38). The responses were considered as mossy fiber fEPSPs only if they were blocked by L-CCG-I application (39, 40). In some experiments, the putative galanin receptor antagonist M35 (26) was added at 1 μM to the perfusion medium.
Statistical Analysis.
Statistical analysis of differences between groups was performed by using Student's unpaired t test. Additionally, repeated-measures ANOVA was used for analysis of differences in kindling development. Differences were considered significant at P < 0.05. Data are presented as mean ± SEM.
Results and Discussion
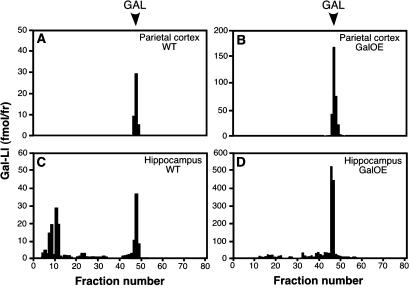
By using RIA, we detected up to 8-fold higher concentrations of galanin in cerebral cortex and hippocampus of GalOE as compared with WT mice (Table 1). HPLC showed a main peak of immunoreactivity corresponding to genuine galanin in samples from these regions (Fig. 1).
Table 1.
Galanin levels in different brain regions of GalOE and WT mice as determined by RIA
| Brain region | Galanin, pmol/mg protein
|
Fold increase | |
|---|---|---|---|
| WT (n = 5) | GalOE (n = 6) | ||
| Frontal cortex | 0.36 ± 0.12 | 2.04 ± 0.28 | 5.7 |
| Parietal cortex | 0.32 ± 0.08 | 2.83 ± 0.48 | 8.8 |
| Piriform cortex | 0.21 ± 0.11 | 1.58 ± 0.36 | 7.5 |
| Hippocampus | 0.92 ± 0.32 | 3.40 ± 0.94 | 3.7 |
Results are presented as mean ± SEM.
Figure 1.
HPLC chromatogram showing galanin-like immunoreactivity (Gal-LI, in fmol per fraction) in parietal cortex (A and B) and hippocampus (C and D) of WT (A and C) and GalOE (B and D) mice. The immunoreactive component elutes in the position of synthetic galanin (GAL) in parietal cortex and hippocampus with a similar appearance in WT and GalOE mice.
Seizure development in response to hippocampal kindling stimulations was markedly retarded in GalOE mice (Fig. 2A; repeated measures ANOVA, P < 0.05). Mean AD duration was also shorter in GalOE mice (Fig. 2B). Compared with WT animals, the GalOE mice needed significantly more stimulations to reach the first generalized (grades 4 and 5) seizures (Fig. 2C). This was because GalOE mice exhibited focal seizures (grades 0–2) considerably longer (17 ± 2 days) than the WT animals (10 ± 2 days; P < 0.05, Student's unpaired t test). The number of days when mice displayed generalized seizures, as well as other kindling characteristics, such as latency to and duration of behavioral seizures, and duration of secondary AD did not differ between GalOE and WT mice. When the already-kindled animals were subjected to new stimulations 4–6 weeks after the last seizure, the threshold for inducing epileptiform activity (AD threshold) was decreased in the WT mice, whereas it remained unchanged in the GalOE animals (Fig. 2D; unpaired t test, P < 0.05). However, both groups had maintained the kindled state, because mean seizure grades in response to these new stimulations were similar in GalOE and WT mice (data not shown).
Figure 2.
Kindling epileptogenesis is suppressed in GalOE mice. (A) GalOE mice show lower mean seizure grades in response to consecutive kindling stimulations as compared with WT animals. (B) Mean afterdischarge (AD) duration in response to five consecutive stimulations during kindling epileptogenesis is shorter in GalOE mice. (C) GalOE mice require more kindling stimulations to reach different seizure grades. (D) AD threshold when kindled animals are subjected to new kindling stimulations after 4–6 weeks (rekindling) is decreased in WT but not in GalOE mice. WT, n = 7; GalOE, n = 8. *, Significant difference between WT and GalOE mice (unpaired t test, P < 0.05). Data are mean ± SEM.
To explore the mechanisms underlying the reduced seizure susceptibility in GalOE mice, we carefully compared the cellular localization of galanin mRNA and protein between the two groups. Basal galanin mRNA expression was not detectable in hippocampal (Fig. 3A) or cortical areas (Fig. 4A) of WT mice, and was low in amygdala (data not shown). In contrast, GalOE animals showed high levels of galanin mRNA in dentate granule cell and CA1–CA3 pyramidal cell layers and hilus (Fig. 3 B and F), and moderate levels in piriform cortex (Fig. 3 C and F). Clusters of radioactive grains were seen over pyramidal neurons in layers 4–5 of the parietal-retrosplenial cortices (Fig. 4B, arrowheads). With the exception of a pronounced up-regulation of galanin mRNA in the piriform cortex in GalOE mice, no other changes of gene expression as compared with basal levels were observed in either animal group at 4 h after the last stimulus-evoked seizure (Fig. 3 D and F).
Figure 3.
GalOE mice express high levels of galanin mRNA in hippocampal and cortical regions, and seizures further increase galanin mRNA levels in piriform cortex. (A and B) Dark-field photomicrographs of in situ hybridization emulsion-dipped sections showing higher galanin mRNA expression under basal conditions in the hippocampus of GalOE mice (B) as compared with WT animals (A). (C and D) Autoradiograms of in situ hybridization films showing expression of galanin mRNA in the piriform cortex of GalOE mice before (C) and after (D) kindling. Arrowheads depict pyramidal layer of piriform cortex. (E and F) Levels of galanin mRNA in dentate granule cell layer (DG) and hilus, CA1–CA3 pyramidal layers, and piriform (Pir) cortex in WT and GalOE mice under basal conditions and after kindling. #, significant difference compared with corresponding WT animals. *, significant difference compared with nonkindled GalOE mice. Data are mean ± SEM. (n = 4 for both WT and GalOE mice; unpaired t test, P < 0.05).
Figure 4.
Basal and seizure-evoked levels of galanin mRNA and immunoreactivity are high in parietal-retrosplenial cortex of GalOE mice. (A and B) Dark-field photomicrographs of in situ hybridization emulsion-dipped sections showing galanin mRNA expression. Note the increased number of radioactive grains over the cell bodies of cortical layer 4–5 pyramidal neurons in GalOE mice (arrowheads). (C and D) Galanin immunoreactivity in parietal-retrosplenial cortex of nonkindled mice. Note the presence of immunoreactivity in dispersed, presumed noradrenergic fibers only in WT mice (C, arrowheads) and in cortical layer 4–5 pyramidal neurons only in GalOE mice (D, arrowheads). (E and F) Galanin immunoreactivity in parietal-retrosplenial cortex of kindled mice. Note the disappearance of the galanin immunoreactive fibers in kindled WT animals (E) and the increased immunoreactivity in cortical layer 4–5 pyramidal neurons of kindled GalOE mice (F, arrowheads). (Scale bar, 30 μm.)
Under basal conditions, galanin-like immunoreactivity (LI) was observed in presumed noradrenergic fibers (6) throughout cortical and hippocampal areas of WT mice (Figs. 4C arrowheads and 5A). Such fibers were absent in cortical areas (Fig. 4D) and were very few in hippocampal areas (Fig. 5B) of GalOE mice. However, in these mice, weak galanin-LI was seen in layer 4–5 pyramidal neurons (Fig. 4D arrowheads) of the parietal–retrosplenial cortices. Most conspicuous in GalOE mice was the high galanin-LI in the dentate granule cell layer and hilus and particularly in the mossy fibers (Fig. 5B), as well as in the outer molecular layer (layer 1a) of the piriform cortex (data not shown).
Figure 5.
Basal and seizure-evoked levels of galanin-LI in the hippocampus of GalOE mice are higher as compared with WT mice. Note that galanin-LI is detectable only in dispersed fibers in WT mice (A). In contrast, galanin-LI is high in granule cell layer, mossy fibers, and hilus of GalOE mice (B). After kindling-induced seizures, galanin-LI is increased in the supragranular layer and stratum lacunosum moleculare of CA1 in WT mice (C) and is much more markedly elevated in granule cell layer, hilus, and mossy fibers of GalOE animals (D).
Twenty-four hours after the last kindling-evoked seizure, galanin-LI in cortical and hippocampal noradrenergic fibers of WT animals had almost completely disappeared (Figs. 4E and 5C). In contrast, galanin-LI was increased in the supragranular layer of the dentate gyrus and stratum lacunosum moleculare of the CA1 area (Fig. 5C). In GalOE mice, kindling led to a more pronounced increase of galanin-LI in the supragranular layer and hilus. The levels of galanin-LI were also more markedly elevated in the mossy fibers of kindled as compared with nonkindled GalOE animals (Fig. 5D). Moreover, kindling increased the staining intensity of galanin-positive cells in cortical areas (Fig. 4F), whereas galanin-LI in the piriform cortex was unchanged in GalOE mice (data not shown).
The highest basal and seizure-evoked levels of galanin-LI in GalOE mice were found in mossy fibers, which have been proposed to play an important role in epileptogenesis (41, 42). We therefore explored whether the ectopic overexpression of galanin affected synaptic transmission at mossy fiber–CA3 cell synapses, in particular glutamate release (25). To address this question, we analyzed so-called frequency facilitation, a form of a short-term synaptic plasticity in which postsynaptic responses are augmented at high frequency stimulations because of increased probability of glutamate release from presynaptic terminals (see ref. 43). Frequency facilitation of fEPSPs in the proximal part of the stratum lucidum of the CA3 area as assessed in hippocampal slices was lower in GalOE mice as compared with WT animals (Fig. 6 A trace 2 and B trace 2). Conversely, in GalOE (Fig. 6 C and E) but not in WT (Fig. 6F) mice, application of the putative galanin receptor antagonist M35 (26) increased frequency facilitation. fEPSPs at low-frequency stimulations were not changed (data not shown). The mGluR II agonist L-CCG-I blocked fEPSPs in CA3 to the same extent in WT and GalOE mice (to 3.9 ± 1.9% and 4.3 ± 2.1% of initial magnitude, respectively; see also Fig. 6 A trace 3 and B trace 3), indicating that mGluR II receptors were functional in GalOE mice, and that the fEPSPs were mostly generated by activation of mossy fibers, which selectively express mGluR II in mice (38, 39).
Figure 6.
Frequency facilitation is decreased in mossy fiber synapses of GalOE mice. (A) Traces of fEPSPs (average of 10) recorded in stratum lucidum of CA3 during low-frequency (0.1 Hz; trace1) and high-frequency (1 Hz; trace 2) stimulation of mossy fibers, and after bath application of L-CCG-I (10 μM; trace 3) in WT animals. Note the increased fEPSPs at high frequency stimulation (trace 2). fEPSPs are blocked by L-CCG-I (trace 3) indicating that they are predominantly generated by mossy fibers. (B) Same recordings as in A in GalOE mice. Note smaller fEPSP during high-frequency stimulation (trace 2) in GalOE mice as compared with WT mice in A. Picrotoxin (100 μM) was present in the bath solution. (C) Frequency facilitation of mossy fiber-CA3 synapse fEPSPs (measured as fEPSP initial slope) expressed as percentage of baseline before and during bath application of the putative galanin receptor antagonist M35, and of L-CCG-I. Dashed lines indicate time lapse of 10 min between the experiments. During L-CCG-I application, stimulation frequency was 0.5 Hz. Note increase and blockade of fEPSPs during M35 and L-CCG-I application, respectively. (D) fEPSP frequency facilitation of mossy fiber–CA3 cell synapses in WT (n = 30 experiments, eight mice) and GalOE (n = 22 experiments, eight mice). (E and F) fEPSP frequency facilitation of mossy fiber–CA3 cell synapses in GalOE (E) and WT (F) mice before and during bath application of M35 (n = 16 experiments, five mice; and 18 experiments, five mice, respectively). Data are means ± SEM. Before fEPSP initial slope measurements were made, traces recorded after bath application of L-CCG-I (3) were always subtracted from those recorded during low (1) or high (2) frequency stimulation of mossy fibers.
This study shows that mice overexpressing galanin under the PDGF-B promoter exhibit marked suppression of seizure development in an animal model of human complex partial seizures. High galanin levels were confirmed by RIA showing up to 8-fold increases in GalOE over WT mice, and HPLC analysis strongly suggests that the overexpressed peptide is identical to synthetic galanin. Galanin overexpression was detected in many neural systems in cerebral cortex and hippocampus, in particular in the granule cell–mossy fiber system, as a consequence of the widespread distribution of the PDGF-B promoter (44, 45). These cortical and hippocampal systems do not express galanin in WT mice under normal conditions. A puzzling finding was the decrease of galanin in noradrenergic fibers originating in the locus ceruleus, which is the only system where we could detect galanin in dorsal cortical and hippocampal areas not only in WT mice but also in rats (6).
The suppression of kindling epileptogenesis in the GalOE mice supports the view that the ectopically overexpressed galanin is released and acts by means of a galanin receptor(s). In these mice, the already high levels of galanin-LI in the mossy fibers were further increased after kindling-evoked seizures. These fibers might be of particular importance in the propagation of seizure activity in the hippocampus (see e.g., ref. 46). Moreover, sprouting of mossy fibers, observed in several animal models of epilepsy (41) and epileptic patients (47), has been proposed to induce increased excitability of hippocampal circuitry and promote epileptogenesis (42). Galanin is not normally expressed in dentate granule cells and mossy fibers (2, 6, 48). However, GalR2 mRNA has been detected in these as well as in locus ceruleus neurons (6, 49, 50), in both cases possibly constituting a presynaptic receptor (51), which normally may be the target for galanin released from noradrenergic fibers (3, 6, 27). We propose that galanin, overexpressed in mossy fibers, is released during epileptic discharges and interacts with presynaptic GalR2 receptors on the mossy fibers themselves. This leads to decreased glutamate release (28) and inhibition of the spread and generalization of focal hippocampal seizures. This hypothesis is supported by our findings that GalOE mice have lower magnitude of frequency facilitation of mossy fiber responses, probably reflecting decreased glutamate release (see, e.g., ref. 43), and that application of the putative galanin receptor antagonist M35 (26) reverses this abnormality. Interestingly, M35 had no effect on mossy fiber fEPSPs of GalOE mice at low-frequency stimulations. This would imply that high-frequency activity is necessary to release galanin from mossy fibers in concentrations sufficient to reach its receptor. If this is the case, it would be in agreement with early studies on peripheral autonomic neurons showing frequency-dependent release of neuropeptides from sympathetic and parasympathetic neurons (52) and with studies on galanin release in response to high-frequency septal stimulation (53). Consequently, galanin overexpression would dampen only high-frequency activity in mossy fibers, e.g., during seizures, but have little effect on normal synaptic transmission at low frequencies, a situation that would be favorable if attempting to develop an antiepileptic agent based on galanin receptor agonism.
The pattern of suppressed kindling epileptogenesis in the GalOE mice already at the early stages of seizure development supports a local hippocampal action of galanin. Shorter duration of ADs and longer time spent in the early stages of kindling compared with WT mice, as well as higher threshold for AD induction in the kindled GalOE animals, further advocate this notion (see ref. 54).
High levels of galanin-LI were found also in the outer molecular layer of the piriform cortex. Because the piriform cortex plays an important role in the transition from focal to generalized seizures during kindling epileptogenesis (55, 56), increased galanin levels in this area could contribute to the suppression of kindling development in GalOE mice. The galanin-LI in the piriform cortex could be located either in the distal apical dendrites of the pyramidal cells or in the nerve terminals originating from the olfactory bulb mitral cells, which innervate these dendrites (57). In rats, galanin-LI has been reported to be present in the tufted cells and short-axon neurons of the olfactory bulb (58). However, in GalOE mice, high levels of galanin were also detected in the mitral cells (unpublished observations), suggesting olfactory bulb origin of the immunoreactivity in the outer molecular layer of the piriform cortex.
We provide here evidence that ectopically expressed galanin in cortical and hippocampal neurons can be regulated by seizures, is released during neuronal activity, and suppresses epileptogenesis by interaction with presynaptic galanin receptors, leading to decreased glutamate release. These findings suggest novel therapeutic strategies, e.g., using gene transfer, to dampen seizure development in epilepsy by ectopic expression of modulatory neuropeptides in excitatory neural systems.
Acknowledgments
We thank Monica Lundahl and Katarina Åman for technical assistance. This work was supported by grants from the Swedish Medical Research Council, the Medical Faculty at the University of Lund, the Royal Physiographic Society, the Wiberg Foundation, the Kock Foundation, the Elsa and Thorsten Segerfalk Foundation, Crafoord Foundation, the Marianne and Marcus Wallenberg Foundation, and the Knut and Alice Wallenberg Foundation, and an Unrestricted Bristol-Myers Squibb Neuroscience Grant. A.N. was supported by a scholarship from the Royal Swedish Academy of Sciences.
Abbreviations
- PDGF-B
platelet-derived growth factor B
- GalRn
galanin receptor n
- SE
status epilepticus
- GalOE
galanin-overexpressing
- WT
wild type
- AD
afterdischarge
- L-CCG-I
(2S,1′S, 2′S)-2-(carboxycyclopropyl)glycine
- galanin-LI
galanin-like immunoreactivity
- mGluR II
metabotropic glutamate receptor group II
- fEPSP
field excitatory postsynaptic potential
- aCSF
artificial cerebrospinal fluid
Footnotes
Holmberg, K., Kahl, U., Lendahl, U., Kokaia, Z., Nanobashvili, A., Lindvall, O., Ekström, P., Bartfai, T. & Hökfelt, T. (2000) Soc. Neurosci. Abstr. 18.5, 26:27.
References
- 1.Tatemoto K, Rökaeus Å, Jörnvall H, McDonald T, Mutt V. FEBS Lett. 1983;164:124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 2.Skofitsch G, Jacobowitz D M. Peptides. 1985;6:509–546. doi: 10.1016/0196-9781(85)90118-4. [DOI] [PubMed] [Google Scholar]
- 3.Melander T, Staines W A, Rökaeus A. Neuroscience. 1986;19:223–240. doi: 10.1016/0306-4522(86)90017-5. [DOI] [PubMed] [Google Scholar]
- 4.Melander T, Hökfelt T, Rökaeus A, Cuello A C, Oertel W H, Verhofstad A, Goldstein M. J Neurosci. 1986;6:3640–3654. doi: 10.1523/JNEUROSCI.06-12-03640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holets V, Hökfelt T, Rökaeus A, Terenius L, Goldstein M. Neuroscience. 1988;24:893–906. doi: 10.1016/0306-4522(88)90076-0. [DOI] [PubMed] [Google Scholar]
- 6.Xu Z-Q, Shi T J, Hökfelt T. J Comp Neurol. 1998;392:227–251. doi: 10.1002/(sici)1096-9861(19980309)392:2<227::aid-cne6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Skofitsch G, Jacobowitz D M. Peptides. 1986;7:609–613. doi: 10.1016/0196-9781(86)90035-5. [DOI] [PubMed] [Google Scholar]
- 8.Melander T, Köhler C, Nilsson S, Hökfelt T, Brodin E, Theodorsson E, Bartfai T. J Chem Neuroanat. 1988;1:213–233. [PubMed] [Google Scholar]
- 9.Habert-Ortoli E, Amiranoff B, Loquet I, Laburthe M, Mayaux J F. Proc Natl Acad Sci USA. 1994;91:9780–9783. doi: 10.1073/pnas.91.21.9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgevin M C, Loquet I, Quarteronet D, Habert-Ortoli E. J Mol Neurosci. 1995;6:33–41. doi: 10.1007/BF02736757. [DOI] [PubMed] [Google Scholar]
- 11.Parker E, Izzarelli D, Nowak H, Mahle C, Iben L, Wang J, Goldstein M. Mol Brain Res. 1995;34:179–189. doi: 10.1016/0169-328x(95)00159-p. [DOI] [PubMed] [Google Scholar]
- 12.Howard A D, Tan C, Shiao L L, Palyha O C, McKee K K, Weinberg D H, Feighner S D, Cascieri M A, Smith R G, Van Der Ploeg L H, Sullivan K A. FEBS Lett. 1997;405:285–290. doi: 10.1016/s0014-5793(97)00196-8. [DOI] [PubMed] [Google Scholar]
- 13.Smith K E, Forray C, Walker M W, Jones K A, Tamm J A, Bard J, Branchek T A, Linemeyer D L, Gerald C. J Biol Chem. 1997;272:24612–24616. doi: 10.1074/jbc.272.39.24612. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Hashemi T, He C, Strader C, Bayne M. Mol Pharmacol. 1997;52:337–343. doi: 10.1124/mol.52.3.337. [DOI] [PubMed] [Google Scholar]
- 15.Wang S, He C, Hashemi T, Bayne M. J Biol Chem. 1997;272:31949–31952. doi: 10.1074/jbc.272.51.31949. [DOI] [PubMed] [Google Scholar]
- 16.Bartfai T, Hökfelt T, Langel U. Crit Rev Neurobiol. 1993;7:229–274. [PubMed] [Google Scholar]
- 17.Hökfelt T, Bartfai T, Crawley J, editors. Galanin: Basic Research Discoveries and Therapeutic Implications, Annuals of the New York Academy of Sciences. New York: N.Y. Acad. Sci.; 1998. [PubMed] [Google Scholar]
- 18.O'Meara G, Coumis U, Ma S, Kehr J, Mahoney S, Bacon A, Allen S, Holmes F, Kahl U, Wang F, et al. Proc Natl Acad Sci USA. 2000;97:11569–11574. doi: 10.1073/pnas.210254597. . (First Published October 3, 2000; 10.1073/pnas.210254597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmes F, Mahoney S, King V, Bacon A, Kerr N, Pachnis V, Curtis R, Priestly J, Wynick D. Proc Natl Acad Sci USA. 2000;97:11563–11568. doi: 10.1073/pnas.210221897. . (First Published October 3, 2000; 10.1073/pnas.210221897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steiner R A, Hohmann J G, Holmes A, Wrenn C C, Cadd G, Jureus A, Clifton D K, Luo M, Gutshall M, Ma S Y, et al. Proc Natl Acad Sci USA. 2001;98:4184–4189. doi: 10.1073/pnas.061445598. . (First Published March 20, 2001; 10.1073/pnas.061445598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gall C, Lauterborn J, Isackson P, White J. Prog Brain Res. 1990;83:371–390. doi: 10.1016/s0079-6123(08)61263-7. [DOI] [PubMed] [Google Scholar]
- 22.Schwarzer C, Sperk G, Samanin R, Rizzi M, Gariboldi M, Vezzani A. Brain Res Rev. 1996;22:27–50. [PubMed] [Google Scholar]
- 23.Vezzani A, Sperk G, Colmers W F. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- 24.Mazarati A M, Liu H, Soomets U, Sankar R, Shin D, Katsumori H, Langel U, Wasterlain C G. J Neurosci. 1998;18:10070–10077. doi: 10.1523/JNEUROSCI.18-23-10070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazarati A M, Hohmann J G, Bacon A, Liu H, Sankar R, Steiner R A, Wynick D, Wasterlain C G. J Neurosci. 2000;20:6276–6281. doi: 10.1523/JNEUROSCI.20-16-06276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartfai T, Fisone G, Langel Ü. Trends Pharmacol Sci. 1992;13:312–317. doi: 10.1016/0165-6147(92)90098-q. [DOI] [PubMed] [Google Scholar]
- 27.Gabriel S M, Knott P J, Haroutunian V. J Neurosci. 1995;15:5526–5534. doi: 10.1523/JNEUROSCI.15-08-05526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zini S, Roisin M P, Langel Ü, Bartfai T, Ben-Ari Y. Eur J Pharmacol. 1993;245:1–7. doi: 10.1016/0922-4106(93)90162-3. [DOI] [PubMed] [Google Scholar]
- 29.Goddard G V, McIntyre D C, Leech C K. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- 30.McNamara J O, Bonhaus D W, Shin C. In: Epilepsy: Models, Mechanisms, and Concepts. Schwartzkroin P A, editor. Cambridge, U.K.: Cambridge Univ. Press; 1993. pp. 27–47. [Google Scholar]
- 31.Collins T, Ginsburg D, Boss J, Orkin S, Pober J. Nature (London) 1985;316:748–750. doi: 10.1038/316748a0. [DOI] [PubMed] [Google Scholar]
- 32.Hogan B, Costanini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1986. [Google Scholar]
- 33.Theodorsson E, Rugarn O. Scand J Clin Lab Invest. 2000;60:411–418. doi: 10.1080/003655100750019323. [DOI] [PubMed] [Google Scholar]
- 34.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 35.Franklin K B J, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. [Google Scholar]
- 36.Racine R J. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 37.Vrontakis M E, Peden L M, Duckworth M L, Friesen H G. J Biol Chem. 1987;262:16755–16758. [PubMed] [Google Scholar]
- 38.Kokaia M, Asztely F, Olofsdotter K, Sindreu C B, Kullmann D M, Lindvall O. J Neurosci. 1998;18:8730–8739. doi: 10.1523/JNEUROSCI.18-21-08730.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castillo P E, Janz R, Südhof T C, Tzounopoulos T, Malenka R C, Nicoll R A. Nature (London) 1997;388:590–593. doi: 10.1038/41574. [DOI] [PubMed] [Google Scholar]
- 40.Kamiya H, Shinozaki H, Yamamoto C. J Physiol. 1996;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ben-Ari Y, Represa A. Trends Neurosci. 1990;13:312–318. doi: 10.1016/0166-2236(90)90135-w. [DOI] [PubMed] [Google Scholar]
- 42.Lynch M, Sutula T. J Neurophysiol. 2000;83:693–704. doi: 10.1152/jn.2000.83.2.693. [DOI] [PubMed] [Google Scholar]
- 43.Cremer H, Chazal G, Carleton A, Goridis C, Vincent J D, Lledo P M. Proc Natl Acad Sci USA. 1998;95:13242–13247. doi: 10.1073/pnas.95.22.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sasahara M, Fries J, Raines E, Gown A, Westrum L, Frosch M, Bonthrin D, Ross R, Collins T. Cell. 1991;64:217–227. doi: 10.1016/0092-8674(91)90223-l. [DOI] [PubMed] [Google Scholar]
- 45.Sasahara M, Sato H, Iihara K, Wang J, Chue C, Takayama S, Hayase Y, Hazama F. Mol Brain Res. 1995;32:63–74. doi: 10.1016/0169-328x(95)00060-6. [DOI] [PubMed] [Google Scholar]
- 46.Rafiq A, DeLorenzo R J, Coulter D A. J Neurophysiol. 1993;70:1962–1974. doi: 10.1152/jn.1993.70.5.1962. [DOI] [PubMed] [Google Scholar]
- 47.Proper E A, Oestreicher A B, Jansen G H, Veelen C W, van Rijen P C, Gispen W H, de Graan P N. Brain. 2000;123:19–30. doi: 10.1093/brain/123.1.19. [DOI] [PubMed] [Google Scholar]
- 48.Melander T, Hökfelt T, Rökaeus A. J Comp Neurol. 1986;248:475–517. doi: 10.1002/cne.902480404. [DOI] [PubMed] [Google Scholar]
- 49.Fathi Z, Cunningham A M, Iben L G, Battaglino P B, Ward S A, Nichol K A, Pine K A, Wang J, Goldstein M E, Iismaa T P, Zimanyi I A. Mol Brain Res. 1997;51:49–59. doi: 10.1016/s0169-328x(97)00210-6. [DOI] [PubMed] [Google Scholar]
- 50.O'Donnell D, Ahmad S, Wahlestedt C, Walker P. J Comp Neurol. 1999;409:469–481. [PubMed] [Google Scholar]
- 51.Ma, X., Tong, Y.-G., Schmidt, R., Brown, W., Payza, K., Hodzic, L., Pou, C., Godbout, C., Hökfelt, T. & Xu, Z.-Q. (2001) Brain Res., in press. [DOI] [PubMed]
- 52.Lundberg J, Hökfelt T. Trends Neurosci. 1983;6:325–333. [Google Scholar]
- 53.Consolo S, Baldi G, Russi G, Civenni G, Bartfai T, Vezzani A. Proc Natl Acad Sci USA. 1994;91:8047–8051. doi: 10.1073/pnas.91.17.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McIntyre D C, Kelly M E, Dufresne C. Epilepsy Res. 1999;35:197–209. doi: 10.1016/s0920-1211(99)00012-1. [DOI] [PubMed] [Google Scholar]
- 55.McIntyre D C, Kelly M E. Ann NY Acad Sci. 2000;911:343–354. doi: 10.1111/j.1749-6632.2000.tb06736.x. [DOI] [PubMed] [Google Scholar]
- 56.Loscher W, Ebert U. Prog Neurobiol. 1996;50:427–481. doi: 10.1016/s0301-0082(96)00036-6. [DOI] [PubMed] [Google Scholar]
- 57.Scott J W, McBride R L, Schneider S P. J Comp Neurol. 1980;194:519–534. doi: 10.1002/cne.901940304. [DOI] [PubMed] [Google Scholar]
- 58.Kasa P, Farkas Z, Balaspiri L, Wolff J R. Neuroscience. 1996;72:709–723. doi: 10.1016/0306-4522(95)00567-6. [DOI] [PubMed] [Google Scholar]