Abstract
The predominant function of the blood-retinal barrier (BRB) is to maintain retinal homeostasis by regulating the influx and efflux between the blood and retina. Breakdown of the BRB occurs in a number of ocular diseases that result in vision loss. Understanding the molecular and cellular pathways involved in the development and maintenance of the BRB is critical to developing therapeutics for these conditions. To visualize the BRB in vivo, we used the transgenic Tg(l-fabp:DBP-EGFP:flk1:mCherry) zebrafish model that expresses vitamin D binding protein (a member of the albumin gene family) tagged to green fluorescent protein. Retinoic acid (RA) plays a number of important roles in vertebrate development and has been shown to play a protective role during inflammation-induced blood-brain barrier disruption. The role of RA in BRB development and maintenance remains unknown. To disrupt RA signaling, Tg(l-fabp:DBP-EGFP:flk1:mCherry) zebrafish were treated with N,N-diethylaminobenzaldehyde and 4-[(1E)-2-[5,6-dihydro-5,5-dimethyl-8-(2-phenylethynyl)-2-naphthalenyl]ethenyl]benzoic acid, which are antagonists of retinal dehydrogenase and the RA receptor, respectively. Treatment with either compound resulted in BRB disruption and reduced visual acuity, whereas cotreatment with all-trans RA effectively rescued BRB integrity. Additionally, transgenic overexpression of Cyp26a1, which catalyzes RA degradation, resulted in breakdown of the BRB. Our results demonstrate that RA signaling is critical for maintenance of the BRB and could play a role in diseases such as diabetic macular edema.—Pollock, L. M., Xie, J., Bell, B. A., Anand-Apte, B. Retinoic acid signaling is essential for maintenance of the blood-retinal barrier.
Keywords: zebrafish, tight junctions, vasculature
The blood-retinal barrier (BRB) is critical for visual function because it isolates the retinal neural tissue from the bloodstream. The BRB is comprised of 2 parts: an inner barrier of endothelial cells (ECs) lining the retinal blood vessels and an outer barrier formed by the retinal pigment epithelium (RPE) separating the retina and the nonneuronal choroid. Tight junctions between cells (ECs or RPE) are crucial to barrier function and integrity (1). Breakdown of the BRB results in vasogenic edema and retinal neural tissue damage, causing loss of vision. BRB breakdown is one of the earliest changes seen in diabetic retinopathy (2) and is observed in age-related macular degeneration, retinal vein occlusions, uveitis, and other chronic retinal diseases (3). The Wnt, sonic hedgehog, and Norrin/Frizzled4 signaling pathways have been postulated to be involved in the formation of the BRB (4). However, the molecular mechanisms involved in maintaining BRB integrity remain poorly understood.
Retinoic acid (RA) is a metabolite of vitamin A, which has multiple functions in the human body (5) and has been shown to regulate the development of the blood-brain barrier (BBB) by promoting the formation of tight junctions (6). However, the role of RA in BRB development and maintenance has not been previously explored. In the RA signaling pathway, vitamin A is stored in the liver in the form of retinyl esters, which can be hydrolyzed to release retinol. Retinol bound to retinol binding protein is taken up by target tissues, such as the retina, and oxidized to retinaldehyde by either alcohol dehydrogenase or retinol dehydrogenase. Retinaldehyde is then further oxidized by retinaldehyde dehydrogenase (RALDH) to form RA. RA binds to CRABP-2 and signals through 3 nuclear RA receptors (RAR-α, RAR-β, and RAR-γ) and the scaffolding retinoid X receptors (RXR-α, RXR-β, and RXR-γ). Upon binding RA, the RAR and RXR receptors dimerize, leading to transcription of genes with an upstream RA response element. In studies of the human cerebral microvascular EC/D3 line, RA treatment led to increased expression of tight junction molecules ZO-1, VE-cadherin, and occludin (6), which are necessary for BBB and BRB integrity (1, 7).
We tested the hypothesis that RA signaling is required for maintenance of BRB integrity. Due to its rapid development and tissue transparency at the embryonic and larval stages, the zebrafish is a good model to study BRB integrity in vivo. Zebrafish embryos develop a functional BRB at 3 d postfertilization (dpf) (8), which is fully enclosed by 5 dpf (9). In this study, we used a double-transgenic zebrafish line, Tg(l-fabp:DBP-EGFP:flk1:mCherry), which expresses enhanced green fluorescent protein (EGFP) in the blood plasma (8), displaying the integrity of the BRB and allowing examination of the functions of RA in BRB maintenance. In the presence of an intact BRB, EGFP is primarily localized within mCherry-tagged retinal vessels. Detection of EGFP outside of the vessels indicates BRB disruption and leakage of plasma proteins into the retina.
By pharmacological and genetic manipulation of the RA signaling pathway in Tg(l-fabp:DBP-EGFP:flk1:mCherry) zebrafish, we demonstrate that RA signaling is essential for maintenance of the BRB at both larval and adult stages.
MATERIALS AND METHODS
Zebrafish
All zebrafish (Danio rerio) studies were conducted using the guidelines of the Cleveland Clinic Animal Care and Use Committee. Zebrafish strains used were Tg(l-fabp:DBP-EGFP) (8), Tg(flk1:mCherry) (10), Tg(hsp70I:cyp26a1) (11), and Tg(fli1a:EGFP-cldn5b) (12). Animals of either sex were used.
N,N-diethylaminobenzaldehyde and 4-[(1E)-2-[5,6-dihydro-5,5-dimethyl-8-(2-phenylethynyl)-2-naphthalenyl]ethenyl]benzoic acid treatment
Tg(l-fabp:DBP-EGFP:flk1:mCherry) embryos were treated from 2 to 7 dpf with varying concentrations of 4-diethylaminobenzaldehyde (DEAB) (MilliporeSigma, St. Louis, MO, USA) or 4-[(1E)-2-[5,6-dihydro-5,5-dimethyl-8-(2-phenylethynyl)-2-naphthalenyl]ethenyl]benzoic acid (BMS493) (Tocris Biosciences, Bristol, United Kingdom) diluted in embryo medium containing 0.6% DMSO (Thermo Fisher Scientific, Waltham, MA, USA). Control embryos were treated from 2 to 7 dpf with 0.6% DMSO in embryo medium. At 7 dpf, fish were anesthetized using ∼0.14% (0.14 mg/ml) ethyl 3-aminobenzoate methanesulfonic acid salt (Acros Organics, Geel, Belgium), and leakage of DBP-EGFP from the hyaloid vessels was examined by fluorescence microscopy (Zeiss Axio Zoom v.16; Carl Zeiss, Oberkochen, Germany).
For treatment of adults, Tg(l-fabp:DBP-EGFP) fish aged 3–5 mo were placed in water containing 5 μM DEAB and 0.6% DMSO for 24 h or 10 μM BMS493 and 0.6% DMSO for 48 h and evaluated for BRB breakdown by confocal scanning laser ophthalmoscopy (SLO).
Confocal SLO imaging of adult zebrafish
Confocal SLO imaging procedures and extravascular space mean signal intensity measurements were performed according to previously published methods (13). Briefly, fish were anesthetized using ∼0.14% ethyl 3-aminobenzoate methanesulfonic acid salt (Acros Organics). Once immobile, animals were placed in a custom holder where a contact lens was affixed to the eye using Systane Ultra hydrating tears (Alcon, Fort Worth, TX, USA). Animals were then positioned for imaging with a confocal scanning ophthalmoscope (HRA2 SLO; Heidelberg Engineering, Heidelberg, Germany).
All-trans RA treatment
Embryos treated with 0.15 μM BMS493 or 10 µM DEAB at 2 dpf were cotreated with all-trans RA (ATRA) (MilliporeSigma) diluted to 0.05 μM in embryo medium containing 0.6% DMSO. BRB integrity was examined at 7 dpf using a fluorescent microscope (Zeiss Axio Zoom v.16; Carl Zeiss).
Quantitative PCR analysis
RNA was isolated from pools of 10 whole zebrafish larvae at 7 dpf using the RNeasy Mini Kit (Qiagen, Hilden, Germany). RNA samples were reverse transcribed using the SuperScript Vilo cDNA Synthesis Kit (Thermo Fisher Scientific). For quantification of mRNA expression, quantitative PCR was carried out using the Radiant Green Hi-Rox Quantitative (q)PCR Kit (Alkali Scientific, Pompano Beach, FL, USA). Reactions were run on a CFX96 Real-Time Detection System (Bio-Rad Laboratories, Hercules, CA, USA) under the following conditions: 2 min at 95°C followed by 40 cycles of 5 s at 95°C and 30 s at 60°C. mRNA expression levels were normalized against actb2 using previously described primer sequences (14). Primer sequences to detect cyp26a1 expression were cyp26a1 forward: 5′-CGAGAACAGCAGAAGAAGTGACG-3′ and cyp26a1 reverse: 5′-CTCTCTGACCTTCTGCACCAC-3′.
Heat shock and genotyping of Tg(hsp70I:cyp26a1) zebrafish
For genotyping, genomic DNA was extracted from Tg(hsp70I:cyp26a1) larvae, and PCR was conducted using primers targeting the hsp70I promoter and cyp26a1 cDNA, HCyp forward: 5′-GAGCAGCCTGACAGGACTTTTC-3′ and HCyp reverse: 5′-CATAAGGGTGTACAGCCCCAT-3′. PCR of genomic DNA from fish carrying the hsp70I:cyp26a1 allele produced a 534 bp band. Embryos and larvae were heat shocked in a 38°C water bath for 20 min once per day from 2 to 6 dpf and analyzed for BRB leakage at 6 dpf.
Whole-mount immunohistochemical staining
Tg(flk1:mCherry) larvae at 7 dpf were fixed with 4% paraformaldehyde in PBS for 6 h at 4°C. After washes in 1× PBS, lenses and attached hyaloid vessels were isolated as previously described (15) and incubated in a blocking solution (1% bovine serum albumin/0.4% Triton X-100/1× PBS) for 1 h at room temperature. The isolated lenses were incubated with mouse anti–claudin-5 (1:250, 18-7364; Zymed, South San Francisco, CA, USA) or rabbit anti–ZO-1 (1:250, 61-7300; Zymed) primary antibody diluted in blocking solution at 4°C overnight. After thorough washes with 0.4% Triton X-100/1× PBS, the samples were incubated in blocking solution with the appropriate secondary antibody (Alexa Fluor 633 goat anti-mouse IgG or goat anti-rabbit IgG, 1:1000; Thermo Fisher Scientific) at room temperature for 1 h. After washes in 1× PBS, samples were mounted on slides with Vectashield (Vector Laboratories, Burlingame, CA, USA) for imaging on a confocal microscope (SP8; Leica, Wetzlar, Germany) using the ×63 objective.
Optokinetic response
Zebrafish larvae at 5–8 dpf were immobilized in 3% methylcellulose, and contrast sensitivity was assessed by previously described methods (16) using a VisioTracker system (302060 Series; TSE Systems, Chesterfield, MO, USA). All optokinetic response (OKR) testing was conducted between 12 and 6 pm.
Statistics
Results are expressed as the means ± sem. Significance was tested using Fisher’s exact test, χ2 test, or Student’s t test. Statistical analysis was completed, and graphs were generated using Prism Software (v.5.02; GraphPad Software, La Jolla, CA, USA). A value of P < 0.05 was considered statistically significant.
RESULTS
RA signaling is necessary for development and early maintenance of BRB structure
To test the role of RA in BRB development and maintenance, we specifically inhibited RA signaling in Tg(l-fabp:DBP-EGFP:flk1:mCherry) zebrafish. Zebrafish in this double-transgenic line have mCherry-tagged ECs and express EGFP-tagged vitamin D binding protein in the blood plasma, allowing the integrity of the BRB to be visualized in vivo (8). Tg(l-fabp:DBP-EGFP:flk1:mCherry) embryos and larvae were exposed from 2 to 7 dpf to embryo medium containing variable concentrations of DEAB or BMS493, which are antagonists of RALDH and RAR, respectively (17, 18). Responses to these treatments were compared with those seen in fish exposed to 0.6% DMSO as a solvent control. No toxic effects were observed during treatment, except cardiac edema at higher doses. Larvae treated with DEAB or BMS493 often had a reduced or absent swim bladder but otherwise developed normal gross morphology (Supplemental Fig. 1A–C). Inhibition of RA signaling beginning at 2 dpf in our study did not significantly affect eye size (Supplemental Fig. 1E).
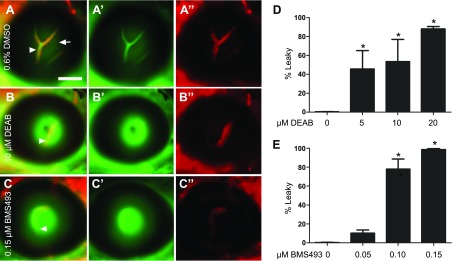
Control embryos (treated with 0.6% DMSO) displayed an intact BRB (Fig. 1A–A″), with DBP-EGFP remaining confined to the lumen of the vasculature. Treatment of the embryos with either DEAB or BMS493 resulted in leakage of blood plasma into the retina, indicated by high DBP-EGFP fluorescence outside the mCherry-tagged vasculature (Fig. 1B–C″). Leaky hyaloid vessels were seen in 88 and 96% of 7 dpf larvae treated with the highest doses of DEAB (20 µM) and BMS493 (0.15 µM), respectively (Fig. 1D, E). Robust leakage was observed beginning at 6 dpf in DEAB-treated larvae and at 4 dpf in BMS493-treated larvae (Supplemental Fig. S2). These results indicate a role for RA signaling in BRB maintenance in larvae and potentially in later stages of BRB development.
Figure 1.
Inhibitors of RA signaling, DEAB and BMS493, disrupt BRB integrity in zebrafish larval stages. A–C″) Merged fluorescent micrographs of eyes from 7 dpf Tg(l-fabp:DBP-EGFP:flk1:mCherry) larvae treated with DMSO or inhibitors of RA signaling starting at 2 dpf. Larvae treated with DMSO alone showed a tight BRB at 7 dpf (A), with EGFP-tagged blood plasma (green, A′) confined within the mCherry tagged hyaloid vessels (red, A″; arrowheads in A). Reflection from the lens (arrow) was detected in the green channel. The majority of embryos treated with DEAB (B–B″) or BMS493 (C–C″) showed leaky hyaloid vessels at 7 dpf, with high DBP-EGFP fluorescence outside the vessels (arrowheads). D, E) Percentage of larvae demonstrating a leaky BRB at 7 dpf after treatment with various doses of DEAB (D) or BMS493 (E). Fisher’s and χ2 tests indicate that DEAB and BMS493 treatments result in significantly increased numbers of larvae showing GFP leakage compared with those treated with DMSO; n = 3 clutches of 50 embryos each/treatment. Scale bar, 50 µm. Error bars indicate sem. *P < 0.05.
RA is required for maintenance of the BRB in adult zebrafish
To determine whether RA signaling is also important for maintenance of the BRB, adult Tg(l-fabp:DBP-EGFP) zebrafish were placed in water containing DMSO, DEAB, or BMS493 for 24–48 h. After treatment, fish were evaluated for BRB breakdown by confocal SLO. This method of imaging BRB breakdown is advantageous because it allows the longitudinal visualization of zebrafish vasculature in the same fish over time. Whereas the BRB remained intact in DMSO-treated fish (Fig. 2A, B), BRB breakdown and leakage of EGFP into the retina was observed as early as 24 h posttreatment with DEAB (Fig. 2C, D) and 48 h posttreatment with BMS493 (Fig. 2E, F). The extravascular space mean signal intensity was significantly increased (P < 0.05 by 1-way ANOVA) after 24 h treatment with DEAB and after 48 h treatment with BMS493, indicating leakage of EGFP into the retina (9) (Fig. 2G). No other toxic effects were observed. These results suggest that RA signaling is likely also involved in BRB maintenance in adult stages.
Figure 2.
RA is required for maintenance of the BRB. A–F) Longitudinal confocal SLO micrograph images from adult (3–5 mo old) Tg(l-fabp:DBP-EGFP) zebrafish before (A, C, E) and 24–48 h after treatment with DMSO (B), DEAB (5 μM; D) or BMS493 (10 μM; F). Vessels in F are obscured due to leakage. Scale bar, 200 µm. G) Quantification of extravascular space mean signal intensity calculated from confocal SLO images taken before treatment (Pretrt), after 24 h of treatment (24 h trt), and after 48 h of treatment (48 h trt) with DMSO, DEAB, or BMS493 (n = 4 fish/treatment). Error bars indicate sem. *P < 0.05.
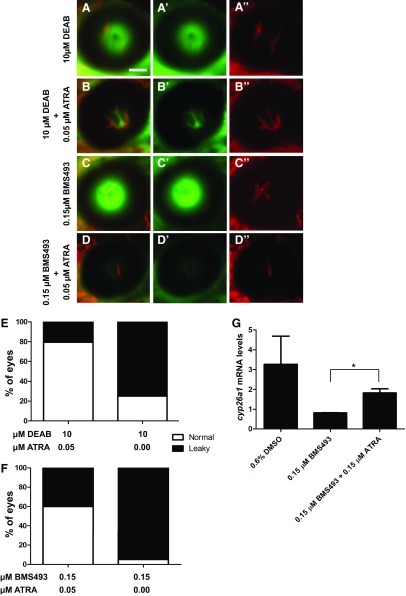
To determine whether the “leaky” phenotype observed with DEAB and BMS493 was a consequence of perturbed RA production, we evaluated whether ATRA could rescue BRB breakdown. Cotreatment of embryos and larvae with DEAB or BMS493 and ATRA from 2 to 7 dpf prevented BRB breakdown (Fig. 3A–D″), decreasing the percentage of larvae with BRB leakage from 78 to 23% in DEAB-treated fish (Fig. 3E) and from 98 to 59% in BMS493-treated fish (Fig. 3F). Rescue of RA signaling in larvae cotreated with BMS493 and ATRA was confirmed by quantitative RT-PCR of cyp26a1 expression, which is regulated by RA signaling (19) (Fig. 3G). Whereas cyp26a1 expression was decreased in larvae treated with BMS493 alone, cyp26a1 expression was rescued in larvae cotreated with BMS493 and ATRA. This indicates that BRB breakdown was a consequence of decreased RA production.
Figure 3.
ATRA can rescue BRB integrity in DEAB- and BMS493-treated zebrafish. A–D″) Merged fluorescent micrographs of eyes from 7 dpf Tg(lfabp:DBP-EGFP:flk1:mCherry) larvae cotreated with 0.05 µM ATRA and 0.15 μM BMS493 or 10 μM DEAB from 2 to 7 dpf. EGFP-tagged blood plasma (green, A′, B′, C′, D′) and mCherry-tagged vasculature (red, A″, B″, C″, D″) are shown. ATRA prevents BRB leakage in DEAB (A–B″) and BMS493-treated (C–D″) larvae. Scale bar, 50 µm. E, F) Percentage of embryos with a leaky BRB. P < 0.001 (Fisher’s exact test for both rescue experiments; n = 50 embryos/treatment). G) Expression of cyp26a1 in whole zebrafish larvae treated with DMSO, BMS493, or BMS493 + ATRA normalized to actb2. *P < 0.05 (Student’s t test).
Cyp26a1 overexpression disrupts BRB development and maintenance
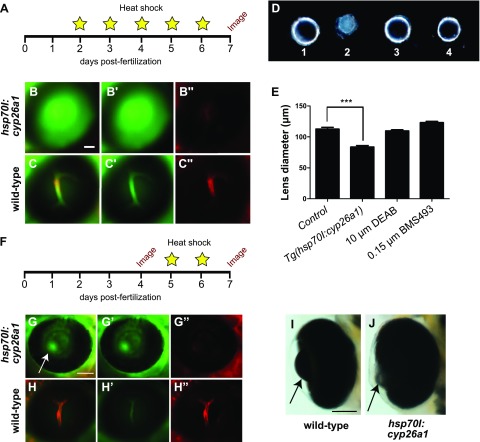
Degradation of RA is catalyzed by Cyp26 enzymes, which are members of the cytochrome P450 family (20). To determine whether increased degradation of RA by Cyp26 enzymes could regulate BRB breakdown, we used a transgenic zebrafish, Tg(hsp70I:cyp26a1), which expresses cyp26a1 driven by the heat shock promoter hsp70 (11). To visualize BRB integrity, Tg(hsp70I:cyp26a1) heterozygous fish were crossed with the Tg(lfabp:DBP:EGFP:flk1:mCherry) line. Embryos from this cross were subject to heat shock (38°C, 20 min) daily from 2 to 6 dpf to induce overexpression of Cyp26a1 (Fig. 4A). After heat shock, BRB integrity was assessed by fluorescent microscopy. Fish were categorized as leaky or nonleaky and then genotyped for the hsp70I:cyp26a1 transgene. Tg(hsp70I:cyp26a1) fish displayed complete BRB breakdown (Fig. 4B–B″), whereas their siblings that did not express the hsp70I:cyp26a1 transgene had an intact and tight BRB (Fig. 4C–C″). Heat-shocked Tg(hsp70I:cyp26a1) larvae also displayed significantly reduced lens size at 7 dpf (Fig. 4D, E), although overall eye size was unaffected (Supplemental Fig. 1E).
Figure 4.
Overexpression of Cyp26a1 prevents BRB development and maintenance. A–C″) Tg(hsp70I:cyp26a1;l-fabp:DBP-EGFP;flk1:mCherry) and Tg(l-fabp:DBP-EGFP;flk1:mCherry) embryos were heat shocked daily from 2 to 6 dpf (38°C for 20 min) and analyzed for BRB disruption at 6 dpf (A) by screening for DBP-EGFP (green) leakage from the vessels (red) under a fluorescent microscope (B–C″). Heat-shocked Tg(hsp70I:cyp26a1;l-fabp:DBP-EGFP;flk1:mCherry) larvae had complete breakdown of their BRBs (B–B″), whereas the BRB of heat-shocked Tg(l-fabp:DBP-EGFP;flk1:mCherry) larvae remained intact (C–C″). D) Isolated lenses from 7 dpf larvae treated with 1) 0.6% DMSO, 2) Tg(hsp70I:cyp26a1) larvae subject to heat shock protocol (A), 3) 10 µM DEAB, and 4) 0.15 µM BMS493 demonstrate decreased lens size in the heat-shocked Tg(hsp70I:cyp26a1) larvae. E) Quantification of lens diameter from 7 dpf larvae from control (0.6% DMSO-treated), heat-shocked Tg(hsp70I:cyp26a1), 10 µM DEAB-treated, and 0.15 µM BMS493-treated groups (n = 10 lenses/treatment group). ***P < 0.0001. Error bars indicate sd. F) To determine whether cyp26a1 overexpression would affect BRB integrity at later larval stages, after BRB development is complete, Tg(hsp70I:cyp26a1;l-fabp:DBP-EGFP;flk1:mCherry) and Tg(l-fabp:DBP-EGFP;flk1:mCherry) larvae were raised to 4 dpf without being subject to heat shock and normal BRB development was confirmed by fluorescent microscopy. Larvae were then heat shocked at 5 and 6 dpf (38°C for 20 min), and BRB integrity was checked again by fluorescent microscopy at 7 dpf. G–H″) Tg(hsp70I:cyp26a1;l-fabp:DBP-EGFP;flk1:mCherry) larvae subjected to this later heat shock protocol also developed complete BRB breakdown and small lenses (arrow, G–G″), whereas heat-shocked larvae that did not express hsp:701:cyp26a1 retained an intact BRB (H–H″). I, J) Light microscopy images of lateral views of Tg(l-fabp:DBP-EGFP;flk1:mCherry) (I) and Tg(hsp70I:cyp26a1;l-fabp:DBP-EGFP;flk1:mCherry) (J) 7 dpf larval zebrafish eyes following the later heat shock protocol (F). Scale bar, 50 µm.
To determine whether Cyp26a1 overexpression could also affect BRB maintenance at later larval stages, we subjected Tg(hsp70I:cyp26a1:lfabp:DBP-EGFP:flk1:mCherry) larvae to heat shock at only 5 and 6 dpf (Fig. 4F), at which point the BRB has been established and is fully enclosed (8, 9). Although the BRB of Tg(hsp70I:cyp26a1) larvae appeared intact at 4 dpf prior to heat shock, after heat shock at 7 dpf the fish displayed a profoundly leaky BRB and reduced lens size (Fig. 4G–J). Tg(hsp70I:cyp26a1) subjected to heat shock often had a reduced or absent swim bladder, but otherwise developed normal gross morphology (Supplemental Fig. 1D). This result indicates that overexpression of Cyp26a1 inhibits BRB development and maintenance, potentially by the increased degradation of RA.
Tight junctions are disrupted in the hyaloid vasculature of larvae exposed to inhibitors of RA signaling
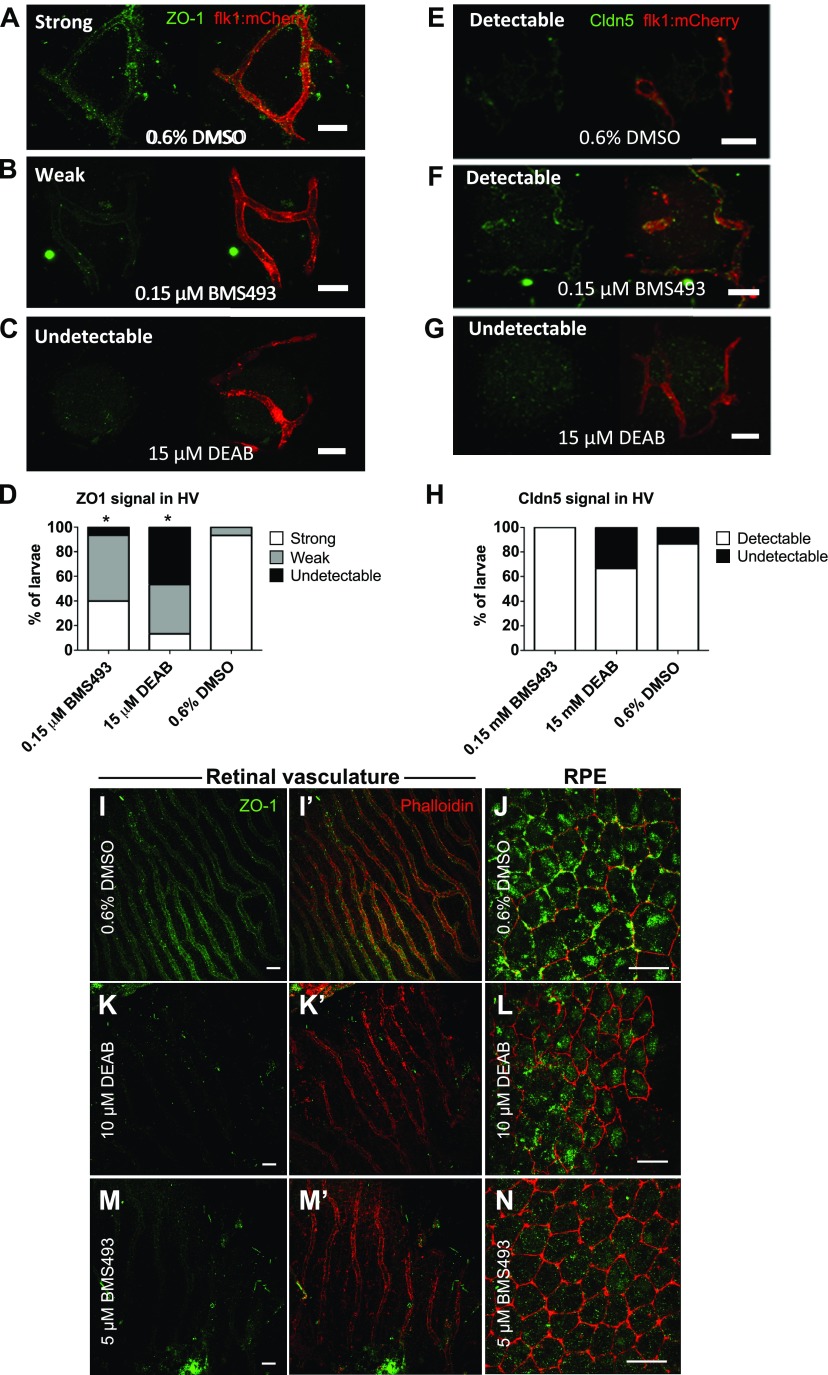
ECs forming the inner BRB are sealed by tight junction proteins, including ZO-1 and Claudin 5 (Cldn5), which are essential to maintaining BRB integrity (3). To determine whether the tight junctions were disrupted in the hyaloid vasculature after DEAB or BMS493 treatment, antibodies recognizing ZO-1 and Cldn5 were used to label RA signaling inhibitor–treated and untreated Tg(flk1:mCherry) larvae at 7 dpf. The specificity of these antibodies in zebrafish has been previously demonstrated (21). The hyaloid vasculature of untreated larvae was strongly labeled by antisera to ZO-1, and, although weaker than ZO-1 staining, Cldn5 labeling could also be clearly detected (Fig. 5A, E). ZO-1 expression was significantly disrupted in larvae treated with either inhibitor of RA signaling (Fig. 5B–D), whereas anti-Cldn5 labeling persisted in BMS493-treated animals (Fig. 5F) and in most DEAB-treated animals (Fig. 5G, H). Although anti-Cldn5 labeling was weak, it matched the transgenic expression pattern of EGFP-Cldn5b observed in Tg(fli1a:EGFP-cldn5b) animals (12) (Supplemental Fig. 3A), indicating that zebrafish Cldn5 protein was labeled by the antisera. EGFP-Cldn5b expression was also not disrupted in larvae treated with DEAB (Supplemental Fig. 3B) or BMS493 (Supplemental Fig. 3C). ZO-1 was disrupted in the retinal vasculature (Fig. 5I, I′, K, K′, M, M′) and RPE (Fig. 5J, L, N) of DEAB- and BMS493-treated adults (Fig. 5I–N).
Figure 5.
ZO1 expression is disrupted in the hyaloid vasculature of larvae treated with DEAB and BMS493. A–C) Confocal micrographs of 7 dpf Tg(flk1:mCherry) zebrafish vasculature (red) labeled with anti-Cldn5 or anti–ZO-1 (green). In fish treated with DMSO alone (A) anti–ZO-1 labeling was robust, whereas in BMS493-treated larvae (B) anti–ZO-1 labeling was weaker and in DEAB-treated larvae (C). ZO-1 was largely undetectable. Scale bars, 20 µm. D) Graph depicting the percentage of fish from each treatment group with strong, weak, or undetectable anti–ZO-1 labeling (n = 15 fish/treatment) P < 0.005 (χ2 test). E–G) Anti-Cldn5 labeling was detected throughout the hyaloid vasculature in fish treated with DMSO (E) or BMS493 (F) and in most fish treated with DEAB (G), Scale bars, 20 µm. H) Graph depicting the percentage of fish from each treatment group with detectable or undetectable anti-Cldn5 labeling (n = 15 fish/treatment). P = not significant. I–N) Confocal micrographs of the retinal vasculature and RPE of adult zebrafish treated with 0.6% DMSO, 10 µM DEAB, or 5 µM BMS493 for 48 h. ZO-1 antibody (green) labels the cellular junctions (labeled in red by fluorescent-coupled phalloidin) in the retinal vessels (I, I′) and RPE (J) of DMSO-treated control fish. Scale bars, 20 µm. K, L) In fish treated with DEAB, ZO-1 expression is disrupted in the retinal vessels (K, K′) and RPE (L). ZO-1 expression is also disrupted in the retinal vessels (M, M′) and RPE (N) of fish treated with BMS493. Scale bars, 10 µm.
Inhibition of RA signaling results in reduced visual acuity in zebrafish larvae
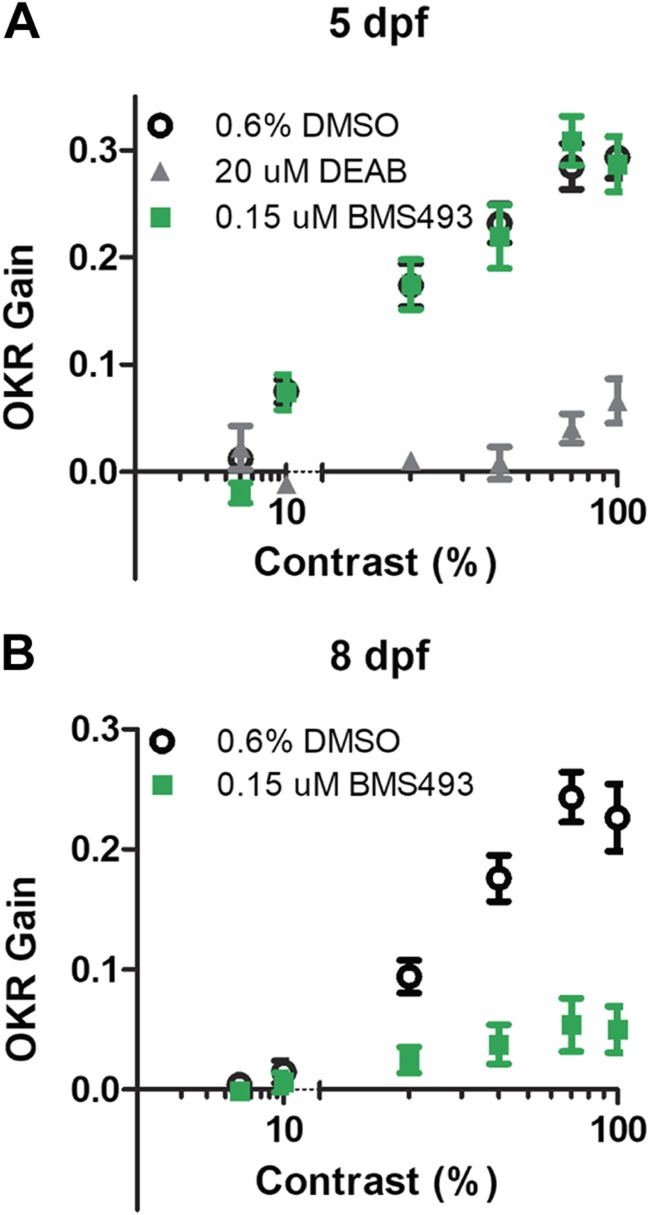
To determine whether RA signaling inhibitor–induced BRB breakdown affects visual function, we evaluated visual acuity in RA signaling inhibitor–treated larvae by OKR testing using the VisioTracker system (22). We measured OKR gain, defined as the ratio of stimulus velocity to eye velocity (23), while varying the contrast of the moving image. Whereas DMSO-treated control larvae showed the expected linear relationship between OKR gain and the logarithm of contrast at 5 dpf (16), larvae treated with DEAB from 2 dpf onward displayed a greatly reduced OKR gain (Fig. 6A). The OKR gain of BMS493-treated larvae was similar to that of DMSO-treated larvae at 5 dpf but was significantly reduced by 8 dpf (Fig. 6B). This indicates that inhibition of RA signaling causes decreased visual acuity in zebrafish larvae.
Figure 6.
Zebrafish larvae treated with DEAB or BMS493 have decreased visual acuity. A) OKR of 5 dpf zebrafish larvae treated from 2 dpf onward with 0.6% DMSO (open circles), 20 µM DEAB (gray triangles), or 0.15 µM BMS493 (green squares) as a function of contrast. B) OKR of 8 dpf zebrafish larvae treated with 0.6% DMSO (open circles) or 0.15 µM BMS493 (green squares) as a function of contrast (n = 15 larvae/group). Error bars indicate sem.
DISCUSSION
Using an in vivo zebrafish model, we show that RA signaling is necessary for BRB maintenance and visual function. The role of RA signaling in BRB integrity has not been previously explored, and our findings provide valuable insight into the molecular signaling pathways involved in the development and maintenance of this complex and poorly understood barrier. Although RA signaling has been previously shown to be involved in development of the BBB (6) and blood–spinal cord barrier (24), this is also the first report of RA signaling being necessary for continued barrier maintenance after development.
Both inhibition of RA synthesis by treatment with DEAB and blocking of RA binding to its nuclear receptors by treatment with BMS493 resulted in profound breakdown of the BRB in zebrafish larvae and adults (Fig. 7). Cotreatment with ATRA rescued BRB integrity in most fish, confirming that BRB breakdown is likely a direct result of the inhibition of RA signaling rather than an off-target effect of the drugs. Whereas BMS493 is a pan-RAR/RXR inverse agonist, ATRA binds to RARα with a 10-fold higher affinity than BMS493 (25). Thus, ATRA can efficiently compete with BMS493 in binding to RAR (26). BRB integrity was rescued in a significant proportion of the fish, although BRB breakdown was still observed in 23 and 59% of fish cotreated with 0.05 μM ATRA and 10 μM DEAB or 0.15 μM BMS493, respectively. A higher concentration of ATRA may be required for complete rescue of BRB integrity. However, treatment with higher doses of ATRA also resulted in teratogenic effects, including reduced eye size and abnormal lens appearance (data not shown), precluding analysis of BRB integrity.
Figure 7.
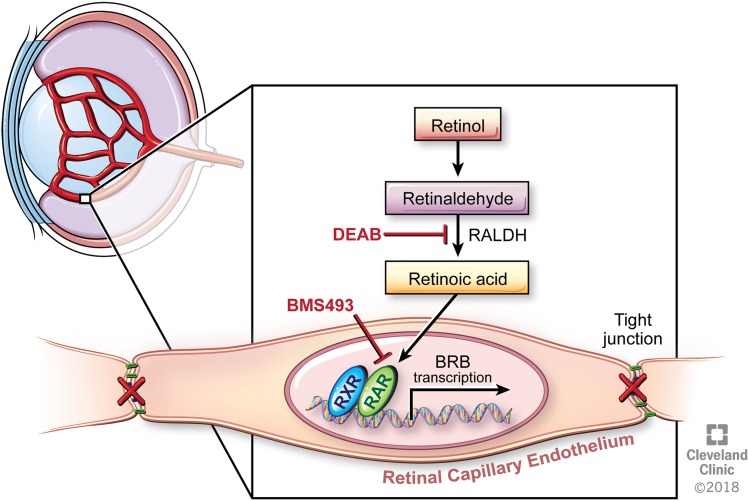
Summary model of RA signaling in the inner BRB. RA binds to RAR receptors in the nuclei of the ECs lining the retinal microvasculature, inducing the expression of BRB genes. When production of RA is blocked by DEAB or RA binding to its receptor is blocked by BMS493, the tight junctions between the ECs are disrupted, leading to BRB breakdown. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 2018. All Rights Reserved.
A role for RA signaling in maintenance of the BRB is further supported by our finding that increased degradation of RA by overexpression of Cyp26a1 also results in BRB breakdown. However, the role of the lens defects that were observed upon Cyp26a1 overexpression is unknown. The lens has been shown to be required for later stages of hyaloid development (9), and the effect of the decreased lens size on BRB development and maintenance in the heat-shocked Tg(hsp70I:cyp26a1) larvae has been unexplored.
RA is essential for retinal development and promotes photoreceptor differentiation (27–30). In the mouse retina, RALDH activity and RA production are very high during embryonic development (31), and these high RA levels may contribute to early BRB development. RALDH activity is also detected throughout the RPE both during development and at postnatal stages (32), where it may contribute to outer BRB development and maintenance. However, precisely which cells express RALDH and RAR/RXR involved in BRB development and maintenance remains unknown. In the developing human BBB, RA is produced by RALDH1/2-positive radial glial cells, and RARβ is expressed by the brain microvasculature (6). In the mouse retina, the Müller glia have been shown to express RALDH1 (33), which may be involved in RA production during BRB maintenance. Although RAR/RXR expression in retinal microvascular ECs has not been determined, all RAR/RXR isotypes are expressed in the developing eye, and RARα, RXRγ, and RXRβ are expressed in the retina both during development and at adult stages (34).
RAR mutations and vitamin A deficiency in early development result in ocular coloboma and microphthalmia, among other eye defects (35). Although inhibition of RA signaling in zebrafish at earlier developmental stages results in disruption or delay of retinal development and microphthalmia (36, 37), inhibition of RA signaling beginning at 2 dpf in our study had no significant effect on eye size (Supplemental Fig. 1E). The role of RA signaling in later ocular development and at adult stages has not been fully explored. Our results suggest that RA signaling plays a significant role in the adult retina through maintenance of the BRB.
Although it is possible that breakdown of the BRB in zebrafish results in reduction of visual acuity, there are other possible factors for this effect. Previous studies have reported that treatment of zebrafish embryos with exogenous RA from 2 to 5 dpf increases the number of rod photoreceptors, whereas pharmacological inhibition of RA synthesis from 2 to 3 dpf decreases the number of cells expressing rod opsin (27). Thus, defective visual acuity in DEAB-treated larvae at 5 dpf could be due to loss of rod photoreceptors. Interestingly, larvae treated with BMS493, which inhibits the RA receptors, display normal visual acuity until 8 dpf, several days after the onset of BRB breakdown. Whether the eventual decline in visual acuity in BMS493-treated larvae is due to direct effects of RA inhibition on the photoreceptors or due to effects of BRB breakdown on the eye remains to be determined.
The disruption of ZO-1 and persistence of Cldn5 at the EC junctions is consistent with previous observations in the developing BBB of BMS493-treated embryonic mice (6). Identification of the signaling pathways involved in RA signaling inhibitor–mediated BRB breakdown may reveal why ZO-1 expression is affected by the inhibition of RA signaling, whereas Cldn5 expression is not. ZO-1 expression is necessary for the assembly and maintenance of functional tight junctions (38), and it is thus possible that altering ZO-1 expression alone is sufficient for BRB disruption. However, the precise molecular mechanism by which tight junction protein expression is disrupted remains to be ascertained. RA may act directly on the microvascular ECs to regulate tight junction protein expression, or it may induce changes in other cells of the neural retina to indirectly regulate BRB integrity. Generation of zebrafish genetic models in which RA signaling is disrupted specifically in ECs may help to answer this question in future studies.
Wnt signaling may play a role in the RA-mediated effects on the BRB and will be an interesting area for future research. ECs in both the BBB and BRB require canonical Wnt signaling for barrier development and maintenance (39). Additionally, RA has been shown to influence Wnt signaling in brain ECs (40). However, the precise signals required for BRB development and maintenance differ in some cases from those required for BBB development and maintenance. For instance, it has been reported that Wnt coreceptor LDL receptor–related protein 5 is required for BRB maintenance, but not for BBB maintenance, and that loss of Wnt receptor Frizzled 4 or its Wnt-like ligand Norrin have more severe effects on the BRB than the BBB (39, 41). Thus, although it has recently been demonstrated that BBB protein expression in vitro is enhanced by pharmacological and not physiologic concentrations of RA and that RA-deficient Rdh10 mutant mice maintain an intact BBB (40), it is possible that the BRB is more sensitive to perturbations in RA signaling than the BBB and/or that there are some species-specific differences in the role of RA signaling between fish and mice.
The role of RA signaling in BRB maintenance could have important therapeutic implications for prevention and treatment of diabetic macular edema. Human patients with type 1 diabetes are reported to have decreased serum levels of vitamin A (42), and decreased levels of tissue vitamin A are associated with obesity and type 2 diabetes (43, 44). Interestingly, treatment of streptozotocin-induced diabetic mice with ATRA prevents retinal vascular leakage (45). Further studies are needed to fully understand the roles of RA signaling in diabetic macular edema.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Mariya Ali (Cleveland Clinic) and Alecia Cutler (Cleveland Clinic) for technical assistance; Dr. Johannes von Lintig (Case Western Reserve University) and Dr. Brian Perkins (Cleveland Clinic) for helpful discussions; Dr. Didier Stainier (Max Planck Institute for Heart and Lung Research, Bad Nauheim, Germany), Dr. Suk-Won Jin (Yale Cardiovascular Research Center, New Haven, CT, USA), and Dr. Neil Chi (University of California, San Diego, La Jolla, CA, USA) for providing the Tg(flk1:mCherry) fish; Dr. Brant Weinstein (National Institutes of Health, Bethesda, MD, USA) for providing the Tg(fli1a:EGFP-cldn5b) fish; and Dr. Nicola Blum and Dr. Gerrit Begemann (both from the University of Bayreuth, Bayreuth, Germany) for providing the Tg(hsp70I:cyp26a1) fish. The authors also thank Amanda Mendelsohn (Center for Medical Art and Photography, Cleveland Clinic) for illustrations. This work was supported by U.S. National Institutes of Health, National Eye Institute Grants EY016490, EY015638, EY026181, T32EY024236, and P30EY025585; a Research to Prevent Blindness Unrestricted/Challenge Grant; and a Foundation Fighting Blindness Center Grant. The authors declare no conflicts of interest.
Glossary
- ATRA
all-trans retinoic acid
- BBB
blood-brain barrier
- BMS493
4-[(1E)-2-[5,6-dihydro-5,5-dimethyl-8-(2-phenylethynyl)-2-naphthalenyl]ethenyl]benzoic acid
- BRB
blood-retinal barrier
- DEAB
N,N-diethylaminobenzaldehyde
- dpf
days postfertilization
- EC
endothelial cell
- EGFP
enhanced green fluorescent protein
- OKR
optokinetic response
- RA
retinoic acid
- RALDH
retinaldehyde dehydrogenase
- RAR
retinoic acid receptor
- RPE
retinal pigment epithelium
- RXR
retinoid X receptor
- SLO
scanning laser ophthalmoscopy
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
L. M. Pollock, J. Xie, and B. Anand-Apte designed the research; L. M. Pollock, J. Xie, and B. A. Bell performed the research and analyzed data; and L. Pollock wrote the paper.
REFERENCES
- 1.Campbell M., Humphries P. (2012) The blood-retina barrier: tight junctions and barrier modulation. Adv. Exp. Med. Biol. 763, 70–84 [PubMed] [Google Scholar]
- 2.Cunha-Vaz J., Faria de Abreu J. R., Campos A. J. (1975) Early breakdown of the blood-retinal barrier in diabetes. Br. J. Ophthalmol. 59, 649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klaassen I., Van Noorden C. J., Schlingemann R. O. (2013) Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog. Retin. Eye Res. 34, 19–48 [DOI] [PubMed] [Google Scholar]
- 4.Díaz-Coránguez M., Ramos C., Antonetti D. A. (2017) The inner blood-retinal barrier: cellular basis and development. Vision Res. 139, 123–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blomhoff R., Blomhoff H. K. (2006) Overview of retinoid metabolism and function. J. Neurobiol. 66, 606–630 [DOI] [PubMed] [Google Scholar]
- 6.Mizee M. R., Wooldrik D., Lakeman K. A., van het Hof B., Drexhage J. A., Geerts D., Bugiani M., Aronica E., Mebius R. E., Prat A., de Vries H. E., Reijerkerk A. (2013) Retinoic acid induces blood-brain barrier development. J. Neurosci. 33, 1660–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott N. J., Patabendige A. A., Dolman D. E., Yusof S. R., Begley D. J. (2010) Structure and function of the blood-brain barrier. Neurobiol. Dis. 37, 13–25 [DOI] [PubMed] [Google Scholar]
- 8.Xie J., Farage E., Sugimoto M., Anand-Apte B. (2010) A novel transgenic zebrafish model for blood-brain and blood-retinal barrier development. BMC Dev. Biol. 10, 76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartsock A., Lee C., Arnold V., Gross J. M. (2014) In vivo analysis of hyaloid vasculature morphogenesis in zebrafish: a role for the lens in maturation and maintenance of the hyaloid. Dev. Biol. 394, 327–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin S. W., Beis D., Mitchell T., Chen J. N., Stainier D. Y. (2005) Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 132, 5199–5209 [DOI] [PubMed] [Google Scholar]
- 11.Blum N., Begemann G. (2012) Retinoic acid signaling controls the formation, proliferation and survival of the blastema during adult zebrafish fin regeneration. Development 139, 107–116 [DOI] [PubMed] [Google Scholar]
- 12.Yu J. A., Castranova D., Pham V. N., Weinstein B. M. (2015) Single-cell analysis of endothelial morphogenesis in vivo. Development 142, 2951–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell B. A., Xie J., Yuan A., Kaul C., Hollyfield J. G., Anand-Apte B. (2014) Retinal vasculature of adult zebrafish: in vivo imaging using confocal scanning laser ophthalmoscopy. Exp. Eye Res. 129, 107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang R., Dodd A., Lai D., McNabb W. C., Love D. R. (2007) Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim. Biophys. Sin. (Shanghai) 39, 384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung S. H., Kim Y. S., Lee Y. R., Kim J. S. (2016) High glucose-induced changes in hyaloid-retinal vessels during early ocular development of zebrafish: a short-term animal model of diabetic retinopathy. Br. J. Pharmacol. 173, 15–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniele L. L., Emran F., Lobo G. P., Gaivin R. J., Perkins B. D. (2016) Mutation of wrb, a component of the guided entry of tail-anchored protein pathway, disrupts photoreceptor synapse structure and function. Invest. Ophthalmol. Vis. Sci. 57, 2942–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wendling O., Dennefeld C., Chambon P., Mark M. (2000) Retinoid signaling is essential for patterning the endoderm of the third and fourth pharyngeal arches. Development 127, 1553–1562 [DOI] [PubMed] [Google Scholar]
- 18.Russo J. E., Hauguitz D., Hilton J. (1988) Inhibition of mouse cytosolic aldehyde dehydrogenase by 4-(diethylamino)benzaldehyde. Biochem. Pharmacol. 37, 1639–1642 [DOI] [PubMed] [Google Scholar]
- 19.Dobbs-McAuliffe B., Zhao Q., Linney E. (2004) Feedback mechanisms regulate retinoic acid production and degradation in the zebrafish embryo. Mech. Dev. 121, 339–350 [DOI] [PubMed] [Google Scholar]
- 20.Thatcher J. E., Isoherranen N. (2009) The role of CYP26 enzymes in retinoic acid clearance. Expert Opin. Drug Metab. Toxicol. 5, 875–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong J.-Y., Kwon H.-B., Ahn J.-C., Kang D., Kwon S.-H., Park J. A., Kim K.-W. (2008) Functional and developmental analysis of the blood-brain barrier in zebrafish. Brain Res. Bull. 75, 619–628 [DOI] [PubMed] [Google Scholar]
- 22.Mueller K. P., Schnaedelbach O. D., Russig H. D., Neuhauss S. C. (2011) VisioTracker, an innovative automated approach to oculomotor analysis. J. Vis. Exp. 56:3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinner O., Rick J. M., Neuhauss S. C. (2005) Contrast sensitivity, spatial and temporal tuning of the larval zebrafish optokinetic response. Invest. Ophthalmol. Vis. Sci. 46, 137–142 [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y., Zheng B., Ye L., Zhang H., Zhu S., Zheng X., Xia Q., He Z., Wang Q., Xiao J., Xu H. (2016) Retinoic acid prevents disruption of blood-spinal cord barrier by inducing autophagic flux after spinal cord injury. Neurochem. Res. 41, 813–825 [DOI] [PubMed] [Google Scholar]
- 25.Germain P., Chambon P., Eichele G., Evans R. M., Lazar M. A., Leid M., De Lera A. R., Lotan R., Mangelsdorf D. J., Gronemeyer H. (2006) International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol. Rev. 58, 712–725 [DOI] [PubMed] [Google Scholar]
- 26.Raverdeau M., Gely-Pernot A., Féret B., Dennefeld C., Benoit G., Davidson I., Chambon P., Mark M., Ghyselinck N. B. (2012) Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc. Natl. Acad. Sci. USA 109, 16582–16587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyatt G. A., Schmitt E. A., Fadool J. M., Dowling J. E. (1996) Retinoic acid alters photoreceptor development in vivo. Proc. Natl. Acad. Sci. USA 93, 13298–13303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelley M. W., Turner J. K., Reh T. A. (1994) Retinoic acid promotes differentiation of photoreceptors in vitro. Development 120, 2091–2102 [DOI] [PubMed] [Google Scholar]
- 29.Kelley M. W., Williams R. C., Turner J. K., Creech-Kraft J. M., Reh T. A. (1999) Retinoic acid promotes rod photoreceptor differentiation in rat retina in vivo. Neuroreport 10, 2389–2394 [DOI] [PubMed] [Google Scholar]
- 30.Stenkamp D. L., Gregory J. K., Adler R. (1993) Retinoid effects in purified cultures of chick embryo retina neurons and photoreceptors. Invest. Ophthalmol. Vis. Sci. 34, 2425–2436 [PubMed] [Google Scholar]
- 31.Dräger U. C., McCaffery P. (1997) Retinoic acid and development of the retina. Prog. Retin. Eye Res. 16, 323–351 [Google Scholar]
- 32.McCaffery P., Mey J., Dräger U. C. (1996) Light-mediated retinoic acid production. Proc. Natl. Acad. Sci. USA 93, 12570–12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDowell R. E., McGahon M. K., Augustine J., Chen M., McGeown J. G., Curtis T. M. (2016) Diabetes impairs the aldehyde detoxifying capacity of the retina. Invest. Ophthalmol. Vis. Sci. 57, 4762–4771 [DOI] [PubMed] [Google Scholar]
- 34.Mori M., Ghyselinck N. B., Chambon P., Mark M. (2001) Systematic immunolocalization of retinoid receptors in developing and adult mouse eyes. Invest. Ophthalmol. Vis. Sci. 42, 1312–1318 [PubMed] [Google Scholar]
- 35.Lohnes D., Mark M., Mendelsohn C., Dollé P., Dierich A., Gorry P., Gansmuller A., Chambon P. (1994) Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development 120, 2723–2748 [DOI] [PubMed] [Google Scholar]
- 36.Le H. G., Dowling J. E., Cameron D. J. (2012) Early retinoic acid deprivation in developing zebrafish results in microphthalmia. Vis. Neurosci. 29, 219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marsh-Armstrong N., McCaffery P., Gilbert W., Dowling J. E., Dräger U. C. (1994) Retinoic acid is necessary for development of the ventral retina in zebrafish. Proc. Natl. Acad. Sci. USA 91, 7286–7290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Umeda K., Ikenouchi J., Katahira-Tayama S., Furuse K., Sasaki H., Nakayama M., Matsui T., Tsukita S., Furuse M., Tsukita S. (2006) ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 126, 741–754 [DOI] [PubMed] [Google Scholar]
- 39.Wang Y., Rattner A., Zhou Y., Williams J., Smallwood P. M., Nathans J. (2012) Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell 151, 1332–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonney S., Siegenthaler J. A. (2017) Differential effects of retinoic acid concentrations in regulating blood-brain barrier properties. eNeuro 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y., Wang Y., Tischfield M., Williams J., Smallwood P. M., Rattner A., Taketo M. M., Nathans J. (2014) Canonical WNT signaling components in vascular development and barrier formation. J. Clin. Invest. 124, 3825–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krempf M., Ranganathan S., Ritz P., Morin M., Charbonnel B. (1991) Plasma vitamin A and E in type 1 (insulin-dependent) and type 2 (non-insulin-dependent) adult diabetic patients. Int. J. Vitam. Nutr. Res. 61, 38–42 [PubMed] [Google Scholar]
- 43.Trasino S. E., Tang X. H., Jessurun J., Gudas L. J. (2015) Obesity leads to tissue, but not serum vitamin A deficiency. Sci. Rep. 5, 15893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trasino S. E., Tang X. H., Jessurun J., Gudas L. J. (2016) Retinoic acid receptor β2 agonists restore glycaemic control in diabetes and reduce steatosis. Diabetes Obes. Metab. 18, 142–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishikiori N., Osanai M., Chiba H., Kojima T., Mitamura Y., Ohguro H., Sawada N. (2007) Glial cell-derived cytokines attenuate the breakdown of vascular integrity in diabetic retinopathy. Diabetes 56, 1333–1340 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.