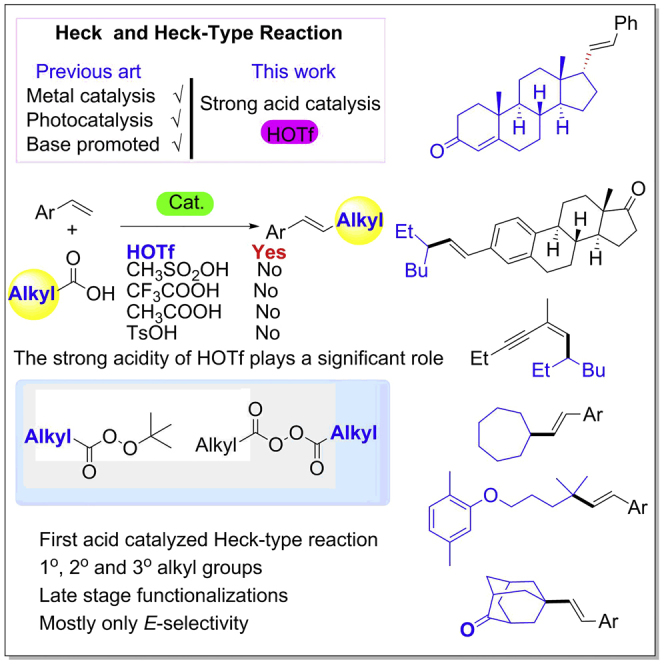
Summary
The Heck reaction, along with other cross-coupling reactions, led to a revolution in organic chemistry. In the last 50 years, metal-catalyzed, photo-induced, or base-mediated Heck and Heck-type reactions have been elegantly developed. Brønsted acid-catalyzed Heck (or Heck-type) reactions are still unknown, however. By introducing alkyl peroxides as the key intermediates, primary, secondary, and tertiary aliphatic carboxylic acids are therefore applied here in a one-pot Brønsted acid-catalyzed Heck-type reaction, to deliver E-alkenes exclusively in most cases. The use of HOTf is vital to the reaction, whose mechanism is supported by both experimental and computational results. This method can be expanded to the direct alkylation of complex natural products.
Subject Areas: Organic Synthesis, Organic Chemistry Methods, Natural Product Synthesis
Graphical Abstract
Highlights
-
•
First acid-catalyzed Heck-type reaction
-
•
Aliphatic acids are utilized as the sources of alkyl functionalities
-
•
E-alkenes exclusively in most cases
-
•
Strong acid effect
Organic Synthesis; Organic Chemistry Methods; Natural Product Synthesis
Introduction
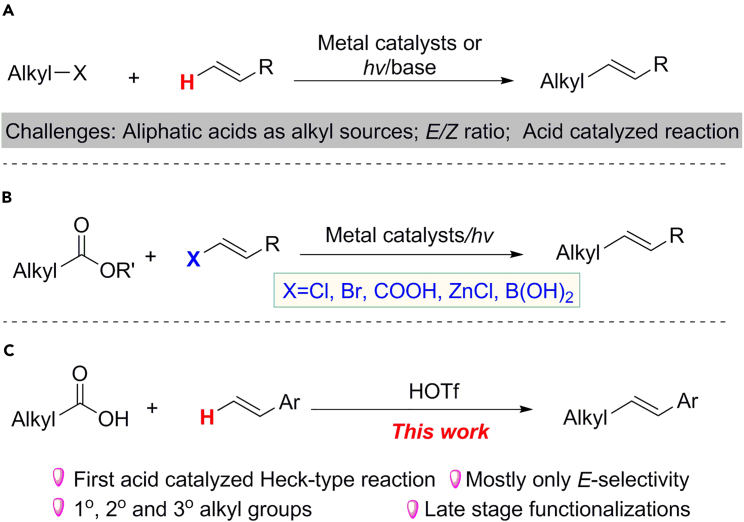
The Heck reaction, pioneered by Heck and Mizoroki in the late 1960s and the early 1970s (Heck, 1968, Mizoroki et al., 1971, Heck and Nolley, 1972), along with other cross-coupling reactions, led to a revolution in organic chemistry (Johansson Seechurn et al., 2012). In the last 50 years, many types of Heck and Heck-type reactions, including metal-catalyzed (Heck, 1968, Mizoroki et al., 1971, Heck and Nolley, 1972, Littke and Fu, 2001, Farrington et al., 2002, Na et al., 2004, Loska et al., 2008, Delcamp et al., 2013, Nishikata et al., 2013, Standley and Jamison, 2013), photo-induced (Iqbal et al., 2012, Liu et al., 2013, Paria et al., 2014, Yu et al., 2014), or base-mediated (Rueping et al., 2011, Shirakawa et al., 2011, Sun et al., 2011) reactions, have been elegantly developed (Beletskaya and Cheprakov, 2000, Dounay and Overman, 2003, Wu et al., 2010, Le Bras and Muzart, 2011, Mc Cartney and Guiry, 2011, Tang et al., 2015). Notwithstanding these classical reaction modes, there is no precedent of Brønsted acid-catalyzed or Brønsted acid-promoted Heck (or type) reaction being realized. Moreover, compared with aryl Heck reactions, the alkyl-Heck (type) reaction has been developed less. This is due mainly to the potential accompanying side reactions. Significant breakthroughs in alkyl-Heck-type reactions have, however, been made (Ikeda et al., 2002, Liu et al., 2012, Liu et al., 2015, Nishikata et al., 2013, Mcmahon and Alexanian, 2014, Zou and Zhou, 2014, Kurandina et al., 2018, Wang et al., 2018) (Scheme 1A), and in this article, we report a Brønsted acid-catalyzed alkyl-Heck-type reaction.
Scheme 1.
Intermolecular Alkyl-Heck-Type Reaction of General Alkyl Groups and Decarboxylative Vinylic Alkylation of Aliphatic Acids
(A) Previous alkyl-Heck-type reactions by Oshima, Alexanian, Zhou, Li, Fu, Lei, and Nishikata.
(B) Previous decarboxylative vinylic alkylation with aliphatic acid derivatives.
(C) This work: Brønsted acid-catalyzed alkyl-Heck-type reaction.
As is well known, alkyl halides are one of the most frequently used alkyl functionalities for alkyl-Heck-type reactions (Kambe et al., 2011, Weix, 2015, Tellis et al., 2016, Choi and Fu, 2017). However, their shortcomings, such as limited availability and perceived instability might prevent more extensive applications (Qin et al., 2016). Furthermore, there are still significant challenges remaining for alkyl-Heck-type reactions such as E/Z selectivity, use of metal catalysis, and diversity of alkyl sources (Scheme 1A). Carboxylic acids are inexpensive, stable, non-toxic, and structurally diverse feedstock chemicals that have been widely used in numerous reactions. For example, they have been utilized in cross-coupling with prefunctionalized alkenes such as vinyl halides or their derivatives to generate alkenes (Mai et al., 2013, Noble et al., 2015, Toriyama et al., 2016, Wang et al., 2016, Edwards et al., 2017, Xu et al., 2017, Zhang et al., 2017) (Scheme 1B). However, the decarboxylative cross-couplings of aliphatic acids or their derivatives with alkenes (X = H) are very rare (Wang et al., 2018). As part of our ongoing interest in the application of aliphatic acids as the alkyl source (Li et al., 2016, Ge et al., 2017, Jian et al., 2017, Qian et al., 2017, Ye et al., 2017, Zhu et al., 2017) and our interests in the discovery of different reaction models of alkyl peroxides, we have developed the first Brønsted acid-catalyzed alkyl-Heck-type reaction of alkenes with aliphatic acids via alkyl peroxide intermediates (Scheme 1C).
Results and Discussion
Optimization Study
We commenced our studies by screening a variety of Brønsted acids for the alkyl-Heck-type reaction of styrene with aliphatic acid. The aliphatic acid was converted into alkyl peresters in the presence of trifluoroacetic anhydride (TFAA) and tert-butyl hydroperoxide (TBHP) and used in situ for the subsequent step (Donchak et al., 2006). The best Brønsted acid was found to be HOTf, which offered the desired alkylated alkene 3 exclusively as a single E-isomer in 88% yield, determined by 1H nuclear magnetic resonance (NMR) analysis (Equation 1 and Table 1, entry 1). Studies of acids showed that Tf2O had a lower efficiency (Table 1, entry 2) and other Brønsted acids such as TsOH⋅H2O, CF3COOH, HOAc, and MeSO3H were ineffective in this reaction (Table 1, entries 3–6). When performed at room temperature (rt), the reaction afforded the desired product in 70% yield (Table 1, entry 7). To exclude the possibility of interference of trace amount of metal in HOTf, the HOTf was used after redistillation and the product was obtained in the same yield (Table 1, entry 8). The role of light was investigated by conducting the reaction in the dark, but no difference in the yield was observed (Table 1, entry 9). In the absence of HOTf, the alkyl peroxide decomposed completely and the styrene remained unchanged (Table 1, entry 10).
Table 1.
Optimizations of Reaction Condition
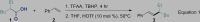 | ||
|---|---|---|
| Entry | Variation from the Standard Conditions | Yield(%)a,b |
| 1 | None | 88(75c) |
| 2 | Tf2O instead of HOTf | 78 |
| 3 | TsOH⋅H2O instead of HOTf | Trace |
| 4 | CF3COOH instead of HOTf | Trace |
| 5 | HOAc instead of HOTf | Trace |
| 6 | MeSO3H instead of HOTf | Trace |
| 7 | Room temperature instead of 50°C | 70 |
| 8 | Fresh distilled HOTf | 88 |
| 9 | In dark | 90 |
| 10 | Without HOTf | Trace |
Reaction conditions: First, 2-ethylhexanoic acid 1 (1.5 mmol), TBHP (1.5 mmol), and TFAA (1.5 mmol) at 0°C–rt for 4 hr, and then THF (2 mL), styrene 2 (0.5 mmol), and HOTf (0.05 mmol) were added. The mixture was stirred at 50°C for 8 hr.
1H NMR yield.
Yield of the isolated product.
Scope of the Investigation
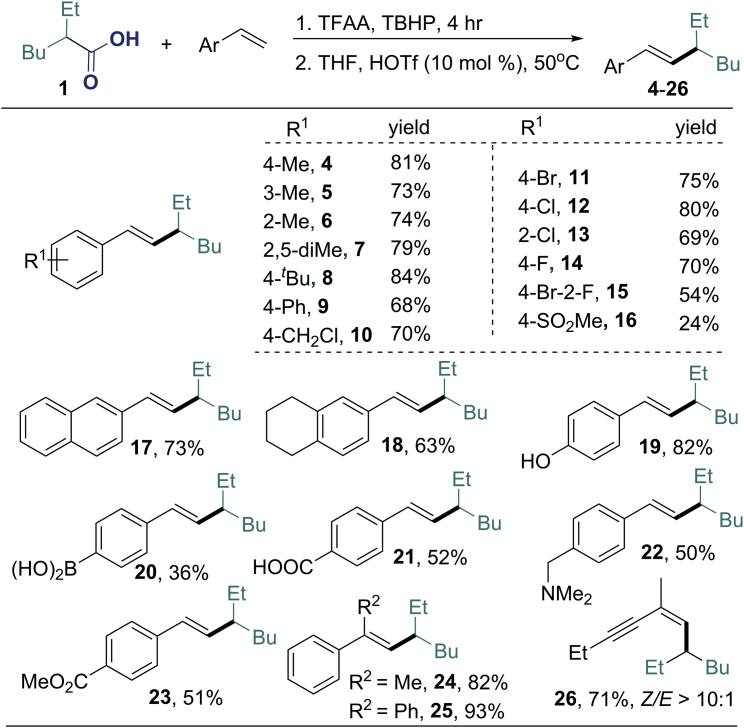
With the identified conditions in hand, we studied the scope of alkenes for this one-pot process (Figure 1). In most of the cases, the products were obtained as a single E-isomer. Reactions of vinyl arenes containing carbon substituents at the o-, m-, and p-positions afforded the corresponding products (4–10) in good yield (68–84%). Vinyl arenes containing halides reacted with 2-ethylhexanoic acid to give the desired products (11–15) in moderate to good yield (54%–80%). Functional groups, such as dimethylaminomethyl, and even free carboxylic acid and boronic acid were compatible with the reaction conditions (20–23). α-Methylstyrene and α-phenylstyrene participated in the reaction smoothly, providing the products (24, 25) in 82% and 93% yields, respectively. Furthermore, an enyne was a suitable substrate for this reaction, and the corresponding terminal-cross-coupled product (26) was obtained in good yield (71%). 1-Octene, an unactivated alkene, examined under the standard reaction conditions was not reactive to this reaction.
Figure 1.
Alkyl-Heck-Type Reaction of Alkenes
Top: One-pot process from aliphatic acid: First, 2-ethylhexanoic acid 1 (1.5 mmol), TBHP (1.5 mmol), and TFAA (2.0 mmol) at 0°C–rt for 4 hr, and then THF (2 mL), alkene (0.5 mmol), and HOTf (0.05 mmol) were added. The mixture was stirred at 50°C for 8 hr; yields of isolated products.
Bottom: HOTf (0.35 mmol) was added for 16, 18, 19, and 21.
The acetyl group on oxygen atom was removed under the reaction conditions for 19.
HOTf (0.75 mmol) was added for 22.
See also Figures S45–S94.
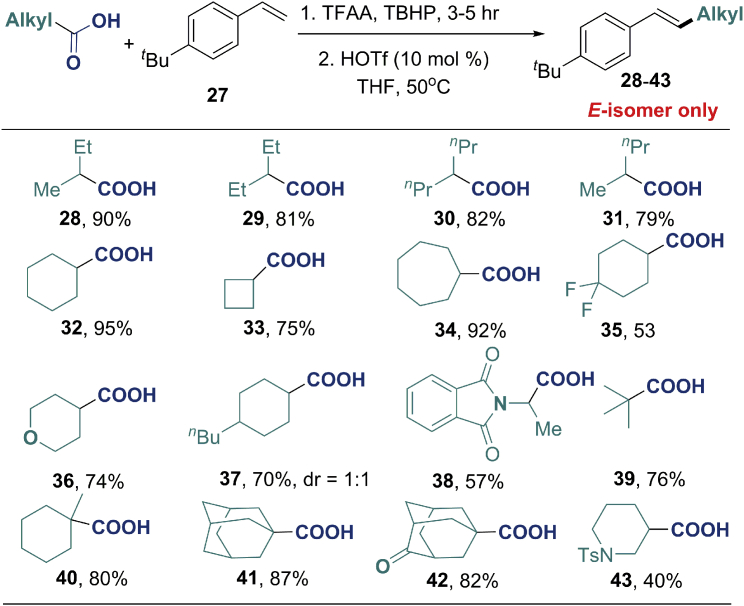
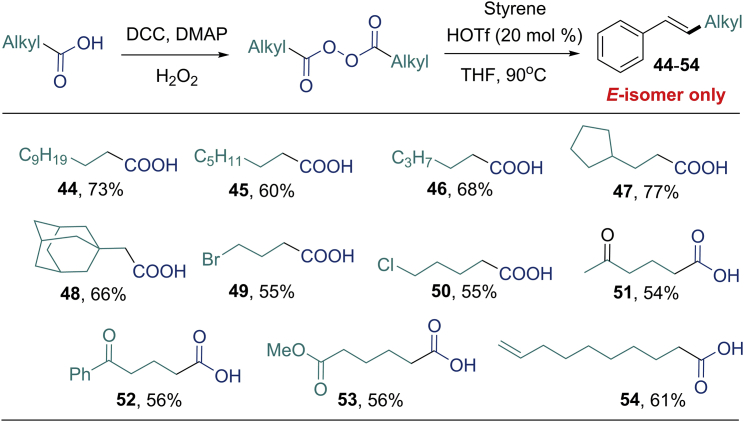
Next, we proceeded to study the scope of the reaction with respect to secondary and tertiary aliphatic carboxylic acids (Figure 2). The desired products (28–43) were obtained in moderate to high yields, using acyclic or cyclic aliphatic acids. The compatibility of various functional groups was good, and many functional groups, such as carbonyl (42), imide (38), amine (43), and ether (36), were tolerated. Most importantly, the E/Z selectivity of this reaction was excellent and only E-alkenes were observed. We then tried to expand this reaction to primary aliphatic acids, but the desired products were obtained in low yields as the methylated vinylic products were observed as by-products (Zhu et al., 2017). To overcome this problem, primary aliphatic acids were converted into alkyl diacyl peroxides and then subjected to the reaction (Figure 3). With the similar reaction conditions (please see Table S4 for details), generic primary aliphatic acids afforded the corresponding products (44–48) in good yields (60–77%). Primary aliphatic acids with functionalities, e.g., the bromide (49), chloride (50), ketones (51 and 52), ester (53), or the alkene (54) were well tolerated in the protocol, delivering the corresponding products in moderate to good yields. In every case, the E-alkene was obtained exclusively.
Figure 2.
Alkyl-Heck-Type Reaction of Secondary and Tertiary Aliphatic Acids
Top: One-pot process from aliphatic acid: First, acid (1.5 mmol), TBHP (1.5 mmol), and TFAA (2.0 mmol) at 0°C–rt for 3–5 hr, and then THF (2 mL), styrene 27 (0.5 mmol), and HOTf (0.05 mmol) were added. The mixture was stirred at 50°C for 8 hr; yields of isolated products.
Bottom: Styrene 27 (0.5 mmol), perester (1.25 mmol), and HOTf (0.1 mmol) at 80°C for 8 hr for 35, 36, 38, 42, and 43.
See also Figures S95–S127.
Figure 3.
Alkyl-Heck-Type Reaction of Primary Aliphatic Acids
Top: Reaction conditions: alkyl diacyl peroxide (synthesized from acid, 1.0 mmol), styrene 2 (0.5 mmol), and HOTf (0.1 mmol) in THF (1 mL); yields of isolated products.
Bottom: Alkyl diacyl peroxide (synthesized from acid, 1.0 mmol), styrene 2 (0.5 mmol), and HOTf (0.25 mmol) in THF (2 mL) for 49 and 50.
See also Figures S128–S149.
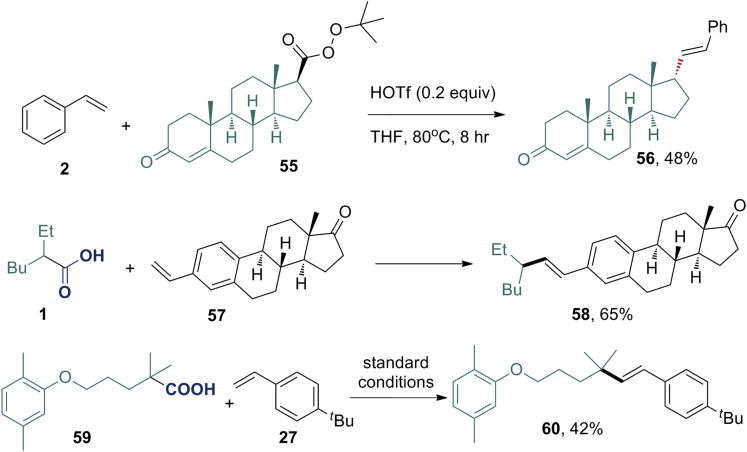
Synthetic Applications
To highlight the synthetic utility of this methodology (Scheme 2), the perester (55), which is readily derived from the corresponding steroidal carboxylic acid, was coupled with styrene in the presence of HOTf. The decarboxylative Heck-type coupling product (56) was obtained in 48% yield as a single isomer. The configuration of the product (56) was reversed, and this was confirmed by X-ray crystallographic analysis (please see Tables S5 and S6 for details). The reaction of 57 afforded the desired product (58) in 65% yield with E-selectivity. Gemfibrozil 59, an oral drug used to lower lipid levels, could also be converted into the vinylated product (60). These examples demonstrated that this reaction is potentially useful for the functionalization of complex molecules in the late stage.
Scheme 2.
Synthetic Applications
See also Figures S150–S155.
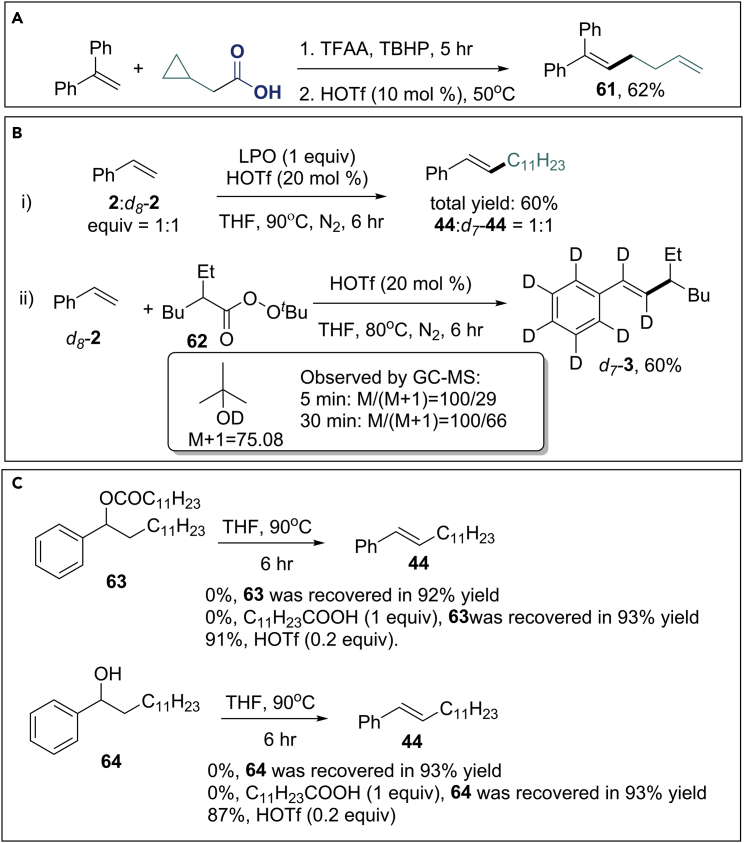
Mechanistic Study
To probe the mechanism of the reaction, a series of control experiments were performed. The reaction of α-phenylstyrene with 2-cyclopropylacetic acid under the standard conditions afforded the ring-opening product (61) in 62% yield (Scheme 3A), supporting the assumption of the involvement of radical species in the reaction. The competitive reaction of styrene and d8-styrene used in 1:1 ratio in the presence of HOTf and lauroyl peroxide (LPO) offered an identical yield of the corresponding products (Scheme 3B). When the reaction of d8-styrene with perester 62 was performed in tetrahydrofuran (THF), the desired product (d7-3) was isolated (Scheme 3B). Interestingly, the deuterated side products d(OD)-butanol were detected by gas chromatography-mass spectrometry (GC-MS). To further explore the mechanism, possible intermediates 63 and 64 were synthesized and tested with or without HOTf (Scheme 3C). Compounds 63 and 64 are thermally stable in the absence or presence of one equivalent of C11H23COOH. Even though compounds 63 and 64 can be converted to the desired alkene products in the presence of 0.2 equivalent of HOTf, it is unlikely that they are competent intermediates because the formation of 63 or 64 was not observed using GC-MS when the corresponding Heck reaction was conducted no matter with or without HOTf (Ge et al., 2017).
Scheme 3.
Mechanism Studies
(A) Radical clock reaction.
(B) Deuterium labeling experiment.
(C) Exclusion of possible intermediates.
See also Figures S156–S164.
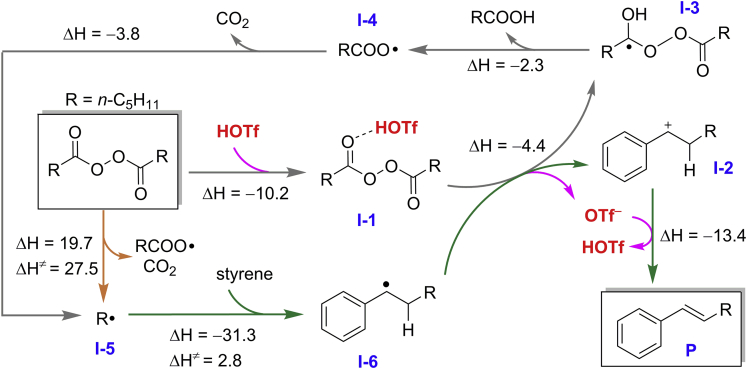
Plausible Reaction Mechanism
As the result shown in entry 10 of Table 1, no desired product was observed in the absence of HOTf, implying that HOTf must play a vital role in the reaction. Density functional theory (DFT) calculations were carried out to gain further insight into the reaction mechanism. As can be seen from Scheme 4, before the catalytic cycle R⋅ radical I-5 can be formed by homolytic dissociation of the alkyl diacyl peroxide, which is a very slow step with a high barrier of 27.5 kcal/mol. However, this is considered as the trigger to invoke the following catalytic cycle. Attack on the styrene substrate by the active species R⋅ radical to form a benzyl radical (I-6) leads to energies lower by 31.3 kcal/mol with a small barrier of 2.8 kcal/mol, indicating that such a reaction is both thermodynamically and kinetically favorable. In the beginning of the catalytic cycle, LPO binding a molecule of HOTf forms a complex I-1 with a strong hydrogen bonding of 10.2 kcal/mol. This complex oxidizes benzyl radical (I-6) to yield a benzyl cation species (I-2), a radical (I-3), and an OTf− anion, which is exothermic by 4.4 kcal/mol. Meanwhile, the generated OTf− deprotonates I-2 to yield the product and regenerate the acid HOTf with a reaction energy of −13.4 kcal/mol. Thus, from the reactions of LPO and I-6 with the product and I-3, a proton-coupled electron transfer process is promoted by HOTf, which serves as the driving force and proton source for the reaction. Thereby, homolytic dissociation of I-3 leads to RCOO⋅ radical (I-4) and RCOOH, which is exothermic by 2.3 kcal/mol without any barrier. Subsequently, C-C cleavage of I-4 is exothermic by 3.8 kcal/mol, which releases the active species R⋅ radical (I-5) and CO2 to close the catalytic cycle. Alternatively, in the absence of HOTf formation of this radical I-4 with carboxylic acid RCOOH requires high energies (>27 kcal/mol, See Scheme S1), indicating that the strong acidity of HOTf plays a significant role in the formation of I-4. A similar mechanism of reaction starting from perester was also calculated and presented in Scheme S2.
Scheme 4.
Plausible Reaction Mechanism
Conclusion
We have developed a Brønsted acid-catalyzed radical alkyl-Heck-type reaction of alkenes with aliphatic acids. This HOTf-catalyzed process has been shown to be an efficient method to deliver only E-alkenes in most cases. Relatively simple and available starting materials are used, and wide substrate scope and good functional group tolerance are observed. Preliminary mechanistic studies illustrated the vital role of HOTf in the reaction, whose proposed mechanism is supported by both the experimental and computational results.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank NSFC (Grant Nos. 21502191, 21672213, 21232001, 21603227, 21573237), Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB20000000), and Haixi Institute of CAS (CXZX-2017-P01) for financial support, and Hundred-Talent Program of the Chinese Academy of Sciences.
Author Contributions
Performed synthetic experiments and analyzed the experimental data: H.Z., L.G., W.J., and Y.L.; theoretical calculations: J.S. and C.L.; performed investigations and prepared the manuscript, H.B.
Declaration of Interests
The authors declare no competing interests.
Published: May 25, 2018
Footnotes
Supplemental Information includes Transparent Methods, 164 figures, 2 schemes, 6 tables, and 3 data files and can be found with this article online at https://doi.org/10.1016/j.isci.2018.04.020.
Contributor Information
Chunsen Li, Email: chunsen.li@fjirsm.ac.cn.
Hongli Bao, Email: hlbao@fjirsm.ac.cn.
Data and Software Availability
The data for the X-ray crystallographic structure of 55 and 56 have been deposited in the Cambridge Crystallographic Data Center under accession number CCDC: 1477011 and CCDC: 1476738 (also see Data S2 and Data S3 in Supplemental Information).
Supplemental Information
References
- Beletskaya I.P., Cheprakov A.V. The heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 2000;100:3009–3066. doi: 10.1021/cr9903048. [DOI] [PubMed] [Google Scholar]
- Choi J., Fu G.C. Transition metal-catalyzed alkyl-alkyl bond formation: another dimension in cross-coupling chemistry. Science. 2017;356 doi: 10.1126/science.aaf7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcamp J.H., Gormisky P.E., White M.C. Oxidative heck vinylation for the synthesis of complex dienes and polyenes. J. Am. Chem. Soc. 2013;135:8460–8463. doi: 10.1021/ja402891m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchak V.A., Voronov S.A., Yur’ev R.S. New synthesis of tert-butyl peroxycarboxylates. Russ. J. Org. Chem. 2006;42:487–490. [Google Scholar]
- Dounay A.B., Overman L.E. The asymmetric intramolecular heck reaction in natural product total synthesis. Chem. Rev. 2003;103:2945–2963. doi: 10.1021/cr020039h. [DOI] [PubMed] [Google Scholar]
- Edwards J.T., Merchant R.R., Mcclymont K.S., Knouse K.W., Qin T., Malins L.R., Vokits B., Shaw S.A., Bao D.H., Wei F.L. Decarboxylative alkenylation. Nature. 2017;545:213–218. doi: 10.1038/nature22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrington E.J., Brown J.M., Barnard C.F.J., Rowsell E. Ruthenium-catalyzed oxidative heck reactions. Angew. Chem. Int. Ed. 2002;41:169–171. doi: 10.1002/1521-3773(20020104)41:1<169::aid-anie169>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Ge L., Li Y., Jian W., Bao H. Alkyl esterification of vinylarenes enabled by visible-light-induced decarboxylation. Chem. Eur. J. 2017;23:11767–11770. doi: 10.1002/chem.201702385. [DOI] [PubMed] [Google Scholar]
- Heck R.F. Acylation, methylation, and carboxyalkylation of olefins by Group VIII metal derivatives. J. Am. Chem. Soc. 1968;90:5518–5526. [Google Scholar]
- Heck R.F., Nolley J.P. Palladium-catalyzed vinylic hydrogen substitution reactions with aryl, benzyl, and styryl halides. J. Org. Chem. 1972;37:2320–2322. [Google Scholar]
- Ikeda Y., Nakamura T., Yorimitsu H., Oshima K. Cobalt-catalyzed heck-type reaction of alkyl halides with styrenes. J. Am. Chem. Soc. 2002;124:6514–6515. doi: 10.1021/ja026296l. [DOI] [PubMed] [Google Scholar]
- Iqbal N., Choi S., Kim E., Cho E.J. Trifluoromethylation of alkenes by visible light photoredox catalysis. J. Org. Chem. 2012;77:11383–11387. doi: 10.1021/jo3022346. [DOI] [PubMed] [Google Scholar]
- Jian W., Ge L., Jiao Y., Qian B., Bao H. Iron-catalyzed decarboxylative alkyl etherification of vinylarenes with aliphatic acids as the alkyl source. Angew. Chem. Int. Ed. 2017;56:3650–3654. doi: 10.1002/anie.201612365. [DOI] [PubMed] [Google Scholar]
- Johansson Seechurn C.C., Kitching M.O., Colacot T.J., Snieckus V. Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012;51:5062–5085. doi: 10.1002/anie.201107017. [DOI] [PubMed] [Google Scholar]
- Kambe N., Iwasaki T., Terao J. Pd-catalyzed cross-coupling reactions of alkyl halides. Chem. Soc. Rev. 2011;40:4937–4947. doi: 10.1039/c1cs15129k. [DOI] [PubMed] [Google Scholar]
- Kurandina D., Rivas M., Radzhabov M., Gevorgyan V. Heck reaction of electronically diverse tertiary alkyl halides. Org. Lett. 2018;20:357–360. doi: 10.1021/acs.orglett.7b03591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bras J., Muzart J. Intermolecular dehydrogenative heck reactions. Chem. Rev. 2011;111:1170–1214. doi: 10.1021/cr100209d. [DOI] [PubMed] [Google Scholar]
- Li Y., Han Y., Xiong H., Zhu N., Qian B., Ye C., Kantchev E.A., Bao H. Copper-catalyzed regioselective 1,2-alkylesterification of dienes to allylic esters. Org. Lett. 2016;18:392–395. doi: 10.1021/acs.orglett.5b03399. [DOI] [PubMed] [Google Scholar]
- Littke A.F., Fu G.C. A versatile catalyst for heck reactions of aryl chlorides and aryl bromides under mild conditions. J. Am. Chem. Soc. 2001;123:6989–7000. doi: 10.1021/ja010988c. [DOI] [PubMed] [Google Scholar]
- Liu C., Tang S., Liu D., Yuan J., Zheng L., Meng L., Lei A. Nickel-catalyzed Heck-type alkenylation of secondary and tertiary alpha-carbonyl alkyl bromides. Angew. Chem. Int. Ed. 2012;51:3638–3641. doi: 10.1002/anie.201108350. [DOI] [PubMed] [Google Scholar]
- Liu Q., Yi H., Liu J., Yang Y., Zhang X., Zeng Z., Lei A. Visible-light photocatalytic radical alkenylation of alpha-carbonyl alkyl bromides and benzyl bromides. Chem. Eur. J. 2013;19:5120–5126. doi: 10.1002/chem.201203694. [DOI] [PubMed] [Google Scholar]
- Liu W., Li L., Chen Z., Li C.J. A transition-metal-free Heck-type reaction between alkenes and alkyl iodides enabled by light in water. Org. Biomol. Chem. 2015;13:6170–6174. doi: 10.1039/c5ob00515a. [DOI] [PubMed] [Google Scholar]
- Loska R., Volla C.M.R., Vogel P. Iron-catalyzed Mizoroki-Heck cross-coupling reaction with styrenes. Adv. Synth. Catal. 2008;350:2859–2864. [Google Scholar]
- Mai W.-P., Song G., Sun G.-C., Yang L.-R., Yuan J.-W., Xiao Y.-M., Mao P., Qu L.-B. Cu/Ag-catalyzed double decarboxylative cross-coupling reaction between cinnamic acids and aliphatic acids in aqueous solution. RSC Adv. 2013;3:19264. [Google Scholar]
- Mc Cartney D., Guiry P.J. The asymmetric Heck and related reactions. Chem. Soc. Rev. 2011;40:5122–5150. doi: 10.1039/c1cs15101k. [DOI] [PubMed] [Google Scholar]
- Mcmahon C.M., Alexanian E.J. Palladium-catalyzed Heck-type cross-couplings of unactivated alkyl iodides. Angew. Chem. Int. Ed. 2014;53:5974–5977. doi: 10.1002/anie.201311323. [DOI] [PubMed] [Google Scholar]
- Mizoroki T., Mori K., Ozaki A. Arylation of olefin with aryl iodide catalyzed by palladium. Bull. Chem. Soc. Jpn. 1971;44:581. [Google Scholar]
- Na Y., Park S., Han S.B., Han H., Ko S., Chang S. Ruthenium-catalyzed Heck-type olefination and Suzuki coupling reactions: studies on the nature of catalytic species. J. Am. Chem. Soc. 2004;126:250–258. doi: 10.1021/ja038742q. [DOI] [PubMed] [Google Scholar]
- Nishikata T., Noda Y., Fujimoto R., Sakashita T. An efficient generation of a functionalized tertiary-alkyl radical for copper-catalyzed tertiary-alkylative Mizoroki-Heck type reaction. J. Am. Chem. Soc. 2013;135:16372–16375. doi: 10.1021/ja409661n. [DOI] [PubMed] [Google Scholar]
- Noble A., Mccarver S.J., Macmillan D.W. Merging photoredox and nickel catalysis: decarboxylative cross-coupling of carboxylic acids with vinyl halides. J. Am. Chem. Soc. 2015;137:624–627. doi: 10.1021/ja511913h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paria S., Kais V., Reiser O. Visible light-mediated coupling of α-bromochalcones with alkenes. Adv. Synth. Catal. 2014;356:2853–2858. [Google Scholar]
- Qian B., Chen S., Wang T., Zhang X., Bao H. Iron-catalyzed carboamination of olefins: synthesis of amines and disubstituted beta-amino acids. J. Am. Chem. Soc. 2017;139:13076–13082. doi: 10.1021/jacs.7b06590. [DOI] [PubMed] [Google Scholar]
- Qin T., Cornella J., Li C., Malins L.R., Edwards J.T., Kawamura S., Maxwell B.D., Eastgate M.D., Baran P.S. A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science. 2016;352:801–805. doi: 10.1126/science.aaf6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueping M., Leiendecker M., Das A., Poisson T., Bui L. Potassium tert-butoxide mediated Heck-type cyclization/isomerization-benzofurans from organocatalytic radical cross-coupling reactions. Chem. Commun. 2011;47:10629–10631. doi: 10.1039/c1cc14297f. [DOI] [PubMed] [Google Scholar]
- Shirakawa E., Zhang X., Hayashi T. Mizoroki-heck-type reaction mediated by potassium tert-butoxide. Angew. Chem. Int. Ed. 2011;50:4671–4674. doi: 10.1002/anie.201008220. [DOI] [PubMed] [Google Scholar]
- Standley E.A., Jamison T.F. Simplifying nickel(0) catalysis: an air-stable nickel precatalyst for the internally selective benzylation of terminal alkenes. J. Am. Chem. Soc. 2013;135:1585–1592. doi: 10.1021/ja3116718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C.L., Gu Y.F., Wang B., Shi Z.J. Direct arylation of alkenes with aryl iodides/bromides through an organocatalytic radical process. Chem. Eur. J. 2011;17:10844–10847. doi: 10.1002/chem.201101562. [DOI] [PubMed] [Google Scholar]
- Tang S., Liu K., Liu C., Lei A. Olefinic C-H functionalization through radical alkenylation. Chem. Soc. Rev. 2015;44:1070–1082. doi: 10.1039/c4cs00347k. [DOI] [PubMed] [Google Scholar]
- Tellis J.C., Kelly C.B., Primer D.N., Jouffroy M., Patel N.R., Molander G.A. Single-electron transmetalation via photoredox/nickel dual catalysis: unlocking a new paradigm for sp(3)-sp(2) cross-coupling. Acc. Chem. Res. 2016;49:1429–1439. doi: 10.1021/acs.accounts.6b00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriyama F., Cornella J., Wimmer L., Chen T.G., Dixon D.D., Creech G., Baran P.S. Redox-active esters in Fe-catalyzed C-C coupling. J. Am. Chem. Soc. 2016;138:11132–11135. doi: 10.1021/jacs.6b07172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.Z., Shang R., Fu Y. Irradiation-induced palladium-catalyzed decarboxylative heck reaction of aliphatic N-(acyloxy)phthalimides at room temperature. Org. Lett. 2018;20:888–891. doi: 10.1021/acs.orglett.8b00023. [DOI] [PubMed] [Google Scholar]
- Wang J., Qin T., Chen T.G., Wimmer L., Edwards J.T., Cornella J., Vokits B., Shaw S.A., Baran P.S. Nickel-catalyzed cross-coupling of redox-active esters with boronic acids. Angew. Chem. Int. Ed. 2016;55:9676–9679. doi: 10.1002/anie.201605463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weix D.J. Methods and mechanisms for cross-electrophile coupling of Csp(2) halides with alkyl electrophiles. Acc. Chem. Res. 2015;48:1767–1775. doi: 10.1021/acs.accounts.5b00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.-F., Anbarasan P., Neumann H., Beller M. From noble metal to Nobel Prize: palladium-catalyzed coupling reactions as key methods in organic synthesis. Angew. Chem. Int. Ed. 2010;49:9047–9050. doi: 10.1002/anie.201006374. [DOI] [PubMed] [Google Scholar]
- Xu K., Tan Z., Zhang H., Liu J., Zhang S., Wang Z. Photoredox catalysis enabled alkylation of alkenyl carboxylic acids with N-(acyloxy)phthalimide via dual decarboxylation. Chem. Commun. 2017;53:10719–10722. doi: 10.1039/c7cc05910h. [DOI] [PubMed] [Google Scholar]
- Ye C., Li Y., Bao H. Copper-catalyzed decarboxylative alkylation of terminal alkynes. Adv. Synth. Catal. 2017;359:3720–3724. [Google Scholar]
- Yu C., Iqbal N., Park S., Cho E.J. Selective difluoroalkylation of alkenes by using visible light photoredox catalysis. Chem. Commun. 2014;50:12884–12887. doi: 10.1039/c4cc05467a. [DOI] [PubMed] [Google Scholar]
- Zhang J.J., Yang J.C., Guo L.N., Duan X.H. Visible-light-mediated dual decarboxylative coupling of redox-active esters with alpha,beta-unsaturated carboxylic acids. Chem. Eur. J. 2017;23:10259–10263. doi: 10.1002/chem.201702200. [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhao J., Bao H. Iron catalyzed methylation and ethylation of vinyl arenes. Chem. Sci. 2017;8:2081–2085. doi: 10.1039/c6sc04274k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Zhou J.S. Palladium-catalyzed intermolecular Heck reaction of alkyl halides. Chem. Commun. 2014;50:3725–3728. doi: 10.1039/c4cc00297k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.